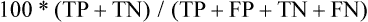Imaging
• Noninvasive medical imaging often is essential to cancer management at multiple times in the course of the illness.
• Imaging currently is used for screening to detect cancer, characterize lesions, perform locoregional and systemic staging, provide prognostic information, assess response during and after therapy, restage after treatment, perform follow-up of patients for recurrence, and precisely guide biopsies and therapies such as external beam or systemic radiation, brachytherapy, or thermal and other ablations.
• More invasive interventional radiologic procedures also can guide and monitor vascular or intraluminal delivery of treatments such as radioactive microspheres, embolic materials, radiofrequency or cryoablation, and therapeutic drugs.
• Imaging methods range from the traditional anatomic methods—radiograph, computed tomography (CT), and ultrasound—to the more functional methods of magnetic resonance imaging (MRI) and nuclear medicine methods, including positron emission tomography (PET), single photon emission computed tomography (SPECT), and planar nuclear imaging. Hybrid methods combining PET and CT, SPECT and CT, and PET and MRI are growing in importance. Optical imaging is promising but is limited by penetration of light through tissues to superficial structures in most cases.
• Plain films and mammography remain useful techniques, with mammography (including digital mammography) being the main imaging method that has been clearly proven capable of reducing cancer deaths when applied in the screening setting.
• CT remains the cornerstone technology for most oncologic imaging, and CT technology that allows for rapid-sequence angiography is finding new applications, as is three-dimensional reconstruction of CT data sets. Screening data with CT-colonography continues to improve, and in some studies it has been found to be comparable with traditional colonoscopy for colon cancer screening. CT scanning for lung cancer screening appears to be capable of reducing lung cancer death rates when applied to high-risk populations. The radiation dose from CT is a concern, and major efforts to reduce this dose from CT scanning have been implemented in newer CT systems.
• MRI is the imaging tool of choice for central nervous system, spinal, and musculoskeletal neoplasms, as well as for assessing vascular and some hepatobiliary and pelvic lesions. MRI also can be used to detect breast cancers, especially in women with dense breasts. Concerns regarding gadolinium-associated nephrogenic systemic fibrosis have led to cautions in the use of MRI contrast medium in patients with impaired renal function. Newer MRI techniques such as diffusion imaging and complement diffusion contrast MRI appear promising in assessing response to tumor treatment.
• Bone scans using single-photon methods (e.g., technetium-99m methylene diphosphonate) remain the dominant procedure for detecting suspected bone metastases; however, the PET agent fluorine-18 sodium fluoride is increasingly being applied. These techniques may be less sensitive for marrow involvement than MRI and other PET techniques for detecting bone metastases of many tumors.
• PET and PET/CT technology using 18F-fluorodeoxyglucose (FDG) continues to grow in a wide variety of applications, and its use is becoming increasingly routine in the management of patients with cancer at varying states of the disease process. PET is used with increasing frequency in the staging and follow-up of lung, colorectal, and head and neck cancers, as well as lymphomas and other types of tumors, and it is now a routine tool in lymphoma management at several points in the disease. PET with non-FDG tracers is a promising research area with growing clinical applications. In particular, progress has occurred in imaging of prostate cancer with several imaging agents, including U.S. Food and Drug Administration (FDA)–approved carbon-11 choline.
• The fusion of anatomic and functional images to create hybrid “anatomolecular images” with software or dedicated instruments such as PET/CT, SPECT/CT, or the newer PET/MRI devices also is seeing rapid growth in applications in cancer imaging. Fully diagnostic CT scans coupled with PET imaging in the form of PET/CT often provide valuable composite imaging for cancer management. PET/MRI is an evolving technology, and several technical approaches are in clinical use at select medical centers.
• Imaging management for staging lung cancer and characterizing solitary pulmonary nodules often includes FDG-PET in addition to CT when the technology is available because PET-CT has high accuracy in lung cancer assessments compared with CT.
• Imaging management of suspected recurrences of colorectal cancer, head and neck cancer, lymphoma, and many other cancers often now includes the use of PET in addition to CT. Response criteria for FDG-avid lymphomas are now mainly PET-based, and PET assessments of treatment response are increasingly applied. Use of PET at earlier stages in the workup is becoming increasingly common, as is the use of PET in early assessments of the efficacy of cancer therapies. Adapting treatments based on the response seen on PET/CT is also increasingly applied.
• In prostate cancer, available imaging methods remain suboptimal for the detection of primary tumor and early determination of local or systemic tumor spread. MRI nodal contrast agents are promising but not yet routinely available, and MR spectroscopy has had only limited success in the prostate. A variety of MRI sequences, including T2 images, diffusion images, and diffusion contrast enhanced MRI may improve upon purely anatomic MRI approaches for lesion detection and detection of extracapsular involvement. A variety of innovative radiotracers for PET show promise for detecting disease recurrence, and 11C choline is now approved by the FDA in the United States for use in persons with prostate cancer.
• Visceral angiography for diagnostic purposes is being supplanted by CT and MRI methods; however, it remains important as a tool for intravascular delivery of therapies such as chemotherapy, coils, or radioactive microspheres.
• CT, ultrasound, fluoroscopy, and innovative MRI systems can guide interventional procedures such as thermal and cryotherapeutic lesion ablations.
• Highly specific probe-reporter systems are being developed to allow for optical and radionuclide imaging of transfected gene biodistribution and function. These approaches face major regulatory challenges when being translated to humans.
• Combined anatomic and functional information is being applied to allow for more precise planning of external beam radiation therapy, including intensity-modulated radiation therapy and conformal therapy, which are methods that potentially allow for increasing dose escalation and minimization of toxicity to normal tissues.
• Emerging imaging methods are proving increasingly useful in providing information on the physiology and molecular characteristics of lesions, which means that a multiparametric biological imaging phenotype for tumors can be obtained, making it possible to display heterogeneities in tumors. This phenotype can more precisely guide individualized tumor treatment to yield a higher probability of success without excessive toxicity for treatment of the selected neoplastic process.
Introduction
Specific clinical questions addressed by imaging include screening for the presence of cancer, characterizing anatomic lesions as malignant or benign, and staging a neoplasm—that is, determining the size and local extent of a primary lesion and determining whether it is localized or locoregionally or systemically metastatic. Such studies are essential for determining whether the patient is a candidate for surgical resection, identifying the extent of the field for radiation therapy, and determining whether systemic chemotherapy is appropriate. Initial staging of tumor size and extent also can provide important prognostic data. During the course of treatment, imaging is used to determine the response of the cancer. Imaging also is often used to monitor patients for recurrence or the development of second malignancies. Imaging is being used more often as a method to assist in the delivery of minimally invasive therapeutic procedures to ablate cancers, guide radiation therapy, and guide the dosing of therapeutic drugs, including radiopharmaceutical agents, more precisely.1
General Considerations
Performance of Imaging Tests
Sensitivity
< ?xml:namespace prefix = "mml" />
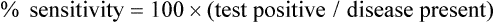
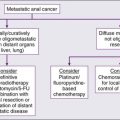
It can sometimes be difficult to judge how “good” a test is by reading the literature. Sensitivity is supposed to be substantially independent of study composition, but as discussed in the next section, certain imaging tests may be insensitive for some very early-stage disease but very, very sensitive for more advanced disease. Each imaging test has a limit of detection threshold below which tumors cannot be detected because they are not distinguishable from the background tissues. Thus the patient population and, very often, the tumor burden and average tumor size can make a difference in the sensitivity of a test for the detection of cancer. Virtually all noninvasive imaging tests are less sensitive for small-volume disease than for large-volume disease. For example, if an imaging test is used in a patient population in which patients have advanced disease before seeking medical attention (e.g., they are symptomatic at presentation), the imaging test may have far greater sensitivity than if it were used in patients with earlier stage, smaller tumors. For example, positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose (FDG) has been reported to be more than 90% sensitive for detecting metastatic melanoma, but it is less than 20% sensitive in detecting early (low tumor volume) nodal metastases of melanoma at initial surgical resection. Mammography has higher sensitivity in women with more radiolucent than radiodense breasts. A test with high sensitivity has a low number of false-negative results.2 The false-negative fraction usually is expressed as 1− sensitivity (in this case, sensitivity being rated on a 0-1 scale).3
Specificity
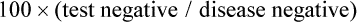
Again, specificity can be calculated on a per-patient basis, a per-lesion basis, or a per-region basis. The per-patient calculations commonly are performed in the screening setting. They also can be done per region of the body (e.g., Is the liver free of tumor? Are the draining lymph nodes free of tumor?). It is technically difficult and sometimes impossible to know exactly how many tumor foci are present, because this depends on the reference gold standard. It is not possible to do “whole body” biopsies antemortem, and thus some very small tumor foci may not be known to be present when disease is diagnosed. Specificity can be affected substantially if the imaging test is used in a population that has a characteristic that can result in false-positive results for the imaging test. For example, inflammatory and infectious lung disease, such as active tuberculosis or sarcoidosis, if present in a patient population, can result in false-positive findings on PET or computed tomography (CT) scans or other imaging methods. In this situation, the specificity of FDG-PET, and likely of CT, for staging the mediastinum for cancer would vary. Thus the specificity of PET for assessing mediastinal lymph nodes may be much lower in areas of the world with endogenous tuberculosis than in developed areas without it. Therefore an imaging test that is very useful in one part of the world may be far less useful in another part of the world. A highly specific test has a low frequency of false-positive results (i.e., a low frequency of positive test results in the patient population that does not have the disease). The ideal imaging test has both high sensitivity and high specificity, although none of our current imaging tests has perfect sensitivity and specificity.2
Positive and Negative Predictive Values
Thus a test that is effective in a patient population with a high prevalence of a disease may be far less valuable in a patient population with a lower prevalence of the same disease, because there would be far too many false-positive results. The most effective use of imaging technology is in groups of patients in whom the imaging characteristics are expected to be robust enough to allow for predictions in individual patients. These challenges are particularly apparent when a test that was developed and validated in a patient population with disease is used to evaluate individuals with a lower prevalence of tumor (i.e., screening). In this situation, the number of false-positive findings may rise dramatically, sometimes nearly completely negating the value of the test.4
Receiver Operator Characteristic Curves
Cancer imaging tests are interpreted by imaging specialists, who are often radiologists. As with all of medicine, considerable science is involved in image interpretation, but the human element, or “art” as it is referred to in some settings, also is involved. In developed countries, medical specialty boards have been established to ensure that practitioners have a base level of training and knowledge, thereby providing some level of uniformity to the interpretation of images. However, even with board certification and extensive training, not all imaging specialists interpret a given imaging study in the same manner. Thus although the goal of an imaging test often is a simple binary “yes, there is tumor” or “no, there is no tumor” answer, varying degrees of certainty exist in the interpretation of an image in most instances. Some readers read with high sensitivity, whereas others read with high specificity. Unless a test is very robust, it is difficult to achieve both high sensitivity and high specificity.5
An example of a receiver operator characteristic (ROC) curve is shown in Figure 18-1. This set of curves reflects the performance of PET imaging in detecting axillary metastases in patients with newly diagnosed breast cancer. The axes of the curves are the true-positive fraction (sensitivity/100), which forms the y axis, and the false-positive fraction (1—specificity/100), which forms the x axis, on a scale of 0 to 1. A perfect diagnostic test would yield no false-positive or false-negative results. The greater the area under an ROC curve, the greater the accuracy of the test.
The results shown in Figure 18-1 are from three readers who graded PET scans using a five-point certainty scale (i.e., not a simple yes/no but a continuum from definitely abnormal to definitely normal). The three readers had similar ROC curves, indicating that they were of generally comparable accuracy. For the same test, however, two readers may be reading at different points on the ROC curve, meaning that one is more sensitive and one is more specific, but both are of equal accuracy.
An excellent reader may have a greater area under the ROC curve than a less skilled reader, meaning that the more experienced (and hopefully more capable) reader is both more sensitive and more specific than a less experienced (and presumably less capable) reader. However, virtually none of our imaging tests is perfect, and varying “cut points” between disease and normalcy often are made, affecting the overall performance of the test. In this study, the area under the curve (AUC) of 0.7 to 0.76 was not viewed as sufficiently good for the task of nodal detection of metastatic cancer spread to the axilla.5 Despite this, a very high sensitivity or a very high specificity can be achieved depending on which part of the curve one operates in. A higher, hypothetical curve, with an AUC of 0.9, is shown for a more robust test, such as a higher resolution PET system devoted to imaging the axilla.
In practice, sentinel node sampling, which often is guided by imaging or a radionuclide-sensitive probe system, is assuming a very important role in this area of tumor staging.6 Practically, if a rather insensitive test has a high positive predictive value, then the test may be of value if positive but of little value if negative. For example, a strongly positive PET scan for axillary metastases may obviate the need for a pretreatment axillary dissection in a patient with newly diagnosed advanced breast cancer in whom neoadjuvant chemotherapy could be given.
Other Approaches to Assessing the Value of Imaging
Although sensitivity, specificity, and accuracy commonly are used to characterize the tumor detection process, other metrics may be of greater importance. For example, some studies have focused on how often imaging substantially changes management. This type of study is of great practical interest, but the optimal methods to assess such changes in treatment decisions are evolving. Ideally one would like to show that the use of imaging, especially a new imaging technology, when applied randomly to half of the study population, provided a reduction in the number of adverse events in the imaged population, improved survival, or had comparable outcomes at lower costs than standard treatments. As an example, a reduction in the number of “futile thoracotomies” has been used as a metric of success for PET versus CT in planning the treatment of newly diagnosed lung cancer.7 Ideally, randomization of patients to imaged versus not imaged groups can be shown to improve survival. Performance of randomized trials in which a portion of the patients undergo imaging and the other patients do not undergo imaging (or they obtain different types of imaging), with an end point of survival, will be of great interest. Unfortunately, such studies are complex or impossible, because management of patients after imaging may be altered markedly on the basis of imaging results. Thus it can be difficult to separate the imaging study effect from the treatment effect. Ultimately, however, for some imaging studies to be adopted, such evaluations of survival will be needed. This point is particularly relevant to screening, as will be discussed later.
Recently, registry data have been applied with substantial benefit to determine if planned or actual patient management is altered through the use of imaging tests. The National Oncologic PET Registry has provided a great deal of information on the use of FDG-PET imaging in the management of patients with a variety of cancers. The National Oncologic PET Registry collected questionnaire data from referring physicians on intended patient management before and after PET. After 1 year, the cohort included data from 22,975 studies (83.7% PET/CT) from 1178 centers. Overall, physicians changed their intended management in 36.5% (95% confidence interval [CI] 35.9-37.2) of cases after PET, supporting the usefulness of PET for cancer imaging in registry cases, which are from a wide range of sources.8
Screening Concepts and Challenges
Screening programs for cancer often have taken the form of laboratory tests such as the Papanicolaou (Pap) smear, or, more recently, blood tests for tumor markers. The success of the Pap smear in reducing mortality rates from cervical cancer is incontrovertible. The use of imaging in screening for cancer is an example of success and considerable interest, but also a source of considerable controversy. As discussed in detail in the chapters on breast cancer (see Chapter 91) and lung cancer (see Chapter 72), screening programs have been shown to be capable of saving lives in women older than 50 years. These programs also may save lives in women 40 to 50 years of age, but the data are less compelling.9
Studies have been initiated in which CT scanning is used in an attempt to detect early lung cancer.10 Screening high-risk populations with CT imaging has recently been proven to reduce lung cancer–specific mortality in the National Lung Cancer Screening Trial (NLST). This finding follows results from the Early Lung Cancer Action Project (ELCAP), a large study screening patients at increased risk of lung cancer with low-dose CT, which reported promising results in 1999.11 The ELCAP showed that lung cancers are detected at a smaller size and that patients whose cancers are detected by screening live longer after diagnosis than do patients whose tumors are not detected by screening. Whether this outcome translates into longer-term survival for the screened population remains unclear. The ELCAP study further evaluated 31,567 asymptomatic persons at risk for lung cancer using low-dose CT from 1993 through 2005 and from 1994 through 2005; 27,456 repeated screenings were performed.12 A diagnosis of lung cancer was made in 484 participants based on screening. Of particular note, 412 patients (85%) had clinical stage I lung cancer. Ten-year survival approached 90% in this group.11,12 This study demonstrated that annual spiral CT screening can detect lung cancer that is curable.
Another large randomized trial of lung cancer screening, the NLST, was reported in 2011.13 In this trial, 53,454 persons at high risk for lung cancer were enrolled from more than 30 U.S. sites. Study participants were randomly assigned to undergo three annual screenings with either low-dose CT (26,722 participants) or single-view posteroanterior chest radiography (26,732). In the CT-screened and chest radiograph–screened groups, 24.2% and 6.9%, respectively, had positive screening studies at some point. A high false-positive screening rate occurred; 96.4% of the positive screening results in the low-dose CT group and 94.5% in the radiography group were false positives. The incidence of lung cancer was significantly higher (1060 vs. 941 cancers) in the low-dose CT group compared with the chest radiograph group. There were 247 deaths from lung cancer per 100,000 person-years in the low-dose CT group and 309 deaths per 100,000 person-years in the radiography group, representing a relative reduction in mortality from lung cancer with low-dose CT screening of 20.0% (95% CI 6.8-26.7; P = .004). The rate of death from any cause was reduced in the low-dose CT group compared with the radiography group by 6.7% (95% CI 1.2-13.6; P = .02).
• The cancer must have a considerable public health effect.
• The disease must have an asymptomatic period in which detection by imaging is possible.
• A therapeutic intervention that should lead to better survival or quality of life must be available.
• The prevalence of the disease must be sufficient in the population being screened to justify screening (especially the cost). Low prevalence of disease lowers the positive predictive value of positive scans.
• Medical treatment, surgery, or other treatment must be available for the early-stage cancer identified by screening.
• The screening test itself must not cause disease at a significant rate.
• There must be a high likelihood that the patients in whom early cancer is identified by image-based screening will go on to have a suitable therapeutic intervention.
Further, the imaging test itself must be acceptable to patients (in terms of level of discomfort and cost), and it must be sufficiently sensitive to identify cancer often and sufficiently specific to minimize false-positive results. Finally, the costs of the screening process—and attendant costs related to false-positive examinations—must be compatible with the economic resources of the society or individual, and the screening procedure must pose little or no risk to the patient.4
Another important consideration in screening programs is lead time bias. This concept, simply stated, indicates that if the natural history of a disease is unchanged, but the diagnosis is made earlier in the course of the illness, the apparent survival will be improved. For example, let us assume that tumor X has a 6-year natural history from its beginning until the death of the patient, and that treatment is ineffective. The disease might become clinically detectable after 4 years and lead to death in 6 years, with a 2-year survival after diagnosis. With screening, if the tumor is detected 3 years after the onset of disease and no improvement in treatment occurs, then the survival in the screened population would appear to increase from 2 to 3 years after diagnosis. This illusion of improved survival in the screened population is a considerable concern and can lead to inappropriate enthusiasm for screening programs.4
Another important consideration in screening is the possibility of length bias, which is a more complex concept, but it may be related to the types of cancer that can be detected by screening programs. A possibility is that very rapidly growing and presumably highly lethal cancers are less likely to be detected by annual screening programs, whereas more slowly growing cancers, which have an intrinsically better prognosis, may be detected more frequently by screening. In fact, some of the early cancers discovered by screening may not be biologically relevant at all. If so, the patients with cancers identified in the screened population could appear to have a better survival than the patients with cancers identified in the unscreened population, as is the case for prostate cancer screening by prostate-specific antigen (PSA), for example, for which major concerns exist regarding overdiagnosis of slow-growing cancers that are presumed to be indolent and might not require surgery.14
A third factor is the selection bias that is difficult to control for in retrospective observational studies—that is, how the patients and their referring physicians determined that a scan should be performed. Selection bias occurs when unintended differences exist between the groups observed that—while associated with the variable used to sort the groups (for example, exposure in case-control studies and outcome in cohort studies)—affect measurement of the study variable.15 For instance, in a case-control design on the effects on disease-specific mortality of a particular screening program, investigators would examine records from patients who have died from the disease in question versus those who have not died, and then determine the rates of the screening intervention in these two populations.
Screening Costs
The determination of whether a screening program is valuable to society is often, in part, based on its cost and benefit. The concept of quality-adjusted life-years (QALYs) often is applied. This concept is defined as the economic cost to society required to result in 1 additional year of quality life for a member of the society. In many Western countries, a figure of $50,000 has been considered a useful guide, with QALYs costing less than this amount considered cost-effective. Such a guideline, however, does not necessarily apply when individuals make their own determinations about whether to pay for a screening test. For example, it is reasonable to expect that persons with greater disposable income would be willing to pay more per QALY than would persons with less disposable income. Thus it can be difficult to generalize about the cost efficacy of screening procedures.16
Size of Detectable Lesions
Noninvasive imaging methods in humans cannot detect and localize a single malignant cell, although flow cytometric methods are very sensitive for finding a very few cancer cells in a patient’s blood, and investigators have a great interest in assays for circulating tumor cells and for tumor DNA and RNA in the blood. Imaging methods are improving, however, and detection of a much smaller number of cells is possible in small animal models. It has been estimated that by the time a tumor reaches 3 to 5 mm in diameter, which is the lower limit in size for detection by the best current noninvasive methods in humans, the tumor has undergone more than 25 doublings and contains 0.1 to 1 billion cells, depending on their size.1 In contrast, a cytologist, on a very good day, or with flow cytometric methods, may be able to identify a single cell as malignant using a microscope.
Major Imaging Modalities
Broadly stated, cancer imaging can be performed using anatomic or functional (“molecular”) imaging methods.1 The traditional imaging of the patient with cancer, and the most established methods, are based on anatomic imaging. However, interest is increasing in more functional methods in cancer imaging. Further, several anatomic imaging methods offer functional components that complement the anatomic method. Hybrid images, derived from and displaying both functional and anatomic data, also are becoming more widely available, often coming from the same hybrid imaging machine, such as PET/CT.17,18 Imaging data are increasingly digital or digitized and suitable for postprocessing and image exchange. The major imaging modalities are discussed in the following section.
Plain Film Radiographs
Plain radiographs offer exceptional resolution but provide relatively little image contrast if not much calcium is present. The radiation dose from a plain film radiograph depends on which portion of the body is being examined. In most centers, plain film radiographs for cancer management are being used less often, with CT scanning increasingly replacing radiographs in the abdomen and MRI in the brain and extremities.19
Mammography
The recent Digital Mammographic Imaging Screening Trial, which included nearly 50,000 women, showed comparable overall accuracy between film screen and digital mammography in the overall study. A higher accuracy for cancer detection in women younger than 50 years, women with radiodense breasts, and pre- and perimenopausal women was seen with digital mammography compared with film-screen mammography.20 Despite this higher accuracy, the reported sensitivity for both techniques for cancers was only 41% with a positive predictive value of 12%, albeit with 98% specificity, which is far from an ideal performance for a screening test. The move to digital mammography is in part to increase diagnostic accuracy but also to allow digital images to be viewed on picture archiving and communication system, as is the case with virtually all other diagnostic imaging methods in more and more imaging centers.20,21
A newer technique, tomosynthesis, is under evaluation in which a number of “slices” of the breast are generated during a mammographic image acquisition, which involves a moving x-ray source. This approach offers considerable promise going forward but is early in its evolution technically. It has recently received approval from the U.S. Food and Drug Administration (FDA) and will likely improve upon the performance of mammography.22 The cost efficacy of such an approach continues to evolve.
Computed Tomography
Although CT is an exceptional technique, it remains a predominantly anatomic imaging method. Although the timing of intravenous (IV) CT contrast enhancement can provide information, such as the ability to estimate tumor blood flow, it is not easily extracted without a substantial radiation dose from repeated images. Thus only a limited number of post-IV contrast images are obtained with CT to limit radiation dose. All CT images are digital. The large amount of image data generated for analysis with CT also poses a major challenge, because fully interpreting the data can be a lengthy process.23
Angiography
Angiography has high resolution but typically delivers a high dose of radiation energy to the patient. The use of angiography remains essential for studies to evaluate gastrointestinal bleeding, but with CT angiography continuing to improve in quality, the use of diagnostic angiography has become less frequent in routine clinical practice.24
Ultrasound
Ultrasound provides real-time imaging capability to guide biopsies and procedures effectively. Ultrasound is less effective in the evaluation of deeper structures and requires access to a sonographic window; thus it has only a modest role in evaluating deep abdominal structures. Ultrasound is used commonly in evaluations of the pelvis, neck (including the thyroid), and gallbladder and liver areas. Agents that can enhance the visualization of vessels or can be specifically retained in clots are under evaluation, suggesting that ultrasound can offer some functional information beyond that which is purely anatomic.25
Ultrasound contrast agents are being applied to a limited extent. Currently, these agents are mainly for intravascular use. High-energy focused ultrasound remains under development as a tool to ablate, or at least deliver thermal energy to, tumors with therapeutic intent. Ultrasound coupled with needle aspiration biopsy has been used in some settings as a minimally invasive procedure to characterize nodal metastases. Ultrasound combined with mammography also is being evaluated as a screening method for breast cancers, but results in high-risk patients have shown disappointingly low detection rates relative to MRI.26
MRI and Magnetic Resonance Spectroscopy
In many clinical settings, MRI, as applied in imaging for most cancers, is used predominantly as an anatomic imaging method that does not use ionizing radiation. MRI offers superb contrast resolution between tissues and excellent spatial resolution. MRI also offers a variety of forms of functional information. However, it does not offer the level of temporal resolution, in general, that is seen with ultrasound, fluoroscopy, or recent-generation, multiple-slice CT scanners. However, MRI technology has moved forward inexorably, and rapid-pulse sequences allowing gating of images now are available for several types of scanners. MR images can be of a variety of pulse sequences, allowing visualization of several parameters. However, visualization of hydrogen nuclei is the major approach with conventional 1.5 tesla (T) and 3 T machines. Visualization of blood (especially with contrast materials such as gadolinium chelates) and of altered vascular permeability is routinely applied.27
In magnetic resonance spectroscopy (MRS), tissue characterization can be achieved by sampling its magnetic spectrum at 1.5 T. More and more scanners now provide a 3 T or higher field strength signal for evaluation, offering the possibility of more refined tissue characterization. Opportunities to detect increased content of choline (which often is increased in tumor foci) versus other substituents can be helpful in separating tumor from nonmalignant tissues in the brain and elsewhere. Spectroscopy also can provide information on lactate concentration and pH, among other parameters. A limitation of spectroscopy is resolution, which typically is not nearly as fine as that of MRI itself. Thus spectroscopy has only limited application in most oncologic practices.28
Recently, diffusion MRI has shown promise in tumor response assessment, which depends on the freer movement of nuclei in areas of necrosis compared with the motion of nuclei in fully viable tumors. This technique is demonstrating considerable potential for response assessment in brain tumors and recently has shown promise in tumors in the bones, breast, prostate, and other tissues.29
Nephrogenic systemic fibrosis has been linked to MRI contrast agents that contain gadolinium.30 This condition has been described in patients with substantially impaired renal function who receive MRI contrast material intravenously. “Black box” warning labels now appear on the product inserts for MRI, and we are seeing increased use of alternative imaging methods to MRI in patients with low creatinine clearance rates followed by sequential imaging studies. Gadolinium may be responsible in part for this process because it has been found in the skin of some of such affected patients.31,32 In 2007, black box warning labels were added to the package inserts related to MRI contrast material containing gadolinium (Box 18-2).
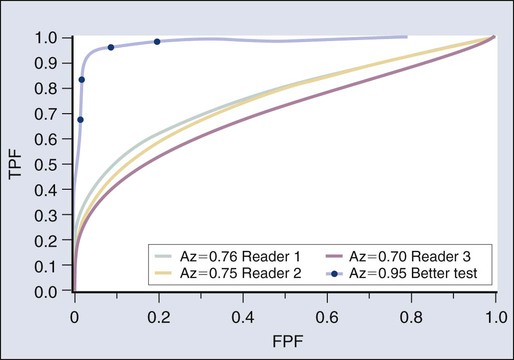
An exciting area of application of MRI technology is in the field of whole-body MRI. Such a test may begin to offer whole-body cancer surveys with no ionizing radiation delivered to the patient. Such applications to date have been less sensitive than whole-body PET imaging, but this area is emerging very rapidly33 (Figs. 18-2 and 18-3).
Nuclear Medicine and PET
With positron emitters, the most commonly used tracer is fluorine-18, which is used as the radiolabel for FDG, an agent that images glycolysis in vivo. Because tumors have increased glycolytic metabolism in general, use of this agent in tumor imaging is increasing very rapidly, especially in lung and colorectal tumors and lymphomas.34
In the past several years, combined PET-MRI devices have also been constructed. These devices can include a PET scanner housed within an MRI scanner that perform both PET and MRI studies at the same time, or adjacent PET and MRI scanners that involve precise alignment of the patient table to allow for sequential imaging using PET and then MRI imaging with preservation of patient alignment. PET-MRI is in evolution; two current limitations are the cost of the systems and challenges associated with precise quantitation of the PET images for radioactivity concentrations in vivo because of the limitations intrinsic to MRI-based attenuation correction algorithms.35
Higher doses of radioactive isotopes also can be therapeutic. For example, iodine-131 is used for the treatment of thyroid cancer, as sodium iodide. The same isotope, conjugated to an anti-CD20 monoclonal antibody, has been approved by the U.S. FDA to treat low-grade and transformed B-cell non-Hodgkin lymphoma that is considered refractory to standard treatments. The tracer doses are used to guide the treatment doses in such instances or at least to determine if the radioantibody has any unexpected targeting behavior.36
Optical Imaging Methods
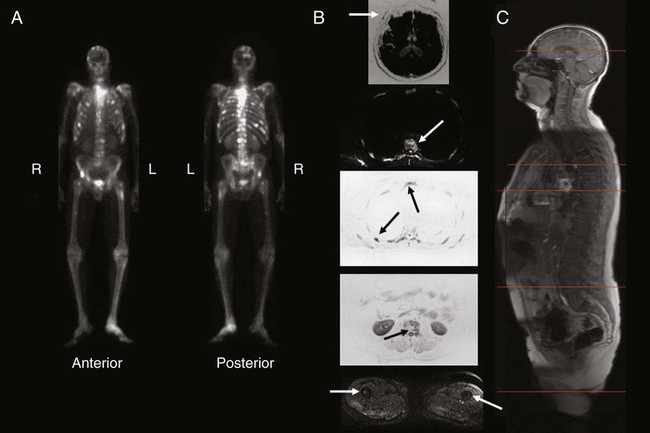
The possibility of constructing light-emitting contrast media is a real one, and optical imaging has the potential to provide remarkable sensitivity and resolution in superficial structures. However, it is not routinely applied in cancer imaging, with the exception of visualization of the interior of the eye, visualization of the cervix, and endoscopy from above and below.37 The combination of light-generating acoustic signals, that is, photoacoustic imaging, also has considerable promise and potential for areas of the body in which light can be delivered at sufficient intensity. The strengths and weaknesses of the major imaging methods are contrasted in Table 18-1.
Table 18-1
| Modality | Resolution | Sensitivity | Specificity | Functional Imaging Ability |
| Magnetic resonance imaging | 1-2 mm | Moderate | Moderate | Moderate with spectroscopy |
| Computed tomography | 1-2 mm | Moderate | Moderate | Low, except angiography |
| Radiographs | 1-2 mm | Low | Moderate | Very little |
| Single photon emission computed tomography | 1 cm | High | Moderate | Excellent |
| Positron emission tomography | 5 mm | High | Relatively high | Excellent |
| Ultrasound | 2 mm | Low | Low | Some, especially with contrast |
| Mammography | 1-2 mm | Moderate | Relatively low | None |
| Angiography | 1-2 mm | Moderate | Moderate | Low |
From Bragg DG, Rubin P, Hricak H. Imaging strategies for oncologic diagnosis and multidisciplinary treatment. In Bragg D, Rubin P, Hricak H, editors. Oncologic imaging. 2nd ed. Philadelphia: WB Saunders; 2002.
Radiation Dose and Imaging
Imaging methods that use ionizing radiation are a major source of population radiation exposure. CT and nuclear medicine procedures (notably cardiac nuclear medicine procedures) are the major sources of the ionizing radiation.38 The most vulnerable population with regard to radiation exposure are children, for whom the risk of radiation-induced cancers is much higher, with a longer time for manifestation, than is seen for cancers in older persons.39 Clearly, caution must be applied when choosing an imaging study using ionizing radiation, because it is quite likely that some increased risks of cancer are associated with a low radiation dose distributed over a very large population of persons.40
It should be noted that many patients with cancer have relatively short life spans because of their underlying disease and the risks of the known cancer greatly exceed the potential risk of cancer from imaging. It is also important to realize that lifetime risks of cancer may carry varying significance; for example, a hypothetically “radiation-induced” cancer that develops 40 years after cure of testicular cancer has a less immediate health care impact than a cancer developing within a few years of radiation.40a Certainly, radiation concerns are appropriate, and every effort should be made to use imaging that includes ionizing radiation only when appropriate and then at the lowest dose possible consistent with adequate image quality.
Anatomic Versus Functional Imaging
Limitations of Anatomic Imaging of Cancer
After surgery, it is even more difficult to assess for the presence of recurrent tumor with anatomic methods. Posttreatment scans are complicated by the need for comparisons with normal anatomy to detect altered morphologic findings as a result of cancer. Anatomic methods do not predict cancer response to treatment and do not quickly document tumors that are responding to therapy.41 Despite these challenges, anatomic images remain routine in cancer management. PET, a functional imaging method, helps to address many of the limitations of anatomic imaging, and when combined with anatomic images in fusion images, it is emerging as a particularly valuable tool that provides both anatomic precision and functional information in a single image set.41
Molecular and Functional Alterations in Cancer
The molecular bases of neoplasia are increasingly becoming well defined. Mutations in genomic DNA precede the development of overt neoplasia.42 With sufficient alterations in genotype, phenotypic changes occur. These genotypic and phenotypic changes in cancer antedate the development of a discrete mass lesion and represent potential targets for innovative imaging agents. The concepts of altered “genome,” “proteome,” and “methylome” resulting in alterations in metabolism, consistent with an altered “metabolosome,” are increasingly recognized as present in cancers, along with a variety of typical cancer “hallmarks.” PET, because of its superb sensitivity to low signal levels, can detect signals from tracers, targeting such alterations that are preferentially present in cancer.
Disease-Specific Imaging Recommendations
Lung Cancer
Chest radiograph screening has not been proven to be effective at reducing mortality from lung cancer. CT-based screening programs are of greater interest; it is clear that these programs can detect lung cancers at an earlier stage, when they are smaller than lung cancers diagnosed by other methods, and these programs appear to prolong survival. Patients with such cancers tend to live longer than do patients with cancers that are diagnosed at a more advanced stage. However, detecting additional cancers is dependent upon detection of small lung nodules, and most of these small nodules do not have a malignant etiology. The costs, both economic and in terms of risks from invasive diagnostic procedures, are considerable. In addition, concerns exist that the cancers detected by such an approach may be the more slowly growing cancers. Results from the NLST support CT screening in a high-risk smoking population. A summary of the results in a format easily understood by patients is included in an article from the New England Journal of Medicine.43
The Fleischner Society has offered guidelines for dealing with small, incidentally detected pulmonary nodules that involve variable imaging follow-up for lesions greater than 4 mm. Growing lesions require additional investigation.44
The morphology of the nodule is investigated to determine whether it has characteristics that suggest malignancy or benignity. The margin and internal density of the lesion are examined. Smooth, well-defined margins suggest a benign nodule, but such margins also are seen in 21% of malignant nodules.45 A lobulated margin suggests cells of different lines with uneven growth, but this margin can be seen in 25% of benign nodules. A spiculated margin is highly suggestive of malignancy. Both benign and malignant nodules can be homogeneous and can cavitate. A cavity with a wall thickness of 4 mm or less is likely benign in 95% of cases, a wall thickness of 5 to 15 mm is indeterminate, and a wall thickness of more than 15 mm is likely malignant in 95% of cases.45 If the lesion contains fat, it is specific for a hamartoma; 50% of hamartomas include fat on CT imaging.
Benign calcifications are central in the lesion, diffuse and solid, laminated, or popcornlike. Calcification is seen in 6% of lung cancers on CT imaging and tends to be eccentric or amorphous.45 Thus calcification alone does not indicate benignity in a lung nodule. If contrast material is administered and the lesion is monitored over 5 minutes, enhancement of less than 15 Hounsfield units (HUs) suggests a benign lesion, and enhancement of greater than 20 HUs suggests a malignant lesion, with reported sensitivity of as high as 98%, specificity of 73%, and accuracy of 85%.46 However, these CT criteria rely on very small changes in CT attenuation levels. These subtle changes may be insufficient to allow for reliable stratification of nodules as malignant or benign, because timing of the CT bolus also is an important consideration. The growth rate of the lesion also can be evaluated, but such evaluation is difficult for subcentimeter lesions. Computer programs for nodule detection are being developed that also provide lesion volume, which may prove useful in follow-up.
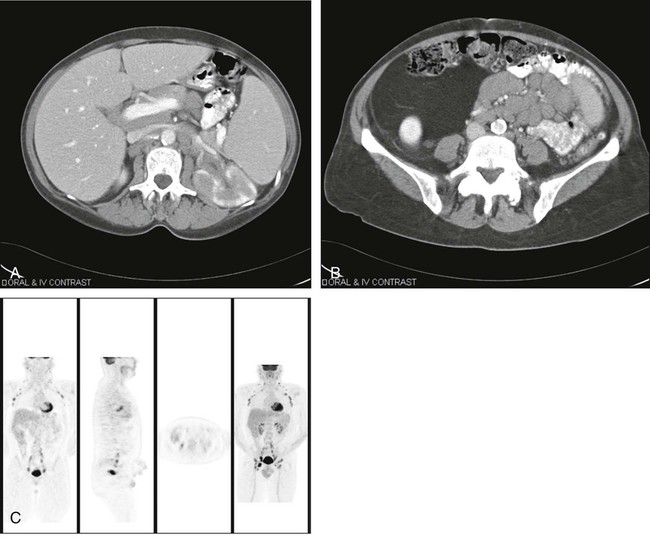
However, for a significant number of patients, the risk of cancer remains intermediate. For such patients, FDG-PET imaging may be useful. PET has been reported to have sensitivity of approximately 96% for detecting cancer in solitary pulmonary nodules (predominantly ≥1 cm in diameter) in a retrospective metaanalysis,47 with specificity of approximately 80%. However, some histologies are less well detected, such as those of bronchioloalveolar carcinomas, which have lower FDG uptake.49–49 Nonetheless, the PET scan can help to determine which patients require immediate biopsy or excision of a nodule (i.e., a nodule with intense FDG uptake) versus those who can be observed (some of those with low or no tracer uptake). The use of PET varies widely, but it can be a valuable tool for helping to determine which patients need invasive procedures for pulmonary nodules. Because false-negative findings occur, however, patients who do not have surgery should be followed up regularly for up to 2 years to ensure that no lesion growth has occurred. Figure 18-4, A, shows “hot” and “cold” nodules in the same patient.
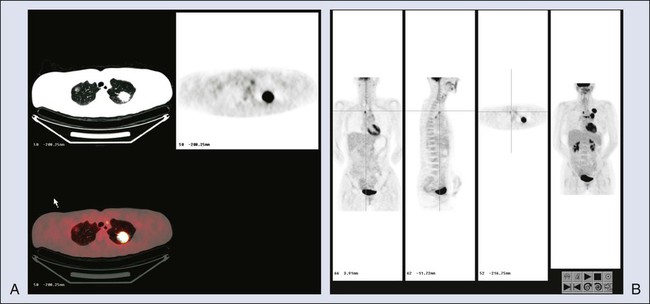
Once lung cancer is diagnosed, an appropriate staging workup should be undertaken. The workup for non–small-cell lung cancer often is performed to determine whether the patient is a candidate for surgery. Because FDG-PET imaging is at least 20% more accurate than CT imaging, PET is commonly recommended as a staging procedure for the mediastinum and for systemic evaluation for metastases.50 In a prospective randomized trial, PET reduced the number of futile thoracotomies by half, from 41% to 21%, versus algorithms in which PET was not performed.51 This reduction occurred, in part, because remote foci of metastatic disease that were not identified by standard staging methods were identified by PET.
Consensus is developing on how PET should be used in staging non–small-cell lung cancer, but many centers routinely perform PET/CT before surgery, with the incremental benefit being most apparent (in terms of detecting additional remote disease from the primary lesion) in patients with larger primary lung cancers. It usually is considered prudent to perform a biopsy on FDG-avid tissues to prove that they represent cancer. Many persons would argue that mediastinoscopy is no longer essential for patients who have negative results of mediastinal PET and CT scans, although in some patients, cancer in nodes is detected only surgically. An example of a positive PET scan with ipsilateral and contralateral mediastinal tumor involvement is shown in Figure 18-4, B. The role of PET/CT in lung cancer is to provide a thorough whole-body screening assessment.
For mediastinal nodes, a short-axis diameter of greater than 1 cm is considered abnormal on CT. Larger nodes can be reactive, and nodes smaller than 1 cm can contain tumor, leading to sensitivity of 40% to 67% and specificity of 79% to 86% for metastatic disease.52
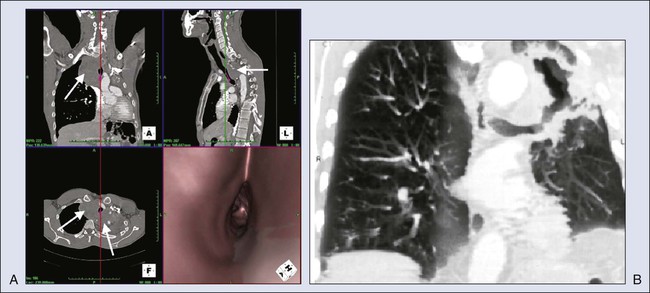
The hybrid PET/CT technology is significantly superior to PET alone for the staging of lung cancer.53 The best algorithm for staging the mediastinum is evolving, but PET and PET/CT are the most accurate noninvasive methods. Some persons argue that mediastinoscopy is necessary in each case, however, because PET may produce false-negative results in patients with a low tumor burden, although the negative predictive value of a negative PET scan and a negative CT scan is approximately 95%. Other persons, however, would argue that patients with a low tumor burden, below the level of detectability with PET and CT, may be suitable candidates for surgery without mediastinoscopy. Positive PET scans for metastases usually require tissue confirmation of the most advanced site of tumor to avoid false-positive imaging findings that indicate a tumor is not resectable. Figure 18-5 illustrates detection of lung cancer on CT and the use of reformatted virtual images.
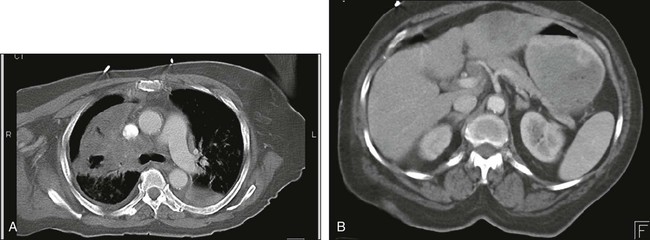
In institutions where PET is not available, a bone scan, MRI of the brain, and CT of the chest and abdomen, including the adrenals, are recommended. The abdominal CT should be performed with contrast to best evaluate the liver. Figure 18-6 shows extensive mediastinal disease and liver metastases on CT. In some centers, PET and diagnostic-quality CT used together provide sufficient diagnostic information, such that no additional studies are required.
For small cell lung cancer, it must be determined whether the disease is extensive or localized before the form of therapy can be decided upon. CT is essential for the chest and upper abdomen. MRI of the brain typically is performed, and a bone scan is performed to search for bone metastases. PET scans have been shown to increase the stage of about 10% of patients with limited stage small cell lung cancer to extensive disease and to find lesions not identified by CT. FDG-PET identifies essentially all lesions detectable with CT.54 Thus many centers are using FDG-PET more routinely for this type of staging, and CMS approves FDG-PET for staging small cell lung cancer.
Evaluation of the Adrenal
Adrenal masses occur in approximately 9% of the population. These masses can be benign adenomas, metastatic disease from primary tumors such as lung cancer, or primary adrenal cortical carcinoma. CT and MRI are used to distinguish adenomas from malignant lesions in the adrenals. Adrenal adenomas have low attenuation on noncontrast CT as a result of elevated lipid content. If a threshold value of 0 HU is used, sensitivity is 47% and specificity is 100% for the diagnosis of adenoma.55 If a threshold value of 10 HU is used, sensitivity is 71% and specificity is 98% for the diagnosis of adenoma.
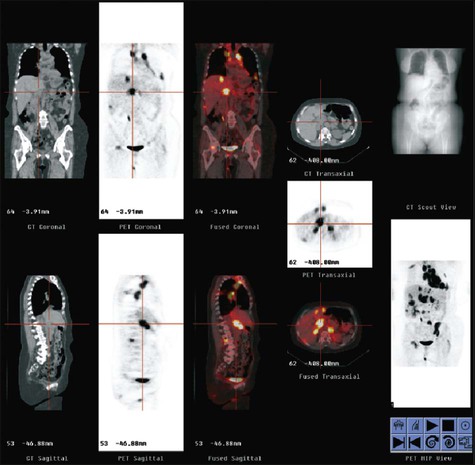
Adenomas take up contrast material, but the washout of contrast material from an adenoma is faster than from a metastatic lesion. On 10-minute delayed images obtained after contrast injection, a greater than 50% decrease in the density of the lesion is specific for an adenoma.55 If the lesion is atypical on CT, chemical shift imaging on MRI is used to determine whether it is an adenoma. An adenoma has both lipid and water content, and decreases in signal are seen on out-of-phase T1-weighted images. PET has a growing role in evaluating the adrenals, with accuracy of ≥90% reported in some series. High FDG uptake typically is seen in metastases to the adrenal. Caution is in order, however, in that some adrenal adenomas can have moderately high FDG uptake. Figure 18-7 illustrates detection of adrenal and systemic metastases on CT and PET.
Breast Cancer
Mammography, the major imaging tool in breast cancer, allows for early detection of tumors. When properly used, mammographic screening programs have been shown to save lives when compared with unscreened populations, and these programs are routinely implemented in many countries.56
Early efforts with tomosynthesis of the breasts, a mammographic method that provides a number of “slices” of the breast for analysis, are promising.57 Recently, breast tomosynthesis devices have received FDA approval, and some studies show superior performance of breast tomosynthesis compared with more standard mammography. Tomosynthesis helps remove overlying structures from the breast image, potentially improving lesion detection. It also is possible that use of IV contrast may enhance mammographic results.58
Although other techniques can detect breast cancer, only ultrasound is used fairly commonly in most imaging centers to help to separate cystic from solid lesions or to help to locate and evaluate palpable but mammographically negative lesions. MRI with gadolinium contrast is used because it is a very sensitive technique and can help to determine whether disease is unifocal or multicentric. An example of a positive mammogram is shown in Figure 18-8, A.
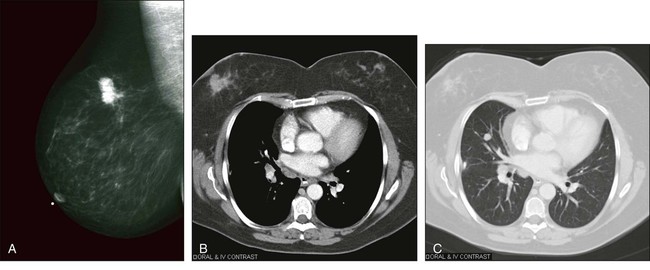
Another concern is that the rate of detection of advanced breast cancers has not changed a great deal since the introduction of mammography, and that some of the improvements in outcomes are due to better overall therapies of breast cancer and not solely due to early detection related to improved diagnosis.59 Although some controversies exist, available data point to regular mammography in women older than 50 years and younger than 75 years as being a valuable tool for reducing the risk of death from breast cancer.
Another method of breast imaging using radionuclides is “molecular breast imaging” (MBI), in which 99mTc methoxyisobutyl isonitrile is injected intravenously and the breasts are imaged with a high-resolution dual-head or single-head gamma camera, optimized for breast imaging. This approach has been evaluated in a number of settings. The radiodense breast is highly problematic for cancer detection by mammography, but it appears the MBI approach is more sensitive. In a study in which MBI was offered as a part of screening for radiodense breasts, of 936 women screened, 11 cancers were identified (one with mammography only, seven with gamma imaging only, two with both combined, and one with neither). Diagnostic yield was 3.2 per 1000 (95% CI 1.1-9.3) for mammography, 9.6 per 1000 (95% CI 5.1-18.2) for gamma imaging, and 10.7 per 1000 (95% CI 5.8-19.6) for both (P = .016 vs. mammography alone).60 The challenge with this method and the positron emission mammography (PEM) method is radiation dose. Major efforts to reduce radiation dose are ongoing. With dose reduction, these approaches may find a growing role in breast cancer screening but must compete directly with MRI approaches, which do not use ionizing radiation.
MRI has been evaluated as a tool for characterizing substantially abnormal mammograms or ultrasounds. In a prospective multicenter National Institutes of Health–sponsored trial, 821 patients referred for a breast biopsy for American College of Radiology category 4 or 5 mammographic assessment or suspicious clinical or ultrasound findings were studied by MRI and showed an AUC of 0.88 in a population with 404 cancers present, with dichotomized data demonstrating sensitivity of 88% and a specificity of 68%. The positive predictive value was about 72%. The high sensitivity was still insufficient to obviate the need for biopsy in these patients, however.61
Recently, MRI has been found to detect breast cancer in the contralateral breast of women with newly diagnosed primary breast cancers in approximately 3% to 4% of cases.62 These additional cancers are found at a rate of about 25% in the lesions identified in this manner (i.e., 75% of the biopsies are negative). In addition, MRI has been used to screen high-risk women (e.g., those with the BRCA1 or BRCA2 mutations) for breast cancer to some advantage. This higher sensitivity comes at the cost of more false-positive results and challenges in biopsy, which have limited deployment of the method. However, increased uniformity of reporting and greater similarities in technique are leading to more use of this methodology, which recently has been comprehensively reviewed.63 PET methods occasionally are applied in the breast, but they are used for diagnosis infrequently because of the low sensitivity of whole-body PET scanners to small tumors. PET is used more commonly in disseminated disease. FDG-PET appears to be superior to CT for detecting disseminated breast cancer.64
Recently, dedicated PET imaging devices for the breast have been increasingly applied for diagnosis. Such PEM devices have relatively good resolution, likely superior to that available from whole-body PET alone. PEM and MRI performed relatively comparably when PEM was directly compared with MRI in a study of 388 women with newly diagnosed breast cancer. Additional cancers were found in 21% of women. In the study, 34% of the 82 breasts with additional cancer were identified with both PEM and MR imaging; 26% with MR imaging only; 17% with PEM only; and 8.5% with mammography and ultrasonography. Of 306 breasts without additional cancer, 279 (91.2%) were correctly assessed with PEM compared with 264 (86.3%) that were correctly assessed with MR imaging (P = .03). The positive predictive value of biopsy prompted by PEM findings (47 [66%] of 71 cases) was higher than that of biopsy prompted by MR findings (61 [53%] of 116 cases) (P = .016).65 MRI was slightly more sensitive, but PEM was more specific in this group. Thus there is promise for the PEM method, especially in women who cannot undergo MRI.
Breast cancer often metastasizes first to locoregional lymph nodes. Current noninvasive imaging methods do not evaluate the axillary nodes effectively. However, imaging can help to define which lymph nodes should be resected for histologic sampling. Sentinel node imaging or detection using a radiation-sensitive probe system is becoming increasingly more important in axillary assessment of patients with breast cancer.66 This procedure is discussed in more detail elsewhere in the text, but imaging is used, especially in European centers, to better localize the axillary nodes for intraoperative assessment and determine atypical routes of lymphatic drainage.
Studies have shown sentinel node sampling procedures to be at least 90% sensitive—which is considered useful sensitivity—relative to axillary dissection, and they are virtually 100% specific.67 FDG-PET is not sufficiently sensitive to detect small metastases and has an accuracy of only about 75% in axillary nodal staging.68
The extent of systemic imaging required in the initial workup of a patient with breast cancer depends on the size of the primary tumor and the status of the axillary nodes at biopsy. It can be argued that the likelihood of systemic metastatic disease is higher for patients with positive nodes; thus more intensive evaluation may be more appropriate. Some clinicians would suggest a baseline bone scan and CT of the chest and abdomen at this time. The frequency of follow-up is debated, given the improbability of curing recurrent systemic disease, and some persons have advocated only limited biochemical follow-up. However, this area is controversial. Examples of primary and metastatic breast cancers imaged on CT are shown in Figure 18-8, B and C. PET is a highly effective tool for evaluating soft tissue involvement and quickly assessing treatment response.69 FDG-PET imaging can detect disseminated cancers; however, the optimal role of PET in imaging is evolving. PET is now approved for monitoring the treatment response of breast cancer.
Prostate Cancer
Evaluation of the Prostate
Ultrasound can be used to detect abnormalities and guide biopsy of the entire prostate because it does an excellent job of defining the overall shape, size, and location of the prostate for purposes of systematic biopsy. A sextant approach often is used to sample the base, middle, and apex of the prostate bilaterally, in addition to taking biopsy samples of any suspicious areas. Although this technique is less than perfectly accurate for detecting prostate cancers, with ultrasound, tumors in the peripheral zone are more readily visible than are tumors in the inner gland. The peripheral zone is echogenic, and tumors that are hypoechoic to it (approximately 60%) can be detected.70 However, hypoechoic lesions also can be caused by inflammatory processes, and the positive predictive value of ultrasound is approximately 18% to 52%.70
Contrast enhancement of the prostate with ultrasound has been used to some advantage to enhance detection rates and positive yields on biopsies of the prostate.71 Similarly, with MRI, cancers that are hypointense on T2-weighted images are seen, but the findings are not specific for tumor. Extension of cancer beyond the prostate capsule is suggested on ultrasound when the capsule margin is irregular or the seminal vesicles are abnormal in morphology; however, sensitivity of as low as 20% has been reported. The sensitivity of MRI for extracapsular invasion is approximately 50%, and specificity is 95%.70 The range of reported accuracies varies widely.
MRI-guided biopsy techniques are also now being applied in research settings for purposes of diagnosis and also as a means of guiding locoregional therapies. Such approaches, when applied by experts, increase the yield of biopsy results from the prostate versus random biopsy approaches.72 This area continues to evolve, but the precise and optimal method of deploying MRI methods, and the precise patients who will benefit most from the method, remain in evolution and practice patterns vary considerably across centers, although expert-based guidelines have been developed.73,74 It is clear that biopsy of findings seen on MRI remains essential.
Patients at intermediate risk for invasion, with a PSA level of 10 to 20 ng/mL and a Gleason score of 5 to 7, may benefit from MRI staging of local extension.70
MRI methods are improving, especially with the use of spectroscopy.70,75 However, spectroscopy is still applied mainly at a few centers with specialized expertise, and thus it is not yet a routine part of management of prostate cancer. It is likely that 3 T methods are superior to 1.5 T methods for spectroscopy. Increased field strength for MRI is now being more systematically assessed and appears promising as a tool to separate benign from malignant prostate tissue by detecting tissue with increased ratios of choline/citrate. To date, it has been hard to prove that spectroscopy greatly improves the diagnosis of prostate cancer.76
CT has no established role in evaluating the prostate itself or local invasion (25% or less sensitivity for capsular invasion), although tumors sometimes can be seen on CT (Figs. 18-9, A, and 18-10). CT is used to detect bladder and rectal invasion, adenopathy, and distant metastases, and MRI is used to assess local extent. Similarly, the sensitivity for detecting nodal metastases has been reported to be as low as 30% with CT. Higher sensitivity can be achieved, but with lower specificity. Pathologic proof is desirable before it is concluded that a patient has metastatic disease to the lymph nodes, and if metastatic disease is demonstrated, radical prostatectomy is considered inappropriate. Large nodal metastases are easily detected on CT imaging, however (Fig. 18-9, B, and Fig. 18-10).
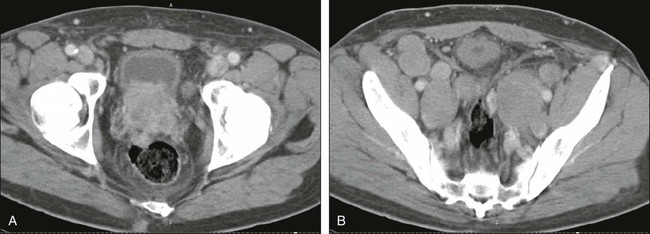
It is not clear that MRI is better than CT for nodal staging; however, recent data on MRI using a node-specific paramagnetic contrast agent have shown very high sensitivity and accuracy in the detection of nodal metastases.75 Considerable hope exists for this or related methods, but MRI-based contrast has not yet been approved by the FDA.77 Given the traditionally low sensitivity of CT and MRI to nodal metastases, it has been recommended that the serum PSA level be at least 20 ng/mL before CT is performed. If the CT findings are positive, then a biopsy can be performed of the enlarged node or nodal sampling can be performed before radical prostatectomy. CT also is the test of choice for visceral metastases.
A 99mTc bone scan is a reasonably sensitive technique relative to radiographs for bone metastases. The results of a bone scan can be positive when radiographs of bone are essentially normal. The current recommendation is that a radionuclide bone scan be performed only if the serum PSA level at presentation is greater than 10 ng/mL. Only very rarely is a bone scan positive for tumor at lower levels at the time of diagnosis. Some persons argue that for patients with large primary tumors or very high Gleason scores, a bone scan still may be appropriate at staging. Bone scans with sodium fluoride (NaF) PET imaging are likely more sensitive than standard bone scans. The precise role for NaF PET in evaluating patients with prostate cancer is uncertain, but it is increasingly available in the United States with FDA approval of NaF for bone imaging. Typically, more lesions are seen on NaF PET than on a standard bone scan or on bone SPECT imaging. For example, in a study of 44 men with high-risk prostate cancer, bone scans (99mTc methylene diphosphonate), SPECT bone scan, and NaF PET scans were compared. Of the 156 18F-fluoride lesions, 81 lesions (52%), including 34 metastases, were overlooked with normal appearance on a planar bone scan. SPECT identified 62% of the lesions overlooked by a planar bone scan. 18F-Fluoride PET/CT was more sensitive and more specific than bone scan (P < .001) and more specific than PET alone (P < .001).78
Thus imaging is used selectively in patients with newly diagnosed prostate cancer. A bone scan commonly is used for recurrent prostate cancer whenever the PSA level begins to rise. New PET methods are promising in prostate cancer but are not in widespread use in the United States. FDG-PET often is falsely negative in prostate cancer, especially for disease in the earliest stages.58 Similarly, MRS has shown promise in evaluating the prostate gland and determining whether the tumor has spread beyond the prostate.
Four independent studies examined a total of 98 patients with elevated blood PSA levels but no sign of recurrent prostate cancer on conventional imaging. After PET imaging with 11C-choline, the patients underwent tissue sampling of the abnormalities detected on the PET scans. In each of the four studies, at least half the patients who had abnormalities detected on PET scans also had recurrent prostate cancer confirmed by tissue sampling of the abnormal areas. PET scan errors also were reported. Depending on the study, falsely positive PET scans were observed in 15% to 47% of the patients. These findings underscore the need for confirmatory tissue sampling of abnormalities detected with 11C-choline injection PET scans.
Other agents are under development for prostate cancer imaging. For example, 18F anti-FACBC, a synthetic amino acid, shows considerable promise.79 Compared with FDA-approved “ProstaScint” for disease detection in the prostate bed, anti-3-18F-FACBC had an accuracy of 83%. Indium-111 Capromab pendetide had an accuracy of 67%. In the detection of extraprostatic recurrence, anti-3-18F-FACBC had an accuracy of 100%. 111In-capromab pendetide had an accuracy of 47%.
An 18F-labeled small molecule (18F DCFBC) that binds to prostate-specific membrane antigen also shows considerable promise in imaging prostate cancer.80 Thus considerable progress has been achieved in the molecular imaging of prostate cancer, with agents approved and under development that are likely to alter practice patterns in prostate cancer, especially metastatic disease assessments.
Colon Cancer
The performance of virtual colonoscopy has been directly compared with that of optical colonoscopy (OC) in a study of more than 6000 patients. Patients were randomized to either an optical or CT colonographic (CTC) group and were compared for the detection of advanced neoplasia and the total number of harvested polyps. Advanced neoplasia was confirmed in 100 of the 3120 patients in the CTC group (3.2%) and in 107 of the 3163 patients in the OC group (3.4%). Seven colonic perforations occurred in the OC group and none occurred in the CTC group. Primary CTC and OC screening strategies resulted in similar detection rates for advanced neoplasia, although the numbers of polypectomies and complications were considerably smaller in the CTC group. CT colonography also was much less expensive than optical colonoscopy.81 Interestingly, the detection rate for polyps requiring biopsy was statistically identical between the two groups of patients.
In a study of 2531 participants (97%) screened by virtual colonoscopy for large adenomas and cancers, the mean (± standard error) per-patient estimates of the area under the receiver-operating-characteristic curve for CT colonography was 0.89 ± 0.02, respectively. The sensitivity of 0.90 (i.e., 90%) indicates that CT colonography failed to detect a lesion measuring 10 mm or more in diameter in 10% of patients. The per-polyp sensitivity for large adenomas or cancers was 0.84 ± 0.04. The per-patient sensitivity for detecting adenomas that were 6 mm or more in diameter was 0.78.82
PET is used very often in this setting and in the setting of a rising carcinoembryonic antigen level after surgery. The precise timing of follow-up studies can be variable, but they often are performed every 6 to 12 months in the early years after surgery. Considerable evidence supports the idea that PET can detect more metastatic foci than CT in the setting of a rising carcinoembryonic antigen level.83 An example of a patient initially believed to have only a limited number of liver metastases is shown in Figure 18-11; however, more extensive disease was identified (Fig. 18-11, B). PET/CT has been shown to offer higher accuracy than PET alone in persons with recurrent colorectal cancer.1,84
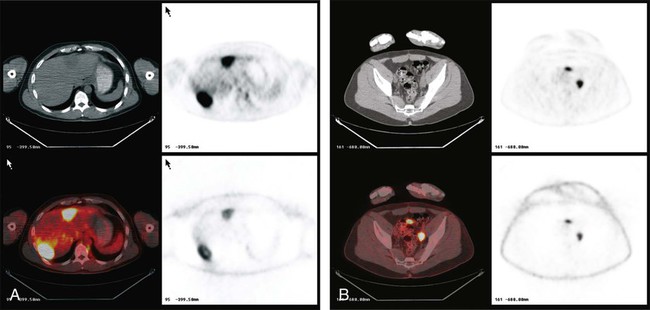
Ultrasound can detect many liver lesions and is a very useful technique for guiding biopsies of the liver.28 For liver metastases, CT is still the most commonly used procedure, but in a metaanalysis, PET was a more robust test to identify the presence and location of hepatic metastases of colorectal cancer compared with CT, MRI, or ultrasound methods.85
MRI methods have continued to improve, and MRI likely can detect more lesions in the liver than CT or PET. Thus for small lesion detection, MRI may offer advantages to PET, but at the cost of increased false-positive results.86
Gynecologic Neoplasms
For cervical carcinoma, screening with use of the Pap smear has a large effect in terms of lowering mortality rates by detecting premalignant changes and early-stage disease. Although much of the staging of cervical carcinoma is performed by physical examination, for larger primary tumors, imaging has an important role. Both CT and MRI are used in the pelvis; however, the use of PET for tumor staging is increasing. As with other tumors, PET appears to be more sensitive than anatomic imaging methods. Emerging data show that PET provides better prognostic value than anatomic imaging in persons with cervical cancer.89–89
For ovarian cancer, no technique is able to detect microscopic metastatic disease. Ultrasound is the main method by which ovarian tumors are identified at their earliest stages; however, these tumors are usually diagnosed at an advanced stage. Imaging can be used in an attempt to determine the extent of the surgical procedure that will be required. Both CT and MRI are used to assess the extent of ovarian cancer, with CT the preferred method (Fig. 18-12). PET is not sensitive to tumor foci smaller than 5 mm, but it is reasonably reliable in detecting larger tumor foci. For this reason, some persons advocate the use of PET to determine whether tumor debulking should be performed. PET has a role in the setting of a rising CA125 level in patients with normal CT findings.90
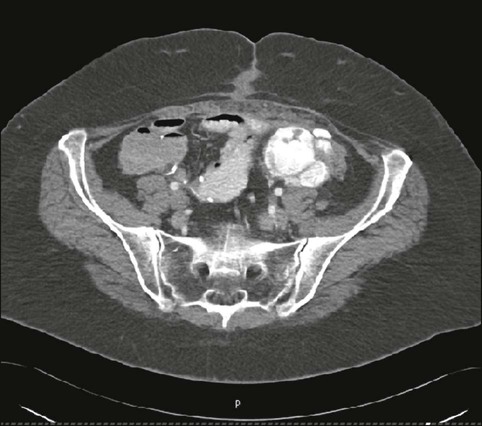
The use of serum markers and imaging is recommended for surveillance after surgery for ovarian carcinoma. The aggressiveness of imaging follow-up depends on the treatments available. PET has an emerging role in this setting, although practice patterns vary widely. PET also has been shown to provide information related to treatment response in preliminary studies.91,92
Lymphoma
For both Hodgkin and non-Hodgkin lymphomas, accurate staging is important. For both types of lymphoma, accurate definition of the tumor burden is needed for effective treatment planning, especially treatment with external beam radiation. CT is the historically accepted method for noninvasive staging of lymphoma, with PET being used increasingly because many studies have shown it to be capable of locating more tumor foci than CT.93 An example of CT imaging of abdominopelvic lymphoma is shown in Figure 18-13. In most lymphoma histologies, FDG-PET is more sensitive than CT, often detecting 20% more lesions than are seen with CT alone.94 PET can detect disease in the bone marrow and spleen in some instances. In fact, marrow involvement seen on FDG-PET often is associated with a negative CT scan.95
Data indicate that gallium-67 scintigraphy can be effective for assessing the viability of residual Hodgkin lymphoma and intermediate- and high-grade non-Hodgkin lymphoma. However, multiple studies now have been published on FDG-PET in this setting, and this imaging modality has essentially replaced 67Ga scintigraphy in assessing the viability of residual masses of lymphoma. If a residual mass of lymphoma shows increased FDG uptake, that usually is indicative of residual viable tumor; however, scans also can be falsely negative, because some tumor foci may be smaller than the resolution of PET imaging.96 False-positive results have been described in patients with Hodgkin lymphoma, however.97
Positive midtreatment 67Ga and PET scans predict a poor outcome from therapy, and positive PET scans at the conclusion of a therapeutic regimen also indicate a poor prognosis. It is increasingly appreciated that PET findings soon after treatment is initiated can be highly predictive of the ultimate outcome of the therapy.98,99
Challenges associated with PET include reactive lymph nodes and nodes involved with inflammatory processes such as sarcoidosis, which can take up FDG very avidly. Similarly, uptake of FDG in brown fat in the neck and thymus can be confusing.100
Although anatomic imaging has been the key for lymphoma assessment, the trend of greater use of PET is growing. PET imaging and PET/CT imaging, which provide additional functional information, are of growing utility. A limitation of PET-only methods had been the lack of a standardized set of response criteria. Tracer activity in PET images generally decreases more rapidly than tumor shrinkage occurs (i.e., anatomic changes of treatment lag behind metabolic changes detectable by PET). For these reasons, PET scans may appear normal before CT scans do. A concern is that strong evidence does not exist to show that a negative PET scan should be used to truncate the duration of lymphoma therapy. For example, if a PET scan became negative after two cycles of treatment, based on current data, this would not justify discontinuing treatment. However, if a treatment involved a standard of four possible courses of treatment and PET became negative after these courses were completed, available data suggest that this outcome portends a very good prognosis compared with a positive PET scan. Recently, new criteria for response including PET were developed for lymphoma (International Workshop Criteria + PET). For FDG-avid lymphomas, a negative PET scan is required to determine a complete response.103–103
It can be argued that performing PET is not essential in all patients with lymphoma. However, the use of PET and PET/CT is becoming increasingly the norm in centers where this technology is available. Because PET can find some lymphomatous tumors that cannot be detected by CT, this method is increasingly finding routine application in the care of patients with lymphoma (Fig. 18-13, C).
An exciting new opportunity for the use of PET in persons with non-Hodgkin lymphoma is in the setting of a “response adaptive” approach. In such treatment approaches, patients who are poorly responding can be identified by PET performed soon after treatment is initiated. This approach can allow segregation of responding from nonresponding patients. In the responding patients, standard treatment is given, whereas in the poorly responding patients, alternative approaches such as stem cell transplants are used. This approach currently is investigational, but it is an area of great promise because it allows the potential for tailoring the therapy to the individual patient’s responsiveness to the treatment algorithm.104 Recently, use of such an approach has been shown to be feasible in a clinical comparative trial on Hodgkin lymphoma.105
Melanoma
The imaging management of melanoma varies based on the stage of disease. Radiologic imaging has no significant role in the diagnosis of primary melanomas. Lymphoscintigraphy—that is, the injection of radiolabeled colloidal material, typically 99mTc sulfur colloid, into the subcutaneous tissues or intradermally to locate lymphatic drainage routes and thus lymph nodes with the potential for metastatic involvement—commonly is performed for primary melanomas of intermediate thickness. Although practice patterns vary, this method typically is used for melanomas that are more than 1-mm thick without other evidence of metastases. If the sentinel node identified by surgery (often using radionuclide guidance) is involved with tumor, additional staging procedures often are performed.106
Recent data suggest that SPECT/CT imaging may improve the accuracy of the sentinel lymph node procedure and reduce the probability of recurrence of disease in a lymph node basin107 versus more standard sentinel node identification procedures. These procedures most commonly include CT of the chest and abdomen and of the pelvis as well if the melanoma affected the lower extremities. If systemic metastases have occurred, brain imaging is performed using MRI with and without gadolinium contrast enhancement.
PET and PET/CT with FDG also are potent methods for detecting metastatic melanoma and often detect more tumor foci than does CT. PET can detect nodal metastases but is not as sensitive as sentinel node imaging and is not a replacement for sentinel node imaging and removal. PET is particularly good for soft tissue metastases but cannot detect microscopic disease. Thus sentinel lymph node biopsy is used in preference to PET for detecting early metastases. Some evidence indicates that ultrasound can detect small nodal metastases of melanoma, but it is not widely applied and is also less sensitive than sentinel node imaging.1 However, PET can detect most tumor foci larger than 6 mm and sometimes can detect smaller tumor foci. CT is a more robust technique for small pulmonary nodules than is PET.108
For systemically metastatic melanoma, PET has been reported to have sensitivity well over 90%. Thus although anatomic imaging dominates, PET has a growing role in melanoma assessment.109 For bone metastases, radionuclide bone scanning also is an important diagnostic procedure. Recently it has been shown that the performance of FDG-PET in melanoma is significantly enhanced if the CT scan portion of PET/CT is analyzed very carefully, which appears to increase both the sensitivity and specificity of the method.110
Bladder Carcinoma
Bladder carcinoma often presents at an early stage, and no imaging evaluation is performed to determine whether there are locoregional or systemic metastases. However, ultrasound has been used to determine the depth of penetration of primary bladder carcinomas. An important consideration in bladder carcinomas is that uroepithelial tumors are often multicentric. Thus intravenous pyelogram examinations to evaluate the entire genitourinary system commonly are performed early in the diagnostic algorithm. Although ultrasound can detect many bladder cancers 5 mm and larger, transurethral sonography is more sensitive; obviously, however, it is invasive. MRI is used more commonly than is CT for assessing primary bladder lesions because of its superior soft tissue contrast characterization abilities.111
MRI probably is more sensitive than CT in detecting nodal metastases, and new contrast agents that accumulate in normal nodes are potentially important for enhancing the diagnostic accuracy of MRI in detecting nodal metastases.112 Initial data with MRI contrast agents support the accuracy of this approach but also point out that the interpreter’s experience is important.113 Such agents are not yet routinely available or approved by the FDA.
FDG-PET has been used and is promising in bladder carcinoma; however, images of the pelvis can be degraded by intense 18F activity in the bladder. The use of PET in bladder cancer is enhanced by PET/CT and iterative reconstruction methods that make for better assessments of the pelvis with less degradation due to the bladder radiotracer activity. For bone metastases, radionuclide bone scan remains the procedure of choice, and MRI also can be sensitive. PET/CT is quite sensitive for metastatic disease beyond the pelvis and is being applied to a greater extent because of the availability of the CMS registry in the United States.114
Head and Neck Cancer
The physical examination is very important in assessing a primary lesion in the head and neck, but both CT and MRI are very potent methods. MRI typically is performed before and after gadolinium contrast is administered; CT scanning usually is performed after contrast enhancement (Fig. 18-14, A). Because MRI is subject to respiratory and motion artifacts, CT is used somewhat more commonly in the initial staging of these tumors.115
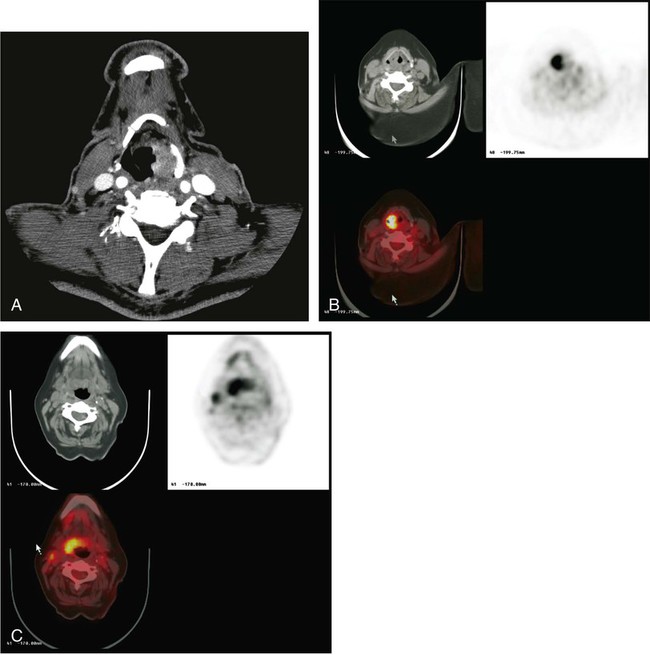
Increasingly, FDG-PET is being applied to the assessment of head and neck cancers. Although several studies have suggested that PET, MRI, and CT have similar sensitivity, more recent studies have suggested that PET is more accurate in staging (Fig. 18-14, B and C). However, PET can detect increased glucose uptake in nonmalignantly involved inflamed nodes (false-positive findings), which can occur in patients with head and neck or gingival infection or the common cold. Defining the precise extent of tumor is important to determine whether surgery or radiation therapy should be performed, because more extensive tumors are less amenable to surgical resection.
Thus for head and neck cancers, MRI offers excellent contrast resolution for soft tissues but can be degraded substantially by motion. For this reason, CT with contrast is much more commonly performed. FDG-PET is assuming a growing role in cancer management, especially for recurrence and for assessment of response to treatment. PET with CT is being used more often to stage and monitor these tumors during and after treatment and may become the standard of care.116 A recent comparison of PET/CT to MRI and PET alone showed superior accuracy of PET/CT compared with the other methods.117
Pancreatic Carcinoma
The standard of care for the imaging diagnosis of pancreatic cancers is CT scanning. Although MRI can be useful, CT, including CT angiography, is the main method used for staging and assessing tumor invasion of vessels (Fig. 18-15). Some studies have shown that FDG-PET is somewhat more sensitive for detecting tumors and has moderately high accuracy—approximately 85%—in characterizing pancreatic lesions as malignant or benign.120–120 Ultrasound is used to assess cystic lesions. After treatment, salvage therapy is ineffective, and these patients often are less aggressively monitored than are patients with other, more treatable cancers.
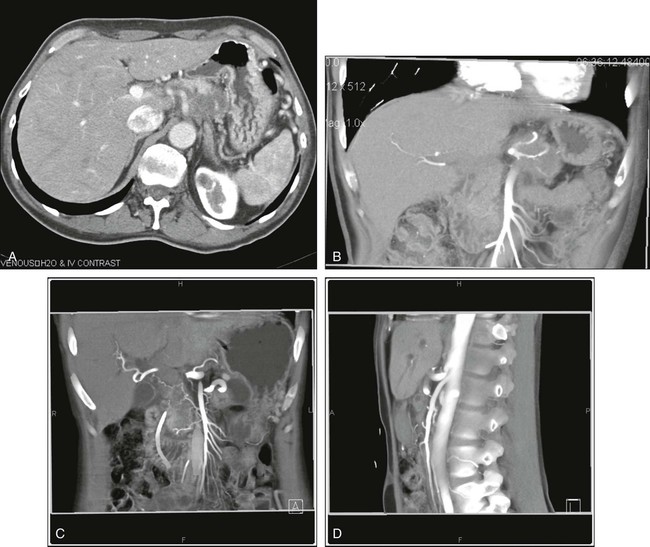
Neuroendocrine tumors of the pancreas often can be detected using nitrogen-111 pentetreotide (Octreoscan), a SPECT procedure. Recently, positron emitter–labeled analogs of somatostatin have been developed and show encouraging biological characteristics compared with single photon agents.121
Liver Cancer
Hepatic malignancies, especially hepatomas, are common worldwide. Both CT and MRI can be very effective in detecting these lesions. They sometimes are challenging to assess, because the appearance of cirrhosis with regenerating nodules and tumors can overlap. Multiphase CT imaging, CT angiography, and MRI with and without gadolinium contrast are commonly used in hepatomas. PET is less reliable, because approximately half—and sometimes more—of hepatomas are not avid for FDG. Some alternative PET tracers such as 11C acetate have been used to image some pancreatic cancers, because some FDG-negative tumors are avid for 11C acetate.122 Ultrasound also can be used to assess the liver and guide biopsies.
Metastatic lesions to the liver also are common, especially in the United States. For most tumors, CT is the initial method used to assess whether tumor is present. However, FDG-PET is more sensitive than CT in detecting liver metastases in common cancers such as colorectal cancer.83,85 Thus PET is seeing greater application in assessing suspected liver metastases, although ultrasound, CT, and MRI also are important methods and still are more commonly applied in many centers (Fig. 18-16).
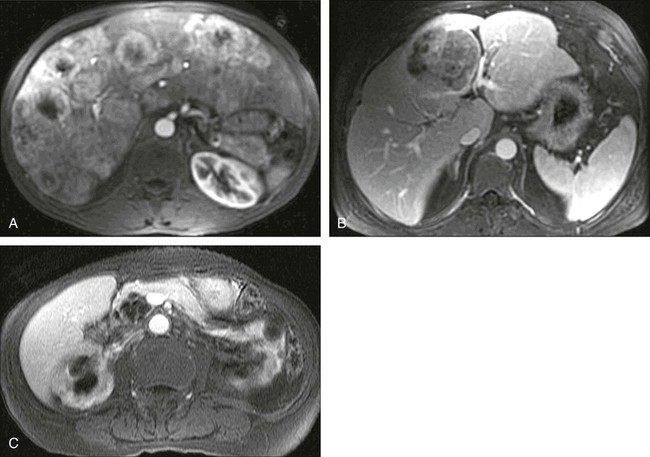
Kidney Cancer
In the past, renal cancers were detected by intravenous pyelograms; currently, however, the most common method for detection is CT. Renal cell cancer commonly is detected incidentally because of the widespread use of cross-sectional imaging. Between 25% and 50% of surgically treated renal cell cancers are discovered incidentally.123 Renal lesions are classified as cysts or solid masses, depending on their characteristics as shown by imaging. Renal cysts are fluid-filled and appear anechoic with increased through-transmission on ultrasound. They show water density without enhancement on CT and appear hyperintense on T2-weighted images, also without enhancement, on MRI.
CT is used to stage the tumor by determining the presence of renal vein invasion, adenopathy, local extension, and distant metastases. The accuracy of CT for staging is 91%.123 For resectable lesions, CT can provide information on whether the lesion is amenable to nephron-sparing surgery or partial nephrectomy. Lesions that are smaller than 4 cm, polar, and cortical and do not involve the renal hilum or collecting system may be candidates for partial nephrectomy.
Although CT, MRI, and ultrasound can all be used to assess renal lesions, CT with contrast is the dominant method (Fig. 18-17). PET is useful only when the tumor is avid for FDG. However, the normal excretion of FDG by the kidneys makes evaluation of the kidneys more challenging than other tissues, and some renal masses are not very avid for FDG. Thus FDG-PET is not currently recommended for renal cancers, at least not for reliably characterizing renal masses as malignant or benign. Metastatic renal cancer is more accurately imaged on FDG-PET than is primary renal cancer.124,125
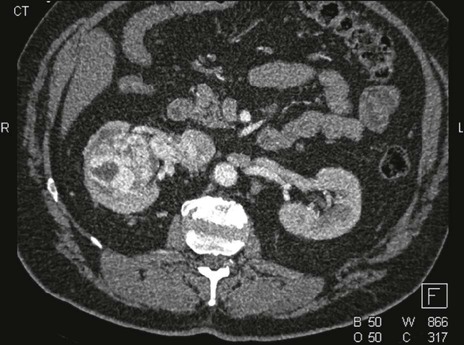
Renal cancers can also be imaged using iodine-124–labeled monoclonal antibodies to carbonic anhydrase IX, which is overexpressed in clear cell renal cancers. A prospective open-label multicenter study of 124I-girentuximab PET/CT in patients with renal masses who were scheduled for resection was performed, and PET/CT and contrast-enhanced CT (CECT) of the abdomen were compared. Complete data sets (histopathological diagnosis and PET/CT and CECT results) were available for 195 patients. The average sensitivity was 86.2% for PET/CT and 75.5% for CECT (P = .023). The average specificity was 85.9% for PET/CT and 46.8% for CECT (P = .005). These data suggest this investigational agent may be useful for noninvasive phenotyping of renal masses as malignant or benign.126
Endocrine Tumors
Imaging is used to study several types of endocrine tumors in a variety of locations. For adrenal tumors, CT is the procedure of choice, with metaiodobenzyl-guanidine 123I (MIBG) scanning and MRI scanning also proving useful for lesion characterization.125 MIBG accumulates selectively in pheochromocytomas. Adrenal masses with low HU values (<10 HU) typically are adenomas, which are rich in lipids.
For the thyroid gland, radioiodine imaging commonly is used. For nonradioiodine-avid thyroid cancers, FDG-PET is very useful for lesion detection and is recommended in the setting of a rising serum thyroglobulin level with a normal 131I or 23I scan, particularly when recombinant thyroid-stimulating hormone stimulation is used (Fig. 18-18).127 For neuroendocrine tumors such as carcinoid tumors, CT and radiolabeled octreotide analogs are very useful.
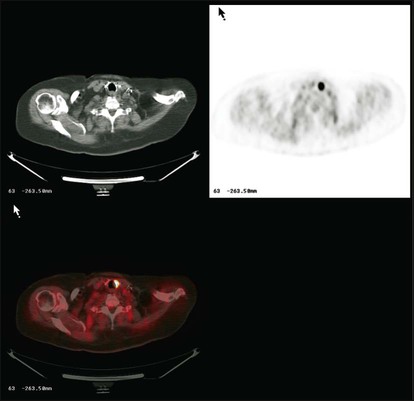
111In pentetreotide is approved by the FDA and used in detecting neuroendocrine tumors. 68Ga-labeled DOTA-Phe(1)-Tyr(3)-Octreotide and related compounds are typically more effective than 111In-labeled compounds and are available in a variety of settings. These agents are typically more accurate than 111In Octreoscan. A recent metaanalysis of the 68Ga peptide imaging literature showed area under the ROC curve in the task of diagnosing somatostatin receptor expressing neuroendocrine tumors in 16 studies including 567 patients. The pooled area under the ROC curve was 0.96. Although these techniques ae not yet approved by the FDA in the United States, these techniques, where available, should be considered as first-line diagnostic imaging methods in patients with suspected neuroendocrine tumors.121,128,129
Brain Tumors
The dominant method for the assessment of brain tumors is the MRI scan, which is the preferred method for initial detection, assessment of extent of disease, and assessment of efficacy of therapy. The superior soft tissue contrast provided by MRI places it ahead of CT for lesion characterization (Fig. 18-19).
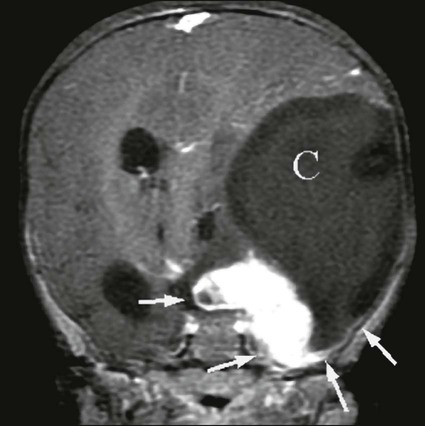
Unfortunately, even sophisticated MRI techniques, which often rely on tumor enhancement using gadolinium, cannot detect microscopic disease. MRI findings, although fairly specific for tumor, are not completely specific. Thus infarcts, infections, and foci of demyelination occasionally can mimic tumor foci on MRI. FDG-PET has a very limited role but can be useful in assessing residual tumor after radiation therapy and determining whether residual masses are caused by tumor or tumor necrosis. It is most accurate in highly aggressive tumors where brain tumor uptake of FDG is greater than that of normal brain tissue. Recently, other tracers such as fluorodopa, fluoroethyl tryosine, and fluorothymidine have shown results more promising than those for FDG.130,131
MRS also can be useful in evaluating this issue because tumors typically have high levels of choline and low levels of N-acetyl-aspartate. Angiography, although a historically useful method, is performed less frequently for diagnostic purposes in brain tumors. MRI, CT, and PET can guide biopsies. Further, MRI can be used intraoperatively to guide therapy.132
Pediatric Tumors
Multiphase CT should be avoided in children unless it is clearly indicated to minimize the radiation dose to the child.133 PET/CT has shown broad utility in pediatric cancers, and the evolving literature suggests that PET/CT often offers improvements over CT alone. Given the radiation dose and long-term risks of carcinogenesis, it is possible that MRI will have a growing role in pediatric cancer imaging because of its nonionizing nature. PET/MRI, a newer technology, may be particularly attractive for use in children.
Esophageal and Gastric Cancer
CT scanning is the method of choice for initial staging of esophageal and gastric cancer. Endoscopic ultrasound is the most sensitive and accurate method for determining whether tumor has invaded the wall of the esophagus or involved the periesophageal nodes. PET scanning is very sensitive in determining whether stage IV disease is present and typically is performed as part of the initial staging evaluation, at least for esophageal cancer, to identify patients who are clearly not candidates for surgery.134 FDG-PET also is being used with increasing frequency to assess treatment response in patients with esophageal cancer, because early changes in glycolysis after treatment appear related to treatment efficacy.135
Sarcomas
MRI is the method of choice for detection and assessment of soft tissue sarcomas. Gadolinium contrast enhancement is commonly used as well. FDG-PET imaging has been useful in assessing primary sarcomas for aggressiveness and defining possible sites for biopsy, as well as for defining prognosis and grade.136,137 It also has been used to assess and predict the response of sarcomas to therapy.138
Gastrointestinal Stromal Tumors
Gastrointestinal stromal tumors are relatively infrequent but have been studied extensively with both CT and PET imaging in the past few years. These tumors often are responsive to imatinib and other tyrosine kinase inhibitors, in part because of their constitutive overactivity of a mutated KIT oncogene. These tumors have been shown to be typically avid for FDG. FDG accumulation declines very rapidly with effective treatments, earlier than changes in tumor size. Of interest is that recently revised CT criteria may allow CT to assess response more quickly to treatment in such tumors, with changes in tumor HU one of the characteristic findings of response. Both PET and CT thus have a role in this somewhat uncommon tumor, especially for the early assessment of treatment response.139
Treatment Response Assessment
Functional methods like PET with FDG or possibly MRI with diffusion assessments may provide earlier indices of response than are seen with size measures alone. Diffusion and diffusion contrast MRI are quite promising methods, but standardization of diffusion MRI is in its early phases, although it seems effective in single center studies, including in brain tumors.140
Systematic response criteria are available for anatomic imaging (most recently the Response Evaluation Criteria In Solid Tumors [RECIST] 1.1 criteria) and criteria have been proposed for PET response—the European Organization for Research and Treatment of Cancer (EORTC) criteria and the more recent PET Response Criteria in Solid Tumors (PERCIST) 1.0 criteria.141,142 To use quantitation in imaging, close attention to detail is required in the imaging process. CT or MRI can provide tumor sizes. PET can provide a standardized uptake value, which can be used to phenotype tumors and to quickly assess treatment response, but such values may vary from device to device and across time, unless careful standardization is followed.143
Guidance Of Radiation Therapy
FDG-PET is beginning to be used to better define the biological tumor volume, often with data from PET/CT. Although this procedure is hardly the norm, it is clear that imaging is key to the optimal planning of radiation therapy ports. Substantial potential exists to target areas of tumor that are not identified on CT (expand port size) or reduce ports to areas that are not involved with tumor, because the goal is to irradiate tumor while not irradiating normal tissues.144 A substantial number of centers now include PET imaging as part of their radiation therapy planning in a broad range of diseases.145 Treatment plans for radiation therapy are discussed in a separate chapter in this book.
Interventional Procedures
Catheter-based delivery systems also are important. For example, angiographic catheters can be used to deliver regional chemotherapy, emboli, or radioactive or chemical microspheres to treat tumors. Radioactive microspheres also are assuming a growing role in interventional oncologic radiology.146
Emerging Opportunities in Imaging
Functional imaging methods such as PET and varying innovative MRI techniques are being used increasingly to assess treatment response early after treatment is begun.29 Beyond this, a variety of imaging methods are being developed to image key aspects of tumor biology (Box 18-3). An exciting area for both MRI and PET is in detection of hypoxia, which is common in a broad range of malignancies and which represents a possible target for cancer treatments.147 In addition to hypoxia, tumor perfusion can be imaged with a variety of approaches.
Even though the process is difficult, interest remains in gene therapeutic approaches, which has led to attempts to determine, through imaging, whether genes, when delivered, actually reach tumors and, more importantly, whether they express in vivo the desired levels of gene product expected to be required to achieve a therapeutic effect. For example, dopamine receptors have been transfected into cells and imaged with radioligands capable of binding to the D2 dopamine receptor. Similarly, genes have been transfected that express varying thymidine kinase activities. This agent is suitable for gene therapy and can be imaged with radiolabeled substrates such as FMAU.148,149
Some methods are of tremendous importance in preclinical studies but will be more difficult to extend to human studies. One example is optical imaging. Such methods are capable of tremendous sensitivity and excellent resolution in vivo in small animals. A variety of approaches can be used, with emitted light, transmitted light, and reflected light.150 Photoacoustic imaging approaches are also applied in which light in transmitted and sound is received.151
With bioluminescence approaches, a very small number of cancer cells can be identified in vivo in small animals. Such approaches, although very potent in vivo in small animals and capable of being combined with radionuclide and other methods, are not as easily translated into use in humans, at least for broad applications, because of the limited penetration in tissue of light photons. However, in a broad range of detection issues, the light can reach detectors or targets, both intraoperatively and endoscopically for superficial structures, and thus this area is likely to be increasingly translated to practice. Detection of sentinel lymph nodes and resection margins are both areas of considerable promise. Thus small animal imaging is a key element of progress allowing for proof of concept and refinement of tumor biological processes before translation to human use.152