Hodgkin Lymphoma
Nancy L. Bartlett and Kelley V. Foyil
• Estimated 9060 new cases in United States in 2012 with 1190 deaths
• U.S. age-adjusted incidence rate of 2.8 per 100,000 per year
• Higher incidence among males than females
• Highest incidence in North America and Western Europe
• Bimodal age distribution (peaks at 15 to 35 years and then again later in life)
• Hodgkin Reed-Sternberg and lymphocyte-predominant (LP) cells derive from germinal center B cells. Hodgkin Reed-Sternberg and LP cells are rare in the lymphoma tissue, and interactions with other cells in the microenvironment may play a role in the pathophysiology of the disease.
• In classic Hodgkin lymphoma (HL), Hodgkin Reed-Sternberg cells express CD30 and have lost expression of B-cell markers.
• Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) represents a distinct clinicopathological entity characterized by scattered large “popcorn” cells that express CD45 and CD20.
• Epstein-Barr virus may be involved in the pathogenesis of some classic HL cases, particularly in tropical areas.
• Physical examination with attention to peripheral nodes and spleen
 Cervical, supraclavicular, mediastinal nodes involved in majority of patients with classic HL
Cervical, supraclavicular, mediastinal nodes involved in majority of patients with classic HL
• History including presence or absence of pruritus, drenching night sweats, fevers, and significant weight loss
• Laboratory evaluation to include complete blood cell count with differential, albumin, and erythrocyte sedimentation rate
• Positron emission tomography/computed tomography (PET/CT)
• Bone marrow biopsy not indicated in early-stage disease and may no longer be needed in advanced-stage disease in patients undergoing PET
• Early-stage non-bulky classic HL
 ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) chemotherapy (two to four cycles) with 20 to 30 Gy of involved-field radiation therapy (IFRT) with number of cycles and dose of radiation dictated by high-risk features
ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) chemotherapy (two to four cycles) with 20 to 30 Gy of involved-field radiation therapy (IFRT) with number of cycles and dose of radiation dictated by high-risk features
 Four to six cycles of ABVD alone results in equivalent overall survival, modestly inferior progression-free survival, and less long-term toxicity compared with combined-modality therapy.
Four to six cycles of ABVD alone results in equivalent overall survival, modestly inferior progression-free survival, and less long-term toxicity compared with combined-modality therapy.
 Interim PET likely to select patients who benefit most from radiation therapy
Interim PET likely to select patients who benefit most from radiation therapy
• Early-stage classic HL with bulky disease
 Combined modality therapy with four to six cycles of ABVD followed by IFRT
Combined modality therapy with four to six cycles of ABVD followed by IFRT
 Standard risk: six cycles of ABVD
Standard risk: six cycles of ABVD
 High risk: six cycles of ABVD or escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone)
High risk: six cycles of ABVD or escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone)
 Interim PET may select patients who benefit most from escalated BEACOPP.
Interim PET may select patients who benefit most from escalated BEACOPP.
 Combined modality therapy with two to four cycles of chemotherapy plus IFRT
Combined modality therapy with two to four cycles of chemotherapy plus IFRT
 Limited data: R-CHOP (rituximab + cyclophosphamide, doxorubicin, vincristine, prednisolone), R-ABVD (rituximab + ABVD), or R-CVP (rituximab + cyclophosphamide, vincristine, prednisolone) are reasonable options.
Limited data: R-CHOP (rituximab + cyclophosphamide, doxorubicin, vincristine, prednisolone), R-ABVD (rituximab + ABVD), or R-CVP (rituximab + cyclophosphamide, vincristine, prednisolone) are reasonable options.
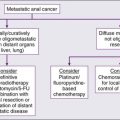
• Salvage chemotherapy followed by autologous stem cell transplant is the standard approach, with 50% to 60% achieving long-term remissions.
• Pretransplant PET/CT is highly predictive of outcomes.
• Brentuximab vedotin, an antibody-drug conjugate, has a 75% response rate in relapsed/refractory HL.
Introduction
Nearly 90% of patients with early-stage Hodgkin lymphoma (HL) and 80% of advanced-stage patients will be cured. Survival rates have continued to increase as a result of enhanced diagnostic techniques, better staging methods, improved understanding of the importance of chemotherapy dose intensity, more effective salvage regimens, and perhaps a greater appreciation of the need to screen for late complications. Although they are indirect evidence, population-based findings of improved survivals since 1980 provide credence to the idea that limiting the number of cycles of chemotherapy and decreasing the use of radiation therapy (RT) has not resulted in worse overall outcomes for patients with HL and support increasing efforts to minimize therapy in an effort to minimize late toxicities.1 With new biological prognostic markers and novel drugs for HL in development, outcomes are likely to continue to improve.
Epidemiology and Etiology
In the United States, an estimated 9060 new cases of HL were diagnosed in 2012 with 1190 deaths.2 The U.S. age-standardized incidence rate (ASR) per 100,000 per year is 2.8, with higher incidence among males. The median age at diagnosis is 38 years, and there is a bimodal age curve in resource-rich countries, showing peaks at 15 to 35 years and then later in life. Worldwide, the incidence of HL is highest in Europe (ASR 2.0), the Americas (ASR 1.5), and the Eastern Mediterranean (ASR 1.4).3 Regions with lower ASRs include Africa (ASR 0.9), Southeast Asia (ASR 0.7), and the Western Pacific (ASR 0.4). In the United States, age-adjusted incidence rates vary among races/ethnicities, with the highest incidence in non-Hispanic whites (ASR 3.6 for males and 2.9 for females), followed by blacks (ASR 3.2 for males and 2.3 for females).2 Although the incidence rates have remained stable for whites, significant annual percentage increases between 1992 and 2009 occurred for blacks and Asian/Pacific Islanders.
HL is likely to have different causes depending on patient age at diagnosis, nationality, and Epstein-Barr virus (EBV) tumor phenotype. Epidemiological studies support both environmental and genetic factors in the etiology of HL. Multiple siblings, higher birth order, crowded living conditions, lower socioeconomic status, and exposure to daycare have all been associated with a decreased risk for HL in young adults. Historically, this has been interpreted as evidence to support late exposure to an infectious agent as an etiologic factor in the development of HL but no causal virus has been found. Additional circumstances associated with an increased risk for HL include a lower level of fecal-oral exposure early in life (e.g., lower incidence in thumb suckers), appendectomy, tonsillectomy, eczema, and smoking.4 As opposed to a specific etiologic infectious agent, others have proposed the “hygiene hypothesis” as a potential cause of HL in young adults. This theory proposes that a reduction in cumulative microbial exposures in childhood may adversely affect immune response development specifically by decreasing interleukin (IL)-12 secretion, which may lead to persistence of the T-helper (Th) type 2 phenotype, resulting in an immature immune response phenotype.4,5
EBV has been long thought to play a role in the pathogenesis of a subset of HL cases because EBV latent membrane protein (LMP-1) and/or EBV-encoded small RNAs are found in Hodgkin Reed-Sternberg cells in approximately one third of HL cases. Serum profiles of antibodies to EBV are significantly altered, both before and after the diagnosis of EBV+ classic HL.6,7 These distinctive serologic responses to EBV latent antigens are also seen in other disorders associated with immune dysfunction such as the acquired immunodeficiency syndrome, rheumatoid arthritis, and inherited immunologic disorders.6 In young adults, a history of infectious mononucleosis is a risk factor for EBV+ classic HL in some series, occurring at a median of 2.9 years after infectious mononucleosis infection.8
Support is building for a familial or inherited component in the development of HL. Mack’s landmark study of twins revealed that monozygotic but not dizygotic twins of patients with HL had a significantly increased risk for developing HL.9 Several reports from the Swedish Family-Cancer Database demonstrated that relatives of HL patients had a fourfold elevated risk for developing HL.10 If the index case was the parent, the standardized incidence ratio (SIR) was 3.05 for both sexes with the highest incidence for a son of a father with HL (SIR 3.64).11 If the affected parent was diagnosed before age 40, the SIR was 6.46. The highest risks were for male-male (SIR 8.00) and female-female (SIR 11.75) sibling pairs.
This increased risk for HL in relatives inspired genomic studies of classic HL, specifically investigating the major histocompatibility complex region (human leukocyte antigen [HLA] system). The HLA class I region is associated with EBV+ HL in numerous studies, with many focusing on the HLA-A gene. Niens and associates reported that the HLA-A*01 haplotype was associated with an increased risk for developing EBV+ HL whereas the HLA-A*02 haplotype had a decreased risk.12 These associations were confirmed in the presence of infectious mononucleosis; the authors suggested that the association between history of infectious mononucleosis and EBV+ lymphoma was not seen in the presence of HLA-A*02 because this allele appeared to “neutralize the effect of infectious mononucleosis.”13 In a genome-wide association study of classic HL and EBV, the associations of the class I region variants with EBV+ classic HL and class II region variants with EBV− classic HL were corroborated.14 Additionally, two loci in the major histocompatibility complex region were associated with classic HL, irrespective of EBV status. This suggests that there may be some components of pathogenesis that are common to both EBV− and EBV+ classic HL. Huang and associates studied HLA and EBV by using a polymerase chain reaction (PCR)-based sequence-specific oligonucleotide probe (SSOP) hybridization approach and discovered significant differences between EBV+ and EBV− classic HL cases for several probes that discriminate between HLA-A*01 and HLA-A*02.15 The authors concluded that these HLA antigens are responsible for the association of EBV with HL, not specific single nucleotide variants shared by multiple alleles.
Pathology and Biology
In classic HL, rare, scattered mononuclear (Hodgkin) and multinucleated (Reed-Sternberg) cells are found in a mixture of inflammatory and accessory cells such as eosinophils, neutrophils, histiocytes, plasma cells, and fibroblasts (Fig. 105-1A and B). T lymphocytes often ring the Hodgkin Reed-Sternberg cells in a rosette-like manner. Hodgkin Reed-Sternberg cells are uniformly positive for CD30 and frequently positive for CD15, both in a membrane pattern with Golgi staining (see Fig. 105-1C). CD20 expression is variable, MUM1 staining is positive, and PAX5 staining is weaker in Hodgkin Reed-Sternberg cells than in reactive B cells. The cells are usually negative for CD45 and CD75. The detection of EBV-encoded RNA is indicative of classic HL and is found in approximately one third of cases.
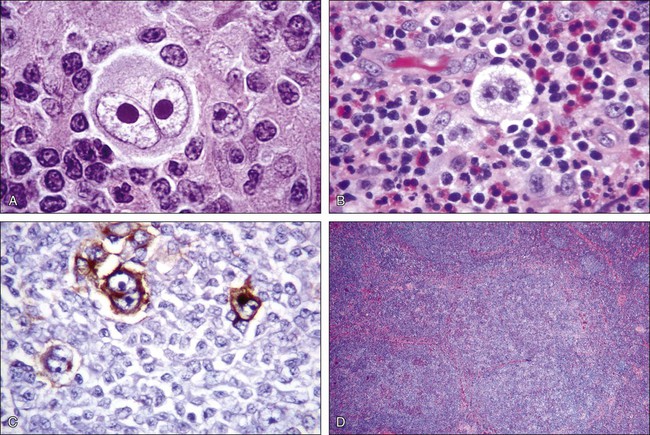
NLPHL comprises numerous large, tightly packed nodules (see Fig. 105-1D). Scattered large “popcorn” or lymphocyte-predominant (LP) cells are CD20+ but rarely CD15+ or CD30+. These LP cells are present with normal lymphocytes and histiocytes within large spherical meshworks of the follicular dendritic network. If the process is entirely diffuse, the diagnosis of T-cell–rich large B-cell lymphoma must be considered.
Both LP and Hodgkin Reed-Sternberg cells are derived from germinal center (GC) B cells.16 LP cells express several typical GC B-cell markers and grow in a follicular dendritic network. In contrast, Hodgkin Reed-Sternberg cells have lost their B-cell signature through transcriptional reprogramming, likely by DNA methylation, upregulation of NOTCH1 and other negative regulators of B cells, and the persistent activation of nuclear factor κB (NF-κB).16 Hodgkin Reed-Sternberg cells, which cannot produce immunoglobulins, should be targeted for apoptosis, but NF-κB is involved in the protection of these cells. This NF-κB protection can result from EBV infection, and these infected cells are then independent of the normal B-cell receptor survival signals.17 Other pathways with deregulated activation in Hodgkin Reed-Sternberg cells include Janus-activated kinase/signal transducer and activator of transcription (JAK/STAT), phosphatidylinositol-3-kinase/Akt (protein kinase B) (PI3K/Akt), AP-1 (activator protein-1), and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk).16,18 Tiacci and colleagues reported the first genome-wide transcriptional analysis of microdissected Hodgkin Reed-Sternberg cells in comparison with classic HL cell lines and normal B-cell subsets.19 This analysis identified two molecular subgroups of classic HL with varying strengths of NOTCH1, MYC, and IRF4 proto-oncogenes. Additionally, BCL-interacting killer (BIK), an apoptosis inducer, and inositol polyphosphate-5-phosphatase, 145kDa (INPP5D), an inhibitor of the PI3K pathway, were silenced in Hodgkin Reed-Sternberg cells.
The tumor microenvironment is critical in the development and proliferation of HL. Reactive cells assist in the proliferation of Hodgkin Reed-Sternberg cells by secreting chemokines and cytokines and creating an immunosuppressive environment. Chetaille and colleagues used gene expression profiles generated by using DNA microarrays to show that variations in the tumor environment correlate with outcome.20 Altered cytokines, chemokines, receptors, and ligands can contribute to classic HL pathogenesis by promoting the Th2 response. Examples include chemokine ligand 17 (CCL17); chemokine ligand 22 (CCL22); chemokine ligand 20 (CCL20); chemokine ligand 9 (CXCL9); chemokine receptor 3 (CXCR3); IL-13, GATA binding protein 3 (GATA3); and chemokine receptor 4 (CCR-4). The aforementioned substances can also suppress the Th1 response (ex: IL-10, transforming growth factor–β [TGF-β], programmed cell death ligand-1 [PD-1L], galectin-1) and can promote the influx of eosinophils (ex: IL-5, IL-9, eotaxin, chemokine ligand 28 [CCL28]), mast cells (IL-9, chemokine ligand 5 [CCL5]), plasma cells (IL-6), neutrophils (IL-8, granulocyte-macrophage colony-stimulating factor), and fibroblasts (IL-13, TGF-β, tumor necrosis factor–α, matrix metalloproteinase [MMP], tissue inhibitor of metalloproteinases 1 and 2 [TIMP1, TIMP2]). 21 Interactions with CD4+ T cells, a mixture of Th cells and regulatory T cells that surround the Hodgkin Reed-Sternberg cell, appear to play a critical role in Hodgkin Reed-Sternberg cell survival.18
Clinical Manifestations, Evaluation, and Staging
Common presenting symptoms include painless peripheral adenopathy (most commonly in the cervical or supraclavicular region), cough or chest pain related to mediastinal disease, and pruritus. B symptoms including recurrent drenching night sweats, recurrent unexplained fever greater than 38° C (100.4° F), and unexplained weight loss (>10% of baseline) occur in about 40% of patients. Bulky mediastinal disease is most common in young women with the nodular sclerosis subtype of classic HL (Fig. 105-2). The mixed cellularity subtype of classic HL more often is seen in older men as peripheral adenopathy or subdiaphragmatic involvement and rarely as mediastinal involvement. Lymphocyte-rich classic HL usually has a very favorable presentation with nonbulky peripheral adenopathy. Lymphocyte-depleted classic HL usually is seen at advanced stage (III to IV) as retroperitoneal lymph node, abdominal organ, and bone marrow involvement. NLPHL patients usually have limited-stage disease with cervical, axillary, or inguinal lymph node involvement.
Staging of HL is based on the Ann Arbor staging system with the “Cotswolds” modifications. Four stages document the extent of lymph node and disseminated disease (Table 105-1) with information on the presence (B) or absence (A) of B symptoms. Additionally, contiguous extranodal involvement is designated E and bulky disease, defined as either a single mass larger than 10 cm or a mediastinal mass exceeding one third of the maximum transverse transthoracic diameter measured to the inside of the ribs on a standard posteroanterior chest radiograph, is noted. Stage I HL involves a single nodal region, stage II HL involves two or more lymph node regions on the same side of the diaphragm, stage III HL involves lymph node regions on both sides of the diaphragm, and stage IV HL diffusely involves one or more extralymphatic organs or sites.
Table 105-1
Modified Ann Arbor Staging System for Hodgkin Lymphoma
| Stage | Involvement |
| I | Single lymph node region (I) or one extralymphatic site (IE).* |
| II | Two or more lymph node regions, same side of the diaphragm (II) or local extralymphatic extension plus one or more lymph node regions or same side of the diaphragm (IIE)* |
| III | Lymph node regions on both sides of the diaphragm (III), which may be accompanied by local extralymphatic extension (IIIE).* |
| IV | Diffuse involvement of one or more extralymphatic organs or sites. |
| A | No B symptoms |
| B | Presence of at least one of the following symptoms: |
| 1. Unexplained weight loss >10% baseline during 6 months before staging | |
| 2. Recurrent unexplained fever >38° C | |
| 3. Recurrent night sweats | |
| Bulky tumor† |
*E lesion: Localized extranodal extension of Hodgkin’s lymphoma from a contiguous or nearby nodal site is noted with the designation E, for example, stage IIEA for asymptomatic disease in the mediastinum with contiguous extension into nearby lung.
†Bulky tumor is defined as either a single mass of tumor tissue exceeding 10 cm in largest diameter or a mediastinal mass exceeding one third of the maximum transverse transthoracic diameter measured to the inside of the ribs on a standard posteroanterior chest radiograph.
Data from Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res 1971;31:1860–1.
Patients should have an excisional biopsy if possible. Core needle biopsies provide limited tissue in which to examine rare Hodgkin Reed-Sternberg cells and architecture, whereas flow cytometry rarely contributes to HL diagnosis. Pretreatment evaluation should include physical examination and history, laboratory studies (complete blood cell count, chemistries, erythrocyte sedimentation rate [ESR]), and whole-body combined positron emission tomography and computer tomography (PET/CT). Limited-stage HL is more common, with approximately 25% of patients with stage I disease, 40% with stage II disease, 20% with stage III disease, and 15% with stage IV disease.2 In addition to determining stage of disease at diagnosis, advanced-stage patients should be classified by the Hasenclever International Prognostic Score (IPS) risk group and limited-stage patients with an unfavorable or favorable status according to the German Hodgkin Study Group or European Organization for Research and Treatment of Cancer criteria (Table 105-2). Risk factor classification provides prognostic information and helps with treatment stratification.
Table 105-2
Prognostic Factors in Hodgkin Lymphoma
| Risk Factors | Early Stage | Advanced Stage | |
| European Organization for Research and Treatment of Cancer | German Hodgkin Study Group | Hasenclever | |
| Bulk | MMR > 1/3 | MMR > 1/3 | — |
| Sites | > Three nodal sites | > Two nodal sites | Stage IV |
| Extranodal sites | |||
| Laboratory | ESR ≥ 50 (if asymptomatic) | ESR ≥ 50 (if asymptomatic) | Hemoglobin < 10.5 g/dL |
| White blood cells ≥ 15,000/µL | |||
| ESR ≥ 30 (if symptomatic) | ESR ≥ 30 (if symptomatic) | Lymphocytes < 600/µL or < 8% of white blood cells | |
| Albumin < 4 g/dL | |||
| Demographics | Age ≥ 50 yr | — | Age ≥ 45 yr |
| Male gender | |||
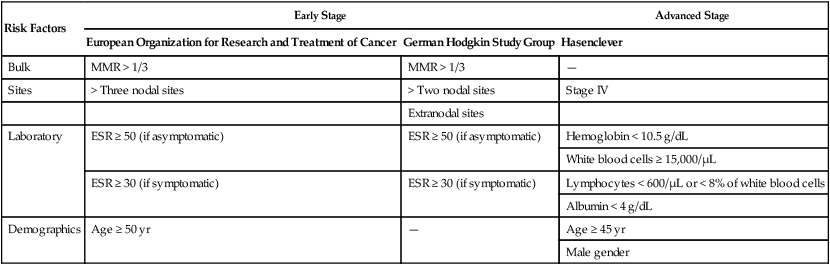
ESR, Erythrocyte sedimentation rate; MMR, mediastinal mass ratio.
Compared with CT alone, PET/CT upstages approximately 15% of patients and downstages less than 5% of patients.22 In two series, staging PET/CT resulted in a change in treatment in 7% to 18% of patients.22,23 Traditionally, bone marrow biopsy has been recommended for patients with advanced disease. El-Galaly and colleagues reported on 454 newly diagnosed patients with HL of whom 18% had focal skeletal lesions on PET/CT and 6% had a positive bone marrow biopsy.24 No patients with stage I or II HL had a positive bone marrow biopsy, and no patients were allocated to a higher clinical risk group based on the results of bone marrow biopsy. Of 27 patients with bone marrow involvement, 23 (85%) had focal bone lesions on PET/CT. Diffuse marrow uptake was never associated with a positive bone marrow biopsy. PET/CT had a negative predictive value of 99% for bone marrow involvement. Bone marrow biopsy is unlikely to change the risk or treatment strategy in the age of PET/CT staging and can likely be eliminated in all patients.
Primary Therapy
Despite the overall excellent prognosis of the majority of patients with HL, the optimal initial treatment for both early- and advanced-stage disease remains controversial. Because of the unique efficacy of salvage therapy for HL, a slightly less effective but less toxic approach as initial therapy may ultimately optimize survivorship.25 Tailoring therapy based on results of an interim PET/CT scan performed after one to three cycles of chemotherapy may represent the optimal approach for most patients. However, mature data from randomized trials testing this concept are not yet available.
Early-Stage Nonbulky Hodgkin Lymphoma
The transition from extended-field radiation therapy (EFRT) to combined-modality therapy with chemotherapy and involved-field radiation therapy (IFRT) in the 1990s represented a major step forward in the treatment of limited-stage HL.26,27 Because of concerns of serious late toxicities (described in detail later), there is universal agreement that the dose and field of radiation should be minimized in the treatment of early-stage HL. DeBruin and associates demonstrated a significant reduction in the incidence of second breast cancers in women treated with mediastinal RT compared with mantle RT for HL, and a meta-analysis of HL trials by Franklin and associates showed a marked decrease in second cancers in patients receiving IFRT compared with EFRT.28,29 However, because mediastinal nodes are involved in the majority of patients with HL, most patients undergoing RT for HL will continue to have at least modest exposure to the heart, lungs, and breasts regardless of efforts to administer only involved-field or even involved-nodal RT. Unfortunately, efforts to completely eliminate radiation for the majority of patients with early-stage HL continue to meet with significant resistance. The 2012 publication of a randomized trial of an ABVD regimen (doxorubicin, bleomycin, vinblastine, dacarbazine) alone versus radiation-based therapy in limited-stage HL showing for the first time a survival advantage in the chemotherapy-alone arm, despite an inferior progression-free survival (PFS), has rekindled the debate.25 Current guidelines and recommendations continue to support either chemotherapy alone or combined-modality therapy in the treatment of nonbulky early-stage HL.30
The specifics of combined-modality therapy for early-stage HL are generally based on the results of two randomized trials from the German Hodgkin Study Group, HD10 and HD11. For patients with the most favorable presentation, two cycles of ABVD and 20 Gy of IFRT resulted in a 5-year freedom from treatment failure (FFTF) of 91% and overall survival rate (OS) of 97%.31 There was no advantage to four cycles of ABVD or 30 Gy of RT in this subset of patients. Importantly, and often misunderstood in the general oncology community, patients eligible for this study (HD10) included only those with disease limited to no more than two sites, no extranodal or bulky disease, and ESR less than 50 if no B symptoms and less than 30 in the presence of B symptoms. Patients with early-stage disease not meeting the eligibility criteria for HD10 were treated on the HD11 trial comparing four cycles of ABVD to four cycles of standard-dose BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) followed by 20 or 30 Gy IFRT.32 The two chemotherapy regimens yielded equivalent results with less toxicity in the ABVD arm, and the outcomes were superior for those receiving 30 Gy compared with 20 Gy, resulting in the recommendation of four cycles of ABVD plus 30 Gy RT in this less favorable subset. At 5 years, FFTF was 85% and OS was 95%.
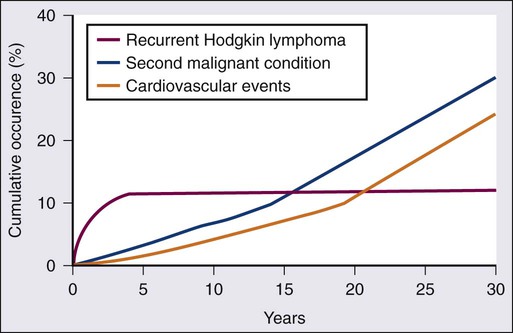
Advocates of chemotherapy alone for nonbulky early-stage disease acknowledge the increased failure rate of 5% to 8% when RT is eliminated.25,33 However, with a follow-up duration of 4 to 5 years, no trial comparing chemotherapy alone to combined-modality therapy in early-stage disease has shown a survival advantage for combined modality therapy, likely because second-line strategies, often including RT, result in durable disease control in nearly half of patients with relapsed disease. As alluded to earlier, the National Cancer Institute of Canada (NCIC) study (HD.6) comparing ABVD alone with subtotal nodal irradiation (STNI), with or without ABVD, in 405 patients with nonbulky stage I to IIA HL showed a 12-year PFS of 87% versus 92% (P = .05) in favor of the RT arm of the study but remarkably a 12-year OS of 94% for ABVD alone compared with 87% for those receiving STNI (P = .04). Importantly, 12-year follow-up does not yet reflect the dramatic increase in cardiovascular disease and second malignancies starting 15 to 20 years after primary therapy with RT (Fig. 105-3).34 Critics condemn this study, owing to the use of EFRT in the control arm, arguing that the OS of the RT arm does not reflect modern RT techniques and fields. Regardless of this criticism, the 12-year event-free survival (EFS) of 87% in the ABVD-alone arm of the study compares favorably with the 5- to 8-year EFS rates of 86% to 87% reported in the HD10 and HD11 trials, respectively, of ABVD in combination with limited dose and field RT. In an effort to more accurately compare the results of these trials because of differences in eligibility, staging, end points, and follow-up duration, investigators performed an individual patient-data comparison of HD10 and HD11 and the ABVD-alone arm of HD.6.35 Patients were included if eligible for one of the combined-modality studies as well as the HD.6 study. In 406 patients, the authors concluded that time to progression (hazard ratio [HR], 0.44, 95% confidence interval [CI], 0.24 to 0.78) and PFS (HR, 0.71; CI, 0.42 to 1.18) but not OS trended to being superior in patients treated with combined-modality therapy (HD10/HD11) versus ABVD alone (HD.6). There were no differences in outcomes for patients achieving a complete response (CR) by CT after two cycles of ABVD. IFRT appeared most beneficial to patients who did not achieve an early CR, which provides support for the role of interim response assessment.
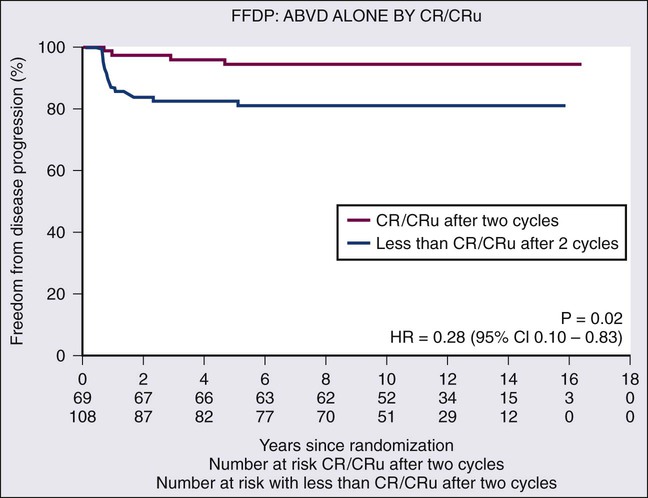
The optimum number of cycles of chemotherapy is not defined for patients receiving chemotherapy alone. In the NCIC HD.6 study, 69/196 (35%) of patients treated with ABVD alone achieved CR by CT criteria after two cycles of ABVD.25 Of these 69 CR patients, 57 received a total of four cycles of ABVD (per protocol) and 12 received six cycles (physician decision) with a 5-year freedom from disease progression of 95%, compared with 81% for the 113 patients not in radiographic CR after two cycles (Fig. 105-4). In the small subset of patients with a CR by CT after two cycles of ABVD, a total of four cycles appears adequate. The German Hodgkin Study Group recently adopted the “2 + 2 regimen” for patients with early unfavorable HL based on the results of the HD14 trial.36 Patients were randomized to four cycles of ABVD (ABVD arm) or two cycles of escalated (esc) BEACOPP followed by two cycles of ABVD (2 + 2 arm). Patients in both arms of the study received 30 Gy IFRT after chemotherapy. The 5-year FFTF was superior in the 2 + 2 arm (94.8%) compared with the ABVD arm (87.7%) (P < .001), but there was no difference in OS between the two arms. Grade 3 and 4 toxicities were significantly more frequent in the 2 + 2 arm compared with the ABVD arm. In univariate and multivariate analyses, only large mediastinal mass and elevated ESR were predictors for PFS events.
Based on compelling data from Gallamini and Hutchings showing a 2-year PFS of 12% for HL patients with a positive PET scan after two cycles of ABVD compared with 95% for patients with a negative interim PET, the use of interim PET has become widespread in standard practice and is the focus of most current clinical trials in both early and advanced HL.37 Current studies are examining the use of early metabolic CR by PET/CT to determine the number of cycles of chemotherapy, the intensity of the chemotherapy regimen, and which patients should receive RT.
It is unclear whether the dramatic predictive value of a positive interim PET applies to patients with early-stage HL. In a small study of 57 patients with stage I or II HL, those patients with a positive PET after two cycles of ABVD still had an approximately 75% chance of durable remission with additional ABVD chemotherapy and consolidative IFRT.38,39 Investigators at the Dana Farber Cancer Institute also reported outcomes for 73 patients with HL, 86% with limited stage, referred for consolidative RT, including 20 of 43 patients with a positive interim PET scan and 13 of 73 patients with a positive PET scan at completion of chemotherapy. After IFRT, the 2-year failure-free survival (FFS) was 85% for patients with a positive interim PET and 69% versus 95% for patients with a positive versus negative PET, respectively, at the completion of all chemotherapy.40
Whether a PET-directed approach will allow us to safely minimize chemotherapy cycles and avoid RT is the subject of several ongoing large randomized trials. As these trials complete accrual and results mature, we hope to have a definitive answer as to whether three to four cycles of ABVD alone will be sufficient therapy for the majority of patients with early-stage HL and whether ABVD + RT or alternatively escBEACOPP + RT will be sufficient therapy for patients with less than a complete metabolic response after two cycles of ABVD. Preliminary results of the first completed randomized trial evaluating interim PET-directed therapy in early-stage HL, the UK RAPID trial, provide the earliest support for this concept.41 Eligibility for the RAPID trial included nonbulky HL without regard to other risk factors. All patients received three cycles of ABVD and were restaged with PET/CT. Patients with a negative PET, as defined by a score of 1 or 2 on the London Deauville visual analog scale, were then randomized to receive IFRT versus observation.42 Patients with a positive interim PET went on to receive one additional cycle of ABVD followed by IFRT. In this study, 74.6% of patients had a negative PET scan after three cycles of ABVD. With a median follow-up of 45.7 months, 91.4% of interim PET-negative patients (384/420) and 86.9% of interim PET-positive patients (126/145) are alive and progression free. The 3-year PFS and OS rates were 93.8% and 97%, respectively, in the 209 PET-negative patients randomized to IFRT and 90.7% and 99.5%, respectively, in the 211 PET-negative patients randomized to no further treatment. The PFS survival risk difference of −2.9% [95% CI, −10.5, 1.4%] marginally exceeded the acceptable noninferiority margin of −7%. These very encouraging results support the use of chemotherapy alone in the 75% of patients with early negative interim PET/CT.
Early-Stage Bulky Hodgkin Lymphoma
Despite a paucity of data, the standard treatment for patients with early-stage bulky HL is combined-modality therapy.27,34 The early-stage bulky presentation of HL is common in young women, the population most at risk for late effects of RT, primarily breast cancer. No randomized trials in early-stage bulky HL have specifically addressed the role of RT. The recent intergroup trial E2496 enrolled 854 patients, including 268 with stage I/II bulky disease.43 The patients were treated with ABVD plus modified IFRT to 36 Gy for patients with bulky mediastinal disease or with the Stanford V regimen plus modified IFRT to 36 Gy for sites larger than 5 cm in maximum transverse dimension or in the presence of macroscopic splenic disease. In the patients with bulky disease, the 5-year FFS and OS were 82% and 94%, respectively, with no difference between the study arms. This subgroup analysis provides the “standard” against which trials investigating a reduction or elimination of RT should be compared to determine if the benefits of RT in early-stage bulky disease outweigh the significant risks. The use of fluorodeoxyglucose (FDG)-labeled PET at the end of treatment to guide consolidative radiation was investigated in patients with residual abnormalities on CT.44 Despite omission of RT in patients with a negative end of treatment PET scan, there was no difference in the 3-year time to progression between the patients with bulky disease and those without bulky disease (86% vs. 91%, P = .71). An Italian study of RT versus observation in 160 patients with “bulky” (≥5 cm) disease and a negative end of treatment PET revealed that 14% of patients who did not receive radiation experienced relapse at a median follow-up of 40 months compared with 4% of patients who received RT.45 Until randomized trials are completed, the standard of care for bulky HL remains combined-modality therapy in most centers.
Advanced-Stage Hodgkin Lymphoma
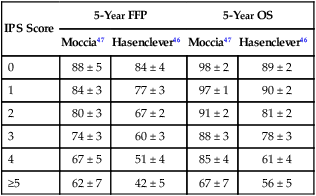
The majority of patients with stage III/IV HL receive six cycles of ABVD chemotherapy, the standard treatment for more than 2 decades. The Hasenclever International Prognostic Score (IPS) is used to predict prognosis of patients with advanced-stage HL treated with ABVD (see Table 105-2).46 In the original publication, the 5-year FFP rates were 70% for those with none to three risk factors compared with 47% for those with four or more high-risk features. A more recent publication examining the outcomes of patients with advanced-stage HL treated in British Columbia between 1980 and 2010 according to the IPS (Table 105-3) showed more favorable 5-year PFS rates of 81% for patients at lower risk (IPS 0 to 3) and 65% for those at high risk (IPS 4 to 7).47 Several reasons are hypothesized for superior PFS rates, including improved diagnostic accuracy resulting in elimination of patients with non-Hodgkin lymphoma, better dose-intensity preservation with current recommendations to treat with full-dose ABVD every 2 weeks regardless of day of treatment neutrophil count, more precise staging with PET/CT resulting in upstaging of perhaps more favorable advanced-stage disease, and anthracycline-based therapy in all patients compared with only 80% of patients in the original series.49–49 The routine use of autologous stem cell transplant (ASCT) in relapsed HL likely accounts for the improvement in OS. Although the IPS continues to delineate outcomes in HL, the narrower range of outcomes makes it more difficult to justify changes in clinical management based on the initial IPS.
Table 105-3
Five-Year Freedom from Progression (FFP) and Overall Survival (OS) by International Prognostic Score (IPS)
| IPS Score | 5-Year FFP | 5-Year OS | ||
| Moccia47 | Hasenclever46 | Moccia47 | Hasenclever46 | |
| 0 | 88 ± 5 | 84 ± 4 | 98 ± 2 | 89 ± 2 |
| 1 | 84 ± 3 | 77 ± 3 | 97 ± 1 | 90 ± 2 |
| 2 | 80 ± 3 | 67 ± 2 | 91 ± 2 | 81 ± 2 |
| 3 | 74 ± 3 | 60 ± 3 | 88 ± 3 | 78 ± 3 |
| 4 | 67 ± 5 | 51 ± 4 | 85 ± 4 | 61 ± 4 |
| ≥5 | 62 ± 7 | 42 ± 5 | 67 ± 7 | 56 ± 5 |
The German Hodgkin Study Group continues to advocate the use of escBEACOPP as initial therapy in all patients with advanced stage HL. Initially reported in 2003, a large randomized trial comparing eight cycles of escBEACOPP to COPP (cyclophosphamide, vincristine, procarbazine, prednisone)/ABVD showed that escBEACOPP resulted in better tumor control and OS.50 Ten-year follow-up of the trial showed FFTF and OS rates of 64% and 75% for COPP/ABVD compared with 82% and 86% for escBEACOPP (P < .0001).51 There was no significant difference in FFTF or OS for patients 60 to 65 years, and escBEACOPP is not recommended in patients 60 years of age or older secondary to unacceptable acute toxicities. Despite the improved FFTF, and perhaps even a survival advantage, physicians outside Germany hesitate to recommend escBEACOPP because of toxicity concerns. Acute hematologic toxicity is high with escBEACOPP, with grade 3 to 4 infections occurring in 22% of patients. Acute myeloid leukemia (AML) developed in 3% of patients on the escBEACOPP arm of the trial. EscBEACOPP results in azoospermia and infertility in the majority of male patients and induces premature menopause in 40% of women younger than age 30 years and 70% of women 30 years of age or older.54–54 An analysis of female survivors treated on the German Hodgkin Study Group HD14 trial revealed a reduced ovarian reserve (as determined by hormone levels) in patients treated with two cycles of escBEACOPP + two cycles of ABVD compared with four cycles of ABVD.55 However, the authors reported that this “2 + 2 regimen” in combination with gonadotropin-releasing hormone (GnRH) analogs did not compromise fertility, because motherhood rates were equivalent to the German normal population. Conflicting reports regarding the use of oral contraceptives and GnRH analogs to protect the ovarian follicle pool have been published.56 A randomized trial of six versus eight cycles of escBEACOPP in advanced-stage HL showed a more favorable toxicity profile and improved 5-year OS with six cycles.57 Secondary AML/MDS (myelodysplastic syndrome) occurred in 0.3% of those receiving six cycles compared with 2.7% for eight cycles, and acute treatment-related deaths were 0.8% with six cycles versus 2.1% for eight cycles.
In three other randomized trials of escBEACOPP versus ABVD, escBEACOPP was not associated with a survival advantage, despite a lower rate of relapse,58,59 All three trials, in an effort to reduce both acute and long-term toxicity, administered a slightly less intensive BEACOPP regimen than the German Hodgkin Study Group with four cycles of escBEACOPP and two cycles of standard BEACOPP in two trials and four cycles of escBEACOPP and four cycles of standard BEACOPP in one study. The lack of a survival difference in these trials is likely due to the better prognosis after second-line therapy for patients initially treated with ABVD compared with escBEACOPP. In one study, 33% (15/45) of the patients who failed ABVD remained in continuous CR after salvage therapy, compared with only 15% (3/20) of the BEACOPP failures at a median follow-up time of 61 months.60 Failure of many physicians to embrace the escBEACOPP regimen is a result of physicians choosing to expose fewer numbers of patients to sterilizing and potentially leukemogenic therapy. The tradeoff of this choice is that a higher percentage of patients will need salvage therapy, including ASCT. ASCT is now associated with less than 2% acute mortality in most centers and in many ways may be a more tolerable treatment than six cycles of escBEACOPP.
An 854-patient U.S. intergroup study compared ABVD with Stanford V chemotherapy in advanced-stage HL and demonstrated no advantage to the Stanford V regimen.43 Five-year FFS was 74% for ABVD compared with 71% for the Stanford V regimen (P = .32). The 5-year OS was 88% in both patient groups. The abbreviated Stanford V regimen was inferior to ABVD in patients with an IPS of 3 to 7 (5-year PFS 57% vs. 67%, P = .02) and was associated with more hematologic toxicity and peripheral neuropathy.
As discussed in treatment of early-stage disease, the interim PET/CT scan may allow therapy to be tailored more accurately than the initial IPS. In the joint report from an Italian-Danish study evaluating the prognostic significance of early interim PET scans after two cycles of ABVD (PET-2), only PET-2 was significant in a multivariate analysis that included all IPS factors.37 Still under investigation is whether altering therapy on the basis of the interim PET will result in more favorable outcomes. Dann and colleagues described 77 patients treated with two cycles of standard-dose BEACOPP followed by a gallium-67 or PET/CT scan.61 Twelve patients had a positive interim gallium-67 or PET/CT scan, and therapy was intensified to escBEACOPP, with only two relapses occurring after intensification of the treatment. In a retrospective review of 154 patients with newly diagnosed advanced-stage HL treated in 2006 and 2007 at one of nine Italian or U.S. centers with two cycles of ABVD followed by escBEACOPP × 4 plus standard BEACOPP × 4 if an interim PET/CT scan was positive, and an additional four cycles of ABVD if PET/CT was negative, the 2-year FFS was 62% for PET-positive patients and 95% for PET-negative patients (P < .0001).62 These results are in stark contrast to the 2-year PFS of 12% reported by Gallamini and colleagues for patients with a positive interim PET/CT who continue treatment with ABVD.37 With approximately 20% of patients expected to have positive interim PET scans, the outcomes in this subset of patients treated with more intensive therapy will be primarily observational. A randomized trial of standard chemotherapy versus intensified therapy for PET-positive patients in advanced HL will not be done, because of ethical concerns based on historical reports. In contrast to the U.S. and U.K. trials in which all patients are treated initially with ABVD and those with a positive interim PET are escalated to more intensive therapy, the German Hodgkin Study Group (HD18, NCT00515554) and Group d’Études des Lymphomes de l’Adulte (NCT01358747) are both conducting clinical trials in advanced-stage HL in which all patients initiate treatment with escBEACOPP and those with a negative interim PET are de-escalated to ABVD (Group d’Études des Lymphomes de l’Adulte) or two additional cycles of escBEACOPP (vs. six additional cycles) (German Hodgkin Study Group). The hope and expectation is that in the coming years physicians will be able to tailor therapy much more effectively by escalating to more toxic therapy only in the small subset of patients with an early positive interim PET and perhaps eliminating cycles or agents such as bleomycin in patients with a negative PET.
Therapy for Hodgkin Lymphoma in Pregnancy
Given the young median age of patients with HL, occasionally women will be diagnosed during pregnancy. Current treatment recommendations are based on a small series of patients with a variety of hematologic malignancies.63 Although guidelines continue to recommend termination for patients diagnosed during the first trimester, many patients decline this option. The slow-growing nature of HL often makes it feasible to delay therapy until the second trimester for those diagnosed early in the course of their pregnancy or until after delivery in those diagnosed during the third trimester. As additional data emerge about the relative safety of standard chemotherapy for women with HL during the second and third trimester of pregnancy, recommendations are often to proceed with standard ABVD chemotherapy in these patients.63,64 In a retrospective analysis of 39 patients diagnosed with HL during pregnancy, 2 patients in the first trimester elected termination, 15 patients deferred therapy (median, 34 weeks’ gestation; range, 6 to 38 weeks) and 21 patients received therapy during pregnancy.64 The majority, 15 of 21, received ABVD chemotherapy; 10 patients started therapy in the second trimester of pregnancy and 5 patients started in the third trimester. The 3-year PFS and OS were 90% and 95%, respectively. Median birth weights were similar among those patients electing treatment and patients deferring treatment. No fetal malformations occurred among the 21 patients receiving treatment during pregnancy. Long-term follow-up of children with prenatal exposure to chemotherapy, including those exposed during the first trimester, has shown no significant differences in physical or cognitive development, cardiac abnormalities, or congenital malformations compared with the general population.65,66 However, prematurity was more common in these children and was associated with impaired cognitive development.65 Full-term delivery in all patients should be the goal.
Therapy for Hodgkin Lymphoma in Older Patients
Fifteen to 20 percent of all HL occurs in patients older than 60 years of age.1 Trends in age-specific, 10-year relative survival rates of patients with HL between 1980 and 2004 show marked increases in all groups, but particularly in patients aged 45 to 59 years (51.4% to 76.2%) and 60 years and older (21.6% to 44.9%).1 However, patients aged 60 years and older still have a much worse prognosis than younger patients. Inferior outcomes are attributable to higher IPS scores at diagnosis, as well as co-morbid conditions that increase the morbidity and mortality associated with therapy.67 Patients older than 60 years are not candidates for aggressive regimens such as escBEACOPP and are often not eligible for salvage regimens incorporating ASCT. Proctor and colleagues reported a 14% toxic death rate during or immediately after completion of treatment in a series of 35 patients 60 years of age and older who received standard ABVD chemotherapy.68 Causes of death were sepsis in four patients and bleomycin pulmonary toxicity in one patient. Evens and associates reported a much higher incidence of bleomycin lung toxicity in patients older than 60 years, especially those receiving concurrent granulocyte colony-stimulating factor.69 In this retrospective analysis of 92 HL patients aged 60 years to 89 years, 32% of patients developed bleomycin lung toxicity, which was associated with a 25% mortality rate. Analysis of the large randomized trial of ABVD versus the Stanford V regimen showed the 5-year PFS for elderly patients was 46% and the OS rate was 56%, both worse than for nonelderly patients.70 ABVD may be a viable option for patients older than 60 years with a good performance status and no underlying cardiopulmonary disease. If results of the phase III U.K. trial evaluating the possible elimination of bleomycin in patients with a negative interim PET/CT after two cycles of ABVD show no adverse effect of abbreviating bleomycin to two doses, this may improve the tolerability of ABVD particularly in patients older than 60 years.
Several alternative regimens to ABVD have been proposed for older patients with HL. Members of the Scotland and Newcastle Lymphoma Group advocate the use of the VEPEMB (vinblastine, cyclophosphamide, prednisolone, procarbazine, etoposide, mitoxantrone, bleomycin) regimen in older patients based on a phase II study in patients 60 years and older.68 The treatment-related mortality rate was 7%, and 3-year PFS rates were 74% in patients with early-stage disease and 58% in those with advanced-stage disease. CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) has also been proposed as an alternative to ABVD; and in a small study of 29 elderly patients treated with CHOP-21, the CR rate was 93% with two treatment-related deaths.71 Three-year OS and PFS rates were 79% and 76%, respectively. The hybrid ChlVPP/ABV regimen (chlorambucil, vinblastine, procarbazine, prednisone/doxorubicin, bleomycin, vincristine) is another well-tolerated alternative regimen in older patients, with decreased relapses and improved survivals when compared with historical results with ChlVPP alone. PVAG (prednisone, vinblastine, doxorubicin, and gemcitabine) was investigated in a phase II study in elderly patients and resulted in a CR rate of 78%.72 One patient died of a treatment-related toxicity, and the 3-year OS and PFS rates were 66% and 58%, respectively. Ongoing efforts to incorporate the very active and well-tolerated drug brentuximab vedotin into first-line therapy may benefit older patients in terms of both improved efficacy and tolerability.
Therapy for Hodgkin Lymphoma in Patients Infected with Human Immunodeficiency Virus
In the era of highly active antiretroviral therapy (HAART), outcomes and therapy recommendations for HL in human immunodeficiency virus (HIV)–infected individuals mirror those of the non–HIV-infected population. Montoto and associates reported on 224 consecutively treated patients with HL diagnosed between 1997 and 2010 at five university hospitals in London, including 93 HIV-positive patients and 131 HIV-negative patients.73 ABVD therapy was administered to 86% of the HIV-positive patients and 73% of the HIV-negative patients. Five-year disease-free survival rate was 85% and 87% for HIV-negative and HIV-positive patients, respectively. Similar favorable results were reported in 108 patients with HIV-associated HL treated with ABVD and IFRT if early stage and six to eight cycles of baseline BEACOPP in those with advanced-stage disease.74 Importantly, in both of these series, patients received concurrent HAART therapy and chemotherapy. Patients with HIV are not candidates for the escBEACOPP regimen.
Therapy for Lymphocyte-Predominant Hodgkin Lymphoma
In contrast to classic HL, there is no universal consensus regarding primary therapy for NLPHL. As previously discussed, it represents a unique pathobiological entity with a distinct clinical presentation and natural history. Historically, treatment of lymphocyte-predominant HL paralleled that of classic HL, with most patients treated using extended- or regional-field RT, with or without chemotherapy. The majority of deaths in patients treated for NLPHL were related to late effects, primarily cardiovascular disease and second malignancies.75 Deaths due to disease were rare. Based on these findings, efforts to develop distinct approaches for NLPHL have emerged. Small patient numbers and very favorable outcomes preclude randomized trials in this subtype of HL.
Current approaches for early-stage disease include observation, most often in patients with a single site that has been resected; IFRT, most commonly neck, axillary, or inguinal RT as mediastinal involvement with NLPHL is rare; single-agent rituximab; or combined-modality therapy. Chen and associates reported on 113 patients with early-stage NLPHL treated at the Dana Farber Cancer Institute between 1970 and 2005. Ten-year PFS rates were 85% (stage I) and 61% (stage II), with OS rates of 94% and 97%, respectively.75 Importantly, PFS and OS did not differ among patients who received IFRT, regional-field RT, or EFRT. Interestingly, six of seven patients who received chemotherapy alone experienced relapse. During this 25-year period, only 3 of 113 patients died of HL and 10 patients died of second malignancy (6 patients), cardiac disease (2 patients), or unrelated causes (2 patients).
The British Columbia Cancer Agency reported on 88 patients with limited-stage NLPHL treated between 1966 and 2009.76 Ten-year PFS rates were 65% for 32 patients treated with RT alone, compared with 91% for the 56 patients treated with ABVD either alone (n = 14) or in combination with RT (n = 42). There were no deaths from NLPHL and 3 deaths related to transformed diffuse large B-cell lymphoma. The current approach at the British Columbia Cancer Agency is ABVD for two cycles followed by PET/CT. If interim PET is negative, patients receive two additional cycles of ABVD and no RT, but if interim PET is positive then IFRT is done. Single-agent rituximab has been used as primary therapy with high response rates but higher rates of early relapse than seen with RT or combined-modality therapy.77,78 A response rate of 100% was reported for 28 patients with stage I NLPHL with a 3-year PFS of 81%.78 Four doses of maintenance rituximab administered during a 2-year period resulted in a nonsignificant increase in PFS (67 months) compared with rituximab induction alone (50 months, P = .7).77
In patients with either advanced-stage or limited-stage disease requiring chemotherapy, the choice of regimen varies across centers. As discussed previously, investigators at the British Columbia Cancer Agency have reported favorable outcomes with ABVD. A review of the 37 NLPHL patients treated with chemotherapy on one of two clinical trials at the Dana Farber Cancer Institute showed 9 of 12 (75%) failures in patients treated with ABVD or EVA (etoposide, vinblastine, doxorubicin) compared with 8 of 25 failures in patients treated with MOPP or MOPP/ABVD, suggesting perhaps an alkylator-based regimen may be superior in LPHL.79 Fanale and associates reported a 100% response rate with R-CHOP (n = 15) or R-CHOP + IFRT (n = 5) and no relapses or transformations at a median follow-up of 42 months.80 The need for an anthracycline in the regimen is unclear. Regardless of the choice of chemotherapy regimen, incorporating rituximab is justified based on the high single-agent response rates. Based on these small series, R-CHOP, R-CVP, and R-ABVD would all represent standard approaches in patients requiring systemic therapy.
Patients with NLPHL are at risk for transformation to diffuse large B-cell lymphoma, with reported transformation rates as high as 15%. Two large retrospective analyses of NLPHL from France and British Columbia determined 10-year rates of transformation to be 12% and 7%, respectively.81,82 Transformation was diagnosed at a median of 4.7 (France) and 8.1 years (British Columbia Cancer Agency) without a plateau in risk for transformation, although few transformations occurred after 25 years. Al-Mansour and colleagues reported that transformation was more likely in patients with splenic involvement at the time of initial NLPHL diagnosis (P = .006).82 The 10-year OS rates from diagnosis of transformation were 60% (France) and 62% (British Columbia Cancer Agency). There is no standard regimen for treatment of transformed NLPHL, although most patients receive R-CHOP or salvage regimens (frequently ICE [ifosfamide, carboplatin, etoposide]) and ASCT. In the French series, the OS rate did not differ between patients who underwent ASCT and those who did not.82 Long-term follow-up is essential in NLPHL because late relapses are common. Additionally, biopsy at the time of recurrence is particularly important to detect transformation.
Treatment and Prognosis of Relapsed Disease
Based on a randomized trial conducted in the 1990s that showed a 7-year FFTF of 49% for patients undergoing ASCT compared with 32% (P = .02) for those undergoing nontransplant salvage, ASCT is the preferred treatment for relapsed HL.83,84 Since this report in 2002, cure rates for relapsed HL with ASCT continue to be approximately 50%.85,86 There is significant heterogeneity among treatment centers in the primary salvage regimen, the transplant preparative regimen, and the use of RT before or after ASCT. The relative rarity of this population likely precludes addressing these issues in a definitive way. However, strikingly similar results across studies and in retrospective comparisons at single centers in this population suggest that, based on currently available treatments, these are not likely to represent major questions.87,88 More important than the precise procedural details of ASCT are how to improve selection of patients for ASCT with precise prognostic models and how to identify alternative novel options for the patients at highest risk and those failing ASCT.
Prognosis at Relapse
Most prognostic models for relapsed HL include not only the disease and patient characteristics at relapse but also the response to initial salvage therapy. In practice, the response to the initial salvage regimen trumps all other prognostic features in the decision to proceed with ASCT. Currently, there are no clinical features at relapse that would prevent administration of standard salvage chemotherapy, with pretransplant response assessment, preferably by PET/CT, as the primary determinant of outcome. Older reports of ASCT results based on response to salvage therapy as determined by CT criteria show 5-year PFS rates of 60% to 69% for patients with a CR, approximately 40% for patients achieving a partial response (PR), and less than 20% for those with no response.88,89
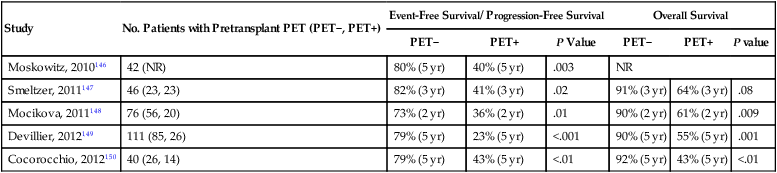
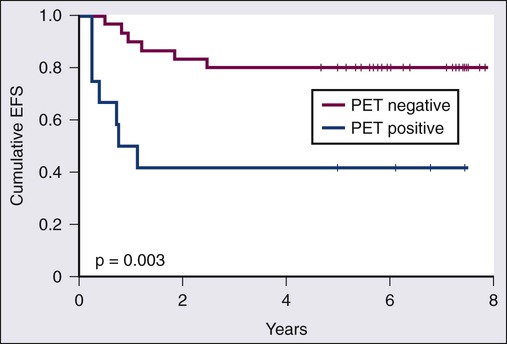
Functional imaging, initially with gallium-60 and more recently FDG-PET, may improve pre-ASCT prognostication, especially in the subset of patients with a PR or stable disease by CT criteria. Table 105-4 summarizes the results of several small series showing ASCT outcomes based on pretransplant PET results. Nearly 80% of patients with a negative PET before a transplant remain in remission 3 to 5 years after ASCT. Because 23% to 41% of patients with a positive pre-ASCT PET will still experience a prolonged remission, most centers proceed to ASCT in this subset of patients, as long as there is no evidence of frank progression. Moskowitz and colleagues administered additional non–cross-resistant salvage therapy before ASCT in patients with a positive PET after first-line salvage.90 Patients achieving a PET-determined CR after first-line salvage therapy and those with a PET-determined CR only after second-line salvage therapy had identical outcomes with an 80% 5-year EFS compared with less than 30% for those who remained positive (Fig. 105-5). Response to second salvage, a refined interpretation of “positive” PET results, or a model incorporating other high-risk clinical features specifically for the PET-positive group may allow better selection of the subgroup for whom alternatives to ASCT should be considered.
Table 105-4
Selected Reports of Outcomes After ASCT Based on Pretransplant PET Results
| Study | No. Patients with Pretransplant PET (PET−, PET+) | Event-Free Survival/ Progression-Free Survival | Overall Survival | ||||
| PET− | PET+ | P Value | PET− | PET+ | P value | ||
| Moskowitz, 2010146 | 42 (NR) | 80% (5 yr) | 40% (5 yr) | .003 | NR | ||
| Smeltzer, 2011147 | 46 (23, 23) | 82% (3 yr) | 41% (3 yr) | .02 | 91% (3 yr) | 64% (3 yr) | .08 |
| Mocikova, 2011148 | 76 (56, 20) | 73% (2 yr) | 36% (2 yr) | .01 | 90% (2 yr) | 61% (2 yr) | .009 |
| Devillier, 2012149 | 111 (85, 26) | 79% (5 yr) | 23% (5 yr) | <.001 | 90% (5 yr) | 55% (5 yr) | .001 |
| Cocorocchio, 2012150 | 40 (26, 14) | 79% (5 yr) | 43% (5 yr) | <.01 | 92% (5 yr) | 43% (5 yr) | <.01 |
NR, not reported. ASCT, Autologous stem cell transplant; PET, positron emission tomography.
Clinical factors at relapse may complement prognostic information provided by the PET response to salvage therapy. Poor prognostic features consistently identified in multivariate analyses include time to relapse (≤12 mo. vs. >12 mo.), advanced clinical stage, anemia, B symptoms, and bulky and extranodal disease.93–93 The use of the IPS developed by Hasenclever for newly diagnosed advanced-stage HL has also been investigated in patients with relapsed HL.89,94 Bierman and associates showed 10-year EFS rates were 38%, 23%, and 7% for patients with none to one (n = 27%), two to three (n = 53%) or four or more (n = 20%) of the seven IPS risk factors (P < .001).94 Sirohi and colleagues reported a 5-year PFS rate of 60% for those with an IPS of 0 to 2 versus 32% for those with a score of 3 or more (P = .0001) immediately before ASCT.89 In a model including B symptoms, extranodal disease, and initial response less than 1 year, 5-year EFS rates of 76% (none to one factor), 35% (two factors), and 8% (three factors) were reported.95 Smith and colleagues stratified patients with relapsed HL into three groups based on risk factors of bulky and extranodal disease.93 Patients with none, one, or two risk factors achieved a 6-year EFS of 65%, 47%, and 24%, respectively.
Pretransplant Salvage Chemotherapy
The ideal pretransplant salvage regimen provides a high response rate, low toxicity, and minimal damage to stem cells, allowing the majority of patients to proceed without delay to ASCT. Overall and complete response rates for selected pretransplant salvage regimens are listed in Table 105-5. Most salvage regimens are designed to incorporate agents that are non–cross resistant to ABVD, such as ifosfamide, platinum, etoposide, gemcitabine, cytarabine, and corticosteroids. Unfortunately, no randomized trials have compared the effectiveness of conventional salvage regimens for relapsed HL. For those not achieving a PET-determined CR with first-line salvage, an alternate regimen should be considered before ASCT. For those receiving initial salvage with ICE, ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin), or DHAP (cisplatin, cytarabine, dexamethasone), a gemcitabine-containing regimen such as GVD (gemcitabine, vinorelbine, liposomal doxorubicin) represents a reasonable second-line salvage option.90,96 Alternatively, single-agent brentuximab vedotin (see later discussion under New Drugs) is approved as second-line salvage with reported response rates of 76% in relapsed HL with a 34% CR rate.97,98 Brentuximab vedotin has been tested primarily in patients who have failed ASCT, which by definition is a more chemosensitive group than the group failing first-line salvage. Brentuximab vedotin as first-line salvage is under investigation in two single-center studies (NCT01393717, NCT01508312).
Table 105-5
Pretransplant Salvage Regimens
| Study | Regimen | No. Patients | Response Rate | Complete Response Rate |
| Aparicio, 1999151 | ESHAP | 22 | 73% | 41% |
| Josting, 2002152 | DHAP | 102 | 89% | 21% |
| Moskowitz, 201290 | ICE/aICE | 97 | NR | 60% by PET |
| Moskowitz, 201095 | ICE/aICE | 105 | 59% | 33% by CT |
| Moskowitz, 200192 | ICE | 65 | 88% | 26% |
| Santoro, 2007153 | IGEV | 91 | 81% | 54% |
| Bartlett, 200796 | GVD | 91 | 70% | 19% |
| Kuruvilla, 200699 | GDP | 34 | 62% | 9% |
| Forero-Torres, 201298 | Brentuximab vedotin* | 20 | 30% | 10% |
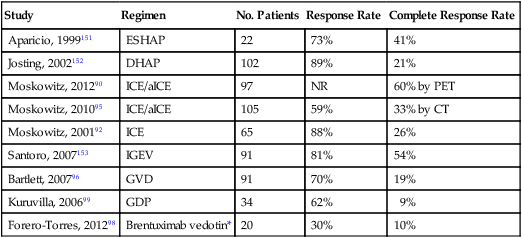
*Brentuximab vedotin is approved for patients who have failed two prior regimens.
In retrospective analyses there appears to be no advantage to aggressive pretransplant regimens such as mini-BEAM (BCNU [carmustine], etoposide, cytarabine, melphalan), which are associated with higher morbidity, mortality, and stem cell toxicity.99,100 Based on retrospective data, Moskowitz and colleagues advocate intensifying salvage regimens for patients with high-risk features at relapse, specifically those with at least two of three poor prognostic features at relapse, including B symptoms, extranodal disease, and remission duration less than 1 year.95 Patients with none to one risk factor received two cycles of standard ICE, those with two risk factors received one cycle of standard ICE and one cycle of augmented ICE (dose of ifosfamide and etoposide doubled), and those with three risk factors received one cycle of high-dose cyclophosphamide followed by one cycle of high-dose ICE requiring stem cell rescue. All responding patients went on to receive ASCT. Five-year EFS rates were approximately 50% for those with two risk factors and 40% for those with three risk factors compared with historical rates of 37% and 8%, respectively. Unfortunately, the complex approach advocated by some centers, with a 10-branch decision tree for a patient with relapsed HL, is difficult to implement across centers.95
Role of Allogeneic Stem Cell Transplantation
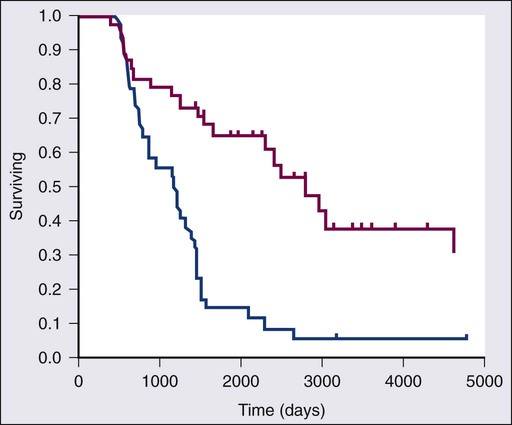
Allogeneic SCT has the theoretical advantages of a tumor-free stem cell source, additional antitumor activity, and a decrease in secondary myelodysplastic syndrome (MDS) and acute leukemia. However, high treatment-related mortality with myeloablative allogeneic SCT has dampened enthusiasm for this approach. Reduced intensity conditioning (RIC-allo) or nonmyeloablative SCT decreases 1-year treatment-related mortality (7% to 25%) but is still associated with significant morbidity related to graft-versus-host disease and an increased risk for relapse (40% to 59%).101 Robinson and associates reported a 3-year PFS of 25% after RIC-allo in 285 patients with relapsed HL, 80% whom had undergone previous ASCT.102 Retrospective analyses suggest that RIC-allo may be superior to conventional salvage therapy in patients for whom ASCT failed, as shown in Figure 105-6.103,104 Chen and colleagues showed very encouraging results of RIC-allo in patients treated with previous brentuximab vedotin.105 Historically, HLA-matched related or matched unrelated donors have been the preferred donor source. Burroughs and associates reported a significantly higher 2-year PFS rate (51% vs. 23% to 29%), decreased relapse rate (40% vs. 56% to 63%), and significantly lower nonrelapse mortality for haploidentical donor transplants compared with HLA-matched transplants.106 Responses after donor lymphocyte infusions provide evidence of graft versus HL effect.109–109
New Drugs
The most promising new therapy for relapsed/refractory HL is brentuximab vedotin (SGN-35), an antibody-drug conjugate composed of an anti-CD30 antibody (cAC10) conjugated to monomethyl auristatin E (MMAE), a potent antimicrotubule agent.110 Brentuximab vedotin was approved by the U.S. Food and Drug Administration in 2011 for patients with relapsed or refractory HL after at least two prior lines of treatment. In a pivotal phase II trial, brentuximab vedotin had an overall response rate of 75% and CR rate of 34% in 102 patients with relapsed or refractory HL after ASCT, including 70% of patients with primary refractory HL.97 Patients received a maximum of 16 cycles unless disease progression or prohibitive toxicity occurred. The median PFS was 5.6 months, and in patients who achieved a CR, the median duration of response was 20.5 months. Common brentuximab vedotin toxicities include peripheral sensory neuropathy (42%), neutropenia (19%), and diarrhea (18%). No febrile neutropenia or treatment-related deaths occurred in the phase II study. Peripheral sensory neuropathy is reversible in most patients. Three cases of progressive multifocal leukoencephalopathy have been reported in patients receiving brentuximab vedotin.111 Brentuximab vedotin is contraindicated in combination with bleomycin given the increased incidence of severe and fatal pulmonary toxicity seen in a phase I study of brentuximab vedotin plus ABVD chemotherapy.112
Re-treatment with brentuximab in patients with relapsed HL who previously responded to brentuximab vedotin resulted in a 60% response rate in 15 patients, including three with complete remissions.113 Brentuximab vedotin may serve as a bridge to RIC-allo in responding patients who failed prior ASCT.105 Additional studies are exploring the use of brentuximab as adjuvant therapy after ASCT, as well as in combination with AVD (ABVD without the bleomycin) as first-line therapy.
Other novel agents under investigation in relapsed/refractory HL include histone deacetylase (HDAC) inhibitors, mammalian target of rapamycin (mTOR) inhibitors, immunomodulatory agents, and the bi-alkylator bendamustine. Panobinostat, a pan-HDAC inhibitor, showed modest activity in a phase II study in relapsed and refractory HL after ASCT.114 The overall response rate was 27% (4% CR) in 129 patients. The median duration of response was 6.9 months, and the median PFS was 6.1 months. The treatment was well tolerated, and the common grade 3 and 4 hematologic adverse events were manageable; only two patients (2%) experienced febrile neutropenia. A phase II trial of the mTOR inhibitor everolimus reported an overall response rate (ORR) of 47% (5% CR) in 19 patients with relapsed HL.115 The median duration of response was 7.1 months, and the median PFS was 6.2 months. Four patients (21%) experienced grade 3 or higher pulmonary toxicity, a known side effect of everolimus, and 2 patients developed infections considered at least possibly related to treatment. Lenalidomide, an immunomodulatory agent, showed modest activity in 38 patients with relapsed/refractory HL.116 The ORR was 18% (3% CR), the median duration of response was 6 months, and the median PFS was 4 months. The most common (≥15%) grade 3 or 4 adverse events were neutropenia and thrombocytopenia; grade 3 or 4 infections occurred in 3 patients (8%). A retrospective analysis of 41 patients treated with bendamustine reported a response rate of 58% (31% CR).117 The median duration of response was 9 months, and the median PFS exceeded 11 months. A prospective phase II study of bendamustine enrolled 36 patients with relapsed/refractory HL and reported an ORR of 57% (33% CR).118 The mean response duration was 5.7 months, but the PFS and OS rates have not been reported. Combinations of new agents with standard therapies are under investigation.
Late Complications of Therapy for Hodgkin Lymphoma
Balancing efficacy with long-term toxicity has long been a goal of HL therapy. This has proven to be an extraordinary challenge, primarily because of the significant delay of more than 10 years, and in many cases 30 years, between HL therapy and its associated complications. Evaluating alterations in therapy requires decades of follow-up to determine if new approaches have indeed accomplished the goal of decreased long-term toxicity. Dissecting the late effects of chemotherapy versus RT when a large percentage of patients receive both modalities presents an additional hurdle. Finally, currently undefined potential host factors in patients with HL, such as subtle immune deficiencies or genetic susceptibilities to cancer, continue to represent potential explanations for at least a portion of the second malignancies. In addition to advances in therapy, research continues on methods to enhance cardiovascular and malignancy screening in HL survivors.119
Second Cancers
Abandonment of the MOPP (mechlorethamine, vincristine, procarbazine, prednisone) regimen and alkylating agents in the primary therapy for HL has virtually eliminated the incidence of secondary acute leukemia related to primary therapy, except in those receiving escBEACOPP. Even with the alkylator-dense escBEACOPP regimen, reducing the number of cycles from eight to six has resulted in many fewer cases of acute leukemia.57 Patients who experience relapse and undergo an ASCT continue to have a significant increased risk for leukemia.89
Second solid tumors are significantly increased in patients treated with regional-field RT or EFRT and based on early follow-up of the HD10 and HD11 trials, likely represent a substantial risk even for those patients receiving a more limited RT field.31,32,34 Hodgson and associates evaluated second cancers among 18,862 HL survivors in 13 population-based cancer registries from 1970 through 1996.120 The 30-year cumulative incidence of cancer was reported according to age at therapy and sex (Table 105-6). Women treated in their 20s had the highest relative risk compared with an age-matched control population (24.3% vs. 4.5% at 30 years). There was no difference in the incidence of solid tumors in patients treated between 1970 and 1984 and 1985 through 1996, despite the likelihood that patients received more limited doses and fields of RT in the second era. In this study, the relative risk for second solid cancers was also increased after chemotherapy alone, perhaps associated with the increased risk for lung and bladder cancers associated with alkylating agents. A British national cohort study of 5002 women treated with RT for HL reported a standardized incidence ratio of 5 for breast cancer risk and a remarkable SIR of 47 for those treated at age 14 years (Table 105-7).121 Risk remained high more than 40 years after treatment and decreased with the use of pelvic RT or alkylating chemotherapy likely because of premature menopause. The risk for lung cancer is also substantially increased after both RT and chemotherapy for HL and occurs most commonly in smokers, but not exclusively.122 In contrast to breast cancer, an increased risk for lung cancer is seen even in the first 5 years after treatment, especially in those treated with alkylating agents. This difference might suggest a different mechanism for lung cancer induction.123 Smoking increases the risk for lung cancer more than 20-fold in HL survivors and appears multiplicative, not additive, in combination with RT.123
Table 105-6
Second Solid Cancers in 5-Year Hodgkin Lymphoma Survivors*
| Age at Diagnosis (yr) | 30-Year Cumulative Incidence of Second Cancer | |||
 -HL -HL |
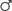 -Control -Control |
 -HL -HL |
 -Control -Control |
|
| 20 | 10.50% | 2.40% | 24.30% | 4.50% |
| 30 | 18.30% | 6.90% | 26.10% | 8.90% |
| 40 | 27.10% | 17.40% | 26.70% | 15.40% |
*Study included 18,862 5-year Hodgkin lymphoma survivors in 13 population-based registries.
From Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol 2007;25:1489–97.
Table 105-7
Cumulative Breast Cancer Risk at 20 and 40 Years After Radiation Therapy (RT)
| Age at RT (yr) | Cumulative Breast Cancer Risk After RT | |
| 20 Yr | 40 Yr | |
| 0-9 | 0 | 0 |
| 10-14 | 6.80% | 34.50% |
| 15-19 | 6.40% | 36.10% |
| 20-24 | 3.50% | 29.20% |
| 25-29 | 7.40% | 17.40% |
| 30-35 | 9.80% | 27.50% |
| MANTLE RT DOSE | ||
| 1-34 Gy | 8.80% | 22.80% |
| 36-39 Gy | 4.30% | 23.20% |
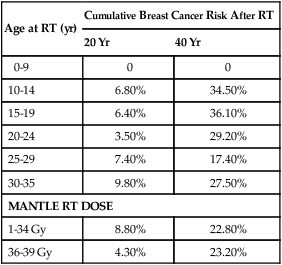
From Swerdlow AJ, Cooke R, Bates A, et al. Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin’s lymphoma in England and Wales: a national cohort study. J Clin Oncol 2012;30:2745–52.
Reducing the dose and field of RT and eliminating alkylating agents from primary therapy will likely result in fewer second cancers, but it not clear that there is a “safe and effective” dose of RT. The Childhood Cancer Survivor study showed a clear dose-response relationship between dose of RT to the breast and the odds ratio of breast cancer.124 Retrospective analysis of pediatric HL survivors treated with doses of 15 to 25.5 Gy with optional 10-Gy boosts to bulky sites, showed a standardized incidence ratio of 22.9 for second cancers with a cumulative incidence of 17% at 20 years.125 Breast cancer risk was substantially decreased with the use of mediastinal versus mantle-field RT and with IFRT compared with EFRT.28,29 The incidence of lung cancer also appears to follow a dose-response relationship.123 In contrast, the German Hodgkin Study Group HD10 trial, which compared limited chemotherapy combined with 20 or 30 Gy IFRT in favorable early-stage HL, reported a 4.6% incidence of second malignancy at a median follow-up of 7.5 years with no difference in the incidence of second malignancies or deaths from second cancers in those receiving 20 versus 30 Gy.31 Recent genome-wide association studies in HL survivors have investigated variants that may be associated with RT-induced second cancers. Allelic variants implicate PRDM1 and FGFR2 in the etiology of second cancer after RT for HL.126,127 The FGFR2 genotype was specifically studied in RT-induced breast cancer risk, whereas PRDM1 variants were studied in all second cancers. Susceptibility to RT-induced cancers may represent a polygenic trait, with cumulative risk established by co-inheritance of multiple low or intermediate penetrance “risk” alleles.119 Future tests may be available for HL patients to determine their genetic susceptibility to RT-induced cancers, possibly influencing the decision to include RT in the treatment of each patient.
Detailed outcomes for patients with second cancers are difficult to investigate. Breast cancer after RT for HL is more likely to be early stage and bilateral and to be diagnosed by screening when compared with sporadic breast cancer.128 HL survivors had a breast cancer–specific mortality similar to matched controls with sporadic breast cancer, but a marked increased risk for a contralateral breast cancer (HR 4.3) and death from any cause. Bilateral mastectomy should be considered in all patients with a diagnosis of breast cancer in the setting of RT for HL.128 Compared with patients with de novo non–small cell lung cancer, HL survivors experienced a 30% to 60% decrease in OS after a lung cancer diagnosis.129
Cardiovascular and Cerebrovascular Complications
Compared with population-based reference rates, Aleman and associates reported a twofold to sevenfold increased risk for myocardial infarction (MI), angina, congestive heart failure (CHF), and valvular disorders in HL survivors treated with mediastinal RT.130 Anthracyclines significantly added to the risk for CHF and valvular disorders, with a 25-year cumulative incidence of CHF after mediastinal RT and anthracyclines of 7.9%. Swerdlow and colleagues found a standardized mortality ratio (SMR) of 2.5 for fatal MI in HL survivors. Risks were independently increased for patients treated with RT, anthracyclines, or vincristine.131 Risk was particularly high for patients receiving ABVD chemotherapy (SMR 9.5), including those who received ABVD but no mediastinal RT (SMR 7.8).131 Hodgson and associates showed a significant increase in cardiac-related hospitalizations in patients treated with ABVD alone compared with the general population, with a 10-year risk for cardiac-related hospitalization of 5.5% compared with 2.2% expected in the general population.132 After a median follow-up of 14.7 years for 1279 patients treated with mediastinal RT, the 10- and 20-year cumulative incidence of cardiac events were 4.5% and 16%, respectively.133 The standardized incidence ratio of stroke in patients treated with neck and mediastinal RT was 2.5 with a 30-year cumulative incidence of stroke or transient ischemic attack of 7% and a median time from RT to stroke of 17.4 years.134 There was a twofold higher risk for those with cardiac disease and an increased risk in patients with hypertension, diabetes, or hypercholesterolemia, supporting recommendations discussed later for aggressive treatment of co-existing cardiovascular risk factors in this population.
Fertility
Because many patients diagnosed with HL are young, maintaining fertility is important in this patient population. Treatment with nonalkyators, such as the ABVD regimen, is considered nonsterilizing.135 ABVD induces transient azoospermia in approximately one third of male patients, but most recover within 12 months. Premature ovarian insufficiency after treatment with ABVD is very rare. In contrast, patients treated with alkylator-based regimens, including escBEACOPP, are at significant risk for infertility. As mentioned previously, use of escBEACOPP results in azoospermia and infertility in the majority of male patients and induces premature menopause in 40% of women younger than 30 years and 70% of women 30 years of age or older.54–54 Conflicting reports regarding the use of oral contraceptives and GnRH to protect the ovarian follicle pool during treatment with alkylators have been published.56 Before initiating therapy for HL, all male patients should be offered cryopreservation and banking of sperm, because this process is noninvasive and effective. Consult with a fertility expert is usually not necessary for young female patients receiving only ABVD, but those who will be treated with alkylators (including escBEACOPP), RT below the diaphragm, or SCT should be referred. Options for fertility preservation include co-treatment with a GnRH analog (protective effects remain unclear) and cryopreservation of embryos, oocytes, or ovarian tissue. Cryopreservation of embryos is the most established and reliable method, but the patient must have a partner or use donor sperm and there must be a treatment delay to allow for controlled ovarian stimulation. After any treatment of HL, patients are strongly encouraged to delay childbearing for at least 2 years, because this is the highest risk period for recurrence.
Screening Recommendations
Unfortunately, screening recommendations for second malignancies and cardiovascular disease are necessarily vague because of the small patient numbers and inability to confirm the benefit of screening. In general, all cancer survivors should follow applicable national guidelines for cancer screening such as those provided by the American Cancer Society, American Society of Clinical Oncology, National Comprehensive Care Network, and the U.S. Preventative Services Task Force.136,137 Evaluations of the risk for a second malignancy in HL survivors should also take into account family history, details of the treatment, environmental factors such as smoking, and co-morbid conditions, particularly diabetes, hypertension, and hypercholesterolemia. Annual breast screening is recommended in all women treated with mediastinal or axillary RT beginning 7 to 10 years after treatment; and in those treated between ages 10 years and 30 years, an annual breast magnetic resonance imaging should be done in conjunction with an annual mammogram.138,139 HL survivors who are current or former smokers and were treated with RT or alkylating agents should consider annual low-dose spiral chest CT starting 5 years after treatment for patients receiving alkylating agents and 10 years after treatment for patients who received supradiaphragmatic RT.137
Screening for cardiovascular disease should include a lipid panel every 3 years with statin therapy recommended for those with a low-density lipoprotein cholesterol value higher than 100, a fasting glucose level checked every 2 to 3 years, annual blood pressure monitoring, and an initial cardiac stress test 5 to 10 years after therapy with continued periodic monitoring.30,140–142 Carotid Doppler imaging is unlikely to be useful as screening for cerebrovascular disease based on the very low incidence of significant stenosis in HL survivors suffering a cerebrovascular accident.134 Patient education, counseling, and surveillance for late complications are essential aspects of life-long follow-up care for HL survivors.
Controversies, Problems, and Challenges
The two primary controversies in the treatment of HL are the use of chemotherapy alone versus combined-modality therapy in early-stage HL and the use of ABVD versus escBEACOPP in advanced-stage disease. Both of these debates hinge on the finding that an inferior PFS does not necessarily equate to an inferior OS. Although use of chemotherapy alone in early-stage disease and use of ABVD in advanced-stage disease result in higher rates of relapse, the acute toxicities and late effects challenge us to look at OS as the primary end point. The need to wait more than 20 years to see the potential life-threatening late effects of therapy requires patience and continued long-term follow-up of all patients with HL. The recent development of novel active agents for relapsed HL, such as brentuximab vedotin, provides the opportunity to incorporate new drugs into first-line regimens in an effort to improve outcomes further without the attendant toxicities associated with RT and dose-intensive approaches. Additional challenges include how best to select patients both for more intensive or novel first-line approaches as well as those who would benefit from less therapy. Although the interim PET/CT scan has proved useful, the specificity of a positive interim scan is inconsistent, and better methods of interpretation are needed. The approach to relapsed HL remains variable with regard to the pretransplant salvage regimen and the role of RIC-allo at first or subsequent relapse. Although outcomes are quite favorable for most patients with HL, we should not give up our “pursuit of perfection” in this disease.143
Future Possibilities and Clinical Trials
The rarity of Hodgkin Reed-Sternberg cells in the diagnostic tissue has slowed progress in studying the molecular characteristics and the genome of HL cells. Gene expression profiling has led to the identification of distinct prognostic groups in non-Hodgkin lymphoma, and efforts are ongoing to develop a similar model in HL. Preliminary data found the overexpression of a macrophage signature in classic HL was associated with the failure of primary therapy.144 This discovery was made more generalizable when immunohistochemical studies showed an increased number of CD68+ cells, a marker of benign macrophages, also correlated strongly with a worse outcome. Unfortunately, efforts to reproduce this result have been inconsistent, perhaps owing to lack of experience with performing and interpreting this stain.145 Efforts are underway to identify other biological markers, both in the serum and tumor, that may enhance or replace the current prognostic models. Ultimately, a combination of biological markers, clinical characteristics, and functional imaging may provide the best prognostic model.