Figure 57-1 Actions of cytokine/growth factors binding to their receptors on the cell surface Cytokines and growth factors are the messengers that mediate intercellular communication. The regulation of cellular and nuclear functions by cytokines and growth factors is initiated through the activation of cell surface receptors. All receptors have a ligand-binding domain that ensures ligand specificity and an effector domain that initiates the generation of the biologic response on ligand binding. The activated receptor may then interact with other cellular components to complete the signal transduction process. Briefly, cytokine binding to receptor subunits induces homo- or heterodimerization resulting in the activation of Jaks that are bound to the receptor chains. The Jaks in turn phosphorylate tyrosine-based docking sites on the receptor. STATs bind via their SH2 domains. The STATs are then phosphorylated, form homo- or heterodimers, and translocate to the nucleus, where they bind target sequences, thereby regulating gene expression.
Table 57-1
Receptors, Natural Antagonists, and Chromosomal Locations of Growth Factors and Cytokines
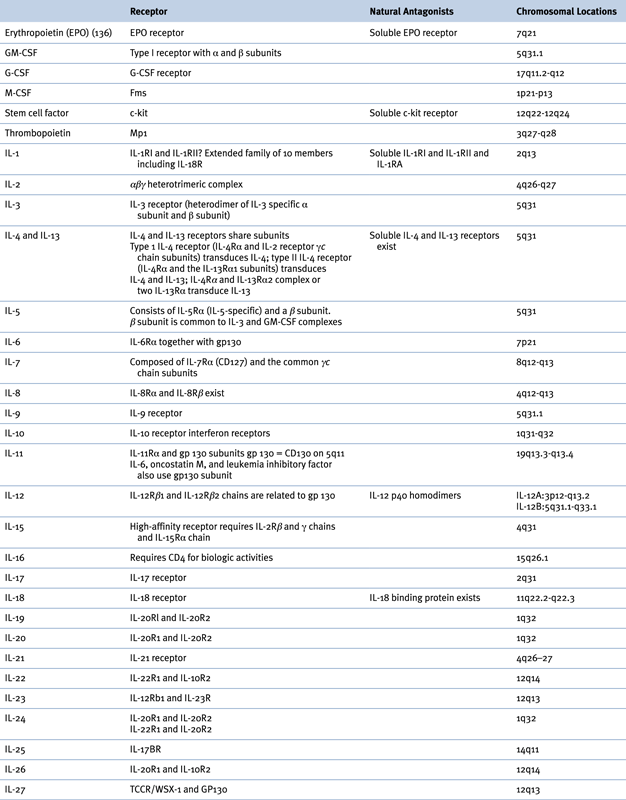
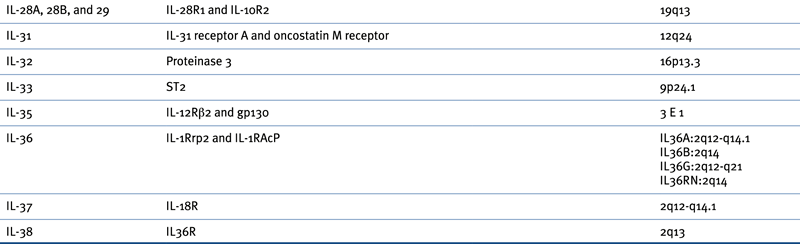
G-CSF, Granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; M-CSF, macrophage colony-stimulating factor.
Erythropoietin-stimulating agents (ESAs) have been shown in clinical trials to decrease the transfusion requirements and increase the hemoglobin in patients with chemotherapy-induced anemia.
5–7 However, these trials have not shown that ESAs prolong survival or improve quality of life in these patients.
8 Moreover, ESAs have been associated with a number of unwanted outcomes in cancer patients, including an increased risk of stroke and venous thromboembolism, worse cancer outcomes, and increased mortality. With these findings of worse outcomes in patients treated with ESAs, the U.S. Food and Drug Administration (FDA) issued warnings against the use of ESA. The 2010 ASH/ASCO (American Society of Hematology/American Society of Clinical Oncology) Guidelines recommend a thorough workup for other causes of anemia before initiation of ESAs as well as discussion of the potential benefits and harms of ESAs.
7
Table 57-2
Growth Factors and Cytokines in the Clinic ∗
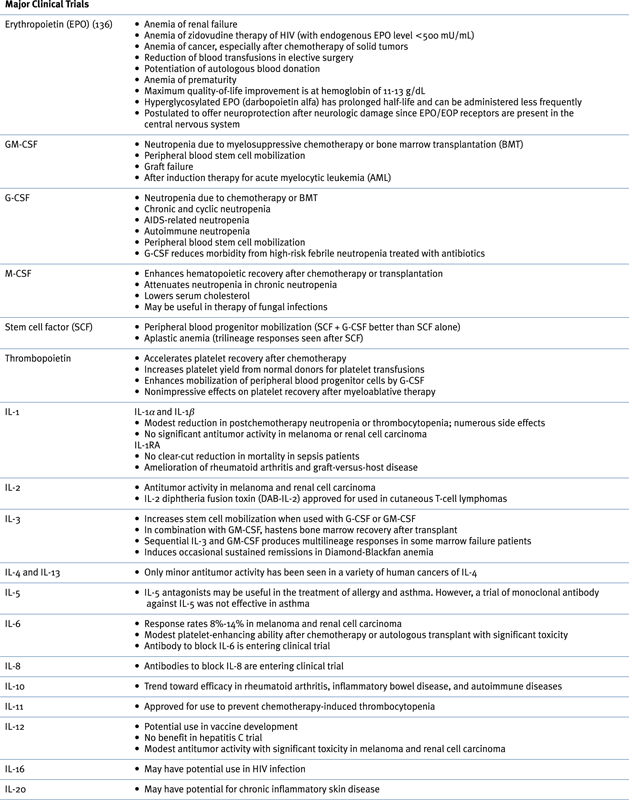
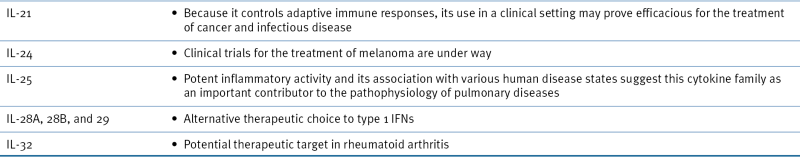
AIDS, Acquired immunodeficiency syndrome; EOP, erythropoietin; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; M-CSF, macrophage colony-stimulating factor; HIV, human immunodeficiency virus; IFN, interferon; IL, interleukin.
∗ Many of the listed applications refer to clinical trials and are not approved uses.