Gestational Trophoblastic Disease
Donald Peter Goldstein and Ross Stuart Berkowitz
• The incidence of complete hydatidiform mole is approximately 1 in 1200 pregnancies in the United States.
• The incidence of partial hydatidiform mole is approximately 1 in 650 pregnancies.
• Repeat moles occur in approximately 1 in 125 pregnancies, and third moles occur in approximately 1 in 5 pregnancies.
• Complete hydatidiform mole is usually due to an androgenetic diploid conception, in which a haploid sperm fertilizes an egg that lacks female chromosomes.
• A partial hydatidiform mole develops when dispermy occurs, and the resulting conceptus is triploid.
• Earlier diagnosis of complete hydatidiform mole due to improved ultrasound and human chorionic gonadotropin (hCG) assays has made the pathological diagnosis of complete hydatidiform mole more difficult because of its resemblance to partial hydatidiform mole and nonmolar abortions.
• Hydatidiform mole is characterized by hydropic villi with trophoblastic hyperplasia.
• Invasive mole is characterized by invasion of myometrium by hydropic villi surrounded by hyperplastic trophoblasts.
• Choriocarcinoma is characterized by sheets of neoplastic cytotrophoblasts and syncytiotrophoblasts invading tissue and is associated with necrosis and hemorrhage. Hematogenous spread occurs early.
• Placental site and epithelioid trophoblastic tumors are rare forms of choriocarcinoma made up of mononuclear cells from intermediate trophoblast at the implantation site that invade the myometrium. Both placental site and epithelioid trophoblastic tumors metastasize late and are relatively resistant to chemotherapy.
• Hydatidiform mole commonly presents in the first trimester with vaginal bleeding.
• Complete hydatidiform mole is usually diagnosed by ultrasound because of the abnormal appearance of the placenta and the absence of a fetus.
• Partial hydatidiform mole can be difficult to diagnose by ultrasound and is usually confirmed pathologically.
• Persistent postmolar gestational trophoblastic neoplasia is usually nonmetastatic and is characterized by a rising hCG level and persistent bleeding due to residual molar tissue.
• Metastatic postmolar gestational trophoblastic neoplasia (GTN) usually involves the lungs and, rarely, the brain, liver, and other distant sites.
• The diagnosis of gestational trophoblastic neoplasia after a miscarriage or term pregnancy is frequently delayed and commonly presents with significant disease.
• The 2002 International Federation of Gynecologists and Obstetricians Staging System combines an anatomic description of the disease (i.e., stages I, II, III, and IV) with a prognostic scoring system.
• Single-agent therapy is usually curative in patients with stage I, II, and III disease who have low prognostic scores (<7). There is evidence that patients with low-risk GTN (scores of 5 and 6) but a large tumor burden (hCG >100,000 mIU/mL) commonly require multiagent chemotherapy to achieve cure.
• Patients with stage II, III, and IV disease who have high-risk scores (≥7) require combination chemotherapy for optimal outcome.
• Survival rates of 100% in patients with stage I, II, and III disease and 80% in patients with stage IV disease should be achieved.
• Response to therapy and remission are determined by hCG levels, which should be tested weekly during chemotherapy.
• Patients with high-risk disease should be treated with three or four consolidation courses after the hCG titer normalizes.
• Toxicity from the chemotherapeutic agents is the most common early complication.
• Other complications relate to the disease and are usually due to internal bleeding.
• Most women are cured, are able to achieve subsequent pregnancy, and are able to return to normal activity soon after attaining a normal hCG titer.
Introduction
Terminology
• It elaborates a reliable and specific tumor marker, human chorionic gonadotropin (hCG).
• It is exquisitely sensitive to chemotherapy.
• It has a unique immunobiological relationship with its host, because it arises from fetal rather than maternal tissue.
Several histologically distinct entities make up GTD:
• Hydatidiform mole: partial (PHM) and complete (CHM)
• Persistent or invasive gestational trophoblastic neoplasia (GTN)
Relevant Historical Issues
An important milestone in the history of GTD occurred in 1928, when Ascheim and Zondek first described a reliable pregnancy test, which became the basis for the early measurements of hCG. In 1963, MacVicor and Donald first used ultrasound in the diagnosis of molar pregnancy. In 1977, Kajii and Ohama1 elucidated the androgenetic origin of hydatidiform moles. Finally, in 1978, Szulman and Surti2 described the genetic basis for PHM as a triploid gestation.
Prior to the introduction of chemotherapy, survival with GTN remained limited and precarious because the only form of treatment consisted of hysterectomy or local excision of metastatic sites where possible. In 1959, Brewer3 reviewed survival with CCA at the Albert Mathieu Chorioepithelioma Registry at Northwestern University. Only 6 of 103 patients with metastatic CCA were free of disease at 5 years after their diagnosis. Brewer’s group also analyzed the 5-year survival rates in patients with nonmetastatic CCA treated by hysterectomy. Only 29 of 70 patients (41%) with presumably localized tumor survived, despite prompt hysterectomy. Metastases developed in the remaining patients after the operative procedure and they died of widely disseminated disease.
A new era in the management of GTN was inaugurated in 1956, when Li and colleagues4 reported the complete regression of metastatic CCA in three women treated with methotrexate. In 1961, Hertz and coworkers5 reported the initial 5-year experience with chemotherapy for metastatic GTN at the National Cancer Institute. Complete remission was achieved with methotrexate in 28 of 63 (47%) patients with metastatic disease. After obtaining dramatic results with chemotherapy in disseminated disease, Hertz and associates6 successfully used chemotherapy for patients with nonmetastatic tumors. It soon became apparent that certain patients with metastatic GTN became resistant to single-agent chemotherapy and experienced a high mortality rate. In 1965, Ross and colleagues7 reported that delay in diagnosis, high hCG levels, and liver and/or brain metastases predisposed patients to a high risk of drug resistance. The subsequent introduction of multiagent chemotherapy for these patients has resulted in substantial improvement in survival.
Incidence and Epidemiology
The incidence and etiologic risk factors that contribute to the development of GTD have been difficult to characterize because of problems in accumulating reliable data, bias, and interpretation and differing methods of expressing incidence in terms of hospital-based versus population-based data. Despite these problems, there are sufficient data to indicate that there are wide regional variations in the incidence of CHM. Estimates from North America, Australia, New Zealand and Europe have shown the incidence of CHM to range from 0.57 to 1.1 per 1000 pregnancies, whereas studies from Southeast Asia and Japan report an incidence approaching 2.0 per 1000 pregnancies.8–12 The incidence of hydatidiform mole in Taiwan is reported to be one in 125 pregnancies.9 In Ireland, the incidence of CHM and PHM has been determined to be 1 in 1945 and 1 in 695 pregnancies, respectively.13 In the United States, molar pregnancy is encountered in 1 in 600 therapeutic abortions and in 1 in 1000 pregnancies. There are data that show an increased incidence of CHM among American Indians, Eskimos, Hispanics, and African Americans.14,15 There is no conclusive evidence that genetic traits, cultural factors, or differences in reporting account for this increase. The etiologic factors that have been linked to the development of CHM are advanced maternal age (>40 years) and prior molar pregnancy.16,17 Familial clusters of biparental CHM have been associated with the NLRP7 gene mutation on chromosome 19q.18 In addition, well-documented nutritional studies have shown an inverse relationship between beta-carotene and animal dietary fat intake and the incidence of CHM.19,20 In this regard, it is of interest that the documented decrease in the incidence of CHM in South Korea has been associated with a gradual Westernization of the Korean diet.21
The epidemiological features of CHM and PHM differ markedly. Parazzini and coworkers16 reported that the risk for partial mole was not associated with maternal age. Additionally, the risk for partial mole has been reported to be associated with the use of oral contraceptives and a history of irregular menstruation but not with dietary factors.22 Therefore, the risk of partial mole appears to be associated with reproductive history rather than dietary factors.23
The overall incidence of invasive mole (IM) has been estimated at 1 in 15,000 pregnancies. Approximately 15% to 29% of hydatidiform moles will result in invasive mole.9 Determining the incidence rate of CCA is even more problematic because of the rarity of this condition and the difficulty in clinically distinguishing postmolar CCA from metastatic mole. In Europe and North America, CCA affects approximately 1 per 40,000 pregnancies, whereas in Southeast Asia and Japan the rates of CCA are 9.2 and 3.3 per 40,000 pregnancies, respectively.24 The gradual decline in the incidence of both CHM and CCA in developing countries over the past 40 years presumably is due in part to improved socioeconomic conditions, earlier completion of childbearing, and improved nutrition.
Risk factors of CCA include prior CHM, ethnicity, and advanced maternal age. CCA is 1000 times more likely to occur after CHM than after another type of pregnancy. The risk is also increased in women of Asian, American Indian, and African descent.24
Etiology and Pathogenesis
PHM are characterized by a triploid karyotype (69 chromosomes) derived from two paternal and one maternal haploid sets of chromosomes.27–27 Most have a 69,XXX or 69,XXY genotype derived from a haploid ovum with dispermic fertilization. The fetus present in a PHM generally exhibits the stigmata of triploidy, including growth retardation and multiple congenital anomalies such as syndactyly, hydrocephaly, omphalocele, and hare lip.
CHM, in contrast, usually have a 46,XX genotype totally derived from duplication of the haploid genome of one sperm. A small percentage of CHM have 46,XY karyotype, consistent with dispermic fertilization. It appears that molar disease, both partial and complete, is associated with excess male genetic composition due to an abnormality of the egg, which is either devoid of maternal chromosomal material or allows for dispermy.28
Several growth factors and oncogenes have been studied in molar tissues and CCA. Increased expression of p53 and c-fms has been observed in CHM, and increased ras and c-myc RNAs have been measured in CCA.31–31 Fulop and associates32,33 have investigated the expression of various growth factors and oncogenes in normal placenta, complete and partial mole, and CCA. CHM and CCA were characterized by overexpression of c-myc, c-erbB-2, and bcl-2, and these oncoproteins could be important in the pathogenesis of GTN. Expression of c-fms protein did not differ between normal placenta and GTN. CHM and CCA were also characterized by increased expression of p53, p21, Rb, and MdM2. The p53 gene was studied to detect any mutation in 22 complete moles and 11 CCAs that had increased expression of p53. Because only one nonsense mutation in p53 was detected by polymerase chain reaction analysis, it is likely that the overexpressed p53 protein was the wild type. Although studies have identified increased expression of several growth factors in GTN, the precise molecular pathogenesis has not been determined. It was observed that the level of expression of epidermal growth factor receptor (EGFR) in CCA and the syncytiotrophoblast and cytotrophoblast of complete mole was significantly greater than the expression of EGFR in syncytiotrophoblast and cytotrophoblast of placenta and partial mole.29 This observation was consistent in both immunohistochemical and in situ hybridization studies. In complete mole, strong expression of EGFR and c-erbB-3 in the extravillous trophoblasts was significantly associated with the development of postmolar tumor. The EGFR-related family of oncogenes might be important in the pathogenesis of GTN.
Extracellular proteinases such as matrix metalloproteinases (MMPs) are thought to be important in modulating both cell-matrix interactions and the degradation of the basement membrane necessary for invasion and metastases. CCA exhibits significantly stronger expression of MMP-1 and MMP-2 and decreased expression of tissue inhibitor of MMP-1 (TIMP-1) than are seen in the syncytiotrophoblast of complete and partial mole and normal placenta.34 The increased expression of MMP-1 and MMP-2 and decreased expression of TIMP-1 in CCA could contribute to the invasiveness of CCA cells.
Certain genes are expressed normally on either the maternal or paternal allele, and this occurrence is described as parental imprinting. Modification of parental imprinting has been associated with tumor formation; both complete moles and CCA have relaxation of parental imprinting.35 Relaxation of parental imprinting could be important in the pathogenesis of GTN.
Pathology
Hydatidiform moles can be categorized as either complete or partial on the basis of gross morphology, histopathology, and karyotype (Table 90-1).
Table 90-1
Features of Complete and Partial Hydatidiform Moles
| Feature | Complete Mole | Partial Mole |
| Fetal or embryonic tissue | Absent | Present |
| Hydropic villi | Diffuse | Focal |
| Trophoblast hyperplasia | Diffuse | Focal |
| Scalloping of chorionic villi | Absent | Present |
| Trophoblastic stromal inclusions | Absent | Present |
| Karyotype | 46,XX; 46,XY | 69,XXY; 69,XXX |
Complete Hydatidiform Mole
CHM is characterized by the following pathological features:
• Vesicular swelling of placental villi.
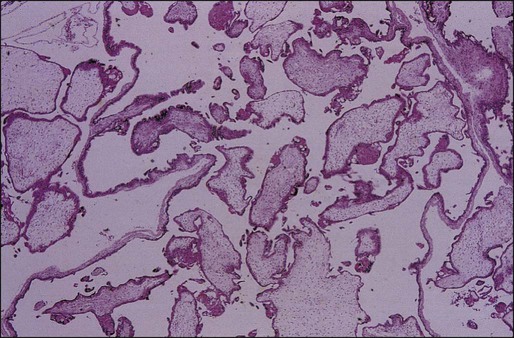
• Proliferation of trophoblastic cells (both cytotrophoblasts and syncytiotrophoblasts) with varying degrees of hyperplasia and dysplasia (Figure 90-1).
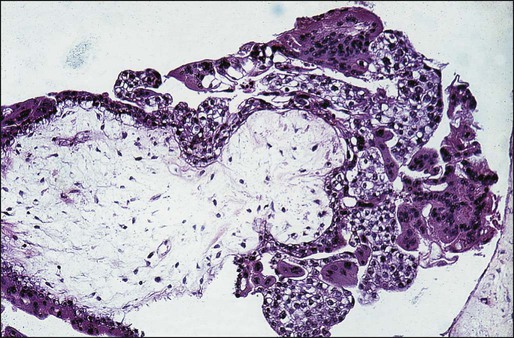
• Fluid-filled and distended chorionic villi with scant or absent blood vessels.
However, the pathological features of CHM have changed significantly over the past two decades owing to earlier diagnosis.36 Whereas cavitation and circumferential trophoblastic proliferation were present in three quarters of complete moles in the past, these findings are now present in fewer than half the cases. Mosher and coworkers37 compared pathological findings of 23 current complete moles (1994 to 1997; mean gestational age: 8.5 weeks) with 20 past complete moles (1969 to 1975; mean gestational age: 17 weeks). Histologically, complete moles that are now encountered have smaller mean maximal villous diameter (5.7 vs. 8.2 mm), less circumferential trophoblastic hyperplasia (39% vs. 75%), more primitive villous stroma (70% vs. 10%), and less global necrosis (22% vs. 54%). Keep and coworkers38 observed that early complete moles were characterized by focal trophoblastic hyperplasia, minimal villous cavitation, and hypercellular primitive stroma. Complete moles are now often characterized by subtle morphologic alterations that could result in their misclassification as partial moles or nonmolar hydropic abortions. DNA ploidy studies or karyotyping are useful adjuncts in these circumstances.26,27 Immunohistochemical tests for maternally expressed genes can also differentiate complete mole from partial mole and hydropic abortion.39,40
Partial Hydatidiform Mole
PHM is characterized by the following pathological features:
Invasive Mole
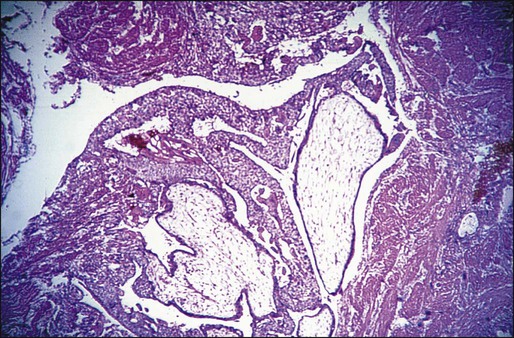
IM is a tumor arising from a molar pregnancy that invades the myometrium by direct extension or by venous channels. Metastases to distant sites occur in about 15% of cases, most commonly to the lungs and vagina.28 The tumor is characterized by swollen placental villi and accompanying hyperplastic trophoblastic tissue, which is usually dysplastic when located in sites outside the uterine cavity (Figure 90-3).
Choriocarcinoma
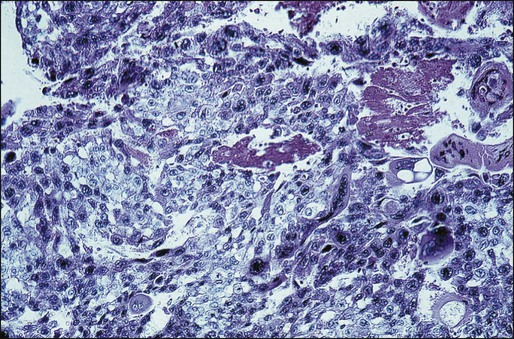
CCA is a highly malignant tumor characterized by abnormal trophoblastic cell hyperplasia and anaplasia, absence of chorionic villi, hemorrhage, and necrosis that invades the uterine wall directly or metastasizes hematogenously to distant sites, most commonly the lungs, vagina, brain, liver, spleen, kidneys, and intestines (Figure 90-4).
Placental Site and Epithelioid Trophoblastic Tumors
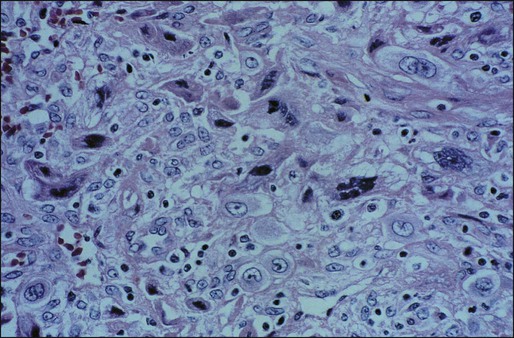
PSTT and ETT are extremely rare, monocellular tumors that arise from the intermediate trophoblast of the implantation site. In the case of PSTT, tumor cells infiltrate the myometrium and grow between smooth muscle cells with vascular invasion (Figure 90-5). ETT characteristically tends to form nodular masses that invade the myometrium. Hemorrhage and necrosis are less evident. Placental villi are absent in both of these tumors. With the use of immunoperoxidase stains, human placental lactogen can be identified prominently in PSTT, whereas hCG is present only sparsely. In both these tumors, serum hCG levels are low, but relatively high levels of free beta subunit of hCG are detectable in patients with PSTT compared with other forms of GTN.41 There appears to be a direct correlation between the mitotic activity of PSTT and outcome.42
Immunobiology
The remarkable curability of GTN might be attributable partly to a host immunologic response to paternal antigens expressed on trophoblastic cells.43 The prognosis of patients with gestational CCA has been related to the intensity of lymphocytic and monocytic infiltration at the tumor-host interface.44 Because the lymphocytes and macrophages that infiltrate gestational CCA are probably exposed to paternal antigens and oncoproteins, the immune cells could become activated. Immunologically active cells might promote the regression of GTN through their release of cytokines. Cytokines have been reported to inhibit the proliferation of CCA cells in vitro and to increase the human leukocyte antigen (HLA) expression of CCA cells in vitro, thereby increasing immunogenicity.45
It has been theorized that the development and progression of GTN could be favored by histocompatibility between the patient and her partner. If the patient and her partner are histocompatible, the trophoblastic tumor that bears paternal antigens might not be immunogenic in the maternal host. The intensity of the host’s immunologic response might depend on the immunogenicity of the trophoblastic tumor. On the other hand, histocompatibility between the patient and her partner does not appear to be a prerequisite for the development of persistent GTN.46 HLA systems could, however, influence the clinical course of rapidly progressive and fatal GTN. Tomoda and colleagues47 reported that drug-resistant CCA was associated with increased histocompatibility between the patient and her partner. Similarly, Morgenson and coworkers observed that histocompatibility between patients and partners was associated with a greater risk of metastatic disease.48 Because all chromosomes in a CHM are paternal in origin, a CHM is a complete allograft and could stimulate a vigorous immune response by the maternal host. There is evidence for both a cellular and a humoral response to CHM. When compared with normal placentas, molar implantation sites have fivefold increased infiltration by helper T cells.49 Circulating immune complexes have also been measured in patients with CHM and have been noted to increase as the patient entered remission.50
Circulating immune complexes in patients with CHM have been demonstrated to contain paternal HLA antigens.51 The maternal host with a CHM is therefore sensitized to paternal HLA antigens. The distribution of HLA antigens in molar chorionic villi has been determined by immunofluorescent assays.52 HLA A, B, and C antigens were detected on the stromal cells of molar chorionic villi but not on the villous trophoblast; however, the molar villous fluid that bathes the stromal cells does not contain soluble HLA antigen.43 The maternal host could therefore be sensitized to paternal HLA antigen when the villous trophoblastic layer is disrupted and HLA-positive villous stromal cells are released into the circulation.
Clinical Presentation
Complete Molar Pregnancy
The clinical presentation of CHM has changed dramatically over the past two decades in the wake of the widespread use of ultrasound and improved methods for hCG measurement. Prior to 1980 CHM was usually diagnosed in the second trimester, whereas the diagnosis is now usually made in the first trimester, before the classic clinical signs and symptoms appear. Table 90-2 summarizes the signs and symptoms of classic CHM and PHM. When patients with CHM present with these classic signs and symptoms, they are at higher risk of developing GTN. Soto-Wright and associates36 compared the clinical presentation and outcome of patients with CHM at the New England Trophoblastic Disease Center (NETDC) between 1988 and 1993 with those of patients between 1965 and 1975 and noted that despite the change in presentation, there was no change in the incidence of GTN.
Table 90-2
Presenting Signs and Symptoms of Classic Complete and Partial Hydatidiform Moles
| Sign or Symptom | Complete Mole (%) (N = 306) | Partial Mole (%) (N = 81) |
| Vaginal bleeding | 97 | 73 |
| Excessive uterine size | 51 | 4 |
| Theca lutein cysts >6 cm | 50 | 0 |
| Preeclampsia | 27 | 3 |
| Hyperemesis | 26 | 0 |
| Hyperthyroidism | 7 | 0 |
| Trophoblastic emboli | 2 | 0 |
Partial Molar Pregnancy
Patients with PHM typically present with symptoms that are indistinguishable from those of a threatened or spontaneous miscarriage, rather than with the classic symptoms of CHM, unless diagnosis is delayed until the second trimester. The diagnosis of PHM is usually suggested by ultrasonography and confirmed by pathological review of curettage material. hCG levels in patients with PHM are usually not as elevated as in patients with CHM. When PHM progresses into the second trimester, the ultrasound diagnosis becomes more accurate because of obvious fetal malformations associated with triploidy and the extent of vesicular change of the placenta. Patients with PHM whose diagnosis is delayed until the second trimester will, in many instances, recapitulate the classical signs and symptoms associated with CHM with regard to the incidence of preeclampsia, hyperthyroidism, trophoblastic embolization, and other complications.55–55 Although patients with CHM are now diagnosed earlier, patients with PHM have not been diagnosed substantially earlier and their clinical symptoms have not changed.22
Gestational Trophoblastic Neoplasia
• A plateau in the level of hCG over more than 3 consecutive weeks
• 10% or greater rise in hCG for 3 or more values over at least 2 weeks
Nonmetastatic Gestational Trophoblastic Neoplasia
Nonmetastatic or locally invasive GTN (NMGTN) develops in about 15% of patients after evacuation of CHM, 1% to 6% after PHM, and infrequently following other pregnancies.22,28,56 Patients with NMGTN present with irregular vaginal bleeding, ovarian theca lutein cysts if hCG levels are markedly elevated, and uterine subinvolution or asymmetrical enlargement due to the presence of tumor. Invasive tumor can erode through the uterine vessels causing vaginal bleeding, or perforate through the myometrium causing intraperitoneal hemorrhage. The presence of bulky necrotic tumor, characteristic of CCA, can serve as a nidus for sepsis, particularly Clostridium welchii. The presence of deep myometrial invasion can be confirmed by ultrasound or imaging studies such as magnetic resonance imaging (MRI).
Metastatic Gestational Trophoblastic Neoplasia
Metastatic GTN (MGTN) occurs in approximately 5% of patients after molar evacuation and infrequently after other gestations. Although IM infrequently metastasizes to distant sites, most MGTN is due to CCA, which characteristically disseminates widely hematogenously. Because trophoblastic tumors are highly vascular, metastatic lesions often present with signs and symptoms of spontaneous bleeding. The most common metastatic sites are the lung (80%), vagina (30%), brain (10%), and liver (10%).9 As a general rule, cerebral and hepatic metastases are uncommon unless there is concurrent involvement of the lungs and/or vagina.
Pulmonary Metastasis
1. Solitary or multiple discrete rounded densities
2. “Snowstorm” or alveolar pattern
3. Embolic pattern resulting from pulmonary artery occlusion
Patients can develop pulmonary hypertension in the absence of substantial parenchymal involvement because of vascular obstruction with tumor. Because the respiratory symptoms and radiographic findings can be striking, patients may be thought to have a primary pulmonary disease, thus delaying the diagnosis. Therefore, it is important to obtain an hCG level in any woman of reproductive age who presents with evidence of metastatic disease without a known primary. Gynecologic symptoms may be minimal or absent in patients with extensive pulmonary involvement. In fact, the reproductive organs are frequently free of disease in patients with widespread metastases. Early respiratory failure requiring mechanical ventilation can develop in patients with extensive pulmonary involvement and can cause death before effective chemotherapy can be started.59–59 Risk factors for early respiratory failure within 1 month of presentation include greater than 50% lung opacification, dyspnea, anemia, cyanosis, and pulmonary hypertension. With chemotherapy, patients could develop bleeding into metastatic sites and potentially worsen pulmonary symptoms and radiologic findings. Kelly and associates57 observed that reducing the initial dose of chemotherapy did not protect against early respiratory failure and recommended administering intensive chemotherapy at the outset.
Vaginal Metastases
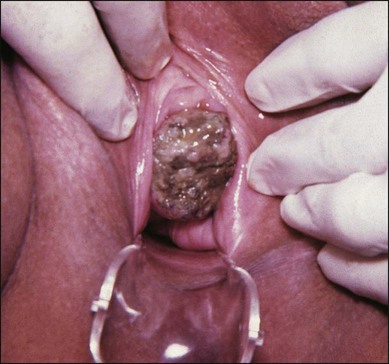
Thirty percent of patients with MGTN have vaginal involvement. Vaginal metastases are most commonly located in the suburethral area or fornices and are due to venous embolization of either IM or CCA. They usually present with either a purulent discharge or irregular bleeding (Figure 90-6). Surgical excision of a vaginal metastasis should be avoided (except in unusual circumstances) because the hemorrhage that results is frequently difficult to control. After chemotherapy is instituted, and tumor shrinkage has occurred, the vaginal lesion can be excised with less risk, if necessary. However, the optimal treatment for bleeding from a vaginal metastasis is arterial embolization.60
Brain Metastases
Cerebral lesions also occur in approximately 10% of patients with MGTN. Similar to hepatic metastases, cerebral involvement is usually seen in postterm patients with advanced disease due to delayed diagnosis. Involvement of the central nervous system (CNS) is usually associated with headache, vomiting, seizures, and focal neurologic signs such as slurred speech, hemiparesis, visual disturbances, or signs of a spinal cord lesion. Neurologic symptoms usually result from increased intracranial pressure or bleeding.64–64
Bagshawe and Harland65 reported that the plasma-to-CSF hCG ratio was <60 in patients with CNS disease. However, a single plasma-to-CSF hCG ratio can be misleading because rapid changes in serum hCG levels due to the effects of treatment on the tumor output of hCG might not be reflected promptly in the CSF.66 Occult asymptomatic CNS metastases are now being detected because of improved imaging techniques (particularly MRI), which allows for earlier therapeutic intervention before neurologic sequelae develop.
Other Metastatic Sites
Gastrointestinal, renal, and splenic metastases usually occur in patients with advanced disease in which the diagnosis has been delayed. Abdominal computed tomography (CT) scanning is the best imaging technique for detecting these metastatic sites, although the use of whole-body 18F FDG-PET scanning is being used more commonly.67
Placental Site and Epithelioid Trophoblastic Tumors
PSTT and ETT are rare tumors that are derived from intermediate implantation site cytotrophoblasts. They can develop from any antecedent pregnancy, but most commonly arise from a term pregnancy or miscarriage and generally present months to years after the antecedent pregnancy with irregular vaginal bleeding or amenorrhea, enlarged uterus, and, rarely, nephrotic syndrome. The serum hCG level is relatively low in relation to tumor volume in comparison to other types of GTN. More than 30% of patients already have metastases at diagnosis.68,69
Laboratory and Imaging Studies
hCG Measurement
A reliable assay for hCG is central to the management of patients with trophoblastic disease. Most kits now used in commercial and hospital-based laboratories accurately measure all portions of the hCG molecule.70,71 Physicians treating patients with GTN, however, must recognize the limitations of the assay they are using and base clinical decisions on the clinical, morphologic, and radiologic findings as well as on hormonal results.
Patients with CHM commonly have markedly elevated preevacuation hCG levels. Genest and coworkers72 noted that 46% of 153 patients with CHM who were managed at the NETDC between 1980 and 1990 had preevacuation hCG levels above 100,000 mIU/mL, whereas the preevacuation hCG levels in patients with PHM tend to be lower. Review of the data from the NETDC noted that only 2 of 30 patients with PHM presented with levels greater than 100,000 mIU/mL.73
False-Positive hCG Tests
False-positive hCG test results can occur because of a substance in the blood that interferes with the hCG assay. Although this is a rare occurrence (estimated at 1 in 10,000 to 1 in 100,000 tests), false-positive tests can be confusing to clinicians when they are attempting to diagnose disorders of pregnancy, including GTN. The misinterpretation of a false-positive test has led to inappropriate surgery and chemotherapy based only on persistently elevated serum hCG levels. False-positive hCG tests should be suspected if the clinical picture and the laboratory results are discordant, if there is no identifiable antecedent pregnancy, or if patients under treatment with low levels of hCG do not respond to treatment appropriately. Heterophile antibodies are thought to be responsible for this problem by interfering in the immunoassay systems used. These patients are also at risk of other false-positive serum test results. To avoid the pitfalls of a false-positive hCG test, it is important for the clinician to remember that the patient’s clinical presentation should correlate with the laboratory results. If there is a discrepancy, then a repeat hCG assay using a different immunoassay and a sensitive urine hCG test should be performed.71 Most commercially available kits have now been corrected for this problem.
Quiescent Gestational Trophoblastic Neoplasia
Quiescent GTN is another rare entity that is associated with persistent low levels of hCG, which can lead to inappropriate treatment. This entity is thought to be due to the presence of highly differentiated, noninvasive syncytiotrophoblastic cells. Characteristically, foci of disease are not otherwise demonstrable clinically, nor do hCG levels fall with therapy, presumably because the growth cycle of these cells is long and comparable to that of normal cells. Close observation of these patients is indicated because 6% to 10% will eventually develop overt GTN, requiring therapy. The hCG of patients with quiescent GTN is not hyperglycosylated, which is useful in correctly identifying these patients.74,75
Ultrasound
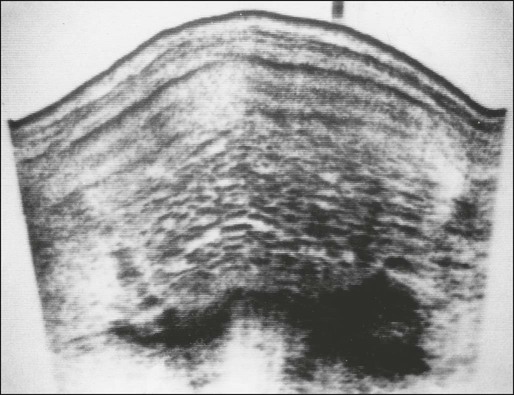
The ultrasound appearance of CHM does not vary considerably between the first and second trimesters. At either stage, complex, echogenic masses with multiple small cystic spaces are visible within the uterus, and no fetus is identifiable (Figure 90-7). Two sonographic features have been described that are characteristic of PHM: focal cystic changes in the placenta and a ratio of the transverse-to-anteroposterior dimension of the gestational sac greater than 1.5.76 Changes in the shape of the gestational sac could be part of the embryopathy of triploidy. On rare occasions, particularly when PHM has progressed into the late first or early second trimester, the sonogram will show the presence of a fetus with multiple congenital abnormalities, focal hydropic changes in the placenta, oligohydramnios, and abnormal placental Doppler flow pattern. These changes might not be visible in the first trimester.
Computed Tomography and Magnetic Resonance Imaging
In patients with NMGTN who are being considered for hysterectomy as primary treatment, a CT scan of the chest rather than a plain radiographic film should be performed, because finding occult metastatic disease would mandate that adjunctive chemotherapy be included in the treatment program.l A full metastatic workup is usually not indicated unless lung involvement is observed because it is unusual for metastatic sites other than the vagina or pelvis to be involved in the absence of pulmonary metastases. When a chest CT is positive, metastatic workup should consist of an abdominal CT scan to assist in the detection of liver, renal, splenic, and/or gastrointestinal involvement, as well as a brain MRI. An initial positive chest CT in a patient with NMGTN should not affect clinical management, because most of these patients are low-risk and do well with single-agent protocols.77
Positron Emission Tomography
Positron emission tomography with [18F]fluorodeoxyglucose (FDG-PET) scanning can aid in identifying residual disease sites in women who relapse from previously treated GTN. Dhillon and colleagues have reported that PET scanning appropriately identified the presence of pulmonary lesions that were successfully resected, when they were equivocal on CT scanning.67 It is important to realize that a false-positive PET scan can occur in nonmalignant conditions such as sarcoid or any benign metabolically active site. Therefore, careful evaluation in combination with other imaging modalities is required to reduce the risk of false-positive results.78
Staging and Prognostic Scoring System
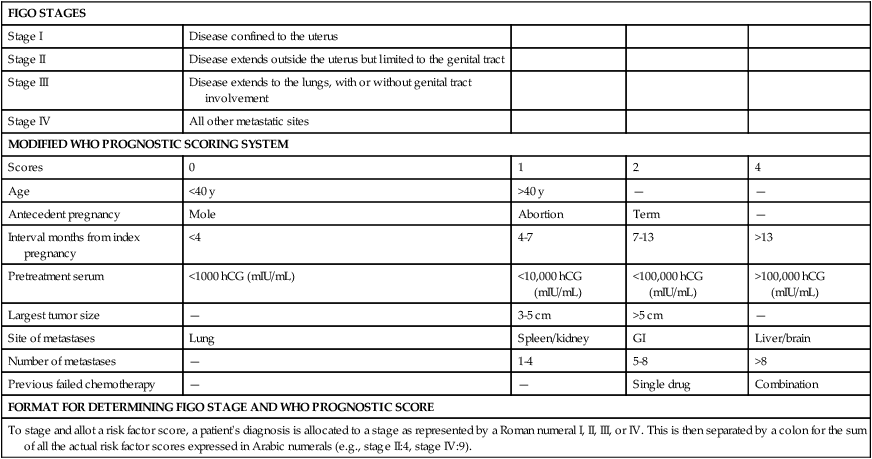
Table 90-3 summarizes the anatomic staging system and prognostic scoring system adopted by the FIGO Cancer Committee in 2002 that is now widely used both as a basis for comparison of treatment outcomes among treatment centers and as a guide for clinicians in the selection of optimal treatment regimens. A patient’s stage and total risk score are described by Roman and Arabic numerals, respectively, separated by a colon (e.g., III:8). The Prognostic Scoring System has proved to be particularly useful in predicting which patients are likely to become drug resistant and require modifications in their treatment regimens to optimize outcome.
Table 90-3
Combined FIGO Staging and WHO Prognostic Scoring System for Gestational Trophoblastic Neoplasm
| FIGO STAGES | ||||
| Stage I | Disease confined to the uterus | |||
| Stage II | Disease extends outside the uterus but limited to the genital tract | |||
| Stage III | Disease extends to the lungs, with or without genital tract involvement | |||
| Stage IV | All other metastatic sites | |||
| MODIFIED WHO PROGNOSTIC SCORING SYSTEM | ||||
| Scores | 0 | 1 | 2 | 4 |
| Age | <40 y | >40 y | — | — |
| Antecedent pregnancy | Mole | Abortion | Term | — |
| Interval months from index pregnancy | <4 | 4-7 | 7-13 | >13 |
| Pretreatment serum | <1000 hCG (mIU/mL) | <10,000 hCG (mIU/mL) | <100,000 hCG (mIU/mL) | >100,000 hCG (mIU/mL) |
| Largest tumor size | — | 3-5 cm | >5 cm | — |
| Site of metastases | Lung | Spleen/kidney | GI | Liver/brain |
| Number of metastases | — | 1-4 | 5-8 | >8 |
| Previous failed chemotherapy | — | — | Single drug | Combination |
| FORMAT FOR DETERMINING FIGO STAGE AND WHO PROGNOSTIC SCORE | ||||
| To stage and allot a risk factor score, a patient’s diagnosis is allocated to a stage as represented by a Roman numeral I, II, III, or IV. This is then separated by a colon for the sum of all the actual risk factor scores expressed in Arabic numerals (e.g., stage II:4, stage IV:9). | ||||
The variables included in the prognostic score include tumor volume (hCG measurement, size, and number of metastases), site of involvement, prior chemotherapy exposure, and duration of disease. Each of these factors is considered an independent variable and is assumed to be additive.79 Patients with prognostic scores of 6 or lower are considered to be at low risk for drug resistance, and are treated initially with single agents. When the prognostic score is >7, multiagent regimens are required to optimize outcome. In general, patients with stage I tumors have low-risk scores, and patients with stage IV tumors have high-risk scores. Therefore, the prognostic scoring system is best applied to patients with stages II and III by identifying those patients who initially require more intensive therapy to reduce the likelihood that they will become drug-resistant CCA after term pregnancy, which is one of the variables associated with poor prognosis.
We reviewed the experience with postterm CCA at the NETDC from 1964 to 1996.80 Seven of 44 patients (16%) presented with clinical evidence of maternal–fetal bleeding that resulted in severe fetal anemia and nonimmune hydrops or third trimester bleeding. Although none of the infants had evidence of metastatic CCA, rare cases of fetal involvement by CCA have been reported and usually have resulted in either fetal or neonatal death. Other risk factors, including the time interval from delivery to diagnosis, sites of metastases, and pretreatment hCG level, were all significant in predicting outcome. All 31 of our patients with a prognostic score <8 survived, whereas 6 of 13 patients (46%) with a score >8 succumbed to their disease.
Treatment
Molar Pregnancy
Surgical Management
Once the patient’s medical condition has stabilized, a decision must be made regarding the most appropriate surgical treatment. If the patient no longer desires to preserve fertility, hysterectomy should be considered, particularly in patients older than age 50 years who have an increased risk for postmolar GTN.81 At the time of hysterectomy, prominent theca lutein cysts can be decompressed and ovarian function preserved. It is important to advise the patient that although hysterectomy eliminates the risk of local invasion, it does not obviate the need for chemotherapy if occult metastases subsequently appear.
Role of Prophylactic Chemotherapy
Patients with CHM characterized by high preevacuation hCG levels (>100,000 mIU/mL) and signs of marked trophoblastic growth (excessive uterine size) are at increased risk for persistent GTN (Table 90-4).28 The prophylactic administration of either methotrexate (MTX) or actinomycin D (ActD) should be considered in patients with high-risk molar disease because it has been shown to significantly reduce the incidence of both metastatic and locally invasive disease.84–84 Prophylaxis should also be considered in situations in which hCG follow-up is either unavailable or unreliable.
Table 90-4
Sequelae of Low- and High-Risk Complete Hydatidiform Mole*
| Outcome | Number of Patients (%) | |
| Low-Risk | High-Risk | |
| Normal involution | 486/506 (96) | 212/352 (60) |
| Persistent GTN | ||
| Nonmetastatic | 17/506 (3.4) | 109/352 (31) |
| Metastatic | 3/506 (0.6) | 31/352 (8.8) |
| Totals | 506/858 (59) | 352/858 (41) |
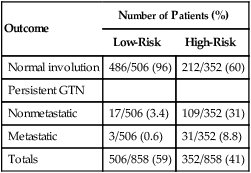
GTN, Gestational trophoblastic neoplasia.
*All patients were managed by evacuation without prophylactic chemotherapy.
Persistent Gestational Trophoblastic Neoplasia
Diagnosis
Persistent GTN can occur after any antecedent pregnancy, but most commonly develops after a molar gestation. The Cancer Committee of FIGO has established the criteria for the diagnosis of postmolar GTN85:
• Four values or more of hCG plateaued over at least 3 weeks
• Increase in hCG of 10% or greater for 3 or more values over at least 2 weeks
When the diagnosis is made on the basis of a rising or plateaued hCG level in the absence of a documented pregnancy or of any clinical, radiologic, or pathological evidence of trophoblastic tissue, it is important to make certain that the assay used is measuring “real” hCG to avoid a false-positive test (so-called phantom hCG).86 The diagnosis of GTN after a nonmolar pregnancy is more difficult, primarily because it is a rare occurrence and usually associated with subtle signs and symptoms. Abnormal bleeding after any pregnancy should be evaluated promptly with an hCG test.
Chemotherapeutic Agents
The treatment of patients with GTN is based on their risk for drug resistance. In general, optimal cure rates are achieved when patients with a low risk of drug resistance (prognostic scores <7) are treated initially with single-agent chemotherapy, and patients at high risk for drug resistance (prognostic scores >7) are treated initially with multiagent regimens. However, there is growing evidence that patients with low-risk GTN who have a large tumor burden as reflected in hCG levels (>100,000 mIU/ml) and/or prognostic scores of 5 and 6 are associated with an increased risk of resistance to single-agent therapy and should be treated primarily with multiagent protocols.87
Single-Agent Chemotherapy
Single-agent chemotherapy with either MTX or ActD has induced comparable and excellent remission rates in both nonmetastatic and low-risk metastatic GTN.88 An optimal regimen should maximize the cure rate while minimizing toxicity. Table 90-5 summarizes the various protocols that are in use for the primary treatment of both nonmetastatic and low-risk metastatic GTN. Several regimens of MTX and ActD have been shown to induce complete remission in 70% to 100% of patients with nonmetastatic GTN and in 50% to 70% of patients with low-risk metastatic GTN. Fortunately, if a patient is resistant to the initial single agent, remission can usually be achieved with the alternative drug.
Table 90-5
Single-Agent Regimens for Low-Risk Gestational Trophoblastic Neoplasm
| METHOTREXATE REGIMENS |
| METHOTREXATE |
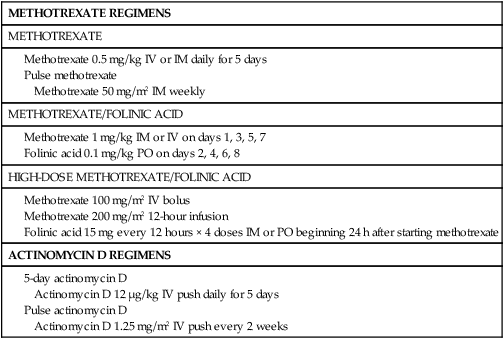
Methotrexate With Folinic Acid Rescue
In 1964 Bagshawe and Wilde89 described administering MTX with folinic acid (FA) to reduce chemotherapeutic toxicity. This regimen remains the initial treatment protocol for nonmetastatic and low-risk metastatic GTN at the Charing Cross Trophoblastic Disease Center as well as at our institution. Although MTX/FA is highly effective, its use is associated with a 20% rate of resistance. At the NETDC, complete remission was achieved in 147 of 163 patients (90%) with stage I and in 15 of 22 patients (68%) with low-risk stage II and III GTN.90 Among the 23 patients who were resistant to MTX/FA, 14 (61%) subsequently achieved remission with ActD, and 9 required multiagent chemotherapy. Thrombocytopenia, granulocytopenia, and hepatotoxicity occurred in only 11 (6%), 3 (2%), and 26 (14%) patients, respectively. One patient required platelet transfusion and developed sepsis due to myelosuppression. No patient developed alopecia. The effectiveness of this protocol is thought to be due to the prolonged exposure to MTX because when MTX is administered at a higher dose (300 mg/m2) and short duration (over 12 hours), the remission rate declines to 69%.91
5-Fluorouracil.
Although MTX and ActD are the two most commonly used single agents for GTN in the United States and Europe, 5-fluorouracil (5-FU) has been the preferred single-agent chemotherapy in China. Sung and associates92 reported that 5-FU induced complete remission in 93% of patients with stage I GTN and in 86% of patients with stage II disease.
Etoposide.
Etoposide used orally is reported to be highly effective for the treatment of patients with nonmetastatic and low-risk metastatic GTN with complete sustained remissions in 56 of 60 patients (93%).93 Etoposide can also be used sequentially in place of ActD when resistance to MTX is encountered.
Multiagent Chemotherapy
Methotrexate–Folinic Acid, Actinomycin D, and Cyclophosphamide (MAC).
Triple chemotherapy with MTX/FA, ActD, and cyclophosphamide (MAC) had been the preferred combination drug regimen at the NETDC in patients with high-risk GTN until superseded by EMACO (etoposide, MTX, ActD, cyclophosphamide, and vincristine) in the 1980s (Table 90-6). Although this regimen is inferior to EMACO in patients with high-risk scores, it is still quite useful in the management of patients with low-risk disease resistant to single agents.
Table 90-6
| Day | Drug | Dose |
| 1 | Methotrexate | 1.0 mg/kg IM or IV |
| Actinomycin D | 12 µg/kg IV push | |
| Cyclophosphamide | 3 mg/kg IV bolus | |
| 2 | Folinic acid | 0.1 mg/kg IM or PO |
| Actinomycin D | 12 µg/kg IV push | |
| Cyclophosphamide | 3 mg/kg IV bolus | |
| 3 | Methotrexate | 1.0 mg/kg IM or IV |
| Actinomycin D | 12 µg/kg IV push | |
| Cyclophosphamide | 3 mg/kg IV bolus | |
| 4 | Folinic acid | 0.1 mg/kg IM or PO |
| Actinomycin D | 12 µg/kg IV push | |
| Cyclophosphamide | 3 mg/kg IV bolus | |
| 5 | Methotrexate | 1.0 mg/kg IM or IV |
| Actinomycin D | 12 µg/kg IV push | |
| Cyclophosphamide | 3 mg/kg IV bolus | |
| 6 | Folinic acid | 0.1 mg/kg IM or PO |
| 7 | Methotrexate | 1.0 mg/kg IM or IV |
| 8 | Folinic acid | 0.1 mg/kg IM or IV |
Emaco and Emaep.
Bagshawe94 reported an 83% remission rate in patients with metastatic GTN including those with high-risk scores using a regimen that included etoposide, MTX, ActD, cyclophosphamide, and vincristine (EMACO). Similarly, Lurain and colleagues reported that 90% of patients with high-risk metastatic GTN achieved remission with EMACO.95 This has become the preferred treatment for patients with high-risk GTN and as salvage therapy for low-risk GTN (Table 90-7). Patients who are resistant to EMACO can still be salvaged by modifying this regimen by substituting etoposide and cisplatin on day 8 (EMAEP; Table 90-8). Bower and colleagues96 reported that EMAEP induced remission either alone or in conjunction with surgery in 16 of 21 patients (76%) who were resistant to EMACO. Unfortunately, the use of etoposide in GTN has been reported to increase the risk of later secondary tumors, including myeloid leukemia, melanoma, colon cancer, and breast cancer, which may not become apparent for 25 years.97 Among all patients who were treated with etoposide, 1.5% subsequently developed leukemia. When patients with nonmetastatic and low-risk metastatic disease prove resistant to single-agent therapy, we now administer triple therapy (MAC) before using regimens that contain etoposide.9
Table 90-7
| Day | Drug | Dose |
| 1 | Etoposide | 100 mg/m2 by infusion in 200 mL saline over 30 min |
| Actinomycin D | 0.5 mg IV push | |
| Methotrexate | 100 mg/m2 IV push | |
| 200 mg/m2 by infusion over 12 h | ||
| 2 | Etoposide | 100 mg/m2 by infusion in 200 mL saline over 30 min |
| Actinomycin D | 0.5 mg IV push | |
| Folinic acid | 15 mg every 12 hours for 4 doses IM or PO beginning 24 h after starting methotrexate | |
| 8 | Cyclophosphamide | 600 mg/m2 by infusion in saline over 30 min |
| Oncovin (vincristine) | 1.0 mg/m2 IV push |
Table 90-8
| Day | Drug | Dose |
| 1 | Etoposide | 100 mg/m2 by infusion in 200 mL saline over 30 min |
| Actinomycin D | 0.5 mg IV push | |
| Methotrexate | 100 mg/m2 IV push | |
| 200 mg/m2 by infusion over 12 h Folinic acid 15 mg/m2 every 12 h × 4 doses PO beginning 24 h after starting methotrexate |
||
| 2 | Etoposide | 100 mg/m2 by infusion in 200 mL saline over 30 min |
| Actinomycin D | 0.5 mg IV push | |
| 8 | Cisplatin | 60 mg/m2 with prehydration |
| Etoposide | 100 mg/m2 by infusion in 200 mL saline over 30 min |
5-Fluorouracil and Floxuridine.
Matsui and colleagues achieved remission in 9 of 11 patients (81.8%) with drug-resistant high-risk GTN using a combination of 5-FU and ActD.101 Two of these patients later relapsed but were subsequently salvaged with other regimens. Similarly, Wan and colleagues reported 100% remissions in 13 drug-resistant patients treated with a floxuridine-containing regimen.102
Other Multiagent Regimens
Regimens containing ifosfamide and paclitaxel have met with some success103,104 as has autologous bone marrow transplantation or stem cell support concurrent with high-dose chemotherapy although its role in GTN has yet to be defined.105,106
Osborne and colleagues described a novel three-drug doublet regimen consisting of paclitaxel, etoposide, and cisplatin (TE/TP) that induced remission in two drug-resistant patients.107
Treatment Protocols and Results
Stage I
Table 90-9 summarizes treatment protocols used for the management of stage I GTN.
Table 90-9
Treatment Protocol for Stage I Gestational Trophoblastic Neoplasm
| INITIAL |
| RESISTANT TO BOTH SINGLE AGENTS |
| FOLLOW-UP hCG |
| Weekly until normal for 3 wk, then monthly until normal for 1 y |
| CONTRACEPTION |
| 12 consecutive months of normal hCG tests |
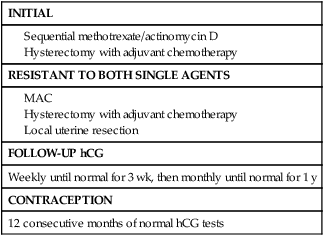
Treatment is either surgical or medical, depending on the patient’s desire to preserve fertility. Primary hysterectomy as initial treatment in patients who desire sterilization reduces the number of courses of chemotherapy required to achieve remission or avoids chemotherapy altogether.108 We advocate the use of a single dose of adjuvant chemotherapy (usually ActD 1.25 µg/m2) at the time of surgery to eradicate any occult metastases. In our experience, its use has not been associated with increased postoperative morbidity.
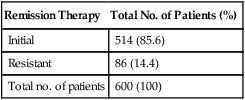
Table 90-10 summarizes the results achieved at the NETDC with stage I GTN. Between July 1965 and December 2011, 600 patients were treated. Complete sustained remission was achieved in 514 patients (85.6%), of whom 468 received sequential MTX/ActD and 45 underwent surgery. The 34 patients who underwent hysterectomy as initial therapy sought sterilization. The one patient who was treated primarily with a multiagent regimen had a prognostic score of 6, which is high for low-risk patients and appears to be associated with a higher risk of resistance to single agents. The remaining 86 patients (14.4%) who were resistant to their initial therapy subsequently were cured with either multiagent chemotherapy or surgical intervention.
Table 90-10
Results of Treatment in Stage I Gestational Trophoblastic Neoplasm (1965-2011)
| Remission Therapy | Total No. of Patients (%) |
| Initial | 514 (85.6) |
| Resistant | 86 (14.4) |
| Total no. of patients | 600 (100) |
Stages II and III
Table 90-11 summarizes the treatment protocols for patients with stages II and III GTN. Patients with low-risk disease (prognostic score <7) are treated with primary single-agent chemotherapy and patients with high-risk disease (prognostic score >6) require primary multiagent regimens to achieve optimal outcomes. Patients resistant to single-agent chemotherapy are switched to multiagent regimens (MAC or EMACO) promptly. Patients resistant to MAC are converted to EMACO, and then to EMAEP when they develop drug resistance. Surgery to remove resistant disease in a wide variety of organs plays an important role in the overall management of these patients.109
Table 90-11
Treatment Protocol for Stages II and III Gestational Trophoblastic Neoplasms
| LOW RISK | |
| Initial | Sequential methotrexate/actinomycin D |
| Resistant to both single agents | MAC or EMACO; |
| Local resection with adjunctive CT | |
| HIGH RISK | |
| Initial | EMACO |
| Resistant | EMAEP; VBP |
| Local resection with adjunctive CT | |
| Follow-up hCG | Weekly until normal for 3 wk, then monthly until normal for 12 mo |
| Contraception | 12 consecutive months of normal hCG tests |
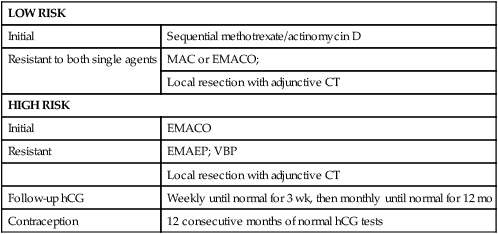
Tables 90-12 and 90-13 summarize the results achieved at the NETDC in treating patients with stage II and III GTN. Between July 1965 and December 2011, all 32 patients with stage II disease achieved remission with either single-agent or multiagent regimens. Single-agent chemotherapy induced complete remission in 19 of 24 (79.2%) patients with low-risk stage II disease, but in only 2 of 4 high-risk patients (50%).
Table 90-12
Results of Treatment in Stage II Gestational Trophoblastic Neoplasm (1965-2011)
| Remission Therapy | Total No. of Patients (%) |
| Low risk | 24 |
| Initial | 19/24 (79.2) |
| Resistant | 5/24 (20.8) |
| High risk | 8 |
| Initial | 6/8 (75) |
| Resistant | 2/8 (25) |
| Total no. of patients | 32 |
Table 90-13
Results of Treatment in Stage III Gestational Trophoblastic Neoplasm (1965-2011)*
| Remission Therapy | Total No. of Patients |
| Low risk | 136 |
| Initial | 108/136 (79.4) |
| Resistant | 28/136 (20.6) |
| High risk | 66 |
| Initial | 57/66 (86.4) |
| Resistant | 8/66 (12.1) |
| Total no. of patients | 202 |
Management of Vaginal and Adnexal Metastases
Vaginal metastases are highly vascular and usually present with heavy bleeding. When bleeding is a problem, embolization can be used effectively.110 After the administration of one or two courses of chemotherapy, the tumor usually regresses and bleeding is no longer a problem. Involvement of the adnexa occurs infrequently and should be monitored by ultrasound when asymptomatic. These tumor deposits usually resolve once chemotherapy is administered; however, surgical intervention is required if signs of intraabdominal bleeding appear.
Management of Lung Metastases
Tomoda and colleagues111 reviewed their experience with pulmonary resection in 19 patients with chemotherapy-resistant GTN and proposed the following criteria for successful resection:
• Primary malignancy is controlled
• No evidence of other metastatic sites
In Tomoda’s series, complete remission was achieved in 14 of 15 patients who met all five criteria but in none of the 4 patients who had one or more unfavorable clinical features. Similarly, Fleming and coworkers109 reported that 10 out of 11 (90.9%) carefully selected patients with drug-resistant pulmonary nodules attained complete remission after lung resection. Several investigators have reported that nondetectable hCG levels within 1 to 2 weeks after resection of a solitary pulmonary nodule are highly predictive of a favorable outcome. Survival after salvage surgery is also influenced by other factors, such as the number of preoperative chemotherapy regimens, the number of disease sites, and the patient’s prognostic score.
Stage IV
Table 90-14 summarizes the treatment protocols for the management of patients with stage IV GTN. All stage IV patients are considered high-risk and should be treated with a multimodal approach that includes chemotherapy, surgery, and radiation where indicated.
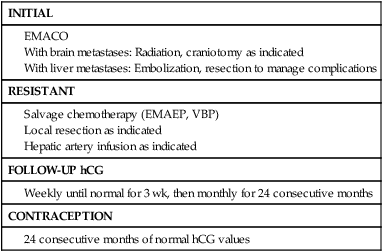
All patients are treated initially with EMACO. When CNS disease is present, the dose of MTX is increased to 1 g/m2 in divided doses to avoid the need for alkalinization of urine. We recommend the use of whole-brain or localized gamma knife radiation promptly coincidentally with chemotherapy to reduce the risk of hemorrhage and for maximal tumoricidal effect. Patients with disease resistant to EMACO should be treated with EMAEP. Yordan and associates112 reported that deaths due to central nervous system involvement occurred in 11 of 25 patients (44%) treated with chemotherapy alone, but none of 18 patients treated with both brain radiation and chemotherapy. On the other hand, Newlands and colleagues have reported complete and sustained remission in 30 of 35 patients (86%) treated with intensive multiagent chemotherapy that included high-dose intravenous and intrathecal methotrexate.113
Management of Hepatic Metastases
The management of liver metastases may be difficult. If a patient is resistant to systemic chemotherapy, hepatic arterial infusion might be effectively used in selected cases. Hepatic resection might be necessary to control acute hepatic bleeding or to remove localized areas of resistant tumor. Grumbine and associates114 reported the use of selective occlusion of the hepatic arteries and concurrent combination chemotherapy in a patient with bleeding liver metastases who ultimately attained remission. Wong and colleagues115 also noted that 9 out of 10 patients with hepatic involvement achieved complete remission with primary intensive multiagent chemotherapy without hepatic irradiation. Bakri and colleagues61 similarly reported that 5 of 8 patients (63%) with liver metastases treated with multiagent chemotherapy alone attained remission.
Table 90-15 summarizes the results obtained in patients with stage IV GTN at the NETDC. Prior to 1975, before the routine use of multiagent protocols for stage IV disease was initiated, only 6 of 20 patients (30%) achieved remission. After 1975, 16 of 19 patients (84%) were cured. This dramatic improvement in survival is a result of recognizing the importance of risk factors, using a multimodal approach to the management of these critically ill patients and the use of support systems for the management of complications both from their disease and treatment.
Table 90-15
Results of Treatment of Stage IV Gestational Trophoblastic Neoplasm: Number of Remissions
| Time Period | Total No. of Patients (%) |
| 1965-1975 | 6/20 (30) |
| After 1975 | 16/19 (84) |
Management of Relapsed and Chemoresistant GTN
When a tumor becomes drug resistant or relapses after treatment with either primary and secondary chemotherapy as manifested either by a plateau or reelevation of the hCG level, it is necessary to reevaluate her status and look for occult metastases. Mutch and colleagues116 reported that recurrences occur after initial remission in 2% of patients with nonmetastatic GTN, 4% of patients with good-prognosis (low-risk) metastatic disease, and 13% of patients with poor-prognosis (high-risk) disease. Relapses developed within 3 and 18 months in 50% and 85% of patients, respectively. At the NETDC, we have observed relapses in 3% of patients with stage I, 8% with stage II, 4% with stage III, and 9% with stage IV disease.117 The mean time to recurrence from the last undetectable hCG level was 6 months, and this rate did not differ among the four FIGO stages. All patients with stage I, II, and III GTN who relapsed were subsequently cured, whereas both of the stage IV patients with recurrent disease succumbed. In contrast to patients with GTN, relapse is rare in patients with molar pregnancy once the hCG level becomes undetectable.118,119
Management of PSTT and ETT
Patients diagnosed with PSTT and ETT are managed similarly. Hysterectomy rather than chemotherapy is the first-line treatment in patients even when metastases are present. At surgery, lymph node sampling should be carried out when there is deep myometrial invasion because, in contrast to CCA, these tumors can spread via lymphatics. The survival rate for patients with nonmetastatic disease is 100%. Patients with metastases may achieve remission if treated with multiagent chemotherapy, particularly if they are diagnosed within 4 years of the antecedent pregnancy.120–123 Risk factors for metastatic disease in patients with PSTT include interval from the previous pregnancy of longer than 2 years, deep myometrial invasion, the presence of tumor necrosis, and mitotic count of more than 6 per high-power field. At present, platinum-containing regimens such as EMAEP are the treatment of choice. Survival rates approach 50% to 60%. In the largest series reported to date, the group from Charing Cross has reported that all 31 patients treated by primary hysterectomy and adjuvant chemotherapy achieved complete remission with no additional therapy.124
Follow-Up
After Evacuation of a Molar Pregnancy
After evacuation of a molar pregnancy, all patients should be followed with weekly quantitative hCG tests until the levels are undetectable for 3 consecutive weeks. Although the current guidelines of the American College of Obstetricians and Gynecologists recommend monthly testing for 6 months before pregnancy is advised, recent reports from the NETDC and other centers indicated that the risk of relapse after achieving undetectable hCG levels is negligible.118,119 We now discontinue testing and allow for pregnancy after 3 consecutive weeks and 3 consecutive months of undetectable hCG tests.
Patients should be counseled to use effective contraception during the entire interval of follow-up. We do not encourage the insertion of intrauterine devices until the patient achieves undetectable hCG levels because of the risk of infection or perforation if residual tumor is present. Unless the patient desires surgical sterilization, her options are either hormonal or barrier methods. Although the incidence of postmolar tumor has been reported to be increased in patients who start oral contraceptives before the hCG level becomes undetectable,125 data from the NETDC and other centers indicate that oral contraceptives do not appear to increase the risk of postmolar GTN.9,126
Subsequent Pregnancy
After Complete Hydatidiform Mole
Table 90-16 summarizes the subsequent pregnancy outcome after CHM at the NETDC. Patients after CHM can anticipate normal reproduction in the future, except for a significant increase in the risk of another molar gestation.
Table 90-16
Subsequent Pregnancy Outcome in Complete Molar Pregnancy (1965-2001)
| Outcome | Number (%) |
| Total pregnancies | 1356 |
| Total deliveries | 1034 |
| Term live | 926 (68.3) |
| Preterm | 101 (7.4) |
| Stillbirth | 7 (0.5) |
| Congenital anomalies | 40/1034 (3.9) |
| Spontaneous abortions | 250 (18.4) |
| Therapeutic abortions | 41 (3.1) |
| Ectopic pregnancy | 11 (0.8) |
| Repeat molar pregnancy | 20 (1.5) |
After Partial Hydatidiform Mole
Table 90-17 summarizes the subsequent pregnancy outcome after PHM at the NETDC. Similar to CHM, patients after PHM can anticipate normal reproduction in the future except for an increased risk of another molar gestation.
Table 90-17
Subsequent Pregnancy Outcome in Partial Molar Pregnancy (1975-2011)
| Outcome | Number (%) |
| Total pregnancies | 327 |
| Total deliveries | 250 |
| Term live | 239 (73.1) |
| Preterm | 6 (1.8) |
| Stillbirth | 1 (0.3) |
| Congenital anomalies | 4/301 (1.3) |
| Spontaneous abortions | 54 (16.5) |
| Therapeutic abortions | 12 (3.7) |
| Ectopic pregnancy | 1 (0.3) |
| Repeat molar pregnancy | 10 (3.1) |
Recurrent Molar Pregnancy
After either a CHM or PHM, patients have an increased risk of another molar pregnancy. Thirty-four (1.6%) of our patients had at least two molar gestations between June 1965 and December 2011. Subsequent molar pregnancy can be either complete or partial regardless of the previous type. Following two molar pregnancies, 34 patients had 35 later conceptions resulting in 20 (57%) full-term normal deliveries, 7 (20%) molar pregnancies (6 complete, 1 partial), 3 spontaneous abortions, 1 ectopic pregnancy, and 1 intrauterine death. Three patients underwent elective termination in the first trimester. These results are similar to those reported by Lurain.127 Bagshawe and colleagues also reported that the risk of a third molar disease after two episodes of molar pregnancy was 15%.128 In six of our cases, we have documented that the patient had different partners at the time of conception of different molar pregnancies.
After Gestational Trophoblastic Neoplasia
Table 90-18 summarizes the subsequent pregnancy outcome in patients treated for GTN. These women can also experience normal reproductive function except that they too have an increased risk of a recurrent molar gestation. It is reassuring that the frequency of congenital anomalies is not increased despite the use of chemotherapeutic agents, which are both teratogenic and mutagenic.
Table 90-18
Subsequent Pregnancy Outcome in Patients Treated for Gestational Trophoblastic Neoplasms (1965-2011)
| Outcome | Number (%) |
| Total pregnancies | 665 |
| Total deliveries | 509 |
| Term live | 443 (66.6) |
| Preterm | 44 (6.6) |
| Stillbirth | 10 (1.5) |
| Congenital anomalies | 12/(2.4) |
| Spontaneous abortions | 123 (18.5) |
| Therapeutic abortions | 28 (4.2) |
| Ectopic pregnancy | 7 (1.1) |
| Repeat molar pregnancy | 10 (1.5) |
Our experience is in general agreement with that of other centers regarding the pregnancy outcome after chemotherapy for GTN. A total of 2657 subsequent pregnancies have been reported, which resulted in 77% full-term live births, 5% premature deliveries, 1% stillbirths, and 14% spontaneous miscarriages.129 Although the frequency of stillbirths appears somewhat increased, congenital anomalies were noted in only 1.8% of patients, which is consistent with the general population. Woolas and associates130 noted that there were no differences in either conception rate or pregnancy outcome between women treated with single agents and those treated with combination chemotherapy. Furthermore, only 7% of women who wished to become pregnant after GTN failed to conceive.
Psychosocial Consequences of Gestational Trophoblastic Neoplasia
Women who develop GTN can experience significant mood disturbance, marital and sexual problems, and concerns over future fertility.131 Because GTN is a consequence of pregnancy, patients and their partners must confront the loss of a pregnancy at the same time that they face concerns regarding malignancy. Patients can experience clinically significant levels of anxiety, fatigue, anger, confusion, sexual problems, and concern for future pregnancy that last for protracted periods of time. Patients with metastatic disease and active disease are particularly at risk of serious psychological disturbances.