Chapter 4
Functional benefits of respiratory muscle training
The rationale for training any muscle is the presence of a functional overload. In Chapter 3 we explored the concept that particular disease conditions are associated with imbalance in the demand / capacity relationship of the respiratory pump muscles, i.e., a functional overload. Disease creates imbalance by altering the resistances and elastances that must be overcome during breathing, and the capacity of the respiratory muscles to meet these mechanical demands. For example, patients with chronic obstructive pulmonary disease (COPD) experience a ‘double whammy’ because their inspiratory muscles are weakened, and the demand for inspiratory work of breathing is increased. However, in other situations inspiratory muscle function may be normal, but the respiratory pump may be operating in the context of a transient increased demand, e.g., mild asthma. Thus, a muscle might be considered ‘normal’ in absolute terms, but if the demands that are placed upon a ‘normal muscle’ are supranormal then it is weak functionally. For example, a morbidly obese person with normal quadriceps muscle strength is functionally weak. Chapter 3 thus explored the rationale for specific training of the respiratory pump muscles in a range of conditions, as well as considering how the work of breathing contributes to exercise limitation in health and disease.
The purpose of the current chapter is to explore how respiratory muscles respond to specific training. It will begin by considering these responses at the structural and functional level of the muscle. A brief review of whole-body responses to respiratory muscle training (RMT) in healthy young athletes will follow, thereby helping to set the scene for responses to RMT in specific disease conditions. Detailed analysis of the different methods of RMT, and the specific adaptations that they elicit, can be found in Chapter 5.
RESPIRATORY MUSCLE RESPONSES TO RMT
Essentially, muscles adapt to training by changing their structure, the result of which is to induce changes in muscle function. For example, when weights are lifted the muscle fibres hypertrophy and the strength of the muscle increases. In contrast, if a muscle is subjected to prolonged, continuous bouts of low-intensity muscle loading, e.g., running, muscle fibres undergo structural and biochemical changes that increase their endurance (see Ch. 2). Broadly speaking, therefore, training can be subdivided into two main types: one that increases strength, and the other that increases endurance. However, there is a great deal of potential for hybridization of training responses in the middle ground between these extremes. In Chapter 5, the training principles of ‘overload’, ‘specificity’ and ‘reversibility’ will be considered in detail, as well as the methods and equipment used to implement respiratory muscle training (RMT). For the purposes of the current chapter, a very brief explanation of training methods follows.
As one might expect, the equipment and methods required to implement strength and endurance training of the respiratory muscles differ considerably. It is akin to comparing a leg press machine with a treadmill: both machines train the leg muscles, but the training and functional outcomes are very different. In the case of specific inspiratory and expiratory muscle strength training, devices are used to impose a resistance to the respiratory muscles at the mouth (like lifting a dumbbell). In contrast, respiratory muscle endurance training consists of hyperventilating for prolonged periods of time (like running on a treadmill). In the case of respiratory muscle endurance training, it is not possible to separate inspiratory and expiratory muscle contributions, so this method trains both sets of muscles simultaneously. Training principles, methods and equipment will be explored in greater detail in Chapter 5. However, in the mean time, the preceding explanation provides a working knowledge of specific RMT.
Structural adaptations to RMT
Patients
At the time of writing, the only study to have obtained inspiratory muscle biopsy samples pre- and post-RMT is that of Ramirez-Sarmiento and colleagues from patients with COPD (Ramirez-Sarmiento et al, 2002). Their randomized controlled trial sampled muscle fibres from the external (inspiratory) intercostal muscles before and after 5 weeks of pressure threshold IMT (see Ch. 5). The training regimen had an endurance training bias (30 minutes training, 5 days per week at a load of 40–50% of maximal inspiratory pressure [MIP]), and patients showed a corresponding increase in the proportion of type I muscle fibres (fatigue-resistant fibres), and a decrease in the proportion of type II fibres. Both fibre types showed an increase in fibre cross-sectional area (hypertrophy), especially the type II fibres. Patients’ inspiratory muscle strength increased, as assessed by MIP (see Ch. 6, ‘Assessment of respiratory muscle function’), as did the ability to breathe continuously against an external load (endurance).
The response of the diaphragm to IMT has also been studied using ultrasound imaging and measurement of diaphragm thickness in patients with chronic heart failure (CHF) and in those with cystic fibrosis (CF). In patients with CHF and inspiratory muscle weakness (MIP ≤ 60% predicted), 4 weeks of pressure threshold IMT (30 minutes daily at 30% of MIP) elicited a 55% increase in diaphragm thickness and a 72% increase in MIP (Chiappa et al, 2008b). The fact that the patients had pre-existing weakness may explain the particularly large improvements observed following IMT in this group. Diaphragm thickness has also been shown to increase after IMT in patients with CF. After 8 weeks of high-intensity incremental flow-resistive loading (80% of MIP), diaphragm thickness increased by 19%, whilst MIP increased by 18% (Enright et al, 2004).
Healthy people
In healthy young people, the only data available on structural adaptations are derived from measuring diaphragm thickness using ultrasound. Contracted diaphragm thickness increases ~ 12% following 4 to 8 weeks of inspiratory muscle resistance training (Enright et al, 2006; Downey et al, 2007). As would be expected, the increase in thickness was accompanied by improvements in MIP (24% and 41% after 4 and 8 weeks of training, respectively). Interestingly, the same magnitude of change in diaphragm thickness was observed after 4 and 8 weeks of training, yet changes in MIP differed. This clearly indicates that diaphragm hypertrophy is not the only source of improvement in MIP, which can also improve through improvements in accessory muscle function as well as neural adaptations. These neural adaptations include an enhanced ability to coordinate the contraction of synergistic muscles, as well as an enhanced ability to maximize the activation of individual muscles. It should also be noted that the 4- and 8-week studies employed different training techniques, i.e., pressure threshold (Downey et al, 2007) and incremental flow-resistive loading (Enright et al, 2006), respectively. See Chapter 5 for details of each training method.
In keeping with its key role as a postural control and core-stabilizing muscle, diaphragm thickness and inspiratory muscle strength have also been shown to increase following 16 weeks of sit-up and bicep curl training (DePalo et al, 2004). The training stimulus to the diaphragm in this study derived from the increases in transdiaphragmatic pressure that resulted from its role as a postural and core-stabilizing muscle. Participants undertook sit-up and bicep curl training 3–4 times per week, for 16 weeks. Expiratory muscle strength increased 37%, which is to be expected as rectus abdominis is an important expiratory muscle. These data highlight the close interrelationship of the breathing and postural stabilizing functions of the trunk muscles, thereby reinforcing the potential benefits of applying functional training techniques to the respiratory muscles (see Ch. 3, ‘Non-respiratory functions of the respiratory muscles’). Diaphragm thickness and respiratory muscle strength have also been shown to be greater in healthy active elderly people than in their more sedentary counterparts (Summerhill et al, 2007). As expected, MIP correlated with diaphragm thickness. These data add further support to the notion that non-respiratory activities generate a training stimulus to the respiratory muscles.
Collectively, these data from patients and healthy people present a picture of the inspiratory muscles as a highly adaptable tissue showing hypertrophy and fibre-type shifts that are consistent with the well-established evidence relating to limb-muscle training. These changes appear to occur similarly in both healthy people and in patients. The data also indicate that respiratory muscles are trained by a variety of overloading stimuli (see also Ch. 5, ‘Methods of respiratory muscle training’). In the following section the functional adaptations to RMT will be considered. The section is divided into subsections addressing different training types (resistance or endurance) and muscle groups (inspiratory, expiratory, or both).
Functional adaptations to RMT
Principally, muscles respond to training by improving their strength, speed of contraction, power output and / or endurance. Because muscles respond to training stimuli in highly specific ways (see Ch. 5, ‘Methods of respiratory muscle training’), different training regimens tend to elicit slightly different changes in each property. For example, training that consists of maximal, static efforts with high force improves strength, but not contraction speed (Romer & McConnell, 2003). However, there is also a good deal of cross-over, such that training regimens that are ostensibly strength orientated can also give rise to improvements in endurance, but generally not vice versa (see Ch. 5, ‘Specificity’). The following section describes what is known about improvements in each functional property following resistance training.
A helpful method of characterizing the functional properties of muscles is to plot a graph of the relationship between strength and contraction speed; the so-called force–velocity relationship (see also Ch. 5, ‘Specificity’). It is also possible to add a third dimension to this in the form of muscle power output, which is the product of force and velocity. Figure 4.1 illustrates these interrelationships for the inspiratory muscles, and encapsulates the three key and interrelated functional properties of muscles (strength, speed and power), all of which can be modified by appropriate training.
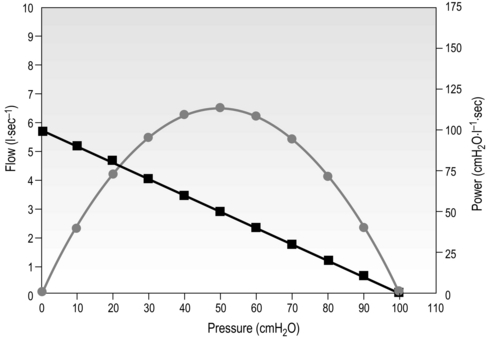
Figure 4.1 Interrelationship of the three key properties of inspiratory muscles: pressure (strength), flow (velocity) and power (strength × velocity). Squares = pressure / flow; circles = pressure / power. (Adapted from McConnell AK, 2011. Breathe strong, perform better. Human Kinetics, Champaign, IL, with permission.)
Inspiratory muscle adaptations to resistance training
The most widely studied muscles are those of inspiration, the functional properties of which have been characterized extensively. As was described above, inspiratory muscle training (IMT) elicits hypertrophy and improvements in strength, and the reader is referred elsewhere for systematic review of the extensive evidence base drawn from both patients and healthy people (Geddes et al, 2008; Gosselink et al, 2011; Hajghanbari et al, 2012; Illi et al, 2012; Plentz et al, 2012; Smart et al, 2012). When IMT is undertaken using moderate loads (~ 60% of MIP) that allow rapid muscle shortening, improvements in strength are accompanied in healthy people by increases in maximal shortening velocity (peak inspiratory flow rate) and maximal power (Tzelepis et al, 1994; Tzelepis et al, 1999; Romer & McConnell, 2003). Moderate loads can typically be sustained for ~ 30 breaths, and have also been shown to improve endurance significantly in healthy people (Caine & McConnell, 1998a). In most studies of resistance IMT a placebo has been used, which typically consists of 30 to 60 breaths at a load of 15% of MIP. The latter has been used repeatedly as an IMT placebo in healthy people as it creates a perceptible resistance, but does not elicit any significant changes in inspiratory muscle function (Volianitis et al, 2001; Romer et al, 2002a; Romer et al, 2002b; Bailey et al, 2010; Brown et al, 2011; Turner et al, 2011; Turner et al, 2012).
Figure 4.2 illustrates the significant changes in strength, peak inspiratory flow and power induced by different IMT regimens in healthy people. It is clear that different types of training induce changes in different functional properties, and this is explored in more detail in Chapter 5 (section on ‘Specificity’), which describes the specificity principle of training, i.e., muscles adapt according to the nature of the training stimulus. For example, it is clear from Figure 4.2 that high-pressure training (maximum static muscle contractions) improves strength, but not velocity of shortening. The opposite was true for high-flow training (unloaded maximal inhalations). No such changes are observed after placebo IMT.
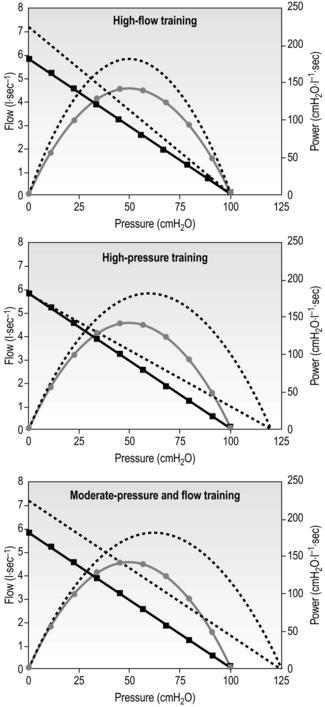
Figure 4.2 Changes in pressure (strength), flow (velocity) and power (strength × velocity) in response to different training stimuli. Dotted lines = post-training; straight lines= pressure / velocity; curved lines = pressure / power. (Data derived from Romer LM, McConnell AK, 2003. Med. Sci. Sports Exerc. 35, 237–244. Adapted from McConnell AK, 2011. Breathe strong, perform better. Human Kinetics, Champaign, IL, with permission.)
As mentioned earlier, strength training can also improve endurance. There is no single accepted method of assessing endurance, but, borrowing the methods used to assess whole-body exercise tolerance, IMT improves inspiratory muscle endurance measured using tests employing continuous incremental and fixed-intensity loading, as well as tests requiring the maintenance of maximal hyperventilation (see below and Ch. 6, section ‘Assessment of respiratory muscle function’). In addition, an improvement in endurance after IMT can be implied from the absence or attenuation of fatigue following a task that had previously induced fatigue, which also occurs after IMT in healthy people (Romer et al, 2002d).
Expiratory muscle adaptations to resistance training
Expiratory muscle training (EMT) has been much less widely studied, but there is good-quality evidence, primarily from patients, that maximal expiratory pressure (MEP) improves significantly in response to specific EMT (19–56%) (Smeltzer et al, 1996; Sapienza et al, 2002; Weiner et al, 2003b; Baker et al, 2005; Chiara et al, 2007; Mota et al, 2007; Kim et al, 2008; Roth et al, 2010). In a particularly comprehensive study on patients with COPD, IMT, EMT and EMT + IMT were studied in three separate, matched groups of patients as well as a fourth placebo control group (Weiner et al, 2003a); this study is unique in that an identical training device and training regimen was used for all of the training. In addition, the IMT and EMT groups received placebo training immediately after their real training session in order to mimic the experience of the EMT + IMT group, who undertook EMT followed immediately by IMT. Following 3 months of IMT the MIP improved significantly by 25%, whereas following EMT the MEP improved significantly by 20%. In the group that undertook EMT + IMT, both MIP and MEP improved significantly by 33%, suggesting that there may be a small potentiation of the training effect when EMT is followed by IMT. Indeed, the IMT and EMT groups both showed small, non-significant increases in maximal pressures for the placebo phase of their training (~ 5%), suggesting a small training effect. In addition, expiratory endurance performance (breathing against an incremental pressure load) improved significantly by 31% following EMT, and significantly by 25% following EMT + IMT, whereas inspiratory endurance improved significantly by 25% following IMT, and significantly by 33% following EMT + IMT. Collectively, these data suggest that, in patients with COPD, expiratory muscles respond in an identical manner to inspiratory muscles when the same training stimulus is applied.
A caveat that is worthy of mention is the ‘purity’, or otherwise, of EMT. A number of studies on patients purporting to undertake EMT have documented improvements in MIP as well as MEP (Roth et al, 2010). On the face of it, this might imply a placebo effect in both groups; however, the effect has been demonstrated in placebo-controlled trials in which there were no significant changes in the placebo groups (Roth et al, 2010). Furthermore, a study that examined the acute responses of inspiratory and expiratory muscles to expiratory loading in healthy people found that it induced a significant decrease in both MIP and MEP, i.e., fatigue (Taylor & Romer, 2009). In other words, expiratory loading also loads the inspiratory muscles. This presumably occurs because of the requirement for inspiratory muscle activation during forceful expiratory efforts. In healthy people, this ‘contamination’ of the training stimulus does not appear to be present during inspiratory loading and IMT, where there is no change in MEP (Romer et al, 2002b; Griffiths & McConnell, 2007), but responses are more variable in patients. For example, patients with spinal cord injury and renal failure show no change in MEP after IMT (Rutchik et al, 1998; Silva et al, 2011), but patients with CHF and multiple sclerosis show a significant increase (Cahalin et al, 1997; Klefbeck & Hamrah Nedjad, 2003). It is possible that these discrepancies are explained by variations in the baseline strength of expiratory muscles and the extent to which they are recruited to lower end-expiratory lung volume during IMT.
Adaptations to simultaneous inspiratory and expiratory muscle resistance training
One might predict that combining IMT and EMT within the same breath cycle (concurrent IMT and EMT) would result in improvements in function in both muscle groups. However, the results of two studies in healthy people that have implemented concurrent training suggest that this approach impairs training responses. For example, a study in young swimmers found no significant change in MIP or MEP despite a strenuous, progressive training regimen (Wells et al, 2005). Similarly, a study on rowers found only modest changes at best in MIP and MEP (Griffiths & McConnell, 2007). Participants generally reported that they found the simultaneous loading of both breath cycles uncomfortable, and that it was impossible to train with maximal effort when both breathing phases are loaded. This may be because of the training stimulus ‘contamination’ effect described in the previous paragraph. However, one study of healthy elderly people has demonstrated significant increases of 22% and 30% in MIP and MEP, respectively, following 8 weeks of concurrent IMT and EMT (Watsford & Murphy, 2008).
Functional adaptations to respiratory endurance training
Pure endurance training of the respiratory muscles is undertaken using a sustained, high-intensity hyperpnoea task (see Ch. 5, ‘Methods of respiratory muscle training’), and has been used much less widely than resistance training, especially in patients. Hyperpnoea training recruits both inspiratory and expiratory muscles simultaneously and, unlike IMT, its nature makes a true placebo difficult to implement. Accordingly, trials have typically included a ‘no training’ control group rather than a placebo group. In healthy people and patients, the training significantly improves the ability to sustain high levels of hyperventilation (an index of endurance) (Scherer et al, 2000; Illi et al, 2012). In healthy people, the training also improves the volume of air that can be respired during a brief maximal burst of hyperventilation, typically 15 seconds (an index of power output) (Verges et al, 2008; Illi et al, 2012). The latter finding is also consistent with improvements in peak velocity of muscle contraction, although there is no direct evidence of this to date. This type of training does not improve the strength of the respiratory muscles in healthy people (Verges et al, 2008; Illi et al, 2012), which is not surprising since the training stimulus required to improve strength must include an increase in the force of muscle contraction (see Ch. 5, section ‘Specificity’). However, in patients with COPD, a small but significant increase in MEP has been shown in one study (Scherer et al, 2000). This may be explained by the presence of expiratory flow limitation in patients with COPD, which creates an expiratory flow resistive load during hyperpnoea. Thus, in the absence of expiratory flow limitation, functional adaptations are typically confined to flow, power and endurance.
Summary
Respiratory muscles respond to training stimuli in the same manner as other skeletal muscles, i.e., by undergoing adaptations to their structure and function that are specific to the training stimulus (see Ch. 5, section ‘Specificity’). Depending upon the training protocol implemented, functional adaptations to respiratory muscle resistance training can include improvement in all four muscle properties, viz., strength, speed of shortening, power output and endurance. Training regimens employing moderate loads (50–60%) sustained to the limit of tolerance (typically 30 breaths) have been shown to generate the widest range of functional benefits (Romer & McConnell, 2003). Expiratory muscle adaptations have been less comprehensively studied, but it is likely that the expiratory muscles respond to training in a similar manner to the inspiratory muscles. Attempts to resistance train both inspiratory and expiratory muscles simultaneously have so far proved impractical, and appear to yield inferior results to loading inhalation and exhalation separately. Specific respiratory muscle endurance training improves speed, power and endurance, but not strength, unless expiratory flow limitation is present thereby creating an expiratory resistive load.
RESPONSES TO RMT IN HEALTHY PEOPLE
In the first section of this chapter, we considered the structural and functional adaptations elicited by specific training of the respiratory muscles. In Chapter 3 we discovered how breathing limits exercise performance in a range of disease conditions, including performance in sports people. In this section we will see how the muscle adaptations described above overcome the limitations to sports performance described in Chapter 3. This will set the scene for a review of the evidence relating to the clinical benefits of RMT for patients in the final section of this chapter.
To get the most from this chapter, it is desirable (but not essential) to have an understanding of breathing-related limitations to exercise performance in healthy people (as described in Ch. 3). In short, there appear to be two major influences arising from the respiratory pump muscles that can cause athletes to slow down or stop exercise: (1) the perception of breathing effort and (2) the consequences of activation of the group III and IV afferents within the inspiratory muscles. The former makes exercise feel more difficult, and it intensifies as the inspiratory muscles become fatigued; the latter reduces limb blood flow, hastens limb fatigue and exacerbates the perception of limb and whole-body effort.
Logically, making the respiratory muscles stronger and more fatigue resistant should delay or abolish the negative influences of breathing upon exercise tolerance. But what is the scientific evidence supporting this, how big are any improvements and what types of activities are improved? The following description of the published literature is subdivided into two subsections, depending upon the type of performance outcome measure tested: (1) time trials and test of endurance, and (2) tests of anaerobic endurance and sprinting. In addition, this section will consider a study of healthy young people in which the physiological responses to hypoxic conditions were examined before and after RMT. The latter has some bearing on patients with hypoxaemia such as those with COPD. For an overview of the entire evidence base relating to RMT in healthy people, readers are referred to Illi et al’s (2012) systematic review of RMT. This detailed analysis of some 46 original studies of RMT included both strength and endurance RMT, revealing ‘significant improvement in performance after RMT, which was detected by constant load tests, time trials, and intermittent incremental tests, but not by [continuous] incremental tests’. Below is a description of the types of performance tests employed in athletes, as well as a summary of the key papers that have evaluated RMT in athletes.
Influence of RMT upon time trials and tests of endurance
Most of the studies of respiratory muscle training (RMT) in healthy people have examined endurance sports, and two types of exercise tests have been used: fixed-intensity exercise undertaken to the limit of tolerance (Tlim) and time trials. The majority have been randomized placebo-controlled trials; unfortunately, some weaker studies with either a simple no-training control or no control at all have found their way into the literature (Illi et al, 2012). However, only placebo-controlled and controlled trials are considered within this section.
There are no competitive events that require athletes to keep going at the same intensity for as long as they can (a Tlim test), but this type of test does provide an excellent laboratory model for studying the effects of ‘ergogenic aids’ such as RMT (i.e., interventions that purport to improve performance). This is because Tlim tests are extremely sensitive to small physiological improvements, they yield large changes (typically greater than 30%) and they allow physiological and perceptual responses to be studied under identical conditions before and after the intervention. In contrast, the obvious advantage of using a time trial to assess performance is that it simulates a race. However, for this very reason the magnitude of the changes that are typically observed after ergogenic interventions is extremely small, typically less than 5% (Currell & Jeukendrup, 2008). In addition, it is impossible to compare physiological responses in a meaningful way before and after the intervention using a time trial because the exercise conditions are not identical. For example, if performance is enhanced post-RMT, athletes will be working at a greater intensity, which cannot then be compared with the pre-intervention test. Both types of tests have provided useful insights into the effects of RMT.
Studies that have used resistance training of the respiratory muscles (see Ch. 5) have done so using cycling, rowing, swimming and running modalities. In the case of cycling and running, this has been undertaken using both Tlim tests and time trials. For rowing and swimming, only time trials have been used. Table 4.1 summarizes the findings of placebo-controlled studies. Many more studies than those presented have been conducted, but their quality is variable (Illi et al, 2012) so the table is limited to those with robust designs.
Table 4.1
Placebo-controlled studies of the influence of resistance breathing muscle training upon endurance performance
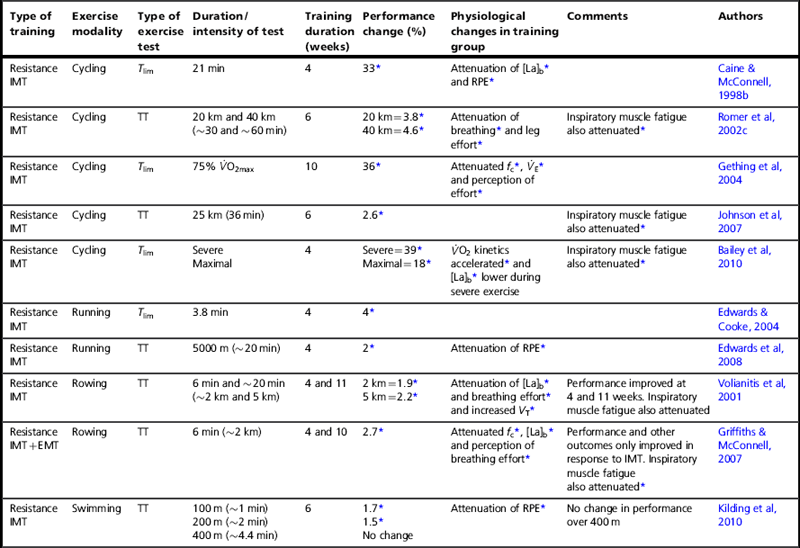
Table 4.1 indicates clearly that inspiratory muscle training (IMT) produces statistically significant improvements in performance, but that expiratory muscle training (EMT) does not. As demonstrated statistically by Illi et al (2012), performance improvements occur whether tested using time trials (1.7% to 4.6% improvement for tests lasting 1 to 60 minutes), or Tlim tests (greater than 30% improvement for a 30-minute test). For higher-intensity, shorter-duration Tlim tests, the improvements are smaller (~ 4% for a test lasting less than 4 minutes). This difference in the size of the improvements occurs because of differences in the factors that lead to people stopping exercise and the rate at which these factors accumulate and lead to intolerance (Currell & Jeukendrup, 2008).
Endurance training of the respiratory muscles involves hyperventilation at high levels for prolonged periods (see Ch. 5). Table 4.2 summarizes the controlled and placebo-controlled trials using endurance RMT. Despite the profound difference in the training method, the results are strikingly similar to those for resistance IMT, a finding confirmed statistically by Illi and colleagues (Illi et al, 2012). Typically, Tlim increases by 20% to 50%, whereas a time trial shows ~ 4% improvement. This similarity is quite unlike the response to whole-body endurance and resistance training, which suggests that breathing muscle training taps into a unique and profoundly important mechanism. As was described in the Chapter 3, the most likely candidate mechanism for this effect is an increase in the threshold for activation of the inspiratory muscle metaboreflex.
Table 4.2
Controlled studies of the influence of endurance breathing muscle training upon endurance performance
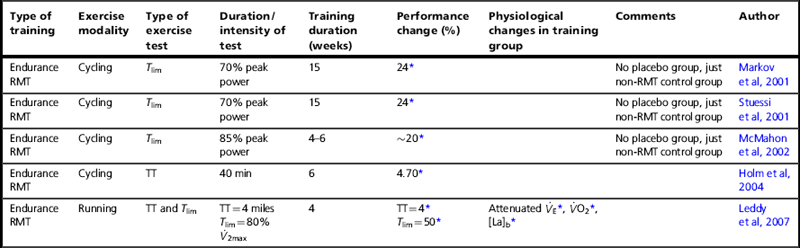
It is impossible to separate the inspiratory and expiratory effects of hyperventilation training, but when this has been done for resistance training the independent roles of the two groups of muscles in performance changes become clear (Griffiths & McConnell, 2007): only IMT improves performance. Indeed, adding EMT to IMT during the same breath cycle seems to impair inspiratory muscle responses to IMT (Griffiths & McConnell, 2007) and to negate any performance benefits (Wells et al, 2005), which provides a strong argument against using this approach or, perhaps, undertaking EMT in healthy young people at all.
Tables 4.1 and 4.2 also summarize some of the physiological changes that accompany RMT, and these help to shed some light on the potential mechanisms that do, and do not, lead to the improvement in performance. Specifically, after IMT there are no changes in the ‘usual suspects’ underpinning improved exercise performance after training, viz., maximal oxygen uptake (![]() O2max) and lactate threshold (see Ch. 2). Since breathing does not limit oxygen diffusion (see Ch. 1), we would not expect
O2max) and lactate threshold (see Ch. 2). Since breathing does not limit oxygen diffusion (see Ch. 1), we would not expect ![]() O2max to improve. However, the absence of a change in lactate threshold is slightly puzzling given that IMT reduces blood lactate concentrations [La]b during exercise at equivalent intensities (see Tables 4.1 and 4.2). It seems that IMT reduces [La]b, but not the intensity of exercise at which accumulation commences, or indeed the critical power, which is a functional correlate of the lactate threshold (Johnson et al, 2007). The mechanism by which steady state [La]b is reduced without an accompanying change in the lactate threshold is an intriguing one. It is common for different people to have different steady-state lactate concentrations but very similar lactate thresholds, so it is clear that the [La]b per se is not a determinant of the lactate threshold. There is good evidence that IMT increases the metabolic potential of the inspiratory muscles, and thus their ability to consume lactate during submaximal exercise (Brown et al, 2011). One might also hypothesize a lower production of lactate by locomotor muscles due to enhanced oxygen delivery, which could arise because of a reduction in sympathetic vasoconstriction. However, improving oxygen delivery in sub-elite athletes by inhaling an oxygen-rich inspirate does not enhance their lactate threshold (Sadowsky et al, 1995). Thus the most likely explanation for the lower steady state [La]b without a change in the lactate threshold is enhanced consumption of lactate by the inspiratory muscles (Peter Brown & Graham Sharpe, personal communication). However, this does not imply that an improved muscle blood flow is redundant. Enhancing muscle blood flow by manipulating the work of breathing reduces exercise-induced locomotor muscle contractile fatigue (Romer et al, 2006). Furthermore, the removal of muscle metabolites may also influence the central contribution to fatigue (see Ch. 1, ‘Mechanisms of fatigue’).
O2max to improve. However, the absence of a change in lactate threshold is slightly puzzling given that IMT reduces blood lactate concentrations [La]b during exercise at equivalent intensities (see Tables 4.1 and 4.2). It seems that IMT reduces [La]b, but not the intensity of exercise at which accumulation commences, or indeed the critical power, which is a functional correlate of the lactate threshold (Johnson et al, 2007). The mechanism by which steady state [La]b is reduced without an accompanying change in the lactate threshold is an intriguing one. It is common for different people to have different steady-state lactate concentrations but very similar lactate thresholds, so it is clear that the [La]b per se is not a determinant of the lactate threshold. There is good evidence that IMT increases the metabolic potential of the inspiratory muscles, and thus their ability to consume lactate during submaximal exercise (Brown et al, 2011). One might also hypothesize a lower production of lactate by locomotor muscles due to enhanced oxygen delivery, which could arise because of a reduction in sympathetic vasoconstriction. However, improving oxygen delivery in sub-elite athletes by inhaling an oxygen-rich inspirate does not enhance their lactate threshold (Sadowsky et al, 1995). Thus the most likely explanation for the lower steady state [La]b without a change in the lactate threshold is enhanced consumption of lactate by the inspiratory muscles (Peter Brown & Graham Sharpe, personal communication). However, this does not imply that an improved muscle blood flow is redundant. Enhancing muscle blood flow by manipulating the work of breathing reduces exercise-induced locomotor muscle contractile fatigue (Romer et al, 2006). Furthermore, the removal of muscle metabolites may also influence the central contribution to fatigue (see Ch. 1, ‘Mechanisms of fatigue’).
So far as the factors that do change after IMT are concerned, these include reductions in [La]b, heart rate, and perception of breathing and limb effort, as well as a speeding of oxygen uptake kinetics during heavy exercise. In addition, breathing becomes deeper and slower, as well as more metabolically efficient (Turner et al, 2012). Perhaps most importantly, IMT delays or abolishes the exercise-induced decrease in MIP observed post-exercise, i.e., IMT attenuates inspiratory muscle fatigue (Romer et al, 2002d). These changes are all consistent with the respiratory-related exercise-limiting factors described in Chapter 3. The attenuation of inspiratory muscle fatigue is indicative of an improvement in the functional capacity of the respiratory pump muscles. Since metabolite accumulation contributes to deficits in the ability to produce muscular force at both a peripheral and a central level (see Ch. 2, ‘Mechanisms of fatigue’), the attenuation of inspiratory muscle fatigue is consistent with a reduction and / or delay in both metabolite accumulation and activation of the inspiratory muscle metaboreflex. Activation of this reflex induces vasoconstriction in the locomotor muscles, but IMT has been shown to increase the intensity of inspiratory muscle work required to activate this reflex (McConnell & Lomax, 2006; Witt et al, 2007; Bailey et al, 2010), and to reduce La production by the inspiratory muscles (Brown et al, 2011). As a result, muscle blood flow may be preserved, and limb fatigue attenuated or delayed (McConnell & Lomax, 2006). This mechanism may also explain the reduction in [La]b, and leg effort, as better-perfused locomotor muscles generate less lactate and have lower levels of metabolites to stimulate the group III and IV afferents that contribute to effort perception (Amann et al, 2010) and fatigue (Gandevia, 2001; Amann et al, 2009) (see Ch. 3). In addition, if inspiratory muscle fatigue is attenuated the perception of breathing effort will be reduced, making it possible to maintain a more efficient deep, slow breathing pattern (Turner et al, 2012). In short, the physiological changes point strongly to the ergogenic effect of IMT being underpinned by preservation of limb blood flow and reduction in breathing effort. These effects are arguably even more important in patients, since exercise tolerance is frequently limited by dyspnoea and / or muscle dysfunctions that are exacerbated by impaired blood flow.
In respect of the inspiratory muscle metaboreflex, there is one particular study of IMT that is worthy of detailed scrutiny because it has particular relevance to patients. The study in question not only demonstrated that IMT extends Tlim, but it also demonstrated that oxygen uptake kinetics were hastened during ‘severe’- and ‘maximal’-intensity cycling (Bailey et al, 2010), a finding that has since been confirmed (Brown et al, 2011). The authors’ interpretation was that their observations were due to ‘increased blood flow to the exercising limbs’, and that this arose because of the absence of inspiratory muscle metaboreflex activation post-IMT (Bailey et al, 2010). These findings are especially relevant to patients since the oxygen uptake kinetics of patients are slowed by disease and inactivity (Chiappa et al, 2008a). The speed of this ‘on transient’ of the oxygen uptake kinetics determines the size of the oxygen deficit during exercise, and thus the size of the anaerobic contribution at the onset of exercise (see Ch. 1). Enhancing the amount of energy liberated from aerobic sources minimizes the production of fatiguing metabolic by-products, and reduces the ventilatory requirement for exercise. In patients for whom walking constitutes ‘severe’-intensity exercise, and who are consequently teetering on the edge of the ‘anaerobic abyss’, this effect on oxygen uptake kinetics may be particularly important and make a disproportionate contribution to improving exercise tolerance. Interestingly, reducing the work of breathing using a low-density inspirate (heliox) speeds oxygen uptake kinetics and improves exercise tolerance in patients with COPD (Chiappa et al, 2009); this response is strikingly similar to that observed in healthy people following IMT (Bailey et al, 2010; Brown et al, 2011).
Finally, there has long been speculation that IMT may enhance the mechanical efficiency of breathing, as some studies have shown small (often non-significant) decreases in the oxygen cost of exercise (Romer et al, 2002a; Griffiths & McConnell, 2007; Turner et al, 2011). A recent study found that 6 weeks of IMT (30 repetitions against a load equivalent to 50% of MIP) reduced the oxygen cost of hyperpnoea by 5–12% (Turner et al, 2012). The greatest decrement in oxygen cost was seen at the highest intensity of respiratory muscle work. This finding has important implications for patients in whom disease-related impairments of breathing mechanics increase the oxygen cost of breathing. Since the latter may account for a considerable proportion of available oxygen uptake, reducing this burden may release oxygen for use by other muscle groups, thereby enhancing exercise tolerance.
In summary, the research using time trials and tests of endurance indicates that, for bouts of exercise involving a sustained effort against the clock of 1 to 60 minutes duration, IMT improves performance significantly by 1.7% to 4.6%. Although there is no direct research evidence that similar improvements will be seen during longer events (e.g., marathons or triathlons), the fact that a decline in MIP has been demonstrated after such events means that performance in these events may be limited by breathing-related factors. Accordingly, there is every reason to believe that IMT will improve performance in events lasting more than 1 hour. The mechanisms that underlie the changes in performance described above are applicable universally and have equal, if not greater, relevance to exercise-limited patients. Indeed, Illi and colleagues’ systematic review revealed that the ergogenic effect of RMT was greatest in the least fit participants (Illi et al, 2012).
Influence of RMT upon tests of anaerobic endurance and sprinting
Sprinting is such a brief activity that the benefits of IMT are not immediately obvious. However, the changes that IMT induces in underlying physiology appear to be so fundamental that it is now clear that performance in sprint tasks can also benefit from IMT. This is relevant to patients because the metabolic demands of activities of daily living can be akin to those of a sprint in a young healthy individual. In the previous section we have already discussed the fact that maximal cycling to the limit of tolerance is extended significantly following IMT, and that this was accompanied by a significant speeding up of oxygen uptake kinetics (Bailey et al, 2010; Brown et al, 2011). Repeated sprinting may not be something that many patients engage in during their everyday lives, but it is encountered in interval training, which is finding increasing favour within rehabilitation programmes (Mador et al, 2009). Thus, the results of studies on repeated sprinting suggest potential benefits of IMT for tolerance to repeated bouts of anaerobic exercise, such as interval training. Improving the tolerability of training interventions has the benefit of enhancing the potency of the training stimulus, and the resulting performance benefits. Indeed, it has been shown that athletes undertaking a period of IMT prior to a programme of interval training were able to train significantly harder, and showed significantly greater improvements in repeated sprint performance, compared with a group that did not receive prior IMT (Tong et al, 2010). Unfortunately, there was no placebo control within this trial.
In the context of team sports, a sense of increased breathing effort between sprints has a profound influence on a player’s ability to sprint again, and in competition this has implications for the quality of the athlete’s contribution to the match or game. For this reason, the first study to examine the effect of IMT on sprinting used perceived rate of recovery during continuous bouts of repeated sprinting (Romer et al, 2002b). The expectation was that IMT would reduce breathing effort between sprints and delay the onset of inspiratory muscle fatigue, thereby making the participants feel as if they had recovered more quickly. However, because the sprint was very brief (3.2 seconds) and punctuated with periods of recovery, there was no expectation that actual sprint performance would improve. These expectations were confirmed; after 6 weeks of IMT (30 repetitions against a load equivalent to 50% of MIP) the athletes showed a significantly faster rate of recovery compared with baseline and placebo, but no change in sprint performance. The potential relevance of these findings for a patient who is limited acutely by dyspnoea is that IMT may reduce the duration that they must spend ‘catching their breath’; this hypothesis awaits evaluation.
Using a slightly different approach, two controlled studies (one of which was placebo controlled) have explored the benefits of IMT to the ability to sustain repeated sprinting, with gradually escalating sprint speed, and short active recovery breaks between sprints (Yo-Yo test) (Bangsbo et al, 2008). Performance in a test of this kind is influenced by both effort perception and factors related to limb blood flow, such as oxygen delivery and metabolite removal. As has been explained previously, both of these factors are potentially influenced by IMT, via its hypothesized effects upon breathing effort and activation of the inspiratory muscle metaboreflex. After 5–6 weeks of IMT (30 repetitions against a load equivalent to 50% of MIP), both studies found that performance in the Yo-Yo test improved significantly by ~ 17% (Tong et al, 2008; Nicks et al, 2009). Accompanying the improvement in performance were significant reductions in perception of breathing and whole-body effort, as well as markers of metabolic stress. In a more recent study, the distribution of blood flow to the legs and respiratory muscles during repeated sprinting has been studied (Tong et al, 2012). As the sprint test progressed, minute ventilation showed a progressive increase and, at the point where respiratory compensation for the developing metabolic acidosis commenced, there was a clear reduction in the oxygenation of both the respiratory and leg muscles. The timing of the two events was correlated significantly. These data are consistent with activation of the inspiratory muscle metaboreflex by the escalating ventilatory demand, followed by vasoconstriction of both the respiratory and leg muscle vasculature. Thus, as suggested for endurance exercise, IMT may improve performance during repeated sprinting by increasing the intensity of respiratory muscle work required to activate the metaboreflex, thereby preserving limb blood flow (McConnell & Lomax, 2006; Witt et al, 2007; Bailey et al, 2010).
Most recently, the effects of combining IMT with a specific inspiratory muscle warm-up (see Ch. 6) have been tested in a placebo-controlled trial on semi-professional football players (Lomax et al, 2011). The same IMT protocol and repeated sprint test were used as in previous studies; after 4 weeks of IMT, sprint performance increased significantly by 12%. At baseline, the warm-up increased sprint performance significantly by 5–7% in both groups, which is consistent with previous findings (Tong & Fu, 2006). When an inspiratory muscle warm-up was added after IMT, performance increased significantly by a further 2.9%. Thus, the benefit of the inspiratory muscle warm-up and IMT were additive (14.9%).
Influence of RMT under hypoxic conditions
The addition of an hypoxic drive to breathe during exercise places a large additional demand upon the respiratory muscles. Therefore, the potential benefits of RMT are arguably even greater to people exercising in an hypoxic environment, or those who have hypoxaemia due to the effects of disease. To date, only one placebo-controlled study has assessed the influence of IMT on exercise tolerance and physiological responses to exercise in hypoxia (Downey et al, 2007). The results of the randomized controlled study were both impressive and surprising. The IMT group trained for 40 breaths, 5 days per week at a load of ~ 50% of MIP, whilst the placebo group used the same regimen at a load of 15% of MIP. The hypoxic environment simulated an altitude of ~ 3500 metres (~ 12 000 feet), which was sufficient to reduce arterial saturation to ~ 93% at rest and ~ 77% at end of exercise. Following 4 weeks of IMT, minute ventilation, cardiac output and oxygen uptake of hypoxic treadmill exercise were reduced significantly in the IMT group by 25%, 14% and 8–12% respectively. Despite the lower minute ventilation, arterial oxygen saturation (SaO2) and lung-diffusing capacity were increased by 4% and 22%, respectively. Perceived exertion and dyspnoea were also reduced significantly. No such changes were observed in the placebo group. The changes in minute ventilation and SaO2 appear to be completely at odds with one another, as an increase in SaO2 normally requires an increase in minute ventilation. The clue to resolving this paradox resides in the improvement in the diffusing capacity of the lungs, which can increase only if the lung diffusion surface area increases. The pulmonary vasculature is responsive to muscle metaboreflex activation (Lykidis et al, 2008). Thus, if IMT were to delay or abolish activation of the inspiratory muscle metaboreflex, it is conceivable that blood flow would be preserved in the pulmonary circulation. Should this occur, an increase in diffusion surface area would result, thereby increasing SaO2. The effect of IMT upon SaO2 in hypoxic conditions has been confirmed under resting conditions in a randomized controlled study of military personnel during an expedition to the Nepali Himalayas (Lomax, 2010). At altitudes of 4880–5550 metres, the resting SaO2 was 6% higher in the IMT group compared with the control group. This protective effect upon SaO2 was not observed at 1400 metres.
DISEASE-SPECIFIC FUNCTIONAL RESPONSES TO RMT
Respiratory disease
Chronic obstructive pulmonary disease
Respiratory muscle training has been studied most extensively in patients with COPD, and the type of intervention implemented has overwhelmingly been inspiratory muscle resistance training (IMT). The first published studies appeared in the late 1970s and the number of studies appearing each year has grown steadily, such that the most recent meta-analysis of this literature included a total of 32 studies (Gosselink et al, 2011); however, these studies represent only the [high-quality] tip of an IMT research iceberg. As was explained in Chapter 3, the rationale for IMT in patients with COPD is the restoration of balance in the demand / capacity relationship of the respiratory pump muscles. Patients with COPD have primary weakness of inspiratory muscles, which is exacerbated by functional weakness. In addition, there is an increased demand for inspiratory muscle work, owing to changes in breathing mechanics and the elevated ventilatory requirement for exercise. The primary exercise-limiting symptom under these conditions is dyspnoea.
The extensive nature of the literature addressing IMT means that there has been a systematic review (Shoemaker et al, 2009) and two meta-analyses (Geddes et al, 2008; Gosselink et al, 2011) published since 2008. Accordingly, it is possible to draw conclusions about the usefulness of IMT in patients with COPD based upon statistical evidence. The three reviews are in agreement, indicating that IMT improves: inspiratory muscle strength (MIP) and endurance, exercise capacity (6-minute walk distance), dyspnoea, and quality of life. Gosselink et al (2011) found the effect sizes of these responses to be ‘medium to large’. In sub-analyses, inspiratory muscle strength training was found to be superior to endurance training for improving exercise capacity and dyspnoea. In addition, patients with the lowest MIP at baseline showed the greatest improvements in MIP and exercise capacity.
An area that has so far received very little attention is the impact of IMT upon the use of healthcare resources. As part of a 12-month placebo-controlled trail of IMT, Beckerman et al (2005) not only noted improvements in MIP, exercise capacity, dyspnoea and quality of life, but also observed significant differences between the IMT group and the placebo group in the number of primary care consultations and days spent in hospital across the intervention. There was also a difference in the number of patients admitted and the total number of admissions, but these failed to reach significance. These data suggest that IMT may reduce the number of exacerbations and hasten recovery from exacerbations. However, further research is needed in order to clarify these potential benefits.
A question that remains unclear at present is the best way to incorporate IMT into a rehabilitation programme. As was described above, there is robust evidence that IMT can be used as a stand-alone intervention to improve inspiratory muscle strength and endurance, exercise capacity, dyspnoea and quality of life. However, it remains unclear whether IMT provides additional improvements when added to a rehabilitation programme that includes whole-body exercise. Nevertheless, based upon the evidence of Lotters et al (2002) and O’Brien et al (2008), addition of IMT to a general exercise programme for patients with inspiratory muscle weakness is recommended by the joint BTS / ACPRC Guidelines (Bott et al, 2009).
A recent systematic review comparing IMT and rehabilitation revealed that, compared with exercise alone, improvement in MIP and one index of exercise tolerance were superior if IMT was added to exercise (O’Brien et al, 2008). Interestingly, when comparing IMT with exercise training, the study also found ‘no significant difference in effect for outcomes of MIP and exercise tolerance among patients with COPD who engage in IMT compared with exercise’ (O’Brien et al, 2008). In other words, IMT appears to improve exercise tolerance as much as exercise. Furthermore, a systematic review of home-based physiotherapy interventions for patients with COPD found that home-based IMT significantly improved dyspnoea (on the Transitional Dyspnoea Index) by 2.36 units compared with the control (Thomas et al, 2010). These findings suggest that IMT is almost certainly a more cost-effective intervention than exercise for improving dyspnoea, as it can be implemented in a domiciliary setting with minimal supervision. It is also pertinent to recall that, in healthy people, implementing IMT prior to whole-body training improved the outcome of the whole-body training (Tong et al, 2010); thus IMT might be considered as ‘pre-habilitation’ for patients awaiting commencement of exercise training.
Expiratory muscle training has received very little attention. To date, there have been only two placebo-controlled studies examining the effect of EMT in patients with COPD: one using specific EMT (Weiner et al, 2003b), and the other comparing responses with EMT, IMT and combined IMT and EMT (Weiner et al, 2003a). In the EMT study, expiratory muscle strength (MEP) and endurance increased significantly, as did exercise capacity. There was no significant change in the sensation of dyspnoea during daily activities. In the second of the two studies (Weiner et al, 2003a), eight patients were assigned to receive EMT, eight received IMT, eight received EMT + IMT and a group of eight was assigned to be a placebo group. There were no changes in the placebo group, but training induced statistically significant, specific increases in MEP and endurance (in the EMT and in the EMT + IMT groups) and in MIP and endurance (in the IMT and EMT + IMT groups). Exercise capacity increased in all three training groups. However, the increase in the IMT and the EMT + IMT groups was significantly greater than that in the EMT group. There was also a decrease in dyspnoea in the IMT and in the EMT + IMT groups, but not in the EMT or placebo groups. It was concluded that the inspiratory and expiratory muscles can be trained specifically to improve both strength and endurance. However, there appears to be no additional benefit gained by combining IMT + EMT, compared with IMT alone.
Finally, there are two specific, less obvious applications of IMT that are worthwhile mentioning. The first relates to the observation that repeated, deep inhalations against an inspiratory load have been found to be twice as effective (as measured by sputum weight) as standardized physiotherapy consisting of postural drainage and the active cycle of breathing technique (Chatham et al, 2004). Thus IMT may also facilitate airway clearance. The second application is in promoting the ability of patients to use inhalers. Dry-powder inhalers (DPI) have been suggested to be more appropriate for patients with coordination difficulties. However, these often have a much higher inherent flow resistance, thus rendering them unusable by very weak patients; one study found that 20% of patients with COPD were unable to generate the required inspiratory flow rate to generate the required flow rate via the Turbohaler® DPI (Weiner & Weiner, 2006). Furthermore, the flow rates achieved through the DPI were correlated with MIP. After the patients had received an 8-week period of IMT, all were able to generate the required flow rate via the Turbohaler®. Thus IMT may be a useful to tool enable and improve DPI use by very weak patients.
In summary, the strength of evidence supporting the efficacy of RMT, and in particular IMT, in patients with COPD has achieved a level where it is possible to recommend IMT as a stand-alone treatment. Improvements in inspiratory muscle strength and endurance, exercise capacity, dyspnoea and quality of life can be expected. Questions remain regarding whether the addition of IMT to exercise training provides any supplemental benefits, but the preliminary indications are that outcomes such as dyspnoea are improved to a greater extent by implementing the two interventions in parallel. The role of EMT is much less clear, owing to a lack of studies. Patients with the weakest inspiratory muscles appear to show the greatest improvements following IMT, but there is no minimum threshold above or below which benefits can, or cannot, be anticipated. Furthermore, acute bouts of loaded breathing may also facilitate airway clearance. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Asthma
Inspiratory muscle training has been studied less extensively in patients with asthma than in those who have COPD. As was presented in Chapter 3, the rationale for IMT in patients with asthma is the restoration of balance in the demand / capacity relationship of the respiratory pump muscles, which are overloaded when airway obstruction is present. The primary symptom under these conditions is dyspnoea. To date, there has been one attempt at a Cochrane systematic review (Ram et al, 2003), which included just five studies. The review concluded that there were insufficient data, and further research was required. However, the analysis was able to confirm that IMT yielded significant improvements in MIP. Unfortunately, since 2003 there has been only one further study of IMT in people with asthma. The findings of the available literature are summarized below, and are also placed in a mechanistic context.
A notable feature of airway obstruction in asthma is the large inter-subject variation in the intensity of dyspnoea for a given fall in FEV1 (Lougheed et al, 1993). Furthermore, women appear to experience higher levels of dyspnoea (Wijnhoven et al, 2003), poorer quality of life and more frequent hospital admission than do men (Weiner et al, 2002c). These observations led Weiner and colleagues to reason that the gender difference might be explained, at least partially, by the fact that women have weaker inspiratory muscles than men (Weiner et al, 2002c). Accordingly, the influence of gender and inspiratory muscle strength (MIP) was examined by comparing the MIP, perception of dyspnoea to threshold loads, and bronchodilator consumption of 22 male and 22 female asthmatic patients with mild-to-moderate bronchoconstriction (FEV1 > 60% of predicted) (Weiner et al, 2002c). For the same FEV1 (% of predicted), the women had significantly weaker inspiratory muscles, whilst dyspnoea during loaded breathing and β2-agonist consumption was significantly higher than in the men. The women were divided randomly into two groups: half received IMT, and the remainder received sham training. After 20 weeks of IMT, the MIP of the women (+ 42%) matched that of the men. Accompanying this change was a reduction in the dyspnoea during loaded breathing and the β2-agonist consumption of the women in the IMT group, compared with that of the placebo group. Furthermore, after IMT, dyspnoea and β2-agonist consumption were no longer different to those in the men.
The strong interrelationship between MIP, dyspnoea and β2-agonist consumption was confirmed in a study designed to examine their interrelationships specifically (Weiner et al, 2002b). There was no correlation between baseline measures of MIP and intensity of dyspnoea during loaded breathing in a sample of 30 patients. However, after IMT both the total medications use and the perception of dyspnoea during loaded breathing showed a direct, quantitative relationship with the increase in MIP. Indeed, 93% of the change in β2-agonist consumption was explained by the change in MIP. These findings support a role for the absolute strength of the inspiratory muscles in determining the consumption of medication, and dyspnoea.
Critics will argue that changes in dyspnoea during loaded breathing are not the same as exertional dyspnoea, and they would be correct. However, there is evidence that exertional dyspnoea is also attenuated after IMT in people with asthma (12.4% reduction), and after as little as 3 weeks of IMT (McConnell et al, 1998). Most recently, a comprehensive study of IMT using a matched double-blind placebo-controlled design confirmed that exertional dyspnoea was reduced (by 16%) after 6 weeks of IMT (Turner et al, 2011); it also noted further benefits, which included an improvement in exercise tolerance, attenuation of exercise-induced decrease in MIP, and a lower oxygen cost of exercise (reduced by 12% at Tlim). The latter was presumed by the authors to derive from improvements in the mechanical efficiency of breathing, which derives some support from their later finding that in healthy people the oxygen cost of voluntary hyperpnoea is reduced by as much 12% after IMT (Turner et al, 2012).
An impressive feature of studies of IMT in patients with asthma is the reduction in β2-agonist consumption that accompanies IMT. In the three studies in which this has been examined (Weiner et al, 1992; Weiner et al, 2000; Weiner et al, 2002c), this reduction has ranged from 38% to 78%, being greatest in those with the highest baseline consumption. Thus, IMT appears to be particularly helpful for patients with high levels of dyspnoea and β2-agonist consumption. Furthermore, a double-blind placebo-controlled trial of IMT conducted over 6 months also observed significant improvements in lung function, asthma symptoms, hospitalizations for asthma and absence from school or work (Weiner et al, 1992).
However, a note of caution is warranted at this point, because there are a small group of patients with asthma for whom further reductions in the intensity of dyspnoea sensation may be life threatening. According to one study, around 26% of patients with asthma have abnormally low perceptions of dyspnoea (Magadle et al, 2002); this was associated with low consumption of medication, increased emergency department visits, hospitalizations, near-fatal asthma attacks, and deaths during follow-up (Magadle et al, 2002). A second study confirmed the association between near-fatal asthma and low perceptions of dyspnoea, as well as blunted hypoxic sensitivity (Kikuchi et al, 1994). It would therefore be inappropriate to implement IMT in this subgroup. However, the available evidence suggests that patients with normal or high sensation of dyspnoea do not become desensitized to bronchoconstriction following IMT. Weiner et al (2000) noted that IMT did not result in exaggerated ablation of dyspnoea, and concluded that IMT was safe, at least for use in patients with mild asthma.
In summary, the strength of evidence supporting the efficacy of IMT in patients with asthma has not yet achieved that of COPD. This is primarily because of the small number of studies available to date. There is some evidence that dyspnoea and use of medication are greatest in those with the weakest inspiratory muscles (lowest MIP). Preliminary evidence also suggests that, irrespective of MIP, IMT induces improvements in inspiratory muscle strength, exercise capacity, dyspnoea and use of medication. Patients with abnormally low dyspnoea perception are unsuitable candidates for IMT, but there appears to be no evidence that inspiratory muscle weakness is a prerequisite to improvements following IMT. Furthermore, acute bouts of loaded breathing may also facilitate airway clearance. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Bronchiectasis
Bronchiectasis is a chronic lung disease that is not normally included within the umbrella of COPD. However, the functional manifestations of bronchiectasis have similarities with those of COPD, including airway obstruction, which leads to detrimental changes in breathing mechanics, attendant exertional dyspnoea and exercise intolerance (Neves et al, 2011). Patients with moderate-to-severe bronchiectasis also have slight respiratory muscle weakness (Moran et al, 2010). It is therefore reasonable to hypothesize that responses to IMT in patients with bronchiectasis would be similar to those of patients with COPD.
To date there have been just two studies in which patients with bronchiectasis have been subjected to IMT. In the first study, moderate-intensity IMT (15 minutes breathing against 30–60% of MIP) was undertaken for 8 weeks in combination with a programme of physical exercise (EX + IMT). The responses of this IMT group were compared with those of a group who received exercise and sham IMT (EX + sham), as well as a control group (Newall et al, 2005). There was a significant increase in MIP in both training groups (18% and 33% for exercise and EX + IMT, respectively), but the increase was not significantly different between the groups. Exercise tolerance improved significantly in both training groups during a treadmill Tlim test and in an incremental shuttle walking test; but the changes were not significantly different between groups. Thus adding IMT to exercise training in these patients did not result in significantly greater improvements in MIP of exercise tolerance. However, a key finding of this study was that, 3 months after cessation of the interventions, exercise tolerance was maintained in the EX + IMT group but not in the EX + sham group. In addition, the EX + IMT group was the only group to show an improved quality of life score, which was also maintained after cessation of training. It is noteworthy that the improvements in all parameters were largest in the EX + IMT group, but it is important to bear in mind that the assessment of IMT was made on an improving baseline of function due to the exercise training, which was implemented in both the IMT and sham groups; the effect of this would be to reduce the effect size of the IMT. Finally, the lack of statistically significant difference between EX + sham and EX + IMT may be due to lack of statistical power, due to the small sample size (~ 10 participants per group). The failure to distinguish an additional benefit of adding IMT to exercise has also been observed in patients with COPD, but a recent systematic review concluded that ‘[in COPD] Results showed significant improvements in maximum inspiratory pressure and maximum exercise tidal volume favoring combined IMT and exercise compared with exercise alone’ (O’Brien et al, 2008). Conclusions regarding other outcomes await further data.
In a more recent study (Liaw et al, 2011), a low-intensity IMT regimen (30 minutes breathing against 30–38% of MIP) was evaluated in 13 patients, and compared with 13 patients who did no training at all. Both MIP and MEP increased in the IMT group (39% and 44%, respectively), and although there was a significant increase in 6-minute walk distance (14.8%), this just failed to be significantly different from that of the control group. Interpretation of these data is hampered by lack of statistical power, and the absence of a placebo control. Taken together, the two studies provide preliminary evidence that IMT may elicit similar improvements to those shown in patients with COPD. The joint BTS / ACPRC Guidelines make the following recommendation in relation to IMT in bronchiectasis: ‘Consider the use of inspiratory muscle training in conjunction with conventional pulmonary rehabilitation to enhance the maintenance of the training effect’ (Bott et al, 2009).
Finally, it is worthwhile mentioning that some IMT studies on patients with CF have reported improvements in expectoration immediately after IMT (Asher et al, 1982; Enright et al, 2004); indeed, repeated, deep inhalations against an inspiratory load have been found to be twice as effective (measured by sputum weight) as standardized physiotherapy consisting of postural drainage and the active cycle of breathing technique (Chatham et al, 2004). Thus IMT may also facilitate airway clearance in patients with bronchiectasis.
In summary, interpretation of the evidence relating to the efficacy of IMT in patients with bronchiectasis is hampered by the very small number of studies. Taken together, the two available studies provide preliminary evidence that IMT may elicit similar improvements to those shown in patients with COPD. Further research on IMT as a stand-alone intervention is justified, as the theoretical rationale for IMT in patients with bronchiectasis is very similar to that for patients with COPD. Furthermore, acute bouts of loaded breathing may also facilitate airway clearance. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Cystic fibrosis
Although there are a handful of studies of RMT in patients with CF, one of these failed to include a control group (Keens et al, 1977) and one utilized an unreliable method of training (Asher et al, 1982). Of the remaining three, only two studies (de Jong et al, 2001; Enright et al, 2004) met the criteria to be included in a recent systematic review (Reid et al, 2008). Although inevitably limited, this review represents the most up-to-date picture of the influence of IMT upon patients with CF (Reid et al, 2008). Analysis of both studies revealed no significant effects of IMT upon lung function or inspiratory muscle strength (MIP). However, these findings cannot be taken at face value as some important features of the two studies differed. In one study, the training regimen was 20–40% of MIP sustained for 20 minutes (de Jong et al, 2001). This constitutes an endurance training regimen; indeed, endurance increased significantly, but not strength – a finding ascribed by the authors to the low intensity of the training. Accordingly, these data should be interpreted cautiously in respect of the efficacy of IMT. In the other study within the review (Enright et al, 2004), a high-intensity inspiratory muscle-strength-training programme was implemented. This high-intensity training elicited improvements in MIP (18%) and endurance, as well as diaphragm thickness. Furthermore, lung function, incremental cycle performance and psychological status were also improved significantly. Whilst seemingly contradictory, the systematic review of these two studies most likely highlights the differing efficacy of the two types of training implemented.
It is not clear why the fifth study was excluded from the systematic review, but this may be because it was not a randomized trial (Sawyer & Clanton, 1993). However, it was a placebo-controlled trial and therefore merits consideration. After 10 weeks of IMT using a moderate load (50–60% of MIP), there were significant improvements in MIP (13%), lung function and exercise tolerance. The greater training intensity and longer duration of the intervention may explain the discrepancy between this study and that of de Jong et al (2001).
Finally, two IMT studies on patients with CF have reported improvements in expectoration immediately after IMT (Asher et al, 1982; Enright et al, 2004); indeed, repeated deep inhalations against an inspiratory load have been found to be twice as effective (measured by sputum weight) as standardized physiotherapy consisting of postural drainage and the active cycle of breathing technique (Chatham et al, 2004). It has been suggested that the effect is similar to that seen after exercise. This finding may have implications for other patient groups in which airway clearance is problematic.
In summary, interpretation of the evidence relating to the efficacy of IMT in patients with CF is hampered by the very small number of studies. When viewed collectively, the data from placebo-controlled trials suggest that, when moderate- to high-intensity loading is used, MIP improves by 13–18% and is accompanied by a range of clinically significant benefits. Further research on IMT as a stand-alone intervention is justified as there is a good theoretical rationale for IMT in patients with CF (see Ch. 3). Furthermore, acute bouts of loaded breathing may also facilitate airway clearance. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Restrictive chest wall disorders
Conditions such as kyphoscoliosis, fibrothorax, thoracoplasty, flail chest and ankylosing spondylitis create a restrictive pulmonary defect in which total respiratory system elastance and resistance are elevated (Donath & Miller, 2009). Furthermore, inspiratory muscle function also tends to be impaired (Lisboa et al, 1985; Cejudo et al, 2009) owing to changes in chest wall and diaphragm configuration.
Given the obvious imbalance in the demand / capacity relationship of the respiratory pump, it is surprising that there has been only one randomized controlled trial of IMT in patients with restrictive chest wall disease (Budweiser et al, 2006). Thirty patients with restrictive lung disorders who were receiving intermittent non-invasive positive-pressure ventilation (NPPV) took part. Half underwent 3 months of endurance RMT (a minimum of 10 minutes of hyperpnoea training), and half sham RMT (incentive spirometry). Surprisingly, endurance RMT induced significant improvements in MIP (27%), which may be because the sample had such low baseline function (42 ± 14.6 cmH2O). Peak ![]() O2, peak cycle power and health-related quality of life also improved significantly compared with the sham group. Pulmonary function, 6-minute walk distance and blood gases remained unchanged. Most recently there has been an uncontrolled study of ‘breathing exercises’ in 22 patients with ankylosing spondylitis. These exercises consisted of diaphragmatic breathing, pursed lip breathing, and thoracic expansion exercises. The patients also used an incentive spirometer equipped with a volume-oriented sustained inhalation (Ortancil et al, 2009). There were significant increases in chest expansion (16%), MIP (9%), MEP (16%) and Bath Ankylosing Spondylitis Functional Index scores after 6 weeks of breathing exercise, but the 6-minute walking distance did not change. The absence of a control group and the unusual nature of the intervention make these data difficult to interpret. The data hint that it may be possible to improve chest wall mobility – which merits further exploration using IMT, which has been shown to increase vital capacity in other conditions. The potential role in improving exertional dyspnoea and exercise tolerance remains unclear as neither study showed a change in 6-minute walking distance. Notwithstanding this, the joint BTS / ACPRC Guidelines make the following recommendation in relation to RMT in patients with kyphoscoliosis: ‘Consider the use of respiratory muscle training’ (Bott et al, 2009).
O2, peak cycle power and health-related quality of life also improved significantly compared with the sham group. Pulmonary function, 6-minute walk distance and blood gases remained unchanged. Most recently there has been an uncontrolled study of ‘breathing exercises’ in 22 patients with ankylosing spondylitis. These exercises consisted of diaphragmatic breathing, pursed lip breathing, and thoracic expansion exercises. The patients also used an incentive spirometer equipped with a volume-oriented sustained inhalation (Ortancil et al, 2009). There were significant increases in chest expansion (16%), MIP (9%), MEP (16%) and Bath Ankylosing Spondylitis Functional Index scores after 6 weeks of breathing exercise, but the 6-minute walking distance did not change. The absence of a control group and the unusual nature of the intervention make these data difficult to interpret. The data hint that it may be possible to improve chest wall mobility – which merits further exploration using IMT, which has been shown to increase vital capacity in other conditions. The potential role in improving exertional dyspnoea and exercise tolerance remains unclear as neither study showed a change in 6-minute walking distance. Notwithstanding this, the joint BTS / ACPRC Guidelines make the following recommendation in relation to RMT in patients with kyphoscoliosis: ‘Consider the use of respiratory muscle training’ (Bott et al, 2009).
Finally, it is worthwhile mentioning that repeated, deep inhalations against an inspiratory load have been found to be twice as effective (measured by sputum weight) as standardized physiotherapy consisting of postural drainage and the active cycle of breathing technique (Chatham et al, 2004). Thus, IMT may also facilitate airway clearance.
In summary, interpretation of the evidence relating to the efficacy of RMT in patients with restrictive chest wall disorders is hampered by the very small number of studies. The single placebo-controlled trial suggests that endurance RMT in patients receiving intermittent NPPV may improve peak exercise capacity and health-related quality of life, but not 6-minute walking distance. Further research on strength IMT as a stand-alone intervention is justified, since there is a good theoretical rationale for IMT in patients with restrictive chest wall disorders (see Ch. 3). Furthermore, acute bouts of loaded breathing may also facilitate airway clearance. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Chronic heart failure
The literature relating to RMT in patients with chronic heart failure (CHF) has been growing steadily over the past decade. The first study to examine RMT in patients with CHF appeared in the literature in 1995 (Mancini et al, 1995). The 3-month intervention combined isocapnic hyperpnoea, maximum static inspiratory efforts and pressure threshold loading. Training produced improvements in the MIP (37%) and endurance of the respiratory muscles (57%), as well as alleviating dyspnoea during activities of daily living. Exercise performance during submaximal and maximal exercise tests was also enhanced. The authors suggest that RMT may provide ‘a simple and useful adjunct to medical therapy’ for patients with CHF. These early findings were replicated either fully or in part over the course of the following decade using pressure threshold IMT (Cahalin et al, 1997; Johnson et al, 1998; Weiner et al, 1999; Martinez et al, 2001). Typically, MIP improves 25–30% over an 8- to 12-week period, whereas the magnitude of changes in exercise tolerance and dyspnoea varies according to the tests used; typically 6-minute walk distance improves by around 20–30% (Mancini et al, 1995; Weiner et al, 1999).
More recently, further studies have consolidated and extended the earlier findings (Laoutaris et al, 2004; Dall’Ago et al, 2006; Padula et al, 2009; Bosnak-Guclu et al, 2011; Mello et al, 2012). Typically, IMT improves MIP and inspiratory muscle endurance, reduces exertional dyspnoea, increases exercise tolerance and improves quality of life. Four systematic reviews have examined the influence of IMT, one focussing upon its effect upon quality of life (Sbruzzi et al, 2012) whereas the other three also examined exercise tolerance (Lin et al, 2012; Plentz et al, 2012; Smart et al, 2012). Sbruzzi and colleagues included four studies in their analysis, concluding that, on balance, these did not support a significant effect of IMT upon quality of life; however, they suggested that further research is required. In contrast, Smart and colleagues included 11 studies (148 IMT participants and 139 placebo or non-training participants). Significant effects were identified for MIP, peak oxygen uptake (1.8 ml·min− 1.kg− 1, 9.2%), 6-minute walk distance (34 metres), quality of life (12.2 units) and ![]() E–
E–![]() CO2 gradient (an index of ventilatory efficiency). Based upon comparison of effect sizes with previous meta-analyses of exercise training in patients with CHF, the authors concluded that: ‘IMT improves cardiorespiratory fitness and quality of life to a similar magnitude to conventional exercise training and may provide an initial alternative to the more severely deconditioned CHF patients who may then transition to conventional [exercise training]’. Similar findings for outcomes relating to MIP, exercise tolerance and dyspnoea were also reported in the systematic reviews by Lin et al (2012) and Plentz et al (2012).
CO2 gradient (an index of ventilatory efficiency). Based upon comparison of effect sizes with previous meta-analyses of exercise training in patients with CHF, the authors concluded that: ‘IMT improves cardiorespiratory fitness and quality of life to a similar magnitude to conventional exercise training and may provide an initial alternative to the more severely deconditioned CHF patients who may then transition to conventional [exercise training]’. Similar findings for outcomes relating to MIP, exercise tolerance and dyspnoea were also reported in the systematic reviews by Lin et al (2012) and Plentz et al (2012).
Recent studies have also shed light on the potential mechanisms underlying improvements in exercise tolerance following IMT (Chiappa et al, 2008b; Stein et al, 2009). The first study is an elegant experiment that examined whether IMT influenced activation of the inspiratory muscle metaboreflex (Chiappa et al, 2008b). Before IMT, breathing against an inspiratory load activated the inspiratory muscle metaboreflex, inducing vasoconstriction in both resting and exercising limbs. In contrast, after 4 weeks of IMT the metaboreflex response to the same loaded breathing task was delayed, and the time to fatigue during subsequent forearm exercise task was extended. The study also documented a 54% increase in contracted diaphragm thickness, which explained 77% of the change in MIP. The finding of a change in the work threshold for activation of the inspiratory muscle metaboreflex after IMT is similar to that in healthy people described above (McConnell & Lomax, 2006; Witt et al, 2007; Bailey et al, 2010), and suggests that this mechanism plays an important role in mediating improvements in exercise tolerance.
The role of improved oxygen delivery in the improvements in exercise tolerance observed after IMT is supported by Stein and colleagues’ retrospective analysis (2009) of the data published by Dall’Ago et al (2006). Stein and colleagues used the oxygen uptake efficiency slope (OUES, or gradient of ![]() O2 vs log
O2 vs log![]() E) (Baba et al, 1996) during submaximal treadmill exercise as an index of the ‘cardiorespiratory functional reserve’ and demonstrated that this was not only improved post-IMT, but that the increase in MIP accounted for 69% of the increase in OUES. The OUES is essentially an index of the rate of increase of
E) (Baba et al, 1996) during submaximal treadmill exercise as an index of the ‘cardiorespiratory functional reserve’ and demonstrated that this was not only improved post-IMT, but that the increase in MIP accounted for 69% of the increase in OUES. The OUES is essentially an index of the rate of increase of ![]() O2 in response to a given
O2 in response to a given ![]() E ; thus it provides an index of the efficiency with which oxygen is being taken up at the lungs, which is in turn affected by the pulmonary diffusion area and alveolar ventilation (
E ; thus it provides an index of the efficiency with which oxygen is being taken up at the lungs, which is in turn affected by the pulmonary diffusion area and alveolar ventilation (![]() A). The OUES also provides an index of systemic perfusion (Baba et al, 1996). Both diffusion area and
A). The OUES also provides an index of systemic perfusion (Baba et al, 1996). Both diffusion area and ![]() A are impaired in patients with CHF, and both have the potential to be improved by IMT. In the case of the diffusion area, the influence of IMT upon the threshold for activation of the inspiratory muscle metaboreflex may enhance pulmonary perfusion (see the section ‘Improvements in hypoxic conditions’, above). Furthermore, data from healthy people suggest that IMT increases VT (Romer et al, 2002a), which theoretically improves
A are impaired in patients with CHF, and both have the potential to be improved by IMT. In the case of the diffusion area, the influence of IMT upon the threshold for activation of the inspiratory muscle metaboreflex may enhance pulmonary perfusion (see the section ‘Improvements in hypoxic conditions’, above). Furthermore, data from healthy people suggest that IMT increases VT (Romer et al, 2002a), which theoretically improves ![]() A, by reducing VD/ VT. Similar changes in patients with CHF after IMT might also contribute to the improved OUES observed by Stein and colleagues (2009). These mechanistic studies provide good evidence that IMT improves exercise tolerance in patients via credible and important physiological mechanisms.
A, by reducing VD/ VT. Similar changes in patients with CHF after IMT might also contribute to the improved OUES observed by Stein and colleagues (2009). These mechanistic studies provide good evidence that IMT improves exercise tolerance in patients via credible and important physiological mechanisms.
The influence of IMT upon autonomic balance has also received attention both in patients with CHF and in those with hypertension. Mello et al (2012) found that 12 weeks of IMT not only yielded significant improvements in MIP (47%), peak oxygen uptake (31%) and quality of life, but also these changes were accompanied by an increase in cardiac vagal tone (increase in the ratio of low-frequency to high-frequency components of heart rate variability (LF / HF) and a decrease in muscle sympathetic nerve activity (27%; assessed using microneurography). The influence of IMT upon sympathetic activity and arterial blood pressure (ABP) has also been examined in hypertensive patients (Ferreira et al, 2011); this randomized, placebo-controlled trial lasted 8 weeks, at the end of which the IMT group exhibited a 46% increase in MIP, which was accompanied by a significant reduction in 24-hour systolic and diastolic blood pressures of 8 mmHg and 5 mmHg, respectively. These changes appeared to be underpinned by improvements in autonomic balance as sympathetic activity was reduced significantly (increase in LF / HF). The authors speculated that this might be due to a reduction in the sympathoexcitatory input from the inspiratory muscle metaboreflex. An alternative explanation is that IMT prompted a slower, deeper breathing pattern, which has also been shown to reduce ABP in hypertensive patients, especially when combined with a small inspiratory load (Jones et al, 2010). Breathing exercises have also been shown to increase cardiac vagal tone in healthy people (Hepburn et al, 2005), which is suggestive of an ability to influence autonomic balance. The magnitude of improvements in ABP after breathing training is comparable to, or better than, those observed following exercise training (2–3 mmHg) (NICE, 2011) and pharmacotherapy (~ 13–17 mmHg) (Wright & Musini, 2009).
A recent double-blind, randomized placebo-controlled study has examined the influence of IMT upon functional balance in patients with CHF, as well as the more traditional indices of function such as exercise tolerance and dyspnoea (Bosnak-Guclu et al, 2011). The beneficial effects upon exercise, dyspnoea and quality of life were confirmed, but the study also identified a significant improvement in the Berg Balance Scale score of 1.36 units. This is consistent with the role of the respiratory muscles in postural control (Hodges & Gandevia, 2000).
A recent consensus statement from the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation concluded that IMT ‘can improve exercise capacity and quality of life, particularly in those who present with inspiratory muscle weakness (IMW). Hence, routine screening for IMW is advisable and specific inspiratory muscle training in addition to standard endurance training might be beneficial’ (Piepoli et al, 2011). This recommendation is echoed by Ribeiro and colleagues in a recent review of the contribution of inspiratory muscles to exercise limitation in patients with CHF (Ribeiro et al, 2012). Perhaps the only contention in respect of current advice regarding implementation of IMT is the restriction to patients with overt inspiratory muscle weakness. The reasons for this are: (1) inspiratory muscle strength needs to be considered in the broader context of the demand / capacity relationship of the respiratory pump (see Ch. 3), and (2) if one accepts that changes to the threshold for activation of the inspiratory muscle metaboreflex contribute to functional improvements after IMT, the similarity of the changes in metaboreflex activation in patients with CHF and in healthy people (McConnell & Lomax, 2006; Chiappa et al, 2008b) supports the notion that similar mechanisms underlie functional improvements. Accordingly, since healthy people have normal inspiratory muscle function and show functional improvements following IMT, it is reasonable to suggest that IMT should also be considered for patients without overt inspiratory muscle weakness.
In summary, the strength of evidence supporting the efficacy of RMT, and in particular IMT, in patients with CHF is growing, and has now achieved a level where it is possible to recommend IMT as a stand-alone treatment. Improvements in inspiratory muscle strength, exercise capacity and dyspnoea can be expected but improvements in quality of life are less consistent. Studies examining limb blood flow and the OUES response to exercise suggest that changes to the inspiratory muscle metaboreflex response may contribute to enhancement of exercise capacity after IMT. Some studies have included only patients with inspiratory muscle weakness, giving rise to the recommendation that this be a prerequisite for IMT. However, there is no direct evidence indicating that patients with normal MIP cannot also benefit. Further research is required to build critical mass within the evidence base, as well as to evaluate the combined benefits of IMT and exercise. The influence of IMT upon autonomic nervous system balance is also worthy of further exploration. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Neurological and neuromuscular disease
Spinal cord injury
Spinal cord injury (SCI) induces profound changes to pulmonary and respiratory muscle function, as well as breathing mechanics, creating imbalance in the demand / capacity relationship of the respiratory pump. Respiratory symptoms are common in patients with SCI, and breathlessness is present in 73% of people with a lesion at C5 but only 29% of those with a lesion below T8 (Spungen et al, 1997). Around two-thirds of the prevalence of dyspnoea has been attributed to inspiratory muscle paralysis (Spungen et al, 1997). Furthermore, respiratory complications remain a major cause of morbidity and mortality in people with SCI (Schilero et al, 2009), the underlying cause of which is poor cough function. Interestingly, MIP shows a higher correlation with cough function than does MEP (Kang et al, 2006).
As poor respiratory muscle function is linked to dyspnoea and cough function, it is reasonable to suppose that specific RMT may have a beneficial influence. There have been three systematic reviews on the topic (Brooks et al, 2005; Van Houtte et al, 2006; Sheel et al, 2008). The first of these reviews examined IMT and addressed the following outcomes: inspiratory muscle strength and endurance, exercise capacity, dyspnoea, pulmonary function and quality of life. Only three studies satisfied the inclusion criteria and were randomized (Loveridge et al, 1989; Derrickson et al, 1992; Liaw et al, 2000); two included a control group (Loveridge et al, 1989; Liaw et al, 2000) whereas the third compared RMT with abdominal muscle training in which the diaphragm was used to lift weights placed on the abdomen (Derrickson et al, 1992). Two studies were undertaken in the acute phase post-injury, whereas the third was undertaken 1 year post-injury (Loveridge et al, 1989). Thus the literature could be described as a ‘mixed bag’. It is perhaps no surprise that the analyses revealed no consistent changes in the outcome variables analysed; even MIP failed to achieve statistical significance between groups, though the difference was significant within groups in all three studies (MIP increased by 29–61%). The latter stems from two factors: first, one study compared IMT with a different form of diaphragm training (Derrickson et al, 1992); secondly, another observed a 30% improvement in MIP in the control group.
A second systematic review was broader, evaluating all forms of RMT (Van Houtte et al, 2006). Only six of 106 studies met the inclusion criteria, and addressed the following outcomes: inspiratory muscle strength and endurance, pulmonary function, respiratory complications, quality of life and exercise performance. Unfortunately, the authors were forced to conclude that due to ‘a limited number of randomized controlled trials, unreported data and heterogeneity … it was impossible to calculate effect sizes and to perform a meta-analysis’. However, they were able to conclude that the data suggested ‘tendencies’ for improved expiratory muscle strength, vital capacity and residual volume after RMT.
The most recent systematic review examined the influence of both IMT and general exercise training upon respiratory muscle and lung function in patients with all levels of SCI (Sheel et al, 2008). Thirteen studies were included in the analysis of IMT but, as with previous systematic reviews, heterogeneity of participants and training methods hampered analysis. The authors concluded that there was insufficient evidence to support IMT as a method of improving inspiratory muscle function, lung function or dyspnoea in people with SCI.
The results of these systematic reviews highlight the difficulties of evaluating any intervention in a highly heterogeneous population, especially when the intervention itself lacks standardization. Since the 2009 review, there have been a handful of further studies published, one of which was a randomized, placebo-controlled trial examining the effects of RMT (hyperpnoea) in the acute phase following SCI (Van Houtte et al, 2008). After 8 weeks of training the RMT group exhibited significant improvements in respiratory muscle strength (25%) and endurance (121%) as well a reduction in respiratory complications. Since the 2008 systematic review, a further three studies using controlled designs have examined the influence of IMT upon the exercise performance of wheelchair athletes (Litchke et al, 2008; West et al, 2009; Goosey-Tolfrey et al, 2010). This subgroup of SCI people are more homogeneous, and also more stable in terms of their underlying physiology. In wheelchair athletes, improvements in MIP ranged from 17% to 44%. In one study, similar changes were seen in MIP and MEP in both the IMT and placebo groups, which suggests either that the baseline measures of function were unreliable or that the placebo training intensity (15% MIP) was sufficient to induce changes in function (Goosey-Tolfrey et al, 2010). This study also had poor training compliance (63%), and found no change in lung function or in repeated wheelchair sprint performance. The results of the study that did not implement any placebo training (Litchke et al, 2008) tend to support the suggestion that placebo IMT may be sufficient to increase MIP; in this study, MIP increased significantly (44%) only in the IMT group. These authors also observed no change in ![]() O2peak post-IMT. The latter is not surprising, as
O2peak post-IMT. The latter is not surprising, as ![]() O2peak represented
O2peak represented ![]() O2max in these athletes, and IMT does not increase
O2max in these athletes, and IMT does not increase ![]() O2max in able-bodied athletes (Romer et al, 2002c). The study by West and colleagues (2009) used a clever placebo, which consisted of using a placebo metered dose inhaler; this study also measured diaphragm thickness. Both MIP and diaphragm thickness improved significantly, and these changes were significantly different from control (23% and 11% respectively). This study also observed a significant increase in arm-cranking power output and VT, as well as a decrease in
O2max in able-bodied athletes (Romer et al, 2002c). The study by West and colleagues (2009) used a clever placebo, which consisted of using a placebo metered dose inhaler; this study also measured diaphragm thickness. Both MIP and diaphragm thickness improved significantly, and these changes were significantly different from control (23% and 11% respectively). This study also observed a significant increase in arm-cranking power output and VT, as well as a decrease in ![]() E /
E / ![]() O2 at peak power during incremental arm-cranking exercise. Similar changes in breathing pattern have been observed in able-bodied athletes (Romer et al, 2002c).
O2 at peak power during incremental arm-cranking exercise. Similar changes in breathing pattern have been observed in able-bodied athletes (Romer et al, 2002c).
The joint BTS / ACPRC Guidelines make the following recommendations in relation to IMT in patients SCI: (1) ‘[IMT] may be considered for patients with upper spinal cord injury to improve respiratory muscle strength’, and (2) ‘[IMT] may be considered for patients with upper spinal cord injury to improve vital capacity and residual volume’ (Bott et al, 2009).
Finally, it is worthwhile mentioning that repeated, deep inhalations against an inspiratory load have been found to be twice as effective (as measured by sputum weight) as standardized physiotherapy consisting of postural drainage and the active cycle of breathing technique (Chatham et al, 2004). Thus, IMT may also facilitate airway clearance.
In summary, the role of RMT in patients with SCI remains unclear, and further, careful, systematic research is required before conclusions can be drawn. Issues that demand investigation include: (1) optimal training methods to enhance respiratory muscle function, (2) optimal timing of training (acute, chronic, or both), and (3) the influence of RMT upon relevant clinical outcomes such as lung function, cough, exercise tolerance and quality of life. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Other NMDs
Despite the fact that respiratory muscle weakness is a common finding, and respiratory complications are a frequent cause of morbidity and mortality in patients with neuromuscular disease (NMD), RMT has been studied comparatively little in this group of patients. This may stem from misconceptions regarding the ability of diseased muscle to respond to a training stimulus, and potentially harmful effects of exercise (Aboussouan, 2009). Generally, the more severe a patient’s disease, the less likely they are to respond to any form of training. Similarly, the more rapid the progress of their disease, the less likely they are to show a training effect. However, the reverse is also true, and large training gains may be possible in patients with mild disease that is progressing slowly.
The umbrella term ‘NMD’ encompasses a wide range of conditions including amyotrophic lateral sclerosis (ALS), stroke, Parkinson’s disease, multiple sclerosis, muscular dystrophy, myasthenia gravis, cerebral palsy, Guillain–Barré syndrome and post-polio syndrome. For many of these conditions, there is a lack of critical mass with respect to studies on the effects of RMT, as well as contradictory evidence. A case in point is Duchenne muscular dystrophy (DMD), where the results have been somewhat contradictory (DiMarco et al, 1985; Martin et al, 1986; Smith et al, 1988; Stern et al, 1989; Vilozni et al, 1994; Wanke et al, 1994; Winkler et al, 2000; Koessler et al, 2001; Topin et al, 2002) and the quality of research design variable, e.g., some lack control groups or randomization. For example, studies fail to agree on fundamental outcomes such as whether respiratory muscle strength and / or endurance improve. However, these discrepancies are most likely methodological in origin, as well as being due to variations in the type of training used. The latter is important, as endurance training does not improve strength, and if strength is the outcome measure of training efficacy then a type II error (falsely accepting the null hypothesis) is the result. Furthermore, some studies contain a diversity of patients with differing conditions and stages of disease progression, making for heterogeneous and diluted outcomes.
In studies in which the patients are either exclusively DMD (Topin et al, 2002), or make up the majority of the sample (Winkler et al, 2000; Koessler et al, 2001), a clearer picture emerges with respect to the influence of IMT. However, this needs to be viewed in the context of the research designs employed. For example, although Topin et al (2002) used a randomized placebo-controlled design, neither Winkler et al (2000) nor Koessler et al (2001) included any control group. The most important observation is that functional improvements appear to be dose dependent, being greatest in those patients who complete the most training sessions. Furthermore, responses are specific to the training stimulus, e.g., where this is endurance biased, improvements in endurance result (46%) with no change in strength (Topin et al, 2002), where a combined protocol is used then both properties improve (Winkler et al, 2000), and where a strength protocol is used then both strength (43%) and endurance (15%) improve (Koessler et al, 2001). The latter arises because of the ‘dual conditioning’ response to strength training (see Chapter 5). Accompanying the increases in inspiratory muscle strength (MIP) are improvements in or maintenance of lung function (Koessler et al, 2001). So far as functional outcomes such as breathing effort perception are concerned, the only study to examine this utilized a randomized placebo-controlled design and a protocol of discrete pressure threshold IMT and EMT (Gozal & Thiriet, 1999). Improvements in both inspiratory and expiratory muscle strength (30% and 47%, respectively) were observed, but without improvement in lung function. However, there was a significant decline in the perception of effort when breathing against an inspiratory load. This study also noted that improvements in respiratory muscle strength were lost 3 months after cessation of training, but that perception of breathing effort remained attenuated. The most recent British Thoracic Society Guideline on management of children with neuromuscular weakness concludes that ‘respiratory muscle training can improve respiratory muscle strength and endurance in children with DMD’ (Hull et al, 2012). Collectively, these data provide preliminary support for the use of RMT in patients with DMD, and specifically inspiratory resistance training, which elicits the widest range of benefits; improvements include lung function and dyspnoea.
Post-polio syndrome is often associated with inspiratory muscle dysfunction. Exercise is limited to some extent by ventilatory insufficiency, as indicated by abnormal blood gas values during exercise (Weinberg et al, 1999). The only study of IMT in post-polio included only seven patients in the training group and three control patients. The study found that an endurance biased programme of IMT improved inspiratory muscle endurance (56%) significantly, and some activities of daily living, but did not change inspiratory muscle strength or lung function (Klefbeck et al, 2000). The latter is to be expected if the training is biased towards endurance. It is also noteworthy that these patients used assisted ventilation at night, and half relied upon an electric wheelchair for mobility. Accordingly, these preliminary data suggest that, even in patients with severely compromised physical function, IMT may provide some benefit to activities of daily living, but further research is required.
Myasthenia gravis (MG) is associated with generalized weakness and fatigue, which includes weakness of the inspiratory muscles, which induces dyspnoea at rest and during exercise (Weiner et al, 1998a). To date, there have been a handful of studies employing respiratory muscle resistance training (Weiner et al, 1998a; Fregonezi et al, 2005) or hyperpnoea endurance training (Rassler et al, 2007, 2011). Methodological quality varied, with most studies failing to include control groups (Weiner et al, 1998a; Rassler et al, 2007; Rassler et al, 2011), and just one employing a randomized placebo-controlled design (Fregonezi et al, 2005). In these studies, strength training resulted in significant improvements in the strength of both inspiratory (35%) and expiratory muscles, and an improvement in endurance (53%) (Weiner et al, 1998a; Fregonezi et al, 2005). Significant improvements in lung function and dyspnoea accompanied these changes in strength and endurance (Weiner et al, 1998a); the former may be the result of the increased thoracic mobility observed post-IMT (Fregonezi et al, 2005). Finally, there were also improvements in health-related quality of life post-IMT (Fregonezi et al, 2005). Predictably, endurance RMT resulted in improvements in respiratory muscle endurance (~ 101–230%), but no significant change in lung function or inspiratory muscle strength (Rassler et al, 2007, 2011). In addition, the Besinger Score of MG symptoms improved after 4 months of training, but not after 4 weeks (Rassler et al, 2007; Rassler et al, 2011). Thus, these preliminary data suggest that RMT may provide some benefit to patients with MG, but further research is required.
Multiple sclerosis (MS) is a progressively debilitating disease in which respiratory muscle weakness is inevitable (Klefbeck & Hamrah Nedjad, 2003). Both IMT (Klefbeck & Hamrah Nedjad, 2003; Fry et al, 2007) and EMT (Smeltzer et al, 1996; Gosselink et al, 2000; Chiara et al, 2007) have been investigated in patients with MS, as well as a combination of IMT and EMT (Olgiati et al, 1989). Methodological quality varied, with some studies failing to include control groups (Olgiati et al, 1989; Chiara et al, 2007), but others using randomized placebo-controlled designs (Klefbeck & Hamrah Nedjad, 2003), placebo-controlled designs (Smeltzer et al, 1996), or randomized controlled designs (Gosselink et al, 2000; Fry et al, 2007). Both IMT and EMT were found to result in improvements in respiratory muscle strength (~ 30–80%), although changes are not always significant (Gosselink et al, 2000), and do not always result in improvements in lung, or other aspects of function (Olgiati et al, 1989; Klefbeck & Hamrah Nedjad, 2003; Chiara et al, 2007). However, the degree of physical disability appears to play a role in activities of daily living, such that improvements are difficult to achieve in patients who are wheelchair or bed bound (Klefbeck & Hamrah Nedjad, 2003). In addition, it may be necessary to increase inspiratory muscle strength considerably (~ 80%) in order to enhance lung function (Fry et al, 2007). In the most recent randomized controlled trial of IMT, patients with moderate disability showed impressive improvements in MIP after IMT (81%) that were accompanied by improvements in lung function (Fry et al, 2007). The data led the authors to speculate that ‘Enhanced pulmonary function in persons with MS may increase effectiveness of cough, improve speech, reduce bouts of pneumonia, improve performance of ADL, increase tolerance for exercise training, and improve quality of life’ (Fry et al, 2007), but this awaits empirical confirmation.
Following stroke, respiratory muscle paralysis may be present on the affected side, resulting in impaired breathing mechanics and dyspnoea (Lanini et al, 2002) as well as impaired lung function (Roth & Noll, 1994). To date, there have been two randomized controlled trials examining the influence of RMT (hyperpnoea) or IMT upon patients following a stroke (Sutbeyaz et al, 2010; Britto et al, 2011). In the study of Sutbeyaz and colleagues (2010), inspiratory muscle strength (MIP) and FVC (forced vital capacity) improved significantly in the IMT group (16% and 7–9%, respectively), but only MIP and MEP improved significantly in the endurance RMT group (14% and 9%, respectively). There were also small, but significant, improvements in MIP (5%) and MEP (5%) in the control group. Furthermore, the IMT group was the only group to exhibit a significant improvement in both exertional dyspnoea and exercise performance during arm cranking, post-intervention (peak ![]() O2, heart rate and
O2, heart rate and ![]() E). In addition, the Barthel Index and Functional Ambulation Categories scores increased significantly in the IMT group, but not in the other two groups. Finally, health-related quality of life scores also improved significantly in the IMT and RMT groups. In their study of IMT, Britto and colleagues (2011) found that both MIP (52%) and inspiratory muscle endurance (53%) improved post-intervention, and changes were significantly different from the placebo group. However, there were no significant changes in exercise tolerance or quality of life. A Cochrane review of these two studies concluded that ‘Further well-designed [randomized controlled trials] are required’ (Xiao et al, 2012), but these data provide some preliminary indications that IMT might be of benefit to patients following a stroke.
E). In addition, the Barthel Index and Functional Ambulation Categories scores increased significantly in the IMT group, but not in the other two groups. Finally, health-related quality of life scores also improved significantly in the IMT and RMT groups. In their study of IMT, Britto and colleagues (2011) found that both MIP (52%) and inspiratory muscle endurance (53%) improved post-intervention, and changes were significantly different from the placebo group. However, there were no significant changes in exercise tolerance or quality of life. A Cochrane review of these two studies concluded that ‘Further well-designed [randomized controlled trials] are required’ (Xiao et al, 2012), but these data provide some preliminary indications that IMT might be of benefit to patients following a stroke.
Parkinson’s disease (PD) patients have a number of pulmonary abnormalities including a reduction in respiratory muscle strength, endurance and mechanical efficiency (Tzelepis et al, 1988; Weiner et al, 2002a), accompanied by a restrictive pattern of lung dysfunction (Sabate et al, 1996). Surprisingly, exertional dyspnoea is uncommon, but this is most likely because of the sedentary lifestyles of most PD patients (Inzelberg et al, 2005). This supposition is supported by the fact that, during loaded breathing, dyspnoea is greater in patients with PD than in control participants (Weiner et al, 2002a). A placebo-controlled trial found that, after IMT, inspiratory muscle strength and endurance improve (26% and 45%, respectively), but without any change in lung function (Inzelberg et al, 2005). These improvements in respiratory muscle function were accompanied by, and correlated with, a reduction in the perception of dyspnoea during a loaded breathing test (22%). To date, no study has evaluated whether the improvements in respiratory muscle function and dyspnoea translate into improved exercise tolerance and quality of life.
Amyotrophic lateral sclerosis (or motor neuron disease; ALS / MND) is a neurodegenerative disease involving progressive weakness of voluntary muscles, including respiratory muscles (Just et al, 2010). A decline in lung function accompanies the inspiratory muscle weakness, which contributes to the development of hypoventilation (Pinto et al, 2009) and ultimately to respiratory failure (Cheah et al, 2009). Indeed, both FVC and respiratory muscle strength correlate with prognosis in ALS (Stambler et al, 1998). To date, two randomized controlled studies have evaluated the influence of IMT upon the function of patients with ALS (Cheah et al, 2009; Pinto et al, 2012). One studied 26 patients in the early stages of the disease (Pinto et al, 2012), whereas the other studied 37 consecutive patients (Cheah et al, 2009). In neither study did IMT induce any statistically significant improvements in respiratory muscle or lung function. However, both sets of data exhibited trends towards improving MIP and lung function (Cheah et al, 2009) or offsetting ongoing deterioration of function (Pinto et al, 2012). In both studies, the statistical power was hampered by sample size, and by the shifting baseline of deteriorating function. It is also noteworthy that both studies implemented a training regimen that would be considered an endurance protocol, i.e., a low relative load sustained for 10 minutes. It is possible that briefer bouts of higher-intensity training might yield improvements in MIP and lung function. The data are therefore inconclusive with respect to the effect of IMT upon patients with ALS.
Finally, it is worthwhile mentioning that repeated, deep inhalations against an inspiratory load have been found to be twice as effective (as measured by sputum weight) as standardized physiotherapy consisting of postural drainage and the active cycle of breathing technique (Chatham et al, 2004). Thus, IMT may also facilitate airway clearance in patients with NMD.
In summary, it is clear from the preceding section that the evidence base for RMT in people with NMD is heterogeneous. There are clear differences between disease conditions in both the quality of the evidence and the ease with which it can be interpreted. Since no evidence-based guidelines exist for exercise prescription in general or RMT in particular for patients with NMD, Aboussouan (2009) offers some pragmatic advice in a review on exercise limitation and pulmonary rehabilitation for patients with NMD, suggesting that the selection of any training modality should be based upon the patients’ limitations. For example, for RMT it is suggested: ‘[RMT] should be considered in patients in whom respiratory muscle weakness or fatigue is contributing to the impairment’. An application that awaits empirical assessment is the prophylactic use of RMT as a means of providing a functional ‘cushion’ against respiratory failure.
Obesity
To date, three studies have examined the influence of RMT in patients with obesity, two of which used hyperpnoea RMT (Frank et al, 2011; Villiot-Danger et al, 2011) and one IMT (Edwards et al, 2012). The premise for the study by Frank and colleagues (2011) was that the attenuating influence of hyperpnoea RMT upon exertional dyspnoea would make exercise ‘more enjoyable’, thereby increasing spontaneous physical activity. The study had a randomized controlled design in which one group undertook just exercise plus nutrition counselling (E + N) whilst the other undertook RMT (30 minutes hyperventilation five times per week) in addition to E + N. There was a 1-month run-in period during which the RMT group trained and the control group received no intervention. This was followed by a 3-month block of supervised RMT and / or E + N, and a second 3-month block of unsupervised RMT and / or E + N. Both groups improved their 12-minute treadmill walk / run distance, but the improvement was significantly greater in the group who also received RMT (8.7% vs 3.6%). Using a case–control design, Villiot-Danger and colleagues (2011) also implemented hyperpnoea RMT (30 minutes of hyperventilation, 3–4 times per week); 10 participants underwent 26 days of inpatient dietary control and exercise plus RMT, whilst the control group received only dietary control and exercise. There were significantly larger improvements in 6-minute walk distance (54 metres, ~ 10%), dyspnoea and quality of life in the group who also received RMT. Furthermore, improvements in 6-minute walk distance were correlated significantly with improvements in respiratory muscle endurance. Finally, Edwards and colleagues (2012) examined the influence of IMT in 15 obese and overweight individuals using a randomized placebo-controlled design. The 4-week training intervention consisted of 30 breaths, twice daily at 55% of MIP. There were no changes in lung function, but the 6-minute walk distance increased significantly (62 metres ~ 20%) in the IMT group, but not in the placebo group. Interestingly, the improvement in 6-minute walk distance correlated positively both with change in MIP and with baseline body mass index.
Ageing
Normal ageing is associated with a number of changes that affect breathing, and the senescent changes to the pulmonary system have been dubbed ‘senile emphysema’ (Janssens et al, 1999). These changes impact upon the demand / capacity relationship of the respiratory muscles, which may contribute to exercise limitation via dyspnoea. Indeed, respiratory muscle strength is independently related to decline in mobility in older people (Buchman et al, 2008). Whether mobility is the chicken or the egg is unclear, but a randomized double-blind placebo-controlled trial has shown that improving inspiratory muscle function through IMT significantly increases participation in moderate-to-vigorous physical activity (Aznar-Lain et al, 2007). The latter may arise because exertional dyspnoea is lower, rendering exercise less uncomfortable.
To date, there have been only a handful of studies of resistance RMT in healthy elderly people (Copestake & McConnell, 1995; Aznar-Lain et al, 2007; Watsford & Murphy, 2008). Collectively, these studies indicate that 6–8 weeks of IMT induce consistent increases in MIP (~ 20–46%), treadmill exercise capacity (peak performance and Tlim duration) and spontaneous physical activity (measured by accelerometry), as well as reducing exertional dyspnoea. One randomized controlled trial implemented IMT + EMT (within the same breath cycle) and demonstrated significant increases in both MIP (20%) and MEP (30%) (Watsford & Murphy, 2008). In addition, the same study demonstrated that, when walking at 5.5 km·h− 1, the heart rate, breathing effort and oxygen uptake were significantly lower in the training group. Similar reductions have also been observed in healthy young people (see the section ‘Responses to RMT in healthy people’). Finally, one randomized controlled trial has examined the effect of 8 weeks of hyperpnoea RMT (Belman & Gaesser, 1988); it observed a significant increase in maximum sustained ventilatory capacity (17%), but no significant improvement in incremental or single-stage treadmill performance, or in breathing effort during exercise.
In summary, specific recommendations regarding the use of RMT to reduce dyspnoea and improve exercise tolerance in older people await further research. However, the small number of studies to date suggest that both IMT and IMT + EMT improve a range of exercise-related outcomes in healthy elderly people, although hyperpnoea RMT does not appear to generate any benefits. The strong theoretical rationale for [strength] RMT in older people, combined with the finding of an apparent relationship between improvement in MIP and participation in moderate-to-vigorous physical activity, also supports its use as a potential means of enhancing physical functioning. The demographic shift towards an increasingly older population makes this a potentially impactful area for future research. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Miscellaneous conditions
Type 1 and 2 diabetes are associated with inspiratory muscle weakness (Heimer et al, 1990; Kaminski et al, 2011) and in type 1 there is also impairment of inspiratory muscle endurance (Heimer et al, 1990), as well as of vital capacity and FEV1 (Innocenti et al, 1994). These factors may contribute to exercise intolerance via their influence on the demand / capacity relationship of the respiratory pump.
To date, there is only one study of IMT in patients with diabetes, and this was undertaken in patients with type 2, who were selected for their inspiratory muscle weakness (Correa et al, 2011). In this randomized placebo-controlled trial, low-intensity IMT (30 minutes breathing against 30% of MIP) was undertaken for 8 weeks. Inspiratory muscle strength and endurance increased significantly over that of the placebo group; indeed, MIP doubled and endurance tripled. Lung function was normal at baseline and did not improve, and incremental treadmill performance (![]() O2peak) also did not improve. The study also evaluated a number of indices of autonomic function, which showed no change. The use of an incremental exercise test is questionable in this study, as peak heart rate data suggest that the patients attained a cardiovascular limited peak at baseline (peak fc = 150 beats · min?1). This being the case, an increase in
O2peak) also did not improve. The study also evaluated a number of indices of autonomic function, which showed no change. The use of an incremental exercise test is questionable in this study, as peak heart rate data suggest that the patients attained a cardiovascular limited peak at baseline (peak fc = 150 beats · min?1). This being the case, an increase in ![]() O2peak post-IMT was extremely unlikely as maximal
O2peak post-IMT was extremely unlikely as maximal ![]() O2 does not increase after IMT. Submaximal constant intensity tests such as the 6-minute walk test are typically most sensitive to the physiological changes elicited by IMT, and might have revealed a change in exercise tolerance in these patients.
O2 does not increase after IMT. Submaximal constant intensity tests such as the 6-minute walk test are typically most sensitive to the physiological changes elicited by IMT, and might have revealed a change in exercise tolerance in these patients.
There is one further relevant, and intriguing, controlled trial that is applicable to patients with diabetes. The premise for the study was the observation that 3 weeks of IMT increased the expression of the glucose transporter GLUT-4 in the diaphragm of sheep (Bhandari et al, 2000). Seven of 14 elderly insulin-resistant patients received 12 weeks of low-intensity IMT (30 minutes breathing against 40% of MIP) (Silva Mdos et al, 2012); IMT increased MIP significantly (25%), and this was accompanied by a decrease in insulin resistance, as indicated by a decrease in the ‘Homeostatic model assessment for insulin resistance’ (HOMA-IR) from 2.9 to 0.9 units. The authors suggested that IMT might offer an important intervention for insulin-insensitive patients who are unable to exercise.
In summary, specific recommendations regarding the use of RMT to reduce dyspnoea and improve exercise tolerance in patients with diabetes await further research. These studies should use tests such as the 6-minute walk test to evaluate functional changes elicited by IMT. Preliminary evidence suggests RMT may reduce insulin resistance, which is also worthy of further research. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Renal failure
Respiratory system involvement in renal failure is extremely complex (Prezant, 1990), deriving from both the disease and its treatment. It has been known for many years that uraemic patients possess impaired inspiratory muscle strength and a restrictive pulmonary defect (Gomez-Fernandez et al, 1984). To date, there have been just three studies of IMT in patients receiving haemodialysis (Weiner et al, 1996; Silva et al, 2011; Pellizzaro et al, 2013). The first (available in English only as an abstract) reported improvements in inspiratory muscle function and functional capacity that were not seen in the sham-training control group (Weiner et al, 1996). Unfortunately, the second study had no control group, and although MIP increased by around 70% after low-intensity IMT (15 minutes breathing against 40% of MIP) this was not statistically significant (Silva et al, 2011). There were no changes in lung function, but the 6-minute walk distance increased significantly (20%) and exertional dyspnoea was reduced significantly (27%). The most recent study (Pellizzaro et al, 2013) was a randomized controlled trial in which RMT was compared with peripheral muscle training (PMT) in 39 patients undergoing haemodialysis (11 RMT, 14 PMT, 14 control). The MIP, MEP and 6-minute walk distance increased significantly in both exercise groups, compared with control, with changes in the RMT group being larger than those in the PMT group.
In summary, specific recommendations regarding the use of RMT to reduce dyspnoea and improve exercise tolerance in patients with renal failure await further research, but are supported by preliminary findings of improved exercise capacity after IMT in two studies. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Myopathic pharmacological agents
Steroid-induced myopathy is a well-established phenomenon in patients receiving high doses of corticosteroids (Perkoff et al, 1959), and the inspiratory muscles are amongst those affected (Weiner et al, 1995). Other agents, e.g., colchicine and statins, have also been linked with iatrogenic myopathy in case studies.
Patients with COPD are routinely prescribed high doses of corticosteroids during exacerbations to control lung inflammation, but their myopathic effects may have unwanted side effects. Weiner and colleagues hypothesized that the inspiratory muscle weakness induced by the corticosteroids might impair lung function (Weiner et al, 1995). They tested this hypothesis by assessing the influence of acute administration of oral corticosteroids upon the inspiratory muscle and lung function of 12 patients (with conditions other than respiratory disease). The spontaneous response to corticosteroid treatment was compared with the response when treatment was accompanied by a programme of IMT. By the end of the 8-week course of corticosteroid treatment, significant reductions in inspiratory muscle strength (~ 30%), endurance (~ 50%) and lung function (forced vital capacity reduced by almost 15%) were observed in the group who did not receive IMT; in contrast, in the group who received IMT during treatment, inspiratory muscle and lung function remained stable. The authors concluded that, in order to prevent the detrimental influence of corticosteroids, particularly in respiratory patients in whom inspiratory muscle weakness is already present, IMT might be implemented.
In summary, where myopathic agents are necessary or desirable modes of treatment, Weiner and colleagues’ study (1995) suggests that their myopathic influence might be minimized by prophylactic IMT. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Surgery
Physical fitness is utilized increasingly as a tool for the risk stratification of patients prior to surgery, with fitter patients showing a lower risk of adverse outcomes (Hennis et al, 2011). The benefit of higher levels of physical fitness prior to surgery has led to the assessment of pre- and post-operative physical training, including IMT (Jack et al, 2011), as tools to enhance outcomes. A recent Cochrane review of preoperative physical therapy for elective cardiac surgery patients concluded that, ‘preoperative physical therapy, especially inspiratory muscle training, prevents some postoperative complications, including atelectasis, pneumonia, and length of hospital stay’ (Hulzebos et al, 2012).
Inspiratory muscle training has been conducted pre-operatively, post-operatively and both pre- and post-operatively. Pre-operative IMT has been undertaken prior to coronary artery by-pass graft surgery (CABG) (Weiner et al, 1998b; Hulzebos et al, 2006a; Hulzebos et al, 2006b), abdominal surgery (Dronkers et al, 2008, 2010; Kulkarni et al, 2010), oesophagectomy (Dettling et al, 2012), open bariatric surgery (Barbalho-Moulim et al, 2011) and thoracic orthopaedic surgery (Takaso et al, 2010). Post-operative treatment has followed open bariatric surgery (Casali et al, 2011) and cardiac sugery (Kodric et al, 2012), whilst pre- and post-operative treatment has been undertaken in patients undergoing CABG surgery (Savci et al, 2011) and pneumonectomy (Weiner et al, 1997). In most cases, these studies have been randomized controlled trials (Weiner et al, 1997; Weiner et al, 1998b; Hulzebos et al, 2006a; Hulzebos et al, 2006b; Dronkers et al, 2008; Dronkers et al, 2010; Kulkarni et al, 2010; Barbalho-Moulim et al, 2011; Casali et al, 2011; Kodric et al, 2012).
Pre-operative treatment duration has typically ranged from 2 to 4 weeks, using a training regimen with a low intensity (15–30 minutes breathing against 10–60% of MIP). Typically, relative to usual care, pre-operative IMT is associated with improvement or maintenance of MIP and lung function. In addition, there is a reduction in the incidence of atelectasis (Hulzebos et al, 2006b; Dronkers et al, 2008), reduction in the number and severity of post-operative pulmonary complications (Hulzebos et al, 2006a) and a reduction in the number of patients requiring mechanical ventilation (Weiner et al, 1998b). In the largest study to date, Hulzebos and colleagues (2006a) planned to recruit 584 patients. However, the study was stopped after 292 patients following interim analysis revealing that the incidence of pneumonia and length of hospital stay were significantly lower in the IMT group, compared with the usual care–control group. The study was halted because the institutional review board ‘thought it was no longer ethical to withhold IMT from the patients in the usual care group’ (Hulzebos et al, 2006a). Post-operative IMT has been associated with a more rapid recovery of lung function following bariatric surgery (Casali et al, 2011). However, Dettling and colleagues found no change in the incidence of pneumonia or length of hospital stay following at least 2 weeks of pre-operative IMT in patients subjected to oesophagectomy, despite a significant increase in MIP (Dettling et al, 2012). However, it is noteworthy that MIP declined by around two-thirds post-operatively in the IMT group (to ~ 37 cmH2O), and by around half in the control group (to ~ 25 cmH2O), but remained significantly higher in the IMT group. This marked decline may be because of the involvement of the diaphragm during oesophageal resection, and it is possible that the superior MIP post-operatively in the IMT group was insufficient, in absolute terms, to provide any influence upon susceptibility to pneumonia. Surgically induced diaphragm dysfunction is also a complication of cardiac surgery. A 1-year randomized, placebo-controlled trial of IMT in patients with post-operative diaphragm paralysis found that 78% of patients receiving IMT (n = 36) showed improved diaphragm mobility, compared with just 12% of placebo patients (n = 16) (Kodric et al, 2012).
In the most recent study to evaluate pre- and post-surgery IMT, functional capacity (6-minute walk test), anxiety score and length of stay in intensive care were all found to improve significantly relative to a group who received usual care in patients who underwent CABG surgery (Savci et al, 2011).
In summary, in patients undergoing surgery to the thorax or abdomen, prophylactic IMT for as little as 2 weeks may provide a functional ‘cushion’ that enhances recovery and minimizes the risk of post-operative complications. The fact that the largest study of pre-operative IMT to date was halted prematurely because it was considered unethical to withhold the treatment from the control group is a strong recommendation for its routine implementation. Similarly, post-operative IMT may speed recovery and enhance exercise capacity. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Mechanical ventilation
Mechanical ventilation can lead to rapid atrophy and loss of respiratory muscle function (Fitting, 1994; Bissett et al, 2012b), precipitating prolongation of mechanical ventilation and difficulty weaning patients from the ventilator (Callahan, 2009; Bissett et al, 2012b). Patients who are weaned successfully from mechanical ventilation have higher MIP than those who do not (20–40 cmH2O vs 40–60 cmH2O) (Epstein et al, 2002; Carlucci et al, 2009). Indeed, MIP has been found to be an independent predictor of delayed weaning (De Jonghe et al, 2007), suggesting that improving MIP might be a therapeutic target in such patients during the weaning process, as well as prophylactically. However, the apparent link between inspiratory muscle weakness and weaning failure is by no means clear-cut, and some studies have failed to find any influence of MIP upon weaning outcome (Meade et al, 2001; Conti et al, 2004). Furthermore, despite the apparent logic of improving MIP, it is far from certain that the inspiratory muscles are ‘trainable’ under conditions of mechanical ventilation, or that improving strength addresses the complex impediments to weaning sufficiently directly (Bissett et al, 2012b).
The majority of studies examining the influence of IMT upon weaning success have been case reports, or uncontrolled trials in small groups of patients (Aldrich & Uhrlass, 1987; Lerman & Weiss, 1987; Aldrich et al, 1989; Martin et al, 2002; Sprague & Hopkins, 2003; Bissett & Leditschke, 2007), including infants (Brunherotti et al, 2012; Smith et al, 2012). In all such trials, patients who had previously failed to wean from a ventilator have been given brief periods of IMT and periods of spontaneous breathing off the ventilator, with the result that MIP improved and successful weaning was achieved.
A handful of randomized controlled trials have examined the influence of IMT upon weaning (Caruso et al, 2005; Cader et al, 2010; Martin et al, 2011; Cader et al, 2012), and a systematic review was published in 2011 (Moodie et al, 2011). The review included 150 patients and demonstrated a significant effect of training upon MIP and a trend in favour of IMT for weaning success and duration, as well as for survival. It is noteworthy that the study by Cader and colleagues (Cader et al, 2010) included ventilated patients who were intubated and in the early phase of mechanical ventilation (Cader et al, 2010), whilst that of Martin and colleagues included tracheostomized patients with weaning failure (Martin et al, 2011). The studies also differed in the training regimen implemented. Cader and colleagues (2010) employed an IMT regimen consisting of 5 minutes at a load equivalent to 30% MIP, twice daily. In contrast, Martin and colleagues employed a regimen of four sets of 6–10 breaths at the highest tolerable load, with mechanical ventilation between each set. Cader and colleagues (2010) found that, in those who did not die or receive a tracheostomy, the time to weaning was significantly shorter in the experimental group than in the control group (1.7 days), whereas Martin and colleagues (2011) found that 71% of their IMT group weaned successfully compared with 47% of the placebo group. In contrast, the study by Caruso et al (2005) found no change in MIP across the intervention; however, training consisted of imposing an inspiratory load by adjusting the pressure trigger of the mechanical ventilator (ranging from 20% of MIP to 40% of MIP). It is therefore possible that the discrepancy with other studies may be explained by the nature of the training stimulus, which would have consisted of a brief threshold load at the very onset of the breath. This is a very different, and inferior, training stimulus compared with inhaling continuously against an inspiratory threshold load. This is emphasized by a recent randomized controlled trial of pressure threshold IMT in intubated, elderly intensive care patients ( n = 28) (Cader et al, 2012). The patients in the IMT group (n = 14) showed a significant increase in MIP (67%) and a significant decrease in Tobin Index, compared with controls (n = 14). The IMT group also had a shorter weaning time (3.64 vs 5.36 days), but this was not significant. However, the time spent in non-invasive positive pressure ventilation was significantly shorter for the IMT group (7.5 vs 23 hours).
Finally, it is pertinent to stress that the available evidence suggests that IMT is safe, even for patients undergoing continuous invasive ventilation (Bissett et al, 2012a), and is well tolerated, even in infants (Brunherotti et al, 2012; Smith et al, 2012). Bissett and colleagues used a prospective cohort study to assess the influence of IMT, without supplemental oxygen, in 10 medically stable ventilator-dependent adult patients. They observed no adverse responses during the 195 IMT sessions assessed. Furthermore, IMT provoked no significant changes in heart rate, mean arterial pressure, oxygen saturation or respiratory rate. Training consisted of three to six sets of six breaths at a training threshold that generated a Borg CR-10 rating of between 6 and 8. Furthermore, no adverse events were reported in the studies of Martin et al (2011) or Cader et al (2010).
In summary, the strong theoretical rationale for IMT appears to be supported by positive case study data and emerging data from randomized controlled trials. When IMT is implemented using a resistance-training device, weaning from mechanical ventilation is enhanced. This conclusion is also supported by the outcomes of the systematic review by Bissett and colleagues (2012b), which suggested that IMT is a useful treatment for patients subjected to mechanical ventilation and those experiencing weaning failure. Importantly, they also highlighted the importance of careful selection of patients for IMT, suggesting they must be ‘stable, alert and co-operative patients who are able to psychologically tolerate the temporary high inspiratory workload of IMT’. Furthermore they recommended that patients should be ‘medically stable and they must not be heavily reliant on high levels of ventilatory support (e.g., PEEP < 10, FiO2 < 60%). Not all critically ill patients will be suitable for IMT, particularly in the most acute phase of their management. However, any patient who is at risk of ventilator-induced respiratory dysfunction, particularly those whose MV has exceeded seven days, should be screened for suitability for IMT.’ Bissett and colleagues have also provided evidence to support the safety of IMT in this vulnerable patient group (Bissett et al, 2012a). The importance of the potential clinical outcomes of IMT in the critical care setting promises to make this a burgeoning area of research.
Obstructive sleep apnoea
Short-term repetition of motor tasks has been shown to induce cortical reorganization in a range of activities, including the practice of diaphragm breathing (Demoule et al, 2008). These changes are consistent with the ability of task repetition to enhance neuromuscular functioning, thereby optimizing reflex coordination of muscles. Furthermore the hyperpnoea of exercise training has been shown to elicit structural adaptions in both the diaphragm and upper airway muscles of rats (Vincent et al, 2002). The structural changes induced by training also influence the compliance of muscle tissue; e.g., the passive stiffness of locomotor muscles increases in a manner that is independent of increases in either mass or strength (Lindstedt et al, 2002).
It may not be immediately apparent why IMT would influence the physiology of the upper airway musculature, but the negative pressure generated within the airways during inhalation tends to collapse the extrathoracic airways. Thus IMT induces activation of upper-airway-stabilizing muscles in order to maintain upper airway patency (How et al, 2007; Cheng et al, 2011). Because these muscles are activated during IMT, it is reasonable to presume that the upper airway muscles are also trained during IMT.
To date, there have been no randomized controlled trials of RMT in patients with OSA; however, there are some data that hint at potential benefits. For example, one study has shown an improvement in nocturnal saturation in patients with COPD following IMT (Heijdra et al, 1996). In addition, RMT using hyperpnoea training has been shown to reduce the incidence of snoring and daytime sleepiness (Furrer et al, 1998; Frank et al, 2011), but did not improve the apnoea–hypopnoea index in obese patients (Frank et al, 2011). Interestingly, a randomized controlled trial of didgeridoo playing showed it to have a beneficial effect upon daytime sleepiness, apnoea–hypopnoea index and partner-reported sleep disturbance (Puhan et al, 2006). In addition, a cross-sectional study of 906 musicians found that musicians who played double-reed instruments had a significantly lower OSA risk than non-wind musicians, and that risk was predicted by the number of hours spent playing (Ward et al, 2012). Further research is needed, but these data provide an impetus for further exploration of RMT in patients with OSA.
In summary, specific recommendations regarding the use of RMT to reduce the severity of OSA awaits further research. However, implementation is supported by preliminary case study evidence and a theoretical rationale. Accordingly, this is an area worthy of future exploration, particularly in light of the growing prevalence of OSA as a co-morbidity of many chronic conditions. In the mean time, IMT could be considered as a safe, simple and inexpensive adjunctive treatment in patients with OSA. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
Vocal cord dysfunction and inspiratory stridor
Traditional treatments for inspiratory stridor are based on speech therapy techniques, and in extreme cases surgery is used to stabilize the upper airway mechanically (Maat et al, 2007). However, two case studies have shown that exercise-induced symptoms of stridor subside after a short period of IMT (30 breaths breathing against 50–60% of MIP for 4 to 11 weeks), without recurrence (Ruddy et al, 2004; Dickinson et al, 2007). In addition, dyspnoea and exercise intolerance due to vocal fold paralysis have also been treated successfully using IMT (Baker et al, 2003a; Baker et al, 2003b).
In summary, specific recommendations regarding the use of RMT to ameliorate IS and VCD await further research. However, implementation is supported by preliminary evidence from case studies and a theoretical rationale. Accordingly, this is an area worthy of future exploration using randomized controlled designs. In the meantime, IMT could be considered as a safe, simple and inexpensive first-line intervention in patients with VCD. For specific recommendations regarding implementation of IMT see Chapters 5 and 6.
References
Aboussouan, L.S. Mechanisms of exercise limitation and pulmonary rehabilitation for patients with neuromuscular disease. Chron. Respir. Dis. 2009;6:231–249.
Aldrich, T.K., Uhrlass, R.M. Weaning from mechanical ventilation: successful use of modified inspiratory resistive training in muscular dystrophy. Crit. Care Med. 1987;15:247–249.
Aldrich, T.K., Karpel, J.P., Uhrlass, R.M., et al. Weaning from mechanical ventilation: adjunctive use of inspiratory muscle resistive training. Crit. Care Med. 1989;17:143–147.
Amann, M., Proctor, L.T., Sebranek, J.J., et al. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J. Physiol. 2009;587:271–283.
Amann, M., Blain, G.M., Proctor, L.T., et al. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol. 2010;109:966–976.
Asher, M.I., Pardy, R.L., Coates, A.L., et al. The effects of inspiratory muscle training in patients with cystic fibrosis. Am. Rev. Respir. Dis. 1982;126:855–859.
Aznar-Lain, S., Webster, A.L., Canete, S., et al. Effects of inspiratory muscle training on exercise capacity and spontaneous physical activity in elderly subjects: a randomized controlled pilot trial. Int. J. Sports Med. 2007;28:1025–1029.
Baba, R., Nagashima, M., Goto, M., et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J. Am. Coll. Cardiol. 1996;28:1567–1572.
Bailey, S.J., Romer, L.M., Kelly, J., et al. Inspiratory muscle training enhances pulmonary O(2) uptake kinetics and high-intensity exercise tolerance in humans. J. Appl. Physiol. 2010;109:457–468.
Baker, S.E., Sapienza, C.M., Collins, S. Inspiratory pressure threshold training in a case of congenital bilateral abductor vocal fold paralysis. Int. J. Pediatr. Otorhinolaryngol. 2003;67:413–416.
Baker, S.E., Sapienza, C.M., Martin, D., et al. Inspiratory pressure threshold training for upper airway limitation: a case of bilateral abductor vocal fold paralysis. J. Voice. 2003;17:384–394.
Baker, S., Davenport, P., Sapienza, C. Examination of strength training and detraining effects in expiratory muscles. J. Speech Lang. Hear. Res. 2005;48:1325–1333.
Bangsbo, J., Iaia, F.M., Krustrup, P. The Yo-Yo intermittent recovery test : a useful tool for evaluation of physical performance in intermittent sports. Sports Med. 2008;38:37–51.
Barbalho-Moulim, M.C., Miguel, G.P., Forti, E.M., et al. Effects of preoperative inspiratory muscle training in obese women undergoing open bariatric surgery: respiratory muscle strength, lung volumes, and diaphragmatic excursion. Clinics. 2011;66:1721–1727.
Beckerman, M., Magadle, R., Weiner, M., et al. The effects of 1 year of specific inspiratory muscle training in patients with COPD. Chest. 2005;128:3177–3182.
Belman, M.J., Gaesser, G.A. Ventilatory muscle training in the elderly. J. Appl. Physiol. 1988;64:899–905.
Bhandari, A., Xia, Y., Cortright, R., et al. Effect of respiratory muscle training on GLUT-4 in the sheep diaphragm. Med. Sci. Sports Exerc. 2000;32:1406–1411.
Bissett, B., Leditschke, I.A. Inspiratory muscle training to enhance weaning from mechanical ventilation. Anaesth. Intensive Care. 2007;35:776–779.
Bissett, B., Leditschke, I.A., Green, M. Specific inspiratory muscle training is safe in selected patients who are ventilator-dependent: a case series. Intensive Crit. Care Nurs. 2012;28:98–104.
Bissett, B., Leditschke, I.A., Paratz, J.D., et al. Respiratory dysfunction in ventilated patients: can inspiratory muscle training help? Anaesth. Intensive Care. 2012;40:236–246.
Bosnak-Guclu, M., Arikan, H., Savci, S., et al. Effects of inspiratory muscle training in patients with heart failure. Respir. Med. 2011;105:1671–1681.
Bott, J., Blumenthal, S., Buxton, M., et al. Guidelines for the physiotherapy management of the adult, medical, spontaneously breathing patient. Thorax. 2009;64(Suppl. 1):i1–i51.
Britto, R.R., Rezende, N.R., Marinho, K.C., et al. Inspiratory muscular training in chronic stroke survivors: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2011;92:184–190.
Brooks, D., O’Brien, K., Geddes, E.L., et al. Is inspiratory muscle training effective for individuals with cervical spinal cord injury? A qualitative systematic review. Clin. Rehabil. 2005;19:237–246.
Brown, P.I., Sharpe, G.R., Johnson, M.A. Inspiratory muscle training abolishes the blood lactate increase associated with volitional hyperpnoea superimposed on exercise and accelerates lactate and oxygen uptake kinetics at the onset of exercise. Eur. J. Appl. Physiol. 2011;12(6):2117–2129.
Brunherotti, M.A., Bezerra, P.P., Bachur, C.K., et al. Inspiratory muscle training in a newborn with anoxia who was chronically ventilated. Phys. Ther. 2012;92:865–871.
Buchman, A.S., Boyle, P.A., Wilson, R.S., et al. Respiratory muscle strength predicts decline in mobility in older persons. Neuroepidemiology. 2008;31:174–180.
Budweiser, S., Moertl, M., Jorres, R.A., et al. Respiratory muscle training in restrictive thoracic disease: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2006;87:1559–1565.
Cader, S.A., Vale, R.G., Castro, J.C., et al. Inspiratory muscle training improves maximal inspiratory pressure and may assist weaning in older intubated patients: a randomised trial. Journal of Physiotherapy. 2010;56:171–177.
Cader, S.A., de Souza Vale, R.G., Zamora, V.E., et al. Extubation process in bed-ridden elderly intensive care patients receiving inspiratory muscle training: a randomized clinical trial. Clinical Interventions in Aging. 2012;7:437–443.
Cahalin, L.P., Semigran, M.J., Dec, G.W. Inspiratory muscle training in patients with chronic heart failure awaiting cardiac transplantation: results of a pilot clinical trial. Phys. Ther. 1997;77:830–838.
Caine, M.P., McConnell, A.K. The inspiratory muscles can be trained differentially to increase strength or endurance using a pressure threshold, inspiratory muscle training device. Eur. Respir. J. 1998;12:58–59.
Caine, M.P., McConnell, A.K. Pressure threshold inspiratory muscle training improves submaximal cycling performance. In: Sargeant A.J., Siddons H., eds. Third Annual Conference of the European College of Sport Science. Manchester: Centre for Health Care Development; 1998:101.
Callahan, L.A. Invited editorial on ‘acquired respiratory muscle weakness in critically ill patients: what is the role of mechanical ventilation-induced diaphragm dysfunction?’. J. Appl. Physiol. 2009;106:360–361.
Carlucci, A., Ceriana, P., Prinianakis, G., et al. Determinants of weaning success in patients with prolonged mechanical ventilation. Crit. Care. 2009;13:R97.
Caruso, P., Denari, S.D., Ruiz, S.A., et al. Inspiratory muscle training is ineffective in mechanically ventilated critically ill patients. Clinics. 2005;60:479–484.
Casali, C.C., Pereira, A.P., Martinez, J.A., et al. Effects of inspiratory muscle training on muscular and pulmonary function after bariatric surgery in obese patients. Obes. Surg. 2011;21:1389–1394.
Cejudo, P., Lopez-Marquez, I., Lopez-Campos, J.L., et al. Factors associated with quality of life in patients with chronic respiratory failure due to kyphoscoliosis. Disabil. Rehabil. 2009;31:928–934.
Chatham, K., Ionescu, A.A., Nixon, L.S., et al. A short-term comparison of two methods of sputum expectoration in cystic fibrosis. Eur. Respir. J. 2004;23:435–439.
Cheah, B.C., Boland, R.A., Brodaty, N.E., et al. INSPIRATIonAL – INSPIRAtory muscle training in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2009;10:1–9.
Cheng, S., Butler, J.E., Gandevia, S.C., et al. Movement of the human upper airway during inspiration with and without inspiratory resistive loading. J. Appl. Physiol. 2011;110:69–75.
Chiappa, G.R., Borghi-Silva, A., Ferreira, L.F., et al. Kinetics of muscle deoxygenation are accelerated at the onset of heavy-intensity exercise in patients with COPD: relationship to central cardiovascular dynamics. J. Appl. Physiol. 2008;104:1341–1350.
Chiappa, G.R., Roseguini, B.T., Vieira, P.J., et al. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J. Am. Coll. Cardiol. 2008;51:1663–1671.
Chiappa, G.R., Queiroga, F., Jr., Meda, E., et al. Heliox improves oxygen delivery and utilization during dynamic exercise in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2009;179:1004–1010.
Chiara, T., Martin, D., Sapienza, C. Expiratory muscle strength training: speech production outcomes in patients with multiple sclerosis. Neurorehabil. Neural Repair. 2007;21:239–249.
Conti, G., Montini, L., Pennisi, M.A., et al. A prospective, blinded evaluation of indexes proposed to predict weaning from mechanical ventilation. Intensive Care Med. 2004;30:830–836.
Copestake, A.J., McConnell, A.K. Inspiratory muscle training reduces exertional breathlessness in healthy elderly men and women. In: International Conference on Physical Activity and Health in the Elderly. Scotland: University of Sterling; 1995:150.
Correa, A.P., Ribeiro, J.P., Balzan, F.M., et al. Inspiratory muscle training in type 2 diabetes with inspiratory muscle weakness. Med. Sci. Sports Exerc. 2011;43:1135–1141.
Currell, K., Jeukendrup, A.E. Validity, reliability and sensitivity of measures of sporting performance. Sports Med. 2008;38:297–316.
Dall’Ago, P., Chiappa, G.R., Guths, H., et al. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J. Am. Coll. Cardiol. 2006;47:757–763.
de Jong, W., van Aalderen, W.M., Kraan, J., et al. Inspiratory muscle training in patients with cystic fibrosis. Respir. Med. 2001;95:31–36.
De Jonghe, B., Bastuji-Garin, S., Durand, M.C., et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit. Care Med. 2007;35:2007–2015.
Demoule, A., Verin, E., Montcel, S.T., et al. Short-term training-dependent plasticity of the corticospinal diaphragm control in normal humans. Respir. Physiol. Neurobiol. 2008;160:172–180.
DePalo, V.A., Parker, A.L., Al-Bilbeisi, F., et al. Respiratory muscle strength training with nonrespiratory maneuvers. J. Appl. Physiol. 2004;96:731–734.
Derrickson, J., Ciesla, N., Simpson, N., et al. A comparison of two breathing exercise programs for patients with quadriplegia. Phys. Ther. 1992;72:763–769.
Dettling, D.S., van der Schaaf, M., Blom, R.L., et al, Feasibility and effectiveness of pre-operative inspiratory muscle training in patients undergoing oesophagectomy: a pilot study. Physiother. Res. Int. 2012; doi:10.1002/pri.1524. [Apr 10. [Epub ahead of print]. Available at:].
Dickinson, J., Whyte, G., McConnell, A. Inspiratory muscle training: a simple cost-effective treatment for inspiratory stridor. Br. J. Sports Med. 2007;41:694–695. [discussion 695].
DiMarco, A.F., Kelling, J.S., DiMarco, M.S., et al. The effects of inspiratory resistive training on respiratory muscle function in patients with muscular dystrophy. Muscle Nerve. 1985;8:284–290.
Donath, J., Miller, A. Restrictive chest wall disorders. Semin. Respir. Crit. Care Med. 2009;30:275–292.
Downey, A.E., Chenoweth, L.M., Townsend, D.K., et al. Effects of inspiratory muscle training on exercise responses in normoxia and hypoxia. Respir. Physiol. Neurobiol. 2007;156:137–146.
Dronkers, J., Veldman, A., Hoberg, E., et al. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: a randomized controlled pilot study. Clin. Rehabil. 2008;22:134–142.
Dronkers, J.J., Lamberts, H., Reutelingsperger, I.M., et al. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clin. Rehabil. 2010;24:614–622.
Edwards, A.M., Cooke, C.B. Oxygen uptake kinetics and maximal aerobic power are unaffected by inspiratory muscle training in healthy subjects where time to exhaustion is extended. Eur. J. Appl. Physiol. 2004;93:139–144.
Edwards, A.M., Wells, C., Butterly, R. Concurrent inspiratory muscle and cardiovascular training differentially improves both perceptions of effort and 5000 m running performance compared with cardiovascular training alone. Br. J. Sports Med. 2008;42:523–527.
Edwards, A.M., Maguire, G.P., Graham, D., et al. Four weeks of inspiratory muscle training improves self-paced walking performance in overweight and obese adults: a randomised controlled trial. Journal of Obesity. 2012;2012:918202.
Enright, S., Chatham, K., Ionescu, A.A., et al. Inspiratory muscle training improves lung function and exercise capacity in adults with cystic fibrosis. Chest. 2004;126:405–411.
Enright, S.J., Unnithan, V.B., Heward, C., et al. Effect of high-intensity inspiratory muscle training on lung volumes, diaphragm thickness, and exercise capacity in subjects who are healthy. Phys. Ther. 2006;86:345–354.
Epstein, C.D., El-Mokadem, N., Peerless, J.R. Weaning older patients from long-term mechanical ventilation: a pilot study. Am. J. Respir. Crit. Care Med. 2002;11:369–377.
Ferreira, J.B., Plentz, R.D., Stein, C., et al. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: A randomized controlled trial. Int. J. Cardiol. 2011. [Oct 8. [Epub ahead of print]].
Fitting, J.W. Respiratory muscles during ventilatory support. Eur. Respir. J. 1994;7:2223–2225.
Frank, I., Briggs, R., Spengler, C.M. Respiratory muscles, exercise performance, and health in overweight and obese subjects. Med. Sci. Sports Exerc. 2011;43:714–727.
Fregonezi, G.A., Resqueti, V.R., Guell, R., et al. Effects of 8-week, interval-based inspiratory muscle training and breathing retraining in patients with generalized myasthenia gravis. Chest. 2005;128:1524–1530.
Fry, D.K., Pfalzer, L.A., Chokshi, A.R., et al. Randomized control trial of effects of a 10-week inspiratory muscle training program on measures of pulmonary function in persons with multiple sclerosis. J. Neurol. Phys. Ther. 2007;31:162–172.
Furrer, E., Baur, S., Boutellier, U. Treatment of snoring by training of the upper airway muscles. Am. J. Respir. Crit. Care Med. 1998;157:A284.
Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001;81:1725–1789.
Geddes, E.L., O’Brien, K., Reid, W.D., et al. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: An update of a systematic review. Respir. Med. 2008;102:1715–1729.
Gething, A.D., Williams, M., Davies, B. Inspiratory resistive loading improves cycling capacity: a placebo controlled trial. Br. J. Sports Med. 2004;38:730–736.
Gomez-Fernandez, P., Sanchez Agudo, L., Calatrava, J.M., et al. Respiratory muscle weakness in uremic patients under continuous ambulatory peritoneal dialysis. Nephron. 1984;36:219–223.
Goosey-Tolfrey, V., Foden, E., Perret, C., et al. Effects of inspiratory muscle training on respiratory function and repetitive sprint performance in wheelchair basketball players. Br. J. Sports Med. 2010;44:665–668.
Gosselink, R., Kovacs, L., Ketelaer, P., et al. Respiratory muscle weakness and respiratory muscle training in severely disabled multiple sclerosis patients. Arch. Phys. Med. Rehabil. 2000;81:747–751.
Gosselink, R., De Vos, J., van den Heuvel, S.P., et al. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur. Respir. J. 2011;37:416–425.
Gozal, D., Thiriet, P. Respiratory muscle training in neuromuscular disease: long-term effects on strength and load perception. Med. Sci. Sports Exerc. 1999;31:1522–1527.
Griffiths, L.A., McConnell, A.K. The influence of inspiratory and expiratory muscle training upon rowing performance. Eur. J. Appl. Physiol. 2007;99:457–466.
Hajghanbari, B., Yamabayashi, C., Buna, T., et al, Effects of respiratory muscle training on performance in athletes: a systematic review with meta-analyses. J. Strength Cond. Res. 2012; doi:10.1519/JSC.0b013e318269f73f. [[Epub ahead of print]. Available at:].
Heijdra, Y.F., Dekhuijzen, P.N., van Herwaarden, C.L., et al. Nocturnal saturation improves by target-flow inspiratory muscle training in patients with COPD. Am. J. Respir. Crit. Care Med. 1996;153:260–265.
Heimer, D., Brami, J., Lieberman, D., et al. Respiratory muscle performance in patients with type 1 diabetes. Diabet. Med. 1990;7:434–437.
Hennis, P.J., Meale, P.M., Grocott, M.P. Cardiopulmonary exercise testing for the evaluation of perioperative risk in non-cardiopulmonary surgery. Postgrad. Med. J. 2011;87:550–557.
Hepburn, H., Fletcher, J., Rosengarten, T.H., et al. Cardiac vagal tone, exercise performance and the effect of respiratory training. Eur. J. Appl. Physiol. 2005;94:681–689.
Hodges, P.W., Gandevia, S.C. Activation of the human diaphragm during a repetitive postural task. J. Physiol. 2000;522(pt 1):165–175.
Holm, P., Sattler, A., Fregosi, R.F. Endurance training of respiratory muscles improves cycling performance in fit young cyclists. BMC Physiol. 2004;4:9.
How, S.C., McConnell, A.K., Taylor, B.J., et al. Acute and chronic responses of the upper airway to inspiratory loading in healthy awake humans: an MRI study. Respir. Physiol. Neurobiol. 2007;157:270–280.
Hull, J., Aniapravan, R., Chan, E., et al. British Thoracic Society guideline for respiratory management of children with neuromuscular weakness. Thorax. 2012;67(Suppl. 1):i1–40.
Hulzebos, E.H., Helders, P.J., Favie, N.J., et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. J. Am. Med. Assoc. 2006;296:1851–1857.
Hulzebos, E.H., van Meeteren, N.L., van den Buijs, B.J., et al. Feasibility of preoperative inspiratory muscle training in patients undergoing coronary artery bypass surgery with a high risk of postoperative pulmonary complications: a randomized controlled pilot study. Clin. Rehabil. 2006;20:949–959.
Hulzebos, E.H., Smit, Y., Helders, P.P., van Meeteren, N.L. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst, Rev 11. 2012.
Illi, S.K., Held, U., Frank, I., et al. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta-analysis. Sports Med. 2012;42:707–724.
Innocenti, F., Fabbri, A., Anichini, R., et al. Indications of reduced pulmonary function in type 1 (insulin-dependent) diabetes mellitus. Diabetes Res. Clin. Pract. 1994;25:161–168.
Inzelberg, R., Peleg, N., Nisipeanu, P., et al. Inspiratory muscle training and the perception of dyspnea in Parkinson’s disease. Can. J. Neurol. Sci. 2005;32:213–217.
Jack, S., West, M., Grocott, M.P. Perioperative exercise training in elderly subjects. Best Pract. Res. Clin. Anaesthesiol. 2011;25:461–472.
Janssens, J.P., Pache, J.C., Nicod, L.P. Physiological changes in respiratory function associated with ageing. Eur. Respir. J. 1999;13:197–205.
Johnson, P.H., Cowley, A.J., Kinnear, W.J. A randomized controlled trial of inspiratory muscle training in stable chronic heart failure. Eur. Heart. J. 1998;19:1249–1253.
Johnson, M.A., Sharpe, G.R., Brown, P.I. Inspiratory muscle training improves cycling time-trial performance and anaerobic work capacity but not critical power. Eur. J. Appl. Physiol. 2007;101:761–770.
Jones, C.U., Sangthong, B., Pachirat, O. An inspiratory load enhances the antihypertensive effects of home-based training with slow deep breathing: a randomised trial. Journal of Physiotherapy. 2010;56:179–186.
Just, N., Bautin, N., Danel-Brunaud, V., et al. The Borg dyspnoea score: a relevant clinical marker of inspiratory muscle weakness in amyotrophic lateral sclerosis. Eur. Respir. J. 2010;35:353–360.
Kaminski, D.M., Schaan, B.D., da Silva, A.M., et al. Inspiratory muscle weakness is associated with autonomic cardiovascular dysfunction in patients with type 2 diabetes mellitus. Clin. Auton. Res. 2011;21:29–35.
Kang, S.W., Shin, J.C., Park, C.I., et al. Relationship between inspiratory muscle strength and cough capacity in cervical spinal cord injured patients. Spinal Cord. 2006;44:242–248.
Keens, T.G., Krastins, I.R., Wannamaker, E.M., et al. Ventilatory muscle endurance training in normal subjects and patients with cystic fibrosis. Am. Rev. Respir. Dis. 1977;116:853–860.
Kikuchi, Y., Okabe, S., Tamura, G., et al. Chemosensitivity and perception of dyspnea in patients with a history of near-fatal asthma. N. Engl. J. Med. 1994;330:1329–1334.
Kilding, A.E., Brown, S., McConnell, A.K. Inspiratory muscle training improves 100 and 200 m swimming performance. Eur. J. Appl. Physiol. 2010;108:505–511.
Kim, J., Davenport, P., Sapienza, C. Effect of expiratory muscle strength training on elderly cough function. Arch. Gerontol. Geriatr. 2008;48(3):361–366.
Klefbeck, B., Lagerstrand, L., Mattsson, E. Inspiratory muscle training in patients with prior polio who use part- time assisted ventilation. Arch. Phys. Med. Rehabil. 2000;81:1065–1071.
Klefbeck, B., Hamrah Nedjad, J. Effect of inspiratory muscle training in patients with multiple sclerosis. Arch. Phys. Med. Rehabil. 2003;84:994–999.
Kodric, M., Trevisan, R., Torregiani, C. Inspiratory muscle training for diaphragm dysfunction after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2012. [(in press).].
Koessler, W., Wanke, T., Winkler, G., et al. 2 years’ experience with inspiratory muscle training in patients with neuromuscular disorders. Chest. 2001;120:765–769.
Kulkarni, S.R., Fletcher, E., McConnell, A.K., et al. Pre-operative inspiratory muscle training preserves postoperative inspiratory muscle strength following major abdominal surgery – a randomised pilot study. Ann. R. Coll. Surg. Engl. 2010;92:700–707.
Lanini, B., Gigliotti, F., Coli, C., et al. Dissociation between respiratory effort and dyspnoea in a subset of patients with stroke. Clin. Sci. (Lond.). 2002;103:467–473.
Laoutaris, I., Dritsas, A., Brown, M.D., et al. Inspiratory muscle training using an incremental endurance test alleviates dyspnea and improves functional status in patients with chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2004;11:489–496.
Leddy, J.J., Limprasertkul, A., Patel, S., et al. Isocapnic hyperpnea training improves performance in competitive male runners. Eur. J. Appl. Physiol. 2007;99:665–676.
Lerman, R.M., Weiss, M.S. Progressive resistive exercise in weaning high quadriplegics from the ventilator. Paraplegia. 1987;25:130–135.
Liaw, M.Y., Lin, M.C., Cheng, P.T., et al. Resistive inspiratory muscle training: its effectiveness in patients with acute complete cervical cord injury. Arch. Phys. Med. Rehabil. 2000;81:752–756.
Liaw, M.Y., Wang, Y.H., Tsai, Y.C., et al. Inspiratory muscle training in bronchiectasis patients: a prospective randomized controlled study. Clin. Rehabil. 2011;25:524–536.
Lin, S.J., McElfresh, J., Hall, B., et al. Inspiratory muscle training in patients with heart failure: a systematic review. Cardiopulm. Phys. Ther. J. 2012;23:29–36.
Lindstedt, S.L., Reich, T.E., Keim, P., et al. Do muscles function as adaptable locomotor springs? J. Exp. Biol. 2002;205:2211–2216.
Lisboa, C., Moreno, R., Fava, M., et al. Inspiratory muscle function in patients with severe kyphoscoliosis. Am. Rev. Respir. Dis. 1985;132:48–52.
Litchke, L.G., Russian, C.J., Lloyd, L.K., et al. Effects of respiratory resistance training with a concurrent flow device on wheelchair athletes. J. Spinal Cord Med. 2008;31:65–71.
Lomax, M. Inspiratory muscle training, altitude, and arterial oxygen desaturation: a preliminary investigation. Aviat. Space Environ. Med. 2010;81:498–501.
Lomax, M., Grant, I., Corbett, J. Inspiratory muscle warm-up and inspiratory muscle training: separate and combined effects on intermittent running to exhaustion. J. Sports Sci. Med. 2011;29:563–569.
Lötters, F., van Tol, B., Kwakkel, G., et al. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur. Respir. J. 2002;20(3):570–576.
Lougheed, M.D., Lam, M., Forkert, L., et al. Breathlessness during acute bronchoconstriction in asthma. Pathophysiologic mechanisms. Am. Rev. Respir. Dis. 1993;148:1452–1459.
Loveridge, B., Badour, M., Dubo, H. Ventilatory muscle endurance training in quadriplegia: effects on breathing pattern. Paraplegia. 1989;27:329–339.
Lykidis, C.K., White, M.J., Balanos, G.M. The pulmonary vascular response to the sustained activation of the muscle metaboreflex in man. Exp. Physiol. 2008;93:247–253.
Maat, R.C., Roksund, O.D., Olofsson, J., et al. Surgical treatment of exercise-induced laryngeal dysfunction. Eur. Arch. Otorhinolaryngol. 2007;264:401–407.
Mador, M.J., Krawza, M., Alhajhusian, A., et al. Interval training versus continuous training in patients with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation and Prevention. 2009;29:126–132.
Magadle, R., Berar-Yanay, N., Weiner, P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002;121:329–333.
Mancini, D.M., Henson, D., La Manca, J., et al. Benefit of selective respiratory muscle training on exercise capacity in patients with chronic congestive heart failure. Circulation. 1995;91:320–329.
Markov, G., Spengler, C.M., Knopfli-Lenzin, C., et al. Respiratory muscle training increases cycling endurance without affecting cardiovascular responses to exercise. Eur. J. Appl. Physiol. 2001;85:233–239.
Martin, A.J., Stern, L., Yeates, J., et al. Respiratory muscle training in Duchenne muscular dystrophy. Dev. Med. Child Neurol. 1986;28:314–318.
Martin, A.D., Davenport, P.D., Franceschi, A.C., et al. Use of inspiratory muscle strength training to facilitate ventilator weaning: a series of 10 consecutive patients. Chest. 2002;122:192–196.
Martin, A.D., Smith, B.K., Davenport, P.D., et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit. Care. 2011;15:R84.
Martinez, A., Lisboa, C., Jalil, J., et al. Selective training of respiratory muscles in patients with chronic heart failure. Rev. Med. Chil. 2001;129:133–139.
McConnell, A.K. Breathe strong, perform better. Champaign, IL: Human Kinetics Publishers; 2011.
McConnell, A.K., Lomax, M. The influence of inspiratory muscle work history and specific inspiratory muscle training upon human limb muscle fatigue. J. Physiol. 2006;577:445–457.
McConnell, A.K., Caine, M.P., Donovan, K.J., et al. Inspiratory muscle training improves lung function and reduces exertional dyspnoea in mild / moderate asthmatics. Clin. Sci. 1998;95:4P.
McMahon, M.E., Boutellier, U., Smith, R.M., et al. Hyperpnea training attenuates peripheral chemosensitivity and improves cycling endurance. J. Exp. Biol. 2002;205:3937–3943.
Meade, M., Guyatt, G., Cook, D., et al. Predicting success in weaning from mechanical ventilation. Chest. 2001;120:400S–424S.
Mello, P.R., Guerra, G.M., Borile, S., et al. Inspiratory muscle training reduces sympathetic nervous activity and improves inspiratory muscle weakness and quality of life in patients with chronic heart failure: a clinical trial. Journal of Cardiopulmonary Rehabilitation and Prevention. 2012;32(5):255–261.
Moodie, L., Reeve, J., Elkins, M. Inspiratory muscle training increases inspiratory muscle strength in patients weaning from mechanical ventilation: a systematic review. Journal of Physiotherapy. 2011;57:213–221.
Moran, F., Piper, A., Elborn, J.S., et al. Respiratory muscle pressures in non-CF bronchiectasis: repeatability and reliability. Chron. Respir. Dis. 2010;7:165–171.
Mota, S., Guell, R., Barreiro, E., et al. Clinical outcomes of expiratory muscle training in severe COPD patients. Respir. Med. 2007;101:516–524.
Neves, P.C., Guerra, M., Ponce, P., et al. Non-cystic fibrosis bronchiectasis. Interactive Cardiovascular and Thoracic Surgery. 2011;13(6):619–625.
Newall, C., Stockley, R.A., Hill, S.L. Exercise training and inspiratory muscle training in patients with bronchiectasis. Thorax. 2005;60:943–948.
NICE: National Clinical Guideline Centre, Hypertension: The clinical management of primary hypertension in adults. Royal College of Physicians: London, 2011. www.nice.org.uk/nicemedia/live/13561/56007/56007.pdf.
Nicks, C.R., Morgan, D.W., Fuller, D.K., et al. The influence of respiratory muscle training upon intermittent exercise performance. Int. J. Sports Med. 2009;30(1):16–21.
O’Brien, K., Geddes, E.L., Reid, W.D., et al. Inspiratory muscle training compared with other rehabilitation interventions in chronic obstructive pulmonary disease: a systematic review update. Journal of Cardiopulmonary Rehabilitation and Prevention. 2008;28:128–141.
Olgiati, R., Girr, A., Hugi, L., et al. Respiratory muscle training in multiple sclerosis: a pilot study. Schweiz. Arch. Neurol. Psychiatr. 1989;140:46–50.
Ortancil, O., Sarikaya, S., Sapmaz, P., et al. The effect(s) of a six-week home-based exercise program on the respiratory muscle and functional status in ankylosing spondylitis. J. Clin. Rheumatol. 2009;15:68–70.
Padula, C.A., Yeaw, E., Mistry, S. A home-based nurse-coached inspiratory muscle training intervention in heart failure. Appl. Nurs. Res. 2009;22:18–25.
Pellizzaro, C.O., Thome, F.S., Veronese, F.V. Effect of peripheral and respiratory muscle training on the functional capacity of hemodialysis patients. Ren. Fail. 2013;35:189–197.
Perkoff, G.T., Silber, R., Tyler, F.H., et al. Studies in disorders of muscle. XII. Myopathy due to the administration of therapeutic amounts of 17-hydroxycorticosteroids. Am. J. Med. 1959;26:891–898.
Piepoli, M.F., Conraads, V., Corra, U., et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur. J. Heart Fail. 2011;13:347–357.
Pinto, S., Turkman, A., Pinto, A., et al. Predicting respiratory insufficiency in amyotrophic lateral sclerosis: the role of phrenic nerve studies. Clin. Neurophysiol. 2009;120:941–946.
Pinto, S., Swash, M., de Carvalho, M. Respiratory exercise in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2012;13:33–43.
Plentz, R.D., Sbruzzi, G., Ribeiro, R.A., et al. Inspiratory muscle training in patients with heart failure: meta-analysis of randomized trials. Arq. Bras. Cardiol. 2012;99:762–771.
Prezant, D.J. Effect of uremia and its treatment on pulmonary function. Lung. 1990;168:1–14.
Puhan, M.A., Suarez, A., Lo Cascio, C., et al. Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. BMJ. 2006;332:266–270.
Ram, F.S., Wellington, S.R., Barnes, N.C. Inspiratory muscle training for asthma. Cochrane Database Syst. Rev. 2003. [CD003792].
Ramirez-Sarmiento, A., Orozco-Levi, M., Guell, R., et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am. J. Respir. Crit. Care Med. 2002;166:1491–1497.
Rassler, B., Hallebach, G., Kalischewski, P., et al. The effect of respiratory muscle endurance training in patients with myasthenia gravis. Neuromuscul. Disord. 2007;17:385–391.
Rassler, B., Marx, G., Hallebach, S., et al. Long-term respiratory muscle endurance training in patients with myasthenia gravis: first results after four months of training. Autoimmune Diseases. 2011;2011:808607.
Reid, W.D., Geddes, E.L., O’Brien, K., et al. Effects of inspiratory muscle training in cystic fibrosis: a systematic review. Clin. Rehabil. 2008;22:1003–1013.
Ribeiro, J.P., Chiappa, G.R., Callegaro, C.C. The contribution of inspiratory muscles function to exercise limitation in heart failure: pathophysiological mechanisms. Revista Brasileira de Fisioterapia. 2012;16(4):261–267.
Romer, L.M., McConnell, A.K. Specificity and reversibility of inspiratory muscle training. Med. Sci. Sports Exerc. 2003;35:237–244.
Romer, L.M., McConnell, A.K., Jones, D.A. Effects of inspiratory muscle training on time-trial performance in trained cyclists. J. Sports Sci. Med. 2002;20:547–562.
Romer, L.M., McConnell, A.K., Jones, D.A. Effects of inspiratory muscle training upon recovery time during high intensity, repetitive sprint activity. Int. J. Sports Med. 2002;23:353–360.
Romer, L.M., McConnell, A.K., Jones, D.A. Effects of inspiratory muscle training upon time trial performance in trained cyclists. J. Sports Sci. Med. 2002;20:547–562.
Romer, L.M., McConnell, A.K., Jones, D.A. Inspiratory muscle fatigue in trained cyclists: effects of inspiratory muscle training. Med. Sci. Sports Exerc. 2002;34:785–792.
Romer, L.M., Lovering, A.T., Haverkamp, H.C., et al. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J. Physiol. 2006;571:425–439.
Roth, E.J., Noll, S.F. Stroke rehabilitation. 2. Comorbidities and complications. Arch. Phys. Med. Rehabil. 1994;75:S42–S46.
Roth, E.J., Stenson, K.W., Powley, S., et al. Expiratory muscle training in spinal cord injury: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2010;91:857–861.
Ruddy, B.H., Davenport, P., Baylor, J., et al. Inspiratory muscle strength training with behavioral therapy in a case of a rower with presumed exercise-induced paradoxical vocal-fold dysfunction. Int. J. Pediatr. Otorhinolaryngol. 2004;68:1327–1332.
Rutchik, A., Weissman, A.R., Almenoff, P.L., et al. Resistive inspiratory muscle training in subjects with chronic cervical spinal cord injury. Arch. Phys. Med. Rehabil. 1998;79:293–297.
Sabate, M., Rodriguez, M., Mendez, E., et al. Obstructive and restrictive pulmonary dysfunction increases disability in Parkinson disease. Arch. Phys. Med. Rehabil. 1996;77:29–34.
Sadowsky, S., Dwyer, J., Fischer, A. Failure of hyperoxic gas to alter the arterial lactate anaerobic threshold. J. Cardiopulm. Rehabil. 1995;15:114–121.
Sapienza, C.M., Davenport, P.W., Martin, A.D. Expiratory muscle training increases pressure support in high school band students. J. Voice. 2002;16:495–501.
Savci, S., Degirmenci, B., Saglam, M., et al. Short-term effects of inspiratory muscle training in coronary artery bypass graft surgery: A randomized controlled trial. Scand. Cardiovasc. J. 2011;45:286–293.
Sawyer, E.H., Clanton, T.L. Improved pulmonary function and exercise tolerance with inspiratory muscle conditioning in children with cystic fibrosis. Chest. 1993;104:1490–1497.
Sbruzzi, G., Dal Lago, P., Ribeiro, R.A., et al. Inspiratory muscle training and quality of life in patients with heart failure: systematic review of randomized trials. Int. J. Cardiol. 2012;156:120–121.
Scherer, T.A., Spengler, C.M., Owassapian, D., et al. Respiratory muscle endurance training in chronic obstructive pulmonary disease: impact on exercise capacity, dyspnea, and quality of life. Am. J. Respir. Crit. Care Med. 2000;162:1709–1714.
Schilero, G.J., Spungen, A.M., Bauman, W.A., et al. Pulmonary function and spinal cord injury. Respir. Physiol. Neurobiol. 2009;166:129–141.
Sheel, A.W., Reid, W.D., Townson, A.F., et al. Effects of exercise training and inspiratory muscle training in spinal cord injury: a systematic review. J. Spinal Cord Med. 2008;31:500–508.
Shoemaker, M.J., Donker, S., Lapoe, A. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: the state of the evidence. Cardiopulmonary Physical Therapy Journal. 2009;20:5–15.
Silva, V.G., Amaral, C., Monteiro, M.B., et al. Effects of inspiratory muscle training in hemodialysis patients. Jornal Brasileiro de Nefrologia. 2011;33:62–68.
Silva Mdos, S., Martins, A.C., Cipriano, G., Jr., et al. Inspiratory training increases insulin sensitivity in elderly patients. Geriatrics and Gerontology International. 2012;12:345–351.
Smart, N.A., Giallauria, F., Dieberg, G. Efficacy of inspiratory muscle training in chronic heart failure patients: A systematic review and meta-analysis. Int. J. Cardiol. 2012. [May 3. [Epub ahead of print]].
Smeltzer, S.C., Lavietes, M.H., Cook, S.D. Expiratory training in multiple sclerosis. Arch. Phys. Med. Rehabil. 1996;77:909–912.
Smith, P.E., Coakley, J.H., Edwards, R.H. Respiratory muscle training in Duchenne muscular dystrophy. Muscle Nerve. 1988;11:784–785.
Smith, B.K., Bleiweis, M.S., Neel, C.R., et al. Inspiratory muscle strength training in infants with congenital heart disease and prolonged mechanical ventilation: a case report. Phys. Ther. 2012. [Mar 30. [Epub ahead of print]].
Sprague, S.S., Hopkins, P.D. Use of inspiratory strength training to wean six patients who were ventilator-dependent. Phys. Ther. 2003;83:171–181.
Spungen, A.M., Grimm, D.R., Lesser, M., et al. Self-reported prevalence of pulmonary symptoms in subjects with spinal cord injury. Spinal Cord. 1997;35:652–657.
Stambler, N., Charatan, M., Cedarbaum, J.M. Prognostic indicators of survival in ALS. ALS CNTF Treatment Study Group. Neurology. 1998;50:66–72.
Stein, R., Chiappa, G.R., Guths, H., et al. Inspiratory muscle training improves oxygen uptake efficiency slope in patients with chronic heart failure. Journal of Cardiopulmonary Rehabilitation and Prevention. 2009;29:392–395.
Stern, L.M., Martin, A.J., Jones, N., et al. Training inspiratory resistance in Duchenne dystrophy using adapted computer games. Dev. Med. Child Neurol. 1989;31:494–500.
Stuessi, C., Spengler, C.M., Knopfli-Lenzin, C., et al. Respiratory muscle endurance training in humans increases cycling endurance without affecting blood gas concentrations. Eur. J. Appl. Physiol. 2001;84:582–586.
Summerhill, E.M., Angov, N., Garber, C., et al. Respiratory muscle strength in the physically active elderly. Lung. 2007;185:315–320.
Sutbeyaz, S.T., Koseoglu, F., Inan, L., et al. Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: a randomized controlled trial. Clin. Rehabil. 2010;24:240–250.
Takaso, M., Nakazawa, T., Imura, T., et al. Surgical correction of spinal deformity in patients with congenital muscular dystrophy. J. Orthop. Sci. 2010;15:493–501.
Taylor, B.J., Romer, L.M. Effect of expiratory resistive loading on inspiratory and expiratory muscle fatigue. Respir. Physiol. Neurobiol. 2009;16:164–174.
Thomas, M.J., Simpson, J., Riley, R., et al. The impact of home-based physiotherapy interventions on breathlessness during activities of daily living in severe COPD: a systematic review. Physiotherapy. 2010;96:108–119.
Tong, T.K., Fu, F.H. Effect of specific inspiratory muscle warm-up on intense intermittent run to exhaustion. Eur. J. Appl. Physiol. 2006;97:673–680.
Tong, T.K., Fu, F.H., Eston, R., et al. Chronic and acute inspiratory muscle loading augment the effect of a 6-week interval program on tolerance of high-intensity intermittent bouts of running. J. Strength Cond. Res. 2010;24(11):3041–3048.
Tong, T.K., Lin, H., McConnell, A., et al. Respiratory and locomotor muscle blood-volume and oxygenation kinetics during intense intermittent exercise. Eur. J. Sport Sci. 2012;12:321–330.
Topin, N., Matecki, S., Le Bris, S., et al. Dose-dependent effect of individualized respiratory muscle training in children with Duchenne muscular dystrophy. Neuromuscul. Disord. 2002;12:576–583.
Turner, L.A., Mickleborough, T.D., McConnell, A.K., et al. Effect of inspiratory muscle training on exercise tolerance in asthmatic individuals. Med. Sci. Sports Exerc. 2011;43:2031–2038.
Turner, L.A., Tecklenburg-Lund, S.L., Chapman, R.F., et al. Inspiratory muscle training lowers the oxygen cost of voluntary hyperpnea. J. Appl. Physiol. 2012;112:127–134.
Tzelepis, G.E., McCool, F.D., Friedman, J.H., et al. Respiratory muscle dysfunction in Parkinson’s disease. Am. Rev. Respir. Dis. 1988;138:266–271.
Tzelepis, G.E., Vega, D.L., Cohen, M.E., et al. Pressure–flow specificity of inspiratory muscle training. J. Appl. Physiol. 1994;77:795–801.
Tzelepis, G.E., Kasas, V., McCool, F.D. Inspiratory muscle adaptations following pressure or flow training in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1999;79:467–471.
Van Houtte, S., Vanlandewijck, Y., Gosselink, R. Respiratory muscle training in persons with spinal cord injury: a systematic review. Respir. Med. 2006;100:1886–1895.
Van Houtte, S., Vanlandewijck, Y., Kiekens, C., et al. Patients with acute spinal cord injury benefit from normocapnic hyperpnoea training. J. Rehabil. Med. 2008;40:119–125.
Verges, S., Boutellier, U., Spengler, C.M. Effect of respiratory muscle endurance training on respiratory sensations, respiratory control and exercise performance: a 15-year experience. Respir. Physiol. Neurobiol. 2008;161:16–22.
Villiot-Danger, J.C., Villiot-Danger, E., Borel, J.C., et al. Respiratory muscle endurance training in obese patients. Int. J. Obes. (Lond). 2011;35:692–699.
Vilozni, D., Bar-Yishay, E., Gur, I., et al. Computerized respiratory muscle training in children with Duchenne muscular dystrophy. Neuromuscul. Disord. 1994;4:249–255.
Vincent, K.R., Braith, R.W., Feldman, R.A., et al. Improved cardiorespiratory endurance following 6 months of resistance exercise in elderly men and women. Arch. Intern. Med. 2002;162:673–678.
Volianitis, S., McConnell, A.K., Koutedakis, Y., et al. Inspiratory muscle training improves rowing performance. Med. Sci. Sports Exerc. 2001;33:803–809.
Wanke, T., Toifl, K., Merkle, M., et al. Inspiratory muscle training in patients with Duchenne muscular dystrophy. Chest. 1994;105:475–482.
Ward, C.P., York, K.M., McCoy, J.G. Risk of obstructive sleep apnea lower in double reed wind musicians. Journal of Clinical Sleep Medicine. 2012;8:251–255.
Watsford, M., Murphy, A. The effects of respiratory-muscle training on exercise in older women. J. Aging Phys. Act. 2008;16:245–260.
Weinberg, J., Borg, J., Bevegard, S., et al. Respiratory response to exercise in postpolio patients with severe inspiratory muscle dysfunction. Arch. Phys. Med. Rehabil. 1999;80:1095–1100.
Weiner, P., Weiner, M. Inspiratory muscle training may increase peak inspiratory flow in chronic obstructive pulmonary disease. Respiration. 2006;73:151–156.
Weiner, P., Azgad, Y., Ganam, R., et al. Inspiratory muscle training in patients with bronchial asthma. Chest. 1992;102:1357–1361.
Weiner, P., Azgad, Y., Weiner, M., et al. Inspiratory muscle training during treatment with corticosteroids in humans. Chest. 1995;107:1041–1044.
Weiner, P., Ganem, R., Zamir, D., et al. Specific inspiratory muscle training in chronic hemodialysis. Harefuah. 1996;130(73–76):144.
Weiner, P., Man, A., Weiner, M., et al. The effect of incentive spirometry and inspiratory muscle training on pulmonary function after lung resection. J. Thorac. Cardiovasc. Surg. 1997;113:552–557.
Weiner, P., Gross, D., Meiner, Z., et al. Respiratory muscle training in patients with moderate to severe myasthenia gravis. Can. J. Neurol. Sci. 1998;25:236–241.
Weiner, P., Zeidan, F., Zamir, D., et al. Prophylactic inspiratory muscle training in patients undergoing coronary artery bypass graft. World J. Surg. 1998;22:427–431.
Weiner, P., Waizman, J., Magadle, R., et al. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin. Cardiol. 1999;22:727–732.
Weiner, P., Berar-Yanay, N., Davidovich, A., et al. Specific inspiratory muscle training in patients with mild asthma with high consumption of inhaled beta(2)-agonists. Chest. 2000;117:722–727.
Weiner, P., Inzelberg, R., Davidovich, A., et al. Respiratory muscle performance and the Perception of dyspnea in Parkinson’s disease. Can. J. Neurol. Sci. 2002;29:68–72.
Weiner, P., Magadle, R., Beckerman, M., et al. The relationship among inspiratory muscle strength, the perception of dyspnea and inhaled beta2-agonist use in patients with asthma. Can. Respir. J. 2002;9:307–312.
Weiner, P., Magadle, R., Massarwa, F., et al. Influence of gender and inspiratory muscle training on the perception of dyspnea in patients with asthma. Chest. 2002;122:197–201.
Weiner, P., Magadle, R., Beckerman, M., et al. Comparison of specific expiratory, inspiratory, and combined muscle training programs in COPD. Chest. 2003;124:1357–1364.
Weiner, P., Magadle, R., Beckerman, M., et al. Specific expiratory muscle training in COPD. Chest. 2003;124:468–473.
Wells, G.D., Plyley, M., Thomas, S., et al. Effects of concurrent inspiratory and expiratory muscle training on respiratory and exercise performance in competitive swimmers. Eur. J. Appl. Physiol. 2005;94:527–540.
West, C.R., Taylor, B.J., Campbell, I.G., et al. Effects of inspiratory muscle training in Paralympic athletes with cervical spinal cord injury. Med. Sci. Sports Exerc. 2009;41:S31–S32.
Wijnhoven, H.A., Kriegsman, D.M., Snoek, F.J., et al. Gender differences in health-related quality of life among asthma patients. J. Asthma. 2003;40:189–199.
Winkler, G., Zifko, U., Nader, A., et al. Dose-dependent effects of inspiratory muscle training in neuromuscular disorders. Muscle Nerve. 2000;23:1257–1260.
Witt, J.D., Guenette, J.A., Rupert, J.L., et al. Inspiratory muscle training attenuates the human respiratory muscle metaboreflex. J. Physiol. 2007;584:1019–1028.
Wright, J.M., Musini, V.M. First-line drugs for hypertension. Cochrane Database Sys. Rev. 2009. [CD001841].
Xiao, Y., Luo, M., Wang, J., et al. Inspiratory muscle training for the recovery of function after stroke. Cochrane Database Sys. Rev. 5, 2012. [CD009360].




