Chapter 3
The respiratory muscles
An important concept that will be explored in this chapter is that of imbalance in the demand / capacity relationship of the respiratory muscles, and, in particular, the inspiratory muscles. Evaluating function in the context of relative demand is a pragmatic method of defining ‘weakness’, since it incorporates context. A muscle might not be considered ‘weak’ in absolute terms, but if the demands that are placed upon a ‘normal muscle’ are excessive then it is rendered ‘weak’ functionally. For example, morbidly obese people with normal quadriceps muscle strength have functional weakness by virtue of their greater body mass. The same principle applies to the respiratory muscles: patients with lung fibrosis may have normal inspiratory muscle strength, but the elevated intrinsic inspiratory load generates functional weakness that manifests as reduced inspiratory muscle endurance (Hart et al, 2002). Traditionally, weakness has been defined by reference to measures of strength, but it is important to appreciate that strength is a one-dimensional index of function, and is just one of a number of important functional properties of muscles (see Ch. 4, Fig. 4.1). Thus weakness and dysfunction are multi-dimensional, and can be primary (sub-normal performance) and functional (inadequate for the prevailing demands). The overriding question is whether the muscles’ capability is ‘fit for purpose’, or whether it induces a functional limitation. The latter can be defined as the inability to undertake a task that would be considered normal. For example, the inability of a middle-aged woman to walk on level ground at 3.96 km·h− 1 (1.10 m·s− 1) (Bohannon & Williams Andrews, 2011) without the need to stop and ‘catch her breath’ defines her as being functionally limited by dyspnoea. In Chapter 1, the underlying physiology of dyspnoea and breathing effort was described. Although dyspnoea is a complex phenomenon, a major contributor to its magnitude is the relative intensity of inspiratory muscle work. This is determined by two factors: (1) the prevailing respiratory system mechanics, and (2) the function of the respiratory muscles; in other words, the resistances and elastances that must be overcome during breathing, as well as the capacity of the respiratory muscles to meet these mechanical demands. An exacerbating factor with respect to this relationship is the prevailing ventilatory demand, which is affected by a wide range of factors including aerobic fitness, ventilation / perfusion matching, diffusing capacity and breathing pattern. To add a further layer of complexity to the demand / capacity relationship of the respiratory muscles, many of these factors are interdependent; for example, altered lung mechanics can precipitate a rapid shallow breathing pattern, which in turn increases the demand for minute ventilation because of its effect on the dead space / tidal volume relationship.
This chapter will explore how disease, exercise and posture interact to influence the demand / capacity relationship of the respiratory muscles. In doing so, a theoretical rationale for specific training will be offered. Evidence relating to the influence of respiratory muscle training upon clinical outcomes is considered in Chapter 4.
CHANGES IN RESPIRATORY MUSCLE FUNCTION AND BREATHING MECHANICS
In the various conditions described below there is either: (1) an imbalance in the demand / capacity relationship of the respiratory muscles that contributes to dyspnoea, exercise limitation and even to respiratory failure, or (2) a contribution to symptoms or morbidity that arises from the respiratory system, including the upper airway. This section will therefore describe the abnormalities of respiratory mechanics, respiratory muscle function and ventilatory demand in a range of situations and, in so doing, establish the rationale for training the respiratory muscles. A comprehensive overview of respiratory muscle disorders can also be found in the excellent review of Laghi & Tobin (2003).
Respiratory disease
The primary symptom and exercise-limiting factor in respiratory disease is dyspnoea. In this section the impact of respiratory disease upon respiratory mechanics, respiratory muscle function and ventilatory demand will be described. Readers wishing to know more about the physiological basis of respiratory disease are referred to Hamid and colleagues’ comprehensive text on the subject (Hamid et al, 2005).
Chronic obstructive pulmonary disease
The hallmark of chronic obstructive pulmonary disease (COPD) is expiratory flow limitation, which results from both reduced lung recoil and airway tethering (see Ch. 1), in addition to intrinsic airway narrowing. Although the most obvious repercussion of airway narrowing for the respiratory muscles is an increased flow resistive work of breathing, this is only the tip of the iceberg. In recent years, the phase ‘dynamic hyperinflation’ has emerged to describe how the loss of lung recoil and airway narrowing disrupt normal breathing mechanics during exercise. Hyperinflation is a pathophysiological manifestation of airway obstruction, and the consequent expiratory flow limitation, which lead to incomplete lung emptying, i.e., expiration is curtailed before the lungs have reached their equilibrium volume (functional residual capacity: FRC). The lungs therefore become hyperinflated. In severe COPD, hyperinflation is present at rest (static hyperinflation), but during exercise even mild obstruction results in a state of dynamic hyperinflation, the severity of which is proportional to the severity of flow limitation and the magnitude of the ventilatory demand.
Figure 3.1 illustrates how, during exercise, expiratory flow limitation stimulates migration of the tidal flow loop towards total lung capacity (TLC), increasing end-expiratory lung volume (EELV) and reducing inspiratory capacity (IC). Although dynamic hyperinflation serves to maximize tidal expiratory flow under conditions of expiratory flow limitation (by moving the tidal flow loop away from the maximum envelope), the requirement to breathe at higher ranges of the TLC increases the elastic load presented to the inspiratory muscles by the lungs and chest wall. This creates a ‘restrictive’ pulmonary defect. The most important mechanical and sensory repercussions of expiratory flow limitation are therefore borne by the inspiratory muscles. However, the repercussions of hyperinflation and / or expiratory flow limitation are not limited to an increase in the elastic work of breathing; inspiratory muscle loading is exacerbated in three further ways:
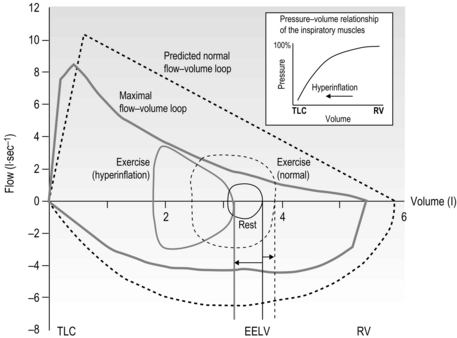
Figure 3.1 Comparison of the response of the exercise tidal flow volume in a person with expiratory flow limitation (EFL) (solid lines), compared with that predicted for someone with normal lungs (dashed lines). Note that in the presence of EFL there is encroachment upon the inspiratory capacity in order to increase minute ventilation (flow volume loop shifts to the left and end-expiratory lung volume (EELV) increases). The person with normal lungs (dashed lines) is able to increase minute ventilation by utilizing both their inspiratory and expiratory reserve volumes (EELV decreases). The inset illustrates the pressure–volume relationship of the inspiratory muscles showing that, as lung volume increases from residual volume (RV) towards total lung capacity (TLC) (as occurs in hyperinflation) the inspiratory muscles become weaker.
• By inducing functional weakening of the inspiratory muscles (see inset to Fig. 3.1). Foreshortening of expiration alters diaphragm geometry, making it flatter and moving the inspiratory muscles to a weaker portion of their pressure–volume relationship (Decramer, 1997).
• By generating intrinsic positive end-expiratory pressure (PEEPi). Expiration ends before all of the forces acting on the lung are in equilibrium, so inspiration is initiated under a positive expiratory load.
• By forcing inspiratory time to shorten. This is another adaptive response, in this case to allow more time for expiration. The cost is to move the inspiratory muscles to a weaker portion of their force–velocity relationship (the faster a muscle contracts, the lower is its force-generating capacity).
Hyperinflation has also been shown to impair respiratory muscle blood flow in a dog model (Kawagoe et al, 1994); in this study, despite an almost two-fold increase in the work of breathing, diaphragm blood flow remained unchanged and accessory muscle blood flow fell during acute hyperinflation. It is not clear whether hyperinflation exerts the same effect in human beings with COPD, but impaired accessory muscle perfusion in the face of an increased demand for muscle work would predispose these muscles to fatigue and / or accumulation of metabolic by-products (see section ‘Respiratory muscle involvement in exercise limitation’).
Thus, COPD-induced changes in respiratory mechanics exert a very potent influence upon dyspnoea because they affect both the demand for inspiratory pressure generation and the capacity of the inspiratory muscles to generate sufficient pressure to meet that demand (see Fig. 3.1). Both phenomena increase the requirement for inspiratory motor drive and intensify dyspnoea (O’Donnell, 2001). However, the inspiratory muscle dysfunction of COPD is not confined to the functional (secondary) weakening precipitated by hyperinflation (Similowski et al, 1991; Polkey et al, 1996). There is also primary dysfunction due to abnormalities within the muscle tissue itself, which lead to declines in strength and endurance (Levine et al, 2003; Barreiro et al, 2005; Ottenheijm et al, 2005). This deterioration of muscle may be in part due to disuse (sedentary lifestyles), but is more likely to be the result of oxidative stress (Barreiro et al, 2005) resulting from the systemic manifestations of COPD, including the chronic inflammatory state. Furthermore, malnutrition causes generalized muscle weakness, which may exacerbate disease-specific respiratory muscle weakness (Decramer, 2001). Finally, the use of oral corticosteroids has been shown to have a myopathic influence upon the respiratory muscles of patients without respiratory disease, who show significant reductions in strength (~ 30%) and endurance (~ 50%) over the treatment period (Weiner et al, 1993; Weiner et al, 1995). Although these changes show some reversal following cessation of corticosteroid treatment, function may take as long as 6 months to normalize (Weiner et al, 1993). Since primary and secondary dysfunction coexist, there is a significant impairment in the capacity of the inspiratory muscles to deliver changes in intrathoracic pressure and tidal volume. Indeed, disease severity correlates negatively with respiratory muscle function (Terzano et al, 2008). Furthermore, hyperinflation leads to changes in chest wall geometry, inducing functional weakening of the accessory inspiratory muscles, which also contributes to a global reduction in the ability of the respiratory pump to generate inspiratory pressure (De Troyer & Wilson, 2009).
Much has been made in recent years of the adaptations that occur within the inspiratory muscles in response to the mechanical changes and increased physical demands described above. The chronically hyperinflated, flattened state of the diaphragm in COPD appears to lead to shortening of the total diaphragm length by around 15% to 25%, depending upon whether this is assessed at functional residual capacity FRC or residual volume (RV) respectively (McKenzie et al, 2009). This adaptation reduces the ability of the diaphragm to shorten during contraction, and thus limits its ability to generate inspiratory flow. However, the adaptations in diaphragm geometry and length appear to have some functional benefits in terms of maintaining its ability to deliver volume excursion, as well as its pressure-generating capacity (McKenzie et al, 2009). In respect of the latter, at equivalent absolute lung volumes the diaphragm pressure-generating capacity of patients with COPD is equal, or superior, to that of control participants (Similowski et al, 1991). However, despite this, the ability of the diaphragm to generate changes in volume at high lung volumes is diminished (McKenzie et al, 2009). It is important to keep in mind that diaphragm function at the same relative lung volumes is impaired in patients with COPD (see above), and that they have a reduced reserve capacity for volume and flow generation.
Change in diaphragm length is not the only chronic adaptation to hyperinflation and chronic inspiratory loading in patients with COPD. There are also changes in diaphragm biochemistry that appear to result from chronic loading (Levine et al, 1997; Ottenheijm et al, 2005). The healthy diaphragm is composed predominantly of two types of muscles fibres: one with high endurance but low power (type I, 45%), the other with low endurance but high power (type II, 55%). Patients with long-standing COPD have an abnormally high proportion of the former (type I 64%, type II 36%), which is an adaptive response to continuous inspiratory muscle loading (Levine et al, 1997).
Studies of the functional properties of the COPD-adapted diaphragm in vitro indicate that the fibres have a smaller cross-sectional area, contain less contractile protein and generate lower forces than those from patients without COPD (Ottenheijm et al, 2005). The dynamic properties of the contractile machinery of the COPD-adapted fibres are also impaired; the fibres appear to be less sensitive to calcium and show slower rates of myosin to actin attachment / detachment (Ottenheijm et al, 2005). Thus there is not only a loss of contractile protein; the protein that remains is also dysfunctional.
On the face of it, a shift towards an endurance-trained phenotype might be considered a positive adaptation; indeed it is cited as a reason for the futility of specific inspiratory muscle training (Polkey et al, 2011). However, it has been suggested that the increase in the proportion of type I fibres might, at least in part, explain the reduction in force-generating capacity (Clanton & Levine, 2009). Thus, depending upon the specific demands placed upon the inspiratory muscles, this adaptation can be either advantageous or disadvantageous. For example, it is advantageous for prolonged, low-intensity work, but disadvantageous for short, high-intensity work. The former is encountered at rest, whereas the latter is encountered during exercise. The diaphragm in patients with COPD therefore appears to be well adapted to generating low flow rates for long periods of time, but this adaptation robs them of the ability to generate the high pressures and flow rates required during exercise.
This suggestion is confirmed by studies of the in vivo strength and endurance of the inspiratory muscles of patients with COPD. For example, compared with control individuals, evoked diaphragm twitch pressure, maximal inspiratory pressure and a measure of endurance during inspiratory loading were all lower in patients with COPD (Barreiro et al, 2005). Furthermore, impairments were proportional to the severity of disease, despite the fact that a concomitant increase in type I fibres, and decrease in capillary to fibre ratio, were also proportional to disease severity. Thus, the shift towards a more endurance-trained phenotype reduced strength and did not appear to protect the inspiratory muscles from global fatigue under conditions of inspiratory loading (Barreiro et al, 2005). This is probably because weaker muscles must operate at a greater proportion of their maximum capacity, which predisposes them to fatigue.
Notwithstanding this apparent predisposition to fatigue, studies have so far failed to demonstrate evidence of exercise-induced contractile fatigue of the diaphragm in patients with COPD using low-frequency phrenic nerve stimulation (Polkey et al, 1995; Mador et al, 2000a; Mador et al, 2000b). However, this finding should not be misinterpreted to indicate that the inspiratory muscles are working within the limits of their capacity to deliver ![]() E, or that they do not impose any limitation upon exercise tolerance. The latter issue will be explored in greater detail in the section ‘Respiratory muscle involvement in exercise limitation’, but in the meantime it is noteworthy that studies where COPD patients walk (Kyroussis et al, 1996) or cycle (Yan et al, 1997) to the limit of tolerance have found a predominance of the rib cage muscle contribution to breathing. By measuring the rate of relaxation of the inspiratory muscles following a sniff effort, it is possible to detect the presence of global inspiratory muscle fatigue. Using this technique, it has been shown that, in patients who walk to the limit of tolerance, there is a slowing of the relaxation rate of oesophageal sniff pressure without any change in diaphragm twitch pressure, which is suggestive of accessory inspiratory muscle fatigue (Kyroussis et al, 1996). Furthermore, there does appear to be a subgroup of COPD patients who display diaphragm fatigue post-exercise (see below) (Hopkinson et al, 2010).
E, or that they do not impose any limitation upon exercise tolerance. The latter issue will be explored in greater detail in the section ‘Respiratory muscle involvement in exercise limitation’, but in the meantime it is noteworthy that studies where COPD patients walk (Kyroussis et al, 1996) or cycle (Yan et al, 1997) to the limit of tolerance have found a predominance of the rib cage muscle contribution to breathing. By measuring the rate of relaxation of the inspiratory muscles following a sniff effort, it is possible to detect the presence of global inspiratory muscle fatigue. Using this technique, it has been shown that, in patients who walk to the limit of tolerance, there is a slowing of the relaxation rate of oesophageal sniff pressure without any change in diaphragm twitch pressure, which is suggestive of accessory inspiratory muscle fatigue (Kyroussis et al, 1996). Furthermore, there does appear to be a subgroup of COPD patients who display diaphragm fatigue post-exercise (see below) (Hopkinson et al, 2010).
Finally, this section would be incomplete without mentioning the expiratory muscles, as well as contextualizing the changes in muscle function induced by COPD. As has already been alluded to, there is generalized muscle weakness, which also affects the expiratory muscles (Gosselink et al, 2000). In COPD patients the voluntary force-generating capacity of the expiratory muscles (maximal expiratory pressure: MEP) is ~ 30% lower than in healthy elderly people (Gosselink et al, 2000). This compares with differences in maximal inspiratory pressure (MIP), handgrip and quadriceps strength of ~ 40%, ~ 20% and 25%, respectively (Gosselink et al, 2000). The slightly larger effect of COPD upon MIP than MEP is most likely a manifestation of the additional influence of secondary weakness, due to the effects of hyperinflation (Gosselink et al, 2000). A recent study examined the influence of symptom limited cycling upon non-voluntary measures of expiratory and inspiratory muscle strength in patients with COPD; a significant exercise-induced fatigue of the abdominal muscles (7.2% fall in twitch gastric pressure) was found, but no change in diaphragm function (Hopkinson et al, 2010). Interestingly, only around one-third of the group exhibited expiratory muscle fatigue (twitch gastric pressure, 21%), and this subgroup also exhibited a significant fall in twitch diaphragm pressure (7.9%). The non-fatiguers exhibited no change in twitch gastric pressure, but a 7.7% increase in twitch diaphragm pressure. Unfortunately, the group was not subdivided to examine the diaphragm fatiguers in more detail. These data suggest that: (1) there is both inspiratory and expiratory muscle overload in at least some patients with COPD, and (2) diaphragm fatigue may be masked by lack of reliability in baseline measurements of twitch diaphragm pressure.
Patients with COPD also experience an increase in the demand for inspiratory muscle work, which arises from an elevated demand for minute ventilation (![]() E), especially during exercise. Ventilation/perfusion mismatching and a higher than normal ratio of dead space to tidal volume (VD / VT) both necessitate an increase in
E), especially during exercise. Ventilation/perfusion mismatching and a higher than normal ratio of dead space to tidal volume (VD / VT) both necessitate an increase in ![]() E in order to minimize changes in blood gases, but hypoxaemia is nevertheless a common finding. Furthermore, patients with COPD also have poor aerobic fitness, which increases the ventilatory demand of exercise (Casaburi et al, 1991), and thus increases inspiratory muscle work still further. Needless to say, these increased ventilatory flow requirements also exacerbate hyperinflation (Somfay et al, 2002).
E in order to minimize changes in blood gases, but hypoxaemia is nevertheless a common finding. Furthermore, patients with COPD also have poor aerobic fitness, which increases the ventilatory demand of exercise (Casaburi et al, 1991), and thus increases inspiratory muscle work still further. Needless to say, these increased ventilatory flow requirements also exacerbate hyperinflation (Somfay et al, 2002).
In summary, patients with COPD have a dramatically increased demand for inspiratory muscle work, but a reduced capacity to supply this demand due to muscle dysfunction. In other words, the demand / capacity relationship is stacked in completely the wrong direction. In the section ‘Respiratory muscle involvement in exercise limitation’, respiratory muscle-induced limitations to exercise tolerance will be considered, and Chapter 4 will review the evidence supporting specific respiratory muscle training for patients with COPD.
Asthma
The mechanical abnormalities in patients with asthma mimic closely those described in COPD; however, there are important differences. For example, there is less reduction in static lung recoil pressure and more widespread intrathoracic airway narrowing in asthma (Pride & Macklem, 1986). In addition, the increased airway collapsibility in patients with COPD is not seen in asthmatics. Furthermore, the reversible nature of airways obstruction in asthma results in relatively short-lived periods of stress upon the inspiratory muscles. The latter means that patients with asthma do not show the same changes in inspiratory muscle length or fibre composition that are expressed in patients with COPD (see above).
There is no clear consensus regarding the presence of primary weakness of the inspiratory muscles in patients with asthma compared with healthy people, as no biopsy data exists. However, the finding that steroid-dependent patients receiving oral corticosteroids show lower inspiratory muscle strength, but similar severity of hyperinflation, suggests that there may be myopathy in steroid-dependent patients with asthma (Akkoca et al, 1999). Generally, respiratory muscle strength and endurance are relatively normal in patients with stable asthma (Hill, 1991).
However, it is accepted universally that bronchoconstriction-induced hyperinflation is associated with secondary weakness of the inspiratory muscles (Fig. 3.1 inset) (Weiner et al, 1990; Perez et al, 1996; Akkoca et al, 1999; Stell et al, 2001; Weiner et al, 2002). As is the case in COPD, the major mechanical consequences of airway narrowing are increased flow resistive work, increased elastic work and PEEPi (resulting from dynamic lung hyperinflation), as well as reduced dynamic lung compliance (Martin et al, 1980; Lougheed et al, 1995). In a study comparing inspiratory muscle function of patients with COPD and asthma, with equivalent severity of hyperinflation, endurance was impaired to a greater degree in patients with asthma (Perez et al, 1996). Interestingly, strength was lower in the COPD patients compared with those with asthma. These data suggest that some of the structural and biochemical adaptations that occur in response to chronic loading in COPD are absent in patients with asthma. Thus, where airway obstruction is present, patients with asthma experience the same acute functional defect in their pulmonary function as those with COPD. However, the reversible nature of the airway obstruction may place patients with asthma at a functional disadvantage, and thus greater vulnerability to functional overload.
In a study of histamine-induced bronchoconstriction (FEV1 49% of baseline), the inspiratory work was found to increase 11-fold, 69% of the increase being due to the elastic component of the work of breathing (Martin et al, 1983). In addition, there also appears to be a prolonged activation of inspiratory muscles during exhalation in the presence of bronchoconstriction-induced hyperinflation (Muller et al, 1980; Muller et al, 1981), which suggests that the work of the total inspiratory muscles may be increased to an even greater extent than inspiratory work alone indicates.
The interrelationship between bronchoconstriction, hyperinflation and dyspnoea has also been studied. Multiple regression analysis indicates that, during methacholine-induced bronchoconstriction, change in inspiratory capacity (an index of dynamic hyperinflation) was the most powerful predictor of dyspnoea during bronchoconstriction – accounting for 74% of the variance in the perceptual rating (Lougheed et al, 1993). These observations are supported by more recent evidence confirming that hyperinflation is a major determinant of dyspnoea in patients with asthma (Martinez-Moragon et al, 2003).
As is the case in COPD, the mechanical changes associated with bronchoconstriction most likely increase the intensity of dyspnoea via their effect upon the magnitude of inspiratory neural drive (see Ch. 1). There is experimental support for this suggestion; Bellofiore et al (1996) found that the strongest determinant of dyspnoea during methacholine-induced bronchoconstriction was inspiratory neural drive (P0.1, mouth occlusion pressure), which explained 82% of the total variance in dyspnoea. More recently, Binks et al (2002) reported that institution of mechanical ventilation during methacholine-induced bronchoconstriction and hyperinflation significantly reduced ratings of ‘effort to breathe’ in people with mild asthma. Furthermore, it has also been shown that gender differences in inspiratory muscle strength may underpin differences in dyspnoea perception, quality of life and consumption of β2-agonist medication (Weiner et al, 2002). These data, along with data from inspiratory muscle training studies (see Ch. 4), support the notion that inspiratory muscle strength, and hence the relative intensity of inspiratory muscle work, makes a fundamental contribution to dyspnoea in people with asthma.
Because exercise is a trigger for asthma in around 90% of people with asthma (Wilkerson, 1998) there is an understandable anxiety regarding exercise that might translate into avoidance of physical activity, and poor aerobic fitness (Welsh et al, 2004). However, there remains no clear consensus regarding levels of physical activity and fitness, especially in children with asthma (Wilkerson, 1998), though there is some evidence to suggest that the aerobic fitness of adults with asthma is generally low (Satta, 2000). Thus, poor aerobic conditioning may exacerbate hyperinflation-related increases in the work of breathing during exercise by increasing the ventilatory requirement and exacerbating hyperinflation.
In summary, patients with asthma have an increased demand for inspiratory muscle work, which is proportional to the severity of their airway obstruction. It is not clear whether they have any primary weakness of their inspiratory muscles, but there is evidence of steroid-induced myopathy of the inspiratory muscles in steroid-dependent asthma. Furthermore, secondary weakness due to the influence of hyperinflation is well established, and linked strongly to dyspnoea. In the section ‘Respiratory muscle involvement in exercise limitation’, respiratory muscle-induced limitations to exercise tolerance will be considered, and Chapter 4 will review the evidence supporting specific respiratory muscle training for patients with asthma.
Bronchiectasis
Bronchiectasis is a chronic lung disease that is not normally included within the umbrella of COPD, but which overlaps with it (Neves et al, 2011); indeed one study found that 50% of patients with COPD also had bronchiectasis (Patel et al, 2004). It is characterized by irreversible widening of the medium-sized airways accompanied by inflammation, chronic infection and destruction of the bronchial walls (Neves et al, 2011). Both the pathology and the functional manifestations of bronchiectasis have similarities with those of COPD, including inflammatory cell profiles, protease release and consequent airway obstruction (Neves et al, 2011). In both conditions, these factors lead to detrimental changes in breathing mechanics, attendant exertional dyspnoea and exercise intolerance. Symptomology is also similar to COPD – including cough, sputum production and wheeze (Neves et al, 2011). Expiratory flow limitation (identified using the negative expiratory pressure technique) is present at rest in 39% of patients with bronchiectasis, which is a lower prevalence than in patients with COPD (Koulouris et al, 2003). The explanation for the latter finding may be that around half of patients had both obstructive and restrictive defects, i.e., restriction acted as a confounding influence; the presence of flow limitation was correlated with the MRC dyspnoea score, which in turn was correlated with exercise tolerance (Koulouris et al, 2003). Thus, the mechanical changes associated with bronchiectasis increase the demand for inspiratory muscle work, which is manifested symptomatically as exertional dyspnoea.
Compared with healthy people of a similar age, patients with moderate-to-severe bronchiectasis exhibit lower maximal inspiratory and expiratory muscle strength (around 20% and 40% lower, respectively) (Newall et al, 2005; Moran et al, 2010). The origin of this weakness is unclear, but is most likely due to a combination of primary weakness and functional weakness due to hyperinflation. Thus, in common with patients with COPD, patients with bronchiectasis have an imbalance in the demand / capacity relationship of the respiratory muscles. This imbalance will also be considered in the section ‘Respiratory muscle involvement in exercise limitation’, and Chapter 4 will review the evidence supporting specific respiratory muscle training for patients with bronchiectasis.
Cystic fibrosis
Respiratory failure is the most common cause of death in patients with cystic fibrosis (CF) (Taylor-Cousar, 2009), and dyspnoea is one of their main complaints (Leroy et al, 2011); it has also been suggested that the deterioration of lung function in patients with CF have is insufficient to explain their exertional dyspnoea. Patients with CF have an elevated work of breathing (Dunnink et al, 2009), and this has been identified as an important contributor to dyspnoea (Leroy et al, 2011). There appears to be no evidence of inspiratory muscle weakness in patients with CF; indeed, some authors have reported that patients with CF have superior strength (Dufresne et al, 2009; Dunnink et al, 2009) and diaphragm thickness (Dufresne et al, 2009). The elevated airway resistance of patients with CF appears to contribute to their diaphragm hypertrophy (Dufresne et al, 2009). However, patients with the lowest fat-free mass exhibit a loss of diaphragm thickness (Ionescu et al, 1998; Enright et al, 2007). Furthermore, although indices of inspiratory muscle strength have been found to be normal or superior in patients with CF, loss of maximal inspiratory muscle work capacity has been reported (Ionescu et al, 1998; Enright et al, 2007), suggesting that there is a deterioration in the metabolic properties of the inspiratory muscles. This finding is suggestive of an imbalance between demand and capacity since the preservation of inspiratory muscle strength is accompanied by an increased demand for inspiratory muscle work, and dyspnoea. The fact that respiratory failure is the primary cause of death highlights the important influence of the imbalance between demand and capacity. This will also be considered in the section ‘Respiratory muscle involvement in exercise limitation’, and Chapter 4 will review the evidence supporting specific respiratory muscle training for patients with CF.
Restrictive chest wall disorders
Conditions such as kyphoscoliosis, fibrothorax, thoracoplasty, flail chest and ankylosing spondylitis all induce chest wall restriction, creating a restrictive pulmonary defect in which total respiratory system elastance and resistance are elevated (Donath & Miller, 2009). In the case of severe kyphosis and / or scoliosis, thoracic volume may also be reduced by collapse of the vertebral column and the cranial displacement of the abdominal contents. As a consequence, breathing pattern tends to be rapid and shallow, creating a higher than normal ratio of dead space to tidal volume (VD / VT) ratio and necessitating an increase in ![]() E. This exacerbates the already elevated work of inhalation (Donath & Miller, 2009), and attendant dyspnoea. Furthermore, inspiratory muscle function also tends to be impaired (Lisboa et al, 1985; Cejudo et al, 2009), owing to changes in chest wall and diaphragm configuration. In kyphoscoliosis, inspiratory muscle strength has been shown to correlate with forced vital capacity (FVC), as well as to arterial blood gases, such that weakest patients exhibited the worst FVC and blood gases (Lisboa et al, 1985). Ultimately, the outcome of these conditions can be respiratory failure and the requirement for mechanical ventilation. The imbalance in the demand / capacity relationship of the respiratory muscles will also be considered in the section ‘Respiratory muscle involvement in exercise limitation’. See Chapter 4 for a description of the evidence supporting breathing exercises.
E. This exacerbates the already elevated work of inhalation (Donath & Miller, 2009), and attendant dyspnoea. Furthermore, inspiratory muscle function also tends to be impaired (Lisboa et al, 1985; Cejudo et al, 2009), owing to changes in chest wall and diaphragm configuration. In kyphoscoliosis, inspiratory muscle strength has been shown to correlate with forced vital capacity (FVC), as well as to arterial blood gases, such that weakest patients exhibited the worst FVC and blood gases (Lisboa et al, 1985). Ultimately, the outcome of these conditions can be respiratory failure and the requirement for mechanical ventilation. The imbalance in the demand / capacity relationship of the respiratory muscles will also be considered in the section ‘Respiratory muscle involvement in exercise limitation’. See Chapter 4 for a description of the evidence supporting breathing exercises.
Interstitial lung disease (ILD) is an umbrella term for a group of lung disorders that share a number of pathophysiological characteristics and clinical features. The principal feature of ILD is exercise intolerance due to exertional dyspnoea and perceptions of fatigue. Exercise intolerance is correlated with quality of life (Holland, 2010), which makes it an important therapeutic target.
The reduced lung compliance in ILD leads to impairment of vital capacity, and a rapid and shallow breathing pattern that worsens during exercise (Javaheri & Sicilian, 1992). This pattern exacerbates the existing ventilation / perfusion (![]() /
/ ![]() ) mismatch, due to its effect upon the VD / VT ratio. There is also an impairment of diffusing capacity, and the combination with
) mismatch, due to its effect upon the VD / VT ratio. There is also an impairment of diffusing capacity, and the combination with ![]() /
/ ![]() mismatching can precipitate substantial arterial desaturation (Miki et al, 2003). These changes also increase the ventilatory demand of exercise and hence the work of breathing.
mismatching can precipitate substantial arterial desaturation (Miki et al, 2003). These changes also increase the ventilatory demand of exercise and hence the work of breathing.
Sarcoidosis involves multiple organs, but pulmonary manifestations typically predominate (Lynch et al, 2007) in the form of an ILD. Dyspnoea is the most common presentation in patients with early to moderately advanced disease (Baydur et al, 2001). Sarcoidosis is associated with reduced inspiratory and expiratory muscle strength (~ 20% reduction), as well as impaired endurance (Wirnsberger et al, 1997; Baydur et al, 2001; Spruit et al, 2005), and respiratory muscle function correlates more closely with dyspnoea during activities of daily living than pulmonary function (Baydur et al, 2001); indeed dyspnoea can be present in the absence of any lung function defects (Baydur et al, 2001). The underlying mechanisms for respiratory muscle dysfunction in sarcoidosis are unclear, but two case reports indicate that granulomatous involvement of respiratory muscles is present (Dewberry et al, 1993; Pringle & Dewar, 1997). At the time of writing there have been no studies of respiratory muscle training in patients with sarcoidosis or other ILD.
Chronic heart failure and pulmonary hypertension
Patients with chronic heart failure (CHF) present with dyspnoea, exercise intolerance and fatigue. Chronic heart failure is a complex condition that generates a number of interrelated pathophysiological changes that affect skeletal muscle, the vasculature, neurohormonal systems and the lungs (Brubaker, 1997).
Inspiratory muscle dysfunction has not been assessed as widely or with the same rigour in CHF as in COPD. For example, there are no biopsy studies of diaphragm composition, and only one study has examined diaphragm twitch pressure (Hughes et al, 1999). Notwithstanding this, the evidence of inspiratory muscle weakness is consistent and compelling (Ribeiro et al, 2009). It has been suggested that inspiratory muscle weakness may be part of the generalized atrophy that is common in CHF, but there is some evidence that there may be selective weakness of the inspiratory muscles (Ribeiro et al, 2009). Indeed, inspiratory muscle strength has been shown to have prognostic value (Frankenstein et al, 2008, 2009), which underscores its importance. Furthermore, inspiratory muscle weakness is correlated with a number of indices of haemodynamic dysfunction, including cardiac output and the severity of pulmonary hypertension (Filusch et al, 2011).
Patients with CHF tend to adopt a rapid, shallow and constrained breathing pattern during exercise (Johnson et al, 2000). This appears to be an adaptive response to changes in the demand / capacity relationship of the inspiratory muscles. Lung compliance is reduced by pulmonary oedema and pulmonary fibrosis (Wright et al, 1990), which increases the work of breathing (Cross et al, 2012). An interesting feature of the rapid, shallow breathing pattern is that it coexists with expiratory flow limitation and a reduction in end-expiratory lung volume. The result is an increase in both the elastic and resistive work of breathing, the latter being present during both phases of respiration, whilst the former is seen only during inspiration (Cross et al, 2012). Unlike patients with COPD and asthma, patients with CHF do not hyperinflate in order to decrease their expiratory flow limitation (Johnson et al, 2000). Instead, unpleasant breathing sensations are minimized by adopting a rapid, shallow breathing pattern. This strategy suggests that the sensations associated with hyperinflation are more unpleasant than those associated with expiratory flow limitation and rapid, shallow breathing. This is entirely reasonable, given that hyperinflation in the presence of pulmonary oedema and fibrosis would increase inspiratory elastic work considerably (Cross et al, 2012). The mechanisms underlying the greater resistive work of breathing in CHF are uncertain, but may be related to worsening of pulmonary congestions and / or bronchoconstriction during exercise (Cross et al, 2012).
There are also a number of abnormalities that increase the ventilatory requirement of exercise in CHF, and thus increase the demands imposed upon the respiratory muscles. For example, diffusion impairment is present in 67% of patients with severe CHF (Wright et al, 1990). This may be due to pulmonary fibrosis, but may also be due to the influence of an impaired cardiac output upon ventilation/perfusion mismatching (Lewis et al, 1996), which elevates the physiological dead space. Furthermore, rapid, shallow breathing increases dead space ventilation further, because it generates a higher than normal ratio of dead space to tidal volume (VD / VT). Higher dead space necessitates an increase in ![]() E in order to minimize changes in blood gases. A further corollary of rapid, shallow breathing is that the higher inspiratory flow rate increases the relative functional demands upon the inspiratory muscles, which must operate on a weaker part of their force–velocity relationship (see Ch. 4, Fig. 4.1).
E in order to minimize changes in blood gases. A further corollary of rapid, shallow breathing is that the higher inspiratory flow rate increases the relative functional demands upon the inspiratory muscles, which must operate on a weaker part of their force–velocity relationship (see Ch. 4, Fig. 4.1).
Elevated peripheral chemoreflex sensitivity is found in as many as 40% of patients with CHF, and may contribute to the exaggerated exercise hyperpnoea, as well as sympathoexcitation (Chua et al, 1997). Recently, it was found that peripheral chemoreflex sensitivity to carbon dioxide is significantly higher in patients with CHF who have inspiratory muscle weakness than in those who do not (Callegaro et al, 2010). The study authors hypothesized that the elevated chemoreflex sensitivity was secondary to the sympathoexcitation resulting from metaboreflex activation in weakened / fatigued inspiratory muscles. This is consistent with the finding that ventilatory and cardiovascular responses to locomotor muscle metaboreflex activation are increased in patients with CHF (Piepoli et al, 1996). Thus, exaggerated responses to chemoreflex and metaboreflex stimulation during exercise most likely conspire to exacerbate an already elevated ventilatory demand.
For reasons that are not yet fully understood, MIP is an independent risk factor for myocardial infarction and cardiovascular disease death (van der Palen et al, 2004). One study has also shown that patient survival was lower in those patients with low MIP (Meyer et al, 2000).
Finally, pulmonary arterial hypertension (PAH) is worthy of mention at this point; the condition is associated with heart failure, but may also be idiopathic. The symptomatology and respiratory manifestations of PAH are similar to those of CHF, including respiratory muscle weakness (Meyer et al, 2005). Furthermore, there is evidence that inspiratory muscle strength may influence exercise tolerance in patients with PAH (Kabitz et al, 2008a).
Thus patients with CHF and / or PAH have an increased demand for inspiratory muscle work and a reduced capacity to supply this demand due to muscle dysfunction. In the section ‘Respiratory muscle involvement in exercise limitation’ the implications of this in the context of respiratory muscle-induced limitations to exercise tolerance will be considered, and Chapter 4 will review the evidence supporting specific respiratory muscle training for patients with CHF.
Neurological and neuromuscular disease
Spinal cord injury
Respiratory complications remain a major cause of morbidity and mortality in people with spinal cord injury (SCI) (Schilero et al, 2009), the underlying cause for these complications is poor cough function, which leads to mucus retention, atelectasis and infections (Schilero et al, 2009). The extent and severity of respiratory system compromise following SCI depend upon a number of factors including the level of the lesion, the completeness of the lesion and the ensuing temporal adaptations to the lesion. Furthermore, there is an elevated prevalence of obstructive sleep apnoea (OSA) in people with high spinal cord lesions (see also the section on OSA below), which may precipitate an increase in cardiovascular disease risk (Schilero et al, 2009).
Figure 3.2 summarizes the spinal innervation levels of the respiratory muscles, as well as indicating the distinctions between paraplegia and tetraplegia. Lesions above the level of the phrenic motor neurons (C3–C5) induce paralysis of all respiratory muscles, whereas in lower cervical lesions (C5–C8) the functions of the diaphragm and sternocleidomastoid are preserved. However, in the latter there is still a loss of inspiratory accessory muscle function (external intercostals and scalenes), as well as the primary muscles of expiration (internal intercostals and abdominals). Lesions in the thoracic region result in progressively less extensive denervation of the intercostal muscles as the level becomes more caudal, but any lesion above T6 results in complete loss of anterior abdominal wall innervation. Lesions between T6 and L3 result in partial denervation of the anterior abdominal wall, becoming less extensive at more caudal levels. Innervation of the posterior abdominal wall originates between T12 and L4, but these muscles make only a minor contribution to breathing.
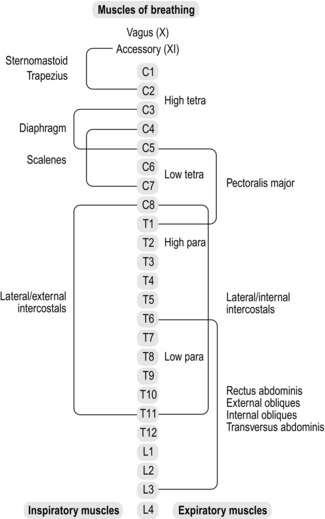
Figure 3.2 Levels of innervation of the respiratory muscles. Para = paraplegia; tetra = tetraplegia.
Maximal respiratory mouth pressures are correlated with the level of lesion for people with complete motor lesions, but not for those with incomplete lesions (Mateus et al, 2007). As one might expect, decrements in strength are greater for the expiratory muscles than for the inspiratory muscles, at equivalent lesion levels, the most severe compromise being for lesions at C4–C5 (maximal expiratory pressure 18% of predicted). In contrast, inspiratory muscle strength is least affected, and for lesions ranging from T1 to L6 is almost normal (85% predicted).
The loss of respiratory pressure-generating capacity has a predictable effect upon lung function, inducing a reduction in forced vital capacity (FVC) to between 49% (C4–C5) and 68% of predicted normal values (T7–L3) (Mateus et al, 2007). Forced expiratory volume in 1 second (FEV1) is also reduced, but this is not an indication of obstruction but rather a reflection of a lower inspiratory capacity (initiating the expiratory effort from a lower lung volume). Indeed, the ratio of FVC to FEV1 is supranormal (90–95%). Notwithstanding this, there is evidence of increased bronchomotor tone, as well as airway hyperresponsiveness, which has been attributed to a loss of sympathetic innervation to the lungs (Schilero et al, 2005).
Respiratory mechanics are altered considerably, especially in tetraplegia. The systems that normally operate to optimize pressure and volume changes during breathing are disrupted, leading to mechanical inefficiency. For example, the normally efficient action of the diaphragm is impaired by paradoxical movement of the rib cage. This paradox may be reduced with time (Scanlon et al, 1989); the improvement has been attributed to ankylosis of the rib cage joints and intercostal spasticity (Estenne & De Troyer, 1985), but the penalty for this is an increased oxygen cost of breathing (Silver, 1963), increased breathing effort perception, a rapid shallow breathing pattern, and possibly increased diaphragm fatigability (Hopman et al, 1997). The increase in abdominal compliance also impairs the efficiency of the diaphragm by reducing the stability of the visceral fulcrum, and inducing a longer resting length. The former also reduces expansion of the lower ribs, generating inhomogeneity of changes in pleural pressure and gas distribution (Estenne & De Troyer, 1985), which is exacerbated by paradoxical movement of the rib cage (Hiraizumi et al, 1986). The resulting ventilation / perfusion mismatching may contribute to inefficient gas exchange.
As one might expect, decrements in pulmonary function are most severe during the acute phase, with some recovery of function over the 12 months following injury. However, recovery shows large inter-individual variation, and has been attributed to improvements in respiratory muscle function and changes in rib cage stability (Schilero et al, 2009).
Respiratory symptoms are common in patients with SCI, with dyspnoea being the most prevalent complaint. Dyspnoea is present in 73% of people with a lesion at C5, but only 29% in those with a lesion below T8 (Spungen et al, 1997). Other symptoms appear to be related to cough and phlegm, which are present in about a quarter of people with SCI, and with no correlation to level of injury. Increasing abdominal compliance, by means of a strapping, in people with lesions between C5 and T6 has been found to reduce breathing effort perception, most likely because of a concomitant increase in diaphragm length and function (Hart et al, 2005).
The prevalence of OSA in patients with SCI is at least twice that observed in the general population, is most prevalent in those with a cervical SCI, and also in the acute phase following injury (Schilero et al, 2009). A number of putative mechanisms have been suggested to explain the high prevalence of OSA in people with SCI, including disruption of the normal coordination between upper airway and respiratory pump muscles, thickening of the oropharyngeal wall and increased adiposity of the neck leading to reduced upper airway patency (Schilero et al, 2009).
The role of the respiratory muscle denervation in exercise tolerance will also be considered in the section ‘Respiratory muscle involvement in exercise limitation’, and Chapter 4 will review the evidence supporting specific respiratory muscle training for patients with SCI.
Other NMDs
Weakness of both inspiratory and expiratory muscles is common in NMD, and leads to a restrictive pattern of pulmonary dysfunction, especially in advanced disease (Gibson et al, 1977). Because physical activity is limited by generalized deterioration of muscle function, dyspnoea is not always present. However, ventilatory limitation due to respiratory muscle weakness may be exacerbated by disease-specific factors that elevate the ventilatory demand, such as an early onset of the anaerobic threshold, particularly where mitochondrial myopathy is present (Flaherty et al, 2001). In addition, the adoption of a rapid, shallow breathing pattern generates a higher than normal ratio of dead space to tidal volume (VD / VT), increasing the demand for minute ventilation (![]() E), and the associated work of breathing. There may also be ventilation / perfusion mismatching and impaired gas exchange (Rochester, 1993). In advanced disease, respiratory muscle weakness, muscle fibrosis and microatelectasis may lead to chronic hypoventilation and hypercapnia. Under these conditions, there is a high risk of inspiratory muscle fatigue in response to small changes in the requirement for inspiratory muscle work, e.g., in the event of pulmonary complications (Kang, 2006). A recent study found that in patients with ALS, a supine Borg score ≥ 3 was associated with impaired inspiratory muscle strength and a lower vital capacity. The authors suggested that this simple assessment might provide a useful test of inspiratory muscle weakness in ALS (Just et al, 2010). Furthermore, respiratory muscle strength correlates with capability of daily living in the self-care and social function domains of a quality of life questionnaire (PEDI) in children with cerebral palsy (Wang et al, 2012), and with physical functioning domains of a quality of life questionnaire (SF-36) in patients with myotonic dystrophy (Araujo et al, 2010). These findings suggest a link between respiratory muscle strength and quality of life in children and adults with NMD.
E), and the associated work of breathing. There may also be ventilation / perfusion mismatching and impaired gas exchange (Rochester, 1993). In advanced disease, respiratory muscle weakness, muscle fibrosis and microatelectasis may lead to chronic hypoventilation and hypercapnia. Under these conditions, there is a high risk of inspiratory muscle fatigue in response to small changes in the requirement for inspiratory muscle work, e.g., in the event of pulmonary complications (Kang, 2006). A recent study found that in patients with ALS, a supine Borg score ≥ 3 was associated with impaired inspiratory muscle strength and a lower vital capacity. The authors suggested that this simple assessment might provide a useful test of inspiratory muscle weakness in ALS (Just et al, 2010). Furthermore, respiratory muscle strength correlates with capability of daily living in the self-care and social function domains of a quality of life questionnaire (PEDI) in children with cerebral palsy (Wang et al, 2012), and with physical functioning domains of a quality of life questionnaire (SF-36) in patients with myotonic dystrophy (Araujo et al, 2010). These findings suggest a link between respiratory muscle strength and quality of life in children and adults with NMD.
Sleep-disordered breathing is also secondary to inspiratory muscle weakness via its influence upon vital capacity (Ragette et al, 2002). Furthermore, upper airway muscle involvement in NMD can result in obstructive respiratory events during sleep (Aboussouan, 2009). Aspiration and difficulties with swallowing are also related to the impairment of bulbar muscle function (Aboussouan, 2009). Poor cough function and respiratory muscle weakness conspire to make respiratory complications a leading cause of morbidity and mortality in NMD (Macklem, 1986).
Acute respiratory failure is a common complication of a number of acute onset neuromuscular conditions, such as Guillain–Barré syndrome, myasthenia gravis and polymyositis (Mehta, 2006), as well as in chronic conditions following development of respiratory complications. Multiple factors underlie the development of respiratory failure, but the principal contributors are weakness and fatigue of upper airway, inspiratory and expiratory muscles, as well as the influence that these impairments have upon cough efficacy and the development of infection (Mehta, 2006). See also the section ‘Mechanical ventilation’, below.
Thus, the picture in NMD is one of multifactorial defects in respiratory and upper airway function, and imbalance in the demand / capacity relationship of the respiratory muscles that can quickly result in respiratory failure (Macklem, 1986). In the section ‘Respiratory muscle involvement in exercise limitation’, the implications of these changes for exercise tolerance will be considered, and Chapter 4 will review the evidence supporting specific respiratory muscle training for patients with NMD.
Obesity
The influence of obesity upon respiratory muscle function stems primarily from the mechanical impedances imposed by fat deposition upon the movement of the chest wall and diaphragm (Salome et al, 2010). Fat deposited on the chest wall decreases respiratory system compliance, creating a restrictive pulmonary defect. Fat deposited within the abdominal cavity reduces its compliance and impedes diaphragm movement into the abdominal compartment. Respiratory system compliance of obese people is approximately half that of lean people, and reduces still further in obese people when supine (Naimark & Cherniack, 1960).
The effect of obesity upon lung volumes is primarily to reduce functional residual capacity (FRC) and end-expiratory lung volumes (EELV) (Babb et al, 2008b), owing to increased respiratory system recoil. This increases the likelihood of expiratory flow limitation (Ferretti et al, 2001). In addition, when breathing closer to residual volume, airway calibre is smaller and thus airway resistance is greater. For example, compared with overweight people (BMI 27 kg·m− 2), airway resistance was 56% greater in obese people (body mass index [BMI] 46 kg·m− 2); airway resistance was also correlated with the reduction in FRC (Zerah et al, 1993). Total lung capacity and residual volume tend to be preserved, but inspiratory reserve volume tends to increase and expiratory reserve volume to decrease (due to the reduction in EELV). However, in extreme obesity TLC may be impaired by the inability of the inspiratory muscles to overcome the increased compliance of the chest wall and abdominal compartment, or by a reduction in thoracic volume due to ingress of adipose tissue (Salome et al, 2010). Overall, the effects of obesity are to roughly double the work of breathing (Naimark & Cherniack, 1960; Pelosi et al, 1996; Kress et al, 1999), with most of the increase deriving from the increased elastic work (Pelosi et al, 1996).
The relative overloading of the respiratory pump in obesity is also reflected in a reduced maximum voluntary ventilation (MVV), the decline being greater with higher BMIs. However, this decline in MVV is greater than the declines in FEV1 and FVC would predict (Weiner et al, 1998), which points strongly to a deficit in the function of the inspiratory muscles. Airway function is impaired slightly, with FEV1 and FVC tending to decrease with increasing BMI (Salome et al, 2010). Since both indices decrease to the same extent, the impairment in FEV1 is most likely secondary to the decrease in FVC, and not to a direct effect of obesity upon airway diameter (Salome et al, 2010). Changes in breathing mechanics mean that obesity leads to a reduction in EELV during exercise, with the consequence that some expiratory flow limitation may result (Rubinstein et al, 1990).
Respiratory muscle strength and endurance appear to be well preserved in some obese adults (Yap et al, 1995; Weiner et al, 1998; Collet et al, 2007), but studies have reported a small (~ 10%) impairment of respiratory muscle strength and endurance (Weiner et al, 1998; Chlif et al, 2005). Interestingly, even in those with relatively well-preserved respiratory muscle function, weight loss following bariatric surgery improves inspiratory and expiratory muscle strength by around 20% (Weiner et al, 1998). One thing is clear: inspiratory muscle function is inversely related to BMI. A significant negative correlation has been observed by some investigators (Chlif et al, 2005), whilst others have noted that inspiratory muscle strength was slightly lower (~ 15%) in patients with a BMI > 49 kg·m− 2 than in those with a BMI < 49 kg·m− 2 (Collet et al, 2007).
Dyspnoea is a common complaint amongst obese individuals, both at rest and during exercise. This may be in part due to inspiratory muscle weakness (Chlif et al, 2007, 2009), but alterations in respiratory system mechanics also contribute. Breathing is associated with a rapid, shallow pattern, an increased ventilatory drive to the inspiratory muscles and an increased inspiratory muscle work (Chlif et al, 2007; Chlif et al, 2009). An increased oxygen cost of breathing has been implicated specifically in the dyspnoea associated with obesity (Babb et al, 2008a).
Another important factor to be borne in mind with regards to the influence of obesity upon breathing is the existence of co-morbidities. For example, it is increasingly common for obesity to be present with COPD (Franssen et al, 2008). There is also a well-established causal relationship between obesity and obstructive sleep apnoea (Schwartz et al, 2010), as well as hypoventilation syndrome (Anthony, 2008). Less well established, but an area of growing interest, is the apparent association between obesity and asthma, with some researchers suggesting that there may be a causal relationship between the two conditions (Sood, 2005), in which obesity is the antecedent (Ford, 2005). A putative underlying mechanism for the development of asthma, as well as the exacerbation of existing disease, is the production of pro-inflammatory cytokines by adipose tissue (Sood, 2010).
Thus, obese patients have an increased demand for inspiratory muscle work, which arises from complex changes in respiratory system mechanics. Furthermore, in those with inspiratory muscle weakness there is also a reduced capacity to supply this elevated demand. In the section ‘Respiratory muscle involvement in exercise limitation’, respiratory muscle-induced limitations to exercise tolerance will be considered, and Chapter 4 will review the evidence supporting specific respiratory muscle training for obese people.
Ageing
The process of normal ageing is associated with a number of changes that affect breathing (Janssens et al, 1999). Thus deterioration in pulmonary mechanics, lung function, locomotor efficiency and respiratory muscle function, as well as remodelling of pulmonary vasculature, all impact upon the demand / capacity relationship of the respiratory muscles.
The senescent changes to the pulmonary system have been dubbed ‘senile emphysema’ (Janssens et al, 1999) and are present from the age of 50 years, becoming most apparent at around 80 years (Britto et al, 2009). The most important of these changes are a decrease in the static recoil of the lung, a decrease in chest wall compliance and a reduction in the strength of the respiratory muscles (Janssens et al, 1999). Accordingly, many of the factors that increase the demand for inspiratory muscle pressure generation in COPD are also common to normal ageing. These include dynamic hyperinflation (Deruelle et al, 2008), and an increase in mechanical ventilatory constraints during exercise (DeLorey & Babb, 1999). Older people also adopt a rapid, shallow breathing pattern, and exhibit a greater dead space to tidal volume ratio (VD / VT), which necessitates an increase in ![]() E (DeLorey & Babb, 1999). There is also remodelling of the pulmonary vasculature, leading to increased vascular stiffness, resistance and pressure (Taylor & Johnson, 2010). These changes reduce pulmonary capillary blood volume and increase heterogeneity in the distribution of ventilation and perfusion. The resultant reduction in membrane diffusing capacity is consistent with a reduction in alveolar–capillary surface area (Taylor & Johnson, 2010). These changes make a small additional contribution to the ventilatory demand. In addition, the mechanical efficiency of exercise appears to be lower in older people (McConnell & Davies, 1992).
E (DeLorey & Babb, 1999). There is also remodelling of the pulmonary vasculature, leading to increased vascular stiffness, resistance and pressure (Taylor & Johnson, 2010). These changes reduce pulmonary capillary blood volume and increase heterogeneity in the distribution of ventilation and perfusion. The resultant reduction in membrane diffusing capacity is consistent with a reduction in alveolar–capillary surface area (Taylor & Johnson, 2010). These changes make a small additional contribution to the ventilatory demand. In addition, the mechanical efficiency of exercise appears to be lower in older people (McConnell & Davies, 1992).
The term sarcopenia first appeared in the literature in the early 1990s to describe the age-related loss of muscle mass (Rogers & Evans, 1993). Respiratory muscles are also affected by this process, and their strength is correspondingly lower in older people (McConnell & Copestake, 1999); indeed, respiratory muscle strength is strongly and independently correlated with hand grip strength (Enright et al, 1994). Furthermore, respiratory muscle strength is also independently related to decline in mobility in older people (Buchman et al, 2008).
Finally, the influence of co-morbidities must also be borne in mind, since the majority of older people are not without disease. Accordingly, the age-related changes described above serve to exacerbate disease-related impairments. The picture in older people is therefore one of an emerging load / capacity imbalance within the respiratory muscles that worsens progressively with advancing age, and is exacerbated by chronic disease. In the section ‘Respiratory muscle involvement in exercise limitation’, the implications of these changes for exercise tolerance will be considered, and Chapter 4 will review the evidence supporting specific respiratory muscle training for older people.
Miscellaneous conditions
The underlying cause of respiratory muscle weakness in the conditions described below is diverse, ranging from the existence of a ‘myopathic muscle milieu’ (e.g., corticosteroid treatment), to profound disuse (mechanical ventilation). Where applicable, the evidence supporting the application of specific respiratory muscle training to these conditions will be described in Chapter 4.
Diabetes
Type 1 and 2 diabetes are associated with inspiratory and expiratory muscle weakness (~ 20% and ~ 10% impairment, respectively) (Heimer et al, 1990; Kaminski et al, 2011); in type 1, there is also impaired inspiratory muscle endurance (Heimer et al, 1990), vital capacity and FEV1 (Innocenti et al, 1994). The reductions in lung volumes are at least partially explained by inspiratory muscle weakness (Wanke et al, 1991). However, loss of lung elasticity, and consequent airway collapse and obstruction, is also implicated (Goldman, 2003). The observation of a reduction in dynamic lung compliance is consistent with peripheral airway obstruction (Mancini et al, 1999). Biochemical changes in lung connective tissue have been suggested to underlie changes in elastic properties (Irfan et al, 2011). There is also a decrease in the pulmonary diffusing capacity, which may have its origins in pulmonary capillary damage induced by microvascular complications (Saler et al, 2009). Functionally, the structural changes to the lung parenchyma result in an elevated work of breathing (Wanke et al, 1992) and there is an increase in the magnitude of the ventilatory response to exercise, which is tachypnoeic in the presence of autonomic neuropathy (Tantucci et al, 1996). These factors most likely contribute to the greater intensity of dyspnoea during exercise (Wanke et al, 1992) and during hypoxia-induced hyperpnoea (Scano et al, 1999). Diabetic neuropathy has also been linked to inspiratory muscle weakness and autonomic dysfunction, as evidenced by a reduction in heart rate variability (Kaminski et al, 2011).
Putative underlying mechanisms for the inspiratory muscle weakness in patients with diabetes are divided broadly into two types: (1) biochemical and (2) neural. For example, inspiratory muscle strength is correlated with carnitine levels (Kilicli et al, 2010), which are lower in people with diabetes. In addition, there is evidence from rodent models of diabetes that the characteristics of resting membrane and action potentials are altered (van Lunteren & Moyer, 2003), and that responsiveness of the diaphragm to magnetic stimulation of the phrenic nerves is impaired in patients with diabetic polyneuropathy (Kabitz et al, 2008b). Impaired endurance of the inspiratory muscles may be explained by the many muscle metabolic abnormalities that arise because of insulin resistance and / or hyperglycaemia (Sun et al, 2008). In the section ‘Respiratory muscle involvement in exercise limitation’, the implications of these changes for exercise tolerance will be considered, and Chapter 4 will review the evidence supporting specific respiratory muscle training for patients with diabetes.
Renal failure
Respiratory system involvement in renal failure is extremely complex (Prezant, 1990), deriving from both the disease and its treatment. It has been known for many years that uraemic patients possess impaired inspiratory muscle strength and a restrictive pulmonary defect (Gomez-Fernandez et al, 1984). The latter is most likely due to the effects of hypervolaemia, which induces pulmonary hypertension and oedema. Impairment of vital capacity is reversible with dialysis, implicating hypervolaemia as a major contributory factor (Kovelis et al, 2008). Since respiratory muscle strength does not appear to improve post-dialysis, primary weakness is implicated. Furthermore, patients who have been receiving dialysis for the longest showed the most impaired inspiratory muscle function (Kovelis et al, 2008). This supports the notion of progressive development of primary weakness (Bark et al, 1988; Karacan et al, 2006; Kovelis et al, 2008). For this reason, estimates of the magnitude of impairment differ between studies, ranging from deficits of ~ 40% and 50% for inspiratory and expiratory muscle strength respectively (Bark et al, 1988), to ~ 10% (Kovelis et al, 2008). Interestingly, respiratory muscle dysfunction is partially reversible following renal transplantation (Guleria et al, 2005). Chapter 4 will review the evidence supporting specific respiratory muscle training for patients with renal failure.
Cancer
One of the most common chronic symptoms in patients with cancer is exertional dyspnoea, which is present in up to 10% of survivors of childhood cancers and up to 70% of patients with advanced cancer (Travers et al, 2008). Generally, pulmonary function is relatively normal in patients with cancer (Travers et al, 2008), and there are no major mechanical abnormalities. However, there are exceptions, and pulmonary function may be impaired in specific cancers affecting the thorax (e.g., lung and breast), especially following locoregional adjuvant radiotherapy (Spyropoulou et al, 2009). There are many ways in which cancer and / or its treatment might cause dyspnoea, but one unifying mechanism that has been suggested to underlie exertional dyspnoea in patients with cancer is respiratory muscle weakness (Feathers et al, 2003; Travers et al, 2008). Since pulmonary function does not correlate with dyspnoea, inspiratory muscle weakness is likely to be an important contributor to the symptom (Travers et al, 2008). In the section ‘Respiratory muscle involvement in exercise limitation’, the implications of these changes for exercise tolerance will be considered. At the time of writing there have been no studies of respiratory muscle training in patients with cancer.
Anorexia nervosa
Anorexia nervosa is associated with generalized muscle wasting and specific weakness of the respiratory muscles (Birmingham & Tan, 2003; Gardini Gardenghi et al, 2009) including the diaphragm (Murciano et al, 1994). In addition, there is evidence of impaired spirometric function that is correlated with body mass (Ziora et al, 2008). Furthermore, malnourishment appears to induce changes to the lung parenchyma resulting in impaired diffusion capacity (Gardini Gardenghi et al, 2009) and / or emphysema-like changes to the lung structure (Coxson et al, 2004), as well as an increase in residual volume (Gonzalez-Moro et al, 2003). Functionally, there is impaired exercise tolerance (Biadi et al, 2001), perhaps due in part to dyspnoea (Birmingham & Tan, 2003). Case study evidence suggests that inspiratory muscle function may take longer to recover following refeeding than other muscles, leading to prolongation of dyspnoea symptoms and exercise intolerance (Birmingham & Tan, 2003).
The underlying causes for the spirometric changes are thought to be a combination of a restrictive defect caused by inspiratory muscle weakness (Ziora et al, 2008) and the effects of undernutrition on the lung parenchyma. Animal models of anorexia have demonstrated decreased production of lung surfactant (D’Amours et al, 1983), as well as the total protein, connective tissue and elastic content of the lungs of young animals (Sahebjami & MacGee, 1985), which is not completely reversible by refeeding (Sahebjami & Domino, 1992). In the section ‘Respiratory muscle involvement in exercise limitation’, the implications of these changes for exercise tolerance will be considered. At the time of writing there have been no studies of respiratory muscle training in patients with anorexia nervosa.
Myopathic pharmacological agents
The myopathic influence of orally administered corticosteroids was discussed briefly in relation to patients with COPD and asthma. Steroid-induced myopathy is a well-established phenomenon in patients receiving high doses of corticosteroids (Perkoff et al, 1959). The first study to examine the independent effects of costicosteroid treatment and disease progression demonstrated that, in non-respiratory patients, 1 to 1.5 mg·kg− 1 per day of prednisolone induced significant reductions in the strength (~ 30%) and endurance (~ 50%) of the inspiratory muscles over an 8-week treatment period (Weiner et al, 1993; Weiner et al, 1995). Although these changes show some reversal following cessation of treatment, function may take as long as 6 months to normalize (Weiner et al, 1993).
There have also been a number of case reports of colchicine-induced myopathy following prolonged treatment (Wilbur & Makowsky, 2004) including one report in which the predominant clinical feature was respiratory muscle weakness, as indicated by severe dyspnoea, orthopnoea, tachypnoea and thoracoabdominal paradox (Tanios et al, 2004). Cessation of treatment resulted in an improvement in respiratory muscle function, which more than doubled from pre-cessation levels of ~ 25–30 cmH2O (Tanios et al, 2004).
Most recently, statin-induced myopathy has come under scrutiny, as it is estimated that 5–10% of patients receiving statins develop myopathy (Rallidis et al, 2011). At least one case study report has demonstrated an association between respiratory symptoms, inspiratory muscle dysfunction and statin administration (Chatham et al, 2009). See Chapter 4 for a description of the evidence supporting specific inspiratory muscle training.
Surgery
Post-operative pulmonary complications (PPC) are common in patients undergoing cardiothoracic, abdominal and other major surgeries. Because of variations in both the nature of surgeries and the definition of PPC, estimates of the incidence vary from 2% to 40% (Canet & Mazo, 2010). The substantial contribution made by PCC to the morbidity and mortality associated with surgery and anaesthesia has led to interest in predicting those patients at risk of developing a PPC. Three main factors contribute to risk, and their interaction appears to determine its level: (1) general health status, (2) effects of anaesthesia and (3) surgical trauma (Canet & Mazo, 2010).
General health status has a predictable influence upon risk, especially where patients have pre-existing respiratory and cardiovascular dysfunction (Canet & Mazo, 2010). Anaesthesia induces a range of pre- and post-operative changes that increase the risk of PCC (Canet & Mazo, 2010). For example, atelectasis is universal, arising from physical compression of the lungs, absorption of alveolar air and impairment of surfactant function. These changes induce ventilation/perfusion mismatching, which increases dead space and hypoxaemia (Canet & Mazo, 2010). Post-operative drugs such as anaesthetics and analgesics also affect upper airway and accessory muscle function, increasing the risk of PCC (Canet & Mazo, 2010). As one might expect, the site of surgery has a potent influence upon the development of PPC. Predictably, thoracic and abdominal surgeries are associated with the highest risk of PPC (10–40%; Duggan & Kavanagh, 2010), and give rise to post-operative respiratory muscle dysfunction. The origin of the resulting muscle dysfunction is complex, but includes factors such as changes in thoracoabdominal mechanics and loss of muscular integrity (Siafakas et al, 1999). In addition, post-operative pain can limit respiratory movements, which can also be impaired by reflex inhibition of respiratory muscle activity, especially the diaphragm (Sharma et al, 1999). The latter arises because of feedback stimulated by mechanical disturbance of the viscera (Canet & Mazo, 2010). Finally, procedures lasting more than 3 hours have a higher risk of PCC (Duggan & Kavanagh, 2010), which may be partially due to the effects of inspiratory muscle inactivity during mechanical ventilation upon inspiratory muscle function (see the section ‘Mechanical ventilation’). See Chapter 4 for a description of the evidence supporting specific inspiratory muscle training for the prevention of PPC.
Mechanical ventilation
The primary reason for admission to intensive care is the need for mechanical ventilation, which imposes a considerable burden upon both patients and healthcare systems (Bissett et al, 2012). Mechanical ventilation is a double-edged sword for the respiratory muscles. On the one hand it can provide much needed rest for overloaded muscles, but on the other the imposed rest can lead to rapid atrophy and loss of respiratory muscle function (Petrof et al, 2010). The latter can lead to prolongation of mechanical ventilation, and difficulty weaning patients from the ventilator (Callahan, 2009). Weaning typically accounts for 40–50% of the total duration of mechanical ventilation and 30% of patients fail to wean at the first attempt (Moodie et al, 2011). Indeed, patients who experience weaning failure are characterized by their rapid, shallow breathing pattern and a diaphragm tension–time index (TTI) that is close to the threshold for fatigue (Karakurt et al, 2011); multiple logistic regression analysis with the weaning outcome as the dependent variable has revealed TTI, and the ratio of breathing frequency to VT, as significant predictors of weaning outcome (Vassilakopoulos et al, 1998). Patients who are weaned successfully from mechanical ventilation have been shown to possess higher MIP than those who do not (Epstein et al, 2002; Carlucci et al, 2009), and low MIP is an independent predictor of prolonged weaning (De Jonghe et al, 2007). However, a recent systematic review failed to find evidence that superior inspiratory muscle strength leads to a shorter duration of mechanical ventilation, improved weaning success or improved survival; the authors highlighted the need for further research (Moodie et al, 2011). It has been suggested that discrepancies relating to the influence of MIP upon these factors may reflect the inadequacy of MIP as a prognostic index of the respiratory muscle contribution to complex phenomena such as weaning (Bissett et al, 2012).
There is mounting evidence that mechanical ventilation leads to pathological changes to the respiratory system that may contribute to prolongation of ventilator dependence and difficulty in weaning (Bissett et al, 2012). The precise time course, as well as the prevalence and incidence of ventilator-acquired respiratory muscle dysfunction, remains unclear, but a primate model suggests a 46% loss of diaphragm strength and a 37% reduction in ventilator endurance after 11 days of mechanical ventilation (Anzueto et al, 1987). Human data are sparse, but one study has compared diaphragm fibre samples from mechanically ventilated brain-dead organ donors with those of control patients who were ventilated for less than 3 hours. Diaphragm fibre cross-sectional area (types I and II) was over 50% lower in the organ donor patients following just 18–69 hours of mechanical ventilation, compared with control (Levine et al, 2008). Interestingly, this atrophy was not present in control muscle biopsies (pectoralis major), suggesting that it was specific to diaphragm inactivity. In another study, twitch diaphragm pressure (TwPdi) was measured serially in mechanically ventilated patients to assess the time course of changes in diaphragm function (Hermans et al, 2010). Increasing duration of mechanical ventilation was associated with a logarithmic decline in TwPdi. This association was also found for cumulative dose of propofol and piritramide, suggesting a potential contribution from factors such as sedatives and analgesics (Hermans et al, 2010).
One of the reasons for the lack of information in this area is the difficulty of assessing respiratory muscle function in mechanically ventilated patients. Voluntary measures of strength such as MIP are considered too unreliable, although a technique in which the patient’s tracheostomy tube is occluded using a one-way valve has been validated and found to be more reliable than conventional MIP (Caruso et al, 1999). Studies using non-volitional measures are difficult to obtain, and hence few and far between. Three such studies utilizing magnetic stimulation of the phrenic nerves found that TwPdi in mechanically ventilated patients was around 30% of that observed in normal people (Watson et al, 2001; Laghi et al, 2003; Hermans et al, 2010).
Underlying mechanisms for the development of ventilator-acquired inspiratory muscle weakness remain unclear. The picture is complex and, although it is one part of the polyneuropathy that has been described in critical illness (Latronico & Bolton, 2011), there appear to be factors that are specific to the respiratory muscles (Levine et al, 2008). These specific factors include the individual and combined effects of disuse atrophy / proteolysis, nutritional factors and pharmacological influences, as well as mechanical responses to positive end-expiratory pressure (PEEP) due to alterations in the length–tension relationship of the diaphragm (Bissett et al, 2012). See Chapter 4 for a description of the evidence supporting specific inspiratory muscle training.
Pregnancy
The increase in intra-abdominal mass during pregnancy impairs normal diaphragm movement, and the effect upon breathing is, to a limited extent, similar to that observed in obesity. The diaphragm is displaced cranially during the latter stages of pregnancy, but continues to move normally during tidal breathing (Laghi & Tobin, 2003). Similarly, respiratory muscle strength remains unchanged (Contreras et al, 1991). However, the hormonal milieu of pregnancy induces an increase in the drive to breathe, and in ![]() E (minute volume) (Jensen et al, 2007); the former is correlated with plasma progesterone levels (Contreras et al, 1991), whereas the latter is achieved exclusively via an increase in VT (tidal volume) (Jensen et al, 2009b). These changes contribute to the increase in breathing effort and dyspnoea that are experienced by around three-quarters of pregnant women (Milne et al, 1978). Although several theories have been put forward to explain gestational dyspnoea, its aetiology remains poorly understood (see also the section ‘Respiratory muscle involvement in exercise limitation’, below), but recent evidence points to its being a ‘normal awareness of increased ventilation’ (Jensen et al, 2009b).
E (minute volume) (Jensen et al, 2007); the former is correlated with plasma progesterone levels (Contreras et al, 1991), whereas the latter is achieved exclusively via an increase in VT (tidal volume) (Jensen et al, 2009b). These changes contribute to the increase in breathing effort and dyspnoea that are experienced by around three-quarters of pregnant women (Milne et al, 1978). Although several theories have been put forward to explain gestational dyspnoea, its aetiology remains poorly understood (see also the section ‘Respiratory muscle involvement in exercise limitation’, below), but recent evidence points to its being a ‘normal awareness of increased ventilation’ (Jensen et al, 2009b).
Another facet of pregnancy in which respiratory muscles play a key role is parturition, which has been shown to induce diaphragm fatigue (Nava et al, 1992). The action of the diaphragm during expulsive efforts plays a very important role in minimizing increases in intrathoracic pressure (which have detrimental haemodynamic effects) and maximizing increases in intra-abdominal pressure. The latter can exceed 150 cmH2O, and is correlated with speed of delivery, i.e., the higher the intra-abdominal pressure that can be maintained the faster the delivery (Buhimschi et al, 2002). Since diaphragm fatigue occurs, it is reasonable to suggest that this may impair the effectiveness of expulsive efforts, which may prolong delivery.
In the section ‘Respiratory muscle involvement in exercise limitation’, the implications of these changes for exercise tolerance will be considered. At the time of writing there have been no studies of respiratory muscle training in pregnant women.
Obstructive sleep apnoea
The contraction of thoracic inspiratory muscles decreases intrapleural pressure, transmitting a sub-atmospheric pressure to the extrathoracic [upper] airways. This creates a transluminal pressure that tends to collapse the upper airways. The magnitude of the transluminal pressure gradient is determined by a number of factors including the airway wall elasticity, intrapleural pressure, as well as how resistance is distributed through the upper airway (Olson et al, 1988). In the absence of bony or cartilaginous structures to maintain upper patency at the pharyngeal level, upper airway patency is dependent entirely upon the activation of over 20 dilator muscles (see Ch. 1), which resist the collapsing effect of negative intraluminal pressure during inspiration (Series, 2002).
Dysfunctions in the coordinated activation of the upper airway and respiratory pump muscles, as well as of the neuromuscular tone of the upper airway muscles, have been identified as key factors in the pathogenesis of obstructive sleep apnoea (OSA) (Steier et al, 2010). Indeed, loss of neuromuscular tone in the upper airway dilator muscles initiates the cascade of events that culminate in airway occlusion (Hudgel & Harasick, 1990). According to the ‘balance of pressures’ concept, upper airway occlusion occurs when the positive dilating pressure from the upper airway musculature is unable to resist the negative intraluminal pressure caused by inspiratory effort (Brouillette & Thach, 1979). Whilst an index of airway dilator muscle function suggests patients with OSA have greater strength, this is accompanied by lower endurance (Eckert et al, 2011). Furthermore, the intrinsic, passive properties of the upper airway and pharynx also contribute to its propensity to collapse (Isono et al, 1997), i.e., the more compliant the walls of the airway, the smaller is the pressure gradient required to induce narrowing, and ultimately occlusion.
Thus, there are two mechanical mechanisms by which the collapsibility of the upper airway could be modified: firstly by improving active neuromuscular tone of the upper airway, and secondly by reducing the passive compliance of the upper airway. The former has two subcomponents, the first being reflex coordination of airway dilator muscles, and the second being the functional properties of these muscles (e.g., strength, fatigue resistance, rate of shortening). Both of these mechanical mechanisms can be influenced by specific training (Lindstedt et al, 2002; Demoule et al, 2008). See Chapter 4 for a description of the evidence supporting specific respiratory muscle training in OSA.
RESPIRATORY MUSCLE INVOLVEMENT IN EXERCISE LIMITATION
Healthy people
In healthy young people, the physical work undertaken by the respiratory muscles during the task of pumping air in and out of the lungs can be immense (Harms & Dempsey, 1999), but even in non-athletes the work of breathing has been shown to influence exercise tolerance. Research suggests that this occurs via two mechanisms: (1) by contributing to perceived effort, and (2) by exacerbating the demands placed upon the circulatory system for blood flow. This section will consider how these factors contribute to exercise limitation, with an emphasis on the role and repercussions of respiratory muscle work.
An important premise in the argument that respiratory muscles limit exercise performance in healthy people is that the muscles operate at, or close to, the limits of their capacity. When muscles work in this way, they become fatigued (see Ch. 1, ‘Mechanisms of fatigue’). Accordingly, without some evidence of exercise-induced respiratory muscle fatigue (RMF), the argument in favour of training these muscles is flimsy at best.
Respiratory muscles are separated functionally according to whether they have inspiratory or expiratory actions. Since breathing demands equal movement of inspiratory and expiratory air, one might predict that fatigue would be present in both groups of muscles under the same conditions. This is not the case. One reason for this is the fact that inspiratory muscle work is always greater than expiratory work (recall the party balloon analogy from Ch. 1 – the stretching of tissues on inhalation assists exhalation); a second reason is the differing training state of the muscles themselves (expiratory muscles are engaged in many postural activities that improve their training status); a third reason is that different exercise conditions overload the inspiratory and expiratory muscles to differing extents.
The earliest reports of inspiratory muscle fatigue (IMF) following competitive sports events appeared in the early 1980s. In this context fatigue was defined as a loss of voluntary force generating capacity post-exercise, and a significant decrease in inspiratory muscle strength (MIP) was measured following marathon running (Loke et al, 1982). Subsequent studies have confirmed this finding (Chevrolet et al, 1993; Ross et al, 2008), as well as showing IMF after ultra-marathon (Ker & Schultz, 1996) and triathlon competitions (Hill et al, 1991).
Under laboratory and field-based research conditions, IMF has been demonstrated following rowing (Volianitis et al, 2001; Griffiths & McConnell, 2007), cycling (Romer et al, 2002b) and swimming (Lomax & McConnell, 2003), as well as a sprint triathlon (Sharpe et al, 1996) and treadmill marathon running (Ross et al, 2008). All of the studies cited above have evaluated IMF using maximal inspiratory pressure (MIP) measured at the mouth, which is a holistic, voluntary surrogate of inspiratory muscle force production (see Ch. 6 for a description of MIP assessment). Although MIP has its merits (being non-invasive, portable, quick and easy to administer, reliable and holistic), it can also be criticized for being susceptible to the influence of changes in effort. In other words, immediately after exercise a lower MIP might be the result of reduced effort, and not due to physiological factors. However, contractile fatigue of the diaphragm has also been confirmed in the laboratory following heavy exercise using electrical and magnetic stimulation of the phrenic nerves (Johnson et al, 1993; Mador et al, 1993; Babcock et al, 1997; Babcock et al, 1998). Thus, not only is there evidence of IMF following real world sports participation and laboratory simulations of competition, but rigorous laboratory trials also demonstrate specific contractile fatigue of the diaphragm after heavy exercise. The observation that the diaphragm was susceptible to exercise-induced fatigue initiated a process that has led to a complete rethink about the role of respiratory muscles in exercise limitation (Romer & Polkey, 2008).
But what of the expiratory muscles? The effect of real world sports activities upon expiratory muscle function has been studied much less extensively, and existing data are currently contradictory. Following marathon running that induced a fall in MIP, no change in maximal expiratory pressure (MEP) was observed post-exercise (Chevrolet et al, 1993; Ross et al, 2008). Similarly, following a triathlon that elicited a significant fall in MIP, there was no change in MEP (Hill et al, 1991). In contrast, following a rowing time trial that simulated a 2000-metre rowing race in the laboratory, a significant decline in MEP was observed (Griffiths & McConnell, 2007). Similarly, under laboratory conditions where cycling exercise was performed to the limit of tolerance (Tlim), MEP was shown to decline (Cordain et al, 1994). In contrast, some authors observed no change in MEP following maximal cycle ergometer exercise, but did observe a fall in MIP (Coast et al, 1999). Non-volitional assessment of expiratory muscle fatigue (EMF) using magnetic stimulation of abdominal muscles has recently demonstrated EMF occurring after high-intensity cycle ergometer exercise to Tlim (Taylor et al, 2006; Verges et al, 2006). Thus, it appears that EMF may be specific to certain exercise modalities and / or intensities of exercise. These conditions appear to be characterized by exercise at maximal intensity, and / or situations in which the expiratory muscles have a key role in propulsive force transmission, such as rowing.
The next question to consider is whether there are any functional repercussions of RMF. This has been studied using a variety of experimental designs, but principally by using two basic approaches: first by inducing RMF and studying its influence upon subsequent exercise, and second by manipulating the work of breathing during exercise to accelerate RMF (by adding a resistance to breathing) or to delay the time to RMF (by using a ventilator to undertake the work of breathing). The effects of prior IMF and EMF are to increase the intensity of breathing effort during subsequent whole-body exercise, and to lead to a shorter time to Tlim during constant power output exercise. For example, one of the first studies to examine the effect of prior IMF on exercise tolerance observed a 23% reduction in the ability to sustain cycling at 90% of maximal oxygen uptake (Mador & Acevedo, 1991). There was an increase in the sensation of effort during exercise, i.e., exercise after IMF felt harder. Using a slightly different experimental design, the effects of prior IMF on isolated plantar flexor exercise have been studied (McConnell & Lomax, 2006). In this case, a more rapid fatigue of the plantar flexor muscles was observed after IMF (the reasons for this are explained later).
More recently, the effects of prior EMF on cycling performance were assessed (Taylor & Romer, 2008). A decrease in Tlim (33%) was observed when exercise followed EMF, as well as an increase in the perceptions of both breathing and leg effort. Leg fatigue was also more severe after EMF. However, a note of caution is required in the interpretation of studies that have pre-fatigued the expiratory muscles. The same authors have also shown that expiratory loading induces simultaneous IMF and EMF (Taylor & Romer, 2009). In other words, the effect of EMF on subsequent exercise performance is ‘contaminated’ by accompanying IMF. This is because the inspiratory muscles are involved in the transmission of expiratory pressure. Thus, any effects of EMF on performance cannot be ascribed solely to the expiratory muscles.
In the first study to explore the impact of the work of breathing on exercise tolerance, the influence of changes in the work of breathing upon leg blood flow during maximal cycle ergometer exercise was examined (Harms et al, 1997). A reciprocal relationship between leg blood flow and the work of breathing was observed, such that when the inspiratory work was undertaken by a ventilator there was a 4.3% increase in leg blood flow. In contrast, when inspiratory work was increased by a flow resistor the leg blood flow decreased by 7%. The changes in leg blood flow were mediated by changes in the neural input to the blood vessels in the limbs, resulting in vasoconstriction when inspiratory work was increased and dilatation when it was reduced. In a series of subsequent studies, it was shown that the stimulus for limb vasoconstriction was a cardiovascular reflex originating within the inspiratory muscles, which became known as the ‘inspiratory muscle metaboreflex’ (St Croix, 2000; Sheel et al, 2001; Sheel et al, 2002). The findings of these studies have been replicated and extended to provide compelling evidence that functional overload of the inspiratory muscles induces reflex cardiovascular adjustments that reduce exercising limb blood flow (McConnell & Lomax, 2006; Katayama et al, 2012). The exercise models employed by early studies suggested that only very-high-intensity exercise was associated with inspiratory muscle metaboreflex activation (Wetter et al, 1999). However, more recent studies have shown that moderate-intensity exercise combined with inspiratory muscle loading also leads to inspiratory metaboreflex activation (Katayama et al, 2012), suggesting that the critical factor is functional overload of the inspiratory muscles, not the whole body muscle intensity.
The ‘inspiratory muscle metaboreflex’ is activated when metabolite accumulation within the inspiratory muscles stimulates afferent nerve fibres (type III and IV) to increase their firing frequency. Stimulation of these fibres precipitates an increase in the strength of sympathetic efferent outflow, which induces a generalized vasoconstriction. Limiting blood flow restricts the supply of oxygen and impairs the removal of exercise metabolites from exercising muscles, with the result that muscles fatigue more quickly and exercise performance is impaired (McConnell & Lomax, 2006). Indeed, this is precisely what has been found; changes in leg blood flow elicited by increasing or reducing the work of inhalation are correlated with changes in the magnitude of exercise-induced leg fatigue (Romer et al, 2006). In other words, increasing the work of inspiration reduces leg blood flow, exacerbates leg fatigue and impairs exercise tolerance, whereas reducing the work of inhalation does the opposite. However, it is important to appreciate that contractile fatigue of the inspiratory muscles is not an essential prerequisite to metaboreflex activation; the prerequisite is metabolite accumulation, which may not necessarily induce contractile fatigue. Indeed, it has been argued that muscle feedback may provide an important protective mechanism that guards against fatigue (Gandevia, 2001). The findings summarized above complete the circle that links RMF with leg fatigue, i.e., metaboreflex activation precipitates limb vasoconstriction, reducing limb blood flow and accelerating limb fatigue. The evidence for an effect of IMT upon metaboreflex activation will be reviewed in Chapter 4.
Earlier, we touched briefly upon the effect of IMF and EMF on breathing and limb effort, i.e., RMF intensifies sense of effort during exercise (Mador & Acevedo, 1991; Taylor & Romer, 2008). The influence upon limb effort should now be clear: IMF reduces limb blood flow, thereby reducing oxygen delivery and accelerating limb fatigue. Although it might seem self-evident that weak or fatigued muscles generate greater perception of effort than fresh or stronger muscles, the neurophysiological mechanism underpinning this reality merits a few words. The human brain is able to judge the size of the outgoing neural drive (McCloskey et al, 1983), and as muscles contract they return information to the brain about the amount of force that is being generated, as well as the speed and range of motion of the movement (Cafarelli, 1982). Heavy objects require high forces, they can be moved only slowly and may impose a limited range of motion; the opposite is true for light objects. The sensory area of the brain compares the size of the drive sent to the muscles with the sensory information coming from the muscles. In doing so, it formulates a perception of effort. The size of the neural drive required to generate a given external force is influenced by a number of factors, but principally by the strength of the muscle. A strong muscle requires a lower neural drive to generate a given force, which is why weights feel lighter after strength training. Similarly, a fatigued muscle requires a higher neural drive to generate a given force, which is why weights feel heavier when muscles are fatigued (Gandevia & McCloskey, 1978).
As was described in Chapter 1, the neurophysiological principles described above apply equally to the respiratory muscles (Campbell, 1966) as they do to other skeletal muscles. Thus, weakness, fatigue, or indeed strengthening, of the respiratory muscles modulates the perception of breathing effort and dyspnoea. Similarly, abnormalities of respiratory mechanics exacerbate perception of breathing effort (Scano et al, 2010). Recently, the term ‘neuromechanical uncoupling’ has been used to describe the imbalance between neural drive and the mechanical events that it produces (see Ch. 1).
Finally, it is worth mentioning some emerging evidence about the influence of group III and IV afferent feedback upon the central perception of effort and central fatigue. Group III and IV fibres project to a number of sites within the central nervous system. Most recently, Amann and colleagues employed a selective μ-opioid receptor agonist to demonstrate, for the first time, that afferent feedback from locomotor group III and IV fibres makes an ‘essential contribution’ to both cardiorespiratory control and perceptual responses in the exercising human being (Amann et al, 2010a). The latter finding is consistent with the observation that strengthening inspiratory muscles attenuates both respiratory and peripheral effort perception (Romer et al, 2002a), which may arise because of reduced feedback from group III and IV afferents in both respiratory and locomotor muscles.
Furthermore, feedback from group III and IV afferents is also implicated in central fatigue mechanisms, via inhibition of central motor output (Gandevia, 2001). It has been suggested that afferent feedback from exercising muscles protects locomotor and respiratory muscles from catastrophic fatigue (Gandevia, 2001). Indeed, Gandevia suggested, ‘An extreme example [of central fatigue] occurs with exercise of the inspiratory muscles in which task failure can occur with minimal peripheral fatigue’ (Gandevia, 2001). The importance of group III and IV afferent feedback in regulating integrated exercise responses was illustrated recently by a study in which a cycle time trial was undertaken with and without the selective μ-opioid receptor agonist fentanyl (Amann et al, 2009). During a self-paced 5 km time trial, intrathecal fentanyl was associated with greater quadriceps fatigue, a higher central motor output and greater perceived exertion compared with placebo. A higher power output in the first half of the fentanyl time trial was offset by a lower power output in the second, resulting in no change in performance time. However, compared with placebo, the decline in quadriceps twitch force was greater with fentanyl (45.6% vs 33.1%) and was associated with ambulatory problems post-exercise. The authors suggest their data ‘emphasize the critical role of locomotor muscle afferents in determining the subject’s choice of the “optimal” exercise intensity that will allow for maximal performance while preserving a certain level of locomotor muscle “functional reserve” at end-exercise’ (Amann et al, 2009). The specific contribution of respiratory muscle group III and IV afferents to central fatigue during whole-body exercise awaits investigation. Given the importance of protecting diaphragm function, it is reasonable to speculate that the inhibitory feedback from diaphragm afferents during exercise influences both respiratory and locomotor central motor output.
Thus, the past decade has seen the emergence of evidence that respiratory muscle work has influences far beyond anything that was thought possible. The respiratory muscles can contribute to exercise limitation through their influence on cardiovascular reflex control, i.e., metaboreflex reduction of limb blood flow. In addition, the respiratory muscles make a potent contribution to the perception of effort during exercise, and may also play a role in central fatigue. The evidence that strengthening the respiratory muscles reduces effort perception and improves exercise tolerance is reviewed in Chapter 4.
In this section we have considered the limitation to exercise tolerance imposed by the respiratory muscles in healthy people. The following section will describe how disease-specific factors limit exercise by exacerbating the breathing-related limitations to exercise tolerance that exist in healthy people. In Chapter 4 we will consider the evidence that rebalancing the relationship between demand and capacity of the respiratory pump muscles using RMT induces beneficial adaptations to respiratory muscle structure and function, as well as clinical benefits.
Respiratory disease
Chronic obstructive pulmonary disease
Patients with chronic obstructive pulmonary disease (COPD) have a complex pattern of disease, and there is an on-going debate regarding the precise exercise-limiting mechanisms(s) (Nici, 2008). However, what is not in doubt is that the primary symptom associated with exercise in patients with COPD is dyspnoea (O’Donnell & Webb, 2008a), the magnitude of which is influenced by both the pulmonary and systemic aspects of the disease (see ‘Changes in breathing mechanics and respiratory muscle function’, above). As O’Donnell & Webb so vividly put it, ‘When you can’t breathe nothing else matters!’ (O’Donnell & Webb, 2008a). Notwithstanding this, patients with COPD also exhibit significant exercise-induced locomotor muscle fatigue, to which they also appear to be more susceptible than healthy age-matched controls (Mador et al, 2003). Thus, peripheral factors also play an important role in exercise limitation, but, as will be discussed below, this influence is potentiated by the effect of elevated respiratory muscle work, which compromises limb oxygen delivery (Amann et al, 2010b).
In patients with COPD, the inspiratory muscle demand / capacity relationship is skewed such that the inspiratory muscles are severely overloaded (Moxham & Jolley, 2009). This imbalance not only affects limb blood flow during exercise (Vogiatzis et al, 2011), it is also a key contributor to dyspnoea via neuromechanical uncoupling (Moxham & Jolley, 2009). As was described in the previous section, COPD patients have primary inspiratory muscle weakness, which is exacerbated by the functional effects of hyperinflation. Hyperinflation increases the operating lung volume thereby raising the demand for inspiratory muscle work due to its influence upon the elastic work of breathing, as well as the inspiratory threshold load imposed by intrinsic positive end-expiratory pressure (PEEPi). The overload of the inspiratory muscles is exacerbated still further during exercise by tachypnoea (rapid shallow breathing) due to tidal volume (VT) restriction, caused by the mechanical consequences of hyperinflation (O’Donnell & Webb, 2008b; O’Donnell et al, 2012). The result of this rapid shallow breathing pattern is an increase in the ventilatory requirement (![]() E), and further functional weakening of the inspiratory muscles due to the increased velocity of inspiratory muscle shortening (O’Donnell & Webb, 2008b). In a retrospective analysis of data from two previous studies, O’Donnell and colleagues stratified patients into four levels of disease severity in order to examine the influence of hyperinflation upon ventilatory constraint and exertional dyspnoea. They observed a strong, negative influence of disease severity upon inspiratory capacity, VT expansion, dyspnoea and exercise tolerance (O’Donnell et al, 2012). During incremental cycling, VT expansion ceased at the same percentage of inspiratory capacity (73–77%), irrespective of disease severity, and dyspnoea escalated rapidly thereafter. The authors concluded that dyspnoea and exercise intolerance are associated with the attainment of ‘critical constraints on VT expansion and attendant increase in dyspnea at a progressively lower ventilation during exercise’ (O’Donnell et al, 2012).
E), and further functional weakening of the inspiratory muscles due to the increased velocity of inspiratory muscle shortening (O’Donnell & Webb, 2008b). In a retrospective analysis of data from two previous studies, O’Donnell and colleagues stratified patients into four levels of disease severity in order to examine the influence of hyperinflation upon ventilatory constraint and exertional dyspnoea. They observed a strong, negative influence of disease severity upon inspiratory capacity, VT expansion, dyspnoea and exercise tolerance (O’Donnell et al, 2012). During incremental cycling, VT expansion ceased at the same percentage of inspiratory capacity (73–77%), irrespective of disease severity, and dyspnoea escalated rapidly thereafter. The authors concluded that dyspnoea and exercise intolerance are associated with the attainment of ‘critical constraints on VT expansion and attendant increase in dyspnea at a progressively lower ventilation during exercise’ (O’Donnell et al, 2012).
Hyperinflation induces extremely high levels of diaphragm activation during exercise in patients with COPD such that, at the end of an incremental exercise test to the limit of tolerance, diaphragm activation can reach 81% (Sinderby et al, 2001). This indicates that the diaphragm (and probably also accessory muscles), work at a very high relative intensity during exercise. Somewhat paradoxically, studies of diaphragm contractile fatigue using phrenic nerve stimulation have failed to demonstrate consistent evidence of diaphragm fatigue following symptom-limited exercise in patients with COPD (Mador et al, 2000a; Mador et al, 2000b), but have observed leg muscle fatigue (Mador et al, 2000a). This apparent paradox was mentioned in the previous section, and is explored further in the final paragraph of the present section.
Poor aerobic fitness and peripheral muscle myopathy also contribute to exercise limitation in COPD, as they lead to a rapid lactic acidosis and a greater ventilatory requirement for exercise. These factors also exacerbate hyperinflation, and its correlates such as inspiratory muscle work. It is therefore no surprise that dyspnoea intensity during exercise in COPD patients correlates significantly with the extent of hyperinflation (O’Donnell & Laveneziana, 2007). Several studies have noted significant decreases in dyspnoea following interventions that reduced the demand for inspiratory muscle work by reducing operational lung volumes, either pharmacologically, surgically or using non-invasive positive airway pressure ventilation (Ambrosino & Strambi, 2004). These studies suggest that modifying the demand side of the inspiratory muscles’ demand / capacity relationship reduces dyspnoea (O’Donnell et al, 2007). It is therefore reasonable to suggest that modifying the capacity side of the relationship should evoke similar attenuation of dyspnoea, which is indeed the case, and the evidence for the latter will be reviewed in Chapter 4.
Reducing the demand for inspiratory muscle work also affects locomotor muscle performance in COPD patients. When the work of breathing is reduced by breathing heliox (a low-density gas), patients with COPD show an improvement in exercise tolerance and a reduction in the magnitude of exercise-induced leg fatigue (Butcher, 2008), which may be secondary to improved limb blood flow (Vogiatzis et al, 2011). Similarly, inspiratory muscle unloading using a proportional assist ventilator improves exercise tolerance, reduces leg effort and improves leg oxygenation in patients with COPD (Borghi-Silva et al, 2008b); the same group has made identical observations in patients with CHF. These data are entirely consistent with earlier observations in healthy young athletes during inspiratory muscle unloading (Harms et al, 1997). There is an emerging consensus that the inspiratory muscle metaboreflex described so extensively in healthy young athletes (Dempsey et al, 2006; see above) also operates to limit limb blood flow and exercise tolerance in patients with COPD (Dempsey et al, 2006; Scano et al, 2006).
These data may also explain the apparent paradox that contractile fatigue of the diaphragm has not been observed consistently in patients with COPD, yet limb fatigue has (Mador et al, 2000a). It is possible that high levels of inspiratory muscle work result in activation of the inspiratory muscle metaboreflex prior to any manifestations of overt diaphragm fatigue. This activation results in a generalized increase in sympathetic outflow that reduces limb blood flow, exacerbating limb fatigue (Romer et al, 2006), but preceding the development of diaphragm fatigue. Indeed, some researchers have suggested that the inspiratory muscle metaboreflex may be part of a protective reflex that preserves diaphragm oxygen delivery (Seals, 2001), sparing inspiratory muscles from fatigue. The emerging evidence that feedback from type III and IV afferents influences effort perception and central fatigue (see section ‘Healthy people’) provides a possible explanation for the absence of diaphragm contractile fatigue during exercise in some groups (Gandevia, 2001).
Another factor that may explain the apparent sparing of the diaphragm is that the work of the diaphragm is increasingly supported by the accessory muscles as exercise progresses, a strategy that is present in both healthy young athletes (Babcock et al, 1996) and patients with COPD (Yan et al, 1997). Unfortunately, there is only one study, in Polish, reporting post-exercise values for MIP, in COPD patients post-exercise; the abstract reports that both MIP and MEP were lower (11% and 10%, respectively) after an incremental treadmill exercise test (Maskey-Warzechowska et al, 2006). However, as mentioned previously, there is also evidence of global inspiratory muscle fatigue in COPD patients who walked to the limit of tolerance (slowing of the relaxation rate of oesophageal sniff pressure (Coirault et al, 1999), which is suggestive of accessory inspiratory muscle fatigue (Kyroussis et al, 1996). These data highlight the dangers of taking a reductionist approach to studying muscle fatigue specifically, and exercise tolerance generally. The inspiratory muscles cannot be discounted as a potential exercise-limiting factor because one, highly reductionist index of function, viz., diaphragm twitch pressure, does not appear to exhibit low-frequency fatigue post-exercise.
However, it is reasonable to ask why contractile fatigue of the diaphragm is present in healthy young adults, but not in patients with COPD. The relationship between diaphragm power output during exercise and subsequent fatigue suggests that the intensity of diaphragm work per se is only one of the factors that govern its propensity to fatigue (Babcock et al, 1995). It has been suggested that factors such as the competition for available blood flow, and the severity of the metabolic acidosis during exercise intensify the fatiguing stimulus of diaphragm work per se (Babcock et al, 1995). In other words, a given level of diaphragm work induces fatigue only in the presence of compromised blood flow and metabolic acidosis. In patients with COPD, dyspnoea limits exercise long before circulatory limitations and metabolic acidosis reach levels commensurate with those observed in healthy young people. Accordingly, although the relative intensity of diaphragm work is high during exercise in patients with COPD, diaphragm blood flow and the extracellular milieu of the muscle fibres may not be sufficiently compromised to induce diaphragm contractile fatigue.
Finally, it is worth considering that studies of exercise responses in patients with COPD have typically been undertaken using stationary cycling as the exercise modality. In a study comparing the ventilatory and metabolic responses of patients with COPD during incremental cycle ergometer exercise and incremental shuttle walking (Palange et al, 2000), the authors found that ![]() E was higher at all levels of
E was higher at all levels of ![]() O2, and increased at a steeper rate during walking. There was also a lower VT during walking. Given the role of the respiratory muscles in maintaining postural control and stability (see the section ‘Non-respiratory functions of the respiratory muscles’), it is likely that the observed alterations in breathing pattern may have been secondary to a compromise to the respiratory function of the respiratory muscles, induced by the necessity for a contribution to postural control during walking. Inspiratory accessory muscles have been shown to contract out of synchrony with the diaphragm during walking in patients with COPD, and dyspnoea was the major symptom limiting exercise tolerance (Delgado et al, 1982). Gosselink and colleagues found that MIP was a significant contributor (along with quadriceps strength) to performance in a 6-minute walk test in patients with COPD (Gosselink et al, 1996), supporting the important contribution of inspiratory muscle function to walking tolerance. Thus, during activities of daily living, patients with COPD are likely to experience severe limitations to their mobility from respiratory muscle-related factors, including the exacerbating compromise to inspiratory muscle function that derives from their non-respiratory role as postural muscles. In Chapter 4 we will consider the evidence that rebalancing the relationship between demand and capacity of the respiratory pump muscles using RMT induces beneficial adaptations to respiratory muscle structure and function, as well as clinical benefits.
O2, and increased at a steeper rate during walking. There was also a lower VT during walking. Given the role of the respiratory muscles in maintaining postural control and stability (see the section ‘Non-respiratory functions of the respiratory muscles’), it is likely that the observed alterations in breathing pattern may have been secondary to a compromise to the respiratory function of the respiratory muscles, induced by the necessity for a contribution to postural control during walking. Inspiratory accessory muscles have been shown to contract out of synchrony with the diaphragm during walking in patients with COPD, and dyspnoea was the major symptom limiting exercise tolerance (Delgado et al, 1982). Gosselink and colleagues found that MIP was a significant contributor (along with quadriceps strength) to performance in a 6-minute walk test in patients with COPD (Gosselink et al, 1996), supporting the important contribution of inspiratory muscle function to walking tolerance. Thus, during activities of daily living, patients with COPD are likely to experience severe limitations to their mobility from respiratory muscle-related factors, including the exacerbating compromise to inspiratory muscle function that derives from their non-respiratory role as postural muscles. In Chapter 4 we will consider the evidence that rebalancing the relationship between demand and capacity of the respiratory pump muscles using RMT induces beneficial adaptations to respiratory muscle structure and function, as well as clinical benefits.
Asthma
Exercise limitation has been much less widely studied in patients with asthma than in those with COPD. However, where significant airway obstruction is present, and hyperinflation results, dyspnoea is a dominating symptom that curtails exercise. The underlying mechanisms have much in common with those present in patients with COPD, except perhaps that the imbalance within the demand / capacity relationship is not quite so severe, due to the relatively normal inspiratory muscle function that is present in asthma (Hill, 1991). Patients with asthma also tend to have better-preserved function of their locomotor muscles (de Bruin et al, 1997) than patients with COPD. Thus, in patients with stable asthma, and in the absence of any exercise-induced bronchoconstriction, the respiratory-related limitations to exercise are similar to those of healthy people. However, where the severity of the condition is less reversible, the respiratory-related limitations become similar to those of COPD. Chapter 4 will review the evidence that inspiratory muscle training improves exercise tolerance in these patients.
Bronchiectasis
The factors limiting exercise tolerance in patients with bronchiectasis are similar to those limiting patients with COPD. Symptomatically, the primary exercise-limiting factor is dyspnoea, which is secondary to expiratory flow limitation and attendant dynamic hyperinflation (Koulouris et al, 2003). As was described above for COPD, dynamic hyperinflation is accompanied by a number of mechanical changes that affect the inspiratory muscles, contributing to dyspnoea and exercise limitation. In addition to the overloading of the inspiratory muscles created by hyperinflation, there is also a contribution to inspiratory muscle work originating from the restrictive defect that is present.
Cystic fibrosis
Patients with cystic fibrosis (CF) have an elevated work of breathing (Dunnink et al, 2009), which is multifactorial in origin; increased dead space, air trapping and airway resistance, as well as decreased lung compliance, all contribute. Peripheral muscle deconditioning also reduces the lactate threshold, which elevates ventilatory demand during exercise. Not surprisingly, the work of breathing has been identified as an important contributor to exertional dyspnoea in patients with CF (Leroy et al, 2011). Whilst indices of inspiratory muscle strength can be normal, or even increased, in patients with CF, loss of maximal inspiratory muscle work capacity has been reported (Ionescu et al, 1998; Enright et al, 2007). In addition, impaired inspiratory muscle endurance has been linked to exertional dyspnoea and alveolar hypoventilation during exercise (Leroy et al, 2011). Thus, both sides of the demand / capacity relationship of the respiratory muscles may be affected during exercise, contributing to intolerance. In Chapter 4, the evidence that IMT improves exercise tolerance in patients with CF will be reviewed.
Restrictive chest wall disorders
Restrictive chest wall disorders influence both sides of the demand / capacity relationship of the respiratory muscles (see the section ‘Changes in breathing mechanics and respiratory muscle function’). Furthermore, in conditions such as scoliosis there is a generalized muscle weakness that has been ascribed to the combined effects of deconditioning, nutritional factors and systemic inflammation (Martinez-Llorens et al, 2010). During the early stages of scoliosis, and in the presence of generalized muscle weakness, the principal exercise-limiting factor may be leg discomfort, particularly if cycle ergometry and incremental protocols are used (Martinez-Llorens et al, 2010). However, as has been mentioned previously, leg discomfort can also be a sign of inspiratory muscle overload due metaboreflex activation (see the section ‘Lesson from the world of sport’). Interestingly, Martinez-Llorens et al (2010) found that maximal cycling performance was predicted most closely by an equation that incorporated both respiratory and peripheral muscle strength. This implies that, during the early stages of scoliosis, exercise limitation is multifactorial. However, as chest wall deformity increases, and the restrictive pulmonary manifestations of the disease(s) predominate, respiratory factors may become the primary exercise-limiting factors (Martinez-Llorens et al, 2010).
In patients with ankylosing spondylitis, exercise tolerance appears to be most closely linked to impairment of pulmonary function; specifically, multiple stepwise regression implicates vital capacity, which explains 55% of the variation in exercise tolerance (Ozdem Yr et al, 2011). It is unlikely that vital capacity per se has a bearing on exercise tolerance; rather, the interrelationship most likely exposes the association between the difficulty in expanding the chest and exercise intolerance. The former has a number of repercussions that can limit exercise tolerance, not least the sense of breathing effort and the repercussions of inspiratory muscle overload. Furthermore, inspiratory muscle strength and endurance have been shown to explain independently around 60% of the variance in peak cycle ergometer work rate and maximal oxygen uptake in patients with ankylosing spondylitis (van der Esch et al, 2004). This strongly suggests that the inspiratory muscles should be a therapeutic target. See Chapter 4 for a description of the evidence supporting breathing exercises for patients with restrictive chest wall disorders.
Sarcoidosis and interstitial lung disease
The pulmonary manifestations of sarcoidosis and interstitial lung disease (ILD) share many clinical features (see the section ‘Changes in breathing mechanics and respiratory muscle function’), including exertional dyspnoea and exercise intolerance (Anderson & Bye, 1984). There are multiple factors underlying exercise intolerance (Markovitz & Cooper, 2010) and this complexity contributes to the poor relationship between resting pulmonary function and exercise intolerance (Cotes et al, 1988).
The exercise hyperpnoea of patients with ILD is tachypnoeic, which has been ascribed to reduced lung compliance (Agusti et al, 1991). Both features increase inspiratory muscle work and contribute to dyspnoea (see Ch. 1). Rapid shallow breathing is a behavioural adaptation to the discomfort associated with expansion of VT during exercise, but the extent to which respiratory mechanics contribute to exercise limitation remains debatable, as not all studies have found ventilatory limitation to be present (Markovitz & Cooper, 2010). However, the appearance of a ‘breathing reserve’ at end-exercise is misleading, and is not necessarily indicative that exercise is limited by non-respiratory factors (see the earlier section ‘Healthy people’).
Furthermore, the abnormally high ratio of dead space to tidal volume (VD / VT) resulting from rapid shallow breathing increases the ventilatory requirement of exercise, and contributes to other hallmarks of ILD, viz., exertional arterial oxygen desaturation and widening of the alveolar to arterial partial pressure gradient for oxygen. The principal underlying mechanism for these changes appears to be ventilation/perfusion (![]() /
/ ![]() ) mismatching, rather than diffusion limitation (Markovitz & Cooper, 2010). Furthermore, end-exercise VD / VT is highly correlated (r = 0.909) with arterial partial pressure of oxygen at end-exercise (Hansen & Wasserman, 1996), which highlights the complex interrelationships between potential exercise-limiting factors.
) mismatching, rather than diffusion limitation (Markovitz & Cooper, 2010). Furthermore, end-exercise VD / VT is highly correlated (r = 0.909) with arterial partial pressure of oxygen at end-exercise (Hansen & Wasserman, 1996), which highlights the complex interrelationships between potential exercise-limiting factors.
In the early stages of ILD, exercise is associated with pulmonary hypertension, which appears to be secondary to arterial hypoxaemia (Markovitz & Cooper, 2010). However, supplemental oxygen does not completely relieve the pulmonary vasoconstriction (Widimsky et al, 1977), which raises a question about whether factors such as muscle metaboreflex activation contribute to the increase in pulmonary arterial pressure (Lykidis et al, 2008). As was discussed in the earlier section ‘Healthy people’, high levels of inspiratory muscle work may be associated with activation of the inspiratory muscle metaboreflex, leading to premature locomotor muscle fatigue. This may be of particularly significance in patients with ILD, as peripheral muscle dysfunction has been demonstrated in patients with some ILDs, and quadriceps weakness is correlated with exercise limitation (Markovitz & Cooper, 2010). Similarly, in patients with sarcoidosis, granulomatous involvement of skeletal muscles contributes to both locomotor and respiratory muscle dysfunction, and quadriceps weakness is associated with exercise intolerance (Spruit et al, 2005). Thus, in patients with both ILD and sarcoidosis, inspiratory muscle weakness combined with high levels of inspiratory muscle work and activation of the inspiratory muscle metaboreflex may exacerbate peripheral muscle fatigue and exercise intolerance by inducing locomotor muscle vasoconstriction. At the time of writing there have been no studies of respiratory muscle training in patients with sarcoidosis or other ILD.
Chronic heart failure and pulmonary hypertension
Dyspnoea and exercise intolerance are hallmarks of chronic heart failure (CHF), with patients experiencing high levels of dyspnoea and limb discomfort at relatively low intensities of exercise (Wilson & Mancini, 1993). The complexity of the syndrome of CHF is such that the contribution of individual abnormalities to exercise intolerance is impossible to isolate. However, since dyspnoea is such a potent contributor to exercise intolerance in CHF, it is clear that the pulmonary contribution is extremely important. More specifically, evidence has emerged over the past decade implicating inspiratory muscle function and the demand / capacity relationship of the inspiratory muscles as important contributors to exercise intolerance in CHF (Ribeiro et al, 2012).
The causes of exertional dyspnoea in CHF and COPD have at least one common denominator: a high demand for inspiratory muscle work. As was described in the earlier section ‘Changes in breathing mechanics and respiratory muscle function’, this increased demand is multifactorial in CHF, being due to an exaggerated ventilatory response, tachypnoeic breathing pattern, increased lung compliance and expiratory flow limitation. Coupled to this increased demand is a reduced capacity for inspiratory muscle work, due to inspiratory muscle dysfunction (Ribeiro et al, 2012). In addition, patients with CHF also have to contend with a severely compromised cardiac output (Pina et al, 2003). The influence of CHF per se upon cardiac output and blood flow is not limited to the pulmonary circulation. During exercise, muscle blood flow is impaired leading to a reduction in oxygen delivery and metabolite removal, with the result that there is a greater reliance upon anaerobic metabolism. This has implications for the inspiratory muscles, as the resulting metabolic acidosis stimulates an increase in ![]() E (Franco, 2011). Patients with CHF also have greater chemoreflex sensitivity, causing an increase in the ventilatory response to chemoreflex activation as well as in muscle sympathetic nerve activity (Ribeiro et al, 2012). Furthermore, there is also evidence that competition for a share of the limited cardiac output results in underperfusion and deoxygenation within the inspiratory muscles during exercise (Mancini et al, 1991; Terakado et al, 1999).
E (Franco, 2011). Patients with CHF also have greater chemoreflex sensitivity, causing an increase in the ventilatory response to chemoreflex activation as well as in muscle sympathetic nerve activity (Ribeiro et al, 2012). Furthermore, there is also evidence that competition for a share of the limited cardiac output results in underperfusion and deoxygenation within the inspiratory muscles during exercise (Mancini et al, 1991; Terakado et al, 1999).
In patients with CHF, the exercise hyperpnoea is associated with a high demand for inspiratory pressure relative to the capacity to generate pressure (MIP), as well as a high level of inspiratory neural drive (Vibarel et al, 1998). Given these conditions, and the evidence that accessory muscle perfusion is compromised (Mancini et al, 1991), one would predict a propensity for inspiratory muscle fatigue in patients with CHF. As is the case in COPD, exercise-related alterations in inspiratory muscle function have been studied relatively little in patients with CHF, and almost exclusively in terms of diaphragm contractile function. One study found no change in twitch diaphragm pressure (TwPdi) after a symptom-limited cycle ergometer test in patients with CHF (Kufel et al, 2002). In this study, four patients reported terminating exercise due to dyspnoea, whilst six stopped because of leg fatigue. The absence of post-exercise diaphragm fatigue was confirmed in a later study employing an incremental cycle test to the limit of tolerance (Dayer et al, 2006). However, these data need to be interpreted carefully as there is evidence that the work of breathing most likely contributes to leg fatigue (Dempsey, 2010). Furthermore, the absence of diaphragm fatigue may also be explained by the support provided by inspiratory accessory muscles in delivering the required exercise hyperpnoea, thereby protecting the diaphragm from fatigue (see above section on COPD). As has been shown for patients with COPD, there is evidence to support the existence of accessory muscle fatigue following exercise in patients with CHF; furthermore, this fatigue appears to be related to their dyspnoea (Hughes et al, 2001). In a study of CHF patients who walked to the point of intolerable dyspnoea, inspiratory muscle relaxation rate was slowed, providing evidence for global inspiratory muscle fatigue (Hughes et al, 2001). These authors concluded that their observations were consistent with an imbalance between the demands placed on the inspiratory muscles and their capacity to meet this demand.
The limiting role of inspiratory muscles in patients with CHF is also supported by evidence that reducing the work of breathing during exercise improves exercise tolerance. When patients with CHF breathed heliox during an incremental exercise test, there was an increase in the time to the limit of tolerance (Mancini et al, 1997). Furthermore, when patients with CHF receive inspiratory assistance during exercise (partial inspiratory muscle unloading with pressure support), the time to the limit of tolerance during a constant load cycle test is increased (O’Donnell et al, 1999). The latter was also accompanied by a reduction in leg discomfort.
These data are similar to those in healthy athletes during assisted breathing, in whom improvements in exercise tolerance are ascribed to the absence of inspiratory muscle metaboreflex activation (Harms et al, 1997). Accordingly, a reduction in perception of leg effort during exercise with assisted breathing is due to improved leg blood flow. Indirect support for this comes from a study that confirmed the finding that inspiratory muscle unloading improves exercise tolerance and reduces leg effort in patients with CHF, but also found that these changes were associated with improved leg oxygenation (Borghi-Silva et al, 2008a). The same group has made identical observations in patients with COPD (Borghi-Silva et al, 2008b). Direct evidence comes from the improvement in limb blood flow observed when patients with CHF cycle (60% peak power) with inspiratory muscle unloading (Olson et al, 2010); however, unlike healthy people, when the inspiratory muscles of people with CHF were loaded, there was no reduction in limb blood flow above that observed during normal breathing. Thus, patients with CHF were unable to intensify the sympathetic output to limb vasculature when the work of breathing was increased, suggesting that this output is already maximal during normal breathing. These data provide support for the role of the inspiratory muscle metaboreflex in exercise limitation in patients with CHF. It is reasonable to suggest that, in patients with a compromised cardiac output, this reflex may play a particularly potent role in exercise limitation.
Resting indices of cardiac function have been shown to be poor predictors of exercise tolerance in patients with CHF; this is in contrast to the excellent predictive power of indices of respiratory function (Faggiano et al, 2001). The primary importance of inspiratory muscle function as a contributor to exercise intolerance in patients with CHF is supported by the observation that peak oxygen uptake is correlated with MIP (but not MEP) (Nishimura et al, 1994; Chua et al, 1995), as well as with inspiratory capacity (Nanas et al, 2003). Inspiratory capacity was also an independent predictor of exercise tolerance, and was correlated with pulmonary capillary wedge pressure (a determinant of lung compliance). The former study (Chua et al, 1995) illustrates the important role of inspiratory muscle capacity, and the latter (Nanas et al, 2003) of the demand for inspiratory muscle work in determining exercise tolerance in patients with CHF.
In Chapter 4, the evidence that inspiratory muscle training improves exercise tolerance in patients with CHF is reviewed.
Neurological and neuromuscular disease
Respiratory symptoms are common in patients with SCI, and dyspnoea is present in 73% of people with a lesion at C5, but only 29% in those with a lesion below T8 (Spungen et al, 1997). However, the extent to which dyspnoea is an exercise-limiting factor is currently unclear.
As was described in the section ‘Changes in breathing mechanics and respiratory muscle function’, SCI induces profound changes to pulmonary and respiratory muscle function, as well as breathing mechanics. The extent of the impact upon breathing is influenced by the level and completeness of the lesion, and the time post-injury. People with high-level, acute lesions exhibit the greatest disruption to their breathing. The result of the changes in respiratory muscle function and breathing mechanics is the potential for an imbalance in the demand / capacity relationship of the inspiratory muscles, predisposing people to dyspnoea and exercise-induced inspiratory muscle fatigue (Taylor et al, 2010).
Relatively few studies have compared directly the cardiorespiratory responses of SCI individuals with those of able-bodied people, but peak exercise values tend to be lower in untrained SCI injured people during arm-crank and wheelchair propulsion (Glaser et al, 1980; Keyser et al, 1999). This may be indicative of the smaller amount of trunk muscle mass, and / or compromised respiratory mechanics. There is also an impairment in the ventilatory equivalent for oxygen, which is lower in people with SCI at equivalent oxygen uptakes, and is also proportional to the level of SCI (Lassau-Wray & Ward, 2000), indicating that the response of minute ventilation is compromised progressively as lesion level becomes more cranial. This impairment may influence the ability to make an effective respiratory compensation for a metabolic acidosis, thereby hastening fatigue (see Ch. 2).
The extent to which exercise is limited by ventilatory factors is unclear. In highly trained Paralympic athletes, cervical SCI did not induce any ventilatory constraint during constant power arm-crank exercise (90% peak power) to the limit of tolerance (Taylor et al, 2010). This was evidenced by a reserve capacity for delivery of volume and flow, as well as the absence of any evidence of inspiratory muscle fatigue. The lack of fatigue may stem from the absence of the normal competition for cardiac output during exercise. The smaller muscle mass involved in exercise in SCI most likely fails to compromise regional blood flow to the extent that oxygen delivery to respiratory muscles is impaired. The majority of the athletes (five of seven) rated arm discomfort greater than dyspnoea. Although none rated either sensation maximally at end-exercise, both ratings were similar in magnitude to those measured at the peak of incremental treadmill exercise in respiratory patients (Hamilton et al, 1996b). In addition, the ventilatory equivalent for oxygen was not reduced in the Paralympians, suggesting that their capacity to deliver minute ventilation was superior to that of non-athletes with cervical SCI (see above). Thus, the extent to which these Paralympic data are relevant to the general population of people with SCI is limited. See Chapter 4 for a description of the evidence supporting specific respiratory muscle training in SCI.
Other NMDs
Because physical activity is limited by generalized deterioration of muscle function in patients with neuromuscular diseases (NMD), dyspnoea is not always present, and may not therefore be the primary exercise-limiting factor (McCool & Tzelepis, 1995). However, this does not preclude respiratory-related limitation; as was discussed in the section ‘Healthy people’, high levels of inspiratory muscle work may be associated with activation of the inspiratory muscle metaboreflex, leading to swift fatigue of already compromised locomotor muscles.
The restrictive pattern of pulmonary dysfunction (Gibson et al, 1977) leads to a rapid, shallow breathing pattern and a higher than normal ratio of dead space to tidal volume (VD / VT). This increases the demand for minute ventilation (![]() E), and is exacerbated by an early metabolic acidosis, which increases the work of breathing during exercise. Although absolute
E), and is exacerbated by an early metabolic acidosis, which increases the work of breathing during exercise. Although absolute ![]() E may not be high in patients with NMD, the relative work of breathing is elevated by respiratory muscle weakness, creating imbalance in the demand / capacity relationship. Thus, in patients with NMD, activation of the inspiratory muscle metaboreflex may contribute to peripheral muscle fatigue and exercise intolerance by inducing locomotor muscle vasoconstriction.
E may not be high in patients with NMD, the relative work of breathing is elevated by respiratory muscle weakness, creating imbalance in the demand / capacity relationship. Thus, in patients with NMD, activation of the inspiratory muscle metaboreflex may contribute to peripheral muscle fatigue and exercise intolerance by inducing locomotor muscle vasoconstriction.
The inspiratory muscle metaboreflex may be a particularly important contributor to exercise limitation in NMD; impairment of the neuronal nitric oxide synthase system (nNOS), which opposes sympathetically mediated vasoconstriction, has been identified in a number of NMDs (Duchenne and Becker muscular dystrophies, as well as other muscle disorders involving limb-girdle muscular dystrophy, congenital muscular dystrophies, myositis and myopathy) (Kobayashi et al, 2008). Accordingly, dysfunction of the nNOS system has been suggested as a common mechanism of locomotor muscle fatigue in patients with NMD (Aboussouan, 2009). Thus, the inspiratory muscle metaboreflex may have a particularly potent effect in these patients, making these muscles an appealing therapeutic target.
In Chapter 4, the evidence that inspiratory muscle training improves exercise tolerance in patients with NMD is reviewed.
Obesity
Exertional dyspnoea is a common symptom in obese patients (Babb et al, 2008a), but it has been studied comparatively little from a mechanistic perspective. Extreme dyspnoea is not a limiting symptom in all obese patients, but a key underlying factor in determining the severity of dyspnoea appears to be the oxygen cost of breathing (Babb et al, 2008a). When the relationship between the oxygen cost of breathing and exertional dyspnoea was examined directly in obese women, the oxygen cost of breathing was found to be considerably and significantly higher (38–70%) in those who were breathless upon exertion (Babb et al, 2008a). Furthermore, around 65% of the variation in dyspnoea was explained by the variation in the oxygen cost of breathing. These findings are consistent with the notion that dyspnoea is directly related to the work of the respiratory muscles. Since work is elevated in the face of relatively normal inspiratory muscle strength, dyspnoea reflects an imbalance between the demand for inspiratory muscle work and the ability of the inspiratory muscles to meet that demand (Campbell, 1966).
An important factor that appears to influence the total oxygen cost of exercise in obese individuals is fat distribution. For example, obese women (mean BMI 40 kg·m− 2) in whom fat was deposited in the upper body (UBD) had a greater oxygen cost of cycling, a higher ![]() E, a more rapid and shallow breathing pattern and a lower anaerobic threshold than women whose fat was distributed in the lower body (Li et al, 2001). This suggests an underlying mechanism related to the higher work of breathing when fat is deposited in and around the trunk. Although dyspnoea was not assessed during this study (Li et al, 2001), it is reasonable to presume that it would have been higher in the UBD group.
E, a more rapid and shallow breathing pattern and a lower anaerobic threshold than women whose fat was distributed in the lower body (Li et al, 2001). This suggests an underlying mechanism related to the higher work of breathing when fat is deposited in and around the trunk. Although dyspnoea was not assessed during this study (Li et al, 2001), it is reasonable to presume that it would have been higher in the UBD group.
The question then arises as to the extent of limitation imposed by dyspnoea and the relationship of this to the performance of the inspiratory muscles. In a study of obese men (mean BMI 42 kg·m− 2), an index of the relative work of breathing has been created by referencing the work of breathing to the capacity of the inspiratory muscles to sustain work (Chlif et al, 2007). In addition, an index of the neural drive to the inspiratory muscles was assessed. The obese men had higher levels of inspiratory neural drive and dyspnoea at the same relative intensity of exercise compared with non-obese men. As is typical, the obese men also adopted a rapid shallow breathing pattern as part of their strategy to minimize respiratory discomfort. However, the strategy did not appear to normalize their dyspnoea, because the relative work of breathing remained too high for the inspiratory muscles, as evidenced by the inspiratory muscle fatigue that was present post-exercise. These data once again reinforce the fact that obese individuals have an imbalance between demand and capacity for inspiratory muscle work.
The inspiratory muscle fatigue observed in the study of Chlif et al (2007) and the high levels of inspiratory muscle work mean that the risk of inspiratory muscle metaboreflex activation is likely to be very high in obese individuals. The repercussions of this were described in the section ‘Healthy people’, and include a reduction in limb blood flow, leading to increased rate of development and magnitude of both limb discomfort and limb muscle fatigue.
In Chapter 4, the evidence that respiratory muscle training improves exercise tolerance in patients with obesity is reviewed.
Ageing
The senescent changes to the pulmonary system have been dubbed ‘senile emphysema’ (Janssens et al, 1999). Accordingly, it is no surprise that exertional dyspnoea is a common complaint amongst the elderly, even in the absence of cardiopulmonary disease (Landahl et al, 1980). Furthermore, the intensity of dyspnoea is higher by 1 to 2 Borg units at a standardized submaximal oxygen uptake during incremental treadmill exercise in healthy elderly (60–80 years) adults compared with younger (40–59 years) adults (Ofir et al, 2008).
However, dyspnoea perception in healthy, active older people does not appear to be correlated with expiratory flow limitation or ![]() E; instead it appears to be related to the relative load upon the inspiratory muscles, i.e., the inspiratory pressure requirement as a percentage of inspiratory pressure-generating capacity – in other words, the state of the demand / capacity relationship (Johnson et al, 1991). The requirement for inspiratory pressure generation during exercise is increased in healthy ageing by a number of factors, including a higher requirement for
E; instead it appears to be related to the relative load upon the inspiratory muscles, i.e., the inspiratory pressure requirement as a percentage of inspiratory pressure-generating capacity – in other words, the state of the demand / capacity relationship (Johnson et al, 1991). The requirement for inspiratory pressure generation during exercise is increased in healthy ageing by a number of factors, including a higher requirement for ![]() E, dynamic lung hyperinflation and mechanical constraints on VT expansion (Jensen et al, 2009a). Furthermore, inspiratory muscle strength declines with advancing age (McConnell & Copestake, 1999).
E, dynamic lung hyperinflation and mechanical constraints on VT expansion (Jensen et al, 2009a). Furthermore, inspiratory muscle strength declines with advancing age (McConnell & Copestake, 1999).
As was described in the earlier section ‘Healthy people’, inspiratory muscle overload can contribute to both respiratory and locomotor muscle fatigue. In healthy people, the symptoms leading to the cessation of exercise fall into two main categories: ‘local’ locomotor muscle fatigue and ‘central’ dyspnoea (Ekblom & Goldbarg, 1971). When the contributions of leg effort and dyspnoea to exercise limitation are assessed, over 60% of healthy people cease exercise because of the combined contribution of the two symptoms (Hamilton et al, 1996a). Interestingly, the Borg CR-10 rating of dyspnoea at exercise cessation in this healthy group was higher (6 units) than the rating in a similar group of patients with respiratory disease (5 units) (Hamilton et al, 1996a). These data indicate that, even in healthy people, dyspnoea is a troubling symptom that makes an important contribution to the decision to stop exercising.
In Chapter 4, the evidence that inspiratory muscle training improves exercise tolerance in healthy older people is reviewed.
Miscellaneous conditions
Diabetes
Patients with diabetes have inspiratory muscle weakness and an increased demand for inspiratory pressure generation during exercise (Wanke et al, 1992); accordingly, the demand / capacity relationship of the respiratory muscles is in a state of imbalance (see ‘Changes in breathing mechanics and respiratory muscle function’). Exercise tolerance is impaired during cycle ergometer exercise compared with healthy people, and the sense of respiratory effort is also elevated (Wanke et al, 1992). The ventilatory response to exercise in diabetes has been studied very little, so it is not clear whether dyspnoea is a primary exercise-limiting factor. However, evidence of neuromechanical uncoupling (see Ch. 1) during hypoxic ventilatory stimulation in diabetic patients (type 1) is indicative of an impairment of the mechanical response to inspiratory drive, and suggests that dyspnoea is a prominent feature of increased inspiratory muscle work (Scano et al, 1999). See Chapter 4 for a description of the evidence supporting specific inspiratory muscle training.
Cancer
One of the most common chronic symptoms in patients with cancer is exertional dyspnoea, which is present in up to 10% of survivors of childhood cancers and up to 70% of patients with advanced cancer (Travers et al, 2008). In general, cancer patients have been shown to have peripheral muscle weakness and a lower lactate threshold compared with healthy people (Travers et al, 2008). Cancer patients with chronic, unexplained exertional dyspnoea also have a lower symptom-limited peak oxygen uptake and minute ventilation compared with cancer patients without chronic dyspnoea, and controls; furthermore, dyspnoea is the primary exercise-limiting factor in ~ 70% of these dyspnoic patients (Travers et al, 2008). Dyspnoic cancer patients appear to be distinguishable by virtue of their rapid shallow breathing pattern during exercise, and their lower inspiratory capacity (Travers et al, 2008). The latter correlates with inspiratory muscle strength, suggesting a causal link (Travers et al, 2008).
The important contribution of inspiratory muscle strength to exercise intolerance was recently shown in patients with thoracic cancer (England et al, 2012). Of a range of physiological and psychological factors explored, only inspiratory muscle strength (MIP) and peripheral muscle power were found to be significant determinants of exercise tolerance (incremental shuttle walking). Dyspnoea made a contribution to exercise cessation in 92% of the patients, which perhaps explains why MIP was a stronger determinant of exercise tolerance than peripheral muscle power. The authors suggested that both factors should be considered as therapeutic targets. At the time of writing there have been no studies of respiratory muscle training in patients with cancer.
Anorexia nervosa
Unfortunately, much of the published literature relating to patients with anorexia nervosa is case study based. However, one study compared 19 young women with anorexia nervosa with matched ‘thin’ (BMI < 19 kg·m− 2) controls; it found that they had lower peak exercise values for all key cardiorespiratory parameters including maximal heart rate and oxygen uptake, lactate threshold, minute ventilation and oxygen pulse (Biadi et al, 2001). The extent to which dyspnoea contributes to exercise limitation is unclear, but case study evidence supports a link between inspiratory muscle weakness, dyspnoea and exercise intolerance (Birmingham & Tan, 2003).
Pregnancy
An increase in breathing effort and dyspnoea are experienced by around three-quarters of pregnant women (Milne et al, 1978). A recent study characterized the ventilatory, metabolic and perceptual response to cycle ergometer exercise in women in their third trimester, and compared these data to those collected 5 months post-partum. The participants were divided into two groups: those with ‘clinically significant activity-related dyspnoea’ (CSB), and those without. The work of breathing was, on average, 43% higher in the third trimester, yet dyspnoea was only higher (45%) in the group with CSB. The CSB group also had an exaggerated ventilatory response to cycle ergometer exercise, both in their third trimester and 5 months post-partum (Jensen et al, 2009b). As there were no differences in central ventilatory control or breathing mechanics, the increase in dyspnoea intensity in the CSB group was ascribed to a ‘normal awareness of increased ventilation’. What is perhaps more intriguing is that a substantial increase in the work of breathing was not accompanied by an increased sense of breathing effort in the group without CSB. At the time of writing there have been no studies of respiratory muscle training in pregnant women.
Vocal cord dysfunction and inspiratory stridor
Inspiratory stridor is an external sign of vocal cord dysfunction (VCD) during exercise. The prevalence of the condition is between 5% and 15%, and it is frequently mistaken for exercise-induced asthma (Kenn & Hess, 2008). However, the fact that symptoms occur suddenly during exercise, and with a loud, rasping, inspiratory wheeze, are cardinal signs of VCD. Notwithstanding this, VCD and asthma can and do coexist (Kenn & Hess, 2008). The precise causes of inspiratory stridor remain unknown, and it probably has a multifactorial aetiology. However, one possible mechanism is fatigue of the muscles that abduct the vocal cords and maintain the upper airway opening.
Since the upper airway musculature is activated in proportion to the magnitude of inspiratory effort, the addition of an external inspiratory load induces increased metabolic activity, and thus a potential training stimulus to the upper airway muscles (How et al, 2007; Cheng et al, 2011). Accordingly, it is possible to train the upper-airway-stabilizing muscles using a resistive inspiratory muscle trainer. See Chapter 4 for a description of the evidence supporting specific respiratory muscle training in VCD.
NON-RESPIRATORY FUNCTIONS OF THE RESPIRATORY MUSCLES
• Core stability: actions that maintain stability of the trunk and lumbopelvic region, protecting the spine from damage and creating a stable platform from which to generate limb movements.
• Postural control: actions that maintain balance in response to destabilizing forces acting upon the body.
Before beginning our discussion, it is helpful to develop our earlier definition of core stability slightly. In simple terms, core stability provides a stable platform from which the limbs can perform movements. Without the stable platform provided by the core muscles, we are just ‘shooting a cannon from a canoe’ (Tsatsouline, 2000), with very similar results!
As mentioned above, the core acts anatomically as a stable base from which the limbs perform movements, and it comprises the spine, pelvis and a myriad of muscles that stabilize these bony structures. The majority of the prime movers and stabilizing muscles of the limbs attach to the pelvis and spine. It has been recognized for many years that breathing is linked functionally to trunk-loading tasks such as lifting, lowering and pushing / pulling. Human beings instinctively take a breath immediately before such movements, and use this to generate a pneumatic stabilizing pressure within the thorax, as well as increasing intra-abdominal pressure (see below). Typically, inspired volume prior to loading is proportional to the magnitude of the load (Hagins & Lamberg, 2006), and people with lower levels of physical fitness appear to require a higher preparatory inspired volume (Lamberg & Hagins, 2012). It is reasonable to suggest that in untrained people, with weaker trunk and abdominal muscles, there is a greater reliance upon intracompartmental pneumatic pressure for stabilization.
The abdominal muscles (transversus abdominis, internal / external obliques, rectus abdominis) are a group of large, relatively superficial muscles that act to stiffen the abdominal compartment and / or increase intra-abdominal pressure, thereby stiffening and stabilizing the spine and pelvis (Hodges et al, 2005). When contracted, these muscles form a rigid cylinder (or ‘corset’) and their ability to increase intra-abdominal pressure is influenced by the state of contraction of the pelvic floor and the diaphragm, which can be considered as the base and lid to the rigid cylinder formed by the abdominal muscles. The increase in intra-abdominal pressure resulting from contraction of the walls of the cylinder is dissipated if the lid (diaphragm) is not held in place. Conversely, if the lid moves downwards into the cylinder the increase in pressure can be magnified (Hodges & Gandevia, 2000). Hence the diaphragm makes a substantial and important contribution to the development of intra-abdominal pressure, and thus to core stability. This role is confirmed by the observations that a programme of weightlifting exercises induces an increase in diaphragm thickness and strength (DePalo et al, 2004) and that weightlifters have thicker, stronger diaphragms than non-weightlifters (McCool et al, 1997). Furthermore, diaphragm contribution to weightlifting tasks is proportional to the weight lifted, and greatest in tasks that induce spinal loading (Al-Bilbeisi & McCool, 2000). Imaging of the diaphragm using MRI (magnetic resonance imaging) during a variety of tasks suggests that human beings are able to voluntarily contract the diaphragm during breath holding, which contributes to stabilization (Kolar et al, 2009). However, this ability varies between individuals, and may explain why some develop low back pain (Kolar et al, 2009). A further interesting finding was that diaphragm contraction was heterogeneous, suggesting that different parts of the diaphragm were involved in stabilization under different conditions, possibly reflecting variations in the interplay between the diaphragm and abdominal musculature (Kolar et al, 2009).
Both the transversus abdominis and the diaphragm are involved in postural control, and contract automatically in anticipation of actions that destabilize and / or load the trunk (Hodges et al, 1997a; Hodges et al, 1997b). The contractions occur irrespective of the phase of breathing, and appear to be superimposed upon the respiratory state of the diaphragm. These feedforward contractions occur approximately 20 milliseconds before EMG (electromyogram) activity is detected in prime mover muscles (Hodges et al, 1997a; Hodges et al, 1997b), acting to stiffen the trunk as part of a strategy to protect the spine, and to exert postural control. The postural challenge employed by Hodges and colleagues was rapid flexion of the shoulder. It is well known that patients with COPD, for example, become breathless during activities of daily living that involve unsupported arm elevation (Breslin, 1992), especially if the movements are overhead, e.g., hair washing. It is not unreasonable to postulate that this may be related to the involvement of the respiratory muscles in stabilizing and balancing tasks. This being the case, it is not surprising that isolated arm resistance training has little effect upon dyspnoea in these patients (Janaudis-Ferreira et al, 2011).
Research has shown that, in situations of increased breathing demand, the diaphragm’s role in breathing always takes precedence over its role in posture (Hodges et al, 2001). So in situations such as exercise the postural role of diaphragm may become compromised, which may lead to an increased risk of injury and / or an increased risk of falling or loss of balance. This has important implications for any activity where breathing demands are high. The conflicting demands of the respiratory and non-respiratory roles of the respiratory muscles are also evidenced by the fact that patients with COPD are able to generate higher respiratory mouth pressures and maximum voluntary ventilation, when the postural role of these muscles has been reduced by leaning forward and taking weight through the arms (Cavalheri et al, 2010). This latter observation also helps to explain why this posture relieves dyspnoea.
Many chronic diseases, including cardiovascular disease and COPD, are associated with an increased falling risk (Lawlor et al, 2003). Furthermore, a recent prospective study by Lawlor and colleagues (2003) found that around one-third of patients with COPD reported a fall during the 6-month trial. The authors concluded that patients with COPD have a high susceptibility to falls, which others have shown is also associated with a worsening of dyspnoea perception (Roig et al, 2011). Intrinsic risk factors for falling in patients with COPD include well-established contributors such as lower limb muscle weakness and deficits of gait and balance (Roig et al, 2009). With respect to the latter factor, specific balance deficits have been identified in patients with COPD, e.g., an increased body sway (Butcher et al, 2004; Chang et al, 2008; Smith et al, 2010), and an impaired ability to maintain balance whilst reaching with the arms (Butcher et al, 2004; Eisner et al, 2008). Hitherto the assumption has been that limb muscle weakness is the source of balance deficits in patients with COPD. However, a recent study suggests that patients with COPD also have an impaired postural control strategy, relying to a greater extent upon ankle proprioceptive feedback (Janssens et al, unpublished work). This strategy is also observed in people with low back pain (Brumagne et al, 2008b), but it is suboptimal and less efficient than the normal, multi-segmental strategy that includes knees, hips and spine (Butcher et al, 2004). Interestingly, when the inspiratory muscles are fatigued, healthy participants experienced larger postural sway when standing on an unstable surface, abandoning their normal multi-segmental strategy in favour of one that relied upon adjustments made around the ankle (Janssens et al, 2010). Thus, in the presence of inspiratory muscle fatigue (IMF), the postural control strategy of healthy people became the same as that of patients with chronic low back pain and COPD. Collectively, these data suggest that people revert to ankle-steered postural control strategy when the contribution of trunk muscles is impaired. In the case of patients with COPD, this may be due to an impaired postural contribution of the inspiratory muscles to balance. This may explain the greater falls risk in this population (Janssens et al, unpublished work).
The interrelationship of multiple factors related to the trunk muscle function was highlighted in a cross-sectional study of over 38 000 Australian women (Smith et al, 2006). Women with disorders of continence and respiration showed a higher prevalence of back pain than women who did not have these disorders. These findings are supported by earlier physiological data showing that the postural function of the diaphragm, abdominal and pelvic floor muscles is reduced by incontinence (Deindl et al, 1994) and respiratory disease (Hodges et al, 2000). Furthermore, people with chronic low back pain exhibit greater breathing-related postural sway than people without back pain (Hamaoui et al, 2002). In addition, when the inspiratory muscles are fatigued in healthy people prior to an isometric trunk extension test (a modified Biering-Sørensen test), fatigue of the back muscles occurs more quickly (Brumagne et al, 2008a). This suggests that inhibiting the contribution of the inspiratory muscles to trunk extension places greater demands upon the back musculature. Collectively, these data support the notion that the ‘discrete’ functions of the trunk muscles (breathing, continence, stabilization, balance) are in fact far from discrete, and are in fact interdependent.
Chapter 7 will consider how to implement functional respiratory muscle training that incorporates both respiratory and stabilizing/postural control challenges.
THE RATIONALE FOR RESPIRATORY MUSCLE TRAINING
The section ‘Changes in breathing mechanics and respiratory muscle function’ provided an overview of the pathophysiology of a range of conditions where demand / capacity imbalance exists. In each case, a theoretical rationale for respiratory muscle training (RMT) was offered. Similarly, in the section ‘Respiratory muscle involvement in exercise limitation’, the contribution of the respiratory muscles to exercise limitation was presented where appropriate, thereby establishing the theoretical rationale for RMT in improving exercise tolerance. Finally, the non-respiratory functions of the respiratory muscles were considered, as these increase the demand for respiratory muscle work, thereby exacerbating any demand / capacity imbalance. In all circumstances, the fundamental rationale of RMT is amelioration of the demand / capacity imbalance. The benefits of this to individual patients will depend upon the clinical manifestations of the imbalance – be they exercise intolerance or respiratory failure. In Chapter 4, the specific evidence for an influence of RMT upon the repercussions of the demand / capacity imbalance is presented. Indications for RMT are considered in Chapter 6 (‘General principles of foundation IMT’).
References
Aboussouan, L.S. Mechanisms of exercise limitation and pulmonary rehabilitation for patients with neuromuscular disease. Chron. Respir. Dis. 2009;6:231–249.
Agusti, A.G., Roca, J., Gea, J., et al. Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1991;143:219–225.
Akkoca, O., Mungan, D., Karabiyikoglu, G., et al. Inhaled and systemic corticosteroid therapies: Do they contribute to inspiratory muscle weakness in asthma? Respiration. 1999;66:332–337.
Al-Bilbeisi, F., McCool, F.D. Diaphragm recruitment during nonrespiratory activities. Am. J. Respir. Crit. Care Med. 2000;162:456–459.
Amann, M., Proctor, L.T., Sebranek, J.J., et al. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. Am. J. Physiol. 2009;587:271–283.
Amann, M., Blain, G.M., Proctor, L.T., et al. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol. 2010;109:966–976.
Amann, M., Regan, M.S., Kobitary, M., et al. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R314–R324.
Ambrosino, N., Strambi, S. New strategies to improve exercise tolerance in chronic obstructive pulmonary disease. Eur. Respir. J. 2004;24:313–322.
Anderson, S.D., Bye, P.T. Exercise testing in the evaluation of diffuse interstitial lung disease. Aust. N. Z. J. Med. 1984;14:762–768.
Anthony, M. The obesity hypoventilation syndrome. Respir. Care. 2008;53:1723–1730.
Anzueto, A., Tobin, M.J., Moore, G., et al. Effect of prolonged mechanical ventilation on diaphragmatic function, a preliminary study of a baboon model. Am. Rev. Respir. Dis. 1987;135:A201.
Araujo, T.L., Resqueti, V.R., Bruno, S., et al. Respiratory muscle strength and quality of life in myotonic dystrophy patients. Rev. Port. Pneumol. 2010;16:892–898.
Babb, T.G., Ranasinghe, K.G., Comeau, L.A., et al. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am. J. Respir. Crit. Care Med. 2008;178:116–123.
Babb, T.G., Wyrick, B.L., DeLorey, D.S., et al. Fat distribution and end-expiratory lung volume in lean and obese men and women. Chest. 2008;134:704–711.
Babcock, M.A., Pegelow, D.F., McClaran, S.R., et al. Contribution of diaphragmatic power output to exercise-induced diaphragm fatigue. J. Appl. Physiol. 1995;78:1710–1719.
Babcock, M.A., Pegelow, D.F., Johnson, B.D., et al. Aerobic fitness effects on exercise-induced low-frequency diaphragm fatigue. J. Appl. Physiol. 1996;81:2156–2164.
Babcock, M.A., Harms, C.A., Pegelow, D.F., et al. Effects of mechanical unloading of inspiratory muscles on exercise-induced diaphragm fatigue. Am. Rev. Respir. Dis. 1997;152:178.
Babcock, M.A., Pegelow, D.F., Taha, B.H., et al. High frequency diaphragmatic fatigue detected with paired stimuli in humans. Med. Sci. Sports Exerc. 1998;30:506–511.
Bark, H., Heimer, D., Chaimovitz, C., et al. Effect of chronic renal failure on respiratory muscle strength. Respiration. 1988;54:153–161.
Barreiro, E., de la Puente, B., Minguella, J., et al. Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005;171:1116–1124.
Baydur, A., Alsalek, M., Louie, S.G., et al. Respiratory muscle strength, lung function, and dyspnea in patients with sarcoidosis. Chest. 2001;120:102–108.
Bellofiore, S., Ricciardolo, F.L., Ciancio, N., et al. Changes in respiratory drive account for the magnitude of dyspnoea during bronchoconstriction in asthmatics. Eur. Respir. J. 1996;9:1155–1159.
Biadi, O., Rossini, R., Musumeci, G., et al. Cardiopulmonary exercise test in young women affected by anorexia nervosa. Ital. Heart J. 2001;2:462–467.
Binks, A.P., Moosavi, S.H., Banzett, R.B., et al. ‘Tightness’ sensation of asthma does not arise from the work of breathing. Am. J. Respir. Crit. Care Med. 2002;165:78–82.
Birmingham, C.L., Tan, A.O. Respiratory muscle weakness and anorexia nervosa. Int. J. Eat. Disord. 2003;33:230–233.
Bissett, B., Leditschke, I.A., Paratz, J.D., et al. Respiratory dysfunction in ventilated patients, can inspiratory muscle training help? Anaesth Intensive Care. 2012;40:236–246.
Bohannon, R.W., Williams Andrews, A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97:182–189.
Borghi-Silva, A., Carrascosa, C., Oliveira, C.C., et al. Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high-intensity exercise in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2465–H2472.
Borghi-Silva, A., Oliveira, C.C., Carrascosa, C., et al. Respiratory muscle unloading improves leg muscle oxygenation during exercise in patients with COPD. Thorax. 2008;63:910–915.
Breslin, E.H. Dyspnea-limited response in chronic obstructive pulmonary disease: reduced unsupported arm activities. Rehabil. Nurs. 1992;17:12–20.
Britto, R.R., Zampa, C.C., de Oliveira, T.A., et al. Effects of the aging process on respiratory function. Gerontology. 2009;55:505–510.
Brouillette, R.T., Thach, B.T. A neuromuscular mechanism maintaining extrathoracic airway patency. J. Appl. Physiol. 1979;46:772–779.
Brubaker, P.H. Exercise intolerance in congestive heart failure: a lesson in exercise physiology. J. Cardiopulm. Rehabil. 1997;17:217–221.
Brumagne, S., Janssen, L., Polspoel, K., et al. Inspiratory muscle fatigue and back muscle fatiguability in persons with and without low back pain. European Respiratory Society, Berlin, 2205. Eur. Respir. J. 2008;32:726S.
Brumagne, S., Janssens, L., Janssens, E., et al. Altered postural control in anticipation of postural instability in persons with recurrent low back pain. Gait Posture. 2008;28:657–662.
Buchman, A.S., Boyle, P.A., Wilson, R.S., et al. Respiratory muscle strength predicts decline in mobility in older persons. Neuroepidemiology. 2008;31:174–180.
Buhimschi, C.S., Buhimschi, I.A., Malinow, A.M., et al. Pushing in labor: performance and not endurance. Am. J. Obstet. Gynecol. 2002;186(6):1339–1344.
Butcher, S.J. Modulation of ventilatory mechanics during exercise in ventilation-limited populations. Appl. Physiol. Nutr. Metab. 2008;33:536–537.
Butcher, S.J., Meshke, J.M., Sheppard, M.S. Reductions in functional balance, coordination, and mobility measures among patients with stable chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 2004;24:274–280.
Cafarelli, E. Peripheral contributions to the perception of effort. Med. Sci. Sports Exerc. 1982;14:382–389.
Callahan, L.A. Invited editorial on ‘acquired respiratory muscle weakness in critically ill patients: what is the role of mechanical ventilation-induced diaphragm dysfunction?’. J. Appl. Physiol. 2009;106:360–361.
Callegaro, C.C., Martinez, D., Ribeiro, P.A., et al. Augmented peripheral chemoreflex in patients with heart failure and inspiratory muscle weakness. Respir. Physiol. Neurobiol. 2010;171:31–35.
Campbell, E.J.M. The relationship of the sensation of breathlessness to the act of breathing. In: Howell J.B.L., ed. Breathlessness. London: Blackwell Scientific; 1966:55–64.
Canet, J., Mazo, V. Postoperative pulmonary complications. Minerva Anestesiol. 2010;76:138–143.
Carlucci, A., Ceriana, P., Prinianakis, G., et al. Determinants of weaning success in patients with prolonged mechanical ventilation. Crit. Care. 2009;13(3):R97.
Caruso, P., Friedrich, C., Denari, S.D., et al. The unidirectional valve is the best method to determine maximal inspiratory pressure during weaning. Chest. 1999;115:1096–1101.
Casaburi, R., Patessio, A., Ioli, F., et al. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am. Rev. Respir. Dis. 1991;143:9–18.
Cavalheri, V., Camillo, C.A., Brunetto, A.F., et al. Effects of arm bracing posture on respiratory muscle strength and pulmonary function in patients with chronic obstructive pulmonary disease. Rev. Port. Pneumol. 2010;16:887–891.
Cejudo, P., Lopez-Marquez, I., Lopez-Campos, J.L., et al. Factors associated with quality of life in patients with chronic respiratory failure due to kyphoscoliosis. Disability Rehabilitation. 2009;31:928–934.
Chang, A.T., Seale, H., Walsh, J., et al. Static balance is affected following an exercise task in chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation and Prevention. 2008;28:142–145.
Chatham, K., Gelder, C.M., Lines, T.A., et al. Suspected statin-induced respiratory muscle myopathy during long-term inspiratory muscle training in a patient with diaphragmatic paralysis. Phys. Ther. 2009;89(3):257–266.
Cheng, S., Butler, J.E., Gandevia, S.C., et al. Movement of the human upper airway during inspiration with and without inspiratory resistive loading. J. Appl. Physiol. 2011;110:69–75.
Chevrolet, J.C., Tschopp, J.M., Blanc, Y., et al. Alterations in inspiratory and leg muscle force and recovery pattern after a marathon. Med. Sci. Sports Exerc. 1993;25:501–507.
Chlif, M., Keochkerian, D., Mourlhon, C., et al. Noninvasive assessment of the tension-time index of inspiratory muscles at rest in obese male subjects. Int. J. Obes. (Lond.). 2005;29:1478–1483.
Chlif, M., Keochkerian, D., Feki, Y., et al. Inspiratory muscle activity during incremental exercise in obese men. Int. J. Obes. (Lond.). 2007;31:1456–1463.
Chlif, M., Keochkerian, D., Choquet, D., et al. Effects of obesity on breathing pattern, ventilatory neural drive and mechanics. Respir. Physiol. Neurobiol. 2009;168:198–202.
Chua, T.P., Anker, S.D., Harrington, D., et al. Inspiratory muscle strength is a determinant of maximum oxygen consumption in chronic heart failure. Br. Heart J. 1995;74:381–385.
Chua, T.P., Ponikowski, P., Webb-Peploe, K., et al. Clinical characteristics of chronic heart failure patients with an augmented peripheral chemoreflex. Eur. Heart J. 1997;18:480–486.
Clanton, T.L., Levine, S. Respiratory muscle fiber remodeling in chronic hyperinflation: dysfunction or adaptation? J. Appl. Physiol. 2009;107:324–335.
Coast, J.R., Haverkamp, H.C., Finkbone, C.M., et al. Alterations in pulmonary function following exercise are not caused by the work of breathing alone. Int. J. Sports Med. 1999;20:470–475.
Coirault, C., Chemla, D., Lecarpentier, Y. Relaxation of diaphragm muscle. J. Appl. Physiol. 1999;87:1243–1252.
Collet, F., Mallart, A., Bervar, J.F., et al. Physiologic correlates of dyspnea in patients with morbid obesity. Int. J. Obes. (Lond.). 2007;31:700–706.
Contreras, G., Gutierrez, M., Beroiza, T., et al. Ventilatory drive and respiratory muscle function in pregnancy. Am. Rev. Respir. Dis. 1991;144:837–841.
Cordain, L., Rode, E.J., Gotshall, R.W., et al. Residual lung volume and ventilatory muscle strength changes following maximal and submaximal exercise. Int. J. Sports Med. 1994;15:158–161.
Cotes, J.E., Zejda, J., King, B. Lung function impairment as a guide to exercise limitation in work-related lung disorders. Am. Rev. Respir. Dis. 1988;137:1089–1093.
Coxson, H.O., Chan, I.H., Mayo, J.R., et al. Early emphysema in patients with anorexia nervosa. Am. J. Respir. Crit. Care Med. 2004;170:748–752.
Cross, T.J., Sabapathy, S., Beck, K.C., et al. The resistive and elastic work of breathing during exercise in patients with chronic heart failure. Eur. Respir. J. 2012;39:1449–1457.
D’Amours, R., Clerch, L., Massaro, D. Food deprivation and surfactant in adult rats. J. Appl. Physiol. 1983;55:1413–1417.
Dayer, M.J., Hopkinson, N.S., Ross, E.T., et al. Does symptom-limited cycle exercise cause low frequency diaphragm fatigue in patients with heart failure? Eur. J. Heart Fail. 2006;8:68–73.
de Bruin, P.F., Ueki, J., Watson, A., et al. Size and strength of the respiratory and quadriceps muscles in patients with chronic asthma. Eur. Respir. J. 1997;10:59–64.
Decramer, M. Hyperinflation and respiratory muscle interaction. Eur. Respir. J. 1997;10:934–941.
Decramer, M. Respiratory muscles in COPD: regulation of trophical status. Verh. K. Acad. Geneeskd. Belg. 2001;63:577–602. [discussion 602–574].
Deindl, F.M., Vodusek, D.B., Hesse, U., et al. Pelvic floor activity patterns: comparison of nulliparous continent and parous urinary stress incontinent women: A kinesiological EMG study. Br. J. Urol. 1994;73:413–417.
De Jonghe, B., Bastuji-Garin, S., Durand, M.C., et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit. Care Med. 2007;35:2007–2015.
Delgado, H.R., Braun, S.R., Skatrud, J.B., et al. Chest wall and abdominal motion during exercise in patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1982;126:200–205.
DeLorey, D.S., Babb, T.G. Progressive mechanical ventilatory constraints with aging. Am. J. Respir. Crit. Care Med. 1999;160:169–177.
Demoule, A., Verin, E., Montcel, S.T., et al. Short-term training-dependent plasticity of the corticospinal diaphragm control in normal humans. Respir. Physiol. Neurobiol. 2008;160:172–180.
Dempsey, J.A. Cardiorespiratory responses to exercise in CHF: a conspiracy of maladaptation. J. Physiol. 2010;588:2683.
Dempsey, J.A., Romer, L., Rodman, J., et al. Consequences of exercise-induced respiratory muscle work. Respir. Physiol. Neurobiol. 2006;151:242–250.
DePalo, V.A., Parker, A.L., Al-Bilbeisi, F., et al. Respiratory muscle strength training with nonrespiratory maneuvers. J. Appl. Physiol. 2004;96:731–734.
Deruelle, F., Nourry, C., Mucci, P., et al. Difference in breathing strategies during exercise between trained elderly men and women. Scand. J. Med. Sci. Sports. 2008;18:213–220.
De Troyer, A., Wilson, T.A. Effect of acute inflation on the mechanics of the inspiratory muscles. J. Appl. Physiol. 2009;107:315–323.
Dewberry, R.G., Schneider, B.F., Cale, W.F., et al. Sarcoid myopathy presenting with diaphragm weakness. Muscle Nerve. 1993;16:832–835.
Donath, J., Miller, A. Restrictive chest wall disorders. Semin. Respir. Crit. Care Med. 2009;30:275–292.
Dufresne, V., Knoop, C., Van Muylem, A., et al. Effect of systemic inflammation on inspiratory and limb muscle strength and bulk in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2009;180:153–158.
Duggan, M., Kavanagh, B.P. Perioperative modifications of respiratory function. Best Pract. Res. Clin. Anaesthesiol. 2010;24:145–155.
Dunnink, M.A., Doeleman, W.R., Trappenburg, J.C., et al. Respiratory muscle strength in stable adolescent and adult patients with cystic fibrosis. J. Cyst. Fibros. 2009;8:31–36.
Eckert, D.J., Lo, Y.L., Saboisky, J.P., et al. Sensori-motor function of the upper airway muscles and respiratory sensory processing in untreated obstructive sleep apnea. J. Appl. Physiol. 2011;111(6):1644–1653.
Eisner, M.D., Blanc, P.D., Yelin, E.H., et al. COPD as a systemic disease: impact on physical functional limitations. Am. J. Med. 2008;121:789–796.
Ekblom, B., Goldbarg, A.N. The influence of physical training and other factors on the subjective rating of perceived exertion. Acta Physiol. Scand. 1971;83:399–406.
England, R., Maddocks, M., Manderson, C., et al. Factors influencing exercise performance in thoracic cancer. Respir. Med. 2012;106:294–299.
Enright, P.L., Kronmal, R.A., Manolio, T.A., et al. Respiratory muscle strength in the elderly Correlates and reference values. Cardiovascular Health Study Research Group. Am. J. Respir. Crit. Care Med. 1994;149:430–438.
Enright, S., Chatham, K., Ionescu, A.A., et al. The influence of body composition on respiratory muscle, lung function and diaphragm thickness in adults with cystic fibrosis. J. Cyst. Fibros. 2007;6:384–390.
Epstein, C.D., El-Mokadem, N., Peerless, J.R. Weaning older patients from long-term mechanical ventilation: a pilot study. Am. J. Crit. Care. 2002;11:369–377.
Estenne, M., De Troyer, A. Relationship between respiratory muscle electromyogram and rib cage motion in tetraplegia. Am. Rev. Respir. Dis. 1985;132:53–59.
Faggiano, P., D’Aloia, A., Gualeni, A., et al. Relative contribution of resting haemodynamic profile and lung function to exercise tolerance in male patients with chronic heart failure. Heart. 2001;85:179–184.
Feathers, L.S., Wilcock, A., Manderson, C., et al. Measuring inspiratory muscle weakness in patients with cancer and breathlessness. J. Pain Symptom. Manage. 2003;25:305–306.
Ferretti, A., Giampiccolo, P., Cavalli, A., et al. Expiratory flow limitation and orthopnea in massively obese subjects. Chest. 2001;119:1401–1408.
Filusch, A., Ewert, R., Altesellmeier, M., et al. Respiratory muscle dysfunction in congestive heart failure – the role of pulmonary hypertension. Int. J. Cardiol. 2011;150:182–185.
Flaherty, K.R., Wald, J., Weisman, I.M., et al. Unexplained exertional limitation: characterization of patients with a mitochondrial myopathy. Am. J. Respir. Crit. Care Med. 2001;164:425–432.
Ford, E.S. The epidemiology of obesity and asthma. J. Allergy Clin. Immunol. 2005;115:897–909. [quiz 910].
Franco, V. Cardiopulmonary exercise test in chronic heart failure: beyond peak oxygen consumption. Curr. Heart Fail. Rep. 2011;8:45–50.
Frankenstein, L., Meyer, F.J., Sigg, C., et al. Is serial determination of inspiratory muscle strength a useful prognostic marker in chronic heart failure? Eur. J. Cardiovasc. Prev. Rehabil. 2008;15:156–161.
Frankenstein, L., Nelles, M., Meyer, F.J., et al. Validity, prognostic value and optimal cutoff of respiratory muscle strength in patients with chronic heart failure changes with beta-blocker treatment. Eur. J. Cardiovasc. Prev. Rehabil. 2009;16(4):424–429.
Franssen, F.M., O’Donnell, D.E., Goossens, G.H., et al. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63:1110–1117.
Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001;81:1725–1789.
Gandevia, S.C., McCloskey, D.I. Interpretation of perceived motor commands by reference to afferent signals. J. Physiol. 1978;283:493–499.
Gardini Gardenghi, G., Boni, E., Todisco, P., et al. Respiratory function in patients with stable anorexia nervosa. Chest. 2009;136:1356–1363.
Gibson, G.J., Pride, N.B., Davis, J.N., et al. Pulmonary mechanics in patients with respiratory muscle weakness. Am. Rev. Respir. Dis. 1977;115:389–395.
Glaser, R.M., Sawka, M.N., Brune, M.F., et al. Physiological responses to maximal effort wheelchair and arm crank ergometry. J. Appl. Physiol. 1980;48:1060–1064.
Goldman, M.D. Lung dysfunction in diabetes. Diabetes Care. 2003;26:1915–1918.
Gomez-Fernandez, P., Sanchez Agudo, L., Calatrava, J.M., et al. Respiratory muscle weakness in uremic patients under continuous ambulatory peritoneal dialysis. Nephron. 1984;36:219–223.
Gonzalez-Moro, J.M., De Miguel-Diez, J., Paz-Gonzalez, L., et al. Abnormalities of the respiratory function and control of ventilation in patients with anorexia nervosa. Respiration. 2003;70:490–495.
Gosselink, R., Troosters, T., Decramer, M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am. J. Respir. Crit. Care Med. 1996;153:976–980.
Gosselink, R., Troosters, T., Decramer, M. Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 2000;20:353–360.
Griffiths, L.A., McConnell, A.K. The influence of inspiratory and expiratory muscle training upon rowing performance. Eur. J. Appl. Physiol. 2007;99:457–466.
Guleria, S., Agarwal, R.K., Guleria, R., et al. The effect of renal transplantation on pulmonary function and respiratory muscle strength in patients with end-stage renal disease. Transplant. Proc. 2005;37:664–665.
Hagins, M., Lamberg, E.M. Natural breath control during lifting tasks: effect of load. Eur. J. Appl. Physiol. 2006;96:453–458.
Hamaoui, A., Do, M., Poupard, L., et al. Does respiration perturb body balance more in chronic low back pain subjects than in healthy subjects? Clin. Biomech. (Bristol, Avon). 2002;17:548–550.
Hamid Q., Shannon J., Martin J., eds. Physiologic basis of respiratory disease. Hamilton: BC Decker, 2005.
Hamilton, A.L., Killian, K.J., Summers, E., et al. Quantification of intensity of sensations during muscular work by normal subjects. J. Appl. Physiol. 1996;81:1156–1161.
Hamilton, A.L., Killian, K.J., Summers, E., et al. Symptom intensity and subjective limitation to exercise in patients with cardiorespiratory disorders. Chest. 1996;110:1255–1263.
Hansen, J.E., Wasserman, K. Pathophysiology of activity limitation in patients with interstitial lung disease. Chest. 1996;109:1566–1576.
Harms, C.A., Dempsey, J.A. Cardiovascular consequences of exercise hyperpnea. Exerc. Sport Sci. Rev. 1999;27:37–62.
Harms, C.A., Babcock, M.A., McClaran, S.R., et al. Respiratory muscle work compromises leg blood flow during maximal exercise. J. Appl. Physiol. 1997;82:1573–1583.
Hart, N., Hawkins, P., Hamnegard, C.H., et al. A novel clinical test of respiratory muscle endurance. Eur. Respir. J. 2002;19:232–239.
Hart, N., Laffont, I., de la Sota, A.P., et al. Respiratory effects of combined truncal and abdominal support in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 2005;86:1447–1451.
Heimer, D., Brami, J., Lieberman, D., et al. Respiratory muscle performance in patients with type 1 diabetes. Diabet. Med. 1990;7:434–437.
Hermans, G., Agten, A., Testelmans, D., et al. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit. Care. 2010;14:R127.
Hill, A.R. Respiratory muscle function in asthma. J. Assoc. Acad. Minor. Phys. 1991;2:100–108.
Hill, N.S., Jacoby, C., Farber, H.W. Effect of an endurance triathlon on pulmonary function. Med. Sci. Sports Exerc. 1991;23:1260–1264.
Hiraizumi, Y., Fujimaki, E., Hishida, T., et al. Regional lung perfusion and ventilation with radioisotopes in cervical cord-injured patients. Clin. Nucl. Med. 1986;11:352–357.
Hodges, P.W., Gandevia, S.C. Changes in intra-abdominal pressure during postural and respiratory activation of the human diaphragm. J. Appl. Physiol. 2000;89:967–976.
Hodges, P.W., Butler, J.E., McKenzie, D.K., et al. Contraction of the human diaphragm during rapid postural adjustments. J. Physiol. 1997;505(pt 2):539–548.
Hodges, P.W., Gandevia, S.C., Richardson, C.A. Contractions of specific abdominal muscles in postural tasks are affected by respiratory maneuvers. J. Appl. Physiol. 1997;83:753–760.
Hodges, P.W., McKenzie, D.K., Heijnen, I., et al. Reduced contribution of the diaphragm to postural control in patients with severe chronic airflow limitation. Respirology. 2000;5(Suppl.):A8.
Hodges, P.W., Heijnen, I., Gandevia, S.C. Postural activity of the diaphragm is reduced in humans when respiratory demand increases. J. Physiol. 2001;537:999–1008.
Hodges, P.W., Eriksson, A.E., Shirley, D., et al. Intra-abdominal pressure increases stiffness of the lumbar spine. J. Biomech. 2005;38:1873–1880.
Holland, A.E. Exercise limitation in interstitial lung disease – mechanisms, significance and therapeutic options. Chron. Respir. Dis. 2010;7:101–111.
Hopkinson, N.S., Dayer, M.J., Moxham, J., et al. Abdominal muscle fatigue following exercise in chronic obstructive pulmonary disease. Respir. Res. 2010;11:15.
Hopman, M.T., van der Woude, L.H., Dallmeijer, A.J., et al. Respiratory muscle strength and endurance in individuals with tetraplegia. Spinal Cord. 1997;35:104–108.
How, S.C., McConnell, A.K., Taylor, B.J., et al. Acute and chronic responses of the upper airway to inspiratory loading in healthy awake humans: an MRI study. Respir. Physiol. Neurobiol. 2007;157:270–280.
Hudgel, D.W., Harasick, T. Fluctuation in timing of upper airway and chest wall inspiratory muscle activity in obstructive sleep apnea. J. Appl. Physiol. 1990;69:443–450.
Hughes, P.D., Polkey, M.I., Harrus, M.L., et al. Diaphragm strength in chronic heart failure. Am. J. Respir. Crit. Care Med. 1999;160:529–534.
Hughes, P.D., Hart, N., Hamnegard, C.H., et al. Inspiratory muscle relaxation rate slows during exhaustive treadmill walking in patients with chronic heart failure. Am. J. Respir. Crit. Care Med. 2001;163:1400–1403.
Innocenti, F., Fabbri, A., Anichini, R., et al. Indications of reduced pulmonary function in type 1 (insulin-dependent) diabetes mellitus. Diabetes Res. Clin. Pract. 1994;25:161–168.
Ionescu, A.A., Chatham, K., Davies, C.A., et al. Inspiratory muscle function and body composition in cystic fibrosis. Am. J. Respir. Crit. Care Med. 1998;158:1271–1276.
Irfan, M., Jabbar, A., Haque, A.S., et al. Pulmonary functions in patients with diabetes mellitus. Lung India. 2011;28:89–92.
Isono, S., Feroah, T.R., Hajduk, E.A., et al. Interaction of cross-sectional area, driving pressure, and airflow of passive velopharynx. J. Appl. Physiol. 1997;83:851–859.
Janaudis-Ferreira, T., Hill, K., Goldstein, R.S., et al. Resistance arm training in patients with COPD: a randomized controlled trial. Chest. 2011;139:151–158.
Janssens, J.P., Pache, J.C., Nicod, L.P. Physiological changes in respiratory function associated with ageing. Eur. Respir. J. 1999;13:197–205.
Janssens, L., Brumagne, S., Polspoel, K., et al. The effect of inspiratory muscles fatigue on postural control in people with and without recurrent low back pain. Spine. 2010;35:1088–1094.
Javaheri, S., Sicilian, L. Lung function, breathing pattern, and gas exchange in interstitial lung disease. Thorax. 1992;47:93–97.
Jensen, D., Webb, K.A., O’Donnell, D.E. Chemical and mechanical adaptations of the respiratory system at rest and during exercise in human pregnancy. Appl. Physiol. Nutr. Metab. 2007;32:1239–1250.
Jensen, D., Ofir, D., O’Donnell, D.E. Effects of pregnancy, obesity and aging on the intensity of perceived breathlessness during exercise in healthy humans. Respir. Physiol. Neurobiol. 2009;167:87–100.
Jensen, D., Webb, K.A., Davies, G.A., et al. Mechanisms of activity-related breathlessness in healthy human pregnancy. Eur. J. Appl. Physiol. 2009;106:253–265.
Johnson, B.D., Reddan, W.G., Seow, K.C., et al. Mechanical constraints on exercise hyperpnea in a fit aging population. Am. Rev. Respir. Dis. 1991;143:968–977.
Johnson, B.D., Babcock, M.A., Suman, O.E., et al. Exercise-induced diaphragmatic fatigue in healthy humans. J. Physiol. 1993;460:385–405.
Johnson, B.D., Beck, K.C., Olson, L.J., et al. Ventilatory constraints during exercise in patients with chronic heart failure. Chest. 2000;117:321–332.
Just, N., Bautin, N., Danel-Brunaud, V., et al. The Borg dyspnoea score: a relevant clinical marker of inspiratory muscle weakness in amyotrophic lateral sclerosis. Eur. Respir. J. 2010;35:353–360.
Kabitz, H.J., Schwoerer, A., Bremer, H.C., et al. Impairment of respiratory muscle function in pulmonary hypertension. Clin. Sci. (Lond.). 2008;114:165–171.
Kabitz, H.J., Sonntag, F., Walker, D., et al. Diabetic polyneuropathy is associated with respiratory muscle impairment in type 2 diabetes. Diabetologia. 2008;51:191–197.
Kaminski, D.M., Schaan, B.D., da Silva, A.M., et al. Inspiratory muscle weakness is associated with autonomic cardiovascular dysfunction in patients with type 2 diabetes mellitus. Clin. Auton. Res. 2011;21:29–35.
Kang, S.W. Pulmonary rehabilitation in patients with neuromuscular disease. Yonsei Med. J. 2006;47:307–314.
Karacan, O., Tutal, E., Colak, T., et al. Pulmonary function in renal transplant recipients and end-stage renal disease patients undergoing maintenance dialysis. Transplant. Proc. 2006;38:396–400.
Karakurt, Z., Fanfulla, F., Ceriana, P., et al. Physiologic determinants of prolonged mechanical ventilation in patients after major surgery. J. Crit. Care. 2011;27(2):221. [e9–221.e16].
Katayama, K., Iwamoto, E., Ishida, K., et al. Inspiratory muscle fatigue increases sympathetic vasomotor outflow and blood pressure during submaximal exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R1167–R1175.
Kawagoe, Y., Permutt, S., Fessler, H.E. Hyperinflation with intrinsic PEEP and respiratory muscle blood flow. J. Appl. Physiol. 1994;77:2440–2448.
Kenn, K., Hess, M.M. Vocal cord dysfunction: an important differential diagnosis of bronchial asthma. Deutsches Arzteblatt International. 2008;105:699–704.
Ker, J.A., Schultz, C.M. Respiratory muscle fatigue after an ultra-marathon measured as inspiratory task failure. Int. J. Sports Med. 1996;17:493–496.
Keyser, R.E., Rodgers, M.M., Gardner, E.R., et al. Oxygen uptake during peak graded exercise and single-stage fatigue tests of wheelchair propulsion in manual wheelchair users and the able-bodied. Arch. Phys. Med. Rehabil. 1999;80:1288–1292.
Kilicli, F., Dokmetas, S., Candan, F., et al. Inspiratory muscle strength is correlated with carnitine levels in type 2 diabetes. Endocr. Res. 2010;35:51–58.
Kobayashi, Y.M., Rader, E.P., Crawford, R.W., et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515.
Kolar, P., Neuwirth, J., Sanda, J., et al. Analysis of diaphragm movement during tidal breathing and during its activation while breath holding using MRI synchronized with spirometry. Physiol. Res. 2009;58:383–392.
Koulouris, N.G., Retsou, S., Kosmas, E., et al. Tidal expiratory flow limitation, dyspnoea and exercise capacity in patients with bilateral bronchiectasis. Eur. Respir. J. 2003;21:743–748.
Kovelis, D., Pitta, F., Probst, V.S., et al. Pulmonary function and respiratory muscle strength in chronic renal failure patients on hemodialysis. J. Bras. Pneumol. 2008;34:907–912.
Kress, J.P., Pohlman, A.S., Alverdy, J., et al. The impact of morbid obesity on oxygen cost of breathing (VO(2RESP) at rest. Am. J. Respir. Crit. Care Med. 1999;160:883–886.
Kufel, T.J., Pineda, L.A., Junega, R.G., et al. Diaphragmatic function after intense exercise in congestive heart failure patients. Eur. Respir. J. 2002;20:1399–1405.
Kyroussis, D., Polkey, M.I., Keilty, S.E., et al. Exhaustive exercise slows inspiratory muscle relaxation rate in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996;153:787–793.
Laghi, F., Tobin, M.J. Disorders of the respiratory muscles. Am. J. Respir. Crit. Care Med. 2003;168:10–48.
Laghi, F., Cattapan, S.E., Jubran, A., et al. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am. J. Respir. Crit. Care Med. 2003;167:120–127.
Lamberg, E.M., Hagins, M. The effects of low back pain on natural breath control during a lowering task. Eur. J. Appl. Physiol. 2012;112(10):3519–3524.
Landahl, S., Steen, B., Svanborg, A. Dyspnea in 70-year-old people. Acta Med. Scand. 1980;207:225–230.
Lassau-Wray, E.R., Ward, G.R. Varying physiological response to arm-crank exercise in specific spinal injuries. J. Physiol. Anthropol. Appl. Human Sci. 2000;19:5–12.
Latronico, N., Bolton, C.F. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10:931–941.
Lawlor, D.A., Patel, R., Ebrahim, S. Association between falls in elderly women and chronic diseases and drug use: cross sectional study. BMJ. 2003;327:712–717.
Leroy, S., Perez, T., Neviere, R., et al. Determinants of dyspnea and alveolar hypoventilation during exercise in cystic fibrosis: impact of inspiratory muscle endurance. J. Cyst. Fibros. 2011;10:159–165.
Levine, S., Kaiser, L., Leferovich, J., et al. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N. Engl. J. Med. 1997;337:1799–1806.
Levine, S., Nguyen, T., Kaiser, L.R., et al. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am. J. Respir. Crit. Care Med. 2003;168:706–713.
Levine, S., Nguyen, T., Taylor, N., et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 2008;358:1327–1335.
Lewis, N.P., Banning, A.P., Cooper, J.P., et al. Impaired matching of perfusion and ventilation in heart failure detected by 133xenon. Basic Res. Cardiol. 1996;91(Suppl. 1):45–49.
Li, J., Li, S., Feuers, R.J., et al. Influence of body fat distribution on oxygen uptake and pulmonary performance in morbidly obese females during exercise. Respirology. 2001;6:9–13.
Lindstedt, S.L., Reich, T.E., Keim, P., et al. Do muscles function as adaptable locomotor springs? J. Exp. Biol. 2002;205:2211–2216.
Lisboa, C., Moreno, R., Fava, M., et al. Inspiratory muscle function in patients with severe kyphoscoliosis. Am. Rev. Respir. Dis. 1985;132:48–52.
Loke, J., Mahler, D.A., Virgulto, J.A. Respiratory muscle fatigue after marathon running. J. Appl. Physiol. 1982;52:821–824.
Lomax, M.E., McConnell, A.K. Inspiratory muscle fatigue in swimmers after a single 200 m swim. J. Sports Sci. 2003;21:659–664.
Lougheed, M.D., Lam, M., Forkert, L., et al. Breathlessness during acute bronchoconstriction in asthma. Pathophysiologic mechanisms. Am. Rev. Respir. Dis. 1993;148:1452–1459.
Lougheed, D.M., Webb, K.A., O’Donnell, D.E. Breathlessness during induced lung hyperinflation in asthma: the role of the inspiratory threshold load. Am. J. Respir. Crit. Care Med. 1995;152:911–920.
Lykidis, C.K., White, M.J., Balanos, G.M. The pulmonary vascular response to the sustained activation of the muscle metaboreflex in man. Exp. Physiol. 2008;93:247–253.
Lynch, J.P., 3rd., Ma, Y.L., Koss, M.N., et al. Pulmonary sarcoidosis. Semin. Respir. Crit. Care Med. 2007;28:53–74.
Macklem, P.T. Muscular weakness and respiratory function. N. Engl. J. Med. 1986;314:775–776.
Mador, M.J., Acevedo, F.A. Effect of respiratory muscle fatigue on subsequent exercise performance. J. Appl. Physiol. 1991;70:2059–2065.
Mador, M.J., Magalang, U.J., Rodis, A., et al. Diaphragmatic fatigue after exercise in healthy human subjects. Am. Rev. Respir. Dis. 1993;148:1571–1575.
Mador, J.M., Kufel, T.J., Pineda, L.A. Quadriceps and diaphragmatic function after exhaustive cycle exercise in the healthy elderly. Am. J. Respir. Crit. Care Med. 2000;162:1760–1766.
Mador, M.J., Kufel, T.J., Pineda, L.A., et al. Diaphragmatic fatigue and high-intensity exercise in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000;161:118–123.
Mador, M.J., Bozkanat, E., Kufel, T.J. Quadriceps fatigue after cycle exercise in patients with COPD compared with healthy control subjects. Chest. 2003;123:1104–1111.
Mancini, D.M., Ferraro, N., Nazzaro, D., et al. Respiratory muscle deoxygenation during exercise in patients with heart failure demonstrated with near-infrared spectroscopy. J. Am. Coll. Cardiol. 1991;18:492–498.
Mancini, D., Donchez, L., Levine, S. Acute unloading of the work of breathing extends exercise duration in patients with heart failure. J. Am. Coll. Cardiol. 1997;29:590–596.
Mancini, M., Filippelli, M., Seghieri, G., et al. Respiratory muscle function and hypoxic ventilatory control in patients with type I diabetes. Chest. 1999;115:1553–1562.
Markovitz, G.H., Cooper, C.B. Rehabilitation in non-COPD: mechanisms of exercise limitation and pulmonary rehabilitation for patients with pulmonary fibrosis / restrictive lung disease. Chron. Respir. Dis. 2010;7:47–60.
Martin, J., Powell, E., Shore, S., et al. The role of respiratory muscles in the hyperinflation of bronchial asthma. Am. Rev. Respir. Dis. 1980;121:441–447.
Martin, J.G., Shore, S.A., Engel, L.A. Mechanical load and inspiratory muscle action during induced asthma. Am. Rev. Respir. Dis. 1983;128:455–460.
Martinez-Llorens, J., Ramirez, M., Colomina, M.J., et al. Muscle dysfunction and exercise limitation in adolescent idiopathic scoliosis. Eur. Respir. J. 2010;36:393–400.
Martinez-Moragon, E., Perpina, M., Belloch, A., et al. Determinants of dyspnea in patients with different grades of stable asthma. J. Asthma. 2003;40:375–382.
Maskey-Warzechowska, M., Przybylowski, T., Hildebrand, K., et al. Maximal respiratory pressures and exercise tolerance in patients with COPD. Pneumonol. Alergol. Pol. 2006;74:72–76.
Mateus, S.R., Beraldo, P.S., Horan, T.A. Maximal static mouth respiratory pressure in spinal cord injured patients: correlation with motor level. Spinal Cord. 2007;45:569–575.
McCloskey, D.I., Gandevia, S., Potter, E.K., et al. Muscle sense and effort: motor commands and judgments about muscular contractions. Adv. Neurol. 1983;39:151–167.
McConnell, A.K., Copestake, A.J. Maximum static respiratory pressures in healthy elderly men and women: issues of reproducibility and interpretation. Respiration. 1999;66:251–258.
McConnell, A.K., Davies, C.T. A comparison of the ventilatory responses to exercise of elderly and younger humans. J. Gerontol. 1992;47:B137–B141.
McConnell, A.K., Lomax, M. The influence of inspiratory muscle work history and specific inspiratory muscle training upon human limb muscle fatigue. J. Physiol. 2006;577:445–457.
McCool, F.D., Tzelepis, G.E. Inspiratory muscle training in the patient with neuromuscular disease. Phys. Ther. 1995;75:1006–1014.
McCool, F.D., Conomos, P., Benditt, J.O., et al. Maximal inspiratory pressures and dimensions of the diaphragm. Am. J. Respir. Crit. Care Med. 1997;155:1329–1334.
McKenzie, D.K., Butler, J.E., Gandevia, S.C. Respiratory muscle function and activation in chronic obstructive pulmonary disease. J. Appl. Physiol. 2009;107:621–629.
Mehta, S. Neuromuscular disease causing acute respiratory failure. Respir. Care. 2006;51:1016–1021. [discussion 1021–1013].
Meyer, F.J., Zugck, C., Haass, M., et al. Inefficient ventilation and reduced respiratory muscle capacity in congestive heart failure. Basic Res. Cardiol. 2000;95:333–342.
Meyer, F.J., Lossnitzer, D., Kristen, A.V., et al. Respiratory muscle dysfunction in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2005;25:125–130.
Miki, K., Maekura, R., Hiraga, T., et al. Impairments and prognostic factors for survival in patients with idiopathic pulmonary fibrosis. Respir. Med. 2003;97:482–490.
Milne, J.A., Howie, A.D., Pack, A.I. Dyspnoea during normal pregnancy. Br. J. Obstet. Gynaecol. 1978;85:260–263.
Moodie, L., Reeve, J., Elkins, M. Inspiratory muscle training increases inspiratory muscle strength in patients weaning from mechanical ventilation: a systematic review. Journal of Physiotherapy. 2011;57:213–221.
Moran, F., Piper, A., Elborn, J.S., et al. Respiratory muscle pressures in non-CF bronchiectasis: repeatability and reliability. Chron. Respir. Dis. 2010;7:165–171.
Moxham, J., Jolley, C. Breathlessness, fatigue and the respiratory muscles. Clin. Med. 2009;9:448–452.
Muller, N., Bryan, A.C., Zamel, N. Tonic inspiratory muscle activity as a cause of hyperinflation in histamine-induced asthma. J. Appl. Physiol. 1980;49:869–874.
Muller, N., Bryan, A.C., Zamel, N. Tonic inspiratory muscle activity as a cause of hyperinflation in asthma. J. Appl. Physiol. 1981;50:279–282.
Murciano, D., Rigaud, D., Pingleton, S., et al. Diaphragmatic function in severely malnourished patients with anorexia nervosa Effects of renutrition. Am. J. Respir. Crit. Care Med. 1994;150:1569–1574.
Naimark, A., Cherniack, R.M. Compliance of the respiratory system and its components in health and obesity. J. Appl. Physiol. 1960;15:377–382.
Nanas, S., Nanas, J., Papazachou, O., et al. Resting lung function and hemodynamic parameters as predictors of exercise capacity in patients with chronic heart failure. Chest. 2003;123:1386–1393.
Nava, S., Zanotti, E., Ambrosino, N., et al. Evidence of acute diaphragmatic fatigue in a ‘natural’ condition. The diaphragm during labor. Am. Rev. Respir. Dis. 1992;146(5 pt 1):1226–1230.
Neves, P.C., Guerra, M., Ponce, P., et al. Non-cystic fibrosis bronchiectasis. Interactive Cardiovascular and Thoracic Surgery. 2011;13(6):619–625.
Newall, C., Stockley, R.A., Hill, S.L. Exercise training and inspiratory muscle training in patients with bronchiectasis. Thorax. 2005;60:943–948.
Nici, L. The major limitation to exercise performance in COPD is inadequate energy supply to the respiratory and locomotor muscles vs. lower limb muscle dysfunction vs. dynamic hyperinflation. Difficulties in determining the primary physiological abnormality that limits exercise performance in COPD. J. Appl. Physiol. 2008;105:760–761.
Nishimura, Y., Maeda, H., Tanaka, K., et al. Respiratory muscle strength and hemodynamics in chronic heart failure. Chest. 1994;105:355–359.
O’Donnell, D.E. Ventilatory limitations in chronic obstructive pulmonary disease. Med. Sci. Sports Exerc. 2001;33:S647–S655.
O’Donnell, D.E., Laveneziana, P. Dyspnea and activity limitation in COPD: mechanical factors. Chronic Obstructive Pulmonary Disease. 2007;4:225–236.
O’Donnell, D., Webb, K. Last word on Point:Counterpoint: The major limitation to exercise performance in COPD is 1) inadequate energy supply to the respiratory and locomotor muscles, 2) lower limb muscle dysfunction, 3) dynamic hyperinflation. J. Appl. Physiol. 2008;105:765.
O’Donnell, D.E., Webb, K.A. The major limitation to exercise performance in COPD is dynamic hyperinflation. J. Appl. Physiol. 2008;105:753–755. [discussion 755–757].
O’Donnell, D.E., D’Arsigny, C., Raj, S., et al. Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am. J. Respir. Crit. Care Med. 1999;160:1804–1811.
O’Donnell, D.E., Banzett, R.B., Carrieri-Kohlman, V., et al. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc. Am. Thorac. Soc. 2007;4:145–168.
O’Donnell, D.E., Guenette, J.A., Maltais, F., et al. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest. 2012;141:753–762.
Ofir, D., Laveneziana, P., Webb, K.A., et al. Sex differences in the perceived intensity of breathlessness during exercise with advancing age. J. Appl. Physiol. 2008;104:1583–1593.
Olson, L.G., Fouke, J.M., Hoekje, P.L., et al. A biomechanical view of upper airway function. In: Mathew O.P., Sant’Ambrogio G., eds. Respiratory function of the upper airway. New York: Marcel Dekker; 1988:359–389.
Olson, T.P., Joyner, M.J., Dietz, N.M., et al. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J. Physiol. 2010;588:2487–2501.
Ottenheijm, C.A., Heunks, L.M., Sieck, G.C., et al. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005;172:200–205.
Ozdem Yr, O., Inanici, F., Hascelik, Z. Reduced vital capacity leads to exercise intolerance in patients with ankylosing spondylitis. European Journal of Physical and Rehabilitation Medicine. 2011;47:391–397.
Palange, P., Forte, S., Onorati, P., et al. Ventilatory and metabolic adaptations to walking and cycling in patients with COPD. J. Appl. Physiol. 2000;88:1715–1720.
Patel, I.S., Vlahos, I., Wilkinson, T.M., et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004;170:400–407.
Pelosi, P., Croci, M., Ravagnan, I., et al. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109:144–151.
Perez, T., Becquart, L.A., Stach, B., et al. Inspiratory muscle strength and endurance in steroid-dependent asthma. Am. J. Respir. Crit. Care Med. 1996;153:610–615.
Perkoff, G.T., Silber, R., Tyler, F.H., et al. Studies in disorders of muscle. XII. Myopathy due to the administration of therapeutic amounts of 17-hydroxycorticosteroids. Am. J. Med. 1959;26:891–898.
Petrof, B.J., Jaber, S., Matecki, S. Ventilator-induced diaphragmatic dysfunction. Curr. Opin. Crit. Care. 2010;16:19–25.
Piepoli, M., Clark, A.L., Volterrani, M., et al. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93:940–952.
Pina, I.L., Apstein, C.S., Balady, G.J., et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–1225.
Polkey, M.I., Kyroussis, D., Keilty, S.E., et al. Exhaustive treadmill exercise does not reduce twitch transdiaphragmatic pressure in patients with COPD. Am. J. Respir. Crit. Care Med. 1995;152:959–964.
Polkey, M.I., Kyroussis, D., Hamnegard, C.H., et al. Diaphragm strength in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996;154:1310–1317.
Polkey, M.I., Moxham, J., Green, M. The case against inspiratory muscle training in COPD. Eur. Respir. J. 2011;37:236–237.
Prezant, D.J. Effect of uremia and its treatment on pulmonary function. Lung. 1990;168:1–14.
Pride, N.B., Macklem, P.T. Lung mechanics in disease. In: Fishman A.P., ed. Handbook of physiology. Bethesda, MD: American Physiological Society; 1986:659–692.
Pringle, C.E., Dewar, C.L. Respiratory muscle involvement in severe sarcoid myositis. Muscle Nerve. 1997;20:379–381.
Ragette, R., Mellies, U., Schwake, C., et al. Patterns and predictors of sleep disordered breathing in primary myopathies. Thorax. 2002;57:724–728.
Rallidis, L.S., Fountoulaki, K., Anastasiou-Nana, M. Managing the underestimated risk of statin-associated myopathy. Int. J. Cardiol. 2011;159(3):169–176.
Ribeiro, J.P., Chiappa, G.R., Neder, J.A., et al. Respiratory muscle function and exercise intolerance in heart failure. Curr. Heart Fail. Rep. 2009;6:95–101.
Ribeiro, J.P., Chiappa, G.R., Callegaro, C.C. The contribution of inspiratory muscles function to exercise limitation in heart failure: pathophysiological mechanisms. Revista Brasileira de Fisioterapia. 2012;16(4):261–267.
Rochester, D.F. Respiratory muscles and ventilatory failure: 1993 perspective. Am. J. Med. Sci. 1993;305:394–402.
Rogers, M.A., Evans, W.J. Changes in skeletal muscle with aging: effects of exercise training. Exerc. Sport Sci. Rev. 1993;21:65–102.
Roig, M., Eng, J.J., Road, J.D., et al. Falls in patients with chronic obstructive pulmonary disease: a call for further research. Respir. Med. 2009;103:1257–1269.
Roig, M., Eng, J.J., MacIntyre, D.L., et al. Falls in people with chronic obstructive pulmonary disease: an observational cohort study. Respir. Med. 2011;105:461–469.
Romer, L.M., Polkey, M.I. Exercise-induced respiratory muscle fatigue: implications for performance. J. Appl. Physiol. 2008;104:879–888.
Romer, L.M., McConnell, A.K., Jones, D.A. Effects of inspiratory muscle training on time-trial performance in trained cyclists. J. Sports Sci. 2002;20:547–562.
Romer, L.M., McConnell, A.K., Jones, D.A. Inspiratory muscle fatigue in trained cyclists: effects of inspiratory muscle training. Med. Sci. Sports Exerc. 2002;34:785–792.
Romer, L.M., Lovering, A.T., Haverkamp, H.C., et al. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J. Physiol. 2006;571:425–439.
Ross, E., Middleton, N., Shave, R., et al. Changes in respiratory muscle and lung function following marathon running in man. J. Sports Sci. 2008;26:1295–1301.
Rubinstein, I., Zamel, N., DuBarry, L., et al. Airflow limitation in morbidly obese, nonsmoking men. Ann. Intern. Med. 1990;112:828–832.
Sahebjami, H., Domino, M. Effects of repeated cycles of starvation and refeeding on lungs of growing rats. J. Appl. Physiol. 1992;73:2349–2354.
Sahebjami, H., MacGee, J. Effects of starvation on lung mechanics and biochemistry in young and old rats. J. Appl. Physiol. 1985;58:778–784.
Saler, T., Cakmak, G., Saglam, Z.A., et al. The assessment of pulmonary diffusing capacity in diabetes mellitus with regard to microalbuminuria. Intern. Med. 2009;48:1939–1943.
Salome, C.M., King, G.G., Berend, N. Physiology of obesity and effects on lung function. J. Appl. Physiol. 2010;108:206–211.
Satta, A. Exercise training in asthma. J. Sports Med. Phys. Fitness. 2000;40:277–283.
Scanlon, P.D., Loring, S.H., Pichurko, B.M., et al. Respiratory mechanics in acute quadriplegia. Lung and chest wall compliance and dimensional changes during respiratory maneuvers. Am. Rev. Respir. Dis. 1989;139:615–620.
Scano, G., Seghieri, G., Mancini, M. Dyspnoea, peripheral airway involvement and respiratory muscle effort in patients with type I diabetes mellitus under good metabolic control. Clin. Sci. (Lond.). 1999;96:499–506.
Scano, G., Grazzini, M., Stendardi, L., et al. Respiratory muscle energetics during exercise in healthy subjects and patients with COPD. Respir. Med. 2006;100:1896–1906.
Scano, G., Innocenti-Bruni, G., Stendardi, L. Do obstructive and restrictive lung diseases share common underlying mechanisms of breathlessness? Respir. Med. 2010;104:925–933.
Schilero, G.J., Grimm, D.R., Bauman, W.A. Assessment of airway caliber and bronchodilator responsiveness in subjects with spinal cord injury. Chest. 2005;127:149–155.
Schilero, G.J., Spungen, A.M., Bauman, W.A., et al. Pulmonary function and spinal cord injury. Respir. Physiol. Neurobiol. 2009;166:129–141.
Schwartz, A.R., Patil, S.P., Squier, S., et al. Obesity and upper airway control during sleep. J. Appl. Physiol. 2010;108:430–435.
Seals, D.R. Robin Hood for the lungs? A respiratory metaboreflex that ‘steals’ blood flow from locomotor muscles. J. Physiol. 2001;537:2.
Series, F. Upper airway muscles awake and asleep. Sleep. Med. Rev. 2002;6:229–242.
Sharma, R.R., Axelsson, H., Oberg, A., et al. Diaphragmatic activity after laparoscopic cholecystectomy. Anesthesiology. 1999;91:406–413.
Sharpe, G.R., Hamer, M., Caine, M.P., et al. Respiratory muscle fatigue during and following a sprint triathlon in humans. J. Physiol. 1996:165P.
Sheel, A.W., Derchak, P.A., Morgan, B.J., et al. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J. Physiol. 2001;537:277–289.
Sheel, A.W., Derchak, P.A., Pegelow, D.F., et al. Threshold effects of respiratory muscle work on limb vascular resistance. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1732–H1738.
Siafakas, N.M., Mitrouska, I., Bouros, D., et al. Surgery and the respiratory muscles. Thorax. 1999;54:458–465.
Silver, J.R. The oxygen cost of breathing in tetraplegic patients. Paraplegia. 1963;1:204–214.
Similowski, T., Yan, S., Gauthier, A.P., et al. Contractile properties of the human diaphragm during chronic hyperinflation. N. Engl. J. Med. 1991;325:917–923.
Sinderby, C., Spahija, J., Beck, J., et al. Diaphragm activation during exercise in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001;163:1637–1641.
Smith, M.D., Russell, A., Hodges, P.W. Disorders of breathing and continence have a stronger association with back pain than obesity and physical activity. Aust. J. Physiother. 2006;52:11–16.
Smith, M.D., Chang, A.T., Seale, H.E., et al. Balance is impaired in people with chronic obstructive pulmonary disease. Gait Posture. 2010;31:456–460.
Somfay, A., Porszasz, J., Lee, S.M., et al. Effect of hyperoxia on gas exchange and lactate kinetics following exercise onset in nonhypoxemic COPD patients. Chest. 2002;121:393–400.
Sood, A. Does obesity weigh heavily on the health of the human airway? J. Allergy Clin. Immunol. 2005;115:921–924.
Sood, A. Obesity, adipokines, and lung disease. J. Appl. Physiol. 2010;108:744–753.
Spruit, M.A., Thomeer, M.J., Gosselink, R., et al. Skeletal muscle weakness in patients with sarcoidosis and its relationship with exercise intolerance and reduced health status. Thorax. 2005;60:32–38.
Spungen, A.M., Grimm, D.R., Lesser, M., et al. Self-reported prevalence of pulmonary symptoms in subjects with spinal cord injury. Spinal Cord. 1997;35:652–657.
Spyropoulou, D., Leotsinidis, M., Tsiamita, M., et al. Pulmonary function testing in women with breast cancer treated with radiotherapy and chemotherapy. In Vivo. 2009;23:867–871.
St Croix, C.M., Morgan, B.J., Wetter, T.J., et al. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J. Physiol. 2000;529(pt 2):493–504.
Steier, J., Jolley, C.J., Seymour, J., et al. Increased load on the respiratory muscles in obstructive sleep apnea. Respir. Physiol. Neurobiol. 2010;171:54–60.
Stell, I.M., Polkey, M.I., Rees, P.J., et al. Inspiratory muscle strength in acute asthma. Chest. 2001;120:757–764.
Sun, Z., Liu, L., Liu, N., Liu, Y. Muscular response and adaptation to diabetes mellitus. Front. Biosci. 2008;13:4765–4794.
Tanios, M.A., El Gamal, H., Epstein, S.K., et al. Severe respiratory muscle weakness related to long-term colchicine therapy. Respir. Care. 2004;49:189–191.
Tantucci, C., Bottini, P., Dottorini, M.L., et al. Ventilatory response to exercise in diabetic subjects with autonomic neuropathy. J. Appl. Physiol. 1996;81:1978–1986.
Taylor, B.J., Johnson, B.D. The pulmonary circulation and exercise responses in the elderly. Semin. Respir. Crit. Care Med. 2010;31:528–538.
Taylor, B.J., Romer, L.M. Effect of expiratory muscle fatigue on exercise tolerance and locomotor muscle fatigue in healthy humans. J. Appl. Physiol. 2008;104:1442–1451.
Taylor, B.J., Romer, L.M. Effect of expiratory resistive loading on inspiratory and expiratory muscle fatigue. Respir. Physiol. Neurobiol. 2009;166:164–174.
Taylor, B.J., How, S.C., Romer, L.M. Exercise-induced abdominal muscle fatigue in healthy humans. J. Appl. Physiol. 2006;100(5):1554–1562.
Taylor, B.J., West, C.R., Romer, L.M. No effect of arm-crank exercise on diaphragmatic fatigue or ventilatory constraint in Paralympic athletes with cervical spinal cord injury. J. Appl. Physiol. 2010;109:358–366.
Taylor-Cousar, J.L. Hypoventilation in cystic fibrosis. Semin. Respir. Crit. Care Med. 2009;30:293–302.
Terakado, S., Takeuchi, T., Miura, T., et al. Early occurrence of respiratory muscle deoxygenation assessed by near-infrared spectroscopy during leg exercise in patients with chronic heart failure. Jpn. Circ. J. 1999;63:97–103.
Terzano, C., Ceccarelli, D., Conti, V., et al. Maximal respiratory static pressures in patients with different stages of COPD severity. Respir. Res. 2008;9:8.
Travers, J., Dudgeon, D.J., Amjadi, K., et al. Mechanisms of exertional dyspnea in patients with cancer. J. Appl. Physiol. 2008;104:57–66.
Tsatsouline, P. Power to the people!: Russian strength training secrets for every American. St Paul, MN: Dragon Door Publications; 2000.
van der Esch, M., van ‘t Hul, A.J., Heijmans, M., et al. Respiratory muscle performance as a possible determinant of exercise capacity in patients with ankylosing spondylitis. Aust. J. Physiother. 2004;50:41–45.
van der Palen, J., Rea, T.D., Manolio, T.A., et al. Respiratory muscle strength and the risk of incident cardiovascular events. Thorax. 2004;59:1063–1067.
van Lunteren, E., Moyer, M. Streptozotocin-diabetes alters action potentials in rat diaphragm. Respir. Physiol. Neurobiol. 2003;135:9–16.
Vassilakopoulos, T., Zakynthinos, S., Roussos, C. The tension-time index and the frequency / tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am. J. Respir. Crit. Care Med. 1998;158:378–385.
Verges, S., Schulz, C., Perret, C., Spengler, C.M. Impaired abdominal muscle contractility after high-intensity exhaustive exercise assessed by magnetic stimulation. Muscle Nerve. 2006;34:423–430.
Vibarel, N., Hayot, M., Pellenc, P.M., et al. Non-invasive assessment of inspiratory muscle performance during exercise in patients with chronic heart failure. Eur. Heart J. 1998;19:766–773.
Vogiatzis, I., Habazettl, H., Aliverti, A., et al. Effect of helium breathing on intercostal and quadriceps muscle blood flow during exercise in COPD patients. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R1549–R1559.
Volianitis, S., McConnell, A.K., Koutedakis, Y., et al. Inspiratory muscle training improves rowing performance. Med. Sci. Sports Exerc. 2001;33:803–809.
Wang, H.Y., Chen, C.C., Hsiao, S.F. Relationships between respiratory muscle strength and daily living function in children with cerebral palsy. Res. Dev. Disabil. 2012;33:1176–1182.
Wanke, T., Formanek, D., Auinger, M., et al. Inspiratory muscle performance and pulmonary function changes in insulin-dependent diabetes mellitus. Am. Rev. Respir. Dis. 1991;143:97–100.
Wanke, T., Formanek, D., Auinger, M., et al. Mechanical load on the inspiratory muscles during exercise hyperpnea in patients with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:425–428.
Watson, A.C., Hughes, P.D., Louise Harris, M., et al. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit. Care Med. 2001;29:1325–1331.
Weiner, P., Suo, J., Fernandez, E., et al. The effect of hyperinflation on respiratory muscle strength and efficiency in healthy subjects and patients with asthma. Am. Rev. Respir. Dis. 1990;141:1501–1505.
Weiner, P., Azgad, Y., Weiner, M. The effect of corticosteroids on inspiratory muscle performance in humans. Chest. 1993;104:1788–1791.
Weiner, P., Azgad, Y., Weiner, M. Inspiratory muscle training during treatment with corticosteroids in humans. Chest. 1995;107:1041–1044.
Weiner, P., Waizman, J., Weiner, M., et al. Influence of excessive weight loss after gastroplasty for morbid obesity on respiratory muscle performance. Thorax. 1998;53:39–42.
Weiner, P., Magadle, R., Beckerman, M., et al. The relationship among inspiratory muscle strength, the perception of dyspnea and inhaled beta2-agonist use in patients with asthma. Can. Respir. J. 2002;9:307–312.
Welsh, L., Roberts, R.G., Kemp, J.G. Fitness and physical activity in children with asthma. Sports Med. 2004;34:861–870.
Wetter, T.J., Harms, C.A., Nelson, W.B., et al. Influence of respiratory muscle work on VO(2) and leg blood flow during submaximal exercise. J. Appl. Physiol. 1999;87:643–651.
Widimsky, J., Riedel, M., Stanek, V. Central haemodynamics during exercise in patients with restrictive pulmonary disease. Bull. Eur. Physiopathol. Respir. 1977;13:369–379.
Wilbur, K., Makowsky, M. Colchicine myotoxicity: case reports and literature review. Pharmacotherapy. 2004;24:1784–1792.
Wilkerson, L.A. Exercise-induced asthma. J. Am. Osteopath. Assoc. 1998;98:211–215.
Wilson, J.R., Mancini, D.M. Factors contributing to the exercise limitation of heart failure. J. Am. Coll. Cardiol. 1993;22:93A–98A.
Wirnsberger, R.M., Drent, M., Hekelaar, N., et al. Relationship between respiratory muscle function and quality of life in sarcoidosis. Eur. Respir. J. 1997;10:1450–1455.
Wright, R.S., Levine, M.S., Bellamy, P.E., et al. Ventilatory and diffusion abnormalities in potential heart transplant recipients. Chest. 1990;98:816–820.
Yan, S., Kaminski, D., Sliwinski, P. Inspiratory muscle mechanics of patients with chronic obstructive pulmonary disease during incremental exercise. Am. J. Respir. Crit. Care Med. 1997;156:807–813.
Yap, J.C., Watson, R.A., Gilbey, S., et al. Effects of posture on respiratory mechanics in obesity. J. Appl. Physiol. 1995;79:1199–1205.
Zerah, F., Harf, A., Perlemuter, L., et al. Effects of obesity on respiratory resistance. Chest. 1993;103:1470–1476.
Ziora, K., Ziora, D., Oswiecimska, J., et al. Spirometric parameters in malnourished girls with anorexia nervosa. J. Physiol. Pharmacol. 2008;59(Suppl. 6):801–807.




