Chapter 6
Implementing respiratory muscle training
GENERAL PRINCIPLES OF FOUNDATION IMT
In Chapters 4 and 5, the functional benefits and practical methods of respiratory muscle training (RMT) were reviewed. From the evidence presented there, it was apparent that the most widely used and validated type of RMT is pressure threshold inspiratory muscle training (IMT). Accordingly, the guidance provided in this chapter is for the application of this type of training.
The diversity of potential applications for IMT makes it impossible to provide specific guidance for all potential applications. Accordingly, this chapter provides guidance that is either generic or applies to the most popular and best-supported application of pressure threshold inspiratory muscle training (IMT), viz., the improvement of inspiratory muscle function for the purposes of alleviating dyspnoea and / or improving exercise tolerance. However, in the context of indications for IMT, readers are encouraged to consider the application of the techniques described in this chapter to alternative settings, e.g., weaning from mechanical ventilation. In this respect, a number of non-exercise-related indications are also provided (see also Chs 3 and 4 and Box 6.1).
Context of RMT in the treatment pathway
The evidence presented in Chapters 3 and 4 identified a wide range of patients in whom: (1) disease can create an imbalance in the demand / capacity relationship of the respiratory pump, and / or (2) IMT has been shown to improve one, or multiple, clinically meaningful outcomes. The diversity of patients, and the healthcare systems that care for them, makes prescriptive guidance on the place of IMT in the care pathway inappropriate. However, the vast majority of patients who could benefit from IMT are living with long-term chronic conditions such as respiratory disease, heart failure, obesity and neuromuscular disease. For these patients, the point at which IMT is delivered will be determined by local policies for the management of chronic disease. At the time of writing, the United Kingdom’s National Health Service (NHS) is undergoing a major restructuring that is shifting the delivery of services to locations that are closer to its service users (patients). This inevitably means that services hitherto delivered in specialist units within secondary care will in future be delivered by ‘up-skilled’ primary care providers, or by local subcontractors to primary care providers. Simultaneously, there is likely to be an increase in the diversity of service providers for services such as rehabilitation, which have typically (but not exclusively) been provided in secondary care by NHS employees. These changes within the NHS are likely to bring its structure and delivery framework closer to that of the private healthcare systems that exist in other countries. The implications of such changes are that IMT could be provided in the patient’s home as part of, for example, a primary care ‘breathing clinic’ service delivered by a nurse specialist, or by a specialist rehabilitation service within secondary care – either of which might be delivered by private sector or NHS providers. In practical terms, these changes should make very little difference to the patients’ experience, provided that the appropriate expertise exists to assess their needs, and to provide the guidance required to support the optimal implementation of IMT whether in their own homes or within an outpatient setting.
The evidence presented in Chapter 4 supports the use of IMT both as a stand-alone intervention, and as part of a multi-dimensional rehabilitation programme, for a wide range of patients. The benign nature of IMT, and the ability of virtually all patients to meet the demands necessary to elicit improved function, make it a versatile intervention for patients with dyspnoea and exercise intolerance, or indeed for those who avoid activity because of their fear of exertional dyspnoea. Furthermore, co-morbidities that preclude exercise training are no barrier to IMT, making it an ideal intervention for severely compromised patients. A systematic review of home-based physiotherapy interventions for patients with chronic obstructive pulmonary disease (COPD) found that home-based IMT significantly improved dyspnoea (Transitional Dyspnoea Index) by 2.36 units, compared with controls (Thomas et al, 2010). Furthermore, a recent systematic review comparing IMT and rehabilitation concluded that the magnitude of improvement in exercise tolerance (and associated parameters) in response to IMT was indistinguishable statistically from that derived following a period of exercise training ‘(O’Brien et al, 2008). In other words, IMT yielded the same benefits as exercise training, making it a very cost-effective stand-alone intervention. The ideal setting for the stand-alone implementation of IMT is primary care. The barrier to implementation in this setting is a general lack of specialist expertise relating to IMT, which is not typically part of the curriculum for respiratory clinicians. This book endeavours to facilitate the acquisition of that expertise and the inclusion of IMT within the clinical curricula of specialist nurses, physiotherapists and physicians.
Finally, the context of IMT needs to be defined in terms of its application as either ‘Foundation IMT’ or ‘Functional IMT’ (see Ch. 7). In this book, the stand-alone implementation of IMT has been termed ‘Foundation IMT’, which is also the first phase of a two-phase process that leads to Functional IMT. Respiratory muscles have a number of important non-respiratory roles (see Ch. 3), which is why patients find walking more ‘dyspnoegenic’ than cycling. A training modality that is applied widely in sport is ‘functional’ training, i.e., the training of functional movements (e.g., a squat), rather than the training of muscles (e.g., leg extensors). This approach has not yet been applied to IMT in patients. However, for people who already have overloaded respiratory muscles, the competing functions of, say, postural control may be sufficient to render an everyday activity impossible because of intolerable dyspnoea. Accordingly, the rationale for implementing a training modality that addresses these competing demands is very strong. The specialist nature of Functional IMT makes its implementation the preserve of rehabilitation specialists and, therefore, probably unsuitable for implementation outside of the rehabilitation setting.
Indications and contraindications for IMT
Chapters 3 and 4 presented the theoretical and evidence-based rationales for RMT, respectively. The rationale for RMT is based upon ameliorating imbalance within the demand / capacity relationship of the respiratory muscles. This information is summarized in Box 6.1. The following section contains a more detailed overview of these issues.
Indications
Chapter 3 summarized the wide range of conditions in which respiratory muscle dysfunction is present, as well as the disease-specific factors that contribute to an imbalance of the demand / capacity relationship of the inspiratory and expiratory muscles. Chapter 4 summarized the evidence for improvement in clinical outcomes following RMT. For the reasons provided in Chapters 4 and 5, the current chapter deals with training of the inspiratory muscles. The simplistic approach to identifying patients who could benefit from rebalancing of the demand/capacity relationship is to assess maximal inspiratory pressure (MIP) and to apply a diagnostic criterion for ‘weakness’ based upon reference to population norms (see Ch. 1), e.g., 60% of the predicted normal value.
However, this approach fails to take account of a number of important issues. First, strength (MIP) is a one-dimensional index of muscle function that is difficult to interpret in limb muscles, but is infinitely more difficult for the respiratory muscles. This is primarily because MIP is merely a surrogate measure of strength, and is several steps removed from the true force-generating capacity of the inspiratory muscles (ATS / ERS, 2002). Secondly, in patients with a tendency to hyperinflate, MIP is not a fixed index of function but one that is dependent upon the prevailing degree of hyperinflation. Thirdly, the interpretation of MIP needs to be made in the context of the prevailing loading conditions, and work requirements, of the inspiratory muscles – in other words, whether the demand / capacity relationship of the inspiratory muscles is in a state of imbalance. In the presence of an increased demand for inspiratory muscle work, a muscle that appears ‘normal’ in absolute terms is rendered weak functionally.
In most chronic conditions, inspiratory muscle dysfunction manifests as dyspnoea and exercise intolerance. Thus, a more pragmatic approach to assessing the extent to which the inspiratory muscles are functionally overloaded is to consider the intensity of dyspnoea that an increased demand for inspiratory muscle work provokes. Any patients who complain of dyspnoea that limits their activities of daily living are therefore candidates for IMT (see also the sections ‘Patient selection’ below, and ‘Assessing patient needs’ in Ch. 7).
Notwithstanding the preceding rationale, the absence of exertional dyspnoea should not be a reason to discount IMT as a potential management tool for exercise intolerance. The inspiratory muscle metaboreflex described in Chapters 3 and 4 is probably the reason that a substantial number of patients with respiratory disease stop exercising because of leg discomfort, rather than dyspnoea (Hamilton et al, 1996). In such cases, improving inspiratory muscle function raises the intensity of inspiratory muscle work associated with metaboreflex activation. This can increase the exercise tolerance of non-dyspnoeic patients by improving leg blood flow.
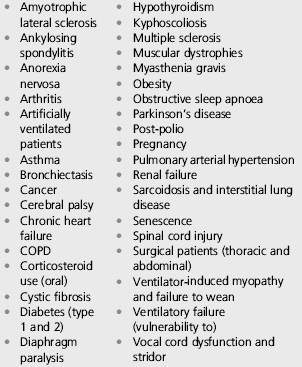
Finally, readers are encouraged to consider the application of the techniques described in this chapter to ameliorating the demand / capacity imbalance of the inspiratory muscles in non-exercise-related contexts. Some indications for these applications are provided in Box 6.1.
Contraindications
Implicitly, the measurement of maximal respiratory pressures is associated with larger pressure changes, so it is perhaps appropriate to consider also the risks associated with these measurements, as they represent a ‘worst case scenario’ for the risk of barotrauma during IMT. The first published measurements of maximal respiratory pressures were made by Black & Hyatt in the late 1960s (Black & Hyatt, 1969), and the first studies of IMT by Leith & Bradley (1976). Thus, the research base spans over 40 years, but there is no mention of any complications or adverse events in any of the studies within the published literature. In other words, of the hundreds of studies of IMT and / or maximal inspiratory mouth pressure measurement (incorporating many thousands of patients), no study has ever reported a patient withdrawal due to an event precipitated by barotrauma. Similarly, there are no case studies of IMT reporting isolated incidents of adverse events during the treatment, or assessment of any person undertaking measurement of maximal inspiratory pressures (MIP), or IMT. Despite the intrathoracic pressure swings, even patients with heart failure experience no deterioration of their cardiac output during training (McConnell et al, 2005).
• A history of spontaneous pneumothorax (i.e., not due to traumatic injury)
• A pneumothorax due to a traumatic injury that has not healed fully
• A burst eardrum that has not healed fully, or any other condition of the eardrum.
There is also an important subgroup of asthma patients for whom IMT is inappropriate, i.e., those with unstable asthma and abnormally low perception of dyspnoea. In these patients, it is the disruption of the normal relationship between dyspnoea and bronchoconstriction that is thought to contribute to their poor adherence to medication and consequent clinical instability (Magadle et al, 2002). Since IMT may reduce their perception still further, it is inadvisable.
Unlike pharmacological treatments, IMT has no side effects or drug interactions.
Patient selection
There remains a perception that only patients with evidence of inspiratory muscle weakness (see section ‘Assessment of respiratory muscle function’) or ventilatory limitation during physical activity can benefit from IMT. The logic of this has always been questionable, particularly given that Olympic standard athletes are known to benefit, and they certainly have no compromise to the function of their ventilatory pump. The misconception is perpetuated by the fact that patients with a MIP < 60 cmH2O appear to show larger improvements than those with stronger inspiratory muscles (Lotters et al, 2002; Gosselink et al, 2011). However, even when inspiratory muscle weakness is not an inclusion criterion, improvements in dyspnoea and exercise performance still follow IMT (Lotters et al, 2002). A pragmatic way to view these observations is that weaker individuals have more to gain from IMT.
In addition, it is also worth noting that research has shown repeatedly that there is a close correlation between the post-IMT decrease in dyspnoea and the improvement in MIP, regardless of MIP at baseline, or whether the participants are patients or healthy young athletes. Thus, it is the extent to which MIP can be improved that dictates the magnitude of improvement in dyspnoea. In this respect also, weaker patients have more to gain, as they appear to show the largest improvements in MIP after IMT (Lotters et al, 2002; Gosselink et al, 2011).
Notwithstanding this, there is nevertheless a dose–response relationship that relates the magnitude of improvement to the number of completed IMT sessions (Winkler et al, 2000), i.e., the more diligently the IMT training regimen is adhered to the better is the outcome. This means that patient motivation is arguably the most important criterion for enrolment. Accordingly, the best candidates for IMT are well-motivated individuals with dyspnoea and / or exercise intolerance.
See Chapter 7 (section ‘Assessing patient needs’) for guidance on assessing dyspnoea and the functional limitations it imposes.
PRACTICAL ISSUES
Posture during training
Recumbent and semi-recumbent postures are known to impair respiratory muscle function, which is optimized in upright positions (Koulouris et al, 1989; Griffiths & McConnell, 2012). Accordingly, posture has an influence on the ability to overcome a given load during IMT. This concept will be developed in Chapter 7 in the context of functional IMT, but posture also has a role to play in obtaining optimal results during the Foundation phase, especially in patients who are unable to adopt an upright position. The premise for this is that larger improvements can be achieved in a shorter time frame if training overload is maximized. Since inspiratory muscle function is optimized in upright positions, the highest training loads can be tolerated when seated or standing, and these represent the ideal positions to commence a period of IMT.
However, there are some caveats to the use of seated or standing postures. The respiratory muscles contribute to postural control, which is a confounding influence during IMT. If the inspiratory muscles are relieved of their postural role, they are able to ‘focus’ upon breathing. Consequently maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP) and maximal voluntary ventilation (MVV) are all higher in a standing, supported, leaning posture (Cavalheri et al, 2010). Thus standing, and leaning forward against the back of a chair, table, window ledge or just by placing the hands on the knees, increases the tolerable training load and the number of repetitions that can be achieved. The logic of this is immediately apparent when one considers what people do when they are breathless: they instinctively lean on something so that their respiratory muscles can be focussed on the act of breathing.
Optimizing breathing technique
Maximizing the training stimulus to the inspiratory muscles, as well as the range of adaptations it elicits, is arguably the most important objective in terms of achieving the best possible training results. Choosing the right type of training device is the first step to maximizing results. In the case of the methods described in this book, the focus is on pressure threshold IMT, for which there are two evidence-based products available (see Ch. 5, Table 5.1). Once the training device has been selected, the second step is to optimize the training stimulus that it generates. Unfortunately, simply breathing through a training device will not achieve this. However, the following section explains the optimal approach for pressure threshold IMT and the physiology that underlies this approach. Firstly, the fundamentals of ‘good’ breathing technique will be considered, i.e., how to develop efficient, comfortable, diaphragm-focussed breathing. Secondly, the optimization of breathing pattern (the combination of respiratory flows, volumes and the timing of breaths) for IMT will be explained.
Diaphragm breathing
Disease-related changes to the mechanics of breathing can lead to a reduction in diaphragm mobility, and, consequently, its contribution to breathing. This phenomenon has been well documented in COPD where it is largely due to hyperinflation (Unal et al, 2000). Diaphragm breathing training programmes have been shown to improve diaphragm mobility and exercise tolerance (Paulin et al, 2007; Yamaguti et al, 2012), as well as breathing pattern and oxygen saturation (Fernandes et al, 2011). However, in patients with COPD who have asynchronous thoracoabdominal motion, diaphragm breathing may exacerbate dyspnoea (Fernandes et al, 2011) and reduce the efficiency of breathing (Gosselink et al, 1995). Consequently, the appropriateness of diaphragm breathing for a specific patient needs to be evaluated on a case-by-case basis. Given that the likely readers of this book are healthcare professionals with an interest, and considerable expertise, in the realm of ‘breathing’, there is a danger of ‘teaching grandmother to suck eggs’ in providing advice on diaphragm breathing. However, relearning normal efficient breathing strategies is the ‘essential groundwork’ that underpins Foundation IMT. Accordingly, expert readers are merely encouraged to ensure that patients receive appropriate diaphragm breathing training as part of their Foundation IMT. For those readers who would welcome some further guidance on teaching diaphragm breathing, please feel free to read on, but also feel free to seek this elsewhere, as the following section is merely included for the sake of completeness.
This first phase of the process of developing efficient breathing can be thought of as ‘getting in touch with the diaphragm’. Human babies breathe almost exclusively with their diaphragm, but most human adults have lost touch with their most important respiratory muscle and become ‘chest breathers’, i.e., they breathe using the accessory muscles of the rib cage. Without re-establishing diaphragm breathing, IMT will neglect the most important inspiratory muscle. However, this emphasis upon diaphragm breathing should not be confused with the objective of focussing the IMT upon the diaphragm; this is not what is being advocated (the reasons for this are explained below in the section ‘Breathing pattern during IMT’). Rather, the objective is to ensure that the complex inspiratory musculature is used holistically, and in concert, during both training and activities of daily living. This section provides guidance on how to reintroduce the diaphragm into the normal, instinctive process of breathing, so that when breathing is challenged, the diaphragm is a subconscious, central part of the response. The mechanism by which isolated, conscious activation of muscles restores their automatic activation is poorly understood, but it has been likened to flipping a rope back into a well-worn groove. Short-term repetition of motor tasks has been shown to induce cortical reorganization in a range of activities including the practice of diaphragm breathing (Demoule et al, 2008). These changes are consistent with the ability of task repetition to enhance neuromuscular functioning. For example, for muscles that are involved in automatic, anticipatory postural adjustments, such as transversus abdominis, it is thought that isolated specific training can normalize previously abnormal patterns of motor activation (Tsao & Hodges, 2007).
Visual and tactile feedback both provide very helpful means of coaching diaphragm breathing. To achieve this, the patient should be positioned in front of a mirror (large enough to see their torso), with palms placed lightly on the ribs with the fingers facing forwards and the tips of the fingers almost touching at the end of a normal exhalation (Fig. 6.1). The exercise should commence by describing (to the patient) the movement of the diaphragm during inhalation and the effect this has on the abdominal wall, i.e., the diaphragm flattens and moves downwards like a piston, pushing the abdominal organs downwards and outwards. A demonstration is helpful, after which the following instructions can be used to guide the patient’s breathing:


Figure 6.1 Placing the hands on the abdomen to feel movement of the abdominal wall during diaphragm breathing: (A) inhalation, (B) exhalation.
• Commence diaphragm breathing at the end of a normal exhalation
• Keep your abdomen, shoulders and chest relaxed, and take a deep, slow inhalation through your nose
• Watch the movement of your abdomen and rib cage in the mirror
• On inhalation, you should see and feel your abdomen bulge forwards and your ribs move sideways and forwards
• Watch your finger tips and try to move them apart using your abdomen as you inhale
• If your chest rises, the diaphragm is not being used properly – relax the shoulders and chest
• Exhalation should be relaxed, with no muscle activity – allow the air to ‘fall out’ of your chest as your lungs and rib cage ‘spring’ back
• You may find it helpful to purse your lips as this helps you to breathe out further, and also slows your exhalation, but be careful not to force the air out – relax and let the air fall out
• Be careful not to hold the breath at the end of the inhalation – relax and let the air fall out.
Use of an external breathing pacer that provides an auditory and visual cue (obtainable via www.physiobreathe.com/apps) can be very helpful for supporting the process of developing an efficient breathing pattern, i.e., deep breaths accompanied by a slow exhalation. In addition, a Theraband®, or similar wide elastic resistance band, wrapped around the lower ribs can help to focus effort on this area. The resistance of the band intensifies the sensation of working with the diaphragm (if the patient still finds the exercise too challenging, see below). Diaphragm breathing should be practised until the patient feels confident about how to activate the diaphragm, and what a diaphragm inhalation feels and looks like. Once this technique has been mastered, it should be tried with eyes closed, focussing on the sensation of the air filling the lungs and the sensation of the diaphragm plunging down into the abdomen.
During the diaphragm breathing exercise, breathing rate should be no more than 12 breaths per minute, and preferably 8. With practice it might be possible to reduce this to 6 breaths per minute. The inhalation phase will typically be slightly longer than exhalation because exhalation is passive, i.e., the only phase that is actively controlled is inhalation. However, this will not be the case in obstructed patients, who typically take longer to exhale. Patients can count the breath in and out. For example, for an obstructed patient, if the objective is 10 breaths per minute this means that each breath should take 6 seconds (60 / 10); count ‘1–2’ during inhalation, and ‘3–4–5–6’ during exhalation. It is vital that the extension of the breath duration is not achieved by breath holding at the end of inhalation or exhalation; the increase in duration should come about through a slower, deeper, more controlled breath so that each phase starts immediately the other ends. Remind patients that exhalation should be passive, and that the air should ‘fall out’ of the lungs as quietly as possible. There are some useful biofeedback applications for smartphones and tablet computers that can facilitate the development of slow, deep breathing, which healthcare professionals can use in a clinic setting and that can be suggested to patients as tools to help them to practise at home (see www.physiobreathe.com/apps). These tools set the duration of each phase of breathing and the breathing frequency, count the number of breaths, and time the practice session.
Developing breath control is a skill that can and should be practised because it maximizes breathing efficiency and minimizes the distracting influence of breathing discomfort. The first stage in achieving this is achieving diaphragm-focussed breathing, which provides the ‘groundwork’ for good breath control; once this becomes second nature the second phase is much easier. Phase two involves developing breath control to overcome the natural desire to breathe as quickly as possible during periods when breathing demand is high and when breathing discomfort / distress is present. By not allowing breathing to become rapid and shallow, voluntary breathing control imposes a deep, slow, calm and efficient pattern. The only time that this can be practised properly is in situations where breathing demand / distress is high, but only when patients are clinically stable. So they should be encouraged to practice deep, slow breath control during periods when they feel dyspnoeic. Keeping breathing calm and relaxed under stressful conditions can help to minimize stress and anxiety, and build a sense of mastery. See also Chapter 7 (section on ‘Breath control’) for some exercises that promote deep, slow, controlled breathing.
Breathing pattern during IMT
Tidal volume
Although muscle is a very adaptable tissue, training adaptations are also highly specific to the nature of the training stimulus. Adaptations elicited by IMT are specific to a number of characteristics of the training stimulus, including the lung volume at which training takes place (see Ch. 5). The practical implication of this is that IMT should be undertaken across the widest range of lung volume possible, i.e., from as close to residual volume as possible to the point at which it is impossible to inhale any more. Failure to do this will lead to suboptimal adaptation at some lung volumes, which is particularly important in patients who hyperinflate when minute ventilation increases. An important consideration in maximizing tidal volume (VT) during IMT is the training load; loading too ‘heavily’ can compromise both the VT that can be achieved and the amount of work that can be undertaken during training, which will also impair the training response (Box 6.2). This impairment occurs because VT has a strong influence upon the amount of work done per breath, and the most important determinant of the ability to inhale deeply is the training load (McConnell & Griffiths, 2010). Functional weakening of the inspiratory muscles during inhalation means that, if the load is too high, the inspiratory muscles are not able to overcome the load at higher lung volumes (where the inspiratory muscles are weaker), despite maximal effort. The heavier the load, the more severely the breath is ‘clipped’.
This means that the training load must be set with these factors in mind. Figure 6.2 illustrates the interrelationships of maximal inspiratory pressure (MIP), lung volume and training load, as well as the effect of fatigue on the VT that can be achieved. Note that the breath volume is clipped progressively earlier in the breath with increasing loads.
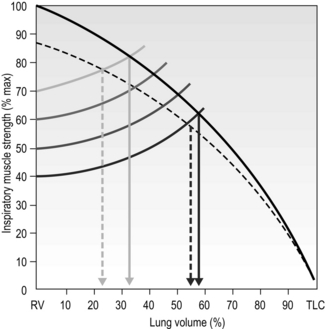
Figure 6.2 The interactions between inspiratory muscle strength (black line), various training loads (40%, 50%, 60% and 70% of maximal inspiratory pressure [faded lines]), and the breath volume that can be achieved during training, as well as the effect of fatigue (dotted lines). For example, at 40% of MIP it is possible to inhale to around 60% of vital capacity, whereas at 70% of MIP it is only possible to inhale to around 35% of vital capacity. (Adapted from McConnell AK, 2011. Breathe strong, perform better. Human Kinetics, Champaign, IL, with permission.)
Inspiratory flow rate
To understand the advice below, it is necessary to return briefly to the force–velocity relationship of muscle. Essentially, this property dictates that the faster a muscle contracts, the less force it is able to generate, and vice versa (see Ch. 4, Fig. 4.1). An example of the force–velocity relationship at work is the difference in force one can exert on the pedals when cycling in a low gear compared with a high gear. This property can be exploited to optimize the training stimulus that the muscle receives. For example, assume that, because of the force–velocity relationship, as the rate of muscle contraction doubles the force it can generate is halved, despite the same [maximal] effort being applied under both conditions. When muscles contract maximally at any speed, the number of muscle fibres that are recruited to the contraction is also maximized, despite the fact that faster contractions result in lower forces. Now consider the effect of doubling the rate of contraction slightly differently: when a muscle is contracting very slowly to move a load that requires, say, half its maximal force-generating capacity, doubling the rate of contraction against the same load now requires ~100% of the muscle’s force-generating capacity (because its ability to generate force has been halved). This means that ~100% of the muscle fibres are recruited for half the force. This can be turned to an advantage during IMT because it means that it is possible to train close to 100% of a muscle’s force-generating capacity no matter what load is being applied, provided that the load is moved as fast as possible (i.e., with maximal effort). Under any given loading condition, fast muscle contractions recruit more muscle fibres than slow contractions (Aagaard et al, 2000). Furthermore, recent evidence suggest that training improvements following bench press training are maximized at higher velocities of muscle shortening (Padulo et al, 2012). Therefore, maximal effort ensures maximum velocity, and the recruitment of the greatest number of muscle fibres.
Accordingly, IMT should be conducted with maximum effort; i.e., each inhalation should be executed as fast as possible. This should take around 1–2 seconds and be accompanied by a loud rushing sound as air is sucked through the valve of the training device at high velocity. Encourage patients to make this sound as loud as possible, because this indicates high flow rates. Be aware that the heavier the relative load, the slower will be the maximal flow rate that can be generated (and the smaller the lung volume; see Fig. 6.2). In contrast to the maximal nature of the inspiratory effort, exhalation should be passive, quiet and take 3 to 4 seconds. Use of an external breathing pacer that provides an auditory cue (obtainable via www.physiobreathe.com/apps) can be very helpful for supporting the process.
Because of the higher-than-normal breath volume and breathing frequency, some light-headedness may result from the hyperventilation-induced hypocapnia. This is harmless for the duration of a 30-breath session, and also seems to lessen in severity as training progresses (but see also ‘Precautions’, above). If it is problematic, ask the patient to pause at the end of exhalation and wait for the urge to breathe in again. For maximal training overload the training breaths should be completed as quickly as possible, but this has to be balanced against the effects of the hypocapnia. A method that can be used to overcome the loss of carbon dioxide is to place the training device inside a bag that has a slit down one side (a supermarket carrier bag is ideal). By rebreathing from the bag, the loss of carbon dioxide is largely abolished and light-headedness is prevented. This allows patients to complete the breaths rapidly, so maximizing the training benefits.
Combining inspiratory and expiratory training
Evidence suggests that loading of the inspiratory and expiratory phases of the same breath produces suboptimal results, and that even healthy young people find this simultaneous loading uncomfortable (Griffiths & McConnell, 2007). Accordingly, this approach is NOT recommended. If expiratory muscle training is to be added to IMT, each should be delivered as a discrete bout of exercise separated by a few minutes of recovery.
Secretions
Repeated, deep inhalations against an inspiratory load have been found to be twice as effective (sputum weight) as standardized physiotherapy consisting of postural drainage and the active cycle of breathing technique (Chatham et al, 2004). These data support anecdotal evidence that patients with conditions such as bronchiectasis or bronchitis experience loosening of secretions following IMT. Accordingly, patients need to be warned of this and given the appropriate advice regarding clearance techniques.
MONITORING PROGRESS
Assessment of respiratory muscle function
A comprehensive description of the assessment of respiratory muscle function is beyond the scope of this book, but for those readers wishing to know more about the topic the joint European Respiratory Society and American Thoracic Society statement on respiratory muscles testing provides comprehensive guidance (ATS / ERS, 2002). For the purposes of gaining a practical understanding of how to assess inspiratory muscle function for monitoring purposes, some basic guidance is provided below.
Maximal respiratory pressures
Respiratory pressures are measured against an occluded airway (incorporating a 1 mm diameter leak to maintain an open glottis) at prescribed lung volumes (see below). Some spirometry equipment incorporates MIP assessment, but there are also hand-held devices that are more convenient for a clinic setting (MicroRPM®, CareFusion Inc.; POWERbreathe® KH1, POWERbreathe International Ltd). The measurement of MIP (and MEP) is subject to the influence of lung volume (Rahn et al, 1946), motivation and skill acquisition (Volianitis et al, 2001a; Hawkes et al, 2007), as well as the effect of repeated measurement upon the excitability of the motor pathway (Hawkes et al, 2007; Ross et al, 2007). Consequently, it is important to control for these confounding factors as far possible by careful coaching, by habituating patients to the measurement, by making repeated measurements until consistency of MIP is achieved (Box 6.3) and by ensuring consistency of lung volume between measurements. In addition, the use of an inspiratory muscle ‘warm-up’ (see “Inspiratory muscle ‘warm-up’ and stretching”, below) has been shown to reduce variability, to reduce the number of measurements required to achieve consistency and to remove the effects of changes in motor pathway excitability (Volianitis et al, 2001a; Lomax & McConnell, 2009).
Care must also be taken to ensure that any task learning and other effects are expressed fully before measured values are recorded. It has been shown that there is a considerable effect of repeated measurement upon MIP, even in experienced participants; in one study (Volianitis et al, 2001a) after 18 repeated trials the MIP was 11.4% higher than the best of the first three measurements made. This effect is large enough to mask changes in MIP due to the effects of inspiratory muscle fatigue and to make detection of training induced improvements statistically difficult. However, this learning effect can be overcome to a large extent by a bout of submaximal inspiratory loading prior to the assessment of MIP (two sets of 30 breaths against an inspiratory threshold load equivalent to 40% of the best MIP measured during the first three efforts; see the section ‘Inspiratory muscle ‘warm-up’ and stretching’, below). Following this prior loading, the difference between the best of the first three efforts and the 18th measurement was only 3%. Thus the time taken to obtain reliable measurements of MIP can be curtailed considerably by implementing a bout of prior loading (see Box 6.3).
The issue of reference values for MIP and MEP is a contentious one. Earlier, it was argued that a diagnosis of ‘weakness’ cannot be made on the basis of MIP (or MEP) alone because this takes no account of the demand side of the demand / capacity relationship. In addition, respiratory pressures are surrogates of true force-generating capacity, and subject to the influence of chest wall geometry, which may change. Perhaps most importantly, the correlation of respiratory pressures with anthropometric indices is extremely poor (Enright et al, 1994; McConnell & Copestake, 1999). Nevertheless, as there is a compulsion to define ‘weakness’ objectively in a clinical setting, some form of reference is required. The largest study to generate reference values for healthy older men and women contained 4443 participants aged 65 years or over (Enright et al, 1994). The resulting reference equations are given below, but it is important to note that the equations account for only between 8% (MIP women) and 18% (MEP women) of the variation in measured values. The ‘lower limits of normal’ for the sample were defined statistically as the fifth percentile of the group.
Sniff inspiratory pressure
Because of the difficulties and limitations of measuring maximal respiratory pressures, alternative indices of global inspiratory pressure generating capacity have been sought. The maximal sniff inspiratory pressure was developed originally in an invasive form, in which gastric and oesophageal pressures were measured with balloon-tipped catheters. However, the test was modified and validated as a non-invasive variant in which pressure is measured in the nostril (Heritier et al, 1994); as nostril pressure reflects nasopharyngeal pressure, it is a reasonable approximation of intrathoracic pressure. Because sniffing is a familiar manoeuvre, the test is argued to be easier for participants to execute maximally. However, maximal sniffs still require practice.
Pressure is typically measured via a catheter that is wedged in one nostril, occluding it. The participant then sniffs through the unoccluded nostril from functional residual capacity. Limitations of the technique are that it is affected by the patency of the nasopharynx, and is therefore susceptible to the effects of nasal congestion, as well as being unsuitable for people with major septal defects (Heritier et al, 1994).
Typically, 5–10 maximal sniff efforts are made ‘until a consistent value’ is obtained (Steier et al, 2007). There are no prediction equations based upon a large cohort of healthy people, but weakness has been defined as a pressure of less than 50 cmH2O for men and 45 cmH2O for women (Steier et al, 2007).
Peak inspiratory flow rate
A very simple index of inspiratory muscle function that has been shown to improve in response to moderate intensity IMT is the peak inspiratory flow rate (Romer & McConnell, 2003; Weiner & Weiner, 2006). This can be measured during spirometry, using a mechanical peak inspiratory flow meter (In-Check®, Clement Clarke Ltd.), or an electronic IMT device (POWERbreathe KH1®, POWERbreathe International Ltd). The measurement requires patients to exhale to residual volume and to inhale as quickly as possible. The emphasis is upon speed and not volume during the manoeuvre; likening the effort to a ‘maximal gasp’ can be helpful for eliciting maximal values. However, it is important to note that the peak flow is achieved at around 50% of vital capacity, so although full lung inflation is not essential it is important that the volume excursion is substantial.
Inspiratory muscle endurance
Endurance is ‘the ability to sustain a specific muscular task over time’ (ATS / ERS, 2002) and is by definition task specific. Although no agreed standards exist for the assessment of inspiratory muscle endurance, the tests fall into two main classes: (1) hyperpnoea tests and (2) inspiratory loaded breathing tests. The latter is subdivided further into (i) the time to the limit of tolerance breathing against a fixed load and (ii) the pressure achieved during an incremental loading task.
A hyperpnoea endurance test is typically designed to identify the maximum sustainable ventilation (MSV), which is expressed as a percentage of the maximum voluntary ventilation (MVV) or, in patients, as a percentage of the predicted MVV (MVV%). In healthy people MSV is 60–80% of MVV. The procedure requires 10–25 minutes, and in obstructed patients should be preceded by a bronchodilator (ATS / ERS, 2002). There is no standardized equipment, but the breathing circuit must have a low inherent flow resistance, maintain isocapnia and provide visual feedback of minute ventilation (![]() E). The test commences with a 12 second MVV, after which one of two methods can be used. In the maximum effort technique, participants are required to sustain 70–90% of their MVV using biofeedback of
E). The test commences with a 12 second MVV, after which one of two methods can be used. In the maximum effort technique, participants are required to sustain 70–90% of their MVV using biofeedback of ![]() E. Participants continue to try to maintain this
E. Participants continue to try to maintain this ![]() E for 8 minutes, and the average
E for 8 minutes, and the average ![]() E sustained for the final minute is taken as the MSV. The maximum incremental technique is analogous to a
E sustained for the final minute is taken as the MSV. The maximum incremental technique is analogous to a ![]() O2max test, and continues until the limit of tolerance. Participants are required to increment their
O2max test, and continues until the limit of tolerance. Participants are required to increment their ![]() E by 10% every 3 minutes, commencing at 20% of their MVV. The MSV is calculated from the last 10 breaths of the final minute of the highest target
E by 10% every 3 minutes, commencing at 20% of their MVV. The MSV is calculated from the last 10 breaths of the final minute of the highest target ![]() E. Reassuringly, the two approaches give very similar values for MSV (Thomas et al, 2010).
E. Reassuringly, the two approaches give very similar values for MSV (Thomas et al, 2010).
Inspiratory loaded breathing tests are designed to identify either the time to the limit of tolerance (Tlim) at a given percentage of MIP or the maximum pressure load that can be achieved during an incremental loading test. Typically, during a fixed-load Tlim test, a load is selected that results in task failure within 5 to 10 minutes (Goldstein et al, 1989). The correct load can be identified by a ‘trial and error’ approach, by using a load that corresponds to around 80% of the load achieved during an incremental test (Hill et al, 2007), or by using a load equivalent to 70% of the MIP (Hart et al, 2002). Another disadvantage of the Tlim endurance test is that, after an intervention, the Tlim may be extended considerably, leading to extremely long post-intervention tests that are typically terminated at 15 minutes by the experimenter (Hill et al, 2007). Incremental inspiratory loading tests typically commence at 10% of MIP, with increases in load of 10% per minute, until task failure (Hill et al, 2007). Performance in the test is expressed as the highest load tolerated for a minimum of 30 seconds.
The problem with both of these tests is the confounding influence of breathing pattern. Participants with the lowest duty cycle (the ratio of inspiratory time to breath duration) tend to have the longest Tlim (Hart et al, 2002). An inevitable consequence of a low duty cycle is a smaller VT, and a lower amount of inspiratory muscle work (McConnell & Griffiths, 2010). Thus, breathing strategy plays a large role in determining performance in both of these tests of inspiratory muscle endurance. Until a method is developed to control the amount of inspiratory muscle work undertaken during inspiratory endurance tests, their results will continue to be a reflection of both behavioural strategies and physiological function.
Evaluating clinical benefits
The parameters selected as clinically significant outcomes will vary enormously between patient populations, or even between individual patients, e.g., in a mechanically ventilated patient the ability to breathe unassisted, or in a patient with COPD the ability to walk continuously for 6 minutes. The outcomes selected therefore need to be specific to the clinical problem being addressed, but selection must also be tempered with realism regarding the clinical factors that are likely to be sensitive to the effects of IMT. For example, IMT will not improve maximal oxygen uptake (![]() O2max) in a patient with heart failure in whom oxygen transport is limited by central factors related to cardiac output. However, the same patient may exhibit an improvement in 6-minute walk distance after IMT, though not because
O2max) in a patient with heart failure in whom oxygen transport is limited by central factors related to cardiac output. However, the same patient may exhibit an improvement in 6-minute walk distance after IMT, though not because ![]() O2max has improved (see Ch. 4).
O2max has improved (see Ch. 4).
The starting point for all outcome assessment is confirmation that respiratory muscle function has improved (see above). Thereafter, the approach is highly specific to the patient and the disease. Table 6.1 contains a summary of disease-specific factors that have been shown to be sensitive to the effects of IMT in a range of clinical groups (see Ch. 4 for details). The outcomes listed in the table are limited to controlled studies or meta-analyses, but where this level of evidence is lacking for a specific condition some suggested outcomes have been provided, based upon the rationale presented in Chapter 3. The specific methods and instruments employed to assess these factors are well-established, disease-specific and beyond the scope of this book. The exception to this is the assessment of dyspnoea, which is described in Chapter 7 in the section ‘Assessing patient needs’ (for functional training of the respiratory muscles).
Table 6.1
Summary of main clinically and statistically significant outcomes (compared with control or placebo) following stand-alone use of IMT for a range of clinical conditions*
| Clinical condition | Outcomes improved significantly | Supporting references** |
| Chronic obstructive pulmonary disease | MIP, IME, ExTol, DyspEX, DyspDL, DyspLB, QoL, UHR | Beckerman et al, 2005; Geddes et al, 2008; Shoemaker et al, 2009; Gosselink et al, 2011 |
| Asthma | MIP, IME, ExTol, DyspEX, DyspLB, MedCon | Weiner et al, 1992; McConnell et al, 1998; Weiner et al, 2000a; Weiner et al, 2000b; Weiner et al, 2002a; Weiner et al, 2002b; Turner et al, 2011 |
| Bronchiectasis | § MIP, IME, ExTol, DyspEX, DyspDL, DyspLB, QoL, UHR, AC | |
| Chronic heart failure | MIP, IME, ExTol, DyspEX, DyspDL, QoL | Cahalin et al, 1997; Johnson et al, 1998; Weiner et al, 1999; Martinez et al, 2001; Laoutaris et al, 2004; Dall’Ago et al, 2006; Padula et al, 2009; Bosnak-Guclu et al, 2011; Lin et al, 2012; Plentz et al, 2012 |
| Neuromuscular disease | MIP, IME, LF, DyspEX, DyspDL, QoL | Weiner et al, 1998a; Winkler et al, 2000; Koessler et al, 2001; Topin et al, 2002; Fregonezi et al, 2005; Inzelberg et al, 2005; Fry et al, 2007; Sutbeyaz et al, 2010; Hull et al, 2012 |
| Obesity | § MIP, ExTol | Edwards et al, 2012 |
| Ageing | MIP, ExTol, DyspEX, PA | Copestake & McConnell, 1995; Aznar-Lain et al, 2007 |
| Cystic fibrosis | MIP, IME, LF, ExTol, AC | Sawyer & Clanton, 1993; de Jong et al, 2001; Chatham et al, 2004; Enright et al, 2004 |
| Restrictive chest wall disorders | § MIP, IME, LF, ExTol, DyspEX, DyspDL, DyspLB, QoL | |
| Sarcoidosis and interstitial lung disease | § MIP, IME, LF, ExTol, DyspEX, DyspDL, DyspLB, QoL | |
| Diabetes | MIP, IME | Correa et al, 2011 |
| Renal failure | MIP, IME, ExTol | Weiner et al, 1996 |
| Cancer | § MIP, IME, ExTol, DyspEX, DyspDL, QoL | |
| Anorexia nervosa | § MIP, IME, LF, ExTol, DyspEX, DyspDL, QoL | |
| Myopathy (iatrogenic) | MIP, IME, LF (reductions offset by IMT) | Weiner et al, 1995 |
| Surgery | MIP, LF, ExTol, PPC | Weiner et al, 1998b; Hulzebos et al, 2006a; Hulzebos et al, 2006b; Dronkers et al, 2008; Casali et al, 2011; Savci et al, 2011; Hulzebos et al, 2012 |
| Mechanical ventilation | MIP, WS^, S^ | Moodie et al, 2011; Cader et al, 2012 |
| Spinal cord injury | MIP, LF^ | Van Houtte et al, 2006; West et al, 2009 |
| Pregnancy | § MIP, IME, ExTol, DyspEX | |
| Obstructive sleep apnoea | § MIP, IME, AHI, QoL | |
| Vocal cord dysfunction | § MIP, IME, ExTol, DyspEX |
AC = airway clearance; AHI = apnoea–hypopnoea index; DyspEX = exertional dyspnoea; DyspDL = dyspnoea during activities of daily living; DyspLB = dyspnoea during loaded breathing; ExTol = exercise tolerance; IME = inspiratory muscle endurance; LF = lung function; MedCon = medication consumption; MIP = maximal inspiratory pressure; PA = physical activity; PPC = post-operative pulmonary complications; QoL = quality of life; S = survival; UHR = use of healthcare resources; WS = weaning success; § = statistically significant evidence is lacking, but there is a physiological rationale supporting a beneficial effect of IMT (see Ch. 3); ^ = trend favouring IMT.
*Where this level of evidence is lacking for a specific condition, some suggested outcomes have been provided, based upon the rationale presented in Chapter 3.
Notwithstanding the disease specificity of outcomes, there are some generic principles for assessing ambulatory patients with dyspnoea and exercise intolerance. In these patients the literature suggests that in most, irrespective of their disease, IMT has the potential to elicit improvements in inspiratory muscle strength and endurance, dyspnoea, exercise tolerance and quality of life. There are well-established, disease-specific techniques for assessing all of these parameters. A notable absentee from the preceding list of outcomes is lung function, since changes appear to be disease specific. For example, there is no evidence that IMT, or indeed exercise training, increases expiratory flow in patients with COPD (Magadle et al, 2007). However, expiratory flow and vital capacity may improve in patients with asthma (Weiner et al, 1992), as well as those in whom inspiratory muscle weakness creates a restrictive defect (Weiner et al, 1998a).
GETTING STARTED
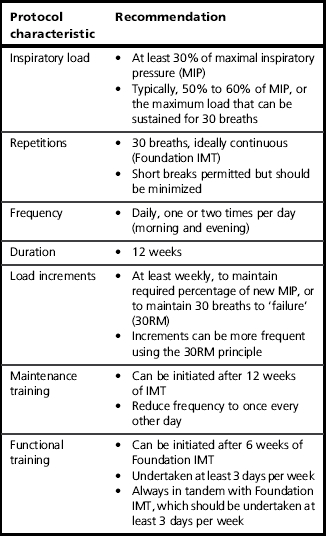
This section provides guidance on the practicalities of initiating a programme of IMT (inspiratory muscle training), and describes the Foundation phase of the training process. A summary of the most important guidance from this section can be found in Table 6.2. In addition, Box 6.4 summarizes the key aspects of Foundation IMT.
Protocol selection
Notwithstanding this, there are some considerations that are worthwhile highlighting, as these may facilitate the process of identifying the most appropriate protocol (see also Chs 3, 4 and 5):
• Most patients lack inspiratory muscle strength, and their apparent lack of endurance is secondary to a lack of strength
• Demand / capacity imbalance within the respiratory pump is rebalanced most effectively by increasing the strength and power of the pump
• Strength-biased training protocols generate the widest range of improvements in inspiratory muscle functional properties (strength, shortening velocity, power and endurance)
• Dyspnoea and sense of breathing effort typically improve only if MIP improves, which requires a strength-biased training protocol
• Strength-biased training protocols are more time efficient and may enhance compliance
• Strength-biased training protocols have been shown to produce the widest range of physiological and functional changes in healthy young people.
Setting the training load
The general principles of training overload were described in Chapter 5, which also included a summary of the evidence relating to different types of training and training regimens. The following guidance is based upon these insights.
Initially, the focus should be on inhaling deeply and forcefully, and exhaling slowly and gently. The objective in the first instance is to develop good breathing technique (see above) and to complete 30 breaths without the need to stop and rest. As soon as 30 breaths can be achieved without stopping, it is time to increase the training load by a quarter turn of the load tensioner (2–3 cmH2O on a typical pressure threshold training device) (see Ch. 5, Fig. 5.1). Note that it may require a number of weeks for some severely compromised patients to achieve this (see the section ‘Progressing training’, below).
Where there is access to equipment for measuring MIP, the training load can be set using objective criteria. The training should commence at a load equivalent to 30–40% of MIP provided that this is tolerable; this is the lowest intensity that has been shown to elicit improvements in function (Lotters et al, 2002). If this load is not tolerated then commence training as described above until 30 breaths can be completed at the lowest setting. Most training devices do not have a calibrated scale printed on them, but many will provide a conversion chart that enables the level settings printed on the device to be cross-referenced to the corresponding load in cmH2O. Once the starting load of 30–40% can be tolerated, the load should be increased by one-quarter turn each day for the next 7–10 days until up to 50–60% of baseline MIP is reached (see ‘Progressing training’).
Many patients experience life-changing results very quickly (see ’Case study‘, below), and this can prompt such enthusiasm that they to want to train more than twice per day. This is definitely inadvisable. Recovery is an important part of the training process, and the inspiratory muscles are already being subjected to a very challenging regimen of twice-daily specific IMT. Patients should therefore be discouraged from training more than twice daily, and they should ensure that the two sessions are separated by at least 6 hours.
In Chapter 7, guidance about the loads to be used during functional training exercises will be divided into ‘light’ and ‘moderate’. These correspond to the following ‘repetition maximum’ and MIP percentage settings:
Repetition failure
Repetition failure is a slightly alien concept to people who have never engaged in any form of weight training. Essentially, it refers to the notion that, at some point during repeated lifting of a weight, fatigue will make it impossible to lift the weight, resulting in ‘failure’. Most weight training is undertaken to the point of failure, because this optimizes the loading conditions that stimulate muscle adaptation (Toigo & Boutellier, 2006). For most muscles, the weakest point in the movement is at, or close to, the starting point. For example, when performing a bicep curl, the biceps are weakest at the onset of the exercise (when the elbow is extended), which means that failure usually occurs at the start of the movement with the result that the repetition cannot be started. In the case of the inspiratory muscles, the opposite is true: they are strongest at the start of the movement (at residual volume), becoming weaker as one inhales (see Fig. 6.2). This means it may be possible to open the valve, but not possible to take a meaningful breath. So how should failure defined for IMT? A pragmatic approach is to tell the patients that once it is impossible to achieve a ‘satisfying breath’ then failure has been reached and the session is complete. Some patients may require some encouragement in order to push themselves to this point, but it is the best way to achieve the results they are working towards. Notwithstanding this, it is not disastrous if patients train only until they have completed 30 breaths, provided that the training load is at least 60% of their current MIP and the breathing technique described above is followed (maximizing inspiratory flow and volume).
Use of training diaries
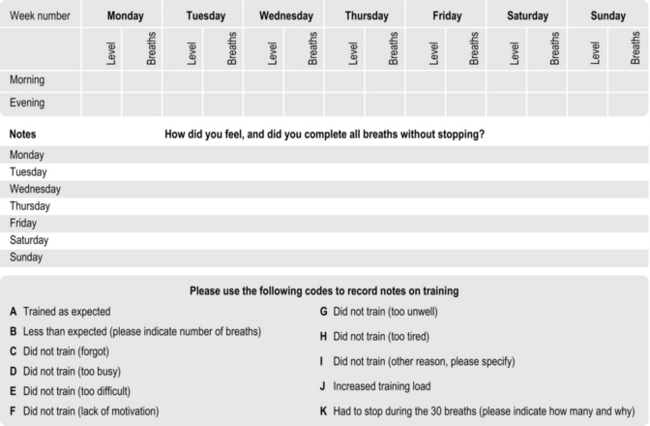
Figure 6.3 provides a template for a training diary that can be reproduced. As well as training load etc., the template provides space for notes on how the training felt and other activities or health-related issues that may impact upon the training, as well as the patient’s response to it. This facilitates cross-referencing of circumstances that may be helpful for the patient and healthcare professional in interpreting sudden up- or down-turns in training and / or symptoms.
PROGRESSING TRAINING
Patients should be encouraged to train beyond 30 breaths if they feel able to and can still achieve a ‘satisfying breath’ (see the section ‘Repetition failure’, above). In this way, they maximize progress. Once 30 continuous breaths can be completed then further increases in the training load should be made once per week, or as soon as 30 breaths can be exceeded. The increase in load should be sufficient to reduce the number of breaths that can be achieved before failure to between 25 and 30. On most training devices this will be about one-quarter to one-half turn on the spring tensioner. Bringing the number of breaths down to 25 is acceptable because within a few days the maximum number of breaths will be back up to 30. As a rough rule of thumb, the training load should be increased by at least one-quarter turn each week for the first 8 to 12 weeks of training. Alternatively, small increases can be made as soon as the maximum number of breaths exceeds 33 to 35. If patients find that increasing the training load is too challenging then they should be encouraged to focus on optimizing their breathing technique during the training, i.e., maximizing inspiratory flow rate and tidal volume (VT). These measures can also increase the training stimulus, by increasing the power output achieved during the inhalation (maximizing flow) and the work done (maximizing volume).
The duration of the intensive, twice-daily Foundation phase of training varies according to the circumstances in which the IMT is being undertaken, and the objectives. The first major consideration is whether the Foundation IMT is being undertaken as a stand-alone intervention or as part of a structured rehabilitation programme (see the section ‘Rehabilitation component’, below). Long-term studies of stand-alone IMT in patients with COPD suggest that improvements in function occur most rapidly over the first 12 weeks, but continue for up to 12 months (Beckerman et al, 2005). These data suggest that the duration of the Foundation phase of IMT should be at least 12 weeks.
1. Continue Foundation training for as long as the patient is motivated to do so. For some patients, changing a routine may create problems with respect to compliance. Alternatively, they may need more than 12 weeks before they feel ready to change.
2. Enter the Maintenance training phase (see next section). Some patients may find the twice-daily Foundation routine onerous, in which case moving to a Maintenance training regimen may retain their compliance.
3. Enter the Functional training phase, which incorporates activities of daily living (see Ch. 7). Motivated patients who have experienced good results during the Foundation phase may feel motivated to develop their training and to incorporate some functional elements in to their daily regimen.
4. Enter a rehabilitation programme, in which IMT is a component. Having undertaken a period of IMT, patients who were previously unable to benefit from exercise training may have recovered sufficient function to be able to enrol in a rehabilitation programme. In these patients, IMT can be continued in whatever form is most appropriate for the programme and the patient (Foundation, Maintenance or Functional). See also the section ‘Incorporating RMT into a rehabilitation programme’.
If the benefits of IMT are to be retained, the intervention must be continued according to one of the options listed above. In circumstances where patients cease IMT completely at the end of the Foundation phase, it is possible to recover the benefits using periodic phases of Foundation IMT. IMT should ideally resume before all of the benefits have regressed, as it is easier to recover post-training gains when starting from an elevated baseline. Data suggest that complete regression of gains in MIP occurs over a period of around 12 months (Weiner et al, 2004). However, it appears that around half of the strength gained is retained for up to 12 weeks. This magnitude of improvement is probably at the threshold of what is required in order to elicit enhancement of exercise tolerance and reduction in dyspnoea. Accordingly, 12 weeks probably represents the maximum period that should be permitted before recommencing IMT. It is also possible, though not proven experimentally, that the duration of the IMT during these periodic bouts of training can be shorter. Thus a design for a periodic training regimen might be 12 weeks of Foundation IMT, followed by repeated cycles of 12 weeks of detraining and 6 weeks of Foundation IMT.
The duration of many pulmonary rehabilitation programmes is typically 6–12 weeks; thus, there may be logistical issues relating to the duration of the Foundation phase and its relationship to the rehabilitation programme. In an ideal scenario where the Foundation phase has a 12-week duration, this can be dovetailed with the rehabilitation programme in a number of ways (see section ‘Incorporating RMT into a structured rehabilitation programme’, below).
MAINTENANCE TRAINING
Once the Foundation phase of training has been completed, some patients are perfectly satisfied with the results that they have achieved and are content to enter a phase of Maintenance training. Research has shown that training frequency can be reduced by as much as two-thirds without any loss of functional benefits (Weiner et al, 2004). In other words, training can switch from twice daily to training once every other day. There is, of course, no reason at all that someone cannot switch directly from Maintenance training to Functional training if they so wish.
INCORPORATING RMT INTO A STRUCTURED REHABILITATION PROGRAMME
Pre-habilitation
A study on athletes showed that implementing IMT prior to whole-body training improved the outcome of the whole-body training (Tong et al, 2010); thus, it is reasonable to suggest that Foundation IMT might be considered as ‘pre-habilitation’ for patients awaiting commencement of exercise training. The improvements in dyspnoea and exercise tolerance that accompany IMT should ease the transition of patients into a programme of whole-body physical activity that most patients will find unfamiliar and challenging. By undertaking IMT before commencing a programme, patients should be able to tolerate higher intensities of exercise (as was the case for the athletes studied by Tong et al, 2010), as well as finding exercise less uncomfortable.
In practice, limited resources may dictate that patients are required to manage this phase of IMT largely autonomously. However, following an initial assessment, some coaching regarding breathing technique, load setting and progression, most patients should be capable of undertaking domiciliary IMT. Indeed, this is generally the model employed in research studies of IMT. Notwithstanding this, where resources permit, weekly reviews of progress including a check of MIP and an increment of the training load (where patients have not increased the load themselves) is desirable (Padula et al, 2009).
Rehabilitation component
Inspiratory muscle training can be incorporated very readily into a structured rehabilitation programme. However, the method of achieving synergy between IMT and exercise training will most likely be a pragmatic combination of what is physiologically desirable and the constraints imposed by local logistics. Table 6.3 represents a suggestion of the ideal synergy between IMT and exercise training.
Table 6.3
Suggested incorporation of IMT into a rehabilitation programme*
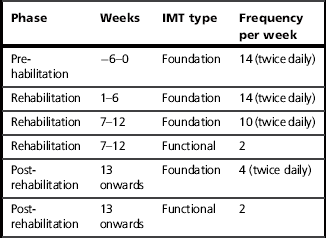
*Assumptions: Patients are enrolled in a 12-week rehabilitation programme, and undergo a pre-enrolment assessment 6 weeks before commencement of a 2 days per week outpatient rehabilitation programme. Immediately after the pre-assessment visit, patients embark upon a pre-habilitation period of Foundation IMT, which is monitored weekly.
The precise form in which the IMT is incorporated into any given rehabilitation session will also be dictated by local factors. For example, where patients exercise in a group environment using a circuit-based approach, IMT can form one station of the circuit. The exercise performed at this station can either be a full 30-breath Foundation IMT workout, a proportion of the 30-breath workout (if performed more than once) or one of the Functional IMT exercises described in Chapter 7. Alternatively, a Foundation IMT workout can be undertaken at the start or end of the rehabilitation session (as part of a warm-up or cool-down) or immediately after a bout of aerobic training; the latter has the benefit of providing a degree of specificity, as the inspiratory muscles may already be slightly fatigued by the preceding exercise, which means that they will receive the greatest relative training overload during IMT.
INSPIRATORY MUSCLE ‘WARM-UP’ AND STRETCHING
The concept of ‘warm-up’ is well established in sport and exercise, and the use of a specific inspiratory muscle ‘warm-up’ has been shown to enhance performance and reduce breathing effort in healthy young people (Volianitis et al, 2001b). Despite the terminology, these effects are unlikely to be temperature-dependent, and are probably due to neural and blood flow effects. There is every reason to anticipate that patients can experience the same benefits of inspiratory ‘warm-up’ prior to physical activity as healthy young athletes, i.e., a reduced intensity of dyspnoea during subsequent activities. Accordingly, the use of a ‘warm-up’ is recommended prior to any physical activity, but especially those exercises precipitating sudden, large increases in breathing requirement.
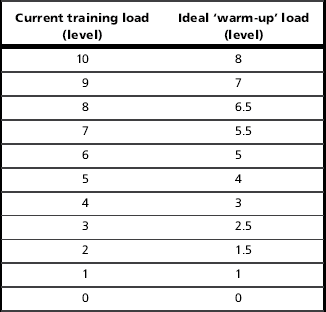
To execute the ‘warm-up’ effectively, it is necessary to find the appropriate loading intensity. This must be sufficiently intense to stimulate the response, but not so intense that it induces fatigue. Research has shown that the correct intensity is around 40% of maximal inspiratory pressure (MIP) (Volianitis et al, 1999). If the patient’s MIP is not known, it is possible to set the load based on the 30-repetition maximum load setting and the characteristics of the spring that loads the valve in the training device; assuming that the training load is 50% to 60% (for a 30-breath regimen), the load setting that corresponds to 40% can be estimated. Table 6.4 is a chart that can be used to look up the correct ‘warm-up’ setting based on the training setting. Once the correct load has been identified, two sets of 30 breaths with 1 minute of rest in between should be completed no more than 10 minutes before a training session.
It is worth noting that the influences of IMT and ‘warm-up’ appear to be additive (Lomax et al, 2011).
Stretching
The trunk and rib cage receive almost no attention when it comes to stretching; however, these areas include numerous muscles, their attachments and associated connective tissue (e.g., the rib cage) that can potentially be a site of great resistance to inhalation. Any resistance to thoracic expansion increases the work of breathing and the associated perception of breathing effort. At least one study has shown that a 4-week programme of thoracic stretching exercises resulted in significant improvements in chest wall expansion (circumferences at three levels) and functional residual capacity (Minoguchi et al, 2002); a number of stretching and mobilization exercises based upon this programme are described in Chapter 7.
INSPIRATORY MUSCLE ‘COOL-DOWN’
As we learned in Chapter 1, the inspiratory muscles are highly aerobic, with a large blood supply, making them ideal candidates for lactate consumption during recovery. Recent research suggests that breathing against a small inspiratory load (the lowest setting on most devices) immediately after exercise accelerates the clearance of lactate (Chiappa et al, 2008), though this is not a universal finding (Johnson et al, 2011). A whole-body active recovery takes around 5 minutes to speed up lactate clearance. In contrast, under conditions where inspiratory loading accelerates lactate clearance, it does so immediately exercise stops, and may also clear the lactate much more quickly (Chiappa et al, 2008). More importantly, when inspiratory loading is undertaken between two bouts of maximal cycling (a Wingate test), performance in the second cycle test has been shown to improve compared with when a passive recovery between the two cycle tests (Chiappa et al, 2009). Finally, research has also shown that trained inspiratory muscles are more effective consumers of lactate during both recovery and exercise (Brown et al, 2008, 2010).
References
Aagaard, P., Simonsen, E.B., Andersen, J.L., et al. Neural inhibition during maximal eccentric and concentric quadriceps contraction: effects of resistance training. J. Appl. Physiol. 2000;89:2249–2257.
ATS/ERS. ATS / ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002;166:518–624.
Aznar-Lain, S., Webster, A.L., Canete, S., et al. Effects of inspiratory muscle training on exercise capacity and spontaneous physical activity in elderly subjects: a randomized controlled pilot trial. Int. J. Sports Med. 2007;28:1025–1029.
Beckerman, M., Magadle, R., Weiner, M., et al. The effects of 1 year of specific inspiratory muscle training in patients with COPD. Chest. 2005;128:3177–3182.
Black, L.F., Hyatt, R.E. Maximal respiratory pressures: normal values and relationship to age and sex. Am. Rev. Respir. Dis. 1969;99:696–702.
Bosnak-Guclu, M., Arikan, H., Savci, S., et al. Effects of inspiratory muscle training in patients with heart failure. Respir. Med. 2011;105:1671–1681.
Brown, P.I., Sharpe, G.R., Johnson, M.A. Inspiratory muscle training reduces blood lactate concentration during volitional hyperpnoea. Eur. J. Appl. Physiol. 2008;104:111–117.
Brown, P.I., Sharpe, G.R., Johnson, M.A. Loading of trained inspiratory muscles speeds lactate recovery kinetics. Med. Sci. Sports Exerc. 2010;42:1103–1112.
Cader, S.A., de Souza Vale, R.G., Zamora, V.E., et al. Extubation process in bed-ridden elderly intensive care patients receiving inspiratory muscle training: a randomized clinical trial. Clinical Interventions in Aging. 2012;7:437–443.
Cahalin, L.P., Semigran, M.J., Dec, G.W. Inspiratory muscle training in patients with chronic heart failure awaiting cardiac transplantation: results of a pilot clinical trial. Phys. Ther. 1997;77:830–838.
Casali, C.C., Pereira, A.P., Martinez, J.A., et al. Effects of inspiratory muscle training on muscular and pulmonary function after bariatric surgery in obese patients. Obes. Surg. 2011;21:1389–1394.
Cavalheri, V., Camillo, C.A., Brunetto, A.F., et al. Effects of arm bracing posture on respiratory muscle strength and pulmonary function in patients with chronic obstructive pulmonary disease. Rev. Port. Pneumol. 2010;16:887–891.
Chatham, K., Ionescu, A.A., Nixon, L.S., et al. A short-term comparison of two methods of sputum expectoration in cystic fibrosis. Eur. Respir. J. 2004;23:435–439.
Chiappa, G.R., Roseguini, B.T., Alves, C.N., et al. Blood lactate during recovery from intense exercise: impact of inspiratory loading. Med. Sci. Sports Exerc. 2008;40:111–116.
Chiappa, G.R., Ribeiro, J.P., Alves, C.N., et al. Inspiratory resistive loading after all-out exercise improves subsequent performance. Eur. J. Appl. Physiol. 2009;106:297–303.
Copestake, A.J., McConnell, A.K. Inspiratory muscle training reduces exertional breathlessness in healthy elderly men and women. In: International Conference on Physical Activity and Health in the Elderly. Scotland: University of Sterling; 1995:150.
Correa, A.P., Ribeiro, J.P., Balzan, F.M., et al. Inspiratory muscle training in type 2 diabetes with inspiratory muscle weakness. Med. Sci. Sports Exerc. 2011;43:1135–1141.
Dall’Ago, P., Chiappa, G.R., Guths, H., et al. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J. Am. Coll. Cardiol. 2006;47:757–763.
de Jong, W., van Aalderen, W.M., Kraan, J., et al. Inspiratory muscle training in patients with cystic fibrosis. Respir. Med. 2001;95:31–36.
Demoule, A., Verin, E., Montcel, S.T., et al. Short-term training-dependent plasticity of the corticospinal diaphragm control in normal humans. Respir. Physiol. Neurobiol. 2008;160:172–180.
Dronkers, J., Veldman, A., Hoberg, E., et al. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: a randomized controlled pilot study. Clin. Rehabil. 2008;22:134–142.
Edwards, A.M., Maguire, G.P., Graham, D., et al. Four weeks of inspiratory muscle training improves self-paced walking performance in overweight and obese adults: a randomised controlled trial. J. Obes. 2012;2012:918202.
Enright, P.L., Kronmal, R.A., Manolio, T.A., et al. Respiratory muscle strength in the elderly. Correlates and reference values. Cardiovascular Health Study Research Group. Am. J. Respir. Crit. Care Med. 1994;149:430–438.
Enright, S., Chatham, K., Ionescu, A.A., et al. Inspiratory muscle training improves lung function and exercise capacity in adults with cystic fibrosis. Chest. 2004;126:405–411.
Fernandes, M., Cukier, A., Feltrim, M.I. Efficacy of diaphragmatic breathing in patients with chronic obstructive pulmonary disease. Chron. Respir. Dis. 2011;8:237–244.
Fregonezi, G.A., Resqueti, V.R., Guell, R., et al. Effects of 8-week, interval-based inspiratory muscle training and breathing retraining in patients with generalized myasthenia gravis. Chest. 2005;128:1524–1530.
Fry, D.K., Pfalzer, L.A., Chokshi, A.R., et al. Randomized control trial of effects of a 10-week inspiratory muscle training program on measures of pulmonary function in persons with multiple sclerosis. J. Neurol. Phys. Ther. 2007;31:162–172.
Geddes, E.L., O’Brien, K., Reid, W.D., et al. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: An update of a systematic review. Respir. Med. 2008;102:1715–1729.
Goldstein, R., De Rosie, J., Long, S., et al. Applicability of a threshold loading device for inspiratory muscle testing and training in patients with COPD. Chest. 1989;96:564–571.
Gosselink, R.A., Wagenaar, R.C., Rijswijk, H., et al. Diaphragmatic breathing reduces efficiency of breathing in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1995;151:1136–1142.
Gosselink, R., De Vos, J., van den Heuvel, S.P., et al. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur. Respir. J. 2011;37:416–425.
Griffiths, L.A., McConnell, A.K. The influence of inspiratory and expiratory muscle training upon rowing performance. Eur. J. Appl. Physiol. 2007;99:457–466.
Griffiths, L.A., McConnell, A.K. The influence of rowing-related postures upon respiratory muscle pressure and flow generating capacity. Eur. J. Appl. Physiol. 2012. [Apr 24 [Epub ahead of print]].
Hamilton, A.L., Killian, K.J., Summers, E., et al. Symptom intensity and subjective limitation to exercise in patients with cardiorespiratory disorders. Chest. 1996;110:1255–1263.
Hart, N., Hawkins, P., Hamnegard, C.H., et al. A novel clinical test of respiratory muscle endurance. Eur. Respir. J. 2002;19:232–239.
Hawkes, E.Z., Nowicky, A.V., McConnell, A.K. Diaphragm and intercostal surface EMG and muscle performance after acute inspiratory muscle loading. Respir. Physiol. Neurobiol. 2007;155:213–219.
Heritier, F., Rahm, F., Pasche, P., et al. Sniff nasal inspiratory pressure. A noninvasive assessment of inspiratory muscle strength. Am. J. Respir. Crit. Care Med. 1994;150:1678–1683.
Hill, K., Jenkins, S.C., Philippe, D.L., et al. Comparison of incremental and constant load tests of inspiratory muscle endurance in COPD. Eur. Respir. J. 2007;30:479–486.
Hull, J., Aniapravan, R., Chan, E., et al. British Thoracic Society guideline for respiratory management of children with neuromuscular weakness. Thorax. 2012;67(Suppl. 1):i1–40.
Hulzebos, E.H., Helders, P.J., Favie, N.J., et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. J. Am. Med. Assoc. 2006;296:1851–1857.
Hulzebos, E.H., van Meeteren, N.L., van den Buijs, B.J., et al. Feasibility of preoperative inspiratory muscle training in patients undergoing coronary artery bypass surgery with a high risk of postoperative pulmonary complications: a randomized controlled pilot study. Clin. Rehabil. 2006;20:949–959.
Hulzebos, E.H., Smit, Y., Helders, P.P., van Meeteren, N.L. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst Rev. 11, 2012.
Inzelberg, R., Peleg, N., Nisipeanu, P., et al. Inspiratory muscle training and the perception of dyspnea in Parkinson’s disease. Can. J. Neurol. Sci. 2005;32:213–217.
Johnson, P.H., Cowley, A.J., Kinnear, W.J. A randomized controlled trial of inspiratory muscle training in stable chronic heart failure. Eur. Heart J. 1998;19:1249–1253.
Johnson, M.A., Mills, D.E., Brown, D.M., et al. Inspiratory loading intensity does not influence lactate clearance during recovery. Med. Sci. Sports Exerc. 2011;44(5):863–871.
Koessler, W., Wanke, T., Winkler, G., et al. 2 years’ experience with inspiratory muscle training in patients with neuromuscular disorders. Chest. 2001;120:765–769.
Koulouris, N., Mulvey, D.A., Laroche, C.M., et al. The effect of posture and abdominal binding on respiratory pressures. Eur. Respir. J. 1989;2:961–965.
Laoutaris, I., Dritsas, A., Brown, M.D., et al. Inspiratory muscle training using an incremental endurance test alleviates dyspnea and improves functional status in patients with chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2004;11:489–496.
Leith, D.E., Bradley, M. Ventilatory muscle strength and endurance training. J. Appl. Physiol. 1976;41:508–516.
Lin, S.J., McElfresh, J., Hall, B., et al. Inspiratory muscle training in patients with heart failure: a systematic review. Cardiopulm. Phys. Ther. J. 2012;23:29–36.
Lomax, M., McConnell, A.K. Influence of prior activity (warm-up) and inspiratory muscle training upon between- and within-day reliability of maximal inspiratory pressure measurement. Respiration. 2009;78:197–202.
Lomax, M., Grant, I., Corbett, J. Inspiratory muscle warm-up and inspiratory muscle training: separate and combined effects on intermittent running to exhaustion. J. Sports Sci. 2011;29:563–569.
Lotters, F., van Tol, B., Kwakkel, G., et al. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur. Respir. J. 2002;20:570–576.
Magadle, R., Bererar-Yanay, N., Weiner, P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002;121:329–333.
Magadle, R., McConnell, A.K., Beckerman, M., et al. Inspiratory muscle training in pulmonary rehabilitation program in COPD patients. Respir. Med. 2007;101:1500–1505.
Martinez, A., Lisboa, C., Jalil, J., et al. Selective training of respiratory muscles in patients with chronic heart failure. Rev. Med. Chil. 2001;129:133–139.
McConnell, A.K. Breathe strong, perform better. Champaign IL: Human Kinetics Publishers; 2011.
McConnell, A.K., Copestake, A.J. Maximum static respiratory pressures in healthy elderly men and women: issues of reproducibility and interpretation. Respiration. 1999;66:251–258.
McConnell, A.K., Griffiths, L.A. Acute cardiorespiratory responses to inspiratory pressure threshold loading. Med. Sci. Sports Exerc. 2010;42:1696–1703.
McConnell, A.K., Caine, M.P., Donovan, K.J., et al. Inspiratory muscle training improves lung function and reduces exertional dyspnoea in mild / moderate asthmatics. Clin. Sci. 1998;95:4.
McConnell, A.K., Romer, L.M., Weiner, P. Inspiratory muscle training in obstructive lung disease; how to implement and what to expect. Breathe. 2005;2:38–49.
Minoguchi, H., Shibuya, M., Miyagawa, T., et al. Cross-over comparison between respiratory muscle stretch gymnastics and inspiratory muscle training. Intern. Med. 2002;41:805–812.
Moodie, L., Reeve, J., Elkins, M. Inspiratory muscle training increases inspiratory muscle strength in patients weaning from mechanical ventilation: a systematic review. Journal of Physiotherapy. 2011;57:213–221.
O’Brien, K., Geddes, E.L., Reid, W.D., et al. Inspiratory muscle training compared with other rehabilitation interventions in chronic obstructive pulmonary disease: a systematic review update. Journal of Cardiopulmonary Rehabilitation and Prevention. 2008;28:128–141.
Padula, C.A., Yeaw, E., Mistry, S. A home-based nurse-coached inspiratory muscle training intervention in heart failure. Appl. Nurs. Res. 2009;22:18–25.
Padulo, J., Mignogna, P., Mignardi, S., et al. Effect of different pushing speeds on bench press. Int. J. Sports Med. 2012;33:376–380.
Paulin, E., Yamaguti, W.P., Chammas, M.C., et al. Influence of diaphragmatic mobility on exercise tolerance and dyspnea in patients with COPD. Respir. Med. 2007;101:2113–2118.
Plentz, R.D., Sbruzzi, G., Ribeiro, R.A., et al. Inspiratory muscle training in patients with heart failure: meta-analysis of randomized trials. Arq. Bras. Cardiol. 2012;99:762–771.
Rahn, H., Otis, A.B., Chadwick, L.E., et al. The pressure–volume diagram of the thorax and lung. Am. J. Physiol. 1946;146:161–178.
Romer, L.M., McConnell, A.K. Specificity and reversibility of inspiratory muscle training. Med. Sci. Sports Exerc. 2003;35:237–244.
Ross, E.Z., Nowicky, A.V., McConnell, A.K. Influence of acute inspiratory loading upon diaphragm motor-evoked potentials in healthy humans. J. Appl. Physiol. 2007;102:1883–1890.
Savci, S., Degirmenci, B., Saglam, M., et al. Short-term effects of inspiratory muscle training in coronary artery bypass graft surgery: A randomized controlled trial. Scand. Cardiovasc. J. 2011;45:286–293.
Sawyer, E.H., Clanton, T.L. Improved pulmonary function and exercise tolerance with inspiratory muscle conditioning in children with cystic fibrosis. Chest. 1993;104:1490–1497.
Shoemaker, M.J., Donker, S., Lapoe, A. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: the state of the evidence. Cardiopulmonary Physical Therapy Journal. 2009;20:5–15.
Steier, J., Kaul, S., Seymour, J., et al. The value of multiple tests of respiratory muscle strength. Thorax. 2007;62:975–980.
Sutbeyaz, S.T., Koseoglu, F., Inan, L., et al. Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: a randomized controlled trial. Clin. Rehabil. 2010;24:240–250.
Thomas, M.J., Simpson, J., Riley, R., et al. The impact of home-based physiotherapy interventions on breathlessness during activities of daily living in severe COPD: a systematic review. Physiotherapy. 2010;96:108–119.
Toigo, M., Boutellier, U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur. J. Appl. Physiol. 2006;97:643–663.
Tong, T.K., Fu, F.H., Eston, R., et al. Chronic and acute inspiratory muscle loading augment the effect of a 6-week interval program on tolerance of high-intensity intermittent bouts of running. J. Strength Cond. Res. 2010;24(11):3041–3048.
Topin, N., Matecki, S., Le Bris, S., et al. Dose-dependent effect of individualized respiratory muscle training in children with Duchenne muscular dystrophy. Neuromuscul. Disord. 2002;12:576–583.
Tsao, H., Hodges, P.W. Immediate changes in feedforward postural adjustments following voluntary motor training. Exp. Brain Res. 2007;181:537–546.
Turner, L.A., Mickleborough, T.D., McConnell, A.K., et al. Effect of inspiratory muscle training on exercise tolerance in asthmatic individuals. Med. Sci. Sports Exerc. 2011;43:2031–2038.
Unal, O., Arslan, H., Uzun, K., et al. Evaluation of diaphragmatic movement with MR fluoroscopy in chronic obstructive pulmonary disease. Clin. Imaging. 2000;24:347–350.
Van Houtte, S., Vanlandewijck, Y., Gosselink, R. Respiratory muscle training in persons with spinal cord injury: a systematic review. Respir. Med. 2006;100:1886–1895.
Volianitis, S., McConnell, A.K., Koutedakis, Y., et al. The influence of prior activity upon inspiratory muscle strength in rowers and non-rowers. Int. J. Sports Med. 1999;20:542–547.
Volianitis, S., McConnell, A.K., Jones, D.A. Assessment of maximum inspiratory pressure. Prior submaximal respiratory muscle activity (‘warm-up’) enhances maximum inspiratory activity and attenuates the learning effect of repeated measurement. Respiration. 2001;68:22–27.
Volianitis, S., McConnell, A.K., Koutedakis, Y., et al. Specific respiratory warm-up improves rowing performance and exertional dyspnea. Med. Sci. Sports Exerc. 2001;33:1189–1193.
Weiner, P., Weiner, M. Inspiratory muscle training may increase peak inspiratory flow in chronic obstructive pulmonary disease. Respiration. 2006;73:151–156.
Weiner, P., Azgad, Y., Ganam, R., et al. Inspiratory muscle training in patients with bronchial asthma. Chest. 1992;102:1357–1361.
Weiner, P., Azgad, Y., Weiner, M., et al. Inspiratory muscle training during treatment with corticosteroids in humans. Chest. 1995;107:1041–1044.
Weiner, P., Ganem, R., Zamir, D., et al. Specific inspiratory muscle training in chronic hemodialysis. Harefuah. 1996;130(73–76):144.
Weiner, P., Gross, D., Meiner, Z., et al. Respiratory muscle training in patients with moderate to severe myasthenia gravis. Can. J. Neurol. Sci. 1998;25:236–241.
Weiner, P., Zeidan, F., Zamir, D., et al. Prophylactic inspiratory muscle training in patients undergoing coronary artery bypass graft. World J. Surg. 1998;22:427–431.
Weiner, P., Waizman, J., Magadle, R., et al. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin. Cardiol. 1999;22:727–732.
Weiner, P., Berar-Yanay, N., Davidovich, A., et al. The perception of dyspnoea in patients with asthma, before and following treatment with inhaled glucocorticosteroids. Respir. Med. 2000;94:161–165.
Weiner, P., Berar-Yanay, N., Davidovich, A., et al. Specific inspiratory muscle training in patients with mild asthma with high consumption of inhaled beta(2)-agonists. Chest. 2000;117:722–727.
Weiner, P., Magadle, R., Beckerman, M., et al. The relationship among inspiratory muscle strength, the perception of dyspnea and inhaled beta2-agonist use in patients with asthma. Can. Respir. J. 2002;9:307–312.
Weiner, P., Magadle, R., Massarwa, F., et al. Influence of gender and inspiratory muscle training on the perception of dyspnea in patients with asthma. Chest. 2002;122:197–201.
Weiner, P., Magadle, R., Beckerman, M., et al. Maintenance of inspiratory muscle training in COPD patients: one year follow-up. Eur. Respir. J. 2004;23:61–65.
West, C.R., Taylor, B.J., Campbell, I.G., et al. Effects of inspiratory muscle training in Paralympic athletes with cervical spinal cord injury. Med. Sci. Sports Exerc. 2009;41:S31–S32.
Winkler, G., Zifko, U., Nader, A., et al. Dose-dependent effects of inspiratory muscle training in neuromuscular disorders. Muscle Nerve. 2000;23:1257–1260.
Yamaguti, W.P., Claudino, R.C., Neto, A.P., et al. Diaphragmatic breathing training program improves abdominal motion during natural breathing in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2012;93:571–577.