Fatigue
Joanna M. Brell and Lee W. Jones
• Fatigue affects most patients with cancer, regardless of the stage of disease or the type of anticancer therapy used.
• Cancer-related fatigue (CFR) affects an estimated 80% to 90% of outpatients with cancer who receive either chemotherapy or radiation therapy.
• CRF can be persistent in cancer survivors.
• Determining the exact incidence requires standardized methods to define, diagnose, and measure CRF.
• Specific pathophysiological mechanisms are unknown at this time but undoubtedly are multifactorial.
• CRF may be caused by the malignancy and/or anticancer therapy.
• CRF is exacerbated by other conditions, some of which are controllable, such as anemia, hypothyroidism, and depression.
• CRF may affect compliance to and tolerance of anticancer therapies.
• CRF impairs function, self-care, ability to work, social relationships, and quality of life.
• No standardized measurement tools exist except for International Classification of Diseases–10 criteria, which have not been widely adopted.
• Using National Comprehensive Cancer Network guidelines, patients report levels of fatigue using a numeric rating scale of 0 to 10. This numeric rating scale is currently used as a screening tool.
• No Food and Drug Administration–approved interventions are currently available.
• All treatable factors of fatigue should be addressed.
• Patients reporting fatigue should be enrolled in clinical studies relating to CRF whenever possible.
• The best evidence to date for improved outcomes related to CRF involve cardiopulmonary fitness through exercise, but further research in this area is needed.
• Stimulants may help severely fatigued patients near the end of life.
Definition
Cancer-related fatigue (CRF) is experienced by most patients with cancer at any point from the time of diagnosis, during treatment, through survivorship, or at disease progression. The National Comprehensive Cancer Network (NCCN) guidelines consensus definition of CRF is a “distressing persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.”1 Fatigue with these characteristics does not appear to occur in the general population. In a large analysis of almost 35,000 patients, fatigue was measured with use of the Functional Assessment of Chronic Illness Therapy (FACIT) questionnaire and comparisons were made between anemic patients with cancer, nonanemic patients with cancer, and a cohort from the general population (with or without medical conditions) (N = 1010). The patients with cancer, regardless of their hemoglobin level, experienced significant fatigue (P > .001) compared with persons without cancer.2 CRF can negatively affect functional status, resulting in limitations in ambulation and exercise, oral intake, socialization with supportive friends and families, ability to maintain a job, and quality of life. Of critical importance, fatigue may prevent full doses of anticancer therapy from being delivered, which could potentially affect survival or tumor control.
Incidence
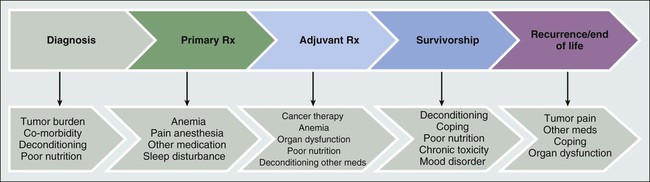
The exact incidence of CRF depends on when in the cancer trajectory it occurs and by what methods patient-reported experience is measured. It does not appear that any time exists on the cancer continuum when patients are not challenged by fatigue (see Fig. 45-1), and CRF occurs with all types of cancers and in all stages. Most likely CRF is underreported, because both patients and health care providers expect fatigue as an inevitable result of a malignancy diagnosis. Discussions between patients and physicians regarding fatigue may be limited, given that few efficacious interventions exist to improve fatigue.
In a review of 27 publications in which CRF was the primary outcome, the incidence ranged from 4% to 91%.3 This wide range probably is the result of significant variation in the patient populations studied, including type of malignancy, stage of disease, receipt of cancer therapy, the type of treatment modality received, and patient gender. In addition, these studies used various survey tools to record patients’ specific description of their fatigue. Currently, 14 validated scales exist to measure CRF, and each has a different construct for evaluating fatigue.4 In all, it has been estimated that 80% to 90% of outpatients with cancer who received either chemotherapy or radiation therapy report the presence of various levels of fatigue.5 Additionally, severe fatigue has been recorded in 75% of hospitalized patients on a palliative care unit.3 One population studied extensively has been breast cancer survivors. In a study of more than 750 breast cancer survivors, almost 20% of patients who were free of disease for 5 to 10 years after completing therapy had significant residual fatigue that impaired their ability to function.6 With new anticancer therapeutic agents causing or associated with fatigue, longer treatment periods, and an increasing number of survivors, CRF is currently and will continue to be a substantial clinical issue.
Etiology
The causes and contributing factors of fatigue are undoubtedly multifactorial, and thus its etiology is not well delineated. Understanding the causative factors is important for finding effective clinical therapies or preventative strategies for CRF. Patients with cancer experience multiple comorbid conditions, in addition to the malignancy itself, that can cause or exacerbate fatigue. This combination of factors may prevent or delay detection of the ultimate contributing disorders. Patients may acquire these medical conditions after receiving therapy or they may have these conditions at the time of presentation with cancer; the term “cancer-related” can encompass all of these factors, because the exact time of onset in the cancer trajectory may be unknown. Defining CRF is quite challenging because many patients with cancer present with fatigue before primary adjuvant therapy is begun. Regardless of the source, clinically recognizing separate causes of fatigue at presentation will help uncover treatable situations, such as anemia, hypothyroidism, depression, concomitant medications, and infection. Remediating potentially reversible conditions is the initial approach to CRF according to NCCN guidelines.1
The literature regarding possible CRF mechanisms contains a few small clinical trials and little preclinical work. The published data suggest multiple nonspecific interactions between cancer, the effects from cancer therapy, and the patients’ responses to both. Unfortunately, the consequences of these interactions have not been convincingly correlated to patient report of fatigue. As well, findings of putative mechanism-directed therapies have not been successful to date. One possible mechanism currently being studied, however, involves the inflammatory response to cancer and its therapy, especially cytokine activation. Clinical studies have revealed increases in interleukin-1 (IL-1) receptor antagonist and C-reactive protein in fatigued patients with cancer who are undergoing radiation therapy.7 CFR has also been correlated with tumor necrosis factor (sTNF-RII), immunoregulatory CD4 lymphocytes, and white blood cell subsets.8 Proinflammatory proteins have been noted in breast cancer survivors with no evidence of disease who have experienced fatigue 3 to 5 years after cancer therapy.9 The reasons why inflammatory responses are still maintained after treatment and resolution of the cancer have not been fully explained. In a study of a group of breast cancer survivors with or without fatigue, at least one cytokine polymorphism at IL-1β–511 (P = .007) was associated with reported fatigue.10
Regardless of the quality or quantity of the pathophysiology involved, patients consistently note multiple sensations that often occur not in isolation but in clusters of complaints. Given the interwoven nature of the facets of CRF, causative perturbations may exert a systemic effect on multiple organs and tissues to obtain the phenotype of fatigue. Clinically, this cluster appears similar to inflammatory-induced symptoms referred to as “sickness behavior” and may encompass depression, sleep disturbances, and cognitive dysfunction.11 A sickness behavior animal model induced by lipopolysaccharide injection has been associated with behavioral changes (e.g., listlessness, decreased oral intake, and sleep disturbances) and increased levels of circulating proinflammatory cytokines.12 Although this preclinical model has been widely used for CRF studies, a lack of valid preclinical models of fatigue remains. The discovery of new animal models of fatigue continues to be a challenging yet key aspect of CRF research.13 Because CFR is subjective, the patients’ description of fatigue (at the bedside) should guide the generation of hypotheses (at the bench) to begin to uncover the etiology or etiologies of CRF, even though some questions surrounding CRF mechanisms do not easily lend themselves to preclinical assessment. Modeling human situations such as lack of motivation to perform, comorbid conditions, and concomitant medications cannot yet be recapitulated. Valid preclinical models can help to accelerate an improved mechanistic understanding of fatigue. This understanding will allow for more precise interventions to be brought into clinical testing. Several grants and research projects are currently exploring inflammation as the primary driver of CRF.
Patients with cancer are often older and commonly present with a diverse range of age-related changes, similar to the general adult population, that limit cardiorespiratory fitness. However, these normal aging consequences are dramatically compounded by the consequences of anticancer therapies, tumor burden in advanced disease, comorbid conditions such as antecedent cardiac disease and pulmonary insufficiency, and decreased activity/deconditioning.14 These situations simultaneously affect the O2 cascade.
A growing body of evidence shows that patients with cancer have significant impairments in cardiorespiratory fitness.15 The data have been collected by methods using objective determination of cardiopulmonary function,16 thus providing reproducible, standardized information. International guidelines defining the proper conduct of cardiorespiratory fitness testing have also been developed.19–19 An incremental cardiopulmonary exercise test with gas exchange measurement to assess peak oxygen consumption (VO2peak) constitutes the gold standard for assessment of cardiorespiratory fitness. Although routine use of cardiopulmonary exercise testing is not feasible, it is a highly informative research tool to measure physiological processes that may deepen the understanding of CRF. One study reported that the mean VO2peak in 346 presurgical patients with non–small cell lung cancer was 25% to 44% below that for age- and sex-matched normative data for sedentary persons.20 As well, the mean VO2peak was 27% below that of age-matched sedentary healthy persons compared with patients with stages I to IV breast cancer, with the lowest mean VO2peak seen in women with metastatic cancer. Additionally, it was determined that a 40-year-old woman with breast cancer has a cardiopulmonary capacity similar to that of a healthy sedentary 70-year-old woman.21 It is not clear if these observed marked reductions in cardiorespiratory fitness are due to limitations in pulmonary, cardiovascular, or peripheral tissues. However, the impairment is probably multifactorial with no one single organ component of O2 transport/utilization yet implicated as being solely responsible.14 It may be that a combination of exercise plus pharmacologic and psychosocial interventions could be tested for their role in initiating and maintaining CFR.
If damage to some aspect of the cardiorespiratory system by cancer or its treatment were contributory, then it follows that either measure of the individual organ components and/or the total functional capacity of these organs (cardiorespiratory fitness) should be correlated with patient-reported fatigue. Jones et al.22 recently reported that in patients with newly diagnosed malignant glioma, prior to therapy, decreased peak VO2peak was correlated with patient-reported fatigue (measured by the FACIT fatigue scale). Also, measures of body composition such as lean body mass, as well as total muscle cross-sectional area by magnetic resonance imaging, were inversely correlated with reported fatigue. In a subsequent study, functional capacity, as measured by a 6-minute walk test, was inversely correlated with fatigue in 171 patients with recurrent malignant glioma.23 Similar findings have been reported in other cancer populations with different measures of cardiorespiratory fitness and functional capacity, including patients with advanced lung cancer24 and early-stage breast cancer.25 These studies support the correlation between CRF and diminished cardiorespiratory activity and strengthen the hypothesis that increasing physical function during or immediately after therapy (primarily through physical exercise) may have a significant effect on reducing CRF.
Evaluation
Fatigue is an experienced sensation. According to NCCN guidance, patients are asked to assess their current level of CRF on a 0 to 10 numeric rating scale (NRS) that is portioned as follows: 0, no fatigue; 1-3, mild fatigue; 4-7, moderate fatigue; and 8-10, severe fatigue.1 This NRS is a screening tool that has also been utilized as an eligibility criteria measurement for fatigue in patients prior to clinical trial enrollment. Based on the results of this screening tool, NCCN has published guidelines for evaluating patients with fatigue that includes reassessment for any changes in fatigue.1 The approach to CRF assessment is similar to the evaluation of pain, a common pathophysiological condition that is defined by patient report. Pain assessment is a high priority in cancer management and offers an opportunity to ask the patient questions regarding fatigue at the same time. Like the sensation of pain, the severity of CRF is influenced by physical and psychosocial factors. As some of these factors fluctuate, constant monitoring with changes in the management plan is essential for both conditions. “Early detection” and intervention for modifiable fatigue-associated conditions is a sound strategy and has clear potential for patient benefit.
To further define CRF and to highlight assessment of other conditions, the Fatigue Coalition has established the International Classification of Diseases–10 (ICD-10) criteria for cancer-related fatigue26 (see Box 45-1). These criteria include four classifications of symptoms (A, B, C, and D). Patients indicate the A1 symptom of significant fatigue, decreased energy, or increased need to rest without activity level change, plus six or more A-level symptoms that have been present every day or nearly every day in the same 2-week period during the past month. The symptoms the patient must have to fulfill the diagnosis for CRF by the ICD-10 criteria are common patient-reported sensations associated with fatigue, such as “…experience of sleep as unrefreshing or nonrestorative.” Additionally, some of the diagnostic criteria could be consistent with concomitant or causative conditions such as depression. Given the overlap of some of the qualities of CRF with mood and other psychiatric disorders and cognitive dysfunction, the extraction of differences is problematic, yet quite clinically significant.
Utilization of the ICD-10 list of criteria in its entirety has not been widely adopted. When applying the full set of criteria (A-D), the incidence of CRF dramatically decreases (2%), as opposed to 37% noted with only the A1 criteria, ten other A-level symptoms, and B-level criteria combined.27 Use of this assessment tool, however, can eliminate known conditions and improve diagnostic accuracy and clinical classification. A harmonious definition of CRF can refine the incidence and define populations/subpopulations for specific clinical trials and for routine clinical practice. The science of CRF will be aided by the development of accurate diagnostic tools with pertinent physiologic assessments and common outcome measures.
Measuring Fatigue
It has been reported that three one-dimensional (focus on physical functioning) patient-reported measures for fatigue have been validated in patients with cancer4: the Brief Fatigue Inventory (BFI),28 the Functional Assessment of Cancer Therapy-Fatigue,29 and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–C30.30,31 Although these tools are used frequently in placebo-controlled intervention clinical trials for CRF, their utility in the clinic has not been established. As well, there is not one agreed-upon measure used in the majority of trials that could allow for comparison between various interventions for fatigue, in part because of the difficulty in integrating the cognitive and emotional aspects of CRF with the physical and functional fatigue in a single validated measure. For specificity, these measures must be validated in the actual cancer population to be studied (e.g., patients with solid tumors who are receiving chemotherapy vs. postoperative immobilized patients without cancer). A future area of research should include devising outcome measures in CRF for clinical practice. Therefore applied research methodology is a critical part of the multidisciplinary approach required to ensure accurate measurement of the patient experience and to advance the science of CRF.
Therapy for CRF
Evidence-based treatments for CRF are scarce. Even after specific conditions for which known therapies exist are identified and properly managed, many patients with cancer still note fatigue. Various interventions have been studied, including pharmacologic agents for symptom palliation (i.e., psychostimulants) and those that target possible etiologies (i.e., serotonin reuptake inhibitors). The complete list of consensus interventions is found in the NCCN guidelines.1 Until improved evidence-based therapies are developed, the best intervention may be enrollment in a fatigue-ameliorating clinical trial when such a trial is available.
Pharmacologic
Finding U.S. Food and Drug Administration (FDA)–approved drug interventions to reduce established cancer-related fatigue has been restricted by the limited mechanism-based evidence to guide the development of rational interventions. As well, the published clinical research in CRF has been inconsistent because of the use of differing eligibility criteria and varied outcome measures. As a result of such heterogeneity, comparison between completed trials cannot be accurately performed. A metaanalysis regarding pharmacologic treatment for CRF, with the additional inclusion of raw data, was updated in 2010.32 A total of 31 randomized, controlled trials assessing fatigue in 7104 adult patients with cancer were included; these trials were chosen because of the use of strong fatigue outcome measures. Four classes of drugs were appraised, based on available data, and the results are outlined in Table 45-1.
Table 45-1
Summary of Drug Therapy for the Management of Cancer-Related Fatigue (Cochrane Database of Systematic Reviews)
| Drug Class (No. of Studies) | Most Common Design (No. of Studies) | Outcomes |
| Psychostimulants (5) | Methylphenidate vs. placebo (4) | Statistically significant for drug, but with small effect size in small trials |
| Antidepressants (3) | Paroxetine vs. placebo (2) | No improvement for drug |
| Progestational steroids (4) | Megestrol acetate vs. placebo (3) | No improvement with drug |
| Erythropoiesis-stimulating agents (15) | Erythropoietin (14) and darbepoetin (4) | Statistically significant for drug, but adverse effects prevent use |
Although it was recognized in the 2007 American Society of Clinical Oncology/American Society of Hematology Guidelines that erythropoiesis-stimulating agents were associated with an increased risk of thromboembolic events,33 later they were also implicated as being associated with decreased survival.34 The 2010 American Society of Clinical Oncology/American Society of Hematology Guidelines therefore state that erythropoiesis-stimulating agents are not indicated for anemic patients with cancer who are not undergoing anticancer therapy,35 representing an example of a promising CFR therapy that cannot be used because of serious adverse effects.
Because no interventions for CRF are currently approved by the FDA, other drugs continue to be investigated. Modafinil and its R-enantiomer armodafinil are considered nontraditional central nervous system stimulants or wakefulness-promoting agents. How they affect the processes involved in the wake/sleep cycle is still unclear; however, multiple effects on central neurotransmitters are observed.36 A blinded randomized controlled trial of modafinil versus placebo was conducted in 631 patients with cancer who noted fatigue of any severity during the first 10 days of the first cycle of chemotherapy.37 It was discovered that only the patients with severe fatigue (rated 7-10 on a scale of 0-10) had a statistically significant improvement in fatigue (P = .033), with this secondary outcome described as a small decrease in the BFI-3 for the modafinil group (score of 7.2; placebo score of 7.6). Other studies of modafinil and CRF are ongoing. Based on these results and other data in the literature, psychostimulants may provide some palliation to patients with severe functional limitations from advanced cancer-associated fatigue, but they do not appear to confer benefit on persons with mild to moderate fatigue, and their routine and long-term use in this population is currently not recommended.1
Nutritional supplements and what have been termed “natural products,” that is, obtainable agents with assumed lower toxicity profiles, have been empirically studied. To date, most of these trials are published in abstract form, are difficult to compare, have very small sample sizes, and are not randomized or controlled. Many are still under investigation (e.g., co-enzyme Q10); others do not appear to be beneficial (e.g., l-carnitine38) or show uncertain activity in early studies (e.g., American ginseng39 and guarana40). Further data together with a mechanistic rationale for the agents and randomized controlled trials will help delineate if any single pharmacologic intervention will help with this multifaceted condition.
Nonpharmacologic
The relationship of CRF to psychosocial stressors and mood disorders is not entirely understood. However, treatments for major depression do not alleviate CRF,41 and there is no known direct correlation between emotional distress and CRF,42 necessitating more research into any connection. A compilation of Cognitive Behavioral Therapies/Behavioral Therapy, Supportive Expressive Therapies, and Psychoeducational Therapies/Educational Therapy has been termed “Psychosocial Interventions” (NCCN1) for CRF. A metaanalysis published in 2009 that assessed psychosocial interventions found that patient education regarding CRF information, coping strategies, and energy conservation plans were of benefit for CRF.43 However, the content and scope of these interventions have not been established.
Although energy conservation and increased sleep quality seem to be intuitive recommendations for a fatigued patient with cancer, their degree of effectiveness is unclear. Sleep disturbances are a common cancer-related problem and are thought to be related to, although separate from, CRF.44 Multiple facets of sleep disturbances have been identified in patients with cancer; however, additional research remains to be performed.45 Although environmental and psychological factors interfering with sleep can be identified, the mechanism by which anticancer therapies can prevent restorative sleep still need to be delineated. Hypnotic agents may add untoward effects and diminish daytime alertness. Interventions such as yoga and massage may provide some symptomatic relief, and research in these areas continues.
Exercise
Consistent with a hypothesis of diminished muscle adenosine triphosphate production or utilization, the NCCN review concluded that moderate exercise is of benefit in persons with CRF.1 Several metaanalyses support NCCN recommendations for exercise as CRF therapy. One metaanalysis performed in 2008 evaluated a total of 28 randomized controlled trials (N = 2083) involving several types of exercise programs during and after anticancer therapy. The exercises yielded statistically significant improvements in fatigue compared with control interventions.46 Another metaanalysis of 44 studies involved 3254 persons with cancer who also varied in diagnosis, stage of treatment, and type of exercise intervention.47 Results indicated that cancer-related fatigue declined to a greater extent in patients undergoing exercise training compared with those receiving usual care. Given the presumed injury to the organs of the cardiopulmonary system by malignancy or cancer therapies, aerobic exercise training interventions that increase the reserve capacity of the entire system may be an effective strategy to mitigate or possibly prevent CRF.
One study involving exercise during cancer therapy investigated the effects of supervised aerobic training, resistance training, or usual care in 121 patients with prostate cancer undergoing androgen deprivation therapy.48 The primary end point was fatigue as measured by the FACIT fatigue scale. Repeated measures indicated that both resistance and aerobic exercise significantly lessened the sensation of fatigue over the short term compared with usual care, and also improved VO2peak. Resistance training was also associated with longer term improvements in fatigue. Whether the favorable changes in cardiorespiratory fitness mediated the favorable changes in fatigue was not analyzed. In another study, postmenopausal patients with breast cancer who had undergone definitive adjuvant therapy at least a year earlier received either 15 weeks of supervised aerobic training or no training to assess fatigue and VO2peak.49 Fatigue was measured by the FACIT fatigue scale. A 9.3-point decrease in fatigue scores was observed in the exercise group compared with the control group. VO2peak also increased after training, whereas only minimal change occurred in the control group. A regression analysis indicated that the improvement in fatigue was associated with improvement in VO2peak.49 These studies provide further supporting evidence for the important role played by cardiorespiratory fitness as an underlying mechanism related to CRF.