Establishing and Maintaining Vascular Access
John C. Mansour and John E. Niederhuber
• Three questions to ask when selecting a catheter system are: (1) Which device best meets the patient’s therapy and lifestyle needs? (2) How is the device most safely inserted and maintained? (3) What are the potential immediate and long-term complication risks?
• Types of central access systems are the traditional central line for short-term use, tunneled central lines for long-term use, surgically implanted infusion ports, and peripherally inserted central catheters.
• Vascular access devices can be placed using a number of anatomic sites to access the superior vena cava or inferior vena cava: the subclavian vein, internal jugular vein, external jugular vein, and femoral vein.
• Insertion can be performed via the Seldinger (closed) technique or by operative exposure of the vein (open technique).
• Short-term complications include vascular laceration, arterial puncture, pneumothorax (2%), hemothorax, and air embolus (overall placement complications should be <5%).
• Long-term complications include catheter exit site or tract infection, catheter-associated sepsis, cardiac arrhythmias, catheter colonization, catheter thrombus (~30%), fibrin sheath, extravasation, occluded catheter, and shearing of the catheter.
• Factors increasing the risk of catheter-associated infection include prolonged duration of indwelling time, multiple-lumen catheters, femoral locations, non–catheter-related bacteremia (in a neutropenic patient), the number of times the system is accessed, difficult catheter placement, and poor technique in catheter or port-site care.
Choosing the Right Device
Surgically Tunneled Central Lines
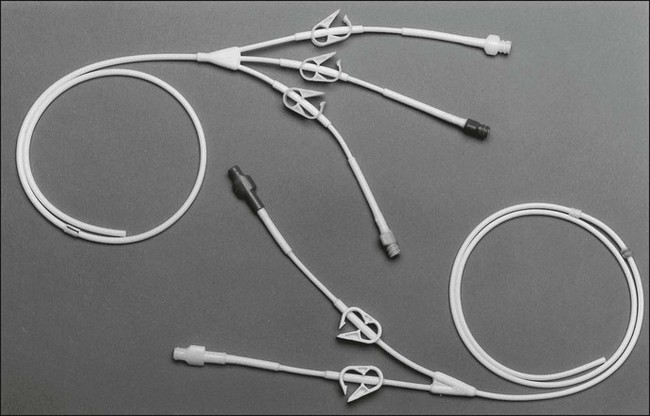
Most patients with cancer need a long-term form of central venous access rather than a traditional short-term central line. Surgically tunneled central lines were developed to increase the distance between the skin entrance site and the puncture of the vein. The hypothesis was that by increasing this distance, the life span of the central access would be increased by decreasing the incidence of infection and thrombosis. Tunneled central lines are commonly referred to by the name of the first brand that was marketed—Hickman. Other examples include Broviac, Quinton, and Groshong (Fig. 26-1). These polymeric silicone rubber venous access catheters are placed via a subcutaneous tunnel that is described in detail later in this chapter. Clinical studies have supported the hypothesis that increasing the distance between the catheter exit site in the skin and the hole in the vein decreases the incidence of externally derived infection.1,2 Studies suggest that the incidence of bloodstream infections associated with tunneled catheters is approximately 1 to 2 per 1000 catheter days.3 The frequency of bacteremia among nontunneled catheters has been reported at between 1.0 and 13.0 per 1000 catheter days.6–6
Surgically Implanted Infusion Ports
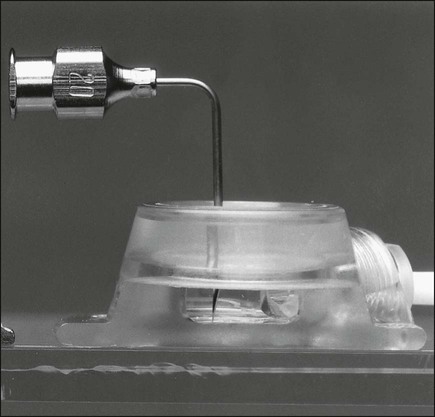
Implantable ports consist of a small injection reservoir with a self-sealing membrane placed entirely beneath the skin. There is no external catheter. An internal catheter runs from the reservoir into the subclavian or internal jugular vein to provide central access. The internal catheter is essentially identical to those used for tunneled central lines. A noncoring (Huber) needle can pass directly through the skin into the reservoir for infusion or aspiration. The self-sealing rubber cap on the reservoir prevents leakage from the reservoir after withdrawal of the Huber needle. The gauge of the noncoring needle—not the catheter—typically limits flow through the port system (Fig. 26-2). The ports can also be used for continuous drug infusion, as shown in Figure 26-3.
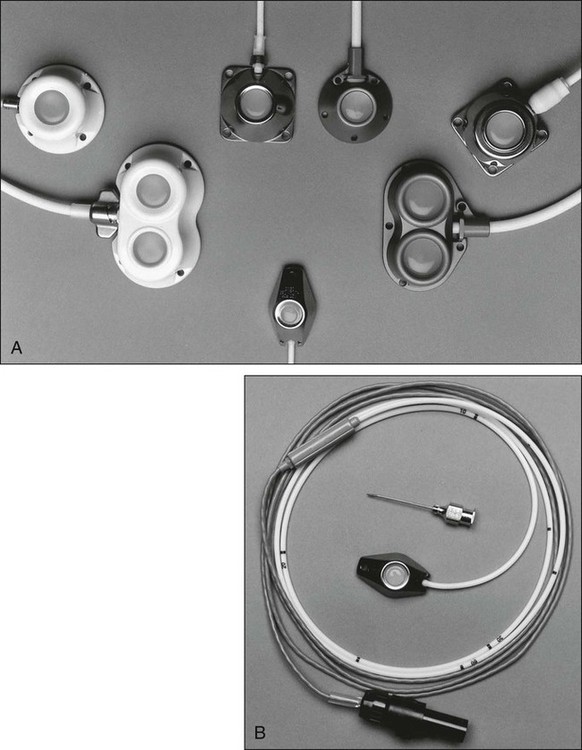
The theoretical concern that bacteria more easily traverse the short distance between the skin and the “neo-vein” or reservoir and cause increased rates of infection does not prove true.7 Sterile technique and site care lead to an infection risk comparable with that of tunneled catheters. With adequate care, the rate of infection could be fourfold to fivefold less than that for tunneled catheters.3 The low rate of infection and extravasation make infusion ports ideally suited for patients with cancer who need long-term single-lumen access and a low-maintenance catheter. However, experience has shown that patients with prolonged periods of neutropenia or significant risk of cutaneous eruptions might not be good candidates for port devices. The visible lump of the port could bother extremely thin patients; however, the port on the anterior chest wall is easily hidden from plain view for most people.
Long-Line Central Access
Also known as a peripherally inserted central catheter (PICC line), the long-line central access vascular access device is becoming increasingly popular. The catheter is inserted into a brachial, cephalic, or antecubital vein and advanced into the subclavian vein or higher. These lines can be placed simply and easily in the outpatient office setting and are well tolerated by patients, with minimal risk. However, many patients with cancer have poor-quality arm veins after undergoing multiple peripheral infusions and are therefore poor candidates for this technique. The 50% risk of catheter-related thrombosis is the greatest drawback to more widespread use of PICC lines in patients with cancer. This problem is a result of the presence of a long length of catheter within the vein and to the catheter tip in the relatively low-flow subclavian vein. Advancing the catheter tip into the SVC can reduce the incidence of thrombosis by more than half.8 Accurate placement of the catheter tip in the SVC can be complicated by the large displacement of the catheter tip (up to 8 cm) with normal arm range of motion.9
Inserting Vascular Access Devices
Providing patients who are undergoing treatment for cancer with appropriate vascular access requires not only a thorough knowledge of available devices but also technical expertise in the procedures for placing these catheters. Although these procedures are sometimes viewed as routine, the risks of poor technique can be devastating for the patient. Before undertaking the procedure, the physician should carefully consider everything from the site of insertion to the dressing to be used at the end of the procedure (Table 26-1).
Table 26-1
General Guidelines for Catheter and Port Use
| Type | Advantages | Uses | Dressings | Flushing | Comments |
| Central line | Placed at bedside | Hospital use only | Sterile transparent dressing | 5 mL heparinized saline solution (10 units/mL) in each lumen daily or after each use | Antibiotic ointment may promote resistance |
| Easy removal | |||||
| One or multiple lumens | Change dressing twice weekly | ||||
| Can change over wire | |||||
| PICC catheter | Safe to insert and remove | 1 wk to 6 mo of IV access | Transparent antimicrobial foam dressing | 3 mL heparinized saline solution bid or after each use | Flush and draw blood slowly to avoid catheter migration |
| Change every 6 days | |||||
| Hickman catheter | Multiple sizes and lumens | Continuous infusion therapy | Newly placed: antimicrobial dressing for 7 to 10 days | 5 mL heparinized saline solution daily or after each use | Clean with chlorhexidine during dressing change |
| Tunneled under skin | Long-term access | ||||
| Gauze or transparent dressing changed 2 times per wk | After 4 wk may clean with antimicrobial soap | ||||
| Groshong catheter | Slit valve requires no heparin flushes | Single- or double-lumen catheter for long-term use | |||
| Smaller, more flexible catheter | |||||
| Port | Completely under skin | One lumen access and low maintenance | None | 5 mL heparinized saline solution daily or after each use | Clean with chlorhexidine before use, access with noncoring needle |
| Minimal care | 20 mL saline solution after blood draw | ||||
| Monthly flush with 5 mL heparinized saline solution (100 units/mL) | Use no needle >20 gauge to access; EMLA cream helpful |
Choosing Insertion Location
The most frequent site for insertion of vascular access devices in the oncology population is the subclavian vein. Clavicular fracture or a previous median sternotomy can alter the anatomy of this location. For a patient with such a history, an alternative site should be considered. Even for patients with standard venous anatomy, the acute angle at the confluence of the subclavian and internal jugular veins at the brachiocephalic vein can complicate the passing of the guide wire into the SVC. A higher incidence of pneumothorax, hemothorax, and catheter malposition occurs among inexperienced operators, and a higher incidence of vein stenosis occurs with subclavian vein placement than with internal jugular placement.10 A subclavian artery puncture during line placement can be difficult to control because of the position of the artery posterior to the clavicle. A catheter placed on the anterior chest wall is more comfortable and easier to cover with clothing, however, than is a line in the neck.
Preparing to Place the Vascular Access Device
Studies suggest that providing a single dose of prophylactic antibiotic to cover common skin flora before inserting the vascular access device reduces central line infection. However, it is difficult to determine how this small benefit affects antibiotic resistance and subsequent infections. Use of central venous catheters impregnated with antibiotics could be more effective in dealing with infectious complications and will be discussed later in this chapter.11
A sterile surgical field with mask, gown, cap, gloves, and a large sterile drape should be used to minimize the risk of line infection.12 The skin of the entire anterior neck and chest should be prepared with chlorhexidine, which is superior to povidone-iodine or alcohol in limiting line infections.13,14
The choice to use ultrasound guidance to identify the vein during cannulation should be addressed before beginning the procedure. Less-experienced operators will likely benefit from the use of ultrasound guidance as an adjunct to the anatomic landmarks technique.15,16 Ultrasound can decrease the incidence of arterial puncture and placement failure. Although a few reports exist in the literature regarding the advantage of these techniques, such superiority is often judged when compared with high rates of complications using the landmark approach as the control group.
The operator must also decide where to position the catheter tip within the central vein. Catheters positioned with the tip in the right atrium will function longer as a source for aspirating blood samples than will those with the tip positioned in the SVC.17,18 A case review of thrombosed catheters documents that the position of the tip of the catheter at the time of thrombosis seems to be the most important contributing factor.19,20 The closer the catheter tip is to the right atrium, the lower is the frequency of thrombosis and infection. The risk of a catheter tip in the right atrial position is primarily that of cardiac arrhythmias when the tip is near the tricuspid valve. An additional risk of right atrial catheter placement is right atrial thrombus or right atrial erosion.20 These risks have led the U.S. Food and Drug Administration to publicly warn operators to avoid placement of the catheter tip within the right atrium. Instead, the catheter tip should sit at the junction of the SVC and the right atrium.
Insertion Technique
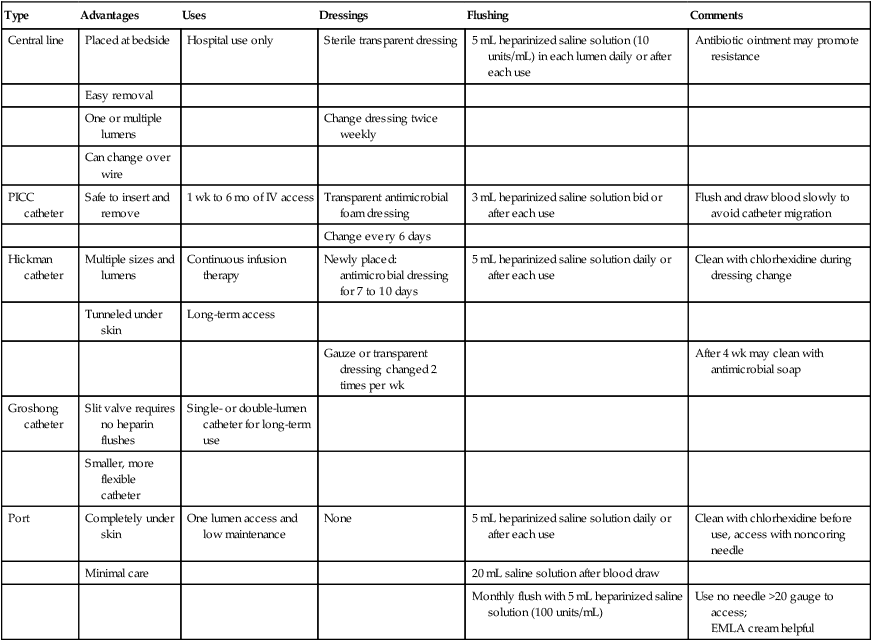
After informed consent is obtained, a rolled towel is placed directly under the vertebral column at the shoulders to extend the clavicles. A peripheral line is established, and the patient is connected to an electrocardiogram monitor and a pulse oximeter. Intravenous sedation is typically established by using small doses of benzodiazepines. The fluoroscopy operating table and the patient are placed in the Trendelenburg position. The skin of the neck and the entire upper anterior thorax is prepared, and sterile drapes are positioned. The skin and deep tissues are anesthetized with 1% lidocaine using a 25-gauge needle for the skin followed by a 22-gauge needle for the anticipated insertion tract (Fig. 26-4, A).
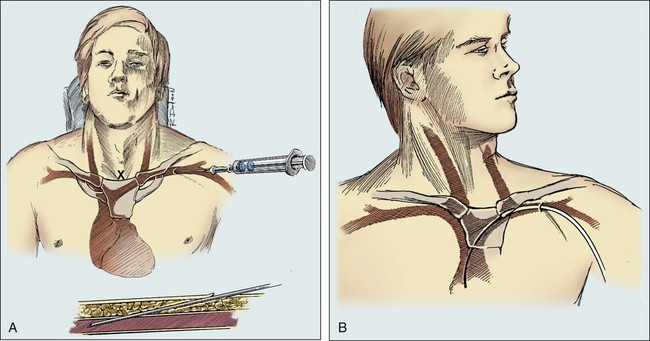
For subclavian vein puncture, the site of skin puncture is usually 1 cm below the angle of the lateral third of the clavicle. The long insertion needle, as depicted in Figure 26-4, A, is slowly inserted below the clavicle, aiming for a point approximately one fingerbreadth above the sternal notch. During insertion, a small amount of negative pressure is maintained on the syringe. With experience, the physician develops a feel for the actual puncture of the vein, and when this occurs, the syringe fills easily with venous blood.
As the position of the needle is being carefully maintained, the syringe is removed, and the guide wire is inserted (Fig. 26-4, B). If the patient experiences discomfort in the neck, the guide wire is partially withdrawn. Turning the patient’s head away from the site of insertion and exerting a gentle downward pull on the ipsilateral arm may facilitate entrance of the catheter or guide wire into the SVC. Minimizing extreme or sudden neck and arm movements can limit the risk of injury to the punctured vein. Cardiac ectopy indicates that the guide wire has entered the right atrium, and the wire should be withdrawn slightly.
When it is necessary to use the veins in the neck, the right internal jugular vein provides more direct access to the SVC and right atrium. In this case, the patient’s head is turned to the opposite side. The insertion site is located just lateral to the carotid artery and approximately two fingerbreadths above the head of the clavicle. Another useful landmark for the insertion site is the angle formed by the sternal and clavicular heads of the sternocleidomastoid muscle (Fig. 26-5).
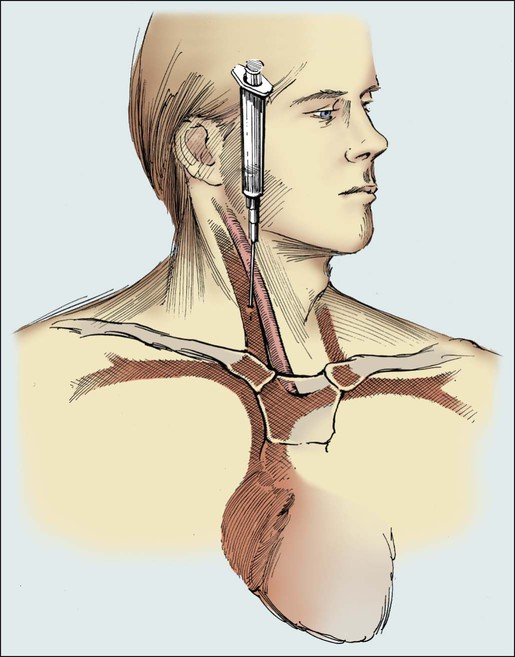
Placement of a permanent Silastic catheter, such as a Hickman catheter, is depicted in Figure 26-6. Placement of the introducing needle and guide wire is as described in previous figures except that a 1-cm incision is made before insertion of the introduction needle. A prophylactic antibiotic is administered before the procedure. It is often beneficial to provide the patient with intravenous sedation.
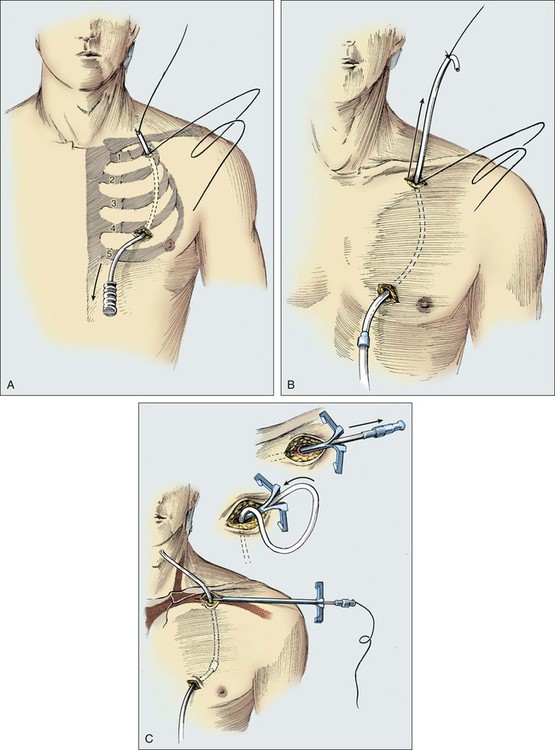
Care is taken to cut the catheter squarely and smoothly. An introducer and a tear-away sheath are passed over the guide wire into the vein (Fig. 26-7). A slight rotary motion facilitates the introduction of the sheath and avoids crimping. To avoid developing a false passage, the guide wire should be withdrawn occasionally as the introducer is inserted. The guide wire is then removed, and a syringe is attached to the introducer to confirm that blood can be aspirated. The introducer is removed, and the thumb is used to control bleeding or air intake through the sheath. It is important to have the catheter tip poised to be inserted as the introducer is removed. The passage of the catheter could meet minimal resistance as it is passed between the clavicle and first rib.
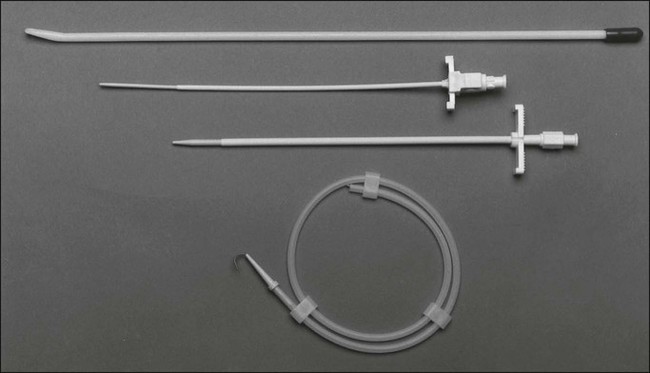
Figure 26-8 illustrates the placement of an implanted injection port. A 1-cm incision is placed below the clavicle at the site planned for subclavian venipuncture. A second, 3-cm incision is placed lower on the chest in a position that provides a relatively flat surface and stability for the port chamber. Local anesthesia is provided via 1% lidocaine with 1:200,000 epinephrine. A subcutaneous pocket just large enough to accommodate the port is dissected inferior to the incision. Ideally, the level of this pocket is over the underlying pectoral fascia. The skin coverage needs to be thick, but the port must be percutaneously accessible. A tunneler is passed subcutaneously from the pocket through the infraclavicular incision. A suture is tied to the tunneler and brought down through the tract. The suture is tied to the end of the port catheter and used to pull the catheter through the tract to the intraclavicular incision. Care should be taken to position with a gentle curve and to avoid catheter angulation. The port is secured in the pocket with three sutures of 0 Prolene. It is necessary to anchor the port adequately to prevent flipping or rotation of the device.
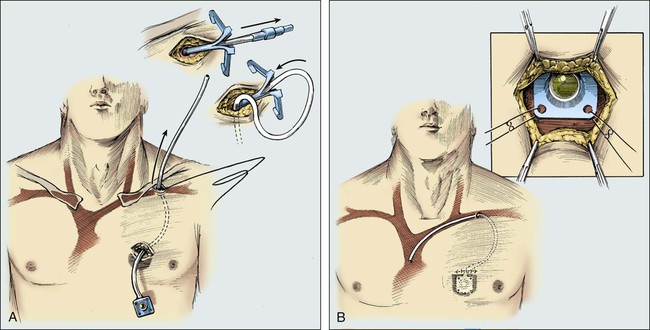
Open insertion methods are quite safe in a skilled surgeon’s hands. The major complication of the open technique is the possibility of air embolus during the actual catheter insertion. This complication is most likely to occur in patients who are hypovolemic, cachectic, or unable to tolerate positioning in the Trendelenburg position. An air embolus happens most frequently when the internal jugular vein is used; however, extreme care with use of the purse-string suture around the insertion site and venous occlusion with vascular clamps should limit the possibility of the introduction of air into the vascular system. With open direct surgical placement—although it takes considerably longer than the closed Seldinger technique—complications should be much lower than 5%.21 The open method results in a complication rate of less than 1%. Although the open method is a safer technique, it requires more training, more experienced operative personnel, and larger incisions. The open method should be used with any patient who has had repeated problems with closed insertions because the open method is the most controlled and safest format for that patient. Sometimes previous operations or radiation therapy in the region of the cardinal veins makes the open operative approach more difficult, but generally such problems are limited.
Complications of Vascular Access Devices
When complication rates are examined as a whole, certain patient factors, catheter factors, and operator factors seem to predict the occurrence of complications. Patient-related factors predicting higher complication rates include the presence of multiple comorbid conditions, atherosclerosis, abnormal anatomy, thrombocytopenia, immunocompromise, prior radiation therapy at the insertion area, recent myocardial infarction, and patient restlessness.22 In addition, multiple-lumen or stiffer catheters carry increased risks of complications.23–26 Factors related to the person performing the insertion of the catheter also influence the risk of complications. Risk increases if the operator has inserted fewer than 50 central venous catheters, if more than two tries at cannulating the vein are required, or if the insertion of the catheter is difficult.9,27 Risk factors for specific complications are discussed in the sections that follow.
Immediate Complications
Pneumothorax
Published complication rates for pneumothorax after jugular vein central line placement are approximately 0.5%, and they are up to four times higher for subclavian vein procedures.9,23,24,28 The risk of this technical complication can be reduced dramatically among experienced operators or among physicians who have experienced supervisors.22
Bleeding
Many patients with cancer are at increased risk of bleeding complications because of thrombocytopenia, uremia, other platelet dysfunction, or anticoagulant therapy. Bleeding complications are associated most frequently with thrombocytopenia.25 For patients with platelet counts less than 50 or an International Normalized Ratio greater than 2.0, we consider administration of platelets or fresh-frozen plasma. Experienced physicians should perform these procedures with access to ultrasound guidance if necessary.
Cardiac Arrhythmias
Disruptions in the normal cardiac conduction pathway can be caused by contact between the catheter and the right atrium. Most of these arrhythmias are short-lived and self-limited.29 These problems are more common with insertion of pulmonary artery catheters than with catheters that are typically inserted for oncologic vascular access. Patients who have recently had a myocardial infarction or who have a history of left bundle branch block are more prone to significant sequelae from the arrhythmias.26,29
Delayed Complications
Infectious Complications
Infectious complications are the most common complications of long-term vascular access devices in the oncology patient population. Two factors make the interpretation of this relatively well-studied topic challenging. First, many of the large randomized controlled trials that have studied central line infections have concentrated on patients in the ICU. The ICU population has different risk factors and susceptibilities from those of the outpatient population of people with cancer. Nevertheless, by carefully reviewing the available data, we can make some conclusions regarding the pathogenesis, prevention, and treatment of line-related infections among patients with cancer. In studies of patients in the ICU with central lines, factors that predispose to line infection have included malignancy, neutropenia, extended duration of indwelling time, and coincident parenteral nutrition. All of these factors can contribute to the incidence of infection among patients with vascular access for oncologic treatment.30
Understanding the pathophysiology of catheter-associated bacteremia can help limit the incidence of this complication. Up to 50% of catheter-associated bacteremia is caused by coagulase-negative staphylococcus. This common skin flora can colonize the catheter during insertion or later. A thrombus that forms at or along the catheter tip can become a nidus for bacterial proliferation, with resultant bacteremia. Thrombosis significantly increases the risks of colonization and infection.23 Bacteria can also be introduced into the bloodstream by hub contamination, by hematogenous seeding from another focus of infection, and, rarely, by the infusate itself.
Factors increasing the risk of catheter infection include prolonged indwelling time, multiple-lumen catheters, femoral vein location, difficult catheter placement, and non–catheter-related bacteremia.30,31 As was mentioned previously, nontunneled catheters are at increased risk of catheter infection compared with tunneled catheters, and totally implantable devices are even less susceptible than are tunneled catheters.32
Multiple-lumen catheters have demonstrated higher infection rates than single-lumen catheters. The addition of each lumen exponentially increases the incidence of infection.33,34 There are two possible explanations for this observation. First, the increased internal lumen diameter of the line is associated with a higher thrombosis rate and accumulation of loose thrombus at the catheter tip. Thrombosis causes more breaking of the line and more line manipulation when declotting is attempted, leading to subsequent infection. Second, the multiple ports invite multiple interruptions of the line for access and result in a greater likelihood of the introduction of bacteria. Despite their greater risk of iatrogenic infection, multiple-lumen catheters have great appeal for patients who need multiple simultaneous infusions of incompatible drugs. Very strict nursing guidelines must be followed in the management of multiple-lumen catheters to prevent iatrogenic infections and thrombosis.35
Diagnosis and management of catheter-related infection differ between nontunneled and tunneled catheters. However, some diagnostic principles apply to both tunneled and nontunneled catheters. Routine surveillance blood cultures should not be performed. When a catheter-related infection is suspected because of fever, chills, or purulence around the catheter site, percutaneous and catheter blood samples should be submitted for culture.36,37 Qualitative culture with continuously monitored differential time to positivity compares the time to positivity for catheter blood cultures with percutaneous peripheral blood cultures. This technique has demonstrated excellent specificity and sensitivity for detecting catheter-related infection in tunneled catheters.38
In most patients with nontunneled central venous catheters, the line should be removed if the patient demonstrates signs of site infection or sepsis or if blood culture results from the catheter and percutaneous blood samples are positive.38 Seven to 10 days of narrow-spectrum antibiotic therapy is generally recommended. In selected cases of clinically stable patients with a single episode of coagulase-negative staphylococcus, a trial of antibiotic therapy might salvage the catheter.34 For any patient with persistent bacteremia despite antibiotic therapy and removal of the infected catheter, a thorough investigation of possible septic sources, such as endocarditis or septic thrombus, is warranted.
Tunneled catheters or infusion ports should be removed in cases of sepsis, complicated infections, tunnel tract infections, or port abscesses.39 A thorough evaluation confirming the surgically implanted catheter as the source of infection should precede the removal of any of these vascular access devices. In the absence of complicated infection, catheter salvage could be indicated. Antibiotic lock therapy is a reasonable approach for attempting to salvage lines with common coagulase-negative staphylococcus, Staphylococcus aureus, or gram-negative bacilli intraluminal infections. This therapy consists of instilling the catheter lumen with high concentrations of antibiotics and leaving them there for several days.40 If salvage therapy fails, the infected catheter should be removed. A new tunneled catheter can be placed after treatment with an appropriate course of antimicrobial therapy until blood cultures are negative.
Catheter Thrombus
The incidence of central venous catheter–related thrombus as demonstrated by ultrasound may be up to 30% for catheters in place longer than 7 days.41 This problem typically presents as progressive difficulty in flushing the catheter. A change in posture or the Valsalva maneuver could allow aspiration of blood. Occasionally, thrombosis can manifest as extremity edema, which can be especially devastating after axillary nodal resection or axillary irradiation. Management of a nonfunctioning catheter is discussed later in this chapter.
Acute line obstruction can also be caused by precipitation of incompatible medications. Common offenders include total parenteral nutrition, etoposide salts, lipid emulsions, calcium salts, antibiotics, and sodium bicarbonate. Infusion of a solution specifically matched to the precipitated material might flush the line.42
Extravasation
Extravasation is defined here as the leaking of infusate into the subcutaneous tissue surrounding a central venous catheter. This complication can be caused by needle displacement from an implanted port, a defect in the catheter tubing, or withdrawal of the catheter from the vein due to inadequate fixation. Additionally, “backtracking” can occur when the catheter is partially occluded and infusate tracks up along the fibrin sleeve and into the subcutaneous tissue. Findings that are suggestive of extravasation include sudden swelling at the line site, increased patient discomfort during infusion, and sudden loss of blood return.43
Clavicular–First Rib Compression
Clavicular–first rib compression is an infrequently discussed complication that may occur up to 1% of the time in long-term indwelling catheters.44 When the subclavian vein is cannulated more medially than usual in the narrow space between the clavicle and first rib, the line can be compressed between the first rib and the clavicle. This compression should be suspected in patients who report difficulty infusing while in the sitting position or when the ipsilateral arm is elevated or abducted. This malposition can be observed on a chest radiograph as kinking of the line over the first rib. The catheter can break free and embolize if the line is not promptly removed. An interventional radiologist using a percutaneous retrograde femoral catheterization approach may be able to retrieve an embolized section of catheter.
Management of Nonfunctioning Catheters
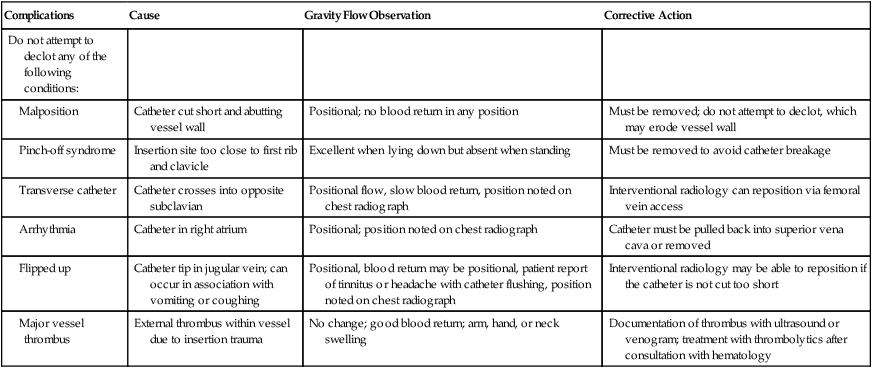
When a central venous catheter does not return blood or infuse solution, the malfunction should be assessed systematically to maximize catheter durability and to minimize the incidence of catheter-related complications. The first step in this assessment is reviewing the most recent chest radiograph to confirm appropriate placement. The next step is checking for gravity flow. Connecting a bag of normal saline solution to gravity flow and asking the patient to change position, cough, and breathe deeply will demonstrate whether flow is positional. If the catheter is patent to gravity flow, the bag should be lowered below the catheter and blood return should be assessed. With this information, the physician can make a decision about whether to remove, reposition, or declot the catheter (Tables 26-2 and 26-3).
Table 26-2
Management of a Suspected Clot
| Complications | Cause | Gravity Flow Observation | Corrective Action |
| Fibrin sheath* | Fibrin collects on the tip or encases the catheter | Excellent in all positions but no blood return in any position | May be declotted by following the declotting procedure |
| Occluded catheter* | Fibrin and platelets collect inside the catheter | Inability to infuse solutions and draw blood | May be declotted by following the declotting procedure; drug precipitate cannot be corrected with tissue plasminogen activator or heparin |
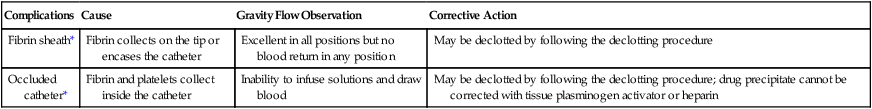
*The fibrin sheath and an occluded catheter are the only two instances in which it is safe to declot after checking the chest radiograph for proper placement.
Table 26-3
| Complications | Cause | Gravity Flow Observation | Corrective Action |
| Do not attempt to declot any of the following conditions: | |||
| Malposition | Catheter cut short and abutting vessel wall | Positional; no blood return in any position | Must be removed; do not attempt to declot, which may erode vessel wall |
| Pinch-off syndrome | Insertion site too close to first rib and clavicle | Excellent when lying down but absent when standing | Must be removed to avoid catheter breakage |
| Transverse catheter | Catheter crosses into opposite subclavian | Positional flow, slow blood return, position noted on chest radiograph | Interventional radiology can reposition via femoral vein access |
| Arrhythmia | Catheter in right atrium | Positional; position noted on chest radiograph | Catheter must be pulled back into superior vena cava or removed |
| Flipped up | Catheter tip in jugular vein; can occur in association with vomiting or coughing | Positional, blood return may be positional, patient report of tinnitus or headache with catheter flushing, position noted on chest radiograph | Interventional radiology may be able to reposition if the catheter is not cut too short |
| Major vessel thrombus | External thrombus within vessel due to insertion trauma | No change; good blood return; arm, hand, or neck swelling | Documentation of thrombus with ultrasound or venogram; treatment with thrombolytics after consultation with hematology |
Techniques for declotting catheters vary from institution to institution but follow the same general principles. We recommend instilling 500 µg of TPA and allowing the infusate to dwell for 1 hour. If patency is not restored after 1 hour, an additional milligram of TPA should be instilled and the infusate should be allowed to dwell for another hour. With use of a very similar technique, the authors of the Cardiovascular Thrombolytic to Open Occluded Lines Trial studied nearly 1000 patients with occluded central venous catheters. These patients in a predominantly oncology population did not undergo prethrombolysis contrast studies. No deaths or major bleeding episodes were directly attributable to the thrombolysis. More than 87% of the catheters were opened with the TPA. At 3-month follow-up, nearly 75% of the catheters were still patent.45 A similar study in the oncologic population reported a success rate greater than 80%.46
Vascular Access Device Maintenance
The risk of complications with a long-term vascular access device does not end with insertion of the device. Proper care and maintenance of each type of central catheter can dramatically reduce the incidence of catheter-related bacteremia, catheter thrombosis, and line failure.47,48 Maintenance is performed not only by qualified staff of oncology centers but also by patients and family members in the outpatient setting. In Table 26-1 and Box 26-1, we have detailed our recommendations for central catheter care after a thorough review of the best available literature.