Epidural and Spinal Analgesia /Anesthesia for Labor and Vaginal Delivery
Cynthia A. Wong MD
Chapter Outline
ANALGESIA/ANESTHESIA FOR VAGINAL DELIVERY
Epidural analgesia and spinal analgesia are the most effective methods of intrapartum pain relief in contemporary clinical practice.1,2 During the first stage of labor, pain results primarily from distention of the lower uterine segment and cervix (see Chapter 20). Painful impulses are transmitted by means of visceral afferent nerve fibers, which accompany sympathetic nerve fibers and enter the spinal cord at the 10th, 11th, and 12th thoracic and 1st lumbar spinal segments. As labor progresses and the fetus descends in the birth canal, distention of the vagina and perineum results in painful impulses that are transmitted via the pudendal nerve to the 2nd, 3rd, and 4th sacral spinal segments. Neuraxial analgesia is the only form of analgesia that provides complete analgesia for both stages of labor. During the first stage of labor, visceral pain impulses entering the spinal cord at T10 to L1 must be blocked. During the late first stage of labor and the second stage of labor, somatic impulses entering the spinal cord from S2 to S4 must also be blocked (see Chapter 20).
In a survey of 1000 consecutive women who chose a variety of analgesic techniques for labor and vaginal delivery (including nonpharmacologic methods, transcutaneous electrical nerve stimulation, intramuscular meperidine, inhalation of nitrous oxide, epidural analgesia, and a combination of these techniques), pain relief and overall satisfaction with the birth experience were greater in patients who received epidural analgesia.2 Similarly, randomized studies that have compared epidural analgesia with systemic opioids and/or inhalation analgesia (i.e., nitrous oxide) have shown that pain scores are lower and patients are more satisfied with neuraxial analgesia.3
The provision of analgesia for labor may result in other benefits. Effective epidural analgesia reduces maternal plasma concentrations of catecholamines (Figure 23-1).4 Decreased alpha- and beta-adrenergic receptor stimulation may result in better uteroplacental perfusion and more effective uterine activity.5,6 Painful uterine contractions result in maternal hyperventilation. The hyperventilation, in turn, leads to maternal respiratory alkalosis, a leftward shift of the oxyhemoglobin dissociation curve, increased maternal hemoglobin affinity for oxygen, and reduced oxygen delivery to the fetus.7 Hypocarbia also leads to hypoventilation between contractions, which may cause a decrease in maternal PaO2. Effective epidural analgesia blunts this “hyperventilation-hypoventilation” cycle.8 Additionally, one study showed that paternal anxiety levels were lower, and both paternal involvement in the childbirth process and paternal satisfaction were greater, in men whose partners received epidural analgesia than in those whose partners did not.9 Finally, the presence of an epidural catheter and effective epidural analgesia facilitate the rapid initiation of epidural anesthesia for emergency cesarean delivery. Neuraxial anesthesia for cesarean delivery is associated with greater overall maternal safety than emergency administration of general anesthesia (see Chapter 26).10
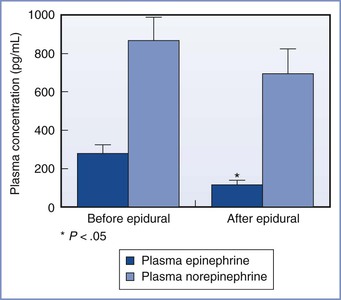
FIGURE 23-1 Influence of epidural analgesia on maternal plasma concentrations of catecholamines during labor. *P < .05 compared with before initiation of epidural analgesia. (Modified from Shnider SM, Abboud TK, Artal R, et al. Maternal catecholamines decrease during labor after lumbar epidural anesthesia. Am J Obstet Gynecol 1983; 147:13-5.)
Neuraxial analgesia is not used by all laboring women, although surveys of obstetric anesthesia practice in the United States have shown that the use of neuraxial analgesia has grown over the past three decades.11 Data collected from the U.S. Standard Certificate of Live Birth in 27 states in 2008 indicated that 61% of women who had a singleton vaginal delivery received neuraxial analgesia.12 The rate was higher among white women than in women of other races/ethnicities, and it was also higher in larger maternal units than in smaller units.11,12 In the United Kingdom, the National Health Service Maternity Statistics for 2010-2011 indicated that approximately one third of parturients chose to receive neuraxial analgesia during childbirth.13
The availability of skilled anesthesia providers influences the neuraxial analgesia rate.11 Other factors include the information and advice provided to pregnant women by obstetricians, nurses, and childbirth education instructors. The personal and cultural expectations of a laboring woman, as well as obstetric complications,2 also affect the childbirth experience and the use of neuraxial analgesia (see Chapters 20 and 21).
In contemporary clinical practice, health care costs have assumed significant importance. Studies using 1998 data estimated that the incremental cost to society for providing epidural labor analgesia was $260 to $340 per parturient (approximately $367 to $481 in 2012 dollars).14,15 A major problem with such analyses is the difficulty of determining the total value of neuraxial analgesia. For example, the ability to rapidly convert epidural analgesia to surgical anesthesia may also have value.
Ideally, the anesthesia provider should tailor the analgesic technique to meet the individual parturient’s needs. Factors that should be considered in formulating an analgesic plan for individual parturients include coexisting maternal disease, the airway examination, fetal status, spontaneous versus induced labor, stage of labor, and anticipated risk for operative delivery. The risks and benefits of the various epidural and spinal analgesic techniques should be assessed for each parturient. Good technique requires thoughtful preparation and meticulous attention to detail to ensure maternal and fetal safety.
The ideal labor analgesic technique is safe for both the mother and the infant, does not interfere with the progress of labor and delivery, and provides flexibility in response to changing conditions. In addition, the ideal technique provides consistent pain relief, has a long duration of action, minimizes undesirable side effects (e.g., motor block), and minimizes ongoing demands on the anesthesia provider’s time. No single technique or anesthetic agent is ideal for all parturients during labor. The American Society of Anesthesiologists (ASA) has published practice guidelines for obstetric anesthesia (see Appendix B),16 as well as guidelines for neuraxial anesthesia in obstetric patients (see Appendix A). Guidelines promulgated by professional organizations in other countries also address obstetric anesthesia care. All obstetric anesthesia providers should review their country’s respective guidelines. Specific neuraxial techniques for labor analgesia, including their advantages, disadvantages, side effects, and complications, are considered in this chapter.
Preparation for Neuraxial Analgesia
Indications and Contraindications
Epidural analgesia is indicated to treat the pain experienced by a woman in labor. In 2008 and 2010, respectively, the American College of Obstetricians and Gynecologists (ACOG)17 and the ASA18 reaffirmed an earlier, jointly published opinion that stated that “in the absence of a medical contraindication, maternal request is a sufficient medical indication for pain relief during labor.” Furthermore, the ACOG19 has stated that “decisions regarding analgesia should be closely coordinated among the obstetrician, the anesthesiologist, the patient, and skilled support personnel.” Neuraxial analgesia is an appropriate treatment for the pain of labor, including early labor (defined as regular uterine contractions that cause progressive effacement and dilation of the uterine cervix). Randomized controlled trials20–24 and a meta-analysis25 have confirmed that initiation of neuraxial analgesia in early labor does not increase the risk for cesarean delivery (see later discussion).
Epidural analgesia may facilitate an atraumatic vaginal breech delivery, the vaginal delivery of twin infants, and vaginal delivery of a preterm infant (see Chapters 34 and 35). By providing effective pain relief, epidural analgesia facilitates blood pressure control in preeclamptic women (see Chapter 36). Epidural analgesia also blunts the hemodynamic effects of uterine contractions (e.g., sudden increase in cardiac preload) and the associated pain response (tachycardia, increased systemic vascular resistance, hypertension, hyperventilation) in patients with other medical complications (e.g., mitral stenosis, spinal cord injury, intracranial neurovascular disease, asthma; see Chapters 42, 49, and 53).
Box 23-1 lists the contraindications to administration of epidural or spinal analgesia. Some anesthesiologists have suggested that systemic maternal infection, preexisting neurologic disease, or severe stenotic heart lesions are relative contraindications to neuraxial analgesia. However, most cases of systemic infection (especially if properly treated), or neurologic or cardiac disease, do not contraindicate the administration of neuraxial analgesia (see Chapters 37, 42, and 49). It is also controversial whether mild or isolated abnormalities in tests of blood coagulation preclude the use of neuraxial analgesia. The dose and timing of administration of drugs administered for thromboprophylaxis must also be considered (see Chapter 44).26 The anesthesia provider should consider the risks and benefits of neuraxial analgesia for each patient individually.
Thorough preparation for neuraxial labor analgesia involves several steps (Box 23-2). These include (1) a review of the parturient’s obstetric history; (2) a focused preanesthetic evaluation that includes maternal obstetric, anesthetic, and health history; and (3) a brief physical examination (i.e., vital signs, airway, heart, lungs, and back).16 Routine measurement of the platelet count is not necessary; however, assessment of the platelet count and other laboratory measurements may be indicated in selected patients.16 Similarly, routine intrapartum blood typing and screening or crossmatching is not necessary in healthy parturients, although consideration should be given to sending a blood sample to the blood bank (to facilitate the rapid availability of blood products in case of emergency need).16 For parturients at increased risk for hemorrhage, intrapartum typing, and screening or crossmatching should be performed. Fetal well-being should be assessed by a skilled provider, and equipment (including resuscitation equipment) should be checked by the anesthesia provider (see Box 12-1). Informed consent should be obtained (see Chapters 12 and 33). Early and ongoing communication among the obstetric and anesthesia providers, nursing staff, and other members of the multidisciplinary team is encouraged.
Types of Neuraxial Analgesia
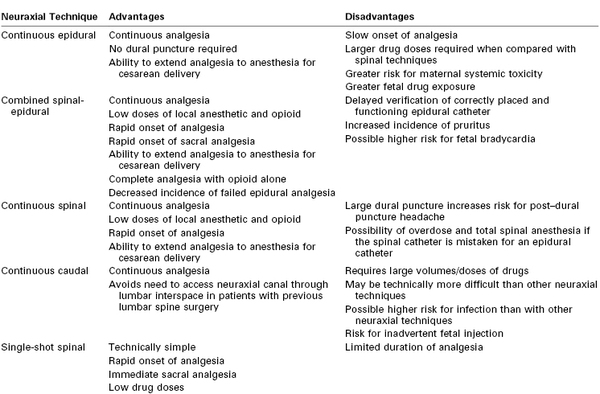
The technical aspects of neuraxial analgesic/anesthetic techniques are discussed in detail in Chapter 12. These techniques include continuous epidural, combined spinal-epidural, and caudal analgesia and continuous and single-shot spinal analgesia. Continuous epidural and combined spinal-epidural analgesia are the most common techniques used for neuraxial labor analgesia. There are advantages and disadvantages to each technique (Table 23-1).
Epidural Analgesia
Continuous lumbar epidural analgesia has been the mainstay of neuraxial labor analgesia for several decades. Placement of an epidural catheter allows analgesia to be maintained until after delivery. No dural puncture is required. The presence of a catheter and effective analgesia allow the conversion to epidural anesthesia should cesarean delivery be necessary. Injection of a local anesthetic in the lumbar epidural space allows both cephalad and caudad spread of the anesthetic solution.
Analgesia is initiated by bolus injection of drug(s) through the epidural needle, catheter, or both. Analgesia is maintained with anesthesia provider- or patient-administered intermittent bolus injections or a continuous epidural infusion, or both. The catheter is removed after delivery when there is no further need for analgesia or anesthesia.
Combined Spinal-Epidural Analgesia
Combined spinal-epidural (CSE) analgesia has become increasingly popular in the past 15 years. Onset of complete analgesia is significantly faster than with epidural techniques (2 to 5 minutes versus 10 to 15 minutes, respectively).27 In a meta-analysis of the onset time of CSE compared with low-dose epidural analgesia,27 the mean difference in onset was −5.4 minutes (95% confidence interval [CI], −7.3 to −3.6). More women with spinal analgesia than with epidural analgesia had effective analgesia at 10 minutes (relative risk [RR], 1.9; 95% CI, 1.5 to 2.5). In particular, the onset of sacral analgesia is significantly slower after the initiation of lumbar epidural analgesia than with spinal analgesia. It may take several hours of lumbar epidural infusion, or several bolus injections of local anesthetic into the lumbar epidural space, to achieve sacral analgesia. Rapid onset of sacral analgesia is advantageous in the parturient in whom analgesia is initiated late in the first stage of labor or in a parous parturient with rapid progress of labor. Spinal analgesia requires significantly lower drug doses to attain effective analgesia than does epidural analgesia; therefore, the risk for local anesthetic systemic toxicity is decreased. In addition, there is less systemic absorption of spinal anesthetic agents into the maternal circulation, so maternal and fetal plasma drug concentrations are lower with spinal than with epidural analgesia.
An additional advantage of spinal analgesia is that complete analgesia for early labor can be accomplished with the intrathecal injection of a lipid-soluble opioid without the addition of a local anesthetic. Thus, motor blockade is avoided and the risk for hypotension is lower.28 This method is ideal for patients who wish to ambulate or for those with preload-dependent cardiac conditions such as stenotic heart lesions. Finally, use of the CSE technique may lower the incidence of failure of epidural analgesia (e.g., a nonfunctioning epidural catheter).29,30 The likelihood of an epidural catheter placed for labor analgesia failing to provide satisfactory anesthesia for a subsequent cesarean delivery was more than five times higher for catheters placed as part of an epidural technique than for catheters placed as part of a CSE technique.31
Cappiello et al.32 have described a technique in which a dural puncture is made with a small-gauge spinal needle but no drug is injected into the subarachnoid space. After injection of epidural local anesthetic and opioid, blockade of sacral dermatomes occurred more frequently in parturients with a dural puncture than in those without, presumably because of enhanced anesthetic solution migration across the dural puncture site.
CSE analgesia has several possible undesirable side effects. Dural puncture is required to initiate CSE analgesia, although puncture with a small-gauge pencil-point needle does not appear to increase the risk for post–dural puncture headache.27 A more serious concern, however, is that dural puncture during labor may be a risk factor for postpartum neuraxial infection, a rare but potentially life-threatening complication (see Chapter 32).
The incidence of pruritus is higher with intrathecal opioid administration than with epidural opioid administration.27 Another potential drawback of CSE analgesia is that it is not clear for 1 to 2 hours after initiation of analgesia whether the epidural catheter is properly sited in the epidural space. Thus, CSE analgesia may not be the technique of choice if a functioning epidural catheter is critical to the safe care of the patient (e.g., a mother with an anticipated difficult airway or a worrisome fetal heart rate [FHR] tracing).
The most common CSE technique for labor analgesia is the needle-through-needle technique in a midlumbar interspinous space (see Chapter 12). Analgesia is maintained via the epidural catheter, as with traditional epidural analgesia.
Continuous Spinal Analgesia
Continuous spinal analgesia is used occasionally for labor analgesia but is not practical for most parturients. Because the available catheters require dural puncture with a large-gauge introducer needle, the technique is associated with an unacceptably high incidence of post–dural puncture headache. However, continuous spinal analgesia is a management option in patients with unintentional dural puncture. Continuous spinal analgesia can readily be converted to surgical anesthesia if necessary.
Caudal Analgesia
Continuous caudal epidural analgesia is used infrequently in modern obstetric anesthesia practice. It is technically more difficult to place a caudal catheter than a lumbar epidural catheter. Large volumes of anesthetic solution are required to extend neuroblockade to the low thoracic spinal segments, resulting in higher maternal plasma concentrations of drug. There is a risk for needle/catheter misplacement and direct injection into the fetus. However, this technique is useful for parturients in whom access to the lumbar spinal canal is not possible (e.g., because of a fused lumbar spine).
Single-Shot Techniques
In general, single-shot techniques (spinal, lumbar epidural, or caudal) are not useful for most laboring women because of their limited duration of action. These techniques may be indicated for parturients who require analgesia or anesthesia shortly before anticipated vaginal delivery or in settings in which continuous epidural analgesia is not possible.33
Informed Consent
Informed consent is an important aspect of preparation for neuraxial labor analgesia (see Chapters 12 and 33). The preanesthetic evaluation and informed consent process allow the physician to allay the patient’s concerns and to demonstrate a commitment to her care. Most laboring women understand the need for informed consent and appreciate the opportunity to participate in decisions about their care.
Equipment and Monitors
Resuscitation equipment, drugs, and supplies must be immediately available for the management of serious complications of neuraxial analgesia (e.g., hypotension, total spinal anesthesia, systemic local anesthetic toxicity) (see Box 12-1).16 Emergency airway equipment should be checked before the administration of neuraxial analgesia.
During the initiation of neuraxial analgesia, the parturient’s oxygen saturation is measured continuously and the blood pressure is assessed every 2 to 3 minutes for 15 to 20 minutes after the neuraxial anesthetic administration, or until the mother is hemodynamically stable (see Chapter 12). The FHR should be monitored before and after the initiation of neuraxial analgesia; it may be difficult to monitor the FHR during the actual procedure.16 During maintenance of neuraxial analgesia, maternal blood pressure is measured every 15 to 30 minutes, or more frequently if hypotension ensues. The sensory level of analgesia and the intensity of motor block (Box 23-3) are assessed after the administration of the test and therapeutic doses of local anesthetics. Subsequently, sensory level, motor block, and pain control are assessed at regular intervals.
Intravenous Hydration
Placement of an intravenous catheter (preferably 18-gauge or larger) and correction of hypovolemia with intravenous hydration are necessary before the initiation of neuraxial analgesia to mitigate hypotension that can result from sympathetic blockade. Data from small studies are conflicting as to whether a fluid bolus administered immediately before the initiation of analgesia decreases the risk for nonreassuring FHR patterns.34–36 Most anesthesia providers administer approximately 500 mL of lactated Ringer’s solution (without dextrose), although the ASA Task Force on Obstetric Anesthesia has stated that a fixed volume of intravenous fluid is not required before neuraxial analgesia is initiated.16 Severe hypotension is less likely with the contemporary practice of administering a dilute solution of local anesthetic for epidural analgesia or an intrathecal opioid for spinal analgesia.
Studies of intravenous hydration and spinal anesthesia for cesarean delivery suggest that there is no advantage to administering the fluid before the initiation of anesthesia (preload) compared with administering the fluid at the time of initiation of anesthesia (co-load).37 Rarely, hydration should be guided by serial assessment of intravascular fluid volume (e.g., central venous pressure, transthoracic echocardiography). Fluid administration should be judicious in parturients at risk for pulmonary edema (e.g., women with severe preeclampsia).
A balanced electrolyte solution (e.g., lactated Ringer’s solution) without dextrose is the most commonly used intravenous fluid for bolus administration. Data are conflicting as to whether the maintenance intravenous infusion of dextrose-containing fluid during labor is associated with a lower incidence of umbilical cord blood acidemia.38,39 One randomized controlled trial demonstrated shorter labor in women who received an intravenous solution of normal saline with dextrose compared with saline without dextrose.40 However, anesthesia and obstetric providers should avoid the bolus administration of dextrose-containing solutions in laboring women.
Maternal Positioning
Either the lateral decubitus or the sitting position can be used during initiation of neuraxial analgesia (see Chapter 12). Factors to consider when positioning the parturient for the procedure include patient comfort, avoidance of aortocaval compression, ability to monitor the FHR, provider comfort and experience, and optimal positioning of the spine and palpation of landmarks. Patient position relative to the baricity of the anesthetic solution should be considered during initiation of spinal analgesia/anesthesia. There is little evidence that patient position influences the extent of neuroblockade during initiation of epidural analgesia/anesthesia. After completion of the procedure, parturients should be assisted to the lateral position for the first 15 to 30 minutes after the neuraxial injection to alleviate aortocaval compression.
Initiation of Epidural Analgesia
A procedure for initiating epidural labor analgesia is outlined in Box 23-4. Commonly, after siting the epidural catheter in the epidural space, a test dose is administered to rule out intrathecal or intravascular placement of the epidural catheter. After a negative test, epidural analgesia is established with the incremental injection of a local anesthetic, usually in combination with a lipid-soluble opioid. Maternal vital signs are monitored and clinical analgesia is verified.
Epidural Test Dose
The purpose of the test dose is to help identify unintentional cannulation of a vein or the subarachnoid space. The test dose should contain a dose of local anesthetic and/or another marker sufficient to allow the recognition of intravenous or subarachnoid injection but not so large as to cause systemic toxicity or total spinal anesthesia. The most common intravascular test dose contains epinephrine (see Chapter 12).
The use of the epinephrine test dose in obstetrics is not without detractors. Some anesthesia providers fear that intravenous injection of epinephrine may decrease uteroplacental perfusion and precipitate fetal compromise. However, there has been no report of adverse neonatal outcome after intravenous injection of an epinephrine-containing test dose. Another argument against routine use of a test dose is that aspiration of multi-orifice catheters is 98% sensitive in identifying their intravascular location.41 (The sensitivity of aspiration is significantly lower for single-orifice catheters.) The epidural test dose contributes to undesirable motor blockade.42,43 Finally, because modern epidural labor analgesia involves the infusion of a low concentration of local anesthetic solution, unintentional intravascular or intrathecal administration is not likely to result in cardiovascular collapse or total spinal anesthesia.
Others argue that the test dose still has a role in obstetric anesthesia practice.44 Large volumes of a concentrated local anesthetic solution are still routinely administered for emergency cesarean delivery. Although not a safety issue, it is easier for the parturient and anesthesia provider to identify a misplaced catheter at the time of initial placement and to replace the catheter at that time rather than identify the misplaced catheter after the sterile field has been breached and the parturient repositioned.
The epinephrine test dose is less specific in laboring women because cyclic changes in maternal heart rate complicate interpretation of its effects.45 For this reason, if used, the test dose should be given immediately after a uterine contraction so there is less confusion as to whether tachycardia is caused by pain or intravenous epinephrine. Other methods of detecting intravascular injection are discussed in Chapter 12.
No matter whether a formal test dose is used or not, it is imperative that the anesthesia provider take the time to look for evidence of unintentional intrathecal injection of local anesthetic. Finally, every anesthesia provider should remember that no single test dose regimen can exclude every case of unintentional intravenous or subarachnoid injection. Box 12-3 summarizes steps that may be taken to decrease the risk for unintentional intravenous or subarachnoid injection of local anesthetic.
Choice of Drugs
The ideal analgesic drug for labor would provide rapid onset of effective analgesia with minimal motor blockade, minimal risk for maternal toxicity, and negligible effect on uterine activity and uteroplacental perfusion. It would undergo limited transplacental transfer and thus have minimal direct effect on the fetus. Finally, this ideal agent would have a long duration of action. Although this perfect analgesic drug does not exist, the combination of a local anesthetic with an opioid allows us to approach this goal.
Traditionally, local anesthetics were administered to block both the visceral pain of labor (lower uterine segment distention and cervical dilation) and the somatic pain (descent of the fetus in the birth canal). Almost 40 years ago, investigators identified dense concentrations of opioid receptors in the dorsal horn of the spinal cord.46 The application of small doses of an opioid to these receptor sites generates a specific and profound opioid response.46 The introduction of neuraxial opioids to the armamentarium of the obstetric anesthesia provider moved us closer to the prediction made by Benjamin Rush in 1805: “A medicine would be discovered which should suspend sensibility altogether and leave irritability or powers of motion unimpaired.”47 Intrathecal opioids effectively relieve the visceral pain of the early first stage of labor, although they must be combined with a local anesthetic to effectively relieve the somatic pain of the late first stage and the second stage of labor. The combination of a local anesthetic with a lipid-soluble opioid allows for the use of lower doses of each agent, thus minimizing undesirable side effects. For example, when used alone without an opioid, the local anesthetic dose required for effective epidural analgesia is associated with an unacceptably high incidence of motor blockade. Similarly, used alone, high doses of epidural opioid are required for satisfactory analgesia during early labor, and such doses are associated with significant systemic absorption and systemic side effects. The addition of an opioid to the local anesthetic also shortens latency,48 an important aspect of labor analgesia, especially with the use of long-acting (and therefore, long-latency) local anesthetics. Thus, contemporary epidural labor analgesia practice most often incorporates low doses of a long-acting local anesthetic combined with a lipid-soluble opioid.
Local Anesthetics
Bupivacaine.
Traditionally, the amide local anesthetic bupivacaine has been the most commonly used agent for epidural labor analgesia. Bupivacaine is highly protein-bound, a feature that limits transplacental transfer. The umbilical vein–to–maternal vein concentration ratio is approximately 0.3.49 After epidural administration of bupivacaine (without opioid) during labor, the patient first perceives pain relief within 8 to 10 minutes,50 but approximately 20 minutes is required to achieve the peak effect. Duration of analgesia is approximately 90 minutes. Bupivacaine 6.25 to 12.5 mg (e.g., 10 to 20 mL of a 0.0625% solution, or 5 to 10 mL of a 0.125% solution) combined with fentanyl or sufentanil is adequate to initiate labor analgesia in most parturients (Table 23-2).
TABLE 23-2
Drugs Used for Initiation of Epidural and Spinal Labor Analgesia
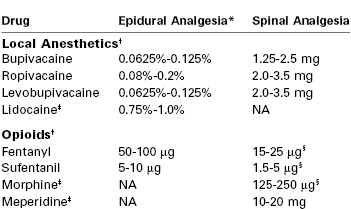
* The volume required to initiate epidural labor analgesia is 10 to 15 mL of local anesthetic solution.
† The local anesthetic dose/concentration and the fentanyl or sufentanil dose are reduced if the drugs are combined or if a local anesthetic-containing epidural test dose is administered before the initial therapeutic dose. The dose of opioid should be reduced or the opioid omitted if the parturient has recently received systemic opioid analgesia.
‡ Lidocaine, morphine, and meperidine are not commonly used for labor analgesia because of their short duration of action (lidocaine), long latency (morphine), and high incidence of nausea and vomiting (morphine and meperidine).
§ Opioids may be administered without local anesthetics when spinal analgesia is induced in early labor. Women in active labor require a higher dose than women in latent labor.
Note: The suggested doses are based on clinical studies, potency ratios, and clinical experience.
NA, not applicable.
The potency of local anesthetics for neuraxial labor analgesia is often assessed by determining the median effective concentration of local anesthetic solution when administered as a 20-mL epidural bolus (this concentration is often referred to as the minimum local anesthetic concentration [MLAC]). It is lower for women in early labor than in late labor,51 and it is also lower when the local anesthetic is combined with a lipid-soluble opioid.52
It is important to consider both the local anesthetic dose and concentration for initiation and maintenance of epidural analgesia. Christiaens et al.53 randomly assigned parturients to receive epidural bupivacaine 20 mg diluted in 4 mL, 10 mL, or 20 mL (0.5%, 0.2%, and 0.1% solutions, respectively). Analgesia in the 10-mL and 20-mL groups was superior to that in the 4-mL group, and duration of analgesia was longest in the 20-mL group. Lyons et al.54 compared the minimum local anesthetic volume (MLAV) and minimum local anesthetic dose (MLAD) for 0.125% and 0.25% bupivacaine for epidural labor analgesia. Bupivacaine 0.125% produced analgesia equivalent to that provided by bupivacaine 0.25%, with a 50% increase in required volume and a 25% reduction in dose (Table 23-3). Stated differently, a dose-sparing effect is achieved by administering a 0.125% solution of bupivacaine rather than a 0.25% solution. Ginosar et al.55 randomized parturients to receive maintenance of analgesia with an epidural infusion of either bupivacaine 0.25% at 5 mL/h or bupivacaine 0.0625% at 20 mL/h (10 mg/h in both groups). The median bupivacaine dose was lower and patient satisfaction was greater with bupivacaine 0.0625% than with bupivacaine 0.25%. Together, these data suggest that epidural analgesia and safety are improved with the use of low concentration–high volume local anesthetic solutions.
TABLE 23-3
Comparison of Epidural Bupivacaine 0.25% and 0.125%: Median Effective Volume and Dose
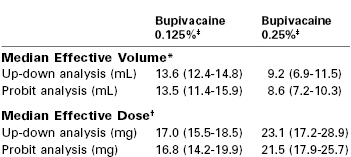
* Median effective volume at a fixed local anesthetic concentration.
† Median effective dose at a fixed local anesthetic concentration.
‡ 95% confidence intervals shown in parentheses, which were calculated using up-down sequential and probit analysis.
Modified from Lyons GR, Kocarev MG, Wilson RC, Columb MO. A comparison of minimum local anesthetic volumes and doses of epidural bupivacaine (0.125% w/v and 0.25% w/v) for analgesia in labor. Anesth Analg 2007; 104:412-5.)
Ropivacaine.
Ropivacaine, a relatively newer amide local anesthetic, is similar to bupivacaine in structure and pharmacodynamics.56 It is a homologue of bupivacaine and mepivacaine, but unlike these other local anesthetics it is formulated as a single-levorotary enantiomer rather than a racemic mixture (see Chapter 13). Studies of pregnant sheep have demonstrated that clinically relevant plasma concentrations of ropivacaine do not adversely affect uterine blood flow.57
Studies in vitro and in vivo have shown that ropivacaine is less cardiodepressant and arrhythmogenic than bupivacaine when doses of equal mass are compared.58,59 Ropivacaine is cleared more rapidly than bupivacaine after intravenous administration in both pregnant and nonpregnant sheep. Consequently, a larger dose of drug—but not a higher plasma concentration—is required to produce systemic toxicity.60 These findings suggest that ropivacaine may have a greater margin of safety than bupivacaine if unintentional intravenous injection occurs in pregnant women. However, many early investigations assumed that ropivacaine and bupivacaine are equipotent; subsequent studies have demonstrated that ropivacaine is 25% to 40% less potent than bupivacaine.61–63 In one study that characterized the full dose-response curves, the slope of the bupivacaine and ropivacaine curves were similar, suggesting that the nature of the drug-receptor interaction is not different between the two drugs.63 When ropivacaine concentrations are adjusted for this difference in potency, there is a less clear advantage for ropivacaine in terms of the risk for systemic toxicity.61 In reality, systemic toxicity is not a major concern with the contemporary administration of a dilute solution of local anesthetic for epidural labor analgesia.
Several studies that compared equal concentrations of ropivacaine and bupivacaine given by patient-controlled epidural analgesia (PCEA) have not found any significant difference in clinical efficacy between the two local anesthetics.64–68 Other studies that adjusted for the potency difference and compared equipotent concentrations (e.g., 0.0625% bupivacaine versus 0.1% ropivacaine) also found no difference in clinical efficacy.69,70 It is important to recognize that potency is an unchanging property of a drug, whereas clinical efficacy is influenced by multiple variables. For example, ropivacaine has a longer duration of analgesia than bupivacaine,61 which may offset its lesser potency when it is administered by continuous epidural infusion.
Early clinical studies suggested that ropivacaine is associated with less motor block than bupivacaine71,72; avoidance of motor blockade is a desirable characteristic of a local anesthetic used for epidural analgesia during labor. However, these studies also compared equal concentrations of ropivacaine and bupivacaine, and the observed lower degree of motor blockade may reflect the lesser potency of ropivacaine. A study of the relative motor-blocking potencies of epidural ropivacaine and bupivacaine showed that ropivacaine was less potent than bupivacaine in terms of motor blockade,73 a finding that corresponded to the relative analgesic potencies of the two drugs.61,62 The differences in potency of motor blockade may not be relevant with the use of low concentrations of local anesthetic. Several clinical studies64,66,74 and a well-conducted meta-analysis of studies that compared epidural ropivacaine and bupivacaine75 did not demonstrate an advantage for ropivacaine in terms of outcome of labor (see later discussion), although the incidence of motor blockade was less in the ropivacaine groups.66,74,75
There is no clear evidence of greater patient safety, lower risk for instrumental vaginal delivery, or other improved outcomes when ropivacaine is used to provide epidural labor analgesia.74,76 A 2010 review concluded that there is no advantage to the routine use of ropivacaine compared with bupivacaine for labor analgesia.76 In contrast, ropivacaine offers greater patient safety in settings in which high concentrations and greater volumes of drugs are administered (e.g., brachial plexus blockade or epidural anesthesia for cesarean delivery).77
Like bupivacaine, ropivacaine is often combined with fentanyl or sufentanil for labor analgesia. Ropivacaine concentrations used to initiate epidural analgesia range from 0.08% to 0.2% (see Table 23-2). Higher concentrations are used if the drug is administered without an opioid.
Levobupivacaine.
Levobupivacaine is the levorotary enantiomer of bupivacaine (which is a racemic mixture). It is not available in the United States. Both preclinical and clinical studies have suggested that, like ropivacaine, levobupivacaine has less potential for cardiotoxicity than bupivacaine when equal doses of the two drugs are compared.78,79 One study found that levobupivacaine was essentially equipotent to bupivacaine with a potency ratio of 0.98; however, the 95% CI was wide (0.67 to 1.41).80 Other studies have suggested that levobupivacaine and ropivacaine have similar potency.81,82 In an MLAC study that compared the motor blocking potency of bupivacaine and levobupivacaine,83 levobupivacaine was less potent than bupivacaine (potency ratio, 0.87; 95% CI, 0.77 to 0.98).83 Beilin et al.66 compared epidural bupivacaine, ropivacaine, and levobupivacaine (0.0625% with fentanyl 2 µg/mL) for labor analgesia. There were no differences among groups in obstetric outcomes, although the incidence of motor blockade was lower in the ropivacaine and levobupivacaine groups. Therefore, although epidural bupivacaine is more potent than ropivacaine for both sensory and motor blockade during labor, and may be more potent than levobupivacaine, there do not appear to be any clinical advantages of one drug over the other two drugs for epidural labor analgesia.
Lidocaine.
Lidocaine is an amide local anesthetic with a duration of action intermediate between those of bupivacaine and 2-chloroprocaine. During labor, the administration of a 0.75% to 1.0% solution of lidocaine typically provides satisfactory analgesia. Lidocaine is not commonly used for initiation or maintenance of epidural labor analgesia, in part because of its shorter duration of action in comparison with bupivacaine, ropivacaine, and levobupivacaine. Lidocaine is less protein-bound than these other amide local anesthetics, and at delivery, the umbilical vein–to–maternal vein lidocaine concentration ratio is approximately twice that of bupivacaine.84 Early studies discouraged the epidural administration of lidocaine in pregnant women because epidural lidocaine was associated with abnormal neonatal neurobehavioral findings.85 Subsequently, larger, more carefully controlled studies have demonstrated that the epidural administration of lidocaine, bupivacaine, and 2-chloroprocaine have similar neonatal outcomes.86,87 Although some investigators have observed subtle differences in neurobehavior between infants exposed to lidocaine and those exposed to other local anesthetics, these differences are within the inherent variability of the examinations and are not clinically significant. Other factors (e.g., mode of delivery) appear to be much more important determinants of neonatal condition.
2-Chloroprocaine.
An ester local anesthetic, 2-chloroprocaine has a rapid onset of action. Epidural administration of 10 mL of 2% 2-chloroprocaine provides effective analgesia for approximately 40 minutes. The short duration of action limits its usefulness during labor. In addition, the epidural administration of 2-chloroprocaine may adversely affect the efficacy of subsequently administered epidural bupivacaine and opioids,88,89 although it is unclear whether the mechanism is related to pharmacokinetic or pharmacodynamic properties of the drug.90,91 In obstetric practice, 2-chloroprocaine is most commonly used for extension of epidural labor analgesia for instrumental vaginal delivery (see later discussion) or emergency cesarean delivery (see Chapter 26).
Opioids
Lipid-Soluble Opioids: Fentanyl and Sufentanil.
Morphine was one of the first opioids to be studied for labor analgesia. However, because of its long latency, side effects, and inconsistent analgesia, morphine has largely been replaced by the lipid-soluble opioids fentanyl and sufentanil (see Chapter 13). The lipid-soluble agents have a rapid onset of action. Permeability (of the dura-arachnoid) is not a rate-limiting factor, and increasing the concentration gradient (by administration of a larger dose) facilitates faster entry into the spinal cord. The high lipid solubility of these agents also results in a shorter duration of action and greater systemic absorption than occurs with water-soluble drugs.
Some investigators have suggested that the improved analgesia results from a supraspinal action rather than a primary spinal action. However, several studies have refuted this theory, including studies of epidural opioid administration by bolus92 and continuous infusion.93 Vella et al.92 observed that the initiation of epidural analgesia with 0.25% bupivacaine with epidural fentanyl 80 µg* resulted in more rapid, complete, and prolonged analgesia than intravenous fentanyl 80 µg, even though plasma fentanyl concentrations were higher in the intravenous group. Similarly, D’Angelo et al.93 demonstrated that a continuous epidural infusion but not an intravenous infusion of fentanyl reduced epidural bupivacaine requirements in laboring women. Polley et al.94 determined that the MLAC of epidural bupivacaine administered as a 20-mL bolus in laboring women was reduced from 0.064% to 0.034% when epidural rather than intravenous fentanyl was co-administered with bupivacaine. Ginosar et al.95 determined that the MLAC of bupivacaine administered by continuous epidural infusion during labor was lower by a factor of three when it was co-administered with an epidural (rather than intravenous) fentanyl infusion. Finally, in a volunteer study,96 lumbar epidural administration of fentanyl resulted in tolerance to experimental pain at a lumbar but not a cranial dermatome, whereas intravenous fentanyl administration resulted in pain tolerance at both dermatomes. These studies strongly suggest that during labor, epidural fentanyl provides analgesia primarily through a spinal site of action.
Epidural fentanyl alone provides moderate analgesia in early labor,48 but the dose needed to provide complete analgesia is accompanied by significant side effects (e.g., pruritus, nausea, maternal sedation, perhaps neonatal depression). In addition, epidural administration of an opioid alone provides inadequate analgesia during the late first stage as well as the second stage of labor. In a study comparing sufentanil alone, sufentanil with bupivacaine, and bupivacaine alone,97 women randomly assigned to the sufentanil-alone (30 µg) group experienced satisfactory analgesia after the initial dose but not after subsequent doses. However, the initial dose was administered after an epidural test dose that contained lidocaine 60 mg. In clinical practice, epidural fentanyl and sufentanil are usually administered with a local anesthetic for the initiation of analgesia (at a minimum, with a local anesthetic–containing epidural test dose).
In clinical practice, either fentanyl or sufentanil is frequently combined with a low-concentration, long-acting amide local anesthetic to initiate epidural labor analgesia. Epidural opioid administration allows the anesthesia provider to use a more dilute solution of local anesthetic to provide epidural labor analgesia.98 Epidural fentanyl and sufentanil decrease epidural bupivacaine requirements during labor in a dose-dependent fashion (Figure 23-2).52,99 The reduction in MLAC by the addition of fentanyl or sufentanil is observed with levobupivacaine,100,101 ropivacaine,101,102 and 2-chloroprocaine103 as well as bupivacaine.
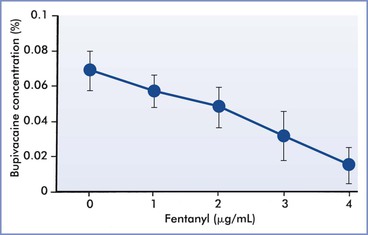
FIGURE 23-2 The effect of epidural fentanyl on the minimum local anesthetic concentration (defined as the effective concentration in 50% of subjects [EC50]) for epidural bupivacaine analgesia during labor. Data are expressed as median concentrations with 95% confidence intervals. (Data from Lyons G, Columb M, Hawthorne L, Dresner M. Extradural pain relief in labour: bupivacaine sparing by extradural fentanyl is dose dependent. Br J Anaesth 1997; 78:493-6.)
The addition of a lipid-soluble opioid to a local anesthetic for neuraxial labor analgesia decreases latency, prolongs the duration of analgesia, and improves the quality of analgesia. For example, Reynolds and O’Sullivan104 showed that epidural bupivacaine 10 mg combined with fentanyl 100 µg was more effective for the treatment of breakthrough pain and had a faster onset and longer duration of action than either bupivacaine 25 mg or fentanyl 100 µg administered alone. Van Steenberge et al.105 observed that the mean (± SD) onset of analgesia was 10.3 ± 3.8 minutes in women randomly assigned to receive bupivacaine 12.5 mg without sufentanil, whereas it was 8.7 ± 2.6 minutes in women who received bupivacaine combined with sufentanil 7.5 µg. Duration of analgesia was longer in the sufentanil group (131 minutes versus 86 minutes without sufentanil).105 In another study,106 the addition of fentanyl 100 µg to 0.125% bupivacaine prolonged the mean duration of analgesia from 55 minutes to 106 minutes.
The quality of analgesia is also better with the addition of an opioid to the local anesthetic. For example, 86% of women rated their analgesia as excellent after epidural analgesia was initiated with bupivacaine combined with sufentanil, compared with 50% of those who received bupivacaine without sufentanil.105 The percentage of women who experienced no or short periods of pain during the first stage of labor was 94% in women who received sufentanil and 76% in women who did not.107 After initiation of analgesia with 0.125% bupivacaine with epinephrine 1.25 µg/mL, 43% of women randomly assigned to receive epidural fentanyl 100 µg rated their analgesia as excellent, compared with 6% in a control group that did not receive fentanyl.106
The dose-sparing effects of fentanyl and sufentanil are also evident when the drugs are combined with a low-concentration solution of bupivacaine used for the maintenance of analgesia throughout labor. For example, the total bupivacaine dose (mean ± SD) was 34 ± 17 mg in laboring women who received 0.125% bupivacaine/epinephrine 1.25 µg/mL with sufentanil, compared with 42 ± 19 mg in those who received bupivacaine/epinephrine without sufentanil.107 Similarly, in women randomly assigned to receive bupivacaine/epinephrine with or without fentanyl 100 µg, the total bupivacaine dose was 55 mg or 110 mg, respectively.106 Advantages of a lower total dose of local anesthetic include (1) decreased risk for local anesthetic systemic toxicity, (2) decreased risk for high or total spinal anesthesia, (3) decreased plasma concentrations of local anesthetic in the fetus and neonate, and (4) decreased intensity of motor blockade.
Several studies have directly compared the administration of epidural fentanyl or sufentanil combined with a local anesthetic for the initiation of labor analgesia. There were no differences in analgesia in women in early labor randomly assigned to receive either fentanyl 100 µg or sufentanil 20 µg immediately after a lidocaine 45-mg/epinephrine 15-µg test dose.108 In contrast, a second study demonstrated slightly better analgesia 20 minutes after injection of 0.125% bupivacaine (15 mg) with sufentanil 15 µg than after the same dose of bupivacaine with fentanyl 75 µg.109
In the United States, fentanyl is more commonly used because historically it has had a lower acquisition cost than sufentanil. Additionally, the concentration of the commercially available sufentanil preparation (50 µg/mL) may make drug errors more likely with sufentanil than with fentanyl because sufentanil doses significantly lower than 50 µg are used for initiation of epidural analgesia.
There are few rigorous dose-response studies of epidural fentanyl or sufentanil combined with bupivacaine for initiation of epidural labor analgesia. Herman et al.110 randomly assigned 100 laboring women with a cervical dilation of 5 cm or less to receive 0.125% bupivacaine 10 mL, combined with fentanyl 0 to 100 µg (in 25-µg increments) or sufentanil 0 to 25 µg (in 5-µg increments), injected after a negative epidural test dose (bupivacaine 7.5 mg with epinephrine 15 µg). Using a probit dose-response analysis, these researchers calculated the effective dose in 95% of subjects (ED95) to be 50 µg for fentanyl and 8 µg for sufentanil; these figures equate to a sufentanil-to-fentanyl potency ratio of 6.3 : 1. Capogna et al.111 sought to determine the median effective analgesic dose (ED50) of epidural fentanyl and sufentanil alone (no local anesthetic) for the initiation of epidural analgesia in nulliparous women with a cervical dilation between 2 and 4 cm. The ED50 of fentanyl was 124 µg (95% CI, 118 to 131) and the ED50 of sufentanil was 21 µg (95% CI, 20 to 22), with a potency ratio of 5.9 : 1. The potency ratio in volunteers subjected to an electrical stimulus was approximately 5 : 1.112 Taken together, these data suggest that the potency ratio of sufentanil to fentanyl administered into the epidural space is approximately 6 : 1.
Several studies have compared bupivacaine combined with fentanyl 50 µg and 100 µg. No differences in the onset, duration, and quality of analgesia were noted in Asian women randomly assigned to receive 0.125% bupivacaine (10 mg) combined with either 50 or 100 µg of fentanyl.113 In contrast, when 0.125% bupivacaine (15 mg) with epinephrine 15 µg was administered to laboring Italian women with either 50 or 100 µg of fentanyl, there was no difference in the onset or duration of analgesia, but more women in the 100-µg group had excellent analgesia.106
There were no differences in latency, duration of analgesia, and quality of analgesia when analgesia was induced with 0.125% bupivacaine (12.5 mg)/epinephrine 12.5 µg and either 7.5 or 15 µg of sufentanil.105 Similarly, after injection of a lidocaine 60-mg/epinephrine 15-µg test dose, another study found no differences in latency and quality of analgesia among epidural sufentanil doses of 5, 10, 20, 30, 40, and 50 µg.114 However, the duration of analgesia was longer after the higher doses of sufentanil.
The range of fentanyl and sufentanil doses used for the initiation of epidural labor analgesia is shown in Table 23-2. Pain and analgesic requirements vary depending on several factors, including parity, stage of labor, presence of ruptured membranes, oxytocin augmentation, and whether the opioid is administered in combination with a local anesthetic. One study reported that the ED50 of epidural sufentanil was higher in women undergoing prostaglandin induction of labor than in women with spontaneous labor.115 Conell-Price et al.116 developed a model of labor pain in nulliparous women and found that the use of oxytocin was associated with 48% more pain at the start of labor. Preliminary evidence suggests that pharmacogenetics may also play a role in dose requirements. Camorcia et al.117 reported that nulliparous women who were heterozygous or homozygous for the single nucleotide polymorphism (SNP) A118G (substitution of adenine for guanine at position 118) of the gene encoding the µ-opioid receptor (OPRM1) had a lower ED50 for epidural sufentanil administered for labor analgesia.
Several early studies suggested that the duration of epidural and spinal analgesia may exhibit circadian rhythm (chronobiology), possibly secondary to human biologic rhythms.118 More recently, however, in a secondary analysis of data from a large study, Scavone et al.119 found no evidence of a circadian response to intrathecal fentanyl or intravenous opioid labor analgesia. In a detailed analysis of data from a study designed to test whether parturient response to intrathecal bupivacaine exhibited a circadian rhythm, Shafer et al.120 demonstrated that external daily rhythms, such as nursing shifts, may contribute to the appearance of biologic rhythm. Thus, whether a circadian response to neuraxial local anesthetic or opioid exists, or is clinically significant, requires further study.
Current evidence supports the administration of epidural opioid doses at the lower end of the dose range for nulliparous women, for women in early labor, or when the opioid is co-administered with a local anesthetic. Higher doses are associated with a higher incidence of maternal side effects and the potential for neonatal depression (see later discussion). The major maternal side effect of epidural fentanyl and sufentanil for labor analgesia is pruritus. Neonatal outcomes do not appear to be adversely affected by the addition of fentanyl or sufentanil to a local anesthetic for epidural analgesia (see later discussion). In fact, the combination of drugs allows lower doses of both drugs to be administered, resulting in lower concentrations of both drugs in the neonate.
Two studies found that the diluent volume (2 to 20 mL) did not affect the onset and duration of epidural labor analgesia when fentanyl was injected into the epidural space after the injection of a local anesthetic solution.121,122
Other Opioids.
Morphine was one of the first opioids used for labor analgesia. Hughes et al.123 compared analgesia using epidural administration of morphine (2.0, 5.0, and 7.5 mg) with that using epidural bupivacaine 0.5%. Morphine was effective in 7 of 11 parturients until the end of the first stage of labor, but all parturients required bupivacaine for adequate analgesia during the second stage of labor. Subsequently, investigators combined morphine with bupivacaine and observed a longer duration of analgesia compared with that for bupivacaine alone.124 However, the inconsistent analgesia, long latency (30 to 60 minutes), and high incidence of side effects of morphine (which continued after delivery), along with the introduction of lipid-soluble opioids and epidural infusion pumps into clinical practice, have made the use of epidural morphine for labor analgesia largely obsolete.
Several studies described the use of alfentanil with bupivacaine for labor analgesia.125,126 Alfentanil has lower lipid solubility than both fentanyl and sufentanil. Only a few small studies have compared alfentanil with other opioids for labor analgesia.
Several groups of investigators have reported the use of epidural hydromorphone for labor analgesia.127–129 The lipid solubility of hydromorphone lies between those of morphine and fentanyl, but is closer to that of morphine.130 In a large prospective observational study, effective labor analgesia was obtained by initiating analgesia with 0.25% bupivacaine (20 to 25 mg) with epinephrine (40 to 50 µg), followed by hydromorphone 100 µg.127 However, Mhyre129 observed that effective labor analgesia could not be provided by 0.035% bupivacaine (7 mg) with hydromorphone 100 to 110 µg. In another trial, parturients were randomly assigned to receive either epidural hydromorphone 300 µg or saline-control immediately after the initiation of analgesia with lidocaine 45 mg, epinephrine 15 µg, and fentanyl 100 µg.128 Duration of analgesia and side effects were similar in the two groups. At the current time, further investigation is required before hydromorphone can be recommended for epidural labor analgesia.
Meperidine may be used effectively alone (without a local anesthetic), in part because it possesses local anesthetic properties.131 When given during labor, epidural meperidine 100 mg provides analgesia similar to that provided by 0.25% bupivacaine, with less motor blockade. However, this dose of epidural meperidine produces more sedation, nausea, and pruritus than epidural bupivacaine. Handley and Perkins132 observed that the addition of meperidine 25 mg to 0.125%, 0.187%, or 0.25% bupivacaine (10 mL) provided adequate analgesia for the first stage of labor. The use of the more concentrated solutions (i.e., 0.187% and 0.25% bupivacaine) did not enhance the quality or duration of analgesia but did shorten latency (10 to 20 minutes versus 20 to 30 minutes for the less concentrated solution). Epidural administration of meperidine effectively prevents or treats the shivering that often occurs during labor.133 Investigators from Saudi Arabia randomly allocated women to receive 0.1% bupivacaine with either meperidine 1 mg/mL or fentanyl 2 µg/mL.134 No differences were noted between groups in analgesic characteristics, except that women in the meperidine group had a higher incidence of nausea and vomiting. Currently there is no evidence that meperidine alone or in combination with bupivacaine has any advantages over a combination of a long-acting amide local anesthetic and a lipid-soluble opioid.
Butorphanol is a lipid-soluble opioid agonist-antagonist, with weak µ-receptor and strong κ-receptor activity. Because κ-opioid receptors appear to be involved in the modulation of visceral pain, κ-receptor agonists should be useful agents for the relief of labor pain, which has a significant visceral component (see Chapter 20).124,135,136 Somnolence is the most prominent side effect of epidural butorphanol. The addition of butorphanol 1, 2, or 3 mg to 0.25% bupivacaine (25 mg) shortened latency and prolonged the duration of analgesia in comparison with epidural bupivacaine alone in one study.135 The investigators concluded that the optimal dose of butorphanol was 2 mg. Of concern was the observation of a transient sinusoidal FHR pattern in the 3-mg group that was not unlike that seen after the intravenous administration of butorphanol.136 However, there was no difference among groups in Apgar scores, umbilical cord blood gas and pH measurements, or neurobehavioral scores. Similarly, Abboud et al.124 observed that the addition of butorphanol 1 or 2 mg to 0.25% bupivacaine resulted in better quality and longer duration of analgesia than the epidural administration of bupivacaine alone, without maternal or neonatal side effects. However, some anesthesia providers have noted that the epidural administration of butorphanol results in somnolence and occasional dysphoria, which are side effects of κ-receptor stimulation.
Diamorphine (heroin) is available for epidural analgesia in the United Kingdom. Using isobolographic analysis, McLeod et al.137 concluded that the combination of diamorphine and levobupivacaine is additive when used for first-stage labor analgesia. Several studies from the United Kingdom have reported diamorphine doses between 250 and 500 µg/h (i.e., diamorphine 25 to 50 µg/mL combined with a low concentration of bupivacaine).125,138 Whether diamorphine offers any advantages over fentanyl or sufentanil has not been studied. It is not available for clinical administration in the United States.
Adjuvants
Although the contemporary mainstay of epidural labor analgesia includes administration of a long-acting amide local anesthetic combined with a lipid-soluble opioid, other drugs may be added as adjuvants. Adjuvants may prolong the duration of analgesia or decrease the required anesthetic dose, thus reducing the risk for specific side effects.
Epinephrine.
Some anesthesia providers add a low dose of epinephrine (1.25 to 5 µg/mL [1 : 800,000 to 1 : 200,000]) to the local anesthetic solution (Table 23-4). The addition of epinephrine shortens the latency and prolongs the duration of epidural bupivacaine analgesia.50,139 The MLAC of bupivacaine with epinephrine (66 µg) is 29% lower than that of bupivacaine without epinephrine,140 perhaps as a result of the stimulation of alpha-adrenergic receptors in the spinal cord.
TABLE 23-4
Adjuncts to Neuraxial Labor Analgesia
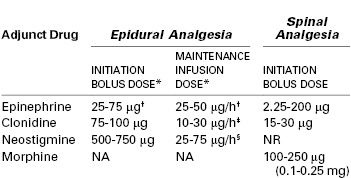
* Adjuncts are usually co-administered with a low-concentration local anesthetic solution (e.g., bupivacaine < 0.08%), often with a lipid-soluble opioid. There is extensive experience with epidural clonidine for labor analgesia in some European countries but less overall experience with epidural neostigmine.
† Usually administered in a 1 : 800,000 to 1 : 200,000 solution (1.25-5 µg/mL).
‡ Administered in a concentration of 0.75-1.5 µg/mL.
§ Administered in a concentration of 4 µg/mL.
NA, not applicable; NR, not recommended.
The addition of epinephrine to the local anesthetic has a variable effect on the systemic uptake of the local anesthetic in obstetric patients.141–143 The systemic absorption of epinephrine may increase maternal heart rate and transiently decrease uterine activity as a result of beta-adrenergic receptor stimulation.50,144,145 However, some studies have shown that the addition of epinephrine to bupivacaine, lidocaine, or levobupivacaine does not result in longer labor than the epidural administration of bupivacaine or lidocaine without epinephrine144,146 or levobupivacaine-sufentanil without epinephrine.147 Epidural administration of an epinephrine-containing local anesthetic solution does not adversely affect intervillous blood flow148 or neonatal outcome.139,141,147 One disadvantage of the use of epinephrine is that it increases the intensity of motor blockade.146,147 The addition of epinephrine may improve the efficacy of epidural opioids,149 but the enhanced effect is insufficient to make use of epidural opioids (without local anesthetic) an attractive regimen for the duration of labor. Finally, the addition of a third drug to the local anesthetic/opioid solution may increase the risk for drug error and contamination. For these reasons, at our institution my colleagues and I do not routinely administer epinephrine-containing local anesthetic solutions during labor. However, other anesthesia providers have a different view, and some consider epinephrine a useful adjuvant, especially when added to a very dilute solution of local anesthetic with an opioid.
Clonidine.
Analgesia is enhanced by the direct stimulation of α2-adrenergic receptors and the inhibition of neurotransmitter release in the dorsal horn of the spinal cord (see Chapter 20). Epidural administration of clonidine alone provides modest analgesia. Studies have evaluated the epidural administration of clonidine as an adjuvant to a local anesthetic alone,150–153 to local anesthetic and opioid combinations,154–158 to fentanyl,159 and to neostigmine (see later discussion).160,161 In an MLAC study,162 clonidine 60 µg, but not 30 µg, decreased the MLAC of ropivacaine by approximately two thirds. In another study,152 clonidine 75 µg and sufentanil 5 µg both reduced the MLAC of ropivacaine by about two thirds.152 Unlike epinephrine, clonidine does not increase the motor blockade that results from the epidural administration of a local anesthetic, but it does potentiate both the quality and duration of analgesia.150,151,154–158 However, in a “black box” warning on the package insert, the manufacturer of Duraclon (the epidural clonidine formulation approved by the U.S. Food and Drug Administration) recommends against its use in obstetric patients because of the risk for hypotension153–155,158,162 and bradycardia. Most studies, however, have found that the hypotension is readily amenable to treatment. An additional side effect is maternal sedation.155,156,162 High doses (> 150 µg) may be associated with FHR changes,154 although no adverse fetal affects have been observed with lower doses.
Clonidine is rarely used for labor analgesia in North America, but it is more widely used in some European countries. It may be particularly useful in women in whom other epidural analgesics are contraindicated or in those who have breakthrough pain with standard local anesthetic/opioid solutions, despite a functioning epidural catheter. In this circumstance, additional local anesthetic will result in motor block but clonidine will not.
Neostigmine.
Neostigmine prevents the breakdown of acetylcholine within the spinal cord. Acetylcholine binds to muscarinic receptors, leading to a reduction in neurotransmitter release and subsequent analgesia. Roelants et al.163 randomly assigned parturients to receive either epidural ropivacaine (20 mg) alone or epidural neostigmine (4 µg/kg) combined with ropivacaine 10 mg, with or without sufentanil 10 µg. The magnitude and duration of analgesia in the ropivacaine/neostigmine group was similar to that of the plain ropivacaine group but less than in the ropivacaine/sufentanil group. Neostigmine is hydrophilic, and the researchers hypothesized that only a small portion of the epidural dose penetrates the spinal cord.163 In a subsequent study, the same researchers compared epidural sufentanil 20 µg with sufentanil 10 µg combined with neostigmine 250, 500, or 750 µg.164 Neostigmine 250 µg with sufentanil was ineffective, but both 500 and 750 µg of neostigmine produced effective analgesia similar in duration to that obtained with sufentanil alone.
Because a synergistic antinociceptive effect of spinal α2-adrenergic agonists and cholinesterase inhibitors is suggested by animal studies,165 researchers have also investigated epidural neostigmine combined with clonidine.160 The combination of clonidine 75 µg with neostigmine 500 or 750 µg provided acceptable analgesia (visual analog scale pain score < 30/100 mm in 30 minutes) in approximately 80% of parturients. Epidural neostigmine 500 µg combined with clonidine 75 µg prolonged labor analgesia initiated with spinal ropivacaine and sufentanil.161
In another study, maintenance of epidural analgesia with a solution of neostigmine 4 µg/mL combined with bupivacaine 0.125% resulted in a 19% reduction in bupivacaine consumption compared with administration of bupivacaine alone.166 However, maternal sedation was noted in the neostigmine group in the first 5 to 20 minutes after initiation of epidural analgesia with bupivacaine and neostigmine 60 µg. Although no significant adverse maternal or neonatal effects were observed in any of the studies, further studies are required to determine the role of epidural neostigmine for routine labor analgesia.167 Neostigmine is not approved for neuraxial injection in the United States.
Summary
Epidural labor analgesia is usually initiated with the bolus injection of a local anesthetic combined with a lipid-soluble opioid. The advantages of the addition of an opioid to an epidural solution of local anesthetic include (1) lower total dose of anesthetic, (2) decreased motor blockade, (3) reduced shivering, and (4) greater patient satisfaction. Some anesthesia providers contend that local anesthetic–opioid techniques result in a lower risk for hypotension, but this belief is unproven. There are no clinically significant differences among the three commonly used, long-acting amide local anesthetics (bupivacaine, ropivacaine, levobupivacaine), nor between fentanyl and sufentanil. Other adjuvants (e.g., epinephrine, clonidine) may prove useful in selected patients, but they currently do not offer any significant advantages to low-dose local anesthetic/lipid-soluble opioid combinations. High-volume/low-concentration local anesthetic solutions compared with low-volume/high-concentration solutions are associated with lower dose requirements and better analgesia.
Initiation of Spinal Analgesia
Initiation of neuraxial analgesia with the intrathecal injection of an opioid, or an opioid combined with a local anesthetic, usually performed as part of a CSE technique, results in a rapid onset of analgesia with a low dose of drug(s) (see Table 23-2). The onset of effective spinal analgesia occurs faster than epidural analgesia, and more women have effective analgesia at 10 minutes.27 Intrathecal opioids can provide complete analgesia during early labor when the pain stimuli are primarily visceral. An intrathecal local anesthetic without an opioid is not commonly used for labor analgesia. Doses high enough to provide analgesia are associated with significant motor blockade, and lower doses either do not provide satisfactory analgesia or are associated with an unacceptably short duration of analgesia.168,169 A lipid-soluble opioid is combined with a local anesthetic (bupivacaine, ropivacaine, or levobupivacaine) when sacral analgesia is necessary for complete analgesia (e.g., initiation of analgesia during the active first stage or the second stage of labor). Studies in animal models support a synergistic interaction between spinal local anesthetics and opioids.170,171 Like the combination of an epidural local anesthetic with an opioid, the combination of an intrathecal opioid with a local anesthetic results in better quality and longer duration of analgesia,172,173 as well as a lower dose requirement for both drugs, compared with either drug used alone.168,169,174
Choice of Drugs
Opioids
Fentanyl and Sufentanil.
The two opioids most commonly used for initiation of spinal labor analgesia are fentanyl and sufentanil. When administered alone in early labor, intrathecal fentanyl and sufentanil provide complete analgesia without a sympathectomy or motor blockade. This is a particularly useful technique for patients in whom a sudden decrease in preload (secondary to neuraxial local anesthetic–induced sympathectomy) might not be well tolerated (e.g., patient with a stenotic heart lesion).
Studies suggest that the ED50 of intrathecal fentanyl varies from 5.5 to 18 µg.175–177 The wide range of published values may be explained by differences in patient population (e.g., parity), cervical dilation at initiation of analgesia, and definition of successful analgesia. Nelson et al.178 hypothesized that acute mixing of fentanyl in cerebrospinal fluid (CSF) may explain the large interindividual variability observed after the injection of intrathecal opioid. In an elegant study, the investigators determined CSF fentanyl concentration at the site of the lumbar injection 60 seconds after the fentanyl injection. CSF fentanyl concentration did not correlate with onset, sensory level, or duration of analgesia. Instead, decreased diastolic and increased systolic blood pressure correlated with duration of analgesia. The authors hypothesized that hemodynamic characteristics may influence the distribution of drug in the CSF and, hence, block characteristics.
Herman et al.177 determined that the ED95 of intrathecal fentanyl for parturients of mixed parity in early labor (cervical dilation ≤ 5 cm) was 17.4 µg (95% CI, 13.8 to 27.1) (Figure 23-3). The duration of analgesia is dose dependent but plateaus at 80 to 90 minutes after administration of 15 to 25 µg of fentanyl (Figure 23-4).175 There does not appear to be any reason to administer doses higher than 25 µg, because side effects (e.g., pruritus, respiratory depression) are also dose dependent.168,175,177
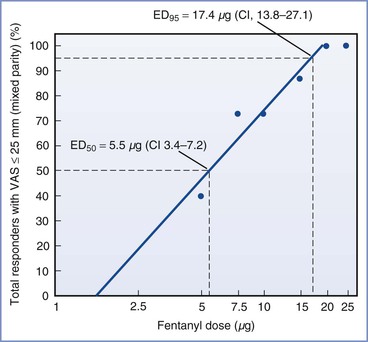
FIGURE 23-3 Dose-response relationship of intrathecal fentanyl in parturients in early labor (≤ 5 cm cervical dilation). The percent response for each dose (plotted on a common log scale) reflects the number of patients with adequate analgesia (visual analog scale [VAS] pain score ≤ 25/100 mm). The intrathecal fentanyl doses, 5, 7.5, 10, 15, and 20 µg, fell on the steep slope of the dose-response curve (between 40% and 100% responders). These data were used to construct the regression line to derive the 50% and 95% effective doses (ED50 and ED95, respectively) by observation. Each data point represents n = 15. CI, 95% confidence interval. (From Herman NL, Choi KC, Affleck AJ, et al. Analgesia, pruritus, and ventilation exhibit a dose-response relationship in parturients receiving intrathecal fentanyl during labor. Anesth Analg 1999; 89:378-83.)
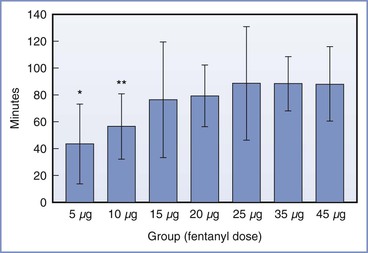
FIGURE 23-4 Duration of intrathecal fentanyl analgesia (mean ± SD) among nulliparous women in active labor who received 5, 10, 15, 20, 25, 35, or 45 µg. Duration of analgesia (time from intrathecal dose to first request for additional analgesia) differed significantly among the groups (analysis of variance [ANOVA], P < .005). *P < .05 versus groups 15 through 45 µg; **P < .05 versus groups 25 through 45 µg. (From Palmer CM, Cork RC, Hays R, et al. The dose-response relationship of intrathecal fentanyl for labor analgesia. Anesthesiology 1998; 88:355-61.)
The reported ED50 of intrathecal sufentanil varies from 1.8 to 4.1 µg,179–182 and the ED95 is 8 to 10 µg.179,182 A comparison of the potencies of fentanyl and sufentanil using ED50 estimates from different studies is difficult because of differences in patient populations and the definition of efficacy. In a single-center study, the relative potency ratio of intrathecal sufentanil to fentanyl for labor analgesia was estimated to be 4.4 : 1.176 When the drugs were administered at twice the ED50 (fentanyl 36 µg, sufentanil 8 µg), the duration of sufentanil analgesia was 25 minutes longer than that of fentanyl analgesia (104 versus 79 minutes), although the incidence of side effects was not different.176
Landau et al.183 investigated the influence of genetic variability of OPRM1 on the ED50 of intrathecal fentanyl for labor analgesia. Nulliparous women who were heterozygous or homozygous for A118G had a lower ED50 for intrathecal fentanyl (18 µg, 95% CI, 13 to 22) than women homozygous for the wild-type allele (A118) (27 µg, 95% CI, 23 to 31). Additionally, women in the group with the A118G allele requested analgesia at a more advanced cervical dilation. However, in a second study,184 there was no difference in the duration of intrathecal fentanyl analgesia in women with the A118G allele compared with women who were homozygous for the wild-type allele. Thus, the clinical implications of genetic polymorphisms of OPRM1 on labor analgesia remain unclear.185
Typically, an intrathecal opioid injection for labor analgesia is administered as part of a CSE technique. Maintenance epidural analgesia is usually initiated soon after initiation of spinal analgesia. Therefore, the duration of intrathecal analgesia is relatively less important. Nelson et al.176 concluded, and we concur, that the longer duration of sufentanil analgesia in comparison with fentanyl analgesia does not necessarily justify the former’s use. Other factors, such as cost and the greater risk for a drug dose error with sufentanil (because of its greater potency), should be considered. In some European countries sufentanil is available in a dilute concentration (5 µg/mL), possibly making it easier and safer to use.
Intrathecal fentanyl (or sufentanil) is often co-administered with an amide local anesthetic (see later discussion), most commonly bupivacaine (see Table 23-2). The addition of a local anesthetic to intrathecal fentanyl or sufentanil markedly decreases the dose of opioid necessary to produce analgesia. Wong et al.168 randomly assigned parous women to receive intrathecal bupivacaine 2.5 mg and intrathecal sufentanil 0, 2.5, 5, 7.5, or 10 µg, followed by a standard epidural test dose. There were no differences among the sufentanil groups in quality and duration of analgesia. These results suggest that a sufentanil dose as small as 2.5 µg is effective when combined with bupivacaine 2.5 mg. In current clinical practice, it is common to combine bupivacaine 2.5 mg with sufentanil 1.5 to 2 µg.186 Stocks at al.173 demonstrated that three different doses of intrathecal fentanyl (5, 15, and 25 µg) led to similar reductions in the ED50 of intrathecal bupivacaine, although both the duration of analgesia and the incidence of pruritus were dose dependent. As with sufentanil, the dose of intrathecal fentanyl is usually reduced when combined with bupivacaine.169 Intrathecal fentanyl 10 to 15 µg, combined with bupivacaine 2.5 mg, provides effective analgesia for most parturients.
Other Opioids.
Early studies demonstrated that the intrathecal administration of 0.5 to 2 mg of morphine reliably produced analgesia during the first stage of labor, but the analgesia was less reliable during the second stage of labor and during instrumental vaginal delivery.187,188 However, intrathecal administration of these relatively large doses of morphine resulted in a high incidence of side effects, including somnolence, nausea and vomiting, pruritus, and respiratory depression. In addition, the onset of analgesia is slower with intrathecal morphine than with lipid-soluble opioids, and the long duration of action may be a disadvantage (i.e., the parturient may deliver before the regression of side effects). Abouleish189 reported a case of life-threatening respiratory depression 1 hour after delivery and 7 hours after the administration of 1 mg of hyperbaric intrathecal morphine.
In several studies,190–192 low-dose morphine (0.1 to 0.25 mg) was successfully combined with intrathecal bupivacaine (2 to 2.5 mg) and fentanyl (12.5 to 25 µg); the combination resulted in short latency of onset and a prolonged duration of analgesia. In contrast, a single study from Sweden193 found no advantage to adding morphine 0.05 or 0.1 mg to bupivacaine 1.25 mg and sufentanil 5 µg. The addition of low-dose morphine to intrathecal bupivacaine and a lipid-soluble opioid may be useful in low-resource settings in which continuous epidural infusion techniques are impractical.33 When used as part of a CSE technique, the addition of intrathecal morphine to bupivacaine and fentanyl has been shown to result in less breakthrough pain during labor190–192 as well as decreased analgesic use in the first 24 hours postpartum, compared with intrathecal bupivacaine and fentanyl without morphine.190,191 The incidence of intrapartum side effects was similar190,192; however, the morphine group had a higher incidence of postpartum nausea (17% versus 0% for no morphine).190
An alternative drug is meperidine. Meperidine is unique among the opioids in that it possesses weak local anesthetic properties,131 and it has been used in large doses (e.g., 1 mg/kg) as the sole agent to provide spinal anesthesia for surgical procedures.194 Intrathecal administration of meperidine (10 to 20 mg) results in effective labor analgesia within 2 to 12 minutes, with a duration of 1 to 3 hours. Honet et al.195 compared the efficacy of intrathecal meperidine 10 mg, fentanyl 10 µg, and sufentanil 5 µg in 65 laboring women. The three drugs were similar in onset of analgesia (< 5 minutes) and duration of effective analgesia (80 to 100 minutes). However, the meperidine group had significantly lower pain scores after cervical dilation had progressed beyond 6 cm. As labor advances, the nature of pain becomes increasingly somatic; only meperidine also functions as a local anesthetic. This fact helps explain why meperidine provided more effective analgesia during advanced labor, including the second stage. Booth et al.196 observed that intrathecal meperidine was associated with a significantly higher incidence of nausea and vomiting than a combination of fentanyl and bupivacaine for labor analgesia. Therefore, intrathecal meperidine does not seem to offer any advantages over bupivacaine-fentanyl for routine intrathecal analgesia, although it may be useful for the rare patient with a contraindication to bupivacaine-fentanyl administration.
In the United Kingdom, some anesthesia providers have advocated the intrathecal administration of diamorphine (heroin) for labor analgesia, although it is not commonly used for this purpose. This drug is not available for clinical use in the United States. Kestin et al.197 observed that the intrathecal administration of diamorphine (0.2 to 0.5 mg) provided good to excellent analgesia in 90% of laboring women. The mean duration of analgesia was approximately 100 minutes. However, 75% of patients had pruritus, nausea, and vomiting. In contrast, Vaughan et al.198 randomly assigned parturients to receive intrathecal bupivacaine 2.5 mg with either fentanyl 25 µg or diamorphine 0.25 mg. Duration of analgesia was longer in the diamorphine group, but the incidence of side effects was low in both groups.
Local Anesthetics
In the late first stage and the second stage of labor, a local anesthetic must be added to the spinal opioid to block somatic stimuli from the vagina and perineum caused by descent of the fetus. The local anesthetic works synergistically with the opioid, so lower doses of both drugs can be used.168,169,173,199 Bupivacaine is most commonly combined with fentanyl or sufentanil. The ED95 of bupivacaine was 3.3 mg when combined with sufentanil 1.5 µg186 and 1.7 mg when combined with fentanyl 15 µg.200 Intrathecal bupivacaine doses between 1.25 and 2.5 mg are commonly used (see Table 23-2). Levobupivacaine and ropivacaine are not usually used for intrathecal injection in the United States. They are less potent than bupivacaine for intrathecal labor analgesia (Figure 23-5).186,201 Spinal lidocaine has not been studied for use in labor analgesia, but it is unlikely to have any advantages compared with other, longer-acting amide local anesthetics. Common doses of spinal local anesthetics are shown in Table 23-2.
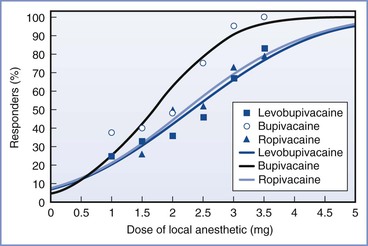
FIGURE 23-5 Predicted (lines) and observed (symbols) dose-response of intrathecal bupivacaine, levobupivacaine, and ropivacaine combined with sufentanil 1.5 µg in 450 laboring women. The dose-response curves were constructed with the use of a probit regression model. The curves were compared with use of likelihood ratio tests. No difference was observed between ropivacaine and levobupivacaine. Significant differences were observed between bupivacaine and ropivacaine (P = .003) and bupivacaine and levobupivacaine (P < .001). (From Van de Velde M, Dreelinck R, Dubois J, et al. Determination of the full dose-response relation of intrathecal bupivacaine, levobupivacaine, and ropivacaine, combined with sufentanil, for labor analgesia. Anesthesiology 2007; 106:149-56.)
Controversy exists as to whether the lower incidence and degree of motor blockade associated with ropivacaine and levobupivacaine66,201 are a result of their inherent difference in potency or of greater sensory-motor separation with the S(−)-enantiomer drugs.186 Camorcia et al.201 have suggested that, especially during intrathecal use, ropivacaine may be associated with less motor blockade than bupivacaine, even when equipotent doses (e.g., 3.6 mg ropivacaine and 2.4 mg bupivacaine) are administered. However, this difference, even if it exists, is unlikely to have any clinical significance during spinal labor analgesia because all local anesthetics administered for this purpose are administered in low doses that lead to minimal motor blockade.
Baricity of the Intrathecal Solution.
The local anesthetic/opioid solutions commonly injected for intrathecal labor analgesia have lower specific gravity relative to that of CSF and hence are hypobaric.202 The extent of cephalad sensory blockade is higher for spinal analgesia initiated with the parturient in the sitting position than in the lateral position.203 Adding dextrose to the solution (opioid alone or opioid with local anesthetic) to make the solution hyperbaric results in less extensive sensory blockade but also in inadequate analgesia.204–206 It is probably necessary for the opioid to penetrate the spinal cord rather than just the nerve roots; therefore, injection of a hyperbaric solution of opioid and local anesthetic below the level of the spinal cord may lead to inadequate analgesia, even though the local anesthetic provides sensory blockade to the T10 dermatome.
Intrathecal Adjuvants
Several drugs have been investigated as adjuvants to local anesthetics, opioids, or combinations of local anesthetics and opioids for intrathecal labor analgesia (see Table 23-4). In one study, the addition of clonidine 30 µg to sufentanil (2.5 to 5 µg) prolonged the duration of analgesia from 104 to 145 minutes without motor block.207 Other investigators have had similar results when clonidine was combined with sufentanil,208 bupivacaine/ropivacaine and sufentanil,209,210 and neostigmine.211 Intrathecal clonidine alone also provides analgesia.212 Unfortunately, a disadvantage of clonidine is the high incidence of maternal hypotension and sedation as well as FHR abnormalities. The slightly longer duration of analgesia provided by the addition of clonidine to bupivacaine and sufentanil is not an advantage when maintenance analgesia is provided by a continuous epidural infusion. Therefore, at present, intrathecal clonidine cannot be recommended for routine spinal labor analgesia, although it might be considered in parturients with contraindications to the use of other drugs.167
Adding intrathecal neostigmine to sufentanil, bupivacaine/sufentanil, or clonidine has been found to potentiate the analgesia and prolong its duration.211,213 However, intrathecal neostigmine was associated with a markedly higher incidence of severe nausea that was unresponsive to standard antiemetics.211,213 Therefore, neostigmine cannot be recommended as an adjuvant for intrathecal labor analgesia.
Analgesia is prolonged by 15 to 40 minutes when epinephrine is added to intrathecal bupivacaine-opioid.214–216 Even an epinephrine dose as low as 2.25 µg prolonged analgesia by 15 minutes.215 However, epinephrine 200 µg combined with bupivacaine 2.5 mg and sufentanil 10 µg resulted in a significant incidence of motor blockade214; epinephrine doses between 12.5 and 100 µg prolonged analgesia without any difference in the quality of analgesia.216
In summary, no adjuvant studied to date prolongs the duration of fentanyl or sufentanil/bupivacaine analgesia long enough to avoid the use of maintenance epidural analgesia for most parturients, and no adjuvant reduces or eliminates the side effects associated with the analgesic drugs used clinically. Therefore, it makes little sense to routinely add adjuvant drugs, because they are associated with higher cost, higher rate or severity of side effects, and probably an increased risk for drug error.
Maintenance of Analgesia
Epidural Analgesia
Painful labor lasts several hours in most parturients; therefore, a single intrathecal or epidural injection of local anesthetic and/or opioid typically does not provide adequate analgesia for the duration of labor. Supplemental doses are needed to maintain analgesia in most women. Neuraxial analgesia is maintained with the intermittent or continuous administration of analgesics, usually a combination of a long-acting amide local anesthetic and a lipid-soluble opioid. By far the most common technique is administration of drugs via a catheter into the epidural space. It is occasionally advantageous to administer drugs via a catheter into the subarachnoid space.
Drugs for the Maintenance of Epidural Analgesia
In the past, epidural labor analgesia was maintained with the intermittent injection or continuous infusion of a neuraxial local anesthetic alone. Currently, most anesthesia providers maintain analgesia with a combination of a low-dose, long-acting amide local anesthetic and a lipid-soluble opioid (Table 23-5). In practice, neither lidocaine nor 2-chloroprocaine is used for maintenance of analgesia. Both have a short duration of action, and tachyphylaxis may develop more quickly with either of these local anesthetics than occurs with the longer-acting local anesthetics. Lidocaine crosses the placenta to a greater extent than bupivacaine, and there is less differentiation between the dose required for sensory and motor blockade.217 There is no evidence that any one of the three long-acting local anesthetics (bupivacaine, ropivacaine, levobupivacaine) has any advantages in terms of clinical outcomes over the other two.66,75,76 Fentanyl is more often detected in umbilical artery blood samples than sufentanil (as discussed earlier)109; however, neonatal outcomes are good after maintenance epidural analgesia with either drug.
TABLE 23-5
Anesthetic Solutions for Maintenance of Epidural Analgesia: Continuous Infusion or Patient-Controlled Epidural Analgesia*
| Drug† | Concentration |
| Local Anesthetics | |
| Bupivacaine | 0.05-0.125% |
| Ropivacaine | 0.08-0.2% |
| Levobupivacaine | 0.05-0.125% |
| Lidocaine‡ | 0.5%-1.0% |
| Opioids | |
| Fentanyl | 1.5-3 µg/mL |
| Sufentanil | 0.2-0.4 µg/mL |
* Local anesthetic is most often combined with an opioid.
† Continuous infusions are usually administered at a rate of 8-15 mL/h into the lumbar epidural space.
‡ Lidocaine is not usually used for maintenance of epidural analgesia because it crosses the placenta to a greater extent than the other amide local anesthetics and may be associated with greater tachyphylaxis.
As with the induction dose, the combination of a local anesthetic with a lipid-soluble opioid allows administration of a lower concentration and a smaller total dose of local anesthetic for maintenance of analgesia. This approach improves safety and leads to less motor blockade and greater patient satisfaction. Chestnut et al.98 demonstrated that maintenance of epidural analgesia by a continuous infusion of 0.0625% bupivacaine with fentanyl 2 µg/mL resulted in comparable maternal and neonatal outcomes, with a lower incidence of motor blockade, compared with maintenance of analgesia by a continuous epidural infusion of 0.125% bupivacaine alone. When administered as intermittent epidural boluses for the maintenance of analgesia, the addition of sufentanil to bupivacaine resulted in better quality analgesia and decreased motor blockade at delivery.105
In contemporary clinical practice, the bupivacaine concentration of maintenance bupivacaine/opioid solutions ranges from 0.05% to 0.125%. Hess et al.218 retrospectively analyzed the use of three solutions at their institution: bupivacaine 0.125% and bupivacaine 0.0625%, both with fentanyl 2 µg/mL, administered at 8 to 12 mL/h, and bupivacaine 0.04% with fentanyl 1.7 µg/mL and epinephrine 1.7 µg/mL, administered at 15 mL/h. There were more interventions for breakthrough pain in the two low-concentration groups and more interventions for hypotension and motor blockade in the high-concentration group. Beilin et al.219 initiated analgesia with intrathecal bupivacaine/fentanyl and an epidural test dose, and then randomly assigned women to receive maintenance epidural analgesia with one of four solutions: bupivacaine 0.125%, bupivacaine 0.0625%, or bupivacaine 0.04% with epinephrine 1.7 µg/mL (all with fentanyl 2 µg/mL) or placebo (saline) at 10 mL/h. The time to request for supplemental analgesia was longest in the bupivacaine 0.125% group; however, this group also had a higher incidence of motor blockade than the other groups. Therefore, to avoid motor blockade, it would seem reasonable to use a bupivacaine concentration less than 0.125%, especially if it is administered via continuous epidural infusion (see later discussion).
The dose-response relationships for fentanyl and sufentanil combined with a local anesthetic for the maintenance of epidural analgesia have not been well studied. The concentration range of fentanyl used in clinical practice is 1.5 to 3 µg/mL, and that of sufentanil, 0.2 to 0.33 µg/mL. The optimal opioid concentration probably varies according to the local anesthetic concentration, the mode of drug delivery (i.e., bolus versus infusion), presence of epinephrine, and the stage of labor, among other factors. Bader et al.220 infused epidural bupivacaine 0.125% with fentanyl 2 µg/mL at 10 mL/h for 1 to 15 hours. Maternal and neonatal fentanyl concentrations, and their ratio, remained constant over the infusion period, and no adverse maternal or neonatal outcomes were noted. Porter et al.221 compared neonatal outcomes in women randomly assigned to receive epidural bupivacaine with fentanyl 2.5 µg/mL or bupivacaine alone to maintain analgesia. There were no differences between groups in measures of neonatal well-being at birth or 24 hours after delivery.
Bernard et al.222 combined sufentanil 0, 0.078, 0.156, 0.312, or 0.468 µg/mL with bupivacaine 0.125% and epinephrine 1.25 µg/mL. Each solution was administered as a 12-mL bolus via PCEA. Sufentanil concentrations lower than 0.156 µg/mL did not provide adequate analgesia for the second stage of labor, and higher doses were associated with an increased incidence of pruritus. Loftus et al.109 compared bupivacaine with sufentanil 0.25 µg/mL or fentanyl 1.5 µg/mL as a continuous epidural infusion at 10 mL/h. Neonates in the fentanyl group had slightly lower 24-hour neuroadaptive capacity scores (NACS) than the sufentanil group.
Administration Techniques
Intermittent Bolus.
Before the introduction of infusion pumps, epidural analgesia was routinely maintained by the intermittent administration of an additional therapeutic bolus dose of local anesthetic when analgesia began to wane. When the patient began to experience recurrent pain, the anesthesia provider assessed the pain relative to the stage of labor and the extent of sensory blockade and then administered another epidural bolus of local anesthetic. Analgesia was usually reestablished with the bolus injection of 8 to 12 mL of a local anesthetic/opioid solution.
The spread and quality of analgesia may change with repeated lumbar epidural injections of local anesthetic. After several injections, blockade of the sacral segments, intense motor blockade, or both may develop.146 The sensory level and the intensity of motor blockade should be assessed and recorded before and after each bolus injection of local anesthetic.
This intermittent bolus technique has several disadvantages, the most salient of which is that pain relief is constantly interrupted by the regression of analgesia. The patient must notify the labor nurse or midwife that she is again uncomfortable and request additional analgesia. In the United States, labor nurses are not allowed to administer additional epidural analgesic drugs223; therefore, the nurse must call the anesthesia provider, resulting in unavoidable delays in administration of additional analgesic drugs and additional pain for the patient.
Continuous Infusion.
Administration of a continuous epidural infusion of a dilute solution of local anesthetic combined with an opioid is a popular technique for the maintenance of epidural analgesia during labor. The potential benefits of a continuous epidural infusion include the maintenance of a stable level of analgesia and a less-frequent need for bolus doses of local anesthetic, which may reduce the risk for systemic local anesthetic toxicity. An additional advantage is a decreased workload for the anesthesia provider.
Published studies, however, have suggested that the continuous epidural infusion and intermittent bolus injection techniques have a comparable safety record. Studies comparing intermittent bolus injections with continuous infusion were performed before the era of neuraxial opioid administration; thus, the studies used concentrations of bupivacaine (0.125% to 0.25%) higher than those typically used in contemporary practice. In theory, maintenance of a constant level of anesthesia should promote maternal hemodynamic stability and improve fetal and neonatal outcome. Only one published study has suggested a trend toward less frequent hypotension and a lower incidence of abnormal FHR patterns during the continuous epidural infusion of bupivacaine than with intermittent bolus injections of bupivacaine; however, neonatal outcomes were similar with the two techniques.224
Randomized trials of intrapartum epidural analgesia maintained by either intermittent bolus injection or continuous infusion of bupivacaine have consistently demonstrated that women require fewer bolus injections administered by the anesthesia provider (i.e., fewer episodes of breakthrough pain) with the continuous infusion technique.224–226 The continuous infusion technique lengthens the time between bolus injections and leads to greater patient satisfaction.226,227 This is advantageous in a busy obstetric anesthesia practice, in which an anesthesia provider may not always be available to give an additional bolus dose of local anesthetic immediately after the onset of recurrent pain.
Most studies suggest that the continuous epidural infusion technique leads to the administration of a larger total dose of bupivacaine,225–228 but such a dose does not seem to result in higher maternal venous or umbilical venous bupivacaine concentrations at delivery.226,228 The continuous epidural infusion of bupivacaine often achieves satisfactory perineal analgesia, obviating the need for a bolus dose of local anesthetic at delivery. Unfortunately, a prolonged epidural infusion of 0.125% bupivacaine at 10 to 14 mL/h may cause significant motor blockade.86,224,225,227,228 Titrating the dose of bupivacaine to meet the individual needs of each patient (rather than administering the same dose to all patients), as well as reducing the total mass of bupivacaine by lowering the local anesthetic concentration and adding an opioid, helps minimize motor blockade while providing effective analgesia.
Migration of the epidural catheter into the subarachnoid, subdural, or intravenous space may occur with either the intermittent bolus injection or continuous infusion technique. If the epidural catheter should migrate into a vein during the continuous epidural infusion of a dilute solution of local anesthetic, it is unlikely that the patient will have symptoms of local anesthetic toxicity; rather, the level of anesthesia will regress. For this reason, the anesthesia provider should suspect the intravenous migration of an epidural catheter when a patient unexpectedly complains of pain during maintenance of analgesia during a continuous epidural infusion.
Migration of the epidural catheter into the subdural or subarachnoid space during an infusion should result in the slow ascent of the level of anesthesia and a greater density of motor blockade. These observations apply to the epidural infusion of a 0.125% solution of bupivacaine at a modest rate (e.g., 5 to 8 mL/h). The continuous infusion of a more concentrated solution or the use of a more rapid rate of infusion most likely narrows the margin of safety.
Patient-Controlled Epidural Analgesia.
The method of delivering the anesthetic solution into the epidural space influences the density of neuroblockade. Given the same concentration of local anesthetic, analgesia maintained by infusion results in greater drug use, a higher degree of motor blockade,225,229 and a higher incidence of instrumental vaginal delivery than intermittent boluses.228 However, intermittent manual bolus administration by the anesthesia provider results in more breakthrough pain, less patient satisfaction, and more work for the anesthesia provider. PCEA is a method of delivering anesthetic solution to the epidural space that overcomes these disadvantages. Since its first description in 1988 by Gambling et al.,230 many studies have consistently found that the analgesia with PCEA is comparable to that with continuous infusion techniques.229,231–237
Van der Vyver et al.238 reported a meta-analysis of nine randomized controlled trials (n = 640) comparing PCEA (without a background infusion) with continuous epidural infusion analgesia. There were fewer anesthetic interventions in the PCEA group, and the total bupivacaine dose was lower, as was the incidence of motor blockade (Figure 23-6). There were no differences in pain scores, patient satisfaction, and maternal and neonatal outcomes between groups.
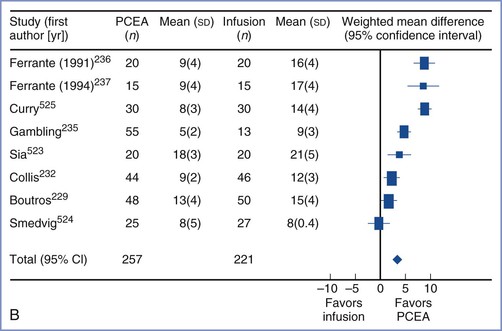
FIGURE 23-6 Meta-analysis of patient-controlled epidural analgesia (PCEA) without background infusion compared with continuous epidural infusion for maintenance of analgesia. A, The number of patients requiring no unscheduled interventions by the anesthesia provider was lower in the PCEA group (risk difference 27%; 95% confidence interval [CI], 18 to 36). B, The dose of local anesthetic (mg/h) was lower in the PCEA group (weighted mean difference, −3.9 mg; 95% CI, −5.4 to −2.4). (From van der Vyver M, Halpern S, Joseph G. Patient-controlled epidural analgesia versus continuous infusion for labour analgesia: a meta-analysis. Br J Anaesth 2002; 89:459-65.)
Data are conflicting as to whether PCEA should include a background infusion.237,239–247 Most studies have reported background infusions of 3 to 4 mL/h. Bupivacaine consumption is higher in PCEA with a background infusion than in a pure PCEA technique without a background infusion.238 A meta-analysis of five studies237,240,241,243,244 reported in the ASA Practice Guidelines for Obstetric Anesthesia16 concluded that a background infusion provides better analgesia than pure PCEA without a background infusion. In a 2009 review, Halpern and Carvalho248 concluded that a background infusion improves analgesia and results in fewer unscheduled interventions by the anesthesia provider. There is no evidence that the higher local anesthetic dose associated with a background infusion increases motor blockade or has adverse effects on obstetric outcome when low-concentration infusion solutions are used. A typical background infusion provides one third to one half of the total hourly dose.248 Sng et al.249 described a novel PCEA system in which they used a computer-integrated infusion pump to modify the background infusion rate based on the previous hour’s requirement for patient-administered bolus doses.
A wide variety of PCEA regimens have been described (Table 23-6). The anesthesia provider can manipulate the infusion solution (local anesthesia/opioid concentration), patient-controlled bolus volume, lockout interval, background infusion rate, and maximum allowable dose per hour. Patient-controlled bolus doses from 2 to 20 mL and lockout intervals from 5 to 30 minutes have been reported235,245–247,250–253; most studies have evaluated patient-controlled bolus doses of 5 to 12 mL. No study has found any differences in unscheduled provider interventions when investigators manipulated the patient-controlled bolus dose and lockout interval. There are no published reports of toxicity with larger bolus volumes, although the study populations were too small to allow determination of safety. Taken together, these studies suggest that there is no ideal bolus dose/volume or lockout interval for labor PCEA.
TABLE 23-6
Sample Patient-Controlled Epidural Analgesia (PCEA) Settings*

* Anesthetic solutions are shown in Table 23-5.
Various local anesthetic concentrations also have been studied. No studies have reported any differences in analgesia efficacy. Use of more-concentrated local anesthetic solutions results in higher local anesthetic consumption241,254–256 and greater motor blockade than use of less-concentrated solutions.245,254,257 Thus, as with continuous infusion epidural analgesia, administration of a dilute local anesthetic solution combined with an opioid results in less local anesthetic consumption and motor blockade without a reduction in analgesia efficacy.
In summary, solutions used for PCEA are identical to those used for continuous epidural infusion analgesia (see Table 23-5). It is suggested that larger bolus volumes be used if PCEA is administered without a background infusion. Early PCEA studies investigated higher-concentration local anesthetic solutions (i.e., 0.125% to 0.25% bupivacaine), smaller bolus volumes (≤ 5 mL), and low background infusion rates (3 to 5 mL/h). Given the more recent data supporting the efficacy of epidural administration of higher volumes of more dilute solutions of local anesthetic, it appears reasonable to apply this principle to PCEA. The safety of large-volume boluses (> 10 mL) has not been determined.
Timed Intermittent Bolus Injection.
Bolus administration of a local anesthetic into the epidural space results in better analgesia than continuous epidural infusion. Likewise, larger volumes of a less concentrated anesthetic solution provide better analgesia than smaller volumes of a more concentrated solution. Presumably, distribution of anesthetic solution in the epidural space is better when larger volumes are administered under high injection pressure.258 Several studies have demonstrated that timed (automated) intermittent boluses (5 to 10 mL every 30 to 60 minutes) administered via a programmable pump result in improved patient satisfaction, less drug use, longer duration of analgesia, and less breakthrough pain than a continuous infusion of the same mass of drug per unit of time.259–263 For example, Wong et al.259 randomly assigned patients to receive either a continuous epidural infusion of a dilute bupivacaine/fentanyl solution at 12 mL/h or 6 mL of the same solution delivered as an automated bolus every 30 minutes. Similarly, Sia et al.263 randomly assigned patients to receive either a continuous epidural infusion of a ropivacaine/fentanyl solution at 5 mL/h or 5 mL of the same solution delivered as an automated bolus every hour. All patients in both studies were allowed PCEA for the treatment of breakthrough pain. The total dose of local anesthetic was smaller in the automated bolus groups than in the continuous infusion groups.
Capogna et al.264 compared motor block and mode of delivery in women randomized to receive an automated timed bolus of levobupivacaine 0.0625% with sufentanil 0.5 µg/mL (10 mL every 60 minutes) or the same solution as a continuous infusion (10 mL/h). Women were able to treat breakthrough pain with PCEA using levobupivacaine 0.125% (5-mL bolus). The incidence of motor block was greater in the continuous infusion group. Of interest, the rate of instrumental vaginal delivery was also higher in the continuous infusion group (7% versus 20%; risk ratio, 2.9; 95% CI, 1.1 to 7.9).
In a systematic review and meta-analysis of nine studies (n = 344), George et al.265 concluded that the intermittent programmed bolus technique was associated with a small decrease in total anesthetic consumption and improved patient satisfaction. Although no difference was found in the need for anesthesia provider intervention and other outcomes, the confidence intervals were wide. The authors concluded that further study is needed to ascertain whether this technique impacts clinically significant anesthetic and obstetric outcomes. Infusion pumps with the ability to deliver this mode of analgesia are now coming on the market.
Patient Monitoring during Maintenance Epidural Analgesia
The use of a continuous epidural infusion technique or PCEA does not abolish the need for frequent assessment of the patient by the anesthesia provider at regular intervals. Assessment should involve determining the quality of analgesia and progress of labor, recording the sensory level and intensity of motor block, and reviewing maternal vital signs and FHR tracings for the previous hour. An inappropriately high level of anesthesia signals the administration of an excessive dose of local anesthetic or subdural or subarachnoid migration of the catheter. A low level of anesthesia may signal intravenous migration of the catheter, movement of the catheter outside the epidural space, or administration of an inadequate dose of local anesthetic.
Equipment
Anesthesia providers should consider the safety of their equipment when choosing a maintenance technique. The use of an infusion pump identical to that used for the intravenous administration of other drugs increases the chance that a nurse or physician will inject oxytocin, magnesium sulfate, or another drug into the epidural space unintentionally. Thus, the use of an infusion pump that is used exclusively for epidural analgesia and that differs from the pumps used for intravenous drug and fluid administration is recommended. The pump should be easy to use, reliable, adjustable, and sturdy. PCEA pumps should differ from patient-controlled intravenous analgesia (PCIA) pumps. The PCEA “buttons” should be labeled with instructions that only the patient (not medical providers or family members) should push the button. If possible, pumps should be preprogrammed with maximum safe limits to prevent errors in pump programming.
The anesthesia provider should use infusion tubing (which connects the pump to the epidural catheter) that is unique for the epidural administration of drugs. Some tubing is color coded (yellow). The presence of an injection side-port increases the likelihood of unintentional epidural administration of the wrong drug; thus, use of tubing that does not have an injection side-port is recommended. The epidural catheter and tubing should be clearly labeled with the word “epidural.” Patient safety experts266 and the U.K. National Patient Safety Agency (NPSA)267 have recommended that syringes, needles, and catheters used for neuraxial injections be modified so that it is not possible to use this equipment for intravenous injections, thus making the possibility of drug administration error less likely.
Each labor unit must have a clear policy as to who may administer and adjust epidural infusion parameters. Anesthesia personnel should be responsible for changes in the content or rate of the infusion and the volume of bolus doses or develop protocols that allow nurses to make changes within the guidelines of the protocol or by order of the anesthesia provider. In the presence of maternal distress or fetal bradycardia, the nurse or obstetrician may discontinue the epidural infusion, but the anesthesia provider should be notified immediately.
Solutions for maintenance of neuraxial analgesia, consisting of dilute local anesthetic and opioid, require careful preparation, because these solutions are not commercially available. A hospital pharmacist or compounding pharmacy should prepare the solution in a clean or sterile environment. Preservative-free drugs and saline should be used to prepare the solutions. Solution contents should always be double-checked for content and expiration date by the anesthesia provider before analgesia is initiated.
Spinal Analgesia
Placement of a catheter in the subarachnoid space allows the anesthesia provider to administer continuous spinal analgesia by intermittent bolus injection or continuous infusion of a local anesthetic combined with an opioid. Continuous spinal analgesia is an option when unintentional dural puncture has occurred (see later discussion). The technique has also been described for use in patients in whom placement of an epidural catheter is difficult (e.g., in patients with morbid obesity or abnormal vertebral anatomy, such as kyphoscoliosis, or in patients with severe cardiac disease who require careful titration of analgesia.)268
Reports of this technique usually describe the use of a standard epidural catheter placed through an 18- or 19-gauge epidural needle. To reduce the risk for post–dural puncture headache, very small (e.g., 28- to 32-gauge) catheters were developed for insertion through small (e.g., 22- to 26-gauge) spinal needles. Unfortunately, several cases of cauda equina syndrome (associated with the use of spinal microcatheters during surgery in nonpregnant patients) prompted the U.S. Food and Drug Administration to remove these microcatheters from the market in 1992.269 The etiology of these neurologic deficits is unclear. Some anesthesiologists have suggested that neurologic injury may result from the maldistribution of local anesthetic within the subarachnoid space.270 The very slow rate of injection through a caudally directed microcatheter may lead to pooling of local anesthetic solution in the terminal part of the dural sac. If the local anesthetic solution is hyperbaric, the neighboring elements of the cauda equina experience prolonged exposure to a high concentration of local anesthetic and a hyperglycemic, hyperosmotic marinade (e.g., 550 to 800 mOsm/L). Permanent neural damage may occur from the combination of tissue dehydration and a toxic concentration of local anesthetic. It is unclear whether this complication is unique to the use of microcatheters.
Arkoosh et al.271 reported a randomized multicenter study comparing continuous spinal labor analgesia (via a 28-gauge catheter) with continuous epidural analgesia. The incidence of neurologic complications was not different between the two groups, and patients in the spinal group had better early analgesia, less motor blockade, and better patient satisfaction. The incidence of post–dural puncture headache also was not different between the two groups (spinal 9%, epidural 4%; P = .10), although a type II statistical error is possible given the size of the study and the low incidence of this outcome. The spinal catheter was associated with a higher incidence of technical difficulties and catheter failures. The researchers concluded that larger studies are needed to determine the safety of the spinal catheter, which is not marketed in the United States.
Continuous spinal analgesia can be initiated with the same drug combination and dose used to initiate CSE analgesia (see Table 23-2).268 For maintenance of analgesia, my colleagues and I administer our standard epidural solution (0.06% bupivacaine with fentanyl 2 µg/mL) at an initial rate of 2 mL/h. The infusion is then titrated to patient needs. We prefer to use our standard PCEA pumps for the continuous infusion, with the PCEA function disabled. This approach allows the anesthesia provider to administer a small (1 to 3 mL) bolus from the infusion bag without disconnecting the spinal catheter from the infusion tubing. Opening the infusion system to air may increase the risk for contamination and drug error. The catheter and pump should be clearly labeled so that all care providers know that the catheter is a spinal, not an epidural, catheter.
Patient-controlled spinal analgesia for labor has been described.272 Continuous spinal analgesia with opioids has also been described for patients with obstructive cardiac lesions.273,274 If intrathecal local anesthetics are used for intrapartum analgesia, the sensory level and the intensity of motor blockade should be monitored. Moreover, the anesthesia provider must be prepared to treat hypotension and other complications associated with high spinal anesthesia.
Ambulatory “Walking” Neuraxial Analgesia
The term “walking” or “mobile” epidural analgesia was first coined to describe low-dose CSE opioid analgesia because motor function was maintained and the ability to walk was not impaired.275 However, the term is more accurately applied to any neuraxial analgesic technique that allows safe ambulation. Initial studies using clinical testing to assess sensory and motor impairment and dorsal column function produced conflicting results. After initiation of epidural analgesia with 15 mL of 0.1% bupivacaine/fentanyl 2 µg/mL, Buggy et al.276 demonstrated that 66% of women had altered proprioception and 38% had impaired vibration sense. In contrast, Parry et al.277 found that dorsal column function was impaired in only 7% of laboring women who received low-dose epidural or CSE analgesia. The same group of investigators then used computerized dynamic posturography to assess balance in nonpregnant women, term pregnant women not in labor, and laboring women after initiation of CSE analgesia with bupivacaine 2.5 mg and fentanyl 5 µg.278 Pregnancy significantly affected balance function, but initiation of CSE analgesia did not further impair function. However, further supplementation of analgesia with the epidural injection of 10 mL of 0.1% bupivacaine/fentanyl 2 µg/mL in a subgroup of patients resulted in impaired balance function. The investigators concluded that the results support the safety of allowing ambulation after low-dose CSE analgesia, but further studies are required to understand the relative contributions of dorsal column function, proprioception, and lower limb motor strength to overall balance and ability to ambulate.278
Several studies have shown that an epidural test dose containing lidocaine 45 mg and epinephrine 15 µg adversely affects the ability to ambulate after initiation of CSE or low-dose epidural analgesia.42,43
The concept of the “walking epidural” is popular in the lay press; however, many women, once comfortable, prefer to rest rather than ambulate. The ability to walk to the toilet or sit in a chair at the bedside, however, remains desirable to many laboring women. In a small study, the ability to walk to the toilet to void resulted in lower postvoid residual volume than voiding on a bedpan.279 Although ambulation per se has not been shown to affect the progress or outcome of labor positively or negatively, dense motor blockade may adversely affect the spontaneous vaginal delivery rate (see later discussion). Thus, the intent of the “walking epidural”— minimization of motor blockade—should be the goal of the anesthesia provider, whether or not the patient wishes to ambulate.
Safe ambulation during labor requires several safeguards (Box 23-5). Prior to ambulation, orthostatic blood pressure and heart rate should be measured and motor function and balance must be assessed. The patient should not ambulate alone.
Analgesia/Anesthesia for Vaginal Delivery
During the second stage of labor, pain results from distention of the pelvic floor, vagina, and perineum. Pain impulses are transmitted to the spinal cord by means of somatic nerve fibers that enter the cord at S2 to S4. These somatic nerve fibers are larger than the visceral afferent nerve fibers that transmit the pain of the first stage of labor. Blockade of these larger nerve fibers may require administration of a more concentrated solution and/or a greater volume of local anesthetic than is required during the first stage of labor51; this need often creates a dilemma for the anesthesia provider. Administration of a more concentrated solution of local anesthetic results in more intense motor blockade at a time when maternal expulsive efforts are helpful.
The continuous epidural infusion of bupivacaine often leads to the gradual development of sacral analgesia. Likewise, several lumbar epidural injections of local anesthetic (given every 60 to 90 minutes) may result in sacral analgesia.146 If analgesia is not adequate for the second stage of labor and delivery, the anesthesia provider can give additional doses of local anesthetic to augment perineal analgesia (Box 23-6). Some anesthesia providers contend that the use of the sitting position helps facilitate the onset of perineal analgesia. Published studies suggest that maternal position does not consistently affect the spread of local anesthetic in the epidural space280,281; rather, the administration of a larger volume of local anesthetic solution facilitates the onset of sacral analgesia.282 Unfortunately, the larger volume also results in a higher (i.e., more cephalad) sensory level of analgesia, so the patient should be observed for evidence of hemodynamic or respiratory compromise.
Dense anesthesia is often required for delivery, especially if the obstetrician performs an episiotomy or a forceps or vacuum-extraction delivery. After administration of a test dose (3 mL of the local anesthetic solution), at our institution my colleagues and I administer 5 to 10 mL of 1% to 2% lidocaine or 2% to 3% 2-chloroprocaine. We inject this “delivery dose” when the fetal head is visible on the perineum during pushing or when the obstetrician has decided to proceed with instrumental vaginal delivery. The anesthesia provider should monitor the maternal blood pressure carefully, especially if excessive blood loss occurs in a patient with extensive anesthesia.
Occasionally a parturient tolerates the pain of labor until late in the first stage (i.e., more than 8 cm cervical dilation) and then requests analgesia. Advanced labor does not preclude initiation of neuraxial analgesia, especially in a nulliparous woman, in whom the second stage of labor may last 2 to 3 hours. However, initiation of lumbar epidural analgesia in the late first stage of labor often results in inadequate sacral analgesia unless large volumes of a concentrated local anesthetic solution are administered. This leads to higher cephalad sensory blockade than necessary and dense motor blockade. Another option is to administer CSE analgesia. The advantages of this technique are that it provides a rapid onset of spinal analgesia with sacral coverage for advanced labor and that it includes the placement of an epidural catheter. Additional local anesthetic can be administered through the epidural catheter if the extent or duration of spinal analgesia is inadequate. One disadvantage is that the correct placement of the epidural catheter in the epidural space cannot be verified until intrathecal analgesia regresses.
A caudal epidural catheter, which facilitates the onset of sacral analgesia, is an option for analgesia late in labor. Caudal analgesia was the first form of neuraxial analgesia used during labor. However, caudal analgesia is used rarely in modern obstetric anesthesia practice. Sacral analgesia adequate for labor and delivery can be achieved with an injection of 12 to 15 mL of 0.25% bupivacaine, 1.0% to 1.5% lidocaine, or 2% 2-chloroprocaine.
Single-shot spinal anesthesia for vaginal delivery may be indicated in a parturient who does not have epidural anesthesia and who requires perineal anesthesia. A so-called saddle block can be administered to achieve blockade of the sacral spinal segments; a small dose of a hyperbaric local anesthetic solution is adequate for this purpose. A saddle block may be advantageous in the patient with a preterm fetus or a vaginal breech presentation. In these cases, dense perineal relaxation may facilitate an atraumatic vaginal delivery. A saddle block performed with the patient in the sitting position with hyperbaric local anesthetic solution provides excellent anesthesia for an outlet/low forceps delivery. A higher level (T10) of anesthesia often is required for a midforceps delivery.
Clear communication between the obstetrician and anesthesia provider is essential. If the obstetrician is certain that the application of forceps (or vacuum extraction) will result in a successful delivery, a saddle block will likely provide satisfactory anesthesia. However, in some cases, the obstetrician will perform a trial of forceps. We alter our technique when giving spinal anesthesia for a trial of forceps. If the trial fails, cesarean delivery must follow. In some cases, we give a dose of local anesthetic appropriate for cesarean delivery. Alternatively, a saddle block can be administered via the CSE technique. If spinal anesthesia is inadequate for the planned procedure, additional local anesthetic can be given through the epidural catheter.
Side Effects of Neuraxial Analgesia
Hypotension
Neuraxial anesthesia–induced sympathetic blockade leads to peripheral vasodilation and increased venous capacitance. Recent data from women undergoing spinal anesthesia for cesarean delivery suggests that the hypotension that occurs after extensive neuroblockade primarily reflects decreased systemic vascular resistance.283 Hypotension is often defined as a 20% to 30% decrease in systolic blood pressure (compared with baseline) or a systolic blood pressure less than 100 mm Hg. Modest hypotension rarely has adverse consequences in young, nonpregnant patients. However, placental circulation has limited autoregulation; thus, maintenance of uteroplacental perfusion largely depends on maintenance of maternal blood pressure (see Chapter 3). Uncorrected hypotension results in decreased uteroplacental perfusion. If hypotension is severe and prolonged, hypoxia and acidosis will develop in the fetus. Blood pressure should be monitored frequently (every 2 to 3 minutes) after initiation of analgesia, until stable blood pressure is ascertained.
The incidence of hypotension after initiation of neuraxial analgesia during labor is approximately 14%.27 Kinsella and Black284 reported that maternal position and the position of the blood pressure cuff markedly influence the measured blood pressure. With laboring patients in the full lateral position, the mean difference in systolic blood pressure between the dependent and upper arm was 10 mm Hg; the mean difference in diastolic pressure was 14 mm Hg. Therefore, the incidence of hypotension may vary with the position of both the patient and the blood pressure cuff.
A meta-analysis of studies comparing low-dose epidural analgesia with CSE analgesia found no difference in the incidence of hypotension between the two techniques.27
The prevention of hypotension includes avoidance of aortocaval compression. Preston et al.285 noted a higher incidence of severe FHR decelerations in women placed in the supine-lateral tilt position than in those in the full lateral position after initiation of epidural analgesia. In contrast, Beilin et al.286 found no difference in maternal blood pressure and FHR decelerations between the two positions.
Traditionally, intravenous “preload” (also known as “prehydration”) with 0.5 to 1.5 L of crystalloid solution has been used to reduce the incidence and severity of hypotension after the initiation of neuraxial labor analgesia. However, several randomized controlled trials have shown that the incidence of hypotension after preload with 0.5 to 1.0 L of fluid is no lower than that after no preload.34,287 In women undergoing spinal anesthesia for cesarean delivery there is no difference in the incidence of hypotension when crystalloid is administered as a rapid bolus prior to the initiation of neuroblockade (preload) compared with administration concurrently with the initiation of anesthesia (co-load).37 Data are inconsistent as to whether a fluid bolus decreases the risk for nonreassuring FHR changes associated with the initiation of neuraxial analgesia (see earlier discussion). Many anesthesia providers omit a fluid bolus. In our practice, my colleagues and I usually administer approximately 500 mL of intravenous crystalloid (co-load) at the time of initiation of neuraxial labor analgesia.
The hypotension associated with neuraxial analgesia is usually easily treated. Treatment includes the administration of additional intravenous crystalloid, placement of the mother in the full lateral and Trendelenburg position, and administration of an intravenous vasopressor. Traditionally, ephedrine 5 to 10 mg has been administered; however, studies in women undergoing spinal anesthesia for elective cesarean delivery have shown that phenylephrine is equally efficacious in restoring blood pressure and is associated with higher umbilical arterial blood pH measurements at birth.288 No differences in neonatal outcome have been noted. Because there is no evidence that the choice of vasopressor influences maternal or neonatal outcome, the use of either drug is acceptable. The FHR should be monitored continuously, and treatment should be more aggressive if nonreassuring FHR patterns are noted or if the mother is symptomatic (e.g., presence of presyncope or nausea). Ephedrine crosses the placenta and may increase both FHR and FHR variability (e.g., saltatory FHR pattern).289,290
Data are conflicting as to whether there is a dose-response relationship between hypotension and intrathecal local anesthetics when these drugs are administered in low doses for labor analgesia. Palmer et al.291 found no difference in blood pressure in women randomly assigned to receive intrathecal fentanyl combined with either 1.25 or 2.5 mg of bupivacaine. In contrast, Lee et al.292 noted a greater decrease in blood pressure at 10 minutes in women who received bupivacaine 2.5 mg than in women who received 1.25 mg. Because 1.25 mg is less than the ED95 for bupivacaine (when combined with fentanyl)200 and there is no apparent advantage to combining bupivacaine 1.25 mg with fentanyl over using fentanyl alone,293 there is little reason to manipulate the dose of intrathecal bupivacaine with the goal of decreasing the incidence and severity of hypotension when low doses are used for labor analgesia.
Pruritus
Pruritus is the most common side effect of epidural or intrathecal opioid administration.294 Intrathecal opioid administration is associated with a higher incidence and severity of pruritus than epidural opioid administration (41.4% versus 1.3% in one study295).27 The incidence of pruritus after intrathecal opioid administration is close to 100% in some studies, although the need for treatment is much lower.168 The incidence and severity of pruritus are dose dependent for both epidural99 and spinal168,177 opioid administration. The co-administration of local anesthetic decreases the incidence of pruritus,199 whereas the co-administration of epinephrine may worsen pruritus.296
The cause of the neuraxial opioid-induced pruritus is poorly understood, but it appears to be unrelated to histamine release. The pruritus appears to be mediated through central µ-opioid receptors, given that µ-opioid receptor antagonists relieve itching.297,298 The pruritus may be caused by a perturbation of sensory input that results from rostral spread of the opioid within the CSF to the level of the trigeminal nucleus in the medullary dorsal horn.297 A disruption of sensory modulation is consistent with the observation that a similar pattern of pruritus is seen in medical conditions in which sensory modulation is disturbed (e.g., diabetic neuropathy, multiple sclerosis).299
Few studies have addressed the treatment of established pruritus (see Chapter 28). The most effective treatment is a centrally acting µ-opioid antagonist (e.g., naloxone or naltrexone) or a partial agonist-antagonist such as nalbuphine (Table 23-7). However, the use of these agents in a bolus or continuous infusion may reverse the analgesia. Antihistamines (e.g., diphenhydramine) are often prescribed but are usually ineffective because the mechanism of pruritus is not related to histamine release. Any observed effect of diphenhydramine is probably related to its sedating effects. Although propofol 10 to 20 mg was found effective for the treatment of pruritus in several studies in nonobstetric patients, its efficacy was no different than placebo in an obstetric study.300 Charuluxananan et al.301 found that the 5-HT3 receptor antagonist ondansetron was more efficacious than placebo for the treatment of established pruritus; however, a second study that compared ondansetron 4 mg to pentazocine (a κ-opioid and partial µ-opioid receptor agonist) 15 mg found that pentazocine was superior to ondansetron.302 Another group of investigators found that ondansetron was no more effective than diphenhydramine.303
A number of drugs have been investigated for prophylaxis against neuraxial opioid–induced pruritus, primarily coincident with neuraxial morphine administration. In a meta-analysis of nine studies, the incidence of pruritus was not reduced with prophylactic 5-HT3 receptor antagonists compared with placebo, although the incidence of severe pruritus requiring treatment was lower.304 Similarly, prophylactic dexamethasone was also not associated with a lower incidence of pruritus after intrathecal morphine administration.305
We do not routinely administer prophylaxis for the pruritus associated with neuraxial administration of opioids for labor analgesia. The pruritus is typically self-limiting; the severity of pruritus usually diminishes markedly in the first hour after opioid administration, and most women do not require treatment. For moderate to severe pruritus that requires treatment, we usually administer nalbuphine 2.5 mg and repeat the dose in 10 to 15 minutes if no improvement is noted. The advantage of nalbuphine is that it is less likely to reverse the intrathecal or epidural opioid analgesia.306
Nausea and Vomiting
Nausea and vomiting occur frequently during labor. It is difficult to determine the incidence of nausea and vomiting directly related to epidural and intrathecal opioid administration. Nausea and vomiting may also be secondary to neuraxial analgesia–induced hypotension. Maternal blood pressure should be measured when the patient complains of nausea in the presence of neuroblockade. Other causes of nausea and vomiting during labor are pregnancy itself, pain, opioid-induced delay of gastric emptying (see later discussion), and systemic opioids, which are sometimes administered before intrathecal or epidural opioids. In one study, the incidences of nausea (7% versus 44%) and vomiting (2% versus 17%) were significantly lower in women randomly assigned to receive intrathecal fentanyl than in those assigned to receive systemic hydromorphone analgesia in early labor.21
The etiology of neuraxial opioid-associated nausea is unclear, but it may be caused by the modulation of afferent input at the area postrema (i.e., the chemoreceptor trigger zone) or at the nucleus of the tractus solitarius, which is a key relay station in the visceral sensory network.307 Of interest, nausea is less common after epidural or intrathecal opioid administration during labor than after the administration of the same drugs for post–cesarean delivery analgesia. Norris et al.295 noted that women who received epidural or intrathecal opioid analgesia during labor had an incidence of nausea of only 1.0% or 2.4%, respectively.