Chapter 14 Endoscopic ultrasound of the biliary tract and pancreas
Imaging and Diagnosis
The diagnosis of benign and malignant diseases of the pancreas and biliary tree has traditionally relied on a detailed history and complete physical examination, with correlation of the results of clinical chemistries. Imaging of the hepatic and pancreatic parenchyma and ductal anatomy has, however, evolved as critical for accurate diagnosis and for guiding therapy. Routine radiography does not provide the soft-tissue resolution required, but ultrasonography (US; see Chapter 13), computed tomography (CT; see Chapter 16), and magnetic resonance imaging (MRI; see Chapter 17) have become important noninvasive modalities in the routine investigation of almost any symptom, physical sign, or laboratory abnormality potentially relating to pathologic conditions of the liver or pancreas.
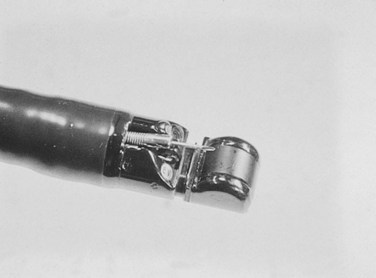
Invasive procedures for imaging the biliary and pancreatic ductal systems, primarily percutaneous cholangiography (PTC) or endoscopic retrograde cholangiopancreatography (ERCP) remain important therapeutically, but with respect to diagnosis, these have been almost entirely replaced by noninvasive modalities. Endoscopic ultrasonography (EUS) has also become an important tool for diagnosis and treatment. Its high-resolution images complement the more general findings of cross-sectional imaging and result in a higher sensitivity for diagnosis of early-stage disease. The new-generation EUS instruments (Figs. 14.1 and 14.2) permit guided passage of needles and devices through the endoscope, allowing biopsies to be obtained and permitting therapeutic interventions. This chapter discusses the techniques of radial and linear endosonography in the diagnosis, staging, and treatment of benign and malignant disease of the pancreas and biliary tree.
Endoscopic Ultrasound Technique
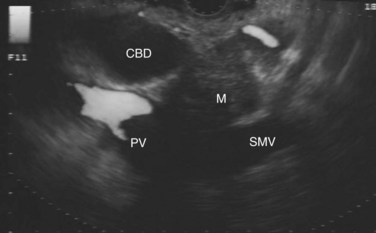
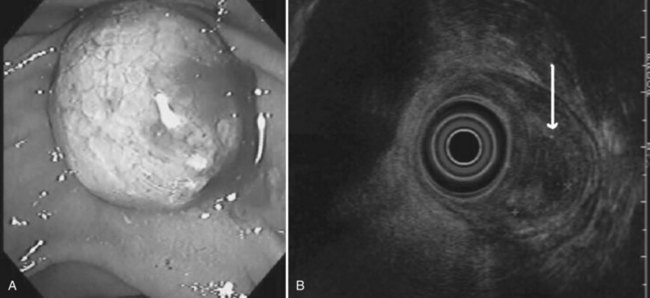
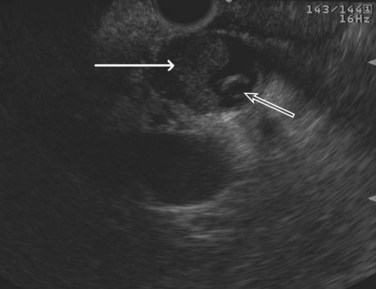
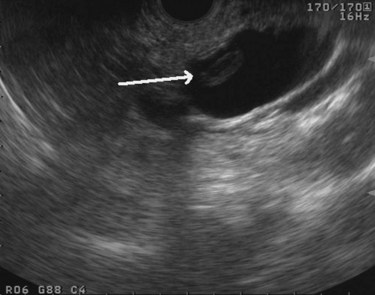
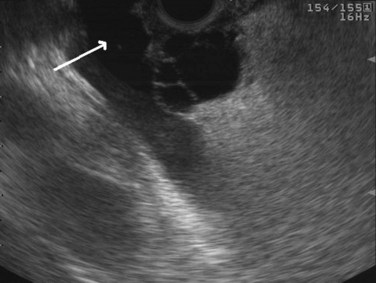
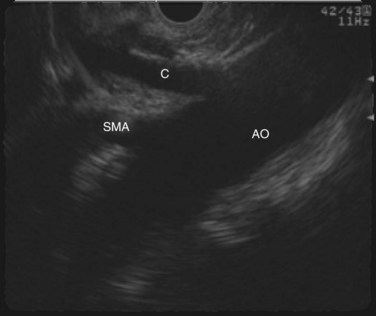
The normal pancreatic parenchyma has a homogeneous echogenic appearance (Fig. 14.3), and tumors usually appear hypoechoic, often with irregular borders, in sharp distinction from normal tissue (Fig. 14.4). Large tumors of the pancreas may be difficult to evaluate completely because of limited penetration of the tumor by US. Conversely, small tumors of the pancreas that are often missed by CT or MRI are readily imaged by EUS. For example, islet cell tumors, which are often encapsulated and small, are frequently detected on EUS and appear as well-demarcated hypoechoic lesions (Fig. 14.5). Other neuroendocrine tumors, such as gastrinomas, may be isoechoic within the pancreatic parenchyma and difficult to identify without careful, tedious, real-time imaging. Ampullary tumors are also often seen and staged on EUS because of their relation to the duodenal wall, CBD, and pancreatic duct (Fig. 14.6). Extrahepatic bile duct tumors can also be detected and described in detail with EUS imaging (Fig. 14.7).
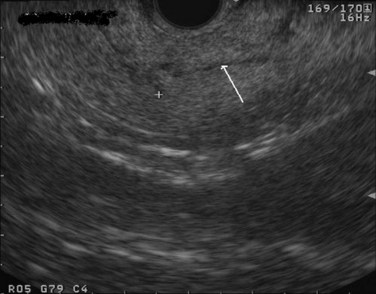
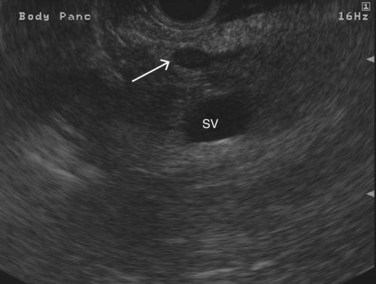
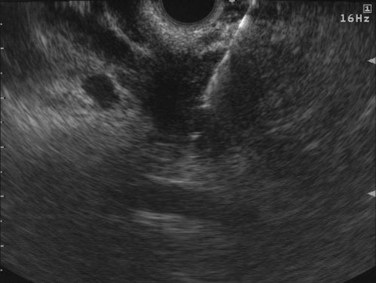
FIGURE 14.3 Normal endoscopic ultrasound image of the body of the pancreas with thin main pancreatic duct (arrow).
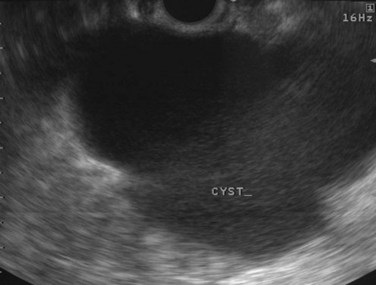
Cysts of the pancreas are generally anechoic and well demarcated and thus easily identified even when small. Some cysts may have internal echoes or solid areas, which raise concern for a mucinous lesion or associated tumor (Fig. 14.8). If the cysts are complex with septa (Fig. 14.9), they are best distinguished from vascular structures using the Doppler flow feature of the linear array echoendoscopes. Serous cystadenomas may also appear isoechoic with the pancreas and require careful imaging for proper identification (Fig. 14.10). Pseudocysts can vary in size and sonographic features but usually lack a discrete wall and septa, particularly if acute. However, internal echoes often are seen as a result of necrotic debris (Fig. 14.11).
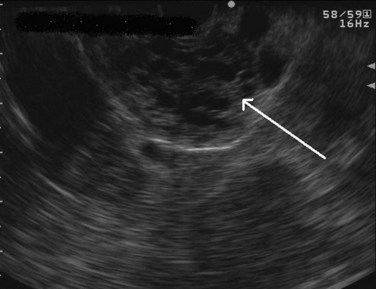
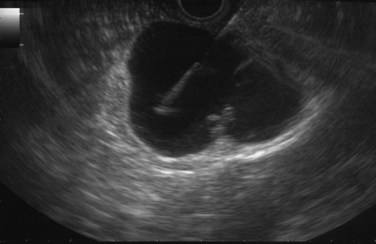
FIGURE 14.10 Typical microcystic appearance of a serous cystadenoma in the head of the pancreas (arrow).
Endoscopic Ultrasound-Guided Fine Needle Aspiration
Endoscopic Ultrasound Fine Needle Aspiration Technique
Within the duodenum or stomach, the US probe is positioned in close proximity to the target lesion, typically less than 3 cm away. The area is then interrogated with Doppler flow US to ensure the absence of significant vascular structures in the needle path. A 25-, 22-, or 19-gauge needle can then be directed into the target lesion. The tip and shaft of the needles used for FNA are manufactured to produce a bright, hyperechoic image. This allows the needle to be followed in real time to ensure precise positioning within the target lesion (Fig. 14.12). Ideally, a cytopathologist or cytotechnologist should be present at the time of the FNA to determine the cellular adequacy of the specimen. Alternatively, multiple punctures (up to seven) should be performed to ensure an adequate cytologic specimen (LeBlanc et al, 2004). Cyst fluid can also be aspirated and sent for cytology, tumor markers, and chemical analysis (Fig. 14.13).
Diagnosis of Pancreatic Cancer
Endoscopic Ultrasound Fine Needle Aspiration of Solid Pancreatic Lesions
Solid masses of the pancreas may represent a primary pancreatic cancer, neuroendocrine tumor, metastatic lesion, or focal pancreatitis. These masses may be difficult to visualize and are often indistinguishable on noninvasive imaging. Percutaneous CT-guided biopsy has a pooled sensitivity of 87% and negative predictive value of only 58% (Hartwig et al, 2009), and it carries the risk of cutaneous or peritoneal tumor seeding. Therefore a percutaneous biopsy is best indicated in patients with unresectable disease to help establish a diagnosis and plan palliative care. Similarly, the sensitivity of cytology obtained from brushings during cholangiography is poor (approximately 40%) and cannot adequately exclude cancer (Wakatsuki et al, 2005).
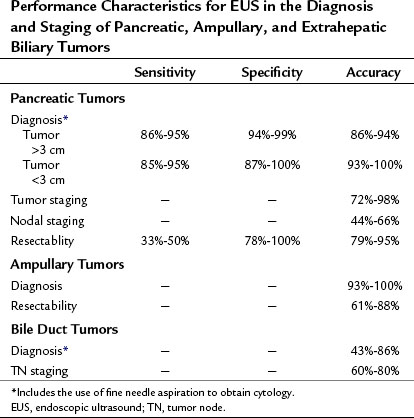
EUS-FNA is highly accurate in diagnosing patients with suspected pancreatic cancer with a sensitivity of 77% to 95%, specificity of 94% to 99%, positive predictive value (PPV) of 98% to 100%, and a negative predictive value (NPV) of 86% to 92% (Wiersema et al, 1997; Yusuf et al, 2009; Turner et al, 2010; Table 14.1). EUS-FNA should be considered as the initial approach to lesions in or adjacent to the pancreas. In a prospective study of 102 patients with suspected pancreatic cancer who had a negative CT-guided biopsy, EUS-FNA had a sensitivity of 95%, specificity of 100%, PPV of 100%, and a NPV of 92% (Gress et al, 2001). With EUS, the issue of needle-tract tumor seeding is minimized, as the FNA is generally performed through a segment of duodenal or stomach wall that will be removed as part of a resection, should the patient be an appropriate surgical candidate. The utility of preoperative EUS-FNA was further demonstrated in a prospective study of 44 patients with resectable pancreatic mass lesions by cross-sectional imaging. EUS with or without FNA precluded surgery in 27% of patients by determining vascular invasion and nodal involvement or by diagnosing lymphoma of the pancreas (Chang et al, 1997).
Endoscopic Ultrasound Fine Needle Aspiration of Pancreatic Cystic Lesions
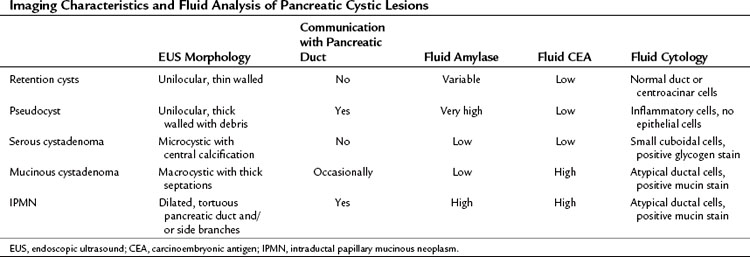
Cystic lesions of the pancreas remain a diagnostic and therapeutic challenge. The differential diagnosis includes retention/simple cysts, pseudocysts, cystic neoplasms, and cystic degeneration of solid masses (Table 14.2). EUS provides detailed information about the entire pancreas—location and number of cysts, size, ductal dilation or communication, signs of chronic pancreatitis, cyst wall thickness, mural nodules, papillary projections, and intracystic structures such as septations, debris, and mass component. Retention cysts are generally small, thin-walled, and unilocular; they carry no malignant potential and can be left untreated. Pseudocysts develop as a result of acute inflammation. On EUS, they are thick-walled as they become chronic and may have debris within the cyst cavity (see Fig. 14.11). They can be seen in or adjacent to the pancreas and often communicate with the pancreatic duct. FNA will yield thick fluid with inflammatory cells but no epithelial cells, and tumor marker levels (carcinoembryonic antigen [CEA], CA19-9) in the cyst fluid are low or undetectable; by contrast, fluid amylase is usually markedly elevated.
Regarding cystic neoplasms, it is important to distinguish mucinous and papillary tumors from serous lesions (see Chapter 57). Serous tumors have little or no malignant potential and do not require resection unless symptoms occur. Typically, serous cystadenomas are composed of a honeycomb of microcysts with no communication with the pancreatic duct (see Fig. 14.10). They can be large and exhibit central stellate calcification on imaging studies. Papillary lesions, solid and pseudopapillary tumors, carry the risk of malignant transformation and usually should be referred for resection. Mucinous lesions—mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN)—also harbor malignant potential that appears to correlate with size; however, universally accepted management criteria are lacking, and treatment recommendations often vary. Mucinous cysts are generally composed of one or more large cystic spaces with thickened walls or septa (see Fig. 14.9). In the case of IPMNs, distinguishing main-duct from side-branch types is important clinically, because the former are associated with a much higher risk of malignant change. Cross-sectional imaging may help in this regard by demonstrating a dilated pancreatic duct characteristic of main-duct IPMN. However, diagnostic uncertainty is not uncommon, and communication with the main pancreatic duct may be demonstrated on EUS.
Although these anatomic characteristics are helpful, EUS cyst morphology alone is insufficient to characterize the lesion (Ahmad et al, 2003; Brugge et al, 2004). Aspiration of cyst fluid via EUS-FNA can further aid in the diagnosis. Cyst fluid can be analyzed for tumor markers, amylase, and molecular markers, and it provides material for cytopathologic assessment. Because of the low cellularity of cyst fluid, the sensitivity and negative predictive value of cytology is low (Centeno et al, 1997; Sedlack et al, 2002). The presence of thick, viscous fluid and a positive mucin stain is suggestive of a mucinous lesion. The concentration of several tumor markers in pancreatic cyst fluid has been examined, and CEA has been found to be the most useful. A cyst-fluid CEA concentration of more than 192 ng/mL predicts a mucinous lesion with a sensitivity of 73%, specificity of 84%, and an accuracy of 79% (Brugge et al, 2004).
Although helpful for diagnosing mucinous lesions, cyst-fluid CEA levels do not correlate with the presence of malignancy. Another limitation of cyst-fluid CEA analysis is that it requires aspiration of at least 1 mL of fluid, which can be difficult if the cavity is small or the fluid is very viscous, making it difficult to pull through a fine needle. More recently, cyst-fluid DNA analysis has shown promise in differentiating mucinous from nonmucinous cysts. This analysis can be done on as little as 200 µL of fluid. The presence of a KRAS mutation, high DNA content, and loss of polymorphic alleles are indicators of mucinous cysts. In a large validation study, early KRAS mutation followed by allelic loss was 96% specific and 37% sensitive for a malignant cyst (Khalid et al, 2009); however, a reliable and sufficiently sensitive marker for malignancy is still lacking.
Complications of Endoscopic Ultrasound Fine Needle Aspiration
EUS-FNA of the pancreas is a safe procedure. The reported rate of pancreatitis is 0% to 2% (Eloubeidi et al, 2004b; Gress et al, 2002a; O’Toole et al, 2001; Williams et al, 1999). In a multicenter analysis of almost 5000 EUS-FNAs of solid pancreatic masses, the incidence of pancreatitis was 0.3%, and the majority of cases were clinically mild (Eloubeidi et al, 2004b). EUS-guided aspiration of pancreatic cysts is similarly safe, with a pancreatitis rate of less than 1% and an overall complication rate of about 2% (Lee et al, 2005). Bacteremia is uncommon following EUS-FNA of solid lesions, with a rate similar to that for diagnostic upper endoscopy; thus prophylactic antibiotic administration is unwarranted (Levy et al, 2003; Janssen et al, 2004). However, early data evaluating EUS-guided cyst aspiration showed increased infectious complications, leading to the recommendation of routine antibiotic prophylaxis for all patients undergoing this procedure (Adler et al, 2005). Major extraluminal hemorrhage is a rare complication and occurs at a rate of less than 1% (Affi et al, 2001).
Staging of Pancreatic Cancer (see Chapter 58B)
The method of staging generally practiced in the United States is that published by the American Joint Committee on Cancer (AJCC). It follows the tumor, node, and metastasis (TNM) staging system and is outlined in Table 14.3.
| TNM Definitions | |
| Primary tumor (T) | |
| TX | Cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor confined to pancreas, ≤2 cm in diameter |
| T2 | Tumor confined to pancreas, >2 cm in diameter |
| T3 | Extrapancreatic extension, no celiac axis or SMA involvement |
| T4 | Involvement of celiac axis or SMA |
| Regional lymph nodes (N) | |
| NX | Cannot be assessed |
| N0 | Regional lymph node metastases absent |
| N1 | Regional lymph node metastases present |
| Distant metastases (M) | |
| MX | Cannot be assessed |
| M0 | Distant metastases absent |
| M1 | Distant metastases present |
| AJCC TNM | |
| Stage 0 | Tis, N0, M0 |
| Stage I | |
| IA | T1, N0, M0 |
| IB | T2, N0, M0 |
| Stage II | |
| IIA | T3, N0, M0 |
| IIB | T1-T3, N1, M0 |
| Stage III | T4, N0-N1, M0 |
| Stage IV | T1-T4, N0-N1, M1 |
TNM, tumor, node, metastasis; AJCC, American Joint Committee on Cancer; SMA, superior mesenteric artery.
From American Joint Commitee on Cancer, 2010: AJCC Cancer Staging Handbook, 7th ed. New York, Springer.
Endoscopic Ultrasound TNM Staging
Some of the first reports of EUS imaging of the pancreas described its ability to detect and diagnose pancreatic and periampullary tumors (Yasuda et al, 1988; Kaufman & Sivak, 1989). However, subsequent studies evaluated the ability of EUS to stage tumors and assess resectability (Tio & Tytgat, 1986; Rosch et al, 1992). Although the general approach to the preoperative evaluation of pancreatic cancer has focused on the TNM stage, the usefulness of such staging is questionable, particularly because T stage does not necessarily correlate with resectability. Initial studies favored EUS over CT in terms of staging accuracy. However, with the development of high-resolution, multidetector technology, CT has equaled if not surpassed the accuracy of EUS and is once again the preferred staging modality. The same can be said for determining vascular invasion and resectability of large pancreatic tumors (>3 cm).
The performance characteristics of EUS for the diagnosis and staging of pancreatic tumors is summarized in Table 14.1. For example, using the TNM system of cancer staging, Muller and colleagues (1994) showed that EUS has an accuracy of 75% for staging pancreatic cancer, as compared with 56% for CT and 57% for MRI. Recently, a prospective study comparing EUS with multidetector CT for diagnosis and staging of pancreatic cancer also found EUS to be more accurate for diagnosis and tumor staging (DeWitt et al, 2004). Of the 80 evaluated patients, the sensitivity for detecting a pancreatic mass was 98% for EUS and 86% for CT (P = .012). Both modalities were equivalent for nodal staging (44% vs. 47%) and for predicting resectability (88% vs. 92%). In contrast, another recent study favored CT, with an accuracy of 74% for tumor staging, 62% for node staging, and 83% for resectability compared with 62%, 65%, and 72%, respectively for EUS (Soriano et al, 2004).
Perhaps the most useful application of EUS is the diagnosis of small pancreatic tumors (<3 cm) in patients with equivocal radiologic imaging (see Table 14.1). Several studies have demonstrated that EUS is superior to CT scanning for evaluation of pancreatic lesions smaller than 3 cm. For example, Muller and colleagues (1994) reported sensitivities of 93% for EUS, 67% for MRI, and 53% for CT using surgical pathology as the gold standard. In addition, EUS far exceeds CT and MRI in the diagnosis and staging of tumors of the ampulla of Vater to determine whether local or radical resection may be appropriate (see Table 14.1). Other reports, as well as our own experience, have shown the usefulness of EUS in evaluating patients with dilated pancreatic and/or bile ducts but no demonstrable cause. EUS in this setting has been useful in the identification of small tumors of the ampulla, pancreas, or bile duct and nonneoplastic causes such as stone disease, chronic pancreatitis, or abnormal anatomy as may be found in patients with chronic narcotic use (Zylberberg et al, 2000). Therefore, when a pancreatic mass is suspected but cannot be identified by standard imaging modalities, EUS should be considered if further clarification is clinically warranted. A recent, large retrospective series reported a high specificity for EUS in the diagnosis of pancreatic cancer, with a negative predictive value of 100%, when the results of EUS showed a normal pancreas (Klapman et al, 2005).
Endoscopic Ultrasound Determination of Vascular Involvement
Some of the earlier descriptions of EUS in assessing pancreatic cancer focused on its accuracy in identifying vascular involvement. In this regard, Yasuda and colleagues (1993) reported an accuracy of 79% for EUS compared with 41% for CT and 72% for angiography. Rosch and colleagues (1992) showed that EUS correctly identified portal vein invasion in 95% of 40 patients undergoing operation for ampullary and pancreatic cancer, compared to 85% for angiography, 73% for CT, and 55% for transcutaneous US. These authors also showed that arterial encasement was less reliably detected by EUS.
Some of these earlier studies were criticized, because the EUS findings were compared with the surgical findings based on manual assessment by the surgeon rather than by pathologic confirmation of vascular invasion. Aslanian and others (2005) carefully studied this issue by retrospectively comparing preoperative EUS findings with the surgical pathology of specimens, including tumors adherent to the vasculature. In this series, the reported sensitivity, specificity, PPV, and NPV of EUS for vascular invasion was 50%, 58%, 28%, and 82%, respectively, but the results were better when only the portal, superior mesenteric, and splenic veins were considered. Another recent report compared EUS and MRI staging of resectability based on arterial invasion or encasement. The sensitivity, specificity, NPV, PPV, and accuracy of MRI were 78%, 96%, 92%, 88%, and 91%, respectively, compared with 33%, 100%, 81%, 100%, and 82% for EUS, but these differences were not statistically significant (P = .13) (Borbath et al, 2005).
Preoperative Reassessment after Neoadjuvant Chemoradiotherapy
Studies on the treatment of pancreatic cancer have recently focused on neoadjuvant treatment with chemotherapy and radiation therapy, followed by attempted radical resection. Several studies have investigated the ability of EUS to reassess patients who have completed neoadjuvant therapy, with most suggesting that EUS is no better than CT for determining resectability. In one report, EUS correctly assessed residual tumor stage in 40% and node status in 90%, but it incorrectly suggested venous invasion in 43% (Bettini et al, 2005). These results suggest that inflammatory changes in the tumor bed, pancreas, and lymph nodes alter the anatomy and blur the distinction between normal tissue planes and between tumor and normal tissue.
Diagnosis and Staging of Cholangiocarcinoma
Primary bile duct cancers (see Chapters 50A through 50D) usually present with painless jaundice. Although transcutaneous US and CT scanning reliably show biliary dilation, they are less accurate for delineating tumors, especially if tumors are less than 2 cm. ERCP is the dominant invasive modality employed to evaluate extrahepatic bile duct strictures. Diagnostic specimens can be obtained by rubbing a cytology brush against the stricture, but the sensitivity is low, with a yield of only 40% to 50% (Wakatsuki et al, 2005; Ponchon et al, 1995). EUS has now emerged as a useful diagnostic and staging technique in this regard through the use of linear and radial echoendoscopes, as well as high-frequency radial miniprobes (passed into the bile duct over a guidewire through the working channel of an ERCP scope), to perform intraductal US.
For detecting benign causes of biliary obstruction, such as common bile duct stones, EUS, with a sensitivity of 89% to 94% and specificity of 94% to 100%, is more accurate than transcutaneous US, CT, MRCP, ERCP, or intraoperative cholangiography (Garrow et al, 2007; Tse et al, 2008). If a biliary stricture is visualized, malignancy is suggested by the presence of an irregular, thickened (>3 mm) bile duct wall (see Fig. 14.7). Hypoechoic infiltration invading through the biliary wall layers or an adjacent pancreatic mass can also be seen. Limited studies have reported on the accuracy of EUS-FNA in the diagnosis of bile duct strictures, but the reported sensitivity is 43% to 86% (DeWitt et al, 2006; Eloubeidi et al, 2004a; Rosch et al, 2004; see Table 14.1). For example, in a study of 24 patients with negative cytology brushings, EUS-FNA of the biliary stricture yielded a diagnosis in 19 patients, or 80% (DeWitt et al, 2006).
Intraductal US can also provide staging information with respect to depth of invasion, the maximal longitudinal extent of the lesion, and the presence of invasion into other organs and major blood vessels, particularly portal vein invasion, with a reported accuracy of 60% to 80% (Tamada et al, 1995, 1998; Inui & Miyoshi, 2005). Standard echoendoscopes are less accurate for tumor staging but can detect regional lymphadenopathy and allow for tissue acquisition via FNA.
Endoscopic Ultrasound–Guided Therapy
Celiac Plexus Neurolysis
In patients with pain as a result of pancreatic cancer, EUS-guided celiac plexus neurolysis has been shown to be a safe and effective alternative to surgical or percutaneous approaches. The celiac axis is easily identified using a linear-array echoendoscope positioned in the stomach (Fig. 14.14), and it provides a quick method of palliating cancer-related pain in patients undergoing staging and diagnosis of pancreas cancer. A recent meta-analysis demonstrated pain relief in 80% of patients at a follow-up range of 1 to 6 months (Puli et al, 2009). In addition, decreased analgesic requirements and fewer opioid-induced side effects can be expected after EUS-guided neurolysis. Recent studies have suggested that bilateral ethanol injection to the right and left of the celiac axis is more effective than unilateral or central injection (Puli et al, 2009; Sahai et al, 2009), and no reports of neurologic complications or pancreatitis have been reported with this technique (Puli et al 2009; O’Toole & Schmulewitz, 2009).
Drainage of Pseudocysts and Peripancreatic Collections
The role for EUS as a minimally invasive and effective technique for guiding drainage of peripancreatic fluid collections after acute pancreatitis (see Chapter 54; Varadarajulu et al, 2007; Lopes et al, 2007) or after pancreatic resection has been established. A fine needle is used to puncture the fluid collection, and one or more plastic catheters are inserted over a guidewire to establish transluminal drainage. Using real-time imaging and Doppler flow, intervening organs and vascular structures can be avoided. Thus there are fewer complications, such as bleeding and perforation, compared with the traditional percutaneous approach. True pseudocysts often contain a large amount of necrotic debris that typically requires debridement and is not effectively drained simply by placing drains. This technique has also been applied to the drainage of peripancreatic fluid collections after distal pancreatectomy (Varadarajulu et al, 2009).
Tumor Localization
Preoperative localization of small pancreatic tumors by fine needle tattooing can be performed safely using EUS (Gress et al, 2002b; Farrell et al, 2009). This precludes the need for intraoperative US and may allow the surgeon to perform a more limited pancreatic resection. A role for EUS-guided intratumoral placement of gold fiducials in localizing pancreatic tumors for targeted radiation therapy has also been demonstrated (Pishvaian et al, 2006; Yan & Van Dam, 2008). This modality of radiotherapy minimizes toxicity to the surrounding tissue by allowing delivery of highly conformal radiation treatments.
Novel Therapeutics
EUS-guided ethanol ablation of cysts has been recently reported (Gan et al, 2005; DeWitt et al, 2009). Patients with asymptomatic, unilocular pancreatic cysts were treated by injection and lavage of the cyst with ethanol through an FNA needle. Compared to saline lavage, a significant decrease in mean cyst size was seen on follow-up imaging. Those that underwent resection showed histologic evidence of epithelial ablation, and 30% to 35% of patients had complete cyst resolution. Acute pancreatitis was observed at a rate of 0% to 4% with no instances of intracystic hemorrhage.
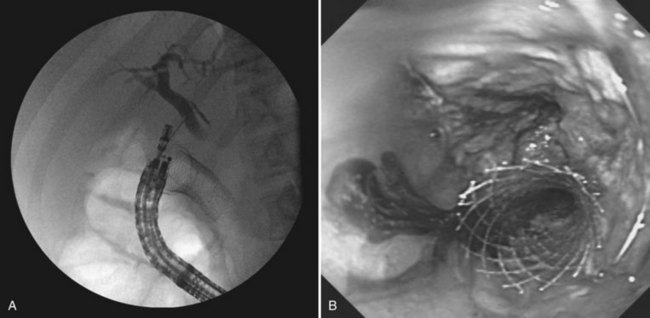
In patients with advanced abdominal malignancies, simultaneous duodenal and biliary obstruction can occur. Traditionally, these patients have required percutaneous or surgical biliary drainage, because transpapillary decompression via ERCP is often not possible. EUS-guided transduodenal or transgastric biliary drainage is a technique in development that may offer an alternate mode of internal decompression. Similar to pseudocyst drainage, the target—in this case, a dilated CBD or left hepatic duct—is localized with EUS and punctured with an FNA needle. A cholangiogram is performed (Fig. 14.15A) followed by guidewire insertion, dilation of the tract, and deployment of a plastic or self-expanding metal stent across the choledochoduodenostomy or hepaticogastrostomy tract (Fig. 14.15B). High technical and clinical success has been reported, with prompt relief of pruritus and jaundice and no major procedural complications (Park et al, 2009; Yamao et al, 2008; Will et al, 2007).
EUS-guided intratumoral therapy of solid masses is being actively investigated. Several therapeutic agents have been evaluated, including activated lymphocyte cultures, viral vectors, oncolytic viruses, and radioactive seeds (Chang et al, 2000; Senzer et al, 2004; Hecht et al, 2003; Jin et al, 2008). Early studies have established safety and demonstrated partial response or disease stabilization in most patients. The feasibility and safety of performing radiofrequency ablation and cryotherapy of the pancreas using an EUS platform has been established in animal models (Goldberg et al, 1999; Carrara et al, 2008).
Adler DG, et al. ASGE guideline: complications of EUS. Gastrointest Endosc. 2005;61(1):8-12.
Affi A, et al. Acute extraluminal hemorrhage associated with EUS-guided fine needle aspiration: frequency and clinical significance. Gastrointest Endosc. 2001;53(2):221-225.
Ahmad NA, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58(1):59-64.
Aslanian H, et al. EUS diagnosis of vascular invasion in pancreatic cancer: surgical and histologic correlates. Am J Gastroenterol. 2005;100(6):1381-1385.
Bettini N, et al. Preoperative locoregional re-evaluation by endoscopic ultrasound in pancreatic ductal adenocarcinoma after neoadjuvant chemoradiation. Gastroenterol Clin Biol. 2005;29(6-7):659-663.
Borbath I, et al. Preoperative assessment of pancreatic tumors using magnetic resonance imaging, endoscopic ultrasonography, positron emission tomography and laparoscopy. Pancreatology. 2005;5(6):553-561.
Brugge WR, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126(5):1330-1336.
Carrara S, et al. Endoscopic ultrasound-guided application of a new hybrid cryotherm probe in porcine pancreas: a preliminary study. Endoscopy. 2008;40(4):321-326.
Centeno BA, et al. Cytologic diagnosis of pancreatic cystic lesions: a prospective study of 28 percutaneous aspirates. Acta Cytol. 1997;41(4):972-980.
Chang KJ, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45(5):387-393.
Chang KJ, et al. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer. 2000;88(6):1325-1335.
DeWitt J, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141(10):753-763.
DeWitt J, et al. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64(3):325-333.
DeWitt J, et al. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc. 2009;70(4):710-723.
Eloubeidi MA, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2(3):209-213.
Eloubeidi MA, et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: a pooled analysis from EUS centers in the United States. Gastrointest Endosc. 2004;60(3):385-389.
Farrell JJ, et al. EUS-guided fine-needle tattooing for preoperative localization of early pancreatic adenocarcinoma. Gastrointest Endosc. 2009;69(1):176-177.
Gan SI, et al. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc. 2005;61(6):746-752.
Garrow D, et al. Endoscopic ultrasound: a meta-analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol. 2007;5(5):616-623.
Goldberg SN, et al. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50(3):392-401.
Gress F, et al. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134(6):459-464.
Gress F, et al. EUS-guided fine-needle aspiration of the pancreas: evaluation of pancreatitis as a complication. Gastrointest Endosc. 2002;56(6):864-867.
Gress FG, et al. Preoperative localization of a neuroendocrine tumor of the pancreas with EUS-guided fine needle tattooing. Gastrointest Endosc. 2002;55(4):594-597.
Hartwig W, et al. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg. 2009;96(1):5-20.
Hecht JR, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9(2):555-561.
Inui K, Miyoshi H. Cholangiocarcinoma and intraductal sonography. Gastrointest Endosc Clin N Am. 2005;15(1):143-155.
Janssen J, et al. Frequency of bacteremia after linear EUS of the upper GI tract with and without FNA. Gastrointest Endosc. 2004;59(3):339-344.
Jin Z, et al. Endoscopic ultrasonography-guided interstitial implantation of iodine-125 seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40(4):314-320.
Kaufman AR, Sivak MVJr. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. Gastrointest Endosc. 1989;35(3):214-219.
Khalid A, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69(6):1095-1102.
Klapman JB, et al. Negative predictive value of endoscopic ultrasound in a large series of patients with a clinical suspicion of pancreatic cancer. Am J Gastroenterol. 2005;100(12):2658-2661.
LeBlanc JK, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59(4):475-481.
Lee LS, et al. EUS-guided fine needle aspiration of pancreatic cysts: a retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. 2005;3(3):231-236.
Levy MJ, et al. Prospective risk assessment of bacteremia and other infectious complications in patients undergoing EUS-guided FNA. Gastrointest Endosc. 2003;57(6):672-678.
Lopes CV, et al. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42(4):524-529.
Muller MF, et al. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190(3):745-751.
O’Toole DL, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53(4):470-474.
O’Toole TM, Schmulewitz N. Complication rates of EUS-guided celiac plexus blockade and neurolysis: results of a large case series. Endoscopy. 2009;41(7):593-597.
Park do H, et al. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009;104(9):2168-2174.
Pishvaian AC, et al. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64(3):412-417.
Ponchon T, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc. 1995;42(6):565-572.
Puli SR, et al. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54(11):2330-2337.
Rosch T, et al. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102(1):188-199.
Rosch T, et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60(3):390-396.
Sahai AV, et al. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104(2):326-329.
Sedlack R, et al. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc. 2002;56(4):543-547.
Senzer N, et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. J Clin Oncol. 2004;22(4):592-601.
Soriano A, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99(3):492-501.
Tamada K, et al. Preoperative staging of extrahepatic bile duct cancer with intraductal ultrasonography. Am J Gastroenterol. 1995;90(2):239-246.
Tamada K, et al. Characterization of biliary strictures using intraductal ultrasonography: comparison with percutaneous cholangioscopic biopsy. Gastrointest Endosc. 1998;47(5):341-349.
Tio TL, Tytgat GN. Endoscopic ultrasonography in staging local resectability of pancreatic and periampullary malignancy. Scand J Gastroenterol Suppl. 1986;123:135-142.
Tse F, et al. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc. 2008;67(2):235-244.
Turner BG, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: a report of accuracy. Gastrointest Endosc. 2010;71(1):91-98.
Varadarajulu S, et al. Role of EUS in drainage of peripancreatic fluid collections not amenable for endoscopic transmural drainage. Gastrointest Endosc. 2007;66(6):1107-1119.
Varadarajulu S, et al. EUS for the management of peripancreatic fluid collections after distal pancreatectomy. Gastrointest Endosc. 2009;70(6):1260-1265.
Wakatsuki T, et al. Comparative study of diagnostic value of cytologic sampling by endoscopic ultrasonography-guided fine-needle aspiration and that by endoscopic retrograde pancreatography for the management of pancreatic mass without biliary stricture. J Gastroenterol Hepatol. 2005;20(11):1707-1711.
Wiersema MJ, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112(4):1087-1095.
Will U, et al. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy. 2007;39(4):292-295.
Williams DB, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44(5):720-726.
Yamao K, et al. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of long-term follow-up. Endoscopy. 2008;40(4):340-342.
Yan BM, Van Dam J. Endoscopic ultrasound-guided intratumoural therapy for pancreatic cancer. Can J Gastroenterol. 2008;22(4):405-410.
Yasuda K, et al. The diagnosis of pancreatic cancer by endoscopic ultrasonography. Gastrointest Endosc. 1988;34(1):1-8.
Yasuda K, et al. Staging of pancreatic carcinoma by endoscopic ultrasonography. Endoscopy. 1993;25(2):151-155.
Yusuf TE, et al. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: a multicenter experience. Endoscopy. 2009;41(5):445-448.
Zylberberg H, et al. Dilated bile duct in patients receiving narcotic substitution: an early report. J Clin Gastroenterol. 2000;31(2):159-161.