Diagnosis, Treatment, and Prevention of Cancer-Associated Venous Thromboembolism
Amer M. Zeidan, Patrick M. Forde and Michael B. Streiff
• Venous thromboembolism (VTE) is a common complication in patients with cancer, affecting approximately 15% of patients during their clinical course. VTE is fivefold to sevenfold more likely to develop in patients with cancer than in patients without cancer.
• The incidence of VTE varies by cancer type and extent of disease. High-risk cancers include pancreatic, brain, and gastric tumors, whereas breast, head and neck, and prostate cancers are associated with a lower risk. Metastatic cancer is associated with a twofold increased risk of VTE.
• Lymphoma and myeloma are also associated with a high risk of VTE.
• VTE is the second most common cause of mortality among patients with cancer and is associated with a threefold increased risk of death compared with patients without cancer.
• Surgery, chemotherapy, hormonal therapy, erythropoietic stimulatory agents, and central venous catheters (CVCs) increase the risk of cancer-associated VTE.
• Risk factors for CVC-associated VTE include left-sided insertion, CVC outer diameter and number of lumens, and catheter tip position above or below the superior vena cava–right atrial junction.
• Pharmacologic VTE prophylaxis is recommended in all surgical and medical oncology patients without contraindications. Optimally managed mechanical prophylaxis should be used when pharmacologic prophylaxis is contraindicated.
• An adjusted dose of warfarin (international normalized ratio 1.3-1.9), a prophylactic dose of nadroparin and semuloparin, and a therapeutic dose of dalteparin have been shown to reduce the risk of VTE in ambulatory patients with cancer who are receiving chemotherapy, although none has improved survival.
• The Khorana risk score that is calculated on the basis of tumor type, prechemotherapy platelet count, and white blood cell count, hemoglobin, use of erythropoietic stimulatory agents, and body mass index can be used to assess the risk of VTE among ambulatory patients with cancer who are starting chemotherapy. This score may help to identify ambulatory medical oncology patients in whom outpatient VTE prophylaxis may be beneficial.
• Enoxaparin, 40 mg daily, and dalteparin, 5000 units daily for 28 days, have been shown to reduce the incidence of VTE compared with prophylaxis for 6 to 10 days in patients with cancer who have undergone surgery.
• In a prospective observational study of more than 2300 patients with cancer who underwent surgery, VTE was responsible for 46% of deaths, making it the most common cause of death within the first 30 days after surgery.
• Extended outpatient pharmacologic VTE prophylaxis should be considered for high-risk surgical oncology patients. Risk factors for VTE in surgical oncology patients include age >60 years, anesthesia time exceeding 2 hours, bed rest exceeding 3 days, advanced cancer stage, and a previous history of VTE.
• Prospective studies have noted that symptomatic central venous catheter thrombosis occurs in 4% of patients with cancer.
• A prophylactic dose of low-molecular-weight heparin and low-dose warfarin are ineffective for CVC-associated deep venous thrombosis (DVT) and should not be prescribed. Adjusted-dose warfarin (international normalized ratio 1.5 to 2) was associated with a reduced incidence of CVC thrombosis at a cost of increased bleeding.
• Diagnosis of VTE in patients with cancer relies primarily on objective imaging with duplex ultrasonography and computed tomography (CT) angiography. In patients with negative duplex studies in whom there is a high suspicion of DVT, CT venography should be considered.
• Low molecular weight heparin is recommended for the initial and long-term treatment of VTE in most patients with cancer who have VTE. Anticoagulation should be continued for at least 3 months or until there is no evidence of active cancer and therapy is completed.
• Catheter-directed pharmacomechanical thrombolysis is a consideration in patients with cancer who do not have contraindications to its use and who have extensive or limb- or life-threatening DVT. Catheter-directed pharmacomechanical thrombolysis is associated with an increased risk of bleeding.
• Systemic thrombolytic therapy should be considered for patients with hemodynamically significant pulmonary embolism (PE).
• Vena cava filters are effective for prevention of PE but are also associated with an increased risk of DVT and inferior vena cava thrombosis. Therefore inferior vena cava filters are primarily recommended for patients who are not candidates for anticoagulation.
• Common causes of recurrent VTE in patients with cancer include local vascular compression, therapeutic resistance (Trousseau syndrome, particularly with vitamin K antagonists) and heparin-induced thrombocytopenia.
• CVC-associated thrombosis generally can be managed by anticoagulation alone without CVC removal. Anticoagulation should be continued for at least 3 months or as long as the CVC is in place.
• Patients with primary and metastatic brain tumors without evidence of hemorrhage generally can be treated safely with anticoagulation for VTE. Metastatic central nervous system tumors at high risk for bleeding include metastatic melanoma, renal cell carcinoma, thyroid carcinoma, and choriocarcinoma.
• Patients with cancer who have stable PE and no signs of hemodynamic compromise can be safely treated as outpatients in the absence of other contraindications to outpatient management. Assessment of right ventricular overload by echocardiography or CT angiography and/or biomarkers can assist with decision making.
• Patients with unsuspected PE should be managed in a similar fashion as patients with symptomatic PE because their outcomes appear to be similar.
Introduction
The seminal description of the association between cancer and venous thromboembolism (VTE) by Trousseau was made almost 150 years ago.1 Consequently, the combination of the cancer and VTE is still commonly referred to as Trousseau syndrome.2 In addition to deep venous thrombosis (DVT) and pulmonary embolism (PE), a wide range of clinically significant thromboembolic events have been observed in patients with cancer, including visceral thrombosis, catheter-related thrombosis, arterial thromboembolism, nonbacterial (marantic) thrombotic endocarditis, migratory thrombophlebitis, hepatic venoocclusive disease (VOD), and disseminated intravascular coagulation (DIC).3 Since the pivotal observation by Trousseau in 1865, our understanding of the prevalence and pathophysiology of VTE in patients with cancer has significantly improved, novel and advanced diagnostic techniques have become readily available for daily clinical use, and effective preventive and therapeutic interventions have been incorporated into standard clinical practice. Nonetheless, the intimate complex bidirectional relationship between VTE and cancer has not been completely deciphered, and VTE continues to be an important and a commonly underrecognized contributor to morbidity and mortality in patients with cancer. In this chapter we will discuss the epidemiology, pathogenesis, diagnosis, prevention, and treatment of cancer-associated VTE.
Epidemiology of Cancer-Associated VTE
Cancer-Associated VTE Is Common
Since the early observations by Trousseau and others, the strong association between malignancy and development of VTE has been confirmed in multiple retrospective and observational prospective studies.4–11 The estimated annual incidence of VTE in patients with cancer is 0.5%, compared with 0.1% in the general population.12 During the clinical course of cancer, the cumulative incidence of symptomatic VTE has been reported to be approximately 15%, with a range of 3.8% to 30.7%.13,14 It has been estimated that up to one fifth of all patients with VTE have an underlying cancer.11,15,16 As a group, malignancies are associated with a fourfold to sevenfold increase in VTE, with a twenty-eightfold increase in risk of VTE in certain types of cancer.5,7,17 Researchers from the Netherlands observed a sevenfold increase in risk of VTE among patients with malignancy compared with persons without cancer.5 In a large population-based study, the presence of a malignant neoplasm was associated with an odds ratio (OR) of 6.5 for VTE compared with control subjects without cancer.17 In addition, incidental asymptomatic VTE is noted on routine imaging in 1.5% to 6.3% of patients.20–20
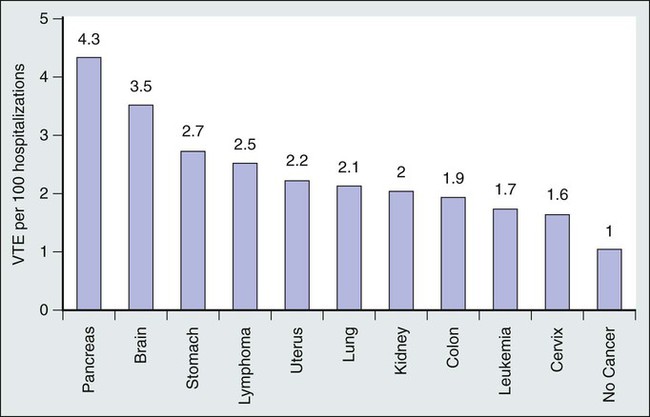
The incidence of VTE is not uniformly distributed among different cancer types.5,21 In general, pancreatic, gastric, brain, ovarian, renal, and hematologic malignancies have been associated with a higher risk of VTE, whereas cancers of the head and neck, breast, prostate, and esophagus are associated with lower VTE rates (Fig. 35-1).4,5,21–24 However, because of the higher incidence and prevalence of lung, colon, prostate, and breast cancers, these malignancies are associated with the highest absolute number of VTEs.21 The histologic type and extent of the malignancy also influence VTE risk. For example, lung adenocarcinoma is associated with a greater risk of VTE than is squamous cell carcinoma of the lung.25 Metastatic cancers are more likely to be associated with VTE than are localized malignancies.4,5,21,25 For the most common cancer types, the relative risk (RR) of symptomatic VTE has been estimated to be fourfold to more than twentyfold higher for metastatic malignancies.4,21,25A Even within the same patient with cancer, VTE risk varies throughout the course of the person’s disease because of effects of other intrinsic and extrinsic factors such as cancer stage, surgery and length of anesthesia, chemotherapeutic and hormonal therapies, age, the presence of indwelling central venous catheters, immobilization, inherited thrombophilia, a previous personal history of VTE, and infections.5,7,8,17,21,26,27 The first year after cancer diagnosis, and especially the first few months, is particularly associated with the development of VTE, although the risk of VTE remains elevated for many years after the initial cancer diagnosis.5 Blom et al.5 noted an adjusted OR for VTE of 53.5 (95% confidence interval [95% CI], 8.6-334.3) during the first 3 months after cancer diagnosis, with a subsequent reduction to 14.3 (95% CI, 5.8-35.2) 3 to 12 months after diagnosis, and a reduction to 3.6 (95% CI, 2.0-6.5) 1 to 3 years after diagnosis, but the increased VTE risk persisted for 15 years after cancer diagnosis.5
Similar to solid tumors, hematologic malignancies are also associated with an increased incidence of cancer-associated VTE.4,5,21,23,24,28–30 In fact, in a population-based study, patients with hematologic malignancies had the highest risk of VTE among different tumor types (OR 28; 95% CI, 4-200).5,8 Among patients with hematologic malignancies, patients with multiple myeloma (MM) are at a higher risk for VTE due to cancer and treatment-related factors.31,32 It has been reported that cancer-associated VTE complicates the course of MM in at least 10% of patients.27,31 In an analysis from the California Cancer Registry, the 1-year cumulative incidence of VTE in persons with acute myeloid leukemia, lymphoma, chronic lymphocytic leukemia, acute lymphoblastic leukemia, and chronic myeloid leukemia were 3.7%, 2.8%, 2.7%, 2.6%, and 1.5%, respectively.11
The Bidirectional Relationship Between Cancer and VTE
The relationship between cancer and VTE is bidirectional; VTE, especially idiopathic VTE, can be a harbinger of an occult malignancy.2 Population-based observational studies have documented an increased risk of malignancy after a first episode of idiopathic VTE.33,34 In an analysis of the Swedish Cancer Registry,33 Baron et al.33 found that at the time of VTE diagnosis or during the first year of follow-up, there was a large increase in the risk for virtually all cancers (standardized incidence ratio of 4.4; 95% CI, 4.2-4.6) The increased risk of future cancer diagnosis in patients with VTE compared with those without VTE persisted for 2 through 25 years after admission to hospital with the index VTE.33 In a large analysis of the Danish Cancer Registry, an increased standardized incidence ratio of 1.3 (95% CI, 1.21-1.33) for cancer diagnosis was found for patients with a VTE episode.34 The risk was substantially elevated only during the first 6 months of follow-up and declined rapidly thereafter to a constant level slightly above 1.0 one year after the VTE episode.34 The risk of cancer was twofold higher for patients diagnosed with idiopathic versus triggered VTE.34 The association was most pronounced for cancers of the pancreas, ovary, liver, and brain.34 Unfortunately, extensive cancer screening at the time of VTE diagnosis is not associated with improved outcome because most cancers associated with VTE are metastatic at the time of VTE diagnosis.35,36 Consequently, the possibility of an underlying cancer among patients presenting with an idiopathic VTE always should be taken into consideration, but cancer screening should be limited to age-appropriate screening procedures in the absence of obvious signs of an underlying malignancy.34
VTE is Associated with Worse Outcomes in Patients with Cancer
Studies have shown that diagnosis of VTE in patients with malignancy is associated with worse outcomes and shortened survival.24,25,28–30,36–39 VTE is the second most common cause of mortality after cancer itself among patients with malignancy.25,40 In fact, 15% of deaths occurring in hospitalized patients with cancer are attributable to PE.15,41 Chew et al.21 analyzed approximately 235,000 patients with cancer in the California Cancer Registry who were linked to the California Patient Discharge Data Set and found in a multivariate analysis that diagnosis of VTE during the first year of follow-up was a significant predictor of death and decreased survival for most cancer types and stages. The 1-year survival of patients with cancer who were diagnosed with VTE is one third (12% versus 36%) that of patients with cancer who do not have VTE.36 Levitan et al.23 noted a threefold increase in 6-month mortality among patients with cancer who had VTE compared with those without VTE.
In addition to symptomatic VTE, unsuspected asymptomatic PE found on routine cancer staging computed tomography (CT) imaging was found to adversely affect survival in patients with cancer.37,39,42 In a retrospective matched cohort study, the hazard ratio (HR) for death among patients who had cancer with unsuspected PE detected on staging CT was 1.51 (95% CI, 1.01-2.27), with the risk attributable to proximal rather than subsegmental unsuspected PE.37 Another retrospective study showed that patients with cancer who were diagnosed with and treated for incidental PE have similar rates of recurrent VTE, bleeding, and mortality compared with patients with cancer who had symptomatic PE.38 Moreover, several studies in patients with cancer have correlated increases in biomarkers of thrombin generation, even in absence of documented VTE, with more aggressive cancer biology and worse outcomes.42–45 Despite ongoing anticoagulation therapy, patients with cancer have a 3.2-fold increased risk of recurrent VTE compared with patients without cancer (12-month cumulative VTE incidence 20.7% vs. 6.8%).46 Additionally, the incidence of major bleeding is 2.2-fold higher in patients with cancer compared with patients without cancer (12.4% vs. 4.9%).46 The risks of recurrent VTE and bleeding appear to correlate with the extent of the malignancy.46 As expected, the development of VTE in patients with cancer is associated with a significant increase in the consumption of health care resources.47
Pathogenesis of Cancer-Associated VTE
Multiple factors contribute to the hypercoagulable state associated with malignancy (Box 35-1). In general terms, these factors can be divided into three broad categories: factors intrinsic to the cancer (tumor-specific factors), patient-related factors (host-specific factors), and environmental factors.
Tumor-Specific Factors
Malignancy is characterized by a bidirectional interrelationship connecting cancer growth, progression, and metastasis with activation of the coagulation cascade and subsequent thrombin generation and inflammation.7,48–50 As discussed earlier, tumor-associated factors such as site, histology, and stage all influence the risk of thrombosis. Cancer cells can disrupt the hemostatic balance via several different pathways, including production of procoagulant, profibrinolytic, proproteolytic, and proaggregating activities, expression of adhesion molecules that mediate direct interactions with host vascular and blood cells, and secretion of proinflammatory and proangiogenic cytokines.7,27,40,50,51 Figure 35-2 depicts some of the tumor-specific factors that contribute to the hypercoagulable state of cancer. Cancer procoagulant is a cysteine protease expressed only by malignant cells that can directly activate factor X independent of factor VII.54–54 In addition, cancer procoagulant has been demonstrated to activate platelets, further adding to its prothrombotic potential.55 Cancer procoagulant has been identified in a wide variety of cancer types and has been noted to have increased activity at disease onset with a subsequent slow decline.7,52–56
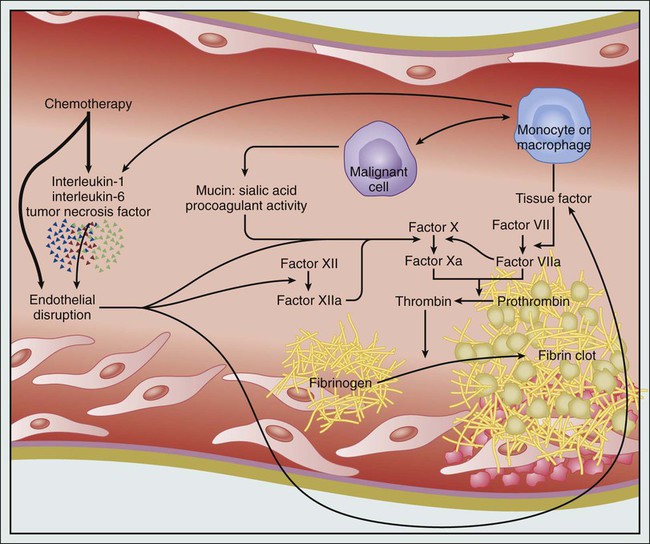
Tissue factor (TF) is the principal initiator of the coagulation protease cascade and is normally expressed as a transmembrane glycoprotein on vascular subendothelial cells.3,7,48,49 Therefore TF is not normally in contact with blood unless vascular endothelial integrity is compromised or if its expression is induced on endothelial cells by inflammatory stimuli.3,7,48,49 Many types of cancer cells such as pancreatic adenocarcinoma and malignant glioma express high levels of TF, which can subsequently lead to activation of both factor X and factor IX, thrombin formation, and ultimately, fibrin clot formation.7,57 TF, which can also be produced by cells in the cancer microenvironment depending on cancer type and context, has been associated with cancer initiation, metastasis, progression, and angiogenesis, in addition to activation of coagulation.49,58–63 Laboratory studies have demonstrated a correlation between increasing TF expression in glioma cells and histologic grade, progression, and the risk of intravascular thrombosis.64,65 In pancreatic adenocarcinoma, it has been shown that fibrinogen and plasminogen activator inhibitors (PAIs) 1, 2, and 3 exist throughout the tumor stroma and that tumor cells stain for TF, PAI, prothrombin, and several other coagulation factors.66 In another study, expression of TF was found to correlate strongly with the degree of histologic differentiation in pancreatic adenocarcinoma. Tumors with stronger immunoreactivity for TF were more poorly differentiated.60 Based on these observations, it has been theorized that local coagulation activation may regulate growth and progression of pancreatic adenocarcinoma.65,66 TF can drive cancer progression by coagulation-dependent and coagulation-independent mechanisms.49,67 The circulating form of TF has been proposed to contribute more significantly to the pathogenesis of cancer-associated VTE than TF expressed on primary cancer cells.49 In a retrospective study, patients with cancer who had circulating TF-expressing microparticles had a 1-year cumulative incidence of VTE of 34.8% versus 0% in those without detectable TF-bearing microparticles.68 This and other similar observations suggest that cancer-derived TF-bearing microparticles are thrombogenic in vivo and are likely to play a central role in the pathogenesis of cancer-associated VTE.49 The clinical utility of this assay has not been demonstrated yet, and it remains an investigational tool at the current time.
Most patients with cancer have been found to exhibit increased levels of coagulation factors V, VIII, IX, XI, and biomarkers of thrombin generation such as prothrombin fragment 1+2 and D dimer.48,59 As mentioned previously, elevated biomarkers of thrombin generation have been associated with aggressive cancer biology and worse clinical outcomes.42–45 The fibrinolytic system can also be significantly dysregulated in patients with cancer.69 Malignant cells can express different proteins in the fibrinolytic system, including urokinase-type and tissue-type plasminogen activators (UPA and TPA, respectively) and PAIs.7,69,70 For example, acute promyelocytic leukemia is often associated at onset with a life-threatening coagulopathy associated with hypofibrinogenemia, increased thrombin generation and fibrin degradation, and prolonged prothrombin and thrombin times.27 The leukemic cells in acute promyelocytic leukemia can express the UPA receptor on their surface, which activates UPA and TPA, contributing to the fibrinolytic state characteristic of the disease.71 Other leukemic cells are also capable of expressing various fibrinolytic and proteolytic enzymes that can mediate the bleeding complications seen in some patients with acute leukemia.27,51,69 Similarly, plasminogen activators, PAIs, and other proteins that regulate the fibrinolytic system are also expressed by solid tumor cells, and the resulting imbalance in fibrinolysis may contribute to the hypofibrinolytic and procoagulant state seen in some affected patients.7,69,70 Additionally, cancers can be associated with deficiencies in the natural anticoagulants, antithrombin, protein C, and protein S, further promoting the cancer-associated thrombogenic state.72,73 The degree of activation of the coagulation cascade and fibrinolysis differs between various tumor types. As noted earlier, some patients with cancer will exhibit clinically evident manifestations of activated coagulation such as DIC and/or venous or arterial thromboembolism, whereas many other patients with cancer will only have laboratory markers of a procoagulant state such as elevated D dimer.74,75
Cancer cells can also modulate the hemostatic balance in an indirect fashion through their interaction with host immune cells such as monocytes and macrophages, leading to activation of platelets and factors X and XII.2 Tumor cells can directly produce inflammatory cytokines or indirectly stimulate their production by host cells (leukocytes and endothelial cells), promoting a hypercoagulable state.2,3 These inflammatory cytokines, such as tumor necrosis factor–alpha (TNF-α), interleukin-1 (IL-1), and vascular endothelial growth factor (VEGF) can induce TF production by endothelial cells and monocytes, stimulate PAI-1 production, and downregulate expression of the natural anticoagulant protein, thrombomodulin, on endothelial cells.3,4A,76,77 In addition, these cytokines can lead to vascular endothelial cell damage and conversion of vascular lining into a thrombogenic surface.2 In addition to activation of TF production by endothelial cells and monocytes, the effects of VEGF include induction of angiogenesis and increased local vascular permeability, therefore increasing the exposure of TF and promoting cancer-associated thrombogenesis.78 The acute phase reactants induced by inflammatory cytokines in patients with cancer include procoagulants such as von Willebrand factor (vWF), factor VIII, and fibrinogen, therefore favoring a thrombogenic hemostatic milieu. Tumor cells can also promote nonenzymatic activation of factor X through the sialic acid moieties of mucin produced by adenocarcinomas.2 Although increased levels of factor VIII, vWF, fibrinogen, PAI, and markers of thrombin generation and fibrin degradation have been associated with more advanced cancers and worse outcomes, these biomarkers have not shown any benefit to date in selecting patients with cancer who might benefit from primary anticoagulant VTE prophylaxis.14,42–45,79
Tumor cells can also directly aggregate platelets and secrete important platelet aggregation agonists such as thrombin and adenosine diphosphate.80 In addition to these biochemical procoagulant mechanisms, direct cell-cell interactions and the local mass effect of tumors contribute to VTE pathogenesis in patients with cancer. Vascular invasion and physical compression by the tumor can mechanically obstruct venous blood flow, leading to venous stagnation, endothelial lining damage, and local activation of the coagulation cascade, all of which predispose to VTE.14,81,82 In patients with myeloma, increased plasma viscosity, elevated levels of circulating immunoglobulins, autoantibodies targeting natural anticoagulants, and secretion of inflammatory mediators with procoagulant activity have all been proposed to contribute to the pathogenesis of VTE.31,32,83
Host-Specific Factors
Studies have shown that some host-specific factors can also modify the risk of VTE. Older age (≥65 years) has been modestly associated with cancer-associated VTE in hospitalized patients.84 Poor performance status in patients with lung cancer who are receiving chemotherapy, which might be a surrogate for limited mobility, has been prospectively associated with increased VTE risk.85 Patient race (African Americans have a higher risk, whereas Asians have a lower risk) and comorbidities such obesity and renal disease have also been associated with increased risk of cancer-associated VTE, whereas gender does not significantly influence risk.5,8,84,86 ABO blood group status, which was found to affect the risk of VTE in the general population, has also been found to modify the risk of VTE in patients with malignant glioma.87,88 Although the pathophysiology of the ABO blood group’s association with VTE is not fully clarified, ABO blood group status does influence factor VIII and vWF levels, which might mediate this effect.89,90
The presence of prothrombotic genetic alterations has been also shown to influence the risk of VTE in patients with cancer. In a large population-based, case-control study of 3220 consecutive patients aged 18 to 70 years in the Netherlands, patients with cancer who were carriers of factor V Leiden or the prothrombin gene 20210A mutation had a fourfold increased risk of VTE compared with patients with cancer who did not have these mutations.5 In contrast, a smaller study from Brazil found no significant difference in the prevalence of four thrombophilic genetic mutations/polymorphisms (factor V Leiden, prothrombin gene 20210A mutation, FXIII Val34Leu polymorphism, and methylenetetrahydrofolate reductase [MTHFR] C677T polymorphism) in patients with cancer who did and did not have VTE.91 A third study in patients with gastrointestinal adenocarcinoma found a significant association between VTE and factor V Leiden mutation but not with prothrombin gene 20210A mutation or the MTHFR C677T polymorphism.92 A matched nested, case-control study of breast cancer prevention using tamoxifen found no significant association between the factor V Leiden mutation or the prothrombin gene 20210A mutation and VTE risk.93
Environmental Factors
Surgery, Radiation Therapy, and Cancer-Associated VTE
Major surgery, a well-recognized VTE risk factor, has been associated with a twofold increased risk of VTE in patients with cancer compared with patients without cancer.94 Furthermore, patients with cancer have a fourfold increased risk of fatal PE after undergoing surgery compared with patients without cancer.15 Factors such as the duration of anesthesia and the procedure, the complexity of the surgery, increasing age, and late mobilization can all modify the risk of postoperative VTE in patients with cancer.3 In contrast to surgery, conflicting data exist regarding the contribution of radiation therapy to the risk of cancer-associated VTE. For example, adjuvant radiation therapy in combination with surgery or chemotherapy was associated with an increased incidence of VTE in patients with glioma or rectal carcinoma, whereas no increased VTE risk was found in otherwise healthy patients with early-stage uterine cervical cancer who received radiation therapy.97–97 In a large observational database study by Blom et al.,4 no additional risk of VTE was conferred by radiation therapy in patients with cancer. These data suggest that radiotherapy is inconsistently associated with VTE8 and that the impact of radiotherapy on VTE risk may be influenced by tumor type and treatment context.
Chemotherapy, Hormonal Therapy, and Cancer-Associated VTE
Chemotherapy, hormonal therapy, and hematopoietic growth factors have all been associated with an increased risk of VTE.2 Patients with cancer who undergo chemotherapy have been found to have a higher risk of VTE and recurrent VTE when compared with patients with cancer who do not undergo chemotherapy.17,98,99 As described earlier, the risk of VTE after cancer therapy depends on the interaction between therapeutic agents, the type and stage of malignancy, and the presence of other VTE risk factors such as advanced age, surgery, immobilization, and the use of central venous catheters.100 Although the causal role of cancer therapies in VTE is well recognized, the pathogenic mechanisms underlying the augmented prothrombotic state and increased risk of VTE are poorly understood despite many years of active research.2,3,100 Multiple mechanisms are likely to be involved depending on the specific chemotherapeutic agent and its interaction with other patient variables. These mechanisms can involve direct vascular endothelial cell damage, increased endogenous procoagulant and decreased anticoagulant levels, altered fibrinolytic activity, platelet activation and aggregation, and increased TF expression by direct and indirect effects.3,100–112 Prechemotherapy platelet count and chemotherapy-associated neutropenia in hospitalized patients with cancer also have been associated with increased VTE risk.9,29,98
The causal relationship between chemotherapy and VTE in patients with cancer has been most studied in the context of breast cancer clinical trials.100 When treated with adjuvant chemotherapy, the risk of DVT in patients with early-stage breast cancer increases from a baseline risk of less than 1% to 2% to 10%.113 In a study of adjuvant epirubicin and cyclophosphamide, a 10% incidence of VTE was found.104 Saphner et al.114 reviewed the records of 2673 patients for the occurrence of vascular complications; these patients received treatment as part of seven consecutive Eastern Cooperative Oncology Group studies of adjuvant therapy for breast cancer. The authors found a significantly higher risk of thrombosis (venous and arterial combined) of 5.4% among patients who received adjuvant therapy in comparison with 1.6% among patients who did not receive adjuvant therapy. Premenopausal patients who received chemotherapy with tamoxifen had a significantly higher rate of VTE than did those who received chemotherapy without tamoxifen (2.8% vs. 0.8%). Postmenopausal patients who received tamoxifen and chemotherapy had a significantly higher rate of VTE than did those who received tamoxifen alone (8% vs. 2.3%) or those who were observed (8% vs. 0.4%). The authors concluded that combining chemotherapy with tamoxifen was associated with more venous and arterial thromboembolic complications than chemotherapy alone in premenopausal patients and with more venous thrombi than tamoxifen alone among postmenopausal patients.114 Other studies confirmed a higher risk of VTE with chemotherapy-hormonal therapy combinations compared with tamoxifen alone in adjuvant therapy for postmenopausal patients with breast cancer.115 In a randomized trial comparing a chemotherapy-hormonal therapy regimen for 12 weeks versus 36 weeks of chemotherapy in patients with stage II breast cancer, VTE developed in 6.8% of treated patients, all during therapy.116 Although aromatase inhibitors such as anastrozole have been associated with a lower risk of VTE than tamoxifen, anastrozole has been associated with a 1% to 2% incidence of VTE as first-line therapy for advanced hormone-receptor positive breast cancer in postmenopausal women.117 In the “Arimidex, tamoxifen, alone or in combination” (ATAC) study evaluating adjuvant endocrine treatment for postmenopausal women with hormone-receptor–positive early breast cancer, the rate of VTE after almost 5.5 years of follow-up was 4.5% for patients who received tamoxifen versus 2.8% for those who received anastrozole.118
The agent 5-fluorouracil, which has been associated with VTE in one out of every seven patients with colorectal cancer who have been treated with the drug, has been proposed to induce a prothrombotic state by decreasing protein C levels, increasing fibrinogen proteolysis, and possibly causing vascular endothelial toxicity.3,119 L-asparaginase has been associated with a 4% to 14% incidence of VTE in adults with acute lymphoblastic leukemia.100,120 L-asparaginase is associated with reductions in fibrinogen, protein C, protein S, antithrombin, plasminogen, and factors IX and XI, whereas it increases levels of factors V and VIII.121,122 Platinum-based regimens have been associated with increased VTE risk in patients with germ cell tumors, non–small cell lung cancer, cervical cancer, and ovarian cancer.85,123–125 Other agents such as bleomycin, mitomycin C, and the use of high-dose chemotherapy conditioning for hematopoietic stem cell transplantation (HSCT) have all been also associated with VTE.126 Corticosteroids, a class of drugs commonly used in cancer therapy, especially for lymphoid malignancies and MM, can increase the risk of VTE, especially when high doses are used in patients with germ cell tumors and MM.123,127 Glucocorticoids have been found to increase factor VII, VIII, XI, and fibrinogen levels, which may contribute to the reported increased risk of VTE in patients using glucocorticoids on a chronic basis.128
Immunomodulatory Agents and Cancer-Associated VTE
In addition to traditional chemotherapy and hormonal therapy, some newer antineoplastic agents have also been associated with increased VTE risk. The immunomodulatory and antiangiogenic agents thalidomide and lenalidomide, which are used for treatment of various cancers, have both been associated with VTE. Thalidomide use in MM as a single agent, whether in newly diagnosed or relapsed/refractory disease, has not been associated with a significantly increased risk of VTE (an incidence of 2% to 4%).31,129–131 In contrast, combinations of thalidomide with dexamethasone or chemotherapy have been associated with an increased risk of VTE in newly diagnosed patients with MM (an incidence of 14% to 26%).31,132 Interestingly, VTE incidence is not increased to the same degree with thalidomide and dexamethasone in patients with relapsed/refractory MM (an incidence of 2% to 8%).133
Similarly, lenalidomide has also been associated with increased VTE risk in patients with MM when used in combination with dexamethasone or chemotherapy, especially in newly diagnosed patients.31,134 In newly diagnosed patients with MM, lenalidomide and high-dose dexamethasone (480 mg/month) have been associated with a 12% to 26% VTE rate compared with 6% to 12% when lenalidomide is used with low-dose dexamethasone (160 mg/kg).134,135 Most cases of VTE associated with lenalidomide occur in the first 3 months of therapy.135 VTE rates as high as 17% have been reported in patients with relapsed or refractory MM who have received treatment with lenalidomide.31,136,137 The etiology of thalidomide- and lenalidomide-associated VTE is not fully understood, although direct vascular endothelial toxicity, acquired protein C resistance, and upregulation of the potent platelet activator cathepsin G have been implicated.31,32,138
Molecularly Targeted Therapies and Cancer-Associated VTE
Some molecularly targeted antineoplastic agents have also been associated with increased VTE risk. The novel antiangiogenic agent bevacizumab, a humanized monoclonal antibody to VEGF that is used in the treatment of several types of cancer, has been variably associated with an increased risk of VTE, arterial thromboembolism, and bleeding.139 In a metaanalysis of 15 randomized trials that included 7956 patients with a variety of advanced solid cancers, patients who received bevacizumab had an incidence of all-grade and high-grade VTE of 11.9% and 6.3%, respectively.140 Therapy with bevacizumab was associated with an RR of VTE of 1.33 (95% CI, 1.13-1.56) compared with control subjects, and that risk was increased for both all-grade and high-grade VTE.140 In contrast, Hurwitz et al.141 found no added risk of VTE among patients treated with regimens containing bevacizumab compared with patients receiving regimens that did not contain bevacizumab (10.9% vs. 9.8%, (OR, 1.14; 95% CI, 0.96 to 1.35; P = .13). Axitinib, an oral receptor tyrosine kinase inhibitor of VEGF and platelet-derived growth factor receptors commonly used in patients with advanced renal cell carcinoma, has been also associated with mesenteric vein thrombosis.142 In a systematic review, two other oral agents targeting angiogenesis, sunitinib and sorafenib, were found to be associated with an increased risk of arterial but not venous thrombosis.143 The safety of continuing VEGF inhibitors in patients who have sustained a VTE while receiving therapy and subsequently undergo anticoagulation is currently unknown.139
Hematopoietic Growth Factors and Cancer-Associated VTE
Hematopoietic growth factor therapy plays an important role in the supportive care of patients with cancer. Erythropoiesis-stimulating agents (ESAs) can reduce the severity of anemia and transfusion requirements in patients with cancer, whereas granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) can reduce neutropenic complications. The use of ESAs has been associated with an increased risk of thrombotic complications in patients with cancer.146–146 Several recent studies have demonstrated an association with tumor progression, mortality, and thrombotic complications, especially VTE, in some patients with solid tumor malignancies who received ESAs.145,146 In a systematic review of 27 randomized trials involving 3287 adult patients with cancer who received darbepoetin or epoetin, Bohlius et al.147 noted that the RR of thromboembolic complications was 1.7 (95% CI, 1.4-2.1) compared with untreated control subjects. In a randomized study of healthy volunteers, G-CSF administration enhanced platelet aggregation by 75% as measured by circulating soluble P-selectin, suggesting that G-CSF use may increase thrombotic risk in patients with cancer.148 Nonetheless, there is currently no conclusive evidence of an association of myeloid growth factor administration with VTE.149 In a retrospective analysis, transfusion of blood products was independently associated with an elevated risk of VTE, arterial thrombosis, and in-hospital mortality in hospitalized patients with cancer.150
Indwelling Venous Catheters and Cancer-Associated VTE
Another important factor that contributes to the predisposition of patients with cancer to VTE is the frequent use of indwelling central venous catheters (CVCs) and peripherally inserted central catheters (PICCs). These catheters facilitate blood sampling and the administration of chemotherapy, intravenous fluids, blood products, and parenteral antibiotics. Thrombosis of the upper extremities has been found to be more common in patients with malignancy and a CVC.151,152 Reported rates of symptomatic CVC-associated VTE in patients with cancer have ranged between 0.3% and 28.3%; recent data from large prospective trials have identified rates of 4% to 8%.153,154 Nonetheless, the majority of CVC- and PICC-associated thrombosis in patients with cancer are actually asymptomatic: one metaanalysis reported that only 12% of CVC-associated VTEs were symptomatic.152,153,155 Several risk factors for CVC-associated thrombosis in patients with cancer have been described. Some of these risk factors include the number of lumens and the outer diameter of the catheter (thrombosis is more common with larger catheter diameter and an increased number of lumens), insertion site (with a higher thrombosis risk for left-sided CVCs), catheter tip position (the right atrial–superior vena caval junction tip position has a lower risk of thrombosis compared with more central or peripheral positions), the material of the catheter, the method of insertion, CVC-related infections, prior personal history of CVC thrombosis, elevated platelet counts, and inherited prothrombotic genetic mutations.152,153,156,157 Tumor type (ovarian carcinoma and lung adenocarcinoma are associated with a higher risk) and the extent of disease (metastatic disease is associated with a higher risk) can also modify the risk of CVC-associated thrombosis in patients with cancer.152,153,156–159 In addition, therapy-related factors can affect the risk of CVC-associated thrombosis in patients with cancer. For example, infusion of sclerosing chemotherapeutic agents and chest radiotherapy have both been associated with increased risk.156,160 Close attention to modifiable risk factors can help to minimize the risk of CVC-associated VTE (see Chapter 26).
Prevention of Cancer-Associated VTE
Prevention of VTE in Hospitalized Medical Oncology Patients
Hospitalized medical oncology patients are at high risk for VTE. Therefore patients with cancer should always receive some form of VTE prophylaxis, preferably pharmacologic prophylaxis given the variable compliance with mechanical prophylaxis in routine care settings.161,162 The American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), and the National Comprehensive Cancer Network (NCCN) all recommend pharmacologic VTE prophylaxis in all hospitalized medical oncology patients, and the American College of Chest Physicians (ACCP) recommend pharmacologic prophylaxis if patients have at least one additional risk factor for VTE.163–168 Because no dedicated randomized trials have been conducted to evaluate VTE prophylaxis in hospitalized medical oncology patients,86 these recommendations are based on the high risk of VTE in this patient population and the supportive data from three large randomized controlled trials in hospitalized general medical patients that included some oncology patients.86,169–171 Although none of these studies reported specific bleeding rates for the subgroup of oncology patients, there was no increase in the overall risk of major bleeding compared with the control arm in any of these trials.86,167,169–171 Therefore until specific data for hospitalized medical oncology patients are available, strategies used for VTE prophylaxis in hospitalized general medical inpatients should be applied to medical oncology patients as well. Box 35-2 describes some of the commonly used pharmacologic thromboprophylaxis regimens, and Box 35-3 lists relative and absolute contraindications to pharmacologic and mechanical VTE prophylaxis. When using graduated compression stockings (GCS) for mechanical prophylaxis in patients with cancer, it is important to fit patients with the correct stocking size and monitor patients closely for skin complications. In a randomized trial of the use of GCS in patients who had a stroke, GCS were associated with a fourfold increased risk of skin ulceration.172
To identify risk factors for VTE among hospitalized patients with cancer, Khorana and colleagues29 examined the University Health System discharge database. Venous thromboembolism occurred in 4.1% of patients, DVT developed in 3.4%, and PE developed in 1.1%. African American patients had the highest rate of VTE (5.1%), whereas white and Hispanic patients had an intermediate rate (4%) and Asians Americans had the lowest rate (3.3%). Patients with pancreatic cancer had the highest rate of VTE (8.1%), followed by other intraabdominal noncolorectal cancers (6.6%), ovarian cancer (5.6%), kidney cancer (5.6%), and myeloma (5%). The lowest VTE rates were found in patients with head and neck cancer (1.4%), prostate cancer (1.9%), and breast cancer (2.3%). Patients receiving chemotherapy were at higher risk (4.9%) than patients not receiving chemotherapy (4%). Medical comorbid conditions such as infections (OR 1.77), renal disease (OR 1.53), pulmonary disease (OR 1.37), and anemia (OR 1.35) were associated with greater risk, as was female gender (1.14) and transfusions (1.35). Mortality was significantly higher among patients who sustained VTE (16.3% vs. 6.3%, P < .0001).29 These factors may be useful for generating a risk stratification model for hospitalized medical oncology patients.
Prevention of VTE in Ambulatory Medical Patients with Cancer
Because most episodes of VTE in patients with cancer occur in the outpatient setting, prevention of VTE in ambulatory medical oncology patients has been the focus of extensive investigation. In 1994, Levine and coworkers173 reported a double-blind, randomized study of very low dose warfarin thromboprophylaxis in patients with stage IV breast cancer. In this study, 152 patients received very low dose warfarin (1 mg adjusted to achieve an INR of 1.3-1.9), whereas 159 patients received placebo. Seven cases of VTE (six DVT and one PE) occurred in the placebo group and one PE occurred in the warfarin group (P = .031), representing an 85% risk reduction. Major bleeding occurred in two placebo recipients and one patient treated with warfarin.173 In the Prophylaxis of Thromboembolism During Chemotherapy Trial (PROTECHT), Agnelli et al.174 randomly assigned 1166 patients in a double-blind fashion to evaluate the clinical benefit of prophylactic-dose nadroparin for thromboprophylaxis in ambulatory patients with cancer who were actively receiving chemotherapy for metastatic or locally advanced solid tumors. Participants with lung, gastrointestinal, pancreatic, breast, ovarian, or head and neck cancers were randomly assigned in a 2 : 1 schema to receive either nadroparin (3800 IU subcutaneously daily, n = 779) or placebo (n = 387) for the duration of chemotherapy up to a maximum of 4 months. The primary end point was a composite of independently adjudicated symptomatic VTE or arterial thromboembolism. In total, 1150 patients (769 patients in the nadroparin arm and 381 patients in the placebo arm) were evaluated for the primary efficacy and safety analyses using a modified intention-to-treat approach. In the nadroparin arm, the primary outcome developed in 15 patients (2%), compared with 15 patients (3.9%) in the placebo group (single-sided P = .02). There was no statistically significant difference in the rates of major bleeding events (0.7% for the nadroparin group vs. 0% in the placebo group, two-sided P = .18) or minor bleeding (7.4% in the nadroparin group compared with 7.9% in placebo arm). In total, 15.7% serious adverse events occurred in the nadroparin group versus 17.6% in the placebo group. The authors concluded that nadroparin reduces the incidence of thromboembolic events in ambulatory patients with metastatic or locally advanced malignancies who are receiving chemotherapy.174 It is worth noting that breast and head and neck cancers are not usually considered high risk for VTE development, and the inclusion of these patients in this study may have accounted for the observed low thromboembolic event rates.86
In another prospective study, the UK-FRAGEM trial, 123 patients with advanced pancreatic carcinoma were randomly assigned to receive either gemcitabine or gemcitabine plus a weight-adjusted therapeutic dose of dalteparin for 12 weeks (200 IU/kg daily × 1 month and then 150 IU/kg daily for months 2 and 3).175 The primary end point was the reduction of all-type VTE during the study period. The incidence of the primary outcome was decreased from 23% to 3.4% (P = .002) in favor of the dalteparin group, with a risk ratio of 0.15 (95% CI, 0.035-0.61), corresponding to an 85% risk reduction. At the end of follow-up, all instances of VTE were also reduced significantly from 28% to 12% (P = .039), with a risk ratio of 0.42 (95% CI, 0.19-0.94), a 58% risk reduction. Lethal VTE occurring earlier than 100 days was seen only in the control arm (8.3% compared with 0%, P = .057, with a risk ratio of 0.092, 95% CI, 0.005-1.635). No difference in overall survival was found. The authors concluded that weight-adjusted dalteparin used as primary VTE prophylaxis for 12 weeks in patients with advanced pancreatic carcinoma who receive gemcitabine is safe and is associated with a significant reduction of all VTEs during the prophylaxis period.175 It should be noted the PROTECHT study used prophylactic-dose nadroparin, whereas the UK-FRAGEM trial used therapeutic-dose dalteparin.86
In the SAVE-ONCO trial, a recently published, double-blind, multicenter randomized study, investigators evaluated the efficacy and safety of the new ultra low molecular weight heparin, semuloparin, for VTE prophylaxis in patients with cancer who were undergoing chemotherapy.176 In this study, 3212 patients with metastatic or locally advanced solid tumors who were starting chemotherapy were randomly assigned to receive subcutaneous semuloparin, 20 mg once daily, or placebo until there was a change of chemotherapy regimen. The primary efficacy outcome of the study was a composite of any symptomatic DVT, any nonfatal PE, and death related to VTE, whereas the primary safety outcome was the incidence of clinically relevant bleeding. The median duration of therapy was 3.5 months. In the semuloparin arm, VTE occurred in 1.2% in comparison with 3.4% in the placebo arm (HR, 0.36; 95% CI, 0.21-0.60; P < .001), with consistent efficacy among subgroups defined based on origin and stage of cancer and the baseline risk of VTE. There was no significant difference in clinically relevant bleeding between the two groups (2.8% vs. 2% in the semuloparin and placebo groups, respectively; HR, 1.40; 95% CI, 0.89-2.21). The authors concluded that semuloparin reduces the incidence of VTE in patients with cancer receiving chemotherapy without a significant increase in major bleeding episodes.176
As discussed earlier, thalidomide and lenalidomide therapy in patients with MM has been associated with an increased incidence of VTE, especially in combination with chemotherapy or high-dose corticosteroids. The 2008 International Myeloma Working Group issued a consensus statement on the prevention of VTE associated with thalidomide and lenalidomide with specific recommendations based on type of therapy and individual VTE and bleeding risks.177 The group developed a risk assessment model based on patient-related (e.g., obesity), myeloma-related (e.g., hyperviscosity) and therapy-related (e.g., high-dose dexamethasone) risk factors for VTE. The panel recommended aspirin for patients with ≤1 VTE risk factor and low molecular weight heparin (LMWH, equivalent to enoxaparin, 40 mg daily) for patients with ≥2 individual or myeloma-related risk factors (Table 35-1).177 LMWH has been recommended for all patients receiving concurrent high-dose dexamethasone or doxorubicin. Full-dose warfarin (INR goal of 2 to 3) has been listed as an alternative to LMWH. The panel acknowledged the limited high-quality data in the literature.177 Subsequent to this consensus statement, two large randomized studies of VTE prophylaxis in patients with MM who were treated with lenalidomide- or thalidomide-based combination regimens have been published.178,179 The first study was a prospective, open-label, randomized study comparing the efficacy and safety of VTE prophylaxis with low-dose aspirin or LMWH in patients with newly diagnosed MM who were treated with lenalidomide and low-dose dexamethasone induction and melphalan-prednisone-lenalidomide consolidation.178 A total of 342 patients without clinical indications or contraindications to antiplatelet or anticoagulant therapy were randomly assigned to receive aspirin, 100 mg daily (n = 176), or enoxaparin, 40 mg daily (n = 166). There was no statistically significant difference in the incidence of VTE between the two groups (2.27% in the aspirin group vs. 1.2% in the LMWH group, corresponding to an absolute risk difference of 1.07%; 95% CI, −1.69-3.83; P = .452). Although PE was observed in 1.7% of patients in the aspirin arm compared with none in the LMWH group, no arterial thrombosis, acute cardiovascular events, major bleeding complications, or sudden deaths were reported. The authors concluded that in newly diagnosed patients with MM who are receiving lenalidomide in conjunction with low-dose dexamethasone, aspirin can offer an effective and less-expensive alternative to LMWH for VTE prophylaxis.178
Table 35-1
Prevention of Venous Thromboembolism in Patients with Myeloma: The 2008 International Consensus Guideline
| VTE Risk Factors | Thromboprophylaxis |
| INDIVIDUAL RISK FACTORS |
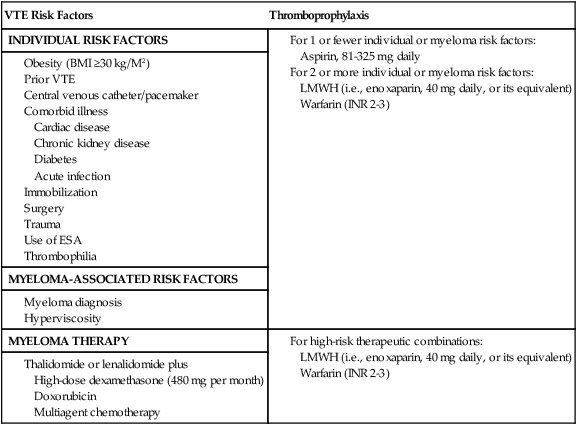
Adapted from Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 2008;22:414–23.
In the second study, aspirin or fixed low-dose warfarin was compared with enoxaparin for VTE prophylaxis in patients with MM who were treated with thalidomide-based regimens.179 In this randomized, open-label, multicenter trial, 667 patients with previously untreated MM with no clinical indication or contraindication for a specific antiplatelet or anticoagulant therapy were randomly assigned to receive low-dose aspirin, 100 mg daily; fixed-dose warfarin, 1.25 mg daily; or enoxaparin, 40 mg daily. The primary outcome was a composite of serious thromboembolic events, acute cardiovascular events, or sudden deaths during the first 6 months of therapy. In total, 659 patients were analyzed, of whom 43 (6.5%) experienced the primary outcome (6.4% in the aspirin group, 8.2% in the warfarin group, and 5% in the enoxaparin group). Compared with enoxaparin, the absolute differences were +1.3% (95% CI, −3%-5.7%; P = .544) in the aspirin group and +3.2% (95% CI, –1.5%-7.8%; P = .183) in the warfarin group. VTE risk was noted to be 1.38 times higher in patients treated with thalidomide without bortezomib. In total, three major (0.5%) and 10 minor (1.5%) bleeding events were recorded. The authors concluded that in patients with MM who were treated with thalidomide-based regimens, aspirin and warfarin showed similar efficacy in reducing serious thromboembolic events, acute cardiovascular events, and sudden deaths compared with enoxaparin, except in elderly patients, in whom warfarin showed less efficacy than enoxaparin.179 It is important to note that none of the patients received highly thrombogenic myeloma regimens, which would presumably be more likely to demonstrate a benefit for more intensive thromboprophylaxis.
Although two large randomized studies of thromboprophylaxis in ambulatory medical oncology patients (PROTECHT and SAVE-ONCO) have demonstrated significant reductions in VTE without an increase in bleeding complications, only the ACCP guidelines recommend thromboprophylaxis for outpatients with cancer if they have other VTE risk factors such as a previous VTE, immobilization, or treatment with an angiogenesis inhibitor.165 The ASCO, ESMO, and NCCN guidelines do not recommend routine prophylaxis in medical oncology outpatients receiving chemotherapy except for high-risk patients with MM who are being treated with thalidomide- or lenalidomide-based combination chemotherapy regimens.8,163,167,168 The NCCN made this decision on the basis of several factors, including the number of patients who need to undergo treatment to prevent one symptomatic VTE, the expense and inconvenience of therapy and its impact on quality of life, and a lack of impact on mortality.168 The NCCN Guideline Committee believes that targeting thromboprophylaxis to the patients at highest risk may make the risk: benefit equation more favorable.168
Assessment of Risk of Cancer-Associated VTE
In addition to the previously mentioned clinicopathological VTE risk factors in patients with cancer, several promising predictive biomarkers have been reported.8,11 Elevated prechemotherapy platelet count (≥350,00/µL), elevated white blood cell count (>11,000/µL), low hemoglobin levels (<10 g/dL), elevated D-dimer and prothrombin fragment 1+2 levels, increased C-reactive protein levels, increased plasma levels of the soluble form of the platelet adhesion molecule P-selectin, increased levels of factor VIII, and elevated levels of TF have all been associated with an increased risk of cancer-associated VTE.125,180–187
Given the highly variable incidence of VTE among ambulatory patients with cancer, identification of the patients at highest risk would maximize the benefit of thromboprophylaxis. To facilitate risk-adaptive VTE thromboprophylaxis, Khorana and colleagues86,180 developed and validated a VTE risk stratification model in medical oncology patients receiving chemotherapy on an outpatient basis using readily available clinical and laboratory data (Table 35-2). The Khorana Risk model was developed in a cohort of 2701 ambulatory patients with cancer in whom chemotherapy was initiated, and it was subsequently validated in an independent cohort of 1365 patients.180 In a stage-adjusted multivariate predictive model, five variables present before chemotherapy initiation were identified: primary site (or type) of malignancy, prechemotherapy platelet count ≥350,000/µL, hemoglobin <10 g/dL and/or the use of ESAs, white blood cell count >11,000/µL, and a body-mass index ≥35 kg/m2.8,180,188 To calculate the score, two points were assigned to very high-risk cancer types (e.g., stomach and pancreas), one point was assigned for high-risk cancers (e.g., lung, lymphoma, gynecologic, testicular, and bladder), and one point was assigned for each of the other four predictive risk factors in the model. Based on the total score, each patient was assigned to a low-risk (score = 0), intermediate-risk (score = 1-2), or a high-risk (score ≥3) category.8,180,188 Over a median of 2.5 months, the rates of VTE in the derivation and validation cohorts were, respectively, 0.8% and 0.3% in the low-risk group, 1.8% and 2% in the intermediate-risk group, and 7.1% and 6.7% in the high-risk group.8,180,188 Using the threshold for the high-risk category (score of 3 points), the model had a negative predictive model of 98.5% for identifying patients at low risk for VTE and was associated with high rate of short-term development of symptomatic VTE (approximately 7%), similar to the VTE rates observed in surgical or hospitalized patients for whom VTE prophylaxis has been demonstrated to be an effective and a safe therapeutic strategy.8,169,180,188 Since it was initially proposed, the Khorana Risk Model has been independently validated by other studies.86,167,189–191
Table 35-2
The Khorana Predictive Model for Risk of Cancer-Associated Venous Thromboembolism in Ambulatory Outpatients Beginning Chemotherapy
| VTE Risk Factor | Points |
| SITE OF CANCER | |
| Very high risk (pancreas, stomach) | 2 |
| High risk (lung, lymphoma, gynecologic, bladder, testicular) | 1 |
| PRECHEMOTHERAPY PLATELET COUNT | |
| >350,000/µL | 1 |
| PRECHEMOTHERAPY WHITE BLOOD CELL COUNT | |
| >11,000/µL | 1 |
| HEMOGLOBIN LEVEL | |
| <10 g/dL or use of erythropoiesis-stimulating agents | 1 |
| BODY MASS INDEX | |
| ≥35 kg/M2 | 1 |
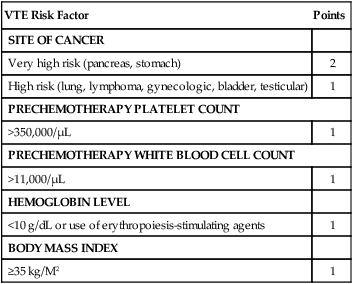
Adapted from Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902–7.
Ay et al.189 prospectively validated the Khorana Risk Model in 819 outpatients with cancer. In their study, VTE developed in 61 patients (7.4%) during a median follow-up of 656 days. The cumulative VTE probability in the Khorana model after 6 months was 17.7% in patients in the high-risk category (score ≥3, n = 93), 9.6% for intermediate risk patients with a score of 2 (n = 221), 3.8% for those with a score of 1 (n = 229), and 1.5% among low-risk patients (score of 0, n = 276).189 Ay and colleagues189 found that measurement of D dimer and P-selectin added further predictive power to their model, the expanded Vienna risk model. The cumulative incidence of VTE probability after 6 months in patients with the highest score (≥5, n = 30) was 35.0%, and it was 10.3% in those with an intermediate score (score 3, n = 130) and 1% in patients with a score of 0 (n = 200).189 The hazard ratio of patients with the highest score compared with those with the lowest score was 25.9 (8.0-84.6).189 The major limitations of the Vienna model compared with the Khorana Risk Model include the need for validation in external cohorts of patients with cancer and the limited availability of P-selectin testing in many centers.86 The availability of easy-to-use risk assessment models for ambulatory medical oncology patients set the stage for risk-appropriate targeted VTE thromboprophylaxis. Khorana and colleagues are currently testing the utility of the Khorana Risk score in targeting VTE thromboprophylaxis in an ongoing prospective, randomized clinical trial (NCT00876915) comparing the safety and efficacy of prophylaxis using dalteparin versus no treatment in reducing VTE in ambulatory patients with cancer with a high Khorana risk score who are beginning chemotherapy. The results of this study will help to determine if routine thromboprophylaxis in high-risk medical oncology outpatients is beneficial. Until these results are available, thromboprophylaxis should be used only in patients with MM who are undergoing thrombogenic chemotherapy regimens.
Prevention of VTE in Hospitalized Surgical Patients with Cancer
Compared with patients without cancer, patients with cancer who undergo major surgery have a twofold increase in VTE and a threefold increase in fatal PE.15,94,192 Several randomized controlled trials have demonstrated the efficacy of unfractionated heparin (UFH), LMWH, and fondaparinux in VTE prevention in patients with cancer who are undergoing major surgery.195–195 In the Enoxacan trial, a prospective international multicenter double-blind randomized study, enoxaparin given 40 mg once daily starting 2 hours prior to surgery was compared with unfractionated low-dose heparin administered thrice daily.193 Eligible patients were older than 40 years and undergoing elective abdominal or pelvic surgery for malignancy. VTE, the primary outcome of the study, was evaluated by mandatory bilateral venography or pulmonary scintigraphy on day 10 ± 2. All patients had clinical follow-up for 3 months. Among 631 evaluable patients, VTE developed in 104 (16.5%) (18.2% in the heparin arm compared with 14.7% in the enoxaparin arm, OR 0.78, 0.51-1.19). Major bleeding and 1-month and 3-month mortality were not significantly different between the two groups. It should be noted that 460 patients were excluded because of inadequate venograms. The authors concluded that both regimens were safe and effective for VTE prophylaxis in patients with cancer undergoing major elective surgery for abdominal or pelvic malignancy.193
Because the risk of VTE remains elevated for weeks after cancer surgery, Bergqvist et al.196 investigated the efficacy of posthospital discharge thromboprophylaxis. In the Enoxacan II trial, the authors conducted a multicenter, randomized double-blind clinical trial in patients with cancer undergoing planned curative open operations for abdominal or pelvic cancers.196 The patients received enoxaparin, 40 mg subcutaneously daily for 6 to 10 days and were subsequently randomly assigned to either enoxaparin or placebo for another 21 days. The primary efficacy outcome, DVT, was assessed by bilateral venograms between days 25 and 31 or sooner if symptoms of concern for DVT occurred. The primary safety outcome was bleeding during the 3 weeks that followed randomization. Using intention-to-treat analysis in 332 patients, DVT rates at the end of the double-blind phase were 12% in the placebo arm compared with 4.8% in the enoxaparin group (P = .02). The difference persisted after 3 months of follow-up (13.8% vs. 5.5%, P = .01). The rates of bleeding and other complications were not different between the two groups. The researchers concluded that 4 weeks of enoxaparin VTE prophylaxis after surgery for abdominal or pelvic cancer is safe and is associated with a significant decrease in radiographically evident DVT compared with 1 week of prophylaxis.196 Similar results were reported in the FAME study, in which patients undergoing major abdominal surgery were randomly assigned to 1 week or 4 weeks of thromboprophylaxis with dalteparin, 5000 IU subcutaneously once daily.195
The Enoxacan trials and the FAME study used routine screening venography or pulmonary scintigraphy to determine the incidence of VTE after elective abdominal or pelvic cancer surgery. In the @RISTOS study, Agnelli et al.6 sought to provide valuable information on the incidence of symptomatic VTE in a wide spectrum of oncologic surgeries. In this prospective observational trial, Agnelli and colleagues examined the incidence of clinically overt VTE occurring up to 30 (±5) days, or longer if the hospital stay was longer than 35 days, after general, urologic, or gynecologic oncologic surgery.6 Among the 2373 patients, 52% underwent general surgery, 29% underwent urologic surgery, and 19% underwent gynecologic surgery. In total, 82% of the patients received in-house VTE prophylaxis and 31% received postdischarge prophylaxis. Independently evaluated symptomatic VTE occurred in 50 patients (2.1%), including DVT (0.4%), nonfatal PE (0.9%), and death (0.8%). The incidence of VTE was 2.8% in general surgery, 2% in gynecologic surgery, and 0.9% in urologic surgery, with 40% of all VTEs occurring more than 21 days after surgery. The overall death rate was 1.7%, of which 46% were attributable to VTE, making VTE the most common cause of death at 30 days after oncologic surgery. Using multivariable analysis, the investigators identified five risk factors for symptomatic VTE: age older than 60 years, a previous history of VTE, advanced cancer stage, anesthesia exceeding 2 hours, and bed rest extending beyond 3 days.6 Based on these findings, ASCO, NCCN, and ESMO guidelines all recommend that high-risk patients with cancer (as defined by the previously identified risk factors) who are undergoing major abdominal or pelvic surgery should be considered for extended VTE prophylaxis.84,163,164,167,168,197
In a metaanalysis of trials evaluating the use of LMWH for VTE prophylaxis in general surgery, LMWH was found to be as effective and safe as UFH.198 It is therefore recommended that providers select VTE prophylaxis on the basis of efficacy, availability, the presence of comorbid diseases, cost, ease of administration, and U.S. Food and Drug Administration (FDA) approval status. In addition, extended VTE prophylaxis should be strongly considered after oncologic surgery, especially in patients with risk factors for VTE. If the patient has a contraindication to pharmacologic VTE prophylaxis, mechanical prophylaxis should be used until pharmacologic prophylaxis is safe to administer. Among surgical patients, the literature suggests that sequential compression devices and intermittent pneumatic compression devices are more effective for mechanical VTE prophylaxis than elastic stockings, and therefore the first two methods are preferred for mechanical prophylaxis.199 Multiple reports in the literature suggest that mechanical VTE prophylaxis is not optimally used (e.g., continuous application in less than 50% of patients), which could account for the less favorable outcomes observed in routine clinical practice in comparison with what has been documented in randomized clinical trials.161,162 Therefore it should be emphasized that institutions need to have established protocols detailing the optimal use of mechanical VTE prophylaxis modalities such as their continuous application and monitoring of application when they are used. Based on these observations, pharmacologic VTE prophylaxis should always be applied when feasible. Several articles reported higher efficacy without compromising safety for combined mechanical and pharmacologic VTE prophylaxis in comparison with either modality alone in several surgical patient populations.200,201 Although no direct evidence supports the use of combined pharmacologic and mechanical VTE prophylaxis in surgical patients with cancer, the use of a combined modality approach is reasonable in high-risk surgical oncology patients because it has been shown to be beneficial in other surgical patient populations.200,202
Prevention of Central Venous Catheter Thrombosis
As discussed earlier, CVC-associated VTE is common in patients with cancer. A prospective study of 444 patients with cancer found that symptomatic VTE developed in 4.3%.157 A prospective study of 2014 patients with PICC catheters found a similar rate of thrombosis (3%).203 As a result, several studies have evaluated the use of VTE prophylaxis with warfarin or LWMH.160,204–206 A randomized study evaluated the use of low-dose warfarin (1 mg daily starting 3 days before CVC placement and adjusted to keep the prothrombin time below 15 seconds) in patients with cancer having a CVC inserted.160 Outcome evaluation was performed with venography when symptoms occurred or at 90 days after insertion. The patients who were treated with warfarin had a significant reduction in VTE (9.5%) compared with those who did not receive warfarin (37.5%, P < .001).160 Monreal et al.205 demonstrated that dalteparin, 2500 IU daily, resulted in a significant reduction in CVC-associated VTE (6%) compared with control subjects (62%, P = .03).
Despite these promising early results, recent large randomized trials of low-dose warfarin and LMWH have shown no benefit of active prophylaxis for CVC-associated DVT.204,206 In a multicenter, double-blind study, consecutive patients with cancer who were scheduled for CVC placement were randomly assigned to receive either enoxaparin, 40 mg subcutaneously daily, or placebo.206 Therapy started 2 hours prior to CVC placement and was continued for 6 weeks. The primary outcomes were DVT (as confirmed by venography performed 6 weeks after randomization), or clinically overt PE (as confirmed by objective testing) during the study drug administration. Among 385 patients, 321 patients (83%) underwent venography, and 155 patients in each treatment group had adequate venograms for evaluation. There was no difference between the two groups in DVT incidence (14.1% in the enoxaparin group vs. 18% in placebo arm, with an RR of 0.78 and a 95% CI of 0.47-1.31) or mortality, and no major hemorrhagic complications occurred. The authors concluded that enoxaparin prophylaxis did not result in a significant reduction in the rate of CVC-associated VTE compared with placebo.206 In another prospective, double-blind, placebo-controlled, multicenter study, no significant difference was found in the frequency of catheter-related complications between the dalteparin prophylaxis group (3.7%) and the placebo arm (3.4%, P = .88).204 One randomized multicenter, placebo-controlled study evaluated the role of thromboprophylaxis for CVC-associated VTE in patients with hematologic malignancies. In this study, 255 patients with cancer (of whom approximately 80% had hematologic malignancies) who required a CVC for at least 7 days were randomly assigned to receive warfarin, 1 mg, or placebo.207 No difference in the incidence of symptomatic CVC-associated VTE was found between the two groups (4.6% in warfarin group vs. 4% in placebo group, HR 1.20; 95% CI, 0.37-3.94).207 Warfarin did not affect the CVC life span, the number of premature CVC removals, or the frequency of major hemorrhagic events.207
Two metaanalyses of CVC prophylaxis have found no evidence of benefit.208,209 However, a recent open-label randomized controlled trial in patients with cancer found that the subgroup treated with adjusted-dose warfarin (INR 1.5-2.0) experienced fewer CVC-associated thromboses than did patients receiving fixed-dose warfarin (13 of 473 [3%] vs. 34 of 471 [7%]; RR 0.38, 95% CI, 0.20-0.71, P = .002). In the separate randomized comparison in the same study of no warfarin (n = 404) versus warfarin (n = 408; 324 [79%] on fixed dose and 84 [21%] on dose adjusted), warfarin did not reduce the incidence of CVC thrombosis (24 [6%] vs. 24 [6%]; RR 0.99, 95% CI, 0.57-1.72, P = .98).210 Warfarin was associated with a trend toward more major bleeding (warfarin vs. no warfarin, 7 vs. 1, P = .07; adjusted dose warfarin versus fixed dose warfarin, 16 vs. 7, P = .09).210 This study suggests that more intensive anticoagulant regimens may prevent CVC thrombosis at the potential cost of more bleeding complications. Until further data supportive of CVC prophylaxis are available, all of the guidelines recommend against the routine use of anticoagulant prophylaxis to prevent CVC-associated DVT.8,163–165,167,168,197
Diagnosis of VTE in Patients with Cancer
Given the high risk of thromboembolism among patients with cancer, health care providers should maintain a high index of suspicion for VTE. In most patients diagnosed with cancer-associated VTE, the diagnosis of cancer precedes the thrombotic event; in some cases, however, patients may have multiple thromboembolic events prior to the diagnosis of cancer.211 Clinically detected VTE occurs in 12.6% of ambulatory patients with cancer within 1 year after commencing chemotherapy and is more common in persons with certain types of cancer, such as pancreatic cancer, in whom the rate is 19.2%.187
Diagnosis of Cancer-Associated VTE
Clinically it is important to note that patients with cancer usually have an elevated pretest probability of DVT and PE because active cancer is a component of the Wells Score and reduced mobility frequently occurs as a result of factors such as fatigue associated with active cancer or cancer treatment.212
D-Dimer Testing in the Diagnosis of VTE
D-dimer testing in conjunction with clinical prediction rules such as the Wells criteria has been useful in excluding VTE without use of objective radiologic testing in patients without cancer.214–214 However, it is important to note that these studies included a limited number of patients with cancer (9% to 14%), and thus the promising results in these studies may not be applicable to patients with cancer.214–214 The utility of clinical prediction rules and D-dimer testing in the diagnosis of VTE in patients with cancer has been examined in two studies.215,216 Ten Wolde215 and colleagues conducted a post-hoc analysis of 217 patients with cancer included in a study of the SimpliRED D-dimer test and Duplex ultrasonography in 1739 consecutive patients presenting for evaluation of suspected DVT. If results of initial D-dimer testing and duplex ultrasonography were negative, patients were followed up without anticoagulation. During 3 months of follow-up, a VTE developed in only one patient with cancer (1.6%; 95% CI, 0.04%-8.5%) and five patients without cancer (0.9%; 95% CI, 0.4%-1.9%).215 Sohne et al.216 conducted a subgroup analysis of a prospective study of the Wells PE clinical model, D-dimer testing, and selective use of spiral contrast computed tomography (CT) in the diagnosis of PE. During 3 months of follow-up, VTE subsequently developed in only one patient with cancer (2%; 95% CI, 0.05%-10.9%) and in five patients without cancer (0.5%; 95% CI, 0.2%-1.1%) who were initially judged not to have PE.216 Unfortunately, many patients with cancer have elevated D-dimer test results at baseline, limiting the utility of D-dimer testing in the diagnosis of VTE. Additional prospective management studies conducted primarily in patients with cancer are warranted to establish the value of D-dimer testing and clinical prediction rules in the diagnosis of VTE in patients with cancer. Until further data supporting this approach are available, diagnosis of VTE in patients with cancer should rely primarily on objective radiologic imaging.
Imaging
Duplex Ultrasonography
Duplex ultrasonography allows assessment of the presence and chronicity of DVT and is the current noninvasive imaging investigation of choice.217 Lack of compressibility of a vein with the ultrasound probe has more than 95% sensitivity and specificity for DVT.218 Patients with initial negative compression ultrasonography and a high Wells score should have a repeat study performed a week later because up to 2% of patients may have a positive repeat test.219 Use of duplex Doppler color flow ultrasound reduces the required duration of the study and demands placed on the patient while maintaining accuracy.220 Important characteristics noted by the ultrasonographer during duplex ultrasound include lack of compressibility of the vein and the change in vein diameter during the Valsalva maneuver, abnormal Doppler color flow, and/or presence of an echogenic band. Compression ultrasonography is less useful for detection of recurrent DVT, proximal lower limb thrombus in the iliac vein, or DVT in patients who have pelvic or arm girdle tumors that may impede flow from distal veins.221,222
Duplex ultrasonography also has limited sensitivity in detection of intrathoracic and intraabdominal thrombosis. If clinical suspicion of a thrombotic event in these anatomic locations is high, then CT or magnetic resonance (MR) venography should be performed.223,224 The REVERSE study was a prospective cohort study aimed at developing criteria for recurrent VTE.225 At the conclusion of therapy, the investigators obtained a baseline duplex study (for patients with DVT) or a PE protocol CT scan. Criteria for recurrent DVT were development of a new noncompressible venous segment or an increase in clot diameter of 4 mm or more. The investigators monitored 646 participants for a median of 11 months (0-58 months). During follow-up, 398 suspected recurrent VTEs developed in 305 patients (240 suspected DVTs, 113 suspected PEs, and 45 suspected DVT + PEs). The following cases were confirmed: 58 of 240 (24%) suspected DVTs, 27 of 113 (24%) suspected PEs, and 21 of 45 (47%) suspected DVT + PEs were confirmed. Of the 284 patients who were not treated with anticoagulation for another reason and did not die of other causes, eight patients experienced a thrombotic event during the 90 days after their initially negative imaging study (2.8%, 95% CI 1.4%-5.5%). The REVERSE study provides an effective strategy for diagnosis of recurrent VTE. The primary limitation of this study is that it excluded patients with malignant disease. Nevertheless, it provides the best available guideline for diagnosis of recurrent VTE.225
Contrast Venography
Contrast venography is considered the gold standard for the diagnosis or exclusion of DVT with >98% sensitivity. However, in practice, because of its invasive nature, it is not recommended as an initial screening test.226 Currently, contrast venography is reserved for situations in which a distal limb venous thrombosis is suspected—for example, calf veins when noninvasive testing is inconclusive or when the clinical impression of DVT is strong despite negative noninvasive testing.227 Contrast venography has been largely replaced by CT and MR venography.
Computed Tomographic Venography
Although CT angiography is the study of choice for diagnosis of PE, it is also a very sensitive and specific diagnostic modality for DVT. In their review of diagnosis of VTE, Saad and Saad228 noted that CT venography has a mean sensitivity and specificity for diagnosis of above-the-knee and calf-vein DVT of 95% (range 89%-100%) and 97% (range 92%-100%). Its diagnostic performance was equivalent to duplex ultrasound for above-the-knee DVT and was superior to duplex ultrasound for calf-vein DVT. Similar to CT angiography for PE diagnosis, CT venography has the advantage of providing superior anatomic information relative to duplex ultrasound regarding other potential medical conditions that could result in the same symptoms as DVT. Indirect CT venography combines examination of the pulmonary vasculature with lower extremity venography. The advantage of this study compared with conventional pulmonary CT angiography is that it provides a global imaging assessment for both PE and DVT. Disadvantages include a larger contrast volume, greater radiation exposure, and higher cost. The sensitivity and specificity of combined indirect CT venography has been estimated to be 89% to 100% and 92% to 100%, respectively.228 CT venography remains a valuable imaging modality for intraabdominal and intrathoracic venous thrombosis and cerebral venous sinus thrombosis.223,229,230
Magnetic Resonance Venography
MR venography has equivalent sensitivity and specificity (>95%) to contrast venography for the detection of DVT with the advantage of being noninvasive.231,232 MR venography is the diagnostic study of choice for cerebral venous sinus thrombosis.230 MR venography also has particular utility in special circumstances such as contrast allergy and diagnosis of recurrent DVT; however, routine use has been limited by high cost and less widespread availability than ultrasound or contrast venography.233
Diagnosis of Cancer-Associated PE
Classic symptoms of acute PE include dyspnea, pleuritic chest pain, and hemoptysis, whereas signs include tachypnea, sinus tachycardia, fourth heart sound, and a prominent P2 component of second heart sound. Clinical diagnosis alone, however, has a relatively low sensitivity (85%) and specificity (51%), necessitating imaging in the vast majority of cases of suspected PE.234
Pulmonary Angiography
Pulmonary angiography is the reference test for the diagnosis of acute PE and is performed by injecting contrast material into a pulmonary artery. Accuracy for detection of PE is approximately 98%.235 With the availability of accurate noninvasive imaging, the routine first-line use of invasive pulmonary angiography has declined because of drawbacks, including the invasive nature of the test and procedure-related mortality and morbidity in 0.5% to 1% of patients and 6% of patients, respectively.236
Ventilation/Perfusion Scanning
Ventilation/perfusion (V/Q) scanning is most accurate when combined with a clinical probability assessment.237 A normal V/Q scan rules out PE, whereas a high-probability V/Q scan is associated with a positive predictive value of greater than 90%. Unfortunately, many patients (50%) fall into the low probability or intermediate probability categories that require further diagnostic testing to establish a diagnosis.237 Patients with cancer in particular may have lung infiltrates or, in some cases, tumors, which may make accurate interpretation of a V/Q scan difficult. A randomized controlled trial of V/Q scanning and CT angiography in the diagnosis of PE found that CT angiography identified significantly more patients with PE than did V/Q scans.238 Consequently, V/Q scans have been replaced by CT angiography in most patients as the diagnostic study of choice for PE. V/Q scans remain useful for the diagnosis of chronic thromboembolic pulmonary hypertension and PE diagnosis in patients with renal insufficiency or life-threatening contrast allergies.
Computed Tomography Pulmonary Angiography
Spiral CT pulmonary angiography (CTPA) is the most frequently used imaging modality for detecting PE in modern clinical practice.239 Since the development of multidetector CTPA, the sensitivity and specificity has increased to 83% to 94% and 94% to 100%, respectively. The negative predictive value of CTPA is 93% to 96%. In a randomized clinical trial comparing V/Q scans and CTPA, CTPA identified PE in significantly more patients than did V/Q scans (19% vs. 14%, absolute difference 5%, 95% CI 1.1%-8.9%). Only two patients (0.4%) who had negative CTPA and lower extremity duplex ultrasound experienced an episode of VTE during 3 months of follow-up compared with six patients (1%) who underwent V/Q scans.238 These results for CTPA are similar to the results of the Christopher study in which 1.3% (95% CI, 0.7%-2%) experienced VTE during 3 months of follow-up and comparable to studies using pulmonary angiography.213,240 The advantages of CTPA include its high sensitivity and specificity, rapid result turnaround, ability to detect other disorders responsible for the patient’s symptoms, and widespread availability. Disadvantages include the higher radiation dose associated with multidetector CTPA and contrast exposure.228 In conjunction with indirect venography, it was shown in one study that CTPA can provide a global assessment for DVT and PE with high sensitivity (90%) and specificity (95%) when combined with optimal use of clinical probability tools such as the Wells score.241 In situations in which there is a significant discrepancy between clinical suspicion and imaging findings, a second investigation such as duplex ultrasonography, pulmonary angiography, or V/Q scanning should be considered to exclude PE.
Management of Cancer-Associated VTE
Management of VTE in patients with cancer is complicated by several patient- and tumor-specific features. Some of these challenges are listed in Box 35-4. The commonly used therapeutic approaches for cancer-associated VTE are described in Box 35-5. Patients with active cancer who experience thrombosis are at a twofold to threefold higher risk of recurrent VTE and a twofold to sixfold higher risk of bleeding than are patients without cancer who are receiving vitamin K antagonists.46,242,243 A large registry of 4709 patients with cancer demonstrated that 4.2% of patients with cancer treated for VTE experienced a bleeding event within 3 months of starting anticoagulation therapy, and cancer-related bleeding risk factors included metastatic disease, renal impairment, recent bleeding, or immobilization.244 Fewer patients bled after 90 days of therapy (0.8%), and the most common sites of bleeding were gastrointestinal (49%), genitourinary (18%), and brain (11%). Bleeding while undergoing anticoagulation therapy carries a poor prognosis for patients with cancer, with 66% of patients dying within a month of a bleeding event; half of the deaths were directly attributed to bleeding.245
Acute Management of Cancer-Associated VTE
Anticoagulation
LMWH is favored for the acute management of VTE in patients with cancer. In a metaanalysis of randomized clinical trials of anticoagulation in patients with cancer, LMWH was associated with reduced mortality (RR, 0.71; 95% CI, 0.52-0.98) but a similar risk of recurrent VTE (0.78; 95% CI, 0.29-2.08) compared with UFH.154 No differences in mortality (RR, 1.27; 95% CI, 0.88-1.84), recurrent VTE (RR, 0.95; 95% CI, 0.57-1.60), or major bleeding (RR, 0.79; 95% CI, 0.39-1.63) were found between fondaparinux and unfractionated heparin.154 The ASCO guideline recommends LMWH for the acute treatment of VTE in patients with cancer.163 The ACCP guideline recommends LMWH or fondaparinux rather than UFH for the acute treatment of VTE.246 The NCCN and ESMO guidelines recommend either LMWH, UFH, or fondaparinux for acute treatment of VTE in patients with cancer.167,197 On November 2, 2012, the FDA approved rivaroxaban for the treatment of DVT and PE. Rivaroxaban was demonstrated to be equally efficacious as LMWH and warfarin in the treatment of DVT and PE in the EINSTEIN-DVT and EINSTEIN-PE studies.247,248 It is worth noting that both studies only included a small number of patients with cancer (5% of participants), and thus the risks and benefits of rivaroxaban are not well characterized in the population of patients with cancer. Given the complex conditions of patients with cancer, we believe that further investigation of rivaroxaban in patients with cancer is warranted.
Thrombolysis
Thrombolytic therapy has been demonstrated to result in more rapid and complete clot lysis than conventional anticoagulation in patients with DVT.249–252 In addition, thrombolytic therapy, in particular catheter-directed thrombolytic therapy, is associated with a lower risk of postthrombotic syndrome.250,251 However, thrombolytic therapy is more costly and associated with a threefold increased risk of major bleeding compared with anticoagulation. Although experience in patients with cancer is limited, catheter-directed thrombolytic therapy appears to be associated with similar outcomes in patients with and without cancer.253 Therefore thrombolytic therapy should be considered in the treatment of DVT in patients with cancer if it is indicated, such as in patients with life- or limb-threatening thrombosis. Although thrombolysis has not been shown to improve survival in patients with acute PE and is associated with increased bleeding risk, it is still considered an important option, particularly in cases of massive PE.246,254,255
Situations in which thrombolysis for patients who have confirmed PE should be considered include hemodynamic instability, severe hypoxemia, extensive clot burden or hypoperfusion on chest imaging, right ventricular dysfunction, free-floating intracardiac thrombus, patent foramen ovale, or situations requiring cardiopulmonary resuscitation.256 In all patients with cancer, CT imaging of the head should be strongly considered prior to thrombolysis to exclude intracranial neoplasm which is considered an absolute contraindication to thrombolysis due to risk of intracranial hemorrhage.257 Other contraindications to thrombolysis include recent intracranial surgery or trauma, active or recent internal bleeding during the prior 6 months, a history of a hemorrhagic stroke, bleeding diathesis, severe uncontrolled hypertension (i.e., systolic blood pressure >200 mm Hg or diastolic blood pressure >110 mm Hg), nonhemorrhagic stroke within the prior 2 months, surgery within the previous 10 days, and thrombocytopenia (i.e., <100,000 platelets/mm3).246
Vena Cava Filters
In the PREPIC study, vena cava filters plus at least 3 months of anticoagulation were demonstrated to result in a significant reduction in PE at 8 years compared with at least 3 months of conventional anticoagulation alone (6.2% vs. 15.1%, HR 0.37, 95% CI 0.17-0.79, P = .008).258 In addition, DVT was higher in the filter group (35.7% vs. 27.5%, HR 1.52, 95% CI 1.02-2.27, P = .04) and mortality was equivalent (48.1% vs. 51%, HR 0.97, 95% CI 0.74-1.28, P = .83).258 Because vena cava filters are associated with an increased risk of thrombotic complications, including DVT and inferior vena cava (IVC) thrombosis and filter or filter leg migration, the NCCN, ASCO, ESMO, and ACCP guidelines recommend their use only in patients with acute VTE who cannot undergo anticoagulation therapy.163,164,167,168,246 In patients with transient contraindications to anticoagulation therapy, the NCCN guideline recommends use of a retrievable filter.168 IVC filters may be considered in patients with PE and cardiopulmonary compromise, as well as in patients with chronic thromboembolic hypertension.
Chronic Management of Cancer-Associated VTE
Patients with cancer who have VTE are at increased risk of recurrent VTE and major bleeding compared with patients who do not have cancer.46,242,243 Recognition of this disparity in outcomes prompted investigation of alternative anticoagulant agents for the chronic management of VTE in patients with cancer. In the landmark CLOT trial, Lee and colleagues demonstrated that weight-based dalteparin (200 IU/kg daily for 1 month followed by 150 IU/kg daily for months 2 to 6) was superior to traditional management with acute parenteral anticoagulation with dalteparin (200 IU/kg daily) followed by adjusted-dose vitamin K antagonist warfarin (INR 2-3) for 6 months.259 Patients treated with dalteparin were almost 50% less likely to experience recurrent VTE (9% LMWH vs. 17% warfarin, P = .002) and had a comparable incidence of major bleeding and early mortality.259 The superiority of LMWH for the chronic treatment of VTE in patients with cancer is supported by several other studies using enoxaparin and tinzaparin,73,260,261 although the CLOT trial remains the largest and most definitive study.
In a systematic review of long-term treatment of VTE in patients with cancer, Louzada and colleagues262 noted that LMWH was associated with a 50% reduction of recurrent VTE (RR 0.53, 95% CI 0.36-0.76, P = .007) with a similar risk of major bleeding (RR 0.98, 95% CI 0.49-1.93, P = .95) and all-cause mortality (RR 0.94, 95% CI 0.80-1.11). They concluded that LMWH was superior to vitamin K antagonist for the treatment of VTE in patients with cancer.262 All the major treatment guidelines recommend LMWH as the preferred option for the long-term treatment of VTE in patients with cancer. In summary, in the absence of contraindications such as significant active bleeding, we suggest treatment dose LMWH for at least 6 months for patients with cancer who have newly diagnosed VTE, because this treatment will reduce the incidence of recurrent VTE and may prolong survival for some patients. In patients who have a contraindication to LMWH, such as heparin-induced thrombocytopenia, the indirect factor Xa inhibitor, fondaparinux, can be used.263 The duration of therapy in patients with cancer is indefinite as long as there is evidence of active disease or ongoing treatment of cancer. Discontinuation of therapy can be considered for patients who have completed therapy and have no radiographic evidence of disease. A clinical prediction tool for assessing the risk of recurrent VTE in patients with cancer has been developed and may aid decision making regarding the duration of anticoagulation.264
Additional issues that should be considered include the goals of care for patients with advanced cancer, in particular the need for daily injections with the use of LMWH or frequent INR monitoring while taking warfarin. Patients with cancer who receive chronic anticoagulation therapy with warfarin are at a high risk of complications because of frequent interactions of warfarin with other drugs frequently used in oncology patients265 (Table 35-3). In general, for patients with cancer and without a contraindication to anticoagulation, we recommend a minimum of 6 months of therapeutic anticoagulation for an initial PE, at which time the indications for continued anticoagulation therapy should be reassessed. Patients with cancer who have non–catheter associated DVT should undergo at least 3 months of anticoagulation therapy or for as long as the CVC is in place, whichever is longer. The risk-benefit ratio of ongoing anticoagulant therapy should be continually reassessed in individual patients, taking into consideration the overall clinical status of the patient, including the quality of life, life expectancy, and goals of care.
Table 35-3
Medications that Can Influence Vitamin K Antagonists
| Increase in the INR | Decrease in the INR |
| Alcohol | Azathioprine |
| Amiodarone | Barbiturates |
| Anabolic steroids | Carbamazepine |
| Broad-spectrum antibiotics | Chlordiazepoxide |
| Capecitabine | Cholestyramine |
| Cimetidine | Griseofulvin |
| Erlotinib | Methimazole |
| Erythromycin | Mitotane |
| Fluorouracil | Nafcillin |
| Fluconazole and other azole antifungal agents | Phenytoin |
| Flutamide | Rifampin |
| Gefitinib | Rifabutin |
| Gemcitabine | Spironolactone |
| Ifosfamide | Sucralfate |
| Imatinib | Vitamin K and vitamin K–rich foods |
| Isoniazid | |
| Metronidazole (Flagyl) | |
| Omeprazole | |
| Piroxicam (Feldene) | |
| Propafenone | |
| Propranolol | |
| Quinidine | |
| Sulfinpyrazone | |
| Trastuzumab | |
| Trimethoprim/sulfamethoxazole |
INR, International normalized ratio.
Derived from Nutescu E, Chuatrisorn I, Hellenbart E. Drug and dietary interactions of warfarin and novel oral anticoagulants: an update. J Thromb Thrombolysis 2011;31:326–43.
Management of Recurrent Cancer-Associated VTE
A high percentage (9% to 17%) of patients with cancer will experience recurrent VTE within 6 months of the initial VTE episode despite undergoing therapeutic anticoagulation.259,266 Patients incidentally diagnosed with unsuspected PE appear to have the same risk of recurrent VTE, bleeding, and mortality as symptomatic patients.38 In any patient presenting with recurrent VTE, anatomic causes of recurrent thrombosis (e.g., tumor or nodal masses compressing the thrombosed vessel, iliac vein compression syndrome, i.e., May-Thurner syndrome, and thoracic outlet syndrome) should be excluded. In patients with anatomic vascular compression, elimination of sluggish blood flow is critical to reduce the risks of recurrent thrombosis. If the patient is in an early stage of treatment, heparin-induced thrombocytopenia should be considered. In patients taking vitamin K antagonists, Trousseau syndrome is a possibility. Subtherapeutic anticoagulation should also be ruled out. In patients taking vitamin K antagonists, switching to an LMWH should be considered. Other options for management of recurrent VTE in patients with cancer include insertion of an inferior vena cava filter, an increased dose of LMWH, switching from once- to twice-daily dosing, or switching to an alternative parenteral agent with a longer half-life (i.e., from LMWH to fondaparinux).168,267–269 Dose escalation of LMWH by 20% to 25% has been studied retrospectively and did not appear to be associated with an increased risk of bleeding; however, prospective studies are required.268 Anti-Xa level monitoring may be useful in the management of patients who experience recurrent VTE while taking LMWH.268 Currently we recommend dose escalation of LMWH by 20% to 25% as the initial management of choice for patients who experience recurrent VTE while taking LMWH. IVC filter use is recommended if there is active bleeding or other absolute contraindications to anticoagulation therapy; however, if the contraindication resolves, then routine anticoagulation therapy with LMWH should be resumed.246 Table 35-4 summarizes some of the management strategies for recurrent VTE in patients with cancer.
Table 35-4
Management of Recurrent Venous Thromboembolism in Patients with Cancer
| Causes | Management |
| Extrinsic vascular compression (causes sluggish flow) | Relieve vascular compression due to tumor, nodal masses, or anatomic abnormalities (i.e., May-Thurner syndrome/iliac vein compression, thoracic outlet syndrome) |
| Intrinsic vascular obstruction | Remove central venous catheter/devices responsible for sluggish flow |
| Trousseau syndrome | Switch to LMWH |
| Heparin-induced thrombocytopenia | Discontinue unfractionated or LMWH and start a direct thrombin inhibitor (preferred) or fondaparinux |
| Therapeutic resistance | If the patient is taking warfarin, switch to LMWH If the patient is taking LMWH, check to ensure the correct weight-based dose is being used, switch to twice-daily dosing (if taking once daily), consider checking LMWH (anti-Xa) level, initiate an empiric 25% dose increase, or switch to fondaparinux |
Management of Cancer-Associated VTE in Special Situations
Central Venous Catheter–Associated VTE
Patients with cancer frequently have indwelling intravenous catheters inserted to allow regular laboratory testing and delivery of chemotherapy without the need for the discomfort of venipuncture. CVC-associated DVT has been noted in as many as 60% of patients with cancer overall, and it is asymptomatic in 75% of patients.270,271 Symptomatic events are less common, occurring in 3% to 25% of patients.153,203,272 Two prospective studies found the incidence of symptomatic CVC-associated DVT to be 3% and 4.3%, respectively.157,203 Placement of PICCs, a history of a previous DVT, catheter infections, and use of subclavian catheters or malpositioned catheters have been identified as risk factors for catheter-associated DVT.273 Implantable ports may have a lower risk for VTE than other vascular access devices.273 Prophylactic anticoagulation therapy is not recommended for prevention of catheter-associated thrombosis.274,275 Symptoms of catheter-associated DVT include thrombophlebitis, extremity edema, or evidence of pulmonary embolism. Clinical prediction tools are available to assist in the assessment of possible VTE in patients with indwelling central vascular access devices.276 Diagnosis may be made using duplex ultrasonography with greater than 90% accuracy for symptomatic patients.277 Duplex ultrasonography has lower sensitivity for asymptomatic thrombi, and venography may be required in this situation.278 Therapeutic anticoagulation, with or without catheter removal, is indicated for patients with acute VTE who do not have a contraindication.246,274 Patients with catheter-induced VTE are less likely to experience postthrombotic complications, and therefore management may be conservative in some cases, for example, catheter removal only (see Chapter 26).279 However, this approach is controversial, and in general we recommend 3 months of therapeutic anticoagulation with LMWH for catheter-associated DVT in patients with cancer.
Cancer-Associated VTE in Patients with Thrombocytopenia
Because of the risk of heparin-induced thrombocytopenia (HIT), platelet counts should be monitored in all patients who are receiving UFH or LMWH.280 For patients receiving therapeutic-dose UFH or LMWH, every-other-day platelet count monitoring is suggested from day 4 to day 14, or until heparin is stopped, whichever occurs first. In patients with cancer, diagnosis of the etiology of thrombocytopenia may be complicated because of the presence of multiple causes of thrombocytopenia that may overlap, including myelosuppression from chemotherapy or bone marrow infiltration by tumor, paraneoplastic phenomena such as disseminated intravascular coagulation, and polypharmacy leading to drug-induced thrombocytopenia. The use of clinical prediction rules for HIT including the 4T score and the HIT Expert Probability score can assist in the determining the pretest probability of HIT.281,282 Management of anticoagulation in patients with platelet counts >50,000/µL who do not have major risk factors for bleeding in general does not require a dose reduction; however, platelet levels should be monitored closely, and HIT should be excluded if the patient is receiving heparin-based products. For patients with a platelet count <50,000/µL, UFH without a bolus and with a low-medium goal activated partial thromboplastin time can be considered; however, evidence is limited for this approach. Platelet transfusion to maintain a platelet count >50,000/µL can also be considered depending on the clinical situation. Nonpharmacologic measures such as a vena cava filter should be considered for patients likely to have persistently low platelet counts that would preclude anticoagulation therapy.163,168 For thromboprophylaxis in thrombocytopenic patients with cancer, the use of mechanical methods such sequential/pneumatic compression devices have been proposed.163,168 Nonetheless, the benefits of these devices in thrombocytopenic patients with cancer are unknown; they are not effective for prevention of CVC-associated VTE and may carry a theoretic risk of soft-tissue bleeding in profoundly thrombocytopenic patients.30
Cancer-Associated VTE in Patients with Central Nervous System Lesions
Patients with primary or secondary brain tumors are at high risk of the development of VTE because they often are in a cancer-induced hypercoagulable state (which in particular is associated with glioblastoma multiforme, occurring in up to 30% of patients),283 frequently undergo neurosurgical procedures, and consequently experience immobility.284,285 Previously, IVC filters were frequently used with such patients because of concerns about an increased risk of intracranial hemorrhage with the use of anticoagulation therapy; however, filters are associated with complications in at least 10% of patients.286 More recently, anticoagulation therapy has been shown to be safe and effective in patients with intracranial tumors and is currently the standard of care.287 However, anticoagulation is contraindicated in patients with active intracranial bleeding. Spontaneous intracranial hemorrhage occurs more commonly in patients with metastatic brain tumors (14%) compared with patients with a primary glioma (0.8%).288 Choriocarcinoma (up to 100% risk), melanoma (as high as 70% risk), and thyroid carcinoma and renal cell carcinoma (40% to 50% risk) have been associated with a higher risk of bleeding in the presence of brain metastases, whereas lung cancer (5%) and breast cancer (1%) are associated with much lower risks. Newer antiangiogenic agents such as bevacizumab that are used in the treatment of primary brain tumors appear to be associated with an increased risk of bleeding and thrombosis.140,289 Anticoagulation with warfarin may not increase the risk of intracranial hemorrhage provided that INR levels are tightly controlled.290 Prophylactic LMWH appears to be safe in patients with primary and secondary brain tumors without an increase in intracranial hemorrhage risk.291 Although no prospective studies have compared warfarin with LMWH in patients with brain tumors, observational studies suggest that the risk of hemorrhage is low. Therefore we recommend therapeutic anticoagulation, preferably with LMWH, for patients with VTE and primary or secondary brain tumors. Patients with evidence of intracranial hemorrhage should not receive anticoagulation therapy and alternative nonpharmacologic methods such as IVC filtration should be considered.12,163,168
Hematopoietic Stem Cell Transplant and VTE
Hematopoietic stem cell transplant (HSCT), which represents the only curative therapeutic modality for some advanced hematologic malignancies, is associated with a significant risk for both VTE and bleeding. In a retrospective analysis, Gerber et al.30 evaluated 1514 patients undergoing inpatient HSCT at a single center (The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins). There was no standardized approach to VTE prophylaxis. By day 180 after HSCT, 75 symptomatic cases of VTE occurred in 70 patients (4.6%; 95% CI, 3.6%-5.8%), of which 55 (3.6%) were catheter associated, 11 (0.7%) were non–catheter-associated DVT, and 9 (0.6%) were PE. Profound thrombocytopenia was not protective against VTE in these patients, because 34% of VTE occurred at a platelet count <50,000/µL and 13% occurred at a platelet count <20,000/µL. In multivariate analysis, VTE was associated with prior VTE and with graft-versus-host disease. Clinically significant bleeding occurred in 230 patients (15.2%), of whom 55 patients (3.6%) had fatal bleeding. Bleeding was associated with anticoagulation (OR 3.1), graft-versus-host disease (OR 2.4), and VOD (OR 2.2). The authors concluded that VTE in patients undergoing HSCT is primarily catheter-related and threefold less common than clinically significant bleeding.30 Another retrospective analysis of 589 patients who underwent HSCT in a single Canadian institution showed a similar percentage of symptomatic VTE occurrence (at 1 year, 3.7%; 95% CI, 2.5-5.6), of whom 1.2% experienced symptomatic non–catheter-related VTE after HSCT (four PE and three DVT).292 Although no thromboprophylaxis was utilized in the Johns Hopkins Hospital study, most patients in the Canadian study who underwent allogeneic HSCT (80%) received enoxaparin, 20 mg daily, for prevention of VOD, starting 6 days before HSCT and lasting for a mean of 22 days. Patient who underwent autologous HSCT did not receive thromboprophylaxis.292
Outpatient Management of Cancer-Associated VTE
PE is associated with a substantial risk of morbidity and mortality. In the ICOPER international observational cohort study of PE, 17.4% (426 of 2454) of patients died within 3 months of their PE.293 In patients with hypotension (systolic blood pressure <90 mm Hg), the 3-month mortality rate exceeded 50% (52.4%; 95% CI, 43.3%-62.1%).293 In the RIETE registry, 3% of patients with nonmassive PE and 9.3% of patients with massive PE experienced a fatal PE within 3 months.294 Risk factors for fatal PE included symptomatic nonmassive PE (OR, 5.4; 95% CI, 3.2-9.2), symptomatic massive PE (OR, 17.5; 95% CI, 7.5-41.2), immobilization >4 days for neurologic disease (OR, 4.9; 95% CI, 2.7-8.8), age >75 years (OR, 2.5; 95% CI, 1.6-3.8), and cancer (OR, 2.04; 95% CI, 1.3-3.2).294 Therefore risk stratification of patients with PE is critical to determining further management. In normotensive patients, a number of approaches to risk stratification have been investigated. Right ventricular hypokinesis or overload as assessed by echocardiography (HR 2.11) or CT angiography (HR 5.17) is associated with a higher risk of adverse outcomes.295 Cardiac biomarkers, which are released from the right ventricular wall when it is under elevated pressures, such as N-terminal pro-brain naturetic peptide, appear to be useful in PE risk stratification (100% negative predictive value; 95% CI, 91-100) for adverse outcome at 3 months when N-terminal pro-brain naturetic peptide is <300 pg/mL.296 The weakness of the aforementioned approaches is that they require additional testing or expertise that may not be available in all health care settings. Two clinical prediction rules have been developed to identify patients with PE who are at low risk for adverse outcomes: the Geneva score and the Pulmonary Embolism Severity Index (PESI) score. The Geneva score was based on a prospective study of outcomes among 296 patients who presented to the University of Geneva hospital with PE.297 Wicki et al.297 identified six independent predictors of outcomes at 3 months. It has been subsequently externally validated in a separate population of patients.298
The PESI score was developed by Aujesky and colleagues299 based on an analysis of 15,531 inpatients discharged with PE from 186 Pennsylvania hospitals. They identified 11 clinical factors that were independently associated with 30-day mortality.299 The PESI score has also been externally validated in several different patient populations and performed better than the Geneva score in identifying patients at low risk for adverse outcomes.302–302 Although the PESI and Geneva scores have been used extensively in general populations of patients with PE, each was derived from study populations that contained a minority of patients with cancer. Both scores consider all cancers as having an equal impact on outcome despite extensive data indicating great variation in VTE risk among cancers. Therefore it remains to be seen whether these scores will be as effective in populations composed exclusively of patients with cancer. Studies examining the utility of these risk-stratification tools are warranted before their use in patients with cancer.
Several recently published reports have noted that as many as 50% of patients with symptomatic PE may be managed as outpatients.303–309 Kovacs et al.305 reported on a retrospective single-center experience of 639 patients who presented to London Health Sciences Centre in Ontario Canada between January 2003 and January 2008. A total of 314 patients who were hemodynamically stable and free of hypoxia or pain requiring parenteral narcotics and deemed at low risk for bleeding were managed as outpatients. Fifty-seven patients with PE (18%) who were managed as outpatients had cancer. Three patients (0.95%; 95% CI, 0.25%-3%) experienced recurrent VTE, three experienced major bleeds (0.95%; 95% CI, 0.25%-3%) and nine deaths occurred (all due to cancer) (2.9%; 95% CI, 1.4%-5.6%) during 3 months of follow-up.305 Erkens et al.308 noted similar results in their retrospective cohort of 473 patients with PE, of whom 260 were treated as outpatients. Eighty-three outpatients (31.9%; 95% CI, 26.3%-38%) had cancer. At 3 months, the mortality rate was 5.0% (95% CI, 2.7%-8.4%), recurrent VTE occurred in 3.8% (95% CI, 1.9%-7%), and major bleeding occurred in 1.5% (95% CI, 0.4%-3.9%).308
In a study by Siragusa et al.,306 patients with cancer who had VTE were treated as outpatients unless they required admission for other problems or were actively bleeding. Outpatient therapy used LMWH followed by warfarin or LMWH alone and was accompanied by an educational program for patients. Among 207 patients, 17% of whom had metastatic disease, LMWH and warfarin were prescribed to 51%, whereas LMWH alone was prescribed to 49%. In total, 127 patients (61%) were entirely treated on outpatient basis. There were no differences between outpatients and hospitalized patients with regard to gender, mean age, site of cancer, presence of metastases, or therapy. After a follow-up of 6 months, no statistically significant differences were found between the patients treated on outpatient basis versus hospitalized patients in recurrent VTE (8.7% vs. 5.6%, P = .58); or major bleeding (2% vs. 1.5%, P = .06).306 After 6 months of follow-up, 33% of hospitalized patients and 26% in the home-treatment group had died. The authors concluded that in patients with cancer who have acute DVT and/or PE, there is no difference between hospitalization and home treatment in terms of major outcomes.306 These studies indicate that clinically stable patients with cancer who have PE and are at low risk for adverse events may be managed safely as outpatients. Box 35-6 lists some of the relative and absolute contraindications for outpatient management of VTE in patients with cancer.
Management of Unsuspected VTE
Unsuspected VTE is a common finding on contrast CT scans, particularly in patients with cancer.18,310 Recent evidence indicates that many of these patients are clinically symptomatic on closer examination310 and have outcomes similar to patients with symptomatic VTE.37–39,42 Therefore patients with cancer with unsuspected VTE should be managed in the same fashion as patients with symptomatic VTE.
Use of Anticoagulants to Improve Survival in Patients with Cancer
Because cancer is associated with a high incidence of VTE and numerous interactions between coagulation factors and platelets and tumor growth and metastasis have been identified, there has long been interest in the therapeutic potential of anticoagulant agents in the treatment of cancer. In the 1980s, a randomized controlled trial of warfarin in patients with small cell lung cancer, colorectal cancer, head and neck cancer, and prostate cancer noted a significant increase in survival among patients with small cell lung cancer who received warfarin in addition to standard chemotherapy (49 weeks vs. 23 weeks, P = .018). No evidence of benefit was noted in the other tumor types.311 Since then, numerous studies have tested the hypothesis that anticoagulants may increase survival in patients with cancer. After the success of the study by Zacharski et al.,311 four other randomized trials of warfarin versus control/placebo were conducted. A metaanalysis by Akl et al.312 of these studies noted no significant difference in mortality at 1 year, 2 years, or 5 years. One study noted an 85% reduction in VTE at a cost of a 4.2-fold increase in major bleeding.
The impact of UFH and LMWH on cancer mortality has also been studied extensively. In the FAMOUS study, Kakkar and colleagues313 randomly assigned 385 patients with advanced cancer to dalteparin, 5000 units daily, or placebo for 1 year. Although survival at 12 months, 24 months, and 36 months in both groups was similar, in a subgroup of patients with a better prognosis who were alive at 17 months, survival among patients treated with dalteparin was better at 2 and 3 years compared with placebo recipients (78% vs. 55% and 60% vs. 36%, respectively; P = .03).313 In a post-hoc analysis of the CLOT trial, Lee and colleagues314 found that patients without metastatic disease who received dalteparin had a lower 12-month mortality rate than did patients treated with warfarin (20% vs. 36%; P = .03). Several other studies have found evidence of a survival benefit associated with LMWH.315,316 A systematic review of parenteral anticoagulants for cancer therapy published in 2011 found modest evidence of a survival benefit at 24 months (RR, 0.92; 95% CI, 0.88-0.97) but not at 12 months (RR, 0.93; 95% CI, 0.85-1.02).317 A recent multicenter open-label randomized study of 2 weeks of a therapeutic dose of nadroparin followed by a half dose of nadroparin in patients with non–small cell lung cancer, hormone-refractory prostate cancer, and pancreatic cancer found no evidence of survival benefit (13.1 months vs. 11.9 months, HR 0.94, 95% CI 0.75-1.18).318 Consequently, at present, although there are tantalizing suggestions that antithrombotic therapy may favorably influence the clinical course of patients with cancer, the data demonstrate insufficient benefit to warrant use of anticoagulant agents for this purpose. Perhaps development of agents targeted at specific procoagulant proteins known to promote tumor growth such as TF may produce greater survival benefits.
Reversal of Anticoagulation
In the event of bleeding, rapid reversal of anticoagulation is often necessary. UFH can be fully reversed by intravenous administration of protamine in a dose of 1 mg per 100 units of heparin. Protamine rarely can be associated with anaphylactoid reactions (0.2%). The risk of anaphylaxis is greater in patients taking neutral protamine hagedorn insulin (3%).319 To reduce the risk of adverse reactions, protamine should not be infused faster than 5 mg per minute. LMWH can also be partially reversed with protamine. The extent of reversal varies by agent. The anti-Xa activity of enoxaparin is reversed by 54%, dalteparin by 74%, and tinzaparin by 85%.320 The dose of protamine is 1 mg/mg enoxaparin or 100 units of dalteparin or tinzaparin. Recombinant human factor VIIa (Novo Seven) has been used in doses of 20 to 30 µg/kg to manage bleeding in patients with excessive LMWH levels.321 Given the thrombogenic potential of this agent, recombinant human factor VIIa should only be used in situations of life-threatening bleeding that is unresponsive to protamine. Fondaparinux-associated bleeding has also been managed with recombinant human factor VIIa.322 Protamine is ineffective for fondaparinux reversal. There is no specific reversal agent for rivaroxaban. A study of human volunteers demonstrated that an inactivated four-factor prothrombin complex concentrate (PCC) could correct coagulation test abnormalities associated with rivaroxaban.323 However, confirmation that a PCC is effective for treatment of clinical bleeding in patients is lacking. A recombinant inactivated form of factor Xa that avidly binds rivaroxaban and other direct and indirect factor Xa inhibitors has been shown in animal models to be effective in restoration of normal hemostasis in anticoagulated study subjects. This agent appears to be a promising antidote for rivaroxaban and other factor Xa inhibitors.324 In patients with life-threatening bleeding, warfarin can be reversed rapidly with administration of a three- or four-factor PCC (25-50 units/kg intravenous). Intravenous vitamin K (10 mg administered over at least 10 minutes) should also be administered. Two units of plasma should be administered if a three-factor PCC is used to provide a source of factor VII. Table 35-5 lists suggested strategies for the reversal of anticoagulation.
Table 35-5
| Anticoagulant | Reversal Strategy |
| Unfractionated heparin | Protamine, 1 mg/100 units IV |
| LMWH | Protamine |
| rhFVIIa, 20-30 µg/kg | |
| Fondaparinux | rhFVIIa, 90 µg/kg |
| Direct thrombin inhibitors (argatroban, bivalirudin, dabigatran, desirudin, lepirudin) | Hemodialysis (effective for lepirudin, bivalirudin, desirudin, and dabigatran) |
| Activated prothrombin complex concentrate (FEIBA 50-100 units/kg) | |
| rhFVIIa, 90 µg/kg | |
| Rivaroxaban | 4 factor prothrombin complex concentrate 50 units/kg |
| Activated prothrombin complex concentrate (FEIBA 50-100 units /kg) | |
| rhFVIIa, 90 µg/kg | |
| Warfarin | IV Vitamin K 10 mg over at least 10 minutes |
| 4 factor prothrombin complex concentrate 25-50 units/kg IV | |
| 3 factor prothrombin complex concentrate 25-50 units/kg IV + 2 units FFP IV |
Adapted from Streiff MB, Bockenstedt PL, Cataland SR, Chesney C, Eby C, Fanikos J, et al. Venous thromboembolic disease. J Natl Compr Canc Netw 2011;9:714–77.