Dermatologic Toxicities of Anticancer Therapy
Lisa Pappas-Taffer, Kachiu Lee, H. William Higgins, Leslie Robinson-Bostom and Charles J. McDonald
• Etiology: Cytotoxic chemotherapy agents target hair follicles that are in the proliferative growing (anagen) phase, causing an “androgen effluvium.” Less common mechanisms include telogen effluvium.
• Incidence: Chemotherapy-induced alopecia (CIA) is common. The incidence differs based on the agent, dose, and frequency of administration.
• Manifestations: Increased hair shedding and hair fragility occur, with a diffuse or patching alopecia that is noticeable when 25% to 40% of scalp hairs are shed. It may be associated with symptoms of pruritus or pain but is most commonly asymptomatic.
• Complications: CIA can dramatically affect patients’ psychosocial health, resulting in significant reductions in quality of life (QoL).
• Treatment: Most treatment recommendations are based on expert opinion, although a few randomized controlled trials (RCTs) exist. Reported preventive strategies include counseling and the use of scalp cooling, ammonium trichloro(dioxoethylene-O,O-) tellurite (AS101), or scalp tourniquets. For acceleration of hair regrowth, minoxidil, 2% twice daily, has been shown to be effective.
• Prognosis: CIA is typically completely reversible, although up to 60% of patients report changes in the texture, thickness, or color of their new hair.
Cutaneous Extravasation Injury
• Etiology: Extravasation injury is divided into irritant versus vesicant reactions. Irritant agents cause inflammation and erythema, whereas vesicant reactions may cause full thickness skin necrosis.
• Incidence: The incidence of irritant reactions is unknown. The incidence of intravenous vesicant reactions is thought to approach 6%.
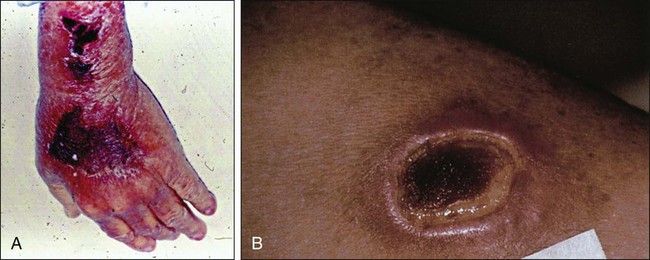
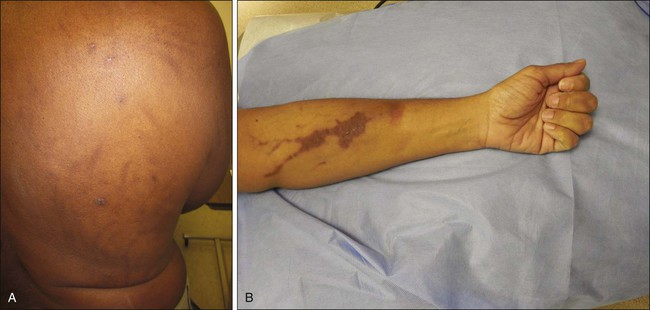
• Manifestations: Extravasation of chemotherapeutics can cause a range of unintended adverse effects, including skin inflammation or necrosis. The severity of tissue injury after unintended extravasation ranges from mild erythema to blisters and full-thickness skin necrosis (Figs. 44-1 and 44-2).
• Complications: Pain can be significant, and rarely, compartment syndrome can result.
• Treatment: The offending drug must be stopped immediately. The subsequent treatment differs based on the chemotherapeutic agent used. A treatment algorithm is presented in Box 44-1 and Table 44-4 based on RCTs and expert opinion.
• Prognosis: Mortality is low, but the QoL impact and related morbidity is severe with vesicant chemotherapeutics.
Chemotherapy-Induced Hyperpigmentation
• Etiology: A variety of patterns of cutaneous hyperpigmentation have been described in association with many different cytotoxic agents.
• Incidence: The overall incidence of hyperpigmentation is unknown.
• Manifestations: Cutaneous hyperpigmentation can be generalized or localized and can occur in drug-specific patterns (Table 44-5, Fig. 44-3). Mucous membranes, hair, teeth, and nails can also be affected by changes in pigmentation produced by cancer chemotherapy.
• Complications: Cutaneous hyperpigmentation may be cosmetically displeasing with reduced QoL.
• Treatment: Limited data are available regarding treatment options.
• Prognosis: Skin hyperpigmentation resolves slowly after discontinuation of therapy, and nail pigmentation grows out distally.
• Etiology: Hand-foot syndrome (HFS) has numerous synonyms, including palmar-plantar erythrodysesthesia and acral erythema. It is caused by a variety of cytotoxic chemotherapeutic agents.
• Incidence: The incidence differs based on the cytotoxic agent used, the dose, and the administration frequency. Overall, the incidence ranges from 3% to 89%.
• Manifestations: The clinical manifestations can vary based on severity, ranging from asymptomatic mild erythema or peeling to extremely painful full-thickness epidermal sloughing with significant functional impairment (Fig. 44-4).
• Complications: Reduced QoL due to pain and functional impairment is common. However, rare complications can include prolonged dysesthesias with loss of fingerprints, rare distal necrosis, or secondary infection.
• Treatment: Drug interruption or dose modification is the most well-documented intervention. In general, wound care to prevent infection, elevation to reduce edema, and symptomatic treatment, including routine use of topical emollients, is recommended. Celecoxib has been shown to be effective in preventing HFS in patients receiving capecitabine. Other preventive treatments suggested to be helpful include regional cooling during infusions, nicotine patches, oral vitamin E, and systemic steroids. Therapies reported to be helpful to reduce symptoms include oral steroids, oral vitamin E, celecoxib, and topical 99% dimethylsulfoxide.
• Prognosis: Severe acral pain, inflammation, and possible blister formation can cause considerable morbidity and poor patient compliance. However, it is typically completely reversible after drug discontinuation. Reaction severity is not thought to be related to the patient’s disease status.
Neutrophilic Eccrine Hidradenitis
• Etiology: Neutrophilic eccrine hidradenitis (NEH) is an acute dermatosis that has been associated with multiple chemotherapeutic agents; in addition to malignancies in the absence of chemotherapy, infections (e.g., human immunodeficiency virus), and nonchemotherapeutic drugs. Hence NEH is considered a reactive process. Eccrine squamous syringoplasia (ESS) is a condition that clinically resembles NEH; however, what skin biopsy reveals is a lack of neutrophils.
• Manifestations: Variable (Fig. 44-5).
• Treatment: Dose reduction or interruption.
• Prognosis: The prognosis for both NEH and ESS is favorable.
• Etiology: Radiation dermatitis is caused by ionizing radiation applied to any area of the skin. The severity is dependent on location, body surface area treated, volume of tissues irradiated, total radiation dose received, and the period over which radiation was administered.
• Incidence: Radiation dermatitis occurs in up to 95% of patients receiving ionizing radiation.
• Manifestations: An acute dermatitis typically occurs within 90 days of exposure. Chronic dermatitis usually occurs after 90 days. Clinical manifestations range from mild erythema resembling a sunburn to chronic ulcerations (Fig. 44-6).
• Complications: Chronic nonhealing or infected ulcerations most commonly occur. However, chronic radiation dermatitis may be associated with the development of nonmelanoma skin cancers and angiosarcomas at irradiated sites.
• Treatment: Treatment is symptomatic. Chronic ulcerations may require persistent wound care to prevent infection and optimize skin healing. Hence guidance from wound care specialists may be necessary.
• Prognosis: Prognosis is generally favorable after cessation of radiation therapy.
• Etiology: Radiation recall occurs in a previously irradiated area after administration of a chemotherapeutic agent. The exact mechanism is unclear.
• Incidence: Incidence is unknown, but it is thought to affect up to 12% of all patients receiving chemotherapy medications after radiation therapy.
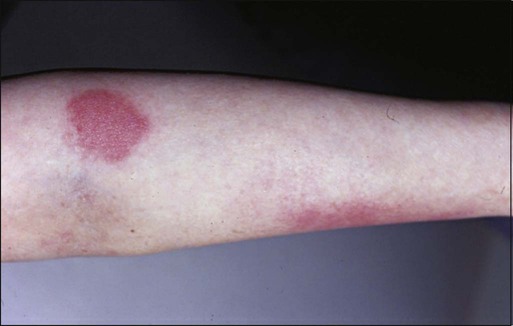
• Manifestations: Clinical manifestation ranges from mild erythema to severe ulceration and necrosis (Fig. 44-7).
• Complications: Chronic nonhealing or infected ulcerations.
• Treatment: Treatment is symptomatic. Severe or chronic ulcerations should be managed in conjunction with wound care specialists.
• Prognosis: Lesions typically resolve with symptomatic management.
• Etiology: Radiation enhancement occurs when chemotherapeutics act as radiation sensitizers to potentiate the effects of radiation. Chemotherapeutics must be administered within 3 weeks of radiation to produce radiation enhancement.
• Incidence: The incidence rate varies with different agents. No prospective studies have been conducted to examine incidence or prevalence rates of radiation enhancement.
• Manifestations: Clinical manifestations include skin findings that resemble radiation dermatitis and radiation recall.
• Complications: Complications can include full-thickness skin necrosis requiring care that includes treatment by a wound care specialist.
• Treatment: Treatment is symptomatic.
• Prognosis: Lesions typically resolve with symptomatic management.
Atypical Vascular Lesions and Angiosarcomas
• Etiology: Atypical vascular lesions (AVLs) and angiosarcomas are long-term sequelae of ionizing radiation, although the mechanism of tumor development is unclear.
• Incidence: AVLs are thought to be extremely rare, with an unclear incidence rate. Angiosarcomas are estimated to occur in approximately 5 in 10,000 patients.
• Manifestations: AVLs can manifest as multiple, scattered, red papules. Angiosarcomas manifest as ecchymotic-appearing patches.
• Complications: AVLs can regress spontaneously. However, recurrent AVLs may progress to angiosarcomas. Angiosarcomas have a 50% rate of metastasis. Even after treatment, 67% of angiosarcomas may recur.
• Treatment: Tumors tend to have an aggressive course, and surgical excision for both types of tumors is recommended.
• Prognosis: AVLs are typically benign, although they have a potential to transform into angiosarcomas. Prognosis for angiosarcoma is poor, with a 5-year disease-free survival rate of 36% in persons without metastatic disease.
• Etiology: Papulopustular eruption (PPE) occurs with use of epidermal growth factor receptor inhibitors (EGFRIs), cetuximab, panitumumab, erlotinib, and gefitinib; it occurs much less commonly with dual EGFR/HER2 inhibitors (e.g., lapatinib), and it occurs infrequently with multikinase inhibitors (MKIs), (e.g., sunitinib and sorafenib). Although historically referred to as “acneiform,” PPE is clinically and pathologically unrelated to acne.
• Incidence: More than 90% of patients treated with EGFRIs experience PPE.
• Manifestations: PPE is marked by itchy/painful papules and pustules most commonly occurring on the face, upper back, and chest (Fig. 44-8).
• Complications: Secondary cutaneous infections develop in 38% of affected patients. PPE is also often complicated by a reduced QoL.
• Treatment: RCT data are limited. See Table 44-14 for a suggested treatment algorithm based on expert opinion.
• Prognosis: Primarily, the presence and severity of EGFRI-associated PPE often correlate with good tumor response and increased patient survival. Prophylactic treatment, with the goal of reducing rash severity, does not adversely affect the inciting agent’s antitumor effect; instead, it may signal an optimized treatment outcome.
• Etiology: Hand-foot skin reaction (HFSR) is caused by MKIs (e.g., sorafenib and sunitinib), and more recently v600E-BRAF inhibitors (e.g., vemurafenib).
• Incidence: HFSR occurs more frequently in patients treated with sorafenib (33.8%) than with sunitinib (19%), with a significant number of patients having severe grade 3 toxicity (6% to 8%). The incidence of HFSR in patients treated with vemurafenib is 6% to 13%.
• Manifestations: HFSR is marked by painful, erythematous-to-hyperkeratotic plaques with a characteristic halo of erythema that occur focally in areas of friction on palmoplantar surfaces ± bullae (Fig. 44-9).
• Complications: Complications include pain and difficulty performing activities of daily living.
• Treatment: Treatment recommendations are largely anecdotal. See Table 44-15 for a proposed treatment algorithm.
• Etiology: Specific (e.g., vemurafenib) and nonspecific (e.g., sorafenib) BRAF inhibitors.
• Incidence: Secondary squamous neoplasms occur in 18% to 31% of patients receiving vemurafenib and 4% to 6% of patients receiving sorafenib.
• Manifestations: Keratoacantomatous carcinomas (KACs), KAC-type invasive squamous cell carcinoma (KAC-type SCCs), and classic SCCs may appear.
• Prognosis/treatment: There are no reported cases of metastatic disease. Treatment is recommended, although spontaneous involution can be seen. No proposed treatment algorithms are found in the literature.
Cutaneous Complications of Cytotoxic Chemotherapy
Cytotoxic chemotherapy agents are drugs that affect cell division or DNA synthesis and function. The major categories include alkylating agents, antimetabolites, anthracyclines, plant alkaloids, and others. These traditional chemotherapy agents cause many dermatologic adverse effects, including alopecia, stomatitis, hyperpigmentation, nail changes, extravasation reactions, hand-foot syndrome (HFS), and neutrophilic eccrine hidradenitis (NEH). A description of all the cutaneous reactions to cancer chemotherapeutic agents is beyond the scope of this book. Instead, we have elected to highlight a group of reactions that are fairly common and yet unique to cancer chemotherapy. Cutaneous complications can be severe and may modify the ultimate course of chemotherapy, whereas others can be treated symptomatically without adversely affecting the treatment course. See Table 44-1 for a summary of cutaneous toxicities to cytotoxic agents.1
Table 44-1
Cutaneous Toxicities Due To Cytotoxic Chemotherapy
| Agent | Indications |
| ANTIMETABOLITES | |
| Pemetrexed | Exanthem, radiation recall, urticarial vasculitis |
| Capecitabine | Hand-foot syndrome, stomatitis, acral hyperpigmentation, palmoplantar keratoderma, pyogenic granuloma, inflammation of actinic keratoses, mucosal hyperpigmentation |
| Fludarabine | Exanthem, mucositis, hand-foot syndrome, paraneoplastic pemphigus |
| Cladribine | Exanthem, toxic epidermolytic necrolysis |
| Tegafur | Hand-foot syndrome, acral hyperpigmentation, melanonychia, brittle nails, photoallergic and photolichenoid eruptions, pityriasis lichenoides et varioliformis acuta |
| Gemcitabine | Alopecia, mucositis, morbilliform exanthem, radiation recall, linear immunoglobulin A bullous dermatitis, scleroderma-like changes, lipodermatosclerosis, pseudocellulitis (resembling erysipelas), pseudolymphoma, Stevens-Johnson syndrome |
| 5-Fluorouracil | Radiation enhancement, radiation recall, photosensitive reactions, cutaneous and nail hyperpigmentation, hand-foot syndrome, inflammation of actinic keratoses, onycholysis |
| TOPOISOMERASE-INTERACTING AGENTS | |
| Irinotecan | Alopecia, mucositis |
| Topotecan | Alopecia, morbilliform exanthem, neutrophilic eccrine hidradenitis |
| Doxorubicin/liposomal doxorubicin | Hand-foot syndrome, radiation recall and enhancement, neutrophilic eccrine hidradenitis, hyperpigmentation, alopecia, mucositis, ultraviolet recall, extravasation reactions, formation of melanocytic macules |
| Liposomal daunorubicin idarubicin | Alopecia, mucositis, extravasation reactions |
| Radiation recall, alopecia, hand-foot syndrome, mucositis, nail hyperpigmentation, extravasation reactions | |
| ANTIMICROTUBULE AGENTS | |
| Paclitaxel | Alopecia, radiation recall, erythema multiforme, onycholysis, hand-foot syndrome, photosensitivity, scleroderma-like changes, subcutaneous lupus erythematosus, subungual hemorrhage |
| Docetaxel | Mucositis, alopecia, erythema, pruritus, desquamation, hand-foot syndrome, fixed erythrodysesthesia plaque, radiation recall, urticaria, exanthems, nail changes, subungual hemorrhage, scleroderma-like changes, subcutaneous lupus erythematosus, extravasations reactions, photosensitivity |
| Vincristine, vinblastine, vinorelbine | Phlebitis, alopecia, hand-foot syndrome, extravasation reactions |
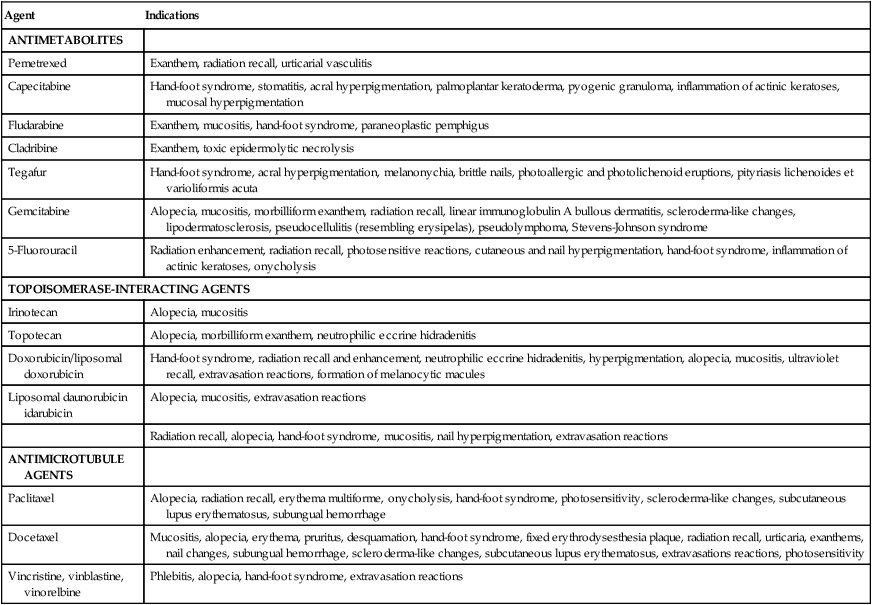
Modified from Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol 2011;65(3):624–35.
Chemotherapy-Induced Alopecia
Etiology and Biocharacteristics
The hair follicle is a highly organized structure within the skin. At the base of every follicle is a collection of rapidly dividing matrix cells that form an outwardly growing hair shaft. Hair follicle formation is complete at birth, with approximately 100,000 terminal hair follicles on the infant scalp.2 Although pharmacologic intervention has been helpful to aid in hair regrowth, hair follicles cannot be regenerated if they are destroyed.4–4 The hair follicle cycles through three phases: growth phase (anagen), a short involuting/regressing phase (catagen), and a resting phase (telogen) that results in hair shedding. On the scalp, 85%, 5%, and 10% of the scalp’s hair, respectively, are in these phases at a given time.5 Stem cells from the hair bulge, a permanent portion of the follicle, act as an endless source of hair matrix cells. The hair matrix cells subsequently divide during the proliferative anagen phase, marking the beginning of the hair cycle.
In general, the most common mechanism for cytotoxic chemotherapy-induced alopecia (CIA) is anagen effluvium—that is, a pattern of hair loss in which anagen hairs are selectively shed.6,7 Because cytotoxic agents target rapidly proliferating cell populations, they attack not only cancer cells but also the rapidly dividing hair matrix cells in anagen phase. This toxic effect on the follicle, as well as the hair shaft itself, leads to anagen hairs falling out with mild pressure, or breaking off at the scalp surface as a result of increased hair fragility (due to weak points in the hair shaft, referred to as Pohl-Pinkus constrictions).6 Molecularly, the mechanism by which these changes occur is largely unknown. However, the nuclear transcription factor p53 is thought to play a crucial role in initiating apoptosis in anagen effluvium-type CIA,2 because CIA does not develop in mice lacking p53 when they are treated with cyclophosphamide.8,9 Fas and c-kit have also been implicated.10 The duration of time until recovery, hair regrowth, and hair repigmentation appears to be dependent on the therapeutic dose and the degree of follicular dystrophy.8,9 Because the permanent pool of stem cells in the follicle is unaffected by most chemotherapy agents, CIA is typically reversible.11
Another mechanism for cytotoxic CIA is telogen effluvium, a type of hair loss that occurs when a greater proportion of anagen hairs transition into the catagen/telogen phase simultaneously. Hair shedding 3 to 4 months after the initial drug exposure is characteristic of persons with telogen effluvium because the duration of the catagen/telogen hair phases last approximately 3 to 4 months. Agents that frequently lead to telogen effluvium include methotrexate, 5-fluorouracil, and retinoids.6 The remainder of this section will discuss anagen effluvium-type CIA.
Epidemiology
The incidence of CIA is unclear given the lack of standardized grading scales or large epidemiological studies.1 However, certain drugs are more commonly associated with CIA, including cyclophosphamide (an alkylating agent), bleomycin and dactinomycin (antitumor antibiotics), doxorubicin (an anthracycline), irinotecan and topotecan (topoisomerase inhibitors), and docetaxel and paclitaxel (taxanes).4,12,13 In addition to the cytotoxic agent, the risk of CIA also depends on the drug route, dose, and frequency of administration.12 For example, high-dose, intermittent, intravenous, and combination chemotherapy regimens are more likely to induce alopecia than are low-dose single agents.4,14,15
Clinical Manifestations
Anagen effluvium-type CIA mainly affects the scalp, given the large percentage (85%) of anagen hairs at this site. Other sites including the eyebrows, eyelashes, beard, genitalia, and axillae can also be affected over time, although much less frequently.2,16 Anagen effluvium typically begins within days to weeks after initial drug exposure and worsens during the next 1 to 2 months.17 Patients may initially describe hairs on their pillowcase in the morning or collecting in their shower. Reduced scalp hairs are noticeable once 24% to 40% of a person’s hair is lost17 and can manifest with diffuse, patchy, or complete hair loss.12 Although the vertex (top) of the scalp is the most common site for hair loss, Yun and Kim7 recently demonstrated that the presence or absence of frontal hairline hair loss (described as a “receding” hairline) appears to differ based on gender. Women are less likely to lose hair at their frontal hairline compared with men, mimicking the gender-based differences seen in androgenetic or male-pattern baldness. In addition, alopecia was found to be asymptomatic in 50% of patients, with the remainder reporting associated pain (15.6%), pruritus (12%), or both (11%).7
Multiple studies, particularly in women undergoing treatment for breast cancer, have demonstrated that CIA dramatically reduces patients’ quality of life (QoL). In a literature review of patients with breast cancer, alopecia was consistently thought of as the most devastating chemotherapy-related adverse effect, resulting in decreased QoL and poor self-image.18 Yeager and Olsen6 summarized the following information: Study patients have reported that losing their hair was a worse experience than losing their breast, that the hair loss was a visible reminder of their cancer to others as well as to themselves, and that the severity of the psychological effect was not related to hair loss severity.6 In fact, some women have refused chemotherapy because of the risk of losing their hair, and in one study, 8% of women considered refusing chemotherapy because of the risk of CIA.18 Although most QoL studies have focused on women receiving chemotherapy for breast cancer, recent studies suggest that men often have similar and equally negative experiences with CIA as women, ranking alopecia as one of the most severe and bothersome chemotherapy adverse effects.6 In fact, an equal number of male patients were found to be receptive to paying increased fees in order to receive a chemotherapy regimen that would reduce their risk of experiencing CIA.19
Workup
No laboratory workup is necessary because CIA is diagnosed clinically. Quantifying the severity of alopecia is recommended. The National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) has proposed a grading scale (Table 44-2), and other scales exist as well.1,6
Table 44-2
Chemotherapy-Induced Alopecia Grading Scale* and Proposed Treatment Algorithm
Camouflage:
Acceleration of hair regrowth:
Infrequent:
Infrequent:
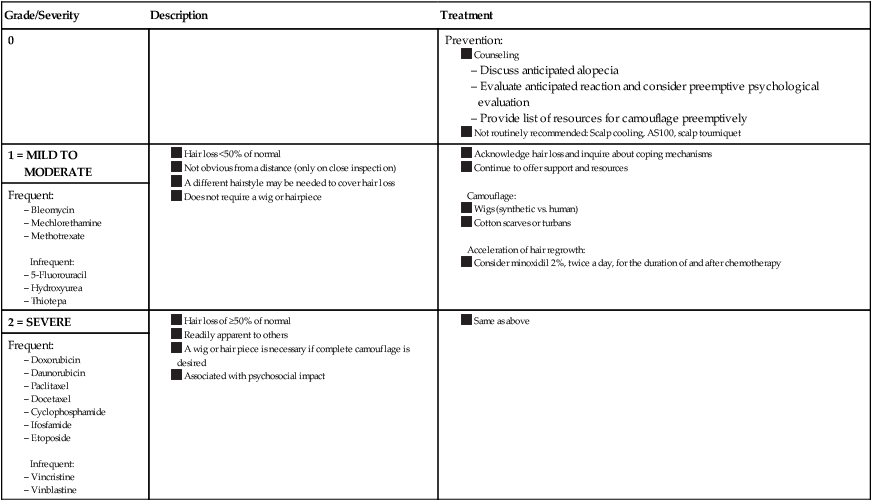
*Common Terminology Criteria for Adverse Events.
Data from Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol 2011;65(3):624–35 and Krause K, Foitzik K. Biology of the hair follicle: the basics. Semin Cutan Med Surg 2006;25(1):2–10.
Although many patients are aware of CIA as an adverse effect of chemotherapy, few are prepared for the QoL and psychological impact of this condition. If depression or other mental health issues are suspected, a referral to psychiatry is recommended.20
Differential Diagnosis
Androgenetic alopecia (or “male-pattern” alopecia) typically presents as gradual hair thinning at the crown and vertex of the scalp.21 It would not normally occur in the setting of cancer drug administration; however, patients with a predisposition toward androgenetic alopecia may experience CIA in locations similar to that of androgenetic alopecia.
Treatment
Preemptive Counseling
It is important for the clinician to fully appreciate the extremely negative and often devastating psychological impact that both male and female patients who undergo chemotherapy can experience with rapid loss of hair. Prior to initiation of chemotherapy, patients should be counseled that CIA will be a likely adverse effect. Physicians should evaluate patients’ perception of CIA and develop a plan for coping with the anticipated adverse effect. A multidisciplinary approach that includes psychiatric services may be necessary. A list of local hair resources such as scarf stores, fitted wigs, and head coverings should be provided. A prescription for a fitted hair prosthesis can be provided; such a prosthesis may or may not be reimbursed by the patient’s health insurance. Internet sites and alopecia support groups, such as the American Cancer Society or the National Alopecia Areata Foundation, can also provide local resources for patients and families.2 Treatment agents to prevent CIA and to promote hair regrowth have been studied. A treatment algorithm based on randomized controlled trial (RCT) data is presented in Table 44-2.
Preventive Treatment
Scalp cooling via the use of ice packs or cooling cap devices is by far the most well-studied and well-published technique for preventing CIA, dating back to the 1970s. Scalp hypothermia is postulated to prevent CIA by reducing blood perfusion and therefore chemotherapy delivery to the scalp, reducing intrafollicular metabolism.6 Despite differences in chemotherapy regimens, patient populations, hair-loss evaluation methods, and cooling mechanisms, a significant advantage in the amount of hair preserved with scalp cooling during chemotherapy was found in six of seven RCTs.2,6 Although cooling cap devices are broadly used in Europe, they were banned in 1990 by the U.S. Food and Drug Administration (FDA) because of safety concerns and lack of reproducible results. Given the concern that cooling could provide a protective environment for micrometastases, the use of scalp cooling caps are not recommended for patients with hematologic malignancies (e.g., mycosis fungoides or acute myeloblastic leukemia), and frequent scalp evaluations are recommended if cooling devices are used.2,6
Beside scalp cooling, other treatments that have been shown to be effective in preventing CIA include scalp tourniquets (in which a sphygmomanometer cuff headband is used to decrease the blood supply to the head) and the use of AS101, a carboplatin-related immunomodulating drug. In a phase 2 RCT of 58 patients, AS101 was found to significantly decrease the incidence and severity of CIA in patients treated with carboplatin/etoposide therapy compared with control subjects.5,21 Its protective effect against CIA is unclear but is thought to be linked to an upregulation of macrophage-derived factors, including interleukin-1.21 In contrast, topical minoxidil 2% and topical calcitriol (a vitamin D3 analog) were found to lack efficacy in preventing CIA.6,22–24
Treatments for Acceleration of Hair Growth After Chemotherapy
Once CIA is noted by the physician, the hair loss should be acknowledged. Treatment intervention may include camouflage16 or agents aimed at accelerating hair regrowth. Three modulators of the hair growth cycle (minoxidil, cyclosporine A, and 17-β-estradiol) have shown promise in animal studies. However, only topical minoxidil has been evaluated in a human RCT. Although topical minoxidil 2% has been shown to be ineffective in preventing the onset of CIA, a topical minoxidil 2% solution applied twice daily was found to shorten the period from hair loss to regrowth from 137 days to 50 days.6
Prognosis
Because chemotherapy effects spare the quiescent stem cells in the hair bulge of follicles, cytotoxic CIA is completely reversible in most cases. The hair follicle resumes normal cycling within a few weeks of treatment cessation, and regrowth becomes apparent 1 to 6 months after withdrawal of the offending medication and may even occur during prolonged cycles of therapy. Many patients report a change in the texture, thickness, or color of the new hair. These alterations may affect up to 60% of patients.7
Permanent alopecia, although rare, can occur. In a retrospective review of 8430 patients, seven patients presented with permanent CIA. Two of these patients were treated with Busulphan, and five patients were treated with taxanes.11 Hence appropriate counseling of patients who are being treated with these agents may be warranted.
Cutaneous Extravasation Injury
Etiology and Biocharacteristics
Extravasation injury occurs as local tissue injury and necrosis from accidental chemotherapy extravasation in surrounding tissues. Nitrogen mustard, doxorubicin, and related anthracyclines were among the first agents reported to cause surrounding soft tissue necrosis and irritation in the 1970s.27–27 In general, when a cytotoxic drug escapes the confines of the cutaneous vasculature and spreads locally to the surrounding tissues, it can cause an inflammatory or destructive reaction, depending on the extravasated drug. Extravasates are categorized as irritant or vesicant reactions (Table 44-3).
Table 44-3
Vesicants and Irritants Causing Extravasation Reactions
| Vesicants | Irritants |
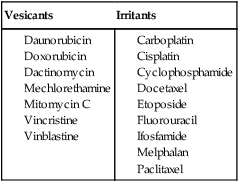
From Langer SW. Extravasation of chemotherapy. Curr Oncol Rep 2010;12(4):242–6.
The type of reaction is based on the agent’s potential for local toxicity. Irritant-type chemotherapy agents are not directly toxic to the tissue, with clinical symptoms thought to be related to the inflammatory response. In contrast, vesicant agents cause direct skin toxicity, leading to more severe topical reactions. Vesicant agents are further divided into DNA-binding and non–DNA-binding categories. DNA-binding agents (e.g., mechlorethamine, doxorubicin, and mitomycin C) bind to DNA in healthy cells, initiating necrosis through cell death. Adjacent cells take up complexes of DNA-bound drug via endocytosis, causing cell necrosis in nearby cells. Non–DNA-binding drugs (e.g., amsacrine, vinblastine, vincristine, and paclitaxel) cause irritation but tend to cause less severe effects.28
Epidemiology
The true incidence of chemotherapy extravasations is unknown. The incidence of intravenous, vesicant chemotherapy extravasation is estimated to be 1% to 6%.1,29,30 The concentration, type, and amount of vesicant, as well as the location of extravasation, can all influence the degree of resultant tissue necrosis.29 Additional risk factors include highly alkaline, acidic, or hypertonic solutions; rigid or indwelling vascular access devices; and patient characteristics, including sclerosed or fragile veins, obesity, an inability to verbalize pain, or an impaired sensory perception.29,30
Clinical Manifestations
After accidental drug extravasation of chemotherapy agents into the surrounding tissue, a wide range of symptoms and manifestations occur. Clinical manifestations can be subdivided into vesicant or irritant extravasation reactions (Figs. 44-1 and 44-2). The clinical manifestation of vesicant drug extravasation can range from local swelling, erythema, burning, or pain to blistering, induration, and progression to skin necrosis. This presentation can also be complicated by deep tissue infection. Symptoms of unintentional extravasation can present immediately after the administration of an agent or therapeutic intervention, or they can develop days to weeks later.28 In contrast, irritant drug extravasation presents with milder symptoms associated with inflammation, including pain, warmth, edema, and erythema. Although irritating, these symptoms typically resolve within weeks and do not have long-term sequelae or necrosis.28
Workup
Extravasation injury should be suspected in any patient with persistent pain, erythema, and swelling, even in the absence of ulceration, and immediate action should be taken. Serial neurovascular examinations and compartment examinations are appropriate to monitor for impending compartment syndrome.28 If the injury is severe or complicated by neurovascular changes, surgical consultation is required.
Treatment
Data regarding the treatment of extravasation injuries is largely limited to case reports and case series. However, several treatment guidelines have been published.29,31–34
Pharmacologic and Surgical Treatment
Prompt recognition and treatment of extravasation reactions is necessary, because delayed management can lead to unnecessary tissue necrosis, scarring, and contracture.28 After recognition of extravasation injury, several steps should be taken. These recommendations are presented in Table 44-4.45 Notably, dexrazoxane, 500 mg, is the first agent approved by the FDA for treatment of chemotherapy extravasation injuries, specifically those associated with anthracyclines. In clinical trials,32,39,40 this medication was shown to definitively decrease the need for surgical intervention.
Table 44-4
Consensus guidelines29
Prospective study38,42,43
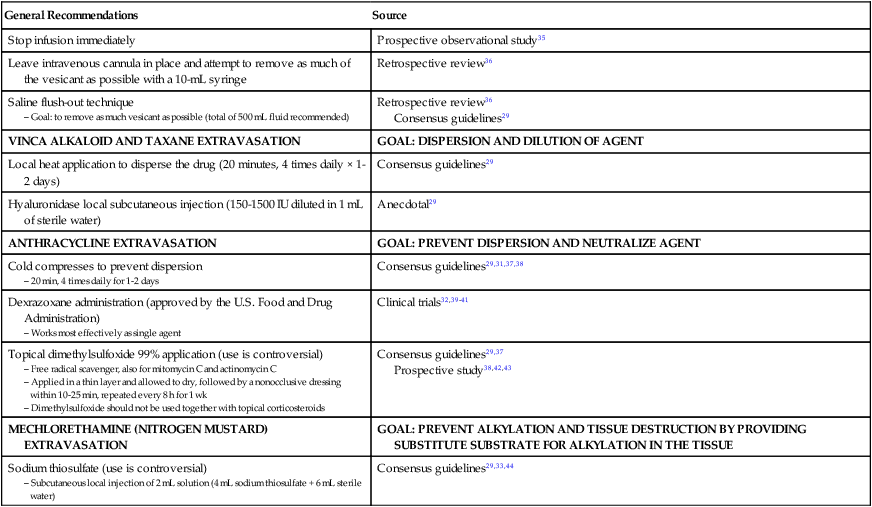
Although routine surgical intervention is not indicated, knowing when to obtain surgical consultation and the timing of surgical intervention when needed are of utmost importance to prevent increases in patient morbidity.46 Severe extravasation injury, blistering, ulceration, and persistent pain may indicate the need for surgical intervention and should prompt surgical consultation.33
Prognosis
The morbidity associated with extravasation injury is high, but mortality is rare.33,34 Intravenous vesicant chemotherapeutic agents can cause severe damage to local tissue, leading to possible tissue necrosis,47 which can result in chronic damage such as scarring. In contrast, irritant drugs cause inflammatory reactions with rare long-term sequelae.30
The degree of discomfort experienced by some patients is severe enough to cause voluntary termination of treatment, and the risk of extravasation injury should be discussed prior to initiation of therapy. The impairment accompanying extravasation injury has no effect on disease status,33,34 and thus there is no reason to stop further treatments with a given agent. Unless extravasation occurs again, retreatment of the patient with the same agent is not associated with recurrence of necrosis.33,34
Chemotherapy-Induced Hyperpigmentation
Etiology and Biocharacteristics
How chemotherapeutic agents act to produce increased melanin deposition is unknown. They may increase pigmentation by a direct stimulatory or toxic effect on melanocytes and/or by slowing the turnover and transit rates of epithelial cells, thus allowing more time for the transfer of melanin to occur.50–50 Other proposed theories for chemotherapy-induced hyperpigmentation have implicated adrenocorticotropic hormone (ACTH) and melanocyte-stimulating hormone. However, no elevations of these hormones were noted in studies evaluating the role of ACTH and melanocyte-stimulating hormone in patients with chemotherapy-induced pigment abnormalities.51,52
Epidemiology
Although it is believed to be fairly common, the rate of pigment alteration in patients treated with cytotoxic chemotherapy agents is unknown. The incidence of hyperpigmentation varies by agent, with certain medications being more commonly associated with pigmentary alterations than others. For example, hyperpigmentation develops in up to 20% of patients treated with bleomycin and in up to 5% of patients treated with 5-fluorouracil (5-FU).53 In addition, chemotherapy-related hyperpigmentation has not been found to correlate with any systemic effects of chemotherapy, with no health threats other than concerns for cosmesis.54,55
Clinical Manifestations
Please see Figure 44-3 and refer to Table 44-5 for specific hyperpigmentation changes related to chemotherapeutic agents.
Table 44-5
Chemotherapeutic Agents Associated with Hyperpigmentation or Discoloration
| Alkylating Agents | Presentation |
| Busulfan |
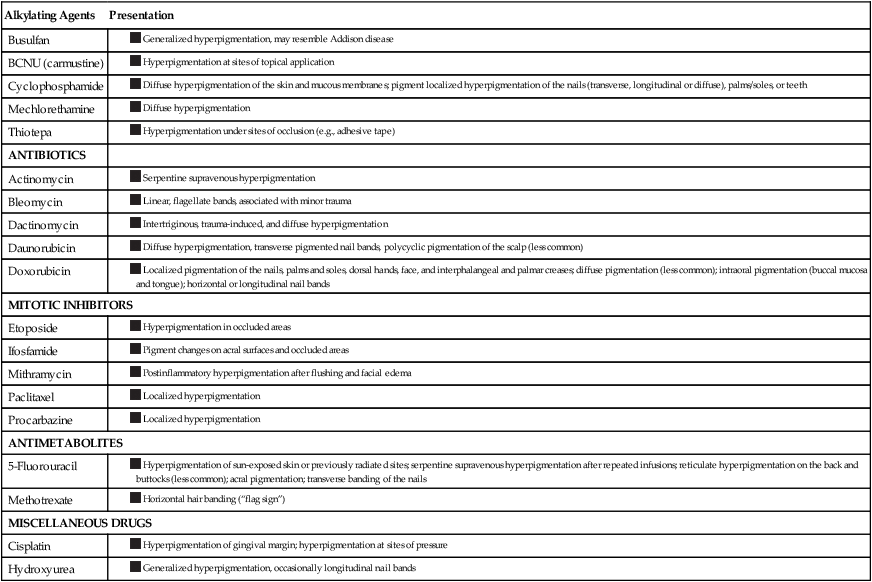
Data from Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol 2011;65(3):624–35 and Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther 2011;24(4):432–42.
Toxic Erythema of Chemotherapy
HFS, NEH, and eccrine squamous syringometaplasia (ESS) are three overlapping cutaneous reactions to cytotoxic chemotherapy agents. Although classically described as three distinct entities, they are all characterized by painful erythema favoring the hands and feet, intertriginous areas, elbows, knees, and ears. In addition, these reactions occur 2 to 3 weeks after administration of chemotherapy, their pathogeneses are presumed to be related to the concentration of cytotoxic agents in the sweat, they are largely self-limited, healing occurs with desquamation and hyperpigmentation, and dose reductions or increased time between cycles is the mainstay for helping to minimize the risk of recurrence.56 Because of their shared characteristics, it has been suggested that these reactions should be reclassified under an umbrella category, termed “toxic erythema of chemotherapy” (TEC).57 HFS, NEH, and ESS are discussed in the following sections as particular patterns of TEC.
Hand-Foot Syndrome
Etiology and Biocharacteristics
HFS is a common, painful, erythematous eruption of the palms and soles associated with cytotoxic chemotherapy agents. In the current literature, this syndrome is referred to as either HFS or palmar-plantar erythrodysesthesia. During the 1980s, HFS was referred to as chemotherapy-induced acral erythema. However, this nonspecific descriptive nomenclature has largely been abandoned.58 Of note, HFS associated with cytotoxic drugs should not be mistaken for hand-foot skin reaction (HFSR), a condition associated with newer, molecularly targeted agents that is described later in this chapter.
Multiple cytotoxic drugs have been reported to cause HFS. Most commonly, these drugs include pyrimidine analogs (e.g., capecitabine, cytarabine, and 5-FU), anthracyclines (pegylated liposomal doxorubicin more than doxorubicin), and taxanes (e.g., docetaxel).59 Less commonly reported causative agents include paclitaxel, hydroxyurea, methotrexate, 6-mercaptopurine, cyclophosphamide, cisplatin, daunorubicin, etoposide, vinorelbine, irinotecan, epirubicin, 6-thioguanine, and tegafur.58
Although the pathogenesis of HFS is unknown, a direct toxic effect of chemotherapeutic agents on the skin is considered the most likely mechanism.60 Factors hypothesized to play a role include the more rapid cell division in palmoplantar skin compared with back skin,61 an increased concentration of capecitabine’s activating enzyme (thymidine phosphorylase) in palmoplantar locations,61,62 an overexpression of cyclooxygenase 2 in the skin as a result of chemotherapy resulting in inflammation,63 and increased drug concentration in the eccrine glands of the palms and soles as a result of drug excretion in sweat.66–66
A role for excretion from sweat glands is supported by the observation that taxanes and liposome-encapsulated doxorubicin can affect areas besides the hands and feet that have an abundance of sweat glands, such as inguinal areas and the axillae. Recent investigations using laser scanning microscopy have shown that liposomal doxorubicin reaches the skin surface via sweat glands and is deposited in the stratum corneum.65,67 However, it is unclear to what degree different cytotoxic drugs share a similar mechanism in causing HFS, and further research is necessary.
Epidemiology
The overall incidence rates for HFS have been reported to vary between 5% and 89%, based on the cytotoxic agent, drug formulation, and administration schedules.59,68 In persons with this condition, symptoms were dose limiting in 30% of patients.69
Risk factors for the development of HFS appear to be related to factors that prolong drug exposure to the cytotoxic agent, including higher doses, greater cumulative doses, drug formulations that increase a drug’s half-life (e.g., liposomal encapsulation of doxorubicin), and increased frequency of administration (e.g., oral daily ingestion of capecitabine, continuous intravenous 5-FU infusions, or a greater number of treatment cycles).58 Although unconfirmed, patient-specific risk factors have been reported to include the occurrence of mucositis, neutropenia, and peripheral neuropathy.70
Clinical Manifestations
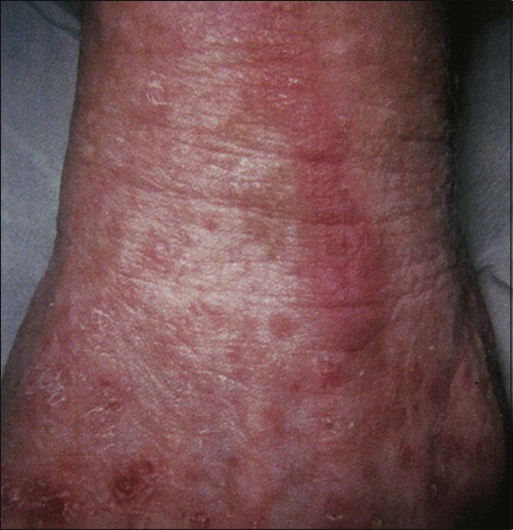
The onset of symptoms is typically within 2 to 3 weeks of drug initiation57 but may range from within a few days to up to 10 months as a result of cumulative exposure.71 Patients initially report a prodrome of tingling sensation or pain in the palms and/or soles. Typically, within a few days, patients progress to the development of painful, symmetric, well-demarcated erythema and edema of palmoplantar skin. The eruption is initially most pronounced over the fat pads of the distal digits, lateral fingers, and the thenar and hypothenar eminences of the palms, but typically generalizes to a diffuse erythema (Fig. 44-4). Skip areas may occur, as can extension to the dorsal surfaces of the extremities. HFS may also rarely involve the penis and scrotum,72 axillae groin, waist, inner side of knees, posterior side of elbows, anterior folding lines of wrists, sacral area, and bra line.71
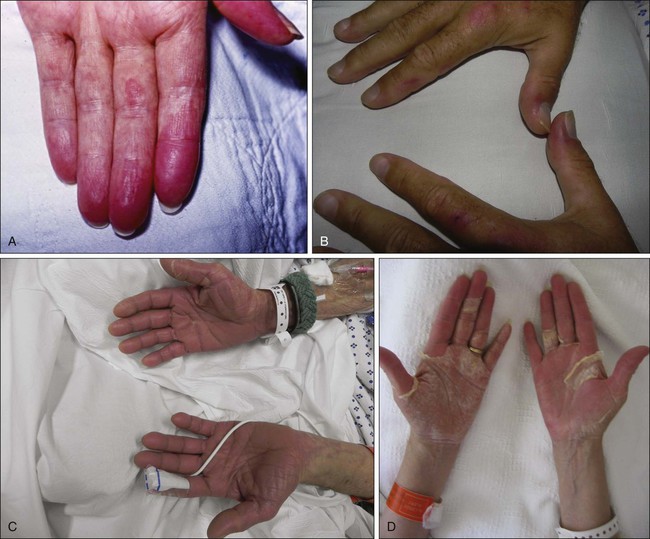
Clinical symptoms can vary by severity, ranging from mild, nontender fine peeling to severe, extremely painful bullous disease with functional impairment. A particularly severe bullous variant that progresses to full-thickness epidermal necrosis and sloughing can occur.68 Nagore et al.68 described the natural history of HFS. The toxicity tends to worsen with continued therapy, with increasing restriction of fine movements of the digits as a result of swelling and pain. The erythema becomes darker or violaceous and diffusely covers the palms and soles. Swelling and severe pain at rest may ultimately occur, with daily activities being limited.
For persons with HFS, clinical manifestations affecting a patient’s daily functional activities may necessitate dose modification.73 The development of the HFS-14, a QoL scale designed specifically to evaluate the impact of HFS, is a promising development that will help standardize research trials.74
Workup
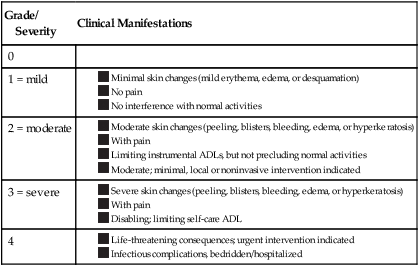
A skin biopsy is not typically indicated because the histologic changes of this toxicity are nonspecific. Treatment of HFS should be guided by severity; however, severity-guided treatment algorithms are lacking the literature. Because HFS is a painful condition and may limit daily activities, functional impairment is a component of the grading system to determine the severity of HFS (Table 44-6).
Table 44-6
Classification/Grading of Hand-Foot Syndrome Severity*
| Grade/ Severity |
Clinical Manifestations |
| 0 | |
| 1 = mild |
Differential Diagnosis
Hand-Foot Skin Reaction
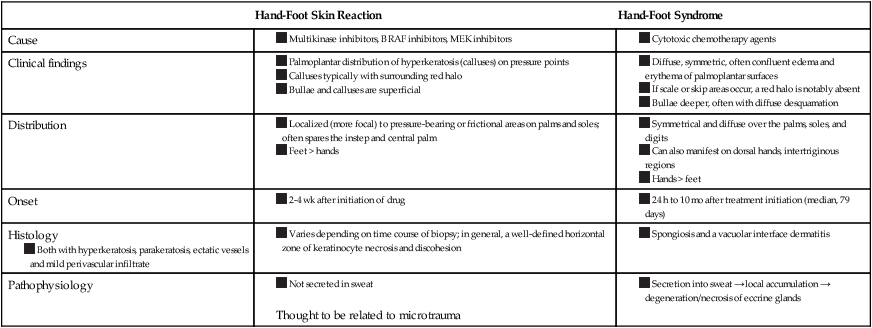
HFSR is a cutaneous toxicity affecting the palms and soles that is associated with molecularly targeted agents, including multikinase inhibitors (MKIs) and BRAF inhibitors (BRAFIs). HFSR differs from HFS in many ways. In contrast to HFSR, HFS affects hands more severely than feet and can affect nonpalmoplantar skin as well; in addition, the eruption is typically more confluent (Table 44-7).60,75
Table 44-7
Comparison of Hand-Foot Skin Reaction and Hand-Foot Syndrome
| Hand-Foot Skin Reaction | Hand-Foot Syndrome | |
| Cause |
Thought to be related to microtrauma
Data from Hoesly FJ, Baker SG, Gunawardane ND, Cotliar JA. Capecitabine-induced hand-foot syndrome complicated by pseudomonal superinfection resulting in bacterial sepsis and death: case report and review of the literature. Arch Dermatol 2011;147(12):1418–23 and Choi JN. Chemotherapy-induced iatrogenic injury of skin: new drugs and new concepts. Clin Dermatol 2011;29(6):587–601.
Acute Graft-Versus-Host Disease
Dermatitis of the palms and soles may occur as the earliest and only sign of acute graft-versus-host disease (GVHD). GVHD is often associated with gastrointestinal abnormalities (including diarrhea, abdominal pain, and elevated serum bilirubin levels). Both diseases can occur concurrently. Unfortunately, acute GVHD can be clinically and histologically indistinguishable from HFS in its early stages. The distinction of acral erythema from GVHD in patients who have undergone a transplant is important because the treatments are distinct.68
Treatment
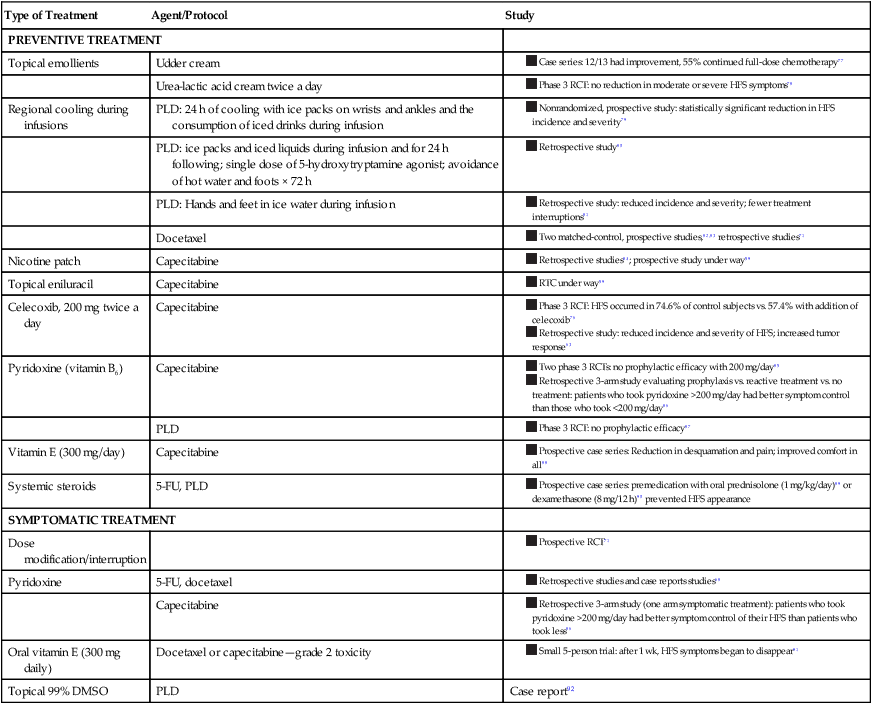
Most treatment recommendations for the management of HFS are based on case reports, case series, and personal experience rather than RCT data. Surprisingly, limited treatment algorithms based on expert consensus exist in the literature. Regarding prevention of HFS, a skin care regimen with emollients and patient education is often described.74 When HFS arises, dose modification or interruption is the goal standard, with symptomatic management for pain or erosions.58,68,71 Recent phase 3 RCT data support the role of prophylactic celecoxib use, which was found to reduce the frequency and severity of HFS, likely because of its antiinflammatory effects.76 Table 44-8 provides a summary of reported treatments in the literature.76–92
Table 44-8
Reported Hand-Foot Syndrome Treatments in the Literature
| Type of Treatment | Agent/Protocol | Study |
| PREVENTIVE TREATMENT | ||
| Topical emollients | Udder cream | |
| Urea-lactic acid cream twice a day | ||
| Regional cooling during infusions | PLD: 24 h of cooling with ice packs on wrists and ankles and the consumption of iced drinks during infusion | |
| PLD: ice packs and iced liquids during infusion and for 24 h following; single dose of 5-hydroxytryptamine agonist; avoidance of hot water and foots × 72 h | ||
| PLD: Hands and feet in ice water during infusion | ||
| Docetaxel |
|
|
| Nicotine patch | Capecitabine |
|
| Topical eniluracil | Capecitabine | |
| Celecoxib, 200 mg twice a day | Capecitabine | |
| Pyridoxine (vitamin B6) | Capecitabine | |
| PLD | ||
| Vitamin E (300 mg/day) | Capecitabine | |
| Systemic steroids | 5-FU, PLD |
|
| SYMPTOMATIC TREATMENT | ||
| Dose modification/interruption | ||
| Pyridoxine | 5-FU, docetaxel | |
| Capecitabine | ||
| Oral vitamin E (300 mg daily) | Docetaxel or capecitabine—grade 2 toxicity | |
| Topical 99% DMSO | PLD | Case report92 |
Prevention
In general, wound care to prevent infection, elevation to reduce edema, and symptomatic treatment of pain are recommended.68 Routine application of topical emollients (e.g., lanolin/petrolatum-based ointments or udder cream) is also recommended.77
Four phase 3 RCTs have been performed to evaluate preventative treatment agents. Only one RCT, which evaluated the efficacy of celecoxib, 200 mg twice daily, demonstrated prophylactic efficacy in persons with HFS, reducing the incidence of HFS from 74.6% in control subjects to 57.4% with the addition of celecoxib.76 In contrast, two RCTs demonstrated no efficacy of pyridoxine, 200 mg/day, in patients treated with pegylated liposomal doxorubicin or capecitabine compared with control subjects.79,80 However, a three-arm retrospective study evaluating prophylactic versus symptomatic pyridoxine compared with control subjects demonstrated that patients who took >200 mg of pyridoxine a day had better symptom control of their HFS than did patients who took a smaller dose.81 These findings suggest that the dose of pyridoxine in the RCTs was insufficient to obtain an effect. Finally, an RCT evaluating the prophylactic efficacy of twice-daily urea-lactic acid cream in patients treated with capecitabine was found to be ineffective as well, suggesting that emollients without these exfoliative properties may be preferable.78
Preventative treatments suggested to be helpful, but that have not been confirmed, include the routine use of topical moisturizers, regional cooling during infusions, nicotine patches, oral vitamin E, and systemic steroids (Table 44-8).
Prognosis
HFS is considered a self-limited reaction after drug discontinuation. If the drug is stopped within a few days of when the reactions appear, a gradual clearing of symptoms will occur over a period of 2 weeks (range, 1 to 5 weeks).67,70 Areas of pallor with blisters develop and eventually desquamate with extensive but superficial exfoliation and healing with hyperpigmentation. Although dose reduction or interruption of treatment after the development of HFS is most common, continued treatment in this scenario has been reported, in which palmoplantar thickening developed (i.e., keratoderma).68
Although HFS is considered self-limited, long-term sequelae have been reported in rare instances. For example, despite cessation of chemotherapy, a persistence of abnormal sensation and an abnormal appearance of the affected digits has been reported.93 Chronic HFS in patients treated with long-term capecitabine therapy has been reported, in which loss of fingerprints impeded international travel.94
Furthermore, although HFS is not typically considered a dangerous condition in itself, the literature includes a few reports of HFS leading to tissue necrosis requiring amputation and one report of death due to pseudomonal superinfection and sepsis.60
Neutrophilic Eccrine Hidradenitis
Etiology and Biocharacteristics
NEH was first described in association with cytarabine given in the setting of acute myelogenous leukemia (AML). The most frequent reported chemotherapy causes of NEH include cytarabine and daunorubicin.95,96 As with HFS and ESS, the pathogenesis is thought to be via eccrine secretion and concentration of chemotherapeutic agents, a supposition that is supported by the association of NEH with chemotherapy.97 However, it has been suggested that NEH may instead represent a neutrophilic dermatosis akin to Sweet syndrome. This suggestion is supported by case reports of NEH being induced with granulocyte colony-stimulating factor (G-CSF), appearing in patients with AML prior to chemotherapy induction, as well as being a prodrome of AML relapse.97–100 Therefore NEH is considered a reactive disorder that may occur in association with malignancy (with or without chemotherapy), infections (human immunodeficiency virus and others), and certain nonchemotherapeutic agents (e.g., acetaminophen, zidovudine, amitriptyline, cloxacillin, gentamicin, fluconazole, methadone, lorazepam, G-CSF, and barbiturates). A localized idiopathic variant in children, called palmoplantar eccrine hidradenitis, has also been reported.101,102
Epidemiology
The incidence of chemotherapy-induced NEH is unclear. The chemotherapy drugs that are most frequently reported to cause NEH are cytarabine and daunorubicin. Other chemotherapeutic agents reported include doxorubicin, vincristine, dacarbazine, bleomycin, cis-platinum, methotrexate, 6-thioguanine, dactinomycin, cyclophosphamide, mitoxantrone, daunoblastina, chlorambucil, 5-FU, VP-16, etoposide, and idarubicin.97,102,103
Clinical Manifestations
Lesions may appear within 2 to 3 days of starting chemotherapy or may go unnoticed until weeks after chemotherapy has started. Classically the eruption begins within 2 to 3 weeks of the initiation of therapy. Affected patients present with a wide variety of cutaneous lesions, and hence the clinical picture is nonspecific (Fig. 44-5). Tender erythematous to purpuric macules, papules, nodules, or plaques can appear on any area during active administration of a cytotoxic agent. Lesions may or may not have central necrosis. The eruption most commonly affects the trunk and extremities but may involve the palms, soles, and even the face. Some unusual presentations include periorbital edema with erythema and injection site reactions. Usual manifestations include hyperpigmented plaques and painful edema of the ears. Fevers may accompany the onset of cutaneous lesions.104
Treatment
Although some studies support the use of systemic corticosteroids to treat NEH, the course or the disease does not appear to be influenced by chemotherapy protocols that often include high dosages of systemic corticosteroids. There are a few case reports of recurrent lesions that have been suppressed with concurrent dapsone administration.106,107