Colorectal Cancer
Sandra Van Schaeybroeck, Mark Lawler, Brian Johnston, Manuel Salto-Tellez, Jack Lee, Paula Loughlin, Richard Wilson and Patrick G. Johnston
• Colorectal cancer (CRC) is the second most common cancer in women and the third most common cancer in men worldwide.
• Within economically developed countries, the lifetime risk of developing CRC is 1 in 20.
• Because of increased screening rates, the incidence of CRC is declining for men and women in the United States.
• The incidence is 15 times higher in adults older than age 50 years, compared with those younger than age 50 years.
• Inherited genetic syndromes (hereditary nonpolyposis colorectal cancer [HNPCC] and familial adenomatous polyposis [FAP]: fewer than 10% of cases) and inflammatory bowel disease (IBD) in concert with dietary and environmental exposures increases risk for CRC.
• The 5-year overall survival rate has greatly improved in the last 2 decades, and is now approximately 65%, with variations across racial and ethnic subgroups.
• Mortality is 35% to 40% higher in men than in women.
Screening and Prevention of CRC
• High level of physical activity decreases the risk of CRC by up to 50%.
• Diets high in fiber and low in red, processed meat may alter risk of CRC.
• Calcium/vitamin D supplementation might have preventive effects.
• Aspirin and cyclooxygenase (COX)-2 inhibitors may prevent polyps and CRC, but are only recommended in high-risk patients.
• Premenopausal hormone replacement therapy reduces the incidence of CRC, but increases the risk of breast cancer and cardiovascular complications.
• There are mixed results in studies of the effects of statins on CRC risk.
• Colonoscopy is the mainstay of screening and a useful tool in the diagnosis of CRC. Sigmoidoscopy may reduce CRC incidence and mortality.
• Fecal occult blood is an acceptable screening tool.
• Virtual colonoscopy, the detection of abnormal DNA within stool sample and capsule endoscopy are potentially new screening tests.
• Screening is based on risk categories that take into account age; race; personal history of IBD, polyps, or cancer; family history of CRC; and presence of hereditary syndromes.
• CRC is often insidious in development, underscoring the importance of screening.
• Altered bowel habits, blood per anum, fatigue, anemia, and weight loss are frequent symptoms.
• Obstruction is the most common acute surgical problem (approximately 30% of left-sided lesions present with an obstruction).
• Approximately 5% of CRC patients will be diagnosed with synchronous cancer. The liver is the most common site for synchronous metastasis.
• Approximately 20% to 40% will have synchronous polyps with cancer primary.
• Computed tomography (CT) scan, magnetic resonance imaging (MRI), and positron emission tomography (PET) are imaging tools used in the staging of CRC.
• Intraoperative ultrasound is the most sensitive method to evaluate liver for metastases.
• Tumor size is not as critical as depth of invasion and nodal status in determining prognosis.
• High histologic grade, lymphatic invasion, venous invasion, and involvement of surgical resection margins are independent adverse prognostic factors.
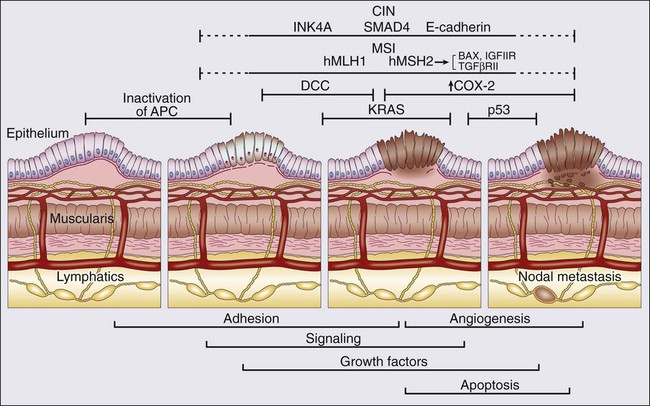
• The “Vogelgram” highlights the involvement of specific oncogenes and tumor suppressor genes in the colorectal adenoma to carcinoma transition, and involves APC, KRAS, TP53, and DCC.
• The APC tumor suppressor gene is defective in more than 80% of colon adenomatous polyps and cancers. KRAS, TP53, and BRAF are mutated in 40% to 50%, approximately 50%, and 8% of CRC, respectively.
• Deletions of 18q21 (location for DCC, SMAD2 and SMAD 4) also play a role in CRC carcinogenesis.
• Chromosomal instability (CIN) and microsatellite instability (MSI) pathways are closely associated to this adenoma-to-carcinoma model, and involve genes in the Wnt signaling and DNA mismatch repair pathways, respectively.
• Defective DNA mismatch repair is responsible for 15% to 20% of CRC; hereditary syndromes (e.g., HNPCC); in 3% to 5% of cases, MSI positivity correlates with the inherited colon cancer HNPCC or Lynch syndrome.
• Integrating genomics and transcriptomics into our standard of care remains a challenge, but is a critical component of a framework to establish a comprehensive personalized medicine strategy for the cancer patient.
• The use of an enhanced recovery program (ERP) is important in the management of patients undergoing elective colonic resections.
• Treatment may consist of en bloc resection of the anatomically defined portions of the colon with in-continuity draining nodes to the root of the mesocolon.
• It is now recommended that, if available, a laparoscopic resection should be offered as an alternative to an open resection where both are available.
• The absence of mechanical bowel preparation is not associated with a greater risk of perioperative morbidity or mortality. Curtailed carbohydrate loading, deep vein thrombosis prophylaxis, and a single dose of prophylactic antibiotics are recommended before surgery.
• Experienced surgeons and the use of high-volume hospitals improve the chance of good surgical outcome.
• The role of surgical resection in management of CRC metastases has led to improved outcomes for patients.
• Eighty percent to 90% of recurrences are detected in the first 3 years following curative surgery. Fewer than 5% of recurrences occur after 5 years.
• Many of these recurrences are solitary or limited, and when resected completely, can still cure at least 35% of patients.
• A high percentage of first recurrence occurs in the liver and lung, are asymptomatic, and can be detected from a rising carcinoembryonic antigen (CEA) or CT imaging.
• Of those patients who are closely followed for evidence of recurrence, 20% are candidates for salvage surgery. These patients have a 5-year disease-free survival rate of 18.6% compared with only 5.6% when metastatic disease is diagnosed because patients became symptomatic.
 Physician exam with CEA testing every 3 to 6 months for the first 2 years, then every 6 months until 5 years.
Physician exam with CEA testing every 3 to 6 months for the first 2 years, then every 6 months until 5 years.
 Full colonoscopy preoperatively or postoperatively, at 1 and 3 years postoperatively, and then every 3 years.
Full colonoscopy preoperatively or postoperatively, at 1 and 3 years postoperatively, and then every 3 years.
 CT scan of the thorax/abdomen/pelvis preoperatively or postoperatively, and then annually for 3 to 5 years.
CT scan of the thorax/abdomen/pelvis preoperatively or postoperatively, and then annually for 3 to 5 years.
Adjuvant Therapy in Early Stage Colorectal Cancer
• Fifty percent to 60% of patients who undergo successful surgery for CRC have residual micrometastatic disease.
• The goal of adjuvant therapy is to eradicate residual local disease. Recommended length for therapy is 6 months.
• Combined 5-fluorouracil (5-FU) plus leucovorin regimens with oxaliplatin have further improved overall survival in patients with stage III CRC.
• Adjuvant 5-FU regimens are also used in high-risk stage II CRC patients. MSI is now considered as a robust prognostic marker, in particular in stage II CRC MSI-H patients, who have a favorable clinical outcome and do not seem to benefit from adjuvant 5-FU-based chemotherapy.
• In contrast to metastatic CRC, all clinical trials investigating a potential role of epidermal growth factor receptor (EGFR)- and vascular endothelial growth factor (VEGF)-targeted therapies have failed in the adjuvant setting.
• Adjuvant radiation therapy has no standard role but may be considered (in conjunction with chemotherapy) in selected cases in which resection of T4 lesions leaves potentially positive circumferential resection margins.
Management of Metastatic Disease
• Patients with metastatic disease, in particular liver metastases, should be evaluated to determine if curative resection with or without perioperative chemotherapy treatment is possible.
• Infusional 5-FU plus leucovorin with irinotecan and EGFR monoclonal antibody (mAB) cetuximab has improved overall survival to 24.9 months in patients with chemo-naive KRAS wild-type CRC tumors.
• Infusional 5-FU plus leucovorin with irinotecan or oxaliplatin in combination with the VEGF mAB bevacizumab has increased median overall survival to more than 25 months.
• The multikinase inhibitor regorafenib has shown to increase survival in patients who have been previously treated with 5-FU, oxaliplatin, irinotecan, anti-VEGF, and anti-EGFR agents.
• KRAS is the first predictive biomarker approved for metastatic CRC. Novel adaptive clinical trial design, incorporating putative prognostic/predictive markers in prospective randomized phase II or III CRC studies, will enable a clinical validation of these biomarkers and facilitate their incorporation into routine medical practice.
Epidemiology of Colorectal Cancer
Incidence
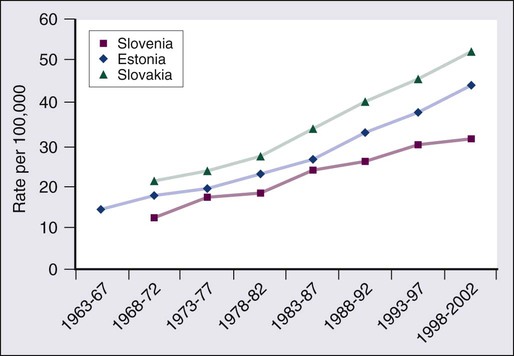
CRC is the second most common cancer in women and the third most common cancer in men worldwide, resulting in more than 1 million cases each year. It is expected that approximately 143,460 new cases of CRC will be diagnosed within the United States and 450,000 in Europe in 2012. In addition, with approximately 51,690 estimated deaths in 2012 in the United States and 232,000 deaths in Europe, CRC remains the second most common cause of cancer death within the Western world.1,2 Countries and regions with a western lifestyle, for example, North America, Europe, Australia, and New Zealand, have traditionally had the highest rates of CRC,3 whereas lowest rates are found in Africa and Asia.3 Within economically developed nations, the lifetime risk of developing CRC is 1/20.2 In economically transitioning countries such as in Eastern Europe, CRC incidence is increasing substantially (Fig. 77-1).4 This increase appears to reflect a change in physical activity and diet. By contrast, the United States is the only country where the incidence is declining for both males and females (Fig. 77-2). The overall incidence of CRC declined by 22% between 1975 and 2000.5 This decline has been attributed largely to increasing screening rates, which expanded from 27% to 46% of the eligible population between 1987 and 2003.6 Celebrity profile and media attention also helped increase the uptake of screening among the general population.7 In 2012, the American Cancer Society released new guidelines in relation to early detection and screening for CRC.8
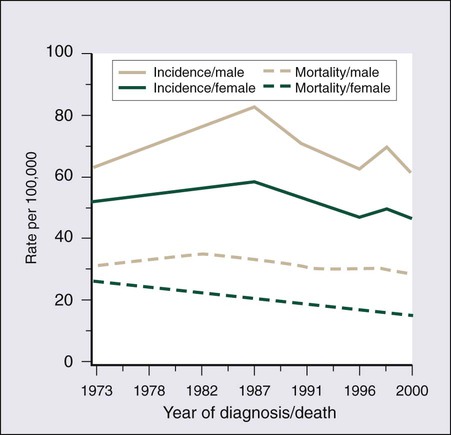
The incidence of CRC is relatively rare in the under 40 years of age group and increases rapidly in older cohorts. The incidence is 15 times greater in adults older than age 50 years compared with those younger than age 50 years.9 CRC is 25% more likely to develop in men than in women, and the rate is 20% higher in African Americans compared with whites.10 The burden of CRC is likely to increase with increasing life expectancy in both males and females.
Mortality
Five-year survival in CRC in the United States is 65%,2 with a 26% decline in CRC-related mortality between 1975 and 2000.5 This reflects the identification and removal of (a) precancerous polyps at screening colonoscopy; (b) CRC at an earlier stage during screening (50% of reduction in mortality); (c) improved risk factor profile (33% reduction in mortality); and (d) improvements in the treatment of CRC (12% reduction in mortality). Mortality is 35% to 40% higher in men than in women. This higher rate is likely to be caused by gender-related hormone differences and gender-related risk factors. In the United Kingdom, 5-year survival rates approach 55%.11 Comparison of CRC survival rates across Europe shows significant intercountry differences.12
Inherited Colon Cancer Syndromes
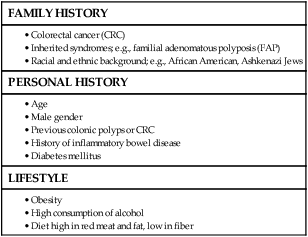
As many as 20% of patients with CRC are likely to have a close relative with the disease and, as such, can be considered as familial in nature.13,14 Indeed, it is likely that 5% to 10% of all CRCs are syndromic. Molecular genetic testing leading to subsequent clinical management recommendations currently exists for patients with a variety of CRC syndromes, including hereditary nonpolyposis colorectal cancer (HNPCC), familial adenomatous polyposis (FAP), MYH-associated polyposis, and the hamartomatous polyposis syndromes (primarily Peutz-Jeghers syndrome, juvenile polyposis, and Cowden disease).17–17 In addition to CRC, these syndromes are accompanied by other extracolonic manifestations, some of which represent extracolonic neoplasias. The risk of CRC in an individual with a first-degree relative with CRC is increased threefold compared with those with no family history. High-risk individuals should undergo genetic counseling, with the potential for genetic testing and a program of screening by colonoscopy.
Hereditary Nonpolyposis Colon Cancer
Approximately 3% to 5% of CRC can be linked to HNPCC (Lynch syndrome).13 The genetic basis is a germline mutation in one of several genes involved in the mismatch repair system, most commonly hMSH2 or hMLH1, but also hPMS1, PMS2, or hMSH6. HNPCC has an autosomal dominant mode of inheritance, with less than 50% penetrance.14 Certain criteria are used to identify high-risk individuals and these are often based on the Amsterdam criteria18 and the revised Bethesda guidelines19 (Table 77-2). Lifetime risk of HNPCC is approximately 66% for men and 43% for women, with the cancer developing 25 to 30 years sooner than the median age of the general population.20 HNPCC is also associated with other cancers, in particular, of the endometrium, ovaries, and stomach. Surveillance by colonoscopy should be started by age 20 to 25 years in individuals who are mutation-proven or at risk, usually at annual intervals. In addition, mutation-proven women should have yearly screening for endometrial cancer, and prophylactic hysterectomy and salpingo-oophorectomy when childbearing is completed should be considered. In view of the strength of the clinical and molecular diagnostic techniques available, as well as a variable penetrance, it has been suggested that testing for HNPCC should be considered in all patients with CRC, regardless of their family history.
Table 77-2
• Colorectal cancer in a patient younger than age 50 years of age
• A second synchronous or metachronous colorectal cancer or cancer associated with HNPCC*
• Presence of high-level microsatellite instability histologically in a patient younger than 60 years of age
• One or more first-degree relative with either colorectal cancer or HNPCC-associated tumor diagnosed when younger than 50 years of age
• Colorectal cancer in two or more first- or second-degree relatives with HNPCC-related tumors at any age
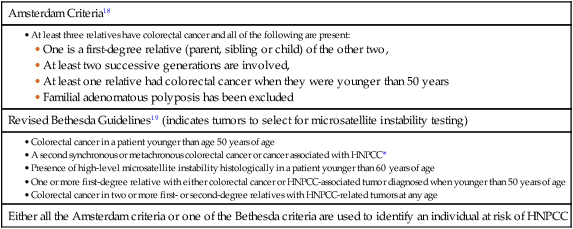
HNPCC, Hereditary nonpolyposis colorectal cancer.
*HNPCC-associated tumors include endometrial, gastric, ovarian, pancreatic, ureter and renal pelvis, biliary tract, small bowel carcinoma, brain
Familial Adenomatous Polyposis
FAP is a rare autosomal dominant disorder that accounts for 1% to 2% of all CRC and is characterized by the presence of hundreds to thousands of adenomas in the colorectal mucosa by 20 to 30 years of age (Fig. 77-3).21,22 An attenuated form of FAP (AFAP) is recognized, with fewer polyps (on the order of 100 or less), and both the presentation and the development of carcinoma are delayed.23
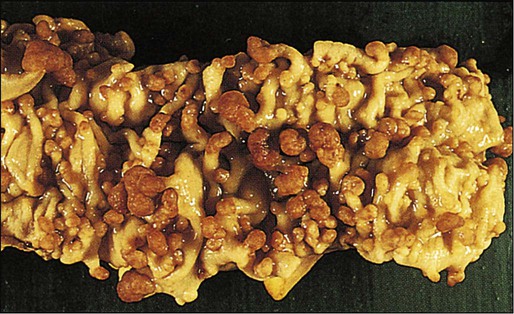
In general, FAP is the result of an inherited defect in one allele of the APC tumor suppressor gene; however, up to 20% of cases could represent de novo mutations without an apparent family history. When the second allele is inactivated through mutation early in childhood, the colonic epithelium begins a process of uncontrolled hyperplasia and polyposis. The lifetime risk of CRC is nearly 100%, with 90% of cases occurring by 45 years of age20; lifetime risk appears to be correlated with the number of adenomas.24 In addition to the risk of CRC, other phenotypic manifestations of the disorder can include tumors of the central nervous system (also known as Turcot syndrome),25 epidermal cysts, osteomas, dental abnormalities, mesenteric or abdominal wall desmoid tumors (also known as Gardner syndrome),26 congenital hypertrophy of the retinal pigmented epithelium, and upper gastrointestinal tumors, especially periampullary duodenal carcinoma. Several of these phenotypic features have been correlated with specific mutation sites.27
MYH-Associated Polyposis
This is a polyposis syndrome characterized by mutation in the MYH gene, which is involved in the DNA proofreading machinery of the genome; its failure allows for the accumulation of further mutations, particularly in the APC gene.30–30 The polyposis of this syndrome tends to be less pronounced than FAP.
Other Genetic Factors
In addition to high-penetrance genes such as those described previously, a variety of low-penetrance polymorphic genes have been investigated for their potential to influence CRC risk, particularly when considered in the context of concomitant dietary or environmental exposures.32 Specifically, polymorphisms associated with an increased risk of CRC included the NAT2 fast phenotype (70% increased risk); the GSTT1 null genotype (40% increased risk); a number of mutant alleles of ALDH2; any of four rare variants of the protooncogene HRAS1; and the a2, a5, and a13 alleles of TNFα. More recent studies using whole-genome scanning approaches have also identified single-nucleotide polymorphisms locus associated with an increased risk of CRC at the 8q24 locus.33,34
Familial clustering of CRC is a common occurrence, even in those without a definable genetic predisposition. For example, twin studies suggest that heritable factors are important in CRC development, even in the absence of known genetic predispositions.35 Two recent meta-analyses of observational studies demonstrated an approximate relative risk of 2.25 in individuals with a single first-degree relative affected by CRC and a 4 to 4.25 relative risk in those with more than one affected relative or with a family member who developed CRC before 45 years of age.36,37 Population lifetime CRC risk estimates for a 50-year-old increased from 1.8% to 3.4% with one affected relative and to 6.9% with two or more affected relatives. Risks were also elevated approximately twofold for individuals with first-degree relatives who harbored colorectal adenomas, further supporting the thesis that colorectal adenomas and carcinomas are linked.
Family History of Colorectal Cancer or Adenomatous Polyps
Screening is recommended for individuals with a first-degree relative who developed CRC before the age of 50 years. Screening should commence at the age of 40 years or 10 years earlier than the earliest cancer.8,38,39 There is newer evidence that having a first-degree relative develop an advanced polyp (>1 cm or villous histology) before the age of 50 years also increases the risk of developing CRC.40 This has led some organizations (including the American College of Gastroenterology) to include it as grounds for screening of targeted individuals.8,41
Prior Polyps; Inflammatory Bowel Disease
The risk of CRC in individuals with a previously diagnosed adenomatous polyp is particularly increased if the polyps are large (>1 cm) or multiple (>2) or advanced (high-grade dysplasia or villous histology) and requires additional follow-up.42,43 With pancolonic inflammatory bowel disease (ulcerative colitis or Crohn disease), the risk of CRC increases with the duration of illness (2% at 10 years, 18% at 30 years).44–47 The extent of the involved colon is an important determinant of risk and no increased risk is seen when it is confined to the rectum.45 Screening is recommended to begin 10 years after the diagnosis is made and the frequency to be increased in subsequent decades or according to the degree of ongoing inflammation.
Diabetes Mellitus; Obesity
There is an association between risk of developing diabetes and CRC. This is partly explained by the shared risk factors of physical inactivity and obesity (see below), but there would appear to be an additional independent risk factor.48
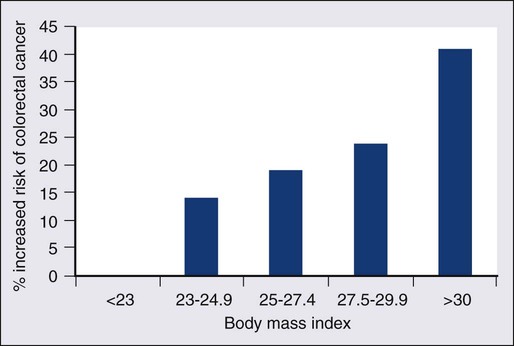
Obesity, particularly abdominal obesity, is a risk factor for CRC, independent of physical activity.49 The association is stronger in men than in women. Men in the highest quintile (body mass index [BMI] >30) have double the risk compared with those in the lowest quintile (BMI <23).50,51 The relationship is linear (Fig. 77-4).52 The mechanism appears to be related to insulin resistance and hyperinsulinism.
Smoking
An association with CRC has been demonstrated, with a relative risk of 1.26 (1.11 to 1.43) compared with nonsmokers, with the risk persisting up to 25 years after stopping smoking.54 It is estimated that 15% to 20% of CRC can be attributed to smoking,55 and the link is strongest for rectal cancer.56
Screening and Prevention of Colorectal Cancer
Genetic Factors
Genetic studies have revealed reductions in CRC risk for polymorphisms in a number of genes, including homozygous carriers of the C677T variant allele of methylenetetrahydrofolate reductase (20% to 30% reduced risk) and heterozygous or homozygous carriers of an intron 3 polymorphism of the p53 gene.32
Contribution of Physical Activity
There is a largely consistent database to support the protective effect of physical activity. High levels of activity decrease the risk of CRC by up to 50%.57,58 This is equivalent for men and women.51,57 Even moderate physical activity (3 to 4 hours brisk walking/week) has been shown to be beneficial.58,59
Contribution of Diet
The Role of Dietary Fiber
Many case-control studies have shown that the high intake of dietary fiber reduces the risk of CRC. Two meta-analyses suggest a reduction of 50% in CRC risk.60,61 By contrast, prospective cohort studies have failed to show this association.64–64 Meta-analysis of these prospective cohort studies with more than 700,000 participants did indicate a modest benefit of increased fiber intake, but this association ceased to be significant when other dietary factors were taken into consideration.65
In contrast to the United States cohort studies outlined above, a European study (European Prospective Investigation into Cancer and Nutrition [EPIC]) of more than half a million participants, revealed a 40% reduction in the risk of CRC among those with the highest fiber intake.66,67 The difference between this European EPIC study and those from the United States could relate to the presence of folate fortification of food in the United States, reducing the absolute benefit of increased fiber. It is also possible that the type of fiber may be important, with unprocessed wheat bran being more effective than the processed forms.67
Resistant starches that are fermented into short-chain fatty acids in the colon, could also reduce the risk of CRC. Some studies have shown such an inverse association,68 but a randomized trial of supplementing resistant starch in European patients with HNPCC failed to note any reduction in neoplasia risk after 4 years of follow-up.69
Decreased Red Meat Consumption
There is a significant body of evidence supporting the relationship between red meat consumption and CRC. This is particularly true of meats that have been cooked at high temperatures for prolonged duration.57 One study demonstrated that the risk of CRC developing in a 50-year-old individual over a 10-year period was 1.7% in the group consuming the most red meat and processed meat compared with 1.3% in those in the lowest category.64,70 Both stimulation of endogenous insulin and consumption of iron or other carcinogenic factors have been suggested as the pathogenic mechanism.
Prevention Strategies
Calcium and Vitamin D
Several studies have demonstrated a protective role for calcium and vitamin D in risk of CRC. Pooled analyses for calcium studies revealed a 22% reduction in CRC risk in the highest quintile of calcium intake compared with the lowest quintile.71 Calcium supplementation significantly reduced adenoma recurrence rates.72 Although not proven to protect against CRC, calcium supplementation has been recommended by some national organizations. For vitamin D, a meta-analysis of prospective studies indicated a 50% reduction in CRC incidence, comparing those with the highest and lowest serum vitamin D levels.73
Folate Supplementation
Although there is evidence that folate plays a protective role, trials of folate supplementation have not shown a benefit in adenoma prevention.74 This may be because of folate supplementation programs where it is added to food. One study found an increased risk of multiple adenomas with additional folate supplementation.75 Additional folate supplementation is not recommended for CRC prevention.
Nonsteroidal Antiinflammatory Drugs, Hormone Replacement Therapy, and Statins
Currently, no medications are recommended for the general population for the prevention of CRC. Cardiovascular protection trials with aspirin have demonstrated an additional benefit of reducing CRC by 50%.77,78 This effect occurred after a lag period of 5 years and was true for high-dose and low-dose aspirin. In the CAPP2 trial, aspirin demonstrated a protective effect in patients with Lynch syndrome, with a CRC incidence ratio of 0.6 (0.32 to 0.99) for those in the aspirin arm of the trial.69,79, The cyclooxygenase (COX)-2 inhibitor celecoxib and the nonsteroidal antiinflammatory drug (NSAID) sulindac have also demonstrated benefit in adenoma regression in FAP.80,81 However, complications can occur, including massive gastrointestinal bleeding or gastric ulceration.
The United States Preventive Services Task Force (USPSTF) recommendations of 2007 are still valid: For average-risk individuals, the harm associated with aspirin or NSAIDs outweighs the benefit in CRC risk reduction.82 A more recent international consensus suggested that aspirin may have a role in high-risk populations.83
Similar to aspirin, statin trials for cardiovascular risk reduction have demonstrated a reduced risk of CRC.84,85 However, other case control studies have not confirmed this finding.86,87 Postmenopausal hormone replacement therapy has also been demonstrated to reduce CRC risk88; however, it is not recommended in this setting because of the associated increased breast cancer and cardiovascular disease risk.89
Screening for Colorectal Cancer
Three key issues for any screening program in cancer are (a) a recognizable predisease state, (b) a simple, effective, and safe test, and (c) evidence that intervention improves survival. All three of these criteria are met for CRC, which progresses from adenoma to carcinoma over a 10- to 15-year period, allowing intervention to occur either before the cancer develops or following the detection of an early, more easily treatable cancer.90
Recommendations regarding frequency of screening for high-risk individuals are contained within guidelines from national and international societies.8,38,39,91 Surveillance for these high-risk patients is via colonoscopy. The rest of this section addresses the average-risk individual and assesses the variety of screening approaches available.
Screening Options
The three most common options for screening the average risk individual are (a) annual fecal occult blood (FOB) testing with high sensitivity tests, (b) sigmoidoscopy every 5 years (± annual FOB testing), and (c) colonoscopy every 10 years. Some national organizations have suggested a preferred screening tool, for example, the American College of Gastroenterology recommends colonoscopy.41 Others have endorsed a wider range of options, including CT colonography and fecal DNA testing.39,91 Programs vary in when they recommend commencing screening (age 50, 55, or 60 years, based on a combination of risk benefit and economic criteria). Most programs suggest stopping at 75 years of age or when life expectancy is less than 10 years.
Screening Tests
Fecal Occult Blood Testing
To date, the most common test has been the guaiac FOB test (gFOBt). This test is not specific for human hemoglobin, making dietary restrictions required before performing the test. The test itself has limited sensitivity when used on a one-off basis (50% for CRC). However, this increases to 90% if used repeatedly over a prolonged period.41,92 The reading and interpretation of the results are important determinants of sensitivity and specificity. The lower the threshold that labels a test as positive, the greater the number of screening colonoscopies that are required.
The first randomized controlled trial of CRC screening by gFOBt was published in 1993 and demonstrated a 33% reduction in mortality among patients undertaking annual gFOBt screening over a 13-year period.93 Two subsequent randomized studies revealed similar effects. A ten-year follow-up of alternate-year gFOBt from Denmark found an 18% reduction in CRC mortality.94 A United Kingdom study, similarly performed over 10 years with testing every 2 years, revealed a 15% reduction in CRC mortality.95 A study looking at “missed” cancers that present symptomatically between the biannual FOBt demonstrated that they accounted for between 30% to 60% of the cancers and that they were more likely to occur in women and on the right side.96 That study recommended adding a one-off flexible sigmoidoscopy as an adjunct to FOB testing.
Fecal Immunohistochemistry Test (FIT)
Limitations of the gFOBt led to the development of a test based on immunochemistry (iFOBt, fecal immunohistochemistry test [FIT]). A randomized comparison between the two demonstrated a positivity rate of 5.5% for iFOBt that was more than double the 2.4% of gFOBt.97 This has resulted in significantly more colonoscopies and in the detection of increased numbers of cancers and advanced adenomas.
iFOBt is a semiquantitative test, therefore the cutoff value can be shifted, depending on the availability of colonoscopy resources and the risk of CRC in any given population. Subject to costs, it is now the recommended methodology for fecal sampling.98
Flexible Sigmoidoscopy
Patients with significant pathology at flexible sigmoidoscopy including high-risk adenomas (multiple, >1 cm or villous histology) proceed to full colonoscopy. There is uncertainty over the management of smaller tubular adenoma (6 to 10 mm). Most clinicians proceed to colonoscopy in these cases.41
Once-only flexible sigmoidoscopy between the ages of 55 and 64 years has been proposed as a strategy to reduce CRC incidence and mortality.99 In a randomized controlled trial, in the United Kingdom with more than 50,000 people in the intervention group, CRC incidence was reduced by 23% and mortality by 31%.100 This was after a follow-up of more than 11 years. An Italian study found an 18% reduction in incidence and a nonsignificant 22% reduction in mortality in a similar study.101
These findings have been replicated in a large Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial of more than 150,000 subjects.102 After a median follow-up of 12 years, there was a 21% reduction in the incidence of CRC, and although this reduction in incidence was seen for both right-sided and left-sided tumors, the overall 26% reduction in CRC mortality came from the reduction of mortality in left-sided CRC. Mortality from right-sided CRC was unaffected. Interim results of the Norwegian Colorectal Cancer Prevention (NORCCAP) study at 6 to 7 years (one-third of the way through the study) revealed no reduction in CRC incidence or mortality.103
Colonoscopy
Strong evidence in favor of colonoscopy comes from the U.S. National Polyp Study (NPS) which demonstrated a very significant reduction in the incidence of CRC, estimated between 76% and 90%.90 A follow-up of that study published in 2012 was also able to demonstrate a reduction in mortality estimated at 53%.104 Although these figures are based on historical comparators and expected incidence-based mortality, they do support colonoscopy as a valid screening tool.
There is increasing evidence of the long-term benefits of screening sigmoidoscopy and colonoscopy. A single flexible sigmoidoscopy reduces the CRC rate for at least 11 years.100 After a negative colonoscopy, the CRC incidence was 2.8 per 100,000 in Year 1 and 13 per 100,000 of the population in Year 4 (expected rate 70.6 per 100,000).105 Most programs recommend repeating colonoscopy 10 years after a negative screening colonoscopy. Subsequent colonoscopy will be sooner in cases where polyps are found and that interval will depend on the specific findings.41,42
Right-Sided Colon Cancers
The lack of reduction in mortality for right-sided tumors raises questions about flexible sigmoidoscopy as a form of screening. However, there has also been a failure in the full colonoscopy studies to demonstrate any reduction in mortality for right-sided colon cancer. A Canadian case control study of colonoscopy showed the reduced cancer mortality being limited to left-sided cancers106 and this limitation has been demonstrated similarly in other studies.107,108
Several explanations have been suggested for the lack of impact of endoscopic screening on right-sided cancers (Table 77-3).109,110 The experience and skill of the endoscopist is key to the success of colonoscopy as a screening tool. In particular, a high adenoma detection rate correlates with a reduced risk of developing an interval cancer.111 Reported adenoma detection rates within the same center can vary from 9% to 33% between endoscopy specialists.112 By contrast, the mortality benefit of FOB testing is equivalent for right- and left-sided lesions.113
Uptake of Colorectal Cancer Screening
A key component of a successful CRC screening program is a high participation rate from the target population. The participation rate was higher for iFOBt (60%) than for gFOBt (47%).97,114 This higher uptake may be the result of requiring fewer samples, easier sample collection, and fewer dietary restrictions. In addition to lack of participation, another factor reducing the efficacy of either FOBt-based screening approach is that 16% of subjects declined follow-up colonoscopy.
One recent study found lower participation rates for colonoscopy screening (38%) than for FOBt (67%).115 There was also a difference depending on the ethnic background of the population. An Italian study initially invited subjects to flexible sigmoidoscopy. Those who failed to respond were then offered iFOBt. The initial response to flexible sigmoidoscopy was between 30% and 40% and an additional 30% of the population who had failed to respond to the invitation to flexible sigmoidoscopy underwent iFOBt screening.116 This stepped approach to a screening strategy increases the population adherence to screening, and the population also benefits from reduction in CRC incidence and mortality.
In 2003, the Council of Europe recommended the implementation of a population-based screening program for CRC, based on the observation from randomized trials that screening contributed to a reduction in colorectal mortality. By the end of 2007, there were screening programs in 19 of 27 European Union countries117; a number of other countries will introduce screening programs in 2012-2013. However, despite the benefits of screening, participation rates in European countries are relatively low (40% to 60%).118
Cost Effectiveness of Colorectal Cancer Screening
All assessments of the cost-effectiveness of CRC screening have shown it to be cost-effective, and in some cases, cost-saving.119 One study comparing gFOBt, iFOBt, and colonoscopy by using accepted screening intervals, suggested that colonoscopy offered the greatest net health benefit, reducing CRC mortality by 83%, but that annual screening using iFOBt offered a better cost-effectiveness ratio of $611.00 per quality-adjusted life-year (QALY). All three of these strategies were identified as cost-effective.120
In a U.S. study looking at annual gFOBt and sigmoidoscopy every 5 years and colonoscopy every 10 years, all three were considered cost-effective.121 A review of the published literature again indicated the consistent message that CRC screening was cost-effective, but the evaluation did not show any consistency with regard to what current screening test offered the greatest cost-effectiveness.122 In addition to actual cost, the choice of screening test will also depend on the availability of resources such as colonoscopy, the general public’s acceptance of the chosen method of screening and the overall costs of the screening, which will differ between countries.
Recently, there have been efforts to standardize reporting of CRC screening to facilitate comparison of programs globally.123,124 Standardization of indicators from these different programs will facilitate interprogram comparisons and help ascertain the best model for the particular circumstances of a country or region, for example, at what age should screening commence? How often should fecal testing be performed? When should any screening be discontinued?
New Technologies in Colorectal Cancer Screening
Computerized tomographic colonography (CTC) involves scanning of the abdomen and pelvis followed by reconstruction in 2D and 3D imaging to create a “virtual colonoscopy.” This technique has a high sensitivity and specificity for detecting large polyps (10 mm or larger), but a lower sensitivity for smaller polyps and does not detect flat lesions that appear to be important in right-sided CRC.125
The addition of oral contrast fecal tagging has helped to improve the interpretation of the procedure. Recognized limitations include the failure to detect smaller lesions, the exposure to ionizing radiation and the need for repeat bowel prep and colonoscopy if a lesion is found (unless the patient can move straight from radiology to endoscopy on the same day). In a study comparing CTC and computerized tomographic colonoscopy, adherence rates were higher for the CTC but computerized tomographic colonoscopy found a higher number of advanced adenomas per attendee, resulting in equivalent numbers of advanced adenomas being detected. CTC detected adenomas that required colonoscopy and overall patient experience was preferable for the direct colonoscopy.126
A second potential screening test for CRC is detection of abnormal DNA within stool samples. It is possible that this will be a future alternative to the detection of blood in stool samples. DNA testing has been improving rapidly with regard to choice of marker panel and use of buffering and preservative solutions. It has proved effective in detecting both cancers and adenoma.127 Similar to the blood-based fecal tests, DNA testing equally detects both proximal and distal neoplastic lesions.128 The greatest current drawback is cost. It has not been found to be cost-effective. However, as the technology develops and the costs per test decrease, DNA testing will emerge as another effective CRC screening tool. More detail on the potential for DNA markers is provided in the section on the molecular basis of CRC.
A third suggested screening modality is capsule endoscopy.129 By swallowing the capsule endoscopy pill, noninvasive images can be obtained of the colon. However, it has not yet demonstrated sufficient sensitivity or specificity to be recommended as a screening tool.
Diagnosis and Staging of Colorectal Cancer
Diagnosis
Staging
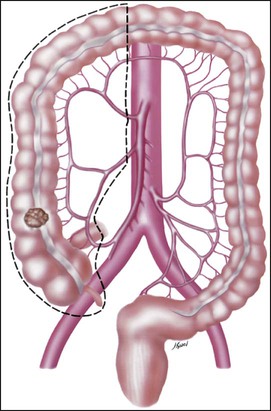
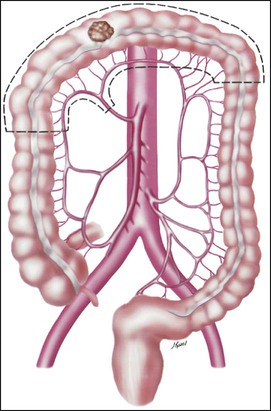
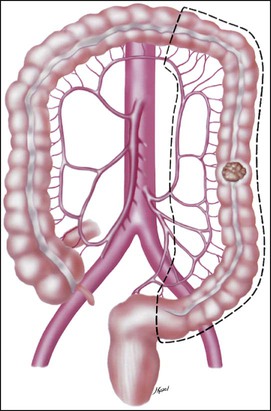
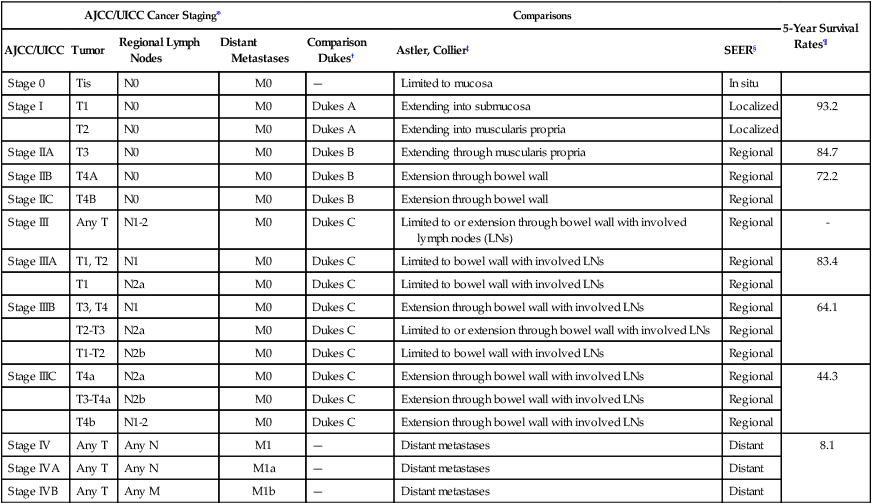
The anatomic extent of disease at presentation (stage) is the strongest predictor of survival for patients with CRC and forms the basis of appropriate patient management. The tumor, node, metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer is considered the international standard for CRC staging.130 In contrast with other staging systems for CRC, the TNM system is continuously updated on the basis of existing data, multidisciplinary in design, allows for the incorporation of all technologic approaches to staging, and has a comprehensive set of rules of application that ensure uniform use. The predictive accuracy of TNM staging can be increased through incorporation of validated prognostic features, such as lymphatic or venous invasion, into the overall assessment process. Indeed, although the basic categories for tumor direct extension, distant metastases, and lymph node involvement have not changed since the last edition, a number of new subdivisions have occurred that may have clinical significance (Table 77-4, Fig. 77-5).
Table 77-4
Surgical Stage and Survival Rates in Colorectal Cancer
| AJCC/UICC Cancer Staging* | Comparisons | 5-Year Survival Rates¶ | |||||
| AJCC/UICC | Tumor | Regional Lymph Nodes | Distant Metastases | Comparison Dukes† | Astler, Collier‡ | SEER§ | |
| Stage 0 | Tis | N0 | M0 | — | Limited to mucosa | In situ | |
| Stage I | T1 | N0 | M0 | Dukes A | Extending into submucosa | Localized | 93.2 |
| T2 | N0 | M0 | Dukes A | Extending into muscularis propria | Localized | ||
| Stage IIA | T3 | N0 | M0 | Dukes B | Extending through muscularis propria | Regional | 84.7 |
| Stage IIB | T4A | N0 | M0 | Dukes B | Extension through bowel wall | Regional | 72.2 |
| Stage IIC | T4B | N0 | M0 | Dukes B | Extension through bowel wall | Regional | |
| Stage III | Any T | N1-2 | M0 | Dukes C | Limited to or extension through bowel wall with involved lymph nodes (LNs) | Regional | – |
| Stage IIIA | T1, T2 | N1 | M0 | Dukes C | Limited to bowel wall with involved LNs | Regional | 83.4 |
| T1 | N2a | M0 | Dukes C | Limited to bowel wall with involved LNs | Regional | ||
| Stage IIIB | T3, T4 | N1 | M0 | Dukes C | Extension through bowel wall with involved LNs | Regional | 64.1 |
| T2-T3 | N2a | M0 | Dukes C | Limited to or extension through bowel wall with involved LNs | Regional | ||
| T1-T2 | N2b | M0 | Dukes C | Limited to bowel wall with involved LNs | Regional | ||
| Stage IIIC | T4a | N2a | M0 | Dukes C | Extension through bowel wall with involved LNs | Regional | 44.3 |
| T3-T4a | N2b | M0 | Dukes C | Extension through bowel wall with involved LNs | Regional | ||
| T4b | N1-2 | M0 | Dukes C | Extension through bowel wall with involved LNs | Regional | ||
| Stage IV | Any T | Any N | M1 | — | Distant metastases | Distant | 8.1 |
| Stage IVA | Any T | Any N | M1a | — | Distant metastases | Distant | |
| Stage IVB | Any T | Any M | M1b | — | Distant metastases | Distant | |
*International Union Against Cancer. UICC TNM classification of malignant tumors. 7th ed. New York: Wiley-Blackwell; 2010.
†Dukes CE. The classification of cancer of the rectum. J Pathol Bacteriol 1932;35:323.
‡Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg 1954;139(6):846–52.
§Steinhorn SC, Kopecky KJ, Myers MH, Ball C. Characteristics of colon cancer patients reported in population-based tumor registries and Comprehensive Cancer Centers. J Natl Cancer Inst 1983;70(4):629–34.
¶O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004;96(19):1420–5.
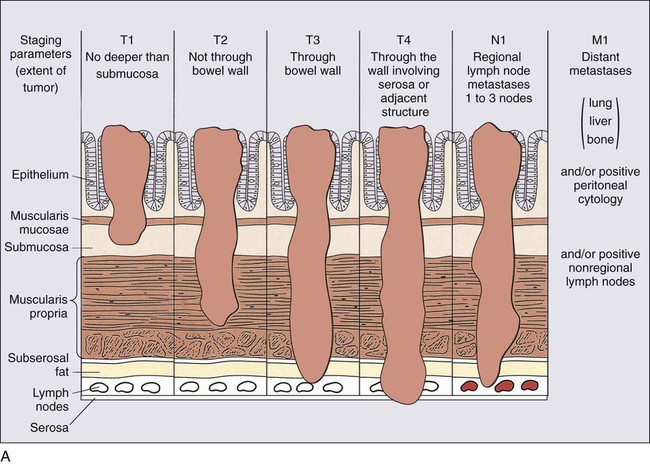
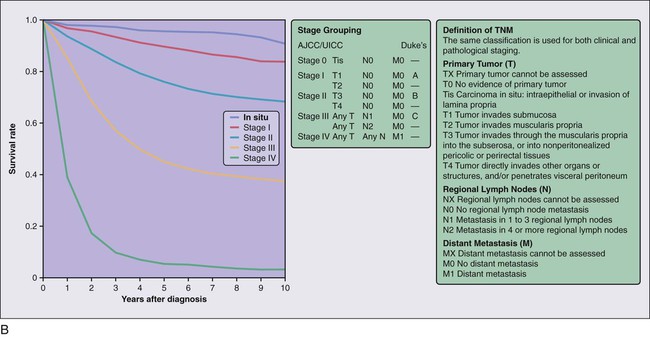
The definitions of the individual TNM categories and stage groupings and stage-related survival are shown in Figure 77-5, and comparisons of the TNM system to other historical staging systems are shown in Table 77-4. All tumors should be verified microscopically as carcinomas to confirm the appropriateness of TNM staging, which applies only to primary colorectal carcinomas, and to plan appropriate treatment.
In detail, these are the individual TNM categories,
(a) Tis: Denotes carcinoma in situ (involving only the mucosa); (b) T1: through the muscularis mucosa and extends into the submucosa; (c) T2: into the muscularis propria; T3: through the muscularis propria and into the external aspects of the colon or rectum; T4a: through the serosa/visceral peritoneum; T4b: attached to or invading into nearby organs.
(a) N0: No cancer in lymph nodes; (b) N1: Involvement of 1 lymph node (N1a), of two to three lymph nodes (N1b) or submucosal satellites with no involved lymph nodes (N1c); (c) N2: involvement of four to six lymph nodes (N2a) or seven or more (N2b).
(a) M0: No distant metastases, M1a in only one organ, M1b in more than one organ.
The TNM categories are translated into the several staging groups, as indicated in Table 77-4.
When establishing the TNM status of a CRC, a number of considerations should apply. Because the colorectal mucosa is architecturally unique and lacks stromal lymphatics, tumor invasion of the lamina propria has no associated risk of regional nodal metastasis (unlike upper gastrointestinal malignancies) and is included in the definition of pTis. Extramural extension of the tumor within lymphatics or veins does not count as local spread of tumor as defined by T3, but is instead denoted as L1 or V1 disease, respectively, in the L or V classification schema of the TNM system. In contrast, discrete smooth-contoured extramural tumor nodules of any size are classified as replaced lymph nodes, and each is counted separately in the N category on the basis of evidence that the number of pericolonic tumor-involved nodes correlates inversely with disease-free survival.131 Among the features that define T4 tumors, serosal penetration is the most severe.132 It is also possible to classify a tumor as pT4 on the basis of positive cytologic specimens of touch preparations from the serosa overlying the primary tumor. Direct invasion of adjacent organs or structures or other segments of the colorectum by way of the serosa or mesocolon (e.g., invasion of the sigmoid colon by carcinoma of the cecum) all should be classified as pT4. In contrast, intramural (longitudinal) extension of tumor from one subsite (segment) of the large intestine into an adjacent subsite or into the ileum (e.g., for a cecal carcinoma) or anal canal (e.g., for a rectal carcinoma) does not affect the pT classification.
Stage-related outcome data are based on pN assignment by conventional histologic staining of lymph nodes that are identified on routine macroscopic examination. Because many nodal metastases in CRC are found in small lymph nodes (<5 mm in diameter), 133 diligent searching for lymph nodes in resection specimens is essential. Indeed, the number of lymph nodes retrieved during the pathological examination of a CRC resection specimen is now considered, by many, to be one of the surrogates of the quality of pathological assessment. Over the last few years, there have been numerous opinions on the number of lymph nodes that represents adequate assessment, anywhere between 7 and 21.134 The number of lymph nodes that are recovered from resection specimens varies widely and depends on several factors: while some are unavoidable (patient factors such as age and anatomic variation), others are part of the quality of care (surgical resection technique or diligence of the pathologist in harvesting all existing nodes).
The consensus statement of the College of American Pathologists135 suggests that 12 lymph nodes be considered the minimum acceptable harvest from a careful specimen dissection. This is also the number suggested by the Royal College of Pathologists in the United Kingdom, which includes a mean number of 12 lymph nodes retrieved as one of the three measures of standard for a satisfactory colorectal pathology service (the other two being a frequency of serosal involvement of at least 20% in colon cancers and 10% for rectal cancers, and a frequency of extramural venous invasion of at least 25%).136 If fewer than 12 nodes are found after careful gross examination, additional techniques (i.e., visual enhancement techniques such as fat clearing) may be considered. It has been further recommended that all grossly negative or equivocal lymph nodes be submitted entirely for microscopic examination and that involvement of grossly positive lymph nodes be confirmed by either complete or partial microscopic examination.
Increasingly, attention is being focused on alternative methods of detection of very small amounts of metastatic tumor in draining lymph nodes, namely isolated tumor cells (ITCs) and micrometastases, and the TNM classification is beginning to focus on these methodologies. To ensure uniform pathological assessment and data collection, small numbers of tumor cells that are detected only by special techniques (including molecular means) or that are seen histologically but measure 0.2 mm or less in diameter are defined as ITCs. According to International Union Against Cancer (TNM) recommendations,130 ITCs are classified as N0, but allow suffixes to discern the presence or absence of these, such as: pN0(i−)—negative morphologic findings for ITC; pN0(i+)—positive morphologic findings for ITC; pN0(mol−)—negative nonmorphologic findings for ITC; and pN0(mol+)—positive for nonmorphologic findings for ITC. In contrast, small amounts of metastatic tumor that measure greater than 0.2 mm but less than 2.0 mm are defined as “micrometastases” and classified as N1 or M1. The number of lymph nodes that are involved by micrometastases or ITCs should be stated in the pathology report. To date, the data on the prognostic impact of micrometastases or ITCs in CRC are conflicting.139–139 Although systematic reviews140 and retrospective studies141 indicate their validity, only when the results of ongoing prospective studies are revealed142 will it be possible to determine their full utility.
Systematic reviews suggest that the use of sentinel lymph node analysis (the lymph node closest to the primary lesion) in colon cancer remains controversial.143,144 In any case, the TNM classification also allows for an analysis of ITCs in sentinel lymph nodes in a similar fashion to regular resections, namely pN0(i−)(sn), pN0(i+)(sn), pN0(mol−)(sn), and pN0(mol+)(sn).
Stage-related prognosis is also modified by the presence of residual tumor in the patient after primary surgical resection. Stage-related prognosis in disease with no (or limited hepatic) distant metastasis at presentation is predicated on complete eradication of all detectable tumor with cancer-directed surgery. The postoperative residual disease status of the patient, reflecting the efficacy of primary treatment, is categorized by a system known as the R classification, as follows130:
The R classification has stage-independent prognostic significance in that R1 and R2 are adverse prognostic factors compared to R0 status at any stage of disease. The relevance of the R classification to the pathologist is primarily related to resection margin evaluation. By convention, tumor that is present microscopically or macroscopically at a resection margin corresponds to R1 or R2, respectively. However, R0 status cannot necessarily be assigned to a case with negative resection margins because R0 refers to absence of residual tumor anywhere in the patient, including distant metastatic sites. Residual tumor is directly linked to the concept of tumor regression after treatment, a concept mentioned in some minimum dataset documents.145 There is emerging evidence that this is predictive of outcome when resection margins are clear,146 but there is uncertainty over the best way for it to be assessed. It is, therefore, recommended that at present, only complete regression, or the presence of minimal residual tumor is recorded.
When the distance between the tumor and the nearest longitudinal margin is 5 cm or more, anastomotic recurrences are very rare.145 In low anterior rectal resection specimens of rectal cancers, however, wide distal cuffs of normal mucosa can be hard to achieve, owing to anatomic constraints. In this circumstance, a margin of 2 cm is accepted as adequate, and in many cases, distal margins of 1 cm or less also prove sufficient, especially for T1 and T2 tumors.
In rectal carcinoma, the CRM has been demonstrated to be the margin of greatest importance in predicting risk of local recurrence, which is itself a strong predictor of survival.147 In fact, multivariate analyses have suggested that tumor involvement of the CRM is the single most critical factor in predicting local recurrence in rectal cancer.148 Emerging data on CRM involvement in the ascending or descending colon suggest a similar relationship to risk of local recurrence.149 Therefore, routine assessment of the CRM is recommended in all applicable CRCs, and measurement of the distance from the tumor to the nearest CRM, representing the “surgical clearance” around the tumor, is suggested.150 On the basis of published data from clinical trials, the risk of local recurrence is strongly increased if tumor is present 1 mm or less from the nonperitonealized surface of the specimen.151 By convention, therefore, a positive CRM is now defined as one that is 1 mm or less from the closest approach of tumor. Some data have suggested that the risk of local recurrence also is significantly increased with clearances of 2 mm or less.152 In contrast, the risk of recurrence is very low with clearance of more than 2 mm and can be considered “negative.”
Other aspects in the pathological analysis that may be of use clinically include:
• Measurement of extramural spread beyond the muscularis propria, both because it is related to prognosis in rectal cancer,153 and because of preoperative imaging auditing purposes.154
• Recording of tumor involvement of the nonperitonealized resection margin to decide on postoperative adjuvant therapy.149 Grading of the surgical plane of resection in rectal cancer specimens.153,155
• Recording of degree of tumor regression in rectal cancer patients with preoperative chemoradiotherapy.156 Recording whether presence and type of tumor perforation within the specimen (serosal vs. retro-/infraperitoneal).
Histopathology
The internationally accepted histologic classification of CRCs proposed by the World Health Organization157 (Table 77-5) is recommended by the College of American Pathologists (CAP). According to this classification, the majority of CRCs are adenocarcinomas of no special type. Special subgroups are worth noting because they may be associated with specific genotypes and prognosis. The mucinous, medullary, and signet ring cell subtypes are more likely to be associated with the microsatellite instability-high (MSI-H) genotype; the signet ring cell variant, when microsatellite stable (MSS) has a more aggressive prognosis. Serrated adenocarcinomas are associated with BRAF mutations and both the serrated and the cribriform phenotypes are more likely to fall into the CpG hypermethylator genotype. Relevant molecular changes and their association with particular subgroups are discussed in more detail below (see “Molecular Pathogenesis [The Molecular Basis of Colorectal Cancer]”). The neuroendocrine carcinomas (NECs) have staging and prediction schemes different from the traditional TNM, with high-grade NECs having an aggressive behavior.
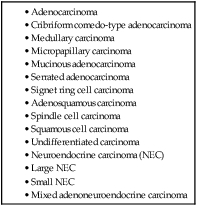
The grading of colorectal carcinoma, overall, is based on both architectural features and cytologic features (e.g., pleomorphism, hyperchromatism, and mucin production), but the degree of gland formation is widely regarded as the most important feature in grading. Most systems stratify tumors into three grades: grade 1 (well differentiated), grade 2 (moderately differentiated), and grade 3 (poorly differentiated). However, the notorious lack of reproducibility, as well as the similar prognosis of well and moderately differentiated, has favored a two-tier system (low-grade and high-grade adenocarcinoma), recommended in a consensus conference sponsored by the CAP.125 These considerations only apply to the conventional adenocarcinoma, as the special subtypes have their own prognosis irrespective of grading. In general, undifferentiated carcinoma is a term of exclusion for those high-grade carcinomas with no clearly defined histologic subgrouping. In any subtype, a MSI-H genotype confers a better prognosis (see below, “Molecular Pathogenesis [The Molecular Basis of Colorectal Cancer]”).
Despite the lack of standardization and interobserver variation in assessment, histologic grade repeatedly has been shown by multivariate analyses to be a stage-independent prognostic factor.160–160 More specifically, high tumor grade has been shown to be an adverse prognostic factor.
Pathology Markers for Colorectal Cancer
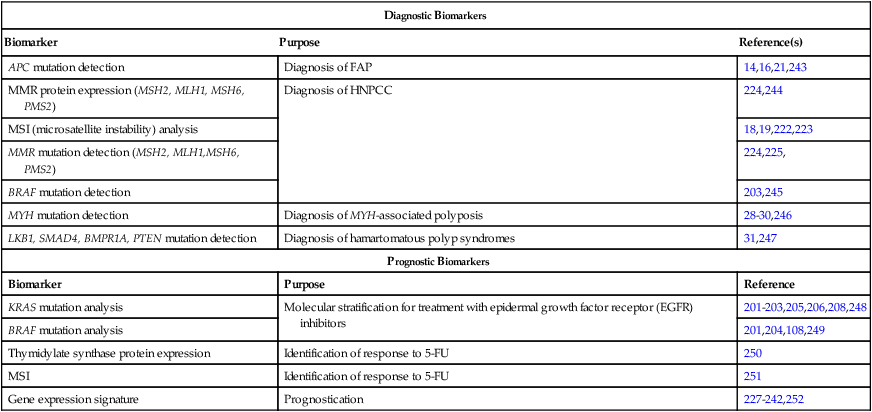
Positive immunostaining for cytokeratin 20 and negative for cytokeratin 7, although nonpathognomonic, are associated with CRC. This association may be of use in the context of a broad differential diagnosis,161 or to detect small clusters or single infiltrating carcinoma cells (<5 cells) at the invasive edge (so-called tumor budding), as the survival rate for stage II patients with tumor budding might not be significantly different from the survival rate for all stage III patients.164–164 Other tests that may be relevant in CRC fall under the framework of molecular diagnostics and/or personalized medicine and are indicated in Table 77-6.
Table 77-6
Molecular Biomarkers used in Clinical Standard-of-Care Decision Making in Colorectal Cancer
| Diagnostic Biomarkers | ||
| Biomarker | Purpose | Reference(s) |
| APC mutation detection | Diagnosis of FAP | 14,16,21,243 |
| MMR protein expression (MSH2, MLH1, MSH6, PMS2) | Diagnosis of HNPCC | 224,244 |
| MSI (microsatellite instability) analysis | 18,19,222,223 | |
| MMR mutation detection (MSH2, MLH1,MSH6, PMS2) | 224,225, | |
| BRAF mutation detection | 203,245 | |
| MYH mutation detection | Diagnosis of MYH-associated polyposis | 28–30,246 |
| LKB1, SMAD4, BMPR1A, PTEN mutation detection | Diagnosis of hamartomatous polyp syndromes | 31,247 |
| Prognostic Biomarkers | ||
| Biomarker | Purpose | Reference |
| KRAS mutation analysis | Molecular stratification for treatment with epidermal growth factor receptor (EGFR) inhibitors | 201–203,205,206,208,248 |
| BRAF mutation analysis | 201,204,108,249 | |
| Thymidylate synthase protein expression | Identification of response to 5-FU | 250 |
| MSI | Identification of response to 5-FU | 251 |
| Gene expression signature | Prognostication | 227–242,252 |
Imaging Modalities for Staging of Colon Cancer
The use of imaging for the staging of colon cancer can be divided into two broad categories: (a) detection and staging of the primary tumor and (b) determination of the extent of metastatic disease. For the detection of the primary cancer and of polyps greater than 1 cm in diameter, colonoscopy is now routinely used. When for technical reasons (e.g., partially obstructing cancer, tortuous colon, poor preparation) it is not possible to complete colonoscopy to the ileocecal valve, air-contrast barium enema or virtual colonoscopy are options for completing the evaluation of the colon, although they lack the capacity for biopsy or removal of polyps (Figs. 77-6 and 77-7). The emerging technology of capsule endoscopy has been found to have low sensitivity for detection of polyps and cancer and although less invasive, has the disadvantage that no biopsy or polyp removal can occur during the examination.129,165 For the staging of potential extracolonic metastatic disease, computed tomography (CT) scan of the thorax/abdomen/pelvis, magnetic resonance imaging (MRI), and positron emission tomography (PET) have been used with varying levels of success.166,167
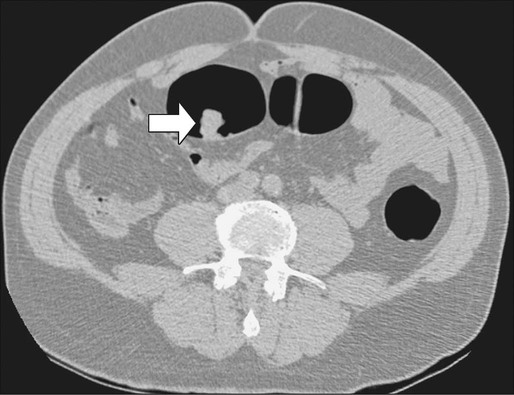
Computed Tomography
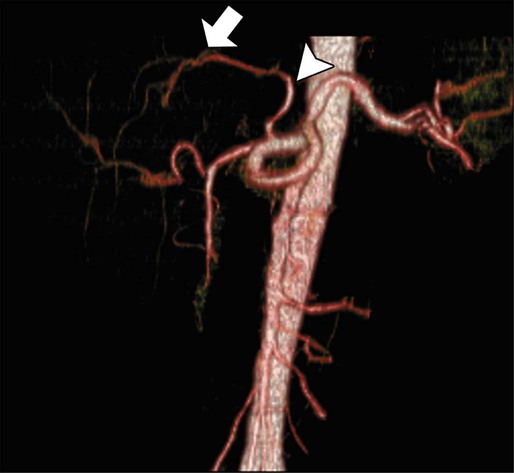
Contrast-enhanced helical CT scan of the thorax/abdomen/pelvis is considered the mainstay of preoperative imaging of colon cancer patients. CT scan can demonstrate regional tumor extension and tumor-related complications (e.g., obstruction, perforation, fistula formation). In addition, CT scan combines a high sensitivity for the detection of lung and liver metastases with availability, safety, and the ability to aid in the detection of peritoneal disease and metastatic lymph nodes. The sensitivity of CT scan is, however, low for abnormalities that adhere to peritoneal and visceral surfaces, such as peritoneal carcinomatosis or low-volume tumor. An added advantage of CT scan as a preoperative imaging modality for hepatic surgery is that CT angiography can be obtained at the same time as a conventional diagnostic CT scan (Figs. 77-8 through 77-11). This can be important in screening for hepatic arterial and venous anomalies prior to hepatic resection or if the surgeon is considering inserting a hepatic artery infusion pump. CT angiography is an excellent modality for determining the hepatic arterial, portal venous, and hepatic venous anatomy and for assessing variants of these vessels.168
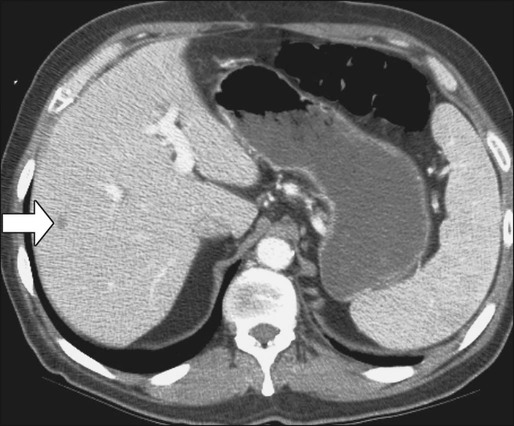
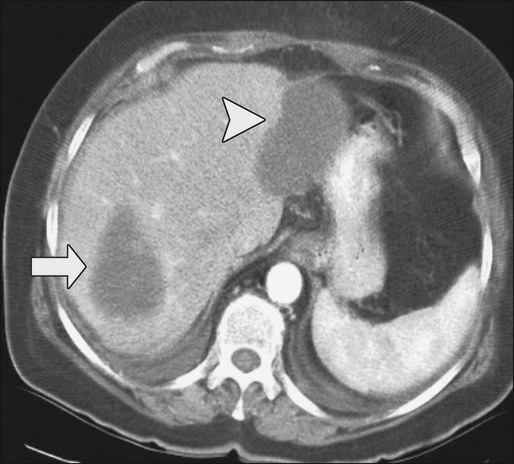
Magnetic Resonance Imaging of the Liver
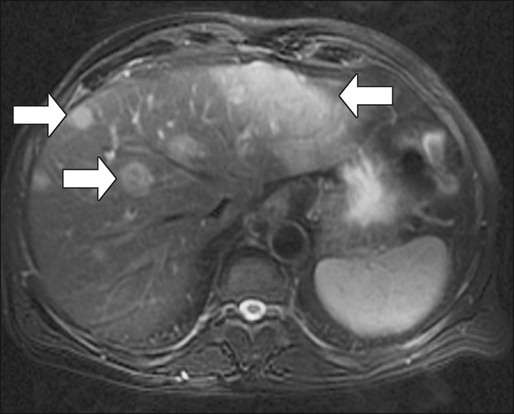
Compared with CT, MRI is probably slightly more sensitive and specific for detection and characterization of colon cancer metastases in the liver.171–171 A recent meta-analysis concluded that MRI was the preferred first-line imaging study for evaluating CRC liver metastases in patients who have not previously undergone therapy.172 MRI is also indicated in evaluating patients with central nervous system symptoms to determine the presence of metastases and for evaluating difficult-to-image pelvic wall recurrences (Fig. 77-12).
Fluorodeoxyglucose Positron Emission Tomography
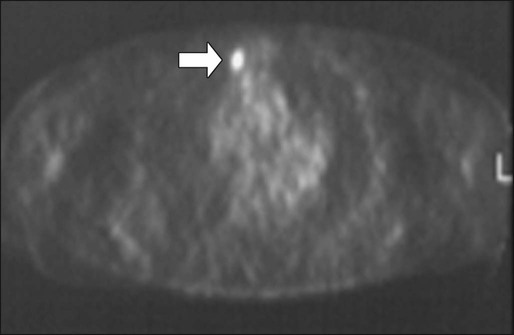
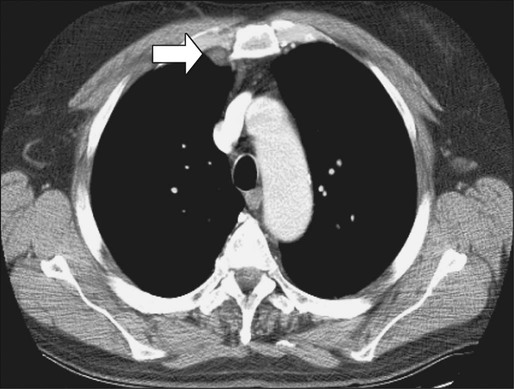
Fluorodeoxyglucose positron emission tomography (FDG-PET) imaging does not appear to add significant information to CT scans for routine preoperative staging.173,174 The main value of PET scanning in patients with CRC is as an adjunct to the other imaging modalities in the setting of:
1. Localizing site(s) of recurrence in patients who have rising CEA and nondiagnostic conventional imaging following primary treatment. In this context, PET scan can potentially detect occult metastatic disease, thereby selecting patients who may benefit from salvage surgery. In a retrospective analysis of 105 patients who underwent PET scanning and subsequent CT scan plus other conventional diagnostic studies, the investigators found a higher sensitivity (87% vs. 66%) and specificity (68% vs. 59%) for PET scanning compared with CT scan plus other conventional studies175 for detection of recurrence/metastases.
2. Evaluation of patients who are thought to be candidates for resection of isolated (liver or other) metastases. Flamen and colleagues reported in their study that PET scanning led to a potentially curative resection in 14 of 50 patients with unexplained rising CEA and completely normal or equivocal conventional diagnostic workup.176
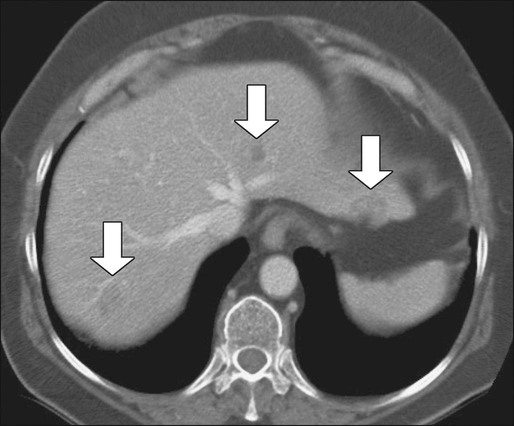
Compared with CT imaging, PET has, however, a lower sensitivity for the detection of hepatic metastases, but a higher sensitivity for the detection of extrahepatic metastases.177 The superiority of FDG-PET compared with CT scan for detection of extrahepatic disease was also suggested in a retrospective analysis that used a scoring system to weigh the individual studies according to the quality of data and the clinical impact of the radiographic findings.178 The pooled sensitivity and specificity for the detection of extrahepatic disease for FDG-PET and CT scan were 91% and 98% and 61% and 91%, respectively. The percent change in clinical management from the performance of PET was 25% (range: 20% to 32%). Consequently, many centers now use a combination of CT and PET scanning to stage patients before potential hepatic resection (Figs. 77-13 and 77-14).179
Intraoperative Ultrasound (Open and Laparoscopic)
Intraoperative ultrasound is considered the most sensitive and specific imaging test for detection of hepatic metastases from colon cancer (Fig. 77-15). It is far more sensitive for the detection of metastatic lesions than are inspection and palpation of the liver by the surgeon, particularly for deep tumors.180,181 Liver metastases as small as 3 mm can be detected routinely by intraoperative ultrasound.182 Additional advantages of intraoperative ultrasound are its ability to guide biopsies of suspicious hepatic lesions and its use as an aid in both liver resection planning and guiding tumor ablation (e.g., cryoablation or radiofrequency ablation) (Fig. 77-16). Intraoperative ultrasound has a very limited role in the evaluation of extrahepatic metastases from colon cancer because of the highly targeted nature of the examination. Occasionally, retroperitoneal lymph nodes and other metastatic deposits can be imaged if specific abnormalities are noted on preoperative imaging or if an abnormality is noted during inspection and palpation of the abdomen.
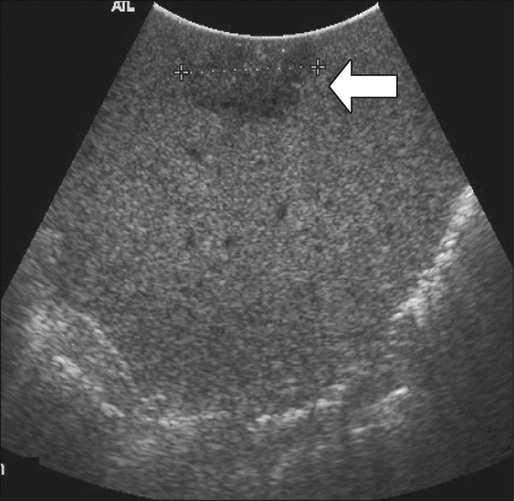
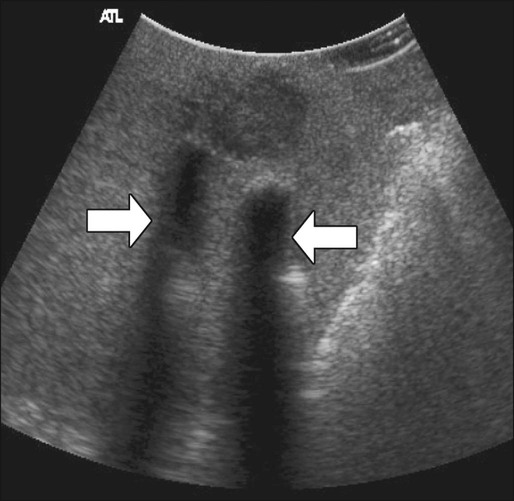
Laparoscopic ultrasound can be used for the same purposes as open intraoperative ultrasound of the liver. Because of lack of access to some areas of the liver, the sensitivity of laparoscopic ultrasound appears to be slightly less than that of open intraoperative ultrasound, but laparoscopic ultrasound has the advantage of being a less invasive approach.183 This approach is particularly useful in patients who have a high likelihood of unresectable liver disease or peritoneal disease or in patients who are to undergo another minimally invasive treatment for known liver metastases, such as tumor ablation.
Molecular Pathogenesis (the Molecular Basis of Colorectal Cancer)
The knowledge of the molecular pathogenesis of CRC is extensive and, yet, paradoxical. Despite the significant amount of genetic information accumulated over the last number of years, we lack a comprehensive, unified molecular classification for CRC. In 1988, Vogelstein and colleagues proposed a multistep model outlining the key pathogenic changes in oncogenes and tumor suppressor genes for CRC development.184 This stepwise model of understanding carcinogenesis, matching phenotypic and genotypic changes, provided a prototype framework for the study of oncogenesis in CRC and many other cancer types, and fuelled our understanding of cancer in general. However, while the “Vogelgram” highlights the involvement of specific oncogenes and tumor suppressor genes in the adenoma to carcinoma transition, the precise sequence of genes proposed (Fig. 77-17) required refinement, as it became clear that other molecular paradigms were necessary to explain CRC carcinogenesis. Currently, our understanding of the molecular etiology of CRC, underpinned by the latest technological advances, is informed by a series of diverse pathways and mechanisms which, it is hoped in the future, will be integrated in a single, coherent model for CRC carcinogenesis.
The Epidermal Growth Factor Receptor Pathway
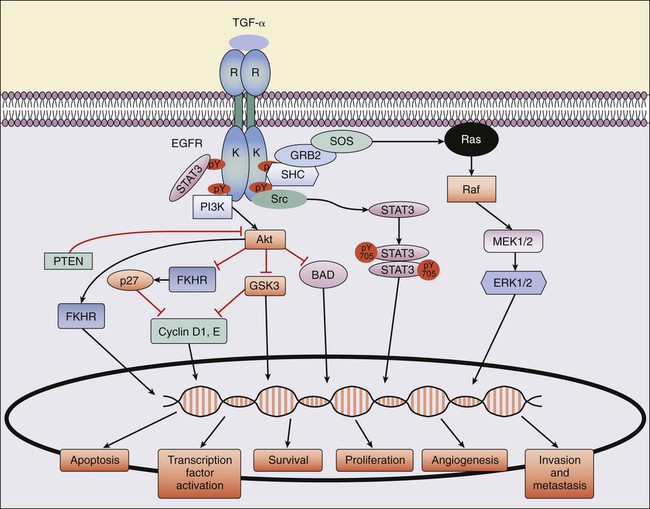
A key pathway in CRC pathogenesis is the epidermal growth factor receptor (EGFR) pathway (Fig. 77-18). EGFR is a transmembrane receptor tyrosine kinase of the ErbB or HER (human epidermal receptor) family, made up of four related receptors: EGFR (ErbB1/EGFR/HER1), ErbB2 (HER2/NEU), ErbB3 (HER3), and ErbB4 (HER4).185,186 EGFR can become activated by ligand-dependent or ligand-independent mechanisms, overexpression, or mutation. Ligands for EGFR include epidermal growth factor (EGF), transforming growth factor alpha (TGF-α), amphiregulin, betacellulin, and epiregulin. Ligand binding to EGFR induces a conformational change in EGFR that results in activation of its tyrosine kinase domain, phosphorylation of its tyrosine residues, and recruitment of several adaptor proteins (Fig. 77-18). Dependent on the genetic background of the tumor, activated EGFR can result in increased signaling through the MAPK, PI3K-Akt, and STAT pathways, mediating multiple cellular responses, including apoptosis, proliferation, invasion, DNA repair, and survival.187 EGFR is overexpressed in 25% to 77% and expressed in 80% to 90% of screened patients with CRC.188 In addition, overexpression of EGFR is associated with poor prognosis but is not predictive for response to EGFR-targeted therapies.
Chromosomal Instability Tumors
The chromosomal instability (CIN) molecular pathway is the one more closely associated with the original adenoma-carcinoma sequence proposed by Vogelstein. It is characterized by the consecutive accumulation of chromosomal abnormalities.190 This pathway is associated with mutations or allelic loss of the APC gene, KRAS mutations, mutations/deletions of the deleted in colon cancer (DCC) gene, and/or TP53 mutations.191 However, although these genes are important in CRC pathogenesis, the ability of the CRC cell to use other cancer-specific molecular pathways, through acquired abnormalities in other key genes or through epigenetic changes, brings not only a significant degree of complexity to the adenoma-carcinoma sequence,192,193 but also provides challenges in targeted therapeutic approaches. The genes that are more likely to be involved in chromosomal instability are predominantly part of the Wnt signaling pathway, highlighting the importance of this pathway in the pathogenesis of early CRC.
A key component of the Wnt signaling cascade, the APC tumor suppressor gene, is defective in both early adenomas and established CRCs.194 Regardless of the study, APC and its partner β-catenin are implicated in a significant proportion (around 80% to 85%) of CRC cases.195,196
APC-β Catenin-Wnt Signaling Pathway
In CRC, the majority of mutations in the APC gene cause a truncation of the APC protein and therefore a loss of its normal function to degrade cytoplasmic β-catenin. This degradation occurs when β-catenin is bound to the APC–AXIN–GSK-3β complex. GSK-3β acts to phosphorylate β- catenin, leading to its conjugation to the ubiquitin protein and degradation. β-Catenin stability is regulated by diacylglycerol-independent protein kinase C-like kinase activity, which is required for β-catenin ubiquitination (Fig. 77-19).197 The absence of a functioning APC protein leads to the accumulation of β-catenin in the cytoplasm and its translocation to the nucleus. β-Catenin plays an important role in cell–cell adhesion by linking cadherin receptors to the actin cytoskeleton.
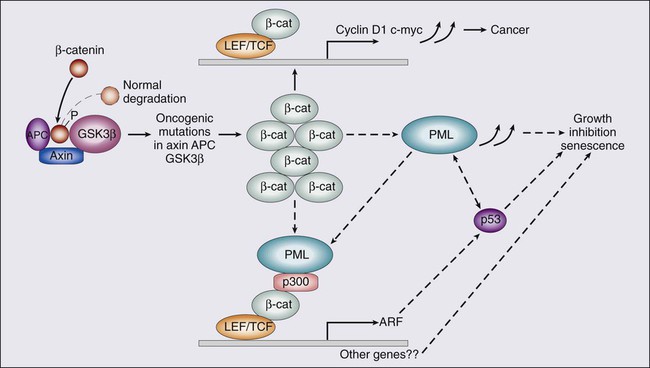
β-Catenin is also related to the Wnt-signaling pathway that determines cell fate, specifically during development. Activation of the Wnt receptor results in phosphorylation and inactivation of GSK-3β, which then prevent GSK-3β from phosphorylating β-catenin.198 The nuclear accumulation of β-catenin causes it to complex with Tcf-LEF (T-cell factor–lymphocyte enhancer factor). The β-catenin LEF protein complex can activate genes with Tcf-LEF promoter recognition sites, including c-Myc, cyclinD1, PPARS, matrilysn, Fra-1, UPAR, c-Jun, PML, and gastrin. These changes result in enhanced cell proliferation and inhibition of apoptosis.199,200
The Protooncogenes KRAS and BRAF
This model of CRC development is dependent on the accumulation of additional genetic and epigenetic aberrations. A critical alteration is a point mutation of the KRAS protooncogene. KRAS is a plasma membrane bound small G protein and acts as a molecular switch, cycling between the inactive, guanosine diphosphate (GDP)-bound state and the active, guanosine triphosphate (GTP)-bound state in response to activation of various growth factor receptors. Oncogenic activating mutations in KRAS occur in 40% to 45% of patients with CRC.184 The majority of mutations occur in exon 2 (codons 12 and 13), while occasional mutations are found in exon 3 (codon 61) and exon 4 (codon 146). The first large Collaborative Kirsten Ras in Colorectal Cancer Collaborative Group (RASCAL) meta-analysis involved more than 2700 patients and reported an increased risk of tumor recurrence and death linked to KRAS mutations.201 A second study (RASCAL II) demonstrated that the key mutation was linked to codon 12 (G12V).202 Although some studies have confirmed a prognostic role for mutant KRAS in CRC,203,204 two recent studies, involving 1321 stages II/III (PETACC3; see also “Adjuvant Irinotecan Combinations”) and 508 stage III (CALGB 89803) CRC, did not find an independent prognostic role for KRAS mutations.205,206
A second important gene in this pathway that is implicated in CRC pathogenesis when mutated is the oncogene BRAF. BRAF is a serine-threonine kinase and is a key regulator of the MAP-kinase/ERK-signaling pathway. The BRAF V600E mutation occurs in approximately 8% to 15% of CRC patients with wild-type KRAS.203,206 Patients with BRAF-mutant tumors have a poor prognosis.208–208 Mutated signaling pathways through KRAS, BRAF, and PI3K can all lead to the development of CRC and may also have predictive relevance in relation to responses to anti-EGFR therapies,208 as discussed in the section “Monoclonal Antibodies Targeting EGFR”.
In addition to the above mutations, hypomethylation of DNA leading to gene activation appears to play a role.211–211 Deletions of 18q21 have also been described,212 and DCC, a tumor suppressor gene, was identified at this chromosomal location.213 In addition, SMAD2 and SMAD4, two other genes that are involved in CRC, have been identified at 18q21.214,215 SMAD2 is a receptor-regulated gene and is activated by TGF-β and activin signaling. Its role in colon cancer appears to be a late event, acting to accelerate progression in the later stages of invasive carcinogenesis.214 Finally, allelic loss or mutation at chromosome 17p, which has been associated with the p53 tumor suppressor gene,184 appears to play a late role in CRC by stimulating invasion. In summary, it has been generally assumed that the accumulation of genomic abnormalities, rather than their precise order, is critical to progression in CRC.
Defective Mismatch Repair Pathway
Microsatellite Instability Tumors
Microsatellites are simple short, repetitive sequences (usually di-, tri- tetra-, or pentanucleotide repeats) that are randomly distributed throughout the human genome. There are approximately 50,000 microsatellites, many of which are especially prone to polymorphic variation, with changes in their repeat number between individuals. Although highly polymorphic between individuals, in principle, a microsatellite should exhibit the same profile within all the cells of an individual. However in CRC, variations were observed in microsatellite repeat number within the tumor of an individual.216,217 This microsatellite instability (MSI) is the result of a defect in one of a number of DNA mismatch repair (MMR) genes.218 Proteins such as MLH1, MSH2, MSH3, MSH6, and PMS2 maintain microsatellite stability in normal cells through surveillance and correction of errors that result from slippage in DNA replication. However, in a subset of CRC patients, mutations in MMR proteins mean that these errors are not accurately repaired, thus leading to the phenomenon of MSI.
Although MSI was originally a marker for MMR-deficient CRC, the discovery that a number of these microsatellites are located within the coding regions of genes, for example, a polymorphic GCG trinucleotide repeat in exon 1 of the TGF-β receptor 1 gene (TGFBR1), a mononucleotide (A)10 repeat in TGFBR2,219 and a polymorphic (G)8 repeat in the proapoptotic BAX,220 indicate a potential functional relevance for MSI. Thus, insertions or deletions in microsatellites located in DNA coding regions can generate frameshift mutations, which can lead to protein truncations.221
Detection of Microsatellite Instability Tumors
MSI can be detected following polymerase chain reaction (PCR) amplification of selected microsatellites using fluorescent primers and automated detection platforms.222 As part of the revised Bethesda criteria (see “Hereditary Nonpolyposis Colon Cancer” and Table 77-2), a panel of three dinucleotide repeats (D2S123, D5S346, D17S250) and two mononucleotide repeats (BAT26, BAT25) were adopted as a standard testing approach for MSI.223 If 2 or more of the 5-marker panel demonstrated MSI, the tumor would be classified as MSI-H. Although this choice of markers remains a gold standard,223,224 new designs of microsatellite testing, including a choice of mononucleotide repeats, are currently being used by many centers. These designs appear to provide a more unequivocal diagnostic picture.
Overall, the laboratory testing around MSI involves three main approaches: MSI testing, immunohistochemistry (IHC) analysis,224,225 for the MMR proteins, and mutation detection in the MMR genes.225 These also remain the key determinants, with clinical and family history, to confirm a diagnosis of HNPCC.
Clinical Relevance of Microsatellite Instability
In CRC, approximately 15% of cases are MSI-positive. The incidence of MSI-H is dependent on tumor stage, with high incidence (22%) in stage II CRC, and significantly lower incidence in stage III (12%) and stage IV (3.5%) CRC.227–227 In 3% to 5% of cases, MSI positivity correlates with the inherited colon cancer syndrome HNPCC (Lynch syndrome).225 In the remaining 10% to 12% of MSI-positive patients, somatic inactivation of both alleles of the relevant MMR gene leads to the development of CRC. In more than 80% of these cases, hypermethylation of the promoter region of the hMLH1 gene, leading to somatic inactivation of this gene, has been demonstrated.228
Epigenetics and Colorectal Cancer
Although somatic changes in oncogenes and/or tumor suppressor genes represent the classical mutational pathway in cancer development, more recently the influence of epigenetic changes (particularly in epigenetic silencing) on tumor development and progression has been clearly demonstrated for a wide number of cancer types, including CRC.229
Epigenetic modulation, either at the gene level (e.g., through methylation of DNA nucleotides) or at the protein level (e.g., through acetylation of histones) can have a profound influence on gene expression. Methylation of the promoter control regions of tumor suppressor genes, for example, can lead to uncontrolled proliferation and the development of malignancy. Gene methylation studies in CRC have characterized a patient subgroup with a higher-than-expected frequency of aberrant methylation, the so-called CpG island methylator phenotype (CIMP) cancers.230, More recently, a classification of CRC based on a combined genetic and epigenetic approach has been proposed.231 Patients are classified into three groups: CIMP1, CIMP2, and CIMP-negative. Although CIMP1 is characterized by MSI (80%) and BRAF mutations (53%), and CIMP2 is associated with 92% KRAS mutations, CIMP-negative cases have a high rate of p53 mutations (71%).
For the practicing oncologist, awareness of methylation is relevant because the detection of “aberrant” methylation may have diagnostic, prognostic, and therapeutic implications.231 Indeed, screening for CRC with methylation-based kits has already been introduced in both the United States and in Europe.127,232,233
Genome-Wide Association Studies and Colorectal Cancer
Genome-wide association studies (GWASs) involve the testing of a large number of genetic variants in a significant cohort of patient samples and controls for their association with a particular disease or disease subtype. In CRC, GWAS has led to the identification of a number of genetic susceptibility loci.234,235 The combination of cost-effective technological advances, large reference sample collections, and a comprehensive array of genetic markers has allowed a precise dissection of the genetic contribution to malignancy. In CRC, the results of these studies have allowed us to revisit the role of the TGF-β pathway in this disease, beyond what was previously suggested.235 While GWASs allow refinement of our understanding of the complexity of cancer and indicate new genetic markers and pathways for further analysis, a genome-wide approach in the clinical setting remains beyond our current technological capacity. However, it has been shown how a combined technical approach of whole-genome sequencing, targeted whole-exome sequencing, and transcriptome sequencing (RNA-Seq) could identify disease-related mutations in a clinically relevant timeframe.236
Gene Expression Profiling and Colorectal Cancer
Although a large number of high-throughput gene expression profile studies have been performed,237–242 overall these studies have had a modest impact on the understanding of the molecular etiology of CRC.238 However, it is likely that gene expression profiling will play a significant role in the prognostication and prediction of stage II and stage III CRC.239–242 Several studies have been published, applying DNA microarray technology to the identification of prognostic gene signatures. The lack of availability of large cohorts of high-quality fresh-frozen material has meant, however, that many of these studies still require validation. Recently, a DNA microarray-based prognostic assay with clinically relevant formalin-fixed paraffin-embedded (FFPE) samples and a novel disease-specific array (DSA) approach has identified a novel prognostic biomarker for stage II colon cancer patients that can be applied to FFPE tumor samples.242 The different proposed prognostic platforms for early-stage CRC, based on gene-expression profiling studies, are discussed in detail in “Indications for Adjuvant Therapy–Clinical and Molecular Risk Factors.”
Molecular Pathology: Translating the Molecular Understanding of Colorectal Cancer to Clinical Application
CRC is arguably one of the most extensively studied (and understood) cancer types from a molecular standpoint, yet a convincing unified taxonomic classification is lacking. However, in the process of understanding its genomic architecture, a series of molecular markers have been identified in CRC that have diagnostic, prognostic, and/or predictive relevance (see Table 77-6).14,16,18,19,21,28–31,201–206,208,222–225,227–242,245–252
Surgical Treatment
Radical resection of the tumor-bearing segment of colon, with wide margins and removal of the lymphatic drainage of the tumor, is the standard for curative therapy. The extent of resection is determined by the tumor size, location, histologic grade, and tumor extension into the colon wall and into adjacent tissue or organs. Historically, resections have been quite radical with respect to the removal of the mesocolon-bearing draining lymphatics at risk for tumor spread; however, there is no major survival advantage to extended lymph node dissection.253 Limited segmental resections are indicated primarily when the surgery is deemed palliative.
Perioperative Clinical Management
The management of patients undergoing elective colonic resection in the perioperative period, has changed significantly in the last decade. Historically, patients were routinely fasted for prolonged periods both before and after surgery, underwent bowel cleansing regimens, and had periods of restricted mobility because of the number of indwelling drains and catheters. In addition, patients routinely received large volumes of intravenous fluids on the basis that significant fluid losses occurred intraoperatively. However, reducing the length of stay in hospital and improving outcomes by using enhanced or fast-track recovery has been responsible for a paradigm shift in what is considered now to be best practice for perioperative care.254
Enhanced Recovery Programs
The use of an enhanced recovery program (ERP) has become the standard approach to the management of patients undergoing elective colonic resections. It encompasses a combination of evidence-based perioperative strategies, which expedite postoperative recovery and, more importantly, reduce morbidity.255,256 Currently, the estimated incidence of morbidity following elective colonic surgery is approximately 25%. There are a multitude of factors responsible for this incidence, and prolonged hospital inpatient stay increases the risk of infectious and thromboembolic complications.
As recently as 2005, the average length of hospital stay in the United States following colectomy was 10.6 days.257 Many of the published series on ERP have shown a reduction to a mean of 4% to 5 days, and as low as 2 days in some cases.258,259 Despite this, readmission rates have not increased to any significant degree and patients return to work more quickly. Central to the successful implementation of ERP is the supportive multidisciplinary team. The number of components in an ERP vary between centers, but most have between 10 and 15. Table 77-7 lists the main components.260
Table 77-7
Summary of Enhanced Recovery Protocol from ASGBI
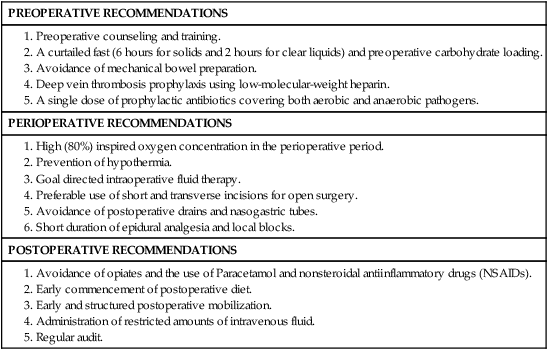
ASGBI, Association of Surgeons of Great Britain and Ireland (ASGBI).
Patient compliance is central to the success of ERP. Proponents encourage involving patients in their own recovery, by the use of daily goal sheets and diaries. Several studies have demonstrated a reduction in postoperative pain, analgesic requirements, and anxiety in those who received preoperative counselling.263–263 Equally important is the cooperation of nursing staff, dieticians, and physiotherapists who are core members of the team involved in perioperative care. Finally, early discharge planning is important, particularly for those who are likely to require either additional support at home after discharge or a period of time in a nursing or residential facility.
Most of the research to date in this area has looked at the benefits of enhanced recovery after open surgery. Although there is evidence that laparoscopic surgery is associated with a reduction in length of stay after colonic resection, it is unclear whether the addition of an enhanced recovery protocol can further reduce hospital stay. In 2012, recruitment into the EnROL (Enhanced Recovery Open versus Laparoscopic) trial commenced; a phase III multicenter randomized trial of conventional versus laparoscopic surgery for CRC within an ERP, with blinding of patients and outcome observers during the first week postoperatively.264 The primary end point is postoperative physical fatigue using the Multidimensional Fatigue Inventory 20 (MFI-20) at 4 weeks postsurgery and the trial is also powered to investigate postoperative hospital stay.
Mechanical Bowel Preparation
Historically, it was believed that mechanical bowel preparation (MBP) was necessary to perform a safe colonic resection. In 1972, Hughes suggested that patients undergoing elective colonic resection may not require MBP; however, this idea was not widely accepted.265,266 In 2011, a Cochrane review, which included 18 studies involving almost 6000 patients, concluded that the absence of MBP is not associated with a greater risk of perioperative morbidity or mortality.267 There was no statistically significant difference in the rate of anastomotic leakage between patients receiving MBP and those who did not. Overall, there was no increase in the risk of intraabdominal or extraabdominal complications; however, there was some evidence that its use may be associated with a greater risk of wound infections. This may be partly the result of a suboptimal bowel cleanse that may leave intraluminal content, which is liquid and, therefore, more likely to spill out intraoperatively. MBP is unpleasant for patients and can result in significant electrolyte imbalance and dehydration.268 Increasingly, MBP is no longer routinely used for elective colonic resection, unless there is a specific indication, such as the need to do an on-table colonoscopy.
Perioperative Nutrition and Fasting and Intraoperative Fluid Management
A period of fasting is a prerequisite for safe general anesthesia to avoid the risk of aspiration of gastric contents. Most anaesthetic associations now advocate the avoidance of solids for at least 6 hours preoperatively and a minimum of 2 hours for clear liquids. This guideline contrasts with the historical prolonged fasting time and “Nil by mouth from midnight” policy. The evidence now clearly shows that prolonged fasting before surgery is detrimental.269 The state of fasting is associated with insulin resistance and there is a correlation between postoperative insulin resistance and length of stay.270 The metabolic response to surgery is one of hypermetabolism and insulin resistance. So, one of the key components of most ERPs is the administration of a carbohydrate drink both the night before surgery and 2 hours before the operation. There is no evidence that this increases the risk of aspiration but it has been shown that in doing so, postoperative insulin resistance is significantly reduced. The implications of this are important, because hyperglycemia postoperatively is associated with increased morbidity and mortality in some patients.
Historically, patients undergoing major colorectal resections received large volumes of intraoperative fluid. There is evidence that excess administration of crystalloids in the perioperative period increases morbidity.271 One of the central components of enhanced recovery is goal-directed fluid replacement. Intraoperatively, fluid requirements can be more accurately determined through the use of an intraesophageal Doppler probe. This approach monitors changes in intraoperative stroke volume, and thus the administration of excess, or inadequate fluid, can be avoided. It facilitates the optimization of cardiac function, thus reducing cardiac morbidity.272,273
Early feeding is a key component of a fast-track recovery program. There is no gain from leaving a nasogastric tube in situ after routine elective surgery. This practice does not reduce the incidence of ileus and it is associated with an increased risk of pneumonia.274 The routine use of intraabdominal drains should be avoided.275
Thromboembolism Prophylaxis
The routine use of thromboembolism prophylaxis is another component of all ERPs. There is a greater risk of thromboembolic complications after colon and rectal surgery than other general surgical procedures. In 1988, a meta-analysis confirmed that the risk of deep vein thrombosis, pulmonary embolism, and resulting fatality is reduced by the routine administration of subcutaneous heparin in general surgical patients.276 Use of adjuncts, including compression stockings and intermittent pneumatic compression, also afford additional protection,277 and early mobilization is paramount.
Laparoscopic Surgery
Approximately 50% of those diagnosed with CRC have potentially curable disease.278 Twenty percent will have metastatic disease at the time of presentation and thus any surgery is likely to be palliative. About one-fifth of CRCs are seen as emergencies. Surgical resection involves removal of the segment of disease-containing colon with a margin of a least 5 cm on either side of the tumor, with the adjacent colonic mesentery containing the regional lymphatic drainage and lymph nodes. In 2000, the National Cancer Institute (NCI) issued guidelines on the surgical management of colon cancer. The resection should include wide excision of the mesentery, with ligation of the feeding artery close to its origin, with a minimum of 12 nodes removed.279 Moreover, the extent of resection must be determined by tumor size, location, histologic grade, and tumor extension into the colon wall and into adjacent tissue or organs.
Until the early 1990s, all colorectal surgery involved a full laparotomy, generally via a midline or paramedian incision. However, since 2000, increasing numbers of CRC resections are being carried out as laparoscopic or laparoscopically assisted procedures. Initially, the use of laparoscopic approach for colorectal resection was considered experimental with the first laparoscopic colonic resection performed in 1991.280 The initial uptake of laparoscopic CRC resection has been slow, a result of initial studies showing higher incidence of port site recurrence.281,282 More recent studies have disproved the higher incidence of port site metastasis associated with laparoscopic CRC surgery. Data from the main randomized, multicenter trials, which included almost 3000 patients, have provided evidence that laparoscopic resection does not compromise outcomes.283,284 Other obstacles to the uptake of this novel surgical approach include higher hospital costs and a long learning curve for surgeons.
The rate of laparoscopic colon cancer resections have increased over the last few years with more than 25% of cases completed laparoscopically. This statistic is compared with 8.3% and 17.2% of major resections of CRC completed laparoscopically in the 2006-2007 and 2007-2008 periods, respectively.285 It is now recommended that if both are available, a laparoscopic resection should be offered as an alternative to an open resection.286 It is anticipated that the laparoscopic approach to CRC resection will continue to increase as more surgical trainees are exposed to this as a routine part of their training. Since 2005, several large, randomized, controlled trials including, the Clinical Outcomes of Surgical Therapy study group (COST),287 the Colon Cancer Laparoscopic or Open Resection (COLOR)288 study, and the Conventional versus Laparoscopic-Assisted Surgery in Colorectal cancer (CLASSIC)284 have been carried out, which have provided the basis for this change in practice. Similar to National Institute for Clinical Excellence (NICE) in the United Kingdom, the American College of Colorectal Surgeons support its use.
Prognostic Factors for Laparoscopic Surgery
Lymph node metastasis is the most important prognostic factor in relation to CRC. Adequate lymph node harvest is, therefore, necessary to facilitate adequate staging and to determine the need for adjuvant therapy (discussed later in “Indications for Adjuvant Therapy”). None of the major trials have shown any significant reduction in lymph node numbers retrieved comparing laparoscopic versus open surgical approach. Furthermore, there is no difference in postoperative mortality or disease-free survival (DFS) after 3 years.288 However, the first randomized trial by Lacy and colleagues in 2002 demonstrated a significant improvement in disease-specific survival for the laparoscopic group in patients with stage II disease.283 Although this benefit was not reproduced in subsequent studies, a total of 23 randomized controlled trials comparing laparoscopic and open colorectal surgery have been performed since 1995 and the overwhelming conclusion is that laparoscopic surgery is at the very least equivalent to open resection.
Benefits of Laparoscopic Surgery
A number of additional benefits have been demonstrated from laparoscopic surgery. Postoperative pain is often a major problem associated with the trauma of a midline laparotomy wound. The use of a laparoscopic technique involves much smaller wounds and there is some evidence that this results in a reduction in the systemic inflammatory response typically associated with major intra-abdominal surgery.289 Many of the randomized studies have shown a reduction in postoperative pain both, both in terms of patient perception and in relation to the requirement for opiate analgesia.290,291 Another feature associated with intraabdominal surgery is a reduction in pulmonary function postoperatively and the resulting risk of postoperative atelectasis and infection which is not seen after laparoscopic resection.292
Cosmesis
Cosmesis is an issue that is of secondary importance compared with operative morbidity and oncologic outcome. Nonetheless, it is an important consideration. Several of the studies have looked at quality of life in the short-term and demonstrated some benefits in relation to laparoscopy; however, this benefit is difficult to measure accurately.293 A systematic review published in 2010 concluded that there was no clinically relevant difference in quality of life between the two approaches.294 Subjectively, it is difficult not to see the cosmetic benefit of a smaller wound.
One criticism of the laparoscopic approach is the increase in cost secondary to longer operating times, and the need for additional equipment and additional training. This must be offset, however, against savings from a shorter hospital stay, reduced analgesic requirements, and the potential for improved quality of life in the short-term. In fact, several studies in the United States looking at laparoscopic resection for benign disease have demonstrated a cost saving.295,296
Conversion to Open Surgical Procedure
Another issue related to laparoscopic surgery is that of conversion to an open procedure. Unsurprisingly, as experience has increased, conversion rates have dropped substantially since the early years when conversion rates as high as 50% were reported. In 2012, Kang and colleagues reported a decrease in conversion rates from 32% to 14% in just longer than 2 years from 2007.297 Initially there were reports of adverse outcomes as a result of conversion, but this was subsequently refuted.298 One important factor is recognition of when a laparoscopic approach is not appropriate.
Laparoscopic Resection Approaches for Colorectal Cancer
Figures 77-20 through 77-23 depict the four classic resections for colon cancer. A right hemicolectomy (Fig. 77-20) is the standard approach for tumors involving the caecum and right colon. The mesocolon and its vascular structures are transected at the base of the mesocolon along the superior mesenteric artery. The ileocolic and right colic arteries are the branches of the superior mesenteric artery that are ligated, along with branches of the middle colic artery. The middle colic artery is usually preserved unless the tumor is located in the hepatic flexure of the colon. In this situation, the resection is extended to include the middle colic artery.
Cancer involving the transverse colon requires mobilization of both the hepatic and splenic flexures. The middle colic artery is transected at the superior mesenteric artery (see Fig. 77-21. When the tumor is located in the splenic flexure region, the surgeon must be more concerned about adequate collateral blood supply through the marginal artery. This concern is increased in elderly patients who have a history of significant vascular disease. Tumors that are located in the left colon are managed by a left hemicolectomy (see Fig. 77-22). The inferior mesenteric artery is ligated near its origin on the aorta. The more distal the location of the cancer, the more mobilization of the upper rectum will be needed. It is not always necessary to mobilize the splenic flexure, but if there is any concern that the anastomosis will be under tension, then it must be performed to provide adequate length. Cancers that involve the sigmoid portion of the colon are resected by what is commonly termed a sigmoid colectomy or a high anterior resection. The more distal sigmoid tumors require resection of the upper rectum to achieve an adequate distal margin.
Figure 77-23 illustrates a subtotal colectomy and ileorectal anastomosis. A subtotal colectomy might be required in patients who harbor synchronous cancer in the right and left colon and patients seen with colon cancer at a young age. Other indications for subtotal colectomy include the presence of an obstructing left-sided cancer, which has led to cecal perforation in the presence of a competent ileocecal valve, extensive diverticular disease, and a right colon lesion with extension into the sigmoid colon or, similarly, a cancer of a redundant sigmoid colon that invades into the right colon.
Restoring Bowel Continuity
The restoration of bowel continuity can be performed in a number of ways. The bowel can be rejoined in an end-to-end, side-to-side, or side-to-end manner. The anastomosis can be fashioned by hand sewing or the use of a stapling device. There is no evidence that one technique is superior to the other. Schwab and colleagues reported the results of an in vitro study comparing initial bursting strength for three different anastomotic techniques.299 These authors performed 10 hand-sutured anastomoses, 10 biofragmentable ring anastomoses, and 10 stapled anastomoses of fresh human colon that was harvested at time of colon cancer surgery. They measured the pressure that was required to burst the anastomosis and found no significant difference. They concluded that because there were no differences, hand-sutured anastomoses were certainly the least expensive and should remain the standard. However, a stapled anastomosis is generally less time-consuming, thus the decision is generally dictated by surgeon preference.
What is of utmost importance in performing an anastomosis is ensuring that there is adequate blood supply, that it is tension free, and that it has an adequate lumen. These conditions reduce the risk of anastomotic dehiscence and the attendant risk of morbidity and mortality. Generally, an anastomotic leak rate of less than 5% is acceptable.300 In addition to technique being critical to the integrity of the anastomosis, factors such as malnutrition, hypoalbuminemia and cardiovascular disease all increase the risk of anastomotic dehiscence.301
Another issue in relation to technique is timing of vascular ligation. Turnbull was one of the early proponents of what is called a “no touch technique.” This technique involves ligation of the lymphovascular pedicle before tumor mobilization to avoid dissemination of tumor cells intraoperatively.302 Although there is evidence that this approach does reduce the number of cells disseminated intravascularly, it has not been demonstrated that this translates into a survival benefit.
Another issue for debate is the use of a cytocidal agent, such as povidone-iodine, for washout of the rectal stump. Theoretically, this could reduce the risk of anastomotic or local recurrence. It is commonly performed by surgeons following anterior resection and before anastomosis of the left colon to the rectal stump. The Association of Coloproctology of Great Britain and Ireland included this recommendation in their 2007 guidelines as good clinical practice. However, this practice is less common in the United States. Although in vitro use of povidone-iodine does effectively kill tumor cells, the presence of blood makes this less effective in vivo.303 In a small study, Agaba and colleagues failed to demonstrate a reduction in local recurrence rates as a result of intraluminal povidone-iodine washout.304
Surgical Management of Lymph Nodes in Colorectal Cancer
It is known, however, that metastases can be found in distant node groups—paraaortic, celiac, and porta hepatis—even when regional nodes appear to be normal. Supporting this observation is a report of administration of 25-mCi 99mtechnetium (Tc)-tagged fab–fragment–antibody to CEA before the patients underwent colon resection. Intraoperatively, a hand-held scintillation probe was used, and surgeons removed all nodes that were identified as suspicious. Seven of 20 patients were upstaged, and metastatic spread was found at distant sites of retroperitoneum and renal hilum.305
The accuracy of regional node analysis and its prognostic value are directly proportional to the thoroughness of the surgical technique in removing all regional nodes and the pathological examination of the resection specimen in identifying and harvesting all regional lymph nodes for microscopic assessment (see “Staging” and Table 77-4). The AJCC and the CAP have recommended examination of at least 12 lymph nodes to assign stage II.306 It has been shown that a minimum of 12 to 18 lymph nodes must be examined to accurately predict regional node negativity in CRC.307–315 For this reason, it has been suggested that 12 lymph nodes be considered the minimal acceptable harvest from a careful specimen dissection, and the National Quality Forum has accepted this recommendation as a quality indicator.316 Increasingly, however, the evidence indicates that the greater the number of nodes that are examined, the greater is the likelihood that metastasis will be found. In one study of T3 tumors, nodal metastases were found in 22% of cases if fewer than 15 lymph nodes were harvested from the specimen, whereas 85% of cases with 15 or greater recovered nodes showed metastasis.307
In a study of more than 2400 pT3 CRCs that were resected at a single institution over 45 years, Goldstein and colleagues showed by mathematical analysis that the predictive probability of identifying a single positive node increased in a continuous manner as the number of recovered lymph nodes increased, suggesting that there is no minimum number of nodes that accurately or reliably stages all patients.309 More importantly, it has been shown that clinical outcome is linked to the lymph node harvest. Numerous studies show that conventional pathological examination of increased numbers of lymph nodes is itself associated with an increased survival advantage in stage II disease,308–314 indicating a positive effect of optimal mesenteric resection by the surgeon, optimal lymph node harvest from the resection specimen by the pathologist, or both.
The large study Intergroup Trial INT-0089, which included 3759 patients with stage III or high-risk stage II disease, showed that overall survival and cause-specific survival both increased as more lymph nodes were examined, suggesting that the number of lymph nodes that are analyzed is itself an independent prognostic variable in CRC.310 In contrast, survival is significantly worse for patients with stage II colon cancer, roughly equivalent to stage III disease, in cases with fewer than 7 to 9 lymph nodes harvested from the resection specimen, suggesting clinically significant understaging of disease when too few nodes are sampled.319–319
Despite the strength of the data demonstrating the critical nature of adequate lymph node assessment in CRC, significant variation in lymph node recovery from resection specimens exists within and among medical centers.322–322 In addition, the average number of lymph nodes examined per specimen is often found to be lower than the minimal recommended number, suggesting that a large number of patients with CRC are staged inadequately.323 The number of lymph nodes that are recovered from resection specimens is dependent on several factors. Surgical technique, surgery volume, and patient factors, such as age and anatomic variation, alter the actual number of nodes in a resection specimen, but the diligence and skill level of the pathology examination in identifying and harvesting lymph nodes in the resection specimen also are major factors.324 Because it has been shown that many nodal metastases in CRC are found in small lymph nodes (<5 mm in diameter), diligent search for lymph nodes is required on gross examination of resection specimens.308
Sentinel node biopsy is an approach that was initially used for melanoma patients and increasingly used for breast cancer patients as a means of improving staging accuracy and reducing the extent of surgical resection needed. However, as previously discussed, sentinel node approaches in CRC have unacceptably high false-negative rates.325 As a result, there is currently no role for sentinel node biopsy in the management of CRC.
Surgical Management of Obstructing Colorectal Cancer
Approximately 20% of patients with a colonic cancer will present as an emergency and bowel obstruction accounts for 80% of these cases.326,327 Most commonly, bowel obstruction is caused by a left-sided tumor but approximately 20% will arise in the right colon. Typically, patients will present with colicky abdominal pain and distention. They may also have a varying degree of alteration in bowel habit and vomiting. A variety of diagnostic investigations are available. These include plain x-ray of abdomen, CT scan of abdomen, and water-soluble contrast enema. It is important to note the diameter of the caecum in this situation; if the ileocecal valve is competent the small bowel will not be distended. In this setting a caecum of greater than 9 to 10 cm is at imminent risk of perforation and intervention should occur without delay. Endoscopy is not recommended as a diagnostic tool in this situation because of the risk of perforation.
• Resection of the tumor containing segment and formation of an end colostomy with the rectal stump being closed off or brought out as a mucous fistula (Hartmann procedure).328 This has the potential for reversal at a later date.
• Resection with on-table lavage of the proximal colon and primary anastomosis. Depending on the physiological state of the patient and the quality of the colon, a defunctioning loop ileostomy may be performed. This is easier to reverse than an end colostomy.
• Subtotal colectomy may be performed if the proximal colon is perforated or ischemic, secondary to the preexisting obstruction. An end ileostomy is then fashioned, or it may be possible to do an ileorectal anastomosis.
• Endoscopic stent placement to relieve the acute obstruction. A planned resection can then be performed once the patient has recovered from the acute event. This also facilitates visualization of the remaining colon in the intervening period, allowing potential synchronous tumors to be picked up.
Although a Hartmann procedure is the treatment of choice in the systemically unwell patient with severe intraabdominal sepsis, it is no longer optimal management in all cases of obstruction. Not only does it necessitate a second operation to restore intestinal continuity, a reversal of the Hartmann procedure is associated with significant morbidity and mortality. A systematic review in 2010, which included more than 6000 patients, confirmed this with morbidity and mortality of up to 50% and 5%, respectively.329 In addition, a recent Spanish multicenter study found that almost 60% of patients having a Hartmann procedure for obstruction never had it reversed.330
Increasingly, there is a move toward performing a primary anastomosis with or without a defunctioning loop ileostomy. In this situation, the proximal colon usually needs to be lavaged either through a proximal enterotomy or the placement of a dedicated lavage device via the distal end. A number of studies confirm the safety of this approach without an increase in the risk of morbidity, and specifically no increase in the rate of anastomotic leak.333–333 Some advocate subtotal colectomy in the setting of obstruction, because it allows for the removal of any synchronous tumors. However, the incidence of such lesions is low at 2% to 4%.334,335 In addition, a randomized study found bowel function after subtotal colectomy and ileorectal anastomosis was generally not as good as after left hemicolectomy.336 To avoid missing any synchronous lesions it is possible to perform on-table colonoscopy after colonic lavage.
Finally, endoscopic placement of a self-expanding metal stent is another option that has become increasingly used in recent years.337–341 By relieving the acute obstruction it can act as a bridge to elective resection for operable tumors, obviating the need for emergency surgery. In doing so, it may facilitate a primary anastomosis and avoidance of a stoma.337,338 Emergency surgery for obstruction is associated with significant morbidity, and mortality in the region of 20%.341–341 However, the use of stents is not without complication and a recent meta-analysis revealed a lower success rate than previously thought and a perforation rate of approximately 6%.337 Tumor perforation is a particular concern given that this automatically converts the tumor stage to a T4 and may influence long-term survival.
Surgical Management with Involvement of Adjacent Organs
When a colon cancer is adherent to an adjacent structure, surgery should involve resection of the attached viscera, partially or fully, in continuity with the tumor. Although it may appear that the tumor is simply adherent to the adjacent viscera via inflammatory adhesions, in more than 50% of cases there will be actual tumor invasion. Colonic tumors most commonly invade the small bowel, urinary bladder, and abdominal wall.342 By performing an en bloc resection, cancer-specific survival is significantly improved. In a follow-up study of more than 150 patients who underwent multivisceral resection for locally advanced disease, no patient who had an R1 or R2 resection survived 5 years.343 In contrast, almost 80% of patients who had an R0 resection survived 5 years. Surgically disrupting these sites of adherence can lead to the spillage of tumor cells into the peritoneal cavity. This spillage accounts for the increase in the risk of recurrence and decrease in survival when this “tumor plane” is disrupted.
When the tumor involves a loop of small bowel, this should be resected in continuity with the tumor. Similarly, a tumor that is adherent to the abdominal wall should be resected along with a portion of the abdominal wall. When involving the bladder, again a partial or possibly even a complete cystectomy is required. Although malignancy accounts for approximately 20% of colovesical fistulae, less than 1% of colonic tumors fistulate to the bladder.344 Depending on the site in the bladder and the extent of bladder involvement, resection may necessitate full cystectomy and formation of an ileal conduit.345
Surgical Management of Perforated Colorectal Cancer
Colonic tumors infrequently present with perforation, with incidence ranging from 2% to 9%.346,347 Perforation secondary to a tumor has a poor prognosis and is associated with greater mortality than colonic perforations caused by other pathologies. Surgical mortality ranges from 30% to 40%.346 The higher mortality is caused by a combination of underlying malignancy and sepsis. Free perforations, in which there is widespread purulent or fecal contamination throughout the peritoneal cavity, occur in one-third of patients. In two-thirds of patients, the perforation is “contained” when there is little in the way of contamination beyond a localized abscess. Patients seen with a perforated colon are often systemically unwell secondary to sepsis, making resuscitation the priority in the first instance.
Historically, the optimal surgical management is resection of the perforated segment with formation of an end colostomy (Hartmann procedure). This procedure remains the treatment of choice in the patient who has widespread contamination and/or is hemodynamically compromised as a result of sepsis. However, similar to cases of obstruction, resection with primary anastomosis and proximal defunctioning is now deemed appropriate in certain cases. Chen and colleagues published data comparing the outcomes for in a group of patients with perforated diverticulitis who were treated with a Hartmann procedure versus primary anastomosis and defunctioning. They demonstrated comparable morbidity and mortality between the two groups, provided anastomosis was performed in a stable patient.348 Although several authors have demonstrated the safety of this approach in perforated diverticular disease,349,350 there is little evidence available regarding its appropriateness in the case of perforated colonic cancer. However, it seems reasonable to apply the same principles to its management.
Surgical Management of the Malignant Colon Polyp
Malignant polyps are defined as adenomas that contain invasive carcinoma which penetrates through the muscularis mucosa into the submucosa. They can be classified as sessile or pedunculated, and are, in essence, pT1 lesions. They do not include polyps that contain intraepithelial or intramucosal carcinoma because these do not carry the potential for metastasis. In various colonoscopic polypectomy series, the incidence of these polyps ranges from 2% to 12%.351,352 With the introduction of screening colonoscopy, the prevalence of these early cancers is likely to increase.353 Two classification systems exist that can assist in determining the likelihood of an individual polyp having associated involved lymph nodes, thus the need for further formal colonic resection can be determined.
The Haggitt classification refers to the depth of invasion, in the stalk of a pedunculated polyp or the submucosa of a sessile polyp. It ranges from 1 to 4. It is estimated that the risk of nodal metastases is less than 1% for Haggitt levels 1, 2, and 3.351,352,354 Consequently, these lesions are potentially adequately treated by polypectomy alone. However, higher rates of lymph node involvement have been reported with Haggitt level 3 lesions.354 Malignant sessile polyps are all Haggitt level 4 and have a much greater incidence of lymph node metastasis, with reports ranging from 10% to 25%.357–357
Kitajima and colleagues described an additional classification system for sessile polyps and those pedunculated polyps that invade the submucosa (Haggitt level 4).358 It is broken down into three groups—SM (submucosa) 1, 2, and 3—depending on the depth of submucosal invasion. The greater the depth, the greater the risk of lymph node metastases. In addition to depth, there are a number of other adverse histologic features which will influence ultimate treatment. These include malignant polyps with pedunculated morphology that have unfavorable histologic criteria (partial polyp resection, poorly differentiated carcinoma, vascular or lymphatic invasion, margin of resection <2 mm, or depth of submucosal invasion ≥3 mm from muscularis mucosae).359,360 For malignant polyps with pedunculated morphology, less than Haggitt level 3 with favorable histologic criteria, polypectomy is considered to be curative; however, close endoscopic surveillance is required.
Surgical Management of Synchronous Metastatic Disease
Approximately 20% of patients with CRC have metastatic disease at the time of diagnosis.361 Metachronous lesions will develop in another 25%. Some authors have suggested that the presence of synchronous lesions predicts a worse outcome than for patients in whom metastases develop at a later date.362,363 The liver is the most common site of distant metastases. Peritoneal disease is rare, but other sites of potential spread include the ovaries, adrenals, lung, bone, brain, and kidneys. Although at one time a diagnosis of liver metastases automatically led to a palliative approach, treatments have now evolved that offer small numbers of these patients a potential for cure.
Surgical Management of Liver Metastases
Patients with liver metastases who have evidence of metastatic disease at other sites are not generally candidates for hepatic resection. However, patients with isolated liver metastases and a resectable primary tumor should be offered the possibility of resection. Approximately 10% to 20% of these patients are candidates for potentially curative liver resection.364 Between 20% and 35% of these patients can expect to live 5 years, and surgical mortality is, in general, less than 5%.361,365–367 Because of its clear impact on overall survival, surgical resection is the treatment of choice when feasible. The criteria for resectability differ between centers and among individual hepatobiliary surgeons. However, absolute resection contraindications include unresectable extrahepatic disease, more than 70% liver involvement, liver failure, and being surgically unfit.368 For resection to be considered, most hepatobiliary surgeons would require radiologic evidence of no tumor involvement in the portal vein, the hepatic artery, major bile ducts, absence of celiac/paraaortic lymph nodes, and adequate predicted functional hepatic reserve following resection.369,370 PET-CT scan is frequently performed to identify extrahepatic unresectable and resectable disease. Preoperative liver MRI and intraoperative ultrasound offer the optimal assessment for the number, size, and proximity of tumors to key vascular and biliary structures. In addition, the primary tumor should have been resected with curative intent.
Although previously only those with unilobar or small volume disease were deemed suitable candidates for resection, the boundaries of what is considered resectable disease continue to expand.371 Table 77-8 lists the current indicators for resection of hepatic CRC metastases. The availability of increasingly effective systemic chemotherapy and biological agents (discussed in detail in “Medical Treatment of Metastatic Colorectal Cancer”) has prompted increasing interest in the preoperative or neoadjuvant use of these agents before liver resection.
Table 77-8
Conversional Versus Modern Indications for Resections of Hepatic Colorectal Cancer Metastases
| Conventional Indications | Modern Approach |
| <4 Metastases, unilobar disease | No limits. Multiple/bilobar metastases acceptable, using neoadjuvant chemotherapy; resection or combined resection–local ablative therapy. |
| Size <5 cm | No limits. |
| No extrahepatic disease | Pulmonary metastases can be resected. |
| Resection margin >1 cm | Resection margin <1 cm managed with ablative therapy of narrow margin (cryosurgery or radiofrequency). |
| Inadequate remnant liver parenchyma | Preoperative portal vein embolization to increase liver remnant volume. |
| Resection of all macroscopic disease | No evidence of disease can be achieved with combination of resection and local ablative therapy. |
| Metachronous liver metastases | Synchronous and metachronous metastases acceptable. |
| Absence of vena cava and hepatic vein confluence invasion | No limits. Caval/hepatic vein resection with reconstruction can be performed. |
| Absence of hepatic pedicle lymph node metastases | In absence of coeliac axis metastases, hepatic pedicle lymph node metastases may be resected for improved 3-year survival. |
Data from Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol 2005;23:8490–9.
Perioperative Chemotherapy
The question as to whether perioperative chemotherapy treatment has an impact on survival in patients undergoing CRC liver metastasectomy was investigated in the European Organization for Research and Treatment of Cancer (EORTC) phase III 40983 (EPOC trial) study. In this trial, 364 patients with up to 4 CRC liver metastases were randomly assigned to perioperative FOLFOX4 (oxaliplatin 85 mg/m2 and LV5FU2), 6 cycles before and 6 cycles after surgery (Chemotherapy Arm), or surgery alone (Surgery Arm); 3-year progression-free survival (PFS) results were presented at the meeting of the American Society of Clinical Oncology (ASCO) in 2007.372 Only 303 patients, evenly balanced between the two Arms, actually underwent resection, and 115 of 151 in the Chemotherapy Arm received postoperative chemotherapy. In the Surgery Arm, 152 patients received resection. The 3-year PFS rate was 28.1% in the Surgery-only Arm versus 35.4% for the patients who were randomly assigned to the Chemotherapy Arm (P = 0.058 for intention-to-treat [ITT] analysis). Excluding the ineligible patients (6%), the PFS rate was 28.1% versus 36.2% (P = 0.041), and for those who actually underwent resection, the 3-year PFS rate was 33.2% versus 42.4% (P = 0.025). The observed median overall survival (OS) was higher in the Chemotherapy Arm (61 months) than in the Surgery Arm (54 months) in all randomly assigned patients, and was 64 months in the Chemotherapy Arm versus 55 months in the Surgery Arm in eligible patients. The 5-year OS rate was 51.2% (Chemotherapy Arm) versus 47.8% (Surgery Arm) (all randomly assigned) and 52.4% (Chemotherapy Arm) versus 48.3% (Surgery Arm) (eligible patients) (hazard ratio [HR] = 0.88; 95% confidence interval [CI], 0.68 to 1.14; P = 0.34) (all randomly assigned).
So, it remains uncertain whether there is a benefit from neoadjuvant chemotherapy treatment compared with initial resection for patients seen with potential resectable CRC liver metastases and the decision is often made on a case-by-case basis. In addition, the optimal chemotherapy regimen to be used before resection of the liver metastases has not been established. In the CRYSTAL study (which is discussed in greater detail in “Monoclonal Antibodies Targeting EGFR–Cetuximab”), the rate of surgery for metastasis and the rate of R0 resection were both higher in patients with KRAS wild-type tumors who received cetuximab plus FOLFIRI compared with FOLFIRI alone (7.9% vs. 4.6%; P = 0.0633 and 5.1% vs. 2.0%; P = 0.0265, respectively).373 In the OPUS trial, when the analysis was limited to patients with KRAS wild-type tumors, resectability was increased from 4% to 10%, but these data are based on very small numbers (3 of 73 with FOLFOX versus 6 of 61 for FOLFOX plus cetuximab).374 Similarly, bevacizumab only improved moderately the resectability of liver metastases when added to XELOX or FOLFOX in a large randomized trial (8.4% vs. 6.1% with chemotherapy alone).375
The role of hepatic intraarterial chemotherapy in conjunction with systemic chemotherapy treatment before liver metastasectomy has only been evaluated in smaller studies.376,377 Although the results of these studies seemed promising, this approach is not commonly used by most clinicians; further large randomized trials are needed to evaluate a potential role of neoadjuvant hepatic intraarterial chemotherapy in the preoperative setting.378
Most patients undergoing liver resection will receive adjuvant chemotherapy treatment. A recent pooled analysis from two phase III studies (Fédération Francophone de Cancérologie Digestive [FFCD] Trial 9002 and the EORTC/National Cancer Institute of Canada Clinical Trials Group/Gruppo Italiano di Valutazione Interventi in Oncologia [ENG] trial), evaluated the role of postoperative chemotherapy (5-fluorouracil [5-FU]+ leucovorin [LV]) following resection of CRC liver or lung metastases.379 A total of 278 patients (Chemotherapy, n = 138; Surgery, n = 140) were included in this analysis. Median PFS was 27.9 months in the Chemotherapy Arm as compared with 18.8 months in the Surgery Arm (HR = 1.32; 95% CI, 1.00 to 1.76; P = 0.058). Median OS was 62.2 months in the Chemotherapy Arm compared with 47.3 months in the Surgery Arm (HR = 1.32; 95% CI, 0.95 to 1.82; P = 0.095). Although statistically not significant, the data provided a proof of principle of benefit for post resection chemotherapy treatment. The chemotherapy used in these studies was considered to be inferior and the more active combination FOLFOX (oxaliplatin plus 5-FU+LV) is now used as adjuvant treatment following resection of CRC liver metastases (see also “Adjuvant Oxaliplatin Combinations”).
Radiofrequency Ablation
Radiofrequency ablation (RFA) is sometimes applied following macroscopically incomplete resection of CRC liver metastases. A recent retrospective review of the RFA literature for CRC liver metastases reported a wide range of 5-year survival (14% to 55%), and local recurrence rates (3.6% to 60%).380 There are no randomized prospective trials in patients with potentially resectable liver metastases, comparing RFA with hepatic resection with or without postoperative chemotherapy treatment. The retrospective comparative series suggest lower local recurrence rates, and better PFS, and, in some cases, better OS, following resection.381 However, these studies are biased, because patients treated with resection alone versus RFA differ across several important clinical and treatment-related variables. Definitive conclusions cannot be made in the absence of prospective randomized trials directly comparing both approaches.
Surgical Management of the Ovaries
Sielezneff and colleagues studied prophylactic oophorectomy in postmenopausal women and found an incidence of only 2.4% having ovarian metastases, with no difference in 5-year survival.382 The Mayo Clinic found no gross or microscopic ovarian involvement in 77 women who were randomly assigned to prophylactic oophorectomy. There was no survival benefit in the oophorectomy arm.383 Overall, the prognosis for those with ovarian metastases is poor, reflecting the presence of metastatic disease. Although a prospective randomized study of prophylactic oophorectomy in CRC did demonstrate a trend toward improved DFS, there was ultimately no statistically significant difference. Currently, there is no evidence to support routine oophorectomy for those patients with a sporadic CRC. However, the risks are greater in HNPCC. Approximately 10% of female carriers will develop ovarian cancer; consequently, prophylactic oophorectomy in this group may be protective.384
Managing Complications of Colorectal Cancer Surgery
Postoperative ileus is one of the most commonly encountered complications following colonic resection. In fact, some degree of ileus is essentially a normal physiological response in most cases, but there is a variation in how clinically significant this is.385 The majority will run a benign self-limiting course. There is no evidence that routine use of a nasogastric tube (NGT) prevents a prolonged ileus. In fact, as discussed previously, early feeding and avoidance of a NGT are key components of most fast track and ERPs. Other contributing factors are avoidance of opiate analgesia and fluid overload.386 The use of such multimodal approaches are associated with a reduction in time to passage of flatus and a shorter length of hospital stay.387 However, in the setting of a prolonged ileus, where the patient has persistent vomiting and is still unable to tolerate oral diet, several days postoperatively, more aggressive measures are appropriate. Importantly, in the first instance, is to exclude an underlying cause, such as a postoperative collection or mechanical obstruction. This is generally best done with the use of CT with oral and intravenous contrast.386 Such patients will generally need a period of fasting and placement of an NGT. The dilemma in these cases is when to instigate total parenteral nutrition. Consensus expert opinion is, that in the absence of preoperative nutritional deficiency, it is reasonable to wait at least 5 days before starting total parenteral nutrition.387,388 Total parenteral nutrition is not without complication, and in particular, there is risk of sepsis from the presence of a central venous catheter to facilitate feeding.
Anastomotic dehiscence is associated with significant morbidity and reported mortality rates range from 6% to 18%.389,390 Although subclinical leaks are probably more common, clinically significant leaks will occur in approximately 4% to 5% of cases.391 The median time of diagnosis is 7 days postoperatively. Anastomotic leak is associated with a poor function in the longer term, as well as increasing the risk of a permanent stoma.392 Patients with an anastomotic leak can present in a variety of ways ranging from severe sepsis with generalized peritonitis, to simply having a prolonged ileus or onset of an arrhythmia such as atrial fibrillation. It is important to have a low index of suspicion to diagnose a leak early, allowing for it to be managed expediently. Although some patients will require reexploration, take down of the anastomosis, and formation of a defunctioning stoma, many can be managed conservatively. Those in whom the leak is “contained,” both clinically and radiologically, can often be managed with intravenous antibiotics or radiologically guided percutaneous drainage if there is evidence of a collection.
Wound infections are a common postoperative problem after colonic resection. Risk factors for wound infection may be patient-dependent or related to operative technique and extent of surgery. Patient factors include obesity, smoking,393,394 diabetes mellitus, and immunosuppression. Poor surgical technique and fecal contamination also increase the risk.395 Currently, wound infection rates vary from 5% to 10%. In the elective setting, the infection rate should be less than 5%. This reduction from historically higher rates is associated with the perioperative administration of antibiotics. Many centers now routinely administer a single dose of a broad-spectrum antibiotic, at the time of induction of anesthesia. There is no benefit from the routine administration of additional postoperative doses.396
Wound infections typically present around the fifth postoperative day with swelling and erythema of the wound with or without systemic signs of sepsis. If there is evidence of cellulitis, intravenous antibiotics should be administered. In some cases, it may be necessary to remove several sutures or staples from the skin to allow drainage of a subcutaneous fluid collection. This type of wound can then generally be managed with daily packing and allowed to heal by secondary intention. If there is a more extensive superficial wound dehiscence with a large volume exudate, it may be appropriate to use a negative pressure vacuum dressing to allow more efficient management of the wound discharge and expedite healing.397 If there is concern that the underling muscle sheath is not fully intact, the use of a vacuum assisted device should be avoided because of the potential risk of forming an enterocutaneous fistula. From the available heterogenous studies, the risk seems to range between 6% and 25%.398 Wound dehiscence is uncommon, but the risk is significantly increased for those undergoing emergency surgery for colon cancer.399
Critical to improving a patient’s outcome in CRC is the surgeon’s training, experience, and operative volume. One such report involved a retrospective analysis of 15,427 admissions in northern Illinois undergoing segmental colon resection between 1994 and 1997 performed at 76 nonfederal hospitals with 514 operating surgeons. The authors determined inpatient mortality, complications of surgery, and hospital length of stay. They found that American Board of Surgery certification and increasing years of experience were associated with reduced mortality. Added colorectal certification and site of training did not factor significantly into the outcomes.400 In contrast, the COST, which was a randomized, controlled trial examining laparoscopic versus open surgery for colon cancer, did not demonstrate any difference in complications or long-term outcomes based on surgical volume.401 But when looking at outcomes in relation to rectal cancer, Borowski and colleagues did demonstrate improved cancer survival for those having their surgery in high volume centers.402
Managing Uncommon Colonic Tumors
GISTs are a type of mesenchymal tumor, previously more commonly known as leiomyomas and leiomyosarcomas. They originate from the interstitial cell of Cajal, the “pacemaker cells” of the gastrointestinal tract, located in the muscularis propria. They all have malignant potential; between 10% and 30% of GISTs are overtly malignant. Tumors less than 2 cm are almost always benign.403 More than 90% have a mutation in the c-kit protooncogene, with the remaining 10% having a mutation in platelet-derived growth factor receptor α (PDGFRα). The mean age of diagnosis is 60 years and GISTs are more common in males. The main criteria that aid in the determination of risk of recurrence and prognosis are, site, size, and mitotic index.404 Tumors less than 2 cm with a mitotic index of less than 5 mitoses per 50 high-powered fields have virtually no risk of recurrence. Unless the tumor is not amenable to resection, preoperative biopsy should be avoided because of the potential risk of bleeding and potential for seeding of tumor cells.405
Historically, the mainstay of treatment was resection with a reported 5-year survival rate of 28% to 35%,406 largely because of the high recurrence rate even with an R0 resection. However, a Japanese study of 60 patients who had a laparoscopic or laparoscopic-assisted resection demonstrated a 5-year survival rate of 89% to 100% for those with tumors between 2 and 5 cm.407 The introduction of imatinib has contributed significantly to the improvement in outcome for metastatic tumors and those not amenable to resection.408
There is also evidence for the use of imatinib as an adjuvant treatment for those tumors with a high risk of recurrence. Colonic GISTs are relatively uncommon, accounting for less than 5% of cases.409,410 Low-risk colonic GISTs generally have a good prognosis after complete resection.411 Those deemed irresectable may undergo downstaging first with a imatinib before attempted resection or debulking.410
Carcinoid tumors of the colon are rare and account for less than 8% of all gastrointestinal carcinoids.413–413 They are neuroendocrine tumors that originate in the Kulchitsky cells of the crypts of Lieberkühn and belong to the amine precursor uptake and decarboxylation system. The majority of colonic carcinoids arise in the caecum and ascending colon and most will require colonic resection.414 Five-year survival rate is approximately 70%, but drops to less than 30% in the presence of distant disease. Less than 10% of these patients will present with carcinoid syndrome.414 Surgery remains the only potentially curative treatment for colonic carcinoid tumors with no distant metastases. If the tumor is not amenable to resection, palliative debulking procedures or intestinal bypass of the tumor can be performed.415
Surgical Management of Tumors of the Appendix
Approximately 1% of appendicectomy specimens contain a neoplasm. Eighty percent to 90% of these are carcinoids, with the remaining 10% to 20% including mucinous cyst adenoma, adenocarcinoma, lymphosarcoma, paraganglionoma, and GISTs.416 Most present as acute appendicitis, the tumor leading to occlusion of the appendix lumen in the same way as a fecalith. The finding of a tumor is usually an incidental one, as a consequence of histologic examination of the surgical specimen.
Carcinoids, which account for 50% to 75% of appendiceal tumors, typically occur at the tip, but can also arise at the base of the appendix. Generally they have low-grade malignant potential and outcomes are good. For tumors with a diameter smaller than 1 to 2 cm, appendicectomy is considered an adequate treatment. However, tumors with diameters larger than 2 cm should be treated with subsequent right hemicolectomy because of the risk of lymph node metastases.417 Adenocarcinoma of the appendix should similarly be treated with right hemicolectomy. Prognosis is typically worse when compared with that for a primary colonic adenocarcinoma. This is because the tumor has often spread to the peritoneal surface by the time of presentation.
Mucinous cyst adenocarcinoma is an uncommon tumor of the appendix that is potentially difficult to manage if it ruptures. When this occurs, it leads to the development of Pseudomyxoma peritonei, which is characterized by the deposition of mucin pools containing tumor cells.418 Pseudomyxoma peritonei can also arise from mucinous ovarian tumors. Patients with this condition typically are seen with a distended abdomen caused by mucinous ascites. Aggressive accumulation of malignant mucin in the peritoneal cavity can lead to intestinal or ureteric obstruction, malnutrition and respiratory compromise.
Management of Pseudomyxoma peritonei is best performed in specialist centers. The now well-established Sugarbaker technique,418 involves complete surgical tumor removal (also known as complete cytoreduction), combined with intraoperative heated chemotherapy, followed by postoperative intraperitoneal chemotherapy. The operation takes approximately 10 hours to complete and includes:
• Removal of the right hemicolon, spleen, gall bladder, greater omentum, and lesser omentum
• Stripping of the peritoneum from the pelvis and diaphragm
• Stripping of tumor from the surface of the liver
One group has reported complete cytoreduction in 66% of patients (n = 289) with Pseudomyxoma peritonei, secondary to a mucinous appendiceal tumor. The reported 5- and 10-year survival rate was 87% and 74%, respectively. This outcome was compared with a poor outcome of 34% (5-year survival) and 3% (10-year survival) for debulking only.419 When complete cytoreduction is not possible, there may still be some benefit from debulking surgery. The majority of patients will be suitable for one of these two approaches.420