Classification and general concepts of CNS neoplasms
CNS NEOPLASMS
Primary CNS neoplasms account for:
Most primary CNS neoplasms are neuroepithelial (Table 34.1). The proportion of CNS neoplasms that is due to spread from a primary neoplasm outside the nervous system varies greatly (14–40%) between reports. Selection bias confounds many epidemiologic studies that rely solely on necropsy data or series from tertiary referral centers.
Most primary CNS neoplasms are sporadic and of unknown etiology. Fewer than 5% are associated with hereditary syndromes that predispose to neoplasia (Table 34.2). Other factors (Table 34.3) are implicated in only a small proportion of cases.
Table 34.3
Factors in the etiology of CNS neoplasms
| 1a | Sex: gliomas are commoner (60:40) in men meningiomas are commoner (67:33) in women |
| 1b | An association exists in women between the development of breast carcinoma and meningioma; both tumors may express sex hormone receptors |
| 2 | Exposure to ionizing radiation has been implicated in the genesis of: meningiomas gliomas nerve sheath tumors meningeal sarcoma |
| 3a | Primary CNS lymphoma is associated with immunodeficiency |
| 3b | Epstein–Barr virus has been found in a very high proportion of primary CNS lymphomas from immunocompromised patients |
| 4 | Nitroso compounds, particularly nitrosoureas, cause CNS neoplasms in experimental animals, yet evidence to implicate these compounds in the genesis of human CNS neoplasms has not been forthcoming |
| 5 | There appears to be an inverse relationship between allergies/autoimmune disease and gliomas. Among autoimmune disorders, diabetes and asthma have the most consistent (negative) relationship |
| 6 | No convincing evidence has linked neoplasms with: trauma occupation diet electromagnetic fields cellular phones |
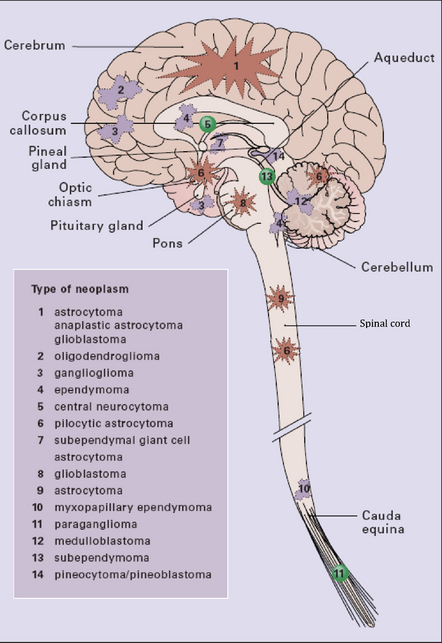
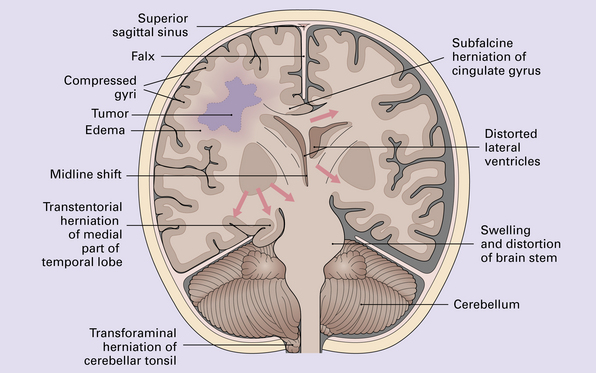
The clinical presentations of CNS neoplasms depend largely on their site (Fig. 34.1) and nature (see Table 34.1). Terminal events are usually related to raised intracranial pressure (Figs 34.2, 34.3).
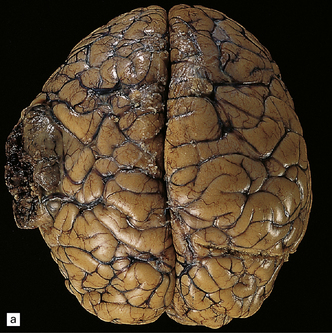
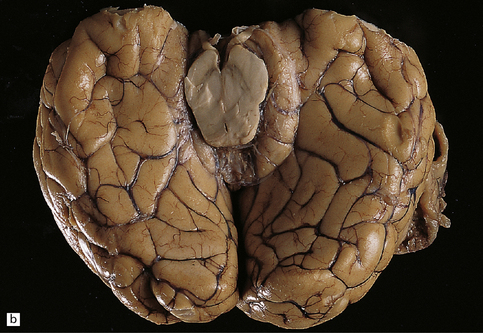
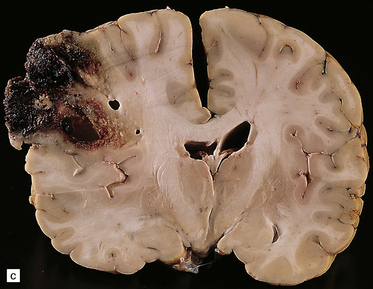
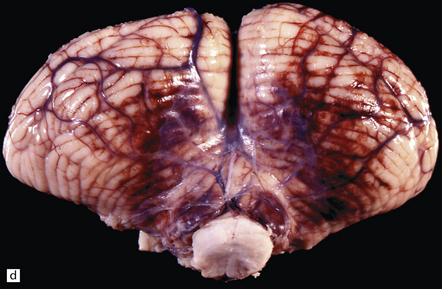
34.2 Raised intracranial pressure associated with a primary CNS neoplasm.
(a) Fatal brain swelling associated with a large left frontoparietal glioblastoma and resulting in both external herniation of involved cerebral tissue and compaction of cerebral gyri. Brain weight was 1680 g. (b) Examination of the undersurface of the brain revealing mild herniation of the right parahippocampal gyrus and marked herniation of the left uncus and parahippocampal gyrus. (c) A coronal section showing the hemorrhagic glioblastoma and asymmetric swelling of the cerebrum. External herniation, displacement of midline structures to the right, downwards displacement of the diencephalon, and distortion of part of the left cingulate gyrus secondary to subfalcine herniation are all evident. Grooves are present (arrows) on the inferomedial aspect of the temporal lobes where these have been indented by the edge of the tentorium. (d) The cerebellar tonsils and swollen brain stem have become compacted in the foramen magnum in this example of tonsillar herniation. Hemorrhage has occurred in the compressed tonsils and spread to the subarachnoid space.
THE PATHOLOGIST AND CNS NEOPLASMS
The clinical details that may help the pathologist to narrow the differential diagnosis include:
 family history (e.g. of syndromes predisposing to CNS neoplasms)
family history (e.g. of syndromes predisposing to CNS neoplasms)
 relevant medical history (e.g. of neoplasia)
relevant medical history (e.g. of neoplasia)
At operation, neurosurgeons can provide information about the macroscopic appearance of a neoplasm and its relationship to adjacent normal structures. It may not be possible to preserve this relationship during surgery and the pathologist is often presented with tissue fragments, which can be interpreted only in the light of an explanation from the surgeon as to their anatomic interrelationship. Useful histologic information, even a firm diagnosis, may be obtained intraoperatively by examination of touch preparations, smears, or cryostat sections (Table 34.4).
Table 34.4
Perioperative pathologic assessment of CNS mass lesions
The aims of a perioperative pathologic assessment of CNS mass lesions are to establish whether:
 sufficient material has been obtained for diagnostic purposes (including supplementary studies, such as electron microscopy)
sufficient material has been obtained for diagnostic purposes (including supplementary studies, such as electron microscopy)
 a neoplastic process is present
a neoplastic process is present
 the course of the operation will be determined by a particular diagnosis
the course of the operation will be determined by a particular diagnosis
 total removal of a neoplasm has been achieved, where this is desired
total removal of a neoplasm has been achieved, where this is desired
Fresh tissue may be examined by three methods:
Touch preparation
A fragment of tissue is dabbed several times on a slide, the preparation is air-dried, and can be stained by various methods (hematoxylin and eosin or toluidine blue or Giemsa). This approach is valuable if only a limited quantity of tissue is available
Smear
A small quantity of tissue is squashed between two slides (Fig. 34.6), and the material is smeared across the touching surfaces of the two slides before brief fixation in alcohol. The tissue is then stained with toluidine blue or hematoxylin and eosin.
Smear preparations are particularly good for assessment of glial tumors
Frozen section
Tissue is snap-frozen in liquid nitrogen, 5 μm sections are cut on a cryostat, and subsequently stained. Hematoxylin and eosin is appropriate for staining frozen sections, but a hematoxylin/van Gieson or a reticulin preparation takes little additional time and may be a useful adjunct.
Frozen sections are particularly good for firm specimens that are difficult or impossible to smear. These include many vasoformative neoplasms and craniopharyngiomas, and some schwannomas and meningiomas.
Freshly resected tissue should be regarded as potentially infectious and handled in a safety cabinet. If the clinical details suggest a significant risk of infection from the specimen, avoid using a cryostat. The preparation of tissue smears in a cabinet carries few hazards and is likely to provide the required information
More detailed examination of the tissue, including immunohistochemistry and electron microscopy, may be undertaken later after it has been fixed. Immunohistochemistry may aid the pathologist by demonstrating the production of proteins indicative of cellular ontogeny or proliferative activity (Figs 34.4, 34.5).
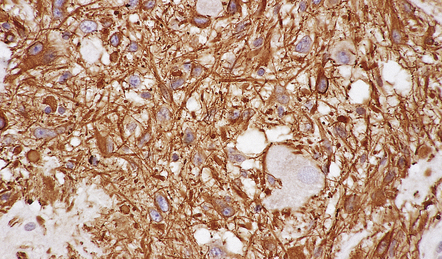
34.4 Immunohistochemistry reveals protein production indicative of cellular ontogeny.
Astrocytic cells in a ganglioglioma are highlighted with an antibody to GFAP.
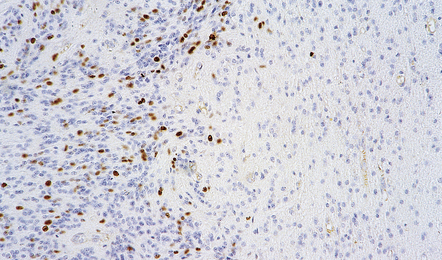
34.5 Immunohistochemistry reveals protein production indicative of proliferative activity.
The growth fraction in different regions of this ependymoma can be assessed with an antibody to Ki-67.
neoplasms. The impact of this on diagnosis and therapy in the future will almost certainly increase. Although molecular genetic analysis is often feasible on paraffin-embedded formalin-fixed tissue, frozen tissue should be retained if sufficient material is available, particularly if there are unusual clinical features or a family history of neoplasia.
Necropsy examination of CNS neoplasms (Table 34.5) provides information on the histologic aspects of the entire neoplasm, the interaction between neoplastic cells and surrounding normal tissues, and the effects of treatment (Table 34.6).
Table 34.5
Necropsy examination of a patient with a CNS neoplasm
A general rather than limited (CNS) examination is desirable, looking for:
 evidence of systemic metastasis from a primary CNS neoplasm
evidence of systemic metastasis from a primary CNS neoplasm
 the location of a primary systemic neoplasm when the CNS neoplasm is secondary
the location of a primary systemic neoplasm when the CNS neoplasm is secondary
 stigmata of any hereditary disorder that has induced a CNS neoplasm
stigmata of any hereditary disorder that has induced a CNS neoplasm
 endocrine effects associated with a pituitary adenoma/sellar tumor (hypopituitarism)
endocrine effects associated with a pituitary adenoma/sellar tumor (hypopituitarism)
The relationship between any CNS neoplasm and adjacent bony structures should be examined
A sellar neoplasm should be studied after removal of the pituitary fossa en bloc The whole specimen can be examined histologically after decalcification
A neoplasm in the auditory meatus should be studied after removal of a wedge of petrous temporal bone containing the internal auditory meatus and inner ear (Fig. 34.7)
The brain and spinal cord should each be removed intact, and fixed by suspension in an appropriate fixative (e.g. 10% neutral buffered formalin) after the fresh brain has been weighed
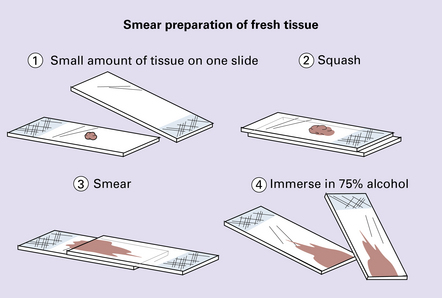
34.6 Smear preparation of fresh tissue.
The preparations can be stained with either toluidine blue or hematoxylin/eosin.
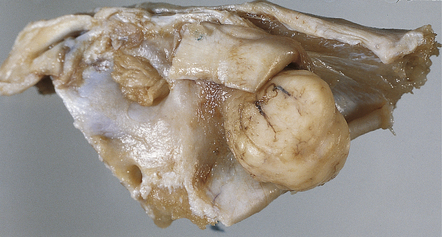
34.7 Assessment of the relationship of a neoplasm to adjacent bony structures.
A wedge of temporal bone containing the internal auditory meatus and showing a schwannoma of the 8th cranial nerve.
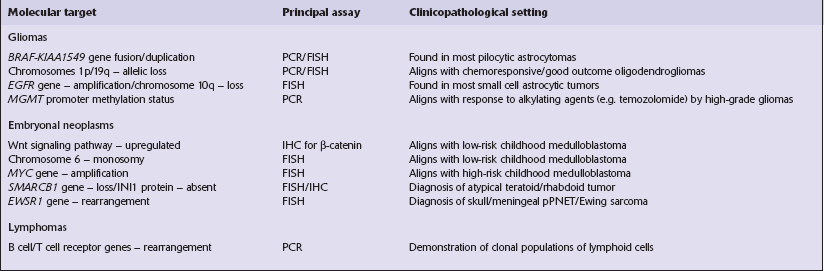
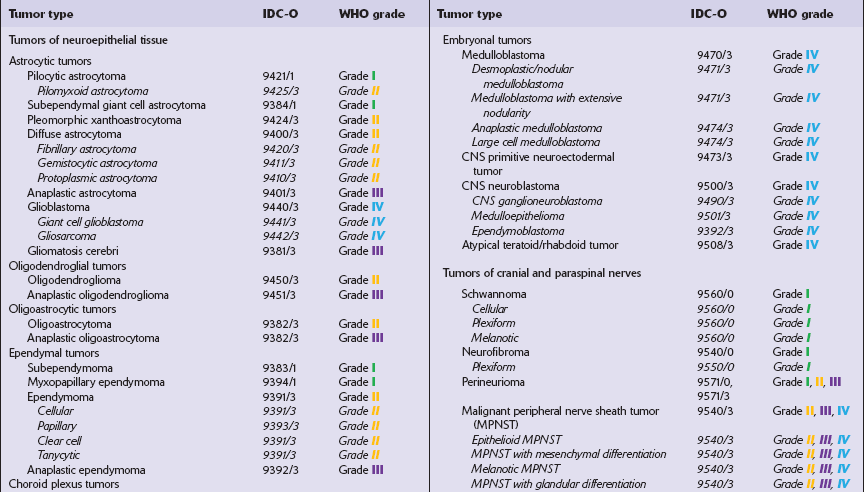
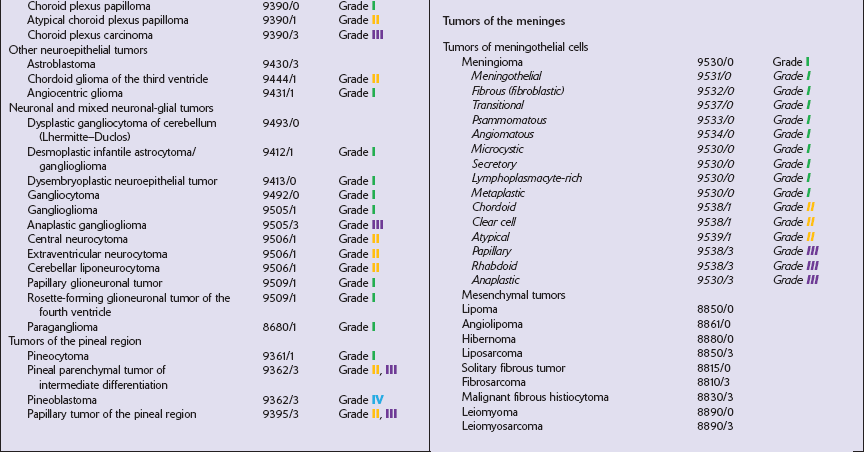
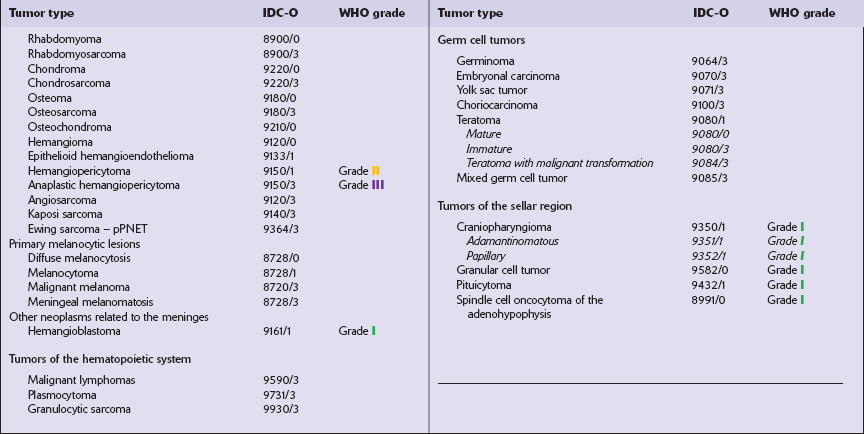
The most practical way for a pathologist to provide information about a neoplasm’s histopathology and anticipated behavior is to conform to a recognized scheme of classifying CNS neoplasms. The World Health Organization’s classification of CNS neoplasms (Table 34.7) is the product of international consensus and combines elements of classifications based on ontogenesis (e.g. that of Bailey and Cushing) and measures of cytologic atypia (e.g. that of Ringertz).
REFERENCES
Asa, S.L. Tumors of the pituitary gland. American Registry of Pathology; 2011.
Brainard, J.A., Prayson, R.A., Barnett, G.H. Frozen section evaluation of stereotactic brain biopsies: diagnostic yield at the stereotactic target position in 188 cases. Arch Pathol Lab Med.. 1997;121:481–484.
Burger, P.C. Smears and frozen sections in surgical neuropathology: A manual. PB Medical Publishing LLC; 2009.
Burger, P.C., Scheithauer, B.W., Vogel, F.S. Surgical pathology of the nervous system and its coverings. London: Churchill Livingstone; 2002.
Kohler, B.A., Ward, E., McCarthy, B.J., et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst.. 2011;103(9):714–736.
Langford, L.A. Central nervous system neoplasms: indications for electron microscopy. Ultrastruct Pathol.. 1996;20:35–46.
Louis, D.N., Ohgaki, H., et al. WHO Classification of tumours of the central nervous system. Geneva: World Health Organization; 2007.
Louis, D.N., Ohgaki, H., et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl).. 2007;114:97–109.
Louis, D.N., Reifenberger, G., et al. Tumours: introduction and neuroepithelial tumours. In: Love S., Louis D.N., Ellison D.W., eds. Greenfield’s neuropathology. London: Hodder Arnold, 2008.
Osborn, A.G., Salzman, K.L., Thurnher, M.M., et al. The New World Health Organization Classification of central nervous system tumors: what can the neuroradiologist really say? AJNR Am J Neuroradiol.. 2011. [Epub:21835942].
Perry, A., Brat, D.J. Practical surgical neuropathology. London: Churchill Livingstone; 2010.
Scheithauer, B.W. Development of the WHO classification of tumors of the central nervous system: a historical perspective. Brain Pathol.. 2009;19:551–564.
Scheithauer, B.W., Burger, P.C. Tumors of the central nervous system. American Registry of Pathology; 2007.
Scheithauer, B.W., Fuller, G.N., VandenBerg, S.R. The 2007 WHO classification of tumors of the nervous system: controversies in surgical neuropathology. Brain Pathol.. 2008;18:307–316.