Chapter 31 Cholecystitis
Acute Cholecystitis
Pathogenesis
The cause of acute cholecystitis is an impacted gallstone in the outlet of the gallbladder, either in the infundibulum or in the cystic duct (Sjodahl et al, 1978). The impacted gallstone results in gallbladder distension and edema with acute inflammation, which eventually can result in venous stasis and obstruction, followed by thrombosis of the cystic artery. Ultimately, ischemia and necrosis of the gallbladder occur. Because the fundus of the gallbladder is the greatest distance from the cystic arterial blood supply, it is more sensitive to ischemia and is the most common location for necrosis of the gallbladder. The acute inflammation of cholecystitis may be complicated by secondary biliary infection. Positive biliary cultures are found in about 20% of patients with acute cholecystitis (den Hoed et al, 1998), the most common of which are gram-negative bacteria of gastrointestinal origin, such as Klebsiella spp. and Escherichia coli.
Diagnostic Evaluation and Imaging
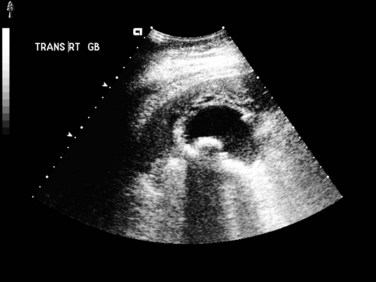
Abdominal US (see Chapter 13) is useful for assessing patients suspected to have acute cholecystitis. Typical findings include gallstones, gallbladder wall thickening (>4 mm), and pericholecystic fluid (Fig. 31.1). In addition, the sonographer can assess for pain and inspiratory arrest when the gallbladder is directly compressed by the US probe (sonographic Murphy sign). Typically, conventional gray scale imaging is used, which is highly sensitive and specific for diagnosing acute cholecystitis, with an overall accuracy of greater than 90% (Soyer et al, 1998). Other techniques of imaging that assess blood flow, such as color velocity imaging, may improve accuracy in selected cases (Soyer et al, 1998; Uggowitzer et al, 1997).
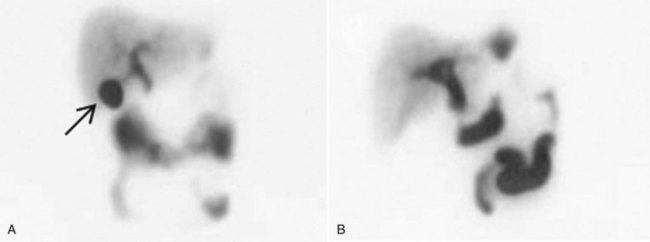
Hepatobiliary scintigraphy (see Chapter 15) is a useful study in selected patients when the diagnosis is uncertain. This nuclear medicine study is performed with derivatives of aminodiacetic acid (hepatoiminodiacetic acid [HIDA], isopropylacetanilido imidodiacetic acid [PIPIDA], or di-isopropylacetanilido iminodiacetic acid [DISIDA]), which are taken up by hepatocytes and secreted in the bile. When the tracer is labeled with technetium, it is possible to visualize biliary function with scintigraphy. A normal scan delineates the biliary tree, including the gallbladder, and shows prompt emptying of the agent into the duodenum. Nonvisualization of the gallbladder on hepatobiliary scan is consistent with acute cholecystitis (Fig. 31.2).
An added utility of this test is that in addition to gallbadder filling or nonfilling, it also evaluates gallbladder emptying. Abnormal emptying is defined as an ejection fraction (EF) of 35% or less on scintigraphy, a finding suggestive of biliary pathology. Middleton and Williams (2001) reported on 141 patients with normal US and scintigraphy with normal gallbladder filling and an EF of 35% or less who underwent cholecystectomy. Of these patients, 95% had significant improvement or complete resolution of symptoms after surgery, and 41% of these patients had cholecystitis on histologic evaluation of the gallbladder.
Hepatobiliary scintigraphy is not useful in patients with poor hepatic function because it requires hepatic excretion of bile, but it is accurate in about 90% of patients and may be more accurate than US alone. Hepatobiliary scintigraphy is more involved, more expensive, and requires a longer time than US; however, it should be reserved for selected cases (Chatziioannou et al, 2000; Kalimi et al, 2001). Mahid and colleagues (2009) have suggested a diagnostic approach that starts with US for patients with biliary symptoms. If no gallstones are definitively identified, this should be followed by esophagogastroduodenoscopy to exclude alternative causes of symptoms, such as peptic ulcer disease or gastritis. If this test result is negative, hepatobiliary scintigraphy should follow.
Computed tomography (CT; see Chapter 16) can also help diagnose acute cholecystitis and provides more detailed anatomic information than US. CT is particularly useful in patients whose symptoms suggest a complication such as pericholecystic abscess or an alternative diagnosis. The CT findings of acute cholecystitis are the same as those seen on US and include wall thickening, pericholecystic stranding, distension of the gallbladder, high-attenuation bile, pericholecystic fluid, and subserosal edema. CT generally is less sensitive than US for diagnosing acute cholecystitis, particularly early in the course, when the imaging findings may be subtle (Fidler et al, 1996; Harvey & Miller, 1999).
Treatment
The definitive treatment for acute cholecystitis is cholecystectomy. From the time this operation was first performed in 1882 by Langenbuch, open cholecystectomy has been the standard of care for patients with acute cholecystitis. With the advent of laparoscopic cholecystectomy in the 1980s, the standard approach has changed such that cholecystectomy is now routinely performed laparoscopically. The benefits of laparoscopic cholecystectomy are discussed in depth elsewhere (see Chapters 33 and 34), but they include a shorter postoperative stay and decreased analgesia requirements (Cox et al, 1993). Although the laparoscopic approach is now standard for most cases, it is interesting to note that two prospective, randomized studies suggested little or no difference in several outcome measures between laparoscopic versus small-incision open cholecystectomy (Majeed et al, 1996; Keus et al, 2008).
Early analysis of the results of laparoscopic cholecystectomy in patients with acute versus chronic cholecystitis showed increased morbidity and mortality rates for patients with simple or complicated acute cholecystitis. Because of the increased morbidity and mortality, acute cholecystitis initially was considered a relative contraindication to laparoscopic cholecystectomy (Flowers et al, 1991). Subsequent reports have shown the improved safety of this technique in the acute setting (Chandler et al, 2000; Johansson et al, 2003; Lai et al, 1998; Lo et al, 1998). The conversion rate to an open procedure is higher for patients with acute cholecystitis compared with patients undergoing elective operations for simple biliary colic (Schirmer et al, 1991), but most patients with acute cholecystitis (>80%) can undergo laparoscopic cholecystectomy successfully (Papi et al, 2004). Retrospective series revealed that risk factors for conversion to open cholecystectomy include obesity (Rosen et al, 2002), elevated white blood cell count (Alponat et al, 1997; Kanaan et al, 2002), and male gender (Kanaan et al, 2002).
Other novel surgical techniques have been proposed to treat patients with acute cholecystitis, including so-called minilaparoscopic cholecystectomy (see Chapter 33), which uses 2- to 3-mm ports (Hsieh, 2003), and minicholecystectomy (Assalia et al, 1997), in which a small (mean, 5.5 cm) incision is used to remove the gallbladder. A prospective, randomized trial demonstrated that minilaparoscopic cholecystectomy resulted in decreased postoperative pain and superior cosmesis when compared with conventional laparoscopic cholecystectomy, with no significant difference in operative time, blood loss, or complications (Novitsky et al, 2005).
Laparoscopic subtotal cholecystectomy (LSC) has also been evaluated as a means of decreasing the conversion rate to open procedure in patients with acute cholecystitis (Horiuchi et al, 2008; Singhal et al, 2009). This procedure involves leaving the posterior wall of the gallbladder when it is significantly difficult to dissect from the liver bed, with cauterization of the remnant mucosa and suturing or stapling of the gallbladder neck in cases of severe inflammation of the triangle of Calot. Indeed, in the face of severe inflammatory change, such an approach is safter than risking injury to the common hepatic duct by pursuing dissection within the porta hepatis. Horiuchi and colleagues (2008) demonstrated a significant decrease in conversion rate to open procedure with the use of these techniques with no increase in postoperative complications. All patients undergoing LSC had temporary subhepatic drains placed in this study. This technique, although innovative, does not have wide clinical applicability because of limited availability of instruments, specialist surgeons trained in their use, or both. Laparoscopic cholecystectomy remains the standard therapy for definitive treatment of patients with acute cholecystitis, with conversion to an open procedure if necessary.
In patients with a high perioperative risk from sepsis or other underlying medical comorbidities, initial treatment of acute cholecystitis with percutaneous cholecystostomy tube placement is preferred (see Chapters 28 and 32). These tubes can be placed using either US or CT guidance (Hatzidakis et al, 2002). This procedure effectively decompresses the gallbladder, evacuating the infected bile and relieving the pain associated with gallbladder distension, and it is associated with a low complication rate (Akyürek et al, 2005; Byrne et al, 2003; Spira et al, 2002; Werbel et al, 1989). In addition, most patients (>80%) improve clinically within a short time (Byrne et al, 2003; Hatzidakis et al, 2002; Vauthey et al, 1993). After stabilization of the patient, and if the clinical situation otherwise warrants, delayed cholecystectomy should be performed, which often can be done laparoscopically (Spira et al, 2002). Akyürek and colleagues (2005) demonstrated decreased hospital stay and cost in high-risk patients undergoing percutaneous cholecystostomy followed by early laparoscopic cholecystectomy compared with those treated conservatively with intravenous (IV) antibiotics and bowel rest followed by delayed cholecystectomy. In high-risk patients in whom general anesthesia is contraindicated, percutaneous stone extraction has been used successfully (Gibney et al, 1987; Wong et al, 1999). More recently, single and repeated percutaneous transhepatic gallbladder aspiration has been shown to be successful in high-risk patients with acute cholecystitis who do not respond to conservative therapies (Tsutsui et al, 2007). This treatment modality is not widely practiced, however.
Timing of Surgery
The optimal interval of time between the diagnosis of acute cholecystitis and definitive treatment with cholecystectomy has been the subject of many prospective randomized trials, nine evaluating open cholecystectomy and five evaluating laparoscopic cholecystectomy (Papi et al, 2004; Siddiqui et al, 2008). The concern in operating on patients with early cholecystitis (typically defined as <3 days) is the potential for increased postoperative complications, including common bile duct injury. The risk of performing cholecystectomy late (weeks after the diagnosis of cholecystitis) is that a subset of patients have recurrent symptoms during the period between diagnosis and surgical treatment, which leads to recurrent hospital admissions and urgent surgery (Papi et al, 2004). In multiple randomized prospective trials evaluating the timing of open cholecystectomy, patients undergoing early operation experienced no increased perioperative morbidity or mortality and had a shorter length of hospital stay compared with patients undergoing delayed operation (Norrby et al, 1983; Van der Linden & Edlund, 1981). In addition, a meta-analysis of these trials demonstrated that more than 20% of patients failed medical management while awaiting definitive treatment, and approximately half of these patients required urgent surgical treatment as a result (Papi et al, 2004). In this same analysis, no increase in morbidity was seen in patients undergoing early definitive treatment with laparoscopic (P = .6) or open (P = .2) cholecystectomy compared with delayed treatment, but a clear difference was seen in length of hospital stay, with patients undergoing delayed treatment requiring a more prolonged hospitalization (Papi et al, 2004). Although it is safe to perform cholecystectomy either early or late after an episode of acute cholecystitis, patients benefit when surgery is performed early.
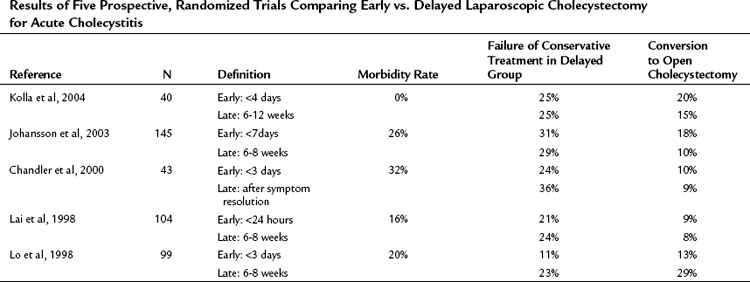
Performing laparoscopic cholecystectomy in patients with acute cholecystitis poses unique challenges to surgeons because of the potential for increased morbidity, including the most feared complication, common bile duct injury. The concern is that the acute inflammation may obscure the anatomy, leading to an increase in postoperative complications. Five prospective randomized trials have evaluated the outcome of patients with acute cholecystitis undergoing early versus late laparoscopic cholecystectomy (Table 31.1). Although a significant increase in operation time was experienced for those undergoing early compared with delayed cholecystecomy (P = .002), the results of these trials uniformly showed no significant difference in postoperative morbidity or mortality, including common bile duct injury, when surgery is performed early (Siddiqui et al, 2008). Additionally, no significant difference was found in the conversion rate to open cholecystectomy, although it was clearly higher (20% to 30%) in patients with cholecystitis compared with prior studies evaluating patients undergoing elective laparoscopic cholecystectomy in the nonacute setting. Perhaps the most important finding was that in all but one study, patients randomized to late cholecystectomy failed conservative management in 15% to 30% of cases. Although patients in the early group generally experienced a longer postoperative hospitalization (P = .004), most of these trials demonstrated a decrease in overall length of hospital stay in the early group versus the delayed group (cumulative P < .001) (Chandler et al, 2000; Johansson et al, 2003; Kolla et al, 2004; Lai et al, 1998; Lo et al, 1998). Early laparoscopic cholecystectomy is therefore the preferred surgical technique for patients with acute cholecystitis. Catena and colleagues (2009) have proposed the use of a harmonic scalpel for improved hemostasis and biliostasis in laparoscopic cholecystectomy, and preliminary data suggest it may decrease the conversion rate to open procedure in patients undergoing laparoscopic cholecystectomy for acute cholecystitis. A prospective, randomized controlled trial is underway to compare the use of harmonic versus monopolar diathermy for laparoscopic cholecystectomy (Catena et al, 2009).
Table 31.1 Results of Five Prospective, Randomized Trials Comparing Early vs. Delayed Laparoscopic Cholecystectomy for Acute Cholecystitis
The majority of trials of early versus delayed laparoscopic cholecystectomy define “early” as within 72 hours of symptom onset. A recent nonrandomized, prospective study by Tzovaras and colleagues (2006) assessed 129 patients undergoing laparoscopic cholecystectomy for acute cholecystitis during the index admission. The patients were divided into three groups regarding the timing of their surgery from symptom onset: within 3 days, between 4 and 7 days, and after 7 days. They found no significant difference in conversion rate, morbidity, or postoperative hospital stay among these groups and thus suggest that the benefits of early cholecystectomy are not limited to patients who present within 72 hours of symptom onset.
Another factor to consider is whether early or delayed laparoscopic cholecystectomy for acute cholecystitis is more cost effective. A meta-analysis of randomized and other studies performed by Lau and colleagues (2006) concluded that early surgery was more cost-effective because of reduced overall length of hospital stay and avoidance of readmissions for recurrent cholecystitis or biliary colic.
Chronic Cholecystitis
Pathogenesis and Clinical Manifestations
There are numerous well-described risk factors for the development of gallstones and subsequent chronic cholecystitis. Patients at particularly high risk include obese women, in whom pathologic changes of chronic inflammation are found even in the absence of gallstones (Calhoun & Willbanks, 1987; Csendes et al, 2003); this may be due to an increase in the cholesterol saturation of bile in obese patients (St. George & Shaffer, 1993; see Chapter 7). These patients are often asymptomatic.
Diagnostic Imaging
US examination of the gallbladder most often reveals circumferential thickening of the gallbladder wall with cholelithiasis. In advanced cases, a small, shrunken gallbladder with a thickened wall and multiple gallstones are seen. Discomfort may be reproduced with direct pressure on the gallbladder with the US probe. Commonly, particularly in obese patients and in those with mild symptoms, the US examination shows no particular gallbladder wall abnormalities (Calhoun & Willbanks, 1987).
Acute Acalculous Cholecystitis
Pathogenesis
Acalculous cholecystitis usually occurs in patients with coexisting major illnesses, such as generalized sepsis, major trauma, or burns or in those undergoing a prolonged recovery from major operations who are unable to tolerate oral intake (Kalliafas et al, 1998). It has been speculated that in such situations, there is no stimulus of the gallbladder to contract, the bile becomes inspissated, and biliary sludge forms. The exact pathogenesis is unknown but likely involves some combination of ischemia, biliary stasis, and sepsis (Hakala et al, 1997; Warren, 1992), and inspissated bile and sludge seem to play some causative role. Although this condition traditionally has been described in the patient groups mentioned earlier, several reports suggest an increase in the de novo presentation of acalculous cholecystitis in the outpatient population, including patients with atherosclerotic vascular disease, such as seen in hypertension and diabetes (Parithivel et al, 1999; Ryu et al, 2003; Savoca et al, 1990). Overall, acalculous cholecystitis represents approximately 5% to 15% of all cases of acute cholecystitis. A male predominance is seen in cases of acalculous cholecystitis, in contrast to acute calculous cholecystitis, which occurs most often in women (Kalliafas et al, 1998; Ryu et al, 2003; Wang et al, 2003).
A prospective study evaluating trauma patients with serial US examinations found that the incidence of acalculous cholecystitis in severely injured patients (injury severity score ≥12, requiring intensive care for >4 days) was 11% (Pelinka et al, 2003), which is similar to other reports (Imhof et al, 1992). In addition, three factors were correlated with an increased risk of developing acalculous cholecystitis in this high-risk population: 1) high injury severity score, 2) increased heart rate, and 3) transfusion requirement at the time of admission. This study suggests that more acutely injured patients, who are expected to require prolonged ventilatory and nutritional support, are at higher risk for the development of acalculous cholecystitis (Pelinka et al, 2003).
Clinical Manifestations
Part of the difficulty in making the diagnosis of acalculous cholecystitis is that many patients presenting with this condition are critically ill and require ventilatory support and sedation. The symptoms and signs are often masked by the patient’s underlying condition or the interventions used to treat it (Kalliafas et al, 1998). In the outpatient population seen with acalculous cholecystitis, the diagnosis is more straightforward, mimicking the signs and symptoms of acute calculous cholecystitis (Ryu et al, 2003).
The most frequent physical and laboratory findings are fever, right upper quadrant pain, leukocytosis, and hyperbilirubinemia. These findings are often nonspecific, however, in the setting of sepsis and critical illness (Kalliafas et al, 1998). Incidence of gangrene and perforation seems to be increased in patients with acalculous cholecystitis compared with acute calculous cholecystitis, likely because of the delay in diagnosis that is common with this disease. Severe gallbladder complications such as gangrene, perforation, and empyema occur more commonly in older patients with an elevated white blood cell count (Ryu et al, 2003; Wang et al, 2003). In many series, the risk of severe gallbladder complications was found to be 50% to 60% (Kalliafas et al, 1998; Swayne, 1986; Wang et al, 2003). This high risk may be the result of the disturbance in capillary microcirculation that has been shown in pathologic studies on gallbladder specimens after cholecystectomy for acalculous cholecystitis (Hakala et al, 1997; Warren, 1992). In addition, partly as a result of the severity of the patient’s underlying condition, the mortality rates are high—15% in some series (Wang et al, 2003).
Diagnostic Evaluation and Imaging
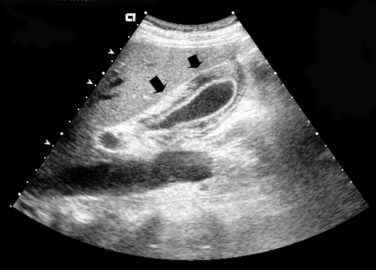
Imaging algorithms for patients with suspected acalculous cholecystitis are similar to algorithms for patients with acute cholecystitis. The initial imaging test is usually US, which classically reveals gallbladder distension, a thickened gallbladder wall, and biliary sludge without stones (Fig. 31.3; Wang et al, 2003). The difficulty with interpreting these findings is that many critically ill, parenteral nutrition–dependent patients have these findings. Partly because of this difficulty, the accuracy of US to diagnose acalculous cholecystitis has been highly institution dependent, with some series showing it to be highly sensitive and specific (Pelinka et al, 2003) and others showing it to be less accurate (Kalliafas et al, 1998). US is widely available and easy to use, even in acutely ill patients, because it can be performed at the bedside, and it is inexpensive. US should be performed as the initial imaging modality for suspected acalculous cholecystitis.
Evaluating the natural history of abnormalities visualized on US in acutely injured patients is problematic; a prospective trial assessed 255 severely injured trauma patients with serial US examinations. In this trial, all patients with US findings consistent with acalculous cholecystitis and major clinical symptoms—abdominal pain or distension or both, hemodynamic instability, or organ failure—underwent cholecystectomy, and all had acalculous cholecystitis on final pathology. A few patients had positive US findings, but no major clinical symptoms were observed. In this group, all US findings returned to normal within 3 weeks. In addition, 15% of patients developed hydrops of the gallbladder without clinical symptoms, and subsequent normalization of the US was observed in all (Pelinka et al, 2003). These findings suggest that although many critically ill patients may develop US abnormalities consistent with acalculous cholecystitis, the combination of clinical symptoms and positive imaging findings will identify most patients who require intervention.
Because of the difficulty establishing a diagnosis in these critically ill patients, CT has been used as an additional imaging test. The advantage of CT is that imaging of the entire chest, abdomen, and pelvis can be obtained; this is particularly important in this patient cohort, in whom clinical signs and symptoms may be misleading or whose signs and symptoms may result from other causes. CT may be more sensitive and specific than US (Blankenberg et al, 1991; Mirvis et al, 1986), but it has the disadvantage of requiring transport of the patient outside of the intensive care unit.
When a patient’s diagnosis is questionable based on physical findings or US evaluation or both, hepatobiliary scintigraphy may be used. In past reports, a high rate of false-positive results was seen in patients with acalculous cholecystitis (Mirvis et al, 1986), but more recent evaluation of this modality has shown improved accuracy in diagnosing acalculous cholecystitis (Kalliafas et al, 1998; Ryu et al, 2003). In some series, scintigraphy has been found to be more sensitive than CT or US in diagnosing acalculous cholecystitis (Kalliafas et al, 1998; Prevot et al, 1999; Puc et al, 2002; Ryu et al, 2003). The specificity of hepatobiliary scintigraphy can be improved by administering morphine to cause constriction of the sphincter of Oddi and improve gallbladder filling. This maneuver decreases the incidence of false-positive studies and improves specificity, but it does not result in improvements in sensitivity compared with conventional scintigraphy (Cabana et al, 1995; Flancbaum et al, 1989; Kalliafas et al, 1998).
Treatment
The definitive treatment for acalculous cholecystitis is cholecystectomy, which can be performed laparoscopically in most cases. In patients who are critically ill, placement of a percutaneous cholecystostomy tube allows for decompression of the gallbladder and drainage of contained, infected bile; this allows time for the patient to recover from the acute illness before considering proceeding with cholecystectomy (McClain et al, 1997; Akyürek et al, 2005). Percutaneous cholecystostomy may be the definitive treatment for acalculous cholecystitis, because there is no chronic obstruction of the gallbladder outlet as in acute cholecystitis (Davis et al, 1999; Vauthey et al, 1993; see Chapters 28 and 32).
Complications of Cholecystitis
Gangrenous Cholecystitis
Gangrenous cholecystitis is a more common finding in diabetic patients with acute cholecystitis who present with a leukocytosis (Fagan et al, 2003). In addition, the risk of gangrenous cholecystitis is higher in patients with acalculous cholecystitis, likely owing to the delay in diagnosis that commonly occurs in this disease (Kalliafas et al, 1998; Swayne, 1986; Wang et al, 2003). As previously mentioned, the most common site for necrosis to occur is in the fundus. Full-thickness necrosis is by definition always present in patients with gangrenous cholecystitis, but this condition may or may not result in free perforation of the gallbladder. In patients with free perforation, bile-stained abdominal fluid is present.
Because these patients are generally ill, imaging with CT scan is often performed. Findings most specific for acute gangrenous cholecystitis on CT scan include air in the wall or lumen, intraluminal membranes, an irregular wall, or pericholecystic abscess. As expected, a contrast-enhanced CT scan may show a lack of mural enhancement in patients with gangrenous cholecystitis (Bennett et al, 2002).
Empyema
In cases of empyema, the gallbladder is filled with purulent bile. This condition usually is associated with acute cholecystitis and occurs in the setting of infected bile and an obstructed cystic duct. Most patients with empyema have calculous cholecystitis, but empyema also can occur in patients with acalculous disease (Tseng et al, 2000). The clinical course can mimic that of an intraabdominal abscess from other causes, and patients are often seen initially with clinical manifestations of sepsis. Patients with gallbladder empyema require urgent cholecystectomy or percutaneous cholecystostomy, depending on the severity of illness at the time of presentation (Tseng et al, 2000). Critically ill patients may be best served by a temporary cholecystostomy tube followed by elective cholecystectomy.
Emphysematous Cholecystitis
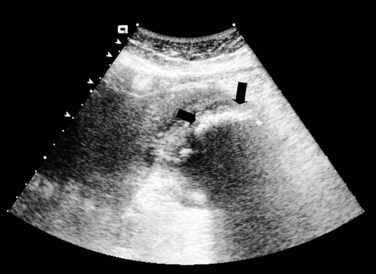
Emphysematous cholecystitis is a rare entity that results from the presence of gas-forming bacteria in the bile. Emphysematous cholecystitis may be seen in association with acute or gangrenous cholecystitis, and it is more common in men and diabetic patients (Tellez et al, 1999). The diagnosis occasionally can be made by simple abdominal radiographs, but more often it is diagnosed on US (Fig. 31.4) or CT scan (Fig. 31.5; Bennett & Balthazar, 2003; Gill et al, 1997; Konno et al, 2002). Patients should receive intravenous antibiotics to include coverage for Clostridium species, followed by emergent cholecystectomy.
Mirizzi Syndrome
Mirizzi syndrome is defined as biliary obstruction secondary to cholecystitis. It occurs in 0.3% to 3% of patients undergoing cholecystectomy (Lai & Lau, 2006). An impacted stone in the gallbladder infundibulum or cystic duct can compress the bile duct, usually at the level of the common hepatic duct (type I), or a stone can erode from the gallbladder or cystic duct into the common hepatic duct, resulting in a cholecystocholedochal fistula (type II). Patients are seen with symptoms of acute cholecystitis but with the additional finding of hyperbilirubinemia and elevated alkaline phosphatase. A laparoscopic approach to this condition has been shown to result in high conversion and complication rates and is not recommended (Lai & Lau, 2006; Antoniou et al, 2009). Open cholecystectomy is the gold standard for treatment when this condition is identified preoperatively. If inflammation has obliterated the triangle of Calot, a partial cholecystectomy with removal of any stones may be all that is possible and usually resolves the condition. In the acute setting, the biliary obstruction often resolves after cholecystectomy and resolution of the inflammatory process. In some cases, however, the chronic inflammation leads to fistulation from the gallbladder to the bile duct, or a biliary stricture results, both of which will complicate the operative procedure and may require biliary reconstruction (see Chapters 42A and 42B).
Cholecystoenteric Fistula
Cholecystoenteric fistula, or perforation of the gallbladder into an adjacent hollow organ, is a rare complication of acute cholecystitis. The duodenum and the transverse colon are the most common sites of fistulation, which results in decompression of the gallbladder and may result in brief symptomatic improvement. This complication occurs most frequently in women in their sixth to seventh decades and has been shown to be associated with Mirizzi syndrome or an additional hepatobiliary abnormality such as gallbladder cancer (Beltran et al, 2008; Chowbey et al, 2006; Costi et al, 2009). Contamination of the biliary tree by enteric organisms may result, and patients may be seen with cholangitis and pneumobilia. Approximately 10% to 15% of patients with cholecystoenteric fistulae will pass gallstones into the small intestine and be seen with small bowel obstruction, termed gallstone ileus. In patients with cholecystocolonic fistula, chronic diarrhea is the most common presenting symptom in nonemergent cases (Costi et al, 2009).
Patients with cholecystoenteric fistula require cholecystectomy with takedown and closure of the fistula. When cholecystoenteric fistula is encountered at the time of surgery, concomitant Mirizzi syndrome should be considered. Patients with gallstone ileus require removal of the obstructing stone via enterotomy (Chowbey et al, 2006), and it is important to perform a thorough examination of the bowel for any other stones. Fistula takedown and cholecystectomy can be performed at the same procedure or at a second, delayed procedure if the patient is too unstable to tolerate a prolonged initial operation, or if the pericholecystic inflammation is so severe as to make initial cholecystectomy unsafe.
Akyürek N, et al. Management of acute calculous cholecystitis in high-risk patients: percutaneous cholecystotomy followed by early laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005;15(6):315-320.
Alponat A, et al. Predictive factors for conversion of laparoscopic cholecystectomy. World J Surg. 1997;21:629-633.
Antoniou SA, et al. Laparoscopic treatment of Mirizzi syndrome: a systematic review. Surg Endosc. 2010;24:33-39. May 23
Assalia A, et al. Emergency minilaparotomy cholecystectomy for acute cholecystitis: prospective randomized trial—implications for the laparoscopic era. World J Surg. 1997;21:534-539.
Beltran MA, et al. The relationship of Mirizzi syndrome and cholecystoenteric fistula: validation of a modified classification. World J Surg. 2008;32:2237-2243.
Bennett GL, Balthazar EJ. Ultrasound and CT evaluation of emergent gallbladder pathology. Radiol Clin North Am. 2003;41:1203-1216.
Bennett GL, et al. CT findings in acute gangrenous cholecystitis. Am J Roentgenol. 2002;178:275-281.
Blankenberg F, et al. Computed tomography as an adjunct to ultrasound in the diagnosis of acute acalculous cholecystitis. Gastrointest Radiol. 1991;16:149-153.
Byrne MF, et al. Percutaneous cholecystostomy in patients with acute cholecystitis: experience of 45 patients at a US referral center. J Am Coll Surg. 2003;197:206-211.
Cabana MD, et al. Morphine-augmented hepatobiliary scintigraphy: a meta-analysis. Nucl Med Commun. 1995;16:1068-1071.
Calhoun R, Willbanks O. Coexistence of gallbladder disease and morbid obesity. Am J Surg. 1987;154:655-658.
Catena R, et al. Prospective analysis of 101 consecutive cases of laparoscopic cholecystectomy for acute cholecystitis operated with harmonic scalpel. Surg Laparosc Endosc Percutan Tech. 2009;19(4):312-316.
Chandler CF, et al. Prospective evaluation of early versus delayed laparoscopic cholecystectomy for treatment of acute cholecystitis. Am Surg. 2000;66:896-900.
Chatziioannou SN, et al. Hepatobiliary scintigraphy is superior to abdominal ultrasonography in suspected acute cholecystitis. Surgery. 2000;127:609-613.
Chowbey PK, et al. Laparoscopic management of cholecystoenteric fistulas. J Laparoendosc Adv Surg Tech A. 2006;16(5):467-472.
Costi R, et al. Cholecystocolonic fistula: facts and myths—a review of the 231 published cases. J Hepatobiliary Pancreat Surg. 2009;16(1):8-18.
Cox MR, et al. Laparoscopic cholecystectomy for acute inflammation of the gallbladder. Ann Surg. 1993;218:630-634.
Csendes A, et al. Histologic findings of gallbladder mucosa in 87 patients with morbid obesity without gallstones compared to 87 control subjects. J Gastrointest Surg. 2003;7:547-551.
Davis CA, et al. Effective use of percutaneous cholecystostomy in high-risk surgical patients: techniques, tube management, and results. Arch Surg. 1999;134:727-731.
den Hoed PT, et al. Infections and bacteriological data after laparoscopic and open gallbladder surgery. J Hosp Infect. 1998;39:27-37.
Fagan SP, et al. Prognostic factors for the development of gangrenous cholecystitis. Am J Surg. 2003;186:481-485.
Fidler J, et al. CT evaluation of acute cholecystitis: findings and usefulness in diagnosis. Am J Roentgenol. 1996;166:1085-1088.
Flancbaum L, et al. Use of cholescintigraphy with morphine in critically ill patients with suspected cholecystitis. Surgery. 1989;106:668-673.
Flowers JL, et al. The Baltimore experience with laparoscopic management of acute cholecystitis. Am J Surg. 1991;161:388-392.
Gibney RG, et al. Combined surgical and radiologic intervention for complicated cholelithiasis in high-risk patients. Radiology. 1987;165:715-719.
Gill KS, et al. The changing face of emphysematous cholecystitis. Br J Radiol. 1997;70:986-991.
Hakala T, et al. Microangiopathy in acute acalculous cholecystitis. Br J Surg. 1997;84:1249-1252.
Harvey RT, Miller WTJr. Acute biliary disease: initial CT and follow-up US versus initial US and follow-up CT. Radiology. 1999;213:831-836.
Hatzidakis AA, et al. Acute cholecystitis in high-risk patients: percutaneous cholecystostomy vs. conservative treatment. Eur Radiol. 2002;12:1778-1784.
Horiuchi A, et al. Delayed laparoscopic subtotal cholecystectomy in acute cholecystitis with severe fibrotic adhesions. Surg Endosc. 2008;22(12):2720-2723.
Hsieh CH. Early minilaparoscopic cholecystectomy in patients with acute cholecystitis. Am J Surg. 2003;185:344-348.
Imhof M, et al. Acute acalculous cholecystitis complicating trauma: a prospective sonographic study. World J Surg. 1992;16:1160-1165.
Johansson M, et al. Management of acute cholecystitis in the laparoscopic era: results of a prospective, randomized clinical trial. J Gastrointest Surg. 2003;7:642-645.
Kalimi R, et al. Diagnosis of acute cholecystitis: sensitivity of sonography, cholescintigraphy, and combined sonography-cholescintigraphy. J Am Coll Surg. 2001;193:609-613.
Kalliafas S, et al. Acute acalculous cholecystitis: incidence, risk factors, diagnosis, and outcome. Am Surg. 1998;64:471-475.
Kanaan SA, et al. Risk factors for conversion of laparoscopic to open cholecystectomy. J Surg Res. 2002;106:20-24.
Keus F, et al. Randomized clinical trial of small-incision and laparoscopic cholecystectomy in patients with symptomatic cholecystitis: primary and clinical outcomes. Arch Surg. 2008;143:371-377.
Kolla SB, et al. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: a prospective randomized trial. Surg Endosc. 2004;18(9):1323-1327.
Konno K, et al. Emphysematous cholecystitis: sonographic findings. Abdominal Imaging. 2002;27:191-195.
Lai PB, et al. Randomized trial of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 1998;85:764-767.
Lai EC, Lau WY. Mirizzi syndrome: history, present and future development. ANZ J Surg. 2006;76(4):251-257.
Lau H, et al. Early versus delayed-interval laparoscopic cholecystectomy for acute cholecystitis: a meta-analysis. Surg Endosc. 2006;20(1):82-87.
Lo CM, et al. Prospective randomized study of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Ann Surg. 1998;227:461-467.
Majeed AW, et al. Randomised, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet. 1996;347:989-994.
Mahid SS, et al. Meta-analysis of cholecystectomy in symptomatic patients with positive hepatobiliary iminodiacetic acid scan results without gallstones. Arch Surg. 2009;144(2):180-187.
McClain T, et al. Laparoscopic cholecystectomy in the treatment of acalculous cholecystitis in patients after thermal injury. J Burn Care Rehabil. 1997;18:141-146.
Middleton GW, Williams JH. Diagnostic accuracy of 99Tcm-HIDA with cholecystokinin and gallbladder ejection fraction in acalculous gallbladder disease. Nucl Med Commun. 2001;22(6):657-661.
Mirvis SE, et al. The diagnosis of acute-acalculous cholecystitis—a comparison of sonography, scintigraphy, and CT. Am J Roentgenol. 1986;147:1171-1175.
Norrby S, et al. Early or delayed cholecystectomy in acute cholecystitis: a clinical trial. Br J Surg. 1983;70:163-165.
Novitsky YW, et al. Advantages of mini-laparoscopic vs conventional laparoscopic cholecystectomy: results of a prospective randomized trial. Arch Surg. 2005;140(12):1178-1183.
Papi C, et al. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol. 2004;99:147-155.
Parithivel VS, et al. Acute acalculous cholecystitis in young patients without predisposing factors. Am Surg. 1999;65:366-368.
Pelinka LE, et al. Acute acalculous cholecystitis after trauma: a prospective study. J Trauma. 2003;55:323-329.
Prevot N, et al. Contribution of cholescintigraphy to the early diagnosis of acute acalculous cholecystitis in intensive-care-unit patients. Eur J Nucl Med. 1999;26:1317-1325.
Puc MM, et al. Ultrasound is not a useful screening tool for acute acalculous cholecystitis in critically ill trauma patients. Am Surg. 2002;68:65-69.
Rosen M, et al. Predictive factors for conversion of laparoscopic cholecystectomy. Am J Surg. 2002;184:254-258.
Ryu JK, et al. Clinical features of acute acalculous cholecystitis. J Clin Gastroenterol. 2003;36:166-169.
Savoca PE, et al. The increasing prevalence of acalculous cholecystitis in outpatients: results of a 7-year study. Ann Surg. 1990;211:433-437.
Schirmer BD, et al. Laparoscopic cholecystectomy: treatment of choice for symptomatic cholelithiasis. Ann Surg. 1991;213:665-676.
Siddiqui T, et al. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: a meta-analysis of randomized clinical trials. Am J Surg. 2008;195(1):40-47.
Singhal T, et al. Laparoscopic subtotal cholecystectomy: initial experience with laparoscopic management of difficult cholecystitis. Surgeon. 2009;7(5):263-268.
Sjodahl R, et al. Pathogenesis of acute cholecystitis. Surg Gynecol Obstet. 1978;146:199-202.
Soyer P, et al. Color velocity imaging and power Doppler sonography of the gallbladder wall: a new look at sonographic diagnosis of acute cholecystitis. Am J Roentgenol. 1998;171:183-188.
Spira RM, et al. Percutaneous transhepatic cholecystostomy and delayed laparoscopic cholecystectomy in critically ill patients with acute calculous cholecystitis. Am J Surg. 2002;183:62-66.
St. George CM, Shaffer EA. Spontaneous obesity and increased bile saturation in the ground squirrel. J Surg Res. 1993;55:314-316.
Swayne LC. Acute acalculous cholecystitis: sensitivity in detection using technetium-99m iminodiacetic acid cholescintigraphy. Radiology. 1986;160:33-38.
Tellez LGS, et al. Acute emphysematous cholecystitis: report of twenty cases. Hepatogastroenterology. 1999;46:2144-2148.
Tseng LJ, et al. Palliative percutaneous transhepatic gallbladder drainage of gallbladder empyema before laparoscopic cholecystectomy. Hepatogastroenterology. 2000;47:932-936.
Tsutsui K, et al. Usefulness of single and repetitive percutaneous transhepatic gallbladder aspiration for the treatment of acute cholecystitis. J Gastroenterol. 2007;42(7):583-588.
Tzovaras G, et al. Timing of laparoscopic cholecystectomy for acute cholecystitis: a prospective nonrandomized study. World J Gastroenterol. 2006;12(34):5528-5531.
Uggowitzer M, et al. Sonography of acute cholecystitis: comparison of color and power Doppler sonography in detecting a hypervascularized gallbladder wall. Am J Roentgenol. 1997;168:707-712.
Van der Linden W, Edlund G. Early versus delayed cholecystectomy: the effect of a change in management. Br J Surg. 1981;68:753-757.
Vauthey JN, et al. Indications and limitations of percutaneous cholecystostomy for acute cholecystitis. Surg Gynecol Obstet. 1993;176:49-54.
Wang AJ, et al. Clinical predictors of severe gallbladder complications in acute acalculous cholecystitis. World J Gastroenterol. 2003;9:2821-2823.
Warren BL. Small vessel occlusion in acute acalculous cholecystitis. Surgery. 1992;111:163-168.
Werbel GB, et al. Percutaneous cholecystostomy in the diagnosis and treatment of acute cholecystitis in the high-risk patient. Arch Surg. 1989;124:782-786.
Wong SK, et al. Percutaneous cholecystostomy and endoscopic cholecystolithotripsy in the management of acute cholecystitis. Surg Endosc. 1999;13:48-52.