Chapter 43 Cholangitis
Cholangitis
Cholangitis is not a single disease with a well-defined clinical appearance but rather a spectrum of disease that presents variably with a wide range of severity. The appearance and course of cholangitis ranges from mild, intermittent, and recurrent episodes of abdominal pain, jaundice, fever, and chills as described by Charcot in 1877 to a rapidly progressive systemic illness that results in shock, coma, and death as described by Reynolds and Dargan (1959).
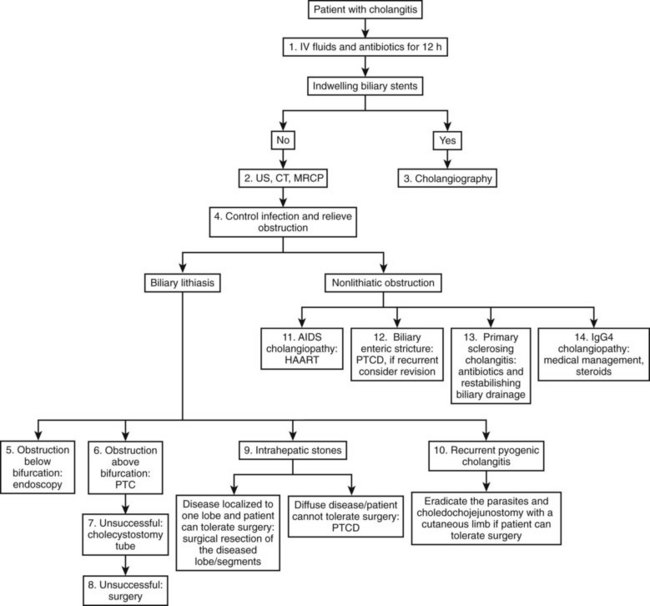
The development of cholangitis requires the presence of three factors: 1) obstruction of bile flow, 2) colonization of the bile with bacteria (bactobilia) or fungi, and 3) elevation of intraductal biliary pressure. The most common causes of biliary obstruction associated with cholangitis are calculi, benign and malignant strictures, obstructed stents, and parasites (Table 43.1; see Chapters 7 and 11). The management of cholangitis should follow three principles: 1) vigorous resuscitation and hemodynamic support, 2) broad-spectrum parenteral antibiotics, and 3) relief of biliary obstruction (decompression). Figure 43.1 provides a suggested management algorithm for patients with cholangitis.
AIDS, acquired immunodeficiency syndrome; ERCP, endoscopic retrograde cholangiopancreatography; PTC, percutaneous transhepatic cholangiography
Modified from Bornman PC, et al, 2003: Management of cholangitis. Hepatobiliary Pancreat Surg 10:406-414.
Pharmacologic Treatment for Cholangitis
The pharmacologic treatment of cholangitis differs from that of acute cholecystitis in the need for more specific and broader antimicrobial therapy dictated by the patient’s underlying pathology and clinical condition. Culture-identified bacteriology of the biliary tree has changed over the past 40 years. Previously, the gram-negative aerobes Escherichia coli and Klebsiella, gram-positive aerobes, and enterococci were the most common isolates identified from patients with cholangitis (Helton, 1987). More recently, infections have been polymicrobial in 30% to 80% of cases (Westphal & Brogard, 1999). In some studies, anaerobes have been detected in more than 15% of patients but rarely as the sole isolate. Bacteroides and Clostridium species are the most frequently cultured anaerobes, and anaerobic bacteria are commonly isolated from biliary tract specimens from patients who have a history of biliary surgery, especially those with a biliary-enteric anastomosis or chronic biliary tract infection and the elderly. Cholangitis arising from anaerobic organisms is reported to be associated with a more severe clinical illness compared with purely aerobic infections (Csendes et al, 1996; see Chapter 11).
Bacteremia in acute cholangitis has been demonstrated to be the result of increased bile duct pressure that favors the reflux of bacteria into the blood and lymphatic circulation (Csendes et al, 1995); it has been reported in 21% to 71% of patients with cholangitis (Cotton et al, 1991; Csendes et al, 1996; see Chapter 7). The organisms isolated reflect a similar distribution to that of biliary cultures, except for anaerobes and enterococci, which are infrequently found in blood cultures.
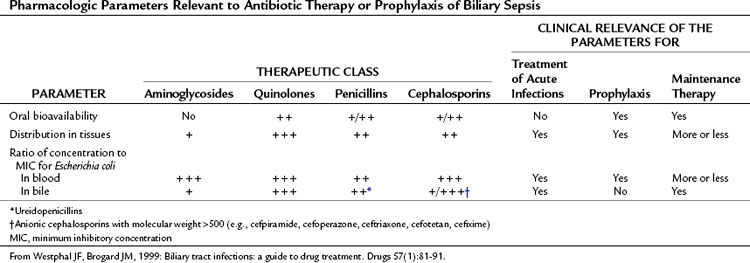
A variety of antibiotic regimens and combinations have been compared in prospective randomized clinical trials to establish efficacy, safety, and toxicity profiles. Monotherapy with broad-spectrum agents, such as a third- and fourth-generation cephalosporins (cefotaxime, cefipime), and a ureidopenicillin (mezlocillin, piperacillin) combined with a β-lactamase inhibitor (ticarcillin-clavulanate, piperacillin-tazobactam) or quinolone (ciprofloxacin) have been reported to be as effective in treating patients with cholangitis as metronidazole or clindamycin in combination with an aminoglycoside or a third-generation cephalosporin and ampicillin (Sung et al, 1995; Thompson et al, 1993).
In a multicenter comparative study of cefepime versus broad-spectrum antibacterial therapy in moderate and severe bacterial infections (Badaro et al, 2002), cefepime was demonstrated to achieve a higher cure rate compared with broad-spectrum combination therapy as an initial empiric treatment for hospitalized patients with moderate to severe community-acquired infections.
Carbapenems, ureidopenicillins, and fluoroquinolones offer good coverage for gram-negative aerobes (Mazuski et al, 2002), but piperacillin offers the advantage of gram-positive coverage, including enterococci, as well as anaerobic coverage (Thompson et al, 1990). Tazobactam, a β-lactamase inhibitor, extends the spectrum to cover organisms that have acquired resistance. These regimens are sufficient for most patients presenting with de novo cholangitis who have not yet been hospitalized, operated upon, or instrumented (Table 43.2). Again, it is emphasized that although hydration and antibiotics may improve the clinical condition in up to 80% of patients with acute cholangitis, 20% with clinical sepsis will require urgent biliary decompression.
For patients with a previously instrumented biliary tract (endoscopic retrograde cholangiopancreatography [ERCP], placement of a biliary stent), a previous biliary operation, or prolonged hospitalization, the cholangitis is likely to be associated with resistant flora such as Pseudomonas or Serratia species. Liver transplant patients who develop cholangitis have been observed to have Candida and/or Enterococcus in the biliary tree. Interestingly, vancomycin-resistant enterococci (VRE) are common in liver transplant patients (Schlitt et al, 1999). When Candida and/or Enterococcus are cultured, these should be treated with amphotericin and one of the streptogramins (quinupristin/dalfopristin [Synercid] or oxazolidinone linezolid [Zyvox], respectively). For all hospitalized patients, antibiotic coverage should always take into consideration the local resistance patterns (“antibiotic-gram”) of specific pathogens.
Duration of Antibiotic Therapy for Cholangitis
No randomized clinical trials have firmly established the duration of antibiotic therapy for the treatment of cholangitis. However, general guidelines are for antibiotic therapy to continue until biliary obstruction is completely relieved, biochemical liver function tests have improved or normalized, and the patient is afebrile for at least 48 hours. After successful biliary drainage by ERCP, a retrospective study comparing short-duration antibiotic therapy (3 days) with longer antibiotic coverage showed that 3 days of antibiotic therapy appears sufficient when adequate drainage has been achieved and fever is abating (van Lent et al, 2002).
Diagnostic Imaging Studies
Transabdominal Ultrasound
TUS (see Chapter 13) is the least expensive and most universally available imaging modality to evaluate the biliary tree and gallbladder. As such, it is the preferred first diagnostic test when evaluating a patient with acute cholecystitis and/or cholangitis. US can reliably demonstrate intrahepatic and extrahepatic biliary dilation, but this can be absent in the case of an acute obstruction. In the presence of gallstones less than 10 mm in size and dilation of the CBD greater than 10 mm, CBD obstruction by gallstones is likely (Abboud et al, 1996). The sensitivity of US to detect CBD stones varies between 20% and 75%, with increased sensitivity in the case of multiple large stones within a dilatated CBD and less sensitivity in the case of stone impaction in the retropancreatic segment of the CBD.
Computed Tomography
Non–contrast-enhanced CT scan (see Chapter 16) overcomes the technical limitations of US, as it can detect impacted stones in the retropancreatic CBD segment; when performed with 2- to 3-mm slices, its sensitivity for detecting small stones is reported to range between 65% and 80% (Neitlich et al, 1997). However, because up to 25% of gallstones will have the same density as bile, the sensitivity of noncontrast CT does not exceed 80%.
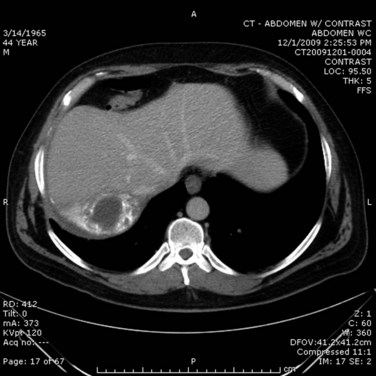
In the case of cholangitis, intravenous (IV) contrast–enhanced CT scan can reveal inflammation and demonstrate thickness of the bile ducts. The arterial phase should reveal peripheral or periductal hypervascularization (Arai et al, 2003). Another important role for CT is to detect the complications of cholangitis, such as hepatic abscess (Fig. 43.2) and pylephlebitis (suppurative thrombosis of the portal vein). Finally, CT can reveal etiologies of biliary obstruction, other than a CBD stone, such as a tumoral mass; however, contrast material is associated with a risk of hypersensitivity or induced renal toxicity, particularly in underresuscitated patients or in the presence of hemodynamic instability.
Magnetic Resonance Cholangiopancreatography
Magnetic resonance cholangiopancreatography (MRCP; see Chapter 17) provides imaging in a noninvasive manner that allows visualization by emphasizing the hyperintensity of stationary liquids, thus the use of this modality precludes the need for contrast injection to fully visualize the biliary system. Images obtained by MRCP are similar to those obtained by cholangiogram: stones appear as hypointense defects (lacking liquid) within the biliary system. However, MRCP cannot differentiate stones from air bubbles, sludge, or blood clots—all these lack liquid. A further limitation of MRCP is that it cannot detect stones less than 3 mm in size, nor can it detect impacted stones in the ampulla, which require evaluation of T1 images before and after gadolinium injection. The overall sensitivity of MRCP to detect CBD stones is 80% to 100%, and its specificity is excellent, varying between 90% and 100% (Hakansson et al, 2002; Kondo et al, 2001; Soto et al, 2000). It is important to recall that MRCP is purely a diagnostic modality, it is not therapeutic.
Endoscopic Ultrasound
Endoscopic ultrasound (EUS; see Chapter 14) is an invasive procedure that requires sedation or general anesthesia. Once it has been passed through the stomach and into the duodenum, the US probe is in close relationship to the CBD; by using a high US frequency (7.5 to 12 MHz), the resolution of EUS is exceptional (<1 mm), making it the gold standard in the detection of small gallstones or other obstructive etiologies. The major limitations of EUS are that it cannot be performed in patients with altered anatomy, such as those with a prior distal gastrectomy or gastric bypass, or in the presence of a significantly calcified pancreas or hilar biliary pathology.
Direct Cholangiography
Although historically considered the reference radiologic modality, direct cholangiography (see Chapter 18) for purely diagnostic purposes has been supplanted by less invasive cross-sectional imaging alternatives, which also provide additional anatomic detail related to adjacent organs to allow a more complete assessment. Retrograde or percutaneous cholangiography is largely limited to the first step of a therapeutic procedure (Gallix et al, 2006).
In addition to its inferior image quality, there are various complications associated with the use of direct cholangiography, including biliary infection, pancreatitis, and hemorrhage (Vidal et al, 2004).
Management of Cholangitis
Extrahepatic Biliary Stone Obstruction: Procedures for Biliary Decompression
When performed in a timely manner, biliary decompression and successful drainage are the cornerstones of acute cholangitis treatment. Several techniques can be used to ensure effective biliary drainage. A review of these techniques with the Tokyo guidelines was recently published (Tsuyuguchi et al, 2007).
The preferred method and route to relieve biliary obstruction, and the ultimate success of biliary drainage, depends upon a number of factors that include availability of the technique (endoscopic, percutaneous, or operative), local expertise, etiology, location and severity of obstruction, and the presence of comorbid medical conditions. Patients who do not respond to initial medical treatment within 12 to 24 hours should undergo emergent, nonoperative decompression of the biliary tract. When the obstruction is believed to be below the bile duct bifurcation, the preferred initial approach is endoscopic (Kumar et al, 2004).
Endoscopic Biliary Decompression (See Chapters 27 and 36)
In cases of difficult biliary cannulation, an endoscopic sphincterotomy (EST) may be performed. A common EST technique is to perform a high-frequency electric surgical incision of the duodenal papilla, using a sphincterotome selectively inserted into the bile duct. In contrast to EST for stone removal, when performed for drainage, only a limited incision is necessary. Bleeding, retroduodenal perforation, and pancreatitis are the most common complications after ERCP and sphincterotomy. Morbidity and mortality rates are much higher with sphincterotomy than with nasobiliary catheter drainage when patients are critically ill (Boender et al, 1995; Chawla et al, 1994; Leese et al, 1986). Complications commonly reported with EST are hemorrhage (2% to 3%) and pancreatitis (1.9% to 5.4%), and mortality rate is 0.4% to 1.3% (Cotton et al, 1991; Freeman et al, 1996).
Contraindications
As stated, emergent sphincterotomy to decompress the bile duct should be avoided in septic patients, and especially in elderly patients, because of potentially high mortality rates (18.8%). In the elderly, endoscopic drainage alone has been observed to result in lower morbidity (16.7%) and mortality (5.6%) rates than surgical (87.5% and 25.0%, respectively) or percutaneous drainage (36.4% and 9.1%, respectively) (Boender et al, 1995; Sugiyama & Atomi, 1997). It is important to emphasize that once control of sepsis has been achieved, sphincterotomy and more optimal biliary imaging can be obtained, thus defining the underlying obstruction and enabling definitive treatment for either a stone, tumor, or stricture (Lee et al, 2002).
Percutaneous Transhepatic Cholangiography and Drainage (See Chapters 28, 32, and 36)
Retrospective analyses and prospective randomized trials have shown that urgent endoscopic drainage is an effective treatment for suppurative cholangitis and results in a better clinical outcome than surgical drainage (Hui et al, 2002); however, in an unstable patient, ERCP should not be performed; percutaneous transhepatic decompression is a better alternative, despite the limitation of not treating the cause of the obstruction (Gould et al, 1985; Kadir et al, 1982).
Percutaneous transhepatic cholangiography and drainage (PTCD) is especially valuable in the presence of a hilar obstruction (Nomura et al, 1997), having been reported to be useful in cases of malignant biliary obstruction, particularly when used in combination with definitive decompressive adjuncts, such as expandable metallic stents. In a series of 233 patients with malignant biliary obstruction requiring biliary drainage, with decompression achieved by PTCD and placement of metallic stents and/or plastic tubes, improvement in survival was reported after either chemotherapy, radiotherapy, or brachytherapy at 1, 3, 6, and 12 months of 97.96%, 95.92%, 89.80%, and 32.59%, respectively. Most notable was the observation that the patency rates for biliary stents at 1, 3, 6, and 12 months were 97.96%, 93.86%, 80.93%, and 56.52%, respectively. These results strongly suggest that for patients with malignant biliary obstruction, biliary decompression with PTCD, using internalized stents for biliary patency, approaches the duration of patient survival (Qian et al, 2006).
Standard Procedure
Although no studies directly compare percutaneous to endoscopic drainage in the setting of acute cholangitis, PTCD should applied to those patients who are not candidates for, or who have failed, endoscopic drainage. PTCD is potentially more morbid, with known complications that include intraperitoneal hemorrhage (2.5%) and biliary peritonitis (1.2%) with a mortality rate as high as 1.7%. In patients with proximal biliary obstruction, percutaneous drainage is clearly preferred, especially in an ill patient (Paik et al, 2009).
Complications from PTCD can be severe and may require additional procedures. The most frequently encountered complications are catheter dislodgement, hemobilia, catheter obstruction, cholangitis, hemoperitoneum, and choleperitoneum (Gazzaniga et al, 1991). The spillage of infected bile into the subdiaphragmatic and perihepatic space may lead to peritonitis and prolonged fever and may eventually require operative drainage.
Mortality rates are substantial after unsuccessful endoscopic drainage of cholangitis from hilar obstruction (Ducreux et al, 1992). Hence, rather than endoscopic drainage, PTCD is indicated for such lesions. If PTCD is unsuccessful or unavailable, the patient should undergo emergent percutaneous cholecystostomy tube decompression, if the cystic duct is patent, and the obstruction is below the cystic duct–CBD union. Because of its high mortality rate (>30%), emergency laparotomy and CBD decompression via a T-tube should only be used as a last resort, when nonoperative modalities are unavailable or unsuccessful (Lai et al, 1992).
Open Common Bile Duct Exploration (See Chapters 29 and 35)
During open CBD exploration, exposure should be obtained along the free border of the lesser omentum, above the duodenum. When dense adhesions are encountered, or the anatomy is unclear, aspiration with a fine needle facilitates the localization of the CBD. Once identified, an anterior vertical incision is made parallel to the long axis of the duct on the distal CBD, with stay sutures placed on either side. It is important to emphasize that a vertical, rather than horizontal, incision should be performed; a horizontal incision is limited in extension, yields a stenotic area when closed, and may interrupt the axial arterial blood supply of the CBD, which courses along the 3 o’clock and 9 o’clock positions along the lateral aspects of the bile duct. A T-tube should be placed in the CBD above the level of the obstruction to externally decompress the biliary system and enable bile duct closure. The benefits of T-tube placement and decompression are maintenance of biliary ductal patency in the setting of edema and allowance of subsequent access to the biliary tract. Usually, the T-tube is kept in place for 4 to 6 weeks and is removed after a normal cholangiogram (Verbesey & Birkett, 2008).
Intrahepatic Biliary Stone Obstruction (See Chapters 39 and 44)
Patients with complex hepatolithiasis represent a difficult problem, as intrahepatic stones are more difficult to access and remove endoscopically and are often associated with intrahepatic biliary strictures. The resulting cholangitis is characterized by high treatment failure and high recurrence rate. In the past, surgical management consisting of hepatic resection and/or hepaticojejunostomy was the only option for patients with complex hepatolithiasis, with the goal of preventing recurrent cholangitis. Currently, a nonsurgical approach consisting of PTCD followed by cholangioscopy, biliary stricture balloon angioplasty, and lithotripsy is preferred to avoid the morbidity and mortality associated with surgical treatment, especially in debilitated elderly patients with significant comorbidities (Chiang et al, 1994). For patients whose intrahepatic stones have been successfully removed, compared with those whose removal failed, the recurrence of cholangitis is delayed (Cheung, 1997; Yeh et al, 1995).
When the percutaneous approach is unsuccessful, and the patient can tolerate a major operation, hepatic resection should still be offered (Yeh et al, 1995). In a selected group of patients with disease localized to one lobe or a few segments, hepatic resection removes the stones and the associated pathologic changes, including ductal stricture, fibrosis, and microabscess (Fig. 43.3). In a series of 174 patients with intrahepatic stones treated with liver resection or percutaneous choledochoscopy (Cheung & Kwok, 2005), the overall success rate in the surgical group was 98.0%, and the 5-year cholangitis recurrence rate was 13.3%. For the percutaneous choledochoscopic–treated group, the overall success rate was 70.5%, and the 5-year cholangitis recurrence rate was 43.2% and 26.4% for those with and without stricture, respectively. Bilateral stones, the presence of stricture, and the presence of hepatic parenchymal atrophy were demonstrated to be significant risk factors for a poor long-term outcome after stone removal alone.
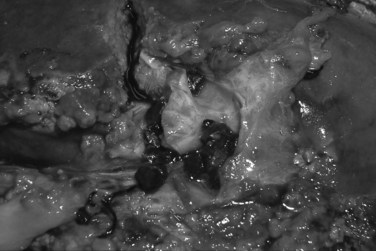
FIGURE 43.3 Intraoperative picture of intrahepatic biliary stones with strictures and microabscesses.
It is generally acceptable to offer liver resection to patients with associated atrophy of the involved segments, mostly those in whom the left lobe was involved, because of the reduced operative risk. Resection of nonatrophic stone-bearing segments is more controversial. In patients with biliary stricture, liver resection can reduce the reported high rate of restenosis; regardless of whether dilation of the stricture was successful, the associated rate of recurrent cholangitis has been reported to be upward of 35% (Jan & Chen, 1995). In patients without stricture yet with numerous stones, resection may be considered to eliminate the risk of future cholangiocarcinoma, which is reported in 5% to 16% of patients (Chen et al, 1993; Chijiiwa et al, 1993; Liu et al, 1998).
Two additional nonsurgical approaches for the treatment of intrahepatic stones are discussed, namely, the transhepatic percutaneous intracorporeal electrohydraulic shock-wave lithotripsy (ICSWL) and yttrium-aluminum-garnet (YAG) laser lithotripsy. In ICSWL, PTCD is placed, and the infection is allowed to resolve. At separate settings, the PTCD catheter is exchanged for larger biliary catheters to enable the formation of a well-formed tract. Once the tract is a sufficient size (at least 14 Fr), downstream stones can be crushed with mechanical lithotriptors or contact shock-wave lithotripsy under direct endoscopic visualization. When stone debris is removed, distal strictures can be traversed with an angioplasty balloon catheter and dilated. As such, multiple treatment sessions are required to successfully and safely clear the duct to avoid sepsis (Bonnel et al, 1991).
Although an important procedure in the armamentarium, fragmentation with ICSWL requires additional therapies for removal of stone fragments in 50% of patients (Bland et al, 1989). It requires general anesthesia or regional blocks that may be avoided with percutaneous techniques. The most obvious advantage is avoidance of the percutaneous biliary catheter, which gives some patients discomfort. When combined with endoscopic removal, ICSWL can achieve stone clearance for 94% to 96% of patients (Harz et al, 1991; White et al, 1998).
A more recent nonsurgical approach involves laser application. This technique consists of percutaneous access to the biliary tree with percutaneous holmium: YAG laser lithotripsy ablation of bile duct stones (Hazey et al, 2007). Depending on the location of the stones and anatomic considerations, the left, right, or both systems may be accessed via percutaneous transhepatic cholangiography (PTC) techniques. The catheter is upsized to 12- or 14-Fr over several weeks to allow for passage of a videocholedochoscope. Passage of an endoscope and direct visualization of the calculi is all that is required to pass energy via the laser and fragment the stones. Once the stones are fragmented, the debris is flushed out the access catheter, or it may pass through the sphincter of Oddi into the duodenum. Frequently, the sphincter has been rendered incompetent by a previous endoscopic sphincterotomy.
The medical literature is replete with studies outlining percutaneous access and removal of primary hepatolithiasis using YAG laser therapy in Asian patients, with clearance rates of 76.8% to 100% (Chen et al, 2005; Cheung et al, 2003; Shamamian & Grasso, 2004). Primary intrahepatic stones have frequently required more treatments (average, 3.9) than secondary intrahepatic calculi (average, 2.6) (Shamamian & Grasso, 2004). The clearance rates diminished significantly for patients with intrahepatic strictures: 58% in the presence of stricture versus 100% in cases with no stricture (Huang et al, 2003). Similarly, presence of stricture is associated with a high recurrence rate of 51.6% (Jan & Chen, 1995) to 63.2% (Huang et al, 2003).
In the Western literature, fewer YAG laser applications for hepatolithiasis are reported. In a relatively small series, Hazey and others (2007) reported that all patients (n = 13) undergoing percutaneous holmium:YAG laser lithotripsy and ablation of their biliary calculi were successfully treated; the average number of treatments required for stone clearance was 1.6 (range, 1 to 3), no patients required more than three treatments, and 3 of the 13 patients were treated solely as outpatients.
Recurrent Pyogenic Cholangitis (See Chapter 44)
A possible association between biliary helminthic infection and hepatolithiasis has been suggested (Huang et al, 2005; see Chapter 45). Ascaris lumbricoides, Clonorchis sinensis, Opisthorchis viverrini, O. felineus, and Fasciola hepatica are pathogens that often lead to the initial epithelial damage (Abdalian & Heathcote, 2006). Ascaris lumbricoides, a round worm, is a common cause of cholangitis in tropical and subtropical countries. Mature adult worms lodged in the biliary system are responsible for a variety of disease processes that include cholangitis, cholecystitis, gallstone formation, and pancreatitis. In the case of cholangitis secondary to Ascaris infection, the worms are located in the CBD in 95% of cases, and they can be seen in 86% of cases during endoscopic examination (Sandouk et al, 1997).
Choledochojejunostomy with a cutaneous limb offers a number of distinct advantages over previous approaches in the management of RPC (see Chapter 29). Following standard cholecystectomy, a portion of the common duct is isolated for choledochoenteric anastomosis. A 60- to 70-cm segment of bowel is used for the Roux-en-Y limb, and a side-to-side choledochojejunostomy is then constructed 10 to 15 cm from the end of the jejunal limb. The blind limb of the jejunal access loop is then brought through the fascia of the abdominal wall in the right upper quadrant at a point that will allow straight access to the biliary tree. Lateral placement of the bowel, just below the skin, will help ensure that the interventional radiologist’s hands and instruments are not in the fluoroscopy beam. Although gross stones are removed, no attempt is made at complete clearance of the CBD or hepatic radicles. After closure of the abdomen, the stoma is matured in a “turn-back” fashion. The availability of the cutaneous stoma greatly facilitates subsequent treatment of the residual stones and strictures. After completion of radiologic treatment, the stoma is mobilized, closed, and left buried in the subcutaneous tissues for future access (Gott et al, 1996).
Clearance of residual stones and dilation of strictures is easily accomplished on an outpatient basis. The treatment of recurrent stones or strictures is greatly simplified by access to the cutaneous jejunal limb, and it avoids the need for high-risk reoperative biliary procedures (Gott et al, 1996).
Nonlithiatic Biliary Obstruction
AIDS Cholangiopathy
First described in 1986 (Margulis et al, 1986), AIDS cholangiopathy occurs in patients with advanced HIV infection. It occurs with acalculous cholecystitis, focal distal biliary stricture at the papilla of Vater, or multifocal biliary strictures similar to what is seen in primary sclerosing cholangitis. These patients are often colonized with cryptosporidia or microsporidia, but it is not clear whether treatment directed at these organisms with albendazole makes any short- or long-term difference in their outcome (Bird et al, 1995).
The prognostic factors in patients with AIDS cholangiopathy have been studied by Ko and colleagues (2003). In this retrospective analysis, the authors evaluated 94 patients with AIDS cholangiopathy who were diagnosed at the San Francisco General Hospital between 1983 and 2001. Patient outcome (death) was subsequently correlated with a number of collected variables via multivariable logistic regression. Mean survival was 9 months in those patients with cholangiopathy, with those on highly active antiretroviral therapy (HAART) having a significantly prolonged duration of survival. The presence of opportunistic infections and an elevated alkaline phosphatase level (>1000 U/L) were negative prognostic indicators of survival. Interestingly, endoscopic sphincterotomy, the type of cholangiopathy, and CD4 lymphocyte counts did not correlate with survival.
The goal of therapy of both is directed toward elevating the CD4 count and decreasing the viral loads, which can only be obtained by HAART. A multidisciplinary approach to this disorder is critical, particularly because the disease rapidly changes, depending on therapy. With the changing modalities of treatment, it is hoped that AIDS cholangiopathy might become a disorder of historical interest only (Enns, 2003).
The overall prognosis of patients with AIDS cholangiopathy is poor, because the disease itself is believed to be a manifestation of advanced HIV. As previously mentioned, the survival of patients is not affected by endoscopic therapy (Cello & Chan, 1995). Survival rates were low, with 1- and 2-year survival as low at 14% to 41% and 8%, respectively, with a mean reported survival of 7 to 12 months (Bouche et al, 1993; Cello & Chan, 1995). For survivors, an association with subsequent cholangiocarcinoma has also been reported (Hocqueloux & Gervais, 2000).
Biliary-Enteric Strictures
Patients with a history of a previous Roux-en-Y hepaticojejunostomy or choledochojejunostomy who are seen with cholangitis present a complex situation. Initially, a PTCD and balloon dilation of the stricture should be performed, as it is a simple and effective procedure. However, the incidence of long-term restenosis (follow-up period of 5 to 7.5 years) is high—up to 45% (Jan et al, 1994). In the case of recurrent cholangitis after PTCD dilation, every consideration should be given to operative revision of the biliary enteric anastomosis, provided that the patient can safely undergo operation.
Primary Sclerosing Cholangitis
PSC (see Chapter 41) is an idiopathic inflammatory process that affects the biliary tree, resulting in patchy areas of fibrosis and obstruction that are often associated with biliary infection. The diagnosis of PSC is based on selection and exclusion criteria. A persistent twofold or threefold elevation of the alkaline phosphatase level is typical (Gordon, 2008). Protoplasmic-staining antineutrophil cytoplasmic antibodies are present in 80% of cases of PSC, but this finding is not specific (Mulder et al, 1993). The radiologic inclusion criteria include the presence of diffusely distributed strictures of the biliary system seen on cholangiography and characteristic hepatic histology. The histology, classified by Ludwig and colleagues (1981), includes four stages: 1) periportal hepatitis, 2) periportal hepatitis and fibrosis, 3) fibrosis extending beyond the limiting plate, and 4) biliary cirrhosis.
Current management of PSC includes medical therapy with the use of choleretic immunosuppressive and antifibrinogenic agents, surgical management for reconstruction of the biliary tract and colon resection in patients with ulcerative colitis, with liver transplantation reserved for end-stage PSC (Gordon, 2008).
Immunoglobulin G4–Associated Cholangitis
Although the systemic diseases associated with immunoglobulin G4 (IgG4) have been recognized since the 1960s (Sarles et al, 1961), the credit for the first description IgG4 cholangitis belongs to Montefusco and colleagues (1984), who described a series of case reports of patients with combined pancreatic and extrapancreatic disease associated with IgG4. The predominant extrapancreatic feature noted was sclerosing cholangitis with PSC-like features.
In the largest reported cohort to date, which includes 53 patients, Ghazale and colleagues (2008) described the most frequent clinical signs and symptoms at presentation of IgG4 cholangitis: jaundice (77%), weight loss (51%), mild to moderate abdominal pain (26%), steatorrhea (15%), and new-onset diabetes (8%). The diagnostic criteria for IgG4 cholangitis are either having a previous diagnosis of IgG4-related pancreatic/biliary disease or two or more of the following: elevated serum IgG4, other manifestation of a systemic disease, or bile duct biopsy showing more than 10 IgG4-positive cells per high-powered field (HPF) with a documented response after 4 weeks of steroid therapy (Ghazale et al, 2008).
Cholangiographic studies do not reveal pathognomonic findings; they show either a picture of intrahepatic bile duct involvement similar to PSC and/or extrahepatic biliary strictures similar to those found with cholangiocarcinoma and pancreatic cancer. As it is difficult to differentiate IgG4-associated cholangitis from PSC, many studies have investigated the clinical and laboratory findings that correlate with the diagnosis of IgG4. A recent review (Alderlieste et al, 2009) reported significant differences between IgG4 and PSC in the age at onset, occurrence of diabetes mellitus and IBD, salivary gland swelling, and elevated serum IgG4 levels (Table 43.3). Liver or biliary biopsy can help to differentiate IgG4 cholangitis from PSC or pancreatic or biliary cancer. Liver histology in IgG4-associated cholangitis is characterized by lymphoplasmacytic infiltrates within and around bile ducts and obliterative phlebitis and fibrosis leading to sclerosis of the bile ducts. Numbers of IgG4-bearing plasma cells, mainly found in the portal tracts, are significantly higher than in patients with other hepatopathies—including PSC, primary biliary cirrhosis, autoimmune hepatitis, and chronic viral hepatitis—and correlate to serum IgG4 levels, decreasing on corticosteroid treatment (Kamisawa, 2008; Umemura et al, 2007). Endoscopic biopsy specimen of biliary epithelium revealed more than 10 IgG4-positive cells/HPF in 88% of 16 patients with IgG4 cholangitis who underwent biopsy (Ghazale et al, 2008). Accurate diagnosis is of utmost importance to prevent unnecessary surgery and avoid delayed diagnosis of a potentially fatal malignancy.
Table 43.3 PSC and IAC: Differences in Clinical Presentation, Immunopathologic Features, and Treatment Response
| PSC | IAC | |
|---|---|---|
| Age (yr) | 25 to 45 | 65 |
| Male gender | 65% | 80% |
| Response to steroids | — | + + + |
| Association with IBD | + + + | — |
| Association with cholangiocarcinoma | + + + | ? |
| Other organ involvement | ? | + + + |
| Histologic findings | Obliterative cholangitis and cirrhosis | Abundant IgG4-positive plasma cells |
| Dominant cholangiographic findings | Bandlike strictures with a beaded appearance | Segmental strictures and distal bile duct strictures |
| Elevated serum IgG4 | 7% to 9%* | ~70% |
IBD, inflammatory bowel disease; IgG4, immunoglobin G4; IAC, immunoglobulin-associated cholangitis; PSC, primary sclerosing cholangitis
* As the authors of this table suggest, within their cohort of PSC patients, some of the patients with elevated IgG4 levels may be misdiagnosed IAC patients.
From Alderlieste YA, et al, 2009: Immunoglobulin G4–associated cholangitis: one variant of immunoglobulin G4–related systemic disease. Digestion 79(4):220-228.
Cholangitis and Intraabdominal Hepatic Abscess
Inadequately managed cholangitis can be complicated by liver, gallbladder, and subphrenic abscesses (see Chapter 66). In general, these abscesses should be treated following standard surgical principles of drainage and broad-spectrum antibiotics, until the fever resolves and liver function tests return to normal (Rintoul et al, 1996).
Untreated pyogenic liver abscesses are almost uniformly fatal. Traditionally, treatment consists of antibiotic administration and drainage of purulent collections. Although this remains the standard approach to the patient with hepatic abscess, some investigators have advocated the use of antibiotics alone in selected patients (Pearce et al, 2003).
Over the past decade, percutaneous aspiration of pyogenic liver abscess without catheter drainage has also gained increased attention. Retrospective series have reported percutaneous aspiration success rate in combination with antibiotic therapy to be in the range of 58% to 88%, which is comparable to the results of catheter drainage (Barakate et al, 1999; Johannsen et al, 2000; Seeto & Rockey, 1996). However, it is important to mention that no randomized trial comparing percutaneous aspiration and catheter drainage of liver abscesses has been performed.
Antibiotics should be started as soon as possible, once the diagnosis of pyogenic liver abscess is suspected. Blood specimens for culture should be obtained before initiating empiric therapy, but delaying therapy until abscess material is obtained is potentially dangerous and ill advised. The suspected origin of infection should be considered when choosing initial antimicrobial therapy, because it will predict the most likely pathogens. Pyogenic liver abscesses that arise in a patient with biliary disease often include enterococci and enteric gram-negative bacilli. Pyogenic liver abscesses from a colonic or pelvic source are more commonly the result of coliforms and anaerobes. Metronidazole should be included in the initial therapy for most cases of pyogenic liver abscess to empirically treat both anaerobes, especially Bacteroides fragilis and Entamoeba histolytica, while the specific etiology is being evaluated. Once microbiologic data are obtained, antibiotic therapy should be tailored to the organisms isolated and their respective antibiotic susceptibility profiles (Johannsen et al, 2000).
Pyogenic liver abscesses are usually treated initially with parenteral antibiotic therapy for 2 to 3 weeks, and patients subsequently complete a 4- to 6-week course of oral antibiotics (Pitt, 1990). In general, abscesses completely resolve after a full course of therapy. On occasion, a residual cavity persists despite prolonged therapy; if the size of this cavity is stable on serial imaging studies, and the patient is asymptomatic, antibiotics can be stopped and the patient observed closely for the development of recurrent fevers or abdominal pain. In these situations, a follow-up CT scan is recommended 1 to 2 months after cessation of therapy.
Abboud PA, et al. Predictors of common bile duct stones prior to cholecystectomy: a meta-analysis. Gastrointest Endosc. 1996;44:450-455.
Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology. 2006;44:1063-1074.
Alderlieste YA, et al. Immunoglobulin G4–associated cholangitis: one variant of immunoglobulin G4–related systemic disease. Digestion. 2009;79:220-228.
Arai K, et al. Dynamic CT of acute cholangitis: early inhomogeneous enhancement of the liver. Am J Roentgenol. 2003;181:115-118.
Badaro R, et al. A multicenter comparative study of cefepime versus broad-spectrum antibacterial therapy in moderate and severe bacterial infections, and the Latin American Antibiotic Research Group. Braz J Infect Dis. 2002;6:206-218.
Barakate MS, et al. Pyogenic liver abscess: a review of 10 years’ experience in management. Aust N Z J Surg. 1999;69:205-209.
Bird GL, Kennedy DH, Forrest JA. AIDS-related cholangitis: diagnostic features and course in four patients. Scott Med J. 1995;40:53-54.
Bland KI, et al. Extracorporeal shock-wave lithotripsy of bile duct calculi: an interim report of the Dornier U.S. Bile Duct Lithotripsy Prospective Study. Ann Surg. 1989;209:743-753. discussion 753-735
Boender J, et al. Endoscopic sphincterotomy and biliary drainage in patients with cholangitis due to common bile duct stones. Am J Gastroenterol. 1995;90:233-238.
Bonnel DH, et al. Common bile duct and intrahepatic stones: results of transhepatic electrohydraulic lithotripsy in 50 patients. Radiology. 1991;180:345-348.
Bouche H, et al. AIDS-related cholangitis: diagnostic features and course in 15 patients. J Hepatol. 1993;17:34-39.
Cello JP, Chan MF. Long-term follow-up of endoscopic retrograde cholangiopancreatography sphincterotomy for patients with acquired immune deficiency syndrome papillary stenosis. Am J Med. 1995;99:600-603.
Chawla YK, Sharma BC, Dilawari JB. Endoscopic nasobiliary drainage in acute suppurative cholangitis. Indian J Gastroenterol. 1994;13:83-85.
Chen C, et al. Reappraisal of percutaneous transhepatic cholangioscopic lithotomy for primary hepatolithiasis. Surg Endosc. 2005;19:505-509.
Chen MF, et al. A reappraisal of cholangiocarcinoma in a patient with hepatolithiasis. Cancer. 1993;71:2461-2465.
Cheung MT. Postoperative choledochoscopic removal of intrahepatic stones via a T tube tract. Br J Surg. 1997;84:1224-1228.
Cheung MT, Kwok PC. Liver resection for intrahepatic stones. Arch Surg. 2005;140:993-997.
Cheung MT, Wai SH, Kwok PC. Percutaneous transhepatic choledochoscopic removal of intrahepatic stones. Br J Surg. 2003;90:1409-1415.
Chiang HJ, Shan TY, Chen CJ. Percutaneous biliary stone removal under fluoroscopy. Zhonghua Yi Xue Za Zhi (Taipei). 1994;54:343-348.
Chijiiwa K, et al. Late development of cholangiocarcinoma after the treatment of hepatolithiasis. Surg Gynecol Obstet. 1993;177:279-282.
Cotton PB, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393.
Csendes A, et al. Bacteriological studies of liver parenchyma in controls and in patients with gallstones or common bile duct stones with or without acute cholangitis. Hepatogastroenterology. 1995;42:821-826.
Csendes A, et al. Counts of bacteria and pyocites of choledochal bile in controls and in patients with gallstones or common bile duct stones with or without acute cholangitis. Hepatogastroenterology. 1996;43:800-806.
Ducreux M, et al. Management of malignant hilar biliary obstruction by endoscopy: results and prognostic factors. Dig Dis Sci. 1992;37:778-783.
Enns R. AIDS cholangiopathy: “an endangered disease”. Am J Gastroenterol. 2003;98:2111-2112.
Freeman ML, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918.
Gallix BP, et al. Acute cholangitis: imaging diagnosis and management [in French]. J Radiol. 2006;87:430-440.
Gazzaniga GM, et al. Percutaneous transhepatic biliary drainage—twelve years’ experience. Hepatogastroenterology. 1991;38:154-159.
Ghazale A, et al. Immunoglobulin G4–associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706-715.
Gordon FD. Primary sclerosing cholangitis. Surg Clin North Am. 2008;88:1385-1407. x
Gott PE, et al. Biliary access procedure in the management of oriental cholangiohepatitis. Am Surg. 1996;62:930-934.
Gould RJ, et al. Percutaneous biliary drainage as an initial therapy in sepsis of the biliary tract. Surg Gynecol Obstet. 1985;160:523-527.
Hakansson K, et al. MR characteristics of acute cholangitis. Acta Radiol. 2002;43:175-179.
Harz C, et al. Extracorporeal shock-wave lithotripsy and endoscopy: combined therapy for problematic bile duct stones. Surg Endosc. 1991;5:196-199.
Hazey JW, et al. Efficacy of percutaneous treatment of biliary tract calculi using the holmium:YAG laser. Surg Endosc. 2007;21:1180-1183.
Helton W. Biliary tract sepsis. In: Root, R, Trunkey, D, Sande, M. New Surgical and Medical Approaches in Infectious Diseases. New York: Churchill Livingstone; 1987:113-132.
Hocqueloux L, Gervais A. Cholangiocarcinoma and AIDS-related sclerosing cholangitis. Ann Intern Med. 2000;132:1006-1007.
Huang MH, et al. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol. 2003;98:2655-2662.
Huang MH, et al. Relation of hepatolithiasis to helminthic infestation. J Gastroenterol Hepatol. 2005;20:141-146.
Hui CK, et al. A randomised controlled trial of endoscopic sphincterotomy in acute cholangitis without common bile duct stones. Gut. 2002;51:245-247.
Jan YY, Chen MF. Percutaneous trans-hepatic cholangioscopic lithotomy for hepatolithiasis: long-term results. Gastrointest Endosc. 1995;42:1-5.
Jan YY, Chen MF, Hung CF. Balloon dilatation of intrahepatic duct and biliary-enteric anastomosis strictures: long-term results. Int Surg. 1994;79:103-105.
Johannsen EC, Sifri CD, Madoff LC. Pyogenic liver abscesses. Infect Dis Clin North Am. 2000;14:547-563. vii
Kadir S, et al. Percutaneous biliary drainage in the management of biliary sepsis. Am J Roentgenol. 1982;138:25-29.
Kamisawa T. Immunoglobulin G4–positive plasma cells in organs of patients with autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2008;6:715. author reply 715
Ko WF, et al. Prognostic factors for the survival of patients with AIDS cholangiopathy. Am J Gastroenterol. 2003;98:2176-2181.
Kondo H, et al. MR cholangiography with volume rendering: receiver operating characteristic curve analysis in patients with choledocholithiasis. Am J Roentgenol. 2001;176:1183-1189.
Kumar R, et al. Endoscopic biliary drainage for severe acute cholangitis in biliary obstruction as a result of malignant and benign diseases. J Gastroenterol Hepatol. 2004;19:994-997.
Lai EC, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582-1586.
Lee DW, et al. Biliary decompression by nasobiliary catheter or biliary stent in acute suppurative cholangitis: a prospective randomized trial. Gastrointest Endosc. 2002;56:361-365.
Leese T, et al. Management of acute cholangitis and the impact of endoscopic sphincterotomy. Br J Surg. 1986;73:988-992.
Liu CL, Fan ST, Wong J. Primary biliary stones: diagnosis and management. World J Surg. 1998;22:1162-1166.
Ludwig J, et al. Morphologic features of chronic hepatitis associated with primary sclerosing cholangitis and chronic ulcerative colitis. Hepatology. 1981;1:632-640.
Margulis SJ, et al. Biliary tract obstruction in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;105:207-210.
Mazuski JE, et al. The Surgical Infection Society guidelines on antimicrobial therapy for intra-abdominal infections: evidence for the recommendations, and the Therapeutic Agents Committee of the Surgical Infections. Surg Infect (Larchmt). 2002;3:175-233.
Montefusco PP, et al. Sclerosing cholangitis, chronic pancreatitis, and Sjögren’s syndrome: a syndrome complex. Am J Surg. 1984;147:822-826.
Mulder AH, et al. Prevalence and characterization of neutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 1993;17:411-417.
Neitlich JD, et al. Detection of choledocholithiasis: comparison of unenhanced helical CT and endoscopic retrograde cholangiopancreatography. Radiology. 1997;203:753-757.
Nomura T, Shirai Y, Hatakeyama K. Cholangitis after endoscopic biliary drainage for hilar lesions. Hepatogastroenterology. 1997;44:1267-1270.
Paik WH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55.
Pearce N, et al. Non-operative management of pyogenic liver abscess. HPB (Oxford). 2003;5:91-95.
Pitt HA. Surgical management of hepatic abscesses. World J Surg. 1990;14:498-504.
Qian XJ, et al. Treatment of malignant biliary obstruction by combined percutaneous transhepatic biliary drainage with local tumor treatment. World J Gastroenterol. 2006;12:331-335.
Reynolds BM, Dargan EL. Acute obstructive cholangitis: a distinct clinical syndrome. Ann Surg. 1959;150:299-303.
Rintoul R, et al. Changing management of pyogenic liver abscess. Br J Surg. 1996;83:1215-1218.
Sandouk F, et al. Pancreatic-biliary ascariasis: experience of 300 cases. Am J Gastroenterol. 1997;92:2264-2267.
Sarles H, et al. Chronic inflammatory sclerosis of the pancreas—an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688-698.
Schlitt HJ, et al. Reconstructive surgery for ischemic-type lesions at the bile duct bifurcation after liver transplantation. Ann Surg. 1999;229:137-145.
Seeto RK, Rockey DC. Pyogenic liver abscess: changes in etiology, management, and outcome. Medicine (Baltimore). 1996;75:99-113.
Shamamian P, Grasso M. Management of complex biliary tract calculi with a holmium laser. J Gastrointest Surg. 2004;8:191-199.
Soto JA, et al. Detection of choledocholithiasis with MR cholangiography: comparison of three-dimensional fast spin-echo and single- and multisection half-Fourier rapid acquisition with relaxation enhancement sequences. Radiology. 2000;215:737-745.
Sugiyama M, Atomi Y. Treatment of acute cholangitis due to choledocholithiasis in elderly and younger patients. Arch Surg. 1997;132:1129-1133.
Sung JJ, et al. Intravenous ciprofloxacin as treatment for patients with acute suppurative cholangitis: a randomized, controlled clinical trial. J Antimicrob Chemother. 1995;35:855-864.
Thompson JEJr, et al. Broad-spectrum penicillin as an adequate therapy for acute cholangitis. Surg Gynecol Obstet. 1990;171:275-282.
Thompson JEJr, et al. Cefepime for infections of the biliary tract. Surg Gynecol Obstet. 1993;177(Suppl):30-34. discussion 35-40
Tsuyuguchi T, et al. Techniques of biliary drainage for acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:35-45.
Umemura T, et al. Immunoglobin G4 hepatopathy: association of immunoglobin G4–bearing plasma cells in liver with autoimmune pancreatitis. Hepatology. 2007;46:463-471.
van Lent AU, et al. Duration of antibiotic therapy for cholangitis after successful endoscopic drainage of the biliary tract. Gastrointest Endosc. 2002;55:518-522.
Verbesey JE, Birkett DH. Common bile duct exploration for choledocholithiasis. Surg Clin North Am. 2008;88:1315-1328. ix
Vidal V, Ho CS, Petit P. Early cholangitis complicating percutaneous biliary drainage [in French]. J Radiol. 2004;85:1707-1709.
Westphal JF, Brogard JM. Biliary tract infections: a guide to drug treatment. Drugs. 1999;57:81-91.
White DM, et al. Extracorporeal shock-wave lithotripsy for bile duct calculi. Am J Surg. 1998;175:10-13.
Yeh YH, et al. Percutaneous trans-hepatic cholangioscopy and lithotripsy in the treatment of intrahepatic stones: a study with 5 year follow-up. Gastrointest Endosc. 1995;42:13-18.