Atherosclerosis, aneurysms and ischaemic heart disease119
14.2 Hypertension, cardiomyopathies and myocarditis122
14.3 Congenital heart disease124
14.4 Valvular heart disease124
14.5 Heart failure125
14.6 Thrombosis, embolism and vasculitis127
14.7 Pericardial disease128
Pathology of the cardiovascular system, the heart and blood vessels, is responsible for a large proportion of deaths in the UK each year, and also causes considerable morbidity through heart failure, stroke and peripheral vascular disease. The major underlying disease processes responsible include atherosclerosis, hypertension and diabetes mellitus. Lifestyle modification can have a significant role in reducing disease risk, particularly in relation to smoking, exercise, body weight and fat consumption. This chapter covers atherosclerosis and its complications, ischaemic heart disease, hypertension, valvular heart disease, cardiomyopathies and congenital heart disease. Many cardiac pathologies result in heart failure, which may initially primarily involve either the left or right ventricle. The basic pathology of vasculitis and vascular tumours is also reviewed.
14.1. Atherosclerosis, aneurysms and ischaemic heart disease
Atherosclerosis
Atherosclerosis (also called atheroma) is an inflammatory, degenerative disease of large and medium-sized arterial vessels. It is characterised by the development of fibrolipid plaques within the intima of the vessel wall. In smaller arteries, including the coronary vessels that supply the myocardium, these plaques can cause severe narrowing (stenosis) of the lumen, with significant impairment of blood flow. The clinical consequences depend on the speed of development of the stenosis, and on whether the affected tissue has any additional source of blood supply (known as collateral supply). In large arteries, such as the abdominal aorta, the inflammatory atherosclerotic process also damages the muscular wall causing weakness and dilatation. This is known as aneurysm formation (see Box 17). As the aneurysm enlarges there is an increasing risk of rupture, with resultant catastrophic haemorrhage.
Box 17
Definition. Abnormal dilatation of a vessel wall, which communicates with the lumen – almost always arterial
Pathogenesis:
• Inflammation: atherosclerotic aneurysm (abdominal aorta, iliac arteries, popliteal arteries); polyarteritis nodosa; Kawasaki’s disease (coronary arteries)
• Infection (mycotic aneurysm): syphilis; direct spread from adjacent infection
• Congenital defect: Berry aneurysm (circle of Willis, see Ch. 27)
• Metabolic: diabetes mellitus (retinal capillary microaneurysms)
• Hypertension: Charcot–Bouchard aneurysms (deep white matter of cerebral hemispheres)
• Trauma
Complications:
• Rupture with haemorrhage (often fatal if aorta or cerebral vessels involved)
• Compression of adjacent structures
• Thrombus formation (vessel occlusion and distal thromboembolism)
• Secondary infection
• Sclerosing periaortitis (dense fibrosis surrounding abdominal aortic aneurysm which can entrap ureters)
Four major risk factors are recognised for atherosclerosis:
• smoking
• hypercholesterolaemia (raised low density lipoproteins)
• hypertension
• diabetes mellitus.
Minor risk factors include increasing age, male sex, obesity, family history and stress.
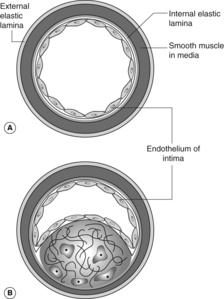
Pathogenesis of the fibrolipid atherosclerotic plaque
Atheromatous plaques are probably initiated by injury to the endothelial cells of the arterial intima. An inflammatory response is evoked to the damage, which results in an infiltrate of macrophages, and proliferation of smooth muscle cells from the media of the vessel wall. These smooth muscle cells migrate into the intima and begin to produce collagen. Both the macrophages and the smooth muscle cells may accumulate lipid within their cytoplasm, giving a vacuolated appearance on light microscopy (‘foam cells’). Free cholesterol and necrotic inflammatory debris also become incorporated within the plaque lesion.
Figure 33 shows a normal muscular artery and a typical atheromatous plaque with a fibrous tissue cap and a lipid-rich, necrotic centre. It is important to realise that the actual composition of individual plaques varies, and can change over time. Plaques that are particularly rich in lipids may be more unstable and susceptible to rupture or haemorrhage. Older plaques can become heavily calcified. The plaque surface is prone to develop superimposed thrombosis, which can rapidly increase the severity of the obstruction to blood flow. At autopsy, it is common to find a recent thrombus complicating an atheromatous coronary artery plaque in patients who died after acute myocardial infarction.
Clinical complications of atherosclerosis
• Cerebral arteries: transient ischaemic attacks, stroke.
• Aorta: abdominal aneurysm formation (risk of rupture and death) (see Box 17).
• Mesenteric arteries: intestinal ischaemia and infarction.
• Renal arteries: renal artery stenosis (hypertension, ischaemic kidney).
• Lower limbs: intermittent claudication, gangrene.
Aortic dissection
Aortic dissection is also called dissecting aneurysm, although this is an inaccurate term as the aorta is not significantly dilated. In aortic dissection, blood tracks into the muscular wall of the blood vessel. The entry point of blood into the aortic media is through an intimal tear, usually within the proximal 10cm of the ascending aorta. Occasionally, there is a second distal luminal tear, through which blood re-enters the circulation, producing a ‘double-barrelled’ aorta. More commonly, however, the haemorrhage extends outwards with vessel rupture and catastrophic extramural haemorrhage into:
• the pericardium, causing cardiac tamponade
• the mediastinum, causing haemothorax
• the abdominal cavity (haematoperitoneum).
The dissection can also involve the great vessels of the neck, compromising cerebral blood flow. Dissection of the coronary arteries is a rare cause of acute myocardial ischaemia.
There is a strong association with systemic hypertension and with Marfan’s syndrome.
Ischaemic heart disease
Ischaemia is due to lack of oxygen, and in the overwhelming majority of ischaemic heart disease (IHD) cases this is due to reduced blood flow through coronary arteries narrowed or occluded by atherosclerosis. However, the left ventricular myocardium is also at risk of ischaemia when there is pathological hypertrophy, for example in systemic hypertension or aortic valve stenosis. Under these conditions, cardiac perfusion may be insufficient to meet the metabolic needs of the increased muscle mass. Rarely, cardiac ischaemia can result from decreased oxygen-carrying capacity of the blood in severe anaemia (even though the coronary arteries may be normal).
Myocardial infarction
Regional (transmural) infarction
Regional (transmural) infarction is caused by occlusion of a single coronary artery. The arterial blockage results from atherosclerosis complicated by thrombosis or by intraplaque haemorrhage, which rapidly expands the atheromatous plaque (see Figure 34). The commonest sites for clinically significant coronary atherosclerosis are:
• proximal left anterior descending artery (up to 50%)
• right coronary artery (30%)
• left circumflex artery (up to 20%)
• left main coronary artery.
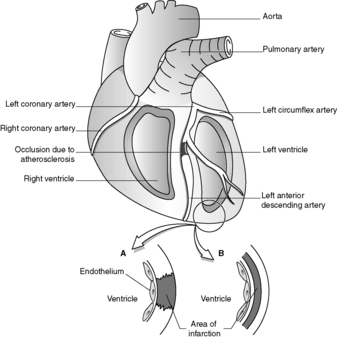 |
| Figure 34 |
Regional infarction most frequently affects part of the anterior wall of the left ventricle, or part of the interventricular septum, with extension into the right ventricle in a small proportion of cases. Isolated right ventricular infarction is very uncommon.
The subendocardial region of the myocardium is the muscle area most vulnerable to hypoxia.
Subendocardial infarction
Subendocardial infarction can occur when there is a global decrease in cardiac blood flow due to systemic hypotension (‘shock’). Myocardial necrosis is usually limited to the inner third of the muscle but can involve the territory of more than one coronary artery.
Unusual causes of myocardial infarction include coronary artery dissection, arteritis or spasm.
Macroscopic and microscopic changes in myocardial infarction
• Less than 24 hours – microscopic changes only (increased eosinophilic staining of cardiac muscle cells, loss of nuclei, muscle cells become buckled).
• From 24 to 72 hours – infarct becomes apparent macroscopically at autopsy as an area of pallor or yellow discoloration, with a peripheral rim of haemorrhage. Microscopically the dead muscle fibres provoke an inflammatory response, initially consisting of neutrophils followed by macrophages. The infarct becomes soft.
Biochemical markers of myocardial infarction
Necrotic cardiac muscle releases enzymes, which can be measured in the serum and are helpful in confirming the diagnosis.
• Heart-specific troponins – released 2–4 hours after cell death, remaining raised for up to 1week. Troponin T and I are highly specific for myocardial damage.
• Creatine kinase (CK) – starts to rise after a few hours of infarction, and falls again within 48 hours. CK is also released from injured skeletal muscle cells; measurement of the specific cardiac isoenzyme is therefore more diagnostically helpful.
• Aspartate aminotransferase (AST) – this is also non-specific as it is released by damaged liver cells.
• Lactate dehydrogenase (LDH) – peaks at 3–6days and may remain elevated for 2weeks. May be useful in patients presenting late after suspicious chest pain.
Complications of myocardial infarction
Immediate:
• arrhythmias
• acute cardiac failure
• cardiogenic shock
• sudden death.
Early:
• infarct rupture (3–7days).
Late:
• mural thrombosis
• cardiac aneurysm.
Dressler’s syndrome (pericarditis associated with circulating antibodies to heart muscle) occurs from 2weeks to 2years after infarction.
Reinfarction can occur at any time. Cardiac failure and arrhythmias may also be late complications. The presence of myocardial scarring increases the risk of sudden death from ventricular arrhythmia.
14.2. Hypertension, cardiomyopathies and myocarditis
You should:
• know the aetiology, risk factors and complications of hypertension, so as to be able to identify patient risk factors amenable to treatment by lifestyle modification, and to investigate patients appropriately for causes of secondary hypertension
• understand the term cardiomyopathy, its classification, major causes and complications.
Hypertension
Chronically raised systemic blood pressure is a major cause of morbidity and mortality. Hypertension can cause, or significantly contribute to:
• atherosclerosis
• hypertensive heart disease (left ventricular hypertrophy)
• chronic renal failure
• cerebrovascular disease (intracerebral haemorrhage, ruptured Berry aneurysm)
• retinopathy.
‘Normal’ blood pressure varies within a population and with age, but evidence suggests that a sustained pressure of 140/90mmHg or greater is associated with increased risk of disease. Persistent diastolic blood pressure in excess of 100mmHg requires treatment. Very high blood pressure (e.g. 240/120mmHg) can result in accelerated disease – ‘malignant hypertension’.
In approximately 10% of cases, systemic hypertension is secondary to another disease (Box 18). The mechanism of primary hypertension is unclear but the following defects have been found in patients:
• abnormal renal excretion of sodium
• abnormal sodium and calcium metabolism in vascular smooth muscle
• abnormalities of renin-angiotensin mechanism, probably in part related to polymorphisms in key genes.
Box 18
Renal disease:
• Glomerulonephritis
• Polycystic kidney disease
• Renal artery stenosis
• Chronic pyelonephritis
• Renal cell carcinoma
Endocrine disease:
• Adrenal cortical tumours
• Cushing’s disease
• Phaeochromocytoma
• Diabetes mellitus
• Acromegaly
Coarctation of the aorta
Iatrogenic:
• Steroid treatment
Note that hypertension can be either a cause or a consequence of chronic renal impairment.
Family history, obesity and excessive alcohol intake are further associated factors. The aetiology of essential hypertension is complex and clearly involves a combination of genetic and environmental factors. Modest, but clinically significant, reductions in blood pressure may be achieved by weight loss, regular physical exercise, moderation of alcohol intake and possibly by reduction of salt in the diet.
Cardiomyopathy
Cardiomyopathy is defined as heart muscle disease not caused by ischaemic, hypertensive, valvular or congenital heart disease. Although uncommon, cardiomyopathies are an important cause of cardiac failure and sudden death in young adults. The aetiology of specific cardiomyopathies is shown in Box 19. Ninety per cent are of the dilated type.
Box 19
Genetic:
• Hypertrophic cardiomyopathy (HOCM)
• Haemochromatosis
Post-infectious:
• Dilated cardiomyopathy (many cases are probably caused by viral infection)
Toxic:
• Alcoholic cardiomyopathy
• Drug induced (chemotherapy agents)
Metabolic:
• Amyloid heart disease
Idiopathic
Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HOCM) is characterised by asymmetrical left ventricular hypertrophy (as opposed to the concentric hypertrophy seen in hypertensive heart disease and aortic valve stenosis). The interventricular septum is particularly thickened. Microscopically, the cardiac muscle cells are enlarged and haphazardly organised (myocyte disarray). There is often fibrosis between muscle cells. Many cases show autosomal dominant inheritance. Genetic defects include cardiac myosin genes on chromosome 14. Complications of hypertrophic cardiomyopathy include:
• atrial fibrillation
• atrial thrombus and systemic embolism
• infective endocarditis of the mitral valve
• cardiac failure
• sudden death.
Dilated cardiomyopathy
Dilated cardiomyopathy is characterised by dilatation of all four cardiac chambers with progressive cardiac failure. The heart is typically enlarged (two to three times normal weight) and flabby at autopsy. Mural thrombi may be present. The microscopic findings are not specific, but there is often patchy ventricular fibrosis. Cardiomyopathy caused by alcohol, chemotherapy agents and haemochromatosis is of the dilated type.
Restrictive cardiomyopathy
Restrictive cardiomyopathy describes a condition of ‘stiff’ ventricles, which fail to relax, impeding diastolic filling. The ventricles are of normal size but the atria are dilated. Microscopically there is non-specific myocardial scarring. Causes include radiation fibrosis and cardiac involvement by amyloid.
Myocarditis
Myocarditis is inflammation of the heart muscle. It may be asymptomatic, or cause:
• acute cardiac failure
• sudden death
• chronic cardiac failure (usually due to dilated cardiomyopathy).
Acute myocarditis may be suggested at autopsy by a pale, flabby heart, but histological examination is necessary for diagnosis. Microscopically there is multifocal myocardial chronic inflammation associated with death of the cardiac muscle cells. The aetiology of myocarditis is shown in Box 20.
Box 20
Infection:
• Viral (Coxsackie, ECHO)
• Bacterial (meningococcus)
• Fungal (Candida)
• Parasitic (Chagas’ disease, toxoplasmosis)
Immune-mediated:
• Post-infective (including rheumatic fever)
• Systemic lupus erythematosus
• Transplant rejection
Idiopathic:
• Sarcoidosis
14.3. Congenital heart disease
Congenital heart disease describes abnormalities of the heart and major vessels that are present at birth. The incidence of congenital heart disease in liveborn infants is approximately 1 : 200. The common lesions include:
• isolated defects in cardiac chamber walls – atrial septal defect, ventricular septal defect
• persistence of embryonic structures – patent foramen ovale, patent ductus arteriosus
• stenosing lesions (‘narrowings’) – aortic valve stenosis, pulmonary stenosis, coarctation of the aorta
• transposition of the great arteries
• complex anomalies – Fallot’s tetralogy.
The aetiology is unknown in many cases but there is evidence of genetic predisposition. A small percentage of cases are associated with chromosome abnormalities, particularly Turner’s syndrome (45XO syndrome), with coarctation of the aorta, and Down’s syndrome (trisomy 21), with atrial and ventricular septal defects. Intrauterine infection with rubella may cause multiple congenital cardiac defects. Atrial septal defects are a feature of fetal alcohol syndrome.
Some congenital heart defects, such as small atrial septal defects, may be clinically inconsequential or present only in later adult life.
Abnormal connections between the cardiac chambers permit shunting of blood from one side of the circulation to the other. In post-natal life, atrial and ventricular septal defects usually cause shunting of blood from the left side of the heart (high pressure) to the right side (low pressure). This increases the work of the right ventricle (pressure and volume overload) and can lead to right ventricular hypertrophy and pulmonary hypertension. Structural narrowing of pulmonary arteries occurs in response to chronically raised pulmonary vascular pressure, and the flow of blood through the shunt may be reversed (i.e. it becomes a right-to-left shunt, known as Eisenmenger syndrome).
Some congenital cardiac defects cause right-to-left blood shunts from their onset. Deoxygenated blood bypasses the lungs and passes directly from the right heart into the systemic circulation. If the shunt is large enough, cyanosis will be clinically apparent as blue discoloration of the skin and nail beds. Cyanotic congenital heart defects include the following.
Fallot’s tetralogy
• Ventricular septal defect
• Outflow obstruction to the right ventricle
• Aorta overriding the ventricular septal defect
• Right ventricular hypertrophy
Transposition of the great vessels
The aorta emerges from the right ventricle and the pulmonary artery arises from the left ventricle. To be compatible with post-natal life, there must also be either an atrial or ventricular septal defect.
14.4. Valvular heart disease
You should:
• understand the pathological causes and pathophysiological consequences of stenosis and incompetence of all the cardiac valves, but particularly the mitral and aortic valves
• understand the pathology of infective endocarditis, so as to be able to identify patients at risk and, when appropriate, ensure prophylactic treatment is given.
Valvular stenosis and incompetence
Damage to the cardiac valves can result in stenosis (narrowing of the valve orifice with obstruction to blood flow) or incompetence (regurgitation of blood back through a leaking valve). Valve disease can be congenital (see Section 14.3) or acquired. In non-congenital cases the clinically important lesions almost always affect the mitral or aortic valves.
Abnormal movement of deformed valves and abnormal blood flow causes characteristic murmurs and added sounds on cardiac auscultation.
Rheumatic heart disease
Rheumatic heart disease (RHD) is a major cause of acquired mitral valve disease. The incidence has dramatically decreased in the UK over recent decades but is still relatively common in the developing countries. The initiating event is usually a pharyngeal infection by group A β-haemolytic streptococci, followed several weeks later by rheumatic fever, an immunologically mediated multisystem disorder. Rheumatic fever (RF) results from the cross-reaction of antistreptococcal antibodies with normal host tissues – direct bacterial infection does not occur. During acute RF there is often a pancarditis with inflammation of pericardium, myocardium and endocardium. Myocarditis can occasionally cause acute heart failure, arrhythmias and death. However, it is recurrent attacks of rheumatic fever that produce the most significant cardiac lesions. Repeated acute inflammation of the endocardium covering heart valves leads to fibrosis and deformity. Valve leaflets become thickened and fused, resulting in ‘fishmouth’ or ‘buttonhole’ mitral valve stenosis. The aortic valve may also be affected. Complications include:
• atrial fibrillation
• valvular and atrial thrombus formation with systemic embolism
• infective endocarditis.
Mitral valve prolapse
Mitral valve prolapse, also known as ‘floppy mitral valve’, is a common condition involving approximately 5% of adults. Young females and individuals with Marfan’s syndrome are particularly affected. One or both mitral valve leaflets are enlarged and prolapse into the left atrium during systole. The condition is usually incidental but occasionally can cause mitral regurgitation, infective endocarditis, valvular thrombosis or arrhythmias.
Infective endocarditis
Infection of the endocardium or vascular endothelium can occur in:
• previously damaged heart valves (e.g. rheumatic heart disease, calcific aortic stenosis)
• congenital heart disease
• prosthetic heart valves or vascular tissue
• normal heart valves (uncommon causes, acute severe endocarditis).
Infective endocarditis (see also Box 21) is usually a chronic/subacute illness caused by low-virulence organisms colonising abnormal tissue. Normal heart valves can be infected by high-virulence bacteria or fungi, especially in intravenous drug users or immunosuppressed individuals (those with acquired immune deficiency syndrome (AIDS) or diabetics, alcoholics, transplant recipients).
Box 21
Acute endocarditis
Acute endocarditis typically presents with fever and acute valvular incompetence. In intravenous drug users the tricuspid valve is often affected (organisms are injected directly into arm or leg veins). Blood cultures will often be positive for the causative virulent organism. Early antibiotic treatment is necessary and emergency valve replacement may be indicated if valve destruction has caused cardiac failure.
Subacute endocarditis
Subacute endocarditis may give rise to non-specific chronic symptoms of malaise, fatigue, weight loss and anorexia. There is often a fluctuating pyrexia and a heart murmur (particularly a regurgitant or changing murmur). Extracardiac clinical features frequently reflect embolisation of cardiac vegetations, immune complex deposition or sepsis, and include:
• splinter haemorrhages in the nails
• petechial haemorrhages in the skin and conjunctivae
• glomerulonephritis
• cerebral infarction (or history of transient ischaemic attacks)
• finger clubbing
• splenomegaly
• disseminated abscesses or infarcts.
Common pathogenetic bacteria in infective endocarditis include:
• Streptococcus viridans – normal flora of upper respiratory tract; low virulence.
• Streptococcus faecalis – normal flora of perineum and gut; low virulence.
• Staphylococcus aureus – can be member of normal mucocutaneous flora; high virulence.
• Staphylococcus epidermidis – normal skin flora; low virulence.
Other organisms include Coxiella, Escherichia coli, Chlamydia and Candida.
Colonisation occurs following transient bacteraemia (bacteria floating in the bloodstream, distinct from septicaemia, which is clinical illness caused by bacteria multiplying in the blood). Such bacteraemia may result from dental work, endoscopy, surgery or established infection elsewhere in the body. Organisms may be introduced in prosthetic material or by intravascular catheters such as central venous pressure lines. Patients known to have damaged or prosthetic heart valves are at risk of developing infective endocarditis after episodes of transient bacteraemia with low virulence organisms, and so require prophylactic antibiotic therapy prior to undergoing any procedure that might seed organisms into the bloodstream.
Layers of microorganisms and fibrinous inflammatory debris build up at the site of colonisation, forming ‘vegetations’, which can be seen on an echocardiogram. Complications include:
• valve perforation with acute incompetence
• valve thrombosis
• perivalvular abscess
• dehiscence of prosthetic valves
• septic embolisation of the vegetations with distant infarction and abscess formation in the brain, kidney, spleen, bone, and elsewhere.
The causes and consequences of valvular heart disease are summarised in Table 19.
| Valve lesion | Causes | Consequences |
|---|---|---|
| Mitral stenosis | Rheumatic heart disease | Increased left atrial pressure; left atrial dilatation, atrial fibrillation Increased pulmonary venous pressure, leading to pulmonary hypertension and right ventricular hypertrophy |
| Mitral incompetence | Rheumatic heart disease Infective endocarditis Left ventricular dilatation Papillary muscle ischaemia, fibrosis or rupture Mitral valve prolapse Leaking prosthetic valve |
Left atrial dilatation Left ventricular hypertrophy, due to volume overload (acute mitral incompetence caused by rupture of necrotic papillary muscle in myocardial infarction can result in acute cardiac failure) |
| Aortic stenosis | Senile calcification of normal tricuspid valve Calcification of congenitally bicuspid valve Rheumatic heart disease |
Left ventricular hypertrophy, due to pressure overload (increased gradient across stenotic valve); ischaemia of hypertrophic ventricular myocardium (angina, arrhythmias, cardiac failure, sudden death) |
| Aortic incompetence | Rheumatic heart disease Infective endocarditis Leaking prosthetic valve Aortic root dilatation (aortic dissection, arthritis, Marfan’s syndrome, syphilis) |
Left ventricular hypertrophy, due to volume overload; left ventricular failure |
14.5. Heart failure
You should:
• understand the pathological causes of heart failure in order to appropriately investigate patients with this condition
• be able to describe the typical changes seen at autopsy in the organs of a patient who has died from cardiac failure
• understand the clinical features of left and right heart failure and be able to relate these to the underlying pathological changes.
Left ventricular failure
Left ventricular failure results from a loss of myocardial contractility, due to the sudden or gradual dysfunction, or death, of cardiac muscle cells. Less frequently, there may be marked impairment of ventricular filling during diastole. The latter situation occurs when the left ventricular wall is abnormally ‘stiff’ and unable to distend adequately.
Loss of myocardial contractility
• Ischaemic heart disease
• Hypertensive heart disease
• Valvular heart disease
• Dilated cardiomyopathy
Inability to fill ventricle adequately
• Massive ventricular hypertrophy (hypertension, hypertrophic cardiomyopathy, aortic stenosis)
• Amyloidosis (restrictive cardiomyopathy)
Other causes
• Pericardial disease that impedes cardiac filling (pericardial effusion or constrictive pericarditis)
• High-output cardiac failure (anaemia or thyrotoxicosis)
Markedly increased blood volume due to iatrogenic fluid overload or renal failure can precipitate or exacerbate cardiac failure.
Right ventricular failure
Right ventricular failure is most often encountered as a consequence of left ventricular dysfunction. Myocardial infarction only infrequently involves the right side of the heart, and then this is usually as an extension of a predominantly left ventricular infarct. Primary right ventricular failure can occur as a result of chronic lung disease. The right ventricle is spared the effects of systemic hypertension. However, raised pressure in the pulmonary circulation will affect the right ventricle, causing hypertrophy. This is recognised by the pathologist at post mortem by an increase in the thickness of the right ventricular wall, which is normally less than 5mm, and an increase in isolated right ventricular weight (normally less than 50–60g; total heart weight depends on sex and body weight, but is usually 250–350g in the absence of any cardiac disease). Lung diseases causing pulmonary hypertension include:
• pulmonary embolism
• obstructive lung diseases
• interstitial lung diseases.
Clinical features of heart failure
In left ventricular failure, these changes first appear in the pulmonary circulation. Back-pressure is transmitted through pulmonary veins into the alveolar capillaries of the lungs. Thus, oedema develops first in the pulmonary air spaces, causing shortness of breath. Patients dying with pulmonary oedema have dark-red, ‘wet’ lungs at post mortem, due to the accumulation of blood and oedema. Lung weights (normally 250–300g) are often doubled or trebled by the fluid, which is easily demonstrated when the lung surfaces are cut with a knife and the fluid squeezed out, like water from a sponge. Microscopically, ‘heart failure cells’ are often prominent – these are alveolar macrophages containing haemosiderin pigment, a marker of previous capillary haemorrhage. Fluid accumulation within the pleural cavities may produce bilateral serous effusions.
In right ventricular failure, the inferior and superior vena cavae and their feeding veins become congested. Clinically this is evident as raised jugular venous pressure in the neck. Congestion may extend into many organs, especially the liver, which becomes enlarged and shows a characteristic patchy, pale and reddish cut surface at post mortem (which pathologists like to describe as ‘nutmeg liver’). Splenomegaly is also common. Oedema develops in the dependent areas of the body, affecting the legs of seated and ambulant patients, and the sacral area in patients confined to bed. Ascites (straw-coloured fluid) may develop in the peritoneal cavity.
In severe left ventricular failure, symptoms and signs will also result from failure to adequately perfuse the systemic organs. The systemic blood pressure is decreased, the skin appears pale and cold as circulation is diverted to conserve vital organs, especially the brain. Intestinal ischaemia can result in haemorrhage or infarction. Renal ischaemia stimulates the renin-angiotensin system, causing fluid retention that may exacerbate oedema. Severe kidney ischaemia causes acute tubular necrosis and acute renal failure. In advanced heart failure, even the cerebral circulation may be compromised sufficiently to cause symptoms of confusion and diminished consciousness. The effects of low cardiac output are confounded as many patients with cardiac failure also have significant atherosclerotic disease in their major visceral arteries, which further limits blood flow to the organs.
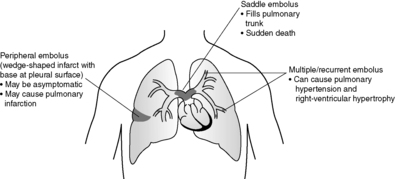
14.6. Thrombosis, embolism and vasculitis
Vasculitis
Inflammation of blood vessels can affect arteries, veins and capillaries. The aetiology most frequently appears to be immunological, with deposition of immune complexes in vessel walls. Some vasculitides are associated with antineutrophil cytoplasmic antibodies (ANCA). These antibodies show two distinct patterns on direct immunofluorescence examination – perinuclear (p-ANCA) and cytoplasmic (c-ANCA). Over 80% of patients with Wegener’s granulomatosis have circulating c-ANCA at some stage of their disease. The pathogenetic role of ANCA is uncertain, but the antibodies are useful in diagnosis and in monitoring disease activity and the effects of treatment.
The more commonly encountered and important vasculitides are shown in Table 20. Vasculitis can also occur in connective tissue disease such as rheumatoid arthritis. Infectious vasculitis occurs when microorganisms invade blood vessels from an adjacent source of sepsis, or via haematogenous spread from distant sites of infection.
| Disease | Vessel type | Clinico-pathological features | Mechanism of disease |
|---|---|---|---|
| Giant cell arteritis | Temporal arteries, also aorta | Segmental granulomatous inflammation, headache, visual impairment | Unknown |
| Polyarteritis nodosa | Medium-sized (muscular) arteries | Focal vessel wall necrosis, aneurysm formation, multiple organ involvement with ulceration, infarcts and haemorrhage | Unknown (possibly immune complex related) |
| Wegener’s granulomatosis | Arteries, arterioles, capillaries, venules | Necrotising vasculitis, granulomatous inflammation of the respiratory tract, glomerulonephritis (often severe) | Immunologically mediated, c-ANCA raised in active disease |
| Kawasaki’s disease | Coronary arteries | Acute illness – necrotising vasculitis, cervical lymphadenopathy, skin rash and erythema; coronary artery aneurysm is a late complication | Unknown (possibly infection, possibly immune system dysfunction) |
| Henoch–Schönlein purpura | Arterioles, capillaries, venules | Haemorrhagic rash on buttocks and legs due to acute necrotising vasculitis, 30% have glomerulonephritis | Immunological (IgA immune complexes) |
| Cutaneous hypersensitivity vasculitis | Arterioles, capillaries, venules | Skin rash, vessel necrosis with acute inflammation (neutrophil polymorphs) | Immunological reaction to drugs, microorganisms or other antigens |
14.7. Pericardial disease
Pericardial serous effusions occur in congestive cardiac failure. Haemopericardium (blood in the pericardium) is seen in ruptured myocardial infarction, aortic dissection and trauma. Rapid accumulation of greater than about 200ml of blood into the pericardium prevents ventricular filling and causes cardiac arrest.
Pericardial inflammation (pericarditis) has various causes:
Infections:
• bacterial
• viral
• tuberculosis
• fungal.
Immunological:
• rheumatic fever
• systemic lupus erythematosus
• post-myocardial infarction (Dressler’s syndrome).
Other causes:
• uraemia
• post-surgery
• neoplasia
• trauma.
14.8. Vascular and cardiac neoplasms
Haemangiomas
Haemangiomas are common benign neoplasms of blood vessels, usually of capillary type. Haemangiomas occur in the skin and in many solid organs.
Kaposi’s sarcoma
Kaposi’s sarcoma is a low-grade vascular proliferation usually classified as a neoplasm, but recently linked to human herpes virus 8 infection. Kaposi’s sarcoma occurs in elderly eastern European men as an indolent disease, and also arises in African children (more aggressive). Kaposi’s sarcoma often develops in patients with AIDS.
Angiosarcoma
Angiosarcoma is an uncommon malignant tumour of endothelial cells, usually arising on the head and neck of elderly patients. It can also arise in solid organs. Hepatic angiosarcoma is associated with industrial exposure to polyvinyl chloride.
Cardiac myxoma
The heart and pericardium can become secondarily involved by malignant tumours, particularly by direct spread of bronchial carcinoma or malignant pleural mesothelioma.
Self-assessment: questions
One best answer questions
2. A 28-year-old man is admitted to hospital with fever and acute left ventricular failure. He admits to regular intravenous drug misuse. He has no previous significant medical history. The likely diagnosis is:
a. viral myocarditis
b. hypertrophic cardiomyopathy
c. congenital heart disease
d. acute endocarditis
e. subacute endocarditis
True-false questions
1. The following investigations may be indicated in a young adult with hypertension:
a. urinary tract ultrasound
b. urinary catecholamine measurement
c. urinary oestrogen measurement
d. renal artery angiography
e. plasma glucose measurement.
2. The following are correctly paired:
a. patent ductus arteriosus – infective endocarditis
b. Fallot’s tetralogy – tricuspid stenosis
c. atrial septal defect – early cyanotic heart disease
d. Turner’s syndrome – coarctation of the aorta
e. ventricular septal defect – pulmonary hypertension
3. Early complications of myocardial infarction include:
a. papillary muscle rupture
b. complete heart block
c. cardiogenic shock
d. ventricular aneurysm
e. Dressler’s syndrome
4. The following conditions can cause a dilated cardiomyopathy:
a. haemochromatosis
b. amyloidosis
c. viral myocarditis
d. alcohol misuse
e. chemotherapeutic agents
5. The following are normal constituents of atherosclerotic plaques:
a. cholesterol
b. smooth muscle cells
c. collagen
d. neutrophil polymorphs
e. foamy macrophages
6. Isolated left ventricular failure causes:
a. pulmonary oedema
b. haemosiderin-laden alveolar macrophages
c. ascites
d. ‘nutmeg’ liver
e. raised jugular venous pressure
7. The following are risk factors for coronary heart disease:
a. alcohol consumption of 1 unit per day
b. raised high density lipoprotein (HDL) cholesterol
c. post-menopausal state
d. modest hypertension
e. diabetes mellitus
8. The following are correctly paired:
a. giant cell arteritis – temporal artery involvement
b. atherosclerosis – granulomatous inflammation
c. Wegener’s granulomatosis – respiratory-tract disease
d. polyarteritis nodosa – c-ANCA
e. Henoch–Schönlein purpura – IgA nephropathy
9. Aortic dissection:
a. is a complication of atherosclerosis
b. often commences distal to the aortic arch
c. is associated with systemic hypertension
d. can occur in patients with inherited connective tissue disorders
e. is inevitably fatal
10. Regarding myocardial infarction:
a. macroscopic changes will be evident in the myocardium after 10 hours
b. the right ventricle alone is affected in 10% of cases
c. the underlying pathology always involves coronary artery atherosclerosis
d. irreversible myocardial cell injury requires at least 20 minutes of anoxia
e. the subepicardial region of the heart muscle is the most susceptible to hypoxia
12. The following are correctly paired:
a. mitral stenosis – left ventricular hypertrophy
b. rheumatic fever – Staphylococcus aureus
c. mitral incompetence – myocardial infarction
d. aortic stenosis – sudden cardiac death
e. mitral valve disease – cerebrovascular accident
13. The following are risk factors for infective endocarditis:
a. rheumatic heart disease
b. prosthetic heart valves
c. immunosuppression
d. endoscopy
e. myocardial infarction
Case history questions
Case history 1
A 67-year-old woman presents with angina. An electrocardiogram (ECG) shows changes of left ventricular hypertrophy. An echocardiogram shows left ventricular thickening and an abnormal bicuspid aortic valve.
1. What are the pathological causes of left ventricular hypertrophy?
2. What is the significance of a bicuspid aortic valve?
3. What other cardiovascular complications might occur in this patient?
Case history 2
A 58-year-old man presents with chest pain. He has a history of hypertension. The blood pressure in his left arm is 180/100mmHg, whereas that in his right arm is 140/90mmHg. A chest X-ray shows minor widening of the mediastinal structures, which may be related to the aorta. Electrocardiogram (ECG) shows no evidence of acute ischaemia. He collapses and dies suddenly and a coroner’s autopsy is performed to ascertain the cause of death. On opening the chest, a haemopericardium is apparent.
1. What are the possible causes of haemopericardium?
2. What is the clinical significance of hypertension and of the blood pressure difference between the arms?
3. What inflammatory conditions can affect the aorta?
Case history 3
A 27-year-old drug misuser is seen in Accident and Emergency after taking an overdose of heroin. Clinical examination and investigations during admission show swinging pyrexia, splinter haemorrhages and a cardiac murmur.
1. Which other investigations are indicated?
2. Of what further cardiovascular complications is the patient at risk?
Case history 4
A 48-year-old woman consults her general practitioner for advice on coronary heart disease prevention. Her mother and paternal grandfather both died of ‘heart attack’ before their seventieth birthday. She has no history of cardiac symptoms herself, and is reluctant to take any regular medications.
1. What lifestyle advice would you give to this patient?
2. In view of her family history, what investigations might be considered?
Viva questions
1. What changes would you expect to see in the organs at the post-mortem examination of a patient with congestive cardiac failure?
2. Discuss the risk factors for pulmonary embolism.
Self-assessment: answers
One best answer
2. d. Whilst all of these conditions can cause left ventricular failure, the history of fever and intravenous drug use is most suggestive of acute infective endocarditis, with high-virulence organisms damaging previously normal heart valves.
True-false answers
1.
a. True. If there are indications from urinalysis (proteinuria, haematuria) or from serum urea, creatinine and electrolyte measurements, that renal disease is present. Renal ultrasound can demonstrate kidney size, scarring and the presence of tumour masses or polycystic renal disease.
b. True. If phaeochromocytoma is suspected (see Ch. 19).
c. False.
d. True. If renal artery stenosis is suspected.
e. True. Fasting hyperglycaemia would suggest an underlying endocrine pathology – diabetes, acromegaly or Cushing’s disease – with secondary hypertension.
2.
a. True.
b. False. There is pulmonary outflow tract stenosis; the tricuspid valve is unaffected.
c. False.
d. True.
e. True. With larger defects, there is a significant left-to-right shunt, increasing pressure in the pulmonary circulation.
3.
a. True. Infarct rupture is most common in the first 3–7days.
b. True.
c. True.
d. False. Ventricular aneurysm develops within the scar that forms as the infarcted muscle is replaced by fibrous tissue; it is a late complication, arising weeks or months after the infarct.
e. False. This is an immunological reaction that develops at least 2weeks after infarction.
4.
a. True.
b. False. Amyloid produces a restrictive cardiomyopathy.
c. True.
d. True.
e. True.
5.
a. True.
b. True.
c. True.
d. False.
e. True.
6.
a. True.
b. True.
c. False.
d. False.
e. False.
7.
a. False. This level of alcohol consumption is probably associated with a lower risk of coronary heart disease than abstinence. However, high alcohol intake is associated with greater cardiovascular morbidity.
b. False.
c. True. Women are relatively protected against ischaemic heart disease during reproductive years.
d. True.
e. True. Diabetes is associated with a twofold increase in ischaemic heart disease.
8.
a. True.
b. False.
c. True.
d. False.
e. True.
9.
a. False.
b. False. The dissection usually commences in the proximal 10cm of the aorta.
c. True.
d. True. There is an increased incidence in patients with Marfan’s syndrome.
e. False.
10.
a. False. Naked-eye changes are unlikely to be evident until at least 24 hours after infarction.
b. False. Isolated right ventricular infarction accounts for only 3% of all infarcts, but the frequency of partial right ventricular involvement in inferior left ventricular infarcts is approximately 40%.
d. True.
e. False. The most sensitive region to hypoxia is the subendocardium.
11.
a. False.
b. False. Herpes virus 8.
c. True. Especially the head and neck of elderly patients.
d. True. Vinyl chloride exposure has been associated with angiosarcoma in the liver.
e. False. They are entirely benign neoplasms.
12.
a. False. The left atrium is usually hypertrophic.
b. False. Rheumatic fever follows Group A β-haemolytic streptococcal infection.
c. True. If the infarct involves the papillary muscle.
d. True.
e. True. Both mitral stenosis and regurgitation can cause atrial enlargement and fibrillation, with a high risk of atrial thrombo-embolism to the brain.
13.
a. True.
b. True.
c. True.
d. True. In patients with previously damaged or prosthetic valves, or certain types of congenital heart disease (e.g. patent ductus arteriosus).
e. False.
Case history answers
Case history 1
1. Pathological causes of left ventricular hypertrophy include:
• hypertension
• aortic stenosis
• mitral valve incompetence
• hypertrophic cardiomyopathy.
The first two are the commonest.
2. Congenitally bicuspid aortic valves occur in 1–2% of the UK population, and are more susceptible to becoming pathologically narrowed due to valve calcification. This occurs at an earlier age than calcific stenosis developing on a previously normal three-cusped aortic valve.
3. A congenitally abnormal valve is a risk factor for infective endocarditis, with its multiple complications. Bicuspid aortic valves can become incompetent as well as stenotic. Left ventricular hypertrophy can cause angina, sudden cardiac death (through a fatal cardiac arrhythmia), or gradual left ventricular failure.
Case history 2
1. Causes of haemopericardium include:
• ruptured myocardial infarction (commonest)
• aortic dissection
• trauma.
2. Hypertension and differing arm blood pressures may be features of aortic dissection. If the dissection involves the aortic arch, blood tracking in the aortic media can partly obstruct the major arterial vessels (carotid and left subclavian arteries), and therefore reduce blood flow and pressure in the upper limbs. Of course hypertension is very common in the UK population, and very few patients presenting with raised blood pressure and chest pain will have aortic dissection. Remember that hypertension is a major risk factor for ischaemic heart disease.
3. Aortic inflammation (vasculitis/aortitis) can occur in giant cell arteritis and in Takayasu disease (a histologically similar but clinically distinct granulomatous vasculitis). In previous decades, tertiary syphilis was a common cause of aortitis with thoracic aortic aneurysm, but this condition is now extremely rare in the UK. Atherosclerosis is, of course, an inflammatory condition that commonly results in distal abdominal aneurysms. Rarely, infective aortitis results in mycotic aneurysm formation.
Case history 3
1. The features are highly suggestive of infective endocarditis. Blood cultures are required for identification of the organism(s) responsible – these are more likely to be positive in acute endocarditis than in subacute cases, where multiple culture specimens can be negative, especially if antibiotics have been started. An echocardiogram will demonstrate valvular vegetations.
2. Comment: Complications are discussed in Box 21, page 125, but include:
• valve incompetence, with or without acute cardiac failure
• septic embolisation and infarction
• glomerulonephritis
• distant organ abscess formation.
Case history 4
Comment: Remember that the four main risk factors for atherosclerosis, and therefore for coronary heart disease, are: smoking, hypertension, diabetes and hypercholesterolaemia. Giving up cigarettes, maintaining a normal body weight, taking regular physical exercise, moderating excess alcohol intake and reducing cholesterol and saturated fats in the diet can all help reduce the risk of ischaemic heart disease.
Heart disease is very common, so it may be mere coincidence that this patient reports a family history. However, hypercholesterolaemia and type 2 diabetes certainly can show familial clustering, so fasting cholesterol and dipstick urine testing or fasting blood glucose might be appropriate. A strong history of multiple young cardiac deaths in the family raises the possibility of a more unusual inherited cause, such as an uncommon form of hyperlipidaemia, hypertrophic cardiomyopathy or an unusual conduction defect.
Viva answers
2. Comment: You need to know what pulmonary embolism is, its clinical consequences, and the risk factors for deep vein thrombosis. Be prepared to discuss the basic pathophysiology of thrombogenesis. This is all covered in Chapters 6 and 15.