general characteristics and carcinogenesis
11.1 Dysplasia and neoplasia94
Neoplasia is abnormal cell growth that does not respond normally to factors controlling growth and differentiation. It is due to genetic abnormalities in the neoplastic cells. The cells form masses (neoplasms or tumours) that may remain confined to the tissue of origin (benign neoplasms) or have the potential to spread to other tissues and organs (malignant neoplasms). Intraepithelial neoplasia is often referred to as dysplasia. Neoplastic transformation (carcinogenesis or oncogenesis) is a multistep process by which cells acquire neoplastic properties and produce tumours.
11.1. Dysplasia and neoplasia
You should:
• define neoplasm
• distinguish benign from malignant neoplasia
• describe biological characteristics of neoplastic cells that are associated with excessive growth, invasion and metastasis
• discuss dysplasia (intraepithelial neoplasia) and give clinically relevant examples.
Neoplasia
Neoplasia literally means ‘new growth’. A definition of neoplasm is given in Box 15. The term ‘tumour’ (which actually means a swelling) is usually used synonymously with ‘neoplasm’.
Box 15
A neoplasm is an abnormal mass of tissue, the growth of which exceeds and is uncoordinated with that of normal tissue. The proliferation is purposeless and continues without regard to its effects on the surrounding tissues or the requirements of the organism. The cells do not respond normally to biological constraints on cell growth. These characteristics are due to genetic changes in the cells.
Our current concept of oncogenesis (the process whereby cells become neoplastic) is that neoplasms are derived from stem cells whose normal control of growth and differentiation has been pathologically changed through genetic defects. Stem cells, which have the capacity to divide continuously as well as produce mature differentiated cells, are found throughout the body in labile and stable cell populations. Differentiated cells have specialised functions which are not present in the precursor stem cell. For example, mucin-secreting goblet cells are derived from epithelial stem cells, and mature lymphocytes are derived from lymphoid stem cells.
Although each somatic cell contains the whole human genome, groups of genes can be switched on or off, and this mechanism determines the differentiation of the cell by controlling which genes will be transcribed. This process is controlled by the genetic programming of the cell and the local environment in which it grows (systemic hormones, local growth factors and matrix proteins).
The normal cell signalling and cell division pathways that maintain normal differentiation and growth are subverted by the genetic abnormalities characteristic of neoplasia. Thus, neoplastic cells continue to multiply inappropriately and may show reduced or abnormal functional specialisation. The genetic events involved are considered in more detail in Section 11.2.
Neoplasms (tumours) consist of neoplastic cells together with the connective tissue framework called the stroma. The blood vessels in the stroma represent the blood supply to the neoplastic cells, and the growth of the neoplasm depends on the ability of the neoplastic cells to induce the growth of these new blood vessels. In malignant neoplasms, the stroma often has a characteristic appearance called ‘desmoplasia’ due to the presence of large numbers of active fibroblasts.
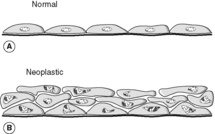
Neoplastic cells show morphological changes that allow them to be recognised by pathologists. Sometimes, pathologists refer to these changes as ‘atypia’ in pathology reports. The features may include one or more of:
• variation in cell shape and size (pleomorphism)
• large nuclei in relation to cytoplasm
• dark-staining nuclei (hyperchromatism)
• irregularities of nuclear contour and chromatin pattern
• loss of normal maturation
• increased mitotic figures – some may be atypical, i.e. of abnormal shape
• enlarged nucleoli.
Neoplastic cells may closely resemble their normal counterparts, in which case they are said to be well differentiated. In contrast, tumour cells that bear little resemblance to normal are poorly differentiated. Occasionally, the cells lose all their differentiating features and are called anaplastic or undifferentiated.
It is possible to grow neoplastic cells in culture in the laboratory. As a result of such work, several important differences in the behaviour of neoplastic cells compared with normal cells have been identified. In particular, neoplastic cells show:
• The transformed phenotype (Figure 26), which means that they:
• do not require extrinsic growth factors
• proliferate to form a colony derived from a single parent stem cell (clone)
• show reduced cell cohesiveness
• show altered surface antigens
• grow to higher cell densities in a haphazard way
• do not show normal cell orientation.
• Tumorigenicity, which means that they will grow into tumours when injected into immunosuppressed animals.
• Immortality, which means that they can undergo indefinite replication without showing senescence. In most cases, this is due to the expression of telomerase, an enzyme normally only found in stem cells that prevents the shortening of the telomeres on the ends of chromosomes. (In untransformed cells, the shortening of the telomeres with each cell division limits the number of times a cell can divide.)
• The ability to evade the host defences against malignant cells. Immune cells, principally natural killer cells and T lymphocytes, are able to recognise neoplastic cells because of altered expression of cell surface antigens. If a neoplasm is to grow successfully, it must be able to avoid immune destruction.
The concepts of benign and malignant
Neoplasms are divided into two main types: benign and malignant. Benign tumours remain confined to their tissue of origin, but malignant neoplasms can spread to other tissues and organs. The term ‘cancer’ means ‘malignant neoplasm’.
By definition, benign tumours do not spread beyond their site of origin. However, they may grow to be extremely large and may distort or disrupt local tissues. Thus, a benign tumour can, by virtue of its position and local effects, kill the patient. For example, a benign tumour in a vital area of the brain can be lethal. Benign neoplasms tend to be:
• well circumscribed or encapsulated
• slow growing (low mitotic rate)
• well differentiated, i.e. resemble the tissue of origin.
The defining characteristic of malignant neoplasms is their ability to invade from the site of origin into other tissues. They often (though not always) show the following features:
• poorly circumscribed
• rapid growth
• may be poorly differentiated
• can metastasise
• extensive disease may be associated with cachexia (generalised body wasting).
Spread of malignant neoplasms
Malignant tumours can show local and metastatic spread.
Local invasion
Local invasion is direct spread into tissues adjacent to the cancer. For example, cervical cancer can invade the uterine body, vagina, rectum, urinary bladder and ureters. A special form of local invasion is perineural spread, in which tumour cells acquire the ability to invade along nerves. Tumours showing this feature often spread rapidly into adjacent tissues, and so pathologists search for perineural invasion when examining malignancies under the microscope.
Metastasis
Metastasis is the spread of the tumour to distant sites away from the original tumour. The metastatic deposits are called secondary tumours, while the original tumour is the primary tumour. Metastases may arise from spread via the lymphatics, via the bloodstream, or across body cavities:
• Haematogenous spread: the most common sites for blood-borne metastases are bone, lung, brain and liver, e.g. liver involvement in gastric cancer and bone metastases from breast cancer.
• Direct seeding across cavities (transcoelomic spread), e.g. colorectal cancer can spread across the peritoneal cavity to produce metastases on the peritoneal surfaces of other organs.
Mechanisms of invasion and metastasis
Malignant neoplasms (cancers) can invade other tissues and spread to distant parts of the body. For invasion to occur, the tumour cells must possess these abilities:
• separation from adjacent cells
• attachment to matrix components
• degradation of extracellular matrix
• migration through extracellular matrix.
An important factor in the poor cohesiveness of cancer cells, allowing them to separate from each other, is abnormal expression of cell adhesion molecules. A cell adhesion molecule often down-regulated in epithelial cancers is E-cadherin. Once separated from their fellows, the cells need to attach to matrix components so they can start to move through the matrix; this is accomplished through expression of various receptors such as integrins. Degradation of matrix is performed by proteases which digest components of the extracellular matrix such as collagen; many of these enzymes are members of the matrix metalloproteinase family. These matrix-degrading enzymes produce corridors for invasion through the tissue. Normal tissues produce protease inhibitors but these may be neutralised by tumour cells.
The process of metastasis through blood or lymph to produce secondary tumours requires the tumour cells to exhibit a number of features:
• invasion through extracellular matrix
• passage through lymphatic or blood vessel into bloodstream (intravasation)
• survival in bloodstream or lymphatic fluid for transfer to a distant site
• passage from vessel lumen into adjacent tissue (extravasation)
• ability to multiply in new tissue
• ability to induce new blood vessel formation (angiogenesis) to supply the enlarging metastasis.
Conditions predisposing to neoplasia
Hyperplasia and metaplasia
In some circumstances, hyperplasia and metaplasia can predispose to the development of neoplasia. However, these conditions should not be confused with each other. Unlike neoplasia, hyperplasia and metaplasia are potentially reversible and are subject to normal constraints on cell growth.
Dysplasia
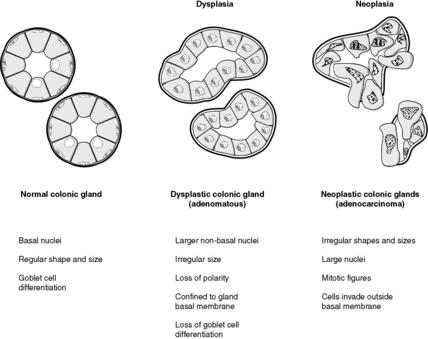
Dysplasia literally means disordered or abnormal growth. However, in general usage it specifically refers to the histological appearances of intraepithelial neoplasia, i.e. neoplastic change in epithelial cells that are still confined by the basement membrane. The dysplastic cells exhibit the morphological changes of neoplasia that were discussed earlier in this section. Dysplasia predisposes to malignancy, which by definition occurs when the neoplastic cells penetrate through the basement membrane and spread into other tissues. As such, dysplasia is a premalignant condition. If recognised, it can be treated before it progresses to cancer.
Dysplasia may arise de novo, or from tissues already showing pathological hyperplasia, metaplasia or chronic inflammation. Early dysplasia may be reversible under certain circumstances if the stimulus is removed. Severe dysplastic changes are sometimes termed ‘carcinoma in situ’.
Clinical examples of the dysplasia to carcinoma sequence
Benign colorectal adenomas
Benign colorectal adenomas are polyps composed of dysplastic epithelial glands. If left in situ, without treatment, many of these will turn into malignant neoplasms over the course of several years (Figure 27). Familial adenomatous polyposis (FAP) is an inherited autosomal dominant disease in which thousands of adenomas develop in the colon. These all have malignant potential and the colon must be removed prophylactically, i.e. to prevent colon cancer occurring.
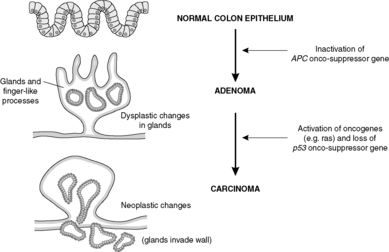 |
| Figure 27 |
Cervical intraepithelial neoplasia
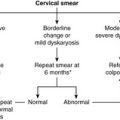
In the uterine cervix, squamous metaplasia associated with human papillomavirus infection may be followed by the development of dysplasia which, in the cervix, is called cervical intraepithelial neoplasia (CIN). If untreated, CIN can develop into cancer. This is the basis of the cervical smear test, designed to identify abnormal cells derived from CIN. Appropriate treatment can then be given to prevent the development of invasive cervical cancer, e.g. excision biopsy of the abnormal area.
Carcinoma in situ of the breast epithelium
Carcinoma in situ of the breast epithelium may be detected using mammography, which forms part of the breast screening programme. Such lesions are surgically excised to prevent the development of breast cancer.
11.2. Carcinogenesis
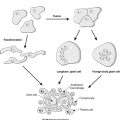
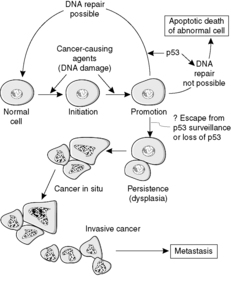
The events that result in the development of a neoplasm are called oncogenesis, tumorigenesis or carcinogenesis (Figure 28). Molecular biology techniques have revolutionised our understanding of the role of genes in this process. The basic principle is that mutations in genes involved with cell growth and differentiation cause the neoplastic phenotype. The risk of a mutation increases with the number of mitotic divisions experienced by a cell, so that tissues with rapidly dividing stem cells (e.g. epithelia) tend to be more prone to neoplastic change.
 |
| Figure 28 |
Transformation is the term given to a cell that has undergone neoplastic change. A transformed cell is not subject to the normal constraints on cell growth, can divide indefinitely without ageing, and can sufficiently evade the process of apoptosis such that the rate of cell production exceeds the rate of cell loss. Thus, transformation is due to genetic changes (mutations) that interfere with the processes of cell division, apoptosis and differentiation. The genetic changes are inherited by the daughter cells, resulting in a clone of cells that forms the tumour mass.
A carcinogen is a substance which can cause neoplasia (Table 16). Most known carcinogens act directly on DNA, causing mutations. For example, polycyclic hydrocarbons can bind covalently to DNA producing molecules called adducts, while radiation damages DNA through its ionising effects. Viruses have a more complex range of effects as discussed in the section on oncogenes. In some circumstances, the effects of a carcinogen may be epigenetic, i.e. there is a change in DNA expression without mutation; anabolic steroids may act this way. However, it appears that epigenetic events alone are unable to produce the full neoplastic phenotype.
| Carcinogen group | Examples | Cancer |
|---|---|---|
| Chemicals | Polycyclic hydrocarbons Azo dyes |
Lung cancer (smoking) Bladder cancer |
| Viruses | Hepatitis B virus Human papilloma virus |
Liver cancer Cervical cancer |
| Radiation | X-rays, UV light Uranium/radon Radioactive iodine |
Skin cancer Lung cancer Thyroid cancer |
| Hormones | Anabolic steroids | Liver cancer |
| Toxins | Aflatoxins | Liver cancer |
| Parasites | Liver fluke | Bile duct cancer |
| Industrial dust | Asbestos Wood dust |
Lung cancer, pleural cancer (mesothelioma) Nasal cancer |
Since rapidly multiplying cells are more at risk of mutational events, factors that increase cell replication may also have a carcinogenetic effect. For example, some infections promote the multiplication of lymphocytes and may thus increase the risk of developing lymphoma.
There may be a long latent interval between exposure to the carcinogen and development of cancer. If more than one carcinogenetic factor is present, the effect is synergistic, i.e. the risk of developing cancer is much higher than with either factor alone.
Genetic changes in neoplasia
Oncogenes
Oncogenes are abnormal genes that are expressed in neoplastic cells. They are derived from proto-oncogenes, which are normal human genes. Proto-oncogenes typically promote growth or inhibit apoptosis under normal circumstances, whereas the oncogenes derived from them do so abnormally.
The genetic abnormalities include point mutations, deletions and translocations. In some cases, the proto-oncogene may be amplified, with multiple copies causing excessive production of gene product (e.g. growth factors). An example is the gene HER2; it encodes a cell surface growth receptor which is over-expressed in about 30% of breast carcinomas. These carcinomas can be treated with an inhibitor for this growth factor receptor.
Alternatively, chromosomal translocation may place the proto-oncogene next to a normally distant promotor DNA sequence which causes activation. Integration of tumorigenic viruses into human DNA may cause activation of proto-oncogenes in a similar way.
Viral oncogenes are derived from certain viruses and their expression causes transformation. Typically, part of the viral genome becomes incorporated into the DNA of human cell and produces proteins that affect the cell cycle or apoptosis. (This is not to be confused with the activation of proto-oncogenes by integrated viral DNA.) Interestingly, oncogenes in retroviruses appear to have arisen during evolution as copies of host proto-oncogenes.
Tumour suppressor genes
Tumour suppressor genes (onco-suppressor genes, anti-oncogenes) are normal genes which act to prevent neoplasia, e.g. by promoting apoptosis or inhibiting cell proliferation. The inactivation of these genes predisposes to cancer.
In human cancer families, where there is a familial tendency to develop cancer, the genetic abnormalities often involve an inherited inactivation of a normal tumour suppressor gene. The best described are the RB-1 gene for familial retinoblastoma and APC in familial adenomatous polyposis (FAP).
Another important anti-oncogene is TP53. Its product, the protein p53, normally causes cell cycle arrest if DNA damage occurs, allowing time for DNA repair enzymes to correct the damage. If repair cannot be effected in reasonable time p53 induces apoptosis. Failure of these mechanisms due to loss of normal p53 function is an important factor in many neoplastic growths (Figure 29).
 |
| Figure 29 |
Self-assessment: questions
One best answer questions
2. Which one of the following is most likely to be found in a malignant neoplasm?
a. abnormal p53 expression
b. inability to spread beyond site of origin
c. normal genome
d. normal expression of cell surface antigens
e. normal regulation of cell cycle
3. The DNA of an epithelial cell combines with a hydrocarbon to produce an adduct. What type of bond characterises this reaction?
a. covalent bond
b. hydrogen bond
c. ionic bond
d. metallic bond
e. van der Waals bond
True-false questions
1. Dysplastic epithelium:
a. rarely progresses to malignancy
b. exhibits cytological abnormalities
c. responds normally to hormonal stimuli
d. may occur in metaplastic epithelium
e. in the cervix may be detected by a smear
2. Oncogenes:
a. are found in neoplastic cells
b. are produced by activation of proto-oncogenes
c. cannot be detected in histological preparations
d. produce mutant oncoproteins
e. may result from chromosomal translocation
3. Compared with normal tissue, malignant neoplastic cells are likely to exhibit:
a. a reduction in the ratio of mitosis to apoptosis
b. increased expression of E-cadherin
c. increased expression of matrix metalloproteinases
d. atypical mitotic figures
e. reduced nuclear size
4. The following are correctly paired:
a. metaplasia – irreversible
b. hepatitis B virus – liver cancer
c. cancer – reduced expression of telomerase
d. asbestos bodies – mesothelioma
e. UV light – skin cancer
Case history questions
Case history 1
A 74-year-old man is admitted to hospital with a cough, producing sputum which is sometimes bloodstained, breathlessness and weight loss. A chest X-ray shows a shadow in the upper part of the left lung. Sputum is sent to the pathology laboratory for examination of any cells coughed up. The pathologist’s report says ‘numerous severely atypical (dysplastic) squamous cells seen’.
1. What is meant by dysplasia? What microscopic features of the cells would lead the pathologist to this diagnosis?
A biopsy is taken from the area of lung shadowing and this shows a malignant neoplasm (carcinoma). The lung is removed surgically. When samples are taken from the bronchial epithelium adjacent to the cancer, squamous metaplasia is seen.
2. Define metaplasia. Give three examples.
3. How might the normal tissue–metaplastic tissue–neoplastic tissue sequence occur in this case?
Self-assessment: answers
One best answer
2. a. Abnormal p53 is common in malignancy. Cell surface antigen expression and regulation of cell cycle are also abnormal in neoplasia. Genetic abnormalities are found in all malignancies. Answer (b) is incorrect because by definition malignant tumours can spread beyond the tissue of origin.
3. a. Adducts are characterised by covalent bonds. This type of bond is strong and stable.
True-false answers
1.
a. False. If it is untreated, the progression of dysplasia to malignancy is common.
b. True. They include pleomorphism, hyperchromatism and other abnormalities in nuclear appearances, loss of polarity (the cells do not show a normal relationship to each other), and loss of normal cytoplasmic differentiation. These cytological changes become more pronounced as the dysplasia progresses towards malignancy.
c. False. Dysplastic epithelium is neoplastic and does not respond normally to constraints on cell growth. That is, any response is only partial.
d. True. Metaplasia may be complicated by dysplasia. For example, Barrett’s metaplasia of the oesophagus is a condition in which the squamous lining of the oesophagus is replaced by intestinalised, glandular epithelium as a result of chronic inflammation due to reflux. Patients with this condition are at risk of developing dysplastic and then malignant changes if not treated.
e. True. Detection of epithelial dysplasia (CIN) by cervical smears is the basis of the screening programme for cervical cancer.
2.
a. True. Oncogenes are pieces of DNA which are present in neoplastic cells and promote the neoplastic phenotype through their effects on the cell cycle, cell signalling mechanisms, etc.
b. True. The proto-oncogene or normal cellular oncogene is abnormally activated or amplified, and thus becomes an oncogene. MYC and RAS are good examples of oncogenes which have well-characterised effects in the early stages of tumorigenesis.
c. False. Using molecular biology techniques, it is possible to apply DNA probes for various oncogenes to tissue sections of normal and neoplastic tissues. This enables the pathologist to visualise the distribution of the abnormal expression of oncogenes.
d. True. When an oncogene is ‘switched on’ this may result in the production of an abnormal gene product or, alternatively, excessively increased amounts of a normal protein product. Oncoproteins are involved in the regulation of cell proliferation, growth factors, growth factor receptors and intracellular signalling mechanisms.
e. True. For example, bcl-2 is a protein that prevents apoptosis by inhibiting caspases. Therefore, the gene BCL-2 that encodes this protein is a proto-oncogene because over-expression of bcl-2 will abnormally inhibit programmed cell death. In some lymphomas, the portion of chromosome 18 containing BCL-2 is translocated to the portion of chromosome 14 containing the antibody heavy chain locus. This t(14;18) translocation places BCL-2 close to the heavy chain gene enhancer. The consequent over-expression of bcl-2 protects the cell from apoptosis.
3.
a. False. In order for neoplasms to grow faster than the surrounding tissues, the number of mitoses relative to the number of apoptoses must be increased. This may be due to an increase in the rate of cell division, a reduction in apoptosis, or (usually) both.
b. False. The reduced cell-cell adhesiveness of malignant tumours is due to reduced expression of molecules responsible for cell-cell adhesion. E-cadherin is a common example.
c. True. These enzymes break down matrix proteins, facilitating invasion by tumour cells.
d. True. The abnormal DNA in malignant cells may show abnormally shaped mitoses.
e. False. Neoplastic cells often have enlarged nuclei, and the nuclear-cytoplasmic ratio is typically increased.
4.
a. False. Metaplasia is essentially a reversible process, although many metaplastic lesions carry the risk of progression to dysplasia.
b. True. Hepatitis B virus is strongly associated with the development of liver cancer. The virus inserts its DNA into the hepatocyte DNA. In the Far East, HBV infection is endemic and liver cancer is very common.
c. False. Telomerase prevents the shortening of telomeres that would otherwise occur at each cell division, allowing a cell to divide indefinitely. Most cancer cells possess telomerase.
d. True. Asbestos fibres are inhaled and can produce lung fibrosis (asbestosis). They can also lead to the development of malignant mesothelioma (a pleural cancer). Most asbestos exposure is occupational and people who have mined asbestos, unloaded it at the docks or worked with it (e.g. lagging boilers) are at high risk of mesothelioma. There is often a long lag period between the time of exposure and onset of tumour. This may be many decades.
e. True. UV light is associated with the development of malignant melanoma, basal cell carcinoma and squamous cell carcinoma, as well as with many kinds of benign proliferative skin lesions. UV light causes damage to DNA, leading to mutations.
Case history answers
Case history 1
1. Dysplasia literally means abnormal growth, and has been used in different ways through the course of medical history. However, in the context of neoplasia it means the histological changes associated with intraepithelial neoplasia. Dysplasia encompasses a constellation of features, including: loss of polarity and tissue organisation, loss of cytoplasmic differentiation, nuclear abnormalities including enlargement, pleomorphism and hyperchromatism, increased mitoses (which may be of abnormal shape), and increased nuclear-cytoplasmic ratio.
2. Comment: Metaplasia is the adaptive change from one adult cell type to another adult cell type in a tissue. When you are asked for examples of metaplasia think of hollow viscera that may become inflamed or obstructed, since these are the commonest sites.
Chronically inflamed transitional epithelium of the urinary bladder will often show areas of squamous metaplasia. Gastro-oesophageal reflux causes glandular metaplasia of the lower oesophagus. The pseudo-stratified, ciliated columnar respiratory epithelium of the bronchus undergoes metaplasia to squamous cells in the presence of chronic irritation by cigarette smoke. When the noxious stimulus is removed (patient gives up smoking!) the epithelium can revert to normal.
3. Comment: There is much research into this area of cell biology. Metaplasia is mediated by gene expression in the stem cells replenishing an epithelium exposed to an adverse environment.
As certain genes are switched on or off, the new cells will have a different complement of intracellular and surface proteins. If the noxious stimulus continues or increases, the new cell type may begin to acquire mutations. This can occur because of increased cell proliferation (at each mitosis there is a risk of mutation), DNA damage caused directly by the noxious stimulus, or both. Ultimately a neoplastic clone may grow out of the epithelium.