Cardiac Effects of Cancer Therapy
Haoyi Zheng, Boris Kobrinsky, Stuart Katz and James L. Speyer
Cardiomyopathy/Chronic Heart Failure
• Anthracyclines: cardiomyopathy/chronic heart failure occurs in 5% to 20% of patients receiving cumulative doxorubicin (more than 450 mg/m2); the incidence is higher in children
• Other antineoplastics (e.g., mitoxantrone and cyclophosphamide): the incidence of cardiomyopathy/chronic heart failure is less than 2%
• Trastuzumab (not common as a single agent; increased risk with anthracycline)
• Radiation therapy: cardiomyopathy/chronic heart failure is common 5 to 20 years after use of a single anteroposterior port
• Arsenic trioxide (a black box warning from the U.S. Food and Drug Administration [FDA])
• Vandetanib (a black box warning from the FDA)
• Nilotinib (a black box warning from the FDA)
• 5-Fluorouracil: 1% to 4.5% with infusion schedules
• Docetaxel, interleukins, interferons, tumor necrosis factor, cytokines, granulocyte-macrophage colony-stimulating factor, monoclonal antibody (CD20)
• Granulocyte-macrophage colony-stimulating factor
• Radiation therapy: effusions in 6% to 30% of patients receiving radiation therapy directed at the chest; constriction in 2% of patients receiving radiation therapy directed at the chest with current techniques
• Anthracycline-induced cardiomyopathy
• HER2 signaling pathway and decreased repair
• Radiation-induced cardiomyopathy
• Radiation-induced myocardial ischemia
• Decreased systemic vascular resistance
• Clinical cardiac symptoms—American Heart Association/American College of Cardiology staging, New York Heart Association classification, and Common Terminology Criteria for Adverse Events for Heart Failure
• Radionuclide scans—serial scans including baseline
• Endomyocardial biopsy—Billingham histopathological grading system
• Echocardiography—pericardial effusion/constriction, left ventricular systolic/diastolic dysfunction
• Cardiac magnetic resonance imaging—left ventricular systolic/diastolic dysfunction, myocardial fibrosis/necrosis detected by late gadolinium enhancement
Cardiomyopathy/Congestive Heart Failure
• Sodium and fluid restriction
• Angiotensin-converting enzyme inhibitors or angiotensin receptor blocking agents
Risk Reduction with Anthracyclines
• Anthracyclines: limit dose (e.g., doxorubicin: <450 mg/m2); use less-toxic analogs
• Unique delivery systems (e.g., liposomes)
• Pegylated liposomal doxorubicin
• Blocking agent: dexrazoxane (Zinecard)
Radiation-Induced Pericardial Disease
• Acute pericarditis: nonsteroidal antiinflammatory drugs, steroids
Introduction
Cardiac disorders are common complications of cancer therapy. A standardized grading system used for reporting adverse events in clinical trials (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf) provides a detailed description of the categories of cardiac disorders associated with cancer therapy.1 The prevalence of cardiac disorders associated with cancer therapy is not well characterized because of limited follow-up time in many clinical trials and inconsistencies in the definitions of cardiac disorders used in the existing medical literature. This chapter proposes the following definitions based on the existing common terminology criteria for adverse events and the pathophysiological model of heart failure associated with cancer therapy presented in the next section.
• Elevation of serum troponin T or troponin I levels above the normal range
• Transient or persistent decrease in left ventricular ejection fraction (LVEF) of >10 percentage points from pretreatment baseline or to a value <55% (or less than the lower limit of normal for the laboratory)
• New myocardial fibrosis and scar detected by the late enhancement of gadolinium with cardiac magnetic resonance imaging (MRI)
• New onset or worsening of diastolic dysfunction detected by echocardiography or cardiac magnetic resonance (CMR)
• Evidence of new or unstable myocardial ischemia
• Symptomatic or asymptomatic arrhythmias (supraventricular and/or ventricular)
• Symptomatic or asymptomatic cardiac conduction system abnormalities
• Symptomatic or asymptomatic abnormal valve structure and/or function
Pathophysiology of Heart Failure
Clinical heart failure occurs when the heart is unable to provide a sufficient oxygen supply to meet the metabolic needs of the body at normal cardiac filling pressures. In the setting of cancer therapy, the pathophysiological process leading to clinical heart failure is initiated by an extrinsic cardiac injury due to radiation, anthracyclines, or other toxins with consequent focal or segmental myocyte death (mediated by necrosis and/or apoptosis). The extrinsic cardiac injury triggers an intrinsic pathological process (ventricular remodeling) characterized by activation of cell repair and regenerative pathways, myocellular hypertrophy, and interstitial fibrosis in the surviving myocardium. The signal transduction pathways that regulate these processes in the heart overlap with pathways for cell growth and proliferation that are known to be important in tumor biology. Initial cellular responses to cardiac injury serve to preserve cardiac output (and oxygen delivery) and therefore are not typically associated with clinical manifestations of heart failure. The progression to chronic heart failure is an indolent process that frequently occurs 10 to 20 years after the initial injury. The development of clinical manifestations of chronic heart failure represents the end stage of a largely occult disease process. With the introduction of modern therapy, there has been a substantial reduction in mortality for patients with systolic heart failure. However, symptomatic heart failure continues to confer a worse prognosis than the majority of cancers in the United States, with 1-year mortality rate as high as 45% in the elderly patient population.2
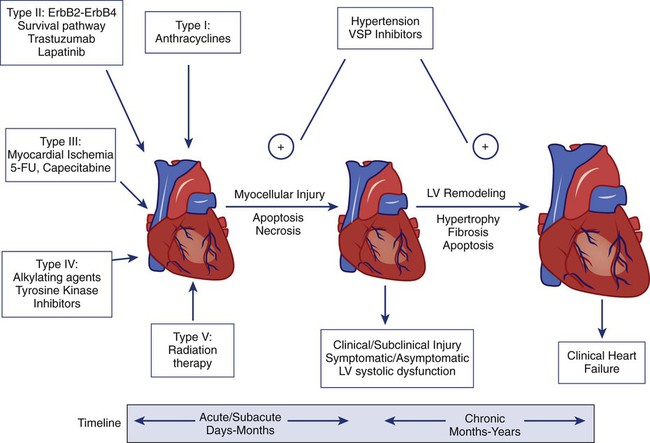
Cardiotoxicity associated with radiation and chemotherapy can be categorized according to the type and clinical manifestations of cardiac injury.3 Five types of myocyte injury have been proposed with different pathophysiological mechanisms (Fig. 59-1). Among these, type I is primarily associated with anthracyclines and is attributable to direct myocyte toxicity with consequent cell death mediated by either apoptosis or necrosis. Type II is primarily associated with trastuzumab and related agents and is attributable to inhibition of specific cardiac repair pathways that may lead to transient myocellular dysfunction or cell death. The notion that trastuzumab-related cardiomyopathy (type II) can be reversed has been challenged by recent studies.4 Because myocytes have a limited repertoire of response to injury, the long-term clinical consequences of all five types of injury follow a final common pathway of pathological cardiac remodeling and progression to heart failure, as previously described.
Anthracyclines (Type I)
The currently approved anthracyclines in the United States are doxorubicin (Adriamycin), liposomal doxorubicin and pegylated doxorubicin (Doxil), epirubicin (Ellence), daunorubicin (Cerubidine), and idarubicin (Idamycin PFS). Anthracyclines have played a major role in the treatment of hematologic malignancies and solid tumors, especially in the treatment of both metastatic and early breast cancer and sarcomas. Their dose-dependent cardiotoxicity is well documented, with the most common clinical presentation being dilated cardiomyopathy with chronic heart failure. Most available information on anthracycline-induced cardiomyopathy is derived from studies with doxorubicin and, to a lesser extent, with epirubicin and daunorubicin. However, other drugs of this class have also been associated with clinically indistinguishable cardiotoxic effects. The clinical presentations of cardiotoxicity can be acute, subacute, and/or chronic. No consensus exists for the definition of these terms. The previous definition for “acute” is the onset cardiac events within a few minutes after administration of anthracycline, which is mainly based on transient arrhythmias and rare cases of acute pericarditis and myocarditis.5 The elevation of troponin, new echocardiographic technology, and CMR allow the early detection of cardiotoxicity. We propose to define these terms as follows (Table 59-1).
Table 59-1
Clinical Presentations of Anthracycline-Induced Cardiotoxicity
| Clinical Presentation | Time Course | Characteristics |
| Acute | Within hours to 1 month of treatment; may be reversible after discontinuation of anthracycline | Elevated troponin, new onset of LV systolic and diastolic dysfunction, pericarditis, myocarditis, transient arrhythmias |
| Subacute | Within 1 month to 1 year of treatment; may persist or progress after discontinuation of anthracycline | Dilated cardiomyopathy; rapid decline in LV function if treatment is continued after the onset of subclinical LV dysfunction |
| Late onset | Years or decades after anthracycline treatment | Dilated cardiomyopathy; restrictive cardiomyopathy |
Data from Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med 1996;125:47-58; and Bristow MR, Mason JW, Billingham ME, Daneils JR. Dose-effect and structure-function relationships in doxorubicin cardiomyopathy. Am Heart J 1981;102:709-18.
Acute Cardiac Toxicity
Acute cardiotoxicity may manifest as acute systolic dysfunction, arrhythmia, and/or clinical pericarditis-myocarditis during and/or soon after the administration of doxorubicin.6,7 These acute forms of cardiotoxicity are rare and usually transient or reversible. The most common form of acute cardiac toxicity is acute cardiomyocyte injury, which is associated with elevated serum troponin levels in the days or weeks after administration of anthracyclines. Elevation of troponin is associated with an increased risk for the development of chronic heart failure.8
Subacute and Chronic Cardiac Toxicity
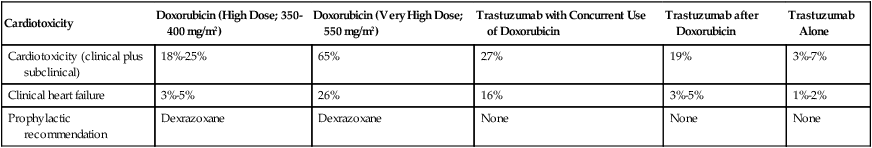
Endomyocardial biopsy specimens have demonstrated that the dose-related cardiotoxicity associated with anthracyclines is biventricular. Retrospective studies indicate that the incidence of clinically recognizable heart failure with doxorubicin is 7% to 15% in patients who have received a cumulative dose greater than 450 mg/m2 to 500 mg/m2 without use of a cardioprotective agent (Table 59-2).9,10 Above this dose, the incidence of CHF rises steeply, with an estimated incidence of CHF of 50% when the cumulative dose of doxorubicin reaches 700 mg/m2. Cumulative cardiotoxic doses associated with the use of other anthracyclines vary, but the shape of the curve that plots cumulative dose against the incidence of CHF is comparable for all anthracyclines that have been studied. Careful surveillance for cardiotoxicity should be maintained from the start of therapy because isolated instances of CHF have been observed at lower cumulative doses.11
Table 59-2
Doxorubicin-Induced and Trastuzumab-Related Cardiotoxicity
| Cardiotoxicity | Doxorubicin (High Dose; 350-400 mg/m2) | Doxorubicin (Very High Dose; 550 mg/m2) | Trastuzumab with Concurrent Use of Doxorubicin | Trastuzumab after Doxorubicin | Trastuzumab Alone |
| Cardiotoxicity (clinical plus subclinical) | 18%-25% | 65% | 27% | 19% | 3%-7% |
| Clinical heart failure | 3%-5% | 26% | 16% | 3%-5% | 1%-2% |
| Prophylactic recommendation | Dexrazoxane | Dexrazoxane | None | None | None |
The incidence of CHF in clinical practice may be higher than that reported from retrospective trials.12,13 Prospective clinical trials indicate that the number of patients with evidence of CHF at cumulative doses of 450 mg/m2 may exceed 25%. Subclinical cardiotoxic effects of anthracyclines are likely present after each dose, with the likelihood of clinically apparent toxicity determined by the cumulative effects from prior doses of anthracyclines or from other co-morbid conditions. The natural history of the chronic anthracycline cardiotoxicity is highly heterogeneous. In its most virulent form, patients demonstrate refractory progressive symptoms despite optimal medical therapy with rapid onset of pump failure and death. Most patients present with permanent reduction in LVEF and persistent symptoms of CHF that progress slowly over time. In some patients, LVEF can improve over time, sometimes for years, with later recurrent deterioration of LVEF and eventually the appearance of CHF.
Risk Factors
The most important risk factor for cardiac toxicity is the total cumulative dose of anthracycline. Other factors associated with an increased risk of anthracycline-induced cardiotoxicity in adults include age greater than 70 years, exposure to ionizing radiation to the chest wall, prior exposure to anthracyclines, and preexisting cardiac disease or traditional cardiovascular risk factors including history of CHF, myocardial infarction, hypertension, and diabetes mellitus. Concomitant use of other agents with cardiotoxicity such as cyclophosphamide and paclitaxel also confer increased risk of anthracycline cardiotoxicity. Concurrent and sequential use of trastuzumab (Herceptin) with anthracycline-based adjuvant chemotherapy in patients with HER 2+ breast cancer significantly increases risk for cardiac toxicity (Table 59-2). The independent contributions of each of these factors (with the exception of trastuzumab) are not well defined, but having multiple risk factors appears to be associated with additive risk.
Heterogeneity in individual patient susceptibility to anthracycline cardiotoxicity suggests that genetic variation may also contribute to risk. Genetic variants in genes encoding proteins related to free radical metabolism, anthracycline transport, and drug biotransformation have been shown to be associated with increased risk in anthracycline-induced cardiotoxicity in both pediatric and adult populations of cancer survivors.16–16 These preliminary findings need to be further replicated and confirmed in larger prospective studies.
Mechanisms
The antitumor effects of anthracyclines are mediated by inhibition of DNA synthesis through direct interactions with DNA (intercalation) and inhibition of topoisomerase II (Top II).17 Top II has two isoenzymes in mammalian cells, Top IIα and Top IIβ. Doxorubicin or daunorubicin binds both DNA and Top IIα to form the topoisomerase IIα-doxorubicin-DNA ternary cleavage complex, which triggers cell death.18 These tumoricidal mechanisms do not likely contribute to cardiac toxicity because Top IIα is overexpressed in tumor cells and is not detectable in quiescent cells.19 Cardiomyocytes are terminally differentiated cells with very low rates of proliferation.
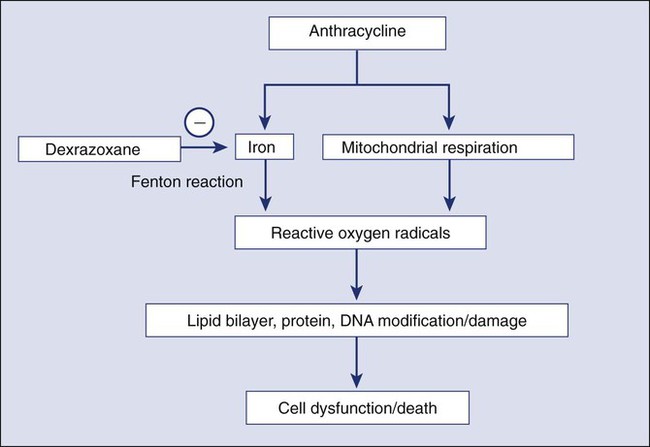
The primary mechanism for anthracycline cardiomyocyte injury was thought to be free radical damage to vital cell structures and subsequent cell death via apoptosis. Reduction of the quinone groups on the B ring of the anthracene structure results in a semiquinone radical before further reduction to the alcohol (e.g., doxorubicinol). Interaction of the semiquinone with oxygen yields superoxide anion (O2−). Dismutation of superoxide anion yields H2O2, which may react further with cardiac iron sources or the semiquinone to produce a reactive intermediate with the chemical characteristics of the hydroxyl radical. These reactions can proceed in either an iron-dependent or iron-independent fashion (Fig. 59-2).20 Doxorubicin also directly increases the labile intracellular iron pool by inhibiting iron regulatory protein–1 with consequent changes in transcription of transferrin receptors and ferritin.21,22 Formation of a Fe3+–doxorubicin complex can also enhance free radical generation via the Fenton reaction. Free radicals can cause damage at a variety of intracellular sites, including the nuclear envelope, cell membrane, mitochondria, DNA, and sarcoplasmic reticulum.23 Increased susceptibility of myocardial cells to free radical injury may be attributable to very low myocardial levels of catalase, one of major cellular enzymatic defenses against free radical injury, in cardiomyocytes when compared with other cells types. Doxorubicin also decreases levels of other antioxidant enzymes, including selenium-dependent glutathione-peroxidase-1 and cytosolic CuZn superoxide dismutase. Recently, Top II–mediated doxorubicin-induced DNA damage was shown to play an important role in cardiotoxicity. As previously mentioned, Top IIα is only expressed in proliferating and tumor cells and is the target of the antitumor effect of anthracyclines. Cardiomyocytes express Top IIβ, which is also a target for doxorubicin. Top IIβ-doxorubicin-DNA ternary complex can induce DNA double-strand breaks, leading to cell death. A recent study showed that cardiomyocyte-specific deletion of Top IIb (encoding Top IIβ) protects cardiomyocytes from doxorubicin-induced DNA double-strand breaks and transcriptome changes that are responsible for defective mitochondrial biogenesis and reactive oxygen species formation. Furthermore, cardiomyocyte-specific deletion of Top IIβ protects mice from the development of doxorubicin-induced progressive heart failure.24 Anthracyclines may also mediate cardiotoxicity through other iron-independent mechanisms related to changes in myofibrillar structure and function (see Fig. 59-2), changes in calcium and energy metabolism, and secondary changes in nitric oxide signaling in response to altered redox state in the myocardium.25
Diagnosis
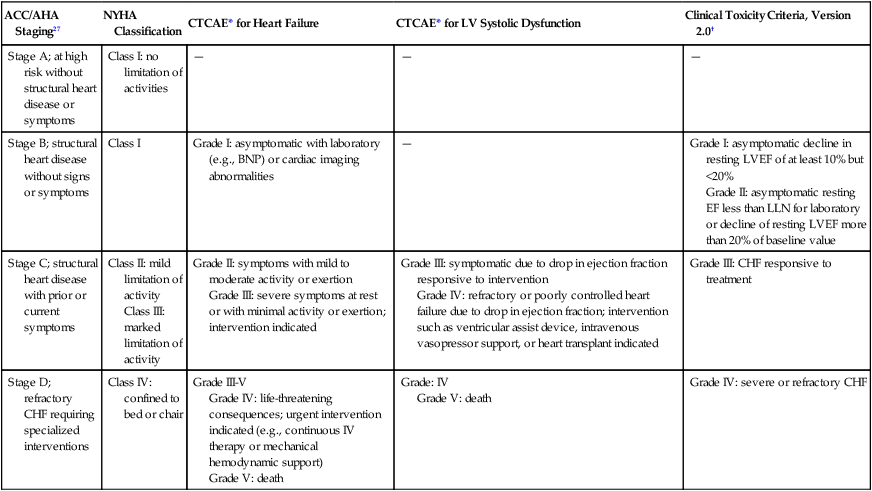
Despite its limitations (discussed in the next section), serial measurement of LVEF remains the most commonly used method for detection of anthracycline cardiotoxicity. CHF may occur with preserved or decreased EF. In patients with preserved EF, heart failure is thought to be primarily attributable to changes in diastolic properties of the heart (increased diastolic LV wall stiffness and/or increased end-diastolic LV volume as a consequence of cardiac injury or overload). The classic clinical signs of congestion associated with heart failure (rales and edema) have both low specificity and low sensitivity. More than half of patients with documented extreme elevation of cardiac filling pressures do not present with rales or radiographic signs of pulmonary congestion. Accordingly, the clinical oncologist must maintain a high level of suspicion in patients receiving chemotherapy associated with potential cardiotoxicity. New onset of otherwise unexplained exertional dyspnea and/or fatigue may be the sole manifestation of heart failure regardless of change in LVEF. Unexplained sinus tachycardia at rest and after exercise in an otherwise oncologically stable patient is another potential early sign of cardiotoxicity. A system of staging anthracycline-induced hemodynamic changes in humans was developed by Bristow.26 Early referral to cardiology is recommended in the setting of suspected cardiotoxicity, even in asymptomatic patients, because accumulating evidence suggests that medical therapy may forestall disease progression. The relationship between staging criteria for heart failure developed by the American Heart Association, American College of Cardiology, and New York Heart Association27 in comparison with the Common Terminology Criteria for Adverse Events is summarized in Table 59-3.
Table 59-3
The Relationship between Staging Criteria for Heart Failure Developed by the American Heart Association, American College Of Cardiology, and New York Heart Association in Comparison with the Common Terminology Criteria for Adverse Events
| ACC/AHA Staging27 | NYHA Classification | CTCAE* for Heart Failure | CTCAE* for LV Systolic Dysfunction | Clinical Toxicity Criteria, Version 2.0† |
| Stage A; at high risk without structural heart disease or symptoms | Class I: no limitation of activities | — | — | — |
| Stage B; structural heart disease without signs or symptoms | Class I | Grade I: asymptomatic with laboratory (e.g., BNP) or cardiac imaging abnormalities | — | Grade I: asymptomatic decline in resting LVEF of at least 10% but <20% Grade II: asymptomatic resting EF less than LLN for laboratory or decline of resting LVEF more than 20% of baseline value |
| Stage C; structural heart disease with prior or current symptoms | Class II: mild limitation of activity Class III: marked limitation of activity |
Grade II: symptoms with mild to moderate activity or exertion Grade III: severe symptoms at rest or with minimal activity or exertion; intervention indicated |
Grade III: symptomatic due to drop in ejection fraction responsive to intervention Grade IV: refractory or poorly controlled heart failure due to drop in ejection fraction; intervention such as ventricular assist device, intravenous vasopressor support, or heart transplant indicated |
Grade III: CHF responsive to treatment |
| Stage D; refractory CHF requiring specialized interventions | Class IV: confined to bed or chair | Grade III-V Grade IV: life-threatening consequences; urgent intervention indicated (e.g., continuous IV therapy or mechanical hemodynamic support) Grade V: death |
Grade: IV Grade V: death |
Grade IV: severe or refractory CHF |
*Adverse events are cardiac general and left ventricular systolic dysfunction; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf.
†Adverse events are cardiovascular (general) and cardiac left ventricular function; http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf.
Routine Monitoring of Cardiac Toxicity
A commonly used strategy for patient monitoring is to obtain a baseline study before therapy commences and then obtain repeat studies at intervals as the cumulative dose increases, with more frequent observations at higher cumulative doses. Scans are examined for sequential changes in wall motion and global LVEF. Any decrease in LVEF, including those that remain within the “normal” range and those that decrease to clearly abnormal values, may indicate anthracycline myocyte damage.26,28 Careful monitoring with serial MUGA scans may permit treatment with greater cumulative doses of doxorubicin beyond the empiric stopping dose of 450 to 500 mg/m.
Endomyocardial Biopsy
Endomyocardial biopsy remains the gold standard for diagnosis because of its high sensitivity and specificity. Anthracyclines cause a unique pattern of histologic damage as originally demonstrated in animal models and confirmed by endomyocardial biopsies in patients.29 Billingham grade has been used as a histopathological scale for doxorubicin-induced cardiomyopathy (Box 59-1).29 A continuum of change is well described, from dilation of vacuoles to mitochondrial swelling, myofibrillar dropout, interstitial fibrosis, and, ultimately, cell death.31–31 The changes seen on endomyocardial biopsy appear to parallel clinical findings.32 The consistency of these findings in nonmammalian species has provided the basis for a number of animal models of anthracycline cardiomyopathy.33 However, endomyocardial biopsy is rarely used in clinical practice because of the risks associated with an invasive procedure, absence of data to demonstrate that the results of the test alter treatment approach or outcome, and the availability of noninvasive alternatives to monitor cardiac toxicity.
Early Detection of Cardiac Toxicity
Cardiac Imaging
Advanced echocardiographic techniques may be useful for earlier detection of cardiac toxicity. Diastolic dysfunction assessed by spectral Doppler echocardiography of mitral valve inflow patterns may be a sensitive method for early detection of cardiac dysfunction.34,35 Stress echocardiography using exercise or dobutamine has also been used to detect subclinical cardiotoxicity.36,37 Strain and strain rate assessment by speckle tracking echocardiography and tissue velocity imaging echocardiography are promising areas for the early detection of cardiac toxicity. In a recent study,38 tissue velocity imaging and strain allowed for early detection of subclinical cardiac dysfunction before conventional echocardiography in patients receiving adjuvant trastuzumab. Another study demonstrated that a decrease in left ventricular longitudinal strain assessed by speckle tracking echocardiography was an independent predictor of later development of cardiotoxicity in patients undergoing chemotherapy.39 Further study utilizing these new echocardiography-derived indexes is required to establish the value of these measures for routine clinical use.
CMR can be used to characterize myocardial tissue and evaluate the LV systolic and diastolic performance. CMR is known to differentiate transient and permanent myocardial injury in various systemic and inflammatory diseases and thus may be able to visualize myocardial tissue changes after chemotherapy before any measurable change in LVEF.40 The presence of late gadolinium enhancement (indicative of scar formation in the myocardium) was reported in patients with breast cancer with trastuzumab-related cardiomyopathy after doxorubicin-based adjuvant chemotherapy.39 The lack of late gadolinium enhancement detected by CMR might predict reversibility of impaired LV function in patients with chemotherapy-induced cardiomyopathy,3 but more confirmatory studies are needed.
Cardiac Biomarkers
Troponin I and troponin T are highly specific and sensitive biomarkers of cardiomyocyte injury from any cause. Measurement of serum troponin can detect chemotherapy-induced cardiac toxicity in its early stage, before the onset of impaired LV function.41 Elevated troponin at 3 days and 1 month after receiving anthracycline-based chemotherapy is a significant prognostic factor for development of a future cardiac event defined as a >15 percentage point reduction of LVEF and CHF.8,41 Elevated troponin has been observed in patients treated with standard doses of anthracycline and even after the administration of the first dose of doxorubicin.42,43 This observation is consistent with the concept that cardiomyocyte injury and/or cell death begins early in the course of anthracycline therapy. In patients with elevated troponin levels after chemotherapy with either a high cumulative dose of anthracycline or standard-dose anthracycline and trastuzumab, treatment with enalapril has been reported to reduce risk of cardiac events.44 Furthermore, in patients with HER2-positive breast cancer treated with trastuzumab after anthracycline-based adjuvant chemotherapy, elevated troponin might help distinguish between reversible and irreversible cardiac damage.45
Strategies to Reduce Risk of Anthracycline Cardiotoxicity
Risk Reduction
Empiric limitation of cumulative doses of doxorubicin to a range of 400 mg/m2 to 450 mg/m2 or to lower doses in patients with other clinical risk factors for cardiotoxicity is a common strategy that can reduce but not eliminate the risk of cardiotoxicity in all patients. The current maximum recommended dose for doxorubicin is 500 mg/m2, and for epirubicin it is 900 mg/m2. The risk and benefit must be carefully assessed for each person because limiting or discontinuing therapy prematurely may deprive patients from receiving the full therapeutic benefit of anthracycline-based chemotherapy. A combination of individualized dose restriction with careful cardiac monitoring may offer the safest practical approach to patients receiving doxorubicin.12
Dose Schedule Changes
Alterations in the dose schedule of anthracyclines are based on the hypothesis that chronic cardiac toxicity is related primarily to peak drug concentration, whereas the antitumor effect is more related to total drug exposure (concentration time, or area under the curve). Several studies have demonstrated decreased cardiac toxicity with prolonged continuous infusions (e.g., 24 to 96 hours) compared with standard rapid infusion schedules. A metaanalysis showed that bolus administration of anthracycline-based chemotherapy was associated with an odds ratio of 4.13 (confidence interval [CI]: 1.75-9.72) for the development of cardiac toxicity versus administration by continuous infusion.50
New Delivery System
Liposomal anthracyclines were designed with the intent of reducing the risk of cardiac toxicity while preserving antitumor efficacy. Pegylated liposomal doxorubicin consists of STEALTH technology-based liposomes containing doxorubicin in an aqueous core. Its stable pegylated cover protects the drug from enzymatic degradation with a dramatic increase in the half-life compared with conventional doxorubicin formulation. The small size of the liposome (100 nm) enables preferential drug penetration through compromised tumor vasculature and accumulation in tumors, thereby producing less systemic and cardiac toxicity. In a phase 3 study of 509 women with metastatic breast cancer, pegylated liposomal doxorubicin demonstrated comparable response rates and survival when compared with conventional doxorubicin with reduced toxicity. Cardiotoxicity (defined as a >20 percentage point reduction in LVEF within the normal range, or a >10 percentage point reduction in LVEF to below the normal range) occurred in 18.8% of patients in the conventional doxorubicin arm versus 3.9% of patients in the pegylated liposomal doxorubicin arm (hazard ratio 3.16, P < .001).46 The reduction in cardiotoxicity was most marked for cumulative doses in excess of 450 mg/m2.47,48
Analogs
Epirubicin is one of most commonly used anthracycline analogs. The results from studies designed to compare the cardiotoxicity between equimolar doses of epirubicin and doxorubicin have yielded inconsistent findings. A metaanalysis of five trials showed no evidence for a significant difference in the occurrence of clinical heart failure between the treatment groups (relative risk [RR] = 0.36; 95% CI 0.12-1.11; P = .07) with no difference in tumor response or overall survival.48 The magnitude of the risk reduction is high, and the lack of statistical significance could be attributable to lack of power as a result of a small number of CHF cases (3 among 521 patients randomized to epirubicin and 12 among 515 patients randomized to doxorubicin). No randomized trials have been conducted regarding the occurrence of combined clinical and subclinical heart failure (change in LVEF) in patients treated with epirubicin versus doxorubicin.
Dexrazoxane
Dexrazoxane (Zinecard), a cyclic derivative of edetic acid, is the only drug approved by the U.S. Food and Drug Administration (FDA) for the prevention of anthracycline-induced cardiotoxicity. The compound is a bisdioxopiperazine that is hydrolyzed intracellularly to form a bidentate chelator that is similar in structure to edetic acid; it effectively binds intracellular free iron and iron bound in anthracycline complexes, thereby preventing free radical generation (Fig. 59-2), which is considered to be the primary mechanism of the cardioprotective effect of dexrazoxane. However, as previously discussed, Top IIβ is expressed in cardiomyocytes and appears to be a critical component of the causal pathway for doxorubicin-induced cardiotoxicity. An in vitro study demonstrated that dexrazoxane degraded Top IIβ in cardiomyocytes, antagonized the formation of Top IIβ DNA cleavage complex, and reduced doxorubicin-induced DNA damage.49 These findings suggest that dexrazoxane may provide cardioprotection via multiple mechanisms of action. In the United States, the current indication for dexrazoxane is for prevention of cardiotoxicity in patients with breast cancer who receive a cumulative dose of doxorubicin greater than 300 mg/m2 or a cumulative dose of epirubicin greater than 550 mg/m2. The recommended dexrazoxane : doxorubicin (or epirubicin) dosage ratio is 10 : 1 (e.g., if 40 mg/m2 of doxorubicin is used, 400 mg/m2 of dexrazoxane should be administered). A reconstituted dexrazoxane solution should be administered intravenously by slow push starting 15 to 30 minutes before intravenous administration of anthracycline. Leukocyte and platelet counts should be regularly monitored because dexrazoxane may potentiate hematologic toxicity induced by chemotherapy or radiation. The drug is generally well tolerated except for a higher incidence of severe leukopenia when compared with placebo.
Dexrazoxane has demonstrated significant cardioprotection in patients receiving anthracycline-based chemotherapy with a reduction of the occurrence of CHF and an asymptomatic decrease in LVEF. Randomized trials in patients with breast cancer,50 lung cancer, sarcoma,51 and leukemia52 have consistently shown a significant reduction of doxorubicin-induced cardiac toxicity as measured by clinical examination, MUGA scan, echocardiography, and endomyocardial biopsy. Clinical trials in pediatric patients with sarcoma, lymphoma, and acute leukemia showed a similar cardiac protective effect. The cardioprotection of dexrazoxane has also been shown in women with breast cancer who were treated with epirubicin53 and patients with sarcoma who were treated with high-dose epirubicin.51 Analysis of randomized trials in persons with breast cancer demonstrates that even when dexrazoxane is added after the sixth course of chemotherapy (300 mg/m2), significant cardioprotection is provided without compromise of the antitumor activity of the regimen.
Despite consistent evidence of its cardioprotective effects in clinical trials, dexrazoxane has not gained widespread acceptance in clinical practice, possibly because of concerns of its potential impact on chemotherapy efficacy and the risk of secondary malignant neoplasms. A single randomized placebo-controlled trial in patients receiving anthracycline chemotherapy reported lower chemotherapy response rates in patients who received dexrazoxane when compared with patients who received placebo.53a Interestingly, the same trial showed an unusually high response rate in the placebo group when compared with other trials. Based on data from this trial, the indication for dexrazoxane in the United States was limited to patients with metastatic breast cancer who have already received a high cumulative dose of doxorubicin. In an updated metaanalysis, no statistically significant difference in antitumor response rate between persons receiving dexrazoxane and persons in control groups was detected.55 Increased risk for development of a secondary malignant neoplasm was reported in a single study in pediatric patients with Hodgkin disease. This study reported secondary malignant neoplasms including acute myeloid leukemia and myelodysplastic syndrome in 8 of the 239 patients who received chemotherapy with dexrazoxane versus 2 of the 239 patients who received chemotherapy without dexrazoxane.54 The authors of this trial speculated that the combination of three topoisomerase inhibitors including doxorubicin, etoposide, and dexrazoxane might increase the risk of secondary malignant neoplasms. On the basis of these findings, the European Medicines Agency has recommended restricting the use of dexrazoxane to adult patients with advanced or metastatic breast cancer who have already received a threshold dose of doxorubicin or epirubicin and contraindicating its use in pediatric patients. However, other trials (see the “Pediatrics” section) and an updated metaanalysis did not show a significant difference in the occurrence of secondary malignant neoplasms between children treated with or without dexrazoxane.55
Other Cardioprotective Agents
No randomized clinical trials have demonstrated a cardiac protective effect of other agents used to treat CHF, including ACE inhibitors, angiotensin receptor blockers, and β-adrenergic receptor blockers. Accordingly, insufficient data are available to develop a consensus recommendation for treatment of anthracycline-induced cardiomyopathy. Once evidence or signs of cardiotoxicity are detected, anthracyclines should be stopped. A prospective cohort study56 reported the longitudinal effects of therapy with enalapril and carvedilol in patients with anthracycline-induced cardiotoxicity; 201 patients with decreased EF ( 45%) as a result of anthracycline exposure were enrolled and followed up for 3 years. Complete EF recovery (defined as EF
45%) as a result of anthracycline exposure were enrolled and followed up for 3 years. Complete EF recovery (defined as EF 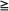 50%) occurred in 42% of patients, 13% had a partial EF response (i.e., an increase in EF by 10 points but <50%), and 45% experienced no recovery (less than a 10-point increase in EF and EF <50%). Earlier initiation of treatment with enalapril and carvedilol (1 to 4 months after administration of anthracycline) was associated with improved response. None of the patients with treatment initiated more than 6 months after administration of anthracycline experienced complete recovery of LVEF. The absence of control subjects limits the interpretation of these findings with regard to strategies for clinical practice. Given the large body of data supporting the use of ACE inhibitors and β-adrenergic receptor blockers in patients with heart failure of diverse etiologies, it is reasonable to initiate treatment with these classes of agents in patients with suspected anthracycline cardiotoxicity and evidence of reduced LVEF.
50%) occurred in 42% of patients, 13% had a partial EF response (i.e., an increase in EF by 10 points but <50%), and 45% experienced no recovery (less than a 10-point increase in EF and EF <50%). Earlier initiation of treatment with enalapril and carvedilol (1 to 4 months after administration of anthracycline) was associated with improved response. None of the patients with treatment initiated more than 6 months after administration of anthracycline experienced complete recovery of LVEF. The absence of control subjects limits the interpretation of these findings with regard to strategies for clinical practice. Given the large body of data supporting the use of ACE inhibitors and β-adrenergic receptor blockers in patients with heart failure of diverse etiologies, it is reasonable to initiate treatment with these classes of agents in patients with suspected anthracycline cardiotoxicity and evidence of reduced LVEF.
Pediatrics
The survival rate for childhood cancer is nearly 80%.57 Anthracycline agents are widely used to treat childhood leukemia and lymphomas, as well as other malignancies. This group of childhood cancer survivors is increasingly confronted with late-onset asymptomatic and symptomatic consequences of early cardiotoxicity.
Children are particularly sensitive to cardiac effects of anthracyclines. Cardiotoxicity risk occurs at lower cumulative doses and appears to increase over time. In a study of 830 children treated in the Netherlands, the incidence of CHF over a median follow-up of 8.5 years was 2.5%, rising to 5.5% at 20 years.58 An update of this cohort of 1362 five-year survivors of childhood cancer treated from 1966 to 1996 revealed that the specific incidence of cardiac events was greater for the combination of anthracyclines and radiation (12.6%) than for anthracyclines (7.3%) or cardiac radiation (4.0%) alone. Of 50 grade 3 and 4 events, 27 were CHF, six were ischemia/infarction, six were valvular dysfunction, eight were arrhythmias, and two were pericarditis.59 More than 7.5% of children exposed to anthracyclines will have CHF diagnosed within 30 years after treatment.60 The cumulative anthracycline dose is the most important risk factor. However, children may experience significant cardiotoxicity at lower doses.61 Younger age at treatment, concomitant radiation exposure, female sex, and tumor type are also risk factors.62
Children are at increased risk for the development of late manifestations of cardiac disease after all types of cancer treatment. In a large retrospective cohort study (Childhood Cancer Survivor Study) of 10,397 survivors of childhood cancer who were followed up for a median of 17 years, the RR of grade 3 or 4 CHF was 15.1 (95% CI 4.8-47.9) and the RR of coronary artery disease was 10.4 (95% CI 4.1-25.9) when compared with their siblings.63 The risk of cardiac disease is not solely attributable to anthracycline exposure. Survivors of childhood cancers have an increased risk of cardiac structural abnormalities, systemic inflammation, and atherosclerotic heart disease even if they were not exposed to anthracyclines and radiation therapy.64 Age-adjusted risk of myocardial infarction, stroke, or coronary death were higher for the childhood cancer survivors compared with population control subjects. A population-based cohort of 17,981 survivors of childhood cancer showed that cardiovascular deaths (including cardiac and cerebrovascular deaths) was 10.7-fold (95% CI 7.8-14.6) during the first 10 years of follow-up and then declined to a plateau and remained roughly at two- to threefold beyond 25 years from diagnosis when compared with population control subjects without a cancer history.65
Echocardiography is routinely used to monitor chemotherapy-induced cardiotoxicity in pediatric patients. As previously discussed for adult populations, the findings of abnormal LV structure and decreased LV systolic and/or diastolic function are typically a late consequence of cardiotoxicity with associated high risk of adverse cardiovascular outcomes. Biomarkers such as troponin and brain natriuretic peptide (BNP) or pro-BNP may hold promise for detecting early cardiotoxicity and for guiding cardioprotective treatment strategies.66
The most important strategy to decrease the risk of cardiotoxicity is to limit the cumulative dose of anthracyclines. The recommended cumulative dose limit of doxorubicin for children and adolescents with high-risk acute lymphoblastic leukemia was 360 mg/m2. This limit was further reduced to 300 mg/m2 on the Dana-Farber Cancer Institute Protocol 95-001.57 Data on the effect of liposomal anthracyclines are lacking in pediatric patients. A randomized trial of continuous (over 48 hours) doxorubicin infusion versus bolus infusion in 121 high-risk pediatric acute lymphoblastic leukemia cases failed to show a difference in cardiotoxicity between groups. 57
The treatment approach is similar to that with adults. Treatment with the ACE inhibitor enalapril for 2 to 3 years did not significantly improve clinical outcomes when compared with placebo in 135 children with anthracycline-induced cardiotoxicity.67 This study had limited power because of the small number of subjects and relatively short length of follow-up. Adverse effects of lightheadedness and fatigue were common in the enalapril group. No controlled trials with β-adrenergic receptor blockers have been conducted in the pediatric population. Dexrazoxane (Zinecard) can prevent or significantly reduce anthracycline-induced cardiac toxicity in pediatric patients. In a randomized trial in 206 children with acute lymphoblastic leukemia treated with doxorubicin, cardiac injury measured by rise in troponin T levels was observed in 21% of children receiving dexrazoxane versus 50% in those receiving doxorubicin alone (P < .001). Moreover, no evidence was found that dexrazoxane compromised the treatment efficacy.52 The same cohort further showed that increased troponin T levels were significantly associated with lower LV mass and LV end-diastolic posterior wall thickness 4 years later.66 In the same cohort assessed 5 years later, the patients receiving doxorubicin alone had worse LV fractional shortening, end-systolic dimension Z scores, LV wall thickness, and thickness to dimension ratio when compared with the group that received dexrazoxane.68 No difference was found in leukemia relapse or secondary malignancies.69
Cardiotoxicity Related to Inhibition of Cardiac Repair Signaling (Type II)
Trastuzumab
Trastuzumab (Herceptin) and related agents that inhibit the HER2 signaling pathway are the chemotherapeutic agents most often associated with cardiotoxicity after anthracyclines. Although the clinical manifestations of cardiotoxicity largely overlap, the clinical course of trastuzumab-induced cardiotoxicity differs from anthracycline-induced cardiotoxicity in several important respects. The cardiotoxicity of trastuzumab does not appear to be dose related and is often reversible. Patients may be successfully rechallenged after recovery of normal cardiac function without evidence of recurrent cardiotoxicity. In recognition of these important differences, Ewer and Lippman9 have proposed a classification system of chemotherapy-induced cardiotoxicity that distinguishes anthracycline-induced cardiac damage (type I) from trastuzumab damage (type II).
The mechanism of trastuzumab cardiotoxicity appears to be related to inhibition of myocellular signaling pathways that play a critical role in the normal homeostatic response to myocellular injury. The HER2 receptor heterodimerizes to other HER receptors, leading to autophosphorylation of the HER2 tyrosine kinase domain.75 This complex (which is critical to the antitumor efficacy of trastuzumab) is activated by neuregulin-1 secreted in paracrine fashion by cardiac endothelial cells under stress.70 Activation of the complex leads to multiple downstream signaling pathways that promote cell survival and hypertrophy. In mice, deletion of HER2 is lethal during embryogenesis because of failed cardiac development. Conditional cardiac-restricted deletion of HER2 in the adult mouse results in a dilated cardiomyopathy phenotype.71 Cardiomyocytes isolated from this mutant strain demonstrate increased sensitivity to anthracycline cardiotoxicity in vitro.72 Chien73 proposed a model of cardiotoxicity in which various types of cardiac stress such as mechanical strain, anthracyclines, or hypoxia trigger two competing pathways of cardiac myocyte survival (mediated by neuregulin-1 or gp130 cytokines) or apoptosis. In this model, treatment with trastuzumab blocks the survival pathway by preventing HER2/HER4 heterodimerization, thus shifting the balance to apoptosis with consequent decreased cardiac contractility and clinical heart failure. This model predicts that inhibitors of HER2 signaling may exacerbate the cardiotoxic effects of other chemotherapy agents with diverse mechanisms of cardiac injury.
The monoclonal antibody to HER2/neu receptor, trastuzumab, is widely used in breast cancers that overexpress Her2/neu and gastric cancer. Cardiotoxicity (cardiomyopathy, decrease in LVEF >10 percentage points, or clinical heart failure) was initially reported in the single-agent phase 2 trial in 4.7% of 222 women with HER2-overexpressing metastatic breast cancer that had progressed after chemotherapy for metastatic disease.74 In the pivotal stage III trial in 469 women with metastatic breast cancer that overexpressed HER2, an unexpectedly higher incidence of cardiotoxicity was observed—27%—when an anthracycline and cyclophosphamide were combined with trastuzumab, compared with 8% when chemotherapy without trastuzumab was utilized. A substantial although smaller magnitude of excess cardiotoxicity (13% vs. 1%) was observed when paclitaxel was combined with trastuzumab in patients who had previously undergone anthracycline therapy.75 Subsequent reports on the incidence of trastuzumab-induced toxicity have yielded a wide range of estimated risk. Seidman and colleagues76 reviewed the records of 1219 patients in seven trastuzumab trials. They report an incidence of cardiac dysfunction of 3% to 7% with trastuzumab alone, 27% when trastuzumab was combined with anthracycline and/or cyclophosphamide, and 13% when trastuzumab was combined with paclitaxel. In the National Surgical Adjuvant Breast and Bowel Project B31 adjuvant breast cancer trials, a 3-year cumulative incidence of grade 3 and 4 cardiac toxicities was reported in 4.1% of patients receiving chemotherapy with trastuzumab compared with 0.8% of patients who received chemotherapy without trastuzumab.77 In a Cochrane overview78 of 11,991 patients enrolled in clinical trials that randomized patients to trastuzumab alone or in combination with chemotherapy, trastuzumab increased the risk of clinical heart failure (RR 5.1; 90% CI 3.00-8.72; P < .00001) and LVEF decline (RR 1.83; 90% CI 1.36-2.47; P = .0008). In two of the eight trials in which trastuzumab was used for less than 6 months, cardiotoxicity was reported, but less frequently.
Additional questions being addressed in several large trials include the relative effects of the duration and sequence of trastuzumab therapy combined with chemotherapy. The addition of carboplatin to paclitaxel and trastuzumab does not appear to increase cardiac toxicity,79 nor does the combination of vinorelbine and trastuzumab.80,81 The lesser cardiotoxic potential of liposomal doxorubicin led to a trial of 30 patients with metastatic breast cancer who were treated with pegylated liposomal doxorubicin (PLD) in combination with trastuzumab. In this relatively small study, 10% of patients experienced asymptomatic decline in LVEF of at least 15%. No cases of symptomatic CHF were observed.82 In another study of 48 patients with HER2-positive metastatic breast cancer treated with pegylated liposomal doxorubicin in combination with cyclophosphamide and trastuzumab, none of the treated patients experienced symptomatic CHF. However, in 16.7% of patients, a grade 2 LV systolic dysfunction was reported to develop.83
The German Breast Group 26/Breast International Group 03-05 trial provided data on the prolonged cardiac effects of trastuzumab in patients with HER2-positive breast cancer who progressed after recent prior trastuzumab treatment. The median duration of previous exposure to trastuzumab was approximately 45 weeks. In that trial, 78 patients were treated with capecitabine alone and 78 patients received trastuzumab, 6 mg/kg every 3 weeks in combination with capecitabine. The median additional exposure to trastuzumab was nine cycles (27 weeks). Four patients (5.19%) in the combination group experienced severe (grade 3-4) cardiac toxicity, and CHF developed in one patient. Two patients (2.7%) in the capecitabine-alone group experienced grade 3-4 cardiac events.84
Lapatinib
Lapatinib (Tykerb, oral) is a small-molecule dual-selective inhibitor of ErbB1 (epidermal growth factor receptor) and ErbB2 (HER2/neu) tyrosine kinases. Because of the reported cardiotoxicity of trastuzumab, cardiac function has been carefully monitored in early clinical trials. Isolated cases of clinical CHF with a reduction in LVEF were reported in early phase 1 and 2 trials. In a retrospective analysis of 3689 patients from all reported trials, Perez et al. 85 reported a 1.3% incidence of asymptomatic CHF and a 0.1% incidence of symptomatic CHF. Because of the possibility of interactions with other agents, cardiac toxicity is still carefully monitored in current trials of this agent.
A phase 3 study of lapatinib plus capecitabine versus capecitabine alone was conducted in 324 patients with metastatic HER2-positive breast cancer who progressed after prior lines of therapy. All patients in this study had normal LVEF at the time of enrollment despite previous exposure to a median of 45 weeks of trastuzumab therapy (all subjects) and prior anthracycline therapy (97%).86 Four patients in the combined therapy arm versus one patient in the monotherapy arm experienced an asymptomatic decrease in LVEF. No symptomatic CHF events were observed.
Pertuzumab
Pertuzumab is an antibody that inhibits HER2 heterodimerization. In a phase 3 trial of the combination of pertuzumab with trastuzumab and docetaxel compared with placebo with trastuzumab and docetaxel in the first-line treatment for HER2-positive metastatic breast cancer, a decrease in LV systolic dysfunction occurred in 1.2% of subjects in the pertuzumab arm and 2.8% of the subjects in the placebo arm.87
Dual blockade of the HER2 pathway may be associated with increased risk of cardiotoxicity. Oral lapatinib in combination with trastuzumab was tested in women with metastatic HER2-positive breast cancer in a phase 3 trial of trastuzumab and lapatinib versus lapatinib alone. Asymptomatic transient decreases in LV function occurred in 3.4% of patients in the combination arm and 1.4% in the single lapatinib arm. The incidence of symptomatic cardiac events was 2% in the combination arm and 0.7% in the lapatinib arm.88
Trastuzumab-DM1
Trastuzumab-DM1 is an antibody-drug conjugate consisting of the trastuzumab molecule conjugated to the maytansinoid DM1 via a thioether linkage (maleimidomethyl cyclohexane-1-carboxylate [MCC]) with significant antineoplastic activity. In a phase 2 trial of trastuzumab-DM1 in 112 patients with HER2-positive metastatic breast cancer whose disease progressed after prior chemotherapy and/or anti-HER2 therapy, no cases of grade 3 or 4 cardiotoxicity and no cases of symptomatic CHF were observed. In two patients, LVEF decreased to a range of 40% to 45%.89
Mitoxantrone
Mitoxantrone was developed primarily as a noncardiotoxic analog of the anthracyclines. It is an anthracenedione that lacks the amino sugar common to anthracyclines. Data from collected single-agent studies and two randomized studies indicate that mitoxantrone may result in a congestive cardiomyopathy, but at a lower incidence than doxorubicin when equimyelotoxic doses are compared.90 The incidence of cardiotoxicity at cumulative doses of greater than 160 mg/m2 is about 5%, although the incidence of clinical congestive heart failure is less than 2%. The clinical presentation is similar to that seen with doxorubicin. The diagnostic evaluation and endomyocardial biopsy changes are similar to those seen with doxorubicin. The biochemical mechanism of damage is not clear. Prior therapy with doxorubicin increases the risk of mitoxantrone cardiac toxicity; changing from one anthracycline to another, or to an analog, may not protect the patient from cardiotoxicity. As stated previously, the mechanism of cardiac toxicity may not be the same as for doxorubicin. A study of 163 patients with multiple sclerosis who were treated with mitoxantrone (mean age 41.9 years and mean cumulative dose 59.7 mg/m2) showed that de novo cardiotoxicity of grade ≥2 (defined as a decline in LVEF) developed in 14% of the patients. The authors concluded that cardiotoxicity developed after cumulative doses that were significantly less than currently recommended maximal doses. In addition, no dose-response cardiotoxicity was observed. The authors emphasized the necessity of appropriate cardiac follow-up of patients treated with mitoxantrone.91
Other Agents with Cardiotoxicity (Type IV)
Phosphoramide Mustards
High-dose intravenous cyclophosphamide infusion (120-240 mg/kg over 1 to 4 days) has been associated with congestive heart failure and death from hemorrhagic myocarditis.92,93 In contrast to cardiac toxicity from anthracyclines, cardiac toxicity from cyclophosphamide is acute and is not related to the cumulative dose. Mortality is high in the face of fulminant hemorrhagic myocarditis; the majority of patients treated with high-dose cyclophosphamide will demonstrate a decrease in QRS voltage and a decrease in systolic function, which often are asymptomatic and reversible. Postmortem studies and an experimental animal model suggest that the loss of systolic function is due to direct endothelial injury resulting in capillary microthrombosis and interstitial fibrin deposition.
Ifosfamide
Ifosfamide also has been associated with the development of CHF in a dose-dependent fashion. In one series of 52 patients receiving a high dose (10-18 g/m2) as part of high-dose chemotherapy with autologous bone marrow transplantation, CHF developed in nine patients (17%; in eight patients the CHF was of sufficient severity to require admission to the intensive care unit) at a mean of 12 days (range, 6-23 days) after therapy.94 Most of these patients had previously been treated with doxorubicin, raising the possibility of sequential stress. Of interest, autopsy data (endomyocardial biopsies were not performed) did not reveal hemorrhagic myocarditis.
Imatinib (Gleevec)
Imatinib (Gleevec)—an oral agent small molecule that competes for BCR-ABL, C-KIT, PDGFR, and ABL-2 protein kinase binding sites by impeding protein kinase phosphorylation and activation of downstream kinases and has significant activity in patients with chronic myelogenous leukemia [CML] and in some patients with acute lymphoblastic leukemia [ALL] and gastrointestinal stromal tumor [GIST]—may also have cardiac adverse effects. Although no cardiac events were noted in a series of 553 patients with CML who were being treated with imatinib,95 two cases of CHF with elevated BNP levels were reported in patients with GIST,96 and Kerkela et al.97 reported CHF in 10 patients (8 with CML, 1 with ALL, and 1 with myelofibrosis). In two patients, pathological whorls suggestive of toxic myopathy (but different from anthracycline effects) were observed in endomyocardial biopsy specimens. A murine model with imatinib revealed similar findings and a dilated cardiomyopathy. The mechanism of imatinib toxicity is uncertain but possibly is mediated through a type II mechanism. The actual incidence or significance of cardiotoxicity remains uncertain but has led to a cautionary warning by the manufacturer.98 An extensive retrospective review of 1276 patients treated with imatinib at M.D. Anderson Cancer Center identified 1.7% patients with possible symptomatic CHF, with 0.6% possibly related to imatinib treatment.99
Sunitinib (Sutent)
Sunitinib is a small molecule, administered orally, that inhibits the PDGFR, VEGFR, KIT, FLT3, CSF-1R, and RET tyrosine kinases that are important for tumor cell growth, survival, metastasis, and angiogenesis. In two studies of 169 patients with metastatic renal cell carcinoma for whom prior cytokine-based therapy had failed, 14% of patients had decline in LVEF below the normal level. In the first study, 4.7% of patients had an LVEF drop of 20 percentage points or more with no reported symptoms.100 In addition, grade 3 hypertension was reported in 6% of subjects. In the second study, dyspnea developed in one patient.101 In another study of 207 patients with GIST, there were no cases of decreased LVEF or CHF.102 In a study of 224 patients treated with sunitinib at M.D. Anderson Cancer Center, 2.6% experienced symptomatic CHF shortly after the initiation of sunitinib. None of the patients had a history of CHF, and five had a normal LVEF prior to treatment with sunitinib.103
Chemotherapy Combinations
Combinations of chemotherapeutic agents have been reported to cause varying degrees of cardiotoxicity. When doxorubicin was first combined with paclitaxel, an 18% incidence of CHF was reported with cumulative doxorubicin doses of 400 mg/m2.104 This high rate was not confirmed in another report.105 When paclitaxel was initially administered as a 24-hour infusion, increased plasma and tissue concentrations of doxorubicin and the doxorubicinol metabolite were observed. Limiting the doxorubicin dose and allowing for an interval between doxorubicin and paclitaxel administration reduced the incidence of CHF to 4.7% of 657 patients, with a higher incidence of 25% in patients who received more than 440 mg/m2.106 The combination of doxorubicin with docetaxel has not been associated with increased cardiac toxicity. In a randomized trial comparing doxorubicin and docetaxel with doxorubicin and cyclophosphamide in 429 women with breast cancer, CHF was not increased (3% vs. 4%), and the decline in LVEF—30 percentage points from baseline—was less (1% compared with 6%).107 The incidence of CHF has been reported to be 6% for the combination of epirubicin with paclitaxel.108 Several trials have raised the possibility of increased cardiac toxicity when doxorubicin is combined with high-dose cyclophosphamide109 in the bone marrow transplant setting.
Radiation-Induced Heart Disease (Type V)
Incidental radiation exposure to the heart that occurs during treatment of adjacent thoracic, chest wall, or breast neoplasms may induce damage to the pericardium, myocardium, cardiac valve leaflets, and coronary arteries. The risk of radiation-induced heart disease is thought to be dose dependent. Cardiac damage can be expected with whole-heart radiation exposure above 30 Gy, but doses <5 Gy have also been associated with increased risk of ischemic disease, pericardial disease, and valvular disease.110 Because myocytes are terminally differentiated cells with a very low turnover rate, radiation-induced heart injury is thought to be primarily mediated through injury to microvascular endothelial cells, with subsequent local ischemic injury and interstitial fibrosis.111 Clinical manifestations of radiation injury to the heart may occur decades after the initial exposure. Biventricular dysfunction is a common although usually asymptomatic finding, occurring 5 to 20 years after radiation therapy. Biventricular dysfunction is especially likely in patients who have been treated through a single anteroposterior port.112 Diastolic dysfunction is common in long-term survivors and is associated with worsened event-free survival.113 In some cases, the radiation injury may induce restrictive cardiomyopathy with clinical heart failure. Pathology studies demonstrate fibrotic lesions that may in some cases be indistinguishable from the effects of atherosclerosis, degenerative valve diseases, and other forms of myocardial and pericardial injury.114 In many cases, the diagnosis of radiation-induced heart disease is determined by the clinical setting. For example, degenerative aortic valve disease in a 45-year-old man with a history of mantle radiation for Hodgkin lymphoma at age 21 years would be presumed to be related to the past radiation exposure in lieu of any other explanation for valve degeneration in this age group.
A meta-analysis derived from 40 randomized clinical trials comparing radiation therapy versus no radiation therapy in nearly 20,000 women with breast cancer demonstrated that radiation therapy was associated with reduced breast cancer mortality but increased vascular mortality.115 A population-based nonrandomized study in 35,000 women showed that left-sided breast cancer radiotherapy increased the risk of cardiac morbidity (ischemic heart disease, pericarditis, and valvular disease) but not cardiac mortality when compared with right-sided radiotherapy.110 A smaller cohort study in 961 patients demonstrated that left-sided radiation for breast cancer was associated with greater risk of myocardial infarction and cardiovascular death when compared with right-sided irradiation in the second decade after treatment.116 The laterality of radiation treatment also corresponds to the location of stenosis in the coronary artery tree.117 With the introduction of cardiac-sparing radiation techniques, risk of death from ischemic heart disease associated with radiation for left-sided breast cancer decreased from 13.1% in patients treated before 1980 to 5.8% in those treated after 1985.118
Standardized incidence ratios for coronary artery disease in Hodgkin lymphoma survivors an average 19 years after chest radiation therapy are three- to fivefold greater than those in an age-matched population.119 Coronary artery disease as a result of radiation is specific to the vessels radiated and typically does not present with clinical symptoms until at least 10 years after treatment.120 In 1132 survivors of Hodgkin lymphoma who were treated with mediastinal radiation before the age of 18 years and were followed up for a median of 32 years, the most common cardiac disease was valvular heart disease.121 The late incidence of all cardiac disease was increased in a dose-dependent manner in the subjects who received whole-heart irradiation >25 Gy. In another series of 1279 survivors of Hodgkin lymphoma who were treated with mediastinal radiation (median follow-up 14.7 years), the cumulative incidence of cardiac disease was 16% at 20 years.122 Coronary artery disease was the most common cardiac disease, but the greatest incremental risk (based on standardized incident ratios) was related to valvular heart disease and pericardial disease. Radiation dose (greater vs. less than 36 Gy) was not a significant predictor of cardiac disease in this population.
Pericardial disease is a well-known adverse effect of thoracic radiation therapy. Effusions have been reported in 6% to 30% of patients treated with radiation therapy to the chest with use of older radiation techniques.123 The incidence of pericarditis has been significantly decreased with modern radiotherapy techniques. Pericarditis may present with pleuritic chest pain, fever, and tachycardia but is asymptomatic in many patients.124 Echocardiography may show pericardial effusion and/or pericardial thickening and, less commonly, cardiac tamponade physiology. Therapy should be tailored to the patient presentation and may include nonsteroidal antiinflammatory agents, corticosteroids, and/or pericardiocentesis. Some patients will experience late manifestations of chronic pericarditis with variable severity ranging from asymptomatic to constrictive pericarditis with refractory heart failure. Signs of constriction may include a pericardial knock, Kussmaul sign, peripheral edema, and evidence of hepatic congestion and ascites. The diagnosis of constrictive physiology can be confirmed by Doppler echocardiography, CMR, and/or cardiac catheterization. In symptomatic patients, pericardiectomy may be warranted; however, the surgery is often difficult and carries substantial morbidity and mortality risk.
No known specific medical treatments exist for radiation-induced heart injury. Revascularization by percutaneous or surgical techniques can be used to treat symptomatic myocardial ischemia. Surgical valve replacement can be used to treat symptomatic valve dysfunction. Advances in the radiotherapy techniques including dose and field size reduction and cardiac shielding have significantly decreased risk of cardiac injury from thoracic irradiation. Other techniques such as tomotherapy, intensity-modulated radiation therapy, mixed electron/photon beams, respiratory gating,125 and prone accelerated partial breast irradiation126 may further decrease risk of radiation-induced heart injury.
Increased caution must be exercised when radiation therapy is combined with anthracycline therapy or other cardiotoxic drugs because of the potential for a synergistic toxic effect on the myocardium, which may occur even if the two therapies are temporally separated by long periods. Prior radiation is a well-recognized risk factor for doxorubicin cardiotoxicity.127 Conversely, radiation can cause a sudden decrease in ventricular function in a patient who either is receiving or has received doxorubicin.
QT Prolongation
Conventional cytotoxic agents such as anthracyclines, cyclophosphamide, and 5-fluorouracil (5-FU) have been reported to be associated with QT prolongation, usually in association with other evidence of severe cardiotoxicity.128,129 The QT prolongation related to these agents is usually dose-dependent and is associated with increased risk of CHF. No guidelines for QT interval monitoring for these agents have been published, but an electrocardiogram is recommended for any patient with suspected cardiotoxicity.
Myocardial depolarization and repolarization are mediated through membrane ion channels that regulate the rapid inflow of sodium and calcium ions (during depolarization) and outflow of potassium ions (during repolarization). Genetic abnormalities and drugs that lead to ion channel malfunction and excess positive intracellular charge extend ventricular repolarization and prolong the QT interval. Almost all drug-induced increases in repolarization are the result of suppression of the rapid component of the delayed rectifier potassium current (IKr) (also known as potassium channel Kv11.1),130 which is responsible for the rapid repolarizing potassium current. The α subunits of Kv11.1 are coded by the human ether-a-go-go–related gene (hERG or KCNH2) located on the long arm of chromosome 7.131 Changes in function of hERG potassium ion channels (hERG K+) are responsible for most, if not all, drug-induced QT prolongation and clinical cases of TdP.132 The net effect of any given drug on the QT interval and risk of arrhythmia is determined by the concentration of the drug, interactions of the drug with the hERG potassium channel (or less often with other sodium, calcium, or other potassium channels), and genetic background relevant to the function of other ion channels on the cardiac membrane.
Agents with QT prolongation potential are listed in Table 59-4. Procedures for use of each specific agent should be followed as described in the package insert. We propose the following general guidelines to optimize patient safety. The risk of fatal arrhythmia is small but not negligible; the oncologist and cardiologist should collaborate to carefully assess the risk/benefit ratio for each patient.
Table 59-4
Chemotherapeutic Agents Causing QT Prolongation
| Medication | Indication | Estimated Incidence (%) | Black Box Warning? | TdP Reported? | Sudden Death Reported? |
| Arsenic trioxide | Acute promyelocytic leukemia | 40-63 | Yes | Yes | Yes |
| Romidepsin | Peripheral T- cell lymphoma | 2 | No | No | No |
| Vorinostat | Cutaneous T- cell lymphoma | 1-4 | No | No | No |
| Pazopanib | Renal cell carcinoma, metastatic | 2 | No | Yes | No |
| Vandetanib | Medullary thyroid carcinoma | 14 | Yes | Yes | Yes |
| Crizotinib | Lung cancer, metastatic | 1.3-3.5 | No | No | No |
| Nilotinib | Chronic myelogenous leukemia, metastatic | >1 | Yes | No | Yes |
| Vemurafenib | Melanoma, metastatic | <1 | No | No | No |
| Eribulin | Breast cancer, metastatic | <1 | No | No | No |
Before initiating therapy with a drug with QT prolongation potential:
1. A 12-lead electrocardiogram should be performed with standardized calculation of the QTc (automated on most modern electrocardiogram systems).
2. Serum electrolytes (i.e., potassium, calcium, and magnesium) and creatinine should be assessed.
3. Preexisting electrolyte abnormalities should be corrected.
4. If possible, drugs that are known to prolong the QT interval should be discontinued (e.g., medications used for supportive care such as methadone, 5-HT3 antagonists and antihistamines, some antibiotics, antifungal drugs, and antivirus agents).
5. Cardiac risk factors for QT prolongation (e.g. CHF, LV hypertrophy, and bradyarrhythmia) should be assessed and treated if possible.
6. For QTc greater than 500 msec, cardiology consultation is recommended for implementation of corrective measures, assessment of arrhythmic risk, and serial monitoring.
1. During therapy, potassium levels should be maintained above 4 mEq/L and magnesium levels should be maintained above 1.8 mg/dL with oral and/or parenteral supplementation as needed.
2. Patients who reach an absolute QT interval value >500 msec should be reassessed and immediate action should be taken to correct concomitant risk factors, if any; in addition, the risk/benefit of continuing versus suspending the treatment should be considered.
3. If syncope, rapid heartbeat, or irregular heartbeat develops, the patient should be hospitalized for monitoring, serum electrolytes should be assessed, and the medication should be temporarily discontinued until the QTc interval decreases to below 460 msec, electrolyte abnormalities are corrected, and symptoms resolve.
4. If QTc prolongation associated with life-threatening signs or symptoms (e.g., ventricular arrhythmia, syncope, and/or TdP) develops, the medication should be stopped permanently.
5. If QTc >500 msec occurs in an asymptomatic patient, cardiology consultation is recommended for implementation of corrective measures, assessment of arrhythmic risk, and serial monitoring.
Vascular Toxicity
Hypertension
The vascular endothelial growth factor (VEGF) family of angiogenic glycoprotein growth factors is known to play an important role in regulation of vasculogenesis and angiogenesis in normal embryogenesis, response to tissue injury and hypoxia, and tumor growth.133 Activation of the VEGF receptor kinase in vascular tissues (VEGFR2) induces nitric oxide release, endothelial cell migration and proliferation, and expression of a distinct profile of adhesion molecules and proteases important in new blood vessel formation.134
VEGF signaling pathway (VSP) inhibitors are widely used in the treatment of patients with cancer. Five agents have been approved by the FDA, including bevacizumab (Avastin), sunitinib (Sutent), sorafenib (Nexavar), pazopanib (Votrient), and vandetanib (Zactima). Bevacizumab, a humanized monoclonal antibody, binds specifically to circulating VEGF. The other four agents are small molecule tyrosine kinase inhibitors that inhibit VEGFR2 kinase and other kinases. Inhibition of the biological effects of VEGF signaling slows tumor growth in part by alteration of vascular structure and function within the tumor tissue.135
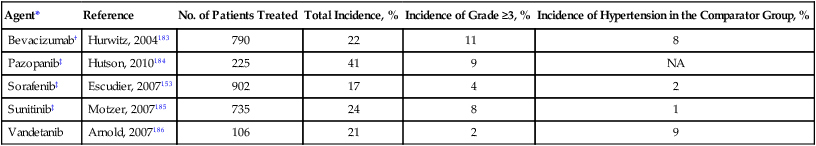
BP elevation is a common adverse effect for all VSP inhibitors (Table 59-5).76,136,137 The incidence of hypertension increases significantly with the use of two VSP inhibitors. The combination of bevacizumab and sunitinib in metastatic renal cell carcinoma was associated with hypertension in 92% of treated patients.138 Drug-induced hypertension may be a marker of anticancer efficacy because overall response rate, progression-free survival, and overall survival are superior in patients with hypertension when compared with patients without hypertension.139 The mechanism of VSP inhibitor-induced hypertension has not been fully characterized. Evidence suggests that vascular endothelial dysfunction, decreased nitric oxide production, increased endothelin-1 production, and microvascular rarefaction contribute to BP elevation.137,140–142
Table 59-5
Incidence of Hypertension in Clinical Trials of Vascular Endothelial Growth Factor Signaling Pathway Inhibitors
| Agent* | Reference | No. of Patients Treated | Total Incidence, % | Incidence of Grade ≥3, % | Incidence of Hypertension in the Comparator Group, % |
| Bevacizumab† | Hurwitz, 2004183 | 790 | 22 | 11 | 8 |
| Pazopanib‡ | Hutson, 2010184 | 225 | 41 | 9 | NA |
| Sorafenib‡ | Escudier, 2007153 | 902 | 17 | 4 | 2 |
| Sunitinib‡ | Motzer, 2007185 | 735 | 24 | 8 | 1 |
| Vandetanib | Arnold, 2007186 | 106 | 21 | 2 | 9 |
NA, Not applicable (no untreated comparator group).
*For each agent, one of the larger studies with that drug and the reported incidence of hypertension by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) is listed. Note the differences in reported rates; grades cannot be accurately compared across trials because of differing clinical settings and the CTCAE versions used.
†Based on CTCAE version 2.0 (grade 3 hypertension is defined as “requiring initiation or increase medication”).
‡Based on CTCAE version 3.0 (grade 3 hypertension is defined as “requiring more than one drug or more intensive therapy than previously”).
Revised from Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst 2010;102:596-604.
Many patients with indications for the use of VSP inhibitors have metastatic disease with limited life expectancy. Active control of hypertension should be incorporated into the overall care plan to increase tolerability and possibly efficacy of VSP inhibitor therapy. The Investigational Drug Steering Committee of the National Cancer Institute recommends assessment of cardiovascular risk and development of a prospective plan for monitoring and management of hypertension before initiation of therapy with VSP inhibitors.134
Hypertension can be treated with ACE inhibitors, diuretics, calcium blockers, and other antihypertensive agents. The VSP inhibitor may be temporarily held for persons with BP higher than 160/100 mm Hg or any symptomatic hypertension despite active management with antihypertensive agents. If BP remains uncontrolled, then discontinuation of the VSP inhibitor should be considered and the patient should be referred to a hypertension specialist. For patients completing a course of VSP inhibitor therapy, the discontinuation or reduction of dose of antihypertensive agents should also be anticipated.134
Coronary Artery Disease (Myocardial Ischemia, Type III Agents)
Fluoropyrimidines are the chemotherapy drugs most commonly associated with myocardial ischemia. The largest number of cases are described in association with treatment with 5-FU.143 A review of more than 1000 patients receiving 5-FU revealed an incidence of cardiac toxicity of 4.5% in patients with previous diagnosis of coronary disease versus an incidence of 1.1% in patients without previous known coronary artery disease. In a prospective series of 910 patients,144 five patients (0.55%) experienced signs and symptoms that were consistent with coronary artery spasm. In a smaller prospective series, resting or exertional angina developed in 9 of 106 patients.145 The mechanism underlying these cardiac events is not well characterized, but drug-mediated endothelial dysfunction is thought to be a contributing factor.146 Because coronary artery spasm may be the underlying mechanism contributing to these clinical events, coronary vasodilating agents such as nitrates and calcium blockers may be protective. Myocardial ischemic events associated with 5-FU may be more common in patients treated with continuous infusion therapy143 and in patients who are receiving concomitant radiation therapy147 or treatment with cisplatin.
Capecitabine (Xeloda), an oral prodrug for 5-FU, has also been associated with cardiac toxicity. In a review of 832 patients, the incidence was similar to that observed with 5-FU (3% for all toxicities and 1% for grades 3 to 4). Individual cases of myocardial infarction, angina pectoris, myocardial ischemia, myocarditis, and tachycardia have been reported.148 It is possible that combination with oxaliplatin may increase this toxicity. In combined data from two trials of 153 patients, cardiac toxicity was observed in 6.5% of patients; 4.6% of these events were related to myocardial ischemia.149
Although the vinca alkaloids also have been reported to precipitate angina and myocardial infarction,150 this observation is strictly anecdotal. The Raynaud phenomenon has been described in patients treated with cisplatin-based therapy.151 It also has been described after therapy with vinblastine, vincristine, and bleomycin. These agents may be similar in mechanism to other ischemic syndromes (e.g., coronary ischemia or cerebral vascular ischemia), which have been described with these agents, as well as with 5-FU.
Myocardial ischemia has been reported with VSP inhibitors, but its incidence and pathophysiology are not well described. Sorafenib-associated multivessel coronary vasospasm on coronary angiography and bevacizumab-associated acute coronary thrombosis have been reported.151,152 In a phase 3 trial of patients with renal cell carcinoma, myocardial ischemia and infarction occurred in 12 patients in the sorafenib group (3%) compared with 2 patients in the placebo group (<1%; P = .01).153
Venous and Arterial Thromboembolism
A high incidence of venous thromboembolism (VTE) has been observed in patients with multiple myeloma treated with thalidomide combined with doxorubicin (a VTE rate of up to 34%)154 and in patients with metastatic renal cancer treated with thalidomide, gemcitabine, and infusional 5-FU (a VTE rate of up to 43%).155 A comparable high incidence of thromboembolic events was reported with the combination of gemcitabine, cisplatin, and semaxanib (SU5416).156 VSP inhibitors were also shown to increase the risk of VTE. A metaanalysis of 7956 patients with a variety of advanced solid tumors who were treated with and without bevacizumab revealed a significantly increased risk of VTE with an RR of 1.31 (P < .001) compared with control subjects. However, the reports for risk of VTE with the use of other VSP inhibitors (small molecules) have been inconsistent.102,157,158
Increased arterial thromboembolic event risk was also reported in up to 7% of patients with prostate cancer who were treated with thalidomide and docetaxel. 159 As is the case for radiation-induced coronary artery disease, clinically significant occlusive lesions in other arteries, such as the carotids,160 have been reported after irradiation.
Edema Formation (Increased Microvasculature Permeability)
Docetaxel (Taxotere), an active taxane analog of paclitaxel (Taxol), is associated with the formation of edema in some patients. This syndrome is progressive with more treatment cycles and does not appear to be of cardiac or renal origin. Premedication with steroids substantially reduces this problem.161
Dasatinib (Sprycel) is an oral agent that is active in 14 of 15 imatinib-resistant BCR-ABL tyrosine kinase mutants and inhibits other tyrosine kinases such as SRC, C-Kit, and PDGFR. In a study of 84 patients with CML and Ph+ ALL, pleural effusion developed in 15%, peripheral edema developed in 19%, pulmonary edema developed in 14%, and pericardial effusion developed in 7% .162 The mechanism is not known.
All-trans retinoic acid, an oral agent that overcomes promyelocyte retinoic acid receptor maturation block caused by a t(15,17)-related protein, has become an important part of acute promyelocytic leukemia treatment. The drug causes retinoic acid syndrome,163 which consists of respiratory distress, hypoxemia, weight gain, pleural and pericardial effusions, pulmonary infiltrates, fever, hypotension, and acute renal failure after a median of 11 days (2 to 47 days) in approximately 25% of treated patients.164 The treatment is high-dose dexamethasone given as soon as the syndrome is suspected165 and temporary discontinuation of all-trans retinoic acid, depending on the clinical situation.
Hypotension
Hypotension has been reported in 6% to 14% of patients treated with alpha interferon.166 The etiology is not clear and may be related in some cases to concomitant cardiac dysrhythmias and/or ischemia. Hypotension is also a common adverse event associated with gamma interferon.167
Hypotension (along with dysrhythmias, ischemia, and decreased contractility) is a common adverse effect of interleukin (IL)-2 and was observed to occur in more than 70% of patients in the early IL2/LAK trials.168 The primary mechanism appears to be decreased vascular resistance.169,170 The clinical presentation may be similar to that of septic shock, with a vascular leak syndrome, fluid retention, and noncardiogenic pulmonary edema. The mechanism may also be related to that of septic shock, with induction of cytokines and effector cells and the release of vasodilatory agents. Reductions in dose and continuous infusion schedules have lessened these toxicities171,172 but also may decrease the antitumor effects.173 In one study,174 oral l-carnitine appeared to decrease these complications. IL-2 toxicity often requires intensive-care management, support with pressors, and, at times, ventilatory support.
Hypotension also has been described with tumor necrosis factor, granulocyte-macrophage colony-stimulating factor,175 and monoclonal antibodies.176 Hypotension is often a dose-limiting adverse effect during treatment with tumor necrosis factor.177 Hypotension with granulocyte macrophage colony-stimulating factor is observed at high doses and can be associated with a capillary leak syndrome and decreased peripheral resistance.178 The mechanism with these two agents is not as well defined. Hypotension also has been reported with IL-1 in phase 1 trials.179
The possibility of cardiac toxicity was raised in early trials of IL-4, especially when one patient was found to have biopsy-proved myocarditis.180 IL-6 has been associated with cardiac dysrhythmias.181 Syncope or near-syncope was reported in 6 of 58 patients in a trial of IL-11 (5 patients at the 50-mg/kg level and 1 patient in the placebo group). Six patients also had documented dysrhythmias.182