Carcinoma of Unknown Primary
Gauri R. Varadhachary, Renato Lenzi, Martin N. Raber and James L. Abbruzzese
• Carcinoma of unknown primary is a diverse group of heterogeneous cancers and accounts for about 2% to 4% of all cancers.
• No universal agreement exists on the extent of evaluation to search for a primary cancer.
• “Adequate,” early biopsy of a metastatic site is recommended to establish the diagnosis and help direct further workup.
• Basic evaluation includes the following:
• Comprehensive history and physical examination (including breast and pelvic examinations in women and testis and prostate examinations in men).
• Routine laboratory tests, chest-abdominal-pelvic computed tomographic scan, and mammography in women.
• Directed invasive tests based on symptomatology and pathological evaluation of the tumor tissue.
• Judicious pathological assessment of the metastatic tumor sample including directed immunohistochemical markers. Additional molecular markers that have a therapeutic intent are based on clinicopathological evaluation (including KRAS mutational status, Her2 (ERBB2) expression, and epidermal growth factor receptor (EGFR) mutation studies).
• The diagnostic utility of positron emission tomography (PET) is poorly defined; it is beneficial in selected patients.
• The role of tissue of origin molecular profiling assays continues to evolve; these tests are beneficial in selected patients.
• Empiric combination cytotoxic therapy and the “one treatment fits all” approach are no longer emphasized.
• Where possible, individualized therapy for the metastatic cancer “profile” is based on detailed clinicopathological evaluation.
• When pathological evaluation falls short (large differential), empiric platinum-based combination therapies are usually selected.
• Taxane, gemcitabine, fluoropyrimidine + platinum doublet is a common “broad spectrum” first-line regimen in good performance status patients.
• Additional lines of therapy depend on performance status, pathological “profile,” and response to first-line therapy.
Introduction
Carcinoma of unknown primary (CUP) is a diverse group of cancers with a poor prognosis. Despite the increasing array of sophisticated diagnostic tools available to establish the diagnosis of human neoplasia, oncologists have struggled to understand a subset of patients with metastatic cancer in whom detailed investigations fail to identify a primary anatomic site. The reported incidence of CUP varies with the practice setting and the definition used, but averages 2% to 4% of all patients who are diagnosed with cancer.1 Because identification of the primary lesion forms the basis for predicting the expected behavior and assigning appropriate therapy for malignant disease, the absence of a defined primary carcinoma poses a major challenge. The inability to identify a primary carcinoma also generates anxiety for the patient, who may feel that the physician’s evaluation has been inadequate or that the prognosis would be improved if a primary site could be established. The management of CUP has been evolving, and recently developed sophisticated diagnostic modalities and novel therapies present both an opportunity and a challenge.
As suggested by the range of incidence statistics, the definition of CUP has not been standardized, varying in published reports with regard to the extent of evaluation required to accept the diagnosis. For the purpose of this chapter, we define patients with CUP as having a biopsy-proven malignancy for which the anatomic origin remains unidentified after history and physical examination (including breast palpation and pelvic examination in women and testicular and prostate examination in men), laboratory studies including liver and renal function tests; hemogram; chest x-ray; computed tomography (CT) of the chest, abdomen, and pelvis; and mammography in women and measurement of prostate-specific antigen (PSA) in men.2 All positive findings on this initial evaluation are then investigated in detail. Depending on the clinical situation, additional studies might include directed invasive studies including upper endoscopy, colonoscopy, or bronchoscopy. To define the patient population further, most investigators have excluded from analysis instances in which soft tissue sarcoma or melanoma present without a definite primary site, because such presentations of these diseases have histology- and stage-specific therapy regardless of the primary. CUP clinical and research efforts concentrate on the vast majority of patients with common epithelial histologies such as adenocarcinoma, carcinoma, squamous carcinoma, and neuroendocrine carcinoma.
Etiology and Epidemiology
Whether specific etiologic factors are relevant to CUP is not known. Although a history of cigarette smoking often can be elicited, the heterogeneity of CUP makes it unlikely that specific etiologic agents will be associated with this disease.3 The fact that numerous occult anatomic sites can give rise to carcinomas that present with only metastatic disease supports the possibility that specific interactions of genetic and environmental insults could give rise to genomic and biochemical changes that lead to the early development of the metastatic phenotype without the associated changes supporting local growth in the organ of origin. Although this concept is highly speculative, the hypothesis can be tested through analysis of available biomarkers such as oncogenes and tumor suppressor genes that have been characterized for cancers with known anatomic origins, such as lung, pancreatic, breast, and colorectal carcinomas. Either the absence of genetic changes typical for malignancies with established primary cancers or the presence of unusual variants of known genetic alterations would support this hypothesis. It is likely that as the genomic and proteomic characterization of malignancies is refined, fewer and fewer malignancies may be assigned to the CUP designation (and we are beginning to see that in this era of novel diagnostics).
Biological Considerations
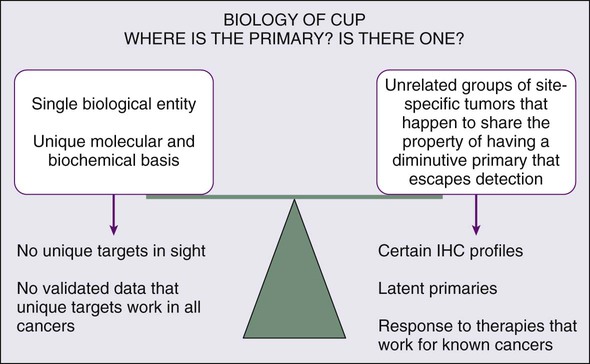
Whether the biology of CUP is fundamentally different from known primary carcinoma with systemic metastases remains controversial (Fig. 94-1). Nystrom and associates have argued that the distribution of metastatic sites in patients with CUP in whom the primary cancer is subsequently found is sufficiently different from known primary carcinoma to support the hypothesis that CUP is biologically unique.4 However, analysis of a series from the MD Anderson Cancer Center demonstrates few significant differences in the pattern of metastases or in overall survival for true CUP versus patients in whom the primary lesion was found.3 However, whether CUP metastases are genetically and phenotypically unique remains to be determined.
The underlying cause for “occult” primary tumors is unknown. Previous investigators have speculated that the primary tumor may remain below the limits of clinical or radiographic detection or that it spontaneously regressed. It is possible that CUP falls along the continuum of cancer presentation in which the primary has been contained or eliminated by the immune system. Alternatively, CUP may represent a specific malignant event that results in an increase in metastatic spread or survival relative to the primary—with immunogenic or antiangiogenic factors that impede local growth.5
Aneuploidy
Aneuploidy, a well-recognized phenomenon occurring in 70% to 90% of solid tumors, is defined as a chromosome complement that is not a simple multiple of the haploid set. Increasing evidence indicates that for many carcinomas, such as breast, prostate, and colorectal cancers, a diploid DNA content is associated with a more favorable prognosis.6 Hedley and associates7 measured the cellular DNA content of tumor biopsy specimens of 152 patients with metastatic adenocarcinoma or undifferentiated carcinoma of unknown primary site to determine favorable subgroups. Aneuploidy was found in the specimens of 70% of the patients. There were no significant differences between men and women, and there was no obvious relationship of ploidy to the various patterns of metastatic involvement. The median survival of patients with diploid tumors was 4.2 months, versus 4.8 months for patients with aneuploid tumors. Of the 46 patients with diploid tumors, 9 (18%) survived for more than 2 years, compared with 10 (9%) of 106 patients with aneuploid tumors. These results indicate that the incidence of aneuploidy in this heterogeneous group of patients is similar to that reported for carcinomas with known primary tumors. However, in contrast to many of these tumor types in this study, metastatic adenocarcinomas of unknown primary origin that were diploid were not associated with a more favorable prognosis than those of known primary origin.
Chromosome Abnormalities
The role of chromosomal abnormalities in CUP has been evaluated in several studies to better understand the biological characteristics of CUP. Abbruzzese and colleagues and Bell and coworkers identified common karyotypic changes in CUP.8,9 The karyotypes of 13 of 20 patients with CUP were determined, and in 12 of the analyzed cell lines, abnormalities were identified in the short arm of chromosome 1. The abnormalities detected included deletion of 1p, translocations, isochromosome 1q, and gene amplification. These findings were consistent with earlier descriptions of chromosome 1p abnormalities in advanced malignancy as described by Atkin29 and Mertens and associates.10
Motzer and associates used karyotyping to determine the frequency of specific abnormalities of chromosome 12 in patients with CUP.11,12 It was hypothesized that patients with undifferentiated carcinoma of unknown primary origin responding to cisplatin-based chemotherapy had unrecognized germ cell tumors and that isochromosome 12p, i(12p), a specific chromosomal marker characterizing germ cell tumors, could be used to identify such patients. Thirty percent of patients had an increased 12p copy number or deletion of the long arm of chromosome 12, which proved predictive of response, validating the hypothesis. Complete response to cisplatin-based therapy was achieved in patients with specific chromosomal aberrations associated with germ cell tumors, and objective responses were achieved in 75% of these patients, compared with 17% of patients without these aberrations. Summersgill and associates and Ilson and associates found similar associations for CUP patients with undifferentiated carcinoma.13,14 Thus for patients with undifferentiated carcinoma, i(12p) correlated with a good response to platinum-based chemotherapy, although lack of i(12p) may not exclude a small percentage of responses; i(12p) occurs in more than 80% of the germ cell tumors and only sparsely in a few other lesions (e.g., acute leukemia, embryonal rhabdomyosarcoma, and neuroepithelioma), and, therefore, determination of the presence or absence of i(12p) may assist in the diagnosis of extragonadal germ cell tumors in patients with CUP.15 These studies were conducted in the 1980s and 1990s; in the current era, pathological evaluation of cancers has significantly improved. Patients with extragonadal germ cell cancers are rarely seen in the CUP clinic and i(12p) testing is almost never needed to make a diagnosis or direct a therapeutic plan.
Oncogenes
The oncogenes ras, MYC, bcl-2, and her-2/neu are overexpressed in a variety of solid tumors. Pavlidis and associates found a high rate of overexpression of MYC (96%), ras (92%), and c-erbB2 (65%) in CUP cancers.16 However, investigators found that the overexpression of these genes was not related to histologic or clinical parameters or and did not have either diagnostic or prognostic value.
Briasoulis and associates studied levels of bcl-2 expression in 40 patients with CUP (8% squamous, 36% adenocarcinoma, and 55.5% poorly differentiated carcinoma).17 Staining was evaluated based on intensity (+1 to +3) and the percentage of positive cells (1% to 100%). Expression of bcl-2 was observed in almost half of the tumors. This finding was not expected, because in most studies, bcl-2 had been found to be upregulated in premalignant lesions rather than in advanced malignancies and also had been associated with a less aggressive phenotype.18,19 In this study, the level of bcl-2 expression, by itself, had no prognostic value. When combined with a high level of expression of TP53, however, high expression of bcl-2 showed a trend toward a higher response to platinum-based chemotherapy.
Hainsworth and associates stained 100 tumor specimens of poorly differentiated adenocarcinoma (PDA) or poorly differentiated carcinoma (PDC) of unknown primary site for Her-2 protein.20 The samples of 10 patients (11%) overexpressed Her-2. These investigators did not observe any major difference in the overall response rate to chemotherapy between the patients whose cancer overexpressed Her-2 and those who did not. Evaluation of the efficacy of trastuzumab in selected patients with CUP with Her-2 overexpression is warranted.
Rashid and associates retrospectively evaluated 100 serial formalin-fixed, paraffin-embedded sections from patients with CUP with biopsies or resections done at MD Anderson Cancer Center.21 Seventy-six samples with adequate tissue were stained by immunohistochemistry for epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF)-A, Cox-2, c-erbB2, and KIT. Seventy-five percent of tumors stained for EGFR and 49% for VEGF-A. EGFR often was present with other markers, and was seen along with VEGF, c-erbB2, or Cox-2 in at least 30% of patients. Three triple-staining patterns of EGFR/c-erbB2/VEGF, EGFR/c-erbB2/Cox-2, and c-erbB2/Cox-2/VEGF were observed. Kaplan-Meier survival by intensity of staining for these proteins, however, failed to demonstrate significant associations.
Tumor Suppressor Genes
It is now known that a large number of gene deletions or allelic inactivation occur in most human cancers. Many of these genetic alterations result in the activation of oncogenes or in the loss of tumor suppressor genes and have been localized to specific chromosomes. To date, TP53 is the best known and most widely studied tumor suppressor gene. TP53 can control tumor development by arresting the cell cycle or initiating apoptosis of damaged cells. TP53 mutations are common and occur in about 55% of all human cancers.24–24
Briasoulis and associates evaluated TP53 expression using immunohistochemistry in 47 cases of CUP (4 squamous carcinoma, 17 adenocarcinoma, 26 PDC). Staining was evaluated based on intensity (+1 to +3) and percentage of positive cells (1% to 100%).25 More than 70% of the tumors expressed TP53; 53% expressed a high level and 47% expressed a low level of immunohistochemical staining. In this study, TP53 expression alone had no prognostic value.
Bar-Eli and associates also investigated the frequency of TP53 mutations in a series of 15 CUP biopsies and 8 cell lines established from CUP.26 Mutations in the conserved regions of TP53 gene were analyzed by single-strand conformation polymorphism analysis of exons 5 to 9 and were verified by direct DNA sequencing of PCR products. The TP53 gene was mutated in 6 of 23 (26%) patients with CUP. Therefore, despite the fact that CUP tumors are often aneuploid, the frequency of TP53 mutation was relatively low in this study. This finding suggests that TP53 mutations may not play a major role in the development and progression of CUP. The discrepancy between the results of these two studies may be due to discordance between the results of immunohistochemical and genetic molecular methods in detecting the TP53 abnormalities as well as the “cancer types” and tissue studied. No research is available on metastasis-suppressor genes in CUP, which seem to play an important role in regulating the growth of disseminated cancer cells at secondary sites.
Microvessel Density
Compelling evidence indicates that angiogenesis, as measured by microvessel density (MVD), correlates with the incidence of metastases in several solid tumors. Hillen and associates aimed to identify a specific biological role for angiogenesis in the metastatic phenotype of CUP by comparing MVD in liver metastasis of CUP with MVD in liver metastasis of colon and breast tumors.27 No difference was found between MVD in liver metastasis of CUP and known primary tumors. In CUP, as in other solid tumors, high MVD correlated with short survival in univariate and multivariate analyses.
Karavasilis and associates investigated angiogenesis by assessing MVD and the tissue expression of VEGF and thrombospondin-1 (TSP-1).28 VEGF is a major stimulator of angiogenesis, and TSP-1 is an intrinsic angiogenic inhibitor. Paraffin-embedded archival material was evaluated from 81 patients diagnosed with CUP. Adenocarcinoma was the predominant histology found in 77% of cases, followed by undifferentiated carcinoma in 18% of cases and squamous cell carcinoma in 5% of cases evaluated. Tissue expression of CD34, VEGF, and TSP-1 was examined immunohistochemically by using specific monoclonal antibodies and was analyzed against clinicopathological data.
Patient Evaluation
Laboratory Studies and Serum Tumor Markers
The potential role of serum tumor markers in the evaluation and management of patients with CUP has been reviewed.29,30 As part of the evaluation of patients with CUP, five markers deserve special recognition. The beta subunit of human chorionic gonadotropin (β-hCG) has been classically associated with nonseminomatous germ cell tumors, and is useful both for diagnosis and during follow-up, often confirming the adequacy of therapy.31 Alpha-fetoprotein (AFP) also is useful for the evaluation of nonseminomatous germ cell tumors as well as hepatocellular carcinoma. Although many previous publications have suggested that the presence of elevated β-hCG or AFP identified patients with marked chemotherapy responsiveness and good survival (see following section on Poorly Differentiated and Undifferentiated Carcinoma), in one study the presence of abnormal plasma levels of AFP or β-hCG did not identify patients with better overall survival.32 Currently, β-hCG and AFP are indicated in patients with a midline tumor and a concern for extragonadal germ cell cancer. AFP is also indicated in patients with liver-predominant presentations and a risk for hepatocellular carcinoma. Measurement of prostate-specific antigen (PSA) is very useful in men with adenocarcinoma and predominantly skeletal metastases. Elevation of PSA can provide a confirmation of metastatic prostate cancer, but the physician should be wary of the occasional coexistence of early prostate cancer with a more aggressive synchronous second neoplasm. Serum measurements of PSA commonly should be coupled with immunohistochemical staining for PSA in tumor tissue, because rare patients have been reported with metastatic cancer and clinical features atypical for metastatic prostate cancer.33,34
Most tumor markers, including carcinoembryonic antigen (CEA), CA-125, CA 19-9, and CA 15-3, are not specific and are thus not helpful in determining the site of the primary tumor.35,36 Similarly, in our view, cytogenetic analysis is not helpful now that immunohistochemical tests are available. In a study by Motzer and colleagues, 40 patients with poorly differentiated carcinoma and CUP underwent cytogenetic analysis. Seventeen patients (42%) were diagnosed by genetic analysis, including 12 (30%) with cytogenetic changes characteristically seen in germ cell tumors (e.g., isochromosome 12p-i [12p], increased 12p copy number, or deletion of the long arm of chromosome 12).37 Pantou and colleagues studied 20 CUP samples, and in 5 patients (4 with lymphoma and 1 with Ewing sarcoma) cytogenetics aided in the diagnosis of the primary tumor. The other samples had multiple complex cytogenetic patterns.38 Unfortunately, these studies are obsolete in the current era of sophisticated immunohistochemical markers.
Pathological Evaluation
General Considerations
Light Microscopy
The initial pathological assessment of the biopsy specimen is by light microscopic examination of paraffin sections stained with hematoxylin and eosin. Based on established cytologic criteria, in the presence of an adequate tissue sample, the pathologist usually can classify the tumor into broad groups such as carcinoma, sarcoma, or lymphoma.39 Additionally, many carcinomas will be immediately recognized as manifesting at least some glandular differentiation (adenocarcinoma). When glandular differentiation is scarce or absent, patients with CUP often are diagnosed with poorly differentiated carcinoma or undifferentiated carcinoma. Other specimens will lack any cytologic distinguishing features, in which case a diagnosis of an undifferentiated malignancy is reported. On light microscopy, about 60% of the cases are reported as adenocarcinoma and 5% as squamous carcinoma; in 35% of cases, light microscopy is not very helpful and poorly differentiated adenocarcinoma, poorly differentiated carcinoma, or poorly differentiated neoplasm is then reported.
Immunohistochemistry
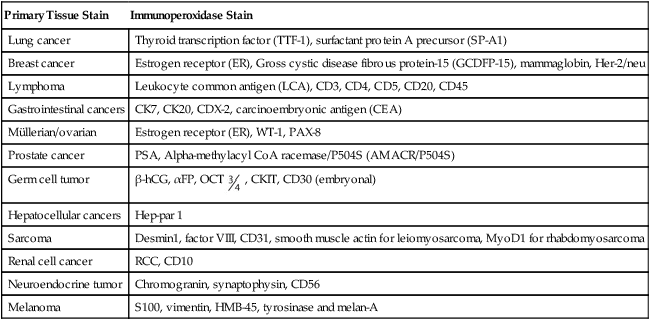
Immunohistochemical markers play a significant role in the diagnosis and workup of CUP (Table 94-1). They help define tumor lineage by using peroxidase-labeled antibody against specific tumor antigens. Direct discussions between the pathologist and clinician are critical to ensure the most accurate pathological characterization possible. Random use of large numbers of tissue markers is rarely helpful for establishing a diagnosis or planning therapy. The role of antibodies against AFP, β-hCG, PSA, and some other markers is well established. More recently, organ-specific stains are gaining more importance as markers for the identification of the origin of the carcinoma.40–46
Table 94-1
Commonly Used Immunoperoxidase Stains to Assist in the Differential Diagnosis of CUP Cancers
| Primary Tissue Stain | Immunoperoxidase Stain |
| Lung cancer | Thyroid transcription factor (TTF-1), surfactant protein A precursor (SP-A1) |
| Breast cancer | Estrogen receptor (ER), Gross cystic disease fibrous protein-15 (GCDFP-15), mammaglobin, Her-2/neu |
| Lymphoma | Leukocyte common antigen (LCA), CD3, CD4, CD5, CD20, CD45 |
| Gastrointestinal cancers | CK7, CK20, CDX-2, carcinoembryonic antigen (CEA) |
| Müllerian/ovarian | Estrogen receptor (ER), WT-1, PAX-8 |
| Prostate cancer | PSA, Alpha-methylacyl CoA racemase/P504S (AMACR/P504S) |
| Germ cell tumor | β-hCG, αFP, OCT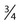 , CKIT, CD30 (embryonal) , CKIT, CD30 (embryonal) |
| Hepatocellular cancers | Hep-par 1 |
| Sarcoma | Desmin1, factor VIII, CD31, smooth muscle actin for leiomyosarcoma, MyoD1 for rhabdomyosarcoma |
| Renal cell cancer | RCC, CD10 |
| Neuroendocrine tumor | Chromogranin, synaptophysin, CD56 |
| Melanoma | S100, vimentin, HMB-45, tyrosinase and melan-A |
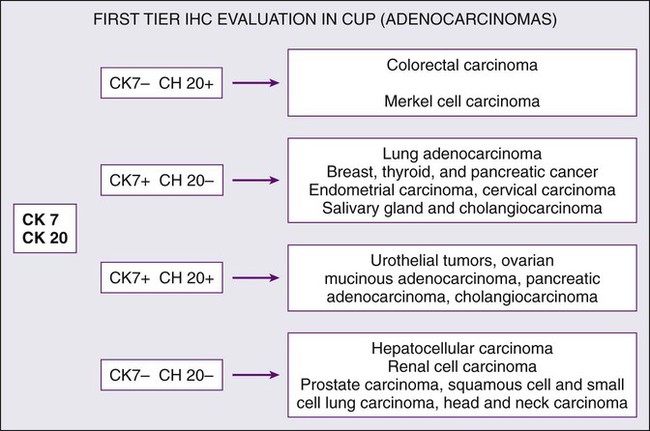
Cytokeratin 20 (CK20) is a low-molecular-weight cytokeratin, which is expressed in normal glands as well as tumors of the GI epithelium, urothelium, and Merkel cell. Cytokeratin 7 (CK7) is found in tumors of the lung, ovary, endometrium, breast, pancreaticobiliary and upper GI tract cancers. TTF-1 is a nuclear protein that plays a role in transcriptional activation during embryogenesis in the thyroid, diencephalon, and respiratory epithelium. TTF-1 staining is typically positive for lung and thyroid cancers. In 2002, Roh and associates described the use of TTF-1 and CK20 in identifying the origin of metastatic carcinomas of cervical lymph nodes.40 They stained 68 specimens with TTF-1 and CK20. The primary sites were lung (29 cases), stomach (13 cases), colorectum (3 cases), and other sites (23 cases). TTF-1 expression was detected in 69% of metastatic lung carcinomas and in none of the GI carcinomas. CK20 expression was detected in 69% of GI tumors and in none of the metastatic lung carcinomas. Jang and associates looked at the use of these markers in identifying the origin of malignant effusions.41 The primary sites of the tumors examined were lung (16 cases), ovary (15 cases), stomach (9 cases), colon (8 cases), and breast (8 cases). The lung adenocarcinomas showed TTF-1 positivity in 81% of the cases (13 of 16), but all of the nonpulmonary adenocarcinomas lacked TTF-1 staining. The CK7−/CK20+ immunophenotype was seen in 63% of colonic adenocarcinomas and in none of the lung, breast, or ovary tumors. The CK7+/CK20− staining was observed in 100% of lung, 88% of breast, and 87% of cancers that originated from the ovary. They concluded that TTF-1 immunostaining was useful in differentiating between pulmonary and nonpulmonary origin of adenocarcinoma in malignant effusions. The combination of CK7−/CK20+ staining is useful in identifying colon adenocarcinoma. In 2000, Rubin and associates looked at the role of CK7 and CK20 in determining the origin of metastatic carcinoma of unknown primary site.42 The nuclear CDX-2 transcription factor, which is the product of a homeobox gene necessary for intestinal organogenesis, is expressed in normal colonic epithelia and most colorectal adenocarcinomas, and often used to aid with the diagnosis of GI adenocarcinomas.43 Some data suggest the role of cytokeratin 5/6 as a marker for squamous cell carcinoma in poorly differentiated tumors if mesothelioma is ruled out. Based on published studies, and with the addition of markers, a simple algorithm can be described (Fig. 94-2) for providing clinicians some guidance on using these immunohistochemical markers to identify the site of origin.
Distinguishing mesothelioma from adenocarcinoma can sometimes prove to be quite challenging.47 Immunohistochemical analysis, rather than electron microscopy, is increasingly used to diagnose mesothelioma; calretinin, Wilms tumor-1, and mesothelin may be useful markers. If the morphologic characteristics are unclear, a combination of immunohistochemical markers such as MOC-31 (or Ber-EP4), estrogen receptor, calretinin, and Wilms tumor-1 are used to help distinguish mesothelioma of the peritoneum from serous papillary carcinomas.48 Expression of hepatocyte paraffin 1 antibody is found primarily in benign and malignant hepatocytes and can aid in the immunohistochemical diagnosis of hepatocellular carcinoma.49,50 GCDFP-15 is a marker of apocrine differentiation that is specifically expressed in breast carcinomas; expression is detected in 62% to 72% of cases.51 UROIII, high-molecular-weight cytokeratin, thrombomodulin, and CK20 are the markers typically used for diagnoses in cases suspected to have a urothelial origin.52,53
Overview of Imaging Studies
Mammography
Bilateral mammography should be a part of the routine evaluation of most women with CUP. Despite the relatively low numbers of occult breast cancer accounting for CUP (4% to 8%), at least one study documented a 7.5% rate of positive examinations, though that was in 1990.54 Even with the apparent low yield from this evaluation, the good response of breast cancer to local and systemic therapy justifies the procedure in this patient population if they have not had screening mammography performed in the last year before their CUP diagnosis. It is clearly indicated in women seen with isolated axillary lymphadenopathy.
Computed Tomography
Computed tomography (CT) of the abdomen and pelvis is routinely performed as part of the diagnostic evaluation of CUP to locate the primary tumor, to evaluate the extent of disease, and to select the most favorable biopsy site. In the 1980s, McMillan and colleagues retrospectively studied the role of abdominal CT in 46 CUP patients with metastatic adenocarcinoma or undifferentiated carcinoma.55 The primary tumor site was ultimately identified in 21 patients. CT was superior to sonography and contrast studies of the urinary and GI tracts. In a study by Abbruzzese and colleagues, latent primary tumors were found in 179 of 879 CUP patients (20%).56 In practice, CT of the chest, abdomen, and pelvis is routinely performed in all patients with CUP cancers; with improved imaging techniques, latent primary cancers have become a rare occurrence.
Magnetic Resonance Imaging
Currently, few prospective or retrospective studies are available to suggest a broad role for MRI in evaluating CUP. One recognized indication is in the evaluation of female patients with isolated axillary lymph node metastases and suspected occult primary breast carcinoma (after negative mammography and sonography). In a reported series of 12 female patients seen with isolated axillary lymphadenopathy pathologically confirmed to contain metastatic adenocarcinoma, 9 had a primary malignancy localized to the breast identified by MRI.57 Olson and colleagues studied 40 women with metastatic disease to the axillary nodes and no primary tumor found by mammography.58 In 28 women (70%), a primary tumor was found on MRI by using a dedicated breast coil. Five of the 12 women with negative breast MRI findings underwent modified radical mastectomy; in 4, no tumor was found in the mastectomy specimen. These studies suggest that MRI is effective at detecting breast cancer in up to 75% of women seen with axillary adenopathy. MRI of the breast can influence surgical management, and these studies suggest that negative breast MRI results are associated with a low yield at mastectomy.
Positron Emission Tomographic Imaging
Positron emission tomography with 18fluoro-2-deoxy-d-glucose (18F-FDG-PET) is a noninvasive nuclear imaging technique that has been proven to be a valuable diagnostic tool in identifying primary malignant tumors and the extent of metastatic disease; it is commonly used in patients with metastatic colorectal cancers and lymphomas. Several studies have been conducted to determine the value of FDG-PET imaging in detecting occult primary tumors after unsuccessful conventional diagnostic evaluation in patients with CUP. Most of the studies consist of a small number of evaluable patients and focus primarily on patients with cervical lymphadenopathy (mostly squamous cell pathology; Table 94-2). In 1998, Kole and associates evaluated the role of FDG-PET imaging in 29 patients with various histologic types of metastasis from CUP after unsuccessful conventional diagnostic workup.59 FDG-PET imaging identified the primary tumor in 7 patients (24%), but survival was not altered by discovery of the primary tumor. In 1999, Lassen and associates prospectively studied 20 patients who underwent an FDG-PET scan after standard evaluation, and the FDG-PET results were verified either histologically or by the clinical course of the disease.60 All the metastatic lesions were visible with FDG-PET. In 13 patients, FDG-PET suggested the site for primary tumor, and this site was verified in 9 patients (45%) either histologically or by the clinical course of the disease. Eight of these patients had primary lung cancer, and one had carcinoma of the base of the tongue. In most patients, FDG-PET had no treatment-related implications. In 2000, Jungehulsing and associates evaluated the use of FDG-PET imaging in 27 patients with head and neck lymphadenopathy and presumed CUP after unsuccessful conventional diagnostic evaluation failed to reveal the primary tumor. FDG-PET imaging revealed a primary tumor in seven patients (24%).61 In 2002 Johansen and associates evaluated 42 patients with squamous cell or undifferentiated metastatic disease from a CUP.62 Potential focal pathological uptake indicated a primary tumor in 20 of 42 cases (48%). After FDG-PET imaging, additional investigations confirmed the primary tumor in 10 patients (24%). Rusthoven and colleagues reviewed 16 FDG-PET studies published between 1994 and 2003 involving 302 patients with cervical metastases from unknown primary tumors.63 Conventional workup included either panendoscopy or CT/MRI, and in 10 of these 16 studies, both of the diagnostic techniques were performed before diagnosis. They reported the overall sensitivity, specificity, and accuracy rates of FDG-PET in detecting unknown primary tumors, to be 88.3%, 74.9%, and 78.8%, respectively. FDG-PET detected approximately 25% of tumors that were not apparent after conventional workup and was sensitive in the detection of previously undetected regional or distant metastases in 27% of patients. FDG-PET had a low specificity for tonsils, with a false-positive rate of 39% in the tonsils. False-positive rates were 21% for the base of the tongue, and 8% for the hypopharynx. Sensitivity for the base of the tongue was 81.5%, and for other sites was 90.5%.
Table 94-2
PET Scan Results in Patients with Cervical Lymphadenopathy from Squamous Carcinoma of Unknown Primary
| First Author (Year) | No. of Patients | Primary Tumor Suggested on PET (%) |
| Kole (1998)59 | 29 | 24 |
| Lassen (1999)60 | 20 | 65 |
| Jungehulsing (2000)61 | 27 | 26 |
| Johansen (2002)62 | 42 | 48 |
| Rusthoven (2004)63 | 302* | 25 |
| Ambrosini (2006)64 | 38 | 53 |
FDG-PET, Fluorodeoxyglucose-positron emission tomography.
*Sixteen FDG-PET studies published between 1994 and 2003 with cervical metastases from unknown primary tumors.63 They reported the overall sensitivity, specificity, and accuracy rates of FDG-PET in detecting unknown primary tumors to be 88.3%, 74.9%, and 78.8%, respectively.
Ambrosini and colleagues reported a 53% primary cancer detection rate in a small study of 38 patients with CUP.64 In a review of 10 FDG-PET studies (involving a total of 221 patients) published between 1998 and 2006, Seve and colleagues found that in 41% of patients, FDG-PET detected primary tumors that were not apparent after conventional workup.65 Most of these patients (94%) were seen with a solitary site of metastasis. In this group of patients, the overall sensitivity, specificity, and accuracy rates of FDG-PET in detecting unknown primary tumors were 91.9%, 81.9%, and 80.5%, respectively. FDG-PET imaging also led to the detection of previously unrecognized metastases in 37% of patients. Lung cancers represented 59% of the detected tumors. FDG-PET had a notably high false-positive rate (58.3%) in tumors of the lower digestive tract, and altered clinical management in 34.7% of patients. A large number of patients in these studies had a single site of metastasis, which may have influenced the study results. Outside of cervical adenopathy with squamous cell cancer, the role of PET scans, especially PET-CT, is evolving, although given that these studies have been small, it is difficult to make firm recommendations.
Larger studies to evaluate the use and cost-effectiveness of PET in the CUP setting (other than for cervical nodes) are warranted, though a challenge. There are clearly some practical applications in which PET-CT scans are useful, and these have not been prospectively studied: first, in the evaluation of the patients presenting with solitary metastatic disease who are candidates for locoregional therapy (including surgery). On occasion, these patients do benefit from a PET scan that may provide a more thorough evaluation before locoregional treatment is applied. Second, patients with osseous predominant CUP require either multiple MRIs or bone scans to evaluate response to therapy. PET-CT scans may suffice as a single study and may be the best modality to image (bony) responses to therapy. PET-CT is also an adequate alternative in patients with severe iodine dye allergy.66
Overview of Tissue-of-Origin Gene Profiling Studies
Ramaswamy and associates subjected 218 tumor tissues spanning 14 common tumor types (80% of all cancers) and 90 normal tissue samples to oligonucleotide microarray gene expression analysis.67 They used the relative levels of expression of 16,063 genes and expressed sequence tags to evolve a predictive support vector machine (SVM) algorithm. The algorithm then was tested on an independent group of 54 tumors, yielding an overall prediction accuracy of 78%. Although greatest accuracy required the use of all 16,063 genes, accuracy was still above 70% with fewer than 50 genes. Of the 54 independent tumors tested, 8 were metastatic tumors, of which 6 were accurately identified, suggesting that the cancers retain the markers of their ToO throughout metastatic evolution, and that gene expression–based approaches to the diagnosis of CUP may be feasible. Most of the tumor types that could not be classified accurately were moderately or poorly differentiated (high-grade) carcinomas. The authors suggest that poorly differentiated tumors may not simply lack a few key markers of differentiation, but rather may have fundamentally distinct gene expression patterns, with a significant implication for the management of patients with these cancers.
Su and associates used a set of 100 primary carcinomas from 10 common tumor types (prostate, breast, lung, ovary, colorectum, kidney, liver, pancreas, bladder/ureter, and gastroesophagus), which collectively account for 70% of all cancer-related deaths in the United States.68 They extracted mRNA from the tumors, and then used an Affymetrix™ oligonucleotide microarray to identify genes that were differentially expressed. A predictive algorithm was developed by using 110 genes of the 9198 genes that were minimally expressed in these tumors. The algorithm was then tested against an additional 75 blinded samples and accurately predicted the tumor of origin in more than 90% of the cases.
Dennis and associates used a different approach to identify predictive markers, starting with published data on differential gene expression by tumor type.69 They identified 61 candidate tumor markers the expression pattern of which was predicted to be characteristic of the site of origin and tested 11 of them against adenocarcinoma samples (breast, ovary, stomach, pancreas, and lung). The actual expression patterns were consistent with those predicted in seven cases (64%), with three agreeing exactly. By extending this approach, it may be possible to identify a smaller subset (10 to 20 genes) of highly predictive markers that could be applicable to more commonly used laboratory techniques such as immunohistochemistry.
Genomic technologies that provide large-scale gene expression profiles based on mRNA or microRNA are currently in commercial application. The data on known metastases has been validated by using independent blinded sets of tumor samples, in which the reference diagnosis is known, with an accuracy of 80% to 90%.70–74 These tests are feasible on small-core biopsy formalin-fixed and paraffin-embedded (FFPE) tissue, which makes this a practical approach for clinic use.
CUP Studies and Molecular Profiling
The MD Anderson Cancer Center, in collaboration with our colleagues at Sarah Cannon Cancer Center, reported the first large CUP series that evaluated the feasibility of a 10-gene RT-PCR assay to identify the ToO in CUP patients.75 Diagnostic FFPE specimens from 120 patients with CUP were studied and ToO assignments rendered by the assay were correlated with clinical and pathological features, and with response to therapy. The assay was successfully performed in 104 patients (87%), and a ToO was assigned in 63 patients (61%). In the remaining 41 patients (39%), the molecular profiles were not specific for the 6 tumor types detectable by this assay. The ToO most commonly identified were lung, pancreas, and colon; most of these patients had clinical and pathological features consistent with these diagnoses.
Monzon and colleagues described a multicenter validation of a 1,550-gene expression profile for identification of tumor ToO.76 Four institutions processed 547 frozen specimens (metastatic, poorly differentiated, undifferentiated primary cancers) representing 15 tissues of origin using oligonucleotide microarrays. The study found overall sensitivity of 88% and overall specificity of 99%. Performance within the subgroup of metastatic cancers was found to be slightly lower than that of the poorly differentiated and undifferentiated primary tumor subgroup. This study did not include patients with CUP cancers.
Two groups have evaluated the role of microRNA expression in ToO profiling. The first study by Ferracin and colleagues evaluated microRNA profiling in 101 FFPE samples from primary cancers and metastases by using a microarray platform.77 Forty samples representing 10 cancer types were used for defining a cancer-type-specific microRNA signature, which was then used for predicting the primary sites of metastatic cancers. A 47-miRNA signature was identified and used to estimate ToO probabilities for each sample. Overall, accuracy reached 100% for primary cancers and 78% for metastases. This signature was applied to an independent, published data set of 170 samples, and prediction was established in 86% of the metastasis cases. This signature was also applied to predict 16 CUP samples; accuracy of the signature was maintained. A second study from MD Anderson exclusively examined CUP patients; FFPE metastatic tissue from 104 CUP patients was reviewed and 87 of these samples contained sufficient tumor for testing.78 The assay quantitated 48 microRNAs and assigned one of 25 tumor diagnoses by using a biologically motivated binary decision tree. The assay predictions were compared with clinicopathological features and, where suitable, to therapeutic response. The assay result was consistent or compatible with the clinicopathological features in 84% of cases processed successfully. The authors concluded that microRNA profiling may be particularly helpful in patients in whom the IHC profile of the metastasis is nondiagnostic or leaves a large differential diagnosis.
Greco and colleagues presented a retrospective study which compared molecular profile results with the latent primary cancer diagnosed over the course of patient’s life/treatment.79 Thirty-eight of 501 patients (7.6%) with CUP (data from 2000 to 2008) had their latent primary site tumor subsequently identified during life. Twenty-eight of these patients (74%) had adequate initial tissue biopsies available for molecular profiling with a RT-PCR assay. The assay was performed on formalin-fixed paraffin-embedded biopsy specimens in a blinded fashion, and the assay results were compared with clinicopathological data and the actual latent primary sites. Twenty of the 28 (71.4%) RT-PCR assays were successfully completed. Fifteen of the 20 assay predictions (75%) were correct corresponding to the actual latent primary sites identified after the initial diagnosis of CUP.
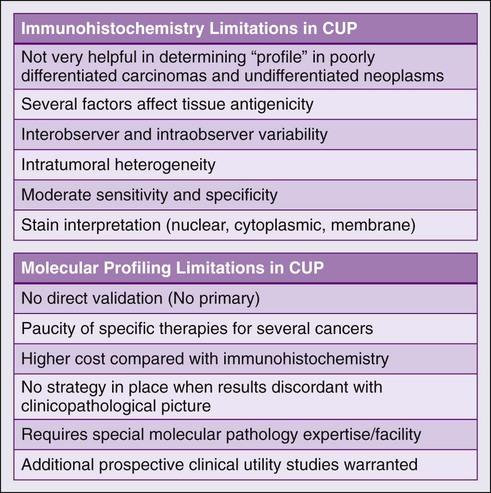
There are several challenges in the field of molecular profiling and CUP (Fig. 94-3) First, because there is no primary cancer (i.e., no direct validation), there is currently no way to resolve a significant discordance between immunohistochemistry and profiling results, especially if the results contradict each other. Second, although useful in some subtypes, the current use of the profiling approach is limited by the paucity of effective drugs for several CUP cancer profiles. As novel therapies are developed for additional known cancers, these treatment programs will significantly affect the appropriate CUP subtypes. Finally, there clearly is a role for ToO gene profiling in patients with inadequate tumor tissue in whom exhaustive immunohistochemistry, if applied, would deplete available tissue resources. Additional studies comparing immunohistochemistry with molecular profiling especially in patients with poorly differentiated cancers will help define the management of these patients.
Treatment Decisions and Emerging Subsets
General Considerations
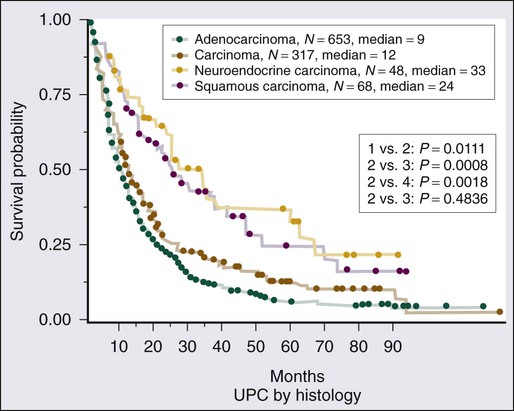
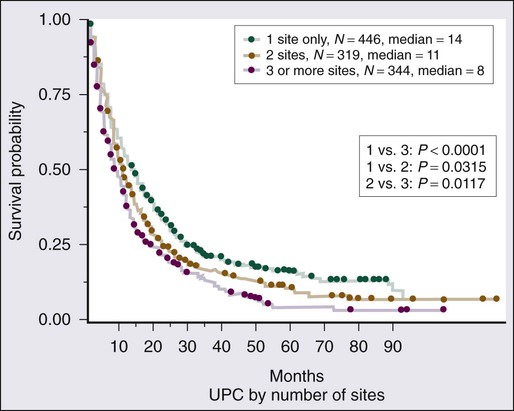
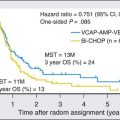
Given the heterogeneity of the CUP cancers, it is difficult to interpret clinical trials and definite benefit of any particular combination in CUP cancers. Lacking are multiinstitutional trials for specific subsets as well as incorporation of emerging targeted therapies in the management of CUP. Median survival for the majority of patients with disseminated CUP is about 6 to 10 months. Although systemic chemotherapy is the main treatment modality in most patients, the careful integration of surgery, radiation therapy, and even periods of observation are important in the overall management of these patients. Prognostic factors include performance status, sites and number of metastases, response to chemotherapy, and serum lactate dehydrogenase (LDH) level. Culine and colleagues recently developed and retrospectively validated a prognostic model using performance status and serum LDH levels that allows the assignment of patients into two subgroups with divergent outcomes.80 Further prospective trials with this prognostic model are warranted. Clinically, some CUP diagnoses fall in favorable prognostic subsets and are discussed below. Others including those with disseminated CUP have a more unfavorable prognosis (Figs. 94-4 and 94-5).
Favorable Clinical Subsets
Squamous Carcinoma Involving Mid-High Cervical Lymph Nodes
High cervical adenopathy with squamous cell carcinoma has been mentioned previously because of its well-defined natural history, high frequency of identification of the primary site, and responsiveness to therapy. With appropriate evaluation, including direct visualization of the hypopharynx, nasopharynx, larynx, and upper esophagus, as well as use of PET-CT scan, an occult primary lesion often will be identified. When no primary site is found, aggressive local therapy is applied to the involved neck.81 Five-year survival rates of 30% to 50% have been reported with radical neck surgery, high-dose radiotherapy, or a combination of both modalities. A potential advantage of radiation therapy is that the suspected primary anatomic sites (nasopharynx, oropharynx, and hypopharynx) can be included in the radiation port. The role of chemotherapy in these patients is unclear. However, one randomized study in 1989 suggested that chemotherapy with cisplatin and 5-fluorouracil improved the response rate and median survival when compared with radiation alone.82
Women With Isolated Axillary Adenopathy
Isolated axillary adenopathy secondary to metastatic adenocarcinoma usually occurs in women and has unique clinical features. In this setting, adequate immunohistochemistry to evaluate for a breast cancer profile is essential. Management is based on the treatment of stage II breast cancer and should include both local and systemic therapies. Prognosis following treatment is comparable to women with stage II breast cancer. However, a reported series of 42 patients suggested that survival was superior in patients receiving systemic chemotherapy, and local control was improved by irradiating the breast and axilla. The actuarial disease-free survival rate in this study was 71% at 5 years and 65% at 10 years. This nonoperative approach has been outlined in a study by Lenzi and colleagues.83
Women With Serous Papillary Peritoneal Carcinomatosis
Women with diffuse (serous) peritoneal carcinomatosis with adenocarcinoma make up another recognized subset. These patients form a distinctive subset because of their clinical similarities to patients with ovarian carcinoma. Often papillary histologic type and elevations in CA125 are found, but exploratory laparotomy fails to document a primary.84 Numerous authors have recognized this patient subset, terming this syndrome peritoneal papillary serous carcinoma or multifocal extraovarian serous carcinoma. These patients often respond to platinum-based chemotherapy. In one study, many patients underwent exploratory laparotomy with surgical debulking followed by chemotherapy. Median survival times are reported to be 16 months to 2 years.85
Poorly Differentiated and Undifferentiated Carcinoma (Extragonadal Germ Cell Cancers)
Approximately one third of patients with CUP will be identified with the histologic picture of poorly differentiated or undifferentiated carcinoma. In this subset, detailed histochemical or immunohistochemical studies are most likely to identify highly treatment-responsive patients with lymphoma (leukocyte common antigen), germ cell (β-hCG, AFP), or neuroendocrine (neuron-specific enolase, chromogranin) neoplasms. Additionally, Greco and Hainsworth identified a group of patients with PDC or PDA who are responsive to platinum-based chemotherapy. Most of these patients had clinical features (e.g., young age, mediastinal/retroperitoneal involvement, and rapid growth) of the extragonadal germ cell syndrome. Many of these patients were male with elevated β-hCG or AFP. Combination chemotherapy regimens specific for germ cell carcinoma of testicular origin were used in the treatment of these patients. In selected patients, these regimens have produced documented complete responses and an actual 10-year disease-free survival rate of 16%.86
Poorly Differentiated Neuroendocrine Carcinoma
Poorly differentiated neuroendocrine carcinoma is a clinicopathological entity recognized primarily for its responsiveness to therapy. There probably is considerable overlap with extrapulmonary small cell carcinomas, anaplastic carcinoid, anaplastic islet cell tumors, Merkel cell tumors, and paragangliomas. Histologically these tumors are very poorly differentiated, but histochemical stains are positive for chromogranin or neuron-specific enolase. These patients often are seen with diffuse hepatic or bone metastases but do not have the indolent histologic or clinical features of typical carcinoid tumors, islet cell tumors, or paragangliomas, and thus observation is not appropriate. These tumors also may be responsive to cisplatin-based chemotherapy.87,88
Colon Cancer Profile Unknown Primary
We have described a subset of the colon cancer profile unknown primary (CCP-CUP) profile based on immunohistochemical markers (CK20+ or CDX-2+ and CK7−) and observed responses to colon cancer chemotherapy regimens.89 In a recently completed study that used RT-PCR profiling in 120 CUP patients, ToO assignments rendered by the assay were correlated with clinical and pathological features, and, retrospectively, with response to therapy. Patients with CCP-CUP ToO profiles showed concordance with their immunohistochemistry profile (i.e., CK20+, CDX-2+, and CK7−). CCP-CUP patients demonstrated better responses to colon cancer–specific therapies than to empiric CUP therapy with taxane/platinum regimens. Patients who are seen with CCP-CUP may have life expectancies that parallel metastatic colon cancer (20 to 24 months), and this profile is a good fit for a “favorable” CUP subset. Interestingly, it is based on immunohistochemistry and profiling compared with the current group of favorable CUP cancers that are defined based on anatomic and histopathological patterns.
Carcinoma of Unknown Primary in Unselected Patients
The encouraging results for the patients with a favorable prognosis described earlier do not apply to the vast majority of patients with disseminated CUP. Interpretation of response and survival data in unselected CUP patients is challenging. Two thirds of patients with CUP have metastatic adenocarcinoma with involvement of two or more visceral sites, usually some combination of liver, lung, lymph nodes, peritoneum, or bone. In addition, many men and women with poorly differentiated carcinoma have none of the clinical features outlined previously and respond poorly to therapy.49 Even in series showing optimistic results for selected patients with poorly differentiated carcinoma or poorly differentiated adenocarcinoma, the overall median survival time remains poor, at 12 months.
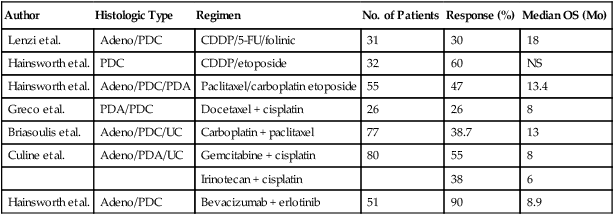
For unselected patients, numerous empiric chemotherapy combinations have been reported (Table 94-3). Traditionally, cisplatin-based combination chemotherapy regimens have been used to treat patients with CUP. Response rates generally range from 20% to 30%, but most responses are partial and brief, resulting in little impact on median survival. Taxane-based regimens evaluated in phase II clinical trials have included the following combinations: (1) paclitaxel/carboplatin/etoposide; (2) paclitaxel/carboplatin; (3) docetaxel/platinum; and (4) paclitaxel/carboplatin/gemcitabine.92–92 Overall response rates with taxane-based regimens have ranged between 24% and 47%. Longer median survival times also have been observed with taxane-based regimens, ranging from 9 to 11 months, compared with a range of 5 to 8 months with older GI and breast cancer chemotherapy regimens. One report that used carboplatin, paclitaxel, and etoposide reported that 25 of 53 patients (47%) had objective responses.93 In this series, seven patients (13%) experienced complete responses. However, the actuarial median survival time for the entire group was 13.4 months. The disappointing aspect of this survival statistic is that it is not substantially different from the 11-month median survival time reported in large consecutive series of patients with CUP.
Table 94-3
Select Chemotherapeutic Trials in Carcinoma of Unknown Primary
| Author | Histologic Type | Regimen | No. of Patients | Response (%) | Median OS (Mo) |
| Lenzi et al. | Adeno/PDC | CDDP/5-FU/folinic | 31 | 30 | 18 |
| Hainsworth et al. | PDC | CDDP/etoposide | 32 | 60 | NS |
| Hainsworth et al. | Adeno/PDC/PDA | Paclitaxel/carboplatin etoposide | 55 | 47 | 13.4 |
| Greco et al. | PDA/PDC | Docetaxel + cisplatin | 26 | 26 | 8 |
| Briasoulis et al. | Adeno/PDC/UC | Carboplatin + paclitaxel | 77 | 38.7 | 13 |
| Culine et al. | Adeno/PDA/UC | Gemcitabine + cisplatin | 80 | 55 | 8 |
| Irinotecan + cisplatin | 38 | 6 | |||
| Hainsworth et al. | Adeno/PDC | Bevacizumab + erlotinib | 51 | 90 | 8.9 |
Gemcitabine is a commonly used first-line drug in CUP cancers. Hainsworth and associates conducted a phase II trial evaluating single-agent gemcitabine in the second-line therapy of patients with CUP.94 Thirty-five patients (90%) had previously received treatment with chemotherapy containing both a platinum agent and a taxane. This study showed an 8% partial response rate (3 of 36 evaluable patients), and 25% (9 patients) had minor responses or stable disease with reduced symptoms. The median time to progression was 5 months. Greco and associates evaluated the efficacy and toxicity of gemcitabine, carboplatin, and paclitaxel in previously untreated patients with CUP.95 Twenty-eight (25%) of 113 assessable patients had a major objective response. The median progression-free survival time was 6 months, with a median survival time for the entire group of 9 months. Actuarial survival at 1 and 2 years was 42% and 23%, respectively. This study showed that combination chemotherapy with gemcitabine, carboplatin, and paclitaxel followed by weekly paclitaxel was well tolerated.
Culine and associates evaluated the efficacy and toxicity of combination chemotherapy consisting of gemcitabine with cisplatin (GC) or irinotecan with cisplatin (IC) in patients with CUP in a phase II clinical trial.96 Eighty patients were assigned to receive either GC or IC. Seventy-eight patients were assessable for efficacy and toxicity. The median number of cycles was four in each arm of the study. Objective responses were observed in 55% of patients in the GC arm and 38% of patients in the IC arm. Treatment had to be discontinued in seven patients in the GC arm and eight patients in the IC arm. Median survival times were 8 and 6 months in the GC and IC arms, respectively, with a median follow-up of 22 months.
Hainsworth and associates have evaluated the efficacy of combined targeted therapy with bevacizumab and erlotinib in 51 patients with CUP.97 Of these, 25% of patients were chemotherapy-naïve, with advanced bone or liver metastasis, and 75% of patients had received prior therapy with one or two chemotherapy regimens. Objective response occurred in 4 patients (8%), and 30 patients (59%) had stable disease or minor response. The median overall survival was 8.9 months, with a median follow-up of 13 months. Median progression-free survival for the entire group was 6.2 months. Forty-two percent of patients were alive at 1 year. The 1-year survival rate for the previously treated versus untreated patients was 41% versus 46%.