Cancer of the Stomach and Gastroesophageal Junction
Leonard L. Gunderson, John H. Donohue, Steven R. Alberts, Jonathan B. Ashman and Dawn E. Jaroszewski
• For stomach cancer in the United States, the expected incidence in 2012 was 21,320 cases and 10,540 deaths.
• These cancers are usually adenocarcinomas.
• In the United States, the site of origin is shifting as more proximal lesions are diagnosed.
• Prognostic factors relate to tumor extent and include nodal involvement and extension beyond the gastric wall.
• Ploidy may be an independent prognostic factor.
• Staging should always include history and physical examination, complete blood cell count, liver chemistries, chest x-ray film, endoscopy with biopsy, ultrasound (determine degree of direct tumor extensions), and computed tomography (CT) of the abdomen (define extragastric disease).
• Additional studies that may help define extent of disease include upper gastrointestinal imaging, CT of the chest (for gastroesophageal junction [GEJ] lesions), laparoscopy (to rule out peritoneal seeding or early liver metastases), and positron emission tomography.
• Surgical resection is the primary therapy of resectable gastric and GEJ cancers.
• Cure rates of 80% or higher are achieved only with early lesions (patients with nodes negative, confined to mucosa or submucosa), which are uncommon in the United States.
• Role for extended node dissection has not been found in randomized trials.
• Adjuvant therapy (chemotherapy, irradiation) is indicated on the basis of patterns of relapse and survival results with surgery alone (high rates of local-regional relapse and distant metastases).
• Adjuvant chemotherapy has a modest, significant benefit and has become the standard in Asia.
• Irradiation alone reduced local-regional relapse and improved overall survival (OS) in a Beijing trial of 370 patients testing preoperative irradiation versus surgery alone (5-year OS 30% vs. 20%, P = 0.009).
• The U.S. intergroup phase III trial of 556 patients found a survival benefit for combined-modality postoperative irradiation plus chemotherapy versus surgery alone (3-year relapse-free survival 48% vs. 31%, P = 0.001; 3-year OS 50% vs. 41%, P = 0.005).
• A British phase III trial of 503 patients demonstrated a survival advantage for perioperative ECF chemotherapy (epirubicin, cisplatin, 5-fluorouracil [5-FU]) when compared with surgery alone (5-year OS 36% vs. 23%, P = 0.009).
• A French phase III trial of 224 patients demonstrated a survival advantage for perioperative cisplatin and 5-FU compared with surgery alone (5-year OS 38% vs. 24%, P = 0.02).
• The POET trial of 120 patients with GEJ lesions tested preoperative chemotherapy versus chemoradiotherapy (CRT); outcomes trends favored preoperative CRT over chemotherapy alone for both OS (P = 0.07) and local control (P = 0.06).
Locally Advanced Disease (Borderline Resectable/Unresectable)
• Combined external beam radiation therapy (EBRT) plus chemotherapy or intraoperative radiation therapy (IORT) produced long-term survival in 10% to 20% of patients in most randomized and nonrandomized trials.
• Neoadjuvant chemotherapy studies reveal possible increase in resection rates but high incidence of local-regional relapse (consider addition of IORT alone or with EBRT and concurrent chemotherapy to neoadjuvant chemotherapy regimens).
• Palliative resection of gastric component of disease may be indicated.
• European phase III trials demonstrate a trend toward improved quality and duration of life with palliative chemotherapy versus supportive care.
Treatment of Metastatic Disease
• Multiple-drug chemotherapy regimens have response rates of 30% to 50%, and provide some improvement in OS, including the two- and three-drug regimens ECF, EOX (epirubicin, oxaliplatin, capecitabine), and DCF (docetaxel, cisplatin, 5-FU).
• A phase III trial of 594 patients showed a significant improvement in OS with the addition of trastuzumab to chemotherapy in patients with HER-2-positive tumors (median OS 13.8 vs. 11.1 months, P = 0.0046)
Epidemiology
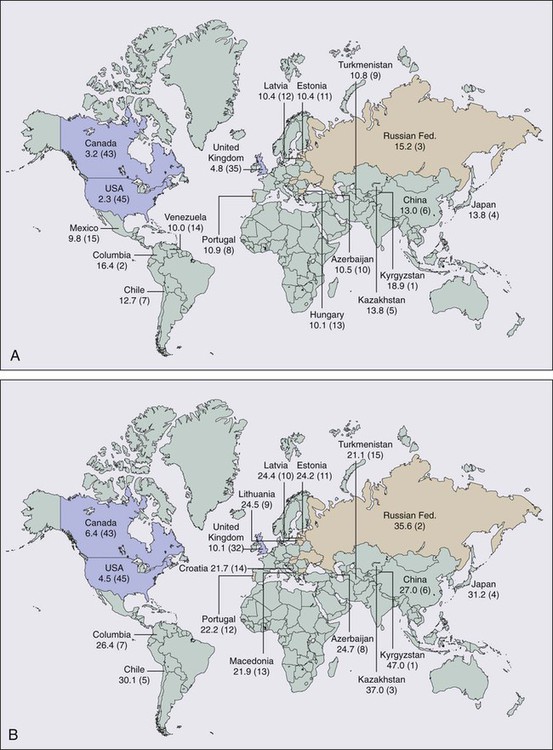
In 2012, cancer of the stomach had an expected incidence in the United States of 21,320 cases and an expected number of 10,540 deaths.1 Age-adjusted gastric cancer death rates have decreased markedly in the United States since 1930 from approximately 28 to 2.3 in 100,000 women and from 38 to 5.2 in 100,000 men. Of the 45 countries in which age-adjusted death rates for gastric cancer were compared for 2000 (E-Fig. 75-1), the United States ranked 45th for both men and women.2 Kyrgyzstan ranked first for both men (47.0 in 100,000) and women (18.9 in 100,000).
Etiology and Biological Characteristics
Etiology
Factors that have been associated with a higher incidence of gastric cancer include smoked or salted foods, foods contaminated with aflatoxin, low intake of fruits and vegetables, low socioeconomic status, and possibly a decreased use of refrigeration.3,4 Possible occupational relationships include coal mining and rubber or asbestos workers. Precursor pathological conditions include pernicious anemia, achlorhydria atrophic gastritis, gastric ulcers, and adenomatous polyps. Between 5% and 10% of individuals with pernicious anemia subsequently develop malignancy. Prior partial gastrectomy for benign gastric or duodenal ulcer disease produces an increased risk of subsequent malignancy in the gastric remnant with latency periods of 20 years or more.5,6
Several studies have shown a threefold to sixfold increased risk of gastric cancer in individuals with Helicobacter pylori infection versus those with no infection, but the precise role of this bacterium in the etiology of gastric cancer remains unknown.9–9 A variety of bacterial, patient, and environment factors most likely act in combination to affect the development of gastric carcinoma. The increased association of H. pylori with gastric cancer seems to be mainly with distal gastric cancers and intestinal-type malignancy. Only a minority of H. pylori–infected individuals develop gastric cancer, and data do not yet exist on the effect of treatment of the H. pylori infection on subsequent malignancy.
Biological Characteristics
Prognostic Factors
The most meaningful prognostic indicators relate to extent of tumor. With either hematogenous metastasis or peritoneal seeding, prognosis is almost always fatal. Recent immunohistochemical analysis of bone marrow aspirates has shown the presence of tumor cells to be an independent predictor of adverse outcome; however, confirmatory studies have yet to be published.10,11 Survival decreases with progressive direct tumor extension both within and beyond the gastric wall.12,13 Lymph node involvement, per se, is not as important as the number and location of nodes.16–16 Minimal lymph node involvement adjacent to the primary lesion results in the most favorable prognosis in node-positive patients, but even micrometastases in regional nodes may adversely impact survival.17 The solitary finding of either involved nodes or complete penetration of the gastric wall is usually not as ominous as the presence of both12,15 (E-Table 75-1).
E-Table 75-1
Extent of Initial Disease Versus Survival Rates in Stomach Cancer*
| Extent of Disease | 5-y OS (%) | >5-y DFS University of Minnesota Reoperation Series13 | |
| Dockerty12† | Kennedy14 | ||
| LYMPH NODES – | |||
| Mucosa only | 100 | 85 | — |
| >Mucosa but within wall | 61 | 52 | — |
| Through wall | 44 | 47 | — |
| LYMPH NODES + | |||
| Lymph node extent | 15 | — | 19 |
| Regional only | — | 17 | — |
| Nonregional | — | 5 | — |
| Extent of primary | |||
| Within wall | — | — | 40 |
| Through wall | — | — | 12 |
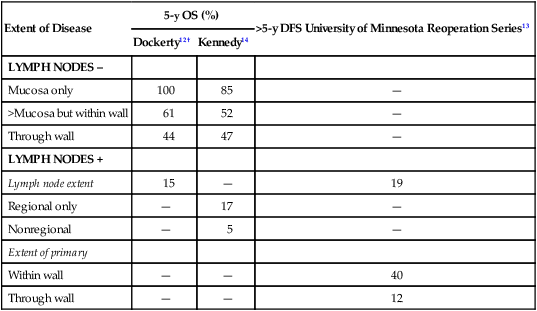
OS, Overall survival; DFS, disease-free survival.
The tumor grade and the gross and histologic pathological appearance of the primary malignancy seem to provide some prognostic information, but none of these factors is a prognostic variable independent of the tumor stage. Prognosis is generally worse with higher grade and diffuse-type carcinomas, which usually present with higher pathological stages of disease (see Fig. 75-1). Borrmann types I and II carcinomas have a relatively favorable 5-year survival rate, but patients with type IV tumors (linitis plastica) fare very poorly.18,19
Some investigators have suggested that tumors of the gastric cardia may have epidemiological factors different from cancers of the distal stomach20,21 and may exhibit different tumor biology.22 The prognosis is worse for cardia lesions,23,24 and flow cytometry reveals a greater incidence of aneuploidy when compared with tumors of the antrum and body.25
Flow cytometry provides valuable prognostic information for gastric cancer and may be an independent prognostic factor.25,26 As noted previously, aneuploidy is associated with unfavorable tumor location such as the cardia25,27 but is also associated with lymph node metastasis26,27 and direct tumor extension.26 Unfavorable DNA flow cytometry characteristics seem to relate closely to an unfavorable prognosis.25,26 In one series in which multivariate analysis of DNA ploidy was analyzed with other known prognostic factors such as stage, age, and sex, DNA ploidy carried statistically significant independent prognostic information.26
The presence of several peptides including estrogen receptor,28 epidermal growth factor receptor,29 the c-erbb2 protein,30 and plasminogen activator inhibitor type 119 seems to affect prognosis adversely. The expression of epidermal growth factor receptor and high levels of epidermal growth factor correlate with a higher incidence of primary tumor infiltration, poor histologic differentiation, and linitis plastica. The pathophysiological relationship between these peptide receptors and poor patient prognosis is not clear. Gastric cancers with class II major histocompatibility complex antigen expression (human leukocyte antigen [HLA]-DR) have a better prognosis, but the loss of expression is not an independent prognostic factor.31
Molecular Biology
Loss of tumor suppressor gene function, especially inactivation of the p53 gene, plays a key role in tumor suppression and cell-cycle regulation.32 The p53 gene puts a brake on DNA replication and triggers programmed cell death in response to DNA damage.33 Loss of p53 function is associated with the development of gastric cancer, impacts the effectiveness of chemotherapy and irradiation,34,35 and predisposes cells to genetic instability.
A second aberration affecting gastric epithelial cells is alterations in mismatch repair genes, including HMSH3 and HMLH1, which account for replication errors throughout the genome. Mutations in these genes generate genetic instability and are associated with an increased tendency for the development of colorectal and gastric tumors.36,37
Two protooncogenes, c-met and K–sam, are associated with scirrhous carcinoma of the stomach. Overexpression of c-met correlates with tumor progression and metastasis, and K-sam encodes a tyrosine kinase receptor family.38 K–sam has a tendency to be activated in women with gastric cancer younger than 40 years of age and c-met to be amplified in men older than 50 years of age.39,40
Modern molecular biology observations confirm the heterogeneity of human gastric cancer.41 Gastric cancers with class II major histocompatibility complex antigen expression (HLA-DR) have a better prognosis, but the loss of expression is not an independent prognostic factor.42
Prevention and Early Detection
Early detection would markedly improve the prognosis of gastric cancer in the United States, because surgical resection has a high cure rate with lesions limited to the mucosa or submucosa. However, the incidence of such early gastric cancers is less than 5% in most U.S. series. In Japan, the incidence of carcinomas confined to the mucosa or submucosa was only 3.8% in the 1955 to 1956 period. However, by 1966 the incidence of early lesions had increased to 34.5% because of vigorous screening procedures, leading to 5-year survival rates of 90.9% in this cohort of patients.43 Although mass screening has been useful in Japan to detect early cancers, defined high-risk populations have not existed in the United States in the past to justify the expense of widespread screening endeavors. Whether screening of individuals with H. pylori infection would be of value is not yet known. Individual practitioners should use upper gastrointestinal (GI) series or preferably endoscopy to screen patients who have occupational or precursor risk factors or individuals with persistent dyspepsia or gastroesophageal symptoms.
Germline mutations in the CDH1 gene, which encodes the E-cadherin protein, have recently been recognized in families with hereditary diffuse gastric adenocarcinoma. Carriers of these mutations have a 70% lifetime risk of developing gastric cancer. Several reports of prophylactic gastrectomy have demonstrated the frequent presence of microscopic intraepithelial carcinomas in patients having regular endoscopic surveillance that includes multiple random biopsies.46–46 Early total gastrectomy has been recommended for this small patient population because of the lack of effective early tumor detection by less aggressive techniques. Microscopic evaluation of the proximal and distal resection margins for complete removal of the gastric mucosa is necessary, because residual gastric mucosa can degenerate and result in a gastric cancer.46
Pathology and Pathways of Spread
The terms gastric cancer and stomach cancer usually refer to adenocarcinoma, which accounts for 90% to 95% of all gastric malignancies. Other histologic types include lymphoma (usually intermediate- or high-grade histologic types), leiomyosarcoma, carcinoid, adenoacanthoma, and squamous cell carcinomas. The site of origin within the stomach has changed in frequency in the United States over recent decades, with more proximal lesions now being diagnosed and treated. The largest percentage of gastric cancers still arises within the antrum or distal stomach (around 40%), are least common in the body of the stomach (around 25%), and are of intermediate frequency in the fundus and esophagogastric junction (around 35%).47
Gastric carcinomas have been categorized by using both microscopic (Fig. 75-1) and gross pathological features. The Lauren classification system includes an intestinal type with improved prognosis that predominates in regions with high prevalence of gastric cancer, as well as a diffuse histologic type, with poor prognosis, which occurs more commonly in countries with low prevalence of stomach cancer.48 Grossly, gastric cancers can be categorized according to Borrmann’s49 five types: I, polypoid or fungating; II, ulcerating lesions surrounded by elevated borders; III, ulceration with invasion of the gastric wall; IV, diffusely infiltrating (linitis plastica); and V, unclassifiable. The Japanese Research Society for Gastric Cancer has a classification system that divides lesions into protruded (I); superficial (II) with elevated (IIa), flat (IIb), and depressed (IIc) subtypes; and excavated (III) types.50
Pathways of Tumor Spread
Direct Extension
Lymphatics
Because of the numerous pathways of lymphatic drainage from the stomach, it is difficult to perform a complete nodal dissection (E-Fig. 75-2). Although initial drainage is usually to lymph nodes along the lesser and greater curvatures (perigastric or N1 nodes using the Japanese Research Society for Gastric Cancer designation), primary node drainage includes nodes along all three branches of the celiac axis (common hepatic, splenic, left gastric) and the celiac artery itself (Japanese N2 nodes).50 Node groups that are more distal include hepatoduodenal, peripancreatic, root of mesentery (N3), periaortic, and middle colic (N4). When proximal gastric lesions extend into the distal esophagus, the paraesophageal nodal system is at risk for involvement.
Clinical Manifestations, Patient Evaluation, Staging
Evaluation of the Patient
The diagnosis of gastric cancer is usually confirmed by upper GI endoscopy, or radiographs (see diagnostic algorithm in Table 75-1). Double-contrast radiographs may reveal small lesions limited to the superficial (inner) layers of the gastric wall. Endoscopy is now the preferred initial diagnostic test, because it allows direct tumor visualization, cytologic testing, and direct biopsy for histology that yield the diagnosis in 90% or more of patients with exophytic lesions. Ulcerated cancers and linitis plastica lesions may be harder to diagnose endoscopically, but multiple biopsies and gastric washings for cytology enhance the probability of accurate diagnosis. Endoscopic ultrasound (EUS) has a high degree of accuracy in determining depth of tumor invasion (i.e., does the lesion extend beyond the muscularis propria?) but is less accurate in detecting regional nodal metastasis.53–53 Ultrasound-guided fine-needle aspiration for cytologic test allows the assessment of regional lymph nodes and some distant metastatic sites (e.g., liver), further enhancing the ability of EUS to determine tumor stage and resectability.
Table 75-1
Diagnostic Algorithm—Gastric/Gastroesophageal Junction Cancer
| Diagnostic Procedure | Diagnosis and Staging Capability | Recommend Routine Use |
| PRIMARY TUMOR/REGIONAL NODES* | ||
| Single-contrast upper GI (UGI) | Useful in detecting and defining primary lesions in stomach | Optional—consider along with double-contrast UGI study |
| Gastroscopy | Very accurate modality to detect and define primary lesion, ≈90% confirmation rate | Yes; use to confirm lesion detected in UGI series and to screen high-risk patients |
| Ultrasound-endoscopy | Most accurate method of determining extension within and beyond gastric wall | Yes; biopsy node(s), if feasible |
| Double-contrast UGI | Useful in detecting early gastric cancers | Consider along with single-contrast |
| CT—abdomen + chest | Most valuable modality to determine degree of extragastric extension and distant metastases | Yes; include CT chest for GE junction cancers |
| METASTATIC TUMORS | ||
| Chest films | Good for detecting metastases | Yes |
| Laparoscopy | May allow visualization of small serosal implants or liver metastases | Optional—recommended if plan preoperative chemotherapy or chemoradiation |
| Positron emission tomography (PET) | Excellent for detecting unsuspected metastases | Optional—recommended if plan preoperative chemo/chemoradiation |
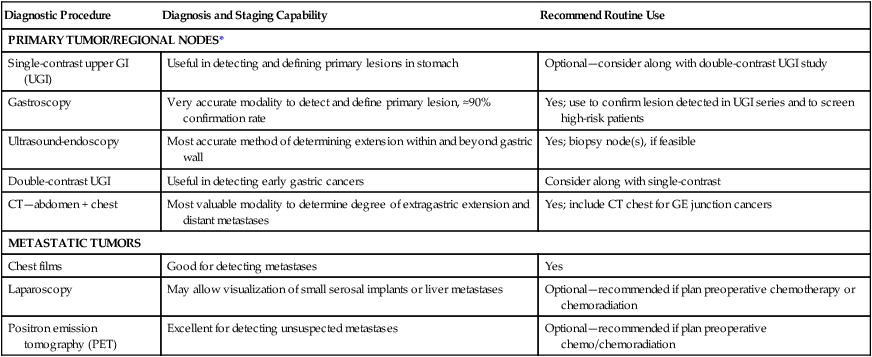
*Laboratory studies: CBC, creatinine, liver function studies (alkaline phosphatase, bilirubin, SGOT, LDH), albumin.
Staging
With the development of laparoscopic general surgery, diagnostic laparoscopy is commonly used to assess for distant metastasis or unresectable locally advanced abdominal cancers. Several groups54,55 have reported the use of laparoscopy in stomach cancer patients. Metastatic disease was documented laparoscopically in 35% to 40% of patients.56–56 The sensitivity for metastases was 85% or greater55,56 and this technique was particularly sensitive in detecting liver and peritoneal disease. Laparoscopy is more sensitive and accurate in staging patients with regard to intraabdominal metastases than either ultrasound or CT scan.56,57 Many surgeons now routinely perform laparoscopy in all gastric cancer patients who are deemed candidates for surgical resection, to avoid nontherapeutic laparotomy.
The current TNM (tumor, lymph node, metastasis) staging system is depicted in Table 75-2 and is acknowledged as the standard system for reporting outcomes in stomach cancer.58 Several comparison studies, including some from Japan,59,60 have shown better prediction of prognosis using the AJCC TNM system compared with other staging systems, including that of the Japanese Research Society for Gastric Cancer.
Table 75-2
TNM Staging for Carcinoma of the Stomach
| Stage | T | N | M |
| O | Tis | 0 | 0 |
| IA | 1 | 0 | 0 |
| IB | 2 | 0 | 0 |
| 1 | 1 | 0 | |
| IIA | 3 | 0 | 0 |
| 2 | 1 | 0 | |
| 1 | 2 | 0 | |
| IIB | 4 | 0 | 0 |
| 3 | 1 | 0 | |
| 2 | 2 | 0 | |
| 1 | 3 | 0 | |
| IIIA | 4a | 1 | 0 |
| 3 | 2 | 0 | |
| 2 | 3 | 0 | |
| IIIB | 4b | 0-1 | 0 |
| 4a | 2 | 0 | |
| 3 | 3 | 0 | |
| IIIIC | 4b | 2 | 0 |
| 4a-b | 3 | 0 | |
| IV | Any | Any | 0 |
| TNM definitions are as follows: | |||
|
Tis: carcinoma in situ; intraepithelial tumor without invasion of the lamina propria. T1: Tumor invades lamina propria, muscularis mucosa or submucosa. T1a: Tumor invades lamina propria or muscularis mucosa; T1b: Tumor invades submucosa T2: Tumor invades the muscularis propria T3: Tumor penetrates subserosal connective tissue without invasion of visceral peritoneum or adjacent structures. T4: Tumor invades serosa (visceral peritoneum) or adjacent structures. T4a: Tumor invades serosa (visceral peritoneum); T4b: tumor invades adjacent structures* N0: No regional lymph node metastasis. N1: Metastasis in 1–6 regional nodes. N2: Metastasis in 7–15 regional nodes. |
|||
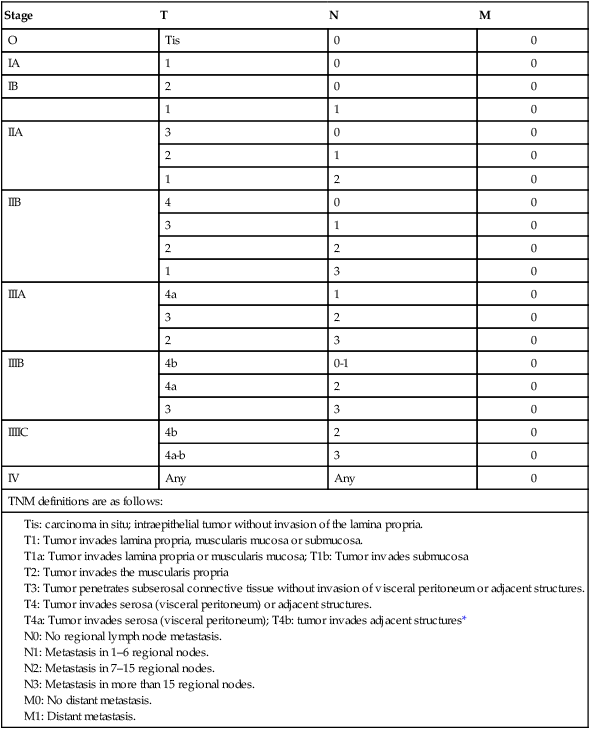
*Adjacent structures include the spleen, transverse colon, liver, diaphragm, pancreas, abdominal wall, adrenal gland, kidney, small intestine, and retroperitoneum.
From Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York: Springer Verlag; 2010. p. 117.
Primary Therapy
Surgical Method—Gastric Cancer
Surgical excision has traditionally been the first treatment for gastric carcinoma. Because many patients have occult distant metastases at presentation and improved results have been demonstrated with preoperative chemotherapy61,62, neoadjuvant treatment is appropriate when preoperative staging is consistent with ≥T3 primary tumors and/or ≥N1 disease. The increasing prevalence of proximal gastric cancers, especially in the developed world, has resulted in more operations encompassing a thoracic component of resection to obtain an adequate proximal margin and remove intrathoracic lymph nodes at risk for metastasis.
Surgical excision of the gastric and nodal components of disease remains the primary therapy for all potentially curable gastric carcinomas. Based on pathological findings, the Japanese Research Society for Gastric Cancer has defined four categories of surgical resection: (1) absolute curative (no peritoneal or hepatic metastases, no serosal involvement, and a level of lymph nodes removed beyond those involved); (2) relative curative (same as category 1 but nodal involvement to the level excised); (3) relative noncurative (complete gross tumor excision but curative criteria not met); and (4) absolute noncurative (residual cancer).50 Most curable tumors can be removed with adequate margins by subtotal gastrectomy; total gastrectomy is used when mandated by proximal cancer location or disease extent. Routine total gastrectomy does not improve survival by providing wider margins and eliminating multicentric disease but it may increase the rates of patient morbidity and mortality. A randomized study63 showed similar survival rates with subtotal and total gastrectomy. Surgical resection alone, including endoscopic mucosal resection in selected patients,64 is an excellent treatment for gastric carcinomas limited to the mucosa or submucosa without nodal involvement (TIS or T1N0M0). These early gastric cancers now occur with an incidence of more than 30% in Japan but still less than 5% in the United States and other Western countries. At least one Japanese report showed similar excellent results for T2 cancers if lymph nodes were uninvolved.65 For the more invasive gastric carcinomas, curative or palliative resection is indicated for 50% to 60% of patients at the time of disease presentation, but only 25% to 40% of these patients will have potentially curative surgical procedures.
Increasingly, subtotal66 and total67 laparoscopic gastrectomies are being performed safely and without apparent compromise of patient outcome. Laparoscopic gastrectomy has reduced morbidity and mortality in controlled trials compared with open resection of mostly clinical early gastric cancers (pathological T1/T2, N0 cancers). Long-term results comparing patient survivals and large controlled trials comparing laparoscopic and open gastrectomy in advanced gastric cancers have yet to be reported.
Only one prospective randomized trial63 exists with regard to the extent of gastric resection, but extensive experience exists with various different surgical procedures, and appropriate generalizations can be made. The preferred treatment for lesions arising in the body or antrum of the stomach is a radical distal subtotal resection (Fig. 75-2). This procedure removes approximately 80% of the stomach along with the first portion of the duodenum, the gastrohepatic and gastrocolic omenta, and the nodal tissue adjacent to the three branches of the celiac axis. Extensive or proximal cancers will require a total gastrectomy to achieve an adequate proximal gastric margin (Fig. 75-3). Total gastrectomy provides no advantage when subtotal gastrectomy will provide a 5-cm clearance of the gross tumor. The propensity for gastric carcinoma to spread via submucosal and subserosal lymphatics dictates the need for a 5-cm surgical resection margin of normal stomach beyond the visible tumor. It may be necessary to extend the resection to include some (or additional) esophagus or duodenum if frozen-section pathological evaluation of the surgical margins fails to confirm the adequacy of proximal and distal resection margins. If total gastrectomy is necessary, a splenectomy is sometimes performed, particularly in gastric cancers of the proximal third of the stomach, and tumors of the body near the greater curvature. These cancers are more apt to metastasize to lymph nodes in the splenic hilum that cannot be completely excised without a splenectomy. Routine splenectomy is no longer practiced because of the increased complications found in randomized controlled68,69 and retrospective studies. A splenectomy should be performed when worrisome, palpable nodes are present in the splenic hilum.
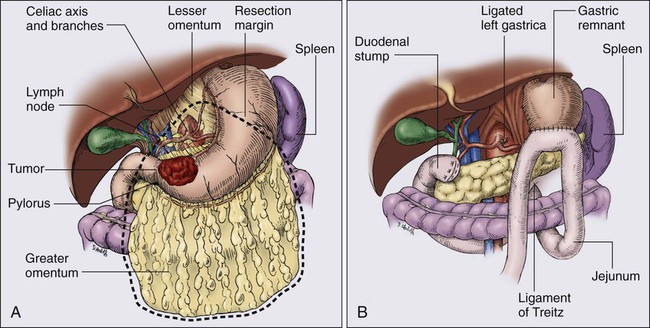
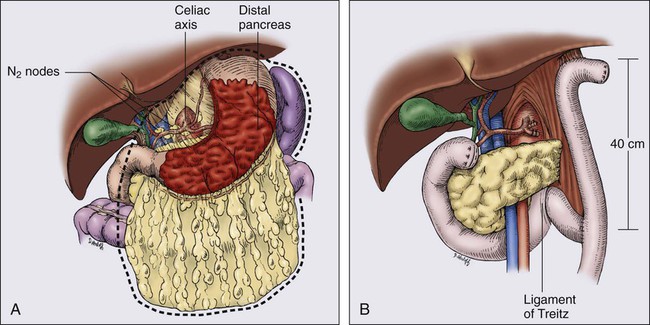
Direct spread beyond the gastric wall should be treated with en bloc extended resection to achieve negative margins of resection, if a curative resection is contemplated.70,71 These extended resections are potentially curative but increase perioperative morbidity and mortality. Common examples of local tumor extension include involvement of the body or tail of the pancreas (treated by distal pancreatectomy and splenectomy), invasion of the transverse mesocolon (often requires transverse colectomy), and involvement of the spleen (splenectomy) or left lobe of the liver (usually requires wedge resection with a 1-cm or wider clearance).
The optimal extent of lymph node dissection for gastric cancer remains controversial. The presence and extent of lymph node metastasis correlate with the depth of primary tumor invasion.72 Japanese surgeons universally advocate regional lymph node removal for all but in situ or intestinal mucosal tumors as a means to improve both local control and survival.73 Because more distal nodes can be involved with metastasis in 11% of patients with negative perigastric nodes, a wider regional nodal dissection is deemed necessary for cure.72 A recent study of sentinel lymph node biopsies in gastric cancer patients in Japan74 demonstrated that 37% of tumors drained to N2 nodes, either in combination with N1 sentinel nodes (32%) or as the sole site of lymphatic drainage (5%). When a radical subtotal gastrectomy and omentectomy is performed, all perigastric (D1) lymph nodes along the lesser curvature and those on the greater curvature distal to the site of transection should be removed. In many major cancer centers, the lymph nodes adjacent to the celiac axis and its branches (D2) are also resected with a limited increase in postoperative morbidity (D2 dissection; see E-Fig. 75-2 regarding D2 dissection; N1, perigastric nodes; N2, nodes along the left gastric, common hepatic, celiac, and splenic arteries). Some surgeons in Japan routinely remove N3 lymph nodes (D3 dissection, usually portal and retropancreatic). A more recent randomized trial comparing a Japan D2 versus extended D2 resections75 shows a very low operative mortality and low morbidity with no significant increase in complications, nor as yet, any improvement in patient survival.
Thus far, randomized trials76,77 have not demonstrated either disease-free or overall survival (OS) advantage for extended lymphadenectomy (D2 dissection). A large multicenter phase III study that accrued 711 curable gastric cancer patients in the Netherlands76 noted significantly higher morbidity and mortality rates with the more extensive nodal dissection (Table 75-3). A randomized study from the United Kingdom that included 400 patients with gastric adenocarcinoma also demonstrated higher morbidity and mortality rates in the extended lymphadenectomy cohort (see Table 75-3).77 Neither the Dutch76 nor the British trial77 demonstrated any improvement in overall or disease-free survival (see Table 75-3). In the Dutch study,76 patients who did not undergo a splenectomy or distal pancreatectomy had an improvement in relapse-free survival ([RFS] 71% vs. 59% at 5 years, P = 0.02). Splenectomy and pancreatectomy had significant adverse impact on survival in both trials.76,77 Preliminary data from a Japanese trial comparing D2 and extended D2/D3 resections75 and an Italian study comparing D1 and D2 resections78 did not show increased morbidity with extended lymphadenectomy. Results for disease-free and long-term OS are not yet available from either study.
Table 75-3
Extent of Surgery: Randomized Trials of D1 Versus D2 Dissection
| No. of Patients | 5-Y Survival (%) | Operative Mortality (%) | ||||
| Series | D1 | D2 | D1 | D2 | P Value | |
| Dutch76 | 711 | 45 | 47 | 4 | 10 | <0.05 |
| MRC77* | 400 | 35 | 33 | 6.5 | 13 | <0.05 |
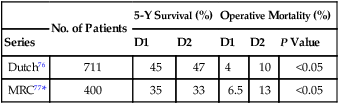
MRC, Medical Research Council.
Both trials showed significantly increased morbidity and mortality with more extensive dissections.
*Defined D1 dissection as resection of AJCC nodes (those within 3 cm of primary tumor).
Any potential survival benefit seen with the extended node dissection performed in Japan may be due to the phenomenon of a stage migration rather than superior surgical therapy.79 In Japan and the United States, gastric resection specimens are handled quite differently.80 Japanese pathologists evaluated an average of 62 nodes in subtotal gastrectomy specimens and as many as 100 in total gastrectomy cases, including lymph nodes less than 3 mm in diameter.81 This number compares with an average of 12 and 13 nodes examined after subtotal and total gastrectomy, respectively, at Memorial Sloan-Kettering.82 Patient survival after a curative operation significantly improves when more than 15 lymph nodes are pathologically examined.83,84 Failure to evaluate an adequate number of regional lymph nodes probably results in the understaging of many gastric cancer patients. N2 nodes cannot be defined as positive if they are not resected and examined; involvement of resected N1 nodes cannot be assessed if the specimen is not thoroughly evaluated by the pathologist. Most patients with more than six lymph node metastases or with lymph node metastasis not adjacent to the primary tumor still have a very poor outcome.16 Extended lymph node dissection seems reasonable for experienced surgeons who can perform this procedure without significantly increased surgical morbidity or mortality, because it improves pathological staging.75,78,85
Endoscopic laser surgery has been used in selected individuals with early gastric cancer.64,86 Small lesions (≤3 cm) that are not ulcerated, do not involve the submucosa, and are well differentiated infrequently have lymph node metastasis (<5%). As many as 75% of these select tumors can be completely removed endoscopically. Although early gastric cancer may have a long natural history before progression, standard surgical resection rather than endoscopic removal is still preferable for most Western patients.
Surgical Method—GEJ Cancer
Although adenocarcinomas arising at or near the GEJ are often classified as esophageal carcinoma, there has been a concerted effort to differentiate treatment approach based on location and cell origin.87–93 Feith, Siewert, and colleagues defined adenocarcinomas of the GEJ into three separate types87,94:
Type I: Adenocarcinoma of the distal esophagus, arising as intestinal metaplasia of the esophagus and possibly infiltrating the GEJ from above
Type II: Adenocarcinoma of the cardia, arising from cardiac epithelium or short segments with intestinal metaplasia at the GEJ
Type III: Subcardial gastric carcinoma, infiltrating the GEJ and distal esophagus from below.
Surgical resection has remained controversial as to the necessary extent of proximal esophageal resection, distal gastric resection, and lymph node dissection.95–101 Complete macroscopic and microscopic resection (R0) provides patients with the best survival.89,94,97,98,100–103 The main goal of lymph node dissection is to optimize staging and reduce locoregional relapse, and lymph node involvement is recognized as a major prognostic factor in GEJ adenocarcinoma.96,99,103,104 Decisions as to the type of surgical resection should be based on achieving an R0 resection and can include transhiatal, transthoracic, partial, and total gastrectomy.
For Type I GEJ cancers, surgical resection should include esophagectomy with at least 6 to 8 cm of proximal margin.89,96,98,100,105 Either transhiatal or transthoracic esophagectomy can be used to achieve an R0 resection.
Both transhiatal and transthoracic esophagectomy include an identical abdominal procedure to assess metastases, mobilize the stomach, and create a gastric tube that will replace the resected esophagus and proximal stomach. Lymph node dissection is also performed along the celiac axis and peripancreatic region. The abdominal portion of the operation can be done either open or with laparoscopy depending on surgeon experience and preference (E-Fig. 75-3A).
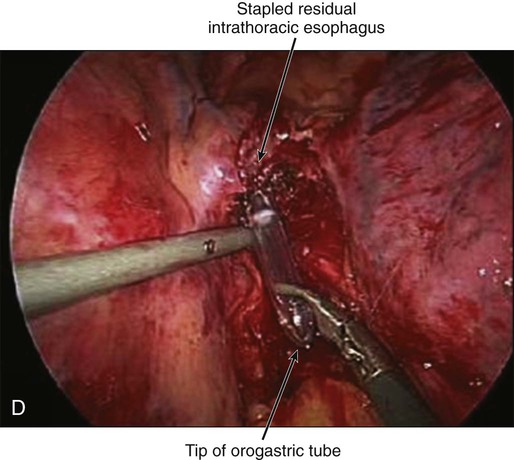
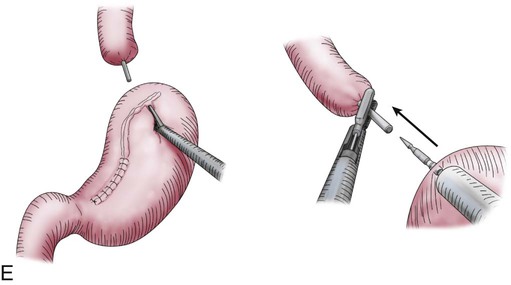
Transthoracic cases will then be completed through a right side of the chest approach. Either open posterolateral thoracotomy or minimally invasive thoracoscopy can be used (E-Fig. 75-3B). The transthoracic approach allows extensive mediastinal lymphadenectomy to be performed as well as resection of periesophageal fat and adhesions. The gastric tube is brought up into the chest through the diaphragm and anastomosed to the proximal esophagus in the upper thorax. Multiple anastomotic techniques have been described.106 For minimally invasive esophagectomy (MIE), we have successfully used an OrVil EEA (U.S. Surgical Corps.) which allows a 25-mm anvil to be advanced through the mouth while attached to an orogastric tube (E-Fig. 75-3C).105,107 The anvil is then pulled through a small opening made in the proximal esophagus (E-Fig. 75-3D) and attached to the pin of the stapler that has been advanced through the back wall of the gastric conduit (E-Fig. 75-3E). There have been no large randomized trials on MIE versus open options for GEJ adenocarcinoma; therefore, evidence-based recommendations cannot be made. There is some suggestion of fewer pulmonary complications and faster recovery with MIE.107–110
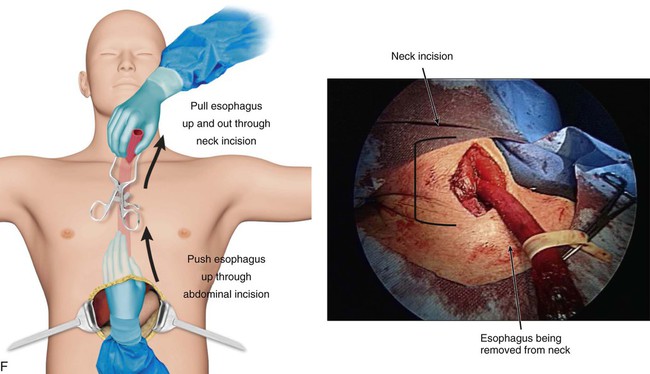
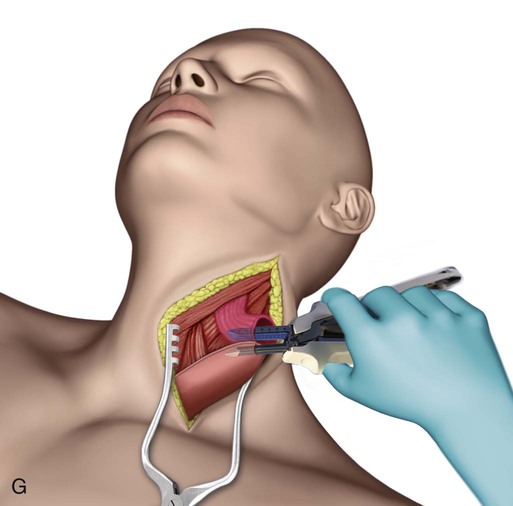
Transhiatal procedures are completed through a cervical incision on the left neck. The esophagus is freed through this incision and hand dissection from below the diaphragm allows the specimen to be brought up through the neck incision (E-Fig. 75-3F). The conduit is anastomosed to the proximal esophagus in the neck. This can be performed with hand sewing or stapling devices (E-Fig. 75-3G). Generally a formal mediastinal lymphadenectomy is not performed with this technique.
Type III GEJ cancers require extensive gastric resection to provide an inferior margin of 2 to 3 cm and a superior margin of 4 to 5 cm.95,111 A total gastrectomy with partial esophagectomy and Roux-en-Y bile diversion through an abdominal approach is generally necessary to achieve R0 resection.95,111,112 These cancers are treated similar to pure gastric cancers.
Survival After Surgery Alone
OS results with surgery alone remain poor, despite improved perioperative treatment, which has resulted in a substantial decline in postoperative mortality rate (median of 4.6% in the 1980s).113 A large review from Europe reported excellent 5-year survival rate for early gastric cancer patients (83%) but a marked diminution in survival for more invasive cancers.114 Excellent survival in excess of 90% has been achieved throughout the world with surgical resection of lesions confined to the mucosa or submucosa.65,115,116 In contrast, for gastric cancers with deeper invasion or nodal involvement, survival decreases proportionally to the degree of invasion or involvement (see E-Table 75-2). When N1 or N2 nodes are involved, Western reports continue to show 5-year survival rates of 10% to 30%,117 whereas Japanese authors report 5-year surgical cure rates of 25% to 60% (vs. <10% with N3 or N4)118,119 (see E-Table 75-2). The improved results seen in Japan compared to the United States likely reflect improved pathological staging with more extensive nodal dissection and possibly differences in tumor biology between the two countries.
E-Table 75-2
Comparison of 5-Year Survivals Following Surgery for Gastric Carcinoma
| Tumor Classification | Nodal Status | 5-Yr Survival (%) | |
| United States | Japan | ||
| T1 | N0 | 90 | 90 |
| T2 | N0 | 52 | 60 |
| T3 | N0 | 47 | 30 |
| T4 | N0 | 15 | 5 |
| — | N1 | 20 | |
| — | N2 | 10 | |
| — | N3 | — | |
| — | N4 | — | |
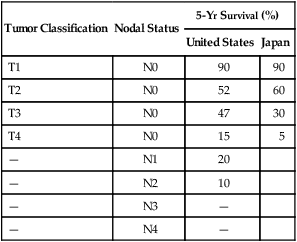
Modified from Noguchi Y, Imada T, Matsumoto A, et al. Radical surgery for gastric cancer. A review of the Japanese experience. Cancer 1989;64:2053.
Recent randomized, controlled trials have shown significantly improved survival for patients with all but the earliest gastric cancers.120 Even in the Far East, patients with locally advanced gastric cancers benefit from multimodality therapy, because cancer-related death occurs in more than 50% of patients following resection alone.
Relapse Patterns after “Curative Resection”
Local regrowth or failure in the tumor bed and regional lymph nodes, or distant failures via hematogenous or peritoneal routes are all common mechanisms of failure after “curative resection” in clinical,121,122 reoperative,13,123 and autopsy123–126 series. For lesions of the GEJ, both the liver and lungs are common sites of hematogenous spread. With gastric lesions that do not extend to the esophagus, the initial site of hematogenous spread is usually the liver, and many relapses could be prevented if an effective “abdominal” therapy could be combined with treatment of the primary tumor and regional lymph nodes.
Local-regional failures occur commonly within the region of the gastric bed and nearby lymph nodes (Table 75-4). Tumor relapse in anastomoses, the gastric remnant, or the duodenal stump is also frequently seen. In a University of Minnesota reoperative analysis,13,123 local-regional failure occurred as the only evidence of relapse in 29% of the 86 patients with relapse (23% of the 105 evaluable patients at risk) and as any component of failure in 88%. More extensive operative procedures including routine splenectomy, omentectomy, and radical lymph node dissection neither improved survival127 nor decreased the incidence of local or regional regrowth in the reoperative analysis.13,123 Subsequent relapse within the scope of the initial node dissection occurred in a high percentage of the patients even when radical node dissections were performed (removal of N1, N2, and sometimes N3 nodes; E-Table 75-3).123,128 This indicates the difficulty of obtaining a complete lymph node excision encompassing this anatomic location and provides a partial explanation for the lack of survival benefit with a D2 versus D1 node dissection in phase III trials previously discussed.75–78,129 In a more recent clinical analysis of patterns of relapse from Memorial Sloan Kettering Cancer Center, 50% of patients with relapse had a local-regional component.122
Table 75-4
Gastric Cancer: Patterns of Local-Regional Failure in Clinical, Reoperation, and Autopsy Series
| Incidence—Any Component | ||||
| MGH121 (Clinical) (N = 130) | UMinn13 (Reoperation) (N = 105) | McNeer et al124 (Autopsy) (N = 92) | Thomson and Robins125 (Autopsy) (N = 28) | |
| Failure Area | No. (%) | No. (%) | No. (%) | No. (%) |
| Gastric bed | 27 (21) | 58 (55) | 48 (52) | 19 (68) |
| Anastomosis or stumps | 33 (25) | 28 (27) | 55 (60) | 15 (54) |
| Abdominal or stab wound | — | 5 (5) | — | — |
| Lymph node(s) | 11 (8) | 45 (43) | 48 (52) | — |
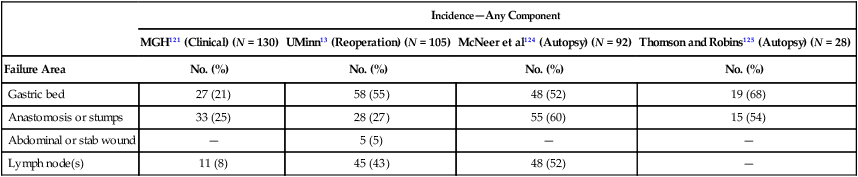
MGH, Massachusetts General Hospital; UMinn, University of Minnesota.
From MacDonald JS, Steele G, Gunderson LL. Carcinoma of the stomach. In DeVita V, Hellman S, Rosenberg SA, editors: Principles and Practices of Oncology. 2nd ed. Philadelphia: JB Lippincott; 1989. p. 675.
E-Table 75-3
Operative Method Versus Patterns of Failure—Reoperation Series*†
| Operative Procedures‡ | No. of Failures/Total at Risk (%) | Local-Regional | Peritoneal Seeding | Distant Metastases | |||
| Alone | Component | Alone | Component | Alone | Component | ||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Method 1 (pre-1950) | 25/36 | 9 (25) | 23 (64) | 1 (3) | 12 (33) | — | 7 (19) |
| Method 2 (1950–1954) | 29/32 | 6 (19) | 24 (75) | 1 (9) | 17 (53) | 3 (9) | 9 (28) |
| Method 3 (1954 on) | 26/37 | 8 (22) | 23 (62) | 1 (3) | 15 (41) | 2 (5) | 7 (19) |
| Totals | 80/105‡ | 23 (22) | 70 (67) | 3 (3) | 44 (42) | 5 (5) | 23 (22) |
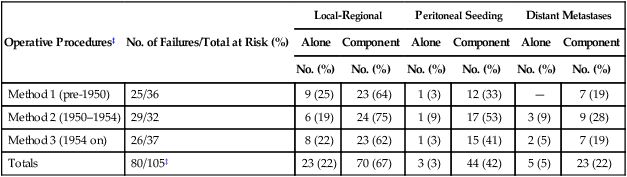
*186 Patients with failure, 80 evaluable by all parameters.
†Data represent number of patients with failure; data in parentheses represent percentage total group at risk who had complete follow-up.
‡Method 1 (pre-1950), subtotal or total gastrectomy, greater omentectomy, regional node dissection; method 2 (1950–1954), method 1 plus splenectomy, total omentectomy, additional node dissection regarding splenic, suprapancreatic, and central celiac axis; method 3 (1954 on), methods 1 and 2 plus extension of node dissection to porta hepatis and pancreaticoduodenal (intent: total lymph node dissection of all primary node areas equivalent to D2 or D3 dissection).
Modified from MacDonald JS, Steele G, Gunderson LL. Carcinoma of the stomach. In DeVita V, Hellman S, Rosenberg SA, editors: Principles and Practices of Oncology. 2nd ed. Philadelphia: JB Lippincott; 1989. p. 765.
Patterns of failure by stage were analyzed in detail in a series of 130 patients who underwent resection performed with curative intent at the Massachusetts General Hospital (MGH).121 Local-regional failure occurred as any component of failure in 49 patients (38%) and as the sole failure in 21 (16% of 130 patients at risk and 24% of the 88 patients with disease progression). The incidence of local-regional failure by stage was in excess of 35% for T3N0, T4N0, T3N1–3, and T4N1–3 lesions. The sites at highest risk for local-regional failure included the gastric bed (27 of 130 patients, 21%) and the anastomosis or gastric remnant (33 of 130 patients, 25%). The true incidence of gastric bed, regional lymph node, and peritoneal failures may be higher, because this was neither a reoperative nor an autopsy series (see comparative findings in Table 75-4 and E-Table 75-3). Some additional information on patterns of relapse by stage exist in both the University of Minnesota reoperation analysis13,123 and the University of Washington autopsy analysis.126 Although patterns of failure data are more accurate in such analyses, patient selection is biased.
Adjuvant and Neoadjuvant Therapy
Neoadjuvant Systemic Chemotherapy
Neoadjuvant therapy has the potential advantage of downstaging gastric cancer and thereby enhancing the potential for resection. It also has the potential to eliminate micrometastatic disease prior to surgery and at a time patients may be more likely to tolerate systemic therapy. A retrospective analysis of 220 patients receiving either 5-fluorouracil (5-FU) alone or 5-FU and cisplatin prior to surgery compared with 100 patients treated with surgery alone suggested a benefit to neoadjuvant therapy.130 Patients receiving neoadjuvant therapy had a significantly longer 5-year survival. However, prospective clinical trials with neoadjuvant therapy are limited.
Several phase II trials of neoadjuvant trials have been reported and have shown mixed results. Ajani and colleagues have performed two phase II studies of preoperative chemotherapy in this setting.131,132 In their first study, 25 patients were treated with two cycles of etoposide, 5-FU, and cisplatin preoperatively.132 Three cycles were administered postoperatively if a positive response to neoadjuvant treatment could be detected endoscopically or radiographically. All 25 patients underwent surgery, and 72% were resected for cure. No pathological complete responses were seen, and the median survival was 15 months overall. Following the report of Wilke and coworkers of pathological complete response to chemotherapy with epirubicin, doxorubicin, and cisplatin (EAP),133 Ajani and associates treated 48 potentially curable patients with three cycles of preoperative EAP and two cycles following surgery if a response to preoperative treatment was observed.131 This trial failed to support the findings of the trial reported by Wilke. Of the 85% of patients who underwent exploration, 77% had resectable cancer. Although 12% of patients achieved a complete clinical response, no pathological complete responders were seen. The overall median survival was 15.5 months.
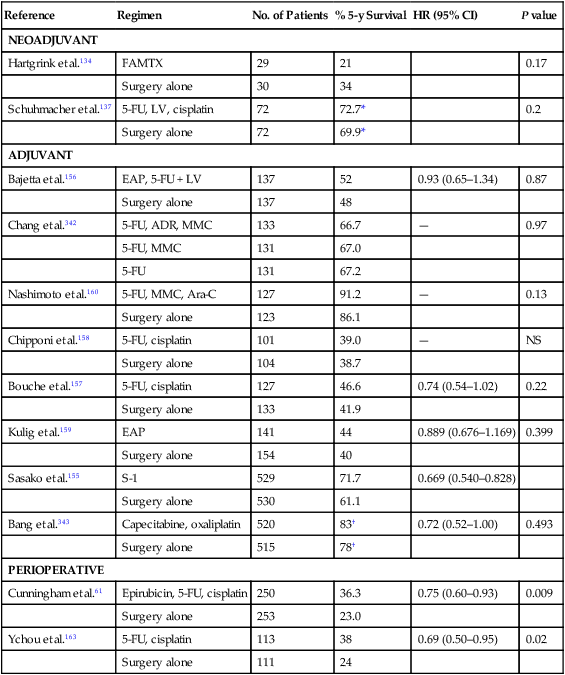
One of the few randomized trials to assess the potential benefit of preoperative chemotherapy to surgery alone in patients with potentially resectable cancer failed to show any benefit in outcomes (Table 75-5).134,135 In this Dutch trial, 59 patients were randomly assigned to either 5-FU, doxorubicin, and methotrexate (FAMTX) followed by surgery (N = 29) or surgery alone. Patients with T1 tumors, tumors arising from the gastric cardia, or evidence of distant metastases were excluded from participation. The trial was closed early as a result of poor accrual. No benefit was seen with the addition of FAMTX before surgery. In a separate phase III trial neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin followed by surgery was compared to surgery alone.136 Patients randomly assigned to chemotherapy received two 48-day cycles of chemotherapy. The trial was closed early for poor accrual. No benefit could be demonstrated for the addition of neoadjuvant therapy as measured by OS (hazard ratio [HR]: 0.84; 95% CI: 0.52, 1.35, P = 0.466) in the 144 patients enrolled after a median follow-up of 4.4 years.
Table 75-5
Gastric Cancer—Randomized Phase III Trials of Neoadjuvant, Adjuvant, or Perioperative Chemotherapy
| Reference | Regimen | No. of Patients | % 5-y Survival | HR (95% CI) | P value |
| NEOADJUVANT | |||||
| Hartgrink et al.134 | FAMTX | 29 | 21 | 0.17 | |
| Surgery alone | 30 | 34 | |||
| Schuhmacher et al.137 | 5-FU, LV, cisplatin | 72 | 72.7* | 0.2 | |
| Surgery alone | 72 | 69.9* | |||
| ADJUVANT | |||||
| Bajetta et al.156 | EAP, 5-FU + LV | 137 | 52 | 0.93 (0.65–1.34) | 0.87 |
| Surgery alone | 137 | 48 | |||
| Chang et al.342 | 5-FU, ADR, MMC | 133 | 66.7 | — | 0.97 |
| 5-FU, MMC | 131 | 67.0 | |||
| 5-FU | 131 | 67.2 | |||
| Nashimoto et al.160 | 5-FU, MMC, Ara-C | 127 | 91.2 | — | 0.13 |
| Surgery alone | 123 | 86.1 | |||
| Chipponi et al.158 | 5-FU, cisplatin | 101 | 39.0 | — | NS |
| Surgery alone | 104 | 38.7 | |||
| Bouche et al.157 | 5-FU, cisplatin | 127 | 46.6 | 0.74 (0.54–1.02) | 0.22 |
| Surgery alone | 133 | 41.9 | |||
| Kulig et al.159 | EAP | 141 | 44 | 0.889 (0.676–1.169) | 0.399 |
| Surgery alone | 154 | 40 | |||
| Sasako et al.155 | S-1 | 529 | 71.7 | 0.669 (0.540–0.828) | |
| Surgery alone | 530 | 61.1 | |||
| Bang et al.343 | Capecitabine, oxaliplatin | 520 | 83† | 0.72 (0.52–1.00) | 0.493 |
| Surgery alone | 515 | 78† | |||
| PERIOPERATIVE | |||||
| Cunningham et al.61 | Epirubicin, 5-FU, cisplatin | 250 | 36.3 | 0.75 (0.60–0.93) | 0.009 |
| Surgery alone | 253 | 23.0 | |||
| Ychou et al.163 | 5-FU, cisplatin | 113 | 38 | 0.69 (0.50–0.95) | 0.02 |
| Surgery alone | 111 | 24 | |||
Adjuvant Systemic Chemotherapy
Initial trials to assess the benefit of adjuvant chemotherapy in the United States were conducted by the Veterans Administration in 1957 testing single agents. Survival was not improved with the adjuvant use of either single-agent 5-fluorodeoxyuridine (FUDR) or triethylenethiophosphoramide (thiotepa) when compared with surgery alone.137,138
In subsequent North American and European trials, the potential benefit of multidrug regimens that had shown benefit in the metastatic setting were evaluated in the adjuvant setting. These regimens included (1) a combination of 5-FU and methyl-chloroethyl-cyclohexyl-nitrosourea (MeCCNU; lomustine); (2) FAM, a combination of 5-FU, doxorubicin (Adriamycin), and mitomycin C; and (3) FAMTX, a combination of 5-FU, doxorubicin, and methotrexate. Although a trial from the Gastrointestinal Tumor Study Group (GITSG) demonstrated a survival advantage for the 71 patients assigned to combination chemotherapy with 5-FU and MeCCNU when compared with an equal number of control patients assigned to surgery alone (P < 0.03),139 subsequent studies performed by the Eastern Cooperative Oncology Group (ECOG), and the Veterans Administration could not confirm a survival advantage for patients treated with the same adjuvant chemotherapy.11,12 Phase III trials testing the use of either FAM or FAMTX in an adjuvant setting also failed to demonstrate a survival advantage when compared with a surgery-alone control arm.140–148 A phase III trial of cisplatin, epirubicin, 5-FU, and leucovorin (PELF) also failed to show any significant benefit.149
Mixed results have been seen in trials performed in Asia. Nakajima and colleagues have performed a series of adjuvant trials in individuals with resected gastric cancer.150–153 In their first trial, mitomycin C was given on a twice-a-week schedule for 5 weeks, but no survival benefit was seen for the whole cohort of patients.150 In a subsequent three-arm adjuvant trial, patients were randomly assigned to surgery alone, mitomycin C alone, or the combination of twice-weekly MFC (mitomycin C, 5-FU, and cytosine arabinoside).153 Forty-two patients were entered in each arm of the trial. At 5 years, a survival benefit for MFC-treated patients was seen when compared with surgical control subjects. No significant benefit was seen for the mitomycin C arm. These same investigators studied the regimen of MFC followed by either long-term oral 5-FU or ftorafur compared with surgery alone.152 A significant survival benefit was seen for individuals with stages I to III disease treated with MFC and oral 5-FU. In a fourth trial, the role of adjuvant mitomycin C and 5-FU followed by oral uracil and tegafur was evaluated in patients undergoing complete resection for early-stage gastric cancer.151 When compared with a control group, adjuvant therapy for this group of patients showed no benefit over surgery alone.
Given the success of the oral fluoropyrimidine S-1 in advanced gastric cancer, a Japanese trial of surgery followed by adjuvant S-1 for 1 year compared with surgery alone for patients with resected stage II or III gastric cancer was performed (Table 75-5).154 A total of 529 patients were randomly assigned to the S-1 group and 530 to the surgery-alone group. The 3-year and 5-year OS improved with the use of S-1 (5-year HR: 0.669; 95% CI: 0.540, 0.793).154,155
Five other phase III trials of adjuvant postoperative chemotherapy have been performed with a surgery-alone control arm, but only two demonstrated a survival benefit from the adjuvant chemotherapy (Table 75-5).156–161 Neri and associates treated surgically resected node-positive patients with the addition of adjuvant epirubicin, 5-FU, and leucovorin (68 patients) and compared them with 69 surgery-alone control patients.161 A significant improvement in 5-year OS was noted in patients receiving the adjuvant chemotherapy (30% vs. 13%, P < 0.01).
In an attempt to better determine the benefit of adjuvant therapy after potentially curative surgery for stomach cancer, several meta-analyses have been performed. A meta-analysis of 17 phase II and III randomized trials showed a significant survival advantage (OS, DFS) to the use of chemotherapy following surgical resection of gastric cancer compared with surgery alone with a median follow-up exceeding 7 years.162
Perioperative Chemotherapy
The use of perioperative chemotherapy was recently addressed in a phase III trial referred to as the MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy) trial (Table 75-5).61 A total of 503 patients with resectable adenocarcinoma of the stomach (372 patients), GEJ (58 patients), or lower esophagus (73 patients) were randomly assigned to either surgery alone or to perioperative chemotherapy with three cycles of epirubicin, cisplatin, and continuous-infusion 5-FU (ECF) before surgery and three cycles of ECF after surgery. With a median follow-up of 4 years, patients randomized to receive perioperative chemotherapy had significantly improved OS when compared with patients randomly assigned to receive surgery alone (5-year OS 36% vs. 23%, HR for death: 0.75, 95% CI: 0.60, 0.93, P = 0.009).
In a separate phase III trial in France, 224 patients with potentially resectable adenocarcinoma of the lower esophagus, GEJ, or stomach were randomly assigned to either perioperative chemotherapy and surgery (n = 113) or to surgery alone (n = 111).163 Chemotherapy consisted of two or three cycles of cisplatin and infusional 5-FU prior to surgery and three to four cycles after surgery. In this trial, perioperative chemotherapy resulted in a significant improvement in 5-year OS (38 vs. 24%, HR: 0.69, 95% CI: 0.50, 0.95, P = 0.02) and DFS (34 vs. 19%, HR: 0.65, 95% CI: 0.48, 0.89, P = 0.003). Grade 3 or 4 toxicity, consisting primarily of neutropenia, occurred in 38% of the patients receiving chemotherapy.
Intraperitoneal Therapy
Building on early promising results from phase II trials, several phase III studies have evaluated the role of intraoperative or postoperative intraperitoneal therapy in patients undergoing potentially curative surgery. In an early study by Dixon and colleagues, patients were randomly assigned to surgery alone or to surgery followed by intraperitoneal thiotepa.137 No significant difference in survival was seen between the two groups. In a more recent study, Sautner and associates examined the use of postoperative intraperitoneal cisplatin compared with surgery alone in a group of 67 patients.164 Although the primary lesion was resected in each case, 21% of the individuals had localized peritoneal carcinomatosis. No survival benefit was seen for the treated patients.
In a phase III trial from Japan, 113 patients were randomly assigned to surgery alone or intraoperative mitomycin C at the time of surgery.165 For patients who underwent curative surgery, intraperitoneal therapy led to a significant improvement in 2- and 3-year OS. No difference in survival was seen for patients with macroscopic peritoneal carcinomatosis. In a separate trial from Japan, 88 patients with peritoneal cytology-positive lavage fluid, but without macroscopic peritoneal metastases, were randomly assigned in the operating room to surgery alone, surgery plus intraoperative peritoneal chemotherapy, or a combination of surgery, intraoperative peritoneal therapy, and “extensive intraoperative peritoneal lavage.”166 The 5-year OS was 43.8% for the extensive intraoperative peritoneal lavage group, compared with 4.6% for those receiving only intraoperative chemotherapy and 0% for those receiving only surgery.
In a single-institution phase III trial from Korea, 248 patients with clinical stage II or III gastric cancer were randomly assigned to surgery alone or to receive intraperitoneal mitomycin C on day 1 and intraperitoneal 5-FU on days 2 to 5.167 Overall, 110 of the 248 patients had either stage I or stage IV disease. When 5-year OS was calculated for the entire group, benefit to adjuvant therapy was seen (P = 0.0278). In a subset analysis, however, the benefit was limited to patients with stage III or IV disease.
Given the small size of most trials of adjuvant intraperitoneal therapy, a meta-analysis was performed.168 Ten of the 13 trials included in the meta-analysis were deemed to be of “fair” quality and 3 to be of “poor” quality. With these recognized limitations, the use of hyperthermic intraoperative intraperitoneal chemotherapy was associated with an improved OS.
Preoperative Systemic and Postoperative Intraperitoneal Chemotherapy
Other investigators have examined the usefulness of combining preoperative chemotherapy and postoperative treatment with intraperitoneal chemotherapy in view of the high peritoneal failure rate following resection of gastric cancer. Initial attempts to use intraperitoneal chemotherapy alone in the adjuvant setting did not show benefit.164,165,167,169–172
Kelsen and associates studied 56 patients with high-risk (clinical stage T3–4) gastric cancer as determined by EUS.171 Patients received three cycles of neoadjuvant FAMTX. Following surgery, patients were treated with intraperitoneal 5-FU and cisplatin along with infusional 5-FU for three cycles. Fifty (89%) patients were explored and 34 (61%) resected for cure. When the initial EUS result was compared to the final pathological staging, 51% of these patients were downstaged. No complete pathological responses were observed, and the median survival was 15.3 months.
Crookes and colleagues updated their promising results with a somewhat similar trial design.169 Fifty-nine patients with potentially resectable cancer received two cycles of 5-FU, leucovorin, and cisplatin preoperatively, and two cycles of intraperitoneal 5-FUDR and cisplatin were given postoperatively to resected patients. Ninety-five percent of the patients underwent exploration, and 68% had a curative resection. The rate of pathological CR was only 9%, but the median survival is estimated to be an impressive 52 months.
In a more recent phase II trial, 32 evaluable patients with locally advanced but potentially resectable stomach cancer received two cycles of systemic cisplatin and irinotecan.172 Twenty-nine patients were able to undergo surgery, and 25 had an R0 resection. Patients with resection subsequently received two cycles of intraperitoneal FUDR and cisplatin. Preoperative chemotherapy led to downstaging in 50% of the patients. With a median follow-up of 28 months, 10 (31%) were alive without relapse, 4 (13%) were alive with relapse, and the remainder had died from either disease (41%) or other causes (16%). Of the 25 patients with an R0 resection, none had a local relapse. A phase III trial of this approach has not yet been reported.
Adjuvant Irradiation
Postoperative Irradiation
Irradiation has only been minimally evaluated as the sole adjuvant treatment following complete surgical resection in randomized phase III trials (Table 75-6). Adjuvant EBRT reduced local-regional failures when compared with the surgery-alone control arm in a British adjuvant trial, but no survival benefits were found.173 Although phase III trials from Japan174 and China175 suggest some survival benefit for IORT versus a surgery-alone control arm, the advantage was found only in subset analyses. At the National Cancer Institute, Sindelar and coworkers176 performed a small randomized trial of IORT versus EBRT following complete surgical resection; this trial demonstrated improved local control with IORT but no survival benefit. A surgery-alone control arm did not exist in the National Cancer Institute trial.
Table 75-6
Surgery ± Adjuvant Therapy for Resected Gastric or Gastroesopheageal Junction Cancer
| Treatment | No. of Patients | Survival | Local-Regional Relapse | Reference | ||||
| Median (mo) | Long-Term (%)* | P Value | No. | Percentage | P Value | |||
| PHASE III TRIALS | ||||||||
| 1. British Stomach Group (3-y) | 15 | 173 | ||||||
| a. Surgery alone | 145 | — | 20 | — | 39 | 27 | — | |
| b. Postop chemo | 138 | — | 19 | — | 26 | 19 | — | |
| c. Postop EBRT | 153 | — | 12 | — | 15 | 10 | — | |
| 2. Japan—Surgery ± IOERT† | S/IOERT | S/IOERT | 174 | |||||
| 110/101 | ||||||||
| a. Stage I | 43/24 | — | 93% vs. 87% | — | — | — | — | |
| b. Stage II | 11/20 | — | 62% vs. 84% | — | — | — | — | |
| c. Stage III | 38/30 | — | 37% vs. 62% | — | — | — | — | |
| d. Stage IV | 18/27 | — | 0 vs. 15% | — | — | — | — | |
| 3. China—Surgery ± IOERT† | 100/100 | 175 | ||||||
| a. Stage III (5-y) | — | — | 30% vs. 65% | <0.01 | — | — | — | |
| b. Stage III (8-y) | — | — | 22% vs. 52% | — | — | — | ||
| 4. Mayo Clinic‡ | 86 | |||||||
| a. Surgery alone | 23 | 15 | 4 | — | — | 54 | — | |
| b. Postop EBRT + 5-FU | 39 | 24 | 23 | 0.05 | — | 39 | — | |
| 5. China-Beijing | 180 | |||||||
| a. Surgery alone | 199 | — | 20 | — | 52 | |||
| b. Preop EBRT | 171 | — | 30 | 0.009 | — | 39 | <0.025 | |
| 6. U.S. GI Intergroup (INT 0116) | (3-y) | RFS, 3-y | 120, 188 | |||||
| a. Surgery alone | 275 | 27 | 41 | — | 31 | |||
| b. Postop EBRT + 5-FU/LV | 281 | 36 | 50 | 0.005 | — | 48 | 0.001 | |
| 7. British MAGIC Trial§ | (3-y) (5-y) | RFS, 3-y | 61 | |||||
| a. Surgery alone | 253 | NA | 31 23 | — | 23 | |||
| b. Perioperative ECF Chemo | 250 | NA | 44 36 | 0.009 | — | 38 | <0.001 | 197 |
| 8. POET Trial (GEJ) | LRFS, 3-y | |||||||
| a. Preop chemotherapy | 60 | 21.1 | 27.7 23 | 59 | ||||
| b. Preop chemoradiation | 60 | 33.1 | 47.4 37 | 0.07 | 76.5 | 0.06 | ||
| 9. French Trial (GE adenocarcinoma) | LRF | 163 | ||||||
| a. Surgery alone | 111 | — | NA 24 | 29 | — | |||
| b. Periop CF Chemo | 113 | — | NA 38 | 0.02 | 28 | — | ||
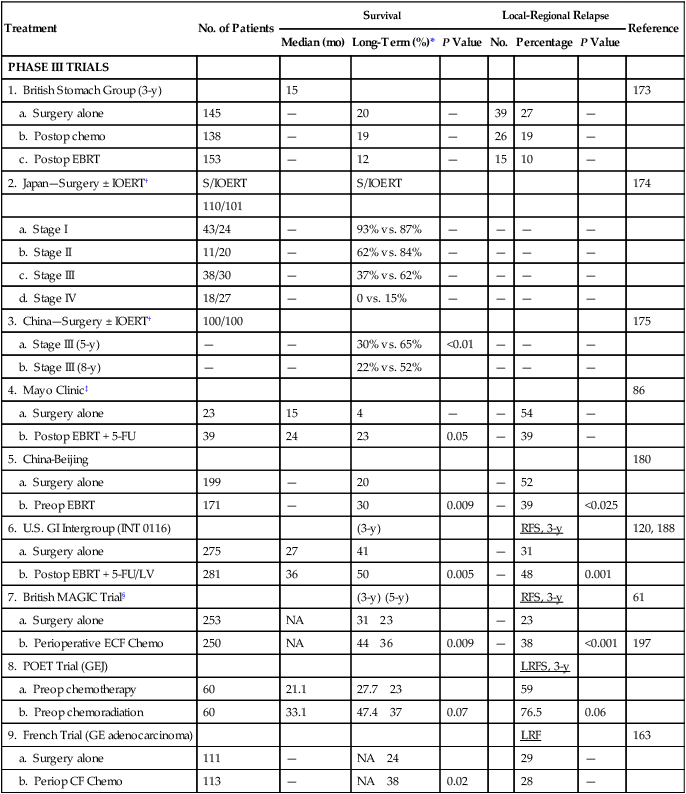
*Long-term survival = 5-year data unless otherwise specified.
†Advantage to IOERT in subset analyses—Japan stages II–IV, China stage III (37% of patients).
‡Survival data based on intent to treat, relapse data on actual treatment.
§MAGIC Trial for gastric, esophagus, GEJ cancers: 3-year survival estimated from published survival curves.
Table 75-6
Surgery ± Adjuvant Therapy for Resected Gastric or Gastroesophageal Junction Cancer
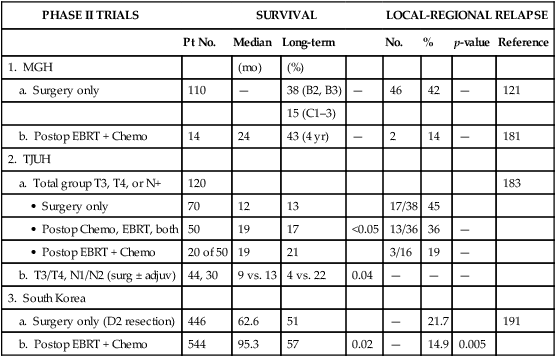
| PHASE II TRIALS | SURVIVAL | LOCAL-REGIONAL RELAPSE | ||||||
| Pt No. | Median | Long-term | No. | % | p-value | Reference | ||
| 1. MGH | (mo) | (%) | ||||||
| a. Surgery only | 110 | — | 38 (B2, B3) | — | 46 | 42 | — | 121 |
| 15 (C1–3) | ||||||||
| b. Postop EBRT + Chemo | 14 | 24 | 43 (4 yr) | — | 2 | 14 | — | 181 |
| 2. TJUH | ||||||||
| a. Total group T3, T4, or N+ | 120 | 183 | ||||||
| • Surgery only | 70 | 12 | 13 | 17/38 | 45 | |||
| • Postop Chemo, EBRT, both | 50 | 19 | 17 | <0.05 | 13/36 | 36 | — | |
| • Postop EBRT + Chemo | 20 of 50 | 19 | 21 | 3/16 | 19 | — | ||
| b. T3/T4, N1/N2 (surg ± adjuv) | 44, 30 | 9 vs. 13 | 4 vs. 22 | 0.04 | — | — | — | |
| 3. South Korea | ||||||||
| a. Surgery only (D2 resection) | 446 | 62.6 | 51 | — | 21.7 | 191 | ||
| b. Postop EBRT + Chemo | 544 | 95.3 | 57 | 0.02 | — | 14.9 | 0.005 | |
The British Stomach Cancer Group completed a prospectively randomized trial of surgery only versus postoperative FAM or EBRT (45 Gy in 25 fractions ± 5-Gy boost).173 A total of 436 patients were randomly assigned and followed for a minimum of 12 months; arms were well balanced with regard to prognostic factors. No patient survival differences by treatment arm were seen (median, 15 months). However, local-regional failure was documented in only 15 of 153 (10%) in the EBRT arm versus 39 of 145 (27%) in the surgery-alone arm, and 26 of 138 (19%) in the FAM group. Interpretation of the results is complicated by the inclusion of 93 patients (21%) with resection but gross residual disease (British Stomach Cancer Group stage IVAi) and 78 (18%) with gross total resection but microscopically positive resection margins. Neither group of patients would be candidates for current gastric surgical adjuvant trials in the United States. In addition, nearly one-third of patients randomly assigned to receive adjuvant treatment did not receive the assigned therapy. Of 153 patients randomly assigned to the EBRT arm, only 104 (68%) received a dose of 40.5 Gy or more, and 36 (24%) received none. Only 62% of patients received six or more cycles of chemotherapy. The results in this study are similar to results seen in the adjuvant treatment of rectal cancer, in which adjuvant pre- and postoperative irradiation as a single adjuvant modality improve local control but do not increase patient survival in most trials, unless combined with chemotherapy.
Intraoperative Irradiation
Takahashi and Abe174 reported results from a large Japanese trial in which 211 patients were randomly assigned on the basis of day of hospital admission to receive either surgery only or surgery plus IORT (28 to 35 Gy). Five-year survival rates for Japanese stages II to IV were improved approximately 15% to 25% in the IORT group versus those treated with surgery alone (stage II, 84% vs. 62%; stage III, 62% vs. 37%; stage IV, 15% vs. 0%). This magnitude of survival improvement correlates nicely with the approximately 20% of patients who fail only local-regionally after complete surgical resection. Although the data are intriguing, this method of randomization is susceptible to bias in treatment selection, and the trial failed to stratify for important prognostic factors.
In an analysis from Beijing, individuals with stage III (serosal involvement or node-positive tumors) or stage IV (unresectable metastasis or adjacent organ involvement) disease were randomly assigned to surgery alone or IORT (single dose, 25–40 Gy).175 In their most recent report of 200 patients, a survival advantage with IORT was demonstrated for only stage III patients (65% vs. 30% 5-year survival; 52% vs. 22% 8-year survival, P < 0.01).
Preoperative Irradiation
Three prospective randomized Russian trials have evaluated preoperative irradiation in potentially resectable gastric cancer.179–179 The first trial randomly assigned 293 patients to receive either surgery alone, surgery after preoperative EBRT (20 Gy in four fractions), or surgery after the same EBRT plus daily hyperthermia. The survival rates at 3 and 5 years were improved in both irradiation arms compared with surgery alone, and the improvement with combined EBRT and hyperthermia was statistically significant at both 3 and 5 years.177 The second trial compared preoperative EBRT (20 Gy) with surgery alone in 279 patients. Both 3- and 5-year survival rates were increased, and no increase in operative morbidity was observed.178 The third trial compared surgery alone with preoperative EBRT (32 Gy with concomitant inhalation of 8% oxygen) plus surgery. A survival advantage was observed with preoperative treatment, and the resection rate was increased by 17%.179 There are some methodologic uncertainties with all three of these trials, and their applicability to Western gastric carcinoma is not clear.
A double-blind randomized trial from Beijing, conducted from 1978 to 1989, compared a surgery-alone control arm (N = 199) with preoperative EBRT plus surgery (N = 171) for individuals with adenocarcinoma of the gastric cardia.180 Irradiation was given with 8-MV photons or cobalt with anteroposterior-posteroanterior (AP-PA) fields to a dose of 40 Gy in 20 fractions of 2 Gy during 4 weeks. Surgery was performed 2 to 4 weeks after completion of irradiation. Both downstaging of disease and improvements in radical resection rates were found with the addition of preoperative EBRT (radical resection rates of 80% vs. 62% with preoperative EBRT vs. surgery alone).
Survival and local-regional disease control were improved in the patients assigned to preoperative EBRT versus surgery alone (see Table 75-6). The 5-year and 10-year survival rates were 30% versus 20% and 20% versus 13%, respectively (P = 0.009, Kaplan-Meier log rank). The divergence in survival curves began in the first year of follow-up and persisted through 9 years. Local-regional disease control were also improved with combined-modality treatment, with local relapse rates of 39% versus 52% (P < 0.025) and regional node relapse rates of 39% versus 54% (P < 0.05). The rates of distant metastases were the same at 24% versus 25%. The improvements in survival and disease control (local-regional) were accomplished with no increase in treatment-related morbidity or mortality rates (operative mortality 0.6% vs. 2.5% with or without preoperative EBRT; intrathoracic leak rates were 1.8% and 4.2%, respectively).






