Cancer of the Rectum
Elin R. Sigurdson, Al B. Benson, III and Bruce Minsky
• Approximately 40,000 new cases of rectal cancer are diagnosed in the United States annually.
• Since 1998, the incidence rate has been decreasing by 2% to 3% per year.
• The peak incidence of rectal cancer is during the fifth decade of life.
• Aspirin and nonsteroidal antiinflammatory drugs have been shown to be effective in the chemoprevention of colorectal cancer by decreasing the risk of adenoma formation as well as the incidence and mortality of colorectal cancer.
Numerous clinical features suggest the presence of rectal cancer, including:
• Located approximately 12 cm from the anal verge
• Rectal bleeding, often bright red and on the surface of the stool
• Subtle changes in bowel habits
• Decreased caliber of stool; mucus in stool
• Sensation of fullness and tenesmus
• Increased straining during defecation
• Synchronous colon cancer (in 2% to 9% of patients with rectal cancer)
• Careful rectal examination yields 67% to 84% accuracy in staging (superficial, mobile, tethered, fixed) and should include pelvic examination for women and prostate examination in men.
• Rigid proctosigmoidoscopy provides the most accurate assessment of distance, size, and position, as well as tethering to surrounding structures.
• Colonoscopy, colonography, or double-contrast barium enema is used to assess for synchronous colon tumors.
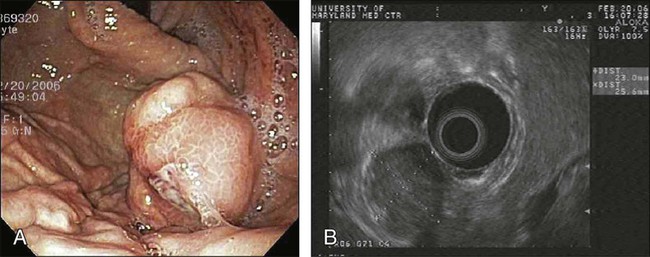
• Endorectal ultrasound can assess the depth of invasion and nodal status. Nodal assessment is less reliable.
• Magnetic resonance imaging (MRI) with endorectal coil and ultrasound are useful to stage rectal cancer and are more sensitive and specific than computed tomography (CT) alone. MRI is used to assess locally advanced or recurrent local disease. CT should be performed on all patients to assess intraabdominal spread. CT or chest x-ray is required to evaluate for synchronous lung metastases.
• The liver is the most frequent site of distant spread, followed by lung, retroperitoneum, ovary, and peritoneal cavity.
• Baseline carcinoembryonic antigen (CEA) levels are assessed and followed postoperatively, even if initially normal.
Differential diagnosis includes:
• Gastrointestinal stromal tumors
• Embryonic tumors (teratomas, chondromas, meningoceles)
• Sacral and presacral tumors (neurogenic tumors, liposarcomas, neurofibromatosis)
• Goals of treatment are cure, local control, and quality of life.
• All retrorectal tumors should be resected, and preoperative biopsy must be avoided.
• Full-thickness local excision is feasible for highly selected patients with T1 mucosal, submucosal, and early invasive cancer, particularly in patients with high-risk comorbidities.
• For T1 to T3 rectal adenocarcinomas, surgical procedures are total mesorectal excision, low anterior resection, low colorectal or coloanal anastomosis with J pouch, and abdominoperineal resection, leaving at least a 2-cm distal margin and clear lateral margins. With surgery, mortality rates are 1% to 7% and morbidity rates are 13% to 46%. The survival rate at 5 years is 74% to 87%.
• Combined therapy cures 50% of N1 patients; 25% of tethered or fixed rectal cancers treated by neoadjuvant chemoradiotherapy are subsequently resected and cured.
• Of patients who die of rectal cancer, 25% fail with pelvic disease only.
Epidemiology
Approximately 40,000 individuals in the United States were diagnosed with rectal cancer in 2012.1 From 1998 through 2006, the incidence rate decreased by 3% per year for men and 2.2% per year for women. Although the incidence rate rises dramatically during the fifth decade of life, the age-adjusted colorectal cancer incidence rates for 1997 to 2006 declined among those age 50 years and older but increased among those younger than 50 years of age.2 Colorectal cancer is the second leading cause of cancer deaths. African Americans are less likely than whites to be diagnosed at a localized stage and have a higher mortality rate than whites for both rectal and colon cancers. In a 32-year period, 1975 to 2007, the gap between the survival rates of African Americans and whites increased from a difference of 6% to 12% for colon cancer and from 3% to 8% for rectal cancer. However, for both colon and rectal cancers, 5-year relative survival rates have significantly increased among all races between 1975 and 1977 and 1999 and 2006.1
Sharpe and colleagues reported an observational study that showed a positive association between cigar smoking and cancer of the rectum. They also noted a weak positive association between cigarette smoking and cancer of the proximal colon.3 Several large cohort studies have shown that cigarette smoking is an independent risk factor for colorectal cancer.4–9 In a large cohort study of more than 22,000 healthy male physicians ages 40 to 84 years who were followed up for more than 12 years, cigarette smoking was an independent risk factor for colorectal cancer incidence, the strongest risk being observed in current smokers of 20 cigarettes or more per day (relative risk: 2.14). Cumulative lifetime exposure and exposure during various periods of life also increased the risk of colorectal cancer.10
One study showed that the increased risk of colorectal cancer associated with cigarette smoking is dependent on the molecular characteristics of the tumor as defined by APC mutation and hMLH1 expression status. The association between frequency of cigarette smoking (for a 5 cigarette/day increment) and colorectal cancer was most apparent and stronger in tumors without a truncating APC mutation, whereas duration of smoking was associated with increased risk in hMLH1-deficient tumors.11
Conversely, aspirin and nonsteroidal antiinflammatory drugs have been shown to be effective in the chemoprevention of colorectal cancer by decreasing the risk of adenoma formation as well as the incidence and mortality of colorectal cancer. Trials have shown that daily aspirin reduces the risk of recurrent colorectal adenoma by 17% to 21% and advanced adenoma by 28%, and that daily aspirin for 5 years reduces incidence and mortality associated with colorectal cancer by 30% to 40% after 20 years of follow-up, and reduces the 20-year risk of all-cause cancer mortality by approximately 20%. Recent evidence also shows that the risk of major bleeding on aspirin diminishes with prolonged use, suggesting that the balance of risk and benefit favors the use of daily aspirin in primary prevention of colorectal and other cancers. In the general population, a significant 26% reduction in colorectal cancer incidence was demonstrated in studies with a 23-year follow-up. In individuals with a history of adenomas, nonsteroidal antiinflammatory drug use was associated with a statistically significant 55% reduction in advanced adenoma incidence and 34% reduction in adenoma recurrence risk.12 Aspirin and celecoxib may be effective in preventing adenomas in patients after polypectomy.13
Clinical Presentation, Evaluation, and Staging
The importance of a detailed history and a thorough physical examination cannot be overstressed. Comorbid conditions and the patient’s physical habitus may preclude major surgery and influence the decision of adjuvant therapy. Physical examination should always include a digital rectal examination to feel for a mass, assess its location and mobility, and feel for enlarged extrarectal lymph nodes (50% accuracy). Depth of invasion and whether the tumor is tethered or fixed can also be assessed during rectal examination with 67% to 84% accuracy.14,15 A careful pelvic examination in women and a prostate assessment in men are essential. A rigid proctosigmoidoscopic examination of the rectum and the anus should follow. The distance of the tumor from the anal verge, anterior/posterior/lateral position, size, morphologic configuration, and extent of circumferential involvement are determined. Tumor mobility and tethering to surrounding structures are ascertained. If not obstructed, patients with rectal cancer should have a preoperative double-contrast barium enema or preferably a colonoscopy to assess for synchronous colon cancer (2% to 9%). Subjective and objective assessment of the patient’s anal sphincter function is desirable. A weak or incompetent sphincter may indicate the need for a colostomy.
Endorectal ultrasound provides valuable preoperative staging (Fig. 78-1), including depth of tumor invasion into the rectal wall (89% to 92% accuracy,16,17 96% sensitivity, 90% specificity, 96% negative predictive value18) and nodal enlargement (79% sensitivity, 74% positive predictive value, 84% negative predictive value19), but confirmation of nodal metastasis with ultrasound-guided needle biopsy is less reliable (77% accuracy, 71% sensitivity, 89% specificity, 92% positive predictive value, and 62% negative predictive value20). Malignant nodes are differentiated from reactive nodes by being hypoechoic, hypervascular, and irregular.21,22 Endorectal ultrasonography and magnetic resonance imaging (MRI) with endorectal coil exhibited similar accuracy and were superior to conventional computed tomographic (CT) scans in preoperative assessment of depth of invasion and adjacent organ invasion.23 High-resolution MRI (83% accuracy, 94% sensitivity, and 67% specificity) and positron emission tomography (PET)-CT (70% accuracy, 61% sensitivity, and 83% specificity) may be helpful in predicting nodal status.24 Improved diagnostic staging information is essential in considering local treatment for rectal cancer, deciding on selective use of preoperative chemoradiotherapy in locally advanced tumors, and choosing between an abdominoperineal and low anterior resection. Both MRI and PET are being investigated for the assessment of pathological response following neoadjuvant therapy.25,26
The liver is the most frequent site of metastasis, followed by the lung, retroperitoneum, ovary, peritoneal cavity, and, rarely, the adrenal glands or bone. Contrast-enhanced CT scan of the abdomen and the pelvis is recommended in all patients with rectal cancer, excluding the very elderly and those with very early cancer, such as cancer within a polyp or T1 rectal cancer. MRI is reserved for patients with locally advanced and recurrent rectal cancer requiring an exenterative procedure. A plain chest radiograph is useful and economical for screening for lung metastasis. Laboratory studies should be ordered as indicated by the patient’s medical condition and anesthetic requirements. Measurement of the carcinoembryonic antigen (CEA) level in combination with imaging can refine the accuracy of preoperative assessment and overall prognosis. Up to 95% of patients with advanced hepatic metastasis will have a CEA level above 20 ng/mL.27 Patients with a normal CEA prior to colorectal resection may still have an elevated CEA with recurrence, so follow-up should include CEA testing postoperatively. Postoperative CEA monitoring may only confer minimal survival advantage. A review of a prospective database of 1900 patients treated for primary colorectal cancer whose follow-up included CEA monitoring found that two-thirds of recurrences were associated with an elevated CEA, which, in turn, was associated with decreased survival. However, of all patients who underwent potentially curative re-resection, only 17% had an elevated CEA.28
Differential Diagnosis
Kaposi sarcoma of the rectum should be suspected in patients with acquired immunodeficiency syndrome who are seen with an unusual or atypical anorectal lesion. It is often associated with proctalgia (62%), hematochezia (50%), and diarrhea (50%).29 Rectal carcinoids are often found incidentally during a screening colonoscopy or typically present with symptoms of bleeding, rectal pain, or constipation. They tend to be more indolent and less aggressive than colonic carcinoids, but as with most gastrointestinal carcinoids, tumor size correlates with the risk of metastasis and survival rates. Endoscopic resection is often adequate. Gastrointestinal stromal tumors of the rectum (Fig. 78-2) are uncommon and often present as a source of lower gastrointestinal bleeding, rectal pain, or constipation. Because of their malignant potential and recent advances in their management with imatinib mesylate (Gleevec), it is imperative that these tumors be correctly diagnosed. Positive immunohistochemical staining with CD34 and CD117 confirms the diagnosis. For large or low-lying rectal gastrointestinal stromal tumors, neoadjuvant therapy with imatinib can facilitate local and sphincter-preserving excision. Except for inflammatory masses, developmental cysts (such as dermoid, epidermoid, duplication, and tailgut cysts) and embryonic tumors (such as teratomas, chondromas, and meningoceles) are the most common retrorectal tumors. Other sacral and presacral tumors include neurogenic tumors, liposarcoma, and neurofibromatosis. Sacral pain and the sensation of fullness in the perirectal area are the most common symptoms of retrorectal lesions.30 Digital rectal examination is the most important diagnostic maneuver. Posteroanterior and lateral radiographs of the sacrum and CT scanning are the preferred methods for characterization and differential diagnosis of retrorectal masses. MRI may also aid in planning the operative approach. Barium enema evaluation will confirm the presence of mass effect. Proctoscopy, although indicated, is usually normal. Most retrorectal lesions should be resected when diagnosed, even if they are asymptomatic and seem benign. Preoperative biopsy is generally not recommended, as it will not change the surgical need for resection and may contaminate the surgical field or lead to abscess formation. Biopsy is reserved for unresectable large retrorectal tumors.
Surgical Treatment of Resectable Rectal Cancer
For most patients with early rectal cancer (T1 to T3), surgical resection is the primary treatment modality. Sound surgical techniques and adjuvant therapy can improve outcomes and maximize local and overall control rates. Tumors in the upper third of the rectum have their lowermost edge 12 cm from the anal verge. Anterior resection or low anterior resection is the primary surgical procedure. Middle and lower-third rectal cancers can be treated with restorative proctectomy with colorectal or coloanal anastomosis or abdominosacral resection with results similar to those that are achieved with abdominoperineal resection and permanent colostomy.31 Overall surgical success depends on the ability to obtain a 2-cm distal margin; surgical expertise in obtaining clear lateral margins; the patient’s body habitus, pelvic width, and prostate size; adequate collateral blood flow through the marginal artery; and whether or not there is associated colonic disease such as diverticulosis. Local approaches may be appropriate for patients with early rectal cancer within 8 cm from the anal verge and in patients with major medical contraindications to radical surgery.
Local Treatment
Selection Factors
Selection factors for full-thickness local excision are the same as or similar to those used for endocavitary radiation therapy. Consequently, this decision is based largely on findings of a digital rectal examination with increasing integration of transrectal ultrasound or MRI with endorectal coil.20,23,32 Patients with T1 tumors without adverse pathological features have a low incidence of local failure (5% to 10%) or lymph node involvement (<10%). With unfavorable pathological features (lymphovascular invasion, high grade, deep submucosal invasion, signet ring cell, or colloid histology)33–36 or evidence of tumor invasion into or through the muscularis propria,35,37,38 the local recurrence rate is at least 17%, and the risk of regional lymph node involvement is at least 10% to 15%.33 In an analysis by the Massachusetts General Hospital of 40 patients who underwent local excision only, patients were categorized according to unfavorable clinical or pathological features.35 Among patients with T1 or T2 cancers following local excision, Blumberg and associates reported positive lymph nodes in 10% of T1 cancers and 17% of T2 cancers.39 In addition, among the total group of 159 patients, the incidence increased with the presence of lymphatic and vascular invasion (14% without vs. 33% with). Even among the 42 patients with the most favorable features (negative lymphatic and vascular space involvement, well- or moderately differentiated T1 cancers), 7% were found to have lymph node involvement. The overall 5-year survival rate for the whole group was 65% with a locoregional recurrence rate of 27%. Hager and associates reported on a series of 20 patients with T2 rectal cancer for which local excision was performed and who were otherwise thought to be “low risk” (well to moderately differentiated, nonmucinous, no lymphovascular invasion, and negative margins), despite which the incidence of locoregional failure was still 17%.37 Others have reported locoregional failure rates as high as 43% following either local or transanal excision in patients with T2 cancers.40 There have been no randomized trials that compared transanal full-thickness local excision alone for T1/T2 with or without adjuvant chemoradiation with low anterior resection or abdominoperineal resection. Recent published results of local excision of T1/T2 rectal lesions without adjuvant therapy show local recurrence rates of 3.4% to 18% for T1 lesions and 27% to 67% for T2 lesions; Table 78-1 summarizes these results.
Table 78-1
Local Excision of T1, T2 Lesions Without Adjuvant Therapy in Selected Series with More Than 50 Patients
| Reference | No. of Patients (Per Stage) | Follow-up Time | LR | Survival Rate | Salvage Surgery for Isolated LR |
| Paty et al.*358 | 125 (T1 = 74, T2 = 51) | 6.7 years | T1 = 17% T2 = 26% | 10-Year OS: T1 = 74%, T2 = 72% | 14/17 |
| Mellgren et al.359 | 108 (T1 = 69, T2 = 39) | 4.4 years | T1 = 21% T2 = 47% | 5-Year OS: T1 = 72%, T2 = 65% | 24/27 |
| Garcia-Aguilar et al.360 | 83 (T1 = 55, T2 = 27) | 54 months | T1 = 18% T2 = 37% | 5-Year: T1 = 98%, T2 = 89% | 17/20 |
| Chakravarti et al.296 | 52 (T1 = 44, T2 = 8) | 52 months | 28% | 5-Year DFS: 66 | NS |
| Steele et al.361 | 59 (T1 = 59) | 48 months | T1 = 5% | 6-Year survival: 85 | 2/2 |
| Kim and Madoff362 | 69 (T1 = 44, T2 = 25) | NS | T1 = 9% T2 = 28% | Cancer-specific 5-year survival: 88% | NS |
| Hager et al.363 | 59 (T1 = 39, T2 = 20) | 33–40.5 months | T1 = 8% T2 = 17% | 5-Year survival: T1 = 90%, T2 = 78% | NS |
| Nelson et al.364 | 124 (T1=60, T2=164) | 5 years | T1=12.5% T2=22% | 5-Year OS: T1 = 77.4%, T2 = 67.6% | NS |
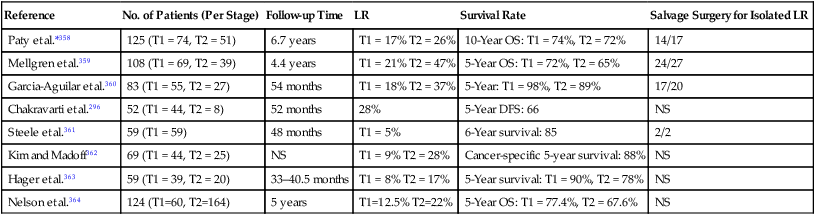
DFS, Disease-free survival; LR, local recurrences; NS, not specified; OS, overall survival.
*In this series, 16 patients received postoperative radiotherapy and 15 additional patients received postoperative 5-FU and radiotherapy; however, local and overall recurrence rates were similar in both groups.
Local Approaches
Optimal candidates for local excision along include small (<4 cm), noncircumferential (<40%), low-lying tumors confined to the muscularis propria, without adverse pathological features (signet ring histology, poor differentiation, lymphovascular invasion). Local excision does not include lymph node evaluation, and adjuvant radiation with or without chemotherapy may be warranted.41
TEM is a minimally invasive surgical technique that was introduced in 1984 by Buess. It incorporates a high-quality binocular operating system and pressure-regulated insufflation with continuous suction. Compared with conventional transanal resection, TEM provides superior intraoperative visualization and the ability to perform full-thickness excision of the tumor with clear margins, together with perirectal fat and adjacent lymph nodes42,43 of tumors higher up in the rectum (4 to 18 cm from the anal verge). The technique is not yet generally established because of the high cost, the necessary special instrumentation and tools, and the unusual technical aspects of the approach.43–51
Treatment of cT2 rectal tumors with TEM combined with preoperative high-dose radiotherapy in 35 patients achieved survival rates similar to those of conventional open surgery.52 Only minor postoperative complications occurred in five (14.3%) patients and included suture-line dehiscence in three patients and stool incontinence in two patients. At a median follow-up period of 38 months, one patient was seen with a local recurrence (2.9%) at 30 months of follow-up, and systemic metastasis developed in four patients (11.4%). The survival and local recurrence rates that were reported in that study led to a prospective multicenter randomized trial (the so-called Urbino trial) to evaluate the efficacy of local excision in T2 tumors that were preoperatively treated by chemotherapy and high-dose radiotherapy versus standard open treatment (low anterior resection or abdominoperineal resection). At a median follow-up period of 56 months (range: 44 to 67 months), the local failure rate (5%) and distant metastasis rate (5%) were equal in the two groups.53
A recent review of the United Kingdom national TEM database from 21 centers since 1993 showed that of 454 rectal cancer patients, 69% underwent TEM with curative intent. The overall morbidity and mortality of TEM were 17.2% and 1.5%, respectively. Pathological staging was as follows: pT0 (1.8%), pT1 (52.9%), pT2 (32.8%), pT3 (9.9%), and pTx (3.1%). Neoadjuvant therapy and adjuvant radiotherapy were administered in 8% and 18% of cases, respectively. Margin positivity (<1 mm) occurred in 20% of cases and was stage dependent. The 5-year disease-free survival rate was 77% for pT1, 74% for pT2, and 35% for pT3, with local recurrence rates of 20%, 25%, and 59%, respectively.54
Radical Resections
Sharp, total mesorectal excision (TME) with autonomic nerve preservation is the radical surgical technique of choice in conjunction with low anterior resection or abdominoperineal resection (APR). The mortality rate is 1% to 7%, and the morbidity rate (including genitourinary dysfunction, fecal incontinence, and permanent colostomy) is 13% to 46%. Locoregional recurrent disease is observed in 4% to 20% of cases, and the 5-year survival rate is 74% to 87%.55–59
Surgical Issues in Radical Resections
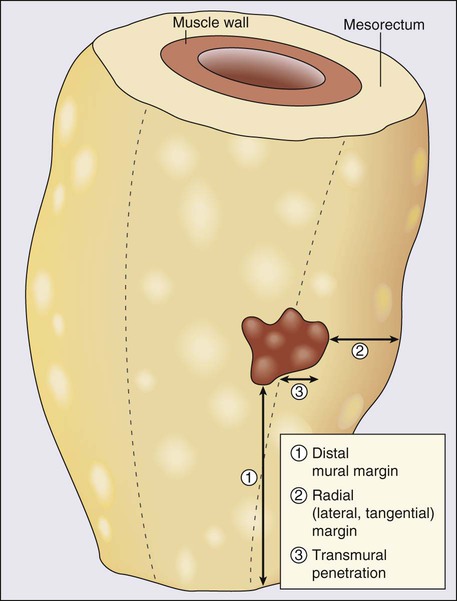
Radial Margins and Total Mesorectal Excision
The ability to obtain a negative lateral circumferential margin is associated with a decreased risk of local recurrence.60–63 In a multivariate analysis, circumferential margin involvement was the most powerful predictor of local recurrence (hazard ratio: 12.2) and of overall cancer mortality (hazard ratio: 3.2; Fig. 78-3). Heald and colleagues have advocated total mesorectal excision (TME) in conjunction with low anterior resection or abdominoperineal resection (APR) as the optimal surgical treatment for rectal cancer.56 This technique involves removal of the entire rectal mesentery, including that distal to the tumor, as an intact unit. Complete distal TME is essential for clearance of any tumor deposits, which occur in 50% of T3 tumors with a maximal distal spread of 4 to 5 cm,64 and is associated with increased frequency of local recurrence and decreased overall survival.65,66
Distal Mucosal Margin
The ability to perform sphincter-preserving surgery in nonirradiated patients is dictated by the requirements of a 2-cm distal margin rather than the traditional 5-cm margin.67–71 Only 2.5% of patients (usually with poorly differentiated and node-positive rapidly disseminating disease) had disease spread greater than 2 cm.31 There is no correlation between risk of local recurrence and the extent of distal margin in excess of 2 cm.72–77
Proximal Extent of Lymph Node Dissection
Proximal lymph node dissection should extend just distal to the origin of the left colic artery. No evidence indicates a relationship between local recurrence and survival and dissection of deep iliac lymph nodes78 or high ligation of inferior mesenteric pedicle.79,80 Patients with pathologically positive nodes along the inferior mesenteric artery have very low 5-year survival rates.81,82
Laparoscopic Surgery
Laparoscopic surgery for curable rectal cancer has become increasingly accepted. Laparoscopic anterior resection with curative intent generates considerably more reservations than does laparoscopic APR, which is technically much easier to perform. Data, including results from randomized trials, on the extent of lymphadenectomy, margins of resection, actuarial survival, and local recurrence rates continue to emerge. The technique of laparoscopic TME is well described by Pikarsky and associates.70 Reports suggest short-term gains of reduced pain, shortened hospital stay, accelerated activity, possible cost reduction, and improved cosmesis.71 Hand-assisted laparoscopic surgery is a new technique that has the potential to overcome many of the existing limitations of pure laparoscopy.83 A prospective nonrandomized single institution trial comparing open versus laparoscopic resection in 191 consecutive patients with low and midrectal cancer demonstrated a conversion rate of 18.4%. In the laparoscopic group, the mean time for complete patient mobilization was shorter (1.7 vs. 3.3 days; P < 0.001), and patients were earlier in passing flatus (2.6 vs. 3.9 days; P < 0.001) and stools (3.8 vs. 4.7 days; P < 0.01) and in resuming oral intake (3.4 vs. 4.8 days; P < 0.001). The mean hospital stay and overall morbidity and mortality rates were similar with no statistically significant differences. Laparoscopic patients had a higher rate of anastomotic fistulas (13.5% vs. 5.1%) and reoperations (6.1% vs. 3.2%), but the difference was statistically nonsignificant. Laparoscopic resection presented a significantly lower local recurrence rate (3.2% vs. 12.6%; P < 0.05). Although the cumulative survival and disease-free rates at 5 years were nonsignificant between both groups (80% and 65.4% after laparoscopic surgery and 68.9% and 58.9% after open surgery), stage-by-stage comparison showed prolonged cumulative survival for stages III and IV cancer in the laparoscopic group (82.5% vs. 40.5%; P = 0.006 and 15.8% vs. 0%; P = 0.013, respectively) and a reduced rate of cancer-related death for stage III in the laparoscopic group (11.4% vs. 51.9%; P = 0.001).84
The U.K. Medical Research Council (MRC) prospective multicenter, randomized, controlled trial of conventional versus laparoscopic-assisted surgery in colorectal cancer included 128 rectal cancer patients in the open group and 253 patients with rectal cancer in the laparoscopy-assisted group with intent to treat. (The actual treatment groups included 132 open and 160 laparoscopy-assisted operations). Approximately 10% more patients underwent TME in the laparoscopy-assisted group than in the open surgery group. The rate of APR was similar (27% in the open surgery group and 25% in the laparoscopic group). Lymph node yield was equally high in the two groups. The intraoperative conversion rate from laparoscopic to open was 34%. In the intent-to-treat population, there was no significant difference in the open versus laparoscopy-assisted group with regards to positive circumferential margin (14% vs. 16%), overall intraoperative complications (14% vs. 18%), overall morbidity (50% vs. 59%), and mortality (5% vs. 4%). Although laparoscopic rectal resection did not adversely affect bladder function, there was a trend toward worse male sexual function, which might be explained by the higher rate of TME in the laparoscopic rectal resection group.85,86
In a 2006 systematic review by the Cochrane group, it was noted that most reported laparoscopic surgery studies for rectal cancer are individual cohort studies, individual case-control studies, or case series with only one reported randomized, controlled trial. Collectively, in 48 studies representing 4224 rectal cancer patients, there appeared to be no significant differences in disease-free survival rate, local recurrence rate, mortality, morbidity, anastomotic leakage, resection margins, or number of lymph nodes harvested. Laparoscopic approaches are generally associated with less blood loss, quicker return to normal diet, less pain, and less narcotic use, but are also associated with longer operative time and higher costs, and no results of quality of life were reported.87
A more recent meta-analysis of trials reporting oncologic outcomes for laparoscopic rectal resections included 1403 laparoscopic and 1755 open rectal resections from 24 publications; 5 of the 24 studies were prospective randomized trials, and long-term survival estimates were reported. This review found that although laparoscopic procedures harvested fewer lymph nodes (10 vs. 12), 3-year overall survival (76% laparoscopic, 49% open) and mean local recurrence (7% laparoscopic, 8% open) were not statistically different. There was also no difference in radial margin positivity.88
Patterns of Failure
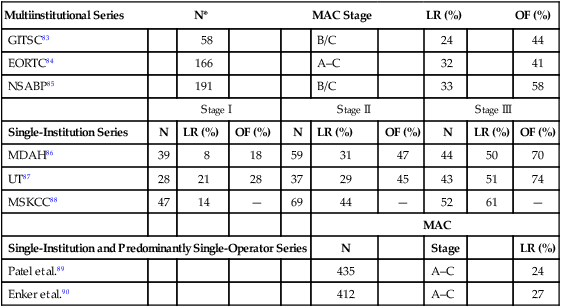
Following potentially curative standard or conventional surgical resection for adenocarcinoma of the rectum, the incidence of locoregional or distant treatment failure is related to the extent of transmural disease and associated involvement of regional lymph nodes by metastases.89–96 The incidence of locoregional failure is 8% to 21% in American Joint Committee on Cancer (AJCC) stage I disease (modified Astler Coller [MAC] stages A/B1), 29% to 44% in AJCC stage II disease (MAC stages B2/B3), and 50% to 61% in AJCC stage III disease (MAC stage C). The incidence of distant failure (as a component of failure) is up to 28% in AJCC stage I disease, up to 47% in AJCC stage II, and up to 74% in AJCC stage III disease. It has been claimed that when one compares these patterns of failure between multiinstitutional trial settings versus single-institutional and predominantly single-operator (surgeon) series, great differences in results can be seen. Table 78-2 summarizes these patterns of failure according to multiinstitutional versus single-institutional/single-operator series.89–96 Although distant metastasis is most likely to be attributed as the cause of death in rectal cancer patients, the potential influence of locoregional failure as an antecedent event to the development of distant metastases is clinically important. Hence, decreasing locoregional failure is an important end point of treatment in rectal cancer. These data and rationale serve as the basis for consideration of adjuvant chemoradiotherapy in the management of rectal cancer and, in particular, as a standard for AJCC stage II (MAC stages B2/B3) and stage III disease (MAC stage C). It is important to note that limited retrospective data identify subsets of patients with stage I disease who may be considered for adjuvant therapy, as well as subsets of patients with T3N0 disease who may not require adjuvant therapy.97,98 Willett and associates identified a subset of patients with stage I disease who have an increased incidence of locoregional failure following APR.97 In an additional review of 117 patients with T3N0 disease, Marks and associates identified a favorable group of patients with moderately or well-differentiated cancers invading less than 2 mm into perirectal fat who had a 10-year actuarial locoregional failure rate of only 5% following surgery alone, compared with 29% in T3N0 patients without these favorable features.99
Table 78-2
Patterns of Locoregional Recurrence and Overall Failure Following Standard/Conventional Surgery Alone for Rectal Cancer
| Multiinstitutional Series | N* | MAC Stage | LR (%) | OF (%) | |||||
| GITSC83 | 58 | B/C | 24 | 44 | |||||
| EORTC84 | 166 | A–C | 32 | 41 | |||||
| NSABP85 | 191 | B/C | 33 | 58 | |||||
| Stage I | Stage II | Stage III | |||||||
| Single-Institution Series | N | LR (%) | OF (%) | N | LR (%) | OF (%) | N | LR (%) | OF (%) |
| MDAH86 | 39 | 8 | 18 | 59 | 31 | 47 | 44 | 50 | 70 |
| UT87 | 28 | 21 | 28 | 37 | 29 | 45 | 43 | 51 | 74 |
| MSKCC88 | 47 | 14 | — | 69 | 44 | — | 52 | 61 | — |
| MAC | |||||||||
| Single-Institution and Predominantly Single-Operator Series | N | Stage | LR (%) | ||||||
| Patel et al.89 | 435 | A–C | 24 | ||||||
| Enker et al.90 | 412 | A–C | 27 | ||||||
Adjuvant Therapy
Treatment Sequencing Issues
Preoperative Versus Postoperative Therapy: Potential Advantages and Disadvantages
Table 78-3 summarizes the advantages and disadvantages of preoperative (typically chemoradiation) versus postoperative adjuvant therapy. The major advantages of preoperative therapy are tumor downstaging with increased resectability and sphincter preservation as well as a reduced incidence of acute and chronic toxicity. Adequate doses of radiation (≥4500 Gy) can sterilize peripheral margins of disease.96 Marginally resectable and unresectable tumors can undergo tumor shrinkage, making them amenable to curative surgical resection, particularly within the confines of the ridged, funnel-shaped bony pelvis, which often limits the potential for adequate circumferential margins of resection.63 Preoperative therapy also allows tumors to be resected with limited longitudinal surgical margins, thereby extending the level to which sphincter-sparing procedures can be performed safely in the distal rectum. These advantages, in turn, are associated with the potential for a significant reduction in a source of tumor spillage associated with locoregional recurrence of disease as well as a reduction of the dissemination during surgery of viable tumor cells increasing the risk for developing distant metastatic foci. The potential therapeutic advantage of preoperative therapy (particularly radiation) with enhanced oxygenation before surgical disruption of tumor blood supply is well established.100,101
Table 78-3
Advantages of Preoperative Versus Postoperative Adjuvant Therapy*
| Advantage | Preoperative Therapy | Postoperative Therapy |
| Tumor downstaging | + | – |
| Increased tumor resectability | + | – |
| Increased sphincter preservation | + | – |
| Treatment based on operative/pathological findings | – | + |
| Decreased locoregional recurrence | ++ | + |
| Increased survival | + | – |
Preoperative therapy also has the potential advantage of reducing the risk of treatment of both chemotherapy- and radiation-related morbidity compared with that seen with postoperative therapy.104–104 Following surgical resection, adhesions often develop and cause loops of bowel to be fixed within the pelvis. These fixed bowel loops often show enhanced tissue reaction with associated bacterial invasion, increasing the risk of severe treatment-related complications. In the preoperative therapy setting, the small bowel is less likely to be fixed within the treatment field and thereby is less prone to both acute and chronic treatment-related injury.
Optimal Timing of Surgery Following Preoperative Therapy
The most common interval between the end of radiation and surgery is 6 to 8 weeks. Until the publication of the Lyon R90–01 randomized trial,105 the optimal timing of surgery following preoperative therapy in rectal cancer was based on hypothesis or retrospective data. This study randomly assigned 201 patients with stage cT2/T3, NX, M0 into two treatment groups: (a) the short-interval group, in whom surgery was performed within 2 weeks of completion of preoperative radiation therapy (39 Gy and 13 fractions) versus (b) the long-interval group, in whom surgery was performed within 6 to 8 weeks after completion of preoperative radiation therapy. At a median follow-up time of 33 months, there was no difference in morbidity, local recurrence, or short-term survival between the two groups. These findings, along with the previously demonstrated findings that rectal cancers undergo slow tumor shrinkage over several months after radiation,106 lend further support to the rationale that a longer delay before surgery, particularly in locally advanced tumors, might be desirable to allow for maximal tumor regression prior to surgery. Current randomized trials are systematically investigating the optimal timing of surgery to as long as 12 weeks following completion of neoadjuvant therapy.
In the most extreme example of delayed surgical intervention, Habr-Gama and colleagues have reported their continuing series of patients in whom they have delayed surgical intervention indefinitely following a clinical complete response (cCR) that was assessed 8 weeks following neoadjuvant chemoradiation by clinical, endoscopic, and radiographic studies.107,108 This approach is considered highly investigational; however, it is thought-provoking in terms of a treatment paradigm mirroring that for patients with cancers of the anal canal, in whom surgery is reserved for salvage of chemoradiation failures in patients who achieve a cCR to initial treatment.
Optimal Timing of Adjuvant Radiation Following Surgery
Lee and associates reported the results of a phase III study of postoperative adjuvant therapy in stages II and III rectal cancer that was designed to define the optimal sequencing of chemotherapy and radiation.109 In this study, 308 patients were randomly assigned to early versus late radiation. Patients received 45 Gy in 25 fractions with 8 monthly cycles of 5-fluorouracil (5-FU)+leucovorin chemotherapy. Radiation began with the start of chemotherapy in the early radiation group versus with the start of the third cycle of chemotherapy in the late radiation group. With a median active follow-up of 37 months, the disease-free survival rate was significantly improved in the early radiation group compared with the late radiation group (81% vs. 71% at 4 years; P = 0.043). This finding was associated with an increase in both distant and locoregional disease recurrence in the late radiation group and with an overall recurrence rate of 17% in the early radiation group versus 27% in the late radiation group (P = 0.047). Although overall survival was not significantly different between the treatment arms, these results suggest that the timing of adjuvant postoperative radiation can have a significant impact on the outcome of patients with rectal cancer.
Radiation Treatment Planning
The relative frequency and sites of pelvic failures were delineated by the seminal work of Gunderson and Sosin.110 In this reoperative series of 75 patients, 69% of locoregional failures occurred in the soft-tissue of the pelvis or the anastomotic site, 42% developed also or exclusively in pelvic lymph nodes, in 25% the perineum was involved. A more contemporary series of 269 patients by Hruby et al. confirmed that the majority of local failures occurred in the posterior central pelvis (47%) or at the anastomosis (21%), whereas anterior recurrences (11%) were mainly restricted to T4 tumors. Perineal recurrences occurred in 16% of patients who underwent APR.111
Irradiation Field Design
The whole pelvic radiation field should adequately cover the primary tumor/tumor bed as well as the primary nodes at risk. The intent of the boost is to treat the primary tumor and not to include the nodes. The exact size is determined by the size and location of the primary tumor. Whole pelvic and boost fields are usually treated with a three-field (posteroanterior and lateral) technique for patients receiving conventional treatment. Field shaping by blocks is used to spare additional small intestine anteriorly and superiorly, the posterior muscle and soft tissue behind the sacrum, and inferior to the symphysis pubis. Standard treatment involves the use of 3D treatment planning which provides the ability to plan and localize the target and normal tissues at all levels of the treatment volume, and to obtain dose volume histogram data. Guidelines for the definition and delineation of the clinical target volumes,112,113 as well as examples of conventional radiation field design,114 are routinely available.
The clinical utility of intensity-modulated radiation therapy (IMRT) treatment planning techniques is being investigated.115,116 IMRT treatment planning techniques can further decrease the volume of small bowel in the field.117 A retrospective analysis of 92 patients treated with either IMRT (n = 31) or conventional chemoradiation (n = 61) at the Mayo Clinic revealed that IMRT was associated with significantly less grade II+ gastrointestinal (GI) toxicity (32% vs. 62%).118 There were no significant differences in hematologic or genitourinary toxicity or in the rate of pathological complete response (pCR). The ultimate clinical benefit of IMRT compared with 3D or conventional treatment delivery is still being investigated.115,119 Figure 78-4 shows examples of 3D and IMRT treatment plans.
Some patients may benefit from being treated on a “belly board” to allow for displacement of the small bowel up and out of the pelvis (Fig. 78-5). Nijkamp et al. showed that in 11 volunteers simulated for IMRT with a full bladder, a belly board had added value in decreasing bowel volume.120 A systematic review of 33 reports revealed that treatment in the prone position resulted in a lower volume of small bowel treated versus a supine position. However, a more significant reduction could be obtained with the use of the prone position plus a belly board.121 Using a 3D planning system, Koelbl and associates found that in patients receiving postoperative radiation, the use of the prone position plus a belly board decreased the small bowel volume treated versus the supine position.122
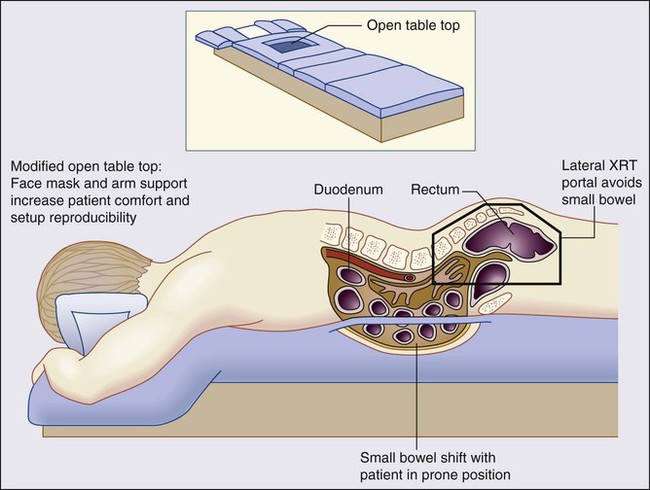
Brierley et al. analyzed the variation of small bowel volume in the pelvis before and during adjuvant pelvic radiation therapy for rectal cancer and reported that the displacement of small bowel from the posterior pelvis by bladder distention was not reliably maintained throughout the treatment course.123 Therefore, any physical maneuver beyond the prone position may not be beneficial in all patients and should be tailored to the individual patient.
The role of hyperfractionated radiation has been examined in phases I/II trials.124 In general, the pCR rates may be improved but at the expense of increased acute toxicity. Coucke et al. treated 250 assessable patients with cT3 to cT4 and/or N+ disease with 41.6 Gy at 1.6 Gy BID and reported a 92% actuarial 5-year local control rate but survival of only 60%.125 Acute grade 3+ toxicity in the 5-FU plus twice-a-day radiation arm of Radiation Therapy Oncology Group (RTOG) R-0012 (1.2 Gy to 45.6 Gy, with a boost of 9.6 Gy to 14.4 Gy) was 42%. Hyperfractionated and accelerated fractionated radiation, especially in combination with chemotherapy, remains investigational.
Other investigational approaches, such as neutrons,126 carbon ions,127 protons,128 and hyperthermia,131–131 have been examined. An analysis of 3D treatment planning techniques suggests that the volume of small bowel in the radiation field is decreased with protons as compared with photons.132 Although treatment plans show improved normal tissue sparing, clinical studies have not shown a clear advantage compared with conventional 3D photon-based pelvic radiation therapy.
Irradiation Dose
A meta-analysis concluded that biologically effective preoperative doses above 30 Gy resulted in a statistically significant reduction in locoregional recurrence.133 With conventional fractionation, the doses necessary to treat microscopic disease are in the range of 45 to 50.4 Gy in 5 to 6 weeks. These doses are necessary to treat microscopic disease.134 A retrospective comparison of 143 patients treated in three phase II trials of chemoradiation with 40 Gy, 46 Gy, and 50 Gy revealed a significant improvement in 2-year survival for those receiving 46 and 50 Gy (94% and 92%, respectively) versus 40 Gy (72%, P = 0.03).135
A boost of 5.4 Gy to the primary tumor or tumor bed may be delivered if the small bowel is excluded from the high-dose field. However, it is not clear that doses greater than 50.4 Gy improve local control. Higher preoperative doses up to 60 Gy are associated with increased pCR rates, however, may also significantly increase acute and long-term morbidity. The RTOG R-0012 phase II randomized trial compared truncated BID preoperative chemoradiation up to 60 Gy (1.2 Gy to 45.6 Gy, with a boost of 9.6 Gy to 14.4 Gy) with conventional fractionation (1.8 Gy to 45 Gy, with a boost of 5.4 Gy to 9.0 Gy) plus 5-FU+irinotecan.136 Both regimens resulted in a 28% pCR rate, but were also associated with a greater than 40% rate of grades 3 to 4 acute toxicity.
In the postoperative setting, if there is incomplete resection (R1 or R2 resection), radiation doses greater than 60 Gy are required. External beam radiotherapy is limited by normal tissue tolerance, and results for patients with residual disease who received postoperative radiation are disappointing.137,138 As is discussed below, intraoperative radiation therapy (IORT) may help to overcome this problem by direct visualization and irradiation of the persistent tumor.
Toxicity of Chemoradiation
Both retrospective and prospective trials reveal that preoperative chemoradiation causes less acute and chronic toxicity compared with the postoperative treatment.103,139 This is likely because small bowel in an unviolated abdomen will be mobile and less likely to be adherent to adjacent pelvic structures. In the German Chirurgische Arbeitsgemeinschaft fur Onkolgie/Arbeitsgemeinschaft Radiologische Onkologie/Arbeitsgemeinschaft Internistische Onkologie (CAO/ARO/AIO)-94 randomized trial of preoperative versus postoperative chemoradiation, grade 3+ GI toxicity was significantly reduced with the preoperative approach (acute: 12% vs. 18%, P = 0.04, and long-term: 9% vs. 15%, P = 0.07).139 Strictures at the anastomotic site were also reduced (4% vs. 12%, P = 0.003). The incidence of small bowel obstruction requiring surgery in the preoperative arm was 2%.
In contrast, in the National Surgical Adjuvant Breast and Bowel Project (NSABP) R-03 trial, patients who received preoperative chemoradiation versus postoperative chemoradiation had a corresponding higher incidence of grade 4+ toxicity (33% vs. 23%), but the incidence of grade 3+ toxicity was lower (41% vs. 50%).140 Although the results are opposite from the German trial, only 267 of the 900 planned patients were accrued, thereby limiting the statistical power.
Cytotoxic agents (5-FU, capecitabine, oxaliplatin, and irinotecan), as well as targeted therapies (bevacizumab and cetuximab), have improved results of patients with colon cancer and are being tested in preoperative chemoradiation programs. Most phases I/II trials suggest higher rates of both pCR and acute toxicity compared with 5-FU. For example, the RTOG R-0012 randomized phase II trial enrolled 106 patients who received preoperative chemoradiation with either continuous infusion (CI) 5-FU plus twice-a-day radiation vs. FOLFIRI plus conventional daily fractionated radiation.136 Although the pCR rates were 26%, the grade 3+ toxicity rates were 42% and 55%, respectively. This was likely a result of the overlapping GI toxicities of pelvic radiation and irinotecan. Three phase III trials have reported significantly higher acute toxicity with no corresponding benefit in the pCR rate with the addition of oxaliplatin to CI 5-FU or capecitabine based chemoradiation.143–143 A fourth trial from Germany reported the opposite results.144 These trials are discussed later.
Complications of pelvic radiation therapy are a function of the volume of the radiation field, overall treatment time, fraction size, radiation energy, total dose, technique, and sequence of radiotherapy.103 Acute side effects, such as diarrhea and increased bowel frequency (small bowel), acute proctitis (large bowel), and dysuria, are common during treatment.145 These conditions are usually transient and resolve within a few weeks following the completion of radiation. The symptoms appear to be a function of the dose rate and fraction size rather than the total dose. The mechanism is primarily the depletion of actively dividing cells in what is otherwise a stable cell renewal system. In the small bowel, loss of the mucosal cells results in malabsorption of various substances, including fat, carbohydrate, protein, and bile salts. Examination during treatment frequently reveals an inflamed, edematous, and friable rectal mucosa. The bowel mucosa usually recovers completely in 1 to 3 months following radiation.
Small bowel–related complications are proportional to the volume of small bowel in the radiation field.146 In patients receiving chemoradiation, the volume of small bowel in the radiation field limited the ability to escalate the dose of 5-FU.103
Delayed complications occur less frequently but are substantially more serious. The initial symptoms commonly occur 6 to 18 months following completion of radiation. Complications may include persistent diarrhea and increased bowel frequency, proctitis, and strictures at the anastomotic site; small bowel obstruction; perineal/scrotal tenderness; delayed perineal wound healing; urinary incontinence; and bladder atrophy/bleeding. Injury to the vascular and supporting stromal tissues is the presumed pathophysiology. Analysis of pooled patients from 1599 patients treated on Swedish rectal cancer trials revealed a 1.5% increase in in-field secondary tumors for those treated with radiation compared with those not receiving radiation.147 However, radiation still had a positive effect on local control.
The most common delayed severe complications are caused by small bowel damage and include small bowel enteritis, adhesions, and small bowel obstruction requiring surgical intervention. The incidence of small bowel obstruction requiring surgery following postoperative pelvic radiation for rectal cancer is 4% to 12% in historical series. In the Massachusetts General Hospital series, the incidence of small bowel obstruction with conventional postoperative radiation therapy was 6% compared with 5% with surgery alone.148 The incidence was 2% in the preoperative chemoradiation arm of the German CAO/ARO/AIO-94 trial.139 Even with appropriate doses and techniques of radiation, approximately 1% of patients will have significant long-term toxicity to pelvic organs. There are a number of radiotherapeutic, surgical, and other general methods to decrease treatment-related toxicity, especially small bowel complications.149
Active inflammatory bowel disease is a contraindication to pelvic radiation although there are some reports of patients who have tolerated it.150,151 Pelvic fractures following pelvic radiation are rare.152,153 Testosterone levels are decreased when the testicles are near or in the radiation field.154,155 Radiation, alone or in combination with surgery can have a negative impact on sexual function.158–158 In the Dutch CKVO trial of short course radiation, patients experienced new or worsening sexual dysfunction following treatment (men: 76%; women: 62%).159 By multivariate analysis, factors that were independent factors included radiation in men and the psychological presence of a stoma in both men and women. The authors concluded that despite the additional effect of radiation, sexual dysfunction was mainly caused by surgery.
Techniques to Reduce and Manage Toxicity
Randomized trials have investigated the use of sucralfate enemas to decrease acute radiation proctitis, olsalazine, and mesalazine to decrease acute enteritis, octreotide acetate to decrease diarrhea,160 and butyric acid to decrease chronic radiation proctitis.161 All of these trials have been negative. The radioprotector WR-2721 did not reduce toxicity in early trials, but there is a suggestion of a benefit in a more recent study.162 Rectally administered amifostine is well tolerated; however, its efficacy remains to be determined.163 Other trials of amifostine have not shown clear benefits.164 The best management of side effects is to decrease their incidence by designing the optimal treatment. There are a number of radiation therapy techniques available to achieve this. Despite their use, toxicities will occur. With appropriate doses and techniques of radiation, 5% to 25% of patients will have acute grade 3+ toxicity and approximately 1% of will have long-term toxicity to pelvic organs.
Surgical techniques to minimize small bowel injury are limited to the postoperative setting. Dexon or Vicryl mesh helps remove the small bowel from the pelvis. However, because the radiation component of postoperative chemoradiation does not begin until at least 4 months after surgery, the mesh may have already resorbed. Other techniques, such as an inflatable pelvic small bowel displacement prosthesis,165 reconstruction of the pelvic floor, construction of an omental pedicle flap, and retroversion of the uterus, have had variable success.
Common treatments by organ sites are as follows:
• Skin: Aquaphor (prophylactically from the start of radiation) in skin folds from the beginning of treatment. During a treatment break 2% Silvadene is preferred; however, it cannot be used during radiation because of secondary electrons from the silver.
• Diarrhea, prescribed in increasing effectiveness: loperamide (Imodium), diphenoxylate and atropine (Lomotil), tincture of opium.
• Dysuria: phenazopyridine (Pyridium).
• Rectal pain, in increasing effectiveness: acetaminophen, acetaminophen with codeine (Tylenol #3), acetaminophen with oxycodone (Percocet).
These treatments as well as careful attention to treatment details will help decrease the acute and long-term toxicity of chemoradiation.
Postoperative Therapy: Results of Randomized Trials
As previously discussed, the major advantage of postoperative therapy in rectal cancer is to recommend treatment on operative/pathological staging (Table 78-4). The National Cancer Institute Consensus Conference concluded in 1990 that chemoradiation was the standard postoperative adjuvant treatment for patients with pT3 and/or N1 to N2 disease.166 This recommendation was based on phase III trials that compared postoperative chemoradiation arms with control arms of either surgery alone or surgery plus postoperative radiation (Mayo Clinic/NCCTG 79-47-51) and demonstrated improvements in both disease-free and overall survival.167 The standard design in U.S. trials was to deliver 6 cycles of bolus 5-FU based chemotherapy, two of which were given with concurrent radiation during cycles 3 and 4.
Table 78-4
Results of GITSG, NCCTG, and NSABP Studies Evaluating Postoperative Adjuvant Therapy for Rectal Cancer
| Type of Therapy | GITSG365 | NCCTG366 | NSABP R-02367 | |||
| LR (%) | 5-Year Survival (%) | LR (%) | 5-Year Survival (%) | LR (%) | 5-Year Survival (%) | |
| Surgery alone | 24 | 44 | — | — | — | — |
| Radiation therapy | 20 | 50 | 25 | 48 | — | — |
| Chemotherapy | 27 | 50 | — | — | 13 | −65 |
| Chemoradiotherapy | 11 | 59 | 13 | 57 | 8 | −65 |
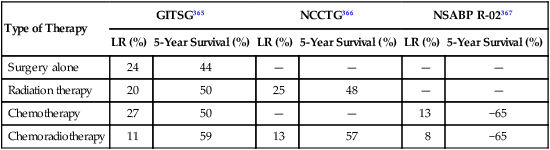
The Intergroup 86-47-51 trial did not demonstrate an incremental benefit to 1-(2-chloroethyl)-3-(4- methylcyclohexyl)-1-nitrosourea (MeCCNU) when added to postoperative radiation plus concurrent and maintenance 5-FU.168 However, a 2×2 component of the study demonstrated a positive benefit for giving CI 5-FU rather than interrupted bolus 5-FU concurrent with pelvic radiation. Patients randomly assigned to receive concurrent CI 5-FU (225 mg/m2 per day; 7 days per week or until intolerance) had improvements in disease control, 4-year disease-free survival (63% vs. 53%, P = 0.01), and 4-year overall survival (70% vs. 60%; P = 0.005).
The follow-up INT 0114 4-arm trial randomly assigned patients with pT3-4N0 and/or TanyN+ rectal cancer to postoperative radiation and bolus 5-FU with or without leucovorin, levamisole, or leucovorin plus levamisole (INT 86-47-51 results were not available before INT 0114 study design and completion). There was no significant difference in local control or survival among the four arms.169 With longer follow-up, the study also revealed that local control and survival results continue to deteriorate after 5 years. At 7 years the local failure rate was 17% and the survival was 56% compared with 14% and 64%, respectively, at 5-years. Patients with high risk (pT3N+ or T4) disease had a lower survival compared with lower risk (pT1-2N+ or T3N0) disease (45% vs. 70%). Further analysis of the INT 0114 trial has revealed that body mass is related to outcome and treatment-related toxicity,170 and both surgeons and hospitals with higher volumes of rectal cancer surgery have improved outcomes compared with those with lower volumes.171
The last postoperative Intergroup trial was INT 0144. This postoperative adjuvant rectal trial was designed to follow up on the positive results achieved with CI 5-FU during radiation in trial 86-47-51.172 Patients were randomly assigned to three arms: Arm 1: bolus 5-FU → CI 5-FU+radiation → bolus 5-FU (the control arm from 86-47-51); Arm 2: CI 5-FU → CI 5-FU+radiation → CI 5-FU; and Arm 3: bolus 5-FU+LV+levamisole → bolus 5-FU+leucovorin (LV)+levamisole+radiation → bolus 5-FU+LV+levamisole. The CI 5-FU arms did not confirm a survival benefit relative to the bolus 5FU+LV+levamisole arm, but Arm 2 did report a lower incidence of grade 3+ hematologic toxicity. Based on these results, when 5-FU is used with either preoperative or postoperative chemoradiation, CI is the preferable standard.
Preoperative Therapy: Results of Clinical Trials
The evolution to preoperative therapy is based on data from two randomized trials in which patients received preoperative therapy followed by TME. The German CAO/ARO/AIO 94 Rectal Cancer Trial compared preoperative versus postoperative chemoradiation (45 Gy to 50.4 Gy in 25 to 28 fractions plus concurrent chemotherapy).139,173 Compared with postoperative chemoradiation, preoperative chemoradiation significantly decreased acute and late toxicity, and significantly increased local control and sphincter preservation. The Dutch CKVO trial compared short-course radiation (25 Gy in 5 fractions) versus surgery alone.174,175 Compared with surgery alone, preoperative short-course radiation significantly increased local control.
Preoperative Chemoradiation
A series of phase III trials have addressed a number of controversies in the use of preoperative chemoradiation for rectal cancer (Table 78-5). These issues are discussed below. When 5-FU is used concurrently with radiation, CI is the conventional regimen.167,176 The NSABP R-04 trial compared preoperative chemoradiation with CI 5-FU versus capecitabine (with or without oxaliplatin). Compared with CI 5-FU, capecitabine had similar rates of pCR (22% vs. 19%), sphincter-sparing surgery (63% vs. 61%), and grade 3 + diarrhea (11%).143 Hofheinz et al. randomly assigned 401 patients with CI 5-FU–based chemoradiation versus capecitabine-based chemoradiation. Patients who received capecitabine had equivalent pCR rates (6% vs. 7%) and their 5-year survival was noninferior (76% vs. 66%, P = 0.0004) compared with CI 5-FU.177 Therefore, CI 5-FU– and capecitabine-based chemoradiation regimens are equivalent.
Table 78-5
Randomized Trials Addressing Controversies in the Adjuvant Management of Rectal Cancer
| Controversy | Trial | Outcome |
| Preoperative versus postoperative chemoradiation | CAO/ARO/AIO94 | Improved local control, toxicity, and sphincter preservation with preoperative chemoradiation. No survival benefit. |
| NSABPR-03 | Survival benefit but no benefit in local control, toxicity, or sphincter preservation with preoperative chemoradiation. | |
| Role of postoperative adjuvant chemotherapy | EORTC22921 FFCD 9203 |
No survival benefit with postoperative chemotherapy. |
| Short- vs. long-course chemoradiation | Polish Trial TROG |
No significant difference in local control or survival. |
| Oxaliplatin-based preoperative chemoradiation | STAR-01 ACCORD NSABP R-04 |
No significant advantage in pCR but a significant increase in grade 3+ toxicity. |
| CAO/ARO/AIO-04 | Significant increase in pCR without an increase in grade 3+ toxicity. | |
| CI 5-FU vs. capecitabine based chemoradiation | NSABP R-04 | No significant difference in pCR, sphincter-sparing surgery, or grade 3+ diarrhea. |
CI, Continuous infusion; 5-FU; 5-fluourouracil; pCR, pathological complete response.
In contrast with chemoradiation, the regimen used in the postoperative adjuvant chemotherapy component of treatment is different. Based on the efficacy demonstrated in patients with stage III colon cancer, the combination of CI 5-FU plus oxaliplatin (FOLFOX) has replaced CI 5-FU as a standard postoperative regimen.178 Other agents, such as irinotecan179 and bevacizumab,180 have not improved survival in the adjuvant setting and, therefore, are not used in the adjuvant management of rectal cancer.
The two randomized trials of preoperative versus postoperative chemoradiation for clinically resectable T3-4Nany rectal cancer (NSABP R-03140 and the German CAO/ARO/AIO 94)139 reported opposite results. The German trial completed the planned accrual of more than 800 patients and randomly assigned patients with rectal cancers smaller than 16 cm from the anal verge to preoperative chemoradiation (with CI 5-FU weeks 1 and 5) versus postoperative chemoradiation.139 Patients were stratified by surgeon and all underwent a TME. Compared with postoperative therapy, patients who received preoperative therapy had a significant decrease in local recurrence (6% vs. 15%, P = 0.006), acute toxicity (27% vs. 40%, P = 0.001), chronic toxicity (14% vs. 24%, P = 0.012), and in those 194 patients judged by the surgeon pretreatment to require an APR, a significant increase in sphincter preservation (39% vs. 20%, P = 0.004). With a median follow-up of 40 months, there was no difference in 5-year survival (74% vs. 76%). A separate analysis revealed that the treatment center, schedule, and gender were independent prognostic factors for 5-year local control.181 The results were updated with a median follow up of 11.2 years. At 10 years, the local control benefit of preoperative versus postoperative therapy was sustained (local failure: 7% vs. 10%, P = 0.048). There was no difference in the 10-year cumulative incidence of distant metastasis (29%) or overall survival (60%).173 As seen with other cancers, both surgeons and hospitals with higher volumes of rectal cancer surgery have improved outcomes compared with those with lower volumes.171
The NSABP R-03 trial accrued only 267 of the planned 900 patients.140 Patients received induction chemotherapy followed by conventional chemoradiation and were randomized to receive it either preoperatively or postoperatively. Some patients underwent a local excision and a TME was not required. Compared with postoperative therapy, patients who received preoperative therapy had a significant improvement in 5-year disease-free survival (65% vs. 53%, P = 0.011), and a borderline significant improvement in 5-year overall survival (75% vs. 66%, P = 0.065). There was no difference in 5-year local recurrence (11%). There was a corresponding higher incidence of grade 4+ toxicity (33% vs. 23%) but the incidence of grade 3+ toxicity was lower (41% vs. 50%). Lastly, based on a prospective office assessment by the operating surgeon, there was no significant improvement in sphincter preservation (48% vs. 39%).
Novel Chemoradiation Regimens
Recent trials have examined the role of different chemoradiation regimens. The NSABP R-04 trial reported equivalent rates of pCR (22% vs. 19%), sphincter-sparing surgery (63% vs. 61%), and grade 3+ diarrhea (11%) with CI 5-FU– and capecitabine-based chemoradiation.143
Four randomized trials examined the role of adding oxaliplatin to 5-FU– or capecitabine-based preoperative chemoradiation (Table 78-6). These include three European trials (Studio Terapia, Adjuvante Retto [STAR]-01,141 Actions Concertees and les Cancers Colorectaux et Digestifs [ACCORD],142 and the German CAO/ARO/AIO-94),144 and one from the U.S. (NSABP R-04).143 Three of the four reported no significant improvement in the pCR rate and a corresponding increase in acute toxicity.141–143,182 Preliminary data from the ACCORD trial revealed no improvement in 3-year local control (4% vs. 5%) or survival (88% vs. 85%) with the addition of oxaliplatin.182 A fifth trial (Pan-European Trial in Adjuvant Colon Cancer [PETACC]-6) is asking a similar question; however, the results are pending.
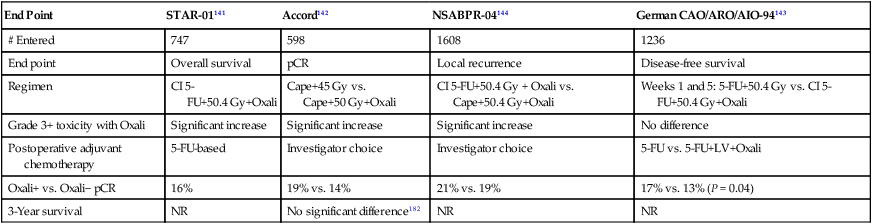
Table 78-6
Phase III Trials of 5-FU– or Capecitabine-Based Chemoradiation ± Oxaliplatin
| End Point | STAR-01141 | Accord142 | NSABPR-04144 | German CAO/ARO/AIO-94143 |
| # Entered | 747 | 598 | 1608 | 1236 |
| End point | Overall survival | pCR | Local recurrence | Disease-free survival |
| Regimen | CI 5-FU+50.4 Gy+Oxali | Cape+45 Gy vs. Cape+50 Gy+Oxali | CI 5-FU+50.4 Gy + Oxali vs. Cape+50.4 Gy+Oxali | Weeks 1 and 5: 5-FU+50.4 Gy vs. CI 5-FU+50.4 Gy+Oxali |
| Grade 3+ toxicity with Oxali | Significant increase | Significant increase | Significant increase | No difference |
| Postoperative adjuvant chemotherapy | 5-FU-based | Investigator choice | Investigator choice | 5-FU vs. 5-FU+LV+Oxali |
| Oxali+ vs. Oxali− pCR | 16% | 19% vs. 14% | 21% vs. 19% | 17% vs. 13% (P = 0.04) |
| 3-Year survival | NR | No significant difference182 | NR | NR |
The benefit of adding targeted biological agents, such as bevacizumab and cetuximab, is being tested. Initial phases I/II trials of preoperative chemoradiation with capecitabine and oxaliplatin (CAPOX) + bevacizumab revealed pCR rates of 18% to 24%.183,184 However, more recent trials report increased acute toxicity and have been closed early as a consequence of increased acute toxicity.185,186 Furthermore, given the lack of a survival benefit in the NSABP C-08 adjuvant colon cancer trial, the ultimate role of bevacizumab in the adjuvant management of rectal cancer remains unclear.180
Phases I/II trials of new preoperative chemoradiation programs have been explored. The STAR-02 trial reported a 21% cCR rate in 60 patients treated with oxaliplatin, 5-FU, panitumumab, and 50.4 Gy.187 Only 8% achieved a pCR with irinotecan, capecitabine, and 50.4 Gy in the Mannheimer Arbeitsgruppe für Gastrointestinale Tumoren (MARGIT) phase II trial.188 Two trials of S-1–based chemoradiation have reported different results. A phase I trial of S-1 plus oxaliplatin and 50.4 Gy had a 13% pCR rate whereas a phase II of S-1 plus irinotecan and 54 Gy (with a limited pelvic radiation field) achieved a pCR of 35%.
The RTOG (RTOG 0247) performed a randomized phase II trial of preoperative chemoradiation (50.4 Gy) with capecitabine plus irinotecan (CAPIRI) versus capecitabine plus oxaliplatin (CAPOX).189 A total of 146 patients were randomly assigned and the pCR rate was higher in those receiving CAPOX (21% vs. 10%); there were no differences in acute toxicity. The CAPOX was the experimental arm of the NSABP R-04 phase III trial which subsequently did not confirm a benefit in pCR compared with capecitabine alone.
Therapy based on KRAS expression is useful in patients with metastatic disease.184 In the adjuvant setting, preliminary results from the phase II EXPERT-C trial (50.4 Gy+CAPOX+cetuximab) revealed a significant increase in 3-year survival in the 90 patients whose tumors were KRAS wild-type and received cetuximab versus those who did not receive cetuximab (96% vs. 81%, P = 0.035).190 The end point of the trial (pCR) was not impacted by the use of cetuximab (11% vs. 7%). Nonetheless, this is the first trial to report that treatment based on a molecular marker had a significant impact on survival in patients with rectal cancer who were treated in the adjuvant setting.
Given the improvements in systemic chemotherapy there may be an opportunity to use preoperative radiation more selectively. In a prospective trial reported in abstract form, Cercek et al. treated 32 selected patients with uT2N1 or uT3N0-1 rectal cancer with neoadjuvant FOLFOX + bevacizumab.191 Of note, patients who required an APR were excluded. Pelvic radiation was reserved for patients who progressed preoperatively or following surgery had pT4, pN2, or positive margins. Of the 30 patients who underwent surgery none required radiation, the pCR rate was 27%, and 2 required postoperative radiation. This approach remains investigational and is being prospectively tested in the phases II/III Alliance N1048 trial.
Preoperative Short-Course Radiation
There are 12 modern randomized trials of preoperative radiation therapy.192 The fractionation varied from 5 Gy in 1 fraction to the more standard 5 Gy in 5 fractions. Most of the trials showed a decrease in local recurrence, and in five of the trials this difference reached statistical significance. Although in some trials a subset analysis revealed a significant improvement in survival, the Swedish Rectal Cancer Trial is the only one that reported a survival advantage for the total treatment group. Two meta-analyses report conflicting results. Although both revealed a decrease in local recurrence, the analysis by Camma et al.193 reported a survival advantage, whereas the analysis by the Colorectal Cancer Collaborative Group133 did not.
In the Swedish Rectal Cancer Trial, patients with cT1-3 rectal cancer were randomly assigned to 25 Gy in 5 fractions followed by surgery 1 week later versus surgery alone.194 With 13-year follow-up, survival was significantly improved (38% vs. 30%, P = 0.008).195 Of note, the local recurrence rate in patients with lymph node-positive disease who underwent surgery alone was 46%, illustrating the inferior results of surgery before the adoption of TME. This trial and the other 10 that preceded it did not mandate TME surgery. Therefore, although interesting from a historical perspective, these trials are not discussed further.
The Dutch CKVO 95-04 trial randomly assigned 1805 patients with cT1-3 disease to TME or 25 Gy in 5 fractions followed by TME.174 Radiation significantly decreased local recurrence (8% vs. 2%) but there was no difference in 2-year survival (82%). With a 12-year median follow-up, 5-year local failure was higher with TME (11%) however, was significantly decreased to 5% with preoperative radiation.196 The acute toxicity in the Dutch CKVO 95-04 trial included 10% neurotoxicity, 29% perineal wound complications, and 12% postoperative leaks.175 In the patients who experienced postoperative leaks, 80% required surgery resulting in 11% mortality. In contrast to the earlier randomized trials of short-course radiation, multiple-field radiation techniques were used. Therefore, whether the increases in morbidity and mortality were a result of the learning curve associated with a new surgical technique, the 1-week interval between the completion of radiation and surgery, or both, is not known.
Preoperative Therapy: 5 Gy × 5 or Chemoradiation?
Historically, the primary reasons for not using short-course radiation is the lack of sphincter preservation, the inability to safely combine it with adequate doses of systemic chemotherapy, and the acute toxicity.197 However, these shortcomings may be mitigated by (a) increasing the interval between the completion of radiation and surgery and (b) delivering chemotherapy sequentially (after radiation) as opposed to concurrently with radiation (Table 78-7).
Table 78-7
Short-Course Radiation Versus Chemoradiation
| Variable | Short-Course Radiation | Chemoradiation |
| Combine with chemotherapy | Sequential | Concurrent |
| Increased sphincter preservation and pCR | No, but Stockholm III trial results pending | Yes, confirmed by the CAO/ARO/AIO-94 trial |
| 3D or IMRT possible | Yes | Yes |
| Clinical stages entered on trials | cT1-3 | cT3 and/or N+ |
| Treatment days to complete radiation | 5 | 28 |
IMRT, Intensity-modulated radiation therapy; RT, radiation therapy.
The Dutch CKVO trial compared short-course radiation (25 Gy in 5 fractions) versus surgery alone.174,175 Compared with surgery alone, preoperative short-course radiation significantly increased local control. The outcomes of these two trials are not comparable because patients selected for treatment with short-course radiation included stages cT1-3, whereas the German trial included stages T3 and/or N+. However, more recent trials of short-course radiation have included patients with stages cT3 and/or N+ as well delivered sequential or postoperative chemotherapy, thereby allowing a more relevant comparison with chemoradiation.
Increasing the Interval Between Short-Course Radiation and Surgery
Increasing the interval between radiation and surgery is being prospectively tested in the Stockholm III trial. This phase III trial will determine whether increasing the interval between short course radiation and surgery from 1 week to 4 weeks improves sphincter preservation and reduces toxicity. Patients are randomized to 5 Gy × 5 followed by surgery 1 week later versus 5 Gy × 5 followed by surgery 4 weeks later versus 2 Gy × 25 (50 Gy) followed by surgery 4 weeks later. Retrospective data from the Royal Marsden Hospital examining 95 patients who received preoperative radiation ± chemotherapy revealed a higher rate of T-downstaging with an increasing interval from the end of treatment to surgery: less than 6 weeks: 33%; 6 to 8 weeks: 38%; and more than 8 weeks: 62%.198
Short-Course Radiation and Sequential Chemotherapy
Because short-course radiation cannot be safely delivered concurrently with systemic chemotherapy, the use of sequential treatment has been examined. In a series from the Dutch Colorectal Group, 50 patients with primary rectal cancer and synchronous resectable metastasis in one or two organs (liver 42, lung 5, both 3) were enrolled in a phase II trial of short-course radiation followed by 6 cycles of CAPOX + bevacizumab (restaging after 2 cycles) and resection of the primary and resection and/or ablation of the metastasis.199 The median time between the completion of radiation and chemotherapy was 11 days (3 to 44 days). The group reported “no toxicity” during radiation. Of the 41 patients brought to surgery, 44% achieved a tumor regression grade of 0 to 2.
Myerson and colleagues have reported preliminary results of short course radiation followed by sequential chemotherapy.200 A total of 60 patients with stages II and III were entered and 44 were evaluable at the time of analysis. Clinical stages included four cT4 and 40 cT3, 32 cN+, and four cM1 disease. Preoperative treatment was 5 fractions (25 Gy to the involved mesorectum and 20 Gy to the pelvic nodes), followed by 4 cycles of modified FOLFOX6 (mFOLFOX6). Postoperative chemotherapy was at the discretion of the medical oncologist. Following surgery, 33 (75%) had ypT0-2 disease, including 13 (30%) who were ypT0 and 14 (32%) were ypN0.
Nonrandomized Trials of Short Course Radiation Versus Chemoradiation
MRC C07
The U.K. Medical Research Trial MRC C07 randomized 1350 patients with clinical stages I to III rectal cancer to 5 Gy × 5 or selective postoperative chemoradiation (45 Gy with concurrent 5-FU), which was delivered only to patients with a histologic CRM of less than 1 mm (12% of all patients with immediate surgery).201 It should be emphasized that this trial did not compare short-course radiation with chemoradiation as only patients with close/positive CRM were selected to receive postoperative treatment. With a median follow-up of 4 years, patients who received preoperative compared with selective postoperative treatment has significantly lower 3-year local recurrence rates (4.4% vs. 10.6%, P < 0.0001), and a higher 3-year disease-free survival (77.5% vs. 71.5%, P = 0.013). A separate quality-of-life analysis revealed that the main adverse effect of treatment was male sexual dysfunction and the primary cause was surgery.197 However, preoperative radiation also adversely impacted sexual and some aspects of bowel function.
Randomized Trials of Short-Course Radiation Versus Chemoradiation
There are two randomized trials of short-course radiation versus chemoradiation. The Polish trial from Bujko et al. and the Intergroup Australian/New Zealand TROG, AGITG, CSSANZ, RACS trial reported by Ngan et al. (Table 78-8).
Table 78-8
Phase III Trials of Preoperative Chemoradiation Versus Short-Course Radiation
| Characteristics | Bjuko et al.202,203 | Ngan et al.204 |
| # Patients | 316 | 326 |
| Stage | cT3–4 | cT3Nany (56% N0) |
| Chemoradiation | 50.4 Gy + 5-FU + LV | 50.4 Gy + CI 5-FU |
| Short-course radiation | 5 Gy × 5 | 5 Gy × 5 |
| Postoperative chemotherapy | Not required | 5-FU+LV (4–6 cycles) |
| Outcome % | ChemoRT | RT | ChemoRT | RT |
| pCR | 16 | 1* | — | — |
| Compliance | 69 | 98 | — | — |
| SP | 58 | 61 | — | — |
| CRM+ | 4 | 13* | — | — |
| Local Failure | 14 | 9 (4-Year) | 4 | 8 (3-Year) |
| Survival | 66 | 67 (4-Year) | 70 | 74 (3-Year) |
| Grades 3-4 late toxicity | — | — | 9 | 8 |
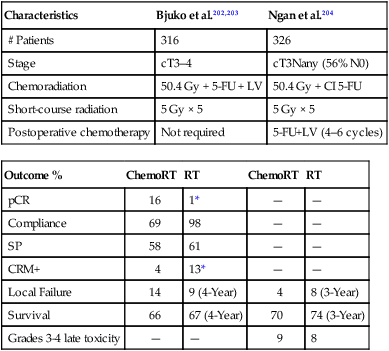
*, Statistically significant; CRM+, positive circumferential radial margins; LV, leucovorin; pCR, pathological complete response; SP, sphincter preservation; RT, radiation therapy.
Polish Trial
Bujko and colleagues randomly assigned 316 patients with cT3 rectal cancer.202,203 All tumors were above the anorectal ring and TME was performed for distal tumors only. Postoperative chemotherapy was at the discretion of the investigator. There was no radiation quality control review. Compared with short-course radiation, patients who received chemoradiation had a higher pCR rate (16% vs. 1%) and a lower incidence of CRM+ (4% vs. 13%, P = 0.017). However, there were no significant differences in sphincter preservation (58% vs. 61%), crude local recurrence (14% vs. 9%), disease-free survival (56% vs. 58%) and 4-year survival (66% vs. 67%). Although acute toxicity was significantly higher with chemoradiation (18% vs. 3%, P < 0.001) there was no difference in postoperative complications.
Australian/New Zealand Intergroup Trial
A similar trial from Australia and New Zealand was reported in abstract form by Ngan et al.204 In this intergroup trial, a total of 326 patients with T3Nany rectal cancer (56% were N0) were randomized to short-course radiation versus chemoradiation (50.4 Gy plus CI 5-FU). In contrast to the trial from Bujko and colleagues, patients in both arms received 4 cycles of postoperative 5-FU+LV adjuvant chemotherapy. The median follow-up was 5.9 years. Comparing short-course radiation with chemoradiation, there were no significant differences in 3-year local recurrence (8% vs. 4%), 5-year distant recurrence-free rates (72% vs. 69%), 5-year survival (74% vs. 70%), and RTOG grades 3 to 4 late toxicity (8% vs. 9%).
The Need for Long-Term Follow-up
Local recurrences can occur late in patients with rectal cancer. In contrast to the results reported in the trials of adjuvant treatment of colon cancer in which 3-year, and possibly 2-year, disease-free survival predicts for 5-year survival,205 the INT 0114 postoperative rectal adjuvant trial confirmed that local control and survival continue to decrease beyond 5 years.169 At 7 years the local recurrence rate was 17% and the survival was 56% compared with 14% and 64%, respectively, at 5-years.
Limiting the analysis to trials where all patients underwent a TME, a similar detriment in outcomes was seen with long-term follow-up. In the German CAO/ARO/AIO-94 trial, patients who received preoperative chemoradiation had an increase in local recurrence (7% vs. 5%) and decrease in survival (60% vs. 74%) at 10 years versus 5 years, respectively.173 The incidence of local recurrence for all patients in the preoperative radiation arm of the Dutch CKVO trial of 5 Gy × 5 increased from 3% at a median follow-up of 3.5 years to 6% at a median follow-up of 6 years.206 These data underscore the importance of long term follow-up, regardless of which preoperative approach is used.
The Role of Postoperative Adjuvant Chemotherapy
Two randomized trials address whether postoperative chemotherapy is beneficial following preoperative therapy. The EORTC 22921 was a four-arm randomized trial of 1011 patients who received preoperative 45 Gy with or without concurrent bolus 5-FU+LV followed by surgery with or without 4 cycles of postoperative 5-FU+LV.207 Only 37% had a TME. The FFCD 9203 was a 2-arm randomized trial of 742 patients randomized to preoperative 45 Gy with or without concurrent bolus 5-FU+LV.208 However, all patients were scheduled to receive postoperative chemotherapy, and 73% did receive it.
The EORTC trial patients who received preoperative chemoradiation versus radiation had a significant decrease in local recurrence (8% to 10% vs. 17%, P < 0.001) but no difference in 5-year survival (65%). However, only 43% received 95% or more of the planned postoperative chemotherapy, which may explain the negative results. Furthermore, a subset analysis of the 785 patients with cT3 to cT4 tumors who had a R0 (complete) resection revealed that those patients who responded to preoperative chemoradiation had a survival benefit from postoperative chemotherapy if they had been downstaged to ypT0-2.209 The FFCD trial reported a similar decrease in local recurrence (8% vs. 17%, P < 0.05), a corresponding increase in pCR (11% vs. 4%, P < 0.05) but no survival benefit (68% vs. 67%).208
A pooled analysis of the two trials with a median follow-up of 5.6 years confirmed that patients who received preoperative chemoradiation compared with radiation had a significant decrease in local recurrence (11% vs. 15%, P = 0.0001); however, there was no difference in 5-year overall survival (66%).210
These results need to be examined in perspective. Given that most patients did not receive adequate doses of postoperative chemotherapy in the European Organization for Research and Treatment of Cancer (EORTC) trial and the FFCD trial tested the impact of only 6 weeks of chemotherapy concurrent with preoperative radiation, the standard practice in many centers remains preoperative chemoradiation followed by surgery and 4 months of postoperative adjuvant chemotherapy. An analysis of the National Comprehensive Cancer Network (NCCN) database revealed that of 810 patients who received preoperative chemoradiation at eight NCCN institutions, 20% did not receive postoperative adjuvant chemotherapy. The most frequent reason for physician refusal was comorbid illness (54%), and the most frequent reason chemotherapy was not received when it was recommended by the medical oncologist was patient refusal (73%).211 Whether the ability to deliver postoperative chemotherapy is more successful in patients who receive short course radiation compared with chemoradiation is unknown.
Beets and associates performed a pooled analysis of 2724 patients who received preoperative chemoradiation at European centers.212 Overall, 41% received postoperative chemotherapy and there was no benefit in disease-free survival in the subsets of patients with ypT0N0 or ypT3-4Nany disease. Patients with ypT1-2N0 disease had the greatest benefit although the hazard ratio (and 95% CI) was 0.45 (0.27 to 0.75).
Two retrospective analyses from the MD Anderson Cancer Center evaluated patients who were treated with neoadjuvant chemoradiation and adjuvant chemotherapy.213,214 In the first series, 117 patients received neoadjuvant chemotherapy and 63% also were treated with 5-FU and LV adjuvant chemotherapy. There was a significant disease free survival advantage for the patients whose tumors were downstaged after neoadjuvant treatment and who received adjuvant chemotherapy compared with those who were downstaged but did not receive the adjuvant treatment. The patients who did not experience tumor downstaging did not appear to benefit from the adjuvant chemotherapy. The second series of 470 patients included 305 who received adjuvant chemotherapy after neoadjuvant chemoradiation and surgery. The pathological tumor stage significantly predicted both the local relapse-free and 5-year overall survival. Those individuals with ypT0-2 disease in particular were noted to have the best outcome with both 5-year disease free and overall survival of 89% and local relapse-free survival of 96%. Because most patients were also treated with adjuvant chemotherapy, it was not possible to determine the added benefit of this approach compared with those treated with neoadjuvant therapy alone. Another retrospective study included 156 cT3 to cT4 rectal cancer patients who received neoadjuvant chemoradiation or neoadjuvant and adjuvant radiation or neoadjuvant chemoradiation and adjuvant chemotherapy with 5-FU. Improvement in both 5-year progression-free and overall survival was noted for the 71 patients in this group who received adjuvant chemotherapy. The group of patients who had significant downstaging after neoadjuvant chemoradiation (ypT0-2) also had improvement in survival compared with those who were not downstaged.215
Nomograms may also be helpful decision making. Valentini et al. pooled data from five major European clinical trials for rectal cancer and developed multivariate nomograms based on Cox regression analysis.216 The nomograms were able to predict events with a c-index for external validation of local recurrence, distant metastases, and overall survival. These may be used as decision support tools by using the three defined risk groups to select patients for postoperative chemotherapy versus close follow-up.
Most investigators feel it is reasonable and use the same adjuvant chemotherapy for adjuvant colon and rectal cancer. For patients selected to receive postoperative adjuvant chemotherapy, 4 months (8 cycles) of mFOLFOX6 is recommended. However, its benefit remains controversial.217
Induction Chemotherapy
Given the difficulty of patients tolerating and/or accepting postoperative adjuvant chemotherapy, the use of neoadjuvant chemotherapy prior to preoperative chemoradiation has been investigated. The Spanish GCR-3 randomized phase II trial compared this approach with conventional preoperative chemoradiation followed by surgery and postoperative chemotherapy.218 A total of 108 patients received preoperative 50.4 Gy plus CAPOX and were randomly assigned to receive 4 months of CAPOX either by induction or adjuvant (postoperative). With the induction approach there was no detriment in the pCR rate (14% vs. 13%), grade 3+ toxicity was lower (17% vs. 51%, P = 0.00004) and the ability to receive all 4 chemotherapy cycles was higher (93% vs. 51%, P = 0.0001).
Garcia-Aguilar and colleagues have tested a similar approach.219 In their randomized phase II trial, 144 patients with cN+ rectal cancer received preoperative chemoradiation (50.4 Gy + CI 5-FU) followed by surgery 6 weeks later vs. chemoradiation, and if they had a clinical response, mFOLFOX6 followed by surgery 11 weeks later. The pCR rate was nonsignificantly higher in the mFOLFOX arm (25% vs. 18%) with no increase in postoperative complications (40% in each arm). The preliminary data suggest that delaying surgery in the patient who has a response to preoperative and delivering additional chemotherapy is not detrimental to the tumor response or surgical complication rates.
Does the Distance from the Anal Verge Impact the Treatment Approach?
The limited data examining the impact of the distance from the anal verge on local recurrence are subset analysis not stratified by distance. There are no prospective randomized data. Furthermore, there are additional variables which may have contributed to differences in local recurrence. For example, TME was standard in the Dutch CVKO and German trials and not in the Swedish trial. All three trials included patients with tumors more than 12 cm from the anal verge in the “upper or high” category. Because the peritoneal reflection varies from 12 to 16 cm, some patients with tumors above the peritoneal reflection (colon cancer) were included in the three trials. Most investigators now limit preoperative treatment to tumors less than 12 cm from the anal verge.220 Lastly, distance measurements with a flexible proctoscope are less accurate than a straight proctoscope. Flexible scopes were used in the Dutch CKVO trial. The German trial used a straight scope. In the Swedish trial proctoscopic information was not mentioned and eligibility was limited to tumors “below the promontory as identified by barium enema.” The Polish trial is not included because all tumors were within reach by digital examination.203
In contrast, there was no significant difference in local recurrence between mid and upper tumors in the German trial.221 Nash and colleagues reported that in a retrospective analysis of 627 patients with stage I-IV rectal cancer treated with either surgery alone or chemoradiation, the pelvic recurrence rate was lower in tumors 7 to 12 cm from the anal verge versus 0 to 6 cm from the anal verge (3% vs. 7%, P = 0.009).222 However, mucosal, distant, and overall recurrences were not significantly different.
Given the conflicting data combined with the report from Guillem et al. confirming that the incidence of positive nodes is the same from 0 to 12 cm from the anal verge,220 treatment decisions, whether with preoperative chemoradiation or short-course radiation, based on the current definitions of low versus mid versus high should not be used regardless if the patient receives preoperative chemoradiation or short course radiation.
Is Clinical Staging Reliable to Select Patients for Preoperative Therapy?
The most common techniques for predicting T stage are transrectal ultrasound and high-resolution MRI. Ultrasound is used most commonly in North America, whereas many European investigators prefer high-resolution MRI. The advantage of MRI is its ability to identify patients likely to have close or positive CRM margins if they underwent initial surgery and therefore, would be better treated with preoperative therapy.223
The overall accuracy in predicting T stage is approximately 50% to 90% with ultrasound224 or high-resolution MRI,225 and 50% to 70% with CT or conventional MRI.226 Although fludeoxyglucose (18F)(FDG)-PET may be more accurate compared with CT for identification of metastatic disease,227 its use to restage patients following preoperative chemoradiation remains controversial.228–231
Overstaging is common, especially when there is a fibrotic thickening of the rectal wall following preoperative chemoradiation. A reasonably high level of accuracy has been observed by phased array MRI for differentiating ypT0-2 from ypT3 disease.232 Both diffusion-weighted MRI and FDG-PET have been used to monitor therapy response and to predict outcome to preoperative therapy.233 With FDG-PET there is a decrease in standardized uptake value on postradiation in responders compared with nonresponders, but the clinical value of this information remains to be determined.234
Identification of positive lymph nodes is more difficult. Overall, the accuracy in detecting positive pelvic lymph nodes with the above techniques is approximately 50% to 75%.235 Although the accuracy of MRI is similar to CT, it is improved with the use of external and/or endorectal coils. Both CT and MRI can identify lymph nodes measuring 1 cm or larger, although enlarged lymph nodes are not pathognomonic of tumor involvement. The accuracy of ultrasound for the detection of involved perirectal lymph nodes may be augmented when combined with fine-needle aspiration.236 Despite these advances, the ability to accurately predict the pathological stage following preoperative chemoradiation with MRI,237,238 ultrasound,239 FDG-PET,228–231 or physical examination240 remains suboptimal.
Does Tumor Response Predict Outcome?
Pathological/Molecular Markers
Although some series show no correlation,241 most series suggest that there is improved outcome with increasing pathological response to preoperative therapy.244–244 A retrospective review of 566 patients who achieved a pCR after receiving a variety of preoperative chemoradiation regimens at multiple European centers was reported by Capirci and associates.243 With a median follow-up of 46 months the local recurrence rate was only 1.6% and the 5-year disease-free and overall survival rates were 85% and 90%, respectively. A pooled analysis 3105 patients from 14 studies confirmed a significant improvement in local recurrence, distant failure, and disease-free and overall survival for the 16% of patients who achieved a pCR (ypT0N0M0) compared to those without a pCR.245
A pooled analysis of 27 articles identified 484 of 3105 patients treated with preoperative chemoradiation who achieved a pCR.245 The overall incidence of pCR was 15% to 27% and the median follow-up was 4 years. Patients who achieved a pCR had a significantly higher 5-year disease survival (83% vs. 66%, P < 0.0001).
Although a number of molecular markers are predictive of outcome in colorectal cancer,246 they have had varying success in identifying patients with rectal cancer who may respond to preoperative therapy. Kuremsky et al. reviewed 1204 studies examining a total of 36 molecular biomarkers which may have predictive value.247 Restricting the analysis to patients treated with preoperative chemoradiation and to gene products examined by five or more studies, only p53, epidermal growth factor receptor (EGFR), thymidylate synthase, Ki-67, p21, and bax/bcl-2 met these criteria. Of these, quantitatively evaluated EGFR or EGFR polymorphisms, thymidylate synthase polymorphisms, and p21 have been identified as promising candidates that should be evaluated in larger prospective trials for their ability to guide preoperative therapy.
More recent trials of preoperative chemoradiation have identified prognostic significance of thymidylate synthase,250–250 nuclear factor kappa B (NF-κB) expression,251 k-RAS, and p53.252 Because the studies are limited retrospective trials and because most do not examine multiple markers, the need for adjuvant therapy should still be based on T and N stage. The presence of acellular mucin following preoperative chemoradiation is seen in approximately 15% of patients and has no prognostic significance.253,254
Imaging
The role of FDG-PET remains controversial. Konski and associates performed pretreatment and posttreatment FDG-PET scans on 53 patients receiving preoperative chemoradiation.255 By multivariate analysis the percent decrease in standardized uptake value was marginally trended in predicting pCR (P = 0.07).
MRI may be the most accurate imaging modality to predict long-term outcome. By multivariate analysis, the posttreatment MRI-based response correlated significantly with recurrence (P = 0.003) in 101 patients treated with preoperative therapy.256 In the MERCURY trial of 111 patients treated with preoperative radiation ± chemotherapy, preoperative MRI-predicted CRM+ independently predicted local recurrence.257,258 The ideal cutoff distance for predicting the MRI-based CRM is 1 mm, and using a distance greater than that does not help identify patients with a higher risk of local recurrence.259 Of note, the nodal status did not predict outcomes leading to the investigators recommending that CRM status based on pretreatment MRI is more predictive than nodal staging. This change has been adopted by other investigators from the United Kingdom.260
The role of PET to predict response after preoperative therapy is controversial. Some investigators have reported a positive predictive ability of posttreatment FDG-PET to identify patients who will have a favorable response and long-term outcome,230,261 whereas a prospective study of 127 patients treated at Memorial Sloan Kettering did not confirm a benefit of serial FDG-PET scans before and after neoadjuvant chemoradiation.25
Pathological Margins
The initial series from Moore and associates reported that following preoperative chemoradiation, distal margins as close as 5 mm were not associated with a higher pelvic recurrence rate.262 This series was updated by Nash et al. and included 627 patients with stages I to IV rectal cancer treated with either surgery alone or chemoradiation with a median follow-up of 5.8 years.222 Distal margins less than 8 mm were associated with a higher rectal mucosal local recurrence rate versus those with margins 8 mm or larger (5% vs. 2%, P = 0.001).222 However, the total pelvic recurrence rate, which included all sites in the pelvis, was not significantly different (6% vs. 4%, P = 0.29). Both a review of 17 published trials263 and a meta-analysis of 21 published series264 confirm that in patients who receive preoperative chemoradiation margins of less than 1 cm, as long as they are negative, are not associated with an increase in local recurrence.
The most significant predictor of local recurrence is the CRM.265 In the Dutch CKVO trial, 17% were CRM+. In a subset analysis by Nagtegaal et al., patients with positive circumferential margins who underwent TME alone had local recurrence rate of 17% after a low anterior resection and 30% after an APR.266 Few centers perform the necessary pathological examination to detect CRM+.267 High-resolution MRI can help identify patients who will have positive margins as well select those who may benefit from preoperative therapy.258,259,268
A CRM+ following preoperative therapy is unfavorable. Compared with 460 patients with negative circumferential margins, Baik et al. reported that the 44 patients with positive margins had higher local recurrence (35% vs. 11%) and decreased survival rates (27% vs. 73%).269 An analysis of more than 17,500 pathological specimens by Nagtegaal and associates reported inferior survival in patients with CRM+ after preoperative treatment compared with immediate surgery (HR 6.3; 95% confidence interval, 3.7 to 16.7 vs. HR 2.0; 95% confidence interval, 1.4 to 2.9, respectively).265
Postoperative treatment has an equally limited ability to control CRM+. In the MRC CR-07 trial, patients with CRM+ were selected to receive postoperative chemoradiation and still had an 11% local recurrence rate.270 Likewise, in a subset analysis of the Dutch CKVO trial, 50 Gy postoperatively did not compensate for positive margins.138
Is Radical Surgery Necessary Following Preoperative Chemoradiation?
Patients who are downstaged to ypT0 following chemoradiation have a 5% incidence of positive nodes and, therefore, a low nodal recurrence rate.271 Are there alternative surgical approaches for those patients?
Habr-Gama and colleagues have questioned the value of radical surgery in patients who had a biopsy proven cCR.272 Of note, their series included patients with cT1 to cT3 disease and has not been reproduced by other investigators. In series limited to patients with cT3 disease who received preoperative chemoradiation, radical surgery is still necessary to fully evaluate the degree of pathological response. Neither posttreatment ultrasound239,273 or physical examination274 are sufficient. Studies on the use of of PET scan230,231,275 and diffusion MRI276 as noninvasive measures of response are being conducted and have reported mixed results. Although Kalff and associates reported FDG-PET identification of residual viable tumor in 63 patients following chemoradiation had a high positive predictive value (0.94; 95% confidence interval, 85% to 99%),277 other groups have reported opposite results.
Glynne-Jones and associates reviewed 218 phase II and 28 phase III trials of preoperative radiation or chemoradiation and confirmed that clinical and/or radiologic response does not sufficiently correlate with pathological response and do not recommend a “wait-and-see” approach to surgery following preoperative therapy.278
Alternate Methods for Sphincter Preservation
Local Excision
Standard therapy for resectable rectal cancer has been radical or conventional surgical resection (inclusive of the possibility of TME) with increasing integration of preoperative or postoperative adjuvant therapy. In highly select patients, this approach has often been challenged by the use of more conservative local measures. Table 78-1 provides a summary of selected series.
Endocavitary Radiation
Endocavitary irradiation alone281–281 has been used for early, noninvasive tumors. For more advanced tumors (cT2 to cT3 and/or lymph node-positive) it is combined with a temporary iridium-192 implant and/or pelvic radiation.282–285 This technique is also known as the Papillion technique or contact radiation. Before delivery, the anus is dilated and a 4-cm proctoscope is introduced. A low-energy x-ray unit is placed through the scope almost against the tumor. Generally, 50-kV x-rays are delivered at 30 Gy per fraction in 3 or 4 fractions over 1 month. Winslow et al. report that patients who develop local failure can successfully undergo surgical salvage.286
Maingon et al. treated 151 patients and the incidence of initial local control and ultimate local control by stage was T1: 78% and 87%; T2: 58% and 79%; and T3: 54% and 69%, respectively.287 The Mayo Clinic treated 29 patients with curative intent with a total dose of up to 155 Gy in 1 to 5 fractions and local control was 76% at 10 years; survival was 65% at 5 years and 42% at 10 years.279 At Washington University, patients received pelvic radiation (20 Gy in 5 fractions for those with cT1 or and the remainder received 45 Gy in 25 fractions) followed 6 to 7 weeks later by two endocavitary treatments of 30 Gy each.285 Results by stage were uT1: 100% disease-free survival; uT2: 85% local control; uT3 (who were not optimal candidates for surgery) or tethered uT2: 56% local control (67% after salvage surgery). Aumock and associates added external beam for T2 to T3 tumors and reported local control rates of T1: 100%, mobile T2: 85%, and T3 or tethered T2: 56%.284 Because the 50 KVp radiation machine is no longer available, there are limited centers which continue to treat patients.
Local Excision Followed by Adjuvant Therapy
Local excision has been performed both before and after radiation therapy. The advantage of performing a local excision prior to radiation is that pathological details can be well characterized. Highly selected patients with pT1 tumors without adverse pathological factors have local failure rates of 5% to 10%. However, when adverse pathological factors are present (high grade, vascular invasion, or signet-ring cells) or the tumor invades into or through the muscularis propria, the local failure rate is at least 17% and the incidence of positive mesorectal and/or pelvic nodes is at least 10% to 15%.288 Nash and associates reported that in 145 patients who underwent radical surgery for cT1N0 disease, 20% were found to be pN+.289
There are a variety of surgical approaches including transanal local excision, posterior proctotomy, and transsphincteric excision. TEM has emerged as another option for local treatment of rectal cancer, either alone for T1 tumors or combined with radiation for selected patients with T2 to T3 disease.290 Regardless of the technique, the excision should be full thickness, nonfragmented, and have negative margins.291
Local Excision Alone
Local excision alone is recommended only for selected patients with pT1 tumors. Paty and colleagues performed a retrospective analysis of patients with cT1N0 disease who underwent local excision (137) or radical surgery (145) and were found to have pT1Nx disease. With a median follow-up of 5 years there was a significantly lower local recurrence rate (3% vs. 13%, P < 0.05) and higher 5-year disease specific survival (96% vs. 87%, P < 0.05) in those who underwent radical surgery versus a local excision.289 By multivariate analysis, both local excision and perineural invasion were independent prognostic factors associated with a lower disease-free survival.
Local Excision Followed by Postoperative Therapy
Table 78-9 summarizes the results of local excision with and without postoperative therapy. It is important to note that most series are retrospective, single-institution studies with varying degrees of integration of chemotherapy (typically 5-FU–based) and relatively limited follow-up.
Table 78-9
Local Excision for T1–T3 Rectal Cancer Plus Postoperative Adjuvant Therapy
| Series | No. of Patients | 5-FU (%) | T3 (%) | Follow-up Time | Survival Rates | Initial Local Control (Salvaged with APR/Local Failures) |
| U. Florida368 | 45 | 4 | 2 | 2 years (minimum) | 88% 5-year disease specific | 89% (1/5) |
| Memorial Sloan-Kettering369 | 39 | 51 | 21 | 41 months (median) | 70% 5-year actuarial | 79% (5/8) |
| MD Anderson370 | 46 | 17 | 33 | 36 months (median) | 93% 3-year overall | 87% (-/4) |
| N.E. Deaconess371 | 48 | 54 | 10 | 41 months (mean) | 94% overall | 92% (3/4) |
| CALGB371 | 51* | 100 | 0 | 48 months (median) | 85% 6-year actuarial | 86% (4/7) |
| MGH372 | 47 | 55 | 0 | 51 months (median) | 74% 5-year disease free | 90% (5/9) |
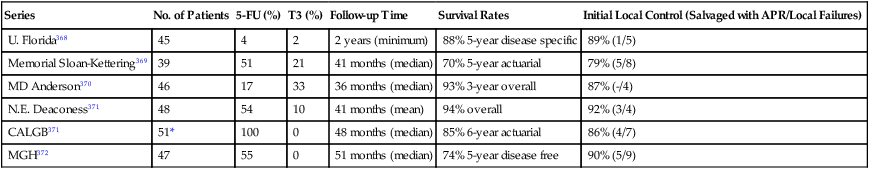
APR, Abdominoperineal resection; MGH, Massachusetts General Hospital; 5-FU, 5-flourouracil.
*Analysis is limited to the 51 of 110 patients with T2 disease who underwent a full-thickness local excision and received postoperative chemoradiotherapy.
When series are combined, the average crude local failure rate increases with T stage: pT1: 5%; pT2: 14%; and pT3: 22%.15,292–301 However, when the analysis is limited to the series with a minimum of 4-year follow-up296,299,301,302 the incidence of local recurrence for pT2 disease is 14% to 24%. Therefore, patients who are treated with local excision and postoperative adjuvant therapy require close follow-up beyond 5 years. Surgical salvage is possible with most series report that approximately half the of patients who undergo an APR can be cured.303
The Cancer and Leukemia Group B (CALGB) performed a phase II trial of local excision and selective postoperative chemoradiation (CALGB 8894).304 A total of 91% of patients underwent a full-thickness local excision. Patients with pT1 disease were observed and patients with pT2 received postoperative 54 Gy plus concurrent 5-FU. With a median follow-up of 4 years, the local recurrence rate in 59 patients with pT1 disease was 5% and with pT2 disease was 14%.
A separate analysis of the 110 patients who met the full eligibility criteria in CALGB 8894 was reported by Greenberg and colleagues.305 These were limited to tumors less than 4 cm, full-thickness excisions, and negative margins. Patients with pT1Nx tumors underwent local excision alone and those with pT2Nx received postoperative chemoradiation. With a median follow-up of 7.1 years, the local recurrence and 10-year actuarial survivals were T1: 8% and 84%, and T2: 18% and 66%, respectively. The median time to failure was T1: 4 years and T2: 2 years.
Sphincter Function
Prospective assessment of functional results is limited. The groups from Memorial Sloan Kettering300 and Gemelli Hospital, Rome,297 report 94% and 100% good-to-excellent function, respectively. Using a different scale, investigators from Fox Chase Cancer Center298 reported 82% good-to-excellent function, the University of Pennsylvania295 reported 92% satisfactory function, and MD Anderson294 reported that all patients were continent. In the preoperative setting sphincter function was reported good to excellent in 88% to 91%.306,307
Preoperative Therapy Followed by Local Excision
Experience with preoperative chemoradiation followed by local excision is more limited.52,306–311 Most series select patients with cT3 disease who were either medically inoperable or refused radical surgery. Local recurrence rates range from 0% to 20% and 5-year survival ranges from 78% to 90% (Table 78-10). Borschitz et al. reported local recurrence rates by pathological stage: ypT1: 2%; ypT2: 6% to 20%.311 The incidence was 43% in ypT3 tumors that did not respond. Kundel et al. examined 320 patients with T2-4N0-1 rectal cancer and reported a subset of 14 patients who underwent a local excision for ypT0 disease.271 With a median follow-up of 48 months, local recurrence or distant failure did not develop in any of the patients. In a compilation of 100 patients reported in 11 series, 7% had local recurrence and 8% had distant failure.
Table 78-10
Results of Preoperative Chemoradiation Followed by Local Excision
| Series | Median | % Recurrence | ||
| Follow-up (months) | # ypT0 | Local | Distant | |
| Schell306 | 48 | 8 | 0 | 12 |
| Hershman373 | 33 | 7 | 0 | 0 |
| Stipa374 | 37 | 7 | 0 | — |
| Ruo310 | 29 | 3 | 0 | 0 |
| Callender313 | 63 | 23 | 5 | 5 |
| Lezoche375 | 84 | 11 | 0 | 0 |
| Huh376 | 91 | 4 | 0 | 25 |
| Caricato377 | 47 (mean) | 3 | 0 | 0 |
| Borschitz311 | 24 | 1 | 0 | 0 |
| Nair378 | 64 | 19 | 5 | 5 |
| Kundel271 | 48 | 14 | 0 | 0 |
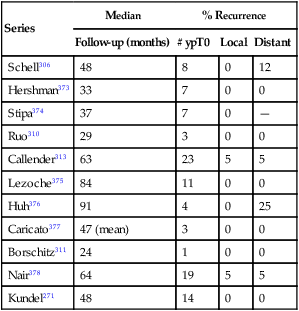
Table modified from Kundel et al.271
This approach has been examined prospectively in a trial from the American College of Surgeons Oncology Group (ACOSOG Z6031).312 Patients with uT2N0 disease received preoperative chemoradiation with CAPOX. Those with stage ypT0-2 and negative margins following a local excision had observation only. Patients with stage ypT3 and/or positive margins underwent radical surgery. A total of 77 patients were enrolled and the pCR rate was 43%. Local recurrence and survival results are pending. A similar trial from France (GRECCAR 2) will accrue 300 patients with cT2 to cT3 disease.
Preliminary data suggest that local excision may be an option in patients who are downstaged to ypT0 following chemoradiation. As illustrated by Callender et al. who reported that two of their five local recurrences occurred at 72 and 76 months, long-term follow-up is necessary.313 More accurate noninvasive methods to predict ypT0 disease are needed.