Cancer of the Esophagus
Lawrence Kleinberg, Ronan Kelly, Stephen Yang, Jean S. Wang and Arlene A. Forastiere
• Esophageal cancer is subdivided into the following four groups: epithelial tumors, metastatic tumors, lymphomas, and sarcomas.
• Cancers of epithelial origin, predominantly squamous cell and adenocarcinomas, are the most common, and other histologic types are rare.
• The appropriate categorization of gastroesophageal junction tumors has been controversial, and patients have been included in clinical trials directed both at esophageal and gastric cancers.
• Within the United States, the incidence of esophageal cancer in persons younger than 80 years is 3.2 per 100,000 persons.
• Historically and internationally, squamous cell tumors are the most common histologic type; however, a dramatic increase in the incidence of adenocarcinoma has been documented in the United States, United Kingdom, and Western Europe.
• The data support the hypothesis that epithelial tumors arise as a result of chronic irritation from a wide variety of sources, including gastric contents in chronic reflux and known carcinogens.
• A strong association of Barrett esophagus and adenocarcinoma is seen, but a benefit to screening endoscopy for those at risk for or with known Barrett esophagus is unknown as the overall risk of cancer-related mortality is low. Studies with longer-term follow-up are needed to clarify this issue. Other identified risk factors are gastroesophageal reflux disease (GERD), obesity, and smoking.
• Squamous cell carcinoma is associated with smoking as well as alcohol use, and the declining incidence has paralleled the decline in smoking.
• Point mutations, increased copy number, and promotor region hypermethylation all appear important in the progression to malignancy.
• Symptoms and demographics will strongly suggest the diagnosis.
• Endoscopy is the best screening examination but esophagram may also be used.
• Diagnosis is made by endoscopy with cytology and biopsy of tumor.
• Transesophageal ultrasound should be used to assess T and N stage to guide optimal definitive therapy.
• Computed tomography (CT) of chest and abdomen is useful in screening for metastatic disease.
• Positron emission tomographic (PET) scan is useful to detect additional cases of metastatic disease before costly and toxic definitive therapy. It may be superior to endoscopic ultrasound (EUS) in detecting intraabdominal lymph nodes, but not periesophageal nodes adjacent to the primary tumor.
• Additional studies include laparoscopy, thoracoscopy, bone scan, and CT of the brain when indicated by clinical circumstances.
• The new AJCC/UICC 7 staging system contains important changes: adenocarcinoma and squamous cell carcinoma are separate; grade of histology is incorporated; the gastroesophageal junction is defined; nodal staging is based on number of involved nodes, similar to gastric cancer; Tis includes high graded dysplasia; and T4 is subcategorized by features suggestive of resectability.
• Staging is based on pathological findings at the time of resection, but therapy is often guided by staging estimated by clinical testing.
• Treatment of premalignant dysplasia is guided by grade of histology. Low-grade dysplasia should be closely followed by endoscopy. High-grade dysplasia is treated with endoscopic therapy or esophagectomy, although close follow-up may be appropriate for selected patients.
• Selection of appropriate treatment for carcinoma depends on tumor stage and patient performance status.
• Surgery is an accepted single-modality therapy for patients with early localized disease (T1-2N0M0) or for patients who may not tolerate combined-modality therapy. The selection of surgical approach depends upon location and experience, but no approach has been demonstrated to lead to superior cure rates.
• Combined chemoradiation leads to prolonged median survival and long-term survival compared with radiation alone used as a definitive nonoperative approach, at the price of increased toxicity. This represents a potentially curative alternative to surgery for squamous cell cancers and is appropriate for most unresectable T4N and M0 lesions of either histology. Because most patients treated on prospective chemoradiation trials had squamous cell carcinomas, the benefits of nonoperative management for adenocarcinoma are not known.
• Randomized trials have not confirmed a survival benefit with surgery added to potentially curative chemoradiation in squamous cell carcinoma, but there was a significant local control benefit. This question has not been well studied for adenocarcinoma, for which definitive chemoradiation is of uncertain curative potential.
• Accumulating evidence convincingly demonstrates that combination therapy with preoperative chemoradiation followed by surgery survival compared with surgery alone for both locally advanced (clinically staged T2-T4 or node positive) adenocarcinoma and squamous cell carcinoma. There is less certainty about the value of preoperative chemotherapy alone.
• Postoperative adjuvant chemotherapy or chemoradiation is less well studied in locally advanced esophageal cancer, but trials in gastric cancer including gastroesophageal junction adenocarcinoma have demonstrated a benefit.
• Combined-modality chemotherapy regimens frequently include 5-fluorouracil (5-FU) or paclitaxel and platinum agents; other commonly used regimens include docetaxel and irinotecan.
• Endoscopic palliative therapy includes laser or electrical fulguration, mucosal resection, photodynamic therapy (PDT), or stenting. Excepting very superficial lesions, these therapies are not alternatives to surgery as they do not address deeper disease or lymphatic spread.
• Radiation therapy, with or without chemotherapy, may be used to palliate local symptoms.
• Chemotherapy may be used for metastatic disease, but response rates and duration of response are modest for most patients. Clinical trials are recommended.
Classification and Location
Esophageal cancer (Table 74-1) is classified based on histologic appearance and cell of origin, as follows: (1) epithelial tumors, (2) metastatic tumors, (3) lymphomas, and (4) sarcomas. Cancers of epithelial cell origin, predominantly squamous cell carcinoma and adenocarcinoma, are the most common. Although squamous cell carcinoma and adenocarcinoma were previously treated as similar entities and grouped together, there is increasing recognition that they should be studied as separate entities whose optimal therapy may diverge in the era of multimodality treatments.
Table 74-1
Classification of Esophageal Cancer
Epithelial
Squamous cell
Ordinary squamous cell
Verrucous squamous cell
Spindle cell (carcinosarcoma)
Adenocarcinoma
Ordinary
Adenoacanthoma
Mucoepidermoid
Adenoid cystic
Small cell
Melanoma
Choriocarcinoma
Metastatic disease
Lymphoma
Sarcoma
Squamous cell cancer usually occurs in the middle third of the esophagus. In a collective review of more than 28,000 cases of squamous cell cancers, Postlethwaite1 estimated the ratio of upper, middle, and lower cancers to be 15 : 50 : 35, respectively. Adenocarcinoma, on the other hand, is most common in the lower third of the esophagus. In a collective review of 4783 cases of esophageal adenocarcinoma, Ming2 noted an upper esophageal location in 4%, middle in 18%, and lower in 67%. Of the rarer primary histologic types, 95% of small cell cancers occur in the middle and lower thirds; both malignant melanoma and choriocarcinoma tend to occur in the lower third. Esophageal sarcomas may occur anywhere along the esophagus as is the case for esophageal lymphomas or metastases from other primary cancers.
Incidence
Tumors of the esophagus other than squamous cell and adenocarcinoma are quite rare. This chapter, therefore, focuses on esophageal squamous cell and adenocarcinoma. Epidemiologic data show that the incidence of esophageal cancer varies considerably from one country to another and often within a single country. This geographic diversity underscores the multifactorial etiologies of esophageal cancer worldwide of which more than 90% are squamous cell cancers. In the United States and other Western industrialized countries,3–9 however, over the past 3 decades there has been a slight decline in squamous cell esophageal cancer likely the result of decreased smoking. However, there has been a dramatic rise in adenocarcinoma of the distal esophagus and GEJ, especially in some Western nations likely related to diet and increased obesity. This histology has increased in incidence approximately sixfold and since the mid-1990s has been the predominant esophageal cancer in Caucasians.7 The absolute incidence in the United States has increased from 3.8 per million in 1973 to 1975 to 23.3 per million in 2001 based on the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database. This rate of increase exceeds that of all other cancers, including lung, breast, prostate, and melanoma. Adenocarcinoma is much less common in African Americans but has increased from 0.4/100,000 to 0.9/100,000, and 0 to 0.2/100,000 in females. In recent years, it appears that although the rate of increase may be slowing in the United States, the incidence of esophageal adenocarcinoma has continued to increase at least through the year 2009 to 25.8 per million,10 with additional evidence that the incidence of GEJ adenocarcinoma in particular may be stabilizing.11
Obesity and gastroesophageal reflux disease (GERD) appear to contribute to this rise in adenocarcinoma incidence. In contrast, the incidence of squamous cell carcinoma decreased in all of these groups during this period, perhaps because of a decline in the prevalence of smoking and increased consumption of fresh fruits and vegetables.12
Pathogenesis
Clinical Risk Factors
Two major risk factors for esophageal squamous cell carcinoma are alcohol use and tobacco smoking, according to epidemiologic studies from various countries worldwide. This relationship is dose-dependent and there is a multiplicative interaction of alcohol intake and tobacco use. In a prospective cohort study from the Netherlands involving more than 120,000 people with 16 years of follow-up, the strongest risk for squamous cell carcinoma was alcohol consumption with a 4.6-fold increased risk, whereas combined exposure with smoking increased the risk more than 8-fold. The risk of squamous cell carcinoma decreased with smoking cessation and alcohol abstinence but only after 1 to 2 decades.13
In adenocarcinoma, smoking but not alcohol is associated with increased risk. A prospective study of tobacco, alcohol, and risk of esophageal cancer in the United States found an increased risk of squamous cell carcinoma among current smokers compared with nonsmokers (hazard ratio: 9.27, 95% confidence interval [CI]: 4.04, 21.29) and also an increased risk for adenocarcinoma (hazard ratio: 3.70, 95% CI: 2.20, 6.22). For people who consumed more than three alcoholic drinks a day compared with one drink, there was increased risk of esophageal squamous cell carcinoma but not adenocarcinoma.14 A pooled analysis from an international consortium composed of 10 population-based case-control studies and 2 cohort studies found strong associations between smoking and adenocarcinoma (odds ratio [OR]: 2.08, 95% CI: 1.83, 2.37).15 However, alcohol was not found to be a risk factor for adenocarcinoma.16
Nutritional and dietary factors have been found to contribute to the risk of both squamous cell carcinoma and adenocarcinoma. Pickled vegetables, processed meat, and other foods containing nitrates have been linked to an increased risk of squamous cell carcinoma. Drinking very hot liquids or eating hot foods such as stewed meat frequently is postulated to cause thermal injury to the esophagus and has been found to be a risk factor for squamous cell carcinoma. Low consumption of fruits and vegetables are risk factors for both squamous cell carcinoma and adenocarcinoma.17
Obesity, defined as a body mass index of 30 or greater, is a strong risk factor for esophageal adenocarcinoma, as demonstrated in a meta-analysis of epidemiologic studies (OR: 2.78, 95% CI: 1.85, 4.16).18 This has clear implications for the increasing obesity rates in the United States and could in part explain the rising incidence of esophageal adenocarcinoma. The exact mechanism for this association of obesity is not understood but might reflect an increased propensity for gastroesophageal reflux. Obesity does not appear to be a risk factor for squamous cell carcinoma.
Adenocarcinoma: Role of GERD and Barrett Esophagus
Gastroesophageal reflux disease is a well-established risk factor for esophageal adenocarcinoma. A population-based case-control study in Sweden demonstrated an OR of 7.7 (95% CI: 5.3, 11.4) for development of esophageal cancer in patients with chronic reflux disease. With longstanding, severe symptoms, the OR for esophageal adenocarcinoma was 43.5 (95% CI: 18.3, 103.5).19 There was no association seen between reflux and squamous cell carcinoma. Interestingly, the increased risk of esophageal adenocarcinoma existed whether or not Barrett esophagus could be identified, leading the authors to speculate that the area of Barrett esophagus in these patients was overgrown by tumor. This theory has been supported by postchemotherapy studies showing a high prevalence of Barrett esophagus in adenocarcinoma patients.
The role of screening for Barrett esophagus is controversial. A nationwide population-based study in Denmark found that patients with known Barrett esophagus comprised only 7.6% of all diagnosed esophageal adenocarcinoma patients.20 Similarly, an analysis of U.S. administrative data found that only 8% of adenocarcinoma patients had been recognized to have Barrett esophagus prior to their cancer diagnosis.21 Therefore, the value of screening endoscopy in the setting of GERD has been questioned by those who point out that Barrett esophagus is uncommon, progression to malignancy is infrequent, and the effect of screening on overall population mortality from esophageal adenocarcinoma appears quite low. A large nationwide case-control study in Sweden found that among patients with adenocarcinoma, 62% had histologic evidence of Barrett esophagus but 40% did not have a history of gastroesophageal reflux.19 Thus screening endoscopy of individuals with symptomatic gastroesophageal reflux will still miss the substantial proportion of individuals with esophageal adenocarcinoma who do not report reflux symptoms. The American Gastroenterological Association and American College of Gastroenterology guidelines state that there is currently insufficient evidence to recommend routine screening for Barrett esophagus in patients with gastroesophageal reflux symptoms and that the decision to screen a patient should be individualized.22,23
The prevalence of Barrett esophagus is estimated to be 1% to 2% of the general population.24 The length of time for progression from Barrett esophagus to dysplasia to adenocarcinoma is unknown. Many advocate lifelong endoscopic surveillance for patients with Barrett mucosa with the goal of treating dysplastic changes and thereby reducing the risk of developing adenocarcinoma. Indeed, tumors that are discovered during surveillance appear to be of an earlier stage and therefore to have a higher chance of cure.25 Still, it is not certain whether surveillance reduces mortality; the overall risk of death from esophageal cancer is relatively low even in this high-risk population. Furthermore, surveillance of Barrett esophagus patients with upper endoscopy is fraught with the problem of sampling error when biopsies are performed during endoscopy and to a high degree of interobserver variability in dysplasia grading.
Several centers have reported results of endoscopic surveillance programs that suggest that progression of Barrett esophagus to adenocarcinoma might be less common than was originally thought, at least in the short term. A nationwide population-based cohort study in Denmark followed up with more than 11,000 patients with Barrett esophagus for a median of 5.2 years and found that the annual risk of adenocarcinoma was 0.12% (95% CI: 0.09, 0.15).20 Meanwhile, a meta-analysis of 57 studies involving more than 11,000 patients with nondysplastic Barrett esophagus found a pooled annual incidence rate of adenocarcinoma of 0.33%.26 Patients with low-grade dysplasia had an incidence rate of adenocarcinoma of 0.5% per year, whereas those with high-grade dysplasia have an incidence rate of adenocarcinoma of 3% to 5% per year.
Molecular Progression to Adenocarcinoma
Molecular genetic data support the histologic observation that there is a progression from normal epithelium to Barrett esophagus to dysplasia to adenocarcinoma.27–32 Although a clearly defined sequence of genetic alterations leading to adenocarcinoma has not been defined, an accumulation of abnormalities27,33–35 has been identified in a wide range of genes that regulate proliferation, apoptosis, invasion, metastasis, angiogenesis, growth, and cell cycle regulation. Tumor suppressor genes have been implicated as early events, as loss of cell cycle checkpoints may be permissive for genetic instability, allowing later transformation in the metaplasia–dysplasia–adenocarcinoma sequence.
In the last decade,36 epigenetic modifications have emerged as heritable and fundamental features of most malignancies, including esophageal adenocarcinoma.37–42 The best-studied epigenetic modification of the DNA is promoter region hypermethylation, an epigenetic modification that is associated with gene inactivation. A meaningful understanding of the molecular events that result in progression to adenocarcinoma will likely require a greater understanding of this phenomenon as it occurs at least as frequently as point mutations. In oncogenesis, hypermethylation is often associated with inactivation of tumor suppressor genes, of genes that suppress metastasis and angiogenesis, as well as of genes that repair DNA. Methylation of DNA occurs mostly at CpG sites in the genome and is catalyzed by a family of three active DNA methyl-transferases that transfer a methyl group from S-adenosyl-methionine to cytosine to form 5-methylcytosine. Because this reaction can be blocked effectively by a drug, 5-azacytidine, which acts as an irreversible inhibitor of the DNA methyltransferases, the therapeutic potential inherent in reversing DNA hypermethylation is significant.43
One of the earliest events that is thought to occur in the molecular progression of Barrett esophagus to adenocarcinoma is inactivation of one of the alleles of the tumor suppressor gene p16 via DNA hypermethylation, loss of heterozygosity (LOH), or mutations. This event is thought to be triggered by chronic inflammation secondary to acid and bile reflux and has been found to occur at the stage of Barrett esophagus with no dysplasia. The p16 gene is located on the short arm of chromosome 9 and is a cyclin-dependent kinase inhibitor which regulates the cell cycle.29,30,44,45 When p16 is inactivated, this promotes phosphorylation of the retinoblastoma protein, leading to proliferation. This clone of cells may expand and subsequently there may be loss of the second p16 allele as a result of LOH and the formation of several p16 null clones. When Barrett cells begin to acquire the hallmarks of cancer, there is clonal expansion of cells that have a selective growth advantage because of the genetic or epigenetic changes they have acquired.
Studies have also documented the importance of p53 inactivation,46–49 the loss of one allele of the tumor suppressor gene, p53, via mutation. Later, loss of the second allele of p53 via LOH may occur, resulting in the inactivation of p53. The gene p53 is located on the short arm of chromosome 17 and is involved in regulating cell cycle control. When cells sustain DNA damage and cannot be repaired, p53 is responsible for inducing apoptosis and preventing the replication of genetic instability. Abnormalities in p53 usually occur when one allele has been deleted (usually via mutation) and the other allele is functionally inactivated, often because of LOH in a two-hit mechanism. Therefore, inactivation of p53 removes the ability to repair DNA damage and leads to the replication of genetic instability. Missense mutations of the p53 gene cause the protein to have a much longer half-life than normal, resulting in accumulation in the nucleus, where its overexpression can then be detected by immunohistochemical staining. However, p53 staining has been found to be inaccurate in some cases, leading to high false-positive and false-negative rates. Meanwhile, detection of p53 LOH appears to be a more accurate marker of p53 gene abnormalities and has been found to be a predictor of progression to cancer. The loss of p53 is thought to be involved in the progression from Barrett esophagus with no dysplasia to low-grade dysplasia. The inactivation of p53 leads to loss of cell cycle regulation, which may promote genomic instability and aneuploidy, leading to additional changes required for progression to high-grade dysplasia and malignancy.
Genomic instability is detected via DNA content abnormalities such as aneuploidy, which refers to gains or losses in parts of chromosomes. Aneuploidy in fact has been one of the most studied markers for neoplastic progression in Barrett esophagus.50 Several prospective studies using flow cytometry within a large cohort of Barrett esophagus patients have demonstrated the presence of aneuploidy during the progression from Barrett esophagus to adenocarcinoma. This promotes the formation of multiple clones, especially as the degree of genetic instability increases, and this clonal diversity ultimately leads to the development of cancer.22
Several recent studies have found that a combination panel of biomarkers is a better predictor of progression to adenocarcinoma than individual biomarkers alone. Recently, a prospective study of Barrett esophagus patients found that a combination of DNA content abnormalities (tetraploidy and aneuploidy), p16 LOH, and p53 LOH provided the best prediction of risk for adenocarcinoma (RR: 38.7, 95% CI: 10.8, 138.5). Patients with all of these findings had at least a 79% adenocarcinoma risk over 10 years, whereas patients with none of these findings had only a 12% risk of adenocarcinoma over 10 years.51 In another prospective study of Barrett esophagus patients, the combination of genetic instability and clonal expansion predicted progression to adenocarcinoma. The investigators defined the size of the clone (x) as the length of the Barrett abnormality multiplied by the portion of the cells in the biopsy flow cytometry specimens that carry the lesion. Taking into account the sizes of clones assessed in this way, relative risks for progression to adenocarcinoma, in comparison with those without the clonal abnormalities, were determined. For p53 LOH, the relative risk (RR) was 1.27x for an x cm clone (95% CI: 1.07, 1.50) and for aneuploidy/tetraploidy the RR was 1.31x (95% CI: 1.07, 1.60). A 5-cm clone containing either p53 LOH or aneuploidy/tetraploidy was associated with an RR of 4.16 (95% CI: 2.01, 8.95) for progression to adenocarcinoma.33
There appears to be a diversity of molecular abnormalities in esophageal cancer caused by actual genetic mutations, epigenetic inactivation, and altered cell regulation. The method of identifying and codifying alterations in these genes into a clinically useful paradigm has not yet proved superior to standard histology for predicting outcome, but more complex analyses using sophisticated molecular techniques such as genomic arrays could be helpful in the future. In the meantime, new molecular abnormalities continue to be identified.52
Overview: the Choice of Therapy
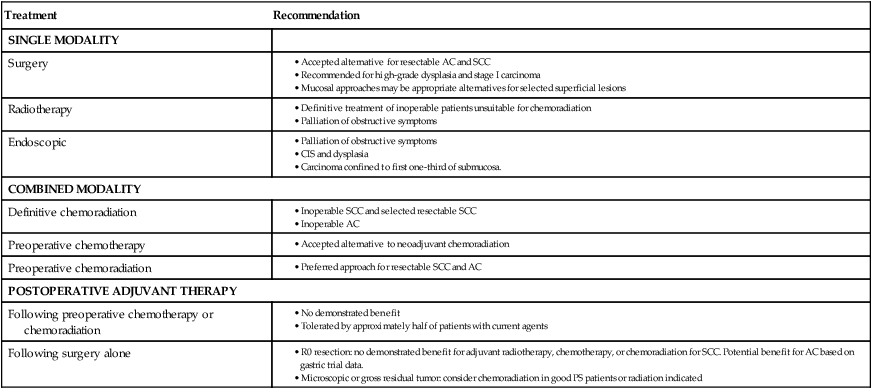
Endoscopic approaches may be curative for very early lesions with no more than superficial submucosal invasion, which are also curable with surgical resection and often radiation. Other early lesions, stage I-IIa, may be appropriately treated with esophagectomy as a single modality for suitable operative candidates. There has been substantial controversy about the optimal management of more advanced but still curable (localized) esophageal cancer where the treatment options include surgery alone, chemotherapy followed by surgery or adjuvant chemotherapy after resection, chemoradiation followed by surgery, and definitive chemoradiation. Radiation alone as definitive treatment aimed at cure is inferior to combined chemoradiation for locally advanced disease and should only be considered when the other options are not feasible. Similarly, radiotherapy used as a single adjuvant or neoadjuvant therapy has not been shown to improve outcome. These options are summarized in Table 74-2.
Table 74-2
Options in the Therapy of Esophageal Carcinoma
| Treatment | Recommendation |
| SINGLE MODALITY | |
| Surgery |
AC, Adenocarcinoma of the distal esophagus; SCC, squamous cell carcinoma.
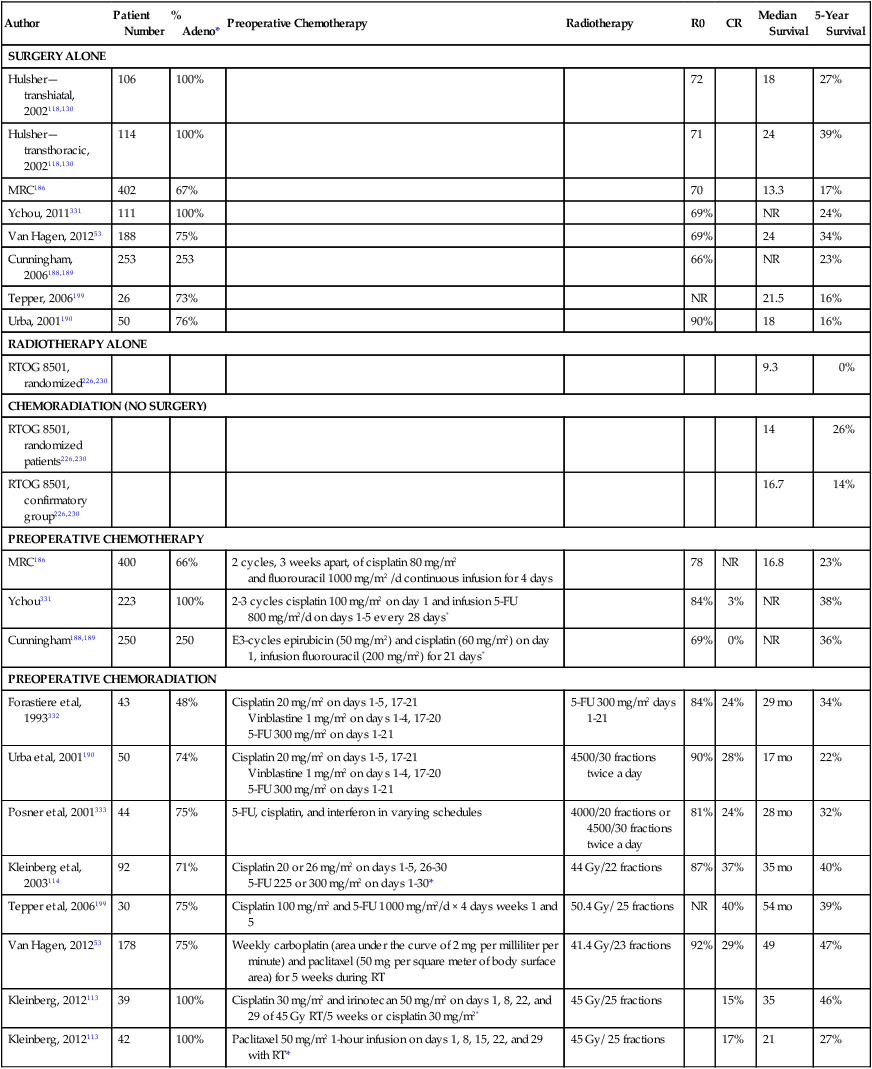
The long-term survival outcome of patients with more locally advanced disease enrolled in selected prospective trials reporting 5-year survival outcomes is summarized in Table 74-3 according to treatment approach. Although patient selection factors may have differed among the trials, there has tended to be better outcome with use of neoadjuvant therapy, either chemoradiotherapy or chemotherapy, compared with the outcome of surgery alone. The benefit of this approach for locally advanced esophageal cancer has been confirmed in randomized trials and is increasingly accepted as the optimal approach. Although never definitively compared in a completed randomized trial, these long-term results suggest a greater benefit to combined neoadjuvant chemoradiation compared with chemotherapy alone, an observation supported by data summarized below. Although radiation as a single therapy is very rarely curative and is generally only useful for palliation, definitive chemoradiation has been demonstrated to be a potentially curative alternative to surgery for locally advanced squamous cell carcinoma, and is appropriate for patients with adenocarcinoma as well who have unresectable primary tumors or inoperable disease because of medical co-morbidities. Adjuvant therapy has been less extensively studied but may be appropriate for patients with locally advanced disease who did not receive preoperative therapy. There are no data to support the concept that chemoradiation is useful to convert an unresectable lesion into a resectable lesion.
Table 74-3
Published Prospective Series With 5-Year Follow-up
| Author | Patient Number | % Adeno* | Preoperative Chemotherapy | Radiotherapy | R0 | CR | Median Survival | 5-Year Survival |
| SURGERY ALONE | ||||||||
| Hulsher—transhiatal, 2002118,130 | 106 | 100% | 72 | 18 | 27% | |||
| Hulsher—transthoracic, 2002118,130 | 114 | 100% | 71 | 24 | 39% | |||
| MRC186 | 402 | 67% | 70 | 13.3 | 17% | |||
| Ychou, 2011331 | 111 | 100% | 69% | NR | 24% | |||
| Van Hagen, 201253 | 188 | 75% | 69% | 24 | 34% | |||
| Cunningham, 2006188,189 | 253 | 253 | 66% | NR | 23% | |||
| Tepper, 2006199 | 26 | 73% | NR | 21.5 | 16% | |||
| Urba, 2001190 | 50 | 76% | 90% | 18 | 16% | |||
| RADIOTHERAPY ALONE | ||||||||
| RTOG 8501, randomized226,230 | 9.3 | 0% | ||||||
| CHEMORADIATION (NO SURGERY) | ||||||||
| RTOG 8501, randomized patients226,230 | 14 | 26% | ||||||
| RTOG 8501, confirmatory group226,230 | 16.7 | 14% | ||||||
| PREOPERATIVE CHEMOTHERAPY | ||||||||
| MRC186 | 400 | 66% | 2 cycles, 3 weeks apart, of cisplatin 80 mg/m2 and fluorouracil 1000 mg/m2 /d continuous infusion for 4 days |
78 | NR | 16.8 | 23% | |
| Ychou331 | 223 | 100% | 2-3 cycles cisplatin 100 mg/m2 on day 1 and infusion 5-FU 800 mg/m2/d on days 1-5 every 28 days* | 84% | 3% | NR | 38% | |
| Cunningham188,189 | 250 | 250 | E3-cycles epirubicin (50 mg/m2) and cisplatin (60 mg/m2) on day 1, infusion fluorouracil (200 mg/m2) for 21 days* | 69% | 0% | NR | 36% | |
| PREOPERATIVE CHEMORADIATION | ||||||||
| Forastiere et al, 1993332 | 43 | 48% | Cisplatin 20 mg/m2 on days 1-5, 17-21 Vinblastine 1 mg/m2 on days 1-4, 17-20 5-FU 300 mg/m2 on days 1-21 |
5-FU 300 mg/m2 days 1-21 | 84% | 24% | 29 mo | 34% |
| Urba et al, 2001190 | 50 | 74% | Cisplatin 20 mg/m2 on days 1-5, 17-21 Vinblastine 1 mg/m2 on days 1-4, 17-20 5-FU 300 mg/m2 on days 1-21 |
4500/30 fractions twice a day | 90% | 28% | 17 mo | 22% |
| Posner et al, 2001333 | 44 | 75% | 5-FU, cisplatin, and interferon in varying schedules | 4000/20 fractions or 4500/30 fractions twice a day | 81% | 24% | 28 mo | 32% |
| Kleinberg et al, 2003114 | 92 | 71% | Cisplatin 20 or 26 mg/m2 on days 1-5, 26-30 5-FU 225 or 300 mg/m2 on days 1-30* |
44 Gy/22 fractions | 87% | 37% | 35 mo | 40% |
| Tepper et al, 2006199 | 30 | 75% | Cisplatin 100 mg/m2 and 5-FU 1000 mg/m2/d × 4 days weeks 1 and 5 | 50.4 Gy/ 25 fractions | NR | 40% | 54 mo | 39% |
| Van Hagen, 201253 | 178 | 75% | Weekly carboplatin (area under the curve of 2 mg per milliliter per minute) and paclitaxel (50 mg per square meter of body surface area) for 5 weeks during RT | 41.4 Gy/23 fractions | 92% | 29% | 49 | 47% |
| Kleinberg, 2012113 | 39 | 100% | Cisplatin 30 mg/m2 and irinotecan 50 mg/m2 on days 1, 8, 22, and 29 of 45 Gy RT/5 weeks or cisplatin 30 mg/m2* | 45 Gy/25 fractions | 15% | 35 | 46% | |
| Kleinberg, 2012113 | 42 | 100% | Paclitaxel 50 mg/m2 1-hour infusion on days 1, 8, 15, 22, and 29 with RT* | 45 Gy/ 25 fractions | 17% | 21 | 27% | |
*Patient either had adenocarcinoma (adeno) or squamous cell carcinoma. R0 is complete resection with negative margins.
Given the lack of randomized comparative data to demonstrate superiority of any one of the combined-modality approaches for resectable disease, clinical decision making for optimal management of locally advanced disease remains complex and controversial. Previously available data from phase II trials, underpowered phase III trials, and metaanalyses described below indicate improved local control and suggest a survival benefit on the order of 10%, which lead to acceptance of the use of neoadjuvant chemoradiation. Recently the results have become available for the well-powered Chemoradiotherapy for Esophageal Cancer Followed by Surgery Study (CROSS)53 trial, described below, which has definitively demonstrated a survival benefit to neoadjuvant paclitaxel, carboplatin, and radiotherapy in a population largely consisting of patients with adenocarcinoma. Commonly used preoperative chemoradiation regimens are associated with substantial toxicity, and therefore, trimodality therapy should be used cautiously in patients with poor performance status or co-morbid conditions that increase the risk of life-threatening toxicity. Such patients might be better treated with surgery alone, or with combined chemoradiation (no surgery) for squamous cell carcinoma, for which this has been well demonstrated to be a potentially curative alternative to resection.
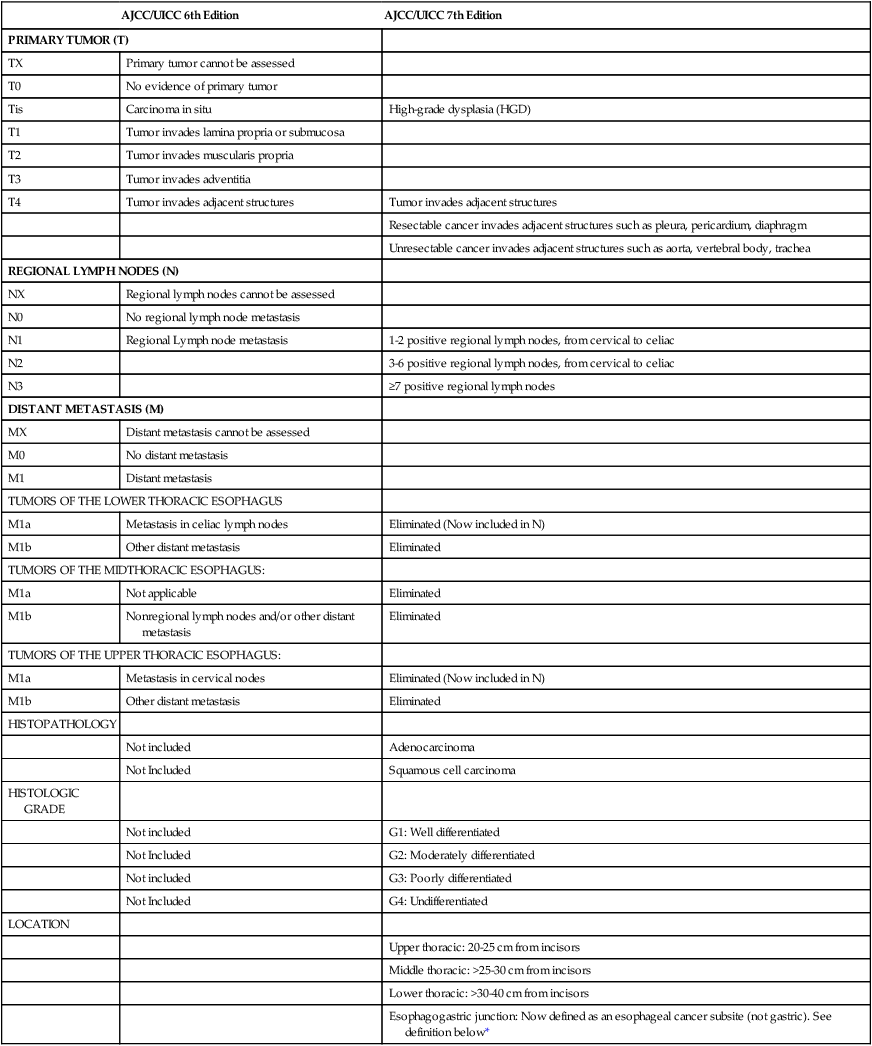
Staging and Diagnosis: American Joint Committee on Cancer/International Union Against Cancer 7, a Substantially Revised Staging System
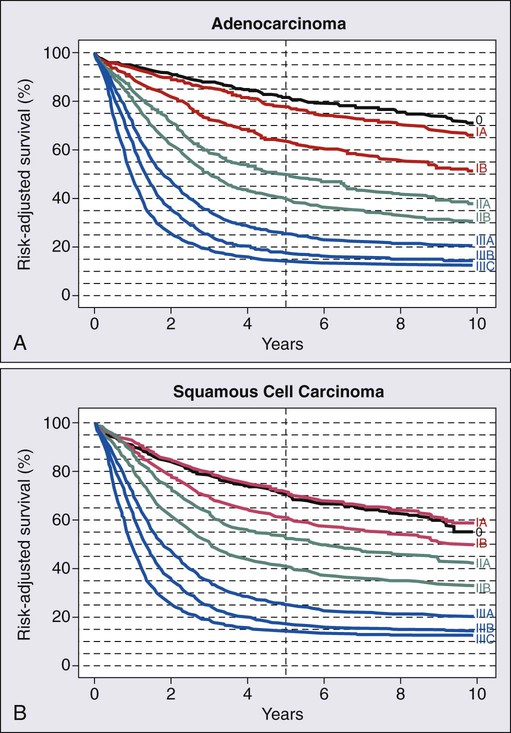
The updated AJCC/International Union Against Cancer (UICC) 7th edition staging system was implemented in 2010.56–56 The 7th edition staging meaningfully differs from the 6th edition AJCC/UICC system. These staging systems and their differences are summarized in Tables 74-4 through 74-6. An important limitation similar to both systems is that they are based on surgical pathology, whereas many patients are treated with neoadjuvant therapies based on clinical and radiographic estimation of the stages. Not only is clinical staging subject to more uncertainty and error, but the use of preoperative therapy may change the prognostic significance of pathological staging criteria in those who are downstaged as a result of response to therapy and in those who progress during treatment. The trial results reported in this chapter are based on the AJCC/UICC 6th or earlier editions of the staging system in effect until recently. Survival by stage for adenocarcinoma and squamous cell carcinoma patients is summarized in Figure 74-1, for patients included in a worldwide database of 4627 surgically treated patients.57
Table 74-4
TNM Staging for Esophagus: Comparison of AJCC 6th and 7th editions
| AJCC/UICC 6th Edition | AJCC/UICC 7th Edition | |
| PRIMARY TUMOR (T) | ||
| TX | Primary tumor cannot be assessed | |
| T0 | No evidence of primary tumor | |
| Tis | Carcinoma in situ | High-grade dysplasia (HGD) |
| T1 | Tumor invades lamina propria or submucosa | |
| T2 | Tumor invades muscularis propria | |
| T3 | Tumor invades adventitia | |
| T4 | Tumor invades adjacent structures | Tumor invades adjacent structures |
| Resectable cancer invades adjacent structures such as pleura, pericardium, diaphragm | ||
| Unresectable cancer invades adjacent structures such as aorta, vertebral body, trachea | ||
| REGIONAL LYMPH NODES (N) | ||
| NX | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastasis | |
| N1 | Regional Lymph node metastasis | 1-2 positive regional lymph nodes, from cervical to celiac |
| N2 | 3-6 positive regional lymph nodes, from cervical to celiac | |
| N3 | ≥7 positive regional lymph nodes | |
| DISTANT METASTASIS (M) | ||
| MX | Distant metastasis cannot be assessed | |
| M0 | No distant metastasis | |
| M1 | Distant metastasis | |
| TUMORS OF THE LOWER THORACIC ESOPHAGUS | ||
| M1a | Metastasis in celiac lymph nodes | Eliminated (Now included in N) |
| M1b | Other distant metastasis | Eliminated |
| TUMORS OF THE MIDTHORACIC ESOPHAGUS: | ||
| M1a | Not applicable | Eliminated |
| M1b | Nonregional lymph nodes and/or other distant metastasis | Eliminated |
| TUMORS OF THE UPPER THORACIC ESOPHAGUS: | ||
| M1a | Metastasis in cervical nodes | Eliminated (Now included in N) |
| M1b | Other distant metastasis | Eliminated |
| HISTOPATHOLOGY | ||
| Not included | Adenocarcinoma | |
| Not Included | Squamous cell carcinoma | |
| HISTOLOGIC GRADE | ||
| Not included | G1: Well differentiated | |
| Not Included | G2: Moderately differentiated | |
| Not included | G3: Poorly differentiated | |
| Not Included | G4: Undifferentiated | |
| LOCATION | ||
| Upper thoracic: 20-25 cm from incisors | ||
| Middle thoracic: >25-30 cm from incisors | ||
| Lower thoracic: >30-40 cm from incisors | ||
| Esophagogastric junction: Now defined as an esophageal cancer subsite (not gastric). See definition below* | ||
AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis.
*Includes cancer with an epicenter in the distal thoracic esophagus, esophagogastric junction, or within the proximal 5 cm of the stomach (cardia) that extend into the esophagogastric junction or distal thoracic esophagus.
From Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721–4.
Table 74-5
Stage Groupings for Esophageal Cancer, AJCC/UICC 6th Edition
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage IIA | T2 | N0 | M0 |
| T3 | N0 | M0 | |
| Stage IIB | T1 | N1 | M0 |
| T2 | N1 | M0 | |
| Stage III | T3 | N1 | M0 |
| T4 | Any N | M0 | |
| Stage IV | Any T | Any N | M1 |
| Stage IVA | Any T | Any N | M1a |
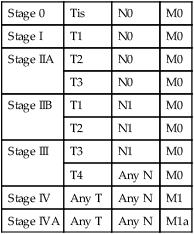
AJCC/UICC, American Joint Committee on Cancer/International Union against Cancer.
From Greene F, Page D, Fleming I. Esophagus. In: American Joint Committee on Cancer (AJCC) cancer staging manual. 6th ed. New York: Springer; 2002. p. 167.
Table 74-6
Stage Groupings for AJCC/UICC 7th Edition, Based on Histology
| Adenocarcinoma | Squamous Cell Carcinoma | ||||||||
| Stage | T | N | M | G | T | N | M | G | Location |
| 0 | Is or HGD | 0 | 0 | 1 | Is or HGD | 0 | 0 | 1 | Any |
| IA | 1 | 0 | 0 | 1-2 | 1 | 0 | 0 | 1 | Any |
| 1 | 0 | 0 | 3 | 1 | 0 | 0 | 2-3 | Any | |
| IB | 2 | 0 | 0 | 1-2 | 2-3 | 0 | 0 | 1 | Lower |
| 2 | 0 | 0 | 3 | 2-3 | 0 | 0 | 1 | Upper, Middle | |
| IIA | 2-3 | 0 | 0 | 2-3 | Lower | ||||
| IIB | 3 | 0 | 0 | Any | 2-3 | 0 | 0 | 2-3 | Upper, Middle |
| 1-2 | 1 | 0 | Any | 1-2 | 1 | 0 | Any | Upper, Middle | |
| IIIA | 1-2 | 2 | 0 | Any | 1-2 | 2 | 0 | Any | Any |
| 3 | 1 | 0 | Any | 3 | 1 | 0 | Any | Any | |
| 4a | 0 | 0 | Any | 4a | 0 | 0 | Any | Any | |
| IIIB | 3 | 2 | 0 | Any | 3 | 2 | 0 | Any | Any |
| IIIC | 4a | 1-2 | 0 | Any | 4a | 1-2 | 0 | Any | Any |
| 4b | Any | 0 | Any | 4b | 1-2 | 0 | Any | Any | |
| Any | N3 | 0 | Any | Any | Any | 0 | Any | Any | |
| IV | Any | Any | 1 | Any | Any | Any | 1 | Any | Any |
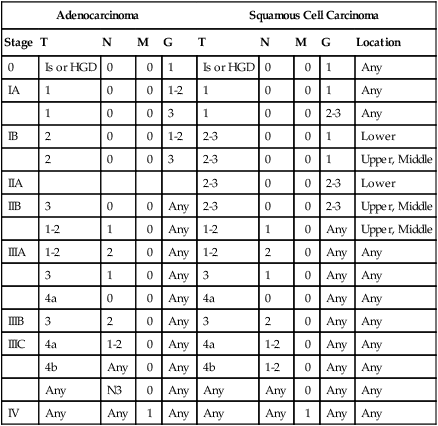
AJCC/UICC, American Joint Committee on Cancer/International Union against Cancer.
From Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: Esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721–4.
There are several important changes based on the new system related to classification of the primary tumor. Now adenocarcinoma and squamous histology are included as elements in esophageal staging. These histologies were originally grouped together because the prognosis appeared similar, surgical approaches were similar, and adenocarcinoma was uncommon. However, in the era of novel and targeted systemic agents, it is likely that treatment strategies and prognosis by stage will diverge. As therapies become more individualized, the difference between adenocarcinoma and squamous cell carcinoma may be more important in therapeutic management. The molecular and biological differences as well as the differing predominant location within the esophagus may lead to differentiation. However, historically surgical and radiotherapeutic management has been similar, with equally poor outcome.58 In contrast, for a group of 164 patients treated with neoadjuvant chemotherapy, generally 5-FU and cisplatin, the outcomes differed for adenocarcinoma and squamous cell carcinoma, including 35% versus 54% for pathological complete response (pCR), 71% versus 100% for 3-year local control, and 61% versus 79% 3-year systemic control, respectively.59 Grade of histology is also considered for stage assignment in the AJCC/UICC 7 system.
The N staging for esophageal cancer, previously stochastic, is significantly different than before. In the previous systems applicable to most of the currently available data, any positive local and regional nodes were categorized as N1 except for supraclavicular or celiac axis nodes, which were categorized as M1a according to specifications defined in Table 74-4. The new system eliminates the M1a category and merges these echelons of nodes into the “count” of involved regional nodes used to define N stage. This is similar to the categorization used for gastric cancer, of importance for GEJ tumors that may have a pattern of lymph node spread characteristic of both esophageal and gastric cancer.
This change in the N staging system is of uncertain importance in developing the treatment pathways at the moment because even pathological N1 patients with 1 to 2 nodes under the new system have sufficiently poor outcomes to justify additional treatment. In a series of 2920 surgically treated patients, 5- and 10-year survivals were 34% and 24% for N1 (1 to 2 nodes) disease, which can motivate use of combined-modality therapies.60 Previously, any patient with nodal disease was staged as N1 and was often, depending on clinical circumstances, considered a candidate for combined-modality therapy. Although it appears reasonable to continue neoadjuvant and adjuvant therapy for all groupings of node-positive patients, more data would be needed to confirm a benefit. Advanced nodal disease, either by celiac/supraclavicular involvement or N3 status by the older or newer staging systems, respectively, appear to have a very poor prognosis, and the potential for cure remains uncertain.
As therapies become more individualized, the difference between adenocarcinoma and squamous cell carcinoma may be more important in therapeutic management. The molecular and biological differences as well as the differing predominant location within the esophagus may lead to differentiation among the approaches in the future (especially as targeted therapies become available). However, historically surgical and radiotherapeutic management has been similar, with equally poor outcome.58 In contrast, for a group of 164 patients treated with neoadjuvant chemotherapy, generally 5-FU and cisplatin, the outcomes differed for adenocarcinoma and squamous cell carcinoma, including 35% versus 54% for pCR, 71% versus 100% for 3-year local control, and 61% versus 79% for 3-year systemic control, respectively.59
Recognition of GEJ Adenocarcinoma as an Entity: AJCC 7 Staging Classification and the Siewert Definition
The issue of how to categorize adenocarcinoma of the distal esophagus and GEJ has been of increasing interest, as the incidence itself increases. Siewert from the Technical University of Munich proposed a definition and an anatomic classification system in the 1980s.61–64 An important aim was to enable clearer communication about therapeutic approaches and treatment results related to the differing anatomical locations affected. This classification approach was based on preoperative assessment.
The classification was based on the anatomical location of the tumor, recognizing that in the region of the distal esophagus this could influence optimal selection of surgical approaches and the pattern of lymph node spread. The determination was made preoperatively using results of barium esophagram, endoscopy with orthograde and retroflexed view of the esophagogastric junction, computed tomography, and also additional intraoperative observations. The actual GEJ itself was considered the “upper end of the typical longitudinal fold of the gastric mucosa.” This anatomic or endoscopic definition was expected to guide decisions made preoperatively, but also recognized that intraoperative findings could provide useful further guidance. The Siewert classification system is described in Table 74-7.
Table 74-7
Classification of Gastroesophageal Junction Adenocarcinoma as Defined by Siewert
| Group | Description |
| I | Adenocarcinoma of the distal esophagus, which usually arises from an area with specialized intestinal metaplasia of the esophagus, i.e., Barrett esophagus, and may infiltrate the esophagogastric junction from above |
| II | Adenocarcinoma of the distal esophagus, which usually arises from an area with specialized intestinal metaplasia of the esophagus, i.e., Barrett esophagus, and may infiltrate the esophagogastric junction from above |
| III | Adenocarcinoma of the distal esophagus, which usually arises from an area with specialized intestinal metaplasia of the esophagus, i.e., Barrett esophagus, and may infiltrate the esophagogastric junction from above |
From Siewert JR, Stein HJ, Feith M. Adenocarcinoma of the esophago-gastric junction. Scand J Surg 2006;95(4):260–9.
Siewert’s group provided data supporting this classification system: 1602 patients63 with 5-year and 10-year survival rates for R0 resected (complete resection with negative margins) patients of 43.2% and 32.7%. For those who had microscopic or macroscopic residual (R1 and R2 resection), the survival rate was 11.1% and 6.2% at 5 and 10 years (P < 0.0001). These data provide not only baseline outcome data relevant to each class but also emphasize the importance of achieving a gross total resection.
Diagnostic and Staging Evaluation
The most common presentation of esophageal carcinoma is solid food dysphagia and weight loss of several months’ duration. Other presentations that occur with esophageal adenocarcinoma in particular include chest pain in the absence of myocardial ischemia, and anemia from a chronic gastrointestinal bleed from the mucosal lesion. These clinical signs and symptoms should prompt endoscopic evaluation and diagnostic imaging. The diagnosis is usually evident by the characteristic narrowing of the esophagus on barium esophagogram, but endoscopy and biopsy are essential for histopathological diagnosis. Endoscopic biopsies and brushings of the lesion will yield the diagnosis in more than 90% of patients. Multiple biopsies may be necessary to obtain the diagnosis of an invasive malignancy that is submucosal or necrotic.65,66 A diagnosis of in situ carcinoma or dysplasia in the face of a large lesion seen on endoscopic or radiographic studies should not be accepted, and biopsy should be repeated to confirm the extent of invasion and guide optimal management.
Once the pathological diagnosis is established, evaluation to determine the extent of disease should include a computed tomographic (CT) scan of the chest and complete abdomen. Although CT is not useful for determining depth and length of invasion within the esophageal wall, it may be quite helpful in assessing for possible invasion into neighboring structures and metastatic disease. The chest CT is useful for evaluating lung parenchyma and mediastinal structures.67 Lymph nodes more than 1 cm in diameter or with necrotic centers suggest metastatic involvement, although infection remains a possible cause and small lymph nodes may still contain deposits of cancer. The chest CT also is helpful for assessing aortic or pericardial invasion of tumor that would preclude esophagectomy. The most common locations for metastatic disease include lung, liver, and bone.
The accuracy of identifying metastases to the liver and celiac axis by abdominal CT depends on the bulk of the disease. Small liver metastases, peritoneal studding, and abdominal nodes will often be undetectable.67–73 For squamous cell lesions of the upper and midthoracic esophagus, a CT scan of the upper abdomen that includes the liver and adrenals is sufficient. For the patient with an adenocarcinoma of the distal esophagus, GEJ, or cardia, a complete abdominal-pelvic CT is recommended to visualize potential areas of nodal metastases. Cancers of this histologic type are more likely to metastasize early to periaortic lymph nodes. A complaint of back pain may signal the presence of enlarged retroperitoneal nodes.
Positron emission tomography (PET) scanning enables the identification of metastatic disease in patients who might otherwise inappropriately receive definitive local therapy and, therefore, is now considered a standard if not mandatory staging test. Studies demonstrated that PET will detect unsuspected metastatic disease in approximately 15% of patients after all other staging tests are completed, although it is not as useful as other techniques in identifying involved regional nodes. A prospective study of 79 patients74 found the specificity and sensitivity of PET for identifying stage IV disease was 90% and 74% versus 47% and 78% for the combination of CT and endoscopic ultrasonography (EUS), with the overall accuracy of identification of stage IV disease of 82% versus 64% (P = 0.004). Furthermore, when PET was added to CT and EUS, 22% of patients had a change in stage that altered their planned treatment (15% upstaged to incurable stage IV disease and 7% downstaged to a stage in which curative therapy would be appropriate). Other investigators have confirmed that PET scanning will change management from curative to palliative in 10% to 20% of patients, while also occasionally demonstrating that suspicious findings on other staging tests did not represent metastatic disease.75,76 Combined PET/CT imaging allows viewing of both sets of images in register and is optimal for accurate identification of smaller volume metastatic tumor.77,78 A consensus panel has confirmed the recommendation for PET imaging in possibly localized esophageal cancer to better detect metastatic disease, although the optimal, most cost-effective timing of this test within the sequence of tests could not be determined based on the extensive literature review.79 The importance of PET scanning lies in providing useful information about regional lymph node spread sufficient to alter therapy, as peritumoral nodes may merge in the imaging with the primary tumor, minimal involvement is not detectible, and the number of positive nodes which is used in the AJCC 7 staging system is not possible to assess. For example, a series of 102 patients clinically staged as T2-3N0M0 by a combination of EUS, CT, and PET were later found to have nodal disease in 70% of patients undergoing an adequate nodal dissection. PET is not valuable in estimating T stage as tissue planes are not visible with this imaging modality.
Accurate determination of the extent of disease has a major impact on therapeutic decision making for single-modality versus multimodality treatment or curative versus palliative intent, and, therefore, it is essential that comprehensive staging is performed. A substantial literature now exists regarding EUS, laparoscopy, and thoracoscopy. The largest and earliest experience was with EUS.80–84 A pooled analysis84 of literature indicates a sensitivity and specificity, respectively, for T1 stage 81.6% and 99.4%, T2 81.4% and 96.3%, T3 91.4% and 99.4%, T3 91.4% and 94.4%, and T4 92.4% and 97.4%. EUS has not been considered accurate in distinguishing in situ cancer from invasive (T1) superficial lesions. For nodal staging based on morphology (size, shape, border, and echo characteristics), the observed sensitivity and specificity was 84.7% and 84.6%, which with the addition of fine needle aspirate improved to 96.7% and 95.5% respectively. EUS is not a reliable technique for diagnosing liver and peritoneal metastases because of the limited depth of penetration of ultrasound.85 However, a recent report86 of 102 clinically staged T2-3N0 patients revealed that 60% had pathologically positive nodes at the time of surgery, suggesting the potential benefit of preoperative therapy in this clinically staged population even when nodal disease cannot be confirmed, which remains our current recommendation. With improvements in technology and technique, EUS may be of increasing value in assessing node positivity, although not necessarily the number of involved nodes. However, evaluation for tracheal involvement with bronchoscopy is necessary for all lesions located at or above the carina.
The indications for minimally invasive surgical staging techniques are not fully defined. Laparoscopic evaluation of abdominal lymph nodes can be achieved with minimal risks when high yield staging information is not obtainable with standard imaging studies. The staging accuracy of laparoscopy for nodal involvement exceeds 95%.87–93 Unsuspected findings such as liver metastases or peritoneal studding that alter treatment occur in 12% to 17% of patients studied. Laparoscopy appears to be most useful for evaluating intraabdominal spread of disease in patients with a bulky distal third or GEJ primary and/or celiac adenopathy. Small hepatic metastases and peritoneal carcinomatosis that are below the limit of resolution of CT and PET imaging may be detected. Laparoscopy is commonly performed at the time of jejunostomy tube placement for patients planning to receive preoperative chemoradiation. Thoracoscopy also has a high level of accuracy, 95% in detecting regional nodal involvement compared with surgical staging.
Choice of Therapeutic Options: Barrett Esophagus and Dysplasia
Barrett esophagus (BE) is a premalignant condition for esophageal and GEJ carcinomas, and is characterized by intestinal metaplastic changes in the esophageal epithelium as confirmed by biopsy. Because of its premalignant nature, it has been recommended that Barrett patients undergo regular endoscopic surveillance, primarily to assess for dysplasia. The risk of progression remains vaguely defined because there are varying selection criteria for the studies and often a substantial number of patients lost to follow-up. Pooled reviews of the literature suggested a risk of progression to high-grade dysplasia or adenocarcinoma of 9.1 to 10.1 cases per 1000 person-years, with 5.3 to 6.5 cases per 1000 patient-years being malignant adenocarcinoma. However, two recent population-based studies suggest that the risk may be significantly lower. A study including 11,028 patients diagnosed with Barrett esophagus in Denmark94 found an incidence of adenocarcinoma of 1.2 (2.6 high-grade dysplasia plus adenocarcinoma) per 1000 patient-years. For those without dysplasia, the risk per 1000 patient-years was 1.0 whereas with low grade dysplasia it was 5.1. Another population-based study conducted in Ireland95 of 8522 patients with median follow-up of 7.0 years demonstrated risk of adenocarcinoma of 0.17% per year: 0.38% per year with specialized intestinal metaplasia versus 0.07% per year with only abnormal columnar epithelium. The risk per year was 1.40% with low-grade dysplasia. These later studies call into question the value of routine endoscopic surveillance for Barrett metaplasia, especially in the absence of dysplasia or specialized intestinal metaplasia as there is not only significant cost but also a competing small risk of injury to consider from the follow-up endoscopies.
The focus in the future may be on better defining the risk of progression for individual patients by using biomarkers and genetic and epigenetic abnormalities. For example, a retrospective assessment of a large multicenter database40 of patients attempted to develop a model of risk assessment based on age and methylation of eight genes previously observed to be associated with progression of Barrett esophagus: HPP1, RUNX3, CDH13, TAC1, NELL1, AKAP12, and SST. A model was developed with a sensitivity of 90% and specificity of 50%, which could create a low-risk group with a 1.7% rate of progression to high-grade dysplasia or adenocarcinoma over 5 years and a high-risk group with a 27% risk with intermediate-risk groups that would also be good candidates for close follow-up. Any study like this requires prospective validation.
The most recent American Gastroenterological Association medical position statement96 on the topic suggests that there may be no need for follow up if the abnormality consists only of columnar epithelium but that follow-up endoscopy should occur in 3 to 5 years for Barrett esophagus with metaplasia, 6 to 12 months if low-grade dysplasia is identified, and every 3 months for untreated high-grade dysplasia for the reasons described below. Patients with high-grade dysplasia should undergo a second endoscopic biopsy surveillance procedure to increase the chance of identifying an undetected early cancer. If no cancer is detected, then patients have several options, including remaining in an endoscopic biopsy surveillance program every 2 to 3 months, esophagectomy, or endoscopic ablative therapy with continued surveillance.
The care of patients with high-grade dysplasia is controversial, and treatment should be individualized, taking into account the patient’s desires, medical fitness to undergo esophagectomy, and willingness to return for frequent lifelong follow-up endoscopies if an option other than esophagectomy is selected. Any patient who will not return at the recommended endoscopic intervals and who has a confirmed diagnosis of high-grade dysplasia should undergo esophagectomy, performed by an experienced esophageal surgeon. Esophagectomy is the only treatment option that allows a patient to safely stop periodic endoscopic biopsy surveillance. However, other alternatives may be optimal for many patients. A recent metaanalysis including 1874 patients97 undergoing esophagectomy for high-grade dysplasia suggested a risk of unsuspected lymph node involvement of only 1% to 2%, suggesting that more localized therapies are indeed appropriate. Validated options include endomucosal resection (EMR), PDT, radiofrequency ablation (RFA), or even close surveillance. However, the patients who choose endoscopic ablative therapy must be willing to undergo endoscopic biopsy surveillance indefinitely.
Indeed, data collected over many years suggest a high enough risk that esophagectomy should be strongly considered. Levine and associates98 reported an endoscopic biopsy protocol that they believe can accurately differentiate high-grade dysplasia from adenocarcinoma. Four-quadrant jumbo forceps biopsies were performed at 2-cm intervals, with additional specimens from any areas of known dysplasia. Based on their series of seven patients where follow-up surgery confirmed the absence of invasive cancer, the authors advocate endoscopic follow-up for patients with high-grade dysplasia alone. In their series, of 22 patients followed up in such a fashion for an average of 32 months (range, 4 to 67 months), in none has a known invasive adenocarcinoma developed. However, another group99 used similar biopsy procedures before esophagectomy, and 10 of 38 were found to have unsuspected invasive adenocarcinoma in the surgical specimen. When high-grade dysplasia is followed up rather than treated, varying risk has been found in several recent large series. This incidence of subsequent diagnosis of adenocarcinoma has been reported to be 59% (5-year cumulative),49 32% (8-year follow-up),100 and 16% over a period of 7.3 years.101 The reason for the varying risks identified is unclear, although in the series with the smallest incidence, no confirmation of diagnosis of high-grade dysplasia was made by a central pathologist. Therefore although intensive endoscopic surveillance may be a reasonable approach, a substantial portion of patients may even have existing adenocarcinoma not detected on the initial biopsy.
Overholt reported a randomized controlled trial of photodynamic therapy of patients who had high-grade dysplasia, demonstrating that although numbers of cancers were cut in half as compared to those patients who did not have ablation, it did not completely eliminate the risk of malignancy.102 Five-year follow-up of the original study confirmed durability of more frequent elimination of dysplasia, that is, 77% compared with 39% of patients in the omeprazole-alone group. In addition, progression to cancer remained significantly lower: 15% versus 29%.103 Limitations of PDT are that it does not allow pathological confirmation that all areas of high-grade dysplasia and/or adenocarcinoma are removed, is associated with a significant rate of stricture, and has a toxicity of photosensitivity.
Radiofrequency ablation after endomucosal resection to remove nodular dysplasia has been evaluated and is an accepted alternative to esophagectomy. EMR allows assessment of pathology and margin status, and can remove nodular high-grade dysplasia. However, there is concern after this limited resection about a high risk of new high-grade dysplasia in the residual Barrett metaplasia, a field that can be addressed afterwards by planned RFA. RFA can address a uniform layer of epithelium of approximately 1 mm, and can be repeated to address residual disease. In a randomized trial for patients with low- and high-grade dysplasia undergoing primary treatment with EMR, a control group received 40 mg of esomeprazole twice a day whereas the experimental group was treated with RFA in addition.104 Patients with nodular high-grade dysplasia were eligible as long as this could be confirmed to be removed by EMR. In this trial, repeat RFA could be performed as needed up to 4 times during the first year. At 1 year after RFA, complete eradication of dysplasia occurred in 90.5% and 81% of those with low- and high-grade dysplasia, respectively, in the ablation group, as compared with 22.7% in the control group (P < 0.001). There was complete eradication of intestinal metaplasia in 77.4% versus only 2.3% of those assigned to esomeprazole alone (P < 0.001). There were fewer cancers (1.2% vs 9.3%, P = 0.045) during follow-up of all patients, reduced from 19% to 2.4% in those with high-grade dysplasia.104,105 A recent international consensus report suggests that this endoscopic approach is preferable to surgery for appropriate candidates as success appears high, and esophagectomy continues to have risk of morbidity and mortality (~2%).106
Choice of Therapeutic Options: Localized Esophageal Cancer
Surgery Alone
Esophagectomy with reconstruction has a clear goal of both achieving local tumor control and restoring swallowing function. Esophageal surgical intraoperative risks, postoperative complications, and length of hospitalization have all decreased to acceptable levels that are now compatible with other major oncologic resections.107–114 Whether surgical resection is performed as the sole therapy, or as part of a combined approach, the surgical principles and techniques are the same with the goal of achieving a gross total resection. Surgery as a single modality is generally only appropriate for curable patients with very early lesions or those medically unfit for combined modality therapy, and cancer cure rates do not appear related to surgical technique used.
When surgery is used, gross total resection with negative margins has been observed to be of critical importance for long-term outcome, even with the availability of adjuvant or salvage chemoradiation. Results from a data set of 1602 patients63 undergoing resection of distal and GEJ esophageal adenocarcinoma demonstrated 5- and 10-year survival rates for R0 resected (complete resection with negative margins) patients of 43.2% and 32.7%, whereas the corresponding figures for patients with incomplete resection with microscopic or macroscopic residual (R1 and R2 resection) were 11.1% and 6.2% (P < 0.0001). The results of a US intergroup trial115 comparing surgery alone with surgery and neoadjuvant chemotherapy found 5-year survival was similar across arms, and that 5-year survival was influenced by achieving an R0 resection (32% vs under 5% for lesser resections) in a population including patients with both adenocarcinoma and squamous cell carcinoma histology
The standard operation in the United States to resect an esophageal cancer includes resecting the involved portion of esophagus, the proximal stomach, and the regional lymph nodes, as illustrated in Figure 74-2. The surgical resection is, therefore, properly termed a partial esophagogastrectomy with regional (or one field) lymphadenectomy. The resected esophagus is replaced with a conduit, usually the stomach or segments of the small or large intestine, which are, in turn, mobilized as a vascularized pedicle and anastomosed to the remaining proximal esophagus.
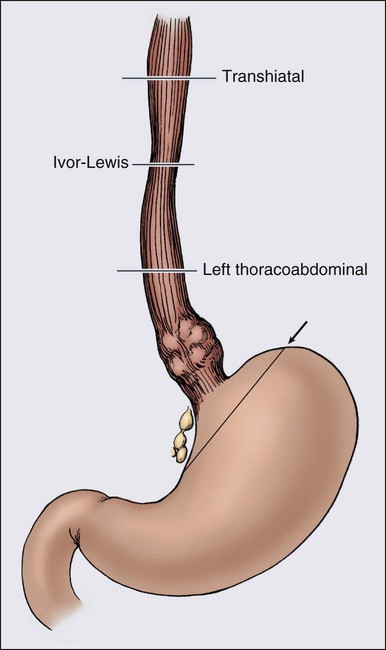
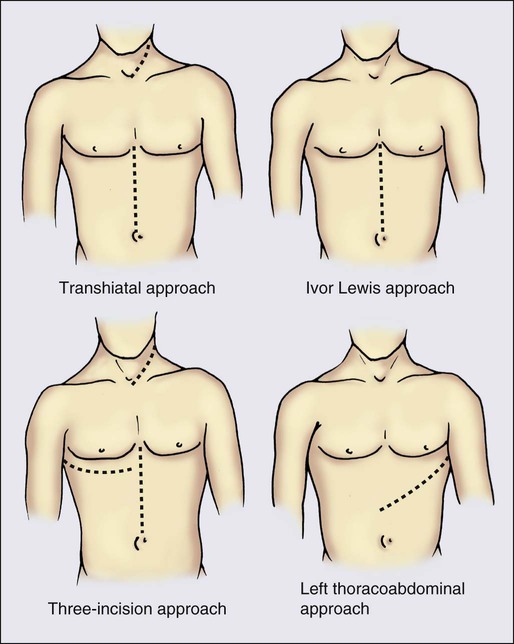
A number of incisional approaches can be successfully used to perform a partial esophagogastrectomy, including the transhiatal, Ivor-Lewis, left thoracoabdominal, and three-incision techniques (Figure 74-3). Other less common techniques or modifications of the approaches are listed. The specific incisional approach used generally determines how much esophagus is removed and where the esophageal anastomosis will be located (Figure 74-2). In the past, various surgeons have argued in support of their preferred techniques, giving the impression that all of these approaches were uniquely different procedures. It is now appreciated, however, that all of these incisional techniques use partial esophagogastrectomy (except segmental esophagectomy with free jejunal grafting, which is discussed separately), and the patient outcome results reported are similar in terms of surgical morbidity and mortality. Long-term disease-specific survival after esophagectomy is related to pathological tumor stage. Prospective studies do not demonstrate a survival advantage related to the surgical esophagectomy technique.93,116–118 The data continue to demonstrate no difference in mortality or survival between transthoracic and transhiatal esophagectomy approaches.119,120 The main variables when performing a partial esophagogastrectomy are which incision(s) to use, the length of esophagus to resect, what to use to replace the esophagus, and which route through the chest this conduit will take.
Choice of Therapeutic Options: Early Esophageal Cancer
Nonsurgical Management of Early-Stage (Tis, Ia) Esophageal Cancer
Radiotherapy alone may be an alternative for some cases of early esophageal cancer. Hishikawa reported 68 patients treated with radiotherapy alone, dose 60 to 72 Gy with external beam radiation or 55 to 60 Gy plus a brachytherapy boost.121 Five-year cause-specific survival and locoregional control rate was 79% and 82%. As locoregional control was only 58% for tumors longer than 5 cm in length, it was recommended that those patients be treated with combined chemoradiation. A more recent series of 54 similarly treated patients also demonstrated similar outcomes of 86% and 79%, respectively, resulting in a recommendation that this radiotherapy option may be appropriate for medically inoperable or elderly patients in this situation.122,123
Endoscopic techniques may be useful for lesions limited to the mucosa. In contrast to radiotherapy including brachytherapy, the effectiveness of PDT is limited to a depth of 4 to 6 mm and the penetration of laser or thermal energy is 6 mm or less. These techniques may potentially be sufficient for the treatment of Barrett esophagus and selected patients with high-grade dysplasia but are not considered curative therapy for invasive disease. Submucosal invasion increases the risk of lymph node involvement, which cannot be addressed by these therapies. In one series, the rate of local recurrence was 29% in patients treated for mucosal confined adenocarcinoma at a median follow-up of 36 months.124 In another report, Tis/T1 lesions had a 44% pCR rate to PDT and T2 lesions had a 28% pCR rate to PDT alone, with control maintained in approximately half of complete responders.125 Although these techniques can be effective for very superficial lesions, esophagectomy is still considered the standard of care, with radiotherapy an accepted alternative for patients unable to undergo surgery. Endoscopic procedures for high-grade dysplasia, in situ carcinoma, or T1a (carcinoma limited to mucosa) are discussed above.
Endomucosal resection (EMR) may be the optimal endoscopic minimally invasive approach for early carcinoma. In contrast with photodynamic therapy and radiofrequency ablation, this approach allows pathological confirmation of depth of invasion. This confirmation is critical because the greater the depth of invasion, the higher is the associated risk of nodal involvement, which requires more extensive therapy for adequate curative management. This is appropriate therapy for disease confined to the mucosa, where there is a probability of lymph node involvement that approaches 0%. This approach may also be appropriate for submucosa involvement up to one-third of the thickness: lymph node involvement is observed in 45% (23/51) of cases with deep submucosal invasion versus 10% (3/29) of middle-third and 7.5% (3/40) of inner-third cancers.126 Therefore, for lesions with invasion beyond the submucosa, standard management including surgical resection is warranted, although EMR may be appropriate under select circumstances with minimal submucosal invasion.
Esophagectomy for Stage I and IIa Tumors
Esophagectomy is the standard therapy. The outcome is favorable, especially for stage I disease, and is demonstrated in Figure 74-1. This procedure has the advantage of pathological confirmation of the extent of disease, to determine whether combined-modality therapy may be warranted. The varying approaches to esophagectomy are discussed in the section on locally advanced esophageal cancer in the following text. Although surgery alone is considered appropriate for patients with stage IIa disease, combined-modality therapy should be considered for appropriate candidates because the long-term survival is less than 50%, and clinical staging may frequently overlook involved lymph nodes discussed previously (Fig. 74-1).
Choice of Therapeutic Options: Locally Advanced Esophageal Cancer
Surgical Approaches
Transhiatal Resection
The transhiatal esophagectomy (THE) is a frequently used approach, “rediscovered” in 1976 by Dr. Mark Orringer, in which the intrathoracic esophagus is mobilized distally through the esophageal hiatus and proximally through a cervical incision. The increased prevalence of adenocarcinoma of the distal esophagus and GEJ has largely been responsible for the widespread popularity among surgeons of the transhiatal approach. Because of their distal esophageal location, these tumors are invariably near the esophagogastric junction and readily accessible for direct-vision dissection through the hiatus. Moreover, the regional lymph nodes for these distal tumors are in the parahiatal and proximal lesser curvature regions, both accessible via laparotomy. The resected esophagus is reconstructed by using the greater curvature of the stomach, vascular pedicle based on the right gastroepiploic artery or long-segment colon, which is passed up into the neck as vascularized grafts to be anastomosed to the proximal cervical esophagus. Although reports exist on the use of jejunum for long-segment esophageal replacement, small bowel is generally not an ideal option for esophageal replacement because of its mesenteric vascular anatomy, unless specialized techniques with vascular augmentation are used.107 The esophageal-replacement conduit is passed through the chest into the neck by one of three routes: (1) subcutaneous, (2) substernal, or (3) posterior mediastinum. The posterior mediastinum is the preferred route when possible. The advantages of THE include avoiding postthoracotomy discomfort, wide proximal esophageal margins to ensure complete resection of tumor and Barrett mucosa, cervical anastomosis where the consequences of anastomotic leak are minimized, and an esophageal reconstruction that results in an excellent quality of swallowing. It is well documented that the approach is acceptable for both benign and malignant esophageal disease.111,120,127 The disadvantages include inability to visualize middle or proximal third tumors, inability to perform extensive intrathoracic regional lymphadenectomy, potential for injury to intrathoracic and cervical structures, and the need for long-segment esophageal replacement. Randomized trials have confirmed similar long-term survival with the use of a transhiatal approach despite a more limited mediastinal lymph node dissection than can be accomplished via thoracotomy.
The transhiatal approach is safe, well tolerated, and associated with infrequent major complications in experienced hands.120 In large series, late functional results have been good or excellent in 73% of surgeries and mortality rates are reported as low as none to 3%.128,129 Recently, Orringer has reported a series of more than 2000 patients who underwent a THE, with a mortality rate of only 1% in those patients who underwent surgery since 1998, an anastomotic leak rate of 9% and 2% pulmonary complications.120 In more than 1000 patients who underwent resection for malignancy, the positive margin rate was only 2%.
A multiinstitutional randomized trial130 conducted at the University of Amsterdam and Erasmus University included patients with Siewert I and II tumors, using an experimental arm of transhiatal resection with reconstruction using the gastric remnant compared with a right thoracic-abdominal approach. In this study, lymph node dissection varied with the two surgical approaches. For all patients, periesophageal lymph nodes were removed, as was the lymph node–bearing region along the left gastric artery. Clinically uninvolved celiac nodes were not resected. However, in the RTA group, a much more extensive dissection was performed, including lower and middle mediastinal, subcarinal, and right-sided paratracheal lymph nodes (dissected en bloc). The aortopulmonary-window nodes were dissected separately. Through a midline laparotomy, the paracardial, lesser-curvature, left-gastric artery (along with lesser-curvature), celiac trunk, common-hepatic artery, and splenic artery nodes were dissected, and a gastric tube was constructed. The cervical phase of the transthoracic procedure was identical to the transhiatal procedure, but a left-sided approach was used. Resection via thoracotomy potentially allowed wider resection of tumor and periesophageal tissues. Despite this, the pattern of recurrence was similar: locoregional recurrence occurred in 14% and 12% of patients, respectively; distant recurrence in 25% and 18%; and both in 18% and 19% (P = 0.60). Perioperative morbidity was substantially less with transhiatal resection, especially pulmonary complications, but in-hospital mortality was similar.
Intriguingly, a subgroup analysis of long-term outcome, though showing no suggestion of survival difference for the groups at large, raised the following issue: although survival was excellent for patients with no positive nodes (86% vs 89%) and was 0% for more than 8 nodes in both arms, there was a significant benefit to the more extensive nodal dissection in patients with 1 to 8 nodes, 23% versus 64% (P = 0.02).118 If this difference is real and not just a function of unplanned subgroup analysis, the use of neoadjuvant chemoradiotherapy potentially may minimize the impact via radiation treatment of these undissected nodal beds.
Another randomized trial testing transhiatal resection,131 conducted in Japan, included only patients with Siewert II and III, in contrast to the trial described above. Transhiatal resection was accompanied by a total gastrectomy with D2 lymphadenectomy (including splenectomy) via a laparotomy. Additional dissection of the lymph nodes along the left inferior phrenic vessels and the paraaortic nodes lateral to the aorta and above the left renal vein was done in curable patients. The procedures were undertaken via laparotomy, and the lower mediastinum was accessed transhiatally. With the transhiatal approach, mediastinal resection included the lower esophagus and only the periesophageal lymph nodes. For the other group randomly assigned to a left thoracic-abdominal (LTA) approach, an oblique incision over the left thorax and the abdomen was made. The same procedure as that for TH was done in the abdominal cavity, including the lymphadenectomy as described above. In the thoracic cavity, a thorough mediastinal nodal dissection below the left inferior pulmonary vein was performed. The study was closed when interim analysis suggested lack of benefit to LTA, including the more extensive mediastinal dissection. In both arms, positive paraaortic nodes were identified in approximately 10% of patients, and positive mediastinal nodes in fewer than 10%.
Ivor Lewis Approach
Partial esophagogastrectomy with an abdominal and right thoracotomy approach was originally described by Welsh thoracic surgeon Ivor Lewis132 in 1945. This was designed to optimize exposure of the intrathoracic esophagus, which passes through the upper two-thirds of the chest along the right posterior mediastinum. Once the involved intrathoracic esophagus is mobilized, a partial esophagogastrectomy is performed and the esophagus is replaced by stomach, colon, or (less frequently) jejunum, which is passed into the chest along the esophageal bed, and anastomosed to the proximal esophagus, usually at or above the level of the azygos arch. The advantages of the technique are the excellent exposure of the mid to upper intrathoracic esophagus, dissection of esophageal pathology from the surrounding mediastinal structures, and the ability to do a mediastinal lymph node dissection. The disadvantages, however, are related to the use of a thoracotomy and associated morbidity, with limits on the proximal resection margin and the potential for an intrathoracic esophageal anastomotic leak, which is a more difficult management problem than a cervical anastomotic leak. Reported complications include respiratory problems in 11% to 20%, anastomotic leak in 3% to 7%, and wound infection in 5%. Operative mortality ranges from none to 4%.107,110,133–135
Left Thoracoabdominal Approach
The left thoracoabdominal approach uses a single incision extending from the left chest onto the abdomen; it provides excellent exposure of the lower third of the esophagus and left upper quadrant of the abdomen.136 This technique is ideal for patients with limited tumors near the GEJ, especially when the extent of gastric invasion is unclear, because it yields superb exposure and maximizes reconstructive options of the lower third of the esophagus. Respiratory complications are the most common postoperative complications with this approach. At least some degree of atelectasis, usually involving the left lower lung, occurs in most patients. Pneumonia is reported to occur in up to 24% of cases. Anastomotic leaks occur in as many as 12% (mean, 3.7%) of cases, leading to a higher mortality rate. Other complications include atrial fibrillation in 10%, wound infection in 1.5% to 5.2%, and (infrequently) empyema and subphrenic abscess. The reported operative mortality is none to 6.2%.136,137
Multiple Incisions
Multiple-incision surgical approaches combine the incisional strategies of the standard techniques. Of these, the three-incision approach using a cervical incision (right or left), right thoracotomy, and midline laparotomy, as described by McKeown,138 is the most common, and is also referred to as the three-incision, three-hole, total esophagectomy or modified McKeown approach. It combines the exposure of the thoracotomy approach for esophageal mobilization or nodal dissection with the advantages of a cervical esophageal anastomosis. Patient outcome results with this technique are similar to those of other approaches, with reported mortality of 3% to 4% and esophageal anastomotic leak rates of 5% or less.139
Radical Resections
The majority of patients with esophageal cancer are first seen with locally advanced (stage II and III) disease. In these patients, survival results are poor with surgery alone. Two approaches attempt to improve survival in these patients. One involves the use of combination therapies, such as preoperative chemoradiation followed by surgery (to be discussed later), and the second involves adding an en bloc, wide-field lymphadenectomy to the standard esophagectomy technique. The esophagus has an extensive regional lymphatic drainage. Arbitrarily, the lymphatic drainage has been divided into three zones or fields—cervical, intrathoracic, and abdominal. Standard esophagectomy techniques involve regional, or one-field, lymphadenectomy. Radical approaches advocate two- or three-field lymphadenectomy in conjunction with esophageal resection and replacement. Hagen and colleagues140,141 believe that proximal hemigastrectomy should also be included as part of an en bloc approach, using colon to replace the resected esophagus. Radical esophagectomy is more complex surgery than standard techniques. This is reflected in morbidity rates as high as 58%.122 Nonetheless, 30-day mortality rates as low as 1.6% to 4.3% are reported.140,142–144 Survival data using radical esophagectomy techniques are conflicting and, therefore, it is unknown whether there is sufficient benefit to outweigh the increased operative morbidity. Hagen and associates140,141 reported an improved survival in early-stage tumors using en bloc esophagectomy compared with a standard transhiatal technique. Although prospective, their trial was not randomized. Also, earlier-stage patients were selected for the en bloc approach and, therefore, biased the results and conclusions. A more recent update of their results continues to suggest excellent local tumor control and improved survival.141 In another nonrandomized series, Altorki143 reported improved survival in patients with stage III disease with radical esophagectomy compared with standard surgical techniques. Nishimaki and colleagues142 reported an overall 5-year survival rate of 41% with extended radical esophagectomy for thoracic esophageal cancer. In contrast, Bumm and coworkers144 demonstrated no difference in overall survival between standard transhiatal and radical THE in which two-field lymphadenectomy is added. Despite the wide surgical dissection with radical techniques, Bhansali and colleagues145 still documented a 21% locoregional cancer recurrence rate. The determination of which cell type and tumor stage, if any, will benefit from radical surgical techniques has yet to be resolved.
Free Jejunal Interposition
With this technique, the reported graft survival rate is 85% to 95%, and the operative mortality is 5%. For those patients with successful grafting, 90% are reported to have an adequate swallowing quality. If graft failure occurs, a second attempt will be successful in 50% to 75% of cases.146–149
Minimally Invasive Esophagectomy
With the advent of minimally invasive surgical techniques, an interest developed in applying thoracoscopic and laparoscopic techniques to esophagectomy.150 Certainly, the techniques of gastric and esophageal mobilization have been well established for other complex minimally invasive surgeries. Minimally invasive esophagectomy (MIE) required that these individual techniques be “spliced” together. Approaches used have included laparoscopic transhiatal resection, combined laparoscopic–thoracoscopic procedures, and laparoscopic creation of gastric tube with thoracotomy and other combinations. The need to convert to an open surgery has been uncommon. The number of lymph nodes removed also appears similar to that achieved with open surgery.151 A steep learning curve exists for these surgeries.
Of late, there has been interest in laparoscopic minimally invasive approaches to esophagectomy.155–155 A variety of MIE approaches exist that use laparoscopic, thorascopic, and robotic approaches. Hybrid techniques have also been performed using these video-assisted methods with the addition of the classic approach through the chest, abdomen, and/or neck. There are numerous cases series, generally with short follow-up, and one significant randomized trial. Luketich152 from the University of Pittsburgh has described results for more than a thousand patients, 76% with adenocarcinoma and 93% with distal and GEJ lesions. In this patient group, 98% had resection with negative margins, and nodal dissection recovered a median of 21 nodes.
There has been a single randomized trial examining the role of MIE.155 In this randomized trial, 115 eligible participants had resectable esophageal cancer (cT1–3, N0–1, M0) of the intrathoracic esophagus and GEJ. The primary objectives included the short-term outcomes of pulmonary infections, pain, and quality-of-life end points of minimally invasive resection versus standard approaches. In this trial, 93% had neoadjuvant therapy including radiation prior to resection. The end points of infection, pain, and quality-of-life measures were indeed superior in the group undergoing minimally invasive surgery. The R0 resection rates were similar, 84% versus 92% demonstrating success at primary tumor removal after neoadjuvant chemoradiation, as was the number of lymph nodes retrieved and pathological stage. This matches the conclusions drawn from other single-institution series. Extensive data about long-term survival outcome with this approach is not available, but a recent aggregate analysis156 of the available data suggests that 1-, 3-, and 5-year survival is similar to the outcome expected for more traditional approaches to esophagectomy. Overall, when comparing MIE with open procedures, anastomotic leaks, stricture rates, and short-term survival seem to be comparable; however, MIE may be associated with a lower intraoperative blood loss, shorter length of stay, and less morbidity, but offset by the longer operative times and costs. A recent review by Schumer et al.157 nicely compared and summarized the available comparative studies, and found no substantial evidence of differences in rates of major complications, mortality, or lymph node harvest.
Survival After Surgery Alone
Cancer-related survival after surgical resection is discussed separately from the description of individual techniques to emphasize the fact that postesophagectomy survival is a function of stage and not of surgical approach. Several points concerning postesophagectomy survival have now become quite clear. The first is that regardless of whether a thoracotomy or nonthoracotomy technique is used, cumulative postoperative survival is the same, at approximately 20% to 25%. This fact has been underscored most graphically by Muller and associates,158 who reviewed the world literature to compare overall postesophagectomy survival by technique and showed no significant difference. Hulscher159 performed a metaanalysis of the English-language literature of transthoracic and transhiatal resection of esophageal cancer and found a higher risk of pulmonary morbidity and mortality with the transthoracic procedure, but a similar 5-year survival of approximately 20%. These investigators also compared limited transhiatal resection with THE with extended en bloc lymphadenectomy in 220 patients with adenocarcinoma of the esophagus. No significant difference was found in survival or operative mortality. Large retrospective series suggest a modest improvement in long-term outlook in recent years, probably the result of lower operative mortality.117,160,161 Gockel162 found that for the periods 1985 to 1995 and 1995 to 2005, 5-year survival increased from 15% to 25%, and 30-day surgical mortality improved from 8.3% to 3.1%. In addition, better patient selection may play an important role in improved outcome. For example, Steyerberg et al. have proposed a simple scale based on important co-morbidities, age, neoadjuvant therapies, and esophagectomy volume at the treating hospital, which divided patients into groups with predicted 30-day mortality of under 4% to approximately 20%.161 There continues to be focus on reducing morbidity and mortality.163,164 It is also expected that more accurate preoperative staging with PET scanning and esophageal ultrasonography may improve surgical outcome by removing some patients with existing gross metastatic disease. The long-term results achieved in some significant prospective trials in the modern era are summarized in Table 74-3.
The second fact is that postoperative survival is stage related. Notably, the majority of patients considered for surgery are found to have stage III disease, and survival for these patients, even with surgery, is poor (approximately 10% to 15%). Hofstetter and associates165 reported results for 1097 consecutive patients undergoing resection and compared outcomes by stage from 1970 through 1985, 1986 through 1996, and 1997 through 2001. Although median survival increased from 7 to 34 months, surgical mortality decreased from 12% to 6%, and the R0 resection rate increased from 78% to 94%, no meaningful difference was found in longer-term survival according to stage. Three-year survival rates through this period were 63%, 52%, and 44% for pathological stage IIA and were 10%, 18%, and 6% for pathological stage III, which did not represent a statistically significant difference over the time periods. Multivariate analysis showed that survival was associated with complete resection and thorough preoperative staging, and that preoperative chemotherapy used in the later years was associated with increased complete resection. For T1N0M0 adenocarcinoma of the esophagus,166,167 survival at 5 and 10 years may be closer to 77% and 68% after surgical resection.
The third point is that thus far postsurgical survival is little influenced by whether the patient has a squamous cell or adenocarcinoma histology of their esophageal cancer. Holscher and coworkers168 documented a postresection survival advantage only for patients with stage I adenocarcinoma. Salazar and colleagues169 reported no difference in cumulative postoperative survival for patients with squamous cell carcinoma and adenocarcinoma. Finally, despite advances in surgical techniques, postesophagectomy survival has remained remarkably stable over time, emphasizing that improvements would likely require effective combined-modality therapies.
Optimizing Surgical Outcome
Evidence-based surgery uses the treatment outcomes of cost, morbidity, mortality, and quality of life to help physicians, health care administrators, and hospitals determine the most appropriate setting and specific management of patients. Data consistently show that increased provider experience improves patient outcome, lowers complication rates, and reduces cost for complex surgeries. In terms of technical difficulty, length of stay, morbidity, and mortality, esophageal surgery is classified as a complex gastrointestinal (GI) operation. Gordon and associates170 reported reduced hospital mortality, length of stay, and cost for a wide range of complex GI procedures including esophagectomy. The institution of standardized patient-care pathways, a product of the evidence-based surgery approach, reduced hospital cost for esophagectomies while keeping surgical mortality low (1.3%).171 Indeed, the use of clinical protocols for postoperative care has the potential to substantially reduce operative mortality resulting from a variety of surgical approaches, perhaps to 1% or less.160,172,173
Dimick and colleagues172,173 documented the importance of surgical volume, hospital experience, and intensive care staffing in optimizing outcome after esophagectomy. These studies underscore the fact that even complex surgical procedures, such as esophagectomy, can be both effective therapy and cost-effective.
In an instructive multiinstitutional analysis174,175 of outcome in Medicare patients over 1998 to 1999, a substantial difference in operative mortality was found to exist based on the number of esophagectomies per year at the institution. This varied from 23% for centers doing fewer than 2 cases per year to 8.1% for high-volume centers performing more than 19 per year. The operative mortality rates reported in this series are higher than those reported in clinical trials, and this may relate not only to particular expertise of the study centers in esophageal cancer treatment but also to patient selection for clinical research and the age of the patients. A subsequent analysis suggested that the variation among hospitals was explained, in part, by lower mortality of individual high-volume surgeons with adjusted operative mortality among Medicare patients of 18% for surgeons performing less than two esophagectomies per year and 9% for those performing more than six.174 Other studies have since confirmed this finding.176,177
Chemotherapy Followed by Surgery: Is Outcome Improved by Systemic Therapy?
Promising results for survival improvement reported from numerous phase II trials of preoperative chemotherapy regimens tested in newly diagnosed patients178–185 prompted two initial large randomized trials with differing results.115,186 These data are summarized below. Although preoperative chemotherapy can be justified based on an advantage demonstrated in the Medical Research Council (MRC) trial, there remains some uncertainty. In fact, a US intergroup trial failed to demonstrate either improved local control or survival with similar preoperative chemotherapy compared with surgery alone. By contrast, there are data indicating survival benefit and improved local control when radiotherapy is added to chemotherapy in a concurrent fashion prior to surgery. Hence, trimodality therapy is favored in the United States over the preoperative chemotherapy approach that is preferred in the United Kingdom.
The US intergroup trial115,187 included 467 patients randomly assigned to receive (1) three courses of cisplatin 100 mg/m2 plus infusional 5-FU 1000 mg/m2 days 1 to 5 before surgery and two courses after surgery (total, five courses) or (2) immediate surgery. No benefit was demonstrated for the addition of chemotherapy in this trial, 45% of patients had squamous cell carcinoma and 55% had adenocarcinoma. There were no differences in resectability or median, 1-, 2-, or 3-year survival rates between treatment groups and between histologic types. The pCR rate was 2.5%. Median and 2-year survivals with and without chemotherapy were 14.9 months and 35% versus 16.1 months and 37%. In both arms, approximately 60% of enrolled patients underwent a gross total resection (R0) and 17% of those patients had a subsequent local recurrence for an ultimate 57% failure to control local disease. There were no differences in surgical morbidity and mortality rates (6%% for both arms). Most patients did not receive the planned two cycles of postoperative chemotherapy: 52% received one cycle and 38% both cycles. An update with longer follow-up reported a 32% disease-free survival rate at 5 years for patients having complete resection and negative margins (R0), whereas only 5% of those with a lesser resection (R1 or R2) were alive.115
The MRC186 conducted an 802-patient randomized trial, testing two cycles of cisplatin 80 mg/m2 and 5-FU 1000 mg/m2/d continuous infusion for 4 days given prior to resection of squamous cell or adenocarcinoma of the esophagus. Patients were required to have resectable tumor, although the staging evaluation was not proscribed by the study. Two-thirds of patients had adenocarcinoma. Median survival was significantly improved from 13.3 to 16.8 months with 2-year survival improved from 34% to 43%. No information about patterns of failure was reported, but similar to the US intergroup trial 57% had a complete resection with negative margins (R0). There is no immediate explanation for the difference between the results of this trial and the US intergroup trial, but these conflicting results may relate to the greater power, less rigorous staging, chance, or unknown differences between the populations in the two studies.
Another trial conducted by the Medical Research Council, the MRC Adjuvant Gastric Cancer Infusional Chemotherapy (MAGIC),188 demonstrated a survival benefit for three cycles of preoperative epirubicin, cisplatin and 5-fluorouracil (ECF) and three cycles of postoperative ECF compared with surgery alone. This trial enrolled patients with gastric, GEJ, and distal esophageal adenocarcinoma (26% of patients). An update reported a persistent 5-year survival benefit of 23% versus 17% (P = 0.03).189 Although not powered for subsite analysis, there did not appear to be heterogeneity in treatment effect for distal esophageal lesions or gastric lesions.
Concomitant Chemotherapy and Radiotherapy Followed by Surgery: Addressing Both Local and Distant Failure to Improve Outcome
The rationale for neoadjuvant chemoradiation or trimodality therapy is the high rate of both local and distant failure seen with surgery alone, such that intensified local and systemic therapies are needed to improve survival outcome. For example, data from the surgery alone control arm of the US Intergroup trial (1990-1995)187 showed a 57% failure to control local disease (including 41% failure to resect all local disease) and 50% distant failure rate in those who did have complete resection.4 A single-institution randomized trial conducted at the University of Michigan190 demonstrated similar survival, local control, and distant first failure rates with surgery alone. Another single-institution study of adenocarcinoma of the distal esophagus and GEJ examined patterns of failure after surgery alone. Thirty-four percent had distant failure, 19% had local failure, 14% had locoregional nodal failure, and 6% had peritoneal seeding.191 These studies show that the rate of complete resection with negative margins and ultimate local control is not improved by the addition of chemotherapy alone prior to surgery. Similarly, local recurrence rate is high (nearly 50%) after definitive chemoradiation.192,193 However, the trimodality approach of chemoradiation followed by surgery results in a significant improvement in local control compared with surgery alone in trials of patients with squamous cell carcinoma and adenocarcinoma.190,193
A number of randomized controlled trials of preoperative chemoradiation compared with surgery alone had been conducted with suggestive but sometimes conflicting results, but a recently reported well-powered trial has now provided convincing supporting evidence. Early randomized trials tested chemoradiation with 5-FU/cisplatin-based regimens followed by surgery compared with surgery alone. Most of these trials were underpowered and the results varied. The results of these trials are summarized in Table 74-8. Two trials, those of Nygaard194 and LePrise,195 used a sequential rather than concurrent chemotherapy and radiotherapy design followed by surgery and failed to demonstrate any significant difference in median, disease-free, or overall survival. These trials will not be further discussed. Notably, the studies published by Walsh,196 Urba,190 and Tepper197 included patients with adenocarcinoma. Those of Bosset,193 Burmeister,198 Walsh,196 Urba,190 and Tepper199 compared concomitant cisplatin-based chemotherapy and radiotherapy followed by surgery with immediate surgery. Walsh and Tepper demonstrated a significant survival benefit whereas Urba reported a significant improvement in local control but the observed survival benefit was not significant. Bosset and colleagues showed an improvement in disease-free survival and fewer deaths from esophageal cancer but not improvement in overall survival. The trial of Burmeister198 did not demonstrate a survival benefit but the chemotherapy and radiotherapy are today considered suboptimal. The trials (Table 74-8) reported similar 3- to 5-year survival rates of 32% to 39% for the investigational combined treatment (trimodality) groups whereas the survival of patients in the surgery control arms varied from 6% to 16%. This difference in outcome in the surgery-alone control arms may relate to selection factors and the rigor of baseline staging. The details of these trials, including the recently reported more definitive CROSS53 trial, are described below.
Table 74-8
Preoperative Chemotherapy and Radiotherapy: Randomized Trials
| % Survival (y) | |||||
| Author | Treatment Arms | Median (mo) | 1 | 2 | 3 |
| Nygaard et al169,184 | S | 13 | 9 | ||
| Cisplatin/bleomycin + 35 Gy + surgery | 23 | 17 | |||
| Le Prise et al195 | S | 10 | 47 | 14 | |
| Cisplatin/5-FU + 35 Gy + surgery | 10 | 46 | 19 | ||
| Bosset et al168 | S | 18.6 | 67 | 42 | 34 |
| Cisplatin + 37 Gy + surgery | 18.6 | 69 | 48 | 37 | |
| Walsh et al171 | S | 11 | 44 | 26 | 6 |
| Cisplatin/5-FU + 40 Gy + surgery | 16 | 52 | 37 | 32 | |
| P = 0.01 | P = 0.01 | ||||
| Urba et al165 | S | 17.5 | 58 | 39 | 16 |
| Cisplatin/5-FU/VBL + 45 Gy + surgery | 16.3 | 70 | 42 | 32 | |
| Burmeister304 | S | 19.3 | P = 0.07 | ||
| Cisplatin/5-FU + 35 Gy + surgery | 22 | ||||
| Tepper172 | S | 22 | 16 (5-y) | ||
| Cisplatin/5-FU + 50.4 Gy + surgery | 48 | 39 (5-y) | |||
| P = 0.02 | |||||
| Van Hagen175 | S | 24 | 34 (5-y) | ||
| Carboplatin/Paclitaxel + 41.4 Gy + surgery | 49 | 47 (5-y) | |||
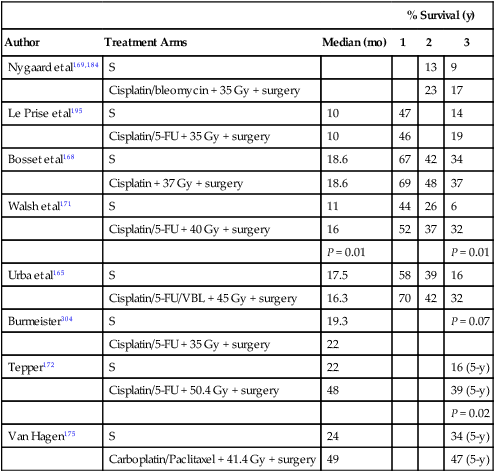
S, squamous cell carcinoma; 5-FU, 5-fluorouracil; VBL, vinblastine.
The trial reported by Walsh196 was limited to adenocarcinoma of the distal esophagus/GEJ and demonstrated significantly improved survival with concurrent 5-FU 15 mg/kg on days 1 to 5, 30 to 35, cisplatin 75 mg/m2 on days 7 and 37, and radiotherapy (RT) 40 Gy in 15 fractions on days 1 to 19. The 3-year survival rate was 32% versus 6% and median survival was 16 months versus 11 months (P = 0.01). The complete response rate for all patients enrolled in the preoperative chemoradiation arm was 22%, and there was evidence of nodal downstaging, with 82% node positive in the surgery arm in contrast with 25% after neoadjuvant therapy (P < .001). This study has been criticized for the relatively small sample size (it closed when an early stopping rule was met), for unexpectedly poor results in the surgery alone arm, and for lack of uniform preoperative staging.
The trial reported by Urba190 randomly assigned 100 patients to surgery (transhiatal esophagectomy) with or without preoperative chemoradiation (cisplatin 20 mg/m2 on days 1 to 5, 17 to 21, vinblastine 1 mg/m2 on days 1 to 4, 17 to 20, 5-FU 300 mg/m2 on days 1 to 21, and RT 1.5 Gy twice a day to 45 Gy). Median survival was 17.6 months with neoadjuvant therapy and 16.9 months with surgery alone, and 3-year survival was 30% versus 16% respectively (P = 0.18). Survival of patients in the trimodality arm was consistent with the Walsh study. The gross total resection rate was in excess of 90% in both arms. The pCR rate was 28%, and did not differ by histology but the number of patients was small (75 adenocarcinoma, 25 squamous cell). Preoperative therapy reduced the incidence of locoregional failure as the site of first failure from 42% in the surgery control arm to 19% (P = 0.0002). Distant failure was not affected, approximately 60% in both arms. Although the improvement in overall survival from 16% to 30% is in an expected range of 10% to 15%, the study was powered to show a much larger difference based on the results of prior phase II trials from this group.
Bosset193 reported the results of a multicenter trial limited to squamous cell carcinoma, stages I and II. These investigators found no difference in overall survival with preoperative therapy although there was a significant improvement in disease-free survival and local recurrence-free survival. The regimen was cisplatin 80 mg/m2 given 0 to 2 days prior to each set of RT treatments and the radiotherapy consisted of two 1-week courses of 18.5 Gy in five 3.7-Gy fractions beginning on days 1 and 22. Postoperative mortality was significantly higher, 12% versus 4% in the combined treatment arm. This trial has been criticized because chemotherapy was often not administered on the same day as radiotherapy, the chemotherapy was less intensive than in the other trials, and an unusual hypofractionated and split-course regimen of radiotherapy was used.
The Trans-Tasman Radiation Oncology Group (TROG) and the Australasian Gastro-Intestinal Trials Group (AGITG) randomly assigned 256 patients to surgery alone or to one cycle of preoperative cisplatin 80 mg/m2 on day 1 and fluorouracil 800 mg/m2 on days 1 to 4, with concurrent radiotherapy, 35 Gy given in 15 fractions.200 Sixty-two percent had adenocarcinoma. No survival benefit was identified but treatment was less intensive with only one cycle of chemotherapy given and a lower radiation dose, although there was a suggestion of benefit for patients with squamous cell carcinoma.
An adequately powered US intergroup trial197 was initiated to definitively assess survival outcome with preoperative cisplatin and 5-FU chemotherapy and concurrent radiation in comparison with surgery alone. Unfortunately, this trial was closed early as a result of poor accrual likely because of the existence of a strong bias among physicians about the benefits of preoperative chemoradiation, lack of consensus about an optimal preoperative regimen, and patient resistance to randomization. Five-year survival was 39% (21%, 57%) versus 16% (5%, 33%), P = 0.005. There was no appreciable difference in surgical complications.
More definitive results in support of combined-modality neoadjuvant therapy are now available from the CROSS53 trial, which accrued patients in 2004 to 2008. Three hundred sixty-eight patients with esophageal and GEJ tumors (82% distal esophagus and GEJ) were randomly assigned to surgery alone or an alternative chemotherapy on days 1, 8, 15, 22, and 29 using carboplatin (AUC 2 mg/mL/min) and paclitaxel 50 mg/m2, along with RT 4140 cGy, a regimen of radiochemotherapy 1 week shorter than the other commonly used approaches. No postoperative chemotherapy was given. Seventy-five percent of patients had adenocarcinoma, 24% had GEJ tumors, and an additional 58% had distal third lesions. Patients were well staged using endoscopy, including ultrasonographic and CT scanning. Ninety-nine percent were clinical stage T2 or greater, and 65% were node positive based on clinical evaluation. No adjuvant chemotherapy was planned.
A randomized phase II trial conducted by the Eastern Cooperative Oncology Group,113 including adenocarcinoma patients only, attempted to assess whether there might be evidence that CPT-11 or paclitaxel-containing platinum-based chemoradiation regimens might lead to superior outcome. Regimens tested included cisplatin 30 mg/m2 and irinotecan 50 mg/m2 on days 1, 8, 22, and 29 of 45 Gy RT for 5 weeks or cisplatin 30 mg/m2 and paclitaxel 50 mg/m2 1-hour infusion days 1, 8, 15, 22, and 29 with RT. Postoperative chemotherapy using the same agents was planned, either cisplatin 30 mg/m2 and irinotecan 65 mg/m2 or cisplatin 75 mg/m2 and paclitaxel 175 mg/m2/d every 21 days × 3. With cisplatin/irinotecan, the pCR rate was 15%, median survival 35 months, and 5-year survival 46%. With cisplatin/paclitaxel, the corresponding results were pCR 17%, median survival 24 months, and 5-year survival 34%. Neither of these regimens appeared superior to the other, nor likely to substantially improve upon the results reported for the regimen used in the CROSS trial or the several studies using 5-FU-containing regimens described above, and there continues to be a need to identify more active systemic agents to improve outcome in this disease with a largely metastatic pattern of failure.
Safety of Esophagectomy after Chemoradiation
The question of whether preoperative chemoradiation increases surgical morbidity and mortality is an area of controversy, but appears to be less relevant in the modern era. The majority of randomized trials discussed previously reported no significant increase in surgical mortality comparing trimodality therapy with surgery alone. The results of 120 patients treated at Johns Hopkins with preoperative chemoradiation were analyzed for surgical morbidity and mortality to evaluate the overall complication rate.201 Surgical mortality was 1%. The complication rate was 59% for squamous cell carcinoma and 31.6% for adenocarcinoma. The higher complication rate observed for squamous cell carcinoma, relative to patients with adenocarcinoma, was attributed to an increased risk of pulmonary complications because of the more proximal location of the primary and potential increased prevalence of chronic lung disease in this population. These results are comparable to the mortality (2.2% to 9.0%) and morbidity (22% to 74%) reported after surgery alone, and suggest that with careful technique and attention to postoperative management, preoperative therapy does enhance surgical morbidity. Operative mortality using transhiatal esophagectomy was 4% with surgery alone and 2% after preoperative therapy. Similar mortality rates have been reported from recent multicenter trials of patients with adenocarcinoma only or including both histologies.113,197 A retrospective comparison of squamous cell carcinoma patients who had neoadjuvant chemoradiation or surgery alone demonstrated operative mortality of 6.3% versus 9% and morbidity of 40.3% and 41%, respectively.202 In contrast, the trial reported by Bosset193 including only patients with squamous cell histology had 12% operative mortality after chemoradiation and 4% after surgery alone.
More recently, data from current approaches have been described for the CROSS randomized trial.53 Most interestingly, morbidity and mortality appeared similar. Pulmonary complications occurred in 46% with preoperative chemoradiation and 44% with surgery alone. Anastomotic leakage occurred in 22% versus 30% respectively. Likewise, cardiac complications, chylothorax, mediastinitis, and anastomotic leakage were similar. Importantly, 30-day mortality was 6% with preoperative therapy and 7% without.
Assessment of Response to Preoperative Therapy to Guide Management
Radiographic and endoscopic assessment of response has not proved accurate enough after chemoradiation to serve as a selection criterion for proceeding to surgery, to guide changing unsuccessful neoadjuvant therapy, or to select patients for more aggressive postoperative therapy. Posttreatment inflammatory changes and thickening are difficult to distinguish from tumor and limit the utility of both CT scanning and endoscopy, even with ultrasonography.190,192,193 Even rigorously performed evaluations of three-dimensional (3D) CT tumor measurements did not correlate with histopathological response of the tumor.203 The lack of predictability is exemplified by one report204 of seven patients with no residual tumor in the resected esophagus (pCR) whose staging with endoscopy and EUS immediately prior to resection indicated persistent advanced disease (T4N1, one patient; T3N1, three patients; T2N1, two patients; and T3N0, one patient). It has been similarly confirmed in more recent reports that EUS stage after neoadjuvant chemoradiation is not accurate for T or N stage and has not been confirmed to predict survival outcome,205 likely the result of disruption of tissue planes by radiation. On the other hand, a recent prospective multicenter trial206 suggested that significant thickness of residual tumor, >6 mm after neoadjuvant therapy, was associated with reduced long-term survival.
Retrospective and prospective207–210 studies suggest that 18F-fluorodeoxyglucose (FDG)-PET imaging performed early in treatment may be an early marker of response that may predict outcome, and potentially create an opportunity to modify therapy early in its course. In a 39-patient prospective study at Memorial Sloan-Kettering Cancer Center,211 a decrease in the standardized uptake value (SUV) of more than 60% compared to baseline was associated with better 2-year disease-free survival rate (67% vs. 38%, P = ns). In a 40-patient trial of preoperative chemotherapy,210 a 35% reduction in SUV on repeat PET after 2 weeks of neoadjuvant chemotherapy was associated with histologic complete tumor response (53% vs 5%, P < 0.001), median time to progression (16 months vs. 9 months, P = 0.01), and overall survival (19 months vs. 13 months, P = 0.04). An expanded study from these same investigators performing FDG-PET imaging 2 weeks after the start of preoperative chemotherapy found that a >35% reduction in metabolic activity was associated with a 70% 3-year survival rate and a 44% pCR response rate, whereas, with a lesser reduction in metabolic activity, the median survival was 24 months and complete histologic response rate 5%.212 Similarly, the results of a prospective Cancer and Leukemia Group B (CALGB) trial supported a possible value of PET imaging.213 Patients were treated with irinotecan and cisplatin (CI) weeks 1, 2, 4, and 5 and then with concurrent CI weeks 7, 8, 10, and 11 and RT. Repeat PET imaging was performed after week 5, prior to beginning RT. A decline in SUV of >22% was associated with a median time to progression of 18.5 months versus 5.5 months. It should be emphasized that these prospective studies assessed the prognostic value of repeat PET after chemotherapy alone where the local inflammatory effects from chemoradiation may be substantial and confounding.214 However, a recently reported series of 61 squamous cell carcinoma patients treated with definitive chemoradiation together, a ≥50% decrease in SUV was associated with 40% 5-year survival versus 0%.215
The phase II MUNICON trials (Metabolic response evalUatioN for Individualisation of neoadjuvant Chemotherapy in oesOphageal and oesophagogastric adeNocarcinoma) for adenocarcinoma patients have explored the potential of using PET to guide therapy. In the MUNICON I216 trial, patients who were found to be responding to neoadjuvant chemotherapy by PET scanning continues a planned three cycles of therapies whereas nonresponders were selected for early surgery rather than continuing a potential ineffective chemotherapy regimen. Fifty-nine percent of enrolled patients were PET responders, of whom 58% had a major pathological response (<10% tumor in specimen), 96% had R0 resection and median time to progression of 29.7 months whereas for PET nonresponders the results were 0% major response, 74% R0 resection, and median survival was not reached for PET responders and was 25.8 months for non-responders (P = 0.015). A follow-up 56-patient trial, MUNICON II,217 allowed a variety of chemotherapy regimens but added neoadjuvant concurrent radiotherapy for patients without early PET response to radiation alone. The results appeared similar for PET responders compared with those achieved in MUNICON I, but the goal of providing evidence for improved R0 resection rate with the addition of radiation in nonresponders was not achieved. Despite a major histologic response rate improved to 26% in nonresponders, R0 resection occurred in only 70% despite addition of radiation. One ongoing randomized trial in the United States, CALGB 80803,218 is exploring whether alteration of the chemotherapy to a non-cross-resistant regimen in PET nonresponders may improve outcome. In this randomized phase II trial, patients receive either a 5-FU oxaliplatin or a carboplatin paclitaxel regimen prior to preoperative radiotherapy. Based on the PET scan response, patients will continue the same agents during concurrent chemoradiation or cross to the other regimen.
Pathological stage at the time of surgery after neoadjuvant therapy is an important predictor of survival outcome that can be used to advise patients and potentially to select high-risk patients for trials of novel adjuvant therapies. In a prospective trial performed at Johns Hopkins and Yale, patients with a pCR had 67% survival at 5 years (median not reached) whereas the remainder of the patients had 5-year survival of 27% (median 21 months) (P < 0.001).114,219 Patients with pathological stage I tumor at the time of surgery had survival times that were similar to those with a complete response to preoperative therapy. Median survivals for patients with pathological stage IIA, IIB, III, and IV disease at the time of surgery were 22, 13.5, 18, and 4.9 months, respectively. An analysis of 276 patients assessed after preoperative chemoradiation similarly suggested that the presence of only limited residual nodal disease, even without a complete response of the primary tumor, could be used as an important prognostic factor. For the group with a complete response, the 3-year survival was 70%. Three-year survival was 70% for T0–1 with up to one positive node, 52% for T2–4 node negative, 32% for T2–4 with one positive node, and 26% for more than 2 nodes regardless of T stage. Those with metastatic disease had worse outcomes.220 As these findings may be influenced by response to the neoadjuvant therapy, there may be implications for applicability of the current staging system based on operative findings based on databases of results observed after surgery alone.