Cancer of the Anal Canal
Karyn A. Goodman, Lisa A. Kachnic and Brian G. Czito
• It was estimated that there were 6230 anal squamous cell carcinomas diagnosed and 780 deaths from this disease in the United States in 2012.
• The age-adjusted incidence rate is 1.7 per 100,000 men and women per year with a female predominance.
• Anal cancer is associated with human papilloma virus (HPV) infection, which mediates transformation of the anal squamous epithelium through a progression of precancerous lesions (anal intraepithelial neoplasia) to invasive cancer.
• American Joint Commission on Cancer (AJCC) tumor–node–metastasis (TNM) staging system for anal cancer is a clinical staging system. Notably, the T stage is based on tumor size rather than depth of invasion as it is in other gastrointestinal cancers.
• Physical examination, evaluating the size, location, mobility, and inguinal nodal involvement, is critical in staging patients and for a baseline comparison to determine response.
• Computed tomography, sigmoidoscopy, endoanal ultrasound, and magnetic resonance imaging or positron emission tomography imaging are helpful in evaluation of the extent of the primary lesion, presence of regional nodal disease or distant metastatic disease.
• Tumors of the anal canal are primarily squamous cell carcinomas.
• Nonsquamous histologies are rare and include anal adenocarcinomas, melanomas, and sarcomas.
• Primary therapy for anal squamous cell carcinomas consists of definitive chemoradiation with concurrent 5-flourouracil, mitomycin-C, and radiotherapy.
• Surgical resection is only used as salvage for patients who have persistent or recurrent disease after chemoradiation.
• Induction chemotherapy, radiation dose-intensification, and maintenance chemotherapy have not been shown to improve outcomes over standard chemoradiation for anal cancer.
• The outcomes for anal squamous cell carcinoma are good, with a colostomy-free survival of 72% and overall survival of 78% at 5 years with pelvic radiotherapy and concurrent 5-fluorouracil and mitomycin-C.
• More advanced tumors are associated with a higher risk of local recurrence and distant failure.
Introduction
Although rare, anal cancer provides a paradigm for organ preservation in the management of cancer. The treatment for anal cancer has evolved from the abdominoperineal resection (APR), which required a permanent colostomy, to sphincter-preserving nonsurgical therapy with concurrent radiation therapy and 5-fluorouracil (5-FU) and mitomycin-C chemotherapy.1,2 However, the favorable disease-free survival associated with definitive chemoradiotherapy has been tempered by a significant rate of both acute and long-term toxicities. Improvements in pelvic radiotherapy techniques to minimize the exposure of normal tissues to potentially damaging radiation doses have demonstrated promising results in reducing acute toxicities. Investigations into alternative chemotherapeutic regimens have not yielded positive results to date. However, studies evaluating targeted agents and less toxic regimens are ongoing. Further studies are warranted to assess intensification of therapy in more advanced anal cancers and reduction of treatment in early-stage disease with the goal of improving both the tolerance of chemoradiotherapy and long-term, disease-free quality of life.
Anatomy
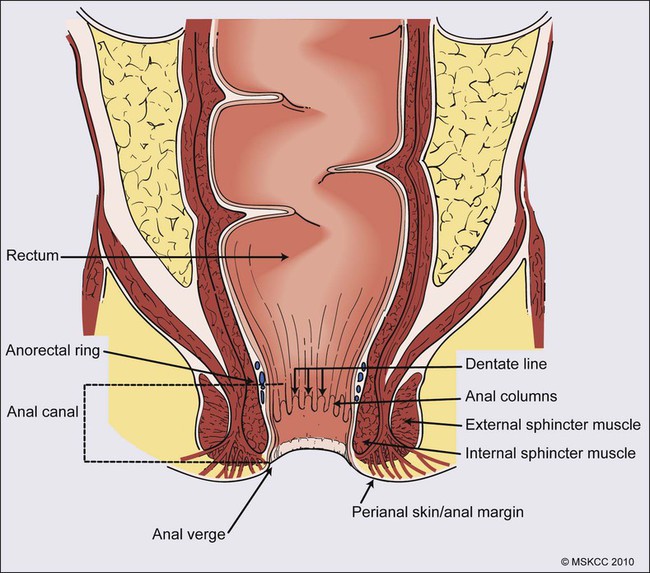
The anal canal is defined as the stratified squamous epithelium that extends from the anal verge to the anorectal ring, measuring approximately 3 to 4 cm in length (Fig. 79-1). The anal margin or perianal skin is the hair-bearing skin within a 5-cm radius immediately beyond the anal verge, and neoplasms involving the anal margin have been traditionally managed as skin cancers.5–5 The anal verge is, therefore, the junction of stratified squamous epithelium of the anal canal and the keratinized squamous epithelium of the perianal skin. The anorectal ring is comprised of a muscular bundle at the junction of the internal sphincter, puborectalis, and external sphincter, and the mucosa overlying the anorectal ring is a columnar epithelium. The columns of mucosa from the anorectal ring extend about 1 cm to the dentate line, the histologic transitional zone between the columnar and stratified squamous epithelium. Tumors arising near the dentate line are referred to as cloacogenic or transitional cell, and are comprised of nonkeratinizing squamous cells; however, such tumors have similar prognoses and are treated in the same fashion as squamous cell cancers of the anal canal.6 Tumors that develop from mucosa (columnar, transitional, or squamous) are true anal canal cancers, whereas tumors that arise from skin distal to the anal verge are termed anal margin or perianal skin tumors.
Epidemiology
Squamous cell carcinoma of the anus is relatively rare and comprises only 4% of all large bowel cancers.7 The National Cancer Institute’s (NCI) Surveillance of Epidemiology and End Results (SEER) Cancer Statistics Review estimated that 6230 new cases will be diagnosed in the United States and 780 deaths from the disease in the United States in 2012.7 From 2005 to 2009, the median age at diagnosis was 60 years of age.8 The age-adjusted incidence rate was 1.7 per 100,000 men and women per year, with a slight female predominance (1.9 per 100,000 women and 1.5 per 100,000 men). There has been a steady increase in incidence of anal cancer in recent years with an annual percent change of 2.2% from 1975 to 2009. Fortunately, at diagnosis, 50% of patients present with localized (stages I/II) disease and only 13% present with metastatic disease. The 5-year overall survival rate for all patients diagnosed between 2002 and 2008 was 65%, with 80% of patients with localized disease surviving 5 years.8
Etiology and Biological Characteristics
There have been several factors that have been implicated in the pathogenesis of anal cancer, including human papilloma virus (HPV), immunosuppression, and cigarette smoking. The most important risk factor for squamous cell anal cancer is infection with HPV, especially types 16 and 18. These high-risk HPV types act as carcinogens in the development of anogenital cancers.9,10 A recent meta-analysis suggests that HPV16 is found more frequently (75%) and HPV18 less frequently (10%) in anal carcinomas than in cervical carcinomas.11 Moreover, approximately 80% of anal cancers demonstrated more than one HPV genotype.11
As in cervical neoplasia associated with HPV, the viral proteins E6 and E7 mediate oncogenic transformation of the anal squamous epithelia. The viral E6 protein binds to the E6-associated protein (E6 AP) and ubiquinates the protein p53, which in turn leads to the proteasomal degradation of this major cellular transcription factor, leading to loss of the cell-cycle arrest and apoptotic mechanisms that allow for deletion of errors in DNA replication.12 The viral E7 protein binds to the product of the retinoblastoma (Rb) gene, and consequently accelerates the cell cycle, allowing cells to progress from the G1 into the S phase of the cell cycle.13
As anal lesions progress from condylomata to low-grade dysplasia and then high-grade anal intraepithelial neoplasia (AIN) and invasive cancer, there is an accumulation of mutant p53 expression, emphasizing its role in tumor development.14,15 A prior history of anal condylomas has been reported in as many as 50% of homosexual and 30% of heterosexual patients diagnosed with anal carcinoma.16 The E2 protein allows HPV to escape intracellular detection by facilitating attachment of the HPV DNA to the host chromatin, an effect that allows steady replication of the virus in tandem with epithelial cells.17 Therefore, through concerted actions of HPV proteins E2, E6, and E7, the anal epithelium accumulates genetic errors leading to proliferation and eventually resulting in carcinogenesis.
Immunosuppression, in particular impaired cellular-mediated immunity, is another important risk factor in the development of anal cancer. The impact of suppressed cell-mediated immunity may be related to a reduced host response that would clear the HPV virus and prevent the establishment of a prolonged viral presence. Support for this finding comes from the observation that anal cancer rates are increased in both human immunodeficiency virus (HIV)–positive patients and patients that undergo renal transplantation where cell-mediated immunity is suppressed.20–20 In one series, there was an approximate 100-fold increased risk of developing anogenital cancer in renal transplant patients.19 In another series, patients receiving chronic steroid therapy for amelioration of autoimmune disorders were also found to be predisposed to HPV-associated anal lesions.21
Cigarette smoking is also associated with the development of anal cancer, much as it is with cervical cancer.22 The risk of anal cancer appears to be related to pack-year history of smoking, with more extensive histories associated with a higher risk. The mechanism of smoking-associated tumors is unclear, but smoking may act as a cocarcinogen in the context of HPV infection.23
Prevention and Early Detection
AIN is the precursor lesion to invasive anal cancer. AIN is subdivided into low- and high-grade AIN, analogous to the classification of low- and high-grade squamous intraepithelial lesions of the cervix. Similar to the Papanicolaou (Pap) smear for cervical cancer, screening for anal cancer can be performed using anal cytology obtained by swabbing the anal canal (“anal Pap smear”). Sensitivity for detection of dysplasia appears higher (approximately 75%) in HIV-positive patients as opposed to HIV-negative patients (approximately 60%).24 In patients with abnormal cytology, anoscopy with administration of 3% acetic acid can then be performed to guide biopsies, much as is done with cervical colposcopy.
Treatment of high-grade AIN includes ablation with anoscopic-directed electrocautery, topical trichloroacetic acid, topical 5-FU, or imiquimod.27–27 Topical applications yield lesion control in the range of 60% to 80%.
To date, however, there are no established guidelines for anal cancer screening using anal Pap smears in high-risk groups as there are for cervical cancer screening, and cost-effective analysis showed no benefit of annual screening in HIV-positive men who have sex with men.28
There has been significant progress in the prevention of HPV-related malignancies with the introduction of the HPV vaccines. Two vaccines (Cervarix and Gardasil) are now approved by the U.S. Food and Drug Administration and have been shown to protect against cervical cancer in women.29,30 The quadrivalent HPV vaccine Gardasil has a high efficacy for prevention of HPV 6-, 11-, 16-, and 18-related genital warts and has been shown to protect against cancers of the anus, vagina, and vulva.31 The quadrivalent HPV vaccine was also studied in men who have sex with men and was found to reduce the rates of AIN.18 Both vaccines have a favorable safety profile. HPV vaccination is now recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control for preteen girls and boys at age 11 or 12 years and girls 13 to 26 years who have not been previously vaccinated.
Pathology and Pathways of Spread
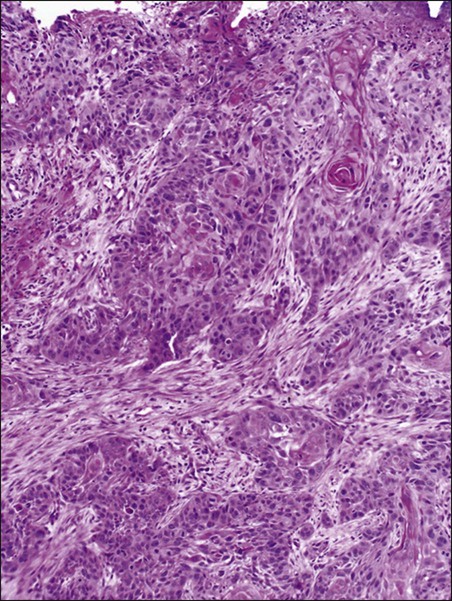
A variety of malignancies can arise within the anal canal. Simplistically, these can be divided into squamous and nonsquamous histologies. Squamous cell tumors are the most common presentation. Historical histologic descriptors such as basaloid, cloacogenic, and junctional (tumors arising in the transitional mucosa of the proximal canal described above) are not as relevant for practical management and are not included in the current World Health Organization classification system for anal cancer. Tumors arising in this area are considered squamous cell tumors (generally nonkeratinizing) and are managed accordingly. Tumors arising below the dentate line are often keratinizing squamous cell carcinomas (Fig. 79-2). Nonsquamous cell tumors including adenocarcinomas, melanomas, lymphomas, neuroendocrine tumors, and sarcomas have been described, but are less common. A suspected anal adenocarcinoma may actually represent extension from a distal rectal adenocarcinoma in some scenarios. Adenocarcinoma arising in the anal canal has a different clinical biology than squamous cell carcinoma and is usually managed like rectal cancer. Ultimately, tumor location within the anal canal is not as important as histologic subtype.
The regional nodes of the anal canal are considered to be inguinal (superficial and deep femoral), internal iliac, external iliac, and perirectal (mesorectal). All other nodal groups represent sites of distant disease. The incidence of involvement of inguinal nodes is directly proportional to the size and extent of the primary tumor. Of all patients presenting with palpable inguinal lymph nodes, only approximately 50% will contain cancer; therefore, fine-needle aspiration is recommended in suspected cases.32
Anal cancer is primarily a locoregional disease, with only approximately 10% of patients diagnosed with distant metastases at presentation. The liver and lungs are the most frequent site of distant spread. After curative treatment, the risk of distant disease varies and depends on the initial tumor size and nodal stage.33
Clinical Manifestations/Patient Evaluation/Staging
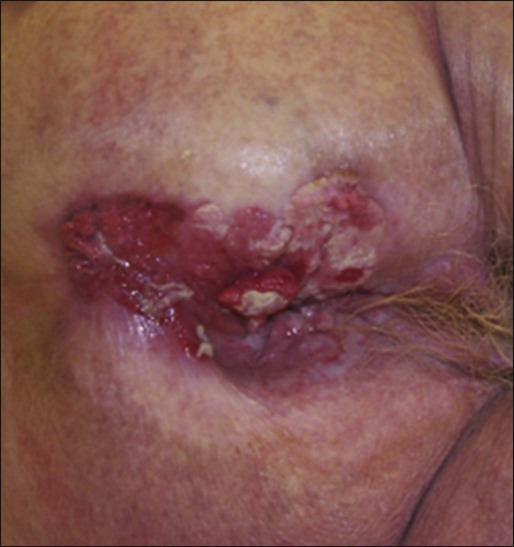
The most common presenting symptom in patients with anal cancer is rectal bleeding, occurring in approximately one-half of patients.34,35 Because bleeding is frequently minor, often in conjunction with a mass palpated at or above the anal sphincter, an initial errant diagnosis of hemorrhoids is common. Additional presenting symptoms include pain from local invasion or a sensation of anorectal fullness, occurring in approximately 30% of patients,20 pruritus, changes in bowel habits, and tenesmus. Frank incontinence is less common but is suggestive of more advanced disease and sphincter destruction. In a minority of presentations, disease is discovered on routine physical examination in an otherwise asymptomatic patient.
Physical examination will often reveal a relatively indurated mass with potential bleeding and ulceration (Fig. 79-3). This may be visible on direct inspection with disease at or distal to the anal verge. On digital examination, tumor location, size, involvement of adjacent organs, and extent should be fully appreciated, including relationship of the mass to the dentate line by anoscopy/endoscopy. Histologic confirmation of malignancy by tissue biopsy is required prior to initiation of tumor-directed therapy, and any suspicious mass should undergo incisional biopsy. If there is clinical suspicion of malignancy prior to biopsy, full local excision should generally be reserved for very small, superficial lesions. In female patients, vaginal examination should be performed to rule out posterior vaginal invasion and fistula, and evaluation of the cervix should be performed to rule out gynecologic malignancy. Given patterns of spread, thorough examination of lymph node areas, including inguinofemoral regions, should be performed. Any suspicious lymphadenopathy should undergo biopsy, generally through fine-needle aspiration, to further facilitate diagnosis and tumor staging. Table 79-1 reviews the standard evaluation for patients with anal cancer.
Table 79-1
Recommended Diagnostic Evaluation in Patients with Anal Cancer

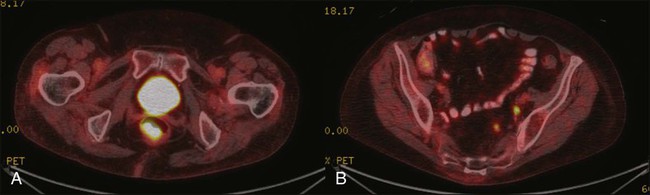
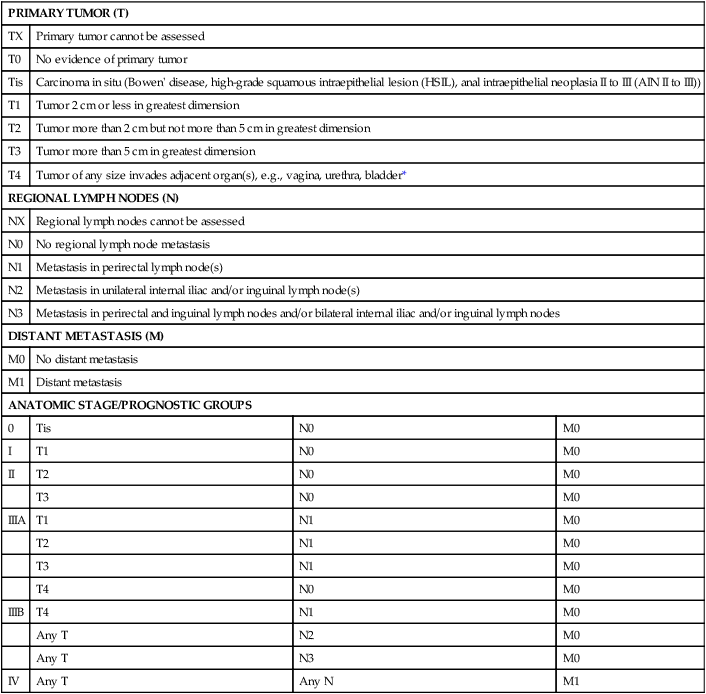
Once diagnosis is established, patients should undergo imaging for staging, including contrasted chest, abdominal and pelvic computed tomography (CT), often in conjunction with positron emission tomography (PET) scan. HIV testing should be performed in patients with established risk factors. Staging, as with most tumors, involves determining local extent of the primary disease, whether or not nodal metastases are present, and whether or not there is evidence for distant spread of disease (outside of the pelvis). PET and PET-CT are now also routinely integrated into the staging algorithm for patients (Fig. 79-4).38–38 In one series, PET-CT appeared to have a higher sensitivity than conventional imaging (CT and/or magnetic resonance imaging [MRI]) for detecting regional lymph node metastases (89% vs. 62%), although for practical reasons not all nodes could be biopsied for a true measure of sensitivity and specificity. PET was found to change planned radiation therapy fields in 13% of patients and thus was worthy of inclusion in the staging process.38 In other reports, PET-CT upstaged 17% to 38% and downstaged 19% to 25% of patients; similarly, there was also change in management in 13% to 29% of patients.39,40 Of note, HIV-positive patients may have falsely positive fluorodeoxyglucose–avid lymph nodes. Biopsy may be necessary in these situations to determine whether or not there is true nodal metastatic disease versus a benign inflammatory process. Table 79-2 outlines the American Joint Commission on Cancer (AJCC) staging system.
Table 79-2
AJCC Staging System for Anal Cancer, Seventh Edition (2010)
| PRIMARY TUMOR (T) | |||
| TX | Primary tumor cannot be assessed | ||
| T0 | No evidence of primary tumor | ||
| Tis | Carcinoma in situ (Bowen’ disease, high-grade squamous intraepithelial lesion (HSIL), anal intraepithelial neoplasia II to III (AIN II to III)) | ||
| T1 | Tumor 2 cm or less in greatest dimension | ||
| T2 | Tumor more than 2 cm but not more than 5 cm in greatest dimension | ||
| T3 | Tumor more than 5 cm in greatest dimension | ||
| T4 | Tumor of any size invades adjacent organ(s), e.g., vagina, urethra, bladder* | ||
| REGIONAL LYMPH NODES (N) | |||
| NX | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastasis | ||
| N1 | Metastasis in perirectal lymph node(s) | ||
| N2 | Metastasis in unilateral internal iliac and/or inguinal lymph node(s) | ||
| N3 | Metastasis in perirectal and inguinal lymph nodes and/or bilateral internal iliac and/or inguinal lymph nodes | ||
| DISTANT METASTASIS (M) | |||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| ANATOMIC STAGE/PROGNOSTIC GROUPS | |||
| 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| T3 | N0 | M0 | |
| IIIA | T1 | N1 | M0 |
| T2 | N1 | M0 | |
| T3 | N1 | M0 | |
| T4 | N0 | M0 | |
| IIIB | T4 | N1 | M0 |
| Any T | N2 | M0 | |
| Any T | N3 | M0 | |
| IV | Any T | Any N | M1 |
Note: cTNM is the clinical classification, pTNM is the pathological classification.
*Direct invasion of the rectal wall, perirectal skin, subcutaneous tissue, or the sphincter muscle(s) is not classified as T4.
Used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer, New York, Inc.
Primary Therapy
Squamous Cell Carcinoma
Surgery
Until the mid-1970s, surgery was the gold standard for the treatment of anal canal cancer.
The standard surgical technique was an APR, requiring a permanent colostomy as well as removal of the rectum, ischiorectal fat, levator sling, perirectal and superior hemorrhoidal nodes, and a wide area of perianal skin. Long-term sexual and urinary dysfunction are potential consequences of an APR. The 5-year overall survival (OS) rate for APR for all patients with anal cancer was approximately 50%.41,42 For superficial lesions without lymph node involvement, the Mayo Clinic reported 5-year OS rates of 90% with radical surgery; however, with muscle invasive, node-positive disease, outcomes were poor, with a 5-year OS of 32%.43 A subsequent review from the Mayo Clinic of 118 anal canal cancers treated with an APR showed an OS rate of 70% and an overall recurrence rate of 40%. Over 80% of those with known recurrence sites had either exclusively local recurrence or a local recurrence component.44 Frost and colleagues45 reported a 5-year OS of 62%, with a 45% rate of failure in the pelvic and inguinal lymph nodes.
Given the poor outcome with radical surgery alone, Nigro evaluated the use of preoperative chemoradiation therapy for anal canal squamous cell cancer. The first report of three patients who received radiation therapy to 30 Gy in 15 fractions, with concurrent 5-FU (25 mg/kg with continuous infusion [CI]) and mitomycin (0.5 mg/kg bolus), was published in 1974.46 Two patients underwent subsequent APR, and one patient refused surgery. At the time of APR, both patients showed pathological complete response, and the patient who refused surgery had no evidence of disease clinically at 14 months.46 Nigro subsequently reported results for a total of 28 patients with anal squamous cell cancer treated in the same manner with preoperative radiation therapy and chemotherapy. Surgery was performed 4 to 6 weeks following the last day of radiation treatment. Twelve patients underwent APR, of whom seven had no residual tumor in the surgical specimen, while one patient had microscopic tumor only. An additional 14 patients had a complete clinical response, and underwent local excision that confirmed pathological complete response. Two other patients were clinically free of tumor without biopsy confirmation.47,48 In a subsequent series using definitive chemoradiation as primary therapy, 38 of 45 patients were cured of disease, with a 5-year OS of 67% and colostomy-free survival of 59%.49 These data suggested that definitive chemoradiation could be used to treat anal canal cancer with equivalent or better tumor control and significantly reduced mortality compared to APR. APR is now rarely used as initial therapy; however, it is still an option for salvage after persistence or recurrence of disease, or for management of complications after combined-modality therapy.50
For early-stage, small (<2 cm), well-differentiated anal canal cancers without other adverse histologic features that are not invading the underlying sphincter and have no clinical lymph node involvement, local excision is considered an option. For these highly selected patients, OS at 5 years has been reported to be over 80%.4,42 However, local excision should only be considered for lesions of the anal margin in which the sphincter can be spared.
Prospective Trials Evaluating Combined-Modality Therapy
Several large randomized multiinstitutional studies, detailed below, have validated definitive chemoradiation with concurrent 5-FU and mitomycin as the primary treatment modality for anal canal cancer.51–56 Table 79-3 summarizes the key findings. Combined-modality therapy has been compared to radiation alone in several trials, which have all demonstrated the superiority of chemoradiation for sphincter preservation. In general, primary radiotherapy alone has lower treatment-related toxicity than chemoradiation, but is associated with a higher local failure rate in tumors greater than 2 cm.
Table 79-3
Summary of Major Randomized Phase III Clinical Trials of Chemoradiotherapy for Anal Cancer
| Study (Reference) | Treatment Arms* | Local-Regional Failure† | Relapse-Free Survival† | Colostomy-Free Survival† | Overall Survival | Acute Toxicity |
| UKCCCR/ACT I51,51a | (1) Radiation | (1) 59.1% | (1) 17.7% | (1) 20.1% | (1) 27.5% | (1) “Severe” skin toxicity = 39%; “severe” GI toxicity = 5% |
| (2) Radiation + 5-FU + MMC | (2) 33.8% (at 12 years) | (2) 29.7% (at 12 years) | (2) 29.6% (at 12 years) | (2) 33.1% (at 12 years) p=NS |
(2) “Severe” skin toxicity = 50%; “severe” GI toxicity = 14% | |
| EORTC53 | (1) Radiation (2) Radiation + 5-FU + MMC |
18% improvement in arm 2 at 5 years | N/A | 32% improvement in arm 2 (colostomy free) | (1) Grades 3–4 diarrhea = 8%; grades 3–4 dermatologic = 50% (2) Grades 3–4 diarrhea = 20%; grades 3–4 dermatologic = 57% |
|
| RTOG/ECOG54 | (1) Radiation + 5-FU | (1) 34% | (1) 51% | (1) 59% | (1) 67% | (1) Grades 4–5 heme = 3%; grades 4–5 non-heme = 4% |
| (2) Radiation + 5-FU + MMC | (2) 16% | (2) 73% (disease-free survival at 4 years) | (2) 73% (at 4 years) | (2) 76% (at 4 years) p=NS |
(2) Grades 4–5 heme = 18%; grade 4–5 non-heme = 7% | |
| RTOG 98-1152,59 | (1) Radiation + 5-FU + MMC | (1) 20% | (1) 68% | (1) 72% | (1) 78% | (1) Grades 3–4 heme = 61%; grades 3–4 non-heme = 74% |
| (2) Cisplatin + 5-FU → radiation + 5-FU + cisplatin | (2) 26% p=NS | (2) 58% (disease-free survival at 5 years) | (2) 65% (colostomy-free survival at 5 years) p=.05 | (2) 71% (at 5 years) | (2) Grades 3–4 heme = 42%; grades 3–4 non-heme = 74% |
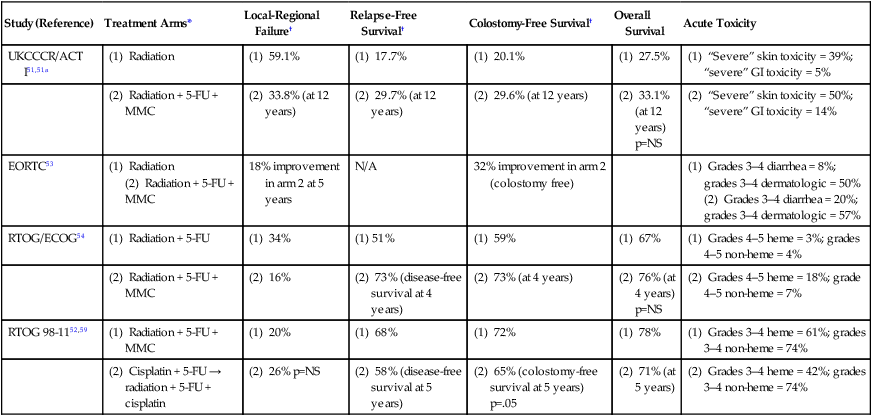
*Details of treatment arms noted in text.
†P values significant for comparisons unless otherwise noted.
The United Kingdom Coordinating Committee on Cancer Research (UKCCCR) Anal Cancer Trial (ACT I) randomized 585 patients with cancer of the anal canal or anal margin to radiotherapy alone with 45 Gy in 20 to 25 fractions versus the same radiotherapy concurrent with 5-FU (1000 mg/m2) CI over 5 days during weeks 1 and 5 of radiation, and mitomycin (12 mg/m2) bolus on day 1.51 Of these, 51% had clinical T3 disease, 20% positive nodes, and 23% anal margin cancers. All patients were restaged 6 weeks after treatment for local failure. Those with less than 50% response had salvage surgery and those with greater than 50% response received a further boost course of radiation therapy: 15 to 20 Gy external beam radiation or brachytherapy. After a median follow-up of 42 months, the 3-year local failure rate was significantly lower in the chemoradiotherapy group (39% for chemoradiation; 61% for radiotherapy alone; P < 0.0001), but the chemoradiotherapy group experienced increased morbidity. Although there was no statistically significant survival advantage (58% vs. 65%; P = 0.25) for the addition of chemotherapy, there was a significant improvement in anal cancer-related mortality with concurrent therapy (28% vs. 39%, P = 0.02).51 The authors concluded that patients with squamous cell carcinoma of the anal canal can be treated with radiotherapy, 5-FU, and mitomycin, with surgery reserved for salvage.
Similarly, in another prospective phase III study conducted by the European Organization for Research and Treatment of Cancer (EORTC), Bartelink and colleagues randomized 110 anal cancer patients to either radiotherapy alone, or radiotherapy with concurrent chemotherapy.53 Notably, 83% of the patients had T3 disease, whereas 48% had involved lymph nodes at the time of diagnosis. The radiation was similar to that administered in the UKCCCR trial. Chemotherapy consisted of 5-FU (750 mg/m2) CI for 5 days on weeks 1 and 5 of radiation, with mitomycin (15 mg/m2) bolus on day 1. Patients showing complete response or partial response received an additional 15 or 20 Gy radiation boost, respectively, and salvage surgery was reserved for patients with less than a partial response. There was a significant advantage of chemoradiotherapy over radiotherapy alone, in terms of complete response (80% for chemoradiotherapy vs. 54% for radiotherapy alone), 5-year local control (68% vs. 50%), and 5-year colostomy-free survival (72% vs. 40% for radiation alone).53 Again, no significant difference in OS was seen between these two arms (3-year survival, 72% vs. 65%, P = 0.17).
Role of Mitomycin-C in Combined-Modality Therapy
Although the previous randomized trials demonstrated a benefit to local tumor control with the addition of chemotherapy to radiotherapy as definitive treatment of anal canal cancer, the acute toxicity associated with the addition of chemotherapy, notably the mitomycin component, is substantial. Therefore the benefit of adding mitomycin to 5-FU was investigated in several trials. The U.S. Intergroup (Radiation Therapy Oncology Group [RTOG] 87-04/Eastern Cooperative Oncology Group [ECOG] 1289) was a prospective, randomized phase III study of 310 patients with stages I to IIIA (T1 to T4, N0 to N1) anal carcinoma randomized to receive radiation therapy (45 to 54.4 Gy) with infusional 5-FU (1000 mg/m2 CI × 4 days) week 1 versus the same regimen with mitomycin (10 mg/m2) bolus weeks 1 and 5. A biopsy was performed 4 to 6 weeks after completion of chemoradiation. If residual disease was seen, patients received an additional 9 Gy radiation boost with concurrent chemotherapy consisting of 5-FU and cisplatin. Biopsy was performed again after salvage therapy; patients without a pathological complete response underwent APR. The authors reported that the addition of mitomycin halved the rate of positive posttreatment biopsies (15% in the 5-FU alone arm vs. 7.7% in the mitomycin arm).54 At 4 years, the mitomycin arm resulted in a significantly lower colostomy rate (9% vs. 22%), higher colostomy-free survival (71% vs. 59%), and higher disease-free survival (73% vs. 51%). There was no difference in OS between the two arms. However, the mitomycin arm also had greater acute toxicity. There was a 23% grade 4 toxicity (notably hematologic and dermatologic) and a 2.7% fatal toxicity (all of which were from neutropenic sepsis).54 Of 25 patients with positive posttreatment biopsy, 12 ultimately had a negative biopsy after “salvage” chemoradiotherapy. An important caveat, however, is the long regression period of squamous cell tumors in some patients after chemoradiotherapy (as long as a year).
Because of the hematologic toxicity reported with mitomycin, less myelosuppressive chemotherapy regimens were investigated. Hung and colleagues from the MD Anderson Cancer Center analyzed the outcome of 92 patients with squamous cell carcinoma of the anus treated with radiation, 5-FU, and cisplatin in place of the mitomycin.57 Radiation consisted of a total dose of 55 Gy to the primary tumor site and macroscopically involved lymph nodes, with elective lymph node regions receiving 30.6 Gy. 5-FU (250 mg/m2 per day) and cisplatin (4 mg/m2 per day) were administered as CI, 5 days each week, throughout the radiation course. Grade 4 toxicity occurred in only 5.4% and no patient developed severe myelosuppression.57 With a median follow-up duration of 44 months, actuarial 5-year OS was 85%, disease-free survival was 77%, and colostomy-free survival was 82%. Local recurrence was noted in 17% and distant metastases in 9%.
Meropol et al. initially reported the results of a Cancer and Leukemia Group B (CALGB) pilot study in 1999 that evaluated the use of induction 5-FU/cisplatin for advanced anal canal cancers (T3-4 or node positive) followed by concurrent chemoradiation therapy with 5-FU/mitomycin and 45 Gy.58 Mitomycin was administered during weeks 1 and 5, with or without a 9 Gy boost and an additional cycle of chemotherapy for persistent primary site disease or bulky N2 or N3 disease at presentation. For these high-risk patients, the 56% colostomy-free survival rate and 80% complete response rate was felt to be very promising.58
Based on the results of these smaller trials and the interest in substituting cisplatin for mitomycin in several other cancer types, the RTOG initiated a large, Phase III, randomized trial (RTOG 98-11) to assess the benefit of induction chemotherapy and the replacement of mitomycin by cisplatin.52 In this study, patients were randomized to a standard arm of concurrent chemoradiotherapy (45 to 59 Gy) with CI 5-FU and bolus mitomycin weeks 1 and 5, or an experimental arm of 2 cycles of induction CI 5-FU and bolus cisplatin on weeks 1 and 5, followed by concurrent chemoradiation with 2 cycles of continuous 5-FU and bolus cisplatin weeks 9 and 13 with radiotherapy (45 to 59 Gy). The primary end point was 5-year disease-free survival, with a secondary intent of reduced acute toxicity. Radiation in this trial included a minimum dose of 45 Gy in 25 fractions of 1.8 Gy each over 5 weeks to the primary tumor using nonconformal radiation delivery techniques with anteroposterior-posteroanterior or multifield arrangements. For T3, T4, node-positive disease or T2 tumors with residual disease after 45 Gy, an additional boost of 10 to 14 Gy could be delivered at the discretion of the treating physician in 2 Gy fractions, for a total dose of 55 to 59 Gy. On initial report, no significant difference was seen in 5-year disease-free survival (60% vs. 54%, P = 0.17), OS (75% vs. 70%, P = 0.10), or locoregional relapse (25% vs. 33%, P = 0.07) rates. However, the 5-year colostomy rate was 10% in patients receiving mitomycin versus 19% in the cisplatin arm (P = 0.02).52 A recent update of the trial results demonstrated a significant improvement in 5-year disease-free survival (67.7% vs. 57.6%, P = 0.005), OS (78.2 % vs. 70.5 %, P = 0.02), and colostomy-free survival (71.8% vs. 64.9%, P = 0.05) with mitomycin.59 While the mitomycin arm showed a significantly increased grade 3+ acute hematologic toxicity rate compared with cisplatin (61% vs. 42%, P < 0.001), the rate of acute nonhematologic toxicity was 74% in both arms (notably dermatologic for the mitomycin group and gastrointestinal for the cisplatin). Both arms showed similar late radiation–related toxicities. Of note, induction chemotherapy did not appear to have any impact on locally advanced disease, as suggested in the CALGB study. Based on these results, 5-FU and mitomycin remain the standard of care for anal canal carcinoma. Furthermore, it is thought that the delay in definitive chemoradiation, associated with induction chemotherapy on the cisplatin arm, led to the poorer outcomes reported on this trial.60
In a more direct comparison of the roles of cisplatin to mitomycin with radiation therapy, preliminary results of a phase III trial by the UKCCCR (the ACT II study) failed to show a benefit for cisplatin over mitomycin.55 This multicenter randomized trial of 950 anal cancer patients used a 2 × 2 factorial design to examine the role of mitomycin versus cisplatin with 5-FU chemoradiation, as well as the utility of maintenance 5-FU and cisplatin chemotherapy following chemoradiation. Patients received the same radiotherapy regimen (50.4 Gy in 1.8 Gy fractions) with either concurrent 5-FU and cisplatin versus 5-FU and mitomycin, followed by a second randomization to receive 2 cycles of 5-FU+cisplatin or no further therapy following the completion of chemoradiation. Grades 3 and 4 acute nonhematologic toxicity was 60.2% in the mitomycin group and 64.6% in the cisplatin group (P = 0.17).55 Not surprisingly, grade 3 and 4 hematologic toxicity was greater in the mitomycin group (24.7% vs. 13.4%; P < 0.001). There was no difference in the primary end point for the first randomization with a similar complete response at 6 months in the two groups (94.5% mitomycin and 95.4% cisplatin). The 3-year colostomy rate was also similar in the two groups (13.7% mitomycin and 11.3% cisplatin). With regard to maintenance therapy, 3-year recurrence-free survival was 75% in both arms. Three-year OS was also similar in the maintenance versus no maintenance groups (85% vs. 84%, respectively).55
Time and Dose Considerations in Chemoradiotherapy for Anal Cancer
Overall treatment time has been shown to impact outcome after radiotherapy in many cancer types. This is of relevance for definitive chemoradiotherapy for anal cancer with regard to both the use of induction chemotherapy and the use of split-course radiotherapy regimens to allow for dose escalation. To further assess whether overall treatment time adversely affects outcomes for anal cancer patients, investigators performed an analysis of two randomized RTOG trials and showed a significant association between overall treatment duration and colostomy failure, local failure, regional failure, and time-to-failure rates. On multivariate analysis, a significant association with local failure and a statistical trend toward an association with colostomy failure and overall treatment duration were seen. The authors concluded that total treatment time seemed to have a detrimental effect on local failure and colostomy rate in anal cancer patients and, in particular, the use of induction chemotherapy on the RTOG 98-11 trial may have compromised local control by delaying “definitive” radiotherapy.60 One hypothesis is that the induction chemotherapy may lead to tumor clonogen “accelerated repopulation.” Nevertheless, the role of neoadjuvant chemotherapy in upregulating pathways that confer radioresistance remains unknown.61
Treatment delays related to toxicity during therapy may also lead to worse outcomes for patients undergoing definitive chemoradiotherapy. In the RTOG 98-11 trial, conventional radiation-therapy techniques were employed to deliver a total dose of 55 to 59 Gy for T3, T4, node-positive disease or T2 tumors with residual disease after 45 Gy without a planned treatment break.52 For patients randomized to the 5-FU+mitomycin arm, acute toxicity was significant (see Table 79-3), most notably grades 3 to 4 skin and gastrointestinal toxicity rates of 48% and 35%, respectively. Treatment breaks because of toxicity were required in 61% of patients randomized to the mitomycin arm, with a median duration of 3 days (range: 0 to 33). Similarly, in the ACT II trial described above, a 61% grades 3 to 4 nonhematologic toxicity rate was reported in the mitomycin arm often leading to treatment breaks.55 Such treatment breaks may compromise treatment efficacy. Investigators at Memorial Sloan-Kettering Cancer Center (MSKCC) demonstrated that patients who did not tolerate chemoradiation (evidenced by the number of break days or duration of therapy) experienced higher rates of disease relapse.62 Similarly, Constantinou et al. report that treatment duration 40 or more days was associated with increased locoregional failure as well as poorer disease-free and OS.63
Dose escalation also remains a point of controversy in the treatment of anal cancer. Some studies suggest that patients with early stage disease treated with an excisional biopsy may be sufficiently treated with 30 Gy postexcisional radiation therapy with concurrent chemotherapy, as demonstrated in the original Nigro studies.64,65 However, local failure remains a challenge, particularly for advanced T-stage disease and, as such, total radiation doses have increased in the past two decades. In this regard, Krieg and colleagues from the University of California at San Francisco have shown significant improvement in local control for anal cancer patients who received more than 54 Gy within 60 days of starting treatment.66 These findings have also been demonstrated from investigators at Boston University Medical Center63 and the MD Anderson Cancer Center.67 The MD Anderson group showed local control rates of approximately 50% for patients receiving 45 to 49 Gy versus 90% for those receiving more than 55 Gy.
RTOG 92-08 investigated dose escalation for anal cancer, but designed the trial with split-course therapy, or a planned treatment break, to reduce radiation-related toxicity. In this phase II trial, 47 patients with anal tumors more than 2 cm in size received 5-FU and mitomycin during weeks 1 and 7 of radiation. The radiation dose used was 59.4 Gy in 1.8 Gy fractions over 9 weeks with a 2-week mandatory rest.68,69 The results were compared with the RTOG 87-04 trial in which patients were treated with 45 Gy in a continuous schedule plus the same chemotherapy regimen.54 With the split-course regimen on RTOG 92-08, 12 (26%) patients suffered grade 4 or higher toxicity, one (2%) of whom died from infection. Although these toxicity profiles were similar to RTOG 87-04, the 2-year colostomy rate with 59.4 Gy and a 2-week break was much higher than expected (30% in the 92-08 trial vs. 9% in the 87-04 trial). Because of this, an additional 20 patients were treated to 59.4 Gy, but without a break.68,69 There were no treatment-related deaths, although morbidity was significant. The main grades 3 and 4 toxicities were dermatologic (78%), hematologic (78%), infectious (17%), and gastrointestinal (28%). The authors concluded that, for higher radiation doses to improve local control, radiation therapy should be given in a continuous fashion with 5-FU and mitomycin, with a resultant further increase in acute toxicity. A further concern with dose escalation to the primary site is that even without a planned treatment break, the higher dose can be associated with significant toxicity necessitating treatment breaks and, potentially, the inability to complete therapy.
The role of dose escalation was further assessed in a phase III randomized study, the French Federation Nationale des Centres de Lutte Contre le Cancer ACCORD (Actions Concertees and les Cancers Colorectaux et Digestifs) 03 trial, in which patients with stages II and III anal cancer were randomized to one of four treatment arms: (1) neoadjuvant 5-FU+cisplatin alone followed by 5-FU+cisplatin+radiation therapy (45 Gy), and a radiation boost (15 Gy) using external beam radiation or brachytherapy techniques; (2) as in arm 1, except high-dose radiation boost (20 to 25 Gy); (3) as in arm 1, but no neoadjuvant chemotherapy; or (4) as in arm 2, but no neoadjuvant chemotherapy. A mandatory 3-week treatment break occurred after completion of 45 Gy, prior to administering the boost dose. At a median follow-up of 50 months, there was no difference between the four arms in 5-year colostomy-free survival rates (ranging from 70% to 82% across the four arms). The overall local failure was 28%, with no significant difference between the arms.56 Cause-specific survival and OS rates were also similar. Although these preliminary results suggest that induction chemotherapy does not improve patient outcomes and question the role of radiation-dose escalation in this disease, the authors concluded that results of the most intensified treatment arm (induction chemotherapy and high-dose radiotherapy boost) were encouraging, with a 5-year local control rate of 88% versus 72% to 83% on the other three arms. Thus, the authors conclude that combinations of induction therapy and radiation dose escalation should be explored further. The split-course delivery of radiotherapy in this study also obscures the interpretation of these data as the delay in the delivery of the radiotherapy may have counteracted any benefit of the higher boost dose.
Intensity-Modulated Radiotherapy for Anal Cancer
Intensity-modulated radiotherapy (IMRT) is a form of advanced, photon-based therapy that uses inverse planning with a computer-optimized algorithm to create radiation-beam characteristics, including beam angles and fluctuating radiation-beam intensities, to meet robust requirements for target volume coverage and normal tissue constraints. The resulting treatment fields conform highly to the target volumes with a steep dose gradient, thereby minimizing the radiation dose to the surrounding normal organs. Because of this steep dose fall-off outside the target volume, adjacent normal tissue may be spared from any significant radiation dose, and thus acute toxicities from radiation or chemoradiation therapy may be reduced. Improvements in treatment-related morbidity have already been described in patients with breast, head and neck, and prostate cancer treated with IMRT, compared with conventionally delivered radiation.72–72
There are several theoretical and practical advantages to the use of IMRT for the treatment of anal cancer. First and foremost, IMRT holds the potential to reduce toxicity, both acute and late. Normal structures such as small bowel, bladder, external genitalia, skin, femoral head and neck, and bone marrow can be spared from the higher radiation doses that are used to treat the primary and gross nodal disease. In turn, with a reduction in the acute toxicity, the use of IMRT may reduce treatment breaks that may negatively influence outcome, as demonstrated in RTOG 92-08.68 Late toxicity, including femoral head and neck fractures73 is also associated with conventional radiation approaches for anal cancer. In the interest of quality of survival, IMRT may have the potential to reduce the incidence of these late effects.
IMRT also may enable dose escalation to high-risk tumors while sparing normal tissues. Based on the recent studies of patterns of failure after chemoradiation for anal cancer, the primary site remains the predominant site of failure, suggesting a potential benefit to dose escalation for advanced anal cancers.74,75 IMRT allows for differential prescription doses to distinct target volumes, such as the primary site, positive nodal disease, and the elective lymph node regions, over the same treatment time. With this “dose painting” approach, the dose to the tumor may also be escalated, while keeping the same dose and fractionation for the nodal regions. This technique is known as an “integrated boost,” and will allow for increased doses to gross tumor and an overall decreased treatment time in comparison to a sequential IMRT approach (Fig. 79-5). Clinical series of IMRT-based therapy of anal cancer have reported reductions in acute treatment-related nonhematologic toxicity and similar disease-related outcomes versus historical controls using 2D and 3D planning.78–78
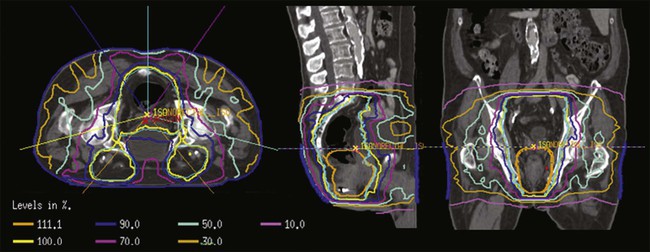
Salama et al. reported on the first multicenter experience of the use of IMRT for patients with anal cancer.79 Fifty-three patients were treated with concurrent chemotherapy and IMRT. The majority of patients in this series were treated with a sequential IMRT approach, whereby the gross tumor and elective nodal volumes received the same daily radiation dose with an initial IMRT plan to 45 Gy, followed by a gross tumor boost to a median dose of 51.5 Gy. Acute grade 3+ gastrointestinal and dermatologic toxicity was reported in 15% and 38% of patients, respectively (compared with 36% and 47% on the 5-FU+mitomycin arm of the RTOG 98-11 trial). The only grade 4 toxicities were hematologic. Treatment breaks occurred in 41.5% of patients. Eighteen-month colostomy-free survival, OS, freedom from local failure, and freedom from distant failure were 83.7%, 93.4%, 83.9%, and 92.9%, respectively.
The results of the only prospective trial of IMRT for anal canal cancer, RTOG 0529, have recently been reported.80 This phase II study combined concurrent 5-FU+mitomycin and “dose-painting” (DP)-IMRT to evaluate the impact of IMRT on acute toxicity among patients receiving definitive chemoradiation. Specifically, the primary end point was to determine if the use of IMRT reduced the combined rate of grade 2 or greater gastrointestinal and genitourinary events by 15% using the data reported in RTOG 98-11 as a benchmark. All patients received 5-FU (1000 mg/m2 per day, 96 hours CI) and mitomycin (10 mg/m2 IV bolus) days 1 and 29. The IMRT doses were based on preliminary experience from Boston University and Massachusetts General Hospital using a DP-IMRT approach.81 Of 63 accrued patients, 52 were analyzable. Stage of cancer was 53% stage II, 25% stage IIIA, and 22% stage IIIB. Rates of grade 3 or greater dermatologic toxicity were superior in the IMRT study (20% vs. 47%, P < 0.0001), as were rates of grade 3 or greater gastrointestinal and genitourinary toxicity (22% vs. 36%, P = 0.014). Median duration of radiotherapy was 42.5 days (range: 32 to 59), compared with 49 days (range: 0 to 102) on the mitomycin arm of RTOG 98-11 (P < 0.0001). A recent update of this study showed that this approach also yielded similar 2-year disease-related outcomes compared with RTOG 98-11.82
Nonsquamous Histologies
Perianal Skin (Anal Margin) Tumors
Tumors that lie distal to the anal verge (i.e., perianal skin cancers) may be managed as skin tumors. Treatment options for squamous cell tumors of the perianal skin include local excision with or without adjuvant radiation, or radiation with or without chemotherapy. The treatment approach must take into account expected morbidity. Chapet et al. reviewed their experience with 26 patients with tumors of the perianal skin (five patients also had involvement of the anal canal).3 Twenty-one tumors were 5 cm or less in diameter. Fourteen patients were treated with definitive radiation (with or without chemotherapy), and 12 patients were treated with radiation after initial local excision. The initial crude local control rate was 61%, increasing to 81% after salvage surgical treatment, with a 5-year cause-specific survival of 88%. Khanfir et al. reported similar results in their series of 45 patients,5 with 29 undergoing local excision before radiation. Five-year locoregional control was 78% and 5-year disease-free survival was 86%.
Balamucki et al. recently updated the University of Florida experience with definitive radiotherapy and chemoradiotherapy for squamous cell tumors of the anal margin.83 Twenty-six patients were treated, two of whom developed local recurrence of disease and two patients had regional lymph node recurrence. Ten-year cause-specific survival was 92%. Of note, two patients with clinically node-negative disease who did not receive prophylactic inguinal nodal irradiation developed groin recurrences.
Anal Canal Adenocarcinoma
Adenocarcinomas of the anal canal are uncommon and in some cases will represent growth of distal rectal adenocarcinomas into the canal. A study of 82 patients from the Rare Cancer Network registry with a diagnosis of anal adenocarcinoma analyzed outcomes based on treatment approach: radiotherapy plus surgery, chemoradiotherapy, and APR.84 Tumor and patient features were evenly distributed across the three groups. Local-regional control at 5 years was highest in the APR-treated patients (80% vs. 64% with chemoradiotherapy and 63% with radiation plus surgery), although the differences were not statistically significant. Five-year survival was improved in the chemoradiotherapy group (58%) as opposed to the radiotherapy plus surgery (29%) and APR (21%) groups. In multivariate analysis, chemoradiotherapy was found to be a positive independent factor for disease-free survival and OS. A review of 165 patients with anal adenocarcinoma from the SEER database yielded differing conclusions.85 Five-year survival for patients treated with APR was 58%, compared with 50% for APR plus radiation and 30% for radiation alone. These differences were statistically significant. Finally, investigators from the MD Anderson Cancer Center analyzed 16 patients with anal adenocarcinoma and compared outcomes with chemoradiotherapy treatment to similarly treated patients with squamous cell tumors.86 Five-year rate of local failure was 54% for the adenocarcinoma group and 18% for the squamous cell group, with corresponding 5-year disease-free survival worse for the adenocarcinoma group (19% vs. 77%), as was OS (64% vs. 85%).
Anal Canal Melanomas
Appropriate local therapy remains a controversial area, although it appears that obtaining a negative surgical margin may be more important than the actual extent of the surgery. Nilsson reviewed 251 presentations from the Swedish National Cancer Registry, and on multivariate analysis the two most important prognostic factors with respect to survival were surgical margin status and tumor stage.87 Patients with an R0 surgery had a 5-year survival rate of 19% as opposed to 6% for those with involved margins. Iddings et al. reviewed data regarding anorectal melanoma from the SEER database from 1973 to 2003.88 One hundred forty-three patients were recorded as having localized disease: 51 underwent APR and 92 underwent local excision. Patients between the two groups had “similar” pathological features, and there were similar outcomes between the two treatments: median survival was 16 months versus 18 months in the APR and local excision groups, respectively, and 5-year survival was 17% versus 19% for APR and local excision, respectively.
Investigators from MD Anderson Cancer Center reported on 23 patients with anorectal melanomas treated to the primary site with local excision, with or without nodal dissection based on clinical presentation.89 Nine patients received some form of systemic therapy. A dose of 30 Gy was delivered in 5 fractions to the primary tumor site (to the level of the bottom of the sacroiliac joints) and inguinal nodes. Four patients received an additional 6 Gy boost dose to the primary site. Local and regional nodal control rates at 5 years were 74% and 84%, respectively. Five-year OS was 31%, and no patients with regional lymph node involvement at presentation were alive at 5 years.
Treatment of Metastatic Disease
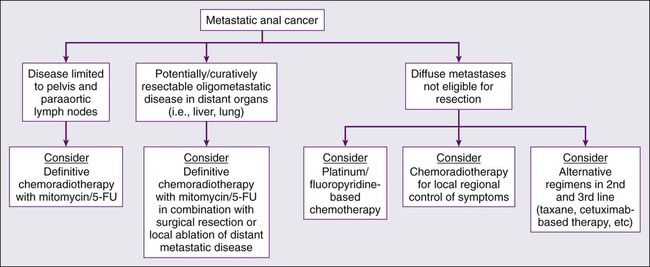
Although squamous cell carcinoma of the anal canal is often cured with combined chemoradiation therapy and patterns of failure primarily consist of locoregional relapse, a minority of patients will develop distant metastases, often in conjunction with locoregional relapse. As an example, in the UKCCCR ACT I trial, extrapelvic metastatic disease was seen in less than 5% of patients at initial presentation and in approximately 15% of patients following a “definitive” radiation-based approach.51 Because of the relative rarity of distant disease development, no universally accepted approach has been established. However, because patients presenting with metastatic disease are often symptomatic from their primary tumor, coupled with the fact that uncontrolled pelvic disease results in significant morbidity and mortality, local regional therapy with radiation in conjunction with chemotherapy should be considered in the context of a multidisciplinary evaluation (Fig. 79-6).
Although less common, and technically considered metastatic disease, a small proportion of patients will present with metastases confined to the retroperitoneal lymph nodes. A report described six patients with anal cancer exhibiting metastases isolated to the paraaortic region. Patients were treated with IMRT to a median dose of 57.5 Gy with concurrent infusional 5-FU and cisplatin. At a median follow up of 25 months, four of six patients were alive and disease free, with 3-year actuarial local control, distant control, and OS rates of 100%, 56%, and 63%, respectively.90 Although this experience is limited, it indicates that a “curative” chemoradiotherapy approach should be considered in patients with metastases isolated to the retroperitoneum, analogous to patients with paraaortic involvement from cervical cancer.
The liver is the most common distant organ involved, with less-common sites including extrapelvic lymph nodes, peritoneal cavity, lungs, as well as bone. Aggressive surgical resection or locally ablative techniques of liver metastases can be considered in selected patients, similar to an approach adopted in patients with oligometastatic colorectal cancer. In one report encompassing eight centers, 52 patients with squamous cell tumors (27 with anal cancer primary tumors) with limited metastatic liver involvement underwent resection, resection plus radiofrequency ablation, or radiofrequency ablation alone.91 Median disease-free survival for the patients with metastatic anal cancer was 9.6 months with an actuarial 5-year OS of 23%. Patients presenting with synchronous metastases, patients with hepatic lesions larger than 5 cm, and patients with involved resection margins had worse survival outcomes. These results indicate that, in selected presentations of liver-only metastatic disease, liver-directed treatments have the potential to play an important role in improving survival outcomes.
Given most published series in the treatment of metastatic squamous cell carcinoma are a small case series, the optimal systemic therapy regimen remains poorly defined. Platinum-based chemotherapy is commonly considered for palliation, although the optimal duration of therapy is unknown. Patients with no contraindications to systemic chemotherapy should generally be treated aggressively with consideration of multidisciplinary management. One of the more established chemotherapy regimens employed in metastatic disease is 5-FU plus cisplatin, which has been demonstrated to prolong OS, with a reported response rate of 66% and complete response rate of 16%.92 A preliminary report from the MD Anderson Cancer Center described 40 evaluable patients with metastatic anal squamous cell carcinoma mostly receiving platinum-based therapy (cisplatin, carboplatin, or oxaliplatin), with five undergoing metastasectomy. Median OS was 38 months, although median time to progression was only 19 weeks. The authors recommended platinum-based chemotherapy as the preferred regimen with consideration of multiple lines of chemotherapy, as well as consideration of chemoradiotherapy for locoregional control and surgery with curative intent in appropriate patients.93 Other chemotherapy agents, including irinotecan, mitomycin, carboplatin, and doxorubicin, also have been employed in this setting.94–97 Additionally, small case series have described clinical activity with single-agent paclitaxel, a combination regimen of carboplatin+paclitaxel+5-FU, and single-agent carboplatin. Newer “targeted” agents, including the epidermal growth factor receptor (EGFR) inhibitor cetuximab, are presently being investigated in the treatment of metastatic disease in combination with traditional chemotherapeutics. However, many of these results are based on very small patient numbers and should be interpreted as such.92 Figure 79-6 illustrates a possible treatment algorithm for patients with metastatic anal cancer. In conclusion, the optimal treatment regimen for the treatment of metastatic squamous cell carcinoma of the anal canal is not firmly established.
Controversies/Problems/Challenges/Future Clinical Trials
Chemoradiation with 5-FU+mitomycin and continuous radiation to a dose of 45 to 59.4 Gy remains the standard practice in the United States for the definitive treatment of stages II and III anal canal cancer. Although many patients treated with chemoradiation for anal canal cancer have adequate outcomes and the use of IMRT has helped to reduce treatment-related morbidity, there remains considerable room for improvement. Five-year outcomes of the RTOG 98-11 trial per individual tumor and nodal stage, presented at the 2011 American Society Of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium, demonstrated that disease-free survival is 40% or less for patients with T4N0 and/or node-positive disease.59 Therefore, trials examining more aggressive and innovative regimens in anal canal cancer are warranted in patients at high-risk for treatment failure to improve outcomes.
Anal cancers strongly express the EGFR.98 Investigators from the RTOG have analyzed EGFR in 190 pretreatment tumor biopsies of patients enrolled in RTOG 98-11, and at a median follow-up of 6.1 years, have shown that EGFR expression is associated with worse outcomes for all clinical end points.99 The EGFR inhibitor cetuximab has been reported to increase locoregional control and OS when combined with radiation for squamous cell head and neck cancers.100 To date, EGFR inhibitors have had limited use in anal cancer. A phase I study of cetuximab in combination with 5-FU, cisplatin, and radiotherapy in patients with locally advanced squamous cell anal carcinoma showed no unexpected toxicities.101 Nine patients with locally advanced anal cancer (T3 to T4 and/or N+ disease) received radiation (45 Gy as 1.8 Gy day × 5 weeks plus a 10-Gy boost to a total dose of 55 Gy) with full-dose cetuximab and escalating dosages of cisplatin and 5-FU. All patients completed full-dose radiation within a median of 68 days (range: 50 to 98 days). Only one patient had a dose-limiting toxicity, grade 4 neutropenic fever. Of nine patients evaluable for pathological response, seven achieved complete clinical response (78%). Building on these results, the AIDS Malignancy Consortium (AMC) and the ECOG recently closed accrual to two companion phase II trials (AMC045 and E-3205) of radiotherapy with concurrent 5-FU, cisplatin, and cetuximab for anal cancer. All patients received cetuximab (400 mg/m2 loading, then 250 mg/m2 per week IV × 6 to 8 weeks) plus cisplatin (75 mg/m2 IV every 28 days × 2) and 5-FU (1000 mg/m2 per day IV infusion on days 1 to 4 every 28 days × 2) concurrently with radiation therapy (45 to 54 Gy) beginning with the second dose of cetuximab. Patients in E-3205 also received two cycles of cisplatin+5-FU alone prior to cetuximab and chemoradiation; this was discontinued after enrollment of 28 patients on the recommendation of the NCI Anorectal Task Force after the results of RTOG 98-11 were disseminated. Both trials were powered to detect a reduction in 3-year locoregional failure rate from 35% to 17.5% (alpha = 0.10; beta = 0.10), the primary end point. No excessive toxicity has been observed in either trial according to planned early stopping rules. Preliminary local failure data show 2-year local failure to be 25.7% in 28 patients for the ECOG study and 17.7% in 45 patients for the AMC trial.102 Further data on these two companion studies are warranted and evaluation within a randomized clinical trial will be required to determine whether patients with anal cancer may specifically benefit from an EGFR-targeted therapeutic agent.
As previously described, there is also a close association between HPV and squamous cell cancer of the anal canal. In squamous cell cancer of the head and neck, the RTOG has established a strong relationship between tumor HPV status, as determined by in situ hybridization, and survival following chemoradiation.103 The RTOG has also correlated the expression of p16, an established biomarker for the function of the HPV E7 oncoprotein, with improved outcome after treatment for head and neck cancer,104 and are in the process of analyzing the pretreatment biopsies of the patients treated in RTOG 98-11 to determine if a similar association with outcome is observed with p16 expression in anal cancer. If p16 expression portends outcome, there are several references in the literature to support the idea that HPV-associated cancers may respond to therapeutic agents or vaccinations based on HPV-16 E7 protein expression.106–106 Investigators from Brown University are organizing a phase I multicenter HPV vaccination trial in combination with 5-FU, mitomycin, and IMRT for locally advanced anal cancer.
Although more aggressive treatment may be warranted for large stage II and stage III anal cancers, there is also an opportunity to examine less-intensified therapy for node-negative early stage disease. Hatfield and colleagues examined external beam radiotherapy (30 Gy in 15 fractions within 3 weeks) and concurrent chemotherapy (bolus mitomycin-C 12 mg/m2) on day 1 to a maximum of 20 mg followed by an infusion of 5-FU, 1000 mg/m2 per 24 hours on days 1 to 4).64 Of the 21 patients, 18 underwent small-volume, involved-field radiotherapy. All were considered to have stage N0 disease radiologically. After a median follow-up of 42 months, only 1 patient (4.7%) had experienced local recurrence and has remained disease free after local excision. Further multiinstitutional efforts exploring reduced volume and dose therapy are warranted.
Another challenge that remains is effective therapy for metastatic disease. As discussed previously, there is currently no standard chemotherapy treatment for metastatic anal cancer, and there is a paucity of published data. The choice of therapy is usually influenced by the patient’s previous treatment for early disease, the disease-free interval, and performance status. Current National Comprehensive Cancer Network (NCCN) guidelines recommend the use of cisplatin and 5-FU as first-line treatment.107 As described above, there is a strong biological rationale for targeting the EGFR pathway in this tumor type. As such, the International Rare Cancer Research Consortium is developing an international randomized phase II effort incorporating an EGFR inhibitor in the metastatic setting.