Cancer in the Elderly
Biology, Prevention, and Treatment
• Cancer in older persons is increasingly common.
• Data about the prevention and management of cancer in older persons are limited.
Epidemiology of Aging and Cancer
• The older population continues to expand as a result of reduced mortality and birth rates. Currently 60% of all malignancies occur in persons aged 65 years and older, and this proportion is expected to rise to 70% by the year 2030. Although cancer-related mortality is declining among younger persons, it is increasing among the oldest persons.
• It is of special interest that cancer appears to affect mainly older persons who are otherwise healthy and would have lived longer were it not for the cancer.
• Carcinogenesis is a time-consuming process, the end-product of which—cancer—is more likely to develop at an advanced age.
• Aging is associated with molecular changes that mimic carcinogenesis; older cells are primed to the effects of environmental carcinogens.
• Aging is associated with environmental phenomena such as immune senescence or proliferative senescence that favor the development of cancer.
• The biology of the tumor cells (e.g., the prevalence of multidrug resistance protein 1 in acute myeloid leukemia increases after age 60 years, causing a worse prognosis).
• The aging of the patient: an age-related increase in circulating concentrations of interleukin-6 (IL-6) may favor the growth of lymphomas, whereas hormonal senescence may inhibit the growth of breast cancer.
Assessment of the Older Person
• Aging involves a progressive shortening of life expectancy and reduction in the functional reserve of multiple organ systems.
• Personal and social resources to cope with stress may become more limited.
• Reduced life expectancy and reduced stress tolerance lessen the benefits and enhance the risks of medical intervention.
• A comprehensive geriatric assessment (CGA) to evaluate the patient’s function, comorbidity, cognition, nutrition, medications, and living resources is a currently available, reliable instrument for predicting life expectancy and the risk of treatment-related complications.
• The CGA may unveil preexisting situations such as undiagnosed disease, poor nutrition, depression, or lack of adequate social support that are remediable and may influence the outcome of treatment.
• A number of laboratory tests, including the circulating levels of IL-6 and d-dimer, along with tests of physical performance, may complement the CGA.
• Older persons may be primary candidates for chemoprevention of cancer, but none of the current chemopreventive agents has demonstrated efficacy definitively.
• Screening asymptomatic patients for cancer of the breast, large bowel, and lung appears reasonable when the life expectancy is 5 years or longer.
• Surgery: Age by itself, until 100 years, does not appear to increase the risk of surgical mortality, although the risk of surgical complications and length of postoperative hospitalization increase with age. Age is a definitive risk factor for mortality related to emergency surgery.
• Radiation therapy: Tolerance for radiation therapy seems to remain high, even for persons aged 80 years and older.
• Cytotoxic chemotherapy: The main pharmacologic changes of age include decreased excretion of drugs and of their active metabolites from the kidneys; decreased volume of distribution of water-soluble drugs, which may in part be accounted for by anemia; increased susceptibility to myelodepression, mucositis, and peripheral and central neuropathy; and cardiomyopathy. The National Cancer Center Network has issued the following guidelines for the management of older patients with cancer:
• Some form of geriatric assessment for individuals 65 and older
• Dose adjustment according to individual glomerular filtration rate for patients aged 65 years and older
• Prophylactic use of filgrastim or pegfilgrastim for patients aged 65 years and older who are treated with combination chemotherapy of dose intensity comparable to that of cyclophosphamide, doxorubicin, vincristine, and prednisone
Introduction
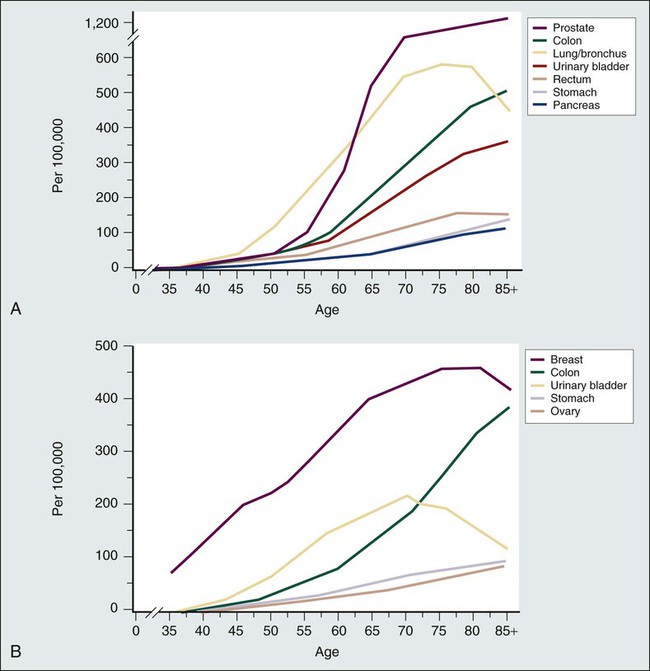
The progressive aging of the population is an epidemiological hallmark of our times. People aged 65 years and older represented 12% of the population in 1990; the percentage in this age group is expected to grow to 20% by 2030.1 The segment of the older population undergoing the most rapid growth is that of persons older than 85 years, the so-called “oldest old.” Aging is the most important risk factor for cancer. The incidence of common malignancies increases with age; currently, more than 50% of all neoplasms occur in persons aged 65 years and older2 (Fig. 63-1), and this proportion is expected to increase to 70% by 2030. Currently cancer is the main cause of death for persons age 85 years or younger.2 Clearly, improved cancer control involves effective management of cancer in older persons.
Biology of Aging
Molecular and Cellular Biology
Reactive oxygen species (ROS) appear to play a role in the genomic changes that occur in aging cells, whereas the deposition of advanced glycosylation end products is responsible for stromal damage.5–5 Advanced glycosylation end products contribute to the production of ROS, and the effects of ROS on cell proliferation and cell death appear to be mediated, in part, by the mammalian target of rapamycin (mTOR).4 Of special interest, recent studies have shown that inhibition of mTOR may prolong the life span and delay the development of cancer in rodents.6,7
One current hypothesis holds that that the basic interactions of cancer and aging occur at the level of so-called “caretaker” and “gatekeeper” genes. The caretaker genes are responsible for repairing the DNA damage, and the gatekeeper genes are responsible for preventing the proliferation and eventually inducing the apoptosis of the aging cells.8 When the function of these genes is preserved, the organism eliminates damaged cells and progressive organ atrophy ensues because of reduced renewal. When these genes are mutated and unable to function, the damaged cells are enabled to enter proliferative cycles and may undergo carcinogenesis through a number of subsequent mutations.
Cellular aging “in vitro” (i.e., cells aging in the plate or test tube) is associated with a number of molecular events, including some that may favor and others that may inhibit the development of cancer. Epigenetic changes that activate oncogenes and inhibit the expression of antiproliferative genes accumulate with aging. These changes include DNA hypermethylation, histone deacetylation, and the activation of microRNAs.9 In addition, genomic changes, such as the formation of DNA adducts and point mutations,10 are also common. These changes mimic the early stages of carcinogenesis and render the aging cell more susceptible to the action of “late-stage carcinogens,” which explains, in part, the association between cancer and age.
Other molecular changes associated with aging, including a progressive reduction in telomere length and telomerase activity11 and activation of the p14 antiproliferative gene, encoding the CDK16 inhibitor, are contrary to those observed in neoplastic cells.12 Paradoxically, proliferative senescence may enhance the risk of cancer; together with the ability to replicate themselves, senescent fibroblasts lose the ability to undergo apoptosis and do acquire immortality.13 Proliferative senescence also is associated with the production of tumor growth factors and of proteolytic enzymes that may favor metastatic spread.13 Recently a number of genetic changes have been recognized in fibroblasts and lymphocytes undergoing proliferative senescence, and the “senescence-associated secretory genotype” has been defined. Some of the genes encode inflammatory cytokines. Whether these genes have any role in the generation of age-related cancer has not yet been established.12,14
Physiology of Aging
Aging involves a progressive reduction in the functional reserve of multiple organ systems, with reduced tolerance of stress, including cancer and cancer treatment. Several factors underlie compromised functional reserve, including organ atrophy, damaged and dysfunctional tissues, chronic inflammation, and chronic diseases.15 The loss of functional reserve may be expressed as allostasis (or loss of homeostasis), which is a decreased ability of the organism to return to a baseline condition after exposure to stress.16 Allostatic elements of special interest include progressive inflammation characterized by increased concentration of inflammatory markers in the circulation17 and an increased concentration of circulating insulin caused by insulin resistance,18 which may be responsible for increased tumor growth in older persons. Catabolic cytokines appear pivotal in physiological aging; their accumulation is associated with increased prevalence of geriatric syndromes,1 increased risk of mortality and functional decline,19 and a generalized catabolic status.20 From the standpoint of cancer treatment, the most significant changes include gastrointestinal, renal, hepatic, hematopoietic, and mucosal alterations that may affect the pharmacokinetics of antineoplastic agents and may increase the risk of complications from cancer treatment.
Biological Interactions of Cancer and Aging
Aging may affect tumor biology at two levels: carcinogenesis and tumor growth.
Aging and Carcinogenesis
The association of aging with carcinogenesis may be explained by at least three mechanisms: duration of carcinogenesis, increased susceptibility of aging tissues to environmental carcinogens,9,10 and changes in body environment, including proliferative senescence,13 endocrine senescence,7 and immune senescence.21 Both experimental and epidemiologic findings support the theory that aging tissues are more susceptible to environmental carcinogens. Several murine tissues, including cutaneous, hepatic, lymphatic, and nervous tissues, have been found to be more likely to develop cancer after exposure to carcinogens if they are obtained from older animals.22 In humans, the incidence of some cancers, such as nonmelanomatous skin cancer and prostate cancer, increases geometrically with age, suggesting enhanced carcinogenesis.2 In addition, the incidence of some neoplasms, including non-Hodgkin lymphomas,23 anaplastic astrocytomas, and glioblastoma multiforme,24 has increased several fold among older individuals during the past 30 years, suggesting that older persons may be more susceptible than younger persons to new environmental carcinogens.17 In a longitudinal study of the aging population in Bruneck, Italy, Willeit et al.25 found that the incidence of cancer was inversely related to the length of leukocytic telomeres. It was threefold higher in those with the shortest telomeres and twofold higher in persons with intermediate-length telomeres. The telomere length was used as an assessment of a person’s physiological age. Age also was a risk factor for chemotherapy-induced acute leukemia and myelodysplasia in women treated with anthracycline-based adjuvant chemotherapy for breast cancer.26
Aging and Tumor Growth
Aging may influence tumor growth at two levels—the neoplastic cell itself and the host environment in which the tumor grows. It is reasonable to expect a concentration of more indolent tumors among older persons (Fig. 63-2) by a process of natural selection, which is certainly the case with breast cancer, as the prevalence of well-differentiated, hormone receptor–rich tumors increases with age.27 The influence of the tumor host on cancer growth was demonstrated in a now-classic experiment by Ershler,28 who demonstrated that the same load of Lewis lung carcinoma and B16 melanoma were associated with shorter survival and a higher incidence of metastasis in younger animals. In successive studies, Ershler demonstrated that the tumor growth rate seemed to decrease for poorly immunogenic tumors and increase for highly immunogenic tumors as the host age increased, highlighting the influence of immune senescence on tumor growth. In humans, Kurtz and coworkers29 demonstrated a reduction in the growth rate of primary breast cancer among older women that was related directly to the degree of mononuclear cell reactions. This observation suggested that immune senescence may mitigate tumor growth in poorly immunogenic tumors.
The clinical behavior of several human malignancies may change with the age of the patient.30 Table 63-127,30–32 demonstrates that the biology of both the tumor cell and the tumor host may influence prognosis. Another important observation is that in some cases, prognosis becomes worse with age, contrary to the generally held view. In any case, age by itself should not be considered a prognostic factor. If it is true that approximately 80% of breast cancers in women aged 70 years and older are rich in hormone receptors, the converse is also true: 20% of cancers of women in this age group are not rich in hormone receptors and may require cytotoxic chemotherapy.
Table 63-1
Age and Changes in Cancer Prognosis
| Neoplasm | Age-related Changes in Prognosis | Mechanism(s) |
| Acute myelogenous leukemia32 | Worse with age: increased resistance to chemotherapy; increased mortality during induction | Neoplastic cell; increased prevalence of cells expressing multi-drug resistance protein 1; increased prevalence of stem cell leukemia |
| Non-Hodgkin lymphoma30,31 | Worse with age: decreased duration of complete remission | Tumor host; increased circulating concentration of interleukin-6 |
| Breast cancer27 | More indolent disease | Neoplastic cell; increased prevalence of hormone receptor-rich, well-differentiated tumors; tumor host; decreased production of sex hormones; immune senescence |
| Celomic ovarian cancer30 | Worse with age: decreased remission duration, decreased survival | Unknown |
| Non–small cell lung cancer30 | Better prognosis with age; presentation at an earlier stage | Unknown |
Clinical Evaluation of the Older Patient
• Is the patient going to die with cancer or of cancer?
• Is the patient going to experience the consequence of cancer during his or her lifetime?
• Is the patient able to tolerate cancer treatment?
• What are the long-term consequences of cancer and cancer treatment in older aged persons?
In addition, the aging of the population and the increased incidence of cancer in this population has a number of social consequences that need to be studied and afforded. In particular, these consequences include the health of the home caregiver and the economic implications of caring for an aging patient. The issue of long-term consequences has become highly relevant as the percentage of older cancer survivors has increased. Several studies summarized in reference 25 reported age as a risk factor for chemotherapy-induced acute leukemia. Age is also a risk factor for anthracycline-induced chronic cardiac dysfunction33 and for cognitive decline after chemotherapy.34
The life expectancy and functional reserve of the older person may be estimated with a comprehensive geriatric assessment (CGA) (Table 63-2) involving the patient’s level of functioning and his or her co-existing medical conditions (e.g., comorbidity, geriatric syndromes, polypharmacy, and malnutrition) and the social resources available to ensure compliance with and safety of cancer treatment.35 In addition, the CGA may unearth unsuspected conditions that interfere with the treatment of cancer, including malnutrition, polypharmacy, cognitive and emotional disturbances, and inadequate social support.35,36
Table 63-2
Comprehensive Geriatric Assessment and Clinical Implications
| Assessment | Clinical Implications |
| FUNCTIONAL STATUS | |
| Activities of daily living and instrumental activities | Relation to life expectancy, functional dependence, and tolerance of stress |
| COMORBIDITY | |
| No. of comorbid conditions and comorbidity indices | Relation to life expectancy and tolerance of stress |
| MENTAL STATUS | |
| Folstein Mini-Mental Status Examination | Relation to life expectancy and functional dependence |
| EMOTIONAL CONDITION | |
| Geriatric Depression Scale | Relation to life expectancy may indicate motivation to receive treatment |
| NUTRITIONAL STATUS | |
| Mini Nutritional Assessment | Reversible condition; possible relationship to survival |
| Polypharmacy | Risk of drug interactions |
| GERIATRIC SYNDROMES | |
| Delirium, dementia, depression, falls, incontinence, spontaneous bone fractures, neglect and abuse, failure to thrive, vertigo | Relation to survival and functional dependence |
General Principles of Geriatric Assessment
In general geriatrics, the CGA has succeeded in reducing the rate of functional dependence and of admission to the hospital and to adult living facilities.35,37 In geriatric oncology, the CGA may unearth conditions that may compromise cancer treatment.37–37 Three studies exploring the effects of CGA in older patients with cancer demonstrated some degree of functional dependence in approximately 70% of those patients, some degree of comorbidity in more than 70%, and dementia, malnutrition, and depression in approximately 20%.40–40 The value of geriatric assessment in directing antineoplastic chemotherapy was demonstrated in a recent study.41 Older patients with large cell lymphoma were treated according to the results of the geriatric assessment or according to the clinical impression of the investigator. The CGA proved more precise in identifying patients at risk for toxicity.
The CGA also helps to make an estimate of the patient’s cancer-independent mortality42 risk and expected tolerance of treatment. Several methods based on function, co-morbidity, activity, and body habits have been developed to assess the risk of mortality over 3 to 9 years for persons 65 years and older.43 One can assess these models free of charge on the Internet site “eprognosis.com.”
More recently, two instruments have been developed to estimate the risk of chemotherapy-related toxicity based on the geriatric assessment. Both the Chemotherapy Risk Assessment Score in High Age Patients (CRASH) and the Cancer and Aging Research Group (CARG) study predict the risk of hematologic and nonhematologic toxicity based on the chemotherapy regimen and a patient’s clinical characteristics (Tables 63-3, 63-4, 63-5, and 63-6).43,44 The CRASH instrument is available online free of charge. Thus the CGA provides a common language for use in the classification of older persons undergoing cancer treatment and for those enrolled in clinical trials.
Table 63-3
Prediction of the Risk of Chemotherapy: CRASH Model
| Item | 0 Point | 1 Point | 2 Point |
| HEMATOLOGIC MODEL | |||
| Diastolic blood pressure | ≥72 | >72 | |
| IADL | 26-29 | 10-25 | |
| LDH | Normal | Above normal | |
| MAX2 | 0.0-0.44 | 0.45-0.57 | >0.57 |
| NONHEMATOLOGIC MODEL | |||
| ECOG PS | 0 | 1-2 | 3-4 |
| Mini Mental Status | 30 | 1 | <30 |
| Mini-nutritional assessment | >27.5 | <27.5 | |
| MAX2 index | 0.0-0.44 | 0.45-0.57 | >0.57 |
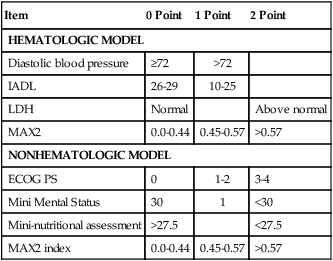
Data from Extermann M, Boler I, Reich R, et al. Predicting the risk of chemotherapy toxicity in older patients. The Chemotherapy Risk Assessment Scale for High Age Patient (CRASH). Cancer 2012;118(13):3377–86.
Table 63-4
Score and Risk of Grade 3-4 Hematologic Toxicity in the CRASH Model
| Score | Incidence of Toxicity (%) |
| HEMATOLOGIC MODEL | |
| 0-1 | 4 |
| 2-3 | 19 |
| 4-5 | 44 |
| >5 | 89 |
| NONHEMATOLOGIC MODEL | |
| 0-1 | 28 |
| 2-3 | 42 |
| 4-5 | 59 |
| >5 | 92 |
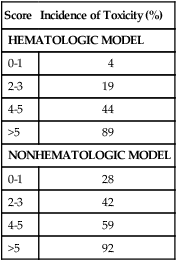
From Extermann M, Boler I, Reich R, et al. Predicting the risk of chemotherapy toxicity in older patients. The Chemotherapy Risk Assessment Scale for High Age Patient (CRASH). Cancer 2012;118(13):3377–86.
Table 63-5
Prediction of the Risk of Chemotherapy: CART Model
| Item | Score |
| Age ≥73 | 2 |
| Gastrointestinal/genitourinary cancer | 3 |
| Standard chemotherapy dose | 3 |
| Polychemotherapy | 2 |
| Hemoglobin (man <11 g/dL; woman <10 g/dL) | 3 |
| Creatinine clearance (Jelliffe) <34 | 3 |
| One or more falls in last 6 months | 3 |
| Hearing impairment (fair or worse) | 2 |
| Limited in walking 1 block | 2 |
| Assistance with medication intake | 1 |
| Decreased social activity | 1 |
Data from Hurria A, Togawa K, Mohile SG, et al. Predictive chemotherapy toxicity in older adult with cancer. A prospective multicenter study. J Clin Oncol 2011;29:3457–65.
Table 63-6
CART Model Score and Risk of Grade 3-4 Toxicity
| Score | Incidence of Toxicity (%) |
| 0-5 | 27 |
| 6-11 | 53 |
| 12+ | 83 |
Data from Hurria A, Togawa K, Mohile SG, et al. Predictive chemotherapy toxicity in older adult with cancer. A prospective multicenter study. J Clin Oncol 2011;29:3457–65.
A recurrent term commonly associated with aging that requires clarification is “frailty.”45 Frailty is defined as a syndrome with multiple causes and involving multiple organ systems and is characterized by increased vulnerability to stress; it is associated with sarcopenia, increased concentrations of inflammatory cytokines in the circulation, and decreased motility and ability to perform complex movements.45 A consensus conference recognized the existence of multiple signs of frailty and agreed to adopt a definition of the frail phenotype that emerged from the Cardiovascular Health Study (CHS).46 Based on five parameters, including weight loss of at least 10 lb over 6 months, reduced energy levels, difficulty in initiating movements, reduced gait speed, and reduced strength of the hands, the CHS recognized three phenotypes of different life expectancy and risk of functional dependence: fit (no abnormalities); prefrail (one or two abnormalities); and frail (three or more abnormalities). It should be emphasized that the definition of frailty will evolve with better insight into the biology of this syndrome. The CHS classification may also be helpful for the classification of patients enrolled in clinical trials. Another common term in geriatrics is “vulnerability,”47 which also refers to a population of older persons at increased risk for adverse outcome such as death, functional dependence, institutionalization, and hospital admission. Although the term is widely used and vulnerable elderly are the subjects of a number of interventional studies,48 no consensus clinical definition has been determined. To some extent, “frailty” and “vulnerability” are overlapping terms, but there is no agreement on whether they represent the same construct.
Clinical Aspects of Geriatric Assessment
Function
Function is assessed based on performance status, activities of daily living (ADLs), and instrumental activities of daily living (IADLs). ADLs include transferring, bathing, dressing, eating, toileting, and continence; dependence on a home caregiver for one or more ADL or institutionalization is associated with a 2-year mortality rate of approximately 40%.37–37 IADLs are the activities necessary to maintain an independent life, including use of transportation, shopping, and the ability to take medications, provide one’s own meals, use the telephone, manage one’s own finances, and take care of laundry and housecleaning. Dependence in one or more IADL (with the exceptions of laundry and housecleaning) is associated with a 2-year mortality rate of 16%37–37 and a 50% risk of developing dementia over 2 years.49 Dependence in some IADLs is an independent risk factor for chemotherapy-induced toxicity.44,45
Co-morbidity
Co-morbidity is an independent cause of mortality for older patients with cancer15 and may be associated with reduced tolerance of cancer treatment. The best way to assess co-morbidity is still being investigated. In general, co-morbidity is associated with increased risk of cancer-unrelated mortality in patients with cancer at early stages, but it does not seem to affect the mortality of patients whose cancer is metastatic.15 Several co-morbidity scales have been devised that take into account the number of conditions and the degree of severity of each condition. Of these, the Cumulative Index of Related Symptoms-Geriatrics (CIRS-G) proved in some studies to be the most sensitive.50 An advantage of the CIRS-G is that its final score may be translated into the score of another scale of common use in epidemiological studies, the Charlson scale.
Anemia is of special interest among the co-morbid conditions,51 because its incidence and prevalence increase with age. Anemia is an independent risk factor for death and for myelosuppression from cytotoxic chemotherapy.51,52 Anemia is a main cause of fatigue and functional dependence51 and may be associated with congestive heart failure and dementia.51 An unsolved question is what level of hemoglobin defines anemia. Of special interest is the Women’s Health Study, which showed that hemoglobin levels lower than 13.4 g/dL were an independent risk factor for death among 556 home-dwelling women aged 65 years and older; these women were followed up prospectively for a period of 8 years.53 Similar findings were reported in the cardiovascular health study.54 In patients with cancer, anemia was associated with reduced survival and increased risk of functional dependence.55
Geriatric Syndromes
Geriatric syndromes include a number of conditions typical of, if not specific to, aging, such as dementia, depression, delirium, incontinence, vertigo, falls, spontaneous bone fractures, failure to thrive, and neglect and abuse. Geriatric syndromes are associated with reduced life expectancy.56 To be considered a geriatric syndrome, these conditions must interfere with a person’s daily life. Dementia must be moderate to severe; delirium must occur as a result of medications or organic diseases that do not commonly affect the central nervous system (e.g., urinary or upper respiratory infections); incontinence must be complete and irreversible; and falls must occur at least three times a month or the fear of falling must prevent regular activities such as walking. Depression is of special interest, because it is associated with decreased life expectancy, even when it is subclinical.57 Depression also interferes with treatment compliance and in many cases may be fully reversible by medication. A simple 15-item questionnaire, the Geriatric Depression Scale 15, speedily and reliably detects subclinical depression and is part of the CGA.57
Social Resources
Pivotal among the social resources is the home caregiver. The ideal caregiver should be able to recognize and manage emergencies, support the patient physically and emotionally, mediate conflicts among family members, and act as spokesperson for the family with the health care provider.58 Under the best of circumstances, the caregiver is the practitioner’s best ally in ensuring compliance with treatment and smooth interactions with the patient. For this reason, it behooves the practitioner to participate in the selection, training, and support of the caregiver. In reality, the caregiver of an older person often is an older spouse with health problems of his or her own or a married daughter who needs to balance her caregiving duties with other family and work responsibilities. The situation of the modern caregiver has been referred to as the “Aeneas syndrome” (hearkening to the Trojan hero Aeneas, who escaped the destruction of his city while carrying his old father on his back and holding his son by the hand, as depicted in a fresco in the “Vatican Stanze” that was painted by Raffaello).59
Nutrition
The prevalence of protein/calorie malnutrition increases with age. Isolation, depression, economic restriction, and reduced appreciation of hunger may all contribute to insufficient food intake, and chronic diseases and inflammatory cytokines may impede the synthesis of new proteins. The Mini Nutritional Assessment is a simple nutritional screening test used worldwide that identifies patients who are malnourished and those at risk of becoming malnourished, thereby permitting the prevention and early reversal of malnutrition.60
Polypharmacy
The prevalence of polypharmacy increases with age, and among patients with cancer aged 70 years and older, it has been found to be as high as 41%.61 The problem of polypharmacy exemplifies a common problem of elderly patients in developed countries: the absence of a primary care provider.62 According to a recent study, more than 50% of persons aged 70 years and older in the United States, Canada, and Israel, although attending multiple specialty clinics, lacked a primary care physician.
Other Forms of Geriatric Assessment
Laboratory Markers of Aging
The recognition that the concentration of catabolic cytokines in the circulation, which increases with age, is correlated with the presence of geriatric syndromes prompted a number of studies aimed at establishing whether these molecules may predict functional dependence and decreased survival. In one recent study, Cohen and coworkers63 demonstrated that increased concentrations of either interleukin-6 (IL-6) or d-dimer were associated with a 50% increase in the risk of functional dependence and death. When the concentration of both substances was elevated (in the upper quartile), however, the risk increased more than threefold.
The length of leukocyte telomeres may also provide an estimate of functional aging.64 Shorter telomeres in persons of the same age are associated with increased prevalence of functional dependence and age-related diseases, as well as increased risk of mortality.65 The main problem is the interindividual variability in telomere length. Longitudinal studies of telomere shortening may provide a more accurate determination of individual aging that is not possible with a single determination of telomere length.
Tests of Physical Performance
Difficulty in performing some activities is considered a predictor of functional dependence and disability. Of particular interest, one recent study has shown that the risk of mortality and functional dependence can be predicted by the “get up and go” test.66 In this test, an older person is asked to get up from an armchair and walk 10 feet forward and back. Both the inability to get up without using the chair arms and requiring more than 10 seconds to walk the distance are highly predictive of functional decline.
Issues Related to the Geriatric Assessment
Who should undergo a CGA? In three prospective studies of patients with cancer aged 70 years and older, the CGA unearthed a number of conditions that were reversible and might interfere with cancer treatment.40–40 In addition, a number of studies showed that a CGA changes the treatment approach to older patients with cancer in at least one third of cases.41,67,68 Based on these findings, the National Cancer Center Network recommended some form of CGA for all patients with cancer aged 70 years and older.36
Is a full CGA necessary in all patients? Because the CGA is time-consuming, more cost-effective alternatives have been explored. Their value has not yet been determined. These alternatives include the use of a screening instrument to identify patients at high risk of functional dependence,36 including questionnaires69 and tests of physical functioning.70 The problem with both of these approaches, however, is that they do not provide any direct assessment of co-morbidity, depression, cognition, and social resources. Another interesting approach, proposed by Ingram and coworkers,40 involved sending a package to the patient’s home including questionnaires about function, co-morbidity, depression, and social resources. At present, one may recommend as a screening test the Vulnerable Elder Survey 13, which was validated with a CGA,69 or the Senior Adult Oncology Program 14 assessment, which is available online free of charge.
Cancer Prevention in Older Persons
Some aspects of aging favor and others interfere with cancer prevention. Based on the fact that the incidence of cancer increases with age, older persons might be the population most likely to benefit from cancer prevention. At the same time, reduced life expectancy and decreased treatment tolerance may lessen the benefits of some types of prevention. The study of cancer prevention in older persons is complicated by a lack of general agreement regarding what represents a meaningful end point. Should it be reduction in cancer-related mortality, as is commonly accepted in prevention trials, or should it instead be an improvement in quality of life, given the limited life expectancy of these persons?71 In this section we provide a brief review of the evidence supporting chemoprevention and early detection of cancer among older persons.
Chemoprevention
At least five groups of substances—the selective estrogen receptor modulators (SERMs) and the aromatase inhibitors for breast cancer, the 5 α-reductase inhibitors for prostate cancer, the retinoids for cancer of the upper airways, and the nonsteroidal antiinflammatory drugs for cancer of the large bowel—have demonstrated cancer preventive activity in randomized clinical trials,72 but these substances are used only to a limited degree in current clinical practice for cancer prevention. The SERMs have a number of potential adverse effects, including endometrial cancer (tamoxifen only), deep vein thrombosis, strokes, and vasomotor and genitourinary manifestations of menopause, the incidence of which increases with age. In a decision analysis, Gail and coworkers73 calculated that tamoxifen may be beneficial for women aged 70 years if their risk of developing breast cancer over 5 years is as high as 7% and if they do not present other contraindications to the drug; the threshold of risk at which this agent may be beneficial increases with the age of the patient. It is not clear whether the aromatase inhibitor exemestane that decreased the incidence of breast cancer in postmenopausal women is more effective or better tolerated than the SERMs.32 Although the 5-α-reductase inhibitors reduce the incidence of prostate cancer in persons undergoing regular screening by 23%,32,74 they do not appear to reduce mortality or improve survival. In addition, these compounds cause a number of unpleasant adverse effects including hot flashes, gynecomastia, and loss of libido. Older persons may represent ideal candidates for future studies of chemoprevention by virtue of their increased risk of cancer, but none of the current options for chemoprevention appears optimal.
Screening and Early Detection
Because the prevalence of common cancers increases with age, one might expect that the positive predictive value of screening tests also would increase.71 However, older persons have in general undergone screening for common cancers earlier in life. Thus previous examinations may eliminate all prevalence cases and minimize the diagnostic yield of subsequent examinations.
Breast Cancer
Most of the randomized controlled studies have established that serial mammograms reduce by 20% to 30% the cancer-related mortality among women aged 50 to 70 years.75 The benefits of mammography after age 70 years have been suggested by three reports. A historically controlled cohort study, the Nijmegen study, showed a reduction in cancer-related mortality until age 75 years;76 a retrospective study of the Survey Epidemiology and End Results (SEER) data showed a more than twofold decline in breast cancer–related mortality for women aged 70 to 79 years who had undergone at least two mammograms after age 70 years;77 and another retrospective analysis of the same data showed that women older than 70 years who had not undergone screening mammography were seen with breast cancer at a more advanced stage than did women who had been screened.78 An important question is the role of clinical examination of the breast (CBE). A Canadian study suggested that CBE may be as effective as screening mammography in women aged 50 to 60 years,79 and the Breast Cancer Detection Demonstration Project showed that mammography was superior to CBE only in the diagnosis of ductal carcinoma in situ.80 CBE appears particularly attractive for older women who undergo multiple clinic visits in the course of the year, because it may be performed with no additional cost and inconvenience. In 2012 the International Society of Geriatric Oncology and the European Society of Breast Cancer Specialists issued a series of guidelines including screening for the management of breast cancer in older women.81 They recommend that women with a life expectancy of 5 years or longer undergo some forms of cancer screening, including biennial mammograms.
Colorectal Cancer
Early detection of cancer of the large bowel reduces the cancer-related mortality for persons aged 50 to 80 years, but controversy lingers concerning the most appropriate screening strategy.82 The issues related to the screening of older individuals for colorectal cancer were recently reviewed.83 The United States Preventive Service Task Force (USPSTF) recommends that screening for colorectal cancer be stopped at age 75 years for persons who had previously undergone regular screening. Persons aged 75 to 85 years who have not received previous screening should be offered screening after potential risks and benefits are discussed. These recommendations should be tempered by the consideration that healthy persons in their 90s still are at increased risk of dying of colorectal cancer.83 Also, the rate of malignant transformation of adenomatous polyps increases with age,84 suggesting that colon cancer may develop in older persons more rapidly than in younger ones; thus, older persons may benefit from continuous screening. According to a recent nationwide study, colonoscopic polypectomy resulted in a decline of more than 50% in the risk of dying of colorectal cancer for patients of all ages.85 In the absence of more data, it appears reasonable to perform some form of screening in older persons with a life expectancy of at least 5 years based on function and comorbidity, irrespective of chronologic age.
Prostate Cancer
The value of screening asymptomatic men for prostate cancer with serial PSA determinations, along with the most cost-effective screening strategies, are controversial topics. The USPSTF concluded that evidence is not sufficient for screening persons of any age and that screening is contraindicated for men 75 years and older.86 These recommendations are supported by current evidence but may be mitigated by the following considerations: new forms of prostate cancer treatment, including intensity-modulated radiation therapy and proton therapy, may have fewer complications than prostatectomy or external beam irradiation and may be well tolerated even by older persons; prostate cancer diagnosed in patients of advanced age tends to be highly malignant,87 suggesting that prostate cancer may become more aggressive in persons with a prolonged life expectancy; and the life expectancy of men is progressively increasing and consequently more persons may experience the problem of untreated prostate cancer during their lifetimes.
Non–Small Cell Lung Cancer
In persons at risk (i.e., current and former smokers) aged 55 to 75 years, annual computed tomography screening has produced a 20% reduction in lung cancer–related mortality and a 6.7% reduction in overall mortality88 during a 5-year follow-up period. Although data for subjects older than 75 years are lacking, it is reasonable to recommend this form of screening for all elderly former smokers with a life expectancy of 5 years or longer, because the risk of lung cancer increases in elderly former smokers.89
Cancer Treatment
Surgery
Although the risk of surgical mortality and other surgical complications increases with the age of the patient, elective surgery appears safe in general, even in patients older than 80 years.90,91 Persons who are 70 years and older are substantially more vulnerable to the complications of emergency surgery, especially surgery related to the digestive tract. Regular screening of these persons for colorectal cancer may minimize the incidence of emergency surgery. A recent case-matched study indicated that the perioperative mortality and long-term survival were the same for persons younger and older than 70 years in a single center experience.92 A predictive model based on geriatric assessment and more traditional preoperative parameters has been validated for estimating the operative risk of persons 70 years and older, the “Preoperative Assessment of Cancer in the Elderly” (PACE).93 A number of recent advances in surgery and anesthesia have rendered cancer surgery safer for older persons. These advances include more limited surgical excisions (e.g., transanal resection of rectal cancer) and the use of anesthetic agents with a shorter half-life and minimal respiratory suppression.
Radiation Therapy
A number of studies94,95 have shown that most forms of radiation therapy are well tolerated in older persons. Combined chemotherapy and radiation therapy in the management of cancer of the head and neck, the esophagus, the bladder, and the lung also appear well tolerated until age 80 years. Among the newer radiation techniques, brachytherapy and high-dose intraoperative radiation are of special interest for treatment of older patients; brachytherapy minimizes the risk of complications to normal tissues, and both techniques eliminate the inconvenience of serial visits. In addition, intensity-modulated radiation therapy, image-guided radiation therapy, and proton therapy may minimize the risk of complications even in the oldest persons. Data on hyperfractionated radiation in older persons are needed.
Among complications of radiation therapy, mucositis is of special concern to older persons, because age is associated with a more limited reserve of mucosal stem cells and increased proliferation of epithelial superficial cells,96 two conditions that predispose to more prolonged and severe mucositis in older persons. In the case of cancer of the upper airways or upper digestive tract, where the risk of mucositis is highest, nutritional management is essential and may involve prophylactic positioning of a percutaneous endoscopic gastrostomy tube.95
Cytotoxic Chemotherapy
Age is associated with changes in the pharmacokinetics and pharmacodynamics of cytotoxic drugs and increased susceptibility of certain organ systems to therapeutic complications.96 The pharmacokinetic changes of major interest involve absorption, renal excretion, and volume of distribution of drugs.
The intestinal absorption of nutrients decreases with age because of a reduction in the surface area for absorption, in the splanchnic circulation, and in gastric motility and secretions;94 however, the bioavailability of oral agents (e.g., capecitabine) does not appear to be compromised with increasing age. Oral drugs are particularly appropriate for older persons because of the convenience of administration and adjustability of doses.
A progressive reduction in glomerular filtration rate is an almost universal consequence of aging, and it may lead to a more prolonged half-life of medications that are excreted by the kidney (e.g., methotrexate, bleomycin, and carboplatin) and of active and toxic metabolites of drugs excreted through other routes.94 These metabolites include daunorubicinol and idarubicinol, which are responsible for approximately 80% of the activity of the parent compounds, and arauridine, which is responsible for the cerebellar toxicity of high-dose cytarabine. Dose adjustment of these agents to the individual’s glomerular filtration rate may improve tolerability.
The volume of distribution of a drug is determined by the body composition and the concentration of serum albumin and of hemoglobin.96 Hemoglobin is important because some antineoplastic agents are bound to red blood cells. In the presence of anemia, the free concentration in plasma and toxicity of these substances increase.51 Correction of anemia may ameliorate the toxicity of chemotherapy. Among the other relevant changes associated with age that can alter anticancer therapy, the most significant are the increased prevalence of multidrug resistance, abnormal intracellular metabolism of drugs, and abnormal repair of DNA damage. Multidrug resistance may be caused by increased expression of the MDR1 gene, as is the case in acute myelogenous leukemia,97 increased prevalence of anoxic tumor cells, and resistance to apoptosis.96 Abnormal drug metabolism and alterations in DNA repair may enhance the toxicity of these agents.96
Myelosuppression and mucositis are particularly common and severe complications of cytotoxic chemotherapy. The risk of neutropenia and neutropenic infections with moderately toxic chemotherapy (such as cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP]) increases with age and appears particularly marked after age 70 years; fortunately, the use of hematopoietic growth factors can prevent this complication in 50% to 75% of persons older than age 70 years.96 Neutropenic infection may be lethal and may occur after the first course of treatment, a fact that prompted the recommendation to use growth factors prophylactically in patients aged 65 years and older.36,98 The risk of mucositis also increases with age, and this complication may be lethal if not treated promptly.99 Keratinocyte growth factor appears promising for the prevention of mucositis.99 Other interventions to ameliorate this complication include substitution of capecitabine for intravenous fluorinated pyrimidines, which is associated with a lower risk of mucositis, and hydration for older persons in whom diarrhea or severe dysphagia develops.
Targeted Therapies
Molecularly targeted therapy has reduced the risk of therapeutic complications in older patients with cancer.100 Some words of cautions are appropriate with respect to the use of these agents in elderly persons:101
• The benefit of some forms of targeted treatment, such as bevacizumab in non–small cell lung cancer, has not been proven in persons older than 70 years.101,102
• A number of complications of these agents may be particularly burdensome to older persons, including the risk of hypertension with bevacizumab; the risk of skin reaction with cetuximab, panitumab, and most small molecule inhibitors of receptor tyrosine kinases; and the risk of myelotoxicity related to lenalidomide and radioimmunotherapy.
Hormonal Treatment
Although hormonal treatment of cancer is considered safer than cytotoxic chemotherapy, the ongoing tendency to institute chemical castration for prostate-specific antigen relapses in men with prostate cancer may have unwanted effects in older persons, including osteoporosis, bone fractures, fatigue, anemia, diabetes, and enhancement of coronary artery disease.103,104
Practical Decisions Related to the Management of Older Persons
Informed decision making is the key to effective and safe treatment of older persons. Any oncologic decision for these patients has two components: the person and the neoplasm. For example, cytotoxic chemotherapy is rarely indicated in a woman aged 90 years or older with stage 1 or 2 breast cancer, given the negligible benefit and the substantial risk of treatment. Randolph and coworkers78 demonstrated that adjuvant chemotherapy is beneficial to an 80-year-old woman when her chances of dying of breast cancer are about 30%, if a 1% reduction in breast cancer–related mortality is desirable; in a 90-year-old woman, however, the risk of dying of breast cancer must be close to 70% to justify the use of chemotherapy. Chemotherapy would appear to be indicated if the same patient has a chemotherapy-responsive disease that may shorten her survival, such as large cell non-Hodgkin lymphoma. Two points that deserve emphasis are (1) the rapidly evolving classification of the physiological status of older persons; and (2) in some circumstances, chemotherapy may represent the best palliation of frail persons. Several agents with minimal toxicity, including capecitabine at low doses, weekly taxanes, gemcitabine, and vinorelbine, may be used safely in these patients.37
National and International Initiatives Related to Cancer and Age
Practice Guidelines
In 1999 the National Cancer Center Network established a panel for the issuance of guidelines for the management of cancer in older persons. The most recent set of guidelines (Box 63-1) was published in 2011.35 These guidelines are based on the clinical evidence reviewed in this chapter and are meant as a frame of reference for clinical practice and as a building block to accommodate emerging data. Other associations, including the European Organization for Research and Treatment of Cancer and the Canadian Cancer Institute, are preparing their own guidelines.