Cancer Cachexia
• Loss of appetite and weight loss are common in patients with advanced, incurable cancer and are associated with a poor prognosis.
• Although patients with advanced, incurable cancer who have the “cachexia syndrome” may appear malnourished, nutritional support with either enteral or parenteral nutrition rarely provides a positive impact on clinical outcomes.
• For palliative purposes, corticosteroids and progestational agents improve appetite in patients with incurable cancer who are experiencing a loss of appetite and weight loss.
• A variety of agents that target muscle, including antimyostatin therapy, are currently being tested in clinical trials to begin to determine their role in treating cancer cachexia syndrome.
Introduction
Any list of end-of-life signs and symptoms in patients with cancer includes loss of appetite and loss of weight. This complex of cancer-associated anorexia and weight loss, referred to herein as “cachexia,” is common, arising in more than 80% of patients with cancer who have advanced disease.1 Moreover, it carries ominous ramifications, having a harmful impact on patients’ functional status and leading to increasing debility, and negatively affecting survival itself. Yet despite the high prevalence of cachexia and its detrimental implications, meaningful interventions that improve the appetite, enhance functionality, and improve survival remain lacking in patients with refractory cancer.
Definitions and Epidemiology
This chapter specifically focuses on patients with incurable, treatment-refractory metastatic disease. These distinctions of incurability and refractory disease are important. First, previous studies have shown that in the setting of potentially curable cancer, severe malnutrition can be treated with nutrition support and result in improved outcomes.2 However, such is not the case in patients with incurable cancer. Although this latter group of patients may appear “malnourished,” caloric repletion clearly does not lead to improved outcomes and in fact leads to a variety of complications that arise directly from implementing nutrition support. Indeed, as far back as 1989, the American College of Physicians provided a position paper on parenteral nutrition in patients with cancer who are receiving chemotherapy, in which it was stated, “….the routine use of parenteral nutrition for patients undergoing chemotherapy should be strongly discouraged, and, in deciding to use such therapy in individual patients whose malnutrition is judged to be life threatening, physicians should take into account the possible exposure to increased risk.”3
To my knowledge, this position statement has not been revised, emphasizing the ongoing concerns for providing nutrition support to patients with cancer who are at or near the end of life. More recently, Baldwin and colleagues4 conducted a systematic review and metaanalysis that included 13 randomized, controlled studies with 1414 patients with cancer in an effort to assess the role of oral nutritional supplementation. Although enteral nutrition appeared to have some beneficial effect on some aspects of quality of life (e.g., emotional functioning, dyspnea, loss of appetite, and global quality of life), it had no effect on mortality (relative risk = 1.06, 95% confidence interval = 0.92 to 1.22, P = .43; I(2) = 0%; P(heterogeneity) = 0.56). As noted, these studies were fraught with clinical and statistical heterogeneity, and hence these investigators concluded that cachectic patients with cancer or those at risk for cachexia do not appear to gain a survival advantage with oral nutritional support. Once again, the fact that these patients with advanced cancer may appear “malnourished” does not translate into their deriving clear benefit from increased calories.
Second, tumor response is often associated with symptom improvement. Geels and colleagues5 showed that among patients with metastatic breast cancer, a higher proportion (91.7%) with a complete or partial response manifested an improvement in anorexia and that none of the patients with a complete response or partial response described a worsening of their appetite.5 Although these results did not reach statistical significance, they point to a phenomenon to which many oncologists can readily attest: tumor response leads to improvements in baseline symptoms in many patients with cancer. Hence this chapter focuses on a commonly seen group of patients with cancer who are cachectic and who experience symptoms and signs that are refractory to cancer treatment and caloric repletion.
From an epidemiological standpoint, is cachexia truly a concern for patients, their families, and health care providers? A classic article from the Eastern Cooperative Oncology Group demonstrates that it is a concern. Dewys and colleagues6 used prospectively gathered data from 12 cancer cooperative group trials that in total included more than 3000 patients with cancer and showed that patients who lost weight lived shorter lives. A patient-reported weight loss of >5% over the preceding months was a powerful predictor of early demise. In multivariate analyses that included cancer type, tumor burden, and patient performance status, patients with weight loss that exceeded this 5% threshold died sooner than did patients who indicated that they had been able to maintain their weight. This study has been replicated by other investigators and demonstrates the strong negative prognostic effect of cachexia.
This same prognostic effect is seen with patient-reported decline in appetite. Quinten and colleagues7 examined 30 randomized controlled trials from the European Organisation for Research and Treatment of Cancer from 1986 through 2004 including more than 1800 patients with cancer who described varying degrees of appetite loss.7 Median survival was directly tied to better appetite; patients with no appetite loss had a much more favorable median survival compared with patients who lost some degree of their appetite. Moreover, these investigators described a fairly direct relationship between extent of appetite loss and shorter survival. Hence not only is weight loss a strong predictor of poor outcome in patients with metastatic cancer, but the same can be said for appetite.
To date, most research has underscored the fact that cancer itself is the main culprit when it comes to losing weight in the setting of advanced malignancy. Several studies point to the predisposition of patients with cancer to lose lean tissue with some slight preservation of adipose tissue, a clinical scenario that stands in stark contrast to what one observes in classic cases of starvation, in which adipose tissue wastes and lean tissue is relatively preserved. Recently, however, findings of a series of studies suggest that some of the newer cancer agents lead to the direct wasting of lean tissue, despite their role in sometimes improving patient survival. For example, Antoun and colleagues8 recently undertook a secondary analysis of data from a placebo-controlled, double-blind trial in patients with metastatic renal cell carcinoma who were treated with sorafenib, 400 mg twice a day. The findings of these investigators, who used retrospective analyses of computed tomographic images, were surprising. As expected, at study entry, muscle wasting was observed in 72% of patients who presented with a body mass index of less than 25 and in 34% of patients with a body mass index greater than 25. However, although patients in the placebo group presented with stable body weight during 6 months with no major changes in body composition, patients who received sorafenib lost on average 2.1 kg of weight over 6 months and 4.2 kg over 1 year. Moreover, patients treated with sorafenib lost skeletal muscle progressively at 6 months (a decrease of 4.9%; P = .01) and 12 months (a decrease of 8.0%; P = .01), and statistically significant decrements in muscle mass were observed even in comparisons against the placebo arm. An emerging literature suggests that some of the newer targeted cancer agents have unexpected and possibly detrimental effects on the body composition of patients with cancer. Further research in this area is indicated.
Biological Characteristics and Pathophysiology
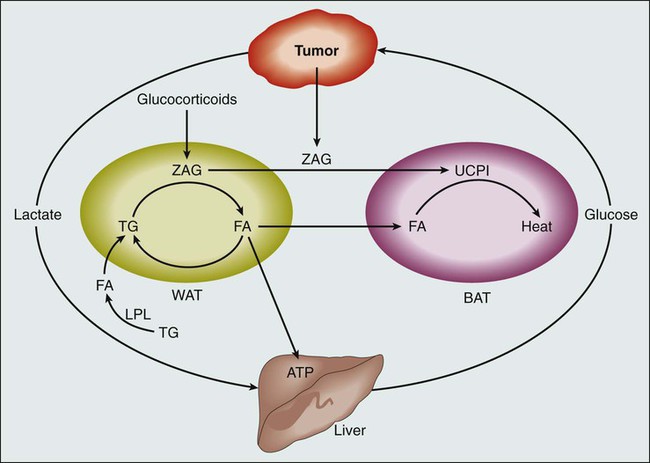
Understanding the biological pathways that lead to cachexia has been difficult (Fig. 41-1). From a pathophysiological standpoint, we know that the clinical picture associated with cachexia represents a disease state as opposed to a potentially readily reversible entity, such as that seen with starvation. Three observations substantiate this statement. First, as alluded to earlier, the changes in body composition in a cancer-laden state are different from those seen during starvation. Cohn and colleagues9 used a variety of body composition assessment techniques, including prompt gamma neutron activation, total body potassium measurement, and total body water assessment by means of tritiated water. Such technology, when applied to patients both with and without cancer, resulted in the following observations: (1) patients with cancer lose both muscle mass and fat mass; (2) patients with cancer appear to retain fat mass to a notable degree; and (3) skeletal muscle is lost appreciably. As noted earlier, the opposite scenario occurs in the setting of classic starvation, in which individuals lose fat and retain lean tissue. Second, for reasons that remain unexplained, some groups of patients with cancer manifest an increase in resting energy expenditure. Results have at times been inconsistent, but several studies indicate that patients with cancer engage in an unintentional, futile utilization of energy stores when they are at rest. In otherwise healthy individuals, in the setting of starvation, these observations do not hold true, and in fact, such individuals manifest a drop in resting energy expenditure, presumably in an effort to retain energy stores. Third, in contrast to simple starvation, cachexia from cancer is associated with a loss of appetite. Patients with cancer who are in the throes of this syndrome generally do not crave food and in fact sometimes have developed an aversion to it. Again, observations such as the three previously outlined draw a sharp distinction between cancer cachexia and simple starvation.
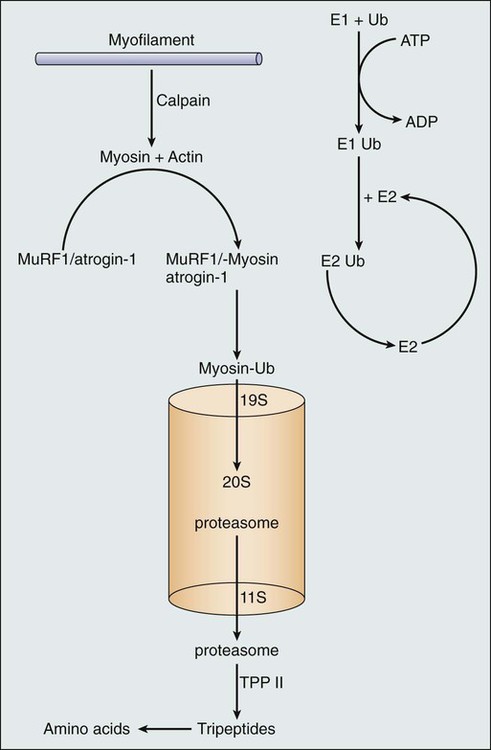
What processes, at a cellular level, explain these observations? Although much work remains to be done to elucidate these processes, a series of laboratory-based studies have suggested that cachexia is driven by inflammatory cytokines, which in turn activate the ubiquitin proteasome system with subsequent muscle breakdown10 (Fig. 41-2). However, small-scale, clinically based interventional studies that have used either cytokine blockade or proteasome inhibition have not demonstrated favorable clinical outcomes.11,12 Other laboratory-based investigators have suggested different mechanisms to explain cancer-associated wasting of lean tissue. For example, a high-profile study described a proteolysis inhibiting factor, but subsequent, more clinically based studies have raised questions regarding the clinical relevance of proteolysis inhibiting factor.13,14 In essence, the pathophysiology of cachexia continues to be studied at a cellular level, but clinical validation of these findings remains elusive.
Patient Evaluation and Staging
A small number of studies attempting to better characterize and stage cancer cachexia have been reported in the literature. For example, Fearon and colleagues15 used a Delphi process for this purpose. This approach yielded the following definition of cachexia: weight loss >5% during the past 6 months; body mass index <20 and any degree of weight loss >2%; or appendicular skeletal muscle index consistent with sarcopenia (males <7.26 kg/m2; females <5.45 kg/m2) and any degree of weight loss >2%. Although some aspects of this definition, such as the >5% weight loss, have been validated,6 other aspects require further endorsement with other clinical data.
Treatment
Nutritional counseling plays an important role in the management of loss of appetite and weight in patients with advanced cancer. In a survey of 199 patients with cancer, Hopkinson and colleagues16 observed that 34% expressed concern about weight loss and 44% expressed concern about decreased caloric intake. However, if a family member helped the patient complete the survey, these numbers changed: concerns about weight loss increased to 59% and concerns about appetite increased to 53%. Moreover, in another study, Holden17 found that although 64% of patients were happy that their care providers were concerned about their oral intake, 28% reported that they were being pushed too hard on this issue with resulting anger and conflict. The point behind these observations is that loss of appetite and weight have far-reaching consequences that not only affect the patient but also deeply affect family members. This situation gives rise to fears, anticipatory grief, and, at times, even family conflict.
Halfdanarson and colleagues18 published a systematic review and metaanalysis that examined nutritional counseling and quality of life in patients with cancer. They examined five randomized trials utilizing a validated quality of life instrument to assess outcomes; the standardized mean difference in quality of life scores among patients who underwent dietary counseling was 0.56 (95% confidence interval, −0.01 to 1.14; P = .06). Hence these investigators concluded that dietary counseling does not appear to improve quality of life in a major way in patients with cancer but that the observed trend suggests a need for further study.
A second palliative approach to help patients cope with cancer cachexia focuses on pharmacologic therapy. Two main classes of drugs have demonstrated efficacy in promoting appetite: corticosteroids and progestational agents. With respect to the former, Moertel and colleagues19 conducted the first randomized, placebo-controlled trial to test corticosteroids in this setting. This study focused on 116 patients with advanced gastrointestinal malignancies. These patients had a poor appetite and were thought to have a life expectancy of only a few months. The patients were randomly assigned to receive dexamethasone, 0.75 mg four times per day; dexamethasone, 1.5 mg four times a day; or a placebo. By the third week, patients assigned to one of the dexamethasone arms described a better appetite, and more than 25% of this cohort also reported an improvement in strength. Of note, this study reported no improvement in survival and no weight gain, and thus, in effect, the palliative effect of corticosteroids was limited to appetite improvement. Nonetheless, for patients with cancer who wish to try a pharmacologic intervention for loss of appetite, a low dose of corticosteroids with dexamethasone in the range of 0.75 mg four times per day, or sometimes even a lower dose, may help with symptom palliation. This approach is further justified by other studies that also demonstrate stimulatory effects of corticosteroids on appetite.
Although several previous trials demonstrate the efficacy of progestational agents in improving appetite in patients with cancer, the largest randomized, placebo-controlled trial was conducted by Loprinzi and colleagues.20 This study included 133 patients, all of whom were symptomatic with loss of appetite and with no potentially hormone-sensitive tumors. These patients were randomly assigned to receive megestrol acetate, 800 mg per day orally, or a placebo. The patients who were assigned to the megestrol acetate arm described a statistically significant improvement in appetite. Moreover, 16% of patients treated with megestrol acetate gained more than 15 lb at some time, in contrast to only 2% (n = 1) in the group that received a placebo. Subsequent body composition studies indicated that this weight gain consisted mostly of adipose tissue; importantly, subsequent studies have suggested that no survival advantage is associated with the use of megestrol acetate for appetite stimulation.21,22
What dose of megestrol acetate should be used for cancer cachexia? Another randomized trial that examined various doses of megestrol acetate for cancer-associated loss of appetite concluded that the 800 mg–per-day oral dose was best, that higher doses provided no additional benefit, and that lower doses yielded less appetite stimulation.23 The availability of an oral suspension form of megestrol acetate might prompt clinicians to recommend a slightly lower dose, in the range of 600 mg per day.
When should one prescribe a corticosteroid, and when should one prescribe a progestational agent? Loprinzi and colleagues24 undertook a direct comparison in a 496-patient trial in which patients were randomly assigned to one of three arms: megestrol acetate, 800 mg per day orally, versus dexamethasone, 0.75 mg four times per day orally, versus an anabolic steroid. The latter proved to be ineffective. Both megestrol acetate and corticosteroids yielded similar rates of appetite improvement, in the range of 60% to 70%. However, the toxicity profiles of these agents help define when to prescribe which agent. Dexamethasone yielded higher rates of peptic ulcer disease, proximal myopathy, and Cushingoid changes; as a result, 36% of patients stopped treatment. In contrast, a higher incidence of thromboembolism (5% versus 1%) occurred with megestrol acetate. Other adverse effects of megestrol acetate include impotence and vaginal bleeding upon withdrawing the agent. Of incidental note, it should be recognized that with either class of agents, abrupt discontinuation can lead to adrenal insufficiency, and the empiric initiation of corticosteroids may be necessary under such circumstances. Hence, taken together, the results of this study suggest that dexamethasone is perhaps best used in patients with a short predicted survival when the adverse effects of the drug do not have time to cause major issues for patients, and megestrol acetate is best used when life expectancy is anticipated to be longer. As a result of its thrombogenic effects, megestrol acetate is contraindicated for appetite stimulation in patients with a history of pulmonary embolism.
As an example, patients with a bowel obstruction who are unable to eat are sometimes considered for parenteral nutrition. Occasionally, multiple sites of obstruction coupled with the adherence of bowel to other structures make successful surgical intervention impossible. Yet some of these patients appear to have indolent cancers and appear viable in every other respect. In this context, Brard and colleagues25 reported on a case series that included 55 previously treated patients with ovarian cancer who had “terminal bowel obstruction.” Within this group, 28 received parenteral nutrition. Patients survived a median of 72 days if they received parenteral nutrition compared with 41 days if they had not received it (P = .01). Admittedly, as is the case with any retrospective study, selection bias alone may account for the improved outcomes. Nonetheless, this study also suggests that exceptions occur, and that parental nutrition, if used sparingly, might benefit a subgroup of patients.
Another example is provided by the Mayo Clinic, which reviewed its 20-year experience with home parenteral nutrition in a group of 52 patients, of whom the majority had incurable cancer.26 This group represented 15% of the total number of patients treated with home parenteral nutrition, an observation that once again makes the point that administering parenteral nutrition under these circumstances should only be done under exceptional circumstances. In general, the parenteral nutrition was well tolerated. Moreover, the median time from starting parenteral nutrition to patient death was 5 months (range, 1 to 154 months), but, importantly, 16 patients lived for longer than a year. Unfortunately, it was not possible to predict which patients could derive the most benefit from parenteral nutrition under such circumstances. Presumably, the selection of good candidates led to favorable outcomes that precluded a careful determination of contributing factors, and small patient numbers also provided inadequate power to make such analyses meaningful. Nonetheless, this study demonstrated that highly selected patients with cancer might benefit from parenteral nutrition.
Future Possibilities and Clinical Trials
Despite a long list of agents that have proved ineffective in palliating cancer cachexia (e.g., dronabinol, fluoxymesterone, infliximab, etanercept, eicosapentaenoic acid, pentoxifylline, hydrazine sulfate, and cyproheptadine [in most circumstances]) and despite a list of agents that have demonstrated only preliminary promise in palliating cancer cachexia (e.g., adenosine triphosphate, melatonin, carnitine, thalidomide, and antidepressants such as mirtazapine), a small number of agents are gaining increasing interest.27–35
Zhou and colleagues36 used cancer cachexia animal models to show that blockade of the ActRIIB, a myostatin receptor, prevented muscle wasting, restored skeletal and cardiac muscle, and led to an improvement in survival among tumor-bearing animals. Importantly, these favorable effects occurred even with continued tumor growth, indicating that the beneficial effects are not tied directly to tumor response. Additionally, these investigators demonstrated that blockade of ActRIIB prevented activation of the ubiquitin-proteasome system and stimulated the growth of muscle stem cells. These findings suggest that blockade of this receptor might open up new avenues of clinical investigation to prevent or treat cancer cachexia. Clinical trials that exploit such mechanisms are ongoing in patients with cancer and might offer palliation of some of the muscle wasting commonly encountered in persons with cancer cachexia.
Recent data from Dalton and colleagues37 provide interesting preliminary data on a compound known as GTx-024. This agent is a nonsteroidal selective androgen receptor modulator—commonly referred to as a “SARM”—that provides tissue-enhancing effects selectively within such tissues as muscle without the more bothersome effects of hirsutism in women and prostatic hyperplasia with urinary obstructive symptoms in men. Building on dose-finding studies with this agent, these investigators went on to test GTx-024 within a 12-week double-blind, placebo-controlled phase 2 trial in 120 healthy elderly men and postmenopausal women.37 With use of dual energy x-ray absorptiometry and other standard methods to capture physical activity, body weight, and other clinical parameters, the investigators observed that the agent resulted in dose-dependent increases in total lean body tissue and improved functionality all within the context of an acceptable safety profile. Although these data were not generated in patients with cancer, the fact that this agent was so well tolerated has given rise to larger studies that are ongoing in patients with cancer.
An oral ghrelin mimetic and growth hormone secretagogue is also being studied and potentially builds on the track record of previous partially successful interventions that have relied on hormonal therapies, such as corticosteroids and progestational agents, to improve outcomes in patients with cancer cachexia. Referred to as anamorelin, this agent has been preliminarily tested in healthy persons with results suggesting that this agent is well tolerated.38 Moreover, these persons also manifested notable weight gain after only 6 days of treatment. Using doses of 50 mg and 75 mg a day, the investigators observed mean increases in weight from baseline of 1.25 kg and 1.16 kg, respectively. Recent preclinical data also demonstrate that this agent does not lead to tumor growth. Large phase 3 trials in patients with cancer are ongoing.