Brain Metastases and Neoplastic Meningitis
Penny K. Sneed, Norbert Kased and James L. Rubenstein
• Central nervous system metastases are common, affecting as many as 25% of patients with cancer.
• Most central nervous metastases involve the brain.
• Less often, the dura, leptomeninges, skull base, or cranial nerves may be affected.
• The terms “neoplastic meningitis” and “carcinomatous meningitis” refer to the dissemination of cancer cells within the leptomeningeal space.
• The most frequent primary tumor types that give rise to brain metastases include lung cancer, melanoma, breast cancer, and renal cell carcinoma.
• Brain metastases are best detected with contrast-enhanced magnetic resonance imaging.
• Metastases generally appear as enhancing, well-circumscribed lesions with or without surrounding vasogenic edema.
• Biopsy or resection may be indicated to confirm the diagnosis, particularly in a patient with a single lesion and no cancer diagnosis or no known metastatic disease.
• Neoplastic meningitis often eludes early detection; meningeal enhancement is only visible on magnetic resonance imaging in about 50% of cases, and cerebral spinal fluid cytology may be negative initially in 40% to 50% of cases.
• The most important factors that predict longer survival of patients with brain metastases include age <65 years, good performance status, control of the primary cancer, and lack of extracranial metastases.
• The most standard treatment for brain metastases is whole-brain radiotherapy (WBRT).
• Patients with a good prognosis and a limited number of brain metastases may benefit from more aggressive therapy such as surgery (especially for a single brain metastasis) or stereotactic radiosurgery (SRS), with or without adjuvant WBRT.
• After WBRT alone, the following observations have been made:
 At least 60% of symptomatic patients improve significantly.
At least 60% of symptomatic patients improve significantly.
 Median survival time is typically 3 to 6 months.
Median survival time is typically 3 to 6 months.
 One third to one half of patients die of brain metastases, and the remainder die of systemic disease.
One third to one half of patients die of brain metastases, and the remainder die of systemic disease.
 Approximately one quarter of brain metastases have a complete response and one third have a partial response.
Approximately one quarter of brain metastases have a complete response and one third have a partial response.
 One-year actuarial local control probability may be as low as 14% or as high as 71%.
One-year actuarial local control probability may be as low as 14% or as high as 71%.
• Among patients with newly diagnosed brain metastases selected for surgery or SRS with or without WBRT, the following observations have been made:
 Median survival time is approximately 9 to 11 months.
Median survival time is approximately 9 to 11 months.
 One-year actuarial local control rates are approximately:
One-year actuarial local control rates are approximately:
 85% for surgery and adjuvant WBRT.
85% for surgery and adjuvant WBRT.
 75% to 90% for SRS with or without adjuvant WBRT.
75% to 90% for SRS with or without adjuvant WBRT.
 The increased local control achievable with surgery plus WBRT or with SRS may only be meaningful in patients likely to live at least 6 to 12 months from the standpoint of their extracranial disease.
The increased local control achievable with surgery plus WBRT or with SRS may only be meaningful in patients likely to live at least 6 to 12 months from the standpoint of their extracranial disease.
 Systemic therapy is not generally used as a primary treatment for brain metastases, but it has some efficacy on its own.
Systemic therapy is not generally used as a primary treatment for brain metastases, but it has some efficacy on its own.
 There is increasing interest in combining systemic agents with radiation therapy.
There is increasing interest in combining systemic agents with radiation therapy.
• Intrathecal chemotherapy plays a major role in the management of neoplastic meningitis, alone or in combination with radiotherapy.
• Craniospinal radiotherapy causes significant acute toxicity and long-lasting myelosuppression.
• Accordingly, neoplastic meningitis may be managed with use of intrathecal chemotherapy combined with more limited radiotherapy.
• Examples of limited radiotherapy include the following:
 Lumbar fields for gross disease in the cauda equina
Lumbar fields for gross disease in the cauda equina
 Skull base fields for cranial nerve involvement
Skull base fields for cranial nerve involvement
 WBRT in patients with hydrocephalus or significant headache stemming from diffuse brain leptomeningeal involvement
WBRT in patients with hydrocephalus or significant headache stemming from diffuse brain leptomeningeal involvement
Brain Metastases
Epidemiology
Brain metastases appear to be more common than primary malignant brain tumors, although the exact incidence is unknown. In a Memorial Sloan Kettering Cancer Center autopsy series, 24% of patients with cancer had CNS metastases and 15% had brain metastases.1 One population-based study in the Netherlands found that brain metastases developed in 8.5% of 2724 patients with melanoma, lung, breast, colorectal, or renal cell carcinoma. The 5-year cumulative incidence of brain metastases was 7.4% in patients with melanoma and 16.3%, 9.8%, 5.0%, and 1.2% for patients with lung, renal cell, breast, or colorectal carcinoma, respectively.2 Similarly, the population-based incidence of brain metastasis among patients with cancer within the Metropolitan Detroit Cancer Surveillance System diagnosed between 1973 and 2001 was 6.9% for patients with melanoma and 19.9%, 6.5%, 5.1%, and 1.8% for patients with lung, renal cell, breast, and colorectal cancer, respectively.3 The incidence of brain metastases appears to be increasing as a result of improvements in cancer management that are leading to longer survival time, as well as increased lesion detection with contrast-enhanced, high-resolution magnetic resonance imaging (MRI),4 although Schouten et al.2 found no evidence of increasing brain metastasis incidence during 1986 through 1995.
The most common primary tumors responsible for brain metastases include lung cancer (constituting approximately 40% to 50% of cases), breast cancer (15%), melanoma (10%), and unknown primary (5% to 10%), followed by renal cell carcinoma, colorectal cancer, gynecologic cancers, and other miscellaneous tumors.5,6 Brain metastases may arise from any primary cancer, but certain tumors such as melanoma and carcinomas of the lung, kidney, and breast have a tendency to spread to the CNS. On the contrary, some tumors rarely metastasize to the brain, such as prostate, oropharyngeal, and skin carcinomas. In children, the most common solid malignancies responsible for brain metastases include sarcomas, neuroblastomas, and germ cell tumors.7
Cancer may spread to the brain at various points in the course of disease. Synchronous brain metastases, found within 1 month of the primary cancer diagnosis, occur in up to one third of patients.2 More commonly, brain metastases are discovered after the diagnosis of cancer, often after other systemic metastases have developed, at a median of less than 1 year after lung cancer diagnosis and 2 to 3 years after diagnosis of melanoma, breast cancer, gynecologic cancer, or renal cell carcinoma.6 Overall, the median time from primary diagnosis to diagnosis of brain metastases is 12 months.6
Pathophysiology
Brain metastases arise primarily from arterial hematogenous spread to the brain. Tumor cells tend to become trapped where blood vessels decrease in caliber at the gray/white matter junction and distal-most vasculature (the border or “watershed” zones between arterial territories).8 Metastatic cells then adhere to the endothelial cells, penetrate into the brain parenchyma, and proliferate. Larger aggregates of tumor cells that gain access to the venous circulation are filtered out in lung capillaries before entering the systemic arterial circulation, but individual tumor cells may pass through to lodge in the brain. Tumor emboli may also break off from lung metastases or primary lung cancers to travel to the brain via the arterial circulation. Cells from pelvic or abdominal cancers may gain access to the posterior fossa or leptomeninges through Batson’s vertebral venous plexus without passing through the lungs.5 Intracranial spread may also occur via direct extension from bone or dural metastases or perineural extension along cranial nerves. The distribution of metastases is roughly proportional to the relative blood flow to different regions; approximately 80% of brain metastases are located in the cerebral hemispheres, 10% to 15% in the cerebellum, and 1% to 5% in the brain stem.5,6 Posterior fossa metastases appear to arise disproportionately from pelvic or abdominal primary tumors.5
Most brain metastases are very well circumscribed. Although extensive associated edema may be present, the tumor cells do not tend to infiltrate into surrounding brain tissue, in contrast to primary malignant brain tumors. Most brain metastases are solid, but they may appear to be heterogeneously enhancing or cystic because of necrosis, keratin deposits in squamous cell carcinoma, or mucin secretion from adenocarcinomas. Brain metastases may be hemorrhagic, particularly from melanoma, renal cell carcinoma, choriocarcinoma, and, less frequently, bronchogenic carcinoma.9
Single brain metastases are more common than multiple metastases in patients with renal cell, gastrointestinal, or unknown primary cancers.6 In the era of computed tomographic (CT) imaging, it appeared that about 50% of patients with brain metastasis had a single brain lesion.5 More recently, in the era of MRI, this number has decreased to approximately 25% to 35%, with improved sensitivity for detecting smaller metastases.10 Of note, the term “solitary brain metastasis” implies that a single brain metastasis is the only known site of metastatic disease.
Clinical Presentation
The possibility of brain metastases should be suspected in any patient with cancer who experiences new neurologic signs or symptoms. Two thirds of patients with cancer found to have brain metastases at autopsy had experienced neurologic symptoms from the metastases,11 and only 10% of a series of 729 patients diagnosed between 1973 and 1993 by CT or MRI were asymptomatic.6 The most common presenting symptoms are headache (24% to 53%), focal weakness (16% to 40%), altered mental status (24% to 31%), seizures (15% to 16%), and ataxia (9% to 20%).6,12 Symptoms may worsen gradually from a growing tumor and associated edema. Less often, acute neurologic symptoms may occur as a result of hemorrhage of a brain metastasis.
Diagnosis
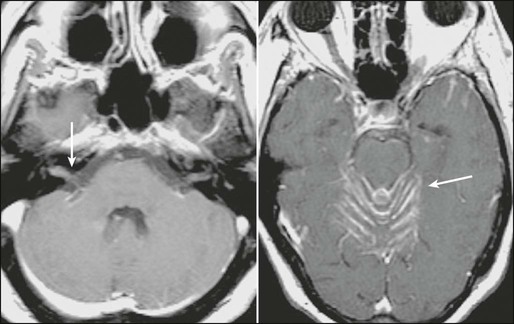
It is widely accepted that MRI is the best diagnostic test to detect brain metastases. Standard imaging includes T2-weighted and pre- and postgadolinium-enhanced T1-weighted sequences; a postcontrast fluid-attenuated inversion-recovery sequence is also helpful in visualizing small metastases near cerebrospinal fluid (CSF) spaces (Fig. 50-1). Gadolinium-enhanced MRI is much more sensitive than nonenhanced MRI or contrast-enhanced CT imaging.13,14 In one study, 17 of 55 patients (31%) with a single metastasis based on contrast-enhanced CT were found to have multiple metastases on contrast-enhanced MRI.15 The sensitivity of MRI is improved by using “triple-dose” gadolinium (0.3 mmol/kg instead of 0.1 mmol/kg gadoteridol)16 and by using contiguous axial 3-mm slices and coronal three-dimensional spoiled gradient echo recovery volume imaging so that small lesions are not missed between slices. Functional imaging techniques such as positron emission tomography, magnetic resonance spectroscopy, and perfusion and diffusion MRI may aid in distinguishing metastatic lesions from necrosis, primary brain tumor, or nonmalignant processes.19–19
The differential diagnosis of an enhancing or hemorrhagic intracranial lesion includes brain metastasis, primary brain tumor, CNS lymphoma, abscess, encephalitis, cerebral infarct or hemorrhage, progressive multifocal leukoencephalopathy, tumefactive demyelinating disease, and radiation necrosis. Factors that aid in making a diagnosis based on imaging include characteristic appearance, a known cancer diagnosis, and a multiplicity of lesions. However, a biopsy may be warranted if there is doubt about the diagnosis or if a single brain lesion is seen in a patient with a history of cancer but no other known metastatic disease. In a study requiring biopsy or surgery before whole-brain radiotherapy (WBRT), 11% of 54 patients with a single brain lesion that was thought to be a metastasis turned out to have glioblastoma, low-grade astrocytoma, abscess, or an inflammatory process.20 A biopsy series in 100 patients with multifocal brain lesions and no known primary cancer found a variety of diagnoses: malignant glioma (37%), primary CNS lymphoma (15%), brain metastases (15%), low-grade glioma (12%), infectious processes (10%), and ischemic lesions (6%).21
Prognostic Factors
In general, brain metastases are associated with a poor prognosis. In the pre-CT era, the median survival time of patients with symptomatic brain metastases was approximately 1 to 2 months without treatment,24–24 2 to 2.5 months with corticosteroid therapy,25 and 3 to 6 months with WBRT.28–28
Despite major advances in cancer diagnosis, cancer treatment, and brain imaging, the overall survival time of unselected patients with brain metastases treated with WBRT has remained at 3 to 6 months since the 1950s.6,29–31 The majority of patients with brain metastases have or will soon experience disseminated systemic disease, and overall survival is often determined by the extent and activity of the extracranial disease. In patients with a relatively short life expectancy, the treatment goal is to achieve rapid palliation and a neurologic symptom-free remission interval commensurate with the life expectancy. However, long-term survival or even cure is possible in a small proportion of patients with brain metastases, and patients with a good prognosis from the standpoint of their extracranial disease may benefit from more aggressive therapy to achieve durable control of their brain metastases.
Prognostic factors have been evaluated in several large series. Lagerwaard et al.32 studied 1292 patients with CT-diagnosed brain metastases treated between 1981 and 1990. The overall median survival time was 3.4 months. The most important parameters prognostic for longer survival included better performance status, limited systemic tumor activity, and a normal serum lactate dehydrogenase level, followed by breast cancer versus other primary sites, age <70 years, and one to two versus three or more brain metastases.32
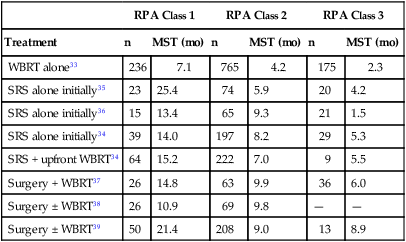
Gaspar et al.33 identified three prognostic groups by using a recursive partitioning analysis (RPA) of more than 1100 patients enrolled in three consecutive Radiation Therapy Oncology Group (RTOG) trials conducted from 1979 through 1993. The median survival time was 7.1 months for RPA class 1, consisting of patients <65 years old with a Karnofsky performance status (KPS) ≥70, controlled primary tumor, and no extracranial metastases. Patients with KPS <70 (RPA class 3) had a median survival time of only 2.3 months; the median survival time was 4.2 months for the remaining patients (RPA class 2) (Table 50-1).33 The prognostic value of the RTOG RPA classes has been validated in patients treated with stereotactic radiosurgery (SRS) ± WBRT or surgery ± WBRT, with median survival times of 10.9 to 25.4 months for RPA class 1, 5.9 to 9.9 months for RPA class 2, and 1.5 to 8.9 months for RPA class 3 (Table 50-1).34–39
Table 50-1
Median Survival Time by Radiation Therapy Oncology Group Recursive Partitioning Analysis Class
| RPA Class 1 | RPA Class 2 | RPA Class 3 | ||||
| Treatment | n | MST (mo) | n | MST (mo) | n | MST (mo) |
| WBRT alone33 | 236 | 7.1 | 765 | 4.2 | 175 | 2.3 |
| SRS alone initially35 | 23 | 25.4 | 74 | 5.9 | 20 | 4.2 |
| SRS alone initially36 | 15 | 13.4 | 65 | 9.3 | 21 | 1.5 |
| SRS alone initially34 | 39 | 14.0 | 197 | 8.2 | 29 | 5.3 |
| SRS + upfront WBRT34 | 64 | 15.2 | 222 | 7.0 | 9 | 5.5 |
| Surgery + WBRT37 | 26 | 14.8 | 63 | 9.9 | 36 | 6.0 |
| Surgery ± WBRT38 | 26 | 10.9 | 69 | 9.8 | — | — |
| Surgery ± WBRT39 | 50 | 21.4 | 208 | 9.0 | 13 | 8.9 |
Sperduto and colleagues40 developed a newer prognostic index for patients with brain metastases termed the Graded Prognostic Assessment, based on a database of 1960 patients accrued to several RTOG brain metastasis protocols. Subscores of 0 to 1 for each factor (age, KPS, number of metastases, and extracranial metastases) were summed to determine a Graded Prognostic Assessment score ranging from 0 to 4 (worst to best prognosis). This system was subsequently validated and refined with diagnosis-specific prognostic indices based on an independent, multi-institutional retrospective analysis of 4259 other patients with brain metastases treated with WBRT and/or SRS.41 Prognostic factors included KPS, age, presence of extracranial metastases, and number of brain metastases for patients with lung cancer; KPS and the number of brain metastases for patients with melanoma and renal cell cancer; tumor subtype, KPS, and age for patients with breast cancer; and only KPS for patients with gastrointestinal cancer.
Treatment
Corticosteroids
Corticosteroid therapy is generally instituted in symptomatic patients as soon as brain metastases are diagnosed to help alleviate symptoms until the brain metastases and edema improve from specific antitumor treatment.42 Corticosteroids reduce the permeability of leaky tumor blood vessels and thereby reduce the mass effect and edema caused by brain metastases.43 The most commonly used steroid is dexamethasone because of its relatively low mineralocorticoid activity; often, a loading dose of 10 mg is used, followed by 4 mg every 6 hours. Lower doses (2 to 4 mg twice daily or 2 to 4 mg three times a day) may be adequate in many situations. Patients usually improve within hours after the first dose, attaining maximal benefit after approximately 3 to 7 days. After patients become asymptomatic or reach maximal benefit, the dose should be gradually tapered and either discontinued or else maintained at the lowest dose level needed to manage symptoms. If headaches recur or neurologic symptoms worsen during the course of the taper, the dose should be increased as needed and then the taper should proceed more gradually. Occasionally patients experience steroid-withdrawal symptoms of depression, fatigue, nausea, or poor appetite, necessitating reinstitution of low-dose dexamethasone or hydrocortisone and a slow taper in the low-dose range.
Anticonvulsant Agents
Seizures are very unlikely to occur from infratentorial metastases, but they may be triggered by supratentorial metastases. About 15% of patients with brain metastases present with seizures when they first are seen by a clinician, and 30% to 40% experience seizures at some point in their disease course.44 An anticonvulsant agent such as phenytoin, carbamazepine, or levetiracetam should be prescribed for any patient with brain metastases who experiences a seizure. Prophylactic anticonvulsant agents are not recommended; prospective and retrospective studies and metaanalyses have failed to demonstrate a benefit for prophylactic anticonvulsant agents in patients with brain metastases.43–46 In addition, anticonvulsant agents may have adverse side effects or cause serious allergic reactions such as Stevens-Johnson syndrome. Importantly, certain anticonvulsant agents such as phenytoin can reduce the efficacy of corticosteroids and can activate the cytochrome P450 enzyme system.47 This latter property can affect patients undergoing chemotherapy by altering the metabolism of some chemotherapeutic agents, thus requiring chemotherapy dose adjustment.
External Beam Radiation Therapy
The most standard treatment for brain metastases consists of WBRT covering the entire intracranial contents while the patient’s eyes are shielded (Fig. 50-2). The benefits of WBRT were first described in the 1950s and 1960s. In these early studies, significant symptomatic improvement was noted in about 60% of patients, and the median survival time ranged from about 3 to 6 months with WBRT28–28 compared with an expected median survival time of 1 to 2 months without treatment.24–24
Selected Randomized Trials of WBRT Alone
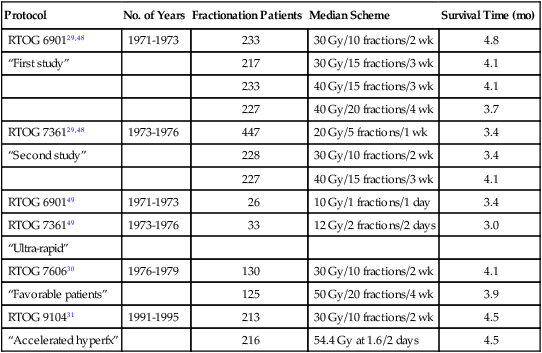
Multiple large phase 3 randomized trials of WBRT have been conducted by the RTOG since 1970 (Table 50-2). The first two trials compared various WBRT fractionation schemes in more than 1800 patients treated from 1971 through 1976 and gathered a wealth of data.29,48 Complete or partial response of specific neurologic symptoms was observed in 60% to 90% of symptomatic patients; 47% to 52% of patients improved to a higher neurologic function class; the median duration of improvement was 10 to 12 weeks; and 75% to 80% of patients’ remaining survival time was spent in an improved or stable neurologic state. The overall median survival times were 4.1 months in the first study and 3.4 months in the second study, and brain metastases were reported to be the cause of death in 49% or 31% of the patients, respectively. There were no significant differences in symptomatic response rates, duration of response, or survival time according to the treatment regimen: 40 Gy in 15 or 20 fractions, 30 Gy in 10 or 15 fractions, or 20 Gy in 5 fractions.29,48 With the ultrarapid fractionation schemes (10 Gy in one fraction or 12 Gy in 2 fractions; Table 50-2), there was some concern that irradiation may have led to herniation and death within 48 hours of treatment in a small number of cases, and time to neurologic progression was shorter than with more protracted regimens.49,50 These findings were in agreement with conclusions of a study of 15 Gy in 2 fractions over 3 days compared with 30 Gy in 15 fractions.51 Two later RTOG trials failed to show any advantage of 50 Gy in 20 fractions or 54.4 Gy at 1.6 Gy twice daily over 30 Gy in 10 fractions (see Table 50-2),30,31 further solidifying 30 Gy in 10 fractions over 2 weeks as the most frequently used WBRT treatment regimen. Other common treatment regimens include 20 Gy in 5 fractions, 35 to 37.5 Gy at 2.5 Gy per fraction, 40 to 50 Gy at 2.0 Gy per fraction, and 45 to 50.4 Gy at 1.8 Gy per fraction. Shorter regimens may be selected in patients with a shorter life expectancy or when WBRT is delaying the chemotherapy needed to treat systemic disease.
Table 50-2
Selected Randomized Trials of Whole-Brain Radiotherapy Alone for Brain Metastases
| Protocol | No. of Years | Fractionation Patients | Median Scheme | Survival Time (mo) |
| RTOG 690129,48 | 1971-1973 | 233 | 30 Gy/10 fractions/2 wk | 4.8 |
| “First study” | 217 | 30 Gy/15 fractions/3 wk | 4.1 | |
| 233 | 40 Gy/15 fractions/3 wk | 4.1 | ||
| 227 | 40 Gy/20 fractions/4 wk | 3.7 | ||
| RTOG 736129,48 | 1973-1976 | 447 | 20 Gy/5 fractions/1 wk | 3.4 |
| “Second study” | 228 | 30 Gy/10 fractions/2 wk | 3.4 | |
| 227 | 40 Gy/15 fractions/3 wk | 4.1 | ||
| RTOG 690149 | 1971-1973 | 26 | 10 Gy/1 fractions/1 day | 3.4 |
| RTOG 736149 | 1973-1976 | 33 | 12 Gy/2 fractions/2 days | 3.0 |
| “Ultra-rapid” | ||||
| RTOG 760630 | 1976-1979 | 130 | 30 Gy/10 fractions/2 wk | 4.1 |
| “Favorable patients” | 125 | 50 Gy/20 fractions/4 wk | 3.9 | |
| RTOG 910431 | 1991-1995 | 213 | 30 Gy/10 fractions/2 wk | 4.5 |
| “Accelerated hyperfx” | 216 | 54.4 Gy at 1.6/2 days | 4.5 |
Hyperfx, Hyperfractionation; RTOG, Radiation Therapy Oncology Group.
Response and Local Control
Nieder et al.52 studied CT response of brain metastases to WBRT (30 Gy in 10 fractions). By lesion, the complete response rate was 24% and the partial response rate was 35%. The overall (complete plus partial) response rates were 81% for small cell carcinoma, 65% for breast cancer, 56% for squamous cell carcinoma, 50% for nonbreast adenocarcinoma, 46% for renal cell carcinoma, and 0% for melanoma. Complete response rates were 39% for solid metastases, 15% for those with less than 50% necrosis, and 11% for those with at least 50% necrosis, and 52%, 39%, 17%, 20%, 5%, and 0% for lesion volumes ≤0.5 mL, 0.6 to 1.0 mL, 1.1 to 3.0 mL, 3.1 to 6.0 mL, 6.1 to 10.0 mL, and >10 mL, respectively.52 In another study by the same group, there was a suggestion that a higher response rate was obtained with 40 Gy at 2 Gy per fraction with or without a partial brain boost to 50 or 60 Gy compared with 30 Gy at 3 Gy per fraction.53 For the WBRT-only arm of an RTOG trial of WBRT ± SRS, the complete response rate was 8%, the partial response rate was 54%, the stable disease rate was 22%, and the progression rate was 17% among 78 patients with imaging follow-up.54 Data on long-term local control of brain metastases after WBRT alone are limited and highly variable. The 1-year actuarial local control probability by patient ranged from 0% to 14% in the WBRT-only arms of randomized trials reported by Kondziolka et al.55 and Patchell et al.20 but as high as 71% in the WBRT-only arm of the RTOG randomized trial of WBRT ± SRS.54
Partial Brain Radiotherapy
Partial brain radiotherapy can be considered for a single metastasis in lieu of WBRT but is generally not advisable because it would complicate or preclude later WBRT if needed (unlike SRS, which delivers a very focal dose). Caution is advised when postoperative radiotherapy to the posterior fossa alone is contemplated because cerebellar metastases appear to be associated with an increased risk of leptomeningeal dissemination after resection.56,57 On the other hand, partial brain radiotherapy may be useful for treating recurrent brain metastases unsuitable for resection or SRS.
Toxicity of WBRT
Acute toxicity of WBRT includes hair loss, fatigue, and modest skin reactions in essentially all patients and mild acute ototoxicity in some patients. The skin reaction resolves by several weeks, and fatigue improves gradually over 1 or several months. Hair generally regrows by 6 months after WBRT, but alopecia may be permanent in a central strip on the top and back of the head from the reduced skin sparing of tangential radiation beams. In patients who are long-term survivors after WBRT, there is a risk of late hearing loss, retinopathy if the retina was included in the radiation field, and permanent neurocognitive toxicity. DeAngelis et al.56 reported an 11% risk of severe radiation-induced dementia among 1-year survivors after resection of a single brain metastasis and postoperative WBRT to 20 to 40 Gy with high-dose daily fractions. A separate report from the same group described 12 patients cured of brain metastases who experienced severe radiation-induced dementia with associated ataxia and urinary incontinence.58 Imaging with CT showed atrophy, ventricular dilatation, and hypodense white matter. The WBRT schemes included mostly mixtures of 3- to 4-Gy fractions with 5- to 6-Gy fractions, but two of the affected patients had received the standard regimen of 30 Gy in 10 fractions.58 Nieder et al.59 reported a 42% 2-year actuarial probability of symptomatic mild, moderate, or severe late radiation toxicity after resection of a single brain metastasis followed by WBRT at 30 Gy in 10 fractions or 40 Gy in 20 fractions. Among 112 patients on the WBRT-only arm of an RTOG randomized trial, Andrews et al.54 reported one case of grade 3 and one case of grade 4 late CNS toxicity and one case of grade 3 late ototoxicity.
Two prospective, randomized controlled studies assessed neurocognitive function in patients with brain metastases treated with SRS ± WBRT. Aoyama and colleagues60 assessed neurocognitive function through the Mini-Mental State Examination (MMSE). After treatment, 53% of the SRS + WBRT group versus 50% of the SRS-alone group had improvement >3 points. However, the average time until MMSE deterioration was 16.5 months in the WBRT + SRS group versus 7.6 months in the SRS-alone group (P = .05); this finding was attributed to the higher rates of brain tumor recurrence in the latter group. The authors concluded that brain tumor control is the most important factor for stabilizing neurocognitive function but that “the long-term adverse effects of WBRT on neurocognitive function might not be negligible.”60
In 2009, a group from the MD Anderson Cancer Center compared neurocognitive function as their primary end point in a prospective, randomized trial of SRS + WBRT versus SRS alone, using the Hopkins Verbal Learning Test–Revised (HVLT-R) total recall at 4 months.61 The authors found that patients treated with SRS + WBRT were significantly more likely to show a decline in learning and memory function at 4 months, and they recommended initial treatment with SRS alone in conjunction with close follow-up to better preserve learning and memory.
Finally, Sun et al.62 compared neurocognitive function in a group of patients with stage III non–small cell lung cancer (SCLC) who completed definitive therapy without progression and were randomly assigned to prophylactic WBRT or observation. The authors used the MMSE, the Activities of Daily Living Scale, and the HVLT to assess neurocognitive function. At 1 year, there were no significant differences in the MMSE or Activities of Daily Living Scale scores, but the WBRT group had a greater decline in both immediate recall (P = .03) and delayed recall (P = .008) on the HVLT.
Surgery
Surgery has important roles to play in certain subsets of patients, including confirming the diagnosis when needed, relieving mass effect from a large, symptomatic lesion, improving the likelihood of durable local control for a single metastasis, and salvaging a metastasis that failed to respond to prior therapy. Surgical resection may also be useful for lesions with considerable peritumoral edema despite use of steroids or for lesions causing refractory seizures, even if the lesion is sufficiently small that SRS would be a therapeutic option.63 Resection of brain metastases has become safer with advances in neuroimaging and neurosurgery such as image guidance, preoperative and intraoperative functional mapping, and intraoperative ultrasound and MRI.64
Randomized Trials of WBRT With or Without Surgery
Three prospective randomized trials have been performed to evaluate the addition of surgery to WBRT (Table 50-3). Patchell et al.20 reported on 48 patients with a single brain metastasis who were randomly assigned to biopsy and WBRT versus resection and WBRT with 36 Gy in 12 fractions. Patients who underwent resection had significantly improved local control (80% for resection and WBRT vs. 48% for biopsy and WBRT; P < .02), duration of functional independence (median, 38 weeks vs. 8 weeks; P < .005), and survival (median, 40 weeks vs. 15 weeks; P < .01). Factors associated with longer survival included younger age, no extracranial disease, surgical resection, and a longer interval from primary diagnosis to brain metastasis diagnosis.20
Table 50-3
Randomized Trials of Whole-Brain Radiation Therapy ± Surgery or Stereotactic Radiosurgery and Surgery or Stereotactic Radiosurgery ± Whole-Brain Radiation Therapy for Brain Metastases
| First Author/Years | Treatment | No. of Patients | Patients With Extracranial Disease (%) | Median Local FFP (mo) | Median Functionally Independent Survival (mo) | Median Survival (mo) |
| Patchell20/ 1985-1988 |
Biopsy + WBRT | 23 | 83 | 4.8 | 1.8 | 3.4 |
| Surgery + WBRT | 25 | 76 | >13.6 | 8.7 | 9.2 | |
| (36 Gy/12 fx) | (P < .0001) | (P < .005) | (P < .01) | |||
| Noordijk65/ 1985-1990 |
WBRT | 31 | 68 | — | 3.5 | 6 |
| Surgery + WBRT | 32 | 69 | — | 7.5 | 10 | |
| (40 Gy at 2 Gy twice a day) | — | (P = .06) | (P = .04) | |||
| Mintz66/ 1989-1993 |
WBRT | 43 | 84 | — | — | 6.3 |
| Surgery + WBRT | 41 | 73 | — | — | 5.6 | |
| (30 Gy/10 fx) | — | (P = NS) | (P = .24) | |||
| Patchell70/ 1989-1997 |
Surgery | 46 | 65 | 6.2 | 8.0 | 9.9 |
| Surgery + WBRT | 49 | 63 | >12.0 | 8.5 | 11.0 | |
| (50.4 Gy/28 fx) | (P < .001) | (P = .61) | (P = .39) | |||
| Kondziolka55/ 1985-1988 |
WBRT | 14 | 71 | 6 | — | 7.5 |
| WBRT + SRS | 13 | 62 | 36 | — | 11.0 | |
| (36 Gy/12 fx) | (P = .0005) | — | (P = .22) | |||
| Andrews54/ 1996-2001 |
WBRT | 167 | 69 | [71% at 1 yr] | — | 5.7 |
| WBRT + SRS | 164 | 68 | [82% at 1 yr] | — | 6.5 | |
| (37.5 Gy/15 fx) | (P = 0.013) | — | (P = .136) | |||
| Aoyama103/ 1999-2003 |
SRS | 67 | 43 | [73% at 1 yr] | [27% at 1 yr] | 8.0 |
| WBRT + SRS | 65 | 37 | [89% at 1 yr] | [34% at 1 yr] | 7.5 | |
| (30 Gy/10 fx) | (P = .002) | (P = .53) | (P = .42) | |||
| Chang61/ 2001-2007 |
SRS | 30 | — | [67% at 1 yr] | — | 15.2 |
| WBRT + SRS | 28 | — | [100% at 1 yr] | — | 5.7 | |
| (30 Gy/12 fx) | — | (P = .012) | — | (P = .003) | ||
| Kocher71/ 1996-2007 |
SRS | 100 | — | [69% at 2 yr] | 10.0 | 10.9 |
| Surgery | 79 | — | [41% at 2 yr] | Combined | Combined | |
| SRS + WBRT | 99 | — | [81% at 2 yr] | 9.5 | 10.7 | |
| Surgery + WBRT | 81 | — | [73% at 2 yr] | Combined | Combined | |
| (30 Gy/10 fx) |
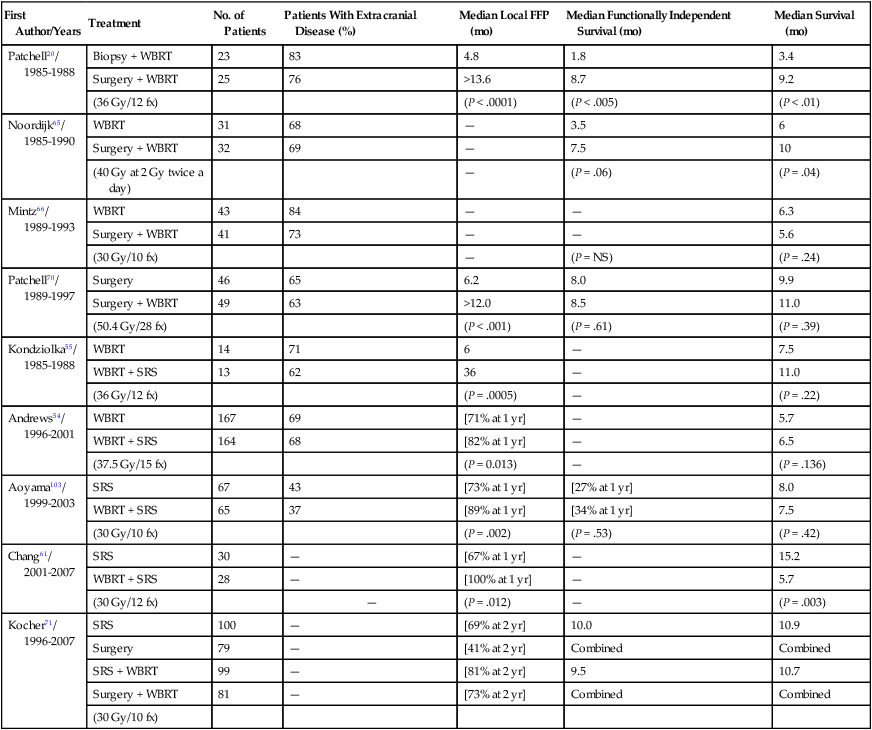
A trial performed in the Netherlands randomly assigned 63 evaluable patients with a single brain metastasis to surgery + WBRT versus WBRT alone (40 Gy at 2 Gy twice daily) (Table 50-3).65 Patients enrolled in the surgery arm of the trial had longer functionally independent survival (median, 7.5 vs. 3.5 months for surgery + WBRT vs. WBRT alone; P = .06) and longer survival time (median, 10 vs. 6 months; P = .04). The survival benefit was seen only in patients without active extracranial disease, in whom the median survival time was 12 months versus 7 months for WBRT alone (P = .02); the median survival time was 5 months for patients with active extracranial disease regardless of the treatment arm. Age >60 years was also confirmed as an unfavorable prognostic factor (P = .003; hazard ratio = 2.74).65
A third trial failed to show a benefit for surgery in addition to WBRT (30 Gy in 10 fractions over 2 weeks) (Table 50-3).66 The median survival times were 5.6 months among 41 patients randomly assigned to surgery + WBRT versus 6.3 months for 43 patients randomly assigned to WBRT alone (10 of whom had surgery before or after WBRT). No difference in the duration of functional independence was found between the two treatment arms.66 However, overall, these studies and previous nonrandomized experience69–69 support the use of surgery in patients with good performance status and a single brain metastasis.
Randomized Trial of Surgery With or Without WBRT
Postoperative WBRT may help prevent recurrence at the resection cavity and prevent appearance of new intracranial metastases by treating microscopic metastases elsewhere in the brain and any tumor cells disseminated at the time of surgery. Patchell et al.70 followed up on retrospective studies suggesting a benefit for WBRT after resection of a brain metastasis by performing a randomized trial (Table 50-3). Ninety-five adults were randomly assigned to observation versus postoperative WBRT with 50.4 Gy in 28 fractions after complete resection of a single brain metastasis. The observation arm had a significantly increased risk of local failure (46% for observation vs. 10% for WBRT), distant brain failure (37% vs. 14%), and any brain failure (70% vs. 18%), along with a shorter time to local failure (median, 6.2 vs. >12 months; P < .001; hazard ratio 6.03) and a shorter time to any brain failure (median, 6.0 vs. >16.1 months; P < .001; hazard ratio 4.94). Patients randomized to observation were more likely to die of neurologic causes (44% vs. 14%; P = .003) but, interestingly, had similar duration of functional independence (median, 8.0 months for observation vs. 8.5 months for WBRT; P = 0.61) and a similar survival time (median, 9.9 vs. 11.0 months; P = 0.39).70
Randomized Trial of Surgery or SRS With or Without WBRT
A phase 3 European Organization for Research and Treatment of Cancer study reported on the outcomes of 359 patients with one to three brain metastases treated with either SRS (n = 199) or surgery (n = 160) who were randomly assigned to WBRT (30 Gy in 10 fractions) versus observation (Table 50-3).71 When observation was compared with WBRT, no significant differences were found in median time to the World Health Organization performance status >2 (10.0 vs. 9.5 months), median overall survival (10.7 vs. 10.9 months), or the percentage of patients alive and functionally independent at 2 years (22.3% vs. 22.7%, respectively). The addition of WBRT to surgery reduced the 2-year relapse rate both at local sites (59% to 27%; P < .001) and at distant sites (42% to 23%; P = .008). In the SRS group, WBRT also significantly decreased local and distant brain failure (local, 31% to 19%, P = .04, and distant, 48% to 33%, P = .023). At 2 years, 22.3% of observation patients versus 22.6% of WBRT patients were alive and functionally independent. Patients enrolled in the observation arm required more salvage therapy (51% of patients) than did patients enrolled in the WBRT arm (16% of patients). Finally, death from intracranial progression was also more frequent in the observation arm relative to the WBRT arm (44% vs. 28%).71
Surgery for Multiple Metastases
Surgical resection may also be used successfully to manage the cases of selected patients with more than one brain metastasis. Bindal et al.72 reported a median survival time of 14 months among 26 patients with multiple brain metastases who underwent resection of all of their brain lesions in a single operation, identical to the survival time of matched patients who had resection of a single metastasis. The complication rate was 9% per craniotomy, the 30-day mortality rate was 4%, and only 6% of symptomatic patients worsened, whereas 83% improved and 11% remained stable.72 A different group describing results of surgery and WBRT noted significantly poorer survival among 18 patients with multiple brain metastases compared with 28 patients with a single metastasis, but apparently only one patient with multiple metastases had gross total resection of all (two) metastases, and this patient survived 46 months.73 In a later series, Paek et al.74 reported a median survival time of 8 months after surgery for approximately 103 patients with a newly diagnosed single metastasis versus 11 months after surgery for 46 patients with newly diagnosed multiple brain metastases (only nine of whom had multiple brain metastases resected); most patients underwent postoperative WBRT. A German group reported on 177 patients with brain metastases who were treated with surgical resection, including 57 with more than one brain metastasis.75 On univariate but not multivariate analysis, resection of all brain metastases was significantly associated with prolonged survival.75
Toxicity of Surgery
The morbidity and mortality associated with surgical resection of brain metastases have decreased over the years as techniques have improved. Lang and Sawaya64 estimated the 30-day mortality rate to be about 4% to 5% after surgery for brain metastasis (essentially identical to the 30-day mortality rate in patients managed with WBRT alone). The most common types of postoperative morbidity include wound infection, hemorrhage, meningitis, pneumonia, deep venous thrombosis, and pulmonary embolism, which occur in about 10% to 15% of patients.64,76 One surgical series reported that 0 to 13% of patients worsened neurologically, 65% to 84% improved, and 11% to 22% remained stable after resection of single or multiple brain metastasis.72 Paek et al.74 reported outcomes in patients who underwent resection of one (n = 191) or multiple (n = 17) newly diagnosed (n = 149) or recurrent (n = 59) brain metastases at a single institution using modern neurosurgical techniques. The 30-day mortality rate was 1.9%, with two deaths from hemorrhage in the resection cavity, one death from a pulmonary embolism, and one death from bowel perforation with sepsis. The median hospital stay was 3 days after surgery; KPS improved in 33%, remained stable in 61%, and decreased in 6% of patients.74
Stereotactic Radiosurgery
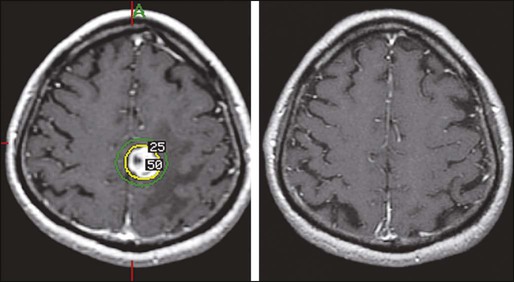
The term SRS implies the delivery of carefully targeted, very focal radiation to one or more intracranial targets, usually with use of a specially adapted linear accelerator,77,78 CyberKnife,79 or Gamma Knife.80 Before the procedure, with use of local anesthesia, a stereotactic frame may be applied to facilitate very precise targeting. Multiple beams or arcs provide for a very steep fall-off of dose outside of the target or targets, minimizing the dose delivered to surrounding normal brain tissue; however, a thin shell of tissue around the target receives a potentially damaging dose of radiation (Fig. 50-3). Because the risk of radiation injury increases with increasing volume, lower doses are generally prescribed for larger target volumes, and single-fraction SRS targets tend to be limited to about 2.5 to 3 cm in diameter.
Retrospective Results of SRS
Table 50-4 summarizes results of selected retrospective series of SRS for single or multiple, newly diagnosed or recurrent brain metastases treated with or without adjuvant WBRT.81–95 For median target volumes ranging from 0.9 to 7.5 mL and median prescribed doses of 15 to 27 Gy in a single fraction, the crude local control rates were 85% to 96%, and 1-year actuarial local control probabilities were 82% to 94% by lesion with a 0 to 17% risk of symptomatic radiation necrosis (average risk, 4%). In most of the series, the median survival times ranged from 7 to 11 months.
Table 50-4
Retrospective Reviews of Stereotactic Radiosurgery ± Whole-Brain Radiation Therapy for Newly Diagnosed or Recurrent Brain Metastases
| Type of Metastases/First Author | No. of Metastases/Patients | Mean or Median Dose (Gy) | Median Target Volume (mL) | % Local Control by Lesion | Median Survival Time (mo) | % Necrosis |
| Single metastases | ||||||
| Auchter81 | 122/122 | 17.0 | 2.7 | 86*/85† | 12.9 | 0 |
| Flickinger82 | 116/116 | 17.5 | — | 85* | 11 | 4 |
| Simonova83 | 237/237 | 21.5 | 7.5 | 95 | 9 | 2.5 |
| Single or multiple metastases | ||||||
| Deinsberger84 | 161/110 | 18.3 | 3.1 | 89* | 12.4 | 2 |
| Flickinger85 | 229/157 | 16.0 | 3.0 | 89* | 10. | 1 |
| Fukuoka86 | >215/130 | >25 | 5.5 | 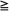 96* 96* |
8 | 5 |
| Gerosa87 | 1307/804 | 20.6 | 4.8 | 94† | 13.5 | — |
| Goodman88 | 682/258 | 18.5 | 1.7 | 82† | 9.1 | — |
| Joseph89 | 189/120 | 26.6 | 5.3 | 94* | 7.4 | 17 |
| Kihlstrom90 | 235/160 | 27.0 | 4.5 | 94* | 7 | 5 |
| Moriarty91 | 643/353 | 15.0 | 2.5 | 88† | 10.5 | 3-6 |
| Petrovich92 | 1305/458 | 18 | 0.9 | 87† | 9 | 4.7 |
| Pirzkall93 | 311/236 | 20 | — | 92* | 5.5 | 2 |
| Sansur94 | 411/193 | 20 | — | 82 by patient | 7.5. | 2 |
| Young95 | 669/250 | — | — | 91* | 7 | <4 |
SRS Dose-Response Relationships
An RTOG dose escalation trial in patients with recurrent primary or metastatic brain tumors used the end point of grade 4-5 or irreversible grade 3 neurotoxicity within 3 months of SRS and concluded that the maximum tolerated doses of single-fraction SRS were 24 Gy for tumors ≤2 cm, 18 Gy for tumors 2.1 to 3.0 cm, and 15 Gy for tumors 3.1 to 4.0 cm in maximum diameter.96 A dose-response analysis specifically in newly diagnosed or recurrent brain metastases ≤2 cm found crude local control rates of 91% by using a SRS dose less than 20 Gy (n = 46), 99% by using a dose of 20 Gy (n = 158), and 96% by using a dose higher than 20 Gy (n = 24) when SRS was combined with planned WBRT.97 Because the risk of complications was 1.9% for a SRS dose ≤20 Gy and 5.9% for a dose >20 Gy, the authors concluded that 20 Gy was the optimal SRS dose for metastases ≤2 cm. Of note, the crude local control rates were 97% for SRS + WBRT versus 87% for SRS alone (P = .0001).97
Based on a data set of 518 newly diagnosed or recurrent brain metastases treated with SRS ± WBRT,88 the 1-year actuarial local freedom from progression probabilities were 88%, 75%, and 29% for doses ≥18 Gy, 15.0 to 17.9 Gy, and <15.0 Gy, and 92%, 83%, 69%, and 37% for maximum target diameters ≤1.0 cm, 1.1 to 2.0 cm, 2.1 to 3.0 cm, and >3.0 cm, respectively. A study of 126 lesions in 80 patients treated with linear-accelerated SRS at the University of Chicago found 1-year local control probabilities of 50%, 97%, and 90% for prescribed doses of 10 to 13.99 Gy, 14 to 17.99 Gy, and  18 Gy, respectively, and 67%, 94%, and 93% for minimum tumor doses of ≤12 Gy, 12.1 to 18 Gy, and >18 Gy, respectively.98
18 Gy, respectively, and 67%, 94%, and 93% for minimum tumor doses of ≤12 Gy, 12.1 to 18 Gy, and >18 Gy, respectively.98
Randomized Trials of WBRT With or Without SRS
The first reported randomized trial of WBRT (30 Gy in 12 fractions) ± SRS boost (16 Gy) was performed in patients with KPS  70 and two to four brain metastases ≤2.5 cm in diameter.55 The study only accrued 27 patients because of early stopping rules based on a significant difference in brain control between the two arms. Compared with WBRT alone, SRS + WBRT yielded significantly improved time to local failure (median, 36 vs. 6 months; P = .0005) and time to any brain failure (median, 34 vs. 5 months; P = .002). Survival was not significantly different for the two arms (median, 7.5 months for WBRT vs. 11 months for WBRT + SRS; P = .22) (Table 50-3). Multiple patients for whom WBRT alone failed underwent salvage SRS.55
70 and two to four brain metastases ≤2.5 cm in diameter.55 The study only accrued 27 patients because of early stopping rules based on a significant difference in brain control between the two arms. Compared with WBRT alone, SRS + WBRT yielded significantly improved time to local failure (median, 36 vs. 6 months; P = .0005) and time to any brain failure (median, 34 vs. 5 months; P = .002). Survival was not significantly different for the two arms (median, 7.5 months for WBRT vs. 11 months for WBRT + SRS; P = .22) (Table 50-3). Multiple patients for whom WBRT alone failed underwent salvage SRS.55
A 2004 RTOG trial randomly assigned 331 patients with KPS 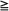 70 and one to three newly diagnosed brain metastases to WBRT (37.5 Gy in 15 fractions) ± SRS boost within 1 week after WBRT from 1996 to 2001.54 Treatment arms were fairly well balanced in comparing WBRT alone to WBRT + SRS, with KPS of 90 to 100 in 63% versus 57%, age <65 years in 60% versus 66%, primary disease controlled or absent in 75% versus 77%, extracranial metastases in 69% versus 68%, and a single brain metastasis in 56% versus 56% of patients, respectively. Patients in the SRS treatment arm had significantly improved KPS at 6 months (P = .033), decreased steroid use at 6 months (P = .016), and improved local control (with 1-year local control probabilities of 71% for WBRT alone vs. 82% for WBRT + SRS; P = .013). Although survival was not significantly different between the two groups overall (5.7 months for WBRT alone vs. 6.5 months for WBRT + SRS; P = 0.136) (Table 50-3), a statistically significant difference in survival was found for patients with a single metastasis (4.9 months for WBRT alone vs. 6.5 months for WBRT + SRS; P = .039).54
70 and one to three newly diagnosed brain metastases to WBRT (37.5 Gy in 15 fractions) ± SRS boost within 1 week after WBRT from 1996 to 2001.54 Treatment arms were fairly well balanced in comparing WBRT alone to WBRT + SRS, with KPS of 90 to 100 in 63% versus 57%, age <65 years in 60% versus 66%, primary disease controlled or absent in 75% versus 77%, extracranial metastases in 69% versus 68%, and a single brain metastasis in 56% versus 56% of patients, respectively. Patients in the SRS treatment arm had significantly improved KPS at 6 months (P = .033), decreased steroid use at 6 months (P = .016), and improved local control (with 1-year local control probabilities of 71% for WBRT alone vs. 82% for WBRT + SRS; P = .013). Although survival was not significantly different between the two groups overall (5.7 months for WBRT alone vs. 6.5 months for WBRT + SRS; P = 0.136) (Table 50-3), a statistically significant difference in survival was found for patients with a single metastasis (4.9 months for WBRT alone vs. 6.5 months for WBRT + SRS; P = .039).54
SRS Without WBRT for Newly Diagnosed Brain Metastases
Because of concern about potential late toxicity of WBRT, multiple groups have tried managing patients by using SRS alone initially, followed by later salvage surgery, SRS, WBRT, or chemotherapy as needed,34,36,93,99–102 laying the groundwork for later randomized trials. Survival was similar for SRS alone initially versus SRS with upfront WBRT for three of five studies (~5 vs. ~6 months;93 11.3 vs. 11.1 months;99 and 8.2 vs. 8.6 months34). In one study, median survival time was thought to be shorter in the WBRT group because of worse prognostic factors in this group,100 and in the fifth study, there was a trend toward longer survival time in patients treated with SRS + WBRT versus SRS alone.102 Multivariate analyses adjusting for known prognostic factors confirmed that the omission of upfront WBRT had no influence on survival time.34,93,99,100 Local control was slightly worse for SRS alone initially, with 1-year actuarial local freedom from progression probabilities of 89% versus 92%,93 71% versus 79%,99 and approximately 62% versus 75%.100 Because of a much greater risk of developing new brain metastases when upfront WBRT was omitted, brain freedom from progression was significantly shorter for SRS alone initially: median, 8.3 versus 15.9 months99 and 9.2 versus 35.1 months.100 In one series, the median brain freedom from progression allowing for salvage therapy was 19.8 months for SRS alone initially versus 18.1 months for SRS with upfront WBRT, and only 26% of patients receiving SRS alone ultimately received WBRT.99
Randomized Trials of SRS With or Without WBRT
The first prospective randomized trial comparing SRS alone (n = 67) to WBRT plus SRS (n = 65) was reported by Aoyama et al.103 Patients with KPS ≥70 and one to four brain metastases <3 cm were accrued between 1999-2003 at 11 institutions. The WBRT dose was 30 Gy in 10 fractions, and SRS-alone doses were 22 to 25 Gy for lesions ≤2 cm and 18 to 20 Gy for lesions >2 cm, with a 30% dose reduction for SRS + WBRT. The median survival times were similar for the two groups (8.0 months for SRS alone vs. 7.5 months for WBRT + SRS; P = .42). Intracranial control was significantly lower in the SRS-alone arm, with 1-year local control probabilities of 73% versus 89% (P = .002), 1-year freedom from new brain metastasis probabilities of 36% versus 58% (P = .003), and 1-year overall brain freedom from progression probabilities of 24% versus 53% (P < .001) for SRS alone versus WBRT + SRS, respectively. Most new brain metastases were asymptomatic. Salvage therapy for brain metastases was given in 29 patients enrolled in the SRS-alone arm versus 10 patients enrolled in the WBRT + SRS arm. There was no significant difference in functional independent survival (27% vs. 34% at 1 year; P = .53) or neurologic preservation (70% vs. 72% at 1 year; P = .99) for SRS alone versus WBRT + SRS, respectively. Deterioration of neurologic function occurred in 21 patients who received SRS alone and in 22 patients who received WBRT + SRS; the deterioration was attributed to new or original brain metastases in 18 patients who received SRS alone versus 13 patients who received WBRT + SRS. Leukoencephalopathy occurred in two patients who received SRS alone versus seven patients who received WBRT + SRS. The authors concluded that SRS alone “could be a treatment option, provided that frequent monitoring of brain tumor status is conducted.”103
Chang et al.61 published results of a prospective, randomized trial assessing outcomes in 58 RPA class 1-2 patients with one to three newly diagnosed brain metastases treated from 2001 to 2007 with SRS ± WBRT, with stratification by RPA class, histology, and number of metastases. The primary end point was neurocognitive function, as discussed in the section on “Toxicity of WBRT.” The WBRT dose was 30 Gy in 12 fractions, and SRS-alone doses were prescribed in accordance with RTOG 90-05. When comparing SRS alone with SRS + WBRT, survival time was longer (median, 15.2 vs. 5.7 months; 1-year survival, 63% vs. 21%; P = .003), whereas local and distant brain control were worse (1-year local control, 67% vs. 100%, P = .012; distant brain tumor control, 45% vs. 73%, P = .02; and 1-year freedom from CNS recurrence, 27% vs. 73%, respectively, P = .0003). Salvage therapy was given in 87% of the patients who received SRS alone versus 7% of patients who received SRS + WBRT. The authors concluded by suggesting that initial treatment with SRS alone for newly diagnosed brain metastases in conjunction with close surveillance could be used as a reasonable treatment strategy.
SRS for Multiple Metastases
The randomized trials assessing SRS ± upfront WBRT excluded patients with more than three to four metastases. However, a multiinstitutional prospective Japanese study (JLGK0901) examined outcomes in patients with 1 to 10 brain metastases who were treated with SRS alone initially.104 This select cohort of patients had newly diagnosed brain metastases, with the largest lesion <10 mL, total tumor volume <15 mL, no evidence of leptomeningeal disease, and KPS >70. Patients were followed up closely with MRI after initial SRS and were treated with salvage SRS or WBRT as deemed appropriate. The trial included 778 patients (280 with 1 lesion [group A], 135 with 2 lesions [group B], 148 with 3 to 4 lesions [group C], 93 with 5 to 6 lesions [group D], and 122 with 7 to 10 lesions [group E]). No significant differences in median survival times were found among the different groups (10.0, 8.3, 8.3, 7.1, and 7.4 months for groups A through E, respectively) or in 1-year functional preservation rate (91%, 93%, 81%, 84%, and 86%, respectively). The 1-year freedom from new metastasis probabilities were 72%, 54%, 44%, 51%, and 66% in groups A through E. Significant differences were found between groups A and B (P = .0003) and groups B and C (P = .0047). This study demonstrated that selected patients with up to 10 newly diagnosed metastases could be reasonably treated with SRS alone initially.
SRS Compared With Surgery
The European Organization for Research and Treatment of Cancer study discussed in the section on “Randomized Trial of Surgery or SRS With or Without WBRT”71 did not directly compare SRS and surgery, and there remains an absence of data to date from prospective randomized trials. Thus several authors have compared results cited in the literature for surgical patients with their own results for similar subsets of patients treated with SRS. Auchter et al.81 reported a four-institution experience of SRS and WBRT in 122 adults who met selection criteria similar to those used in randomized trials of WBRT with or without surgical resection: KPS ≥70 and newly diagnosed, surgically resectable single brain metastases with “nonsensitive” histology (excluding lymphoma, leukemia, multiple myeloma, SCLC, and germ cell tumors) and no urgent indication for resection. Local control was achieved in 86% of lesions with a 1-year actuarial local control probability of 85%, a median duration of functional independence of 10.1 months, and median survival time of 12.9 months (Table 50-4), comparable with the results of the “surgery + WBRT” arms of the randomized trials reported by Patchell et al.20 and Noordijk et al.65 (Table 50-3).
Three other retrospective comparisons of SRS and surgery are summarized in this section with somewhat differing results.76,105,106 Bindal et al. 105 reported 61% versus 87% crude local control rates for SRS (± WBRT) versus surgery (± WBRT) and median survival times of 7.5 months versus 16.4 months, respectively. Muacevic et al.76 compared outcomes for patients with single metastases ≤3.5 cm in diameter treated with SRS alone (56 patients) or with surgery with WBRT (52 patients). One-year local freedom from progression probabilities were 83% for SRS versus 75% for surgery with WBRT (P = .49); new brain metastases developed in 11 (20%) of the patients treated with SRS versus 6 (12%) of the patients treated with surgery + WBRT, but salvage SRS was successful in all 6 patients treated with SRS who were offered salvage therapy. Median survival times were 8.0 months after SRS versus 15.6 months after surgery with WBRT. Death rates from neurologic causes and complication rates were similar for the two treatment approaches, and steroid requirements tended to be less among the patients treated with SRS.76
A third retrospective study compared patients with single metastases who were candidates for either surgery or SRS.106 Most patients received adjuvant WBRT. Pretreatment performance status was worse in the SRS group. Crude local control rates were 100% versus 85% (P = .02) and 1-year survival probabilities were 56% versus 62% for SRS versus surgery (univariate P = .15; multivariate P = .62 with adjustment for age, performance status, and systemic disease status). Short-term and long-term complications occurred in 0% versus 13.5% and 17.4% versus 17.6% of patients treated with SRS versus surgery.106
Toxicity of SRS
Acute complications of SRS occur in about 10% of patients, including seizures, headaches, exacerbation of preexisting neurologic deficits, nausea, and hemorrhage.76,93,107 Complications may include transient perifocal edema responding to a short course of steroids in 7% to 18% of patients76 or symptomatic late radiation effect in an average of 4% of patients (Table 50-4), causing headaches, seizures, or neurologic deficits. Symptoms usually respond to steroids, but surgery may be needed if a patient requires a prolonged course of steroids, tolerates steroids poorly or continues to have symptoms while taking steroids, or if there is uncertainty about whether a lesion represents a progressive tumor versus a radiation effect.
Brachytherapy
Brachytherapy—that is, the insertion of radioactive sources directly inside a tumor or tumor bed—allows delivery of a high dose of radiation to the target with steep dose fall-off. Temporary brachytherapy has been applied to brain metastases by using high-activity sources, often within afterloading catheters inserted into a gross tumor or a resection cavity, or by using a balloon within a resection cavity inflated with radioactive liquid. Permanent brachytherapy is generally accomplished by lining a tumor bed with low-activity radiation sources intraoperatively after resection of a metastasis. Several groups have described outcomes using permanent brachytherapy.108–115 In patients treated for recurrent brain metastases, crude local freedom from progression ranged from 60% to 95% and median survival from 6 to 14.8 months. Among patients treated for newly diagnosed brain metastases, local freedom from progression ranged from 80% to 95% and median survival ranged from 8 to 17 months (except for an outlier median survival of 68.2 months in five patients). The incidence of symptomatic radiation necrosis ranged from 0 to 30%.
Systemic Therapy
Chemotherapy
Chemotherapy has generally been considered to be relatively ineffective for brain metastases, presumably because the blood-brain barrier prevents adequate access of chemotherapy to these tumors. However, some chemotherapeutic agents partially or even readily cross the blood-brain barrier; the fact that brain metastases enhance with contrast material is proof that the blood-brain barrier is broken down within metastases. The chemosensitivity of the primary tumor is another critical factor in determining the potential efficacy of chemotherapy for brain metastases.116,117 The responsiveness of brain metastases to chemotherapy is similar to that of the primary tumor and extracranial metastases. Primary SCLC, lymphoma, germ cell tumors, and breast cancer are relatively chemosensitive,118 whereas non–small cell lung carcinoma (NSCLC) and melanoma are less chemosensitive.
The role of primary systemic therapy in patients with brain metastases is generally limited to persons with a chemosensitive primary tumor who have good performance status and multiple, small, asymptomatic lesions.119 Although systemic treatment in conjunction with radiation therapy improves imaging response rates, it has not been shown to improve survival in patients with brain metastases.120 More commonly, systemic agents are used as salvage after more conventional treatment (e.g., surgery, SRS, and WBRT) have failed. A comprehensive review on the role of chemotherapy in brain metastases is beyond the scope of this chapter. The reader is directed to the selected references for trials assessing chemotherapy for brain metastases from a variety of primary sites,121–129 as well as specific primary sites including NSCLC,130–140 SCLC,141–146 melanoma,122,147–152 and breast cancer.121–123,125,126,153–159
Molecularly Targeted Therapy
Several molecularly targeted agents are being investigated for the treatment of brain metastases. These agents include antiangiogenic drugs, immunomodulatory agents, and inhibitors of epidermal growth factor receptor (EGFR), BRAF, Gamma-secretase/notch, histone deacetylases, poly(adenosine diphosphate-ribose) polymerase, mammalian target of rapamycin, and protein kinase C beta.160 The use of some of these agents as they relate to specific histologies is discussed in this section.
The antiangiogenic agent bevacizumab has been studied in the treatment of brain metastases from NSCLC. One group reported the safety and moderate efficacy in terms of imaging and clinical response in this setting.161 Also, sunitinib and sorafenib (which are antiangiogenic agents) have been used in patients with brain metastases from renal cell carcinoma, albeit with limited efficacy.162,163 The use of the small-molecule EGFR inhibitor erlotinib has also been used because patients with brain metastases from NSCLC may have activating mutations of EGFR. One group was able to demonstrate encouraging objective CNS radiographic responses by using erlotinib in patients with EGFR-mutant metastatic lung cancer.164
In patients with metastatic HER2-positive breast cancer to the brain, lapatinib (a small-molecule dual inhibitor of HER2 and EGFR) has been used with some efficacy, although it remains investigational.155,165 A newer immunomodulatory agent, ipilimumab, has been investigated in the management of brain metastases from melanoma. It functions as a negative regulator of the antitumor T-cell response by blocking cytotoxic T-lymphocyte antigen-4 and thus acts as a T-cell potentiator.119 The results of a phase 2 study investigating the use of ipilimumab as a single agent for patients with brain metastases from melanoma were recently reported and describe objective responses in some patients.166
Follow-up and Salvage Therapy
In patients who are doing reasonably well from the standpoint of their systemic disease, we recommend follow-up MRI every 3 months after treatment for brain metastases to detect new or recurrent brain metastases that may need to be addressed with salvage therapy. Of note, a contrast-enhancing lesion seen after high-dose external beam radiation, SRS, or brachytherapy could represent radiation necrosis rather than recurrent tumor, and additional analyses may be needed before considering retreatment.167 The same therapeutic options available for newly diagnosed brain metastases may be considered for new, progressive, or recurrent metastases, although the type of previous therapy given may influence therapeutic options at recurrence. In general, retreatment options include WBRT, SRS, surgery (especially for a single large or symptomatic lesion), perhaps with permanent brachytherapy to help prevent local recurrence, and chemotherapy. If repeat WBRT is given, lower doses and smaller fraction sizes are generally used, such as 20 to 25 Gy in 10 fractions or 30 Gy at 1.0 Gy twice daily. Results are similar to those reported for a first course of WBRT, with symptomatic improvement in 42% to 75% of patients and median or mean survival of 3.2 to 5 months after reirradiation.168–171
Neoplastic Meningitis
Epidemiology
Virtually any type of cancer can disseminate into the leptomeningeal space. Acute leukemias and intermediate or high-grade lymphomas are common causes of neoplastic meningitis. Among solid tumors, melanoma and small cell carcinoma exhibit the strongest propensity for leptomeningeal dissemination, with this complication developing in up to 25% of patients with metastatic disease from these diagnoses. Ultimately, carcinomatous meningitis develops in between 2% to 5% of patients with breast cancer. Leptomeningeal dissemination has also been documented in less common neoplasms such as sarcomas, squamous cell carcinomas, and germ cell tumors. Primary tumors of the central nervous system such as medulloblastoma or ependymoma often seed the leptomeningeal space as well.172–176
Pathophysiology
The biological basis for dissemination and multifocal seeding of the leptomeninges by malignant cells is not well understood. Several distinct pathways have been proposed: (1) The most obvious pathway for leptomeningeal contamination is as a consequence of drop metastases that may occur during resection of metastatic foci within the brain, especially after resection of posterior fossa tumors; (2) direct extension from the cerebrum; (3) infiltration through arachnoid vessels or the choroid plexus after hematogenous dissemination of the tumor; (4) direct extension along peripheral nerves to the subarachnoid space or perivenous spread from the bone marrow within the skull; (5) extension along perineural or perivascular lymphatics; and (6) subependymal or choroid plexus metastases with subsequent escape into the CSF.177 Spread of tumor cells along the meningeal surface is facilitated by bulk CSF flow. In turn, meningeal deposits may also invade parenchyma, as well as cranial or spinal nerve roots. The most frequently affected regions of the CNS are the basilar cisterns, the posterior fossa, and the cauda equina, where gravity promotes deposition of circulating cells.
Clinical Presentation
Leptomeningeal dissemination of cancer elicits several distinct neurologic presentations. First, local tumor infiltration in the brain or spinal cord may cause headache, alterations in mental status, cranial nerve deficits causing diplopia, hearing loss, decreased taste, and problems with swallowing and hoarseness, as well as incontinence, lower motor neuron weakness, and back or radicular pain. Second, metabolic dysfunction caused by disturbances in regional blood flow in the affected nervous tissue may cause seizures, isolated neurologic deficits, including strokelike symptoms, and even generalized encephalopathy. Third, obstruction of normal CSF flow pathways by focal tumor deposits may cause increased intracranial pressure and hydrocephalus.172–176,178
Diagnosis
CSF Evaluation
A high index of suspicion is required to make an early diagnosis of neoplastic meningitis; a detailed examination of CSF ultimately is required. Routine measurements include opening pressure, cell count, differential, protein, glucose, and cytologic evaluation of the CSF.172 Patients with carcinomatous meningitis typically have elevated CSF opening pressure, increased CSF protein, and decreased CSF glucose.179 Although the CSF is abnormal in terms of protein or glucose concentration in most patients with neoplastic meningitis, cytologic evaluation of the CSF, the gold standard, is an insensitive test; 40% to 50% of patients with neoplastic meningitis have negative CSF cytology on initial lumbar puncture.172 Although repeat CSF cytological evaluations over time will increase diagnostic sensitivity, the eventual conversion to positive cytology usually occurs in pace with neurologic deterioration as a result of overt tumor progression. Because of the importance of early diagnosis and intervention, there has been significant effort to identify surrogate biomarkers for CNS and leptomeningeal metastases. For example, Posner’s group demonstrated that carcinoembryonic antigen, as well as the enzymatic activity of beta-glucuronidase, could be detected in the CSF in patients with brain and leptomeningeal metastases.180 The presence of these markers was shown to precede clinical detection of neoplastic meningitis and to rise and fall in parallel with the clinical course. Several other biomarkers have been examined in patients with carcinomatous meningitis, including lactate dehydrogenase, beta-human chorionic gonadotropin, alkaline phosphatase, vascular endothelial growth factor, myelin basic protein, creatinine kinase, and others. The utility of these biomarkers is still under investigation.179 The use of other diagnostic tools such as polymerase chain reactions, fluorescence in-situ hybridization, immunohistochemical examinations of the CSF, and cytogenetic analysis may also enhance detection.181
Radiologic Features
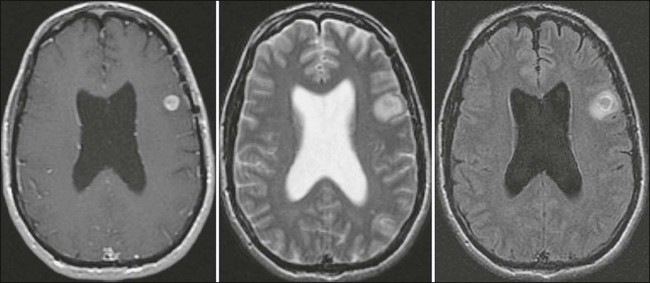
Generally, an MRI of the entire neuraxis is advisable. The most frequent radiographic presentation is hydrocephalus without an identifiable mass lesion. Leptomeningeal contrast enhancement is suggestive of neoplastic meningitis (Fig. 50-4) but may also be seen after lumbar puncture or with infection, inflammatory disease, trauma, subdural hematoma, or changes occurring after a craniotomy.172 The enhancement pattern may be focal (including tumor nodules) or diffuse.181 Although gadolinium-enhanced MRI is more sensitive than CT in identifying leptomeningeal enhancement, only approximately 50% of patients with neoplastic meningitis and spinal symptoms have abnormal imaging studies.172
Treatment
Radiation Therapy
Radiation therapy is the most effective means of palliation, with the focus on symptomatic sites and regions where imaging studies have demonstrated bulk disease. Craniospinal axis irradiation results in substantial acute toxicity, with nausea, vomiting, marked fatigue, and myelosuppression. A negative impact on bone marrow function of long duration also occurs, compromising the safe administration of subsequent myelosuppressive chemotherapy. One strategy is to selectively apply external beam irradiation to symptomatic sites of disease and to rely on intrathecal chemotherapy to suppress the remainder of the disease in the neuraxis. For example, in patients presenting with cranial nerve deficits, one approach is to treat only the base of the skull with radiation. In patients who present with cauda equina syndrome, external beam irradiation may be directed to the lumbosacral spine. The best palliation for patients who present with seizure or hydrocephalus caused by extensive cranial leptomeningeal disease may be achieved with whole-brain irradiation.173–176
Intrathecal Chemotherapy
The most reliable means of administering intrathecal chemotherapy is to use an implanted subcutaneous reservoir and ventricular catheter (Ommaya device). Although subarachnoid injections of chemotherapy result in high local CSF concentrations, studies of lumbar administration suggest that 10% to 15% of lumbar punctures fail to completely deliver all of the drug to the subarachnoid space.176 In addition, retrospective analysis suggests that intraventricular administration may result in prolonged remission in patients with leptomeningeal leukemia compared with administration by lumbar puncture.182 Chemotherapeutic agents administered into the ventricle are carried through the neuraxis by bulk CSF flow. CSF flow abnormalities are common in patients with leptomeningeal metastases, who frequently present with hydrocephalus and increased intracranial pressure as a result of disease that impedes CSF flow. Radionuclide ventriculography in patients with neoplastic meningitis has demonstrated that as many as 70% have ventricular outlet obstruction, abnormal flow in the spinal canal, or impaired flow over the CSF convexities.183 These CSF flow abnormalities may be reversed with local irradiation. Because of the potential risk of irreversible neurotoxicity from high sustained concentrations of intrathecal chemotherapy, a CSF flow study is recommended for every patient beginning intrathecal chemotherapy via a ventricular catheter.173,174
Cytosine arabinoside (Ara-C) is also commonly used but may have less efficacy in the treatment of neoplastic meningitis. Ara-C is inactivated by deamination by the enzyme cytidine deaminase. Low CNS levels of this enzyme result in relatively slow deamination of Ara-C within the brain and CSF, resulting in an extended half-life in the CNS compartment.176 Thiotepa, a cell cycle nonspecific alkylating agent, is another commonly used intrathecal drug.181 Other intrathecal agents that have been studied in this context include mafosphamide,184 topotecan,185 etoposide,186 interferon alpha,187 and 5-fluoro-2′ deoxyuridine.188 Further investigation of these agents is warranted.
Treatment-Related Toxicity
Placement of an intraventricular catheter is associated with <1% risk of perioperative hemorrhage. Extended use of the device is associated with a ≥5% risk of infection, usually with Staphylococcus epidermidis or Staphylococcus aureus. Impaired CSF flow of chemotherapy as a result of obstruction may result in seizures, as well as acute arachnoiditis, characterized by nausea, vomiting, and mental status changes. For this reason many practitioners obtain a radionuclide CSF flow study before initiation of intra-Ommaya chemotherapy. Ultimately the most significant toxicity associated with the treatment of leptomeningeal carcinomatosis is the development of necrotizing leukoencephalopathy, which is most common in patients who have been treated with intrathecal methotrexate after cranial irradiation. Initial findings are imaging changes, usually symmetric abnormalities in white matter. Many of these patients subsequently experience progressive dementia that can progress to substantial debility and death.176
Systemic Chemotherapy and New Approaches
A significant fraction of contrast-enhancing tumor visualized on neuroimaging studies is theoretically accessible by systemic chemotherapy that is able to reach this fraction of tumor supplied by an abnormally permeable neovasculature. However, water-soluble chemotherapy drugs are limited by the intact blood-brain barrier and systemic therapy fails to treat microscopic, nonenhancing disease both in brain parenchyma and in the subarachnoid space. One exception is the use of high-dose systemic administration of methotrexate that results in therapeutic levels in the CSF for a longer duration than the intrathecal route. This therapeutic strategy has been shown to be active in persons with neoplastic meningitis in both lymphoma and in solid tumors. Moreover, systemic administration of methotrexate at high doses overcomes the problems associated with CSF flow obstruction that may compromise subarachnoid administration. However, because administration of high-dose methotrexate requires detailed inpatient monitoring of fluid status, urine alkalinization, and renal function, systemic administration of methotrexate at high doses is not appropriate or practical for all patients.189
Finally, in the current era of targeted therapeutics, there is increasing interest in the use of biological therapies for leptomeningeal disease, particularly small molecule protein kinase inhibitors or monoclonal antibodies against tumor-associated cell surface molecules. An increasing amount of evidence indicates that when these agents are administered systemically they penetrate the leptomeningeal space inefficiently. For example, relatively low CSF levels of monoclonal antibodies that target CD20 in B-cell lymphomas or of small molecules that inhibit the bcr-abl tyrosine kinase have been documented after systemic administration.190,191 Direct intra-CSF administration of monoclonal antibodies and immunotoxins is an area of current early-phase investigation in the treatment and/or prophylaxis of neoplastic meningitis.190,192 A phase 1 dose escalation study formally evaluated the safety and efficacy of intrathecal administration of an anti-CD20 antibody in 10 patients with recurrent CNS and intraocular lymphoma.193 Responses were identified in patients with meningeal, parenchymal, and intraocular disease. Another group reported promising results of targeted iodine-131–radiolabeled monoclonal antibodies administered to 52 patients with neoplastic meningitis.194 Some groups have also documented responses to trastuzumab, a humanized antibody directed to HER2, in patients with leptomeningeal carcinomatosis from HER2-overexpressing primary breast cancer.197–197