Bladder Cancer
Angela Smith, Arjun V. Balar, Matthew I. Milowsky and Ronald C. Chen
• Worldwide, bladder cancer is diagnosed in approximately 380,300 cases each year and causes 150,200 deaths. In the United States, it is the sixth most common cancer, with an estimated 73,510 diagnoses and 13,750 deaths in 2012.
• Median age at diagnosis is 73 to 74 years. Many patients have comorbid illnesses such as cardiovascular disease at diagnosis.
• Cigarette smoking is the most significant risk factor, accounting for approximately 50% of bladder cancers in the United States. Occupational chemical exposure to polycyclic aromatic hydrocarbons and aromatic amines represents the next most important risk factor.
• Urothelial cancer (previously called transitional cell carcinoma) is the most common histologic type in the United States and Europe.
• Low-grade noninvasive versus high-grade bladder cancers have distinct molecular pathways and signatures.
• There is no clear role for screening.
• The primary manifesting symptom is painless gross hematuria. All patients with unexplained gross hematuria require evaluation.
• Cystoscopy with transurethral resection and urine cytology are the mainstay of diagnosis. Photodetection during cystoscopy is increasingly used to improve sensitivity of detecting bladder tumors.
• Upper tract evaluation is necessary to detect additional urothelial tumors and obstruction.
• Patients with muscle-invasive bladder cancer (MIBC) require metastatic workup.
• Transurethral resection of bladder tumor (TURBT) is the initial procedure and used to determine the clinical stage that drives subsequent treatment approaches.
• For patients with noninvasive bladder cancer (Ta, Tis, or T1), a complete TURBT may be sufficient. The addition of intravesical therapy reduces the risks of recurrence and progression to muscle-invasive cancer.
• Bacillus Calmette-Guerin (BCG) is the most effective agent for intravesical therapy in patients with high-grade noninvasive disease. An induction course of 6-weekly treatments, followed by maintenance therapy every 6 months for 2 to 3 years, may be used.
• For patients with MIBC, radical cystectomy with urinary diversion is the most commonly used treatment approach in the United States. However, there is significant undertreatment of elderly patients with MIBC likely because of concerns about tolerability of cystectomy.
• Trimodality bladder preservation therapy (TURBT followed by concurrent chemoradiation) is a well-tolerated and effective alternative for patients with MIBC, including elderly patients. Overall, 75% to 80% of patients maintain their native bladders long-term.
• Effective radiosensitizing chemotherapy agents include cisplatin-based regimens or 5-fluorouracil (5-FU) with mitomycin C.
Neoadjuvant and Adjuvant Therapy
• Despite aggressive local treatment, up to 50% of MIBC patients eventually develop local or distant recurrences.
• Neoadjuvant cisplatin-based chemotherapy prior to cystectomy provides a 5% to 10% absolute benefit in overall survival over cystectomy alone.
• Data on the potential benefit of adjuvant chemotherapy are conflicting, and treatment decisions should be individualized.
• Patients with pathological T3–T4 cancers and those with positive surgical margins are especially likely to develop local recurrences after cystectomy. Adjuvant radiation therapy may be considered for these patients.
• Cisplatin-based combination therapy is the standard treatment for patients with advanced bladder cancer. Gemcitabine/cisplatin (GC) or methotrexate/vinblastine/Adriamycin/cisplatin (MVAC) have similar efficacy, but the latter regimen is more toxic.
• Short-course radiation therapy can achieve a significant palliative benefit.
Epidemiology
Worldwide in 2008, 386,300 new cases of bladder cancer were diagnosed and 150,200 deaths occurred.1 The majority of bladder cancers occur in males; bladder cancer is the 7th most common cancer in men and 17th most common cancer in women. The highest incidence rates are found in Europe, North America, and Northern Africa.1 Worldwide, the cumulative risk of developing bladder cancer and dying from it by age 75 are 1.9% and 0.5%, respectively, in more developed areas.1 In less developed areas, the risks are 0.6% and 0.3%, respectively.
In the United States, bladder cancer is the sixth most common cancer. In 2012, there were 73,510 diagnoses and 14,880 deaths.2 The most common histologic type in the United States and Europe is urothelial carcinoma (previously called transitional cell carcinoma). Bladder cancer disproportionally affects the elderly, with median age of diagnosis 73 years for men, and 74 years for women.3 It is a smoking-related disease, and many patients diagnosed with bladder cancer have comorbid illnesses such as cardiovascular disease.4,5 For all disease stages combined, five-year survival for bladder cancer has increased in the United States from 1975 to 2007.2
Etiological and Biological Characteristics
Etiology
Tobacco use is the most significant risk factor for bladder cancer as well as many of the accompanying comorbid illnesses including cardiovascular disease, peripheral vascular disease, and pulmonary disease that make the management of patients with bladder cancer particularly challenging. In a recent evaluation of the association between tobacco smoking and bladder cancer including men and women in the National Institutes of Health-AARP (NIH-AARP) Diet and Health Study cohort, former smokers (119.8 per 100,000 person-years; HR 2.22; 95% CI, 2.03 to 2.44) and current smokers (177.3 per 100,000 person-years; HR 4.06; 95% CI, 3.66 to 4.50) had higher risks of bladder cancer than never smokers (39.8 per 100,000 person-years).6 These results were higher than a pooled estimate of U.S. data from cohorts initiated between 1963 and 1987 (HR 2.94; 95% CI, 2.45 to 3.5). The population attributable risk for ever smoking in the NIH-AARP study was 0.50 (95% CI, 0.45 to 0.54) in men and 0.52 (95% CI, 0.45 to 0.59) in women. Additional data from the New England Bladder Cancer Study, a population-based case–control study, supports a strengthening association between smoking and bladder cancer in spite of the decreased prevalence of bladder cancer in the U.S. population.7 Changes in cigarette composition including higher concentrations of certain carcinogens such as β-naphthylamine and specific nitrosamines may be responsible for this increased association of smoking with bladder cancer risk. For equivalent total pack-years of cigarettes smoked, smoking fewer cigarettes over a longer time appears more harmful than smoking more cigarettes over a shorter time.7 Inherited polymorphisms in the carcinogen detoxification gene NAT2 is associated with smoking and bladder cancer risk. Specifically, aromatic amines, the major carcinogen in tobacco smoke are detoxified by NAT2; NAT2 slow acetylators as compared to rapid acetylators have an increased relative risk from smoking for the development of bladder cancer. Recent data support the contention that the NAT2 slow acetylator phenotype interacts with smoking as a function of exposure intensity (i.e., at least 40 cigarettes per day).8 Continued tobacco use in patients with bladder cancer is associated with worse disease-associated outcomes than patients who quit smoking.9
Occupational exposures to polycyclic aromatic hydrocarbons and aromatic amines represent the next most important risk factor for bladder cancer after smoking.10 Occupational risk has generally been associated with exposure to paint, dyes, metals and petroleum products. Specific occupations include dyestuffs workers, painters, leather workers, truck drivers, aluminum workers and workers in the dry cleaning industry. In a recent population-based survey examining exposure to aromatic amines, diesel engine exhaust, hairdressers/barbers, mineral oils, polycyclic aromatic hydrocarbons, and paint, the occupational attribution for men to bladder cancer was 7.1% with no clear attribution seen for women.11 Environmental exposure to arsenic in drinking water has been associated with an increased risk of bladder cancer. In a well-studied arsenic exposed area in Northern Chile, a long-term impact of arsenic exposure was observed 20 years after the end of exposure with an increase in bladder cancer mortality as compared with the rest of the country (HR 3.6; 95% CI, 3.0 to 4.7).12
Several medications have been associated with an increased risk of bladder cancer, including phenacetin, cyclophosphamide, and pioglitazone. In a population-based case control study evaluating phenacetin and other analgesic and nonsteroidal anti-inflammatory drugs (NSAIDs), an elevated odds ratio (OR) was seen with phenacetin-containing medications used for a longer duration (>8 years OR = 3.00, 95% CI, 1.4 to 6.5).13 Medical use of the alkylating agent cyclophosphamide for indications including cancer and rheumatologic disease has been associated with an increase in risk for bladder cancer. In a Danish study of patients with Wegener granulomatosis treated with cyclophosphamide, the standardized incidence ratio (SIR) for bladder cancer was significantly increased (SIR 3.6, 95% CI 1.2 to 8.3).14 The association was seen with high cumulative cyclophosphamide doses, and malignancies occurred 6.9 to 18.5 years after initiation of cyclophosphamide. Most recently, pioglitazone, a thiazolidinedione, has been demonstrated to increase the risk of incident bladder cancer in individuals with type 2 diabetes. In a retrospective cohort study of people with type 2 diabetes who were newly treated with oral hypoglycemic agents, ever use of pioglitazone was associated with an increased rate of bladder cancer (rate ratio 1.83, 95% CI, 1.10 to 3.05), with the rate increasing with duration of use and with a higher cumulative dose.15 Ionizing radiation has also been associated with the development of bladder cancer. An increased risk has been seen after radiation for survivors of germ cell testicular cancer.16
Chronic irritation of the bladder is associated with bladder cancer. Infection with Schistosoma haematobium, a trematode that is prevalent in certain parts of Africa and the Middle East, is associated with an increased risk of both squamous and urothelial carcinomas of the bladder. Spinal cord injury patients are at an increased risk for the development of squamous and urothelial carcinomas of the bladder. Squamous cell carcinoma is more common in patients with chronic indwelling catheters; the neurogenic bladder itself may also pose an increased risk.17,18
Genome-wide association studies have identified multiple susceptibility loci associated with bladder cancer risk.19 One such susceptibility locus within SLC14A1, a urea transporter gene on chromosome 18q12.3, is involved in regulation of cellular osmotic pressure.20 Hereditary nonpolyposis colorectal cancer (HNPCC) is an autosomal dominant inherited disorder caused by germline mutations in DNA mismatch repair genes including MLH1, MSH2 and MSH6. In addition to a high risk for colon and endometrial cancer, other less common cancers seen in mutation carriers include urothelial cancers of the bladder and upper urinary tract.21
Molecular Biology
Divergent molecular pathways in urothelial tumorigenesis lead to the distinct clinical phenotypes of low-grade noninvasive disease and high-grade invasive disease.22 Low-grade noninvasive tumors account for 80% of urothelial carcinomas and although these tumors are often multifocal and recurrent, progression to muscle-invasive disease is uncommon. In contrast, invasive tumors often develop in the absence of a preexisting lesion or in the setting of high-grade carcinoma in situ (CIS) and are associated with a lethal phenotype, with greater than 50% of patients developing metastases and dying from urothelial cancer despite aggressive therapy.
Deletion of chromosome 9 is one of the earliest events in urothelial tumorigenesis. Low-grade noninvasive papillary tumors are characterized by mutations in the HRAS gene and fibroblast growth factor receptor 3 gene (FGFR3) indicating that receptor tyrosine kinase-Ras activation has an early and major role in bladder cancer tumorigenesis. HRAS was the first human oncogene identified in urothelial carcinomas, with mutations most commonly seen in codons 12, 13, and 61 leading to constitutive activation of the HRAS protein with transformation of the NIH-3T3 cell line.23 Recent studies indicate that HRAS mutations occur in 10% to 20% of urothelial cancers. However, there is still continued controversy regarding the true HRAS mutation frequency, with the need for studies using sensitive detection assays.24 Activation of Ras signaling also occurs as a result of overexpression of HRAS in the urothelium, which occurs in more than 50% of urothelial cancers.22 In addition to HRAS mutations and protein overexpression, constitutive activation of several receptor tyrosine kinases occurring upstream of RAS leads to activation of Ras signaling. Most notable in bladder cancer are mutations in the FGFR3 gene.25 In the case of bladder cancer, FGFR3 mutations are found predominantly in low-stage/grade disease with a frequency of 50% to 67%; however, these mutations also occur in 16% of invasive tumors.28–28 There are conflicting data with regard to the prognostic value of FGFR3 mutation status.28,29 Mutations in phosphatidylinositol 3-kinase, catalytic, alpha polypeptide (PIK3CA), also represent a common event in early bladder carcinogenesis and have been associated with FGFR3 mutations in noninvasive papillary bladder tumors.30
High-grade invasive tumors are characterized by defects in the p53 and retinoblastoma protein (RB) tumor suppressor genes. p53 nuclear overexpression independently predicts for progression and decreased survival.31 In a series of 80 patients with bladder cancer who underwent radical cystectomy and pelvic lymph node dissection, immunohistochemical expression for p53, p21, pRB, and p16 demonstrated that altered expression of each of these cell cycle regulators was associated with bladder cancer outcome, with p53 as the strongest predictor, followed by p21—suggesting a pivotal role of the p53/p21 pathway in bladder cancer progression.32 A phase III study of molecularly targeted therapy in locally advanced urothelial cancer of the bladder based on p53 status failed to confirm the prognostic or the predictive value for chemotherapy in p53-positive tumors.33 A recent report described frequent mutations in a variety of chromatin remodeling genes including UTX, MLL-MLL3, CREBBP-EP300, NCOR1, ARID1a, and CHD6 in urothelial cancer.34 Advances in genomics have allowed for the identification of novel tumor subgroups of urothelial carcinoma with the promise of a better understanding of tumor biology with the potential for the identification of novel therapeutic targets.35 A recent example involves the use of whole-genome sequencing to investigate a long-term durable response in a patient with metastatic bladder cancer treated with the mammalian target of rapamycin (mTOR) pathway inhibitor, everolimus, demonstrating a loss of function mutation in TSC1 (tuberous sclerosis complex 1), a regulator of mTOR pathway activation.36
Prevention and Early Detection
Urothelial bladder cancer represents a common malignancy with significant public health implications. Primary prevention programs seek to target those exposures most closely linked to bladder cancer etiology. This includes smoking, followed by occupational exposure to aromatic amines and polycyclic hydrocarbons. These exposures account for approximately 70% of all cases, and primary prevention programs targeting these risks may have the largest public health impact.10 Smoking cessation counseling by both bladder cancer specialists and general practitioners is important. Other primary prevention programs target occupational exposures in industrial settings. Safety measures have been implemented to aid in prevention of disease related to occupational exposures, but recent population-based surveys still describe occupational attribution at approximately 7%.10 Industrial companies that use dyes, chemicals, and rubber products should clearly explain the risks of prolonged exposure to their workers. Workers should also be tested periodically with at least cytology and urine examination as well as wear protective masks during working hours.37
Early detection complements primary prevention as another important strategy to reduce bladder cancer mortality in the population. Although screening has been suggested to aid early diagnosis, screening programs have been widely criticized as ineffective and largely disregarded. As a prerequisite for all screening tests, the World Health Organization requires the availability of a valid, reliable, and inexpensive test with reasonable sensitivity and specificity and high positive predictive value. In a 2010 update of the 2004 U.S. Preventive Services Task Force (USPSTF) evidence review, no study was found that adequately evaluated the sensitivity or specificity of hematuria tests, urinary cytology, or urinary biomarkers in asymptomatic individuals.38
However, three observational studies were included in the UPSTSF review that did demonstrate an association between screening and decreased risk of bladder cancer mortality and/or lower stage at diagnosis.41–41 Unfortunately, these studies were difficult to interpret because of significant methodologic shortcomings. No randomized trials or high-quality controlled observational studies were found that evaluated clinical outcomes associated with bladder cancer screening including the harms associated with treatment for screen-detected bladder cancer compared to no treatment. These findings, along with new epidemiological data suggesting the possible benefits of screening, led to the UPSTF’s Level “I” statement, concluding that the current evidence is insufficient to assess the benefits and harms of screening for bladder cancer in asymptomatic adults.38 Before screening can be widely accepted as a public health measure, several research gaps require attention, including a need to evaluate the natural history of early-stage, untreated bladder cancer as well as the diagnostic accuracy of urine screening tests, including newer urine-based tests such as UroVysion (fluorescence in situ hybridization [FISH]), ImmunoCyt, and nuclear matrix protein (NMP)-22 in representative populations.
Pathology and Natural History
Bladder urothelium is lined by transitional cells that can transform into a variety of malignant tumors. In the United States and Europe, the significant majority of bladder cancers demonstrate urothelial origin followed by less common histologic types such as sarcoma, signet ring cell carcinoma, squamous cell carcinomas, small cell carcinoma, and adenocarcinomas.42 The latter nonurothelial malignancies are rare and often manifest as high-grade, high-stage tumors with poor prognosis. As urothelial cancer represents the vast majority of bladder cancer pathology, this section will focus on its histologic appearance, classification, and pathways of spread.
Understanding the pathology of urothelial carcinoma is critical in determining prognosis, as the most important risk factor for progression is tumor grade rather than stage.43 The 2004 World Health Organization (WHO) classification of bladder tumors represents the standard classification system used clinically (Table 83-1).44 The “low grade” versus “high grade” distinction replaced the older system (grades 1 to 3)—eliminating grade 2 cancers that were often the source of significant interobserver variation. Furthermore, papillary Ta grade 1 cancers are now described as benign because of indolent growth rates.
Table 83-1
2004 World Health Organization Classification of Noninvasive and Invasive Urothelial Neoplasia
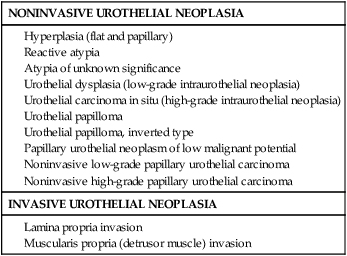
Adapted from Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. Int J Surg Pathol 2005;13:143-153.
The prognoses of several types of noninvasive tumors are summarized in Table 83-2. Of noninvasive tumors, the least aggressive is papillary urothelial neoplasm of low-malignant potential. PUNLMP is in a distinct category from papillomas because of the higher recurrence rates noted with the former.44 Similar to a benign papilloma, PUNLMP shows minimal cytologic atypia but has the addition of a thicker cell layer and larger nuclei with occasional mitotic figures as shown in Figure 83-1. Progression to invasive or metastatic disease is rare, but recurrence after resection can be seen in up to one-third of patients.46–46 Despite the essentially benign appearance of PUNLMP, the WHO recommends that pathology reports contain the statement “Patients with these tumors are at risk of developing new bladder tumors (‘recurrence’), usually with similar histology. However, occasionally, these subsequent lesions manifest as urothelial carcinoma such that follow-up of the patient is warranted.”47
Table 83-2
Prognoses of Urothelial Papillary Lesions
| Papilloma | Papillary Neoplasm of Low Malignant Potential | Low-Grade Papillary Carcinoma | High-Grade Papillary Carcinoma | |
| Recurrence | 0%-8% | 27%-47% | 48%-71% | 55%-58% |
| Grade Progression | 2% | 11% | 7% | Not applicable |
| Stage Progression | 0% | 0%-4% | 2%-12% | 15%-40% |
| Survival | 100% | 93%-100% | 82%-96% | 74%-90% |

Adapted from Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. Int J Surg Pathol 2005;13:143-153.
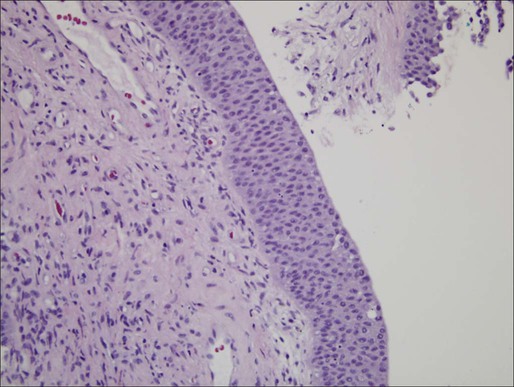
Distinct from the benign clinical course associated with PUNLMP, CIS is characterized by higher rates of progression and is a flat, high-grade tumor within the superficial epithelium.44 Morphologic diagnosis requires the presence of severe nuclear anaplasia, loss of cellular features, and a noncohesive structure as demonstrated in Figure 83-2. Tumor cells tend to be large with moderate to abundant cytoplasm and coarse, clumped chromatin. By definition, CIS represents high-grade disease and is associated with genetic alterations that are similar to those found in other high-grade urothelial subtypes. Abnormal expression of cytokeratin 20, presence of the NMP-22, and abnormal expression of p53 and RB proteins may correlate with progression and response to Bacillus Calmette-Guerin (BCG) therapy.48
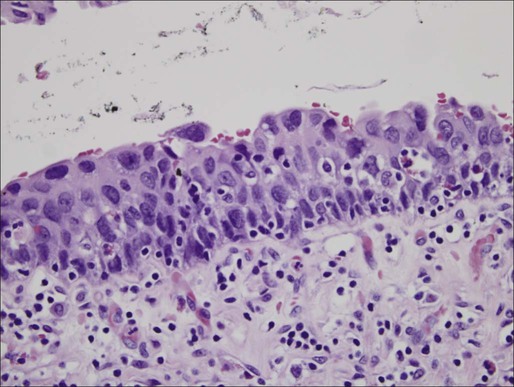
CIS can be distinguished from other high-grade tumors by its cystoscopic appearance. CIS is characterized by a reddish, velvety appearance and can often be mistaken for the edematous changes of radiation cystitis. Despite its unassuming name, CIS can progress to invasive disease and also demonstrate pagetoid spread to the ureters and prostatic urethra in some cases.49
Distinct from the high-grade classification of CIS, low-grade papillary urothelial carcinoma demonstrates less nuclear atypia with a fibrovascular stalk and papillary branching (Figure 83-3).47 Genetic abnormalities are different from high-grade lesions and most commonly include deletion of 9q.50 These tumors are also immunoreactive for cytokeratin-20 and CD-44, but a spectrum of cytologic and architectural abnormalities may exist within a single lesion lending importance to examination of the entire specimen and noting the highest grade of abnormality.51 Progression is rare with these tumors, and most exhibit a solitary lesion.51
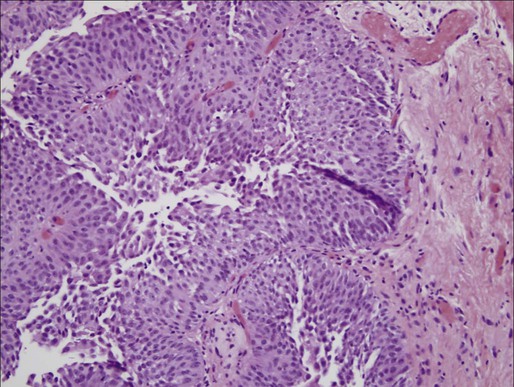
Higher progression and mortality rates are noted with high-grade disease, believed to be secondary to genetic abnormalities distinct from low-grade lesions, which confer the ability to invade the underlying stroma. High-grade tumors are composed of fused papillary stalks with undifferentiated cancer in the urothelial layer as illustrated in Figure 83-4A. Numerous mitotic figures are present along with a disordered growth pattern and pleomorphic cells with abnormal nuclei. If untreated, more than 80% of high-grade tumors will invade the underlying stroma. Gross specimens can demonstrate invasive disease into the perivesical fat and/or ureter in some cases (Figure 83-5).
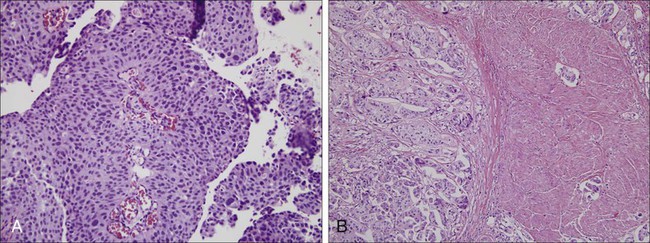
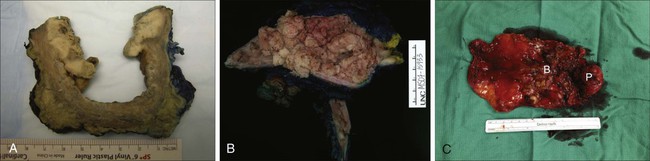
The urologic provider should be aware of several less common high-grade histologic variants, many of which denote increased risk for understaging. Two of the more common variants include micropapillary and nested variants, both of which portend relatively poor prognoses.52 The micropapillary variant consists of infiltrating papillary clusters of tumor cells that lack central vascular cores and are surrounded by retraction artifact that may mimic vascular invasion (Figure 83-4B). Pathologists should report the percentage of micropapillary component, as this is inversely related to survival.52 More than 95% of these tumors are muscle invasive at diagnosis, and lymph node metastases are present in more than one-third of patients.53,54 Upstaging at cystectomy can occur in 50% to 80% of patients with micropapillary features.
The “nested variant” of urothelial carcinoma is underdiagnosed as it has deceptively benign-appearing superficial nests without major atypia, commonly confused with benign von Brunn nests. However, it has been shown to correlate with upstaging, much more than that of high-grade urothelial cancer. Compared with standard high-grade histology, the nested variant more frequently demonstrates muscle-invasive disease on transurethral resection (70% vs. 31%), extravesical disease (83% vs. 33%), and metastatic disease (67% vs. 19%).55
Staging Classification
Tumor, nodes, and metastases (TNM) staging classification is determined by the American Joint Commission on Cancer (AJCC) in collaboration with the Union for International Cancer Control (UICC). The most recent AJCC staging system describes Ta disease as noninvasive papillary, whereas T1 and T2 represent invasion into the subepithelial connective tissue and muscularis propria, respectively (Table 83-3).56 Muscle-invasive disease is subdivided into T2a and T2b, which represent invasion into the inner and outer halves of the muscularis propria, respectively. Although this is used in official pathological AJCC staging, there is no discrete evidence that depth of muscle invasion predicts overall or disease-free survival.57
Table 83-3
American Joint Committee on Cancer (AJCC) TNM Staging System for Bladder Cancer (7th ed., 2010)
| PRIMARY TUMOR (T) | |||
| TX | Primary tumor cannot be assessed | ||
| T0 | No evidence of primary tumor | ||
| Ta | Noninvasive papillary carcinoma | ||
| Tis | Carcinoma in situ: “flat tumor” | ||
| T1 | Tumor invades subepithelial connective tissue | ||
| T2 | Tumor invades muscular propria | ||
| pT2a | Tumor invades superficial muscularis propria (inner half) | ||
| pT2b | Tumor invades deep muscularis propria (outer half) | ||
| T3 | Tumor invades perivesical tissue | ||
| pT3a | Microscopically | ||
| pT3b | Macroscopically (extravesical mass) | ||
| T4 | Tumor invades any of the following: prostatic stroma, seminal vesicles, uterus, vagina, pelvic wall, abdominal wall | ||
| T4a | Tumor invades prostatic stroma, uterus, vagina | ||
| T4b | Tumor invades pelvic wall, abdominal wall | ||
| REGIONAL LYMPH NODES (N) | |||
| Regional lymph nodes include both primary and secondary drainage regions. All other nodes above the aortic bifurcation are considered distant lymph nodes. | |||
| NX | Lymph nodes cannot be assessed | ||
| N0 | No lymph node metastasis | ||
| N1 | Single regional lymph node metastasis in the true pelvis (hypogastric, obturator, external iliac, or presacral lymph node) | ||
| N2 | Multiple regional lymph node metastasis in the true pelvis (hypogastric, obturator, external iliac, or presacral lymph node metastasis) | ||
| N3 | Lymph node metastasis to the common iliac lymph nodes | ||
| DISTANT METASTASIS (M) | |||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| ANATOMIC STAGE/PROGNOSTIC GROUPS | |||
| Stage 0a | Ta | N0 | M0 |
| Stage 0is | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage II | T2a | N0 | M0 |
| T2b | N0 | M0 | |
| Stage III | T3a | N0 | M0 |
| T3b | N0 | M0 | |
| T4a | N0 | M0 | |
| Stage IV | T4b | N0 | M0 |
| Any T | N1-3 | M0 | |
| Any T | Any N | M1 | |
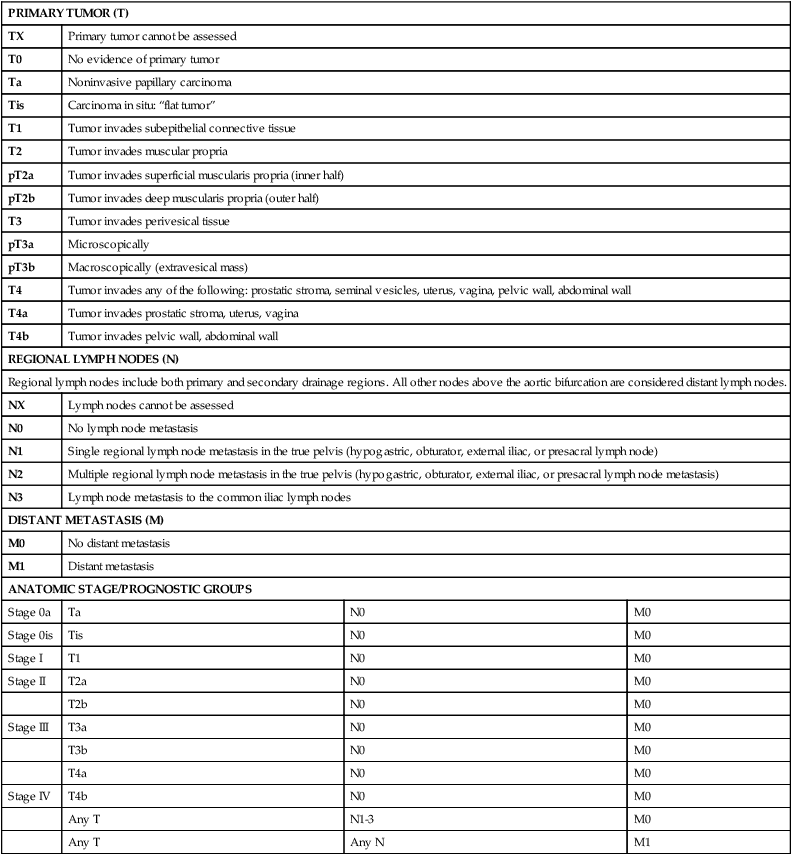
From Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010.
Clinical staging follows a similar scheme, but understaging is common. Approximately 27% of T1 tumors are upstaged after radical cystectomy, and some series have reported that more than 50% of T2 tumors are upstaged to T3.58 Further, in two large studies, 19% to 35% of patients with clinically node-negative muscle-invasive bladder cancer were found to have positive nodes on cystectomy pathology.59,60 High rates of upstaging have also been shown after repeat transurethral resection. Of those patients with T1 disease on the first resection, 30% were found to have T2 cancer: 15% if muscle was present in the original specimen, but up to 40% if no muscle was present. These high rates of upstaging have led to the American Urological Association guideline recommendation for repeat transurethral resection in patients with T1 disease, even if muscularis propria is present in the resection specimen and uninvolved.61
Clinical Manifestations
Bladder cancer is most often associated with the classic symptom of gross, painless hematuria, occurring in approximately 85% of patients, with the remainder exhibiting microhematuria.62 Patients presenting with gross hematuria should undergo evaluation for bladder cancer with particular attention to the characterization of hematuria as initial, terminal, or total to better localize the source. Terminal hematuria is more likely to represent a bladder source of the bleeding. Hematuria can be intermittent, and thus a subsequent negative urinalysis does not rule out the presence of bladder cancer. A negative urinalysis and lack of subsequent workup for patients who present with hematuria can lead to delayed diagnosis and may partially explain worse outcomes and advanced stages in women, who may misinterpret hematuria as a gynecologic source.
Of those without gross hematuria, asymptomatic microscopic hematuria may be the only sign of potential underlying malignancy. However, although asymptomatic hematuria is present in up to 13% of the general population, only 0.4% have urothelial carcinoma.63 Other symptoms from bladder cancer can include those related to the lower urinary tract such as urinary frequency, urgency, dysuria—which can be associated with both CIS or invasive cancer.64 Less common symptoms relate to locally invasive or metastatic disease, with patients complaining of flank pain due to ureteral obstruction, lower extremity edema, pelvic fullness, weight loss, and bone pain.
Patient Evaluation
Evaluation for bladder cancer is usually prompted by hematuria (Figure 83-6). All patients with unexplained gross hematuria warrant evaluation. Further, the American Urological Association recently released guidelines for the evaluation of asymptomatic microscopic hematuria.65 Key items include the definition of asymptomatic microscopic hematuria as ≥3 red blood cells per high power field on formal (not dipstick) urinalysis. If hematuria is confirmed, further workup is indicated.
A relatively new enhancement to white light cystoscopy involves photodetection using 5-aminolevulinic acid (5-ALA), first described in 1994.66 After intravesical instillation, 5-ALA induces enhancement of protoporphyrin IX with a strongly fluorescent dye in the mucosa of cancerous lesions. This fluorescence becomes visible with blue light (375 to 400 nm) and is visible using a filter in the cystoscope eyepiece.67 Photodetection has high sensitivity for detecting early-stage bladder cancer (up to 96% in some series), but has less specificity because of cross-reactivity with inflammatory, noncancer pathology. It is therefore best used in untreated urothelium rather than after intravesical treatment, which may promote false positives. Photodetection is being increasingly used in part because of lower recurrence-free survival noted in a single-center randomized study.68 However, without larger, multicenter trials, its usefulness needs to be further studied.
Evaluation for bladder cancer also involves urine cytology, which is the pathological evaluation of changes in disaggregated cells under light microscopy.64 Although cytology can be used to detect a variety of bladder lesions (including sarcomatous, squamous, glandular, and small cell lesions), it is most commonly applied to the detection of high-grade urothelial carcinoma. For this reason, cytology is often performed during cystoscopic evaluation, through vigorous barbotage of urine performed immediately after cystoscopy (but before urine is evacuated). If no bladder lesion is noted during local cystoscopy but cytology is positive, concern for upper tract disease is appropriate and selective urine cytologies with the possible addition of ureteroscopy are necessary.
As upper tract disease is an additional concern in the setting of hematuria, an essential addition to cystoscopic evaluation includes upper tract evaluation for urothelial tumors and to assess possible obstruction of the upper tract due to bladder cancer. The upper urinary tract collecting system can be evaluated using intravenous pyelogram (IVP), retrograde pyelogram (performed under anesthetic), or most commonly computed tomography (CT) or magnetic resonance (MR) urogram. Fewer than 2% of patients with a papillary bladder lesion will develop an upper tract tumor; however, stage, grade, and tumor volume do not correlate with the risk of upper tract cancer, illustrating the importance of upper tract imaging studies as part of the workup for all patients.69,70
If hematuria workup uncovers muscle-invasive disease, further evaluation is warranted to search for possible metastatic disease. This includes a complete blood count, chemistry profile including alkaline phosphatase (for bone metastasis), chest imaging, as well as an abdominal/pelvic CT or MRI. If alkaline phosphatase is elevated or the patient has symptoms suggestive of metastatic disease, a bone scan is warranted.71
Primary Therapy
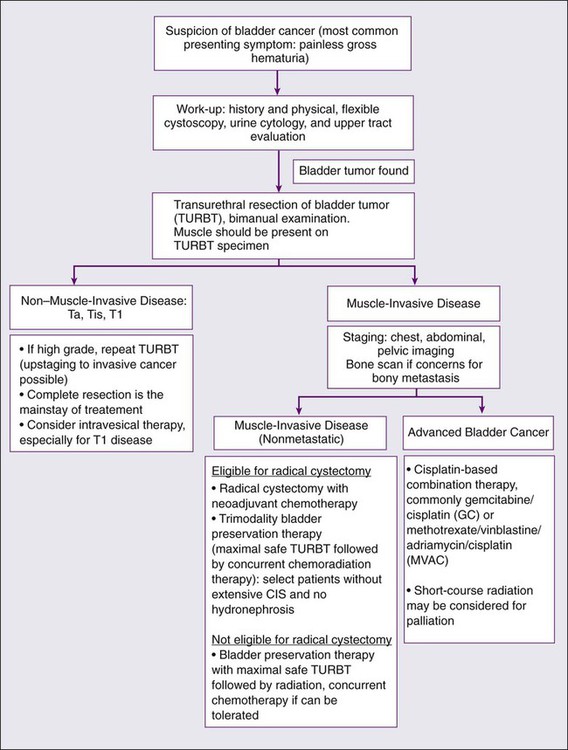
Primary therapy for bladder cancer relies on initial staging from a transurethral resection of bladder tumor (TURBT) (see Figure 83-6). A patient’s clinical stage, which is determined by pathological evaluation of the TURBT specimen, drives subsequent treatment approaches. In general, bladder cancer can be categorized into three groups: non–muscle-invasive disease (including Ta, Tis and T1 cancers), muscle-invasive localized disease, and advanced cancer. Treatment approaches for each will be described in detail.
Treatment for Non–Muscle-Invasive Disease
TURBT may be supplemented with intravesical therapy to reduce the risks of recurrence and progression to muscle-invasive cancer. Intravesical therapy was first introduced in the 1950s, and therapeutic agents used today include mitomycin C, doxorubicin, thiotepa, valrubicin, interferon, and Bacillus Calmette-Guerin (BCG).72 Of these agents, the most commonly used agents for low- and high-grade diseases are mitomycin C and BCG, respectively.
Intravesical Bacillus Calmette-Guerin
BCG is an attenuated mycobacterium first developed as a vaccine in tuberculosis that has been proved to have antitumor activity in multiple cancers, including urothelial carcinoma.73 Use as an intravesical agent was first proposed in 198274; since then, BCG has been used in the treatment of noninvasive high-grade disease as well as recurrent low-grade disease. BCG is reconstituted with 50 mL saline, instilled through a urethral catheter under gravity, retained in the bladder for approximately 2 hours, and drained. Fluid restriction prior to administration is recommended to avoid dilution during treatment. Although the mechanism for its efficacy remains unknown, an intact host immune response appears to be necessary. Treatment regimens usually begin 2 to 4 weeks after tumor resection, to allow adequate healing and limit bacterial intravasation.
An induction course of BCG involves six weekly administrations, followed by cystoscopic surveillance. For partial responders, a second course of BCG may be considered; alternatively, cystectomy can be considered because of a high risk of progression into muscle-invasive disease in this patient population.75 For complete responders, maintenance BCG is offered with three weekly instillations every 6 months for 2 to 3 years. Four weeks after the last instillation, a repeat cystoscopy is recommended. BCG dose reduction may be required for some patients because of poor tolerance. However, the efficacy of diminished dosage schemes is unknown with limited evaluation in the literature.
BCG has been shown in multiple studies to decrease the risk of tumor recurrence by 30% to 40%.74,76 In addition, BCG also has been shown to reduce the risk of progression into muscle-invasive disease by approximately 25% when evaluating patients undergoing maintenance therapy.77,78 In a Southwest Oncology Group (SWOG) randomized trial, patients who were randomized to receive maintenance therapy had a median recurrence-free survival of 76.8 months compared to 35.7 months for those randomized to the control group.79 Of note, only 16% of patients tolerated the full-dose regimen because of side effects.
For some patients, including those with BCG refractory disease, BCG may be combined with interferon, a glycoprotein produced in response to antigenic stimuli with multiple antitumor activities. Although there is no clear efficacy of interferon therapy alone, there is evidence that its combination with BCG may lead to reduced recurrence rates as well as the potential to decrease BCG dosage, thus lessening side effects.80 There is also a suggestion that the combination may be useful in some BCG nonresponders.
Intravesical Therapy with Chemotherapeutic Agents
The most common chemotherapeutic agent used as intravesical therapy for non–muscle-invasive bladder cancer is mitomycin C. Mitomycin C is an alkylating agent that inhibits DNA synthesis and is most efficacious within 24 hours of TURBT. For patients with low-grade bladder cancer, a single dose of 40 mg mitomycin C can be administered after TURBT. For recurrent low-grade disease, an induction dose is given within 24 hours of TURBT, followed by weekly treatment for 6 to 8 weeks at doses between 20 and 60 mg. Mitomycin C causes significantly fewer lower urinary tract side effects than other intravesicle agents because of its high molecular weight and minimal systemic absorption. Efficacy has been shown to improve with urine alkalinization and microwave warming.83–83
Other intravesical chemotherapeutic agents include thiotepa as well as doxorubicin derivatives such as epirubicin and valrubicin. Thiotepa is an alkylating agent and represents the only chemotherapeutic agent approved by the FDA specifically for intravesical treatment of bladder cancer. Hematopoietic side effects are occasionally seen because of thiotepa’s low molecular weight and systemic absorption. Doxorubicin derivatives, on the other hand, are anthracycline antibiotics that inhibit topoisomerase II and protein synthesis. Epirubicin has been shown to decrease recurrence as a single post-TURBT dose as well as in a full 8-week course; however, it is only available in Europe at the present time. Valrubicin is a semisynthetic analog of doxorubicin and has been FDA-approved for BCG-refractory CIS in those unable to tolerate cystectomy. In this particular patient population, approximately 20% achieve complete response with this treatment.84
Treatment for Muscle-Invasive Localized Disease
The most commonly used treatment in the United States for muscle-invasive bladder cancer is radical cystectomy, with or without neoadjuvant cisplatin-based chemotherapy.85 There are also several bladder-preserving treatment approaches, including partial cystectomy, TURBT followed by chemotherapy, and concurrent chemoradiation (“trimodality therapy”)—with the latter having the most published long-term experience from prospective trials.
Muscle-invasive bladder cancer (MIBC) is an aggressive but potentially curable cancer. Recently published population-based patterns of care studies have consistently demonstrated an underutilization of potentially curative therapies for patients with this disease, especially in elderly patients.85,86 Across the United States, the proportions of patients who undergo radical cystectomy for MIBC decrease dramatically with age: for patients 50 years and under, 63% receive cystectomy; this decreases to 55% for those aged 61 to 70, 45% aged 71 to 80, and 22% 81 to 90.85 This is likely due to concerns from physicians and patients about the tolerability of cystectomy in elderly bladder cancer patients, especially because this is a smoking-related cancer and a significant proportion have comorbidities such as cardiovascular disease.4,5 However, the use of other potentially curative treatments such as radiation therapy do not as dramatically increase for elderly patients; as a result, one-third to one-half of elderly patients with MIBC receive no potentially curative treatment.
Radical Cystectomy
Radical cystectomy with bilateral pelvic and iliac lymphadenectomy is the standard surgical procedure for MIBC. Radical cystectomy involves removal of the bladder and perivesical soft tissue, including the prostate and seminal vesicles in men and the ovaries, uterus, cervix, and anterior vagina in women. Sparing of the anterior vagina may be considered in sexually active women, but must be used judiciously with oncologic control in mind. Furthermore, full anterior pelvic exenteration may be unnecessary in many women because involvement of these organs is uncommon, avoiding postmenopausal symptoms such as hot flashes.87
Radical cystectomy is a morbid procedure associated with 90-day estimated complication rates of 64% and mortality of 1.5% in recent large series.89 The most common complications include gastrointestinal morbidity (ileus) and infectious and wound-related complications.89 Older age is significantly associated with higher rates of complications90 and perioperative mortality.91 In a population-based study, Liberman et al. reported a 2% mortality rate within 90 days of cystectomy in patients 69 years or younger, 5.4% in those aged 70 to 79, and 9.2% among those aged 80 years or older.91
Additional intraoperative concerns involve ureteral and urethral margins. There is considerable debate whether the presence of CIS in ureteral margins require resection. Although atypia and dysplasia do not require further resection, many recommend that the surgeon attempt to achieve a negative margin in the case of CIS. However, if this compromises ureteral length (necessitating nephrectomy), further resection may be stopped. It is important to note that CIS of the ureter has not been shown to be independently associated with poor outcomes after cystectomy.92
In terms of the urethral margin, urethrectomy should be considered in men with diffuse CIS within the prostatic urethra or ducts, or tumor invasion of the prostatic stroma. Selective biopsies prior to cystectomy can be performed—and if positive, preoperative counseling may be offered regarding urethrectomy and its implications. The probability of a secondary urothelial carcinoma of the urethra is only 7% and lower with orthotopic compared with cutaneous diversion, possibly related to judicious evaluation of the urethral margin prior to selecting diversion type.93 For females undergoing cystectomy, the urethra is often removed if cutaneous diversion is selected, but in the case of orthotopic neobladder, evaluation of the urethral margin is necessary and neobladder is contraindicated in the presence of urethral tumor or T4 tumor involving the anterior vaginal wall.
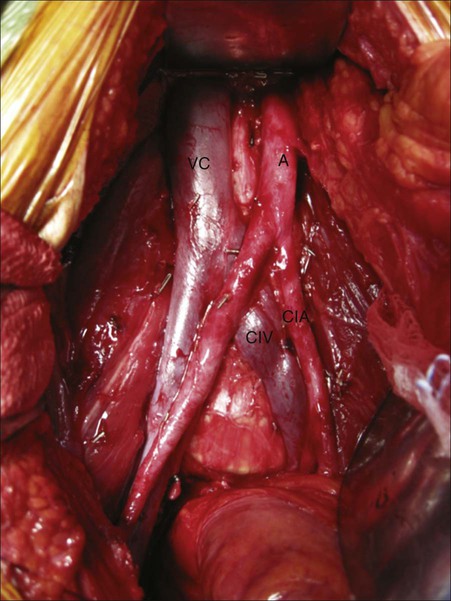
Pelvic and iliac lymph node dissection is an integral part of primary surgical treatment of muscle-invasive bladder cancer. Although the minimum number of lymph nodes necessary for removal is undefined, several studies suggest that the total number of nodes removed and the percentage of positive nodes are independent predictors of recurrence and survival.94 Node counts may be dependent on several variables, including the meticulous nature of the pathological evaluation, the number of packets submitted to the pathologist, as well as the surgeon and the nodal template.
Pelvic and iliac node dissections can be performed using a standard or extended template. Although both nodal templates use the same lateral and distal margins (genitofemoral nerve and node of Cloquet, respectively), the superior extent differs. The standard template uses the bifurcation of the common iliac arteries into the internal and external as the superior most extent, whereas the extended template proceeds as superior as the presacral nodal packet and the bifurcation of the aorta (Figure 83-7). More extensive node dissection can result in improved local-regional cancer control. Furthermore, identification of nodal disease can lead patients to adjuvant chemotherapy, with potential improvement in overall survival. However, patient age and comorbidities (including the presence of atherosclerotic disease and inability to tolerate increased operative time) may affect the surgeon’s decision to pursue standard or extended node dissection. The survival benefit related to template selection is an area of active research, with a randomized study currently enrolling patients.
Urinary Diversion
Cutaneous Incontinent Urinary Diversion
Cutaneous incontinent diversions can employ various bowel segments, including stomach, jejunum, ileum, or colon, and numerous techniques for conduit construction exist. The most frequently performed procedure is an ileal conduit, involving the harvest of a 10- to 15-cm segment of ileum approximately 10 to 15 cm proximal to the ileocecal valve. Once the segment is harvested and the bowel brought back into continuity, ureteral anastomoses are performed, involving tunneled or untunneled anastomoses, performed as separate anastomoses or a single anastomosis joining both ureters (Figure 83-8A). Several stomal techniques exist, with the majority involving a rosebud formation on-end, situated in the right lower quadrant (Figure 83-8B).
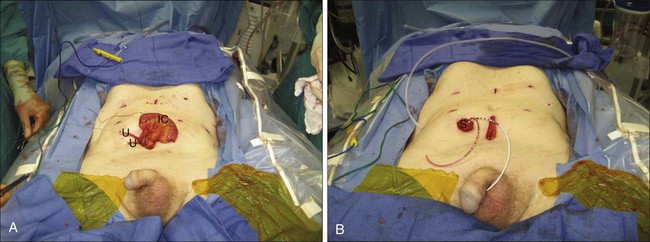
Complications of an ileal conduit include leakage of bowel contents, urine leakage, abdominal abscesses, fistulas, sepsis, hemorrhage, anastomotic ureteral stenosis, and metabolic acid–base abnormalities such as hyperchloremic, hypokalemic metabolic acidosis. Fortunately, major complications are uncommon, but ileus and other infectious complications can occur in up to 20% to 25% of patients undergoing the procedure. Long-term complications also may include stomal stenosis as well as renal deterioration, which can occur in up to 60% during long-term follow-up.95
Orthotopic Neobladder
Although there are a variety of surgical techniques for orthotopic neobladder construction, all have similar goals. A neobladder must be formed through detubularization of the bowel with reconstruction into a spheric shape that can eventually accommodate 400 to 500 mL at low pressure. Careful patient selection is necessary as urethral margins should be negative to proceed. The most significant risk factor for urethral recurrence in men and women is the presence of prostatic stromal invasion and bladder neck or anterior vaginal involvement, respectively.93 Intraoperative frozen section is therefore recommended for an accurate assessment of the patients’ candidacy for this approach. Similar to ileal conduit, the need for antireflux mechanisms for ureteral anastomosis remains controversial, with long-term results for both showing good upper tract preservation.
Functional results of orthotopic diversion include considerations of continence and urinary retention. Daytime continence often develops over a period of 6 months, in which patients use timed voiding to expand the capacity of the neobladder. Approximately 80% to 90% of patients will achieve daytime continence, whereas persistent nocturnal incontinence is much more common in upwards of 50% of patients.96 On the other hand, urinary retention has been reported in approximately 15% to 25% of patients. Worse functional outcomes have been noted with increased age, and non–nerve-sparing surgical techniques.
Complications specific to orthotopic neobladder include metabolic abnormalities due to the absorptive properties of the bowel segment, urethral stricture and pouch rupture, the latter of which requires emergent surgical repair. Regular follow-up imaging is necessary to identify upper tract obstruction, nephrolithiasis (as well as pouch stones), and should involve urethral cytology to identify urethral recurrence, which is more commonly noted in men compared to women.97
Preoperative or Postoperative Chemotherapy
Despite definitive local treatment with radical cystectomy, up to 50% of patients with MIBC will ultimately develop distant metastases and die from this disease, underscoring the need to incorporate systemic treatment with local therapy.88 The identification of chemotherapeutic agents effective in the metastatic setting and the proven efficacy of perioperative chemotherapy in other solid tumor malignancies such as breast and colon cancers have inspired investigators to study this approach in muscle-invasive urothelial cancer.
Adjuvant Chemotherapy
Overall, radical cystectomy alone is associated with a 50% to 60% long-term survival rate; however, patients with disease invading beyond the muscle or who have lymph node involvement have poor long-term survival estimated at 40% and 10%, respectively.98 This observation provides compelling rationale for the study of adjuvant chemotherapy in these high-risk patients. Promising small single-center studies and retrospective experiences have led to larger multi-institutional randomized trials but have been hampered by slow accrual, inadequate delivery of assigned therapy, and early termination.101–101 Today, despite the widespread use of adjuvant chemotherapy, there is limited and conflicting data to support its use after radical cystectomy in patients with invasive urothelial cancer.
Two early trials demonstrated a survival benefit with adjuvant chemotherapy, but were relatively small, single-center experiences and in one study, patients randomized to the observation arm did not receive salvage therapy at the time of relapse.99,100 A systematic review that included 8 randomized trials of adjuvant therapy demonstrated no survival benefit with adjuvant chemotherapy, even for those with extravesical or lymph node involvement.102 However, an analysis of an international collaborative database of patients who underwent radical cystectomy without neoadjuvant chemotherapy demonstrated a significant improvement in survival associated with the use of adjuvant chemotherapy, particularly in those patients with high-risk features.103 Larger and more contemporary randomized trials not included in previous meta-analyses have attempted to definitively establish the role for adjuvant chemotherapy; however, each was closed prematurely because of poor accrual.
An Italian multicenter study that randomized patients to adjuvant gemcitabine and cisplatin (GC) versus observation and a European Organization for Research and Treatment of Cancer (EORTC) study that randomized patients to observation versus adjuvant methotrexate, vinblastine, Adriamycin, and cisplatin (MVAC) or high-dose MVAC or GC both terminated early because of poor accrual. However the EORTC study was recently reopened following a futility analysis and results are expected in 2013. Notably, for the 194 patients who were enrolled in the Italian multicenter study, no significant difference in disease-free survival or overall survival was detected.104
A trial led by the Southwest Oncology Group (SWOG) and the University of Southern California that randomized patients with p53 overexpression by immunohistochemistry (IHC) to adjuvant MVAC chemotherapy versus observation was also terminated early after slow accrual and a high rate of noncompliance with the study design. An analysis of the 499 patients enrolled neither demonstrated a survival benefit with adjuvant chemotherapy nor demonstrated a prognostic or predictive value to p53 testing.33
In the Spanish Oncology Group Trial 99/01, a randomized trial of adjuvant chemotherapy in patients with invasive urothelial cancer at high risk for recurrence did provide some evidence that adjuvant chemotherapy can perhaps improve survival in select patients. This trial randomized high-risk patients with pT3–T4 or lymph node–positive urothelial cancer of the bladder after radical cystectomy to observation versus paclitaxel, gemcitabine, and cisplatin (PGC) for four cycles with a planned enrollment of 340 patients. Although it was closed early because of poor accrual in 2007, a preliminary analysis of the 142 patients enrolled with median follow-up of 51 months demonstrated a significant improvement in overall survival at 5 years with adjuvant PCG (60% vs. 30%, HR 0.44, P < 0.0009).105 Despite accruing less than half the planned enrollment, this trial demonstrated a survival benefit with adjuvant chemotherapy, perhaps because it selected for patients with the highest risk for recurrence, and thus, the highest likelihood of deriving benefit from adjuvant chemotherapy. Interpretation of this trial is limited by the sample size and incomplete accrual. However, it does provide a basis for considering adjuvant chemotherapy in select high-risk patients.
Neoadjuvant Chemotherapy
The rationale and the clinical evidence supporting the use of neoadjuvant chemotherapy in MIBC are more compelling, though this treatment approach remains relatively underutilized.106,107 Data from two large randomized studies of neoadjuvant cisplatin-based therapy have established definitive evidence of a survival benefit. The Medical Research Council of the United Kingdom (MRC) and EORTC conducted a multicenter phase III trial (BA06) that randomized 976 patients to cisplatin, methotrexate, and vinblastine (CMV) versus no therapy prior to definitive treatment for muscle-invasive urothelial cancer of the bladder. The choice of definitive treatment included radical cystectomy or radiotherapy and was determined by institutional practice. The trial was powered to detect a 10% absolute improvement in survival at 3 years, and for the 484 patients who underwent radical cystectomy, initial results reported at a median follow-up of 4 years did not demonstrate a significant difference in survival: 3-year survival rate of 55.5% for the chemotherapy arm versus 50% in the no chemotherapy arm (P = 0.075).108 However, a recently updated long-term report at a median follow-up of more than 8 years demonstrated a statistically significant reduction in the risk of death (HR 0.84; 95% CI, 0.72 to 0.99; P < 0.037) corresponding to an increase in 10-year survival from 30% to 36% for patients receiving neoadjuvant CMV.109 In the Southwest Oncology Group (SWOG)/Intergroup 8710 study, 317 patients with pT2-4aN0 urothelial cancer of the bladder were enrolled over 11 years to receive three cycles of MVAC or no therapy prior to radical cystectomy.110 At a median follow-up of over 8 years there was an improvement in overall survival (5 year: 57% vs. 43%) and an increase in median survival (77 vs. 46 months) favoring neoadjuvant MVAC; however, it was not statistically significant. A stratified proportional-hazards model showed that patients treated with cystectomy alone had a statistically significantly greater risk of death than those treated with combination therapy (HR 1.33; 95% CI, 1.00 to 1.76). Further, stratification by clinical T stage showed that all patients benefited from neoadjuvant chemotherapy; however, the benefit was greatest in patients with clinical T3 or greater disease. The rate of complete response (pT0) to neoadjuvant chemotherapy (38% for neoadjuvant chemotherapy vs. 15% for no chemotherapy; P < 0.001) strongly predicted improved long-term survival, with 85% of patients with pT0 still alive at 5 years. This observation highlights the prognostic value of in vivo assessment of response to neoadjuvant chemotherapy. Lastly, two large meta-analyses of randomized trials of neoadjuvant chemotherapy have also confirmed the benefit of this approach in improving survival for patients with MIBC.102,111 Thus neoadjuvant cisplatin-based combination chemotherapy represents the standard of care for patients with this disease. The magnitude of absolute long-term survival benefit is estimated between 5% and 10%.
The regimen of gemcitabine and cisplatin (GC), which is commonly used in metastatic disease, has never been prospectively evaluated in the neoadjuvant setting. A retrospective report from a single institution demonstrated a similar rate of pT0 between MVAC and GC, suggesting that survival outcomes may also be similar.112 In practice, MVAC, CMV, and GC are all commonly used cisplatin-based regimens as neoadjuvant chemotherapy. Novel approaches currently being evaluated in phase II neoadjuvant trials include dose-dense chemotherapy and molecularly targeted therapy added to standard therapy.
Preoperative or Postoperative Radiation Therapy
Some patients with MIBC who undergo radical cystectomy as initial treatment may also have significant risks of local recurrence (in addition to distant recurrence), so either preoperative or postoperative radiation therapy may be reasonable to consider. However, the use of radiation therapy with cystectomy is not well studied. In a secondary analysis of patients treated on the SWOG/Intergroup 8710 trial (radical cystectomy with vs. without neoadjuvant MVAC chemotherapy), patients with pathological T3–T4 cancers and those with positive surgical margins had significant risks for developing local recurrences (32% and 68%, respectively).113 In an older phase III trial from Egypt, 236 patients with pathological T3–T4 bladder cancers after cystectomy were randomized to no further treatment versus conventionally fractionated radiation therapy versus hypofractionated radiotherapy.114 Postoperative radiation was associated with higher rates of 5-year local control (87% to 93%) compared with cystectomy alone (50%), as well as improved disease-free survival (44% to 49% vs. 25%).
Few modern studies have directly compared patient outcomes of those undergoing radical cystectomy alone versus cystectomy with preoperative radiation therapy. In a retrospective study, patients with clinical T3b cancers had a significant reduction of pelvic failure rates with preoperative radiation therapy (5-year rates 28% without vs. 9% with radiation, P = 0.003), but no benefit was seen in patients with T2 or T3a disease.115 This use of preoperative or postoperative radiation therapy is worth further study in future clinical trials.
Partial Cystectomy
Instead of radical cystectomy, the surgical removal of a portion of the bladder wall may be suitable for a limited number of patients with a solitary high-grade lesion in which a sufficient margin can be obtained. In a small case series of highly select patients, the overall 5-year survival was reported at 69%.116 Selection criteria for this approach are critical and include solitary tumors located in the bladder dome with minimal or no CIS and no history of multifocal bladder cancer.117 These criteria represent a minority of patients with MIBC. A recent study demonstrated that there is likely overuse of this treatment approach for MIBC in some patient settings and populations.118
Trimodality Bladder Preservation Therapy
Organ-preserving approaches using radiation therapy have been studied and applied to many common cancers, including breast, prostate, head and neck, sarcoma, and anal cancers. For patients with MIBC who are not cystectomy candidates, or those motivated to preserve their native bladders, trimodality therapy offers a well-studied alternative.119,120 This treatment regimen involves a maximally safe TURBT, followed by concurrent chemoradiation therapy. Many published trials have stopped the radiation at approximately 40 Gy to allow an early cystoscopic evaluation of tumor response. Patients who have an incomplete response and are suitable for surgery proceed to immediate cystectomy, whereas the remainder receive additional chemoradiation with total radiation dose of 64 to 65 Gy. After treatment completion, patients with a preserved native bladder undergo long-term cystoscopic surveillance, with salvage cystectomy performed for invasive recurrences.
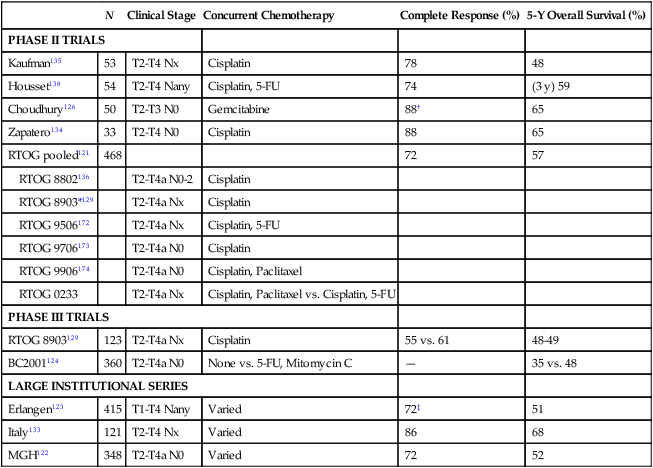
Multiple prospective trials have been performed of trimodality bladder preservation therapy (Table 83-4). The majority of trials have reported complete response rates of more than 70% at cystoscopic evaluation; therefore, less than 30% of patients who have received trimodality therapy were unsuccessful in keeping their native bladders and required immediate cystectomy. Five-year overall survival rates in published trials range from 48% to 65%. Recently, a pooled analysis of six cooperative group trials from the Radiation Therapy Oncology Group (RTOG) was completed, which included 468 patients with a median age of 66 years at enrollment.121 After a median follow-up of 7.8 years for survivors, 5-year overall survival was 57%, including 62% for patients with clinical T2 disease and 49% for clinical T3–T4 disease. In this study and also in a large institutional series from the Massachusetts General Hospital,122 disease-specific survival was found to be almost identical for patients younger than 75 years versus those 75 and older—suggesting that trimodality therapy is similarly effective in younger and older patients. Further, several studies that have had long-term follow-up have reported minimal changes in the rates of invasive recurrences123,124 and disease-specific survival beyond 5 years,121,122 demonstrating stability of disease control outcomes long-term after trimodality therapy.
Table 83-4
Select Prospective Clinical Trials and Large Institutional Studies of Trimodality Bladder Preservation Therapy for Muscle-Invasive Bladder Cancer
| N | Clinical Stage | Concurrent Chemotherapy | Complete Response (%) | 5-Y Overall Survival (%) | |
| PHASE II TRIALS | |||||
| Kaufman135 | 53 | T2-T4 Nx | Cisplatin | 78 | 48 |
| Housset138 | 54 | T2-T4 Nany | Cisplatin, 5-FU | 74 | (3 y) 59 |
| Choudhury126 | 50 | T2-T3 N0 | Gemcitabine | 88† | 65 |
| Zapatero134 | 33 | T2-T4 N0 | Cisplatin | 88 | 65 |
| RTOG pooled121 | 468 | 72 | 57 | ||
| RTOG 8802136 | T2-T4a N0-2 | Cisplatin | |||
| RTOG 8903*129 | T2-T4a Nx | Cisplatin | |||
| RTOG 9506172 | T2-T4a Nx | Cisplatin, 5-FU | |||
| RTOG 9706173 | T2-T4a N0 | Cisplatin | |||
| RTOG 9906174 | T2-T4a N0 | Cisplatin, Paclitaxel | |||
| RTOG 0233 | T2-T4a Nx | Cisplatin, Paclitaxel vs. Cisplatin, 5-FU | |||
| PHASE III TRIALS | |||||
| RTOG 8903129 | 123 | T2-T4a Nx | Cisplatin | 55 vs. 61 | 48-49 |
| BC2001124 | 360 | T2-T4a N0 | None vs. 5-FU, Mitomycin C | — | 35 vs. 48 |
| LARGE INSTITUTIONAL SERIES | |||||
| Erlangen123 | 415 | T1-T4 Nany | Varied | 72‡ | 51 |
| Italy133 | 121 | T2-T4 Nx | Varied | 86 | 68 |
| MGH122 | 348 | T2-T4a N0 | Varied | 72 | 52 |
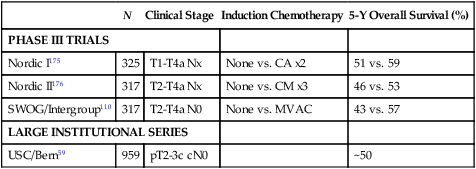
Comparisons of outcomes among published studies of trimodality bladder preservation therapy (with salvage cystectomy at recurrence) versus upfront cystectomy are difficult because of the lack of a modern randomized trial comparing these two treatment approaches. Patients who undergo bladder preservation treatments are commonly older and have more comorbidities than those in cystectomy trials. Also, clinical staging (which is reported by all bladder preservation trials) is not directly comparable with pathological/surgical staging (reported by most cystectomy studies). (See section on Staging Classification.) With these limitations in mind, trimodality bladder preservation therapy appears to achieve similar long-term overall survival outcomes as radical cystectomy. Table 83-5 summarizes results from cystectomy studies that have provided clinical staging information. Five-year overall survival rates ranged from 43% to 59% overall, and 53% to 59% for patients who received neoadjuvant chemotherapy.
Table 83-5
Select Prospective Clinical Trials and Large Institutional Series of Radical Cystectomy for Muscle-Invasive Bladder Cancer
| N | Clinical Stage | Induction Chemotherapy | 5-Y Overall Survival (%) | |
| PHASE III TRIALS | ||||
| Nordic I175 | 325 | T1-T4a Nx | None vs. CA x2 | 51 vs. 59 |
| Nordic II176 | 317 | T2-T4a Nx | None vs. CM x3 | 46 vs. 53 |
| SWOG/Intergroup110 | 317 | T2-T4a N0 | None vs. MVAC | 43 vs. 57 |
| LARGE INSTITUTIONAL SERIES | ||||
| USC/Bern59 | 959 | pT2-3c cN0 | ~50 | |
Effective Radiosensitizing Chemotherapy Agents
Most of the published trials have used cisplatin-based concurrent chemotherapy, either cisplatin alone, cisplatin with 5-FU, or with paclitaxel (Table 83-4). In a retrospective study from Erlangen, outcomes of 415 patients treated with radiation-based bladder preservation therapy between 1982 and 2000 were examined. Complete response rates differed significantly among patients who received radiation alone (61%), radiation plus carboplatin (66%), radiation with cisplatin (82%), and radiation with cisplatin and 5-FU (87%, multivariate P = 0.05).123 Five-year overall survival (40%, 45%, 62%, 65%, respectively) also approached significance (multivariate P = 0.06). There are no published data to support the effectiveness of carboplatin in this setting.
For patients who are non-cisplatin candidates either because of poor renal function or concerns about cisplatin toxicity, 5-FU and mitomycin C is an alternative regimen based on level 1 evidence. In the BC2001 trial, 360 patients with MIBC were randomized to receive either radiation therapy alone, or radiation plus 5-FU and mitomycin C.124 The median age of patients on this trial was 72 years, and 11% were 80 years or older. The treatment regimen was well tolerated in this older patient group, with more than 95% of patients randomized to the chemoradiation arm completing the radiation treatment, and approximately 80% completing chemotherapy. After a median follow-up of 70 months, chemoradiation resulted in significantly better outcomes than radiation alone. Two-year local-regional disease-free survival, the primary end point of this trial, was 67% in patients randomized to chemoradiation, and 54% for radiation alone (P = 0.03). Concurrent chemoradiation also appeared to reduce the need for cystectomy (2-year rates 11.4% vs. 16.8% for radiation alone, P = 0.07) and improve overall survival (5-year survival 48% vs. 35% for radiation alone, P = 0.16). However, the trial was not fully powered to detect differences in these secondary endpoints.
Gemcitabine as a radiosensitizing agent for MIBC has been studied in phase I125 and II studies126, and is currently being compared to cisplatin plus 5-FU in RTOG 0712, a randomized phase II trial. In addition, a phase III trial has compared treatment with radiation alone versus radiation with current hypoxia modifiers carbogen (2% carbon dioxide and 98% oxygen at 15 L/min for 5 minutes before and during radiotherapy) and oral nicotinamide.127 Median overall survival was 30 months for radiation alone, and 54 months for combined therapy (P = 0.04). This trial demonstrates a potential new treatment option for MIBC and should be further studied.
There is currently no clear role for neoadjuvant chemotherapy prior to concurrent chemoradiation. In the BA06 trial, patients with MIBC received definitive local treatment (either cystectomy or radiation therapy by patient and physician choice), and were randomized to receive versus not receive three cycles of neoadjuvant cisplatin, methotrexate, vinblastine (CMV) chemotherapy.128 Patients randomized to the neoadjuvant chemotherapy arm had improved overall survival, which on subgroup analysis appeared to apply to both cystectomy and radiation therapy patients. However, patients in this trial received radiation therapy alone but not concurrent chemoradiation. In RTOG 8903, patients received radiation therapy with concurrent cisplatin, and were randomized to receiving versus not receiving two cycles of neoadjuvant CMV chemotherapy.129 Overall survival was identical in the two arms, but patients in the neoadjuvant chemotherapy arm developed increased hematologic toxicity and nausea/vomiting.
Morbidity, Bladder Function, and Quality of Life after Trimodality Bladder Preservation Therapy
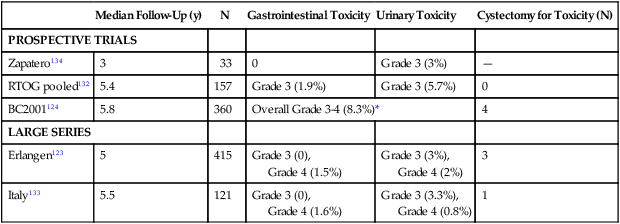
Approximately 75% to 80% of long-term survivors after trimodality therapy preserve their native bladders, which tend to function well, and patients maintain a high quality of life.130,131 Long-term significant morbidity is uncommon (Table 83-6). In a pooled analysis of four RTOG trials of long-term survivors after trimodality therapy, after a median follow-up of 5.4 years, a total of 7% of patients experienced long-term grade 3 toxicity, including 5.7% urinary and 1.9% gastrointestinal toxicity.132 In the BC2001 randomized trial, late grade 3 to 4 toxicity was 8%.124 Multiple other studies have reported similarly low rates.123,133,134 It is rare for patients to require a cystectomy due to bladder toxicity. Further, in a prospective phase II study of patients treated with radiation and concurrent cisplatin plus 5-FU chemotherapy, urinary symptoms improved from baseline to 24 months after treatment,131 potentially due to tumor shrinkage and improved bladder function.
Table 83-6
Long-Term Toxicity After Trimodality Therapy for Muscle-Invasive Bladder Cancer
| Median Follow-Up (y) | N | Gastrointestinal Toxicity | Urinary Toxicity | Cystectomy for Toxicity (N) | |
| PROSPECTIVE TRIALS | |||||
| Zapatero134 | 3 | 33 | 0 | Grade 3 (3%) | — |
| RTOG pooled132 | 5.4 | 157 | Grade 3 (1.9%) | Grade 3 (5.7%) | 0 |
| BC2001124 | 5.8 | 360 | Overall Grade 3-4 (8.3%)* | 4 | |
| LARGE SERIES | |||||
| Erlangen123 | 5 | 415 | Grade 3 (0), Grade 4 (1.5%) |
Grade 3 (3%), Grade 4 (2%) |
3 |
| Italy133 | 5.5 | 121 | Grade 3 (0), Grade 4 (1.6%) |
Grade 3 (3.3%), Grade 4 (0.8%) |
1 |
A study by Zietman et al. evaluated long-term survivors after trimodality therapy who retained their native bladders and were disease free, at a median of 6.3 years after treatment.130 Of 32 patients who underwent a urodynamic study, 24 (75%) were judged to have normally functioning bladders. A total of 48 patients completed patient-reported outcomes at a median age of 68 years. Overall, 6% reported symptoms related to urinary flow, 15% urgency, and 19% incontinence. Approximately 22% reported bowel symptoms and 54% of men had erections sufficient for intercourse. Overall quality of life was high.
Ideal Candidates for Trimodality Bladder Preservation Therapy
Tumor-associated hydronephrosis has been found in multiple studies to be associated with a lower likelihood for achieving complete response to chemoradiation therapy and bladder preservation,129,135 and worse overall survival.129,134–136 In RTOG 8903, patients who had versus did not have hydronephrosis had lower rates of complete response (38% vs. 64%, P = 0.02), and worse overall survival approaching statistical significance (5-year survival 33% vs. 54%, P = 0.06).129
A visibly complete TURBT prior to chemoradiation can also increase the probability for bladder preservation,122,123,129,133 although an inability for complete tumor resection does not preclude successful bladder preservation for MIBC. In a series from the Massachusetts General Hospital, 57% of patients with TURBT that was not visibly complete achieved a complete response to chemoradiation therapy.122 In another study from Erlangen University, complete response rate was 54% for patients who had an R2 resection. These results suggest that patients with an incomplete TURBT may still have a greater than 50% chance of achieving bladder preservation.
In addition, concurrent chemotherapy with radiation compared to radiation alone increases the complete response rate,123 reduces cystectomy and local-regional recurrence,124 and potentially improves overall survival.123,124 Patients with extensive CIS137 and whose bladders are poorly functioning or have little capacity may also not be ideal candidates for trimodality bladder preservation therapy.
Salvage Cystectomy After Trimodality Bladder Preservation Therapy
For patients who do not achieve a complete response to concurrent chemoradiation therapy, an immediate cystectomy can be performed without significant delay. However, incomplete response has been consistently found to be a poor prognostic factor,122,123,133,135,138 indicating that these patients have biologically aggressive disease that is unlikely curable. In the Erlangen series, 5-year disease-specific survival (from time of cystectomy) was 21% for patients who required a cystectomy for incomplete response.123 A similarly low rate (28.5%) was reported in another series.133 These rates are dramatically lower than those reported for trimodality bladder preservation therapy overall.
On the other hand, for patients who initially achieve a complete response to trimodality therapy and subsequently develop an invasive bladder recurrence, salvage cystectomy offers an effective, potentially curative treatment modality. In one series, 42 patients after radiation-based bladder preservation therapy underwent radical cystectomy for an invasive local recurrence.123 Disease-specific survival rates were 50% at 5 years and 45% at 10 years, assessed from the time of salvage cystectomy. Another study from Italy has also reported a 5-year disease-specific survival rate of 50%.133 Further, at high volume centers, salvage cystectomy can be performed safely. In the Massachusetts General Hospital experience of 91 patients with a median age of 69 years, 50 underwent cystectomy for incomplete response after chemoradiation therapy and 41 for invasive local recurrence.139 Tissue healing complications were reported in 12% of the former group and 35% of the latter. The overall 90-day complication rate was 69%, including 16% major complications; mortality was 2.2%. These rates were slightly higher but comparable with those reported from nonirradiated patients operated on at a large tertiary center.90
Treatment for Locally Advanced and Metastatic Disease
Systemic Therapy
Early clinical trials of single-agent chemotherapy in the 1970s demonstrated urothelial cancer to be a chemotherapy-sensitive disease. Cisplatin and methotrexate were the most active agents with reported response rates of approximately 30%.140 Other active agents included doxorubicin, 5-FU, vinblastine, vincristine, and mitomycin C having single-agent response rates of approximately 15%. These single-agent trials provided the basis for the development of combination regimens such as CMV, MVAC, and CIsCA (cisplatin, cyclophosphamide, and doxorubicin) in an effort to achieve higher response rates and improve survival. An initial phase II trial of MVAC reported by Memorial Sloan-Kettering Cancer Center demonstrated a 72% response rate (including 18% complete response) in 121 evaluable patients.141 Median survival was 13.3 months and 20% of patients achieved long-term disease-free survival. In a phase III trial comparing MVAC with CISCA, the response rate for MVAC was 65% (vs. 46% for CISCA), and median survival for MVAC was 11 months (vs. 10 months for CISCA).142 From these trials, MVAC emerged as a standard therapy for patients with metastatic urothelial cancer.
Early trials of MVAC chemotherapy also identified a subset of patients who could achieve long-term disease-free survival. MVAC chemotherapy resulted in major responses in a small proportion of patients, most commonly in those with locally advanced disease, converting initially unresectable tumors to resectable tumors, allowing for consolidative surgery. An analysis of advanced urothelial cancer patients treated across multiple trials of MVAC chemotherapy who underwent postchemotherapy surgery found that patients with unresectable primary tumors or with metastatic disease restricted to regional lymph nodes were most likely to benefit from consolidative surgery.143 With the potential for similar outcomes with modern cisplatin-containing regimens, patients with locally advanced disease who achieve a major response to cisplatin-based therapy should be considered for consolidative surgery.
MVAC is associated with significant toxicity. However, an international randomized trial demonstrated that cisplatin alone was inferior to MVAC for response rate and overall survival.144 Investigators then attempted to improve on the efficacy of MVAC chemotherapy by reducing the interval between treatments (increasing dose density). The rationale for increasing the dose density of chemotherapy was based on evidence suggesting solid tumors were composed of two cancer cell populations: a highly proliferative and chemotherapy-sensitive fraction and another, slower growing and more resistant fraction.145 More frequent administration of chemotherapy would more effectively treat both populations and ultimately lead to higher response rates and potentially improved long-term survival. The EORTC conducted a phase III trial of standard MVAC versus high-dose (HD) MVAC in which the high-dose regimen was, in fact, standard-dose MVAC administered every 2 weeks (increased dose density) with granulocyte colony-stimulating factor (GCSF) support. This trial was powered to detect a 50% improvement in median overall survival. The initial results reported in 2001 demonstrated a 12% absolute improvement in response rate (62% vs. 50%; P = 0.06) and 13.1% absolute improvement in 2-year progression-free survival (24.7% vs. 11.6%) though neither met statistical significance.146 Long-term results reported at a median follow-up of 7 years demonstrated a 15.1 months and 14.9 months median overall survival for HD-MVAC and standard MVAC, respectively.147
The emergence of the newer chemotherapeutic agents paclitaxel and gemcitabine and their demonstrated activity as single agents have led to combination trials with cisplatin, the most active drug in the urothelial cancer armamentarium. Three phase II trials of gemcitabine and cisplatin (GC) demonstrated similar response rates and survival outcomes compared to MVAC.150–150 A seminal multicenter phase III trial conducted in both North America and Europe randomized metastatic urothelial cancer patients to either GC or MVAC.151 Response rates (GC 49.4% vs. MVAC 45.7%) and median survival (GC 13.8 months vs. MVAC 14.8 months) were similar for both regimens; however, GC was far more tolerable with significantly better chemotherapy delivery than MVAC. This trial established the GC regimen as the preferred treatment standard in metastatic urothelial cancer over MVAC.
Taxanes, such as paclitaxel and docetaxel, have also demonstrated activity in urothelial cancer. Promising results from phase II trials of paclitaxel as a single agent and in combination led to EORTC 30987, a phase III trial comparing GC with paclitaxel, cisplatin, and gemcitabine (PCG) in 626 patients with metastatic urothelial cancer.152–155 PCG was associated with a significantly higher response rate (55.5% vs. 43.6%; P = 0.0031); however, a 3.1-month improvement in median survival did not reach statistical significance (PCG 15.8 months vs. GC 12.7 months; P = 0.075).155 PCG was reasonably well tolerated and represents another standard option in the treatment of metastatic disease, particularly for young patients with excellent performance status who may better tolerate triple-agent chemotherapy.
A more recent phase III trial led by the Hellenic Oncology Cooperative Group compared dose-dense (DD) GC to DD MVAC chemotherapy. Initial results reported at the 2011 ASCO Annual Meeting demonstrated comparable response rates (MVAC 62.7% vs. GC 65.3%) and median survival (MVAC 19 months vs. GC 18 months) for the two treatment arms.156 DD GC was better tolerated than DD MVAC, similar to outcomes in the original phase III trial comparing GC to MVAC. The investigators concluded that DD GC also represents a standard treatment option in metastatic disease.
Cisplatin-based chemotherapy is associated with a survival benefit in metastatic urothelial cancer. However, it is estimated that up to 50% of patients will be ineligible for cisplatin-based therapy because of impaired renal function, poor performance status, or other medical illnesses.157 Treatment options for these patients are limited and no firmly established standard therapy exists. Carboplatin-based regimens are commonly used in the community and recommended in clinical practice guidelines but are inferior to cisplatin.158 The EORTC 30986 phase II/III trial randomized 238 cisplatin-ineligible patients (defined as having an estimated glomerular filtration rate [GFR] <60 mL/min and/or ECOG performance status 2) to either gemcitabine and carboplatin (GCa) or the older regimen of methotrexate, carboplatin, and vinblastine (M-CAVI). At a median follow-up of 4.5 years, there was no significant difference in response (GCa 41.2% vs. M-CAVI 30.3%) or median survival (GCa 9.3 months vs. M-CAVI 8.1 months) between the two treatment arms, though GCa was better tolerated.159 This trial provided the first Level 1 evidence for a treatment regimen in this patient population.
There are currently no approved therapies for metastatic urothelial cancer patients after failure of first-line platinum-based therapy. Phase II trials in pretreated patients of single-agent paclitaxel and the novel antifolate, pemetrexed, have demonstrated response rates of 10% to 28% and are commonly used agents in the second-line setting in the United States.160,161 Vinflunine is approved only in Europe for second-line treatment of metastatic urothelial cancer based on a phase III trial comparing vinflunine with best supportive care (BSC), which demonstrated a 9% response rate for vinflunine and 2.3-month improvement in median survival (6.9 months vinflunine vs. 4.6 months BSC) which was not statistically significant.162
The prognosis of metastatic urothelial cancer is dismal with an estimated median survival in the range of 13 to 15 months with cisplatin-based therapies. A retrospective analysis of 203 metastatic urothelial cancer patients treated with MVAC chemotherapy identified poor performance status and visceral metastatic disease as prognostic factors for shortened survival.163 Patients with both risk factors had a median survival of 9.3 months whereas those with none had a median survival of 33 months. A similar study of patients who failed platinum-based therapy found that liver metastases, poor performance status, and low hemoglobin predicted for poor survival in the second-line setting.164 Despite several decades of research of novel agents and combinations, survival in metastatic urothelial cancer remains unchanged and novel therapies are desperately needed.
Current experimental treatments under investigation in metastatic urothelial cancer also include molecularly targeted agents as single agents and in combination with standard therapy. Angiogenesis has long been implicated in urothelial cancer tumorigenesis and progression, and recent phase II clinical trials of agents targeting the vascular endothelial growth factor (VEGF) pathway—the best described mediator of angiogenesis—have been promising. Active agents in this class such as bevacizumab (a monoclonal antibody targeting circulating VEGF) and pazopanib (a small-molecule multitargeted receptor tyrosine kinase inhibitor of VEGF receptor and other targets) are currently in clinical development in advanced urothelial cancer.167–167 Agents targeting the human epidermal growth factor receptor-2 (HER2) such as trastuzumab and lapatinib are also being investigated.
Radiation Therapy
Radiation therapy can have a significant palliative effect.168 In a phase III trial, patients with muscle-invasive bladder cancer causing local symptoms and deemed unsuitable for curative treatment either due to advanced disease and/or comorbidities received radiation treatment, randomized to 35 Gy in 10 fractions versus 21 Gy in 3 fractions.169 Median age of patients was 81 years. Of 500 patients recruited, data on symptom improvement at 3 months was available for 272. Hematuria improved in 88% of patients, urinary frequency in 82%, dysuria in 72%, and nocturia in 64%. Median survival for this cohort was 7.5 months, and approximately 25% had died by the 3-month time point. These data demonstrate that a short course of radiation treatment is effective in palliating local symptoms in the majority of patients with MIBC.
Future Trials
In addition, there are emerging data using biomarkers to predict patient outcomes after bladder-preservation radiotherapy. A study from Leeds Cancer Center (United Kingdom) examined the expression of several proteins potentially important in the detection and repair of double-stranded DNA damage and cell cycle checkpoints by immunohistochemistry in pretreatment tumor specimens.170 In an initial cohort of 86 patients, low tumor MRE 11 (meiotic recombination 11 homolog) expression was associated with worse cancer-specific survival compared to high expression (3-year survival 41% vs. 69%, P = 0.012). In the same study, this finding was confirmed in a separate cohort of 93 patients who received definitive radiation therapy, and was not associated with outcome in cystectomy patients. Similar results were also reported in a separate study from the University of Erlangen.171 A future trial to prospectively validate predictive biomarkers for patients receiving trimodality bladder preservation therapy is being planned, and can lead to the ability of individualizing patient treatment decision making using information available at diagnosis. Further, next-generation genomics has led to the identification of potentially targetable molecular alterations in patients with advanced disease.