CHAPTER 212 Birth Head Trauma
Incidence and Risk Factors
The incidence of major birth trauma such as fractures, paralysis, and lacerations is between 1 and 11.7 per every 1000 to 2000 live births.1–3 Perinatal death secondary to birth trauma alone is a rare event that occurs in 1 in 1000 to 2000 births.1 Many factors related to the fetus, the mother, and the birthing process have been correlated with an increased incidence of traumatic birth. Such factors include macrosomia, growth retardation, preterm labor, breech presentation, and multiparity.1,2,4–7
The mode of delivery has also been implicated in birth trauma. There is much debate in the obstetric literature regarding the efficacy and safety of assistive devices for vaginal delivery, such as forceps and vacuum extractors.8–11 As with all medical instruments, these instruments are safe when used in the appropriate manner by trained physicians. The indications for assisted vaginal delivery are delay, malrotation, or fetal distress in the second stage of labor. The cervix should be fully dilated, and the fetal head should be at the level of the ischial spines or below. There is, however, an increased risk for certain types of birth trauma associated with their use (discussed later).12–17 Operative vaginal delivery, either by forceps, vacuum, or converted cesarean section performed during labor, was associated with an increased incidence of intracranial hemorrhage when compared when spontaneous vaginal delivery.18 One would assume that having a lower threshold to proceed with cesarean section would lower the rate of birth trauma. An extensive study conducted by Puza and colleagues showed that this assumption was true because of the trend toward delivering neonates by cesarean section.3
Scalp Injury
In a newborn, scalp injury occurs in three distinct areas: the outer layers, the potential subgaleal space, and the subperiosteal plane. The most common scalp injury is caput succedaneum.4 This occurs in virtually all children, normally at the vertex. Rather than being a true injury, it is edema of the outer layers of the scalp, and it usually collects in the portion of the scalp that leads the way down the birth canal. To the obstetrician, it is known as chignon and is commonly associated with the use of vacuum extraction.4 It is generally noted immediately after birth and resolves within 24 hours. Rarely, it may have associated skin ecchymoses, abrasions, or lacerations.19 Soft cups used for vacuum extraction cause scalp injury less frequently than do rigid cups.8
Although rare, the most life-threatening scalp injury is a hematoma in the subgaleal plane.12,16,20 Plauche reported a mortality of 22.8% associated with this neonatal emergency.16 The galea aponeurotica extends from the supraorbital rims to the hairline posteriorly and laterally to the level of the ears. This sizable potential space can easily accommodate the entire blood volume of a neonate.
Three conditions put newborns at risk for the development of subgaleal hematoma.12 A coagulation defect or vitamin K deficiency after a routine or complicated delivery increases the risk for subgaleal hematoma. The third condition—and the most common—is a difficult delivery in which vacuum extraction is used. In Plauche’s review of 123 patients with subgaleal hematomas, vacuum extraction was used in 49% of the cases and forceps in 14%.16
The last type of scalp injury is cephalohematoma, which occurs in 1% to 2% of all births.21,22 It is an accumulation of blood between the periosteum and bone; therefore, the cranial sutures limit its expansion. The hemorrhage is thought to occur when the forces of labor acting on the neonatal head shear the periosteum away from the bone.21 Both the bone and the undersurface of the periosteum bleed, and a collection forms. The most common location is in the parietal region, but cephalohematoma can occur anywhere over the skull.
Usually, cephalohematomas are of no clinical significance and resolve spontaneously within a few weeks to months. Rarely, hyperbilirubinemia, anemia, or hemodynamic instability develops. There are a few reported cases of cephalohematomas that became infected.22–25 Infection may occur spontaneously, as a complication of fetal monitoring, or after sepsis or meningitis. Infection of a cephalohematoma can also lead to osteomyelitis, meningitis, and sepsis in the newborn. The most common infecting organism is Escherichia coli, and the infection usually occurs within 3 weeks. In this situation, a diagnostic tap or open irrigation and drainage are indicated.22 However, drainage of the hematoma is not generally advised because of the risk of introducing bacteria. In less than 5% of patients, the cephalohematoma calcifies. If it is large or cosmetically unpleasant, the parents may opt to have it removed. To do so, one merely burs the calcified lesion down to the outer layer of the skull. All bleeding should be judiciously controlled with bone wax and electrocautery.
Skull Fractures
Three types of skull fractures can occur in newborns: linear, depressed or “ping-pong,” and occipital osteodiastasis. The cause of skull fracture is a narrow pelvic passage or pressure against the promontory of the sacrum by forceps and vacuum extractors. The exact incidence in newborns is difficult to determine. Although skull fractures are demonstrable on plain radiographs and CT bone window scans, linear fractures can be difficult to visualize. It has been reported that linear skull fractures occur in as many as 10% of all births.21 Most linear skull fractures involve the parietal bone, although they can occur in the occipital and frontal bones as well. They usually heal within 2 to 3 months and require only a follow-up skull radiograph to rule out a growing skull fracture.13–15 Growing fractures are associated with underlying dural laceration and brain injury (see Chapter 214). Such fractures associated with birth trauma are rare and have been reported in only a few cases.13 Vacuum extractions can rupture suture lines and result in a growing fracture.26
Anywhere from 5.4% to 25% of linear skull fractures have an associated cephalohematoma.4,27,28 In such patients, CT should be performed to rule out an epidural hematoma, which can occasionally require surgical removal (discussed in the next section).
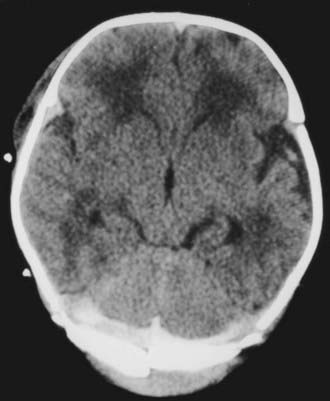
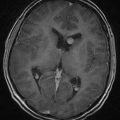
Depressed skull fractures are unique to the newborn period. Because of the thin, pliable nature of a newborn’s skull, the bone cortex indents or buckles inward. True indentation is rare, however, and skull fracture is often observed on CT scans or at surgical repair of a depressed fracture (Fig. 212-1). As with linear fractures, the parietal bone is the most common location.
FIGURE 212-1 Axial computed tomography bone window scan showing a depressed fracture caused by forceps.
For all children with such lesions, CT of the head should be performed to rule out an associated intracranial hemorrhage. There is debate whether these lesions need to be surgically elevated in an otherwise asymptomatic child. There are case reports in the literature of a breast pump, digital pressure, and vacuum extractor being used to elevate these fractures.29–32 However, depressed fractures in newborns often improve and become elevated spontaneously. Loeser and coworkers recommend surgical intervention only for children who have bone fragments in the cerebrum, neurological deficits with or without increased intracranial pressure, or an associated dural tear with leakage of cerebrospinal fluid beneath the galea.33 They would also consider surgical elevation in neonates who failed conservative management, had poor cosmetic results, or had unreliable long-term follow-up.
During a difficult delivery, particularly after a forceps breech delivery, it is theorized that the synchondrosis opens between the squamous and condylar portions of the occipital bone.12,34,35 In this manner, the squamous bone is able to slide anteriorly, thereby allowing excessive vertical molding of the skull. This separation of the occipital bones is known as occipital osteodiastasis. Although it allows the fetal head to pass more easily through the birth canal, it is stressful on the intracranial contents and often results in tentorial and falcine tears, with damage to draining venous structures. In some cases, the squamous portion may sustain a vertical fracture resulting from compression at breech delivery (Fig. 212-2).
Intracranial Hemorrhage
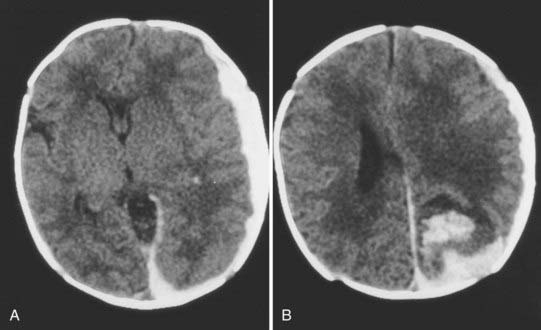
An epidural hematoma may occur without an associated skull fracture in 30% to 40% of cases.29 Because of its pliable nature, the skull may distort without fracturing, thereby tearing the dura away from the undersurface of the cranium. Bleeding may occur from torn emissary dural veins or, less likely, from branches of the middle meningeal artery.29,36 Because of the expansibility of the skull and the compliance of the brain of newborns, an epidural hematoma may initially go undetected (Fig. 212-3). Only after it has reached a significant size do signs of increased intracranial pressure develop in neonates. Initially, they may exhibit irritability with a full fontanelle and increasing head circumference. Later, signs of brain or brainstem compression with pupillary changes may develop.
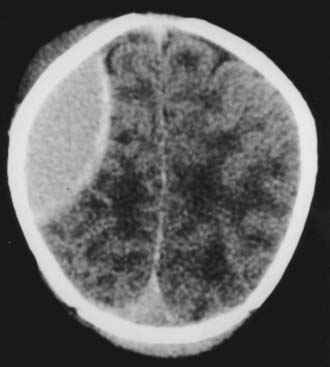
FIGURE 212-3 Axial computed tomography scan showing a large epidural hematoma over the cerebral convexity in a neonate.
A small epidural hematoma less than 1 cm thick can be managed conservatively with serial surveillance CT scans. Surgical treatment consists of open craniotomy with evacuation of the hematoma. The dura is then tacked up to the cranium to prevent reaccumulation of blood. Long-term residual deficits from these lesions are rare. Aspiration of epidural hematomas either with or without ultrasound guidance has been described but has not been compared with the standard of treatment in large series.37,38
Subdural hematomas in newborns may occur over the convexity or adjacent to the tentorium. Hemorrhages overlying the convexity are usually secondary to rupture of cortical veins; those above or below the tentorium are the result of a torn tentorium, falx, or dural sinus.29,39 All such hemorrhages are thought to be caused by excessive vertical molding of the head, and infrequently there is associated occipital osteodiastasis.40 Elongation and distortion of the head, which are part of the physiologic process of parturition, can cause excessive stress during descent through the birth canal. Subdural hygromas discovered because of increased head circumference have been suggested to be a consequence of birth trauma.41
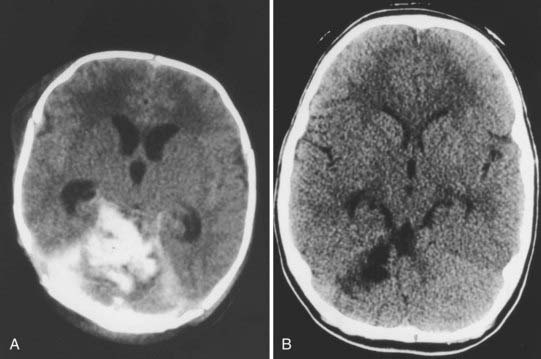
In the cerebral convexity, excessive cranial molding causes stretching of the bridging veins and may tear them. Subdural hematomas in the cerebral convexity are often minimal and subclinical. At times, however, these hemorrhages may be sizable, which can make it difficult to distinguish between a large subdural hematoma and intraparenchymal hemorrhage (Fig. 212-4). These lesions are rare in premature newborns because of underdeveloped bridging veins.39
During passage through the birth canal, the head undergoes compressive fronto-occipital foreshortening. As a result, the distance between the skull base and the vertex increases. Excessive vertical cranial molding causes stretching of the tentorium at the junction of the falx and tentorium, near the vein of Galen. This skull molding is often aggravated by suction delivery. The excessive vertical skull molding and stretching of the tentorium and posterior falx may result in tentorial laceration. The tentorial laceration may extend into the vein of Galen or the straight sinus and result in a posterior fossa subdural hematoma (PFSH). Some hemorrhage may originate from injured cerebellar bridging veins or tentorial leaflets at the attachment to the lateral sinus.34 Most posterior fossa hemorrhages are of venous origin. Rarely, deformation in the region of the incisura causes disruption of the posterior cerebellar artery, the superior cerebellar artery, or their branches. This causes not only hemorrhage but also posttraumatic arterial aneurysm.42 PFSHs can occur in both term and premature newborns.39
Neonates with PFSHs usually exhibit symptoms within 24 hours of birth. In the study by Perrin and associates, symptoms occurred in 14 of 15 neonates within 24 hours of birth; only one child was seen in delayed fashion at 41 days of age.40 The most common initial symptoms are irritability, lethargy, a full fontanelle, hypotonia, poor oral intake, and unstable vital signs. Perinatal seizures may also be associated with PFSH.
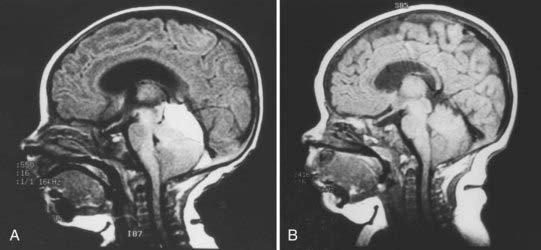
There is much debate among pediatric neurosurgeons regarding the appropriate management of PFSH in neonates.40 One group advocates aggressive surgical drainage of any subdural hematoma causing signs of increased intracranial pressure or brainstem compression.43,44 Drainage may consist of needle aspiration through the lambdoid suture, bur hole placement, or open craniotomy with clot evacuation. It is often difficult to gain access to a newborn baby’s small posterior fossa and to control intraoperative hemorrhage because its source and location are invariably at the region of the superior vermis and tentorial hiatus. Others strongly believe that the friable nature of the neonatal brain is not amenable to surgical intervention and thus surgery should be avoided.12,43 PFSHs often dissolve spontaneously over time (Fig. 212-5). Aggressive intervention is recommended only when signs of serious brainstem compression develop, such as apnea and bradycardia. Hydrocephalus is common in association with PFSHs. An external ventricular drain or cerebrospinal fluid reservoir may be used to manage this obstructive hydrocephalus, which usually resolves within a few weeks of birth (Fig. 212-6). Only rarely does progressive hydrocephalus requiring permanent cerebrospinal fluid diversion develop in these children.45 Most neonates with PFSH have a good outcome when attended to promptly. Children with poorer outcomes have been shown, in retrospect, to have had more extensive traumatic injury, especially of the cerebral hemisphere.
Intraparenchymal hemorrhages usually occur in conjunction with subdural hematomas. Intracerebellar hematomas may be due to mechanical deformation of the intraoccipital synchondrosis during delivery when the lower lip of the squamous portion of the occipital bone is depressed and causes cerebellar contusion or laceration. These hematomas are associated with forceps or breech delivery.17 Neonates have symptoms similar to those of a subdural hematoma alone. They may initially be irritable with a full fontanelle, which may progress to lethargy, seizures, hemiparesis, or bradycardia. An ultrasonographic or CT scan demonstrates the lesion. Small clots causing few or no symptoms can be treated conservatively; larger ones causing signs of brain or brainstem compression require surgical evacuation. As with all surgical interventions in newborns, an experienced surgical and anesthesia team is mandatory, with careful attention paid to hemostasis and blood replacement.
Doumouchtsis SK, Arulkumaran S. Head injuries after instrumental vaginal deliveries. Curr Opin Obstet Gynecol. 2006;18:129-134.
Heyman R, Heckly A, Magagi J, et al. Intracranial epidural hematoma in newborn infants: clinical study of 15 cases. Neurosurgery. 2005;57:924-929. discussion 924-929
Loeser JD, Kilburn HL, Jolley T. Management of depressed skull fracture in the newborn. J Neurosurg. 1976 Jan;44(1):62-64.
Perlow JH, Wigton H, Hart J, Strassner HT, et al. Birth trauma. A five-year review of incidence and associated perinatal factors. J Reprod Med. 1996;41:754-760.
Whitby EH, Griffiths PD, Rutter S, et al. Frequency and natural history of subdural haemorrhages in babies and relation to obstetric factors. Lancet. 2004;363:846-851.
1 Geirsson RT. Birth trauma and brain damage. Baillieres Clin Obstet Gynecol. 1988;2:195-212.
2 Perlow JH, Wigton T, Hart J, et al. Birth trauma: a five-year review of incidence and associated perinatal factors. J Reprod Med. 1996;41:754-760.
3 Puza S, Roth N, Macones OA, et al. Does cesarean section decrease the incidence of major birth trauma? J Perinatol. 1998;18:9-12.
4 Curran JS. Birth-associated injury. Clin Perinatol. 1981;8:111-129.
5 Kolderup LB, Laros RYJr, Musei TJ. Incidence of persistent birth injury in macrosomic infants: association with mode of delivery. Am J Obstet Gynecol. 1997;177:37-41.
6 Koo MR, Dekker GA, van Geijn HP. Perinatal outcome of singleton term breech deliveries. Eur J Obstet Gynecol Reprod Biol. 1998;78:19-24.
7 Mikulandra F, Stojnic E, Perisa M, et al. Fetal macrosomia—pregnancy and delivery. Zentralbl Gynakol. 1993;115:553-561.
8 Kuit JA, Eppinga HG, Wallenburg HCS, et al. A randomized comparison of vacuum extraction delivery with a rigid and a pliable cup. Obstet Gynecol. 1993;82:280-284.
9 Thompson JP. Forceps deliveries. Clin Perinatol. 1995;22:953-972.
10 Williams MC. Vacuum-assisted delivery. Clin Perinatol. 1995;22:933-952.
11 Williams MC, Knuppel RA, O’Brien WF, et al. A randomized comparison of assisted vaginal delivery by obstetric forceps and polyethylene vacuum cup. Obstet Gynecol. 1991;78:789-794.
12 Govaert P, Vanhaesebrouck P, De Praeter C, et al. Vacuum extraction, bone injury and neonatal subgaleal bleeding. Eur J Pediatr. 1992;151:532-535.
13 Hes R, de Jong TH, Pazy Geuze DH, et al. Rapid evolution of a growing skull fracture after vacuum extraction in case of fetal hydrocephalus. Pediatr Neurosurg. 1997;26:269-274.
14 Hickey K, McKenna P. Skull fracture caused by vacuum extraction. Obstet Gynecol. 1996;88:671-673.
15 Papaefthymiou G, Oberbauer R, Pendl O. Craniocerebral birth trauma caused by vacuum extraction: a case of growing skull fracture as a perinatal complication. Childs Nerv Syst. 1996;12:117-120.
16 Plauche WC. Subgaleal hematoma: a complication of instrumental delivery. JAMA. 1980;244:1597-1598.
17 Riihsinghani A, Belsare TJ. Neonatal intracerebellar hemorrhage after forceps delivery: report of a case without neurological damage. J Reprod Med. 1997;42:127-130.
18 Towner D, Castro MA, Eby-Wilkens E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
19 Matheson GW, Davajan V, Mischell DRJr. The use of the vacuum extractor: a reappraisal. Acta Obstet Gynecol Scand. 1968;47:155-165.
20 Benaron DA. Subgaleal hematoma causing hypovolemic shock during delivery after failed vacuum extraction: a case report. J Perinatol. 1993;13:228-231.
21 Hovind KH. Traumatic birth injuries. In: Raimondi AJ, Choux M, Di Rocco C, editors. Head Injuries in the Newborn and Infant. New York: Springer Verlag; 1985:87-109.
22 LeBlanc CM, Allen UD, Ventureyra E. Cephalhematomas revisited. Clin Pediatr (Phila). 1995;34:86-89.
23 Blom NA, Vreede WB. Infected cephalohematomas associated with osteomyelitis, sepsis, and meningitis. Pediatr Infect Dis J. 1993;12:1015-1017.
24 Ellis SS, Montgomery JR, Wagner ME, et al. Osteomyelitis complicating neonatal cephalhematoma. Am J Dis Child. 1974;127:100-102.
25 Miedema CJ, Ruige M, Kimpen JLL. Primarily infected cephalohematoma and osteomyelitis in a newborn. Eur J Med Res. 1999;22:8-10.
26 Hansen KN, Pedersen H, Petersen MB. Growing skull fracture: rupture of the coronal suture caused by vacuum extraction. Neuroradiology. 1987;29:502.
27 Kendall N, Woloshin H. Cephalohematoma associated with fracture of the skull. J Pediatr. 1952;41:125-132.
28 Zelson C, Pearl M. The incidence of skull fractures underlying cephalohematomas in newborn infants. J Pediatr. 1974;85:371-373.
29 Faix RG, Donn SM. Immediate management of the traumatized infant. Clin Perinatol. 1983;10:487-505.
30 Raynor R, Parsa M. Nonsurgical elevation of depressed skull fracture in an infant. J Pediatr. 1968;72:262-264.
31 Schrager GO. Elevation of depressed skull fracture with a breast pump. J Pediatr. 1970;77:300-301.
32 Tan KL. Elevation of congenital depressed fractures of the skull by the vacuum extractor. Acta Paediatr Scand. 1974;63:562-564.
33 Loeser JD, Kilburn HL, Jolley T. Management of depressed skull fracture in the newborn. J Neurosurg. 1976;44:62-64.
34 Govaert P, Calliauw L, Vanhaesebrouck P, et al. On the management of neonatal tentorial damage: eight case reports and a review of the literature. Acta Neurochir (Wien). 1990;106:52-64.
35 Hemsath FA. Birth injury of the occipital bone with a report of thirty-two cases. Am J Obstet Gynecol. 1934;27:194-203.
36 Takagi T, Nagai R, Wakabayashi S. Extradural hemorrhage in the newborn as a result of birth trauma. Childs Brain. 1978;4:306-318.
37 Heyman R, Heckly A, Magagi J, et al. Intracranial epidural hematoma in newborn infants: clinical study of 15 cases. Neurosurgery. 2005;57(5):924-928.
38 Mathur A, Vachharjani A. Ultrasound-guided needle aspiration of cranial epidural hematoma in a neonate: treating a rare complication of vacuum extraction. Am J Perinatol. 2002;19:8-401.
39 Towbin A. Central nervous system damage in the human fetus and newborn infant. Am J Dis Child. 1970;119:529-542.
40 Perrin RG, Rutka JT, Drake JM, et al. Management and outcomes of posterior fossa subdural hematomas in neonates. Neurosurgery. 1997;40:1190-1200.
41 Ney J, Keven J, Mitchell M. Late subdural hygromas from birth trauma. Neurology. 2005;65:517.
42 Piatt JH, Clunie DA. Intracranial arterial aneurysm due to birth trauma: case report. J Neurosurg. 1992;77:799-803.
43 Ravenel SD. Posterior fossa hemorrhage in the term newborn: report of two cases. Pediatrics. 1979;64:39-42.
44 Serfontein GL, Rom S, Stein S. Posterior fossa subdural hemorrhage in the newborn. Pediatrics. 1980;65:40-43.
45 Tanaka Y, Sakamoto K, Kobayashi S, et al. Biphasic ventricular dilatation following posterior fossa subdural hematoma in the full-term neonate. J Neurosurg. 1988;68:211-216.