CHAPTER 278 Treatment of Disk and Ligamentous Diseases of the Cervical Spine
Anatomy AND Pathophysiology
Pathophysiology of Spondylosis
As part of normal aging, degenerative biochemical changes occur in hydrophilic proteoglycan molecules that result in loss of adsorbed water. This eventually leads to a decrease in viscoelasticity of the nucleus pulposus and a reduction in its overall volume and disk height. As a result, the stress under an axial load is translated to the annulus fibrosus and it bulges, with eventual wear and tear causing thinning and further fibrosis. The weakened lamellae allow the fibrotic nuclear material to dissect through the fibers and disrupt the attachment of Sharpey’s fibers to the edges of the vertebral body bone. Subsequently, this process stimulates reactive bony growth and is the origin of early osteophyte formation. In a more acute scenario, dissection of nuclear material through the weakened annulus causes disk herniation. The recent literature shows that the delamination process of the annulus during progressive cervical disk herniation occurs within the lamellae rather than between them, without evidence of any annulus rupture or failure.1
Early reactive bone growth ultimately causes osteophyte spur and ridge formation, which leads to infolding and peeling of the posterior longitudinal ligament from the bone and secondary hypertrophy and ossification. In addition, the loss of disk space height causes initial straightening of the normally lordotic sagittal curvature of the cervical spine. As the center of axial loading shifts anteriorly, a cycle of further degeneration contributes to the chronic compressive vertebral body changes that eventually result in a kyphotic deformity.2 These changes lead to abnormal cervical spine biomechanics and subsequently to hypertrophy or laxity of the facet joint and ligamentum flavum. The cervical spine hypermobility or instability is pathologically stabilized by osteophyte bar formation and uncovertebral joint degeneration. Multiple studies have demonstrated a significant decrease in mobility with advanced age.3 In a more recent study by Miyazaki and colleagues, magnetic resonance imaging (MRI) was performed on patients with degrees of spondylosis ranging from very mild to very severe.4 The early degenerative process affected the mobility of the functional spinal unit, which changed from a normal disk to a more unstable phase with increased mobility. As the degeneration entered later phases, the motion segment stabilized and became more ankylosed. The C4-5 and C5-6 segments were shown to contribute most of the total angular mobility, but their contribution to total angular mobility decreased significantly after severe degeneration. This degenerative cascade eventually leads to neuroforaminal or spinal canal stenosis, or both.
Pathophysiology of Pain
Patients can have clinically significant neurological symptoms or objective findings during any of the aforementioned stages. Symptoms can range from mild axial neck pain to severe cervical myelopathy. Even though the actual source of pain in cervical spondylosis is controversial, there is consensus that it originates from degeneration of the cervical disk or facet joint (or both).5 Multiple studies have demonstrated very rich innervation, both somatic and autonomic, of the cervical intervertebral disk and facet joint.
As the cervical nerve root exits the neuroforamina, it splits into ventral and dorsal rami, which are the source of somatic, proprioceptive, and nociceptive input. The small branches (somatic root) of the ventral ramus join the vertebral nerve (autonomic root) to form the sinuvertebral nerve. The vertebral nerves are formed by the gray rami communicantes, which are branches of the sympathetic trunk and the stellate ganglion. The sinuvertebral nerve arborizes superiorly and inferiorly as it enters the cervical canal through a neuroforamen and supplies the posterior aspect of the annulus (up to the posterior third), the posterior longitudinal ligament, and the dura. The anterior longitudinal ligament and anterior annulus are innervated by the sympathetic trunk and recurrent branches of the gray rami communicantes. It is reasonable to conclude that an annular tear can cause increased afferent output that can be the source of axial neck pain. Moreover, Bogduk and coworkers have proposed that intervertebral disks, especially when under significant stress, can be the source of pain even without obvious annular rupture or herniation.6 An internal annular rupture would suffice because it can cause enough inflammatory changes as it encroaches on the deep and rich innervation of the annulus fibrosus.
In addition to the cervical disk being a source of axial neck pain, the cervical facet joint can also be an important source. The dorsal rami of the cervical nerve roots supply most of the innervation to the facet joints, which are rich in mechanoreceptors and nociceptive nerve endings. Studies by Dwyer and associates have confirmed that stimulation of the facet joint produces a clinically distinguishable, characteristic pattern of pain that enables the construction of pain charts.7 Practitioners can use these charts to isolate the symptomatic joint in patients with cervical zygapophyseal pain. Some authors believe that facet joint pain could be of primary importance, especially in neck pain associated with whiplash injury.8
Pathophysiology of Radiculopathy
The pathologic changes in patients with cervical radiculopathy can be divided into acute and chronic. Acute radiculopathy is usually secondary to soft disk herniation and occurs in a younger patient group. The inflammatory process, which involves a cytokine-mediated response, leads to a decrease in the number of large-diameter myelinated axons. This finding can be seen within the first week9 and results in relatively more prominent motor findings. Chronic radiculopathy, in contrast, usually causes predominantly sensory complaints and is more commonly seen in an older patient group. Chronic radiculopathy is associated with cervical spondylosis. Jancalek and Dubovy showed that changes include thickening of the dura mater and arachnoid membrane around the affected nerve root with associated alteration of the blood-nerve barrier, which eventually leads to nerve root dysfunction as a result of chronic compression.10
Pathophysiology of Myelopathy
Multiple pathologic processes that eventually lead to similar pathologic cervical cord changes in the white and gray matter can cause cervical myelopathy. Even acute cervical cord injury (despite representing a different mechanism and manifestation) has been shown to result in similar pathologic cord findings as those of indolent cervical spondylosis.11
The pathogenesis of myelopathy in patients with cervical spondylosis should be subdivided into three main components that are responsible for the final cord changes.12 The first consists of static factors, which are processes that lead to cervical canal stenosis and cord compression. The second consists of dynamic factors caused by repetitive movement of the compressed cord, which is associated with decreased elasticity and susceptibility to stretch-associated injury. The third component is the final cord changes that are seen histopathologically and include vascular rearrangement, arterial- or venous-induced ischemia and infarction (or both), oligodendrocyte apoptosis, and other cytotoxic cell changes.
The static factors that contribute to decreased canal diameter include acquired spondylosis of the disk, facet, vertebral bodies, and ligaments and subsequent loss of the lordotic curvature. In addition, other less common conditions can contribute, such as congenital cervical stenosis, ossification of the posterior longitudinal ligament, and ossification of the ligamentum flavum. Patients with congenital cervical stenosis are initially asymptomatic but eventually progress to severe cord compression and usually become symptomatic by the third decade. The normal sagittal cervical canal diameter has been identified as being approximately 17 to 18 mm. Based on multiple studies, the canal is considered stenotic when smaller than 13 mm,13 which is associated with a high risk for the development of cord compression with myelopathic changes.
The dynamic factors resulting in cervical cord injury are multifactorial. First, the movements of a severely compressed cord are restricted. Second, flexion of the spine results in overstretching of the cord. This effect is more severe in the setting of a prominent ventral osteophyte complex or with a kyphotic deformity. Conversely, extension causes posterior cord compression by buckling of the ligamentum flavum and shingling of the laminae. Some studies, including MRI, show both flexion- and extension-induced compression, with extension-associated injury causing more severe compression. Panjabi and White13a showed that axial rotation and lateral bending do not cause as significant cord indentations as do sagittal movements. As mentioned earlier, the spondylotic spine can become unstable and result in repetitive cord injury during flexion-extension movements. Furthermore, the spinal cord’s ability to tolerate stretch decreases with age, and thus older adults become extremely susceptible to stretch injury.14 In animal studies, Shi and Pryor confirmed three types of conduction block resulting from and correlating with the extent of stretch injury in the absence of pathologic variables related to vascular damage15: an immediate, spontaneously reversible component that may result from a transient increase in membrane permeability that affects ionic distribution; a second component that may be due to perturbation of the myelin sheath and was reversible with the use of a potassium channel blocker; and a third component that was irreversible and resulted from profound axolemmal disruption.
Clinical Findings
Cervical Radiculopathy
Diagnostic Studies
Diskography
Diskography in the cervical spine remains very controversial, and there is weak medical evidence to support fusion of diskography-positive disks for the treatment of axial neck pain. In patients with chronic axial neck pain but without any definite or conclusive imaging findings to implicate a specific level, diskography may add useful information. The specific indication for the test should be to confirm or evaluate suspected one- or two-level disk disease, generally in patients with de novo axial neck pain that fails conservative treatment. To be used appropriately, concordant pain should be carefully correlated with both the clinical and imaging findings. A recent systematic literature review by Buenaventura and associates showed strong evidence that intradiskal distention (by injecting a small amount of fluid) can produce pain and moderate evidence supporting its use in identifying patients with chronic cervical diskogenic pain.16
Nonoperative Management
Epidemiology
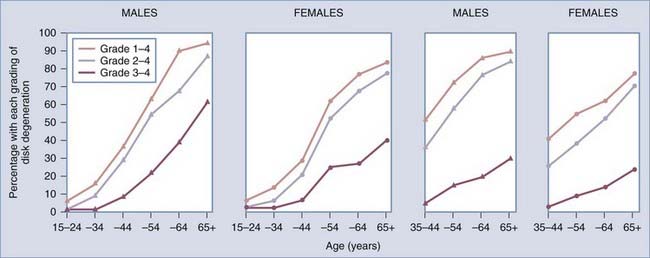
The goal of any treatment should first of all be guided by the natural history of the disease. Cervical spondylosis is a chronic degenerative process, so one should expect to see its prevalence increase significantly with age. Multiple radiographic studies have looked into the epidemiology of cervical spondylosis, and one of the earliest, by Lawrence in 1969, showed that the prevalence based on cervical plain radiographs increases with age17: the prevalence of spondylosis (grades 1 to 4) increased from 15% at 34 years of age, to 60% at 54 years, and to 90% at 65 years and older, with moderate to severe spondylosis (grades 2 to 4) in 8%, 55%, and 85%, respectively (Fig. 278-1). The peak prevalence of symptomatic pain, defined as neck-shoulder-brachial pain, was 9%. A history of nerve root involvement was noted in just 1.7% of the 662 patients studied with moderate to severe cervical spondylosis. Other studies have confirmed the increasing prevalence of cervical spondylosis with age. More recently, Boden and coworkers substantiated the earlier findings with an MRI-based study. Evidence of cervical degeneration was observed in 90% of men older than 50 years and 90% of women older than 60.18
Cervical Pain
The symptoms of chronic neck pain or neurological dysfunction, or both, are less common than the correlative imaging findings. The prevalence of chronic neck pain (duration longer than 6 months in most studies) is approximately 10% in large population studies from Norway and Finland.19 As mentioned earlier, degeneration of the disk and facet joint is probably responsible for the chronic pain in most cases, with other possible causes including radiculopathy, myelopathy, and spondylotic instability. C3 and C4 radiculopathy can also be manifested as trapezoidal and posterior scapular pain, which may require surgical decompression. Confirmatory findings on imaging usually include the dehydrated disk with or without annular tears or facet joint hypertrophy. Treatment is almost exclusively conservative. A nonsteroidal anti-inflammatory drug (NSAID) is a good initial choice. If the pain becomes severe, opioid analgesics and muscle relaxants may be started. Most types of axial neck pain should respond to medical management, especially in those with a history of traumatic whiplash. If there is still no relief and significant disability is present, further differentiation is needed. Provocative diskography and facet joint anesthetic blocks can identify the sensitive component. Based on the findings, one should proceed with either facet joint injection or disk blocks. Physical therapy, primarily isometric exercises, have also been shown to help. If the pain is posterior (dorsal rami) in origin and has a good response to facet joint injection, radiofrequency ablation may provide longer relief of pain. Only patients who fail extensive conservative treatment with correlative imaging and diskographic findings should be considered for surgery. These authors emphasize, however, that there is little medical evidence to support the use of cervical fusion for the treatment of axial neck pain (even if thought to be due to disk degeneration).
Cervical Radiculopathy
Cervical radiculopathy is less common than axial neck pain and occurs in 0.5% to 3% of individuals, with a peak incidence around the age of 50. In 1963, Lees and Turner showed that in about half of patients, the radicular symptoms resolve even though some of these patients might have one temporary recurrence.20 About 25% had improved but persisting symptoms, and in about 20% of patients the symptoms persist or worsen. In a prospective nonrandomized multicenter study by Sampath and colleagues, 246 patients with a primary symptom of cervical radiculopathy were monitored.21 Of these, 155 (63%) returned for follow-up, with 58 (37%) undergoing surgical treatment. When comparing the results in both groups, the surgically treated patients had better overall outcomes with greater average patient recovery despite having significantly worse symptomatology and overall disability initially. Patient satisfaction was higher in the surgically treated patients (80% versus 60%), and even though 75% of the surgically treated patients were reported as being “cured” of their radiculopathy, 26% continued to report significant pain with some neurological deficits, thus limiting their work ability. Also, Boden and coauthors reported a high incidence of asymptomatic disk herniation, with true disk herniation found in 10% of asymptomatic patients younger than 40 years as opposed to just 5% of those older than 40.18 One should conclude that the natural course of radiculopathy is more benign than that of myelopathy, but not as benign as cervical pain alone. A significant proportion of radiculopathy patients, especially those with milder symptoms, readily respond to an initial course of conservative treatment.
Cervical Myelopathy
Because the pathophysiology of cervical myelopathy includes three components—static, dynamic, and cord ischemia—minimizing any of these components would slow progression of the myelopathy. Most of the literature in support of conservative treatment recommends intermittent cervical immobilization for at least 8 hours a day to affect the dynamic component, with some variations.22–24 These studies were conducted over a very short period with a small number of patients. They showed marginal improvement in some patients, with worsening symptoms developing in a significant proportion. In one frequently quoted example, Matsumoto and coworkers in 2001 subjected 27 patients to conservative treatment, with 17 of the 27 showing improvement in Japanese Orthopedic Association scores from 16.2 to 13.6, but 10 patients underwent surgery because of neurological worsening.22 These results certainly raise questions about the utility of intermittent cervical immobilization to treat a lifelong progressive disease.
Lees and Turner20 in 1963 and Nurick25 in 1972 showed the progressive nature of cervical myelopathy, especially in the group older than 60 years. Instead of steady worsening, most patients exhibited long quiescent periods with phases of deterioration. Notably, a significant number of patients with very mild symptoms did not progress over very long-term follow-up (up to 14 years). There have been no good prospective randomized studies that evaluated surgical versus conservative treatment of cervical myelopathy. A prospective nonrandomized trial by Sampath and colleagues showed decreased overall pain and improved functional outcome with surgical treatment, but no comparative neurological improvement.26 However, multiple retrospective studies have demonstrated neurological improvement after surgery. Based on these findings, one cannot predict the course of symptoms for a single patient with mild cervical myelopathy. Careful follow-up studies and neurological examination are therefore necessary, and patients who progress should be considered for surgery.
Operative Management
Cervical Pain
The sinuvertebral nerve arborizes over the posterior longitudinal ligament and extends at least one level above and one level below as it enters the cervical canal through the neuroforamen at every level. As a result, pain originating from three adjacent disks can potentially be contained in one specific sinuvertebral nerve complex, or conversely, one disk can be innervated by two or three overlapping nerves. Such a diffuse distribution of neuronal sensory output is probably the reason for the overlapping pain pattern results seen with diskometry. It has also been shown that stimulation of the sinuvertebral nerve causes a spasmodic response of the surrounding musculature, thereby complicating the interpretation of results. Nonetheless, discordant pain within normal-appearing adjacent disks is not inconsistent with good surgical outcomes for fusion of disks with concordant pain.27,28 Furthermore, patients who undergo analgesic diskography (injection of lidocaine into the disk) and have relief of symptoms may be good candidates for surgery. In a study of axial neck pain published in 1976, Roth reported 93% good or excellent results 2 years after surgery.29 This group of patients with primarily diskogenic pain who failed conservative treatment and had characteristic imaging findings can be considered for one- or two-level anterior cervical diskectomy and fusion (ACDF). In some patients, however, facet joint disease can be partially responsible for the axial pain. The reproducible regional pattern of pain generated on stimulation of a facet joint includes an axial component of pain. Unfortunately, even though facet blocks or other ablative (percutaneous radiofrequency neurotomy) procedures have been shown to be somewhat beneficial, considerable controversy remains. In patients who are shown to have a facet joint pain pattern, posterior fusion could be considered. Nonetheless, despite some of its drawbacks, diskography is our primary method of evaluating axial neck pain clinically. In most centers, however, fusion is seldom recommended for the treatment of axial neck pain without associated radiculopathy or myelopathy.
Cervical Radiculopathy
Posterior Approach for Diskectomy
The posterolateral laminoforaminotomy approach has a good track record, with some of its first proponents, Murphey and Simmons, having reported in 1966 that 90% of their patients achieved relief of their radicular pain.30 More recently, Zeidman and Ducker reported a success rate of 97% in treating radicular pain.31 Because disk herniation is a ventral pathology, the indications for a posterior approach are intuitively limited. Appropriate patient selection for posterolateral laminoforaminotomy becomes critical, unlike an anterior approach, which can be used to treat multiple ventral pathologies. The ideal surgical candidate for posterior foraminotomy is a patient with a one- or possibly two-level lateral or far lateral (foraminal) disk herniation with consistent physical and imaging findings. The posterior approach can also be considered in any patient who may be at risk for the complications associated with an anterior approach. A patient with a history of previous surgery and with dysphagia or vocal cord paralysis is one example.
Cadaver studies have shown that up to 50% of the cervical facet can be safely resected without causing postoperative instability, especially when operating unilaterally.32,33 This will expose up to 5 mm of the nerve root and allow direct visualization of the laterally positioned disk fragment in the nerve root axilla just lateral to the dura of the spinal cord. The posterior approach has multiple advantages over ACDF, including direct visualization of the root, preservation of the remaining disk and motion segment, and avoidance of the specific complications related to the anterior approach, such as recurrent laryngeal nerve injury and dysphagia. The posterior approach also possibly prevents degenerative complications in adjacent segments related to the anterior fusion. Even though posterolateral laminoforaminotomy has multiple advantages, ACDF is a more frequently used approach that has broader indications for the treatment of cervical radiculopathy, myeloradiculopathy, or both.
Experience based on analogous lumbar procedures led to the belief that small paramedian muscle-splitting approaches instead of a midline subperiosteal approach might result in a marked reduction in postoperative pain and muscle spasms. The new technique of microendoscopic posterior cervical laminoforaminotomy for diskectomy was developed on the basis of this concept. Indications for the minimally invasive paramedian approach are nearly identical to those for an open procedure unless another intraoperative maneuver is being contemplated or it is a complex reoperative case. Using a 16-mm operative cylinder, Adamson in 2001 reported 97% excellent or good results (97 of 100 patients), with patients returning to their preoperative employment and baseline level of physical activity.34 Only 2 patients with persistent paresthesias were reported, 1 with disabling neck pain, as well as 2 patients with intraoperative dural tears but without any further intervention other than the application of Gelfoam. More significantly, 90% of the patients went home the same day, 84% did not require additional prescription medication after the first 7 days, and 60% returned to work or to their preoperative daily activities 1 week or less after surgery. Ruetten and colleagues in 2007 reported similar results, but with a new fully endoscopic technique for cervical posterior foraminotomy consisting of a dedicated endoscopic set of instruments and working sheaths with an outer diameter of 7.9 mm.35 The goal of the minimally invasive procedure is to produce the good results and low risk profile of the well-established open procedures while trying to minimize trauma to surrounding tissue. With a significantly decreased operative field, the minimally invasive approaches require appropriate surgeon training and mastery of the local anatomy.
Anterior Cervical Diskectomy with or without Fusion
The advantages of the anterior approach introduced by Smith and Robinson in 1958 were realized immediately.36 From their initial experience, these authors concluded that the posterolateral disk-osteophyte complex was not the primary cause of the problem, even in light of the cord or nerve root compression, as suggested by the physical and roentgenographic findings. In their view, the primary goal of surgery was to decrease motion at the diseased level by fusion; the osteoarthritic spurs were then expected to be resorbed. During the diskectomy portion of the surgery, the posterior cortex of the vertebral bodies was left intact to prevent posterior movement of the iliac bone graft. Cloward37 in 1958 and others subsequently modified the technique, with greater emphasis on a more comprehensive diskectomy and resection of the actual offending elements causing the neurological disability.
Another area of evolution has been in the technique of fusion, with early methods using autologous iliac crest. Subsequently, the use of allograft was adopted to avoid donor site complications. Today, many surgeons now use fusion cages made of PEEK (polyetheretherketone) or titanium, often combined with off-label use of bone morphogenetic protein, to achieve excellent results and avoid the need for allograft or autograft bone.38
The complications of ACDF can be separated into early and late ones. In the largest and most recent study investigating the early complications of ACDF, Fountas and associates in 2007 reported an overall morbidity rate of 19.3% (196 of 1015 patients) and a mortality rate of 0.1% (1 of 1015 patients); the death occurred as a result of esophageal perforation.39 The most common complication was the development of isolated postoperative dysphagia, which was observed in 9.5% of the patients. Postoperative hematoma occurred in 5.6%, but only 2.4% of the patients required surgical intervention. Clinically significant recurrent laryngeal nerve palsy was seen in 3.1% of the patients. Other less common complications included dural penetration in 0.5%, esophageal perforation in 0.3%, worsening of preexisting myelopathy in 0.2%, Horner’s syndrome in 0.1%, instrumentation backout in 0.1%, and superficial wound infection in 0.1%.
Late complications of ACDF include adjacent-segment disease, adjacent-level ossification, pseudarthrosis, subsidence, and late implant malfunction. Even though there is some disagreement on the prevalence of adjacent-segment disease, the consensus would suggest that it occurs in approximately 2.9% of patients per year over a 10-year period.40 About half of patients with this complication are asymptomatic, with the other half requiring surgical intervention because of either neurological deterioration or progressive axial pain. Some authors believe that adjacent-segment disease is the result of progressive degeneration at the level above and below and would have occurred with or without fusion at that level.41 Increasingly, however, mounting clinical data and numerous spine kinematic studies are supporting the concept that adjacent-segment degeneration is largely the result of fusion. Another less well-recognized consequence of ACDF is adjacent-segment ossification. Park and colleagues recently concluded that any adjacent-level ossification within the first 12 months postoperatively (especially that occurring within 3 to 6 months) had a substantial likelihood of progression to advanced ossification by 24 months.42 However, levels with no evidence of ossification at 12 or 24 months or with mild ossification at 24 months are rarely seen to progress to severe ossification. In addition, patients with a plate-to-disk distance (the distance between the tip of the plate and the adjacent disk) of less than 5 mm had a much higher rate of adjacent-level ossification. Unfortunately, the clinical correlation between these progressive changes and the actual pathologic symptomatology has not been well studied.
Other Anterior Approaches (Cervical Disk Arthroplasty, Anterior Endoscopic Microforaminotomy)
Evolution in anterior cervical spine surgery includes the use of disk arthroplasty implants (discussed further in Chapter 293) and anterior endoscopic techniques. Although overall motion may not decrease after one-level ACDF, motion at the level above and below is significantly altered.43,44 To maintain physiologic motion and perhaps avoid adjacent-level disease related to fusion, interest in cervical arthroplasty has increased. Recently, Cheng and associates studied motion in patients who underwent C5-6 ACDF and in normal controls.45 They showed that during 20-degree flexion to 15-degree extension, the average relative angles at the adjacent levels of C6-7 and C4-5 were 13.4 and 8.8 degrees in the fused patients versus 3.7 and 4.8 degrees in the healthy (non-ACDF) individuals. Differences at C3-4 averaged only about 1 degree. Further studies by the same group showed that an artificial cervical disk replacement group exhibited kinematic and kinetic results similar to the healthy adult group.46 The first pilot study of the original Cummins cervical disk implant (Prestige cervical disk, Medtronic Sofamor Danek Memphis, TN) showed that 89% (16 of 18) of patients had both long-term clinical improvement and preservation of motion.47 The original design was a metal-on-metal implant with a ball-and-socket articulation. With better understanding of cervical spine biomechanics, multiple changes were introduced to emphasize anterior-posterior translation and flexion-extension motion. This led to the stainless steel ball-and-trough articulation of the Prestige ST artificial disk, which allows unconstrained motion comparable to that provided by a normal cervical spine segment. The utility of this implant has been confirmed by multiple studies with at least 24 months’ follow-up.48,49
In 2007, Auerbach and colleagues wanted to determine what percentage of their typical patients who undergo ACDF could be candidates for cervical disk arthroplasty.50 For the purposes of their study, they used the published indications and contraindications listed in the trials of four different cervical disk arthroplasty devices. They found that 43% of their patients (167 patients) would have been candidates for cervical disk arthroplasty or 47% if those with adjacent-segment disease were included. Some surgeons might argue against some of the indications cited in this study, but there were no absolute contraindications included. One can therefore see that unlike lumbar disk arthroplasty, total disk replacement may play a greater role in cervical degenerative disease in the future.
Because of the recent introduction and U.S. Food and Drug Administration approval of cervical disk arthroplasty, long-term data are still lacking. In 2007, Mummaneni and coauthors reported the results of a prospectively randomized multicenter study in which cervical disk arthroplasty was compared with ACDF in 541 patients being treated for symptomatic single-level cervical degenerative disk disease.51 Follow-up data were collected for 2 years in 80% (of 276) of those treated by arthroplasty and 75% (of 265) of those treated by ACDF. The arthroplasty group showed a 2-point greater improvement in the neck disability index score than the control group (statistically significant at 1 and 3 months, but not at 12 and 24 months) and also had a statistically higher rate of neurological success and returned to work 16 days earlier. Rates of secondary revision surgery (0 versus 5 patients) and supplemental fixation (0 versus 9 patients) were significantly lower after arthroplasty. The rate of adjacent-segment reoperation was also significantly less in the investigational group (3 [1.1%] versus 11 [3.4%] patients). The implant removal rate was lower in the investigational group than in the control group (1.8% versus 3.4%), but this difference was not statistically significant. Postoperative radiculopathy was the primary reason for implant removal in the arthroplasty group (4 of 5 patients), and all these patients required ACDF. The cervical disk implant maintained segmental sagittal angular motion that averaged more than 7 degrees based on dynamic studies. The arthroplasty group did not report any cases of implant failure or migration.
Besides posterior, completely endoscopic cervical laminoforaminotomy, an anterior endoscopic cervical microforaminotomy and diskectomy technique has been described. Jho and colleagues reported a consecutive series of 104 patients with radiculopathy secondary to either medial or lateral disk herniation or a spur (or both) who underwent one- to two-level anterior endoscopic microforaminotomy.52 Ninety-eight percent of the patients had either good or excellent results, 31% were discharged the same day, and 67% were discharged on postoperative day 1. Of these patients, diskitis developed in 1, transient Horner’s syndrome in 2, and transient postoperative hemiparesis in 1 that resolved in 6 weeks. The authors also performed dynamic cervical radiography, which showed preserved motion in all cases except the patient with diskitis. Furthermore, in addition to treating the pathology causing the radiculopathy, the authors applied a similar approach with minor adjustments to the treatment of both soft and hard disk herniation or osteophytes causing cervical myelopathy. The group also monitored 40 patients who underwent one- to four-level anterior endoscopic microforaminotomy, 30 of whom were included in the final survey.53 Good or excellent results were seen in 90% of the patients, and 87.5% were discharged on the same day or the day after the surgery. In all the patients, dynamic imaging showed preservation of cervical spinal stability.
Cervical Myelopathy
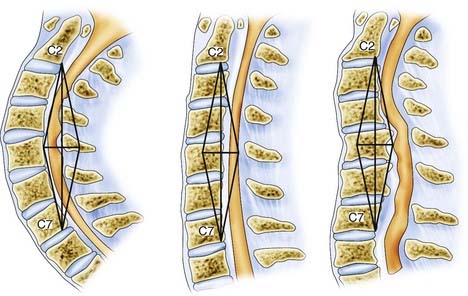
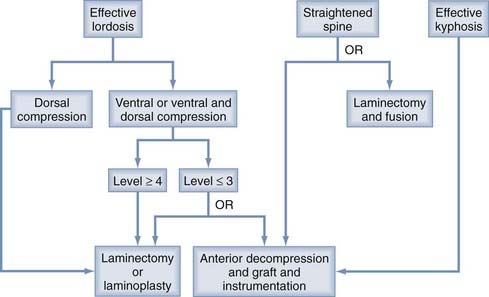
In choosing an approach for any patient with cervical myelopathy, a few important factors have to be evaluated. The most important are the sagittal curvature and the site of compressive pathology. Naderi and associates defined the difference in sagittal cervical curvature (from effective lordosis, to straightened, to a kyphotic spine).54 In evaluating the intrinsic curvature, a line is drawn from the dorsocaudal aspect of the C2 vertebral body to the same C7 point to create the associated gray zone (Fig. 278-2). Effective lordosis is defined when the shaded gray zone is posterior to any of the vertebral bodies. A straightened cervical spine occurs when the posterior-most aspect of the vertebral body is within the gray zone. Effective kyphosis is seen when any portion of the vertebral bodies is posterior to the shaded gray area. In addition to the sagittal curvature, the location of the compressive pathology is critical (Fig. 278-3). In a patient with fewer than three levels of ventral disease, the anterior approach is preferred. Patients with more than three levels of compression are generally treated by posterior decompression, especially with preserved lordotic curvature. A multilevel laminectomy is less demanding overall than an equivalent anterior approach and is associated with shorter operative times and fewer perioperative complications. Laminoplasty should be considered when multilevel posterior decompression is planned, particularly if lordosis is preserved. Preoperative dynamic cervical radiographs should be obtained. In patients with marked stiffening or ankylosis (as commonly seen in older adults), posterior decompression alone might suffice. Younger patients with full range of motion in flexion and extension are at risk for a delayed swan neck deformity after laminectomy, so the surgeon should consider including concomitant lateral mass fixation and posterolateral fusion. Additionally, patients with mobile subluxation at one or several levels should be considered for posterior instrumentation and fusion when multilevel laminectomy is performed.
The presence of posterior ligamentous hypertrophy is directly dealt with during posterior decompression. In the presence of cervical lordosis, posterior decompression will lead to dorsal migration of the spinal cord away from the ventral pathology and result in indirect decompression (see Fig. 278-2). The posterior approach is contraindicated in patients with kyphosis but can be used as additional stabilization in those who require aggressive, long-segment (greater than two-level vertebrectomies) anterior correction and decompression. Clinical and surgical judgment is critical in assessing patients with cervical myelopathy (see Fig. 278-3). The major advantages and disadvantages of the anterior and posterior approaches are summarized in Table 278-1.
TABLE 278-1 Advantages and Disadvantages of the Anterior and Posterior Approaches to Cervical Myelopathy
| ADVANTAGES | DISADVANTAGES | |
|---|---|---|
| Anterior approach | Direct decompression Stabilization with arthrodesis Correction of deformity Axial lengthening of the spinal column Good axial pain relief |
Technically demanding Graft complications Need for postoperative bracing Loss of motion Adjacent-segment degeneration |
| Posterior approach | Less loss of motion Not as technically demanding Less bracing needed Avoids graft complications |
Indirect decompression Preoperative kyphosis Instability limitations Inconsistent axial pain results Late instability |
From Clark CR, ed. The Cervical Spine, 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
, Benzel EC, editor. Spine Surgery: Techniques, Complication Avoidance, and Management. Philadelphia: Churchill Livingstone, 2004.
, Benzel EC, Todd JS. Cervical spondylosis. eds. Neurosurgery, 60, supp 1. 2007.
, Clark CR, editor. The Cervical Spine, 4th ed, Philadelphia: Lippincott Williams & Wilkins, 2004.
, White AA, Panjabi MM. Clinical Biomechanics of the Spine, 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1990.
1 Tampier C, Drake JD, Callaghan JP, et al. Progressive disc herniation: an investigation of the mechanism using radiologic, histochemical, and microscopic dissection techniques on a porcine model. Spine. 2007;32:2869-2874.
2 Shedid D, Benzel EC. Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery. 2007;60:S7-13.
3 Kuhlman KA. Cervical range of motion in the elderly. Arch Phys Med Rehabil. 1993;74:1071-1079.
4 Miyazaki M, Hong SW, Yoon SH, et al. Kinematic analysis of the relationship between the grade of disc degeneration and motion unit of the cervical spine. Spine. 2008;33:187-193.
5 Ahn NU, Ahn UM, Ipsen B, et al. Mechanical neck pain and cervicogenic headache. Neurosurgery. 2007;60:S21-S27.
6 Bogduk N, Windsor M, Inglis A. The innervation of the cervical intervertebral discs. Spine. 1988;13:2-8.
7 Dwyer A, Aprill C, Bogduk N. Cervical zygapophyseal joint pain patterns. I: a study in normal volunteers. Spine. 1990;15:453-457.
8 Bogduk N. The anatomy and pathophysiology of neck pain. Phys Med Rehabil Clin N Am. 2003;14:455-472. v
9 Yoshizawa H, Kobayashi S, Morita T. Chronic nerve root compression. Pathophysiologic mechanism of nerve root dysfunction. Spine. 1995;20:397-407.
10 Jancalek R, Dubovy P. An experimental animal model of spinal root compression syndrome: an analysis of morphological changes of myelinated axons during compression radiculopathy and after decompression. Exp Brain Res. 2007;179:111-119.
11 Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine. 1998;23:2730-2737.
12 Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6:190S-197S.
13a White AA, Panjabi MM. Clinical Biomechanics of the Spine, 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1990.
13 Yue WM, Tan SB, Tan MH, et al. The Torg-Pavlov ratio in cervical spondylotic myelopathy: a comparative study between patients with cervical spondylotic myelopathy and a nonspondylotic, nonmyelopathic population. Spine. 2001;26:1760-1764.
14 Bilston LE, Thibault LE. The mechanical properties of the human cervical spinal cord in vitro. Ann Biomed Eng. 1996;24:67-74.
15 Shi R, Pryor JD. Pathological changes of isolated spinal cord axons in response to mechanical stretch. Neuroscience. 2002;110:765-777.
16 Buenaventura RM, Shah RV, Patel V, et al. Systematic review of discography as a diagnostic test for spinal pain: an update. Pain Physician. 2007;10:147-164.
17 Lawrence JS. Disc degeneration. Its frequency and relationship to symptoms. Ann Rheum Dis. 1969;28:121-138.
18 Boden SD, McCowin PR, Davis DO, et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:1178-1184.
19 Bovim G, Schrader H, Sand T. Neck pain in the general population. Spine. 1994;19:1307-1309.
20 Lees F, Turner JW. Natural history and prognosis of cervical spondylosis. Br Med J. 1963;2:1607-1610.
21 Sampath P, Bendebba M, Davis JD, et al. Outcome in patients with cervical radiculopathy. Prospective, multicenter study with independent clinical review. Spine. 1999;24:591-597.
22 Matsumoto M, Chiba K, Ishikawa M, et al. Relationships between outcomes of conservative treatment and magnetic resonance imaging findings in patients with mild cervical myelopathy caused by soft disc herniations. Spine. 2001;26:1592-1598.
23 Matz PG. Does nonoperative management play a role in the treatment of cervical spondylotic myelopathy? Spine J. 2006;6:175S-181S.
24 Nakamura K, Kurokawa T, Hoshino Y, et al. Conservative treatment for cervical spondylotic myelopathy: achievement and sustainability of a level of “no disability.”. J Spinal Disord. 1998;11:175-179.
25 Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101-108.
26 Sampath P, Bendebba M, Davis JD, et al. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. 2000;25:670-676.
27 Garvey TA, Transfeldt EE, Malcolm JR, et al. Outcome of anterior cervical discectomy and fusion as perceived by patients treated for dominant axial-mechanical cervical spine pain. Spine. 2002;27:1887-1895.
28 Palit M, Schofferman J, Goldthwaite N, et al. Anterior discectomy and fusion for the management of neck pain. Spine. 1999;24:2224-2228.
29 Roth DA. Cervical analgesic discography. A new test for the definitive diagnosis of the painful-disk syndrome. JAMA. 1976;235:1713-1714.
30 Murphey F, Simmons JC. Ruptured cervical disc. Experience with 250 cases. Am Surg. 1966;32:83-88.
31 Zeidman SM, Ducker TB. Posterior cervical laminoforaminotomy for radiculopathy: review of 172 cases. Neurosurgery. 1993;33:356-362.
32 Raynor RB. Anterior or posterior approach to the cervical spine: an anatomical and radiographic evaluation and comparison. Neurosurgery. 1983;12:7-13.
33 Raynor RB, Pugh J, Shapiro I. Cervical facetectomy and its effect on spine strength. J Neurosurg. 1985;63:278-282.
34 Adamson TE. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg. 2001;95:51-57.
35 Ruetten S, Komp M, Merk H, et al. A new full-endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9-mm endoscopes: prospective 2-year results of 87 patients. Minim Invasive Neurosurg. 2007;50:219-226.
36 Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40:607-624.
37 Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
38 Tumialan LM, Pan J, Rodts GE, et al. The safety and efficacy of anterior cervical discectomy and fusion with polyetheretherketone spacer and recombinant human bone morphogenetic protein-2: a review of 200 patients. J Neurosurg Spine. 2008;8:529-535.
39 Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine. 2007;32:2310-2317.
40 Clark CR. The Cervical Spine, 4th ed. Philadelphia: Lippincott Williams & Wilkins. 2004.
41 Goffin J, Geusens E, Vantomme N, et al. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Discord Tech. 2004;17(2):79-85.
42 Park JB, Watthanaaphisit T, Riew KD. Timing of development of adjacent-level ossification after anterior cervical arthrodesis with plates. Spine J. 2007;7:633-636.
43 Baba H, Furusawa N, Imura S, et al. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine. 1993;18:2167-2173.
44 Williams JL, Allen MBJr, Harkess JW. Late results of cervical discectomy and interbody fusion: some factors influencing the results. J Bone Joint Surg Am. 1968;50:277-286.
45 Cheng JS, Liu F, Komistek RD, et al. Comparison of cervical spine kinematics using a fluoroscopic model for adjacent segment degeneration. Invited submission from the Joint Section on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2007;7:509-513.
46 Liu F, Cheng J, Komistek RD, et al. In vivo evaluation of dynamic characteristics of the normal, fused, and disc replacement cervical spines. Spine. 2007;32:2578-2584.
47 Cummins BH, Robertson JT, Gill SS. Surgical experience with an implanted artificial cervical joint. J Neurosurg. 1998;88:943-948.
48 Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine. 2005;3:417-423.
49 Sasso RC, Smucker JD, Hacker RJ, et al. Artificial disc versus fusion: a prospective, randomized study with 2-year follow-up on 99 patients. Spine. 2007;32:2933-2940.
50 Auerbach JD, Jones KJ, Fras CI, et al. The prevalence of indications and contraindications to cervical total disc replacement. Spine J. 2008;8:711-716.
51 Mummaneni PV, Burkus JK, Haid RW, et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198-209.
52 Jho HD, Kim WK, Kim MH. Anterior microforaminotomy for treatment of cervical radiculopathy: part 1—disc-preserving “functional cervical disc surgery.”. Neurosurgery. 2002;51:S46-S53.
53 Jho HD, Kim MH, Kim WK. Anterior cervical microforaminotomy for spondylotic cervical myelopathy: part 2. Neurosurgery. 2002;51:S54-S59.
54 Naderi S, Benzel EC, Baldwin NG. Cervical spondylotic myelopathy: surgical decision making. Neurosurg Focus. 1996;1(6):e1.