Chapter 45 Biliary parasitic disease
Parasitic Infections
Parasitic infections of the biliary tract are a common cause of biliary obstruction in tropical developing countries and less frequently in developed countries. These infections are important, because they can lead to serious complications such as cholelithiasis (see Chapters 30, 35, and 39), recurrent pyogenic cholangitis (Chapter 44), cirrhosis (Chapter 70A, Chapter 70B ), pancreatitis (Chapter 53), and cholangiocarcinoma (Chapters 50A and 50B). The most common parasites of the biliary tract reported in humans are Fasciola, Opistorchis, Clonorchis, and Ascaris species. They have a wide geographic distribution, complicated life cycles, and various clinical manifestations with several diagnostic options available to detect them and multidisciplinary treatments. Millions of people are infected by these parasites, and surgical complications occur quite often.
Fascioliasis
Fascioliasis, or distomatosis, is a zoonosis caused by Fasciola hepatica or F. gigantica (Trematoda: Fasciolidae). The infection is distributed globally on all continents, but F. hepatica predominates in temperate zones, whereas F. gigantica is found in most tropical regions. In approaching a patient with suspected fascioliasis, epidemiologic (Table 45.1), clinical (Table 45.2), and imaging features (Table 45.3) can provide clues for the diagnosis before ordering a diagnostic test to confirm the infection. An algorithm for diagnosis and management is recommended and summarized in Figure 45.1.
| Geographic Area | Risk Factors | Population at Risk |
|---|---|---|
| Latin America (Andean region) Europe Africa Asia Australia |
Watercress Drinking alfalfa juice Green vegetables Contaminated water Travel to endemic areas Living close to irrigation canals Eating salads |
Children in endemic areas Travelers Women Vegetarians |
Table 45.3 Clinical Manifestations, Laboratory Data, and Imaging in Fascioliasis
| Clinical Picture | Imaging and Laboratory Results |
|---|---|
| Acute | |
| Prolonged fever (weeks or months) Abdominal pain (mostly upper abdomen) Hepatomegaly Weight loss Urticaria Ectopic lesions* |
Eosinophilia (any cell count) Anemia Anicteric hepatitis Biliary hemorrhage or hemobilia Subscapular hematoma or hepatic rupture (seen on CT) Hepatic abscesses Tracklike lesions on CT |
| Chronic | |
| Abdominal pain in right upper quadrant Biliary colic Nausea and vomiting Recurrent or intermittent jaundice Uritcaria |
Eosinophilia (sometimes) Cholestasis Hepatic abscesses Liver fibrosis and ultimately cirrhosis Necrotic granuloma (increased ALT and AST levels) Cystic tumors Cholangitis caused by Klebsiella, Escherichia coli, Enterococcus spp. Choledocolithiasis Eosinophilic cholecystitis Achalcolous cholecystitis |
CT, computed tomography; ALT, alanine aminotransferase; AST, aspartate aminotransferase
* Ectopic migration and other clinical manifestations. Acute stage: migratory nodule under the skin or peritoneal cavity, arthralgias, lymphadenopathies, hemolytic anemia, seizures, pleural effusion. Chronic stage: subcutaneous nodules and gastric nodules.
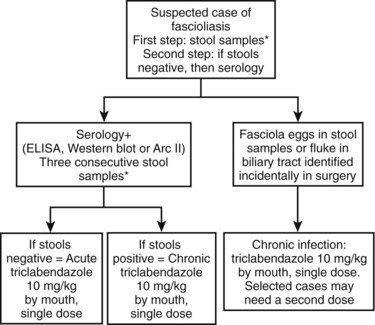
FIGURE 45.1 Summary of management and treatment of a suspected patient with fascioliasis. *Three consecutve stool samples must be examined by a sedimentation technique (Lumbreras, 1962) before ruling out the infection. Stool examination is preferred over serology because of cost effectiveness and availability. In highly suspected cases, a trial of triclabendazole is warranted. Single-dose triclabendazole 10 mg/kg has a cure rate greater than 90%. A second single dose may be used in selected cases (e.g., high intensity of infection in feces, large numbers of parasites in surgery, refractory cases). ELISA, enzyme-linked immunosorbent assay.
Epidemiology
F. hepatica infection was first documented in the Gallo-Roman period (Da Rocha et al, 2006). Today, the estimated number of human infections ranges from 2.4 million to 17 million, and 91.1 million are at risk of infection around the world (Keiser & Utzinger, 2005). In the past, fascioliasis was limited to specific and typical geographic areas, but it is now widespread throughout the world. According to the reported cases, F. hepatica transmission has increased in Europe, the Americas, and Oceania, and in Africa and Asia, where F. gigantica and F. hepatica overlap. The geographical distribution is determined by the intermediate host (Lymnaea spp.) and certain other conditions such as climate, alimentary behaviors, and poverty.
Examples of countries with estimates of the infected population include 830,000 in Egypt, 742,000 in Peru, 360,000 in Bolivia, 37,000 in Yemen, 20,000 in Ecuador, and 10,000 in Iran (Haseeb et al, 2002). Furthermore, other countries that have reported a significant number of cases in years include Argentina (Kleiman et al, 2007), Venezuela (Incani et al, 2003), Chile (Llanos et al, 2006), Ecuador (Trueba et al, 2000), Mexico (Cruz-Lopez et al, 2006), Turkey (Kaya et al, 2006; Turhan et al, 2006), Thailand (Aroonroch et al, 2006), Japan (Inoue K, et al, 2007), Korea (Lee & Kim, 2006), the United States (Fullerton et al, 2006; Graham et al, 2001), Tunisia (Khelifi et al, 2006), and Lebanon (Birjawi et al, 2002), among others. The majority of reported cases in these countries are related to complications from the infection, and the current number of cases is undoubtedly underestimated. On the other hand, globalization and migration of populations from rural areas to large cities have led to a number of cases of fascioliasis in nonendemic areas. In fact, because of clinicians’ lack of familiarity with this parasitic infection in nonendemic areas, the diagnosis can be delayed, which increases the rate of complications (Kang, 2008).
Fascioliasis is distributed globally, with the most affected area in the world being the Andean region of South America. In fact, more than half of the population may carry the infection in some selected regions, with prevalence ranging from 6% to 68% (Marcos et al, 2005b, 2007a; Parkinson et al, 2007). New evidence has shown that proximity of medium- to high-income industrialized cities to rural areas creates a potential source of infection in nonendemic areas because of the importation of contaminated vegetables to the high-consuming markets of big cities. Thus it is not uncommon to see cases of fascioliasis in nonendemic areas. Another factor that contributes significantly to the dissemination of the infection to new areas is the highly adaptable capacity of both the parasite and the lymnaeid snail hosts to challenging meteorologic conditions (e.g., 4200 meters above sea level, Andean Altiplano). Paleoparasitologic studies have shown that the introduction of F. hepatica and its snail host from Europe into the Americas has been relatively recent. Today, fascioliasis as a result of F. hepatica is the vector-borne disease with the widest latitudinal, longitudinal, and altitudinal distribution known. These key factors explain the wide geographical distribution of the disease (World Health Organization [WHO], 2007).
The epidemiologic pattern of transmission of fascioliasis can be classified as follows (Mas-Coma et al, 1999): 1) cases imported to areas where neither human nor animal fascioliasis is transmitted; 2) autochthonous, isolated, nonconstant cases of sporadic infection in areas where animal fascioliasis is present; 3) endemic fascioliasis (hypoendemic ≤1%, mesoendemic 1% to 10%, hyperendemic ≥10%); 4) epidemic fascioliasis (i) in animal endemic areas and (ii) in human endemic areas. Epidemiology is an important determinant of the initial evaluation of a patient with probable fascioliasis.
Life Cycle
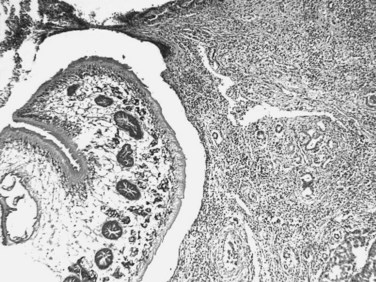
The adult F. hepatica flukes are large, flat, brown, and leaf shaped with a broad anterior portion covered with scalelike spines. Flukes measure approximately 25 to 30 mm by 10 to 15 mm, although F. gigantica can measure up to 75 mm. The adult fluke lives in the common and hepatic bile ducts of the human or animal host, and animals susceptible to becoming reservoir hosts for Fasciola species include mainly cattle, sheep, pigs, buffaloes, and donkeys, although it has been also reported in horses, dogs, goats, llamas, alpacas, dromedaries, and camels. The eggs are oval, yellowish brown, and measure approximately 130 to 150 by 60 to 90 μm (Fig. 45.2).
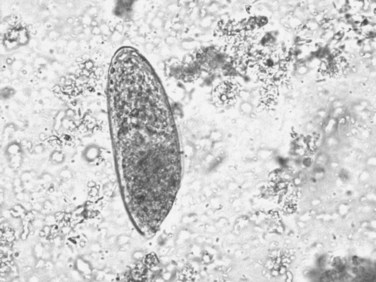
FIGURE 45.2 Adult egg of Fasciola hepatica in microscopic examination of stools by the rapid sedimentation technique.
Sometimes the larvae deviate to other locations; these are called extrahepatic forms or ectopic infections. Maturation from juvenile larvae into adult flukes takes approximately 3 to 5 months, during which time the larvae mature and migrate through the liver into the large hepatic and common bile ducts (Fig. 45.3). Mature flukes consume hepatocytes and duct epithelium and reside for years in the hepatic and common bile ducts and occasionally in the gallbladder. Adult fluke worms produce eggs within 4 months after infection (range, 3 to 18 months); these eggs traverse the sphincter of Oddi and intestine and then continue the cycle of infection. Of note, acute and chronic stages can overlap; this is commonly seen in endemic areas, and it is not unusual to find eggs in the stool samples of patients with acute infection.
Risk Factors
The main source of infection is the consumption of metacercariae-contaminated raw vegetables—such as watercress, lettuce, alfalfa juice, and mixed green salads—or contaminated water from man-made irrigation channels (Marcos et al, 2004, 2005a). Because of epidemic obesity, alimentary habits have changed; for example, higher consumption of green vegetables has led to an increase in the risk for fascioliasis, because it requires vegetables imported from endemic areas (Marcos et al, 2007b). The infection has been also seen in people who have eaten salads in luxury restaurants or hotels in endemic countries, and watercress has traditionally been the most common known source of infection. Nonetheless, a large variety of plants have been described in recent years, such as Medicago sativa (alfalfa in juice) in Peru; Taraxacum dens leonis (dandelion leaves), Valerianella olitoria (lamb’s lettuce), and Mentha viridis (spearmint) in France; green leafy Nasturtium spp. and Mentha spp. in Iran; Juncus andicola and J. ebracteatus (Juncaceae), Mimulus glabratus (Scrophulariaceae), and Nostoc species (Cianofitas) in the Bolivian Altiplano (Bjorland et al, 1995; Mas-Coma et al, 1999).
More women are affected than men, with higher prevalence rates, more severe infections, and with more reported liver or biliary complications (Marcos et al, 2006). Although, the differences are not well understood, alimentary habits, immunologic factors, and proximity to contaminated sources may play a role; for example, children are affected more than adults (Marcos et al, 2007c) likely because of alimentary habits. Reinfection is commonly seen in highly endemic areas, and the parasite can live in the biliary tract for a long time—between 9 and 13.5 years.
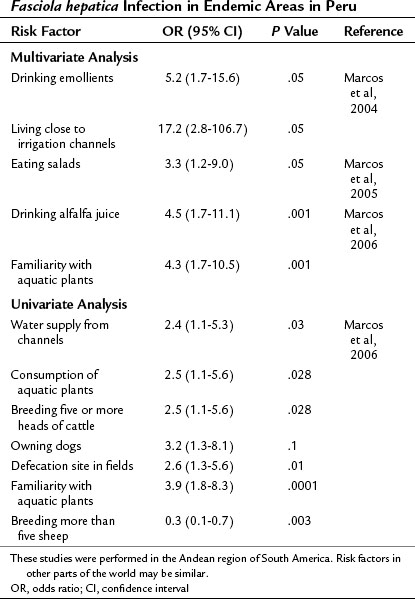
Epidemiologic studies have been carried out in endemic areas to measure the impact of alimentary habits on the acquisition of infection. Drinking beverages made from watercress or alfalfa leaves, called emollients, and living close to irrigation channels were found to be risk factors in a multivariate analysis in the Andean region (Marcos et al, 2004). Eating salads is the common factor among infected families, and it carries a 3.3-fold increased risk of acquiring the infection (Marcos et al, 2005b). In a logistic regression analysis, an age- and gender-matched case-control study comparing 60 infected children found that drinking alfalfa juice carries a 4.5-fold increase in the risk of acquiring fascioliasis, and familiarity with aquatic plants carries a 4.3-fold increased risk (Marcos et al, 2006). Therefore aquatic plants—watercress, alfalfa, and others—and the irrigation channels that carry the metacercariae play a key role in the transmission of fascioliasis in Andean endemic areas. Hypothetically, exportation of plants or other products could lead to transmission in nonendemic areas. Treatment of contaminated plants with high doses of potassium permanganate decreases metacercariae viability and could be used to prevent infection (Ashrafi et al, 2006), although its impact in endemic areas has not yet been tested. A summary of risk factors with studies reporting odds ratios is presented in Table 45.4.
| Ultrasonography | Computed Tomography | Magnetic Resonance Imaging |
|---|---|---|
| Focal areas Multiple nodules Irregular lesions Variable or increased echogenicity Single complex mass Complex cystic mass Parasites moving in gallbladder |
Multiple hepatic metastatic-like lesions Change in position, attenuation, shape in time Abscess-like lesions Low-density serpiginous tortuous tunnel-like branching Subscapular hematoma Cystic calcifications Glisson’s capsule contrast enhancement Single non–contrast-enhanced hypodense irregular mass |
Homogenous hyperintense T2-weighted turbo spin-echo image Subscapular multiple hypointense areas Hypointense T1-weighted 3D gradient-echo image |
3D, three-dimensional
Clinical Manifestations
Fascioliasis has two distinct clinical phases: acute and chronic. Signs and symptoms depend on the worm burden, duration, and phase of infection. In general, the chronic infection is usually diagnosed in epidemiologic studies in endemic areas as a cause of biliary obstruction or in routine stool tests for other reasons. On the other hand, the acute infection has a more florid clinical picture that brings the patient to the emergency room. The clinical manifestations are so variable that mild right upper quadrant pain may call for a step-by-step workup that can lead to the final diagnosis of fascioliasis (Behar et al, 2009).
Acute Infection
The first acute or invasive phase lasts from 3 to 5 months and is caused by the migration of the immature larvae from the duodenum to the liver. Finally reaching the bile ducts, parasites migrate through the liver parenchyma and digest hepatic tissue, causing intense inflammation and hemorrhage proportionate to the number of worms. Migration tracks can be observed in histologic sections, and flukes sometimes die, leaving cavities filled with necrotic debris; these are eventually replaced by scar tissue. Symptoms include prolonged fever, hepatomegaly with abdominal pain, and mild eosinophilia (early infection) or hypereosinophilia (mid- or late-acute infection). Multiple hypodense lesions can be seen on CT scan, similar to metastases (MacLean & Graeme-Cook, 2002). Of note, one of the most frequent manifestations in this acute phase is hypereosinophilia, which is seen in almost all cases. If no eosinophilia is detected at the initial visit, it may be too early in the acute infection; a repeated cell blood count 3 to 5 days later will detect a significant increase in the eosinophil count. Absence of persistent eosinophilia reduces the suspicion for acute infection. In summary, acute fascioliasis is a clinical syndrome similar to acute cholecystitis with significant eosinophilia.
The acute phase presents itself with subcapsular hematomas, hepatic cysts, residual hepatic calcifications, and severe anemia. Hyperbilirubinemia is absent in the acute phase (Marcos et al, 2008b), which distinguishes it from other forms of acute hepatitis. Other manifestations are anorexia, weight loss, nausea, vomiting, cough, diarrhea, urticaria, lymphadenopathies, and arthralgias (Marcos et al, 2005c). Occasionally, the juvenile larvae reach other anatomic locations, such as the subcutaneous tissue, pancreas, eye, brain, and stomach wall, among others (Rana et al, 2007).
Chronic Infection
The chronic phase begins approximately 3 to 6 months after the consumption of the metacercariae, when the parasite reaches the bile ducts. By macroscopic examination, the liver has large, dilated, thick-walled, and calcareous bile ducts with yellowish brown bile. By microscopic examination, the bile ducts have a thickened hyperplastic wall with marked fibrosis (Haridy et al, 1999; Marcos et al, 2007c). Symptoms usually reflect biliary obstruction with colicky pain in the right upper quadrant, epigastric area, or upper abdomen (Rana et al, 2007; Maco et al, 2003; Jimenez et al, 2001). It can also be a silent, potential threat: the parasites can survive for longer than 10 years, and infection is commonly asymptomatic (Marcos et al, 2004).
Liver function tests during this phase are consistent with an extrahepatic cholestasis syndrome (Dobrucali et al, 2004) that can lead to surgery to treat the biliary obstruction (Jimenez et al, 2001). Moreover, an increase in liver enzymes can be present with minimal symptoms in endemic areas. In Egypt, it was found that patients with fascioliasis had significant liver enzyme abnormalities: elevation of alanine aminotransferase in 21.5% of the patients, aspartate aminotransferase in 21.9%, total bilirubin in 16.5%, gamma-glutamyltransferase in 80.6%, and alkaline phosphatase in 76.4% was reported. Excluding viral liver infections, F. hepatica infection is a significant cause of cholestasis in endemic areas (P < .05) (El-Shazly et al, 2005).
Imaging abnormalities were also found on ultrasound (US), including hepatomegaly, splenomegaly, periportal fibrosis, thickened gall bladder wall, dilated common bile duct, parasites in the gallbladder and common bile duct, stones in the gallbladder, stones in bile duct, cystic lesions in the liver, focal lesions in the liver, and ascites (El-Shazly et al, 2001).
Fasciola may also cause acute eosinophilic cholecystitis (Umac et al, 2006) along with pruritus and intermittent jaundice (Umac et al, 2006; Marcos et al, 2002). The parasites appear as small intrahepatic cystic lesions (Aroonroch et al, 2006) or as a large, multiloculated cyst that causes abscesses. On imaging parasites may appear very similar to echinococcosis (Maeda et al, 2008). Bacterial superinfection of Fasciola cysts is a complication of the chronic phase. Recent studies in a rat model have shown a significantly increased risk of bacterobilia in the chronic infection (Valero et al, 2006) and with gallstones (Valero et al, 2003). Even after successful treatment, abdominal pain and weight loss may still be present in approximately 2% to 4% of patients for several months (Rondelaud et al, 2006), and morbidity in these patients is significant.
Eosinophilia is absent in approximately half of the chronic cases. Upon admission to a tertiary health center, 47% of 277 complicated cases had eosinophilia (Blancas et al, 2004). A similar percentage was found in 101 chronic cases from the Andean region and other endemic areas: 48% had eosinophilia above normal levels, and only 14% had more than 1000 eosinophils/mL (Alban et al, 2002). In another study, about half of a group of 61 children in the Peruvian Altiplano with chronic fascioliasis had eosinophilia (Marcos et al, 2002). In Turkey, two (11%) of 18 subjects with fascioliasis had eosinophilia (Turhan et al, 2006).
Eosinophilia is present in a minority of cases of fascioliasis in the chronic phase. If present, it is generally mild (Gil-Gil et al, 2006). Few cases in the chronic phase have high-grade eosinophilia, in contrast with the acute phase, which presents with hypereosinophilia in almost all cases. Finally, a wide variety of other infectious agents are associated with eosinophilia, such as Strongyloides stercoralis, Ascaris lumbricoides, and hookworms or other helminths. Despite the fact that these are the most common parasitic causes of eosinophilia, they do not typically cause hepatic lesions, nor do they reach the high levels that we observe in patients with acute fascioliasis.
Another presentation of the chronic infection is hemobilia as a result of ulcerative lesions in the biliary tract caused by the adult parasite (see Chapter 105; Bahçecioglu et al, 2007). A granulomatous chronic inflammation may also be triggered by parasite ova in the liver or in other anatomic locations (Marcos & Terashima, 2007; Naresh et al, 2006). New data developed in an animal model have demonstrated a persistent immune suppression in advanced chronic infection (Gironès et al, 2007), suggesting that the infected host may be susceptible during the chronic phase to any Th2-suppression–dependent infection. Hence, a chronic immunosuppression may predispose to bacterial infection that can be life threatening.
An association exists between fascioliasis and liver fibrosis. It appears that hepatic fibrosis may evolve in some susceptible hosts, depending on the time and burden of infection. For instance, almost 50% of cattle infected chronically by fascioliasis had cirrhosis (Marcos et al, 2007c). In addition, hepatic cirrhosis has been reported in infected children (Almendras-Jaramillo et al, 1997; Marcos et al, 2005a) and adults (Heredia et al, 1984; Sanchez-Sosa et al, 2000), especially those with high-density infections. New data on the pathogenesis of hepatic involvement associated with F. hepatica infection have implicated cathepsin L1 and its collagenolytic function associated with tissue-invasion (Stack et al, 2007), with proteolytic activity leading to collagen type I expression and ultimately to hepatic fibrosis. The chronic infection can also have a negative impact on the nutrition and health of children in endemic areas.
Imaging Studies
The most common imaging findings in fascioliasis are summarized in Table 45.3.
Abdominal Ultrasound
Findings in the acute phase include focal areas of increased echogenicity, multiple nodular or irregular lesions of variable echogenicity, or a single, complex mass in the liver that resembles malignancy mimicking malignancy (Cosme et al, 2001, 2003). In frequent travelers, an abnormal liver US showing a complex cystic lesion warrants a workup for fascioliasis or other parasitic disease, such as echinococcal infection. In the chronic phase, US is even less specific, although the adult parasites can be visualized in the gallbladder (Bonniaud et al, 1984; Kabaalioglu et al, 1999; Gonzales-Carbajal et al, 2001). Overall, the detection rate is extremely low if used as the sole diagnostic tool. For instance, of 76 subjects with chronic fascioliasis evaluated by means of abdominal US, only 11 patients (14%) had visualized parasites, and in only two cases (2.6%), the parasites were spontaneously moving into the gallbladder. Therefore, the detection-rate of F. hepatica chronic infection by US is disappointingly low (Richter et al, 1999) and not specific (Turhan et al, 2006).
Computed Tomography
The most common computed tomography (CT) findings include multiple hepatic metastasis-like lesions that change in position, attenuation, and shape over time (Marcos et al, 2008). Initial lesions may be easily confused with hepatic metastases. Other findings are hepatomegaly, tract-like hypodense lesions with subcapsular location, subcapsular hematoma, and cystic calcifications (Marcos et al, 2008; Loja et al, 2003). The hepatic lesions correlate with the time of infection. Early infection is associated with contrast enhancement of the Glisson capsule as a result of inflammation stimulated as the juvenile parasite penetrates the liver capsule. This occurs in the early stage of the acute infection (first month of infection). In the intermediate stage (after the first month), multiple hypodense nodular areas (abscesslike lesions) or low-density serpiginous, tortuous, tunnel-like branching lesions that range from 2 to 10 mm are created by parasite migration through the liver and are typically visualized in the subcapsular region (MacLean & Graeme-Cook, 2002; Gonzalo-Orden et al, 2003). In the late stage (≥3 months), a necrotic granuloma is seen that appears as a single, non–contrast-enhanced hypodense irregular mass in the liver parenchyma, more central than peripheral (Kim KA et al, 1999; Noyer et al, 2002). Finally, part of the differential diagnosis of liver calcification is fascioliasis, which generally means an old infection of at least 6 months’ duration. Although the characteristics of these cyst calcifications seem to be unique to fascioliasis, this finding adds a new agent to the list of infectious diseases associated with tissue calcifications, such as echinococcosis, paragonimiasis, histoplasmosis, toxoplasmosis, and others.
Magnetic Resonance Imaging (MRI)
Only a few cases visualized on MRI imaging have been reported. T2-weighted turbo spin-echo MRI showed a homogeneous hyperintense area in a subcapsular location containing multiple hypointense areas. A T1-weighted three-dimensional gradient-echo image displayed homogeneous contrast enhancement (Orlent et al, 2007). The hypodense lesions observed in the CT scan are of hypointense signal in T1-weighted imaging and hyperintense in T2 (Kabaalioglu et al, 2000; Aksoy et al, 2006).
Diagnosis
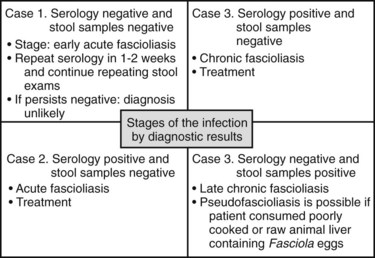
The diagnosis of the acute phase of fascioliasis is confirmed by serology or response to therapy. Accurate identification of early disease has been difficult because of the lack of a confirmatory test; the serologic test that requires medium-high technology is not available in most endemic areas. Nonetheless, if the pretest probability or clinical suspicion is unequivocally high, an antiparasitic trial is warranted. Diagnostic criteria are significant clinical improvement and decreasing levels of eosinophils in the 3 to 5 days after a trial of triclabendazole (Marcos, 2008b). The results of the serology and stool sampling, the possible stage of the infection, and the clinical significance are illustrated in Figure 45.4.
Acute Phase
Among the serologic tests developed in the last few years, an enzyme-linked immunoabsorbent assay (ELISA) detects the antibodies against cathepsin L1 and is highly sensitive and specific for the acute phase of fascioliasis. It is also called Fas2-ELISA (cathepsin L1–based antibody), and it is more specific than Western blot and Arc II (Espinoza et al, 2005, 2007). This ELISA has been tested in 634 infected children from endemic areas in Peru and has been found to have a sensitivity of 92.4% and a specificity of 83.6%, with a negative predictive value of 97.2% (Espinoza, 2007). Acute F. hepatica infection must be considered in patients with eosinophilia, prolonged fever, abdominal pain, multiple hepatic abscesses, or metastases-like lesions in the peritoneum or liver before starting multiple diagnostic tests. Recognizing the clinical scenario early may allow timely and noninvasive identification of this infection.
Chronic Phase
The gold standard test for the chronic infection is the visualization of the parasite eggs in the stool of the host. A serologic test is used when an adequate stool test is persistently negative despite a high pretest probability. A sedimentation technique must be performed on serial stool specimens (at least three) from different days to increase the likelihood of detecting the eggs in the stools, and an experienced technician is required. The intermittent deposition of parasite ova in the biliary duct can decrease the sensitivity of the sedimentation technique. Again, a high pretest probability warrants a trial of triclabendazole. The sedimentation technique that can be performed in any laboratory in the world is the rapid sedimentation technique (RST) described by Lumbreras and colleagues (1962) in Peru. This test is inexpensive, easy to perform, and highly sensitive, with a higher sensitivity than the ether-formalin concentration method (Marcos et al, 2007a). The Kato-Katz technique may be used to measure the intensity of infection (Katz et al, 1972), and it can be also helpful for the diagnosis.
Fasciola and Liver Fibrosis
F. hepatica infection causes bile duct hyperplasia (Hamir & Smith, 2002), increased levels of proline (Campbell et al, 1981; Wolf-Spengler & Isseroff, 1983), and type I and III collagen in the liver (Mark & Isseroff, 1983); these anomalies are similar to the progression observed in cirrhosis and other pathologic conditions, including wound healing. However, few studies have attempted to identify factors associated with liver fibrosis, which is a significant clinical outcome of the infection (Marcos et al, 2007c; Perez et al, 1999; Phiri et al, 2006; Shirai et al, 2006). In addition, liver cirrhosis has been reported in both children and adults with fascioliasis (Almendras-Jaramillo et al, 1997; Heredia et al, 1984; Marcos et al, 2005a; Sanchez-Sosa et al, 2000). In cirrhotic patients in an endemic country, 9.1% were positive for Fas2-ELISA (Marcos et al, 2009b). In conclusion, the chronicity of the infection can lead to liver fibrosis.
Diagnosis of Fascioliasis by Surgery
In the cases reported in the literature, the majority that underwent a surgical or invasive procedure were patients in the chronic phase of the infection whose initial clinical problem was biliary obstruction and choledocholithiasis (Kim et al, 2006). The detection rate of the adult parasite in operations of the biliary tract is low. In a series of 162 cases of cholecystectomy in an endemic area, only 1.2% had F. hepatica in the gallbladder (Alban et al, 2002); however, several cases have been diagnosed in the operating room incidentally.
Because the chronic phase may be seen with or without very mild eosinophilia, a high level of suspicion should always be maintained in cases of biliary obstruction in endemic areas or in immigrants or travelers from endemic areas. Nine cases have been diagnosed and managed by endoscopic retrograde cholangiopancreatography (ERCP), and only two had mild eosinophilia (Bahçecioglu et al, 2008; Diaz Fernandez et al, 2005; Gulsen et al, 2006). A single dose of triclabendazole must be given after the procedure to ensure elimination of any parasites missed by endoscopy or remaining in other anatomic locations. The adult parasite can also be found during an elective laparoscopic cholecystectomy (Bulbuloglu et al, 2007).
Even in highly endemic areas, the diagnosis can be missed initially, and cases of parasites accidentally obstructing percutaneous biliary drainage catheters have been reported (Maco et al, 2003). Clinical judgment is mandatory when biliary obstruction is associated with cholangitis that requires surgical intervention. In cases of biliary obstruction, both surgical intervention to manage the obstruction and antiparasitics are the cornerstones in the treatment when clinically indicated.
Interestingly, fascioliasis can be misdiagnosed as echinococcal because the former may have a strong cross-reactivity in the ELISA. Patients may end up with surgical intervention to remove the pseudocyst of Echinococcus, when parasites of F. hepatica were causing an intrahepatic cyst (Das et al, 2007). In other situations, adult parasites were found when trying to remove a possible malignancy or metastasis-like lesion from the colonic wall (Makay et al, 2007), neck (Marcos et al, 2009a), epidural space (Vatsal et al, 2006), eye (Dalimi & Jabarvand, 2005), and breast (Naresh et al, 2006).
When fascioliasis affects the pancreatic duct (Parsak et al, 2007), the management, either surgical or conservative, should be individualized. However, when indicated, drainage can be a reasonable approach, followed up by a trial of triclabendazole with antispasmodics to reduce the severe abdominal pain experienced after the death of the parasite. Complications such as pancreatic duct obstruction and consequent pancreatitis may be life threatening, but they are rare (Echenique-Elizondo et al, 2005).
In cases of cholangitis (Ait Ali et al, 2002), emergent percutaneous drainage is recommended, because despite the antiparasitic medication, the dead parasites will remain in the biliary duct for a few days before being released, thus interrupting the drainage through the ampulla of Vater. The most common organisms identified in an animal model were Escherichia coli (45%), Enterococcus faecalis (45%), and Klebsiella pneumonaie (10%) (Valero et al, 2006). No data in humans are available, but the fact that E. faecalis is present in almost half of the cases of bacterobilia in the animal model should be taken with special consideration for antibiotic choice. It is recommended that a single antibiotic be given, such as a β-lactam/lactamase inhibitor for mild to moderate infection or a carbapenem for severe infection. A combination therapy of cephalosporin or fluoroquinolone is also acceptable. Ideally, antimicrobial therapy should be adjusted according to the organism identified, with susceptibilities to common antimicrobials.
Treatment
Triclabendazole is the treatment of choice for both phases of Fasciola infection (Keiser & Utzinger, 2007a). The cure rate exceeds 90% for acute stages after a single dose of 10 mg/kg (Marcos et al, 2009), and similar results have been obtained in the therapy of chronic infection (Apt et al, 1995; Talaie et al, 2004; El-Tantawy et al, 2007). This drug has a better absorption with a fatty meal, and the most frequent adverse event is biliary colic caused by the passage of dead or dying parasites through the bile ducts. Triclabendazole was introduced in the early 1980s for the treatment of F. hepatica infections in livestock, and it has been placed on the WHO List of Essential Medicines (http://www.who.int/medicines/publications/essentialmedicines/en/) because of its efficacy and cost effectiveness.
Treatment of fascioliasis with triclabendazole was shown to reduce the prevalence significantly, from 5.2% to 1.2% in 7 years (Abdussalam et al, 1995). However, the intensive use of triclabendazole has resulted in the development of resistance in animals (Mottier et al, 2006; Alvarez-Sanchez et al, 2006). On the other hand, in places where triclabendazole for humans is not available, the veterinary form can be effective as well. In Peru, a single dose of 10 mg/kg cured 96%, whereas 10 mg/kg for 2 days cured 100% with no major adverse effects registered (Terashima et al, unpublished data). The most important adverse effect was abdominal pain or biliary colic during the first week of treatment. This pain is likely caused by the passage of dead or dying parasites through the bile ducts (Millan et al, 2000; Richter et al, 1999). Antispasmodics may decrease or completely alleviate the transitory episodes of abdominal pain and should be used in most cases at the beginning of the therapy or even before the first dose.
A single dose of triclabendazole is effective and tolerable, without major side effects, and it may also be used as a diagnostic tool (Marcos et al, 2007c). However, given the unlikelihood of any new drugs against F. hepatica being developed in the foreseeable future, the emergence of resistance represents an important threat (Alvarez-Sanchez et al, 2006), although resistance in humans has not been reported.
Many years ago, parenteral dehydroemetine at doses of 1 mg/kg for 10 days was used. Then, bithionol was applied at doses of 30 to 50 mg/kg every third day for a total of 10 to 15 doses, although it is cardiotoxic and very expensive. Other drugs, such as nitazoxanide, have been evaluated for fascioliasis, but cure rates have been disappointingly low. Adult patients who received a 7-day course of nitazoxanide had a cure rate of 60%, but the rate was only 40% in children (Favennec et al, 2003). Because of this, and because of the increasing triclabendazole resistance in animals, new drugs have been evaluated and tested in animals with fascioliasis. For instance, artesunate, artemether, and OZ78 have fasciocidal properties in animals, and they are promising drugs for the near future, should resistance to triclabendazole become a problem (Keiser et al, 2007a, 2009). Albendazole, metronidazole, and praziquantel are not recommended for fascioliasis treatment.
Future Directions and Vaccines
Because fascioliasis constitutes a major economic impact, development of effective vaccines would be an important advance. Preliminary studies in animals have reported significant advances (McManus & Dalton, 2006; Spithill & Dalton, 1998). Cysteine proteinases released by F. hepatica play a key role in parasite feeding and migration through host tissues and in immune system evasion. A recombinant cysteine proteinase (CPFhW) expressed as inclusion bodies in E. coli was used for enteral vaccination of rats against fascioliasis. In that study, oral vaccination reduced the parasite burden by 78% to 80% after a challenge with metacercariae (Kesik et al, 2007). The glutathione transferase superfamily (GST) from liver fluke plays phase II detoxification and housekeeping roles and has been shown to contain protective vaccine candidates (Chemale et al, 2006). Promising future research will yield meaningful immunologic targets to prevent the infection, especially in the well-recognized endemic areas and particularly in children, but so far the vaccines are targeted only to animals.
Clonorchiasis and Opisthorchiasis
C. sinensis and O. viverrini, small Asian liver flukes 8 to 15 mm in length, are very similar in adult morphology and genetics but differ in geographic distribution (Park, 2007). There are 601 million and 79.8 million people at risk of infection with Clonorchis and Opisthorchis, respectively, and C. sinensis is endemic in northeast China, southern Korea, Japan, Taiwan, northern Vietnam, and the far eastern part of Russia (Rim, 2005), and O. viverrini is endemic in Laos, Thailand, Vietnam, and Cambodia. O. felineus infection is the most prevalent foodborne liver fluke infection of humans in Russia, Ukraine, and Kazakhstan. In Thailand, approximately 6 million people are infected with O. viverrini (Kaewpitoon et al, 2008). In China, Clonorchis infections have more than tripled over the last decade, with 15 million people infected in 2004 (Lun et al, 2005). Similar to fascioliasis, the geographic pattern of these small flukes is not uniform. For instance, in Thailand, the greatest prevalence of opisthorchiasis is in the north (19.3%) and northeast (15.7%) compared with the central (3.8%) and southern regions (0%). In general, the infection is acquired by eating raw or uncooked cyprinoid fish products in rural areas or dishes such as koi-pla (Sayasone et al, 2007). Some cases are commonly documented in North America, mainly imported by Asian immigrants.
Clinical Manifestations
Clonorchis sinensis
Acute infection by C. sinensis is usually asymptomatic, but some patients may have fever, rash, malaise, and right upper quadrant abdominal discomfort. Chronic infection reflects the worm burden and may appear as recurrent pyogenic liver cholangitis (see Chapters 43 and 44), cholecystitis (see Chapter 31), obstructive jaundice, hepatomegaly, cholecystitis, multiple hepatic tumours (Liao et al, 2006), and cholelithiasis (Park & Son, 2008; Stunell et al, 2006). An association between clonorchiasis and cholangiocarcinoma has also been reported (Choi et al, 2006). Severe C. sinensis infection is a significant risk factor for malignant changes in bile ducts and surrounding liver tissues occurring as a result of direct contact with C. sinensis worms and their excretory-secretory products.
Opisthorchis felineus
Infection with O. felineus usually follows consumption of raw, slightly salted, and frozen fish (“stroganina”). Acute symptoms occur 2 to 4 weeks after eating raw fish. These include high-grade fever, nausea, vomiting, abdominal pain, malaise, arthralgias, lymphadenopathy, and skin rash (Tselepatiotis et al, 2003). Peripheral eosinophilia is a common finding, especially during the first 2 to 6 weeks of the infection, together with raised liver enzymes. In chronic infection, eosinophilia is usually milder, but patients may present with suppurative cholangitis and liver abscess as a result of biliary obstruction.
Consequences of Chronicity of Infection
The higher the intensity of anti–O. viverrini antibody titers, the higher the risk for cholangiocarcinoma (Honjo et al, 2005). The International Agency for Research on Cancer recognizes this parasite as a category I carcinogen. The lesions that predispose to malignant changes in O. viverrini are evident as a dilation of subcapsular medium and large bile ducts with a prominent fibrotic wall, periductal inflammatory cell infiltration, goblet cell metaplasia, epithelial and adenomatous hyperplasia, and periductal fibrosis. The pathogenesis of O. viverrini–mediated hepatobiliary changes may be due to mechanical irritation or to its metabolic products (Sriamporn et al, 2004). Several N-nitroso compounds and their precursors occur at low levels in fermented food, such as preserved mud fish paste (pla ra), a condiment ubiquitous in the cuisine of northeastern Thailand and Laos (Sripa et al, 2007). The study of O. viverrini genes should expedite molecular studies of cholangiocarcinogenesis and accelerate research focused on developing new interventions, drugs, and vaccines that might help in controlling O. viverrini and related flukes (Laha et al, 2007). Similarly, recent studies show a strong association between C. sinensis and the development of cholangiocarcinoma (Choi et al, 2006). For instance, a recent epidemiologic study in Korea correlates the prevalence of C. sinensis and the incidence rate of cholangiocarcinoma: C. sinensis prevalence was 2.1% in Chuncheon, 7.8% in Chungju, and 31.3% in Haman; cholangiocarcinoma incidence rate was 0.3, 1.8, and 5.5 per 100,000 population, respectively (Lim et al, 2006). Hepatocellular carcinoma also has been associated with clonorchiasis, along with hepatitis B virus and alcohol consumption as cofactors (Tan et al, 2008). It seems plausible that cholangiocarcinogenesis associated with clonorchiasis is the cumulative end result of a multifactorial carcinogenic mechanism, although the mechanisms involved are not completely understood.
Improving diagnosis with new serologic tests may be helpful, but such tests cannot distinguish between recent or past infection. Currently, the Ov-CP-1–based ELISA shows a sensitivity of 95% and specificity of 96% in serum coinfected with hookworm, minute intestinal fluke, S. stercoralis, Taenia spp., Giardia lamblia, and E. coli infection (Watthanakulpanich et al, 1997). The sensitivity and specificity are similar to other studies using an ELISA based on recombinant trematode cysteine protease such as C. sinensis (sensitivity 81.3% to 96%, specificity 92.6% to 96.2%) (Nagano et al, 2004; Zhao et al, 2004).
Human clonorchiasis and opisthorchiasis are primarily diagnosed by the detection of eggs in feces. The Kato-Katz method is accepted as the best for fecal examination, although sometimes the eggs may not be detected because of biliary obstruction or intermittent egg excretion similar to that encountered with fascioliasis. In light infections, with less than 10 adult worms in the biliary tract, a polymerase chain reaction (PCR) detecting the DNA of the adult parasite in stools may be helpful (Duenngai et al, 2008). Intrahepatic duct dilation is the most common finding on US imaging (76% of patients), and increasing periductal echogenicity and gallbladder sludge are seen only in patients with heavy infection (Choi et al, 2005). Recently, Ruangsittichai and colleagues (2006) reported high sensitivity and specificity using a recombinant eggshell protein with potential for the serodiagnosis of human opisthorchiasis. However, detection of O. viverrini DNA is expensive and requires skillful personnel.
Treatment
Praziquantel, a derivative of pyrazino isoquinoline, is the drug of choice for O. viverrini, O. felineus, and C. sinensis treatment. For O. viverrini, a single dose (40 to 50 mg/kg) of praziquantel treatment is indicated, with a cure rate between 91% and 97%. For clonorchiasis, the recommended dose of praziquantel is 25 mg/kg three times at 5-hour intervals in 1 day (total dose 75 mg/kg), with a cure rate of 83% to 85% (Rim, 2005).
Other Parasitosis of the Biliary Tract
Occasionally, ERCP may diagnose parasitosis of the biliary tract. For example, of 3548 ERCPs performed for extrahepatic cholestasis, cholangitis, and choledocolithiasis (see Chapters 31, 43, and 44) in a moderate endemic area in Eastern Europe, only 24 (0.66%) were found to show biliary parasitosis, such as hydatid cystic disease (n = 16; see Chapter 68); in addition, 8 showed partial obstruction of the biliary tract, and 8 had ruptured cysts; F. hepatica (n = 4) and A. lumbricoides (n = 4) were also found. Endoscopic sphincterotomy was performed, after which the choledochus was examined carefully using a balloon catheter and basket procedure. ERCP is very useful in the therapy of biliary parasitic infections. The treatment for Ascaris in the biliary tract is elimination of the adult parasite through the endoscope, followed up by a single, oral dose of albendazole (400 mg).
Hepatic hydatid cyst rupture into the biliary tree occurs in 5% to 25% of patients and constitutes the most common complication of hepatic echinococcal cysts (see Chapter 68). In this setting, ERCP plays a pivotal role in the therapeutic management of the disease, even as a definitive therapy in some cases. However, oral albendazole (400 mg) twice a day can be started prior to the procedure. Typically, the ERCP will show a global dilation of the biliary tree with several laminated defects occupying the distal common bile duct with multiple white germinative membranes; a sphincterotomy will help eliminate the membranes causing the obstruction. The treatment of choice for hepatic echinococcosis usually involves antihelmintic therapy and surgical resection or percutaneous aspiration. However, when hydatid material, comprising daughter cysts and hydatid membranes, is released into the biliary tree through a fistulous tract, an ERCP is mandatory before surgery to ensure the retrieval of hydatid biliary material to treat or prevent biliary obstruction complications, mainly acute cholangitis. Regardless of management, antihelmintic drugs should always be started prior to endoscopic or surgical therapy to inactivate intracystic material and minimize allergic disorders or postoperative recurrence.
Abdussalam M, Kaferstein FK, Mott KE. Food safety measures for the control of foodborne trematode infections. Food Control. 1995;6:71-79.
Ait Ali A, et al. Hepatobiliary distomatosis: a mistaken cause of cholangitis. Gastroenterol Clin Biol. 2002;26:541.
Aksoy DY, et al. Fasciola hepatica infection: clinical and computerized tomographic findings of ten patients. Turk J Gastroenterol. 2006;17:40-45.
Alban M, Jave J, Quispe T. Fasciolasis in Cajamarca. Rev Gastroenterol Peru. 2002;22:28-32.
Almendras-Jaramillo M, et al. Hepatic fascioliasis in children: uncommon clinical manifestations. Arq Gastroenterol. 1997;34:241-247.
Alvarez-Sanchez MA, et al. Resistance of Fasciola hepatica to triclabendazole and albendazole in sheep in Spain. Veterinary. 2006;159:424-425.
Apt W, Aguilera X, Vega F. Treatment of human chronic fascioliasis with triclabendazole: drug efficacy and serologic response. Am J Trop Med Hyg. 1995;52:532-535.
Aroonroch R, et al. Hepatic fascioliasis due to Fasciola hepatica: a two-case report. J Med Assoc Thai. 2006;89:1770-1774.
Ashrafi K, et al. Plant-borne human contamination by fascioliasis. Am J Trop Med Hyg. 2006;75:295-302.
Bahçecioglu IH, Ataseven H, Aygen E, et al. Fasciola hepatica case with hemobilia. Acta Medica (Hradec Kralove). 2007;50:155-156.
Bahçecioglu IH, et al. Biliary fasciolosis: a report of three cases diagnosed by ERCP. Turkiye Parazitol Derg. 2008;32:375-378.
Behar JM, Winston JS, Borgstein R. Hepatic fascioliasis at a London hospital—the importance of recognising typical radiological features to avoid a delay in diagnosis. Br J Radiol. 2009;82:189-193.
Birjawi GA, et al. Biliary fascioliasis: case report and review of the literature. J Med Liban. 2002;50:60-62.
Bjorland J, et al. An outbreak of acute fascioliasis among Aymara Indians in the Bolivian Altiplano. CID. 1995;21:1228-1233.
Blancas G, et al. Fasciolosis humana y compromiso gastrointestinal: estudio de 277 pacientes en el Hospital Nacional Cayetano Heredia, 1970-2002. Rev Gastroenterol Peru. 2004;24:143-157.
Bonniaud P, et al. Ultrasound aspect of fascioliasis of the biliary tract. J Rad. 1984;65:589-591.
Bulbuloglu E, et al. Diagnosis of Fasciola hepatica cases in an operating room. Trop Doct. 2007;37:50-52.
Campbell AJ, et al. Proline biosynthesis by Fasciola hepatica at different developmental stages in vivo and vitro. Mol Biochem Parasitol. 1981;3:91-101.
Chemale G, et al. Proteomic analysis of glutathione transferases from the liver fluke parasite, Fasciola hepatica. Proteomics. 2006;6:6263-6273.
Choi D, et al. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. J Hepatol. 2006;44:1066-1073.
Choi MS, et al. Correlation between sonographic findings and infection intensity in clonorchiasis. Am J Trop Med Hyg. 2005;73:1139-1144.
Cosme A, et al. Fasciola hepatica study of a series of 37 patients. Gastroenterol Hepatol. 2001;24:375-380.
Cosme A, et al. Sonographic findings of hepatic lesions in human fascioliasis. J Clin Ultrasound. 2003;31:358-363.
Cruz-Lopez O, et al. Fasciolosis hepatica diagnosticada en fase de estado. Rev Gastroenterol Mex. 2006;71:59-62.
Dalimi A, Jabarvand M. Fasciola hepatica in the human eye. Trans R Soc Trop Med Hyg. 2005;99:798-800.
Da Rocha GC, et al. Paleoparasitological remains revealed by seven historic contexts from “Place d’Armes,” Namur, Belgium. Mem Inst Osw Cruz. 2006;5:43-52.
Das K, et al. Non-resolving liver abscess with Echinococcus cross-reactivity in a non-endemic region. Indian J Gastroenterol. 2007;26:92-93.
Diaz Fernandez R, et al. Obstructive jaundice, Fasciola hepatica: a new case report. Rev Cubana Med Trop. 2005;57:151-153.
Dobrucali A, et al. Fasciola hepatica infestation as a very rare cause of extrahepatic cholestasis. World J Gastroenterol. 2004;10:3076-3077.
Duenngai K, et al. Improvement of PCR for detection of Opisthorchis viverrini DNA in human stool samples. J Clin Microbiol. 2008;46:366-368.
Echenique-Elizondo M, Amondarain J, Lirón de Robles C. Fascioliasis: an exceptional cause of acute pancreatitis. JOP. 2005;6:36-39.
El-Shazly AM, et al. Clinico-epidemiological study of human fascioliasis in an endemic focus in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol. 2001;31:725-736.
El-Shazly AM, et al. Cholestasis in human fascioliasis in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol. 2005;35:83-94.
El-Tantawy WH, Salem HF, Mohammed Safwat NA. Effect of fascioliasis on the pharmacokinetic parameters of triclabendazole in human subjects. Pharm World Sci. 2007;29:190-198.
Espinoza JR, Timoteo O, Herrera-Velit P. Fas2-ELISA in the detection of human infection by Fasciola hepatica. J Helminthol. 2005;79:235-240.
Espinoza JR, et al. Evaluation of Fas2-ELISA for the serological detection of Fasciola hepatica infection in humans. Am J Trop Med Hyg. 2007;76:977-982.
Favennec L, et al. Double-blind, randomized, placebo-controlled study of nitazoxanide in the treatment of fascioliasis in adults and children from northern Peru. Aliment Pharmacol Ther. 2003;17:265-270.
Fullerton JK, Vitale M, Vitale GC. Therapeutic endoscopic retrograde cholangiopancreatography for the treatment of Fasciola hepatica presenting as biliary obstruction. Surg Innov. 2006;13:179-182.
Gil-Gil F, et al. Hepatobiliary fasciolasis without eosinophilia. Rev Clin Esp. 2006;206:464.
Gironès N, et al. Immune suppression in advanced chronic fascioliasis: an experimental study in a rat model. J Infect Dis. 2007;195:1504-1512.
Gonzales-Carbajal PM, et al. Imagenología y fasciolasis de vías biliares: reporte de 4 casos. Rev Gastroenterol Peru. 2001;21:234-238.
Gonzalo-Orden M, et al. Diagnostic imaging in sheep hepatic fascioliasis: ultrasound, computer tomography and magnetic resonance findings. Parasitology Research. 2003;90:359-364.
Graham CS, Brodie SB, Weller PF. Imported Fasciola hepatica infection in the United States and treatment with triclabendazole. CID. 2001;33:1-5.
Gulsen MT, et al. Fascioliasis: a report of five cases presenting with common bile duct obstruction. Neth J Med. 2006;64:17-19.
Hamir AN, Smith BB. Severe biliary hyperplasia with liver fluke infection in an adult alpaca. Vet Pathol. 2002;39:592-594.
Haridy FM, et al. Fascioliasis an increasing zoonotic disease in Egypt. J Egypt Soc Parasitol. 1999;29:35-48.
Haseeb AN, et al. A review on fascioliasis in Egypt. J Egypt Soc Parasito. 2002;32:317-354.
Heredia D, et al. Gallbladder fascioliasis in a patient with liver cirrhosis. Med Clin (Barc). 1984;82:768-770.
Honjo S, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, Northeast Thailand. Int J Cancer. 2005;117:854-860.
Incani RN, et al. Human infection by Fasciola hepatica in Venezuela: report of a geriatric case. Invest Clin. 2003;44:255-260.
Inoue K, et al. A case of human fasciolosis: discrepancy between egg size and genotype of Fasciola sp. Parasitol Res. 2007;100:665-667.
Jimenez J, et al. Fasciolasis hepática ¿un problema diagnóstico? Rev Gastroenterol Peru. 2001;21:148-152.
Kabaalioglu A, et al. US-guided gallbladder aspiration: a new diagnostic method for biliary fascioliasis. Eur Radiol. 1999;9:880-882.
Kabaalioglu A, et al. Fascioliasis: US, CT, and MRI findings with new observations. Abdom Imaging. 2000;25:400-404.
Kaewpitoon N, Kaewpitoon SJ, Pengsaa P. Opisthorchiasis in Thailand: review and current status. World J Gastroenterol. 2008;14:2297-2302.
Kang ML, et al. Fasciola hepatica in a New Zealander traveler. J Travel Med. 2008;15:196-199.
Katz N, Chavez A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev do Inst Med Trop Sâo Paulo. 1972;14:397-402.
Kaya S, et al. Seroprevalence of fasciolosis and the difference of fasciolosis between rural area and city center in Isparta, Turkey. Saudi Med J. 2006;27:1152-1156.
Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg Infect Dis. 2005;11:1507-1514.
Keiser J, Utzinger J. Food-borne trematodiasis: current chemotherapy and advances with artemisinins and synthetic trioxolanes. Trends Parasitol. 2007;23:555-562.
Keiser J, Utzinger J. Artemisinins and synthetic trioxolanes in the treatment of helminth infections. Curr Opin Infect Dis. 2007;20:605-612.
Keiser J, et al. Activity of artemether and OZ78 against triclabendazole-resistant Fasciola hepatica. Trans R Soc Trop Med Hyg. 2007;101:1219-1222.
Keiser J, et al. Clonorchicidal properties of the synthetic trioxolane OZ78. J Parasitol. 2007;93:1208-1213.
Keiser J, et al. Anthelmintic activity of artesunate against Fasciola hepatica in naturally infected sheep. Res Vet Sci. 2010;88:107-110.
Kesik M, et al. Enteral vaccination of rats against Fasciola hepatica using recombinant cysteine proteinase (cathepsin L1). Vaccine. 2007;25:3619-3628.
Khelifi S, et al. Common bile duct distomatosis managed by coelioscopic approach: one case report. Tunis Medicine. 2006;84:385-386.
Kim JB, et al. A human case of invasive fascioliasis associated with liver abscess. Korean J Parasitol. 1999;33:395-398.
Kim JC, et al. Fasciola hepatica in the common bile duct. Gastrointest Endosc. 2006;63:501.
Kim KA, et al. Necrotic granuloma of the liver by human fascioliasis: imaging findings. Abdom Imaging. 1999;24:462-464.
Kleiman F, et al. Dynamics of Fasciola hepatica transmission in the Andean Patagonian valleys, Argentina. Vet Parasitol. 2007;145:274-286.
Laha H, et al. Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics. 2007;8:189.
Lee OJ, Kim TH. Indirect evidence of ectopic pancreatic fascioliasis in a human. J Gastroenterol Hepatol. 2006;21:1631-1633.
Liao WC, et al. Multiple hepatic nodules: rare manifestation of clonorchiasis. J Gastroenterol Hepatol. 2006;21:1497-1500.
Lim MK, et al. Clonorchis sinensis infection and increasing risk of cholangiocarcinoma in the Republic of Korea. Am J Trop Med Hyg. 2006;75:93-96.
Llanos C, et al. Systemic vasculitis associated with Fasciola hepatica infection. Scand J Rheumatol. 2006;35:143-146.
Loja OD, et al. Hematoma hepático subcapsular por fasciola. Rev Gastroenterol Peru. 2003;23:142-148.
Lumbreras H, Cantella R, Burga R. Acerca de un procedimiento de sedimentación rápida para investigar huevos de Fasciola hepatica en las heces, su evaluación y uso en el campo. Rev Med Peru. 1962;31:167-174.
Lun ZR, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31-41.
MacLean JD, Graeme-Cook FM. Case records of the Massachusetts General Hospital weekly clinicopathological exercises: case 12-2002—a 50-year-old man with eosinophilia and fluctuating hepatic lesions. N Engl J Med. 2002;346:1232-1239.
Maco V, et al. Obstrucción de dren de Kehr por Fasciola hepatica en una paciente postcolecistectomizada por colangitis aguda. Parasitol Latinoamericana Día. 2003;58:152-158.
Maeda T, et al. Unusual radiological findings of Fasciola hepatica infection with huge cystic and multilocular lesions. Intern Med. 2008;47:449-452.
Makay O, et al. Ectopic fascioliasis mimicking a colon tumor. World J Gastroenterol. 2007;13:2633-2635.
Marcos LA, Terashima A. Update on human fascioliasis in Peru: diagnosis, treatment and clinical classification proposal. Neotrop Helminthol. 2007;1:85-103.
Marcos LA, Terashima A, Gotuzzo E. Update on hepatobiliary flukes: fascioliasis, opisthorchiasis and clonorchiasis. Curr Opin Infect Dis. 2008;21:523-530.
Marcos LA, et al. Características clínicas de la infección crónica por Fasciola hepatica en niños. Rev Gastroenterol Peru. 2002;22:228-233.
Marcos LA, et al. Hiperendemicidad de fasciolosis humana en el Valle del Mantaro: factores de riesgo de la infección por Fasciola hepatica. Rev Gastroenterol Peru. 2004;24:158-164.
Marcos L, et al. Altas tasas de prevalencia de fasciolosis humana en el Perú: una enfermedad emergente. Rev Per Enf Infec Trop. 2005;3:8-13.
Marcos LA, et al. Fascioliasis in relatives of patients with Fasciola hepatica infection in Peru. Rev Inst Med Trop Sao Paulo. 2005;47:219-222.
Marcos LA, et al. Reporte de casos de fasciolosis en el Instituto Especializado de Salud del Niño (1988-2003). Rev Gastroenterol Peru. 2005;25:198-205.
Marcos LA, et al. Risk factors for Fasciola hepatica infection in children: a case-control study. Trans R Soc Trop Med Hyg. 2006;100:158-166.
Marcos LA, et al. Fasciola hepatica infection in Peru: an emergent disease. Rev Gastroenterol Peru. 2007;27:389-396.
Marcos LA, et al. Zonas hiperendémicas y mesoendémicas de la infección por Fasciola hepatica aledañas a la ciudad de Lima: una enfermedad emergente? Rev Gastroenterol Peru. 2007;27:21-26.
Marcos LA, et al. Hepatic fibrosis and Fasciola hepatica infection in cattle. J Helminth. 2007;25:1-6.
Marcos LA, et al. Natural history, clinico-radiologic correlates and response to triclabendazole in acute massive fascioliasis. Am J Trop Med Hyg. 2008;78:222-227.
Marcos LA, et al. Cervical tumor caused by the sexually mature stage of Fasciola hepatica: a case report. Trans Roy Soc Trop Med Hyg. 2009;103:318-320.
Marcos LA, et al. Detection of antibodies against Fasciola hepatica in cirrhotic patients from Peru. J Helminthol. 2009;83:23-26.
Mark LG, Isseroff H. Levels of type I and type III collagen in the bile duct of rats infected with Fasciola hepatica. Mol Biochem Parasitol. 1983;8:253-262.
Mas-Coma MS, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: a review and proposed new classification. Bull World Health Organization. 1999;77:340-346.
McManus DP, Dalton JP. Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology. 2006;133:43-61.
Millan JC, et alTriclabendazole Study Group. The efficacy and tolerability of triclabendazole in Cuban patients with latent and chronic Fasciola hepatica infection. Am J Trop Med Hyg. 2000;63:264-269.
Mottier L, et al. Resistance-induced changes in triclabendazole transport in Fasciola hepatica: ivermectin reversal effect. J Parasitol. 2006;92:1355-1360.
Nagano I, et al. Molecular expression of a cysteine proteinase of Clonorchis sinensis and its application to an enzyme-linked immunosorbent assay for immunodiagnosis of clonorchiasis. Clin Diagn Lab Immunol. 2004;11:411-416.
Naresh G, et al. Fasciolosis (liver fluke) of the breast in a male patient: a case report. Breast. 2006;15:103-105.
Noyer CM, et al. Hypereosinophilia and liver mass in an immigrant. Am J Trop Med Hyg. 2002;66:774-776.
Orlent H, et al. Clinical challenges and images in GI Fasciola hepatica infection and Von Hippel–Lindau disease type 1 with pancreatic and renal involvement. Gastroenterology. 2007;132:467-468.
Park DH, Son HY. Clonorchis sinensis, images in clinical medicine. N Engl J Med. 2008;358:16.
Park GM. Genetic comparison of liver flukes, Clonorchis sinensis and Opisthorchis viverrini, based on rDNA and mtDNA gene sequences. Parasitol Res. 2007;100:351-357.
Parkinson M, O’Neill SM, Dalton JP. Endemic human fascioliasis in the Bolivian Altiplano. Epidemiol Infect. 2007;135:669-674.
Parsak CK, et al. Surgery in Fasciola hepatica pancreatitis: report of a case and review of literature. Z Gastroenterol. 2007;45:313-316.
Perez J, et al. Pathological and immunohistochemical study of the liver and hepatic lymph nodes in goats infected with one or more doses of Fasciola hepatica. J Comp Pathol. 1999;120:199-210.
Phiri AM, et al. Comparative fluke burden and pathology in condemned and non-condemned cattle livers from selected abattoirs in Zambia. Onderstepoort J Vet Res. 2006;73:275-281.
Rana SS, et al. Parasitic infestations of the biliary tract. Curr Gastroenterol Rep. 2007;9:156-164.
Richter J, et al. Fascioliasis: sonographic abnormalities of the biliary tract and evolution after treatment with triclabendazole. Trop Med Intern Health. 1999;4:774-781.
Rim HJ. Clonorchiasis: an update. J Helminthol. 2005;79:269-281.
Rondelaud D, Dreyfuss G, Vignoles P. Clinical and biological abnormalities in patients after fascioliasis treatment. Med Mal Infect. 2006;36:466-468.
Ruangsittichai J, et al. Opisthorchis viverrini: identification of a glycine-tyrosine rich eggshell protein and its potential as a diagnostic tool for human opisthorchiasis. Int J Parasitol. 2006;36:1329-1339.
Sanchez-Sosa S, et al. Massive hepatobiliary fascioliasis. Rev Gastroenterol Mex. 2000;65:179-183.
Sayasone S, et al. Epidemiology of Opisthorchis viverrini in a rural district of southern Lao PDR. Trans R Soc Trop Med Hyg. 2007;101:40-47.
Shirai W, et al. Anatomicopathological study of vascular and biliary systems using cast samples of Fasciola-infected bovine livers. J Vet Med A Physiol Pathol Clin Med. 2006;53:239-245.
Spithill TW, Dalton JP. Progress in development of liver fluke vaccines. Parasitol Today. 1998;14:224-228.
Sriamporn S, et al. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int Health. 2004;9:588-594.
Sripa B, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:201.
Stack CM, et al. The major secreted cathepsin L1 protease of the liver fluke, Fasciola hepatica: a leu-12 to pro-12 replacement in the non-conserved C-terminal region of the prosegment prevents complete enzyme autoactivation and allows definition of the molecular events in prosegment removal. J Biol Chem. 2007;282:16532-16543.
Stunell H, et al. Recurrent pyogenic cholangitis due to chronic infestation with Clonorchis sinensis. Eur Radiol. 2006;16:2612-2614.
Talaie H, et al. Randomized trial of a single, double and triple dose of 10 mg/kg of a human formulation of triclabendazole in patients. Clin Exp Pharmacol Physiol. 2004;31:777-782.
Tan SK, et al. Evaluation of the risk of clonorchiasis inducing primary hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi. 2008;16:114-116.
Trueba G, et al. Detection of Fasciola hepatica infection in a community located in the Ecuadorian Andes. Am J Trop Med Hyg. 2000;62:518.
Tselepatiotis E, et al. A case of Opisthorchis felineus infestation in a pilot from Greece. Infection. 2003;6:430-432.
Turhan O, et al. Seroepidemiology of fascioliasis in the Antalya region and uselessness of eosinophil count as a surrogate marker and portable ultrasonography for epidemiological surveillance. Infez Med. 2006;14:208-212.
Umac H, et al. Pruritus and intermittent jaundice as clinical clues for Fasciola hepatica infestation. Liver Int. 2006;26:752-753.
Valero M, et al. Risk of gallstone disease in advanced chronic phase of fascioliasis: an experimental study in a rat model. J Inf Dis. 2003;188:787-793.
Valero MA, et al. High risk of bacterobilia in advanced experimental chronic fasciolosis. Acta Trop. 2006;100:17-23.
Vatsal DK, et al. Ectopic fascioliasis in the dorsal spine: case report. Neurosurgery. 2006;59:706-707.
Watthanakulpanich D, et al. Evaluation of Bithynia funiculata snail antigens by ELISA-serodiagnosis of human opisthorchiasis. Southeast Asian J Trop Med Public Health. 1997;28:593-598.
Wolf-Spengler ML, Isseroff H. Fascioliasis: bile duct collagen induced by proline from the worm. J Parasitol. 1983;69:290-294.
World Health Organization (WHO), 2007: Control of foodborne trematode infections: report of a WHO study group. WHO Technical Report Series 849.
Zhao QP, et al. Evaluation of Clonorchis sinensis recombinant 7-kilodalton antigen for serodiagnosis of clonorchiasis. Clin Diagn Lab Immunol. 2004;11:814-817.