Chapter 7 Bile secretion and pathophysiology of biliary tract obstruction
Overview
Bile secretion is one of the major functions of the liver, and it serves two major purposes: 1) the excretion of hepatic metabolites—including bilirubin, cholesterol, drugs, and toxins—and 2) the facilitation of intestinal absorption of lipids and fat-soluble vitamins. Alterations in bile secretion may contribute to cholelithiasis (see Chapter 30) and its potential complications, such as cholecystitis (see Chapter 31) and choledocholithiasis (see Chapters 35, 36, and 37). On the other hand, obstruction of bile flow results in alterations of coagulation, the immune system, and all organ function. This chapter will discuss the physiology of bile secretion, the pathophysiology of bile obstruction, and the management of obstructive jaundice.
Bile Secretion
Bile Composition
The components of hepatic and gallbladder bile are essentially the same, but the concentration varies considerably because of the ability of the gallbladder to absorb water (Table 7.1). The gallbladder absorbs water both actively via sodium–hydrogen (Na+/H+) pumps and passively through aquaporin channels. Both chloride (Cl−) and bicarbonate (HCO3−) are absorbed by the gallbladder epithelium via the cystic fibrosis transmembrane regulator (CFTR; Swartz-Basile et al, 2007). The secretion of hydrogen ions and the absorption of bicarbonate by the gallbladder alter the acid-base balance from basic in hepatic bile to acidic in gallbladder bile.
| Characteristics* | Hepatic Bile | Gallbladder Bile |
|---|---|---|
| Sodium | 160 | 270 |
| Potassium | 5 | 10 |
| Chloride | 90 | 15 |
| Bicarbonate | 45 | 10 |
| Calcium | 4 | 25 |
| Magnesium | 2 | 4 |
| Bilirubin | 1.5 | 15 |
| Protein | 150 | 200 |
| Bile acids | 50 | 150 |
| Phospholipids | 8 | 40 |
| Cholesterol | 4 | 18 |
| Total solids | — | 125 |
| pH | 7.8 | 7.2 |
Significant ranges may be seen.
* All determinations are milliequivalents per liter, except for pH.
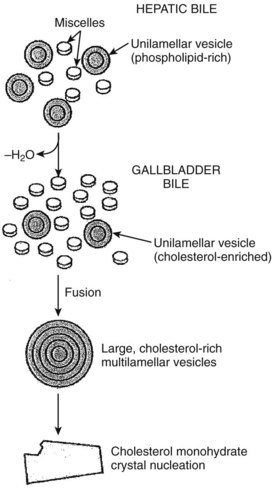
The gallbladder mucosa also absorbs the calcium (Ca++) and magnesium (Mg++) cations. However, calcium absorption is not as efficient as the absorption of sodium and water, which leads to a significantly greater relative increase in the concentration of calcium in the gallbladder. Similarly, the concentration of bilirubin, which is not actively absorbed by the gallbladder, may be as high as 10-fold. Thus precipitation of calcium bilirubinate crystals, the major component of pigment gallstones, is much more likely to occur within the gallbladder. In addition, the biliary lipids, bile salts, phospholipids, and cholesterol all become more concentrated in the gallbladder. As the gallbladder bile becomes concentrated, several changes occur in the capacity of bile to solubilize cholesterol. The solubility in the micellar fraction is increased, but the stability of the phospholipid–cholesterol vesicles is greatly decreased. Because cholesterol crystal precipitation occurs preferentially by vesicular, rather than micellar, mechanisms, the net effect of concentrating bile is an increased tendency to form cholesterol crystals (Klein et al, 1996).
Bile Salt Secretion
Bile is secreted from the hepatocyte into canaliculi, which drain their contents into small bile ducts. Secretion of bile salts is the major osmotic force for the generation of bile flow. Bile acids are formed at a rate of 500 to 600 mg per day. The bulk of the bile salt pool is maintained in the gallbladder, followed by the liver, the small intestine, and the extrahepatic bile ducts. Bile acids are synthesized from cholesterol via two main pathways: a classic pathway leads to the formation of cholic acid, and an alternate pathway results in the synthesis of chenodeoxycholic acid. The classic pathway is the predominant mode of bile acid synthesis in humans. As a result, approximately 70% of the bile acid pool consists of cholic acid and its metabolite deoxycholic acid, with chenodeoxycholic acid occurring less commonly in human bile (Kullack-Ublick, 2004).
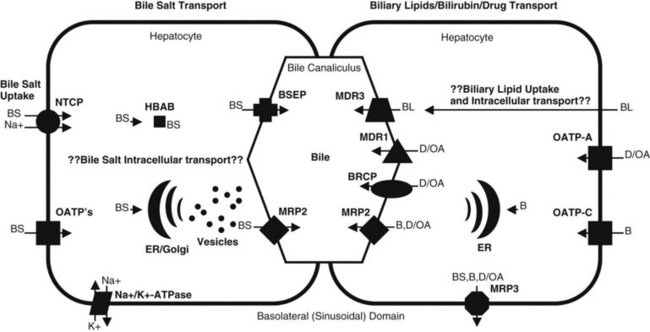
In plasma, bile acids circulate bound to either albumin or lipoproteins. In the space of Disse within the liver, bile salt uptake into the hepatocytes is very efficient. This process is mediated by sodium-dependent and sodium-independent mechanisms. The sodium-dependent pathway accounts for more than 80% of taurocholate uptake but less than 50% of cholate uptake (Meier & Stieger, 2002). In recent years a number of transport proteins have been identified that play a key role in this process (Fig. 7.1). The bile salt transporter is termed the sodium-taurocholate cotransporting polypeptide (NTCP), and it is exclusively expressed in the liver and located in the basolateral membrane of the hepatocyte. Sodium-independent hepatic uptake of bile acids is mediated primarily by a family of transporters termed the organic anion transporting polypeptides (OATPs). In contrast to NTCP, these transporters have a broader substrate affinity and transport a variety of organic anions, including the bile salts. OATP-C is the major sodium-independent bile salt uptake system, but OATP-A also takes up bile acids, and OATP-8 mediates taurocholate uptake.
Intracellular bile acid transport occurs within a matter of seconds. Two mechanisms may be responsible for bile acid transcellular movement: one involves transfer of bile acids from the basolateral membrane to the canalicular membrane via bile acid–binding proteins (Crawford, 1996), the other moves cellular bile salt through vesicular transport. In contrast, the transport of bile salts across the canalicular membrane of hepatocytes represents the rate-limiting step in the overall secretion of bile salts from the blood into bile.
Bile salt concentrations are 1000-greater within the canaliculi than in the hepatocytes. This gradient necessitates an active transport mechanism, which is an ATP-dependent process. This bile salt export pump (BSEP) is closely related to the proteins encoded by the multidrug resistance (MDR) gene family of ATP binding cassette (ABC) transporters (Kullack-Ublick, 2004). The ABC transporters mediate the transport of metabolites, peptides, fatty acids, cholesterol, and lipids in the liver, intestines, pancreas, lungs, kidneys, brain, and in macrophages. Although BSEP is the major transporter for monovalent bile salts into the canaliculus, MDR-related protein-2 (MRP2), a member of the multidrug resistance protein family, also transports sulfated and glucuronidated bile salts into the canaliculus. MRP2 also mediates the export of multiple other organic anions, including conjugated bilirubin, leukotrienes, glutathione disulfide, chemotherapeutic agents, uricosurics, antibiotics, toxins, and heavy metals (Gerk & Vore, 2002).
Biliary Lipid Secretion
Compared with bile salts, the biliary lipids, phospholipids, and cholesterol play a secondary role in the formation of bile. Phospholipids and cholesterol are formed primarily from low-density lipoproteins circulating in plasma and from de novo synthesis by hepatocytes. Less is known about the secretion of biliary lipids compared with bile salt secretion; however, biliary lipid secretion is key for cholesterol disposal, intestinal absorption of dietary lipids, and cytoprotection against bile acid–induced hepatocyte and cholangiocyte injury (Arrese & Accatino, 2002).
Phospholipid secretion involves the delivery of phospholipids to the inner leaflet of the canalicular plasma membrane (Elferink & Groen, 2000). In humans the MDR3 transporter translocates phospholipids from the inner to the outer leaflet of the canalicular membrane. Humans with an MDR3 deficiency develop progressive familial intrahepatic cholestasis type 3 (Kullak-Ublick, 2004). These patients have no phosphatidylcholine in bile and therefore do not form mixed micelles with bile salts. As a result, toxic bile salts injure the biliary epithelium, resulting in neonatal cholestasis, cholestasis of pregnancy, and cirrhosis in adults.
Less is known about the role of transporter proteins in cholesterol secretion, but the ABC transporters, ABCG5 and ABCG8, have been demonstrated to be involved in the elimination of plant steroids (Lee, 2004). Cholesterol is highly nonpolar and insoluble in water; thus it is insoluble in bile. The key to maintaining cholesterol in solution is the formation of micelles, a bile salt–phospholipid–cholesterol complex. Bile salts are amphipathic compounds that contain both a hydrophilic and hydrophobic portion. In aqueous solutions, bile salts are oriented with the hydrophilic portion outward. Phospholipids are incorporated into the micellar structure, allowing cholesterol to be added to the hydrophobic central portion of the micelle. In this way, cholesterol can be maintained in solution in an aqueous medium. The concept of mixed micelles as the only cholesterol carrier has been challenged by the demonstration that much of the biliary cholesterol exists in a vesicular form. Structurally, these vesicles are made up of lipid bilayers of cholesterol and phospholipids. In their simplest and smallest form, the vesicles are unilamellar, but an aggregation may take place, leading to multilamellar vesicles. Present theory suggests that in states of excess cholesterol production, these large vesicles may also exceed their capability to transport cholesterol, and cystal precipitation may occur (Fig. 7.2).
Bilirubin Secretion
In the sinusoidal membrane of the hepatocyte, bilirubin is taken up by OATP-C, a membrane transporter belonging to the OATP family (Cui et al, 2001). OATP-C is involved with the uptake of both conjugated and unconjugated bilirubin, but unconjugated bilirubin also can cross hepatic sinusoidal membranes by a diffusion process. In the hepatocyte, bilirubin binds to a driver of gluthathione-S-transferase and is catalyzed by bilirubin uridine-5-diphosphate (UDP)-glycosyltransferase to form bilirubin glucuronides. Mutations in the gene encoding bilirubin UDP-glycosyltransferase are associated with the unconjugated hyperbilirubin syndromes, Crigler-Najjar and Gilbert syndromes (Iganagi, 1998).
Enterohepatic Circulation
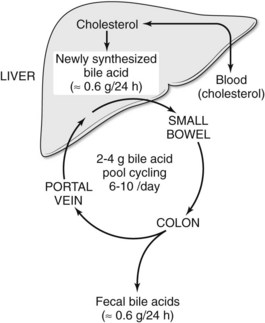
Bile salts are synthesized and conjugated in the liver; secreted into bile; stored temporarily in the gallbladder; passed from the gallbladder into the duodenum; absorbed throughout the small intestine, especially in the ileum; and returned to the liver via the portal vein. This cycling of bile acids between the liver and the intestine is referred to as the enterohepatic circulation (Fig. 7.3). The total amount of bile acids in the enterohepatic circulation is defined as the circulating bile pool. In this highly efficient system, nearly 95% of bile salts are reabsorbed. Thus, of the total bile salt pool of 2 to 4 g, which recycles through the enterohepatic cycle 6 to 10 times daily, only about 600 mg is actually excreted into the colon. Bacterial action in the colon on the two primary bile salts, cholate and chenodeoxycholate, results in the formation of the secondary bile salts, deoxycholate and lithocholate. Although some deoxycholate is reabsorbed passively by the colon, the remainder is lost in fecal waste; however, the physiology of bile salt, biliary lipid, bilirubin, bile flow, and the enterohepatic circulation are dramatically altered when the bile ducts become obstructed.
Biliary Obstruction
Causes of Jaundice
Jaundice may result from 1) increased production of bilirubin, 2) impaired hepatocyte uptake of bilirubin, 3) impaired conjugation of bilirubin, 4) impaired transport or excretion of bilirubin into the bile canaliculus, or 5) obstruction of the intrahepatic or extrahepatic biliary tree (Table 7.2). Overproduction, impaired uptake, and impaired conjugation of bilirubin all lead to a predominently unconjugated hyperbilirubinemia. Impaired transport and excretion and biliary ductal obstruction result in hyperbilirubinemia that is primarily conjugated. Some patients have multiple defects in normal metabolism. For example, a patient with biliary obstruction from a tumor may develop secondary hepatocellular dysfunction. Therefore these classification systems may be simplifications of more complex disease processes.
| Defect in Bilirubin Metabolism | Predominant Hyperbilirubinemia | Examples |
|---|---|---|
| Increased production | Unconjugated | Congenital hemoglobinopathies, hemolysis, multiple transfusions, sepsis, burns |
| Impaired hepatocyte uptake | Unconjugated | Gilbert disease, drug induced |
| Reduced conjugation | Unconjugated | Neonatal jaundice, Crigler-Najjar syndrome |
| Impaired transport and excretion | Conjugated | Hepatitis, cirrhosis, Dubin-Johnson syndrome, Rotor syndrome |
| Biliary obstruction | Conjugated | Choledocholithiasis, benign strictures, chronic pancreatitis, sclerosing cholangitis, periampullary cancer, cholangiocarcinoma |
Diseases causing bile duct obstruction may be further divided into conditions causing 1) complete obstruction, 2) intermittent obstruction, 3) chronic incomplete obstruction, or 4) segmental duct obstruction (Table 7.3). Patients with complete biliary obstruction will have clinical jaundice, and those with intermittent obstruction may develop symptoms (pain, pruritus, fevers) and biochemical changes without necessarily developing clinical jaundice. Patients with chronic incomplete obstruction can eventually develop hepatic fibrosis (see Chapter 6) and biliary cirrhosis (see Chapter 70A).
Table 7.3 Lesions Commonly Associated with Biliary Tract Obstruction
| Type I Complete Obstruction |
Pathophysiology
Hepatobiliary
The biliary system normally has a low pressure (5 to 10 cm H2O); however, in the setting of complete or partial biliary obstruction, biliary pressure can approach 30 cm H2O. As biliary pressure increases, the tight junctions between hepatocytes and bile duct cells are disrupted, resulting in an increase in bile duct and canalicular permeability. Bile contents can freely reflux into liver sinusoids, causing a marked polymorphonuclear leukocyte infiltration into the portal triads. This inflammatory response is followed by increased fibrinogenesis with deposition of reticulin fibers, which undergo conversion to type I collagen (see Chapter 6). The extrahepatic bile ducts exhibit mucosal atrophy and squamous metaplasia followed by inflammatory infiltration and fibrosis in the subepithelial layers of the bile duct (Karsten et al, 1991).
Several authors have reported impairment of both macrovascular and microvascular perfusion of the liver in obstructive jaundice. Intravital fluorescence microscopy has shown a significant increase in the number of nonperfused sinusoids after 3 days of extrahepatic obstruction. Moreover, in perfused sinusoids, a 35% decrease in the mean diameter and a 25% decrease in flow velocity were noted (Koeppel et al, 1997). This alteration in hepatic perfusion may help explain the increased risk of hepatocellular dysfunction when performing liver resections in patients with obstructive jaundice (see Chapters 50C and 93A). Extrahepatic biliary obstruction and jaundice can also alter important secretory, metabolic, and synthetic functions of the liver. When biliary pressure rises above 20 cm H2O, hepatic bile secretion is diminished, and hepatocytes cannot excrete efficiently against the high pressure. As a result, excretory products of the hepatocytes reflux directly into the vascular system, leading to systemic toxicity.
Jaundiced patients have a decreased capacity to excrete drugs, such as antibiotics, that are normally secreted into bile (Blenkharn et al, 1985). The increased concentration of bile acids associated with obstructive jaundice results in inhibition of the hepatic cytochrome P450 enzymes and therefore a decrease in the rate of oxidative metabolism in the liver. In addition, bile acids in abnormally high concentrations can induce apoptosis (programmed cell death) in hepatocytes (Patel et al, 1994). The synthetic function of the hepatocyte is also decreased with obstructive jaundice, as evidenced by decreased plasma levels of albumin, clotting factors, and secretory immunoglobulins (IgA).
Kupffer cells are tissue macrophages that are the predominant cell type of the hepatic reticuloendothelial system (see Chapter 9). Normally, infectious agents, damaged blood cells, cellular debris, fibrin degradation products, and endotoxin absorbed or formed in the portal circulation are effectively filtered by Kupffer cells and removed from the systemic circulation. Kupffer cells also play an interactive role with hepatocytes, modulating synthesis of hepatic proteins. Obstructive jaundice has been shown to have profound effects on Kupffer cells, including decreased endocytosis, phagocytosis, clearance of bacteria and endotoxin, and expression of the major histocompatibility complex class II antigen with a consequent diminished ability to process antigen (Nehez & Anderson, 2002; Puntis & Jiang, 1996). Biliary obstruction also has been shown to increase levels of proinflammatory cytokines, including TNF-α and IL-6 (see Chapters 9 and 10).
Cardiovascular
In addition to hepatic dysfunction, obstructive jaundice may cause severe hemodynamic and cardiac disturbances. Experimental animals with obstructive jaundice tend to be hypotensive and exhibit an exaggerated hypotensive response to hemorrhage. Studies in experimental animals have demonstrated that bile duct ligation 1) decreases cardiac contractility; 2) reduces left ventricular pressures; 3) impairs response to β-agonist drugs, such as isoproterenol and norepinephrine; and 4) decreases peripheral vascular resistance (Ma et al, 1999). In a study of 9 patients with obstructive or cholestatic jaundice, Lumlertgul and colleagues (1991) showed a significantly blunted response in left ventricular ejection fraction compared with normal volunteers following the infusion of the positive inotrope dobutamine. Padillo and others (2001) also have shown a negative correlation between serum bilirubin and left ventricular systolic work in 13 patients with biliary obstruction and no previous history of heart, lung, or kidney disease. Successful internal biliary drainage in patients was associated with a significant increase in cardiac output, compliance, and contractility. The combination of depressed cardiac function and decreased total peripheral resistance most likely makes the jaundiced patient more susceptible to the development of postoperative shock than nonjaundiced patients.
Renal
The association between jaundice and postoperative renal failure has been known for many years. The reported incidence of postoperative acute renal failure has been reported to be as high as 10% but varies depending on the nature of the procedure. Moreover, the mortality rate in jaundiced patients who develop renal failure has been reported to be as high as 70% (Fogarty et al, 1995). Important factors that may play a role in the development of renal failure in obstructive jaundice include 1) depressed cardiac function, 2) hypovolemia, and 3) endotoxemia.
The decreased cardiac function associated with obstructive jaundice leads to a decrease in renal perfusion. Decreased cardiac output may also result in stretching of the atrium and increased production of atrial natriuretic peptide (ANP), a hormone known to cause natriuresis, to counter the action of water- and sodium-retaining hormones, to inhibit the thirst mechanism, and to produce peripheral vasodilation. Plasma levels of ANP have been shown to be increased in both experimental animals and in patients with extrahepatic biliary obstruction (Padillo et al, 2001).
In addition to the direct effects of jaundice on the heart and peripheral vasculature discussed above, the increased serum levels of bile acids associated with obstructive jaundice have a direct diuretic and natriuretic effect on the kidney that results in significant extracellular volume depletion and hypovolemia. In dogs, the infusion of bile into the renal artery results in increased urine flow, natriuresis, and kaliuresis. This diuretic effect may be mediated by increased prostaglandin E2 (PGE2) production by the kidney (Green & Better, 1994).
The third factor in the development of renal failure is endotoxemia (Fig. 7.4). Approximately 50% of patients with obstructive jaundice have endotoxin in their peripheral blood (Hunt et al, 1982). This phenomenon may be the result of decreased hepatic clearance of endotoxin by Kupffer cells and a lack of bile salts in the gut lumen that normally prevent absorption of endotoxins and inhibit anaerobic bacterial growth. Endotoxin also causes renal vasoconstriction and redistribution of renal blood flow away from the cortex, and disturbances in coagulation that include the activation of complement, macrophages, leukocytes, and platelets (Hunt et al, 1982). As a result, glomerular and peritubular fibrin is deposited. This factor, in combination with reduced renal cortical blood flow, results in the tubular and cortical necrosis observed in jaundiced patients with renal failure. Mechanisms of the pathophysiology of renal failure in obstructive jaundice are summarized in Figure 7.3.
Coagulation
In experimental animals, endotoxin affects metabolism of factors XI and XII and causes platelet and direct endothelial damage (Hunt et al, 1982). Moreover, endotoxin release in jaundiced patients results in a low-grade, disseminated intravascular coagulation with increased fibrin degradation products. Hunt and colleagues have shown that jaundiced patients with circulating endotoxin or increased fibrin degradation product levels before surgery are at increased risk for hemorrhagic complications. In addition to problems with endotoxemia, patients with coexisting cirrhosis often have additional problems related to thrombocytopenia from hypersplenism and fibrinolysis.
Immune System
Surgery in jaundiced patients is associated with a higher rate of postoperative septic complications compared with those without jaundice, due in large measure to defects in cellular immunity that make them more prone to infection (see Chapters 9 and 11). Cainzos and colleagues (1992) have demonstrated an association between jaundice and altered delayed-type hypersensitivity. Only 16% of 118 jaundiced patients were immunocompetent, compared with 76% of 59 healthy controls, when tested with a panel of seven skin antigens. Several authors have shown impaired T-cell proliferation (Thompson et al, 1990), decreased neutrophil chemotaxis (Andy et al, 1992), and defective bacterial phagocytosis (Scott-Conner et al, 1993) following bile duct ligation in rats. As mentioned earlier, the ability of the reticuloendothelial system, specifically Kupffer cells, to clear bacteria and endotoxin from the circulation also is reduced in obstructive jaundice.
The absence of bile from the intestinal tract also plays a role in the infectious complications seen in patients with obstructive jaundice. It has been shown that bacterial translocation from the gut is increased in the setting of bile duct obstruction (Deitch et al, 1990). Obstruction causes a disruption of the enterohepatic circulation and results in loss of the emulsifying antiendotoxin effect of bile acids; therefore a larger pool of endotoxin is available within the intestine for absorption into the portal circulation. The combination of reduced or absent bile in the intestine and impaired cellular immunity and reticuloendothelial cell function appears to be a major factor contributing to more frequent infective complications in the jaundiced patient.
Acute cholangitis is a bacterial infection of the biliary ductal system, and it varies in severity from mild and self-limited to severe and life threatening (see Chapter 43). The clinical triad associated with cholangitis—fever, jaundice, and pain—was first described in 1877 by Charcot. Cholangitis results from a combination of two factors: significant bacterial concentrations in the bile and biliary obstruction. Although bile from the gallbladder and bile ducts is usually sterile, in the presence of common bile duct stones or other obstructing pathology, the incidence of positive cultures increases; likewise, instrumentation of the biliary tree also greatly increases rates of bile colonization. The most common organisms recovered from the bile in patients with cholangitis include Escherichia coli, Klebsiella pneumonia, the enterococci, and Bacteroides fragilis (Thompson et al, 1990). However, even in the presence of high biliary bacterial concentrations, clinical cholangitis and bacteremia will not develop, unless obstruction causes elevated intraductal pressures (Lipsett & Pitt, 1993).
The most common causes of biliary obstruction are choledocholithiasis (see Chapter 35), benign strictures (see Chapter 42A, Chapter 42B ), biliary-enteric anastomotic strictures (see Chapter 29, Chapter 42A, Chapter 42B ), and periampullary or proximal biliary cancers (see Chapter 49, Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D, Chapter 58A, Chapter 58B, Chapter 59 ). Prior to 1980, choledocholithiasis was the cause of approximately 80% of the reported cases of cholangitis. In recent years, however, malignant strictures have become a frequent cause, particularly at tertiary referral centers. Endoscopic cholangiography, percutaneous transhepatic cholangiography, and stent placement via either the endoscopic or percutaneous route are all known to cause bacteremia, and these procedures are frequently performed in patients with a presumptive diagnosis of malignant obstruction.
Wound Healing
Delayed wound healing and a high incidence of wound dehiscence and incisional hernia have been observed in jaundiced patients undergoing surgery. Patients with obstructive jaundice have decreased activity of the enzyme propylhydroxylase in their skin. Propylhydroxylase is necessary for the incorporation of proline amino acid residues into collagen, and its activity has been used as a measure of collagen synthesis. Grande and colleagues (1990) measured skin propylhydroxylase activity in 95 patients with extrahepatic bile duct obstruction and 123 nonjaundiced control patients undergoing cholecystectomy. The jaundiced patients had only 11% of the skin propylhydroxylase activity of the controls. In the subgroup of patients with jaundice secondary to malignancy, propylhydroxylase activity was less than 7% of controls. With relief of obstruction, the activity increased to 22% of controls. Interestingly, in patients with jaundice secondary to benign obstruction, the activity increased to 100% of controls.
Other Factors
Several recent observations suggest that the many physiologic derangements induced by obstructive jaundice take a long time to reverse. For example, Koyama and colleagues (1981) have shown that hepatic mitochondrial function does not return to normal even 7 weeks after relief of obstruction. This same prolonged effect of obstructive jaundice has been noted with lymphocyte, polymorphonuclear, and Kupffer cell function. Therefore even patients who have had temporary relief of biliary obstruction via percutaneous or endoscopic stents are likely to remain at risk for significant complications following surgery, because derangements in hepatic function are likely still present at the time of operation.
Management
In the past, the only option for the relief of obstructive jaundice was operative intervention; however, with the development of therapeutic techniques such as percutaneous (see Chapters 18, 28, and 50D) and endoscopic stenting (see Chapters 18 and 27), balloon dilation, and endoscopic sphincterotomy, many nonoperative options for the relief of obstructive jaundice are now available. The surgeon must determine the safest and most effective therapy for each individual patient and must adequately prepare each patient for surgery or nonoperative therapeutic intervention.
Patients with obstructive jaundice and those with hepatocellular disease severe enough to cause jaundice are prone to develop many secondary problems. Jaundiced patients are at increased risk for the development of renal failure, gastrointestinal bleeding, infections, and wound complications (see the earlier section on the pathophysiology of jaundice). Cardiac, pulmonary, and renal function must be considered in every patient undergoing major abdominal surgery. In addition, special attention must be focused on the jaundiced patient’s nutritional status, coagulability, immune function, and presence or absence of biliary sepsis. Patients with chronic liver disease and cirrhosis also may develop complications related to portal hypertension, such as ascites, varices, and encephalopathy, and these may require specific treatment (see Chapter 70B, Chapter 74, Chapter 75A, Chapter 75B, Chapter 75C ).
Cardiopulmonary
In assessing cardiopulmonary status, the patient’s age, history of recent myocardial infarction, and the presence of congestive heart failure, significant valvular aortic stenosis, or a disturbance of normal cardiac rhythm have all been correlated with increased operative risk (Goldman et al, 1977). In addition, patients with severe pulmonary disease may not be candidates for extensive abdominal surgery.
Renal
Certain oral bile salts have been shown to be efficacious in preventing the development of postoperative renal dysfunction. In a study by Cahill (1983) 54% of 24 jaundiced patients not given oral bile salts before surgery were found to have systemic endotoxemia, which was associated with renal impairment in two thirds of the cases. In comparison, none of the 8 jaundiced patients given 500 mg of sodium deoxycholate every 8 hours for 48 hours before surgery had portal or systemic endotoxemia. Moreover, none of these 8 patients had evidence of renal impairment.
Nutrition
Malnutrition is a significant risk factor for surgery in the setting of obstructive jaundice (see Chapter 24). Halliday and colleagues (1988) noted that patients who died in the postoperative period following surgery for obstructive jaundice had had a significant reduction in body weight, midarm circumference, total body potassium, and reactivity to skin test antigens prior to operation. In a study from Italy (Foschi et al, 1986), enteral hyperalimentation was found to significantly decrease operative morbidity and mortality in a group of patients treated with 20 days of preoperative percutaneous biliary drainage. Although most patients with benign biliary problems are adequately nourished, various degrees of malnutrition are frequently present in patients with malignant obstruction; therefore patients with malignant obstructive jaundice should be evaluated for evidence of malnutrition and have nutritional support instituted if necessary.
Cholangitis
Biliary sepsis also has been identified as a major risk in jaundiced patients (see Chapter 43). Cholangitis occurs when partial or complete obstruction of the bile duct exists, resulting in increased intraluminal pressure and infected bile behind the obstruction. Patients with cholangitis present with right upper quadrant abdominal pain, fever, and jaundice (Charcot triad). Patients with “toxic” cholangitis, Charcot triad plus shock and mental confusion (Reynold pentad), have significant mortality with appropriate antibiotic therapy alone and therefore require emergent biliary decompression.
Gigot and colleagues (1989) identified seven prognostic factors that are indications for urgent biliary decompression: 1) acute renal failure, 2) liver abscess, 3) cirrhosis, 4) high malignant stricture, 5) percutaneous transhepatic cholangiography, 6) female gender, and 7) advanced age. However, emergent surgical treatment is associated with significant morbidity and mortality; therefore both percutaneous and endoscopic biliary drainage have been proposed as effective therapy for the 5% to 10% of patients with cholangitis who are unresponsive to conservative therapy. Lai and colleagues (1992) have shown in a series of 82 patients with severe acute cholangitis that endoscopic drainage is associated with a lower morbidity (34% vs. 66%) and mortality (10% vs. 32%) than operative drainage. A more complete discussion of the bacteriology and antibiotic management of patients with cholangitis can be found in Chapters 11 and 43.
Preoperative Drainage
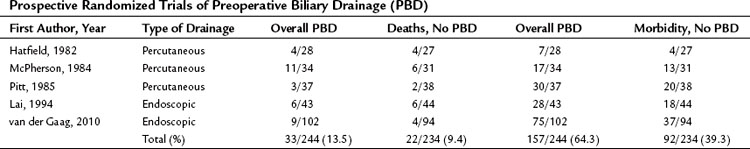
The preoperative relief of jaundice and the reversal of its systemic effects by either endoscopic or transhepatic biliary decompression has been proposed as a method to decrease the risk of surgery in jaundiced patients. However, several prospective randomized studies have shown that the routine use of preoperative biliary drainage does not reduce operative morbidity or mortality in patients with obstructive jaundice (Hatfield et al, 1982; Lai et al, 1994; McPherson et al, 1984; Pitt et al, 1985; van der Gaag et al, 2010; Table 7.4). In addition, a meta-analysis also concluded that preoperative biliary drainage increased, rather than decreased, overall complications from surgery and the drainage procedure and provided no benefit in terms of reduced mortality or decreased hospital stay (Sewnath et al, 2002). In fact, several retrospective studies have documented a higher incidence of infectious complications (wound infection, pancreatic fistula) and even mortality in patients undergoing pancreatic or biliary tract resection after preoperative biliary decompression (Sewnath et al, 2002; Sohn et al, 2000).
A criticism of some of the prospective studies is that the duration of preoperative drainage (10 to 18 days) may have been inadequate to reverse the multiple metabolic and immunologic abnormalities associated with severe obstructive jaundice. Both animal and human studies demonstrate that the recovery of various metabolic and immune functions requires at least 6 weeks to pass after the relief of biliary obstruction (Clements et al, 1993; Kennedy et al, 1994; Thompson et al, 1990). Similarly, animal studies strongly suggest that return of bile to the intestinal tract has significant advantages over external biliary drainage (Roughneen et al, 1986).
Although the data suggest that routine preoperative biliary drainage may be of limited benefit, it may have some value in selected patients with advanced malnutrition, biliary sepsis, and hilar malignancies that require liver resection. Regarding the latter, it should be emphasized that most of the published data regarding preoperative biliary drainage focuses on patients with periampullary malignancy. Patients with proximal biliary obstruction undergoing major hepatic resection represent an entirely different subgroup, and although no adequately powered study has addressed this issue specifically in this population, some evidence does suggest a benefit of preoperative biliary drainage of the future liver remnant (see Chapters 50B, 50C, and 93A). Preoperatively placed catheters also may be of value in the operating room during difficult biliary dissections in patients with proximal biliary tumors or strictures, and they may aid in the placement of long-term transhepatic stents.
Andy OJ, et al. Peritoneal neutrophil chemotaxis is impaired in biliary obstruction. Am Surg. 1992;58:28-31.
Arrese M, Accatino L. From blood to bile: recent advances in hepatobiliary transport. Ann Hepatol. 2002;1:64-71.
Blenkharn JI, et al. Decreased biliary excretion of piperacillin after percutaneous relief of extrahepatic obstructive jaundice. Antimicrob Agents Chemother. 1985;28:778-780.
Cahill CJ. Prevention of postoperative renal failure in patients with obstructive jaundice: the role of bile salts. Br J Surg. 1983;70:590-595.
Cainzos M, et al. Hyperbilirubinemia, jaundice and anergy. Hepatogastroenterology. 1992;39:330-332.
Clements WD, et al. Effects of extrahepatic obstructive jaundice on Kuppfer cell clearance capacity. Arch Surg. 1993;128:200-204.
Crawford JM. Role of vesicle-mediated transport pathways in hepatocellular bile secretion. Semin Liver Dis. 1996;16:169-189.
Cui Y, et al. Hepatic uptake of bilirubin and its conjugates by the human organic anion transport SLC21A6. J Biol Chem. 2001;276:9626-9630.
Deitch EA, et al. Obstructive jaundice promotes bacterial translocation from the gut. Am J Surg. 1990;159:79-84.
Elferink O, Groen AK. Mechanisms of biliary lipid secretion and their role in lipid homeostasis. Semin Liver Dis. 2000;20:293-305.
Fogarty BJ, et al. Renal dysfunction in obstructive jaundice. Br J Surg. 1995;82:877-884.
Foschi D, et al. Hyperalimentation of jaundiced patients on percutaneous transhepatic biliary drainage. Br J Surg. 1986;73:716-719.
Gerk PM, Vore M. Regulation of expression of the multidrug resistance–associated protein 2 (MRP2) and its role in drug disposition. J Pharmacol Exp Ther. 2002;302:407-415.
Gigot JF, et al. Acute cholangitis: multivariate analysis of risk factors. Ann Surg. 1989;209:435-438.
Goldman L, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845-850.
Grande L, et al. Obstructive jaundice and wound healing. Br J Surg. 1990;77:440-442.
Green J, Better OS. Circulatory disturbance and renal dysfunction in liver disease and in obstructive jaundice. Isr J Med Sci. 1994;30:48-65.
Halliday AW, Benjamin IS, Blumgart LH. Nutritional risk factors in major hepatobiliary surgery. J Parent Ent Nutr. 1988;12:43-48.
Hatfield AR, et al. Preoperative external biliary drainage in obstructive jaundice. Lancet. 1982;2:896-899.
Hunt DR, et al. Endotoxemia, disturbance of coagulation, and obstructive jaundice. Am J Surg. 1982;144:325-329.
Iyanagi T, et al. Biochemical and molecular aspects of genetic disorders of bilirubin metabolism. Biochim Biophys Acta. 1990;1407:173-184.
Karsten TM, et al. Morphological changes of extrahepatic bile ducts during obstruction and subsequent decompression by endoprosthesis. Surgery. 1991;111:562-568.
Kennedy JA, et al. Modulation of immune function and weight loss by L-arginine in obstructive jaundice in the rat. Br J Surg. 1994;81:1199-1201.
Klein A, et al. Liver, biliary tract, and pancreas. In: O’Leary, J, editor. Physiologic Basis of Surgery. Baltimore: Wilkins & Williams; 1996:441-478.
Koeppel TA, et al. Extrahepatic biliary obstruction impairs microvascular perfusion and increases leukocyte adhesion in rat liver. Hepatology. 1997;26:1085-1091.
Koyama K, et al. Experimental and clinical studies on the effect of biliary drainage in obstructive jaundice. Am J Surg. 1981;142:293-299.
Kullak-Ublick GA, et al. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322-342.
Lai ECS, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582-1586.
Lai ECS, et al. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195-1198.
Lee MH, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79-83.
Lippset PA, Pitt HA. Biliary infection: prophylaxis and treatment. In: Toouli, J, editor. Surgery of the Biliary Tract. Edinburgh: Churchill Livingstone; 1993:59-70.
Lumlertgul D, et al. The jaundiced heart: evidence of a blunted response to positive inotropic stimulation. Ren Fail. 1991;13:15-22.
Ma Z, et al. Differential effects of jaundice and cirrhosis on β-adrenoceptor signaling in three rat models of cirrhotic cardiomyopathy. J Hepatol. 1999;30:485-491.
McPherson GAD, et al. Preoperative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984;71:371-375.
Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635-661.
Nehez L, Anderson R. Compromise of immune function in obstructive jaundice. Eur J Surg. 2002;168:315-328.
Padillo J, et al. Improved cardiac function in patients with obstructive jaundice after internal biliary drainage: hemodynamic and hormonal assessment. Ann Surg. 2001;234:652-656.
Patel T, Bronk SF, Gores GJ. Increase in intracellular magnesium promotes glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest. 1994;94:2183-2192.
Pitt HA, et al. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201:545-552.
Puntis MCA, Jiang WG. Plasma cytokine levels and monocyte activation in patients with obstructive jaundice. J Gastroenterol Hepatol. 1996;11:7-13.
Roughneen PT, et al. Impaired specific cell-mediated immunity in experimental biliary obstruction and its reversibility by internal biliary drainage. J Surg Res. 1986;41:113-125.
Scott-Conner CE, et al. Impaired bacterial killing in early obstructive jaundice. Am J Surg. 1993;166:308-310.
Sewnath ME, et al. Meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17-27.
Sohn TA, et al. Do preoperative biliary stents increase postpancreaticoduodenectomy complications? J Gastrointest Surg. 2000;4:258-268.
Swartz-Basile DA, et al. Leptin regulates gallbladder genes related to absorption and secretion. Am J Physiol. 2007;293:84-90.
Thompson JE, et al. Broad spectrum penicillin as adequate therapy for acute cholangitis. Surg Gyn Obst. 1990;171:275-282.
Thompson RL, et al. Development and reversibility of T lymphocyte dysfunction in experimental obstructive jaundice. Br J Surg. 1990;77:1229-1232.
Van der Gaag NA, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129-137.