Aspiration
Risk, Prophylaxis, and Treatment
Geraldine O’Sullivan MD, FRCA, M. Shankar Hari Dip. Epi., MD, FRCA, FFICM
Chapter Outline
INCIDENCE, MORBIDITY, AND MORTALITY
History
In 1848, Sir James Simpson first suggested aspiration as a cause of death during anesthesia. Hannah Greener, a 15-year old given chloroform for a toenail extraction, became cyanotic and “sputtered” during the anesthetic. A “rattling in her throat” then developed, and she soon died. Her physician administered water and brandy by mouth. Simpson1 contended that it was the aspiration of water and brandy, and not the adverse effects from the chloroform, that caused her death. In 1940, Hall published a report of 15 cases of aspiration, 14 of which occurred in mothers receiving inhalation anesthesia for a vaginal or cesarean delivery.2 Among the 14 obstetric cases, 5 mothers died.
Subsequently, Curtis Mendelson, in a landmark paper, reported a series of animal experiments that clearly described the clinical course and pathology of pulmonary acid aspiration.3 In the same paper, Mendelson also audited 44,016 deliveries at the New York Lying-In Hospital between 1932 and 1945. He identified 66 (0.15%) cases of aspiration, of which the aspirated material was recorded in 45 cases; 40 mothers aspirated liquid, and 5 aspirated solid food. Importantly, no mothers died of acid aspiration, but 2 mothers died of asphyxiation caused by the aspiration of solid food. At this time general anesthesia usually involved the inhalation of ether, often as Mendelson observed, by “a new and inexperienced intern.” Mendelson therefore advocated (1) the withholding of food during labor, (2) the greater use of regional anesthesia, (3) the administration of antacids, (4) the emptying of the stomach before administration of general anesthesia, and (5) the competent administration of general anesthesia. This advice became the foundation of obstetric anesthesia practice during subsequent decades.
Incidence, Morbidity, and Mortality
Maternal mortality from pulmonary aspiration of gastric contents has declined to almost negligible levels in the past 3 decades (Figure 29-1).4–6 This decline can probably be attributed to the following factors: (1) the greater use of neuraxial anesthesia; (2) the use of antacids, histamine-2 (H2) receptor antagonists, and/or proton-pump inhibitors; (3) the use of rapid-sequence induction of general anesthesia; (4) an improvement in the training of anesthesia providers; and (5) the establishment and enforcement of nil per os (NPO) policies. Arguably, the common use of neuraxial analgesic/anesthetic techniques, both during labor and for cesarean delivery, is the single most important factor in this remarkable decline in maternal mortality from pulmonary aspiration.
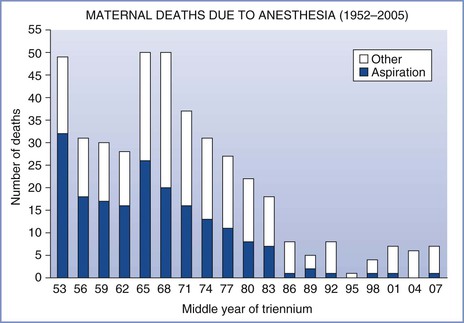
FIGURE 29-1 Maternal mortality from anesthesia and pulmonary aspiration in the United Kingdom, 1952-2008. (Compiled from data from references 4 through 6.)
The reported incidence of aspiration pneumonitis depends on the criteria used for making the diagnosis. The relative risk for aspiration in pregnant versus nonpregnant women can best be estimated from comparisons within single-study populations. Olsson et al.7 reported an overall incidence of aspiration of 1 in 2131 in the general population undergoing anesthesia and 1 in 661 in women undergoing cesarean delivery (i.e., a threefold higher aspiration risk). In two other surveys related to aspiration (one a retrospective review of 172,334 consecutive patients undergoing general anesthesia and the other a review of 133 cases of aspiration from the Australian Anaesthetic Incident Monitoring Study [AIMS]), there were no cases of pulmonary aspiration in women undergoing either elective or emergency cesarean delivery.8,9 However, in the latter two studies, emergency surgery was a significant predisposing factor for aspiration; this finding may be relevant for the practice of obstetric anesthesia, given that many obstetric surgical procedures are performed on an urgent or emergency basis. The AIMS study also implicated obesity as a significant risk factor for aspiration; others have noted that obesity is associated with an increased risk for maternal mortality.4,5
Morbidity and mortality associated with aspiration vary according to (1) the physical status of the patient, (2) the type and volume of aspirate, (3) the therapy administered, and (4) the criteria used for making the diagnosis. Since 1952, the Department of Health in the United Kingdom has published detailed triennial reports on all maternal deaths. Data from these reports, now administered by the body Mothers and Babies—Reducing Risk through Audits and Confidential Enquiries across the UK (MBRRACE-UK), indicate that death from pulmonary aspiration in obstetrics is vanishingly rare (see Figure 29-1).4–6 In the last five reports, which cover the 15-year period from 1994 to 2008, there were three maternal deaths from aspiration; one was an obese parturient, the second was a mother anesthetized 3 days after delivery, and the third was a women with a placenta previa who required an emergency cesarean delivery after eating a full meal and aspirated on emergence from general anesthesia. Although the number of anesthetics, particularly general anesthetics, administered to parturients during this 15-year period is unknown, there were approximately 10.5 million deliveries, indicating that the mortality rate from aspiration was less than 1 in 3.5 million deliveries.
Data on pulmonary aspiration in obstetrics in the United States are more difficult to evaluate. Despite the establishment of an ongoing National Pregnancy Mortality Surveillance System by the Centers for Disease Control and Prevention (CDC), it is often difficult to obtain adequate and detailed information about every maternal death. Prior to 1990, aspiration was the most common cause of anesthesia-related maternal death in the United States; it has been calculated that at that time there were 17 deaths related to general anesthesia for every one death related to regional anesthesia.10 By the early 1990s, this ratio had improved to 6 to 1. By 2002, death rates for both general and regional anesthesia were similar.11 However, mortality statistics are generally a poor predictor of maternal morbidity; several studies have indicated that perioperative aspiration can be associated with important morbidity in obstetric patients,12,13 and thus all possible measures still must be taken to prevent pulmonary aspiration in obstetric patients.
Gastroesophageal Anatomy and Physiology
Esophagus
In adults, the esophagus is approximately 25 cm long and the esophagogastric junction is approximately 40 cm from the incisor teeth. In humans, the proximal one third of the esophagus is composed of striated muscle but the distal end contains only smooth muscle. Muscular sphincters at both ends are normally closed. The cricopharyngeal or upper esophageal sphincter prevents the entry of air into the esophagus during respiration, and the gastroesophageal or lower esophageal sphincter prevents the reflux of gastric contents. The lower esophageal sphincter is characterized anatomically and manometrically as a 3-cm zone of specialized muscle that maintains tonic activity. The end-expiratory pressure in the sphincter is 8 to 20 mm Hg above the end-expiratory gastric pressure. The lower esophageal sphincter is kept in place by the phrenoesophageal ligament, which inserts into the esophagus approximately 3 cm above the diaphragmatic opening (Figure 29-2). The lower esophageal sphincter is not always closed; transient relaxations occur that account for the gastroesophageal reflux that healthy subjects experience.14
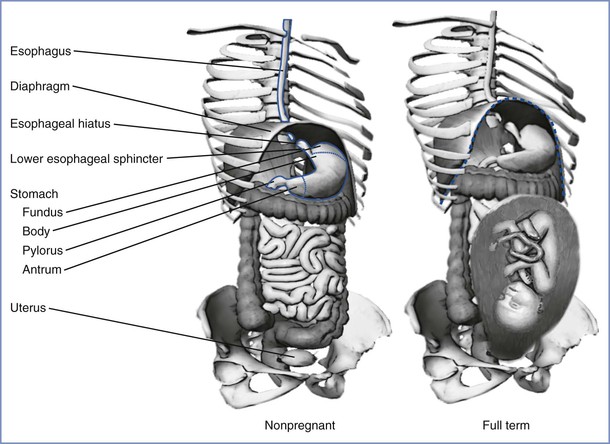
FIGURE 29-2 The stomach and its relationship to the diaphragm in nonpregnancy (left) and pregnancy (right). The stomach consists of a fundus, body, antrum, and pylorus. The function of the lower esophageal sphincter depends on the chronic contraction of circular muscle fibers, the wrapping of the esophagus by the crus of the diaphragm at the esophageal hiatus, and the length of the esophagus exposed to intra-abdominal pressure. The gravid uterus may encroach on the stomach and alter the effectiveness of the lower esophageal sphincter. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Gastrointestinal Motility
Differences in fasting and fed patterns of gut motility are now firmly established. During fasting, the main component of peristalsis is the migrating motor complex (MMC).15 Each MMC cycle lasts 90 to 120 minutes and comprises four phases: phase I has little or no electrical spike activity and thus no measurable contractions, phase II has intermittent spike activity, phase III has spikes of large amplitude and is associated with strong contractile activity, and phase IV is a brief period of intermittent activity leading back to phase I. The MMC first appears in the lower esophageal sphincter and stomach, followed by the duodenum, and finally the terminal ileum, at which time a new cycle begins in the lower esophageal sphincter and stomach. The phase of the MMC at the time of administration of certain drugs can affect absorption and thereby the onset of therapeutic effect.16 Eating abolishes the MMC and induces a pattern of intermittent spike activity that appears similar to that in phase II. The duration of the fed pattern is determined both by the calorie content and the type of nutrients in the meal.
The stomach, through the processes of receptive relaxation and gastric accommodation, can accept 1.0 to 1.5 L of food before intragastric pressure begins to increase. The contraction waves that propel food into the small intestine begin in the antrum. The pylorus closes midway through the contraction wave, allowing some fluid to exit into the duodenum but causing the remaining fluid to move retrograde toward the body of the stomach.17 The jet of fluid that exits the pylorus contains primarily liquid and fine particles. Large particles that lag behind are caught in the retrograde flow of fluid, which assists in their disintegration. Therefore, the manner by which individual components of a meal pass through the stomach depends on the particle size and the viscosity of the suspension. Small particles and fluids exit the stomach faster than larger particles.17 The outlet of the stomach—the pylorus—limits outflow by means of both its chronic tone and its anatomic position. The pylorus is higher than the most dependent portion of the stomach in both the supine and standing positions.17
Gastric Secretion
In one day, the stomach produces as much as 1500 mL of highly acidic fluid containing the proteolytic enzyme pepsin.18 Normal individuals can produce a peak acid output of 38 mmol/h.19 Acid is secreted at a low basal rate of approximately 10% of maximal output, even when the stomach is empty.19,20 There is diurnal variation in this basal rate of gastric acid secretion, with the lowest and highest outputs occurring in the morning and evening, respectively.
The stomach lining has two types of glands: pyloric and oxyntic. The pyloric glands contain chief cells, which secrete pepsinogen, the precursor for pepsin. The oxyntic glands contain the oxyntic cells, which secrete hydrochloric acid. Water molecules and carbon dioxide in the oxyntic cells combine to form carbonic acid, which dissociates into hydrogen ions and bicarbonate. The bicarbonate leaves the cell for the bloodstream, and the hydrogen ions are actively exchanged for potassium ions in the canaliculi connecting with the lumen of the oxyntic gland. The secretions of the oxyntic cell can contain a hydrochloric acid concentration as great as 160 mmol/L (pH 0.8).18 Proton pump inhibitors (PPIs) block the hydrogen ion pump on the canaliculi to decrease acid production.21
The pylorus contains G cells, which secrete gastrin into the bloodstream when stimulated by the vagus nerve, stomach distention, tactile stimuli, or chemical stimuli (e.g., amino acids, certain peptides). Gastrin binds to gastrin receptors on the oxyntic cell to stimulate the secretion of hydrochloric acid. Acetylcholine binds to muscarinic (M1) receptors on the oxyntic cell to cause an increase in intracellular calcium ion concentration, which results in hydrochloric acid secretion. Histamine potentiates the effects of both acetylcholine and gastrin by combining with H2 receptors on the oxyntic cell to increase the intracellular cyclic adenosine monophosphate concentration, leading to a dramatic increase in the production of acid.18 H2-receptor antagonists (e.g., ranitidine, famotidine) prevent histamine’s potentiation of acid production (Figure 29-3).
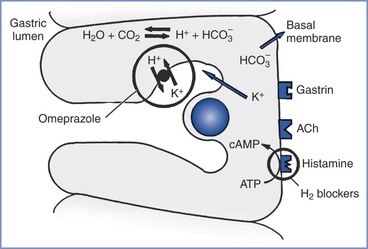
FIGURE 29-3 The oxyntic cell produces hydrogen ions that are secreted into the gastric lumen and bicarbonate ions that are secreted into the bloodstream. H2-receptor antagonists (e.g., ranitidine, famotidine) and proton-pump inhibitors (e.g., omeprazole) act on the oxyntic cell to reduce gastric acid secretion. H2-receptor antagonists block the histamine receptor on the basal membrane to decrease hydrogen ion production in the oxyntic cell. Omeprazole blocks the active transport of the hydrogen ions into the gastric lumen. ACh, acetylcholine; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; CO2, carbon dioxide; H+, hydrogen ion; HCO3−, bicarbonate; H2O, water; K+, potassium.
Ingestion of Food
When a meal is eaten, the mechanisms that control the secretion of gastric juice and the motility and emptying of the stomach interact in a complex manner to coordinate the functions of the stomach. The response to eating is divided into three phases: cephalic, gastric, and intestinal. Chewing, tasting, and smelling cause an increase in the vagal stimulation of the stomach, which in turn increases gastric acid production. This represents the cephalic phase of digestion.18 In this phase, gastric acid output increases to approximately 55% of peak output.22 The gastric phase begins with the release of gastrin. Gastric acid secretion depends on antral distention, vagal activity, gastrin concentration, and the composition of the meal.20,22,23 Gastric acid secretion during a mixed-composition meal increases to approximately 80% of peak acid output.19 The intestinal phase begins with the movement of food into the small intestine and is largely inhibitory. Hormones (e.g., gastrin, cholecystokinin, secretin) and an enterogastric reflex further modulate gastric acid secretion and motility depending on the composition and volume of the food in the duodenum.18,24 This inhibition of gastric emptying by food in the duodenum enables the duodenal contents to be processed before more material is delivered from the stomach.
After the ingestion of a meal, gastric emptying depends on (1) the pre-meal volume, (2) the volume ingested, (3) the composition of the meal, (4) the size of the solids, (5) the amount of gastric secretion, (6) the physical characteristics of the stomach contents entering the duodenum, and (7) patient position.20,24–26 A mixture of liquids and solids passes through the stomach much more slowly than liquids alone. Gastric emptying is slowed by high lipid content, high caloric load, and large particle size.20,27,28 Thus, predicting an exact time for the passage of liquids and solids through the stomach is very difficult. For non-nutrient liquids (e.g., normal saline), the gastric volume decreases exponentially with respect to time.26 In one study, 90% of a 150-mL saline meal given to fasting adults in the sitting position passed through the stomach in a median time of 14 minutes; however, in adults in the left lateral position, the median time for gastric emptying was 28 minutes.25 In another study, 100% of a 500-mL saline meal given to fasting adults passed through the stomach within 2 hours, as determined by a polyethylene glycol marker.20 However, despite complete emptying of the saline test meal, the mean residual gastric volume at the end of 2 hours was 46 mL; this was because of greater secretion of gastric acid. Progressively less complete gastric emptying and higher mean residual gastric contents were observed with meals containing amino acids, glucose, and glucose with fat.20 These studies indicate that the volume and composition of the test meal, as well as the resulting gastric secretions, strongly affect gastric emptying and residual gastric content. For example, the subject described in Figure 29-4 responded to the test meal by secreting 800 mL of gastric juice and consequently the volume in the stomach remained high for almost 2 hours despite early, rapid emptying.29
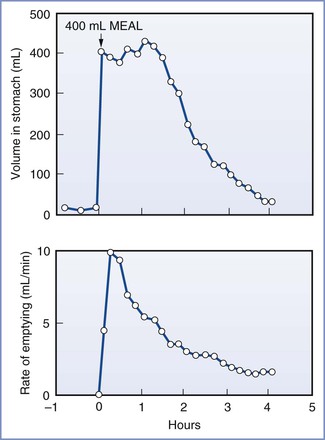
FIGURE 29-4 Volume of gastric contents and rate of gastric emptying in a subject eating a 400-mL meal of steak, bread, and vanilla ice cream. (From Malagelada JR, Longstreth GF, Summerskill WHJ, et al. Measurements of gastric functions during digestion of ordinary solid meals in man. Gastroenterology 1976; 70:203-10.)
Effects of Pregnancy on Gastric Function
Gastroesophageal reflux, resulting in heartburn, is a common complication of late pregnancy. Pregnancy compromises the integrity of the lower esophageal sphincter; it alters the anatomic relationship of the esophagus to the diaphragm and stomach, raises intragastric pressure, and in some women limits the ability of the lower esophageal sphincter to increase its tone.30–33 Progesterone, which relaxes smooth muscle, probably accounts for the inability of the lower esophageal sphincter to increase its tone.34 Lower esophageal pH monitoring has shown a higher incidence of reflux in pregnant women at term, even in those who are asymptomatic, than in nonpregnant controls. Therefore, at term gestation the pregnant woman who requires anesthesia should be regarded as having an incompetent lower esophageal sphincter. These physiologic changes return to their prepregnancy levels by 48 hours after delivery.33
Serial studies assessing gastric acidity during pregnancy have proved difficult to perform because pregnant women do not usually wish to swallow nasogastric tubes repeatedly for research purposes. However, in the most comprehensive study of gastric acid secretion during pregnancy, basal and histamine-augmented gastric acid secretion was measured in 10 controls and 30 pregnant women equally distributed throughout the three trimesters of pregnancy.35 No significant differences in basal gastric acid secretion were seen between the pregnant and nonpregnant women. However, when the women were divided into groups according to gestational age, the mean rate of gastric acid secretion was found to be reduced during the second trimester. The maximal response to histamine was significantly lower in women in the first and second trimesters than in women who were either not pregnant or in the third trimester of pregnancy.35
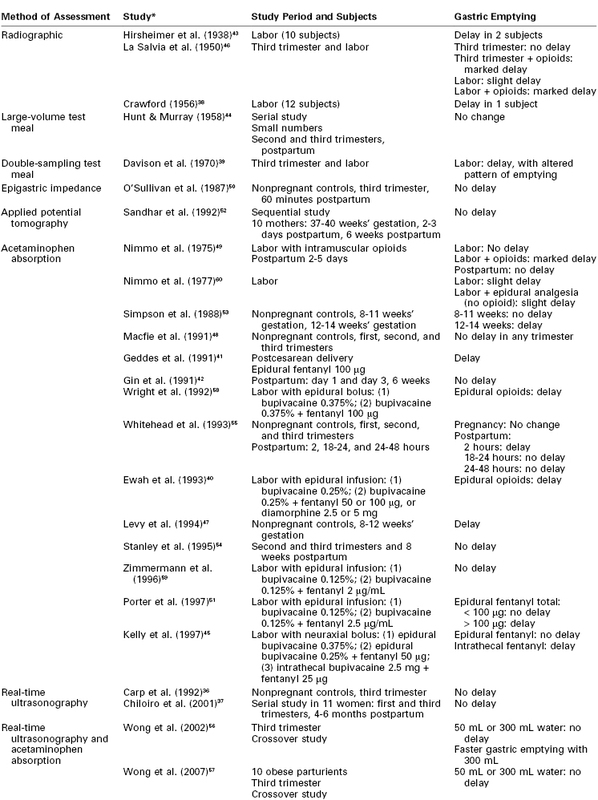
Assessment of gastric emptying during pregnancy and labor presents technical and ethical challenges, and a variety of techniques have been used (Table 29-1).36–60 Pregnancy does not significantly alter the rate of gastric emptying.39 In addition, gastric emptying was not found to be delayed in either obese or nonobese term pregnant women who ingested 300 mL of water after an overnight fast.56,57 However, management of obese parturients should take into account the possible presence of other associated problems in this group of patients (e.g., hiatal hernia or difficult airway). Gastric emptying appears to be normal in early labor but becomes delayed as labor advances49; the cause is uncertain. Pain is known to delay gastric emptying, but even when labor pain is abolished with epidural analgesia using a local anesthetic alone, the delay still occurs.60 Parenteral opioids cause a significant delay in gastric emptying, as do bolus doses of epidural and intrathecal opioids.40,45,49,51,58 Continuous epidural infusion of low-dose local anesthetic with fentanyl does not appear to delay gastric emptying until the total dose of fentanyl exceeds 100 µg.51
The plasma concentration of the gastrointestinal hormone motilin is decreased during pregnancy.61 Studies have shown either no change30,32,62 or an increase63 in the plasma concentration of gastrin.
Risk Factors for Aspiration Pneumonitis
Mendelson3 divided aspiration pneumonitis into two types: liquid and solid. Whereas the aspiration of solids could result in asphyxiation, Mendelson demonstrated that the sequelae from the aspiration of liquids were more severe clinically and pathologically when the liquid was highly acidic (Figure 29-5). His observations, together with the results from other investigations,64–72 suggest that the morbidity and mortality of aspiration depend on the following three variables: (1) the chemical nature of the aspirate, (2) the physical nature of the aspirate, and (3) the volume of the aspirate. Aspirates with a pH less than 2.5 cause a granulocytic reaction that continues beyond the acute phase.72 Aspiration of particulate material can engender a clinical picture with severity equal to or greater than that caused by the aspiration of acidic liquid.71 Aspiration of small volumes of neutral liquid results in a very low rate of mortality. However, aspiration of large volumes of neutral liquid results in a high mortality rate, presumably as a result of the disruption of surfactant by the large volume of liquid or from a mechanism similar to that seen in “near drowning.”67
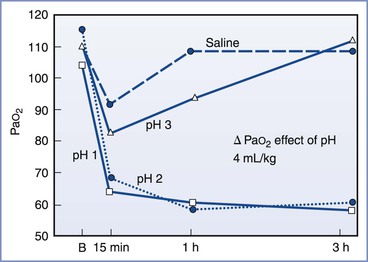
FIGURE 29-5 Relationship between acidity and PaO2. In this study, 4 mL/kg of fluid of varying pH was instilled into the tracheas of dogs. The severity of the hypoxemia correlated with the pH of the aspirate. A maximal decrease in PaO2 occurred with aspirates with a pH of less than 2.5. B, baseline. (From Awe WC, Fletcher WS, Jacob SW. The pathophysiology of aspiration pneumonitis. Surgery 1966; 60:232-9.)
Historically, anesthesia providers have considered a nonparticulate gastric fluid with a pH less than 2.5 and a gastric volume greater than 25 mL (i.e., 0.4 mL/kg) as risk factors for aspiration pneumonitis.64,69,70 No human study has directly addressed the relationship between preoperative fasting, gastric acidity and volume, and the risk for pulmonary aspiration during anesthesia.73,74 There appears to be a reasonable scientific basis using a gastric pH cut-off value of less than 2.5 as a risk factor. In animal experiments, the risk for aspiration pneumonitis clearly increased with decreasing pH of the tracheal aspirate.64,67 Awe et al.64 illustrated this concept in a graph of PaO2 versus time for aspirates of varying pH (see Figure 29-5).
Animal studies have also demonstrated that an increase in the volume of tracheal aspirate is associated with a higher risk for aspiration pneumonitis.67 However, the volume of aspirated material associated with risk has been disputed. The commonly accepted volume of 0.4 mL/kg (approximately 25 mL in a 70-kg adult) originated from an experiment in a single rhesus monkey in which 0.4 mL/kg of an acidic liquid was administered into the right mainstem bronchus and resulted in the animal’s death.70 The investigators made the assumption that this entire volume, if contained in the stomach, could be aspirated. However, Raidoo et al.75 demonstrated variability in the response of juvenile monkeys to different volumes of an acidic tracheal aspirate. Death was seen with aspirate volumes of 0.8 mL/kg and 1.0 mL/kg but not with volumes of 0.4 mL/kg and 0.6 mL/kg. Similarly, Plourde and Hardy76 refuted the assumption that all the gastric contents would be aspirated and demonstrated that gastric volumes of 0.4 mL/kg did not increase the risk for aspiration. Hence the gastric volume that puts a patient at risk for aspiration pneumonitis has not been determined. However, a reasonable goal of prophylactic therapy would be a gastric pH greater than 2.5 and a gastric volume as low as possible.
Pathophysiology
Aspiration pneumonitis (Mendelson’s syndrome) describes a chemical injury to the tracheobronchial tree and alveoli caused by the inhalation of sterile acidic gastric contents, whereas aspiration pneumonia may be regarded as an infectious process of the respiratory tract caused by the inhalation of oropharyngeal secretions that are colonized by pathogenic bacteria. Aspiration of gastric contents could therefore result in acid injury to the lung with or without bacterial and particulate matter–related effects.
Aspiration of acidic liquid injures the alveolar epithelium and results in an alveolar exudate composed of edema, albumin, fibrin, cellular debris, and red blood cells,3,69,71,72 whereas the aspiration of neutral, nonparticulate liquid leads to an alveolar exudate with minimal damage to the alveoli. The phospholipid and apoprotein composition of surfactant changes, exerting a negative effect on its surface-active properties.77 This effect leads to an increase in intraalveolar water and protein content and a loss of lung volume, resulting in a decrease in pulmonary compliance and intrapulmonary shunting of blood. The cellular debris and bronchial denuding cause bronchial obstruction. The exudative pulmonary edema, bronchial obstruction, reduced lung compliance, and shunting result in hypoxemia, increased pulmonary vascular resistance, and increased work of breathing. After the direct acid-mediated injury of the respiratory tract, an intense inflammatory response ensues from macrophage activation and secretion of cytokines, interleukins (IL) IL-1, IL-6, IL-8, and IL-10, and tumor necrosis factor-alpha (TNF-α).78 These inflammatory mediators lead to the chemotaxis, accumulation, and activation of neutrophils in the alveolar exudate, up-regulation of adhesion molecules within the pulmonary vasculature, and activation of the complement pathways. The neutrophils subsequently release oxidants, proteases, leukotrienes, and other proinflammatory molecules.78 Amplification of these inflammatory processes may result in the development of acute lung injury or acute respiratory distress syndrome (ARDS) (Figure 29-6).77–79
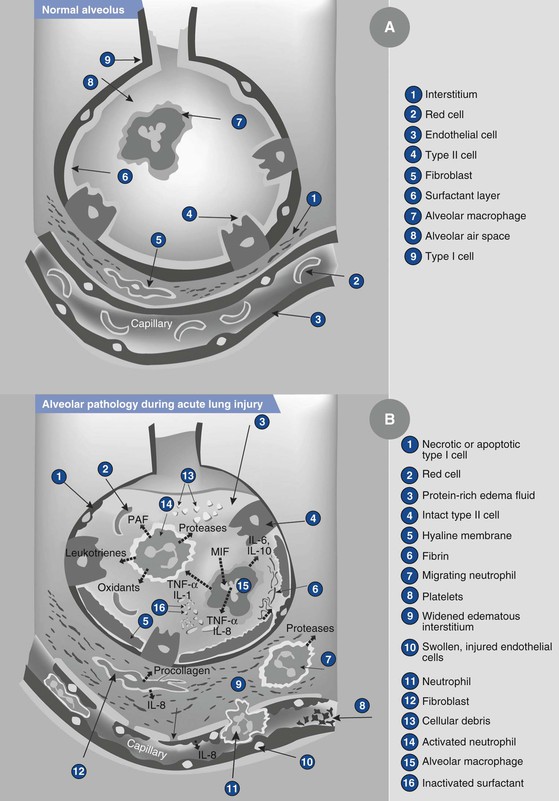
FIGURE 29-6 Illustration showing the normal alveolus (A) and the injured alveolus (B) during acute lung injury. In the acute phase of acute lung injury there is formation of protein-rich hyaline membranes on the denuded basement membrane. Neutrophils are marginating through the interstitium into the air space. Alveolar macrophages secrete interleukin (IL)-1, 6, 8, and 10, as well as tumor necrosis factor-alpha (TNF-α), that stimulate and activate neutrophils. Neutrophils release proinflammatory molecules (oxidants, proteases, leukotrienes, platelet-activating factor [PAF ]). The influx of protein-rich edema fluid into the alveolus has led to the inactivation of surfactant and, together with unresolved fibrin depositions, fibrin-rich hyaline membranes are formed. MIF, müllerian inhibiting factor. (From Dahlem P, van Aalderen WMC, Bos AP. Pediatric acute lung injury. Paediatr Respir Rev 2007; 8:348-62.)
The acidic contents of the stomach prevent the growth of bacteria under normal conditions. However, gastric contents may become colonized with pathogenic gram-negative bacteria in patients receiving antacid therapy or with enteral feeding tubes, gastroparesis, or intestinal obstruction. The bacterial content adds to the inflammatory response to acid aspiration.80
Aspiration of particulate matter in the supine position most commonly involves injury to the posterior segments of the upper lobes and the apical segments of the lower lobes, whereas aspiration in the semirecumbent or upright position typically leads to injury to the lower lobes. The right lower lobe is the most common site of aspiration injury because the right mainstem bronchus has larger and more vertical architecture compared with the left mainstem bronchus. Obstruction of the bronchus or bronchioles results in bronchial denudation and collapse of the bronchopulmonary segments. Persistent or unresolved collapse can lead to lung abscesses and cavitation.80
After the acute period, the process resolves through the proliferation and differentiation of surviving type II pneumocytes in the alveolar epithelial cells.78,79 The type II pneumocytes actively transport sodium out of the alveolus, and water follows passively. Soluble proteins are removed by paracellular diffusion and endocytosis, and insoluble proteins are removed by macrophages. Neutrophils are removed by programmed cell death and subsequent phagocytosis by macrophages. Type II pneumocytes gradually restore the normal composition of the surfactant. In a subset of patients with ARDS, the injury progresses to a fibrosing alveolitis—an accumulation of mesenchymal cells, their products, and new blood vessels.
Bronchospasm and disruption of surfactant likely account for the slight decrease in PaO2 and increase in shunting that are observed.72 Aspiration of large solid particles may cause atelectasis by obstructing large airways.3 Aspiration of smaller particulate matter causes an exudative neutrophilic response at the level of the bronchioles and alveolar ducts; the clinical picture is similar after the aspiration of acidic liquid.66,71,72
Clinical Course
In most cases of aspiration during anesthesia, the anesthesia provider witnesses regurgitation of gastric contents into the hypopharynx.3 Patients who aspirate while breathing spontaneously have a brief period of breath-holding followed by tachypnea, tachycardia, and a slight respiratory acidosis. Significant aspiration always results in some hypoxemia caused by greater shunting and frequent bronchospasm.
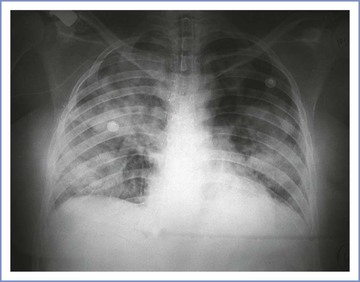
An abnormality on a chest radiograph can be seen in 85% to 90% of patients who aspirate gastric contents.68,81 Because these chest radiographic findings may lag behind clinical signs by as much as 12 to 24 hours, the initial radiograph may appear normal.81 In mild cases, alveolar infiltrates are seen in the dependent portions of the lungs. Severe aspiration results in diffuse bilateral infiltrates without signs of heart failure (i.e., engorged pulmonary vasculature and/or enlarged cardiac silhouette) (Figure 29-7).
These symptoms and signs may progress to satisfy the Berlin Definition for ARDS; the criteria are as follows82:
Treatment
Management of Aspiration
Management principles include rigid bronchoscopy, appropriate use of antibiotics, and management of hypoxemia with CPAP in nonintubated patients. Common treatments that lack evidence to support their use are the administration of corticosteroids and lung lavage with saline and bicarbonate.80
Rigid Bronchoscopy and Lavage
Suction of the upper airway followed by tracheal intubation and suction of the primary bronchi commonly precedes rigid bronchoscopy. Rigid bronchoscopy is useful for removing large food particles that cause airway obstruction. Lung lavage with saline or bicarbonate does not reduce the parenchymal damage caused by acid aspiration and can worsen preexisting hypoxemia.80
Antibiotics
Prophylactic antibiotics are not efficacious for aspiration and may lead to the development of infection with resistant organisms. Infection is not a component of acute pulmonary aspiration of sterile gastric contents.80 Antibiotics should be administered only in the presence of clinical findings that suggest infection (e.g., fever, worsening infiltrates on chest radiographs, leukocytosis, positive result of Gram stain of sputum, clinical deterioration).
In patients who are intubated, a nonbronchoscopic bronchoalveolar lavage sample can be sent for laboratory analysis. Tracheal sputum samples may be insufficient to identify a bacterial pathogen, and some authorities recommend sampling of the lower respiratory tract with a protected specimen brush.80
Empirical antibiotic therapy is appropriate in patients with suspected bacterial colonization of gastric contents. The “at risk” group (see earlier discussion) includes patients who have gastroparesis or bowel obstruction and those who are receiving enteral feeding or antacid therapy. Empirical antibiotic therapy is also appropriate in patients with aspiration pneumonitis that fails to resolve within 48 hours. The choice of antibiotic depends on the observed local patterns of antibiotic resistance. The target pathogens are gram-positive organisms (e.g., Streptococcus pneumoniae, Staphylococcus aureus) and some gram-negative organisms (e.g., Haemophilus influenzae, Escherichia coli, Enterobacteriaceae) when the diagnosis is made less than 48 hours after hospital admission (i.e., community-acquired pneumonia). Pseudomonas aeruginosa is a common pathogen in cases of nosocomial (hospital-acquired) aspiration pneumonia. Anaerobes are no longer believed to be present in the majority of cases.80 Pharmacologic therapy should be altered when specific pathogens and their antibiotic sensitivities are determined.
Treatment of Hypoxemia
Exudation of fluid into the alveoli, decreased surface activity of surfactant, and atelectasis all result in intrapulmonary shunting and hypoxemia. The administration of CPAP in patients breathing spontaneously or the administration of PEEP in patients undergoing mechanical ventilation restores functional residual capacity, reduces pulmonary shunting, and reverses hypoxemia. Supplemental oxygen should be given as required.
Corticosteroids
Despite decades-long use of corticosteroids in the management of aspiration pneumonitis, animal and human studies have failed to demonstrate a beneficial effect on pulmonary function, lung injury, alveolar-capillary permeability, or clinical outcomes after acid aspiration.80 Thus, the administration of corticosteroids for aspiration pneumonitis cannot be recommended.
Management of Respiratory Failure
Aspiration of gastric contents can result in activation of inflammatory intrapulmonary pathways80 consistent with the pathophysiology observed in ARDS.79,82 The basic tenets of management of ARDS include the use of “lung-protective” ventilation strategies, the judicious management of fluids, and the application of basic critical care algorithms. The management of severe ARDS and hypoxemia resistant to conventional management involves the use of rescue therapies (e.g., prone positioning, high-frequency oscillatory ventilation, extracorporeal membrane oxygenation) usually used in the critical care setting83 and thus will not form part of this overview. The following section outlines the basic principles in immediate management and stabilization. Readers are referred to extensive reviews of management of ARDS for further information.83–86
Mechanical Ventilation
The key principles governing mechanical ventilation in ARDS involve limiting the inspiratory plateau pressure to 30 cm H2O and providing the lowest effective tidal volume to prevent alveolar overdistention and tidal (cyclic) recruitment-derecruitment. Such “lung-protective” strategies correlated with improved outcomes in a prospective multicenter trial of the management of “early” ARDS; the use of initial tidal volumes and plateau pressures of 6 mL/kg and 30 cm H2O or less was compared with the use of 12 mL/kg and 50 cm H2O or less.87
Positive End-Expiratory Pressure
PEEP is a recommended component of the initial ventilator settings for ventilatory support in the setting of ARDS. A randomized clinical trial of ARDS (n = 549) funded by the National Institutes of Health (NIH) compared the effects of low and intermediate PEEP levels set according to predetermined combinations of PEEP and FIO2 in the setting of a lung-protective mechanical ventilation strategy.88 There were no significant differences in hospital mortality (24.9% versus 27.5%, respectively) or days to unassisted breathing (14.5 days versus 13.8 days, respectively) between the two groups. However, the role of comparatively higher levels of PEEP in protective ventilation strategies for ARDS is far from clear.89–91
Fluid Management
In a 2 × 2 factorial trial design, the ARDS Clinical Trials Network research group evaluated the use of conservative versus liberal fluid strategy and the value of guiding this intervention with central venous pressure or pulmonary artery wedge pressure measurements.92 The group that received conservative fluid management, whether guided by central venous pressure and/or pulmonary capillary wedge pressure measurements, had much lower net fluid balance, better lung function, and a shorter duration of mechanical ventilation and intensive care unit stay. Further, there appeared to be no increase in the incidence or duration of shock or need for dialysis in the conservative fluid management arm of the trial. Thus, early management of ARDS (after initial resuscitation) focuses on limiting iatrogenic insult with conservative fluid management strategy.
Basic Critical Care Algorithms
To minimize the risk for sepsis, central venous catheters and other invasive hemodynamic monitors should be removed as early as is clinically feasible. Aseptic precautions should be used during care, and infections should be treated with antibiotics specific to the bacterial pathogen for 3 to 7 days. Whether tight and rigorous glycemic control should be employed is controversial. Occasional withdrawal of sedation and the use of prophylaxis for gastrointestinal bleeding and thromboembolic events are currently considered the standard of care in any critically ill patient (see Chapter 55).93
Corticosteroids
Recovery from ARDS depends on the functional resolution of the underlying pulmonary disorder, which may follow one of two courses: (1) rapid improvement in lung function with an uncomplicated recovery or (2) slow improvements in lung function, oxygenation, and ventilation with prolonged weaning and recovery.
The corticosteroid controversy in ARDS in the context of aspiration is still unresolved.80 Although a recent systematic review suggested potential benefit from corticosteroids,94 their use did not appear to improve lung function or recovery in patients with ARDS in two of the well-conducted randomized clinical trials95,96 and may be associated with longer-term side effects. To further add to the controversy, a 2007 randomized controlled trial reported an improvement in outcomes in patients with ARDS randomized to receive a methylprednisolone infusion versus placebo.97 In our opinion, there appears to be no benefit from giving corticosteroids with the aim of attenuating lung injury after aspiration. However, if corticosteroids are being used for other reasons (e.g., bronchospasm, steroid replacement), then corticosteroid therapy could be considered.
Prophylaxis
The risk for aspiration is extremely low when gastric emptying is normal and patients, including parturients, are appropriately fasted. Factors predisposing to regurgitation, particularly in obstetrics, include emergency surgery, difficult/failed tracheal intubation, light anesthesia, and gastroesophageal reflux. The risk for failed intubation is 3 to 11 times greater in pregnant patients than in nonpregnant patients98,99 (see Chapter 30). Airway edema, breast enlargement, obesity, and the high rate of emergency surgery can all contribute to the risk for failed intubation in pregnant women. Aspiration pneumonitis is often associated with difficult or failed intubation during the induction of general anesthesia. In a survey conducted by the Society for Obstetric Anesthesia and Perinatology, intubation was recorded as difficult in 14 of 19 cases of aspiration in which tracheal intubation was used.100 Moreover, Warner et al.9 reported that the risk for aspiration during emergence from anesthesia was almost as high as that during induction of anesthesia. Thus, prophylactic regimens must provide protection during both induction of, and emergence from, general anesthesia. Although the risk for aspiration during elective, as opposed to emergency, surgery under general anesthesia is very low, parturients undergoing cesarean delivery or other surgical procedures should receive pharmacologic prophylaxis.
Because the incidence of aspiration pneumonitis is low, the efficacy of prophylactic regimens is measured by their ability to alter gastric pH and volume. In 30% to 43% of pregnant women the fasting gastric volume is greater than 25 mL and the gastric fluid pH is less than 2.5.101,102 However, the percentage of term parturients at risk may not differ from that of patients undergoing elective abortion, postpartum sterilization, or gynecologic surgery (Table 29-2).103–105 Gastric volume and acidity at term gestation are similar to gastric volume and acidity during early pregnancy, during the postpartum period, and in nonpregnant patients.101–108 Decreased lower esophageal sphincter tone and a higher risk for difficult intubation are the primary factors that increase the risk for aspiration during pregnancy and the immediate postpartum period, and these are the factors that mandate the need for pharmacologic prophylaxis.
Preoperative Oral Fluid Administration
Multiple studies have described no increase in gastric volume or acidity after the oral administration of 150 mL of fluid (e.g., coffee, tea, water, other clear liquids, orange juice without pulp) in nonpregnant adults 2 hours before elective surgery.109,110 The patients in these studies all fasted overnight and should have had a low gastric volume when the test meal was given. Lewis and Crawford111 noted that in women undergoing elective cesarean delivery, those who had been allowed to consume a meal of both tea and toast 2 to 4 hours preoperatively had an increase in gastric volume and a decrease in gastric pH compared with a control group. Consumption of tea without toast resulted in an increase in gastric volume but it did not alter gastric pH. Particulate material was aspirated from the stomachs of 2 of the 11 patients who consumed both tea and toast. The investigators did not state the volume of tea consumed by these patients.
In addition, when gastric emptying of both 50 mL and 300 mL of water was assessed in nonlaboring term parturients, the gastric emptying half-time for 300 mL was significantly shorter than that for 50 mL.56 When a similar study was conducted in obese nonlaboring parturients term (mean [±SD] prepregnancy body mass index 41 ± 9 kg/m2), the gastric emptying time for 300 mL was not longer than that for 50 mL.57 The latter finding suggests that the American Society of Anesthesiologists (ASA) Guidelines for Obstetric Anesthesia,73 which state that “the uncomplicated patient undergoing elective cesarean delivery may have modest amounts of clear liquids up to 2 h before induction of anesthesia” could also be applied to healthy, obese pregnant women presenting for elective surgery. However, factors other than the rate of gastric emptying can influence the rate of pulmonary aspiration, particularly in obese subjects. Obesity is associated with a higher incidence of gastroesophageal reflux and difficulty with airway management (both intraoperatively and postoperatively) (see Chapter 50). Moreover, the cesarean delivery rate is higher and the success rate of trial of labor after cesarean delivery is lower in obese parturients.112,113
Choice of Anesthesia
The Obstetric Anesthesia Work Force Survey demonstrated that the use of neuraxial anesthesia for cesarean delivery rose dramatically from 1981 to 2001, with the use of general anesthesia accounting for less than 5% of elective procedures.114 A review of procedures performed at a large tertiary care obstetric facility showed that from 1990 to 1995 the use of general anesthesia for cesarean delivery decreased from 7.2% to 3.6%. The yearly incidence of difficult intubation ranged from 1.3% to 16.3%, with one maternal death resulting from a failed intubation.115 Hawkins et al.10 reported 67 maternal deaths resulting from complications of general anesthesia and 33 maternal deaths resulting from complications of neuraxial anesthesia in the United States during the years 1979 to 1990. Approximately 73% of general anesthesia–related maternal deaths were due to airway problems, primarily failed intubation and/or aspiration. However, data collected by Hawkins et al. for the more recent period spanning the years 1997 to 2002 indicate that the mortality rates for cesarean delivery are similar for general and regional anesthesia.11 Studies reviewing failed intubation during the periods 1993 to 1998 and 1999 to 2003 in the United Kingdom showed that whereas the rate of failed intubation during this period had not declined there were no deaths from this potentially fatal complication.116,117 Although this relative change in maternal mortality from the complications of general anesthesia is very encouraging, other factors such as the worldwide obesity epidemic have increased the challenges presented to anesthesia providers, particularly with respect to emergency operative deliveries. Therefore, techniques for preventing pulmonary aspiration of gastric contents will remain or even become increasingly pertinent to clinical practice.
Antacids
The ASA Practice Guidelines for Obstetric Anesthesia state, “Before surgical procedures (i.e., cesarean delivery, postpartum tubal ligation) practitioners should consider the timely administration of nonparticulate antacids, H2-receptor antagonists, and/or metoclopramide for aspiration prophylaxis.”73 Particulate antacids should not be used as prophylaxis because when aspirated they cause pulmonary shunting and hypoxemia of magnitude similar to that caused by acid aspiration and greater than that caused by saline, alkalinized saline, or sodium citrate.118 Therefore, nonparticulate antacids (e.g., 0.3 M sodium citrate, Bicitra, Alka-Seltzer effervescent) should be used; their efficacy depends on the baseline gastric volume and acidity.119,120 A total of 30 mL of sodium citrate neutralizes 255 mL of hydrochloric acid with a pH of 1.0. The effective duration of action of sodium citrate is variable and depends on the rate of gastric emptying.121,122 O’Sullivan and Bullingham121,122 used radiotelemetry pH pills to perform noninvasive assessments of the efficacy of sodium citrate in pregnant women. After the administration of 15 mL of sodium citrate to women in the third trimester of pregnancy, the time that the pH remained greater than 3.0 was less than 30 minutes.121 When the same study was repeated in laboring women,122 the mean time that the pH remained greater than 3.0 was 57 minutes in subjects who had received no analgesia and 166 minutes in those who had received meperidine. Nonparticulate antacids should be administered within 20 minutes of the induction of general anesthesia, particularly if the procedure is an emergency and there is insufficient time for a co-administered H2-receptor antagonist to be effective.
Histamine-2 Receptor Antagonists
The ASA Task Force on Obstetric Anesthesia concluded that H2-receptor antagonists are efficacious in reducing gastric acidity and volume.73 H2-receptor antagonists block histamine receptors on the oxyntic cell and thus diminish gastric acid production, leading to a slight reduction in gastric volume in the fasting patient. When given intravenously, an H2-receptor antagonist begins to take effect in as little as 30 minutes, but 60 to 90 minutes are required for maximal effect.102 After oral administration, gastric pH is higher than 2.5 in approximately 60% of patients at 60 minutes and in 90% at 90 minutes.123 The duration of action is sufficiently long to cover emergence from general anesthesia for a cesarean delivery.
Cimetidine (given in doses of 200 to 400 mg intravenously or orally) reduces gastric acidity within 60 to 90 minutes.102,123 Therapeutic plasma concentrations are sustained for approximately 4 hours. Cimetidine may decrease the rate of plasma clearance of certain drugs, including some local anesthetics (e.g., lidocaine), by binding to the cytochrome P450 system in the hepatocyte and by reducing hepatic blood flow.124 Cimetidine crosses the placenta, but this does not appear to have harmful effects.125 Because arrhythmias and cardiac arrest have been reported with the rapid intravenous administration of cimetidine,126 a slow rate of intravenous administration or the oral route of administration is recommended. The use of cimetidine in obstetric anesthesia has largely been replaced by the use of other H2-receptor antagonists.
Ranitidine, a chemically substituted amino-alkyl furan, has been evaluated after the administration of an intravenous or intramuscular dose of 50 to 100 mg or an oral dose of 150 mg.127–129 These studies have noted that the administration of ranitidine results in a gastric pH greater than 2.5 within 1 hour and sustained therapeutic concentrations for approximately 8 hours.127–129 Ranitidine does not have any major interaction with the cytochrome P450 system130 and does not alter plasma concentrations of lidocaine or bupivacaine after their epidural administration.131
Nizatidine (given in doses of 150 to 300 mg orally) and famotidine (given in doses of 20 to 40 mg orally or intravenously) are alternative H2-receptor antagonists.132,133 Both have a duration of action greater than 10 hours and do not interfere with the metabolism of other drugs by the cytochrome P450 system.132,133
Tramadol, a synthetic 4-phenyl-piperidine analogue of codeine with a low affinity for µ-opioid receptors, has the additional property of inhibiting type 3 muscarinic receptors that mediate gastric gland secretion and smooth muscle contraction. In one study, 60 healthy parturients undergoing elective cesarean delivery under general anesthesia were randomly assigned to receive either intramuscular tramadol 100 mg or famotidine 20 mg 1 hour before surgery.134 The median (range) gastric fluid pH after induction of anesthesia was 6.4 (1.7 to 7.2) in the tramadol group and 6.3 (1.9 to 8.1) in the famotidine group. Two patients in each group had a gastric volume greater than 0.4 mL/kg with a pH less than 2.5. Parturients in the tramadol group had better pain scores and used less analgesia during the first 24 hours after delivery. Neonatal well-being was similar in the two groups. Further investigation is required before such a novel method of antacid prophylaxis is adopted into everyday clinical practice.
Proton-Pump Inhibitors
Omeprazole (20 to 40 mg orally) and lansoprazole (15 to 30 mg orally) are substituted benzimidazoles that inhibit the hydrogen ion pump on the gastric surface of the oxyntic cell. Purported advantages of proton pump inhibitors (PPIs) are a long duration of action, low toxicity, and the potential for low maternal and fetal blood concentrations at the time of delivery.21,135,136 For emergency cesarean delivery, studies have suggested that H2-receptor antagonists and PPIs administered intravenously are equally effective adjuncts to sodium citrate for reducing gastric acidity and volume.136 However, a meta-analysis has indicated that premedication with ranitidine is more effective than PPIs in reducing the volume of gastric secretion and increasing gastric pH.137
Metoclopramide
Metoclopramide is a procainamide derivative that is a peripheral cholinergic agonist and a central dopamine receptor antagonist. An intravenous dose of metoclopramide 10 mg increases lower esophageal sphincter tone and reduces gastric volume by increasing gastric peristalsis. Metoclopramide can have a significant effect on gastric volume in as little as 15 minutes.101 Unfortunately, prior administration of an opioid or atropine antagonizes the effect of metoclopramide.138 Extrapyramidal effects are a major potential side effect of metoclopramide. Metoclopramide crosses the placenta, but studies have reported no significant effects on the fetus or neonate.139
A Cochrane review of antacid prophylaxis concluded that there was no evidence to support the routine administration of drugs to women in normal labor to reduce the incidence of pulmonary aspiration or Mendelson’s syndrome.140 This conclusion reflects the low incidence of pulmonary aspiration of gastric contents and the absence of high-quality studies of antacid prophylaxis, rather than the presence of studies demonstrating negative results; the Cochrane review cited only three studies, published in 1971, 1980, and 1984. One study assessed the use of metoclopramide and perphenazine in women receiving meperidine in labor, and the other two studies focused on the use of particulate antacids. An audit of acid aspiration prophylaxis during labor in the United Kingdom showed a decreasing number of institutions with policies to administer routine antacid prophylaxis to all laboring women. However, many institutions attempted to identify women at high risk for an emergency cesarean delivery, to whom they gave oral ranitidine 150 mg at 6-hour intervals throughout labor.141
Sellick Maneuver and Induction of Anesthesia
Sellick demonstrated that the occlusion of the esophagus by cricoid pressure in cadavers prevented the flow of barium from the stomach to the pharynx.142 He also reported the successful use of this maneuver in 26 cases to prevent the passive regurgitation of gastric contents into the airway. For proper application of cricoid pressure, the head should be fully extended; it may help to have a trained assistant place a hand behind the patient’s neck, so that the cervical vertebrae and esophagus are brought forward, making it easier to occlude the latter. The trained assistant should place the thumb and middle finger on either side of the cricoid cartilage; no more than light pressure should be applied while the patient is awake, to prevent coughing, straining, retching, and esophageal rupture. After denitrogenation (preoxygenation) and administration of induction drugs, an increasingly firm downward pressure is applied to the cricoid cartilage as loss of consciousness occurs. Full application of cricoid pressure requires a force of 30 Newtons (N), 1 N being the force required to accelerate a mass of 1 kg by 1 m/s2. (As a practical clinical guide to the amount of force to apply, 10 N is approximately equivalent to the downward force exerted by a mass of 1 kg.) Vanner and Pryle143 demonstrated that 30 N of cricoid force prevented regurgitation of saline in cadavers with esophageal pressures as high as 40 mm Hg. They recommended a modest cricoid force (10 N) before loss of consciousness, increasing to 30 N after loss of consciousness; their data suggested that such pressure should be sufficient to prevent passive regurgitation of esophageal contents during induction of general anesthesia in most patients.144,145 Cricoid pressure is maintained until the endotracheal tube cuff is inflated and correct endotracheal tube position is confirmed.
The value of cricoid pressure has been questioned. A study employing magnetic resonance imaging of 22 healthy volunteers of mixed sex noted that the resting position of the esophagus was lateral relative to the cricoid cartilage in 53% of the subjects without cricoid pressure and in 91% with cricoid pressure.146 In addition, cricoid pressure displaced the esophagus relative to its initial resting position to the left and right in 68% and 21% of the subjects, respectively. The authors suggested that cricoid pressure may lead to airway displacement and an inability to reliably produce midline esophageal compression; these factors could limit the protective effect of the maneuver against passive reflux and make the intubation process more difficult. However, Rice at al.147 challenged these conclusions in a subsequent magnetic resonance imaging study investigating the efficacy of cricoid pressure. They demonstrated that the hypopharynx, rather than the esophagus, lies behind the cricoid cartilage (Figure 29-8). The relationship of the cricoid and laryngeal cartilages is constant and is maintained by their connecting ligaments and muscles. Because the cricoid cartilage and postcricoid hypopharynx are constantly related, they will behave as a unit when compressed against the cervical spine. They contended that the sealing of the hypopharynx is therefore independent of the position of the esophagus and the actual position of the esophagus is irrelevant to the successful application of cricoid pressure.
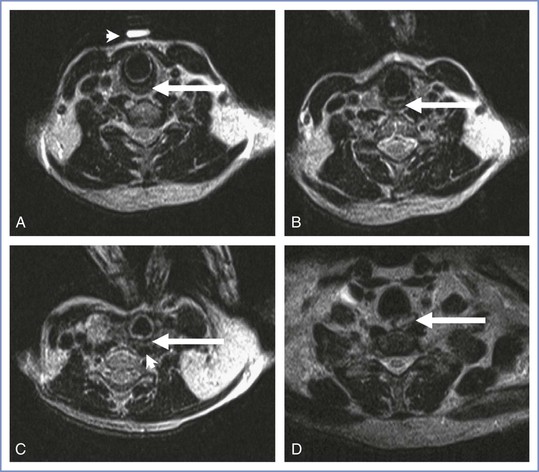
FIGURE 29-8 Axial magnetic resonance images in the sniffing position, without (A) and with (B) cricoid pressure. A, Postcricoid hypopharynx (arrow) and the vitamin E marker (arrowhead) placed by the anesthesiologist before imaging. C, Example of postcricoid hypopharynx compression (arrow) lateral to the vertebral body with cricoid pressure. In this image, the postcricoid hypopharynx is compressed against the longus colli muscle group (arrowhead). D, Image 2 cm inferior to the cricoid ring distinctly shows the cervical esophagus (arrow) lateral to the vertebral body. In B and C, the anesthesiologist’s thumb and index finger can be seen pushing on the cricoid cartilage. The axial image chosen for each study (A-C) was the image at the most inferior level of the cricoid cartilage. (From Rice MJ, Mancuso AA, Gibbs C, et al. Cricoid pressure results in compression of the postcricoid hypopharynx: the esophageal position is irrelevant. Anesth Analg 2009; 109:1546-52.)
When the technique of rapid-sequence induction of anesthesia with tracheal intubation was first described in detail, it was recommended that the trunk be elevated 30 degrees to prevent reflux and aspiration.148 Over subsequent years, others argued the advantages of both the supine and head-down positions for induction of anesthesia. Hignett et al.149 demonstrated that the functional residual capacity of healthy term parturients was increased in the 30-degree head-up position compared with the supine position. Moreover, in the 30-degree head-up position, the esophageal pressure is lower and thus the force applied to the cricoid could be reduced to 20 N143; this reduction in force may reduce the incidence of airway problems, because these problems are often proportional to the force applied.150 Further work is required to determine whether the increase in functional residual capacity in the 30-degree head-up position prolongs the time to oxyhemoglobin desaturation during the apnea phase of rapid-sequence induction and whether this position should be routinely adopted for induction of general anesthesia in obstetric patients.
Should the incorrect application of cricoid pressure distort the laryngeal inlet and cause difficulty with laryngoscopy and/or intubation, the cricoid pressure should be promptly released.
Recommendations for Cesarean Delivery
When possible, all mothers should be encouraged to have neuraxial anesthesia for cesarean delivery. Awake fiberoptic intubation should be considered for parturients with a potentially difficult airway who require general anesthesia. For elective cesarean delivery, a suitable antacid regimen may include the oral administration of an H2-receptor antagonist (e.g., ranitidine 150 mg or famotidine 20 mg) or a PPI (e.g., omeprazole 40 mg) at bedtime and again 60 to 120 minutes before the induction of anesthesia. However, given that parturients are now encouraged to drink clear fluids until 2 hours before elective surgery, the morning dose can be taken with the final preoperative drink. Some practitioners also give metoclopramide 10 mg orally at the same time as the H2-receptor antagonist or intravenously at least 15 minutes before the induction of anesthesia.
For emergency cesarean delivery under general anesthesia, 30 mL of sodium citrate should be administered just after transfer of the patient to the operating room. This timing is important because sodium citrate has a relatively short duration of action, except in those mothers in whom gastric emptying has been delayed by the administration of an opioid. In addition, ranitidine 50 mg (or famotidine 20 mg or omeprazole 40 mg) and metoclopramide 10 mg should be given intravenously when time allows. Administration of these drugs may not reduce gastric volume or acidity at the time of intubation but will decrease the risk for aspiration at the time of extubation. Some units administer an H2-receptor antagonist orally every 6 hours during labor to all mothers considered to be at risk for an operative delivery.
The evidence that H2-receptor antagonists or PPIs reduce maternal morbidity and mortality has not been conclusively demonstrated; however, increasing the pH and reducing the volume of gastric contents should assist in limiting damage if pulmonary aspiration occurs. The use of cricoid pressure as part of a rapid-sequence induction technique remains standard practice.
Oral Intake during Labor
Women in the third trimester of pregnancy exhibit a state of “accelerated starvation” if denied food and drink.151 Fasting results in the production of ketones, primarily beta-hydroxybutyrate and acetoacetic acid, and the nonesterified fatty acids from which they are derived. These changes are exacerbated by the metabolic demands of labor and delivery. Consequently, some obstetricians and nurse-midwives have suggested that maternal oral intake policies should be liberalized during labor.152 It is argued that allowing mothers to eat and drink during labor prevents ketosis and dehydration and subsequently improves obstetric outcome. The widespread use of neuraxial analgesia has resulted in a reduction in the use of systemic opioids for labor analgesia114; thus, fewer women may be at risk for opioid-induced delays in gastric emptying (with its inherent potential for aspiration). This trend has increased the demand to liberalize NPO policies during labor.
A randomized study examined the effect of a light diet on the maternal metabolic profile, the residual gastric volume, and the outcome of labor.153 Women presenting in early uncomplicated labor at term were stratified by parity and randomly assigned to receive either a light diet or water only. The results showed that mothers who consumed a light diet did not have the increase in beta-hydroxybutyrate and nonesterified acid levels seen in the mothers who consumed water only. However, the gastric volumes as measured by ultrasonography were significantly larger in those who had eaten. Thus, mothers who consume a light diet during labor could be at greater risk for aspiration if general anesthesia is required. The same study design was used in another group of mothers, but isotonic “sport drinks” were administered instead of solid food154; it was found that these drinks reduced ketosis without increasing intragastric volume.
O’Sullivan et al.155 evaluated the effect of food intake during labor on obstetric outcome in a randomized controlled study. A total of 2443 low-risk nulliparous women in labor were randomly assigned to either an “eating” or a “water only” group. Intention-to-treat analysis was performed. No significant differences were found in (1) the normal vaginal delivery rate, (2) the instrumental vaginal delivery rate, (3) the cesarean delivery rate, or (4) the incidence of vomiting (Figure 29-9). Similarly, there was no difference between groups in the duration of labor; the geometric mean (GM) labor duration was 597 minutes in the “eating” group and 612 minutes in the “water only” group (ratio of GM, 0.975; 95% confidence interval, 0.927 to 1.025).
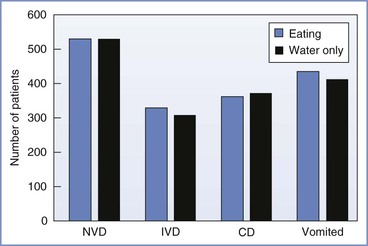
FIGURE 29-9 The effect of eating during labor on maternal obstetric outcome. CD, cesarean delivery; IVD, instrumental vaginal delivery; NVD, normal vaginal delivery. (Based on data from O’Sullivan G, Liu B, Hart D, et al. Effect of food intake during labour on obstetric outcome: randomised controlled trial. BMJ 2009; 338:b784.)
Maternal death from Mendelson’s syndrome is now extremely rare, and its decline probably owes more to the widespread use of neuraxial anesthesia than to NPO policies. Rigid NPO policies are therefore no longer appropriate on the labor and delivery unit, and women should be allowed to alleviate thirst during labor by consuming ice chips and clear fluids (e.g., isotonic sports drinks, fruit juices without pulp, black tea, and coffee). In some high-risk pregnancies, it will remain appropriate to achieve hydration by the intravenous route, and such cases must be managed individually. The American College of Obstetricians and Gynecologists (ACOG) has stated that adherence to a predetermined fasting period before nonelective surgical procedures (i.e. cesarean delivery) is not possible. They therefore concluded that solid food should be avoided in laboring patients.156 European guidelines also discourage women from eating solid food during labor. However the European guidelines acknowledge the current low incidence of aspiration in obstetrics and therefore state that low-risk women could consume low residue foods (biscuits, toast, cereals) during labor.157 To date, countries with a more liberal attitude to eating during labor (e.g., the Netherlands, the United Kingdom, Australia) have not witnessed a higher incidence of maternal deaths from pulmonary aspiration. Further audit, research, and observation are required to fully inform the guidelines for oral intake during labor.
References
1. Simpson JY. Remarks on the alleged case of death from the action of chloroform. Lancet. 1848;51:175–176.
2. Hall CC. Aspiration pneumonitis: an obstetric hazard. JAMA. 1940;114:728–733.
3. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191–205.
4. Report on Confidential Enquiries into Maternal and Child Health. Why Mothers Die 2000-2002: The Sixth Report of the Confidential Enquiries into Maternal Death in the United Kingdom. RCOG Press: London; 2004.
5. Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203.
6. Lewis G, Drife J. Why Mothers Die 1997-1999: The Confidential Enquiries into Maternal Deaths in the United Kingdom. RCOG Press: London; 2001.
7. Olsson GL, Hallen B, Hambraeus-Jonzon K. Aspiration during anaesthesia: a computer-aided study of 185,358 anaesthetics. Acta Anaesthesiol Scand. 1986;30:84–92.
8. Kluger MT, Short TG. Aspiration during anaesthesia: a review of 133 cases from the Australian Anaesthetic Incident Monitoring Study (AIMS). Anaesthesia. 1999;54:19–26.
9. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56–62.
10. Hawkins JL, Koonin LM, Palmer SK, Gibbs CP. Anesthesia-related deaths during obstetric delivery in the United States, 1979-1990. Anesthesiology. 1997;86:277–284.
11. Hawkins JL, Chang J, Palmer SK, et al. Anesthesia-related maternal mortality in the United States: 1979-2002. Obstet Gynecol. 2011;117:69–74.
12. Catanzarite V, Willms D, Wong D, et al. Acute respiratory distress syndrome in pregnancy and the puerperium: causes, courses, and outcomes. Obstet Gynecol. 2001;97:760–764.
13. Søreide E, Bjørnestad E, Steen PA. An audit of perioperative aspiration pneumonitis in gynaecological and obstetric patients. Acta Anaesthesiol Scand. 1996;40:14–19.
14. Dodds WJ, Dent J, Hogan WJ, et al. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 1982;307:1547–1552.
15. Code CF, Marlett JA. The interdigestive myo-electric complex of the stomach and small bowel of dogs. J Physiol. 1975;246:289–309.
16. Schurizek BA, Kraglund K, Andreasen F, et al. Gastrointestinal motility and gastric pH and emptying following ingestion of diazepam. Br J Anaesth. 1988;61:712–719.
17. Meyer JH. Motility of the stomach and gastroduodenal junction. Johnson LR. Physiology of the Gastrointestinal Tract. Raven Press: New York; 1987:613–629.
18. Spencer G, Metz DC. Gastric acid secretion and hormones. Talley NJ, DeVault KR, Fleischer DE. Practical Gastroenterology and Hepatology: Esophagus and Stomach. Wiley-Blackwell: Hoboken, NJ; 2010:16–21.
19. Feldman M, Richardson CT. Total 24-hour gastric acid secretion in patients with duodenal ulcer: comparison with normal subjects and effects of cimetidine and parietal cell vagotomy. Gastroenterology. 1986;90:540–544.
21. Ewart MC, Yau G, Gin T, et al. A comparison of the effects of omeprazole and ranitidine on gastric secretion in women undergoing elective caesarean section. Anaesthesia. 1990;45:527–530.
22. Mayer G, Arnold R, Feurle G, et al. Influence of feeding and sham feeding upon serum gastrin and gastric acid secretion in control subjects and duodenal ulcer patients. Scand J Gastroenterol. 1974;9:703–710.
23. Bergegårdh S, Olbe L. Gastric acid response to antrum distension in man. Scand J Gastroenterol. 1975;10:171–176.
24. DeVault KR, Bouras EP, Talley NJ. Esophageal and gastric motor function. Talley NJ, DeVault KR, Fleischer DE. Practical Gastroenterology and Hepatology: Esophagus and Stomach. Wiley-Blackwell: Hoboken, NJ; 2010:9–15.
25. Anvari M, Horowitz M, Fraser R, et al. Effects of posture on gastric emptying of nonnutrient liquids and antropyloroduodenal motility. Am J Physiol. 1995;268:G868–G871.
26. Hunt JN, Macdonald I. The influence of volume on gastric emptying. J Physiol. 1954;126:459–474.
27. Collins PJ, Horowitz M, Maddox A, et al. Effects of increasing solid component size of a mixed solid/liquid meal on solid and liquid gastric emptying. Am J Physiol. 1996;271:G549–G554.
28. Jian R, Vigneron N, Najean Y, Bernier JJ. Gastric emptying and intragastric distribution of lipids in man: a new scintigraphic method of study. Dig Dis Sci. 1982;27:705–711.
29. Malagelada JR, Longstreth GF, Summerskill WH, Go VL. Measurement of gastric functions during digestion of ordinary solid meals in man. Gastroenterology. 1976;70:203–210.
30. Hey VM, Cowley DJ, Ganguli PC, et al. Gastro-oesophageal reflux in late pregnancy. Anaesthesia. 1977;32:372–377.
31. Lind JF, Smith AM, McIver DK, et al. Heartburn in pregnancy—a manometric study. Can Med Assoc J. 1968;98:571–574.
32. Van Thiel DH, Gavaler JS, Joshi SN, et al. Heartburn of pregnancy. Gastroenterology. 1977;72:666–668.
33. Vanner RG, Goodman NW. Gastro-oesophageal reflux in pregnancy at term and after delivery. Anaesthesia. 1989;44:808–811.
34. Van Thiel DH, Gavaler JS, Stremple J. Lower esophageal sphincter pressure in women using sequential oral contraceptives. Gastroenterology. 1976;71:232–234.
35. Murray FA, Erskine JP, Fielding J. Gastric secretion in pregnancy. J Obstet Gynaecol Br Emp. 1957;64:373–381.
36. Carp H, Jayaram A, Stoll M. Ultrasound examination of the stomach contents of parturients. Anesth Analg. 1992;74:683–687.
37. Chiloiro M, Darconza G, Piccioli E, et al. Gastric emptying and orocecal transit time in pregnancy. J Gastroenterol. 2001;36:538–543.
38. Crawford JS. Some aspects of obstetric anaesthesia. Br J Anaesth. 1956;28:201–208.
39. Davison JS, Davison MC, Hay DM. Gastric emptying time in late pregnancy and labour. J Obstet Gynaecol Br Commonw. 1970;77:37–41.
40. Ewah B, Yau K, King M, et al. Effect of epidural opioids on gastric emptying in labour. Int J Obstet Anesth. 1993;2:125–128.
41. Geddes SM, Thorburn J, Logan RW. Gastric emptying following caesarean section and the effect of epidural fentanyl. Anaesthesia. 1991;46:1016–1018.
42. Gin T, Cho AM, Lew JK, et al. Gastric emptying in the postpartum period. Anaesth Intensive Care. 1991;19:521–524.
43. Hirsheimer A, January DA, Daversa JJ. An X-ray study of gastric function during labor. Am J Obstet Gynecol. 1938;36:671–673.
44. Hunt JN, Murray FA. Gastric function in pregnancy. J Obstet Gynaecol Br Emp. 1958;65:78–83.
45. Kelly MC, Carabine UA, Hill DA, Mirakhur RK. A comparison of the effect of intrathecal and extradural fentanyl on gastric emptying in laboring women. Anesth Analg. 1997;85:834–838.
46. La Salvia LA, Steffen EA. Delayed gastric emptying time in labor. Am J Obstet Gynecol. 1950;59:1075–1081.
47. Levy DM, Williams OA, Magides AD, Reilly CS. Gastric emptying is delayed at 8-12 weeks’ gestation. Br J Anaesth. 1994;73:237–238.
48. Macfie AG, Magides AD, Richmond MN, Reilly CS. Gastric emptying in pregnancy. Br J Anaesth. 1991;67:54–57.
49. Nimmo WS, Wilson J, Prescott LF. Narcotic analgesics and delayed gastric emptying during labour. Lancet. 1975;1:890–893.
50. O’Sullivan GM, Sutton AJ, Thompson SA, et al. Noninvasive measurement of gastric emptying in obstetric patients. Anesth Analg. 1987;66:505–511.
51. Porter JS, Bonello E, Reynolds F. The influence of epidural administration of fentanyl infusion on gastric emptying in labour. Anaesthesia. 1997;52:1151–1156.
52. Sandhar BK, Elliott RH, Windram I, Rowbotham DJ. Peripartum changes in gastric emptying. Anaesthesia. 1992;47:196–198.
53. Simpson KH, Stakes AF, Miller M. Pregnancy delays paracetamol absorption and gastric emptying in patients undergoing surgery. Br J Anaesth. 1988;60:24–27.
54. Stanley K, Magides A, Arnot M, et al. Delayed gastric emptying as a factor in delayed postprandial glycaemic response in pregnancy. Br J Obstet Gynaecol. 1995;102:288–291.
55. Whitehead EM, Smith M, Dean Y, O’Sullivan G. An evaluation of gastric emptying times in pregnancy and the puerperium. Anaesthesia. 1993;48:53–57.
56. Wong CA, Loffredi M, Ganchiff JN, et al. Gastric emptying of water in term pregnancy. Anesthesiology. 2002;96:1395–1400.
57. Wong CA, McCarthy RJ, Fitzgerald PC, et al. Gastric emptying of water in obese pregnant women at term. Anesth Analg. 2007;105:751–755.
58. Wright PM, Allen RW, Moore J, Donnelly JP. Gastric emptying during lumbar extradural analgesia in labour: effect of fentanyl supplementation. Br J Anaesth. 1992;68:248–251.
59. Zimmermann DL, Breen TW, Fick G. Adding fentanyl 0.0002% to epidural bupivacaine 0.125% does not delay gastric emptying in laboring parturients. Anesth Analg. 1996;82:612–616.
60. Nimmo WS, Wilson J, Prescott LF. Further studies of gastric emptying during labour. Anaesthesia. 1977;32:100–101.
61. Christofides ND, Ghatei MA, Bloom SR, et al. Decreased plasma motilin concentrations in pregnancy. Br Med J (Clin Res Ed). 1982;285:1453–1454.
62. O’Sullivan G, Sear JW, Bullingham RE, Carrie LE. The effect of magnesium trisilicate mixture, metoclopramide and ranitidine on gastric pH, volume and serum gastrin. Anaesthesia. 1985;40:246–253.
63. Attia RR, Ebeid AM, Fischer JE, Goudsouzian NG. Maternal fetal and placental gastrin concentrations. Anaesthesia. 1982;37:18–21.
64. Awe WC, Fletcher WS, Jacob SW. The pathophysiology of aspiration pneumonitis. Surgery. 1966;60:323–329.
65. Exarhos ND, Logan WD Jr, Abbott OA, Hatcher CR Jr. The importance of pH and volume in tracheobronchial aspiration. Dis Chest. 1965;47:167–169.
66. Hamelberg W, Bosomworth PP. Aspiration pneumonitis: experimental studies and clinical observations. Anesth Analg. 1964;43:669–677.
67. James CF, Modell JH, Gibbs CP, et al. Pulmonary aspiration—effects of volume and pH in the rat. Anesth Analg. 1984;63:665–668.
68. LeFrock JL, Clark TS, Davies B, Klainer AS. Aspiration pneumonia: a ten-year review. Am Surg. 1979;45:305–313.
69. Roberts RB, Shirley MA. Reducing the risk of acid aspiration during cesarean section. Anesth Analg. 1974;53:859–868.
70. Roberts RB, Shirley MA. Antacid therapy in obstetrics. Anesthesiology. 1980;53:83.
71. Schwartz DJ, Wynne JW, Gibbs CP, et al. The pulmonary consequences of aspiration of gastric contents at pH values greater than 2.5. Am Rev Respir Dis. 1980;121:119–126.
72. Teabeaut JR 2nd. Aspiration of gastric contents; an experimental study. Am J Pathol. 1952;28:51–67.
73. American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia. Anesthesiology. 2007;106:843–863.
74. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 2011;114:495–511.
75. Raidoo DM, Rocke DA, Brock-Utne JG, et al. Critical volume for pulmonary acid aspiration: reappraisal in a primate model. Br J Anaesth. 1990;65:248–250.
76. Plourde G, Hardy JF. Aspiration pneumonia: assessing the risk of regurgitation in the cat. Can Anaesth Soc J. 1986;33:345–348.
78. Matthay MA. Conference summary: acute lung injury. Chest. 1999;116:119S–126S.
79. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349.
80. Marik PE. Pulmonary aspiration syndromes. Curr Opin Pulm Med. 2011;17:148–154.
81. Landay MJ, Christensen EE, Bynum LJ. Pulmonary manifestations of acute aspiration of gastric contents. AJR Am J Roentgenol. 1978;131:587–592.
82. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533.
83. Pipeling MR, Fan E. Therapies for refractory hypoxemia in acute respiratory distress syndrome. JAMA. 2010;304:2521–2527.
84. Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131:913–920.
85. Kacmarek RM. Ventilatory management of ARDS: high frequency oscillation and lung recruitment!. Crit Care. 2006;10:158.
86. Pierrakos C, Karanikolas M, Scolletta S, et al. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J Clin Med Res. 2012;4:7–16.
87. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308.
88. Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336.
89. Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–873.
90. Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–645.
91. Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655.
92. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575.
93. Takala J, Dellinger RP, Koskinen K, et al. Development and simultaneous application of multiple care protocols in critical care: a multicenter feasibility study. Intensive Care Med. 2008;34:1401–1410.
94. Tang BM, Craig JC, Eslick GD, et al. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37:1594–1603.
95. Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317:1565–1570.
96. Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684.
97. Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963.
98. Lyons G. Failed intubation. Six years’ experience in a teaching maternity unit. Anaesthesia. 1985;40:759–762.
99. Rocke DA, Murray WB, Rout CC, Gouws E. Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology. 1992;77:67–73.
100. Gibbs CP, Rolbin SH, Norman P. Cause and prevention of maternal aspiration. Anesthesiology. 1984;61:111–112.
101. Cohen SE, Jasson J, Talafre ML, et al. Does metoclopramide decrease the volume of gastric contents in patients undergoing cesarean section? Anesthesiology. 1984;61:604–607.
102. McCaughey W, Howe JP, Moore J, Dundee JW. Cimetidine in elective Caesarean section: effect on gastric acidity. Anaesthesia. 1981;36:167–172.
103. Blouw R, Scatliff J, Craig DB, Palahniuk RJ. Gastric volume and pH in postpartum patients. Anesthesiology. 1976;45:456–457.
104. James CF, Gibbs CP, Banner T. Postpartum perioperative risk of aspiration pneumonia. Anesthesiology. 1984;61:756–759.
105. Wyner J, Cohen SE. Gastric volume in early pregnancy: effect of metoclopramide. Anesthesiology. 1982;57:209–212.
106. Coté CJ, Goudsouzian NG, Liu LM, et al. Assessment of risk factors related to the acid aspiration syndrome in pediatric patients-gastric pH and residual volume. Anesthesiology. 1982;56:70–72.
107. Rennie AL, Richard JA, Milne MK, Dalrymple DG. Post-partum sterilisation—an anaesthetic hazard? Anaesthesia. 1979;34:267–269.
108. Vaughan RW, Bauer S, Wise L. Volume and pH of gastric juice in obese patients. Anesthesiology. 1975;43:686–689.
109. Agarwal A, Chari P, Singh H. Fluid deprivation before operation: the effect of a small drink. Anaesthesia. 1989;44:632–634.
110. Kallar SK, Everett LL. Potential risks and preventive measures for pulmonary aspiration: new concepts in preoperative fasting guidelines. Anesth Analg. 1993;77:171–182.
111. Lewis M, Crawford JS. Can one risk fasting the obstetric patient for less than 4 hours? Br J Anaesth. 1987;59:312–314.
112. Barau G, Robillard PY, Hulsey TC, et al. Linear association between maternal pre-pregnancy body mass index and risk of caesarean section in term deliveries. BJOG. 2006;113:1173–1177.
113. Durnwald CP, Ehrenberg HM, Mercer BM. The impact of maternal obesity and weight gain on vaginal birth after cesarean section success. Am J Obstet Gynecol. 2004;191:954–957.
114. Bucklin BA, Hawkins JL, Anderson JR, Ullrich FA. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103:645–653.
115. Tsen LC, Pitner R, Camann WR. General anesthesia for cesarean section at a tertiary care hospital 1990-1995: indications and implications. Int J Obstet Anesth. 1998;7:147–152.
116. Barnardo PD, Jenkins JG. Failed tracheal intubation in obstetrics: a 6-year review in a UK region. Anaesthesia. 2000;55:690–694.
117. Rahman K, Jenkins JG. Failed tracheal intubation in obstetrics: no more frequent but still managed badly. Anaesthesia. 2005;60:168–171.
118. Gibbs CP, Schwartz DJ, Wynne JW, et al. Antacid pulmonary aspiration in the dog. Anesthesiology. 1979;51:380–385.
119. Chen CT, Toung TJ, Haupt HM, et al. Evaluation of the efficacy of Alka-Seltzer Effervescent in gastric acid neutralization. Anesth Analg. 1984;63:325–329.
120. Gibbs CP, Spohr L, Schmidt D. The effectiveness of sodium citrate as an antacid. Anesthesiology. 1982;57:44–46.
121. O’Sullivan GM, Bullingham RE. The assessment of gastric acidity and antacid effect in pregnant women by a non-invasive radiotelemetry technique. Br J Obstet Gynaecol. 1984;91:973–978.
122. O’Sullivan GM, Bullingham RE. Noninvasive assessment by radiotelemetry of antacid effect during labor. Anesth Analg. 1985;64:95–100.
123. Johnston JR, McCaughey W, Moore J, Dundee JW. Cimetidine as an oral antacid before elective Caesarean section. Anaesthesia. 1982;37:26–32.
124. Somogyi A, Gugler R. Drug interactions with cimetidine. Clin Pharmacokinet. 1982;7:23–41.
125. Howe JP, McGowan WA, Moore J, et al. The placental transfer of cimetidine. Anaesthesia. 1981;36:371–375.
126. Lineberger AS 3rd, Sprague DH, Battaglini JW. Sinus arrest associated with cimetidine. Anesth Analg. 1985;64:554–556.
127. Dammann HG, Muller P, Simon B. Parenteral ranitidine: onset and duration of action. Br J Anaesth. 1982;54:1235–1236.
128. Francis RN, Kwik RS. Oral ranitidine for prophylaxis against Mendelson’s syndrome. Anesth Analg. 1982;61:130–132.
129. Maile CJ, Francis RN. Pre-operative ranitidine: effect of a single intravenous dose on pH and volume of gastric aspirate. Anaesthesia. 1983;38:324–326.
130. Kirch W, Hoensch H, Janisch HD. Interactions and non-interactions with ranitidine. Clin Pharmacokinet. 1984;9:493–510.
131. Dailey PA, Hughes SC, Rosen MA, et al. Effect of cimetidine and ranitidine on lidocaine concentrations during epidural anesthesia for cesarean section. Anesthesiology. 1988;69:1013–1017.
133. Pattichis K, Louca LL. Histamine, histamine H2-receptor antagonists, gastric acid secretion and ulcers: an overview. Drug Metabol Drug Interact. 1995;12:1–36.
134. Elhakim M, Abd El-Megid W, Metry A, et al. Analgesic and antacid properties of i.m. tramadol given before Caesarean section under general anaesthesia. Br J Anaesth. 2005;95:811–815.
135. Levack ID, Bowie RA, Braid DP, et al. Comparison of the effect of two dose schedules of oral omeprazole with oral ranitidine on gastric aspirate pH and volume in patients undergoing elective surgery. Br J Anaesth. 1996;76:567–569.
136. Yau G, Kan AF, Gin T, Oh TE. A comparison of omeprazole and ranitidine for prophylaxis against aspiration pneumonitis in emergency caesarean section. Anaesthesia. 1992;47:101–104.
137. Clark K, Lam LT, Gibson S, Currow D. The effect of ranitidine versus proton pump inhibitors on gastric secretions: a meta-analysis of randomised control trials. Anaesthesia. 2009;64:652–657.
138. Hey VM, Ostick DG, Mazumder JK, Lord WD. Pethidine, metoclopramide and the gastro-oesophageal sphincter: a study in healthy volunteers. Anaesthesia. 1981;36:173–176.
139. Bylsma-Howell M, Riggs KW, McMorland GH, et al. Placental transport of metoclopramide: assessment of maternal and neonatal effects. Can Anaesth Soc J. 1983;30:487–492.
140. Gyte GM, Richens Y. Routine prophylactic drugs in normal labour for reducing gastric aspiration and its effects. Cochrane Database Syst Rev. 2006;(3).
141. Calthorpe N, Lewis M. Acid aspiration prophylaxis in labour: a survey of UK obstetric units. Int J Obstet Anesth. 2005;14:300–304.
142. Sellick BA. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2:404–406.
143. Vanner RG, Pryle BJ. Regurgitation and oesophageal rupture with cricoid pressure: a cadaver study. Anaesthesia. 1992;47:732–735.
144. Vanner RG, O’Dwyer JP, Pryle BJ, Reynolds F. Upper oesophageal sphincter pressure and the effect of cricoid pressure. Anaesthesia. 1992;47:95–100.
145. Vanner RG, Pryle BJ, O’Dwyer JP, Reynolds F. Upper oesophageal sphincter pressure and the intravenous induction of anaesthesia. Anaesthesia. 1992;47:371–375.
146. Smith KJ, Dobranowski J, Yip G, et al. Cricoid pressure displaces the esophagus: an observational study using magnetic resonance imaging. Anesthesiology. 2003;99:60–64.
147. Rice MJ, Mancuso AA, Gibbs C, et al. Cricoid pressure results in compression of the postcricoid hypopharynx: the esophageal position is irrelevant. Anesth Analg. 2009;109:1546–1552.
148. Stept WJ, Safar P. Rapid induction-intubation for prevention of gastric-content aspiration. Anesth Analg. 1970;49:633–636.
149. Hignett R, Fernando R, McGlennan A, et al. A randomized crossover study to determine the effect of a 30 degrees head-up versus a supine position on the functional residual capacity of term parturients. Anesth Analg. 2011;113:1098–1102.
150. Hartsilver EL, Vanner RG. Airway obstruction with cricoid pressure. Anaesthesia. 2000;55:208–211.
151. Metzger BE, Ravnikar V, Vileisis RA, Freinkel N. “Accelerated starvation” and the skipped breakfast in late normal pregnancy. Lancet. 1982;1:588–592.
152. O’Sullivan G, Shennan A. Labour—a gastronomic experience!. Int J Obstet Anesth. 2002;11:1–3.
153. Scrutton MJ, Metcalfe GA, Lowy C, et al. Eating in labour: a randomised controlled trial assessing the risks and benefits. Anaesthesia. 1999;54:329–334.
154. Kubli M, Scrutton MJ, Seed PT, O’Sullivan G. An evaluation of isotonic “sport drinks” during labor. Anesth Analg. 2002;94:404–408.
155. O’Sullivan G, Liu B, Hart D, et al. Effect of food intake during labour on obstetric outcome: randomised controlled trial. BMJ. 2009;338:b784.
156. American College of Obstetricians and Gynecologist. Oral intake during labor. [ACOG Committee Opinion No. 441. Washington, DC, September 2009 (Reaffirmed 2011)] Obstet Gynecol. 2009;114:714.
157. Smith I, Kranke P, Murat I, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556–569.