Antepartum and Postpartum Hemorrhage
Barbara M. Scavone MD
Chapter Outline
Obstetric hemorrhage is the most common cause of maternal mortality worldwide, accounting for 25% of maternal deaths.1 The World Health Organization estimates that severe hemorrhage complicates 10.5% of live births globally and carries with it a case-fatality rate of 1%.1 The rates of maternal death and death due to hemorrhage vary widely throughout various regions of the world (see Chapter 40).1–3 In the United States, hemorrhage accounts for 12.5% of pregnancy-related deaths (1.8 pregnancy-related deaths due to hemorrhage per 100,000 live births).4 Data from the United Kingdom indicate death from peripartum hemorrhage occurs in 0.39 per 100,000 maternities.5 Hemorrhage is the most common cause for admission of an obstetric patient to an intensive care unit and is a risk factor for myocardial ischemia and infarction and stroke.6–8 A 2010 investigation demonstrated that organ dysfunction complicates 16% of cases of major obstetric hemorrhage (defined as transfusion of 5 or more units of packed red blood cells [PRBCs]).9
Evidence indicates hemorrhage rates and severe morbidity due to hemorrhage are increasing in the United States and other high-resource countries, owing primarily to increases in postpartum, rather than antepartum, hemorrhage.10–13 The explanation for this acceleration is not entirely clear but appears to be related to rising rates of postpartum uterine atony as well as increases in abnormal placentation coincident with the rise in cesarean delivery rates.10,11,14,15
The majority of hemorrhage-related adverse outcomes are considered preventable.5,16,17 Common provider-related shortcomings include failure to recognize risk factors, failure to accurately estimate the extent of blood loss, and failure to initiate treatment in a timely fashion. It is essential that clinicians develop an appreciation for the rapidity with which obstetric patients can become unstable, and that anesthesiologists—often the only physicians on the labor and delivery unit with specific training in resuscitation and critical care—become involved early in the care of bleeding patients. Timely and effective communication among all obstetric caregivers is imperative.
Mechanisms of Hemostasis
Uterine contraction, stimulated by endogenous oxytocic substances released after delivery, represents the primary mechanism for controlling blood loss at parturition. Uterine tetany creates shearing forces that cleave the placenta from the uterine wall through the layer of the uterine decidua (see Figure 4-3). In addition, uterine contraction constricts the spiral arteries and placental veins spanning the myometrium and supplying the placental bed.
After disruption of vascular integrity, mechanisms of coagulation include (1) platelet aggregation and plug formation, (2) local vasoconstriction, (3) clot polymerization, and (4) fibrous tissue fortification of the clot. Platelet activation and aggregation occur rapidly after endothelial damage. Activated platelets release adenosine diphosphate (ADP), serotonin, catecholamines, and other factors that promote local vasoconstriction and hemostasis. These factors also activate the coagulation cascade. The end result of the cascade is conversion of fibrinogen to fibrin and stabilization of the blood clot (see Chapter 44).
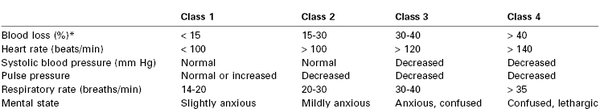
Anesthesia providers, obstetricians, midwives, and labor nurses frequently underestimate blood loss at delivery.18 Heavy bleeding is associated with larger errors in estimated blood loss, and this underestimation may lead to inadequate replacement of intravascular volume.18 Tachycardia and hypotension are late signs of hypovolemia, particularly in healthy young patients (Table 38-1); therefore, constant vigilance is necessary to ensure accurate estimation of blood loss and adequate resuscitation. Fluid and transfusion therapy is best guided by continual reassessment of maternal vital signs, urine output, hemoglobin concentration, and acid-base balance.
Antepartum Hemorrhage
Antepartum vaginal bleeding may occur in as many as 25% of pregnant women; fortunately, only a fraction of these patients experience life-threatening hemorrhage.19 The majority of cases occur during the first trimester. The causes of antepartum hemorrhage range from cervicitis to abnormalities in placentation, including placenta previa and placental abruption. The greatest threat of antepartum hemorrhage is not to the mother but to her fetus. Several decades ago, vaginal bleeding during the second and third trimesters was associated with perinatal mortality rates as high as 80%. More recent data suggest that antepartum bleeding secondary to placenta previa and placental abruption is responsible for perinatal mortality rates of 2.3% and 12%, respectively.20–22
Placenta Previa
Placenta previa is present when the placenta implants in advance of the fetal presenting part. Further classification can be made on the basis of the relationship between the placenta and the cervical os. A total placenta previa completely covers the cervical os. A partial placenta previa covers part but not all of the os. A marginal placenta previa lies within 2 cm of, but does not cover, the cervical os (Figure 38-1).
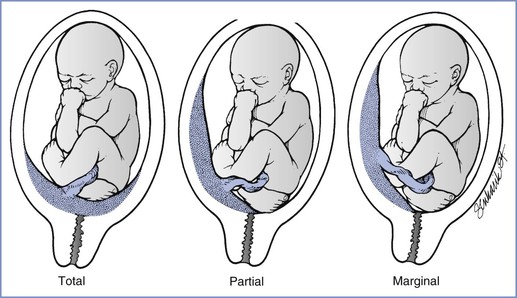
FIGURE 38-1 Three variations of placenta previa. (From Benedetti TJ. Obstetric hemorrhage. In Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and Problem Pregnancies. 4th edition. New York, Churchill Livingstone, 2001:516.)
Epidemiology
The incidence of placenta previa is 4.0 per 1000 pregnancies.23,24 The exact cause is unclear, but prior uterine trauma (e.g., scar from prior cesarean delivery) is a common element. The placenta may implant in the scarred area, which typically includes the lower uterine segment. Conditions associated with placenta previa include multiparity, advanced maternal age, smoking history, male fetus, previous cesarean delivery or other uterine surgery, and previous placenta previa.23 The presence of placenta previa increases the likelihood that the patient will require a peripartum hysterectomy.25
Diagnosis
Transvaginal ultrasonography has become the gold standard for diagnosis of placenta previa; the distance from the placental edge to the internal os is measured and predicts the likelihood of antepartum hemorrhage and need for cesarean delivery.26,27 Advances in ultrasonography have made the double setup examination (i.e., vaginal examination with all personnel ready for immediate cesarean delivery) nearly obsolete in modern obstetric practice. Magnetic resonance imaging (MRI) is also useful for the diagnosis of placenta previa, but its use is not practical in most cases of antepartum hemorrhage.
The classic clinical sign of placenta previa is painless vaginal bleeding during the second or third trimester. The first episode of bleeding typically occurs preterm and is not related to any particular inciting event. The lack of abdominal pain and/or absence of abnormal uterine tone helps distinguish this event from placental abruption. The absence of these factors does not exclude abruption, however, and patients with placenta previa are at risk for coexisting placental abruption.24
Obstetric Management
Obstetric management is based on the severity of vaginal bleeding and the maturity and status of the fetus. Active labor, persistent bleeding, a mature fetus (≥ 36 weeks’ gestational age), or nonreassuring fetal status should prompt delivery.28 The fetus is at risk from two distinct pathophysiologic processes: (1) progressive or sudden placental separation that causes uteroplacental insufficiency and (2) preterm delivery and its sequelae. The first episode of bleeding characteristically stops spontaneously and rarely causes maternal shock or fetal compromise. Expectant management in the hospital has been shown to prolong pregnancy by an average of 4 weeks after the initial bleeding episode.28 Maternal vital signs are assessed frequently, and the hemoglobin concentration is checked at regular intervals. Fetal evaluation involves frequent performance of a nonstress test or biophysical profile, ultrasonographic assessment of fetal growth, and fetal lung maturity studies as indicated. Hemorrhage may be prevented by limitations on physical activity and avoidance of vaginal examinations and coitus, although the evidence supporting these measures is limited.
Outpatient management has resulted in good outcome in carefully selected patients.29 Outpatient management is reserved for stable patients without bleeding in the previous 48 hours who have both telephone access and the ability to be transported quickly to the hospital. Expectant management requires immediate access to a medical center with 24-hour obstetric and anesthesia coverage and a neonatal intensive care unit.28
In most cases of placenta previa diagnosed between 24 and 34 weeks’ gestation, a corticosteroid (e.g., betamethasone) is administered to accelerate fetal lung maturity.28 A significant number of patients with placenta previa have preterm labor, which may provoke bleeding. Obstetricians may administer tocolytic therapy to decrease preterm contractions, with the goal to stabilize antepartum bleeding. Ritodrine has been shown to prolong pregnancy in women with placenta previa, but no studies have confirmed any decrease in the frequency or severity of vaginal bleeding.28,30 Obstetricians must balance the potential cardiovascular consequences of tocolytic therapy in the event of maternal hemorrhage against the consequences of preterm delivery. Tocolytic therapy is not recommended for patients with uncontrolled hemorrhage or those in whom placental abruption is suspected. Although expectant management reduces the risk for prematurity, it does not eliminate it, and prematurity remains the most common cause of neonatal mortality and morbidity, especially if bleeding begins before 20 weeks’ gestation.31
Fetuses of women with placenta previa may be at risk for other complications, including asymmetric fetal growth restriction (also known as intrauterine growth restriction).32 Several factors may account for the association between placenta previa and fetal growth restriction. First, the lower uterine segment may be less vascular than normal sites of placental implantation. Second, the placenta often is adherent to an area of fibrosis tissue. Third, patients with placenta previa have a higher incidence of first-trimester bleeding, which may promote a partial placental separation, reducing the surface area for placental exchange. Fourth, although the blood loss from placenta previa is almost entirely maternal, trauma to the placenta with vaginal examination or coitus may result in some fetal blood loss, which could retard fetal growth.32 Some studies have reported a higher incidence of congenital anomalies in the fetuses of women with placenta previa.20
Anesthetic Management
All patients admitted with antepartum vaginal bleeding should be evaluated by an anesthesia provider on arrival. Special consideration should be given to the airway examination, intravascular volume assessment, and history of previous cesarean delivery or other procedures that create a uterine scar. Volume resuscitation should be initiated using a non–dextrose-containing balanced salt solution (e.g., lactated Ringer’s, normal saline). Women with placenta previa may remain hospitalized for some time prior to delivery, and at least one intravenous catheter should be maintained if bleeding is recurrent or imminent delivery is anticipated. For women without recurrent bleeding, consideration may be given to either deferring venous access entirely or placing a peripherally-inserted central catheter (PICC) for drug administration so that other peripheral veins are preserved for large-bore intravenous catheter access in the event of hemorrhagic emergency. Hemoglobin concentration measurement may be indicated after a bleeding episode. A blood type and screen, and for women who are actively bleeding, a blood type and crossmatch, should be maintained. The American Association of Blood Banks (AABB) recommends repeating such tests every 3 days in pregnant women owing to the small but finite risk for developing a new alloantibody during pregnancy.33 This recommendation is written into the U.S. Code of Federal Regulations.34 The use of lower-extremity sequential compression devices may decrease the risk for venous thromboembolism in patients on bed rest. Pharmacologic prophylaxis is not commonly used because of the risk for bleeding.
Double Setup Examination.
The accuracy of ultrasonography for the identification of placenta previa has almost eliminated the need for double setup examination, but a few patients still require it (e.g., morbidly obese patients who cannot be adequately evaluated with ultrasonography). The examination is performed in the operating room. All members of the obstetric care team, including the anesthesia provider, obstetrician, and pediatrician, make full preparation for cesarean delivery. Full preparation consists of application of maternal monitors, insertion of two large-bore intravenous cannulas, administration of a nonparticulate antacid, and sterile preparation and draping of the abdomen. The obstetrician subsequently performs a careful vaginal examination. A cesarean delivery is performed if significant bleeding occurs or if the obstetrician confirms the presence of total placenta previa in a woman with a mature fetus.
Cesarean Delivery.
Experts recommend that women with a placental edge-to-internal os distance greater than 1 cm be offered a trial of labor; the risk for antepartum hemorrhage and need for cesarean delivery during labor are low in this setting.26 Parturients with total previa, placental edge-to-internal os distance less than 1 cm, and/or significant bleeding will require abdominal delivery, as will some patients with nonreassuring fetal status. The choice of anesthetic technique depends on the indication and urgency for delivery, the severity of maternal hypovolemia, and the obstetric history (e.g., prior cesarean delivery).
Surveys of obstetric anesthesiologists show that neuraxial anesthesia is preferred in patients with placenta previa without active bleeding or intravascular volume deficit.35 Patients who have placenta previa—without active preoperative bleeding—remain at risk for increased intraoperative blood loss for at least three reasons. First, the obstetrician may injure an anteriorly located placenta during uterine incision. Second, after delivery, the lower uterine segment implantation site, lacking uterine muscle compared with the fundus, does not contract as well as the normal fundal implantation site. Third, a patient with placenta previa is at increased risk for placenta accreta, especially if there is a history of previous cesarean delivery (Table 38-2).25 For these reasons, two large-bore intravenous cannulas should be placed before the start of either elective or emergency cesarean delivery. No consensus exists on the need for blood product availability in these patients, but it seems prudent to order at least a blood type and screen. If preoperative imaging indicates the possibility of a placenta accreta, preparation for massive blood loss should be undertaken (see later discussion).
TABLE 38-2
Risk for Placenta Accreta in Patients with Placenta Previa: Relationship to Number of Prior Cesarean Deliveries
| Number of Prior Cesarean Deliveries | % of Patients with Placenta Accreta |
| 0 | 3 |
| 1 | 11 |
| 2 | 40 |
| 3 | 61 |
| 4 or more | 67 |
Modified from Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol 2006; 107:1226-3.
A randomized controlled trial comparing epidural with general anesthesia for cesarean delivery in women with placenta previa in the absence of active bleeding demonstrated that epidural anesthesia was associated with (1) more stable blood pressure after delivery and (2) lower transfusion rates and transfusion volumes with similar hematocrit measurements the day after surgery.36 Operative times, estimated blood loss, urine output, and neonatal Apgar scores were similar in the two groups.36 Combined spinal-epidural anesthesia, or even single-shot spinal anesthesia, is considered acceptable for patients without active bleeding provided that there is both a low risk for placenta accreta and a low risk for difficult airway management should intraoperative conversion to general anesthesia become necessary.
Patients with placenta previa and active preoperative bleeding represent a significant challenge for the anesthesia care team. Frequently, such patients have just presented to the hospital and there is minimal time for evaluation. In these cases, patient evaluation, resuscitation, and preparation for operative delivery all proceed simultaneously. Because the placental site is the source of hemorrhage, the bleeding may continue unabated until the placenta is removed and the uterus contracts. Preoperative evaluation requires careful assessment of the parturient’s airway and intravascular volume. Two large-bore intravenous catheters should be placed, and blood products should be ordered as necessary. Blood administration sets, fluid warmers, and equipment for invasive monitoring should be immediately available. Initially, non–dextrose-containing crystalloid or colloid is infused rapidly. In some cases, the patient requires transfusion before completion of the blood crossmatch, and type-specific blood or type O, Rh-negative blood must be administered.
Rapid-sequence induction of general anesthesia is the preferred technique for bleeding patients. The choice of intravenous induction agent depends on the degree of cardiovascular instability. In patients with severe hypovolemic shock, tracheal intubation may be accomplished without an induction agent, although this situation is rare. A low dose of propofol should be administered in women with ongoing hemorrhage; in the case of severe ongoing hemorrhage it may be best to avoid propofol. Ketamine and etomidate are useful alternative induction agents for hemodynamically unstable patients. Ketamine 0.5 to 1.0 mg/kg has an excellent record of safety and efficacy in obstetric anesthesia practice. Emergence phenomena such as hallucinations and nightmares are uncommon when the dose does not exceed 1 mg/kg. Ketamine may cause myocardial depression, which may result in hypotension in patients with severe hypovolemia. Etomidate 0.3 mg/kg causes minimal cardiac depression and is safe for use in obstetric patients.37 A low dose is appropriate in patients with severe hemorrhage. Disadvantages of etomidate include venous irritation, myoclonus, and possible adrenal suppression.38
The agent(s) chosen for maintenance of anesthesia depends on maternal cardiovascular stability. In patients with modest bleeding and no fetal compromise, 50% nitrous oxide in oxygen can be administered with a low concentration of a volatile halogenated agent before delivery to prevent maternal awareness. The concentration of nitrous oxide can be reduced or omitted in cases of severe maternal hemorrhage or fetal compromise. In these cases, scopolamine or a benzodiazepine such as midazolam may be administered to ensure amnesia.
Oxytocin should be administered by intravenous infusion immediately after delivery. The relatively amuscular lower uterine segment implantation site does not contract as efficiently as the uterine fundus. If bleeding continues, it may be best to discontinue the volatile halogenated agent after delivery and to substitute 70% nitrous oxide and an intravenous opioid or ketamine. These drugs, along with small doses of midazolam, can be administered without causing significant uterine relaxation or cardiovascular depression. A low-dose infusion of propofol and/or ketamine may be considered, with the caution that propofol causes decreased uterine contractility in a dose-dependent manner.39,40 Some anesthesia providers contend that bispectral index (BIS) monitoring may be useful in lowering the risk for intraoperative awareness in cases in which the volatile anesthetic agent has been discontinued, although this issue is a matter of some dispute.
If the placenta does not separate easily, a placenta accreta may exist. In such cases, massive blood loss and the need for cesarean hysterectomy should be anticipated (see later discussion). The need for invasive hemodynamic monitoring varies among patients. An indwelling arterial catheter is useful for patients with hemodynamic instability or for those who require frequent determination of hematocrit and blood gas measurements. Coagulopathy rarely occurs with placenta previa.
Placental Abruption
Placental abruption is defined as complete or partial separation of the placenta from the decidua basalis before delivery of the fetus. Maternal hemorrhage may be revealed by vaginal bleeding or may be concealed behind the placenta. Fetal compromise occurs because of the loss of placental surface area for maternal-fetal exchange of oxygen and nutrients.41
Epidemiology
Placental abruption complicates 0.4% to 1.0% of pregnancies, and the incidence is increasing, particularly among African-American women in the United States.21,42–44 The causes are not well understood, but several conditions are known risk factors for abruption (Box 38-1).42,43 Patients hospitalized for both acute and chronic respiratory diseases are at risk for placental abruption for unclear reasons.45
Diagnosis
The classic presentation of abruption consists of vaginal bleeding, uterine tenderness, and increased uterine activity; however, not all of these symptoms are always present. In cases of concealed abruption, vaginal bleeding may be absent and gross underestimation of maternal hypovolemia can occur. Bleeding may be painless.41 In some cases, abruption may manifest as idiopathic preterm labor. Patients may have a variety of nonreassuring fetal heart rate (FHR) patterns, including bradycardia, late or variable decelerations, and/or loss of variability.41 The diagnosis of placental abruption is primarily clinical, but in a subset of cases, ultrasonography may help confirm it. Ultrasonography is highly specific for placental abruption (96%), but it is not very sensitive (24%).46 It is also useful for determining placental location, which can exclude placenta previa as a cause of vaginal bleeding.41 The ultrasonographic examination can ascertain whether retroplacental or subchorionic hematoma is present. Normal findings do not exclude the diagnosis of placental abruption.
Pathophysiology
Complications of placental abruption include hemorrhagic shock, coagulopathy, and fetal compromise or demise. One third of coagulopathies in pregnancy are attributable to abruption, and coagulopathy is associated with fetal demise.47 Placental tissue displays tissue factor and other procoagulant substances on cell membranes, and it is surmised that when bleeding at the decidual-placental interface (i.e., abruption) occurs, these thromboplastic substances are released into the central circulation, resulting in consumptive coagulopathy and disseminated intravascular coagulation (DIC).48
Although some cases of abruption occur acutely (e.g., in the setting of trauma), many abruptions complicate chronic, long-standing placental abnormalities. Investigators have noted strong associations between abruption, fetal growth restriction, and preeclampsia, and all three conditions share similar risk factors.49 Naeye et al.50 prospectively studied more than 53,000 deliveries and found that decidual necrosis at the placental margin and large placental infarcts were the most common abnormalities among patients who suffered placental abruption and fetal demise. Infants who died had 14% less placental weight, 8% less body weight, and 3% shorter body length than surviving control infants of the same gestational age. In addition, histologic evidence of shallow trophoblastic invasion of the spiral arteries supports the conclusion that “ischemic placental disease” may underlie chronic placental hypoxia, leading to preeclampsia, fetal growth restriction, and abruption.49
The major risks for the fetus are hypoxia and prematurity. Fetal oxygenation depends on adequate maternal oxygen-carrying capacity, uteroplacental blood flow, and transplacental exchange. Separation of all or part of the placenta reduces gas exchange surface area and can lead to fetal death. The risk for intrauterine fetal demise increases as the detachment area increases, particularly when the location of bleeding is retroplacental rather than subchorionic.41,51,52 Inadequate transplacental oxygen exchange is exacerbated by maternal hypotension, which decreases uteroplacental blood flow. Ananth and Wilcox21 reviewed outcomes for 7.5 million pregnancies in the United States; the perinatal mortality rate associated with placental abruption was 12%. The high mortality rate is due in large part to the fact that infants of mothers with placental abruption are five times more likely to be delivered preterm.21
Obstetric Management
If the diagnosis of abruption is suspected, the practitioner should insert a large-bore intravenous catheter and obtain blood for assessment of hematocrit, coagulation status, and type and crossmatch. When assessing volume status, the clinician must remain aware of the possibility of hemorrhage concealed behind the placenta. Placement of a urethral catheter to monitor urine output may help the physician assess the adequacy of renal perfusion. The definitive treatment is delivery of the infant and placenta, but the degree of maternal and fetal compromise and estimated gestational age determine the timing and route of delivery.41 If the fetus is at or near term and both maternal and fetal status are reassuring, vaginal delivery may be appropriate. If the patient is preterm, the extent of abruption is minimal, and the mother and fetus show no signs of compromise, the patient may be hospitalized and the pregnancy allowed to continue to optimize fetal maturation. The obstetrician may administer a corticosteroid to promote fetal lung maturity. If the mother develops hemodynamic instability or coagulopathy, or fetal status becomes nonreassuring, urgent cesarean delivery may become necessary. Vaginal delivery is preferred for patients with intrauterine fetal demise.41
Anesthetic Management
The anesthesia provider should consider the severity of the abruption and the urgency of delivery in planning anesthetic management.
Labor and Vaginal Delivery.
Neuraxial labor analgesia may be offered in the setting of abruption provided that hypovolemia has been treated and coagulation status is normal. The appropriateness of neuraxial analgesia with its accompanying sympathectomy in patients at risk for extension of abruption and further hemorrhage has been questioned; however, the risk that neuraxial analgesia will worsen hemorrhage-associated tachycardia and hypotension can be mitigated by appropriate intravascular volume replacement and use of vasopressors. Close monitoring is required for evidence of further bleeding and changes in intravascular volume status. A coagulopathic patient may present for vaginal delivery, particularly in the setting of fetal demise. In this case, intravenous patient-controlled opioid analgesia should be offered.
Cesarean Delivery.
Similar anesthetic considerations pertain to the administration of neuraxial anesthesia for cesarean delivery. Spinal, combined-spinal epidural, or epidural anesthesia may be administered in stable patients in whom intravascular volume status is adequate and coagulation studies are normal. General anesthesia is preferred for most cases of urgent cesarean delivery accompanied by unstable maternal status, a nonreassuring FHR pattern, or both. Propofol may precipitate severe hypotension in patients with unrecognized hypovolemia; ketamine and etomidate may represent better options for the patient with unknown or decreased intravascular volume.
Aggressive volume resuscitation is critical. Either crystalloid or colloid may be used; the choice is less important than adequate restoration of intravascular volume. In cases of severe hemorrhage, insertion of an intra-arterial catheter may aid prompt recognition of hypotension and allow for frequent blood sampling and assessment of anemia and coagulation status. Patients with abruption are at risk for persistent hemorrhage after delivery from uterine atony or coagulopathy; after delivery, oxytocin should be infused promptly to prevent uterine atony. Persistent uterine atony requires the administration of other uterotonic drugs (see later discussion). Red blood cells (RBCs) and coagulation factors should be replaced as indicated by laboratory studies. Experts recommend aggressive monitoring and early replacement of coagulation factors, especially fibrinogen, to minimize the developing coagulopathy.53
Most parturients recover quickly and completely after delivery. A minority of postpartum patients, notably those who have prolonged hypotension or coagulopathy, and who need massive blood volume and blood product replacement, are best monitored in a multidisciplinary intensive care unit.
Uterine Rupture
Epidemiology
Rupture of the gravid uterus can be disastrous for both the mother and the fetus. Fortunately, it does not occur often. Previous uterine surgery (e.g., cesarean delivery or myomectomy) increases the risk, but the incidence of true uterine rupture after cesarean delivery is still low, occurring at a rate of less than 1%.54,55 Uterine rupture is rare in the primigravid woman or the woman with an unscarred uterus, but it does occur.55 Box 38-2 lists additional conditions that have been associated with uterine rupture.55,56 Very rarely, uterine rupture occurs without explanation.57
Rupture of a previous uterine scar may occur in the absence of labor. In a review of records from a large multistate hospital system, nearly half of all true uterine ruptures occurred in the absence of a history of cesarean delivery, and 22% of ruptures occurred in the absence of labor.58 Lydon-Rochelle et al.59 undertook a population-based retrospective analysis of more than 20,000 women who had undergone one previous cesarean delivery. The risk for rupture among nonlaboring women was 1.6 per 1000. Among women in spontaneous labor the risk increased approximately threefold to 5.2 per 1000; among women undergoing induction of labor the risk increased nearly fivefold to 7.7 per 1000; and among women undergoing prostaglandin induction the risk increased almost 16-fold to 24.5 per 1000. Additional risk factors for uterine rupture during a trial of labor after cesarean (TOLAC) include post-term gestation (≥ 42 weeks), birth weight greater than 4000 g, maternal age older than 35 years, and maternal height greater than 164 cm.54
Because of variation in nomenclature and severity, accurate determination of maternal and fetal morbidity secondary to uterine rupture is difficult. The most common variety of uterine scar disruption is separation or dehiscence, some cases of which are asymptomatic. Uterine scar dehiscence is defined as a uterine wall defect that does not result in excessive hemorrhage or FHR abnormalities and does not require emergency cesarean delivery or postpartum laparotomy. In contrast, uterine rupture, less common than dehiscence, refers to a uterine wall defect with maternal hemorrhage and/or fetal compromise sufficient to require emergency cesarean delivery or postpartum laparotomy.
The rupture of a classical uterine incision scar (a vertical incision involving the muscular uterine fundus) is associated with greater morbidity and mortality than rupture of a low transverse uterine incision scar because the anterior uterine wall is highly vascular and may include the area of placental implantation. Lateral extension of the rupture can involve the major uterine vessels and is typically associated with massive bleeding. Maternal death secondary to uterine rupture is rare, although there were three deaths attributed to uterine rupture in the 2006 to 2008 triennial report from the United Kingdom.5 In Sweden between 1983 and 2001, Kaczmarczyk et al.54 estimated that the neonatal mortality rate associated with uterine rupture was approximately 5%.
Diagnosis
The variable presentation of uterine rupture may cause diagnostic difficulty. Abdominal pain and an abnormal FHR pattern are the two most common presenting signs of uterine rupture,60 but neither is 100% sensitive. One retrospective study reported the occurrence of abdominal pain in 17% of patients; an FHR abnormality was the first sign of uterine rupture in 87% of patients (see Chapter 19).61 Other presenting signs include vaginal bleeding, uterine hypertonia, cessation of labor, maternal hypotension, loss of the fetal station, decrease in cervical dilation, or a change in fetal presentation. Breakthrough pain during neuraxial labor analgesia may also indicate uterine rupture.62
Obstetric Management
Treatment options for uterine rupture include repair of the uterus, arterial ligation, and hysterectomy. Uterine repair is appropriate for most cases of separation of a prior low transverse uterine scar and for some cases of rupture of a classical incision. However, the risk for rupture in a future pregnancy remains. A disadvantage of arterial ligation is that it may not control the bleeding and may delay definitive treatment. Hysterectomy may be required for some cases of uterine rupture.56
Anesthetic Management
Patient evaluation and resuscitation are initiated while the patient is being prepared for emergency laparotomy. If rupture has occurred antepartum, fetal compromise is likely. General anesthesia is often necessary, except in some stable patients with preexisting epidural labor analgesia. Aggressive volume replacement is essential, and transfusion may be necessary. Urine output should be monitored. Invasive hemodynamic monitoring may be appropriate if there is uncertainty about the intravascular volume status.
Vasa Previa
Vasa previa is defined as the velamentous insertion of the fetal vessels over the cervical os (i.e., the fetal vessels traverse the fetal membranes ahead of the fetal presenting part). Thus, the fetal vessels are not protected by the placenta or the umbilical cord. Rupture of the membranes is often accompanied by tearing of a fetal vessel, which may lead to exsanguination of the fetus.
Epidemiology
Vasa previa occurs rarely (1 in 2500 to 1 in 5000 deliveries).28 Because it involves the loss of fetal blood, vasa previa is associated with a high fetal mortality rate (nearly 60% if vasa previa is unrecognized).63 The blood volume of the fetus at term is approximately 80 to 100 mL/kg. Therefore, the amount of blood that can be lost without fetal death is small. In addition, the vulnerable fetal vessels may be compressed by the fetal presenting part, resulting in fetal hypoxia and death. Risk factors for vasa previa include the presence of placenta previa or low-lying placenta in the second trimester, placental accessory lobes, in vitro fertilization, and multiple gestation.28
Diagnosis
Ultrasonography can be used to visualize the velamentous insertion of the vessels, and transvaginal color Doppler imaging can confirm the diagnosis.28,63 Vasa previa should be suspected whenever bleeding occurs with rupture of membranes, particularly if the rupture is accompanied by FHR decelerations or fetal bradycardia. Hemorrhage can also occur without rupture of membranes, making the diagnosis more difficult. Rarely, vasa previa can be diagnosed via digital cervical examination or amnioscopy. The diagnosis of vasa previa can be confirmed through examination of the shed blood for evidence of fetal hemoglobin (e.g., Kleihauer-Betke test); however, when bleeding occurs, the emergency nature of vasa previa usually precludes such diagnostic confirmation.
Obstetric Management
Prenatal diagnosis confers a neonatal survival benefit. Oyelese et al.63 conducted a retrospective study of 155 pregnancies complicated by vasa previa. Neonatal mortality was 3% when the vasa previa was diagnosed antenatally and 56% when it was not. The authors recommended ultrasonographic examination with transvaginal color Doppler in patients at risk for vasa previa. The management of vasa previa is directed solely toward ensuring fetal survival. Some authors advocate hospitalization of the patient between 30 and 32 weeks’ gestation to ensure prompt delivery if rupture of membranes should occur; consideration should be given to the administration of a corticosteroid to promote fetal lung maturity.28 Timing of delivery reflects a balance between the risks of preterm delivery and the risk for vessel rupture if the pregnancy is allowed to continue. Robinson and Grobman64 compared delivery timing strategies for women with vasa previa and calculated that the best fetal outcomes occurred with elective delivery between 34 and 35 weeks’ gestation. They further determined that confirmation of fetal lung maturity via amniocentesis was not necessary.64
Ruptured vasa previa is a true obstetric emergency that requires immediate delivery of the fetus, almost always by the abdominal route. Neonatal resuscitation requires immediate attention to neonatal volume replacement with colloid, balanced salt solutions, and blood.
Anesthetic Management
The choice of anesthetic technique depends on the urgency of the cesarean delivery. In many cases, general anesthesia is necessary for prompt delivery.
Postpartum Hemorrhage
Conflicting definitions of postpartum hemorrhage exist; however, the most commonly accepted definition is more than 500 mL blood loss after vaginal delivery or more than 1000 mL after cesarean delivery.1,65 These values may have low clinical utility because they are only slightly higher than the average blood loss for each type of delivery. Postpartum hemorrhage can also be inferred clinically (albeit retrospectively) from a 10% decrease in hematocrit from admission to the postpartum period or the need to administer PRBCs owing to postpartum blood loss.
Postpartum hemorrhage is the most common cause of maternal mortality worldwide and an important contributor to maternal death in the United States.1,2,4 The incidence of postpartum hemorrhage varies widely throughout different regions of the world2; in the United States the current rate of postpartum hemorrhage is approximately 3%.10,11 Postpartum hemorrhage, severe postpartum hemorrhage, and the attendant morbidity and mortality from hemorrhage are increasing in incidence.10,11 Between 1994 and 2006 the transfusion rate for postpartum hemorrhage more than doubled.10 The explanation for this acceleration is not entirely clear but appears to be related to rising rates of postpartum uterine atony as well as increases in the incidence of abnormal placentation, both coincident with the rise in cesarean delivery rates.10,11,14,15 Other factors may include the rising rates of obstetric interventions, such as induction and augmentation of labor,66–68 and the increasing prevalence of obesity,69–71 multiple gestation,67,72 hypertensive diseases of pregnancy,73 and advanced maternal age.12,74 However, the rising prevalence of these risk factors does not entirely explain the upward trend in postpartum hemorrhage that has been observed.10,11
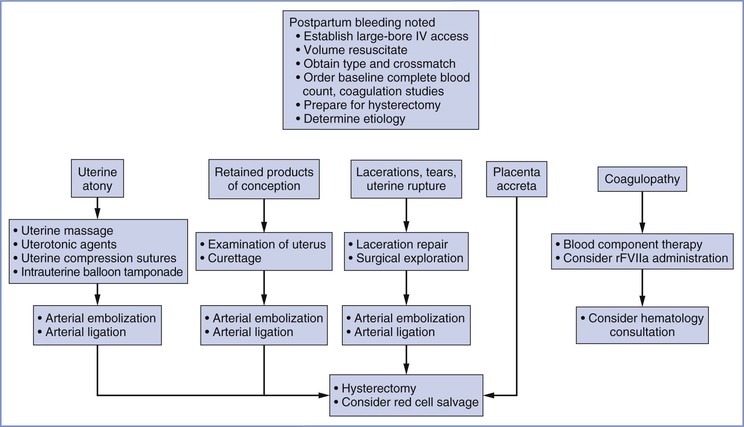
Primary postpartum hemorrhage occurs during the first 24 hours, and secondary postpartum hemorrhage occurs between 24 hours and 6 weeks after delivery.75 Primary postpartum hemorrhage is more likely to result in maternal morbidity or mortality. Figure 38-2 provides an overview of the obstetric management of postpartum hemorrhage.
Uterine Atony
Epidemiology
Uterine atony is the most common cause of severe postpartum hemorrhage, accounting for approximately 80% of cases.10,11 In addition to normal hemostatic mechanisms, postpartum hemostasis involves the release of endogenous uterotonic agents—primarily oxytocin and prostaglandins—that contract the uterus and constrict uterine vessels. Uterine atony represents a failure of this process. In addition, parturients with obstetric hemorrhage may have uterine arteries that are relatively unresponsive to vasoconstrictor substances.76 Box 38-3 lists conditions associated with uterine atony.
Diagnosis
A soft, poorly contractile uterus and vaginal bleeding are the most common findings in patients with uterine atony. The absence of vaginal bleeding does not exclude this disorder because the atonic, engorged uterus may contain more than 1000 mL of blood. Unrecognized bleeding may manifest initially as tachycardia; worsening hypovolemia eventually leads to hypotension (see Table 38-1).
Obstetric and Anesthetic Management
Prophylaxis.
The American College of Obstetricians and Gynecologists (ACOG) recommends prophylactic administration of uterotonic agents to prevent uterine atony.75 Active management of the third stage of labor, including uterine massage and oxytocin administration, decreases blood loss and transfusion requirements compared with expectant management.77,78
Oxytocin is the first-line drug for prophylaxis and treatment of uterine atony after delivery of a third-trimester pregnancy. The number of high-affinity receptors for oxytocin increases greatly near term; alternative uterotonics are more effective in the first and second trimesters of pregnancy. Endogenous oxytocin is a 9-amino acid polypeptide produced in the posterior pituitary. The exogenous form of the drug (Pitocin, Syntocinon) is a synthetic preparation with a rapid onset and short half-life. Unfortunately, exogenous oxytocin can be associated with serious side effects, including tachycardia, hypotension, myocardial ischemia, and, rarely, death, especially in hypovolemic or other hemodynamically compromised women79–83; many of these adverse effects are directly related to the dose of oxytocin.84,85 Preeclamptic women may be less able to tolerate high doses of oxytocin than healthy women.86 In addition, high doses of oxytocin administered concomitantly with large volumes of intravenous fluids, especially those containing free water, can lead to hyponatremia, seizures, and coma because of oxytocin’s structural similarity to vasopressin.87
The dose of oxytocin required to generate satisfactory uterine tone after delivery is lower than previously thought (see Chapter 26). In a study of nonlaboring women undergoing elective cesarean delivery, the ED90 of bolus dose oxytocin for satisfactory uterine tone within 3 minutes of delivery was 0.35 international units (IU)88; The ED90 was approximately 3 IU in laboring women undergoing cesarean delivery for labor arrest after labor augmentation with oxytocin.89 The ED90 of oxytocin administered via infusion without a bolus dose in nonlaboring women was approximately 0.3 IU/min for 1 hour.90 Munn et al.91 randomized women undergoing a cesarean delivery during labor to receive a prophylactic infusion of oxytocin at 2.67 IU/min or 0.33 IU/min for 30 minutes after delivery; the higher dose was associated with less need for secondary uterotonics (19% versus 39%, respectively; P < .001); however, the high dose may be associated with clinically significant tachycardia and hypotension (see later discussion).
Oxytocin is rapidly metabolized by hepatic oxytocinases and cleared in the urine and bile, resulting in a half-life of less than 6 minutes. Consequently, a prolonged intravenous infusion may be more effective than bolus administration in preventing uterine atony. In an international randomized, controlled trial, Sheehan et al.92 found that the addition of a 4-hour maintenance infusion of 0.17 IU/min (after an initial 5-IU bolus dose) decreased the need for secondary uterotonics compared with a 5-IU bolus dose alone.92 King et al.93 studied women at high risk for postcesarean uterine atony and demonstrated that administering a 5-IU bolus of oxytocin before a 1.3-IU/min infusion did not provide benefit compared with an infusion without a bolus. Administration of phenylephrine with oxytocin can mitigate the adverse hemodynamic consequences of oxytocin,94 but phenylephrine may not be necessary as long as an oxytocin bolus dose is avoided and the infusion rate is maintained below 1 IU/min, the threshold at which hemodynamic consequences become apparent.84
Data demonstrating lower oxytocin dose requirements than previously assumed and awareness of the dangers of high-dose administration call into question the common practice of injecting 10 to 40 IU of oxytocin into a 1-liter crystalloid solution and infusing the solution at an unspecified rate, often “wide open” (i.e., gravity-dependent flow). The doses administered with this method may approach those achieved with bolus administration. At my institution, my colleagues and I administer prophylactic oxytocin at a rate of 0.3 IU/min (the ED90) and increase the rate to 0.6 IU/min (twice the ED90) if there is inadequate response. The maximum beneficial oxytocin infusion rate to treat persistent uterine atony is unknown.
Carbetocin is an alternative synthetic oxytocin-receptor agonist available in Canada, the United Kingdom, and other developed countries but not the United States. A meta-analysis comparing carbetocin with oxytocin suggests that carbetocin reduces the need for secondary uterotonics95; this difference may reflect the fact that equipotent dosing regimens have not been determined. Carbetocin has a longer duration of action than oxytocin; therefore, prolonged infusion is not necessary.
Treatment.
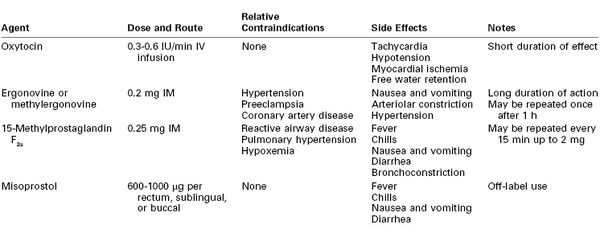
Despite preventive measures, postpartum uterine atony may occur. A multidisciplinary response to atony is imperative. General resuscitative measures include (1) additional large-bore intravenous access, (2) intravenous administration of crystalloid and colloid solutions and vasopressors, (3) laboratory determination of hemoglobin concentration or hematocrit and assessment of coagulation status, and (4) blood bank preparation of blood products for transfusion. Bimanual compression and massage of the uterus and continued infusion of oxytocin may be helpful in restoring uterine tone. Unfortunately, few high-quality data exist to guide therapy if these management strategies fail; current practice relies on expert opinion and clinical judgment. In the case of inadequate response to oxytocin, additional uterotonic agents should be employed. Three classes of drugs are currently available for the treatment of uterine atony: oxytocin, ergot alkaloids, and prostaglandins (Table 38-3).
The ergot alkaloids comprise one class of drugs used for the treatment of uterine atony. The natural ergot alkaloids are produced by a fungus that commonly infests rye and other grains. Ergonovine and methylergonovine (a semisynthetic preparation) are the two ergot alkaloids currently available for use; their pharmacologic profiles are identical. Ergot alkaloids are unstable unless they are refrigerated.96 Both drugs are dispensed in ampules containing 0.2 mg. They have a rapid onset when administered via the intramuscular route. Bolus intravenous administration is not recommended. The uterotonic effect usually lasts for 2 to 4 hours.
Both drugs rapidly produce tetanic uterine contractions and for this reason are restricted to postpartum use. The mechanism of action is poorly understood, but the uterotonic effect is most likely mediated by alpha-adrenergic receptor stimulation.97 Parenteral administration of an ergot alkaloid is associated with a high incidence of nausea and vomiting.98 Administration by any route may cause serious cardiovascular system derangements, including vasoconstriction, hypertension,98 myocardial ischemia and infarction due to coronary vasospasm,99–101 cerebrovascular accidents,102 seizures,102 and even death.99,103 Patients at greatest risk are those with preexisting hypertension; however, sudden and marked hypertension may also occur in previously normotensive patients. The combination of an ergot alkaloid followed by a vasopressor has been reported to lead to exaggerated hypertension.104 Relative contraindications to the use of ergot alkaloids include hypertension, preeclampsia, peripheral vascular disease, and ischemic heart disease. Treatment of ergot-induced vasoconstriction and hypertension may require administration of a potent vasodilator such as nitroglycerin or sodium nitroprusside. Blood pressure and the electrocardiogram should be monitored closely after administration.
Prostaglandins of the E and F families have gained wide acceptance as escalation therapy when high-dose oxytocin is inadequate. Concentrations of endogenous prostaglandins increase during labor, but levels do not peak until the time of placental separation. It is hypothesized that uterine atony may represent a failure of prostaglandin concentrations to increase during the third stage of labor in some women.105,106 Prostaglandins increase myometrial intracellular free calcium concentration,107 ultimately leading to an increase in myosin light-chain kinase activity. Common side effects noted after administration of prostaglandins include fever, chills, diarrhea, nausea, and vomiting.108,109
A prostaglandin commonly used for the treatment of refractory uterine atony is 15-methyl prostaglandin F2α, or carboprost; its administration may succeed in controlling hemorrhage when all other pharmacologic treatments have failed.108,110 The recommended dose is 0.25 mg (250 µg) administered intramuscularly, which may be repeated every 15 to 30 minutes; the total dose should not exceed 2 mg (eight doses). Unfortunately, this valuable agent may precipitate bronchospasm, abnormal ventilation-perfusion ratio, increased intrapulmonary shunt fraction, and hypoxemia in susceptible patients.111,112
Misoprostol is a prostaglandin E1 analogue that has been used successfully for cervical ripening and induction of labor. Misoprostol is thermostable in tropical conditions and does not require intravenous access for administration; prophylactic misoprostol administration reduced the incidence of postpartum hemorrhage compared with placebo.113 These characteristics make it an attractive alternative to oxytocin and ergot alkaloids in low-resource areas, where the rate of maternal mortality from hemorrhage is high1,2,113; however, parenteral oxytocin is more effective for postpartum hemorrhage prophylaxis than misoprostol (relative risk [RR] of hemorrhage, 1.34; 95% confidence interval [CI], 1.16 to 1.55).113
Whether misoprostol can also decrease bleeding in patients with postpartum uterine atony unresponsive to conventional uterotonics is unclear.114 A large international randomized controlled trial failed to identify any benefit of misoprostol 600 µg administered sublingually in addition to oxytocin for treatment of postpartum hemorrhage.109 A second randomized controlled trial suggested that misoprostol may be less effective than the combined administration of ergometrine and oxytocin for the treatment of postpartum hemorrhage.115 A dose of 600 to 1000 µg per rectum is commonly administered; administration via the oral, buccal, and sublingual routes has been described.75,113,114 Like other prostaglandins, misoprostol may be associated with fever, chills, nausea, vomiting, and diarrhea.109,113,114 Misoprostol may have a more favorable side effect profile than ergonovine or 15-methyl prostaglandin F2α in patients with hypertension and/or reactive airway disease.
If hemorrhage and atony persist despite aggressive administration of multiple classes of uterotonic drugs, invasive techniques must be considered. Invasive techniques include intrauterine balloon tamponade, uterine compression sutures, embolization of the arteries supplying the uterus, surgical ligation of arteries, and cesarean hysterectomy (see later discussion).
Genital Trauma
The most common childbirth injuries are lacerations and hematomas of the perineum, vagina, and cervix. Most injuries have minimal consequence, but some puerperal lacerations and hematomas are associated with significant hemorrhage, either immediate or delayed.116 Prompt recognition and treatment can minimize morbidity and mortality.116 Genital tract lacerations should be suspected in all patients who have vaginal bleeding despite a firm, contracted uterus. The cervix and vagina must be inspected carefully in these patients. Computed tomography (CT) and/or MRI may be useful in detecting the presence, location, and extent of suspected hematoma.117 Pelvic hematomas may be divided into four types: vaginal, vulvar, vulvovaginal, and retroperitoneal.116
Vaginal hematomas result from soft tissue injury during delivery, and they may involve bleeding from the descending branch of the uterine artery.116,118 The use of forceps or vacuum extraction increases the risk.118 A study in Sweden of all cases of vaginal hematoma from 1987 to 2000 found a prevalence of approximately 1 in 1240 deliveries.119 The investigators identified nulliparity, advanced maternal age, and neonatal birth weight exceeding 4000 g as risk factors for vaginal hematoma. Other risk factors may include prolonged second stage of labor, multiple gestation, preeclampsia, and vulvovaginal varicosities.116
Vulvar hematomas commonly involve branches of the pudendal artery.116 Injury is usually suggested by extreme pain or clinical manifestations of hypovolemia secondary to blood loss.116 Small vaginal or vulvar hematomas that are not enlarging may be observed and treated conservatively with ice packs and oral analgesics. Large hematomas should be incised and evacuated. Bleeding vessels should be ligated. Often no specific bleeding source can be identified. The successful use of arterial embolization to decrease bleeding and aid in surgical management of genital tract hematomas has recently been reported.118 Volume resuscitation and transfusion may be necessary.116
Retroperitoneal hematomas are the least common and most dangerous hematomas associated with childbirth. A retroperitoneal hemorrhage occurs after laceration of one of the branches of the hypogastric artery. Injury typically occurs during cesarean delivery or rarely after rupture of a low transverse uterine scar during labor. These hematomas may be large and may extend as far as the kidneys.
The symptoms of concealed bleeding depend on the size of the hematoma and the rate at which it forms. In some instances, abrupt hypotension may be the first sign of bleeding. The diagnosis of a retroperitoneal hematoma must be considered whenever a postpartum patient has an unexpected decrease in hematocrit or unexplained tachycardia and hypotension. Other signs and symptoms are restlessness, lower abdominal pain, a tender mass above the inguinal ligament that displaces a firm uterus to the contralateral side, and vaginal bleeding with hypotension out of proportion to the external blood loss. Ileus, unilateral leg edema, urinary retention, and hematuria also may occur.116 A high index of suspicion is needed; in obese women it may be especially difficult to examine the abdomen for signs of retroperitoneal hematoma.
Occasionally, a retroperitoneal hematoma may be self-limiting and need no surgical intervention. Life-threatening hematomas require exploratory laparotomy and ligation of the hypogastric vessels. Fliegner120 reported that 38 of 39 patients with a broad ligament hematoma received a blood transfusion. The average amount administered was 4000 mL. Eight (21%) of the patients required a hysterectomy.
Anesthetic Management
Choice of anesthetic technique for the repair of genital lacerations and evacuation of pelvic hematomas depends on the affected area, surgical requirements, volume/hemodynamic status of the patient, and urgency of the procedure. Local infiltration and a small dose of intravenous opioid suffice for drainage of some vulvar hematomas; however, repair of extensive lacerations and drainage of vaginal hematomas require significant levels of analgesia or anesthesia. Pudendal nerve block may not be technically feasible because of anatomic distortion or severe pain from the hematoma. Low doses of ketamine (10-mg boluses, not exceeding a total dose of 0.5 mg/kg) may suffice to produce sedation and analgesia with minimal risk for altering airway reflexes. Spinal or epidural anesthesia may be necessary, although the clinician should exercise caution initiating (or extending) a neuraxial block in a hypovolemic patient. In some cases, general anesthesia with tracheal intubation may be necessary. Exploratory laparotomy for a retroperitoneal hematoma typically requires the administration of general anesthesia.
Retained Placenta
Retained placenta is defined as failure to deliver the placenta completely within 30 minutes after delivery of the infant and occurs in approximately 3% of vaginal deliveries.121,122 Retained placenta is a leading cause of both primary and secondary postpartum hemorrhage. The risk for postpartum hemorrhage increases significantly if the interval between delivery of the infant and the placenta exceeds 30 minutes.121 The severity of bleeding ranges from minimal to severe and can be life-threatening and require transfusion.122 Risk factors for retained placenta include history of retained placenta, preterm delivery, oxytocin use during labor, preeclampsia, and nulliparity.121,122
Obstetric Management
Treatment of retained placenta during the early postpartum period often involves manual removal and inspection of the placenta. Curettage may be required. After removal of the placenta, uterine tone should be augmented with oxytocin and the patient should be observed for evidence of recurrent hemorrhage.
Anesthetic Management
Choice of anesthetic technique depends on the degree of hemorrhage. In some cases, the administration of small amounts of sedatives and analgesics is adequate to allow examination and manual placental extraction by a skilled obstetrician. Neuraxial anesthesia may be considered in patients who are not bleeding severely and who are hemodynamically stable. This may be accomplished with either administration of additional local anesthetic through an existing labor epidural catheter or initiation of spinal anesthesia. General anesthesia sometimes becomes necessary, particularly in patients who are hemodynamically unstable.
In some cases, the obstetrician requires uterine relaxation to facilitate manual removal of the placenta. Historically, anesthesia providers have performed rapid-sequence induction of general anesthesia, followed by the administration of a high dose of volatile halogenated agent to relax the uterus. Equipotent doses of halothane, sevoflurane, and desflurane depress uterine contractility equally and in a dose-dependent manner. In a study of isolated human uterine muscle, an equi-anesthetic concentration of isoflurane was a less effective uterine relaxant than the other volatile agents.123 Uterine contractility is decreased by 50% with administration of approximately 1.5 minimum alveolar concentration (MAC) of a volatile anesthetic agent.123 The induction of general anesthesia and administration of a volatile halogenated agent results in rapid onset of uterine relaxation, and discontinuation of the volatile agent results in rapid offset when uterine relaxation is no longer necessary. However, induction of general anesthesia in a parturient entails risk for failed ventilation, failed tracheal intubation, and/or aspiration of gastric contents.
Alternatively, nitroglycerin may be administered for uterine relaxation. Nitroglycerin provides a rapid onset of reliable smooth muscle relaxation and a short plasma half-life (2 to 3 minutes).124,125 Nitroglycerin has been administered for various obstetric emergencies without clinically significant side effects.125 Peng et al.126 described successful removal of retained placenta in 15 parturients after administration of intravenous nitroglycerin 500 µg. DeSimone et al.127 used a substantially smaller dose of nitroglycerin (50 to 100 µg) with similar results; all patients were managed successfully without the need for induction of general anesthesia. Nitroglycerin may also be administered sublingually via spray or tablet. A double-blind, randomized, controlled study compared sublingual nitroglycerin with placebo for management of retained placenta128; the placenta was delivered successfully within 5 minutes in all 12 of the parturients who received nitroglycerin, compared with only 1 of the 12 who received placebo. Nitroglycerin most likely produces uterine smooth muscle relaxation by releasing nitric oxide; it may require the presence of placental tissue to be effective.129
Uterine Inversion
Epidemiology
Uterine inversion, or the turning inside-out of all or part of the uterus, is a rare but potentially disastrous event. It is associated with severe postpartum hemorrhage, and hemodynamic instability may be worsened by concurrent vagal reflex–mediated bradycardia. Inversions may be acute or chronic, but only acute peripartum inversions involve the obstetric anesthesia provider. The reported incidence of this disorder varies widely; recent reports suggest an incidence of approximately 1 in 2500 deliveries.130
Risk factors for uterine inversion include uterine atony, a short umbilical cord, uterine anomalies, and overly aggressive management of the third stage of labor, including maneuvers such as inappropriate fundal pressure or excessive umbilical cord traction.130 Uterotonic therapy can convert a partial inversion to a complete inversion. An abnormally implanted placenta (i.e., placenta accreta) may be first recognized when uterine inversion occurs.
Diagnosis
Many cases of uterine inversion are obvious because of hemorrhage and a mass in the vagina, but others may not be readily apparent. Inversion should be suspected in all cases of postpartum hemorrhage. Ultrasonographic examination may show characteristic findings, such as an echolucent zone within an echogenic mass filling the uterine cavity on transverse view.131 Historically, obstetricians have stated that the shock is out of proportion to the blood loss, but an underestimation of obstetric hemorrhage is more likely. An incomplete inversion not protruding through the introitus is more likely to result in missed or delayed diagnosis.116
Obstetric Management
Immediate replacement of the uterus, even before removal of the placenta, is the treatment goal, but it may be difficult to achieve. The appropriate technique for correcting an inversion has been described.130 All uterotonic drugs should be discontinued immediately. The obstetrician should attempt to right the inversion by applying pressure through the vagina to the uterine fundus; ring forceps may be used on the cervix to apply countertraction. Early diagnosis and prompt correction may reduce the morbidity and mortality associated with uterine inversion.
Anesthetic Management
Often, uterine tone precludes replacement of the uterus, and uterine relaxation is necessary for successful uterine reduction.130 The use of nitroglycerin to facilitate relaxation and replacement of the uterus has been reported.132,133 Fairly large intravenous doses (200 to 250 µg) may be required, and the anesthesiologist typically will need to support the circulation with intravenous fluids and vasopressors. Administration of general anesthesia with a volatile halogenated agent may become necessary, not only for uterine relaxation but also to prepare for laparotomy should it become necessary to correct the inversion. Once the uterus has been replaced, a firm, well-contracted uterus is desired.130 Oxytocin should be infused, and additional uterotonic drugs may be needed.
Placenta Accreta
Placenta accreta is defined as a placenta that in whole or in part invades the uterine wall and is inseparable from it.134 Three types of placenta accreta occur (Figure 38-3). Placenta accreta vera is defined as adherence of the basal plate of the placenta directly to uterine myometrium without an intervening decidual layer. Placenta increta refers to a placenta in which chorionic villi invade the myometrium. Placenta percreta represents invasion through the myometrium into serosa and sometimes into adjacent organs, most often the bladder.134
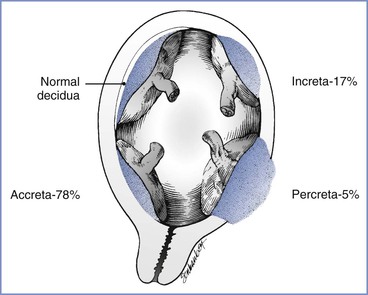
FIGURE 38-3 Uteroplacental relationships found in abnormal placentation. (From Francois KE, Foley MR. Antepartum and postpartum hemorrhage. In Gabbe SG, Niebyl JR, Simpson JL, et al., editors. Obstetrics: Normal and Problem Pregnancies. 6th edition. Philadelphia, Elsevier Saunders, 2012:424.)
Epidemiology
Evidence suggests that the incidence of placenta accreta is rising, primarily because of the increasing cesarean delivery rate. Between 1994 and 2007 the rate of peripartum hysterectomy for abnormal placentation increased by 20% in the United States, an increase entirely explained by adjustment for the rising prevalence of previous cesarean delivery among childbearing women.15 The percentage of U.S. cesarean deliveries increased from 20.7% in 1997 to 32.9% in 2009, owing to increases in both primary and repeat cesarean deliveries and a decrease in the TOLAC rate.135–137 Fortunately, the cesarean delivery rate remained stable between 2009 and 2012.138
Previous cesarean delivery or other uterine surgery increases the risk for both placenta previa and accreta. The combination of placenta previa with previous cesarean delivery synergistically increases the risk for coexisting placenta accreta, particularly if the placenta is anterior and overlies the uterine scar. In a prospective multicenter observational study, Silver et al.25 determined the relationship between placenta accreta, placenta previa, and previous cesarean delivery. Placenta previa with no prior uterine surgery conferred a 3% risk for placenta accreta. In women with placenta previa and one previous cesarean delivery, the risk for placenta accreta was 11%. In patients with placenta previa and a history of two previous cesarean deliveries, the incidence of placenta accreta was increased to 40%. The incidence of placenta accreta was over 60% in women with placenta previa and a history of three or more previous cesarean deliveries (see Table 38-2). Another study noted a relationship between the extent of uterine wall invasion and the number of previous cesarean deliveries.139
Diagnosis
In some cases of placenta accreta the condition is first suspected at vaginal delivery, when the obstetrician notes difficulty in separating the placenta. The definitive diagnosis is then made at laparotomy. Antenatal diagnosis of placenta accreta facilitates effective planning. Antenatal diagnosis is associated with less maternal and neonatal morbidity, including decreased blood loss at delivery and transfusion of fewer units of blood products.140 Ultrasonography is a useful screening tool in patients with placenta previa and/or previous cesarean delivery and is the primary imaging modality used for making the diagnosis of placenta accreta. However, among women at risk for placenta accreta, ultrasonography has imperfect sensitivity and specificity.134 Some evidence suggests that MRI may help confirm the diagnosis in at-risk patients with inconclusive ultrasonographic examinations.141
Obstetric Management
The ACOG advises that providers working at small hospitals without adequate blood bank supplies consider transferring patients with placenta accreta to a tertiary care facility.134 Patients treated at institutions with a 24-hour in-house obstetrician and anesthesiologist, immediate availability of a gynecologic oncologist, a fully stocked blood bank, and interventional radiology services suffer less morbidity than those treated at hospitals without these services.142 Planned delivery with all of the necessary multidisciplinary collaborators present compared with emergency delivery is associated with less maternal morbidity, including fewer transfusions, complications, and intensive care unit admissions. Some cases of vaginal bleeding remote from term resolve spontaneously, and expectant management may prolong the duration of pregnancy, at least into the third trimester. However, the risk for severe antenatal bleeding increases as gestational length increases.142 Timing of delivery therefore involves balancing this risk against the neonatal risks of preterm delivery. Institutions that manage women with suspected placenta accreta expectantly must have the capacity to mobilize the entire perioperative team at any time.
Most patients with known placenta accreta should undergo planned preterm cesarean delivery and hysterectomy with the placenta left in situ because attempts to remove the placenta are likely to initiate hemorrhage. Because the positive predictive value of ultrasonography may be low, the ACOG advises that it is reasonable to await spontaneous placental delivery in some cases, but manual removal of the placenta should be avoided.134 Preoperative placement of ureteral stents may minimize urinary tract injury.134 A midline vertical skin incision may provide optimal surgical exposure; it may be necessary to modify the uterine incision to avoid cutting through the placenta.
The preoperative insertion of internal iliac artery balloon catheters is controversial. Optimally, the balloons are inflated after delivery as a means to provide a less bloody surgical field and decrease blood loss and transfusion requirements. Retrospective cohort studies have reported conflicting data regarding effects of balloon catheter placement on estimated blood loss, transfusion requirements, and duration of the surgical procedure.143–146 The largest of these studies compared 59 patients who had placement of preoperative balloon catheters with 58 who did not and determined there was lower mean estimated blood loss, fewer cases with estimated blood loss greater than 2500 mL, and fewer massive transfusions in the balloon group.146
Multiple complications can arise from the placement of these devices, some of them involving serious disruptions of the vasculature and lower extremity ischemia.144,146–148 Introduction of the arterial catheters (without inflation) can cause fetal bradycardia, necessitating emergency delivery.149 If employed, therefore, internal iliac artery balloon catheters should be placed in the operating room to avoid dislodgement during transport and to allow for rapid delivery should fetal compromise occur. Authors of a recent review of the literature opined that current evidence, confined to case reports, case series, and small retrospective studies, is inadequate to guide clinical decision-making.150 The Society of Maternal-Fetal Medicine (SMFM) recommends reserving the use of prophylactic intra-arterial balloon catheters for well-counseled women with a strong desire for fertility preservation, those who decline blood products, and those with unresectable placenta percreta.151
Two forms of conservative therapy for placenta accreta have been described. In selected patients with a partial placenta accreta, small focal areas of placental invasion may be managed by curettage and oversewing. Alternatively, it may be possible to leave the intact placenta in situ, close the uterus and abdomen, and await spontaneous placental involution.152–154 However, conservatively managed patients often require additional therapies such as internal iliac artery balloon inflation, embolization, or methotrexate. Some patients treated in this way have subsequently had successful pregnancies152; however, complications have been reported, including secondary hemorrhage, subsequent need for hysterectomy, and sepsis.152,154 The ACOG considers planned peripartum hysterectomy to be the management of choice for patients with placenta accreta and cautions the obstetrician to reserve uterine conservation strategies for hemodynamically stable patients who strongly desire future fertility.134
Anesthetic Management
Anesthetic management is similar to other cases of severe postpartum hemorrhage and peripartum hysterectomy (see later discussion). Preoperative suspicion for placental implantation abnormalities should alert the anesthesia provider to the potential for massive blood loss. One group of investigators reported that estimated blood loss exceeded 2000 mL in 66% of cases of placenta accreta, 5000 mL in 15%, 10,000 mL in 6.5%, and 20,000 mL in 3% of cases.155 Initial blood loss may be minimal but can rapidly become torrential if the placental bed is disturbed or if the surgeons encounter unavoidable placental tissue during the course of the hysterectomy.
Invasive Treatment Options
Regardless of the cause of obstetric hemorrhage, first-line conservative measures may fail to control bleeding. In these cases, invasive procedures must be performed promptly to avoid severe morbidity and mortality. Second-line options include intrauterine balloon tamponade, uterine compression sutures, angiographic arterial embolization, and uterine artery and/or internal iliac artery ligation, performed in an attempt to avoid hysterectomy. Unfortunately, no randomized controlled trials assessing relative efficacy and safety of these options exist to guide management.156,157
Intrauterine balloon tamponade is a conservative method for controlling postpartum hemorrhage, especially when uterine atony or lower uterine segment bleeding is suspected.75 A 2007 systematic review reported the technique is successful (no need for additional therapy) 84% of the time,156 and a prospective evaluation reported a success rate of 81%.158 A variety of devices have been used, including a balloon specifically designed for this purpose.159 An intrauterine balloon can be deployed quickly, requires minimal analgesia for both insertion and removal, and preserves fertility. Failure is easily recognized and commonly attributed to prolapse though a partially open cervix; in such cases, the balloon may be replaced and secured by applying bilateral ring forceps to the cervix160 or by placing a cervical cerclage.161 Few complications have been reported, although concerns for infection exist.156,157 A case report described uterine rupture after curettage and subsequent tamponade balloon placement.162
Uterine compression sutures (e.g., B-Lynch suture163) are most useful in cases of refractory uterine atony but have also been used in cases of retained placenta and accreta.156,157 A systematic review—based mostly on case reports and case series and therefore subject to reporting bias—estimated a 92% success rate for this procedure,156 but the success rate (avoidance of hysterectomy) in a prospective population-based trial was more modest (75%).164 The suture may slip off of the uterine fundus and fail to provide compression.157 Placement of compression sutures may preserve fertility; however, data are lacking on the long-term effects on fertility and pregnancy outcomes. Complications include infection, uterine necrosis, and suture erosion.157
Angiographic arterial embolization may be appropriate if moderate blood loss continues and if the patient is stable for transport to the interventional radiology suite. The uterine arteries, which are branches of the anterior trunk of the internal iliac arteries, provide the primary blood supply to the uterus. The ovarian and vaginal arteries also make a sizable contribution to uterine blood flow during pregnancy. During angiography the radiologist can identify the vessels responsible for bleeding and embolize these vessels effectively with gelatin sponge pledgets (Gelfoam). A small percentage of cases may require placement of a metallic coil in addition to gelatin sponges. The gelatin sponge is a temporary occlusive agent, and flow through these vessels returns over time, preserving both the uterus and fertility.165 Published success rates in emergently controlling postpartum hemorrhage with this approach vary between 70% and 100%.156,157 Successful treatment of acute postpartum hemorrhage with this modality requires rapid access to an angiography facility and a skilled interventional radiologist. The patient must be observed and monitored carefully while undergoing the procedure. Ischemic complications of embolization therapy have been reported, but the risk is reduced with the use of selective techniques.156,166,167
Bilateral surgical ligation of the uterine arteries (O’Leary sutures) may be used to control bleeding at laparotomy. In the case of failure to control bleeding, the surgeon may proceed with a more complex procedure that involves ligation of the tubo-ovarian and ascending and descending uterine arteries. Internal iliac artery ligation may also be considered, although it is more difficult to perform.157 Reported success rates are highly variable, and it appears that arterial ligation is being used less often than in the past.75 The rich collateral circulation of the uterus at term most likely contributes to failure to control bleeding, as does the challenging nature of the procedure itself. Engorgement of pelvic viscera, variability in vascular anatomy, and the increased blood flow during pregnancy contribute to the risk of complications when this approach is used. Successful surgical ligation permits preservation of fertility. Ischemic complications and neuropathy have been reported.156
Peripartum Hysterectomy
Peripartum hysterectomy is the definitive treatment for postpartum hemorrhage unresponsive to medical and other invasive therapies. The two most common indications for this procedure are uterine atony and placenta accreta.15 Between 1994 and 2007 the overall rate of peripartum hysterectomy increased by 15% in the United States, because of a 130% increase in hysterectomy for atony and a 23% increase in hysterectomy for placental implantation abnormalities.15 The increase in hysterectomy for placental abnormalities is almost entirely explained by an increase in the cesarean delivery rate.15 Parturients with a history of previous cesarean delivery are more than five times as likely to require a peripartum hysterectomy than those without this history, and risk for hysterectomy rises progressively with an increasing number of previous cesarean deliveries.15,25 The incidence of peripartum hysterectomy due to uterine rupture has declined in the United States because the rate of TOLAC has declined.15 Elective peripartum hysterectomy may also be undertaken for concurrent gynecologic abnormalities, especially malignancies.
Peripartum hysterectomy is a technically challenging operation; the uterus is enlarged, exposure may be difficult, the vessels are engorged, and the pregnant uterus receives a rich collateral blood supply. The presence of dense adhesions from previous surgeries can further complicate the surgery. A 2010 systematic review of emergency postpartum hysterectomy for hemorrhage revealed a perioperative morbidity rate of 56% and a mortality rate of 2.6%.168 Compared with nonobstetric hysterectomy, patients undergoing obstetric hysterectomy are more likely to suffer postoperative hemorrhage and require blood transfusion, have intraoperative urinary tract injury, and experience perioperative complications such as wound infection, venous thromboembolism, and cardiovascular and other medical complications.169 Mortality is more than 25 times higher in peripartum than nonperipartum hysterectomy.169
Transfusion is required in 44% or more of patients.168 Emergency peripartum hysterectomy is associated with increased blood loss, worse coagulopathy, and increased transfusion rates compared with elective peripartum hysterectomy.170 A multicenter review showed that the mean blood loss for emergency obstetric hysterectomy was 2526 mL, with a mean transfusion requirement of 6.6 units of PRBCs; in elective procedures, the mean blood loss was 1319 mL and the average replacement was 1.6 units of PRBCs (Table 38-4).170
TABLE 38-4
Operative Management and Complications of Elective versus Emergency Obstetric Hysterectomies
NS, not significant.
Values are n or mean ± SD.
Modified from Chestnut DH, Dewan DM, Redick LF, et al. Anesthetic management for obstetric hysterectomy: a multi-institutional study. Anesthesiology 1989; 70:607-10.
Owing to the challenging technical aspects of the procedure, the obstetrician may elect to perform a subtotal hysterectomy, wherein the cervix is left in situ. Subtotal approaches are associated with fewer urinary tract and other operative injuries and a shorter length of stay,168,169 but greater mean transfusion requirements and more frequent rates of reoperation than total hysterectomy.169 Subtotal hysterectomy is not appropriate for patients with bleeding from the cervix, lower uterine segment, or both (e.g., implantation of the placenta on the lower uterine segment).
Manual compression of the aorta can be a lifesaving procedure in the event of catastrophic obstetric hemorrhage.171 Effective aortic compression against a vertebral body in the upper abdomen should decrease blood flow to the pelvis, thereby allowing hemodynamic and hemostatic resuscitation and surgical control.172 An aortic cross-clamp requires vascular surgery expertise and retroperitoneal dissection but may be necessary to achieve hemostasis. Mild cardiac and renal dysfunction have been noted in nonobstetric patients if the aortic cross-clamp time exceeds 50 minutes173; if a prolonged clamp time is required, the anesthesiologist should prepare for lactic acidosis and hemodynamic instability at the time the clamp is released. Advanced surgical techniques to control friable, engorged blood vessels include felt or Teflon pledgets to buttress sutures, the rapid application of straight clamps, and the application of high-pressure surgical sealants.172,174
Anesthetic Management.
Anesthesia for peripartum hysterectomy is frequently challenging because massive blood loss may occur unpredictably.175 An experienced, skilled team is invaluable and critical to a successful outcome. An experienced team may elect neuraxial anesthesia in a properly prepared patient. Intraperitoneal manipulation, dissection, and traction may exceed similar maneuvers required with cesarean delivery alone, leading to pain, nausea, and vomiting. Maintenance of a T4 sensory level of anesthesia and judicious sedation may reduce the need for intraoperative conversion to general anesthesia. In a multicenter study of peripartum hysterectomy, none of the 12 patients who received continuous epidural anesthesia for elective or emergency hysterectomy required intraoperative induction of general anesthesia.170
Single-shot spinal anesthesia is unlikely to provide anesthesia of sufficient duration for an unanticipated hysterectomy. Indications for induction of general anesthesia at the beginning of a cesarean hysterectomy include anticipated difficult airway management, antepartum hemorrhage with hemodynamic instability, and known placenta percreta, which may increase the risk for massive hemorrhage and complex surgery.
Patients who have delivered vaginally with preexisting epidural labor analgesia may be managed successfully with extension of epidural blockade, but careful consideration of hemodynamic status should precede the administration of local anesthetics into the epidural space. Animal models suggest that sympatholysis established before the onset of hemorrhage reduces excessive catecholamine response to blood loss and may improve survival.176 However, the induction of sympatholysis during hemorrhage may compromise end-organ perfusion and even precipitate cardiopulmonary arrest.
As the magnitude of blood loss increases, general anesthesia becomes the anesthetic technique of choice. First, severely hypotensive patients may require tracheal intubation for airway protection. Second, large fluid shifts and massive transfusion may adversely affect oxygenation so that control of ventilation via an endotracheal tube becomes necessary. Third, these same fluid shifts increase airway edema, potentially making failed ventilation/failed tracheal intubation more likely as the surgery proceeds. Fourth, the massive transfusion of blood products often results in the need for coadministration of potent vasopressors and calcium chloride and, thus, central venous access; the placement of a central venous catheter may be more easily accomplished after the induction of general anesthesia. In all cases, patients at risk for peripartum hysterectomy managed with neuraxial anesthesia should be informed in advance that intraoperative discomfort or severe hemorrhage may mandate the intraoperative induction of general anesthesia.
The induction of general anesthesia in the setting of severe hemorrhage requires careful use of small doses of nondepressant induction agents such as ketamine or etomidate. The circulation should be supported with replacement of intravascular volume and vasopressors as needed. A review of maternal deaths from postpartum hemorrhage in France revealed that 5 of 38 deaths followed cardiac arrest on induction of general anesthesia.177
Regardless of the anesthetic technique used, two or more large-bore intravenous catheters should be inserted. Invasive blood pressure monitoring may aid in the prompt recognition of hypotension and provide access for frequent blood draws. The blood bank should be alerted to the possible need for massive transfusion. At least 4 units of PRBCs should be immediately available, with additional blood products, including plasma and cryoprecipitate, readily available without delay. The ACOG recommends consideration of intraoperative blood salvage in cases of placenta accreta (see later discussion).75,134 Vasoactive drugs (e.g., phenylephrine, epinephrine) should be available. Fluid warmers, a forced-air body warmer, and equipment for rapid infusion of fluids and blood products should be accessible if the care team is anticipating and managing significant blood loss. Teamwork and precise communication are indispensable.
Response to Hemorrhage
Prevention of Mortality
Data from the U.K. Confidential Enquiry into Maternal and Child Health and the French Confidential Enquiry into Maternal Death reveal that maternal deaths from hemorrhage in high-resource settings are often preventable and associated with substandard care.5,177 Berg et al.16 reported that whereas only 40% of all pregnancy-related deaths in the state of North Carolina between 1995 and 1999 were considered preventable, a staggering 93% of deaths due to hemorrhage were judged preventable. The most important factor in preventability of deaths from hemorrhage was management that did not meet the expected standard of care the review committee believed should have been available.16
Delays in diagnosis and treatment of postpartum hemorrhage increase the severity of hemorrhage.17,178,179 In a retrospective structured chart review of 63 women who suffered severe obstetric hemorrhage (defined by > 3 units blood transfused and intensive care unit admission), Della Torre et al.17 identified clinically significant delays in the diagnosis of hemorrhage in 23% of cases and delays in treatment in 38% of cases. A multicenter cohort study in France evaluated all women who developed postpartum hemorrhage due to uterine atony after vaginal birth to determine risk factors for progression to severe postpartum hemorrhage (defined by a decrease in hemoglobin concentration > 4 g/dL).179 Patients were more likely to develop severe hemorrhage if oxytocin administration, manual exploration of the uterus, or both were delayed. Delays of more than 10 minutes in summoning either an obstetrician or an anesthesiologist also increased the odds of severity, presumably by delaying treatment. Interestingly, epidural labor analgesia protected against severe hemorrhage, in all likelihood by allowing for more rapid and thorough uterine exploration.179
Delays in care most likely result from several factors, including difficulties in accurately estimating blood loss and in diagnosing maternal hypovolemia and shock, lack of aggressive monitoring and treatment of coagulopathy, and poor coordination of team responses.
Practitioners often underestimate the amount of blood loss, with the degree of underestimation increasing as blood loss volume increases (Figure 38-4).180–183 Accuracy of blood loss estimation can be improved with the use of calibrated collection drapes.18,184 Clinician education using simulated scenarios with known blood volumes enhances estimation accuracy.181,183,185 Separation of nonblood fluids (i.e., switching the suction canister after evacuation of amniotic fluid) and weighing pads and bedding may improve assessment. It is imperative that clinicians have a low threshold for the diagnosis of postpartum hemorrhage.
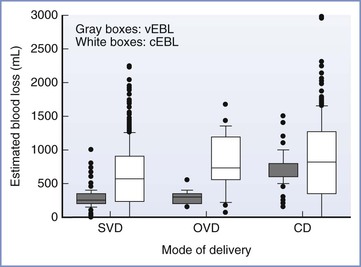
FIGURE 38-4 The visually estimated blood loss (vEBL) and calculated estimated blood loss (cEBL) by mode of delivery. The boxes illustrate the 25th, 50th, and 75th percentiles and the whiskers illustrate the 10th and 95th percentiles. Calculated estimated blood loss was derived by multiplying the calculated maternal blood volume by the percent of blood volume lost, where calculated maternal blood volume = 0.75 × {[maternal height in inches × 50] + [maternal weight in pounds x 25]} and percent of blood volume lost = {predelivery hematocrit − postdelivery hematocrit}/predelivery hematocrit. vEBL was statistically different from cEBL for each degree of laceration and for all modes of delivery, demonstrating an underestimation of vEBL with increasing cEBL. SVD, spontaneous vaginal delivery; OVD, operative vaginal delivery; CD, cesarean delivery. (From Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obstet Gynecol 2008; 199:519.e1-7.)
A second potential explanation for delays in diagnosis and treatment relates to difficulties in diagnosing hypovolemic shock in healthy young patients. The American College of Surgeons Trauma Committee’s Advanced Trauma Life Support (ATLS) system defines four stages of hypovolemic shock based on degree of blood loss (see Table 38-1).186 Obstetric patients are often mildly tachycardic, and worrisome rates of tachycardia (> 120 beats per minute) may not develop until the patient has lost 30% to 40% of her total blood volume. Similarly, hypotension and mental status changes are late signs. Findings such as these have led to the use of a modified early obstetric warning system (MEOWS) to detect impending adverse events (Table 38-5).187 Physiologic variables such as vital signs, oxygenation, and mental status are monitored regularly, and thresholds that trigger clinical evaluation are defined. MEOWS is not specific to hemorrhage. It has a positive predictive value of 39% and a negative predictive value of 98% in predicting maternal morbidity.187 It is not known whether hemorrhage-specific modifications to the MEOWS would improve outcomes in the setting of obstetric hemorrhage.
TABLE 38-5
Trigger Thresholds for MEOWS Parameters
| Red Trigger* | Yellow Trigger* | |
| Temperature (° C) | < 35 or > 38 | 35-36 |
| Systolic blood pressure (mm Hg) | < 90 or > 160 | 150-160 or 90-100 |
| Diastolic blood pressure (mm Hg) | > 100 | 90-100 |
| Heart rate (beats/min) | < 40 or > 120 | 100-120 or 40-50 |
| Respiratory rate (breaths/min) | < 10 or > 30 | 21-30 |
| Oxygen saturation (%) | < 95 | — |
| Pain score† | — | 2-3 |
| Neurologic response‡ | Unresponsive, pain | Voice |
* A trigger is defined as a single markedly abnormal observation (red trigger) or the simultaneous combination of two mildly abnormal observations (yellow trigger). A trigger prompts urgent medical assessment.
† Pain scores: 0 = no pain at rest or movement; 1 = no pain at rest, slight pain on movement; 2 = intermittent pain at rest, moderate pain on movement; 3 = moderate pain at rest, severe pain on movement.
‡ Neurologic responses: Alert: patient is alert and conscious; Verbal: patient responds to verbal stimulation; Pain: patient responds to painful stimulation; Unresponsive: patient is unresponsive to any stimulus.
MEOWS, modified early obstetric warning system.
From Singh S, McGlennan A, England A, Simons R. A validation study of the CEMACH recommended modified early obstetric warning system (MEOWS). Anaesthesia 2012; 67:12-8.
Another issue that may lead to delays in care is the speed with which coagulopathy develops in the setting of obstetric hemorrhage. The rapid consumption of coagulation factors, especially fibrinogen, during obstetric hemorrhage has prompted experts to recommend early monitoring for, and aggressive treatment of, coagulopathy, especially hypofibrinogenemia.53,188 Postpartum hemorrhage and other placental bed bleeding such as abruption appear to trigger a coagulopathy disproportionate to the amount of blood loss or dilution of coagulation factors (see later discussion). Once coagulopathy develops, the requirement for additional resources (e.g., blood products) accelerates. The institutional system(s) by which personnel and resources are rapidly activated must be clearly defined in advance.171,189
Protocols and Team Approach
Because early delays in care during postpartum hemorrhage worsen outcomes, protocols have been introduced that focus on early recognition and treatment; specifically, protocols emphasize team responses, the accurate estimation of blood loss, recognition of early signs of hypovolemic shock, early monitoring for anemia and coagulopathy, and appropriate transfusion of blood products. A number of protocols have been implemented and published, including a detailed obstetric hemorrhage “kit” by the California Maternal Quality Care Collaborative.190
The combination of protocols and team practice drills that emphasize these elements has been shown to decrease the severity of hemorrhage and, in some cases, mortality.191–193 Shields et al.193 introduced a postpartum hemorrhage protocol with practice drills and subsequently performed a follow-up study to determine whether introduction of the protocol reduced severity of hemorrhage and/or need for transfusion. All obstetric patients were assessed on admission to the hospital for risk for bleeding, and patients deemed at increased risk had blood samples sent to the blood bank for type and screen or type and crossmatch. Blood loss after delivery was estimated by weighing all absorbent material, adding the liquid contents of fluid collection devices, and subtracting the nonblood fluid volume (e.g., amniotic fluid). After delivery, each patient was assigned to one of four stages of increasing acuity, depending on estimated blood loss and changes in hemodynamic status. Higher stages of acuity directed the team of caregivers to summon help, to advance progressively through pharmacologic and surgical interventions to control the source of bleeding, and to simultaneously intensify hemodynamic and hemostatic support. In the preprotocol period, only 33% of patients were successfully treated in the earliest stage of hemorrhage (stage 1), compared with 82% in the postprotocol period (P = .02). Similarly, among patients who progressed to moderate hemorrhage (stage 2), 8% were successfully treated at that stage in the preprotocol period, compared with 50% in the postprotocol group (P = .02). Transfusion rates declined and there was a trend toward less DIC in the postprotocol period.193
Introduction of these types of protocols and drills has positive effects on sentinel events and malpractice payments.194 Additionally, in situ drills may uncover latent systems errors that can subsequently be corrected.195,196 The Joint Commission has called for clinicians to identify triggers that warn of excessive blood loss and hemodynamic instability early in the course of hemorrhage and to develop protocols for responding to such triggers; they recommend drills for obstetric hemorrhage, noting that these team exercises can be used for training staff as well as identifying and fixing systems problems.197
Transfusion Therapy
Despite advances in the prevention, diagnosis, and treatment of the hemorrhagic complications of pregnancy, the potential for significant blood loss remains. All physicians who provide care for pregnant women should understand the indications, risks, and benefits of transfusion. Transfusion may be indicated for treatment of severe anemia after moderate obstetric hemorrhage or to preserve life during massive hemorrhage.
The AABB recommends restrictive transfusion strategies after moderate hemorrhage in otherwise hemodynamically stable adults.198 This advice is based on consideration of the risks of anemia compared to the risks of transfusion. Randomized controlled trials have failed to demonstrate benefits for liberal compared with restrictive RBC transfusion strategies. Additional considerations arise from concern about resource utilization and the costs associated with transfusion.
Risks and Benefits
Risk of Anemia
Blood oxygen content and oxygen delivery to the tissues are a function of hemoglobin concentration; however, compensatory physiologic responses offset the negative effect of anemia on oxygen transport, especially if euvolemia is maintained with crystalloid or colloid intravascular volume expansion after moderate hemorrhage. Tachycardia and increased stroke volume combine to increase cardiac output, and blood viscosity and systemic vascular resistance decrease, augmenting blood flow to the tissues. Additionally, tissue oxygen extraction increases. Experience with patients who refuse transfusion indicate that these compensatory mechanisms are usually adequate to compensate for moderate blood loss; no increase in mortality is observed as long as hemoglobin concentration remains greater than 5.0 g/dL.199 Weiskopf et al.200 studied the effects of acute normovolemic hemodilution on oxygen delivery and extraction. As hemoglobin concentration fell, systemic vascular resistance decreased and heart rate, stroke volume, and cardiac index increased. Oxygen transport rate and mixed venous oxyhemoglobin saturation did not decrease until hemoglobin concentration levels reached 5.0 g/dL. Plasma lactate did not accumulate, leading the authors to conclude that transport of oxygen was not compromised during anemia of this magnitude. These measurements were made in healthy, nonpregnant patients and volunteers who were at rest; extrapolation of these results to sick or pregnant patients may not be warranted. Anemia carries increased risks in patients with cardiovascular disease.201
Postpartum anemia is associated with fatigue. However, fatigue is transient, and within 1 week fatigue and health-related quality of life scores are similar between postpartum patients who were anemic at discharge and those who were not.202 Transfusion of 1 or 2 units of PRBCs to moderately anemic parturients has no effect on length of hospital stay.203 Jonsson et al.204 investigated the correlation between postoperative hematocrit and wound tissue oxygenation and wound collagen deposition. There was no correlation until the hematocrit fell below 15%, most likely because compensatory responses to anemia mitigate the effects of low oxygen content. The effect of moderate anemia on breast-feeding is unknown.
Risks of Transfusion
Blood product transfusion is associated with known risks (see Table 55-2). Transfusion-associated circulatory overload (TACO) occurs in 1% to 6% of transfused patients.205–207 TACO sometimes develops in young patients and may follow the transfusion of as little as 1 unit of PRBCs.208 The likelihood of TACO increases with larger amounts of plasma transfused205 and may occur during the correction of the coagulopathy that accompanies severe hemorrhage and massive transfusion.
Transfusion-related acute lung injury (TRALI) is defined as a new acute lung injury (ALI) that occurs within 6 hours of transfusion in a patient without an alternative risk factor for ALI. It is accompanied by hypoxemia and radiographic evidence of pulmonary edema in the absence of circulatory overload (Box 38-4).209 TRALI results when human leukocyte antigen (HLA) class I and II neutrophil or possibly monocyte antibodies in donor plasma prime and activate recipient white blood cells (WBCs) to cause an ALI. This WBC activation leads to increased pulmonary microvascular permeability and interstitial and alveolar edema and extravasated neutrophils in the alveolar spaces.210 Multiparous female donors are more likely to carry the offending antibodies.211 In 2006, U.S. blood collection agencies instituted male-only donor plasma transfusion policies, and the TRALI rate has fallen to approximately one third of its prior level (approximately 1 case per 12,000 transfused units).211 Multicomponent apheresis collection techniques also decreased the risk for TRALI.210
Allogeneic RBC administration also risks transfusion-related immunomodulation (TRIM). The mechanisms for development of TRIM are incompletely understood, but subsequent to RBC transfusion, the host develops immune tolerance and a period of generalized immune suppression. Consequences of immune tolerance and suppression include an increased incidence of nosocomial infection, postoperative infection, and cancer recurrence.212 In addition, microchimerism, whereby donor cells/DNA persist in the host for several years, occurs and predisposes the recipient to autoimmune illnesses.212 There is some suggestion that TRIM increases the lifetime risk for nonsolid cell malignancies.213 These consequences are more likely to manifest as time passes and therefore may be more likely to affect young obstetric patients than older patients. Leukoreduction may have a modest role in mitigating TRIM.210
The risk for viral transmission because of allogeneic blood transfusion continues to decrease with thorough donor screening and use of nucleic acid amplification testing (nucleic acid technology [NAT]).214 The residual risk for transmission after both NAT and serologic testing of donor blood is approximately 1 in 2 million for both human immunodeficiency virus (HIV) and hepatitis C virus (HCV).198,214,215 NAT is not routinely used to test for hepatitis B virus (HBV). Instead, donor screening for hepatitis B surface antigen (HBsAg) and anti-core (HBc) antibody have greatly reduced the risk for transmission of HBV; the current risk for transmission with a blood transfusion is 1 in 350,000.198,214 The transfusion transmission of Creutzfeldt-Jakob disease has been reported. Because of the long incubation period of this prion, symptoms may not be evident for several years after transfusion.216 Levels of infectivity are believed to be very low.214 The first case of West Nile virus transmitted via a blood transfusion was identified in 2002; since 2003, routine blood screening has been implemented, virtually eliminating this risk.214 Cytomegalovirus (CMV) is carried in the monocytes of asymptomatic donors and may be transmitted to uninfected recipients of blood transfusions.217 Most of the subsequent infections are asymptomatic or mild, but CMV infection of an immunocompromised patient and/or fetus or neonate can lead to serious sequelae. The transmission rate may be as high as 30% without preventive techniques. The risk for transmission of CMV is reduced to 1.3% if seronegative blood is transferred218 and to 2.5% with the use of leukodepleted PRBCs.219 In 2000, a Canadian consensus conference concluded that these two methods of preventing post-transfusion CMV infection can be used interchangeably.217
Bacterial contamination occasionally occurs. Because platelets may undergo conformational changes at temperatures below 18° C, they are stored at 20° C to 24° C. This warmer storage temperature (compared with that used for PRBCs) increases the risk for bacterial proliferation. In 2004, the AABB mandated testing of all platelets for bacterial contamination. Use of culture-negative platelets has resulted in a reduction in the risk of septic transfusion to 1 in 75,000.220
Hemolytic transfusion reaction is a rare complication; it occurs most commonly as a result of accidental administration of ABO-incompatible blood.198 Acute intravascular hemolysis typically results in fever, chills, nausea, flushing, and chest and flank pain. These symptoms are masked by general anesthesia. Signs that may manifest during general anesthesia include hypotension, tachycardia, DIC, and hemoglobinuria.221 Immediate supportive care consists of discontinuation of the transfusion, treatment of hypotension and hyperkalemia, administration of a diuretic, and alkalinization of the urine. Assays for urine and plasma hemoglobin concentration and antibody screening confirm the diagnosis. A second crossmatch must be performed.
The biochemical and additional changes that occur during blood storage can lead to complications in the recipient, particularly when blood products are infused rapidly, as during massive transfusion for severe hemorrhage. The anticoagulant used for blood collection and storage contains citrate, which binds ionized calcium. Citrate is rapidly metabolized in the liver and typically does not lead to significant hypocalcemia. In patients who are hypothermic, have liver disease, or require rapid infusion of multiple units of blood products, however, citrate may accumulate and cause a decrease in ionized calcium. The concentration of citrate is seven times higher in fresh frozen plasma (FFP) and platelets than in PRBCs.222 Hypocalcemia results in reduced cardiac contractility, hypotension, and elevated central venous pressure.223
Plasma potassium concentration increases in stored blood. Transfused potassium usually moves intracellularly or is excreted in the urine. However, rapid infusion of multiple units of blood can lead to hyperkalemia, particularly in the hypothermic acidotic patient.223 Blood maintained at 4° C also can contribute to hypothermia, especially if the patient is anesthetized in a cold operating room. The decreased pH of stored blood is caused by the addition of citrate-phosphate-dextrose and the accumulation of lactic and pyruvic acids as a result of RBC metabolism and glycolysis. Despite the lower pH, transfusion of large amounts of stored blood rarely causes acidosis as long as tissue perfusion remains normal.223
Transfusion Strategies
Several randomized controlled trials have compared the use of restrictive and liberal transfusion practices, based on lower or higher hemoglobin triggers.224 These trials uniformly failed to demonstrate benefit to a liberal strategy and suggested that using higher hemoglobin triggers may cause harm.224–227 A 2012 meta-analysis that examined the effect of restrictive compared with liberal transfusion triggers on various outcomes228 found that the use of lower hemoglobin triggers reduced the risk of receiving an RBC transfusion (RR, 0.61; 95% CI, 0.52 to 0.72), reduced the volume of RBC transfused, and did not impact the rate of adverse events such as myocardial infarction, other cardiac events, or stroke. Furthermore, restrictive strategies were associated with a reduction of in-hospital mortality (RR, 0.77; 95% CI, 0.62 to 0.95) and a trend toward lower 30-day mortality (RR, 0.85; 95% CI, 0.70 to 1.03). There are no published randomized controlled trials examining low versus high transfusion triggers in obstetric patients. Among a series of 117 women with major obstetric hemorrhage (5 units or more of packed PRBCs transfused), a hematocrit nadir below 20% was associated with end organ injury.9
The indications for, and appropriateness of, RBC transfusion have been recently reevaluated because of (1) concerns for transfusion-related complications, (2) appreciation of the body’s mechanisms for compensating for anemia, and (3) the results of the randomized trials indicating no benefit to early transfusion. The AABB recommends that RBC transfusion not be considered in euvolemic, hemodynamically stable patients until the hemoglobin concentration reaches 7 g/dL, or 8 g/dL in patients with cardiovascular disease.198 The AABB further advises that transfusion be guided by symptoms and not by hemoglobin concentration triggers alone.198
Practices vary widely229 and often deviate from these guidelines. In a retrospective chart review, Butwick et al.230 found no specific documented indication for transfusion in 34% of obstetric patients who received a transfusion. Additionally, 18% had a pretransfusion hemoglobin concentration greater than 8 g/dL. Parker et al.231 documented that 31% of obstetric transfusions administered over a 1-year period occurred despite a hemoglobin concentration greater than 7 g/dL in the absence of ongoing bleeding or symptomatic anemia. Similarly, Fong et al.232 reported that 24% of transfusions administered to patients undergoing cesarean delivery were inappropriate, based on a preadministration hemoglobin concentration higher than 7 mg/dL. Finally, in a review of 33,795 obstetric-related admissions from 1994 to 2002, Silverman et al.233 found that 32% of RBC transfusions were not appropriate when judged against institutional guidelines.
The AABB guidelines do not address transfusion in parturients.198 Similarly, the ACOG is silent on this issue. Given current evidence in nonobstetric patients and the lack of data in obstetric patients (who are generally young and healthy), it seems reasonable that transfusion should be considered in obstetric patients with a hemoglobin concentration less than 7 g/dL or clinical evidence of inadequate oxygen-carrying capacity. Moreover, ongoing blood loss should prompt transfusion in some patients with a hemoglobin concentration greater than 7 g/dL.
The need to obtain a blood type and screen for all parturients on admission is controversial. Some clinicians suggest that this test is not necessary in patients with no identifiable risk factors for peripartum hemorrhage. Ransom et al.234 found that only 0.8 per 1000 low-risk patients undergoing cesarean delivery required a blood transfusion. They concluded that a routine type and screen in low-risk patients is not cost-effective. However, many anesthesia providers believe that the potential need for transfusion, and the occasional patient who develops an antibody from fetal antigen exposure during pregnancy, warrants the routine performance of a blood type and screen. The American Society of Anesthesiologists (ASA) Practice Guidelines for Obstetric Anesthesia state that the decision to perform a type and screen should be based on maternal history, anticipated hemorrhagic complications, and local institutional policies.235 It is not necessary for all parturients to undergo a type and crossmatch, although it may be prudent to obtain a crossmatch for patients with risk factors for bleeding.235 In addition, patients with a positive antibody screen should have a blood sample sent for crossmatch to avoid a delay in obtaining blood products should the need arise.
If blood is required quickly and the results of antibody screening are not available, the safest option is to administer ABO- and Rh-specific blood. If the blood type is unknown and blood products are required immediately, type O Rh-negative blood can be administered.
Blood Conservation Techniques
Iron deficiency is common in childbearing women because erythropoiesis occurs in the fetus at the expense of maternal iron stores. Oral iron therapy is a mainstay of anemia prevention and treatment in pregnant women. Unfortunately, oral therapy is not well-tolerated and therefore many patients may be noncompliant; others may have reduced gastrointestinal absorption.236 Vitamin C enhances iron absorption.236 In cases of malabsorption, intravenous iron administration may be required. Intravenous therapy more quickly and reliably corrects anemia than oral iron therapy,237 including anemia in postpartum women.238 Low-molecular-weight iron dextran is recommended for this purpose because it is associated with few serious adverse effects, in contrast to older iron preparations.239 Erythropoietin stimulates bone marrow erythropoiesis but has been associated with venous thromboembolism in vulnerable populations.240 Its use is not well studied in pregnancy.
The potential advantages of autologous blood transfusion include avoiding the risks of some transfusion-related adverse events and reduction of demands on the blood supply. The three methods of autologous transfusion are (1) preoperative (antepartum) donation, (2) normovolemic hemodilution, and (3) intraoperative blood salvage. Preoperative autologous donation causes anemia, may not reduce the risk of allogeneic transfusion, cannot be used in emergencies, and is not cost-effective because of difficulties in predicting transfusion need in obstetric patients, even those with traditional risk factors for hemorrhage.241,242 Normovolemic hemodilution may also induce anemia and may not reduce the risk of allogeneic transfusion.242,243 Experts do not endorse the routine use of either of these two techniques in obstetric patients.242
Intraoperative blood salvage is a technique of scavenging blood lost during surgery, processing it by centrifugation, washing and filtering, and administering the scavenged, autologous RBCs back to the patient. Red blood cells that are salvaged, processed, and transfused have an excellent survival rate. This procedure, which can rapidly provide large quantities of autologous blood, is widely used in cardiovascular and general surgery and is acceptable to many Jehovah’s Witness patients.244 Employment of cell salvage diminishes the need for allogeneic transfusion and postoperative anemia after cesarean delivery.245
Contamination of salvaged blood with bacteria, fat, bowel contents, or various pharmacologic agents represents a relative contraindication to the technique.246 In the past, the use of intraoperative blood salvage in obstetric patients has been limited, in part, by concern that blood processing may not adequately remove amniotic fluid, fetal debris, or fetal cells and that reinfusion might precipitate amniotic fluid embolism. However, modern salvaging processes efficiently remove these contaminants.246,247 In vitro studies demonstrate that washing and filtration remove tissue factor, which is implicated in the pathophysiology of amniotic fluid embolism, from salvaged products.248 In addition, combining these processes with the use of a leukocyte-depletion filter greatly reduces fetal squames and other fetal debris.249,250 Waters et al.250 demonstrated that fetal squamous cell concentration in postfiltration scavenged blood is equal to that in maternal venous blood. In all cases of potential contamination, a double suction setup is recommended.246,247 In this setup, one suction line, connected to the general suction system, removes grossly contaminated fluid immediately after amniotomy and delivery, before a second suction line delivers blood to the salvaging system.
The cell scavenging system does not distinguish between maternal and fetal red cells, and the transfusion of washed blood is likely to expose the mother to a greater amount of fetal RBCs than commonly occurs during delivery.249,250 In one series, the median fetal red cell transfusion (contamination) volume was 0.8 mL, ranging from 0.2 to 12.9 mL.251 Isoimmunization of the mother is possible, and anti-D immune globulin should be administered as guided by Kleihauer-Betke testing.246,247
Reports of clinical experience with cell salvage in obstetric patients have accumulated over recent years; more than 650 cases have been published describing its use in obstetric patients without adverse sequelae.251–253 Few controlled trials have been published. Rebarber et al.254 performed a retrospective, multicenter study of 139 patients in whom autologous blood transfusion was performed during cesarean delivery between 1988 and 1997. The volume of autotransfused blood ranged from 200 to 11,250 mL. The investigators identified no cases of acute respiratory distress syndrome or amniotic fluid embolism, and the incidence of other complications (e.g., DIC) in these patients was not different from the incidence in 87 control patients who underwent similar surgical procedures without autotransfusion in the same hospitals. In a randomized study of women undergoing cesarean delivery, Rainaldi et al.245 observed that 34 patients who underwent intraoperative salvage and re-infusion of autologous blood (mean ± SD volume, 363 ± 153 mL) required less allogeneic blood and had a shorter hospital stay than the control group. The authors of one case report attributed maternal death to cell salvage-induced amniotic fluid embolism.255 Many authorities do not accept the cause of death as amniotic fluid embolism because the patient was quite ill with severe preeclampsia, HELLP syndrome, and coagulopathy and the postmortem examination was inconclusive concerning the diagnosis of amniotic fluid embolism.
Intraoperative blood salvage during cesarean delivery is widely regarded as safe in the United Kingdom242,247 and is gaining acceptance in the United States. The ACOG has stated that in cases of suspected placenta accreta, “cell saver technology should be considered if available.”75 The ASA Practice Guidelines for Obstetric Anesthesia recommend that “in cases of intractable hemorrhage when banked blood is not available or the patient refuses banked blood, intraoperative cell salvage should be considered if available.”235
Many authorities advocate this technique as a potential solution to the worsening shortage of banked blood, increased cost of allogeneic blood transfusion, and concern about transfusion-related infections and clerical errors.246,247,249 Waters et al.256 performed an economic analysis of the use of cell salvage in obstetrics and calculated that its cost-effectiveness may depend, in part, on an institution’s case volume and the expected volume of blood lost per case. Fong et al.232 have suggested that the use of intraoperative blood salvage might reduce exposure to allogeneic blood in almost half of obstetric patients who require transfusion and might eliminate exposure to allogeneic blood altogether in 14% to 25% of patients.
Treatment of Massive Blood Loss
In the initial resuscitation of the hemorrhaging patient, warmed non–dextrose-containing crystalloid (e.g., lactated Ringer’s solution, normal saline) and/or colloid (e.g., 5% albumin) solutions are acceptable choices for volume replacement. During massive hemorrhage, blood replacement therapy becomes necessary. A coagulopathy can develop rapidly in the bleeding obstetric patient, because of hemostatic factor consumption, exsanguination, dilution, or hyperfibrinolysis. Dilutional coagulopathy results from the replacement of blood loss with crystalloid and PRBCs, which dilutes coagulation factors and platelets. Sustained hemorrhage may also lead to DIC with simultaneous accelerated consumption and fibrinolysis, resulting in worsening hemorrhage (see later discussion). Pregnancy-related pathologic processes commonly associated with DIC include amniotic fluid embolism, placental abruption, uterine infection, intrauterine fetal demise, and severe postpartum hemorrhage.
Blood Products
Allogeneic blood can be transfused as whole blood or component products. Whole blood would be an ideal choice for maintaining intravascular volume in the setting of massive hemorrhage. Alexander et al.257 performed a population-based observational study of 1540 obstetric patients who required transfusion. The authors compared outcomes among patients who received only whole blood (43%), only PRBCs (39%), or multicomponent therapy (19%). Whole blood was associated with lower rates of acute tubular necrosis than the two other transfusion practices. However, few donor units are kept as whole blood in the modern blood bank. The high demand for blood components such as platelets, FFP, and cryoprecipitate necessitates that more than 90% of donor blood be fractionated into blood components. Blood component therapy provides the patient with only those products that are required and helps extend the shelf-life of each unit of donor blood because derivatives from one unit of blood can be used to treat several patients. Characteristics of commonly administered blood products are summarized in Table 38-6. During massive resuscitation, care must be taken to avoid hypothermia, acidosis, and hypocalcemia, because these conditions contribute to coagulopathy.
TABLE 38-6
Characteristics of Blood Components
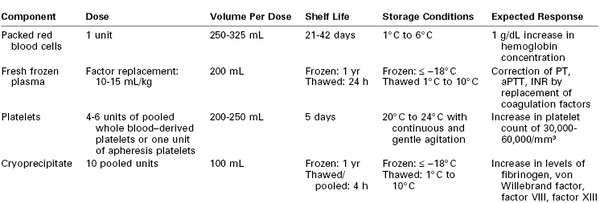
aPTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time.
Modified from Sanford K, Roseff S. A surgeon’s guide to blood banking and transfusion medicine. In: Spiess BD, Spence RK, Shander A, editors. Perioperative Transfusion Medicine, 2nd edition. Philadelphia, Lippincott Williams and Wilkins, 2006: 179-98.
PRBC units are prepared by removing plasma from whole blood and replacing it with additives to improve red cell survival. These units are packaged with preservatives and anticoagulant (citrate, phosphate, dextrose, adenine) and have a 42-day shelf-life. Each unit has volume of approximately 300 mL with a hematocrit of 70%. Transfusion of 1 unit of PRBC increases the hemoglobin concentration by approximately 1 mg/dL in the absence of ongoing bleeding.
A unit of fresh frozen plasma has a volume of approximately 250 mL and contains coagulation factors. Transfusion of FFP is indicated when replacement of coagulation factors is necessary to achieve hemostasis, as may occur during massive transfusion or in the presence of DIC. Administration of FFP may be considered for correction of microvascular bleeding if the prothrombin time (PT) is more than 1.5 times normal, the international normalized ratio (INR) is greater than 2.0, or the activated partial thromboplastin time (aPTT) is more than two times normal.221 FFP should not be used to treat hypovolemia or as a protein supplement. One unit of FFP per 20 kg of body weight (or 10 to 15 mL/kg body weight) is an appropriate initial dose.221 The prophylactic use of FFP is not effective for decreasing blood loss in patients at risk for massive blood loss.222
Cryoprecipitate is prepared from thawed FFP and contains fibrinogen, factor VIII, von Willebrand factor, fibronectin, and factor XIII. In the setting of postpartum hemorrhage, cryoprecipitate is used to replace fibrinogen, which is rapidly consumed during obstetric hemorrhage. Normal pregnancy is a hypercoagulable state,258,259 and coagulation activity peaks at the time of parturition260,261 because of an increase in circulating tissue factor concentration and enhancement of the tissue factor–dependent coagulation pathway.262 It is postulated that tissue factor and other procoagulant substances are released from the placental implantation site at the time of placental separation, augmenting thrombin formation and serving an important hemostatic function after delivery.48 In the setting of continued bleeding from the placental bed, these thromboplastic substances may continue to enter the circulation and, in severe cases, may lead to a consumptive coagulopathy and DIC.48
The consumption of fibrinogen appears to play a central role in the pathophysiology of peripartum hemorrhage.263 Charbit et al.188 prospectively identified 128 patients who had postpartum hemorrhage and classified the hemorrhage as severe (decrease in hemoglobin concentration of ≥ 4 g/dL, transfusion of ≥ 4 units PRBCs, or invasive hemostatic intervention required) or nonsevere.188 At the time postpartum hemorrhage was diagnosed, patients who subsequently developed severe hemorrhage had lower fibrinogen, prothrombin, factor V, and antithrombin levels compared with patients without severe hemorrhage. These early differences were most marked for fibrinogen. A fibrinogen concentration less than 200 mg/dL at the time hemorrhage was diagnosed had a 100% positive predictive value for severe hemorrhage; a fibrinogen concentration greater than 400 mg/dL had a 79% negative predictive value for severe hemorrhage. The coagulation changes were consistent with a consumptive coagulopathy because they were accompanied by increases in thrombin-antithrombin complexes and D-dimer levels, both markers of excessive coagulation. Furthermore, because the fall in fibrinogen concentration was twice the fall in hemoglobin concentration, it was believed that dilution did not account for the difference in fibrinogen levels between the two groups. Other investigators have confirmed that decreases in fibrinogen correlate better than other hemostatic measures with the severity of hemorrhage.264,265
The alarmingly rapid consumption of coagulation factors, especially fibrinogen, during obstetric hemorrhage has prompted experts to recommend early monitoring for, and aggressive treatment of, hypofibrinogenemia with rapid central laboratory or point-of-care testing.266–268 During active hemorrhage, clinicians should attempt to maintain the fibrinogen concentration higher than 150 to 200 mg/dL.268 Each dose of cryoprecipitate supplied by most blood banks contains 5 to 10 single-donor units of cryoprecipitate, and each unit of cryoprecipitate contains approximately twice the fibrinogen of 1 unit of FFP. Therefore, the most efficient method to replace fibrinogen during obstetric hemorrhage may be to administer cryoprecipitate.269 In a multicenter prospective cohort study of trauma victims (n = 1175), administration of cryoprecipitate compared with large doses of FFP was associated with a decreased risk for multiorgan failure.270 In a small case series of obstetric hemorrhage associated with hypofibrinogenemia, fibrinogen concentrate was used to restore fibrinogen levels.271 This product is currently approved for the treatment of acute bleeding in individuals with congenital hypofibrinogenemia. Further study is required to clarify its role in the treatment of the acquired hypofibrinogenemia associated with obstetric hemorrhage.263,270
Thrombocytopenia may develop after massive transfusion secondary to dilution or in association with obstetric comorbidities such as HELLP syndrome. Platelet transfusion may be necessary if hemorrhage is accompanied by a platelet count less than 50,000/mm3.221 In nonbleeding patients, a transfusion trigger of 20,000/mm3 has traditionally been suggested, although many clinicians prefer to administer platelets before the platelet count decreases to this value.272 Platelet dysfunction associated with bleeding may also necessitate platelet administration.221 One unit of donor platelets increases the platelet count by 5000 to 10,000/mm3 in the average adult. The blood bank typically provides pooled random-donor platelets or single-donor apheresis platelets obtained from an ABO- and Rh-compatible donor, although ABO compatibility is not essential. One unit of apheresis platelets is equivalent to 4 to 6 units of pooled platelets.
The optimal FFP : PRBC transfusion ratio remains a topic of research and debate. Borgman et al.273 sought to characterize the relationship between this ratio and survival in a retrospective review of combat victims in Iraq between 2003 and 2005. The investigators identified 246 patients who required massive transfusion and separated them into low (median ratio 1 : 8), medium (median ratio 1 : 2.5), and high (median ratio 1 : 1.4) FFP : PRBC ratio groups. A high FFP : PRBC ratio was independently associated with increased odds of survival after correcting for confounders (odds ratio, 8.6; 95%, CI 2.1 to 35.2). After publication of these data, some experts recommended a 1 : 1 FFP : PRBC ratio during massive hemorrhage. However, many authors,274 including Borgman et al.,273 pointed out the well-known limitations of retrospective data, which include the potential existence of unidentified confounders. These authors have articulated the fact that deaths in the low-ratio group occurred much earlier than deaths in the high-ratio group, raising the possibility that those in the low-ratio group had more severe injuries and died before FFP could be thawed and administered (e.g., survivor bias). Indeed, if the analysis is adjusted for survivor bias, the survival benefit associated with high FFP : PRBC ratio disappears.274,275 Furthermore, a study in civilian trauma victims identified a FFP : PRBC ratio of 1 : 2 to 1 : 3 as optimal for survival.276 However, the extrapolation of data from young male trauma victims to bleeding parturients seems fraught with potential for error.
A 2013 publication described the retrospective review of records from 142 women who had postpartum hemorrhage and required transfusion within 6 hours of delivery.277 Patients were divided into two groups based on their response to sulprostone therapy: those in whom bleeding was controlled with sulprostone alone and those who required advanced interventional procedures. Propensity score analysis revealed that a high FFP : PRBC ratio (> 1 : 2) was associated with fewer requirements for interventional procedures. Retrospective studies such as these can only demonstrate an association between the FFP : PRBC ratio and outcome and cannot infer cause and effect. Investigators have uniformly called for high-quality randomized trials to adequately define the optimal FFP : PRBC ratio during resuscitation for massive hemorrhage.273,275–278
Many blood banks have in place massive transfusion protocols whereby blood products are delivered to the operating room in fixed ratios, sometimes in a cooler or refrigerator.279 Such protocols allow the blood bank to more quickly provide component products280 and may encourage clinicians to more effectively prevent and/or treat coagulopathy. A massive transfusion protocol for postpartum hemorrhage has been described (Figure 38-5).281
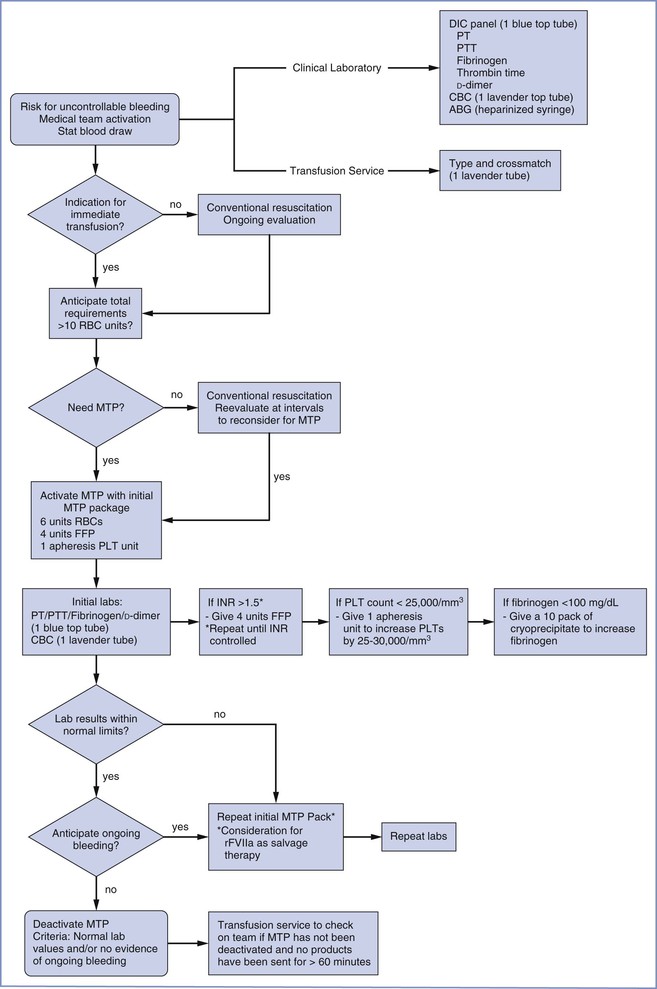
FIGURE 38-5 Sample algorithm for a massive transfusion protocol for the labor and delivery unit. ABG, arterial blood gas; CBC, complete blood cell count; DIC, disseminated intravascular coagulation; FFP, fresh frozen plasma; INR, international normalized ratio; MTP, massive transfusion protocol; PLT, platelet; PT, prothrombin time; RBC, red blood cells; rFVIIA, recombinant activated factor VII. (Modified from Burtelow M, Riley E, Druzin M, et al. How we treat: management of life-threatening primary postpartum hemorrhage with a standardized massive transfusion protocol. Transfusion 2007; 47:1564-72.)
Recombinant Activated Factor VII
A number of reports have described the administration of recombinant activated factor VII (rFVIIa) for hemorrhage and coagulopathy unresponsive to conventional blood product resuscitation.282 Factor VIIa binds not only to tissue factor but also with low affinity to the thrombin-activated platelet. This low-affinity binding to the activated platelet allows direct activation of factor X to Xa on the surface of the activated platelet, bypassing the normal need for factors VIIIa and IXa. Activation of factor X leads to a small thrombin burst. This thrombin, in turn, activates more platelets. Additionally, rFVIIa enhances platelet aggregation and adhesion. Recombinant activated factor VII is currently approved in the United States for the treatment and prophylaxis of bleeding in patients with hemophilia A and B with inhibitors to factors VIII and IX, acquired hemophilia, and congenital factor VII deficiency. However, its use has been reported off-label for multiple clinical scenarios, including the following: platelet disorders; severe liver disease; major trauma; cardiac, prostate, and liver surgery; stroke; and postpartum hemorrhage.236,283 Greater numbers of patients are being treated with rFVIIa each year, and the majority of treated patients are receiving the drug for off-label indications.284
No randomized controlled trials investigating the use of rFVIIa in the setting of obstetric hemorrhage have been published; most recommendations are therefore based on case report or case series data. Franchini et al.285 compiled 9 reports of 272 obstetric patients who received rFVIIa. A 10th report described a registry of 105 patients who received rFVIIa for postpartum hemorrhage in Australia and New Zealand.286 Of the 377 patients included in these 10 reports, 82% were judged by their practitioners to have had a positive treatment response (bleeding decreased or stopped) after one or more doses of rFVIIa. These data are difficult to interpret. Variation in underlying pathology existed; 49% of patients underwent cesarean delivery, and 51% underwent vaginal delivery. Nineteen percent had vaginal or uterine lacerations, 25% had placental abnormalities, and 8% had retained placenta. Additionally, the doses were not standardized and there were no criteria for the administration of rFVIIa, nor were there criteria for what constituted a positive response. Most patients received simultaneous therapy with other traditional therapies such as FFP and cryoprecipitate administration. These circumstances make judgments regarding response to rFVIIa unreliable and uncontrolled; thus, a high likelihood for bias exists. Several authors have called for randomized placebo-controlled trials of rFVIIa use for postpartum hemorrhage, but such a trial will be difficult to undertake given the low incidence and unpredictability of massive obstetric hemorrhage.
There is concern that rFVIIa may increase the likelihood of thromboembolic events. The true incidence is not known. Arterial events (e.g., myocardial infarction, cerebral thrombosis) are more likely than venous events287,288 and are dose287 and age dependent.288 Among the 377 reported cases of rFVIIa administered for postpartum hemorrhage, eight patients developed venous thromboembolism, one suffered myocardial infarction, and one had a thrombotic cerebrovascular accident, suggesting a thrombotic complication frequency of 2.7% in the postpartum period. However, severe obstetric hemorrhage and peripartum hysterectomy are known to increase the risk for thrombotic complications; thus, this frequency cannot support or refute any causal relationship with rFVIIa. Leighton et al.289 advise against rFVIIa administration in the setting of amniotic fluid embolism because tissue factor may play a role in its pathophysiology and thrombotic complications may be increased.
Given the unknown efficacy and safety of rFVIIa, as well as its high cost, rFVIIa is not recommended for routine use in obstetric practice. In 2005, an American panel, based on type II evidence (obtained from well-designed, nonrandomized trials or cohort or case-control analytic trials), judged the use of rFVIIa for treatment of postpartum hemorrhage as appropriate only when bleeding continued despite clotting factor replacement.290 The ASA guidelines recommend consideration of rFVIIa therapy if “traditional well-tested options for treating microvascular bleeding (i.e., coagulopathy) have been exhausted.”221 In addition, maintenance of normothermia and correction of acidosis are necessary for optimal rFVIIa activity; optimization of platelet count, fibrinogen, and serum calcium are also advisable as rFVIIa requires these substances to be effective.
Antifibrinolytic Therapy
It has been suggested that antifibrinolytic therapies such as tranexamic acid may be useful in treating postpartum hemorrhage–associated coagulopathy.291 A meta-analysis of six randomized trials comparing tranexamic acid with placebo administered shortly after birth demonstrated that tranexamic acid was associated with a nonsignificant reduction in blood loss of 33 mL.292 In five of the six trials, tranexamic acid was administered prophylactically. The sixth trial enrolled women who had lost at least 800 mL after vaginal delivery; tranexamic acid reduced the total blood loss over the subsequent 6 hours by 50 mL (170 mL versus 221 mL, P = .04).293 A large international placebo-controlled trial of tranexamic acid therapy in the setting of postpartum hemorrhage is ongoing.294 Tranexamic acid may be most beneficial for women who demonstrate hyperfibrinolysis based on hemostatic monitoring such as thromboelastography.263,268
References
1. AbouZahr C. Global burden of maternal death and disability. Br Med Bull. 2003;67:1–11.
2. Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074.
3. Mhyre JM. Maternal mortality. Curr Opin Anaesthesiol. 2012;25:277–285.
4. Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–1309.
5. Norman J. Haemorrhage. Lewis G. Centre for Maternal and Child Enquiries (CMACE): Saving Mothers’ Lives: Reviewing Maternal Deaths to Make Motherhood Safer—2006-2008 The Eighth Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. CMACE: London; 2011:73–78.
6. Karpati PC, Rossignol M, Pirot M, et al. High incidence of myocardial ischemia during postpartum hemorrhage. Anesthesiology. 2004;100:30–36.
7. Crozier TM, Wallace EM. Obstetric admissions to an integrated general intensive care unit in a quaternary maternity facility. Aust N Z J Obstet Gynaecol. 2011;51:233–238.
8. James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106:509–516.
9. O’Brien D, Babiker E, O’Sullivan O, et al. Prediction of peripartum hysterectomy and end organ dysfunction in major obstetric haemorrhage. Eur J Obstet Gynecol Reprod Biol. 2010;153:165–169.
10. Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol. 2010;202:353 e1–6.
11. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110:1368–1373.
12. Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55.
13. Roberts CL, Ford JB, Algert CS, et al. Trends in adverse maternal outcomes during childbirth: a population-based study of severe maternal morbidity. BMC Pregnancy Childbirth. 2009;9:7.
14. Kuklina EV, Meikle SF, Jamieson DJ, et al. Severe obstetric morbidity in the United States: 1998-2005. Obstet Gynecol. 2009;113:293–299.
15. Bateman BT, Mhyre JM, Callaghan WM, Kuklina EV. Peripartum hysterectomy in the United States: nationwide 14 year experience. Am J Obstet Gynecol. 2012;206:63.e1–63.e8.
16. Berg CJ, Harper MA, Atkinson SM, et al. Preventability of pregnancy-related deaths—results of a state-wide review. Obstet Gynecol. 2005;106:1228–1234.
17. Della Torre M, Kilpatrick SJ, Hibbard JU, et al. Assessing preventability for obstetric hemorrhage. Am J Perinatol. 2011;28:753–759.
18. Toledo P, McCarthy RJ, Hewlett BJ, et al. The accuracy of blood loss estimation after simulated vaginal delivery. Anesth Analg. 2007;105:1736–1740.
19. Smits LJ, North RA, Kenny LC, et al. Patterns of vaginal bleeding during the first 20 weeks of pregnancy and risk of pre-eclampsia in nulliparous women: results from the SCOPE study. Acta Obstet Gynecol Scand. 2012;91:1331–1338.
20. Crane JM, van den Hof MC, Dodds L, et al. Neonatal outcomes with placenta previa. Obstet Gynecol. 1999;93:541–544.
21. Ananth CV, Wilcox AJ. Placental abruption and perinatal mortality in the United States. Am J Epidemiol. 2001;153:332–337.
22. Tikkanen M, Luukkaala T, Gissler M, et al. Decreasing perinatal mortality in placental abruption. Acta Obstet Gynecol Scand. 2013;92:298–305.
23. Faiz AS, Ananth CV. Etiology and risk factors for placenta previa: an overview and meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2003;13:175–190.
24. Iyasu S, Saftlas AK, Rowley DL, et al. The epidemiology of placenta previa in the United States, 1979 through 1987. Am J Obstet Gynecol. 1993;168:1424–1429.
25. Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226–1232.
26. Vergani P, Ornaghi S, Pozzi I, et al. Placenta previa: distance to internal os and mode of delivery. Am J Obstet Gynecol. 2009;201:266 e1–5.
27. Oppenheimer LW, Farine D. A new classification of placenta previa: measuring progress in obstetrics. Am J Obstet Gynecol. 2009;201:227–229.
28. Rao KP, Belogolovkin V, Yankowitz J, Spinnato JA 2nd. Abnormal placentation: evidence-based diagnosis and management of placenta previa, placenta accreta, and vasa previa. Obstet Gynecol Surv. 2012;67:503–519.
29. Wing DA, Paul RH, Millar LK. Management of the symptomatic placenta previa: a randomized, controlled trial of inpatient versus outpatient expectant management. Am J Obstet Gynecol. 1996;175:806–811.
30. Sharma A, Suri V, Gupta I. Tocolytic therapy in conservative management of symptomatic placenta previa. Int J Gynaecol Obstet. 2004;84:109–113.
31. McShane PM, Heyl PS, Epstein MF. Maternal and perinatal morbidity resulting from placenta previa. Obstet Gynecol. 1985;65:176–182.
32. Dommisse J. Placenta praevia and intra-uterine growth retardation. S Afr Med J. 1985;67:291–292.
33. Downes K, Shulman I. Pretransfusion testing. Roback J, Grossman B, Harris T, Hillyer C. Technical Manual. 17th edition. American Association of Blood Banks: Bethesda, MD; 2011:441–442.
34. CFR, Title 21: Food and Drugs. § 606.151(b): Current good manufacturing practice for blood and blood components.
35. Bonner SM, Haynes SR, Ryall D. The anaesthetic management of Caesarean section for placenta praevia: a questionnaire survey. Anaesthesia. 1995;50:992–994.
36. Hong JY, Jee YS, Yoon HJ, Kim SM. Comparison of general and epidural anesthesia in elective cesarean section for placenta previa totalis: maternal hemodynamics, blood loss and neonatal outcome. Int J Obstet Anesth. 2003;12:12–16.
37. Downing JW, Buley RJ, Brock-Utne JG, Houlton PC. Etomidate for induction of anaesthesia at caesarean section: comparison with thiopentone. Br J Anaesth. 1979;51:135–140.
38. Jabre P, Combes X, Lapostolle F, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374:293–300.
39. Shin YK, Kim YD, Collea JV. The effect of propofol on isolated human pregnant uterine muscle. Anesthesiology. 1998;89:105–109.
40. Thind AS, Turner RJ. In vitro effects of propofol on gravid human myometrium. Anaesth Intensive Care. 2008;36:802–806.
41. Oyelese Y, Ananth CV. Placental abruption. Obstet Gynecol. 2006;108:1005–1016.
42. Tikkanen M, Nuutila M, Hiilesmaa V, et al. Clinical presentation and risk factors of placental abruption. Acta Obstet Gynecol Scand. 2006;85:700–705.
43. Ananth CV, Oyelese Y, Srinivas N, et al. Preterm premature rupture of membranes, intrauterine infection, and oligohydramnios: risk factors for placental abruption. Obstet Gynecol. 2004;104:71–77.
44. Ananth CV, Oyelese Y, Yeo L, et al. Placental abruption in the United States, 1979 through 2001: temporal trends and potential determinants. Am J Obstet Gynecol. 2005;192:191–198.
45. Getahun D, Ananth CV, Peltier MR, et al. Acute and chronic respiratory diseases in pregnancy: associations with placental abruption. Am J Obstet Gynecol. 2006;195:1180–1184.
46. Glantz C, Purnell L. Clinical utility of sonography in the diagnosis and treatment of placental abruption. J Ultrasound Med. 2002;21:837–840.
47. Saftlas AF, Olson DR, Atrash HK, et al. National trends in the incidence of abruptio placentae, 1979-1987. Obstet Gynecol. 1991;78:1081–1086.
48. Thachil J, Toh CH. Disseminated intravascular coagulation in obstetric disorders and its acute haematological management. Blood Rev. 2009;23:167–176.
49. Ananth CV, Vintzileos AM. Ischemic placental disease: epidemiology and risk factors. Eur J Obstet Gynecol Reprod Biol. 2011;159:77–82.
50. Naeye RL, Harkness WL, Utts J. Abruptio placentae and perinatal death: a prospective study. Am J Obstet Gynecol. 1977;128:740–746.
52. Oyelese Y, Smulian JC. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol. 2006;107:927–941.
53. Mercier FJ, Van de Velde M. Major obstetric hemorrhage. Anesthesiol Clin. 2008;26:53–66.
54. Kaczmarczyk M, Sparen P, Terry P, Cnattingius S. Risk factors for uterine rupture and neonatal consequences of uterine rupture: a population-based study of successive pregnancies in Sweden. BJOG. 2007;114:1208–1214.
55. Walsh CA, Baxi LV. Rupture of the primigravid uterus: a review of the literature. Obstet Gynecol Surv. 2007;62:327–334.
56. Plauché WC, Von Almen W, Muller R. Catastrophic uterine rupture. Obstet Gynecol. 1984;64:792–797.
57. Walsh CA, O’Sullivan RJ, Foley ME. Unexplained prelabor uterine rupture in a term primigravida. Obstet Gynecol. 2006;108:725–727.
58. Porreco RP, Clark SL, Belfort MA, et al. The changing specter of uterine rupture. Am J Obstet Gynecol. 2009;200:269.e1–269.e4.
59. Lydon-Rochelle M, Holt VL, Easterling TR, Martin DP. Risk of uterine rupture during labor among women with a prior cesarean delivery. N Engl J Med. 2001;345:3–8.
60. Zwart JJ, Richters JM, Ory F, et al. Uterine rupture in The Netherlands: a nationwide population-based cohort study. BJOG. 2009;116:1069–1078.
61. Bujold E, Gauthier RJ. Neonatal morbidity associated with uterine rupture: what are the risk factors? Am J Obstet Gynecol. 2002;186:311–314.
62. Rowbottom SJ, Critchley LA, Gin T. Uterine rupture and epidural analgesia during trial of labour. Anaesthesia. 1997;52:486–488.
63. Oyelese Y, Catanzarite V, Prefumo F, et al. Vasa previa: the impact of prenatal diagnosis on outcomes. Obstet Gynecol. 2004;103:937–942.
64. Robinson BK, Grobman WA. Effectiveness of timing strategies for delivery of individuals with vasa previa. Obstet Gynecol. 2011;117:542–549.
65. Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22:999–1012.
66. Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Effects of onset of labor and mode of delivery on severe postpartum hemorrhage. Am J Obstet Gynecol. 2009;201:273.e1–273.e9.
67. Rossen J, Okland I, Nilsen OB, Eggebo TM. Is there an increase of postpartum hemorrhage, and is severe hemorrhage associated with more frequent use of obstetric interventions? Acta Obstet Gynecol Scand. 2010;89:1248–1255.
68. Grotegut CA, Paglia MJ, Johnson LN, et al. Oxytocin exposure during labor among women with postpartum hemorrhage secondary to uterine atony. Am J Obstet Gynecol. 2011;204:56.e1–56.e6.
69. Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25:1175–1182.
70. Bhattacharya S, Campbell DM, Liston WA. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health. 2007;7:168.
71. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–490.
72. Smulian JC, Ananth CV, Kinzler WL, et al. Twin deliveries in the United States over three decades: an age-period-cohort analysis. Obstet Gynecol. 2004;104:278–285.
73. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–1306.
74. Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1–21.
75. American College of Obstetricians and Gynecologists. Postpartum hemorrhage. ACOG Practice Bulletin No. 76. [Washington, DC (Reaffirmed 2011)] Obstet Gynecol. 2006;108:1039–1047.
76. Nelson SH, Suresh MS. Lack of reactivity of uterine arteries from patients with obstetric hemorrhage. Am J Obstet Gynecol. 1992;166:1436–1443.
77. Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2011;(11).
78. Cotter AM, Ness A, Tolosa JE. Prophylactic oxytocin for the third stage of labour (up-to-date 2010). Cochrane Database Syst Rev. 2001;(4).
79. Pinder AJ, Dresner M, Calow C, et al. Haemodynamic changes caused by oxytocin during caesarean section under spinal anaesthesia. Int J Obstet Anesth. 2002;11:156–159.
80. Archer TL, Knape K, Liles D, et al. The hemodynamics of oxytocin and other vasoactive agents during neuraxial anesthesia for cesarean delivery: findings in six cases. Int J Obstet Anesth. 2008;17:247–254.
81. Svanstrom MC, Biber B, Hanes M, et al. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during Caesarean section. Br J Anaesth. 2008;100:683–689.
82. Jonsson M, Hanson U, Lidell C, Norden-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose: a randomised controlled trial. BJOG. 2010;117:76–83.
83. Thomas TA, Cooper GM. Maternal deaths from anaesthesia: an extract from Why Mothers Die 1997-1999, the Confidential Enquiries into Maternal Deaths in the United Kingdom. Br J Anaesth. 2002;89:499–508.
84. Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing Caesarean section. Br J Anaesth. 2007;98:116–119.
85. Sartain JB, Barry JJ, Howat PW, et al. Intravenous oxytocin bolus of 2 units is superior to 5 units during elective Caesarean section. Br J Anaesth. 2008;101:822–826.
86. Langesaeter E, Rosseland LA, Stubhaug A. Hemodynamic effects of oxytocin during cesarean delivery. Int J Gynaecol Obstet. 2006;95:46–47.
87. Bergum D, Lonnee H, Hakli TF. Oxytocin infusion: acute hyponatraemia, seizures and coma. Acta Anaesthesiol Scand. 2009;53:826–827.
88. Carvalho JC, Balki M, Kingdom J, Windrim R. Oxytocin requirements at elective cesarean delivery: a dose-finding study. Obstet Gynecol. 2004;104:1005–1010.
89. Balki M, Ronayne M, Davies S, et al. Minimum oxytocin dose requirement after cesarean delivery for labor arrest. Obstet Gynecol. 2006;107:45–50.
90. George RB, McKeen D, Chaplin AC, McLeod L. Up-down determination of the ED(90) of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing Cesarean delivery. Can J Anaesth. 2010;57:578–582.
91. Munn MB, Owen J, Vincent R, et al. Comparison of two oxytocin regimens to prevent uterine atony at cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2001;98:386–390.
92. Sheehan SR, Montgomery AA, Carey M, et al. Oxytocin bolus versus oxytocin bolus and infusion for control of blood loss at elective caesarean section: double blind, placebo controlled, randomised trial. BMJ. 2011;343:d4661.
93. King KJ, Douglas MJ, Unger W, et al. Five unit bolus oxytocin at cesarean delivery in women at risk of atony: a randomized, double-blind, controlled trial. Anesth Analg. 2010;111:1460–1466.
94. Dyer RA, Reed AR, van Dyk D, et al. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology. 2009;111:753–765.
95. Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2012;(2).
96. Hogerzeil HV, Walker GJ. Instability of (methyl)ergometrine in tropical climates: an overview. Eur J Obstet Gynecol Reprod Biol. 1996;69:25–29.
97. de Groot AN, van Dongen PW, Vree TB, et al. Ergot alkaloids: current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gynaecology. Drugs. 1998;56:523–535.
98. Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database Syst Rev. 2007;(2).
99. Hayashi Y, Ibe T, Kawato H, et al. Postpartum acute myocardial infarction induced by ergonovine administration. Intern Med. 2003;42:983–986.
101. Li Y, Honye J, Takayama T, Saito S. Generalized spasm of the right coronary artery after successful stent implantation provoked by intracoronary administration of ergonovine. Int J Cardiol. 2007;119:251–254.
102. Abouleish E. Postpartum hypertension and convulsion after oxytocic drugs. Anesth Analg. 1976;55:813–815.
103. Lin YH, Seow KM, Hwang JL, Chen HH. Myocardial infarction and mortality caused by methylergonovine. Acta Obstet Gynecol Scand. 2005;84:1022.
104. Casady GN, Moore DC, Bridenbaugh LD. Postpartum hypertension after use of vasoconstrictor and oxytocic drugs: etiology, incidence, complications, and treatment. J Am Med Assoc. 1960;172:1011–1015.
105. Fuchs AR, Husslein P, Sumulong L, Fuchs F. The origin of circulating 13,14-dihydro-15-keto-prostaglandin F2α during delivery. Prostaglandins. 1982;24:715–722.
106. Noort WA, van Bulck B, Vereecken A, et al. Changes in plasma levels of PGF2α and PGI2 metabolites at and after delivery at term. Prostaglandins. 1989;37:3–12.
107. Izumi H, Garfield RE, Morishita F, Shirakawa K. Some mechanical properties of skinned fibres of pregnant human myometrium. Eur J Obstet Gynecol Reprod Biol. 1994;56:55–62.
108. Hayashi RH, Castillo MS, Noah ML. Management of severe postpartum hemorrhage with a prostaglandin F2α analogue. Obstet Gynecol. 1984;63:806–808.
109. Widmer M, Blum J, Hofmeyr GJ, et al. Misoprostol as an adjunct to standard uterotonics for treatment of post-partum haemorrhage: a multicentre, double-blind randomised trial. Lancet. 2010;375:1808–1813.
110. Bigrigg A, Chissell S, Read MD. Use of intra myometrial 15-methyl prostaglandin F2α to control atonic postpartum haemorrhage following vaginal delivery and failure of conventional therapy. Br J Obstet Gynaecol. 1991;98:734–736.
111. Andersen LH, Secher NJ. Pattern of total and regional lung function in subjects with bronchoconstriction induced by 15-methyl PGF2α. Thorax. 1976;31:685–692.
112. O’Leary AM. Severe bronchospasm and hypotension after 15-methyl prostaglandin F2α in atonic postpartum haemorrhage. Int J Obstet Anesth. 1994;3:42–44.
113. Alfirevic Z, Blum J, Walraven G, et al. Prevention of postpartum hemorrhage with misoprostol. Int J Gynaecol Obstet. 2007;99(Suppl 2):S198–S201.
114. Blum J, Alfirevic Z, Walraven G, et al. Treatment of postpartum hemorrhage with misoprostol. Int J Gynaecol Obstet. 2007;99(Suppl 2):S202–S205.
115. Lokugamage AU, Sullivan KR, Niculescu I, et al. A randomized study comparing rectally administered misoprostol versus Syntometrine combined with an oxytocin infusion for the cessation of primary post partum hemorrhage. Acta Obstet Gynecol Scand. 2001;80:835–839.
116. You WB, Zahn CM. Postpartum hemorrhage: abnormally adherent placenta, uterine inversion, and puerperal hematomas. Clin Obstet Gynecol. 2006;49:184–197.
117. Lee NK, Kim S, Lee JW, et al. Postpartum hemorrhage: clinical and radiologic aspects. Eur J Radiol. 2010;74:50–59.
118. Fargeaudou Y, Soyer P, Morel O, et al. Severe primary postpartum hemorrhage due to genital tract laceration after operative vaginal delivery: successful treatment with transcatheter arterial embolization. Eur Radiol. 2009;19:2197–2203.
119. Saleem Z, Rydhstrom H. Vaginal hematoma during parturition: a population-based study. Acta Obstet Gynecol Scand. 2004;83:560–562.
120. Fliegner JR. Postpartum broad ligament haematomas. J Obstet Gynaecol Br Commonw. 1971;78:184–189.
121. Combs CA, Laros RK Jr. Prolonged third stage of labor: morbidity and risk factors. Obstet Gynecol. 1991;77:863–867.
122. Endler M, Grunewald C, Saltvedt S. Epidemiology of retained placenta: oxytocin as an independent risk factor. Obstet Gynecol. 2012;119:801–809.
123. Yoo KY, Lee JC, Yoon MH, et al. The effects of volatile anesthetics on spontaneous contractility of isolated human pregnant uterine muscle: a comparison among sevoflurane, desflurane, isoflurane, and halothane. Anesth Analg. 2006;103:443–447.
124. Caponas G. Glyceryl trinitrate and acute uterine relaxation: a literature review. Anaesth Intensive Care. 2001;29:163–177.
125. Chandraharan E, Arulkumaran S. Acute tocolysis. Curr Opin Obstet Gynecol. 2005;17:151–156.
126. Peng AT, Gorman RS, Shulman SM, et al. Intravenous nitroglycerin for uterine relaxation in the postpartum patient with retained placenta. Anesthesiology. 1989;71:172–173.
127. DeSimone CA, Norris MC, Leighton BL. Intravenous nitroglycerin aids manual extraction of a retained placenta. Anesthesiology. 1990;73:787.
128. Bullarbo M, Tjugum J, Ekerhovd E. Sublingual nitroglycerin for management of retained placenta. Int J Gynaecol Obstet. 2005;91:228–232.
129. Segal S, Csavoy AN, Datta S. Placental tissue enhances uterine relaxation by nitroglycerin. Anesth Analg. 1998;86:304–309.
130. Mirza FG, Gaddipati S. Obstetric emergencies. Semin Perinatol. 2009;33:97–103.
131. Smulian JC, Defulvio JD, Diven L, Terrazas JL. Sonographic findings in acute uterine inversion. J Clin Ultrasound. 2013;41:453–456.
132. Hong RW, Greenfield ML, Polley LS. Nitroglycerin for uterine inversion in the absence of placental fragments. Anesth Analg. 2006;103:511–512.
133. Dufour P, Vinatier D, Puech F. The use of intravenous nitroglycerin for cervico-uterine relaxation: a review of the literature. Arch Gynecol Obstet. 1997;261:1–7.
134. American College of Obstetricians and Gynecologists. Placenta accreta. ACOG Committee Opinion No. 529. [Washington, DC] Obstet Gynecol. 2012;120:207–211.
135. Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326.e1–326.e10.
136. MacDorman MF, Menacker F, Declercq E. Cesarean birth in the United States: epidemiology, trends, and outcomes. Clin Perinatol. 2008;35:293–307.
137. MacDorman M, Declercq E, Menacker F. Recent trends and patterns in cesarean and vaginal birth after cesarean (VBAC) deliveries in the United States. Clin Perinatol. 2011;38:179–192.
138. Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. Natl Vital Stat Rep. 2013;62:1–33.
139. Palacios Jaraquemada JM, Bruno CH. Magnetic resonance imaging in 300 cases of placenta accreta: surgical correlation of new findings. Acta Obstet Gynecol Scand. 2005;84:716–724.
140. Warshak CR, Ramos GA, Eskander R, et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet Gynecol. 2010;115:65–69.
141. Warshak CR, Eskander R, Hull AD, et al. Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of placenta accreta. Obstet Gynecol. 2006;108:573–581.
142. Eller AG, Bennett MA, Sharshiner M, et al. Maternal morbidity in cases of placenta accreta managed by a multidisciplinary care team compared with standard obstetric care. Obstet Gynecol. 2011;117:331–337.
143. Tan CH, Tay KH, Sheah K, et al. Perioperative endovascular internal iliac artery occlusion balloon placement in management of placenta accreta. AJR Am J Roentgenol. 2007;189:1158–1163.
144. Shrivastava V, Nageotte M, Major C, et al. Case-control comparison of cesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters for placenta accreta. Am J Obstet Gynecol. 2007;197:402 e1–5.
145. Angstmann T, Gard G, Harrington T, et al. Surgical management of placenta accreta: a cohort series and suggested approach. Am J Obstet Gynecol. 2010;202:38 e1–9.
146. Ballas J, Hull AD, Saenz C, et al. Preoperative intravascular balloon catheters and surgical outcomes in pregnancies complicated by placenta accreta: a management paradox. Am J Obstet Gynecol. 2012;207.
147. Greenberg JI, Suliman A, Iranpour P, Angle N. Prophylactic balloon occlusion of the internal iliac arteries to treat abnormal placentation: a cautionary case. Am J Obstet Gynecol. 2007;197:470 e1–4.
148. Bishop S, Butler K, Monaghan S, et al. Multiple complications following the use of prophylactic internal iliac artery balloon catheterisation in a patient with placenta percreta. Int J Obstet Anesth. 2011;20:70–73.
150. Dilauro MD, Dason S, Athreya S. Prophylactic balloon occlusion of internal iliac arteries in women with placenta accreta: literature review and analysis. Clin Radiol. 2012;67:515–520.
151. Publications Committee Society of Maternal-Fetal Medicine, Belfort MA. Placenta accreta. Am J Obstet Gynecol. 2010;203:430–439.
152. Kayem G, Davy C, Goffinet F, et al. Conservative versus extirpative management in cases of placenta accreta. Obstet Gynecol. 2004;104:531–536.
153. O’Brien JM, Barton JR, Donaldson ES. The management of placenta percreta: conservative and operative strategies. Am J Obstet Gynecol. 1996;175:1632–1638.
154. Sentilhes L, Ambroselli C, Kayem G, et al. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol. 2010;115:526–534.
155. Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210–214.
156. Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv. 2007;62:540–547.
157. Rath W, Hackethal A, Bohlmann MK. Second-line treatment of postpartum haemorrhage (PPH). Arch Gynecol Obstet. 2012;286:549–561.
158. Doumouchtsis SK, Papageorghiou AT, Vernier C, Arulkumaran S. Management of postpartum hemorrhage by uterine balloon tamponade: prospective evaluation of effectiveness. Acta Obstet Gynecol Scand. 2008;87:849–855.
159. Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139–142.
160. Kawamura A, Kondoh E, Hamanishi J, et al. Cervical clamp with ring forceps to prevent prolapse of an intrauterine balloon in the management of postpartum hemorrhage. J Obstet Gynaecol Res. 2013;39:733–737.
161. Jain V. Placement of a cervical cerclage in combination with an intrauterine balloon catheter to arrest postpartum hemorrhage. Am J Obstet Gynecol. 2011;205:E15–E17.
162. Ajayi OA, Sant M, Ikhena S, Bako A. Uterine rupture complicating sequential curettage and Bakri balloon tamponade to control secondary PPH. BMJ Case Rep. 2013;2013.
163. B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372–375.
164. Kayem G, Kurinczuk JJ, Alfirevic Z, et al. Uterine compression sutures for the management of severe postpartum hemorrhage. Obstet Gynecol. 2011;117:14–20.
165. Eriksson LG, Mulic-Lutvica A, Jangland L, Nyman R. Massive postpartum hemorrhage treated with transcatheter arterial embolization: technical aspects and long-term effects on fertility and menstrual cycle. Acta Radiol. 2007;48:635–642.
166. Porcu G, Roger V, Jacquier A, et al. Uterus and bladder necrosis after uterine artery embolisation for postpartum haemorrhage. BJOG. 2005;112:122–123.
167. Cottier JP, Fignon A, Tranquart F, Herbreteau D. Uterine necrosis after arterial embolization for postpartum hemorrhage. Obstet Gynecol. 2002;100:1074–1077.
168. Rossi AC, Lee RH, Chmait RH. Emergency postpartum hysterectomy for uncontrolled postpartum bleeding: a systematic review. Obstet Gynecol. 2010;115:637–644.
169. Wright JD, Devine P, Shah M, et al. Morbidity and mortality of peripartum hysterectomy. Obstet Gynecol. 2010;115:1187–1193.
170. Chestnut DH, Dewan DM, Redick LF, et al. Anesthetic management for obstetric hysterectomy: a multi-institutional study. Anesthesiology. 1989;70:607–610.
171. Belfort MA, Zimmerman J, Schemmer G, et al. Aortic compression and cross clamping in a case of placenta percreta and amniotic fluid embolism: a case report. Am J Perinatol Rep. 2011;1:33–36.
172. Rich NM, Mattox KL, Hirschberg A. Vascular Trauma. 2nd edition. Elsevier Saunders: Philadelphia; 2004.
173. Georgakis P, Paraskevas KI, Bessias N, et al. Duration of aortic cross-clamping during elective open abdominal aortic aneurysm repair operations and postoperative cardiac/renal function. Int Angiol. 2010;29:244–248.
174. Natour E, Suedkamp M, Dapunt OE. Assessment of the effect on blood loss and transfusion requirements when adding a polyethylene glycol sealant to the anastomotic closure of aortic procedures: a case-control analysis of 102 patients undergoing Bentall procedures. J Cardiothorac Surg. 2012;7:105.
175. Wright JD, Pri-Paz S, Herzog TJ, et al. Predictors of massive blood loss in women with placenta accreta. Am J Obstet Gynecol. 2011;205:38 e1–6.
176. Shibata K, Yamamoto Y, Murakami S. Effects of epidural anesthesia on cardiovascular response and survival in experimental hemorrhagic shock in dogs. Anesthesiology. 1989;71:953–959.
177. Bonnet MP, Deneux-Tharaux C, Bouvier-Colle MH. Critical care and transfusion management in maternal deaths from postpartum haemorrhage. Eur J Obstet Gynecol Reprod Biol. 2011;158:183–188.
178. Bouvier-Colle MH, Ould El Joud D, Varnoux N, et al. Evaluation of the quality of care for severe obstetrical haemorrhage in three French regions. BJOG. 2001;108:898–903.
179. Driessen M, Bouvier-Colle MH, Dupont C, et al. Postpartum hemorrhage resulting from uterine atony after vaginal delivery: factors associated with severity. Obstet Gynecol. 2011;117:21–31.
180. Razvi K, Chua S, Arulkumaran S, Ratnam SS. A comparison between visual estimation and laboratory determination of blood loss during the third stage of labour. Aust N Z J Obstet Gynaecol. 1996;36:152–154.
181. Dildy GA 3rd, Paine AR, George NC, Velasco C. Estimating blood loss: can teaching significantly improve visual estimation? Obstet Gynecol. 2004;104:601–606.
182. Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obstet Gynecol. 2008;199:519 e1–7.
183. Toledo P, McCarthy RJ, Burke CA, et al. The effect of live and web-based education on the accuracy of blood-loss estimation in simulated obstetric scenarios. Am J Obstet Gynecol. 2010;202:400 e1–5.
184. Patel A, Goudar SS, Geller SE, et al. Drape estimation vs. visual assessment for estimating postpartum hemorrhage. Int J Gynaecol Obstet. 2006;93:220–224.
185. Bose P, Regan F, Paterson-Brown S. Improving the accuracy of estimated blood loss at obstetric haemorrhage using clinical reconstructions. BJOG. 2006;113:919–924.
186. Guly HR, Bouamra O, Little R, et al. Testing the validity of the ATLS classification of hypovolaemic shock. Resuscitation. 2010;81:1142–1147.
187. Singh S, McGlennan A, England A, Simons R. A validation study of the CEMACH recommended modified early obstetric warning system (MEOWS). Anaesthesia. 2012;67:12–18.
188. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5:266–273.
189. Pri-Paz S, Devine PC, Miller RS, et al. Cesarean hysterectomy requiring emergent thoracotomy: a case report of a complication of placenta percreta requiring a multidisciplinary effort. J Reprod Med. 2012;57:58–60.
190. California Maternal Quality Care Collorative. OB hemorrhage tool kit. [Available at] http://www.cmqcc.org/ob_hemorrhage [Accessed July 2013] .
191. Rizvi F, Mackey R, Barrett T, et al. Successful reduction of massive postpartum haemorrhage by use of guidelines and staff education. BJOG. 2004;111:495–498.
192. Skupski DW, Lowenwirt IP, Weinbaum FI, et al. Improving hospital systems for the care of women with major obstetric hemorrhage. Obstet Gynecol. 2006;107:977–983.
193. Shields LE, Smalarz K, Reffigee L, et al. Comprehensive maternal hemorrhage protocols improve patient safety and reduce utilization of blood products. Am J Obstet Gynecol. 2011;205:368 e1–8.
194. Grunebaum A, Chervenak F, Skupski D. Effect of a comprehensive obstetric patient safety program on compensation payments and sentinel events. Am J Obstet Gynecol. 2011;204:97–105.
196. Hamman WR, Beaudin-Seiler BM, Beaubien JM, et al. Using in situ simulation to identify and resolve latent environmental threats to patient safety: case study involving operational changes in a labor and delivery ward. Qual Manag Health Care. 2010;19:226–230.
197. The Joint Commission. Sentinel Event Alert, Issue 44: Preventing Maternal Death. [Available at] http://www.jointcommission.org/sentinel_event_alert_issue_44_preventing_maternal_death/ [Accessed June 2013] .
198. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58.
199. Viele MK, Weiskopf RB. What can we learn about the need for transfusion from patients who refuse blood? The experience with Jehovah’s Witnesses. Transfusion. 1994;34:396–401.
200. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–221.
201. Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060.
202. Jansen AJG, Duvekot JJ, Hop WCJ, et al. New insights into fatigue and health-related quality of life after delivery. Acta Obstet Gynecol Scand. 2007;86:579–584.
203. Palo R, Ahonen J, Salo H, et al. Transfusion of red blood cells: no impact on length of hospital stay in moderately anaemic parturients. Acta Anaesthesiol Scand. 2007;51:565–569.
204. Jonsson K, Jensen JA, Goodson WH 3rd, et al. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg. 1991;214:605–613.
205. Li G, Rachmale S, Kojicic M, et al. Incidence and transfusion risk factors for transfusion-associated circulatory overload among medical intensive care unit patients. Transfusion. 2011;51:338–343.
206. Popovsky MA, Audet AM, Andrzejewski C Jr. Transfusion-associated circulatory overload in orthopedic surgery patients: a multi-institutional study. Immunohematology. 1996;12:87–89.
207. Bierbaum BE, Callaghan JJ, Galante JO, et al. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81:2–10.
208. Popovsky MA. Transfusion-associated circulatory overload: the plot thickens. Transfusion. 2009;49:2–4.
209. Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–1789.
210. Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology. 2011;115:635–649.
211. Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–1767.
212. Raghavan M, Marik PE. Anemia, allogenic blood transfusion, and immunomodulation in the critically ill. Chest. 2005;127:295–307.
213. Cerhan JR, Wallace RB, Dick F, et al. Blood transfusions and risk of non-Hodgkin’s lymphoma subtypes and chronic lymphocytic leukemia. Cancer Epidemiol Biomarkers Prev. 2001;10:361–368.
214. Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14:671–676.
215. Dodd RY, Notari EPt, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. 2002;42:975–979.
216. Llewelyn CA, Hewitt PE, Knight RS, et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421.
217. Laupacis A, Brown J, Costello B, et al. Prevention of posttransfusion CMV in the era of universal WBC reduction: a consensus statement. Transfusion. 2001;41:560–569.
218. Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103:2003–2008.
219. Blajchman MA, Goldman M, Freedman JJ, Sher GD. Proceedings of a consensus conference: prevention of post-transfusion CMV in the era of universal leukoreduction. Transfus Med Rev. 2001;15:1–20.
220. Eder AF, Kennedy JM, Dy BA, et al. Bacterial screening of apheresis platelets and the residual risk of septic transfusion reactions: the American Red Cross experience (2004-2006). Transfusion. 2007;47:1134–1142.
221. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105:198–208.
222. O’Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11–28.
223. Santoso JT, Saunders BA, Grosshart K. Massive blood loss and transfusion in obstetrics and gynecology. Obstet Gynecol Surv. 2005;60:827–837.
224. Carson JL, Carless PA, Hebert PC. Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA. 2013;309:83–84.
225. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417.
226. Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559–1567.
227. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462.
228. Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;(4).
229. Jansen AJ, van Rhenen DJ, Steegers EA, Duvekot JJ. Postpartum hemorrhage and transfusion of blood and blood components. Obstet Gynecol Surv. 2005;60:663–671.
230. Butwick AJ, Aleshi P, Fontaine M, et al. Retrospective analysis of transfusion outcomes in pregnant patients at a tertiary obstetric center. Int J Obstet Anesth. 2009;18:302–308.
231. Parker J, Thompson J, Stanworth S. A retrospective one-year single-centre survey of obstetric red cell transfusions. Int J Obstet Anesth. 2009;18:309–313.
232. Fong J, Gurewitsch ED, Kang HJ, et al. An analysis of transfusion practice and the role of intraoperative red blood cell salvage during cesarean delivery. Anesth Analg. 2007;104:666–672.
233. Silverman JA, Barrett J, Callum JL. The appropriateness of red blood cell transfusions in the peripartum patient. Obstet Gynecol. 2004;104:1000–1004.
234. Ransom SB, Fundaro G, Dombrowski MP. Cost-effectiveness of routine blood type and screen testing for cesarean section. J Reprod Med. 1999;44:592–594.
235. American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia. Anesthesiology. 2007;106:843–863.
236. Goodnough LT, Shander A. Current status of pharmacologic therapies in patient blood management. Anesth Analg. 2013;116:15–34.
237. Van Wyck DB, Mangione A, Morrison J, et al. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49:2719–2728.
238. Van Wyck DB, Martens MG, Seid MH, et al. Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia: a randomized controlled trial. Obstet Gynecol. 2007;110:267–278.
239. Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmen J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378–382.
240. Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–924.
241. Andres RL, Piacquadio KM, Resnik R. A reappraisal of the need for autologous blood donation in the obstetric patient. Am J Obstet Gynecol. 1990;163:1551–1553.
242. Catling S. Blood conservation techniques in obstetrics: a UK perspective. Int J Obstet Anesth. 2007;16:241–249.
243. Bryson GL, Laupacis A, Wells GA. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta-analysis: the International Study of Perioperative Transfusion. Anesth Analg. 1998;86:9–15.
245. Rainaldi MP, Tazzari PL, Scagliarini G, et al. Blood salvage during caesarean section. Br J Anaesth. 1998;80:195–198.
246. Esper SA, Waters JH. Intra-operative cell salvage: a fresh look at the indications and contraindications. Blood Transfus. 2011;9:139–147.
247. Allam J, Cox M, Yentis SM. Cell salvage in obstetrics. Int J Obstet Anesth. 2008;17:37–45.
248. Bernstein HH, Rosenblatt MA, Gettes M, Lockwood C. The ability of the Haemonetics 4 Cell Saver System to remove tissue factor from blood contaminated with amniotic fluid. Anesth Analg. 1997;85:831–833.
249. Catling SJ, Williams S, Fielding AM. Cell salvage in obstetrics: an evaluation of the ability of cell salvage combined with leucocyte depletion filtration to remove amniotic fluid from operative blood loss at caesarean section. Int J Obstet Anesth. 1999;8:79–84.
250. Waters JH, Biscotti C, Potter PS, Phillipson E. Amniotic fluid removal during cell salvage in the cesarean section patient. Anesthesiology. 2000;92:1531–1536.
251. Ralph CJ, Sullivan I, Faulds J. Intraoperative cell salvaged blood as part of a blood conservation strategy in Caesarean section: is fetal red cell contamination important? Br J Anaesth. 2011;107:404–408.
252. Liumbruno GM, Meschini A, Liumbruno C, Rafanelli D. The introduction of intra-operative cell salvage in obstetric clinical practice: a review of the available evidence. Eur J Obstet Gynecol Reprod Biol. 2011;159:19–25.
253. Brearton C, Bhalla A, Mallaiah S, Barclay P. The economic benefits of cell salvage in obstetric haemorrhage. Int J Obstet Anesth. 2012;21:329–333.
254. Rebarber A, Lonser R, Jackson S, et al. The safety of intraoperative autologous blood collection and autotransfusion during cesarean section. Am J Obstet Gynecol. 1998;179:715–720.
255. Oei SG, Wingen CBM, Kerkkamp HEM. Cell salvage: how safe in obstetrics? Int J Obstet Anesth. 2000;9:143–144.
256. Waters JR, Meier HH, Waters JH. An economic analysis of costs associated with development of a cell salvage program. Anesth Analg. 2007;104:869–875.
257. Alexander JM, Sarode R, McIntire DD, et al. Whole blood in the management of hypovolemia due to obstetric hemorrhage. Obstet Gynecol. 2009;113:1320–1326.
258. Cerneca F, Ricci G, Simeone R, et al. Coagulation and fibrinolysis changes in normal pregnancy: increased levels of procoagulants and reduced levels of inhibitors during pregnancy induce a hypercoagulable state, combined with a reactive fibrinolysis. Eur J Obstet Gynecol Reprod Biol. 1997;73:31–36.
259. Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114:409–414.
260. Andersson T, Lorentzen B, Hogdahl H, et al. Thrombin-inhibitor complexes in the blood during and after delivery. Thromb Res. 1996;82:109–117.
261. Epiney M, Boehlen F, Boulvain M, et al. D-Dimer levels during delivery and the postpartum. J Thromb Haemost. 2005;3:268–271.
262. Boer K, den Hollander IA, Meijers JC, Levi M. Tissue factor–dependent blood coagulation is enhanced following delivery irrespective of the mode of delivery. J Thromb Haemost. 2007;5:2415–2420.
263. Butwick AJ. Postpartum hemorrhage and low fibrinogen levels: the past, present and future. Int J Obstet Anesth. 2013;22:87–91.
264. Cortet M, Deneux-Tharaux C, Dupont C, et al. Association between fibrinogen level and severity of postpartum haemorrhage: secondary analysis of a prospective trial. Br J Anaesth. 2012;108:984–989.
265. de Lloyd L, Bovington R, Kaye A, et al. Standard haemostatic tests following major obstetric haemorrhage. Int J Obstet Anesth. 2011;20:135–141.
266. Huissoud C, Carrabin N, Audibert F, et al. Bedside assessment of fibrinogen level in postpartum haemorrhage by thrombelastometry. BJOG. 2009;116:1097–1102.
267. Chandler WL, Ferrell C, Trimble S, Moody S. Development of a rapid emergency hemorrhage panel. Transfusion. 2010;50:2547–2552.
268. Solomon C, Collis RE, Collins PW. Haemostatic monitoring during postpartum haemorrhage and implications for management. Br J Anaesth. 2012;109:851–863.
269. Levy JH, Szlam F, Tanaka KA, Sniecienski RM. Fibrinogen and hemostasis: a primary hemostatic target for the management of acquired bleeding. Anesth Analg. 2012;114:261–274.
270. Watson GA, Sperry JL, Rosengart MR, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67:221–227.
271. Bell SF, Rayment R, Collins PW, Collis RE. The use of fibrinogen concentrate to correct hypofibrinogenaemia rapidly during obstetric haemorrhage. Int J Obstet Anesth. 2010;19:218–223.
272. Beutler E. Platelet transfusions: the 20,000/µL trigger. Blood. 1993;81:1411–1413.
273. Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813.
274. Ho AM, Dion PW, Yeung JH, et al. Prevalence of survivor bias in observational studies on fresh frozen plasma:erythrocyte ratios in trauma requiring massive transfusion. Anesthesiology. 2012;116:716–728.
275. Snyder CW, Weinberg JA, McGwin G Jr, et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66:358–362.
276. Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270.
277. Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg. 2013;116:155–161.
278. Mohan D, Milbrandt EB, Alarcon LH. Black Hawk down: the evolution of resuscitation strategies in massive traumatic hemorrhage. Crit Care. 2008;12:305.
279. Schuster KM, Davis KA, Lui FY, et al. The status of massive transfusion protocols in United States trauma centers: massive transfusion or massive confusion? Transfusion. 2010;50:1545–1551.
280. O’Keeffe T, Refaai M, Tchorz K, et al. A massive transfusion protocol to decrease blood component use and costs. Arch Surg. 2008;143:686–690.
281. Gutierrez MC, Goodnough LT, Druzin M, Butwick AJ. Postpartum hemorrhage treated with a massive transfusion protocol at a tertiary obstetric center: a retrospective study. Int J Obstet Anesth. 2012;21:230–235.
282. Franchini M, Lippi G, Franchi M. The use of recombinant activated factor VII in obstetric and gynaecological haemorrhage. BJOG. 2007;114:8–15.
283. Yank V, Tuohy CV, Logan AC, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med. 2011;154:529–540.
284. Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med. 2011;154:516–522.
285. Franchini M, Franchi M, Bergamini V, et al. The use of recombinant activated FVII in postpartum hemorrhage. Clin Obstet Gynecol. 2010;53:219–227.
286. Phillips LE, McLintock C, Pollock W, et al. Recombinant activated factor VII in obstetric hemorrhage: experiences from the Australian and New Zealand Haemostasis Registry. Anesth Analg. 2009;109:1908–1915.
287. Diringer MN, Skolnick BE, Mayer SA, et al. Risk of thromboembolic events in controlled trials of rFVIIa in spontaneous intracerebral hemorrhage. Stroke. 2008;39:850–856.
288. Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–1800.
289. Leighton BL, Wall MH, Lockhart EM, et al. Use of recombinant factor VIIa in patients with amniotic fluid embolism: a systematic review of case reports. Anesthesiology. 2011;115:1201–1208.
290. Shander A, Goodnough L, Ratko T, et al. Consensus recommendations for the off-label use of recombinant human factor VIIa (NovoSeven®) therapy. Pharmacotherapeutics. 2005;30:644–658.
291. Gülmezoglu AM, Souza JP, Chou D, et al. WHO guidelines for the management of postpartum haemorrhage and retained placenta. [Available at] http://whqlibdoc.who.int/publications/2009/9789241598514_eng.pdf [Accessed June 2013] .
293. Ducloy-Bouthors AS, Jude B, Duhamel A, et al. High-dose tranexamic acid reduces blood loss in postpartum haemorrhage. Crit Care. 2011;15:15.
294. Shakur H, Elbourne D, Gulmezoglu M, et al. The WOMAN Trial (World Maternal Antifibrinolytic Trial): tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind placebo controlled trial. Trials. 2010;11:40.