Anesthesia for Fetal Surgery and Other Intrauterine Procedures
Mark D. Rollins MD, PhD, Mark A. Rosen MD
Chapter Outline
Anesthesia for Minimally Invasive and Percutaneous Procedures
Fetal therapy originated in 1963 with Sir William Liley’s successful intraperitoneal blood transfusion to a fetus with erythroblastosis fetalis.1 This was followed by many years of discouraging attempts to transfuse blood via direct cannulation of fetal vessels through a small uterine incision.2 In 1981, after careful experimentation and practice in sheep3,4 and rhesus monkeys,5 the first successful human fetal surgery, a vesicostomy, was performed in a fetus with bilateral hydronephrosis due to a lower urinary tract obstruction.6
Advances in prenatal diagnostic technology, particularly in the resolution of imaging, contribute to increasing sophistication in diagnosis of fetal disorders, principally anatomic anomalies. Fetal therapy is largely nonsurgical (e.g., administration of medications, nutrients, blood, stem cells) (see Chapter 6). Some identified disorders are amenable to intrauterine fetal surgery, but most anatomic malformations diagnosed in utero remain unsuitable for antenatal intervention. Prenatal diagnosis of serious malformations (e.g., those that are uncorrectable and incompatible with normal postnatal life) allows the choice of pregnancy termination. Most correctable malformations are best managed after delivery at term gestation, but antepartum recognition allows time for the coordination of appropriate prenatal and postnatal care, including transfer of peripartum care to an appropriate medical center while the fetus is in utero rather than as a newly delivered, fragile neonate. Some defects, especially those that cause airway obstruction, can be treated with an intrapartum intervention, in which the fetus undergoes repair of the defect and/or the airway is secured during birth, while the uteroplacental unit remains functional.
Fetal surgery is reasonable only with informed consent and only if (1) the lesion is diagnosed accurately, (2) the lesion’s severity is assessed correctly, (3) the associated anomalies that contraindicate intervention are excluded, (4) the maternal risk is acceptably low, and (5) the neonatal outcome would be better with in utero surgery than with surgery performed after delivery. With all fetal surgical procedures, an emphasis also must be placed on maternal welfare to guard against undue maternal risk.7,8
Fetal surgical interventions can be broadly categorized into three kinds of procedures, namely, open surgical procedures, minimally invasive procedures, and intrapartum procedures. Open surgical procedures involve both maternal laparotomy and hysterotomy with use of pharmacologic agents to maintain uterine relaxation. These procedures are typically performed near mid gestation and entail greater maternal and fetal risks compared with the minimally invasive techniques, including a significant risk for preterm premature rupture of membranes (PROM), preterm labor and delivery, uterine dehiscence, oligohydramnios, hemorrhage, pulmonary edema, and fetal mortality.9,10 In addition, after an open surgical procedure, a cesarean delivery is required for the subsequent delivery and all future deliveries owing to the location of the hysterotomy and the associated risks for uterine dehiscence or rupture. Open fetal surgical procedures have been used to repair fetal myelomeningoceles, resect congenital pulmonary airway abnormalities, and debulk sacrococcygeal teratomas.
The third kind of procedure involves a modification of cesarean delivery to allow intrapartum fetal therapy while the fetus remains supported by placental gas exchange. These delivery techniques are termed EXIT procedures, for ex utero intrapartum therapy.11 EXIT procedures are most often employed (1) to secure the airway by endotracheal intubation, bronchoscopy, or tracheostomy or (2) to perform other fetal procedures while gas exchange continues in the placenta (placental bypass). The EXIT procedure enables the prevention of postnatal asphyxia in newborns with lesions such as cystic hygroma, lymphangioma, cervical teratoma, and congenital syndromes, in whom securing an airway after birth can be problematic. The procedure is also used as a bridge to extracorporeal membrane oxygenation (ECMO) for a fetus with cardiopulmonary disease at risk for postnatal cardiac failure or failure of adequate gas exchange in the lungs.
Fetal surgery is a reasonable therapeutic intervention for certain correctable fetal anomalies with predictable, life-threatening, or serious developmental consequences. If untreated, these lesions can interfere with fetal organ development or result in cardiac failure; if corrected in utero, irreversible organ damage and fetal demise may be prevented.
Indications and Rationale for Fetal Surgery
Bilateral Hydronephrosis–Obstructive Uropathy
Congenital obstructive uropathy occurs in approximately 0.1% of pregnancies.12 Congenital bilateral hydronephrosis results from fetal urethral obstruction at the bladder outlet, most often by posterior urethral valves in male fetuses or urethral obstruction in females. Other causes of fetal obstructive uropathy include obstruction at the ureteropelvic or vesicoureteric junction and a number of complex disorders in females (e.g., cloacal plate anomalies). These uropathies are easily detected by ultrasonography, which is often performed to investigate oligohydramnios from diminished fetal urine output. Severe obstructive lesions may lead to progressive renal dysplasia and dysfunction, bladder distention, and oligohydramnios and ultimately result in devastating developmental consequences, such as limb and facial deformities and pulmonary hypoplasia (Figure 7-1).13 Preterm delivery allows early urinary tract decompression ex utero, but fetal pulmonary immaturity limits the efficacy of this approach. Early intrauterine intervention with placement of a vesicoamniotic shunt allows drainage of urine from the fetal bladder into the amniotic cavity, thereby decompressing the urinary tract. In animal models, in utero relief of obstructive uropathy improves dysplastic renal histology, restores normal urine flow and amniotic fluid volume, and results in improved lung growth and development.14 The applicability of these findings to human fetal obstructive uropathy remains unclear and controversial.15
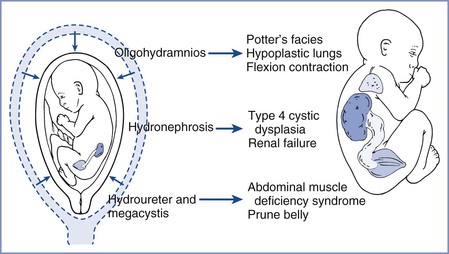
FIGURE 7-1 Developmental consequences of fetal urethral obstruction. Obstructed fetal urinary flow results in hydronephrosis, hydroureter, megacystis, oligohydramnios, and pulmonary hypoplasia. (Redrawn from Harrison MR, Filly RA, Parer JT, et al. Management of the fetus with a urinary tract malformation. JAMA 1981; 246:635-9.)
Vesicoamniotic catheter shunts have been used for intrauterine treatment of bilateral hydronephrosis since the early 1980s.16 These valveless, double-coiled catheters are placed percutaneously with ultrasonographic guidance, with one coil being left in the urinary bladder and the other in the amniotic space. Common problems associated with these catheters include (1) difficult placement, occlusion, and displacement; (2) fetal trauma, iatrogenic abdominal wall defects, and amnioperitoneal leaking; and (3) preterm PROM, preterm labor, and infection.17 Neonatal survival rates after fetal vesicoamniotic shunting (performed from the 1980s to 2001) varied from 50% to 90%, with approximately half of the survivors having normal renal function.15,18,19 Currently a multicenter, randomized controlled trial is underway comparing the perinatal mortality and renal function of fetuses with lower urinary tract obstruction treated by either vesicoamniotic shunting or conservative noninterventional care.20
Fetal cystoscopy is a more recent treatment option that allows direct visualization of the fetal urethra. Although not a viable treatment for urethral atresia, fetal cystoscopy facilitates diagnosis and treatment of lower urinary tract obstruction due to posterior urethral valves.21 Fetal cystoscopy with ablation of posterior urethral valves appears to provide a survival advantage over conservative therapy but has not been demonstrated to improve perinatal survival over vesicoamniotic shunting.22
Current evidence supports fetal surgery for the correction of obstructive uropathy in selected fetuses in an effort to restore amniotic fluid volume, prevent pulmonary hypoplasia, and decrease perinatal mortality. However, the effects on long-term renal function and other morbidities remain unclear, and additional evidence is needed.
Congenital Diaphragmatic Hernia
Approximately 1 of 2500 live newborns has a congenital diaphragmatic hernia (CDH).23 Without fetal intervention, this anomaly causes significant mortality from pulmonary hypoplasia and insufficiency. Survival rates have improved to between 60% and 85% over the past 20 years24–27 and are closely associated with the degree of pulmonary hypertension and dysfunction.24 Significant mortality occurs despite optimal postnatal surgical management at tertiary care medical centers (i.e., procedures involving removal of the herniated viscera from the chest, administration of surfactant, ventilation techniques that minimize lung trauma, use of ECMO, and closure of the diaphragm). Intrauterine correction of CDH has the potential to prevent the development of pulmonary hypoplasia and allow the fetal lung to develop before delivery.
The use of a fetal lamb model demonstrates that parenchymal hypoplasia and associated pulmonary vascular changes can be reversed by correction in utero.3 Primary repairs of human CDH in utero have been undertaken only for fetuses with severe disease, with limited success but many lessons learned, including the development of minimally invasive approaches.28,29
Fetal lungs contribute to amniotic fluid volume by secreting more than 100 mL/kg/day of fluid that exits the trachea and mouth. Tracheal occlusion impedes the normal egress of fetal lung fluid and results in expansion of the hypoplastic lung, thereby inducing lung growth and cellular maturation in fetuses with CDH.30,31 This occlusion technique, termed “plug the lung until it grows” (i.e., PLUG),32,33 replaced primary repair in utero for the correction of the pulmonary hypoplasia associated with CDH. It is a less extensive, palliative fetal surgical procedure that enhances lung growth to improve postnatal survival, with postponement of the definitive repair until after birth.30,34 Once the trachea is occluded, fetal pulmonary fluid slowly accumulates and expands the lung, pushing the viscera out of the thorax. A small detachable balloon for endoluminal tracheal occlusion is placed in the trachea via percutaneous endoscopic endotracheal intubation and is either left in place until delivery or deflated and removed earlier (Figure 7-2).35,36
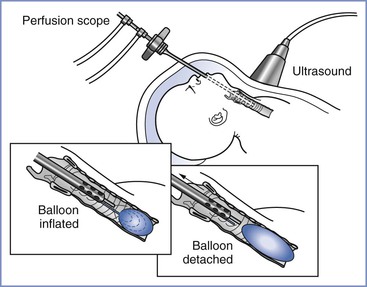
FIGURE 7-2 Schematic of fetal tracheal occlusion using a balloon. (From Harrison MR, Albanese CT, Hawgood SB, et al. Fetoscopic temporary tracheal occlusion by means of detachable balloon for congenital diaphragmatic hernia. Am J Obstet Gynecol 2001; 185:730-3.)
A prospective randomized trial (1999-2001) evaluated fetal tracheal occlusion for intrauterine treatment of severe CDH.37 Inclusion criteria included (1) a gestational age of 22 to 28 weeks, (2) left-sided herniation of the liver into the hemithorax, and (3) a low lung-to-head ratio (LHR) (i.e., < 1.4). The LHR is a ratio of the contralateral lung size compared with head circumference and is correlated with the severity of pulmonary hypoplasia and survival for a given gestational age.38 The trial was closed early (n = 11); fetal tracheal occlusion resulted in no improvement in survival compared with control (77% versus 73%) and no reduction in morbidity at 90 days. The rates of preterm PROM and preterm delivery were higher in the fetal intervention group.37 However, the survival rate was unexpectedly high in the control group. It is possible that the LHR criterion of less than 1.4 was not sufficiently restrictive and allowed inclusion of fetuses in the study that were likely to survive with standard postnatal tertiary medical care. Table 7-1 notes outcomes of left-sided CDH fetuses treated in utero or with standard postnatal care.
TABLE 7-1
Postnatal Survival in Fetuses with Left-Sided Congenital Diaphragmatic Hernia and Intrathoracic Liver Herniation Based on Fetal Lung-to-Head Ratio (LHR)
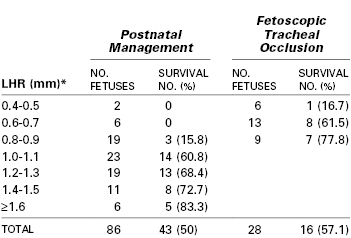
* LHR measurements in the table were obtained at 23 to 29 weeks’ gestation.
Modified from Jani JC, Nicolaides KH, Gratacos E, et al. Fetal lung-to-head ratio in the prediction of survival in severe left-sided diaphragmatic hernia treated by fetal endoscopic tracheal occlusion (FETO). Am J Obstet Gynecol 2006; 195:1646-50.
More recently in Europe, the Fetal Endoscopic Tracheal Occlusion (FETO) Task Force began a collaboration among three medical centers for treatment of severe cases of CDH with a high risk for death.39 FETO intervention criteria for fetuses at high risk included both LHR less than 1.0 and liver herniation into the hemithorax.40 Use of smaller-gauge endoscopes and reversal of the tracheal occlusion before birth appear to show great promise for reduction in the risk for preterm delivery due to preterm PROM.41,42 Owing to concern for tracheal damage by very early tracheal balloon placement,43 the tracheal balloon is placed between 26 and 28 weeks’ gestation and removed before birth by a second fetoscopic procedure near 34 weeks (if the fetus is still in utero).44 This second procedure is performed to minimize the risk of preterm labor, avoid the need for the EXIT procedure, and potentially improve lung growth and minimize the reduction of type II alveolar cells associated with prolonged tracheal occlusion. Outcomes for 210 cases (through 2008) of fetuses with a mean gestational age of 27 weeks, LHR less than 1.0, and primarily left-sided CDH (84%) were compared with those for historic postnatal treatment controls (1995-2004). Use of FETO significantly improved the survival rate (47% versus 20%), and delivery occurred at a median gestational age of 35.3 weeks.39 However, the comparative results may represent selection bias or improvement in technique and clinical care over time. A more recent (2008-2010) randomized, controlled, single-institution trial compared cases of severe CDH (LHR < 1.0 with liver herniation) randomized to either FETO (n = 21) at 26 to 30 weeks’ gestation or standard postnatal care (n = 20).45 The overall survival rate with severe CDH was significantly greater in the FETO intervention group than in the expectant management group (52.6% versus 5.3%). In 2009, a randomized Tracheal Occlusion To Accelerate Lung growth trial (TOTAL) was started.46 It compared postnatal management to late (30 to 32 weeks’ gestation) FETO intervention for moderate lung hypoplasia and earlier FETO intervention (27 to 30 weeks’ gestation) for severe lung hypoplasia. In addition, it is now understood that LHR depends on gestational age47 and that a ratio of observed to expected LHR is a better expression of CDH severity and likelihood of survival.48,49 This ratio is used as part of the ongoing TOTAL trial. Results of this trial will help determine if and when FETO should be offered for cases of severe CDH.
Congenital Pulmonary Airway Malformation
Congenital pulmonary airway malformations (CPAM) are pulmonary tumors with cystic and solid components; these malformations were previously described as congenital cystic adenomatoid malformations (CCAM).50 The incidence is approximately 1 in 25,000 pregnancies.51 The classification scheme for CPAM includes five subtypes, based on cyst size, characteristics of the epithelial lining, cyst wall thickness, and the presence of mucous cells, cartilage, and skeletal muscle.50,52 Lesions are assessed by ultrasonography and described as containing cysts larger (macrocystic) and smaller (microcystic) than 5 mm in diameter. Lesions can either regress with minimal associated morbidity or progressively enlarge, often resulting in hydrops fetalis (fetal heart failure). Small lesions detected in utero or in the newborn are treated after birth by surgical excision of the affected pulmonary lobe. Large lesions can cause mediastinal shift, hydrops, and pulmonary hypoplasia and can interfere with fetal or neonatal survival; fetuses with untreated lesions associated with hydrops fetalis have a survival rate of less than 5%.53 In a retrospective single-institution review of 71 cases, the initial antenatal ultrasonographic ratio of CPAM volume to fetal head circumference (CVR) was evaluated for the formation of hydrops fetalis and postnatal outcomes.54 Fetuses with a CVR less than 0.56 were noted to have no adverse postnatal outcomes, whereas a CVR greater than 0.56 had a positive predictive value for adverse postnatal outcome of 33%. In addition, a CVR greater than 1.6 was associated with a greater risk for hydrops fetalis.55
Depending on lesion size, location, and characteristics, CPAMs can be managed with either fetal intervention or postnatal resection. Macrocystic lesions have been decompressed in utero by placement of shunt catheters between the cysts and the amniotic cavity, resulting in sustained decompression and resolution of hydrops56; these procedures are followed by postnatal surgery. However, not all lesions can be decompressed successfully because the cysts are not always contiguous (i.e., in communication with each other) and can refill rapidly. In addition, these thoracoamniotic shunts have associated risks, including malfunction, displacement, fetal hemorrhage, and chorioamnionitis.57 CPAMs inappropriate for drainage can be resected with open fetal surgery. Fetal pulmonary lobectomy for lesions associated with hydrops fetalis has resulted in a 30-day postnatal survival rate of 50%, with tumor resection allowing for compensatory lung growth and resolution of hydrops fetalis.58 Maternal administration of betamethasone also has been noted to improve hydrops fetalis and overall outcome in selected fetuses with CPAM.59,60 A retrospective review of 24 fetuses with predominantly microcystic CPAM and the presence of hydrops fetalis found that corticosteroid treatment resulted in better survival than resection with open fetal surgery.61
Intralobar and extralobar pulmonary sequestrations (bronchopulmonary sequestrations) are rarer congenital lung anomalies than CPAM and involve nonfunctional lung tissue (disconnected from the bronchial tree). As with CPAM, therapeutic options depend on fetal morbidities including hydrops fetalis and pulmonary hypoplasia. Thoracoamniotic shunts have been successfully placed to decompress massive congenital pleural effusions caused by fetal chylothorax that otherwise result in hydrops fetalis, pulmonary compression, and fetal or neonatal death.62
Sacrococcygeal Teratoma
The prevalence of sacrococcygeal teratoma (SCT) is approximately 1 in 20,000 to 40,000.63 Some fetuses with SCT undergo massive tumor enlargement, experience hydrops fetalis and placentomegaly, and die in utero. These tumors function as large arteriovenous fistulas, and fetal demise results from high-output cardiac failure. Management of these tumors requires close surveillance because they can grow rapidly and reach a size as large as 1000 cubic centimeters.63 Fetuses with large lesions are at risk for intrapartum dystocia or tumor rupture and hemorrhage; these fetuses may require cesarean delivery. Fetuses with lesions diagnosed before 30 weeks’ gestation have a poor prognosis but may benefit from surgical debulking in utero; surgical techniques have not reached the necessary level of sophistication to allow complete resection of lesions that deeply invade the pelvis. In utero radiofrequency ablation and thermocoagulation have been used to reduce the tumor blood supply, but the benefit remains unclear.64 Catheterization of a fetal hand or umbilical cord vein for blood and crystalloid transfusion during tumor resection may be needed. To date, there has been no significant improvement in outcome with intervention in cases of SCT with hydropic fetalis.
Some SCT cases are accompanied by “maternal mirror syndrome” or Ballantyne syndrome, a hyperdynamic state (i.e., hypertension, peripheral and pulmonary edema) in which the maternal physiology mirrors the abnormal circulatory physiology of the hydropic fetus.65 This syndrome is associated with a substantial increase in fetal mortality and maternal morbidity and requires aggressive management similar to that used for severe preeclampsia, a disease from which it must be distinguished. Platelet count, aspartate aminotransferase, alanine aminotransferase, and haptoglobin are typically unaffected in maternal mirror syndrome and may serve as diagnostic clues to rule out severe preeclampsia and HELLP (hemolysis, elevated liver enzymes, low platelets). Unfortunately, maternal mirror syndrome typically does not resolve quickly, even with rapid correction of the fetal pathophysiology, and severe maternal complications including pulmonary edema occur in about 20% of cases.65
Myelomeningocele
Although not lethal, a myelomeningocele is a protrusion of the meninges and spinal cord through a congenital defect in the vertebrae and overlying muscles and skin. It can result in lifelong morbidity and disability, including paraplegia, bowel and bladder incontinence, hydrocephalus, Arnold-Chiari II malformation, and impaired cognition.66 Myelomeningocele has an incidence of about 1 in 2000 live births but is becoming less common owing to folate supplementation in the maternal diet. In addition, detection by ultrasonography and alpha-fetoprotein screening of maternal blood has allowed for earlier diagnosis (i.e., second trimester) and consideration of pregnancy termination.
The specific cause of myelomeningocele remains unknown. Animal models have demonstrated improved neonatal neurologic function with fetal closure of the defect in utero.67,68 The results associated with defect closure support a “two-hit” disease model in which the pathology is produced by failure of the fetal neural tube to form combined with prolonged exposure to the uterine amniotic fluid.69 Mutations of the PAX3 gene and direct cord trauma may also play a role in the pathophysiology associated with a myelomeningocele.70
The 5-year mortality of myelomeningocele is approximately 8% for live births; if it is not corrected in utero, surgical closure must be performed within a few days after birth.71 Ventriculoperitoneal shunting is required in 85% to 90% of uncorrected cases; however, despite successful shunting, permanent deficits such as central hypoventilation, vocal cord dysfunction, and oromotor and swallowing dysfunction can still occur from the associated Arnold-Chiari malformation.72 The average intelligence quotient in myelomeningocele patients who require ventriculoperitoneal shunting is 80 (low normal).73
The purpose of fetal surgery for myelomeningocele is to improve function later in life.69 Fetal surgery is primarily performed through an open fetal surgical technique. Preliminary results suggest that in utero repair successfully reverses the hindbrain herniation of the Arnold-Chiari II malformation, probably through normalization of cerebrospinal fluid flow, and decreases the need for ventriculoperitoneal shunt placement before 1 year of age.74 More recently, a randomized, prospective, multicenter clinical trial completed between 2003 and 2010 examined the risks and benefits of open fetal surgery for myelomeningocele repair compared with standard postnatal repair in 183 patients.75 Open fetal repair reduced the need for ventriculoperitoneal shunting and improved motor function at 30 months of age, but increased the risk for preterm birth and a partial or complete uterine dehiscence. Two perinatal deaths occurred in each group. Table 7-2 displays a subset of outcome measures that were significantly different between the prenatal and postnatal repair groups. Further data from the trial will include assessment of the long-term benefit from the prenatal intervention.
TABLE 7-2
Maternal and Fetal or Neonatal Complications for MOMS Trial Patients*
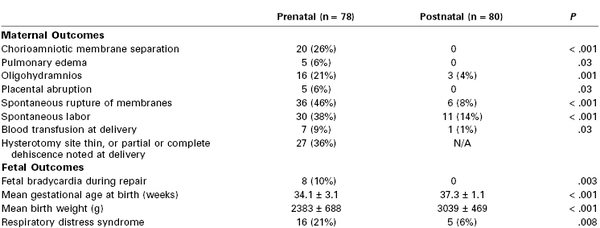
* The table lists maternal and fetal/neonatal complications that were significantly different (P < .05) between the prenatal and postnatal repair groups in the Management of Myelomeningocele Study (MOMS). Other outcomes were evaluated, but only those that were different between the two groups are included. Data for each group are shown as both an absolute number and as a percentage.
Modified from Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011; 364:993-1004.
A recent study of endoscopic intrauterine myelomeningocele repair resulted in an extraordinarily high complication rate for both mothers and fetuses.76 Of the 19 study patients, three fetuses died intraoperatively and another three procedures were stopped owing to severe hemorrhage from the procedure. Although this intervention was associated with spinal segmental neuroprotection, it resulted in significantly more complications; the authors concluded that, pending further advances, this technique is unsuitable as standard care.76
Twin-to-Twin Transfusion Syndrome
An abnormal connection of chorionic blood vessels in the placenta between two monochorionic twins can result in twin-to-twin transfusion syndrome (TTTS). TTTS complicates 10% to 15% of monochorionic pregnancies, usually manifests at 15 to 26 weeks’ gestation, and is typically recognized at 20 to 21 weeks’ gestation.77,78 Intertwin transfusion is common between monochorionic twins and is usually balanced by the presence of arterioarterial (AA) and venovenous (VV) connections; the presence of AA connections is associated with a ninefold reduction in TTTS.79 By contrast, unidirectional and imbalanced blood flow through arteriovenous (AV) chorionic vessels results in TTTS. In normal fetoplacental vasculature, the umbilical artery branches at the placenta surface and traverses and then descends into the tissue, where it further branches into capillary divisions for gas and nutrient exchange. The arterial system is “paired” with venous vasculature, which returns blood to the umbilical cord. In TTTS, the umbilical artery similarly descends into the placenta and cotyledon, but rather than connecting with a paired vein it connects with a vein that transports blood to the other twin.80
The twin serving as the recipient demonstrates polycythemia, polyuria, polyhydramnios, and hypertrophic cardiomyopathy; this twin is at risk for hydrops fetalis and fetal death. The twin serving as the donor is typically hypovolemic, growth restricted, and pressed against the endometrium in an oligohydramniotic sac (hence the designation “stuck” or “pump” twin) and often has a velamentous cord insertion; this twin is at risk for neonatal renal failure, tubular dysgenesis and dysfunction, and high cardiac output hydrops fetalis. Diagnosis is currently based on ultrasonographic findings that focus on differences in fetal size or amniotic sac fluid volume, presence of cardiac dysfunction in the recipient twin, umbilical cord size, and abnormal umbilical arterial flow velocity.77,81 Twin size discordance is not always present and is no longer considered necessary to confirm the diagnosis. For unclear reasons, fetuses with TTTS are at risk for neurologic injury with white-matter lesions and long term disability; poor neurodevelopmental outcomes are associated with increased TTTS severity, delayed therapeutic interventions, and preterm delivery.82 If TTTS is untreated, it carries a greater than 80% mortality with 15% to 50% risk for significant morbidity in surviving neonates.83
A variety of therapeutic management techniques have been developed, including (1) serial amnioreduction to control polyhydramnios and reduce the risk for preterm labor, (2) surgical septostomy to equalize amniotic pressures, (3) selective feticide to allow the other fetus to survive, and (4) selective fetoscopic laser photocoagulation (SFLP) of the vascular anastomoses between the two twins. Serial amnioreduction was demonstrated to improve placental perfusion and decrease the occurrence of preterm delivery.84 In a retrospective review of 223 twin sets with TTTS, amnioreduction resulted in an overall birth survival rate of 78%, with 65% of recipient twins and 55% of donor twins alive at 1 month of age.85 In a prospective randomized trial comparing serial amnioreduction to septostomy, there was no difference in the rate of survival between the two techniques.86 Septostomy is rarely used for treatment because the creation of a single amniotic sac can increase the risk for umbilical cord entanglement.
The laser used for SFLP is typically inserted percutaneously through an endoscope (≤ 2.0 mm diameter) into the recipient twin’s amniotic sac. Maternal anesthesia is commonly managed with either neuraxial blockade or local anesthetic infiltration from skin to myometrium. Fetoscope placement is determined by placental location and is guided by ultrasonography. Vessels that cross the dividing membrane separating the amniotic sacs are visualized, and abnormal connecting vessels are selectively coagulated with the laser.77,80 On occasion, based on anatomic constraints created by the location of the fetuses and placenta, nonselective laser ablation is performed; however, this type of ablation is associated with higher rates of intrauterine fetal demise.77,87 Ablation of all abnormal connecting vasculature is not needed for success of the procedure.88 After completion of the SFLP, amniotic fluid may be removed to reduce the degree of polyhydramnios and possibly decrease the risk for preterm labor.
A 2004 randomized multicenter trial compared laser therapy to amnioreduction for treatment of severe TTTS diagnosed between 15 and 26 weeks’ gestation.89 Rates of at least one twin survival were significantly higher in the laser treatment group at both 28 days (76% versus 56%, P < .01) and 6 months of life (76% versus 51%, P < .01). In addition, neurologic outcomes were better in the laser treatment group. A subsequent prospective study of a large subgroup of survivors from this trial observed these children for 6 years and found no additional change in survival or long-term neurologic outcome from the original 6-month data.90 A meta-analysis of studies published between 1997 and 2007 noted that treatment of TTTS with laser ablation resulted in a higher overall rate of fetal survival than amnioreduction91; similar findings were demonstrated in a Cochrane review of treatment for TTTS.92
A more recent variation of the SFLP technique requires vascular laser ablation in a specific sequence. First, ablation occurs at the donor-to-recipient AV anastomoses, then at the recipient- to-donor AV anastomoses, then at the AA superficial anastomoses, and finally at the VV superficial anastomoses. The order of the procedure is designed to reduce the chance of hemodynamic compromise and hypotension during the procedure in the donor twin93; however, it is associated with longer operative times and increased case difficulty, particularly with an anterior placenta. In a single-institution study of consecutive SFLP for treatment of TTTS, twins treated with this sequence had better survival rates than twins whose SFLP procedure was performed without a specific sequence.93 A prospective multicenter trial found a significantly improved 30-day survival of both fetuses and improved donor survival with this sequential technique when compared with a cohort control group.94
The most common complication of SFLP is preterm PROM with subsequent preterm labor and delivery. Other possible complications include placement of the trocar through the placenta, hemorrhage, and possible membrane perforation resulting in limb entrapment and ischemia.77 In conclusion, trial results and meta-analyses provide evidence that SFLP results in superior outcomes than amnioreduction for the treatment of TTTS. Further research is needed to determine optimal techniques and timing of interventions for the treatment of TTTS.
Twin Reversed Arterial Perfusion Sequence
In monozygotic twins, one twin can also perfuse the other by retrograde blood flow though AA anastomoses. Twin reversed arterial perfusion (TRAP) sequence affects 1% of monozygotic twins and 1 in 30 triplets. Inadequate perfusion of the recipient twin via retrograde flow results in the development of a lethal set of anomalies that include acardia and acephalus. The normal (“pump”) twin perfuses both itself and the nonviable twin and is at risk for high-output congestive heart failure, polyhydramnios, and preterm birth. If untreated, TRAP sequence is associated with a risk for intrauterine death of the pump twin exceeding 50%.95 Diagnosis is confirmed with ultrasonographic demonstration of reverse flow to the acardiac twin via the umbilical artery. Cardiovascular failure in the pump twin is the indication for intervention, and early diagnosis is beneficial for optimal treatment.
The goal of therapy is to interrupt the vascular communication between the two twins. In contrast to the treatment of TTTS, treatment of TRAP sequence results in the death of the anomalous nonviable fetus. Percutaneous endoscopic laser or radiofrequency coagulation of the umbilical cord and/or placental vascular anastomoses is the most viable therapeutic option.96,97 Alternative therapies include sectio parva (selective cesarean delivery of one of multiple fetuses), percutaneous thrombosis of the acardiac twin’s umbilical cord with coils or other thrombogenic material, and alcohol-impregnated suture cord ligation. A retrospective review of 60 TRAP sequence cases from multiple European centers using endoscopic laser coagulation noted overall survival rates approaching 80% and a median gestational age of 37.4 weeks at delivery.98 An additional study, using radiofrequency coagulation in 26 TRAP sequence cases at a single U.S. medical center, demonstrated a 92% survival rate of the viable twin with a mean gestational age of 35.6 weeks at delivery.96 Both procedures are typically performed with infiltration of local anesthesia at the insertion site of the ablation device, although neuraxial anesthesia has also been used. The procedures are guided by ultrasonography, and absence of flow to the nonviable acardiac twin is confirmed with Doppler imaging at the end of the procedure and again 12 to 24 hours later.
Congenital Heart Defects
The most commonly performed closed fetal cardiac intervention for a congenital heart defect is an aortic valvuloplasty for mid-gestational aortic stenosis with evolving hypoplastic left heart syndrome. Technical success as high as 75% has been reported using an angioplasty balloon over a guidewire inserted percutaneously through an access cannula.99 Approximately 40% of successful cases result in aortic regurgitation and minimal subsequent left ventricular growth; however, the physiology of the left ventricle improves and leads to improved aortic and mitral valvular growth. In about 30% of the successful cases, biventricular circulation is present at birth. Other congenital heart defects that may benefit from antepartum closed fetal cardiac intervention include (1) hypoplastic left heart syndrome with an intact or highly restrictive atrial septum and (2) evolving hypoplastic right heart syndrome with pulmonary atresia or stenosis without a ventricular septal defect.100,101
More complex congenital cardiac defects that might benefit from open repair have only been repaired in animal models and require fetal extracorporeal circulatory bypass; the use of fetal cardiac bypass induces a catecholamine-mediated fetal stress response that increases vascular resistance and cardiac afterload and is poorly tolerated by the immature fetal myocardium. Fetal cardiac bypass can also result in severe placental dysfunction when the high-capacitance, low-resistance placenta is incorporated as the oxygenator, resulting in endothelial dysfunction and leukocyte and complement activation. After fetal cardiac bypass, increases in placental vascular resistance, reduced blood flow, impaired gas exchange, and fetal acidosis are frequently observed.102,103 Correction of complex congenital cardiac defects, either open or closed, requires careful anesthetic and pharmacologic strategies for myocardial protection.
Surgical Benefits and Risks
The primary goal of intrauterine fetal surgery is to improve neonatal outcomes over that of surgery performed after a preterm or term delivery. The intrauterine environment supports rapid wound healing (i.e., without scarring before mid gestation),104 and the umbilical circulation meets nutritional and respiratory needs without outside assistance. The poorly developed fetal immune surveillance system may facilitate certain invasive procedures. However, continued refinement of surgical and anesthetic techniques, reduction of maternal and fetal risk, and appropriate clinical trials for each intervention must occur before fetal surgery can be performed on a more routine basis for a given congenital anomaly.
Serious maternal complications from intrauterine fetal surgery are relatively uncommon. Maternal risks include blood loss, infection, placental abruption, and pulmonary edema secondary to tocolytic therapy and fluid overload from absorption of significant amounts of pressurized crystalloid uterine irrigation during fetoscopic techniques.105,106 Open fetal surgery involves a hysterotomy that is not in the lower uterine segment, and therefore all future deliveries must occur via a cesarean procedure. Maternal welfare must always be emphasized.7
The fetal risks of intrauterine surgery remain relatively high. The most common postoperative complications are fetal central nervous system injuries, postoperative amniotic fluid leaks, membrane separation, preterm PROM, and preterm labor and delivery. Preterm delivery accounts for significant morbidity and mortality among fetuses that might otherwise have benefited from the therapeutic interventions. Chorioamniotic membrane separation can cause amniotic bands, umbilical cord strangulation, and fetal demise.107 Improved techniques for sealing the membranes are being devised, including different surgical techniques, fibrin glue, and intra-amniotic injection of platelets and cryoprecipitate.108,109
Anesthetic Management
Fundamental considerations for the anesthetic management of fetal surgery are similar to those for nonobstetric surgery during pregnancy (see Chapter 17). Maternal safety is paramount. Anesthesiologists must participate in preoperative maternal assessment and exclude women when their perioperative risk is not acceptably low given the potential fetal benefit. To ensure both maternal and fetal safety, the anesthesiologist must understand the physiologic impact of pregnancy on anesthetic management (see Chapter 2) and must take an active role as a member of the multidisciplinary team. Imaging studies should be reviewed for placental location, anatomic information about the congenital lesion, and estimated fetal weight.
Unlike other surgical procedures performed during pregnancy in which the fetus is an innocent bystander (e.g., maternal appendectomy), fetal surgery involves two surgical patients. This requires the anesthesiologist to balance the anesthetic needs of both patients, as well as control uterine tone throughout the perioperative period. Complete uterine relaxation is necessary during open fetal surgical procedures.
Maternal analgesia and anesthesia can involve local infiltration, intravenous sedation, neuraxial anesthesia, general anesthesia, or a combination of these techniques, depending on the procedure, location of the placenta, and maternal co-morbidities. Fetal analgesia and anesthesia can be achieved via placental transfer of anesthetic agents given to the mother, via direct fetal intravenous or intramuscular administration of agents, or by a combination of these methods. An appropriate method of fetal monitoring and the potential requirement for fetal intravenous access or fluid resuscitation should be determined preoperatively. The operative team should be prepared for emergency situations such as maternal hemorrhage, fetal deterioration requiring aggressive intrauterine resuscitation (e.g., fetal epinephrine administration), and/or delivery and resuscitation of the viable newborn infant.110
Anesthesia for Minimally Invasive and Percutaneous Procedures
Local anesthetic infiltration of the abdominal wall is sufficient to reduce maternal discomfort for many percutaneous procedures (e.g., amniocentesis, cordocentesis, intrauterine blood transfusion, needle aspiration of cysts, shunt placement into the fetal bladder or thorax, SFLP for TTTS). Supplemental maternal analgesia and anxiolysis can be achieved by maternal administration of an opioid, a benzodiazepine, or a low-dose propofol infusion, which may confer some fetal immobility and analgesia via placental transfer.111 However, when larger needles or multiple attempts are necessary for percutaneous procedures or a minilaparotomy, maternal comfort can be difficult to achieve with local infiltration, even when supplemented with sedation. In these circumstances, neuraxial anesthesia is recommended.77,91 General anesthesia is rarely necessary for percutaneous procedures unless placental location makes the procedure significantly challenging or uterine exteriorization is needed.
Fetal movement may be hazardous for the fetus in cases of intrauterine transfusion, cord blood sampling, or thoracic shunt placement, because displacement of the needle or catheter may lead to trauma, bleeding, or compromise of the umbilical circulation. Placental transfer of maternally administered opioids and benzodiazepines can reduce fetal movement89 but cannot ensure fetal immobility for more stimulating procedures. A randomized controlled trial of the use of a maternal remifentanil infusion (0.1 µg/kg/min) demonstrated improved fetal immobility and operating conditions during fetoscopic surgery, when compared with maternal administration of diazepam.112 Fetal immobility can be safely achieved with direct fetal intramuscular or umbilical venous administration of pancuronium or vecuronium (0.3 mg/kg intramuscularly or 0.1 to 0.25 mg/kg intravenously) using ultrasonographic guidance. The onset of fetal paralysis occurs in 2 to 5 minutes, with an approximate duration of 1 to 2 hours.113 For procedures that can cause noxious stimulation to the fetus, such as shunt catheter placement or cardiac septoplasty, an opioid (e.g., fentanyl 10 to 20 µg/kg) can be administered to the fetus intramuscularly or intravenously.114,115 When general anesthesia is employed, placental transfer of a volatile halogenated agent is usually sufficient to immobilize and anesthetize the fetus.
The fetal surgical team should be prepared for treatment of fetal compromise with immediate availability of appropriate doses of atropine and epinephrine. The obstetric team should be prepared to perform an emergency cesarean delivery if the gestational age is compatible with extrauterine viability. The anesthesiologist should be prepared to emergently provide general anesthesia if required.
Tocolysis typically is unnecessary after cordocentesis or intrauterine transfusion. For more invasive percutaneous procedures (e.g., shunt catheter placement, endoscopic techniques), some fetal surgery groups administer prophylactic tocolytic agents.
Anesthesia for Open Fetal Surgery
When corrective fetal surgery or an intrauterine procedure requires surgical access through a hysterotomy, general anesthesia is typically administered. Unique considerations for open fetal procedures include the need for profound uterine relaxation, intraoperative fetal monitoring, fetal anesthesia or analgesia, and postoperative maternal analgesia and uterine tocolysis (Table 7-3). In addition, significant maternal and fetal blood loss may occur, and the anesthesiologist must be prepared to achieve maternal and fetal resuscitation. A high concentration of a volatile halogenated agent is typically administered to provide both maternal and fetal anesthesia as well as uterine relaxation; adequate uterine relaxation may require greater than twice the minimum alveolar concentration (MAC) of a volatile halogenated agent.116
TABLE 7-3
Perioperative Considerations for Open Fetal Surgery

CO2, carbon dioxide; FIO2, fraction of inspired oxygen; IM, intramuscular; IV, intravenous.
Preoperatively, the mother receives agents for aspiration prophylaxis and uterine tocolysis (e.g., rectal indomethacin) and an epidural catheter is placed for postoperative analgesia. Minimal doses of preanesthetic and adjuvant anesthetic agents are given to allow significant doses of a volatile halogenated agent to be administered to achieve effective uterine relaxation; this approach may also reduce the occurrence of hypotension. The patient is placed in the supine position with left uterine displacement. After administration of 100% oxygen and denitrogenation of the lungs, a rapid-sequence induction of general anesthesia with cricoid pressure and endotracheal intubation is performed. Fetal heart rate (FHR) and umbilical cord blood flow are often monitored with ultrasonography during induction.
Initially, anesthesia is maintained with a low concentration of a volatile halogenated agent while further preparations for surgery are undertaken, including (1) obtaining additional maternal vascular access, (2) prophylactic antibiotic administration, (3) urinary bladder catheterization, and (4) ultrasonographic assessment of fetal presentation and placental location. Medications to provide fetal analgesia (e.g., fentanyl 10 to 20 µg/kg), immobility (e.g., vecuronium 0.3 mg/kg), and resuscitation (atropine 0.02 mg/kg, epinephrine 1 and 10 µg/kg, and crystalloid 10 mL/kg) are prepared in sterile labeled syringes; each syringe should contain a single weight-based unit dose. Crossmatched blood should be available for maternal transfusion. In addition, O-negative, cytomegalovirus (CMV)-negative, irradiated, leukocyte-depleted, maternally crossmatched blood should be available for fetal transfusion. For open procedures that have a high risk for significant fetal blood loss (e.g., mass resection), an intravenous catheter should be placed in the fetus to provide access for blood and fluid transfusions. An arterial catheter should be placed for maternal blood pressure monitoring if uterine tocolysis with a nitroglycerin infusion is planned. Intraoperative maternal intravenous fluids are restricted (< 2 L) to reduce the risk for postoperative pulmonary edema; some fetal surgery centers choose to limit fluids even further (< 500 mL), although no clinical trials have proven a benefit of fluid restriction in this setting.117 The use of tocolytic agents such as magnesium sulfate and nitroglycerin has been associated with maternal pulmonary edema in patients undergoing fetal surgery.
A final discussion (i.e., surgical time-out), prophylactic antibiotic administration, and an increase in the concentration of the volatile halogenated agent should occur before skin incision. Maternal blood pressure is maintained with a mean arterial pressure within 10% of baseline values and greater than 65 mm Hg; a phenylephrine infusion provides titratable blood pressure control with minimal changes in the fetal acid-base status.118,119 Ephedrine or glycopyrrolate boluses also can be administered to maintain maternal heart rate and improve cardiac output.120 Maternal administration of a nondepolarizing muscle relaxant is usually not needed owing to the profound depth of anesthesia but may be used to improve operative conditions. The intrauterine location and position of the fetus is confirmed with ultrasonography just before hysterotomy to optimize the incision location.
Before uterine incision, it is important to achieve an increased end-tidal concentration of the volatile halogenated agent to provide both fetal anesthesia and uterine relaxation (typically ≥ 2 MAC). Fetal well-being can be assessed with pulse oximetry, ultrasonography for FHR monitoring, echocardiography to assess fetal ventricular contractility, and electrocardiography (ECG).
The uterus is assessed both visually and by palpation for contractions or increased tone; further tocolysis can be achieved with the administration of additional halogenated agent (≤ 3 MAC) or intravenous nitroglycerin as an infusion or in small boluses (50 to 200 µg).121 Use of desflurane at 1.5 MAC with supplemental intravenous propofol and remifentanil has provided adequate uterine relaxation in one retrospective study.122 For circumstances in which volatile halogenated agents or general anesthesia must be avoided (e.g., family history of malignant hyperthermia), a spinal or epidural anesthetic can be used with an intravenous infusion of nitroglycerin in doses up to 20 µg/kg/min.121 This technique does not have a clear advantage for fetal outcome and may be associated with more morbidity. Nitroglycerin administration during open fetal surgery has been associated with maternal pulmonary edema.117,123 Fetal intraventricular and periventricular hemorrhage and cerebral ischemia can result from changes in fetal cerebral blood flow, and concern has been raised regarding the use of tocolytic agents, which may affect vascular tone.124
An opioid and a muscle relaxant are administered to the fetus intramuscularly, either before uterine incision with ultrasonographic guidance or after uterine incision with direct vision. Some anesthesiologists administer atropine at this time in an effort to prevent opioid-induced fetal bradycardia; a muscle relaxant with vagolytic effects also can be chosen to minimize this type of bradycardia. Further studies are needed to determine the optimal anesthetic technique for ensuring maternal and fetal cardiovascular stability, optimal uteroplacental perfusion, and adequate fetal anesthesia to cause immobility and blockade of the fetal stress response.
A small uterine incision is created remote from the location of the placenta. A stapling device with absorbable synthetic copolymer (Lactomer) staples is used to extend the incision to seal the membranes to the endometrium and prevent excessive bleeding.125 During surgery, the exposed fetus and uterus are bathed with warmed fluids. The intrauterine temperature is closely monitored to prevent fetal circulatory compromise associated with hypothermia.126
When uterine closure is initiated at the conclusion of the procedure, a loading dose of magnesium sulfate is administered (4 to 6 g intravenously over 20 minutes), followed by an intravenous infusion of 1 to 2 g per hour. As magnesium potentiates neuromuscular relaxation, close monitoring of twitch recovery is needed if a nondepolarizing muscle relaxant was administered. The volatile halogenated agent can be significantly decreased or discontinued after the magnesium sulfate bolus has been administered. The epidural analgesia can be initiated and maternal anesthesia is maintained with additional fentanyl, nitrous oxide, and/or propofol.
Postoperative concerns include maternal and fetal pain, preterm PROM, preterm labor, infection, and a variety of potential fetal complications, including heart failure, intracranial hemorrhage, constriction of the ductus arteriosus from indomethacin, and fetal demise. Postoperative maternal analgesia can be maintained with a continuous epidural infusion of a dilute solution of local anesthetic and opioid for several days. Effective analgesia may help prevent postoperative preterm labor.127,128 Intravenous opioids can also be used for analgesia; however, decreased FHR variability may occur.129
Management of postoperative preterm labor has been the “Achilles heel” of fetal surgery.7 Tocolysis is typically provided by an infusion of magnesium sulfate for at least 24 hours; however, supplemental agents may include indomethacin, terbutaline, or nifedipine. Magnesium most likely competes with calcium at voltage-operated calcium channels, indomethacin blocks the synthesis of prostaglandins, and beta-adrenergic agonists activate adenylate cyclase in the uterine muscle, thereby reducing intracellular calcium levels. Frequently two tocolytic agents are required to create uterine quiescence. Uterine activity and FHR are monitored closely during the first 2 to 3 postoperative days. The fetus is evaluated postoperatively by ultrasonography, and if indomethacin is used, periodic fetal echocardiography is conducted to determine whether premature closure of the ductus arteriosis has occurred.
Patients recovering from open fetal surgery should remain near the fetal treatment center after being discharged. These patients are at high risk for preterm PROM, preterm labor, infection, and uterine rupture. Unless preterm labor occurs, cesarean delivery is typically planned at 37 weeks’ gestation.
Anesthesia for the Ex Utero Intrapartum Treatment Procedure
Initially described as a method to remove the iatrogenic airway obstruction created for intrauterine treatment of CDH, the EXIT procedure has evolved into a technique useful for a number of fetal disorders that compress the airway and/or render neonatal tracheal intubation difficult or impossible. It is also useful when resuscitation and surgical intervention are required immediately before birth, while the fetus is still supported by the placentofetal circulation. Cases appropriate for an EXIT procedure include (1) thoracotomy for congenital pulmonary airway malformations and (2) tracheostomy and the removal of neck masses such as fetal teratoma. The use of an EXIT procedure may also assist the transition to ECMO for pulmonary insufficiency or the stabilization of conjoined twins prior to separation.11,130,131 Similar to open fetal surgery procedures, sustained uterine relaxation and delay of placental separation are necessary for a successful EXIT procedure.
Anesthesia for EXIT procedures is most commonly performed with the use of general anesthesia, although neuraxial anesthesia, typically combined with intravenous nitroglycerin infusion to achieve uterine relaxation, may also be used. Multiple reviews of the surgical and obstetric considerations associated with the EXIT procedure have been published.132–134
The conventional anesthetic approach is a modification of the general anesthetic technique used for cesarean delivery. Preparation for fetal monitoring, airway management, fetal/neonatal resuscitation, and postdelivery care should be completed before entering the operating room. A sterile pulse oximeter probe and an end-tidal carbon dioxide indicator or gas analyzer are used for fetal monitoring during the procedure; basic ultrasonography can be added to assess the fetal heart. Unit doses of atropine, epinephrine, and calcium for intramuscular fetal injection are transferred sterilely to the scrub nurse for possible emergency intraoperative resuscitation. Supplemental fetal anesthetic agents for intramuscular injection are also prepared and transferred to the scrub nurse (see later discussion). A sterile ventilation bag with an air/oxygen source and manometer is available for the fetus, along with multiple endotracheal tube sizes and devices for fetal tracheal intubation. Catheters for intravenous access as well as crystalloid and blood (O-negative, CMV-negative, leukocyte depleted, irradiated, maternally crossmatched) should be available for fetal volume resuscitation if needed.
A maternal epidural catheter may be placed preoperatively for postoperative analgesia. Anesthetic considerations for the mother are similar to those for cesarean delivery but should also include large-bore intravenous access, availability of uterotonic agents and crossmatched blood, and the ability to quickly obtain invasive maternal monitoring if needed.
Techniques of induction and tracheal intubation do not differ from those typically used for cesarean delivery. Although techniques for maintenance of anesthesia vary between medical centers, administration of 2 to 3 MAC of a volatile halogenated agent is often needed to achieve and maintain adequate uterine relaxation. Occasionally, nitroglycerin administered intravenously as a bolus dose (50 to 200 µg) or as an infusion is required to obtain appropriate uterine relaxation. Fetal anesthesia from the halogenated agent transferred across the placenta can be supplemented by direct fetal (intramuscular) administration of an opioid (fentanyl 5 to 15 µg/kg) and a paralytic agent (rocuronium 1 to 3 mg/kg or pancuronium 0.1 to 0.3 mg/kg); some practitioners also administer intramuscular atropine (20 µg/kg) to prevent fetal bradycardia.132,134 Intramuscular agents can be administered to the fetus either before uterine incision with ultrasonographic guidance or after incision with direct visualization. Significant variability in serum fentanyl concentrations among fetuses has been documented in umbilical cord blood during EXIT procedures,135 and similar variability may exist with muscle relaxants and other agents.
When adequate uterine relaxation has been achieved, the placental location and edges are confirmed by intraoperative ultrasonography. Similar to open fetal procedures, the uterine incision is extended with a stapling device to minimize blood loss. The fetal head and shoulders are delivered in preparation for tracheal intubation. For more extensive procedures, such as fetal thoracotomy, or when there is fetal bradycardia suggestive of umbilical cord compression, the fetus can be completely delivered and placed on the maternal chest and abdomen. In an effort to maintain fetoplacental circulation, warmed fluids are continuously irrigated into the uterine cavity and care is taken to avoid manipulation of the umbilical cord. The warmed irrigant maintains fetal euthermia and helps prevent decreased uterine volume, placental separation, and spasm of the cord vessels. The fetus is initially monitored with (1) a pulse oximeter probe placed on the fetal hand, (2) periodic ultrasonography, and (3) direct visualization.
The duration of the fetal procedure can range from a few minutes (e.g., bronchoscopy or intubation) to several hours (e.g., neck or thoracic mass resection, tracheostomy, or central intravascular access). Although the majority of procedures require less than an hour, the anesthetic technique should be capable of providing maternal, fetal, and uteroplacental stability over several hours.136,137 Once surgery is completed and the trachea secured, surfactant is given if indicated. Fetal oxygen saturation is typically 40% to 60% at this time138 but increases significantly to above 90% with ventilation of the fetal lungs. Failure to achieve a fetal saturation of at least 90% is an indication for the use of ECMO before clamping the umbilical cord and delivering the fetus.139
After the umbilical cord is clamped, the maternal anesthetic technique is altered to help achieve uterine tone and diminish the risk for postpartum hemorrhage.132,140 This is accomplished by (1) substantially reducing the inspired concentration of the volatile halogenated agent; (2) adding nitrous oxide, propofol, and/or an opioid to maintain anesthesia; (3) administering oxytocin and other uterotonic agents, if needed; and (4) initiating epidural analgesia via the epidural catheter that was placed before surgery.
An alternative technique for EXIT procedures involves the use of neuraxial anesthesia.141–143 Although this approach avoids many of the risks associated with general anesthesia, large doses of intravenous nitroglycerin (1 to 10 µg/kg/min) are often required to achieve adequate uterine relaxation. Nitroglycerin can cross the placenta; however, a significant amount is metabolized at the placental interface, resulting in minimal fetal effects.121,143 If nitroglycerin is to be used at significant doses for a prolonged period, an arterial catheter is recommended and the patient should be observed for evidence of pulmonary edema. Fetal analgesia and immobility can be achieved with fetal administration of intramuscular drugs (see earlier discussion); maternally administered intravenous remifentanil undergoes significant transfer across the placenta and may serve as an adjuvant for fetal analgesia and immobility.144,145 Prospective trials are necessary to delineate the advantages and disadvantages of these various anesthetic techniques for the EXIT procedure.
Fetal Response to Surgical Stimulation
The subjective phenomenon of pain has not been, and perhaps cannot be, assessed adequately in human fetuses. Pain is a multidimensional, subjective, psychological construct that can exist in the absence of physical stimuli (e.g., phantom limb pain), and it includes emotional and affective components that require higher-level cortical processing. As such, although pain is commonly associated with physical noxious stimuli, it is more than nociception or a simple reflex activity associated with a withdrawal response.146 Attempts have been made to correlate pain with surgical stress; however, the physiologic responses are not equivalent and reductions in stress hormones should not be interpreted as an indicator of adequate analgesia.147 The stress response is mediated primarily in the spinal cord, brainstem, and/or basal ganglia, without involvement of the cortex. This distinction acknowledged, pioneering studies of preterm neonates undergoing surgery with minimal anesthesia have revealed circulatory, sympathoadrenal, and pituitary adrenal responses characteristic of stress (e.g., increased release of catecholamines, growth hormone, glucagon, cortisol, aldosterone, and other corticosteroids; decreased secretion of insulin).148,149 Administration of adequate anesthesia in neonates blunts this stress response,150 and attenuation of the stress response in preterm neonates has been associated with improved outcomes.151
In studies of intrauterine blood transfusion in the human fetus, surgical needling of the intrahepatic vein (in contrast to the insensate umbilical cord) is associated with evidence of a stress response, including increases in plasma beta-endorphin and cortisol concentrations and decreases in the middle cerebral artery pulsatility index (determined by Doppler imaging). These responses, which may redistribute blood flow to vital organs, including the brain,152 can be blunted by the administration of fentanyl 10 µg/kg.114 Human fetuses elaborate pituitary-adrenal, sympathoadrenal, and circulatory stress responses to noxious stimuli as early as 18 weeks’ gestation.153–156 During late gestation, fetuses can respond to environmental stimuli such as noise, light, music, pressure, touch, and cold.157
Nerve terminals for the detection of touch, temperature, and vibration (not pain) are present deep within the human skin at 6 weeks’ gestation and become more numerous and superficial (e.g., toward the skin surface) by 10 weeks’ gestation.158 Immature skin nociceptors likely begin to emerge at 10 weeks’ gestation and are present by 17 weeks’ gestation159; in internal organs, nociceptors develop slightly later.
Peripheral nerve fibers that control movement grow into the spinal cord at about 8 weeks’ gestation. When these fibers connect with nociceptors is unknown, but the timing of these connections is delayed when compared with other sensory inputs in nonhuman, mammalian models. One human study suggested that nociceptive nerve fibers do not enter the spinal cord before about 19 weeks’ gestation.160 The cerebral cortex develops after the fetal spinal cord and brainstem. Thalamocortical axons reach the somatosensory cortex at 24 to 26 weeks’ gestation.146
The developing cerebral cortex consists of transient fetal zones where neuronal proliferation, cell migration, apoptosis, axonal outgrowth, and synaptogenesis occur according to a highly specific timetable (see Chapter 10). Originating as a smooth layer without sulci and gyri, the cerebral cortex, like the thalamus, has no internal cellular organization.158 The cortical subplate is a temporary structure that serves as a waiting and organizing zone for various afferents destined for the cortex; it develops about 13 weeks’ gestation and recedes after 32 to 34 weeks’ gestation. The insular cortex starts developing in humans at approximately 15 weeks’ gestation, with the cortical plate eventually developing into the six layers of the cerebral cortex.161–163
The first fibers from the thalamus reach the subplate between 12 and 18 weeks’ gestation and remain until the maturation of the cortical plate. The gestational age at which thalamic pain fibers reach the human cortex only can be estimated from histologic studies of other thalamocortical circuits. Thalamic projections reach the visual subplate at 20 to 22 weeks’ gestation,162,164 the visual cortex at 23 to 27 weeks’ gestation,165 and the auditory cortical plate at 26 to 28 weeks’ gestation.166 The subplate becomes thinner in the insula and in areas of the brain where early cortical folding occurs. Extensive brain growth and maturation occur after 34 weeks’ gestation, resulting in cortical sulci and gyri and an extensive network of pathways within the cortex and to the thalamus, midbrain, and spinal cord. Prior to the completion of the thalamocortical system, the midbrain reticular system is possibly responsible for pain awareness, as it is for consciousness.162,167
Studies of fetal electroencephalograms (EEGs) at 24 weeks’ gestation demonstrate electrical activity only 2% of the time, predominantly in 20-second bursts with periods of inactivity lasting up to 8 minutes. At 30 weeks’ gestation, EEGs begin showing patterns of wakefulness and sleep, but these are not concordant with fetal behavior. By 34 weeks’ gestation, electrical activity is present 80% of the time, with more distinct wakefulness and sleep cycles similar to adult patterns.
Low levels of oxygenation in utero and endogenous neural inhibitors such as adenosine and pregnenolone may preclude optimal functioning of neural tissues and networks.168 However, two studies using near-infrared spectroscopy in preterm infants demonstrated differences in cerebral oxygenation over the somatosensory cortex with noxious and non-noxious stimulation. This appears to indicate that noxious information is at least transmitted to the infant cortex by 25 weeks’ gestation169,170; similarly, preterm neonates also have demonstrated cortical evoked potentials after a heel lance.171
Although initial fetal reactions to nociceptive stimulation are purely reflexive, and cortical processes can occur only after thalamocortical connections have been completed, nociceptive stimuli can activate a number of subcortical mechanisms; moreover, stress responses may influence the maturation of both the thalamocortical pathways and the cerebral cortex. Although the data on the consequences of fetal exposure to stressful stimuli are incomplete, recognition should be given to the possibility that noxious stimuli, which can be attenuated or blocked by anesthesia, may be associated with adverse long-term neurodevelopmental consequences.172 For example, circumcision in nonanesthetized neonates has been associated with increased pain responses to injections performed 6 months later173; in addition, fetal stress has been associated with long-term adverse hormonal effects in young monkeys.174
The exact onset of fetal sentience, the capacity to feel pain, is unknown (see Chapter 5). Because of this uncertainty, it seems best to err on the side of administering adequate fetal anesthesia.175 Altogether, clinical observations of fetal and neonatal behavior, information about the development of mechanisms of pain perception, and studies of fetal and neonatal responses to noxious stimuli provide a compelling physiologic and philosophic rationale for the provision of adequate fetal anesthesia, especially after 24 to 26 weeks’ gestation. Noxious stimulation during fetal life causes a stress response, which could have both short- and long-term adverse effects on the developing central nervous system. Although the link between the stress response and pain is not always predictable, the threshold for pain relief is typically below that for stress response ablation, and the stress response to noxious stimulation is clear evidence that the fetal nervous system is reactive.176 Administration of fetal anesthesia has been the standard practice worldwide since the inception of fetal surgery more than 30 years ago.5,6,177,178 The importance of fetal immobility, cardiovascular homeostasis, analgesia, and perhaps amnesia have always been emphasized in fetal surgery practice.
Effects of Anesthesia on the Fetus
In fetal lambs, the concentration of halothane required to prevent movement in response to painful stimuli (1.0 MAC) is less than 40% of the concentration required in adult sheep.179 Despite rapid placental transfer of volatile halogenated agents, experiments in sheep models have shown that fetal concentrations of these agents remain lower than maternal concentrations for significant periods after maternal administration (Figure 7-3).180
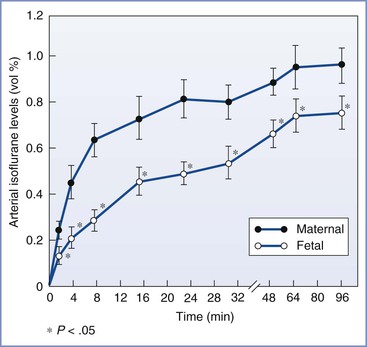
FIGURE 7-3 Maternal and fetal arterial isoflurane concentrations in sheep during maternal administration of 2.0% isoflurane (mean ± SE). (From Biehl DR, Yarnell R, Wade JG, Sitar D. The uptake of isoflurane by the foetal lamb in utero: effect on regional blood flow. Can Anaesth Soc J 1983; 30:581-6.)
Experimental studies of the fetal effects of maternal administration of a volatile halogenated agent in sheep have not produced uniform results.180–183 Maternal administration of isoflurane and halothane resulted in variable changes in fetal blood pressure, heart rate, oxygen saturation, cardiac output, and acid-base status. A retrospective analysis of cardiac imaging from both open fetal cases and EXIT procedures noted severe left ventricular systolic dysfunction in the fetus with use of high concentrations of desflurane.122
Maternal-fetal sheep studies, with no surgical stimulus to either the mother or fetus, show that deep maternal inhalation anesthesia (> 2.0 MAC) results in progressive fetal acidosis. By contrast, lower maternal inhalation anesthesia (1.0 MAC) also may be undesirable because it does not adequately block the fetal response to a painful stimulus, which includes increased fetal catecholamines, vasoconstriction, and redistribution of fetal blood flow.184 However, the combined effect of adequate fetal anesthesia with a halogenated agent, intrauterine manipulation, and fetal stress on fetal cardiovascular stability and regional blood flow remains unknown. Brief fetal exposure to deep maternal inhalation anesthesia (i.e., 2.0 to 3.0 MAC) does not appear significantly detrimental to the fetus, with no fetal hypoxia, hypercarbia, or acidosis observed even after exposures of 2 hours if maternal arterial pressure is maintained.136 However, others have seen acidosis after 45 minutes of fetal exposure to anesthesia.185
Another concern is that anesthetic agents may result in neuronal apoptosis in the developing fetal brain (see Chapter 10). Initial evidence was found in 2003, when an anesthetic consisting of midazolam, nitrous oxide, and isoflurane was shown to alter neurons in the developing brain of 7-day-old rats and to cause long-term impairment of brain function.186 Additional in utero rat studies noted that fetal exposure to isoflurane for 4 hours near mid gestation resulted in abnormal spatial memory acquisition.187 Primate studies have demonstrated significant neurodegeneration in the neonatal period after exposure to isoflurane.188 It is not currently known if anesthetic agents similarly affect human fetuses or newborns. Several retrospective human studies have produced inconclusive results. One retrospective cohort trial noted that a single short anesthetic exposure in children younger than age 2 years did not have long-term cognitive implications; however, exposure to multiple anesthetics was a significant risk factor for development of learning disabilities.189 Another retrospective study examined anesthetic exposure among twins and found no causal relationship between early anesthetic exposure and learning disabilities.190 Although there is concern for neurotoxicity in human fetuses or children exposed to anesthetic drugs, current findings remain inconclusive about the long-term effects of anesthetic agents on brain function in humans. A 2007 U.S. Food and Drug Administration advisory committee concluded that no change in clinical practice is justifiable based on current data.191,192 The neurocognitive effect of anesthetic exposure on fetuses during fetal surgery remains unknown.
Fetal Monitoring
Maternal and fetal anesthesia, uterine incision, fetal manipulation, and surgical stress may adversely affect uteroplacental and fetoplacental circulation by several mechanisms. Maternal hypotension, increased uterine activity, and maternal hyperventilation and hypocarbia impair uteroplacental and/or umbilical blood flow. Fetal manipulation may affect fetal cardiac output, regional distribution of cardiac output, and umbilical blood flow. Direct compression of the umbilical cord, inferior vena cava, and mediastinum adversely affects fetal circulation. Current methods of intraoperative fetal monitoring include FHR monitoring, pulse oximetry, ultrasonography (including echocardiography and Doppler assessment of umbilical cord blood flow), and blood gas and acid-base analysis.
Intraoperative monitoring of the FHR during open fetal surgery was initially attempted with a standard fetal corkscrew electrode processed by a standard FHR cardiotachometer, but signal failure was frequent, secondary to low signal amplitude and movement artifact. With use of atrial pacing wires sutured to the fetus, proximal wire shielding, increased gain to allow signal amplification, and filter modification, a more reliable display of fetal ECG with visible P waves and QRS complexes was possible. Unfortunately, this technique did not eliminate motion artifact. Analysis of the fetal ECG using ST waveform analysis may prove beneficial for fetal surgery.193 A fetal scalp electrode has been used successfully as part of an EXIT procedure to monitor FHR after the head was exposed.194
Plethysmography combined with pulse oximetry has proved to be very useful, particularly for the EXIT procedure. The predictive value of pulse oximetry may be superior to FHR monitoring; bradycardia has been found to be a late sign of fetal compromise in fetal lambs subjected to umbilical cord compression (Figure 7-4).195 However, bradycardia can also precede oxyhemoglobin desaturation during human fetal surgery (Figure 7-5).196
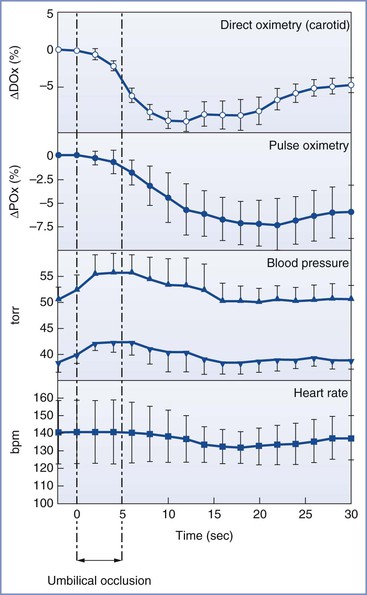
FIGURE 7-4 Response to 5 seconds of umbilical cord occlusion in the fetal lamb. Direct oximetry and pulse oximetry are expressed as delta saturation (Tx − T0). (From Luks FI, Johnson BD, Papadakis K, et al. Predictive value of monitoring parameters in fetal surgery. J Pediatr Surg 1998; 33:1297-1301.)
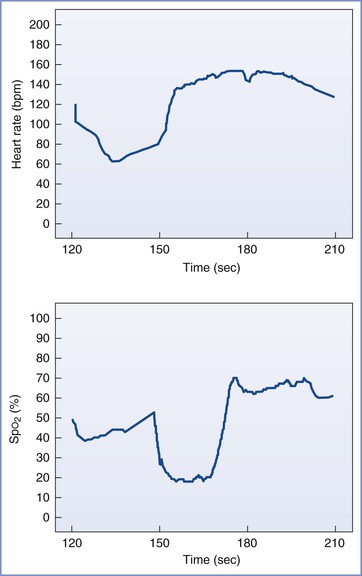
FIGURE 7-5 Graphic representation of monitoring data from a fetus at 24 weeks’ gestation undergoing open diaphragmatic hernia repair. Top graph shows fetal heart rate (bpm) and bottom graph shows oxygen saturation (SpO2) detailed over a 2-minute period. The tracings demonstrate an acute decrease in fetal heart rate (FHR) with an associated decrease in fetal SpO2. The onset of the desaturation detected in the fetal hand is delayed in relation to the onset of the bradycardia. Similarly, the recovery in FHR precedes the onset of the rapid increase in saturation. This pattern most likely represents a transport delay of the blood from the heart to the fetal hand. (From Rosen MA. Anesthesia for fetal surgery. In Hughes SC, Levinson G, Rosen MA, editors. Shnider and Levinson’s Anesthesia for Obstetrics. 4th edition. Philadelphia, Lippincott Williams & Wilkins, 2002.)
Ultrasonography is a crucial intraoperative fetal monitoring device. The FHR can be determined with visualization of the heart or with Doppler assessment of umbilical cord blood flow. Fetal cardiac contractility and volume also can be assessed qualitatively by echocardiography. Unfortunately, the sterile transducer often cannot be positioned continuously because its location interferes with surgery.
New devices may allow monitoring of (1) fetal blood pressure and EEG; (2) fetal arterial blood oxygen saturation, PO2, and PCO2; and (3) fetal cerebral oxygenation, blood volume, and blood flow with near-infrared spectroscopy.
The Future of Fetal Therapy
Successful diagnosis and management of complex congenital anomalies and other fetal conditions amenable to prenatal intervention relies on a multidisciplinary team whose members communicate and work together to improve fetal outcome without incurring substantive maternal risk. Well-organized, multidisciplinary, professional, and comprehensive fetal treatment programs at academic medical centers facilitate the sustained effort to innovate new techniques, challenge dogma, and ensure ongoing success. More collaborative clinical investigation among international research centers will benefit these efforts and guide the evolution of prenatal fetal therapy. Advances in technology will continue to drive improvement and availability of fetal intervention. For example, dynamic tracheal occlusion for CDH rather than complete occlusion, using devices that have pressure-sensitive valves to allow egress of fetal lung fluid, may improve outcome by more closely imitating normal developmental physiology and result in more normal lung function than that seen with current techniques.
With continued advances and miniaturization of invasive techniques and decreases in maternal and fetal risk, fetal intervention for a wider variety of procedures is the likely future for fetal therapy. Endoscopic repair of myelomeningocele has already been attempted. Other procedures with future potential include (1) placement of a cardiac pacemaker to restore sinus rhythm and improve survival in fetuses with complete heart block or a cardiac arrhythmia, when treatment is refractory to transplacental administration of medication; (2) repair of craniofacial anomalies, gastroschisis, and cleft lip and palate; and (3) corrections of skeletal anomalies by allogenic bone grafting. Innovations focused on tissue engineering, using stem cells and gene therapies, are exciting possibilities for future fetal therapy.
Although the rationale for prenatal fetal therapy seems straightforward, many issues remain problematic. Questions remain regarding the manner in which fetal development is modulated by intrauterine intervention. Other questions revolve around maternal and fetal rights, safety, efficacy, long-term outcomes, cost-effectiveness, and societal resource allocation. Societal expectations and the availability of therapy must be balanced against the budgetary constraints in contemporary health care. In addition, there is concern about the sensitivity, specificity, and appropriate use of diagnostic testing. Fetal therapy raises complex social, ethical, and legal issues that go beyond those customary for therapeutic intervention.197 In some cases, distinguishing innovative therapy from experimentation is difficult. The ethical framework for the transition from innovation to clinical trials to offering fetal surgery as a standard of care must be managed thoughtfully and responsibly. A bioethics committee with representatives from both the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists has provided recommendations for medical institutions offering fetal surgery.198
Fetal therapy must be evaluated carefully in properly conducted trials and undertaken only with great caution and informed maternal consent. Publication in scientific journals and open communication with colleagues nationally and internationally facilitates the moral obligation of researchers to report all results to allow peer review of the merits and liabilities of fetal surgery. The principal concept of primum non nocere argues that it is unethical to undertake human trials until a procedure is appropriately tested in animals.199,200
Training programs are necessary to safely expand access and services from academic medical centers to local units. There is debate whether results of trials from tertiary academic centers such as the MOMS trial75 are applicable to institutions that did not participate. Outcomes from new fetal centers with little experience and patients outside strict inclusion criteria may have less favorable outcomes with greater morbidity.201 Fetuses that may benefit from invasive therapy must be carefully distinguished from those that will not. Intervention should be undertaken only when there is a reasonable probability of long-term benefit and minimal maternal risk. Despite more than 3 decades of experience, there remains much to study, a great deal to learn, and, it is hoped, a lot to achieve.
References
1. Liley AW. Intrauterine transfusion of foetus in haemolytic disease. Br Med J. 1963;2:1107–1109.
2. Adamsons K Jr. Fetal surgery. N Engl J Med. 1966;275:204–206.
3. Harrison MR, Bressack MA, Churg AM, de Lorimier AA. Correction of congenital diaphragmatic hernia in utero. II. Simulated correction permits fetal lung growth with survival at birth. Surgery. 1980;88:260–268.
4. Harrison MR, Jester JA, Ross NA. Correction of congenital diaphragmatic hernia in utero. I. The model: intrathoracic balloon produces fatal pulmonary hypoplasia. Surgery. 1980;88:174–182.
5. Harrison MR, Anderson J, Rosen MA, et al. Fetal surgery in the primate. I. Anesthetic, surgical, and tocolytic management to maximize fetal-neonatal survival. J Pediatr Surg. 1982;17:115–122.
6. Harrison MR, Golbus MS, Filly RA, et al. Fetal surgery for congenital hydronephrosis. N Engl J Med. 1982;306:591–593.
7. Golombeck K, Ball RH, Lee H, et al. Maternal morbidity after maternal-fetal surgery. Am J Obstet Gynecol. 2006;194:834–839.
8. Longaker MT, Golbus MS, Filly RA, et al. Maternal outcome after open fetal surgery: a review of the first 17 human cases. JAMA. 1991;265:737–741.
9. Sutton LN, Adzick NS, Bilaniuk LT, et al. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA. 1999;282:1826–1831.
10. Tulipan N, Bruner JP, Hernanz-Schulman M, et al. Effect of intrauterine myelomeningocele repair on central nervous system structure and function. Pediatr Neurosurg. 1999;31:183–188.
11. Mychaliska GB, Bealer JF, Graf JL, et al. Operating on placental support: the ex utero intrapartum treatment procedure. J Pediatr Surg. 1997;32:227–230.
12. Estes JM, MacGillivray TE, Hedrick MH, et al. Fetoscopic surgery for the treatment of congenital anomalies. J Pediatr Surg. 1992;27:950–954.
13. Harrison MR, Filly RA, Parer JT, et al. Management of the fetus with a urinary tract malformation. JAMA. 1981;246:635–639.
14. Glick PL, Harrison MR, Adzick NS, et al. Correction of congenital hydronephrosis in utero. IV. In utero decompression prevents renal dysplasia. J Pediatr Surg. 1984;19:649–657.
15. Lissauer D, Morris RK, Kilby MD. Fetal lower urinary tract obstruction. Semin Fetal Neonatal Med. 2007;12:464–470.
16. Golbus MS, Harrison MR, Filly RA, et al. In utero treatment of urinary tract obstruction. Am J Obstet Gynecol. 1982;142:383–388.
17. Wu S, Johnson MP. Fetal lower urinary tract obstruction. Clin Perinatol. 2009;36:377–390.
18. Freedman AL, Johnson MP, Smith CA, et al. Long-term outcome in children after antenatal intervention for obstructive uropathies. Lancet. 1999;354:374–377.
20. Kilby M, Khan K, Morris K, et al. PLUTO trial protocol: percutaneous shunting for lower urinary tract obstruction randomised controlled trial. BJOG. 2007;114:904–905.
21. Quintero RA, Hume R, Smith C, et al. Percutaneous fetal cystoscopy and endoscopic fulguration of posterior urethral valves. Am J Obstet Gynecol. 1995;172:206–209.
22. Morris RK, Ruano R, Kilby MD. Effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for lower urinary tract obstruction: a systematic review. Ultrasound Obstet Gynecol. 2011;37:629–637.
23. Danzer E, Hedrick HL. Neurodevelopmental and neurofunctional outcomes in children with congenital diaphragmatic hernia. Early Hum Dev. 2011;87:625–632.
24. de Buys Roessingh AS, Dinh-Xuan AT. Congenital diaphragmatic hernia: current status and review of the literature. Eur J Pediatr. 2009;168:393–406.
25. Mayer S, Klaritsch P, Petersen S, et al. The correlation between lung volume and liver herniation measurements by fetal MRI in isolated congenital diaphragmatic hernia: a systematic review and meta-analysis of observational studies. Prenat Diagn. 2011;31:1086–1096.
26. Samangaya RA, Choudhri S, Murphy F, et al. Outcomes of congenital diaphragmatic hernia: a 12-year experience. Prenat Diagn. 2012;32:523–529.
27. Waag KL, Loff S, Zahn K, et al. Congenital diaphragmatic hernia: a modern day approach. Semin Pediatr Surg. 2008;17:244–254.
28. Harrison MR, Adzick NS, Flake AW, et al. Correction of congenital diaphragmatic hernia in utero. VI. Hard-earned lessons. J Pediatr Surg. 1993;28:1411–1417.
29. Esteve C, Toubas F, Gaudiche O, et al. [Evaluation of 5 years of experimental in utero surgery for the repair of diaphragmatic hernia]. Ann Fr Anesth Reanim. 1992;11:193–200.
30. Wilson JM, DiFiore JW, Peters CA. Experimental fetal tracheal ligation prevents the pulmonary hypoplasia associated with fetal nephrectomy: possible application for congenital diaphragmatic hernia. J Pediatr Surg. 1993;28:1433–1439.
31. Alcorn D, Adamson TM, Lambert TF, et al. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat. 1977;123:649–660.
32. Hedrick MH, Estes JM, Sullivan KM, et al. Plug the lung until it grows (PLUG): a new method to treat congenital diaphragmatic hernia in utero. J Pediatr Surg. 1994;29:612–617.
33. Bealer JF, Skarsgard ED, Hedrick MH, et al. The ‘PLUG’ odyssey: adventures in experimental fetal tracheal occlusion. J Pediatr Surg. 1995;30:361–364.
34. DiFiore JW, Fauza DO, Slavin R, et al. Experimental fetal tracheal ligation reverses the structural and physiological effects of pulmonary hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg. 1994;29:248–256.
35. Deprest JA, Evrard VA, Van Ballaer PP, et al. Tracheoscopic endoluminal plugging using an inflatable device in the fetal lamb model. Eur J Obstet Gynecol Reprod Biol. 1998;81:165–169.
36. Harrison MR, Albanese CT, Hawgood SB, et al. Fetoscopic temporary tracheal occlusion by means of detachable balloon for congenital diaphragmatic hernia. Am J Obstet Gynecol. 2001;185:730–733.
37. Harrison MR, Keller RL, Hawgood SB, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–1924.
38. Metkus AP, Filly RA, Stringer MD, et al. Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg. 1996;31:148–151.
39. Jani JC, Nicolaides KH, Gratacos E, et al. Severe diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol. 2009;34:304–310.
40. Jani J, Nicolaides KH, Keller RL, et al. Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol. 2007;30:67–71.
41. Jani J, Gratacos E, Greenough A, et al. Percutaneous fetal endoscopic tracheal occlusion (FETO) for severe left-sided congenital diaphragmatic hernia. Clin Obstet Gynecol. 2005;48:910–922.
42. Deprest J, Jani J, Cannie M, et al. Prenatal intervention for isolated congenital diaphragmatic hernia. Curr Opin Obstet Gynecol. 2006;18:355–367.
43. Jani J, Valencia C, Cannie M, et al. Tracheal diameter at birth in severe congenital diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Prenat Diagn. 2011;31:699–704.
44. Nelson SM, Hajivassiliou CA, Haddock G, et al. Rescue of the hypoplastic lung by prenatal cyclical strain. Am J Respir Crit Care Med. 2005;171:1395–1402.
45. Ruano R, Yoshisaki CT, da Silva MM, et al. A randomized controlled trial of fetal endoscopic tracheal occlusion versus postnatal management of severe isolated congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2012;39:20–27.
46. Dekoninck P, Gratacos E, Van Mieghem T, et al. Results of fetal endoscopic tracheal occlusion for congenital diaphragmatic hernia and the set up of the randomized controlled TOTAL trial. Early Hum Dev. 2011;87:619–624.
47. Peralta CF, Cavoretto P, Csapo B, et al. Assessment of lung area in normal fetuses at 12-32 weeks. Ultrasound Obstet Gynecol. 2005;26:718–724.
48. Jani JC, Nicolaides KH, Gratacos E, et al. Fetal lung-to-head ratio in the prediction of survival in severe left-sided diaphragmatic hernia treated by fetal endoscopic tracheal occlusion (FETO). Am J Obstet Gynecol. 2006;195:1646–1650.
49. Jani JC, Benachi A, Nicolaides KH, et al. Prenatal prediction of neonatal morbidity in survivors with congenital diaphragmatic hernia: a multicenter study. Ultrasound Obstet Gynecol. 2009;33:64–69.
50. Shimohira M, Hara M, Kitase M, et al. Congenital pulmonary airway malformation: CT-pathologic correlation. J Thorac Imaging. 2007;22:149–153.
51. Kim YT, Kim JS, Park JD, et al. Treatment of congenital cystic adenomatoid malformation-does resection in the early postnatal period increase surgical risk? Eur J Cardiothorac Surg. 2005;27:658–661.
52. Stocker JT. Congenital and developmental diseases. Hammar S. Pulmonary Pathology. Springer-Verlag: New York; 1994:155–190.
53. Harrison MR, Adzick NS, Jennings RW, et al. Antenatal intervention for congenital cystic adenomatoid malformation. Lancet. 1990;336:965–967.
54. Yong PJ, Von Dadelszen P, Carpara D, et al. Prediction of pediatric outcome after prenatal diagnosis and expectant antenatal management of congenital cystic adenomatoid malformation. Fetal Diagn Ther. 2012;31:94–102.
55. Mann S, Wilson RD, Bebbington MW, et al. Antenatal diagnosis and management of congenital cystic adenomatoid malformation. Semin Fetal Neonatal Med. 2007;12:477–481.
56. Wilson RD, Baxter JK, Johnson MP, et al. Thoracoamniotic shunts: fetal treatment of pleural effusions and congenital cystic adenomatoid malformations. Fetal Diagn Ther. 2004;19:413–420.
57. Lakhoo K. Management of congenital cystic adenomatous malformations of the lung. Arch Dis Child Fetal Neonatal Ed. 2009;94:F73–F76.
58. Grethel EJ, Wagner AJ, Clifton MS, et al. Fetal intervention for mass lesions and hydrops improves outcome: a 15-year experience. J Pediatr Surg. 2007;42:117–123.
59. Curran PF, Jelin EB, Rand L, et al. Prenatal steroids for microcystic congenital cystic adenomatoid malformations. J Pediatr Surg. 2010;45:145–150.
60. Peranteau WH, Wilson RD, Liechty KW, et al. Effect of maternal betamethasone administration on prenatal congenital cystic adenomatoid malformation growth and fetal survival. Fetal Diagn Ther. 2007;22:365–371.
61. Loh KC, Jelin E, Hirose S, et al. Microcystic congenital pulmonary airway malformation with hydrops fetalis: steroids vs open fetal resection. J Pediatr Surg. 2012;47:36–39.
62. Rodeck CH, Fisk NM, Fraser DI, Nicolini U. Long-term in utero drainage of fetal hydrothorax. N Engl J Med. 1988;319:1135–1138.
63. Wilson RD, Hedrick H, Flake AW, et al. Sacrococcygeal teratomas: prenatal surveillance, growth and pregnancy outcome. Fetal Diagn Ther. 2009;25:15–20.
64. Hirose S, Farmer DL. Fetal surgery for sacrococcygeal teratoma. Clin Perinatol. 2003;30:493–506.
66. Adzick NS. Fetal myelomeningocele: natural history, pathophysiology, and in-utero intervention. Semin Fetal Neonatal Med. 2010;15:9–14.
67. Julia V, Sancho MA, Albert A, et al. Prenatal covering of the spinal cord decreases neurologic sequelae in a myelomeningocele model. J Pediatr Surg. 2006;41:1125–1129.
68. Yoshizawa J, Sbragia L, Paek BW, et al. Fetal surgery for repair of myelomeningocele allows normal development of anal sphincter muscles in sheep. Pediatr Surg Int. 2004;20:14–18.
69. Fichter MA, Dornseifer U, Henke J, et al. Fetal spina bifida repair—current trends and prospects of intrauterine neurosurgery. Fetal Diagn Ther. 2008;23:271–286.
70. Hirose S, Farmer DL. Fetal surgery for myelomeningocele. Clin Perinatol. 2009;36:431–438.
71. Bol KA, Collins JS, Kirby RS. Survival of infants with neural tube defects in the presence of folic acid fortification. Pediatrics. 2006;117:803–813.
72. Shaer CM, Chescheir N, Schulkin J. Myelomeningocele: a review of the epidemiology, genetics, risk factors for conception, prenatal diagnosis, and prognosis for affected individuals. Obstet Gynecol Surv. 2007;62:471–479.
73. Oakeshott P, Hunt GM. Long-term outcome in open spina bifida. Br J Gen Pract. 2003;53:632–636.
74. Bruner JP. Intrauterine surgery in myelomeningocele. Semin Fetal Neonatal Med. 2007;12:471–476.
75. Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004.
76. Verbeek RJ, Heep A, Maurits NM, et al. Fetal endoscopic myelomeningocele closure preserves segmental neurological function. Dev Med Child Neurol. 2012;54:15–22.
77. Chalouhi GE, Essaoui M, Stirnemann J, et al. Laser therapy for twin-to-twin transfusion syndrome (TTTS). Prenat Diagn. 2011;31:637–646.
78. El Kateb A, Ville Y. Update on twin-to-twin transfusion syndrome. Best Pract Res Clin Obstet Gynaecol. 2008;22:63–75.
79. Crombleholme TM, Shera D, Lee H, et al. A prospective, randomized, multicenter trial of amnioreduction vs selective fetoscopic laser photocoagulation for the treatment of severe twin-twin transfusion syndrome. Am J Obstet Gynecol. 2007;197:396 e1–9.
80. Bajoria R, Wigglesworth J, Fisk NM. Angioarchitecture of monochorionic placentas in relation to the twin-twin transfusion syndrome. Am J Obstet Gynecol. 1995;172:856–863.
81. Habli M, Lim FY, Crombleholme T. Twin-to-twin transfusion syndrome: a comprehensive update. Clin Perinatol. 2009;36:391–416.
82. Lopriore E, Ortibus E, Acosta-Rojas R, et al. Risk factors for neurodevelopment impairment in twin-twin transfusion syndrome treated with fetoscopic laser surgery. Obstet Gynecol. 2009;113:361–366.
83. Yamamoto M, El Murr L, Robyr R, et al. Incidence and impact of perioperative complications in 175 fetoscopy-guided laser coagulations of chorionic plate anastomoses in fetofetal transfusion syndrome before 26 weeks of gestation. Am J Obstet Gynecol. 2005;193:1110–1116.
84. Saunders NJ, Snijders RJ, Nicolaides KH. Therapeutic amniocentesis in twin-twin transfusion syndrome appearing in the second trimester of pregnancy. Am J Obstet Gynecol. 1992;166:820–824.
85. Mari G, Roberts A, Detti L, et al. Perinatal morbidity and mortality rates in severe twin-twin transfusion syndrome: results of the International Amnioreduction Registry. Am J Obstet Gynecol. 2001;185:708–715.
86. Moise KJ Jr, Dorman K, Lamvu G, et al. A randomized trial of amnioreduction versus septostomy in the treatment of twin-twin transfusion syndrome. Am J Obstet Gynecol. 2005;193:701–707.
87. Quintero RA, Dickinson JE, Morales WJ, et al. Stage-based treatment of twin-twin transfusion syndrome. Am J Obstet Gynecol. 2003;188:1333–1340.
88. Lewi L, Jani J, Cannie M, et al. Intertwin anastomoses in monochorionic placentas after fetoscopic laser coagulation for twin-to-twin transfusion syndrome: is there more than meets the eye? Am J Obstet Gynecol. 2006;194:790–795.
89. Senat MV, Deprest J, Boulvain M, et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med. 2004;351:136–144.
90. Salomon LJ, Ortqvist L, Aegerter P, et al. Long-term developmental follow-up of infants who participated in a randomized clinical trial of amniocentesis vs laser photocoagulation for the treatment of twin-to-twin transfusion syndrome. Am J Obstet Gynecol. 2010;203:444 e1–7.
91. Rossi AC, D’Addario V. Laser therapy and serial amnioreduction as treatment for twin-twin transfusion syndrome: a metaanalysis and review of literature. Am J Obstet Gynecol. 2008;198:147–152.
92. Roberts D, Gates S, Kilby M, Neilson JP. Interventions for twin-twin transfusion syndrome: a Cochrane review. Ultrasound Obstet Gynecol. 2008;31:701–711.
93. Chmait RH, Khan A, Benirschke K, et al. Perinatal survival following preferential sequential selective laser surgery for twin-twin transfusion syndrome. J Matern Fetal Neonatal Med. 2010;23:10–16.
94. Chmait RH, Kontopoulos EV, Korst LM, et al. Stage-based outcomes of 682 consecutive cases of twin-twin transfusion syndrome treated with laser surgery: the USFetus experience. Am J Obstet Gynecol. 2011;204:393 e1–6.
95. Moore TR, Gale S, Benirschke K. Perinatal outcome of forty-nine pregnancies complicated by acardiac twinning. Am J Obstet Gynecol. 1990;163:907–912.
96. Lee H, Wagner AJ, Sy E, et al. Efficacy of radiofrequency ablation for twin-reversed arterial perfusion sequence. Am J Obstet Gynecol. 2007;196:459 e1–4.
97. Nakata M, Sumie M, Murata S, et al. Fetoscopic laser photocoagulation of placental communicating vessels for twin-reversed arterial perfusion sequence. J Obstet Gynaecol Res. 2008;34:649–652.
98. Hecher K, Lewi L, Gratacos E, et al. Twin reversed arterial perfusion: fetoscopic laser coagulation of placental anastomoses or the umbilical cord. Ultrasound Obstet Gynecol. 2006;28:688–691.
99. McElhinney DB, Tworetzky W, Lock JE. Current status of fetal cardiac intervention. Circulation. 2010;121:1256–1263.
100. Bacha EA. Impact of fetal cardiac intervention on congenital heart surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2011;14:35–37.
101. Oepkes D, Moon-Grady AJ, Wilkins-Haug L, et al. 2010 Report from the ISPD Special Interest Group fetal therapy: fetal cardiac interventions. Prenat Diagn. 2011;31:249–251.
102. Ikai A, Riemer RK, Ramamoorthy C, et al. Preliminary results of fetal cardiac bypass in nonhuman primates. J Thorac Cardiovasc Surg. 2005;129:175–181.
103. Zhou CB, Zhuang J, Chen JM, et al. Decrease in inflammatory response does not prevent placental dysfunction after fetal cardiac bypass in goats. J Thorac Cardiovasc Surg. 2012;143:445–450.
104. Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126:1172–1180.
105. Robinson MB, Crombleholme TM, Kurth CD. Maternal pulmonary edema during fetoscopic surgery. Anesth Analg. 2008;107:1978–1980.
106. Hering R, Hoeft A, Putensen C, et al. Maternal haemodynamics and lung water content during percutaneous fetoscopic interventions under general anaesthesia. Br J Anaesth. 2009;102:523–527.
107. Sydorak RM, Hirose S, Sandberg PL, et al. Chorioamniotic membrane separation following fetal surgery. J Perinatol. 2002;22:407–410.
108. Deprest J, Emonds MP, Richter J, et al. Amniopatch for iatrogenic rupture of the fetal membranes. Prenat Diagn. 2011;31:661–666.
109. Cortes RA, Wagner AJ, Lee H, et al. Pre-emptive placement of a presealant for amniotic access. Am J Obstet Gynecol. 2005;193:1197–1203.
110. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: neonatal resuscitation guidelines. Pediatrics. 2006;117:e1029–e1038.
112. Van de Velde M, Van Schoubroeck D, Lewi LE, et al. Remifentanil for fetal immobilization and maternal sedation during fetoscopic surgery: a randomized, double-blind comparison with diazepam. Anesth Analg. 2005;101:251–258.
113. Leveque C, Murat I, Toubas F, et al. Fetal neuromuscular blockade with vecuronium bromide: studies during intravascular intrauterine transfusion in isoimmunized pregnancies. Anesthesiology. 1992;76:642–644.
114. Fisk NM, Gitau R, Teixeira JM, et al. Effect of direct fetal opioid analgesia on fetal hormonal and hemodynamic stress response to intrauterine needling. Anesthesiology. 2001;95:828–835.
115. Rosen MA. Anesthesia for fetal procedures and surgery. Yonsei Med J. 2001;42:669–680.
116. Kafali H, Kaya T, Gursoy S, et al. The role of K(+) channels on the inhibitor effect of sevoflurane in pregnant rat myometrium. Anesth Analg. 2002;94:174–178.
117. DiFederico EM, Burlingame JM, Kilpatrick SJ, et al. Pulmonary edema in obstetric patients is rapidly resolved except in the presence of infection or of nitroglycerin tocolysis after open fetal surgery. Am J Obstet Gynecol. 1998;179:925–933.
118. Ngan Kee WD, Khaw KS, Tan PE, et al. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–512.
119. Ngan Kee WD, Lee A, Khaw KS, et al. A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: the effects on fetal acid-base status and hemodynamic control. Anesth Analg. 2008;107:1295–1302.
120. Habib AS. Review article: a review of the impact of phenylephrine administration on maternal hemodynamics and maternal and neonatal outcomes in women undergoing cesarean delivery under spinal anesthesia. Anesth Analg. 2012;114:377–390.
121. Rosen MA, Andreae MH, Cameron AG. Nitroglycerin for fetal surgery: fetoscopy and ex utero intrapartum treatment procedure with malignant hyperthermia precautions. Anesth Analg. 2003;96:698–700.
122. Boat A, Mahmoud M, Michelfelder EC, et al. Supplementing desflurane with intravenous anesthesia reduces fetal cardiac dysfunction during open fetal surgery. Paediatr Anaesth. 2010;20:748–756.
123. DiFederico EM, Harrison M, Matthay MA. Pulmonary edema in a woman following fetal surgery. Chest. 1996;109:1114–1117.
124. Bealer JF, Raisanen J, Skarsgard ED, et al. The incidence and spectrum of neurological injury after open fetal surgery. J Pediatr Surg. 1995;30:1150–1154.
125. Bond SJ, Harrison MR, Slotnick RN, et al. Cesarean delivery and hysterotomy using an absorbable stapling device. Obstet Gynecol. 1989;74:25–28.
126. Aboud E, Neales K. The effect of maternal hypothermia on the fetal heart rate. Int J Gynaecol Obstet. 1999;66:163–164.
127. Santolaya-Forgas J, Romero R, Mehendale R. The effect of continuous morphine administration on maternal plasma oxytocin concentration and uterine contractions after open fetal surgery. J Matern Fetal Neonatal Med. 2006;19:231–238.
128. Tame JD, Abrams LM, Ding XY, et al. Level of postoperative analgesia is a critical factor in regulation of myometrial contractility after laparotomy in the pregnant baboon: implications for human fetal surgery. Am J Obstet Gynecol. 1999;180:1196–1201.
129. Smith CV, Rayburn WF, Allen KV, et al. Influence of intravenous fentanyl on fetal biophysical parameters during labor. J Matern Fetal Med. 1996;5:89–92.
130. Garcia PJ, Olutoye OO, Ivey RT, Olutoye OA. Case scenario: anesthesia for maternal-fetal surgery: the Ex Utero Intrapartum Therapy (EXIT) procedure. Anesthesiology. 2011;114:1446–1452.
131. MacKenzie TC, Crombleholme TM, Flake AW. The ex-utero intrapartum treatment. Curr Opin Pediatr. 2002;14:453–458.
132. Abraham RJ, Sau A, Maxwell D. A review of the EXIT (Ex utero Intrapartum Treatment) procedure. J Obstet Gynaecol. 2010;30:1–5.
133. Hirose S, Farmer DL, Lee H, et al. The ex utero intrapartum treatment procedure: looking back at the EXIT. J Pediatr Surg. 2004;39:375–380.
134. Olutoye OO, Olutoye OA. EXIT procedure for fetal neck masses. Curr Opin Pediatr. 2012;24:386–393.
135. Tran KM, Maxwell LG, Cohen DE, et al. Quantification of serum fentanyl concentrations from umbilical cord blood during ex utero intrapartum therapy. Anesth Analg. 2012;114:1265–1267.
136. Hirose S, Sydorak RM, Tsao K, et al. Spectrum of intrapartum management strategies for giant fetal cervical teratoma. J Pediatr Surg. 2003;38:446–450.
137. Rahbar R, Vogel A, Myers LB, et al. Fetal surgery in otolaryngology: a new era in the diagnosis and management of fetal airway obstruction because of advances in prenatal imaging. Arch Otolaryngol Head Neck Surg. 2005;131:393–398.
138. Khazin AF, Hon EH, Hehre FW. Effects of maternal hyperoxia on the fetus. I. Oxygen tension. Am J Obstet Gynecol. 1971;109:628–637.
139. Kunisaki SM, Barnewolt CE, Estroff JA, et al. Ex utero intrapartum treatment with extracorporeal membrane oxygenation for severe congenital diaphragmatic hernia. J Pediatr Surg. 2007;42:98–104.
140. Noah MM, Norton ME, Sandberg P, et al. Short-term maternal outcomes that are associated with the EXIT procedure, as compared with cesarean delivery. Am J Obstet Gynecol. 2002;186:773–777.
141. Clark KD, Viscomi CM, Lowell J, Chien EK. Nitroglycerin for relaxation to establish a fetal airway (EXIT procedure). Obstet Gynecol. 2004;103:1113–1115.
142. Benonis JG, Habib AS. Ex utero intrapartum treatment procedure in a patient with arthrogryposis multiplex congenita, using continuous spinal anesthesia and intravenous nitroglycerin for uterine relaxation. Int J Obstet Anesth. 2008;17:53–56.
143. George RB, Melnick AH, Rose EC, Habib AS. Case series: combined spinal epidural anesthesia for Cesarean delivery and ex utero intrapartum treatment procedure. Can J Anaesth. 2007;54:218–222.
144. Kan RE, Hughes SC, Rosen MA, et al. Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology. 1998;88:1467–1474.
145. Fink RJ, Allen TK, Habib AS. Remifentanil for fetal immobilization and analgesia during the ex utero intrapartum treatment procedure under combined spinal-epidural anaesthesia. Br J Anaesth. 2011;106:851–855.
146. Lee SJ, Ralston HJ, Drey EA, et al. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294:947–954.
147. Derbyshire SW. Locating the beginnings of pain. Bioethics. 1999;13:1–31.
148. Anand KJ, Brown MJ, Bloom SR, Aynsley-Green A. Studies on the hormonal regulation of fuel metabolism in the human newborn infant undergoing anaesthesia and surgery. Horm Res. 1985;22:115–128.
149. Anand KJ, Brown MJ, Causon RC, et al. Can the human neonate mount an endocrine and metabolic response to surgery? J Pediatr Surg. 1985;20:41–48.
150. Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. Lancet. 1987;1:62–66.
151. Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317:1321–1329.
152. Giannakoulopoulos X, Sepulveda W, Kourtis P, et al. Fetal plasma cortisol and beta-endorphin response to intrauterine needling. Lancet. 1994;344:77–81.
153. Giannakoulopoulos X, Teixeira J, Fisk N, Glover V. Human fetal and maternal noradrenaline responses to invasive procedures. Pediatr Res. 1999;45:494–499.
154. Gitau R, Fisk NM, Teixeira JM, et al. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86:104–109.
155. Teixeira J, Fogliani R, Giannakoulopoulos X, et al. Fetal haemodynamic stress response to invasive procedures. Lancet. 1996;347:624.
156. Teixeira JM, Glover V, Fisk NM. Acute cerebral redistribution in response to invasive procedures in the human fetus. Am J Obstet Gynecol. 1999;181:1018–1025.
157. Liley AW. The foetus as a personality. Aust N Z J Psychiatry. 1972;6:99–105.
159. Terenghi G, Sundaresan M, Moscoso G, Polak JM. Neuropeptides and a neuronal marker in cutaneous innervation during human foetal development. J Comp Neurol. 1993;328:595–603.
160. Konstantinidou AD, Silos-Santiago I, Flaris N, Snider WD. Development of the primary afferent projection in human spinal cord. J Comp Neurol. 1995;354:11–12.
161. Kostovic I, Judas M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec. 2002;267:1–6.
162. Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470.
163. Mrzljak L, Uylings HB, Kostovic I, Van Eden CG. Prenatal development of neurons in the human prefrontal cortex. I. A qualitative Golgi study. J Comp Neurol. 1988;271:355–386.
164. Hevner RF. Development of connections in the human visual system during fetal mid-gestation: a DiI-tracing study. J Neuropathol Exp Neurol. 2000;59:385–392.
165. Kostovic I, Rakic P. Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J Neurosci. 1984;4:25–42.
166. Krmpotic-Nemanic J, Kostovic I, Kelovic Z, et al. Development of the human fetal auditory cortex: growth of afferent fibres. Acta Anat (Basel). 1983;116:69–73.
167. Merker B. Consciousness without a cerebral cortex: a challenge for neuroscience and medicine. Behav Brain Sci. 2007;30:63–81.
168. Mellor DJ, Diesch TJ, Gunn AJ, Bennet L. The importance of ‘awareness’ for understanding fetal pain. Brain Res Brain Res Rev. 2005;49:455–471.
169. Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122:109–117.
170. Slater R, Cantarella A, Gallella S, et al. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–3666.
171. Slater R, Worley A, Fabrizi L, et al. Evoked potentials generated by noxious stimulation in the human infant brain. Eur J Pain. 2010;14:321–326.
172. Lowery CL, Hardman MP, Manning N, et al. Neurodevelopmental changes of fetal pain. Semin Perinatol. 2007;31:275–282.
173. Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet. 1997;349:599–603.
174. Clarke AS, Wittwer DJ, Abbott DH, Schneider ML. Long-term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Dev Psychobiol. 1994;27:257–269.
175. Glover V, Fisk N. Do fetuses feel pain? We don’t know; better to err on the safe side from mid-gestation. BMJ. 1996;313:796.
176. White MC, Wolf AR. Pain and stress in the human fetus. Best Pract Res Clin Anaesthesiol. 2004;18:205–220.
177. Rosen MA. Anesthesia and tocolysis for fetal intervention. Harrison MR, Golbus MS, Filly RA. The Unborn Patient: Prenatal Diagnosis and Treatment. Grune & Stratton: Orlando, FL; 1984:417–434.
178. Van de Velde M, Jani J, De Buck F, Deprest J. Fetal pain perception and pain management. Semin Fetal Neonatal Med. 2006;11:232–236.
179. Gregory GA, Wade JG, Beihl DR, et al. Fetal anesthetic requirement (MAC) for halothane. Anesth Analg. 1983;62:9–14.
180. Biehl DR, Yarnell R, Wade JG, Sitar D. The uptake of isoflurane by the foetal lamb in utero: effect on regional blood flow. Can Anaesth Soc J. 1983;30:581–586.
181. Biehl DR, Cote J, Wade JG, et al. Uptake of halothane by the foetal lamb in utero. Can Anaesth Soc J. 1983;30:24–27.
182. Biehl DR, Tweed WA, Cote J, et al. Effect of halothane on cardiac output and regional flow in the fetal lamb in utero. Anesth Analg. 1983;62:489–492.
183. Palahniuk RJ, Shnider SM. Maternal and fetal cardiovascular and acid-base changes during halothane and isoflurane anesthesia in the pregnant ewe. Anesthesiology. 1974;41:462–472.
184. Sabik JF, Assad RS, Hanley FL. Halothane as an anesthetic for fetal surgery. J Pediatr Surg. 1993;28:542–546.
185. Gaiser RR, Kurth CD, Cohen D, Crombleholme T. The cesarean delivery of a twin gestation under 2 minimum alveolar anesthetic concentration isoflurane: one normal and one with a large neck mass. Anesth Analg. 1999;88:584–586.
186. Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882.
187. Palanisamy A, Baxter MG, Keel PK, et al. Rats exposed to isoflurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology. 2011;114:521–528.
188. Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841.
189. Flick RP, Katusic SK, Colligan RC, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–e1061.
190. Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12:246–253.
191. Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509–520.
192. Stratmann G. Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–1179.
193. Rosen KG, Amer-Wahlin I, Luzietti R, Noren H. Fetal ECG waveform analysis. Best Pract Res Clin Obstet Gynaecol. 2004;18:485–514.
194. Kaneko M, Tokunaga S, Mukai M, et al. Application of a fetal scalp electrode for continuous fetal heart rate monitoring during an ex utero intrapartum treatment. J Pediatr Surg. 2011;46:e37–e40.
195. Luks FI, Johnson BD, Papadakis K, et al. Predictive value of monitoring parameters in fetal surgery. J Pediatr Surg. 1998;33:1297–1301.
196. Izumi A, Minakami H, Sato I. Fetal heart rate decelerations precede a decrease in fetal oxygen content. Gynecol Obstet Invest. 1997;44:26–31.
197. Dickens BM, Cook RJ. Legal and ethical issues in fetal surgery. Int J Gynaecol Obstet. 2011;115:80–83.
198. Maternal-fetal intervention and fetal care centers. Pediatrics. 2011;128:e473–e478.
199. Chervenak FA, McCullough LB. Ethics of fetal surgery. Clin Perinatol. 2009;36:237–246.
200. Shurtleff D. Fetal endoscopic myelomeningocele repair. Dev Med Child Neurol. 2012;54:4–5.
201. Danzer E, Johnson MP, Adzick NS. Fetal surgery for myelomeningocele: progress and perspectives. Dev Med Child Neurol. 2011;54:8–14.