Anemias
Red Blood Cell Morphology and Approach to Diagnosis
After completion of this chapter, the reader will be able to:
1. Define anemia and recognize laboratory results consistent with anemia.
2. Discuss the importance of the history and the physical examination in the diagnosis of anemia.
3. Describe clinical signs and symptoms of anemia and recognize them in clinical scenarios.
4. List procedures that are commonly performed for the detection and diagnosis of anemia.
5. Distinguish among effective, ineffective, and insufficient erythropoiesis when given examples.
6. Discuss the importance of the reticulocyte count in the evaluation of anemia.
7. Characterize the three groups of anemias involving decreased production that are categorized on the basis of mean cell volume (MCV) and give one example of each.
8. Recognize the importance of reviewing the peripheral blood film when assessing anemias and distinguish the important findings.
9. Describe the use of the red blood cell distribution width (RDW) in the diagnosis of anemias.
10. Briefly explain how the body adapts to anemia over time and the impact on the patient’s experience of the anemia.
11. Use an algorithm incorporating the reticulocyte count, MCV, and RDW to narrow the differential diagnosis of anemia.
12. Classify given examples of variations in red blood cell morphology as inclusions, shape changes, volume changes, or color changes.
Case Study
1. Why did the physician want the patient to come to the office before she prescribed therapy?
2. How do the MCV and reticulocyte count help determine the classification of the anemia?
3. Why is the examination of the peripheral blood film important in the work-up of an anemia?
Red blood cells (RBCs) perform the vital physiologic function of oxygen delivery to the tissues. The hemoglobin within the erythrocyte has the remarkable capacity to bind oxygen in the lungs and then release it appropriately in the tissues.1 The term anemia is derived from the Greek word anaimia, meaning “without blood.”2 A decrease in the number of RBCs, or the amount of hemoglobin in the RBCs, results in decreased oxygen delivery and subsequent tissue hypoxia. Anemia is a commonly encountered condition affecting an estimated 1.62 billion people worldwide.3 Anemia should not be thought of as a disease, but rather as a manifestation of other underlying disease processes.4,5 Therefore, the cause of all anemias should be thoroughly investigated. This chapter provides an overview of the diagnosis, mechanisms, and classification of anemia. In the following chapters, each anemia is discussed in detail.
Definition of Anemia
Anemia is defined operationally as a reduction, from the baseline value, in the total number of RBCs, amount of circulating hemoglobin, and RBC mass for a particular patient. In practice, this definition is not applicable, because a patient’s baseline value is rarely known.5,6 A more conventional definition is a decrease in RBCs, hemoglobin, and hematocrit below the reference range for healthy individuals of the same age, sex, and race, under similar environmental conditions.4–8 Problems with this conventional definition may occur for several reasons. The reference ranges are derived from large pools of “normal” individuals; however, the definition of normal is different for each of these data sets. This has led to the development of different reference ranges, depending on which pool of individuals was used. Furthermore, these pools of individuals lack the heterogeneity required to be universally applied to all the different populations.6
Clinical Findings
The history and physical examination are important components in making a clinical diagnosis of anemia. The classic symptoms associated with anemia are fatigue and shortness of breath. If oxygen delivery is decreased, then patients will not have enough energy to perform their daily functions. Obtaining a good history requires carefully questioning the patient, particularly with regard to diet, drug ingestion, exposure to chemicals, occupation, hobbies, travel, bleeding history, ethnic group, family history of disease, neurologic symptoms, previous medication, jaundice, and various underlying diseases that produce anemia.4,7–9 Although inquiry in these areas can reveal common conditions that can lead to anemia, there are numerous other possibilities as well. Therefore, a thorough discussion is required to elicit any potential cause of the anemia. For example, iron deficiency can lead to an interesting symptom called pica.10 Patients with pica have cravings for unusual substances such as ice (pagophagia), cornstarch, or clay. Alternatively, individuals with anemia may be asymptomatic, as can be seen in mild or slowly progressive anemias.
Certain features should be evaluated closely during the physical examination to provide clues to hematologic disorders, such as skin (for pallor, jaundice, petechiae), eyes (for hemorrhage), and mouth (for mucosal bleeding). The examination should also look for sternal tenderness, lymphadenopathy, cardiac murmurs, splenomegaly, and hepatomegaly.4,7–9 Jaundice is important for the assessment of anemia, because it may be due to increased RBC destruction, which suggests a hemolytic component to the anemia. Measuring vital signs is also a crucial component of the physical evaluation. Patients experiencing a rapid fall in hemoglobin concentration typically have tachycardia (fast heart rate), whereas if the anemia is long-standing, the heart rate may be normal due to the body’s ability to compensate for the anemia.
Moderate anemias (hemoglobin concentration of 7 to 10 g/dL) may not produce clinical signs or symptoms if the onset of anemia is slow.4 Depending on the patient’s age and cardiovascular state, however, moderate anemias may be associated with pallor of conjunctivae and nail beds, dyspnea, vertigo, headache, muscle weakness, lethargy, and other symptoms.4,7–9 Severe anemias (hemoglobin concentration of less than 7 g/dL) usually produce tachycardia, hypotension, and other symptoms of volume loss, in addition to the symptoms listed earlier. The severity of the anemia is gauged by the degree of reduction in RBC mass, cardiopulmonary adaptation, and the rapidity of progression of the anemia.4
Physiologic Adaptations
Reduced delivery of oxygen to tissues caused by reduced hemoglobin causes an increase in erythropoietin secretion by the kidneys. Erythropoietin stimulates the RBC precursors in the bone marrow, which leads to the release of more RBCs into the circulation (see Chapter 8). With persistent anemia, the body implements physiologic adaptations to increase the oxygen-carrying capacity of a reduced amount of hemoglobin. Heart rate, respiratory rate, and cardiac output are increased for a more rapid delivery of oxygenated blood to tissues. In addition, the tissue hypoxia triggers an increase in RBC 2,3-bisphosphoglycerate that shifts the oxygen dissociation curve to the right (decreased oxygen affinity of hemoglobin) and results in increased delivery of oxygen to tissues (see Chapter 10).11 This is a significant mechanism in chronic anemias that enables patients with low levels of hemoglobin to remain relatively asymptomatic. With persistent and severe anemia, however, the strain on the heart can ultimately lead to cardiac failure.
Mechanisms of Anemia
The life span of the RBC in the circulation is about 120 days. In a healthy individual with no anemia, each day approximately 1% of the RBCs are removed from circulation due to senescence, but the bone marrow continuously produces RBCs to replace those lost. Hematopoietic stem cells develop into erythroid precursor cells, and the bone marrow appropriately releases reticulocytes that mature into RBCs in the peripheral circulation. Adequate RBC production requires several nutritional factors, such as iron, vitamin B12, and folate. Globin synthesis also must function normally. In conditions with excessive bleeding or hemolysis, the bone marrow must increase RBC production to compensate for the increased RBC loss. Therefore, the maintenance of a stable hemoglobin concentration requires the production of functionally normal RBCs in sufficient numbers to replace the amount lost.4,7,8
Ineffective and Insufficient Erythropoiesis
Erythropoiesis is the term used for marrow erythroid proliferative activity. Normal erythropoiesis occurs in the bone marrow (see Chapter 8).4 When erythropoiesis is effective, the bone marrow is able to produce functional RBCs that leave the marrow and supply the peripheral circulation with adequate numbers of cells.
Ineffective erythropoiesis refers to the production of erythroid progenitor cells that are defective. These defective progenitors are often destroyed in the bone marrow before their maturation and release into the peripheral circulation. Several conditions, such as megaloblastic anemia, thalassemia, and sideroblastic anemia, are characterized by ineffective erythropoiesis. In these anemias, the peripheral blood hemoglobin is low despite an increase in RBC precursors in the bone marrow. The effective production rate is considerably less than the total production rate, which results in a decreased number of normal circulating RBCs. Consequently, the patient becomes anemic.12
Insufficient erythropoiesis refers to a decrease in the number of erythroid precursors in the bone marrow, resulting in decreased RBC production and anemia. Several factors can lead to the decreased RBC production, including a deficiency of iron (inadequate intake, malabsorption, excessive loss from chronic bleeding); a deficiency of erythropoietin, the hormone that stimulates erythroid precursor proliferation and maturation (renal disease); loss of the erythroid precursors due to an autoimmune reaction (aplastic anemia, acquired pure red cell aplasia) or infection (parvovirus B19); or suppression of the erythroid precursors due to infiltration of the bone marrow with granulomas (sarcoidosis) or malignant cells (acute leukemia).12
Acute Blood Loss and Hemolysis
Anemia can also develop as a result of acute blood loss (such as traumatic injury) or premature hemolysis resulting in a shortened RBC life span. (Note that chronic blood loss leads to iron deficiency and is covered under insufficient erythropoiesis in the previous section.) With acute blood loss and excessive hemolysis, the bone marrow is able to increase production of RBCs, but the level of response is inadequate to compensate for the excessive RBC loss. Numerous causes of hemolysis exist, including intrinsic defects in the RBC membrane, enzyme systems, or hemoglobin, or extrinsic causes such as antibody-mediated processes, mechanical fragmentation, or infection-related destruction.4,8,12
Laboratory Diagnosis of Anemia
Complete Blood Cell Count with Red Blood Cell Indices
To detect the presence of anemia, the medical laboratory professional performs a complete blood count (CBC) using an automated hematology analyzer to determine the RBC count, hemoglobin concentration, hematocrit, RBC indices, white blood cell (WBC) count, and platelet count. The RBC indices include the mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC) (see Chapter 14).13 The most important of these indices is the MCV, a measure of the average RBC volume in femtoliters (fL). Reference ranges for these determinations are listed on the inside front cover of the text. Automated hematology analyzers also provide an RBC histogram and the red blood cell distribution width (RDW). A relative and absolute reticulocyte count, described subsequently, should be performed for every patient when anemia is found. Automated analyzers are available to perform reticulocyte counts with greater accuracy and precision than manual counting methods.
The RBC histogram is an RBC volume frequency distribution curve with the relative number of cells plotted on the ordinate and RBC volume in femtoliters on the abscissa. With a normal population of RBCs, the distribution is approximately gaussian. Abnormalities include a shift in the curve to the left (microcytosis) or to the right (macrocytosis), and a widening of the curve caused by a greater variation of RBC volume about the mean or by the presence of two populations of RBCs with different volumes (anisocytosis). The histogram complements the peripheral blood film examination in identifying variant RBC populations.12 (A discussion of histograms with examples can be found in Chapter 39.)
The RDW is the coefficient of variation of RBC volume expressed as a percentage.13 It indicates the variation in RBC volume within the population measured and correlates with anisocytosis on the peripheral blood film. Automated analyzers calculate the RDW by dividing the standard deviation of the RBC volume by the MCV and then multiplying by 100 to convert to a percentage. The usefulness of the RDW is discussed later.
Reticulocyte Count
The reticulocyte count serves as an important tool to assess the bone marrow’s ability to increase RBC production in response to an anemia. Reticulocytes are young RBCs that lack a nucleus but still contain residual ribonucleic acid (RNA). Normally, they circulate peripherally for only 1 day while completing their development. The adult reference range for the reticulocyte count is 0.5% to 1.5% expressed as a percentage of the total number of RBCs.13 The newborn reference range is 1.5% to 5.8%, but these values change to approximately those of an adult within a few weeks after birth.7–9 An absolute reticulocyte count is determined by multiplying the percent reticulocytes by the RBC count. The reference range for the absolute reticulocyte count is 25 to 75 × 109/L, based on a normal adult RBC count.4 A patient with a severe anemia may seem to be producing increased numbers of reticulocytes if only the percentage is considered. For example, an adult patient with 1.5 × 1012/L RBCs and 3% reticulocytes has an absolute reticulocyte count of 45 × 109/L. The percentage of reticulocytes is above the reference range, but the absolute reticulocyte count is within the reference range. For the degree of anemia, however, both of these results are inappropriately low. In other words, production of reticulocytes within the reference range is inadequate to compensate for an RBC count that is approximately one third of normal.
The reticulocyte count may be corrected for anemia by multiplying the reticulocyte percentage by the patient’s hematocrit and dividing the result by 45 (the average normal hematocrit). If the reticulocytes are released prematurely from the bone marrow and remain in the circulation 2 to 3 days (instead of 1 day), the corrected reticulocyte count must be divided by maturation time to determine the reticulocyte production index (RPI). The RPI is a better indication of the rate of RBC production than is the corrected reticulocyte count (Table 18-1).4
TABLE 18-1
Formulas for Reticulocyte Counts and Red Blood Cell Indices
| Test | Formula | Adult Reference Range |
| Absolute reticulocyte count (× 109/L) | = [reticulocytes (%)/100] × RBC count (× 1012/L) | 25-75 × 109/L |
| Corrected reticulocyte count (%) | = reticulocytes (%) × patient’s Hct (%)/45 | — |
| Reticulocyte production index (RPI) | = corrected reticulocyte count/maturation time | In anemic patients, RPI should be >3 |
| Mean cell volume (MCV) (fL) | = Hct (%) × 10/RBC count (× 1012/L) | 80-100 fL |
| Mean cell hemoglobin (MCH) (pg) | = Hb (g/dL) × 10/RBC count (× 1012/L) | 26-32 pg |
| Mean cell hemoglobin concentration (MCHC) (g/dL) | = Hb (g/dL) × 100/Hct (%) | 32-36 g/dL |
Analysis of the reticulocyte count plays a crucial role in determining whether an anemia is due to an RBC production defect or to a premature destruction and shortened survival defect. If there is shortened RBC survival, as in the hemolytic anemias, the bone marrow tries to compensate by increasing RBC production. This increased production of RBCs results in the release of more reticulocytes into the peripheral circulation and a higher reticulocyte count. Although an increased reticulocyte count can also be observed in acute blood loss, it is more commonly observed in the hemolytic anemias.4,13 Chronic blood loss, on the other hand, does not lead to an appropriate increase in the reticulocyte count, but rather leads to iron deficiency and a subsequent low reticulocyte count. An inappropriately low reticulocyte count results from decreased production of normal RBCs, due to either insufficient or ineffective erythropoiesis. Reticulocytes and related calculations are discussed in Chapter 14.
Peripheral Blood Film Examination
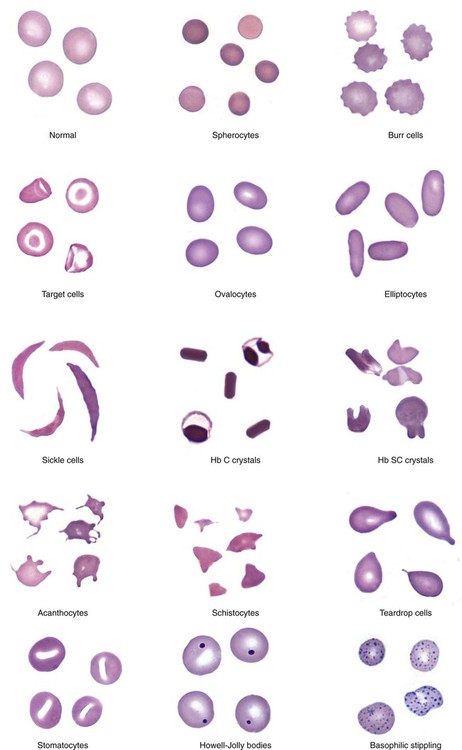
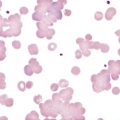
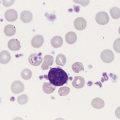
An important component in the evaluation of an anemia is examination of the peripheral blood film, with particular attention to RBC diameter, shape, color, and inclusions. The peripheral blood film also serves as a quality control to verify the results produced by automated analyzers. Normal RBCs on a Wright-stained blood film are nearly uniform, ranging from 6 to 8 µm in diameter. Small or microcytic cells are less than 6 µm in diameter, and large or macrocytic RBCs are greater than 8 µm in diameter. Certain shape abnormalities of diagnostic value (such as sickle cells, spherocytes, schistocytes, and oval macrocytes) and RBC inclusions (such as malarial parasites, basophilic stippling, and Howell-Jolly bodies) can be detected only by studying the RBCs on a peripheral blood film (Tables 18-2 and 18-3). Examples of abnormal shapes and inclusions are provided in Figure 18-1.
TABLE 18-2
Description of Red Blood Cell (RBC) Abnormalities and Commonly Associated Disease States
| RBC Abnormality | Cell Description | Commonly Associated Disease States |
| Anisocytosis | Abnormal variation in RBC volume or diameter | Hemolytic, megaloblastic, iron deficiency anemia |
| Macrocyte | Large RBC (>8 µm in diameter), MCV >100 fL | Megaloblastic anemia Myelodysplastic syndrome Chronic liver disease Bone marrow failure Reticulocytosis |
| Oval macrocyte | Large oval RBC | Megaloblastic anemia |
| Microcyte | Small RBC (<6 µm in diameter), MCV <80 fL | Iron deficiency anemia Anemia of chronic inflammation Sideroblastic anemia Thalassemia HbE disease and trait |
| Poikilocytosis | Abnormal variation in RBC shape | Severe anemia Certain shapes helpful diagnostically |
| Spherocyte | Small, round, dense RBC with no central pallor | Hereditary spherocytosis Immune hemolytic anemia Extensive burns (along with schistocytes) |
| Elliptocyte, ovalocyte | Elliptical (cigar-shaped), oval (egg-shaped), RBC | Hereditary elliptocytosis or ovalocytosis Iron deficiency anemia Thalassemia major Myelophthisic anemias |
| Stomatocyte | RBC with slitlike area of central pallor | Hereditary stomatocytosis Rh deficiency syndrome Acquired stomatocytosis (liver disease, alcoholism) Artifact |
| Sickle cell | Thin, dense, elongated RBC pointed at each end; may be curved | Sickle cell anemia Sickle cell–β-thalassemia |
| Hb C crystal | Hexagonal crystal of dense hemoglobin formed within the RBC membrane | Hb C disease |
| Hb SC crystal | Fingerlike or quartzlike crystal of dense hemoglobin protruding from the RBC membrane | Hb SC disease |
| Target cell (codocyte) | RBC with hemoglobin concentrated in the center and around the periphery resembling a target | Liver disease Hemoglobinopathies Thalassemia |
| Schistocyte (schizocyte) | Fragmented RBC due to rupture in the peripheral circulation | Microangiopathic hemolytic anemia* (along with microspherocytes) Traumatic cardiac hemolysis Extensive burns (along with microspherocytes) |
| Helmet cell (keratocyte) | RBC fragment in shape of a helmet | Same as schistocyte |
| Folded cell | RBC with membrane folded over | Hb C disease Hb SC disease |
| Acanthocyte (spur cell) | Small, dense RBC with few irregularly spaced projections of varying length | Severe liver disease (spur cell anemia) Neuroacanthocytosis (abetalipoproteinemia, McLeod syndrome) |
| Burr cell (echinocyte) | RBC with blunt or pointed, short projections that are usually evenly spaced over the surface of cell; present in all fields of blood film but in variable numbers per field† | Uremia Pyruvate kinase deficiency |
| Teardrop cell (dacryocyte) | RBC with a single pointed extension resembling a teardrop or pear | Primary myelofibrosis Myelophthisic anemia Thalassemia Megaloblastic anemia |
Hb, Hemoglobin; MCV, mean cell volume.
*Such as thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, disseminated intravascular coagulation.
†Cells with similar morphology that are unevenly distributed in a blood film (not present in all fields) are likely due to a drying artifact in blood film preparation; these artifacts are sometimes called crenated RBCs.
TABLE 18-3
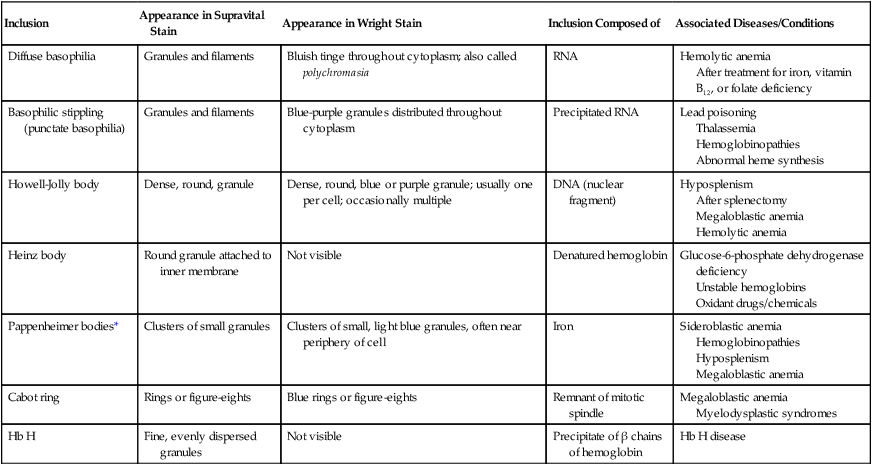
Erythrocyte Inclusions: Description, Composition, and Some Commonly Associated Disease States
| Inclusion | Appearance in Supravital Stain | Appearance in Wright Stain | Inclusion Composed of | Associated Diseases/Conditions |
| Diffuse basophilia | Granules and filaments | Bluish tinge throughout cytoplasm; also called polychromasia | RNA | Hemolytic anemia After treatment for iron, vitamin B12, or folate deficiency |
| Basophilic stippling (punctate basophilia) | Granules and filaments | Blue-purple granules distributed throughout cytoplasm | Precipitated RNA | Lead poisoning Thalassemia Hemoglobinopathies Abnormal heme synthesis |
| Howell-Jolly body | Dense, round, granule | Dense, round, blue or purple granule; usually one per cell; occasionally multiple | DNA (nuclear fragment) | Hyposplenism After splenectomy Megaloblastic anemia Hemolytic anemia |
| Heinz body | Round granule attached to inner membrane | Not visible | Denatured hemoglobin | Glucose-6-phosphate dehydrogenase deficiency Unstable hemoglobins Oxidant drugs/chemicals |
| Pappenheimer bodies* | Clusters of small granules | Clusters of small, light blue granules, often near periphery of cell | Iron | Sideroblastic anemia Hemoglobinopathies Hyposplenism Megaloblastic anemia |
| Cabot ring | Rings or figure-eights | Blue rings or figure-eights | Remnant of mitotic spindle | Megaloblastic anemia Myelodysplastic syndromes |
| Hb H | Fine, evenly dispersed granules | Not visible | Precipitate of β chains of hemoglobin | Hb H disease |
Finally, a review of the WBCs and platelets may help show that a more generalized bone marrow problem is leading to the anemia. For example, hypersegmented neutrophils can be seen in vitamin B12 or folate deficiency, whereas blast cells and decreased platelets may be an indication of acute leukemia. (See Chapter 15 for a complete discussion of the peripheral blood film evaluation.) Additional information from the blood film examination always complements the data from the automated hematology analyzer.
Bone Marrow Examination
The cause of many anemias can be determined from the history, physical examination, and results of laboratory tests on peripheral blood. When the cause cannot be determined, however, or the differential diagnosis remains broad, a bone marrow aspiration and biopsy may help in establishing the cause of anemia.4,8 A bone marrow examination is indicated for a patient with an unexplained anemia associated with or without other cytopenias, fever of unknown origin, or suspected hematologic malignancy. A bone marrow examination evaluates hematopoiesis and can determine if there is abnormal infiltration of the marrow. Important findings in the marrow that can point to the underlying cause of the anemia include abnormal cellularity of the marrow (e.g., hypocellularity in aplastic anemia); evidence of ineffective erythropoiesis and megaloblastic changes (e.g., vitamin B12 deficiency or myelodysplastic syndrome); lack of iron on iron stains of the bone marrow (the gold standard for diagnosis of iron deficiency); and the presence of granuloma, fibrosis, infectious agents, and tumor cells that may be inhibiting normal erythropoiesis.
Other tests that can assist in the diagnosis of anemia can be performed on the bone marrow sample as well, including flow cytometry, cytogenetic studies, and molecular analysis to detect abnormal cells, specific gene mutations, and chromosome abnormalities. (Chapter 16 discusses bone marrow procedures and bone marrow examination in detail.)
Other Laboratory Tests
Other laboratory tests that can assist in establishing the cause of anemia include routine urinalysis (to detect hemoglobinuria or an increase in urobilinogen) with a microscopic examination (to detect hematuria or hemosiderin) and analysis of stool (to detect occult blood or intestinal parasites). Also, certain chemistry studies are very useful, such as renal and hepatic function tests. More recently, copper deficiency has been identified as another nutritional cause of anemia.14
After the hematologic laboratory studies are completed, the anemia may be classified based on reticulocyte count, MCV, and peripheral blood film findings. Iron studies (including serum iron, total iron-binding capacity, transferrin saturation, and serum ferritin) are valuable if an inappropriately low reticulocyte count and a microcytic anemia are present. Serum vitamin B12 and serum folate assays are helpful in investigating a macrocytic anemia with a low reticulocyte count, whereas a direct antiglobulin test can differentiate autoimmune hemolytic anemias from hemolytic anemias with other causes. Because of the numerous potential etiologies of anemia, the cause needs to be determined before initiation of therapy.7,12
Approach to Evaluating Anemias
The approach to the patient with anemia begins with taking a complete history and performing a physical examination.4,5,7–9 For example, new-onset fatigue and shortness of breath suggest an acute drop in the hemoglobin concentration, whereas a lack of symptoms suggests a long-standing or congenital condition. A strict vegetarian may not be getting enough vitamin B12 in the diet, whereas an individual with alcoholism may not be getting enough folate. A large spleen may be an indication of hereditary spherocytosis, whereas a stool positive for occult blood may indicate iron deficiency. The complete history and physical examination can yield information to narrow the possible cause or causes of the anemia and thus lead to a more rational approach to ordering the appropriate diagnostic tests.
The first step in the laboratory diagnosis of anemia is detecting its presence by the accurate measurement of the hemoglobin, hematocrit, and RBC count and comparison of these values with the reference range for healthy individuals of the same age, sex, race, and environment. Knowledge of previous hematologic results is often extremely valuable. A reduction of 10% or more in hematologic values may be the first clue that an abnormal condition may be present.4,6,15
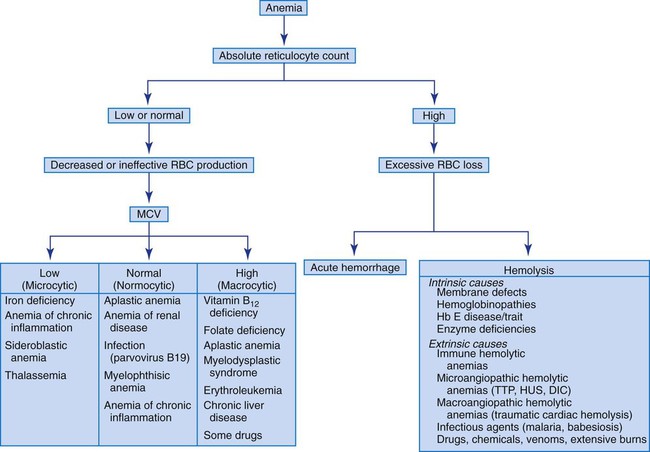
Reticulocyte Count and Anemia Classification
The absolute reticulocyte count is useful in initially classifying anemias into the categories of decreased RBC production (decreased reticulocyte count) and shortened RBC survival (increased reticulocyte count). When the reticulocyte count is decreased, the MCV can further classify the anemia into three subgroups: (1) normocytic anemias, (2) microcytic anemias, and (3) macrocytic anemias. Figure 18-2 presents an algorithm that illustrates how anemias can be evaluated based on the reticulocyte count and MCV.
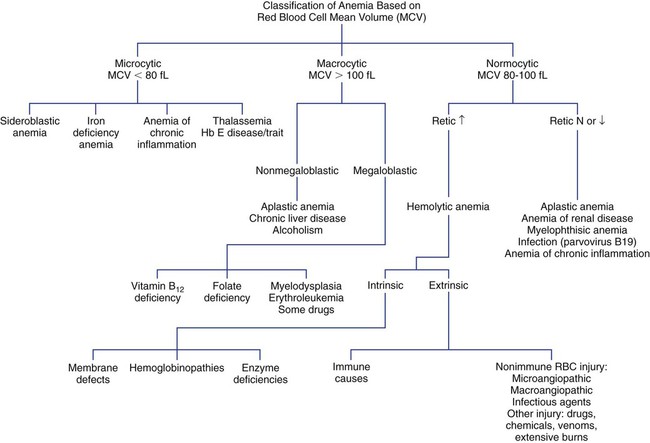
Mean Cell Volume and Anemia Classification
Microcytic anemia is characterized by an MCV of less than 80 fL with small RBCs (less than 6 µm in diameter). Hypochromia, characterized by an MCHC of less than 32 g/dL and increased central pallor in the RBCs, may accompany the microcytosis. Microcytic anemias are caused by conditions that result in reduced hemoglobin synthesis: iron deficiency, inability to utilize iron (chronic inflammatory states), globin synthesis defect (thalassemia), and heme synthesis defect (sideroblastic anemia, lead poisoning). The most common microcytic anemia results from an iron level that is insufficient for maintaining normal erythropoiesis; it is characterized by abnormal results on iron studies. Early iron deficiency is manifested only by reduced iron stores without anemia or microcytosis. The causes of iron deficiency vary in infants, children, adolescents, and adults, and it is imperative to find the cause before beginning treatment (see Chapter 19).
Macrocytic anemia is characterized by an MCV greater than 100 fL with large RBCs (greater than 8 µm in diameter). Macrocytic anemias may be megaloblastic or nonmegaloblastic. Megaloblastic anemias are caused by conditions that impair synthesis of deoxyribonucleic acid (DNA), such as vitamin B12 and folate deficiency or myelodysplasia. Nuclear maturation lags behind cytoplasmic development as a result of impaired DNA synthesis. This asynchrony between nuclear and cytoplasmic development results in larger cells. All cells of the body are ultimately affected by the defective production of DNA (see Chapter 20). Pernicious anemia is one cause of vitamin B12 deficiency, whereas malabsorption secondary to inflammatory bowel disease is one cause of folate deficiency. A megaloblastic anemia is characterized by oval macrocytes and hypersegmented neutrophils in the peripheral blood and by megaloblasts or large nucleated RBC precursors in the bone marrow. The MCV in megaloblastic anemia can be markedly increased (up to 150 fL), but modest increases (100 to 115 fL) occur as well.
Normocytic anemia is characterized by an MCV in the range of 80 to 100 fL. The RBC morphology on the peripheral blood film must be examined to rule out a dimorphic population of microcytes and macrocytes that can yield a normal MCV. The presence of a dimorphic population can also be verified by observing a bimodal distribution on the RBC histogram produced by an automated CBC instrument (see Chapter 39). Some normocytic anemias develop due to the premature destruction and shortened survival of RBCs (hemolytic anemias), and they are characterized by an elevated reticulocyte count. Other normocytic anemias develop due to a decreased production of RBCs and are characterized by a decreased reticulocyte count. Figure 18-3 presents an algorithm for initial evaluation of anemia based on the MCV.
Red Blood Cell Distribution Width
The RDW can help determine the cause of an anemia when used in conjunction with the MCV. Each of the three MCV categories mentioned previously (normocytic, microcytic, macrocytic) can also be subclassified by the RDW as homogeneous (normal RDW) or heterogeneous (increased or high RDW), according to Bessman et al.16,17 For example, a low MCV with a high RDW is suggestive of iron deficiency (Table 18-4). This classification is not absolute, however, because there can be an overlap of RDW values among some of the conditions in each MCV category.
TABLE 18-4
| MCV LOW | MCV NORMAL | MCV HIGH | |||
| RDW Normal | RDW High | RDW Normal | RDW High | RDW Normal | RDW High |
| Heterozygous thalassemia Anemia of chronic inflammation Hb E disease/trait |
Iron deficiency Sickle cell–β-thalassemia |
Anemia of chronic inflammation Anemia of renal disease Acute hemorrhage Hereditary spherocytosis |
Early iron, folate, or vitamin B12 deficiency Mixed deficiency of iron + vitamin B12 or folate Sickle cell anemia Hb SC disease |
Aplastic anemia Chronic liver disease Alcoholism |
Folate or vitamin B12 deficiency Myelodysplastic syndrome Chemotherapy Cold agglutinin disease Chronic liver disease |
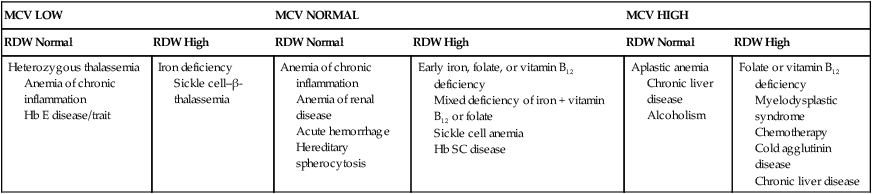
Hb, Hemoglobin; RDW, red blood cell distribution width.
Modified from Bessman JD, Gilmer PR, Gardner FH: Improved classification of anemias by MCV and RDW, Am J Clin Pathol 80:324, 1983; and Marks PW, Glader B: Approach to anemia in the adult and child. In Hoffman R, Benz EJ, Shattil SJ, et al, editors. Hematology: basic principles and practice, ed 5, Philadelphia, 2009, Churchill Livingstone.
Hemolytic Anemias
In cases of an elevated reticulocyte count caused by shortened survival of RBCs, an investigation for a hemolytic anemia is required. As indicated previously, numerous intrinsic and extrinsic causes of hemolysis exist. A direct antiglobulin test helps differentiate immune-mediated destruction from the other causes. In the other hemolytic anemias, reviewing the peripheral blood film is vital for determining the cause of the hemolysis (see Table 18-2 and Figure 18-1). Hemolytic anemias are discussed in Chapters 22 to 26.
Pathophysiologic Classification
In a pathophysiologic classification of anemia, related conditions are grouped by the mechanism causing the anemia. In this classification scheme, the anemias caused by decreased RBC production (e.g., disorders of DNA synthesis) are distinguished from the anemias caused by increased RBC destruction or loss (intrinsic and extrinsic abnormalities of RBCs). Box 18-1 presents a pathophysiologic classification of anemia based on the causes of the abnormality and gives one or more examples of an anemia in each classification.
Summary
• Anemia is defined conventionally as a decrease in RBCs, hemoglobin, and hematocrit below the reference range for healthy individuals of the same age, sex, and race, under similar environmental conditions.
• Clinical diagnosis of anemia is based on history, physical examination, signs, symptoms, and laboratory test results.
• Many anemias have common manifestations. Careful questioning of the patient may reveal contributing factors, such as diet, medications, occupational hazards, and bleeding history.
• A thorough physical examination is valuable in determining the cause of anemia. Some of the areas that should be evaluated are skin, nail beds, eyes, mucosa, lymph nodes, heart, and size of the spleen and liver.
• Moderate anemias may not manifest clinical symptoms if the onset is slow. Severe anemias (hemoglobin concentration of less than 7 g/dL) usually produce pallor, dyspnea, vertigo, headache, muscle weakness, lethargy, hypotension, and tachycardia.
• Laboratory procedures helpful in the diagnosis of anemia include CBC with RBC indices and RDW, reticulocyte count, examination of the peripheral blood film with emphasis on RBC morphology, and bone marrow examination, if indicated. Other tests are indicated based on the RBC indices, history, and physical examination (e.g., serum iron, total iron-binding capacity, serum ferritin, serum folate and vitamin B12, and direct antiglobulin test).
• The reticulocyte count and MCV play crucial roles in investigation of the cause of an anemia.
• Subclassifications of anemias based on MCV include normocytic, microcytic, and macrocytic anemias.
• The MCV, when combined with the RDW, also can aid in diagnosing anemia.
• The peripheral blood film plays an important role in the diagnosis of hemolytic anemias.
• Anemias may have more than one pathophysiologic cause.
• The cause of anemia should be determined before treatment is initiated.
Review Questions
1. Common clinical signs and symptoms of anemia include:
2. The RBC index that is used to describe the average RBC volume is the:
3. Variation in RBC volume is expressed by the:
4. An anemia develops because the bone marrow becomes fibrotic and the amount of active bone marrow, including RBC precursors, is diminished. The cells that are present are normal in appearance, but there are too few to meet the demand for blood cells, and anemia develops. The reticulocyte count is low. The anemia would be described as:
5. An increase in which one of the following suggests a reduced RBC life span and a hemolytic anemia?
6. An increased MCV, along with a high RDW, suggests:
7. Which of the following is detectable only by examination of a peripheral blood film?
8. Refer to Figure 18-2 and Table 18-4. Which of the following would be within the differential diagnosis of a patient with an MCV of 115 fL and an RDW of 20% (reference range 11.5% to 14.5%)?
9. When anemia is long-standing, which of the following is among the adaptations of the body?
b. Reduced oxygen affinity of hemoglobin
d. Reduction in the volume of blood ejected from the heart with each contraction
10. Which of the following patients would be considered anemic with a hemoglobin value of 14.5 g/dL? Refer to reference ranges inside the front cover of this text.