Aerosol Drug Therapy
After reading this chapter you will be able to:
 Describe how particle size, motion, and airway characteristics affect aerosol deposition.
Describe how particle size, motion, and airway characteristics affect aerosol deposition.
 Describe how aerosols are generated.
Describe how aerosols are generated.
 List the hazards associated with aerosol drug therapy.
List the hazards associated with aerosol drug therapy.
 Describe how to select the best aerosol drug delivery system for a patient.
Describe how to select the best aerosol drug delivery system for a patient.
 Describe how to initiate and modify aerosol drug therapy.
Describe how to initiate and modify aerosol drug therapy.
 State the information patients need to know to self-administer drug aerosol therapy properly.
State the information patients need to know to self-administer drug aerosol therapy properly.
 Describe how to assess patient response to bronchodilator therapy at the point of care.
Describe how to assess patient response to bronchodilator therapy at the point of care.
 Describe how to apply aerosol therapy in special circumstances.
Describe how to apply aerosol therapy in special circumstances.
 Describe how to protect patients and caregivers from exposure to aerosolized drugs.
Describe how to protect patients and caregivers from exposure to aerosolized drugs.
An aerosol is a suspension of solid or liquid particles in gas. Aerosols occur in nature as pollens, spores, dust, smoke, smog, fog, and mist.1 A primary function of the upper airway and respiratory tract is to protect the lungs from invasion by these aerosols. In the clinical setting, medical aerosols are generated with atomizers, nebulizers, and inhalers—devices that physically disperse matter into small particles and suspend them into a gas. Aerosols can be used to deliver bland water solutions to the respiratory tract (see Chapter 35) or to administer drugs to the lungs, throat, or nose for local and systemic effect. This chapter focuses on the principles of aerosol drug therapy.
The aim of medical aerosol therapy is to deliver a therapeutic dose of the selected agent (drug) to the desired site of action. The indication for any specific aerosol is based on the need for the specific drug and the targeted site of delivery.1 For patients with pulmonary disorders, administration of drugs by aerosol offers higher local drug concentrations in the lung with lower systemic levels compared with other forms of administration. Improved therapeutic action with fewer systemic side effects provides a higher therapeutic index.2
Characteristics of Therapeutic Aerosols
Particle Size
Aerosol particle size depends on the substance for nebulization, the method used to generate the aerosol, and the environmental conditions surrounding the particle.3 It is impossible to determine visually whether a nebulizer is producing an optimal particle size. The unaided human eye cannot see particles less than 50 to 100 µm in diameter (equivalent to a small grain of sand). The only reliable way to determine the characteristics of an aerosol suspension is laboratory measurement. The two most common laboratory methods used to measure medical aerosol particle size distribution are cascade impaction and laser diffraction. Cascade impactors are designed to collect aerosols of different size ranges on a series of stages or plates. The mass of aerosol deposited on each plate is quantified by drug assay, and a distribution of drug mass across particle sizes is calculated. In laser diffraction, a computer is used to estimate the range and frequency of droplet volumes crossing the laser beam.
Deposition
When aerosol particles leave suspension in gas, they deposit on (attach to) a surface. Only a portion of the aerosol generated and emitted from a nebulizer (emitted dose) may be inhaled (inhaled dose). A fraction of the inhaled dose is deposited in the lungs (respirable dose). Inhaled mass is the amount of drug inhaled. The proportion of the drug mass in particles that are small enough (fine-particle fraction) to reach the lower respiratory tract is the respirable mass. Not all aerosol delivered to the lung is retained, or deposited. A small percentage (1% to 5%) of inhaled drug may be exhaled. Whether aerosol particles that are inhaled into the lung are deposited in the respiratory tract depends on the size, shape, and motion of the particles and on the physical characteristics of the airways and breathing pattern. Key mechanisms of aerosol deposition include inertial impaction, gravimetric sedimentation, and brownian diffusion.1,3
Inertial Impaction
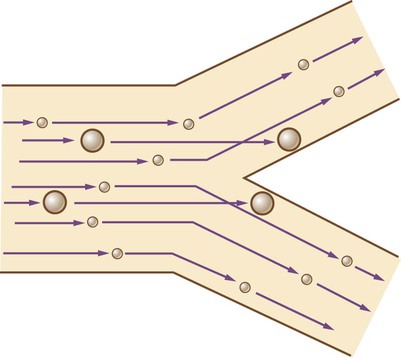
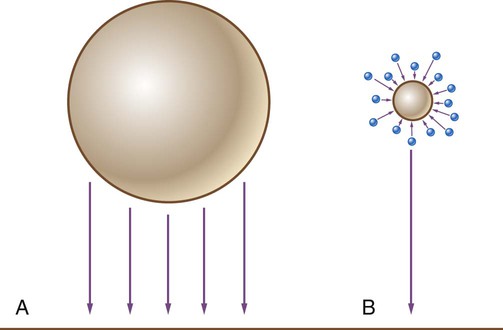
Inertial impaction occurs when suspended particles in motion collide with and are deposited on a surface; this is the primary deposition mechanism for particles larger than 5 µm. The greater the mass and velocity of a moving object, the greater its inertia, and the greater the tendency of that object to continue moving along its set path (Figure 36-1). When a particle of sufficient (large) mass is moving in a gas stream and that stream changes direction, the particle tends to remain on its initial path and collide with the airway surface.
Sedimentation
Sedimentation occurs when aerosol particles settle out of suspension and are deposited owing to gravity. The greater the mass of the particle, the faster it settles (Figure 36-2). During normal breathing, sedimentation is the primary mechanism for deposition of particles 1 to 5 µm. Sedimentation occurs mostly in the central airways and increases with time, affecting particles 1 µm in diameter. Breath holding after inhalation of an aerosol increases the residence time for the particles in the lung and enhances distribution across the lungs and sedimentation. A 10-second breath hold can increase aerosol deposition 10% and increase the ratio of aerosol deposited in lung parenchyma to central airway by fourfold.4
Diffusion
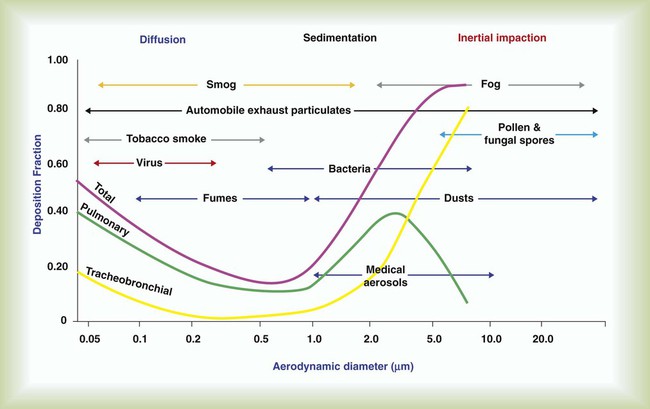
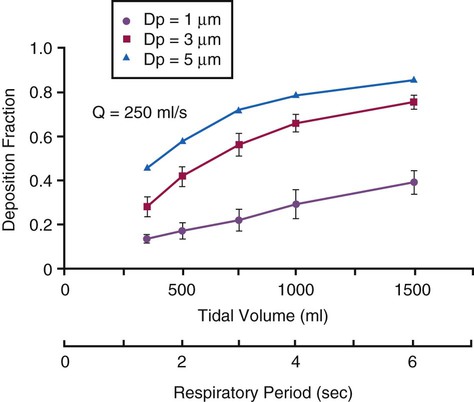
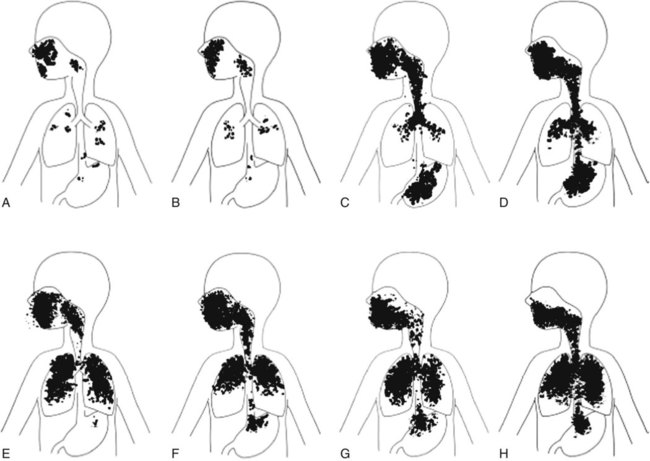
Figure 36-3 summarizes the relationships between particle size and aerosol deposition in the respiratory tract. The depth of penetration and deposition of a particle in the respiratory tract tend to vary with size and tidal volume (VT).5 With this knowledge, it may be possible to target aerosol deposition to specific areas of the lung by using the proper particle size and breathing pattern.
Aging
Aerosol particles can change size as a result of either evaporation or hygroscopic water absorption. The relative rate of particle size change is inversely proportional to the size of a particle, so small particles grow or shrink faster than large particles. Small water-based particles shrink when exposed to relatively dry gas. Aerosols of water-soluble materials, especially salts, tend to be hygroscopic, absorbing water and growing when introduced into a high-humidity environment.5
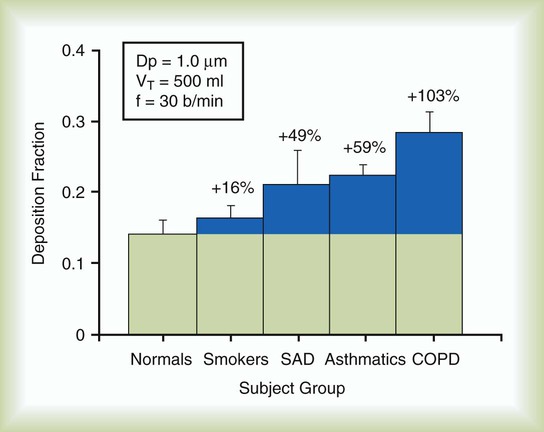
Particle size is not the only determinant of deposition. Inspiratory flow rate, flow pattern, respiratory rate, inhaled volume, ratio of inspiratory time to expiratory time (I : E ratio), and breath holding all influence where a particle of any specific size is deposited. The presence of airway obstruction is one of the greatest factors influencing aerosol deposition. It has been shown that total pulmonary deposition is greater in smokers and patients with obstructive airway disease than in healthy persons (Figure 36-4). Similarly, when inspiratory flow rates are constant, the deposition fraction of monodisperse aerosols increases with increased VT, length of respiratory (inspiratory) period, and particle size (Figure 36-5).
Quantifying Aerosol Delivery
One approach used to quantify aerosol deposition to the human body (in vivo) involves scintigraphy, in which a drug is “tagged” with a radioactive substance (e.g., technetium), aerosolized, and inhaled. A scanner (similar to scanners used in nuclear medicine) measures the distribution and intensity of radiation across the device and the patient’s head and thorax. The result is a radiation map of aerosol deposition in the upper airway, the lungs (central and peripheral airways), and the stomach. This information is used to calculate the percentage of drug retained by the device and delivered to various areas in the patient.6
A less direct approach relates the systemic pharmacokinetic profile of a drug delivered by aerosol to an assay of the drug in a patient’s blood or urine over time. This method does not estimate actual lung delivery, but it provides insight into systemic drug levels achieved after aerosol administration. Care must be taken to differentiate drug absorbed through the lungs from drug absorbed through the gastrointestinal tract. Simple laboratory, or in vitro, models, which simulate a range of VT values, inspiratory flow rates, I : E ratios, and respiratory rates, have been useful in predicting inhaled mass of drug and relative performance of nebulizers.7
Hazards of Aerosol Therapy
The primary hazard of aerosol drug therapy is an adverse reaction to the medication being administered (see Chapter 32). Other hazards to the patient include infection, airway reactivity, systemic effects of bland aerosols, drug concentration, and eye irritation. Care providers and bystanders risk these hazards as a result of exposure to secondhand aerosol drugs.
Infection
Aerosol generators can contribute to nosocomial infections by spreading bacteria by the airborne route.8 The most common sources of bacteria are patient secretions, contaminated solutions (i.e., multiple-dose drug vials), and caregivers’ hands. Offending organisms are primarily gram-negative bacilli, in particular, Pseudomonas aeruginosa and Legionella pneumophila (the cause of the highly virulent legionnaires’ disease).9
Various procedures can help reduce contamination and infection associated with respiratory care equipment. Guidelines from the U.S. Centers for Disease Control and Prevention (CDC) state that nebulizers should be sterilized between patients, frequently replaced with disinfected or sterile units, or rinsed with sterile water (not tap water) and air dried every 24 hours (see Chapter 4).
Airway Reactivity
Cold air and high-density aerosols can cause reactive bronchospasm and increased airway resistance, especially in patients with preexisting respiratory disease.10 Medications such as acetylcysteine, antibiotics, steroids, cromolyn sodium, ribavirin, and distilled water have been associated with increased airway resistance and wheezing during aerosol therapy. Administration of bronchodilators before or with administration of these agents may reduce the risk or duration of increased airway resistance.
The risk of inducing bronchospasm always should be considered when aerosols are administered. Monitoring for reactive bronchospasm should include peak flow measurements or percentage forced expiratory volume in 1 second (%FEV1) before and after therapy; auscultation for adventitious breath sounds; observation of the patient’s breathing pattern and overall appearance; and, most essential, communicating with the patient during therapy to determine the perceived work of breathing.11
Pulmonary and Systemic Effects
Preliminary assessment should balance the need versus the risk of aerosol therapy, especially among patients at high risk, such as infants, patients who are prone to fluid and electrolyte imbalances, and patients with atelectasis or pulmonary edema. For patients unable to clear their own secretions, suctioning or other airway clearance techniques may be indicated as an adjunct to aerosol therapy. Care must be taken to ensure that patients are capable of clearing secretions when the secretions are mobilized by aerosol therapy. Appropriate airway clearance techniques should accompany any aerosol therapy designed to help mobilize secretions (see Chapter 40).
Drug Concentration
During nebulization, the evaporation, heating, baffling, and recycling of drug solutions undergoing jet or ultrasonic nebulization increase solute concentrations.12 This process can expose the patient to increasingly higher concentrations of the drug over the course of therapy and result in a larger concentration of drug remaining in the nebulizer at the end of therapy. This increase in concentration usually is time-dependent; the greatest effect occurs when nebulization of medications occurs over extended periods, as in continuous aerosol drug delivery.
Eye Irritation
Aerosol administration via a face mask may deposit drug in the eyes and cause eye irritation. In very rare cases, anticholinergic medications (see Chapter 32) have been suspected to worsen preexisting eye conditions, such as forms of glaucoma. Caution should be exercised when a face mask is used during aerosol drug therapy. In addition, special mask designs that have been shown to reduce drug deposition in the eyes or mouthpieces should be considered for at-risk patients.13,14
Secondhand Exposure to Aerosol Drugs
Workplace exposure to aerosol may be detectable in the plasma of bystanders and health care providers. Repeated secondhand exposure to bronchodilators is associated with increased risk of occupational asthma. Institutions should develop and implement an occupational health and safety policy to minimize the risk of secondhand aerosol exposure for care providers and bystanders.15–17 Unless filters are placed in the expiratory limb, 40% of aerosol produced during mechanical ventilation is exhausted to the air of the intensive care unit.18 Implementation of an occupational health and safety policy could include using systems that introduce less aerosol to the atmosphere (pressurized metered dose inhalers [pMDIs], dry powder inhalers [DPIs], and breath-actuated nebulizers), filtering exhalation to contain aerosol, and using environmental controls.
Aerosol drug Delivery Systems
Effective aerosol therapy requires a device that quickly delivers sufficient drug to the desired site of action with minimal waste and at a low cost.19 Aerosol generators in use include pMDIs with or without spacers or holding chambers, DPIs, small and large volume (jet) nebulizers, hand-bulb atomizers (including nasal spray pumps), ultrasonic nebulizers (USNs), and vibrating mesh (VM) nebulizers as well as numerous emerging technologies.2
Clinicians are often exposed to competing and sometimes conflicting claims about the different delivery systems and may not be provided the information they need to select the correct system for a given situation. Because device selection can make the difference between successful and unsuccessful therapy, clinicians must have in-depth knowledge of the operating principles and performance characteristics of these various systems and how best to select and apply them.20
Metered Dose Inhalers
The pMDI is used to administer bronchodilators, anticholinergics, and steroids. More formulations of these drugs are available for use by pMDIs than for use with nebulizers. Properly used, pMDIs are at least as effective as other nebulizers for drug delivery. For this reason, pMDIs often are the preferred method for delivering bronchodilators to spontaneously breathing patients and patients who are intubated and undergoing mechanical ventilation.21
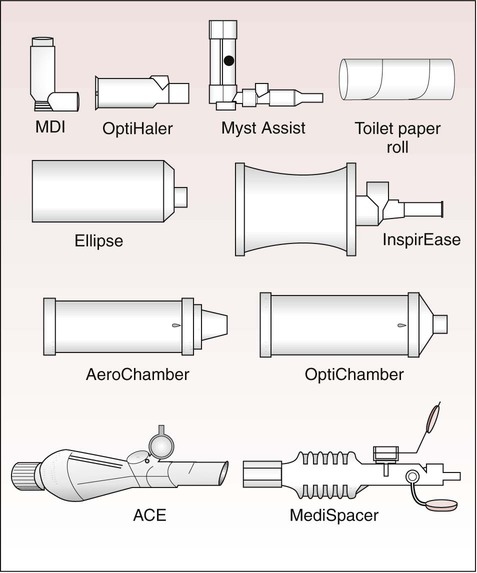
Most pMDIs are “press and breathe,” but there is increasing presence of a variation known as breath-actuated pMDIs. The basic components of pMDI are similar regardless of type, manufacturer, or active ingredient; commonly used pMDIs are shown in Figure 36-6.
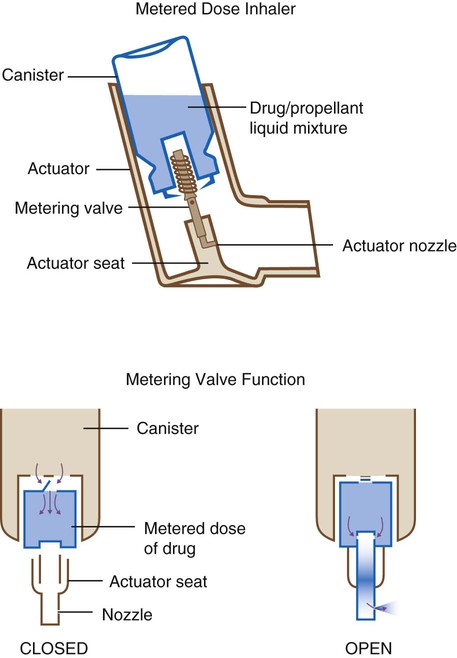
A pMDI is a pressurized canister that contains the prescribed drug (a micronized powder or aqueous solution) in a volatile propellant combined with a surfactant and dispersing agent (Figure 36-7). When the canister is inverted (nozzle down) and placed in its actuator, or “boot,” the volatile suspension fills a metering chamber that controls the amount of drug delivered. Pressing down on the canister aligns a hole in the metering valve with the metering chamber. The high propellant vapor pressure quickly forces the metered dose out through this hole and through the actuator nozzle.
From their inception in the mid-1950s to the beginning of the twenty-first century, chlorofluorocarbons (CFCs) such as Freon were the propellants used in pMDIs. Manufacture of CFCs for most applications was prohibited because of the effect of these compounds on global warming, with a period of transition provided for pMDIs. A consortium of eight pharmaceutical companies developed hydrofluoroalkane (HFA)-134a to be more environment-friendly and possibly clinically safer than CFCs.22 Redesign of key components of the pMDI has resulted in improved performance.23
Before initial use and after storage, every pMDI should be primed by shaking and actuating the device to atmosphere one to four times (see label for the specific device). Without priming, the initial dose actuated from a new pMDI canister contains less active substance than subsequent actuations.20 This “loss of dose” from a pMDI occurs when drug particles rise to the top of the canister over time (“cream”). A reduction in emitted dose with the first actuation commonly occurs with a pMDI after storage, particularly with the valve pointed in the downward position. Loss of prime is related to valve design and occurs when propellant leaks out of the metering chamber during periods of nonuse (e.g., 4 hours). The result is reduced pressure and drug released with the next actuation.20 Improved designs of metering valves developed for use with HFA propellants reduce these losses. It is recommended that a single dose be wasted before the next dose is inhaled when a CFC pMDI has not been used for 4 to 6 hours. An HFA pMDI requires no wasting of dose for periods exceeding 2 days.
Breath-Actuated Pressurized Metered Dose Inhaler
A variation of a pMDI is a breath-actuated model, which incorporates a trigger that is activated during inhalation. This trigger theoretically reduces the need for the patient or caregiver to coordinate MDI actuation with inhalation.24 However, patients may stop breathing when the MDI is actuated (“cold Freon effect”) or have suboptimal inspiration. Evaluation of the efficacy of breath-actuated pMDIs in children younger than 6 years is limited, and their use should be restricted to older children and adults. Oropharyngeal deposition of steroids using these devices is still very high.
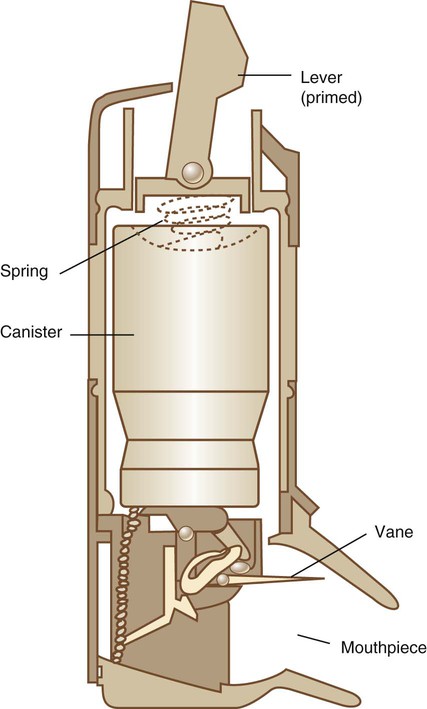
The Aerocount Autohaler is a flow-triggered pMDI developed and marketed by the 3M Corporation (St. Paul, MN) (Figure 36-8). The device is designed to eliminate the need for hand-breath coordination by automatically triggering in response to the patient’s inspiratory effort.24 To use the Autohaler, the patient cocks a lever on the top of the unit, which sets in motion a downward spring force. Using the closed-mouth technique, the patient draws through the mouthpiece. When the patient’s flow rate exceeds 30 L/min, a vane releases the spring, which forces the canister down and triggers the pMDI. In the United States, the Autohaler is available only with pirbuterol, a bronchodilator similar to albuterol. Current data indicate that the device reduces pharyngeal impaction and enhances lung deposition. A possible limitation of the device is that it can be breath actuated only. Patients experiencing an acute exacerbation of bronchospasm may be unable to generate sufficient flows to trigger the Autohaler. This theoretical concern has not been widely observed in clinical studies of patients with severe exacerbation of asthma receiving treatment in emergency departments. Nevertheless, caution may be appropriate in ordering breath-triggered pMDIs for small children and patients prone to severe levels of airway obstruction.
The Easihaler (GlaxoSmithKline, Philadelphia) is a breath-actuated pMDI that has been developed with a range of medications and is currently available in Europe and Canada. Release in the United States is anticipated in the near future. A new generation of pMDIs such as the Tempo (MAP Pharmaceuticals, Mountain View, CA) have been designed to be breath actuated with lower force of the plume exiting the mouthpiece, reducing oropharyngeal deposition and increasing lung dose (Figure 36-9).
Dose Counters
A serious limitation of pMDIs is the lack of a “counter” to indicate the number of doses remaining in the canister. After the number of label doses have been administered, the pMDI may seem to give another 20 to 60 doses, which may deliver little or no medications as the doses “tail-off.” Tail-off effect refers to variability in the amount of drug dispensed toward the end of the life of the canister. The result of tail-off is swings from normal to almost no dose emitted from one breath to the next with no reliable indicator to the user. Without a dose counter, there is no viable method to determine remaining drug in a pMDI other than manually keeping a log of every dose taken. The U.S. Food and Drug Administration (FDA) is requiring all new pMDIs to have a counter technology to track pMDI actuations remaining. Third-party dose counters may be added to older pMDI models but may not have the accuracy of built-in technology (Figure 36-10).
Factors Affecting Pressurized Metered Dose Inhaler Performance and Drug Delivery
Temperature
Decreased temperature (<10° C) has been shown to decrease the output of CFC pMDIs. Patients with cold air–induced bronchospasm who keep their pMDIs in outer coat pockets when outside in cold winter weather may receive only a small percentage of drug compared with that administered with the same pMDI at 25° C. This problem has been less serious with the newer HFA pMDIs.20
Timing of Actuation Intervals
Manufacturers recommend 30 seconds to 1 minute between actuations. When propellants are released, the device cools, changing aerosol output. The pause allows the device to return to room temperature and recover normal output. However, Fink and colleagues25 showed that pMDI output is similar at 15-second intervals. Very rapid actuation of multiple puffs per breath reduces inhaled drug per puff.
Aerosol Delivery Characteristics
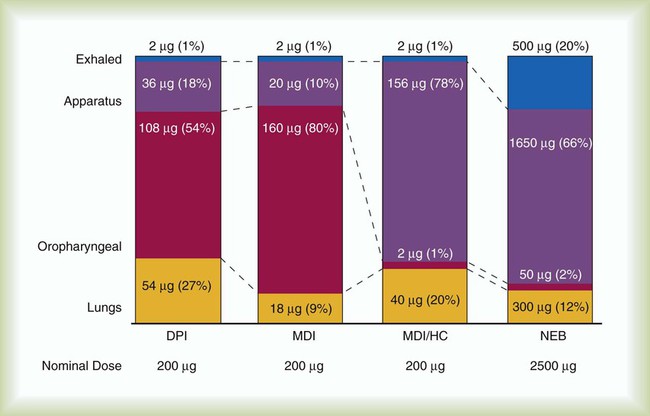
Although pMDIs can produce particles in the respirable range (MMAD 2 to 6 µm),20 the initial velocity and dispersion of the aerosol plume generate larger particles that decrease in size as they leave the pMDI, resulting in approximately 80% of the dose leaving the actuator to impact and become deposited in the oropharynx. A significant proportion of this oropharyngeal deposition is swallowed and may be a factor in systemic absorption of some drugs. Pulmonary deposition ranges from 10% to 20% in adults and larger children (less in infants).26 The exact amount of drug delivered to an individual patient is unpredictable because of high variability between patients and because pMDI drug administration is technique-dependent.
Technique
The successful administration of aerosol drugs by pMDI is highly technique-dependent. Two-thirds of patients and health care professionals who should teach pMDI use do not perform the procedure properly.27 Box 36-1 outlines the recommended steps for self-administering a bronchodilator by simple pMDI. Thorough preliminary patient instruction can last 10 to 30 minutes and should include demonstration, practice, and confirmation of patient performance (demonstration pMDIs with placebo are available from manufacturers for this purpose). Repeated instruction improves performance; repeat instruction is done most appropriately with follow-up clinic or home visits. Demonstration and return demonstration must occur several times for best patient adherence to device use.
Most pMDI labels call for placing the mouthpiece between the lips. However, research has shown that positioning the outlet of the pMDI approximately 4 cm (two fingerbreadths) in front of the mouth improves lung deposition by decreasing oropharyngeal impaction.28 Holding the canister outside the open mouth (at two fingerbreadths) provides a space for the particles to decelerate while evaporating, allowing particle size to reduce to respirable size. Use of the open-mouth technique with a low inspiratory flow rate can result in a doubling of the dose delivered to the lower respiratory tract of an adult from approximately 7% to 10% to 14% to 20%. However, this technique is more difficult for patients to perform reliably than the closed-mouth technique. Although it may reduce oropharyngeal deposition, the technique has not been shown to improve the clinical response to pMDI bronchodilators.
The high percentage of oropharyngeal drug deposition with use of steroid pMDIs can increase the incidence of opportunistic oral yeast infection (thrush) and changes in the voice (dysphonia). Rinsing the mouth after steroid use can help avoid this problem, but most pMDI steroid aerosol impaction occurs deep in the hypopharynx, which cannot be easily rinsed with gargling. For this reason, steroid pMDIs should not be used alone but always in combination with a spacer or valved holding chamber. See Box 36-2 for instructions for determining dosage left in the pMDI.
Pressurized Metered Dose Inhaler Accessory Devices
Spacers and Holding Chambers
Basic concepts for spacer devices include (1) small volume adapters, (2) open tube designs, (3) bag reservoirs, and (4) valved holding chambers (Figure 36-11). More than a dozen different devices with volumes ranging from 15 to 750 ml have been developed over the past 30 years.
A spacer is a simple valveless extension device that adds distance between the pMDI outlet and the patient’s mouth. This distance allows the aerosol plume to expand and the propellants to evaporate before the medication reaches the oropharynx. Larger particles leaving the pMDI tend to impact on the spacer walls. In combination, this phenomenon reduces oropharyngeal impaction and increases pulmonary deposition. Proper use of a simple open-tube spacer still requires some hand-breath coordination because a momentary delay between triggering and inhaling the discharged spray results in a substantial loss of drug and reduced lung delivery. Exhalation into a simple spacer after pMDI actuation clears the aerosol from the device and wastes most of the dose to the atmosphere. This reduction in dose also occurs with small volume reverse-flow design spacers if there is no provision for “holding” the aerosol in the device.30
Holding chambers produce a finer, slower moving, more “respirable” aerosol with less impaction of drug in the oropharyngeal area (1% of dose) than simple spacers (10%) or a pMDI alone (80% of dose). Deposition after inhalation of a radiolabeled pMDI solution aerosol from the AeroChamber, compared with deposition from the same pMDI inhaled with the open-mouth technique, showed a 10-fold to 17-fold decrease in the amount of radioactivity deposited in the oropharyngeal-laryngeal area while a similar lung dose was maintained. This finding was true for both healthy subjects and patients with chronic obstructive pulmonary disease (COPD).31 The advantage of reduced oropharyngeal deposition is fewer side effects from steroid aerosols, as shown in numerous published clinical trials. If multiple actuations of one or more drugs are placed into a spacer, both the total dose and the respirable dose of drug available for inhalation are reduced. The extent of these losses may vary for different drugs and spacer designs.20
Even with a holding chamber, respirable particles containing drug settle out and become deposited within the device, causing a whitish buildup on the inner chamber walls. This residual drug poses no risk to the patient but may be rinsed out periodically. Drug output from plastic spacers has been shown to decrease owing to the presence of an electrostatic charge. With these devices, a buildup of material can be seen on the walls of the chamber. As more material builds up on the wall of the chamber, the charge is dissipated, and more drug is inhaled by the patient. Washing the chamber with water (without soap) causes the electrostatic charge to be reestablished, making the device less effective for the next few puffs, until the static charge in the chamber (which attracts small particles) is again reduced.32 Optimal technique is outlined in Box 36-3.
Use of conductive metal or nonelectrostatic plastic chambers or washing the plastic chamber periodically with deionizing detergent (liquid dishwashing soap) can overcome the loss of fine-particle mass owing to electrostatic charge and increase the inhaled mass from 20% to 50% of the emitted dose of the pMDI, even in children (Figure 36-12).32 The effect of washing the chamber with conventional dishwashing soap reduces this static charge for up to 30 days. All valved holding chambers and spacers should be cleaned regularly, typically monthly, as recommended by the manufacturer. Use of dilute liquid dishwashing soap, with or without rinsing, and allowing to air dry are recommended.
The addition of a one-way valve to convert an open tube into a reservoir for the aerosol, the incorporation of the actuator in the pMDI, the shape of the device, flow of air through the device, edge effects, masks, and manufacturing materials all affect aerosol characteristics and yield. The inhalation valve, which is used to contain the aerosol, also acts as a baffle to reduce oropharyngeal deposition. This valve must be able to withstand the initial pressure from the pMDI when the device is triggered to retain aerosol and have sufficiently low resistance to open readily when the user inhales, in particular, when the user is a child or an infant. Exhalation valves in a face mask attached to a spacer device must also provide low resistance. Issues of spacer volume, VT, frequency of breathing, and mechanical dead space between the spacer and mouth are of particular concern when these devices are used by children.33 Differences of twofold to threefold in the amount of drug available at the mouth have been measured among spacers used at the present time to treat infants. Clinicians should determine the delivery efficiencies of spacer devices before using the devices in the care of a particular population.
Accessory devices are used with either the manufacturer-designed boot that comes with the pMDI or with a “universal adapter” that triggers the pMDI canister. Different formulations of pMDI drugs operate at different pressures and have a different-sized orifice in the boot that is specifically designed by the manufacturer for use exclusively with that pMDI. The output characteristics of a pMDI change when an adapter with a different-sized orifice is used. With HFA pMDIs, the diameter of the actuator orifice is smaller, and the spray is predictably finer. When the HFA pMDI is used in an actuator designed for use with CFC pMDIs, output is reduced. When these HFA formulations are used with any particular spacer, it is important to know how comparable the available dose and particle size distribution are to the dose and particle size from an existing CFC pMDI.20
Dry Powder Inhalers
A DPI is typically a breath-actuated dosing system. With a DPI, the patient creates the aerosol by drawing air though a dose of finely milled drug powder with sufficient force to disperse and suspend the powder in the air. DPIs are inexpensive, do not need propellants, and do not require the hand-breath coordination needed for pMDIs. However, dispersion of the powder into respirable particles depends on the creation of turbulent flow in the inhaler. Turbulent flow is a function of the ability of the patient to inhale the powder with a sufficiently high inspiratory flow rate (Figure 36-13). In terms of both lung deposition and drug response, DPIs are as effective as pMDIs.34
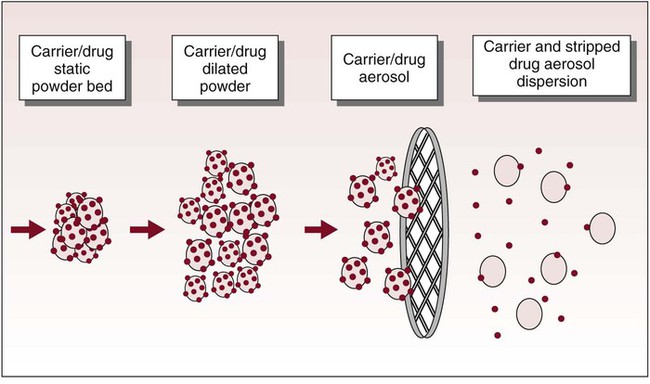
Equipment Design and Function
As shown in Figure 36-14, there are numerous DPIs on the market, which can be divided into three categories based on the design of their dose containers: (1) unit-dose DPI, (2) multiple unit-dose DPI, and (3) multiple dose drug reservoir DPI.
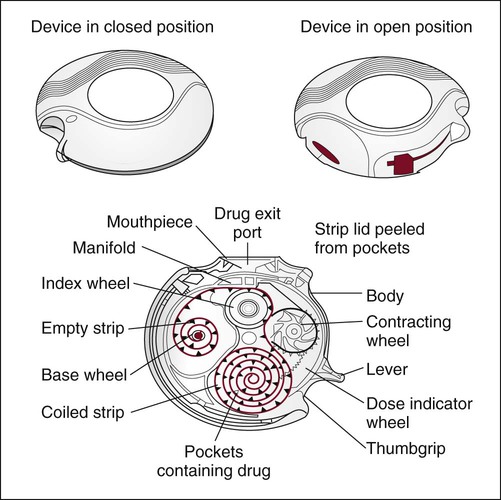
Unit-dose DPIs, such as the Aerolizer (Schering-Plough, Kenilworth, NJ) and the HandiHaler (Boehringer Ingleheim, Ingelheim am Rhein, Germany), dispense individual doses of drug from punctured gelatin capsules. Multiple unit-dose DPIs (Diskhaler; GlaxoSmithKline, Philadelphia) contain a case of four or eight individual blister packets of medication on a disk inserted into the inhaler. Multiple dose DPIs include the Twisthaler (Schering-Plough), Flexhaler (AstraZeneka, London), and the Diskus (GlaxoSmithKline). The Twisthaler and Flexhaler have a multidose reservoir powder system preloaded with a quantity of pure drug sufficient for dispensing 120 doses of medication, and the Diskus incorporates a tape system that contains up to 60 sealed single doses (Figure 36-15).
Factors Affecting Dry Powder Inhaler Performance and Drug Delivery
Performance of DPIs can be affected by the materials used in production and manufacturing.
Patient’s Inspiratory Flow Ability
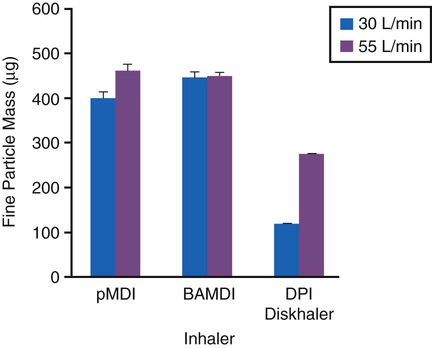
The high peak inspiratory flow rates (>60 L/min) required to dispense the drug powder from most current DPI designs result in a pharyngeal dose comparable to the dose received from a typical pMDI without an add-on device. If inhalation is not performed at the optimal inspiratory flow rate for a particular device, delivery to the lung decreases as the dose of drug dispensed decreases and the particle size of the powder aerosol increases (Figure 36-16).34
Technique
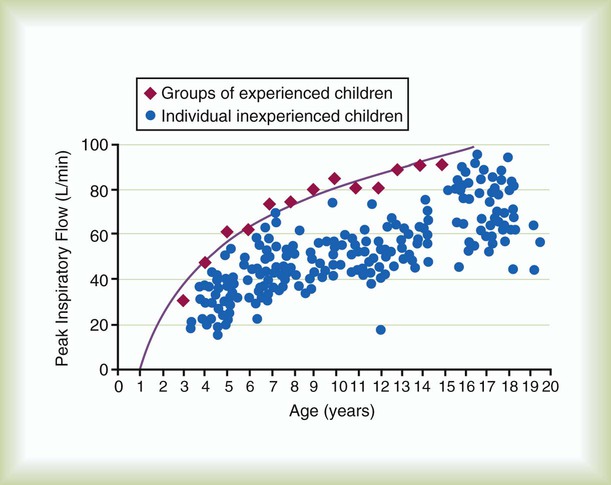
As with pMDIs, to derive the maximum benefit from a DPI, proper technique is essential. Box 36-4 outlines the basic steps for ensuring optimal drug delivery. The most critical factor in using a passive DPI is the need for high inspiratory flow. Patients must generate an inspiratory flow rate of at least 40 to 60 L/min to produce a respirable powder aerosol. Because infants, small children (<5 years old) (Figure 36-17), and patients who are unable to follow instructions cannot develop flow this high, these patients cannot use DPIs. Also, patients with severe airway obstruction may be unable to achieve the required flow; DPIs should not be used in the management of acute bronchospasm.
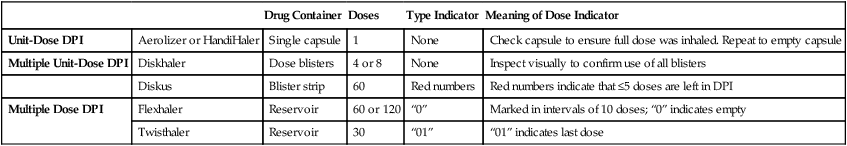
Although hand-breath coordination is not as important with DPIs as it is with pMDIs, exhalation into the device before inspiration can result in loss of drug delivery to the lung. Some devices also require assembly, which can be cumbersome or difficult for some patients, especially in an emergency. It is important that patients receive demonstrations with their inhalers and have the opportunity to assemble and use the DPI (return demonstration) before self-administration. Although the DPI may require cleaning in accordance with the product label, the device should never be submerged in water. Moisture in the device dramatically reduces available dose. Based on the different types of DPIs and the various drug container closure systems, Table 36-1 provides methods to determine the dose in the DPI.
TABLE 36-1
Determining Doses Left in the Dry Powder Inhaler
| Drug Container | Doses | Type Indicator | Meaning of Dose Indicator | ||
| Unit-Dose DPI | Aerolizer or HandiHaler | Single capsule | 1 | None | Check capsule to ensure full dose was inhaled. Repeat to empty capsule |
| Multiple Unit-Dose DPI | Diskhaler | Dose blisters | 4 or 8 | None | Inspect visually to confirm use of all blisters |
| Diskus | Blister strip | 60 | Red numbers | Red numbers indicate that ≤5 doses are left in DPI | |
| Multiple Dose DPI | Flexhaler | Reservoir | 60 or 120 | “0” | Marked in intervals of 10 doses; “0” indicates empty |
| Twisthaler | Reservoir | 30 | “01” | “01” indicates last dose |
Nebulizers
Nebulizers generate aerosols from solutions and suspensions. The three categories of nebulizers include (1) pneumatic jet nebulizers, (2) USNs, and (3) VM nebulizers. Nebulizers are also described in terms of their reservoir size. Small volume nebulizers (SVNs) most commonly used for medical aerosol therapy hold 5 to 20 ml of medication. Large volume nebulizers, also known as jet nebulizers, hold up to 200 ml and may be used for either bland aerosol therapy (see Chapter 35) or continuous drug administration.
Pneumatic (Jet) Nebulizers
Gas-powered jet nebulizers (Figure 36-18, A) have been in clinical use for longer than 100 years. Most modern jet nebulizers are powered by high-pressure air or oxygen (O2) provided by a portable compressor, compressed gas cylinder, or 50-psi wall outlet.
Factors Affecting Nebulizer Performance
Nebulizer design, gas pressure, gas density, and medication characteristics affect SVN performance (Box 36-5).
Nebulizer Design
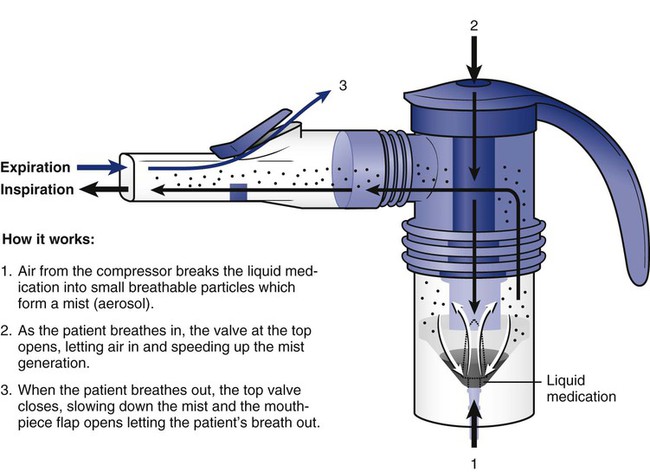
As shown in Figure 36-18, B, a typical SVN is powered by a high-pressure stream of gas directed through a restricted orifice (the jet). The gas stream leaving the jet passes by the opening of a capillary tube immersed in solution. Because it produces low lateral pressure at the outlet, the high jet velocity draws the liquid up the capillary tube and into the gas stream, where it is sheared into filaments of liquid that break up into droplets. This primary spray produces a heterodisperse aerosol with droplets ranging from 0.1 to 500 µm.35
Baffles are key elements in nebulizers; well-designed baffling systems decrease both the MMAD (size) and the GSD (range of sizes) of the generated aerosol. Atomizers operate with the same basic principles as nebulizers without baffling and produce aerosols with larger MMAD and GSD. Unintentional baffles are created by the angles within delivery tubing, by interfaces with other devices outside the aerosol generator, and by the surfaces of the upper airway itself.36
Residual drug volume, or dead volume, is the medication that remains in the SVN after the device stops generating aerosol and “runs dry.”37 The residual volume of a 3-ml dose can range from 0.5 to more than 2.2 ml, which can be more than two-thirds of the total dose. The greater the residual drug volume, the more drug that is unavailable as aerosol, and the less efficient the delivery system. Residual volume also depends on the position of the SVN. Some SVNs stop producing aerosol when tilted 30 degrees from vertical. Increasing the fill volume allows a greater proportion of active medication to be used for nebulization. In a nebulizer with a residual volume of 1.5 ml, a fill of 3 ml would leave only 50% of the nebulizer charge (nominal dose) available for nebulization. In contrast, a fill of 5 ml would make 3.5 ml, or more than 70% of the medication, available to be inhaled. The unit-dose volumes of drugs were based on clinical response of patients using nebulizers with substantial residual drug volumes. Although increasing dose volume may increase available dose, it should be considered off-label administration, and no significant difference in clinical response has been shown to date with varying diluent volumes and flow rates.
Density
Gas density affects both aerosol generation and delivery to the lungs. The lower the density of a carrier gas, the less turbulent the flow (i.e., the lower the Reynolds number), resulting in less aerosol impaction. This phenomenon has been shown with low-density helium-O2 mixtures (heliox). The lower the density of a carrier gas, the less aerosol impaction occurs as gas passes through the airways, and the greater the deposition of aerosol in the lungs.38 However, when heliox is used to drive a jet nebulizer at standard flow rates, aerosol output is substantially less than with air or O2, and aerosol particles are considerably smaller. When driving a nebulizer with heliox, twofold to threefold greater flow is required to produce a comparable aerosol output. Heliox concentrations of 40% or greater have been shown to improve aerosol deposition.39
Humidity and Temperature
Humidity and temperature can affect particle size and the concentration of drug remaining in the nebulizer. Evaporation of water and adiabatic expansion of gas can reduce the temperature of the aerosol to 10° C less than ambient temperature. This cooling may increase solution viscosity and reduce the nebulizer output, while decreasing particle MMAD.40 Aerosol particles entrained into a warm and fully saturated gas stream increase in size. These particles also can coalesce (stick together), increasing the MMAD further and, in the case of a DPI, can severely compromise the output of respirable particles. How much these particles enlarge depends primarily on the tonicity of the solution. Aerosols generated from isotonic solutions probably maintain their size as they enter the respiratory tract. Hypertonic solutions tend to enlarge, whereas evaporation can cause hypotonic droplets to evaporate and shrink.
Small Volume Nebulizers
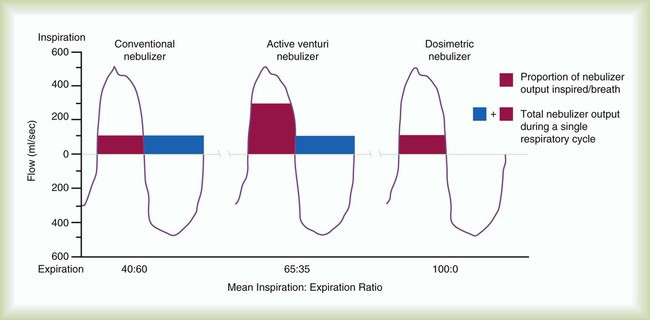
Four categories of jet SVNs include (1) continuous nebulizer with simple reservoir, (2) continuous nebulizer with collection reservoir bag, (3) breath-enhanced nebulizer, and (4) breath-actuated nebulizer (Figure 36-19). The most commonly used SVN is the constant output design. Supplemental air is entrained across the top of the device and dilutes the aerosol produced within the nebulizer as it exits toward the patient. Aerosol is generated continuously, with 30% to 60% of the nominal dose being trapped as residual volume in the nebulizer, and more than 60% of the emitted dose is wasted to the atmosphere. Continuous nebulization wastes medication because the aerosol is produced throughout the respiratory cycle and is largely lost to the atmosphere, as shown in Figure 36-20. Patients with an I : E ratio of 40 : 60 (or 1 : 1.5) lose 60% of the aerosol generated to the atmosphere. If 50% of the total dose is emitted from the nebulizer, and 50% of that aerosol is in the respiratory range and 40% of that is inhaled by the patient, less than 10% deposition is commonly measured in adults receiving continuous nebulizer therapy. In neonates and infants, given the small minute volumes and small airways with increased impaction and reduced sedimentation, deposition can be only 0.5%.
Aerosolized medication can also be conserved with reservoirs.41 A reservoir on the expiratory limb of the nebulizer conserves drug aerosol.
Small Volume Nebulizer With a Reservoir
Many types of disposable SVNs are packaged with a 6-inch (15-cm) piece of aerosol tubing to be used as a reservoir (see Figure 36-18, A). This may increase inhaled dose by 5% to 10% or increase the inhaled dose from 10% to approximately 11% with the reservoir tube.
Continuous Small Volume Nebulizer With Collection Bag
Bag reservoirs hold the aerosol generated during exhalation and allow the small particles to remain in suspension for inhalation with the next breath, while larger particles rain out, attributed to a 30% to 50% increase in inhaled dose.41 A collection bag is attached on the expiratory side of the nebulizer “T,” which collects aerosol leaving the SVN when the patient is not actively inhaling. Some of the aerosol in the bag is inhaled with the next inspiration, increasing total dose efficiency. The patient inhales aerosol from the SVN and reservoir through a one-way valve with exhalation through a second valve to the atmosphere.
Breath-Enhanced Nebulizers
Breath-enhanced nebulizers generate aerosol continuously, using a system of vents and one-way valves to minimize aerosol waste.42 In the Pari LC Sprint (Pari, Midlothian, VA) breath-enhanced nebulizer (Figure 36-21), an inspiratory vent allows the patient to draw in air through the nebulization chamber generating and containing aerosolized drug. On exhalation, the inlet vent closes, and aerosol exits by a one-way valve near the mouthpiece; this process can increase inhaled mass by 50% over standard continuous nebulizers and reduces aerosol waste to the atmosphere.
Breath-Actuated Nebulizers
AeroEclipse (Trudell Medical International, London, Ontario, Canada) is a breath-actuated SVN. A unique, spring-loaded, one-way valve design draws the jet to the capillary tube during inspiration and causes nebulization to cease when the patient’s inspiratory flow decreases below the threshold or the patient exhales into the device (Figure 36-22). Expiratory pressure on the valve at the initiation of exhalation moves the nebulizer baffle away from its position directly above the jet orifice, reduces the pressure, and stops aerosolization. Because aerosol is generated only during inhalation, exhaled aerosol and contamination of the environment during the expiratory phase of the breathing cycle are largely eliminated.
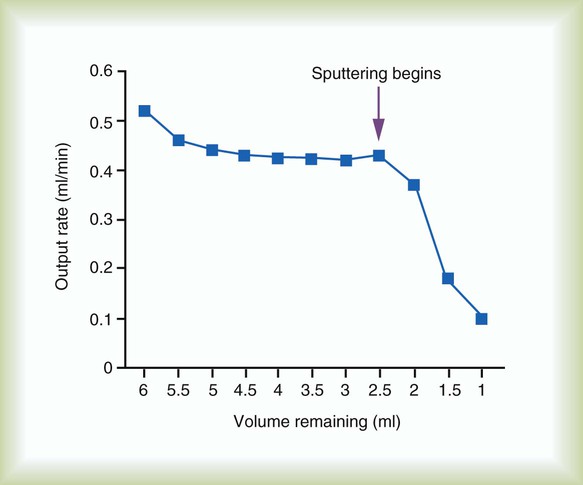
It can be difficult to determine when a nebulizer treatment is complete. Malone and colleagues43 found that with three different fill volumes, albuterol delivery from the nebulizer ceased after the onset of inconsistent nebulization (sputtering) (Figure 36-23). Aerosol output declined one-half within 20 seconds of the onset of sputtering. The concentration of albuterol in the nebulizer cup increased significantly when the aerosol output declined, and further weight loss in the nebulizer was caused primarily by evaporation. The authors concluded that aerosolization past the point of initial nebulizer sputter is ineffective.
Table 36-2 summarizes some of these key factors for many commercially available SVNs.44 Numerous SVNs are on the market, and they vary widely in design and performance. SVNs of the same design and lot number can exhibit variable performance, even to the point that some nebulizers of the same model number do not work at all.45 Managers and clinicians always must evaluate SVNs carefully before purchasing or using them. Manufacturers should provide data on the performance of their nebulizers under common use conditions.
TABLE 36-2
Comparison of Different Nebulizers
| Jet | Ultrasonic | Vibrating Mesh | |
| Features | |||
| Power source | Compressed gas or electrical mains | Electrical mains | Batteries or electrical mains |
| Portability | Restricted | Restricted | Portable |
| Treatment time | Long | Intermediate | Short |
| Output rate | Low | Higher | Highest |
| Residual volume | 0.8-2.0 ml | Variable but low | ≤0.2 ml |
| Environmental Contamination | |||
| Continuous use | High | High | High |
| Breath-activated | Low | Low | Low |
| Performance variability | High | Intermediate | Low |
| Formulation Characteristics | |||
| Temperature | Decreases* | Increases† | Minimum change |
| Concentration | Increases | Variable | Minimum change |
| Suspensions | Low efficiency | Poor efficiency | Variable efficiency |
| Denaturation | Possible‡ | Probable‡ | Possible‡ |
| Cleaning | Required, after single use | Required, after multiple use | Required after single use |
| Cost | Very low | High | High |
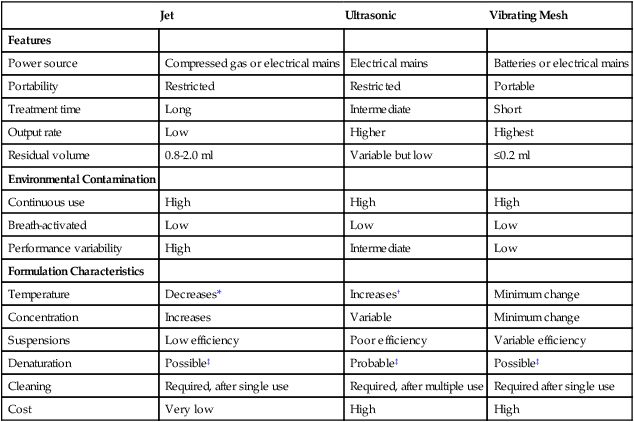
*For jet nebulizers, the temperature of the reservoir fluid decreases about 15° C during nebulization because of evaporation.
†For ultrasonic nebulizers, vibration of the reservoir fluid causes a temperature increase during aerosol generation, which can be 10° C to 15° C.
‡Denaturation of DNA occurs with all the nebulizers.
Modified from Dolovich MB, Dhand R: Aerosol drug delivery: developments in device design and clinical use. Lancet 377:1032, 2011.
Technique
Box 36-6 outlines the optimal technique for using an SVN for aerosol drug delivery. Use of an SVN is less technique-dependent and device-dependent than use of a pMDI or DPI delivery system. Slow inspiratory flow optimizes SVN aerosol deposition. However, deep breathing and breath holding during SVN therapy do little to enhance deposition over normal tidal breathing.46 Because the nose is an efficient filter of particles larger than 5 mm, many clinicians prefer not to use a mask for SVN therapy. As long as the patient is mouth breathing, there is little difference in clinical response between therapy given by mouthpiece and therapy given by mask. The selection of delivery method (mask or mouthpiece) should be based on patient ability, preference, and comfort.
Infection Control Issues
The CDC recommends that nebulizers be cleaned and disinfected, or rinsed with sterile water, and air dried between uses. Oie and Kamiya,47 studying microbial contamination of antibiotic aerosol solutions, found that after 7 days, five of six solutions were contaminated. The contamination appeared to have been caused by storage of multiple dose solutions at room temperature instead of in a refrigerator and reuse of syringes for measuring the solution. Refrigerating solutions and discarding syringes every 24 hours eliminated bacterial contamination.
Large Volume Jet Nebulizers
The high-output extended aerosol respiratory therapy nebulizers HEART (Cardinal Health, Dublin, OH), and HOPE (B & B Medical Technologies, Carlsbad, CA) are examples of devices designed for this purpose. These nebulizers have a reservoir greater than 200 ml that produces an aerosol with an MMAD of 2.2 to 3.5 µm. Actual output and particle size vary with the pressure and flow at which the nebulizer operates. A potential problem with continuous bronchodilator therapy (CBT) is increase in drug concentration. Patients receiving CBT need close monitoring for signs of drug toxicity (e.g., tachycardia and tremor). An additional strategy is to use an intravenous infusion pump to drip premixed bronchodilator solution into a standard SVN. Although an equipment-intensive approach, this technique can provide dosing equivalent to every 15 minutes.48
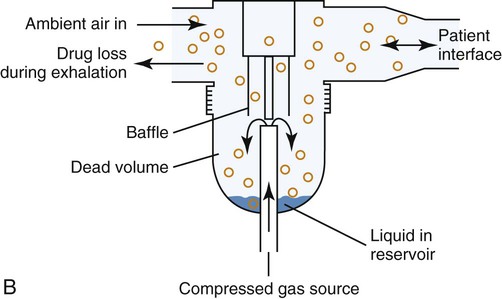
Another special-purpose large volume nebulizer is a small particle aerosol generator (SPAG) (Figure 36-24). The SPAG was manufactured by ICN Pharmaceuticals specifically for administration of ribavirin (Virazole) to infants with respiratory syncytial virus infection. The device is unique in clinical respiratory care practice in that it incorporates a drying chamber with its own flow control to produce a stable aerosol. The SPAG reduces medical gas source from the normal 50 pounds per square inch gauge (psig) line pressure to 26 psig with an adjustable regulator. The regulator is connected to two flowmeters that separately control flow to the nebulizer and drying chamber. The nebulizer is located within the glass medication reservoir, the fluid surface and wall of which serve as primary baffles. As it leaves the medication reservoir, the aerosol enters a long, cylindrical drying chamber. Here the second (separate) flow of dry gas is entrained, reducing particle size by evaporation, creating a monodisperse aerosol with an MMAD of 1.2 to 1.4 µm. Nebulizer flow should be maintained at approximately 7 L/min with total flow from both flowmeters not less than 15 L/min. The latest model operates consistently even with back pressure and can be used with masks, hoods, tents, or ventilator circuits.
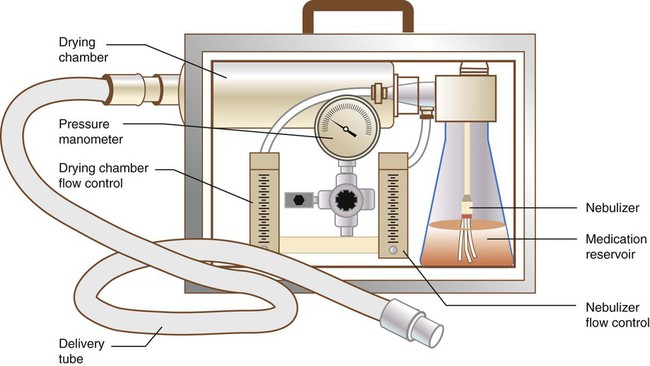
Two specific problems are associated with SPAG use to deliver ribavirin. The first is caregiver exposure to the drug aerosol. Approaches to limit caregiver exposure are discussed later (see the section on Controlling Environmental Contamination). The other problem occurs only when the SPAG is used to deliver ribavirin through a mechanical ventilator circuit. Drug precipitation can jam breathing valves or occlude the ventilator circuit. This problem can be overcome by (1) placing a one-way valve between the SPAG and the circuit and (2) filtering out the excess aerosol particles before they reach the exhalation valve, changing filters frequently to avoid increasing expiratory resistance.46
Hand-Bulb Atomizers and Spray Pumps
Hand-bulb atomizers and nasal spray pumps are used to administer sympathomimetic, anticholinergic, antiinflammatory, and anesthetic aerosols to the upper airway, including nasal passages, pharynx, and larynx (see also Chapter 32). These agents are used to manage upper airway inflammation and rhinitis, to provide local anesthesia, and to achieve systemic effects. Guidelines for the delivery of drugs to the upper airway have been developed by the American Association for Respiratory Care (AARC).49
Ultrasonic Nebulizers
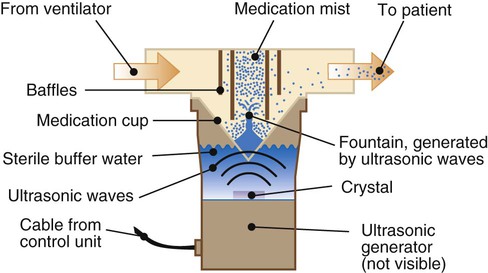
The USN uses a piezoelectric crystal to generate an aerosol. The crystal transducer converts an electrical signal into high-frequency (1.2- to 2.4-MHz) acoustic vibrations. These vibrations are focused in the liquid above the transducer, where they disrupt the surface and create oscillation waves (Figure 36-25). If the frequency of the signal is high enough and its amplitude strong enough, the oscillation waves form a standing wave that generates a geyser of droplets that break free as fine aerosol particles.
Large Volume Ultrasonic Nebulizers
Large volume USNs (used mainly for bland aerosol therapy or sputum induction) incorporate air blowers to carry the mist to the patient (see Chapter 35). Low flow through the USN is associated with smaller particles and higher mist density. High flow yields larger particles and less density. In contrast to jet nebulizers, the temperature of the solution placed in a USN increases during use. As the temperature increases, the drug concentration increases, as does the likelihood of undesired side effects.
Small Volume Ultrasonic Nebulizers
Many small volume USNs have been marketed for aerosol drug delivery (see Figure 36-25). In contrast to the larger units, some of these systems do not use a couplant compartment; the medication is placed directly into the manifold on top of the transducer. The transducer is connected by a cable to a power source, often battery-powered to increase portability. These devices have no blower; the patient’s inspiratory flow draws the aerosol from the nebulizer into the lung.
Small volume USNs have been promoted for administration of a wide variety of formulations ranging from bronchodilators to antiinflammatory agents and antibiotics.50 Use of a small volume USN may increase available respirable mass for designs with less residual drug volume than SVNs; this may reduce the need for a large quantity of diluent to ensure delivery of the drugs. The contained portable power source adds a great deal of convenience in mobility. Both theoretical advantages of the ultrasonic devices are outweighed by relatively high purchase costs and poor reliability.
Small volume USNs have been used to administer undiluted bronchodilators to patients with severe bronchospasm.50 Because the nebulizers have minimal residual drug volume, the treatment time is reduced with smaller volumes; however, it may be increased with standard dosing volumes. Use of undiluted bronchodilators has been described in the literature, but this is not included at the present time in the manufacturer’s label on product dosing information. Some ventilator manufacturers (e.g., Maquet, Rastatt, Germany) have promoted the use of USNs for administration of aerosols during mechanical ventilation. In contrast to SVNs, USNs do not add extra gas flow to the ventilator circuit during use. This feature reduces the need to change and reset ventilator and alarm settings during aerosol administration.51
Vibrating Mesh Nebulizers
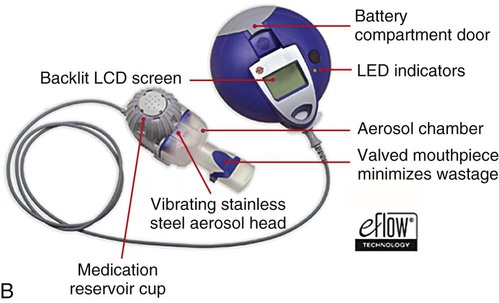
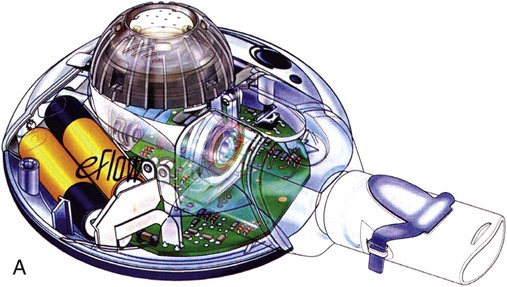
Two types of VM nebulizers, active and passive, are available commercially.52 Active VM nebulizers use a dome-shaped aperture plate, containing more than 1000 funnel-shaped apertures. This dome is attached to a plate that is also attached to a piezoceramic element that surrounds the aperture plate. Electricity applied to the piezoceramic element causes the aperture plate to be vibrated at a frequency of approximately 130 kHz (or one-tenth that of a USN), moving the aperture plate up and down by 1 µm or 2 µm, creating an electronic micropump. The plate actively pumps the liquid through the apertures, where it is broken into fine droplets. The exit velocity of the aerosol is low (<4 m/sec), and the particle size can range from 2 to 3 µm (MMAD), varying with the exit diameter of the apertures (Figure 36-26). Examples of an active VM nebulizer include the Aeroneb Go, Pro, and Solo nebulizers (Aerogen, Inc, Galway, Ireland) and the eFlow (Pari, Midlothian, VA). An active VM nebulizer can provide nebulization with single drops 15 mcl of formulations containing small and large molecules, suspensions, microsuspensions, and liposomes.
New-Generation Nebulizers
New Nebulizer Designs for Liquids
New nebulizer designs are available for delivery of liquids.53
Respimat
The Respimat soft mist inhaler (Boehringer, Ingelheim am Rhein, Germany) is a small hand-held inhaler that uses mechanical energy to create an aerosol from liquid solutions to produce a low-velocity spray (10 mm/sec) that delivers a unit dose of drug in a single actuation. To operate the device, patients twist the body of the device to load an internal spring, place the mouthpiece of the Respimat between the lips, and press a button to release the drug through a uniblock to create spray, which is released over 1.1 to 1.4 seconds, depending on the formulation configuration. The Respimat device requires hand-breath coordination on the part of the patient, as does a pMDI, but because of the longer spray time, it seems more likely to get a greater percent of emitted dose despite coordination issues. Because of the small particle size and low-velocity spray, pulmonary deposition of 40% is independent of inspiratory flows with oral deposition (40%) half the oral dose used with most pMDIs and DPIs (80%). The Respimat is currently available with several drugs in Europe and is slated for introduction with tiotropium in the United States.54
Smart Nebulizers
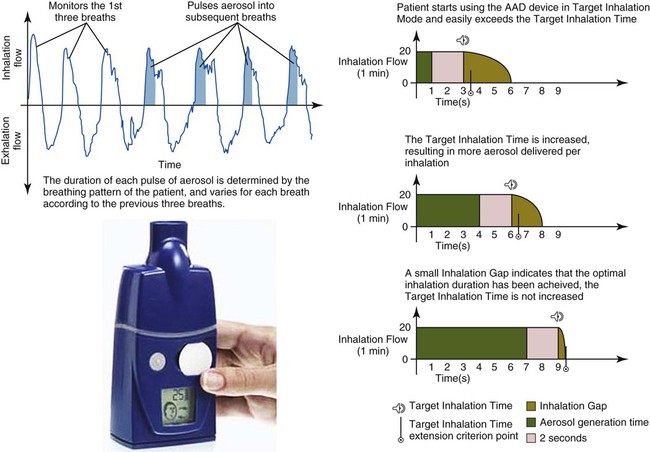
The I-Neb (Phillips Respironics, Murrysville, PA) is a breath-actuated passive VM nebulizer with adaptive aerosol delivery that monitors pressure changes and inspiratory time for the patient’s first three consecutive breaths (Figure 36-27).55 Drug is then aerosolized over 50% of the inspiratory maneuver during the fourth and all subsequent breaths. Targeted inhalation mode guides the patient to take serially longer inspirations to achieve optimal inhalation duration, reducing the time for administration. When the prescribed emitted dose has been aerosolized, the system provides an audible signal indicating the treatment should be stopped and the remaining medication discarded. Built-in electronics monitor patient treatment schedules and delivered doses with the goal to improve compliance with therapy. The I-Neb has been released for delivery of prostacyclin.
The Akita (Activaero, Gemuenden/Wohra, Germany) allows controlled inhalation of aerosol produced by either a jet or VM nebulizer. The Akita controls inspiratory flow to keep it slow (12 to 15 L/min) reducing impaction loss of aerosols in the upper airways. Patient pulmonary function is stored on a smart card programmed to tell the device when to generate aerosol during inspiration. Aerosol generated early targets distal airways, whereas aerosol generated later in the breath targets larger, more central airways.56 Smart nebulizers can track the actual time, duration, and dose administered for each treatment and provide logs of use that can be downloaded for the medical or research record.
Advantages and Disadvantages of Aerosol Systems
Knowledge of the advantages and disadvantages of various aerosol drug delivery systems is crucial for proper selection and application. Table 36-3 compares pMDI, DPI, SVN, and USN delivery systems.
TABLE 36-3
Advantages and Disadvantages of Aerosol Drug Delivery Systems
| Advantages | Disadvantages |
| MDI | |
| Convenient Inexpensive Portable No drug preparation required Difficult to contaminate |
Patient coordination required Patient activation required High percentage of pharyngeal deposition Risk of abuse Difficult to deliver high doses Not all medications are available Most units still use ozone-depleting CFCs |
| MDI With Accessory Device | |
| Less patient coordination required Less pharyngeal deposition No drug preparation required |
More complex for some patients More expensive than MDI alone Less portable than MDI alone Not all medications available |
| DPI | |
| Less patient coordination required Breath-activated Breath hold not required Can provide accurate dose counts No CFCs |
Requires high inspiratory flow Most units are single dose Risk of pharyngeal deposition Not all medications are available Difficult to deliver high doses |
| SVN | |
| Inexpensive Less patient coordination required High doses possible (even continuous) No CFC release |
Wasteful Drug preparation required Contamination possible if device not cleaned carefully Not all medications available Pressurized gas source required Long treatment times |
| USN | |
| Moderate residual volume Quiet Smaller residual drug volume than SVN Aerosol accumulates during exhalation |
Expensive Prone to electrical or mechanical breakdown Not all medications available Drug preparation required |
| VM Nebulizer | |
| Low residual volume Quiet Does not require gas or propellant Flow-independent delivery Shorter treatment times |
Expensive Not all medications available Drug preparation required |
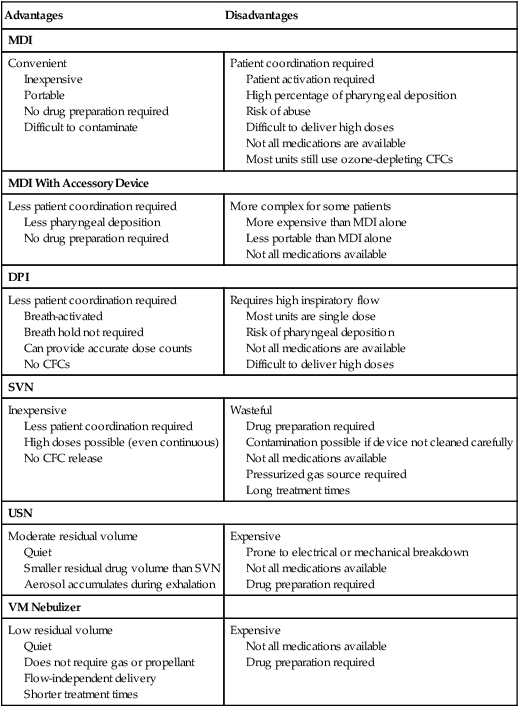
Modified from Hess D: Aerosol delivery. Respir Care Clin N Am 1:235, 1995.
Special Medication Delivery Issues for Infants and Children
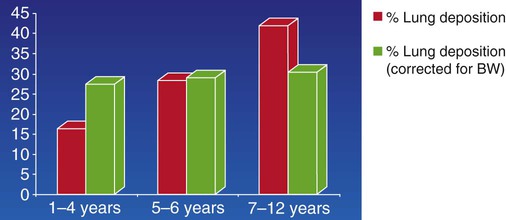
Children and infants have a smaller airway diameter than adults. In addition, their breathing rate is faster, nose breathing filters out large particles and deposits more medication in the upper airway, and mouthpiece administration often cannot be used before 3 years of age. Patient cooperation and ability vary with age and developmental ability. Finally, infants and small children have lower minute volumes than adults and so inhale a smaller proportion of the output of a continuous nebulizer than adults.7
Normal tidal breathing is the most effective method for administering aerosols to an infant. Mouth breathing enhances medication delivery to the airways of adults, but there is little evidence to show that this is true for infants, who are preferential nose breathers up to 1 year of age. Aerosols should never be administered to a crying child. Crying greatly reduces lower airway deposition of aerosol medication (Figure 36-28).
For infants and children who can tolerate a mask, a medication nebulizer can be fitted to an appropriately sized aerosol mask. There is no difference in clinical response between mouthpiece and close-fitting mask treatment, so patient tolerance, compliance, and preference should guide selection of the device. There is evidence that the aerosol available to the patient is substantially less when a loosely fitting mask (>1-cm leak) is used rather than a snug mask or mouthpiece with either a nebulizer or a pMDI with a holder chamber.57,58 If a patient cannot tolerate mask treatment (e.g., will not wear a close-fitting mask), a commonly used strategy is “blow-by” technique, in which the practitioner directs the aerosol from the nebulizer toward the patient’s nose and mouth from a distance of several inches from the face. There are no peer-reviewed published data supporting the use of the blow-by technique. Studies suggest that almost no drug enters the airway with this method. Rather than “blow-by,” it may be more efficient to take the time to condition the infant or child to tolerate the mask without crying or to deliver medication with a close-fitting mask when the patient is asleep.59
Spontaneous breathing in all patients, including pediatric and neonatal patients, results in greater deposition of aerosol from an SVN than occurs with positive pressure breaths (e.g., intermittent positive pressure ventilation). This mode of ventilation reduces aerosol deposition more than 30% compared with the effect of spontaneously inhaled aerosols.60
Selecting an Aerosol Drug Delivery System
The American College of Chest Physicians commissioned an extensive evidence-based review of the literature to determine which type of aerosol delivery system is superior. It was concluded that pMDIs, DPIs, and nebulizers all work with comparable clinical results, as long as they are prescribed for the appropriate patients and are used properly.61 Consequently, clinicians need to know the strengths and limitations of each type of device, match the device to each patient, and ensure that the patient or caregiver is trained to use the device properly.62
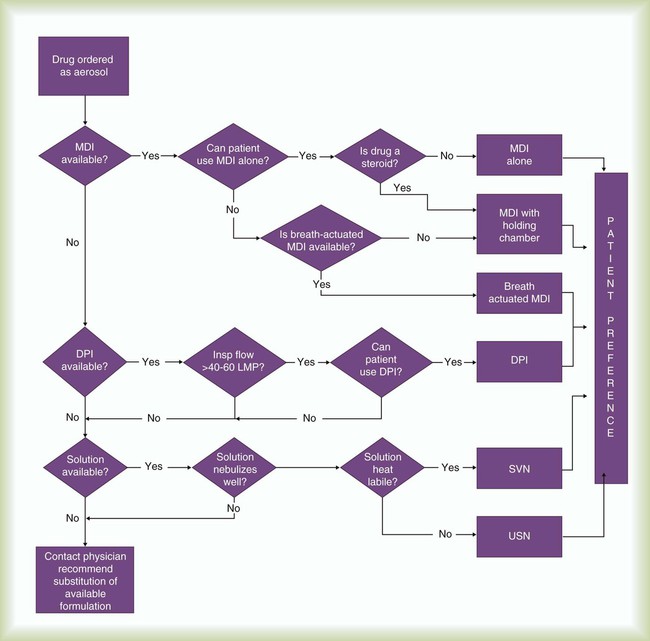
To guide practitioners in selecting the best aerosol delivery system for a given clinical situation, the AARC has published relevant clinical practice guidelines for aerosol delivery to the upper airway,49 to the lung parenchyma,63 and to neonatal and pediatric patients.64 Figure 36-29 is a selection algorithm that provides guidance regarding device selection.
Regardless of the device used, the clinician must be aware of the limitations of aerosol drug therapy. First, depending on the device and patient, 10% or less of drug emitted from an aerosol device may be deposited in the lungs (Figure 36-30). As indicated in Box 36-7, additional reductions in lung deposition can occur in many clinical situations that sometimes necessitate the use of higher dosages. Clinical efficacy varies according to both patient technique and device design. For these reasons, the best approach to aerosol drug therapy is to use an assessment-based protocol that emphasizes individually tailored therapy modified according to patient response.
Assessment-Based Bronchodilator Therapy Protocols
Sample Protocol
Figure 36-31 is an algorithm underlying a bronchodilator therapy protocol for acutely ill adults or children admitted to an emergency department.65 The protocol relies heavily on bedside assessment of the severity of airway obstruction based on the patient’s response to varying drug dosages.
Assessing Patient Response
Careful, ongoing patient assessment is key to an effective bronchodilator therapy protocol. To guide practitioners in implementing effective bedside assessment, the AARC has published Clinical Practice Guideline: Assessing Response to Bronchodilator Therapy at Point of Care.66
Use and Limitations of Peak Flow Monitoring
Because the peak flow measurement is effort-dependent and volume-dependent, evaluation of patient performance is subjective, and there are no good acceptability criteria. In addition, agreement between conventional spirometry values, such as forced vital capacity (FVC) and FEV1, and bedside PEFR values may be poor for individual patients. Although peak flow measurement can be used at the bedside to assess treatment effectiveness and to monitor trends, conventional spirometry remains the standard for determining bronchodilator response.66
Some peak flowmeters are more accurate and reliable than others. Even different units of the same model may give variable results. For this reason, the AARC recommends that when monitoring trends, the same unit be used for a given patient and that the patient’s range be reestablished if a different flowmeter is used.66
Other Components of Patient Assessment
All patients with acute airway obstruction should be monitored for oxygenation status with pulse oximetry. This value can be used in conjunction with observational assessment to titrate the level of inspired O2 given to the patient (see Chapter 35). Arterial blood gases are not essential for determining patient response to bronchodilator therapy but may be needed for patients in severe distress to assess for hypercapnic respiratory failure.
Frequency of Patient Assessment
How frequently patients should undergo assessment for bronchodilator therapy depends primarily on the acuity of the condition. A patient in unstable condition and in acute distress should undergo closer and more frequent scrutiny than a patient in stable condition. Box 36-8 provides guidance regarding the frequency of assessment according to acuity.
Special Considerations
Acute Care and Off-Label Use
Continuous Nebulization for Refractory Bronchospasm
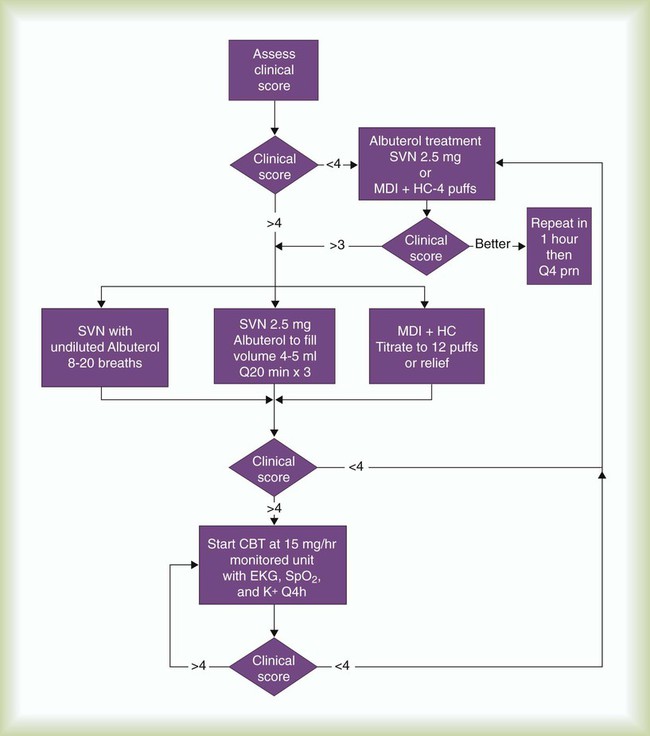
Patients in the emergency department with severe exacerbation of asthma or acute bronchospasm often have been taking standard doses of their bronchodilators for 24 to 36 hours before admission without response. Giving nebulizer treatments with standard bronchodilator doses and repeating the treatments until the symptoms are relieved can require hours of staff time. Administering higher doses of albuterol in short time frames can be accomplished by nebulization of undiluted albuterol (8 to 20 breaths) or by protocol titration with a pMDI and holding chamber (up to 12 puffs). If these strategies fail to provide relief, CBT with albuterol nebulization doses ranging from 5 to 20 mg/hr have proved safe and effective for adult and pediatric patients (Figure 36-32).
Figure 36-33 is a treatment algorithm for high-dose therapy and CBT for pediatric patients with status asthmaticus who are unable to perform peak flow maneuvers.67 Candidates for this protocol are children who, despite frequent beta agonist treatments, remain in extremis with bronchospasm, dyspnea, cough, chest tightness, and diminished breath sounds.
According to this protocol, children older than 6 years with tachypnea, hypoxemia, increased work of breathing, and restlessness who do not respond to standard therapy are given CBT with a large volume nebulizer or SVN at a dose rate of 15 mg/hr (see the accompanying Mini Clini “CBT Dosage Computations” for dosage computations). A standardized asthma score is used to evaluate children younger than 6 years for the severity of the condition (Table 36-4). Patients with an asthma score of 4 or higher are given CBT.
TABLE 36-4
| Score | |||
| Indicator | 0 | 1 | 2 |
| PaO2 | >70 mm Hg (air) | <70 mm Hg (air) | <70 mm Hg (40% O2) |
| SpO2 | >94% (air) | <94% (air) | <94% (40% O2) |
| Cyanosis | No | Yes | Yes |
| Breath sounds | Equal | Unequal | Absent |
| Wheezing | None | Moderate | Marked |
| Accessory muscle use | None | Moderate | Marked |
| Level of consciousness | Alert | Agitated or depressed | Comatose |
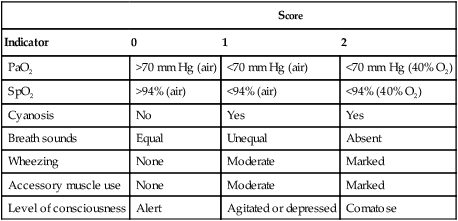
Modified from Volpe J: Therapist-driven protocols for pediatric patients. Respir Care Clin N Am 2:117, 1996.
The patient has responded poorly to CBT if any of the indicators listed in Table 36-4 worsens. The patient must be observed for adverse drug responses, including worsening tachycardia, palpitations, and vomiting. In these situations, the attending physician must be contacted immediately.
As an alternative to large volume drug nebulizers, some protocols are based on high-dose pMDI therapy (12 to 24 puffs per hour).68 To provide an extra margin of safety, some clinicians recommend that patients receiving CBT undergo continuous electrocardiogram monitoring and measurement of serum potassium level every 4 hours.
Aerosol Administration to Mechanically Ventilated Patients
Since the advent of modern mechanical ventilation, clinicians have administered aerosols to patients with the sickest of lungs. Four primary forms of aerosol generator are used to deliver aerosols during mechanical ventilation: SVN, USN, VM nebulizer, and pMDI with third-party adapter. Table 36-5 summarizes the factors affecting aerosol drug delivery to mechanically ventilated patients. Techniques to optimize delivery to patients receiving ventilatory support are described.69
TABLE 36-5
Factors Affecting Aerosol Drug Delivery During Mechanical Ventilation
| Category | Factor |
| Ventilator-related | Mode of ventilation |
| VT | |
| Respiratory rate | |
| Duty cycle | |
| Inspiratory waveform | |
| Breath-triggering mechanism | |
| Circuit-related | Size of endotracheal tube |
| Type of humidifier | |
| Relative humidity | |
| Density and viscosity of inhaled gas | |
| Device-related MDI | Type of spacer or adapter used |
| Position of spacer in circuit | |
| Timing of MDI actuation | |
| SVN | Type of nebulizer used |
| Fill volume | |
| Gas flow | |
| Cycling: inspiration vs. continuous | |
| Duration of nebulization | |
| Position in circuit | |
| Patient-related | Severity of airway obstruction |
| Mechanism of airway obstruction | |
| Presence of dynamic hyperinflation | |
| Spontaneous ventilation | |
| Disease process | |
| Drug-related | Dose |
| Aerosol particle size | |
| Targeted site for delivery | |
| Duration of action |
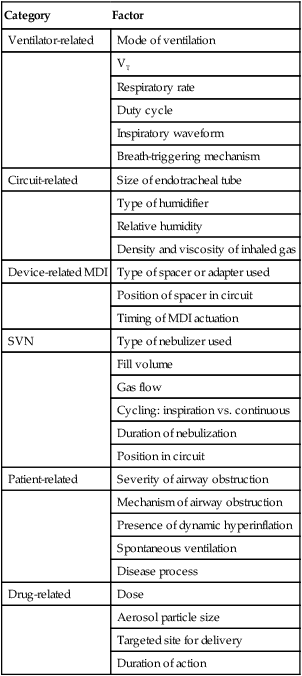
Techniques for assessing the response to a bronchodilator in intubated patients undergoing mechanical ventilation differ from techniques used in the care of spontaneously breathing patients because expiration is passive during mechanical ventilation, and forced expiratory values (PEFR, FVC, FEV1) cannot normally be obtained. Additional techniques can be used for mechanically ventilated patients because (1) a change in the differences between peak and plateau pressures (the most reliable indicator of a change in airway resistance during continuous mechanical ventilation) can be measured, (2) automatic positive end expiratory pressure levels may decrease in response to bronchodilators (see Chapter 41), and (3) breath-to-breath variations make measurements more reliable when the patient is not actively breathing with the ventilator.70
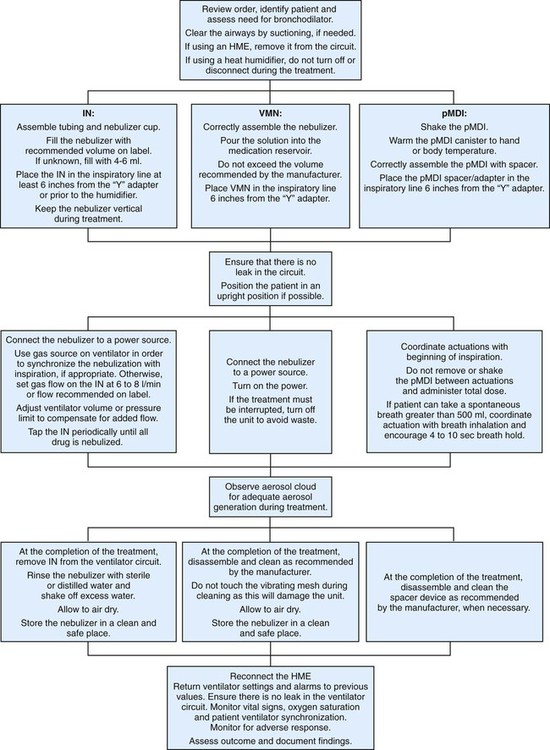
Techniques for aerosol administration vary by type of aerosol generator and device used. The optimal technique for drug delivery to mechanically ventilated patients with each type of aerosol generator is described in Box 36-9.
Use of a Small Volume Nebulizer During Mechanical Ventilation
The aerosol administered by SVN to intubated patients receiving mechanical ventilation tends to be deposited mainly in the tubing of the ventilator circuit and expiratory filter. Under normal conditions with heated humidification and standard jet nebulizers, pulmonary deposition ranges from 1.5% to 3.0%.70,71 When nebulizer output, humidity level, VT, flow, and I : E ratio are optimized, deposition can increase to 15%.
Use of a Pressurized Metered Dose Inhaler During Mechanical Ventilation
Results of in vitro studies show that effective aerosol delivery by pMDIs during mechanical ventilation can range from 2% to 30%. Direct pMDI actuation by simple elbow adapters typically results in the least pulmonary deposition, with most of the aerosol impacting in either the ventilator circuit or the tracheal airway. Higher aerosol delivery percentages occur only when an actuator or spacer is placed in-line in the ventilator circuit. These spacers allow an aerosol “plume” to develop before the bulk of the particles impact on the surface of the circuit or endotracheal tube. The result is a more stable aerosol mass that can penetrate beyond the artificial airway and be deposited mainly in the lung. This situation leads to a better clinical response at lower doses.52
Aerosol Generator Placement
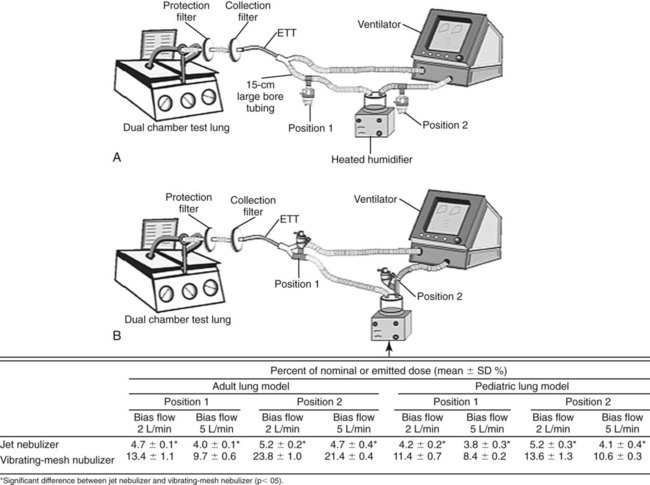
Placement of aerosol generators in the ventilator circuit can have a substantial impact on the available lung dose of drug. During adult ventilation without bias flow, placement of aerosol generators distal to the patient in the inspiratory limb may increase inhaled dose for jet nebulizers, where continuous gas flow acts to charge the inspiratory limb of the ventilator circuit with aerosol increasing the inhaled dose. In contrast, pMDI, USN, and VM nebulizer devices were more efficient when placed proximal to the patient.72 With continuous or bias flow through the ventilator circuit, the delivery is reduced as flow increases, whereas placement of a VM nebulizer near the ventilator increases delivery (Figure 36-34).73
Placement During Noninvasive Ventilation
Noninvasive ventilation may be administered with standard and bilevel ventilators. Bilevel ventilators often use a flow turbine, with a fixed valve or leak in the circuit that permits excess flow to vent to atmosphere. Placement of the aerosol generator between the leak and the patient’s airway seems to provide the highest aerosol delivery efficiency.74 A VM nebulizer delivers a greater fine-particle dose than an SVN during noninvasive ventilation presumably because of the lower residual drug volume and lower total flow in the circuit.75
Placement During High-Flow Nasal Oxygen
Researchers used a VM nebulizer to simulate the delivery of aerosol via a high-flow nasal O2 setup using infant, pediatric, and adult cannulas, with inhaled dose ranging from 8% to 28%.76 Figure 36-35 shows such a setup, including the location of the VM nebulizer. In addition to the type and location of the nebulizer used with high-flow nasal O2, the inhaled dose seems to vary based on cannula size, respiratory pattern, and O2 flow. Heliox (80 : 20) appears to improve aerosol delivery at higher flow rates with these setups.77
Placement During Intrapulmonary Percussive Ventilation
Intrapulmonary percussive ventilation provides high-frequency oscillation of the airway while administering aerosol particles. During intrapulmonary percussive ventilation, the aerosol generator should be placed in the circuit as close to the patient’s airway as practical. Aerosol administration during intrapulmonary percussive ventilation has been compared with a standard jet nebulizer. The MMAD was smaller with intrapulmonary percussive ventilation than with the jet (0.2 µm vs. 1.89 µm), and the fine-particle fraction was lower (16.2% vs. 67.5%). However, lung dose was similar (2.49% with intrapulmonary percussive ventilation vs. 4.2% with the jet nebulizer). It was concluded that intrapulmonary percussive ventilation was too variable and too unpredictable to recommend for drug delivery to the lung.78
Placement During High-Frequency Oscillatory Ventilation
When used in conjunction with high-frequency oscillatory ventilation, administration of albuterol sulfate via a VM nebulizer placed between the ventilator circuit and the patient airway has been reported to deliver greater than 10% of dose to both infants and adults.79,80 A pMDI with adapter placed immediately proximal to the endotracheal tube achieved similar results in adult patients ventilated via high-frequency oscillatory ventilation.81
Controlling Environmental Contamination
Drugs for nebulization that escape from the nebulizer into the atmosphere or are exhaled by the patient can be inhaled by anyone in the vicinity of the treatment. The risk imposed by this environmental exposure is clear and is associated with a range of drugs and patients with infectious disease. Pentamidine and ribavirin were associated with health risks to health care providers even when used in conjunction with filters on exhalation ports of nebulizers, containment and scavenger systems, and high-efficiency particulate air (HEPA) filter hoods and ventilation systems (Figure 36-36).
A survey found that RTs were more than twice as likely as physical therapists to develop asthma-like symptoms during the course of their careers. The authors associated this with administration of ribavirin and exposure to gluteraldehyde.17 There have been anecdotal reports of respiratory care clinicians who have developed a sensitivity to secondhand aerosol from bronchodilators. Further research is required to understand more thoroughly the hazards of secondhand exposure to aerosols in the clinical setting. Most of nebulizer therapy currently delivered does not include filtering systems.
Patients with infectious and resistant organisms, such as tuberculosis, severe acute respiratory syndrome, and H1N1 virus, require respiratory isolation, and caregivers require protection. RTs have a duty to take appropriate steps to protect themselves and their patients. In these cases, the aerosols generated by the patient from coughing, speaking, or laughing can transmit disease. These aerosols can travel substantial distances between rooms and even floors in institutions. Although exposure to secondhand aerosol generated by a nebulizer is undesirable, it poses less risk than the infected aerosols produced by mucosa of patients.82 In essence, it is unlikely that any medical aerosol that is inhaled by the patient would be contaminated. Nonetheless, during the severe acute respiratory syndrome outbreak, some centers outlawed use of medical aerosols to reduce exposure. Efforts should be taken to reduce transmission of both nebulizer aerosols and patient-generated aerosols to the environment.
Various techniques are available for protecting patients and caregivers from environmental exposure during aerosol drug therapy. The greatest occupational risk for RTs has been associated with the administration of ribavirin and pentamidine. Conjunctivitis, headaches, bronchospasm, shortness of breath, and rashes have been reported among individuals administering these drugs.83 Patients given aerosolized ribavirin or pentamidine must be treated in a private room, booth, or tent or at a special station designed to minimize environmental contamination.
Booths and Stations
A variety of booths and specially designed stations are available for delivery of pentamidine or ribavirin. The Emerson containment booth (Figure 36-37) is an example of a system that completely isolates the patient during aerosol administration. The AeroStar Aerosol Protection Cart (Respiratory Safety Systems, San Diego, CA) is a portable patient isolation station for administration of hazardous aerosolized medication. It has been used during sputum induction and for pentamidine treatment. The patient compartment is collapsible with a swing-out counter and three polycarbonate walls. Captured aerosols are removed with a HEPA filter. A prefilter is used to retain larger dust particles and to prevent early loading of the more expensive HEPA filter.
Personal Protective Equipment
Personal protective equipment is recommended when caring for any patient with a disease that can be spread by the airborne route.84 The greatest risk is communication of tuberculosis or chickenpox. Although environmental controls should be instituted in the care of these patients, standard and airborne precautions should also be implemented. Various masks and respirators have been recommended for use when caring for a patient with tuberculosis or other respiration-transmitted diseases. Traditional surgical masks, particulate respirators, disposable and reusable HEPA filters, and powered air-purifying respirators have been used. No data are available for determining the most effective and most clinically useful device to protect health care workers and others, although the U.S. Occupational Safety and Health Administration requires specific levels of protection (HEPA filters and powered air-purifying respirators). Guidelines from the World Health Organization recommend surgical masks for all patient care with the exception of N95 masks for aerosol-generating procedures such as sputum induction. Evidence from laboratory studies of potential airborne spread of influenza from contagious patients indicates that guidelines related to the current 1-m respiratory zone may need to be extended to a larger respiratory zone and include eye protection.85