Adrenarche and Adrenopause
Adrenarche
The adrenal glands are unique components of the endocrine system in displaying morphologic and functional characteristics that change dramatically according to different stages of prenatal and postnatal development. The onset of increased production of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) by the zona reticularis of the adrenals between 6 and 8 years of age defines the phenomenon of adrenarche. This appears to be a recapitulation of the steroidogenic pattern of the fetal adrenal during the second half of pregnancy, characterized by a massive increase in size of the adrenals relative to other fetal organs. Such a pattern of fetal and childhood adrenarche is observed only in humans and nonhuman primates. The triggers for the onset of adrenarche may differ among primate species such as the human, rhesus macaques (Old World primate), and marmosets (New World primate). The increase in DHEA is a common feature whose study can shed light on zona reticularis function in health and disease.1
The Fetal Adrenal Gland
The fetal adrenal develops by the fourth week of gestation as a thickening of the celomic epithelium adjacent to the urogenital ridge.1 Cells destined to become steroid secreting in both the adrenals and gonads are derived from the same migratory cells from the primitive mesonephros. The fetal adrenal has the capacity to produce steroids by 6 to 8 weeks of gestation, a fact that dictates the need to start dexamethasone early for the prenatal treatment of congenital adrenal hyperplasia (see Chapter 8).
Trophic Control of the Fetal Adrenal
Adrenocorticotropic hormone (ACTH) appears to be the principal trophic factor controlling growth and function of the fetal adrenal.2 Fig. 9-1 illustrates data on combined adrenal weights according to gestational age in normal fetuses compared with anencephaly and congenital adrenal hyperplasia. There is a clear demarcation outside the normal range for anencephaly and congenital adrenal hyperplasia, indicating the ACTH-dependent growth of the fetal adrenal. Biochemical monitoring of treated pregnancies at risk of congenital adrenal hyperplasia shows the ACTH dependence of fetal adrenal steroidogenesis.3 Growth is mainly by hypertrophy of the fetal zone, which renders the fetal adrenal as large as the kidney by 20 weeks’ gestation and 20- to 30-fold larger than the adult adrenal by 30 weeks. The definitive zone grows mainly by hyperplasia and is controlled by a number of growth factors acting in concert with ACTH. These include basic fibroblast growth factor, epidermal growth factor, insulin-like growth factors, transforming growth factors, and the activins.1
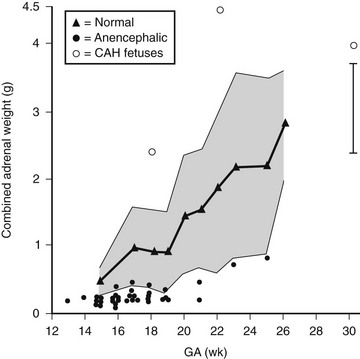
FIGURE 9-1 Combined fetal adrenal weights according to gestational age. (Data from Young MC, Laurence KM, Hughes IA: Relationship between fetal adrenal morphology and anterior pituitary function, Horm Res 32:130–135, 1989.)
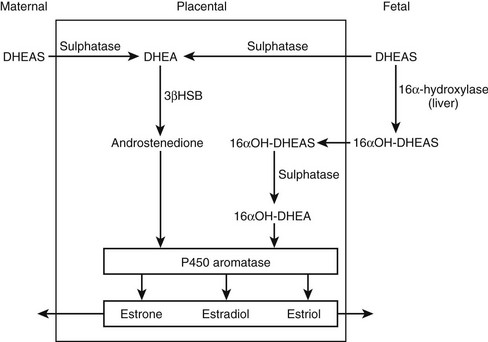
In concert with the morphologic changes in the fetal adrenal, there is a vast output of steroids in the form of DHEA, pregnenolone, 170H-pregnenolone, and their respective sulfated conjugates. The steroid output amounts to about 200 mg per day, of which 60% is DHEAS.4 This C19 fetal steroid is a major substrate for estrogen biosynthesis via the fetoplacental unit (Fig. 9-2). Key enzymes are 16α-hydroxylase to form 160H-DHEAS in the liver, placental sulfatase to produce free DHEA, and the action of placental P450 aromatase to synthesize the estrogens, estrone, estradiol, and estriol, from their respective C19 androgen substrates. The abundance of C19 steroids, which are normally aromatized by the placenta, is illustrated by the profound virilizing effects on the mother and her female fetus in the presence of an inactivating mutation of the CYP19 gene.5
Postnatal Adrenal Morphology and Function
A precipitous fall in adrenal weight by about 50% occurs after birth as a result of involution of the fetal zone. The definitive zone remains and is the template for development into the characteristic zones of the adult adrenal cortex. It is proposed that a combination of proliferation and migration of progenitor cells underlies this characteristic tissue zonation.6 The rapid reduction in adrenal gland size within weeks of birth is an apoptotic process rather than due to hemorrhage or necrosis.7 It appears to be independent of ACTH and is induced by the action of activin and transforming growth factor alpha (TGF-α). Involution of the fetal zone is reflected biochemically in a concomitant decrease in DHEAS levels, which remain low until the increase characteristic of adrenarche from ages 6 to 8. There is thickening of the zona reticularis evident by age 3.8 Longitudinal studies of 24-hour urinary androgen excretion and serum DHEAS concentrations suggest there is already a gradual increase in C19 steroid output from the postnatal adrenal gland from early childhood.9,10 This recrudescence of the pattern of fetal adrenal steroidogenesis indicates a common mechanism involving modulating factors operating within the adrenals to increase C19 steroid production independent of ACTH.
Mechanisms of Androgen Secretion at Adrenarche
DHEAS is the most abundant endogenous steroid produced within the endocrine system and circulates in micromolar concentrations. The pattern of secretion throughout prenatal and postnatal life is depicted in Fig. 9-3, and the prerequisites for DHEA and DHEAS synthesis are shown in Fig. 9-4.
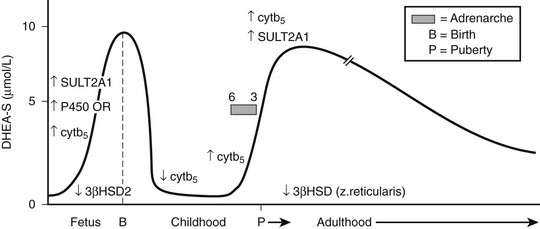
FIGURE 9-3 Schematic of dehydroepiandrosterone sulfate (DHEAS) production from fetal to adult life. The arrows indicate temporal changes in factors controlling DHEAS (see text).
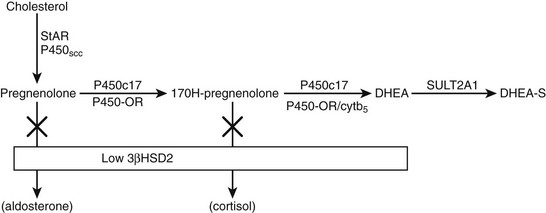
FIGURE 9-4 Pathway of dehydroepiandrosterone sulfate (DHEAS) synthesis in fetal adrenal and adult zona reticularis. Also depicted is the effect of low expression of 3β-hydroxysteroid dehydrogenase 2 (3β-HSD2) in these tissues.
The initial step in converting cholesterol to pregnenolone is common to all steroid-producing glands and is rate limiting.11 The key steroidogenic junction is at the step mediated by the P450c17 enzyme and defined as the qualitative regulator of steroidogenesis.12 This enzyme uniquely performs two catalytic activities called 17α-hydroxylase and 17,20-lyase. It is the latter enzyme activity that preferentially ensures C19 steroid production and, in particular, DHEA synthesis and adrenarche. A number of factors enhance the differential activities of P450c17. These include posttranslational regulation of P450c17 by phosphorylation on serine/threonine residues and the role of electron transfer proteins.13–15 The principal electron donor to microsomal P450 enzymes, including P450c17, is nicotinamide adenine dinucleotide phosphate (NADPH) cytochrome P450 oxidoreductase. This enzyme cofactor augments both 17α-hydroxylase and 17,20-lyase activities but is not the principal determinant of increased DHEA synthesis. However, mutations in the P450 oxidoreductase gene lead to a biochemical profile reminiscent of combined partial 17α-hydroxylase and 21-hydroxylase deficiencies with virilization in affected girls and undermasculinization in affected boys.16,17 The Antley-Bixler syndrome, a condition characterized by severe skeletal abnormalities, genital anomalies, and adrenal dysfunction, is also a manifestation of this enzyme deficiency.18 Enhancement of 17,20-lyase activity is linked specifically to the action of cytochrome b5 in the presence of adequate P450 oxidoreductase.12 This effect is far more substantial with Δ5 substrates such as 170H-pregnenolone as compared with a Δ4 substrate such as 170H-progesterone. Hence, the predominance of DHEA synthesis. Coupled to the promotion of DHEA synthesis is a relative deficiency of 3β-hydroxysteroid dehydrogenase (HSDB2) in the fetal adrenal and postnatal zona reticularis. This enzyme would normally compete with P450c17 to convert Δ5 to Δ4 steroids.19
More than 99% of DHEA is sulfated to DHEAS through the action of dehydroepiandrosterone sulfatase (SULT2A1).20 Sulfated steroids are also unavailable as substrates for HSDB2 activity, thus maintaining the high levels of DHEAS. Immunohistochemical studies in the fetal adrenal and in age-related postnatal adrenal glands have demonstrated a developmental pattern of enzyme and cofactor expression that may explain the intraadrenal control of adrenarche.21–23 In summary, the pattern of increased DHEAS secretion is associated with increased expression of P450c17, P450 oxidoreductase, cytochrome b5, SULT2A1, and decreased expression of HSDB2 in the fetal adrenal and again in the postnatal zona reticularis after age 5. In contrast, a reversal of this pattern occurs in the postnatal adrenal gland between infancy and age 5. Expression of cytochrome b5 and SULT2A1 is maintained beyond the adult age when DHEAS levels start to decline. The zona reticularis becomes smaller with increasing age, suggesting that the decline in DHEAS levels is associated with a decrease in cell number rather than a change in enzyme content. What factors stimulate the changes in expression of key proteins controlling DHEAS synthesis remain to be determined. HSDB2 has the important function of determining the ratio of Δ5 and Δ4 steroid production. A family of orphan nuclear receptors (nerve growth factor–induced clone B [NGF1B]) is expressed in parallel with HSDB2 and directly act on the promoter to up-regulate the HSD3B2 gene.24 It is possible that decreased expression of this and other transcriptional regulators of HSD3B2 at key developmental stages allows the increased production of DHEAS during fetal life and at adrenarche.
Extraadrenal Control of Adrenarche
There is sufficient dissociation between changes in cortisol and DHEAS secretion to indicate that ACTH is not the primary trophic factor responsible for the rise in DHEAS levels at adrenarche. Nevertheless, ACTH may play a permissive role because DHEAS levels are either undetectable or significantly decreased in familial glucocorticoid deficiency associated with ACTH resistance.25 Corticotropin-releasing factor (CRH) has been suggested as a specific adrenal androgen secretagogue in view of the direct effect of CRH on human fetal adrenal cells in stimulating DHEAS secretion and following CRH infusion in normal men.26,27 However, the interpretation of such in vivo data is compounded by the concomitant effect of increased ACTH secretion.
Numerous other factors have been postulated to have effects on adrenal androgen secretion, mainly based on in vitro studies using isolated human fetal adrenal cells. These include prolactin, estrogens, TGFs, cytokines, insulin-like growth factors (IGFs), and a fragment of pro-opiomelanocortin (POMC). None has proven to be a specific adrenal androgen secretagogue. Leptin differentially regulates 17,20-lyase activity in an adrenocortical carcinoma cell line that expresses the leptin receptor.28 There is also an association between DHEAS and leptin levels at the time of adrenarche in obese children.29 Overall, the adrenarche results from an alteration of expression of CYP17, HSD3B2, CYB5 (cytochrome b5), and SULT2A1, intertwined with changes in protein kinase A and insulin signaling pathways.
Clinical Facets of Adrenarche
The term adrenarche defines the rise in adrenal androgens (DHEA, DHEAS, and androstenedione) that occurs between 6 and 8 years of age as a result of the modulation of adrenal steroidogenesis previously described; it is thus a biochemical definition for an event that is not usually translated into clinical signs. Adrenarche is often used interchangeably with pubarche, which describes the growth of sexual hair in the suprapubic area and axillae. Adrenal androgens are the stimuli for public and axillary hair growth in females, and hair growth typically starts on the labia. In boys, the distinction is less clear because the growth of pubic and axillary hair is primarily stimulated by the rise in testicular testosterone production. Adrenarche (or pubarche) is considered premature when there is onset of pubic hair growth before age 8 in girls and before age 9 in boys. Because all children have an increase in adrenal androgen production between 6 and 8 years of age, it remains perplexing why hair growth is expressed only in a small minority. Premature adrenarche is more common in girls than boys, and there is an association with low birth weight.30 However, this sex dimorphism is not apparent in normal children in whom a relationship between lower birth weight and higher adrenal androgen levels is present in both sexes.31 Children with premature adrenarche are typically taller than average, may have increased body odor and acne, and exhibit a bone age advanced by 1 to 2 years. In a study of girls with premature adrenarche, increased linear growth was already evident in the first 2 years of life and associated with higher IGF-1 levels.32 The taller stature later is probably the result of increased androgen levels, yet the mini–growth spurt in normal children that also occurs around ages 6 to 8 is probably not a function of adrenarche.33 Obesity is generally associated with taller stature in childhood, and the body mass index (BMI) tends to be higher in premature adrenarche, but the BMI does not correlate with androgen levels.29
Table 9-1 lists the conditions that should be excluded when assessing a child with early pubic hair development and the appropriate investigations to perform in such cases. Imaging using ultrasound or computed tomography (CT) will readily exclude an adrenal tumor. Furthermore, if the cause is an adrenal tumor, signs of virilization are more profound. These signs include clitoromegaly, hirsutism, voice deepening, and in boys, penile growth.34 The main condition to exclude is late-onset congenital adrenal hyperplasia (CAH) which is found in about 5% of children with early onset of pubic hair. Baseline measurements of 170H-progesterone, androstenedione, testosterone, and DHEAS are usually sufficiently sensitive and specific to distinguish the two conditions.35 Further confirmation rests with mutational analysis of the CYP21 gene. Premature adrenarche has generally been regarded as a benign variant with a normal age of menarche in girls and no negative effect on adult height.36 However, more detailed longitudinal studies of premature adrenarche suggest an increased risk of later ovarian hyperandrogenism in adolescent girls and hyperinsulinism.37 It is possible that premature adrenarche is a risk factor for later development of the metabolic syndrome in adult life. Daughters of mothers with polycystic ovary syndrome are at higher risk of developing this syndrome, and a significant number exhibit exaggerated adrenarche.38 The role of sulfotransferases in providing adrenal substrates for androgen production is vividly illustrated by a child with premature adrenarche who subsequently progressed to features of the polycystic ovary syndrome.39 Investigations showed compound heterozygous mutations in the gene encoding for a sulfate donor required for sulfation of DHEA to DHEAS by DHEA sulfotransferase. The net result is an increase in unconjugated DHEA, which provides the substrate for increased adrenal androgen production. Currently, there is no indication that the use of insulin sensitizers should be considered in a child presenting with typical clinical and biochemical features of premature adrenarche, but it is apposite to counsel parents that affected girls may later experience menstrual disturbances in adolescence. Early intervention after menarche with insulin sensitizers may prevent progression to polycystic ovarian syndrome and reduce the risk of long-term cardiovascular problems.40
Role of Adrenal Androgens in Adults
The physiologic role of adrenal androgens, including DHEAS, is not well understood. The association of adrenarche with pubic and axillary hair development suggests a role for DHEAS as a substrate for androgen synthesis. There is evidence that DHEAS is converted to sex steroids (testosterone, estradiol) in peripheral tissues via several enzymatic steps. Circulating, adrenally derived DHEAS is first converted to DHEA by sulfatase, which is further converted to androstenedione by 3β-hydroxysteroid dehydrogenase (3β-HSD); in turn, androstenedione is modified to testosterone or estradiol by isozymes of 17β-HSD or P450 aromatase, respectively (Fig. 9-5). It has been suggested that such conversion, occurring intracellularly via these enzymes that are widely expressed in many peripheral tissues, constitutes an intracrine mechanism for local generation of sex steroids from DHEA. This mechanism might also account for the observation that DHEA administration can result in androgenic/estrogenic effects without significant changes in circulating levels of these steroids.41 There is also evidence to suggest that DHEA can act as a neurosteroid to exert effects in the central nervous system. Steroidogenic enzymes mediating DHEA synthesis are expressed in the brain, and it has effects on neuronal growth and differentiation.42 DHEA has also been shown to modulate neurotransmitter signaling, acting as an allosteric antagonist at the γ-aminobutyric acid (GABA) receptor but as an agonist at the N-methyl-d-aspartate (NMDA) receptor.43 DHEA may have a further potential role as an antiglucocorticoid. It has been shown to antagonize glucocorticoid-induced thymic involution or corticosterone-mediated neurotoxicity to hippocampal cells.44,45
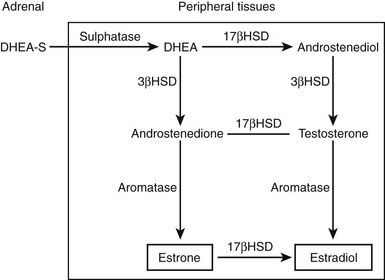
FIGURE 9-5 Pathways by which dehydroepiandrosterone sulfate (DHEAS) is converted intracellularly to sex steroids (testosterone, estradiol) in peripheral target tissues. Elevation in levels of androgen metabolites (e.g., androstanediol glucuronide) following DHEA treatment provides evidence for such peripheral sex steroid synthesis.
To date, a specific receptor that mediates the action of DHEA has not been identified, and whether it acts at the cell surface or intracellularly remains to be elucidated. DHEA has been shown to stimulate nitric oxide synthesis in endothelial cells via a specific G protein–coupled membrane receptor46 and to activate the mitogen-activated protein kinase (MAPK) pathway in vascular smooth muscle cells independently of androgen and estrogen receptors.47 Microarray studies of human peripheral blood mononuclear cells indicate that DHEA induces a gene expression profile that is distinct from glucocorticoid and testosterone, supporting the notion that it may act via an independent pathway.48
Adrenopause
Following peak levels in early adulthood, there is an inexorable decline in DHEA production such that by the age of 80 years, circulating levels are only 10% to 20% of those in young adults,49 whereas adrenal glucocorticoid and mineralocorticoid synthesis is relatively unchanged. The cause of this age-related decrease in DHEAS levels, termed the adrenopause, is not understood, but it is accompanied by involution of the zona reticularis and diminished adrenal 17α-hydroxylase enzyme activity. In both sexes, there is a progressive reduction in DHEA production of about 2% per year,50 with absolute levels being lower in women than men in the age range of 50 to 89 years.51 Interestingly, caloric restriction in monkeys attenuates the age-related fall in DHEAS,52 and smoking has been shown to maintain DHEAS levels in men.53
Cross-sectional epidemiologic studies have documented an association between the decline in DHEAS levels and various adverse effects of aging. An inverse relationship has been found between DHEAS levels and cardiovascular disease and mortality in elderly men,54 but this association was not observed in women.55 Low DHEAS levels have been correlated with heightened risk of breast cancer in premenopausal women.56 There is a positive correlation between reduced serum DHEAS levels and lower bone mineral density at the spine, hip, and radius in women 45 to 69 years of age57 but no such effect in men.58 In the central nervous system, lower DHEAS levels are associated with depressed mood in older women but not men,59 and a higher cortisol/DHEA ratio correlates with cognitive decline in both sexes.60 There are conflicting reports of lower DHEAS levels in Alzheimer’s disease.61,62 DHEAS levels are lower in men with type 2 diabetes mellitus,63 and insulin-sensitizing drug therapy enhances DHEAS levels, suggesting an inverse relationship with insulin resistance. However, a major caveat of such observational data is that falling DHEAS levels may simply be a marker of the aging process and therefore associated with its morbidities, rather than there being a causal relationship.
DHEA Replacement in Aging
Pharmacokinetic studies have established appropriate dose ranges for DHEA replacement in human aging: 50 mg of oral DHEA administration in men 49 to 70 years restored circulating DHEAS to young adult levels, whereas 100 mg daily was supraphysiologic.64 Administration of 25 or 50 mg of DHEA in elderly men and women achieved steady-state physiologic levels in 8 days.65 Interestingly, the half-life of circulating DHEA was more than 20 hours, similar to its longer-lived sulfated metabolite, suggesting that there may be significant back conversion of DHEAS in vivo. Circulating testosterone and estrogens also rose following DHEA administration, but to levels within the young adult range.
A randomized, double-blind, placebo-controlled trial examined the effect of 50 mg of DHEA treatment for 6 months in 13 men and 17 women 40 to 70 years of age.66 In addition to restoration of young adult DHEAS levels, there was a marked increase in physical and psychological well-being in both sexes (67% of men and 81% of women), with no change in libido. This effect was only associated with increased insulin-like growth factor 1 (IGF-1) and reduced IGF-binding protein 1 (IGFBP-1) levels, with no change in circulating sex hormone–binding globulin (SHBG), estrogens, or lipids. Extending these observations, the same researchers studied 100 mg of DHEA replacement for 6 months in elderly subjects (9 men, 10 women, aged 50 to 65),67 showing restoration of circulating DHEAS and the cortisol/DHEAS ratio to young adult levels. There was a decrease in fat mass and enhanced muscle strength in men but not women. Circulating androgens (androstenedione, testosterone, dihydrotestosterone) rose above young adult levels in women but not men, suggesting gender-specific differences in the response to DHEA administration. However, another study of 100 mg DHEA for 3 months in elderly men showed no effect on body composition, serum prostate-specific antigen, or urologic function.68
A major randomized, placebo-controlled trial was conducted of 50 mg DHEA replacement for 12 months in 280 men and women aged 60 to 79 years.69 Although young adult DHEAS levels were restored in both sexes, these researchers observed gender differences with other parameters as well. Serum testosterone and androstanediol glucuronide rose to slightly supraphysiologic levels at 6 months in 21% of women but not in men. Bone mineral density improved at the femoral neck (60- to 69-year age group) and radius (70- to 79-year age group), with a fall in serum collagen telopeptide, selectively in women. Likewise, only women reported an increase in libido, sexual function, and satisfaction. The skin changes seen in both sexes included increased skin hydration, diminished facial pigmentation and epidermal atrophy, and enhanced sebum production (particularly in women over 70). There were no changes in vascular function as assessed by ultrasonographic methods. A placebo-controlled trial evaluated DHEA supplementation for 24 months in 29 men and 27 women over 60 years, showing no effect on body composition, physical performance, insulin sensitivity, or quality of life but an improvement in bone mineral density at the ultradistal radius (women) or femoral neck (men).70
Following menopause, testosterone and androstenedione levels fall by 50%, and the decline in DHEAS with aging leads to a further fall in circulating androgens. This knowledge has prompted treatment of postmenopausal women with DHEA as a form of androgen replacement. Treatment of 14 women from the ages of 60 to 70 for 12 months with 10% DHEA cream applied topically successfully raised serum DHEA levels 10-fold.71 In conjunction with this, increased sebum production and an estrogenic effect on vaginal epithelium was observed. Bone mineral density at the hip was enhanced, with increased osteoblastic (osteocalcin) and decreased osteoclastic (bone alkaline phosphatase, urinary hydroxyproline) marker activity. Other changes included a 10% reduction in skinfold thickness and lower blood glucose and insulin levels, with no adverse effects on the lipid profile.72 Subjects also reported improved well-being. In contrast, 50 mg DHEA administered orally in 60 perimenopausal women (ages 45 to 55) had less effect. Despite a twofold rise in DHEAS levels, there was no effect on mood, cognition, quality of life, or libido.73 The addition of DHEA to exercise training in postmenopausal women showed no further benefit in physical performance, insulin sensitivity, or lipid profile.74
Exposure to DHEA augments natural killer cell–mediated cytotoxicity in vitro,75 and DHEA supplementation in postmenopausal women or aging men increases natural killer cell number and function.76,77 Although DHEA treatment does not influence the immune response to influenza vaccination,78 future studies of immune function following DHEA replacement which examine better in vivo immunologic correlates of its activity might reveal additional effects. Finally, there may be a pharmacologic role for DHEA in certain contexts. Although short-term (2 weeks’ duration) placebo-controlled studies have shown no effect of DHEA treatment on cognitive function in the elderly,79 a recent study has shown that DHEA therapy for 6 weeks had a significant beneficial effect in major depression.80
DHEA and Adrenal Insufficency
In comparison to aging, circulating DHEAS levels in adrenal failure are very low or undetectable. Such deficiency is observed in secondary as well as primary adrenal insufficiency (Addison’s disease), implying that production of this adrenal steroid, like glucocorticoid synthesis, is pituitary dependent. Glucocorticoid and mineralocorticoid deficiencies in adrenal insufficiency are life threatening and require oral replacement therapy, but the associated near-total failure of DHEA synthesis is not usually corrected. Despite optimal therapy with conventional steroids, patients with Addison’s disease report persistent fatigue and reduced well-being,81,82 with specific impairment in subscales of health status.83,84 These observations have prompted trials of DHEA replacement.
In 10 patients with hypopituitarism, 50 mg of DHEA replacement restored DHEAS, androstenedione, and testosterone to young adult levels, whereas a 200 mg daily dose was supraphysiologic.85 In young normal subjects in whom endogenous adrenal steroidogenesis had been suppressed with dexamethasone, 50 mg of DHEA daily was also an appropriate dose.86
A randomized, double-blind, placebo-controlled trial of 50 mg DHEA replacement was conducted in 24 women with adrenal insufficiency (14 primary, 10 secondary).87 Physiologic levels of DHEAS and androstenedione were restored, and serum testosterone rose from below to within low-normal range. Following 4 months of therapy, psychological testing showed a significant reduction in scores for depression and anxiety, together with improvement in overall well-being and mood. Patients also reported markedly increased sexual thoughts and interest, with enhanced mental and physical sexual satisfaction. Serum testosterone rose from below to within low-normal range, with a fall in SHBG; 19 subjects developed some cutaneous androgenic side effects. A later study of DHEA replacement in nine women with Addison’s disease also reported increased apocrine sweat secretion and acne, with no difference between 50 or 200 mg daily dosage.88
In a randomized, placebo-controlled trial in 15 men and 24 women with Addison’s disease, the authors observed similar restoration of DHEAS and androstenedione levels in both sexes following 3 months of treatment with 50 mg of DHEA, together with a rise in serum testosterone and fall in SHBG in women but not men.89 There was an overall trend of enhanced well-being, with particular improvement in self-esteem. Mood and fatigue also improved significantly, with benefit being evident in the evenings. The authors found no effects on cognitive or sexual function. No significant adverse effects were seen in patients. Beneficial psychological effects in males, independent of changes in circulating testosterone, supported the notion of a direct central nervous system effect of DHEA rather than it simply being a substrate for peripheral androgen biosynthesis. DHEA replacement for 12 or 24 weeks in adrenal insufficiency has no effect on vascular and endothelial function90,91 but is significantly immunomodulatory.92
Administration of lower doses (20 to 30 mg) of DHEA for 6 months to 38 women with hypopituitarism was associated with increased axillary or pubic hair, together with improved alertness, stamina, and initiative, as reported by their spouses.93 A recent study has shown similar benefit in adolescent girls with central adrenal insufficiency.94 A further benefit in female hypopituitarism is that addition of DHEA treatment reduces the dose of growth hormone replacement required to maintain IGF-1 levels.95 A longer term, 9-month trial of administering 25 mg of DHEA to 39 women with adrenal insufficiency showed no beneficial effects on subjective health status or sexual function, but the study may have been underpowered.96,97 In a trial with a larger cohort of over 100 patients with Addison’s disease, the authors found that treatment with 50 mg of DHEA for 12 months was associated with beneficial effects in well-being and fatigue, enhancement of lean body mass, and improvement in femoral neck bone mineral density. Adverse androgenic side effects were observed in older females, suggesting that a lower dose of DHEA might be more appropriate in this age group.83
References
1. Abbott, DH, Bird, IM. Non-human primates as models for human adrenal androgen production: function and dysfunction. Rev Endocr Metab Disord. 2009;10:33–42.
2. Young, MC, Laurence, KM, Hughes, IA. Relationship between fetal adrenal morphology and anterior pituitary function. Horm Res. 1989;32:130–135.
3. Coleman, MA, Honour, JW. Reduced maternal dexamethasone dosage for the prenatal treatment of congenital adrenal hyperplasia. Br J Obstet Gynaecol. 2004;111:176–178.
4. Rehman, KS, Carr, BR, Rainey, WE. Profiling the steroidogenic pathway in human fetal and adult adrenals. J Soc Gynecol Investig. 2003;10:372–380.
5. Jones, ME, Boon, WC, McInnes, K, et al. Recognizing rare disorders: aromatase deficiency. Nat Clin Pract Endocrinol Metab. 2007;3:414–421.
6. Wolkersdorf, G, Bornstein, SR. Tissue remodelling in the adrenal gland. Biochem Pharmacol. 1998;56:163–171.
7. Spencer, SJ, Mesiano, S, Lee, JY, et al. Proliferation and apoptosis in the human adrenal cortex during the fetal and perinatal periods: implications for growth and remodelling. J Clin Endocrinol Metab. 1999;84:1110–1115.
8. Dhom, G. The prepubertal and pubertal growth of the adrenal (adrenarche). Beitr Pathol. 1973;150:357–377.
9. Remer, T, Manz, F. Role of nutritional status in the regulation of adrenarche. J Clin Endocrinol Metab. 1999;84:3936–3944.
10. Palmert, MR, Hayden, DL, Mansfield, MJ, et al. The longitudinal study of adrenal maturation during gonadal suppression: evidence that adrenarche is a gradual process. J Clin Endocrinol Metab. 2001;86:4536–4542.
11. Stocco, DM, Clark, BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–244.
12. Miller, WL. Androgen biosynthesis from cholesterol to DHEA. Mol Cell Endocrinol. 2002;198:7–14.
13. Zhang, L, Rodriguez, H, Ohno, S, et al. Serine phosphorylation of human P450c17 increases 17,20 lyase activity: implications for adrenarche and for the polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1995;92:10619–10623.
14. Lin, D, Black, SM, Nagahama, Y, et al. Steroid 17a-hydroxylase and 17,20-lyase activities of P450c17: Contributions of serine 106 and P450 reductase. Endocrinology. 1993;132:2496–2506.
15. Lee-Robichaud, P, Wright, JN, Akhtar, ME, et al. Modulation of the activity of human 17a-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem J. 1995;308:901–908.
16. Arlt, W, Walker, EA, Draper, N, et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: Analytical study. Lancet. 2004;363:2128–2135.
17. Miller, WL. P450 oxidoreductase deficiency: a new disorder of steroidogenesis with multiple clinical manifestations. Trends Endocrinol Metab. 2004;15:311–315.
18. Flück, CE, Tajima, T, Pandey, AV, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36:228–230.
19. Conley, AJ, Birm, IM. The role of cytochrome P450 17α-hydroxylase and 3β-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the Δ5 and Δ4 pathway of steroidogenesis in mammals. Biol Reprod. 1997;56:789–799.
20. Strott, CA. Steroid sulfotransferases. Endocr Rev. 1996;17:670–697.
21. Suzuki, T, Sasano, H, Takeyama, J, et al. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol. 2000;53:739–747.
22. Rainey, WE, Carr, BR, Sasano, H, et al. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–239.
23. Auchus, RJ, Rainey, WE. Adrenarche-physiology, biochemistry and human disease. Clin Endocrinol. 2004;60:288–296.
24. Bassett, MH, Suzuki, T, Sasano, H, et al. The orphan nuclear receptor NGF1B regulates transcription of 3β-hydroxysteroid dehydrogenase: implications for the control of adrenal functional zonation. J Biol Chem. 2004;279:37622–37630.
25. Weber, A, Clark, AJ, Perry, LA, et al. Diminished adrenal androgen secretion in familial glucocorticoid deficiency implicates a significant role for ACTH in the induction of adrenarche. Clin Endocrinol. 1997;46:431–437.
26. Smith, R, Mesiano, S, Cheng, EC, et al. Corticotropin-releasing hormone directly and preferentially stimulates Dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J Clin Endocrinol Metab. 1998;83:2916–2920.
27. Ibanez, L, Potau, N, Marcos, MV, et al. Corticotropin-releasing hormone as adrenal androgen secretagogue. Pediatr Res. 1999;46:351–353.
28. Biason-Lauber, A, Zachmann, M, Schoenle, EJ. Effect of leptin on CYP17 enzymatic activities in human adrenal cells: new insight in the onset of adrenarche. Endocrinology. 2000;141:1446–1454.
29. l’Allemand, D, Schmidt, S, Rousson, V, et al. Associations between body mass, leptin, IGF-1 and circulating adrenal androgens in children with obesity and premature adrenarche. Eur J Endocrinol. 2002;146:537–543.
30. Charkaluk, M-L, Trivin, C, Brauner, R. Premature pubarche as an indicator of how body weight influences the onset of adrenarche. Eur J Pediatr. 2004;163:89–93.
31. Ong, KK, Potau, N, Petry, CJ, et al. Opposing influences of prenatal and postnatal weight gain on adrenarche in normal boys and girls. J Clin Endocrinol Metab. 2004;89:2647–2651.
32. Utrainen, P, Voutilainen, R, Jääskeläinen, J. Girls with premature adrenarche have accelerated early childhood growth. J Pediatr. 2009;154:882–887.
33. Remer, T, Manz, F. The midgrowth spurt in healthy children is not caused by adrenarche. J Clin Endocrinol Metab. 2001;86:4183–4186.
34. Ribeiro, RC, Figueiredo, B. Childhood adrenocortical tumours. Eur J Cancer. 2004;40:1117–1126.
35. Armegaud, J-B, Charkaluk, M-L, Trivin, C, et al. Precocious pubarche: distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. J Clin Endocrinol Metab. 2009;94:2835–2840.
36. Ibanez, L, Virdis, R, Potau, N, et al. Natural history of premature pubarche: an auxological study. J Clin Endocrinol Metab. 1992;74:254–257.
37. Ibanez, L, Diaz, R, Lopez-Bermejo, A, et al. Clinical spectrum of premature pubarche: links to metabolic syndrome and ovarian hyperandrogenism. Rev Endocr Metab Disord. 2009;10:63–76.
38. Maliqueo, M, Sir Petermann, T, Perez, V, et al. Adrenal function during childhood and puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1923–1930.
39. Noordam, C, Dhir, V, McNelis, C, et al. Inactivating PAPSS2 mutations in a patient with premature adrenarche. N Engl J Med. 2009;360:2310–2318.
40. Ibanez, L, Ferrer, A, Ong, K, et al. Insulin sensitisation early after menarche prevents progression from precocious pubarche to polycystic ovary syndrome. J Pediatr. 2004;144:4–6.
41. Labrie, F, Luu-the, V, Labrie, C, et al. Endocrine and intracrine sources of androgens in women: Inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24:152–182.
42. Mellon, S, Griffin, L. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43.
43. Majewska, MD. Neuronal actions of dehydroepiandrosterone. Possible roles in brain development, aging, memory, and affect. Ann N Y Acad Sci. 1995;774:111–120.
44. May, M, Holmes, E, Rogers, W, et al. Protection from glucocorticoid induced thymic involution by dehydroepiandrosterone. Life Sci. 1991;46:1627–1631.
45. Kimonides, VG, Spillantini, MG, Sofroniew, MV, et al. Dehydroepiandrosterone (DHEA) antagonises the neurotoxic effects of corticosterone and translocation of SAPK3 in hippocampal primary cultures. Neuroscience. 1999;89:429–436.
46. Liu, D, Dillon, JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Gai2,3. J Biol Chem. 2002;277:21379–21388.
47. Williams, MR, Ling, S, Dawood, T, et al. Dehydroepiandrosterone inhibits human vascular smooth muscle cell proliferation independent of ARs and ERs. J Clin Endocrinol Metab. 2002;87:176–181.
48. Maurer, M, Trajanoski, Z, Frey, G, et al. Differential gene expression profile of glucocorticoids, testosterone, and dehydroepiandrosterone in human cells. Horm Metab Res. 2001;33:691–695.
49. Vermeulen, A. Dehydroepiandrosterone sulfate and aging. Ann N Y Acad Sci. 1995;774:121–127.
50. Orentreich, N, Brind, JL, Rizer, RL, et al. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555.
51. Laughlin, GA, Barrett-Connor, E. Sexual dimorphism in the influence of advanced aging on adrenal hormone levels: The Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:3561–3568.
52. Lane, MA, Ingram, DK, Ball, SS, et al. Dehydroepiandrosterone sulfate: a biomarker of primate ageing slowed by calorie restriction. J Clin Endocrinol Metab. 1997;82:2093–2096.
53. Salvini, S, Stampfer, MJ, Barbieri, RL, et al. Effects of age, smoking and vitamins on plasma DHEAS levels: a cross-sectional study in men. J Clin Endocrinol Metab. 1992;74:139–143.
54. Barrett-Connor, E, Khaw, K-T, Yen, SSC. A prospective study of dehydroepiandrosterone sulfate, mortality and cardiovascular disease. New Engl J Med. 1986;315:1519–1524.
55. Barrett-Connor, E, Goodman-Gruen, D. Dehydroepiandrosterone sulfate does not predict cardiovascular death in postmenopausal women, The Rancho Bernardo Study. Circulation. 1995;91:1757–1760.
56. Helzlsouer, KJ, Gordon, GB, Alberg, AJ, et al. Relationship of prediagnostic serum levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate to the risk of developing premenopausal breast cancer. Cancer Res. 1992;52:1–4.
57. Szathmari, M, Szucs, J, Feher, T, et al. Dehydroepiandrosterone sulphate and bone mineral density. Osteoporos Int. 1994;4:84–88.
58. Greendale, GA, Edelstein, S, Barrett-Connor, E. Endogenous sex steroids and bone mineral density in older women and men: The Rancho Bernardo Study. J Bone Miner Res. 1997;12:1833–1843.
59. Barrett-Connor, E, von Muhlen, D, Laughlin, GA, et al. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc. 1999;47:685–691.
60. Kalmijn, S, Launer, LJ, Stolk, RP, et al. A prospective study on cortisol, dehydroepiandrosterone sulfate and cognitive function in the elderly. J Clin Endocrinol Metab. 1998;83:3487–3492.
61. Nasman, B, Olsson, T, Backstrom, T, et al. Serum dehydroepiandrosterone sulfate in Alzheimer’s disease and in multi-infarct dementia. Biol Psychiatry. 1991;30:684–690.
62. Schneider, LS, Hinsey, M, Lyness, S. Plasma dehydroepiandrosterone sulfate in Alzheimer’s disease. Biol Psychiatry. 1992;31:205–208.
63. Barrett-Connor, E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med. 1992;117:807–811.
64. Arlt, W, Haas, J, Callies, F, et al. Biotransformation of oral dehydroepiandrosterone in elderly men: significant increase in circulating estrogens. J Clin Endocrinol Metab. 1999;84:2170–2176.
65. Legrain, S, Massien, C, Lahlou, N, et al. Dehydroepiandrosterone replacement administration: Pharmacokinetic and pharmacodynamic studies in healthy elderly subjects. J Clin Endocrinol Metab. 2000;85:3208–3217.
66. Morales, AJ, Nolan, JJ, Nelson, JC, et al. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360–1367.
67. Morales, AJ, Haubrich, RH, Hwang, JY. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol. 1998;49:421–432.
68. Flynn, MA, Weaver-Osterholtz, D, Sharpe-Timms, KL, et al. Dehydroepiandrosterone replacement in aging humans. J Clin Endocrinol Metab. 1999;84:1527–1533.
69. Baulieu, EE, Thomas, G, Legrain, S, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci U S A. 2000;97:4279–4284.
70. Nair, KS, Rizza, RA, O’Brien, P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659.
71. Labrie, F, Diamond, P, Cusan, L, et al. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina and endometrium in postmenopausal women. J Clin Endocrinol Metab. 1997;82:3498–3505.
72. Diamond, P, Cusan, L, Gomez, J-L, et al. Metabolic effects of 12-month percutaneous dehydroepiandrosterone replacement therapy in postmenopausal women. J Endocrinol. 1996;150:S43–S50.
73. Barnhart, KT, Freeman, E, Grisso, JA, et al. The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health-related quality of life. J Clin Endocrinol Metab. 1999;84:3896–3902.
74. Igwebuike, A, Irving, BA, Bigelow, ML, et al. Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in postmenopausal women. J Clin Endocrinol Metab. 2008;93:534–538.
75. Solerte, SB, Fioravanti, M, Vignati, G, et al. Dehydroepiandrosterone sulfate enhances natural killer cell cytotoxicity in humans via locally generated immunoreactive insulin-like growth factor I. J Clin Endocrinol Metab. 1999;84:3260–3267.
76. Casson, PR, Andersen, RN, Herrod, HG, et al. Oral dehydroepiandrosterone in physiologic doses modulates immune function in postmenopausal women. Am J Obstet Gynecol. 1993;169:1536–1539.
77. Khorram, O, Vu, L, Yen, SS. Activation of immune function by dehydroepiandrosterone (DHEA) in age-advanced men. J Gerontol. 1997;52:M1–M7.
78. Danenberg, HD, Ben-Yehuda, A, Zakay-Rones, Z, et al. Dehydroepiandrosterone treatment is not beneficial to the immune response to influenza in elderly subjects. J Clin Endocrinol Metab. 1997;82:2911–2914.
79. Wolf, OT, Kirschbaum, C. Wishing a dream came true: DHEA as a rejuvenating treatment? J Endocrinol Invest. 1998;21:133–135.
80. Wolkowitz, OM, Reus, VI, Keebler, A, et al. Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry. 1999;156:646–649.
81. Reidel, M, Weise, A, Schurmeyer, TH, et al. Quality of life in patients with Addison’s disease: effects of different cortisol replacement modes. Exp Clin Endocrinol. 1993;101:106–111.
82. Baker, SJK, Hunt, PJ, Wass, JAH. Assessing the potential for finetuning the management of Addison’s disease/steroid replacement therapy. J Endocrinol. 1997;155(Suppl):P2.
83. Gurnell, EM, Hunt, PJ, Curran, SE, et al. Long-term DHEA replacement in primary adrenal insufficiency: a randomised, controlled trial. J Clin Endocrinol Metab. 2008;93:400–409.
84. Hahner, S, Loeffler, M, Fassnacht, M, et al. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab. 2007;92:3912–3922.
85. Young, J, Couzinet, B, Nahoul, K, et al. Panhypopituitarism as a model to study the metabolism of dehydroepiandrosterone (DHEA) in humans. J Clin Endocrinol Metab. 1997;82:2578–2585.
86. Arlt, W, Justl, H-G, Callies, F, et al. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young females after dexamethasone suppression. J Clin Endocrinol Metab. 1998;83:1928–1934.
87. Arlt, W, Callies, F, van Vlijmen, JC, et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. New Engl J Med. 1999;341:1013–1020.
88. Gebre-Medhin, G, Husebye, ES, Mallmin, H, et al. Oral dehydroepiandrosterone (DHEA) replacement therapy in women with Addison’s disease. Clin Endocrinol. 2000;52:775–780.
89. Hunt, PJ, Gurnell, EM, Huppert, FA, et al. Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison’s Disease in a randomized, double blind trial. J Clin Endocrinol Metab. 2000;85:4650–4656.
90. Christiansen, JJ, Andersen, NH, Sorensen, KE, et al. Dehydroepiandrosterone substitution in female adrenal failure: no impact on endothelial function and cardiovascular parameters despite normalization of androgen status. Clin Endocrinol (Oxf). 2007;66:426–433.
91. Rice, SP, Agarwal, N, Bolusani, H, et al. Effects of dehydroepiandrosterone replacement on vascular function in primary and secondary adrenal insufficiency: a randomized crossover trial. J Clin Endocrinol Metab. 2009;94:1966–1972.
92. Coles, AJ, Thompson, S, Cox, AL, et al. Dehydroepiandrosterone replacement in patients with Addison’s disease has a bimodal effect on regulatory (CD4+CD25hi and CD4+FoxP3+) T cells. Eur J Immunol. 2005;35:3694–3703.
93. Johannsson, G, Burman, P, Wiren, L, et al. Low dose dehydroepiandrosterone affects behavior in hypopituitary androgen-deficient women: a placebo-controlled trial. J Clin Endocrinol Metab. 2002;87:2046–2052.
94. Binder, G, Weber, S, Ehrismann, M, et al. Effects of dehydroepiandrosterone therapy on pubic hair growth and psychological well-being in adolescent girls and young women with central adrenal insufficiency: a double-blind, randomized, placebo-controlled phase III trial. J Clin Endocrinol Metab. 2009;94:1182–1190.
95. Brooke, AM, Kalingag, LA, Miraki-Moud, F, et al. Dehydroepiandrosterone (DHEA) replacement reduces growth hormone (GH) dose requirement in female hypopituitary patients on GH replacement. Clin Endocrinol (Oxf). 2006;65:673–680.
96. Lovas, K, Gebre-Medhin, G, Trovik, ST, et al. Replacement of dehydroepiandrosterone in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab. 2003;88:1112–1118.
97. Arlt, W, Alolio, B. DHEA. Replacement in adrenal insufficiency. J Clin Endocrinol Metab. 2003;88:4001–4002.