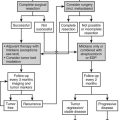
Adrenal Surgery
Prior to 1992, adrenalectomies were performed exclusively by open techniques. These were mainly performed through an open anterior approach via midline or subcostal incision, or through a posterior approach through the 11th or 12th ribs. In 1992, the laparoscopic technique was described by Gagner in Canada and by Higashihara in Japan.1,2 Initially, laparoscopic approaches to adrenalectomy were limited to small benign lesions, but indications continue to expand. Currently, laparoscopy is the gold standard for excision of the majority of benign adrenal lesions.3–5 The use of laparoscopy for lesions greater than 10 cm or in clearly malignant lesions remains under debate. Recently, robotic surgery has been applied to adrenalectomy, although the role of this technology remains unclear.
In parallel to advances in surgical techniques, imaging technology has become increasingly sophisticated, allowing a greater understanding of endocrinologic disorders. CT and MRI have allowed excellent visualization of the retroperitoneal space and both adrenal glands. Enhanced CT and MRI virtually eliminated the necessity of retroperitoneal exploration in situations such as familial pheochromocytoma, allowing the use of a unilateral approach and subsequent periodic imaging to evaluate possible recurrence.6
Indications and Contraindications
Adrenal masses are common and are frequently found incidentally on imaging obtained for other reasons.7 The workup of an adrenal mass is straightforward and is focused on determining if a mass is functional or may represent malignancy, both indications for adrenalectomy (Table 15-1). The specifics of this workup are discussed in detail elsewhere in this text. Although the majority of adrenal masses are benign, the finding of malignant adrenal masses is not uncommon, and as many as 15% of incidentally found adrenal masses are functionally active.8 Benign adrenal masses include the nonfunctioning (“incidentaloma”) and the functioning adrenal mass (Conn’s Syndrome, Cushing’s tumor, pheochromocytoma, or an androgen-secreting tumor). Primary malignancies of the adrenal may have their own characteristic imaging finding; however, this is often difficult to determine with certainty preoperatively, and size of the mass is used as an important predictor. A final class of adrenal masses includes metastatic lesions.
Table 15-1
Mass
Hyperplasia (Bilateral)
Of the benign lesions mentioned, pheochromocytoma has an increased chance of being bilateral or ectopic. Careful evaluation should be made prior to the initial operation, documenting the location of ectopic tissue. Previously, MEN2 patients underwent bilateral adrenalectomy and retroperitoneal exploration. In 1993, Wells and colleagues advocated that if a unilateral lesion is found, it may be reasonable to resect it, followed by surveillance of the remaining adrenal.6 Only 50% of patients with unilateral pheochromocytoma in the MEN2 population will require contralateral adrenalectomy within 5 years, thereby decreasing their exposure to exogenous corticosteroid replacement for that time. This allows for less invasive, more direct approaches, including flank incision (and later, laparoscopy), with greatly reduced morbidity and mortality.
Contraindications to adrenal surgery after appropriate selection are relatively few. It has been emphasized that prior to considering resection, several factors must be taken into account. For example, a 2-cm, nonfunctioning incidentaloma should not (in general) be surgically removed, whereas a 6-cm lesion should.9 A 2-cm functioning tumor should be removed, whereas a 2-cm metastasis to the adrenal should be carefully considered with regard to prognosis. An asymptomatic angiomyelolipoma of almost any size need not be resected if the diagnosis is certain. Therefore, although the surgical capability to perform adrenalectomy is usually available, it must be tempered by presurgical judgment. If the laparoscopic approach is to be considered, it is clear that experience is needed both in endocrine surgery and in laparoscopy. Caution should be used in the appropriate choice of technique for adrenal or retroperitoneal procedures.
Laparoscopy has emerged as the standard for surgical removal of most lesions of the adrenal gland.3–5 Although no prospective randomized trial has been reported comparing laparoscopic adrenalectomy to open adrenalectomy, in multiple reviews, laparoscopy has proven to be as safe and effective as the open approach and offers less pain, shorter hospital stay, and more rapid convalescence.5 Gagner et al. had suggested that increased intraoperative catecholamine secretion occurs during laparoscopic surgery, possibly related to CO2 insufflation.10 Other studies have not shown that CO2 had any different effect than helium insufflation, and most recent studies have confirmed the intraoperative safety of the technique.11
Metastatic or regionally advanced carcinoma of the adrenal, or the presence of regionally metastatic pheochromocytoma, makes the laparoscopic approach less appropriate.12 Such a clinical situation might require en bloc resection, possibly involving kidney or other organs as well as regional lymph nodes. Despite advances in minimally invasive techniques, it still is believed that adrenal masses that demonstrate evidence of local invasion should be approached with an open resection, and reported series of laparoscopic adrenalectomy have excluded these patients.
The size of the adrenal lesion may affect the choice of operative approach. There are two primary concerns regarding resecting large adrenal lesions through minimally invasive techniques. The first is the technical difficulty of safely dissecting and removing bulky lesions. The second major concern is the possibility of laparoscopically resecting a known or unknown adrenocortical carcinoma, which remains a debated practice. The likelihood of an undiagnosed lesion harboring malignancy increases dramatically with the size of the lesion. According to the NIH consensus statement for the management of undiagnosed adrenal masses, the incidence of adrenocortical carcinomas increases from 2% in lesions ≤40 mm, to 6% in lesions 41 to 60 mm, to 25% in lesions >60 mm.13
Adrenocortical carcinomas are rare and have poor prognoses, with 12% to 38% 5-year survival.14 Complete resection offers the only hope of cure (see Chapter 11). Therefore, patients with carcinomas often require more extensive resection, which may include local lymph nodes, kidney, spleen, and/or partial resection of the pancreas or liver. The standard approach for documented adrenal malignancy has been an open laparotomy. Early, limited reports of laparoscopic resections of primary adrenal malignancies were associated with very high rates of early recurrence, including in laparoscopic port sites as well as peritoneal carcinomatosis.15 It was believed that insufflation, which is necessary for laparoscopy, contributed to disease spread. This belief now has been replaced by an understanding that the operation must follow basic oncologic principles regardless of the technique employed for resection. As additional experience has accumulated, improved outcomes are now reported with laparoscopy for primary adrenal malignancy.15 Table 15-2 summarizes 15 publications that included laparoscopic resections for primary adrenal malignancies, all published between 2002 and 2008 (adapted from McCauley15). This aggregate includes 60 primary adrenal malignancies; the low number is reflective of the rarity of these tumors. In this analysis, six of the reported tumor recurrences and all five instances of port-site recurrence and carcinomatosis occurred in a single series.16 Of note, those five patients represent a highly selected group, all of whom presented with recurrent disease prior to the resection reported in this series, identifying them as high risk for aggressive tumor behavior. Even including these five patients, oncologic results in the overall group compare favorably to open series; 35% had recurrence during approximately 3 years of mean follow-up. This compares well with results reported in open series which report similar or higher recurrence rates.15,16
Table 15-2
Summary of Series Reporting Laparoscopic Adrenalectomy for Primary Adrenal Malignancies from 2002 to 2008
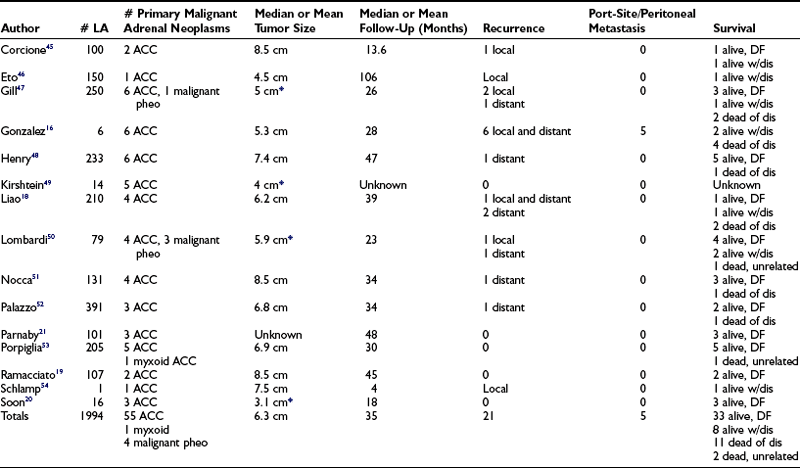
*Indicates that size includes all tumor types for these series.
Data from McCauley and Nguyen.15
Although the question is not settled, there is a growing consensus in the literature that minimally invasive techniques may be employed for excision of primary adrenal malignancy in appropriately selected cases. It also is now becoming evident that laparoscopy provides similar long-term results to open adrenalectomy when employed for solitary metastases to the adrenal.15 Appropriate planning and patient selection are imperative. If preoperative imaging suggests invasion into surrounding structures or vena caval thrombus, one should proceed with open resection. Principles of resection that apply in open surgery must be maintained in laparoscopy, including preservation and dissection of tissue planes and avoidance of fracture or rupture of the lesion. The specimen should be removed from the body in an impermeable retrieval bag to avoid exposure of the port site. It is obvious that the surgeon must be experienced in both laparoscopy and endocrine surgery. Because malignancy may be undiagnosed prior to resection, all of these principles should be applied to laparoscopic and open adrenalectomy in general, with particular attention in large or suspicious lesions.
There remains debate in the literature regarding how large of a lesion should be approached laparoscopically, both for oncologic and technical reasons. A large lesion makes the operation more challenging. Most series reporting resection of large masses, including those in Table 15-1, emphasize the lateral transperitoneal technique, which allows the greatest visualization and operative space. Even with this approach, however, visualization can be a challenge in large lesions. Large lesions also may have aberrant vascular supply, thus leading to potential for operative bleeding. While lesions as large as 15 cm have been resected laparoscopically,17 the appropriate size restrictions for laparoscopic adrenalectomy has not been universally agreed upon. Multiple series have now been published of laparoscopic adrenalectomy for lesions over 6 cm.15,18–21 Hospital length of stay, operative blood loss, and overall morbidity are not necessarily worsened following laparoscopic resections of large lesions. Rosoff’s review concludes that tumor size alone should not preclude laparoscopic approaches, but that larger lesions should be managed by experienced laparoscopic surgeons who also are adept at the open procedure, should conversion be required. The review also emphasizes the importance of adhering to the oncologic principles discussed earlier.22 Previous upper-abdominal surgery such as nephrectomy, splenectomy, or hepatic resection is not an absolute contraindication to laparoscopy but does make the procedure more difficult. This could necessitate conversion to an open procedure, potentially in an urgent fashion if difficult dissection leads to operative bleeding. Retroperitoneal laparoscopic approach has been reported to circumvent these problems, but this procedure is technically challenging and not widely practiced.
Robotic surgery is a fairly recent development in adrenal surgery. Robotic computer-assisted telemanipulation systems were developed to overcome some of the limitations of standard laparoendoscopic techniques and to facilitate surgeon hand motions in limited operating spaces. The surgeon operates from a remote console, and hand motions are reproduced in scaled proportion through robotically controlled microwrist instruments inserted through the body wall. Purported advantages of robotic-assisted laparoscopic surgery over conventional laparoscopy include hand motions intuitive even to the nonlaparoscopic surgeon,23 seven degrees of freedom of motion (compared to four with standard laparoscopy), three-dimensional image projection, tremor suppression, motion scaling, and the potential to perform remote “telesurgery.”
Robotic approaches have been applied in a wide variety of urologic, cardiothoracic, gynecologic, and general surgical procedures. The first report of robotic adrenalectomy was by Piazza et al. in 1999.24 Hanly and colleagues reported 30 robotic adrenalectomies without any conversions to open procedures.25 They felt that the robotic system permitted improved identification and control of the multiple adrenal arteries. Although all other reports of robotic adrenalectomy have been for benign lesions, a robotic resection of an 8-cm adrenal lesion that proved to be adrenocortical carcinoma with clean margins has now been reported, with good early results.26
Several reports have compared robotic to laparoscopic adrenalectomy. No difference was found in perioperative quality of life measures between laparoscopic and robotic adrenalectomy.27 An earlier randomized series compared one hospital’s first 10 robotic adrenalectomies to 10 laparoscopic adrenalectomies28 and reported that laparoscopy was superior to robotics in terms of morbidity and cost. More recently, a retrospective review of prospectively collected data compared 50 patients undergoing robotic unilateral adrenalectomy to 59 patients undergoing laparoscopic unilateral transperitoneal adrenalectomy.29 They found that the robotic approach was associated with lower blood loss but longer operative times early in their series. Interestingly, they reported a learning curve of 20 robotic adrenalectomies, after which the difference in operative time was absent. Furthermore, operative times were not affected by obesity or large tumors in the robotic group, while these factors led to significantly longer operative times in the laparoscopic group. Conversion rate, morbidity, and hospital stay were similar between groups.
Preoperative History and Evaluation
The patient with pheochromocytoma deserves particular attention (see Chapter 14). Once this diagnosis is made, medical management must address hypertension, decreased intravascular volume, and possible cardiac arrhythmias. The use of phenoxybenzamine, a long-acting α-adrenoceptor blocker, is usually suggested for 1 to 3 weeks prior to surgery (see also Chapter 14). More recently, some groups have reported the use of preoperative blockade with selective postsynaptic α1-adrenergic receptor antagonists such as prazosin hydrochloride or doxazosin. Such selective agents are thought not to produce reflex tachycardia and have a shorter half-life. These agents may avoid postoperative hypotension that are associated with the longer-acting drugs. These are important considerations, yet phenoxybenzamine remains a standard and time-tested preoperative blockade. The use of calcium channel blockers or ACE inhibitors has also gained acceptance. Because these medications are given in the preoperative period, attention should be given to oral salt and fluid intake to address expansion of intravascular volume prior to surgery. This preoperative regimen must be closely watched. During this time, patients will retain fluid and demonstrate side effects of the medication, possibly including sinus discomfort and orthostatic hypotension. Additionally, a small subset of patients may have arrhythmias which may require β-adrenoceptor blockade. Effective β-blockers include propranolol and nadolol.30 Our group has had excellent experience with metyrosine, 1 to 4 g/day for 10 to 14 days prior to surgery, in addition to phenoxybenzamine. Metyrosine is a tyrosine hydroxylase inhibitor and is very effective in reducing blood pressure; it is associated with fatigue and sinus discomfort. Patients need to be instructed carefully about its side effects.
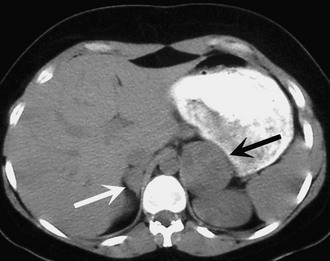
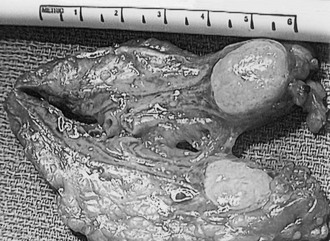
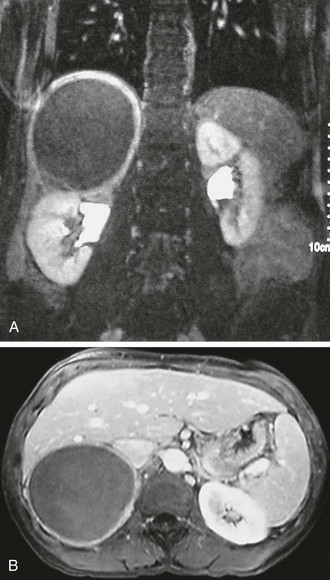
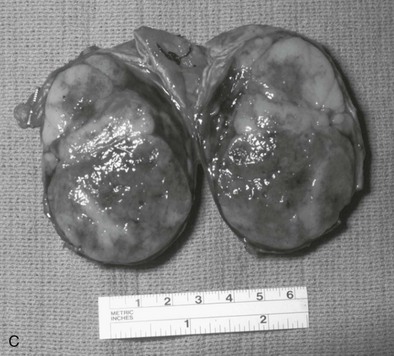
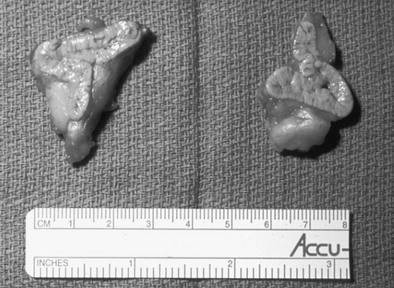
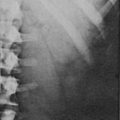
Preoperative imaging must be evaluated by expert interpretation and also should be studied by the operating surgeon. CT and MRI form the cornerstones of workup and preoperative diagnosis. These films must be of adequate technique and quality to allow investigation of the retroperitoneum and the contralateral adrenal, as well as any other anatomy relevant to the diagnostic workup (e.g., the lung fields, in the case of metastasis). In the case of aldosterone excess, the diagnosis of a solitary adrenal mass must be made to secure the diagnosis of Conn’s syndrome. If both adrenals are not investigated, occasional macronodular bilateral hyperplasia might present with a unilateral nodule that, although appearing to be dominant, might draw attention away from less discrete nodularity in the contralateral gland (Figs. 15-1, 15-2). This situation might require bilateral adrenal vein sampling for aldosterone levels (see Chapter 12). In the case of the pheochromocytoma, multicentric tumors in the retroperitoneum and the contralateral adrenal must be excluded. Any suspicious masses may require MIBG radionuclide study or T1- and T2-weighted MRI scanning (Fig. 15-3).
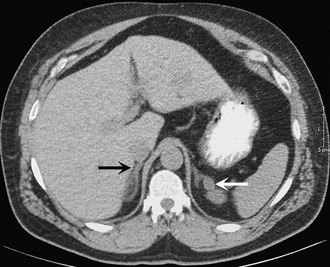
FIGURE 15-1 CT scan of 36-year-old female with hypertension and hypokalemia. Initial workup demonstrated a 1.2-cm left adrenal mass (white arrow). However, subsequent targeted imaging suggests a mass in the right adrenal (black arrow), raising the question of bilateral macromodular hyperplasia.
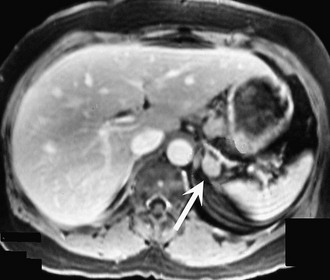
FIGURE 15-2 MRI scan shows an enlarged left adrenal mass in a patient with Conn’s syndrome (arrow). Right adrenal is seen behind the vena cava and is normal size.
Surgical Anatomy, Approaches, and Techniques
Surgical Approaches
Open Transabdominal (Anterior) Approach
Until 20 years ago, this was the classic surgical approach, particularly in the case of familial pheochromocytoma. It allows access to both adrenals and full exploration of intraabdominal and retroperitoneal extraadrenal deposits of tumor. The midline or bilateral subcostal incision is employed, allowing access to the peritoneal cavity with the patient positioned supine. The right adrenal gland is approached by mobilizing the hepatic flexure of the colon and right lobe of the liver. A generous Kocher maneuver is performed, mobilizing the duodenum medially (Fig. 15-4). In this manner, a full view is gained of the vena cava from the level of the right renal vein to the diaphragm. The adrenal gland is usually dissected from a lateral position, mobilizing it off of the kidney and from the retroperitoneal tissues; the gland is mobilized medially until the right adrenal vein is seen and securely ligated.
Open Lateral Approach
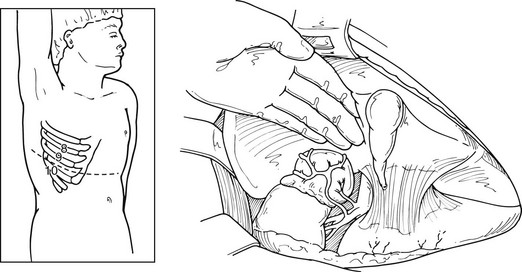
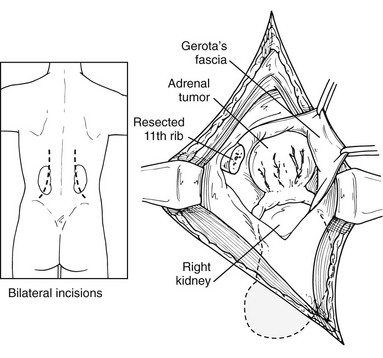
This approach has been used more commonly since the 1980s and before the advances in laparoscopic procedures. After inducing general anesthesia, the patient is placed in the lateral decubitus position, with the table flexed just above the anterior superior iliac spine; this accentuates the area between the costal margin and the iliac crest on the side of the lesion. The patient is rolled forward slightly, which has the added advantage of allowing the abdominal pannus to fall out of the surgical field, allowing excellent exposure of the costoiliac area. In patients with Cushing’s syndrome, this maneuver is particularly helpful. The tip of the 11th or 12th rib can be palpated fairly easily in this position even if the patient is somewhat obese. A curvilinear incision is employed on the 11th rib. The periosteum is stripped off of the rib, which is then resected at the base. Care must be used to preserve the intercostal neurovascular bundle. By incising through the medial aspect of this incision, the abdominal musculature can be divided, and the peritoneum can be pushed anteriorly and not entered. The pleura can be usually demonstrated and pushed superiorly. If the pleura is entered, it can be repaired under positive-pressure ventilation, which usually does not require a chest tube. Immediately inferior to the edge of the diaphragm is the area of perinephric fat at the superior aspect of the kidney; Gerota’s fascia is encountered here and incised. This area is entered, and by proceeding medially, the surgeon can identify either the adrenal tumor or normal-appearing cortex of the adrenal, which stands out in contrast to the surrounding fat (Fig. 15-5). These procedures can be performed through an 8- to 10-cm incision, employing self-retaining retractors to gain optimal exposure. On the left, the surgeon must be careful about vigorous retraction on the spleen; on the right, similar care must be used on the liver. The dissection of each adrenal gland proceeds as described for the anterior open approach.
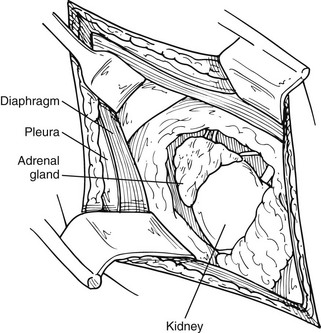
FIGURE 15-5 Lateral open approach to an adrenal mass on the right through an 11th-rib resection. Diaphragm and pleura are retracted superiorly, and Gerota’s fascia is incised, allowing exposure of the mass. (From Scott HW, Liddle GW, Mulherin JL et al: Surgical experience with Cushing’s disease, Ann Surg 185:524, 1977.)
Open Posterior Approach
The patient is placed in a prone position, with the operating table in a flex position. Hyperflexion of the lower back area is achieved by positioning the table and by the use of pillows under the abdomen. In this fashion, the area between the posterior iliac crest and the posterior costal margin is accentuated. The advantage of this position is that it allows access to both adrenals. The disadvantage is very limited exposure; therefore, the incision and consequent dissection must be carefully planned. A curvilinear incision starting parallel to and about 3 inches lateral to the spine and turning sharply out over the 12th rib is preferred. This allows exposure to the latissimus dorsi muscle and the sacrospinal fascia. The latissimus dorsi and the lateral aspect of the sacrospinal are divided, and the 12th rib is identified. The periosteum is stripped along the entire length of the rib, taking care to preserve the intercostal neurovascular bundle. The rib is removed, and the bed of the rib is incised. This allows access to the retroperitoneum, exposing the perinephric fat. Superior retraction identifies the parietal pleura and the lateral arcuate ligament of the diaphragm. The pleura should be reflected upward, allowing reasonable exposure to the perinephric fat. Dissection through the fat exposes the upper pole of the kidney, which is retracted inferiorly. It is at this time that distinct limitations in visibility can be appreciated if the appropriate dissection has not been done (Fig. 15-6). The gland on either the right or left side should be identified in the perinephric fat. Superior dissection usually allows enough mobilization to gain access to the adrenal vein, coursing into the vena cava (on the right side) or coursing inferiorly into the left adrenal vein (on the left side).
Lateral Laparoscopic Transperitoneal Approach
This is the most commonly employed approach to laparoscopic adrenalectomy.5 The patient is placed in the lateral decubitus position, as previously described. Again, the appropriate side should be hyperextended, maximizing the space between the costal margin and the iliac crest. The operating surgeon and camera driver should be on the patient’s abdominal side. The initial skin incision, approximately 1 cm in length, is made 2 cm below and parallel to the costal margin, medial to the anterior axillary line. The port is placed, and the abdomen is insufflated with CO2, either by a Veress needle or by placing a 10-mm Hassan trocar under direct vision. Through this initial incision, a 30-degree, 10-mm laparoscope is placed into the peritoneal cavity, and the peritoneal cavity is then visualized. On the left side, two additional 5- or 10-mm trocars are inserted under direct laparoscopic visualization (Fig. 15-7A). The first is placed inferiorly and slightly medial to the tip of the 11th rib; the other is inserted slightly anterior and medial to the initial trocar. The two most lateral ports are used for the dissecting instruments, and the visualizing laparoscope is placed at the most medial of these. On the left side, this allows good mobilization of the splenic flexure, and dissection then proceeds as described for the left adrenalectomy. After the spleen is fully mobilized it will fall medially, and the retroperitoneum can be almost completely visualized (see Fig. 15-7B, C, and D). Occasionally, a fourth trocar can be inserted 3 to 5 cm posterior to the previous lateral port. This trocar could be used to retract the spleen and kidney or surrounding fat for better exposure. Laparoscopic ultrasound can be used if there is difficulty in finding the gland or the lesion within the gland. Careful dissection is then needed in the space between the aorta and the superior pole of the adrenal. Our preferred method is to isolate the left adrenal vein at its junction into the renal vein and divide it securely between clips (see Fig. 15-7E). Dissection can then be continued superiorly, mobilizing the adrenal from the surrounding perinephric fat. After ligation of the adrenal vein, almost all the other left adrenal vasculature can be coagulated using cautery or the harmonic scalpel.
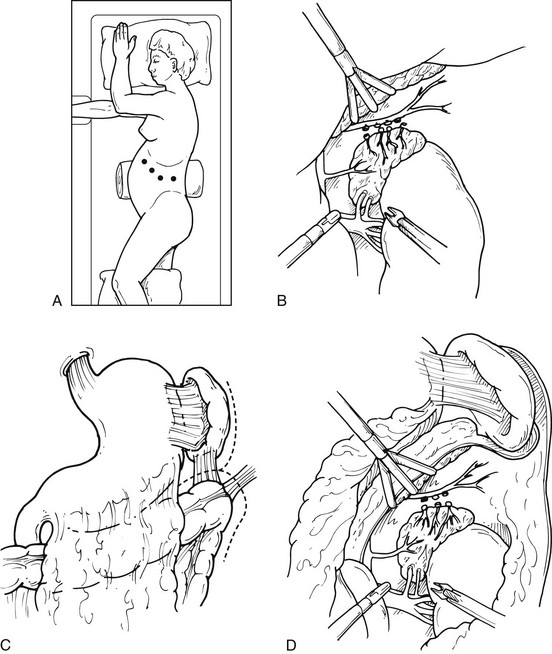
FIGURE 15-7 Lateral laparoscopic transperitoneal approach to the left adrenal gland. A, Trocar site placement. B, C, D, Mobilization of spleen, splenic flexure, and tail of pancreas allows excellent exposure. E, Intraoperative view during laparoscopic transperitoneal adrenalectomy of a left pheochromocytoma, demonstrating the adrenal vein (white arrowhead), the renal vein (white arrow), the phrenic vein (black arrow), and the adrenal lesion (being swept up by grasper). (From Fernandez-Cruz L, Sachz A, Benarroch G et al: Laparoscopic unilateral and bilateral adrenalectomy for Cushing’s syndrome: transperitoneal and retroperitoneal approaches, Ann Surg 224:727–736, 1996.)
For a laparoscopic transperitoneal right adrenalectomy, the patient is placed in the right lateral decubitus position, with the surgeon and his assistant on the patient’s abdominal side. The peritoneum is insufflated in the same fashion as previously described; the laparoscope is placed, and the abdomen is inspected. Three additional 5- or 10-mm trocars are placed under the ribs in the same position as previously described (Fig. 15-8A). The dissecting instruments are used through the most lateral trocar sites. Under laparoscopic visualization, the hepatic flexure of the colon is taken down, and the right lobe of the liver is retracted medially. A fan-type liver retractor can be inserted through a medial port to reflect the right hepatic lobe medially. This necessitates taking down the right lateral hepatic attachments and the triangular ligament, using laparoscopic scissors or the harmonic scalpel. This dissection is usually carried up to the level of the diaphragm, allowing full visualization of the area behind the liver and in the retroperitoneum (see Fig. 15-8B, C, and D). The perinephric fat appears in the area between the diaphragm and the superior pole of the kidney. The inferior portion of the adrenal can be immobilized using electrocautery and the grasper dissector. If this dissection is carried medially and superiorly, the lateral edge of the vena cava is identified. Ultrasound can be used to identify adrenal tissue or masses. The right adrenal vein is almost always in the midportion of the body of the adrenal, coursing transversely. As one carefully dissects in this area, the right adrenal vein will almost always be encountered, and it can be clipped at this time. At least two clips should remain on the vena cava side. If the vein is not found in this position, careful search for a superior route into the hepatic vein must be considered. Once the adrenal vein is divided, the adrenal gland can be mobilized more easily, and dissection should be carried superiorly. Smaller branches from the inferior phrenic vessels will be encountered, and these should be divided. Occasionally, particularly in larger adrenal masses, there will be increased vascularity and attachments encountered on the posterior surface of the liver. These must be carefully dissected, particularly in the case of hypercortisolism, in an effort to remove all cortical tissue. After complete mobilization, the adrenal gland can be placed within a sterile nylon bag and removed through one of the 10-mm laparoscopic ports. Closure of the individual trocar sites is performed as previously described.
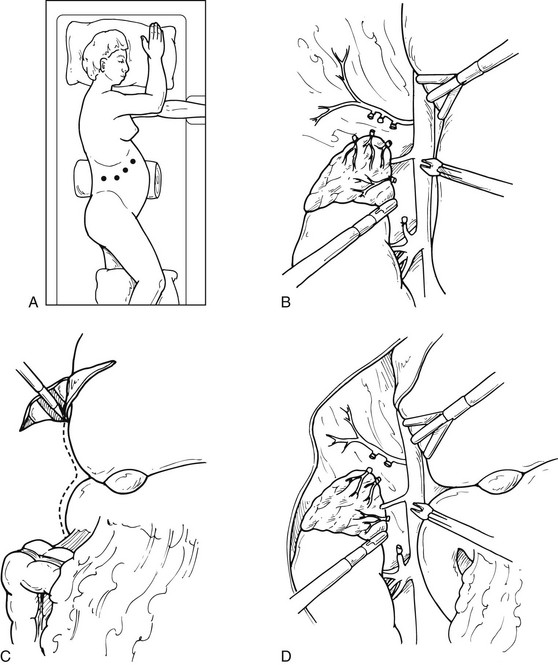
FIGURE 15-8 Lateral laparoscopic transperitoneal approach to the right adrenal gland. A, Trocar site placement. B-D, Exposure of the right adrenal by mobilization of the right lobe of liver and visualization of the vena cava. (From Fernandez-Cruz L, Sachz A, Benarroch G et al: Laparoscopic unilateral and bilateral adrenalectomy for Cushing’s syndrome: transperitoneal and retroperitoneal approaches, Ann Surg 224:727–736, 1996.)
Endoscopic Retroperitoneal Adrenalectomy
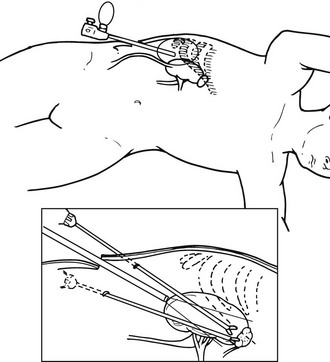
The patient is placed under general anesthesia, and then is placed in lateral decubitus position, with the affected side exposed. As described previously, the OR table is then flexed until the space between the costal margin and iliac crest is maximized (Fig. 15-9). A 2-cm, muscle-splitting incision is made in the midaxillary line just beneath the tip of the 11th rib. The surgeon’s finger is placed through this incision and into the retroperitoneal space, palpating the lower pole of the kidney. A transparent, disposable dissecting balloon is introduced and insufflated under visual control to create a retroperitoneal working space. The balloon must be directly visualized and placed posteriorly to prevent penetration of the peritoneal sac. After this insufflation technique is completed, the balloon is deflated and replaced by a Hasson trocar. The CO2 is then insufflated to a pressure of 12 to 15 mm Hg, and a 10-mm laparoscope is introduced. A second 5-mm trocar is inserted immediately adjacent to the 12th rib, through which a dissecting instrument can be inserted. Continued dissection of the retroperitoneal space allows the introduction of third and fourth trocar sites to the medial side of the first trocar site. Again, every effort must be made not to perforate the peritoneal sac. At this time, the lateral conal fascia appears as a thin, avascular, white-gray membrane that connects the peritoneum to the quadratus lumborum muscle; this is opened. This allows the identification of the upper renal pole. On the left, the inferior rim of the adrenal gland, as well as the ventral and lateral aspects of the adrenal gland, are exposed through this view. As previously described, dissection must now proceed carefully, without damaging the surface of the gland, and gaining access to the left adrenal vein before complete removal can be accomplished. After this dissection is complete, the adrenal gland can be removed in a Silastic bag. All trocar sites are closed in a method described previously.
Robotic Adrenalectomy
Right adrenalectomy is performed with the patient in the left lateral decubitus position. The first port, a 12-mm camera port, is placed midway between the umbilicus and the right costal margin. Two robotic instrument ports, both 8 mm, are then placed along a line two fingerbreadths from the costal margin. A 10-mm liver retraction port is placed in the midline in the epigastrium. A 10-mm accessory port (fifth trocar) is placed in the right abdomen, through which the assistant can use a clip applier. Left adrenalectomy is performed with the patient in the right lateral decubitus position. Positioning is the mirror image of that for the right adrenalectomy described earlier. The role of the assistant at the operating table is to change the robotic instruments as necessary, to use the clip applier for division of the adrenal vein, and to manipulate the suction. Otherwise, the sequential steps for dissection and excision of the gland are the same as those for a lateral transperitoneal adrenalectomy by traditional laparoscopic techniques described previously.29
Partial Adrenalectomy
Classically, the finding of an adrenal mass on one side has resulted in the complete removal of that adrenal gland. More recently, there has been some interest in preserving adrenal function in the case of benign tumors. For patients requiring bilateral adrenalectomy, adrenal-sparing procedures may avoid the need for lifelong steroid replacement and avoid the significant risk of Addisonian crisis. Even in patients undergoing unilateral adrenalectomy, there may be inadequate hormonal reserve to respond to stress.31 Therefore, it may be in the patient’s best interest to preserve adrenal tissue when possible. This technique is generally applied to those patients with Conn’s syndrome, a cortisol-producing adenoma, and hereditary pheochromocytoma. Partial adrenalectomy is not appropriate when there is significant concern for malignancy.
Partial adrenalectomy may be performed through any of the surgical approaches described. A large series of posterior endoscopic adrenalectomies reported by Walz et al. in 2006 included 149 partial adrenalectomies in 139 patients.32 This group has reported 100% biochemical cure which persisted through follow-up. Other groups have also reported excellent cure rates, with very low rates of recurrence and negative surgical margins.
Familial pheochromocytoma has usually been associated with bilateral adrenalectomy and exogenous steroid dependency. Recent experience has documented the use of partial adrenalectomy, preserving a portion of normal adrenal tissue and its blood supply. This can be done through either an open or a laparoscopic approach. However, long-term follow-up is mandatory because recurrence is a distinct possibility. Lee described partial adrenalectomy, sparing cortical tissue, in 14 patients with MEN2 and von Hippel-Lindau syndrome.33 Thirteen of these patients (93%) had normal cortisol levels after surgery and were free of exogenous steroids. Nine patients showed no recurrent tumor at 90 months after surgery. In 1999, Neumann et al.34 and Walther et al.35 reported series of 39 and 13 patients, respectively, with excellent results and little increased morbidity. More recently, Diner et al. reported the National Cancer Institute’s experience with 33 patients with hereditary pheochromocytoma treated with partial adrenalectomy.36 Although 15% of these patients required steroid replacement in the early postoperative period, by 3 months only 3% continued to be dependent on exogenous steroid, and all patients experienced normalization of catecholamine levels. One issue particular to partial adrenalectomy for pheochromocytoma is that there is a greater degree of gland manipulation than during total adrenalectomy, which may increase the risk of hypertensive crisis, although this has not been observed in reported series.
Recurrence is an obvious potential risk in partial adrenalectomy. In the National Cancer Institute experience with hereditary pheochromocytoma, 6% of patients had recurrence, requiring completion adrenalectomy.36 Reviews of other series have shown that recurrent tumors developed in 15% to 20% of patients with von Hippel-Lindau at a median of 18 to 40 months of follow-up, and in 33% of patients with MEN2 at 58 to 84 months follow-up.37 In patients with aldosterone-producing adenomas, aldosterone may be produced by other, nondominant, nodules within the gland. Although excellent results generally are reported, there is a definite incidence of persistent hyperaldosteronism, in one series occurring in 7% of partial adrenalectomies.38
Although the body of literature employing this approach has grown, partial adrenalectomy remains controversial. Issues remaining to be resolved include patient selection, the amount of tissue to excise, which technical method to use to divide the gland, and whether or not to divide the adrenal vein.37 Some groups performing partial adrenalectomy have reported that it is not necessary to preserve the adrenal vein so long as at least a third of the gland volume is preserved,32 but others have found that adrenal vein preservation is essential.39
Special Circumstances
Intraoperative Management of Hypertension
Pheochromocytoma deserves special comment in this regard. In the usual circumstance, the patient has received pharmacologic blockade prior to surgery. It is important that adequate peripheral and central venous access be available and also that a radial artery catheter be placed preoperatively for continuous hemodynamic monitoring. Additionally, a Swan-Ganz catheter may be placed, as required, because of cardiac disease or other cardiopulmonary indications. Intraoperatively, manipulation of a pheochromocytoma can result in acute hypertension. To control hypertension, sodium nitroprusside is an excellent choice by the anesthesiologist (Fig. 15-10): it has a direct-acting vasodilator effect and a rapid half-life. Therefore, within seconds of discontinuing the infusion, its effect ends, allowing for precision in dealing with possible rapid fluctuations of hypertension and hypotension. Other potent vasodilators, such as nitroglycerin, can be used with or instead of nitroprusside. Dysrhythmias may occur before or after tumor removal and can be treated with propranolol, esmolol, or lidocaine. Esmolol has the shortest half-life, and is gaining popularity as the preferred β-adrenoceptor blocker intraoperatively.40
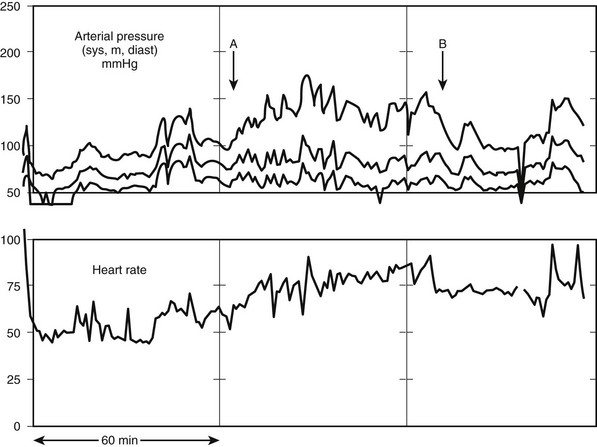
FIGURE 15-10 On-line arterial pressure measurements during pheochromocytoma resection. A, The intraperitoneal dissection and tumor manipulation begins, with systolic and diastolic blood pressure peaks. These are rapidly and effectively treated with esmolol and nitroprusside infusions. B, The adrenal vein is ligated and the tumor removed. No further infusions are necessary or administered.
Postoperative Care
Conn’s Syndrome
Patients with Conn’s syndrome (Fig. 15-11) most likely have been on significant antihypertensive medication as well as potassium supplementation. After successful adrenal surgery, the patient will be able to decrease this potassium medication relatively rapidly; however, blood pressure evaluation needs to continue. Lo’s group reported on 46 consecutive patients undergoing removal of an aldosterone-secreting tumor between 1983 and 199441; there was no operative mortality. In patients undergoing long-term follow-up, 10 of 44 patients (23%) remained on medical therapy for hypertension. Interestingly, younger patients (aged 44 versus 52 years) and patients who responded to preoperative spironolactone antihypertensive therapy were statistically likely to have a better blood pressure response to surgery. Hypokalemia was corrected in all patients.
Pheochromocytoma
Patients with pheochromocytoma (Fig. 15-12) also have been on significant antihypertensive medication as well as preoperative blockade. The preoperative α- and β-adrenoceptor blockade can be immediately discontinued; in fact, it may be dangerous if taken after surgery. The hypertension of a pheochromocytoma quickly resolves after resection. The patient needs to be educated in terms of blood pressure monitoring during convalescence. These patients may have significant fluid shifts postoperatively, and they require careful monitoring, especially those with a preoperative cardiac history. Brunt’s group has reported results using the laparoscopic approach for familial pheochromocytoma42; from 1993 to 2001, 21 such patients had surgery. Operative times averaged 216 ± 57 minutes, and average blood loss was 168 mL. No blood transfusion was necessary in any patient. Fifteen patients (71%) had intraoperative hypertension and/or tachycardia requiring medication; all responded to nitroprusside and/or nitroglycerin and labetalol. Only two (10%) had intraoperative systolic hypertension exceeding 200 mm Hg.
Cushing’s Syndrome
Cushing’s disease patients who have failed pituitary surgery and for whom bilateral adrenalectomy is being considered (Fig. 15-13) also have special postoperative circumstances. Some of these patients may have panhypopituitary syndromes. Their medications may include pituitary replacement medications such as desmopressin. These patients must be carefully evaluated preoperatively, and the extent of any pituitary deficiency as a result of previous surgery should be completely assessed. Postoperatively, these patients can have significant fluid and electrolyte abnormalities and may require replacement with desmopressin. After successful bilateral adrenalectomy, close monitoring of glucocorticoid and mineralocorticoid replacement must be continued for life. Patients with Cushing’s disease are also at increased risk of complications due to obesity, impaired healing, increased susceptibility to infection, and increased risk of thromboembolism, compared to those undergoing adrenalectomy for other indications.43 In some centers, preoperative treatment with adrenolytic therapy may be instituted for 1 to 3 months before surgery.
Future Directions
Virtually every part of the medical field has been affected by technologic changes, and surgery has been affected as much as any other. Continued advancements in imaging, miniaturization, and computer enhancement techniques will drive the continued application of minimally invasive surgery. Yet the thought processes, anatomy, and indications for surgical approach will remain the same. For example, with wider application of high-resolution axial imaging, adrenal incidentalomas may be found in as many as 4.4% of all abdominal CT scans, vastly increasing the number of patients being brought to the attention of physicians.7 However, the workup and the indications for removal remain the same despite the temptation to apply novel surgical techniques. Dr. Sam Wells has warned “… the availability of a technique for easily removing these lesions is not necessarily an indication for doing so.”44
The future may not be so bright for adrenal malignancy. Little progress has been made over the last 20 years in treatment (see Chapter 11). Continued efforts will be needed if any breakthrough in altering the grim prognosis of this disease is to be expected. Perhaps genetic mapping will allow the discovery of a proto-oncogene, as has been discovered for medullary carcinoma of the thyroid, allowing earlier surgical intervention. In any event, advances in technology, as well as increased understanding of normal and altered adrenal physiology, are sure to continue to have an impact on surgical approaches, as has been seen over the last 20 years.
References
1. Gagner, M, Lacroix, A, Bolte, E. Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. N Engl J Med. 1992;327(14):1033.
2. Higashihara, E, Tanaka, Y, Horie, S, et al. A case report of laparoscopic adrenalectomy. Nippon Hinyokika Gakkai Zasshi. 1992;83(7):1130–1133.
3. Assalia, A, Gagner, M. Laparoscopic adrenalectomy. Br J Surg. 2004;91(10):1259–1274.
4. Smith, CD, Weber, CJ, Amerson, JR. Laparoscopic adrenalectomy: new gold standard. World J Surg. 1999;23(4):389–396.
5. Gumbs, AA, Gagner, M. Laparoscopic adrenalectomy. Best Pract Res Clin Endocrinol Metab. 2006;20(3):483–499.
6. Lairmore, TC, Ball, DW, Baylin, SB, et al. Management of pheochromocytomas in patients with multiple endocrine neoplasia type 2 syndromes. Ann Surg. 1993;217(6):595–601.
7. Bovio, S, Cataldi, A, Reimondo, G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298–302.
8. Mantero, F, Terzolo, M, Arnaldi, G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85(2):637–644.
9. Siren, J, Tervahartiala, P, Sivula, A, et al. Natural course of adrenal incidentalomas: seven-year follow-up study. World J Surg. 2000;24(5):579–582.
10. Gagner, M, Breton, G, Pharand, D, et al. Is laparoscopic adrenalectomy indicated for pheochromocytomas? Surgery. 1996;120(6):1076–1079.
11. Fernandez-Cruz, L, Saenz, A, Taura, P, et al. Helium and carbon dioxide pneumoperitoneum in patients with pheochromocytoma undergoing laparoscopic adrenalectomy. World J Surg. 1998;22(12):1250–1255.
12. Gagner, M, Pomp, A, Heniford, BT, et al. Laparoscopic adrenalectomy: lessons learned from 100 consecutive procedures. Ann Surg. 1997;226(3):238–246.
13. NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”). NIH Consens State Sci Statements. 2002;19(2):1–25.
14. Paton, BL, Novitsky, YW, Zerey, M, et al. Outcomes of adrenal cortical carcinoma in the United States. Surgery. 2006;140(6):914–920.
15. McCauley, LR, Nguyen, MM. Laparoscopic radical adrenalectomy for cancer: long-term outcomes. Curr Opin Urol. 2008;18(2):134–138.
16. Gonzalez, RJ, Shapiro, S, Sarlis, N, et al. Laparoscopic resection of adrenal cortical carcinoma: a cautionary note. Surgery. 2005;138(6):1078–1085.
17. Kebebew, E, Siperstein, AE, Duh, QY. Laparoscopic adrenalectomy: the optimal surgical approach. J Laparoendosc Adv Surg Tech A. 2001;11(6):409–413.
18. Liao, CH, Chueh, SC, Lai, MK, et al. Laparoscopic adrenalectomy for potentially malignant adrenal tumors greater than 5 centimeters. J Clin Endocrinol Metab. 2006;91(8):3080–3083.
19. Ramacciato, G, Mercantini, P, La Torre, M, et al. Is laparoscopic adrenalectomy safe and effective for adrenal masses larger than 7 cm? Surg Endosc. 2008;22(2):516–521.
20. Soon, PS, Yeh, MW, Delbridge, LW, et al. Laparoscopic surgery is safe for large adrenal lesions. Eur J Surg Oncol. 2008;34(1):67–70.
21. Parnaby, CN, Chong, PS, Chisholm, L, et al. The role of laparoscopic adrenalectomy for adrenal tumours of 6 cm or greater. Surg Endosc. 2008;22(3):617–621.
22. Rosoff, JS, Raman, JD, Del Pizzo, JJ. Laparoscopic adrenalectomy for large adrenal masses. Curr Urol Rep. 2008;9(1):73–79.
23. Bentas, W, Wolfram, M, Brautigam, R, et al. Laparoscopic transperitoneal adrenalectomy using a remote-controlled robotic surgical system. J Endourol. 2002;16(6):373–376.
24. Piazza, L, Caragliano, P, Scardilli, M, et al. Laparoscopic robot-assisted right adrenalectomy and left ovariectomy [case reports]. Chir Ital. 1999;51(6):465–466.
25. Hanly, EJ, Talamini, MA. Robotic abdominal surgery. Am J Surg. 2004;188(4A Suppl):19S–26S.
26. Zafar, SS, Abaza, R. Robot-assisted laparoscopic adrenalectomy for adrenocortical carcinoma: initial report and review of the literature. J Endourol. 2008;22:985–989.
27. Brunaud, L, Bresler, L, Zarnegar, R, et al. Does robotic adrenalectomy improve patient quality of life when compared to laparoscopic adrenalectomy? World J Surg. 2004;28(11):1180–1185.
28. Morino, M, Beninca, G, Giraudo, G, et al. Robot-assisted vs laparoscopic adrenalectomy: a prospective randomized controlled trial. Surg Endosc. 2004;18(12):1742–1746.
29. Brunaud, L, Bresler, L, Ayav, A, et al. Robotic-assisted adrenalectomy: what advantages compared to lateral transperitoneal laparoscopic adrenalectomy? Am J Surg. 2008;195(4):433–438.
30. Grant, CS. Pheochromocytomas. In: Clark OH, Duh QY, eds. Textbook of Endocrine Surgery. ed 1. Philadelphia: Saunders; 1997:579–582.
31. Nakada, T, Kubota, Y, Sasagawa, I, et al. Therapeutic outcome of primary aldosteronism: adrenalectomy versus enucleation of aldosterone-producing adenoma. J Urol. 1995;153(6):1775–1780.
32. Walz, MK, Alesina, PF, Wenger, FA, et al. Posterior retroperitoneoscopic adrenalectomy—results of 560 procedures in 520 patients. Surgery. 2006;140(6):943–948.
33. Lee, JE, Curley, SA, Gagel, RF, et al. Cortical-sparing adrenalectomy for patients with bilateral pheochromocytoma. Surgery. 1996;120(6):1064–1070.
34. Neumann, HP, Bender, BU, Reincke, M, et al. Adrenal-sparing surgery for phaeochromocytoma. Br J Surg. 1999;86(1):94–97.
35. Walther, MM, Keiser, HR, Choyke, PL, et al. Management of hereditary pheochromocytoma in von Hippel-Lindau kindreds with partial adrenalectomy. J Urol. 1999;161(2):395–398.
36. Diner, EK, Franks, ME, Behari, A, et al. Partial adrenalectomy: the National Cancer Institute experience. Urology. 2005;66(1):19–23.
37. Disick, GI, Munver, R. Adrenal-preserving minimally invasive surgery: update on the current status of laparoscopic partial adrenalectomy. Curr Urol Rep. 2008;9(1):67–72.
38. Ishidoya, S, Ito, A, Sakai, K, et al. Laparoscopic partial versus total adrenalectomy for aldosterone producing adenoma. J Urol. 2005;174(1):40–43.
39. Jeschke, K, Janetschek, G, Peschel, R, et al. Laparoscopic partial adrenalectomy in patients with aldosterone-producing adenomas: indications, technique, and results. Urology. 2003;61(1):69–72.
40. Ulchaker, JC, Goldfarb, DA, Bravo, EL, et al. Successful outcomes in pheochromocytoma surgery in the modern era. J Urol. 1999;161(3):764–767.
41. Lo, CY, Tam, PC, Kung, AW, et al. Primary aldosteronism. Results of surgical treatment. Ann Surg. 1996;224(2):125–130.
42. Brunt, LM, Lairmore, TC, Doherty, GM, et al. Adrenalectomy for familial pheochromocytoma in the laparoscopic era. Ann Surg. 2002;235(5):713–720.
43. Poulin, EC, Schlachta, CM, Burpee, SE, et al. Laparoscopic adrenalectomy: pathologic features determine outcome. Can J Surg. 2003;46(5):340–344.
44. Wells, SA, Merke, DP, Cutler, GB, Jr., et al. Therapeutic controversy: the role of laparoscopic surgery in adrenal disease. J Clin Endocrinol Metab. 1998;83(9):3041–3049.
45. Corcione, F, Miranda, L, Marzano, E, et al. Laparoscopic adrenalectomy for malignant neoplasm: our experience in 15 cases. Surg Endosc. 2005;19(6):841–844.
46. Eto, M, Hamaguchi, M, Harano, M, et al. Laparoscopic adrenalectomy for malignant tumors. Int J Urol. 2008;15(4):295–298.
47. Gill, IS. The case for laparoscopic adrenalectomy. J Urol. 2001;166(2):429–436.
48. Henry, JF, Sebag, F, Iacobone, M, et al. Results of laparoscopic adrenalectomy for large and potentially malignant tumors. World J Surg. 2002;26(8):1043–1047.
49. Kirshtein, B, Yelle, JD, Moloo, H, et al. Laparoscopic adrenalectomy for adrenal malignancy: a preliminary report comparing the short-term outcomes with open adrenalectomy. J Laparoendosc Adv Surg Tech A. 2008;18(1):42–46.
50. Lombardi, CP, Raffaelli, M, De Crea, C, et al. Role of laparoscopy in the management of adrenal malignancies. J Surg Oncol. 2006;94(2):128–131.
51. Nocca, D, Aggarwal, R, Mathieu, A, et al. Laparoscopic surgery and corticoadrenalomas. Surg Endosc. 2007;21(8):1373–1376.
52. Palazzo, FF, Sebag, F, Sierra, M, et al. Long-term outcome following laparoscopic adrenalectomy for large solid adrenal cortex tumors. World J Surg. 2006;30(5):893–898.
53. Porpiglia, F, Fiori, C, Tarabuzzi, R, et al. Is laparoscopic adrenalectomy feasible for adrenocortical carcinoma or metastasis? BJU Int. 2004;94(7):1026–1029.
54. Schlamp, A, Hallfeldt, K, Mueller-Lisse, U, et al. Recurrent adrenocortical carcinoma after laparoscopic resection. Nat Clin Pract Endocrinol Metab. 2007;3(2):191–195.