Acute Abdomen, Bowel/Biliary Obstruction, and Fistula
Fumito Ito and Alfred E. Chang
• Perforation affects approximately 20% of patients with acute abdominal emergencies.
• Bowel perforation can be due to spontaneous tumor rupture, tumor necrosis secondary to chemotherapy, radiation therapy, drugs (e.g., steroids), or inflammatory conditions.
• Operative intervention is mandated unless the patient’s overall prognosis is poor.
• Bleeding affects approximately 15% of patients with acute abdominal emergencies.
• Bleeding is more commonly seen in patients with leukemia or lymphoma who are undergoing chemotherapy.
• Endoscopy is critical to identify the source of bleeding and can even be therapeutic.
• Nonoperative therapy is successful for many patients.
• Also termed necrotizing enterocolitis, neutropenic enterocolitis typically affects the terminal ileum, cecum, and ascending colon in patients with chemotherapy-induced neutropenia.
• Most patients respond to conservative management with broad-spectrum antibiotics and bowel rest.
• Surgical intervention should be considered for perforation, uncontrolled sepsis, or persistence of symptoms despite correction of neutropenia.
• Obstruction affects approximately 40% of patients with cancer who experience acute abdominal emergencies.
• One fourth to one third of patients who require surgical intervention have a benign cause of their obstruction.
• Partial bowel obstruction can initially be treated nonoperatively, which is successful 25% of the time.
• Cross-sectional imaging gives valuable information about the location and etiology of malignant biliary obstruction and resectability of tumor.
• Manifesting symptoms rarely match those of intraabdominal malignancy and more commonly represent complications after surgery or radiation therapy or both.
• Medical management consisting of nutritional support and bowel rest allows spontaneous closure of most enterocutaneous fistulas.
• Causes for persistence of a fistula include undrained infection, luminal obstruction distal to the fistula, prior radiation, epithelialization of the fistulous tract, cancer within the tract, presence of a foreign body, and malnutrition.
Acute Abdomen: General Considerations
The classic general surgery approach to a patient with acute abdominal pain entails identification of a potentially life-threatening problem and evaluation for emergency exploratory laparotomy. In patients with cancer, the etiology of acute abdominal pain may be directly related to a malignant process, but seemingly unrelated processes must be considered as well. Some GI cancers can manifest as abdominal emergencies that require surgical intervention. Problems that require surgical consultation include obstruction, perforation, hemorrhage, inflammatory processes, and other miscellaneous problems such as fistulae (Box 47-1).1,2 In a prospective analysis of more than 1000 consecutive palliative procedures in patients with cancer, more than 50% were performed on the GI system, with obstruction and bleeding making up more than 75% of the manifesting symptoms. The procedures were either operative (70%) or endoscopic (30%) intervention, resulting in initial symptom resolution in 80% of patients, although they were associated with significant morbidity (40%), mortality (10%), and limited anticipated survival (approximately 6 months).3 The decision to intervene with an invasive procedure is often difficult because of abnormal physiological responses to injury and inflammation, complications associated with previous cancer therapies, and competing risks due to extent or stage of the underlying cancer. It requires a multidisciplinary approach, and in some instances, medical management or use of palliative measures makes up the mainstay of therapy. Interactions among the patient, the family, and the surgeon (palliative triangle) are important to guide individual decisions regarding care. Abdominal pain is the most common symptom in the patient with an acute abdomen. In patients with a diagnosed intraabdominal malignancy who are undergoing chemotherapy and/or radiation therapy, abdominal pain must be investigated carefully. Care must be taken not to automatically attribute these complaints to cancer progression. Important considerations in the evaluation of the patient with an acute abdominal process include hemodynamic stability and deterioration of symptoms during the course of examination and workup. Patients taking corticosteroids or who have an altered sensorium warrant special attention because of a potentially unreliable physical examination. Other abdominal complaints such as vomiting, distention, lack of flatus, and fever should raise the examiner’s index of suspicion that an emergent problem is present.
Ultimately, the surgeon must make the determination as to whether the patient has an acute abdominal process that requires surgical intervention. Decisions to operate on acutely ill patients with cancer are difficult and often require a high level of surgical decision making. Due consideration must be given to the stage and prognosis of the cancer itself, because the surgeon’s ability to offer a procedure with curative intent versus palliation only may alter the decision-making process. The role of palliative procedures has increased tremendously, with as many as 6% to 21% of cancer operations being classified as palliative in nature.3,4 Even without curative intent, surgeons have a great deal to offer in terms of symptom relief and overall improvement in quality of life.3 However, attempts at successful palliation must be balanced with inappropriately aggressive attempts, which may carry unacceptable rates of morbidity and mortality.
Gastrointestinal Perforation
Perforation of the GI tract mandates operative intervention unless the patient’s condition is judged to be extremely poor. Excluding iatrogenic causes of bowel perforation, the causes of perforation can be categorized into the following subgroups: spontaneous tumor rupture or erosion into bowel, drug-induced or associated perforations, and inflammatory conditions. GI tract cancers may present with perforation as the precipitating event, but this presentation is rare and is associated with high mortality rates. Primary colon cancers can present as localized perforations or can cause obstruction with subsequent perforation. Whether perforation is associated with poorer long-term overall survival is unclear; the perioperative mortality rate after presentation with colon cancer perforation, either at or proximal to the tumor site, remains high, ranging from 17% to 48%.5,6
Spontaneous perforation of GI tumors is more common in patients with lymphomas involving the GI tract, although this diagnosis is rare. Large malignant retroperitoneal lymphomas, renal cell carcinomas, and testicular cancers metastatic to aortocaval nodes can invade the adjacent duodenum and cause perforation. Patients with GI lymphomas can have symptoms at presentation or can experience symptoms related to regression of their primary tumor during treatment with chemotherapy and/or radiation therapy. Perforation or bleeding from treatment-induced tumor lysis occurs in approximately 3% and 5% of patients, respectively. Although resection of larger, higher grade GI lymphomas can be associated with increased morbidity, the mortality rate is greater than 50% when surgical intervention is urgently required for perforation or bleeding in the setting of neutropenia and thrombocytopenia.7,8
Gastrointestinal Bleeding
The true incidence of significant GI bleeding in patients with cancer is not well defined. An estimated 10% to 15% of patients with abdominal emergencies requiring operative intervention had abdominal hemorrhage, most of them resulting from intraluminal bleeding.1 Patients with lymphoma or leukemia who are treated with combination chemotherapy regimens can experience GI tract bleeding. Although a GI tumor itself could be the source of bleeding, the differential diagnosis should include non–tumor-related sources as well. The most common causes of upper GI tract bleeding in patients with cancer are peptic ulcer disease or stress ulceration and complications of anticoagulation or thrombocytopenia.2 Rates are likely to be lower in clinical practice, given the availability and increasingly widespread use of H2-receptor blockers and proton pump inhibitors. Other less common causes of bleeding include Candida esophagitis, Mallory-Weiss mucosal tears, hemorrhage from inflammatory conditions (e.g., neutropenic enterocolitis), or radiation-associated complications (e.g., arterial-enteric fistulae and radiation enteropathy).
The pathogenesis of upper GI bleeding can be multifactorial. Gastritis or peptic ulcers can be associated with a variety of agents, such as aspirin, alcohol, steroids, indomethacin, or phenylbutazone. Chemotherapeutic agents can depress platelet production, as well as damaging or irritating the mucosal surfaces. Although the correlation between thrombocytopenia and bleeding is well described, it is difficult to predict which patients might experience bleeding that requires transfusion or life-threatening hemorrhage. Overall, the incidence of bleeding is low, with one group reporting bleeding episodes with 9% of chemotherapy cycles.9 The vast majority of episodes were mild (e.g., epistaxis), but major hemorrhage (including GI bleeding) was seen in approximately 3% of cycles. In that study, administration of certain chemotherapy agents (e.g., cisplatin, carboplatin, carmustine, and lomustine) was noted to be significantly related to bleeding episodes. Other investigators have more recently reported thrombocytopenia, hemorrhage, and hemolysis with oxaliplatin.10 Prophylactic platelet transfusions should be considered on a case-by-case basis, especially if bleeding has occurred previously.
Hepatic arterial infusion of fluorodeoxyuridine using an implanted pump for the treatment of liver tumors has been reported to result in a significant incidence of upper GI toxicity with biliary sclerosis, gastritis, peptic ulcers, and intraabdominal bleeding due to catheter displacement.11 Other unusual causes of bleeding include hemobilia secondary to the presence and/or treatment of hepatobiliary tumors. Arterial-enteric fistulization may result in severe intraluminal hemorrhage, and operative management should be approached with joint vascular surgery consultation. Communication with vascular structures should always be considered in previous surgical sites and areas of prior radiation treatment.
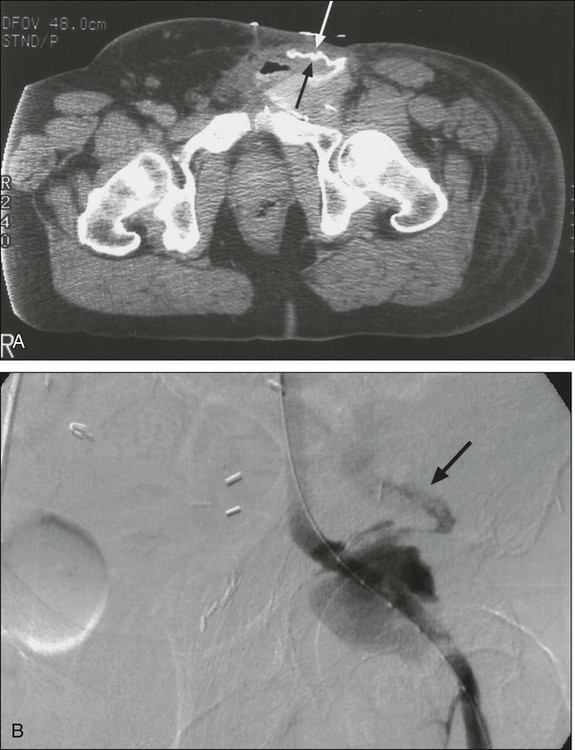
Intraabdominal hemorrhage is usually seen as a result of tumor rupture (Fig. 47-1), but spontaneous splenic rupture in patients with hematologic malignancies can be seen as well. Rapid tumor necrosis with subsequent hemorrhage has been reported during chemotherapy and is most commonly seen in patients with GI lymphomas. The incidence of significant GI bleeding in this patient population as a result of tumor lysis from therapy is approximately 5%.12 Surgical resection of GI lymphoma before systemic or radiation therapy could be beneficial for lesions that are amenable to resection with minimal morbidity.
Adverse Events with Cytotoxic Agents Leading to Bleeding or Perforation
Special consideration should be given to patients receiving targeted molecular therapy agents. Bevacizumab (Avastin), a monoclonal antibody targeting the vascular endothelial growth factor receptor, has been shown to significantly improve overall and progression-free survival rates in patients with metastatic colorectal cancer when used with combination chemotherapy compared with chemotherapy alone.13,14 Results of these randomized trials have led to the widespread use of bevacizumab in patients with metastatic colorectal cancers and in selected patients with other solid-organ cancers. Because this drug targets tumor angiogenesis, rare but serious complications, such as bowel perforation, can occur in about 1.5% to 1.7% of patients during treatment not only for colorectal cancer but also for other malignancies that lack disease within the peritoneal cavity.13,15 Risk factors for perforation are difficult to define but include the presence of an intact primary, abdominal irradiation, nonsteroidal antiinflammatory drug use, diverticulosis, and recent endoscopy.15
Another targeted molecular agent associated with bowel perforation and GI bleeding is small-molecule tyrosine kinase inhibitors including imatinib (Gleevec) and sunitinib (Sutent). Imatinib is commonly used in the treatment of chronic myelogenous leukemia and GI stromal tumors (GISTs). Sunitinib is used for GISTs that are refractory to imatinib, advanced renal cell carcinomas, and pancreatic neuroendocrine tumors. GI bleeding or intraabdominal hemorrhage in patients with GISTs has been reported to be 3% to 5% with imatinib and 3% to 9% with sunitinib.16–20 Although it is difficult to determine whether an emergency event is associated with the treatment effect or disease progression, one report showed high mortality associated with emergency surgery.20 Close follow-up would be recommended, especially for patients with necrotic or cystic degeneration shown on imaging studies during treatment. With the expanding indications for use of immunotherapeutic agents such as interleukin-2 (IL-2) for metastatic melanoma and renal cell cancer, clinicians should be alert to occasional cases of colonic infarction and perforation.21 The causes of these perforations are unknown, but it has been postulated that they arise from impaired perfusion due to hypotension, tissue edema, and vasoconstriction as a result of the use of pressor agents. A recent report suggests that bowel perforation might be more commonly seen when IL-2 is used in combination with anticytotoxic T-lymphocyte antigen 4 antibody, and that association can be seen in conjunction with autoimmune colitis.22
Bowel necrosis with perforation has been described with the use of cytosine arabinoside. In 50 patients treated for leukemia, 7 patients were found to have bowel necrosis and peritonitis at autopsy.23 Paclitaxel (Taxol), which is commonly used to treat patients with ovarian, breast, and non–small cell lung cancers, has also been reported as a chemotherapeutic agent that can cause bowel perforation unrelated to tumor lysis.24 In patients with advanced ovarian cancer, this complication is rare but serious, and its presentation is associated with a 43% mortality rate.25 Noncytotoxic drugs are the presumed cause of perforation when bowel wall injury cannot be associated with tumor necrosis or other specific factors in patients with cancer who are undergoing drug therapy. Immunosuppressed patients have a blunted inflammatory response and may have a more subtle presentation of GI perforation. The drugs that are most commonly implicated are corticosteroids, which can give rise to ulcers and perforations in various portions of the GI tract.
Inflammatory Conditions in the Patient with Cancer
Increasingly, aggressive chemotherapeutic regimens are being used in the treatment of patients with cancer. These treatments can expose patients to life-threatening complications related to bone marrow suppression and neutropenia. The incidence of acute illnesses necessitating surgical intervention in the setting of the neutropenic patient with cancer is approximately 7%.26 Unique inflammatory abdominal problems in patients with cancer who are receiving aggressive therapies are reviewed in this section.
Neutropenic Enterocolitis
Neutropenic enterocolitis is a clinicopathological syndrome that involves the GI tract of patients receiving chemotherapy for hematologic and solid malignancies. The clinical condition has been given a variety of names in the past, including typhlitis, ileocecal syndrome, and necrotizing enteropathy. Most commonly seen in pediatric patients who are undergoing treatment for leukemia, neutropenic enterocolitis is still a relatively rare occurrence in adults, with an incidence of 5% in this patient population.27
The clinical presentation of neutropenic enterocolitis is extremely variable, and no specific criteria are available with which to make the diagnosis. Furthermore, the symptoms are nonspecific and can be similar to those of a number of other GI processes. Affected patients typically present with fever, abdominal pain and distension, and diarrhea. Abdominal tenderness is frequently localized to the right lower quadrant but can also be diffused. Peritonitis suggests intestinal perforation. Portal venous gas, low serum bicarbonate levels, and generalized peritonitis are ominous findings and suggest a poor outcome.28 A right lower quadrant mass might be palpable, indicating a dilated cecum or a focal inflammatory phlegmon or abscess. Advanced cases can present with systemic sepsis and multiorgan failure.
No specific laboratory or radiologic findings are diagnostic for neutropenic enterocolitis. Pneumatosis intestinalis is often seen and is not itself an indication for surgical intervention.28 The initial treatment for neutropenic colitis is supportive, with the administration of broad-spectrum antibiotics, nasogastric decompression, intravenous fluids, bowel rest, and serial abdominal examinations. In most patients, these measures are sufficient, and symptoms resolve after correction of the neutropenia. Surgical intervention is rarely helpful, but sound surgical judgment should be exercised in determining which patients require surgery (Box 47-2). Specific situations that dictate surgery include uncontrollable GI bleeding, intestinal perforation, and deteriorating clinical course on medical management.
Appendicitis
Other intraabdominal inflammatory conditions, such as appendicitis, can be indistinguishable from neutropenic enterocolitis in patients receiving chemotherapy. In a large series of children with leukemia or other malignancies, the incidence of appendicitis has been reported to be between 0.2% and 2%, which is equivalent to the incidence in the general pediatric population.29 Typically, acute appendicitis manifests with right lower quadrant pain and localized tenderness. “Classic” symptoms and clinical findings of appendicitis can be followed by a CT scan to confirm diagnosis. Whereas the treatment of neutropenic enterocolitis is primarily medical, the treatment of acute appendicitis is surgical. Nonoperative management of appendicitis (e.g., intravenous antibiotics with interval appendectomy) is associated with high mortality in patients with leukemia. Delay in treatment results in a higher incidence of perforation, peritonitis, and death. Appendectomy is the treatment of choice for appendicitis. Cases that are complicated by perforation or abscess formation could necessitate the placement of drains, and the surgical incision should be left to close by secondary intention.
Pancreatitis
Pancreatitis is a recognized complication of a number of antineoplastic chemotherapeutic agents, although the drug with which it is most commonly reported is l-asparaginase. Other antineoplastic drugs that are known to induce pancreatitis are corticosteroids, didanosine, and, less commonly, cytarabine, cisplatin, IL-2, vincristine, methotrexate, mitomycin C, cyclophosphamide, doxorubicin, and ifosfamide.30 The clinical course of patients with chemotherapy-induced pancreatitis is most often mild and self-limiting but can progress to necrotizing pancreatitis or pseudocyst formation. Pancreatitis is also a complication of other cancer treatments. Associated procedures, such as endoscopic retrograde cholangiopancreatography, pancreatectomy, or splenectomy, can also result in pancreatitis. Transarterial embolization of the liver for primary or metastatic tumors can cause pancreatitis by misperfusion of chemotherapeutic agents.
Perianal and Perirectal Infections in Patients with Cancer
The exact pathogenesis of perianal infections in the patient with cancer is not well defined, although most patients have an underlying neutropenia or immunosuppression. Many patients have a history of preexisting anorectal problems, including previous perianal or perirectal abscesses, fistula in ano, anal fissures, or hemorrhoids. Once established, the infection can spread into the ischiorectal fossa, supralevator space, retroperitoneum, or perineum. Perianal and perirectal infections develop most frequently in patients with acute leukemia who are being treated with chemotherapy but can also be encountered in some patients with solid tumors or after HSCT. In most series in the literature, the incidence of perianal infections in patients with leukemia is between 2% and 8%.31,32
Bowel and Malignant Biliary Obstruction
Complete or partial obstruction of the GI tract is a common problem in patients with cancer. The typical presentation includes abdominal pain, nausea, and vomiting. Intestinal obstruction is one of the most common indications for emergency laparotomy.1,3 Intestinal obstruction can occur at any site along the GI tract, and symptoms are often dictated by the level of obstruction. Initial symptoms may be identical to those of adynamic ileus, a condition that needs to be distinguished from obstruction. Adynamic ileus in patients with cancer can be related to chemotherapeutic agents or can be a result of other metabolic problems. Vincristine sulfate and the vinca alkaloids are known to produce peripheral neuropathies and a paralytic ileus. Drug-induced ileus is estimated to occur in 10% of patients receiving these agents and could be due to their neurotoxic effects.33 Clinical presentation and radiologic studies are very helpful in differentiating adynamic ileus from a mechanical obstruction. With an adynamic ileus, generalized small bowel and colonic distension is apparent on plain films without the multiple air-fluid levels and absence of colonic gas that are seen with mechanical obstruction. Cross-sectional imaging with CT is now the adjunctive study of choice, because it can provide information simultaneously about the presence, level, severity, and cause of obstruction.
Therapeutic options include nasogastric decompression with bowel rest (e.g., nothing by mouth with intravenous fluid support). Adjunctive treatment with analgesics, antiemetics, somatostatin, or motility agents have been used with varying success. Patients with a history of previous malignancy who present with bowel obstruction should be treated like any other patient with an intestinal obstruction.34 In the case of a first presentation with obstructive symptoms, the etiology is benign (e.g., due to adhesions or herniation) in up to one third of patients. Many patients who require a laparotomy to treat their obstructions are found to have benign disease, and some even have a new primary malignancy. Given a good baseline performance status, surgical exploration for benign causes of obstruction have a high rate of success.
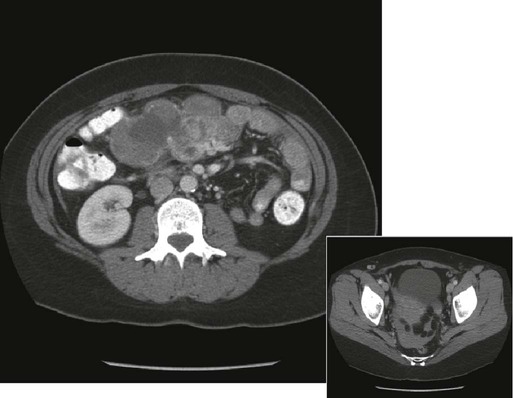
Recurrence of cancer, either locally or as diffuse peritoneal disease, is a more common problem and, unfortunately, is more difficult to treat. Surgical intervention is required if bowel strangulation and infarction are suspected because of fever, leukocytosis, and localized peritoneal tenderness. Radiographic evidence of complete bowel obstruction with loss of air in the distal large bowel and absence of flatus may likewise prompt an urgent laparotomy. Patients with partial obstructions can be managed expectantly. Further evaluation to include a CT scan or other contrast studies may be helpful in delineating level(s) or extent of obstruction (Fig. 47-2).
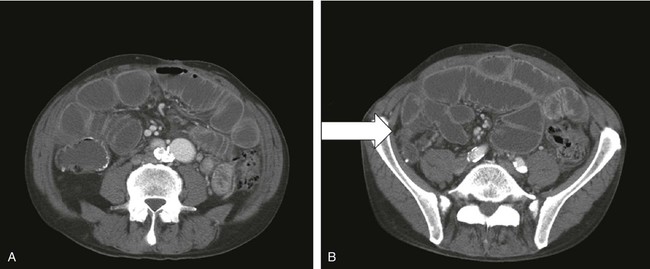
Successful nonoperative management of malignant bowel obstruction is reported in up to 29% of cases; however, recurrent episodes of obstruction are commonplace. Operative mortality and successful intervention rates for patients with cancer undergoing exploratory laparotomy for intestinal obstruction are difficult to measure, because patient selection bias tends to skew the reported results. The extent of disease and failing performance status are associated with high perioperative morbidity and mortality.35 Even with early relief of symptoms, durability of palliation can be short.
Stomach and Duodenum
Benign, obstructive ulcer disease is uncommonly seen in the modern era of H2-receptor blockers and proton pump inhibitors. Operative therapy is generally required after initial stabilization, with fluid resuscitation and nasogastric decompression in uncontrolled cases. Vagotomy with pyloroplasty is the treatment of choice, particularly for patients with cancer who might be debilitated. For obstructing primary gastric carcinomas, a curative resection with gastrojejunostomy reconstruction is recommended if the patient is stable and if the procedure is technically feasible. In cases of advanced disease, gastrostomy tube placement, with or without gastrojejunostomy (intestinal bypass), may be the best course of action. When gastric outlet obstruction is due to an unresectable pancreatic or biliary tract neoplasm, prognosis is typically poor because of the extent of disease. Creation of a gastrojejunostomy via an open or laparoscopic approach can be considered for bypassing the obstruction and maintaining GI continuity, but outcomes are not uniform. Many patients have poor emptying of stomach contents even with a mechanically patent anastomosis. Therapeutic endoscopic options are in their infancy, and success has been seen with endoscopically deployed metal endoluminal stents. This procedure is gaining widespread acceptance, and reports show 70% relief of symptoms with low complication rates. Common complications include perforation (0% to 15%) and stent migration (0% to 40%).36
Palliation Issues in Bowel Obstruction
Because of the nature of the underlying disease process, surgical resection and/or bypass might not yield durable palliation. Proper patient selection is paramount to successful palliative efforts. Recent series have reported effective palliation in up to 80% of patients with obstruction.3 Another approach that has been described for the therapy of these patients is the use of a decompressive gastrostomy tube in conjunction with either enteral or parenteral fluids. Management of malignant bowel obstruction must be coordinated with end-of-life care in some patients. Time to mortality with malignant bowel obstruction is short; small series report mean durations of survival ranging from 35 to 64 days regardless of intervention.38–38
Malignant Biliary Obstruction
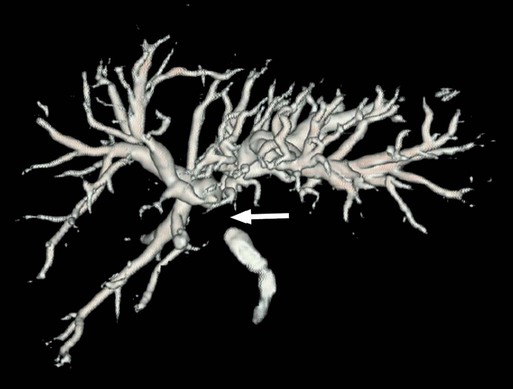
Painless jaundice in elderly patients should be considered to be due to malignancy until proved otherwise. The diagnostic approach to patients with obstructive jaundice begins with a careful history and physical examination, along with laboratory studies. Although ultrasonography is noninvasive, portable, and relatively inexpensive, CT gives more valuable information about the location and etiology of malignant biliary obstruction (hilum: hilar cholangiocarcinoma (Klatskin tumor), gallbladder carcinoma, and hepatoma; distal: pancreatic head, ampullary, duodenal cancer, or distal cholangiocarcinoma) and resectability of the tumor. If biliary obstruction is suspected from malignant disease, a multidisciplinary approach is required. Resectability of tumor can be further assessed with endoscopic ultrasound or duplex ultrasound for distal and proximal bile duct obstruction, respectively. Although preoperative endoscopic biliary drainage with endoscopic retrograde cholangiopancreatography (ERCP) would be beneficial in relieving jaundice and preventing complications from cholestasis and may allow time for neoadjuvant therapy for patients with borderline resectable or locally advanced cancer, routine use of preoperative endoscopic biliary drainage for resectable pancreaticobiliary disease is debated. Several studies showed that it increases perioperative complications in persons with proximal and distal malignant biliary obstruction.39,40 Magnetic resonance cholangiopancreatography is a noninvasive technique and a potential alternative to ERCP, allowing visualization of the level of obstruction and providing information about the extent of tumor and vascular and nodal involvement without risk of biliary intubation (Fig. 47-3). For patients with unresectable cancer causing distal biliary obstruction, ERCP with self-expanding metal stents would be preferable compared with surgical bypass or endoscopic plastic stents.41 Endoscopic metal stenting also would be the preferred palliative drainage modality for proximal bile duct obstruction from unresectable tumor, and percutaneous transhepatic cholangiodrainage can be used as backup in the event of failure.
Gastrointestinal Problems Following Hematopoietic Stem-Cell Transplantation
Evaluation of acute abdominal complaints in patients who have had allogeneic HSCT represents a major diagnostic challenge for the surgeon. Virtually all patients who have undergone HSCT will experience GI problems at some point in the posttransplant period; these problems can include nausea, vomiting, alterations in liver function tests, watery diarrhea, and abdominal pain.35 Such findings in an acutely ill patient after HSCT may herald a broad spectrum of acute abdominal processes. However, several disease entities are unique to the patient who has undergone HSCT and warrant special consideration.
Three common causes of abdominal complaints in the patients who have undergone HSCT are unique:
1. High-dose induction chemotherapy or chemoradiation therapy given before transplantation
2. Acute intestinal graft-versus-host disease (GVHD) in recipients of allogeneic transplants
3. Infections of the gut that occur before bone marrow recovery
Infections of the gut caused by bacteria and fungus are most often seen before posttransplant day 30, whereas viral infections are usually seen after 30 days, although there are reports of late (5 months to 1 year) reactuation of varicella zoster infection associated with acute abdominal pain, hepatitis and pancreatitis, or disseminated disease. Mortality rates are from 50% to 100%.42,43
GVHD is a process in which donor T cells react to recipient cells; it develops in 30% to 50% of patients receiving allogeneic grafts.44 Despite the prominence of intraabdominal organs and symptoms in the manifestations of GVHD, it is rare that surgical intervention is necessary. The most common indications for abdominal surgery in patients with GVHD are GI bleeding and obstruction.44 Perforation of the bowel is uncommon. The finding of pneumatosis intestinalis on abdominal radiography has been reported to be present in as many as 18% of patients who have undergone HSCT with acute GVHD45; the majority of these cases do not require surgery.42,46 Although free air in the peritoneal cavity usually is an indication for surgery, it need not be associated with a frank bowel perforation, and this problem has been reported to be managed without an operation.
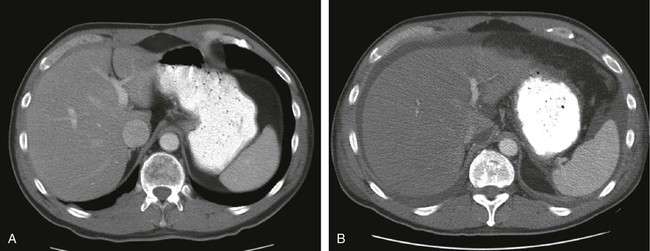
Another intraabdominal complication of HSCT that manifests with abdominal pain is venoocclusive disease (VOD) of the liver.47 The etiology of VOD is thought to be damage to the endothelium of hepatic venules and centrilobular hepatic necrosis, leading to fibrosis and occlusion of the central hepatic veins. VOD is associated with significant mortality. The differential diagnosis of VOD is lengthy and includes any disorder that could result in right upper quadrant pain, jaundice, or ascites. A partial list would include such disorders as hepatitis, drug- or parenteral nutrition–induced liver dysfunction, acute cholecystitis, cholangitis, or liver abscess; careful workup should be undertaken. The diagnosis of VOD can usually be made on the basis of clinical signs, with liver biopsy being reserved for patients in whom the diagnosis is not certain (Fig. 47-4). Because of underlying hematologic disorders, liver biopsies are associated with acute bleeding and must be performed with care. The treatment of VOD is supportive, with emphasis on maintaining intravascular volume and renal perfusion while limiting the amount of sodium and extravascular fluid accumulation.
Fistulae
Fistulae are seen as complications in patients with cancer because multiple risk factors for fistula development are often present in these patients. Fistulae are rarely the manifesting symptom of an intraabdominal malignancy; they most often manifest as complications during or after treatments. Most clinicians agree that contributing factors include prior abdominal surgery, inflammatory bowel disease, use of radiation therapy, cancer, malnutrition, and intraabdominal sepsis. Unfortunately, many of these factors are prevalent in patients with cancer and are not often reversible. Despite the use of total parenteral nutrition, enteral nutrition, antibiotics, and highly sophisticated bioengineered wound care adjuncts, mortality and morbidity rates from GI fistulae remain high. Although mortality rates as low as 7% are reported, numbers in the range of 10% to 30% are more representative.48,49 The exact incidence of GI fistula formation in patients with cancer is not known but is likely quite low.
Enterocutaneous fistulae commonly occur in patients who have received radiation therapy. Direct invasion with subsequent tumor necrosis can result in an abnormal connection between viscera and the skin. Ischemic necrosis as a result of neoplastic vascular invasion or small vessel occlusion from host reaction and sclerosis likewise can lead to fistula formation. Finally, tumor-induced perforation with abscess and subsequent erosion can result in fistula formation. This latter mechanism is particularly troublesome because it can lead to diagnostic confusion and can complicate management. Ionizing radiation could have both acute and chronic effects on the GI tract. The acute effects—a result of the depletion of rapidly proliferating mucosal cells causing diarrhea, nausea, vomiting, abdominal pain, and GI tract bleeding—are generally self-limiting. However, chronic radiation damage can become evident as early as 1 month after radiation therapy, or it might not be clinically apparent for as long as 30 years after treatment.50 Although radiation injury to the bowel can arise as a complication in as many as 15% of patients receiving treatment in abdominal fields, fistulae occur more rarely. Factors related to the incidence of radiation-induced injury include the total radiation dose and fractionation, the sensitivity of exposed normal organs within the radiation port, anatomic considerations, the presence of comorbid conditions, and concomitant administration of other drugs.
A number of factors decrease the likelihood of spontaneous closure of a fistula. The most common are undrained infections and luminal obstruction distal to the fistula. Other factors include prior radiation or chemotherapy, epithelialization of the fistula tract, cancer within the tract, granulomatous disease (e.g., inflammatory bowel disease or mycobacterial infection) within the fistula, presence of a foreign body, and malnutrition. Although predictive of morbidity due to fistulae, fistula output does not necessarily appear to be related to the probability of spontaneous fistula closure. Given proper nutritional support and attention to these factors, as many as 60% of GI fistulae can close spontaneously.48,49
Patients who exhibit signs of sepsis must be evaluated aggressively to identify the source. Intraabdominal and perifistular abscesses are common and must be identified quickly. Thorough physical examination (including digital examination of the rectum, vagina, stomas, and wounds) is absolutely necessary. CT scanning of the abdomen and pelvis using intravenous and enteric contrast and contrast in the rectum, fistula tract, and drainage tubes is often the most enlightening radiologic study (Fig. 47-5). Any undrained foci of infection must be addressed aggressively and drained either percutaneously or operatively. Once the acute electrolyte imbalances have been corrected and the fistula output has stabilized, intravenous fluid and nutritional infusions may be combined to simplify fluid management. Definitive treatment of fistulae ultimately requires the reestablishment of normal skin integrity; macerated skin and large open wounds complicate and delay spontaneous or operative closure. Techniques such as sump drainage, stoma bag application, and barrier protection of the skin using special adhesives or pastes should be used liberally.