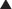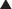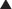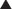Chapter 18 Infectious Diseases
Introduction to Antibiotics
Suggestions
 Know which category the individual drugs belong to, and learn the category as a whole. For example, ceftriaxone is a third-generation cephalosporin. Therefore learn about cephalosporins in general, and then know the general differences among first-, second-, third-, and fourth-generation cephalosporins. In some circumstances there might be one or two extra important bits of information pertaining to individual drugs that are important to learn.
Know which category the individual drugs belong to, and learn the category as a whole. For example, ceftriaxone is a third-generation cephalosporin. Therefore learn about cephalosporins in general, and then know the general differences among first-, second-, third-, and fourth-generation cephalosporins. In some circumstances there might be one or two extra important bits of information pertaining to individual drugs that are important to learn. Antibiotics can usually be classified in terms of the categories of bacteria they kill. These categories include the following:
Antibiotics can usually be classified in terms of the categories of bacteria they kill. These categories include the following:
Important Considerations
 It is required that you learn the mechanism of action (MOA) of a drug and also the mechanism of resistance, which enables an organism to live in the presence of the drug. This is important because when you are selecting an antibiotic, if your first choice does not work, you will have a better understanding of why it did not work and will be better educated to select a different antibiotic that will have a greater probability of killing the organism. For example, if a penicillin (a β-lactam) was given and the bug is known to produce β-lactamase, a third-generation cephalosporin (another β-lactam) might be a poor next choice.
It is required that you learn the mechanism of action (MOA) of a drug and also the mechanism of resistance, which enables an organism to live in the presence of the drug. This is important because when you are selecting an antibiotic, if your first choice does not work, you will have a better understanding of why it did not work and will be better educated to select a different antibiotic that will have a greater probability of killing the organism. For example, if a penicillin (a β-lactam) was given and the bug is known to produce β-lactamase, a third-generation cephalosporin (another β-lactam) might be a poor next choice. Make sure that the drug you select can get to the tissue in which the infection is located. Special examples include the following:
Make sure that the drug you select can get to the tissue in which the infection is located. Special examples include the following:
 Gut (intraintestinal, not intraperitoneal) infections are often best treated with oral medications that cannot be absorbed into the body (because they remain in the gut, which is where the infection is)—for example, oral vancomycin for C. difficile colitis.
Gut (intraintestinal, not intraperitoneal) infections are often best treated with oral medications that cannot be absorbed into the body (because they remain in the gut, which is where the infection is)—for example, oral vancomycin for C. difficile colitis. The choice of antibiotic (oral versus intravenous, broad-spectrum versus narrow-spectrum agents) is usually determined by how sick the patient is and how certain you are about which bacterium is causing the infection. If you are uncertain, choose a broad-spectrum drug.
The choice of antibiotic (oral versus intravenous, broad-spectrum versus narrow-spectrum agents) is usually determined by how sick the patient is and how certain you are about which bacterium is causing the infection. If you are uncertain, choose a broad-spectrum drug. If you can do cultures, try to do them before the patient starts taking antibiotics, because after you administer antibiotics the drug will be in the patient’s system and will inhibit growth of specimens collected from the patient after that point in time.
If you can do cultures, try to do them before the patient starts taking antibiotics, because after you administer antibiotics the drug will be in the patient’s system and will inhibit growth of specimens collected from the patient after that point in time. Broad-spectrum drugs tend to be more expensive and more “powerful” and should be reserved for situations in which narrow-spectrum agents are not indicated. Development of resistance is a very real risk every time an antibiotic is used. Always using the powerful drugs will result in bacterial resistance to them. Then what will you use?
Broad-spectrum drugs tend to be more expensive and more “powerful” and should be reserved for situations in which narrow-spectrum agents are not indicated. Development of resistance is a very real risk every time an antibiotic is used. Always using the powerful drugs will result in bacterial resistance to them. Then what will you use?Viruses are technically not alive, so antivirals are usually not referred to as antimicrobials.
Advanced Killing Techniques
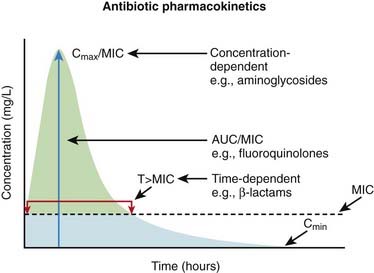
Knowledge of pharmacokinetics is required to enable a clinician to be really good at knowing how to kill off an infection. Understanding some very important fundamental concepts are required (Figure 18-1).
 Minimum inhibitory concentration (MIC): The MIC of an antimicrobial is the minimum concentration that will inhibit growth of a pathogen. Obviously, it is desirable to have the concentration in the patient’s infected tissues to be greater than the MIC. If drug A has a lower MIC than drug B, then drug A kills the pathogen at a lower concentration of drug and would therefore be better at killing that particular pathogen, assuming all other factors are identical. When the MIC level of a drug for a given pathogen is low, the pathogen is considered sensitive to the drug (i.e., the drug will kill it). When the MIC is a moderate value, the pathogen’s sensitivity to the drug will be intermediate, and when the MIC is high, the pathogen will be resistant to that drug. Laboratory values often report sensitivities as S, I, or R to report sensitive, intermediate, and resistant, respectively.
Minimum inhibitory concentration (MIC): The MIC of an antimicrobial is the minimum concentration that will inhibit growth of a pathogen. Obviously, it is desirable to have the concentration in the patient’s infected tissues to be greater than the MIC. If drug A has a lower MIC than drug B, then drug A kills the pathogen at a lower concentration of drug and would therefore be better at killing that particular pathogen, assuming all other factors are identical. When the MIC level of a drug for a given pathogen is low, the pathogen is considered sensitive to the drug (i.e., the drug will kill it). When the MIC is a moderate value, the pathogen’s sensitivity to the drug will be intermediate, and when the MIC is high, the pathogen will be resistant to that drug. Laboratory values often report sensitivities as S, I, or R to report sensitive, intermediate, and resistant, respectively.Now, exactly how the concentration of the antimicrobial stays above the MIC in the body is very important and is different for different drugs. The most important concepts are illustrated in Figure 18-1 and include:
 Time dependence is the total length of time that a drug level stays above the MIC. What matters is the total duration that the concentration is above the MIC. These drugs are called time-dependent antimicrobials. β-Lactams (penicillins, cephalosporins, and carbapenems) all fall into this category. Note that it does not matter if the drug level is just barely above the MIC or is 10 times the MIC.
Time dependence is the total length of time that a drug level stays above the MIC. What matters is the total duration that the concentration is above the MIC. These drugs are called time-dependent antimicrobials. β-Lactams (penicillins, cephalosporins, and carbapenems) all fall into this category. Note that it does not matter if the drug level is just barely above the MIC or is 10 times the MIC. CMAX/MIC is the maximum concentration of the drug compared with the MIC. In other words, some drugs kill better when the maximum concentration of the drug is very high. These drugs are called concentration-dependent antimicrobials. As a general rule, it is important to measure drug concentrations of these drugs. Vancomycin and aminoglycosides fall into this category and it is common to measure levels of both of them. Note that it does not matter how long the concentration stays above the MIC for these drugs, which is in complete contrast to the time-dependent drugs.
CMAX/MIC is the maximum concentration of the drug compared with the MIC. In other words, some drugs kill better when the maximum concentration of the drug is very high. These drugs are called concentration-dependent antimicrobials. As a general rule, it is important to measure drug concentrations of these drugs. Vancomycin and aminoglycosides fall into this category and it is common to measure levels of both of them. Note that it does not matter how long the concentration stays above the MIC for these drugs, which is in complete contrast to the time-dependent drugs.Penicillins
Moa (Mechanism of Action)
 All β-lactams (penicillins, carbapenems, and cephalosporins) act through this common sequence of events.
All β-lactams (penicillins, carbapenems, and cephalosporins) act through this common sequence of events.Binding
Cell Wall Destruction
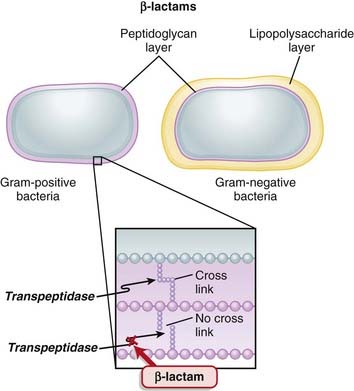
 Transpeptidases are enzymes that cross-link peptidoglycan molecules in bacterial cell walls. Cross-linking these molecules gives strength to the cell wall.
Transpeptidases are enzymes that cross-link peptidoglycan molecules in bacterial cell walls. Cross-linking these molecules gives strength to the cell wall. β-Lactams work by inhibiting transpeptidase, preventing it from forming cross-links. This results in a bacterium with a structurally deficient cell wall, typically leading to bacterial lysis (Figure 18-2).
β-Lactams work by inhibiting transpeptidase, preventing it from forming cross-links. This results in a bacterium with a structurally deficient cell wall, typically leading to bacterial lysis (Figure 18-2). Gram-negative bacteria have a thinner peptidoglycan layer, but external to this layer is a lipopolysaccharide layer. This lipopolysaccharide layer protects the peptidoglycan layer from β-lactam activity, and therefore gram-negative bacteria are significantly more resistant to β-lactams.
Gram-negative bacteria have a thinner peptidoglycan layer, but external to this layer is a lipopolysaccharide layer. This lipopolysaccharide layer protects the peptidoglycan layer from β-lactam activity, and therefore gram-negative bacteria are significantly more resistant to β-lactams. β-Lactamase inhibitors are added to some β-lactam antibiotics to overcome resistance caused by β-lactamase. Although β-lactamase inhibitors do contain a β-lactam, they are not toxic to the bacteria; they merely bind to β-lactamase. Examples of β-lactamase inhibitors include the following:
β-Lactamase inhibitors are added to some β-lactam antibiotics to overcome resistance caused by β-lactamase. Although β-lactamase inhibitors do contain a β-lactam, they are not toxic to the bacteria; they merely bind to β-lactamase. Examples of β-lactamase inhibitors include the following:
 Narrow-spectrum penicillins contain a larger molecule on the penicillin molecule side chain that confers steric hindrance: the inability to twist the molecule into other stereoisomers. This results in these penicillins being resistant to β-lactamase but at the same time restricts their spectrum of activity (thus they are said to be narrow-spectrum agents).
Narrow-spectrum penicillins contain a larger molecule on the penicillin molecule side chain that confers steric hindrance: the inability to twist the molecule into other stereoisomers. This results in these penicillins being resistant to β-lactamase but at the same time restricts their spectrum of activity (thus they are said to be narrow-spectrum agents). Aminopenicillins have an added amino group (NH2) that makes the molecule more hydrophilic and thus able to cross the lipopolysaccharide layer more easily. Therefore aminopenicillins have greater activity against gram-negative bacteria.
Aminopenicillins have an added amino group (NH2) that makes the molecule more hydrophilic and thus able to cross the lipopolysaccharide layer more easily. Therefore aminopenicillins have greater activity against gram-negative bacteria. Broad-spectrum penicillins are modifications of aminopenicillins: nitrogen and carbon atoms are added to the molecule. This increases the range of bacteria that are sensitive to the antibiotic. These penicillins are usually coadministered with a β-lactamase inhibitor because they are β-lactamase sensitive (a common example is “Pip/Tazo,” which is piperacillin and tazobactam).
Broad-spectrum penicillins are modifications of aminopenicillins: nitrogen and carbon atoms are added to the molecule. This increases the range of bacteria that are sensitive to the antibiotic. These penicillins are usually coadministered with a β-lactamase inhibitor because they are β-lactamase sensitive (a common example is “Pip/Tazo,” which is piperacillin and tazobactam).Mechanisms of Resistance
 Gram-negative bacteria, as described previously, inherently have an outer protective lipopolysaccharide layer that guards the peptidoglycan layer from attack by some β-lactams.
Gram-negative bacteria, as described previously, inherently have an outer protective lipopolysaccharide layer that guards the peptidoglycan layer from attack by some β-lactams. The main mechanisms of resistance to β-lactams include the following:
The main mechanisms of resistance to β-lactams include the following:
 Production of β-lactamase, which enzymatically destroys the four-carbon β-lactam ring
Production of β-lactamase, which enzymatically destroys the four-carbon β-lactam ring
Pharmacokinetics
 Eighty percent of penicillin is cleared by the kidneys within 4 hours. Therefore it is very quickly eliminated from the body, which is an undesirable characteristic if the goal is to expose the bacterial infection to prolonged concentrations of the drug.
Eighty percent of penicillin is cleared by the kidneys within 4 hours. Therefore it is very quickly eliminated from the body, which is an undesirable characteristic if the goal is to expose the bacterial infection to prolonged concentrations of the drug. Probenecid, a uricosuric, competes with penicillin in the organic acid transporter in the kidney and therefore decreases renal clearance of penicillin, thereby prolonging high tissue concentrations and a longer half life. It is coadministered with penicillin.
Probenecid, a uricosuric, competes with penicillin in the organic acid transporter in the kidney and therefore decreases renal clearance of penicillin, thereby prolonging high tissue concentrations and a longer half life. It is coadministered with penicillin. Most penicillins are renally cleared, and the dose must be adjusted in patients with renal dysfunction. However, cloxacillin is hepatically cleared and does not require dose adjustment in patients with renal dysfunction.
Most penicillins are renally cleared, and the dose must be adjusted in patients with renal dysfunction. However, cloxacillin is hepatically cleared and does not require dose adjustment in patients with renal dysfunction.Important Notes
FYI
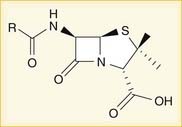
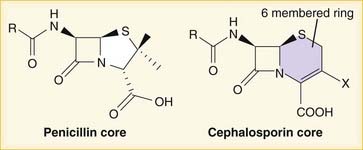
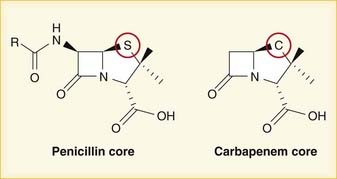
 A lactam is a chemical ring. A β-lactam is a four-molecule ring (three carbons and one nitrogen) and is the nucleus of the penicillin molecule. The penicillin nucleus is shown in Figure 18-3. Modifications to the five-membered ring are the basis on which cephalosporins and carbapenems (two other β-lactam antibiotics) were derived.
A lactam is a chemical ring. A β-lactam is a four-molecule ring (three carbons and one nitrogen) and is the nucleus of the penicillin molecule. The penicillin nucleus is shown in Figure 18-3. Modifications to the five-membered ring are the basis on which cephalosporins and carbapenems (two other β-lactam antibiotics) were derived. Peptidoglycans are polymers of sugars and amino acids. Cross-linking of these polymers results in a rigid crystal structure.
Peptidoglycans are polymers of sugars and amino acids. Cross-linking of these polymers results in a rigid crystal structure. The clinically significant difference between amoxicillin and ampicillin is that amoxicillin is generally administered orally whereas ampicillin is generally administered IV.
The clinically significant difference between amoxicillin and ampicillin is that amoxicillin is generally administered orally whereas ampicillin is generally administered IV. It would be nice for memorization if penicillin G were given in the gut and penicillin V were given IV; unfortunately, it is the other way around.
It would be nice for memorization if penicillin G were given in the gut and penicillin V were given IV; unfortunately, it is the other way around.Cephalosporins
Prototypes and common drugs
Moa (Mechanism of Action)
 All β-lactams (penicillins, carbapenems, and cephalosporins) act through this common sequence of events.
All β-lactams (penicillins, carbapenems, and cephalosporins) act through this common sequence of events.Binding
Cell Wall Destruction
 Transpeptidases are enzymes that cross-link peptidoglycan molecules in bacterial cell walls. Cross-linking these molecules gives strength to the cell wall (see Figure 18-2).
Transpeptidases are enzymes that cross-link peptidoglycan molecules in bacterial cell walls. Cross-linking these molecules gives strength to the cell wall (see Figure 18-2).Mechanisms of Resistance
 The main mechanisms of resistance to β-lactams include the following:
The main mechanisms of resistance to β-lactams include the following:
 Production of β-lactamase, which enzymatically destroys the four-carbon β-lactam ring
Production of β-lactamase, which enzymatically destroys the four-carbon β-lactam ring
Pharmacokinetics
 Penetration to the brain through the blood-brain barrier is a very important factor for antibiotics because it will determine whether or not an antibiotic will be effective against bacterial meningitis. Third-generation cephalosporins have good CNS penetration.
Penetration to the brain through the blood-brain barrier is a very important factor for antibiotics because it will determine whether or not an antibiotic will be effective against bacterial meningitis. Third-generation cephalosporins have good CNS penetration.Side Effects
Important Notes
FYI
 Note how the four-membered ring (the β-lactam) is the same as in penicillins; however, the other ring is six-membered, whereas in penicillins it is five-membered. The diagram shows the core nucleus of cephalosporins. There are currently four generations of cephalosporins (Figure 18-4).
Note how the four-membered ring (the β-lactam) is the same as in penicillins; however, the other ring is six-membered, whereas in penicillins it is five-membered. The diagram shows the core nucleus of cephalosporins. There are currently four generations of cephalosporins (Figure 18-4). Cephalosporins were first isolated from Cephalosporium acremonium from the sea near a sewer outlet in 1948. They were initially found to cure Staph. aureus infection and typhoid.
Cephalosporins were first isolated from Cephalosporium acremonium from the sea near a sewer outlet in 1948. They were initially found to cure Staph. aureus infection and typhoid. The suffix -penia means low cell counts. WBCs are granulocytes. The most abundant type of WBC is the neutrophil. Thus a low WBC count can be referred to as granulocytopenia or neutropenia.
The suffix -penia means low cell counts. WBCs are granulocytes. The most abundant type of WBC is the neutrophil. Thus a low WBC count can be referred to as granulocytopenia or neutropenia. Eosinophilia occurs in drug hypersensitivity reactions and also in other conditions. The mnemonic CHINA can help you remember the common causes of eosinophilia:
Eosinophilia occurs in drug hypersensitivity reactions and also in other conditions. The mnemonic CHINA can help you remember the common causes of eosinophilia:
Carbapenems
Moa (Mechanism of Action)
 All β-lactams (penicillins, carbapenems, and cephalosporins) act through this common sequence of events.
All β-lactams (penicillins, carbapenems, and cephalosporins) act through this common sequence of events.Binding
Cell Wall Destruction
 Transpeptidases are enzymes that cross-link peptidoglycan molecules in bacterial cell walls. Cross-linking these molecules gives strength to the cell wall (Figure 18-5).
Transpeptidases are enzymes that cross-link peptidoglycan molecules in bacterial cell walls. Cross-linking these molecules gives strength to the cell wall (Figure 18-5).Mechanisms of Resistance
 The main mechanisms of resistance to β-lactams include the following:
The main mechanisms of resistance to β-lactams include the following:
 Production of β-lactamase, which enzymatically destroys the four-carbon β-lactam ring
Production of β-lactamase, which enzymatically destroys the four-carbon β-lactam ring
Pharmacokinetics
 Imipenem is hydrolyzed by renal tubular dipeptidase. Imipenem is therefore always combined with cilastatin, which inhibits this breakdown. Other carbapenems do not require coadministration with cilastatin because they are not metabolized by dipeptidase.
Imipenem is hydrolyzed by renal tubular dipeptidase. Imipenem is therefore always combined with cilastatin, which inhibits this breakdown. Other carbapenems do not require coadministration with cilastatin because they are not metabolized by dipeptidase. Doses must be reduced in patients with renal dysfunction. With imipenem, there is increased risk for seizures in patients with renal dysfunction.
Doses must be reduced in patients with renal dysfunction. With imipenem, there is increased risk for seizures in patients with renal dysfunction.Side Effects
 Fever: This can cause diagnostic dilemmas because carbapenems are administered to patients with suspected infections, who usually already have a fever. If the drug starts to create a fever, then it can be very difficult to know when the infection is gone (which is usually accompanied by a normalization of the patient’s temperature).
Fever: This can cause diagnostic dilemmas because carbapenems are administered to patients with suspected infections, who usually already have a fever. If the drug starts to create a fever, then it can be very difficult to know when the infection is gone (which is usually accompanied by a normalization of the patient’s temperature).Important Notes
 MRSA is an important resistant strain of S. aureus. It is not sensitive to carbapenems. It is treated with vancomycin.
MRSA is an important resistant strain of S. aureus. It is not sensitive to carbapenems. It is treated with vancomycin. VRE is another important resistant strain. About 50% of VRE infections are sensitive to carbapenems.
VRE is another important resistant strain. About 50% of VRE infections are sensitive to carbapenems. Carbapenems are very broad-spectrum agents. They are considered “big guns” and are used only in patients who are very sick or suspected of having resistant organisms. They are generally not used as first-line treatment.
Carbapenems are very broad-spectrum agents. They are considered “big guns” and are used only in patients who are very sick or suspected of having resistant organisms. They are generally not used as first-line treatment.FYI
 Carbapenems differ structurally from penicillins in that the five-membered ring that is attached to the β-lactam ring contains a carbon atom instead of a sulfur atom. The name carbapenem comes from carbon and penicillin. Figure 18-6 shows the core structure of a carbapenem but does not show the side chains.
Carbapenems differ structurally from penicillins in that the five-membered ring that is attached to the β-lactam ring contains a carbon atom instead of a sulfur atom. The name carbapenem comes from carbon and penicillin. Figure 18-6 shows the core structure of a carbapenem but does not show the side chains.Glycopeptides
Moa (Mechanism of Action)
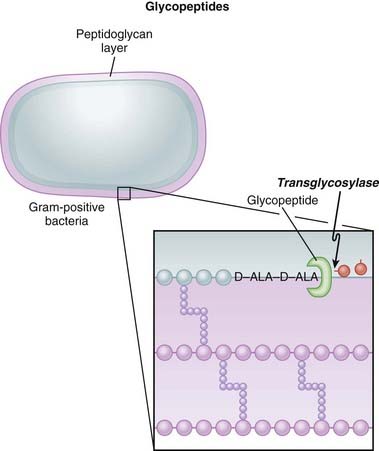
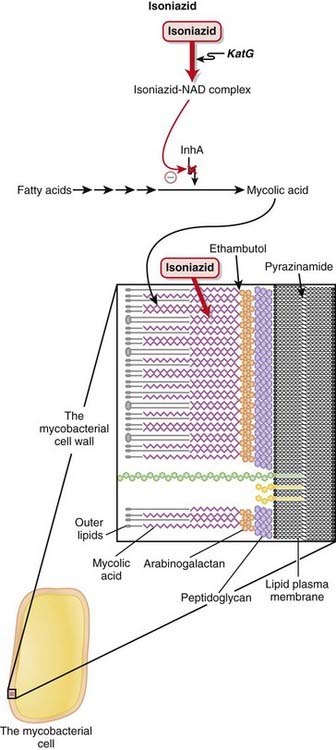
 Glycopeptides inhibit cell wall synthesis by attaching to the end of the peptidoglycan precursor units (a short four– or five–amino acid sequence called the d-alanyl-d-alanine [D-ALA-D-ALA] terminus) that are required to be laid down into the matrix. This step is catalyzed by transglycosylase. Because the glycopeptide binds to the end of the precursor, the precursor is not released from the carrier and thus peptidoglycan synthesis stops (Figure 18-7).
Glycopeptides inhibit cell wall synthesis by attaching to the end of the peptidoglycan precursor units (a short four– or five–amino acid sequence called the d-alanyl-d-alanine [D-ALA-D-ALA] terminus) that are required to be laid down into the matrix. This step is catalyzed by transglycosylase. Because the glycopeptide binds to the end of the precursor, the precursor is not released from the carrier and thus peptidoglycan synthesis stops (Figure 18-7). Glycopeptides are bactericidal in organisms that are dividing, because a dividing bacterium requires new cell wall synthesis, and the absence of the new cell wall results in death of the organism.
Glycopeptides are bactericidal in organisms that are dividing, because a dividing bacterium requires new cell wall synthesis, and the absence of the new cell wall results in death of the organism. As a result of targeting the peptidoglycan layer, glycopeptides are effective only against gram-positive organisms. This is because gram-negative bacteria possess a thick outer layer of lipopolysaccharide that covers the peptidoglycan layer.
As a result of targeting the peptidoglycan layer, glycopeptides are effective only against gram-positive organisms. This is because gram-negative bacteria possess a thick outer layer of lipopolysaccharide that covers the peptidoglycan layer.Mechanisms of Resistance
 A change to the end of the amino acid precursor (which has a d-alanyl-d-alanine terminus) can result in the drug not binding to the precursor. This is the most common method by which Enterococcus becomes resistant (and is then called VRE, vancomycin-resistant Enterococcus).
A change to the end of the amino acid precursor (which has a d-alanyl-d-alanine terminus) can result in the drug not binding to the precursor. This is the most common method by which Enterococcus becomes resistant (and is then called VRE, vancomycin-resistant Enterococcus).Pharmacokinetics
 The half-life of vancomycin is 6 hours, whereas the half-life of teicoplanin is much longer, as high as 100 hours (assuming normal kidney function).
The half-life of vancomycin is 6 hours, whereas the half-life of teicoplanin is much longer, as high as 100 hours (assuming normal kidney function). Glycopeptides are very poorly absorbed from the GI tract. If the infection that is being treated is inside the GI tract (for example, C. difficile colitis, also called “C diff colitis,” or pseudomembranous colitis), then administering the drug orally provides the highest exposure of antibiotic to the infection.
Glycopeptides are very poorly absorbed from the GI tract. If the infection that is being treated is inside the GI tract (for example, C. difficile colitis, also called “C diff colitis,” or pseudomembranous colitis), then administering the drug orally provides the highest exposure of antibiotic to the infection. If the infection is anywhere other than inside the GI tract (e.g., blood, soft tissues, brain, heart), then the drug must be administered via the intravenous route—or, for teicoplanin, also intramuscularly.
If the infection is anywhere other than inside the GI tract (e.g., blood, soft tissues, brain, heart), then the drug must be administered via the intravenous route—or, for teicoplanin, also intramuscularly. They are renally cleared, and in patients who have renal dysfunction or renal failure, the frequency of administration must be dramatically reduced (sometimes as infrequently as one dose every 2 to 3 days) and should be guided by drug levels in the blood. Because teicoplanin has a longer half-life, it can actually be administered once a week in patients without any renal function.
They are renally cleared, and in patients who have renal dysfunction or renal failure, the frequency of administration must be dramatically reduced (sometimes as infrequently as one dose every 2 to 3 days) and should be guided by drug levels in the blood. Because teicoplanin has a longer half-life, it can actually be administered once a week in patients without any renal function. Glycopeptides are cleared by dialysis. This is important because patients on dialysis usually get dialyzed three times a week. The best time to administer vancomycin would be just after dialysis, which would result in the drug remaining at high levels in the blood and tissues for the full 2 to 3 days until next dialysis. It would not be logical to give the drug right before dialysis.
Glycopeptides are cleared by dialysis. This is important because patients on dialysis usually get dialyzed three times a week. The best time to administer vancomycin would be just after dialysis, which would result in the drug remaining at high levels in the blood and tissues for the full 2 to 3 days until next dialysis. It would not be logical to give the drug right before dialysis.Side Effects
 Flushing: When vancomycin is administered quickly, the blood pressure can fall because of histamine release. Therefore vancomycin must be administered slowly (usually over 1 hour). The flushing caused by histamine release has been called red man syndrome and is not an allergy but a predictable response to rapidly administered vancomycin.
Flushing: When vancomycin is administered quickly, the blood pressure can fall because of histamine release. Therefore vancomycin must be administered slowly (usually over 1 hour). The flushing caused by histamine release has been called red man syndrome and is not an allergy but a predictable response to rapidly administered vancomycin. Ototoxicity is very rare, unless vancomycin is administered with another ototoxic agent such as aminoglycosides.
Ototoxicity is very rare, unless vancomycin is administered with another ototoxic agent such as aminoglycosides.Important Notes
 Although vancomycin can kill S. aureus organisms that are resistant to cloxacillin (or methicillin [i.e., MRSA]), cloxacillin has a lower MIC than vancomycin for strains that are β-lactam susceptible. Therefore cloxacillin is a better killer of S. aureus when there is no resistance. Vancomycin is not a stronger antibiotic. It is simply a different but paradoxically slightly weaker one that avoids β-lactam resistance.
Although vancomycin can kill S. aureus organisms that are resistant to cloxacillin (or methicillin [i.e., MRSA]), cloxacillin has a lower MIC than vancomycin for strains that are β-lactam susceptible. Therefore cloxacillin is a better killer of S. aureus when there is no resistance. Vancomycin is not a stronger antibiotic. It is simply a different but paradoxically slightly weaker one that avoids β-lactam resistance.Fluoroquinolones
MOA (Mechanism of Action)
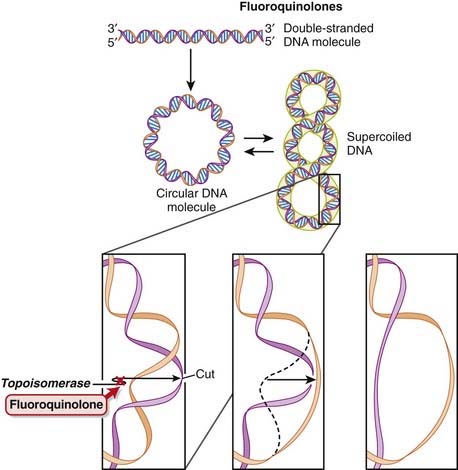
 DNA is normally supercoiled. Supercoiled DNA is under too much tension to be separated, so an extra step is required before replication and transcription can occur. DNA gyrase relaxes supercoiled DNA by cutting it, allowing rotation to occur, and then reattaching it.
DNA is normally supercoiled. Supercoiled DNA is under too much tension to be separated, so an extra step is required before replication and transcription can occur. DNA gyrase relaxes supercoiled DNA by cutting it, allowing rotation to occur, and then reattaching it. Fluoroquinolones bind to and inhibit DNA gyrase (also called topoisomerase II) and topoisomerase IV. Fluoroquinolones inhibit DNA gyrase in gram-negative organisms and topoisomerase IV in gram-positive organisms (Figure 18-8).
Fluoroquinolones bind to and inhibit DNA gyrase (also called topoisomerase II) and topoisomerase IV. Fluoroquinolones inhibit DNA gyrase in gram-negative organisms and topoisomerase IV in gram-positive organisms (Figure 18-8). The fluoroquinolones inhibit DNA gyrase after the cutting step, preventing reattachment from occurring. At high doses this leads to the release of these broken segments of DNA. It is thought that the accumulation of these DNA fragments leads to cell death, accounting for the bactericidal action of fluoroquinolones.
The fluoroquinolones inhibit DNA gyrase after the cutting step, preventing reattachment from occurring. At high doses this leads to the release of these broken segments of DNA. It is thought that the accumulation of these DNA fragments leads to cell death, accounting for the bactericidal action of fluoroquinolones.Pharmacokinetics
 Fluoroquinolones can enter human cells easily and therefore are often used to treat intracellular pathogens.
Fluoroquinolones can enter human cells easily and therefore are often used to treat intracellular pathogens. Fluoroquinolones are absorbed very well from the gut. Therefore if a person can tolerate oral medication, he or she can usually be switched from an intravenous form to an oral form.
Fluoroquinolones are absorbed very well from the gut. Therefore if a person can tolerate oral medication, he or she can usually be switched from an intravenous form to an oral form.Important Notes
 Originally, quinolones were mainly effective against gram-negative bacteria, but newer agents are useful against gram-positive cocci as well.
Originally, quinolones were mainly effective against gram-negative bacteria, but newer agents are useful against gram-positive cocci as well.FYI
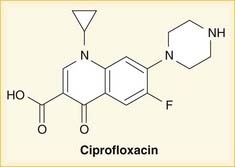
 As the name suggests, fluoroquinolones possess a fluorine ion. They all contain two six-membered rings. Ciprofloxacin is shown in the diagram (Figure 18-9). Earlier generations of fluoroquinolones were not fluorinated and were simply called quinolones. They are infrequently used now.
As the name suggests, fluoroquinolones possess a fluorine ion. They all contain two six-membered rings. Ciprofloxacin is shown in the diagram (Figure 18-9). Earlier generations of fluoroquinolones were not fluorinated and were simply called quinolones. They are infrequently used now. An isomerase converts a molecule from one isomer to another isomer. Topo- refers to topographic, or surface shape. Therefore topoisomerase enzymes convert DNA molecules from one shape to another shape.
An isomerase converts a molecule from one isomer to another isomer. Topo- refers to topographic, or surface shape. Therefore topoisomerase enzymes convert DNA molecules from one shape to another shape.Aminoglycosides
MOA (Mechanism of Action)
 The production of proteins from mRNA is called translation; this step requires ribosomes, transfer RNA (tRNA), and messenger RNA (mRNA) (Figure 18-10).
The production of proteins from mRNA is called translation; this step requires ribosomes, transfer RNA (tRNA), and messenger RNA (mRNA) (Figure 18-10). mRNA contains the sequencing code based on DNA; tRNA contains single amino acids and binds to a three-base sequence of mRNA; ribosomes are like assembly line processing machinery, bringing the mRNA and tRNA together to assemble sequences of amino acids.
mRNA contains the sequencing code based on DNA; tRNA contains single amino acids and binds to a three-base sequence of mRNA; ribosomes are like assembly line processing machinery, bringing the mRNA and tRNA together to assemble sequences of amino acids. The ribosomes are different sizes:
The ribosomes are different sizes:
 Ribosomes have different binding sites, named:
Ribosomes have different binding sites, named:
 There are two theories about how aminoglycosides work:
There are two theories about how aminoglycosides work:
 Many other protein synthesis inhibitors are bacteriostatic (only inhibit replication of bacteria versus killing bacteria). The action of aminoglycosides on the outer bacterial membrane, in addition to its protein synthesis inhibition, is thought to be the reason that aminoglycosides are bactericidal.
Many other protein synthesis inhibitors are bacteriostatic (only inhibit replication of bacteria versus killing bacteria). The action of aminoglycosides on the outer bacterial membrane, in addition to its protein synthesis inhibition, is thought to be the reason that aminoglycosides are bactericidal.Pharmacokinetics
 Penetration of biologic membranes is poor because of the drug’s polar structure. Therefore all aminoglycosides are poorly absorbed in the GI tract and are not administered orally, and intracellular concentrations are usually low.
Penetration of biologic membranes is poor because of the drug’s polar structure. Therefore all aminoglycosides are poorly absorbed in the GI tract and are not administered orally, and intracellular concentrations are usually low.
 The most common route of administration is the intravenous route, but tobramycin can be inhaled (for treatment of severe pneumonia), and some aminoglycosides are available as topical drops for the ears or eyes.
The most common route of administration is the intravenous route, but tobramycin can be inhaled (for treatment of severe pneumonia), and some aminoglycosides are available as topical drops for the ears or eyes. Aminoglycosides are rapidly excreted by glomerular filtration in the kidney, resulting in a plasma half-life of 2 hours in a patient with normal renal function, but up to 30 to 60 hours in patients who have nonfunctioning kidneys. Therefore repeat doses in patients with renal dysfunction will lead to very high levels in the body and will cause toxicity.
Aminoglycosides are rapidly excreted by glomerular filtration in the kidney, resulting in a plasma half-life of 2 hours in a patient with normal renal function, but up to 30 to 60 hours in patients who have nonfunctioning kidneys. Therefore repeat doses in patients with renal dysfunction will lead to very high levels in the body and will cause toxicity.
 Aminoglycosides have a narrow therapeutic index. Toxicity occurs at a serum concentration just slightly higher than the therapeutic concentration.
Aminoglycosides have a narrow therapeutic index. Toxicity occurs at a serum concentration just slightly higher than the therapeutic concentration. Paromomycin is effective against GI parasites and is poorly absorbed in the GI tract. Administering this aminoglycoside results in high levels of drug inside the GI tract (where the parasite exists) with negligible systemic absorption.
Paromomycin is effective against GI parasites and is poorly absorbed in the GI tract. Administering this aminoglycoside results in high levels of drug inside the GI tract (where the parasite exists) with negligible systemic absorption.Side Effects
 Nephrotoxicity
Nephrotoxicity
 Drug accumulates in proximal tubule cells, leading to mitochondrial poisoning and cell membrane disruptions. If the serum creatinine starts to rise during administration, then there must be a very high suspicion that kidney damage secondary to the aminoglycoside is occurring. The aminoglycoside should be immediately stopped.
Drug accumulates in proximal tubule cells, leading to mitochondrial poisoning and cell membrane disruptions. If the serum creatinine starts to rise during administration, then there must be a very high suspicion that kidney damage secondary to the aminoglycoside is occurring. The aminoglycoside should be immediately stopped.Important Notes
 Serum levels of aminoglycosides must be monitored. Peak and trough levels help determine required doses.
Serum levels of aminoglycosides must be monitored. Peak and trough levels help determine required doses. Amikacin is particularly effective when used against bacteria that are resistant to other aminoglycosides, because its chemical structure makes it less susceptible to inactivating enzymes.
Amikacin is particularly effective when used against bacteria that are resistant to other aminoglycosides, because its chemical structure makes it less susceptible to inactivating enzymes.Advanced
 Neuromuscular blockade occurs because of a reduction in acetylcholine release. These drugs do not paralyze patients, but under anesthesia in patients who are already receiving a neuromuscular blocking drug (such as rocuronium or vecuronium), the duration of blockade can be longer than normal. Other conditions in which neuromuscular blockade could become problematic are:
Neuromuscular blockade occurs because of a reduction in acetylcholine release. These drugs do not paralyze patients, but under anesthesia in patients who are already receiving a neuromuscular blocking drug (such as rocuronium or vecuronium), the duration of blockade can be longer than normal. Other conditions in which neuromuscular blockade could become problematic are:
 In patients on dialysis, about 65% to 75% of a given dose will be removed by a single treatment of dialysis. The objective with antibiotics is to maintain a concentration in the blood that will kill bacteria (and not be toxic to the patient); if the drug is immediately removed after it is administered, this objective will not be met. Common sense dictates, therefore, that the drug should be given after a dialysis run and not before.
In patients on dialysis, about 65% to 75% of a given dose will be removed by a single treatment of dialysis. The objective with antibiotics is to maintain a concentration in the blood that will kill bacteria (and not be toxic to the patient); if the drug is immediately removed after it is administered, this objective will not be met. Common sense dictates, therefore, that the drug should be given after a dialysis run and not before.FYI
 Nomenclature:
Nomenclature:
 The suffix –mycin is for drugs that are derived from Streptomyces, a genus of bacteria commonly found in soil. Streptomycin is named after Streptomyces, but it is no longer commonly used.
The suffix –mycin is for drugs that are derived from Streptomyces, a genus of bacteria commonly found in soil. Streptomycin is named after Streptomyces, but it is no longer commonly used. The suffix –micin is for drugs that are derived from Micromonospora, also a genus of bacteria found in soil.
The suffix –micin is for drugs that are derived from Micromonospora, also a genus of bacteria found in soil. The S in 50S and 23S refers to Svedberg units. A Svedberg unit is a measurement of time and equals 10-13 seconds. It is used to describe speed of sedimentation during centrifuging. Combining two molecules (30S + 50S) and predicting the combined Svedberg units is not simply an additive process because the surface area changes. Thus in the bacterial ribosome, 30S + 50S = 70S (and not 80).
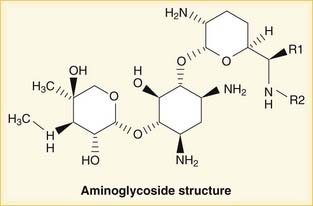
The S in 50S and 23S refers to Svedberg units. A Svedberg unit is a measurement of time and equals 10-13 seconds. It is used to describe speed of sedimentation during centrifuging. Combining two molecules (30S + 50S) and predicting the combined Svedberg units is not simply an additive process because the surface area changes. Thus in the bacterial ribosome, 30S + 50S = 70S (and not 80). Glycosides are molecules produced in nature that contain sugar molecules. Therefore aminoglycosides contain amino groups connected to sugar molecules. Gentamicin, with three sugar molecules and five amino groups, is shown in Figure 18-11.
Glycosides are molecules produced in nature that contain sugar molecules. Therefore aminoglycosides contain amino groups connected to sugar molecules. Gentamicin, with three sugar molecules and five amino groups, is shown in Figure 18-11. Tobramycin is available in an inhaled form and is used for patients with cystic fibrosis who develop chronic gram-negative infections (particularly Pseudomonas). Inhaled administration increases the dose of drug delivered to the site of infection.
Tobramycin is available in an inhaled form and is used for patients with cystic fibrosis who develop chronic gram-negative infections (particularly Pseudomonas). Inhaled administration increases the dose of drug delivered to the site of infection.Lincosamides
MOA (Mechanism of Action)
 The production of proteins from mRNA is called translation; this step requires ribosomes, tRNA, and mRNA.
The production of proteins from mRNA is called translation; this step requires ribosomes, tRNA, and mRNA. mRNA contains the sequencing code based on DNA; tRNA contains single amino acids and binds to a three-base sequence of mRNA. Ribosomes are like “assembly line” processing machinery, bringing the mRNA and tRNA together to assemble sequences of amino acids (Figure 18-12).
mRNA contains the sequencing code based on DNA; tRNA contains single amino acids and binds to a three-base sequence of mRNA. Ribosomes are like “assembly line” processing machinery, bringing the mRNA and tRNA together to assemble sequences of amino acids (Figure 18-12). The ribosomes are different sizes:
The ribosomes are different sizes:
 Ribosomes have different binding sites, named:
Ribosomes have different binding sites, named:
 Lincosamides bind the 23S rRNA molecule of the 50S RSU and inhibit peptidyl transferase, blocking the transfer of the new amino acid onto the growing chain.
Lincosamides bind the 23S rRNA molecule of the 50S RSU and inhibit peptidyl transferase, blocking the transfer of the new amino acid onto the growing chain. Inhibition of protein synthesis does not typically kill bacteria cells, so these agents are generally bacteriostatic but in high concentrations can be bactericidal.
Inhibition of protein synthesis does not typically kill bacteria cells, so these agents are generally bacteriostatic but in high concentrations can be bactericidal. Clindamycin, because of its action on protein synthesis, has been postulated to be beneficial in toxin-producing infections; most bacterially produced toxins are proteins, so an added benefit of a protein synthesis inhibitor is reduced toxin production, in addition to slowed bacterial growth.
Clindamycin, because of its action on protein synthesis, has been postulated to be beneficial in toxin-producing infections; most bacterially produced toxins are proteins, so an added benefit of a protein synthesis inhibitor is reduced toxin production, in addition to slowed bacterial growth.Mechanisms of Resistance
 Mutation of the ribosomal receptor site or modification of the receptor by a methylase enzyme results in decreased binding to the 50S subunit.
Mutation of the ribosomal receptor site or modification of the receptor by a methylase enzyme results in decreased binding to the 50S subunit. Some bacteria possess an efflux-based pump mechanism (which lowers bacterial intracellular drug levels and confers resistance) to other ribosomal inhibitors, such as macrolides. However, bacteria that are resistant to macrolides because of the presence of these efflux pumps are not resistant to clindamycin. Even though these drugs act very similarly, they are chemically different, and clindamycin is unaffected by the pump.
Some bacteria possess an efflux-based pump mechanism (which lowers bacterial intracellular drug levels and confers resistance) to other ribosomal inhibitors, such as macrolides. However, bacteria that are resistant to macrolides because of the presence of these efflux pumps are not resistant to clindamycin. Even though these drugs act very similarly, they are chemically different, and clindamycin is unaffected by the pump.
Pharmacokinetics
 Clindamycin is well absorbed orally and is metabolized by the liver. Dose adjustments are not required in patients with renal dysfunction but are required in those with severe hepatic dysfunction.
Clindamycin is well absorbed orally and is metabolized by the liver. Dose adjustments are not required in patients with renal dysfunction but are required in those with severe hepatic dysfunction. Clindamycin penetrates bone well and is therefore effective for dental infections that might have bony involvement.
Clindamycin penetrates bone well and is therefore effective for dental infections that might have bony involvement. Clindamycin does not penetrate into the brain very well; therefore, it should not be used for CNS infections.
Clindamycin does not penetrate into the brain very well; therefore, it should not be used for CNS infections. Coadministration of two different antibiotic classes that both bind the 50S subunit (e.g., lincosamides, oxazolidinones, macrolides) is not recommended because although the drugs bind different sites on the subunit, they can displace each other and result in being less effective than if administered alone.
Coadministration of two different antibiotic classes that both bind the 50S subunit (e.g., lincosamides, oxazolidinones, macrolides) is not recommended because although the drugs bind different sites on the subunit, they can displace each other and result in being less effective than if administered alone.Side Effects
 Pseudomembranous colitis (aka C. difficile colitis or “C diff” colitis) may occur. Bacterial flora of the colon changes with antibiotic administration. Some antibiotics (clindamycin is the worst offender) wipe out the normal flora and enable pathologic flora to grow, resulting in inflammation of the colon and diarrhea. This is a major problem and requires a second antibiotic (usually metronidazole or vancomycin) to be used to treat the diarrhea, which can be moderate to severe in intensity. C. difficile infections can sometimes be very difficult to eradicate.
Pseudomembranous colitis (aka C. difficile colitis or “C diff” colitis) may occur. Bacterial flora of the colon changes with antibiotic administration. Some antibiotics (clindamycin is the worst offender) wipe out the normal flora and enable pathologic flora to grow, resulting in inflammation of the colon and diarrhea. This is a major problem and requires a second antibiotic (usually metronidazole or vancomycin) to be used to treat the diarrhea, which can be moderate to severe in intensity. C. difficile infections can sometimes be very difficult to eradicate.Important Notes
 Although a very effective antibiotic for gram-positive infections, the extremely high incidence of C. difficile colitis severely limits the use of clindamycin when other, alternative antibiotics can be used effectively.
Although a very effective antibiotic for gram-positive infections, the extremely high incidence of C. difficile colitis severely limits the use of clindamycin when other, alternative antibiotics can be used effectively. Clindamycin is commonly used in infections in and around the oral cavity because of the high preponderance of anaerobic bacteria causing these infections.
Clindamycin is commonly used in infections in and around the oral cavity because of the high preponderance of anaerobic bacteria causing these infections. Toxic shock syndrome is an inflammatory response resulting from a Staphylococcus or Streptococcus infection by a strain of bacteria that produces proteins that function as superantigens and trigger a full-blown inflammatory response. Clindamycin is thought to be helpful with these infections because it inhibits the production of the superantigen protein.
Toxic shock syndrome is an inflammatory response resulting from a Staphylococcus or Streptococcus infection by a strain of bacteria that produces proteins that function as superantigens and trigger a full-blown inflammatory response. Clindamycin is thought to be helpful with these infections because it inhibits the production of the superantigen protein. Necrotizing fasciitis is also called flesh-eating disease and is caused by group A streptococci (GAS). GAS is also a toxin-producing strain and produces a superantigen that can cause a dramatic, amplified, and virtually unregulated inflammatory cascade resulting in life-threatening illness. In addition, the infection causes severe necrosis of soft tissues and spreads faster than antibiotics can treat (because blood vessels are destroyed in the area of the infection and intravenous antibiotics cannot actually be delivered to the site of the infection). Management requires surgical removal of the infected tissue (sometimes limb amputation) and high-dose antibiotics.
Necrotizing fasciitis is also called flesh-eating disease and is caused by group A streptococci (GAS). GAS is also a toxin-producing strain and produces a superantigen that can cause a dramatic, amplified, and virtually unregulated inflammatory cascade resulting in life-threatening illness. In addition, the infection causes severe necrosis of soft tissues and spreads faster than antibiotics can treat (because blood vessels are destroyed in the area of the infection and intravenous antibiotics cannot actually be delivered to the site of the infection). Management requires surgical removal of the infected tissue (sometimes limb amputation) and high-dose antibiotics.FYI
 The S in 50S and 23S refers to Svedberg units. A Svedberg unit is a measurement of time and equals 10-13 seconds. It is used to describe speed of sedimentation during centrifuging. Combining two molecules (30S + 50S) and predicting the combined Svedberg units are not simply an additive process because the surface area changes. Thus in the bacterial ribosome, 30S + 50S = 70S (and not 80).
The S in 50S and 23S refers to Svedberg units. A Svedberg unit is a measurement of time and equals 10-13 seconds. It is used to describe speed of sedimentation during centrifuging. Combining two molecules (30S + 50S) and predicting the combined Svedberg units are not simply an additive process because the surface area changes. Thus in the bacterial ribosome, 30S + 50S = 70S (and not 80).Tetracyclines
MOA (Mechanism of Action)
 The production of proteins from mRNA is called translation; this step requires ribosomes, transfer RNA (tRNA), and messenger RNA (mRNA) (Figure 18-13).
The production of proteins from mRNA is called translation; this step requires ribosomes, transfer RNA (tRNA), and messenger RNA (mRNA) (Figure 18-13). mRNA contains the sequencing code based on DNA; tRNA contains single amino acids and binds to a three-base sequence of mRNA; ribosomes are like “assembly line” processing machinery, bringing the mRNA and tRNA together to assemble sequences of amino acids.
mRNA contains the sequencing code based on DNA; tRNA contains single amino acids and binds to a three-base sequence of mRNA; ribosomes are like “assembly line” processing machinery, bringing the mRNA and tRNA together to assemble sequences of amino acids. The ribosomes are different sizes:
The ribosomes are different sizes:
 Ribosomes have different binding sites, named:
Ribosomes have different binding sites, named:
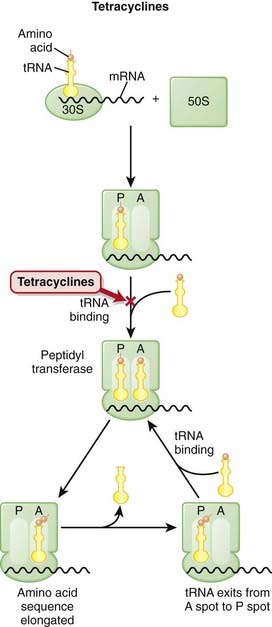
 Tetracyclines must first enter the microorganism before they can exert their antimicrobial effects. Microbes that actively take in tetracycline develop increased susceptibility to the drug because intracellular levels of the drug are high.
Tetracyclines must first enter the microorganism before they can exert their antimicrobial effects. Microbes that actively take in tetracycline develop increased susceptibility to the drug because intracellular levels of the drug are high. Tetracyclines bind reversibly to the 16S subunit of the 30S RSU and inhibit translation (protein synthesis). Binding of tetracycline to the ribosome weakens the ribosome-tRNA interaction; this prevents addition of amino acids to the growing peptide. This is in contrast to antibiotics that bind the 23S subunit and inhibit the initiation of translation.
Tetracyclines bind reversibly to the 16S subunit of the 30S RSU and inhibit translation (protein synthesis). Binding of tetracycline to the ribosome weakens the ribosome-tRNA interaction; this prevents addition of amino acids to the growing peptide. This is in contrast to antibiotics that bind the 23S subunit and inhibit the initiation of translation. Because tetracyclines stop protein synthesis, they are bacteriostatic. When drug levels fall, the protein synthesis can continue.
Because tetracyclines stop protein synthesis, they are bacteriostatic. When drug levels fall, the protein synthesis can continue. Mammalian cells lack the active transport system that bacteria use to take up tetracycline; this provides part of the basis of selectivity of tetracyclines on microbes and not the host. Different ribosome shapes and sizes also confer selectivity of bacterial versus human ribosome binding.
Mammalian cells lack the active transport system that bacteria use to take up tetracycline; this provides part of the basis of selectivity of tetracyclines on microbes and not the host. Different ribosome shapes and sizes also confer selectivity of bacterial versus human ribosome binding. Demeclocycline has a further action, which is to inhibit the binding of antidiuretic hormone (ADH) to its receptor. This is clinically important and is the basis for using this drug for treatment of a condition in which ADH levels are too high.
Demeclocycline has a further action, which is to inhibit the binding of antidiuretic hormone (ADH) to its receptor. This is clinically important and is the basis for using this drug for treatment of a condition in which ADH levels are too high.Mechanisms of Resistance
 There are three commonly recognized mechanisms by which tetracycline resistance is conferred to organisms:
There are three commonly recognized mechanisms by which tetracycline resistance is conferred to organisms:
Pharmacokinetics
 Tetracyclines are bound and inactivated by divalent cations such as calcium and magnesium, and co-ingestion of these agents (e.g., in the form of calcium supplements or antacids) interferes with the effectiveness of tetracyclines. Therefore, tetracyclines should be taken on an empty stomach.
Tetracyclines are bound and inactivated by divalent cations such as calcium and magnesium, and co-ingestion of these agents (e.g., in the form of calcium supplements or antacids) interferes with the effectiveness of tetracyclines. Therefore, tetracyclines should be taken on an empty stomach. All tetracyclines are excreted in urine and bile; doses should be reduced in patients with advanced renal dysfunction. Doxycycline is less dependent on renal excretion, because a lower fraction is excreted renally.
All tetracyclines are excreted in urine and bile; doses should be reduced in patients with advanced renal dysfunction. Doxycycline is less dependent on renal excretion, because a lower fraction is excreted renally.Side Effects
 GI: Significant nausea, vomiting, and diarrhea are caused by direct irritation to the GI tract. This is more of a problem with tetracycline than the other tetracyclines.
GI: Significant nausea, vomiting, and diarrhea are caused by direct irritation to the GI tract. This is more of a problem with tetracycline than the other tetracyclines. Mottling of teeth: Because of binding with calcium, this is a particular problem in newborns; therefore it is contraindicated in pregnancy and lactation. Furthermore, it can permanently stain teeth in children whose adult teeth are still being formed and is therefore contraindicated in children under the age of 8.
Mottling of teeth: Because of binding with calcium, this is a particular problem in newborns; therefore it is contraindicated in pregnancy and lactation. Furthermore, it can permanently stain teeth in children whose adult teeth are still being formed and is therefore contraindicated in children under the age of 8. Photosensitivity: This results in skin damage caused by sunlight (ultraviolet-A in particular). It resembles an exaggerated sunburn and can involve blistering. The exact mechanism is not fully elucidated. Absorbed photons are converted into chemical energy, such as oxygen free radicals. These species then result in chemical damage to nearby tissues. Of particular interest is the fact that tetracycline is used for malaria prophylaxis, and many regions in which malaria is endemic are very sunny.
Photosensitivity: This results in skin damage caused by sunlight (ultraviolet-A in particular). It resembles an exaggerated sunburn and can involve blistering. The exact mechanism is not fully elucidated. Absorbed photons are converted into chemical energy, such as oxygen free radicals. These species then result in chemical damage to nearby tissues. Of particular interest is the fact that tetracycline is used for malaria prophylaxis, and many regions in which malaria is endemic are very sunny. Superinfection: Superinfection is a rare but serious side effect. Because of the wide spectrum of activity, tetracyclines can wipe out normal flora, which provides an opportunistic environment in which pathogens can grow. This complication is more common in patients who are also immunocompromised.
Superinfection: Superinfection is a rare but serious side effect. Because of the wide spectrum of activity, tetracyclines can wipe out normal flora, which provides an opportunistic environment in which pathogens can grow. This complication is more common in patients who are also immunocompromised. Diabetes insipidus: Demeclocycline blocks ADH and therefore can cause diabetes insipidus (water wasting in the urine because of impaired water reabsorption in the collecting duct) if it is used in patients who do not have syndrome of inappropriate ADH secretion (SIADH).
Diabetes insipidus: Demeclocycline blocks ADH and therefore can cause diabetes insipidus (water wasting in the urine because of impaired water reabsorption in the collecting duct) if it is used in patients who do not have syndrome of inappropriate ADH secretion (SIADH).Important Notes
 Although demeclocycline is classified as an antibiotic, its clinical use is pretty much limited to treating patients diagnosed with SIADH. Remember that a diuretic produces increased urine volume, so ADH works to decrease urine volume (via reabsorption of water).
Although demeclocycline is classified as an antibiotic, its clinical use is pretty much limited to treating patients diagnosed with SIADH. Remember that a diuretic produces increased urine volume, so ADH works to decrease urine volume (via reabsorption of water). Tigecycline is a new tetracycline and is classified as a glycylcycline; antibiotics of this class are derivatives of tetracyclines and were designed to overcome the two primary methods of resistance—namely, efflux pumps and ribosomal protection.
Tigecycline is a new tetracycline and is classified as a glycylcycline; antibiotics of this class are derivatives of tetracyclines and were designed to overcome the two primary methods of resistance—namely, efflux pumps and ribosomal protection. Tetracycline spontaneously degrades over time (before it is ingested). Ingesting outdated tetracycline is dangerous because the degradation products are nephrotoxic and can cause a condition called Fanconi’s syndrome, which is characterized by damage to the proximal tubules, resulting in impaired reabsorption of glucose, amino acids, and other compounds from the ultrafiltrate within the tubule.
Tetracycline spontaneously degrades over time (before it is ingested). Ingesting outdated tetracycline is dangerous because the degradation products are nephrotoxic and can cause a condition called Fanconi’s syndrome, which is characterized by damage to the proximal tubules, resulting in impaired reabsorption of glucose, amino acids, and other compounds from the ultrafiltrate within the tubule.Advanced
 Porphyria cutanea tarda is a form of porphyria that affects the skin. Porphyria is a group of diseases caused by enzyme deficiencies resulting in accumulation of porphyrins, which are precursors to heme. Tetracycline can cause a rare form of phototoxicity that mimics porphyria and is called pseudoporphyria (but does not involve porphyrins).
Porphyria cutanea tarda is a form of porphyria that affects the skin. Porphyria is a group of diseases caused by enzyme deficiencies resulting in accumulation of porphyrins, which are precursors to heme. Tetracycline can cause a rare form of phototoxicity that mimics porphyria and is called pseudoporphyria (but does not involve porphyrins). Four other ribosomal proteins (S3, S8, S14, and S19) are important for tetracycline binding, but these are low affinity binding.
Four other ribosomal proteins (S3, S8, S14, and S19) are important for tetracycline binding, but these are low affinity binding. Stenotrophomonas is a genus of bacteria that was originally classified as Pseudomonas. It is usually susceptible to sulfa antibiotics, but if it is resistant to sulfa antibiotics it is sometimes susceptible only to minocycline.
Stenotrophomonas is a genus of bacteria that was originally classified as Pseudomonas. It is usually susceptible to sulfa antibiotics, but if it is resistant to sulfa antibiotics it is sometimes susceptible only to minocycline.FYI
 The class of tetracyclines was first discovered in the 1940s, but tetracycline itself was not the first drug in this class.
The class of tetracyclines was first discovered in the 1940s, but tetracycline itself was not the first drug in this class. Chlortetracycline was the first tetracycline (and thus the true prototype). However, it is no longer used clinically.
Chlortetracycline was the first tetracycline (and thus the true prototype). However, it is no longer used clinically. A modification of tetracyclines has resulted in a new class of antibiotic called glycylcyclines (tigecycline). These chemicals contain an N-O-N group added to the tetracycline. They were introduced in 2006, and this class of antibiotics can overcome some forms of tetracycline resistance.
A modification of tetracyclines has resulted in a new class of antibiotic called glycylcyclines (tigecycline). These chemicals contain an N-O-N group added to the tetracycline. They were introduced in 2006, and this class of antibiotics can overcome some forms of tetracycline resistance. Humans carry a 30S ribosome subunit within their mitochondria. However, the side effects of tetracyclines do not involve the mitochondria.
Humans carry a 30S ribosome subunit within their mitochondria. However, the side effects of tetracyclines do not involve the mitochondria. Tetracycline is produced by Streptomyces bacteria. Some tetracyclines are natural, and some are modified or synthetic.
Tetracycline is produced by Streptomyces bacteria. Some tetracyclines are natural, and some are modified or synthetic. The S in 50S and 23S refers to Svedberg units. A Svedberg unit is a measurement of time and equals 10-13 seconds. It is used to describe speed of sedimentation during centrifuging. Combining two molecules (30S + 50S) and predicting the combined Svedberg units are not simply an additive process because the surface area changes. Thus in the bacterial ribosome, 30S + 50S = 70S (and not 80).
The S in 50S and 23S refers to Svedberg units. A Svedberg unit is a measurement of time and equals 10-13 seconds. It is used to describe speed of sedimentation during centrifuging. Combining two molecules (30S + 50S) and predicting the combined Svedberg units are not simply an additive process because the surface area changes. Thus in the bacterial ribosome, 30S + 50S = 70S (and not 80).Macrolides
MOA (Mechanism OF Action)
 The production of proteins from mRNA is called translation; this step requires ribosomes, transfer RNA (tRNA), and messenger RNA (mRNA) (Figure 18-14).
The production of proteins from mRNA is called translation; this step requires ribosomes, transfer RNA (tRNA), and messenger RNA (mRNA) (Figure 18-14). mRNA contains the sequencing code based on DNA; tRNA contains single amino acids and binds to a three-base sequence of mRNA; ribosomes are like assembly line processing machinery, bringing the mRNA and tRNA together to assemble sequences of amino acids.
mRNA contains the sequencing code based on DNA; tRNA contains single amino acids and binds to a three-base sequence of mRNA; ribosomes are like assembly line processing machinery, bringing the mRNA and tRNA together to assemble sequences of amino acids. The ribosomes are different sizes:
The ribosomes are different sizes:
 Ribosomes have different binding sites, named:
Ribosomes have different binding sites, named:
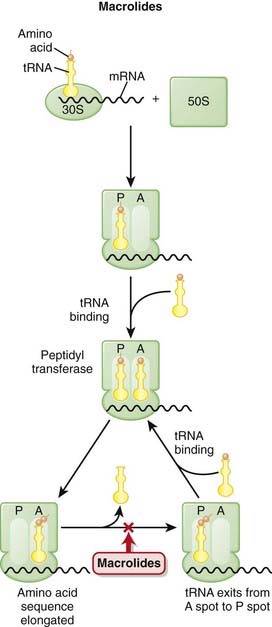
 Macrolides bind the 23S rRNA molecule of the 50S RSU and inhibit peptidyl transferase, blocking the transfer of the new amino acid onto the growing chain.
Macrolides bind the 23S rRNA molecule of the 50S RSU and inhibit peptidyl transferase, blocking the transfer of the new amino acid onto the growing chain. Inhibition of protein synthesis does not typically kill bacteria cells, so these agents are generally bacteriostatic, but in high concentrations they can be bactericidal.
Inhibition of protein synthesis does not typically kill bacteria cells, so these agents are generally bacteriostatic, but in high concentrations they can be bactericidal. Macrolides are phagocytosed by macrophages, which is a benefit because WBCs preferentially travel to sites of infection, thereby theoretically delivering the drug to the site at which it is needed. This is very convenient.
Macrolides are phagocytosed by macrophages, which is a benefit because WBCs preferentially travel to sites of infection, thereby theoretically delivering the drug to the site at which it is needed. This is very convenient.Mechanisms of Resistance
 Modification of the RSU binding site either by chromosomal mutation or through methylation via methylase greatly decreases the efficacy of macrolides; bacterial methylase can be produced constitutively (all the time) or can be induced.
Modification of the RSU binding site either by chromosomal mutation or through methylation via methylase greatly decreases the efficacy of macrolides; bacterial methylase can be produced constitutively (all the time) or can be induced. Reduced intracellular concentrations are found within the bacterium, through either reduced permeability of cell membrane to macrolides or, probably more important, increased efflux of macrolides via active pumps.
Reduced intracellular concentrations are found within the bacterium, through either reduced permeability of cell membrane to macrolides or, probably more important, increased efflux of macrolides via active pumps.Pharmacokinetics
 Half-lives of erythromycin, clarithromycin, and azithromycin are 1.5, 6, and 68 hours, and administration is four times daily, twice daily, and once daily, respectively. At high doses, clarithromycin is sometimes administered once a day.
Half-lives of erythromycin, clarithromycin, and azithromycin are 1.5, 6, and 68 hours, and administration is four times daily, twice daily, and once daily, respectively. At high doses, clarithromycin is sometimes administered once a day. Azithromycin contains an additional nitrogen molecule in the macrolide ring to make it a 15-atom ring. This imparts increased stability of the ring. A very high amount of drug is distributed intracellularly; although distribution into tissues is excellent, it is not distributed into CNS (and therefore is not useful for CNS infections). The intracellular distribution contributes to its long half-life.
Azithromycin contains an additional nitrogen molecule in the macrolide ring to make it a 15-atom ring. This imparts increased stability of the ring. A very high amount of drug is distributed intracellularly; although distribution into tissues is excellent, it is not distributed into CNS (and therefore is not useful for CNS infections). The intracellular distribution contributes to its long half-life. Erythromycin and clarithromycin are significant CYP450 enzyme inhibitors and are metabolized by the liver. Drug interactions with other CYP450 inhibitors should be monitored.
Erythromycin and clarithromycin are significant CYP450 enzyme inhibitors and are metabolized by the liver. Drug interactions with other CYP450 inhibitors should be monitored. Erythromycin is unstable in gastric acid and therefore must be administered with salts or esters or via enteric-coated tablets when administered orally.
Erythromycin is unstable in gastric acid and therefore must be administered with salts or esters or via enteric-coated tablets when administered orally. The addition of a methyl group to erythromycin creates clarithromycin, and the addition of a methylated nitrogen to erythromycin creates azithromycin; both are stable in gastric acid and very well absorbed orally.
The addition of a methyl group to erythromycin creates clarithromycin, and the addition of a methylated nitrogen to erythromycin creates azithromycin; both are stable in gastric acid and very well absorbed orally. Because of the long duration of action of azithromycin, a 5-day, oral, once-a-day course for most sensitive infections is considered a treatment of adequate duration.
Because of the long duration of action of azithromycin, a 5-day, oral, once-a-day course for most sensitive infections is considered a treatment of adequate duration.Indications
 In addition to antibacterial activity, erythromycin specifically is used to enhance GI motility. Through direct stimulation of the GI tract, this mechanism is responsible both for the enhanced motility that is of benefit in patients who have dysmotility and also, unfortunately, for the GI intolerance (side effects) in patients who have normal motility.
In addition to antibacterial activity, erythromycin specifically is used to enhance GI motility. Through direct stimulation of the GI tract, this mechanism is responsible both for the enhanced motility that is of benefit in patients who have dysmotility and also, unfortunately, for the GI intolerance (side effects) in patients who have normal motility.
Important Notes
 The short duration of treatment, good oral absorption, once-a-day administration, and low side effect profile are all favorable characteristics for an antibiotic and have contributed to azithromycin’s popularity.
The short duration of treatment, good oral absorption, once-a-day administration, and low side effect profile are all favorable characteristics for an antibiotic and have contributed to azithromycin’s popularity.FYI
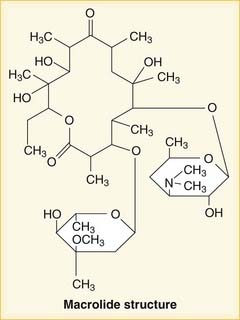
 A macrolide ring is a 12-, 14-, or 16-atom ring made of 11 carbon and three oxygen molecules. Two sugar molecules are attached to the macrolide ring. Erythromycin is shown in Figure 18-15.
A macrolide ring is a 12-, 14-, or 16-atom ring made of 11 carbon and three oxygen molecules. Two sugar molecules are attached to the macrolide ring. Erythromycin is shown in Figure 18-15. Nomenclature of macrolides is similar to that of aminoglycosides (gentamicin) except that for macrolides, thro precedes the mycin.
Nomenclature of macrolides is similar to that of aminoglycosides (gentamicin) except that for macrolides, thro precedes the mycin. Tacrolimus is technically also a macrolide, but it is not an antibiotic. It is an immunosuppressive.
Tacrolimus is technically also a macrolide, but it is not an antibiotic. It is an immunosuppressive.Oxazolidinones
MOA (Mechanism of Action)
 The production of proteins from mRNA is called translation; this step requires ribosomes, transfer RNA (tRNA), and messenger RNA (mRNA) (Figure 18-16).
The production of proteins from mRNA is called translation; this step requires ribosomes, transfer RNA (tRNA), and messenger RNA (mRNA) (Figure 18-16). mRNA contains the sequencing code based on DNA; tRNA contains single amino acids and binds to a three-base sequence of mRNA; ribosomes are like assembly line processing machinery, bringing the mRNA and tRNA together to assemble sequences of amino acids.
mRNA contains the sequencing code based on DNA; tRNA contains single amino acids and binds to a three-base sequence of mRNA; ribosomes are like assembly line processing machinery, bringing the mRNA and tRNA together to assemble sequences of amino acids. The ribosomes are different sizes:
The ribosomes are different sizes:
 Ribosomes have different binding sites, named:
Ribosomes have different binding sites, named:
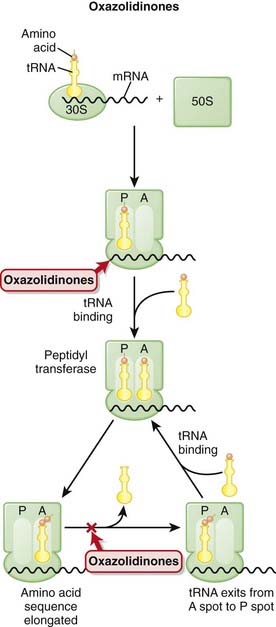
 Linezolid blocks the translocation step of protein synthesis by binding the 23S rRNA of the 50S RSU.
Linezolid blocks the translocation step of protein synthesis by binding the 23S rRNA of the 50S RSU.Pharmacokinetics
Side Effects
 Serotonin syndrome is a condition characterized by high levels of serotonergic activity in the brain. It occurs in patients who are taking drugs that increase the amount of available serotonin in the brain. Drugs in the selective serotonin reuptake inhibitor (SSRI) class of antidepressants are the most common offenders, but drugs that possess MAOI activity can also produce the syndrome.
Serotonin syndrome is a condition characterized by high levels of serotonergic activity in the brain. It occurs in patients who are taking drugs that increase the amount of available serotonin in the brain. Drugs in the selective serotonin reuptake inhibitor (SSRI) class of antidepressants are the most common offenders, but drugs that possess MAOI activity can also produce the syndrome. Hyperlactatemia and metabolic acidosis are probably caused by mitochondrial inhibition. The lactate is produced by the cells that cannot undergo aerobic metabolism because of the mitochondrial suppression, and the accumulation of lactate, which is an acid, produces metabolic acidosis.
Hyperlactatemia and metabolic acidosis are probably caused by mitochondrial inhibition. The lactate is produced by the cells that cannot undergo aerobic metabolism because of the mitochondrial suppression, and the accumulation of lactate, which is an acid, produces metabolic acidosis. Nerve damage: Central and peripheral neuropathies have been reported. The nerve damage has occurred at the same time as the lactic acidosis and therefore could also be mediated by mitochondrial suppression.
Nerve damage: Central and peripheral neuropathies have been reported. The nerve damage has occurred at the same time as the lactic acidosis and therefore could also be mediated by mitochondrial suppression. Hematologic: Bone marrow suppression (myelosuppression) characterized by low blood counts (platelets, RBCs, or WBCs) has been reported in patients treated with linezolid for at least 21 days. It is usually mild and is reversible within 4 weeks of stopping the drug. It is not common and usually occurs around 1 month after a patient begins to take the drug.
Hematologic: Bone marrow suppression (myelosuppression) characterized by low blood counts (platelets, RBCs, or WBCs) has been reported in patients treated with linezolid for at least 21 days. It is usually mild and is reversible within 4 weeks of stopping the drug. It is not common and usually occurs around 1 month after a patient begins to take the drug.Important Notes
 Linezolids were approved in 2000, and linezolid resistance was first reported in 2001. New drugs often have low rates of resistance, but over time, increased evolutionary pressure from exposure of the drug to bacteria results in increased levels of resistance.
Linezolids were approved in 2000, and linezolid resistance was first reported in 2001. New drugs often have low rates of resistance, but over time, increased evolutionary pressure from exposure of the drug to bacteria results in increased levels of resistance. Currently their use is generally limited to infections caused by multidrug-resistant gram-positive bacteria. Liberal use of antibiotics is associated with resistance, and therefore antibiotics that are effective against resistant organisms should not be used against organisms that are still sensitive to other antibiotics:
Currently their use is generally limited to infections caused by multidrug-resistant gram-positive bacteria. Liberal use of antibiotics is associated with resistance, and therefore antibiotics that are effective against resistant organisms should not be used against organisms that are still sensitive to other antibiotics:
Sulfonamides and Other Folate Synthesis Inhibitors
MOA (Mechanism of Action)
 Most bacteria must synthesize their own folate for survival; they cannot use external sources. In contrast, humans do not synthesize folate but rather obtain it through the diet.
Most bacteria must synthesize their own folate for survival; they cannot use external sources. In contrast, humans do not synthesize folate but rather obtain it through the diet. A few steps are required in the synthesis of folate. Specifically, sulfonamides are competitive inhibitors with p-aminobenzoic acid (PABA) in the first reaction of the folate synthesis pathway, catalyzed by the enzyme dihydropteroate synthase. Inhibiting the synthesis of tetrahydrofolate (THF) results in reduced DNA synthesis, which stops bacterial growth (a bacteriostatic effect).
A few steps are required in the synthesis of folate. Specifically, sulfonamides are competitive inhibitors with p-aminobenzoic acid (PABA) in the first reaction of the folate synthesis pathway, catalyzed by the enzyme dihydropteroate synthase. Inhibiting the synthesis of tetrahydrofolate (THF) results in reduced DNA synthesis, which stops bacterial growth (a bacteriostatic effect). Trimethoprim inhibits the last reaction of folate synthesis (catalyzed by dihydrofolate reductase) and is commonly administered in conjunction with sulfonamides for greater antibiotic efficacy. Inhibition of the folate synthesis pathway at two different steps results in a bactericidal action and is the reason why trimethoprim and sulfamethoxazole (TMP-STP) are combined and commonly used clinically for many infections.
Trimethoprim inhibits the last reaction of folate synthesis (catalyzed by dihydrofolate reductase) and is commonly administered in conjunction with sulfonamides for greater antibiotic efficacy. Inhibition of the folate synthesis pathway at two different steps results in a bactericidal action and is the reason why trimethoprim and sulfamethoxazole (TMP-STP) are combined and commonly used clinically for many infections.Mechanisms of Resistance (Figure 18-17)
 Bacteria that do not synthesize their own folate (similar to humans) are inherently completely resistant to sulfonamides.
Bacteria that do not synthesize their own folate (similar to humans) are inherently completely resistant to sulfonamides. Enzyme mutation of dihydropteroate synthase leads to reduced affinity for sulfas and results in resistance.
Enzyme mutation of dihydropteroate synthase leads to reduced affinity for sulfas and results in resistance. Lower levels of drug inside bacterial cells because of either decreased membrane permeability to sulfas or active efflux of the drug also confer resistance to organisms.
Lower levels of drug inside bacterial cells because of either decreased membrane permeability to sulfas or active efflux of the drug also confer resistance to organisms.Pharmacokinetics
 Metabolism of sulfonamides is via the liver and excretion by the kidney. In patients with advanced renal dysfunction, doses should be reduced.
Metabolism of sulfonamides is via the liver and excretion by the kidney. In patients with advanced renal dysfunction, doses should be reduced. Sulfadoxine has a long half-life (7 to 9 days), whereas the half-life of sulfamethoxazole, a commonly prescribed sulfa drug, is 10 to 12 hours.
Sulfadoxine has a long half-life (7 to 9 days), whereas the half-life of sulfamethoxazole, a commonly prescribed sulfa drug, is 10 to 12 hours.Side Effects
 Precipitation in urine: Sulfa molecules might precipitate at neutral or acidic pH, leading to crystalluria (crystals in the urine), and possibly hematuria (blood in urine secondary to reaction from crystals).
Precipitation in urine: Sulfa molecules might precipitate at neutral or acidic pH, leading to crystalluria (crystals in the urine), and possibly hematuria (blood in urine secondary to reaction from crystals). Hypersensitivity: Cross-reactivity can occur with other sulfone-containing drugs, including hydrochlorothiazide and sulfonylureas. There is little evidence to support cross-reactivity, which is usually not severe and limited to rash.
Hypersensitivity: Cross-reactivity can occur with other sulfone-containing drugs, including hydrochlorothiazide and sulfonylureas. There is little evidence to support cross-reactivity, which is usually not severe and limited to rash.Rare and Serious
 Stevens-Johnson syndrome is a rare but very serious (life-threatening) hypersensitivity reaction involving skin and mucous membranes.
Stevens-Johnson syndrome is a rare but very serious (life-threatening) hypersensitivity reaction involving skin and mucous membranes.Important Notes
 Common clinical infections treated by sulfonamides include the following:
Common clinical infections treated by sulfonamides include the following:
 Sulfasalazine contains a sulfa component but is used clinically as an antiinflammatory (because of the salicylate component) for the GI tract (because it is very poorly absorbed and thus has exposure only to the GI tract). It is described in the section on salicylates.
Sulfasalazine contains a sulfa component but is used clinically as an antiinflammatory (because of the salicylate component) for the GI tract (because it is very poorly absorbed and thus has exposure only to the GI tract). It is described in the section on salicylates.FYI
 A sulfone is an 0=S=0 group connected to two R groups. A sulfonamide is created when one of the R groups is an amide (NH2). Sulfonamides are also called sulfa drugs.
A sulfone is an 0=S=0 group connected to two R groups. A sulfonamide is created when one of the R groups is an amide (NH2). Sulfonamides are also called sulfa drugs. Sulfa drugs were discovered in the 1930s and were the only antibiotics available before penicillin. They were therefore the first antibiotics.
Sulfa drugs were discovered in the 1930s and were the only antibiotics available before penicillin. They were therefore the first antibiotics. PABA was found in sunscreens but has been replaced largely by padimate O, a derivative of PABA. It is interesting that the sulfonamides increase the risk of photosensitivity and that PABA was used to protect the skin, despite their similar chemical structures.
PABA was found in sunscreens but has been replaced largely by padimate O, a derivative of PABA. It is interesting that the sulfonamides increase the risk of photosensitivity and that PABA was used to protect the skin, despite their similar chemical structures.Metronidazole
MOA (Mechanism of Action)
 Metronidazole is a prodrug. The nitrogen group must be reduced (addition of electron) before the chemical obtains its antiinfective function. It is reduced by a nitroreductase enzyme called a ferredoxin (an iron- and sulfur-containing enzyme). The extra nitrogen side chain is reduced in this reaction.
Metronidazole is a prodrug. The nitrogen group must be reduced (addition of electron) before the chemical obtains its antiinfective function. It is reduced by a nitroreductase enzyme called a ferredoxin (an iron- and sulfur-containing enzyme). The extra nitrogen side chain is reduced in this reaction. With aerobic bacteria the electron transport chain does not require these special enzymes because oxygen is the terminal electron acceptor; therefore the prodrug is not converted to the active form of the drug. However, with anaerobic bacteria, with which oxygen is absent, these special enzymes are present. Therefore metronidazole is not activated with aerobic bacteria but is particularly effective against anaerobic bacteria.
With aerobic bacteria the electron transport chain does not require these special enzymes because oxygen is the terminal electron acceptor; therefore the prodrug is not converted to the active form of the drug. However, with anaerobic bacteria, with which oxygen is absent, these special enzymes are present. Therefore metronidazole is not activated with aerobic bacteria but is particularly effective against anaerobic bacteria. Reduction of the prodrug metronidazole results in the production of toxic products (hydroxylamine) and other free radicals that damage DNA.
Reduction of the prodrug metronidazole results in the production of toxic products (hydroxylamine) and other free radicals that damage DNA.Mechanisms of Resistance
 Some bacteria are facultatively anaerobic (can live with or without oxygen) or are microaerophiles (can live in the presence of very little oxygen). In these bacteria, the genes required to produce ferredoxin can be switched off; this would result in metronidazole not becoming reduced, and therefore it would remain in the inactive prodrug state.
Some bacteria are facultatively anaerobic (can live with or without oxygen) or are microaerophiles (can live in the presence of very little oxygen). In these bacteria, the genes required to produce ferredoxin can be switched off; this would result in metronidazole not becoming reduced, and therefore it would remain in the inactive prodrug state.Pharmacokinetics
 Metronidazole is metabolized by the liver. Dose adjustments are not required in patients with renal dysfunction but are required in those with hepatic dysfunction.
Metronidazole is metabolized by the liver. Dose adjustments are not required in patients with renal dysfunction but are required in those with hepatic dysfunction.Contraindications
FYI
 Metronidazole contains the five-member imidazole ring (three carbons + two nitrogen atoms), but there is a nitrogen side chain; it is therefore a nitroimidazole. It is in a drug category all on its own (Figure 18-19).
Metronidazole contains the five-member imidazole ring (three carbons + two nitrogen atoms), but there is a nitrogen side chain; it is therefore a nitroimidazole. It is in a drug category all on its own (Figure 18-19).Introduction to Antimycobacterials
The main anti-TB drugs and their abbreviations include the following:
Mutation Rate
 Severe, advanced pulmonary TB has approximately 1012 bacteria in the body, resulting in approximately:
Severe, advanced pulmonary TB has approximately 1012 bacteria in the body, resulting in approximately:
Standard Regimen
 Treatment involves an induction phase (high dose, more drugs) followed by a maintenance phase (fewer drugs).
Treatment involves an induction phase (high dose, more drugs) followed by a maintenance phase (fewer drugs).Ethambutol
MOA (Mechanism of Action) (Figure 18-20)
 Ethambutol disrupts formation of the tuberculous cell wall by blocking arabinosyl transferases. These enzymes attach the arabinose residue of arabinogalactan to mycolic acid.
Ethambutol disrupts formation of the tuberculous cell wall by blocking arabinosyl transferases. These enzymes attach the arabinose residue of arabinogalactan to mycolic acid.Side Effects
 Neurologic:
Neurologic:
 Optic neuritis: <1% occurrence. Inflammation of the optic nerve results in decreased visual acuity (blurred vision). Recovery usually occurs after discontinuation of the drug. This is a dose-related effect and occurs more commonly with higher doses. One theory for the MOA of ocular toxicity is related to its zinc chelating effect, but the mechanism has not been fully established.
Optic neuritis: <1% occurrence. Inflammation of the optic nerve results in decreased visual acuity (blurred vision). Recovery usually occurs after discontinuation of the drug. This is a dose-related effect and occurs more commonly with higher doses. One theory for the MOA of ocular toxicity is related to its zinc chelating effect, but the mechanism has not been fully established.
Important Notes
 Vision tests for acuity and color discrimination should be performed at the start of administration of ethambutol and should be repeated periodically, or immediately if visual problems are reported.
Vision tests for acuity and color discrimination should be performed at the start of administration of ethambutol and should be repeated periodically, or immediately if visual problems are reported.Isoniazid
Description
Isoniazid is a first-line anti-TB drug. It is used in combination with other anti-TB drugs.
MOA (Mechanism of Action) (Figure 18-21)
 The chemical steps are a bit complicated; the following is a simplified version:
The chemical steps are a bit complicated; the following is a simplified version:
 The activated compound reacts with nicotinamide adenine dinucleotide (NAD) to form an INH-NAD complex.
The activated compound reacts with nicotinamide adenine dinucleotide (NAD) to form an INH-NAD complex.Pharmacokinetics
 Isoniazid is distributed in serum, in cerebrospinal fluid (CSF), and within caseous granulomas (which often surround TB). Caseous means having a cheeselike consistency.
Isoniazid is distributed in serum, in cerebrospinal fluid (CSF), and within caseous granulomas (which often surround TB). Caseous means having a cheeselike consistency.Side Effects
 Hepatitis, which can be initially asymptomatic and detected only by hepatic enzyme elevation. Significant enzyme elevation might mandate that isoniazid be discontinued in that patient. Note that the term hepatitis here does not refer to viral hepatitis, but rather a noninfectious inflammation of the liver that is directly drug induced.
Hepatitis, which can be initially asymptomatic and detected only by hepatic enzyme elevation. Significant enzyme elevation might mandate that isoniazid be discontinued in that patient. Note that the term hepatitis here does not refer to viral hepatitis, but rather a noninfectious inflammation of the liver that is directly drug induced.Important Notes
 Baseline measurements of hepatic enzymes are required because of the risk of hepatitis. Repeat measurements as follows:
Baseline measurements of hepatic enzymes are required because of the risk of hepatitis. Repeat measurements as follows:
Advanced
 Deficiency mutations cause loss of an enzyme. Therefore if two genes (one normal and one mutated) are present, the mutated gene will be recessive because the normally functioning gene will replace the missing enzyme. Recessive INH-resistant genes include katG, ndh, msh, and nat.
Deficiency mutations cause loss of an enzyme. Therefore if two genes (one normal and one mutated) are present, the mutated gene will be recessive because the normally functioning gene will replace the missing enzyme. Recessive INH-resistant genes include katG, ndh, msh, and nat. Overexpression mutations cause too much enzyme. Therefore if two genes (one normal and one mutated) are present, the mutated gene will be dominant because the mutated gene will continue to pump out excessive enzyme even in the presence of the normal gene. Dominant INH-resistant genes include InhA, which is inhibited by INH-NAD. It is more difficult to inhibit an enzyme that is produced in high quantity.
Overexpression mutations cause too much enzyme. Therefore if two genes (one normal and one mutated) are present, the mutated gene will be dominant because the mutated gene will continue to pump out excessive enzyme even in the presence of the normal gene. Dominant INH-resistant genes include InhA, which is inhibited by INH-NAD. It is more difficult to inhibit an enzyme that is produced in high quantity.FYI
 Isoniazid was the first anti-TB drug and was discovered in 1952. The name isoniazid is derived from isonicotinic acid hydrazide.
Isoniazid was the first anti-TB drug and was discovered in 1952. The name isoniazid is derived from isonicotinic acid hydrazide.Pyrazinamide
MOA (Mechanism of Action) (Figure 18-22)
 Pyrazinamide is a prodrug. It must first be converted to pyrazinoic acid by a mycobacterial enzyme called pyrazinamidase.
Pyrazinamide is a prodrug. It must first be converted to pyrazinoic acid by a mycobacterial enzyme called pyrazinamidase. Pyrazinoic acid probably exerts its antituberculous activity via inhibition of an enzyme called fatty acid synthase I that is involved in cell wall synthesis.
Pyrazinoic acid probably exerts its antituberculous activity via inhibition of an enzyme called fatty acid synthase I that is involved in cell wall synthesis. Fatty acid synthase I activity is required for fatty acid synthesis, but the exact role that it plays in mycobacterial growth and structure is not fully elucidated.
Fatty acid synthase I activity is required for fatty acid synthesis, but the exact role that it plays in mycobacterial growth and structure is not fully elucidated.Indications
Side Effects
More Common (Less Serious)
 Increased uric acid: PZA inhibits the excretion of urate (uric acid salt) and therefore increases the probability of precipitating gout (which causes joint pain and inflammation via deposition of uric acid crystals in joints, especially the great toe).
Increased uric acid: PZA inhibits the excretion of urate (uric acid salt) and therefore increases the probability of precipitating gout (which causes joint pain and inflammation via deposition of uric acid crystals in joints, especially the great toe).Rifamycins
MOA (Mechanism of Action) (Figure 18-23)
 RNA polymerase reads DNA to produce an mRNA copy of the genetic code. Without RNA, protein synthesis cannot occur.
RNA polymerase reads DNA to produce an mRNA copy of the genetic code. Without RNA, protein synthesis cannot occur. Rifampin inhibits bacterial RNA synthesis by binding prokaryotic (bacterial) RNA polymerase. It prevents initiation of RNA synthesis (but not elongation of RNA).
Rifampin inhibits bacterial RNA synthesis by binding prokaryotic (bacterial) RNA polymerase. It prevents initiation of RNA synthesis (but not elongation of RNA). Rifampin does not bind human RNA polymerase, so its action on RNA synthesis is limited to bacterial RNA, which serves to limit its side effects.
Rifampin does not bind human RNA polymerase, so its action on RNA synthesis is limited to bacterial RNA, which serves to limit its side effects. Rifampin is bactericidal for extracellular and intracellular bacteria, but despite this, it is not commonly used as a solo agent for treatment of established infections because of the high rates of resistance that develop.
Rifampin is bactericidal for extracellular and intracellular bacteria, but despite this, it is not commonly used as a solo agent for treatment of established infections because of the high rates of resistance that develop. Rifampin is highly lipophilic. This is extremely important because it confers the property that the drug can easily cross lipophilic membranes:
Rifampin is highly lipophilic. This is extremely important because it confers the property that the drug can easily cross lipophilic membranes:
 Mycobacterial cell walls contain mycolic acids, which are long fatty acids and therefore lipids. Rifampin therefore easily and readily enters mycobacterial cells.
Mycobacterial cell walls contain mycolic acids, which are long fatty acids and therefore lipids. Rifampin therefore easily and readily enters mycobacterial cells. Biofilms (inert films that bacteria can live in) created by some bacteria generate a barrier that antibiotics generally cannot enter. However, rifampin can enter the biofilms because of its lipophilic nature.
Biofilms (inert films that bacteria can live in) created by some bacteria generate a barrier that antibiotics generally cannot enter. However, rifampin can enter the biofilms because of its lipophilic nature.Pharmacokinetics
 Rifamycins are primarily metabolized by the liver and eliminated in the bile. Does adjustments are not required in liver or renal dysfunction.
Rifamycins are primarily metabolized by the liver and eliminated in the bile. Does adjustments are not required in liver or renal dysfunction. Rifamycins are potent inducers of a number of CYP enzymes. Therefore patients on drugs broken down by CYP enzymes will require higher doses of these drugs. Important examples include warfarin, an anticoagulant; human immunodeficiency virus (HIV) medications (protease inhibitors and nonnucleoside reverse transcription inhibitors); and methadone.
Rifamycins are potent inducers of a number of CYP enzymes. Therefore patients on drugs broken down by CYP enzymes will require higher doses of these drugs. Important examples include warfarin, an anticoagulant; human immunodeficiency virus (HIV) medications (protease inhibitors and nonnucleoside reverse transcription inhibitors); and methadone.
 Aminosalicylic acid may delay the absorption of rifampin and cause inadequate plasma concentrations. If both drugs need to be administered, it is important to space out administration by as many hours as possible.
Aminosalicylic acid may delay the absorption of rifampin and cause inadequate plasma concentrations. If both drugs need to be administered, it is important to space out administration by as many hours as possible.Side Effects
Common and Mild
 Discoloration of secretions: Because of its lipophilic nature, it enters secretions readily and colors them orange or red. Such secretions include tears, urine, sweat, stool, sputum, and saliva. Contact lenses can be stained. Patients should be warned about this so that they do not become unnecessarily alarmed when it happens.
Discoloration of secretions: Because of its lipophilic nature, it enters secretions readily and colors them orange or red. Such secretions include tears, urine, sweat, stool, sputum, and saliva. Contact lenses can be stained. Patients should be warned about this so that they do not become unnecessarily alarmed when it happens. Cholestatic jaundice and hepatitis: Levels of liver enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and bilirubin can be elevated. Elevations in bilirubin usually resolve after 10 days. Rifampin blocks bilirubin excretion, and liver enzyme production increases to compensate. Isolated mild elevations in bilirubin are not an indication to stop treatment.
Cholestatic jaundice and hepatitis: Levels of liver enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and bilirubin can be elevated. Elevations in bilirubin usually resolve after 10 days. Rifampin blocks bilirubin excretion, and liver enzyme production increases to compensate. Isolated mild elevations in bilirubin are not an indication to stop treatment.Important Notes
 Staphylococcal infections (S. epidermidis and S. aureus) are commonly associated with biofilm production. Because of the lipophilic nature of rifampin, it absorbs into and crosses biofilms and can be more effective against these infections.
Staphylococcal infections (S. epidermidis and S. aureus) are commonly associated with biofilm production. Because of the lipophilic nature of rifampin, it absorbs into and crosses biofilms and can be more effective against these infections. Rifampin is indicated as a prophylactic measure in the case of exposure to a patient with meningitis caused by N. meningitidis.
Rifampin is indicated as a prophylactic measure in the case of exposure to a patient with meningitis caused by N. meningitidis.Advanced
 Many guidelines recommend rifampin as a second drug to be used in combination for some staphylococcal and streptococcal infections. However, a meta-analysis found that only orthopedic infections (bone, joint, or joint hardware) with Staphylococcus showed a significant improvement in cure rates with combination treatment.
Many guidelines recommend rifampin as a second drug to be used in combination for some staphylococcal and streptococcal infections. However, a meta-analysis found that only orthopedic infections (bone, joint, or joint hardware) with Staphylococcus showed a significant improvement in cure rates with combination treatment. Rifampin is an inducer of the P-glycoprotein (Pgp) transport system. This can result in decreased efficacy of other drugs that are transported by the Pgp system.
Rifampin is an inducer of the P-glycoprotein (Pgp) transport system. This can result in decreased efficacy of other drugs that are transported by the Pgp system.CCR5 Antagonists
MOA (Mechanism of Action)
 Several steps are involved in the replication of HIV. The virus must fuse to the host cell, uncoat, enter and be transcribed by reverse transcriptase, become incorporated into the host genome, and be transcribed to viral RNA, which is then translated into polyproteins (Figure 18-24).
Several steps are involved in the replication of HIV. The virus must fuse to the host cell, uncoat, enter and be transcribed by reverse transcriptase, become incorporated into the host genome, and be transcribed to viral RNA, which is then translated into polyproteins (Figure 18-24). Entry of HIV into the host cell begins with the attachment of the HIV envelope glycoprotein (gp120) to the CD4 T-cell receptor. This interaction produces a conformational change in gp120 that allows it to bind to a chemokine co-receptor, either CCR5 or CXCR4.
Entry of HIV into the host cell begins with the attachment of the HIV envelope glycoprotein (gp120) to the CD4 T-cell receptor. This interaction produces a conformational change in gp120 that allows it to bind to a chemokine co-receptor, either CCR5 or CXCR4. Once gp120 is bound to either CCR5 or CXCR4, further conformational changes occur that allow the HIV envelop gp41 fusion peptide to insert into the cell membrane, causing the virion to fuse with the cell membrane.
Once gp120 is bound to either CCR5 or CXCR4, further conformational changes occur that allow the HIV envelop gp41 fusion peptide to insert into the cell membrane, causing the virion to fuse with the cell membrane.Pharmacokinetics
Side Effects
 Antiretrovirals are typically administered in combination regimens, which can make it unclear how each individual drug contributes to side effects.
Antiretrovirals are typically administered in combination regimens, which can make it unclear how each individual drug contributes to side effects. Postural hypotension and syncope: Caution is advised in patients taking antihypertensives or who have a history of syncope. The mechanism is not established.
Postural hypotension and syncope: Caution is advised in patients taking antihypertensives or who have a history of syncope. The mechanism is not established.Important Notes
 In clinical trials, virologic failure typically occurred in patients exhibiting a switch in HIV-1 from CCR5 to CXCR4 or dual-mixed. However, once patients had discontinued maraviroc, they typically reverted back to CCR5.
In clinical trials, virologic failure typically occurred in patients exhibiting a switch in HIV-1 from CCR5 to CXCR4 or dual-mixed. However, once patients had discontinued maraviroc, they typically reverted back to CCR5. The switching of HIV-1 to CXCR4 after therapy with CCR5 antagonists has prompted the search for CXCR4 antagonists. These agents have not been successfully brought to market yet, because of concerns over toxicity and lack of response.
The switching of HIV-1 to CXCR4 after therapy with CCR5 antagonists has prompted the search for CXCR4 antagonists. These agents have not been successfully brought to market yet, because of concerns over toxicity and lack of response.FYI
 The development of CCR5 antagonists was inspired by the observation that individuals who did not express CCR5 because of a gene deletion were resistant to HIV infection.
The development of CCR5 antagonists was inspired by the observation that individuals who did not express CCR5 because of a gene deletion were resistant to HIV infection. The name chemokine comes from the ability of these agents to act as chemotaxic cytokines. Chemokines induce chemotaxis in responding cells and thus direct the movements of various cells within the body. Once a chemokine binds to a chemokine receptor, that cell begins to migrate toward a site of injury or maturation. Certain viruses, including HIV, also bind to chemokine receptors, as a means for gaining entry into host cells.
The name chemokine comes from the ability of these agents to act as chemotaxic cytokines. Chemokines induce chemotaxis in responding cells and thus direct the movements of various cells within the body. Once a chemokine binds to a chemokine receptor, that cell begins to migrate toward a site of injury or maturation. Certain viruses, including HIV, also bind to chemokine receptors, as a means for gaining entry into host cells.Fusion Inhibitors
MOA (Mechanism of Action) (Figure 18-25)
 Several steps are involved in the replication of HIV. The virus must fuse to the host cell, uncoat, enter and be transcribed by reverse transcriptase, become incorporated into the host genome, and be transcribed to viral RNA, which is then translated into polyproteins.
Several steps are involved in the replication of HIV. The virus must fuse to the host cell, uncoat, enter and be transcribed by reverse transcriptase, become incorporated into the host genome, and be transcribed to viral RNA, which is then translated into polyproteins.Indications
Integrase Inhibitors
MOA (Mechanism of Action)
 Several steps are involved in the replication of HIV. The virus must fuse to the host cell, uncoat, enter and be transcribed by reverse transcriptase, become incorporated into the host genome, and be transcribed to viral RNA, which is then translated into polyproteins (Figure 18-26).
Several steps are involved in the replication of HIV. The virus must fuse to the host cell, uncoat, enter and be transcribed by reverse transcriptase, become incorporated into the host genome, and be transcribed to viral RNA, which is then translated into polyproteins (Figure 18-26). Specifically, integrase binds to viral DNA and joins it with host DNA. The divalent cations in the catalytic core of integrase enable it to form covalent bonds with DNA. This is followed by cellular repair activities that seal the viral DNA into the chromosome.
Specifically, integrase binds to viral DNA and joins it with host DNA. The divalent cations in the catalytic core of integrase enable it to form covalent bonds with DNA. This is followed by cellular repair activities that seal the viral DNA into the chromosome. Integrase inhibitors prevent the formation of covalent bonds with host DNA. This prevents incorporation of HIV into the host genome.
Integrase inhibitors prevent the formation of covalent bonds with host DNA. This prevents incorporation of HIV into the host genome.Pharmacokinetics
 The main route of elimination for raltegravir is hepatic; however, dose adjustments are not required in patients with mild to moderate hepatic impairment. Adjustments in severe hepatic impairment have not been studied. Because renal elimination plays a relatively minor role, no adjustments are required in patients with renal impairment.
The main route of elimination for raltegravir is hepatic; however, dose adjustments are not required in patients with mild to moderate hepatic impairment. Adjustments in severe hepatic impairment have not been studied. Because renal elimination plays a relatively minor role, no adjustments are required in patients with renal impairment.Side Effects
Important Notes
 During clinical trials, there was a slight, non–statistically significant increase in the incidence of cancer in patients treated with raltegravir versus placebo-treated patients. Although these differences were not statistically significant and raltegravir is not considered to be a carcinogen, rates of cancer are being monitored closely in the postmarketing period.
During clinical trials, there was a slight, non–statistically significant increase in the incidence of cancer in patients treated with raltegravir versus placebo-treated patients. Although these differences were not statistically significant and raltegravir is not considered to be a carcinogen, rates of cancer are being monitored closely in the postmarketing period.Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
MOA (Mechanism of Action)
 Several steps are involved in the replication of HIV. The virus must fuse to the host cell, uncoat, enter and be transcribed by reverse transcriptase, become incorporated into the host genome, and be transcribed to viral RNA, which is then translated into polyproteins.
Several steps are involved in the replication of HIV. The virus must fuse to the host cell, uncoat, enter and be transcribed by reverse transcriptase, become incorporated into the host genome, and be transcribed to viral RNA, which is then translated into polyproteins. Reverse transcriptase is an enzyme that transcribes viral RNA into viral DNA (hence the term reverse).
Reverse transcriptase is an enzyme that transcribes viral RNA into viral DNA (hence the term reverse). Once they have entered the host cell, NRTIs are converted to nucleotides by host cell kinases. These nucleotides then compete with endogenous nucleosides for incorporation into viral DNA in the reaction catalyzed by reverse transcriptase (Figure 18-27).
Once they have entered the host cell, NRTIs are converted to nucleotides by host cell kinases. These nucleotides then compete with endogenous nucleosides for incorporation into viral DNA in the reaction catalyzed by reverse transcriptase (Figure 18-27). NRTIs therefore act as competitive inhibitors of reverse transcriptase. Once they incorporate themselves into DNA, NRTIs cause termination of the DNA chain, in a manner analogous to that seen with the nucleoside analogues such as acyclovir. Chain termination halts the process of viral replication.
NRTIs therefore act as competitive inhibitors of reverse transcriptase. Once they incorporate themselves into DNA, NRTIs cause termination of the DNA chain, in a manner analogous to that seen with the nucleoside analogues such as acyclovir. Chain termination halts the process of viral replication. NRTIs also inhibit host cell DNA polymerase, although this likely contributes more to their toxic effects than their efficacy. Inhibition of DNA polymerase in mitochondria leads to depletion of mitochondrial DNA and subsequent depletion of mitochondrial RNA and peptides involved in oxidative phosphorylation. This leads to mitochondrial dysfunction, and this is believed to be the cause of several of the important toxicities associated with drugs of this class.
NRTIs also inhibit host cell DNA polymerase, although this likely contributes more to their toxic effects than their efficacy. Inhibition of DNA polymerase in mitochondria leads to depletion of mitochondrial DNA and subsequent depletion of mitochondrial RNA and peptides involved in oxidative phosphorylation. This leads to mitochondrial dysfunction, and this is believed to be the cause of several of the important toxicities associated with drugs of this class.Pharmacokinetics
 Zidovudine can be given orally or IV, whereas the rest of the NRTIs are available only in oral dosage forms.
Zidovudine can be given orally or IV, whereas the rest of the NRTIs are available only in oral dosage forms. Zidovudine and didanosine are metabolized by the liver, whereas the others rely on the kidneys for their elimination, therefore dose adjustments for these agents should be considered in patients with renal impairment.
Zidovudine and didanosine are metabolized by the liver, whereas the others rely on the kidneys for their elimination, therefore dose adjustments for these agents should be considered in patients with renal impairment.Side Effects
 Myalgia: One of the most common and earliest side effects associated with NRTIs, myalgia was also the first indication of the mitochondrial toxicity that has become associated with these agents. The mitochondrial toxicity is believed to be caused by the inhibition of DNA polymerase by the NRTI.
Myalgia: One of the most common and earliest side effects associated with NRTIs, myalgia was also the first indication of the mitochondrial toxicity that has become associated with these agents. The mitochondrial toxicity is believed to be caused by the inhibition of DNA polymerase by the NRTI. Diarrhea is most associated with didanosine, likely as a result of the buffers used in oral formulations, some of which contain magnesium, a known laxative.
Diarrhea is most associated with didanosine, likely as a result of the buffers used in oral formulations, some of which contain magnesium, a known laxative.Serious
 Lactic acidosis: The impairment of mitochondrial function leads to a reliance on anaerobic metabolism, which produces excessive amounts of lactate.
Lactic acidosis: The impairment of mitochondrial function leads to a reliance on anaerobic metabolism, which produces excessive amounts of lactate. Peripheral neuropathy (didanosine, stavudine, zalcitabine) is likely caused by mitochondrial toxicity. Typically this effect will improve or resolve completely as long as the drug is stopped as soon as the symptoms appear.
Peripheral neuropathy (didanosine, stavudine, zalcitabine) is likely caused by mitochondrial toxicity. Typically this effect will improve or resolve completely as long as the drug is stopped as soon as the symptoms appear.Unique
 Hypersensitivity (abacavir), characterized by fever, GI problems including abdominal pain, rash, malaise, and fatigue may occur. If fever, abdominal pain, and rash occur within 6 weeks of initiation of therapy, the drug should be discontinued.
Hypersensitivity (abacavir), characterized by fever, GI problems including abdominal pain, rash, malaise, and fatigue may occur. If fever, abdominal pain, and rash occur within 6 weeks of initiation of therapy, the drug should be discontinued.Important Notes
 Lamivudine, emtricitabine, and tenofovir are also active against hepatitis B virus. This presents an opportunity to treat two indications (HIV and hepatitis B) with one drug, but also means that care must be taken when withdrawing these agents from hepatitis B co-infected patients. These patients may experience a rebound and worsening of hepatitis B.
Lamivudine, emtricitabine, and tenofovir are also active against hepatitis B virus. This presents an opportunity to treat two indications (HIV and hepatitis B) with one drug, but also means that care must be taken when withdrawing these agents from hepatitis B co-infected patients. These patients may experience a rebound and worsening of hepatitis B.Advanced
Drug Interactions
 Didanosine is easily degraded in an acidic environment and is therefore formulated with buffers such as calcium carbonate and magnesium hydroxide. Agents that bind to divalent cations (e.g., calcium and magnesium) should be administered separately from didanosine. Examples of these agents include fluoroquinolones such as ciprofloxacin. The same is true for agents whose dissolution is pH dependent, such as itraconazole.
Didanosine is easily degraded in an acidic environment and is therefore formulated with buffers such as calcium carbonate and magnesium hydroxide. Agents that bind to divalent cations (e.g., calcium and magnesium) should be administered separately from didanosine. Examples of these agents include fluoroquinolones such as ciprofloxacin. The same is true for agents whose dissolution is pH dependent, such as itraconazole.FYI
 Individual antiretrovirals are commonly referred to by three letter or digit acronyms. Aside from the fact that the abbreviation of drug names can lead to dispensing errors, the acronyms typically reflect the chemical rather than the generic name of the drug and are thus very confusing. See examples in Table 18-1.
Individual antiretrovirals are commonly referred to by three letter or digit acronyms. Aside from the fact that the abbreviation of drug names can lead to dispensing errors, the acronyms typically reflect the chemical rather than the generic name of the drug and are thus very confusing. See examples in Table 18-1.TABLE 18-1 Origins of the Abbreviations for Various Antiretrovirals
| Generic Name | Chemical Name | Abbreviation |
|---|---|---|
| Zidovudine | Azidothymidine | AZT |
| Lamivudine | 3-Thiacytidine | 3TC |
| Emtricitabine | Fluorothiacytidine | FTC |
| Didanosine | Dideoxyinosine | ddI |
Nonnucleoside Reverse Transcriptase Inhibitors (nNRTIs)
Description
nNRTIs, used in the treatment of HIV infection, inhibit HIV by preventing its transcription.
MOA (Mechanism of Action)
 Several steps are involved in the replication of HIV. The virus must fuse to the host cell, uncoat, enter and be transcribed by reverse transcriptase, become incorporated into the host genome, and be transcribed to viral RNA, which is then translated into polyproteins (Figure 18-28).
Several steps are involved in the replication of HIV. The virus must fuse to the host cell, uncoat, enter and be transcribed by reverse transcriptase, become incorporated into the host genome, and be transcribed to viral RNA, which is then translated into polyproteins (Figure 18-28). Reverse transcriptase is an enzyme that transcribes viral RNA into viral DNA (hence the term reverse).
Reverse transcriptase is an enzyme that transcribes viral RNA into viral DNA (hence the term reverse). The nNRTIs bind to a site distant from the active site of the reverse transcriptase, and induce a conformational change in the enzyme. This conformational change greatly reduces the activity of the enzyme.
The nNRTIs bind to a site distant from the active site of the reverse transcriptase, and induce a conformational change in the enzyme. This conformational change greatly reduces the activity of the enzyme. Also, because the binding of nNRTIs to the reverse transcriptase is very specific, nNRTIs act specifically on the HIV-1 strain and lack activity against HIV-2. Conversely, the NRTIs indirectly inhibit reverse transcriptase and are therefore not specific for HIV-1.
Also, because the binding of nNRTIs to the reverse transcriptase is very specific, nNRTIs act specifically on the HIV-1 strain and lack activity against HIV-2. Conversely, the NRTIs indirectly inhibit reverse transcriptase and are therefore not specific for HIV-1.Pharmacokinetics
 Nevirapine crosses the placenta and is therefore recommended for prevention of mother-child transmission of HIV.
Nevirapine crosses the placenta and is therefore recommended for prevention of mother-child transmission of HIV.Side Effects
 Rash: Macular or papular rash, often pruritic and also self-limiting, may occur with continued drug administration. In a minority of individuals, rash can progress to more serious Stevens-Johnson syndrome.
Rash: Macular or papular rash, often pruritic and also self-limiting, may occur with continued drug administration. In a minority of individuals, rash can progress to more serious Stevens-Johnson syndrome.Important Notes
 The nNRTIs do not inhibit DNA polymerase and therefore do not exhibit the same mitochondrial toxicities as the NRTIs. Inhibition of DNA polymerase in mitochondria leads to depletion of mitochondrial DNA and subsequent depletion of mitochondrial RNA and peptides involved in oxidative phosphorylation. This leads to mitochondrial dysfunction, and this is believed to be the cause of several of the important toxicities associated with the NRTIs.
The nNRTIs do not inhibit DNA polymerase and therefore do not exhibit the same mitochondrial toxicities as the NRTIs. Inhibition of DNA polymerase in mitochondria leads to depletion of mitochondrial DNA and subsequent depletion of mitochondrial RNA and peptides involved in oxidative phosphorylation. This leads to mitochondrial dysfunction, and this is believed to be the cause of several of the important toxicities associated with the NRTIs. Etravirine is the first of the second-generation nNRTIs. It was developed through rational drug design, by testing a variety of compounds against known mutations of the HIV-1 reverse transcriptase. This means that etravirine was designed to exhibit minimal cross-resistance with existing nNRTI mutants. However, unique resistant mutations to etravirine have developed.
Etravirine is the first of the second-generation nNRTIs. It was developed through rational drug design, by testing a variety of compounds against known mutations of the HIV-1 reverse transcriptase. This means that etravirine was designed to exhibit minimal cross-resistance with existing nNRTI mutants. However, unique resistant mutations to etravirine have developed.