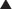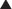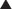Chapter 19 Musculoskeletal System
Bisphosphonates (BPs)
MOA (Mechanism of Action)
 The structural integrity of bone is determined to a large extent by the balance between the activity of osteoclasts, which break down bone (resorptive), and the activity of osteoblasts, which build bone.
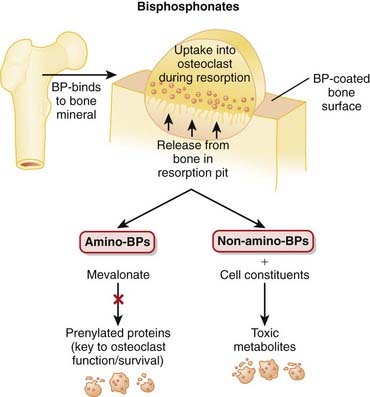
The structural integrity of bone is determined to a large extent by the balance between the activity of osteoclasts, which break down bone (resorptive), and the activity of osteoblasts, which build bone. Bisphosphonates (BPs) inhibit osteoclast activity through a variety of mechanisms, some better understood than others (Figure 19-1).
Bisphosphonates (BPs) inhibit osteoclast activity through a variety of mechanisms, some better understood than others (Figure 19-1). Inside the osteoclast the aminobisphosphonates disrupt the mevalonate pathway, a pathway involved in the posttranslational modification of proteins that are involved in cellular signaling. Disruption of the mevalonate pathway interrupts osteoclast function and leads to apoptosis of the osteoclast.
Inside the osteoclast the aminobisphosphonates disrupt the mevalonate pathway, a pathway involved in the posttranslational modification of proteins that are involved in cellular signaling. Disruption of the mevalonate pathway interrupts osteoclast function and leads to apoptosis of the osteoclast. The non-aminobisphosphonates work by increasing the accumulation of cytotoxic metabolites within osteoclasts, interfering with their function and possibly leading to osteoclast cell death.
The non-aminobisphosphonates work by increasing the accumulation of cytotoxic metabolites within osteoclasts, interfering with their function and possibly leading to osteoclast cell death. The clawlike chemical structure of BPs facilitates their attachment to bone. The multiple oxygen atoms around the perimeter of the BP molecule bind to divalent cations such as Ca2+ within bone matrix. The BPs remain within the matrix until the acids released by the osteoclasts break down the matrix and liberate the BPs. Ironically, the activity of the osteoclasts seals their own fate!
The clawlike chemical structure of BPs facilitates their attachment to bone. The multiple oxygen atoms around the perimeter of the BP molecule bind to divalent cations such as Ca2+ within bone matrix. The BPs remain within the matrix until the acids released by the osteoclasts break down the matrix and liberate the BPs. Ironically, the activity of the osteoclasts seals their own fate!Pharmacokinetics
 BPs have very low oral bioavailability (<10%), and their absorption is further reduced by food and by divalent cations such as calcium. It is therefore recommended that BPs be taken on an empty stomach, with plain water.
BPs have very low oral bioavailability (<10%), and their absorption is further reduced by food and by divalent cations such as calcium. It is therefore recommended that BPs be taken on an empty stomach, with plain water.Contraindications
Side Effects
All
Serious
 Esophagitis or esophageal erosion is more commonly seen with the aminobisphosphonates. It may result from a direct irritant effect from tablets lodged in the esophagus or from reflux of gastric acid including the acidic form of the BP. Patients are advised to avoid reclining for at least 30 minutes after taking a BP, reducing the chance of tablet staying in the esophagus or reflux.
Esophagitis or esophageal erosion is more commonly seen with the aminobisphosphonates. It may result from a direct irritant effect from tablets lodged in the esophagus or from reflux of gastric acid including the acidic form of the BP. Patients are advised to avoid reclining for at least 30 minutes after taking a BP, reducing the chance of tablet staying in the esophagus or reflux. Osteonecrosis of the jaw is typically only seen at higher doses. The mechanism has not been established; however, the fact that BPs alter bone turnover is thought to play a role. Jaw bone may have a higher rate of turnover than other areas of the body, perhaps explaining why this side effect is localized to this area.
Osteonecrosis of the jaw is typically only seen at higher doses. The mechanism has not been established; however, the fact that BPs alter bone turnover is thought to play a role. Jaw bone may have a higher rate of turnover than other areas of the body, perhaps explaining why this side effect is localized to this area.Important Notes
 The non-aminobisphosphonates were the original members of this drug class, whereas the aminobisphosphonates are newer, more potent agents.
The non-aminobisphosphonates were the original members of this drug class, whereas the aminobisphosphonates are newer, more potent agents. BPs have an established history as adjuncts in cancer therapy. They have demonstrated ability to reduce bone pain secondary to metastases and prevent treatment-induced bone loss.
BPs have an established history as adjuncts in cancer therapy. They have demonstrated ability to reduce bone pain secondary to metastases and prevent treatment-induced bone loss. Etidronate can cause bone demineralization; therefore unlike the other BPs, which are typically taken once daily, etidronate is typically administered in 90-day cycles, with 14 days on treatment and 76 days off treatment. During the off-treatment period, calcium tablets are typically administered, and this 90-day treatment cycle (etidronate followed by calcium) is marketed in one kit.
Etidronate can cause bone demineralization; therefore unlike the other BPs, which are typically taken once daily, etidronate is typically administered in 90-day cycles, with 14 days on treatment and 76 days off treatment. During the off-treatment period, calcium tablets are typically administered, and this 90-day treatment cycle (etidronate followed by calcium) is marketed in one kit. Estrogens appear to have a role in decreasing bone resorption; therefore one of the benefits of hormone replacement therapy in postmenopausal women is to maintain integrity of bone, hopefully leading to fewer fractures.
Estrogens appear to have a role in decreasing bone resorption; therefore one of the benefits of hormone replacement therapy in postmenopausal women is to maintain integrity of bone, hopefully leading to fewer fractures.Evidence
Risedronate versus Placebo or Calcium and Vitamin D or Both in Postmenopausal Osteoporosis
 A 2008 Cochrane review (7 trials, N = 14,049 females) found no statistically significant effects for risedronate with respect to primary prevention of vertebral and nonvertebral fractures. For secondary prevention, risedronate demonstrated statistically significant relative risk reductions (RRRs) of vertebral fractures (39%), nonvertebral fractures (20%), and hip fractures (26%). The corresponding absolute risk reductions were small: 5%, 2%, and 1%, respectively. No statistically significant differences were found for adverse events.
A 2008 Cochrane review (7 trials, N = 14,049 females) found no statistically significant effects for risedronate with respect to primary prevention of vertebral and nonvertebral fractures. For secondary prevention, risedronate demonstrated statistically significant relative risk reductions (RRRs) of vertebral fractures (39%), nonvertebral fractures (20%), and hip fractures (26%). The corresponding absolute risk reductions were small: 5%, 2%, and 1%, respectively. No statistically significant differences were found for adverse events.Etidronate versus Placebo and/or Calcium and Vitamin D in Postmenopausal Osteoporosis
 A 2008 Cochrane review (11 trials, N = 1248 females) found no statistically significant effects of etidronate with respect to primary prevention of any fractures. A statistically significant RRR of 47% was found for secondary prevention of vertebral fractures but not for nonvertebral, hip, or wrist fractures. No statistically significant differences were found for adverse events.
A 2008 Cochrane review (11 trials, N = 1248 females) found no statistically significant effects of etidronate with respect to primary prevention of any fractures. A statistically significant RRR of 47% was found for secondary prevention of vertebral fractures but not for nonvertebral, hip, or wrist fractures. No statistically significant differences were found for adverse events.BPs versus Placebo or No Treatment in Myeloma
 A 2002 Cochrane review (11 trials, N = 2183 patients) found that BPs prevented pathologic vertebral fractures (number needed to treat [NNT] = 10) and relieved pain (NNT = 11). BPs did not affect mortality, nonvertebral fractures, or hypercalcemia. No significant adverse events were associated with the BPs.
A 2002 Cochrane review (11 trials, N = 2183 patients) found that BPs prevented pathologic vertebral fractures (number needed to treat [NNT] = 10) and relieved pain (NNT = 11). BPs did not affect mortality, nonvertebral fractures, or hypercalcemia. No significant adverse events were associated with the BPs.FYI
 One of the earliest indications that the BPs had potential in the treatment of bone diseases was the observation that they inhibit the dissolution of hydroxyapatite crystals.
One of the earliest indications that the BPs had potential in the treatment of bone diseases was the observation that they inhibit the dissolution of hydroxyapatite crystals. Because of their ability to localize in bone, one of the early uses for BPs was as bone scanning agents, used in the detection of malignancies and other skeletal lesions.
Because of their ability to localize in bone, one of the early uses for BPs was as bone scanning agents, used in the detection of malignancies and other skeletal lesions. The bisphosphonates are so named because of the two phosphate (P) groups that form the backbone of these molecules.
The bisphosphonates are so named because of the two phosphate (P) groups that form the backbone of these molecules.Vitamin D Replacement
MOA (Mechanism of Action)
 Vitamin D is an important regulator of calcium and phosphate homeostasis and bone metabolism. It works in conjunction with PTH. The overall effect of vitamin D is to increase serum calcium concentrations. These effects are mediated via the following:
Vitamin D is an important regulator of calcium and phosphate homeostasis and bone metabolism. It works in conjunction with PTH. The overall effect of vitamin D is to increase serum calcium concentrations. These effects are mediated via the following:
 Vitamin D is lipophilic (it is one of the fat-soluble vitamins—A, D, E, and K) and thus freely crosses the cytoplasmic membrane.
Vitamin D is lipophilic (it is one of the fat-soluble vitamins—A, D, E, and K) and thus freely crosses the cytoplasmic membrane. Intracellularly, it binds vitamin D receptors (VDRs) and binds DNA, where it regulates transcription of genes in the intestine, bone, kidney, and parathyroid gland.
Intracellularly, it binds vitamin D receptors (VDRs) and binds DNA, where it regulates transcription of genes in the intestine, bone, kidney, and parathyroid gland. Vitamin D also has actions in macrophages and T cells and in proliferation and differentiation of a large number of cells, including cancer cells. Through these actions, it has immunomodulating and potentially, anticancer actions. Also, these actions are the basis of the mechanism whereby it is effective in psoriasis.
Vitamin D also has actions in macrophages and T cells and in proliferation and differentiation of a large number of cells, including cancer cells. Through these actions, it has immunomodulating and potentially, anticancer actions. Also, these actions are the basis of the mechanism whereby it is effective in psoriasis.Important Notes
 Vitamin D is synthesized in the skin, liver, and kidney. Vitamin D supplementation is therefore frequently required in patients with renal failure.
Vitamin D is synthesized in the skin, liver, and kidney. Vitamin D supplementation is therefore frequently required in patients with renal failure. Rickets is a childhood disease characterized by impeded growth and deformity (curvature) of the long bones caused by vitamin D deficiency. The fortification of milk with vitamin D has dramatically reduced the incidence of rickets in developed countries.
Rickets is a childhood disease characterized by impeded growth and deformity (curvature) of the long bones caused by vitamin D deficiency. The fortification of milk with vitamin D has dramatically reduced the incidence of rickets in developed countries. Osteomalacia is a condition of bone softening resulting from abnormality in the mineralization of the organic portion of the bone matrix called osteoid. It can be caused by vitamin D deficiency and is like an adult form of rickets. It is characterized by proximal muscle weakness, pain, and bone fragility.
Osteomalacia is a condition of bone softening resulting from abnormality in the mineralization of the organic portion of the bone matrix called osteoid. It can be caused by vitamin D deficiency and is like an adult form of rickets. It is characterized by proximal muscle weakness, pain, and bone fragility. Osteoporosis is characterized by reduced bone mineral density (BMD) and increased bone fragility. It is caused by abnormal osteoblastic and osteoclastic activity. The bone is porous, hence the name of the disease.
Osteoporosis is characterized by reduced bone mineral density (BMD) and increased bone fragility. It is caused by abnormal osteoblastic and osteoclastic activity. The bone is porous, hence the name of the disease.Advanced
 Vitamin D supplements are sometimes used in the treatment of psoriasis, a common skin condition involving rapid turnover and inflammation of the skin. The evidence for this use, however, is not strongly conclusive.
Vitamin D supplements are sometimes used in the treatment of psoriasis, a common skin condition involving rapid turnover and inflammation of the skin. The evidence for this use, however, is not strongly conclusive.| Common Name | Drug Name | Abbreviation |
|---|---|---|
| Vitamin D2 | Ergocalciferol | D2 |
| 1-Hydroxyvitamin D2 | Doxercalciferol | 1(OH)D2 |
| Vitamin D3 | Cholecalciferol | D3 |
| 25-Hydroxyvitamin D3 | Calcifediol | 25(OH)D3 |
| 1,25-Dihydroxyvitamin D3 | Calcitriol | 1,25(OH)2D3 |
| 24,25-Dihydroxyvitamin D3 | Secalcifediol | 24,25(OH)2D3 |
Evidence
Vitamin D Plus Calcium and Bone Fractures in the Elderly
 The same meta-analysis in 2008 showed that vitamin D plus calcium supplements do reduce hip fractures in the elderly (8 trials, N = 46,658 participants, relative risk [RR] 0.84). Hypercalcemia is significantly more common in people receiving vitamin D or an analogue, with or without calcium (18 trials, N = 11,346 participants, RR 2.35). There is a significant but modest increase in gastrointestinal symptoms (RR 1.04) and a small but significant increase in renal disease (RR 1.16).
The same meta-analysis in 2008 showed that vitamin D plus calcium supplements do reduce hip fractures in the elderly (8 trials, N = 46,658 participants, relative risk [RR] 0.84). Hypercalcemia is significantly more common in people receiving vitamin D or an analogue, with or without calcium (18 trials, N = 11,346 participants, RR 2.35). There is a significant but modest increase in gastrointestinal symptoms (RR 1.04) and a small but significant increase in renal disease (RR 1.16).FYI
 The concept that vitamin D comes from the sun is inaccurate; the inert precursor (7-dehydrocholesterol) is present in the skin, and exposure to ultraviolet light converts it to cholecalciferol, which is then isomerized to vitamin D3. Reduced exposure to sunlight is one cause of vitamin D deficiency.
The concept that vitamin D comes from the sun is inaccurate; the inert precursor (7-dehydrocholesterol) is present in the skin, and exposure to ultraviolet light converts it to cholecalciferol, which is then isomerized to vitamin D3. Reduced exposure to sunlight is one cause of vitamin D deficiency.Parathyroid Hormone
MOA (Mechanism of Action)
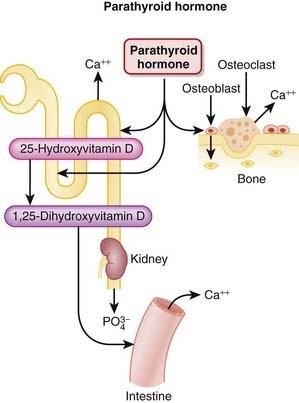
 PTH is released from the parathyroid gland. It regulates calcium and phosphate flux across cell membranes in bone and kidney. The key effects of PTH are as follows:
PTH is released from the parathyroid gland. It regulates calcium and phosphate flux across cell membranes in bone and kidney. The key effects of PTH are as follows:
 The effects on osteoclasts are indirect. PTH increases activity of the RANK (receptor activator of nuclear factor κ) ligand (RANKL). RANKL regulates osteoclast activity (see the discussion of RANKL inhibitors in this chapter). Increasing the activity of RANKL in turn stimulates an increase in the activity and number of osteoclasts.
The effects on osteoclasts are indirect. PTH increases activity of the RANK (receptor activator of nuclear factor κ) ligand (RANKL). RANKL regulates osteoclast activity (see the discussion of RANKL inhibitors in this chapter). Increasing the activity of RANKL in turn stimulates an increase in the activity and number of osteoclasts. The stimulation of osteoclasts increases bone remodeling. PTH increases both bone resorption and formation; however, the net effect of excess PTH is to increase bone resorption (Figure 19-3).
The stimulation of osteoclasts increases bone remodeling. PTH increases both bone resorption and formation; however, the net effect of excess PTH is to increase bone resorption (Figure 19-3). Low levels of intermittent PTH, however, can enhance bone formation. The actions of PTH are largely mediated through the PTH-1 receptor. The anabolic effects are mediated by direct effects of PTH on osteoblasts, increasing their number and inhibiting their apoptosis. PTH also stimulates insulin-like growth factor (IGF-1) in osteoblasts, and IGF-1 also has anabolic effects on bone.
Low levels of intermittent PTH, however, can enhance bone formation. The actions of PTH are largely mediated through the PTH-1 receptor. The anabolic effects are mediated by direct effects of PTH on osteoblasts, increasing their number and inhibiting their apoptosis. PTH also stimulates insulin-like growth factor (IGF-1) in osteoblasts, and IGF-1 also has anabolic effects on bone. It is still not clear why high, sustained PTH has a catabolic effect, whereas low, intermittent administration has an anabolic effect on bone.
It is still not clear why high, sustained PTH has a catabolic effect, whereas low, intermittent administration has an anabolic effect on bone.Pharmacokinetics
Contraindications
Side Effects
Important Notes
 The greatest safety concern associated with teriparatide is osteosarcoma (malignant bone cancer). However, this concern is based on observations in rodents exposed to prolonged high doses and on the ability of teriparatide to stimulate osteoblasts. So far, there does not appear to be an elevated risk of osteosarcoma with teriparatide use in humans.
The greatest safety concern associated with teriparatide is osteosarcoma (malignant bone cancer). However, this concern is based on observations in rodents exposed to prolonged high doses and on the ability of teriparatide to stimulate osteoblasts. So far, there does not appear to be an elevated risk of osteosarcoma with teriparatide use in humans. Because of the concerns over osteosarcoma, use of teriparatide is limited to 1.5 to 2 years. The reason for this cutoff is that the safety of teriparatide has not been assessed beyond 2 years in clinical trials.
Because of the concerns over osteosarcoma, use of teriparatide is limited to 1.5 to 2 years. The reason for this cutoff is that the safety of teriparatide has not been assessed beyond 2 years in clinical trials. Most of the agents used to manage osteoporosis are antiresorptive and work by inhibiting bone turnover. Teriparatide works by promoting bone turnover; thus there is concern that patients switching from antiresorptive agents, particularly potent agents such as BPs, might experience reduced efficacy with teriparatide. Therefore some clinicians suggest that there should be a washout period when switching from the BPs to teriparatide. The benefits of a washout should be balanced against the risk of no treatment, particularly in patients with severe disease.
Most of the agents used to manage osteoporosis are antiresorptive and work by inhibiting bone turnover. Teriparatide works by promoting bone turnover; thus there is concern that patients switching from antiresorptive agents, particularly potent agents such as BPs, might experience reduced efficacy with teriparatide. Therefore some clinicians suggest that there should be a washout period when switching from the BPs to teriparatide. The benefits of a washout should be balanced against the risk of no treatment, particularly in patients with severe disease.Evidence
Bisphosphonates or Teriparatide in Postmenopausal Women
 A 2005 systematic review (90 trials) compared all BPs and teriparatide with calcium, calcium plus vitamin D, calcitriol, hormone replacement therapy, exercise, and placebo or no treatment. They found that only teriparatide and risedronate reduced the risk of nonvertebral fracture in women with severe osteoporosis and adequate calcium intake.
A 2005 systematic review (90 trials) compared all BPs and teriparatide with calcium, calcium plus vitamin D, calcitriol, hormone replacement therapy, exercise, and placebo or no treatment. They found that only teriparatide and risedronate reduced the risk of nonvertebral fracture in women with severe osteoporosis and adequate calcium intake.RANKL Inhibitors
MOA (Mechanism of Action)
 RANKL is a cytokine member of the tumor necrosis factor (TNF) superfamily and is an important regulator of osteoclast activity.
RANKL is a cytokine member of the tumor necrosis factor (TNF) superfamily and is an important regulator of osteoclast activity. The primary function of osteoclasts is to break down bone; their counterparts are osteoblasts, which function to build up bone.
The primary function of osteoclasts is to break down bone; their counterparts are osteoblasts, which function to build up bone. The binding of RANKL to RANK results in increased bone resorption through the differentiation, activation, and prolonged survival of osteoclasts.
The binding of RANKL to RANK results in increased bone resorption through the differentiation, activation, and prolonged survival of osteoclasts. Osteoclast activity is an important factor in the development of osteoporosis and is also strongly implicated in bone destruction associated with rheumatoid arthritis, metastatic cancer, multiple myeloma, and sex hormone deprivation (menopause, aromatase inhibitor therapy, and androgen deprivation therapy).
Osteoclast activity is an important factor in the development of osteoporosis and is also strongly implicated in bone destruction associated with rheumatoid arthritis, metastatic cancer, multiple myeloma, and sex hormone deprivation (menopause, aromatase inhibitor therapy, and androgen deprivation therapy).Important Notes
 Osteoporosis can be evaluated through plain x-ray films of bones, but the gold standard is dual-energy x-ray absorptiometry (DXA).
Osteoporosis can be evaluated through plain x-ray films of bones, but the gold standard is dual-energy x-ray absorptiometry (DXA).
 Bones with decreased BMD are at increased risk for fracture. A score of less than 2.5 (standard deviations below normal) is the diagnostic criterion for osteoporosis.
Bones with decreased BMD are at increased risk for fracture. A score of less than 2.5 (standard deviations below normal) is the diagnostic criterion for osteoporosis.FYI
 As per naming convention for monoclonal antibodies (mAbs), the –os– refers to bone and the –u– refers to fully humanized.
As per naming convention for monoclonal antibodies (mAbs), the –os– refers to bone and the –u– refers to fully humanized. Osteopenia is the term for a mild reduction in BMD. Osteoporosis is the term for a more advanced reduction in BMD. The suffix -penia usually refers to a low cell count (e.g., thrombocytopenia means a low thrombocyte or platelet count); therefore osteopenia is somewhat of a misnomer when used in this setting when referring to BMD.
Osteopenia is the term for a mild reduction in BMD. Osteoporosis is the term for a more advanced reduction in BMD. The suffix -penia usually refers to a low cell count (e.g., thrombocytopenia means a low thrombocyte or platelet count); therefore osteopenia is somewhat of a misnomer when used in this setting when referring to BMD.Colchicine
MOA (Mechanism of Action)
 Most of the pharmacologic effects of colchicine result from colchicine binding to tubulin (Figure 19-4). Tubulin is required for microtubule assembly, and colchicine prevents microtubule assembly. Microtubules are important components of the cytoskeleton and are involved with the following cellular functions:
Most of the pharmacologic effects of colchicine result from colchicine binding to tubulin (Figure 19-4). Tubulin is required for microtubule assembly, and colchicine prevents microtubule assembly. Microtubules are important components of the cytoskeleton and are involved with the following cellular functions:
 Gout is a disease that occurs when uric acid levels in the blood are elevated and uric acid crystals precipitate in joints; the crystals induce an intense inflammatory response resulting in arthritis, a condition referred to as crystal-induced arthritis.
Gout is a disease that occurs when uric acid levels in the blood are elevated and uric acid crystals precipitate in joints; the crystals induce an intense inflammatory response resulting in arthritis, a condition referred to as crystal-induced arthritis.Pharmacokinetics
Side Effects
 Gastrointestinal: Diarrhea is virtually a guaranteed side effect when colchicine is given in doses suitable for acute attacks of gout and occurs in many patients treated for prevention (preventative doses are lower than those for acute attacks).
Gastrointestinal: Diarrhea is virtually a guaranteed side effect when colchicine is given in doses suitable for acute attacks of gout and occurs in many patients treated for prevention (preventative doses are lower than those for acute attacks).Important Notes
 Gout is a common disorder of uric acid metabolism whereby uric acid levels in the blood are elevated, which results in uric acid crystals being deposited into joints, causing inflammation and intense pain. The great toe is the joint most commonly affected.
Gout is a common disorder of uric acid metabolism whereby uric acid levels in the blood are elevated, which results in uric acid crystals being deposited into joints, causing inflammation and intense pain. The great toe is the joint most commonly affected. Colchicine, because of its high risk of toxicity, is not a first-line therapy for gout. It should be reserved for situations in which first-line therapy (nonsteroidal antiinflammatory drugs [NSAIDs]) is contraindicated or ineffective. Other drugs used to treat gout include uricosurics (drugs that increase elimination of renal uric acid) and xanthine oxidase inhibitors (which decrease uric acid formation).
Colchicine, because of its high risk of toxicity, is not a first-line therapy for gout. It should be reserved for situations in which first-line therapy (nonsteroidal antiinflammatory drugs [NSAIDs]) is contraindicated or ineffective. Other drugs used to treat gout include uricosurics (drugs that increase elimination of renal uric acid) and xanthine oxidase inhibitors (which decrease uric acid formation). Although doses are not routinely described in this textbook, overdose of colchicine can be fatal, and therefore it is important to highlight that there are maximum dose recommendations.
Although doses are not routinely described in this textbook, overdose of colchicine can be fatal, and therefore it is important to highlight that there are maximum dose recommendations. Multisystem failure and death: One study showed that eight of nine overdoses over a 15-year period resulted in death.
Multisystem failure and death: One study showed that eight of nine overdoses over a 15-year period resulted in death. The FDA reported 33 deaths associated with intravenous colchicine from 1985 to 1997. The manufacture of intravenous colchicine in the United States was halted in February 2008.
The FDA reported 33 deaths associated with intravenous colchicine from 1985 to 1997. The manufacture of intravenous colchicine in the United States was halted in February 2008.Evidence
Acute Gout Pain
 A Cochrane review in 2006 (one RCT, N = 43), compared with placebo, colchicine demonstrated an absolute reduction of 34% for pain scales and a 30% reduction for tenderness, swelling, redness, and pain. The NNT to reduce pain was three. All participants treated with colchicine experienced gastrointestinal side effects (diarrhea and/or vomiting), and the number needed to harm (NNH) with colchicine versus placebo was one. Note: The sample size was very small, and only one study met inclusion criteria in this review.
A Cochrane review in 2006 (one RCT, N = 43), compared with placebo, colchicine demonstrated an absolute reduction of 34% for pain scales and a 30% reduction for tenderness, swelling, redness, and pain. The NNT to reduce pain was three. All participants treated with colchicine experienced gastrointestinal side effects (diarrhea and/or vomiting), and the number needed to harm (NNH) with colchicine versus placebo was one. Note: The sample size was very small, and only one study met inclusion criteria in this review.Nonsteroidal Antiinflammatory Drugs (NSAIDs)
Description
NSAIDs are antiinflammatory drugs that do not possess a steroidal structure (nonsteroidal).
MOA (Mechanism of Action)
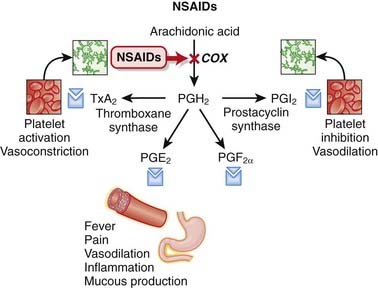
 Cyclooxygenase (COX) is an enzyme that catalyzes the conversion of arachidonic acid to prostaglandin (PG) G (PGG) and PGH. These intermediaries are then converted to a variety of important PGs as well as thromboxane A2 (TXA2).
Cyclooxygenase (COX) is an enzyme that catalyzes the conversion of arachidonic acid to prostaglandin (PG) G (PGG) and PGH. These intermediaries are then converted to a variety of important PGs as well as thromboxane A2 (TXA2). Each of these eicosanoids binds to its respective receptor, mediating the physiologic effects summarized in Figure 19-5.
Each of these eicosanoids binds to its respective receptor, mediating the physiologic effects summarized in Figure 19-5. There are actually two major isoforms of the COX enzyme: COX-1 and COX-2. COX-1 is a constitutive enzyme, meaning that its levels remain relatively constant, and it is distributed widely throughout the body. COX-1 is thus believed to play a maintenance or protective role, responsible for production of cytoprotective mucus in the stomach and for platelet aggregation (clotting).
There are actually two major isoforms of the COX enzyme: COX-1 and COX-2. COX-1 is a constitutive enzyme, meaning that its levels remain relatively constant, and it is distributed widely throughout the body. COX-1 is thus believed to play a maintenance or protective role, responsible for production of cytoprotective mucus in the stomach and for platelet aggregation (clotting). COX-2 is an inducible enzyme, meaning that its levels and activity can increase rapidly and significantly in response to a stimulus. The main stimuli for COX-2 induction are inflammatory mediators, and thus COX-2 is typically associated with inflammation.
COX-2 is an inducible enzyme, meaning that its levels and activity can increase rapidly and significantly in response to a stimulus. The main stimuli for COX-2 induction are inflammatory mediators, and thus COX-2 is typically associated with inflammation. The theory behind selective inhibition of COX-2 enzymes is therefore to preserve the gastric cytoprotective effects mediated through COX-1 while maximizing the antiinflammatory effects mediated through COX-2.
The theory behind selective inhibition of COX-2 enzymes is therefore to preserve the gastric cytoprotective effects mediated through COX-1 while maximizing the antiinflammatory effects mediated through COX-2. An unanticipated consequence of selective COX-2 inhibition is a pro-platelet effect. TXA2 and prostacyclin (PGI2) have opposite effects on platelets: TXA2 activates platelets, and PGI2 inhibits platelet activation. Of the two enzymes, COX-1 is primarily responsible for generating TXA2, whereas COX-2 is responsible for generating PGI2. Therefore, selective COX-2 inhibition removes this “check” on the platelet-activating actions of TXA2.
An unanticipated consequence of selective COX-2 inhibition is a pro-platelet effect. TXA2 and prostacyclin (PGI2) have opposite effects on platelets: TXA2 activates platelets, and PGI2 inhibits platelet activation. Of the two enzymes, COX-1 is primarily responsible for generating TXA2, whereas COX-2 is responsible for generating PGI2. Therefore, selective COX-2 inhibition removes this “check” on the platelet-activating actions of TXA2.Pharmacokinetics
 Many nonselective COX inhibitors have short to intermediate half-lives (2 to 12 hours), requiring frequent administration. The newer COX-2 selective inhibitors typically have longer half-lives and are administered once daily. One exception is celecoxib, which is administered twice daily.
Many nonselective COX inhibitors have short to intermediate half-lives (2 to 12 hours), requiring frequent administration. The newer COX-2 selective inhibitors typically have longer half-lives and are administered once daily. One exception is celecoxib, which is administered twice daily.Side Effects
Nonselective
 Gastrointestinal effects occur because of the inhibition of COX-1–mediated production of cytoprotective mucus in the stomach.
Gastrointestinal effects occur because of the inhibition of COX-1–mediated production of cytoprotective mucus in the stomach.All
 Central nervous system (CNS): Confusion, dizziness, depression, and hallucinations may occur. The mechanism is not confirmed, but COX-2 is the most abundant COX isoform in the CNS, and COX-2 may play a role in neurotransmission. The frequency of CNS side effects appears to be higher with COX-2–selective inhibitors and possibly with indomethacin. These reactions are uncommon.
Central nervous system (CNS): Confusion, dizziness, depression, and hallucinations may occur. The mechanism is not confirmed, but COX-2 is the most abundant COX isoform in the CNS, and COX-2 may play a role in neurotransmission. The frequency of CNS side effects appears to be higher with COX-2–selective inhibitors and possibly with indomethacin. These reactions are uncommon.Important Notes
 Indomethacin is considered to be a particularly potent COX inhibitor with very strong antiinflammatory effects. However, this enhanced efficacy is balanced with an increased severity of adverse effects, particularly gastrointestinal effects. Indomethacin is therefore typically reserved for use in more severe inflammatory conditions rather than everyday analgesia.
Indomethacin is considered to be a particularly potent COX inhibitor with very strong antiinflammatory effects. However, this enhanced efficacy is balanced with an increased severity of adverse effects, particularly gastrointestinal effects. Indomethacin is therefore typically reserved for use in more severe inflammatory conditions rather than everyday analgesia. Diclofenac is marketed in a fixed-dose combination with the PGE analogue misoprostol. The theory is that adding a PGE analogue helps to replace the PGE that is lost through COX inhibition.
Diclofenac is marketed in a fixed-dose combination with the PGE analogue misoprostol. The theory is that adding a PGE analogue helps to replace the PGE that is lost through COX inhibition. The extent of selectivity of COX-2 inhibitors varies significantly, and the distinction between COX-2 selective and nonselective inhibitors is not as clear as one would expect. Rofecoxib is by far the most COX-2 selective among currently marketed agents, with approximately 10 times the selectivity of celecoxib. Diclofenac and etodolac are also relatively selective for COX-2, whereas ketorolac is a relatively selective COX-1 inhibitor.
The extent of selectivity of COX-2 inhibitors varies significantly, and the distinction between COX-2 selective and nonselective inhibitors is not as clear as one would expect. Rofecoxib is by far the most COX-2 selective among currently marketed agents, with approximately 10 times the selectivity of celecoxib. Diclofenac and etodolac are also relatively selective for COX-2, whereas ketorolac is a relatively selective COX-1 inhibitor. Acetylsalicylic acid (ASA; aspirin) is a nonselective COX inhibitor with all the key features of other NSAIDs (antipyretic, analgesic, antiinflammatory activity), but it is often classified separately. Reasons include the fact that it irreversibly inhibits the COX enzyme and as a result has prolonged antiplatelet activity.
Acetylsalicylic acid (ASA; aspirin) is a nonselective COX inhibitor with all the key features of other NSAIDs (antipyretic, analgesic, antiinflammatory activity), but it is often classified separately. Reasons include the fact that it irreversibly inhibits the COX enzyme and as a result has prolonged antiplatelet activity. The antiplatelet effects of ASA are observed at doses much lower than required for antiinflammatory or other activities. This is thought to be the case because the antiplatelet effects are mediated by the irreversible inhibition of COX-1 by the parent ASA. Salicylic acid, the metabolite of ASA, is a reversible inhibitor of COX, just like the other NSAIDs. Because the antiplatelet effects of ASA are not affected by first pass, a much lower dose can be used to achieve platelet inhibition.
The antiplatelet effects of ASA are observed at doses much lower than required for antiinflammatory or other activities. This is thought to be the case because the antiplatelet effects are mediated by the irreversible inhibition of COX-1 by the parent ASA. Salicylic acid, the metabolite of ASA, is a reversible inhibitor of COX, just like the other NSAIDs. Because the antiplatelet effects of ASA are not affected by first pass, a much lower dose can be used to achieve platelet inhibition.Advanced
 A potential role for COX inhibitors in the treatment of cancer has been under investigation for many years. The COX-2 enzyme, which may stimulate cell division, has been selectively targeted in indications such as colon cancer. Inhibition of COX-2 may promote apoptosis, inhibit angiogenesis, and inhibit cell growth.
A potential role for COX inhibitors in the treatment of cancer has been under investigation for many years. The COX-2 enzyme, which may stimulate cell division, has been selectively targeted in indications such as colon cancer. Inhibition of COX-2 may promote apoptosis, inhibit angiogenesis, and inhibit cell growth.Evidence
Alone or in Combination with Opiates for Treatment of Cancer Pain
 A 2005 Cochrane review (42 trials, N = 3084 patients) found that NSAIDs were more effective than placebo for cancer pain. No conclusions could be drawn about the efficacy of NSAIDs relative to one another. The combination of opiates and NSAIDs was slightly and statistically better than either agent alone in 9 of 14 trials and was no different in 4 of 14 trials. The authors noted that the generalizability of the findings was limited by the short term of the studies.
A 2005 Cochrane review (42 trials, N = 3084 patients) found that NSAIDs were more effective than placebo for cancer pain. No conclusions could be drawn about the efficacy of NSAIDs relative to one another. The combination of opiates and NSAIDs was slightly and statistically better than either agent alone in 9 of 14 trials and was no different in 4 of 14 trials. The authors noted that the generalizability of the findings was limited by the short term of the studies.Versus Opiates for Treatment of Acute Renal Colic
 A 2006 Cochrane review (20 trials, N = 1613 patients) compared NSAIDs with opiates for treatment of renal colic. Results of the trials were too heterogeneous to perform a meta-analysis. The authors concluded that both NSAIDs and opiates can significantly relieve pain in acute renal colic. Opiates appeared to cause more adverse effects, particularly vomiting, and particularly pethidine.
A 2006 Cochrane review (20 trials, N = 1613 patients) compared NSAIDs with opiates for treatment of renal colic. Results of the trials were too heterogeneous to perform a meta-analysis. The authors concluded that both NSAIDs and opiates can significantly relieve pain in acute renal colic. Opiates appeared to cause more adverse effects, particularly vomiting, and particularly pethidine.FYI
 The antiplatelet effects of aspirin were first identified in the 1940s, after a physician noted increased bleeding in children who chewed aspirin gum after tonsillectomy. Speculating that a “blood-thinning” effect could reduce cardiovascular risk, he began giving aspirin to high-risk patients. Despite claims of success, the FDA did not accept the antiplatelet effects of aspirin until 1980.
The antiplatelet effects of aspirin were first identified in the 1940s, after a physician noted increased bleeding in children who chewed aspirin gum after tonsillectomy. Speculating that a “blood-thinning” effect could reduce cardiovascular risk, he began giving aspirin to high-risk patients. Despite claims of success, the FDA did not accept the antiplatelet effects of aspirin until 1980. The use of COX-2 inhibitors in cancer led to one of the largest drug withdrawals in the history of pharmaceutical development. During a very large trial in cancer prevention, it was discovered that there was a higher incidence of cardiovascular events in the rofecoxib-treated group versus placebo. The study was halted, the news hit the media, and this top-selling drug was withdrawn from the market.
The use of COX-2 inhibitors in cancer led to one of the largest drug withdrawals in the history of pharmaceutical development. During a very large trial in cancer prevention, it was discovered that there was a higher incidence of cardiovascular events in the rofecoxib-treated group versus placebo. The study was halted, the news hit the media, and this top-selling drug was withdrawn from the market.Uricosurics
MOA (Mechanism of Action)
 Gout is a condition whereby uric acid crystals precipitate in joints and the crystals induce an intense inflammatory reaction within the synovial space, leading to severe pain. Uric acid is an organic acid and a byproduct of purine metabolism.
Gout is a condition whereby uric acid crystals precipitate in joints and the crystals induce an intense inflammatory reaction within the synovial space, leading to severe pain. Uric acid is an organic acid and a byproduct of purine metabolism. Uric acid levels in the blood are elevated in gout, and one treatment strategy is to lower uric acid levels by enhancing uric acid excretion, which is what uricosurics do.
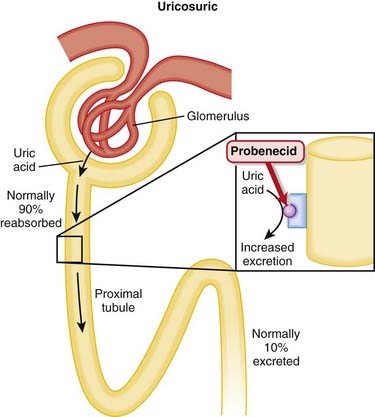
Uric acid levels in the blood are elevated in gout, and one treatment strategy is to lower uric acid levels by enhancing uric acid excretion, which is what uricosurics do. Uric acid is freely filtered at the glomerulus and is also both reabsorbed and secreted in the proximal tubule. The amount excreted usually is about 10% of that filtered.
Uric acid is freely filtered at the glomerulus and is also both reabsorbed and secreted in the proximal tubule. The amount excreted usually is about 10% of that filtered. In the proximal tubule, a transporter that exchanges urate for either an organic or an inorganic anion is the channel responsible for uric acid reabsorption; uricosuric drugs compete with urate for this brush-border transporter, thereby inhibiting its reabsorption. Probenecid is completely reabsorbed by the proximal tubule (Figure 19-6).
In the proximal tubule, a transporter that exchanges urate for either an organic or an inorganic anion is the channel responsible for uric acid reabsorption; uricosuric drugs compete with urate for this brush-border transporter, thereby inhibiting its reabsorption. Probenecid is completely reabsorbed by the proximal tubule (Figure 19-6). As the urinary excretion of uric acid increases, the amount of urate in the body decreases, although the plasma concentration may not be greatly reduced.
As the urinary excretion of uric acid increases, the amount of urate in the body decreases, although the plasma concentration may not be greatly reduced. With the increase in uric acid excretion, a predisposition to renal stone (urate stone) formation is increased. Therefore the urine volume should be maintained at a high level to help reduce urate concentrations in the urine and to promote flow, both acting to minimize precipitation within the renal system.
With the increase in uric acid excretion, a predisposition to renal stone (urate stone) formation is increased. Therefore the urine volume should be maintained at a high level to help reduce urate concentrations in the urine and to promote flow, both acting to minimize precipitation within the renal system.Pharmacokinetics
Contraindications
Important Notes
 Alcohol and high protein intake will increase uric acid levels in the blood. Therapy for gout must include dietary modification.
Alcohol and high protein intake will increase uric acid levels in the blood. Therapy for gout must include dietary modification. Paradoxically, an acute attack can occur in up to 20% of gouty patients treated with probenecid alone. Therefore concomitant NSAIDs are indicated early in the course of therapy to avoid precipitating an attack of gout.
Paradoxically, an acute attack can occur in up to 20% of gouty patients treated with probenecid alone. Therefore concomitant NSAIDs are indicated early in the course of therapy to avoid precipitating an attack of gout. Low-dose aspirin causes net retention of uric acid. It should not be used for analgesia in patients with gout.
Low-dose aspirin causes net retention of uric acid. It should not be used for analgesia in patients with gout. Fenofibrate (lipid-lowering agent) and losartan (angiotensin receptor blocker) both have uricosuric properties.
Fenofibrate (lipid-lowering agent) and losartan (angiotensin receptor blocker) both have uricosuric properties. Transport across the urate transporter is bidirectional, and depending on dose, a uricosuric drug may either decrease or increase the excretion of uric acid. Decreased excretion usually occurs at a low dose, whereas increased excretion is observed at a higher dose.
Transport across the urate transporter is bidirectional, and depending on dose, a uricosuric drug may either decrease or increase the excretion of uric acid. Decreased excretion usually occurs at a low dose, whereas increased excretion is observed at a higher dose. In 2006 fewer than 5% of patients treated for gout were treated with probenecid in the United States. The low rate of use of this drug was speculated to be a result of the availability of other medications for gout, the lack of efficacy in patients with renal dysfunction, and the propensity for renal calculi.
In 2006 fewer than 5% of patients treated for gout were treated with probenecid in the United States. The low rate of use of this drug was speculated to be a result of the availability of other medications for gout, the lack of efficacy in patients with renal dysfunction, and the propensity for renal calculi.FYI
 Tophaceous means stonelike. Tophaceous gout is a condition in which large stony deposits of uric acid accumulate around the joint.
Tophaceous means stonelike. Tophaceous gout is a condition in which large stony deposits of uric acid accumulate around the joint.Xanthine Oxidase Inhibitors
MOA (Mechanism of Action)
 Allopurinol is a purine analogue of hypoxanthine and is a substrate for, and inhibitor of, the enzyme xanthine oxidase.
Allopurinol is a purine analogue of hypoxanthine and is a substrate for, and inhibitor of, the enzyme xanthine oxidase. Xanthine oxidase converts hypoxanthine to xanthine to uric acid; inhibition therefore reduces the production of uric acid (Figure 19-7).
Xanthine oxidase converts hypoxanthine to xanthine to uric acid; inhibition therefore reduces the production of uric acid (Figure 19-7). Allopurinol’s primary metabolite, oxypurinol, also inhibits xanthine oxidase. Oxypurinol has a long half-life in tissues and is responsible for much of the pharmacologic activity of allopurinol.
Allopurinol’s primary metabolite, oxypurinol, also inhibits xanthine oxidase. Oxypurinol has a long half-life in tissues and is responsible for much of the pharmacologic activity of allopurinol. Allopurinol competitively inhibits xanthine oxidase at low concentrations and is a noncompetitive inhibitor at high concentrations.
Allopurinol competitively inhibits xanthine oxidase at low concentrations and is a noncompetitive inhibitor at high concentrations. Allopurinol also results in the accumulation of xanthine and hypoxanthine; these molecules can cause feedback inhibition of purine synthesis.
Allopurinol also results in the accumulation of xanthine and hypoxanthine; these molecules can cause feedback inhibition of purine synthesis. The net effect of xanthine oxidase inhibition is a lower production of uric acid and a decrease of total body uric acid (including dissolved and precipitated uric acid).
The net effect of xanthine oxidase inhibition is a lower production of uric acid and a decrease of total body uric acid (including dissolved and precipitated uric acid). Xanthine oxidase inhibitors facilitate the dissolution of tophi (nodular crystal deposits) and prevent the development or progression of chronic gouty arthritis by lowering the uric acid concentration in plasma below the limit of its solubility.
Xanthine oxidase inhibitors facilitate the dissolution of tophi (nodular crystal deposits) and prevent the development or progression of chronic gouty arthritis by lowering the uric acid concentration in plasma below the limit of its solubility.Pharmacokinetics
 Allopurinol increases the half-life of probenecid and enhances its uricosuric effect, whereas probenecid increases the clearance of oxypurinol, thereby increasing dose requirements of allopurinol. The clinical relevance of this interaction is that both drugs are used to treat gout and therefore are potentially coadministered.
Allopurinol increases the half-life of probenecid and enhances its uricosuric effect, whereas probenecid increases the clearance of oxypurinol, thereby increasing dose requirements of allopurinol. The clinical relevance of this interaction is that both drugs are used to treat gout and therefore are potentially coadministered. Mercaptopurine, azathioprine, and theophylline are metabolized by xanthine oxidase, and coadministration with allopurinol will dramatically increase levels of these drugs. Coadministration should be avoided or should be performed with great caution and should include large dose reductions (down to 25% of the normal dose). Clinical relevance of this interaction is in the treatment of transplant patients because they are treated with immunosuppressants such as mercaptopurine and azathioprine.
Mercaptopurine, azathioprine, and theophylline are metabolized by xanthine oxidase, and coadministration with allopurinol will dramatically increase levels of these drugs. Coadministration should be avoided or should be performed with great caution and should include large dose reductions (down to 25% of the normal dose). Clinical relevance of this interaction is in the treatment of transplant patients because they are treated with immunosuppressants such as mercaptopurine and azathioprine.Indications
Side Effects
Allopurinol
 Skin reactions: Both mild and severe reactions can occur.
Skin reactions: Both mild and severe reactions can occur.
 The rash is predominantly a pruritic, erythematous, or maculopapular eruption (itchy, red, flat or slightly raised).
The rash is predominantly a pruritic, erythematous, or maculopapular eruption (itchy, red, flat or slightly raised). Rarely, toxic epidermal necrolysis or Stevens-Johnson syndrome occurs, which can be fatal. These are very severe skin reactions. The risk for Stevens-Johnson syndrome is probably limited to within the first 2 months of treatment.
Rarely, toxic epidermal necrolysis or Stevens-Johnson syndrome occurs, which can be fatal. These are very severe skin reactions. The risk for Stevens-Johnson syndrome is probably limited to within the first 2 months of treatment. Liver reactions: Severe hepatic reactions including elevations of liver enzymes, fever, eosinophilia, and rash may occur. Allopurinol should be stopped immediately if a hypersensitivity reaction is suspected.
Liver reactions: Severe hepatic reactions including elevations of liver enzymes, fever, eosinophilia, and rash may occur. Allopurinol should be stopped immediately if a hypersensitivity reaction is suspected.Important Notes
 Paradoxically, urate-lowering therapy can cause a flare-up of gout in the early months of treatment. Therefore:
Paradoxically, urate-lowering therapy can cause a flare-up of gout in the early months of treatment. Therefore:
 Because allopurinol has no antiinflammatory effects, it has no role in the treatment of acute gouty arthritis.
Because allopurinol has no antiinflammatory effects, it has no role in the treatment of acute gouty arthritis. Complete blood counts, liver function test results, and renal function test results should be assessed periodically, especially during the first few months of treatment, to monitor for liver and renal injury.
Complete blood counts, liver function test results, and renal function test results should be assessed periodically, especially during the first few months of treatment, to monitor for liver and renal injury. The development of a skin rash mandates immediate assessment by a physician; a mild rash can be a precursor to a severe skin reaction.
The development of a skin rash mandates immediate assessment by a physician; a mild rash can be a precursor to a severe skin reaction. Lack of effectiveness of allopurinol is generally attributed to inadequate dose or duration of treatment, meaning that patients are treated with a dose that is too small or for too short a time to be effective. Precipitation of acute gout attacks early in the course of therapy is a common cause of discontinuation.
Lack of effectiveness of allopurinol is generally attributed to inadequate dose or duration of treatment, meaning that patients are treated with a dose that is too small or for too short a time to be effective. Precipitation of acute gout attacks early in the course of therapy is a common cause of discontinuation.FYI
 Allopurinol initially was synthesized as a candidate antineoplastic agent but was found to lack antineoplastic activity. Subsequent testing showed it to be an inhibitor of xanthine oxidase that was useful clinically for the treatment of gout.
Allopurinol initially was synthesized as a candidate antineoplastic agent but was found to lack antineoplastic activity. Subsequent testing showed it to be an inhibitor of xanthine oxidase that was useful clinically for the treatment of gout.