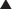Chapter 20 Neoplasia
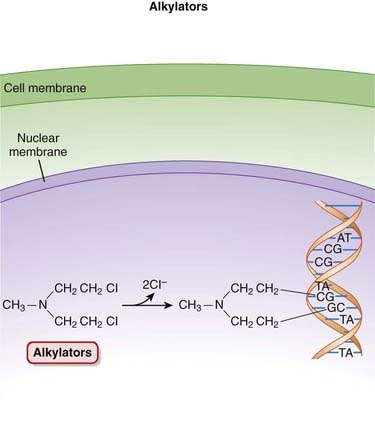
Alkylators
MOA (Mechanism of Action)
 The alkylating agents transfer alkyl (chemical) groups to DNA. DNA alkylation in the nucleus leads to the death of the cell.
The alkylating agents transfer alkyl (chemical) groups to DNA. DNA alkylation in the nucleus leads to the death of the cell. Once in the cell, the alkylating agents undergo a structural rearrangement that results in the formation of an unstable intermediate, an ethylene immonium ion.
Once in the cell, the alkylating agents undergo a structural rearrangement that results in the formation of an unstable intermediate, an ethylene immonium ion. This ion either directly, or via another intermediate, a carbonium ion, transfers alkyl groups to nucleic acids such as guanine or to other cellular constituents.
This ion either directly, or via another intermediate, a carbonium ion, transfers alkyl groups to nucleic acids such as guanine or to other cellular constituents. Alkylation of guanine or other bases results in abnormal base pairing as well as the excision of these bases, which in turn leads to strand breakage.
Alkylation of guanine or other bases results in abnormal base pairing as well as the excision of these bases, which in turn leads to strand breakage. Alkylating agents are considered to be cell cycle phase nonspecific, with cells in G1 and S phases being most susceptible (Figure 20-1).
Alkylating agents are considered to be cell cycle phase nonspecific, with cells in G1 and S phases being most susceptible (Figure 20-1). The connection between DNA alkylation and death of the cancer cell has not been established; however, one of the likely mechanisms is damage to DNA that is sufficient to activate proapoptotic proteins such as p53, leading to cell death.
The connection between DNA alkylation and death of the cancer cell has not been established; however, one of the likely mechanisms is damage to DNA that is sufficient to activate proapoptotic proteins such as p53, leading to cell death. Nitrosoureas have an additional mechanism of action. Nitrosoureas undergo another reaction, referred to as carbamoylation, with lysine residues of proteins. Carbamoylation is the transfer of carbamoyl (NH2CO) groups to an amino group, such as that found in amino acids.
Nitrosoureas have an additional mechanism of action. Nitrosoureas undergo another reaction, referred to as carbamoylation, with lysine residues of proteins. Carbamoylation is the transfer of carbamoyl (NH2CO) groups to an amino group, such as that found in amino acids.Pharmacokinetics
 Oral dosage forms are available for cyclophosphamide, melphalan, chlorambucil, and busulfan. Lomustine is available only in oral dosage forms. The rest are all intravenous.
Oral dosage forms are available for cyclophosphamide, melphalan, chlorambucil, and busulfan. Lomustine is available only in oral dosage forms. The rest are all intravenous.Side Effects
 Nausea, vomiting: These are common side effects with cytotoxic agents, which tend to target rapidly dividing cells, including those of the GI tract.
Nausea, vomiting: These are common side effects with cytotoxic agents, which tend to target rapidly dividing cells, including those of the GI tract. Alopecia: Cytotoxic chemotherapy agents tend to target tissues with rapidly dividing cells, such as those in hair follicles. This effect is seen most often with cyclophosphamide.
Alopecia: Cytotoxic chemotherapy agents tend to target tissues with rapidly dividing cells, such as those in hair follicles. This effect is seen most often with cyclophosphamide.Serious
 Myelosuppression: Typical of cytotoxic agents, alkylators tend to target cells that are actively dividing, such as those found in the bone marrow.
Myelosuppression: Typical of cytotoxic agents, alkylators tend to target cells that are actively dividing, such as those found in the bone marrow. Hemorrhagic cystitis (cyclophosphamide and ifosfamide) is caused by acrolein, a metabolite of cyclophosphamide and ifosfamide. This effect can be managed by increasing fluid intake and by administering sulfhydryl donors such as N-acetylcysteine or mesna (an antioxidant). These agents bind with acrolein and form a nontoxic compound.
Hemorrhagic cystitis (cyclophosphamide and ifosfamide) is caused by acrolein, a metabolite of cyclophosphamide and ifosfamide. This effect can be managed by increasing fluid intake and by administering sulfhydryl donors such as N-acetylcysteine or mesna (an antioxidant). These agents bind with acrolein and form a nontoxic compound.Important Notes
 A number of the alkylating agents (cyclophosphamide, ifosfamide, estramustine, melphalan, and chlorambucil) are also known as nitrogen mustards. These agents are related to mustard gas, a biologic warfare agent used during World War I.
A number of the alkylating agents (cyclophosphamide, ifosfamide, estramustine, melphalan, and chlorambucil) are also known as nitrogen mustards. These agents are related to mustard gas, a biologic warfare agent used during World War I. Cyclophosphamide has a significant effect on lymphocytes; therefore it is also used as an immunosuppressant to prevent rejection of transplanted organs, as well as in conditions such as rheumatoid arthritis and nephrotic syndrome. Cyclophosphamide has a steroid-sparing effect, meaning that it can reduce the need for use of corticosteroids.
Cyclophosphamide has a significant effect on lymphocytes; therefore it is also used as an immunosuppressant to prevent rejection of transplanted organs, as well as in conditions such as rheumatoid arthritis and nephrotic syndrome. Cyclophosphamide has a steroid-sparing effect, meaning that it can reduce the need for use of corticosteroids. Estramustine is a combination of estrogen and a mustine called chlormethine. Accordingly, it has both cytotoxic and hormonal actions and is used primarily in prostate cancer.
Estramustine is a combination of estrogen and a mustine called chlormethine. Accordingly, it has both cytotoxic and hormonal actions and is used primarily in prostate cancer.FYI
 The first rationally designed anticancer agent was a nitrosourea (methyl nitrosourea), developed in 1898.
The first rationally designed anticancer agent was a nitrosourea (methyl nitrosourea), developed in 1898. The U.S. Army played a role in the development of nitrogen mustards, deriving the prototype to this class (mechlorethamine) from the mustard gases used in the first World War. After an accidental exposure, it was discovered that nitrogen mustard produced lymphopenia, inspiring its use in malignant proliferative disorders.
The U.S. Army played a role in the development of nitrogen mustards, deriving the prototype to this class (mechlorethamine) from the mustard gases used in the first World War. After an accidental exposure, it was discovered that nitrogen mustard produced lymphopenia, inspiring its use in malignant proliferative disorders.Anthracyclines
MOA (Mechanism of Action)
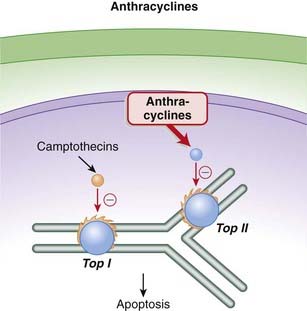
 Topoisomerase II (Top II) plays a key role during DNA synthesis, nicking and resealing the DNA helix so that it does not become tangled during replication.
Topoisomerase II (Top II) plays a key role during DNA synthesis, nicking and resealing the DNA helix so that it does not become tangled during replication. The anthracyclines prevent the resealing step from occurring by intercalating into and inhibiting the DNA–topoisomerase II complex after the nicking phase. This results in a large number of DNA fragments, eventually prompting the cancer cell to undergo apoptosis (Figure 20-2).
The anthracyclines prevent the resealing step from occurring by intercalating into and inhibiting the DNA–topoisomerase II complex after the nicking phase. This results in a large number of DNA fragments, eventually prompting the cancer cell to undergo apoptosis (Figure 20-2). As a secondary mechanism, anthracyclines produce free radicals, and these free radicals in turn damage cell membranes, proteins, and lipids. The generation of free radicals is also believed to mediate an important toxicity associated with this class (see Side Effects).
As a secondary mechanism, anthracyclines produce free radicals, and these free radicals in turn damage cell membranes, proteins, and lipids. The generation of free radicals is also believed to mediate an important toxicity associated with this class (see Side Effects). In addition anthracyclines are DNA intercalators, meaning that they insert themselves into DNA structure, inhibiting transcription and replication.
In addition anthracyclines are DNA intercalators, meaning that they insert themselves into DNA structure, inhibiting transcription and replication.Pharmacokinetics
 Doxorubicin was the first anthracycline to be encapsulated in liposomes to facilitate targeted delivery of the drug and therefore avoid cardiotoxicity (see Side Effects). One of the key mechanisms for this targeted delivery relies on the fact that the liposomes readily extravasate in tissues that have a disrupted vasculature, such as that found in tumors. Blood vessels in tumors tend to be “leaky” owing to the constant angiogenesis that is taking place. Conversely, the liposomes have difficulty exiting vessels in tissues such as the myocardium.
Doxorubicin was the first anthracycline to be encapsulated in liposomes to facilitate targeted delivery of the drug and therefore avoid cardiotoxicity (see Side Effects). One of the key mechanisms for this targeted delivery relies on the fact that the liposomes readily extravasate in tissues that have a disrupted vasculature, such as that found in tumors. Blood vessels in tumors tend to be “leaky” owing to the constant angiogenesis that is taking place. Conversely, the liposomes have difficulty exiting vessels in tissues such as the myocardium.Contraindications
Side Effects
 Nausea and vomiting are common side effect with cytotoxic agents, which tend to target rapidly dividing cells, including those of the GI tract.
Nausea and vomiting are common side effect with cytotoxic agents, which tend to target rapidly dividing cells, including those of the GI tract. Alopecia: Cytotoxic chemotherapy agents tend to target tissues with rapidly dividing cells, such as those in hair follicles. The hair loss is reversible.
Alopecia: Cytotoxic chemotherapy agents tend to target tissues with rapidly dividing cells, such as those in hair follicles. The hair loss is reversible. Mucositis or stomatitis is an inflammatory condition of the mouth. Cytotoxic chemotherapy agents tend to target tissues with rapidly dividing cells, such as those in the oral mucosa.
Mucositis or stomatitis is an inflammatory condition of the mouth. Cytotoxic chemotherapy agents tend to target tissues with rapidly dividing cells, such as those in the oral mucosa. Soft tissue necrosis: If the anthracyclines become extravascular, they can damage surrounding tissue. Infusions should be carried out slowly to reduce the risk of extravasation.
Soft tissue necrosis: If the anthracyclines become extravascular, they can damage surrounding tissue. Infusions should be carried out slowly to reduce the risk of extravasation.Serious
 Cardiotoxicity: The free radicals generated by the anthracyclines cause peroxidation of the cardiac sarcoplasmic reticulum, leading to a Ca2+-dependent cardiac necrosis. The reason this toxicity is selective for cardiac tissue is that catalase, able to neutralize these free radicals, is not found in cardiac tissue.
Cardiotoxicity: The free radicals generated by the anthracyclines cause peroxidation of the cardiac sarcoplasmic reticulum, leading to a Ca2+-dependent cardiac necrosis. The reason this toxicity is selective for cardiac tissue is that catalase, able to neutralize these free radicals, is not found in cardiac tissue.Important Notes
 Cardiotoxicity associated with anthracyclines can occur both acutely and chronically. Acute toxicity is characterized by abnormal electrocardiograms (ECGs) and reductions in systolic function. Chronic toxicity is cumulative and dose related. It manifests as congestive heart failure, and once it has reached this point it has a very high mortality rate. This chronic cardiotoxicity is of greater concern, and it is addressed using a number of strategies, including limitations on doses used, as well as use of liposomal formulations and adjuvant agents, as described later.
Cardiotoxicity associated with anthracyclines can occur both acutely and chronically. Acute toxicity is characterized by abnormal electrocardiograms (ECGs) and reductions in systolic function. Chronic toxicity is cumulative and dose related. It manifests as congestive heart failure, and once it has reached this point it has a very high mortality rate. This chronic cardiotoxicity is of greater concern, and it is addressed using a number of strategies, including limitations on doses used, as well as use of liposomal formulations and adjuvant agents, as described later. Mitoxantrone has a chemical structure that is distinct from that of the anthracyclines, and it is believed to be less cardiotoxic than the anthracyclines. It does not generate free radicals.
Mitoxantrone has a chemical structure that is distinct from that of the anthracyclines, and it is believed to be less cardiotoxic than the anthracyclines. It does not generate free radicals.Advanced
 Strategies to minimize cardiotoxicity include the use of a cardioprotective drug such as dexrazoxane. The generation of free radicals by anthracyclines is iron dependent. Dexrazoxane chelates iron that is bound in anthracycline complexes, and this prevents the formation of the free radicals that damage the myocardium. Dexrazoxane does not appear to impair the antitumor activity of the anthracyclines.
Strategies to minimize cardiotoxicity include the use of a cardioprotective drug such as dexrazoxane. The generation of free radicals by anthracyclines is iron dependent. Dexrazoxane chelates iron that is bound in anthracycline complexes, and this prevents the formation of the free radicals that damage the myocardium. Dexrazoxane does not appear to impair the antitumor activity of the anthracyclines.Drug Interactions
 It appears that the cardiotoxic effects of anthracyclines may be worsened by concurrent administration of trastuzumab. Trastuzumab is a monoclonal antibody that is used in the treatment of breast cancers expressing the HER2/neu receptor. A number of anthracyclines are used in treating breast cancer.
It appears that the cardiotoxic effects of anthracyclines may be worsened by concurrent administration of trastuzumab. Trastuzumab is a monoclonal antibody that is used in the treatment of breast cancers expressing the HER2/neu receptor. A number of anthracyclines are used in treating breast cancer.Antimetabolites
MOA (Mechanism of Action)
Folate Analogues
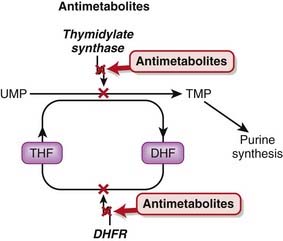
 Tetrahydrofolate (THF) is an essential cofactor in the transformation of 2’-deoxyuridylate (dUMP) to 2’-deoxythymidylate (dTMP). This is a required step in the synthesis of purines and thus DNA.
Tetrahydrofolate (THF) is an essential cofactor in the transformation of 2’-deoxyuridylate (dUMP) to 2’-deoxythymidylate (dTMP). This is a required step in the synthesis of purines and thus DNA.Pyrimidine Analogues
 Fluorouracil is a uracil analogue. It is converted to FdUMP (fluorodeoxyuridine monophosphate) and although it interacts with thymidylate synthetase, it cannot be converted to dTMP because of the fluoro component and therefore results in a deficiency of dTMP. Without dTMP, DNA synthesis cannot occur. It is considered to be a fraudulent nucleotide.
Fluorouracil is a uracil analogue. It is converted to FdUMP (fluorodeoxyuridine monophosphate) and although it interacts with thymidylate synthetase, it cannot be converted to dTMP because of the fluoro component and therefore results in a deficiency of dTMP. Without dTMP, DNA synthesis cannot occur. It is considered to be a fraudulent nucleotide. Inhibitors of other enzymes important in dTMP synthesis include raltitrexed (thymidylate synthetase inhibitor) and pemetrexed (thymidylate transferase inhibitor).
Inhibitors of other enzymes important in dTMP synthesis include raltitrexed (thymidylate synthetase inhibitor) and pemetrexed (thymidylate transferase inhibitor). Cytarabine, also known as cytosine arabinoside, is an analogue of 2’-deoxycytidine (essentially, cytosine). On entering the cell, it is converted to cytosine arabinoside triphosphate by the same phosphorylation reaction that 2’-deoxycytidine undergoes. The cytosine arabinoside triphosphate competitively inhibits DNA polymerase, inhibiting both DNA synthesis and repair.
Cytarabine, also known as cytosine arabinoside, is an analogue of 2’-deoxycytidine (essentially, cytosine). On entering the cell, it is converted to cytosine arabinoside triphosphate by the same phosphorylation reaction that 2’-deoxycytidine undergoes. The cytosine arabinoside triphosphate competitively inhibits DNA polymerase, inhibiting both DNA synthesis and repair.Purine Analogues
 Fludarabine inhibits DNA polymerases, leading to inhibition of DNA synthesis and repair. It also incorporates into DNA and may also promote apoptosis by an undetermined mechanism.
Fludarabine inhibits DNA polymerases, leading to inhibition of DNA synthesis and repair. It also incorporates into DNA and may also promote apoptosis by an undetermined mechanism. Pentostatin inhibits adenosine deaminase, an enzyme that transforms adenosine to inosine. This leads to accumulation of intracellular adenosine and deoxyadenosine, which can block DNA synthesis by inhibiting ribonucleotide reductase. Ribonucleotide reductase catalyzes the formation of deoxyribonucleotides from ribonucleotides. Deoxyribonucleotides are used in the synthesis of DNA.
Pentostatin inhibits adenosine deaminase, an enzyme that transforms adenosine to inosine. This leads to accumulation of intracellular adenosine and deoxyadenosine, which can block DNA synthesis by inhibiting ribonucleotide reductase. Ribonucleotide reductase catalyzes the formation of deoxyribonucleotides from ribonucleotides. Deoxyribonucleotides are used in the synthesis of DNA.Pharmacokinetics
 Methotrexate can be administered orally, intravenously, intramuscularly, or intrathecally. It is not able to cross the blood-brain barrier, so entry into the central nervous system (CNS) can be achieved only by intrathecal administration.
Methotrexate can be administered orally, intravenously, intramuscularly, or intrathecally. It is not able to cross the blood-brain barrier, so entry into the central nervous system (CNS) can be achieved only by intrathecal administration. Only a small fraction of methotrexate is metabolized; most of it is eliminated unchanged in the urine. Because of its potential for nephrotoxicity, dose adjustments must be considered in patients with renal impairment.
Only a small fraction of methotrexate is metabolized; most of it is eliminated unchanged in the urine. Because of its potential for nephrotoxicity, dose adjustments must be considered in patients with renal impairment. The purine analogues mercaptopurine and thioguanine are available in oral dosage forms. They are metabolized by the liver. One of the enzymes responsible for mercaptopurine metabolism is xanthine oxidase; therefore inhibitors of this enzyme, such as allopurinol, can lead to toxicity. The elimination half-life of mercaptopurine is relatively short (<1 hour).
The purine analogues mercaptopurine and thioguanine are available in oral dosage forms. They are metabolized by the liver. One of the enzymes responsible for mercaptopurine metabolism is xanthine oxidase; therefore inhibitors of this enzyme, such as allopurinol, can lead to toxicity. The elimination half-life of mercaptopurine is relatively short (<1 hour). Fludarabine and cladribine can be administered intravenously or orally, and pentostatin is administered intravenously. All are primarily eliminated by renal excretion. Dose adjustments should be considered in patients with renal impairment.
Fludarabine and cladribine can be administered intravenously or orally, and pentostatin is administered intravenously. All are primarily eliminated by renal excretion. Dose adjustments should be considered in patients with renal impairment. Cytarabine is administered intravenously or subcutaneously, and 5-fluorouracil is administered intravenously or topically. Cytarabine (cytarabine triphosphate) and 5-fluorouracil (5-fluorodeoxyuridine monophosphate and triphosphate) are converted to active metabolites in the body. Both are largely metabolized by the liver, and dose adjustments should be considered in patients with hepatic impairment.
Cytarabine is administered intravenously or subcutaneously, and 5-fluorouracil is administered intravenously or topically. Cytarabine (cytarabine triphosphate) and 5-fluorouracil (5-fluorodeoxyuridine monophosphate and triphosphate) are converted to active metabolites in the body. Both are largely metabolized by the liver, and dose adjustments should be considered in patients with hepatic impairment.Side Effects
Folate Analogues
 Myelosuppression: Conventional chemotherapy agents work by targeting rapidly dividing cells. This nonspecific effect can also target rapidly dividing cells in other areas of the body, including bone marrow and the GI tract.
Myelosuppression: Conventional chemotherapy agents work by targeting rapidly dividing cells. This nonspecific effect can also target rapidly dividing cells in other areas of the body, including bone marrow and the GI tract.Purine Analogues
Important Notes
 High-dose regimens of methotrexate may require rescue with folinic acid, also known as leucovorin. Folinic acid is a reduced form of folic acid. Methotrexate is an “antifolate” drug, and patients who are taking high doses or receiving chronic therapy are likely to experience severe symptoms of folate deficiency unless they are treated adjunctively with leucovorin.
High-dose regimens of methotrexate may require rescue with folinic acid, also known as leucovorin. Folinic acid is a reduced form of folic acid. Methotrexate is an “antifolate” drug, and patients who are taking high doses or receiving chronic therapy are likely to experience severe symptoms of folate deficiency unless they are treated adjunctively with leucovorin. The drug interaction between mercaptopurine and allopurinol is particularly relevant because patients taking conventional chemotherapy are predisposed to development of uric acid crystals, also known as gout. Allopurinol is one of the key drugs used for treating gout, thus increasing the likelihood that these agents may be administered simultaneously.
The drug interaction between mercaptopurine and allopurinol is particularly relevant because patients taking conventional chemotherapy are predisposed to development of uric acid crystals, also known as gout. Allopurinol is one of the key drugs used for treating gout, thus increasing the likelihood that these agents may be administered simultaneously.Advanced
Pharmacogenetics
 Thiopurine methyltransferase (TPMT) plays a role in the metabolic inactivation of mercaptopurine. Approximately 15% of Caucasians have reduced activity of this enzyme, and these individuals are at greater risk for toxicity. TPMT genotyping is now readily available, and genotype-based dosage recommendations are available.
Thiopurine methyltransferase (TPMT) plays a role in the metabolic inactivation of mercaptopurine. Approximately 15% of Caucasians have reduced activity of this enzyme, and these individuals are at greater risk for toxicity. TPMT genotyping is now readily available, and genotype-based dosage recommendations are available.Bleomycin
Description
Bleomycin belongs to a family of glycopeptides that exert a cytotoxic effect by damaging DNA.
MOA (Mechanism of Action)
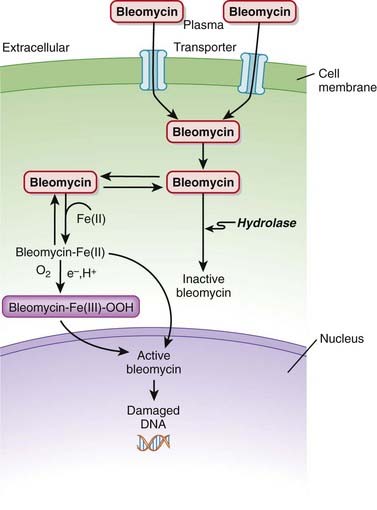
 Bleomycin is a DNA intercalator. An intercalator is a molecule that binds DNA and inserts itself into DNA structure.
Bleomycin is a DNA intercalator. An intercalator is a molecule that binds DNA and inserts itself into DNA structure. Bleomycin binds to Fe2+, and this complex leads to free radical formation when oxygen is added. These free radicals then intercalate between DNA strands, which produces single- and double-stranded breaks in DNA (Figure 20-4).
Bleomycin binds to Fe2+, and this complex leads to free radical formation when oxygen is added. These free radicals then intercalate between DNA strands, which produces single- and double-stranded breaks in DNA (Figure 20-4). Once in the cell, bleomycin either translocates to the nucleus or is broken down by bleomycin hydrolase, an enzyme that is found in both normal and malignant cells but is found in decreased concentrations in lung and skin. It is believed that the toxicities seen in lung and skin with these agents are the result of the lack of hydrolase activity in these regions.
Once in the cell, bleomycin either translocates to the nucleus or is broken down by bleomycin hydrolase, an enzyme that is found in both normal and malignant cells but is found in decreased concentrations in lung and skin. It is believed that the toxicities seen in lung and skin with these agents are the result of the lack of hydrolase activity in these regions.Pharmacokinetics
 Bleomycin is a large cation and therefore has difficulty penetrating cell membranes. It enters cells through a special membrane binding protein.
Bleomycin is a large cation and therefore has difficulty penetrating cell membranes. It enters cells through a special membrane binding protein.Side Effects
 Cutaneous side effects appear to be more common than with other conventional cytotoxic agents, likely because of the lack of hydrolase activity and relative buildup of bleomycin in skin:
Cutaneous side effects appear to be more common than with other conventional cytotoxic agents, likely because of the lack of hydrolase activity and relative buildup of bleomycin in skin:
 Nausea, vomiting: Cytotoxic agents tend to target rapidly dividing cells, including those of the GI tract, contributing to irritation of the GI tract.
Nausea, vomiting: Cytotoxic agents tend to target rapidly dividing cells, including those of the GI tract, contributing to irritation of the GI tract.Platinum Compounds
MOA (Mechanism of Action)
 Platinum (Pt) compounds are named for their central Pt ion, surrounded by chloride (Cl−) atoms and ammonia groups.
Platinum (Pt) compounds are named for their central Pt ion, surrounded by chloride (Cl−) atoms and ammonia groups. The platinum compounds cross-link DNA strands, thereby inhibiting DNA synthesis and function. If the DNA is damaged enough, the cell will undergo apoptosis (Figure 20-5).
The platinum compounds cross-link DNA strands, thereby inhibiting DNA synthesis and function. If the DNA is damaged enough, the cell will undergo apoptosis (Figure 20-5). On entering the cell, the Cl− ions dissociate, leaving behind a reactive complex that interacts with DNA.
On entering the cell, the Cl− ions dissociate, leaving behind a reactive complex that interacts with DNA.Side Effects
 Nausea and vomiting can be severe. Cytotoxic anticancer drugs target actively dividing cells, such as those found in the GI tract, leading to GI side effects.
Nausea and vomiting can be severe. Cytotoxic anticancer drugs target actively dividing cells, such as those found in the GI tract, leading to GI side effects. Nephrotoxicity is believed to be primarily mediated by mitochondrial toxicity. Preventive measures such as generous hydration with normal saline to promote diuresis should be undertaken. Nephrotoxicity is more of a problem with cisplatin than with the other agents in this class.
Nephrotoxicity is believed to be primarily mediated by mitochondrial toxicity. Preventive measures such as generous hydration with normal saline to promote diuresis should be undertaken. Nephrotoxicity is more of a problem with cisplatin than with the other agents in this class. Myelosuppression is less severe than with other conventional anticancer drugs. Conventional anticancer drugs target actively dividing cells, such as those found in the bone marrow, leading to bone marrow suppression.
Myelosuppression is less severe than with other conventional anticancer drugs. Conventional anticancer drugs target actively dividing cells, such as those found in the bone marrow, leading to bone marrow suppression. Tinnitus and hearing loss result from promotion of apoptosis in cochlear cells and appear to be less severe with oxaliplatin.
Tinnitus and hearing loss result from promotion of apoptosis in cochlear cells and appear to be less severe with oxaliplatin.Important Notes
 The 5-HT3 antagonists (e.g., ondansetron) have proven effective in managing the nausea and vomiting associated with this drug class.
The 5-HT3 antagonists (e.g., ondansetron) have proven effective in managing the nausea and vomiting associated with this drug class. Compared with cisplatin, carboplatin (a derivative of cisplatin) causes more myelosuppression but less nephrotoxicity, neurotoxicity, ototoxicity, nausea, and vomiting.
Compared with cisplatin, carboplatin (a derivative of cisplatin) causes more myelosuppression but less nephrotoxicity, neurotoxicity, ototoxicity, nausea, and vomiting.Taxanes
MOA (Mechanism of Action)
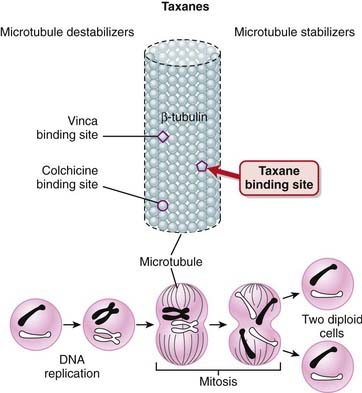
 Microtubules perform several important functions in the cell, the key being the segregation of chromosomes during mitosis.
Microtubules perform several important functions in the cell, the key being the segregation of chromosomes during mitosis. Microtubules are composed of tubulin, a heterodimer composed of a-tubulin and ß-tubulin. The polymerization of tubulin leads to formation of microtubules.
Microtubules are composed of tubulin, a heterodimer composed of a-tubulin and ß-tubulin. The polymerization of tubulin leads to formation of microtubules. Microtubules are in a dynamic equilibrium with the intracellular pool of α-tubulin and β-tubulin. Microtubules are constantly growing and shortening, and this process, known as dynamic instability, leads to the polar movement of chromosomes during anaphase.
Microtubules are in a dynamic equilibrium with the intracellular pool of α-tubulin and β-tubulin. Microtubules are constantly growing and shortening, and this process, known as dynamic instability, leads to the polar movement of chromosomes during anaphase. The taxanes bind to β-tubulin, stabilizing the microtubule and preventing its disassembly. This stabilization interferes with the segregation of chromosomes during mitosis, which leads to cell death. This is believed to be the main mechanism for the cytotoxic effects of taxanes (Figure 20-6).
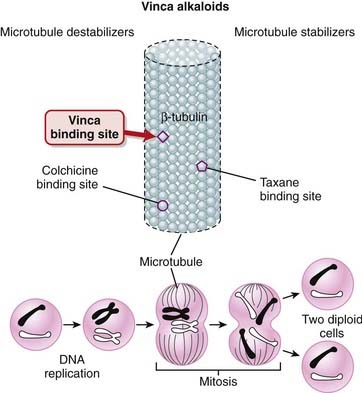
The taxanes bind to β-tubulin, stabilizing the microtubule and preventing its disassembly. This stabilization interferes with the segregation of chromosomes during mitosis, which leads to cell death. This is believed to be the main mechanism for the cytotoxic effects of taxanes (Figure 20-6). Vinca alkaloids are also microtubule inhibitors, and they also bind to β-tubulin; the difference is that they prevent polymerization of β-tubulin into microtubules and thus destabilize the microtubule.
Vinca alkaloids are also microtubule inhibitors, and they also bind to β-tubulin; the difference is that they prevent polymerization of β-tubulin into microtubules and thus destabilize the microtubule. Taxanes, specifically paclitaxel, are also used in drug-eluting vascular stents (often for coronary artery disease) to reduce the incidence of restenosis. Restenosis occurs, in part, because of the growth of new cells in the area of the stent. The taxanes inhibit cell division, thus preventing the growth of these new cells.
Taxanes, specifically paclitaxel, are also used in drug-eluting vascular stents (often for coronary artery disease) to reduce the incidence of restenosis. Restenosis occurs, in part, because of the growth of new cells in the area of the stent. The taxanes inhibit cell division, thus preventing the growth of these new cells.Pharmacokinetics
Side Effects
 Myelosuppression is a side effect commonly seen with conventional chemotherapy, which targets rapidly dividing cells, including those in the bone marrow.
Myelosuppression is a side effect commonly seen with conventional chemotherapy, which targets rapidly dividing cells, including those in the bone marrow. Neurotoxicity: Microtubules are believed to play an important role in neuronal function, and this is the likely reason why microtubule inhibitors are associated with neurotoxicity. This manifests itself as a variety of neuropathies:
Neurotoxicity: Microtubules are believed to play an important role in neuronal function, and this is the likely reason why microtubule inhibitors are associated with neurotoxicity. This manifests itself as a variety of neuropathies:
 Resistant fluid retention: Pretreatment with oral dexamethasone tends to mitigate the fluid retention. Docetaxel tends to produce more edema than the other agents in this class.
Resistant fluid retention: Pretreatment with oral dexamethasone tends to mitigate the fluid retention. Docetaxel tends to produce more edema than the other agents in this class. Hypersensitivity is likely to happen with either agent, and therefore pretreatment with corticosteroids and antihistamines is recommended. This effect results not from the agents themselves but from the solvents (polyoxyethylated castor oil or polysorbate) that each drug has to be dissolved in because of its low solubility.
Hypersensitivity is likely to happen with either agent, and therefore pretreatment with corticosteroids and antihistamines is recommended. This effect results not from the agents themselves but from the solvents (polyoxyethylated castor oil or polysorbate) that each drug has to be dissolved in because of its low solubility.Important Notes
 Other microtubule binding agents include the cytotoxic vinca alkaloids and colchicine, which is most commonly used for gout.
Other microtubule binding agents include the cytotoxic vinca alkaloids and colchicine, which is most commonly used for gout.Evidence
For Adjuvant Treatment of Early Breast Cancer
 A 2007 Cochrane review (12 studies, N = 18,304 women) compared taxane-containing with non–taxane-containing regimens as adjuvant treatment for premenopausal or postmenopausal women with early breast cancer. Overall survival (Hazard ratio [HR] 0.81) was improved with taxane-based regimens, as was disease-free survival (HR 0.81).
A 2007 Cochrane review (12 studies, N = 18,304 women) compared taxane-containing with non–taxane-containing regimens as adjuvant treatment for premenopausal or postmenopausal women with early breast cancer. Overall survival (Hazard ratio [HR] 0.81) was improved with taxane-based regimens, as was disease-free survival (HR 0.81).For Treatment of Metastatic Breast Cancer
 A 2005 Cochrane review (12 studies, N = 3643 women) compared taxane-containing with non–taxane-containing regimens in women with metastatic breast cancer. The authors found that overall survival was improved with taxane-based regimens versus nontaxane regimens (HR 0.93), although this difference was not statistically significant when taxane regimens were used first line. Time to progression was also improved (HR 0.92), as was overall response (odds ratio [OR] 1.34), although with considerable heterogeneity for overall response.
A 2005 Cochrane review (12 studies, N = 3643 women) compared taxane-containing with non–taxane-containing regimens in women with metastatic breast cancer. The authors found that overall survival was improved with taxane-based regimens versus nontaxane regimens (HR 0.93), although this difference was not statistically significant when taxane regimens were used first line. Time to progression was also improved (HR 0.92), as was overall response (odds ratio [OR] 1.34), although with considerable heterogeneity for overall response.Docetaxel versus Vinca Alkaloids for Treatment of Advanced Non–Small Cell Lung Cancer
 A 2007 systematic review (7 trials, N = 2867 participants) compared docetaxel with vinca alkaloid–based chemotherapy regimens (both are tubulin inhibitors) for treatment of advanced non–small cell lung cancer. Vinorelbine was the comparator in six trials, and vindesine in the remaining trial. The authors found improved overall survival with docetaxel (HR 0.89). The incidence of neutropenia was significantly reduced with docetaxel compared with the vinca alkaloids (OR 0.59), as was the incidence of febrile neutropenia (OR 0.57). There were also fewer serious adverse events with docetaxel compared with the vinca alkaloids.
A 2007 systematic review (7 trials, N = 2867 participants) compared docetaxel with vinca alkaloid–based chemotherapy regimens (both are tubulin inhibitors) for treatment of advanced non–small cell lung cancer. Vinorelbine was the comparator in six trials, and vindesine in the remaining trial. The authors found improved overall survival with docetaxel (HR 0.89). The incidence of neutropenia was significantly reduced with docetaxel compared with the vinca alkaloids (OR 0.59), as was the incidence of febrile neutropenia (OR 0.57). There were also fewer serious adverse events with docetaxel compared with the vinca alkaloids.Topoisomerase Inhibitors
MOA (Mechanism of Action)
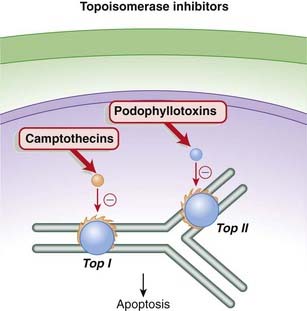
 The camptothecins are topoisomerase I inhibitors, whereas the podophyllotoxins are topoisomerase II inhibitors. Topoisomerase is an enzyme that cuts and reseals DNA strands, a process that is essential for DNA synthesis.
The camptothecins are topoisomerase I inhibitors, whereas the podophyllotoxins are topoisomerase II inhibitors. Topoisomerase is an enzyme that cuts and reseals DNA strands, a process that is essential for DNA synthesis. During the process of DNA replication or transcription, a significant amount of torsional strain is placed on the DNA helix. The strain is analogous to twisting a rubber band or a telephone cord.
During the process of DNA replication or transcription, a significant amount of torsional strain is placed on the DNA helix. The strain is analogous to twisting a rubber band or a telephone cord. The torsional strain is relieved by breaks in DNA strands that are created by the enzymes topoisomerase I (single-stranded DNA) and topoisomerase II (double-stranded DNA). The analogy would be relieving the strain in a twisted rubber band by making small breaks in the band, allowing the band to untwist, then resealing the breaks. The topoisomerase enzymes also reseal the breaks after the tension has been relieved (Figure 20-7).
The torsional strain is relieved by breaks in DNA strands that are created by the enzymes topoisomerase I (single-stranded DNA) and topoisomerase II (double-stranded DNA). The analogy would be relieving the strain in a twisted rubber band by making small breaks in the band, allowing the band to untwist, then resealing the breaks. The topoisomerase enzymes also reseal the breaks after the tension has been relieved (Figure 20-7).Pharmacokinetics
 All topoisomerase inhibitors are available in intravenous formulations. Etoposide and topotecan are also available in an oral formulation.
All topoisomerase inhibitors are available in intravenous formulations. Etoposide and topotecan are also available in an oral formulation. Topotecan is eliminated renally; therefore dose adjustments may be required in patients with renal impairment. The elimination half-life is 2 to 3 hours.
Topotecan is eliminated renally; therefore dose adjustments may be required in patients with renal impairment. The elimination half-life is 2 to 3 hours. Irinotecan is metabolized by the liver; therefore dose adjustments may be required in patients with hepatic impairment. The parent is metabolized by CYP3A4, and it also has an active metabolite, SN-38. SN-38 has much greater antitumor activity than the parent compound, and it is metabolized by glucuronidation via UGT1A1.
Irinotecan is metabolized by the liver; therefore dose adjustments may be required in patients with hepatic impairment. The parent is metabolized by CYP3A4, and it also has an active metabolite, SN-38. SN-38 has much greater antitumor activity than the parent compound, and it is metabolized by glucuronidation via UGT1A1.Contraindications
Side Effects
 Nausea, vomiting: Cytotoxic agents tend to target rapidly dividing cells, including those of the GI tract, contributing to irritation of the GI tract.
Nausea, vomiting: Cytotoxic agents tend to target rapidly dividing cells, including those of the GI tract, contributing to irritation of the GI tract. Alopecia: Cytotoxic chemotherapy agents tend to target tissues with rapidly dividing cells, such as those in hair follicles.
Alopecia: Cytotoxic chemotherapy agents tend to target tissues with rapidly dividing cells, such as those in hair follicles.Serious
 Bone marrow suppression may lead to severe neutropenia, thrombocytopenia, and/or anemia. These have caused fatalities. The mechanism is the same as that seen with other cytotoxic chemotherapy agents, namely destruction of actively dividing cells in the marrow.
Bone marrow suppression may lead to severe neutropenia, thrombocytopenia, and/or anemia. These have caused fatalities. The mechanism is the same as that seen with other cytotoxic chemotherapy agents, namely destruction of actively dividing cells in the marrow. Interstitial lung disease can be fatal. Signs and symptoms include cough, fever, dyspnea, and/or hypoxia. More common with topotecan than irinotecan.
Interstitial lung disease can be fatal. Signs and symptoms include cough, fever, dyspnea, and/or hypoxia. More common with topotecan than irinotecan.Irinotecan
 Diarrhea can be either early onset or late onset with irinotecan. Late-onset diarrhea can be particularly severe and life-threatening. Early onset diarrhea is caused by cholinergic activation (irinotecan is a cholinesterase inhibitor) and can therefore be managed with atropine. Late-onset diarrhea must be treated promptly with loperamide.
Diarrhea can be either early onset or late onset with irinotecan. Late-onset diarrhea can be particularly severe and life-threatening. Early onset diarrhea is caused by cholinergic activation (irinotecan is a cholinesterase inhibitor) and can therefore be managed with atropine. Late-onset diarrhea must be treated promptly with loperamide.Important Notes
 Although topoisomerase inhibitors are used as anticancer treatments, topoisomerase inhibition has been linked to promoting some cancers, particularly leukemia. The reason is that some of the cells with fragmented DNA survive. The fragmented DNA in these surviving cells can recombine, forming mutations that may then lead to development of neoplasms.
Although topoisomerase inhibitors are used as anticancer treatments, topoisomerase inhibition has been linked to promoting some cancers, particularly leukemia. The reason is that some of the cells with fragmented DNA survive. The fragmented DNA in these surviving cells can recombine, forming mutations that may then lead to development of neoplasms.Advanced
Pharmacogenetics
 Patients with the UGT1A1*28 polymorphism may have reduced activity of this enzyme, reducing their ability to metabolize SN-38, the active metabolite of irinotecan. This polymorphism is seen in approximately 10% of North Americans, and patients with it may be at greater risk of serious toxicities, including fatal hematologic toxicities such as neutropenia.
Patients with the UGT1A1*28 polymorphism may have reduced activity of this enzyme, reducing their ability to metabolize SN-38, the active metabolite of irinotecan. This polymorphism is seen in approximately 10% of North Americans, and patients with it may be at greater risk of serious toxicities, including fatal hematologic toxicities such as neutropenia.Vinca Alkaloids
MOA (Mechanism of Action)
 Tubulin exists in the cell as a heterodimer consisting of one molecule of α-tubulin and one molecule of β-tubulin. The polymerization of tubulin leads to formation of microtubules, which play an important role in spindle formation. Formation of the mitotic spindle is a key step in the segregation of chromosomes.
Tubulin exists in the cell as a heterodimer consisting of one molecule of α-tubulin and one molecule of β-tubulin. The polymerization of tubulin leads to formation of microtubules, which play an important role in spindle formation. Formation of the mitotic spindle is a key step in the segregation of chromosomes. When the vinca alkaloids bind β-tubulin, they inhibit its polymerization into microtubules, preventing spindle formation in dividing cells. This prevents chromosomes from aligning as they normally do, resulting in a disordered dispersion of chromosomes throughout the nucleus. This causes cell cycle arrest at metaphase, and apoptosis (Figure 20-8).
When the vinca alkaloids bind β-tubulin, they inhibit its polymerization into microtubules, preventing spindle formation in dividing cells. This prevents chromosomes from aligning as they normally do, resulting in a disordered dispersion of chromosomes throughout the nucleus. This causes cell cycle arrest at metaphase, and apoptosis (Figure 20-8). Other cellular activities that involve microtubules are also inhibited by these agents, including leukocyte phagocytosis and chemotaxis, as well as axonal transport in neurons.
Other cellular activities that involve microtubules are also inhibited by these agents, including leukocyte phagocytosis and chemotaxis, as well as axonal transport in neurons.Pharmacokinetics
 The vinca alkaloids have long elimination half-lives (24 to 48 hours). They are administered intravenously. The main route of elimination for the vinca alkaloids is hepatic, and dose adjustments should be considered in patients with hepatic impairment.
The vinca alkaloids have long elimination half-lives (24 to 48 hours). They are administered intravenously. The main route of elimination for the vinca alkaloids is hepatic, and dose adjustments should be considered in patients with hepatic impairment.Contraindications
 Neurologic diseases that might be worsened by the neurotoxic effects of the vinca alkaloids, particularly vincristine, are contraindications.
Neurologic diseases that might be worsened by the neurotoxic effects of the vinca alkaloids, particularly vincristine, are contraindications. Intrathecal administration: The intrathecal route (into the cerebral spinal fluid via a spinal injection) is fatal.
Intrathecal administration: The intrathecal route (into the cerebral spinal fluid via a spinal injection) is fatal.Side Effects
 Myelosuppression: Conventional chemotherapy agents tend to target rapidly dividing cells, such as those found in the bone marrow.
Myelosuppression: Conventional chemotherapy agents tend to target rapidly dividing cells, such as those found in the bone marrow. Alopecia: Conventional chemotherapy agents also tend to target rapidly dividing cells found in hair follicles, leading to hair loss.
Alopecia: Conventional chemotherapy agents also tend to target rapidly dividing cells found in hair follicles, leading to hair loss.Evidence
Docetaxel versus Vinca Alkaloids in Advanced Non–Small Cell Lung Cancer
 A 2007 systematic review (seven trials, N = 2867 participants) compared docetaxel with vinca alkaloid–based chemotherapy regimens (both are tubulin inhibitors) in advanced non–small cell lung cancer. Vinorelbine was the comparator in six trials, and vindesine in the remaining trial. The authors found improved overall survival with docetaxel (HR 0.89). The incidence of neutropenia was significantly reduced with docetaxel compared with the vinca alkaloids (OR 0.59), as was the incidence of febrile neutropenia (OR 0.57). There were also fewer serious adverse events with docetaxel compared with the vinca alkaloids.
A 2007 systematic review (seven trials, N = 2867 participants) compared docetaxel with vinca alkaloid–based chemotherapy regimens (both are tubulin inhibitors) in advanced non–small cell lung cancer. Vinorelbine was the comparator in six trials, and vindesine in the remaining trial. The authors found improved overall survival with docetaxel (HR 0.89). The incidence of neutropenia was significantly reduced with docetaxel compared with the vinca alkaloids (OR 0.59), as was the incidence of febrile neutropenia (OR 0.57). There were also fewer serious adverse events with docetaxel compared with the vinca alkaloids.Gonadotropin-Releasing Hormone (GnRH) Analogues
MOA (Mechanism of Action)
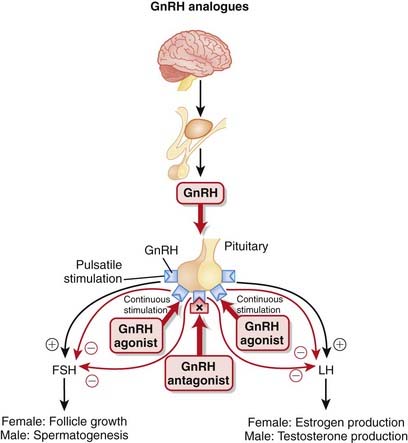
 GnRH is synthesized in the hypothalamus and controls the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. FSH and LH stimulate the gonads (ovaries in females, testes in males), leading to the production of androgens in males.
GnRH is synthesized in the hypothalamus and controls the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. FSH and LH stimulate the gonads (ovaries in females, testes in males), leading to the production of androgens in males. GnRH is released in a pulsatile manner. This intermittent release is crucial for the proper synthesis and release of FSH and LH (Figure 20-9).
GnRH is released in a pulsatile manner. This intermittent release is crucial for the proper synthesis and release of FSH and LH (Figure 20-9).Pharmacokinetics
Indications
Contraindications
Side Effects
Rare, Serious
 Ovarian hyperstimulation syndrome (OHSS): Through extensive luteinization (transformation of a follicle into a corpus luteum), estrogen, progesterone, and cytokine levels increase, resulting in leaky capillaries and in fluid shifts from the vascular compartment to the extravascular compartments. This can result in edema, ascites, and intravascular hypovolemia.
Ovarian hyperstimulation syndrome (OHSS): Through extensive luteinization (transformation of a follicle into a corpus luteum), estrogen, progesterone, and cytokine levels increase, resulting in leaky capillaries and in fluid shifts from the vascular compartment to the extravascular compartments. This can result in edema, ascites, and intravascular hypovolemia.Important Notes
 The use of GnRH agonists results in an initial increase in testosterone release, commonly referred to as a flare. This flare can last anywhere from days to weeks. Once the receptors have become desensitized, GnRH is inhibited, and the antiandrogenic effects of the drug are seen. The flare can result in a temporary worsening of the clinical condition before benefits are seen.
The use of GnRH agonists results in an initial increase in testosterone release, commonly referred to as a flare. This flare can last anywhere from days to weeks. Once the receptors have become desensitized, GnRH is inhibited, and the antiandrogenic effects of the drug are seen. The flare can result in a temporary worsening of the clinical condition before benefits are seen. One potential advantage of GnRH antagonists over GnRH agonists is that because they antagonize the GnRH receptor, this flare does not occur.
One potential advantage of GnRH antagonists over GnRH agonists is that because they antagonize the GnRH receptor, this flare does not occur. Another difference between GnRH agonists and antagonists is that antagonists consistently block both FSH and LH secretion, whereas FSH levels tend to rise after a period of time with agonist therapy. The clinical significance of this difference is not known.
Another difference between GnRH agonists and antagonists is that antagonists consistently block both FSH and LH secretion, whereas FSH levels tend to rise after a period of time with agonist therapy. The clinical significance of this difference is not known. Prostate cancer tends to be a very hormone-dependent cancer, growing under the stimulation of androgens. The growth stimulating effects of androgens can be reduced in two ways, by either surgical (removal of both testes) or “medical castration.” Medical castration is essentially androgen deprivation therapy.
Prostate cancer tends to be a very hormone-dependent cancer, growing under the stimulation of androgens. The growth stimulating effects of androgens can be reduced in two ways, by either surgical (removal of both testes) or “medical castration.” Medical castration is essentially androgen deprivation therapy.Evidence
Preoperative GnRH Analogues in Treatment of Uterine Fibroids
 A 2001 Cochrane review (26 trials) evaluated the role of pretreatment with GnRH analogues before hysterectomy or myomectomy for uterine fibroids. The authors found that the use of GnRH analogues for 3 to 4 months before fibroid surgery reduced uterine volume and fibroid size. The authors also found that midline incisions could be avoided because of a reduction in the size of the uterus.
A 2001 Cochrane review (26 trials) evaluated the role of pretreatment with GnRH analogues before hysterectomy or myomectomy for uterine fibroids. The authors found that the use of GnRH analogues for 3 to 4 months before fibroid surgery reduced uterine volume and fibroid size. The authors also found that midline incisions could be avoided because of a reduction in the size of the uterus.GnRH Agonists versus Antagonists for Assisted Conception
 A 2006 Cochrane review (27 trials) evaluated the efficacy of GnRH antagonists compared with the standard long protocol of GnRH agonists for controlled ovarian hyperstimulation in assisted conception. The pregnancy rate was lower with antagonist therapy (OR 0.84), but there was a reduction in the incidence of severe OHSS (OR 0.61).
A 2006 Cochrane review (27 trials) evaluated the efficacy of GnRH antagonists compared with the standard long protocol of GnRH agonists for controlled ovarian hyperstimulation in assisted conception. The pregnancy rate was lower with antagonist therapy (OR 0.84), but there was a reduction in the incidence of severe OHSS (OR 0.61).FYI
 Leutin is Latin, meaning yellow. The corpus luteum is yellow endocrine tissue released from the graafian follicle in the ovary.
Leutin is Latin, meaning yellow. The corpus luteum is yellow endocrine tissue released from the graafian follicle in the ovary. Myo– refers to muscle. Myome– refers to myometrium, the middle muscular layer of the uterus. Myomectomy is surgical removal of a fibroid (which is composed of myometrium).
Myo– refers to muscle. Myome– refers to myometrium, the middle muscular layer of the uterus. Myomectomy is surgical removal of a fibroid (which is composed of myometrium).Angiogenesis Inhibitors
Description
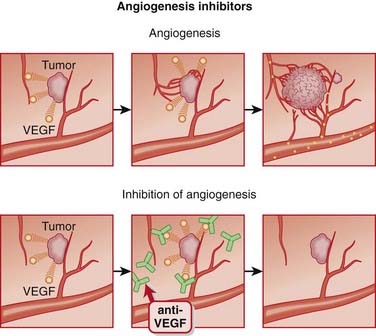
Angiogenesis inhibitors are agents that inhibit the growth of new blood vessels (angiogenesis).
MOA (Mechanism of Action)
 Angiogenesis is essential to the growth of solid tumors. Neoplastic tissue, which is highly metabolically active, must have access to the nutrients in blood in order to continue growing.
Angiogenesis is essential to the growth of solid tumors. Neoplastic tissue, which is highly metabolically active, must have access to the nutrients in blood in order to continue growing. It is believed that complete blockade of angiogenesis will not only prevent the growth of new vessels, but also promote instability and degradation of existing vessels within the tumor.
It is believed that complete blockade of angiogenesis will not only prevent the growth of new vessels, but also promote instability and degradation of existing vessels within the tumor.Pharmacokinetics
Side Effects
Bevacizumab
 GI perforation: VEGF blockade may lead to ischemia in regions of the GI tract, promoting perforation.
GI perforation: VEGF blockade may lead to ischemia in regions of the GI tract, promoting perforation. Hemorrhage, particularly pulmonary hemorrhage, may be fatal in some cases. This is because of the nonspecific destabilization of blood vessels secondary to VEGF blockade.
Hemorrhage, particularly pulmonary hemorrhage, may be fatal in some cases. This is because of the nonspecific destabilization of blood vessels secondary to VEGF blockade. Hypertension: VEGF tends to have a vasorelaxant effect, and it is believed that blockade of VEGF leads to elevation in blood pressure, which becomes clinically significant in some patients.
Hypertension: VEGF tends to have a vasorelaxant effect, and it is believed that blockade of VEGF leads to elevation in blood pressure, which becomes clinically significant in some patients.Important Notes
 Bevacizumab is being used off-label in many jurisdictions for the management of age-related macular degeneration. Because it is typically administered in large doses systemically for cancer, small doses of bevacizumab administered locally are much cheaper than ranibizumab.
Bevacizumab is being used off-label in many jurisdictions for the management of age-related macular degeneration. Because it is typically administered in large doses systemically for cancer, small doses of bevacizumab administered locally are much cheaper than ranibizumab. Because of its effects on wound healing, bevacizumab should not be initiated within 4 weeks after surgery, and it should be discontinued at least 6 weeks before surgery.
Because of its effects on wound healing, bevacizumab should not be initiated within 4 weeks after surgery, and it should be discontinued at least 6 weeks before surgery. Ranibizumab was specifically designed to be used in age-related macular degeneration and is not used in cancer. It is administered locally, by intravitreal injection, and therefore lacks many of the side effects of bevacizumab.
Ranibizumab was specifically designed to be used in age-related macular degeneration and is not used in cancer. It is administered locally, by intravitreal injection, and therefore lacks many of the side effects of bevacizumab. Verteporfin photodynamic therapy (PDT) is another approach used to treat age-related macular degeneration (AMD) and in fact was the favored new therapy before the approval of the angiogenesis inhibitors. This drug was administered intravenously and activated by a laser shone into the eye. The laser stimulated the production of free radicals, which then damaged blood vessels. This was an early antiangiogenic therapy, although it was primitive compared with the newer angiogenesis inhibitors.
Verteporfin photodynamic therapy (PDT) is another approach used to treat age-related macular degeneration (AMD) and in fact was the favored new therapy before the approval of the angiogenesis inhibitors. This drug was administered intravenously and activated by a laser shone into the eye. The laser stimulated the production of free radicals, which then damaged blood vessels. This was an early antiangiogenic therapy, although it was primitive compared with the newer angiogenesis inhibitors.Evidence
Age-Related Macular Degeneration
 A 2008 Cochrane review (five trials, N = 2500 participants) investigated anti-VEGF therapies for age-related macular degeneration and included three trials of ranibizumab. Efficacy was measured by the ability to prevent a reduction in visual acuity of 15 letters or more, and ranibizumab prevented vision loss versus verteporfin PDT (number needed to treat [NNT = 3]) and sham (NNT = 3); the combination of ranibizumab and verteporfin PDT prevented vision loss versus verteporfin alone (NNT = 4). Ranibizumab also improved vision by 15 letters or more versus each of these agents.
A 2008 Cochrane review (five trials, N = 2500 participants) investigated anti-VEGF therapies for age-related macular degeneration and included three trials of ranibizumab. Efficacy was measured by the ability to prevent a reduction in visual acuity of 15 letters or more, and ranibizumab prevented vision loss versus verteporfin PDT (number needed to treat [NNT = 3]) and sham (NNT = 3); the combination of ranibizumab and verteporfin PDT prevented vision loss versus verteporfin alone (NNT = 4). Ranibizumab also improved vision by 15 letters or more versus each of these agents.Metastatic Colorectal Cancer
 A 2009 Cochrane review investigated the efficacy and toxicity of targeted antiangiogenic therapies in addition to chemotherapy in patients with metastatic colorectal cancer. The authors found five trials of bevacizumab (N = 3101 patients). The combination of bevacizumab and first-line chemotherapy significantly improved overall survival (HR 0.81) and progression-free survival (HR 0.61) versus first-line chemotherapy alone. The results for combining bevacizumab with second-line chemotherapy were not conclusive owing to significant heterogeneity.
A 2009 Cochrane review investigated the efficacy and toxicity of targeted antiangiogenic therapies in addition to chemotherapy in patients with metastatic colorectal cancer. The authors found five trials of bevacizumab (N = 3101 patients). The combination of bevacizumab and first-line chemotherapy significantly improved overall survival (HR 0.81) and progression-free survival (HR 0.61) versus first-line chemotherapy alone. The results for combining bevacizumab with second-line chemotherapy were not conclusive owing to significant heterogeneity.FYI
 One of the most notorious drugs ever developed, thalidomide, is an angiogenesis inhibitor. When it first came out in the 1950s, it was marketed as an antinauseant for pregnant women. Tragically, thalidomide turned out to be one of the worst teratogens ever developed and caused countless birth defects. Many babies were born with malformed limbs; this was a result of impaired vascular development, an effect of thalidomide that was not known at the time of its development.
One of the most notorious drugs ever developed, thalidomide, is an angiogenesis inhibitor. When it first came out in the 1950s, it was marketed as an antinauseant for pregnant women. Tragically, thalidomide turned out to be one of the worst teratogens ever developed and caused countless birth defects. Many babies were born with malformed limbs; this was a result of impaired vascular development, an effect of thalidomide that was not known at the time of its development.Tyrosine Kinase Inhibitors
MOA (Mechanism of Action) (Figure 20-11)
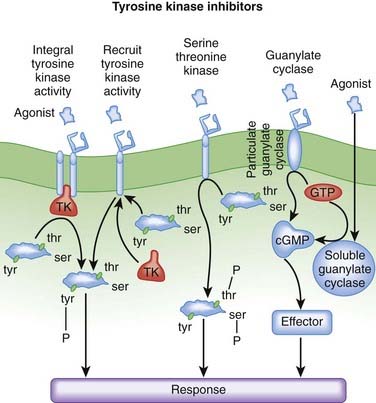
 Phosphorylation is a process whereby a protein kinase transfers a phosphate group from adenosine triphosphate (ATP) or guanosine triphosphate (GTP) to free hydroxyl groups of amino acids. In doing so, they modify protein function. Most protein kinases phosphorylate serine and threonine residues, but a subset of protein kinases phosphorylate tyrosine residues.
Phosphorylation is a process whereby a protein kinase transfers a phosphate group from adenosine triphosphate (ATP) or guanosine triphosphate (GTP) to free hydroxyl groups of amino acids. In doing so, they modify protein function. Most protein kinases phosphorylate serine and threonine residues, but a subset of protein kinases phosphorylate tyrosine residues. The protein tyrosine kinases are subdivided into receptor tyrosine kinases (RTKs) and nonreceptor (cytoplasmic) tyrosine kinases.
The protein tyrosine kinases are subdivided into receptor tyrosine kinases (RTKs) and nonreceptor (cytoplasmic) tyrosine kinases. Tyrosine kinase receptors regulate growth and cell differentiation. Therefore ligands for these receptors include several growth factors, and they play an important role in neoplasia. The RTK family includes growth factor receptors such as epidermal growth factor receptor (EGFR).
Tyrosine kinase receptors regulate growth and cell differentiation. Therefore ligands for these receptors include several growth factors, and they play an important role in neoplasia. The RTK family includes growth factor receptors such as epidermal growth factor receptor (EGFR). Under normal circumstances, frequent binding of growth factors to these tyrosine kinase receptors leads to receptor down-regulation. This blunts the activity of these growth factors, preventing abnormal growth from occurring. In cancer, mutation of these receptors prevents this down-regulation, removing an important check on uncontrolled growth.
Under normal circumstances, frequent binding of growth factors to these tyrosine kinase receptors leads to receptor down-regulation. This blunts the activity of these growth factors, preventing abnormal growth from occurring. In cancer, mutation of these receptors prevents this down-regulation, removing an important check on uncontrolled growth.EGFR/HER
 The epidermal growth factor (EGF) pathway plays an important role in neoplasia. Four receptors belong to the EGF pathway: EGFR and human epidermal growth factors (HER2, HER3, HER4).
The epidermal growth factor (EGF) pathway plays an important role in neoplasia. Four receptors belong to the EGF pathway: EGFR and human epidermal growth factors (HER2, HER3, HER4).BCR-ABL/src
 Chronic myelogenous leukemia (CML) is most frequently caused by a chromosomally abnormal hematopoietic stem cell. The BCR (break point cluster) gene of one chromosome and the ABL (Abelson) gene of another chromosome translocate, moving from separate chromosomes onto the same chromosome, fusing to become one gene, known as BCR-ABL. BCR-ABL is referred to as a fusion oncoprotein, because these two genes, when joined together, are associated with the development of cancer.
Chronic myelogenous leukemia (CML) is most frequently caused by a chromosomally abnormal hematopoietic stem cell. The BCR (break point cluster) gene of one chromosome and the ABL (Abelson) gene of another chromosome translocate, moving from separate chromosomes onto the same chromosome, fusing to become one gene, known as BCR-ABL. BCR-ABL is referred to as a fusion oncoprotein, because these two genes, when joined together, are associated with the development of cancer. Important to drug therapy, ABL codes for tyrosine kinase receptors. BCR-ABL creates a tyrosine kinase receptor that is constitutively active, meaning that it is continually active. Tyrosine kinase receptors are growth factor receptors, meaning that their continual activation leads to dysregulated growth of myeloid cells.
Important to drug therapy, ABL codes for tyrosine kinase receptors. BCR-ABL creates a tyrosine kinase receptor that is constitutively active, meaning that it is continually active. Tyrosine kinase receptors are growth factor receptors, meaning that their continual activation leads to dysregulated growth of myeloid cells.Other Kinases
 Drugs that inhibit multiple kinases were originally thought to be disadvantageous because of an increased likelihood of side effects. Instead, it is now believed that targeting multiple kinases addresses the redundancy that is found among signaling pathways, perhaps leading to enhanced efficacy. Sorafenib and sunitinib are examples of multikinase inhibitors, and their targets include:
Drugs that inhibit multiple kinases were originally thought to be disadvantageous because of an increased likelihood of side effects. Instead, it is now believed that targeting multiple kinases addresses the redundancy that is found among signaling pathways, perhaps leading to enhanced efficacy. Sorafenib and sunitinib are examples of multikinase inhibitors, and their targets include:
Pharmacokinetics
 The small molecule tyrosine kinase inhibitors used in cancer are all orally administered. The monoclonal antibodies are administered by intravenous infusion.
The small molecule tyrosine kinase inhibitors used in cancer are all orally administered. The monoclonal antibodies are administered by intravenous infusion. The bioavailability of sorafenib is reduced with a high-fat meal; therefore it is recommended that it either be taken on an empty stomach or with a low- or moderate-fat meal.
The bioavailability of sorafenib is reduced with a high-fat meal; therefore it is recommended that it either be taken on an empty stomach or with a low- or moderate-fat meal. Sunitinib and its active metabolites have a long elimination half-life (40 to 60 and 80 to 110 hours, respectively).
Sunitinib and its active metabolites have a long elimination half-life (40 to 60 and 80 to 110 hours, respectively). Sunitinib, sorafenib, imatinib, dasatinib, and erlotinib are all metabolized by CYP3A4. Interactions with known inhibitors or inducers of these isozymes are to be expected, and concomitant use should be avoided if possible. Because these agents are primarily eliminated by hepatic metabolism, no dose adjustments are required in patients with renal impairment.
Sunitinib, sorafenib, imatinib, dasatinib, and erlotinib are all metabolized by CYP3A4. Interactions with known inhibitors or inducers of these isozymes are to be expected, and concomitant use should be avoided if possible. Because these agents are primarily eliminated by hepatic metabolism, no dose adjustments are required in patients with renal impairment.Side Effects
Serious
Sorafenib, Imatinib
 Left ventricular dysfunction: Cardiotoxicity with imatinib may be caused by mitochondrial dysfunction. Cardiomyocytes are highly energy dependent. The mechanism is not clear with sorafenib, but because the drug is a multikinase inhibitor, it might be inhibiting a kinase involved in myocardial function.
Left ventricular dysfunction: Cardiotoxicity with imatinib may be caused by mitochondrial dysfunction. Cardiomyocytes are highly energy dependent. The mechanism is not clear with sorafenib, but because the drug is a multikinase inhibitor, it might be inhibiting a kinase involved in myocardial function.Dasatinib, Imatinib
Important Notes
 The tyrosine kinase inhibitors are collectively referred to as targeted therapies, a reflection of the fact that they target growth factors that are active only in cancer. The theoretical advantage of targeted therapies is enhanced selective toxicity and avoidance of disabling side effects such as GI effects, myelosuppression, and alopecia. It is important to note, though, that these side effects still occur with many of these targeted therapies but at a lower incidence than with conventional chemotherapy.
The tyrosine kinase inhibitors are collectively referred to as targeted therapies, a reflection of the fact that they target growth factors that are active only in cancer. The theoretical advantage of targeted therapies is enhanced selective toxicity and avoidance of disabling side effects such as GI effects, myelosuppression, and alopecia. It is important to note, though, that these side effects still occur with many of these targeted therapies but at a lower incidence than with conventional chemotherapy. Although the multikinase inhibitors both attack similar targets, sunitinib appears to have stronger binding affinity for many of these targets and therefore enhanced efficacy over sorafenib.
Although the multikinase inhibitors both attack similar targets, sunitinib appears to have stronger binding affinity for many of these targets and therefore enhanced efficacy over sorafenib.Advanced
Pregnancy
 The extent of risk for all the tyrosine kinase inhibitors in pregnancy is approximately the same. Because they inhibit growth factors, in many cases angiogenic growth factors, there is serious concern about their effects on fetal development. These agents are therefore not recommended in pregnancy unless the withdrawal of therapy represents unacceptable risk to the mother.
The extent of risk for all the tyrosine kinase inhibitors in pregnancy is approximately the same. Because they inhibit growth factors, in many cases angiogenic growth factors, there is serious concern about their effects on fetal development. These agents are therefore not recommended in pregnancy unless the withdrawal of therapy represents unacceptable risk to the mother.FYI
 The translocation that results in the formation of the BCR-ABL fusion oncoprotein is called the Philadelphia chromosome. It was named after the city in which it was discovered.
The translocation that results in the formation of the BCR-ABL fusion oncoprotein is called the Philadelphia chromosome. It was named after the city in which it was discovered. The tyrosine kinase inhibitors ending in -nib (e.g., erlotinib, imatinib) are often referred to as small-molecule tyrosine kinase inhibitors to distinguish them from the tyrosine kinase inhibitors that are monoclonal antibodies. Because monoclonal antibodies are proteins, they have a much larger structure.
The tyrosine kinase inhibitors ending in -nib (e.g., erlotinib, imatinib) are often referred to as small-molecule tyrosine kinase inhibitors to distinguish them from the tyrosine kinase inhibitors that are monoclonal antibodies. Because monoclonal antibodies are proteins, they have a much larger structure.