36 Zygapophysial Joint Pain
Procedures for Diagnosis and Treatment
Zygapophysial joint (ZJ) pain stems from synovial ZJs, which are formed by adjacent articular processes (or apophyses) of the vertebrae. In the past, these joints were commonly called “facet joints” but that term is inappropriate because most synovial joints of the body (such as those of the elbows, wrists, and hands) have facets. The term “zygapophysial joints” is the correct name for the spinal synovial joints in current anatomic nomenclature.1
The phenomenon of lumbar ZJ pain was first mooted in 19112 and gradually gained acceptance,3,4 but since the 1930s when intervertebral disc surgery became feasible as a treatment for spinal pain, ZJ pain has been overshadowed in the minds of many by disc pain. Cervical ZJ pain has been established scientifically for only the last few decades.5,6 Over that time, a great deal of scientific research has been done and a considerable body of literature has been produced. ZJ pain has also generated considerable interest in clinical, funding, and medicolegal fields. Controversies have arisen about the etiology of ZJ pain and about the reliability, validity, and effectiveness of methods used to address it. Against that background, it is important for clinicians to appreciate the methods available for the diagnosis and treatment of ZJ pain and the scientific evidence on which they are based.
Zygapophysial Joints
Anatomy
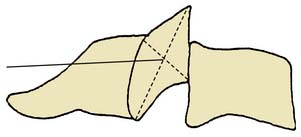
The ZJs are paired synovial joints that link the posterior elements of the spine from the C2-3 level down to the lowest spinal motion segment, L5-S1.7 At each of these cervical, thoracic, and lumbar spinal levels, the two adjacent vertebrae are linked by three articulations: at the front is a synarthrodial interbody joint linking the anterior spinal elements (the vertebral bodies) and at the back are two diarthrodial (synovial) ZJs, one on the left and one on the right, linking the posterior spinal elements (the neural arches of the vertebrae that enclose the spinal canal8) as indicated in Figure 36-1.
A ZJ is made up of two bony processes or apophyses, a superior and an inferior articular process, each of which has an articular surface (or facet) lined with hyaline cartilage about 1 to 2 mm. thick.9 Under the cartilage is a thickened layer of the subchondral bone. The joint surfaces are enveloped by a collagenous articular capsule which has superior and inferior capsular recesses above and below the main joint space.10,11 The joint space, including the recesses, is typically of about 1 mL in volume.12 The capsule is lined internally with synovial membrane, which with the cartilaginous joint surfaces, encloses the joint space. Within the joint space but outside the synovial membrane there are often intraarticular inclusions, the most common being adipose tissue pads and fibroadipose meniscoids.13 The morphologic features of specific cervical, thoracic and lumbar joints will be addressed in the following section on biomechanics and the sections on the various interventional procedures.
The nerve supply of the ZJs is via the medial branches of the dorsal rami of the spinal nerves.5,14,15 More specifically, the ZJs are supplied by articular branches of the medial branches; these articular branches are simply groups of medial branch dendrites that join the main trunks of their respective medial branches in the posterior parts of their courses. The joint capsules are richly innervated by sensory afferent fibers (first order neurons) which transmit neural impulses from each joint via the medial branch nerves to their cell bodies in the dorsal root ganglia and then on to synapse with second order neurons in the dorsal horn of the spinal cord; from there impulses are transmitted via central pathways to the sensory cortex.
Most ZJs are supplied by two medial branches, those of the spinal nerves above and below the joint. In the cervical spine from C3-4 down the two medial branches that supply each joint are those numbered accordingly. For example the C5-6 ZJ is supplied by the C5 and C6 medial branches. Because there are eight cervical spinal nerves, the C7-T1 ZJ is supplied by the C7 and C8 medial branches. Then from T1-2 downward the medial branches that supply the thoracic and lumbar ZJs are numbered one less than the corresponding joint. Thus, for example, the T1-2 ZJ is supplied by the C8 and T1 medial branches, the T8-9 ZJ by the T7 and T8 medial branches and the L4-5 ZJ by the L3 and L4 medial branches. To reduce confusion in the designation of ZJs and medial branches the International Spine Intervention Society (ISIS), which sets standards of practice and publishes practice guidelines for spinal interventions, has established a convention for denoting joints by the use of a hyphen (e.g., the left L4-5 ZJ) and denoting nerves by the use of a comma (e.g., the L3,4 medial branches).16 This convention is recommended for use in all written records.
The medial branches lie close to the bone of the joint partners and are mostly bound down to it by fascia. Over the medial branches are the deep layers of the paraspinal muscles. The anatomic relationships of the nerves to the bones vary with the morphology of the cervical, thoracic, and lumbar regions. Generally speaking, the cervical medial branches run obliquely across the waists of the articular pillars of the joint partners to the intervertebral foramina where they join the dorsal rami. The thoracic and lumbar medial branches run obliquely across the articular pillars and over the tops of the transverse processes of their vertebrae. The courses of the medial branches will be considered in more detail in the later sections on medial branch blocks.
The facets of the ZJs have surface areas of about 100-160 square mm. Their surfaces are curved and their anatomic orientations vary with the morphology of the cervical, thoracic, and lumbar regions. The orientations and their functional significance in each part of the spine will be considered in the section on biomechanics. As stated above, the joints properly termed zygapophysial are those of the twenty-three spinal motion segments from C2-3 down to L5-S1. Above C2-3 are the two uppermost spinal segments designated C0-1 and C1-2. The anatomy of those segments is specialized and their synovial joints that correspond to the ZJs lower down are designated by their anatomic joint partners, as the atlantooccipital joints (at C0-1) and the lateral atlantoaxial joints (at C1-2). These joints are also paired, with a left and a right joint at each level. The atlantooccipital joints involve two superior articular processes of the C1 vertebra (also called the atlas) which have concave facets that articulate with the convex facets of the occipital condyles of the base of the skull. The atlantoaxial joints, between the C1 vertebra (or atlas) and the C2 vertebra (the axis) are actually three in number. Anteriorly is the median atlantoaxial joint, a single synovial trochoid joint in which the odontoid process (or dens) of the axis rotates between the anterior arch of the atlas (in front of it) and the cruciate ligament (behind it). The lateral atlantoaxial joints (LAAJs) are paired synovial joints, one on each side, between the inferior articular processes of the atlas and the superior articular processes of the axis.17
The nerve supplies of the atlantooccipital and LAAJs are different from those of the ZJs. The atlantooccipital joints are supplied by the C1 nerve roots via their ventral rami, which follow curved courses around the outside of the arch of the atlas on each side.17 The LAAJs are supplied by the C2 spinal nerves, each of which lies immediately behind the LAAJ on that side bound to its inferior joint partner (the superior articular process of the axis) by fascia.17 The C2 spinal nerve divides behind the LAAJ into its ventral and dorsal rami; the ventral ramus passes across the back of the joint where it receives articular branches that form the LAAJ’s sensory supply; the dorsal ramus passes inferiorly and posteriorly, and its dorsal root ganglion lies behind the medial aspect of the lower part of the LAAJ.18 Another important anatomic relation of the LAAJ is the vertebral artery which lies immediately beside the joint’s lateral margin.
Biomechanics
Mobility of the spine is achieved primarily by the ways in which the ZJs move so as to make each pair of adjacent vertebrae a spinal motion segment. Every individual ZJ has the potential for the movements of translation (or gliding) and rotation in each of the three main anatomic planes—the sagittal, coronal (or frontal), and horizontal (or transverse) planes.19 Some of the six potential movements of each joint can only be achieved passively but all contribute to the mobility of the motion segment. The mobility of the spine as a whole is augmented by the ways in which the various spinal motion segments move in relation to each other giving the vertebral column ranges of extension and flexion in the sagittal plane, left and right side-bending in the coronal plane (also called lateral bending) and left and right rotation in the horizontal plane (also called axial rotation).
The shapes and orientations of the facets of the ZJs determine their contributions to stability and mobility. The typical cervical ZJs have surfaces that are more or less flat with a slight curvature so that from the side the upper facet looks slightly concave and the lower one slightly convex; the cervical joints are oriented at 90 degrees to the sagittal plane and at angles of about 45 degrees to both the coronal and horizontal planes so that the upper facet of each (that on the inferior articular process of the upper vertebra) faces forward and downward,19,20 as in Figure 36-2.
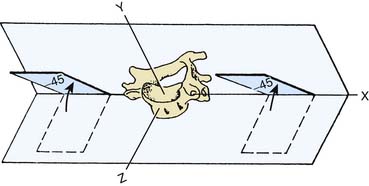
Figure 36-2 Orientation of the articular facets of a typical cervical zygapophysial joint.
(From White AA III, Panjabi MM: The basic kinematics of the human spine. A review of past and current knowledge. Spine 1978;3:12-20, with permission).
The facets of the thoracic ZJs are almost flat and oriented at an angle of about 20 degrees to the coronal plane. In this orientation, the upper facet of each faces forward,19,20 as in Figure 36-3.
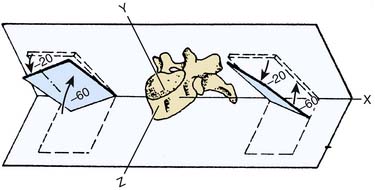
Figure 36-3 Orientation of the articular facets of a typical thoracic zygapophysial joint.
(From White AA III, Panjabi MM: The basic kinematics of the human spine. A review of past and current knowledge. Spine 1978;3:12-20, with permission).
The lumbar ZJs have correspondingly curved surfaces so that the upper facet of each is concave and the lower facet that matches it is convex. The lumbar joints are oriented mainly in the sagittal plane so that the concave upper facet faces laterally and slightly anteriorly, and the convex lower facet faces medially and slightly posteriorly,19,20 as in Figure 36-4.
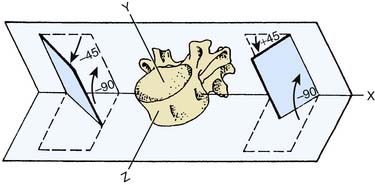
Figure 36-4 Orientation of the articular facets of a typical lumbar zygapophysial joint.
(From White AA III, Panjabi MM: The basic kinematics of the human spine. A review of past and current knowledge. Spine 1978;3:12-20, with permission.)
The cervical spine is the most mobile of the spinal regions because of its articular shapes and orientations, and the laxity of its joint capsules. The ranges of active movements of the cervical spine have been measured as up to 70 degrees each of extension and flexion (as determined by a radiographic study21), about 45 degrees of side-bending to each side and up to 40 degrees of rotation in either direction (as determined by a study using electric goniometers22). The ranges of side-bending and rotation are “coupled” so that they occur together, and to the same side, in the lower cervical spine (from C3 to C7; e.g., right side-bending is accompanied by right rotation). The passive ranges of cervical spinal movement have been measured as about 10 degrees more than the active ranges quoted in extension, flexion and side-bending to either side, and up to 50 degrees more in rotation to either side.23 Active and passive ranges of movement decrease as age increases.23
The movements of extension and flexion, side-bending, and rotation of each spinal motion segment occur around a central point known as the instantaneous axis of rotation (IAR).24 The position of the IAR in the segment determines the way the facets move in relation to each other as a ZJ goes through a range of movement. The positions of the IARs of cervical ZJ segmental motion have been determined—for example, the IAR of physiologic segmental extension in the cervical spine is located in the lower vertebral body of the segmental pair, near its upper end plate.24–,26
The thoracic spine is much less mobile and more stable than the cervical and lumbar spinal regions. The stability of the thoracic spine is enhanced by the coronal orientation of its ZJs, lesser degrees of laxity of their capsules, the supporting spinal ligaments, closer interlocking of the thoracic vertebrae, their long spinous processes, and the buttressing effect of the rib cage. The ranges of movement of the thoracic spine are difficult to measure experimentally in living subjects because the overlying ribs inhibit dynamic radiographic studies. A post mortem study of 10 specimens yielded an average range of about 60 degrees in the sagittal plane (i.e., of extension and flexion combined).27 The ranges are hard to discern clinically and in clinical practice the ranges of movement of the thoracic spine are usually assessed in conjunction with those of the lumbar spine.28
The ranges of active movements of the lumbar spine have been measured as up to 15 degrees of extension and 50° of flexion (as determined by several radiographic studies29–31), about 20° of side-bending to each side and up to 10 degrees of rotation in either direction.31,32 The movements of side-bending and rotation are coupled so that they occur together, and to opposite sides, in the upper lumbar spine (from L1 to L4); e.g., left side-bending is accompanied by right rotation in those motion segments.32 Side-bending and rotation occur to the same side in the L5-S1 segment and either way at L4-5 in different individuals.32 Extension and flexion also tend to be coupled with side-bending and rotation in the lumbar spine, but to varying extents in different subjects so that the coupling is much less predictable.32 As in the other spinal regions, the active ranges of lumbar movement decrease with increasing age.33,34
In the lumbar spine, as elsewhere, the movements of extension and flexion, side-bending, and rotation of the spinal motion segments each occur around an instantaneous axis of rotation (IAR). The positions of the IARs in the lumbar spine have been determined so, for example, the IAR of physiologic extension and flexion of a typical lumbar motion segment is located on the upper end plate of the lower vertebra of the segmental pair.35
The lumbar spine is stabilized by the mainly sagittal orientation of its ZJs (which limits side-bending and rotation), relatively taut ZJ capsules (compared to those of the cervical region), the anterior longitudinal ligament (which limits extension), the posterior longitudinal ligament, ligamentum flavum, and interspinous ligaments (which all limit flexion) the intertransverse and iliolumbar ligaments (which limit side-bending), and by the spinous processes (which limit extension). The resultant stability of the lumbar spine enables it to support the weight of the upper body in various postures at rest and during movements.
Static loading of the cervical spine is caused by the weight of the head and the tensions in cervical ligaments and muscles. The static load varies with posture; for example, it is low in lying with the head and neck supported, higher in upright sitting with the head and neck in the anatomic (neutral) position, and higher still with the head and neck at the end-ranges of extension and flexion.36
Static loading of the thoracic and lumbar spines results from the weight of the upper body, the tensions in thoracic and lumbar ligaments and muscles, and the effect of any additional load such as an object held in the hand. As in other spinal regions, static loading varies with posture. Lumbar static loading is relatively very low in supported supine lying, quite low in relaxed upright standing, higher in relaxed upright sitting, and progressively higher in standing with lumbar flexion and sitting with lumbar flexion.37
Dynamic loading of the thoracic and lumbar spines is a combination of static loading and any additional force(s) caused by active bodily movement or by the passive effects of loads applied with movement from external sources. For example, during ordinary walking, the dynamic loading of the lumbar spine varies with the phases of the gait cycle and is greatest at toe-off.38 In lifting an object while standing, dynamic loading of the lumbar spine is greater if the lumbar spine is flexed than if the back is kept straight, because the flexed position involves additional moments of force related to the distances of the upper body and the lifted object from the center of gravity.39,40
Zygapophysial Joint Pain
Mechanisms
Pain is defined “as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”.41 The unpleasant emotional experience is mediated by unpleasant sensation, which begins with stimulation of sensory fibers of the peripheral nervous system. Peripheral sensory nerves have Ad and c nerve fibers that are sensitive to pain. Ad and c fibers are polymodal: they are sensitive to touch, vibration, proprioception, and thermal change as well as pain. In their role as pain sensors they are called nociceptors and the neurologic process of encoding and processing noxious stimuli which evoke pain is called nociception.42,43
ZJs have afferent sensory innervation including Ad and c fibers and so are capable of generating pain.5,15 Both the synovium and the capsule of each ZJ are richly supplied with nociceptor terminals. When they are stimulated in certain ways, pain will be generated from that joint. Pain generation was demonstrated in a study of normal volunteers; when ZJs were distended by injections of contrast medium, pain was evoked in patterns of distribution that were found to be reliable, recognizable, and joint-specific.12
Etiology
The unpleasant experience of pain begins with sensation that the brain interprets as actual or potential tissue damage.41 ZJ pain is initiated by mechanical loading of the tissues of one or more ZJ(s) at levels close to, or beyond, their load-bearing capacity. In such circumstances, the joint tissues are strained as the load is applied and if the load exceeds their capacity to resist, they will fail. In both phases of strain and then failure, nociceptors are stimulated, evoking pain.
The damaging forces that occur in motor vehicle accidents have been studied most. A cineradiographic in vivo study of cervical ZJs under loading in a simulated rear-end collision44 showed that under accident conditions the instantaneous axis of rotation moved upward and forward from its normal position in the lower vertebral body of a spinal motion segment to a position in the upper vertebral body of the pair. Movement around this “crash IAR” caused the ZJ facets to clash in unusual ways—often so that the posterior edge of the upper facet gouged into the surface of the lower facet. Other phenomena observed included the upper facet making impact on the anterior edge of the lower facet with shearing and compressive forces on both, and the facets gliding to different extents causing rupture of the joint restraints.
Another study using human cadaver head-neck complexes subjected to simulated motor accident conditions45 produced similar findings. The effects observed were consistent with what has been found in studies of the pathology of ZJ injuries, as set out in the next section.
Pathology
Several pathoanatomic studies have been undertaken to investigate the consequences of spinal structures being subjected to excessive forces.46–54 These studies have been based on post mortem examinations, some from autopsies conducted on individuals who had died in circumstances likely to have injured their spines, and some from autopsies conducted on those who died in other circumstances but were known to have had spinal pain from injuries sustained previously, some many years before death. Most of the studies also included radiologic examination of the spine to investigate any relationship between pathoanatomic findings and radiographic appearances.
In the cervical spine, these studies showed that injuries of the cervical ZJs and interbody joints are common consequences of spinal trauma but many of the injuries found involved only the soft tissues of the joints and were undetected by plain radiography.48,50–53 In one study of 22 spines, 19 (86%) were found to have ZJ injuries, many of which were at multiple levels. Sixty-nine injured ZJs were observed; of these only three subjects (14% of the study population) were found by radiography to have ZJ injuries, and in each case, only one ZJ injury was detected radiographically. So of the 69 injuries observed at autopsy, 66 (96%) went undetected by radiographic studies.50 In another study of 45 spines from people who died in motor vehicle accidents, the investigators found soft tissue injuries of the cervical ZJs in 72% of the sample but identified fractures in only 28% of them.51 The results of the other studies were similar. Studies of the cervical spines of those known to have suffered chronic neck pain and to have died in circumstances requiring autopsy (but unrelated to neck trauma) showed lesions in the cervical ZJs consistent with the long-term effects of the types of injuries observed in acute cases.51
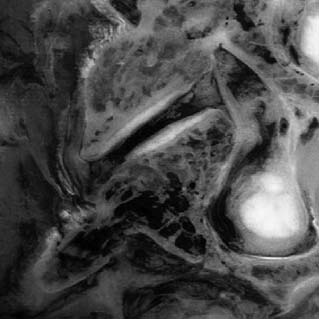
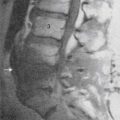
The cervical ZJ pathology observed in these studies was predominantly of soft tissues, which explains why most lesions were not detected by radiographic imaging. A range of pathologic lesions was found, including tears, splits, and partial loss of articular cartilage. In some cases, the full thickness of the articular cartilage was gouged away and this was often associated with injury of the surface layers of the subchondral bone (termed infraction), a lesion obvious on direct visualization or by special staining on microscopic preparations but not of such depth as to deform the bony surface grossly, so not detected radiographically. An example of full-thickness cartilage loss and infraction is shown in Figure 36-5. Other lesions observed included tearing of ZJ capsules and small undisplaced fractures of facet tips or of articular processes which did not appear on radiographs. In the chronic cases, the spines of those who had suffered spinal pain and died later of some other cause, pathologic changes observed included distortion of the joint surfaces with thinning of the articular cartilage and disruption of the articular cartilage.51,53
In the atlantooccipital and LAAJs, the pathologic lesions found were again mainly of articular soft tissues, the articular cartilages, and joint capsules. Also observed in conjunction with some soft tissue injuries at these levels were undisplaced fractures of the anterior and/or posterior arches of the atlas, of the odontoid process (or dens), and/or the arches of the axis.51,54
Pathoanatomic autopsy studies of the lumbar spine show that injuries of the lumbar ZJs are common consequences of spinal trauma as well. Again many of the injuries observed on dissection involve only the soft tissues of the joints and are undetected by plain radiography. In one study of 31 lumbar spines of individuals killed in motor vehicle accidents or other traumatic circumstances, 24 (77%) were found to have ZJ injuries, many of which involved more than one ZJ. Healed injuries of similar types were found in the spines of others who died of other causes but had been known to have a history of lumbar spinal pain since previous accidents. As in the studies of cervical injuries,48,50–54 few of the acute or chronic lumbar injuries were detected by plain radiography.47 Other studies have shown such injuries are not detected reliably by more sophisticated imaging modalities either (see later, in the section “Diagnosis of Zygapophysial Joint Pain”).
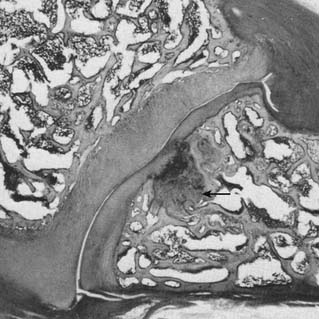
The lumbar ZJ pathology observed in the autopsy studies was also predominantly of soft tissues, which again explains why lesions were not detected by radiologic examination. The pathologic lesions found in lumbar ZJs were similar to those described in the cervical region: tears, splits, and gouging away of articular cartilage, infraction of subchondral bone (Fig. 36-6), tearing of joint capsules, and small undisplaced fractures of facet tips or of articular processes that did not show radiologically. In the spines of those who died after suffering lumbar spinal pain for a long time, pathologic changes observed in the ZJs included thinning of the articular cartilage, irregularity of the joint surfaces, and disruption of the joint capsule.47,49,51
Epidemiology
ZJ pain is common. It is one of the more common causes of spinal pain related to the neck, upper back, or lower back, and must be considered as a diagnostic possibility whenever a patient presents with any such symptom.55
The 12-month prevalences of chronic neck pain in the general adult population have been assessed as 1.7% (for more intense chronic neck pain that limits ability to work), 2.4% (for chronic neck pain that limits social activities), and 11.5% (for chronic neck pain that limits some activity).56 Studies show the prevalence of cervical ZJ pain among patients with chronic neck pain after motor vehicle accidents or similar trauma is at least 50%55–59 and in some circumstances may be as high as 80%.60 In those with lower neck pain the C5-6 level is most commonly affected and C6-7 is the next most commonly affected.58 Among patients whose dominant symptom after a motor vehicle accident is headache, the prevalence of pain stemming from the C2-3 ZJ has been assessed as between 37% and 68% with a mean of 53%.61,62
Chronic upper back pain related to the thoracic spinal region has been estimated to have annual prevalences in adult populations of between 5% and 10%.63,64 The prevalence of chronic thoracic ZJ pain among those with thoracic spinal pain has been determined as 48%.64
Chronic low back pain is very common in all countries. The point prevalence of chronic low back pain in general adult populations has been estimated as about 10% in Western countries such as Australia,65 New Zealand,66 the United Kingdom,67,68 and the United States69 and as high as 34% in the rural population of Tibet.70 The annual prevalence has been reported as between 13% and 49% in Western countries,71 42% in the rural population of Tibet,70 60% among Finnish reindeer herders,72 and from 60% to 83% among rural workers in China.73 The prevalence of lumbar ZJ pain has been determined as 30% to 40% among adults with chronic back pain in the United States.55,74 Age-related prevalences of lumbar ZJ pain have been measured in Australia, in studies based on definitive diagnosis by criterion standard methods, as 15% of younger adults with a history of injury and attending a pain clinic75 and 40% of older adults attending a rheumatology clinic.76 Both these studies showed the L5-S1 level is most commonly affected in the lumbar region, and the L4-5 level is the next most commonly affected.
Diagnosis of Zygapophysial Joint Pain
Clinical assessment, taking the medical history and performing a physical examination, may help the clinician to form an impression of the likely cause of a patient’s pain and should be carried out carefully and systematically in every case.77,78 However, there are no clinical features that are pathognomonic of ZJ pain at any spinal level, so the decision to test for ZJ pain cannot be validated on clinical grounds alone.
Some impression can be gained of the likelihood of a ZJ being the source of a patient’s pain by an informed consideration of the symptoms, and especially of the pain distribution and pain quality.77 The distribution of pain generated from a particular structure is called a “pain map.” Studies based on joint provocation in normal subjects and anesthetization of specific joints in those with chronic pain have resulted in pain maps being plotted for ZJs at all cervical,12,79–81 thoracic,82 and lumbar4,83–85 levels, and for the atlantooccipital and LAAJs.86,87 The quality of pain provides another useful clue. ZJ pain is typically dull and aching in quality, of the type called somatic pain (in a local distribution near the source) and somatic referred pain when it is perceived in more distant regions.88,89 Other spinal structures may also generate somatic pain, so it is not specific to ZJs, but it is quite different from radicular pain, the sharp, shooting, and sometimes “electric” pain associated with nerve root irritation.90
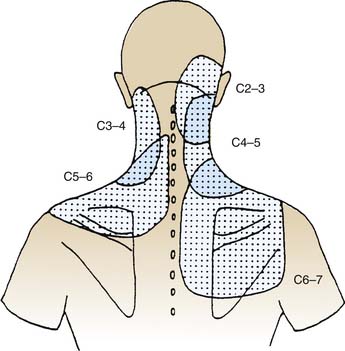
The pain maps of cervical ZJs are depicted in Figure 36-7. If a patient presents with somatic and/or somatic referred pain in the neck, in the lower neck and shoulder, or in the upper neck and head (so-called cervicogenic headache91,92) in a distribution like one of those described in Figure 36-7, it may be suspected to be of cervical ZJ origin. The pain maps provide clues to the level of a joint that may be a pain source but do not identify a level specifically because the maps overlap considerably: each part of a patient’s neck lies in at least two, and perhaps as many as four, of the areas to which pain may be referred from particular joints.
When a patient presents with what is suspected to be cervicogenic headache, the lateral atlantoaxial (C1-2) and atlanto-occipital (C0-1) joints must also be considered as possible sources. The pain maps of those joints are depicted in Figures 36-8 and 36-9. The pain maps of the C0-1, C1-2, C2-3, and C3-4 joints also overlap considerably, so although they provide clues to the possible origin of cervicogenic headache, they do not, in themselves, enable identification of the source. In the thoracic spinal region the pain maps overlap as well. The distribution of pain from a thoracic ZJ is to an adjacent zone of the upper back, lateral to the joint and from half a spinal segment higher to a full segment lower as shown in Figure 36-10.
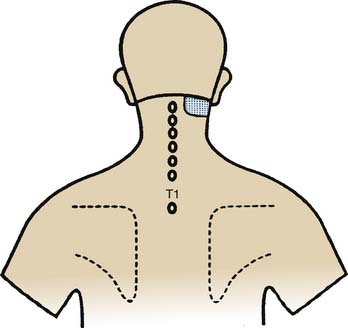
Figure 36-8 Pain map showing the pattern of distribution of lateral atlantoaxial (C1-2) joint pain.
(From Dreyfuss P, Michaelsen M, Fletcher D: Atlanto-occipital and lateral atlanto-axial joint pain patterns. Spine 1994;19:1125-1131, with permission.)
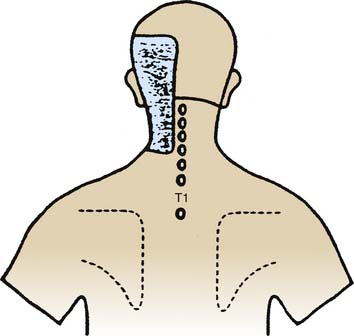
Figure 36-9 Pain map showing the pattern of distribution of atlantooccipital (C0-1) joint pain.
(From Dreyfuss P, Michaelsen M, Fletcher D: Atlanto-occipital and lateral atlanto-axial joint pain patterns. Spine 1994;19:1125-1131, with permission.)
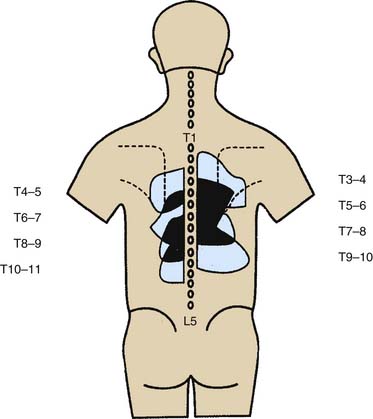
Figure 36-10 Pain maps showing patterns of distribution of thoracic zygapophysial joint pain.
(From Dreyfuss P, Tibiletti C, Dreyer SJ: Thoracic zygapophyseal joint pain patterns. A study in normal volunteers. Spine 1994;19:807-811, with permission.)
Lumbar ZJ pain maps are comparable. Somatic pain from a lumbar ZJ occurs in an adjacent zone of the lower back, lateral to the joint, and from about one spinal segment higher to one segment lower, with somatic referred pain extending down the back of the leg. The patterns overlap to a considerable extent with each joint generating pain to a slightly lower level than that of the joint above it. The overlap makes it difficult to depict individual maps in a single diagram but the composite pattern of distribution is as shown in Figure 36-11.
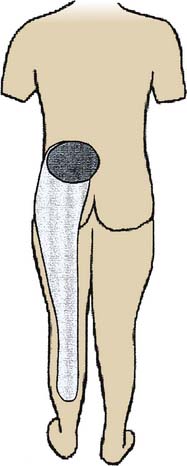
Figure 36-11 Pain map showing patterns of distribution of somatic and somatic referred pain of lumbar zygapophysial joint origin.
(From Bogduk N, McGuirk B: Medical management of acute and chronic low back pain: an evidence-based approach. Amsterdam, Elsevier, p 9, 2002, with permission.)
Physical examination is a traditional component of clinical assessment and it is valuable for helping the clinician develop an impression of the patient and the problem. However, clinical examination findings provide few clues to the likelihood of pain being of ZJ origin and there is no physical sign, or combination of signs, that enables identification of a particular ZJ as a source of pain. By convention, physical examination of the musculoskeletal system includes inspection, palpation, and testing of movements (which includes assessing active, passive, and accessory ranges of movement, and challenging the restraints to movement).78 If the clinician examines the patient systematically and addresses all these domains, many signs will be elicited but care must be exercised in interpreting them in the light of the scientific evidence of their reliability and validity.
Data on the reliability of inspection of the various regions of the spine for lordosis, kyphosis, and scoliosis show interobserver agreement to be low, with Kappa scores from 0.13 to 0.39.93,94 Data on the reliability of palpation of the spine for tenderness at specific sites also show interobserver agreement to be low, with Kappa scores from 0.11 to 0.53.95–97 The reliability of assessing gross ranges of active spinal movement seems somewhat better, in some ranges at least, with, for example, reported Kappa scores of 0.40 for cervical rotation and 0.56 for cervical extension,95 from 0.35 to 0.74 for lumbar extension,94 but only from 0.11 to 0.43 for lumbar side-bending.94 The reliability of testing passive intervertebral movements is very poor, with Kappa scores in negative ranges.93,98
Reliability is one thing but reliability data only show the consistency of observations, not what those observations can be interpreted to mean. Such interpretation must be based on evidence of validity. There is very little evidence of the validity of physical examination in the diagnosis of ZJ pain. There are no sound data on the validity of inspection or testing gross ranges of movement in the diagnosis of ZJ pain in the cervical, thoracic, or lumbar regions. There are no sound data on the validity of palpation in the diagnosis of thoracic or lumbar ZJ pain. Such data as exist on the validity of physical examination relate to palpation for specific physical signs in the assessment of patients with neck pain. There are data99 showing certain physical tests are valid, with positive likelihood ratios of from 2.7 to 12.8, for confirming that pain is of spinal origin but not for identifying the specific source of that pain.
The data from two validity studies show that manual examination is not valid for diagnosis of cervical ZJ pain. A small set of data from a preliminary study100 seemed to show that manual examination was valid for the identification of a painful cervical ZJ and much faith was placed on that evidence by manual therapists for some time thereafter. However, the data set was small and the authors of that early study called for further research to be done before their results were generalized. In response to that call, a larger study101 was done and its results showed clearly that manual examination is not valid for the diagnosis of cervical ZJ joint pain; the positive likelihood ratios were only from 1.4 to 1.8. Reassessment of the data of the earlier study, in the light of later knowledge of aspects such as the rate of false-positive results, showed the results of both studies are consistent and both actually show manual examination is invalid for the purpose. The summary of the evidence on clinical assessment is that some guidance is provided by established patterns of distribution of pain from individual joints, the so-called spinal pain maps, but there are no clinical methods that are valid for identifying specific painful ZJs.
Imaging studies provide information about the spine and traditionally imaging has been used in the assessment of patients with spinal pain, but imaging results do not identify painful spinal joints. The imaging modalities used most often are plain radiographs, computer assisted tomographic (CAT or CT) scanning, magnetic resonance imaging (MRI), and isotopic bone scans. These frequently show radiologic appearances of spondylosis or osteoarthrosis, including joint space irregularity and narrowing, subchondral bony sclerosis, subchondral cysts, periarticular bony hypertrophy, and the presence of osteophytes. These changes, sometimes incorrectly called “degenerative changes,” reflect the normal responses of the articular cartilage and the subchondral bone to the repeated biomechanical stresses of daily living.102,103 It is often assumed that there is a causal relationship between these imaging changes and pain generation but the scientific evidence suggests otherwise. The fact that the changes are age-related is clearly demonstrated by the results of numerous studies showing direct correlation between their prevalence and increasing age.104–109 The fact that they are not regularly associated with pain is borne out by the results of numerous studies showing no correlation between the presence of pain and spondylotic changes shown by plain radiography,110–116 CT scanning,117 and MRI.118–121
No other radiologic appearances seen on plain radiography,122,123 CT scanning,124 MRI,125,126 or any other imaging modality have been proved to be correlated with ZJ pain. Thus, after comprehensive clinical assessment and imaging, the clinician may gain the impression that a patient’s pain may be of ZJ origin, but that impression cannot be confirmed by clinical features and/or imaging results—separately or in combination.
The only way of determining that a ZJ is (or is not) a source of pain is to test it by anesthetic blockade of that joint alone, to see if the pain is abolished by the blockade: such tests are called diagnostic joint blocks. If a decision to undertake ZJ blocks is made, the next issue is which joint to test first. That decision will depend on the clinical impression; further guidance may be obtained from the prevalence data set out earlier under “Epidemiology”. As stated there, in the cervical spine, the ZJs most often involved in cervicogenic headache are those at the C2-3 level58 and the joints most often associated with lower neck pain are those at C5-6.62 There are no prevalence data for specific segmental origins of thoracic ZJ pain. In the lumbar spine, the ZJs most often involved in pain generation are those at the L5-S1 level.75
Indications for Interventions
When contemplating specific indications for interventions in the management of ZJ pain, the first consideration is the duration of the condition. By convention, conditions are classified by their time courses as acute and chronic.127 Acute pain is defined as that of short duration which is likely to settle spontaneously by natural healing of the causative condition. Chronic pain is defined as that which persists beyond the normal time of natural healing.128,129 After much discussion among clinicians and researchers about how long should be allowed for natural healing, acute pain was defined as pain present for up to 3 months and chronic pain was defined as pain present for more than 3 months.130
Acute spinal pain, in most cases, will resolve by natural healing if just left alone.131–133 Clinicians who do not appreciate this phenomenon often use interventions to treat acute spinal pain when to do so is unlikely to shorten the course of the condition and may even lengthen it. Active interventions are actually contraindicated for most acute spinal pain. What patients with acute spinal pain need is explanation of the favorable natural history, reassurance, pain relief (by analgesic medication or some other method) and encouragement to remain active; beyond that all they need is follow-up until the pain settles.131–133
Chronic spinal pain usually persists until effective treatment is applied, so intervention is indicated in virtually every case. Some clinicians seem unaware of the evidence in this regard and manage chronic spinal pain as if its cause is unidentifiable and only general measures can be applied to help the patient live with the problem. That approach is outmoded. These days the causes of most chronic spinal pain can be identified precisely and many of those identifiable causes can be treated effectively. That is certainly the case for chronic ZJ pain. The key to the management of chronic spinal pain is precise diagnosis.134
The specific indications for a diagnostic intervention in the management of chronic ZJ pain are (1) the patient is likely on the basis of their clinical presentation to have chronic ZJ pain, (2) the intervention planned is known to be useful for determining whether ZJ pain is present or not, and if so from which joint(s), and (3) the intervention is justified in terms of its safety, therapeutic utility, personal utility for the patient and cost-effectiveness, and the patient has given informed consent.
Medial Branch Blocks
Development
Medial branch blocks are local anesthetic blocks of the articular nerves (the medial branches) that transmit sensory information including nociceptive signals from ZJs (as described earlier under “Mechanisms”). Historically, the first method used to test a ZJ as a possible source of pain was injection of local anesthetic into the cavity of a lumbar ZJ84; such tests are called intraarticular blocks (IABs). Comparable techniques were developed for cervical135 and thoracic136 ZJs as well. Later, less invasive methods were developed to achieve blockade of a specific joint by blocking the articular nerve(s) outside the joint137,138; such tests are designated by the particular nerves involved as medial branch blocks (MBBs) and third occipital nerve blocks (TONBs). Because articular nerve blocks are less invasive and are performed more often to investigate the common sources of chronic spinal pain, they will be described first.
Cervical Medial Branch Blocks C3 to C6—Procedure
A cervical medial branch block procedure involves a lot more than the needling and injection commonly associated with the name. This is also true for other such procedures. Each diagnostic procedure involves three phases, a preoperative phase, an operative needling phase, and a postoperative observational phase. The full test process involves a fourth phase, the interpretation of individual block results and the integration of the information they provide in the overall process of diagnosis. If a medial branch block is thought of mainly as the needling part, the other phases may be given less emphasis than they deserve and the whole process may be compromised.
Each cervical ZJ from C3-4 down to C6-7 is supplied by two medial branches, the medial branches of the cervical dorsal rami above and below the joint. Thus, the C5-6 ZJ is supplied by the C5 medial branch and the C6 medial branch. The procedure to test the C5-6 ZJ as a pain source involves blocking each of those two nerves. The courses of the medial branches have been plotted by cadaveric dissection studies and shown to lie more or less horizontally across the middle parts of the articular pillars that join the superior and inferior articular processes of each vertebra.139 The courses of these medial branches are illustrated in Figure 36-12.
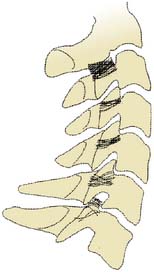
Figure 36-12 Plots of the courses of cervical medial branches across the articular pillars of the vertebrae.
(From Lord SM, Barnsley L, Bogduk N: Neurosurgery Quarterly 1998;8:288-308, with permission.)
These nerves are the targets at which cervical medial branch blocks (MBBs) are aimed. The nerves themselves are not seen on fluoroscopy, but by appreciating the ranges of their courses it can be understood how particular medial branches can be anesthetized. They are accessible for injection around them as they pass over the “waists” of the articular pillars. Needles can be placed onto them at that site readily and safely from lateral approaches, as depicted in Figure 36-13.
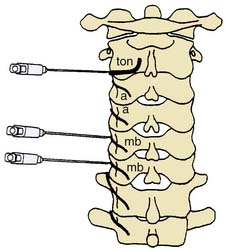
Figure 36-13 Target points for cervical medial branch blocks indicated by needles introduced via lateral approaches.
(From Bogduk N: Back pain: Zygapophyseal blocks and epidural steroids. In: Cousins MJ, Bridenbaugh PO [eds]: Neural Blockade in Clinical Anesthesia and Management of Pain, 2nd ed. Philadelphia, JB Lippincott, 1988, 935-954, with permission.)
The target point for each MBB injection is the centroid or geometric center of the relevant articular pillar (Fig. 36-14). Medial branch blockade is achieved by placing the tip of a spinal needle at that point and injecting a small volume of local anesthetic, just sufficient to reach the highest and lowest of the known courses of the nerve in question. The volume of the injectate must be large enough to achieve that coverage reliably but not so large as to spread to and block other nerves because that would compromise the specificity of the test. Local anesthetic preparations suitable for the purpose are lidocaine 2% or bupivacaine 0.5% and the volume of injectate sufficient to achieve the desired spread has been found to be no more than 0.5 mL.137
The placing of a needle tip at a specific point on the spine requires particular facilities and equipment. MBBs and other such procedures should be performed in a procedure suite or operating room where infection control measures are undertaken regularly. The patient’s safety is a prime consideration and it is crucial for safety that the operator knows precisely where the needle is at all times. The procedure must be done with radiologic guidance, preferably that provided by a fluoroscope with a high-resolution image intensifier and a C-arm that allows the X-ray beam to be directed at different angles to provide anteroposterior (AP), oblique, and lateral views without moving the patient. A radiolucent x-ray table is also required, preferably one with a mobile plinth that can be moved to modify the fluoroscopic views. Also needed in the procedure suite are spinal needles, extension tubes, and syringes for the block- injections, local anesthetic agents, sterile layouts on which the equipment for the procedure can be placed, skin preparation trays with swabs, forceps and suitable antiseptics, sterile drapes, sterile towels and sterile gloves for the operator, and a scrub sink adjacent. The suite should also be equipped for resuscitation and supportive care in the rare event of a serious complication arising.
The procedure begins with the preoperative phase. When a patient arrives at the test facility, he or she is admitted by a nurse who is trained in pain assessment. The nurse answers any questions the patient may have, checks for any contraindications such as allergy, infection, anticoagulant therapy, or pregnancy, and makes sure the patient has given informed consent (as described in the earlier section “Indications for Interventions”). After that, the nurse asks the patient to describe the pain for which the test is to be done; that pain is designated the “index pain.” For example, if a patient has headache and lower neck pain, the lower neck pain may be identified as the index pain for that day’s test. The nurse asks the patient about the intensity and distribution of the index pain, and those attributes are recorded on appropriate charts. The intensity is assessed by the patient as a score out of 10 or 100, using a printed visual analog scale (VAS) or some similar device such as a pain ruler, and recorded by the nurse on a pain chart. The distribution is recorded by the patient marking it on a body chart. The selection of the joint to be tested is checked by comparing the pain pattern drawn by the patient with the pain maps shown in Figure 36-7 to 36-11. The patient then changes into a hospital gown and at the appropriate time is taken to the procedure suite, preferably for the patient’s safety and convenience in a wheelchair.
The operative phase begins when the patient reaches the procedure room. The patient is helped onto the x-ray table and positioned lying comfortably on their nonpainful side so that the painful side is uppermost (Fig.36-15).
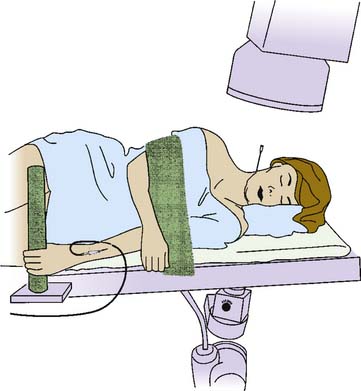
Figure 36-15 The position in which a patient should lie on an x-ray table for a right cervical medial branch block.
Their gown is tucked away from the neck and shoulder on that side. The skin of the area is sponged with a suitable antiseptic such as povidone-iodine or chlorhexidine and alcohol, allowed to dry and then draped with a sterile fenestrated drape. The operator, who should be wearing operating room scrubs, a surgical cap, operating mask and protective leads, should scrub their hands and don sterile gloves. The needles and other equipment for the procedure should be laid out on a sterile set-up (Fig. 36-16).

Figure 36-16 A sterile set-up with skin preparation tray and equipment required for a cervical medial branch block.
(Image courtesy of Pendlebury Clinic, Newcastle, Australia.)
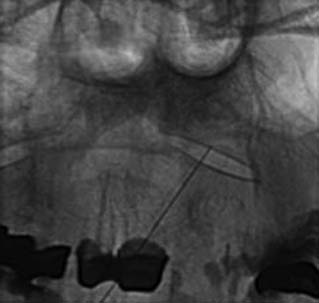
Next the operator and the radiographer confer to ascertain the first target nerve. For example, if the patient is to have C5-6 MBBs, the first target nerve will be the C5 medial branch and its target point is at the centroid of the C5 articular pillar. When the first target is agreed, the radiographer obtains a clear lateral view of the target region and cones the image to it. Coning is important to minimize the exposure to the patient, the operator, and the radiographer to ionizing radiation. In the directly lateral view, the left and right articular pillars of the target vertebra will overlie each other and the target point will have a wide margin of bone around it, which enhances the safety of the procedure. Having obtained this view, the operator and the radiographer stand or sit so they can see the monitors clearly and carry out the procedure comfortably as in Figure 36-17.
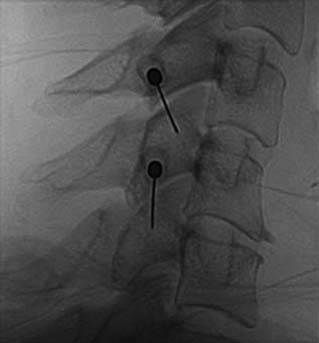
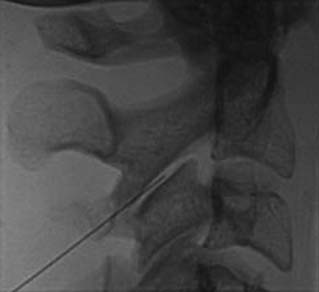
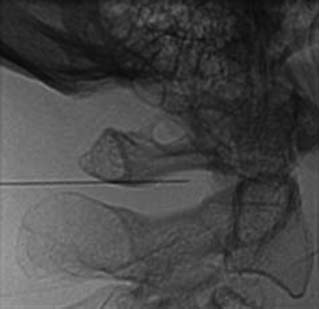
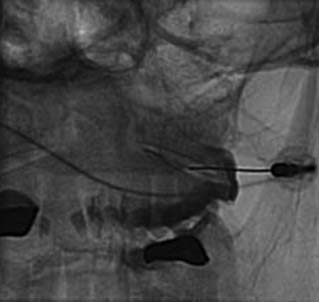
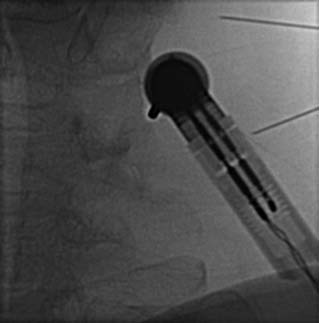
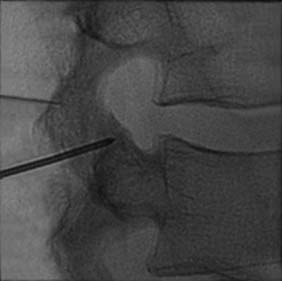
The MBB needling procedure should be performed in accordance with the protocol recommended by the International Spine Intervention Society (ISIS) and described fully in the relevant practice guidelines.140,141 It is important for the operator to talk to the patient to explain what is happening at each stage of the procedure, including what might be felt. The operator selects a suitable needle (usually a 25- or 26-gauge spinal needle at least 3.5 inches or 88 mm in length) and with the plastic needle guard still on, places it on the skin of the patient’s neck to determine, by intermittent fluoroscopic images, the point on the patient’s skin directly over the target point; that will be the needle insertion site. Then the operator takes the guard off the needle, places its tip carefully on the patient’s skin at the insertion site and checks its position there with another intermittent image (Fig. 36-18).
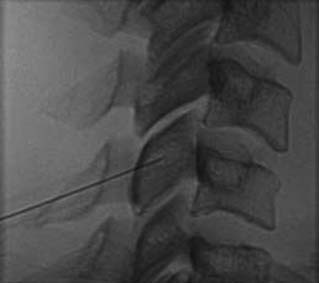
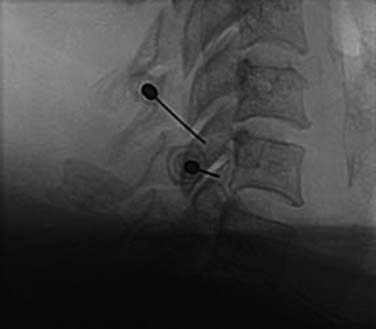
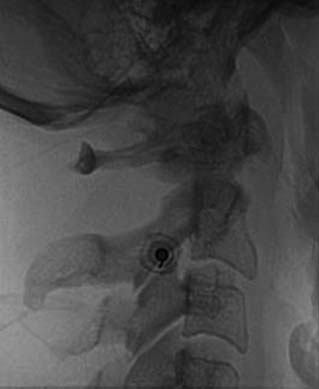
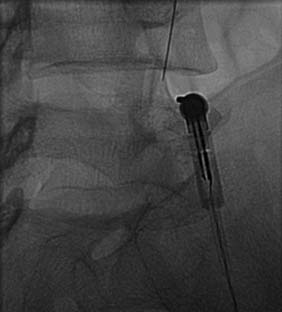
The operator stands the needle up in alignment with the fluoroscopic beam, warning the patient it will be felt, and inserts the needle through the skin as gently as possible. When the needle has purchase (i.e., when it has been inserted deeply enough to stand up without being held) its position is checked on another lateral view. Then the operator guides the needle through the patient’s neck muscles toward the target point, steering it by moving its hub, shaft and/or bevel in ways that are learned in practical training. Before each adjustment of the needle its position is checked in an intermittent fluoroscopic view; continuous screening is avoided to keep the x-ray exposure to a minimum. When the needle tip reaches bone at the target point, its position there is checked and recorded on a fluoroscopic image, as in Figure 36-19.
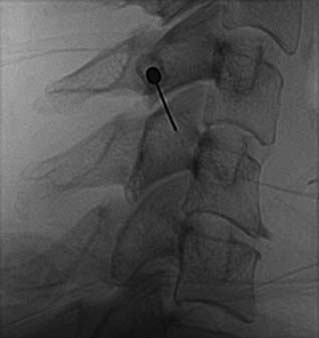
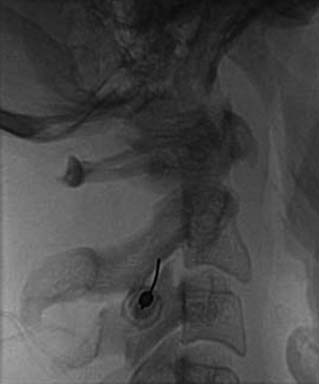
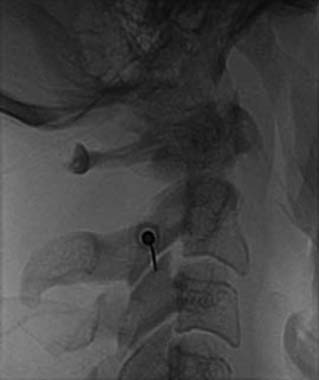
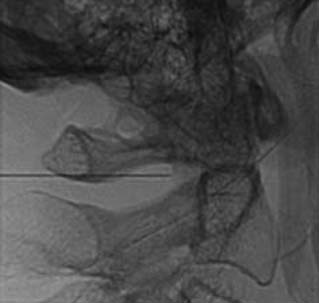
The patient is then told the first of two blocks has been done and the same procedure will be followed to block the second nerve. The radiographer adjusts the fluoroscope to obtain a lateral view of the second target. The insertion point is determined and another needle is inserted in a similar manner, until its tip is resting on bone at the second target point. The position of the second needle at its target is checked and recorded on a fluoroscopic image, as in Figure 36-20, and 0.5 mL of local anesthetic is injected over the second medial branch. The patient is told both blocks are in place and the needles are about to be withdrawn, then that is done as smoothly as possible to complete the operative part of the MBB procedure.
After the needles are out, the drape is removed and the antiseptic is sponged gently from the patient’s skin. Dressings are not usually required if 25- or 26-gauge needles have been used because they do not usually leave any mark, but if there is any bleeding small adhesive dressings may be applied. While still lying down, the patient is asked if they feel lightheaded or dizzy. If they do, they can be reassured that such symptoms are quite normal after a cervical nerve block and they should be allowed to lie quietly on the table until the dizziness settles, which may take a minute or two. When the patient is not dizzy, they are allowed to sit up with their legs over the side of the table, being supported by the operator and/or a nurse in case the change of posture makes them lightheaded. When they feel ready, the patient is helped from the table to a wheelchair and taken from the fluoroscopy suite to a room where they can rest and be observed by a nurse who is trained in pain assessment.
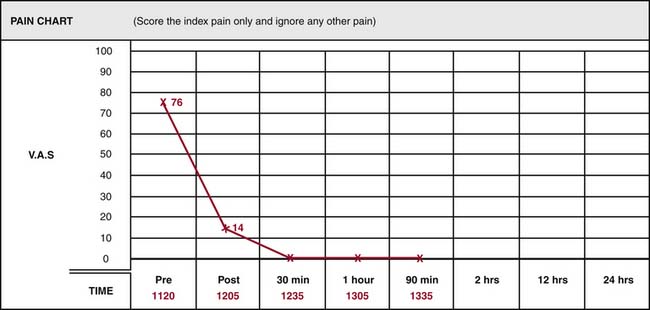
During the observational phase, the treating physician should stay away from the patient to avoid any interaction that might influence their responses. At the end of the observation period, the pain chart may be reviewed to determine the result of the test (Fig. 36-21).
Cervical Medial Branch Block—Interpretation of Results
Accurate interpretation of test results depends on understanding and applying the standards set out in the literature and specified in the relevant practice guidelines.140,141 A single MBB test result is deemed positive only if the index pain is relieved completely (i.e., its VAS score goes down to zero) in the postblock period, as shown on the pain chart in Figure 36-21. Any other result showing the index pain was still present, even if at a reduced level of intensity, must be considered negative or inconclusive. A treating physician may be tempted to try to interpret a reduced pain score, say if a patient’s index pain is reduced from 86/100 to 14/100, but rational interpretation of such a result will be confounded by the possibilities. The reduced score may be because the joint tested is contributing to, but not wholly responsible for, the pain or because the pain is actually stemming from an adjacent joint and one of its medial branches was blocked in the test, or because of some bias in recording.
Another factor to be taken into account in interpreting MBB responses is the possibility of false-positive and false-negative results. A study of cervical MBBs showed 27% of responses to single blocks are false-positive and 5% of responses are false-negative.142 That means even if the index pain is relieved completely after an MBB, the pain is only 73% likely to be stemming from the joint blocked and if the index pain is not relieved after an MBB, the pain is only 95% likely to be not from that joint. The same study considered the positive predictive values (PPVs) of positive single block responses. PPVs vary with the prevalence of the condition; if the prevalence is as high as 70%, the PPV of a positive block response is only 89%.
The liabilities of false-positive single block results are overcome by performing comparative blocks.143 If a patient has a positive response to an MBB, a second block is performed on a separate occasion to test whether the first result was true-positive. The second block is done following the same procedure but using another local anesthetic agent with a different duration of action. If the patient again has complete relief of the index pain after the second block, the durations of the responses can be compared. If the duration of pain relief was longer with the longer acting anesthetic (bupivacaine) than with the shorter acting (lidocaine), that joint is positively identified as the source of the index pain and the result is described as “comparative block positive and concordant,” which is the criterion standard for definitive diagnosis of cervical ZJ pain.140,141 Any other combination of results makes a comparative block test negative or inconclusive.
C7 Medial Branch Block—Procedure
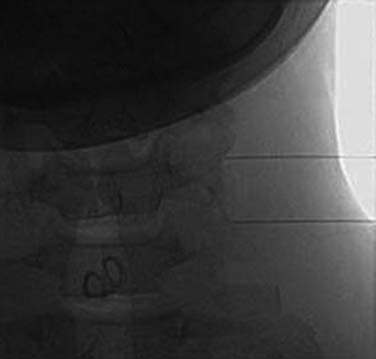
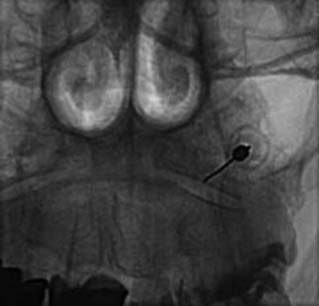
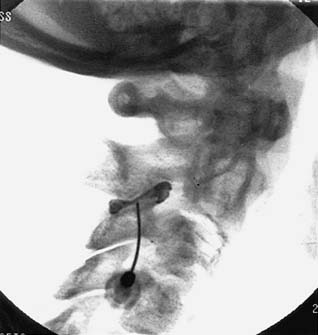
Blockade of the C7 medial branch involves a slightly different technique because the nerve runs over a different part of its vertebra and has a wider range of anatomic variation in its course (see Fig. 36-12).139 It usually passes forward over the bony surface of the tip of the superior articular process of the C7 vertebra, somewhere between its peak and the root of the transverse process, but in some individuals it is superficial to the bone rather than immediately adjacent to it because the nerve is separated from the bone by a slip of the semispinalis capitis muscle. To ensure blockade of the nerve, wherever it happens to lie, requires injections of 0.3 mL of local anesthetic at each of two target points, one on the lateral aspect of the curved surface of the articular process up near its peak and the other 4 mm superficial to that. The first part of the procedure is similar to that followed at other cervical levels. When the needle tip reaches bone on the C7 vertebra at the initial target site, its position is checked (first) in a lateral view (Fig. 36-22).
The C-arm is then rotated 90 degrees for a direct AP view. In that view, the needle position is checked again to ensure its tip is on the “skyline,” (i.e., on the lateral edge of the articular process, not around on the anterior or posterior parts of the bone’s curved surface) and that the tip is above the transverse process (Fig. 36-23). If the needle tip is in the correct position, the first injection of 0.3 mL of local anesthetic is done while the needle tip is on bone. The needle is then withdrawn 4 mm and the position of its tip is recorded again off bone in an AP view before the second injection is made. The rest of the procedure is as described for the C3-4 to C5-6 levels.
Third Occipital Nerve Block—Procedure
The C2-3 ZJ is supplied by a single nerve, the third occipital nerve (TON) on that side which is the medial branch of the dorsal ramus of the C3 spinal nerve. Accordingly, the test procedure at the C2-3 level that corresponds to MBBs at lower cervical levels is called a third occipital nerve block (TONB). The TON is thicker than other medial branches and that must be taken into account in consideration of procedures directed at it. Also, the TON carries some sensory fibers from a small patch of skin over the occipital region behind the ear on that side. Other medial branches generally do not carry skin sensation and this makes a difference to the observations after a TONB.
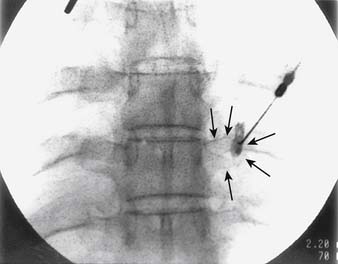
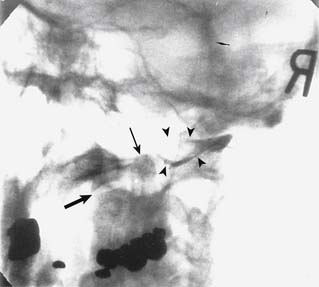
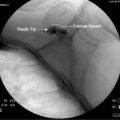
The general course of the TON is forward over the C2-3 ZJ in the pericapsular fascia to join its dorsal ramus in the C2-3 intervertebral foramen. The course varies between individuals so the nerve passes somewhat horizontally across the joint at a level that ranges between the top and just below the bottom of the intervertebral foramen (see Figure 36-12).139 To ensure blockade of the TON wherever it happens to lie in this range requires injections of 0.3 mL of local anesthetic at each of three target points, which lie in a vertical line over the middle of the joint—a high one at the level of the apex of the C3 superior articular process; a low one at the level of the bottom of the C2-3 intervertebral foramen; and a middle one halfway between the other two (Fig. 36-24).
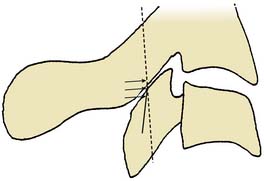
Figure 36-24 The three target points for a third occipital nerve block(arrows).
(From Barnsley L, Bogduk N: Medial branch blocks are specific for the diagnosis of cervical zygapophysial joint pain. Reg Anesth 1993;18:343-350, with permission.)
In other respects, the procedure is similar to that used for cervical MBBs at lower levels, although in the operative phase, only one needle is inserted and it is moved to each of the target points in turn. The patient is positioned on the x-ray table lying on his/her side with the painful side up. The insertion site is determined by the needle being placed on the skin of the patient’s neck over the middle target point (Fig. 36-25).
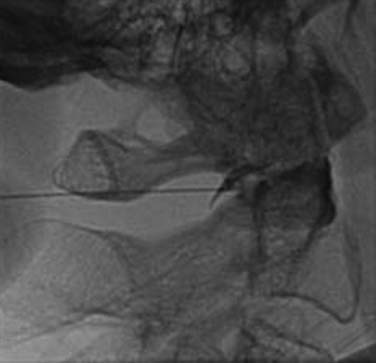
The operator inserts the needle and steers it to each of the three target points in turn. At each target, the needle tip position is checked and recorded on a lateral fluoroscopic image (Figs. 36-26–36–28) and 0.3 mL of local anesthetic is injected.
Cervical Medial Branch Blocks—Validity
Face validity refers to whether a test or instrument appears, on the face of it, to assess what it is meant to assess. Cervical MBBs depend on the effects of blocking specific target nerves (and no others). Their face validity depends on whether they appear to block the target nerves only, (i.e., on their target-specificity). A study of the target-specificity of cervical MBBs137 showed that when the blocks are performed in the manner described, in accordance with the standard guidelines and injecting the volumes specified, the injectates will cover the target nerves but will not spread to any other nerves. Therefore, cervical MBBs have face validity.
Predictive validity refers to whether an instrument such as a diagnostic test is of value in predicting responses to treatment. It is directly related to therapeutic utility and of the greatest significance in the clinical application of diagnostic procedures like MBBs. The predictive validity of cervical MBBs depends on whether their results predict the likelihood of treatment being effective for relieving conditions diagnosed by the test. Positive and concordant, comparative MBB or TONB results identify pain stemming from a specific cervical ZJ on the basis of that pain being transmitted via the medial branch(es) blocked in the test procedure. Percutaneous radiofrequency cervical medial branch neurotomy is a therapeutic procedure established as effective for the treatment of cervical pain mediated via specific medial branches by the data of studies139,144,145 that used positive and concordant, comparative MBB, or TONB results as the indications for the treatment procedure. Hence, comparative cervical MBBs and TONBs, performed in those studies as described, have predictive validity. Because they have face validity, construct validity, and predictive validity all based on sound, published data, cervical medial branch blockade is described in the ISIS practice guidelines141 as an established procedure.
There are many other publications on cervical blocks that touch on aspects of validity, including papers on procedures that sound like MBBs but are done in ways that differ from the standard guidelines, and the data from all those publications are extensive. Authors have described using placebo injections as an additional arm of a comparative block protocol146; studies of the efficacy of cervical radiofrequency neurotomy139,144,145 show that the results of comparative cervical MBBs using two local anesthetics and those of placebo-controlled comparative blocks have the same predictive validity for the outcomes of medial branch neurotomy treatment. Other authors have described MBB procedures that include the injection of contrast medium to confirm injectate spread147 but their results do not contradict those of the earlier study137 showing the target-specificity of MBBs done as described earlier. Placebo controls and contrast injection may be of value in specific circumstances, such as in research or for medicolegal purposes, but MBB and TONB procedures as laid down in the standard guidelines140,141 (and as described in this chapter) are of known, established validity for use in regular clinical practice.
The data on validity in the foundation literature, on which the guidelines are based, are applicable to MBBs and TONBs performed as described. Those data cannot be generalized to blocks done in other ways, so such blocks cannot be assumed to be valid on that basis. In the absence of specific scientific evidence, whether blocks that are not performed in accordance with the standard guidelines are valid or not is simply unknown. Confusion about this has given rise to controversies about the reliability and validity of diagnostic block procedures in general; such controversies would diminish if block procedures were evaluated on the basis of the specific scientific evidence that applies to them.
Thoracic Medial Branch Blocks—Procedure
The thoracic medial branches run forward from above and below the thoracic ZJs they supply and over the transverse processes of the joint partners to join their respective dorsal rami in the intervertebral foramina. The numbering of the thoracic medial branches is one less than the vertebrae over which they run as explained in the earlier under “Anatomy”. This numeric variation need not be confusing if its pattern is appreciated. The T3-4 ZJ is supplied by the T2 and T3 medial branches, which run over the T3 and T4 transverse processes, respectively. The procedure to test the T3-4 ZJ as a source of pain involves blocking each of those two nerves. Even if they appreciate the numeric relationships between nerves and joints, it is important for clinicians to realize the potential for confusion and to be consistent in describing any procedure by the joint, or nerves, involved. The ISIS convention for designating joints by the use of a hyphen16 (e.g., the left T7-8 ZJ), is recommended for all written records.
The courses of the thoracic medial branches have been plotted by cadaveric dissection studies.148 The upper and lower thoracic medial branches are reliably found on the bony surfaces of the thoracic transverse processes but the midthoracic medial branches are usually suspended in the soft tissues of the intertransverse spaces, as shown in Figure 36-29.
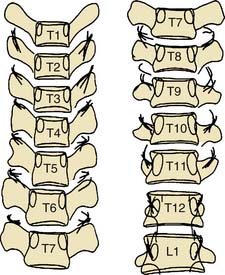
Figure 36-29 Plots of the courses of thoracic medial branches across the transverse processes of the vertebrae.
(From Chua WH: Clinical Anatomy of the Thoracic Dorsal Rami [thesis]. Newcastle, Australia, University of Newcastle, 1994, with permission.)
A thoracic MBB procedure should be performed in accordance with the protocol described in the relevant practice guidelines.140,149 The needling part of the procedure must be undertaken with particular care because thoracic needling carries the risk of pleural puncture and pneumothorax. To minimize that risk, the guidelines must be followed meticulously.
Having obtained a suitable view, the operator selects a suitable needle, determines the insertion point, inserts the needle, and directs it to the target site as described for cervical MBBs. It is important to keep the needle tip over the bone of the transverse process at all times, to avoid the danger of pleural puncture. Before each adjustment of the needle, its path toward the target point should be checked on intermittent AP views, and the depth of insertion should be checked on intermittent lateral views. When the needle tip reaches bone at the target point, or the point just above the transverse process and behind the rib for T5 to T8 medial branches, its position is checked and recorded first in a lateral view and then in an AP view (Fig. 36-30). The first injection, of 0.5 mL of local anesthetic, is made at that site. Then the second target point is injected similarly.
Thoracic Medial Branch Blocks—Interpretation of Results
As with cervical MBBs, a single thoracic MBB test result is positive only if the index pain is relieved completely (i.e., its VAS score goes down to zero) in the postblock period. Any other result, with postblock index pain scores of more than zero must be considered negative or inconclusive. If the first block is positive, the issue arises as to whether it is true-positive or false-positive. This is even more important than it is for cervical blocks, because a study has shown single thoracic MBBs have a false-positive rate of 58%.64 Comparative blocks must be done before any sensible conclusion can be drawn from thoracic MBB results. Only two comparative block results that are both positive and concordant can be interpreted as identifying a specific thoracic ZJ as a pain generator.
Lumbar Medial Branch Blocks—Procedure
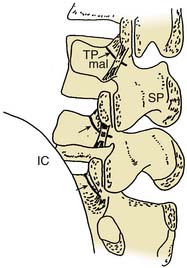
Each lumbar ZJ has sensory innervation via two nerves. The joints from L1-2 to L4-5 are each supplied by articular fibers of two medial branches, numbered like the thoracic medial branches and for the same reason, as one less than the joint they supply. Thus, the L4-5 ZJ has sensory innervation via the L3 and L4 medial branches. The L5-S1 ZJ has its innervation by articular fibers of the L4 medial branch above it and articular fibers that go directly to the L5 dorsal ramus below. The L1 to L4 medial branches receive articular fibers from the tops and bottoms of adjacent ZJs and run through the mamillo-accessory notches, under the mamillo-accessory ligaments, and then across the bases of the superior articular processes near where they join the transverse processes. The numbering is such that the L1 medial branch runs across the superior articular process of the L2 vertebra near where it joins the L2 transverse process. The L5 dorsal ramus runs across the superior articular process of the S1 sacral segment near where it joins the ala of the sacrum (which is analogous to a transverse process). These nerves are accessible for blocking because they run across the bases of the superior articular processes, at the points marked by arrows in Figure 36-31. These points each correspond to the “eye” of a Scottie dog configuration as seen in an oblique fluoroscopic view. If the variation of joint and nerve numbering seems confusing, it may help to remember that the target points for a lumbar ZJ are near the roots of the transverse processes of the same designation (i.e., the target points for MBBs of the L4-5 ZJ are near the roots of the L4 and L5 transverse processes). As with thoracic structures, to avoid ambiguity when referring to a procedure targeting lumbar ZJs and medial branches, it is important to describe it by the joint or the nerves involved, explicitly.
The lumbar MBB procedure, like those of cervical and thoracic MBBs, involves a preoperative phase, an operative (needling) phase, and a postoperative (observational) phase, and when results are at hand, their interpretation which may be considered a fourth phase. The phases are much the same as those described for the other spinal regions. To ensure validity, the procedure should be carried out in accordance with the standard guidelines.140,150
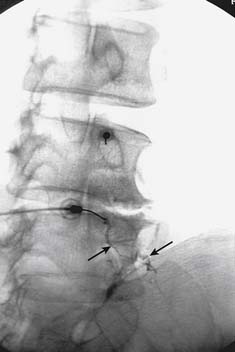
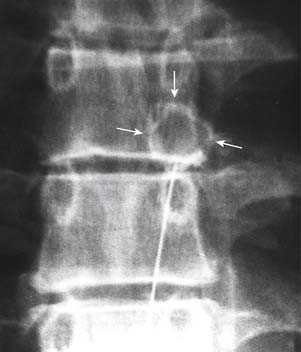
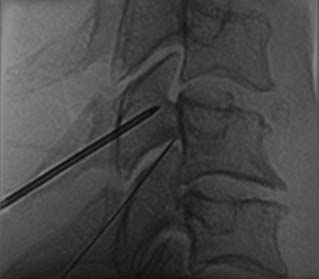
The patient is positioned lying prone on the x-ray table and the skin of their lumbar region is exposed, swabbed, and draped. The operator and radiographer ascertain the spinal level to be tested and the radiographer obtains an AP view of the region. On that view, both operator and radiographer should count down the lumbar vertebrae from the last thoracic vertebra that has a rib attached. If there seem to be six lumbar vertebrae because the first sacral segment is lumbarized, or if any other anatomic variation is noted, the joint to be tested should be confirmed. When the target level is agreed, the radiographer swings the C-arm along the patient to focus on the end-plates of the vertebrae of that segment in an AP view, and then moves it across to obtain an ipsilateral oblique (Scottie dog) view of the target region and cones to it. The target point will be the “dog’s eye.”
To block each nerve the operator takes a suitable needle, determines the insertion point, inserts the needle and directs it to the target site as described for cervical MBBs. It is important to keep the needle tip over bone at all times to avoid overshooting the target. Before each adjustment of the needle, its path toward the target point should be checked on intermittent oblique views. When the needle tip reaches bone at the target point, its position is checked and recorded in an oblique view (Fig. 36-32). Prior to the injection of local anesthetic, 0.5 mL of nonionic contrast medium should be injected to test for intravascular placement. This is done because a study has shown that at the target points for lumbar MBBs, the needle tip is intravascular in 8% of cases151 and if so, any injectate would be carried away in the blood and produce a false-negative result. If the contrast shows the needle tip is not intravascular, 0.5 mL of local anesthetic is injected.
Lumbar Medial Branch Blocks—Interpretation of Results
If the first block is positive, the question arises as to whether that is a true-positive or false-positive result. One study showed that single lumbar MBBs have a false-positive rate of 38%152; other studies have shown false-positive rates between 25% and 41%.74,153 The practical effect of those rates is that if the true-positive to false-positive ratio is simplified conservatively as 2:1, of every three positive lumbar MBB results only two will be truly positive. When considered in conjunction with the relatively low prevalence of lumbar ZJ pain, the high false-positive rate renders the positive predictive value of a single test unacceptably low.152 To compensate for these liabilities, controlled, comparative blocks must be performed, as in other spinal regions, before valid conclusions can be drawn. For maximal diagnostic confidence based on lumbar MBB results, because of the high false-positive rate and the low prevalence, these comparative blocks must include placebo controls.154 Placebo injections must only be given with informed consent but that is usually obtained readily if patients are told about the value of the extra control to the interpretation of the test results. Two comparative MBB results that are both positive and concordant, and a negative placebo control result, can be interpreted as identifying a specific lumbar ZJ as a pain generator.
Lumbar Medial Branch Blocks—Validity
The face validity of lumbar MBBs depends on whether they appear prima facie to test what they are meant to test. Evidence of their face validity is provided by a study of the target-specificity of lumbar MBBs which showed that 0.5 mL injectates at lumbar MBB target sites cover the medial branches and do not spread to any other diagnostically significant structures.151
The construct validity of lumbar MBBs depends on the extent to which positive or negative block results reflect that a lumbar ZJ tested by them is, or is not, a pain source. A randomized, controlled trial showed lumbar MBBs anesthetize painful lumbar ZJs in 89% of cases.155 The issue of false-positive results is addressed by performing placebo-controlled, comparative blocks, as described earlier. False-negative results of lumbar MBBs have been shown to occur at a rate of 8% due to intravascular injection151 but that issue is addressed by preinjection of contrast medium. When performed as described, with contrast injection to check for intravascular needle placement and in comparative series with placebo controls, lumbar MBBs do have construct validity.
The most practical domain of validity is predictive validity or therapeutic utility. Lumbar MBBs have been shown to have predictive validity by studies of percutaneous radiofrequency lumbar medial branch neurotomy. These studies show that therapeutic procedure is effective for the relief of lumbar ZJ pain when it is performed on the basis of positive and concordant, controlled, comparative, lumbar MBBs.156,157
Diagnostic Intraarticular Blocks
Development
Intraarticular blocks (IABs) of ZJs were developed long before MBBs and for some years were used as diagnostic tests for ZJ pain. In the light of the current scientific literature, IABs of ZJs must now be considered obsolete for diagnostic purposes. Medial branch blocks are much more appropriate as diagnostic tests because they are easier to perform, less invasive, safer and validated by the comparative block protocol set out in the standard guidelines. By contrast, IABs of ZJs are more difficult to perform, especially when the joint space is narrowed or occluded by osteophytes. MBBs involve needling of skin, subcutaneous tissues, muscles, and periosteum only, whereas IABs involve intrusion into the joint where the needle may damage delicate intraarticular structures. In MBB procedures, the needle tip can be kept over bone, which prevents inadvertent intrusion into nontarget structures but when IABs of ZJs are done, it is possible for the needle to pass right through the joint and into the adjacent spinal canal, where it may damage the spinal cord or nerve roots. When comparative MBBs are performed in accordance with the standard guidelines they are of proven validity, whereas there are no data on the validity of ZJ IABs. Even if comparative IABs were performed, the differential effects of anesthetic agents inside joints are not known. For these reasons diagnostic IABs of ZJs are not recommended and they will not be described in this chapter.
The lateral atlantoaxial (C1-2) and atlantooccipital (C0-1) joints have nerve supplies quite different from those of the ZJs, as described under “Anatomy”. The lateral atlantoaxial (C1-2) and atlantooccipital (C0-1) joints are supplied via the ventral rami of their respective spinal nerves, not via the medial branches and dorsal rami as are the ZJs. Thus, the procedures of MBBs have no application for the joints of the C0-1 and C1-2 spinal motion segments. Diagnostic blocks of the ventral rami are not practical because of the other structures those nerves supply. Therefore, the lateral atlantoaxial (C1-2) and atlantooccipital (C0-1) joints are tested as sources of pain by intraarticular blockade. Diagnostic IABs of the LAAJs were first described in 1987.158 LAAJ blocks are described in the ISIS practice guidelines as an established procedure159 and will be described here. Diagnostic IABs of the atlantooccipital joints are not described at all in the guidelines, not even as an emerging procedure, because they are not supported by evidence of safety and validity; accordingly, they will be addressed only briefly in this chapter.
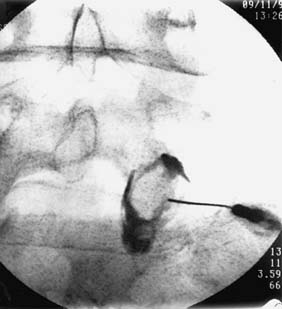
Lateral Atlantoaxial (C1-2) Joint Blocks—Procedure
The LAAJs have been shown to be injured in traumatic events62 and are reported as sources of cervicogenic headache94,95,160 in the distribution illustrated in Figure 36-8. LAAJ blocks should be considered in the diagnosis of a patient with headache in that distribution and a history that suggests the possibility of LAAJ injury. Headache in that distribution may also stem from the C2-3 and C3-4 ZJs, and cervicogenic headache is believed to be more commonly associated with those upper ZJs, so it is prudent to test the C2-3 and C3-4 ZJs first. The main indication for diagnostic LAAJ blocks is for the investigation of cervicogenic headache when C2-3 and C3-4 blocks have proved negative, or when there is residual cervicogenic headache after C2-3 and/or C3-4 headache has been treated effectively.
Intraarticular blocks of the LAAJs are performed via a posterior approach. As for any invasive procedure, it is vital for the operator to have sound knowledge of the regional anatomy. In relation to an LAAJ block, the operator must be aware of two important anatomic structures, the vertebral artery and the C2 dorsal ramus, which lie very close to the target joint. The vertebral artery is usually located immediately lateral to the LAAJ but within the normal range of anatomic variation it may be posterior to the lateral part of the LAAJ. The dorsal ramus of the C2 spinal nerve passes superomedially across the back of the superior articular process of the axis (i.e., just behind the lower process of the LAAJ). To avoid inadvertent puncture of either of those structures, the operator must be meticulous in following the guidelines for the procedure.159 The most appropriate local anesthetic for the block is 2% lidocaine. Longer-acting local anesthetics such as bupivacaine should not be used at the atlantoaxial level because if it should leak from the capsule, it could cause prolonged high spinal block.
The radiographer obtains an AP view of the patient’s upper neck and moves the C-arm longitudinally until the LAAJs are seen as ellipsoid structures on either side of the odontoid process near its base. Both the operator and the radiographer should identify the image of the arch of the axis, which can lie over the LAAJs in an AP view. If necessary, the radiographer angles the x-ray beam so the image of the arch of the atlas is well above the target joint and there is a clear view of the upper margin of the LAAJ in its middle third, which is the initial target point. The radiographer then cones to that area. The operator takes a spinal needle and locates the insertion site on skin over the target (Fig. 36-33).
The needle is inserted and guided through the muscles as described for other blocks. Intermittent AP fluoroscopic views are used to keep the needle tip over the target zone and lateral views are used to check the depth of penetration (Fig. 36-34). When the needle tip reaches bone at the initial target point, on the upper margin of the LAAJ in the middle third of the inferior articular process of the atlas, its position is checked and recorded on AP and lateral fluoroscopic images (Figs. 36-35 and 36-36).
The needle is then withdrawn slightly, directed downward and inserted into the joint. Another intermittent lateral view should be obtained to check the needle tip is intraarticular. A syringe of nonionic contrast medium is connected to the needle by an extension tube and the needle tip placement confirmed by injection of about 0.2 mL of contrast medium to produce an arthrogram of the LAAJ (Fig. 36-37). The C-arm is then rotated 90 degrees and the confirmatory arthrogram recorded on an AP view as well (Fig. 36-38).
Lateral Atlantoaxial (C1-2) Joint Blocks—Validity
Lateral atlantoaxial joint blocks have face validity on the grounds of the confirmatory arthrograms recorded during the procedure. Those arthrograms show that LAAJ blocks are target specific. The construct validity of LAAJ blocks would depend on the extent to which positive or negative block results reflect that the joint tested by them is, or is not, a pain source. There are no numeric data on these aspects of LAAJ blocks.
The predictive validity or therapeutic utility of LAAJ blocks is problematic because there is no simple, validated treatment for LAAJ pain. Intraarticular steroid injections are used by some but the evidence for their effectiveness is weak and based on practice audits95,161; there are no data from randomized, controlled trials. The only other treatment described is surgical fusion of the atlantoaxial segment162,163; although there is a place for such a procedure, there are no rigorous data to show that it is validly indicated by positive LAAJ blocks.
Atlantooccipital (C0-1) Joint Blocks
The atlantooccipital (C0-1) joints have been reported to be pain sources94,161,164 and a pattern of cervicogenic headache associated with them has been described.94 Consideration of the morphology and biomechanics of the atlantooccipital (AO) articulations suggests that they are less likely to be injured, and so to give rise to articular pain, than other spinal joints. The most likely mechanism of injury would be distraction, as the AO joints have powerful restraints to other biomechanical threats. Be that as it may, the prevalence of AO joint pain is unknown. Diagnostic intraarticular blocks of the AO joints are possible in theory but are seldom performed in practice. It is certainly not a procedure to be undertaken because it can be. The technique of injecting an AO joint is a highly specialized one carrying significant risks of damage to vital structures including the vertebral artery, the internal jugular vein, and the vagus nerve, all of which lie adjacent to the joint.
If an AO joint injection is considered necessary and the risks are worth taking, a lateral oblique approach is used with very careful control of the needle course on intermittent oblique fluoroscopic views and monitoring of the depth of insertion on open-mouth AP views. A posterior approach must not be used because the vertebral arteries cross the atlantooccipital joints posteriorly; any attempt to inject an AO joint from behind carries a serious risk of injuring the vertebral artery, with potentially disastrous consequences. Diagnostic AO joint blocks should not be attempted by anyone without rigorous practical training in the procedure. If needle position is confirmed by an arthrogram (Fig. 36-39) and a diagnostic block of the joint is achieved, the interpretation of the result is subject to the same liabilities as those of LAAJ joint blocks. The validity of diagnostic AO joint blocks is also limited like that of LAAJ blocks. If a confirmatory arthrogram is recorded, a block can be said to have face validity (target specificity). There are no data on the construct validity of AO joint blocks and very little data on their predictive validity or therapeutic utility—only two small data sets based on practice audits.161,164
Treatment of Zygapophysial Joint Pain
Development
ZJ pain can be diagnosed definitively, specifically and validly, by the careful application of medial branch block procedures. When it is, the question arises as to how to treat it. The treatment of pain of (or presumed to be of) ZJ origin has a long and colorful history. An extensive range of treatments has been put forward over the years. Many of treatments are still done by a variety of practitioners to relieve pain thought to stem from ZJ impairment of some sort, and variously believed to be due to injury, disease, mechanical dysfunction (including locking, displacement and/or hypermobility) and a host of other putative mechanisms. The forms of treatment still offered include physical modalities (heat, cold, infrared, ultrasound, laser, electrotherapy, massage, mobilization, manipulation), exercise programs, analgesic and anti-inflammatory medications, dry needling, “prolotherapy,” acupuncture, transcutaneous electrical nerve stimulation, other forms of neuromodulation such as spinal cord stimulation, spinal surgery (in particular arthrodesis), functional restoration, “back schools”, and psychological interventions such as cognitive behavioral therapy. These are only some of the “mainstream” treatments: the total range is vast and only limited by human imagination.
Therapeutic Intraarticular Joint Blocks—Procedure
The procedure for each of these intraarticular injections involves three phases like those described for cervical MBBs. In the operative phase the patient lies on the table in the appropriate position (laterally, with the painful side up for cervical injections or prone for thoracic and lumbar procedures). All precautions for aseptic technique are followed, as described for MBBs. The operator and radiographer confirm the target joint and the radiographer obtains a suitable view and cones to the target area. The operator introduces the needle and the guides it through the muscles, taking care to check its path and depth of penetration on intermittent fluoroscopic views, until its tip reaches bone near the target joint. The position of the tip on the joint margin is confirmed on AP and lateral views. Then the tip is guided into the joint cavity. Its position intraarticularly is checked on further AP and lateral views and contrast is injected for a confirmatory arthrogram as described for diagnostic blocks of the LAAJs. Typical cervical, thoracic, and lumbar ZJ arthrograms are shown in Figures 36-40–36–42.
Therapeutic Intraarticular Joint Blocks—Effectiveness
Therapeutic intraarticular joint blocks have been used for the treatment of spinal joint pain for some years and remain in common use. The evidence of their effectiveness is not compelling. The reports of uncontrolled, observational studies,165–168 mainly of intraarticular corticosteroid injection of lumbar joints, gave the impression that the treatment is effective for ZJ pain. These reports encouraged the use of corticosteroid injections for pain from spinal joints at all levels, including ZJs, LAAJs, and atlantooccipital joints.
The results of randomized, controlled trials do not confirm the utility of the treatment. A randomized, controlled trial of cervical intraarticular corticosteroid injections for ZJ pain169 showed the treatment is not more effective than placebo. A randomized, controlled trial of lumbar intraarticular corticosteroid injections for ZJ pain170 also showed no benefit of steroid injections when compared to saline controls. There are no sound data from randomized, controlled trials for the effectiveness of intraarticular corticosteroid injections at other spinal levels.
Radiofrequency Cervical Medial Branch Neurotomy—Development
Radiofrequency medial branch neurotomy (RFMBN) is a treatment that evolved from earlier concepts of trying to relieve ZJ pain by interrupting conduction in the nerves that transmit nociceptive information from the joints. Early attempts at denervation of ZJs by injection of phenol,171 by cryotherapy172 and by surgical neurectomy173 either failed to achieve their purposes or had unacceptable unwanted effects, or both. In particular neurectomy, if it could be achieved, caused neuroma formation and deafferentation, both of which caused worse pain than that which the neurectomy was designed to relieve. In the 1970s, radiofrequency energy was explored as a means of treating nerves to prevent conduction of pain from ZJs.174 Success was claimed in descriptive studies of the technique but it was called into question when its stated targets were found to be inaccurate anatomically.175 Further research lead to the development of more useful techniques.
In its evolution over the last 3 decades, RFMBN has appeared in several different forms. The most effective form, that set out in the ISIS practice guidelines176 and described in this chapter, involves placing an RF electrode parallel to, and closely up against, each medial branch nerve to be treated. Radiofrequency energy is then applied in such a way as to “cook” (i.e., coagulate by heat) a length of the nerve in the accessible part of its course. The purpose of medial branch neurotomy (MBN) is to change the chemical structure of the nerve fibers so they do not conduct nociceptive signals. Nerves treated by thermal RF undergo chemical change but their structure remains as before (like a hard-boiled egg). The maintenance of neural structure obviates the risk of neuroma formation, which occurred after neurectomy. To perform RF cervical MBN properly, making multiple heat lesions over the course of each target nerve from different directions, takes about an hour for each nerve treated, so a two-nerve procedure such as is necessary to treat each of the cervical ZJs (other than that at C2-3) takes about 2 hours.
The equipment required for MBN includes, in addition to the facilities and equipment described for MBBs, an RF generator capable of producing a conventional, thermal RF field, a matched earth plate, connecting leads, and an RF electrode. A large-diameter (18 or 16 gauge) RF electrode should be used to avoid the inadequate effects that were reported in earlier times when smaller electrodes were used.177
Radiofrequency Cervical Medial Branch Neurotomy—Procedure
For the operative phase the patient is positioned laterally on the x-ray table with the side to be treated up (see Fig. 36-15). They are made as comfortable as possible with padding under their lower shoulder and hip, and reassured that they will be kept informed of the progress of the procedure. Full aseptic precautions are observed to ensure a sterile field. The operator should scrub and put on a sterile gown and gloves, the image intensifier should be covered with a sterile cover, and the skin of the side and back of the patient’s neck should be swabbed widely with antiseptic and allowed to dry, then sterile drapes should be applied so the patient’s upper body is covered, except for the side and back of the neck, and the face. It is important to keep the face clear of drapes so the patient can see adequately, breathe easily, and communicate with the operator. The operator and the radiographer confer to confirm the nerves to be treated and the one to be treated first. Then the radiographer obtains a clear lateral view of the target joint and cones to it.
The C-arm is then swung though 90 degrees for an AP view in which the tip of the block needle is seen on the waist of the articular pillar of the target vertebra. A second spinal needle is inserted via a posterior approach so its tip meets that of the block needle. That second needle will lie along the path to be followed by the electrode in its first (sagittal) pass. A short-acting local anesthetic, such as lidocaine 1%, is injected through the second needle as it is withdrawn slowly to anesthetize the electrode path. Then the electrode is introduced and directed along a sagittal course, with the guidance of intermittent AP and lateral views, until it rests on the articular pillar as shown in Figure 36-43. The position of the electrode is confirmed on an AP view to check it is lying snugly against bone, as in Figure 36-44.
Radiofrequency Cervical Medial Branch Neurotomy—Effectiveness
The efficacy of RF cervical MBN has been demonstrated by the results of a rigorously designed, randomized, placebo-controlled clinical trial of the procedure done as described.144 The trial involved a relatively small number of subjects (N = 49) but the design of the study gave it immense statistical power (100%) which is far more important than the sample size. The method used in that trial was judged by objective assessors as the standard for the procedure178 and is the method described in the standard practice guidelines.176 Percutaneous RF cervical MBN, when validly indicated and performed in accordance with those guidelines using 18- or 16-gauge electrodes to make multiple overlapping lesions over the medial branches from sagittal and oblique passes, is an effective treatment for established cervical ZJ pain. Most patients will have complete relief of pain from the joint treated and of any disability caused by that pain.144,145 Any psychological distress associated with the pain can also be expected to resolve.179 If the patient has any residual neck pain after the postoperative discomfort has dissipated, it will be from another pain generator, possibly another injured ZJ that can be investigated and treated similarly.
The effect of RF lesions made as described can be expected to last for an extended period. Data from studies with long follow-up show that after cervical MBN, total pain relief usually lasts for between 270 and 400 days (9 to 13 months).144,145,180,181 The treated nerves are coagulated but not destroyed anatomically and they will recover from the lesions over time. When they do, the pain will return but the RF treatment can then be repeated as needed to restore control of the pain and pain-related disability for as long as the problem persists. RF cervical MBN, when performed correctly and followed appropriately, can completely relieve patients of cervical ZJ pain.
No other report has been published of a rigorously-designed, randomized, controlled trial of MBN for cervical ZJ pain, although there have been publications describing cervical RF procedures done in other ways. As with other spine interventions, it is strongly recommended that RF cervical MBN be undertaken in accordance with the standard method described in the standard guidelines, the method that has been proven effective.
The establishment of a standard necessarily implies other methods are less acceptable. The data of the rigorous controlled trial of MBN144 cannot be generalized to “RF neurotomy” performed in other ways, such as procedures using smaller gauge electrodes, those aimed at different target sites and/or those in which fewer lesions are made over each nerve. Such procedures are likely to achieve less satisfactory outcomes. Systematic reviews that include reports of diverse procedures, all called “radiofrequency neurotomy” but done in different ways, will inevitably show a diversity of outcomes which can be perplexing for anyone trying to gauge the effectiveness of proper MBN treatment. Failure to appreciate the differences between procedures that are given similar names, but vary in fundamental ways, can lead to confusion and controversy. If so, the problem lies with the understanding, not with the standard method. This issue is addressed further under “Radiofrequency Lumbar Medial Branch Neurotomy—Effectiveness”.
Radiofrequency Lumbar Medial Branch Neurotomy—Procedure
Percutaneous thermal RF lumbar MBN is an established procedure for the treatment of lumbar ZJ pain. It is performed in a way similar to that described for RF cervical MBN, using large-diameter (18 or 16 gauge) RF electrodes placed parallel to, and very close to, each of the medial branches that supply the target joint. The treatment should be undertaken in accordance with the protocol laid down in the standard practice guidelines182 for maximal safety and effectiveness.
The RF lumbar MBN procedure involves three phases like those of the cervical treatment, a preoperative phase, an operative phase, and a postoperative, recovery phase. In the operative phase, the patient is positioned lying prone on the table and is made comfortable with suitable padding. Aseptic conditions are observed to ensure a sterile field. Local anesthesia is established and the block needle is left in place to mark the target zone. An RF electrode is introduced in an oblique direction from below and lateral to the target nerve, and aimed toward the target indicated by the block needle. The electrode is directed carefully through the muscles until its tip lies at the base of the superior articular process that the medial branch courses over, and on the posterior part of the adjacent transverse process, with the shaft of the electrode parallel to the nerve. The position and orientation of the active tip of the electrode is critical to the success of the procedure.183 When the electrode is in place at the target site, its position there is checked and recorded on AP, oblique and lateral views, as shown in Figures 36-45 and 36-46.
Radiofrequency Lumbar Medial Branch Neurotomy—Effectiveness
The therapeutic value of percutaneous RF lumbar MBN is controversial. The controversy, like that associated with cervical MBN, is related more to the diversity of RF procedures described in the literature than to the outcomes of valid lumbar MBN treatments. There are many more publications on lumbar RF procedures than on cervical ones, so the potential for confusion and controversy is much greater when lumbar treatments are being considered.
When properly indicated (by positive, controlled, comparative MBBs) and performed in the manner described in the standard practice guidelines,182 percutaneous RF lumbar MBN is an effective treatment for chronic lumbar ZJ pain. In most cases, thermal RF treatment will relieve the pain from the target joint completely and free the patient of any disability related to that pain. If the patient has any residual ZJ pain after the procedure, it will be from another source that may be treated similarly. The published evidence of the effectiveness of thermal RF lumbar MBN is compelling but that is clear only when it is separated from the large amounts of data relating to other RF procedures. These may seem similar superficially but they are essentially dissimilar if they are done to treat different conditions, and/or are based on different indications, and/or involve different techniques, and/or are aimed at different targets. The potential for confusion is considerable.
The effectiveness of RF lumbar MBN was demonstrated first by the report of a randomized, controlled clinical trial published in 1999,184 which showed substantial benefits of the RF procedure over placebo. Although the pain relief was significantly greater in the treatment group (showing that coagulating nerves is better than a sham treatment), the results of the trial are compromised by two factors. First, it included patients on the basis of 50% or more relief of pain after single MBBs (so the study population would have been mixed) and it used a technique in which the electrodes were placed perpendicular to the target nerves, not parallel to them (so only short lengths of nerve were likely to have been coagulated and, in many cases, the relief was only of brief duration). The trial data count as evidence of the effectiveness of medial branch coagulation but do not count as evidence for the full RF lumbar MBN procedure as described in the standard practice guidelines.
Another controlled trial published in 2007,185 used a valid RF technique with electrodes placed parallel to the medial branches. It again showed that conventional, thermal RF coagulation is more effective than placebo, and also more effective than a “pulsed RF” technique which was also tested in the study. The inclusion criteria for this trial were clinical features and a single positive MBB only, so again the study population would have been mixed. The thermal RFMBN outcomes of this study were better and lasted longer than those of the earlier controlled trial. These data again show the effectiveness of medial branch coagulation but because of the inclusion criteria, they do not count as evidence for the full RF lumbar MBN procedure.
A further randomized, controlled clinical trial of RF lumbar MBN published in 2008,157 used positive, comparative MBBs as the diagnostic criterion and employed the RF technique recommended in the standard guidelines. It showed clinically (and statistically) significant relief of pain and improvements in quality-of-life indicators after the active treatment. These data are evidence of the effectiveness of the RF lumbar MBN procedure done in accordance with the standard guidelines. Another controlled clinical trial of RF lumbar MBN published in 2009,186 used a valid, parallel electrode technique and produced further evidence of the effectiveness of the treatment but it used a combination of a positive MBB and a positive intraarticular block as the indication for the treatment, so in that respect it did not conform to the standard criteria.
The data from these four randomized, controlled trials are reinforced by the results of two descriptive outcome studies156,187 of RF lumbar MBN using positive, comparative MBB results as the indication for the treatment and valid, parallel electrode techniques in the therapeutic procedure. Both these studies showed that RF lumbar MBN, when properly indicated and performed according to the standard guidelines, is an effective treatment for lumbar ZJ pain. These data are supported by the conclusions of two systematic reviews of the literature published in 2002188 and 2009,189 which both found evidence in favor of the effectiveness of the procedure.
This clear evidence of the effectiveness of RF lumbar MBN is overshadowed by the reports of several other studies and “systematic reviews” (including one review190 within the framework of the Cochrane Collaboration) which seem to show that the weight of evidence is against the effectiveness of lumbar RF procedures. As intimated earlier, confusion about different types of RF treatment has lead to controversy. Both the confusion and the controversy are easily resolved by considering the different methods used in lumbar RF treatments with specific focus on their validity (i.e., whether those methods achieve what they are intended to achieve). In this regard, the significant elements of any lumbar RF procedure are (1) its target, (2) its indications and (3) how that target is treated. In evaluating the evidence of the effectiveness of RF lumbar MBN for the treatment of ZJ pain, it is important to consider all reports of the outcomes of procedures that (1) target the lumbar medial branches, (2) were indicated by positive, controlled, comparative MBBs and (3) used a valid technique involving multiple lesions made by thermal RF fields applied with 18 or 16 gauge electrodes correctly positioned parallel to the target nerves. The 2008 randomized, controlled trial157 and the two descriptive studies156,187 showing effective RF treatment met all those criteria. Significantly, there are no other published studies that meet those criteria and have unsatisfactory outcomes. Thus, the evidence of the effectiveness of RF lumbar MBN for the treatment of ZJ pain is incontrovertible.
The evidence on the effectiveness of lumbar RF treatment provides a good example of how controversies about interventions arise and how they are resolved. From a scientific point of view, skepticism is always to be encouraged and any doubts raised should be dispelled by considering all relevant scientific evidence. That is the rationale of evidence-based medicine. Current principles of evidence-based medicine flowed from the ideas of Archie Cochrane,191 a Scottish physician who practiced from the 1930s until the 1980s. Cochrane advocated the evaluation of medical interventions by consideration of the scientific evidence of their outcomes. His ideas lead to development of the systematic review, a process in which all relevant, scientific evidence is collated and analyzed to produce a summary of current knowledge of the topic, on which rational management decisions can be based so patients have the best treatment available. After his death, the Cochrane Collaboration, a movement to encourage systematic reviews for that purpose, was named in his honor.
The published literature on lumbar RF procedures includes numerous reports of case series, clinical trials, and systematic reviews showing various outcomes including total pain relief, partial pain relief, only short-term pain relief, and no relief after what are all described as “lumbar radiofrequency” treatments. None of the individual studies other than those described earlier156,157,187 are of procedures performed in the manner recognized as the standard for RF lumbar MBN, so strictly speaking, they are irrelevant to it. These irrelevant publications include reports of procedures targeting the lumbar dorsal root ganglia rather than the medial branches, procedures using pulsed radiofrequency rather than thermal radiofrequency fields, and procedures done for various indications including partial relief of low back pain after single, uncontrolled intraarticular injections. Some clinicians fail to understand the difference between these studies and those of valid RF lumbar MBN, perhaps simply because the words “lumbar” and “radiofrequency” appear in their titles. The confusion is made worse by the publication of what purport to be systematic reviews, which include dissimilar studies as if they are comparable. The authors of such reviews are supposed to understand methodology and to be discriminating in selecting study reports for comparison, yet there are three so-called “systematic reviews”190,192,193 of RF procedures for spinal pain that lump together the data of widely disparate interventions and draw unwarranted conclusions. These conclusions are essentially irrelevant to RF lumbar MBN but the confusion they engender results in some patients with chronic ZJ pain being denied the treatment they need for relief, contrary to what Archie Cochrane strove to achieve.
1. Federative Committee on Anatomical Terminology. Terminologica Anatomica. New York: Thieme Medical Publishers; 1998
2. Goldthwaite J.E. The lumbo-sacral articulation: An explanation of many cases of “lumbago,” “sciatica,” and paraplegia. Boston Med J. 1911;164:365-372.
3. Ghormley R.K. Low back pain with special reference to the articular facets, with presentation of an operative procedure. J Am Med Assoc. 1933;101:1773-1777.
4. Mooney V., Robertson J. The facet syndrome. Clin Orthop Relat Res. 1976;115:149-156.
5. Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine. 1982;7:319-330.
6. Bogduk N., Marsland A. The cervical zygapophysial joints as a source of neck pain. Spine. 1988;13:610-617.
7. Hollinshead W.H. Anatomy for Surgeons, 3rd ed. Philadelphia: Harper & Row; 1982. 77-105
8. Nordin C.C., Levangie P.K. Joint Structure and Function. A Comprehensive Analysis, 2nd ed. Philadelphia: F.A. Davis; 1992. 128-129
9. Delmas A., Ndjaga-Mba M., Vannareth T. Le cartilage articulaire de L4-5 et L5-S1. Comptes Rendus de l’Association des Anatomistes. 1970;147:230-234.
10. Bland J.H., Boushey D.R. Anatomy and physiology of the cervical spine. Semin Arthritis Rheum. 1990;20:1-20.
11. Lewin T., Moffet B., Viidik A. The morphology of the lumbar synovial intervertebral joints. Acta Morphol Neerlando-Scandinav. 1962;4:299-319.
12. Dwyer A., Aprill C., Bogduk N. Cervical zygapophyseal joint pain patterns: I: A study in normal volunteers. Spine. 1990;15:453-457.
13. Engel R., Bogduk N. The menisci of the lumbar zygapophysial joints. J Anat. 1982;135:795-809.
14. Wyke B. Articular neurology—a review. Physiotherapy. 1972;58:94-99.
15. Bogduk N., Wilson A.S., Tynan W. The human lumbar dorsal rami. J Anat. 1982;134:383-397.
16. International Spine Intervention Society. Lumbar medial branch blocks. In: Bogduk N., editor. Practice Guidelines for Spinal Diagnostic and Treatment Procedures. San Francisco: International Spine Intervention Society; 2004:47-65.
17. Bogduk N. Local anesthetic blocks of the second cervical ganglia: a technique with application in occipital headache. Cephalalgia. 1981;1:41-50.
18. Lu J., Ebraheim N.A. Anatomic considerations of C2 nerve root ganglion. Spine. 1998;23:649-652.
19. White A.A.III, Panjabi M.M. The basic kinematics of the human spine. A review of past and current knowledge. Spine. 1978;3:12-20.
20. Panjabi M.M., Oxland T., Takata K., et al. Articular facets of the human spine: Quantitative three-dimensional anatomy. Spine. 1993;18:1298-1310.
21. Johnson R.M., Hart D.L., Simmons E.F., et al. Cervical orthoses. A study comparing their effectiveness in restricting cervical motion in normal subjects. J Bone Joint Surg Am. 1977;59A:332-339.
22. Alund M., Larsson S.E. Three-dimensional analysis of neck motion: A clinical method. Spine. 1990;15:87-91.
23. Dvorák J., Antinnes J.A., Panjabi M.M., et al. Age and gender related normal motion of the cervical spine. Spine. 1992;17(Suppl):S393-S398.
24. Amevo B., Worth D., Bogduk N. Instantaneous axes of rotation of the typical cervical motion segments: A study in normal volunteers. Clin Biomech. 1991;6:111-117.
25. van Mameren H., Sanches H., Beursgens J., Drukker J. Cervical spine motion in the sagittal plane II. Position of segmental averaged instantaneous centers of rotation: A cineradiographic study. Spine. 1992;17:467-474.
26. Nowitzke A., Westaway M., Bogduk N. Cervical zygapophysial joints: geometrical parameters and relationship to cervical kinematics. Clin Biomech. 1994;9:342-348.
27. White A.A. III: Analysis of the mechanics of the thoracic spine in man: An experimental study of autopsy specimens. Acta Orthop Scand. 1969;127(Suppl):1-105.
28. Greene W.B., Heckman J.D., editors. The Clinical Measurement of Joint Motion. Rosemont, Ill: American Academy of Orthopædic Surgeons. 1994:69-98.
29. Pearcy M., Portek I., Shepherd J. Three-dimensional x-ray analysis of normal movement in the lumbar spine. Spine. 1984;9:294-297.
30. Pearcy M.J. Stereo-radiography of lumbar spine motion. Acta Orthop Scand. 1985;212(Suppl):1-45.
31. Dvorák J., Panjabi M.M., Chang D.G., et al. Functional radiographic diagnosis of the lumbar spine: Flexion-extension and lateral bending. Spine. 1991;16:562-571.
32. Pearcy M.J., Tibrewal S.B. Axial rotation and lateral bending in the normal lumbar spine measured by three-dimensional radiography. Spine. 1984;9:582-587.
33. Loebl W.Y. Measurement of spinal posture and range of spinal movement. Ann Phys Med. 1967;9:103-110.
34. Fitzgerald G.K., Wynveen K.J., Rheault W., et al. Objective assessment with establishment of normal values for lumbar spine range of motion. Phys Ther. 1983;63:1776-1781.
35. Pearcy M.J., Bogduk N. Instantaneous axes of rotation of the lumbar intervertebral joints. Spine. 1988;13:1033-1041.
36. King A.I., Prasad P., Ewing C.L. Mechanism of spinal injury due to caudocephalad acceleration. Orthop Clin North Am. 1975;6:19-31.
37. Nachemson A., Morris J.M. In vivo measurements of intradiscal pressures. J Bone Joint Surg Am. 1964;46:1077-1092.
38. Cappozzo A. Compressive loads in the lumbar vertebral column during normal level walking. J Orthop Res. 1984;1:292-301.
39. Andersson G.B.J., Ortengren R., Nachemson A. Quantitative studies of back loads in lifting. Spine. 1976;1:178-184.
40. Farfan H.F. The biomechanical advantage of lordosis and hip extension for upright activity. Man as compared with other anthropoids. Spine. 1978;3:336-342.
41. Merskey H., Bogduk N., editors. Classification of Chronic Pain. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms, 2nd ed. Seattle: International Association for the Study of Pain Press. 1994:210.
42. Merskey H., Bogduk N., editors. Classification of Chronic Pain. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms, 2nd ed. Seattle: International Association for the Study of Pain Press. 1994:213.
43. Loeser J.D., Treede RD. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008;137:473-477.
44. Kaneoka K., Ono K., Inami S., Hayashi K. Motion analysis of cervical vertebrae during whiplash loading. Spine. 1999;24:763-770.
45. Cusick J.F., Pintar F.A., Yoganandan N. Whiplash syndrome: Kinematic factors influencing pain patterns. Spine. 2001;26:1252-1258.
46. Bucholz R.W., Burkhead W.Z., Graham W., Petty C. Occult cervical spine injuries in fatal traffic accidents. J Trauma. 1979;19:768-771.
47. Twomey L.T., Taylor J.R., Taylor M.M. Unsuspected damage to lumbar zygapophyseal (facet) joints after motor vehicle accidents. Med J Aust. 1989;151:210-217.
48. Rauschning W., McAfee P.C., Jónsson H. Pathoanatomical and surgical findings in cervical spinal injuries. J Spinal Disord. 1989;2:213-222.
49. Taylor J.R., Twomey L.T., Corker M. Bone and soft tissue injuries in post mortem lumbar spines. Paraplegia. 1990;28:119-129.
50. Jónsson H.Jr., Bring G., Rauschning W., Sahlstedt B. Hidden cervical spine injuries in traffic accident victims with skull fractures. J Spinal Disorders. 1991;4:251-263.
51. Kakulas B.A., Taylor J.R. Pathology of injuries of the vertebral column and spinal cord. Frankel H.L., editor. Handbook of Clinical Neurology, Vol. 17. Amsterdam: Elsevier. 1992:21-50.
52. Taylor J.R., Twomey L.T. Acute injuries to cervical joints: An autopsy study of neck sprain. Spine. 1993;18:1115-1122.
53. Taylor J.R. The pathology of whiplash. BC Med J. 2002;44:252-256.
54. Schonstrom N., Twomey L.T., Taylor J.R. Lateral atlanto-axial joint injuries. J Trauma. 1993;35:886-892.
55. Manchikanti L., Boswell M.V., Singh V., et al. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;5:15.
56. Hogg-Johnson S., van der Velde G., Carroll L.J., et al. The burden and determinants of neck pain in the general population: Results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(Suppl):S39-S51.
57. Barnsley L., Lord S.M., Wallis B.J., Bogduk N. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine. 1995;20:20-26.
58. Lord S., Barnsley L., Wallis B.J., Bogduk N. Chronic cervical zygapophysial joint pain after whiplash: A placebo-controlled prevalence study. Spine. 1996;21:1737-1745.
59. Bogduk N., Lord S.M. Cervical zygapophysial joint pain. Neurosurg Quarterly. 1998;8:107-117.
60. Gibson T., Bogduk N., Macpherson J., McIntosh A. Crash characteristics of whiplash associated chronic neck pain. J Musculoskeletal Pain. 2000;8:87-95.
61. Lord S.M., Bogduk N., Barnsley L. Prevalence of third occipital headache following whiplash. J Bone Joint Surg. 1993;75B(Suppl 1):74.
62. Lord S., Barnsley L., Wallis B., Bogduk N. Third occipital nerve headache: a prevalence study. J Neurol Neurosurg Psychiatry. 1994;57:1187-1190.
63. Linton S.J., Hellsing A.L., Hallden K. A population-based study of spinal pain among 35-45-year-old individuals. Prevalence, sick leave, and health care use. Spine. 1998;23:1457-1463.
64. Manchikanti L., Singh V., Pampati V., et al. Evaluation of the prevalence of facet joint pain in chronic thoracic pain. Pain Physician. 2002;5:354-359.
65. Blyth F.M., March L.M., Brnabic A.J., et al. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127-134.
66. Taylor W. Musculoskeletal pain in the adult New Zealand population: prevalence and impact. N Z Med J. 2005;118:U1629.
67. Power C., Frank J., Hertzman C., et al. Predictors of low back pain onset in a prospective British study. Am J Public Health. 2001;91:1671-1678.
68. McBeth J., Jones K. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007;21:403-425.
69. Manchikanti L., Singh V., Datta S., et al. Comprehensive review of epidemiology, scope and impact of spinal pain. Pain Physician. 2009;12:E35-E70.
70. Hoy D., Toole M.J., Morgan D., Morgan C. Low back pain in rural Tibet. Lancet. 2003;361:225-226.
71. Bressler H.B., Keyes W.J., Rochon P.A., et al. The prevalence of low back pain in the elderly: A systematic review of the literature. Spine. 1999;24:1813-1819.
72. Nayha S., Videman T., Laasko M., Hassi J. Prevalence of low back pain and other musculoskeletal symptoms and their association with work in Finnish reindeer herders. Scand J Rheumatol. 1991;20:406-413.
73. Barrero L.P., Yi-Hsiang H., Terwedow H., et al. Prevalence and physical determinants of low back pain in a rural Chinese population. Spine. 2006;31:2728-2734.
74. Manchikanti L., Pampati V., Fellows B., Bakhit C.E. Prevalence of lumbar facet joint pain in chronic low back pain. Pain Physician. 1999;2:59-64.
75. Schwarzer A.C., Aprill C.N., Derby R., et al. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine. 1994;19:1132-1137.
76. Schwarzer A.C., Wang S., Bogduk N., et al. Prevalence and clinical features of lumbar zygapophysial joint pain: a study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54:100-106.
77. King W. Medical History. In: Schmidt R.F., Willis W.D.Jr., editors. Encyclopedic Reference of Pain. Berlin: Springer-Verlag; 2007:1112-1115.
78. King W. Musculoskeletal Examination. In: Schmidt R.F., Willis W.D.Jr., editors. Encyclopedic Reference of Pain. Berlin: Springer-Verlag; 2007:1230-1232.
79. Aprill C., Dwyer A., Bogduk N. Cervical zygapophyseal joint pain patterns. II: A clinical evaluation. Spine. 1990;15:458-461.
80. Fukui S., Ohseto K., Shiotani M., et al. Referred pain distribution of the cervical zygapophyseal joints and cervical dorsal rami. Pain. 1996;68:79-83.
81. Cooper G., Bailey B., Bogduk N. Cervical zygapophysial joint pain maps. Pain Med. 2007;8:344-353.
82. Dreyfuss P., Tibiletti C., Dreyer S.J. Thoracic zygapophyseal joint pain patterns. A study in normal volunteers. Spine. 1994;19:807-811.
83. McCall I.W., Park W.M., O’Brien J.P. Induced pain referred from posterior lumbar elements in normal subjects. Spine. 1979;4:441-446.
84. Fairbank J.C.T., Park W.M., McCall I.W., O’Brien J.P. Apophyseal injections of local anaesthetic as a diagnostic aid in primary low-back pain syndromes. Spine. 1981;6:598-605.
85. Fukui S., Ohseto K., Shiotani M., et al. Distribution of referred pain from the lumbar zygapophyseal joints and dorsal rami. Clin J Pain. 1997;13:303-307.
86. Dreyfuss P., Michaelsen M., Fletcher D. Atlanto-occipital and lateral atlanto-axial joint pain patterns. Spine. 1994;19:1125-1131.
87. Aprill C., Axinn M.J., Bogduk N. Occipital headaches stemming from the lateral atlanto-axial (C1-2) joint. Cephalalgia. 2002;21:15-22.
88. Murphy P.M. Somatic Pain. In: Schmidt R.F., Willis W.D.Jr., editors. Encyclopedic Reference of Pain. Berlin: Springer-Verlag; 2007:2190-2192.
89. Roselt D. Somatic Referred Pain. In: Schmidt R.F., Willis W.D.Jr., editors. Encyclopedic Reference of Pain. Berlin: Springer-Verlag; 2007:2192-2196.
90. Govind J. Radicular Pain, Diagnosis. In: Schmidt R.F., Willis W.D.Jr., editors. Encyclopedic Reference of Pain. Berlin: Springer-Verlag; 2007:2081-2083.
91. Bogduk N., Corrigan B., Kelly P., et al. Cervical headache. Med J Aust. 1985;143:202-207.
92. Fredriksen T.A., Hovdal H., Sjaastad O. “Cervicogenic headache”: clinical manifestation. Cephalalgia. 1987;7:147-160.
93. Fjellner A., Bexander C., Faleij R., Strender L.E. Interexaminer reliability in physical examination of the cervical spine. J Manipulative Physiol Ther. 1999;22:511-516.
94. Strender L.E., Sjoblom A., Sundell K., Ludwig R., Taube A. Interexaminer reliability in physical examination of patients with low back pain. Spine. 1997;22:814-820.
95. Viikari-Juntura E. Interexaminer reliability of observations in physical examination of the neck. Phys Ther. 1987;67:1526-1532.
96. Waddell G., Main C.J., Morris E.W., et al. Normality and reliability in the clinical assessment of backache. Br Med J. 1982;284:1519-1523.
97. McCombe P.F., Fairbank J.C.T., Cockersole B.C., Punsent P.B. Reproducibility of physical signs in low-back pain. Spine. 1989;14:908-918.
98. Phillips D.R., Twomey L.T. A comparison of manual diagnosis with a diagnosis established by a uni-level lumbar spinal block procedure. Man Ther. 1996;1:82-87.
99. Sandmark H., Nisell R. Validity of five common manual neck pain provoking tests. Scand J Rehabil Med. 1995;27:131-136.
100. Jull G., Bogduk N., Marsland A. The accuracy of manual diagnosis for cervical zygapophysial joint pain syndromes. Med J Aust. 1988;148:233-236.
101. King W., Lau P., Lees R., Bogduk N. The validity of manual examination in assessing patients with neck pain. Spine J. 2007;7:22-26.
102. Taylor J.R., Twomey L.T. Age changes in the subchondral bone of human lumbar apophyseal joints. J Anat. 1985;143:233.
103. Taylor J.R., Twomey L.T. Age changes in lumbar zygapophysial joints. Spine. 1986;11:739-745.
104. Lawrence J.S., Bremner J.M., Bier F. Osteoarthrosis. Prevalence in the population and relationship between symptoms and x-ray changes. Ann Rheum Dis. 1966;25:1-24.
105. Boden S.D. The use of radiographic imaging studies in the evaluation of patients who have degenerative disorders of the lumbar spine. J Bone Joint Surg Am. 1996;78:114-124.
106. Kalichman L., Li L., Kim D.H., et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine. 2008;33:2560-2565.
107. Yoshimura N., Muraki S., Oka S., et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Min Metab. 2009;27:620-628.
108. Okada E., Matsumoto M., Ichihara D., et al. Aging of the cervical spine in healthy volunteers: a 10-year longitudinal magnetic resonance imaging study. Spine. 2009;34:706-712.
109. Van Tulder M.W., Assendelft W.J.J., Koes B.W., Bouter L.M. Spinal radiographic findings and non-specific low back pain. A systematic review of observational studies. Spine. 1997;22:427-434.
110. Fridenberg Z.B., Miller W.T. Degenerative disc disease of the cervical spine. A comparative study of asymptomatic and symptomatic patients. J Bone Joint Surg Am. 1963;45:1171-1178.
111. Magora A., Schwartz A. Relation between the low back pain syndrome and x-ray findings. 1. Degenerative osteoarthritis. Scand J Rehabil Med. 1976;8:115-125.
112. Torgerson W.R., Dotter W.E. Comparative roentgenographic study of the asymptomatic and symptomatic lumbar spine. J Bone Joint Surg Am. 1976;58:850-853.
113. Heller C.A., Stanley P., Lewis-Jones B., Heller R.F. Value of x-ray examinations of the cervical spine. Brit Med J. 1983;287:1276-1278.
114. Frymoyer J.W., Newberg A., Pope M.H., et al. Spine radiographs in patients with low-back pain. An epidemiological study in men. J Bone Joint Surg Am. 1984;66:1048-1055.
115. Gore D.R., Sepic S.B., Gardner G.M. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;II:521-524.
116. Roh J.S., Teng A.L., Yoo J.U., et al. Degenerative disorders of the lumbar and cervical spine. Orthop Clin North Am. 2005;36:255-262.
117. Wiesel S.W. A study of computer-assisted tomography. 1. The incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984;9:549-551.
118. Teresi L.M., Lufkin R.B., Reicher M.A., et al. Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology. 1987;164:83-88.
119. Boden S.D., Davis D.O., Dina T.S., et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:1178-1184.
120. Boden S.D., McCowin P.R., Davis D.G., et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects: A prospective investigation. J Bone Joint Surg. 1990;72A:1178-1184.
121. Jensen M.C., Bran-Zawadzki M.N., Obucjowski N., et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73.
122. Van der Donk J., Schouten J.S.., Passchier J., et al. The associations of neck pain with radiological abnormalities of the cervical spine and personality traits in a general population. J Rheumatol. 1991;18:1884-1889.
123. Symmons D.P.M., van Hemert A.M., Vandenbroucke J.P., Valkenburg H.A. A longitudinal study of back pain and radiological changes in the lumbar spines of middle aged women: II. radiographic findings. Ann Rheum Dis. 1991;50:162-166.
124. Schwarzer A.C., Wang S.C., O’Driscoll D., et al. The ability of computed tomography to identify a painful zygapophysial joint in patients with chronic low back pain. Spine. 1995;20:907-912.
125. Ronnen H.R., de Korte P.J., Brink P.R.G., et al. Acute whiplash injury: Is there a role for MR imaging? A prospective study of 100 patients. Radiology. 1996;201:93-96.
126. Fukuda K., Kawakami G. Proper use of MR imaging for evaluation of low back pain (radiologist’s view). Semin Musculoskelet Radiol. 2001;5:133-136.
127. Merskey H., Bogduk N., editors. Classification of Chronic Pain. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms, 2nd ed., Seattle: International Association for the Study of Pain Press, 1994:xi-xii.
128. Bonica J.J. The Management of Pain. Philadelphia: Lea and Febiger; 1953.
129. Sternbach R.A. Pain Patients: Traits and Treatments. New York: Academic Press; 1974.
130. King W. Acute, Subacute and Chronic Pain. In: Schmidt R.F., Willis W.D.Jr., editors. Encylopedic Reference of Pain. Berlin: Springer-Verlag; 2007:35-36.
131. Borghouts J.A.J., Koes B.W., Bouter L.M. The clinical course and prognostic factors of non-specific neck pain: A systematic review. Pain. 1998;77:1-13.
132. Indahl A., Velund L., Reikerås O. Good prognosis for low back pain when left untampered. Spine. 1995;20:473-477.
133. McGuirk B., King W., Govind J., et al. Safety, efficacy, and cost-effectiveness of evidence-based guidelines for the management of acute low back pain in primary care. Spine. 2001;26:2615-2622.
134. Bogduk N., Derby R., Aprill C., et al. Precision diagnosis of spinal pain. Seattle: International Association for the Study of Pain Press; 1996:. In 8th World Congress on Pain, Refresher Course Syllabus313-323
135. Hove B., Gyldensted C. Cervical analgesic facet joint arthrography. Neuroradiology. 1990;32:456-459.
136. Wilson P.R. Thoracic facet syndrome: A clinical entity? Pain. 1987;4(suppl):S87.
137. Barnsley L., Bogduk N. Medial branch blocks are specific for the diagnosis of cervical zygapophysial joint pain. Reg Anesth. 1993;18. 343-350
138. Dreyfuss P., Schwarzer A.C., Lau P., Bogduk N. Specificity of lumbar medial branch and L5 dorsal ramus blocks. A computed tomographic study. Spine. 1997;22:895-902.
139. Lord S.M., McDonald G.J., Bogduk N. Percutaneous radiofrequency neurotomy of the cervical medial branches: A validated treatment for cervical zygapophysial joint pain. Neurosurg Q. 1998;8:288-308.
140. Bogduk N. International Spinal Injection Society guidelines for the performance of spinal injection procedures. Part 1: Zygapophysial joint blocks. Clin J Pain. 1997;13:285-302.
141. International Spine Intervention Society. Cervical medial branch blocks. In: Bogduk N., editor. Practice Guidelines: Spinal Diagnostic and Treatment Procedures. San Francisco: International Spine Intervention Society; 2004:112-137.
142. Barnsley L., Lord S.M., Wallis B.J., Bogduk N. False-positive rates of cervical zygapophysial joint blocks. Clin J Pain. 1993;9:124-130.
143. Barnsley L., Lord S., Bogduk N. Comparative local anaesthetic blocks in the diagnosis of cervical zygapophysial joint pain. Pain. 1993;55:99-106.
144. Lord S.M., Barnsley L., Wallis B.J., et al. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335:1721-1726.
145. Govind J., King W., Bailey B., Bogduk N. Radiofrequency neurotomy for the treatment of third occipital headache. J Neurol Neurosurg Psychiatry. 2003;74:88-93.
146. Lord S.M., Barnsley L., Bogduk N. The utility of comparative local anesthetic blocks versus placebo-controlled blocks for the diagnosis of cervical zygapophysial joint pain. Clin J Pain. 1995;11:208-213.
147. Verrills P., Mitchell B., Vivian D., et al. The incidence of intravascular penetration in medial branch blocks: cervical, thoracic, and lumbar spines. Spine. 2008;33:E174-E177.
148. Chua W.H., Bogduk N. The surgical anatomy of thoracic facet denervation. Acta Neurochir. 1995;136:140-144.
149. International Spine Intervention Society. Thoracic medial branch blocks. In: Bogduk N., editor. Practice Guidelines: Spinal Diagnostic and Treatment Procedures. San Francisco: International Spine Intervention Society; 2004:330-347.
150. International Spine Intervention Society. Lumbar medial branch blocks. In: Bogduk N., editor. Practice Guidelines: Spinal Diagnostic and Treatment Procedures. San Francisco: International Spine Intervention Society; 2004:47-65.
151. Dreyfuss P., Schwarzer A.C., Lau P., Bogduk N. The target specificity of lumbar medial branch and L5 dorsal ramus blocks: A computed tomography study. Spine. 1997;22:895-902.
152. Schwarzer A.C., Aprill C.N., Derby R., et al. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain. 1994;58:195-200.
153. Manchikanti L., Pampati V., Fellows B., Bakhit C.E. The diagnostic validity and therapeutic value of lumbar facet joint nerve blocks with or without adjuvant agents. Curr Rev Pain. 2000;4:337-344.
154. Bogduk N. On the rational use of diagnostic blocks for spinal pain. Neurosur Q. 2009;19:88-100.
155. Kaplan M., Dreyfuss P., Halbrook B., Bogduk N. The ability of lumbar medial branch blocks to anesthetize the zygapophysial joint. A physiologic challenge. Spine. 1998;23:1847-1852.
156. Dreyfuss P., Halbrook B., Pauza K., et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine. 2000;25:1270-1277.
157. Nath S., Nath C.A., Pettersen K. Percutaneous lumbar zygapophysial (facet) joint neurotomy using radiofrequency current, in the management of chronic low back pain. A randomized double-blind trial. Spine. 2008;33:1291-1297.
158. McCormick C.C. Arthrography of the atlanto-axial (C1-C2) joints: Technique and results. J Intervent Radiol. 1987;2:9-13.
159. International Spine Intervention Society. Lateral atlanto-axial joint blocks. In: Bogduk N., editor. Practice Guidelines: Spinal Diagnostic and Treatment Procedures. San Francisco: International Spine Intervention Society; 2004:138-151.
160. Ehni G., Benner B. Occipital neuralgia and the C1-2 arthrosis syndrome. J Neurosurg. 1984;61:961-965.
161. Busch E., Wilson P.R. Atlanto-occipital and atlanto-axial injections in the treatment of headache and neck pain. Reg Anesth. 1989;14(suppl 2):45.
162. Joseph B., Kumar B. Gallie’s fusion for atlantoaxial arthrosis with occipital neuralgia. Spine. 1994;19:454-455.
163. Ghanayem A.J., Leventhal M., Bohlman H.H. Osteoarthrosis of the atlanto-axial joints. Long-term follow-up after treatment with arthrodesis. J Bone Joint Surg Am. 1996;78:1300-1307.
164. Dreyfuss P., Rogers J., Dreyer S., Fletcher D. Atlanto-occipital joint pain. A report of three cases and description of intraarticular joint block technique. Reg Anesth. 1994;19:344-351.
165. Lippit A.B. The facet joint and its role in spine pain. Management with facet joint injections. Spine. 1984;9:746-750.
166. Lau L.S.W., Littlejohn G.O., Miller M.H. Clinical evaluation of intra-articular injections for lumbar facet joint pain. Med J Aust. 1985;143:563-565.
167. Lynch M.C., Taylor J.F. Facet joint injection for low back pain. A clinical study. J Bone Joint Surg Br. 1986;68:138-141.
168. Murtagh F.R. Computed tomography and fluoroscopy guided anesthesia and steroid injection in facet syndrome. Spine. 1988;13:686-689.
169. Barnsley L., Lord S.M., Wallis B.J., Bogduk N. Lack of effect of intra-articular corticosteroids for chronic pain in the cervical zygapophyseal joints. N Engl J Med. 1994;330:1047-1050.
170. Carette S., Marcoux S., Truchon R., et al. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med. 1991;325:1002-1007.
171. Peterson T.H. Injection treatment for back pain. Am J Orthop. 1963;5:320-325.
172. Schuster G.D. The use of cryoanalgesia in the painful facet syndrome. J Neurol Orthop Surg. 1982;3:271-274.
173. Rees W.E.S. Multiple bilateral percutaneous rhizolysis. Med J Aust. 1975;1:536-537.
174. Shealy C.N. Percutaneous radiofrequency denervation of spinal facets. Treatment for chronic back pain and sciatica. J Neurosurg. 1975;43:448-451.
175. Bogduk N., Long D. The anatomy of the so-called “articular nerves” and their relationship to facet denervation in the treatment of low-back pain. J Neurosurg. 1979;51:172-177.
176. International Spine Intervention Society. Percutaneous radiofrequency cervical medial branch neurotomy. In: Bogduk N., editor. Practice Guidelines: Spinal Diagnostic and Treatment Procedures. San Francisco: International Spine Intervention Society; 2004:249-284.
177. Lord S.M., Barnsley L., Bogduk N. Percutaneous radiofrequency neurotomy in the treatment of cervical zygapophyseal joint pain: a caution. Neurosurgery. 1995;36:732-739.
178. Bassett K., Sibley L.M., Anton H., et al. Percutaneous radio-frequency neurotomy treatment of chronic cervical pain following whiplash injury: Reviewing evidence and needs. Vancouver: University of British Columbia Centre for Health Services and Policy Research; 2001. British Columbia Office of Health Technology Assessment monograph report 01:5T
179. Wallis B.J., Lord S.M., Bogduk N. Resolution of psychological distress of whiplash patients following treatment by radiofrequency neurotomy: a randomised, double-blind, placebo-controlled trial. Pain. 1997;73:15-22.
180. McDonald G.J., Lord S.M., Bogduk N. Long-term follow-up of patients treated with radiofrequency neurotomy for chronic neck pain. Neurosurgery. 1999;45:61-68.
181. Barnsley L. Percutaneous radiofrequency neurotomy for chronic neck pain: outcomes in a series of consecutive patients. Pain Med. 2005;6:282-286.
182. International Spine Intervention Society. Percutaneous radiofrequency lumbar medial branch neurotomy. In: Bogduk N., editor. Practice Guidelines: Spinal Diagnostic and Treatment Procedures. San Francisco: International Spine Intervention Society; 2004:188-218.
183. Lau P., Mercer S., Govind J., Bogduk N. The surgical anatomy of lumbar medial branch neurotomy (facet denervation). Pain Med. 2004;5:289-298.
184. van Kleef M., Barendse G.A., Kessels A., et al. Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine. 1999;24:1937-1942.
185. Tekin I., Mirzai H., Ok G., et al. A comparison of conventional and pulsed radiofrequency denervation in the treatment of chronic facet joint pain. Clin J Pain. 2007;23:524-529.
186. Burnham R.S., Holitski S., Dinu I. A prospective outcome study on the effects of facet joint radiofrequency denervation on pain, analgesic intake, disability, satisfaction, cost, and employment. Arch Phys Med Rehabil. 2009;90:201-205.
187. Gofeld M., Jitendra J., Faclier G. Radiofrequency denervation of the lumbar zygapophysial joints: 10-year prospective clinical audit. Pain Physician. 2007;10:291-300.
188. Manchikanti L., Singh V., Vilims BD., et al. Medial branch neurotomy in management of chronic spinal pain: systematic review of the evidence. Pain Physician. 2002;5:405-418.
189. Datta S., Lee M., Falco F.J., et al. Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician. 2009;12:437-460.
190. Niemistö L., Kalso E., Malmivaara A., et al. Radiofrequency denervation for neck and back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine. 2003;16:1877-1888.
191. Cochrane A.L. Effectiveness and Efficiency. Cambridge, UK: Cambridge University Press; 1972.
192. Geurts J.W., van Wijk R.M., Stolker R.J., Groen G.J. Efficacy of radiofrequency procedures for the treatment of spinal pain: a systematic review of randomized clinical trials. Reg Anesth Pain Med. 2001;26:394-400.
193. Slipman C.W., Bhat A.L., Gilchrist R.V., et al. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3:310-316.