6 Complications of Common Selective Spinal Injections
Prevention and Management
Selective spinal injections are being performed with increasing frequency in the management of acute and chronic pain syndromes.1–3 Because these procedures require placing a needle in or around the spine, a risk of complications is always present. Therefore, knowledge about prevention of complications, and early recognition and management when they do occur, are paramount to appropriate patient care. This requires adequate physician training and appropriate patient preparation and monitoring. This chapter will discuss physician training, patient preparation and monitoring, and specific complications and their treatment (Appendix II).
Physician Training
Although it is true that uncomplicated lumbar procedures (in an otherwise healthy population) do not require the degree of training and expertise that high-risk procedures performed in a medically unstable population do, certain standards must still be met. Currently, the American Academy of Physical Medicine and Rehabilitation (AAPM&R) has adopted guidelines that recommend a minimum level of documented didactic and clinical training in complication prevention, recognition and management, spinal injection technique, and patient selection, such as that provided in an appropriate fellowship or residency program.4 This program must provide specific training in spinal injections and the recognition, prevention and treatment of related complications; and advanced cardiac life support (ACLS) certification.
Patient Preparation
Patient preparation issues include patient education,5,6 informed consent statement, NPO (nil per os; “nothing by mouth”) status, IV access, certainty that no procedural contraindications exist, patient positioning, sterile preparation and draping, supplemental intravenous (IV) fluids and oxygen, and plans for appropriate recovery following the procedure. Depending on the procedure and patient status, prophylactic antibiotics may also be included.
Patient education should include a thorough description of the procedure, including potential risks, benefits, alternatives, and likely outcomes.6 An informed consent statement, confirming the conversation, should be executed. The statement should include signatures of the patient, the doctor, and a witness.
Prior to the procedure, the patient should be NPO for 12 hours for solid foods and for 8 hours for fluids, preoperatively, to ensure that all gastric contents are distal to the ligament of Treitz.7
If the patient has coexisting medical problems (e.g., has chronic obstructive pulmonary disease [COPD], heart disease, etc.), clearance from the patient’s primary care or specialty doctor should be obtained. Depending on the patient’s problem, preprocedure laboratory work may include a complete blood count with differential diagnosis, liver function tests, urinalysis, chest radiograph, ECG, blood culture and sensitivity, urine culture and sensitivity, and erythrocyte sedimentation rate.
Care must be taken to ensure there is no region of neural compression or stretch, particularly if sedating medication will be used. Areas that are particularly vulnerable to neural compression or stretch include the ulnar nerve at the elbow and the brachial plexus.8 If necessary, use an arm board, tape, strapping or padding to make the patient more comfortable, enable him or her to hold the appropriate position, and prevent the patient’s hands from inadvertently compromising the sterile field.
General Complications of Spinal Injections
Infectious Complications
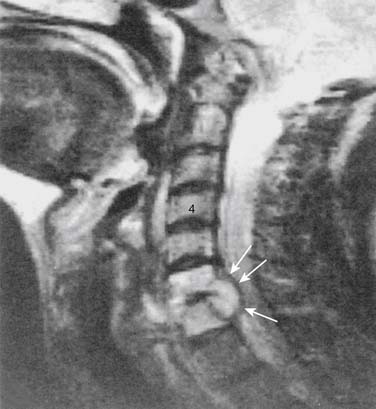
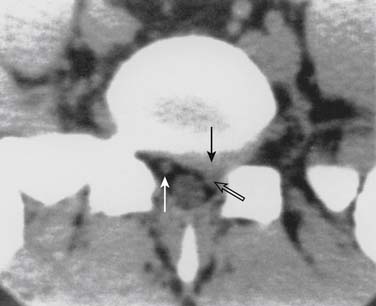
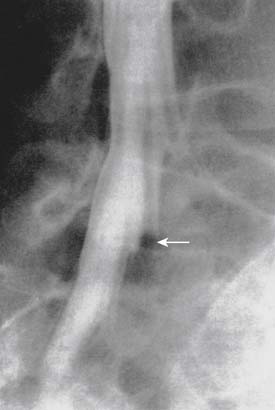
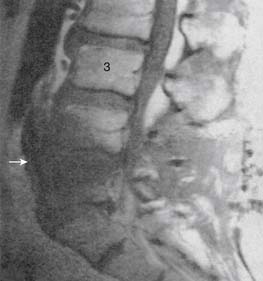
Infections, ranging from minor to severe conditions such as meningitis,9,10 epidural abscess,11–13 and osteomyelitis (Figs. 6-1 and 6-2),14,15 occur in 1% to 2% of spinal injections. Severe infections are rare and occur in from 1 in 1000 to 1 in 10,000 spinal injections. Severe infections may have far-reaching sequelae, such as sepsis, spinal-cord injury, or spreading to other sites in the body via Batson plexus or direct contiguous spreading. Poor sterile technique is the most common cause of infection. Staphylococcus aureus is the most common offending organism causing infection from skin structures.
If the infection appears to progress to spinal structures or spaces, or if the patient is infirm or otherwise predisposed to infection, in-patient evaluation and care with appropriate IV antibiotics is usually required. If epidural abscess occurs, emergent surgical drainage must be considered to avoid neural damage or other complications.16 Early detection and treatment of epidural or intrathecal infection is necessary to avoid morbidity and mortality. It usually manifests with severe back or neck pain, fever, and chills, with a leukocytosis developing on the third day following the injection.13
Cardiovascular Complications
Bleeding is a risk inherent to all injection and surgical procedures. The potential for bleeding during spinal injection is increased by liver disease, the consumption of warfarin or other anticoagulants,5,17,18 certain inherited anemias (such as G6PD deficiency or sickle-cell anemia), coagulopathy from any cause, and venous puncture.
The epidural vasculature is injured in 0.5% to 1% of spinal injections on average, and is more common with placement of the needle in the lateral portion of the spinal canal than the midline.19 Significant epidural bleeding may cause the development of an epidural hematoma. Clinically-significant epidural hematomas are rare, with a reported incidence of less than 1 in 4000 to 1 in 10,000 lumbar epidural cortisone injections; and may lead to irreversible neurologic compromise if not surgically decompressed within 24 hours.19–25 Retroperitoneal hematomas may occur following spinal injection if the large vessels are inadvertently penetrated. These hematomas are usually self-limited but may be a cause of acute hypovolemia or anemia. In addition to bleeding, a variety of dysrhythmias may occur. When a dysrhythmia occurs, treatment should be initiated immediately. The entire team of primary care physicians (PCPs) must be able to function synergistically when treating a dysrhythmia.
ACLS code scenarios should be run in the procedure facility no less than quarterly; all PCPs should know how to alert other staff and extended PCPs immediately; and everyone should know their specific roles in such situations. In addition, all PCPs should know where emergency care equipment is located and how to use it within the limits of their roles. Treatment of individual dysrhythmias is beyond the scope of this chapter; however, the reader is directed to the Emergency Cardiac Care Algorithms included in Appendix I and other sources for more detailed information.26,27
Neurologic Complications
Studies demonstrate that fluoroscopically-guided spinal injections are less apt to cause inadvertent neural injury or injection into a vascular structure.28 A pertinent neurologic review of symptoms and a physical examination should be performed immediately if a neurologic complication is suspected.
Respiratory Complications
Respiratory arrest occurs when a patient becomes apneic for greater than 1 minute, due to lack of central respiratory drive or paralysis of the muscles of respiration.29 Respiratory arrest may occur from a variety of causes, including oversedation, central nervous system trauma, and intrathecal or epidural injection of a sufficient amount of local anesthetic to cause spinal anesthesia.
The true incidence of respiratory depression due to spinal opioid administration is unknown. Factors that may cause respiratory depression include the use of sedatives, parenteral or spinal opioids, and local anesthetics. One of the main advantages of spinal versus parenteral opioid administration is the lack of respiratory depression with the former.30 It should be emphasized that respiratory rate alone is inadequate to establish the presence or lack of respiratory depression. The measurement of blood gases remains the preferred option.29
When a pneumothorax does occur, it is usually self-limited and causes only minor collapse(s) of the lung (10%).31 Treatment includes close observation with supportive care, usually in a hospital, and serial chest radiographs. A chest tube should be placed if the pneumothorax advances significantly over 25% or the patient develops shortness of breath or other signs of respiratory distress.
Urological Complications
The application of local anesthetics and/or opioids to the lumbar and sacral nerve roots results in higher incidence of urinary retention.32 This side-effect of lumbar epidural nerve block is seen more commonly in elderly males, multiparous females, and patients who have undergone inguinal and perineal surgery. Overflow incontinence may occur if such a patient is unable to void or bladder catheterization is not utilized. All patients undergoing lumbar epidural nerve block should demonstrate the ability to void the bladder prior to discharge from the pain center.
Dural Puncture
In the hands of the experienced interventional spine specialist, inadvertent dural puncture during lumbar epidural injections should occur in <0.5% of cases (or 1 in 200 epidural injections).33 This occurs when the dura mater is violated by the epidural needle, and a sufficient amount of cerebrospinal fluid leaks out from the thecal sac, causing a positional headache.34–37 The rare occurrence of postdural puncture (spinal-tap) headache is an annoying side effect, but is generally benign for the most part and will pass without permanent harm or morbidity to the patient.
Rarely, with dehydration and severe nausea and vomiting, uncal herniation may occur, with associated brainstem involvement and potentially death.38 If a needle is placed subdurally and epidural doses of local anesthetics are administered, the signs and symptoms are similar to subarachnoid injection.39 The subdural or subarachnoid injection of large doses of local anesthetics may cause total spinal anesthesia, loss of consciousness, hypotension, cardiovascular arrest, apnea, and even death. This condition requires immediate resuscitative measures and support of all vital signs until the condition resolves. Intubation is usually required to adequately control the airway and ventilate the patient.
Fluoroscopic Exposure
Epidural injections performed without fluoroscopy are not always placed into the epidural space, at the desired vertebral interspace; or the medication does not get to the desired target organ due to anatomic abnormality, as noted in various sources.40–48 For this reason, most spine-management specialists recommend fluoroscopic direction and the use of nonionic or low ionic contrast agents for epidural injections. This helps confirm accurate needle placement and the delivery of the injected solution to the appropriate target organ.48
Medication Reactions
Lidocaine and bupivacaine are the most common local anesthetics used during spinal injections. Awareness of their potential central nervous system (CNS) effects, cardiovascular toxicity, and side effects is very important. Strict cardiovascular and neurologic monitoring is required before, during, and after the procedure. Although most anaphylactic reactions typically occur within 2 hours after the epidural injection, they have been known to occur up to 6 hours later.49
Central nervous-system toxicity by 1% lidocaine has an onset at plasma concentrations of 5-10 mcg/mL, which is slightly more than 400 mg (or 40 mL) of total intravenous bolus. Bupivacaine is about four times more toxic than lidocaine, with a toxic bolus of 100 mg (or 10 mL).50
Methylprednisolone, triamcinolone, and betamethasone are the most commonly used corticosteroid preparations. Side effects are uncommon but could include headache, dizziness, insomnia, facial erythema, rash and pruritus, low-grade “fever” (<100° F), hyperglycemia, transient hypotension and hypertension, increased back or limb pain, fluid retention, mood swing, euphoria, menstrual irregularity, and gastritis.17 Other rare side effects include elevation of cerebrospinal-fluid protein levels, septic or aseptic meningitis, worsening of multiple sclerosis symptoms, sclerosing spinal pachymeningitis, exacerbation of latent infection, near-fatal septic meningitis (intrathecal injection), hypercorticism, and congestive heart failure.
Anaphylactic and Allergy Reactions
Anaphylactoid (without histologic immune response) and anaphylaxis (with a histologic immune response) occur most often within 2 hours after the epidural injection, and have been known to develop up to 6 hours later.49 These usually cause fatalities by respiratory-related complications involving mechanical airway obstruction. Therefore, monitoring patients closely for approximately 30 minutes after the procedure is recommended. Informing the patient about possible risks and side effects can also expedite early identification of complications.
Bleeding Complications
Epidural hematoma formation following injection is extremely rare. Bleeding usually occurs because of damage to the veins in the highly vascular epidural space. Medications that interfere with the clotting mechanism include heparin, warfarin, aspirin, and most nonsteroidal anti-inflammatory drugs (NSAIDs).5,17,18
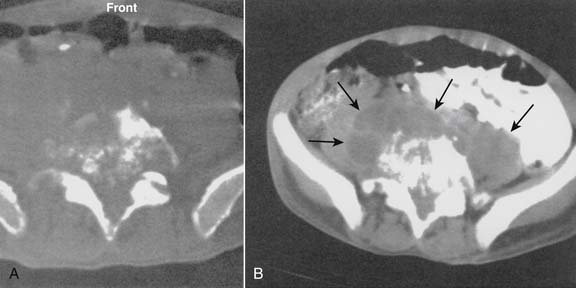
Patients usually present with severe neck or back pain, associated with any significant neurologic complaint, right after the procedure.17 An immediate physical examination, followed by a computer tomography (CT) scan or MRI (magnetic resonance imaging) scan, is essential for patients thought to have an epidural hematoma because early surgical intervention can limit or even prevent permanent neurologic damage (Fig. 6-3).
Specific Complications of Selective Spinal Injections
Lumbar Epidural Injections
The lumbar epidural space is highly vascular. Inadvertent intravenous placement of the epidural needle occurs in approximately 0.5% to 1% of patients undergoing lumbar epidural anesthesia.33 This rare complication is mostly seen with distended epidural veins, such as those present in pregnant patients and patients with large abdominal tumor masses.
If the misplacement is unrecognized, injection of a large volume of local anesthetic directly into an epidural vein may result in significant local anesthetic toxicity.51 Careful four-quadrant aspiration (aspiration in all four quadrants by rotating the needle), prior to injection of drugs into the epidural space, is mandatory in identifying the vascular placement of the needle when performing a “blind” (nonfluoroscopically-guided) epidural injection.
Neurologic complications of lumbar nerve block are uncommon if proper technique is used. Usually, these complications are associated with a preexisting neurologic lesion or with surgical or obstetric trauma, rather than with the lumbar block itself.32
Direct trauma to the spinal cord or nerve roots is usually accompanied by pain. Any significant pain that occurs during placement of the epidural needle or catheter, or during injection, should warn the injectionist to pause and confirm needle placement before proceeding.33 The use of deep intravenous sedation or general anesthesia prior to initiation of epidural nerve block may reduce the patient’s ability to provide accurate verbal feedback if needle misplacement occurs. Therefore, conscious sedation or general anesthesia prior to epidural nerve block should be employed with caution.52
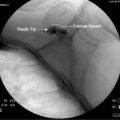
When the patient’s lower-extremity neurologic status deteriorates rapidly or when a cauda equina syndrome is suspected within 24 to 48 hours following an epidural procedure, an expanding epidural hematoma should be considered.53 If the injectionist suspects that diagnosis, an immediate and complete clinical evaluation is mandatory. If the diagnosis is still suspected following the clinical evaluation, a lumbar CT scan or MRI scan should be obtained (Figs. 6-4 through 6-6). If the diagnosis is confirmed, an emergent surgical consult to consider decompression should be arranged.
Caudal Epidural Injections
Incorrect needle placement during caudal epidural injection occurs 25% to 40% of the time.54,55 The needle may be placed outside the sacral canal, resulting in injection of air or fluid into the subcutaneous tissues, periosteum, sacrococcygeal ligament, sacral marrow cavity, and/or pelvic cavity, possibly entering both the rectum and/or vaginal vault.
The application of local anesthetic and opioids to the sacral nerve roots results in increased incidence of urinary retention, especially in elderly males and multiparous females, and after inguinal and perineal surgery. The use of smaller doses of local anesthetic will help avoid these burdensome complications, without adversely affecting the efficacy of caudal epidural steroid injections when treating painful conditions.56
Cervical Epidural Injections
Because of the potential for hematogenous spread via Batson plexus, local infection and sepsis represent absolute contraindications to the cervical approach to the epidural space. Anticoagulation and coagulopathy also represent absolute contraindications to cervical epidural nerve block because of the risk of epidural hematoma.5,6,17,18
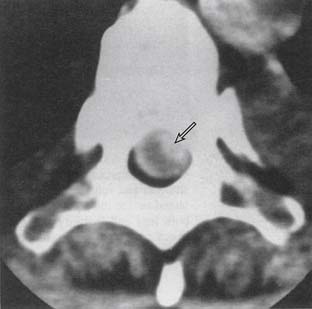
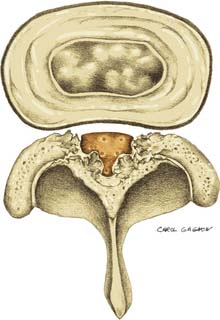
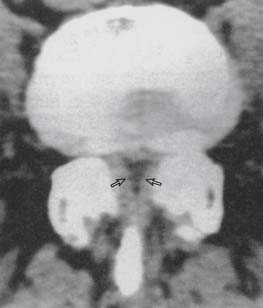
Because the spinal cord lies within the spinal canal, there is increased risk for spinal cord injury with the injection technique, as compared to lower- or midlumbar injections. Central canal stenosis, either from bony eburnation, central disc herniation, or congenital shortening of the pedicles, represents an absolute contraindication to performing an interlaminar epidural injection at that level (Figs. 6-7 and 6-8).57,58
Thoracic Epidural Injections
Thoracic epidural techniques are similar to lumbar techniques; but the presence of the narrow epidural space, and proximity to the spinal cord in the thoracic vertebral canal, makes spinal cord trauma more likely. The incidence of spinal cord damage is unknown, although the incidence of infection is increased in the thoracic spine when compared to the lumbar spine.59
The presence of the lungs on either side of the spine makes a pneumothorax a potential complication not usually considered with either a cervical or lumbar injection. The injection of local anesthetic in the mid-thoracic epidural space may cause inhibition of the cardiac accelerator zone, causing hypotension and bradycardia and its potential sequelae. In addition, a thoracic motor block from either the epidural or intrathecal injection of local anesthetic can cause up to 50% reduction in tidal volume, making adequate ventilation of a patient with pulmonary disease difficult.
Selective Nerve Root Blocks
Selective nerve root blockade has been used interchangeably as a spinal nerve block, selective epidural, or an anterior ramus block.48 In this brief discussion we will deal with each separately.
Discography
The most common severe complication after discography is infection of the disc, commonly referred to as discitis. This should occur no more frequently than in 1 in 500 to 1 in 750 discs injected.60,61 The most common organisms infecting the lumbar disc are S. aureus and Staphylococcus epidermidis.60,62
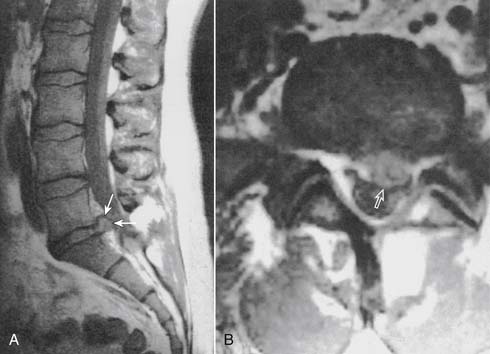
Discitis usually manifests as an increase in spine pain 5 to 14 days following discography. Acutely, there should be no change in the patient’s neurologic status. An elevated sedimentation rate will be seen within the first week to 10 days.63–65 The preferred option in the detection of discitis is now considered to be magnetic resonance imaging (MRI), which was found to be superior to bone scanning with a 92% sensitivity, 97% specificity, and 95% overall accuracy (Fig. 6-9).66–68
The incidence of thoracic discitis following a thoracic discogram is unknown, but the organisms infecting those discs following discography should be similar to those involving the lumbar discs. Similarly, the incidence of pneumothorax and large-vessel damage following thoracic discography is also unknown. In one series of 230 outpatient thoracic discograms, Schellhas reported a zero incidence of pneumothorax.69 Although this is encouraging, the complication does still occur; thus, the procedure should not be attempted without substantial experience and training.
In addition to infectious complications, pneumothorax may occur after cervical and thoracic discography. This complication should rarely occur if appropriate techniques are used. Most pneumothoraces following cervical or thoracic discography are small (10% to 15% of lung volume) and can often be treated conservatively. However, all pneumothoraces must be taken seriously and observed overnight, with serial chest radiographs and close monitoring of vital signs and blood gases. If the pneumothorax progresses, a chest tube must be placed.
Intercostal Nerve Blocks
Given the proximity of the pleural space, pneumothorax after intercostal nerve blocks is a distinct possibility. The incidence of the complication is less than 1% (0.082%),70 but it occurs with greater frequency in patients with chronic obstructive pulmonary disease. Because of the proximity to the intercostal nerve and artery, when analgesia is produced from the intercostal block, the compensatory vasoconstriction eases, and the patient may become hypotensive. In a similar manner, intercostal blocks can lead to respiratory failure when pain relief from the block unmasks the ventilatory depression of previously administered, but ineffective, parenteral narcotics.71
Facet Joint Nerve Blocks
The problem cited most often with these procedures is a transient exacerbation in pain (about 2% incidence), lasting as long as 6 weeks to 8 months in some cases.17,72,73 Spinal anesthesia may occur after facet joint injection if the needle is positioned within the thecal sac, or if there is an abnormal communication between the facet joint capsule and the thecal sac. Chemical meningitis after lumbar facet block has been reported.73–75 These complications are thought to have occurred after inadvertent dural puncture. Facet-capsule rupture also occurs, especially if more than 2.0 mL of injectate is used for intraarticular injections.48
Third occipital nerve blocks can cause transient ataxia and unsteadiness due to partial blockade of the upper cervical proprioceptive afferents and the righting response.76,77 In one study of cervical facet joint radiofrequency denervation, 13% of patients complained of postprocedure pain that resolved in 2 to 6 weeks. Four percent of patients complained of occipital hypesthesia, probably due to a lesion of the third occipital nerve, which resolved in 3 months.77 No persistent motor or sensory deficits occurred.
Sympathetic Nerve Blocks
In the cervicothoracic (stellate ganglion) block, acute, potentially life-threatening complications may occur, including seizure, spinal block, hypotension, or pneumothorax.78–82 Additional complications could include block or injury to the recurrent laryngeal nerve, phrenic nerve, sympathetic trunk, apex of the lung, or brachial plexus.
In the lumbar sympathetic block, potential complications include intravascular injections, intradural injections with spinal anesthesia or postural headaches, hypotension, lumbar-plexus block, renal puncture, or genitofemoral neuralgia.79,83,84 Other risks include injury to the spleen, intestines, and/or liver, and injection of large volumes of local anesthetic into the aorta or inferior vena cava.
1. Falco F.J. Lumbar spine injection procedures in the management of low back pain. Occup Med. 1998;13(1):121-149.
2. Moskovich R. Epidural injections for the treatment of low back pain. Bull Hosp Jt Dis. 1996;55(4):178-184.
3. Kinard R.E. Diagnostic spinal injection procedures. Neurosurg Clin N Am. 1996;7(1):151-165.
4. PASSOR: PASSOR Fellowship Guidelines. Endorsed by the AAPM&R Board of Governors; 1998.
5. PASSOR: PASSOR Educational Guidelines for Interventional Spinal Procedures. Accepted by the PASSOR Board of Governors and AAPM&R Board of Governors; June, 2001; Update, 2004.
6. North American Spine Society. Epidural Steroid Injections. General Information. 2008-2009.
7. Demling R. Preoperative Care. In: Way L.W., editor. Current Surgical Diagnosis and Treatment. Conn. Appleton & Lange: Norwalk, 1991. p.9.8
8. Sunderland S. Nerves and Nerve Injuries, 2nd ed. Edinburgh: Churchill Livingstone; 1978.
9. Dougherty J.H.Jr, Fraser R.A. Complications following intraspinal injections of steroids: Reports of two cases. J Neurosurg. 1978;48:1023-1025.
10. Gutknecht D.R. Chemical meningitis following injections of epidural steroids. Am J Med. 1987;82(3):570. [Letter]
11. Shealy C.N. Dangers of spinal injections without proper diagnosis. JAMA. 1966;197:1104-1106.
12. Goucke C.R., Graziotti P. Extradural abscess following local anesthetic and steroid injection for chronic low back pain. Br J Anaesth. 1990;65(3):427-429.
13. Chan S.T., Leung S. Spinal epidural abscess following steroid injection for sciatica. Case report. Spine. 1984;14(1):106-108.
14. Tham E.J., Stoodley M.A., Macintyre P.E., Jones N.R. Back pain following postoperative epidural analgesia: An indicator of possible infection. Anaesth Intensive Care. 1997;25(3):297-301.
15. Cooper A.B., Sharpe M.D., et al. Bacterial meningitis and cauda equina syndrome after epidural steroid injections. Can J Anaesth. 1996;43(5 Part 1):471-474.
16. Baker A.S., Ojemann R.G., Swartz M.N., Richardson E.P.Jr. Spinal epidural abscess. N Engl J Med. 1975;293:463-468.
17. University of Rochester Spine Center; Department of Orthopaedic Surgery; Department of Physical Medicine & Rehabilitation; University of Rochester School of Medicine; Strong Memorial Health System: What Are Possible Side Effects and Complications of Spine Procedures? 2007.
18. Derby R., Baker R.M., Lee C.H., Anderson P.A. Evidence-informed management of chronic low back pain with intradiscal electrothermal therapy. Spine J. 2008;8:80-95.
19. Bonica J.J. Diagnostic and therapeutic blocks. A reappraisal based on 15 years’ experience. Anesth Analg. 1958;37:58-68.
20. Odoom J.A., Sih I.L. Epidural analgesia and anticoagulant therapy: Experience with one thousand cases of continuous epidurals. Anesthesia. 1983;38:254-259.
21. Rao T.L., El-Etr A.A. Anticoagulation following placement of epidural and subarachnoid catheters: an evaluation of neurologic sequelae. Anesthesiology. 1981;55:618-620.
22. Delaney T.J., Rowlingson J.C., Carron H., et al. Epidural steroid effects on nerves and meninges. Anesth Analg. 1980;59:610-614.
23. Knight C.L., Burnell J.C. Systemic side-effects of extradural steroids. Anaesthesia. 1980;35:593-594.
24. Goebert H.W.Jr., Jallo S.J., Gardner W.J., Wasmuth C.E. Painful radiculopathy treated with epidural injections of procaine and hydrocortisone acetate: results in 113 patients. Anesth Analg. 1961;40:130-134.
25. Knutsen O., Ygge H. Prolonged extradural anesthesia with bupivicaine at lumbago and sciatica. Acta Orthop Scand. 1971;42:338-352.
26. American Heart Association. Textbook of Advanced Cardiac Life Support. 3rd ed. 1993.
27. American Heart Association. Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiac Care. JAMA. 1992;268(16):2171-2302.
28. Racz G. Personal communication. Lubbock, Texas.
29. Rawal N., Wattwil M. Respiratory depression following epidural morphine: An experimental and clinical study. Anesth Analg. 1984;63:8-14.
30. Rawal N., Arntr S., Gustafsson L.L., Allvin R. Present state of epidural and intrathecal opiate analgesia in Sweden. A nationwide follow-up survey. Br J Anaesth. 1987;59:791-799.
31. Bridenbaugh P.O., DuPen S.L., Moore D.C., et al. Post-operative intercostal nerve block analgesia versus narcotic analgesia. Anesth Analg. 1973;52:81-85.
32. Armitage E.N. Lumbar and thoracic epidural. In: Wildsmith J.A.W., Armitage E.N., editors. Principles and Practice of Regional Anesthesia. New York: Churchill Livingstone; 1987:109.
33. Bromage P.R. Complications and contraindications. In: Bromage P.R., editor. Epidural Analgesia. Philadelphia: WB Saunders; 1978:654-711.
34. Benzon H. Epidural steroid injections for low back pain and lumbosacral radiculopathy. Pain. 1986;24:277-295.
35. Swerdlow M., Sayle-Creer W. A study of extradural medication in the relief of the lumbosciatic syndrome. Anaesthesia. 1970;25:341-345.
36. Warr A., Wilkinson J.A., Burn J.M., et al. Chronic lumbosciatic syndrome treated by epidural injection and manipulation. Practitioner. 1972;209:53-59.
37. Kepes E.R., Duncalf D. Treatment of backache with spinal injections of local anesthetics, spinal and systemic steroids: A review. Pain. 1985;22(1):33-47.
38. Deisenhammer E. Clinical and experimental studies on headaches after myelography. Neuroradiology. 1975;9:99-102.
39. Waldman S.D. Subdural injection as a cause of unexplained neurological symptoms. Reg Anesth. 1992;17:55.
40. El-Khoury Ehara S, Weinstein J.N., et al. Epidural injections: A procedure ideally performed with fluoroscopic control. Radiology. 1988;168:554-557.
41. Mehta M., Salmon N. Extradural block. Confirmation of the injection site by x-ray monitoring. Anaesthesia. 1985;40:1009-1012.
42. Dreyfuss P. Epidural steroid injections: A procedure ideally performed with fluoroscopic control and contrast media. ISIS Newsletter. 1(5), 1993.
43. Stewart H.D., Quinnell R.C., Dann N. Epidurography in the management of sciatica. Br J Rheumatol. 1987;26:424-429.
44. Forrest J.B. The response to epidural steroid injections in chronic dorsal root pain. Can Anaesth Soc J. 1980;27:40-46.
45. White A.H., Derby R., Wynne G. Injection techniques for the diagnosis and treatment of low back pain. Spine. 1980;5:78-86.
46. Renfrew D.L., Moore T.E., Kathol M.H., et al. Correct placement of epidural steroid injections: Fluoroscopic guidance and contrast administration. AJNR. 1991;12:1003-1007.
47. White A.H. Injection techniques for the diagnosis and treatment of low back pain. Orthop Clin North Am. 1983;14:553-567.
48. Hildebrandt J. Relevance of nerve blocks in treating and diagnosing low back pain—is the quality decisive? Schmerz. (article in German). 2001;15(6):474-483.
49. Simon D.L., Kunz R.D., German J.D., et al. Allergic or pseudoallergic reaction following epidural steroid deposition and skin testing. Reg Anesth. 1989;14:253-255.
50. Covino BG. Clinical pharmacology of local anesthetic agents. In: Cousins MJ, et al, editors. Neural Blockade in Clinical Anesthesia and Management of Pain. Philadelphia: Lippincott; 1988:111-114.
51. Braid D.P., Scott D.B. The systemic absorption of local analgesic drugs. Br J Anaesth. 1965;37:394-404.
52. Cousins M.J., et al. Epidural neural blockade. In: Cousins M.J., Bridenbaugh M.J., Phillip O., editors. Neural Blockade. Philadelphia: Lippincott; 1988:340-341.
53. Cousins M.J. Hematoma following epidural block. Anesthesiology. 1972;37:263.
54. Waldman S.D., Winnie A.D. Caudal epidural nerve block. In: Waldman S.D., editor. Interventional Pain Management. Philadelphia: WB Saunders; 1996:381-382.
55. Derby R., Bogduk N., Kine G. Precision percutaneous blocking procedures for localizing spinal pain. Part 2. The lumbar neuroaxial compartment. Pain Digest. 1993;3:175-188.
56. Dincer U., Kiralp M.Z., Cakar E., et al. Caudal epidural injection versus non-steroidal anti-inflammatory drugs in the treatment of low back pain accompanied with radicular pain. Joint Bone Spine. 2007;74(5):467-471.
57. Lerner SM, Gutterman P, Jenkins F, et al. Epidural hematoma and paraplegia after numerous lumbar punctures. Anesthesiology. 1973;39:550-551.
58. Waldman S.D. Complications of cervical epidural nerve blocks with steroids: a prospective study of 790 consecutive blocks. Reg Anesth. 1989;11:149-151.
59. Redekop G.J., Del Maestro R.F. Diagnosis and management of spinal epidural abscess. Can J Neurol Sci. 1992;19:180-187.
60. Fraser R.D., Osti O.L., Vernon-Roberts B. Discitis after discography. J Bone Joint Surgery. 1987;69:26-35.
61. Crock H. Practice of Spinal Surgery. New York: Springer-Verlag; 1983.
62. Guyer R.D., Collier R., Stith W.J., et al. Discitis after discography. Spine. 1988;13:1352-1354.
63. Fernand R., Lee C.K. Post-laminectomy disc space infection. A review of the literature and a report of three cases. Clin Orthop Relat Res. 1986;209:215-218.
64. Lindholm T.S., Pylkkanen P. Discitis following removal intervertebral disc. Spine. 1982;7:618-622.
65. Thibodeau A.A. Closed space infection following the removal of lumbar intervertebral disc. J Bone Joint Surg Am. 1968;50:400-410.
66. Arrington J.A., Murtagh F.R., Silbiger M.L., et al. Magnetic resonance imaging of postdiscogram discitis and osteomyelitis in the lumbar spine: case report. J Fla Med Assoc. 1986;73:192-194.
67. Modic M.T., Feiglin D.H., Piraino D.W., et al. Vertebral osteomyelitis: Assessment using MR. Radiology. 1985;157:157-166.
68. Szypryt E.P., Hardy J.G., Hinton C.E., et al. A comparison between magnetic resonance imaging and scintigraphic bone imaging in the diagnosis of disc space infection in an animal model. Spine. 1988;13:1042-1048.
69. Schellhas KP, Pollei K. Thoracic disc degeneration: Correlation of MR imaging and discography. Presented at the 8th Annual Assembly of the North American Spine Society. San Diego, CA; oct 14-16.
70. Moore D.C., Bridenbaugh L.D. Oxygen: the antidote for systemic toxic reactions from local anesthetic drugs. JAMA. 1960;174:842-847.
71. Cory P.C., Mulroy M.F. Post-operative respiratory failure following intercostal block. Anethesiology. 1981;54:418-419.
72. Falco F.J., Irwin L., Zhu J. Lumbar spine injection and interventional procedures in the management of low back pain. Clin Occup Environ Med. 2006;5(3):655-702. vii-viii
73. Bous R.A. Facet joint injections. In: Stanton-Hicks M., Bous R., editors. Chronic Low Back Pain. New York: Raven Press; 1982:199-211.
74. Thomson S.J., Lomax D.M., Collett B.J., et al. Chemical meningism after lumbar facet joint nerve block with local anesthetic and steroids. Anaesthesia. 1991;46:563-564.
75. Berrigan T. Chemical meningism after lumbar facet joint block. Anaesthesia. 1992;47:905-906.
76. Pawl R.P. Headache, cervical spondylosis, and anterior cervical fusion. Surg Annu. 1977;9:391-408.
77. Bogduk N., Marsland A. The cervical zygapophysial joints as a source of neck pain. Spine. 1988;13:610-617.
78. Carron H., Litwiller R. Stellate ganglion block. Anesth Analg. 1975;54:567-570.
79. Lofstrom J., Cousins M.J. Sympathetic neural blockade of upper and lower extremity. In: Cousins M., editor. Neural Blockade in Clinical Management of Pain. Philadelphia: Lippincott; 1988:461.
80. Malmqvist E., Bengtsson M., Sorensen J., et al. Efficacy of stellate ganglion block: A clinical study with bupivacaine. Reg Anesth. 1992;17:340-347.
81. Sachs B.L., Zindrick M.R., Beasley R.D. Reflex sympathetic dystrophy after operative procedures on the lumbar spine. J Bone Joint Surg Am. 1993;75:721-725.
82. Wallace M.S., Milholland A.V. Contralateral spread of local anesthetic with stellate ganglion block. Reg Anesth. 1993;18:55-59.
83. Schmidt S.D., Gibbons J.J. Postdural puncture headache after fluoroscopically-guided lumbar paravertebral sympathetic block. Anesthesiology. 1993;78:198-200.
84. Sprague R.S., Ramamurthy S. Identification of the anterior psoas sheath as a landmark for lumbar sympathetic block. Reg Anesth. 1990;15:253-255.
85. Waldman S.D., Winnie A.P. Interventional Pain Management. W B Saunders; 1996.
86. Kricun R., Kricun M.E. Computed Tomography of the Spine: Diagnostic Exercises. Aspen Publications. 1987.
87. Daffner R.H. Clinical Radiology: The Essentials. Williams & Wilkins; 1993.