Weaning from Mechanical Ventilation
Mechanical ventilation is often lifesaving, but it is associated with numerous complications.1,2 Accordingly, it is imperative to disconnect patients from the ventilator at the earliest feasible time. Deciding the right time to initiate this disconnection process, usually referred to as weaning, is one of the greatest challenges in critical care medicine.3 If a physician is too conservative and postpones the initiation of weaning, the patient is placed at an increased risk of life-threatening, ventilator-associated complications. Conversely, if weaning is begun prematurely, the patient may suffer cardiopulmonary or psychological decompensation of sufficient severity to set back a patient’s clinical course.4
Pathophysiology of Weaning Failure
After patients have been disconnected from the ventilator, up to 25% experience respiratory distress severe enough to necessitate the reinstitution of mechanical ventilation.5,6 The pathophysiologic mechanisms of weaning failure can be divided into those occurring at the level of the respiratory control system, mechanics of the lung and chest wall, the respiratory muscles, the cardiovascular system, and gas-exchange properties of the lung.7
Control of Breathing
Many weaning-failure patients develop hypercapnia. Accordingly, it had been thought that these patients experience an acute decrease in minute ventilation consequent to a decrease in respiratory center output.3 Measurements of respiratory motor output, using mean inspiratory flow or airway occlusion pressure (P0.1), have consistently revealed an increase, not a decrease, in respiratory drive in weaning-failure patients.4,8,9
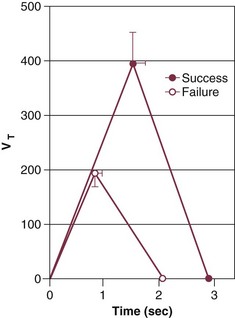
Weaning-failure patients, however, exhibit marked abnormalities in respiratory timing, specifically marked shortening of inspiratory time (TI), which is coupled with shortening of expiratory time (TE). The decrease in both TI and TE means that respiratory frequency (f) is markedly elevated. The shortening of TI combined with a normal mean inspiratory flow (VT/TI) results in a marked decrease of tidal volume (VT).8 This combination (elevated f and decreased VT) is referred to as rapid shallow breathing—now recognized as the physiologic hallmark of weaning failure (Fig. 43.1).3
Respiratory Mechanics
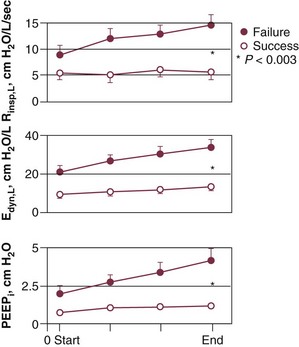
The most detailed study of respiratory mechanics during weaning trials was carried out by Jubran and Tobin.10,11 Immediately before commencement of a trial of spontaneous breathing, patients who went on to tolerate or fail the trial showed little or no difference in detailed measurements of passive respiratory mechanics.11 Resistance, elastance, and intrinsic positive end-expiratory pressure (PEEPi) were equivalent in the two groups.
Over the course of the trial, all of these variables became more abnormal in the weaning-failure patients than in the weaning-success patients (Fig. 43.2).10 Respiratory resistance increased progressively, reaching about seven times the normal value at the end of the trial. Pulmonary elastance increased, reaching five times the normal value. Intrinsic PEEP more than doubled over the course of the trial. A similar pattern has been observed by other investigators.12 The observation that respiratory mechanics were equivalent in weaning-success and weaning-failure patients immediately before a weaning trial but deteriorated immediately in the weaning-failure patients as soon as they began to breathe spontaneously indicates that some mechanism associated with the act of spontaneous breathing causes the worsening of respiratory mechanics that leads to weaning failure.
Patient Effort
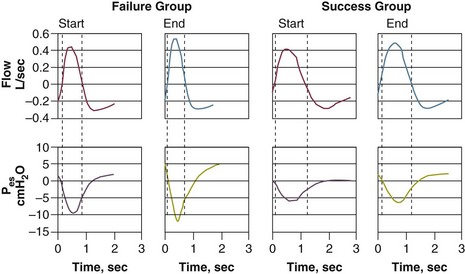
To compensate for the marked worsening of respiratory mechanics, patients need to make a greater inspiratory effort. It had been thought that weaning-failure patients make weaker inspiratory efforts than do weaning-success patients.7 On the contrary, direct measurements of work of breathing and pressure-time product13,14 show that weaning-failure patients consistently make a greater inspiratory effort than do weaning-success patients (Fig. 43.3).10,15
Respiratory Muscles
Numerous research groups have shown that maximal inspiratory pressure (PImax), a measure of respiratory muscle strength, does not discriminate between weaning-success and weaning-failure patients.7 These findings led to the belief that respiratory muscle weakness is not an important determinant of weaning outcome. PImax, however, can misrepresent respiratory muscle strength because the values are heavily influenced by patient motivation and cooperation.13 A more objective measure of diaphragmatic strength is obtained by stimulation of the phrenic nerves and recording the resulting transdiaphragmatic pressure (Pdi). Weaning-failure patients have twitch Pdi values below 10 cm H2O, whereas values of 35 to 39 cm H2O are observed in healthy subjects.16 These data suggest that weaning-failure patients may have considerable muscle weakness.
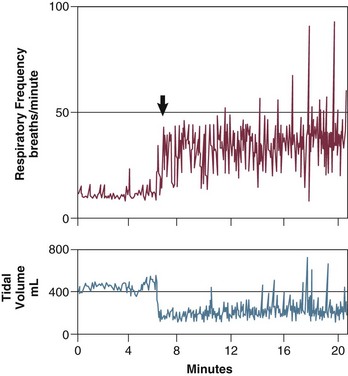
Stimulation of the phrenic nerves and recording of the resulting Pdi also provides the most direct measure of diaphragmatic fatigue.13 Laghi and coworkers17 employed this technique in 11 weaning-failure and 8 weaning-success patients before and after a T-tube trial. No patient in either group exhibited a fall in twitch pressure. This result was surprising. Related analyses disclosed why. Failure patients became progressively distressed during the trial, leading clinicians to reinstate ventilator support before patients had breathed long enough to develop fatigue (Fig. 43.4).17,18 In other words, monitoring clinical signs of distress provides sufficient warning to avoid respiratory muscle fatigue.
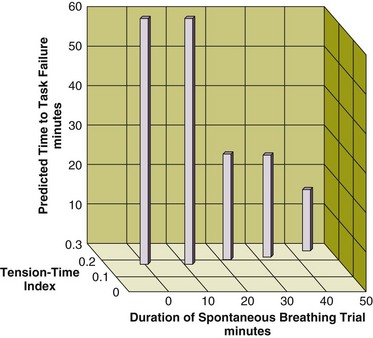
Figure 43.4 Interrelationship between the duration of a spontaneous breathing trial, tension-time index of the diaphragm, and predicted time to task failure in nine patients who failed a trial of weaning from mechanical ventilation. The patients breathed spontaneously for an average of 44 minutes before a physician terminated the trial. At the start of the trial, the tension-time index was 0.17, and the formula of Bellemare and Grassino18 predicted that patients could sustain spontaneous breathing for another 59 minutes before developing task failure. As the trial progressed, the tension-time index increased and the predicted time to development of task failure decreased. At the end of the trial, the tension-time index reached 0.26. That patients were predicted to sustain spontaneous breathing for another 13 minutes before developing task failure clarifies why patients did not develop a decrease in diaphragmatic twitch pressure. In other words, physicians interrupted the trial on the basis of clinical manifestations of respiratory distress, before patients had sufficient time to develop contractile fatigue. (Redrawn from Laghi F, Tobin MJ: Disorders of the respiratory muscles. Am J Respir Crit Care Med 2003;168:10-48.)
Cardiovascular Performance
During a weaning trial, patients can experience substantial increases in right and left ventricular afterload.19,20 These afterload increases most likely result from associated increases in negative swings of intrathoracic pressure. At the completion of a weaning trial, the level of oxygen consumption is equivalent in weaning-success and weaning-failure patients. How the cardiovascular system meets the oxygen demand differs in the two groups of patients. In weaning-success patients, oxygen demand is met through an increase in oxygen delivery, mediated by the expected increase in cardiac output on discontinuation of positive-pressure ventilation.20 In weaning-failure patients, oxygen demand is met through an increase in oxygen extraction; these patients have a relative decrease in oxygen delivery.20 The greater oxygen extraction causes a substantial decrease in mixed venous oxygen saturation, contributing to the arterial hypoxemia that occurs in some patients.20
Gas Exchange
Studies employing the multiple inert-gas technique have revealed that the ventilation-perfusion maldistribution and acute hypercapnia observed in weaning-failure patients is produced primarily by shallow breathing (low VT).7 About half of weaning-failure patients experience an increase in PaCO2 of 10 mm Hg or more over the course of a spontaneous breathing trial.10 The hypercapnia is not usually a consequence of a decrease in minute ventilation. Instead, it results from rapid, shallow breathing, which causes an increase in dead space ventilation. In a small proportion of weaning-failure patients, primary depression of respiratory motor output may be responsible for the hypercapnia.10
Weaning-Predictor Testing
In randomized controlled trials (RCTs) of different weaning techniques, most patients who had received mechanical ventilation for a week or longer were able to tolerate ventilator discontinuation on the first day that weaning-predictor tests were measured.5,6 Many of these patients probably would have tolerated extubation a day or so earlier. As such, one of the main sources of weaning delay is the failure of the physician to think that the patient just might come off the ventilator. Psychological research suggests that much of this delay in ventilator weaning results from clinicians being overconfident in their intuition that a patient is not ready for a weaning trial.4 Another source of error is the failure of clinicians to pay close attention to pretest probability—they fail to recognize the importance of bayesian principles in clinical decision making. When taking care of a ventilator-supported patient, physicians should be mindful of these cognitive processes and employ compensatory tactics, specifically the use of screening tests, to spot a patient’s readiness for weaning. By alerting an unsuspecting physician to a patient’s readiness to tolerate unassisted ventilation—hours or days before he or she would otherwise order a spontaneous breathing trial—weaning-predictor tests circumvent the cognitive errors inherent in clinical decision making.4
Pitfalls in Use of Weaning-Predictor Tests
Physicians commonly view diagnostic testing in monolithic terms: a test is a test is a test. In reality, diagnostic testing has to satisfy two very different tasks: one is screening, the other is confirmation.21 The characteristics of these test types differ, and a single diagnostic test rarely fulfills both functions.21
The fundamental job of a weaning-predictor test is screening.4 Because the goal is to not miss anybody with the condition under consideration, a good screening test has a low rate of false-negative results; to achieve this goal, a higher false-positive rate is acceptable. Thus an ideal screening test has a high sensitivity.4,21
Weaning involves the use of three diagnostic tests in sequence: measurement of predictors, a weaning trial, and a trial of extubation.4 The sequential nature of the testing gives rise to particular problems in studies undertaken to investigate the reliability of a predictor test. One is spectrum bias. This occurs when a new study population contains fewer (or more) sick patients than the population in which a diagnostic test was originally developed.21,22 A second is test-referral bias. This occurs when the results of a test under evaluation are used to select patients for a reference-standard test, such as use of a weaning-predictor test to select patients for a reference-standard test (passing a weaning trial that leads to extubation).21,22
A third factor that affects studies of the reliability of a predictor test is base-rate fallacy.22,23 Consider a diagnostic test for a disease that has a false-positive rate of 5% and false-negative rate of 0%, and the incidence of the disorder (under consideration) is 1 per 1000 persons. A randomly selected person undergoes diagnostic testing. The result comes back positive. What is the chance this person has the disease? More than 80% of physicians answer 95%. The correct answer is 1.96%.23 Physicians who answer 95% are failing to take into account the pretest probability of the disorder. Thus they fall into the trap of base-rate fallacy.
Pretest probability is a physician’s estimate of the likelihood of a particular condition (weaning outcome) before a diagnostic test is undertaken.4 Post-test probability (typically expressed as positive or negative predictive value) is the new likelihood after the test results are obtained. A good diagnostic test achieves a marked increase (or decrease) in the post-test probability (over pretest probability). For every test in every medical subspecialty, the magnitude of change between pretest probability and post-test probability is determined by Bayes’s theorem.22 Three factors (alone) determine the magnitude of the pretest to post-test change: sensitivity, specificity, and pretest probability. Sensitivity and specificity are commonly assumed to remain constant for a test. In truth, test-referral bias, a common occurrence in studies of weaning tests, leads to major changes in sensitivity and specificity.21 Likewise, major changes in pretest probability arise as a consequence of spectrum bias.21 All of these factors need to be carefully considered when reading a study that evaluates the reliability of a weaning-predictor test.
Respiratory Frequency/Tidal Volume Ratio
The ratio of respiratory frequency to tidal volume (f/VT) is measured during 1 minute of spontaneous breathing (Fig. 43.5).24 Measurements of f/VT in the presence of pressure support or continuous positive airway pressure (CPAP) will result in inaccurate predictions of weaning outcome.4 The higher the f/VT ratio, the more severe the rapid, shallow breathing and the greater the likelihood of unsuccessful weaning. An f/VT ratio of 100 best discriminates between successful and unsuccessful attempts at weaning.24
The initial evaluation of f/VT was reported in 1991.24 Since then, this test has been evaluated in more than 25 studies. Reported sensitivity ranges from 0.35 to 1.22 Specificity ranges from 0 to 0.89.22 At first glance, this wide scatter suggests that f/VT is an unreliable predictor of weaning outcome. This was also the viewpoint of an Evidence-Based Medicine Task Force that undertook a meta-analysis of the studies.25,26 The Task Force, however, failed to take into account test-referral bias and spectrum bias.4 In consequence, the Task Force committed at least 15 major errors—any one of which was sufficient to scupper their conclusions.27,28 In contrast, when data from the studies (included in the meta-analysis) were compared against the test characteristics in the original 1991 report, taking into account bayesian pretest probability, the weighted Pearson correlation coefficient was 0.86 (P < 0.0001) for positive predictive value and 0.82 (P < 0.0001) for negative predictive value (Figs. 43.6 and 43.7).22
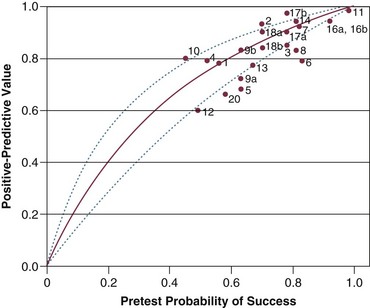
Figure 43.6 Positive predictive value (post-test probability of successful outcome) for f/VT plotted against pretest probability of successful outcome. The curve is based on the sensitivity and specificity originally reported by Yang and Tobin24 and Bayes’s formula for 0.01-unit increments in pretest probability between 0.00 and 1.00.22 The lines represent the upper and lower 95% confidence intervals for the predicted relationship of the positive predictive values against pretest probability. The observed positive predictive value in a study is plotted against the pretest probability of weaning success (prevalence of successful outcome). (Redrawn from Tobin MJ, Jubran A: Variable performance of weaning-predictor tests: Role of Bayes’ theorem and spectrum and test-referral bias. Intensive Care Med 2006;32:2002-2012.)
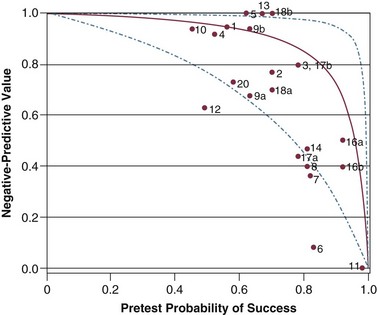
Figure 43.7 Negative predictive value (post-test probability of unsuccessful outcome) for f/VT. The curve, its 95% confidence intervals, and placement of a study on the plot are described in the legend of Figure 43.6. The observed negative predictive value in a study is plotted against the pretest probability of weaning success (prevalence of successful outcome). (Redrawn from Tobin MJ, Jubran A: Variable performance of weaning-predictor tests: Role of Bayes’ theorem and spectrum and test-referral bias. Intensive Care Med 2006;32:2002-2012.)
The primary job of a weaning-predictor test is screening, which requires a high sensitivity.4,21 The average sensitivity in all of the studies on f/VT was 0.89, and 85% of the studies reveal sensitivities higher than 0.90.22 This sensitivity compares well with commonly used diagnostic tests.4
The whole purpose of diagnostic screening is to perform a simple test at a time when a physician’s pretest probability is low (less than 50%).4 A screening test should be cheap, easy to perform, pose minimal risk to patients, and provide a quick answer. A spontaneous breathing trial that involves 30 to 120 minutes of monitored performance is the antithesis of a screening test. Yet, the Evidence-Based Medicine Task Force recommends that clinicians should start weaning with a spontaneous breathing trial (a confirmatory test), and use the initial few minutes of the trial as a screening test.25 This is analogous to saying that when you suspect diabetes, start with a glucose tolerance test and then, as the test gets under way, ask the patient for a urine sample in order to do a dipstick.29
Weaning Trials
When a screening test is positive, the clinician proceeds to a confirmatory test.21 The goal of a positive result on a confirmatory test is to rule in a condition19: The likelihood of a patient tolerating a trial of extubation is high. An ideal confirmatory test has a low rate of false-positive results (i.e., a high specificity).21 Unfortunately, the specificity of a spontaneous breathing trial is not known. Indeed, its specificity will never be known because its determination would require an unethical experiment: extubating all patients who fail a weaning trial and counting how many require reintubation.4
Multiple T-Tube Trials
Of the four methods available for conducting a weaning trial, the use of repeated T-tube trials, several times a day, is the oldest method.3 The patient receives an enriched supply of oxygen through a T-tube circuit. Initially 5 to 10 minutes in duration, T-tube trials are extended and repeated several times a day until the patient can sustain spontaneous ventilation for several hours. This approach has become unpopular because it requires considerable time on the part of intensive care staff.
Intermittent Mandatory Ventilation
For many years, intermittent mandatory ventilation (IMV) was the most popular method of weaning.3 With IMV, the mandatory rate from the ventilator is reduced in steps of 1 to 3 breaths per minute, and an arterial blood gas value is obtained about 30 minutes after each rate change.30 Unfortunately, titrating the number of breaths from the ventilator in accordance with the results of arterial blood gases can produce a false sense of security. As few as two to three positive-pressure breaths per minute can achieve acceptable blood gas values, but these values provide no information regarding the patient’s work of breathing (which may be excessive).4 At IMV rates of 14 breaths per minute or fewer, patient inspiratory efforts are increased to a level likely to cause respiratory muscle fatigue.31,32 Moreover, this occurs not only with the intervening spontaneous breaths but also with ventilator-assisted breaths. Consequently, use of IMV may actually contribute to the development of respiratory muscle fatigue or prevent its recovery.7
Pressure Support
When pressure support is used for weaning, the level of pressure is reduced gradually (decrements of 3 to 6 cm H2O) and titrated on the basis of the patient’s respiratory frequency.33 When the patient tolerates a minimal level of pressure support, he or she is extubated. What exactly constitutes a “minimal level of pressure support” has never been defined.34
Once-Daily T-Tube Trials
The fourth method of weaning is to perform a single daily T-tube trial, lasting for 30 to 120 minutes. If this trial is successful, the patient is extubated. If the trial is unsuccessful, the patient is given at least 24 hours of respiratory muscle rest with full ventilator support before another trial is performed.4
Comparison of Weaning Methods
Until the early 1990s, it was widely believed that all weaning methods were equally effective, and the physician’s judgment was regarded as the critical determinant.3 The results of RCTs have revealed that the period of weaning is as much as three times as long with IMV as with trials of spontaneous breathing.5,6 In a study involving patients with respiratory difficulties on weaning, trials of spontaneous breathing halved the weaning time as compared with pressure support6; in another study, the weaning time was similar with the two methods.5 Performing trials of spontaneous breathing once a day is as effective as performing such trials several times a day but much simpler.6 In patients not expecting to pose any particular difficulty with weaning, a half-hour trial of spontaneous breathing is as effective as a 2-hour trial.35
Weaning by Protocol Versus Usual Care
Six groups of investigators have undertaken RCTs comparing the use of protocols versus usual care in the management of weaning. Three groups—Namen and colleagues,36 Randolph and associates,37 and Krishnan and coworkers38—found that protocolized weaning was without benefit. Data from the other three studies are sometimes viewed as supportive of the superiority of protocolized weaning. The studies by Kollef and colleagues39 and by Ely and associates,40 however, contain internal-validity problems of such magnitude that the data cannot be accepted as valid evidence on which to base a claim that protocols per se expedite weaning. The third study, by Marelich and coworkers,41 revealed no benefit in one of the two ICUs in the study. In summary, only half of one study out of six studies revealed valid support for protocolized weaning, with the remainder providing no evidence of benefit.
Tanios and colleagues42 undertook an RCT to determine whether the inclusion of f/VT in a weaning protocol influenced weaning time. In the f/VT-protocol group, patients could proceed to a weaning trial (CPAP and pressure support) if—and only if—they had an f/VT of less than 106. In the second study arm, clinicians did not follow the protocolized approach to weaning. The duration of weaning was longer in the f/VT-protocol group than in the nonprotocol group.
The study has two major problems. First, the investigators assume that physicians managing patients in the control group did not calculate f/VT. Physicians, however, are highly aware that respiratory frequency and tidal volume are key variables in deciding whether a patient will tolerate weaning and extubation.3 Once this knowledge has crept into a physician’s brain, it cannot be surgically extirpated at the point of commencing an RCT. To ensure that physicians did not employ breathing pattern in the decision making, Tanios and colleagues42 would have had to have taken steps to hide or occlude the display of frequency and tidal volume on the bedside monitor and ventilator screen.
Second, f/VT was one component in a weaning protocol. It is important to make a distinction between the use of a protocol in conducting a research study and its use in everyday clinical practice. In the research protocol of Tanios and colleagues,42 patients who had an f/VT of 105 or less progressed to a weaning trial, whereas patients with an f/VT of 106 or higher were returned to the ventilator. When conducting research, this is exactly how a protocol must be specified and followed. No flexibility is permitted. A competent clinician, however, would think it daft to slavishly comply with a protocol that decided an entire day of ventilator management on a one-unit difference in a single measurement of f/VT.43 Rather, intelligent physicians customize knowledge to the particulars of each patient and are expected to outperform the inflexible application of a protocol—as has been shown in numerous studies of weaning protocols.
Extubation
Decisions about weaning and decisions about extubation are commonly combined.44 When a patient tolerates a weaning trial without distress, a clinician feels reasonably confident that the patient will be able to sustain spontaneous ventilation after extubation. Before removing the endotracheal tube, however, the clinician must also judge whether or not the patient will be able to maintain a patent upper airway after extubation.
Of patients who are expected to tolerate extubation without difficulty, approximately 10% to 20% fail and require reintubation.5,6 The mortality rate among patients who require reintubation is more than six times as high as the mortality rate among patients who can tolerate extubation.35 The reason for the higher mortality rate is unknown. It might be related to the development of new problems after extubation or to complications associated with reinsertion of a new tube. A more likely explanation is that the need for reintubation reflects greater severity of the underlying illness.42
Many physicians find it convenient to extubate a patient once he or she can breathe comfortably on a pressure support of about 7 cm H2O and PEEP 5 cm H2O based on the belief that such “minimal ventilator settings” are simply overcoming the resistance engendered by an endotracheal tube.45 This claim ignores the inflammation and edema that develop in the upper airways after an endotracheal tube has been in place for a day or more. On removal of the tube, the mucosal swelling produces an increase in upper airway resistance. Straus and associates46 demonstrated experimentally that the respiratory work dissipated against the supraglottic airway after extubation is almost identical to the work dissipated against an endotracheal tube before extubation. Thus, applying any level of pressure support causes physicians to underestimate the respiratory resistance a patient will encounter after extubation. The addition of a small amount of pressure support produces surprisingly large reductions in inspiratory work in ventilated patients: 5 cm H2O decreases inspiratory work by 31% to 38% and 10 cm H2O decreases work by 46% to 60%.34,47 Independently, the addition of 5 cm H2O of PEEP can decrease the work of breathing by as much as 40% in ventilated patients.4 In the case of a patient who might experience cardiorespiratory difficulties after extubation, it is incumbent on a physician to ensure that the patient is able to breathe comfortably for about 30 minutes in the complete absence of pressure support or PEEP before removal of the endotracheal tube.45
References
1. Tobin, MJ. Advances in mechanical ventilation. N Engl J Med. 2001; 344:1986–1996.
2. Tobin MJ, ed. Principles and Practice of Mechanical Ventilation, 3rd ed, New York: McGraw-Hill, 2012.
3. Tobin, MJ. Remembrance of weaning past: The seminal papers. Intensive Care Med. 2006; 32:1485–1493.
4. Tobin, MJ, Jubran, A. Weaning from mechanical ventilation. In Tobin MJ, ed. : Principles and Practice of Mechanical Ventilation, 3rd ed, New York: McGraw-Hill, 2012.
5. Brochard, L, Rauss, A, Benito, S, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994; 150:896–903.
6. Esteban, A, Frutos, F, Tobin, MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995; 332:345–350.
7. Tobin, MJ, Laghi, F, Jubran, A. Ventilatory failure, ventilator support, and ventilator weaning. Compr Physiol. 2012; 2:1–51.
8. Tobin, MJ, Perez, W, Guenther, SM, et al. The pattern of breathing during successful and unsuccessful trials of weaning from mechanical ventilation. Am Rev Respir Dis. 1986; 134:1111–1118.
9. Sassoon, CS, Te, TT, Mahutte, CK, et al. Airway occlusion pressure. An important indicator for successful weaning in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1987; 135:107–113.
10. Jubran, A, Tobin, MJ. Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Respir Crit Care Med. 1997; 155:906–915.
11. Jubran, A, Tobin, MJ. Passive mechanics of lung and chest wall in patients who failed or succeeded in trials of weaning. Am J Respir Crit Care Med. 1997; 155:916–921.
12. Vassilakopoulos, T, Zakynthinos, S, Roussos, C. The tension-time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med. 1998; 158:378–385.
13. Tobin, MJ, Laghi, F. Monitoring of respiratory muscle function. In: Tobin MJ, ed. Principles and Practice of Intensive Care Monitoring. New York: McGraw-Hill; 1998:497–544.
14. Tobin, MJ. Monitoring respiratory mechanics in spontaneously breathing patients. In: Tobin MJ, ed. Principles and Practice of Intensive Care Monitoring. New York: McGraw-Hill; 1998:617–654.
15. Laghi, F, Cattapan, SE, Jubran, A, et al. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med. 2003; 167:120–127.
16. Tobin, MJ, Laghi, F, Jubran, A. Ventilator-induced respiratory muscle weakness. Ann Intern Med. 2010; 153:240–245.
17. Laghi, F, Tobin, MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003; 168:10–48.
18. Bellemare, F, Grassino, A. Effect of pressure and timing of contraction on human diaphragm fatigue. J Appl Physiol. 1982; 53:1190–1195.
19. Lemaire, F, Teboul, JL, Cinotti, L, et al. Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology. 1988; 69:171–179.
20. Jubran, A, Mathru, M, Dries, D, et al. Continuous recordings of mixed venous oxygen saturation during weaning from mechanical ventilation and the ramifications thereof. Am J Respir Crit Care Med. 1998; 158:1763–1769.
21. Feinstein, AR. Clinical Epidemiology: The Architecture of Clinical Research. Philadelphia: WB Saunders; 1985.
22. Tobin, MJ, Jubran, A. Variable performance of weaning-predictor tests: Role of Bayes’ theorem and spectrum and test-referral bias. Intensive Care Med. 2006; 32:2002–2012.
23. Casscells, W, Schoenberger, A, Graboys, TB. Interpretation by physicians of clinical laboratory results. N Engl J Med. 1978; 299:999–1001.
24. Yang, KL, Tobin, MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991; 324:1445–1450.
25. MacIntyre, NR, Cook, DJ, Ely, EW, Jr., et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: A collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001; 120(Suppl 6):375S–395S.
26. Meade, M, Guyatt, G, Cook, D, et al. Predicting success in weaning from mechanical ventilation. Chest. 2001; 120:400S–424S.
27. Tobin, MJ, Jubran, A, Hines, E, Jr. Four questions for Dr. MacIntyre on his editorial. Crit Care Med. 2008; 36:2709.
28. MacIntyre, N. Four questions for Dr. MacIntyre on his editorial (author reply). Crit Care Med. 2008; 36:2709–2710.
29. Tobin, MJ. The new irrationalism in weaning. J Bras Pneumol. 2011; 37:571–573.
30. Sassoon, CS. Intermittent mechanical ventilation. In Tobin MJ, ed. : Principles and Practice of Mechanical Ventilation, 2nd ed, New York: McGraw-Hill, 2012.
31. Marini, JJ, Smith, TC, Lamb, VJ. External work output and force generation during synchronized intermittent mechanical ventilation. Effect of machine assistance on breathing effort. Am Rev Respir Dis. 1988; 138:1169–1179.
32. Imsand, C, Feihl, F, Perret, C, et al. Regulation of inspiratory neuromuscular output during synchronized intermittent mechanical ventilation. Anesthesiology. 1994; 80:13–22.
33. Brochard, L. Pressure-support ventilation. In Tobin MJ, ed. : Principles and Practice of Mechanical Ventilation, 2nd ed, New York: McGraw-Hill, 2012.
34. Jubran, A, Van de Graaff, WB, Tobin, MJ. Variability of patient-ventilator interaction with pressure support ventilation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995; 152:129–136.
35. Esteban, A, Alia, I, Tobin, MJ, et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1999; 159:512–518.
36. Namen, AM, Ely, EW, Tatter, SB, et al. Predictors of successful extubation in neurosurgical patients. Am J Respir Crit Care Med. 2001; 163:658–664.
37. Randolph, AG, Wypij, D, Venkataraman, ST, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: A randomized controlled trial. JAMA. 2002; 288:2561–2568.
38. Krishnan, JA, Moore, D, Robeson, C, et al. A prospective, controlled trial of a protocol-based strategy to discontinue mechanical ventilation. Am J Respir Crit Care Med. 2004; 169:673–678.
39. Kollef, MH, Shapiro, SD, Silver, P, et al. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997; 25:567–574.
40. Ely, EW, Baker, AM, Evans, GW, et al. The prognostic significance of passing a daily screen of weaning parameters. Intensive Care Med. 1999; 25:581–587.
41. Marelich, GP, Murin, S, Battistella, F, et al. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses: Effect on weaning time and incidence of ventilator-associated pneumonia. Chest. 2000; 118:459–467.
42. Tanios, MA, Nevins, ML, Hendra, KP, et al. A randomized, controlled trial of the role of weaning predictors in clinical decision making. Crit Care Med. 2006; 34(10):2530–2535.
43. Tobin, MJ. Of principles and protocols and weaning. Am J Respir Crit Care Med. 2004; 169:661–662.
44. Tobin, MJ, Laghi, F. Extubation. In Tobin MJ, ed. : Principles and Practice of Mechanical Ventilation, 2nd ed, New York: McGraw-Hill, 2012.
45. Tobin, MJ. Extubation and the myth of “minimal ventilator settings. ”. Am J Respir Crit Care Med. 2012; 185(4):349–350.
46. Straus, C, Louis, B, Isabey, D, et al. Contribution of the endotracheal tube and the upper airway to breathing workload. Am J Respir Crit Care Med. 1998; 157:23–30.
47. Sassoon, CS, Light, RW, Lodia, R, et al. Pressure-time product during continuous positive airway pressure, pressure support ventilation, and T-piece during weaning from mechanical ventilation. Am Rev Respir Dis. 1991; 143:469–475.