Vitamin deficiencies
THIAMINE DEFICIENCY AND WERNICKE’S ENCEPHALOPATHY
MACROSCOPIC APPEARANCES
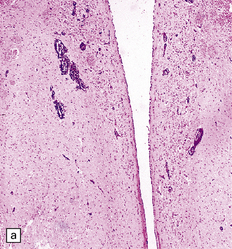
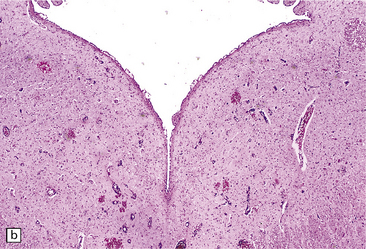
Lesions are usually discernible in the mamillary bodies (Fig. 21.1), but may also involve other parts of the hypothalamus, the medial thalamic nuclei, the floor of the third ventricle, the periaqueductal region (Fig. 21.1), the colliculi (Fig. 21.1), the nuclei in the pontomedullary tegmentum (Fig. 21.1) (particularly the dorsal motor nuclei of the vagus), the inferior olives, and the cerebral cortex. Typically, the involved regions are slightly shrunken and show brown discoloration due to hemosiderin deposition, and there may be petechial hemorrhages. The periventricular and periaqueductal lesions often spare a slender strip of subependymal tissue. In some patients, particularly those with previously treated disease, the mamillary bodies may be only mildly discolored and the brain may appear macroscopically normal.
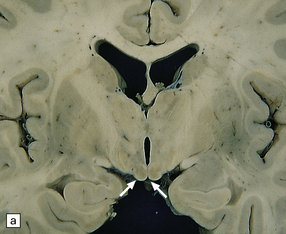
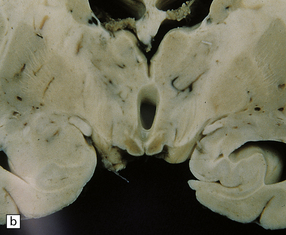
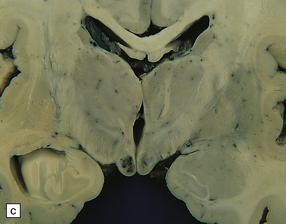
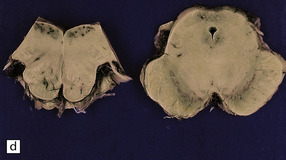
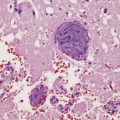
21.1 Wernicke’s encephalopathy.
Compare the shape and color of (a) normal mamillary bodies (arrows), with (b) the shrunken, brown mamillary bodies, and (c) the presence of petechial hemorrhages in cases of Wernicke’s encephalopathy. (d) Brown discoloration and petechial hemorrhages in the periaqueductal region, colliculi, and floor of the fourth ventricle.
MICROSCOPIC APPEARANCES
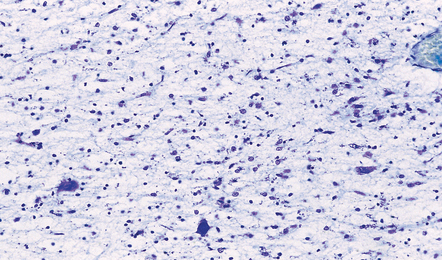
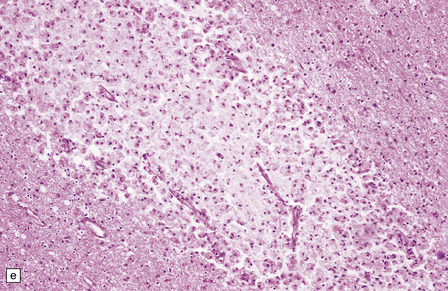
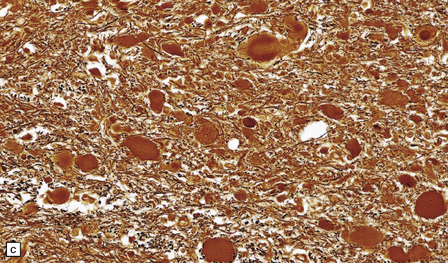
Acute lesions are edematous with relative preservation of neurons, variable necrosis of intervening tissue (Fig. 21.2), and loss of myelinated fibers. Capillaries may appear strikingly prominent due to endothelial hyperplasia and cuffing by macrophages (Fig. 21.2), but this is not a constant feature. There may be petechial hemorrhages and hemosiderin-laden macrophages (Fig. 21.3). Astrocytes show reactive changes.
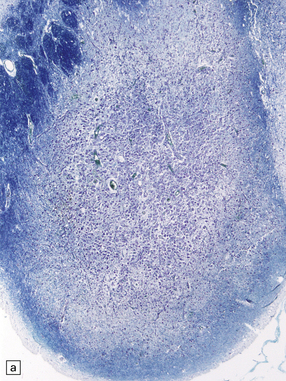
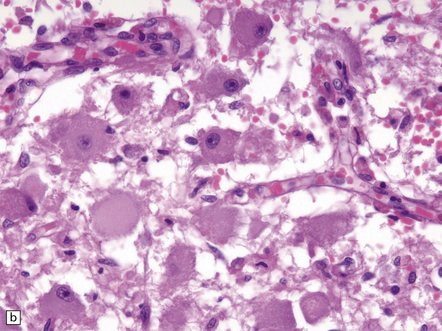
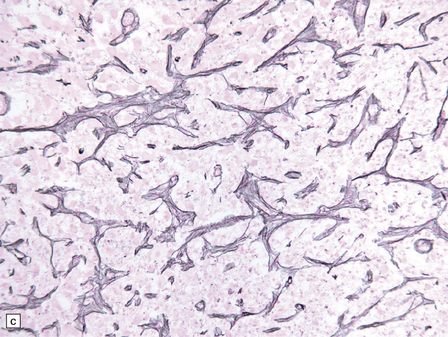
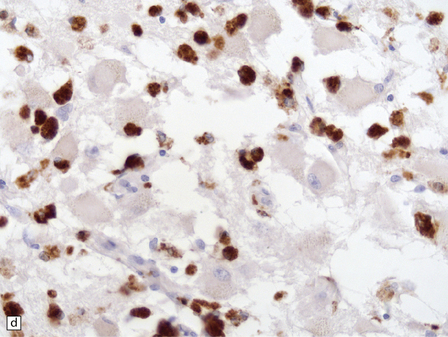
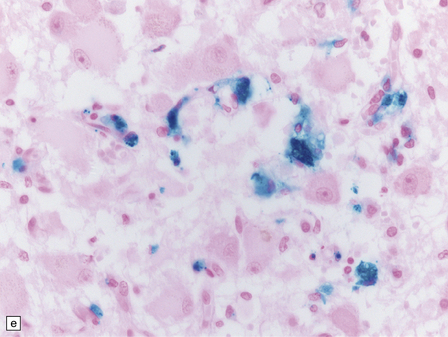
21.2 Active Wernicke’s encephalopathy.
(a) The mamillary body shows loss of myelin and an increase in cellularity. (b) Neuronal somata are relatively preserved, although some neurons are abnormally swollen. (c) The increased prominence of capillaries is well demonstrated by silver impregnation of the capillary basement membranes. (d) The increase in cellularity results mostly from infiltration by macrophages, immunopositive for CD68. (e) Some of the macrophages contain hemosiderin pigment, stained here by Perls’ method.
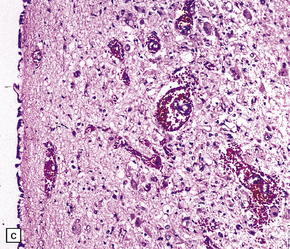
21.3 Hypothalamic and periaqueductal lesions in acute Wernicke’s encephalopathy.
(a) Petechial hemorrhages in the hypothalamus, and (b) periaqueductal gray matter. (c) Higher magnification view of petechial hemorrhages in the hypothalamus.
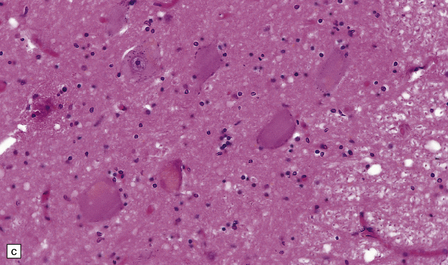
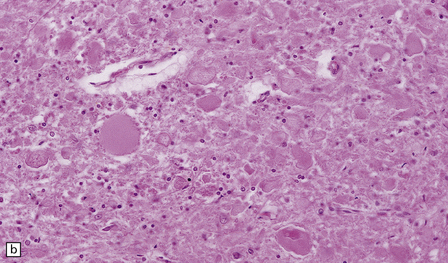
Chronic lesions usually appear gliotic and slightly spongiotic, with mild loss of neurons, depletion of myelinated fibers, and scattered hemosiderin-laden macrophages and astrocytes (Figs 21.4, 21.5).
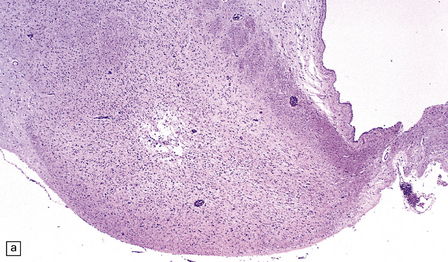
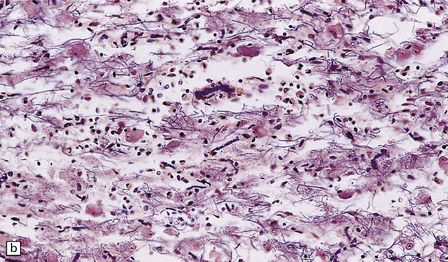
21.4 Chronic Wernicke’s encephalopathy.
(a) Mild focal spongiosis of the mamillary body. (b) Higher magnification of a gliotic mamillary body.
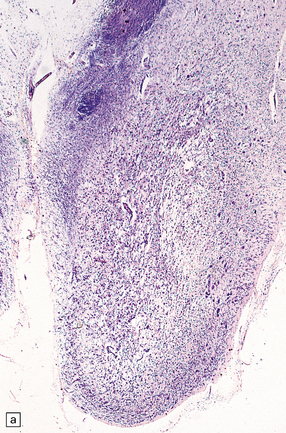
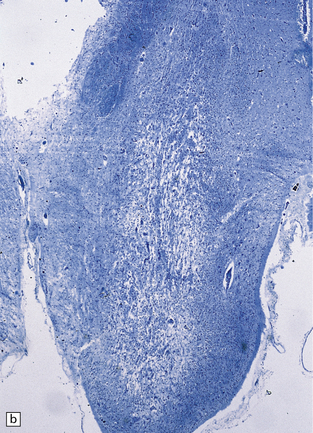
21.5 Chronic Wernicke’s encephalopathy.
(a) Marked shrinkage and spongy gliosis of the mamillary body. (b) The mamillary body is depleted of myelinated fibers.
The histologic changes in Wernicke’s encephalopathy and Wernicke–Korsakoff syndrome are essentially identical. Studies suggest that Korsakoff’s psychosis occurs in those patients who have lesions involving the medial dorsal (or possibly other medial thalamic) nuclei (Fig. 21.6).
NICOTINIC ACID DEFICIENCY AND PELLAGRA
MACROSCOPIC AND MICROSCOPIC APPEARANCES
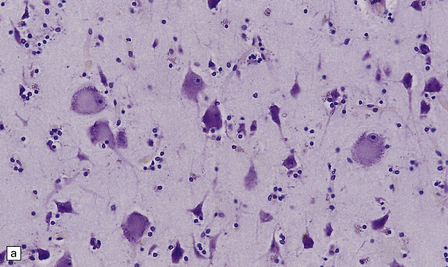
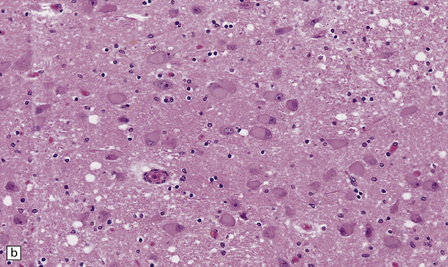
The brain and spinal cord appear macroscopically normal. Histologic changes in the CNS occur predominantly in the later stages of pellagra. Betz cells and neurons in the pontine and cerebellar dentate nuclei show striking chromatolysis without associated microglial or astrocytic changes (Fig. 21.7). Other neurons in the brain stem and the anterior horn cells of the spinal cord (Fig. 21.7) may also be affected. Symmetric degeneration of the dorsal columns, especially the gracile funiculi, and, to a lesser extent, of the corticospinal tracts, has been observed.
PYRIDOXINE (VITAMIN B6)
Pyridoxine is present in most foods, and nutritional deficiency is rare. A functional deficiency can occur in patients receiving isoniazid or other pyridoxine antagonists (see Chapter 25).
VITAMIN B12 (COBALAMIN) DEFICIENCY AND SUBACUTE COMBINED DEGENERATION (SACD)
MICROSCOPIC APPEARANCES
Early lesions consist of spongy vacuolation and degeneration of myelin sheaths in the thoracic region, initially in the posterior columns and later in the corticospinal and spinocerebellar tracts in the lateral columns (Figs 21.8, 21.9). The lesions are approximately symmetric. The disease is not a system degeneration in the sense of involving specific nuclei and their related pathways, and the extent of individual lesions in cross-sections through the cord does not necessarily correspond to that of specific fiber tracts. As the disease progresses myelin breakdown is followed by axonal degeneration, macrophage infiltration, and astrocytic gliosis (Figs 21.8, 21.9). The severity of the lesions usually diminishes towards the cervical and lumbar regions, but these show changes of secondary ascending and descending tract degeneration. In severe cases the anterior columns are also involved (Fig. 21.9). Rarely, the lesions extend rostrally into the medulla.
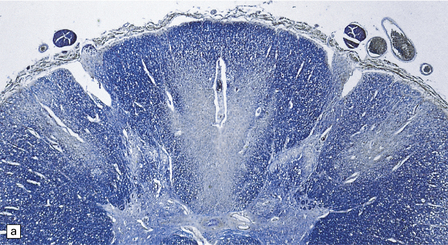
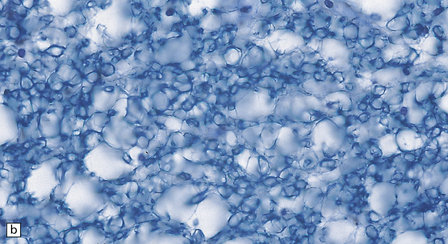
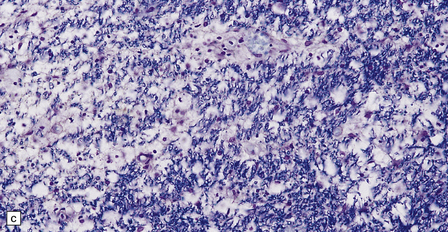
21.8 Early SACD.
(a) There is symmetric loss of myelin staining in part of the posterior and lateral columns. (b) Vacuolation within the myelin sheaths in the posterior columns is the earliest histologic abnormality in SACD. (c) These lesions show early infiltration by macrophages.
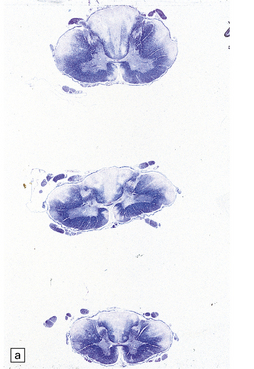
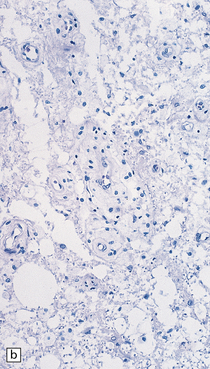
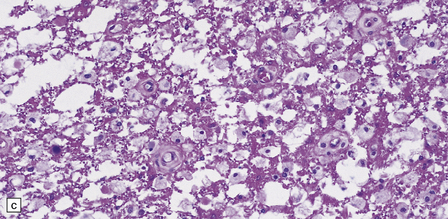
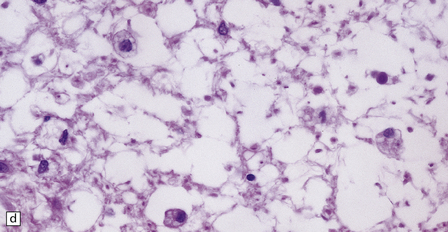
21.9 Advanced SACD.
(a) Myelin pallor in the posterior and lateral columns in the cervical and upper thoracic spinal cord in advanced SACD. There is also some anterior column involvement. (b) Higher magnification view showing spongy vacuolation of white matter in the posterior column, loss of myelin staining, perivascular macrophages, and some reactive astrocytes. Note the punctate cross-sectional profiles of some axons that are still intact, particularly towards the bottom of the illustration. (c,d) show coarse vacuolation of the affected spinal white matter, and infiltration by macrophages. (e) This case shows involvement of the anterior columns as well as the posterior and lateral columns. (f) Perivascular lesion with accumulation of foamy macrophages in the cerebral white matter of a patient with SACD.
The cerebral white matter and optic nerves may contain small, often perivascular, foci of demyelination or fiber degeneration with an accumulation of lipid-laden macrophages (Fig. 21.9). Rarely, the cerebral lesions are extensive.
VITAMIN E (α-TOCOPHEROL)
MACROSCOPIC AND MICROSCOPIC APPEARANCES
The brain and spinal cord appear macroscopically normal. Histology reveals swelling and degeneration of the distal part of longer axons in peripheral nerves and the posterior spinal, spinocerebellar, and corticospinal tracts (see Chapter 25). Axonal swellings are particularly prominent in the gracile and, to a lesser extent, cuneate funiculi and nuclei (Fig. 21.10), but may also be seen in other brain stem nuclei and in the basal ganglia. The axons show dystrophic ultrastructural changes, with accumulation of filaments, membranes, tubules, degenerate mitochondria, and osmiophilic debris.
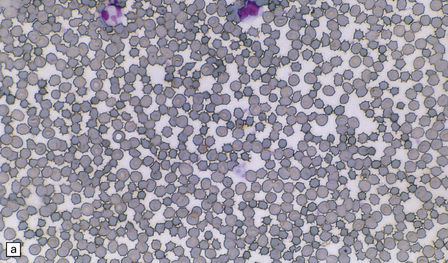
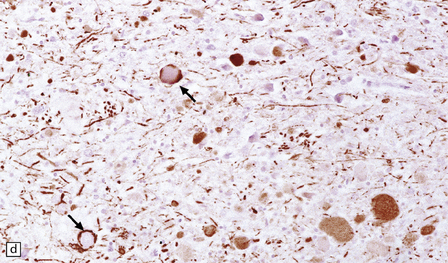
21.10 Vitamin E deficiency.
(a) Acanthocytosis due to vitamin E deficiency. This blood smear shows many of the red blood cells to have characteristic spiny protuberances. (b) Axonal swellings in the rostral part of the gracile funiculus. (c) The swollen axons in the gracile funiculus are moderately argyrophilic. (d) They also show variable neurofilament immunopositivity, in some cases confined to the subsarcolemmal region (arrows).
REFERENCES
Abu-Kishk, I., Rachmiel, M., Hoffmann, C., et al. Infantile encephalopathy due to vitamin deficiency in industrial countries. Childs Nerv Syst.. 2009;25:1477–1480.
Black, M.M. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull.. 2008;29:S126–S131.
Casella, E.B., Valente, M., de Navarro, J.M., et al. Vitamin B12 deficiency in infancy as a cause of developmental regression. Brain Dev.. 2005;27:592–594.
Chatterjee, A., Yapundich, R., Palmer, C.A., et al. Leukoencephalopathy associated with cobalamin deficiency. Neurology.. 1996;46:832–834.
Cook, C.C., Hallwood, P.M., Thomson, A.D. B Vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol.. 1998;33:317–336.
Czeizel, A.E. Primary prevention of neural-tube defects and some other major congenital abnormalities: recommendations for the appropriate use of folic acid during pregnancy. Paediatr Drugs.. 2000;2:437–449.
Dror, D.K., Allen, L.H. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev.. 2008;66:250–255.
Ghenimi, N., Beauvieux, M.C., Biran, M., et al. Vitamin A deficiency in rats induces anatomic and metabolic changes comparable with those of neurodegenerative disorders. J Nutr.. 2009;139:696–702.
Hahn, J.S., Berquist, W., Alcorn, D.M., et al. Wernicke encephalopathy and beriberi during total parenteral nutrition attributable to multivitamin infusion shortage. Pediatrics.. 1998;101:E10.
Harding, A.E. Vitamin E and the nervous system. Crit Rev Neurobiol.. 1987;3:89–103.
Harper, C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol.. 2009;44:136–140.
Harper, C. Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe. Eur J Neurol.. 2006;13:1–6.
Holmøy, T., Moen, S.M. Assessing vitamin D in the central nervous system. Acta Neurol Scand Suppl.. 2010;190:88–92.
Lanska, D.J. Historical aspects of the major neurological vitamin deficiency disorders Overview and fat-soluble vitamin A. Handb Clin Neurol.. 2009;95:435–444.
Levenson, C.W., Figueirôa, S.M. Gestational vitamin D deficiency: long-term effects on the brain. Nutr Rev.. 2008;66:726–729.
Linnell, J.C., Bhatt, H.R. Inherited errors of cobalamin metabolism and their management. Baillieres Clin Haematol.. 1995;8:567–601.
McCann, J.C., Ames, B.N. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J.. 2008;22:982–1001.
Metz, J. Cobalamin deficiency and the pathogenesis of nervous system disease. Annu Rev Nutr.. 1992;12:59–79.
Morley, J.E. Nutrition and the brain. Clin Geriatr Med.. 2010;26:89–98.
Nelson, J.S. Neuropathological studies of chronic vitamin E deficiency in mammals including humans. Ciba Found Symp.. 1983;101:92–105.
Serdaru, M., Hausser-Hauw, C., Laplane, D., et al. The clinical spectrum of alcoholic pellagra encephalopathy. A retrospective analysis of 22 cases studied pathologically. Brain.. 1988;111:829–842.
Smolders, J., Damoiseaux, J., Menheere, P., et al. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol.. 2008;194:7–17.
Snodgrass, S.R. Vitamin neurotoxicity. Mol Neurobiol.. 1992;6:41–73.
Surtees, R. Biochemical pathogenesis of subacute combined degeneration of the spinal cord and brain. J Inherit Metab Dis.. 1993;16:762–770.
Thomson, A.D. Mechanisms of vitamin deficiency in chronic alcohol misusers and the development of the Wernicke-Korsakoff syndrome. Alcohol Alcohol Suppl.. 2000;35:2–7.
Ueda, K., Takada, D., Mii, A., et al. Severe thiamine deficiency resulted in Wernicke’s encephalopathy in a chronic dialysis patient. Clin Exp Nephrol.. 2006;10:290–293.
Zuccoli, G., Gallucci, M., Capellades, J., et al. Wernicke encephalopathy: MR findings at clinical presentation in twenty-six alcoholic and nonalcoholic patients. AJNR Am J Neuroradiol.. 2007;28:1328–1331.