Varicose Vein: Current Management
Chronic venous insufficiency can be found in 15% to 20% of the population. The prevalence goes up to 50% if small telangiectasias are included [1]. Venous ulcers are observed in 2% of patients with chronic venous insufficiency, and the treatments of these ulcers alone carry a significant cost [2]. Several risk factors for the development of varicose veins have been identified, which include age, female gender, multiparity, family history, obesity, and job activities that involve prolonged standing. Obesity seems to be a risk factor only in women but not in men. Exercise activity seems to be protective in men but not in women. In at least one study, however, trunk varices were observed to be more prevalent in men [1].
Etiology
The etiology of chronic venous insufficiency is believed to involve one or a combination of the following: venous obstruction, valvular insufficiency, and calf muscle pump dysfunction. Valvular insufficiency is the most common cause, and most valvular insufficiency cases involve the superficial veins of the lower extremity. Deep vein thrombosis (DVT) is a primary cause of valvular insufficiency and obstruction in the deep system. Calf muscle pump dysfunction leads to the inability of the blood column to properly exit the lower extremity. Similar to all are stasis and persistent venous hypertension, which eventually result in the sequelae of chronic venous insufficiency [2].
Signs, symptoms, evaluation, and treatment
Common complaints of patients with chronic venous disease include pain, swelling, leg heaviness or throbbing, itching, and cramps. Skin changes (hyperpigmentation, eczema, lipodermatosclerosis, or atrophie blanche) and ulcer formation are seen in more advanced presentations of the disease. Varicose veins are defined as dilated (>3 mm) subcutaneous veins that are visible and palpable. These veins can elongate and have significant tortuosity. Varicose veins can be trunk varices or those limited to branches [2,3].
The evaluation of a patient with chronic venous disease should incorporate the CEAP classification, which classifies the patient’s disease severity based on clinical signs, etiology, anatomic location, and pathophysiology (Box 1) [4]. The severity of the patient’s symptoms is evaluated using the Venous Clinical Severity Score (VCSS) and it is the best determination of treatment effectiveness over time. The VCSS classifies disease severity based on pain, presence plus extent of varicose veins, edema, inflammation, skin pigmentation, as well as the number, size, and duration of ulcers. The use of compression stockings is also assessed within the VCSS [5].
Venous duplex ultrasonography (US) has become the primary diagnostic tool in the evaluation of chronic venous disease. Significant reflux is defined as reflux longer than 0.5 seconds with rapid release of distal compression, and greater than 1 second with the use of manual compression. US examination should also include the small saphenous vein because it can be a source of significant and clinically relevant reflux [2]. Duplex US plays a prominent role not only in the evaluation of patients before and after treatment but also during interventions as is discussed later. There are 4 different emerging treatment modalities for varicose veins. The traditional open surgery consisting of high ligation and stripping of the greater saphenous vein (GSV) has been largely replaced by either form of endothermal vein ablation in the United States or the use of foam sclerotherapy (FS) in Europe.
Radiofrequency ablation
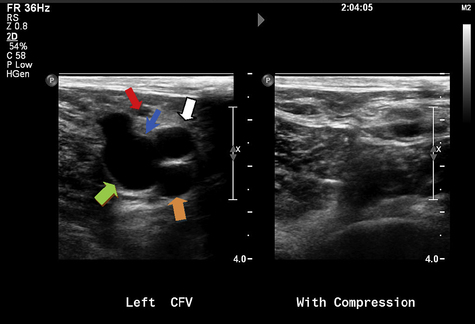
Preoperative duplex US is obtained to determine the presence of superficial saphenous vein reflux and the absence of DVT (Fig. 1). Duplex US is also helpful in preoperative planning of the length of vein to be treated and choice of catheter length to be used. The ClosureFAST system comes in either a 60-cm or 100-cm length. Treatment can certainly be done under local anesthesia and with monitored sedation, but general anesthesia can also be used to provide optimal pain control. The patient is positioned appropriately depending on the vein segment being treated, GSV (supine) or small saphenous vein (prone). During the initial phase of the operation, the patient is positioned in a reverse Trendelenburg position to dilate the veins and allow easy percutaneous access and guidewire placement. A scrubbed ultrasonographer is also present in the operating room to aid in mapping the GSV, to aid in proper catheter placement, and to confirm a successful intervention, but many interventionalists perform the intraoperative duplex imaging themselves.
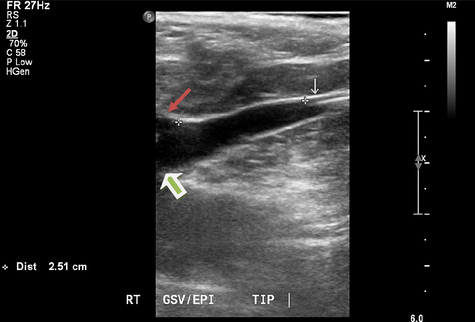
The intraoperative duplex imaging allows the surgeon to choose an appropriate segment of the vein for percutaneous placement of the 7F sheath. A standard Cook needle is used to access the vein under ultrasound guidance. If the access site involves a small caliber vein, a micropuncture needle can be used, and the microsheath is exchanged for the 7F sheath. Cutting down access to the saphenous is always an alternative. The 0.025-in guidewire is passed through the needle and into the common femoral vein (CFV) if easily advanced. The 7F sheath is inserted and a Bernstein catheter is used to help position the guidewire into the CFV if not previously positioned. The ClosureFAST catheter is introduced over the wire to below the superficial epigastric vein and 2 cm below the SFJ (Fig. 2). Appropriate positioning of the catheter tip is achieved with ultrasound guidance. The patient is then placed into the Trendelenburg position to decompress the vein. Tumescence anesthesia is injected into the saphenous compartment under ultrasound guidance. The RF catheter can be visualized within the vein by US and presents a confirmation of proper infusion and venous compression around the catheter. The recommended amount of tumescence used is 10 mL/cm of vein being treated, but, in reality, it is intended to fill the saphenous compartment and compress the vein around the catheter. The typical tumescence solution consists of lactated Ringer and buffered lidocaine with epinephrine (Box 2) [6]. The manufacturer recommends achieving a distance of at least 1 cm filled with tumescence fluid between the vein being treated and the skin. Tumescence serves several functions, including compressing the vein, preventing thermal injury to the skin, decreasing nerve injury, and providing local anesthesia.
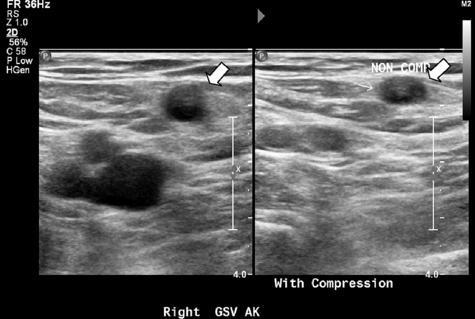
After injection of tumescence fluid, the first segment closest to the SFJ is treated twice. Significant external compression is applied during RF treatment to allow for maximal vein wall compression around the catheter electrode. The process is repeated in 7-cm lengths until the entire course of the vein has been treated. Careful recognition of the catheter markings must be observed to prevent thermal treatment within the sheath or near the skin surface as the last segment of vein is treated. The mechanism of action is heating of the vein wall, which essentially causes localized injury to the treated site. This process leads to the denaturation of the collagen matrix and eventual fibrotic sealing of the lumen. Both the duration of treatment and actual tissue temperature achieved determine the total vein shrinkage [6]. The ablation is then completed with a duplex US examination of the treated area to ensure that there is no thrombosis involving the SFJ or the deep veins (Fig. 3). Presence of DVT after this procedure requires acute treatment with anticoagulation. The device instructions for use discourages immediate retreatment of an acutely treated vein and recommends duplex US within 72 hours postprocedure for DVT detection. In general, the authors ask their patients to return within 1 week for follow-up and a repeat duplex US study. The patients are also instructed to maintain the compression dressing for at least 72 hours and refrain from strenuous activities or any heavy lifting; however, normal ambulation is encouraged.
The evolution of the RFA treatment of varicose vein is nicely detailed by Lohr and Kulwicki [6]. The initial use of endoluminal RFA for treatment of varicose veins was reported in Bern, Switzerland in 1998. This procedure was first combined with high ligation of the GSV. However, several studies later showed no statistical difference between combining RFA with saphenous vein high ligation and RFA alone in terms of recurrence or symptom improvement. In fact, there is some thought that maintaining pelvic drainage via the superficial epigastric vein is beneficial, and there may be less neovascularization without the addition of high ligation. Various modifications of this technique have been used since this initial experience. The use of tumescence anesthesia was introduced in 1999, and perivenous injection of tumescence fluid under ultrasound guidance later became routine. Access of the saphenous vein also evolved into the percutaneous technique. Target treatment temperature changed to the current recommended temperature of 120°C. As mentioned earlier, prior versions of the RFA catheter required a controlled pullback technique, which has been eliminated with the ClosureFAST catheter. RFA treatment of incompetent perforator veins is now available with the ClosureRFS Stylet (VNUS Medical Technologies, San Jose, CA, USA) [6].
Results
RFA treatment of varicose veins is quite effective. The reported immediate success rate is up to 98% [6,7]. In one study using an earlier version of the Closure catheter, the success rate was 93% and absence of reflux was documented in 90% of treated limbs at 2 years. The same study reported a satisfaction rate of 95% among treated patients at 2 years [8]. The 5-year follow-up data revealed absence of reflux in 83.8% of treated limbs and vein occlusion rate of 87.2%. Those patients deemed to have anatomic failure (groin reflux or flow in a segment of the treated vein) still had a clinical improvement rate of up to 80% [9]. Proebstle and colleagues [10] published the first clinical experience with the ClosureFAST catheter and found an occlusion rate of greater than 99% up to 6 months posttreatment. Another study using the ClosureFAST showed an occlusion rate of 97% of the GSV trunk at 1 year [11].
Anatomic failures are classified into 3 types. Type I failures are those veins that never occluded and remained patent during follow-up. Type II failure involves recanalization of an initially occluded vein. Type III failures involve the presence of reflux at the groin as seen on duplex US with an occluded vein trunk. A patent accessory vein likely contributes to the groin reflux noted in the later cases. Recurrence of visible varicose veins is the risk in each situation [12].
RFA compares favorably to stripping and vein ligation. In a recent randomized trial of RFA versus high ligation and stripping, Subramonia and Lees [13] report that RFA-treated patients had less pain, greater satisfaction, and increased quality of life compared with the surgical group. The investigators used the ClosurePLUS (VNUS Medical Technologies, San Jose, CA, USA) RFA catheter system with tumescent anesthesia. The median number of days that the patients in the RFA group required to return to work was 10 days versus 18.5 days for those in the surgery group. The median number of days that patients in the RFA group took to return to normal activity was 3 days versus 12.5 days for those in the surgery group. These differences were statistically significant, favoring the RFA group. Cutaneous sensory complaints were also higher in the surgery group. The RFA procedure took longer to perform than conventional surgery, but this was thought to be due to the use of tumescent anesthesia and extensive duplex imaging.
The aforementioned findings confirmed results from earlier studies. In 2003, Lurie and colleagues [14] reported the results of a prospective randomized trial between RFA and conventional surgery for the treatment of varicose veins. The overall complication rates, including ecchymosis and hematoma formation, were lower for the RFA group compared with the conventional surgery patients. The RFA patients were also able to return to normal activity and work earlier than the surgery group. However, the paresthesia rate was more common from those patients who underwent RFA, which may have been because of lack of tumescent anesthesia use in this study. In a 2-year follow-up, the RFA group maintained better quality-of-life measurements and pain scores than the surgery group. The recurrence rate at 2 years was 14% for the RFA patients compared with 21% in the surgery patients; this difference was not statistically significant [15].
Complications
Described complications after RF procedures include DVT, pulmonary embolism (PE), superficial thrombophlebitis, paresthesia, skin burns, hematoma formation, ecchymosis, and seroma formation. Access-related problems can certainly occur, and direct cutting down of the vein to gain access can be done. The use of tumescent anesthesia has decreased the incidence of skin burn and nerve injury. Limiting the treatment to mostly the above-knee segment of the GSV should minimize nerve injury. Saphenous nerve injury is most commonly noted when the below-knee GSV is treated because this is the location where the saphenous nerve travels in close proximity to the vein. Perhaps the most significant complications of RFA are PE and DVT, therefore, repeat duplex US in the perioperative period is highly recommended [6,12]. Extension of thrombus into the CFV, also known as endovenous heat-induced thrombus (EHIT), is a widely recognized complication of endothermal vein ablation. Kabnick and colleagues [16] described 4 different types of EHIT (Box 3) based on location, size, and vein patency. The investigators recommended treatment of EHIT 2 to 4 with anticoagulation, whereas EHIT 1 can be relegated to observation and close follow-up. There is recent evidence that a prior history of DVT can significantly increase the risk of new DVT formation after treatment with RFA [17].
Endovenous laser surgery
Approval of endovenous laser surgery (EVLS) by the Food and Drug Administration (FDA) for treatment of varicose veins came in 2002. Although great and small saphenous veins are common targets, perforator veins can also be treated using a dedicated laser system. There are several different companies that supply a variety of laser generators. The available wavelengths for treatment range from 810 nm to 1470 nm [18]. Most recently, there is report of a 1500-nm wavelength laser generator that, similar to other higher-wavelength devices, should preferentially allow absorption by the water molecules in the vein wall cells rather than by blood [19]. The contraindications to the use of EVLS are similar to those of RFA (Box 4). Relative contraindications may include the inability to traverse the vein segment (tortuosity and prior treatment), ineffective treatment (large vein diameter), and increased risk of DVT (hormone replacement therapy [HRT]). Anticoagulation therapy may increase the risk of vein recanalization [18,20,21].
The mechanism of action of endovenous laser therapy involves causing thermal injury to the vein wall endothelium. Steam bubbles are generated at the tip of the laser fibers, and there is a direct correlation between the amount of steam bubbles produced and the laser energy being used. The delivery of 60 to 100 J/cm of vein treated is thought to be both safe and effective. The total energy delivered per centimeter of vein is directly related to the power used and the pullback velocity. Similar to RFA, it is important to empty the vein segment being treated of blood by placing the patient in the Trendelenburg position. Although heated blood has been suggested to result in the homogenous distribution of thermal injury, the presence of blood within the segment being treated may actually prevent the generation of adequate levels of injury to the vein wall [18,20,21].
As with RFA, treatment of the GSV is generally limited to the above-knee portion because of the increased risk of nerve injury associated with treatment of the below-knee segment. Concurrent stab phlebectomies and sclerotherapy can be performed to address more superficial varicosities. The procedure can be performed under sedation, and patient positioning depends on the vein being treated. The patient may be placed prone or in a lateral position when treating the small saphenous vein. There are also commercially available devices for endovenous laser ablation treatment of perforator veins. After sterile preparation and draping, the course of the saphenous vein is mapped with US. An appropriate access site is chosen with the use of US, and the vein is accessed with a micropuncture needle. A small amount of nitropaste can be used to affect local vasodilation to facilitate the access. In certain situations, a small incision can be made and the vein can be elevated out of the subcutaneous space using a hook or a clamp. A guide wire is advanced through the needle, and a microsheath is introduced over the wire. This microsheath is later exchanged for a treatment sheath, which is usually included with the manufacturer’s kit. The size of the sheath varies depending on the system being used. The tip of the sheath is placed 15 to 20 mm distal to the SFJ below the superficial epigastric vein, and the laser fiber is then advanced through the sheath. The laser fiber is marked such that it protrudes from the sheath for a length sufficient to prevent laser damage to the sheath during activation and treatment. Similar to RFA, tumescent anesthesia is injected into the saphenous compartment under ultrasound guidance. The patient is placed in the Trendelenburg position, and the laser is activated to start the treatment process. Manual pressure should be applied to the area of the saphenous vein near the SFJ during initial treatment and over the length of the vein as the laser advances through it. The ideal pullback speed is 1 to 3 mm per second with the goal of delivering 60 to 80 J/cm of vein being treated. The proximal portion of the vein should be treated slower (closer to 1 mm/sec) than the more distal aspect. Circumferential compression dressings are placed on the affected limb from the ankle to the groin for the next several days. The activity limitations are similar to those in the RFA-treated patients [18,20].
Results
The occlusion rate for EVLS ranges from 77% to 100%, but the published studies differ in the type of laser wavelength and follow-up in addition to other factors. Recanalization rates in the literature also vary. Disselhof and colleagues [22] used an 810-nm laser and reported a recanalization rate of 16% at 3 months, but none at 1- and 2-year follow-up. In this study, there were 85 patients with 100 limbs treated. The patients were symptomatic with ultrasound-proven GSV reflux. The recanalization rate at 3 months was 16%, but only about half of these patients required surgical retreatment. The remainder of the recanalized limbs were asymptomatic at 1- and 2-year follow-up. Those with successful ablation initially did not have recurrent reflux or recanalization at 1 and 2 year follow-up. The investigators determined that a history of superficial thrombophlebitis involving the saphenous vein may result in the inability to traverse the vein. They also pointed out that occlusion rates are directly related to the amount of energy delivered and that a continuous mode with slower pullback speed should improve the occlusion rate. The goal of the treatment is to achieve nonthrombotic occlusion secondary to vein wall injury.
A study by Proebstle and his colleagues [23] found an early recanalization rate of less than 10%. The administration of anticoagulation as well as body mass index greater than 35 kg/m2 may have increased the risk of early recanalization, although the difference did not achieve statistical significance. A higher-wavelength laser theoretically allows for preferential heat absorption by the water molecules in the vein wall, thus causing a more direct thermal wall injury improving the vein occlusion rate. Using a 1500-nm laser with a continuous method of energy delivery versus the typical pulsed laser treatment, Vuylsteke and associates [19] achieved 93.3% occlusion rate at 6 months. When comparing their patient cohort with another group of patients treated with a 980-nm diode laser in a pulsed fashion, the investigators found similar occlusion rates. The complication rates, however, favored the 1500-nm laser.
Complication
Access-related complications include the inability to place the needle percutaneously, thus requiring a small cutting down for vessel access. Venous anatomy at times may prevent a smooth advancement of the wire or catheter or render the vein segment completely nontraversable. In cases of tortuous veins with large branches, intraoperative US may ensure that the main axial trunk is being treated versus large collaterals. Other possible complications include dysrhythmias, ecchymosis, anesthesia, hyperesthesia, infection, phlebitis, skin burn, and discoloration. Lymphatic leak at the site of the venous access has also been reported. Ecchymosis is essentially self-resolving but has been reported to occur in 23% to 100% of patients treated with EVLS. Both vein wall perforation and injection of tumescent anesthesia are believed to cause ecchymosis postoperatively. Saphenous nerve injury occurs more commonly when the below-the-knee segment of saphenous vein is included in the treatment. Avoidance of this area and liberal use of tumescent anesthesia can help protect from nerve injury. Superficial thrombophlebitis occurs between 1% and 12% of the cases and is most commonly managed with nonsteroidal medication, compression, and ambulation [18–22].
DVT is a serious adverse event after EVLS. The reported rate of DVT is up to 7.7% of cases [24]. A follow-up venous duplex US should be performed within 3 days or so of the operation. The classification and treatment of thrombus within the unablated portion of the saphenous vein near the SFJ (EHIT) are detailed earlier [16]. Some investigators have observed these thrombi to undergo retraction within a period of about 1 week. Combined with the fact that there have not been any reports of PE in the patients treated with EVLS, some investigators have suggested that anticoagulation treatment in these patients may not be required. However, close and frequent follow-up with duplex US must be done if one takes this approach [18]. Those patients with a prior history of DVT or thrombophilia may be at higher risk of DVT after EVLS, and prophylactic treatment with low–molecular-weight heparin may be necessary in the postoperativeperiod [25]. Patients older than 50 years may also be at a higher risk of DVT formation after EVLS and, some investigators believe, should be treated with perioperative thromboprophylaxis [26].
RFA versus EVLS
There are 2 recent randomized controlled trials comparing RFA ClosureFAST with EVLS. Almeida and colleagues [27] reported a multicenter single-blinded randomized clinical trial comparing RFA using the ClosureFAST system with a 980-nm laser system in continuous energy delivery mode. The inclusion criteria include venous reflux consistent with reversal of flow on duplex US for more than 0.5 seconds during compression and age between 18 and 80 years. Those who have known malignancy, previous GSV treatment, and thrombus in the vein being treated and those who were pregnant or on anticoagulation were excluded. Tumescent anesthesia was used. RFA and EVLS treatment followed the standard protocols with an energy delivery of 80 J/cm in the EVLS group. Compression dressings were applied after the procedure, and the patients were told to wear compression stockings for 2 weeks postoperatively. Follow-up visits were scheduled at 48 hours, 1 week, 2 weeks, and 1 month after procedure. Patients treated with RFA had less pain and tenderness at 48 hours, 1 week, and 2 weeks postoperatively than those in the EVLS group. The differences during these time points reached statistical significance. The RFA group also reported less pain and tenderness at 1 month after the procedure; however, this difference did not reach statistical significance. Ecchymosis defined as a percentage of the treated area was also significantly less in the RFA group at all time points during the follow-up period. Complications of the procedures included hyperpigmentation, phlebitis, infection, erythema, paresthesia, and thromboembolism. These complications were more common in the EVLS group. There were also more individuals in the EVLS group with multiple complications compared with the RFA group. As can be expected from the improved pain and complication rates, the RFA patients also had better quality-of-life scores at all time points up to 1 month postprocedure. At 1-month postprocedure, both groups had similar scores. The occlusion rate for both procedures was 100% throughout the entire follow-up period. This study was particularly designed to look at the pain and complication profiles after each procedure, and it seems that RFA with the ClosureFAST offers less complications and improved pain symptoms compared with EVLS during the immediate postoperative period.
Shepherd and colleagues [28] also conducted a single-blinded randomized controlled trial between the ClosureFAST catheter and a 980-nm laser endovenous treatment performed at a single center. Concomitant stab phlebectomies were preformed on those patients with branch varicosities. Tumescent anesthesia was used, and the laser treatment was delivered in a continuous mode at greater than 60 J/cm of energy per vein treated. Compression bandage was applied immediately, then exchanged for thromboembolic deterrent stockings before discharge. The patients were encouraged to maintain continuous use of the stockings for 1 week. The pain scores at 3 and 10 days were better for the RFA group. These patients also took less pain medication. The difference, however, did not translate to better quality-of-life scores or earlier return to work for the RFA group. The assessment of quality of life and symptom severity was determined only once at 6 weeks postoperatively. There was no difference in the complication rates between the 2 groups, unlike the previous study discussed, but ecchymosis was not included in this report. There was 1 reported PE case in the RFA group in a patient without evidence of DVT and 1 patient with lymphatic leak at the access site in the EVLS group. PE has only been reported in the RFA literature, but the incidence of DVT is generally accepted to be similar in the RFA- and EVLS-treated patients.
Goode and associates [29] performed a randomized study using an 810-nm laser and the Olympus Celon Rfitt (Olympus, Teltow, Germany) RFA system. Both pain and bruising were significantly less in the RFA group. The quality-of-life scores and period of return to activity were similar in both groups. The rates of vein occlusion at 10 days and 9 months were also similar between the 2 treatments. In another study comparing EVLS and an older version of the RFA Closure catheter, the recanalization rates for EVLS and RFA were 1.7% and 5.5%, respectively. The primary occlusion rate was 85% for RFA and 92% for EVLS at 500 days in a Kaplan-Meier life table analysis [30]. Similarly, Gale and colleagues [31] reported a randomized controlled trial comparing an 810-nm laser and the ClosurePLUS catheter, which is also an older version of the current Closure catheter system. The investigators found the rate of ecchymosis to be significantly less in the RFA group at 1 week, but the difference disappeared by 1 month. There were also more recanalized veins in the RFA group at 1 month. The recanalization, however, did not affect the patients’ quality-of-life and symptom severity scores because most of the anatomic failures in both groups had improved scores at 1-year follow-up. In comparing the RFA and the EVLS group, severity scores demonstrated greater improvement in the RFA group at 1 week postoperatively. By 1 year, however, there was no difference in the score improvement between the 2 groups. Quality-of-life scores improved after the procedures in all patients, and there was no difference between the treatment groups. The investigators also discovered that there was a subjective preference for the RFA procedure at 1 month among those with bilateral disease who received both treatment modalities. This preference did not exist at the 1-year follow-up.
Sclerotherapy
Sclerotherapy has been used to treat small and isolated branch varicosities as well as telangiectasias for many years. The development of FS has allowed for the treatment of larger-diameter veins including, the GSV and small saphenous vein. This technique only needs needle access to the vein or a branch that flows to the vein that is to be treated to be a viable treatment option. By moving the extremity above or below the heart, the foam can be directed cephalad or distal. Compression is also used to help control the movement of the sclerotherapy agent. The currently approved sclerosing agent in the United States is sodium tetradecyl sulfate, whereas polidocanol has also been widely used in Europe. These are surfactants that form foam when combined with air in a syringe [32]. The foam displaces blood, permitting contact between the sclerosant and the vein wall endothelium. Varying concentrations of surfactant can be used (0.25%–3%) depending on the target vein. The actual preparation technique consisting of manual mixing of sclerosant with air in a syringe produces a wide range of foam bubble sizes, thus, significantly altering the concentration of sclerosing agent being delivered. Injection of foam sclerosant into the vein is accomplished with ultrasound guidance. In Europe, FS has become a common treatment of both truncal and branch varicosities [33]. At present, there is no foam sclerosant agent that is FDA approved in the United States.
Box 5 lists some of the known complications of FS. Guex and associates [34] found that visual disturbances were the most commonly reported adverse event after FS. Jia and colleagues [35] reported the incidence of adverse events after FS, including PE, DVT, embolic stroke, and skin necrosis, to be less than 1%. The European Consensus Meeting on Foam Sclerotherapy published a series of recommendations regarding the use of FS in 2006. Some of these recommendations include varied volume of sclerosant based on the size of the vein being treated, with larger veins requiring higher concentrations and treating reticular veins with small volume and low concentration of sclerosant. Telangiectases and reticular veins should be treated with liquid sclerosant first, and small-volume/low-concentration FS is to be used only in case of ineffective initial treatment. The recommended maximum volume per session is 10 mL. A patient with symptomatic patent foramen ovale (PFO) is an absolute contraindication for FS, whereas a patient with a known asymptomatic PFO can be treated only if certain precautions are followed. Compression stocking should be applied only after waiting a few minutes (1–10 minutes) posttreatment and should be used for up to 4 weeks. Microthrombectomy (small skin incision to allow drain of the clot) may be necessary in superficial veins afflicted with thrombophlebitis after treatment to decrease the risk of hyperpigmentation [36].
Smith [37] reported an occlusion rate of 88% in GSV and 82% in small saphenous vein in patients with more than 6 months of follow-up. However, close to half of the patients required a second treatment to actually obliterate the target vein. A 5-year prospective study of ultrasound-guided FS showed 12% recurrence at 12 months and 64% recurrence at 5 years. Of the 64% recurrences, 47% had both recurrence of reflux in the target vein and reflux in new vessels seen by US, whereas another 17% had new vessel reflux only. The rate of clinical recurrence in this study was only 4%, and 100% of the patients believed the procedure to be a success [38]. FS may be of significant importance in treating elderly patients with nonhealing venous ulcers because many of these patients may not be able to tolerate more invasive procedures. Recurrent varicosities can also be treated with FS, especially in cases in which access for either RFA or EVLS is an issue [33]. There is also some evidence that RFA and EVLS become less suitable as patient age increases perhaps because of changes in venous anatomy [39]. Although there is no current FDA-approved foam sclerosant in the United States, Varisolve (BTG International Ltd, London, United Kingdom) is undergoing clinical trials in the United States. It is polidocanol endovenous microfoam with consistent bubble size, and initial studies showed the recurrence rate to be less than 10% with 90% of treated patients free of reflux at 3 months [40].
Transilluminated powered phlebectomy system
Powered phlebectomy has been offered as an option to the traditional stab phlebectomy for the treatment of branch varicosities. The TRIVEX System (Smith and Nephew, Inc, Endoscopy, Andover, MA, USA) is described as a powered phlebectomy system combined with a transilluminator and tumescent anesthesia infuser. The transilluminator cannula is first inserted through a small (2–3 mm) incision, and tumescent anesthesia is infused into the subcutaneous space adjacent to the target varicosities. The powered resector cannula is then inserted through a second small incision into the subcutaneous plane adjacent to the target vein but slightly more superficial than the transilluminator cannula. After resection of the varicosity, which is much like a directed liposuction, tumescent anesthesia is again infused to prevent hematoma formation. The reported complications of powered phlebectomy include ecchymosis, pain, hematoma, infection, swelling, paresthesia, and hyperesthesia [41].
Chetter and associates [42] reported a randomized clinical trial comparing manual phlebectomy and powered phlebectomy. Their results indicated that patients in the powered phlebectomy group had fewer incisions. However, these patients suffered from significant bruising, pain, and decreased quality-of-life scores compared with those who underwent stab phlebectomy. Recurrence has also been shown to be higher in the powered phlebectomy patients [43]. Patients seem to favor the traditional phlebectomy over powered phlebectomy if they are to undergo a repeat procedure [44]. These findings do not bode well for widespread application of this technique.