Recent Advances in the Diagnosis and Treatment of Gastrointestinal Carcinoids
Carcinoid tumors were first described by Lubarsch in 1888 [1]. In 1907, Oberndorfer [2] was the first to recognize these tumors as distinct from carcinomas and coined the term, Karzinoide, to describe the carcinoma-like appearance of these tumors as well as what was originally thought a relatively benign course. Since that time, the malignant potential of carcinoid tumors has become apparent. Currently, carcinoid tumors account for 0.49% of all malignancies [3]. Although these tumors are relatively uncommon, their incidence has been increasing. A recent database analysis of 13,715 carcinoid tumors revealed a 43.1% increase in carcinoid tumors compared proportionally with other cancers [3]. The most common location for carcinoid tumors is the gastrointestinal tract followed by the pulmonary system. Within the gastrointestinal tract, the highest frequency of tumors occurs in the small intestine followed by the rectum, colon, and appendix [3].
Etiology
Carcinoid tumors, derived from neuroendocrine (NE) cells, can secrete several active substances, including serotonin, corticotropin, histamine, dopamine, substance P, neurotensin, prostaglandins, and kallikrein [4]. There are at least 13 NE cell types within the gut, including enterochromaffin cells, which give rise to small bowel carcinoids, and the enterochromaffin-like gastric cells associated with certain types of gastric carcinoid [5]. In the World Health Organization classification updated in 2000, NE disease was classified based on histologic grade [6]. The distinction was made between well-differentiated NE tumors (benign disease), well-differentiated NE carcinoma (low-grade malignancy), and poorly differentiated NE carcinomas (high-grade malignancy) [7]. The term, carcinoid, applies to well-differentiated NE tumors and well-differentiated NE carcinomas.
Carcinoids can also be classified based on their embryologic origin and secretory products. Foregut tumors include those that arise from the respiratory tract, esophagus, stomach, and proximal duodenum. Tumors in this location typically produce low levels of serotonin [4]. Carcinoid tumors of the midgut include the distal duodenum, jejunum, ileum, appendix, and ascending colon and have a greater tendency to produce high levels of serotonin [8]. Hindgut carcinoid tumors arise from the distal colon and rectum. These tumors generally do not produce serotonin; however, they can produce other hormones, such as somatostatin, peptide YY, and 5-hydroxytryptophan (5-HTP) [8].
Gastric carcinoids are further divided into 3 subtypes. Type I gastric carcinoids account for 70% to 80% of gastric carcinoids and are associated with autoimmune-related pernicious anemia, atrophic gastritis, and parietal cell loss resulting in hypergastrinemia [9]. Type II gastric carcinoids are also associated with hypergastrinemia; however, in this situation it is related to Zollinger-Ellison syndrome and in some cases multiple endocrine neoplasia, type I. A duodenal gastrinoma is most often responsible for the hypergastrinemia in type II disease [9]. Type III gastric carcinoids are sporadic and not associated with an elevation in gastrin levels. Type I and II gastric carcinoids have an excellent prognosis whereas type III tumors tend to follow a more aggressive course and are often metastatic at the time of diagnosis [10].
Carcinoid syndrome
Carcinoid syndrome occurs in less than 10% of patients. Symptoms include diarrhea, bronchospasm, myopathy, arthropathy, and edema [7]. Cutaneous manifestations also occur and include flushing, pellagra, and scleroderma, of which scleroderma has also been shown to be a poor prognostic indicator [11]. Fibrosis can be associated with carcinoid syndrome and may affect retroperitoneal, pleural, pulmonary, dermal, and cardiac sites [12]. Carcinoid heart disease occurs in up to 70% of patients with carcinoid syndrome and, in many patients, results in death [13,14]. Carcinoid heart disease is typically right sided and is characterized by plaque-like deposits of fibrous tissue on the valvular cusps and leaflets as well as the right atrium and right ventricle [15]. Typically, carcinoid syndrome most commonly results from midgut tumors with metastatic disease, whereas foregut and occasionally hindgut tumors are more likely to produce an atypical carcinoid presentation [8]. Carcinoid tumor products that are suspected of contributing to carcinoid syndrome include serotonin, 5-HTP, histamine, dopamine, kallikrein, substance P, prostaglandin, and neuropeptide K. Although several drugs are available for the relief of various individual symptoms, severe carcinoid syndrome is best managed by medical therapy in combination with surgical treatment of metastatic disease. These treatment strategies are discussed later.
Diagnosis and staging
History and physical presentation
The majority of carcinoid tumors are asymptomatic and found incidentally during endoscopy or surgery. When symptoms do occur, they vary depending on the site of disease. Symptoms from gastric carcinoids are more likely to occur in type III rather than type I or II tumors and can include abdominal pain, gastrointestinal bleeding, and weight loss [16]. Patients with disease of the small intestine can present with symptoms of obstruction or ischemia, including abdominal pain and intermittent diarrhea [17]. Appendiceal carcinoid tumors are most likely to present as acute appendicitis [18]. The large diameter of the colon frequently results in the absence of symptoms until the tumor has reached an advanced state. When present, the most common signs and symptoms include weight loss, diarrhea, abdominal tenderness, rectal bleeding, and a palpable abdominal mass [19]. Rectal carcinoids are frequently detected during screening of asymptomatic patients. Complaints attributable to these tumors include rectal bleeding, localized pain, change in bowel habits, and weight loss [20].
Laboratory studies
In patients clinically suspected of having a carcinoid tumor, laboratory studies can aid in the diagnosis. Serotonin produced by carcinoid tumors is metabolized in the liver and lung and converted to 5-hydroxyindoleacetic acid (5-HIAA). An elevation of 24-hour urine 5-HIAA levels has a specificity of approximately 88%, although the sensitivity is significantly lower [21]. Chromogranin A, which is secreted by many NE tumors, has greater sensitivity and is elevated in more than 80% of patients with carcinoid tumor [22]. A combination of elevated serum chromogranin A and 24-hour 5-HIAA levels seems to provide the best diagnostic approach. Other biochemical markers that have been studied include bradykinin, substance P, neurotensin, human chorionic gonadotropin, neuropeptide K, and neuropeptide PP; however, these are more complex to measure and not as accurate as chromogranin A and 5-HIAA [7]. In the case of gastric carcinoids, the additional measurement of gastrin levels assists in determining type [23].
Imaging
Multiple imaging modalities are available for the evaluation of carcinoid tumors. CT scan is commonly used as an initial technique and has a sensitivity of approximately 80% [24]. Various protocols can increase the sensitivity, such as the use of intraluminal water, rapid intravenous contrast administration, multiplanar reconstructions, and, in the case of liver metastases, triple-phase contrast [25,26]. Single-photon emission CT (SPECT) and CT enteroclysis can also improve the accuracy of carcinoid tumor detection (Fig. 1) [26]. Features noted on CT scan include the presence of a polypoid intraluminal lesion, bowel wall thickening, and, in cases of mesenteric extension, a spiculated mesenteric mass [25]. MRI can be used as an alternative or adjunctive imaging modality; however, trials comparing MRI to CT have not demonstrated significant differences [24].
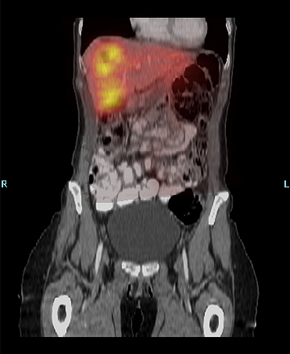
Fig. 1 SPECT/CT fusion study demonstrating carcinoid liver metastases.
(Courtesy of Dr Adrian Dawkins, Elizabeth Cheatham, Lexington, KT and Staci Allen, University of Kentucky, Lexington, KT.)
Somatostatin receptor scintigraphy can further characterize primary or metastatic disease. Somatostatin acts through membrane-bound receptors, 5 of which have been cloned and are designated somatostatin receptor types 1 through 5. Somatostatin receptor type 2 tends to be the most strongly expressed in neoplastic tissue [27]. Multiple analogs targeting these receptors are used in somatostatin receptor scintigraphy (eg, In-111 diethylenetriamine pentaacetic acid [DTPA]-octreotide, In-111 DTPA-lanreotide, and technetium Tc 99m depreotide). In-111 DTPA-octreotide, also known as OctreoScan, is the most commonly used agent for scintigraphy and has a reported sensitivity as high as 90% [24,28]. Indium In 111 DTPA-lanreotide has a lower sensitivity than its octreotide counterpart and is, therefore, generally not used for carcinoid tumors [29]. Technetium Tc 99m depreotide is approved specifically for lung cancer. It is less effective in the detection of abdominal carcinoid tumors due to the high level of background activity in the abdomen and the tracer’s short half-life [30].
Traditional fludeoxyglucose (FDG)-positron emission tomography (PET) is ineffective for carcinoid tumors due to their relatively slow rate of growth. PET has received increasing attention as a result of the introduction of multiple novel radionuclides, however, which may allow for more accurate imaging. Determining the effectiveness of these tracers has proved difficult due to the wide variety of available peptides, variations in production methods, and lack of adequately designed studies [30]. Radioactive carbon [11C]5-HTP and 18F-DOPA are 2 recently introduced radioligands that are reported as more sensitive than somatostatin receptor scintigraphy. The instability of these products, however, necessitates their synthesis near the location where they are administered [28]. Radiogallium-labeled ligands also have promising characteristics, including their high affinity for somatostatin receptor types 2 and 5 and their rapid clearance from the circulatory system [31]. Two of these, [68Ga-DOTA0, Tyr3]octreotide and [68Ga-DOTA0,Tyr3]octreotate, have received particular attention due to their high affinity for somatostatin receptor subtype 2, their relative ease of production, and the existence of counterparts used in peptide receptor radionuclide therapy [28]. Overall, PET scanning can be considered for use in the diagnosis and staging of carcinoid disease; however, further studies are required to appropriately delineate the optimal technique and its exact role in clinical practice.
Barium studies are sometimes performed during the work-up of symptoms related to carcinoid disease, such as diarrhea or abdominal pain. The radiologic appearance of small bowel carcinoids in these studies is nonspecific. Features include submucosal nodules, barium-filled craters known as target lesions resulting from mucosal ulceration, and thickening of the bowel wall from tumor infiltration and ischemia [25]. The use of angiography and high-resolution ultrasound has been described; however, these techniques are not routinely performed due to the frequency of nonspecific findings [8]. Metaiodobenzylguanidine imaging has also been studied, but this method is less accurate than OctreoScan and generally not indicated in the diagnosis of carcinoids [32].
Surgical treatment
Surgical treatment of localized disease
The treatment of gastric carcinoid tumors is based on type (summarized in Table 1). Because of the excellent prognosis associated with types I and II gastric carcinoids, these tumors have increasingly been treated with more conservative measures. Regular endoscopic surveillance or endoscopic resection, which consists of polypectomy or endoscopic mucosal resection, is appropriate for tumors less than 1.0 cm in size [9,23]. Surgical resection should be considered if a tumor is greater than 1 cm or increasing in size, if there is concern for gastric adenocarcinoma on biopsy, or if a patient is unable to undergo endoscopic surveillance [23]. Surgery may consist of partial or total gastrectomy depending on the extent of tumor involvement [16]. In type I gastric carcinoids, antrectomy is an additional treatment option, particularly when multiple tumors make complete resection difficult [16]. Antrectomy removes the majority of G cells of the stomach, thus resulting in the reversal of enterochromaffin-like hyperplasia. This technique is effective in more than 80% of cases [33]. Type III gastric carcinoids are considerably more aggressive and should be treated with radical gastric resection and lymph node removal [16].
Table 1 Surgical treatment of gastrointestinal carcinoid tumors
| Stomach Type I or II | <1.0 cm | Endoscopic surveillance versus resection (polypectomy, EMR) |
| >1.0 cm, increasing size, suspicion of adenocarcinoma, or unable to undergo surveillance | Partial versus total gastrectomy; consider antrectomy for type I | |
| Type III | Any size | Radical gastric resection with lymphadenectomy |
| Duodenum | <1.0 cm 1–2 cm >2 cm |
Endoscopic resection Open, transduodenal excision Segmental resection versus pancreaticoduodenectomy; also consider for periampullary tumors |
| Small Bowel | Any size | Wide, en bloc resection |
| Appendix | <1.0 cm | Appendectomy |
| 1–2 cm | Appendectomy versus right hemicolectomy | |
| >2 cm | Right hemicolectomy | |
| Colon | <1 cm, no lymphatic invasion >1 cm or lymphatic invasion |
Endoscopic resection Colonic resection with lymphadenectomy |
| Rectum | <1 cm, no invasion beyond muscularis propria, no lymph node involvement | Endoscopic resection (EMR vs ESD) |
| 1–2 cm, unable to completely resect endoscopically | Transanal excision versus transanal endoscopic microsurgery | |
| >2 cm, invasion beyond muscularis propria, atypical histology, lymph node involvement | LAR, APR | |
| Advanced Disease | Liver metastases | Resect when feasible; consider RFA, hepatic artery embolization, radioembolization for unresectable disease |
| Mesenteric disease | Aggressive surgical resection/debulking |
Abbreviations: APR, abdominoperineal resection; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; LAR, low anterior resection; RFA, radio frequency ablation.
Duodenal carcinoid tumors are rare, which makes a standardized treatment protocol difficult to develop. Tumors less than 1 cm can generally be treated with endoscopic resection [34]. For tumors between 1 cm and 2 cm, achieving complete endoscopic resection may be more difficult; therefore, an open, transduodenal excision is often a more appropriate approach [34]. In a recent study, lymph node metastases were detected even in tumors less than 1 cm that were confined to the submucosa [35]. This did not necessarily correlate with distant metastatic disease or survival, however. Although the investigators agreed with treatment dictated by the size of the tumor, based on these findings, they argued that lymph node dissection should be performed for all patients with radiographic suspicion of lymph node involvement as well as in patients undergoing laparotomy. This approach was recommended regardless of tumor size on the grounds that the small sample size in available studies makes it difficult to determine the exact impact of lymph node metastases on outcome. An additional caveat to conservative management of small tumors is that periampullary tumors seem to behave more aggressively; therefore, more extensive resection may be warranted in these tumors [36,37]. Tumors greater than 2 cm are treated with a segmental resection or pancreaticoduodenectomy, depending on the location and extent of disease [34].
Small bowel carcinoids have a greater tendency to metastasize than many other carcinoids. Although there is an increasing incidence of metastasis as the size of the tumor increases, even small tumors less than 1.0 cm have been shown to have metastatic potential [38]. Therefore, carcinoid tumors of the small intestine should be managed with wide en bloc resection of the primary tumor as well as removal of the associated lymphatic drainage. Furthermore, small bowel carcinoids are frequently associated with both carcinoid and noncarcinoid synchronous tumors [3,39]. As a result, a thorough intraoperative examination should be performed to identify potential additional lesions.
Appendiceal carcinoids can be treated either by simple appendectomy or right hemicolectomy. The risk of metastatic disease with appendiceal carcinoids increases as the size increases. As a result, size plays an important role in procedure selection. Appendiceal carcinoids less than 1.0 cm are associated with minimal risk of metastasis [8]. Therefore, tumors of this size are adequately treated with simple appendectomy. Alternatively, carcinoids larger than 2 cm are associated with lymph node metastasis in 25% to 50% of patients [4,40]; these lesions are treated with a right hemicolectomy so as to include the associated lymphatic drainage. Controversy exists regarding the extent of surgery for appendiceal carcinoids that are 1 cm to 2 cm in size. In a large study of 150 patients with appendiceal carcinoids, there was no evidence of metastases in the 127 patients with tumors smaller than 2 cm [40]. Select studies have shown rare lymph node involvement, however, in appendiceal carcinoids 1 cm to 2 cm in size, particularly in cases involving the mesoappendix, causing some investigators to argue for right hemicolectomy [41,42].
Patients with a colonic carcinoid tumor generally develop symptoms later than carcinoids of other locations, owing to the large diameter of the colon. This later presentation has typically coincided with more advanced disease, an occurrence that may be partially mitigated by an increase in the prevalence of screening colonoscopy [8,19,43]. In a recent study, colonic carcinoids less than 1 cm in size and without evidence of lymphatic invasion on pathology did not have any lymph node metastases. When one of these risk factors was present (ie, size >1 cm or lymphatic invasion), the incidence of lymph node metastasis was 16%; when both risk factors were present, the incidence increased to 77% [44]. Therefore, it is reasonable that colonic carcinoids smaller than 1 cm may be treated with endoscopic resection. If a lesion is greater than 1 cm in size or there is evidence of lymphatic invasion after endoscopic removal, colonic resection with lymphadenectomy should be performed.
Small rectal carcinoids, especially those less than 1 cm without evidence of muscular invasion or lymph node metastasis, can be managed by endoscopic resection [45]. A high percentage of rectal carcinoid tumors extend into the submucosa; therefore, a polypectomy often does not result in a complete resection [46]. Endoscopic mucosal resection, particularly in tumors less than 5 mm in size, can achieve a complete resection [45]. Endoscopic submucosal dissection is a technique that is potentially even more effective in achieving total removal; however, it may be associated with a higher rate of perforation [45,47]. Rectal carcinoids up to 2 cm in size that are unable to be completely resected by endoscopy can be managed by local excision. Transanal excision is a technique that has commonly been used for local resection and is associated with low morbidity and good outcomes [48,49]. Transanal endoscopic microsurgery is a more recently developed alternative that may offer several advantages, including fewer positive margins and the ability to excise lesions located higher in the rectum [50]. Rectal carcinoids larger than 2 cm, invading beyond the muscularis propria, displaying atypical histologic features, or having evidence of lymph node involvement are associated with a poorer outcome [3,20]. In general, these tumors are managed by low anterior resection or abdominoperineal resection, although several retrospective studies have not shown a survival benefit [20,51,52].
Surgical treatment of metastatic disease
Resection of hepatic metastases is often palliative in symptomatic patients and may even improve survival, especially in cases where greater than 90% of the disease is removed [53–55]. Hepatic resection can be performed simultaneously with operation for the primary tumor while maintaining similar complication rates when compared with a staged procedure [53,56]. Although resection is safe and effective, disease recurs in approximately half of patients after their first liver operation [54]. Recurrent disease may be treated with repeated resection; however, there is a greater chance of recurrence with each additional procedure. In one study, 90% of patients undergoing their third liver operation experienced a recurrence [54]. This lack of durable disease control should be kept in mind when contemplating reoperation. Cholecystectomy should also be considered at the time of operation due to the possibility of cholelithiasis associated with subsequent somatostatin therapy [17,56].
Hepatic artery embolization, radiofrequency ablation (RFA), and radioembolization are alternative treatments for unresectable disease. Hepatic artery embolization has a response rate of over 50%; however, the duration of these results tends to be limited [57,58]. RFA can be used for smaller lesions in cases with unresectable or recurrent hepatic disease [54,59]. Radioembolization, which uses radioactive isotopes that are delivered to the tumor through the hepatic artery, may be useful as well. In the largest series to date, radioembolization resulted in a partial response (decrease in size on imaging of at least 30%) in 60.5% of patients and a complete response (disappearance of all known lesions confirmed at 4 weeks) in an additional 2.7% of patients [60]. Liver transplant has been suggested as a therapeutic option, but the recurrence rate seems prohibitively high. Currently, transplantation should not be performed except in the context of an investigational trial.
Surgical intervention for mesenteric involvement is often beneficial as well. In a study of 75 patients with advanced abdominal carcinoid tumors, median survival was 139 months in those treated with surgical debulking as compared with only 69 months without debulking [61]. In patients presenting with symptoms of small bowel obstruction or ischemia, surgery can also result in significant improvement and sometimes even complete resolution of symptoms [17,62]. Caution should be exercised when attributing symptoms to nonoperable disease. Symptoms thought to be related to recurrent, terminal disease may actually be the result of adhesions from prior surgery that are treatable with adhesiolysis [63]. Additionally, abdominal pain and intermittent diarrhea resulting from small bowel obstruction or ischemia can be misinterpreted as carcinoid syndrome and are actually treatable with operative intervention [17,62]. Moreover, encasement of mesenteric vasculature is not an absolute contraindication to surgery because this is often a constrictive entrapment rather than invasion of the vessel wall and can be relieved with careful dissection to free the entrapment [62,63]. In cases where resection is unable to be performed, intestinal bypass can be considered for relief of obstruction [17,56].
Carcinoid heart disease can occur in the presence of metastatic tumors and is associated with significant morbidity and mortality. The effects are typically confined to the right side of the heart and frequently consist of stenosis or regurgitation of the tricuspid and pulmonary valves [15]. Medical therapy is generally ineffective in the treatment of cardiac disease; however, surgical intervention can result in functional improvement and may potentially increase survival [64,65]. Surgical indications include development of cardiac symptoms, increasing right ventricular size, decreasing right ventricular function, and anticipation of major hepatic resection in patients with elevated systemic venous pressures secondary to tricuspid or pulmonary valve dysfunction [65]. The optimal timing of surgery remains to be determined.
Medical treatment
Somatostatin analog therapy
In general, somatostatin can be considered the universal off-switch in the body. Somatostatin treatment inhibits the release of the majority of intestinal hormones and decreases secretion and intestinal motility. These effects are mediated through the binding to one of the 5 G-protein–coupled somatostatin receptors [66]. Somatostatin receptors are found in the majority of carcinoid tumors [67]; this has led to the successful use of somatostatin analogs in the treatment of carcinoid disease. Multiple somatostatin analogs are clinically available (Table 2). Octreotide, a relatively long-acting somatostatin analog with a half-life of approximately 1.5 hours, is safe and effective in the relief of symptoms related to carcinoid syndrome, and should also be administered before invasive procedures to prevent carcinoid crisis [68]. Octreotide LAR provides a more convenient dosing regimen and is administered by an intramuscular depot injection every 28 days as compared with every-8-hour subcutaneous injections for octreotide [69]. Lanreotide, an alternative treatment option to octreotide, is a longer-acting somatostatin analog with a half-life of 2.5 hours and similar efficacy to octreotide [69,70]. A depot preparation, known as lanreotide Autogel, has been developed and also requires only monthly dosing. Pasireotide has a half-life of 12 hours and a broader receptor binding profile with high affinity for somatostatin receptors 1, 2, 3, and 5 [71]. This broader spectrum may make it effective in disease refractory to other somatostatin analogs [71]. In addition to the use of somatostatin analogs for treatment of symptoms related to carcinoid syndrome, these compounds might also have antiproliferative effects. A recent double-blind, randomized controlled trial showed that octreotide LAR significantly increased time to tumor progression compared with placebo [72]. Interferon alpha has been evaluated, both alone and in combination with somatostatin analogs, but the side effects are substantial and the benefit remains controversial [70].
| Drug | Binding affinity | Dosing |
|---|---|---|
| Octreotide | Highest affinity for 2 and 5 | Every 8 hours |
| Octreotide LAR | Highest affinity for 2 and 5 | Monthly |
| Lanreotide | Highest affinity for 2 and 5 | Every 10–14 days |
| Lanreotide Autogel | Highest affinity for 2 and 5 | Monthly |
| Pasireotide | 1, 2, 3, and 5 | Twice daily |
Encouraging clinical results have been demonstrated for radiolabeled somatostatin analogs. In-111 octreotide, which is commonly used in somatostatin imaging, was one of the first radiolabeled analogs to be studied as a therapeutic agent; however, evidence of its ability to decrease tumor size was minimal [73,74]. The introduction of β-emitting radionuclides was met with greater success. Yttrium-90-labeled somatostatin analogs were the first such radionuclides to be developed and demonstrated tumor regression of 50% or more in up to 33% of patients [75]. Lutetium-177-labeled analogs have since become available. In a study of 310 patients with gastroenteropancreatic NE tumors treated with this analog, complete response was achieved in 2% of patients and partial response (at least a 30% reduction in tumor size on subsequent imaging) in an additional 28% [76]. Side effects of these radionuclide-labeled analogs included renal insufficiency, hematological toxicity, and hepatic toxicity, although the renal effects can be minimized with the use of renal protective strategies [28].
Cytotoxic chemotherapy
Cytotoxic chemotherapy has been met with only limited success. Single-agent therapies, such as 5-fluorouracil, streptozocin, and doxorubicin, are associated with response rates of approximately 20% [77]. Combination therapy has only modestly improved their effectiveness. Several streptozocin-based combination therapies have been evaluated with response noted in up to one-third of patients. Their usefulness is limited, however, by toxicity and any survival benefit is questionable [77,78]. Other agents, such as dacarbazine, paclitaxel, docetaxel, and gemcitabine, have been ineffective as well [77].
Molecular targeted therapy
Given the generally poor response to cytotoxic therapy, efforts have been made to identify specific molecular targets that can be used for therapy in carcinoid tumors. Therapeutic targets include the inhibition of tyrosine kinases, angiogenesis, and the phosphatidylinositol 3-kinase (PI3K)-Akt-mTOR pathway. Receptor tyrosine kinases identified as potential targets include epidermal growth factor receptor, stem cell factor c-KIT, and platelet-derived growth factor receptor [79]. Gefitinib, a small molecule inhibitor of epidermal growth factor receptor, is one such drug that has demonstrated modest clinical benefit. In a phase II clinical trial including 40 patients with carcinoid tumors, 61% showed progression free survival at 6 months [80]. There was only one partial response (>30% decrease in size) and one minor response (20%–29% decrease in size) in this study. Imatinib, another tyrosine kinase inhibitor, has shown disappointing results in phase II clinical trials thus far [79].
Antiangiogenic therapies are also an attractive option for carcinoid tumors, owing to their vascular nature and tendency to express proangiogenic molecules, such as vascular endothelial growth factor (VEGF) [81]. Multiple antiangiogenic compounds have been developed and work through a variety of mechanisms. These include inhibition of VEGF, targeting of intracellular domains of VEGF receptors, and use of antiangiogenic mechanisms unrelated to VEGF [82]. Bevacizumab, an anti-VEGF monoclonal antibody, is one such drug that has shown promise in clinical trials. In a phase II trial of 44 patients with advanced carcinoid tumors on stable doses of octreotide randomized to either additional therapy with bevacizumab or pegylated interferon alpha-2b, those receiving bevacizumab had a progression-free survival of 95% at 18 weeks as compared with 68% in the alternate group [83]. The effectiveness of bevacizumab as well as other antiangiogenic therapies continues to be studied both as single agents and in combination with other available treatments [79].
Mammalian targets of rapamycin (mTOR) inhibitors, initially used for immunosuppressant therapy, are now being studied as antitumor agents. The PI3K-Akt-mTOR pathway plays a role in the regulation of cell growth, proliferation, motility, and survival as well as transcription and protein synthesis [82]. There are two mTOR complexes, designated mTORC1 and mTORC2. Rapamycin and its derivatives primarily exert their effects through inhibition of mTORC1. Drugs in this category include everolimus and temsirolimus. Everolimus, in particular, has shown promise in clinical trials. In a phase II study that included 30 patients with carcinoid tumors treated with everolimus in combination with octreotide LAR, 17% of patients demonstrated a partial response (>30% decrease in size) [84]. Phase III trials are currently under way.
Surveillance
According to the National Comprehensive Cancer Network guidelines [85], all patients should have a complete history and physical with associated imaging studies, such as a CT or MRI, within the first 3 to 12 months after resection and annually thereafter. Elevated chromogranin A levels may suggest recurrence and, therefore, can be used as a tumor marker during follow-up. Twenty-four hour urinary 5-HIAA levels may also be considered. Imaging studies should be performed as indicated based on clinical suspicion. In the case of gastric carcinoids with associated hypergastrinemia, surveillance includes a history and physical every 6 to 12 months for the first 3 years followed by annual examinations thereafter. Esophagogastroduodenoscopy may also be performed at similar intervals. Appendiceal and rectal tumors smaller than 1 cm do not require follow-up. For rectal tumors between 1 and 2 cm, proctoscopy should be performed at 6 and 12 months and then as indicated.
Prognosis
The 5-year survival rate for all carcinoid tumors, irrespective of location, is approximately 67% [3]. Carcinoid tumors localized to the primary site and able to be completely resected have a survival rate that approaches 100%. When metastatic disease is present, 5-year survival drops significantly, ranging from 39% to 60% [86]. As discussed previously, many other factors contribute to overall prognosis, including tumor size, location, depth of invasion, presence of carcinoid heart disease, and in some cases histologic subtype. Although advanced disease has a poorer outcome, many patients can still derive long-term benefit from treatment with regards to both palliation and survival.
References
[2] S. Oberndorfer. Karzinoide tumoren des dunndarms. Frankf Z Pathol. 1907;1:425-429. [in German]
[4] M.H. Kulke, R.J. Mayer. Carcinoid tumors. N Engl J Med. 1999;340(11):858-868.
[31] Y. Krausz, N. Freedman, R. Rubinstein, et al. (68)Ga-DOTA-NOC PET/CT Imaging of Neuroendocrine Tumors: comparison with (111)In-DTPA-Octreotide (OctreoScan(R)). Mol Imaging Biol. 2010. Available at: http://www.springerlink.com/content/6t7074318377645j/ Accessed March 16, 2011
[41] J.R. Anderson, B.G. Wilson. Carcinoid tumours of the appendix. Br J Surg. 1985;72(7):545-546.
[85] National Comprehensive Cancer Network (NCCN) Guidelines for neuroendocrine tumors. Available at: www.nccn.org, 2010. Accessed April 18, 2011